Abstract
Pyrroloquinoline quinone is a quinone described as a cofactor for many bacterial dehydrogenases and is reported to exert an effect on metabolism in mammalian cells/tissues. Pyrroloquinoline quinone is present in the diet being available in foodstuffs, conferring the potential of this compound to be supplemented by dietary administration. Pyrroloquinoline quinone’s nutritional role in mammalian health is supported by the extensive deficits in reproduction, growth, and immunity resulting from the dietary absence of pyrroloquinoline quinone, and as such, pyrroloquinoline quinone has been considered as a “new vitamin.” Although the classification of pyrroloquinoline quinone as a vitamin needs to be properly established, the wide range of benefits for health provided has been reported in many studies. In this respect, pyrroloquinoline quinone seems to be particularly involved in regulating cell signaling pathways that promote metabolic and mitochondrial processes in many experimental contexts, thus dictating the rationale to consider pyrroloquinoline quinone as a vital compound for mammalian life. Through the regulation of different metabolic mechanisms, pyrroloquinoline quinone may improve clinical deficits where dysfunctional metabolism and mitochondrial activity contribute to induce cell damage and death. Pyrroloquinoline quinone has been demonstrated to have neuroprotective properties in different experimental models of neurodegeneration, although the link between pyrroloquinoline quinone-promoted metabolism and improved neuronal viability in some of such contexts is still to be fully elucidated. Here, we review the general properties of pyrroloquinoline quinone and its capacity to modulate metabolic and mitochondrial mechanisms in physiological contexts. In addition, we analyze the neuroprotective properties of pyrroloquinoline quinone in different neurodegenerative conditions and consider future perspectives for pyrroloquinoline quinone’s potential in health and disease.
Keywords: metabolism, mitochondria, neurodegenerative disease, neuroprotection, pyrroloquinoline quinone, retinal diseases
Introduction
Pyrroloquinoline quinone (PQQ) is a quinone first described in the 1960s as a cofactor of several bacterial dehydrogenases, including alcohol and sugar dehydrogenases (Hauge, 1964). The importance of PQQ in regulating physiological processes from fungi to mammals has been identified (Jonscher et al., 2021). Although PQQ cannot be synthesized de novo in mammals, trace amounts of PQQ have been measured in a concentration range from picomolar to nanomolar in many human and rodent tissues, suggesting that this compound can easily reach the body systems through external sources (Kumazawa et al., 1992). The presence of PQQ in many types of foods confers the potential for this compound to be supplemented and introduced in the organism by dietary consumption. PQQ’s nutritional role in mammalian health is further supported by the wide range of defects in reproduction, growth, and immunity resulting from the dietary absence of PQQ (Akagawa et al., 2016b). For these reasons, PQQ has been considered as a “new vitamin,” although this concept remains controversial and still to be properly established (Akagawa et al., 2016b). Despite this controversy to include PQQ in the long list of vitamins, the multitude of PQQ beneficial effects for human health involving a different range of physiological properties has been demonstrated in a wide range of studies. In particular, PQQ seems to be involved in cell signaling pathways regulating metabolism and mitochondrial mechanisms in many experimental models, thus providing a rationale for considering PQQ as a vital compound regulating key processes for life (Rucker et al., 2009). By regulating a wide range of mechanisms, PQQ might improve clinically relevant conditions where dysfunctional metabolism and mitochondrial activity may be the main cause or contribute together with other processes to cell stress and damage (Rucker et al., 2009; Akagawa et al., 2016b). In this context, PQQ has been demonstrated to exert neuroprotective effects in animal models of damage in the nervous system, providing evidence of the potential of this compound as a putative therapeutical tool for neurodegenerative diseases (Rucker et al., 2009; Akagawa et al., 2016b). Here, we review the general characteristics of PQQ and its modulation of metabolic and mitochondrial mechanisms. In addition, we focus our attention on analyzing the neuroprotective role of PQQ in different neurodegenerative diseases and discuss considerations for future perspectives for PQQ in health and disease.
Search Strategy
The literature search was performed based on the PubMed database to find papers related to the topic using the following keywords: “pyrroloquinoline quinone” and “neuroprotection,” “ATP,” “mitochondrial biogenesis,” “deficiency,” “metabolism,” or “NAD synthesis.” The search was limited to articles published between 1964 and 2023, including research papers, review articles, and clinical trials. Given the relevance of some older papers or the limited number of papers on some topics included in this review, year filtration for the most recent papers was performed when possible. The search results were further screened according to the relevance to the topic. Articles non-relevant to the topic were excluded from the analyses.
Molecular Properties of Pyrroloquinoline Quinone and Its Role in Nutrition
Molecular structure and dietary sources of PQQ
4,5-Dihydro-4,5-dioxo-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid (PQQ) is an aromatic o-quinone that can continuously undergo redox reactions, reducing to pyrroloquinoline quinol (PQQH2) through a semiquinone intermediate in a two-electron reduction mechanism (Cordell and Daley, 2022). This reaction occurs with the presence of organic substrates such as ascorbate, NAD(P)H, and glutathione (Cordell and Daley, 2022). PQQH2 is then reoxidized by the transfer of electrons to molecular oxygen and the formation of superoxide anion, which is subsequently dismutated to hydrogen peroxide (Cordell and Daley, 2022). As this reaction of redox cycling can occur continuously, only picomoles of PQQ are required to generate micromolar amounts of products, being more efficient than many enediols (e.g., ascorbic acid or menadione), isoflavonoids, phytoalexins, and polyphenols (Paz et al., 1996; Stites et al., 2000). PQQ efficiency in redox cycling is further promoted by the fact that PQQ is not easily self-oxidized or exhausted into inactive forms (Jonscher et al., 2021). The reactions of redox cycling can be performed by both the oxidized and the reduced form of PQQ (Jonscher et al., 2021). As redox cofactor, PQQ can catalyze redox reactions involving oxidation of different substrates as thiols, riboflavin, ubiquinone, terminal cytochromes, α-tocopheroxyl radicals, and nicotinamide adenine dinucleotide cofactors (Stites et al., 2000; Ouchi et al., 2013; Várnai et al., 2018; Chan et al., 2021). For instance, PQQ also catalyzes the oxidation of primary amines to aldehydes or ketones by reacting with the ε-amino group of lysine residues in proteins such as elastin and collagen under aerobic conditions, creating covalent cross-links (Akagawa et al., 2016b). Moreover, PQQ can react with amino acids in biological tissues leading to the formation of imidazole derivatives, such as imidazolopyrroloquinoline quinone, known to have biological activities (Akagawa et al., 2016b). In addition, PQQ can act as a free radical scavenger (Ouchi et al., 2013). The protonated form of PQQ shows only partial water solubility. Due to this, a different chemical form provided as a disodium salt (PQQNa2; available as BioPQQTM) was developed by Mitsubishi Gas Chemical Co., Inc. (Tokyo, Japan) to improve the solubility of this compound in water and advantage its usage in experimental conditions (Akagawa et al., 2016b).
PQQ can be derived from numerous nutritional and dietary sources, such as tea, fermented soybeans, kiwi, pepper, and parsley (Kumazawa et al., 1995; Mitchell et al., 1999). Different methods and assays for evaluating the presence of PQQ in foods have been developed, although the content of PQQ described from these is highly variable due to PQQ’s tendency to produce derivatives and condensation products with other nutrients (Ameyama et al., 1985; Bergethon, 1990; Kumazawa et al., 1995; Mitchell et al., 1999; Noji et al., 2007). Analyzing the PQQ content in various dietary sources by a method based on gas chromatography/mass spectrometry, it has been estimated a PQQ content ranging from 3.7–61 ng/g wet weight (or ng/mL for liquid foods) (Kumazawa et al., 1995). Another study using a reliable liquid chromatography and electrospray-ionization tandem mass spectrometry established that PQQ was contained in food at an amount of 0.19–7.02 ng/g fresh weight or ng/mL in liquid foods, with mustard, parsley, and natto (fermented soybeans) containing the highest amount (Noji et al., 2007). In addition, a recent study combining enzymatic and mass spectrometric analyses indicated that vinegar has higher levels of PQQ than beer (Kato et al., 2018). PQQ has also been found in human milk in an estimated concentration of 20–30 or 140–180 μg/L if considered without or with its various derivatives created from the reaction with non-branched chain amino acids (Jonscher et al., 2021). The estimated amount of PQQ consumed per day is 0.1–1 mg based on the available data of food composition (Harris et al., 2013).
Nutritional role of PQQ: is PQQ a “new vitamin”?
Although reported in over hundreds of different prokaryotes, the biosynthesis of PQQ has not been demonstrated in higher organisms, suggesting that the major source of this compound in plants and animals is either from microflora or from the diet. Since the common strains of bacteria in the human gut seem to have little capacity for synthesizing PQQ, a likely route for PQQ to enter human tissues is dietary consumption (Akagawa et al., 2016b). The presence of PQQ in several different foods gives the possibility to introduce this compound through the diet and provides the potential of a putative supplementation in case of deficiency. In this respect, the nutritional importance of PQQ in regulating mammalian health has been demonstrated under PQQ nutritional deficiency, where deficits in reproduction, growth, development, and immune performance occur (Steinberg et al., 1994., 2003). In mice fed with a PQQ-deficient diet, reproductive performance in terms of conception (the percentage of females giving birth to living pups) and fertility (indicated as the percentage of births) were impaired together with a decreased number and survival rate of pups per litter (Steinberg et al., 1994, 2003). Offspring from mice fed in the absence of PQQ seems to have a reduced growth rate, which has been demonstrated to require a dose of 300 ng/g of dietary PQQ to be optimal for survival (Steinberg et al., 1994). The reduced growth rate was further supported by the associated decrease in mRNA levels of Type I procollagen α1-chains in skin and lungs and a lower lysyl oxidase accumulation in neonatal mice, indicating a likely reduced capacity of neonatal extracellular matrix production and maturation in the absence of dietary PQQ (Steinberg et al., 2003). Mice raised in PQQ-deficient conditions displayed reduced response to mitogens by splenic cells, further supporting the likely role of PQQ as an essential nutrient for supporting animal growth (Steinberg et al., 1994). As support to a reduced immune performance in the absence of PQQ, neonates from female mice fed with diets devoid of PQQ tend to have reduced levels of interleukin 2, an autocrine and paracrine growth factor important for T-cell proliferation (Steinberg et al., 1994). Considering the nutritional importance of PQQ in regulating all these different aspects of mammalian health, this compound has previously been considered a novel vitamin. In 2003, Kasahara and Kato cloned a mouse homolog of the yeast 2-amminoadipic acid reductase, called U26, and hypothesized its action as a PQQ-dependent dehydrogenase involved in metabolic degradation of dietary lysine (Kasahara and Kato, 2003). Since the authors of this paper identified a putative PQQ-binding motif believed to be conserved in PQQ-dependent bacterial dehydrogenases, they concluded that PQQ may be qualified as a newcomer to the vitamins belonging to the B group (Kasahara and Kato, 2003). Despite these findings, a current view of PQQ as a new vitamin has been questioned, since the conclusive evidence of a mammalian PQQ-dependent enzyme is still lacking, although Akagawa and colleagues recently identified some potential candidates (Felton and Anthony, 2005; Rucker et al., 2005; Bauerly et al., 2006; Akagawa et al., 2016a). Nonetheless, the clear evidence of the regulation of several aspects of mammalian health mediated by PQQ shows how this compound may have a nutritional importance regardless of its formal inclusion in the list of vitamins.
The possibility of using PQQ as a supplement also at high doses is further suggested by its safety demonstrated by pre-clinical and clinical studies of toxicity. Different genotoxic assays in vitro of PQQNa2, represented by Ames test, in vitro chromosomal aberration tests, provided negative or weak positive results at high dosages, confirming the relative safety of PQQ (Nakano et al., 2013). Some genotoxic assays were also performed in vivo by micronucleus assay in mice, reporting no PQQ toxicity in bone marrow erythrocytes at doses up to 2000 mg/kg (Nakano et al., 2013). The acute and subchronic toxicity of oral PQQ was studied in vivo in rats administered with oral doses of this compound (Nakano et al., 2014, Liang et al., 2015). Nakano and colleagues tested the acute toxicity of BioPQQTM by treating rats through oral gavage for a 14-day preliminary and a 28-day repeated-dose study, documenting at 14 days a sex-specific increase in relative kidney weights with associated histopathology, which resulted in augmented urinary proteins and crystals after 28 days of treatment (Nakano et al., 2014). Notably, these morphological signs of disease were reversible and disappeared after a period of recovery (suggesting the PQQ can be rapidly cleared) (Nakano et al., 2014). In addition, a subchronic assessment of 13 weeks of treatment was performed in the same study, identifying no signs of evident toxicity, as histopathological changes observed in the PQQ-treated group were not dose-dependent and happened similarly to control untreated groups (Nakano et al., 2014). The authors of this study set the median lethal dose at a range of 0.5–2.0 g/kg and the no-observed-adverse-effect-level at 100 mg/kg per day (Nakano et al., 2014). Liang et al. (2015) assessed a subchronic oral toxicity of 13 weeks of treatment confirming no evident signs of toxicity in rats, determining the no-observed-adverse-effect-level at 400 mg/kg per day, the highest dose the authors tested. Data on PQQ safety have been assessed also in humans through placebo-controlled, double-blinded safety studies in healthy patients, where 20 or 60 mg/d PQQ was administered for 4 weeks (Akagawa et al., 2016b). No adverse effects were reported in standard clinical blood tests. Urinary concentration of markers of renal damage was not detected at both doses, further supporting that PQQ can be a safe compound if administered orally (Akagawa et al., 2016b).
Despite the potential of PQQ’s possible supplementation and safety, the bioavailability of this compound in several body systems after oral administration seems to be low. Smidt et al. (1991) estimated the absorption of PQQ by treating Swiss-Webster mice with oral [14]C PQQ to evaluate its absorption, tissue distribution, and excretion. The authors of this study indicated that PQQ can be readily absorbed in the lower intestine (62%) and excreted by the kidney (81%) within the first 24 hours from the administration (Smidt et al., 1991). However, the only tissues retaining a relevant abundance of PQQ after 24 hours were the skin and kidney, with some tissues such as the adrenal gland and brain displaying almost no presence of PQQ already after 6 hours from treatment, suggesting its poor bioavailability in these organs (Smidt et al., 1991). The peak of PQQ in human serum was observed after 3 hours of administration and its clearance in the serum parallels the change in urine (Harris et al., 2013).
Pyrroloquinoline Quinone Influence on Metabolism and Mitochondrial Mechanisms
There is evidence for the capacity of PQQ to potentially modulate the basic cell metabolism under physiological contexts. PQQ may regulate cell metabolism acting on different metabolic steps, which can be modulated either singularly or concomitantly to ultimately improve metabolic processes and increase ATP production. These mechanisms seem to be regulated in a very context-dependent manner. For instance, PQQ has been reported to modulate pathways providing metabolic substrates fueling glycolysis or tricarboxylic acid cycle and oxidative phosphorylation (OXPHOS) to increase ATP production (Owen et al., 2002). In this respect, PQQ may be responsible for regulating several pathways connected to the metabolism of amino acids and lipids. PQQ deficiency alters lysine metabolism and plasma levels of amino acids such as threonine, serine, and glycine in vivo (Bauerly et al., 2006). In addition, the treatment with PQQ in healthy tissues such as the liver and optic nerve impacted amino acid metabolism, further supporting the role of PQQ in modulating amino acid metabolism in several tissues (Bauerly et al., 2006; Canovai et al., 2023). PQQ might be involved in regulating lipid metabolism since the deficiency of this compound results in altered plasma lipid composition and expression of enzymes connected to lipid metabolism in the liver and heart (Bauerly et al., 2011).
Another mechanism by which PQQ may influence metabolic processes is through its capacity to act as a cofactor for metabolic enzymes including lactate dehydrogenases (LDH; Akagawa et al., 2016a). Akagawa and colleagues identified LDH-A as a mammalian PQQ-binding protein in mouse NIH/3T3 fibroblasts and further characterized the reaction catalyzed by this enzyme in vitro using a purified rabbit muscle LDH. In this study, the authors showed that PQQ can bind LDH as a cofactor and promote the oxidation of NADH to NAD+, catalyzing the reverse reaction converting lactate to pyruvate (Akagawa et al., 2016a). This latter substrate can in turn enhance energy production by promoting the mitochondrial tricarboxylic acid cycle and OXPHOS, leading to increased ATP synthesis (Arnold and Finley, 2023).
Increasing intracellular content of metabolic cofactors, such as NAD+, is another mechanism potentially improving cell metabolism and likely being regulated by PQQ. In this respect, the incubation with PQQ in vitro has been reported to increase intracellular NAD+ in cell lines such as HepG2 and NIH/3T3 cells (Zhang et al., 2015; Saihara et al., 2017). An enhanced expression in the nicotinamide phosphoribosyltransferase (NAMPT) gene (a key enzyme involved in NAD synthesis) has been identified concurrently with increased NAD+ activity after the incubation of PQQ in HepG2 cell cultures, suggesting that in some contexts PQQ may enhance NAD production through the promotion of its biosynthetic pathways (Zhang et al., 2015). As a support, the promoted increase in total NAD has been further documented in vivo, where Canovai et al. (2023) report an increased NAD content following PQQ administration in some districts of the visual system, such as the superior colliculus, in healthy mice.
PQQ has been reported to modulate mitochondrial mechanisms, particularly by acting on mitochondrial biogenesis. In effect, there are many studies documenting that PQQ can induce mitochondrial biogenesis through a likely influence on the activity of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and the expression of its target genes involved in the generation of new mitochondria (e.g., nuclear respiratory factor 1 and 2 and mitochondrial transcription factor A [TFAM]). These effects have been demonstrated in many different cell lines, including NIH/3T3 fibroblasts, 3T3-L1 adipocytes, SK-N-SH, HepG2 and Hepa1-6 cells, where the incubation of PQQ without the presence of stressors could promote the activation of PGC-1α and result in increased mitochondrial content and activity (Chowanadisai et al., 2010; Zhang et al., 2015; Saihara et al., 2017; Yamada et al., 2020; Ishak et al., 2021). However, the effects of PQQ on regulating the mitochondrial biogenesis in these cell lines are very context-dependent, with a wide range of effect intensity in different lines depending on the doses and incubation times used in the experiment. For instance, nanomolar doses of PQQ may promote the nuclear translocation of PGC-1α without affecting its total protein levels, which seem to be increased by the administration of micromolar doses (Chowanadisai et al., 2010; Zhang et al., 2015; Saihara et al., 2017; Yamada et al., 2020; Ishak et al., 2021). Across different cell lines, the same magnitude of effect (e.g., PGC-1α overexpression) has been documented under incubation with different concentrations of PQQ in different contexts (Yamada et al., 2020). Moreover, the same concentration of PQQ may result in different outcomes depending on the cell line, as suggested by an increase in total PGC-1α protein identified in Hepa1-6 but not in Hep2G cells following the incubation with 30 µM PQQ (Chowanadisai et al., 2010; Zhang et al., 2015). The context dependency of PQQ effects on mitochondrial mechanisms in vitro is further demonstrated in studies where PQQ administration was not able to induce mitochondrial biogenesis in normal human epidermal keratinocytes and dermal fibroblasts (Gruber and Holtz, 2022). The capacity of PQQ in regulating physiological mitochondrial content was further confirmed in in vivo systems, where the deficiency of PQQ leads to reduced mitochondrial number and function in the liver whilst having no apparent effect in the heart (Stites et al., 2006; Bauerly et al., 2011). In a recent study by Canovai et al. (2023), only a mild effect of PQQ administration on mitochondrial content in healthy retinal tissues was demonstrated after neither a short nor a long-term treatment, reporting only a small variation in NADH:Ubiquinone Oxidoreductase Subunit B8 (NDUFB8; a mitochondrial complex I marker) expression without any evident activation of mitochondrial biogenesis machinery. Taken together, these in vivo studies further suggest the context dependency of PQQ effectiveness in increasing mitochondrial content previously shown in in vitro experiments.
The regulation of metabolic substrate mobilization, the increased activity of metabolic enzymes and cofactors, and the improvement of OXPHOS through mitochondrial mechanisms can all enhance ATP production. By modulating these different mechanisms, PQQ can exert an ATP-boosting activity, which has been identified in several contexts in vitro and in vivo. ATP-boosting activity has been demonstrated by the dose-dependent induction of ATP synthesis in NIH/3T3 cells resulting from the exposure of PQQ in vitro (Akagawa et al., 2016a). This ATP-boosting effect has been further confirmed in other different cell lines and primary cultures, where the augmentation of cofactors and mitochondrial mechanisms seem to favor an increase in ATP pools (Saihara et al., 2017; Ebeling et al., 2020). In another study, Canovai et al. (2023) identified an increase in ATP content in in vitro models of dissociated cells from the brain cortex and visual system tissues (e.g., dissociated retina, optic nerve, and superior colliculus). The authors further confirmed this ATP-boosting activity in vivo in healthy retinal ganglion cell-related tissues, showing a different temporal variation of ATP content among the retina, optic nerve, superior colliculus, and brain cortex (Canovai et al., 2023). In the specific context of retinal ganglion cell-related tissues, the authors attributed a likely moderate effect on mitochondrial mechanisms and the capacity of PQQ to change metabolic profiles of non-diseased retinal ganglion cell tissues, although other mechanisms could not be excluded and further investigated (Canovai et al., 2023). Considered as a whole, all these studies suggest the variability of mechanisms possibly regulated by PQQ showing the potential of this compound to influence basic cellular metabolism at different levels.
Neuroprotective Role of Pyrroloquinoline Quinone
Neuronal ATP depletion induced by metabolic and mitochondrial dysfunctions is a common feature of neurodegenerative diseases (Procaccini et al., 2016; Ferrington et al., 2020; Muddapu et al., 2020; Muench et al., 2021; Chen et al., 2022). In this context, neurons are extremely susceptible to metabolic fluctuations and require well-regulated ATP content control to sustain their high energy demand and guarantee their structural integrity and physiological activity. Slight imbalances in the mechanisms providing ATP synthesis under stress render neurons susceptible to additional disease–related stressors and trigger neurodegeneration. Given its capacity to regulate the basic cellular metabolism, PQQ may be a good candidate to improve neuronal resilience under stress by regulating all the metabolic processes promoting ATP production, thus increasing cell viability and favoring neuroprotection. In this respect, many studies have reported the neuroprotective capacity of PQQ in several neurodegenerative contexts with diverse types of acute and chronic injuries displaying different temporal and spatial dynamics. The investigation of the mechanisms by which PQQ exerts this neuroprotective action in some neurodegenerative contexts is still partially undefined, especially in determining a clear correlation between neuroprotection and metabolic regulation of PQQ under stress. There is emerging evidence of PQQ-driven improvement of metabolic processes concomitantly with neuroprotective properties. In other experiments, there is only a demonstration of neuroprotection without any data on metabolic mechanisms. Nonetheless, the neuroprotective efficacy of PQQ has been reported by many in vitro and in vivo studies.
PQQ and neuroprotection: in vitro evidence
Several in vitro studies suggest a neuroprotective potential of PQQ through the modulation of metabolism and mitochondrial mechanisms. There is mounting evidence documenting the efficacy of PQQ in counteracting in vitro neurodegeneration of primary cultures and cell lines under metabolic stress induced by the mitochondrial complex I inhibitor rotenone. In these studies, PQQ has been demonstrated to prevent the apoptosis of rotenone-treated SH-SY5Y cells and primary cultured midbrain neurons by restoring mitochondrial membrane potential, increasing mtDNA, and upregulating gene expression of mitochondrial complex I subunits, thus improving mitochondrial health and activity (Zhang et al., 2014, 2016; Qin et al., 2015). The administration of PQQ in rotenone-treated human SH-SY5Y cells can improve mitochondrial morphology under damage and prevent the decrease in biogenesis- and fission/fusion-related markers such as PGC-1α, TFAM, dynamin-related protein 1, and mitofusin 2 (Lu et al., 2018; Cheng et al., 2021). In these studies, ameliorated mitochondrial processes resulting from PQQ administration prevented neuronal stress-related dysfunctional features such as microtubular destabilization, tyrosine residue nitration, and dopamine redistribution (Qin et al., 2015; Zhang et al., 2016). In this context, several signaling pathways such as extracellular signal-regulated protein kinase 1/2 and AMP-activated protein kinase pathways appear to regulate the action of PQQ on mitochondrial mechanisms and antioxidant activity (Zhang et al., 2014; Cheng et al., 2021). The improved neuronal metabolism and mitochondrial function can reduce also oxidative stress, which can be a consequence of dysfunctional OXPHOS leading to increased toxic reactive oxygen species (ROS). In this respect, a reduced production of ROS concomitantly with an improved mitochondrial function and integrity has been demonstrated under rotenone injury (Zhang et al., 2014, 2016; Qin et al., 2015). The antioxidant activity of PQQ under pro-oxidative stressors seems to be also related to other mechanisms, such as the stimulation of many signaling pathways promoting the antioxidant defense and scavenging dangerous ROS, as phosphatidylinositol 3-kinase/Akt, Nrf2, and DJ-1 pathways, or by the modulation of ROS-producing receptors, as N-methyl-D-aspartate (NMDA) receptors (Aizenman et al., 1992, 1994; Scanlon et al., 1997; Zhang and Rosenberg, 2002; Nunome et al., 2008; Zhang et al., 2011, 2012b; Guan et al., 2015). These different mechanisms have been documented in different neuronal cell lines and primary cultures, such as rat primary forebrain neurons, primary hippocampal neurons, neural stem cells and progenitor cells, SHSY-5Y cells, neuroblastoma cell line (SK-N-H cells), where the administration of PQQ under pro-oxidative stress was neuroprotective. Whether these mechanisms act secondarily or in concomitance with the implementation of metabolic processes and enhance the benefits of PQQ in providing neuroprotection still needs to be clearly established. In addition, PQQ and its derivatives have been demonstrated to induce the production of nerve growth factor, a neurotrophic factor important for neuronal viability and considered to improve neuronal mitochondrial function (Yamaguchi et al., 1993, 1996; Urakami et al., 1995; Martorana et al., 2018; Ding et al., 2020). Besides the regulation of metabolic processes and antioxidant mechanisms, PQQ may exert neuroprotective effects secondarily by reducing the accumulation of toxic byproducts responsible for neuronal death. PQQ has been demonstrated to reduce in vitro aggregation and accumulation of aggregated amyloid-β (Aβ)25–35, a toxic product inducing neuronal death in Alzheimer’s disease, and α-synuclein, a protein involved in Parkinson’s disease (PD) pathogenesis (Kobayashi et al., 2006; Zhang et al., 2009; Kim et al., 2010; Li et al., 2021, 2022a). Taken together, these studies demonstrate the neuroprotective potential of PQQ in vitro, showing the complex interplay of different mechanisms by which PQQ may act to reduce neuronal cell death and provide neuroprotection. The wide range of mechanisms also depicts the context-dependent and the heterogeneity of these mechanisms, dictating the necessity to investigate PQQ mechanisms in disease- or system-specific contexts.
PQQ in neurodegenerative diseases: evidence in in vivo models
The neuroprotective properties of PQQ have also been assessed in several in vivo models of neurodegenerative disease where neuronal mitochondrial activity and energetical deficits occur (Folbergrová and Kunz, 2012; Moon and Paek, 2015; Hiebert et al., 2015; Kang et al., 2017; Park et al., 2018; Liu et al., 2018; Wang et al., 2020; Roberts, 2021; Bhatia et al., 2022; Slater et al., 2022; Li et al., 2022b; Fizíková et al., 2023). In some contexts, clear evidence of improved metabolic processes concomitantly with increased neuronal viability has been reported, whereas in some other diseases, this correlation has still to be established. The summary of in vivo studies testing the neuroprotection of PQQ, and the overall outcomes are detailed in Table 1.
Table 1.
Summary of in vivo studies testing PQQ neuroprotection in models of neurodegenerative diseases
| Disease | Species | PQQ dose | Route | Overall outcome | Reference |
|---|---|---|---|---|---|
| AD | Mouse | 6, 12 mg/kg/d (given as Li3PQQ) | Gastric gavage daily for 8 wk | Improved cognitive performance and hippocampal synaptic plasticity; reduced accumulation of amyloid and phosphorylated tau | Zhao et al., 2014 |
| AD | Rat | 2 mg/kg for water administration + 20 mg/kg/d supplementation | Water from weaning to 6 mon + supplementation by oral gavage for 30 d | Prevented cognitive impairment and improved synaptosomes’ bioenergetic capacity | Martino Adami et al., 2017 |
| Epileptic seizures | Rat | 20 mg/kg | i.p. injection 30 min before the induction of seizures | Reduced behavioral seizures | Sanchez et al., 2000 |
| Hypoxia/ischemic brain | Rat | 10, 20 mg/kg | i.p. injection either before or after hypoxia | Reduced infarct size | Jensen et al., 1994 |
| ICH | Rat | 5, 10 mg/kg | i.p. injection every 24 h for 2 wk before IHC and for different time points after injury (1, 2, 3, 5, 7 d) | Improved locomotor function; reduced hematoma volume and brain edema; decreased ROS and apoptosis | Lu et al., 2015 |
| PD | Rat | 6 μL of 333 μM (low dose) or 4 μL 1.5 mM (high dose) | Intracerebral injection together with rotenone (inducer of PD) | Prevented cognitive decline; decreased neural loss; increased antioxidant ability; increased expression of mitochondrial complex I markers, tyrosine hydroxylase, and vesicular monoamine transporter 2 | Qin et al., 2015 |
| PD | Rat | 0.4, 2, 10 mg/kg/d | i.p. injection daily for 8 wk | Prevented cognitive decline; decreased neural loss; increased antioxidant ability; increased expression of mitochondrial complex I Ndufs1/4 and tyrosine hydroxylase | Zhang et al., 2016 |
| PD | Rat | 0.4, 2, 10 mg/kg/d | i.p. injection daily for 8 wk | Increased levels of PGC-1α, TFAM, Drp-1 and Mfn2 | Lu et al., 2018 |
| PD | Mouse | 0.8, 4, 20 mg/kg/d | i.p. injection daily for 1, 2, or 3 wk | Reduced locomotor deficits and nigral dopaminergic neuron loss; diminished reduction of mitochondria number and their morphological disruption; blocked reduction in the expression of PGC-1α and TFAM | Cheng et al., 2021 |
| PD | Fruit fly | 0.3 mM | Supplemented in the cornmeal-agar medium for 25 d | Increased PPL1 dopaminergic neurons and mitochondrial area | Cheng et al., 2021 |
| Retinal ganglion cell-related stress | Mouse | 20 mg/kg/d | i.p. injection daily | Reduced retinal ganglion cell loss | Canovai et al., 2023 |
| Reversible middle cerebral artery occlusion | Rat | 1, 3, 10 mg/kg | Intravenous injection at the initiation or after ischemia | Reduced brain infarct size and improved neurobehavioral scores | Zhang et al., 2006 |
| Schizophrenia | Mouse | 0.2, 2, 20 μg/kg/d | i.p. injection for 60 d | Reduced stereotypical behaviors, ataxia, learning and memory deficits | Zhou et al., 2014 |
| Schizophrenia | Mouse | 5 μg/mL | Drinking water during pregnancy and after birth of the pups | Reduced stereotypical behaviors, ataxia, learning and memory deficits, social interaction disorders, depression | Peng et al., 2022 |
| SCI | Rat | 5 mg/kg | i.p. injection after the injury | Improved locomotor activity and neuronal morphology; reduced inflammation and apoptosis | Zhou et al., 2021 |
| SCI | Rat | 5 mg/kg | i.p. injection immediately after injury and once every 24 h for 7 d | Recovered locomotor function; reduced lesion size; increased axonal density | Hirakawa et al., 2009 |
| TBI | Rat | 2 mM | Microinjected intracerebrally after TBI | Reduced neuronal apoptosis; improved electroencephalographic responses | Zhang et al., 2017 |
| TBI | Rat | 1, 2 mM | Not specified | Reduced brain apoptosis | Ye et al., 2017 |
| TBI | Rat | 5, 7, 10 mg/kg | i.p. injection for 3 d before TBI and consecutively until the end of the experiment (9 d) | Improved behavioral performances and reduced brain injury | Zhang et al., 2012a |
AD: Alzheimer’s disease; Drp-1: dynamin-related protein-1; ICH: intracerebral hemorrhage; Mfn2: mitofusin 2; Ndufs: NADH:ubiquinone oxidoreductase core subunit; PD: Parkinson’s disease; PGC-1α: peroxisome proliferator-activated receptor-gamma coactivator-1α; PPL1: protocerebral posterior lateral 1; ROS: reactive oxygen species; SCI: spinal cord injury; TBI: traumatic brain injury; TFAM: mitochondrial transcription factor A.
Traumatic brain insults and ischemia
Brain functional impairment resulting from different types of insults, such as traumatic brain injury (TBI), intracerebral hemorrhage, and ischemic damage occur as a consequence of neuronal alterations when mitochondrial dysfunction is present (Hiebert et al., 2015; Liu et al., 2018; Li et al., 2022a). In these systems, mitochondrial impairment leads to energy depletion, ATP exhaustion, and increased ROS, triggering neuronal death. In this context, promoting neuronal metabolism by PQQ may improve neuronal energetic efficiency triggered by injury thus delaying the progression of neurodegeneration. The first study identifying PQQ neuroprotection under TBI was documented in a rat model of TBI where PQQ dose-dependently prevented behavioral deficits and morphological alterations in brain tissues (Zhang et al., 2012a). Neuroprotective properties of PQQ were further demonstrated by improved electroencephalogram and reduced apoptosis after administration of PQQ under brain damage (Ye et al., 2017; Zhang et al., 2017). Evidence of PQQ exerting neuroprotective effects has been reported in a rat model of intracerebral hemorrhage, where PQQ treatment inhibited the decline in locomotor activity, the formation of hematomas and brain edemas, reducing ROS and neuronal apoptosis following intracerebral hemorrhage (Lu et al., 2015). Treatment with PQQ has been also reported to reduce brain infarct size and neurobehavioral impairment in in vivo models of cerebral ischemia (Jensen et al., 1994; Zhang et al., 2006). Although the neuroprotection of PQQ has been demonstrated in all these models, the clear association of improved neuronal viability with a promoted metabolic activity still needs to be rigorously and empirically tested.
Epilepsy and schizophrenia
As metabolic and mitochondrial OXPHOS provide the vast majority of ATP for neuronal activity, the dysfunction in these mechanisms may strongly alter neuronal excitability and synaptic transmission. These are affected by many neurological and psychiatric disorders, such as epilepsy and schizophrenia (Folbergrová and Kunz, 2012; Ni et al., 2022; Fizíková et al., 2023). A growing body of evidence in the last decade reports the involvement of mitochondrial dysfunction and energetic deficit in these diseases, and the treatment with PQQ may improve clinical symptoms associated with these disorders. As support to this, PQQ has been reported to have a beneficial effect in animal models where epilepsy or schizophrenia is modeled pharmacologically. PQQ has been reported to reduce behavioral seizures in vivo after the induction of epileptic injury by pentylenetetrazol or bicuculline methiodide injection (Sanchez et al., 2000). In addition, the treatment with PQQ in a model of schizophrenia induced by the injection of non-competitive NMDA antagonist MK-801 was effective in reducing stereotypical behavior, ataxia, and cognitive impairment even if supplemented from pregnancy (Zhou et al., 2014, Peng et al., 2022). These beneficial effects of PQQ still need to be correlated with its capacity to modulate metabolic and mitochondrial processes, and secondary mechanisms affected by the administration of this compound cannot be excluded. Given the involvement of dysregulated activity of NMDA receptors in these disorders (Balu, 2016; Kapur, 2018) and the capacity of PQQ to modulate its activity, it is likely that the protective effect of this compound in these contexts may be favored in part by such mechanism.
Spinal cord injury
In the early stages of axonal degeneration following spinal cord injury, mitochondrial dysfunction occurs resulting in energy deficiency and ATP depletion. This promotes and exacerbates neuronal death (Liu et al., 2022; Cheng et al., 2023). PQQ administration may improve metabolism and mitochondrial function, delaying axonal degeneration and preserving spinal cord neuron activity. Functional recovery of spinal cord neurons after hemi-transection was documented following PQQ administration in a rat model of spinal cord injury, accompanied by reduced size of the lesion with increased density of axons, improved morphological features, and reduced neuronal apoptosis (Hirakawa et al., 2009; Zhou et al., 2021). Further work is needed to correlate this suggested neuroprotective property of PQQ with an ameliorated bioenergetic balance and mitochondrial function.
Alzheimer’s disease
Metabolic alterations and mitochondrial dysfunctions have been reported to occur in the complex interplay of pathophysiological mechanisms influencing neuronal cell death in Alzheimer’s disease (Kang et al., 2017; Bhatia et al., 2022). In this context, it has been reported that PQQ partially restores bioenergetic deficits of hippocampal synaptosomes in hemizygous transgenic McGill-R-Thy1-APP rats by improving mitochondrial function and reducing oxidative stress, thus resulting in reduced cognitive impairment (Martino Adami et al., 2017). The neuroprotective efficacy of PQQ in Alzheimer’s disease is further suggested by another study in the APP/PS1 mice model, where the administration of PQQ in combination with lithium reduced the deposition of cerebral Aβ1–42 and phosphorylated tau, resulting in decreased impairment of learning and memory and improved hippocampal synaptic plasticity (Zhao et al., 2014).
Parkinson’s disease
Mitochondrial dysfunction and oxidative stress have been considered to have a central role in the establishment of PD pathogenesis and resulting in the selective death of dopaminergic neurons of the substantia nigra pars compacta (Wang et al., 2020). There is evidence suggesting the ability of PQQ to protect dopaminergic neurons from death occurring in PD by ameliorating mitochondrial processes. The administration of PQQ in a rat PD model (obtained by injecting rotenone in the medial forebrain bundle) reduced the decline in cognitive functions and neuronal loss, improved ROS scavenging and the expression of mitochondrial complex subunits together with tyrosine hydroxylase and vesicular monoamine transporter 2 (Qin et al., 2015; Zhang et al., 2016). In this respect, the treatment with PQQ in this rat PD model could inhibit the decline in the levels of mitochondrial dynamics-related markers such as PGC-1α, TFAM, dynamin-related protein 1, and mitofusin 2 in the midbrain following rotenone injection (Lu et al., 2018). Similarly, in a mouse model of PD obtained by intraperitoneal injection of rotenone, PQQ dose-dependently reduced locomotor deficits and nigral dopaminergic neuron loss, whilst preventing mitochondrial loss and morphological impairment and increasing the levels of PGC-1α and TFAM (Cheng et al., 2021). The pharmacological stimulation of the PGC-1α ortholog spargel in Drosophila by PQQ reduced dopaminergic neural loss and increased mitochondrial area in PD flies (Ng et al., 2017). Taken together, these studies suggest the potential of PQQ as a novel compound to improve mitochondrial function and treat neuronal degeneration occurring in PD.
Retinal degenerations
Only in the last few years, the potential of PQQ as a novel therapeutic in retinal diseases has been raised. There are only a few reports describing the beneficial effects of this compound in treating retinal alterations induced by metabolic dysfunctions occurring in different retinal pathologies. A recent paper from Ebeling and colleagues is, to our knowledge, the first study showing that PQQ may have a beneficial role in treating the dry form of age-related macular degeneration, a retinal disease where photoreceptors degenerate following retinal pigment epithelium degeneration that occurs as a consequence of mitochondrial and metabolic dysfunction (Ferrington et al., 2020). In these studies, PQQ was reported to upregulate mitochondrial proteins and improve mitochondrial function and ATP production in retinal pigment epithelium cells from age-related macular degeneration donors, providing evidence of the potential of PQQ for additional studies in retinal diseases (Ebeling et al., 2020). This potential of PQQ in treating retinal neurodegenerative diseases has been further investigated in other studies focusing on disorders involving another cell type, such as retinal ganglion cells, which degenerate in several diseases including glaucoma, autosomal dominant optic atrophy, and Leber’s hereditary optic neuropathy. Since in these pathological contexts, mitochondrial dysfunction and metabolic disruption concur in rendering retinal ganglion cells susceptible to damage and death, it was hypothesized that PQQ might confer bioenergetic support and protect these cells from degeneration induced by stressors. For this reason, Canovai and colleagues investigated the neuroprotective potential of PQQ in different models of retinal ganglion cell stress and studied the metabolic mechanisms by which this compound may alter the retinal bioenergetic balance. The authors of this study demonstrated that PQQ was neuroprotective in two different models of retinal ganglion cell stress, where either acute axonal damage or mitochondrial dysfunction induced an impaired bioenergetic balance with ATP depletion resulting in retinal ganglion cell death (Canovai et al., 2023). They identified an ATP-boosting activity and alteration of metabolic profiles in non-diseased retinal ganglion cell-related tissues, suggesting a likely mechanism by which PQQ exerts neuroprotective effects in retinal ganglion cell contexts, although a clear correlation between neuroprotection and improved metabolic processes still needs to be established, e.g., via specific genetic knockouts in mice (Canovai et al., 2023). Taken together, these data suggest the potential of PQQ as a novel adjuvant for the treatment of retinopathies, especially those that are characterized by metabolic dysfunction and altered bioenergetic balance.
Evidence of PQQ benefits in neuronal districts in humans
Evidence of PQQ neuronal benefits has been provided also in humans through several clinical trials describing the effect of PQQ supplementation on improving memory. A randomized, placebo-controlled, double-blinded study investigated the capacity of PQQ to improve cognitive function in elderly healthy patients by supplementing PQQ for 12 weeks and demonstrating functional improvements in attention and working memory (Itoh et al., 2016). Itoh and colleagues identified an increased prefrontal cortex blood flow and oxygen metabolism (Itoh et al., 2016; Nakano et al., 2016). The capacity of PQQ to improve cognitive function in healthy humans was further confirmed in additional clinical trials, where PQQ has been reported to improve brain function in both younger and older adults (Shiojima et al., 2022; Tamakoshi et al., 2023).
Future Perspectives
Considering the wide range and the strict context dependency of mechanisms related to metabolism and regulated by PQQ, the study of its effects in a specific system and disease is always recommended before drawing wide conclusions. Regarding neurodegenerative diseases, the necessity of fully elucidating the mechanisms behind which PQQ exerts its neuroprotective properties is fundamental. This is especially important if one considers that metabolic processes are characterized by a complex interplay of different components, which can be modulated by this compound either singularly or synergistically. In this respect, there is a need to further study what aspects of metabolism are regulated in different neurodegenerative disorders and if these mechanisms are shared among diseases or just typical of single pathological contexts for specific cell types. In addition, for several contexts, the clear correlation between neuroprotective properties of PQQ and its capacity to improve neuronal metabolism and mitochondrial function needs to be further investigated and confirmed. This correlation is required as PQQ may have many other different secondary effects, which might contribute to protecting neurons from degeneration or may be the downstream consequence of improved metabolism and neuronal viability. The molecular pathways affected by PQQ are still to be extensively characterized, although the involvement of AMP-activated protein kinase, serine–threonine liver kinase B1, phosphatidylinositol 3-kinase/Akt, and PGC-1α has been suggested in some contexts but not confirmed in others (Chowanadisai et al., 2010; Zhang et al., 2014, 2015; Saihara et al., 2017; Lu et al., 2018; Yamada et al., 2020; Cheng et al., 2021; Ishak et al., 2021; Canovai et al., 2023). To further study what pathways may be involved in PQQ mechanisms, knockout lines, and model animal tools are essential to dissect the different actors playing in the complex regulation of such compounds. In this respect, it may be fundamental to study if PQQ binds to some specific targets and the type of interactions they establish. Moreover, it is of paramount importance to assess if the mechanisms that PQQ is able to regulate in basic non-disease conditions are still influenced by the treatment with this compound under stress conditions and vice versa. For instance, Canovai and colleagues reported an ATP-boosting activity of PQQ in healthy retinal ganglion cell-related tissues with a concomitant moderate effect on mitochondrial mechanisms and variations in the whole metabolome (Canovai et al., 2023). It would be interesting to assess whether the same mechanisms are regulated by PQQ in these tissues under stress conditions or if the treatment with PQQ in the presence of stressors results in different outcomes.
Since PQQ can modulate metabolic processes in different aspects, all the neurodegenerative diseases characterized by metabolic dysfunction as one of the pathophysiological events determining neuronal death may benefit from PQQ administration. Dysfunctional metabolism and OXPHOS have been reported as common mechanisms occurring in different neuronal disorders in different districts of the nervous system, such as amyotrophic lateral sclerosis, Huntington’s disease, and multiple sclerosis (Procaccini et al., 2016, Muddapu et al., 2020). Consequently, testing the efficacy of PQQ in providing neuroprotection in such contexts may be a good strategy for studying a novel therapeutic tool to delay the progress of the disease. In retinal diseases, there is a wide range of disorders where metabolic disruption and mitochondrial dysfunction play a critical role in determining retinal cell death. In particular, retinal ganglion cells are very susceptible retinal neurons with a fine-tuned metabolism requiring a strictly controlled ATP production for their activity. The ATP-boosting capacity and the neuroprotective capacity of PQQ might be fundamental to protect retinal ganglion cells from death, as the preliminary data of PQQ protection in models of acute retinal ganglion cell stress from Canovai and colleagues might suggest (Canovai et al., 2023). Future investigations in specific models of these diseases will be essential to further assess and confirm the neuroprotective potential of PQQ as a novel adjuvant therapy to treat such disorders.
Another issue to solve with PQQ is represented by its poor bioavailability, especially in neuronal tissues. Pharmacological studies are needed to assess the exact PQQ bioavailability and its pharmacokinetics in brain and retinal tissues, as well as the assessment of putative derivatives, which might be involved in the effects identified from the treatment of this compound. There is the necessity of finding a feasible and easy administration route with a proper bioavailability to reach the neuroprotective effect with a non-invasive administration. Dietary/oral administration, which may be the most practicable way to give PQQ since it is contained in food, has not been demonstrated to give a sufficient bioavailability in neuronal tissues from the studies held so far. Oral administration of PQQ in mice resulted in a poor brain bioavailability after 6 and 24 hours from the administration (Smidt et al., 1991). In addition, previous work by Canovai and colleagues identified a negligible effect of orally given PQQ (in the form of BioPQQ, the disodium salt) in modulating ATP and NAD content in tissues from the visual system and brain cortex, suggesting that the dietary administration at that dose could not reach an effective bioavailability in such tissues (Canovai et al., 2023). Further development is required to establish a proper dose sufficient to provide a good PQQ bioavailability to have metabolic effects and provide neuroprotection if PQQ needs to be administered by oral routes. Strategies of drug delivery systems might be useful to further improve the bioavailability of PQQ by favoring its mobility across body barriers and increasing the concentration of PQQ in body systems (e.g., absorption in the gastrointestinal tract and blood-brain barrier permeability). The possibility to synthetize chemically engineered stabilized versions or include PQQ in carries such as liposomes or nanoparticles may improve its stability and increase its bioavailability, resulting in a potentiated effect. Alternatively, the design of slow-release or packaged formulations or a combinatory treatment with other compounds, which may synergistically act with PQQ might represent different strategies to further improve the outcomes and reach a better neuroprotection.
Taken together, although promising, there is a lot of work to do before considering the translation of PQQ to clinical settings, especially in the context of neurodegenerative disease. A deep understanding of PQQ mechanisms, the assessment of its efficacy in complex models of neurodegeneration, and the study of its pharmacology in the body systems are fundamental steps that need to be done before starting the administration of PQQ in the clinical management of neurodegenerative diseases. Studies assessing the correct absorption, distribution, metabolism, and excretion in humans may add more information about PQQ pharmacology and help to design further studies evaluating its efficacy and mechanisms. Identifying the optimal route of administration and the optimal dosage of PQQ in order to have significant effects with low invasiveness and high compliance in humans would be a reasonable aim in the future to favor the use of PQQ in clinical settings.
Conclusions
The capacity of PQQ to regulate basic cellular metabolism suggests that PQQ has the potential to be a good neuroprotective compound in the pathological contexts where metabolic imbalances and ATP depletion occur (Figure 1). Improved metabolic and mitochondrial metabolism and homeostasis under treatment with PQQ may improve neuronal resilience and cell viability, ultimately delaying the progression of neurodegeneration. Although further studies are needed to better characterize the metabolic processes regulated by PQQ in several neurodegenerative diseases, the potential of PQQ as a novel adjuvant neuroprotectant supplementing the existing therapies is supported by several studies. The presence of PQQ in food and the possibility to administer this compound by oral supplementation presents an opportunity to use PQQ as a future tool in the clinic with a low risk of side effects if optimized for dosage and delivery system in humans.
Figure 1.
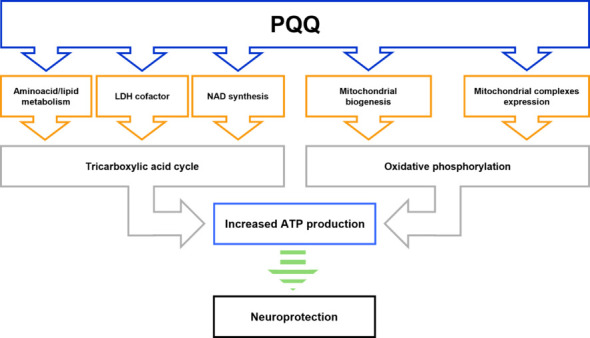
Schematic diagram depicting the metabolic processes that are potentially regulated by PQQ under physiological conditions and may provide neuroprotection.
PQQ has been reported to influence many metabolic mechanisms fueling tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS), thus regulating basic cell metabolism. In particular, PQQ can modulate cell metabolism by influencing amino acid and lipid metabolism, acting as lactate dehydrogenase (LDH) cofactor, promoting nicotinamide adenine dinucleotide (NAD) synthesis, inducing mitochondrial biogenesis and complexes expression. These mechanisms can be regulated by PQQ either singularly or synergistically, in a very context-dependent manner among different systems. The modulation of these metabolic processes can improve TCA cycle and potentiate the oxidative phosphorylation (OXPHOS), thus resulting in increased adenosine 5′-triphosphate (ATP) production. The neuroprotective properties of PQQ documented in many different models of neurodegeneration may be related to its capacity to promote neuronal metabolism, although in some contexts this strict association needs to be further confirmed (and hence indicated in the figure by the green dotted arrow). Created with Microsoft PowerPoint. PQQ: Pyrroloquinoline quinone.
Additional file: Open peer review report 1 (78.8KB, pdf) .
Funding Statement
Funding: PAW was supported by Karolinska Institutet in the form of a Board of Research Faculty Funded Career Position, by St. Erik Eye Hospital philanthropic donations, and Vetenskapsrådet 2022-00799.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: The data are available from the corresponding author on reasonable request.
Open peer reviewer: Robert B. Rucker, University of California, Davis, USA.
P-Reviewer: Rucker RB; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- Aizenman E, Hartnett KA, Zhong C, Gallop PM, Rosenberg PA. Interaction of the putative essential nutrient pyrroloquinoline quinone with the N-methyl-D-aspartate receptor redox modulatory site. J Neurosci. 1992;12:2362–2369. doi: 10.1523/JNEUROSCI.12-06-02362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman E, Jensen FE, Gallop PM, Rosenberg PA, Tang LH. Further evidence that pyrroloquinoline quinone interacts with the N-methyl-D-aspartate receptor redox site in rat cortical neurons in vitro. Neurosci Lett. 1994;168:189–192. doi: 10.1016/0304-3940(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Akagawa M, Minematsu K, Shibata T, Kondo T, Ishii T, Uchida K. Identification of lactate dehydrogenase as a mammalian pyrroloquinoline quinone (PQQ)-binding protein. Sci Rep. 2016a;6:26723. doi: 10.1038/srep26723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagawa M, Nakano M, Ikemoto K. Recent progress in studies on the health benefits of pyrroloquinoline quinone. Biosci Biotechnol Biochem. 2016b;80:13–22. doi: 10.1080/09168451.2015.1062715. [DOI] [PubMed] [Google Scholar]
- Ameyama M, Nonobe M, Shinagawa E, Matsushita K, Adachi O. Method of enzymatic determination of pyrroloquinoline quinone. Anal Biochem. 1985;151:263–267. doi: 10.1016/0003-2697(85)90174-5. [DOI] [PubMed] [Google Scholar]
- Arnold PK, Finley LWS. Regulation and function of the mammalian tricarboxylic acid cycle. J Biol Chem. 2023;299:102838. doi: 10.1016/j.jbc.2022.102838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT. The NMDA receptor and schizophrenia: from pathophysiology to treatment. Adv Pharmacol. 2016;76:351–382. doi: 10.1016/bs.apha.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerly KA, Storms DH, Harris CB, Hajizadeh S, Sun MY, Cheung CP, Satre MA, Fascetti AJ, Tchaparian E, Rucker RB. Pyrroloquinoline quinone nutritional status alters lysine metabolism and modulates mitochondrial DNA content in the mouse and rat. Biochim Biophys Acta. 20061760:1741–1748. doi: 10.1016/j.bbagen.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Bauerly K, Harris C, Chowanadisai W, Graham J, Havel PJ, Tchaparian E, Satre M, Karliner JS, Rucker RB. Altering pyrroloquinoline quinone nutritional status modulates mitochondrial, lipid, and energy metabolism in rats. PLoS One. 2011;6:e21779. doi: 10.1371/journal.pone.0021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergethon PR. Amperometric electrochemical detection of pyrroloquinoline quinone in high-performance liquid chromatography. Anal Biochem. 1990;186:324–327. doi: 10.1016/0003-2697(90)90089-r. [DOI] [PubMed] [Google Scholar]; Erratum. Anal Biochem. 194:449. [Google Scholar]
- Bhatia S, Rawal R, Sharma P, Singh T, Singh M, Singh V. Mitochondrial dysfunction in Alzheimer’s disease: opportunities for drug development. Curr Neuropharmacol. 2022;20:675–692. doi: 10.2174/1570159X19666210517114016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canovai A, Tribble JR, Jöe M, Westerlund DY, Amato R, Trounce IA, Dal Monte M, Williams PA. Pyrroloquinoline quinone drives ATP synthesis in vitro and in vivo and provides retinal ganglion cell neuroprotection. Acta Neuropathol Commun. 2023;11:146. doi: 10.1186/s40478-023-01642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SI, Chuankhayan P, Reddy Nareddy PK, Tsai IK, Tsai YF, Chen KH, Yu SS, Chen CJ. Mechanism of pyrroloquinoline quinone-dependent hydride transfer chemistry from spectroscopic and high-resolution x-ray structural studies of the methanol dehydrogenase from methylococcus capsulatus (Bath) J Am Chem Soc. 2021;143:3359–3372. doi: 10.1021/jacs.0c11414. [DOI] [PubMed] [Google Scholar]
- Chen Y, Coorey NJ, Zhang M, Zeng S, Madigan MC, Zhang X, Gillies MC, Zhu L, Zhang T. Metabolism dysregulation in retinal diseases and related therapies. Antioxidants (Basel) 2022;11:942. doi: 10.3390/antiox11050942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Cai B, Lu D, Zeng H. The role of mitochondrial energy metabolism in neuroprotection and axonal regeneration after spinal cord injury. Mitochondrion. 2023;69:57–63. doi: 10.1016/j.mito.2023.01.009. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Chen J, Guo H, Lu JL, Zhou J, Guo XY, Shi Y, Zhang Y, Yu S, Zhang Q, Ding F. Pyrroloquinoline quinone promotes mitochondrial biogenesis in rotenone-induced Parkinson’s disease model via AMPK activation. Acta Pharmacol Sin. 2021;42:665–678. doi: 10.1038/s41401-020-0487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowanadisai W, Bauerly KA, Tchaparian E, Wong A, Cortopassi GA, Rucker RB. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression. J Biol Chem. 2010;285:142–152. doi: 10.1074/jbc.M109.030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell GA, Daley SK. Pyrroloquinoline quinone chemistry, biology, and biosynthesis. Chem Res Toxicol. 2022;35:355–377. doi: 10.1021/acs.chemrestox.1c00340. [DOI] [PubMed] [Google Scholar]
- Ding XW, Li R, Geetha T, Tao YX, Babu JR. Nerve growth factor in metabolic complications and Alzheimer’s disease: physiology and therapeutic potential. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165858. doi: 10.1016/j.bbadis.2020.165858. [DOI] [PubMed] [Google Scholar]
- Ebeling MC, Polanco JR, Qu J, Tu C, Montezuma SR, Ferrington DA. Improving retinal mitochondrial function as a treatment for age-related macular degeneration. Redox Biol. 2020;34:101552. doi: 10.1016/j.redox.2020.101552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton LM, Anthony C. Biochemistry: role of PQQ as a mammalian enzyme cofactor? Nature. 2005;433:E10–12. doi: 10.1038/nature03322. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Fisher CR, Kowluru RA. Mitochondrial defects drive degenerative retinal diseases. Trends Mol Med. 2020;26:105–118. doi: 10.1016/j.molmed.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizíková I, Dragašek J, Račay P. Mitochondrial dysfunction, altered mitochondrial oxygen, and energy metabolism associated with the pathogenesis of schizophrenia. Int J Mol Sci. 2023;24:7991. doi: 10.3390/ijms24097991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folbergrová J, Kunz WS. Mitochondrial dysfunction in epilepsy. Mitochondrion. 2012;12:35–40. doi: 10.1016/j.mito.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Gruber JV, Holtz R. Pyrroloquinoline Quinone Disodium (PQQ2Na) has an NLRP inflammasome-induced caspase-1 release influence in UVB-irradiated but not ATP-treated human keratinocytes but has no influence in increasing skin cell mitochondrial biogenesis in either human keratinocytes or fibroblasts. Clin Cosmet Investig Dermatol. 2022;15:107–115. doi: 10.2147/CCID.S343123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S, Xu J, Guo Y, Ge D, Liu T, Ma X, Cui Z. Pyrroloquinoline quinone against glutamate-induced neurotoxicity in cultured neural stem and progenitor cells. Int J Dev Neurosci. 2015;42:37–45. doi: 10.1016/j.ijdevneu.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Harris CB, Chowanadisai W, Mishchuk DO, Satre MA, Slupsky CM, Rucker RB. Dietary pyrroloquinoline quinone (PQQ) alters indicators of inflammation and mitochondrial-related metabolism in human subjects. J Nutr Biochem. 2013;24:2076–2084. doi: 10.1016/j.jnutbio.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Hauge JG. Glucose dehydrogenase of bacterium anitratum: an enzyme with a novel prosthetic group. J Biol Chem. 1964;239:3630–3639. [PubMed] [Google Scholar]
- Hiebert JB, Shen Q, Thimmesch AR, Pierce JD. Traumatic brain injury and mitochondrial dysfunction. Am J Med Sci. 2015;350:132–138. doi: 10.1097/MAJ.0000000000000506. [DOI] [PubMed] [Google Scholar]
- Hirakawa A, Shimizu K, Fukumitsu H, Furukawa S. Pyrroloquinoline quinone attenuates iNOS gene expression in the injured spinal cord. Biochem Biophys Res Commun. 2009;378:308–312. doi: 10.1016/j.bbrc.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Ishak NSM, Ikemoto K, Kikuchi M, Ogawa M, Akutagawa K, Akagawa M. Pyrroloquinoline quinone attenuates fat accumulation in obese mice fed with a high-fat diet, Daphnia magna supplied with a high amount of food, and 3T3-L1 adipocytes. ACS Food Sci Technol. 2021;1:1979–1989. [Google Scholar]
- Itoh Y, Hine K, Miura H, Uetake T, Nakano M, Takemura N, Sakatani K. Effect of the antioxidant supplement pyrroloquinoline quinone disodium salt (BioPQQ™) on cognitive functions. Adv Exp Med Biol. 2016;876:319–325. doi: 10.1007/978-1-4939-3023-4_40. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Gardner GJ, Williams AP, Gallop PM, Aizenman E, Rosenberg PA. The putative essential nutrient pyrroloquinoline quinone is neuroprotective in a rodent model of hypoxic/ischemic brain injury. Neuroscience. 1994;62:399–406. doi: 10.1016/0306-4522(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Jonscher KR, Chowanadisai W, Rucker RB. Pyrroloquinoline-quinone is more than an antioxidant: a vitamin-like accessory factor important in health and disease prevention. Biomolecules. 2021;11:1441. doi: 10.3390/biom11101441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Lee YH, Lee JE. Metabolism-centric overview of the pathogenesis of Alzheimer’s disease. Yonsei Med J. 2017;58:479–488. doi: 10.3349/ymj.2017.58.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J. Role of NMDA receptors in the pathophysiology and treatment of status epilepticus. Epilepsia Open. 2018;3:165–168. doi: 10.1002/epi4.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara T, Kato T. Nutritional biochemistry: a new redox-cofactor vitamin for mammals. Nature. 2003;422:832. doi: 10.1038/422832a. [DOI] [PubMed] [Google Scholar]
- Kato C, Kawai E, Shimizu N, Mikekado T, Kimura F, Miyazawa T, Nakagawa K. Determination of pyrroloquinoline quinone by enzymatic and LC-MS/MS methods to clarify its levels in foods. PLoS One. 2018;13:e0209700. doi: 10.1371/journal.pone.0209700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Harada R, Kobayashi M, Kobayashi N, Sode K. The inhibitory effect of pyrroloquinoline quinone on the amyloid formation and cytotoxicity of truncated alpha-synuclein. Mol Neurodegener. 2010;5:20. doi: 10.1186/1750-1326-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Kim J, Kobayashi N, Han S, Nakamura C, Ikebukuro K, Sode K. Pyrroloquinoline quinone (PQQ) prevents fibril formation of alpha-synuclein. Biochem Biophys Res Commun. 2006;349:1139–1144. doi: 10.1016/j.bbrc.2006.08.144. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Seno H, Urakami T, Matsumoto T, Suzuki O. Trace levels of pyrroloquinoline quinone in human and rat samples detected by gas chromatography/mass spectrometry. Biochim Biophys Acta. 19921156:62–66. doi: 10.1016/0304-4165(92)90096-d. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Sato K, Seno H, Ishii A, Suzuki O. Levels of pyrroloquinoline quinone in various foods. Biochem J. 1995;307:331–333. doi: 10.1042/bj3070331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Noroozifar M, Zhou J, Kerman K. Electrochemical flow injection analysis of the interaction between pyrroloquinoline quinone (PQQ) and α-synuclein peptides related to Parkinson’s disease. Analyst. 2021;146:4545–4556. doi: 10.1039/d1an00698c. [DOI] [PubMed] [Google Scholar]
- Li S, Raja A, Noroozifar M, Kerman K. Understanding the inhibitory and antioxidant effects of pyrroloquinoline quinone (PQQ) on copper(II)-induced α-synuclein-119 aggregation. ACS Chem Neurosci. 2022a;13:1178–1186. doi: 10.1021/acschemneuro.1c00703. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu H, Tian C, An N, Song K, Wei Y, Sun Y, Xing Y, Gao Y. Targeting the multifaceted roles of mitochondria in intracerebral hemorrhage and therapeutic prospects. Biomed Pharmacother. 2022b;148:112749. doi: 10.1016/j.biopha.2022.112749. [DOI] [PubMed] [Google Scholar]
- Liang C, Zhang X, Wang W, Song Y, Jia X. A subchronic oral toxicity study on pyrroloquinoline quinone (PQQ) disodium salt in rats. Food Chem Toxicol. 2015;75:146–150. doi: 10.1016/j.fct.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Liu C, Liu Y, Ma B, Zhou M, Zhao X, Fu X, Kan S, Hu W, Zhu R. Mitochondrial regulatory mechanisms in spinal cord injury: a narrative review. Medicine (Baltimore) 2022;101:e31930. doi: 10.1097/MD.0000000000031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Lu J, Manaenko A, Tang J, Hu Q. Mitochondria in ischemic stroke: new insight and implications. Aging Dis. 2018;9:924–937. doi: 10.14336/AD.2017.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Shen J, Song X, Ge J, Cai R, Dai A, Jiang Z. Protective effect of pyrroloquinoline quinone (PQQ) in rat model of intracerebral hemorrhage. Cell Mol Neurobiol. 2015;35:921–930. doi: 10.1007/s10571-015-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Chen S, Shen M, He Q, Zhang Y, Shi Y, Ding F, Zhang Q. Mitochondrial regulation by pyrroloquinoline quinone prevents rotenone-induced neurotoxicity in Parkinson’s disease models. Neurosci Lett. 2018;687:104–110. doi: 10.1016/j.neulet.2018.09.031. [DOI] [PubMed] [Google Scholar]
- Martino Adami PV, Quijano C, Magnani N, Galeano P, Evelson P, Cassina A, Do Carmo S, Leal MC, Castaño EM, Cuello AC, Morelli L. Synaptosomal bioenergetic defects are associated with cognitive impairment in a transgenic rat model of early Alzheimer’s disease. J Cereb Blood Flow Metab. 2017;37:69–84. doi: 10.1177/0271678X15615132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorana F, Gaglio D, Bianco MR, Aprea F, Virtuoso A, Bonanomi M, Alberghina L, Papa M, Colangelo AM. Differentiation by nerve growth factor (NGF) involves mechanisms of crosstalk between energy homeostasis and mitochondrial remodeling. Cell Death Dis. 2018;9:391. doi: 10.1038/s41419-018-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AE, Jones AD, Mercer RS, Rucker RB. Characterization of pyrroloquinoline quinone amino acid derivatives by electrospray ionization mass spectrometry and detection in human milk. Anal Biochem. 1999;269:317–325. doi: 10.1006/abio.1999.4039. [DOI] [PubMed] [Google Scholar]
- Moon HE, Paek SH. Mitochondrial dysfunction in Parkinson’s disease. Exp Neurobiol. 2015;24:103–116. doi: 10.5607/en.2015.24.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddapu VR, Dharshini SAP, Chakravarthy VS, Gromiha MM. Neurodegenerative diseases - Is metabolic deficiency the root cause? Front Neurosci. 2020;14:213. doi: 10.3389/fnins.2020.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench NA, Patel S, Maes ME, Donahue RJ, Ikeda A, Nickells RW. The influence of mitochondrial dynamics and function on retinal ganglion cell susceptibility in optic nerve disease. Cells. 2021;10:1593. doi: 10.3390/cells10071593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M, Suzuki H, Imamura T, Lau A, Lynch B. Genotoxicity of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™) Regul Toxicol Pharmacol. 2013;67:189–197. doi: 10.1016/j.yrtph.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Nakano M, Takahashi H, Koura S, Chung C, Tafazoli S, Roberts A. Acute and subchronic toxicity studies of pyrroloquinoline quinone (PQQ) disodium salt (BioPQQ™) in rats. Regul Toxicol Pharmacol. 2014;70:107–121. doi: 10.1016/j.yrtph.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Nakano M, Murayama Y, Hu L, Ikemoto K, Uetake T, Sakatani K. Effects of antioxidant supplements (BioPQQ™) on cerebral blood flow and oxygen metabolism in the prefrontal cortex. Adv Exp Med Biol. 2016;923:215–222. doi: 10.1007/978-3-319-38810-6_29. [DOI] [PubMed] [Google Scholar]
- Ng CH, Basil AH, Hang L, Tan R, Goh KL, O’Neill S, Zhang X, Yu F, Lim KL. Genetic or pharmacological activation of the Drosophila PGC-1α ortholog spargel rescues the disease phenotypes of genetic models of Parkinson’s disease. Neurobiol Aging. 2017;55:33–37. doi: 10.1016/j.neurobiolaging.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Ni P, Ma Y, Chung S. Mitochondrial dysfunction in psychiatric disorders. Schizophr Res. 2022 doi: 10.1016/j.schres.2022.08.027. doi: 10.1016/j.schres.2022.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji N, Nakamura T, Kitahata N, Taguchi K, Kudo T, Yoshida S, Tsujimoto M, Sugiyama T, Asami T. Simple and sensitive method for pyrroloquinoline quinone (PQQ) analysis in various foods using liquid chromatography/electrospray-ionization tandem mass spectrometry. J Agric Food Chem. 2007;55:7258–7263. doi: 10.1021/jf070483r. [DOI] [PubMed] [Google Scholar]
- Nunome K, Miyazaki S, Nakano M, Iguchi-Ariga S, Ariga H. Pyrroloquinoline quinone prevents oxidative stress-induced neuronal death probably through changes in oxidative status of DJ-1. Biol Pharm Bull. 2008;31:1321–1326. doi: 10.1248/bpb.31.1321. [DOI] [PubMed] [Google Scholar]
- Ouchi A, Ikemoto K, Nakano M, Nagaoka S, Mukai K. Kinetic study of aroxyl radical scavenging and α-tocopheroxyl regeneration rates of pyrroloquinolinequinol (PQQH2, a reduced form of pyrroloquinolinequinone) in dimethyl sulfoxide solution: finding of synergistic effect on the reaction rate due to the coexistence of α-tocopherol and PQQH2. J Agric Food Chem. 2013;61:11048–11060. doi: 10.1021/jf4040496. [DOI] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- Park JS, Davis RL, Sue CM. Mitochondrial dysfunction in parkinson’s disease: new mechanistic insights and therapeutic perspectives. Curr Neurol Neurosci Rep. 2018;18:21. doi: 10.1007/s11910-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz MA, Martin P, Flückiger R, Mah J, Gallop PM. The catalysis of redox cycling by pyrroloquinoline quinone (PQQ), PQQ derivatives, and isomers and the specificity of inhibitors. Anal Biochem. 1996;238:145–149. doi: 10.1006/abio.1996.0267. [DOI] [PubMed] [Google Scholar]
- Peng Y, Xu D, Ding Y, Zhou X. Supplementation of PQQ from pregnancy prevents MK-801-induced schizophrenia-like behaviors in mice. Psychopharmacology (Berl) 2022;239:2263–2275. doi: 10.1007/s00213-022-06113-9. [DOI] [PubMed] [Google Scholar]
- Procaccini C, Santopaolo M, Faicchia D, Colamatteo A, Formisano L, de Candia P, Galgani M, De Rosa V, Matarese G. Role of metabolism in neurodegenerative disorders. Metabolism. 2016;65:1376–1390. doi: 10.1016/j.metabol.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Qin J, Wu M, Yu S, Gao X, Zhang J, Dong X, Ji J, Zhang Y, Zhou L, Zhang Q, Ding F. Pyrroloquinoline quinone-conferred neuroprotection in rotenone models of Parkinson’s disease. Toxicol Lett. 2015;238:70–82. doi: 10.1016/j.toxlet.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Roberts RC. Mitochondrial dysfunction in schizophrenia: with a focus on postmortem studies. Mitochondrion. 2021;56:91–101. doi: 10.1016/j.mito.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker R, Storms D, Sheets A, Tchaparian E, Fascetti A. Biochemistry: is pyrroloquinoline quinone a vitamin? Nature. 2005;433:E10–12. doi: 10.1038/nature03323. [DOI] [PubMed] [Google Scholar]
- Rucker R, Chowanadisai W, Nakano M. Potential physiological importance of pyrroloquinoline quinone. Altern Med Rev. 2009;14:268–277. [PubMed] [Google Scholar]
- Saihara K, Kamikubo R, Ikemoto K, Uchida K, Akagawa M. Pyrroloquinoline quinone, a redox-active o-quinone, stimulates mitochondrial biogenesis by activating the SIRT1/PGC-1α Signaling Pathway. Biochemistry. 2017;56:6615–6625. doi: 10.1021/acs.biochem.7b01185. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Wang C, Gardner G, Orlando L, Tauck DL, Rosenberg PA, Aizenman E, Jensen FE. Novel role for the NMDA receptor redox modulatory site in the pathophysiology of seizures. J Neurosci. 2000;20:2409–2417. doi: 10.1523/JNEUROSCI.20-06-02409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon JM, Aizenman E, Reynolds IJ. Effects of pyrroloquinoline quinone on glutamate-induced production of reactive oxygen species in neurons. Eur J Pharmacol. 1997;326:67–74. doi: 10.1016/s0014-2999(97)00137-4. [DOI] [PubMed] [Google Scholar]
- Shiojima Y, Takahashi M, Takahashi R, Moriyama H, Bagchi D, Bagchi M, Akanuma M. Effect of dietary pyrroloquinoline quinone disodium salt on cognitive function in healthy volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. J Am Nutr Assoc. 2022;41:796–809. doi: 10.1080/07315724.2021.1962770. [DOI] [PubMed] [Google Scholar]; Erratum. J Am Nutr Assoc. :1. PMID: 34415830. [Google Scholar]
- Slater PG, Domínguez-Romero ME, Villarreal M, Eisner V, Larraín J. Mitochondrial function in spinal cord injury and regeneration. Cell Mol Life Sci. 2022;79:239. doi: 10.1007/s00018-022-04261-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidt CR, Unkefer CJ, Houck DR, Rucker RB. Intestinal absorption and tissue distribution of [14C]pyrroloquinoline quinone in mice. Proc Soc Exp Biol Med. 1991;197:27–31. doi: 10.3181/00379727-197-43219. [DOI] [PubMed] [Google Scholar]
- Steinberg FM, Gershwin ME, Rucker RB. Dietary pyrroloquinoline quinone: growth and immune response in BALB/c mice. J Nutr. 1994;124:744–753. doi: 10.1093/jn/124.5.744. [DOI] [PubMed] [Google Scholar]
- Steinberg F, Stites TE, Anderson P, Storms D, Chan I, Eghbali S, Rucker R. Pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp Biol Med (Maywood) 2003;228:160–166. doi: 10.1177/153537020322800205. [DOI] [PubMed] [Google Scholar]
- Stites TE, Mitchell AE, Rucker RB. Physiological importance of quinoenzymes and the O-quinone family of cofactors. J Nutr. 2000;130:719–727. doi: 10.1093/jn/130.4.719. [DOI] [PubMed] [Google Scholar]
- Stites T, Storms D, Bauerly K, Mah J, Harris C, Fascetti A, Rogers Q, Tchaparian E, Satre M, Rucker RB. Pyrroloquinoline quinone modulates mitochondrial quantity and function in mice. J Nutr. 2006;136:390–396. doi: 10.1093/jn/136.2.390. [DOI] [PubMed] [Google Scholar]
- Tamakoshi M, Suzuki T, Nishihara E, Nakamura S, Ikemoto K. Pyrroloquinoline quinone disodium salt improves brain function in both younger and older adults. Food Funct. 2023;14:2496–2501. doi: 10.1039/d2fo01515c. [DOI] [PubMed] [Google Scholar]
- Urakami T, Tanaka A, Yamaguchi K, Tsuji T, Niki E. Synthesis of esters of coenzyme PQQ and IPQ, and stimulation of nerve growth factor production. Biofactors. 1995;5:139–146. [PubMed] [Google Scholar]
- Várnai A, Umezawa K, Yoshida M, Eijsink VGH. The pyrroloquinoline-quinone-dependent pyranose dehydrogenase from coprinopsis cinerea drives lytic polysaccharide monooxygenase action. Appl Environ Microbiol. 2018;84:e00156–18. doi: 10.1128/AEM.00156-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhao F, Ma X, Perry G, Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol Neurodegener. 2020;15:30. doi: 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Nishii K, Kuwata K, Nakamichi M, Nakanishi K, Sugimoto A, Ikemoto K. Effects of pyrroloquinoline quinone and imidazole pyrroloquinoline on biological activities and neural functions. Heliyon. 2020;6:e03240. doi: 10.1016/j.heliyon.2020.e03240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Sasano A, Urakami T, Tsuji T, Kondo K. Stimulation of nerve growth factor production by pyrroloquinoline quinone and its derivatives in vitro and in vivo. Biosci Biotechnol Biochem. 1993;57:1231–1233. doi: 10.1271/bbb.57.1231. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Tsuji T, Uemura D, Kondo K. Cyclooxygenase induction is essential for NGF synthesis enhancement by NGF inducers in L-M cells. Biosci Biotechnol Biochem. 1996;60:92–94. doi: 10.1271/bbb.60.92. [DOI] [PubMed] [Google Scholar]
- Ye Y, Zhang P, Qian Y, Yin B, Yan M. The effect of pyrroloquinoline quinone on the expression of WISP1 in traumatic brain injury. Stem Cells Int. 2017;2017:4782820. doi: 10.1155/2017/4782820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Meruvu S, Bedi YS, Chau J, Arguelles A, Rucker R, Choudhury M. Pyrroloquinoline quinone increases the expression and activity of Sirt1 and -3 genes in HepG2 cells. Nutr Res. 2015;35:844–849. doi: 10.1016/j.nutres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Zhang RF, Meng XK. Protective effect of pyrroloquinoline quinone against Abeta-induced neurotoxicity in human neuroblastoma SH-SY5Y cells. Neurosci Lett. 2009;464:165–169. doi: 10.1016/j.neulet.2009.08.037. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu J, Cheng C, Yuan Y, Yu B, Shen A, Yan M. The neuroprotective effect of pyrroloquinoline quinone on traumatic brain injury. J Neurotrauma. 2012a;29:851–864. doi: 10.1089/neu.2011.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Ye Y, Qian Y, Yin B, Zhao J, Zhu S, Zhang L, Yan M. The effect of pyrroloquinoline quinone on apoptosis and autophagy in traumatic brain injury. CNS Neurol Disord Drug Targets. 2017;16:724–736. doi: 10.2174/1871527316666170124164306. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shen M, Ding M, Shen D, Ding F. The neuroprotective action of pyrroloquinoline quinone against glutamate-induced apoptosis in hippocampal neurons is mediated through the activation of PI3K/Akt pathway. Toxicol Appl Pharmacol. 2011;252:62–72. doi: 10.1016/j.taap.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ding M, Gao XR, Ding F. Pyrroloquinoline quinone rescues hippocampal neurons from glutamate-induced cell death through activation of Nrf2 and up-regulation of antioxidant genes. Genet Mol Res. 2012b;11:2652–2664. doi: 10.4238/2012.June.27.3. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang J, Jiang C, Qin J, Ke K, Ding F. Involvement of ERK1/2 pathway in neuroprotective effects of pyrroloquinoline quinine against rotenone-induced SH-SY5Y cell injury. Neuroscience. 2014;270:183–191. doi: 10.1016/j.neuroscience.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chen S, Yu S, Qin J, Zhang J, Cheng Q, Ke K, Ding F. Neuroprotective effects of pyrroloquinoline quinone against rotenone injury in primary cultured midbrain neurons and in a rat model of Parkinson’s disease. Neuropharmacology. 2016;108:238–251. doi: 10.1016/j.neuropharm.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rosenberg PA. The essential nutrient pyrroloquinoline quinone may act as a neuroprotectant by suppressing peroxynitrite formation. Eur J Neurosci. 2002;16:1015–1024. doi: 10.1046/j.1460-9568.2002.02169.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feustel PJ, Kimelberg HK. Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible middle cerebral artery occlusion in the adult rat. Brain Res. 20061094:200–206. doi: 10.1016/j.brainres.2006.03.111. [DOI] [PubMed] [Google Scholar]
- Zhao L, Gong N, Liu M, Pan X, Sang S, Sun X, Yu Z, Fang Q, Zhao N, Fei G, Jin L, Zhong C, Xu T. Beneficial synergistic effects of microdose lithium with pyrroloquinoline quinone in an Alzheimer’s disease mouse model. Neurobiol Aging. 2014;35:2736–2745. doi: 10.1016/j.neurobiolaging.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Jin H, Shi N, Gao S, Wang X, Zhu S, Yan M. Inhibit inflammation and apoptosis of pyrroloquinoline on spinal cord injury in rat. Ann Transl Med. 2021;9:1360. doi: 10.21037/atm-21-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Chen Q, Hu X, Mao S, Kong Y. Pyrroloquinoline quinone prevents MK-801-induced stereotypical behavior and cognitive deficits in mice. Behav Brain Res. 2014;258:153–159. doi: 10.1016/j.bbr.2013.10.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


