Abstract
Disentangling the factors influencing the climate sensitivity of trees is crucial to understanding the susceptibility of forests to climate change. Reducing tree-to-tree competition and mixing tree species are two strategies often promoted to reduce the drought sensitivity of trees, but it is unclear how effective these measures are in different ecosystems. Here, we studied the growth and physiological responses to climate and severe droughts of silver fir and Douglas-fir growing in pure and mixed conditions at three sites in Switzerland. We used tree-ring width data and carbon (δ13C), oxygen (δ18O) and hydrogen (δ2H) stable isotope ratios from tree-ring cellulose to gain novel information on water relations and the physiology of trees in response to drought and how tree species mixture and competition modulate these responses. We found significant differences in isotope ratios between trees growing in pure and mixed conditions for the two species, although these differences varied between sites, e.g. trees growing in mixed conditions had higher δ13C values and tree-ring width than trees growing in pure conditions for two of the sites. For both species, differences between trees in pure and mixed conditions regarding their sensitivity to temperature, precipitation, climatic water balance and vapor pressure deficit were minor. Furthermore, trees growing in pure and mixed conditions showed similar responses of tree-ring width and isotope ratios to the past severe droughts of 2003, 2015 and 2018. Competition had only a significantly negative effect on δ13C of silver fir, which may suggest a decrease in photosynthesis due to higher competition for light and nutrients. Our study highlights that tree species mixture may have only moderate effects on the radial growth and physiological responses of silver fir and Douglas-fir to climatic conditions and that site condition effects may dominate over mixture effects.
Keywords: Abies alba, carbon isotope, drought, hydrogen isotope, oxygen isotope, Pseudotsuga menziesii
Introduction
Given the rapid changes in climate conditions and their impacts on forest ecosystems worldwide (Allen et al. 2015), it is a priority to better understand the growth and physiological responses of trees to climate change. However, these responses in uncontrolled environments are complex due to many interacting effects. For example, it is still unclear how tree species diversity and competition modulate the climate sensitivity of trees and if their modifications via forest management can reduce the drought susceptibility of trees.
Tree-ring width (TRW) is a valuable and widely used proxy to investigate tree responses to climate change, allowing the quantification of yearly stem radial growth over time (Fritts 1976). In addition, carbon (δ13C), oxygen (δ18O) and hydrogen (δ2H) stable isotope ratios in tree-ring cellulose are suitable indicators for inferring the physiological responses of trees to past biotic and abiotic effects (Siegwolf et al. 2022). These stable isotope ratios can provide information on water-use efficiency and gas exchange strategy of trees (McCarroll and Loader 2004).
The drivers of carbon and oxygen isotope fractionation in plant materials are well known (McCarroll and Loader 2004; Gessler et al. 2014). During water stress (either from competition and/or drought), δ13C and δ18O are expected to increase. While photosynthetic and stomatal conductance rates are reflected in tree-ring δ13C, δ18O mainly provides insights into the source water of the trees and transpiration or stomatal conductance rates at the leaf level (McCarroll and Loader 2004; Song et al. 2022). Although δ2H provides information about the source water, its signal is also modified by biochemical processes like the use of stored carbohydrates (Lehmann et al. 2022). δ18O and δ2H in tree-ring cellulose carry different physiological and climatic signals (Vitali et al. 2022) and their decoupling under stress conditions could be related to increased carbohydrate storage use (Vitali et al. 2023). Analyzing δ18O and δ2H simultaneously can thus potentially provide complementary information on tree responses to climatic stress and how tree species mixture and competition modulate these responses.
So far, studies using stable isotopes in tree rings to analyze mixture effects on the response of trees to climate have focused on δ13C (e.g. Grossiord et al. 2014b; Bonal et al. 2017; Schwarz and Bauhus 2019) and also, though less, on δ18O (e.g. Vannoppen et al. 2020). None of them has, however, included δ2H measurements. Only recently, new analytical methods have made the measurement of δ2H in tree rings more accessible (Lehmann et al. 2022). The combined analysis of TRW, δ13C, δ18O and δ2H can provide complementary climatic and physiological information that is useful to understand the effects of tree species mixture on tree growth and climate sensitivity of trees.
The theory behind the expected positive effects of species diversity on tree growth and climate sensitivity is that trees growing in multispecific stands can benefit from reduced competition compared with trees growing in monospecific stands (competitive reduction; Forrester and Bauhus 2016). This competitive reduction can be explained by different root structures or temporal water use, i.e. through resource partitioning (Forrester and Bauhus 2016). However, this species diversity effect on tree radial growth response to drought is not always observed in natural environments where various external factors can modulate or hinder growth (Grossiord 2019; Haberstroh and Werner 2022).
Tree-ring isotopes can help to understand species diversity and competition effects on tree physiology. For example, Vannoppen et al. (2020) observed for European beech (Fagus sylvatica L.) that species diversity mitigated the drought response based on δ13C data, but the signal from δ18O was not sensitive to species diversity. The authors reported that during drought, the increase in δ13C was lower in beech trees growing in multispecific than monospecific stands, which indicated enhanced stomatal conductance and growth in mixtures. Competition, which we consider here as the interaction of individuals relying on the same resources, may be recorded in the isotope ratios since competition for light or water affects tree physiology, and hence isotope ratios (Marshall et al. 2022). Therefore, δ13C, δ18O and δ2H should be lower in trees growing in mixed conditions, where competitive reduction occurs, than in pure conditions with high intraspecific competition.
In this study, we focus on silver fir (Abies alba Mill.) and non-native Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), two important coniferous tree species promoted in Central Europe as a substitute for Norway spruce (Picea abies (L.) H. Karst.) due to their higher drought tolerance (Lévesque et al. 2014; Vitali et al. 2017; Vitasse et al. 2019). This drought tolerance is favored by the deep rooting system and the isohydric behavior of the two species (McMinn 1963; Bastien 2019; Vitasse et al. 2019). However, with the rapidly changing climatic conditions, the potential of silver fir and Douglas-fir to thrive in a warmer and drier climate is becoming uncertain (Vejpustková and Čihák 2019; Piedallu et al. 2023). Therefore, analyzing how these species responded to past severe droughts and understanding how competition and species diversity modulated this response are highly relevant for developing adaptive forest management strategies.
The main aim of our study was to analyze the growth and physiological responses to climate of silver fir and Douglas-fir in pure and mixed conditions. We asked the following research questions. (i) Are there differences in isotope ratios and TRW of silver fir and Douglas-fir between trees growing in pure and mixed conditions for the period 2000–2020? (ii) What are the responses of δ13C, δ18O, δ2H and TRW of silver fir and Douglas-fir growing in pure and mixed conditions to climate and past severe droughts? (iii) How does the local tree neighborhood (competition, tree species mixture and species diversity) influence the physiological responses and the radial growth response of silver fir and Douglas-fir to vapor pressure deficit?
Materials and methods
Data collection and dendrochronological methods
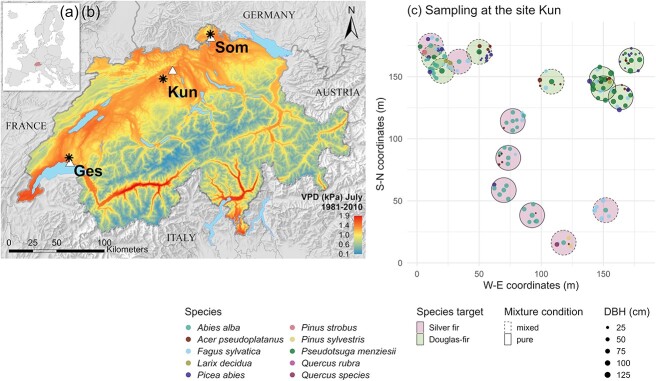
We selected three sites in Switzerland based on the co-occurrence of mature silver firs and Douglas-firs, growing in pure and mixed conditions and with comparable age for a given species at a given site (Fig. 1, Tables 1 and 2). At each site, all the sampled trees irrespective of their group had comparable site conditions (i.e. soil, topography). At the three sites, Douglas-fir trees were planted in small groups or as single individuals in mixtures with other species originating from natural regeneration. Silver fir trees likely originate from natural regeneration and, as for Douglas-fir, grow in pure small groups or in mixtures with other species. In the last decades, all the stands have been managed according to close-to-nature silvicultural practices, consisting of low-intensity thinning interventions and continuous cover forestry. The climate of the three sites is temperate, with an average annual temperature between 9.5 and 9.9°C and an annual precipitation sum between 917 and 1094 mm for the period 2000–2020 (Table 1, Fig. S1 available as Supplementary data at Tree Physiology Online). Three severe summer droughts (i.e. 2003, 2015, and 2018) occurred during this period (Fig. S2 available as Supplementary data at Tree Physiology Online).
Figure 1.
Map of (a) the location of Switzerland (red) within Europe (© EuroGeographics for the administrative boundaries) and of (b) the location of the three study sites in Switzerland. The white triangles indicate the location of the meteorological stations. Colors indicate the average VPD in July over the period 1981–2010, calculated based on the data of air temperature and relative air humidity provided by Huber et al. (2015) and based on MeteoSwiss data. The background map is from the Federal Office of Topography swisstopo. (c) Example of the sampling design of silver fir (pink shaded areas) and Douglas-fir (green shaded areas) at the site Kun (Küngoldingen). Each target tree is represented in the center of the 10 m radius circle and surrounded by its neighbor trees (colored dots, see the legend for the corresponding species). The dashed line and solid line circles represent the classification of the target trees as either in mixed or pure conditions, respectively.
Table 1.
Description of the sites with the climate variables for the period 2000–2020. The full names of the sites are given together with their three-letter abbreviation (used elsewhere in the text and figures). For latitude, longitude, and aspect of the slope, north (N), south (S), west (W) and east (E) refer to the cardinal points. The mean annual temperature, annual precipitation sum and CWB (based on April through September data) are given as mean ± standard deviation.
| Site | Latitude (N) | Longitude (E) | Elevation (m a.s.l.) | Aspect | Slope (°) | Temperature (°C) | Precipitation (mm) | CWB (mm) |
|---|---|---|---|---|---|---|---|---|
| Bois des Gésiaux (Ges) | 46° 33′ 20" | 6° 39′ 12" | 760 | S | 5 | 9.9 ± 6.4 | 1094 ± 219 | 6 ± 27 |
| Küngoldingen (Kun) | 47° 18′ 6" | 7° 56′ 50" | 480 | W | 8 | 9.8 ± 6.7 | 942 ± 144 | −3 ± 20 |
| Sommerwies (Som) | 47° 43′ 6" | 8° 36′ 41" | 550 | NE | 10 | 9.5 ± 6.8 | 917 ± 137 | −8 ± 18 |
Table 2.
Description of the sampled trees. DBH, tree height, estimated age, TRW (tree-ring width of the period 2000–2020), competition (Hegyi index) and intraspecific competition are given with mean ± standard deviation of four trees for each mixture group.
| Species | Site | Mixture | DBH (cm) | Tree height (m) | Est. age (years) | TRW (mm) | Competition (unitless) | Intraspecific competition (%) |
|---|---|---|---|---|---|---|---|---|
| Silver fir | Ges | Pure | 49.1 ± 2.9 | 27.4 ± 2.9 | 38 ± 7 | 7.36 ± 2.36 | 1.5 ± 0.1 | 88 ± 3 |
| Mixed | 50.1 ± 3.8 | 26.0 ± 0.7 | 38 ± 10 | 5.54 ± 2.70 | 1.1 ± 0.4 | 8 ± 5 | ||
| Kun | Pure | 68.7 ± 10.8 | 36.0 ± 3.3 | 103 ± 19 | 2.01 ± 1.23 | 0.8 ± 0.2 | 84 ± 7 | |
| Mixed | 72.3 ± 1.7 | 38.4 ± 2.2 | 92 ± 8 | 4.09 ± 3.07 | 0.6 ± 0.2 | 3 ± 6 | ||
| Som | Pure | 39.4 ± 11.7 | 30.9 ± 3.9 | 46 ± 10 | 4.12 ± 2.02 | 1.8 ± 0.6 | 59 ± 10 | |
| Mixed | 56.7 ± 8.7 | 35.6 ± 1.6 | 66 ± 15 | 5.98 ± 3.32 | 0.8 ± 0.2 | 0 ± 0 | ||
| Douglas-fir | Ges | Pure | 99.1 ± 7.4 | 51.6 ± 1.8 | 103 ± 8 | 2.78 ± 1.04 | 0.8 ± 0.1 | 63 ± 7 |
| Mixed | 109.9 ± 16.2 | 50.4 ± 3.4 | 105 ± 7 | 3.27 ± 2.14 | 0.7 ± 0.2 | 7 ± 7 | ||
| Kun | Pure | 81.4 ± 12.6 | 50.7 ± 3.3 | 102 ± 9 | 3.56 ± 1.86 | 1.0 ± 0.2 | 55 ± 2 | |
| Mixed | 90.3 ± 11.7 | 48.8 ± 2.6 | 96 ± 7 | 4.69 ± 2.30 | 0.6 ± 0.2 | 0 ± 0 | ||
| Som | Pure | 71.6 ± 8.6 | 48.0 ± 3.4 | 88 ± 15 | 3.45 ± 1.21 | 0.7 ± 0.2 | 82 ± 7 | |
| Mixed | 84.9 ± 16.8 | 43.7 ± 4.8 | 88 ± 19 | 4.06 ± 1.53 | 0.5 ± 0.1 | 19 ± 17 |
From the 20 silver fir and 20 Douglas-fir trees sampled at each study site in Charlet de Sauvage et al. (2023), we selected eight silver firs and eight Douglas-firs, all healthy and dominant/co-dominant (hereafter target trees) for isotope analysis. The trees were sampled between the end of August 2020 and May 2021. Trees were selected according to their neighborhood considering the level of tree species mixture. We selected the four individuals growing in the purest conditions (i.e. surrounded by their conspecifics) and the four individuals growing in the most mixed conditions (i.e. surrounded by trees from other species) for each species and at each site (see an example of sampling design in Fig. 1c and for all sites in the Supplementary data). Trees were carefully selected to be of similar age within a site and species to ensure comparability.
Two increment cores were sampled per tree at ca 50 cm from the ground and perpendicularly to the slope, with a 40- or 60-cm long increment borer (5.15 mm core diameter; Haglöf, Sweden). Tree cores were air dried, mounted on wooden holders, and sanded. Tree-ring widths were measured to the nearest 0.01 mm with either CooRecorder (v9.6, Cybis, Sweden) on scanned images or with TSAP-Win (v4.81) on a Lintab five measuring table (both from RINNTECH, Heidelberg, Germany). Both methods provided accurate and comparable measurements. Crossdating of TRWs was first performed visually and then confirmed statistically using COFECHA (Holmes 1983). We focused our analyses on the period 2000–2020 to include the dry years 2003, 2015 and 2018 and to study the most recent two decades with reliable neighborhood information since we measured the neighborhood in 2020 and used a static competition index (see Neighborhood Data). The diameter of the target trees (hereafter diameter) was reconstructed for the period 2000–2020 based on the diameter at coring height measured in the field and the TRWs. The age of the trees was estimated with the number of tree rings on the longest increment core for each tree plus the missing rings to the pith estimated with the software CooRecorder 9.6 (Cybis, Sweden).
Sample preparation for isotope analysis
For each tree, we selected the core with the best quality (i.e. not broken, no missing rings, correctly crossdated) and with the highest correlation with the site chronology for isotope analysis. The tree cores, glued on wooden holders with wood glue (base of polyvinyl acetate, Geistlich, Switzerland), were soaked in hot water and detached from their support. The remaining glue was manually scraped off with a scalpel blade under a stereomicroscope. A simple test processing dried glue separately through the cellulose extraction steps revealed that the glue dissolved and was washed out during the chemical extraction so any glue residues on the wood samples would be removed.
Individual tree rings were split with a scalpel under a stereomicroscope into thin slices to facilitate cellulose extraction. We analyzed whole rings because Douglas-fir and silver fir have a gradual early- to latewood transition that makes the boundary between early- and latewood arbitrary. Additionally, the use of stored carbohydrates from previous years is of relatively minor importance for earlywood formation in conifers (Monson et al. 2018). Furthermore, the presence of narrow rings justified the use of the whole rings to ensure enough material for the isotope measurements. All tree rings were processed individually for the 48 trees and for each year for the period 2000–2020, leading to a total of 1008 samples.
The wood samples were packed into fiber filter bags (F57, Ankom Technology, USA), and the holocellulose (hereafter cellulose) was extracted following Boettger et al. (2007), modified according to Weigt et al. (2015). The cellulose extracted yielded 51.6 ± 4.1% and 54.5 ± 3.1% (mean ± standard deviation) of the original wood mass of the tree rings for silver fir and Douglas-fir, respectively. Cellulose samples were homogenized with an ultrasonic device (UP200S, Hielscher Ultrasonics, Germany) in ca 1 mL of distilled water, following Laumer et al. (2009). Samples were freeze-dried for up to 2 days (Beta 1–8 LD plus, Christ, Germany) to remove the water left from homogenization. Then, 1 ± 0.05 mg of cellulose was packed into silver capsules (3.3 × 5 mm, Säntis Analytical, Switzerland). To determine the hydrogen isotope ratios of carbon-bound hydrogen in the cellulose samples, the samples were equilibrated following procedures and calculations in Schuler et al. (2022). Finally, the samples were converted to H2 and CO by thermal decomposition at 1420 °C with a TC/EA (Pyrocube, Elementar, Hanau, Germany), and all isotope ratios were measured with an isotope-ratio mass spectrometer (MAT 253, Thermo, Germany). The precision of the analysis was 0.2‰ for δ13C and δ18O, and 1.5‰ for δ2H. Values of δ13C were corrected for changes in atmospheric δ13C (Belmecheri and Lavergne 2020) but not for changes in atmospheric CO2 concentration because this correction is too subjective (McCarroll et al. 2009; Treydte et al. 2009). This should not have biased our results because our study period was common to all the studied trees and covered only 21 years.
Neighborhood data
Within a 10-m radius of each target tree, all the trees with a diameter at breast height (DBH) above 10 cm (hereafter neighbor trees) were measured. The DBH, species and distance to the target tree were recorded for each neighbor tree. The neighbor trees included, in descending order of abundance, silver fir, Norway spruce, Douglas-fir, European beech, sycamore maple (Acer pseudoplatanaus L.) and other less occurrent species (see Fig. 1c, Table S1 available as Supplementary data at Tree Physiology Online, and Supplementary data showing the maps of all the sampled trees). The distances between the target and neighbor trees were measured horizontally, from tree center to tree center, with a Vertex IV measuring device (Haglöf, Sweden).
To quantify the competition around each target tree, we calculated a distance-dependent competition index according to Hegyi (1974):
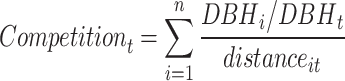 |
(1) |
with t referring to the target tree and i to the neighbor trees and their respective DBH. n is the number of neighbors for a given target tree. The distanceit refers to the distance between the target tree and a given neighbor.
To evaluate the effects of intra- and inter-specific competition and quantify the degree of mixture, we calculated the percentage of intraspecific competition:
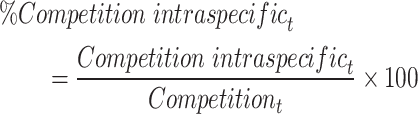 |
(2) |
with t referring to the target tree. Competition intraspecifict is the competition calculated according to Eq. (1) including only the neighbor trees of the same species as the target tree. Competitiont refers to the total competition of a target tree calculated according to the Eq. (1). Trees were classified as either in mixed or pure conditions (categorical variable) based on the percentage of intraspecific competition calculated with Eq. (2), if the value was below or above 50%, respectively.
To estimate the tree species diversity in the neighborhood of each target tree, we calculated the Shannon index (Shannon 1948) using the function diversity from the R package vegan (v2.6-2; Oksanen et al. 2022):
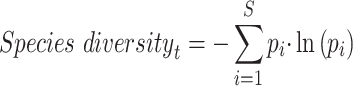 |
(3) |
with 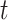 referring to a given target tree,
referring to a given target tree, 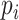 the proportional abundance of species i and S the number of species within the neighborhood of the target tree.
the proportional abundance of species i and S the number of species within the neighborhood of the target tree.
We focused the analyses on the period 2000–2020 because we measured the neighborhood in 2020–2021 and decided to consider a static neighborhood and competition instead of attempting to reconstruct the neighborhood back in time based on the TRW of the target trees. For easier reference, the variables used to characterize the neighborhood of the target trees are summarized in Table 3.
Table 3.
Variables used to characterize the neighborhood of the target trees.
| Variable name | Reference | Definition | Variable type (with unit) |
|---|---|---|---|
| Competition | Eq. (1) | Quantifies the total amount of competition around a target tree independently of the species. | Continuous (unitless) |
| Percentage of intraspecific competition | Eq. (2) | Portion of competition coming from intraspecific trees around the target tree. It is a percentage, thus the value does not reflect the absolute quantity of competition. | Continuous (%) |
| Mixed/pure | Based on the percentage of intraspecific competition | It is based on the percentage of intraspecific competition: mixed ≤ 50%; pure > 50%. | Categorical |
| Species diversity | Eq. (3) | It reflects the diversity of species around a target tree, including species richness and evenness. | Continuous (unitless) |
Climate data
Hourly average temperature, precipitation sum and relative humidity for the period 1999–2020 were retrieved from MeteoSwiss meteorological stations located near the study sites, which allowed us to calculate the daily minimum and maximum values. The stations were located 3 to 13 km from the study sites (Fig. 1, Table S2 available as Supplementary data at Tree Physiology Online). Temperature values were corrected for the differences in elevation between the sites and the meteorological stations with published monthly temperature lapse rates from Lotter et al. (2002).
Monthly vapor pressure deficit (VPD; kPa) was calculated with the monthly average of daily minimum and maximum temperature and relative humidity values (Eq. S1 available as Supplementary data at Tree Physiology Online). To answer research question (iii), we averaged the VPD for each year over the period April to September to include the entire growing period (Etzold et al. 2022; see also Response to climate and severe drought of trees in mixed and pure conditions). We chose to focus on VPD and not on other climatic variables in research question (iii) because VPD indicates the actual evaporative capacity of the atmosphere (Allen et al. 1998) and directly influences stomatal conductance (Grossiord et al. 2020), and thus isotopic variations in tree rings (McCarroll and Loader 2004).
We also estimated the climatic water balance (CWB; mm), which corresponds to precipitation minus potential evapotranspiration. The potential evapotranspiration was calculated from monthly average temperatures and the latitude of the sites following Thornthwaite (1948) with the R package SPEI (v1.8.1; Beguería and Vicente-Serrano 2023).
Statistical analyses
To test the potential differences in isotope ratios and TRWs between pure and mixed trees (research question i), we performed Wilcoxon tests for each species, site and tree-ring variable (δ13C, δ18O, δ2H and TRW).
To evaluate the climate sensitivity of the species (research question ii), correlation analysis was performed between the tree-ring chronologies and seasonal climatic variables (VPD, temperature, precipitation sum and CWB) for the period 2000–2020. Ring-width indices (RWI) were obtained by applying a detrending with a cubic smoothing spline with a rigidity of 16 years on individual TRW series using the R package dplR (v1.7.4; Bunn et al. 2022). δ13C, δ18O and δ2H chronologies were calculated by averaging the yearly values per species, site and group. Seasonal climatic variables included 3-month averages, from June of the year before tree-ring formation to August of the year of tree-ring formation. Bootstrapped Pearson’s correlations were calculated with the function dcc from the R package treeclim (v2.0.6.0, Zang and Biondi 2015).
To assess the response of trees to drought (research question ii) and the potential differences in drought response between trees in pure and mixed conditions, we conducted superposed epoch analysis (SEA) based on the three driest years of the study period, i.e. 2003, 2015 and 2018 (Fig. S1 available as Supplementary data at Tree Physiology Online). SEA allowed us to evaluate the departure of tree-ring variables from mean values during superposed drought events. We ran the SEA with detrended and standardized chronologies of δ13C, δ18O, δ2H and RWI, with one chronology per species, site and mixture conditions. For this purpose, we applied the same spline detrending to the isotope series as for TRW. We restricted the analysis to 2 years prior to and after the drought to include the dry year 2018, which had only 2 years of post-drought data. The SEA was performed with a resampling of 1000 bootstrap samples to calculate the significance of the departure from the mean during and prior/after the drought year (P ≤ 0.05), using the function sea from the R package dplR (v1.7.4; Bunn et al. 2022).
To analyze the effects of competition, species diversity, tree species mixture, and their interaction with VPD (research question iii), we used linear mixed-effects models (one model per species and per tree-ring variable) using the function lme from the R package nlme (v3.1-157; Pinheiro et al. 2022):
 |
(4) |
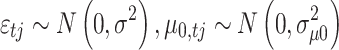 |
where 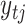 is the response variable (δ13C, δ18O, δ2H or TRW) of a target tree t in year j, and the β are the coefficients. We log-transformed the response variable TRW and used non-detrended TRW series to retain the individual variability inherent to each tree. The years j included 2000 to 2020. Diameter refers to the reconstructed diameter of tree t in year j and is included in the model to account for tree size effect. VPDApr.–Sep. is the average VPD from April to September. In the results, we show fitted values for VPD values of 0.6, 0.7 and 0.8 kPa, which were in the range of our data (see Fig. S1c available as Supplementary data at Tree Physiology Online). Species diversity, competition and % competition intraspecific are described in Table 3. The normality of the errors ε and random effect μ0 (only calculated for the intercept) were assessed with visual plots. We did not include random slopes in the models because they prevented the models from converging. We tested for autocorrelation in our response variables and included an autocorrelation parameter of order 1 to account for temporal autocorrelation only for the model explaining TRW. We used tree identity as a grouping factor, nested in sites, for the random effect and the autocorrelation parameter. All predictor variables were scaled by subtracting their mean and dividing them by their standard deviation to allow comparison of effect sizes of the predictors.
is the response variable (δ13C, δ18O, δ2H or TRW) of a target tree t in year j, and the β are the coefficients. We log-transformed the response variable TRW and used non-detrended TRW series to retain the individual variability inherent to each tree. The years j included 2000 to 2020. Diameter refers to the reconstructed diameter of tree t in year j and is included in the model to account for tree size effect. VPDApr.–Sep. is the average VPD from April to September. In the results, we show fitted values for VPD values of 0.6, 0.7 and 0.8 kPa, which were in the range of our data (see Fig. S1c available as Supplementary data at Tree Physiology Online). Species diversity, competition and % competition intraspecific are described in Table 3. The normality of the errors ε and random effect μ0 (only calculated for the intercept) were assessed with visual plots. We did not include random slopes in the models because they prevented the models from converging. We tested for autocorrelation in our response variables and included an autocorrelation parameter of order 1 to account for temporal autocorrelation only for the model explaining TRW. We used tree identity as a grouping factor, nested in sites, for the random effect and the autocorrelation parameter. All predictor variables were scaled by subtracting their mean and dividing them by their standard deviation to allow comparison of effect sizes of the predictors.
Statistical analyses were performed with the software R (v4.2.1; R Core Team 2022), and all figures were plotted with ggplot2 (v3.3.6, Wickham 2016), except for the map in Fig. 1 (ArcGIS Desktop v10.8).
Results
Differences in δ13C, δ18O, δ2H and TRW between trees in pure and mixed conditions
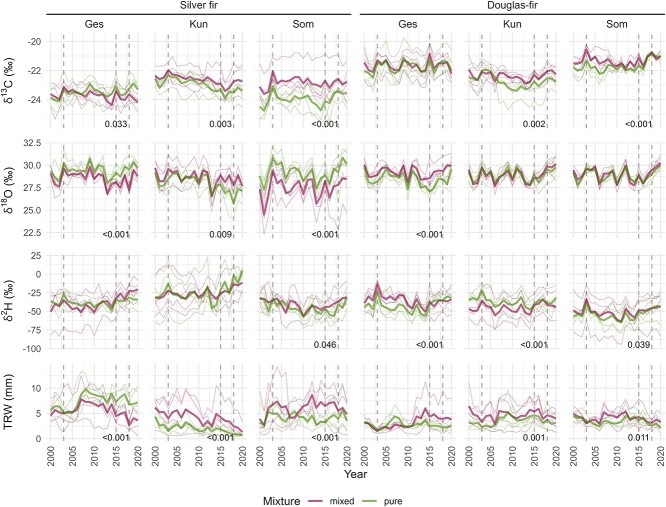
Silver fir and Douglas-fir trees growing in mixed conditions had significantly higher δ13C values than those in pure conditions at the sites Kun and Som (Fig. 2). In contrast, silver fir trees in pure conditions had significantly higher δ13C values than trees in mixed conditions at the site Ges, and there was no difference for Douglas-fir. δ18O in silver fir was significantly higher for trees growing in pure conditions than for trees in mixed conditions at the sites Ges and Som (Fig. 2). On the contrary, silver firs and Douglas-firs showed significantly higher δ18O values in mixed than in pure conditions at the sites Kun and Ges, respectively. δ2H values in silver fir were similar between pure and mixed conditions at the site Ges and Kun, whereas silver fir in pure conditions had lower δ2H values at the site Som (Fig. 2). For Douglas-fir, trees in mixed conditions had significantly higher δ2H values than those in pure conditions at the sites Ges and Som but lower values at the site Kun. Tree-ring width values were significantly higher for trees in mixed compared with pure conditions for both species at the sites Kun and Som (Fig. 2). At the site Ges, the TRW values of silver fir were lower in mixed than in pure conditions and not significantly different for Douglas-fir. At all sites and for both species, we observed the same significant differences for δ13C and TRW between trees in pure and mixed conditions. When δ13C values were higher in pure than in mixed conditions, the same was observed for TRW.
Figure 2.
Time series of δ13C, δ18O, δ2H and TRW of silver fir and Douglas-fir growing in pure and mixed conditions at the three study sites (Ges: Bois des Gésiaux, Kun: Küngoldingen, Som: Sommerwies). Thick lines represent the average of four trees and thin lines the individual series of each tree. Significant differences (Wilcoxon test) between mixed and pure trees are indicated by P-values (≤0.05) at the bottom right corner of each subplot. For all P-values, see Table S3 available as Supplementary data at Tree Physiology Online. δ13C values were corrected for changes in atmospheric δ13C. Vertical dotted lines highlight the drought years 2003, 2015 and 2018 (see also Fig. S1 available as Supplementary data at Tree Physiology Online).
Response to climate and severe drought of trees in mixed and pure conditions
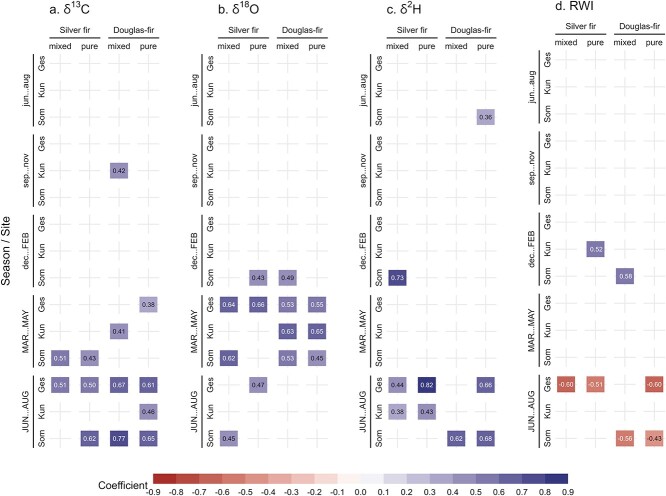
We found no clear differences in the sensitivity of all four tree-ring variables to temperature, precipitation, CWB, and VPD between trees growing in pure and mixed conditions (Fig. 3 and Supplementary Figs S3–S5 available as Supplementary data at Tree Physiology Online). Additionally, for both silver fir and Douglas-fir in pure and mixed conditions, the strength of the correlations varied among sites (the trees at the site Kun were the least climate-sensitive). Positive correlations occurred between δ13C and VPD in spring and particularly summer of the current year (Fig. 3a). δ18O correlated positively with VPD in spring, with Douglas-fir in pure and mixed conditions having significant correlations at all sites (Fig. 3b). δ2H correlated positively in summer, with correlation coefficients up to 0.82 for silver fir in pure conditions (Fig. 3c). RWI correlated negatively with VPD in summer of the current year (Fig. 3d).
Figure 3.
Bootstrapped Pearson’s correlation coefficients between mean seasonal VPD and tree-ring chronologies of (a) δ13C, (b) δ18O, (c) δ2H and (d) RWI for the period 2000–2020 at the three study sites (Ges, Kun, Som). Months in capital letters indicate months of the year of tree-ring formation. See Table 1 and Fig. 1 for the full names of the sites and their location. The colored cells with the values indicate significant correlations (P-value ≤ 0.05).
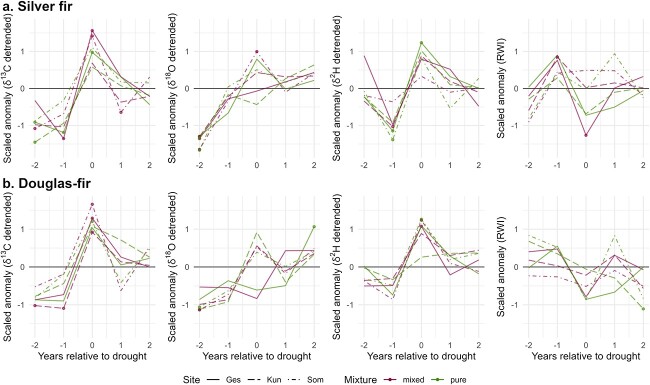
For each tree-ring variable, the SEA of the drought years 2003, 2015, and 2018 revealed no clear differences between trees in pure and mixed conditions for silver fir and Douglas-fir (Fig. 4). During the drought years, the isotope ratios were generally high, particularly for δ13C and δ2H, and the RWI values were low. We observed stronger significant deviations from the mean for δ13C and δ2H compared with δ18O and RWI (Fig. 4). Most tree-ring variables were close to average values in the year following the severe droughts, although isotope ratios, particularly δ18O, remained slightly higher in the following 2 years after the drought events. In addition, RWI of Douglas-fir remained low after 2 years at some sites.
Figure 4.
Superposed epoch analysis (SEA) for detrended δ13C, δ18O and δ2H and RWI of (a) silver fir and (b) Douglas-fir considering the drought years 2003, 2015 and 2018 (see Figs S1 and S2 available as Supplementary data at Tree Physiology Online). Significant departures (P ≤ 0.05) from 1000 random simulations are represented with filled circles.
Competition and species diversity effects on δ13C, δ18O, δ2H and TRW
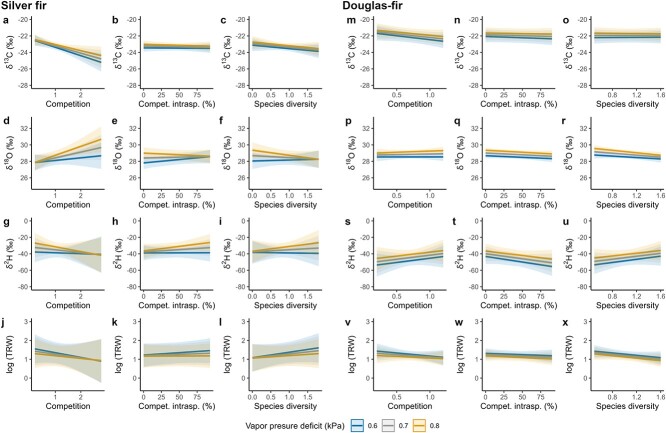
Competition significantly and negatively influenced δ13C values of silver fir (Fig. 5a and Table S4 available as Supplementary data at Tree Physiology Online). We also observed some significant interactions between VPD and competition, between VPD and the percentage of intraspecific competition, or between VPD and species diversity for silver fir (Table S4 available as Supplementary data at Tree Physiology Online). For example, under dry climatic conditions (high VPD values) and high competition, δ18O values increased (Fig. 5d). Also, δ18O decreased under higher species diversity and drier conditions (Fig. 5f). δ2H of silver fir increased during drier conditions and for trees with rather pure neighborhoods (higher intraspecific competition; Fig. 5h). Apart from the main effect of competition on δ13C of silver fir, none of the main effects representing the neighborhood of the target trees (i.e. competition, percentage of intraspecific competition and species diversity) significantly influenced the tree-ring variables. For Douglas-fir, we found no significant main effect of competition, percentage of intraspecific competition, and species diversity nor significant interaction with VPD on the tree-ring variables (Fig. 5 and Table S4 available as Supplementary data at Tree Physiology Online). We only found a significant main effect of VPD on all tree-ring variables of Douglas-fir, also observed for silver fir.
Figure 5.
Effects of competition, percentage of intraspecific competition, and species diversity on δ13C, δ18O, δ2H and TRW of silver fir (a–l) and Douglas-fir (m–x) for the period 2000–2020. One model was used per response variable (i.e. δ13C, δ18O, δ2H and TRW) and per species following Eq. (4). The statistical outputs are shown in Table S4 available as Supplementary data at Tree Physiology Online. Vapor pressure deficit was included in the model as the average per year and per site for the period April to September. The fitted values are represented by the solid lines with 95% confidence intervals for moist (VPD = 0.6 kPa), intermediate (VPD = 0.7 kPa) and dry climatic conditions (VPD = 0.8 kPa).
Discussion
Differences in δ13C, δ18O, δ2H and TRW between trees in pure and mixed conditions
We found some significant differences in isotope ratios and TRW between trees growing in pure and mixed conditions, but the results were inconsistent across sites. At two out of the three studied sites (Kun and Som), silver fir and Douglas-fir trees growing in mixed conditions had higher δ13C values and TRW than trees growing in pure conditions, indicating higher photosynthetic rate and radial growth of the trees growing in mixed conditions. The higher TRW of the trees growing in mixed conditions could, however, also be due to the lower competition experienced by these trees in our study (Table 2).
Silver fir δ18O values in pure conditions were significantly higher than in mixed conditions at two of the three sites (Ges and Som). Assuming that δ18O in tree-ring cellulose is driven by the source water isotope signal modulated by leaf and soil water evaporative enrichment (Treydte et al. 2023), this suggests that silver fir in pure conditions likely relies more on a shallower soil water pool enriched in 18O (Treydte et al. 2014) than in mixed conditions. On the contrary, silver fir in mixed conditions would rely on a deeper soil water pool, which should, therefore, be an advantage during drought, as suggested by Gazol and Camarero (2016) and Lebourgeois et al. (2013). However, the tree-ring isotope signal is also influenced by leaf-level processes and by further re-exchange of oxygen atoms on the pathway from photosynthesis to wood formation, affecting the final δ18O signal in tree-ring cellulose (Gessler et al. 2013; Martínez-Sancho et al. 2023). Therefore, also differences in transpiration between trees growing in pure and mixed conditions could modify the δ18O values.
At first sight, the higher δ2H values of Douglas-fir trees in mixed conditions observed at two sites (Ges and Som) may indicate a preferential use of shallower soil water pools compared with trees in pure conditions since soil surface water tends to be more enriched in 2H in comparison to deeper soil horizons (Dawson et al. 2002). However, the opposite was observed at the third site (Kun). Mechanisms driving 2H fractionation until it is fixed in the tree rings are not yet fully understood (Lehmann et al. 2022), but there is clear evidence that tree-ring δ2H carries a strong physiological and biochemical signal besides source water signatures (Augusti et al. 2006; Lehmann et al. 2021; Vitali et al. 2022, 2023; Wieloch et al. 2022). Thus, higher δ2H values could indicate that trees in mixed conditions at the sites Ges and Som rely more on the use of 2H-enriched stored carbohydrates, while trees in pure conditions use more fresh 2H-depleted assimilates (Lehmann et al. 2021, 2022). Overall, the isotope ratios give some insights in photosynthetic rates (δ13C), source water uptake and rooting depth (δ18O), and use of stored carbohydrates (δ2H), although the differences between trees growing in mixed or pure conditions are not very clear.
Response to climate and severe drought of trees in mixed and pure conditions
Previous studies suggested that the effects of tree species mixture on the drought response of trees range from negative to positive or even are absent depending on the drought severity (e.g. Haberstroh and Werner 2022). It is thus relevant to compare the climate sensitivity and response to severe drought of trees growing in pure and mixed conditions. Overall, we observed similar responses to VPD, temperature, precipitation and CWB between trees growing in pure and mixed conditions. The sensitivity of silver fir and Douglas-fir to interannual climate variability varied more among sites than between mixture conditions. Although we observed differences in isotope ratios between pure and mixed groups, as discussed in Differences in δ13C, δ18O, δ2H and TRW between trees in pure and mixed conditions, it does not necessarily imply that their sensitivity to interannual climate variability is also different. However, some studies, although based on TRW only and not stable isotopes, found that Douglas-fir in mixed conditions was less sensitive to climate than in pure conditions (Thurm et al. 2016). Similar results were observed for silver fir under extreme drought in Spain (Gazol and Camarero 2016).
Trees growing in mixed conditions could indeed be expected to be less affected by drought than trees growing in pure conditions due to enhanced resource-use efficiency and complementarity among species (Gazol and Camarero 2016; Thurm et al. 2016). This concept is based on the idea that species with contrasting functional traits may use resources differently in space and time. This is especially relevant when resources are limited, such as water during drought. For example, tree species with different root architecture can access water from different soil depths (Forrester and Bauhus 2016). In an experiment in Germany, Grossiord et al (2014a) analyzed the water uptake depth of young trees by spraying water labeled with deuterium on the soil surface during a dry summer. Young European beech, sessile oak (Quercus petraea (Matt.) Liebl.), Douglas-fir and Norway spruce were growing together, and the authors found that Douglas-fir trees did not show differences in the depth of soil water extraction in relation to the percentage of conifers in their neighborhood. This study found some differences only for European beech, with individuals growing within a higher percentage of conifers having a higher soil water extraction depth. Here, our two investigated species have taproots (McMinn 1963; Magh et al. 2020), allowing them to access water pools from deep soil horizons and rely less on surface water during drought. Although the differences in δ18O or δ2H time series between trees in pure and mixed conditions suggested some differences in the depth of main soil water access at some sites (Differences in δ13C, δ18O, δ2H and TRW between trees in pure and mixed conditions, Fig. 2), we did not observe a clear difference in the δ18O and δ2H responses to the severe 2003, 2015 and 2018 droughts between trees in pure and mixed conditions. Therefore, it is possible that under severe droughts, our investigated trees used a variety of soil water sources irrespective of their neighborhood conditions.
Although we did not find clear differences in climate sensitivity between trees in mixed and pure conditions, our results provide general insights into climate factors and seasonality driving the different tree-ring variables. Irrespective of the mixture conditions and tree species, we found that δ18O was significantly influenced by spring VPD or precipitation, whereas δ2H was significantly influenced by summer VPD or temperature. These seasonal differences support the often reported mismatch between δ18O and δ2H signals in tree rings (Lehmann et al. 2022) and emphasize the complementarity of δ18O and δ2H for inferring the climate response of trees. In addition, the drought signal in δ13C and δ2H, which may reflect a physiological response, was more pronounced than in δ18O and RWI (Fig. 4). The investigated severe droughts occurred in summer (see Fig. S2 available as Supplementary data at Tree Physiology Online) and we saw earlier that δ13C and δ2H were correlated to summer climatic conditions while δ18O was correlated to spring conditions (Fig. 3). This could explain why δ13C and δ2H showed a higher sensitivity to the summer droughts of 2003, 2015 and 2018 than δ18O and RWI in the SEA. Hartl-Meier et al. (2015) also observed a stronger summer drought signal in δ13C than in δ18O or TRW of Norway spruce, European beech and European larch (Larix decidua Mill.).
The results of the SEA further showed that silver fir and Douglas-fir mostly reached average values of δ13C, δ18O, δ2H and RWI in the year following the severe drought events irrespective of mixture conditions. This indicates that the physiology and growth of both species can overall recuperate after drought, as previously observed in comparable climatic regions by Lévesque et al. (2014) for Douglas-fir and Vitali et al. (2017) for silver fir and Douglas-fir. However, we still observed at some sites that 1 or 2 years after the drought some isotope values (and RWI) were still high (respectively low), indicating some lag effects from droughts.
Competition and species diversity effects on δ13C, δ18O, δ2H and TRW
As opposed to research questions (i) and (ii), where we considered the mixture as a categorical variable (i.e. pure or mixed conditions), we considered tree species mixture as a continuous variable to answer research question (iii), by including the percentage of intraspecific competition and species diversity, and further included the competition effect in our linear mixed-effects models. For silver fir, we observed a significant negative effect of competition on δ13C (Fig. 5a). In general, δ13C increases (enrichment) when a tree experiences stressful conditions, as it is the case during drought and associated stomatal closure (Gagen et al. 2022). Following competition for resources, we would have then expected an increase in δ13C when neighborhood competition increases, but we found an opposite response. Although such a result seems counterintuitive at first sight, this response has also been reported for European beech (Mölder et al. 2011) and in a meta-analysis on the thinning effect on carbon isotope discrimination (Marshall et al. 2022). Tree-ring δ13C reflects changes in photosynthetic assimilation and stomatal conductance rates or water-use efficiency (McCarroll and Loader 2004). In our study, the observed decrease in δ13C under higher competition could be due to decreased photosynthetic assimilation rates because of competition for light and nutrients. Alternatively, the denser canopy cover likely associated with higher competition could limit evapotranspiration from the individual trees and mitigate their drought stress. Additionally, we observed a significant and positive interaction between competition and VPD on δ13C (Fig. 5a), which supports the results from Marshall et al. (2022), who found that the response of δ13C to lowered competition through thinning varied depending on the precipitation regime. In our case, the decrease in δ13C with increasing competition was stronger under moister climatic conditions (low VPD).
Regarding the effects of species diversity and mixture, we found several significant interactions between VPD and species diversity or the percentage of intraspecific competition for silver fir. From these interactions, it seemed that tree species mixture influenced the responses of δ18O, δ2H and TRW to VPD. However, only the interactions were significant (i.e. not the main effect), and the effects were small. For Douglas-fir, we found no significant effect of competition, species diversity or the percentage of intraspecific competition on tree-ring variables, indicating that the neighborhood of the trees did not influence their tree-ring isotope ratios nor TRW. Similar to the effects of tree species mixture on TRW (Grossiord 2019), the literature reports variable effects on isotope ratios. For example, Schwarz and Bauhus (2019) found no mixture effect on δ13C and drought resilience indices of silver fir and European beech at three sites in Germany and one site in Croatia. Vannoppen et al. (2020) observed a mixture effect on δ13C but not on δ18O in European beech in two temperate forests in Belgium. For Douglas-fir, the results from the literature are also variable. Thurm et al. (2016) found a reduced climate sensitivity for Douglas-fir in mixed stands, while Vitali et al. (2018) observed no effect of mixture on the radial growth of Douglas-fir during normal years but an increase in drought stress during dry years.
Limitations of the study
We focused our analysis on the last 21 years of data (2000–2020) to control for important changes in stand conditions through time that could act as confounding factors. During the study period, the stand conditions (tree density) did not change much through management or natural mortality, as indicated by the visual assessment of the stumps during sampling. However, the stand conditions have likely changed throughout the ontogeny of the target trees, especially for older trees, and this might have influenced, for example, the root development and soil water uptake source of the trees over time. Information about past forest management and natural mortality at the time of tree establishment that may have influenced the stand dynamics around the target trees could have been helpful but was unavailable.
We sampled trees of different age classes across sites and species. For example, at the sites Ges and Som, silver fir and Douglas-fir trees did not have the same age. Although these age differences imply that the two species have established at different times within a site, the silver firs and Douglas-firs growing in mixed conditions did not necessarily grow together. Any other species could surround them (see details of the sampling in the Supplementary data). Since we did not directly compare the two species within a site but rather the groups of trees growing in pure and mixed conditions within a species, the age differences did not cause bias in our comparison.
Conclusions
We used a triple-isotope approach and TRW measurements to better understand the growth and physiological responses of silver fir and Douglas-fir to climate and drought and how tree species mixture and competition modulate these responses. We found that the effects of tree species mixture and competition on tree-ring variables varied between species and among sites. Our results highlight that tree species mixture had only a weak or no effect on climate and drought sensitivity of Douglas-fir and silver fir, possibly because other confounding and uncontrolled factors (e.g. microsite conditions, belowground competition, species identity) can interplay and mask such mixture effects. To evaluate the effects of mixing different tree species, it might be insightful to look at specific species combinations to precisely test the expected benefits of mixture types on drought sensitivity. From a physiological point of view and at the individual tree level, we found few differences between trees growing in pure and mixed conditions. However, studying these effects at the population level might result in different response patterns of mixed and pure stands to drought. Finally, although our study did not find a lower drought sensitivity of trees in mixed conditions, promoting diverse tree species and favoring mixed forests are still efficient silvicultural options to lower the risk of pest attacks (Brockerhoff et al. 2017), promote the conservation of forest biodiversity (Cavard et al. 2011) and sustainably provide a wide range of ecosystem services.
Supplementary Material
Acknowledgments
We thank Loïc Schneider for his valuable support in the lab, Annette Temperli for her help with the sample preparation and Manuela Oettli for her support with the isotope measurements. We also thank two anonymous reviewers for their valuable comments.
Contributor Information
Justine Charlet de Sauvage, Silviculture Group, Institute of Terrestrial Ecosystems, ETH Zurich, Universitätstrasse 16, CH-8092 Zurich, Switzerland.
Kerstin Treydte, Forest Dynamics, Swiss Federal Institute for Forest, Snow and Landscape Research WSL, Zürcherstrasse 111, CH-8903 Birmensdorf, Switzerland; Oeschger Centre for Climate Change Research, University of Bern, Hochschulstrasse 4, CH-3012 Bern, Switzerland.
Matthias Saurer, Forest Dynamics, Swiss Federal Institute for Forest, Snow and Landscape Research WSL, Zürcherstrasse 111, CH-8903 Birmensdorf, Switzerland.
Mathieu Lévesque, Silviculture Group, Institute of Terrestrial Ecosystems, ETH Zurich, Universitätstrasse 16, CH-8092 Zurich, Switzerland.
Funding
This research project was funded by the Federal Office for the Environment FOEN of Switzerland (Contract numbers: 16.0072.PJ/S174-0684 and 19.0051.PJ/7438E15A4).
Conflict of interest
None declared.
Data availability
The dataset is available at doi: 10.16904/envidat.527 (Charlet de Sauvage et al. 2024).
References
- Allen CD, Breshears DD, McDowell NG. 2015. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere. 6(8):art129. 10.1890/ES15-00203.1. [DOI] [Google Scholar]
- Allen RG, Pereira LS, Raes D, Smith M. 1998. Crop evapotranspiration-guidelines for computing crop water requirements-FAO irrigation and drainage paper 56. Rome, Italy: FAO – Food and Agriculture Organization of the United Nations, p 300.others. [Google Scholar]
- Augusti A, Betson TR, Schleucher J. 2006. Hydrogen exchange during cellulose synthesis distinguishes climatic and biochemical isotope fractionations in tree rings. New Phytol. 172(3):490–499. 10.1111/j.1469-8137.2006.01843.x. [DOI] [PubMed] [Google Scholar]
- Bastien JC. 2019. Potential of Douglas-fir under climate change. 3.2. In: Spiecker H, Lindner M, Schuler J (eds), Douglas-fir: an option for Europe. Joensuu, Finland: European Forest Institute, pp 40–45. [Google Scholar]
- Beguería S, Vicente-Serrano SM. 2023. SPEI: calculation of the standardized precipitation-evapotranspiration index. https://CRAN.R-project.org/package=SPEI
- Belmecheri S, Lavergne A. 2020. Compiled records of atmospheric CO2 concentrations and stable carbon isotopes to reconstruct climate and derive plant ecophysiological indices from tree rings. Dendrochronologia. 63:125748. 10.1016/j.dendro.2020.125748. [DOI] [Google Scholar]
- Boettger T, Haupt M, Knöller K, Weise SM, Waterhouse JS, Rinne KT, Loader NJ, Sonninen E, Jungner H, Masson-Delmotte V, et al. . 2007. Wood cellulose preparation methods and mass spectrometric analyses of δ13C, δ18O, and nonexchangeable δ2H values in cellulose, sugar, and starch: an interlaboratory comparison. Anal Chem. 79(12):4603–4612. 10.1021/ac0700023. [DOI] [PubMed] [Google Scholar]
- Bonal D, Pau M, Toigo M, Granier A, Perot T. 2017. Mixing oak and pine trees does not improve the functional response to severe drought in central French forests. Ann For Sci. 74(4):72. 10.1007/s13595-017-0671-9. [DOI] [Google Scholar]
- Brockerhoff EG, Barbaro L, Castagneyrol B, Forrester DI, Gardiner B, González-Olabarria JR, Lyver PO, Meurisse N, Oxbrough A, Taki H, et al. . 2017. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers Conserv. 26(13):3005–3035. 10.1007/s10531-017-1453-2. [DOI] [Google Scholar]
- Bunn A, Korpela M, Biondi F, Campelo F, Mérian P, Qeadan F, Zang C. 2022. dplR: dendrochronology program library in R. https://CRAN.R-project.org/package=dplR
- Cavard X, Macdonald SE, Bergeron Y, Chen HYH. 2011. Importance of mixedwoods for biodiversity conservation: evidence for understory plants, songbirds, soil fauna, and ectomycorrhizae in northern forests. Environ Rev. 19(NA):142–161. 10.1139/a11-004. [DOI] [Google Scholar]
- Charlet de Sauvage J, Bugmann H, Bigler C, Lévesque M. 2023. Species diversity and competition have minor effects on the growth response of silver fir, European larch and Douglas fir to drought. Agric For Meteorol. 341:109664. 10.1016/j.agrformet.2023.109664. [DOI] [Google Scholar]
- Charlet de Sauvage J, Saurer M, Treydte K, Lévesque M. 2024. Carbon, oxygen and hydrogen isotopes in tree-ring cellulose of silver fir and Douglas-fir in Switzerland. EnviDat. 10.16904/envidat.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. 2002. Stable isotopes in plant ecology. Annu Rev Ecol Syst. 33(1):507–559. 10.1146/annurev.ecolsys.33.020602.095451. [DOI] [Google Scholar]
- Etzold S, Sterck F, Bose AK, Braun S, Buchmann N, Eugster W, Gessler A, Kahmen A, Peters RL, Vitasse Y, et al. . 2022. Number of growth days and not length of the growth period determines radial stem growth of temperate trees. Ecol Lett. 25(2):427–439. 10.1111/ele.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester DI, Bauhus J. 2016. A review of processes behind diversity—productivity relationships in forests. Curr For Rep. 2(1):45–61. 10.1007/s40725-016-0031-2. [DOI] [Google Scholar]
- Fritts HC (ed). 1976. Tree rings and climate. London, UK: Academic Press. 10.1016/B978-0-12-268450-0.50006-9. [DOI] [Google Scholar]
- Gagen M, Battipaglia G, Daux V, Duffy J, Dorado-Liñán I, Hayles LA, Martínez-Sancho E, McCarroll D, Shestakova TA, Treydte K. 2022. Climate signals in stable isotope tree-ring records. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M (eds), Stable isotopes in tree rings: inferring physiological, climatic and environmental responses. Cham: Springer International Publishing, pp 537–579. [Google Scholar]
- Gazol A, Camarero JJ. 2016. Functional diversity enhances silver fir growth resilience to an extreme drought. J Ecol. 104(4):1063–1075. 10.1111/1365-2745.12575. [DOI] [Google Scholar]
- Gessler A, Brandes E, Keitel C, Boda S, Kayler ZE, Granier A, Barbour M, Farquhar GD, Treydte K. 2013. The oxygen isotope enrichment of leaf-exported assimilates – does it always reflect lamina leaf water enrichment? New Phytol. 200(1):144–157. 10.1111/nph.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler A, Ferrio JP, Hommel R, Treydte K, Werner RA, Monson RK. 2014. Stable isotopes in tree rings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol. 34(8):796–818. 10.1093/treephys/tpu040. [DOI] [PubMed] [Google Scholar]
- Grossiord C. 2019. Having the right neighbors: how tree species diversity modulates drought impacts on forests. New Phytol. 228(1):42–49. 10.1111/nph.15667. [DOI] [PubMed] [Google Scholar]
- Grossiord C, Buckley TN, Cernusak LA, Novick KA, Poulter B, Siegwolf RTW, Sperry JS, McDowell NG. 2020. Plant responses to rising vapor pressure deficit. New Phytol. 226(6):1550–1566. 10.1111/nph.16485. [DOI] [PubMed] [Google Scholar]
- Grossiord C, Gessler A, Granier A, Berger S, Bréchet C, Hentschel R, Hommel R, Scherer-Lorenzen M, Bonal D. 2014a. Impact of interspecific interactions on the soil water uptake depth in a young temperate mixed species plantation. J Hydrol. 519:3511–3519. 10.1016/j.jhydrol.2014.11.011. [DOI] [Google Scholar]
- Grossiord C, Granier A, Ratcliffe S, Bouriaud O, Bruelheide H, Chećko E, Forrester DI, Dawud SM, Finér L, Pollastrini M, et al. . 2014. b. Tree diversity does not always improve resistance of forest ecosystems to drought. Proc Natl Acad Sci USA. 111(41):14812–14815. 10.1073/pnas.1411970111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstroh S, Werner C. 2022. The role of species interactions for forest resilience to drought. Plant Biol. 24(7):1098–1107. 10.1111/plb.13415. [DOI] [PubMed] [Google Scholar]
- Hartl-Meier C, Zang C, Buntgen U, Esper J, Rothe A, Gottlein A, Dirnbock T, Treydte K. 2015. Uniform climate sensitivity in tree-ring stable isotopes across species and sites in a mid-latitude temperate forest. Tree Physiol. 35(1):4–15. 10.1093/treephys/tpu096. [DOI] [PubMed] [Google Scholar]
- Hegyi F. 1974. A simulation model for managing Jack-pine stands. In: Fries J (ed), Growth models for tree and stand simulation. Stockholm, Sweden: Royal College of Forestry, pp 74–90. [Google Scholar]
- Holmes RL. 1983. Computer-assisted quality control in tree ring dating and measurement. Tree Ring Bull. 43:69–78. [Google Scholar]
- Huber B, Zischg A, Frehner M, Carraro G, Burnand J. 2015. Neu entwickelte Klimakarten für den Wald im Klimawandel. Schweiz Z Forstwes. 166:432–434. [Google Scholar]
- Laumer W, Andreu L, Helle G, Schleser GH, Wieloch T, Wissel H. 2009. A novel approach for the homogenization of cellulose to use micro-amounts for stable isotope analyses: stable isotope analyses of micro-amounts of cellulose. Rapid Commun Mass Spectrom. 23(13):1934–1940. 10.1002/rcm.4105. [DOI] [PubMed] [Google Scholar]
- Lebourgeois F, Gomez N, Pinto P, Mérian P. 2013. Mixed stands reduce Abies alba tree-ring sensitivity to summer drought in the Vosges mountains, western Europe. For Ecol Manage. 303:61–71. 10.1016/j.foreco.2013.04.003. [DOI] [Google Scholar]
- Lehmann MM, Vitali V, Schuler P, Leuenberger M, Saurer M. 2021. More than climate: hydrogen isotope ratios in tree rings as novel plant physiological indicator for stress conditions. Dendrochronologia. 65:125788. 10.1016/j.dendro.2020.125788. [DOI] [Google Scholar]
- Lehmann MM, Schuler P, Cormier MA, Allen ST, Leuenberger M, Voelker S. 2022. The stable hydrogen isotopic signature: from source water to tree rings. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M (eds), Stable isotopes in tree rings: inferring physiological, climatic and environmental responses (Vol. 8). Cham: Springer International Publishing, p. 331. 10.1007/978-3-030-92698-4_11. [DOI] [Google Scholar]
- Lévesque M, Rigling A, Bugmann H, Weber P, Brang P. 2014. Growth response of five co-occurring conifers to drought across a wide climatic gradient in Central Europe. Agric For Meteorol. 197:1–12. 10.1016/j.agrformet.2014.06.001. [DOI] [Google Scholar]
- Lotter AF, Appleby P, Bindler R, Dearing J, Grytnes J-A, Hofmann W, Kamenik C, Lami A, Livingstone D, Ohlendorf C, et al. . 2002. The sediment record of the past 200 years in a Swiss high-alpine lake: Hagelseewli (2339 m asl). J Paleolimnol. 28(1):111–127. 10.1023/A:1020328119961. [DOI] [Google Scholar]
- Magh R-K, Eiferle C, Burzlaff T, Dannenmann M, Rennenberg H, Dubbert M. 2020. Competition for water rather than facilitation in mixed beech-fir forests after drying-wetting cycle. J Hydrol. 587:124944. 10.1016/j.jhydrol.2020.124944. [DOI] [Google Scholar]
- Marshall JD, Brooks JR, Talhelm AF. 2022. Forest management and tree-ring isotopes. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M (eds), Stable isotopes in tree rings: inferring physiological, climatic and environmental responses. Cham: Springer International Publishing, pp 651–673. [Google Scholar]
- Martínez-Sancho E, Cernusak LA, Fonti P, Gregori A, Ullrich B, Pannatier EG, Gessler A, Lehmann MM, Saurer M, Treydte K. 2023. Unenriched xylem water contribution during cellulose synthesis influenced by atmospheric demand governs the intra-annual tree-ring δ18O signature. New Phytol. 240(5):1743–1757. 10.1111/nph.19278. [DOI] [PubMed] [Google Scholar]
- McCarroll D, Loader NJ. 2004. Stable isotopes in tree rings. Quat Sci Rev. 23(7–8):771–801. 10.1016/j.quascirev.2003.06.017. [DOI] [Google Scholar]
- McCarroll D, Gagen MH, Loader NJ, Robertson I, Anchukaitis KJ, Los S, Young GHF, Jalkanen R, Kirchhefer A, Waterhouse JS. 2009. Correction of tree ring stable carbon isotope chronologies for changes in the carbon dioxide content of the atmosphere. Geochim Cosmochim Acta. 73(6):1539–1547. 10.1016/j.gca.2008.11.041. [DOI] [Google Scholar]
- McMinn RG. 1963. Characteristics of Douglas-fir root systems. Can J Bot. 41(1):105–122. 10.1139/b63-010. [DOI] [Google Scholar]
- Mölder I, Leuschner C, Leuschner HH. 2011. δ13C signature of tree rings and radial increment of Fagus sylvatica trees as dependent on tree neighborhood and climate. Trees. 25(2):215–229. 10.1007/s00468-010-0499-5. [DOI] [Google Scholar]
- Monson RK, Szejner P, Belmecheri S, Morino KA, Wright WE. 2018. Finding the seasons in tree ring stable isotope ratios. Am J Bot. 105(5):819–821. 10.1002/ajb2.1083. [DOI] [PubMed] [Google Scholar]
- Oksanen J, Simpson GL, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Solymos P, Stevens MHH, et al. . 2022. Vegan: community ecology package. https://CRAN.R-project.org/package=vegan
- Piedallu C, Dallery D, Bresson C, Legay M, Gégout J-C, Pierrat R. 2023. Spatial vulnerability assessment of silver fir and Norway spruce dieback driven by climate warming. Landsc Ecol. 38(2):341–361. 10.1007/s10980-022-01570-1. [DOI] [Google Scholar]
- Pinheiro J, Bates D, R Core Team . 2022. Nlme: linear and nonlinear mixed effects models. https://CRAN.R-project.org/package=nlme
- R Core Team . 2022. R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Schuler P, Cormier M, Werner RA, Buchmann N, Gessler A, Vitali V, Saurer M, Lehmann MM. 2022. A high-temperature water vapor equilibration method to determine non-exchangeable hydrogen isotope ratios of sugar, starch and cellulose. Plant Cell Environ. 45(1):12–22. 10.1111/pce.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JA, Bauhus J. 2019. Benefits of mixtures on growth performance of silver fir (Abies alba) and European beech (Fagus sylvatica) increase with tree size without reducing drought tolerance. Front For Glob Change. 2:79. 10.3389/ffgc.2019.00079. [DOI] [Google Scholar]
- Shannon CE. 1948. A mathematical theory of communication. Bell Syst Tech J. 27(3):379–423. [Google Scholar]
- Siegwolf RTW, Brooks JR, Roden J, Saurer M, editors. 2022. Stable isotopes in tree rings: inferring physiological, climatic and environmental responses (Vol. 8). Cham: Springer International Publishing. 10.1007/978-3-030-92698-4 [DOI] [Google Scholar]
- Song X, Lorrey A, Barbour MM. 2022. Environmental, physiological and biochemical processes determining the oxygen isotope ratio of tree-ring cellulose. In: Siegwolf RTW, Brooks JR, Roden J, Saurer M (eds), Stable isotopes in tree rings: inferring physiological, climatic and environmental responses. Cham: Springer International Publishing. p. 311–329. [Google Scholar]
- Thornthwaite CW. 1948. An approach toward a rational classification of climate. Geogr Rev. 38(1):55–94. [Google Scholar]
- Thurm EA, Uhl E, Pretzsch H. 2016. Mixture reduces climate sensitivity of Douglas-fir stem growth. For Ecol Manage. 376:205–220. 10.1016/j.foreco.2016.06.020. [DOI] [Google Scholar]
- Treydte K, Frank DC, Saurer M, Helle G, Schleser GH, Esper J. 2009. Impact of climate and CO2 on a millennium-long tree-ring carbon isotope record. Geochim Cosmochim Acta. 73(16):4635–4647. 10.1016/j.gca.2009.05.057. [DOI] [Google Scholar]
- Treydte K, Boda S, Graf Pannatier E, Fonti P, Frank D, Ullrich B, Saurer M, Siegwolf R, Battipaglia G, Werner W, et al. . 2014. Seasonal transfer of oxygen isotopes from precipitation and soil to the tree ring: source water versus needle water enrichment. New Phytol. 202(3):772–783. 10.1111/nph.12741. [DOI] [PubMed] [Google Scholar]
- Treydte K, Liu L, Padrón RS, Martínez-Sancho E, Babst F, Frank DC, Gessler A, Kahmen A, Poulter B, Seneviratne SI, et al. . 2023. Recent human-induced atmospheric drying across Europe unprecedented in the last 400 years. Nat Geosci. 17(1):58–65. 10.1038/s41561-023-01335-8. [DOI] [Google Scholar]
- Vannoppen A, Treydte K, Boeckx P, Kint V, Ponette Q, Verheyen K, Muys B. 2020. Tree species diversity improves beech growth and alters its physiological response to drought. Trees. 34(4):1059–1073. 10.1007/s00468-020-01981-0. [DOI] [Google Scholar]
- Vejpustková M, Čihák T. 2019. Climate response of Douglas fir reveals recently increased sensitivity to drought stress in Central Europe. Forests. 10(2):97. [Google Scholar]
- Vitali V, Büntgen U, Bauhus J. 2017. Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south-western Germany. Glob Chang Biol. 23(12):5108–5119. 10.1111/gcb.13774. [DOI] [PubMed] [Google Scholar]
- Vitali V, Forrester DI, Bauhus J. 2018. Know your neighbours: drought response of Norway spruce, silver fir and Douglas fir in mixed forests depends on species identity and diversity of tree neighbourhoods. Ecosystems. 21(6):1215–1229. 10.1007/s10021-017-0214-0. [DOI] [Google Scholar]
- Vitali V, Martínez-Sancho E, Treydte K, Andreu-Hayles L, Dorado-Liñán I, Gutierrez E, Helle G, Leuenberger M, Loader NJ, Rinne-Garmston KT, et al. . 2022. The unknown third – hydrogen isotopes in tree-ring cellulose across Europe. Sci Total Environ. 813:152281. 10.1016/j.scitotenv.2021.152281. [DOI] [PubMed] [Google Scholar]
- Vitali V, Peters RL, Lehmann MM, Leuenberger M, Treydte K, Büntgen U, Schuler P, Saurer M. 2023. Tree-ring isotopes from the Swiss Alps reveal non-climatic fingerprints of cyclic insect population outbreaks over the past 700 years. Tree Physiol. 43(5):706–721. 10.1093/treephys/tpad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitasse Y, Bottero A, Rebetez M, Conedera M, Augustin S, Brang P, Tinner W. 2019. What is the potential of silver fir to thrive under warmer and drier climate? Eur J For Res. 138(4):547–560. 10.1007/s10342-019-01192-4. [DOI] [Google Scholar]
- Weigt RB, Bräunlich S, Zimmermann L, Saurer M, Grams TEE, Dietrich H-P, Siegwolf RTW, Nikolova PS. 2015. Comparison of δ18O and δ13C values between tree-ring whole wood and cellulose in five species growing under two different site conditions. Rapid Commun Mass Spectrom. 29(23):2233–2244. 10.1002/rcm.7388. [DOI] [PubMed] [Google Scholar]
- Wickham, H. (2016). ggplot2: elegant graphics for data analysis. New York: Springer-Verlag. https://ggplot2.tidyverse.org [Google Scholar]
- Wieloch T, Grabner M, Augusti A, Serk H, Ehlers I, Yu J, Schleucher J. 2022. Metabolism is a major driver of hydrogen isotope fractionation recorded in tree-ring glucose of Pinus nigra. New Phytol. 234(2):449–461. 10.1111/nph.18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang C, Biondi F. 2015. Treeclim: an R package for the numerical calibration of proxy-climate relationships. Ecography. 38(4):431–436. 10.1111/ecog.01335. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset is available at doi: 10.16904/envidat.527 (Charlet de Sauvage et al. 2024).