Abstract
The scientific literature dealing with alcohol and alcoholic beverages revealed that these drinks possess an adverse impact on periodontal tissues. Additionally, other principal risk factors include tobacco, smoking, poor oral hygiene, etc. It has been observed that among chronic alcoholics, there are further issues, such as mental, social, and physical effects, that promote alcoholism. These people may have weak immunity for defense against pathogenic organisms and bacteria. Thus, chances of gingival bleeding, swollen gums, bad breath, and increased bone loss are there. Different alcoholic beverages in the market cause less salivation; these beverages contain sugars that promote acid production in the oral cavity by pathogens that demineralize the enamel and damage gum and teeth. This chronic alcohol consumption can progress into different types of oral disorders, including cancer, halitosis, and caries, and is also associated with tobacco and smoking. Chronic alcohol consumption can cause alteration of the oral microbiome and increase oral pathogens, which lead to periodontal disease and an environment of inflammation created in the body due to malnutrition, diminished immunity, altered liver condition, brain damage, and gut microbiota alteration. Heavily colored alcoholic beverages produce staining on teeth and, due to less saliva, may cause other toxic effects on the periodontium. Over-dependency on alcohol leads to necrotizing lesions such as necrotizing gingivitis, necrotizing periodontitis, and necrotizing stomatitis. These pathological impairments instigate severe damage to oral structures. Therefore, proper counseling by the attending dental surgeon and related health professionals is urgently required for the patient on the basis that the individual case needs to go away from the regular heavy consumption of alcohol.
Keywords: necrotizing periodontal diseases, alcohol-induced carcinoma, tooth decay, tooth cavity, alveolar or bundle bone, normal gamma-glutamyl transferase (ggt), periodontitis, gum disease, liquor, booze
Introduction and background
Periodontal diseases include inflammatory conditions that affect the supporting structures of the teeth, including the gingiva and bone, which may lead to tooth loss and other systemic inflammation [1,2]. It has been reported that worldwide, 5-50% of the population suffering from periodontitis eventually lose their teeth [3-5]. Some of the behavioral factors that can cause periodontal diseases include smoking [6-8], chronic alcohol consumption [9,10], nutritional deficiency [11,12], psychological issues [13,14], and systemic diseases [15-17].
In this narrative review, the authors are going to discuss the effects of alcohol consumption on oral tissues that lead to periodontal diseases. A regular and considerable amount of alcohol consumption is more commonly seen in middle and older age groups compared to younger ones [18,19]. Nonetheless, recently, the scenario started changing in the younger community frequently, with a sizable portion consuming alcoholic drinks globally, which also includes India [20-23]. Alcohol causes more negative effects on older people because of biological changes, especially in the brain [24], conciliation renal physiology [25], pharmacokinetic and pharmacodynamic changes [26], and increased need for medication because of the aging process [27,28]. Alcoholism-induced periodontal disease can be associated with poor oral hygiene [29]. In most cases, poor periodontal health is due to poor oral hygiene or not taking care of dental care rather than alcohol use [4,30,31].
Yuan et al. reported that two healthy régime features comprise refraining from substantial alcohol drinking and persistent eating and drinking practices reformed Mediterranean diet [32]. Multiple studies reported that drinking alcohol, high body mass index (BMI), cigarette smoking, unwholesome diet, and physical sedentariness remain as principal modifiable risk factors of lifestyle that cause multiple diseases [33,34]. For a considerable portion of the population globally, including in low-and-middle-income countries (LMICs), consistently consuming alcohol becomes one of the critical reasons for seriously damaged health [35,36]. These patients often take less interest in dental health care and do not think seriously about any advice dental health professionals provide [37-39].
Problem statement
Chronic alcoholism possesses the potential to harm the general health and well-being of a person [40,41]. Alcohol necessity and habit are categorized by primary transformations in the brain's compensation (incentive) and anxiety (worry) systems that are discernable as withdrawal symptoms when alcohol drinking is ceased or noticeably cut down. These transformations are also ostensible to fire impetus to enroll again in extreme alcohol consumption rate [42,43]. In 2016, it was reported that alcohol abuse was the seventh leading cause of disability and premature mortality [44,45]. Chronic high amounts of alcohol consumption frequently lead to hypertension, cardiac and hepatic disorders, stroke, and gastrointestinal diseases. Additionally, there is a strong association in developing the mouth, breast, esophagus, throat, voice box, liver, colon, and rectal carcinoma [46]. Chronic and heavy alcohol drinking indeed distresses the mouth cavity, oral mucosa, and teeth [47]. Additionally, it has been observed that a substantial portion of alcohol consumption is associated with solid periodontal health. It has been clarified by several biotic probabilities, such as an increased possibility of severe infection (ruins neutrophil, macrophage, and T-cell physiological function) due to compromised immune systems and unhealthy oral hygiene practices [48-52]. Priyanka et al. 2017 reported that the "prevalence of periodontitis was higher (89.61%) in alcohol-dependent subjects compared to controls (78.67%). Prevalence of mucosal lesions among alcohol-dependent subjects was 31.5%, which was higher than the controls (25%)" [29]. The underlying mechanisms for the causation of periodontal diseases in alcoholics and the association of alcoholism with other harmful habits also need further investigation.
Objectives of the study
This paper intends to evaluate and describe the effect of alcoholism on periodontal tissues. Additionally, this study plans to appraise the role of alcoholism in the pathogenesis of periodontal diseases and assess the relationship of alcoholism with other harmful habits, such as smoking, on periodontal tissues. This narrative essay forms an opinion of the role of alcohol-containing mouthwashes in maintaining proper oral hygiene.
Review
Materials and methods
This review considered data in a database (PubMed, Google Scholar, Elton B. Stephens Company (EBSCO)), year (2000 to 2023), language (English), and study design (Systematic Reviews, Narrative Reviews, Observational Studies). Researchers utilized keywords such as "Periodontal diseases," AND "Alcohol misuse," AND "Gingiva," AND "Alveolar bone," AND "Weak immunity," AND "Tobacco," "Smoking," AND "Cancer," AND "Caries," AND "Necrotizing Lesions." Inclusion criteria are studies describing alcohol's toxic effects on periodontal tissues, association with smoking, tobacco, and poor oral hygiene, mainly focusing on the gingiva, bone, and soft tissues (Figure 1). Exclusion criteria are studies in other languages, animal and in vitro studies, and studies involving other medically compromised patients.
Figure 1. Schematic diagram illustrating the methodology of the study.
Note: This figure was drawn using the premium version of BioRender (https://biorender.com/ (Accessed on 25th April 2024)) with the agreement license number VO26QPXSCW [53].
Image credit: Susmita Sinha
Discussion
Gamma-glutamyl transferase (GGT) is a liver enzyme. Nevertheless, this enzyme is integrated into "the plasma membranes of most cells and organ tissues" [52]. GGT levels are substantially raised among individuals drinking alcohol in excessive quantity and persistently [54,55] and denote the severity of periodontal disease [56,57]. In some studies, the use of alcohol is associated with self-reported periodontal disease, especially among tobacco smokers [58-60]. A robust relationship was detected among tobacco smokers in males and females who drink alcohol more than 30 g and 20 g daily, respectively [58]. Individuals who meet a major portion of total dietary energy intake through excessive alcohol consumption are often found to suffer from primary and secondary malnutrition [61-63]. In primary malnutrition, alcohol supplants or substitutes some nutritional intake - triggering a paucity of essential elements, vitamins, minerals, and proteins [61,63,64]. In secondary malnutrition, alcohol leads to gastrointestinal problems and causes malabsorption and malnutrition [65].
Consistent alcohol intake increases the possibility of infections [66]. High levels of alcohol drinking for a prolonged time often lead to protein deficiency [67,68]. Complement instigation is the principal issue of the innate immune system, which plays an imperative function in protecting against microbial infectious diseases, restoring the host, and clearing the inflammatory damage products [69,70]. Complement insufficiencies are caused by the specific complement protein deficiency [71]. Chronic heavy alcohol is frequently found with C3 and C4 protein deficiency [72,73]. Persistent heavy alcohol consumers repeatedly found defective neutrophil function, macrophage, and T-cell function [74]. Thus, chronic alcoholics are highly prone to communicable diseases (bacterial and viral) that include periodontal infections [75-77]. Individuals consuming a high quantity of alcohol relentlessly promote dysbiosis of the oral microbiome [78] and encourage periodontal pathogenic microbes' accumulation [79] and tooth pocket formation more than non-alcoholics [10]. Porphyromonas gingivalis and Fusobacteria nucleatum are the most notorious periodontal pathogenic microbes [80]. It has been reported that the accumulation of these pathogens (P. gingivalis and F. nucleatum) was substantially higher among heavy alcoholic drinkers than in non or occasional alcoholics [28,81,82]. Chronic heavy alcohol consumers have a higher overall quantity of red (i.e., P. gingivalis) and orange-complex (i.e., F. nucleatum) pathogens than non-alcoholic subjects [83]. P. gingivalis and F. nucleatum repeatedly cause severe periodontitis with tooth loss [84,85].
Effects of alcohol on alveolar bone
A J-curve has been anticipated regarding the implication between alcohol consumption and springing up and the evolution of periodontal infectious diseases. It was explained that consuming alcohol in small quantities is prone to minor evolution and high doses with a more hostile and destructive advancement of the disease. Therefore, adverse outcomes are dose-dependent [86-89]. Long-standing drinking of alcohol blights the equilibrium of microbiota in the digestive tract, causing the gastrointestinal physiological impediment [78,90,91], the liver's capability to decontaminate microbial yields and to spawn a well-adjusted cytokine ambiance, and the brain's authority to control inflammation in the periphery. While these protection lines are compromised, systemic inflammation proceeds [90].
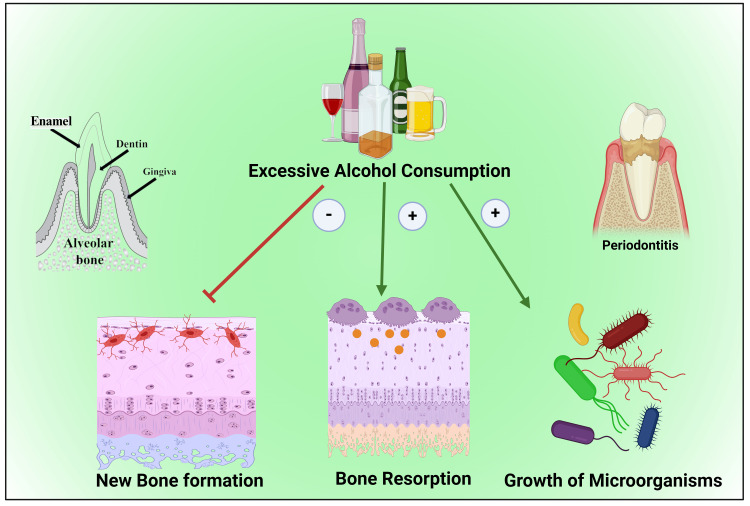
Moreover, the sugar content of alcohol attracts pathogenic microbes to accumulate in the oral cavity [79,92,93]. The prolonged high amount of alcohol drinking raises the severity of periodontitis in a dose-dependent mode [88,89] (Figure 2) by raising the scale of the inflammatory reaction in a confined area [48]. Those include receptor activator of nuclear factor kappa-Β ligand (RANKL) immunoreactivity outline and the number of tartrate-resistant acid phosphatase (TRAP) positive cells (TRAP+cells), and tumor necrosis factor-α (TNF-α), which provide a route to more prominent alveolar bone knocking down and a lower pulpal blood flow (PBF) [48,94,95].
Figure 2. Illustration showing the detrimental effect of alcohol consumption on alveolar bone.
Note: This figure was drawn using the premium version of BioRender (https://biorender.com/ (Accessed on 19th May 2024)), which has the agreement license number CR26U3IFYP [53].
Image credit: Susmita Sinha
Alcohol on periodontal tissues
Heavy regular alcohol drinking directly affects the bone anabolism [12,95]. Alcohol instigates the clampdown of osseous tissue construction because of its toxic consequence on osteoblastic endeavor and multiplying [96,97]. Alcohol inhibits osteoblastic pursuit but promotes osteoclastic accomplishment [97-99]. Additionally, alcohol affects the gastrointestinal system, causing malnutrition [63,65]; thereby, multiple essential nutritional components for the growth and proliferation of bone are not absorbed [61]. Moreover, heavy alcoholic beverage consumers often meet their energy needs up to 60% [100]. Therefore, essential nutritional intake is usually deficient, which affects bone growth [100,101].
Alcohol and the development of dental caries
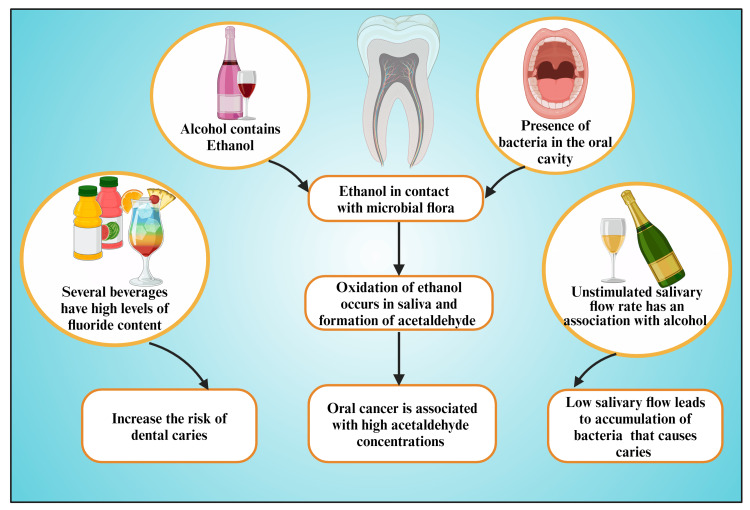
Alcohol has been reported to trigger many oral health disorders. These include periodontal disorder, tooth deterioration, caries, oral malignancy, halitosis, damage, and tarnishing [102]. It has been reported that Chinese colorless liquor named Baijiu promotes dental caries by distressing the oral microbial ecosystem, exclusively by forming dental pathogens biofilms, because Baijiu contains excessive alcohol [103]. Almost all alcoholic beverages or drinks contain sugar [104]; nevertheless, some possess relatively high amounts (five teaspoons of sugar in 700 mL pack) [105], e.g., sherries, fortified wines, cider, and pre-mixed drinks like alcopops and liqueurs [105]. World Health Organization (WHO) reported that "free sugars are the essential dietary factor in developing dental caries" [106]. Sheiham and James reported a solid log-linear association between dental caries and sugar consumption among individuals who consume 0-10% of their energy intake with sugar [107]. Pathogenic microbes within the oral cavity and supragingival plaque utilize sugar as energy through fermentation, producing acid through the Embden-Meyerhof-Parnas (EMP) pathway. This acid demineralized teeth enamel dentin and formed cavities [108-111]. It is not only sugar-containing alcoholics that cause dental carries formation; nonetheless, all sugar-sweetened beverages (SSBs) cause a similar phenomenon [112,113]. WHO reported that fluoride consumption has equally advantageous properties - in dropping the frequency of dental caries - and adverse consequences - in instigating tooth enamel and skeletal fluorosis after long-standing high subjection [114]. Certain alcoholic drinks frequently contain high levels of fluoride [115,116], which increases the risk of caries.
A considerable portion of acute and long-standing alcohol consumption severely impacts oral physiological executions [117]. Alcohol in substantial amounts acts as a diuretic [118,119], causing increased urination with the loss of all essential cellular features and succeeding dehydration [118-120]. Hence, alcohol intake escalates thirstiness and causes xerostomia (dry mouth) [117]. Alcohol-induced thirst perception and oral aridness are primarily initiated by the instigation of impairment in the midline of the brain (dedicated to neuroepithelial configuration) around the third and fourth ventricles [117]. This brain area communicates directly with blood and cerebrospinal fluid (circumventricular organs) [117]. Therefore, through that area, polypeptide hypothalamic hormones depart the brain, exclusive of interrupting the blood-brain barrier (BBB) and hypothalamus with a diminished rate of salivary excretion [117]. This lower salivation flow changes pH toward the acidic side [121,122] than normal individuals. Additionally, low salivary flow leads to more microbial accumulation on enamel surfaces [123] and synthesizing more acid that causes caries [124] (Figure 3).
Figure 3. Diagram showing the possible mechanisms by which alcohol consumption results in dental caries.
Note: This figure was drawn using the premium version of BioRender (https://biorender.com/ (Accessed on 25th April 2024)), which has the agreement license number HU26QQ80TS [53].
Image credit: Susmita Sinha
Alcohol-instigated carcinoma
Alcohol-instigated cancer incidence rates were among males (3%) and females (0.5%), and death rates were 2.8% (male) and 0.1% (female) in Korea, respectively [125]. The principal cause of mortality among people of the Republic of Korea is cancer, accounting for 26.5% of all fatal outcomes. These cancers are robustly associated with lifestyle issues, e.g., chronic heavy alcohol consumption, high-level tobacco smoking, high BMI level, and low physical activity. This study also revealed that poor style issues increase the possibility of cancer by 1.47 times more threat than those who maintain a healthy lifestyle [126]. A substantial proportion of cancer is frequently ascribable to alcohol consumption, especially among regular and heavy in-takers who often cross the upper limits of the recommended dose in Western Europe. Alcohol-caused cancer in European males and females was detected with incidence rates of 10% and 3%, respectively. These cases were erstwhile and existing heavy and alcohol consumers [127]. There is an increased risk (15%) of developing oral, pharyngeal [128,129], esophageal, and colorectum cancer [130] among people who drink alcohol more than 10 g/day [128]. Bagnardi et al., in their meta-analysis, reported that the relative risk of oral and pharyngeal carcinoma was swelled up from 1.13 to 5.13 for existing alcohol consumption around 12.5 g and above 50 g alcohol daily, respectively [131]. Population attributable fraction (PAF) refers to the proportion of all cases with a particular outcome in a population that could be prevented by eliminating a specific exposure [132]. The PAF due to alcohol is 29.3% for oral cavity cancer, 43.3% for pharyngeal cancer, and 25.8% for laryngeal cancer in men. In women, PAF is 4.2% for colorectal cancer and 0.2% for breast cancer [125]. There is three times more permanent teeth loss among alcoholics than among the healthy population [29]. Thus, alcoholics have a lesser number of teeth [133].
Association between tobacco and alcohol
Smoking is the second principal hazardous aspect for mortality and disability-adjusted life-years (DALYs), which has elicited over 200 million people expiries prematurely over 30 years [134,135]. Tobacco chewing is considered independently as a risk factor for oral cancer [136,137]. Tobacco smokers or chewers, along with betel nut and leaf, who concurrently drink alcoholic beverages have a greater probability of oral and esophageal carcinoma [138], as well as head and neck cancer (HNC), especially in Southeast Asia [139,140], and become snowballing public health apprehension [141]. Another study revealed that alcohol and tobacco have give-and-take impacts on despot hankering, "subjective responses to fixed-dose alcohol or nicotine administration and self-administration" [142]. Smoking and alcohol ingestion have been stated as considerable risk features for cardiovascular diseases (CVDs), cancers, type 2 diabetes mellitus (T2DM), obesity, bronchial asthma, stress, stroke, and other lifestyle-related noncommunicable diseases, and increasing mortalities [143-145]. WHO reported annually that around the globe, 7 and 3 million people died because of tobacco and alcohol consumption, respectively [146,147]. McCambridge and Morris revealed that alcohol assassinates smaller people around the globe than tobacco. It has been estimated that roughly 5% of total mortalities are attributed to alcohol (ethanol) compared to 8% for cigarettes and other tobacco products) [148].
Alcohol affecting the brain
Long-lasting, heavy alcohol drinking consequences in anatomical alterations that lead to potentially permanent brain impairment with its neurotoxic possessions [149-152]. Straightforwardly, alcohol causes neuro-inflammation and neuronal damage of brain tissue [153,154]. Additionally, alcohol produces an adverse impact on the gastrointestinal microbiome colonies, triggering hepatic encephalopathy [154-156]. The alcohol-persuaded brain damage led to alcohol-induced persisting amnestic syndrome (Wernicke-Korsakoff syndrome (WKS)) and alcohol-related dementia (ARD), which manifest as forgetfulness, intense perplexity, and visual disturbance [151,157-160]. Wernicke encephalopathy (WE) is an acute neuropsychiatric brain ailment instigated by a thiamine absence or deprivation of vitamin B1 [151].
Alcohol impedes the brain's signaling route and upsets the brain's anatomy and physiology [161,162]. Alcohol affects brain cells and converts brain tissues firmer and smaller, especially those areas responsible for "controlling balance, memory, speech, and judgment to do their jobs," raising a greater probability of physical damage and other adverse consequences [161]. Over time, a substantial portion of drinking alcohol has been reported to cause the wasting of brain tissue, fall of brain mass, impairment of neurons, and compromised physiology function of the white matter fiber [161,162]. Similar findings were also reported when drinking alcohol in mild to moderate portions [163-167]. Alcohol has a negative impact on the hippocampus area of the brain by exerting an adverse impact on neural stem cells and adult neurogenesis [168], causing diminished cognitive function. Thereby, among chronic alcoholics, the transfer of memoirs from short-term to long-term storage is impaired. These patients often are unable to recall many events when they are heavily drunk [169-172]. The memory-transferring phenomenon is known as memory consolidation [173]. Long-term drinking of alcohol often led to modifications in moods, feelings (autocorrelation (emotional inertia)), and individuality, in addition to compromised awareness, discernment, acquiring knowledge, studying, and memorial function [162,174].
Alcohol causing more stains on teeth
Certain alcoholic beverages heavily contain coloring agents. Sunset Yellow FCF (Tartrazine, Orange Yellow S, or FD+C Yellow No. 6) is an orange-yellow azo dye usually added to alcoholic beverages. Nevertheless, it also added many SSBs, fat-based desserts, candies, fruit-flavored snacks, drink mixes/powders, and many more foods [175,176]. Sunset Yellow FCF is also used in many pharmaceutical products, such as paracetamol and ibuprofen, which cause tooth discoloration among the pediatric population [177]. Several alcohol-containing cocktails comprise chromogens that produce attractive colors of these alcoholic drinks. These chromogens fasten to dental enamel and discolored teeth habitual consumers (Figure 4) [178].
Figure 4. Comparison of healthy teeth with diseased teeth because of misuse of alcoholic beverages.
Note: This figure was drawn using the premium version of BioRender (https://BioRender.com/ (Accessed on 23rd April 2024)), which has the agreement license number BQ26QFMZQZ [53].
Image credit: Utsav Gandhi
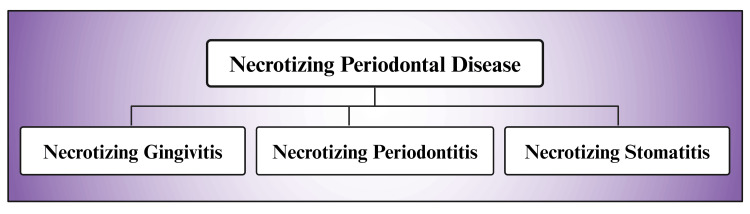
Necrotizing periodontal diseases
Over-dependency on alcohol, along with its toxic effects, undernourished and poor level of immunity, leads to necrotizing periodontal diseases (NPDs) [9,15,179,180]. Other considerable, affecting aspects causing NPDs include HIV infection leading to compromised immune function, bad oral cleanliness, extraordinary emotional stress, deficient sleep, being Caucasian, age range (18-21 years), tobacco use, vitamin C deficiency, chronic considerable portion alcohol drinking, and recent severe diseases [9, 181,182]. Another study reported that NPDs are interrelated to host immunity status [183]. NPDs are of three types (Figure 5) [180]. NPDs are a group of chronic noncommunicable microbial dysbiotic inflammatory oral/dental illnesses. NPD is portrayed by a speedy commencement of inflammation, soreness, ache, gum hemorrhage, interdental or papilla necrosis, and licked or pounded crater-like contusion of the papilla interdentalis [9,184-190]. NPDs differ from other chronic gingivitis through the presence of considerable pain, gingival necrosis amid the teeth, cankerous papilla, and gum bleeding [191]. The precise pathological process of NPDs yet remains elusive [180].
Figure 5. Diagram showing the types of necrotizing periodontal diseases.
Note: This figure was drawn using the premium version of BioRender (https://biorender.com/ (Accessed on 25th April 2024)), which has the agreement license number NQ26QQA71P [53].
Image credit: Susmita Sinha
Alcohol-containing mouthwashes
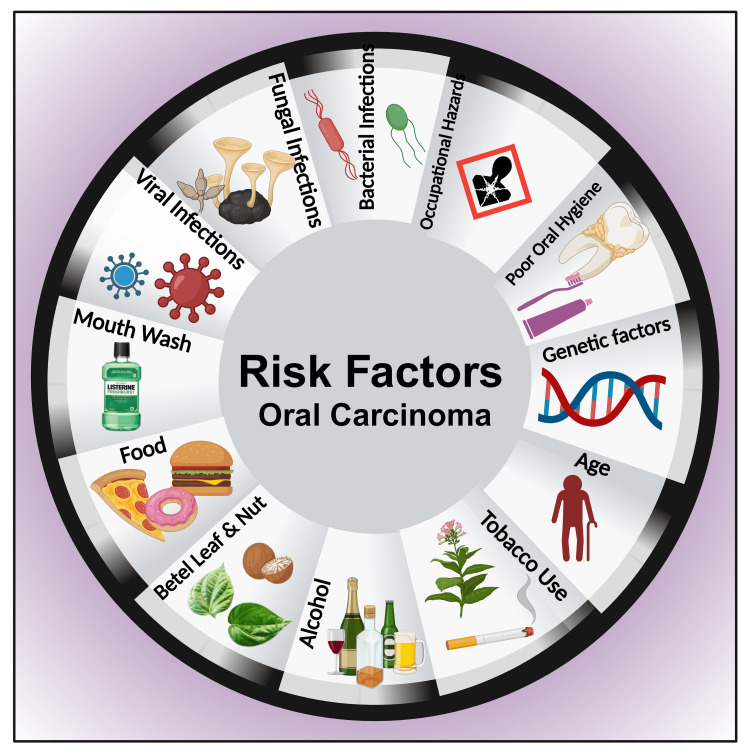
Several brand mouthwashes contain alcohol. It has been reported that ethanol concertation perchance escalated to 26% [192]. Multiple studies earlier reported that containing ethanol mouthwashes increases the possibility of developing oral cancer [193,194]. However, it has been reported that alcohol alone does not comply as an independent risk factor for evolving oral carcinoma; nonetheless, the probability does intensify with the presence of other risk features of mouth cavity malignancies [195-198] (Figure 6). Furthermore, ethanol-containing mouthwashes do not possess antiplaque properties [199]. Additionally, almost all mouthwashes are inept at pulling out biofilms and ineffective in eliminating the total microbial content of biofilm [200]. Moreover, multiple times rinsing utilizing such high alcohol-containing cleansing agents exposes the oral cavity much more times than those who consume alcoholic beverages and increases the risk of "hyperkerastosic lesions" [199].
Figure 6. Schematic diagram showing the risk factors for oral carcinoma.
Note: This figure was drawn using the premium version of BioRender (https://biorender.com/ (Accessed on 17th May 2024)), which has the agreement license number EQ26TU4XE2 [53].
Image credit: Susmita Sinha
Rajendiran et al. reported that the usual active constituents in dental gel or cream and mouth rinse comprise "chlorhexidine, cetylpyridinium chloride, sodium fluoride, stannous fluoride, stannous chloride, zinc oxide, zinc chloride, and two herbs - licorice and curcumin" [201]. Flossing and toothbrushing to conserve oral cleanness and periodontal health are the best endorsed and effectual procedures to prevent plaque formation [201-203]. The areas of the oral cavity that are to a lesser extent reachable to flossing and toothbrushing; chemical plaque control agents (chlorhexidine, cetylpyridinium chloride, amine fluoride/stannous fluoride, stannous chloride, delmopinol hydrochloride, hexetidine, triclosan, phenolic compounds, etc.) containing in dental gel, cream, pastes, and mouthwashes can prevent the evolution of plaque biofilm [201,204-207].
Limitations of the study
The results of this review need to be generalizable, and more clinical and histological studies with large sample sizes across various ethnic populations are required. An increased number of longitudinal studies and systematic reviews need to be carried out to assess the association between alcoholism and periodontal disease. Systemic factors and psychosocial assessment of alcoholism need to be evaluated.
Conclusions
Alcohol is either directly or indirectly associated with periodontal diseases. It is also one of the significant factors that may cause clinical attachment loss, pocket formation, and dental caries. There is an imbalance of microbes within the oral cavity of alcohol consumers, and the presence of sugar in alcoholic beverages further promotes the build-up of bacteria that aggravate the periodontal disease. Alcohol over-dependency, its toxic effects, undernourishment, and poor immunity may lead to NPD. Human beings who are consuming alcohol for a long time can develop some toxic effects. Alcohol deteriorates an individual's physical, social, psychological, and financial condition. If it is associated with other factors, such as using tobacco, smoking, or excessive sugar intake, it worsens the conditions. An unhealthy oral cavity would contribute to an individual’s reduced quality of life. It is of great importance that the consumption of alcohol is limited and oral health is maintained with flossing, tooth brushing, and, if needed, dental gel, cream, paste, and mouthwash to remove harmful elements within the oral cavity. Public health workers, policymakers, physicians, and dentists need to impart knowledge regarding the health-deteriorating effects of alcohol consumption and need to encourage alcohol consumers to pay regular visits to dentists to keep periodontal diseases at bay.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Mainul Haque, Utsav H. Gandhi, Amit Benjamin, Shreya Gajjar, Tanvi Hirani, Bansariben B. Suhagia, Rahnuma Ahmad, Susmita Sinha, Santosh Kumar, Khushboo Desai
Acquisition, analysis, or interpretation of data: Mainul Haque, Utsav H. Gandhi, Amit Benjamin, Shreya Gajjar, Tanvi Hirani, Bansariben B. Suhagia, Rahnuma Ahmad, Susmita Sinha, Santosh Kumar, Khushboo Desai
Drafting of the manuscript: Mainul Haque, Utsav H. Gandhi, Amit Benjamin, Shreya Gajjar, Tanvi Hirani, Bansariben B. Suhagia, Rahnuma Ahmad, Susmita Sinha, Santosh Kumar, Khushboo Desai
Critical review of the manuscript for important intellectual content: Mainul Haque, Utsav H. Gandhi, Amit Benjamin, Shreya Gajjar, Tanvi Hirani, Bansariben B. Suhagia, Rahnuma Ahmad, Susmita Sinha, Santosh Kumar, Khushboo Desai
Supervision: Utsav H. Gandhi, Amit Benjamin, Shreya Gajjar, Tanvi Hirani, Bansariben B. Suhagia, Rahnuma Ahmad, Susmita Sinha, Santosh Kumar, Khushboo Desai
References
- 1.Periodontal diseases. Kinane DF, Stathopoulou PG, Papapanou PN. Nat Rev Dis Primers. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 2.Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Chapple IL, Mealey BL, Van Dyke TE, et al. J Periodontol. 2018;89 Suppl 1:0–84. doi: 10.1002/JPER.17-0719. [DOI] [PubMed] [Google Scholar]
- 3.A public health approach for prevention of periodontal disease. Janakiram C, Dye BA. Periodontol 2000. 2020;84:202–214. doi: 10.1111/prd.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevalence of periodontal disease, its association with systemic diseases and prevention. Nazir MA. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5426403/pdf/IJHS-11-72.pdf. Int J Health Sci (Qassim) 2017;11:72–80. [PMC free article] [PubMed] [Google Scholar]
- 5.Current concepts in the management of periodontitis. Kwon T, Lamster IB, Levin L. Int Dent J. 2021;71:462–476. doi: 10.1111/idj.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Association between smoking and periodontal disease in South Korean adults. Sim KY, Jang YS, Jang YS, Nerobkova N, Park EC. Int J Environ Res Public Health. 2023;20:4423. doi: 10.3390/ijerph20054423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The impact of smoking on subgingival microflora: from periodontal health to disease. Jiang Y, Zhou X, Cheng L, Li M. Front Microbiol. 2020;11:66. doi: 10.3389/fmicb.2020.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The role of cigarette smoking in periodontal disease and treatment outcomes of dental implant therapy. Apatzidou DA. Periodontol 2000. 2022;90:45–61. doi: 10.1111/prd.12449. [DOI] [PubMed] [Google Scholar]
- 9.Necrotising periodontal diseases and alcohol misuse - a cause of osteonecrosis? Tkacz K, Gill J, McLernon M. Br Dent J. 2021;231:225–231. doi: 10.1038/s41415-021-3272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcohol use and the development of periodontal pockets: an 11-year follow-up study. Sankaranarayanan R, Saxlin T, Knuuttila M, Ylöstalo P, Suominen AL. J Periodontol. 2020;91:1621–1631. doi: 10.1002/JPER.19-0602. [DOI] [PubMed] [Google Scholar]
- 11.Nutrition as a key modifiable factor for periodontitis and main chronic diseases. Martinon P, Fraticelli L, Giboreau A, Dussart C, Bourgeois D, Carrouel F. J Clin Med. 2021;10:197. doi: 10.3390/jcm10020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietary factors affecting the prevalence and impact of periodontal disease. Santonocito S, Polizzi A, Palazzo G, Indelicato F, Isola G. Clin Cosmet Investig Dent. 2021;13:283–292. doi: 10.2147/CCIDE.S288137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Periodontal health and psychiatric disorders. Kisely S. Curr Oral Health Rep. 2023;10:111–116. [Google Scholar]
- 14.Mental health and periodontal and peri-implant diseases. Ball J, Darby I. Periodontol 2000. 2022;90:106–124. doi: 10.1111/prd.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Periodontal health and systemic conditions. Lim G, Janu U, Chiou LL, Gandhi KK, Palomo L, John V. Dent J (Basel) 2020;8:130. doi: 10.3390/dj8040130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Periodontal inflammation and systemic diseases: an overview. Martínez-García M, Hernández-Lemus E. Front Physiol. 2021;12:709438. doi: 10.3389/fphys.2021.709438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Periodontal disease, tooth loss, and systemic conditions: an exploratory study. Chatzopoulos GS, Jiang Z, Marka N, Wolff LF. Int Dent J. 2024;74:207–215. doi: 10.1016/j.identj.2023.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Differences in alcohol use between younger and older people: results from a general population study. Veerbeek MA, Ten Have M, van Dorsselaer SA, Oude Voshaar RC, Rhebergen D, Willemse BM. Drug Alcohol Depend. 2019;202:18–23. doi: 10.1016/j.drugalcdep.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Drinking patterns vary by gender, age and country-level income: cross-country analysis of the International Alcohol Control Study. Chaiyasong S, Huckle T, Mackintosh AM, et al. Drug Alcohol Rev. 2018;37 Suppl 2:0–62. doi: 10.1111/dar.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binge drinking in young adults: data, definitions, and determinants. Courtney KE, Polich J. Psychol Bull. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young people, young adults and binge drinking. LA Fauci V, Squeri R, Spataro P, Genovese C, Laudani N, Alessi V. J Prev Med Hyg. 2019;60:0–85. doi: 10.15167/2421-4248/jpmh2019.60.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negative alcohol-related consequences experienced by young adults in the past 12 months: Differences by college attendance, living situation, binge drinking, and sex. Patrick ME, Terry-McElrath YM, Evans-Polce RJ, Schulenberg JE. Addict Behav. 2020;105:106320. doi: 10.1016/j.addbeh.2020.106320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A study on alcohol use and its related health and social problems in rural Puducherry, India. Ramanan VV, Singh SK. J Family Med Prim Care. 2016;5:804–808. doi: 10.4103/2249-4863.201175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Effect of alcohol consumption on kidney function: population-based cohort study. Lee YJ, Cho S, Kim SR. Sci Rep. 2021;11:2381. doi: 10.1038/s41598-021-81777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alcohol in the aging brain - the interplay between alcohol consumption, cognitive decline and the cardiovascular system. Mende MA. Front Neurosci. 2019;13:713. doi: 10.3389/fnins.2019.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Mangoni AA, Jackson SH. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risks of combined alcohol/medication use in older adults. Moore AA, Whiteman EJ, Ward KT. Am J Geriatr Pharmacother. 2007;5:64–74. doi: 10.1016/j.amjopharm.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcohol and aging - an area of increasing concern. White AM, Orosz A, Powell PA, Koob GF. Alcohol. 2023;107:19–27. doi: 10.1016/j.alcohol.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Impact of alcohol dependency on oral health - a cross-sectional comparative study. Priyanka K, Sudhir KM, Reddy VC, Kumar RK, Srinivasulu G. J Clin Diagn Res. 2017;11:0–6. doi: 10.7860/JCDR/2017/26380.10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The association between oral hygiene and periodontitis: a systematic review and meta-analysis. Lertpimonchai A, Rattanasiri S, Arj-Ong Vallibhakara S, Attia J, Thakkinstian A. Int Dent J. 2017;67:332–343. doi: 10.1111/idj.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oral hygiene practices and oral health knowledge among students in Split, Croatia. Tadin A, Poljak Guberina R, Domazet J, Gavic L. Healthcare (Basel) 2022;10:406. doi: 10.3390/healthcare10020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A prospective evaluation of modifiable lifestyle factors in relation to peripheral artery disease risk. Yuan S, Damrauer SM, Håkansson N, Åkesson A, Larsson SC. Eur J Vasc Endovasc Surg. 2022;64:83–91. doi: 10.1016/j.ejvs.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Ng R, Sutradhar R, Yao Z, Wodchis WP, Rosella LC. Int J Epidemiol. 2020;49:113–130. doi: 10.1093/ije/dyz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Healthy lifestyle and cancer risk: modifiable risk factors to prevent cancer. Marino P, Mininni M, Deiana G, et al. Nutrients. 2024;16:800. doi: 10.3390/nu16060800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Advancing alcohol research in low-income and middle-income countries: a global alcohol environment framework. Walls H, Cook S, Matzopoulos R, London L. BMJ Glob Health. 2020;5:0. doi: 10.1136/bmjgh-2019-001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harmful alcohol consumption: prevalence, trends, health burden, reduction strategy. Грузева ТС, Дуфинец ВА, Замкевич ВБ. https://pubmed.ncbi.nlm.nih.gov/27487531/ Wiad Lek. 2016;69:183–189. [PubMed] [Google Scholar]
- 37.Dental care and oral disease in alcohol-dependent persons. Khocht A, Schleifer SJ, Janal MN, Keller S. J Subst Abuse Treat. 2009;37:214–218. doi: 10.1016/j.jsat.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barriers to accessing primary dental care in adults with alcohol dependence: a qualitative study. Bowes C, Breckons M, Holmes RD, Durham J, Bareham BK. JDR Clin Trans Res. 2024;0:42. doi: 10.1177/23800844231169642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oral health-care needs among clients receiving alcohol and other drugs treatment-a scoping review. Poudel P, Kong A, Hocking S, Whitton G, Srinivas R, Borgnakke WS, George A. Drug Alcohol Rev. 2023;42:346–366. doi: 10.1111/dar.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vulnerability to alcohol-related problems: a policy brief with implications for the regulation of alcohol marketing. Babor TF, Robaina K, Noel JK, Ritson EB. Addiction. 2017;112 Suppl 1:94–101. doi: 10.1111/add.13626. [DOI] [PubMed] [Google Scholar]
- 41.Awakening to the alcohol epidemic - need of the hour. Shah DS. Indian J Public Health. 2017;61:205–207. doi: 10.4103/ijph.IJPH_226_16. [DOI] [PubMed] [Google Scholar]
- 42.Alcohol dependence, withdrawal, and relapse. Becker HC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3860472/pdf/arh-31-4-348.pdf. Alcohol Res Health. 2008;31:348–361. [PMC free article] [PubMed] [Google Scholar]
- 43.Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Becker HC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3860383/pdf/arcr-34-4-448.pdf. Alcohol Res. 2012;34:448–458. [PMC free article] [PubMed] [Google Scholar]
- 44.Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. GBD 2016 Alcohol Collaborators. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. GBD 2016 Alcohol and Drug Use Collaborators. Lancet Psychiatry. 2018;5:987–1012. doi: 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alcohol consumption and risks of more than 200 diseases in Chinese men. Im PK, Wright N, Yang L, et al. Nat Med. 2023;29:1476–1486. doi: 10.1038/s41591-023-02383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oral mucosal disease: dilemmas and challenges in general dental practice. A Atkin P, Cowie R. Br Dent J. 2024;236:269–273. doi: 10.1038/s41415-024-7080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chronic consumption of alcohol increases alveolar bone loss. de Almeida JM, Pazmino VF, Novaes VC, et al. PLoS One. 2020;15:0. doi: 10.1371/journal.pone.0232731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcohol consumption is associated with periodontitis. A systematic review and meta-analysis of observational studies. Pulikkotil SJ, Nath S, Dharamarajan L, Jing KT, Vaithilingam RD. Community Dent Health. 2020;37:12–21. doi: 10.1922/CDH_4569Pulikkotil10. [DOI] [PubMed] [Google Scholar]
- 50.Alcohol consumption increases periodontitis risk. Pitiphat W, Merchant AT, Rimm EB, Joshipura KJ. J Dent Res. 2003;82:509–513. doi: 10.1177/154405910308200704. [DOI] [PubMed] [Google Scholar]
- 51.Alcohol’s effect on host defense. Szabo G, Saha B. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4590613/pdf/arcr-37-2-159.pdf. Alcohol Res. 2015;37:159–170. [PMC free article] [PubMed] [Google Scholar]
- 52.Gamma-glutamyltransferase-friend or foe within? Kunutsor SK. Liver Int. 2016;36:1723–1734. doi: 10.1111/liv.13221. [DOI] [PubMed] [Google Scholar]
- 53.BioRender. [ Apr; 2024 ]. 2024. https://biorender.com https://biorender.com
- 54.Gamma-glutamyl transferase: a useful marker of habitual drinking in cases of alcohol-associated osteonecrosis of the femoral head. Hamada H, Ando W, Takao M, Sugano N. Alcohol Alcohol. 2021;56:175–180. doi: 10.1093/alcalc/agaa117. [DOI] [PubMed] [Google Scholar]
- 55.Serum gamma-glutamyltransferase, daily alcohol consumption, and the risk of chronic kidney disease: the Kansai Healthcare Study. Shibata M, Sato KK, Uehara S, et al. J Epidemiol. 2020;30:163–169. doi: 10.2188/jea.JE20180240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Associations between periodontal status and liver function in the Japanese population: a cross-sectional study. Fujii T, Aoyama N, Kida S, Taniguchi K, Yata T, Minabe M, Komaki M. J Clin Med. 2023;12:4759. doi: 10.3390/jcm12144759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assessment of gingival crevicular fluid levels of gamma glutamyl transferase in chronic periodontitis patients before and after non-surgical periodontal therapy: a clinico-biochemical study. G K, Shankar SM, Nagesh U, Gururaj SB, Chidambar CK, Bhushan K. J Oral Biol Craniofac Res. 2022;12:481–485. doi: 10.1016/j.jobcr.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alcoholic beverage consumption, smoking habits, and periodontitis: a cross-sectional investigation of the NutriNet-Santé study. Hamdi Z, Detzen L, Fessi S, et al. J Periodontol. 2021;92:727–737. doi: 10.1002/JPER.20-0192. [DOI] [PubMed] [Google Scholar]
- 59.Alcohol use and periodontal pocket development: findings from a 4-yr longitudinal study. Sankaranarayanan R, Saxlin T, Ylöstalo P, Khan S, Knuuttila M, Suominen AL. Eur J Oral Sci. 2019;127:232–240. doi: 10.1111/eos.12610. [DOI] [PubMed] [Google Scholar]
- 60.Testing the association between tobacco smoking, alcohol consumption, and risk of periodontitis: a Mendelian randomization study. Baumeister SE, Freuer D, Nolde M, et al. J Clin Periodontol. 2021;48:1414–1420. doi: 10.1111/jcpe.13544. [DOI] [PubMed] [Google Scholar]
- 61.Development, prevention, and treatment of alcohol-induced organ injury: the role of nutrition. Barve S, Chen SY, Kirpich I, Watson WH, McClain C. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513692/pdf/arcr-38-2-289.pdf. Alcohol Res. 2017;38:289–302. [PMC free article] [PubMed] [Google Scholar]
- 62.The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Sebastiani G, Borrás-Novell C, Casanova MA, Pascual Tutusaus M, Ferrero Martínez S, Gómez Roig MD, García-Algar O. Nutrients. 2018;10:1008. doi: 10.3390/nu10081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Relationships between nutrition, alcohol use, and liver disease. Lieber CS. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6668875/pdf/220-231.pdf. Alcohol Res Health. 2003;27:220–231. [PMC free article] [PubMed] [Google Scholar]
- 64.Nutrition and chronic alcohol abuse. Moreno Otero R, Cortés JR. https://pubmed.ncbi.nlm.nih.gov/18714405/ Nutr Hosp. 2008;23 Suppl 2:3–7. [PubMed] [Google Scholar]
- 65.The influence of alcohol consumption on intestinal nutrient absorption: a comprehensive review. Butts M, Sundaram VL, Murughiyan U, Borthakur A, Singh S. Nutrients. 2023;15:1571. doi: 10.3390/nu15071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alcohol use as a risk factor in infections and healing: a clinician’s perspective. Trevejo-Nunez G, Kolls JK, de Wit M. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4590615/pdf/arcr-37-2-177.pdf. Alcohol Res. 2015;37:177–184. [PMC free article] [PubMed] [Google Scholar]
- 67.Mechanisms underlying muscle protein imbalance induced by alcohol. Kimball SR, Lang CH. Annu Rev Nutr. 2018;38:197–217. doi: 10.1146/annurev-nutr-071816-064642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dysregulation of skeletal muscle protein metabolism by alcohol. Steiner JL, Lang CH. Am J Physiol Endocrinol Metab. 2015;308:0–712. doi: 10.1152/ajpendo.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Complement system part II: role in immunity. Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Front Immunol. 2015;6:257. doi: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Complement system part I - molecular mechanisms of activation and regulation. Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Front Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mollah F, Tam S. Treasure Island, FL: StatPearls Publishing [Internet]; 2024. Complement Deficiency. [Updated 2023 Mar 13] [PubMed] [Google Scholar]
- 72.Roles of the complement system in alcohol-induced liver disease. Zhou Y, Yuan G, Zhong F, He S. Clin Mol Hepatol. 2020;26:677–685. doi: 10.3350/cmh.2020.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Complement system in alcohol-associated liver disease. Santiesteban-Lores LE, Carneiro MC, Isaac L, Bavia L. Immunol Lett. 2021;236:37–50. doi: 10.1016/j.imlet.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Immune cells in alcohol-related liver disease. Xu H, Wang H. Liver Res. 2022;6:1–9. [Google Scholar]
- 75.Alcohol use and the risk of communicable diseases. Morojele NK, Shenoi SV, Shuper PA, Braithwaite RS, Rehm J. Nutrients. 2021;13:3317. doi: 10.3390/nu13103317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Relationship between alcohol consumption and periodontal tissue condition in community-dwelling elderly Japanese. Suwama K, Yoshihara A, Watanabe R, Stegaroiu R, Shibata S, Miyazaki H. Gerodontology. 2018 doi: 10.1111/ger.12335. [DOI] [PubMed] [Google Scholar]
- 77.Alcohol intake and periodontitis in adults aged ≥30 years: NHANES 2009-2012. Gay IC, Tran DT, Paquette DW. J Periodontol. 2018;89:625–634. doi: 10.1002/JPER.17-0276. [DOI] [PubMed] [Google Scholar]
- 78.Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Fan X, Peters BA, Jacobs EJ, et al. Microbiome. 2018;6:59. doi: 10.1186/s40168-018-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.The oral microbiome in alcohol use disorder: a longitudinal analysis during inpatient treatment. Barb JJ, Maki KA, Kazmi N, et al. J Oral Microbiol. 2022;14:2004790. doi: 10.1080/20002297.2021.2004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.The roles and interactions of Porphyromonas gingivalis and Fusobacterium nucleatum in oral and gastrointestinal carcinogenesis: a narrative review. Wang B, Deng J, Donati V, Merali N, Frampton AE, Giovannetti E, Deng D. Pathogens. 2024;13:93. doi: 10.3390/pathogens13010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fusobacterium nucleatum in biopsied tissues from colorectal cancer patients and alcohol consumption in Korea. Kim M, Lee ST, Choi S, et al. Sci Rep. 2020;10:19915. doi: 10.1038/s41598-020-76467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evaluation of the subgingival microbiota of alcoholic and non-alcoholic individuals. Amaral Cda S, da Silva-Boghossian CM, Leão AT, Colombo AP. J Dent. 2011;39:729–738. doi: 10.1016/j.jdent.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 83.Differences in the subgingival microbial composition associated with alcohol intake: a systematic review. Oliveira LM, Antoniazzi RP, Demarco FF, Zanatta FB. J Oral Biol Craniofac Res. 2023;13:259–266. doi: 10.1016/j.jobcr.2023.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pathogenic mechanisms of Fusobacterium nucleatum on oral epithelial cells. Groeger S, Zhou Y, Ruf S, Meyle J. Front Oral Health. 2022;3:831607. doi: 10.3389/froh.2022.831607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Porphyromonas gingivalis in the development of periodontitis: impact on dysbiosis and inflammation. Gasmi Benahmed A, Kumar Mujawdiya P, Noor S, Gasmi A. Arch Razi Inst. 2022;77:1539–1551. doi: 10.22092/ARI.2021.356596.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ethanol binge drinking exposure affects alveolar bone quality and aggravates bone loss in experimentally-induced periodontitis. Frazão DR, Maia CD, Chemelo VD, et al. PLoS One. 2020;15:0. doi: 10.1371/journal.pone.0236161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.The relationship between alcohol consumption and health: J-shaped or less is more? Tsai MK, Gao W, Wen CP. BMC Med. 2023;21:228. doi: 10.1186/s12916-023-02911-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alcohol consumption and periodontal disease. The Third National Health and Nutrition Examination Survey. Tezal M, Grossi SG, Ho AW, Genco RJ. J Clin Periodontol. 2004;31:484–488. doi: 10.1111/j.1600-051X.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 89.Risk variables in the association between frequency of alcohol consumption and periodontitis. Lages EJ, Costa FO, Lages EM, et al. J Clin Periodontol. 2012;39:115–122. doi: 10.1111/j.1600-051X.2011.01809.x. [DOI] [PubMed] [Google Scholar]
- 90.Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. Wang HJ, Zakhari S, Jung MK. World J Gastroenterol. 2010;16:1304–1313. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gut microbiota dysbiosis: the potential mechanisms by which alcohol disrupts gut and brain functions. Chen G, Shi F, Yin W, Guo Y, Liu A, Shuai J, Sun J. Front Microbiol. 2022;13:916765. doi: 10.3389/fmicb.2022.916765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.The oral microbiome impacts the link between sugar consumption and caries: a preliminary study. Pang L, Zhi Q, Jian W, Liu Z, Lin H. Nutrients. 2022;14:3693. doi: 10.3390/nu14183693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Does high sugar intake really alter the oral microbiota?: a systematic review. Angarita-Díaz MD, Fong C, Bedoya-Correa CM, Cabrera-Arango CL. Clin Exp Dent Res. 2022;8:1376–1390. doi: 10.1002/cre2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.RANKL-induced M1 macrophages are involved in bone formation. Huang R, Wang X, Zhou Y, Xiao Y. Bone Res. 2017;5:17019. doi: 10.1038/boneres.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gut microbiota is involved in alcohol-induced osteoporosis in young and old rats through immune regulation. Cheng M, Tan B, Wu X, Liao F, Wang F, Huang Z. Front Cell Infect Microbiol. 2021;11:636231. doi: 10.3389/fcimb.2021.636231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Influence of chronic alcohol use on osteoblastic differentiation of bone marrow cells, bone properties, and hepatic and renal morphology of rats. Cardoso de Sousa M, Vegian MR, Biserra MA, et al. Sci World J. 2018;2018:2494918. doi: 10.1155/2018/2494918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Impact of alcohol on bone health, homeostasis and fracture repair. Eby JM, Sharieh F, Callaci JJ. Curr Pathobiol Rep. 2020;8:75–86. doi: 10.1007/s40139-020-00209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alcohol's harmful effects on bone. Sampson HW. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6761900/pdf/arh-22-3-190.pdf. Alcohol Health Res World. 1998;22:190–194. [PMC free article] [PubMed] [Google Scholar]
- 99.Cellular and molecular mechanisms of alcohol-induced osteopenia. Luo Z, Liu Y, Liu Y, Chen H, Shi S, Liu Y. Cell Mol Life Sci. 2017;74:4443–4453. doi: 10.1007/s00018-017-2585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alcohol contribution to total energy intake and its association with nutritional status and diet quality in eight Latin American countries. Brenes JC, Gómez G, Quesada D, et al. Int J Environ Res Public Health. 2021;18:13130. doi: 10.3390/ijerph182413130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alcohol consumption, bone mineral density, and risk of osteoporotic fractures: a dose-response meta-analysis. Godos J, Giampieri F, Chisari E, et al. Int J Environ Res Public Health. 2022;19:1515. doi: 10.3390/ijerph19031515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.The relevance of alcohol to dental practice. Grocock R. Br Dent J. 2018;223:895–899. doi: 10.1038/sj.bdj.2017.997. [DOI] [PubMed] [Google Scholar]
- 103.Relationship between Chinese Baijiu consumption and dental caries among 55- to 74-year-old adults in Guangdong, southern China: a cross-sectional survey. Huang X, Liang Y, Fan W, Liu W, Wu B, Li J. BMC Geriatr. 2021;21:506. doi: 10.1186/s12877-021-02453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.The sugars in alcohol cocktails matte. Wakabayashi KT, Greeman EA, Barrett ST, Bevins RA. ACS Chem Neurosci. 2021;12:3284–3287. doi: 10.1021/acschemneuro.1c00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Action on Sugar: ‘Ready to drink’ alcoholic beverages. [ May; 2024 ]. 2024. https://www.actiononsugar.org/surveys/2020/ready-to-drink-alcoholic-beverages/#d.en.764846 https://www.actiononsugar.org/surveys/2020/ready-to-drink-alcoholic-beverages/#d.en.764846
- 106.World Health Organization: Sugars and dental caries. [ May; 2024 ]. 2017. https://www.actiononsugar.org/surveys/2020/ready-to-drink-alcoholic-beverages/#d.en.764846 https://www.actiononsugar.org/surveys/2020/ready-to-drink-alcoholic-beverages/#d.en.764846
- 107.A reappraisal of the quantitative relationship between sugar intake and dental caries: the need for new criteria for developing goals for sugar intake. Sheiham A, James WP. BMC Public Health. 2014;14:863. doi: 10.1186/1471-2458-14-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.The role of sucrose in cariogenic dental biofilm formation--new insight. Paes Leme AF, Koo H, Bellato CM, Bedi G, Cury JA. J Dent Res. 2006;85:878–887. doi: 10.1177/154405910608501002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.The evolving microbiome of dental caries. Spatafora G, Li Y, He X, Cowan A, Tanner AC. Microorganisms. 2024;12:121. doi: 10.3390/microorganisms12010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.The role of bacteria in the caries process: ecological perspectives. Takahashi N, Nyvad B. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 111.Biology of oral streptococci. Abranches J, Zeng L, Kajfasz JK, et al. Microbiol Spectr. 2018;6:5. doi: 10.1128/microbiolspec.gpp3-0042-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.A narrative review of the effects of sugar-sweetened beverages on human health: a key global health issue. Haque M, McKimm J, Sartelli M, Samad N, Haque SZ, Bakar MA. J Popul Ther Clin Pharmacol. 2020;27:0. doi: 10.15586/jptcp.v27i1.666. [DOI] [PubMed] [Google Scholar]
- 113.Effect of sugar-sweetened beverages on oral health: a systematic review and meta-analysis. Valenzuela MJ, Waterhouse B, Aggarwal VR, Bloor K, Doran T. Eur J Public Health. 2021;31:122–129. doi: 10.1093/eurpub/ckaa147. [DOI] [PubMed] [Google Scholar]
- 114.World Health Organization: Preventing disease through healthy environments. Inadequate or excess fluoride: A major public health concern. [ May; 2024 ]. 2024. https://iris.who.int/bitstream/handle/10665/329484/WHO-CED-PHE-EPE-19.4.5-eng.pdf https://iris.who.int/bitstream/handle/10665/329484/WHO-CED-PHE-EPE-19.4.5-eng.pdf
- 115.Fluoride content in alcoholic drinks. Goschorska M, Gutowska I, Baranowska-Bosiacka I, Rać ME, Chlubek D. Biol Trace Elem Res. 2016;171:468–471. doi: 10.1007/s12011-015-0519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beer as a rich source of fluoride delivered into the body. Styburski D, Baranowska-Bosiacka I, Goschorska M, Chlubek D, Gutowska I. Biol Trace Elem Res. 2017;177:404–408. doi: 10.1007/s12011-016-0888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thirst sensation and oral dryness following alcohol intake. Inenaga K, Ono K, Hitomi S, Kuroki A, Ujihara I. Jpn Dent Sci Rev. 2017;53:78–85. doi: 10.1016/j.jdsr.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.The diuretic action of weak and strong alcoholic beverages in elderly men: a randomized diet-controlled crossover trial. Polhuis KC, Wijnen AH, Sierksma A, Calame W, Tieland M. Nutrients. 2017;9:660. doi: 10.3390/nu9070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mechanism of dehydration following alcohol ingestion. Roberts KE. Arch Intern Med. 1963;112:154–157. doi: 10.1001/archinte.1963.03860020052002. [DOI] [PubMed] [Google Scholar]
- 120.Alcohol's impact on kidney function. Epstein M. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6826793/pdf/arhw-21-1-84.pdf. Alcohol Health Res World. 1997;21:84–92. [PMC free article] [PubMed] [Google Scholar]
- 121.Salivary characteristics and dental caries: evidence from general dental practices. Cunha-Cruz J, Scott J, Rothen M, Mancl L, Lawhorn T, Brossel K, Berg J. J Am Dent Assoc. 2013;144:0–40. doi: 10.14219/jada.archive.2013.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reduced salivary flow and caries status are correlated with disease activity and severity in patients with diffuse cutaneous systemic sclerosis. Parat K, Radić M, Perković D, Lukenda DB, Kaliterna DM. J Int Med Res. 2020;48:300060520941375. doi: 10.1177/0300060520941375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.The role of natural salivary defences in maintaining a healthy oral microbiota. Lynge Pedersen AM, Belstrøm D. J Dent. 2019;80 Suppl 1:0. doi: 10.1016/j.jdent.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 124.Microbial etiology and prevention of dental caries: exploiting natural products to inhibit cariogenic biofilms. Chen X, Daliri EB, Kim N, Kim JR, Yoo D, Oh DH. Pathogens. 2020;9:569. doi: 10.3390/pathogens9070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Attributable fraction of alcohol consumption on cancer using population-based nationwide cancer incidence and mortality data in the Republic of Korea. Park S, Shin HR, Lee B, et al. BMC Cancer. 2014;14:420. doi: 10.1186/1471-2407-14-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Combinations of lifestyle behaviors and cancer risk among Korean adults. Luu NM, Bui TT, Tran TP, Nguyen TH, Oh JK. Sci Rep. 2023;13:13765. doi: 10.1038/s41598-023-40819-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort study. Schütze M, Boeing H, Pischon T, et al. BMJ. 2011;342:0. doi: 10.1136/bmj.d1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.World Cancer Research Fund/American Institute for Cancer Research: Diet, Nutrition, Physical Activity, and Cancer: A Global Perspective. [ May; 2024 ];https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf 2018 6:2024. [Google Scholar]
- 129.Alcohol and cancer: epidemiology and biological mechanisms. Rumgay H, Murphy N, Ferrari P, Soerjomataram I. Nutrients. 2021;13:3173. doi: 10.3390/nu13093173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alcohol as a risk factor for cancer: existing evidence in a global perspective. Roswall N, Weiderpass E. J Prev Med Public Health. 2015;48:1–9. doi: 10.3961/jpmph.14.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Bagnardi V, Rota M, Botteri E, et al. Br J Cancer. 2015;112:580–593. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Recommendation on unbiased estimation of population attributable fraction calculated in "prevalence and risk factors of active pulmonary tuberculosis among elderly people in China: a population based cross-sectional study". Khosravi A, Mansournia MA. Infect Dis Poverty. 2019;8:75. doi: 10.1186/s40249-019-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dental diseases and loss of teeth in a group of Finnish alcoholics: a radiological study. Enberg N, Wolf J, Ainamo A, Alho H, Heinälä P, Lenander-Lumikari M. Acta Odontol Scand. 2001;59:341–347. doi: 10.1080/000163501317153176. [DOI] [PubMed] [Google Scholar]
- 134.Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. GBD 2019 Tobacco Collaborators. Lancet. 2021;397:2337–2360. doi: 10.1016/S0140-6736(21)01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burden of smoking on disease-specific mortality, DALYs, costs: the case of a high-income European country. Farcher R, Syleouni ME, Vinci L, Mattli R. BMC Public Health. 2023;23:698. doi: 10.1186/s12889-023-15535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tobacco and oral squamous cell carcinoma: a review of carcinogenic pathways. Jiang X, Wu J, Wang J, Huang R. Tob Induc Dis. 2019;17:29. doi: 10.18332/tid/105844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Relationship between type of smokeless tobacco and risk of cancer: a systematic review. Gupta S, Gupta R, Sinha DN, Mehrotra R. Indian J Med Res. 2018;148:56–76. doi: 10.4103/ijmr.IJMR_2023_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tobacco-smoking, alcohol-drinking, and betel-quid-chewing behaviors: development and use of a web-based survey system. Hsu KY, Tsai YF, Huang CC, et al. JMIR Mhealth Uhealth. 2018;6:0. doi: 10.2196/mhealth.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tobacco smoking, chewing habits, alcohol drinking and the risk of head and neck cancer in Nepal. Chang CP, Siwakoti B, Sapkota A, Gautam DK, Lee YA, Monroe M, Hashibe M. Int J Cancer. 2020;147:866–875. doi: 10.1002/ijc.32823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.The magnitude of tobacco smoking-betel quid chewing-alcohol drinking interaction effect on oral cancer in South-East Asia. A meta-analysis of observational studies. Petti S, Masood M, Scully C. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0078999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Trends in concurrent tobacco use and heavy drinking among individuals 15 years and older in Mongolia. Pengpid S, Peltzer K. Sci Rep. 2022;12:16639. doi: 10.1038/s41598-022-21094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Verplaetse TL, McKee SA. Am J Drug Alcohol Abuse. 2017;43:186–196. doi: 10.1080/00952990.2016.1189927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shared lifestyle-related risk factors of cardiovascular disease and cancer: evidence for joint prevention. Masoudkabir F, Mohammadifard N, Mani A, et al. Sci World J. 2023;2023:2404806. doi: 10.1155/2023/2404806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.The combination of cigarette smoking and alcohol consumption synergistically increases reactive carbonyl species in human male plasma. Mure K, Tomono S, Mure M, et al. Int J Mol Sci. 2021;22:9043. doi: 10.3390/ijms22169043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.The combined effect of smoking tobacco and drinking alcohol on cause-specific mortality: a 30 year cohort study. Hart CL, Davey Smith G, Gruer L, Watt GC. BMC Public Health. 2010;10:789. doi: 10.1186/1471-2458-10-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.World Health Organization: Tobacco. [ May; 2024 ]. 2020. https://www.who.int/news-room/fact-sheets/detail/tobacco https://www.who.int/news-room/fact-sheets/detail/tobacco
- 147.World Health Organization: Alcohol. [ May; 2024 ]. 2020. https://www.who.int/health-topics/alcohol#tab=tab_1 https://www.who.int/health-topics/alcohol#tab=tab_1
- 148.Comparing alcohol with tobacco indicates that it is time to move beyond tobacco exceptionalism. McCambridge J, Morris S. Eur J Public Health. 2019;29:200–201. doi: 10.1093/eurpub/cky227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Neuropathology of alcoholism. Sutherland GT, Sheedy D, Kril JJ. Handb Clin Neurol. 2014;125:603–615. doi: 10.1016/B978-0-444-62619-6.00035-5. [DOI] [PubMed] [Google Scholar]
- 150.Alcoholism and the brain: an overview. Oscar-Berman M, Marinkovic K. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6668884/pdf/125-133.pdf. Alcohol Res Health. 2003;27:125–133. [PMC free article] [PubMed] [Google Scholar]
- 151.Incidence and mortality of alcohol-related dementia and Wernicke-Korsakoff syndrome: A nationwide register study. Palm A, Vataja R, Talaslahti T, et al. Int J Geriatr Psychiatry. 2022;37:75. doi: 10.1002/gps.5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ethanol as a neurotoxin. Leonard BE. Biochem Pharmacol. 1987;36:2055–2059. doi: 10.1016/0006-2952(87)90129-8. [DOI] [PubMed] [Google Scholar]
- 153.Chronic alcohol-induced neuroinflammation involves CCR2/5-dependent peripheral macrophage infiltration and microglia alterations. Lowe PP, Morel C, Ambade A, et al. J Neuroinflammation. 2020;17:296. doi: 10.1186/s12974-020-01972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Effects of alcohol on the brain in cirrhosis: beyond hepatic encephalopathy. Davis BC, Bajaj JS. Alcohol Clin Exp Res. 2018;42:660–667. doi: 10.1111/acer.13605. [DOI] [PubMed] [Google Scholar]
- 155.Microbiome as a therapeutic target in alcohol-related liver disease. Sarin SK, Pande A, Schnabl B. J Hepatol. 2019;70:260–272. doi: 10.1016/j.jhep.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 156.The pathogenesis of gut microbiota in hepatic encephalopathy by the gut-liver-brain axis. Zhu R, Liu L, Zhang G, Dong J, Ren Z, Li Z. Biosci Rep. 2023;43:524. doi: 10.1042/BSR20222524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Alcohol-related dementia: an update of the evidence. Ridley NJ, Draper B, Withall A. Alzheimers Res Ther. 2013;5:3. doi: 10.1186/alzrt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alcohol’s effects on the brain: neuroimaging results in humans and animal models. Zahr NM, Pfefferbaum A. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513685/pdf/arcr-38-2-183.pdf. Alcohol Res. 2017;38:183–206. [PMC free article] [PubMed] [Google Scholar]
- 159.Akhouri S, Kuhn J, Newton EJ. Treasure Island, FL: StatPearls Publishing [Internet]; 2024. Wernicke-Korsakoff Syndrome. [Updated 2023 Jun 26] [PubMed] [Google Scholar]
- 160.Risk of dementia and alcohol and wine consumption: a review of recent results. Letenneur L. Biol Res. 2004;37:189–193. doi: 10.4067/s0716-97602004000200003. [DOI] [PubMed] [Google Scholar]
- 161.National Institute on Alcohol Abuse and Alcoholism: Alcohol and the Brain: An Overview. [ May; 2024 ]. 2022. https://www.niaaa.nih.gov/publications/alcohol-and-brain-overview. https://www.niaaa.nih.gov/publications/alcohol-and-brain-overview [PMC free article] [PubMed]
- 162.Impairments of brain and behavior: the neurological effects of alcohol. Oscar-Berman M, Shagrin B, Evert DL, Epstein C. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6826797/pdf/arhw-21-1-65.pdf. Alcohol Health Res World. 1997;21:65–75. [PMC free article] [PubMed] [Google Scholar]
- 163.Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Daviet R, Aydogan G, Jagannathan K, et al. Nat Commun. 2022;13:1175. doi: 10.1038/s41467-022-28735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brain-behavior relations and effects of aging and common comorbidities in alcohol use disorder: a review. Sullivan EV, Pfefferbaum A. Neuropsychology. 2019;33:760–780. doi: 10.1037/neu0000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Human alcohol-related neuropathology. de la Monte SM, Kril JJ. Acta Neuropathol. 2014;127:71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Structural and microstructral imaging of the brain in alcohol use disorders. Zahr NM. Handb Clin Neurol. 2014;125:275–290. doi: 10.1016/B978-0-444-62619-6.00017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Light to moderate alcohol use is associated with increased cortical gray matter in middle-aged men: a voxel-based morphometric study. Sachdev PS, Chen X, Wen W, Anstey KJ. Psychiatry Res. 2008;163:61–69. doi: 10.1016/j.pscychresns.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 168.Detrimental effects of alcohol-induced inflammation on brain health: from neurogenesis to neurodegeneration. Anand SK, Ahmad MH, Sahu MR, Subba R, Mondal AC. Cell Mol Neurobiol. 2023;43:1885–1904. doi: 10.1007/s10571-022-01308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Longitudinal effects of alcohol consumption on the hippocampus and parahippocampus in college students. Meda SA, Hawkins KA, Dager AD, et al. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:610–617. doi: 10.1016/j.bpsc.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Effect of alcohol on hippocampal-dependent plasticity and behavior: role of glutamatergic synaptic transmission. Mira RG, Lira M, Tapia-Rojas C, Rebolledo DL, Quintanilla RA, Cerpa W. Front Behav Neurosci. 2019;13:288. doi: 10.3389/fnbeh.2019.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Pitel AL, Beaunieux H, Witkowski T, et al. Alcohol Clin Exp Res. 2007;31:1169–1178. doi: 10.1111/j.1530-0277.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Distinctions in alcohol-induced memory impairment: a mixed methods study of en bloc versus fragmentary blackouts. Miller MB, Merrill JE, DiBello AM, Carey KB. Alcohol Clin Exp Res. 2018;42:2000–2010. doi: 10.1111/acer.13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Consolidation and reconsolidation: two lives of memories? McKenzie S, Eichenbaum H. Neuron. 2011;71:224–233. doi: 10.1016/j.neuron.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.The effects of alcohol on emotion in social drinkers. Sayette MA. Behav Res Ther. 2017;88:76–89. doi: 10.1016/j.brat.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sunset Yellow: physical, thermal, and bioactive properties of the widely employed food, pharmaceutical and cosmetic orange azo-dye material. Leulescu M, Pălărie I, Rotaru A, et al. J Therm Anal Calorim. 2023;148:1265–1287. [Google Scholar]
- 176.Prevalence of artificial food colors in grocery store products marketed to children. Batada A, Jacobson MF. Clin Pediatr (Phila) 2016;55:1113–1119. doi: 10.1177/0009922816651621. [DOI] [PubMed] [Google Scholar]
- 177.Evaluation of the effect of pediatric drugs and an oral rinse on primary teeth discoloration. Yılmaz N, Baygin O, Tüzüner T, Turgut SN, Erbek ŞM. Dent Med Probl. 2022;59:225–231. doi: 10.17219/dmp/133406. [DOI] [PubMed] [Google Scholar]
- 178.North Sydney Dental Practice: Effect of alcohol on dental health. North Sydney NSW 2060. [ May; 2024 ];North Sydney Dental Practice. https://www.northsydneydentalpractice.com.au/effect-alcohol-dental-health/ 2023 10:2024. [Google Scholar]
- 179.Necrotising periodontal diseases. Bermejo-Fenoll A, Sánchez-Pérez A. http://www.medicinaoral.com/pubmed/medoralv9suppl_i_p114.pdf. Med Oral Patol Oral Cir Bucal. 2004;9:108–114. [PubMed] [Google Scholar]
- 180.Gasner NS, Schure RS. Treasure Island, FL: StatPearls Publishing [Internet]; 2024. Necrotizing Periodontal Diseases. [Updated 2023 May 8] [PubMed] [Google Scholar]
- 181.Necrotizing ulcerative gingivitis, periodontitis, and stomatitis: clinical staging and predisposing factors. Horning GM, Cohen ME. J Periodontol. 1995;66:990–998. doi: 10.1902/jop.1995.66.11.990. [DOI] [PubMed] [Google Scholar]
- 182.Prevalence and treatment of necrotizing ulcerative gingivitis (NUG) in the British Armed Forces: a case-control study. Dufty J, Gkranias N, Petrie A, McCormick R, Elmer T, Donos N. Clin Oral Investig. 2017;21:1935–1944. doi: 10.1007/s00784-016-1979-9. [DOI] [PubMed] [Google Scholar]
- 183.Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. Herrera D, Retamal-Valdes B, Alonso B, Feres M. J Clin Periodontol. 2018;45 Suppl 20:0–94. doi: 10.1111/jcpe.12941. [DOI] [PubMed] [Google Scholar]
- 184.Periodontitis: from microbial immune subversion to systemic inflammation. Hajishengallis G. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Non-communicable diseases and oral health: an overview. Wolf TG, Cagetti MG, Fisher JM, Seeberger GK, Campus G. Front Oral Health. 2021;2:725460. doi: 10.3389/froh.2021.725460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.The role of the oral microbiota in chronic non-communicable disease and its relevance to the Indigenous health gap in Australia. Handsley-Davis M, Jamieson L, Kapellas K, Hedges J, Weyrich LS. BMC Oral Health. 2020;20:327. doi: 10.1186/s12903-020-01308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.The oral-microbiome-brain axis and neuropsychiatric disorders: an anthropological perspective. Bowland GB, Weyrich LS. Front Psychiatry. 2022;13:810008. doi: 10.3389/fpsyt.2022.810008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Aaron SL, DeBlois KW. Treasure Island, FL: StatPearls Publishing [Internet]; 2024. Acute Necrotizing Ulcerative Gingivitis. [Updated 2023 Jul 20] [PubMed] [Google Scholar]
- 189.Necrotising periodontal diseases: an update on classification and management. Ogunleye R, Ukoha O, Nasterska W, McColl E, Dantata F, Adetula I. Br Dent J. 2022;233:855–858. doi: 10.1038/s41415-022-5201-y. [DOI] [PubMed] [Google Scholar]
- 190.Necrotizing gingivitis: microbial diversity and quantification of protein secretion in necrotizing gingivitis. Gerhard N, Thurnheer T, Kreutzer S, Gmür RD, Attin T, Russo G, Karygianni L. Antibiotics (Basel) 2021;10:1197. doi: 10.3390/antibiotics10101197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Periodontal diseases as bacterial infection. Bascones-Martínez A, Figuero-Ruiz E. Med Oral Patol Oral Cir Bucal. 2004;9 Suppl:101–107. doi: 10.4321/s1699-65852005000300002. [DOI] [PubMed] [Google Scholar]
- 192.Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. Lachenmeier DW. J Occup Med Toxicol. 2008;3:26. doi: 10.1186/1745-6673-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Are alcohol containing mouthwashes safe? Werner CW, Seymour RA. Br Dent J. 2009;207:488–489. doi: 10.1038/sj.bdj.2009.1014. [DOI] [PubMed] [Google Scholar]
- 194.The role of type of tobacco and type of alcoholic beverage in oral carcinogenesis. Castellsagué X, Quintana MJ, Martínez MC, et al. Int J Cancer. 2004;108:741–749. doi: 10.1002/ijc.11627. [DOI] [PubMed] [Google Scholar]
- 195.Mouthwash and oral cancer risk: an update. La Vecchia C. Oral Oncol. 2009;45:198–200. doi: 10.1016/j.oraloncology.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 196.Does the use of alcohol mouthwash increase the risk of developing oral cancer? Carr E, Aslam-Pervez B. Evid Based Dent. 2022;23:28–29. doi: 10.1038/s41432-022-0236-0. [DOI] [PubMed] [Google Scholar]
- 197.Alcohol-based mouthwash as a risk factor of oral cancer: a systematic review. Ustrell-Borràs M, Traboulsi-Garet B, Gay-Escoda C. Med Oral Patol Oral Cir Bucal. 2020;25:0. doi: 10.4317/medoral.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mouthwash with alcohol and oral carcinogenesis: systematic review and meta-analysis. Aceves Argemí R, González Navarro B, Ochoa García-Seisdedos P, Estrugo Devesa A, López-López J. J Evid Based Dent Pract. 2020;20:101407. doi: 10.1016/j.jebdp.2020.101407. [DOI] [PubMed] [Google Scholar]
- 199.Alcohol-containing mouthwashes, and oral cancer. Critical analysis of literature. Carretero Peláez MA, Esparza Gómez GC, Figuero Ruiz E, Cerero Lapiedra R. http://www.medicinaoral.com/pubmed/medoralv9_i2_p120.pdf. Med Oral. 2004;9:120–123. [PubMed] [Google Scholar]
- 200.Evidence on the use of mouthwash for the control of supragingival biofilm and its potential adverse effects. Takenaka S, Sotozono M, Ohkura N, Noiri Y. Antibiotics (Basel) 2022;11:727. doi: 10.3390/antibiotics11060727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Recent development of active ingredients in mouthwashes and toothpastes for periodontal diseases. Rajendiran M, Trivedi HM, Chen D, Gajendrareddy P, Chen L. Molecules. 2021;26:2001. doi: 10.3390/molecules26072001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Biological plaque control: novel therapeutic approach to periodontal disease. Sugano N. J Oral Sci. 2012;54:1–5. doi: 10.2334/josnusd.54.1. [DOI] [PubMed] [Google Scholar]
- 203.Flossing for the management of periodontal diseases and dental caries in adults. Sambunjak D, Nickerson JW, Poklepovic Pericic T, Johnson TM, Imai P, Tugwell P, Worthington HV. Cochrane Database Syst Rev. 2019;4:0. doi: 10.1002/14651858.CD008829.pub2. [DOI] [PubMed] [Google Scholar]
- 204.Human exposure to triclosan via toothpaste does not change CYP3A4 activity or plasma concentrations of thyroid hormones. Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G. Basic Clin Pharmacol Toxicol. 2009;105:339–344. doi: 10.1111/j.1742-7843.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 205.Can chemical mouthwash agents achieve plaque/gingivitis control? Van der Weijden FA, Van der Sluijs E, Ciancio SG, Slot DE. Dent Clin North Am. 2015;59:799–829. doi: 10.1016/j.cden.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 206.Revisiting oral antiseptics: microorganism targets and effectiveness. Garrido L, Lyra P, Rodrigues J, Viana J, Mendes JJ, Barroso H. J Pers Med. 2023;13:1332. doi: 10.3390/jpm13091332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Plaque bacteria counts and vitality during chlorhexidine, meridol and listerine mouthrinses. Netuschil L, Weiger R, Preisler R, Brecx M. Eur J Oral Sci. 1995;103:355–361. doi: 10.1111/j.1600-0722.1995.tb01857.x. [DOI] [PubMed] [Google Scholar]