Abstract
Background
Yellow fever (YF) is an acute viral haemorrhagic disease prevalent in tropical Africa and Latin America. The World Health Organization (WHO) estimates that there are 200,000 cases of YF and 30,000 deaths worldwide annually. Treatment for YF is supportive, but a live attenuated virus vaccine is effective for preventing infection. WHO recommends immunisation for all individuals > 9 months living in countries or areas at risk. However, the United States Advisory Committee on Immunization Practices (ACIP) advises that YF vaccine is contraindicated in individuals with HIV. Given the large populations of HIV‐infected individuals living in tropical areas where YF is endemic, YF vaccine may be an important intervention for preventing YF in immunocompromised populations.
Objectives
To assess the risk and benefits of YF immunisation for people infected with HIV.
Search methods
We used standard Cochrane methods to search electronic databases and conference proceedings with relevant search terms without limits to language.
Selection criteria
Randomised controlled trials and cohort studies of individuals with HIV infection who received YF vaccine (17DD or 17D‐204).
Data collection and analysis
Two authors screened abstracts of references identified by electronic or bibliographic searches according to inclusion and exclusion criteria as detailed in the protocol. We identified 199 references and examined 19 in detail for study eligibility. Data were abstracted independently using a standardised abstraction form.
Main results
Three cohort studies were included in the review. They examined 484 patients with HIV infection who received YF immunisation. Patients with HIV infection developed significantly lower concentrations of neutralising antibodies in the first year post immunisation compared to uninfected patients, though decay patterns were similar for recipients regardless of HIV infection. No study patient with HIV infection suffered serious adverse events as a result of YF vaccination.
Authors' conclusions
YF vaccination can produce protective levels of neutralising antibodies in HIV patients. Immunogenicity of YF vaccine is slightly less in HIV‐infected patients compared to HIV‐uninfected patients. No serious adverse events related to YF vaccine were observed in HIV‐infected study participants. At time of immunisation, higher CD4 cell counts and lower HIV RNA levels in patients with HIV infection seem to be key determinants for development of protective titres of neutralising antibodies. The quality of the evidence for all outcomes was low to very low. YF vaccine may potentially be used safely in HIV‐infected patients, although our conclusions are limited by small numbers of patients who have been reported. To assure maximum effectiveness YF vaccine should be given to HIV‐infected patients after HIV replication has been suppressed.
Keywords: Adult; Child; Humans; Antibodies, Neutralizing; Antibodies, Neutralizing/immunology; Cohort Studies; HIV Infections; HIV Infections/immunology; Vaccination; Yellow Fever; Yellow Fever/immunology; Yellow Fever/prevention & control; Yellow Fever Vaccine; Yellow Fever Vaccine/administration & dosage; Yellow Fever Vaccine/adverse effects; Yellow Fever Vaccine/immunology
Plain language summary
Yellow fever vaccine for patients with HIV infection
In the United States of America, current guidelines do not recommend YF vaccine for individuals with HIV infection or AIDS; these recommendations, however, are targeted mostly at travellers to the parts of Latin America and Africa where YF occurs and who have the option of not going. For HIV‐infected patients living in these areas where exposure is inevitable, it is important to weigh the risks of vaccination against the risk of developing YF. There are no known medicines for YF, further highlighting the importance of vaccine. The purpose of this review was to assess the risks and benefits of YF vaccine for people living with HIV. We found three cohort studies that addressed this question. One study in children, from a time before effective widespread use of antiretroviral drugs, found that YF vaccine worked much less well in children with HIV than it did in those without HIV. Two studies in adults found that the immune response to yellow fever vaccine was slightly lower in HIV‐infected patients. No severe adverse events were observed in patients in these studies. However, because the numbers of people with HIV who have received YF vaccine is small, and serious side effects are uncommon in people without HIV infection, we are not positive about its safety. When it does need to be used, it should be given to people whose viral loads are low and CD4 counts are high.
Summary of findings
Summary of findings for the main comparison. Immunologic response to YF vaccine in HIV‐infected adults before HIV diagnosis, vs. immunologic response to YF vaccine in HIV‐infected adults after HIV diagnosis.
| Immunologic response to YF vaccine in HIV‐infected adults before HIV diagnosis, vs. immunologic response to YF vaccine in HIV‐infected adults after HIV diagnosis | ||||||
| Patient or population: Adults with HIV infection Settings: France Intervention: YF vaccine in HIV‐infected adults before HIV diagnosis, vs. YF vaccine in HIV‐infected adults after HIV diagnosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | YF vaccine in HIV‐infected adults before HIV diagnosis, vs.YF vaccine in HIV‐infected adults after HIV diagnosis | |||||
| Immunogenicity: Adequate antibody response (NT ≥1:10), follow‐up 1 yr | 962 per 1000 | 876 per 1000 (818 to 943) | RR 0.91 (0.85 to 0.98) | 364 (1 study) | ⊕⊕⊝⊝ low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Immunologic response to YF vaccine in HIV‐infected children vs. immunologic response to YF vaccine in HIV‐uninfected children.
| Immunologic response to YF vaccine in HIV‐infected children vs. immunologic response to YF vaccine in HIV‐uninfected children | ||||||
| Patient or population: Children with HIV infection Settings: Côte d’Ivoire Intervention: YF vaccine in HIV‐infected children vs.YF vaccine in HIV‐uninfected children | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | YF vaccine in HIV‐infected children vs.YF vaccine in HIV‐uninfected children | |||||
| Adequate antibody response (NT ≥1:10), follow‐up median 29 months | 737 per 1000 | 169 per 1000 (59 to 472) | RR 0.23 (0.08 to 0.64) | 75 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Pre‐antiretroviral era. 2 Very few participants. Grade down by 2.
Summary of findings 3. Immunologic response to YF vaccine in HIV‐infected adults vs. immunologic response to YF vaccine in HIV‐uninfected adults.
| Immunologic response to YF vaccine in HIV‐infected adults vs. immunologic response to YF vaccine in HIV‐uninfected adults | ||||||
| Patient or population: Adults with HIV infection Settings: Switzerland Intervention: YF vaccine in HIV‐infected adults vs.YF vaccine in HIV‐uninfected adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | YF vaccine in HIV‐infected adults vs.YF vaccine in HIV‐uninfected adults | |||||
| Immunogenicity: Adequate antibody response (NT ≥1:10), follow‐up 1 yr | 970 per 1000 | 834 per 1000 (747 to 931) | RR 0.86 (0.77 to 0.96) | 144 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Duration: Adequate antibody response (NT ≥1:10), follow‐up 1‐10 yrs | 880 per 1000 | 775 per 1000 (669 to 898) | RR 0.88 (0.76 to 1.02) | 162 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Retrospective analysis of a prospective cohort. Graded down by 1. 2 Very few participants. Graded down by 2.
Background
Yellow fever (YF) is an acute viral haemorrhagic disease prevalent in tropical Africa and Latin America (WHO 2013a). The World Health Organization (WHO) estimates that there are 200,000 cases of YF and 30,000 deaths worldwide annually. Treatment for YF is supportive, but a live attenuated virus vaccine is effective for preventing infection (WHO 2013b). WHO recommends immunisation for all individuals >9 months living in countries or areas at risk (WHO 2013a). However, in the United States of America the Advisory Committee on Immunization Practices (ACIP) advises that YF vaccine is contraindicated in individuals with HIV, although these recommendations are primarily aimed at Americans travelling to endemic areas (CDC 2010).
Presently, more than 35 million people are infected with HIV (UNAIDS 2013). In 2013, UNAIDS reported that about 25 million people living with HIV reside in sub‐Saharan Africa, while 1.5 million people living with HIV reside in Latin America, both largely tropical, often densely populated regions where YF is endemic (UNAIDS 2013). Given this context, YF vaccine may be an important intervention for preventing YF in HIV‐infected persons.
While YF vaccine is highly effective, the literature is sparse regarding its safety and effectiveness in patients with HIV infection. We review the risks and benefits of YF immunisation in HIV‐infected individuals and consider its use in preventing incident infection in patients infected with HIV.
Description of the condition
YF is an acute flavivirus infection primarily transmitted by mosquitoes. In jungles and forests in Africa, it is transmitted by Aedes africanus; in Latin America, it is transmitted by Haemagogus andSabethes species, with New World primates as the primary hosts (WHO 2013b).
Although jaundice is sometimes observed as a symptom of YF, it is often difficult to diagnosis as its clinical presentation is similar to viral hepatitis, malaria, leptospirosis, typhus and other hemorrhagic fevers (WHO 2013b).
There are no known effective medications for YF.
YF vaccine
17D vaccines (17DD and 17D‐204) are highly immunogenic and provide an estimated 40 years of protection. More than 600 million YF immunisations have been given worldwide. WHO recommends immunisation for all individuals from 9 months to 59 years of age. It discourages immunisation for individuals ≥60 years as a precaution against risk of severe adverse events.
Reports of severe adverse events caused by YF vaccine are rare. However, neurologic conditions such as encephalitis, myelitis, encephalomyelitis and viscerotropic conditions, such as multiorgan failure of the liver, kidneys and heart, have been reported (WHO 2013b). Since the initial cases of multiorgan failure were published in 2001, more than 50 confirmed and suspected cases of YF vaccine viscerotropic disease (YEL‐AVD) have been reported throughout the world (CDC 2010).
Description of the intervention
YF vaccine given to individuals infected with HIV.
Inclusion criteria
Randomised controlled trials, or observational cohort studies with comparators
Compares HIV‐infected and uninfected individuals who received YF vaccine
Compares baseline characteristics among HIV‐infected patients who failed to develop protective titres of YF neutralising antibodies as a result of YF vaccination to those who did develop protective levels of neutralising antibody
Exclusion criteria
Case reports and case series without comparison groups
How the intervention might work
The intervention works by stimulating humoral immunity (neutralising antibodies) to YF.
Why it is important to do this review
In light of the fact that YF is endemic in tropical Africa and Latin America where large populations of HIV‐infected individuals reside, identifying effective YF prevention strategies for people infected with HIV is critical to help prevent disease. Although the U.S. ACIP has stated that YF vaccine is contraindicated in individuals with HIV infection or AIDS, its recommendations are targeted predominantly at travellers from non‐endemic areas to endemic areas, who have the option of not going. For HIV‐infected patients residing in endemic areas in whom exposure is inevitable, it is important to weigh the risks of vaccination against the risk of developing YF.
Objectives
To assess the risk and benefits of YF vaccination for people infected with HIV.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials
Observational cohort studies with comparators
Types of participants
HIV‐infected individuals (males and females) between 6 months and 60 years old
Types of interventions
YF immunisation
Comparator
HIV‐uninfected patients
HIV‐infected patients with different baseline predictor variables
Types of outcome measures
Primary outcomes
YF
YF neutralising antibody titres (NT) ≥1:10
-
YF vaccine‐associated viscerotropic disease (YEL‐AVD)
Multiorgan failure of the liver, kidneys, heart, and circulation
-
YF vaccine‐associated neurologic disease (YEL‐AND)
Encephalitis
Myelitis
Encephalomyelitis
Secondary outcomes
•Mortality
Search methods for identification of studies
See search methods used in reviews by the Cochrane Collaborative Review Group on HIV Infections and AIDS.
Electronic searches
We formulated a comprehensive search strategy to identify all relevant studies regardless of language or publication status (published, unpublished, in press and in progress). Full details of the Cochrane HIV/AIDS Review Group methods are published in the section on Collaborative Review Groups in The Cochrane Library.
Journal Databases
We searched the following electronic databases from the period of 1 January 1980 through 23 December 2013:
CENTRAL (Cochrane Central Register of Controlled Trials)
EMBASE
PubMed
Web of Science
World Health Organization (WHO) Global Health Library (http://www.globalhealthlibrary.net), which includes references from AIM (AFRO), LILACS (AMRO/PAHO), IMEMR (EMRO), IMSEAR (SEARO), and WPRIM (WPRO)
Searching other resources
Conference Databases
We searched the Aegis archive of HIV/AIDS conference abstracts, which includes abstracts for the following conferences up to 2008:
Conferences on Retroviruses and Opportunistic Infections (CROI)
International AIDS Society, International AIDS Conferences (IAC)
International AIDS Society, Conferences on HIV Pathogenesis, Treatment and Prevention (IAS)
We searched the conference web sites for abstracts from 2008 through 2013. Additionally, we examined the references of included studies and reviews that we identified.
Data collection and analysis
The methodology for data collection and analysis was based on the guidance of Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008).
Selection of studies
One author (THH) performed a broad first examination of all downloaded material from the electronic searches to exclude citations that were plainly irrelevant. Two authors (HB, THH) read the titles, abstracts and descriptor terms of the remaining downloaded citations to identify potentially eligible reports. Full text articles were obtained for all citations identified as potentially eligible, and two authors (HB, THH) independently inspected these to establish the relevance of the article according to the pre‐specified criteria. If there was uncertainty about the eligibility of the record, the full article was obtained.
Two authors (HB, THH) independently applied the inclusion criteria, and any differences were resolved by discussion. Studies were reviewed for relevance based on study design, types of participants and outcome measures.
Data extraction and management
Two authors (HB, GWR) independently extracted data into a standardised, pre‐piloted data extraction form. The following characteristics were extracted from each included study:
Administrative details: trial identification number; author(s); published or unpublished; year of publication; number of studies included in paper; year(s) in which study was conducted; details of other relevant papers cited
Details of the study: study design; type, duration and completeness of follow‐up; location/orientation of study (e.g. higher‐income vs. low or middle‐income country; stage of HIV epidemic)
Details of participants: age range, sex, or sexual orientation if appropriate; clinical characteristic if appropriate, risk for HIV infection, risk for YF
Details of intervention: venue; stage of HIV infection when given YF vaccine
Details of outcomes
Details necessary for risk of bias or methodological quality assessment
Assessment of risk of bias in included studies
Two review authors (HB, GWR) independently assessed risk of bias for each study using the bias assessment tool described in the Cochrane Handbook (Higgins 2008). We resolved any disagreement by discussion or by involving a neutral third party.
The Cochrane approach assesses risk of bias in individual studies across six domains: allocation concealment, blinding, incomplete outcome data, selective reporting, sequence generation and other forms of potential bias.
Allocation concealment (checking for selection bias)
Adequate: participants and the investigators enrolling participants cannot foresee assignment
Inadequate: participants and investigators enrolling participants can foresee upcoming assignment (e.g., an open random allocation schedule, a list of random numbers), or envelopes were unsealed, non‐opaque or not sequentially numbered
Unclear: insufficient information to permit judgement of the allocation concealment or the method not described.
Blinding (checking for performance bias and detection bias)
Adequate: blinding of the participants, key study personnel and outcome assessor and unlikely that the blinding could have been broken. Not blinding in the situation where non‐blinding is unlikely to introduce bias.
Inadequate: no blinding or incomplete blinding when the outcome is likely to be influenced by lack of blinding.
Unclear: insufficient information to permit judgment of adequacy or otherwise of the blinding.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Adequate: no missing outcome data, reason for missing outcome data unlikely to be related to true outcome or missing outcome data balanced in number across groups.
Inadequate: reason for missing outcome data likely to be related to true outcome, with either imbalance in number across groups or reasons for missing data.
Unclear: insufficient reporting of attrition or exclusions.
Selective reporting
Adequate: a protocol is available which clearly states the primary outcome is the same as in the final trial report.
Inadequate: the primary outcome differs between the protocol and final trial report.
Unclear: no trial protocol is available or there is insufficient reporting to determine if selective reporting is present.
Sequence generation (checking for selection bias)
Adequate: investigators described a random component in the sequence generation process, such as the use of random number table, coin tossing, card or envelope shuffling.
Inadequate: investigators described a non‐random component in the sequence generation process, such as the use of odd or even date of birth, algorithm based on the day or date of birth, hospital or clinic record number.
Unclear: insufficient information to permit judgment of the sequence generation process
Other forms of bias
Adequate: there is no evidence of bias from other sources.
Inadequate: there is potential bias present from other sources (e.g., early stopping of trial, fraudulent activity, extreme baseline imbalance or bias related to specific study design).
Unclear: insufficient information to permit judgment of adequacy or otherwise of other forms of bias.
For blinding and incomplete outcome data, multiple entries would have been made if more than one outcome (or time points) had been involved.
We used the Newcastle‐Ottawa Quality Assessment Scale for Cohort Studies (Newcastle‐Ottawa) to assess methodological quality in the included non‐randomised studies. Specifically, the scale uses a star system to judge three general areas: selection of study groups, comparability of groups and ascertainment of outcomes (in the case of cohort studies). This instrument can thus be used in a systematic review to assess the quality of non‐randomised studies.
Assessment of Quality of Evidence Across Studies
We assessed the quality of evidence with the GRADE approach (Guyatt 2008), defining evidence quality for each outcome as “the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest” (Higgins 2008). When data for the same outcome from two or more studies can be pooled, evidence quality for that outcome is assessed across the studies providing those data. The quality rating has four levels: high, moderate, low and very low. Data from RCTs are initially considered to provide high quality evidence, but this evidence can be downgraded. Data from non‐randomised studies are initially considered to provide low quality evidence, but this evidence can be upgraded or further downgraded. Factors that can decrease evidence quality include limitations in design, indirectness of evidence, unexplained heterogeneity or inconsistency of results, imprecision of results typically due to small numbers of events or high probability of publication bias. Factors that can increase evidence quality include a large magnitude of effect, all plausible confounding leading to an underestimation of effect, or a dose‐response gradient.
Measures of treatment effect
We used Review Manager 5.1 (RevMan 2011) provided by the Cochrane Collaboration to prepare the review and for statistical analysis. We summarised dichotomous outcomes for effect using risk ratios (RR), with 95% confidence intervals (CI). We calculated summary statistics and present findings in regard to evidence quality in GRADE summary of findings tables, for all outcomes of interest.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
Study authors would have been contacted to obtain missing data if necessary.
Assessment of reporting biases
We minimised the potential for publication bias by using comprehensive search strategies, which include searching scientific literature from a wide range of databases, published or unpublished, written in any language.
Data synthesis
Meta‐analysis would have been conducted when appropriate. Since meta‐analysis was not possible, we conducted a narrative synthesis of studies. Data were also presented using the GRADEpro software (GRADEpro 2011). We generated GRADE evidence profiles and summary of findings tables.
Subgroup analysis and investigation of heterogeneity
If data had allowed, we had planned to perform subgroup analyses. These analyses could have included subgroup analyses based on age, degree of immunosuppression, study region, middle‐income vs. low‐income country, characteristics of key populations or other factors.
Sensitivity analysis
If the data had allowed, we had planned to examine the contributions of individual studies to overall heterogeneity by removing them one at a time.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
We identified three cohort studies.
Results of the search
Searches produced 199 titles after 84 duplicates were removed. We initially excluded 100 titles, and 99 titles and abstracts were reviewed more closely. Applying the inclusion criteria relevant to study design, participant characteristics, intervention characteristics and outcome characteristics, two authors independently examined the titles, abstracts, and descriptor terms of all references.
We examined 19 full‐text articles closely and identified three cohort studies meeting our inclusion criteria for data extraction and possible meta‐analysis (Sibailly 1997,Veit 2009, Pacanowski 2012). See Figure 1 for a flowchart depicting our screening process.
1.
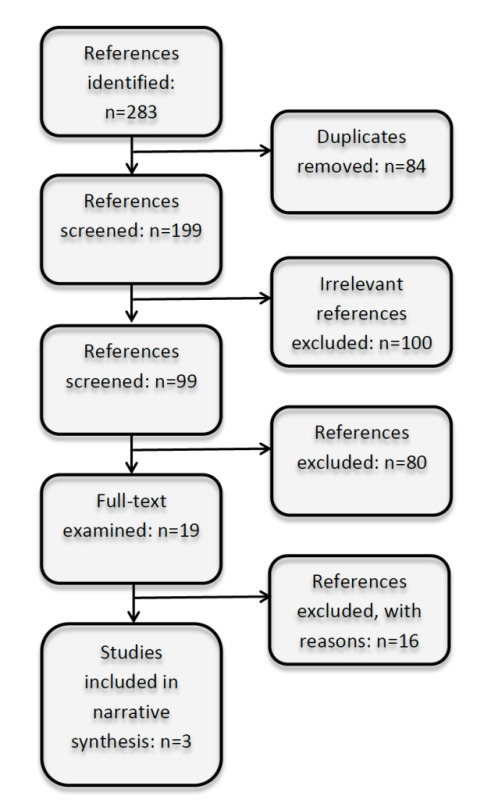
Flow chart depicting screening process.
Included studies
Sibailly 1997: This was a prospective cohort study conducted in Côte d’Ivoire, primarily focused on prevention of mother‐to‐child HIV transmission (PMTCT). The study took place in 1991‐1993, and thus predates the widespread availability of antiretroviral therapy (ART) in Africa. The mean age of participants was 10 months (range 7 months to 14 months); 18 HIV‐infected children and 57 HIV‐uninfected children received a single 0.5 ml dose of 17D YF vaccine. At a median of 29 months of follow‐up, three (17%) of the 18 HIV‐infected children had an adequate YF neutralising antibody response (NT ≥1:10) compared with 42 (74%) of 57 HIV‐uninfected children (relative risk [RR] 0.23, 95% confidence interval [CI] 0.08 to 0.64). No child developed YF, and no adverse events were reported, although these were not systematically ascertained.
Veit 2009: This was a retrospective cohort study embedded within the prospective Swiss HIV Cohort Study. Cohort participants at 4 of 7 participating centres were asked if they had travelled to a tropical country between 1996 and 2005; those reporting that they had were asked if they had received a YF vaccine. Receipt of vaccine was confirmed by chart review. The authors compared the development of titres of NT ≥1:10 after YF immunisation among 102 HIV‐infected participants in the Swiss HIV Cohort Study with 209 uninfected individuals of similar age in Germany, who had been previously studied using the same laboratory methods (Niedrig 1999) Both groups received 17DV YF vaccine. Of the 102 patients in the Swiss cohort, 54 (53%) were male, the median age was 34.7 years (range 28.1‐41.5 years), and the median baseline CD4 cell count was 512 cells/µL (range 368‐664 cells/µL). Among 84 patients for whom viral load was reported, 41 (48%) had HIV RNA levels <50 copies/mL. Within the first year of vaccination, 13 (17%) of 78 individuals infected with HIV had not developed NT ≥1:10, while two (3%) of 66 individuals not infected with HIV had NT ≤1:10 (p=0.01). An even higher proportion of nonreactive NTs (19%) was seen among the 63 patients who had been vaccinated for the first time against YF (p=0.004, compared with HIV‐uninfected individuals). One to ten years following vaccination, 11 (16%) of 70 patients infected with HIV had NT ≤1:10 compared to 11 (12%) of 92 HIV‐uninfected individuals. NT decay patterns were similar in HIV‐infected and HIV‐uninfected patients (p=0.07). Higher NTs during the first year after vaccination were associated with undetectable HIV RNA levels, increasing CD4 cell count and female sex. The study included no data on effectiveness of immunisation in preventing clinical YF. No severe adverse events were reported.
Pacanowski 2012: This was a prospective cohort study conducted at a single referral hospital in France that analysed risk factors for failure to develop protective NT following YF immunisation in patients infected with HIV. They assessed 364 patients; 124 had been immunised before HIV diagnosis and 240 immunised after HIV diagnosis. Baseline characteristics were compared to NT outcomes. Because patients immunized before and after their HIV diagnosis differed significantly in baseline factors such as age at immunization, frequency of origin from an area of YF endemicity, number of injections of vaccine and time between immunization and NT determination, the authors stratified the analysis.
The 124 patients immunised before HIV diagnosis had a median age of 29.0 years (SD=9.0 years) at time of immunisation and a median age of 42 years (SD=9.8 years) at time of NT determination. Of the 124, 76 (61%) were male, and 60 (48%) were from YF‐endemic regions. There was no report of median CD4 cell count and HIV RNA levels at time of immunisation for these patients. At NT determination, median CD4 cell count was 459 cell/µL, and 37 (30%) of 125 had HIV RNA viral loads <400 copies/mL. Among these patients, the time from immunization to diagnosis of HIV infection was shorter in those with NT <1:10 compared to those with NT ≥1:10 (−3.7 years vs.−6,9 years, p= 0.04). This equates to a 1.12‐fold increased risk of vaccine failure per year of delay (95% CI: 0.98 to 1.28). The authors speculated that patients with shorter time periods between immunization and NT determination were more likely to be infected and have uncontrolled viral replication, which is consistent with the other group studied. The 204 patients immunised after HIV diagnosis had a median age of 40.7 years (SD=9.2 years) at time of immunisation and a median age of 44 years (SD=9.0 years) at time of NT determination. Of the 240, 123 (55%) were male, and 149 (62%) were from regions endemic for YF. At time of immunisation, median CD4 cell count was 451 cells/µL, and 80 of 240 (33%) had HIV RNA levels <400 copies/mL. At NT determination, median CD4 cell count was 429 cells/µL, and 55 (23%) had HIV RNA viral loads <400 copies/mL. Among 79 patients who were vaccinated for the first time after diagnosis of HIV infection, higher HIV RNA at immunisation was the unique independent risk factor for NT <1:10 (adjusted odds ratio [aOR] 3.73 per log10 increase in viral load, 95% CI 1.14‐12.28). The authors concluded that lower NT was independently associated with a shorter duration of undetectable plasma HIV RNA (aOR = 1.05 per year, 95% CI 1.005 to 1.09) and higher plasma HIV RNA (aOR = 0.91 per log10, 95% CI 0.84 to 0.99) at immunisation. This study also included no data on effectiveness of immunisation in preventing clinical YF. No severe adverse events were reported.
Excluded studies
We excluded six studies (Osinusi 1990; Monath 2002; Camacho 2004; Camacho 2007; Ripoll 2008; Roukens 2008) because the study population did not include participants with HIV infection. We excluded an additional eight articles (Goujon 1995; Kengsakul 2002, Receveur 2000; Tattevin 2004; Pistone 2007; Ho 2008; Pistone 2010; Sidibe 2012) because they were case reports or case series without comparators. We excluded two articles (Veit 2010; Thomas 2012) because they were review articles.
Risk of bias in included studies
We used the Cochrane Collaboration tool for assessing the risk of bias for each individual study and present results in a summary table (see Figure 2). Because of their observational nature, all three studies were at high risk of bias overall.
2.
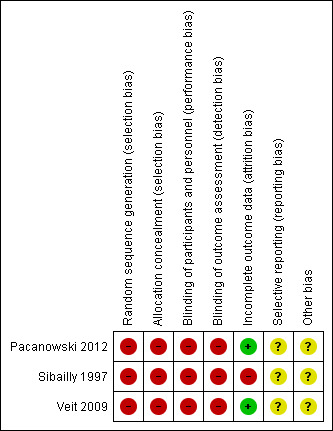
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We used the Newcastle‐Ottawa Scale (Newcastle‐Ottawa) to assess methodological quality in the three included studies. Quality overall was high, bearing in mind that these were observational studies at inherently high risk of bias. The cohort in Sibailly 1997 was representative of HIV‐infected children in Côte d’Ivoire at the time of the study. The cohorts in Veit 2009 and Pacanowski 2012 were representative of HIV‐infected European residents who travelled to YF‐endemic regions. Sibailly 1997 and Veit 2009 compared outcomes in HIV‐infected participants with those in HIV‐uninfected participants, and, as each study aimed to make this comparison, there were no problems in regard to participant selection or comparability of the cohorts. Outcome assessment in all studies was performed adequately. See Figure 3.
3.
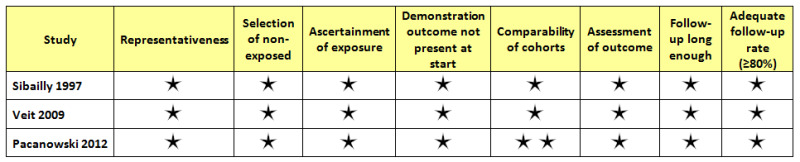
Newcastle‐Ottawa assessment.
Effects of interventions
See: Table 1; Table 2; Table 3
In one prospective cohort from the pre‐ART era, embedded in a PMTCT study, substantially fewer HIV‐infected children had YF NT titres of ≥1:10 compared to HIV‐uninfected children (RR 0.23, 95% CI 0.08 to 0.64).
In one retrospective cohort study that compared response to YF vaccine in HIV‐infected adults to YF vaccine in HIV‐uninfected adults, slightly fewer HIV‐infected adults had YF antibody response of NT ≥1:10 compared to HIV‐uninfected adults (RR 0.86 95% CI 0.77 to 0.96). From 1 to 10 years after vaccination there was no difference in YF antibody response between the groups (RR 0.88, 95% CI 0.76, 1.02.p=0.07).
In one prospective cohort study, comparing YF immunisation in adults before HIV diagnosis with YF immunisation after HIV diagnosis, slightly fewer patients receiving YF vaccine before HIV diagnosis had NTs of ≥1:10 compared to patients receiving YF vaccine after HIV diagnosis (RR 0.91, 95% CI 0.85 to 0.98). In a mean 8.4 years of follow‐up, 340 of 364 (93.4%) patients overall retained an adequate YF antibody response.
In the two adult studies, YF vaccine in HIV‐infected patients was associated with better primary immunologic response in those patients whose CD4 counts were higher and whose HIV RNA levels were suppressed.
Discussion
Summary of main results
Three cohort studies were included in this review (Sibailly 1997, Pacanowski 2012 and Veit 2009) that examined immunologic responses to YF immunisation among 484 patients with HIV infection. Sibailly 1997 compared NT responses in HIV‐infected and uninfected African children; Veit 2009 compared NT responses in HIV‐infected and uninfected European adults and predictors of a protective response to immunisation among HIV‐infected patients; Pacanowski 2012 compared HIV‐infected patients with different baseline predictor variables in French adults. The two studies in adults found that higher CD4 cell counts and suppressed HIV RNA levels at time of immunisation were associated with better immunologic response. Pacanowski found that delaying until after HIV diagnosis was associated with a 1.12‐fold increased risk of vaccine failure per year of delay.
Additionally there are several other case series and case reports that we did not include in our systematic review because they did not meet our inclusion criterion of needing a comparison group (Goujon 1995; Kengsakul 2002, Receveur 2000; Tattevin 2004; Pistone 2007; Ho 2008; Pistone 2010; Sidibe 2012). In these studies, which included 89 patients, there was a single case of YEL‐AND reported from Thailand in a patient who developed meningoencephalitis shortly after receiving a 17D immunisation (Kengsakul 2002). Unfortunately, no laboratory data were available to confirm that this patient's subsequent death was caused by the 17D virus.
Quality of the evidence
The quality of the evidence in this literature is low to very low. Evidence quality for outcomes in Sibailly 1997 and Veit 2009 was graded down for imprecision due to small numerators. Evidence quality for outcomes in Veit 2009 was further graded down for indirectness (as it was a retrospective analysis). Evidence quality for the outcome in Pacanowski 2012 was not graded down, but because it was an observational study, quality of evidence was rated low for the outcomes they reported.
Potential biases in the review process
Potential biases were minimised by not limiting the search by language and by performing a comprehensive search of databases and conference proceedings.
Authors' conclusions
Implications for practice.
YF vaccine may potentially be used safely in HIV‐infected patients, although our conclusions are limited by the small numbers of patients who have been reported. To assure maximum effectiveness YF vaccine should be given after HIV replication has been suppressed and immunologic recovery is underway. Because our conclusions regarding the safety of YF vaccine in patients with HIV infection is based on very low quality data, it may be most prudent to restrict its use to those unable to avoid endemic areas.
Implications for research.
Additional research is needed to assess the efficacy and immunogenicity of YF vaccination in patients with HIV infection. Research analysing the timing of ART with respect to successful YF immunisation could be of interest. With regard to safety, ongoing registers of HIV‐infected patients who have been immunised against YF would be one way to collect data on rare outcomes such as YEL‐AND and YEL‐AVD and to better understand if risks of serious adverse events following YF immunisation are greater in those who are HIV‐infected than in those who are not.
Acknowledgements
We thank Gavrilah Wells for obtaining PDFs of articles.
Appendices
Appendix 1. PubMed search strategy, modified and adapted for use in the other databases
| Search | PubMed search strategy |
| #3 | Search (yellow fever vaccine‐associated neurologic disease[tw] OR YEL‐AND[tw] OR yellow fever vaccine‐associated viscerotropic disease[tw] OR YEL‐AVD[tw] OR Yellow Fever Vaccine[MeSH] OR YFV 17D[tw] OR YFV17DD[tw] OR YFV17D[tw] OR YFV 17DD[tw]OR (yellow fever[ti] AND vaccin*[ti])) |
| #2 | Search (yellow fever[tw] OR fievre jaune[tw] OR febre amarela[tw] OR fiebre amarilla[tw] OR vomi noir[tw] OR vomito negro[tw] OR yellow jack[tw] OR Yellow Fever[MeSH]) |
| #1 | Search (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR double‐blind method[mh] OR single‐blind method[mh] OR clinical trial[pt] OR trial[tw] OR clinical trials[mh] OR (clinical trial [tw]) OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR placebos[mh] OR placebo*[tw] OR random*[tw] OR cohort[tw] OR (case[tw] AND control[tw]) OR observation*[tw] OR nonrandom*[tw]) |
Data and analyses
Comparison 1. Immunologic response to YF vaccine in HIV‐infected children vs. immunologic response to YF vaccine in HIV‐uninfected children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adequate antibody response (NT ≥1:10), follow‐up median 29 months | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.08, 0.64] |
1.1. Analysis.
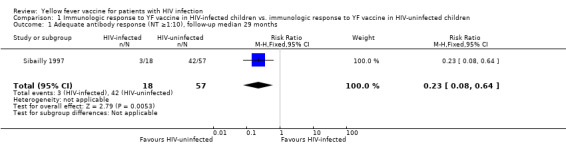
Comparison 1 Immunologic response to YF vaccine in HIV‐infected children vs. immunologic response to YF vaccine in HIV‐uninfected children, Outcome 1 Adequate antibody response (NT ≥1:10), follow‐up median 29 months.
Comparison 2. Immunologic response to YF vaccine in HIV‐infected adults vs. immunologic response to YF vaccine in HIV‐uninfected adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Immunogenicity: Adequate antibody response (NT ≥1:10), follow‐up 1 yr | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.96] |
| 2 Duration: Adequate antibody response (NT ≥1:10), follow‐up 1‐10 yrs | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.76, 1.02] |
2.1. Analysis.
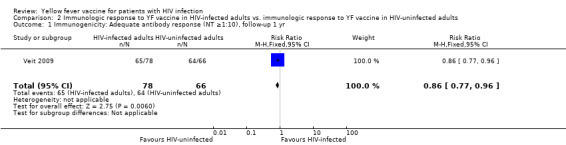
Comparison 2 Immunologic response to YF vaccine in HIV‐infected adults vs. immunologic response to YF vaccine in HIV‐uninfected adults, Outcome 1 Immunogenicity: Adequate antibody response (NT ≥1:10), follow‐up 1 yr.
2.2. Analysis.
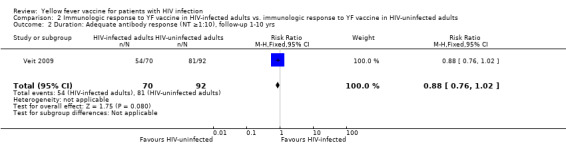
Comparison 2 Immunologic response to YF vaccine in HIV‐infected adults vs. immunologic response to YF vaccine in HIV‐uninfected adults, Outcome 2 Duration: Adequate antibody response (NT ≥1:10), follow‐up 1‐10 yrs.
Comparison 3. Immunologic response to YF vaccine in HIV‐infected adults before HIV diagnosis, vs. immunologic response to YF vaccine in HIV‐infected adults after HIV diagnosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Immunogenicity: Adequate antibody response (NT ≥1:10), follow‐up 1 yr | 1 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.85, 0.98] |
3.1. Analysis.
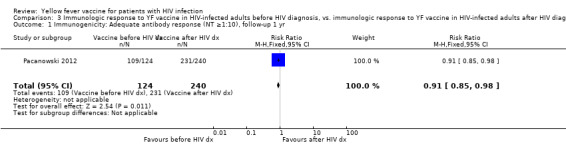
Comparison 3 Immunologic response to YF vaccine in HIV‐infected adults before HIV diagnosis, vs. immunologic response to YF vaccine in HIV‐infected adults after HIV diagnosis, Outcome 1 Immunogenicity: Adequate antibody response (NT ≥1:10), follow‐up 1 yr.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Pacanowski 2012.
| Methods | A prospective cohort study analysing a cohort of 364 patients diagnosed with HIV infection who were given YF vaccination. | |
| Participants | 364 patients diagnosed with HIV infection who were given YF vaccination ‐ 124 patients were immunised before HIV diagnosis and 204 patients were immunised after HIV diagnosis. All patients attended Saint‐Antoine Hospital in Paris, France. | |
| Interventions | Arm 1: Received YF vaccination before diagnosis of HIV infection. Arm 2: Received yellow fever vaccination after diagnosis of HIV infection. |
|
| Outcomes | YF antibody response (immunogenicity and duration) | |
| Notes | Of note: At time of YF immunisation, 14 patients vaccinated after HIV diagnosis had CD4 cell count <200 cell/mm3. Among these, 6/14 had HIV RNA load >400 copies/mL. Of the 14, 4 received primary vaccination while 7 received booster injection (data were missing for the remaining three patients). After a mean delay of 4.6 years after immunisation, these 11 patients exhibited a NT>1:10. They had no serious adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational study |
| Allocation concealment (selection bias) | High risk | Observational study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Observational study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observational study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Yes' or 'No'. |
| Other bias | Unclear risk | None detected |
Sibailly 1997.
| Methods | Observational cohort study (embedded in a PMTCT cohort study) | |
| Participants | 18 HIV‐infected children and 57 HIV‐uninfected children in Abidjan, Côte d'Ivoire | |
| Interventions | Between June 1991 and June 1993 all children received a single 0.5 ml dose of 17D YF vaccine and measles vaccine at a mean of 10 months of age (range 7 months to 14 months). Children were followed for a median 29 months. | |
| Outcomes | Adequate YF antibody response. | |
| Notes | Pre‐ART era | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational study |
| Allocation concealment (selection bias) | High risk | Observational study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Observational study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observational study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Adverse events were not systematically ascertained |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Yes' or 'No'. |
| Other bias | Unclear risk | Study predates antiretroviral therapy. Patient immunologic and virologic markers not provided. |
Veit 2009.
| Methods | A prospective double cohort study analysing a cohort of 102 patients infected with HIV given YF vaccination compared with serologic data from 209 patients not infected with HIV, also given YF vaccination. | |
| Participants | 102 HIV‐infected patients who reported a journey to a tropical destination from the Swiss Cohort study and 209 HIV‐uninfected patients in Germany who were similar in age range to Swiss Cohort study. All participants received YF vaccination. | |
| Interventions | Arm 1: Patients infected with HIV receive YF vaccination (n=102) Arm 2: Patients not infected with HIV receive YF vaccination (n=209) |
|
| Outcomes | YF antibody response (immunogenicity and duration) | |
| Notes | 4/102 HIV‐infected patients showed nonreactive NTs at first analysis but developed reactive NTs during follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational study |
| Allocation concealment (selection bias) | High risk | Observational study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Observational study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observational study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment of 'Yes' or 'No'. |
| Other bias | Unclear risk | None detected |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Camacho 2004 | Study population HIV‐uninfected |
| Camacho 2007 | Study population HIV‐uninfected |
| Goujon 1995 | Case series |
| Ho 2008 | Case series |
| Kengsakul 2002 | Case report |
| Monath 2002 | Study population HIV‐uninfected |
| Osinusi 1990 | Study population HIV‐uninfected |
| Pistone 2007 | Case series |
| Pistone 2010 | Case series |
| Receveur 2000 | Case reports |
| Ripoll 2008 | Study population HIV‐uninfected |
| Roukens 2008 | Study population HIV‐uninfected |
| Sidibe 2012 | Case series |
| Tattevin 2004 | Case series |
| Thomas 2012 | Review article |
| Veit 2010 | Review article |
Differences between protocol and review
None.
Contributions of authors
Draft protocol: HB. Conduct searches: THH. Screen references: HB, THH. Abstract data: HB, GWR. Risk of bias assessment: HB, GWR, THH. Enter data: THH. Write review: HB, GWR, THH. Interpretation and analysis: HB, GWR, THH. GRADE analyses: THH, GWR.
Sources of support
Internal sources
Global Health Sciences, University of California, San Francisco (in kind support), USA.
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies included in this review
Pacanowski 2012 {published data only}
- Pacanowski J, Lacombe K, Campa P, Dabrowska M, Poveda JD, Meynard JL, Poirot JL, Fonquernie L, Girard PM. Plasma HIV‐RNA is the key determinant of long‐term antibody persistence after yellow fever immunization in a cohort of 364 HIV‐infected patients. J Acquir Immune Defic Syndr. 2012 Apr 1;59(4):360‐67. [DOI] [PubMed] [Google Scholar]
Sibailly 1997 {published data only}
- Sibailly TS, Wiktor SZ, Tsai TF, Cropp BC, Ekpini ER, Adjorlolo‐Johnson G, Gnaore E, et al. Poor antibody response to yellow fever vaccination in children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1997 Dec;16(12):1177‐79. [DOI] [PubMed] [Google Scholar]
Veit 2009 {published data only}
- Veit O, Niedrig M, Chapuis‐Taillard C, Cavassini M, Mossdorf E, Schmid P, Bae H‐G, Litzba N, Staub T, Hatz C, Furrer H, Swiss HIV Cohort Study. Immunogenicity and safety of yellow fever vaccination for 102 HIV‐infected patients. Clin Infect Dis. 2009 Mar 1;48(5):659‐66. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Camacho 2004 {published data only}
- Camacho LA, Freire Mda S, Leal Mda L, Aguiar SG, Nascimento JP, Iguchi T, et al. Immunogenicity of WHO‐17D and Brazilian 17DD yellow fever vaccines: a randomized trial. Rev Saude Publica. 2004 Oct;38(5):671‐8. [DOI] [PubMed] [Google Scholar]
Camacho 2007 {published data only}
- Collaborative Group for Studies with Yellow Fever Vaccine. Randomized, double‐blind, multicenter study of the immunogenicity and reactogenicity of 17DD and WHO 17D‐213/77 yellow fever vaccines in children: implications for the Brazilian National Immunization Program. Vaccine. 2007 Apr 20;25(16):3118‐23. [DOI] [PubMed] [Google Scholar]
Goujon 1995 {published data only}
- Goujon C, Tohr M, Feuillie V, Coulaud P, Dupont B, Sansonetti P. Good tolerance and efficacy of yellow fever vaccine among carriers of human immunodeficiency virus. J Trav Med 1995;2:145. [Google Scholar]
Ho 2008 {published data only}
- Ho YL, Enohata T, Lopes MH, Sousa Dos Santos S. Vaccination in Brazilian HIV‐infected adults: a cross‐sectional study. AIDS Patient Care STDs. 2008 Jan;22(1):65‐70. [DOI] [PubMed] [Google Scholar]
Kengsakul 2002 {published data only}
- Kengsakul, K, Sathirapongsasuti K, Punyagupta S. Fatal myeloencephalitis following yellow fever vaccination in a case with HIV infection. J Med Assoc Thailand 2002;85:131‐4. [PubMed] [Google Scholar]
Monath 2002 {published data only}
- Monath TP, Nichols R, Archambault WT, Moore L, Marchesani R, Tian J, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF‐VAX) in a phase III multicenter, double‐blind clinical trial. Am J Trop Med Hyg. 2002 May;66(5):533‐41. [DOI] [PubMed] [Google Scholar]
Osinusi 1990 {published data only}
- Osinusi K, Akinkugbe FM, Akinwolere OA, Fabiyi A. Safety and efficacy of yellow fever vaccine in children less than one‐year‐old. West Afr J Med. 1990 Jul‐Sep;9(3):200‐3. [PubMed] [Google Scholar]
Pistone 2007 {published data only}
- Pistone T, Verdiere C‐H, Receveur M‐C, Ezzedine K, Lafon ME, Malvy D. Immunogenicity and tolerance to yellow fever vaccine in travelers living with HIV, France [Immunogenicite et tolerance du vaccin amaril chez le voyageur vivant avec le VIH, France]. Bull Epidemiol Hebdomadaire 2007;25/26:238‐40. [Google Scholar]
Pistone 2010 {published data only}
- Pistone T, Verdiere CH, Receveur MC, Ezzedine K, Lafon ME, Malvy D. Immunogenicity and tolerability of yellow fever vaccination in 23 French HIV‐infected patients. Curr HIV Res. 2010 Sep;8(6):461‐6. [DOI] [PubMed] [Google Scholar]
Receveur 2000 {published data only}
- Receveur MC, Thiebaut R, Vedy S, Malvy D, Mercie P, Bras ML. Yellow fever vaccination of human immunodeficiency virus‐infected patients: report of 2 cases. Clin Infect Dis. 2000 Sep;31(3):E7‐8. [DOI] [PubMed] [Google Scholar]
Ripoll 2008 {published data only}
- Ripoll C, Ponce A, Wilson MM, Sharif N, Vides JB, Armoni J, et al. Evaluation of two yellow fever vaccines for routine immunization programs in Argentina. Hum Vaccin. 2008 Mar‐Apr;4(2):121‐6. [DOI] [PubMed] [Google Scholar]
Roukens 2008 {published data only}
- Roukens AH, Vossen AC, Bredenbeek PJ, Dissel JT, Visser LG. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: a randomized controlled non‐inferiority trial. PLoS One. 2008 Apr 23;3(4):e1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sidibe 2012 {published data only}
- Sidibe M, Yactayo S, Kalle A, Sall AA, Sow S, Ndoutabe M, et al. Immunogenicity and safety of yellow fever vaccine among 115 HIV‐infected patients after a preventive immunisation campaign in Mali. Trans R Soc Trop Med Hyg. 2012 Jul;106(7):437‐44. [DOI] [PubMed] [Google Scholar]
Tattevin 2004 {published data only}
- Tattevin P, Depatureaux AG, Chapplain JM, Dupont M, Souala F, Arvieux C, et al. Yellow fever vaccine is safe and effective in HIV‐infected patients. AIDS. 2004 Mar 26;18(5):825‐7. [DOI] [PubMed] [Google Scholar]
Thomas 2012 {published data only}
- Thomas RE, Lorenzetti DL, Spragins W, Jackson D, Williamson T. The safety of yellow fever vaccine 17D or 17DD in children, pregnant women, HIV+ individuals, and older persons: systematic review. Am J Trop Med Hyg 2012;86(2):359‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veit 2010 {published data only}
- Veit O, Hatz C, Niedrig M, Furrer H. Yellow fever vaccination in HIV‐infected patients. HIV Ther. 2010 Jan;4:17‐26. [Google Scholar]
Additional references
CDC 2010
- Centers for Disease Control and Prevention (CDC). Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report (MMWR) 2010;59(RR‐7):1‐27. [PubMed] [Google Scholar]
GRADEpro 2011 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro, Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2011.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schünemann HJ, GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008 Apr 26;336(7650):924‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley‐Blackwell, 2008. [Google Scholar]
Newcastle‐Ottawa
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 7 January 2014].
Niedrig 1999
- Niedrig M, Lademann M, Emmerich P, Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health. 1999 Dec;4(12):867‐71. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
UNAIDS 2013
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global Report, 2013. Available from: http://www.unaids.org/en/resources/campaigns/globalreport2013/ [accessed 7 January 2014] 2013.
WHO 2013a
- World Health Organization (WHO). Health Topics: Yellow Fever. Available from: http://www.who.int/topics/yellow_fever/en/ [accessed 17 December 2013].
WHO 2013b
- World Health Organization (WHO). Global and Alert Response (GAR): Vaccines and vaccination against yellow fever. Available from: http://www.who.int/csr/resources/publications/yellowfever/ [accessed 7 January 2013].


