Thromboembolism and anticoagulant related bleeding are major life threatening complications in patients with valvar heart disease and those with prosthetic heart valves. In these patients effective and safe antithrombotic therapy is indicated to reduce the risks of thromboembolism while keeping bleeding complications to a minimum.
Assessment
Risk factors that increase the incidence of systemic embolism must be considered when defining the need for starting antithrombotic therapy in patients with cardiac valvar disease and prosthetic heart valves. These factors include age, smoking, hypertension, diabetes, hyperlipidaemia, type and severity of valve lesion, presence of atrial fibrillation, heart failure or low cardiac output, size of the left atrium (over 50 mm on echocardiography), previous thromboembolism, and abnormalities of the coagulation system including hepatic failure.
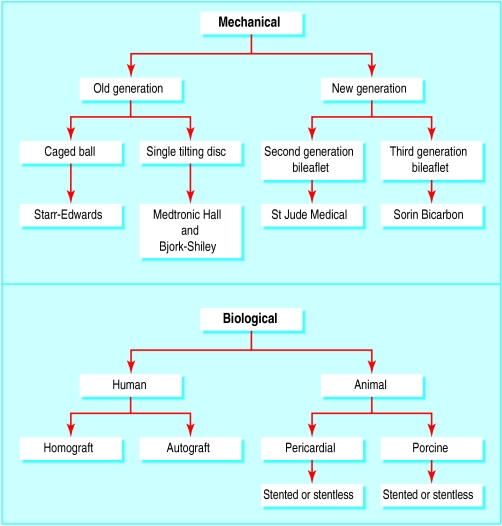
Secondly, the type, number, and location of prostheses implanted must be considered. For example, mechanical prostheses are more thrombogenic than bioprostheses or homografts, and hence patients with mechanical valves require lifelong anticoagulant therapy. However, the intensity of treatment varies according to the type of mechanical prosthesis implanted. First generation mechanical valves, namely the Starr-Edwards caged ball valve and Bjork-Shiley standard valves, have a high thromboembolic risk; single tilting disc valves have an intermediate thromboembolic risk; and the newer (second and third generation) bileaflet valves have low thromboembolic risks.
Considerations for antithrombotic therapy in patients with valve disease
Assessment of risk for thromboembolic events, which may be patient related or valve prosthesis related
Indications for starting treatment
Choice of antithrombotic agent
Duration of treatment and optimal therapeutic range
Antithrombotic therapy in special circumstances (surgical procedures, pregnancy, and resistance to oral anticoagulants)
Management of treatment failures and complications
In patients with a bioprosthesis in sinus rhythm, antithrombotic therapy with an antiplatelet drug may suffice, whereas patients with homografts in sinus rhythm may not need any antithrombotic therapy. Thromboembolic events are commoner with prosthetic mitral valves than aortic valves and in patients with double replacement valves compared with those with single replaced valves. Moreover, the risk of thromboembolic events is greatest in the first three months after implantation.
Types of prosthetic valves and thrombogenicity
| Type of valve | Model | Thrombogenicity |
| Mechanical | ||
| Caged ball | Starr-Edwards | ++++ |
| Single tilting disc | Bjork-Shiley,Medtronic Hall | +++ |
| Bileaflet | St Jude Medical,Sorin Bicarbon,Carbomedics | ++ |
| Bioprosthetic | ||
| Heterografts | Carpentier-Edwards,Tissue Med (Aspire), Hancock II | + to ++ |
| Homografts | + |
Choice of antithrombotic agent
Warfarin is the most used oral anticoagulant, and its dose is guided by achieving a target international normalised ratio (INR) range. The use of heparin is confined to short periods when anticoagulant cover is needed and oral anticoagulants are stopped. The dose of heparin is adjusted to achieve at least twice normal level of activated partial thromboplastin time (APTT) regardless of cardiac rhythm and type or position of prosthesis. Fixed weight-adjusted low molecular weight heparin may be used as an alternative to heparin. Antiplatelet drugs, such as low dose aspirin or dipyridamole, are used in patients with bioprosthesis in sinus rhythm and in addition to anticoagulants in the high risk patients with mechanical valves.
Risk factors for patients with bioprostheses include previous thromboembolic events, atrial fibrillation, enlarged left atrial cavity, and severe cardiac failure
Patients with mechanical valves and those with bioprostheses and associated risk factors require lifelong anticoagulant cover. In patients with a bioprosthetic valve in sinus rhythm anticoagulant cover with warfarin for the first three postoperative months may suffice, followed by low dose aspirin treatment for life. Alternatively, some surgeons give only low dose aspirin after surgery in patients with bioprostheses in sinus rhythm (providing aspirin is not contraindicated). Patients with homografts usually do not require any antithrombotic therapy.
Indications for antithrombotic therapy
Native valve disease
Oral anticoagulant treatment is indicated in all patients who have established or paroxysmal atrial fibrillation with native valve disease regardless of the nature or severity of the valve disease. In patients with mitral stenosis in sinus rhythm, treatment is guided by the severity of stenosis, the patient's age, size of the left atrium, and the presence of spontaneous echocontrast or echocardiographic evidence of left atrial appendage thrombus. In these patients a target INR of 2.5 (range 2-3) is recommended. Similarly, in patients with mitral regurgitation treatment is indicated in the presence of congestive cardiac failure, marked cardiomegaly with low cardiac output, and an enlarged left atrium. In the absence of cardiac failure, previous thromboemboli, or heart failure, antithrombotic therapy is not indicated in patients with isolated aortic or tricuspid valve disease.
Mitral valve prolapse per se does not require anticoagulant cover, although sometimes aspirin is recommended because of the association with cerebrovascular events.
Percutaneous balloon valvuloplasty
In patients with mitral stenosis, the presence or absence of left atrial thrombus is first confirmed by transoesophageal echocardiography. In the presence of thrombus, valvuloplasty is deferred and anticoagulant treatment started for two months before the procedure, with a target INR range of 2-3. In the absence of atrial thrombus but in the presence of risk factors— namely, previous thromboembolism, enlarged left atrium, spontaneous echocontrast, or atrial fibrillation—oral anticoagulant treatment should be started a month before the procedure.
Comparison of mechanical and biological valve prostheses
| Mechanical | Biological |
| Durable—valves lasting 20-30 years | Limited life span—10% of homografts and 30% of heterografts fail within10-15 years |
| Thrombogenic—patients require lifelong anticoagulant therapy | Low thrombogenic potential—lifelong anticoagulation is not required |
| Preferred in younger patients with >10-15 years life expectancy | Preferred in older patients with <10-15 years life expectancy |
| Preferred in patients who require lifelong anticoagulant therapy | Preferred in those who cannot (or will not) take lifelong anticoagulant therapy |
During the procedure, intravenous heparin (2000-5000 IU bolus) should be given to all patients immediately after trans-septal catheterisation. After the procedure, subcutaneous heparin should be given for 24 hours and oral anticoagulant treatment restarted 24 hours after the procedure in patients with risk factors, especially in the presence of atrial fibrillation or spontaneous echocontrast.
Patients in sinus rhythm who are undergoing aortic valvuloplasty do not need long term anticoagulant treatment. However, treatment with heparin during the procedure is required.
Mitral valve repair
After mitral valve repair, oral anticoagulation (target INR 2.5) is needed for the first six weeks to three months, and thereafter treatment is guided by the presence or absence of risk factors such as atrial fibrillation, heart failure, and enlarged left atrium.
Heart valve replacement
Antithrombotic therapy in patients with replaced heart valves is guided by the type of prosthesis implanted (mechanical or biological), position of the implant, associated risk factors (such as atrial fibrillation), previous thromboembolism, bleeding risk, and the patient's age.
Patients with porcine or pericardial bioprostheses in sinus rhythm may be started on lifelong antiplatelet treatment with low dose aspirin as soon as they can swallow the drugs. However, many centres start oral anticoagulant treatment the day after implantation, maintaining an INR range of 2-3 for the first three months. Lifelong anticoagulant treatment is recommended for patients with associated risk factors. These factors are previous thromboembolism, left atrial thrombus, marked cardiomegaly, heart failure, dilated left atrium, or spontaneous echocontrast.
Intensity of anticoagulation guidelines for Europe
| European Society of Cardiology 1995 INR range | British Society of Haematology 1998 INR target | |
| Mechanical valves* | ||
| Aortic: | ||
| First generation | 3.0-4.5 | 3.5 |
| Second generation | 2.5-3.0 | 3.5† |
| Third generation | 2.5-3.0 | 3.5† |
| Mitral | 3.0-3.5 | 3.5 |
| Bioprosthetic valves | ||
| In sinus rhythm: | ||
| Aortic | 2.5-3.0 for three months | No anticoagulation‡ |
| Mitral | 3.0-3.5 for three months. No anticoagulation after three months | 2.5 for three months. No anticoagulation after three months |
| In atrial fibrillation: | ||
| Rheumatic valvar heart disease | 3.0-4.5 | 2.5 |
| Patients with recurrent emboli under adequate anticoagulation | 3.0-4.5 + 100 mg aspirin | — |
| Non-valvar atrial fibrillation with risk factors | 2.0-3.0 | 2.5 |
*First generation valves include Starr-Edwards and Bjork-Shiley; second generation valves include St Jude Medical and Medtronic Hall; and third generation valves include the Sorin Bicarbon bileaflet valve
†For second and third generation mechanical aortic valves a target INR of 2.5 is used
‡Low dose aspirin is used by most centres in the United Kingdom
Patients with mechanical heart valves require lifelong anticoagulant treatment, and patients with first generation valves (with the highest thromboembolic risk) need a higher target INR than patients with single tilting disc prostheses (intermediate thromboembolic risk) or the newer bileaflet prosthesis (lower thromboembolic risk).
Most centres start (or restart) oral anticoagulant treatment the day after implantation, with or without heparinisation. As the thromboembolic risk is highest in the early postoperative period, it is advisable to give heparin and to continue it until the oral anticoagulant treatment achieves the target INR. The dose of heparin should be adjusted to achieve twice the normal level of APTT regardless of cardiac rhythm and type or position of the valve.
The European and North American guidelines have minor differences. The duration of antithrombotic therapy also varies according to a number of factors. Lifelong anticoagulant treatment is indicated for patients with mechanical valves and those with bioprosthetic valves or native valve disease with additional risk factors.
Antithrombotic therapy in special circumstances
Modification of anticoagulant treatment may be required in patients who have prosthetic valves and are undergoing non-cardiac surgical procedures, who are are pregnant, or who have resistance to oral anticoagulants.
Surgical procedures
For minor procedures, such as certain dental surgery or cryotherapy, where blood loss is expected to be minimal and easily manageable, anticoagulant treatment may be continued. After dental extraction bleeding can be stopped with oral tranexamic acid (4.8%) mouth wash. However, before a planned minor surgical procedure, the INR should be adjusted to between 1.5 and 2.0. This can be achieved by stopping or adjusting oral anticoagulant treatment one to three days before the procedure depending on the drug used. In most cases, resumption of oral anticoagulant treatment is possible on the same day as the procedure, and interim heparin treatment is not needed. Patients undergoing endoscopic procedures and in whom an endoscopic biopsy is anticipated should be managed in the same way as patients needing major non-cardiac surgical procedures.
Intensity of anticoagulation guidelines for North America
| AHA and ACC 1998 INR range | ACCP 2001 INR (target range) | |
| Mechanical valves | ||
| First, second, and third generation valves: | ||
| Aortic | 2.0-3.0 | 2.5 (2.0-3.0) |
| Mitral | 2.5-3.5 | 3.0 (2.5-3.5) |
| Bioprosthetic valves | ||
| In sinus rhythm: | ||
| Aortic | Aspirin80-100 mg/day | 2.5 (2.0-3.0) for three months |
| Mitral | Aspirin80-100 mg/day.No anticoagulation after three months | 2.5 (2.0-3.0) for three months |
| In atrial fibrillation: | ||
| Aortic | 2.0-3.0 | 2.5 (2.0-3.0) |
| Mitral | 2.5-3.5 | 2.5 (2.0-3.0) |
AHA=American Heart Association; ACC=American College of Cardiology; ACCP=American College of Chest Physicians
For major non-cardiac surgical procedures, in which there is a substantial risk of bleeding, anticoagulation should be discontinued for several days (generally four to five days) before surgery and the INR should be normalised at 1.0. The risk of thromboembolism increases, and so interim heparin treatment should be given in a dose that prolongs the APTT to twice the control value. However, heparin should be stopped in time to bring the APTT down to near normal at the time of operation and resumed as soon as possible postoperatively. An alternative approach would be to use therapeutic fixed weight-adjusted doses of low molecular weight heparin.
Pregnancy
In pregnant women with prosthetic valves, the incidence of thromboembolic complications is increased. Hence, adequate antithrombotic therapy is particularly important. Warfarin use in the first trimester of pregnancy is associated with a substantical risk of embryopathy and fetal death, and so warfarin should be stopped when a patient is trying to become pregnant or when pregnancy is detected. Instead, twice daily subcutaneous heparin should be given to prolong the APTT to twice the control value, and this treatment may be continued until delivery. Alternatively, heparin may be given until the thirteenth week of pregnancy, then a switch made to warfarin treatment until the middle of the third trimester. Then warfarin can be stopped and heparin resumed until delivery. Because low dose aspirin is safe for mother and child, it may be used in conjunction with anticoagulant treatment in women at high risk of thromboembolism. Low molecular weight heparin does not cross the placental barrier and may be an alternative to unfractionated heparin in this setting, although there are limited data on its efficacy or safety in pregnancy.
Indications for lifelong oral anticoagulation in valve disease
Mechanical prostheses
Chronic or paroxysmal atrial fibrillation in the presence of native valve disease, bioprosthesis, valve repair, or valvuloplasty
Native valve disease and previous thromboembolism
Mitral valve stenosis, irrespective of rhythm, in association with high transmitral valve gradient, left atrial thrombus, spontaneous echocontrast, large left atrium (>50 mm), low cardiac output, or congestive heart failure
Management of temporary interruption of oral anticoagulants
Discontinue oral anticoagulation five days before procedure
Measure INR three days before procedureIf INR <2 start low molecular weight heparin in therapeutic dosesIf INR >2.5 consider giving Vitamin K1 1-2 mg orally and start low molecular weight heparin in therapeutic doses. Repeat INR measurement the day before procedure
Continue low molecular weight heparin until evening before procedure (last injection not less than 12 hours preprocedure)
Restart warfarin night of or day after procedure
Restart low molecular weight heparin 12-24 hours after procedure and when haemostasis is established
Further reading
Bonow RO, Carobello D, de Leon AC, Edmunds LH Jr, Fedderly BJ, Freed MD, et al. ACC/AHA guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol 1998; 32:1486-8
Gohlke-Barwölf C. Anticoagulation in valvular heart disease: new aspects and management during non-cardiac surgery.
Gohlke-Barwölf C, Acar J, Oakley C, Butchart E, Durckhardt D, Delahaye JP, et al. Guidelines for prevention of thromboembolic events in valvular heart disease. Eur Heart J 1995;16:1320-30
Figure.
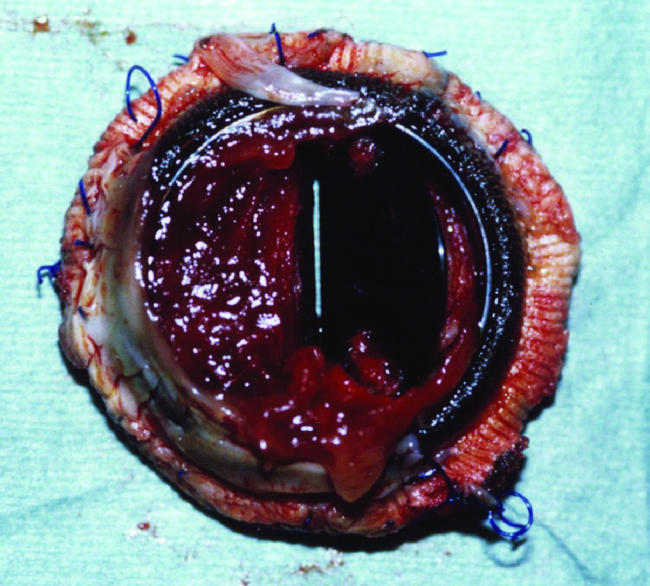
Valve thrombosis of a bileaflet prosthetic mitral valve
Figure.
Types of heart valve prostheses
Figure.
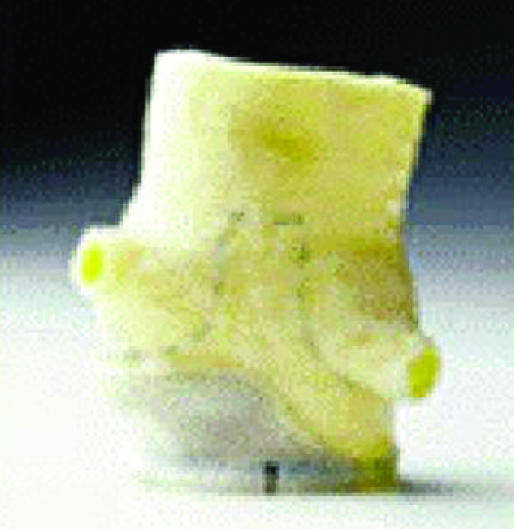
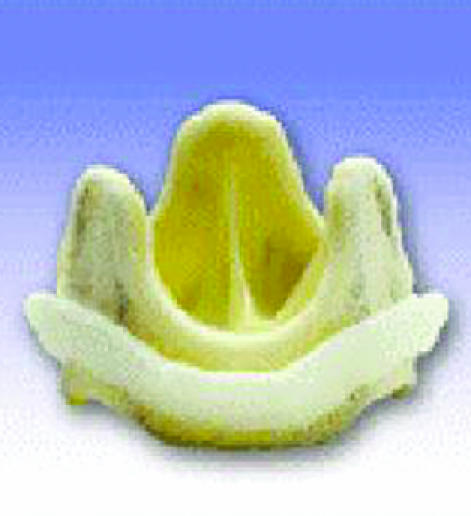
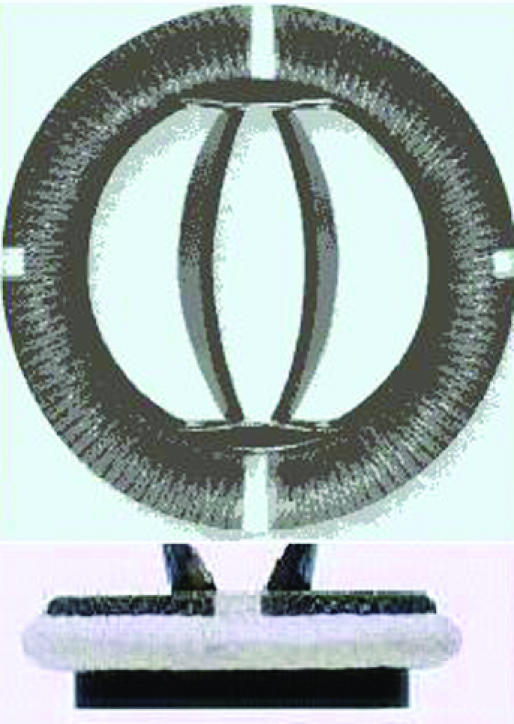
Examples of biological and mechanical valve protheses: (left) stentless porcine valve, (middle) stented porcine valve, (right) Sorin Bicarbon valve
Footnotes
Alexander G G Turpie is professor of medicine, McMaster University, Hamilton Health Sciences Corporation, Hamilton, Canada; Ira Goldsmith is research fellow in cardiothoracic surgery and Gregory Y H Lip is professor of cardiovascular medicine at the haemostasis thrombosis and vascular biology unit, university department of medicine, City Hospital, Birmingham.
The ABC of antithrombotic therapy is edited by Gregory Y H Lip and Andrew D Blann, senior lecturer in medicine, haemostasis thrombosis and vascular biology unit, university department of medicine, City Hospital, Birmingham. The series will be published as a book in spring 2003.