Abstract
In addition to the CCR5 and CXCR4 chemokine receptors, a subset of primary human immunodeficiency virus type 1 (HIV-1) isolates can also use the seven-transmembrane-domain receptor APJ as a coreceptor. A previously identified ligand of APJ, apelin, specifically inhibited the entry of primary T-tropic and dualtropic HIV-1 isolates from different clades into cells expressing CD4 and APJ. Analysis of apelin analogues demonstrated that potent and specific antiviral activity was retained by a 13-residue, arginine-rich peptide. Antiviral potency was influenced by the integrity of methionine 75, which contributes to APJ-binding affinity, and by the retention of apelin residues 63 to 65. These studies demonstrate the ability of a small peptide ligand to block the function of APJ as an HIV-1 coreceptor, identify apelin sequences important for the inhibition, and provide new reagents for the investigation of the significance of APJ to HIV-1 infection and pathogenesis.
Infection of humans with human immunodeficiency virus type 1 (HIV-1) results in the depletion of host CD4+ T lymphocytes, culminating in AIDS (3, 9, 21, 23). HIV-1 infection of target cells requires the sequential binding of envelope glycoproteins to CD4 and a seven-transmembrane-domain receptor (7TMR). The major 7TMRs utilized by primary HIV-1 viruses as coreceptors are the chemokine receptors CCR5 and CXCR4 (1, 8, 11, 14, 15, 22). Macrophage-tropic (M-tropic) or R5 viruses primarily use CCR5 and exhibit a non-syncytium-inducing (NSI) phenotype (1, 8, 11, 14, 15); in contrast, T-cell-line-tropic (T-tropic) or X4 viruses use CXCR4 and exhibit a syncytium-inducing (SI) phenotype in T-cell lines (22). Dualtropic or R5/X4 viruses can use both CCR5 and CXCR4. R5 viruses account for most of the cases of horizontal and vertical transmission and are recovered early in the course of disease. In subsequent years after infection, both X4 and R5/X4 viruses are recovered in approximately 50% of HIV-1-infected individuals (6, 10). The emergence of R5/X4 or X4 viruses is usually correlated with rapid progression to AIDS (6, 10, 29, 39).
All primary HIV-1 strains studied to date use CCR5, CXCR4, or both molecules as a coreceptor; however, a subset of primary HIV-1 viruses can use other 7TMRs as alternative coreceptors. Nine alternative coreceptors have been demonstrated to support HIV-1 infection, albeit not as efficiently as CCR5 and CXCR4 (4, 5, 12, 13, 17, 32, 35). In certain individuals, disease progression is associated with expansion of coreceptor usage to alternative coreceptors in vitro (6, 10, 13, 32). However, the role of alternative coreceptors in AIDS or other sequelae of HIV-1 infection is unknown. Alternative coreceptors could potentially play an important role in HIV-1 pathogenesis, depending on their level of expression and tissue distribution, as well as how broadly and efficiently they support viral entry. For example, the alternative coreceptor CCR3 is postulated to play a role in central nervous system (CNS) infection in patients with HIV-1-induced dementia. CCR3 is expressed in the microglia, which are the major target cells of HIV-1 in the CNS, and can facilitate HIV-1 infection of these cells (26).
The alternative coreceptor APJ might also play a role in HIV-1 neuropathogenesis. APJ, a homologue of the angiotensin receptor, is a 7TMR widely expressed in the brain (33, 34). APJ mRNA was detected in glial cells, astrocytes, and neuronal subpopulations, as well as in activated peripheral blood mononuclear cells (PBMC), the spleen, and the thymus (7, 17, 33, 36). APJ is used efficiently by several primary R5, X4, and dualtropic (R5/X4) HIV-1 and simian immunodeficiency viruses (7, 17, 40, 43). Some brain-derived viruses have also been reported to use APJ as a coreceptor (1, 38). Taken together, these observations suggest that APJ might contribute to HIV-1 pathogenesis, especially in the development of CNS disease. In addition, its frequent use by dualtropic HIV-1 isolates and its sequence similarity to both CCR5 and CXCR4 raise the possibility that APJ might facilitate the adaptation of R5 viruses to CXCR4 (7, 18).
The identification of specific inhibitors of APJ-mediated HIV-1 entry would greatly assist efforts to understand the in vivo role of APJ. A novel technique was employed to isolate apelin, the ligand of the human APJ receptor, from bovine stomach tissue extracts (28, 41). Northern and in situ hybridization analyses revealed coexpression of human and rat apelin and APJ in the brain (30). Apelin was also detected in a variety of tissues, including the mammary gland, and in colostrum and breast milk (25, 30). The human and bovine apelin cDNA encodes a preprotein consisting of 77 amino acids (Fig. 1). Amino acid sequence analysis showed that the sequence of the apelin peptide extracted from bovine stomach tissue was encoded in the C-terminal region of the preprotein. Based on this observation, a 36-amino-acid apelin peptide (apelin-36), which includes most of the C-terminal portion, was predicted to comprise the mature form. When synthesized, apelin-36, as well as some shorter derivatives, promoted extracellular acidification in Chinese hamster ovary (CHO) cells expressing the APJ receptor but not in parental CHO cells, suggesting that apelin is an endogenous ligand for APJ (41).
FIG. 1.
Amino acid sequence of human preproapelin and the synthetic apelins. The arrowhead indicates the predicted cleavage site of a putative signal peptide. [<Glu65]apelin-13, apelin-15, apelin-36 were synthesized using an automatic peptide synthesizer (model 430; PE Biosystems) as described previously (41).
The elucidation of the biologic role of CCR5 and CXCR4, as well as their role as major coreceptors of HIV-1, was aided by the identification of their natural ligands. With the discovery of apelin as the natural ligand of the HIV-1 coreceptor APJ, we can now study the biologic role of APJ, as well as its role in mediating HIV-1 infection. In the work reported here, we investigate whether apelin can inhibit the entry of primary HIV-1 viruses mediated by the APJ coreceptor. We also study the structural features of apelin analogues that contribute to anti-HIV-1 activity.
To test the anti-HIV-1 activity of apelin, we used an env-complementation assay, in which HIV-1 envelope glycoproteins expressed in trans complement a single round of replication of an env-deleted provirus expressing the chloramphenicol acetyltransferase (CAT) gene (8, 27). We generated recombinant viruses by pseudotyping HIV-1 expressing the CAT reporter gene with the envelope glycoproteins of the primary R5/X4 HIV-1 clade B isolate, 89.6. The HIV-1 isolate 89.6 was used because it was previously shown to use APJ, in addition to CCR2b, CCR3, CCR5, and CXCR4, as a coreceptor (7, 8, 14). As target cells, we used the canine thymocyte cell line, Cf2Th, which is not susceptible to HIV-1 infection unless transfected with plasmids expressing human CD4 and chemokine receptors specific for particular viral strains (8). Previously, we showed that Cf2Th cells expressing both human CD4 and human APJ allowed entry of recombinant viruses expressing envelope glycoproteins from the 89.6 strain and from several other primary HIV-1 isolates (7). Prior to infection, synthetic apelins (Fig. 1) shown previously to induce acidification of APJ-expressing cells (41) were added to CD4+ Cf2Th target cells expressing APJ. The efficiency of infection was determined by measuring the CAT activity in the target cells 3 days after infection.
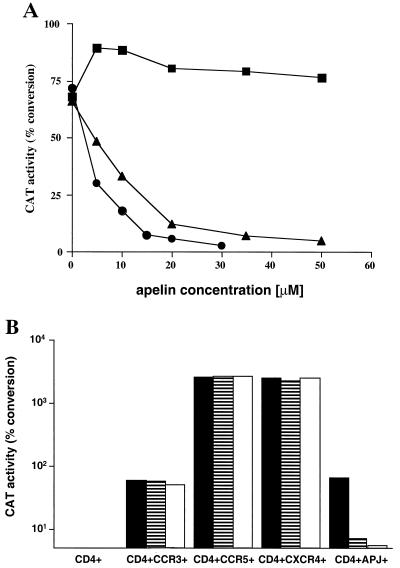
The results in Fig. 2 show that two of the synthetic apelins, apelin-15 and apelin-36, but not [<Glu65]apelin-13, inhibited entry of the primary R5/X4 89.6 recombinant virus in a dose-dependent manner. Apelin-36 (50% inhibitory concentration [IC50] = 7 μM) was a more potent inhibitor of HIV-1 entry than apelin-15 (IC50 = 12 μM) (Fig. 2A). [<Glu65]apelin-13 did not inhibit HIV-1 entry, even though this peptide, like apelin-15 and apelin-36, is potent in inducing extracellular acidification in APJ-expressing CHO cells (41). The data shown in Fig. 2A suggest that the basic amino acid stretch (R63R64Q65) in the N-terminal end of apelin-15 is critical for inhibiting HIV-1 entry.
FIG. 2.
Inhibition of HIV-1 viruses containing 89.6 envelope glycoproteins by apelins. (A) Apelin inhibits HIV-1 entry into cells expressing human CD4 and human APJ. Cf2Th-CD4 cells were transfected with plasmids expressing APJ by the calcium phosphate method (8). Transfectants were replated in six-well plates at 2 × 105 per well and incubated with either [<Glu65]apelin-13 (■), apelin-15 (▴), or apelin-36 (●) at the indicated concentrations for 1 h prior to incubation with recombinant HIV-1 containing the envelope glycoproteins of the primary R5/X4 89.6 HIV-1 clade B isolate. The recombinant virus containing the CAT reporter was produced by cotransfection of the pSVIIIenv plasmid expressing the envelope glycoproteins of interest with the pHXBH10ΔenvCAT plasmid into 293T cells. Approximately 40,000 cpm of the reverse transcriptase (RT) activity of the recombinant virus was used to infect the target cells. The CAT activity, calculated as a percentage conversion of chloramphenicol to acetylated forms in target cell lysates, is shown. All values have been normalized to a given amount of lysate. The results shown are representative of two independent experiments. (B) Apelin preferentially inhibits HIV-1 entry mediated by APJ. Cf2Th-CD4 cells transfected with plasmids expressing CCR3, CCR5, CXCR4 or APJ were mock treated (■) or were incubated with either 35 μM apelin-15 (▤) or 20 μM apelin-36 (□) for 1 h prior to addition of 40,000 cpm (RT activity) of recombinant HIV-1 virus containing envelope glycoproteins of the primary R5/X4 89.6 HIV-1 clade B isolate. The CAT activity is indicated as the percent conversion of chloramphenicol to acetylated forms for a given amount of lysate. The figure shown is representative of two independent experiments.
To examine the specificity of the anti-HIV-1 activity of the apelins, we tested the ability of the apelins to inhibit the infection of cells expressing other HIV-1 coreceptors such as CCR3, CCR5, and CXCR4. Both apelin-15 and apelin-36, when used at concentrations of 35 and 20 μM, respectively, dramatically inhibited the infection of APJ-expressing cells by the 89.6 HIV-1 recombinant virus (Fig. 2B). The same concentrations of apelins did not significantly inhibit the entry of the 89.6 HIV-1 recombinant virus into CD4+ Cf2Th-expressing CCR3, CCR5, or CXCR4. No infection was detected in the control Cf2Th cells expressing CD4 alone (Fig. 2B). These results suggest that apelin-15 and apelin-36 specifically inhibit HIV-1 entry that requires APJ as a coreceptor.
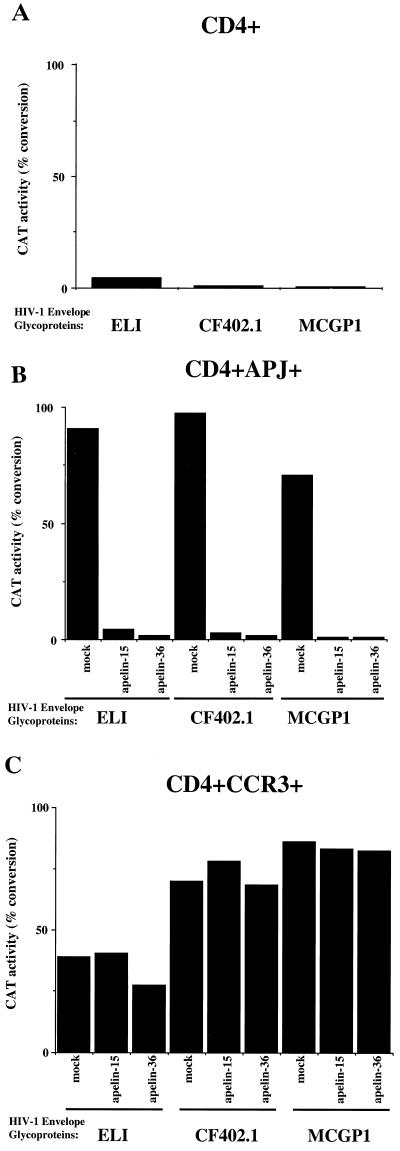
It is possible that the observed inhibition of HIV-1 entry by the apelins might be strain or clade specific. To address this issue, we examined the ability of apelin-15 and apelin-36 to inhibit other primary HIV-1 strains and clades. We generated recombinant HIV-1 viruses containing the envelope glycoproteins of ELI (an X4, clade B primary HIV-1 isolate), CF402.1 (an X4, clade E primary HIV-1 isolate), and MCGP1 (an R5/X4, clade C primary HIV-1 isolate). The clade E env clone, CF402.1, was kindly provided by Feng Gao and Beatrice Hahn (24). MCGP1 env was cloned from a provirus isolated from the PBMC of an Ethiopian patient. Phylogenetic tree analysis of the env sequence classified MCGP1 as an HIV-1 clade C virus (unpublished data). These viruses utilize APJ, in addition to CCR3, CXCR4, and/or CCR5 (reference 7 and unpublished data). Figure 3B shows that apelin-15 and apelin-36 significantly abrogated APJ coreceptor usage by all three viruses. The inhibition by both apelins is specific for the APJ coreceptor in that usage of CCR3 by these viruses was not significantly affected by either apelin (Fig. 3C). Although in some experiments apelin-36 slightly inhibited the infection of CCR3-expressing cells by viruses with the ELI envelope glycoproteins, this effect was not consistently observed. Thus, apelin-15 and apelin-36 specifically inhibit infection of APJ-expressing cells by a diverse group of primary HIV-1 viruses.
FIG. 3.
Apelin inhibits entry of other primary HIV-1 viruses from different clades. HIV-1 recombinant viruses expressing envelope glycoproteins from a primary T-tropic clade B virus, ELI, a primary dualtropic clade C virus, MCGP1, and a primary T-tropic clade E virus, CF402.1, were generated and used to infect Cf2Th-CD4 cells transfected with either a pcDNA3.1 control (A), a plasmid expressing APJ (B), or a CCR3 expression plasmid (C). Transfected cells were mock treated or were incubated with either 35 μM apelin-15 or 20 μM apelin-36 for 1 h prior to the addition of 40,000 cpm (RT activity) of recombinant virus. The CAT activity present in a given amount of target cell lysate is indicated. Similar results were obtained in two additional independent experiments.
To investigate the structural requirements for the anti-HIV-1 activity of apelin, the abilities of a group of apelin analogues to inhibit the infection of CD4+ APJ+ cells by a recombinant HIV-1 containing the 89.6 envelope glycoproteins were determined (Table 1). Apelin-19, which includes the carboxy-terminal 19 residues of apelin, was as potent as apelin-36 in this assay. Consistent with the results described above, apelin-15 exhibited weaker antiviral activity than the longer apelin variants. However, apelin-15-(63-75)-peptide was as potent as apelin-36 and apelin-19 in this assay. This result indicates that the simultaneous removal of apelin residues 59 to 62 and 76 to 77 is less detrimental to the anti-HIV-1 activity than the removal of residues 59 to 62 only. The integrity of the methionine residue at position 75 is important for antiviral potency, as indicated by the relative potencies of apelin-15-(63-75)-peptide, apelin-15-(63-74)-peptide, and [Met(O)75]apelin-15-(63-74)-peptide. In the latter two analogues, the terminal methionine at position 75 is, respectively, deleted or oxidized.
TABLE 1.
HIV-1 inhibition by apelin analogues
| Analogue | Sequencea | IC50 (μM)b |
|---|---|---|
| Apelin-36 | 42LVQPRGSRNGPGPWQGGRRKFRRQRPRLSHKGPMPF77 | 7 |
| Apelin-19 | 59RRKFRRQRPRLSHKGPMPF77 | 4.7 |
| Apelin-15 | 63RRQRPRLSHKGPMPF77 | 12 |
| Apelin-15-(63-75)-peptide | RRQRPRLSHKGPM | 3.3 |
| [Met(O)75]apelin-15-(63-75)-peptide | RRQRPRLSHKGPM* | 45 |
| Apelin-15-(63-74)-peptide | RRQRPRLSHKGP | 37 |
| [<Glu65]apelin-13) | pERPRLSHKGPMPF | >50 |
The amino acid sequence of the apelin analogues is shown in single-letter code. The residue numbers refer to the sequence of preproapelin. The “pE” in [<Glu65]apelin-13 indicates pyroglutamine. The asterisk in the [Met(O)75]apelin-15-(63-75)-peptide indicates that the methionine at position 75 is oxidized.
That is, the concentration of each analogue required to inhibit 50% of the infection of the Cf2Th cells expressing CD4 and APJ by recombinant HIV-1 viruses containing the envelope glycoproteins of the 89.6 isolate. The IC50 values shown represent the results obtained from a typical experiment.
In a separate study, the affinity of these apelin analogues for the APJ receptor was determined. All of the analogues bound APJ at least as well as apelin-36, indicating that binding to the APJ protein is not sufficient for potent antiviral activity. In addition, no correlation was evident between the anti-HIV-1 activity of the apelin analogues and the ability to affect cyclic AMP levels in APJ-expressing cells (data not shown).
All of the apelin derivatives used in this study have been shown to interact with the APJ protein and to promote extracellular acidification in APJ-expressing CHO cells (41). Two of the apelins, apelin-15 and apelin-36, inhibited infection of cells expressing CD4 and APJ by viruses pseudotyped with envelope glycoproteins from several different primary HIV-1 isolates. The observed inhibition is specific because infection of target cells expressing CD4 and other HIV-1 coreceptors was not significantly affected by either apelin-15 or apelin-36.
Surprisingly, a 13-residue analogue, apelin-15-(63-75)-peptide retained the potent anti-HIV-1 activity of significantly longer apelin variants, such as apelin-36. The simultaneous deletion of both the amino and the carboxyl termini of apelin contributed to this retention of potent activity. Further deletion, however, affecting residues 63 to 65 or methionine 75 resulted in significant decreases in anti-HIV-1 activity. The detrimental effects of deletion of the arginine residues at positions 63 and 64 suggest that particular arginine residues in the peptide may be important for the specific ability to block HIV-1 entry via the APJ coreceptor. It is possible that some of these arginines interact with the acidic, sulfotyrosine-containing amino-terminal segment of APJ (20). The analogous region on CCR5 has been shown to be important in HIV-1 entry (16, 19, 20).
The identification of specific APJ ligands that inhibit HIV-1 infection allows further exploration of the role of the APJ receptor in HIV-1 pathogenesis. This role is presently unclear, although the expression of APJ in the CNS raises the possibility that this protein might contribute to AIDS dementia. APJ is not expressed in microglia, the major target for HIV-1 infection in the CNS. However, APJ is expressed in astrocytes and neurons, two cell types that demonstrate apoptotic changes in autopsied brain specimens from children and adults with AIDS (37, 42). CNS injury in HIV-1-infected individuals is believed to occur through indirect mechanisms, which might include envelope glycoprotein-APJ interactions. These possibilities warrant further investigation.
Acknowledgments
We thank Paul Gorry and Dana Gabuzda for helpful discussion and Yvette McLaughlin for manuscript preparation. We also thank Feng Gao and Beatrice Hahn (University of Alabama) for providing the CF402.1 env clone.
M.C. is a recipient of a Ford Foundation Fellowship and is an Albert J. Ryan Fellow. This work was supported by the National Institute of Health (AI24755 and AI41851), the G. Harold and Leila Y. Mathers Foundation, the Friends 10, the late William McCarty-Cooper, and Douglas and Judi Krupp. We also thank the Ethio-Netherlands AIDS Research Programme (ENARP), which is supported by The Netherlands Ministry of Foreign Affairs and the Ethiopian Ministry of Health.
REFERENCES
- 1.Albright A V, Shieh J T, Itoh T, Lee B, Pleasure D, O'Connor M J, Doms R W, Gonzalez-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Barre-Sinoussi F, Chermann J C, Rey F, Nugeyre M T, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 4.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl. A):S3–S16. [PubMed] [Google Scholar]
- 5.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 6.Bjorndal A, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyo E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choe H, Farzan M, Konkel M, Martin K, Sun Y, Marcon L, Cayabyab M, Berman M, Dorf M E, Gerard N, Gerard C, Sodroski J. The orphan seven-transmembrane receptor APJ supports the entry of primary T-cell-line-tropic and dualtropic human immunodeficiency virus type 1. J Virol. 1998;72:6113–6118. doi: 10.1128/jvi.72.7.6113-6118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 9.Clavel F, Guetard D, Brun-Vezinet F, Chamaret S, Rey M A, Santos-Ferreira M O, Laurent A G, Dauguet C, Katlama C, Rouzioux C, et al. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986;233:343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- 10.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major coreceptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Dimitrov D S, Xiao X, Chabot D J, Broder C C. HIV coreceptors. J Membr Biol. 1998;166:75–90. doi: 10.1007/s002329900450. [DOI] [PubMed] [Google Scholar]
- 13.Doms R W, Peiper S C. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 14.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta- chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 15.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC- CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 16.Dragic T, Trkola A, Lin S W, Nagashima K A, Kajumo F, Zhao L, Olson W C, Wu L, Mackay C R, Allaway G P, Sakmar T P, Moore J P, Maddon P J. Amino-terminal substitutions in the CCR5 coreceptor impair gp120 binding and human immunodeficiency virus type 1 entry. J Virol. 1998;72:279–285. doi: 10.1128/jvi.72.1.279-285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edinger A L, Clements J E, Doms R W. Chemokine and orphan receptors in HIV-2 and SIV tropism and pathogenesis. Virology. 1999;260:211–221. doi: 10.1006/viro.1999.9819. [DOI] [PubMed] [Google Scholar]
- 18.Edinger A L, Hoffman T L, Sharron M, Lee B, Yi Y, Choe W, Kolson D L, Mitrovic B, Zhou Y, Faulds D, Collman R G, Hesselgesser J, Horuk R, Doms R W. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998;72:7934–7940. doi: 10.1128/jvi.72.10.7934-7940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farzan M, Choe H, Vaca L, Martin K, Sun Y, Desjardins E, Ruffing N, Wu L, Wyatt R, Gerard N, Gerard C, Sodroski J. A tyrosine-rich region in the N terminus of CCR5 is important for human immunodeficiency virus type 1 entry and mediates an association between gp120 and CCR5. J Virol. 1998;72:1160–1164. doi: 10.1128/jvi.72.2.1160-1164.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farzan M, Mirzabekov T, Kolchinsky P, Wyatt R, Cayabyab M, Gerard N P, Gerard C, Sodroski J, Choe H. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell. 1999;96:667–676. doi: 10.1016/s0092-8674(00)80577-2. [DOI] [PubMed] [Google Scholar]
- 21.Fauci A S, Macher A M, Longo D L, Lane H C, Rook A H, Masur H, Gelmann E P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984;100:92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 23.Gallo R C, Salahuddin S Z, Popovic M, Shearer G M, Kaplan M, Haynes B F, Palker T J, Redfield R, Oleske J, Safai B, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 24.Gao F, Robertson D L, Morrison S G, Hui H, Craig S, Decker J, Fultz P N, Girard M, Shaw G M, Hahn B H, Sharp P M. The heterosexual human immunodeficiency virus type 1 epidemic in Thailand is caused by an intersubtype (A/E) recombinant of African origin. J Virol. 1996;70:7013–7029. doi: 10.1128/jvi.70.10.7013-7029.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta. 1999;1452:25–35. doi: 10.1016/s0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 26.He J, Chen Y, Farzan M, Choe H, Öhagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 27.Helseth E, Kowalski M, Gabuzda D, Olshevsky U, Haseltine W, Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990;64:2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinuma S, Habata Y, Fujii R, Kawamata Y, Hosoya M, Fukusumi S, Kitada C, Masuo Y, Asano T, Matsumoto H, Sekiguchi M, Kurokawa T, Nishimura O, Onda H, Fujino M. A prolactin-releasing peptide in the brain. Nature. 1998;393:272–276. doi: 10.1038/30515. [DOI] [PubMed] [Google Scholar]
- 29.Jansson M, Popovic M, Karlsson A, Cocchi F, Rossi P, Albert J, Wigzell H. Sensitivity to inhibition by beta-chemokines correlates with biological phenotypes of primary HIV-1 isolates. Proc Natl Acad Sci USA. 1996;93:15382–15387. doi: 10.1073/pnas.93.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee D K, Cheng R, Nguyen T, Fan T, Kariyawasam A P, Liu Y, Osmond D H, George S R, O'Dowd B F. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 31.Li J T, Halloran M, Lord C I, Watson A, Ranchalis J, Fung M, Letvin N L, Sodroski J G. Persistent infection of macaques with simian-human immunodeficiency viruses. J Virol. 1995;69:7061–7067. doi: 10.1128/jvi.69.11.7061-7067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Littman D R. Chemokine receptors: keys to AIDS pathogenesis? Cell. 1998;93:677–680. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto M, Hidaka K, Akiho H, Tada S, Okada M, Yamaguchi T. Low stringency hybridization study of the dopamine D4 receptor revealed D4-like mRNA distribution of the orphan seven-transmembrane receptor, APJ, in human brain. Neurosci Lett. 1996;219:119–122. doi: 10.1016/s0304-3940(96)13198-0. [DOI] [PubMed] [Google Scholar]
- 34.O'Dowd B F, Heiber M, Chan A, Heng H H, Tsui L C, Kennedy J L, Shi X, Petronis A, George S R, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 35.Rucker J, Doms R W. Chemokine receptors as HIV coreceptors: implications and interactions. AIDS Res Hum Retrovir. 1998;14(Suppl. 3):S241–S246. [PubMed] [Google Scholar]
- 36.Sabri F, Tresoldi E, Di Stefano M, Polo S, Monaco M C, Verani A, Fiore J R, Lusso P, Major E, Chiodi F, Scarlatti G. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology. 1999;264:370–384. doi: 10.1006/viro.1999.9998. [DOI] [PubMed] [Google Scholar]
- 37.Shi B, De Girolami U, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Investig. 1996;98:1979–1990. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shieh J T, Albright A V, Sharron M, Gartner S, Strizki J, Doms R W, Gonzalez-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh A, Besson G, Mobasher A, Collman R G. Patterns of chemokine receptor fusion cofactor utilization by human immunodeficiency virus type 1 variants from the lungs and blood. J Virol. 1999;73:6680–6690. doi: 10.1128/jvi.73.8.6680-6690.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou M X, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 42.Vallat A V, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith T W, Gabuzda D. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y J, Dragic T, Cao Y, Kostrikis L, Kwon D S, Littman D R, KewalRamani V N, Moore J P. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J Virol. 1998;72:9337–9344. doi: 10.1128/jvi.72.11.9337-9344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]