Abstract
Iron is an essential nutrient and a constituent of ferroproteins and enzymes crucial for human life. Generally, nonmenstruating individuals preserve iron very efficiently, losing less than 0.1% of their body iron content each day, an amount that is replaced through dietary iron absorption. Most of the iron is in the hemoglobin (Hb) of red blood cells (RBCs); thus, blood loss is the most common cause of acute iron depletion and anemia worldwide, and reduced hemoglobin synthesis and anemia are the most common consequences of low plasma iron concentrations. The term iron deficiency (ID) refers to the reduction of total body iron stores due to impaired nutrition, reduced absorption secondary to gastrointestinal conditions, increased blood loss, and increased needs as in pregnancy. Iron deficiency anemia (IDA) is defined as low Hb or hematocrit associated with microcytic and hypochromic erythrocytes and low RBC count due to iron deficiency. IDA most commonly affects women of reproductive age, the developing fetus, children, patients with chronic and inflammatory diseases, and the elderly. IDA is the most frequent hematological disorder in children, with an incidence in industrialized countries of 20.1% between 0 and 4 years of age and 5.9% between 5 and 14 years (39% and 48.1% in developing countries). The diagnosis, management, and treatment of patients with ID and IDA change depending on age and gender and during pregnancy. We herein summarize what is known about the diagnosis, treatment, and prevention of ID and IDA and formulate a specific set of recommendations on this topic.
INTRODUCTION
Iron is an essential nutrient and a constituent of ferroproteins and enzymes that are crucial for human life. Generally, nonmenstruating individuals preserve iron very efficiently, losing less than 0.1% of their body iron content each day, an amount that is replaced through dietary iron absorption. Most of the iron is in the hemoglobin (Hb) of red blood cells (RBCs); thus, blood loss is the main cause of iron deficiency (ID) worldwide, and reduced hemoglobin synthesis and anemia are the most common consequences of low plasma iron concentrations. Severe ID can also affect the synthesis of ferroproteins in nonerythroid cell types, causing cellular dysfunction and leading to additional manifestations including epithelial changes in nails, tongue, and esophagus, deficits in cognitive function and muscle performance, and impaired adaptive immune response. 1
The term ID refers to the reduction of total body iron stores due to (a) decreased iron intake because of impaired nutrition, reduced absorption secondary to gastrointestinal diseases, and use of proton pump inhibitors, (b) increased utilization (e.g., pregnancy), or (c) increased iron losses, usually because of bleeding. Heavy menstrual bleeding (HMB) in women is defined as the regular loss of more than 80 ml of blood during a menstrual period, exceeding iron intake, and is considered the most common cause of iron deficiency (ID). Absolute ID occurs when total body iron stores are insufficient to meet the needs of the individual. In functional ID, total body iron is preserved but iron is maldistributed. Functional ID is explained by reduced iron export via ferroportin, which is controlled by hepcidin‐dependent and independent mechanisms in response to inflammation. 1 Consequently, iron absorption from the gastrointestinal system is inhibited, and iron is trapped in macrophages, resulting in reduced circulating iron levels. 2 Iron deficiency can lead to chronic fatigue, poor concentration, impaired exercise performance, and poor quality of life. 3 As ID becomes more severe, it will cause microcytic anemia. 3
Iron deficiency affects more than 2 billion people worldwide, with iron deficiency anemia (IDA) remaining the main cause of anemia. In clinical practice, the current oral iron treatments are often inadequate because of suboptimal effectiveness and side effects that lead to poor compliance and premature therapy discontinuation. In ID, iron storage must be severely depleted before anemia occurs since, while in modest iron stores' reduction, the recycling of iron from the daily RBC turnover provides sufficient iron for erythropoiesis and hemoglobin production. 4
IDA is defined as low Hb or hematocrit associated with microcytic (low mean corpuscular volume, MCV) and hypochromic (low mean corpuscular hemoglobin, MCH) erythrocytes and low RBC count. 4 IDA most commonly affects children, women of reproductive age, patients with chronic and inflammatory diseases, and the elderly. 4 IDA is the most frequent hematological disorder in children, with an incidence in industrialized countries of 20.1% between 0 and 4 years of age and 5.9% between 5 and 14 years (39 and 48.1% in developing countries). 4 The response to IDA includes increased EPO secretion to stimulate erythropoiesis and decreased hepcidin production to increase intestinal iron uptake and mobilization of iron stores.
As discussed in the subsequent paragraphs, the diagnosis, management, and treatment of patients with ID and IDA should be tailored according to the age and gender and underlying conditions, like during pregnancy. We herein summarize what is known about the diagnosis, treatment, and prevention of ID and IDA and formulate a specific set of recommendations on this topic.
METHODS
The following set of recommendations is based on a systematic literature search. All published articles in the literature that address different aspects of ID and IDA, including causes, diagnosis, and treatment strategies, were identified by PubMed, Online Mendelian Inheritance in Man, and Textbook search, including all the additional relevant references cited in the articles found. The key search terms “iron deficiency” and “iron deficiency anemia” were used. The examined period was from 1980 to 2022. Conference abstracts were included if deemed to be of relevance. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method was used to evaluate levels of evidence level and assess the strength of recommendations (Figure 1). The GRADE criteria can be found at http://www.gradeworkinggroup.org. This recommendation paper was produced in collaboration with the European Hematology Association (EHA), including the Red Cell and Iron Specialized Working Group members.
Figure 1.

Recommendations and relative consensus.
DIAGNOSIS
ID and IDA can be diagnosed by evaluating specific hematological and iron biomarkers. In otherwise healthy individuals, ferritin levels reflect iron stores but are rarely informative about actual iron availability for erythropoiesis. For this reason, other parameters such as transferrin saturation (TSAT), soluble transferrin receptor (sTfR), percentage of hypochromic erythrocytes (%HYPO), and reticulocyte hemoglobin content (CHr) are useful to identify an inadequate iron supply to erythropoiesis.
A Hb level below the lower limit of normally indicates IDA. Iron status can be adequately characterized using multiple complementary parameters, and its clinical relevance can be assessed.
-
✓
1a. How is the diagnosis of iron deficiency (ID) or iron deficiency anemia (IDA) established across different age groups, including children, adolescents, adults, and during pregnancy? Which tests are recommended for diagnosing patients with ID/IDA?
Hematological and biochemical markers support the diagnosis of ID/IDA. The absence (in ID) and presence of anemia (in IDA) are confirmed by Hb concentration, as shown by a complete blood count (CBC). According to the World Health Organization (WHO, 2011), anemia is defined as a Hb level of <130 g/L in men, <120 g/L in nonpregnant women, and <110 g/L in both pregnancy and children >5 years. Specific thresholds at various stages of childhood and pregnancy are also commonly used (WHO 2011) (Figures 2 and 3). ID is the most common cause of anemia in pregnancy due to the growing fetus and placenta, and those with untreated ID are unnecessarily at risk of anemia. Anemia in pregnancy is generally defined as a hemoglobin concentration <110 g/L in the first trimester, <105 g/L in the second trimester, and <110 g/L in the third trimester. To define ID in pregnancy, there are no standardized serum ferritin thresholds. Ferritin is an acute phase reactant and may be elevated as a result of pregnancy itself. While a low ferritin invariably indicates ID in this population, a normal ferritin cannot reliably exclude it.
Figure 2.
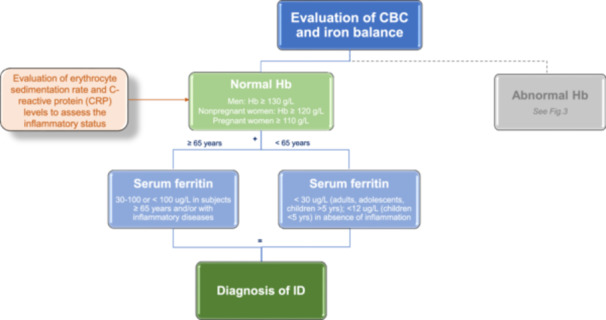
Iron deficiency (ID) diagnosis. Flow chart showing the crucial steps to make a diagnosis of ID.
Figure 3.
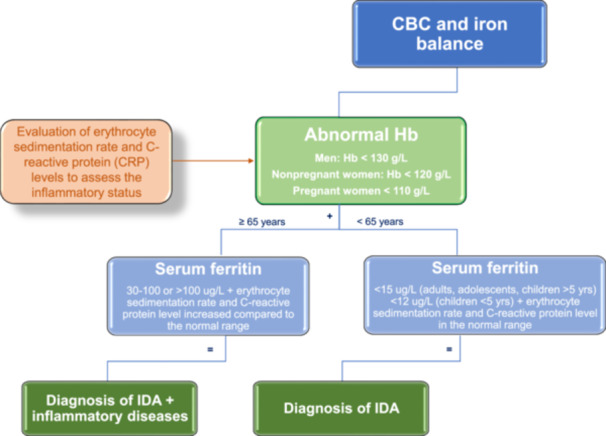
Iron deficiency anemia (IDA) diagnosis. Flow chart showing the crucial steps to make a diagnosis of IDA.
The evaluation of the blood smear, which typically shows microcytosis, hypochromia, and pencil forms in IDA, is very useful. Additionally, it serves as an important, cost‐effective, and readily available diagnostic tool. It is important to note that microcytosis visible on the peripheral smear may be detected before abnormalities are evident on the complete blood cell count.
Measuring ferritin, serum iron, and TSAT is a routine method for diagnosing ID in various conditions. In the absence of inflammation, specifically determined by C‐reactive protein (CRP) and erythrocyte sedimentation rate dosage as reliable indicator of infectious and inflammatory diseases, ferritin is the most accurate biomarker that correlates with total body iron stores, and it is the mainstay for diagnosing absolute ID. 5 A low serum ferritin concentration is a sensitive indicator for ID uncomplicated by other concurrent diseases long before changes are observed in blood Hb concentrations. The WHO defines ID as serum or plasma ferritin levels <15 ug/L in children older than 5 years, adolescents, and adults and less than 12 ug/L in children younger than 5 years (WHO, 2022). Many authors suggested that the diagnostic accuracy of ferritin could be improved by increasing the cutoff to 30 μg/L. 6 , 7 , 8 Such a value has a higher sensitivity (from 85% to 92%) and unchanged specificity (98%), and it is the most accepted threshold used to establish a diagnosis of ID, even in the mildest cases. 9 However, little evidence is available from high‐quality studies to justify specific thresholds. Published cutoffs are often based on older studies that (i) were conducted without international standards or (ii) use assays different from those currently used. 10 , 11 Altogether, this confounds the interpretation of ferritin in clinical practice.
The diagnosis of absolute ID is controversial in the elderly population, with high prevalence of comorbidities, in whom the classical cutoff of ferritin <15–30 μg/L has been claimed as too stringent. In some small studies on older patients, a serum ferritin level <45–50 μg/L showed greater reliability in predicting ID than conventional cutoff values. For this reason, some authors suggest that ferritin thresholds of at least 45 μg/L, if not 100 μg/L, could be reasonable in people aged >65 years, mainly when specific comorbidities occur, such as advanced chronic kidney disease (CKD) or chronic heart failure (CHF). 11 , 12 , 13
An important issue in the diagnosis of ID and IDA is the differential diagnosis with beta thalassemia carriers. To confirm ID or IDA, transferrin saturation along with ferritin levels are recommended parameters. An increase in red cell distribution width (RDW), reflecting variation in RBC size (anisocytosis), is typical in IDA. In contrast, thalassemia carriers exhibit RDW values within or close to the reference interval due to consistent red cell size (microcytes). High RBC count relative to the degree of anemia is typically encountered in thalassemia carriers in contrast to IDA where RBC count is low and commensurate with the degree of anemia. Blood smear is very useful also in this differential diagnosis showing target cells, fine basophilic stippling, nucleated RBCs in thalassemia carriers, and pencil forms in iron deficiency.
When ID/IDA is diagnosed, a thorough investigation of etiology is mandatory, in part because it may reveal underlying causes that are an even greater threat to health than ID/IDA. If the causes can be actively treated, recurrence will be avoided, and long‐term resolution of ID/IDA will be more likely. 1 , 2 , 3
-
✓
1b. How can iron deficiency (ID) or iron deficiency anemia (IDA) be diagnosed in patients with chronic disease, inflammation, or malignancy? How should the diagnostic criteria for ID/IDA be adjusted in the presence of inflammation?
In patients with functional ID, withholding iron from the plasma promotes iron‐deficient erythropoiesis and anemia despite adequate body iron stores. 14 , 15 This process is common in patients with inflammation, malignancy, chronic infections, parasitic infections, such as hookworm infestations, malaria, iatrogenic blood loss from procedures, and blood sampling. 16 In the developed world, this disease is easily identified and treated but frequently overlooked by physicians. In contrast, it is a health problem that affects major portions of the population in underdeveloped countries; indeed, local economics generally dictate the level of nutrition worldwide. Overall, the prevention and successful treatment for iron deficiency anemia remains woefully insufficient worldwide, especially among underprivileged women and children. ID may be further exacerbated by increased demand for iron like in patients receiving erythropoiesis‐stimulating agents. 16 Particularly in African children, malaria and iron deficiency (ID) are common and interrelated public health issues. Observational data indicate that interrupting malaria transmission can lead to a reduction in the prevalence of ID. 4
The traditional gold standard test for absolute ID is the finding of absent stainable bone marrow iron. Patients with functional ID have detectable stainable bone marrow iron unless they have concomitant absolute ID. Bone marrow aspiration is invasive and never done routinely to diagnosis ID, but it remains helpful in complex cases. In current practice, ID and IDA are usually diagnosed by blood biomarkers. Red cell indices can indicate anemia, microcytic, and hypochromic RBCs with an increased red blood cell distribution width (anisocytosis) and elongated (pencil‐shaped) cells. 17 It's worth mentioning that in older individuals, there's a common occurrence of vitamin B12 and folate deficiency, which can cause an increase in MCV, resulting in normocytic anemia. This can make interpreting laboratory data challenging. Consequently, relying solely on MCV assessment isn't reliable for ruling out iron deficiency anemia in the elderly, particularly if they have accompanying comorbidities. 13
Because ferritin is an acute‐phase protein, the diagnosis of ID (based on ferritin alone) can be obscured by inflammation. 18 , 19 , 20 Strategies for adjusting ferritin concentration cutoffs in inflammation include raising of the ferritin threshold (WHO, 2020) or developing a regression equation based on the correlation between ferritin and inflammatory markers. When there is evidence of systemic inflammation, such as an increased erythrocyte sedimentation rate or elevated C‐reactive protein levels, the WHO defines ID at a ferritin concentration of less than 30 µg/L in children under five years and less than 70 µg/L in older children and adults (WHO, 2020). Algorithms to correct ferritin for inflammation are not universally applicable in part because the changes in markers of inflammation vary with the etiology of inflammation and severity of the underlying disease. 18 , 21 Diagnosing absolute ID in patients with inflammation is important to identify the underlying factors (such as bleeding) and for population estimates of ID; however, treatment approaches should also consider coexistent functional ID. Ferritin concentrations can also increase in liver disease, including nonalcoholic fatty liver disease. 22 Moreover epidemiological data suggest that population ferritin concentrations are increasing with increasing obesity rates. 17 Serum iron concentration is reduced in ID and inflammation; hypoferremia alone does not indicate absolute ID. Transferrin saturation (e.g., less than 15% in adult and less than 7% in pediatric subjects) helps define low plasma iron availability to tissues in both absolute and functional ID in adult subjects. Soluble transferrin receptor (sTfR) is an index of tissue iron needs and of erythropoietic activity. The sTfR:log(ferritin) ratio has been a useful predictive index for bone marrow iron stores, especially in patients with inflammation. 23 Its utility is limited by low clinical availability and different thresholds between sTfR assays. 24
Several modern automated hematology analyzers can measure reticulocyte‐specific hemoglobin content and related indices. 25 The percentage of hypochromic red blood cells (%HYPO RBC) reflects iron‐restricted erythropoiesis during the preceding 2–3 months. 26 The reticulocyte hemoglobin content (CHr) reflects iron availability for erythropoiesis of the previous 3–4 days before testing. 27 Both parameters are useful to detect iron‐restricted erythropoiesis due to absolute or functional ID and evaluate response to therapy. 28 , 29
Measurement of hepcidin concentration is under investigation as a test for ID and for distinguishing absolute from functional ID. 30 Hepcidin concentration has been studied in pregnant and nonpregnant women, in children, and in patients with rheumatoid arthritis, inflammatory bowel disease, cancer‐related anemia, or critical illness. 30 Suppressed hepcidin concentrations indicate a physiological iron need, predict responsiveness to iron, and enable personalization of the route of iron replenishment. 31 In the absence of inflammation, the hepcidin/TSAT ratio has been proven to be an effective tool to identify patients with iron‐refractory iron deficiency anemia (IRIDA) due to variants in the TMPRSS6 gene among patients with chronic IDA. 32 , 33 Measurement of hepcidin is limited primarily to research settings and rarely used in the clinical setting in some European hospital laboratories. Using commutable calibration materials with human plasma or serum will allow for a standardization methodology that is essential to enable routine clinical hepcidin testing. 34
Iron deficiency is the presenting manifestation of various pathological processes, and investigation to exclude serious pathology and define the underlying cause is essential. Serological testing for coeliac disease should be considered in patients with nonanemic ID and is recommended for all adult patients with IDA. 35 Men and postmenopausal women with IDA are at high risk of bleeding gastrointestinal lesions and should be considered for upper and lower gastrointestinal endoscopy. 36 Assessment for autoimmune gastritis and H. pylori should be considered in all patients with ID or IDA, especially in those who do not adequately respond to oral iron. 4 Furthermore, it's crucial to consider the involvement of gut bacteria and their interactions with the host in shaping iron acquisition. Bacterial activities impact the host's iron absorption, whereas the host's iron intake and levels affect the composition and function of gut bacteria, thereby influencing their virulence. Alterations in the host's innate immune system and circulating factors such as hepcidin, lipocalin 2, and lactoferrin are associated with metabolic disorders occurring at the interface between the host and the microbiota. 37
Premenopausal women with IDA should be considered for bidirectional endoscopy if they have symptoms of gastrointestinal disease (e.g., altered bowel habit or overt bleeding), a personal history or a first‐degree relative with a history of colorectal cancer, or if they do not have a clear explanation for ID, such as HMB. 37 Fecal occult blood testing should not be used to suggest endoscopy in patients with ID. CT colonography can be considered when colonoscopy is contraindicated but does not have the sensitivity for smaller mucosal lesions (less than 6 mm) and does not permit biopsy or polypectomy. Endoscopy is not recommended as a routine procedure in patients with nonanemic ID unless there are other concerns for gastrointestinal malignancy or if ID is recurrent. 37 If upper and lower endoscopic studies exclude substantial pathology, it is reasonable to withhold further gastrointestinal investigation unless there is recurrent, refractory, or severe IDA. 35 Small intestinal investigation can be accomplished by video capsule endoscopy (a noninvasive imaging approach) or enteroscopy (an endoscopic approach enabling tissue sampling and therapeutic maneuvers).
For reproductive‐aged women, the most common causes of ID and IDA are the symptoms of HMB and unreplenished losses from previous pregnancy. 36 , 38 , 39 As discussed previously, HMB has a prevalence much higher than that generally perceived from healthcare system‐based data; survey‐based studies indicate that up to 53% of women of reproductive age may experience the symptom at any given time putting them at high risk for ID and IDA. 40 , 41 , 42 , 43 , 44 This risk is exemplified by evaluating iron‐dependent erythropoiesis in women with and without HMB, 2 and a Finnish study of women with HMB showed that 27% of its participants had IDA, 90% with a serum ferritin less than 30 µg/L, and 60% with serum ferritin levels below 15 µg/L. 45 Effective diagnostic and therapeutic strategies exist for the varying causes of the symptoms of HMB 46 , 47 ; failure to identify and address this issue will prolong or even prevent the sustained normalization of iron stores. Moreover, it is of importance to screen females experiencing HMB and recurrent or refractory IDA without uterine organic lesions for congenital bleeding disorders (CBDs) such as platelet function disorders and von Willebrand disease (VWD). CBDs are present in approximately 20%–30% of females with HMB and can result in unnecessary hysterectomy.
Recommendation 1
The diagnosis of ID/IDA is based on evaluating several hematological and biochemical markers, such as Hb, ferritin, and TSAT levels. The cutoff for each specific marker is based on age, sex, and pregnancy status (cutoff for Hb: men Hb ≥ 130 g/L; nonpregnant women ≥ 120 g/L; pregnant women ≥ 110 g/L; and children < 5 yrs ≥ 110 g/L). In the absence of inflammation, ferritin is the most specific marker correlating with total body iron stores (cutoff for serum ferritin: adults, adolescents, children > 5 yrs < 30 ug/L; children < 5 yrs < 12 ug/L). In the context of multiple comorbidities, such as inflammation, ferritin thresholds <100 μg/L or higher values are suggested in combination with TSAT. In elderly patients (>65 years) with chronic kidney disease (CKD) or chronic heart failure (CHF), ferritin thresholds of at least 45 μg/L can be used. The evaluation of individuals identified with ID/IDA should also consider the reason for the deficiency, with concomitant investigation and treatment appropriate for identified causes or contributors.
CLINICAL MANIFESTATIONS
-
✓
2.a What are the common clinical manifestations of ID/IDA?
ID refers to iron deficiency without affecting hematopoiesis. Therefore, neither hemoglobin, MCV, nor MCH are abnormal. 48 Because anemia is a very late consequence of ID, iron deprivation can also affect various cellular processes, including myoglobin synthesis (skeletal muscle and cardiomyocytes), DNA synthesis, mitochondrial respiration, heme, and nonhemic enzyme synthesis. In addition to serum ferritin, TSAT is recommended as a marker to identify ID, 49 particularly in chronic inflammatory conditions. Zinc protoporphyrin, 48 sTFR, hepcidin, CHr, and %HYPO RBC can be used alternatively, although not widely available. 23 Invasive determination of bone marrow iron content should be reserved for rare situations. ID has been described in different age groups or disease conditions. Typically, these populations involve individuals whose dietary iron intake does not meet their needs (Table 1). Patients at risk of developing ID should be screened. Identifying these patients early, before developing IDA would prevent the development of severe complications. In adults with ID, iron supplementation is associated with a reduction in self‐reported fatigue but not with objective measures of physical capacity, despite a significant increase in hemoglobin concentration. 50 In children and adolescents, oral iron positively impacted intelligence test scoring and correlated with dosage but did not significantly affect attention, short‐term memory, long‐term memory, or school performance. 51 Iron deficiency, diagnosed in early pregnancy, typically reflects ID before conception, a circumstance that poses risks to the mother and the developing fetus.
Table 1.
Target population and causes of iron deficiency (ID).
|
A recent Cochrane review concluded that current evidence is insufficient to demonstrate the benefit of intravenous (IV) iron preparations for treating nonanemic ID in various patient populations. 52 However, in some indications, particularly heart failure with reduced ejection fraction, iron replacement therapy (particularly IV) is indicated even in the absence of anemia. 53 As for IDA, the underlying cause of ID should always be sought and managed. Biofortification of food has been proposed in several countries and for different target populations to prevent ID, including its nonanemic form, and is empirically recommended in guidelines despite the lack of a universal consensus. 50 , 51
Iron deficiency can cause symptoms both in the presence and absence of anemia. Because many of its symptoms can be nonspecific, physicians and patients do not always recognize that ID/IDA is present. Subsequently, a diagnosis is not made, and the condition is left untreated and is thus further exacerbated. 17 , 54 , 55 Common signs and symptoms of ID/IDA include fatigue, lethargy, chills, dizziness, dyspnea, tinnitus, pallor, heart palpitations, restless legs syndrome, and headache. 17 , 54 , 55 Other presentations commonly seen in these patients include alopecia, dry hair or skin, koilonychia, and atrophic glossitis.
The relevance of ID identified in early pregnancy lies in its frequent progression to IDA due to increasing maternal and fetal iron requirements as gestation progresses. Recent evidence suggests that these effects are most significant when IDA is diagnosed in the first trimester rather than the third. 56 , 57 Neonates born to women with ID and IDA are themselves iron deficient, with ongoing risks of cognitive impairment and delayed motor and cognitive development. 56 , 57 However, controlled studies on the consequences of ID in children are scarce, with most conducted in low‐income countries where unfavorable socioeconomic conditions may also impair cognitive development. Therefore, the efficacy of routine antenatal iron supplementation on offspring neurodevelopment remains uncertain. Indeed, the physiological requirement for iron during the period of rapid and critical brain development in young infants should be carefully evaluated, considering the risks associated with supplementing nonanemic infants with high iron levels.
In adults, ID is associated with decreased physical performance and quality of life; in the elderly, it is often associated with cognitive decline. 58 , 59 Moreover, several medical and chronic inflammatory conditions, including heart failure, ischemic heart disease, inflammatory bowel disease, and chronic kidney disease, can be further exacerbated when ID/IDA is present, thus worsening the prognosis and impairing the overall quality of life. 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 This is particularly evident in elderly patients who suffer from multiple morbidities, whereby even mild anemia can increase the chance of mortality. 68
Moreover, pica that refers to the craving or consumption of nonfood items, such as dirt, clay, or ice, is considered another symptom of ID and IDA, particularly in young children and pregnant African women. This unusual eating behavior can sometimes be a manifestation of the body's attempt to obtain missing nutrients, including iron, although the exact cause of pica is not fully understood. In the context of ID and IDA, pica may be an indicator of severe ID and should prompt further evaluation and treatment.
Recommendation 2.a
Common symptoms and signs of ID and IDA to evaluate are fatigue, lethargy, chills, dizziness, dyspnea, tinnitus, pallor, heart palpitations, restless legs syndrome, and headache. Other presentations to assess include alopecia, dry hair or skin, koilonychia, and atrophic glossitis. In children with ID, it is also important to analyze the motor and cognitive development. In adults, ID is associated with decreased physical performance and quality of life; in the elderly, it is often related to cognitive decline.
-
✓
2.b What is the significance of maternal ID/IDA during pregnancy, at the time of delivery, and postpartum?
The maternal consequences of ID/IDA may manifest during pregnancy, at the time of delivery, or during the postpartum period and may or may not include any signs and symptoms. 69 Common pregnancy symptoms often overlap with ID/IDA symptoms and can thus prevent the recognition of anemia, thus increasing the risk of maternal morbidity and mortality. 70 In a recent systematic review, maternal IDA was associated with a significantly increased risk of cesarean delivery, postpartum anemia, and blood transfusion. 71 Maternal consequences of ID/IDA also include abnormal thyroid function, placental abruption, pre‐eclampsia, and eclampsia. 72 , 73 , 74 Studies have also identified anemia as a significant risk factor for postpartum hemorrhage. 75 , 76 , 77 In fact, one study showed that the risk of death for women who experience a postpartum hemorrhage is almost seven‐fold higher if they are anemic at the onset of pregnancy. 78
Iron deficiency anemia in pregnancy may also adversely impact the fetus, resulting in an increased risk of prematurity, low birth weight, physical developmental delay, and morbidity. 79 , 80 These infants also show an increased risk of developing neurocognitive developmental dysfunctions such as reduced recognition, difficulty processing, and slower processing speed, as well as neurological disorders such as autism spectrum disorder, attention deficit disorder, and other intellectual disabilities. 38 , 81 , 82 , 83 These impacts should be seen as permanent as available evidence reveals their effects persist through the third decade of life. 36 It is apparent that the fetal vulnerability to the adverse impacts of ID is greatest in the first trimester of pregnancy and that these associated neurocognitive disorders persist into adult life. Considering these risks, identification and appropriate treatment of ID in women before conception becomes imperative.
Maternal or prepartum IDA and excessive blood loss at delivery are the leading causes of postpartum anemia. 73 , 84 , 85 Reduced milk production and resultant shortening of lactation periods are also characteristic of ID/IDA during the postpartum period. The emotional well‐being of postpartum women with IDA can also be seriously affected, with an increased risk of postpartum anxiety and depression and a decreased quality of life. 85 , 86
Recommendation 2.b
In periconceptual and pregnancy‐related ID/IDA, it is crucial to evaluate the possible fetal developmental delay and neurocognitive disorders in the newborn. ID and IDA are also linked to increased risks of thyroid dysfunction, preterm labor, placental abruption, pre‐eclampsia, eclampsia, cesarean delivery, postpartum anemia, and blood transfusion. Preconceptual normalization of iron status and prompt, effective treatment of IDA identified during pregnancy or postpartum should be urgent priorities for healthcare delivery systems.
THERAPY/MANAGEMENT
-
✓
3.1 What oral/IV iron formulations are available? What are the advantages and disadvantages of oral versus IV? How is the iron made available from these formulations for systemic use? Are some formulations preferred compared to others in different clinical settings? What are the potential side effects?
During absolute ID empty liver iron stores and low transferrin saturation are regulatory signals that reduce the mRNA expression of the iron‐regulated hormone hepcidin. Consequently, iron export from duodenal enterocytes increases, promoting the uptake of dietary iron and supplemented oral iron into the bloodstream. Although ferrous iron preparations are better absorbed than ferric iron preparations because of the low solubility of ferric iron and the physiology of iron absorption, ferrous iron is more irritating to mucosal surfaces and less well tolerated by patients than ferric iron, prompting a resurgence of interest in ferric iron therapy. The absorption of iron includes heme iron sourced from animal‐based foods and nonheme iron found in plant‐based foods and supplements. Heme iron, which is abundant in meats, poultry, and seafood, is absorbed more efficiently and has greater bioavailability compared to nonheme iron. Iron is mainly available as ferric iron and must be reduced by the ferrireductase dCytb to be transported into the duodenal enterocyte via the divalent metal transporter (DMT1). Iron is exported from the enterocyte via ferroportin. Likewise, low hepcidin levels enable efficient ferroportin‐mediated iron export from macrophages that recycle iron from senescent red blood cells and efficiently phagocytose and digest parenteral iron products. 87
Oral iron products are well established in the clinic and include ferrous salts; other iron salts include ferrous fumarate, glycine sulfate, bisglycinate, ascorbate, carbonate, tartrate, iodine, chloride, sodium citrate, aspartate. or succinate (Table 2). Oral iron is widely available, inexpensive, and safe. However, nonadherence to therapy is considered one of the most significant causes of nonresponse or recurrence of ID during iron replacement therapy. Main adverse events include abdominal pain, constipation, nausea, vomiting, and diarrhea. 88 More recently, novel oral therapies with improved absorption properties and lesser gastrointestinal side effects have been found on the market. These are generally carriers bound to ferric iron, such as ferric maltol (now approved for the treatment of IDA in Europe and the USA); sucrosomial iron (assessed in patients with cancer, kidney disease, and inflammatory bowel disease); iron hydroxide adipate tartrate, a medication currently being tested in children in developing countries; or ferric citrate. 89 , 90 , 91 , 92 , 93 Treatment of ID has changed by the availability of iron preparations that are applied intravenously. These circumvent the gastrointestinal issues experienced with oral iron preparations and can be used in higher doses. IV iron preparations consist of a carbohydrate shell with an iron core at its center. Iron sucrose or iron gluconate consists of less stable shells, which limits the amount of iron that can be infused. More stable shells that hallmark ferumoxytol, ferric carboxymaltose, and ferric derisomaltose release iron slowly, thus permitting the administration of higher iron doses. Avni et al. performed a systematic review of clinical trials testing parenteral iron formulations and concluded that serious adverse side effects, severe infusion reactions, or a higher prevalence of infections are rare. 92 Of note, IV administration of ferric carboxymaltose may cause hypophosphatemia due to increased FGF23 levels that induce phosphaturia, although it can be observed at lower frequency, also during treatment with other IV iron preparations. Hypophosphatemia is usually of short duration (8–10 weeks), but severe cases have been reported after chronic treatment. 94 , 95 Importantly, parenteral iron should not be applied in patients with sepsis as bacterial growth may be stimulated.
Table 2.
Oral iron formulations.
| Formulations | Accessibility to therapy and possible side effects |
|---|---|
| Ferrous ascorbate | Affordable and readily accessible but often associated with gastrointestinal side effects. |
| Ferrous fumarate | Affordable and readily accessible but often associated with gastrointestinal side effects. |
| Ferrous gluconate | Affordable and readily accessible but often associated with gastrointestinal side effects. |
| Ferrous sulfate | Affordable and readily accessible but often associated with gastrointestinal side effects. |
| Polysaccharide‐iron complex | With a reduced likelihood of gastrointestinal discomfort and a more favorable taste profile. |
| Carbonyl iron | Cost‐effective with no discernible advantage in terms of efficacy or side effects when compared. |
| Iron proteinsuccinylate | There are some data suggesting potential improvements in tolerability and efficacy compared to ferrous salts. However, it is unsuitable for individuals with hypersensitivity to milk protein. |
| Iron amino acid chelates (ferrousbisglycinate, ferrictrisglycinate) | Less prone to dietary interactions but potentially higher in cost compared to ferrous salts. |
Generally, oral iron is the first line treatment in uncomplicated cases of ID. Parenteral iron is applied in cases of moderate to severe anemia, when the response to oral iron is poor, in patients intolerant to oral iron, or when a rapid response to iron is required (e.g., in the perioperative setting). The administration of IV iron is more efficient in improving Hb values. 95 Higher costs of IV iron formulations are a clear disadvantage compared to oral preparations.
Recommendation 3.a
The treatment of ID and IDA comprises both oral iron formulations and IV iron preparations. Oral iron formulations include ferrous salts, such as ferrous sulfate or iron polymaltose. However, patient compliance is poor due to gastrointestinal adverse events, such as constipation, nausea, and diarrhea. More recently, novel oral therapies with improved absorption properties and lesser gastrointestinal side effects have reached the market, such as sucrosomial iron (assessed in patients with cancer, kidney disease, and inflammatory bowel disease) iron hydroxide adipate tartrate, a medication currently being tested in children in developing countries, or ferric citrate (mainly used in patients with chronic kidney disease where it also functions as a phosphate binder).
IV iron preparations consist of a carbohydrate shell with an iron core. Parenteral iron is applied in cases with moderate to severe anemia or when the response to oral iron is poor. Intravenous iron application is more efficient in improving hemoglobin values, but the higher costs of intravenous iron formulations are a clear disadvantage compared to oral preparations.
-
✓
3.2 What is the optimal schedule/dosing strategy for PO iron supplementation and dietary co‐adjuvants? What are the minimal safety conditions (environment of administration) for IV iron administration? What are the best markers for monitoring the response to iron replacement therapy?
Patients with IDA should receive iron replacement therapy (IRT). Furthermore, the benefit of treating ID, even in the absence of anemia, is increasingly recognized in patients with some comorbidities, such as chronic heart failure (CHF). 96
The choice of an iron compound and the route of administration (oral vs. IV) primarily depend on the presence and degree of anemia, underlying cause, clinical status (age, symptoms, long‐standing vs. recent onset, comorbidities), and, in some instances, patient preference.
Traditionally, oral iron has been administered at 100–200 mg daily in adults and 3‐6 mg/kg in children, in 2–3 divided doses, preferably without food. However, the rapid increase of hepcidin in response to iron administration, which persists for up to 48 hours, has a negative influence on the absorption of the subsequent doses. Indeed, studies measuring the absorption of an iron isotope in nonanemic ID women demonstrated that less frequent administration (from daily to alternate day and from multiple to single doses) and lower dosages (40–80 mg Fe) could improve efficacy and tolerability of oral iron treatment, by maximizing fractional iron absorption, reducing gastrointestinal side effects, and potentially increasing compliance. In a randomized trial comparing treatment regimes in subjects with IDA, 60 mg of elemental iron two times a day produced Nb increments similar to 120 mg on alternate days after the same total dose, with a lower prevalence of nausea. 97
Although the absorption of oral iron is theoretically favored by an acidic environment, administration of vitamin C is not recommended, based on a large randomized clinical trial (RCT) demonstrating that vitamin C neither enhances the hematological response nor diminishes the side effects. 98
In anemic patients, oral iron should be continued until the Hb normalizes, which may take 6–12 weeks (depending on the severity of anemia). After Hb restoration, oral supplements should be continued for at least three months to adequately replenish iron stores (with an ideal target of ferritin >100 μg/L). 91 , 99
Hb response to oral iron should be checked in the first four weeks when a rise in Hb of 20 g/L or into the normal range is considered an optimal response. 100
The optimal follow‐up protocol after IRT remains to be established, but periodic monitoring is advised, given the possibility of recurrences. Monitoring Hb periodically (every 3 months for 12 months and then every 6 months for 2–3 years) appears appropriate. Although ferritin is a reliable measure of total body iron stores, there are insufficient data to recommend its routine use for monitoring. 99 , 100
In patients who do not respond to IRT (i.e., anemic patients with Hb increase <10 g/L after 2–4 weeks of oral iron) despite adequate adherence, further investigations for unrecognized causes of anemia/ID are warranted. 101 , 102
No ideal markers can predict which patients will respond to oral iron. Low serum hepcidin levels could help identify patients in whom a response is probable. 31 Other studies have indicated that a rise in the Hb content of reticulocytes (CHr) may provide an early prediction of response to oral iron. 29 , 103
Indications for IV iron are expanding, thanks to the increased awareness that modern compounds are safer and better tolerated than the “old” preparations. 13 , 104 , 105 , 106 However, based on postmarketing reports, the European Medicine Agency (EMA) recommendations are still restrictive, suggesting that the relationship between risks and benefits should always be evaluated, and several rules should be adopted when considering parenteral iron. 107
-
✓
3.3 How to treat ID/IDA in adults?
Management and work‐up for determination of the underlying etiology of ID/IDA are summarized in Figures 4 and 5.
Figure 4.

Management of adult patients with iron deficiency (ID)/iron deficiency anemia (IDA). Flow chart showing the crucial steps to manage adult patients with ID/IDA. AUB, abnormal uterine bleeding; FIT, fecal immunochemical testing; HMB, heavy menstrual bleeding; GI, gastrointestinal; ID, iron deficiency; IDA, iron deficiency anemia; IRT, iron replacement therapy. *Oral IRT may interfere with colon preparation of colonoscopy by causing constipation. **May be delayed in elderly un‐fit populations or those with severe co‐morbidities, or CT cholangiography may be an alternative to colonoscopy.
Figure 5.
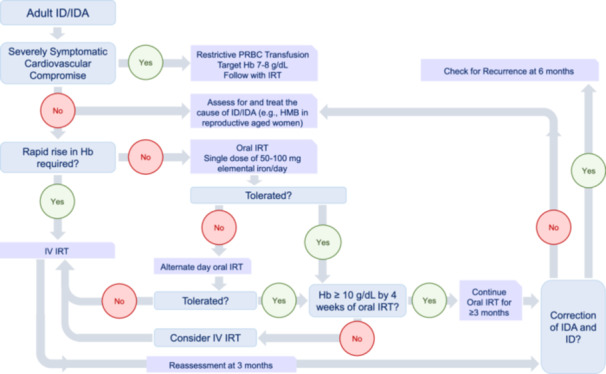
Treatment of iron deficiency (ID)/iron deficiency anemia (IDA) in adult patients. Flow chart showing the possible therapeutic options in adult patients with ID/IDA.
Transfusion with packed red blood cells is only reserved for severely symptomatic patients with cardiovascular complications. Slow infusion of restrictive transfusion should be followed with IRT. 99
Oral IRT with iron salts is the standard first‐line treatment in otherwise healthy and asymptomatic patients. Recent results indicate that lower doses and every‐other‐day regimens have an equivalent or even better iron absorption than daily dosing with fewer adverse events and increased tolerability. 99 , 108 , 109 Initiating oral ferrous salts once daily and if not tolerated alternating to once every other day is recommended. The oral iron formulations are summarized in Table 2.
Oral iron salts are inexpensive and, therefore, advantageous for under‐sourced areas. They are generally effective with high tolerability problems due to gastrointestinal (GI) side effects. These GI effects are more common with ferrous sulfate formulations and within the elderly population. In older adults, considering regimens of not more than once daily and even once every other day may be preferable to decrease GI effects, and intravenous IRT may be considered earlier.
Oral iron salts, including ferrous sulfate, ferrous gluconate, and ferrous fumarate, are available in liquids, tablets, and capsules containing various amounts of elemental iron with pills and capsules ranging from 30 to just over 100 mg. To facilitate absorption, iron salts should be taken on an empty stomach, as calcium‐containing food and drinks especially, but those containing phosphates, phytates, and tannates, as well as tea or coffee, can impair iron absorption. 110 The absorption of ferrous iron also depends on gastric acidity which maintains the solubility of iron; therefore, antacids, histamine receptor blockers, and proton pump inhibitors decrease the absorption of ferrous salts.
Other oral formulations include heme iron polypeptide (HIP), polysaccharide iron complex (PIC), and ferric citrate, which should be taken with meals. Of these, HIP and PIC are expensive but have better tolerability; however, there are limited clinical data on both. 100 On the other hand, due to unpredictable absorption, enteric‐coated or sustained‐release formulations are usually not recommended.
The duration of treatment is at least three months to correct anemia, but it should also be extended to 6 months to replenish iron stores. To check for recurrence due to ongoing blood losses, cessation after three months of oral IRT and follow‐up of the patient is applicable.
Intravenous IRT is recommended for specific indications. The risk of allergic reactions, anaphylaxis, and shock are less commonly encountered with current formulations. Particularly, IV iron should be administered only by staff trained to evaluate and manage anaphylactic and anaphylactoid reactions in a suitable location with rapid access to resuscitation facilities. Iron infusion should always be slow, especially in the first minutes of administration, and the patient should be carefully monitored. Patients with a history of allergies should be carefully evaluated before treatment. Due to the lack of safety data, women in the first trimester of pregnancy should be excluded from IV treatment. The intravenous iron formulations are summarized in Table 3.
Table 3.
Intravenous iron formulations.
| Formulation | Amount per dose (mg) | Infusion time |
|---|---|---|
| LMW‐iron dextran | 25 mg initial test dose | 2–6 hours |
| 100 mg/dose | ||
| Iron sucrose | 200–300 mg/dose | 100 mg/30 min |
| Ferrous gluconate | 125 mg | 12.5 mg/min |
| Ferumoxytol | 510 mg | 15 min |
| Ferric carboxymaltose | 750–1000 mg (differs according to brand) | 15 min |
| Iron isomaltoside | Differs according to brand | Differs according to brand |
Abbreviations: LMW, low‐molecular‐weight; min, minute.
Indications for intravenous iron treatment include 99 , 100 , 111 , 112 , 113 , 114 , 115 :
-
1.
Intolerance to oral IRT (including daily and alternate‐daily dosing)
-
2.
Inadequate response to oral IRT (Hb < 10 g/dL by the 4th week of oral IRT)
-
3.
Rapid iron replacement is required (moderately symptomatic patient or preoperative anemic patient whenever less than six weeks is available up to surgery)
-
4.
Inflammatory bowel disease
-
5.
Chronic kidney disease
-
6.
Chronic heart failure
-
7.
In patients with intestinal malabsorption like short bowel syndrome, allergic enteritis, atrophic gastritis
-
8.
After bariatric surgery or
-
9.
Ongoing abnormal uterine bleeding in case gynecological intervention is delayed
-
10.
IDA in the second or third trimester of pregnancy
-
11.
IRIDA.
Low‐molecular‐weight iron dextran is recommended to be applied after a test dose with an infusion time of 2–6 hours. In the other formulations, a test dose is not required. Premedication is not recommended before infusions except for patients with asthma or a history of drug allergy. 116 Ganzoni formula may be used to determine the amount of iron that will be infused but is no longer used for new formulations like ferric carboxymaltose and ferric derisomaltose. 117 , 118 The total amount of iron that will be infused=Patient's weight in kg x (target Hb‐patient's Hb in g/dL) x 2.4 + storage iron. Target Hb for patients below and above 35 kg is 13 and 15 g/dL, respectively. Storage iron is calculated as 15 mg/kg for patients below 35 kg. Storage iron is considered 500 mg for patients above 35 kg.
Ferric carboxymaltose and iron isomaltoside are two formulations that give the opportunity to apply higher doses of iron at a single time. A dose of 1000 mg of ferric carboxymaltose or iron isomaltoside can be infused with re‐evaluation after four weeks to determine the need for additional doses. Additional doses may be required, especially in patients with ongoing bleeding and inflammatory bowel disease.
For other formulations, the total amount of iron calculated is given at divided doses every 1 to 2 weeks until iron stores are replenished. Before each infusion, Hb, serum ferritin, and reticulocytes Hb are measured. Reassessment of the patient three months after the final infusion dose is recommended to evaluate for recurrence of ID/IDA.
Recommendation 3.b
The choice of an iron compound and the route of administration (oral vs. IV) largely depend on the presence and degree of anemia, underlying cause, clinical status (age, symptoms, long‐standing vs. recent onset, comorbidities), and, in some instances, patient preference. Traditionally, oral iron is administered at 100–200 daily in adults and 3‐6 mg/kg in children, in 2–3 divided doses, preferably without food. However, recent results indicate that lower doses (e.g., 60–80 mg) and every‐other‐day regimens have equivalent or even better iron absorption than daily dosing with fewer adverse events and increased tolerability. In anemic patients, oral iron should be continued until the Hb normalizes, which may take 6–12 weeks (depending on the severity of the anemia). After Hb restoration, oral supplements should be continued for at least three months to adequately replenish iron stores (with an ideal target of ferritin > 100 μg/L).
Intravenous IRT is recommended for specific indications such as intolerance or inadequate response to oral IRT; requirement of rapid iron replacement; inflammatory bowel disease; chronic kidney disease; chronic heart failure; in patients with intestinal malabsorption like allergic enteritis and atrophic gastritis; after bariatric surgery; in women with abnormal uterine bleeding; during the second or third trimester of pregnancy in women with IDA (only if highly necessary and strictly monitored); and in patients with IRIDA. It is important to evaluate the risk of allergic reactions, anaphylaxis, and shock. Due to the lack of safety data, women in the first trimester of pregnancy should be excluded from IV treatment.
-
✓
3.4 How to treat ID/IDA in infants, children, and adolescents?
The nutritional status must be assessed; indeed, prolonged exclusive breastfeeding in infants or the insufficient intake of iron‐rich foods considering the growth velocity at any age or menstruation and epistaxis in adolescent girls, vegan diet, and obesity may be the etiology bases of IDA. Moreover, low iron stores in the neonatal period may be due to a short gestation duration in the case of a preterm birth or a low birthweight, a maternal IDA, or an early cord clamping.
Transfusion with packed red blood cell transfusion is only reserved for severely symptomatic patients with cardiovascular compromise and for those who have Hb below 5 g/dL. Slow infusion (3–4 hours) of restrictive (4–5 mL/kg) transfusion should be followed with IRT.
Oral IRT is recommended as a first‐line treatment in infants, children, and adolescents. Ferrous sulfate or other iron salts at a dose of 3–6 mg elemental iron/kg/day is recommended. In adolescents, 65–130 mg of elemental iron, once daily, is suggested. There are limited data on the efficacy of alternate‐day use of oral iron salts in the pediatric age group. Oral iron salts should be given on an empty stomach; calcium‐containing food and drinks, such as milk and other dairy products, should not be taken with oral iron salts. Liquid iron salts may stain the teeth; the family must be warned about this. Rinsing the mouth and brushing the teeth after iron salt ingestion is recommended. Patients who are intolerant to GI effects may be advised to use alternate day dosing at lower doses; however, the data on the efficacy of this application in children and adolescents are limited.
A follow‐up visit with blood testing to assess the response is recommended. Patients with a Hb below 9 g/dL at diagnosis may have an earlier control of Hb by 2nd week of oral IRT initiation, and at least 1 g/dL of Hb rise is targeted to be considered as a responder at that time. It is usually difficult to catch the reticulocyte crisis which may be as early as 3rd day of treatment initiation and may vary in different patients; therefore, it is not recommended routinely. Oral IRT is recommended to be continued for at least 3 months. By the 3rd month of treatment initiation, hemogram analyses is recommended for a decision to stop iron. Serum ferritin may also be ordered on a healthy day to evaluate whether the iron stores were replenished.
Intravenous IRT is reserved for patients severely intolerant to oral IRT, malabsorption, inflammatory bowel disease, chronic kidney disease, and IRIDA. Iron sucrose is the most commonly preferred formulation (100 mg/infusion in children and 200 mg/infusion in adolescents) 119 ; other options include ferric gluconate, low‐molecular‐weight iron dextran, and ferric carboxymaltose. There are limited data on the use of ferric carboxymaltose in children. Only low‐molecular‐weight iron dextran requires a test dose. 120 Premedication is not recommended in any formulation unless the patient has asthma or a previous drug allergy history.
Potential causes of nonresponse or relapse ID include chronic inflammation, celiac disease, allergic enteritis, inflammatory bowel disorders, and menorrhagia in adolescent females. A rare genetic cause of IDA called IRIDA usually presents in childhood. Caution should be placed to not misdiagnose thalassemia carriers with IDA as both present with hypochromic microcytic anemia.
Finally, it is important to consider the potential usefulness of iron‐fortified formula in preventing iron deficiency in infants. A cross‐sectional observational study conducted in primary care pediatrician offices throughout France from 2016 to 2017 included consecutively infants aged 24 months for a food survey and blood sampling. Associations between consumption of iron‐fortified formula and serum ferritin (SF) levels were studied using multivariable regression after adjusting for sociodemographic, perinatal, and dietary characteristics, including other sources of dietary iron. The study revealed that the use of infant formulas was associated with a low prevalence of iron deficiency in infants aged 24 months. 121
-
✓
3.5 How to treat ID/IDA in pregnant women?
It is suggested by the American College of Obstetricians and Gynecologists and the Centers for Disease Control and Prevention that all pregnant women initiate oral iron supplementation to reduce the risk of ID/IDA during pregnancy. 122 Once daily or every other day, applications of single‐dose oral iron salts are recommended. Every other day dosing has been reported to increase iron absorption with higher tolerability to GI side effects in nonpregnant women. Oral iron salts are the only IRT formulations recommended during the first trimester of pregnancy. Due to a lack of safety data during the 1st trimester, intravenous iron formulations are only used during the 2nd or 3rd trimester. 74 Iron sucrose, low‐molecular‐weight iron dextran, ferric carboxymaltose, ferumoxytol, and iron isomaltoside could be used during these trimesters of pregnancy (doses are similar to those used in adults). Of note, European Medicines Agency currently recommend that all pregnant women with ID should be monitored while they are receiving IV iron because of the risk of fetal bradycardia. Intravenous iron should therefore not be used during pregnancy unless clearly necessary. Treatment should be confined to the second or third trimester, provided the benefits of treatment clearly outweigh the risks to the unborn baby. It also recommended further activities, including yearly reviews of allergic reaction reports and a study to confirm the safety of intravenous iron medicines.
There is no evidence‐based screening time for ID/IDA during pregnancy. Whenever IDA is diagnosed during pregnancy, oral IRT should be used for treatment in the first trimester (initial 14 weeks of gestation). For patients who were diagnosed with IDA during 2nd or 3rd trimesters, intravenous IRT is recommended. Four to 6 weeks after IRT initiation, testing for serum ferritin is recommended.
-
✓
3.6 How to treat ID/IDA in particular conditions? How to manage ID and IDA in the setting of patient blood management for major surgery?
In some conditions, there is a particular need to optimize patients' iron status to guarantee blood preservation. In the setting of major surgery, the risks of significant blood loss can be high. Allogenic blood transfusions (ABT) are commonly used as “life‐saving” measures but are frequently overused. Concerns about conserving patients' blood started with the introduction of the so‐called “bloodless surgery” to accommodate Jehovah's Witnesses request for treatment without ABT. 122 This concept evolved with generalized modalities to preserve all patients' blood, standing on three pillars: minimizing surgical and iatrogenic blood losses, managing coagulopathic bleeding, and focusing on diagnosing and timely treating anemia and ID. In 2005, Isbister introduced the term “patient blood management” (PBM), 123 recently defined as a “patient‐centered, systematic, evidence‐based approach to improve patient outcomes by managing and preserving patients' own blood”. 123 In 2017, the European Commission proposed PBM as a standard of care procedure. 124 In 2021, WHO released an awareness policy about the urgent need to implement PBM. 125 A set of clinical and research recommendations was then established through an international consensus. 126 There is still an unmet need to formally prove the long‐term clinical benefits or cost‐effectiveness of PBM. 127 Nevertheless, preoperative iron deficiency anemia (IDA) is common, is associated with poorer postoperative outcomes, and is a major predictive factor of peri‐operative ABT. 128 Good clinical practice dictates that the underlying cause of IDA should be diagnosed and treated appropriately. The main challenge in PBM is how to do it promptly, particularly when the interval between diagnosis and surgery is too short. Another challenge is the management of ID without anemia. While difficult to determine, it probably represents a much higher population than IDA, but many of those patients probably do not need ABT, although they may need PBM.
Recommendation 3.c
In the setting of major surgery, we recommend systematically screening for ID, offering oral iron before and after surgery in IDA patients, and considering intravenous iron for patients with IDA who cannot tolerate or absorb oral iron or if the interval between the diagnosis and surgery is too short for oral iron be effective. It is also recommended that larger, preferably multi‐institutional/multinational surveillance prospective studies be performed to further support PBM implementation.
AUTHOR CONTRIBUTIONS
Achille Iolascon, Immacolata Andolfo, Roberta Russo, Mayka Sanchez, Fabiana Busti, Dorine Swinkels, Patricia Aguilar Martinez, Rayan Bou‐Fakhredin, Martina U. Muckenthaler, Sule Unal, Graça Porto, Tomas Ganz, Antonis Kattamis, Lucia De Franceschi, Maria Domenica Cappellini, Malcolm G. Munro, and Ali Taher all took part in the data synthesis and writing of the recommendations.
CONFLICT OF INTEREST STATEMENT
Patricia Aguilar Martinez: Nothing to Disclose. Dorine Swinkels: Nothing to Disclose. Sule Unal: Nothing to Disclose. Fabiana Busti: Nothing to Disclose. Myka Sanchez: Co‐Founder of SME BLOODGENETICS SL (a genetic company www.bloodgenetics.com); Participation in 2 clinical trials one for IRIDA (KEROS THERAPEUTICS), one for atransferrinemia (SanquinBlood Netherland). Achille Iolascon: Nothing to Disclose. Ali Taher—Outside Work: Novartis Pharmaceuticals: Consultancy, Research funding; Bristol‐Myers Squibb (Celgene): Consultancy, Research funding; Ionis Pharmaceuticals: Consultancy, Research Funding; Vifor: Consultancy, Research Funding; Agios: Consultancy. Rayan Bou Fakhredin: Nothing to Disclose. Graça Porto: Nothing to Disclose. Immacolata Andolfo: Nothing to Disclose. Roberta Russo: Nothing to Disclose. Martina Muckenthaler Silence Therapeutics PLC, Editorial Board/Blood Journal/HemaSphere. Malcolm. G. Munro: Consultancies Pharmacosmos, Vifor, Daichi‐Sankyo, Shield Therapeutics, Myovant, Abbvie; Immediate Past Chair, FIGO Menstrual Disorders Committee. Tomas Ganz: shareholder and scientific advisor of Intrinsic LifeSciences, and consultant for Ionis Pharmaceuticals, Disc Medicine, Silence Therapeutics, Chugai, Vifor, Akebia, Dexcel, and Avidity Bio.
FUNDING
NextGenerationEU and Italian Ministry of Research PNRR ‐ National Center for gene therapy and Drugs based on RNA technology ‐ Spoke 1‐CN3 “Genetic Diseases” (E63C22000940007).
Contributor Information
Achille Iolascon, Email: achille.iolascon@unina.it.
Immacolata Andolfo, Email: immacolata.andolfo@unina.it.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
REFERENCES
- 1. Nemeth E, Ganz T. Hepcidin and iron in health and disease. Annu Rev Med. 2023;74:261‐277. 10.1146/annurev-med-043021-032816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123‐130. 10.1111/ejh.13892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koleini N, Shapiro JS, Geier J, Ardehali H. Ironing out mechanisms of iron homeostasis and disorders of iron deficiency. J Clin Invest. 2021;131:e148671. 10.1172/JCI148671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camaschella C. Iron‐deficiency anemia. N Engl J Med. 2015;372:1832‐1843. 10.1056/NEJMra1401038 [DOI] [PubMed] [Google Scholar]
- 5. Peyrin‐Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102:1585‐1594. 10.3945/ajcn.114.103366 [DOI] [PubMed] [Google Scholar]
- 6. Daru J, Colman K, Stanworth SJ, De La Salle B, Wood EM, Pasricha SR. Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr. 2017;106:1634S‐1639S. 10.3945/ajcn.117.155960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rohr M, Brandenburg V, Brunner‐La Rocca HP. How to diagnose iron deficiency in chronic disease: a review of current methods and potential marker for the outcome. Eur J Med Res. 2023;28:15. 10.1186/s40001-022-00922-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naveed K, Goldberg N, Shore E, et al. Defining ferritin clinical decision limits to improve diagnosis and treatment of iron deficiency: a modified Delphi study. Int J Lab Hematol. 2023;45:377‐386. 10.1111/ijlh.14016 [DOI] [PubMed] [Google Scholar]
- 9. Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45‐51. 10.1093/clinchem/44.1.45 [DOI] [PubMed] [Google Scholar]
- 10. Blackmore S, Hamilton M, Lee A, et al. Automated immunoassay methods for ferritin: recovery studies to assess traceability to an international standard. Clin Chem Lab Med. 2008;46:1450‐1457. 10.1515/CCLM.2008.304 [DOI] [PubMed] [Google Scholar]
- 11. Swinkels DW. Iron metabolism. In: Rifai, ed. Tietz Textbook of Laboratory Medicine, 7th ed. Tietz; 2022. Chapter 40.
- 12. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011‐1023. 10.1056/NEJMra041809 [DOI] [PubMed] [Google Scholar]
- 13. Girelli D, Marchi G, Camaschella C. Anemia in the elderly. HemaSphere . 2018;2:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood. 2018;131:505‐514. 10.1182/blood-2017-07-746446 [DOI] [PubMed] [Google Scholar]
- 15. Marques O, Weiss G, Muckenthaler MU. The role of iron in chronic inflammatory diseases: from mechanisms to treatment options in anemia of inflammation. Blood. 2022;140:2011‐2023. 10.1182/blood.2021013472 [DOI] [PubMed] [Google Scholar]
- 16. Ganz T. Anemia of inflammation. N Engl J Med. 2019;381:1148‐1157. 10.1056/NEJMra1804281 [DOI] [PubMed] [Google Scholar]
- 17. Pasricha SR, Tye‐Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. 2021;397:233‐248. 10.1016/S0140-6736(20)32594-0 [DOI] [PubMed] [Google Scholar]
- 18. Williams AM, Ladva CN, Leon JS, et al. Changes in micronutrient and inflammation serum biomarker concentrations after a norovirus human challenge. Am J Clin Nutr. 2019;110:1456‐1464. 10.1093/ajcn/nqz201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop‐Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta‐analysis. Am J Clin Nutr. 2010;92:546‐555. 10.3945/ajcn.2010.29284 [DOI] [PubMed] [Google Scholar]
- 20. Darton TC, Blohmke CJ, Giannoulatou E, et al. Rapidly escalating hepcidin and associated serum iron starvation are features of the acute response to typhoid infection in humans. PLoS Neglected Trop Dis. 2015;9:e0004029. 10.1371/journal.pntd.0004029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suchdev PS, Williams AM, Mei Z, et al. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am J Clin Nutr. 2017;106:1626S‐1633S. 10.3945/ajcn.117.155937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Porto G, Vicente C, Fraga J, da Silva BM, de Sousa M. Importance of establishing appropriate local reference values for the screening of hemochromatosis: a study of three different control populations and 136 hemochromatosis family members. Hemochromatosis Clinical and Research Group. J Lab Clin Med. 1992;119:295‐305. [PubMed] [Google Scholar]
- 23. Infusino I, Braga F, Dolci A, Panteghini M. Soluble transferrin receptor (sTfR) and sTfR/log ferritin index for the diagnosis of iron‐deficiency anemia. A meta‐analysis. Am J Clin Path. 2012;138:642‐649. 10.1309/AJCP16NTXZLZFAIB [DOI] [PubMed] [Google Scholar]
- 24. Thorpe SJ, Heath A, Sharp G, Cook J, Ellis R, Worwood M. A WHO reference reagent for the serum transferrin receptor (sTfR): international collaborative study to evaluate a recombinant soluble transferrin receptor preparation. Clin Chem Lab Med. 2010;48:815‐820. 10.1515/CCLM.2010.167 [DOI] [PubMed] [Google Scholar]
- 25. Piva E, Brugnara C, Spolaore F, Plebani M. Clinical utility of reticulocyte parameters. Clin Lab Med. 2015;35:133‐163. 10.1016/j.cll.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 26. Urrechaga E, Boveda O, Aguayo FJ, et al. Percentage of hypochromic erythrocytes and reticulocyte hemoglobin equivalent predictors of response to intravenous iron in hemodialysis patients. Int J Lab Hematol. 2016;38:360‐365. 10.1111/ijlh.12496 [DOI] [PubMed] [Google Scholar]
- 27. Ullrich C, Wu A, Armsby C, et al. Screening healthy infants for iron deficiency using reticulocyte hemoglobin content. JAMA. 2005;294:924‐930. 10.1001/jama.294.8.924 [DOI] [PubMed] [Google Scholar]
- 28. Thomas DW, Hinchliffe RF, Briggs C, Macdougall IC, Littlewood T, Cavill I. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161:639‐648. 10.1111/bjh.12311 [DOI] [PubMed] [Google Scholar]
- 29. van Santen S, de Mast Q, Oosting JD, van Ede A, Swinkels DW, van der Ven AJAM. Hematologic parameters predicting a response to oral iron therapy in chronic inflammation. Haematologica. 2014;99:e171‐e173. 10.3324/haematol.2014.106799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016;127:2809‐2813. 10.1182/blood-2015-12-639112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict non responsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol. 2013;88:97‐101. 10.1002/ajh.23354 [DOI] [PubMed] [Google Scholar]
- 32. van der Staaij H, Donker AE, Bakkeren DL, et al. Transferrin saturation/hepcidin ratio discriminates TMPRSS6‐related iron refractory iron deficiency anemia from patients with multi‐causal iron deficiency anemia. Int J Mol Sci. 2022;23:1917. 10.3390/ijms23031917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heeney MM, Guo D, De Falco L, et al. Normalizing hepcidin predicts TMPRSS6 mutation status in patients with chronic iron deficiency. Blood. 2018;132:448‐452. 10.1182/blood-2017-03-773028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diepeveen LE, Laarakkers CMM, Martos G, et al. Provisional standardization of hepcidin assays: creating a traceability chain with a primary reference material, candidate reference method and a commutable secondary reference material. Clin Chem Lab Med. 2019;57:864‐872. 10.1515/cclm-2018-0783 [DOI] [PubMed] [Google Scholar]
- 35. Al‐Toma A, Volta U, Auricchio R, et al. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten‐related disorders. United European Gastroenterol J. 2019;7:583‐613. 10.1177/2050640619844125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Milman N. Postpartum anemia I: definition, prevalence, causes, and consequences. Ann Hematol. 2011;90:1247‐1253. 10.1007/s00277-011-1279-z [DOI] [PubMed] [Google Scholar]
- 37. Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309‐1316. 10.1136/gut.2010.228874 [DOI] [PubMed] [Google Scholar]
- 38. Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW. Prevalence and risk factors for early postpartum anemia. Eur J Obstet Gynecol Reprod Biol. 2010;150:126‐131. 10.1016/j.ejogrb.2010.02.030 [DOI] [PubMed] [Google Scholar]
- 39. Breymann C, Auerbach M. Iron deficiency in gynecology and obstetrics: clinical implications and management. Hematology. 2017;2017:152‐159. 10.1182/asheducation-2017.1.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fraser IS, Mansour D, Breymann C, Hoffman C, Mezzacasa A, Petraglia F. Prevalence of heavy menstrual bleeding and experiences of affected women in a European patient survey. Int J Gynecol Obstet. 2015;128:196‐200. 10.1016/j.ijgo.2014.09.027 [DOI] [PubMed] [Google Scholar]
- 41. Schoep ME, Nieboer TE, van der Zanden M, Braat DDM, Nap AW. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol. 2019;220:569.e1‐569.e7. 10.1016/j.ajog.2019.02.048 [DOI] [PubMed] [Google Scholar]
- 42. Henry C, Ekeroma A, Filoche S. Barriers to seeking consultation for abnormal uterine bleeding: systematic review of qualitative research. BMC Womens Health. 2020;20:123. 10.1186/s12905-020-00986-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Henry C, Filoche S. Reflections on access to care for heavy menstrual bleeding: past, present, and in times of the COVID‐19 pandemic. Int J Gynecol Obstet. 2023;162:23‐28. 10.1002/ijgo.14945 [DOI] [PubMed] [Google Scholar]
- 44. Sinharoy SS, Chery L, Patrick M, et al. Prevalence of heavy menstrual bleeding and associations with physical health and wellbeing in low‐income and middle‐income countries: a multinational cross‐sectional study. Lancet Global Health. 2023;11:e1775‐e1784. 10.1016/S2214-109X(23)00416-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peuranpää P, Heliövaara‐Peippo S, Fraser I, Paavonen J, Hurskainen R. Effects of anemia and iron deficiency on quality of life in women with heavy menstrual bleeding. Acta Obstet Gynecol Scand. 2014;93:654‐660. 10.1111/aogs.12394 [DOI] [PubMed] [Google Scholar]
- 46. Jain V, Munro MG, Critchley HOD. Contemporary evaluation of women and girls with abnormal uterine bleeding: FIGO Systems 1 and 2. Int J Gynecol Obstet. 2023;162:29‐42. 10.1002/ijgo.14946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. MacGregor B, Munro MG, Lumsden MA. Therapeutic options for the management of abnormal uterine bleeding. Int J Gynecol Obstet. 2023;162:43‐57. 10.1002/ijgo.14947 [DOI] [PubMed] [Google Scholar]
- 48. Clénin G, Cordes M, Huber A, et al. Iron deficiency in sports ‐ definition, influence on performance and therapy. Swiss Med Wkly. 2015;145:w14196. 10.4414/smw.2015.14196 [DOI] [PubMed] [Google Scholar]
- 49. Al‐Naseem A, Sallam A, Choudhury S, Thachil J. Iron deficiency without anaemia: a diagnosis that matters. Clin Med. 2021;21:107‐113. 10.7861/clinmed.2020-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non‐anaemic iron‐deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8:e019240. 10.1136/bmjopen-2017-019240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen Z, Yang H, Wang D, et al. Effect of oral iron supplementation on cognitive function among children and adolescents in low‐ and middle‐income countries: a systematic review and meta‐analysis. Nutrients. 2022;14:5332. 10.3390/nu14245332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miles LF, Litton E, Imberger G, Story D. Intravenous iron therapy for non‐anaemic, iron‐deficient adults. Cochrane Database Syst Rev. 2019;2019:CD013084. 10.1002/14651858.CD013084.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rizzo C, Carbonara R, Ruggieri R, Passantino A, Scrutinio D. Iron deficiency: a new target for patients with heart failure. Front Cardiovasc Med. 2021;8:709872. 10.3389/fcvm.2021.709872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cappellini MD, Comin‐Colet J, de Francisco A, et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am J Hematol. 2017;92:1068‐1078. 10.1002/ajh.24820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cappellini MD, Musallam KM, Taher AT. Iron deficiency anaemia revisited. J Intern Med. 2020;287:153‐170. 10.1111/joim.13004 [DOI] [PubMed] [Google Scholar]
- 56. Falkingham M, Abdelhamid A, Curtis P, Fairweather‐Tait S, Dye L, Hooper L. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta‐analysis. Nutr J. 2010;9:4. 10.1186/1475-2891-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Andro M, Le Squere P, Estivin S, Gentric A. Anaemia and cognitive performances in the elderly: a systematic review. Eur J Neurol. 2013;20:1234‐1240. 10.1111/ene.12175 [DOI] [PubMed] [Google Scholar]
- 58. Halawi R, Moukhadder H, Taher A. Anemia in the elderly: a consequence of aging? Expert Rev Hematol. 2017;10:327‐335. 10.1080/17474086.2017.1285695 [DOI] [PubMed] [Google Scholar]
- 59. Gingoyon A, Borkhoff CM, Koroshegyi C, et al. Chronic iron deficiency and cognitive function in early childhood. Pediatrics. 2022;150:e2021055926. 10.1542/peds.2021-055926 [DOI] [PubMed] [Google Scholar]
- 60. Manceau H, Ausseil J, Masson D, et al. Neglected comorbidity of chronic heart failure: iron deficiency. Nutrients. 2022;14(15):3214. 10.3390/nu14153214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Siddiqui SW, Ashok T, Patni N, Fatima M, Lamis A, Anne KK. Anemia and heart failure: a narrative review. Cureus. 2022;14:e27167. 10.7759/cureus.27167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lam CSP, Doehner W, Comin‐Colet J. Iron deficiency in chronic heart failure: case‐based practical guidance. ESC Heart Failure. 2018;5:764‐771. 10.1002/ehf2.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Savarese G, von Haehling S, Butler J, Cleland JGF, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Eur Heart J. 2023;44(1):14‐27. 10.1093/eurheartj/ehac569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shah Y, Patel D, Khan N. Iron deficiency anemia in IBD: an overlooked comorbidity. Expert ev Gastroenterol Hepatol. 2021;15:771‐781. 10.1080/17474124.2021.1900730 [DOI] [PubMed] [Google Scholar]
- 65. Maas LA, Krishna M, Parian AM. Ironing it all out: a comprehensive review of iron deficiency anemia in inflammatory bowel disease patients. Dig Dis Sci. 2022;68:357‐369. 10.1007/s10620-022-07599-1 [DOI] [PubMed] [Google Scholar]
- 66. Alnuwaysir RIS, Grote Beverborg N, Hoes MF, et al. Additional burden of iron deficiency in heart failure patients beyond the cardio‐renal anaemia syndrome: findings from the BIOSTAT‐CHF study. Eur J Heart Fail. 2022;24:192‐204. 10.1002/ejhf.2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guedes M, Muenz D, Zee J, et al. Serum biomarkers of iron stores are associated with worse physical health‐related quality of life in nondialysis‐dependent chronic kidney disease patients with or without anemia. Nephrol Dial Transplant. 2021;36:1694‐1703. 10.1093/ndt/gfab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841‐3846. 10.1182/blood-2005-10-4308 [DOI] [PubMed] [Google Scholar]
- 69. James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663‐674. 10.1097/AOG.0000000000004559 [DOI] [PubMed] [Google Scholar]
- 70. Igbinosa I, Berube C, Lyell DJ. Iron deficiency anemia in pregnancy. Curr Opin Obstet Gynecol. 2022;34:69‐76. 10.1097/GCO.0000000000000772 [DOI] [PubMed] [Google Scholar]
- 71. Jung J, Rahman MM, Rahman MS, et al. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: a systematic review and meta‐analysis. Ann NY Acad Sci. 2019;1450:69‐82. 10.1111/nyas.14112 [DOI] [PubMed] [Google Scholar]
- 72. Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru‐Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799‐2806. 10.1111/trf.13252 [DOI] [PubMed] [Google Scholar]
- 73. Obuchowska A, Standyło A, Obuchowska K, Gorczyca K, Kimber‐Trojnar Ż, Leszczyńska‐Gorzelak B. Iron deficiency anemia in pregnancy: evaluation and management. J Educ Health Sport. 2022;12:168‐174. 10.12775/JEHS.2022.12.09.021 [DOI] [Google Scholar]
- 74. Benson AE, Shatzel JJ, Ryan KS, et al. The incidence, complications, and treatment of iron deficiency in pregnancy. Eur J Haematol. 2022;109:633‐642. 10.1111/ejh.13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Faysal H, Araji T, Ahmadzia HK. Recognizing who is at risk for postpartum hemorrhage: targeting anemic women and scoring systems for clinical use. Am J Obstet Gynecol. 2023;5:100745. 10.1016/j.ajogmf.2022.100745 [DOI] [PubMed] [Google Scholar]
- 76. Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita ATN. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. Am J Obstet Gynecol. 2013;209:P51.E1‐51.E6. 10.1016/j.ajog.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Briley A, Seed P, Tydeman G, et al. Reporting errors, incidence and risk factors for postpartum haemorrhage and progression to severe PPH: a prospective observational study. BJOG Int J Obstet Gynaecol. 2014;121:876‐888. 10.1111/1471-0528.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tort J, Rozenberg P, Traoré M, Fournier P, Dumont A. Factors associated with postpartum hemorrhage maternal death in referral hospitals in Senegal and Mali: a cross‐sectional epidemiological survey. BMC Pregnancy Childbirth. 2015;15:235. 10.1186/s12884-015-0669-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Means RT. Iron deficiency and iron deficiency anemia: implications and impact in pregnancy, fetal development, and early childhood parameters. Nutrients. 2020;12:447. 10.3390/nu12020447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McArdle HJ, Gambling L, Kennedy C. Iron deficiency during pregnancy: the consequences for placental function and fetal outcome. Proc Nutr Soc. 2014;73:9‐15. 10.1017/S0029665113003637 [DOI] [PubMed] [Google Scholar]
- 81. Kemppinen L, Mattila M, Ekholm E, et al. Gestational iron deficiency anemia is associated with preterm birth, fetal growth restriction, and postpartum infections. J Perinat Med. 2021;49:431‐438. 10.1515/jpm-2020-0379 [DOI] [PubMed] [Google Scholar]
- 82. Tran TD, Biggs BA, Tran T, et al. Impact on infants' cognitive development of antenatal exposure to iron deficiency disorder and common mental disorders. PLoS One. 2013;8:e74876. 10.1371/journal.pone.0074876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Beard JL, Hendricks MK, Perez EM, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135:267‐272. 10.1093/jn/135.2.267 [DOI] [PubMed] [Google Scholar]
- 84. Milman N. Postpartum anemia II: prevention and treatment. Ann Hematol. 2012;91:143‐154. 10.1007/s00277-011-1381-2 [DOI] [PubMed] [Google Scholar]
- 85. Kemppinen L, Mattila M, Ekholm E, et al. Gestational anemia and maternal antenatal and postpartum psychological distress in a prospective FinnBrain Birth Cohort Study. BMC Pregnancy Childbirth. 2022;22:704. 10.1186/s12884-022-05032-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Corwin EJ, Murray‐Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133:4139‐4142. 10.1093/jn/133.12.4139 [DOI] [PubMed] [Google Scholar]
- 87. Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168:344‐361. 10.1016/j.cell.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tolkien Z, Stecher L, Mander AP, Pereira DIA, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side‐effects in adults: a systematic review and meta‐analysis. PLoS One. 2015;10:e0117383. 10.1371/journal.pone.0117383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pergola PE, Fishbane S, Ganz T. Novel oral iron therapies for iron deficiency anemia in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:272‐291. 10.1053/j.ackd.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 90. Pereira DIA, Mohammed NI, Ofordile O, et al. A novel nano‐iron supplement to safely combat iron deficiency and anaemia in young children: the IHAT‐GUT double‐blind, randomised, placebo‐controlled trial protocol. Gates Open Res. 2018;2:48. 10.12688/gatesopenres.12866.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Girelli D, Ugolini S, Busti F, Marchi G, Castagna A. Modern iron replacement therapy: clinical and pathophysiological insights. Int J Hematol. 2018;107:16‐30. 10.1007/s12185-017-2373-3 [DOI] [PubMed] [Google Scholar]
- 92. Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter‐Gvili A. The safety of intravenous iron preparations. Mayo Clin Proc. 2015;90:12‐23. 10.1016/j.mayocp.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 93. Vandemergel X, Vandergheynst F. Potentially life‐threatening phosphate diabetes induced by ferric carboxymaltose injection: a case report and review of the literature. Case Rep Endocrinol. 2014;2014:843689. 10.1155/2014/843689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Klein K, Asaad S, Econs M, Rubin JE. Severe FGF23‐based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018:2018;2018:bcr2017222851. 10.1136/bcr-2017-222851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Clevenger B, Gurusamy K, Klein AA, Murphy GJ, Anker SD, Richards T. Systematic review and meta‐analysis of iron therapy in anaemic adults without chronic kidney disease: updated and abridged Cochrane review. Eur J Heart Fail. 2016;18:774‐785. 10.1002/ejhf.514 [DOI] [PubMed] [Google Scholar]
- 96. Osman M, Syed M, Balla S, Kheiri B, Faisaluddin M, Bianco C. A meta‐analysis of intravenous iron therapy for patients with iron deficiency and heart failure. Am J Cardiol. 2021;141:152‐153. 10.1016/j.amjcard.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kaundal R, Bhatia P, Jain A, et al. Randomized controlled trial of twice‐daily versus alternate‐day oral iron therapy in the treatment of iron‐deficiency anemia. Ann Hematol. 2020;99:57‐63. 10.1007/s00277-019-03871-z [DOI] [PubMed] [Google Scholar]
- 98. Li N, Zhao G, Wu W, et al. The efficacy and safety of vitamin C for iron supplementation in adult patients with iron deficiency anemia: a randomized clinical trial. JAMA Network Open. 2020;3:e2023644. 10.1001/jamanetworkopen.2020.23644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Snook J, Bhala N, Beales ILP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021;70:2030‐2051. 10.1136/gutjnl-2021-325210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Okam MM, Koch TA, Tran M‐H. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101:e6‐e7. 10.3324/haematol.2015.129114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Okam MM, Koch TA, Tran M‐H. Iron supplementation, response in iron‐deficiency anemia: analysis of five trials. Am J Med. 2017;130:991.e1‐991.e8 10.1016/j.amjmed.2017.03.045 [DOI] [PubMed] [Google Scholar]
- 102. Lopez A, Cacoub P, Macdougall IC, Peyrin‐Biroulet L. Iron deficiency anaemia. The Lancet. 2016;387:907‐916. 10.1016/S0140-6736(15)60865-0 [DOI] [PubMed] [Google Scholar]
- 103. Parodi E, Giraudo MT, Ricceri F, Aurucci ML, Mazzone R, Ramenghi U. Absolute reticulocyte count and reticulocyte hemoglobin content as predictors of early response to exclusive oral iron in children with iron deficiency anemia. Anemia. 2016;2016:7345835. 10.1155/2016/7345835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Auerbach M, Adamson J, Bircher A, et al. On the safety of intravenous iron, evidence trumps conjecture. Haematologica. 2015;100:e214‐e215. 10.3324/haematol.2014.121004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang C, Graham DJ, Kane RC, et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA. 2015;314:2062‐2068. 10.1001/jama.2015.15572 [DOI] [PubMed] [Google Scholar]
- 106. Muñoz M, Gómez‐Ramírez S, Bhandari S. The safety of available treatment options for iron‐deficiency anemia. Expert Opin Drug Saf. 2018;17:149‐159. 10.1080/14740338.2018.1400009 [DOI] [PubMed] [Google Scholar]
- 107. Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017;31:225‐233. 10.1016/j.blre.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 108. Schrier SL. So you know how to treat iron deficiency anemia. Blood. 2015;126:1971. 10.1182/blood-2015-09-666511 [DOI] [PubMed] [Google Scholar]
- 109. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice‐daily doses in iron‐depleted young women. Blood. 2015;126:1981‐1989. 10.1182/blood-2015-05-642223 [DOI] [PubMed] [Google Scholar]
- 110. Cook J, Reddy M. Efficacy of weekly compared with daily iron supplementation. Am J Clin Nutr. 1995;62:117‐120. 10.1093/ajcn/62.1.117 [DOI] [PubMed] [Google Scholar]
- 111. Ning S, Zeller MP. Management of iron deficiency. Hematology. 2019;2019:315‐322. 10.1182/hematology.2019000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Avni T, Bieber A, Steinmetz T, Leibovici L, Gafter‐Gvili A. Treatment of anemia in inflammatory bowel disease–systematic review and meta‐analysis. PLoS One. 2013;8:e75540. 10.1371/journal.pone.0075540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ratcliffe LEK, Thomas W, Glen J, et al. Diagnosis and management of iron deficiency in CKD: a summary of the NICE guideline recommendations and their rationale. Am J Kidney Dis. 2016;67:548‐558. 10.1053/j.ajkd.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 114. Macdougall IC, Bock A, Carrera F, et al. The FIND‐CKD study—a randomized controlled trial of intravenous iron versus oral iron in non‐dialysis chronic kidney disease patients: background and rationale. Nephrol Dial Transplant. 2014;29:843‐850. 10.1093/ndt/gft424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Auerbach M, Muñoz M, Macdougall IC. Intravenous iron: out of sight, out of mind. Lancet Haematol. 2018;5:e10‐e12. 10.1016/S2352-3026(17)30230-2 [DOI] [PubMed] [Google Scholar]
- 116. Auerbach M, Deloughery T. Single‐dose intravenous iron for iron deficiency: a new paradigm. Hematology. 2016;2016:57‐66. 10.1182/asheducation-2016.1.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ganzoni AM. Intravenous iron‐dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr. 1970;100:301‐303. [PubMed] [Google Scholar]
- 118. Koch TA, Myers J, Goodnough LT. Intravenous iron therapy in patients with iron deficiency anemia: dosing considerations. Anemia. 2015;2015:1‐10. 10.1155/2015/763576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Crary SE, Hall K, Buchanan GR. Intravenous iron sucrose for children with iron deficiency failing to respond to oral iron therapy. Pediatr Blood Cancer. 2011;56:615‐619. 10.1002/pbc.22930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Plummer ES, Crary SE, McCavit TL, Buchanan GR. Intravenous low molecular weight iron dextran in children with iron deficiency anemia unresponsive to oral iron. Pediatr Blood Cancer. 2013;60:1747‐1752. 10.1002/pbc.24676 [DOI] [PubMed] [Google Scholar]
- 121. Sacri AS, Bocquet A, de Montalembert M, et al. Young children formula consumption and iron deficiency at 24 months in the general population: a national‐level study. Clin Nutr. 2021;40:166‐173. 10.1016/j.clnu.2020.04.041 [DOI] [PubMed] [Google Scholar]
- 122. Anemia in Pregnancy . ACOG practice bulletin, number 233. Obstet Gynecol. 2021;138:e55 ‐ e64. 10.1097/AOG.0000000000004477 [DOI] [PubMed] [Google Scholar]
- 123. Shander A, Hardy JF, Ozawa S, et al. A global definition of patient blood management. Anesth Analg. 2022;135:476‐488. 10.1213/ANE.0000000000005873 [DOI] [PubMed] [Google Scholar]
- 124. Gombotz H, Kastner P, Nørgaard A, et al. European Commission, Consumers, Health, Agriculture and Food Executive Agency, Supporting Patient Blood Management (PBM) in the EU: a practical implementation guide for hospitals, Publications Office, 2017.
- 125. World Health Organization . The urgent need to implement patient blood management: policy brief. WHO; 2021. [Google Scholar]
- 126. Mueller MM, Van Remoortel H, Meybohm P, et al. Patient Blood Management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA. 2019;321:983‐997. 10.1001/jama.2019.0554 [DOI] [PubMed] [Google Scholar]
- 127. Roman MA, Abbasciano RG, Pathak S, et al. Patient blood management interventions do not lead to important clinical benefits or cost‐effectiveness for major surgery: a network meta‐analysis. Br J Anaesth. 2021;126:149‐156. 10.1016/j.bja.2020.04.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Muñoz M, Gómez‐Ramírez S, Campos A, Ruiz J, Liumbruno GM. Pre‐operative anaemia: prevalence, consequences and approaches to management. Blood Transfusion. 2015;13:370‐379. 10.2450/2015.0014-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.


