Abstract
The increasing environmental and human health concerns about lead in the environment have stimulated scientists to search for microbial processes as innovative bioremediation strategies for a suite of different contaminated media. In this paper, we provide a compressive synthesis of existing research on microbial mediated biogeochemical processes that transform lead into recalcitrant precipitates of phosphate, sulfide, and carbonate, in a genetic, metabolic, and systematics context as they relate to application in both laboratory and field immobilization of environmental lead. Specifically, we focus on microbial functionalities of phosphate solubilization, sulfate reduction, and carbonate synthesis related to their respective mechanisms that immobilize lead through biomineralization and biosorption. The contributions of specific microbes, both single isolates or consortia, to actual or potential applications in environmental remediation are discussed. While many of the approaches are successful under carefully controlled laboratory conditions, field application requires optimization for a host of variables, including microbial competitiveness, soil physical and chemical parameters, metal concentrations, and co-contaminants. This review challenges the reader to consider bioremediation approaches that maximize microbial competitiveness, metabolism, and the associated molecular mechanisms for future engineering applications. Ultimately, we outline important research directions to bridge future scientific research activities with practical applications for bioremediation of lead and other toxic metals in environmental systems.
Keywords: Phosphate solubilizing bacteria, sulfate reducing microorganism, microbial induced carbonate precipitation, microbial remediation, lead, heavy metals
1. Introduction
Environmental lead (Pb) contamination is ranked at the top of the World’s Worst Pollution Problems based on a global assessment of human health risks at hazardous waste sites (Cross, 2012). Lead is highly poisonous, causing severe damage to the brain and kidneys and, ultimately, death. It has been long recognized that exposure to Pb may result in significant health impacts. About 1% of the total global burden of disease is attributed to mild intellectual disability and cardiovascular problems caused by Pb exposure (Fewtrell et al., 2004). Children are exceptionally vulnerable because their bodies absorb four to five times as much Pb as adults. Major anthropogenic activities causing Pb pollution includes Pb smelting, mining and ore processing, Pb-acid battery recycling and manufacturing. In the United States, reducing human exposure to Pb is a priority for the U.S. Environmental Protection Agency (EPA) as stipulated in the December 2018 Federal Action Plan to Reduce Childhood Lead Exposures and Associated Health Impacts (U.S. EPA, 2019a). Most recently, the emphasis is placed on reducing Pb exposures and disparities in U.S. communities (U.S. EPA, 2022).
The increasing environmental and human health concerns over Pb in the environment have stimulated scientists to search for innovative, eco-friendly, and cost-effective technologies for remediation of Pb contaminated sites. Conventional remediation methods for Pb contaminated sites rely on physical removal of material and engineering control (U.S. EPA, 2019b), yet these traditional techniques often require soil excavation and transportation, which in turn, generates high cost and energy input along with negative impacts on soil structure and existing ecosystems. In-situ site stabilization methods involve chemical addition to reduce Pb acute toxicity and bioavailability, thereby controlling the risk of Pb exposure (U.S. EPA, 2019b). Phosphate containing compounds such as rock phosphate and triple superphosphate are especially effective to immobilize Pb in contaminated sites due to the formation of highly recalcitrant Pb phosphate precipitates such as pyromorphite (Bradham et al., 2018; Scheckel et al., 2013). Carbon-based materials, including pristine or modified biochars, have been shown to be effective sorbents of Pb in both aqueous substrate and contaminated soils through a number of adsorption mechanisms (Cao et al., 2011; Cao et al., 2009; Ding et al., 2016; Uchimiya et al., 2012; Wang et al., 2019a). Additionally, bioremediation has received considerable attention as an alternative technology for Pb-contaminated sites (Muthusaravanan et al., 2018), such as sustainable biomineralization and biocement technologies (Sharma et al., 2022; Yu and Zhang, 2023) or the use of a consortia of cyanobacteria, algae or diatoms for metal recovery and restoration by leveraging their biosorption properties in industrial applications (Adey et al., 1996; Ajayan et al., 2018; Safonova et al., 2004).
The capability of microorganisms to mobilize or immobilize heavy metals is fundamental to the biogeochemical cycles of metals, providing the basis for the development of effective environmental bioremediation technologies to aid in heavy metal sequestration or removal from the environment. The functionality of microorganisms to influence and/or mediate metal mobilization or immobilization processes stems from their ability to modulate the balance of metal species between soluble and insoluble phases. Microorganisms attain an ecological advantage in Pb contaminated environments, primarily due to extracellular and intracellular strategies that either exclude or sequester Pb in nontoxic forms (Jaroslawiecka and Piotrowska-Seget, 2014; Naik and Dubey, 2013; Pan et al., 2017). Mobilization of metals can be achieved by protonation, chelation, and chemical transformation; however, immobilization occurs by precipitation or crystallization of insoluble organic or inorganic compounds or by sorption, uptake, and intracellular sequestration. In such a context, solubilization processes may enable removal from solid matrices, such as soils, sediments, dumps, and industrial wastes. Alternatively, immobilization processes may enable metals to be transformed in situ into insoluble and chemically inert forms and are also applicable to removing metals from aqueous solution.
Immobilization of toxic Pb into insoluble precipitates is an important strategy to reduce Pb bioavailability and toxicity to acceptable levels. Microbial mediated precipitates such as Pb phosphate, sulfide, and carbonate derivatives, can sequester bioavailable Pb in water, soil, and sediment. Once formed, these recalcitrant compounds render less Pb bioavailability or even reduce or eliminate its toxicity, thereby remediating the affected media. Furthermore, because precipitates can be more easily recovered than unbound Pb, these same mechanisms can be engineered for industrial bioremediation purposes. Phosphate solubilizing bacteria (PSB), sulfate reducing microorganisms (SRM), and carbonate synthesizers for microbial induced carbonate precipitation (MICP) are the primary microbial groups that can immobilize Pb in the presence of other heavy metals in a variety of environmental media (Fig. 1). In recent years, much research has been done to characterize these microorganisms’ isolation and selection, taxonomy, functionalities, and mechanisms for immobilization of Pb in the environment (Fuchida et al., 2020; He et al., 2019; Neculita et al., 2008; Niu et al., 2018; Park et al., 2011a; Park et al., 2011b; Teng et al., 2019; Yin et al., 2020b; Zheng et al., 2019). It is well documented that the efficiency of microbial-mediated immobilization of environmental Pb depends on not only the particular taxa but also the ambient environment of contaminated sites (Sevak et al., 2021; Shan et al., 2021). In addition, state-of-the-art research on these microbial mediated processes emphasizes practical applications of bioremediation. For example, biogenic compounds, such as schwertmannite (iron-oxyhydroxysulphate) precipitated by Acidithiobacillus ferrooxidans in high sulfate/Fe acidic environments, immobilized the heavy metals Pb, As, and Cd (Chai et al., 2016; Liao et al., 2009; Min et al., 2017). Biogenic iron (oxyhydr)oxide-nano-silica composites removed aqueous Sb (Sb+3 and Sb+5) and Cr (Seo and Roh, 2015; Xu et al., 2022a). The sulfate reducing bacterium, Desulfovibrio desulfuricans subsp. desulfuricans, mediated FeS-kaolin formation which effectively immobilized multiple heavy metals including Cd, Pb, Cu, Zn, As, and Sb (Xu et al., 2022b). Clostridium sp. produced bio-magnetite and bio-Fe-S/siderite, both of which immobilized Cr and have the potential to bind Pb (Seo et al., 2013). Biocement technologies also are under investigation to incorporate various waste materials such as sandstone waste (Sharma et al., 2022), carbide sludge (Yu and Zhang, 2023), or sulfate reducing bacteria containing granules (Chetty et al., 2022). Practical applications of microorganism-based environmental remediation may benefit from a systematic synthesis of the existing literature on this subject.
Fig. 1.
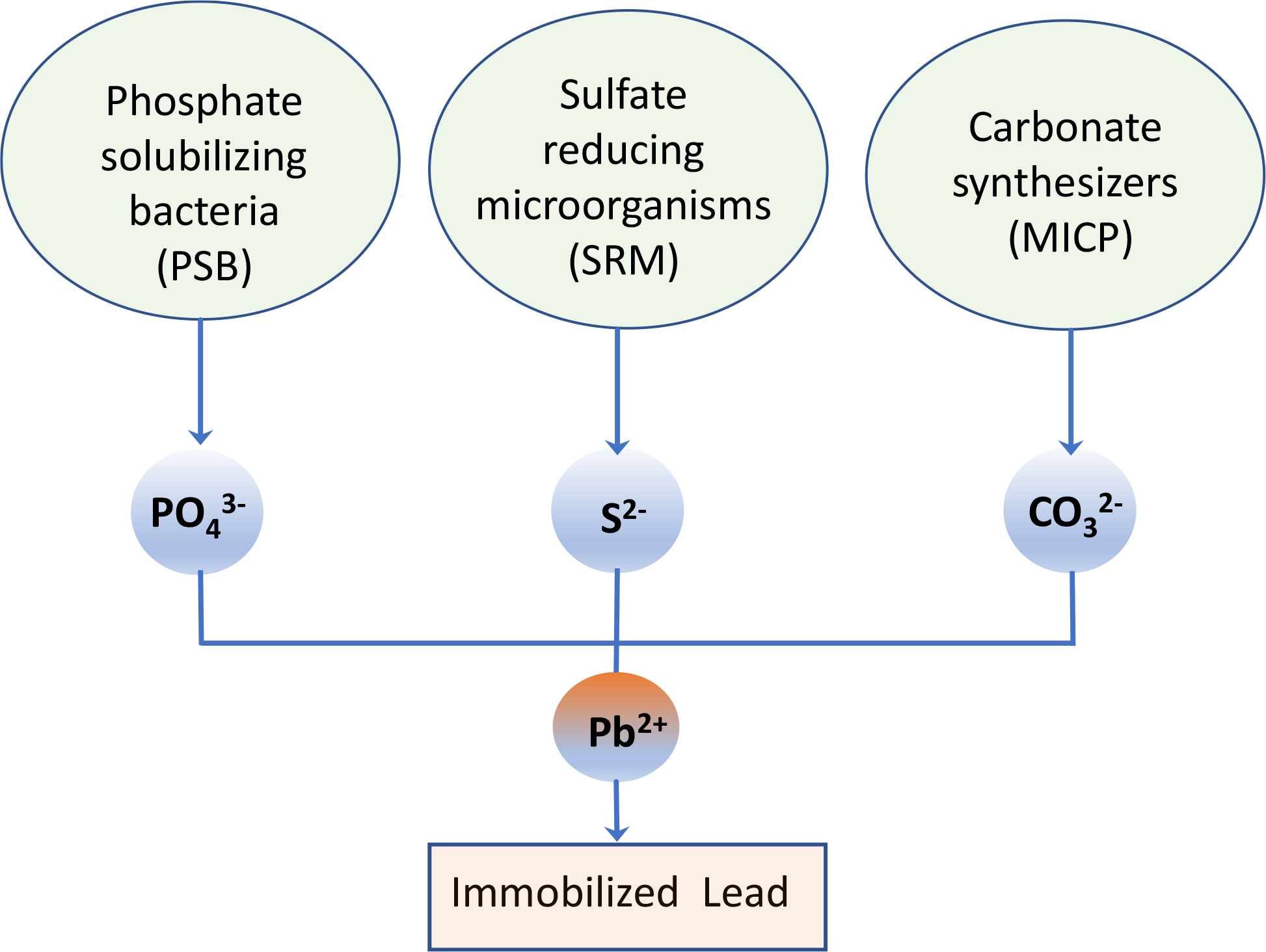
Schematic representation of immobilization of environmental lead mediated by microorganisms through phosphate solubilization, sulfate reduction, and microbially induced carbonate precipitation (MICP).
The objective of this work is, through a thorough review of the existing literature, to understand the important microbiological processes of environmental Pb immobilization which are of significance in actual or potential applications in environmental remediation. Specifically, we focus on microbial functionalities of phosphate solubilization, sulfate reduction, and carbonate synthesis in mediating the immobilization process of environmental Pb. Ultimately, we outline some important future directions to bridge the scientific research with practical applications for bioremediation.
2. Immobilization of Lead by Phosphate Solubilizing Bacteria
The term phosphate solubilizing bacteria (PSB) refers to diverse beneficial bacteria capable of mobilizing P from insoluble compounds, resulting in elevated levels of available phosphate in the environment. Since the pioneering work of Pikovskaya (1948) that linked mobilization of P in soils with vital activities of some microbial species, PSB and their roles in P cycling have been a focal research area with a primary emphasis on identifying strains for agronomic applications as inoculants to increase soil available P content by releasing P bound by Al, Fe, and Ca (Rodríguez and Fraga, 1999). Therefore, PSB traditionally refer to bacteria that solubilize inorganic P compounds. Phosphate solubilizing bacteria are widely distributed in soils, water, and sediments (Liu et al., 2014; Zhang et al., 2015). While microbial communities vary significantly with the ambient environment, up to 50% of the total number of bacteria in soil are PSB, concentrated in the rhizosphere where they are metabolically active. PSB are especially active under abiotic stress such as drought, low or high pH, salinity, and temperature (Sukweenadhi et al., 2015). In the context of phosphate-based remediation of Pb-contaminated soils and wastes (Scheckel et al., 2013), scientists have paid increasing attention to the value of PSB in immobilizing Pb in soils and water (Pagnout et al., 2018; Park et al., 2011a; Park et al., 2011b; Teng et al., 2019; Yuan et al., 2017; Zhang et al., 2019a). A large number of studies isolated PSB strains that convert either inorganic or organic P into reactive phosphate that binds Pb (Table 1). Some examples of such PSB include Bacillus subtilis (Bai et al., 2014), Pantoea sp. (Chen and Liu, 2019; Park et al., 2011b), Enterobacter sp. (Chen et al., 2019; Li et al., 2018; Park et al., 2011a), Acinetobacter pittii (Wan et al., 2020), Serratia marcescens (Zhu et al., 2019a), Leclercia adecarboxylata and Pseudomonas putida (Teng et al., 2019). Fungal strains, such as Penicillium chrysogenum, also have been found to be capable to solubilizing phosphate to form Pb-phosphate minerals (Povedano-Priego et al., 2017). In this section, we summarize the mechanisms of phosphate solubilization used by PSB in the context of immobilization of Pb and the processes and practical considerations of using PSB for remediation.
Table 1.
Summary results of selected studies on immobilization of lead by phosphate solubilizing bacteria (ND, not determined)
| Source | Microorganism | Lead Resistance | P Solubilization capacity (mg/L) | P Source | P solubilization effects | Pb immobilization mechanism | References |
|---|---|---|---|---|---|---|---|
| China: Pb-Zn concentrator tailing-waste contaminated soil: Pb, 43.8 mg/kg Cd, 0.33 mg/kg Cu, 11.9 mg/kg |
Serratia marcescens OPDB3–6-1 |
1.9 mM | 167.6 (15 d incubation) |
Organic P in growth media | Production of alkaline matter pH increase (7.0->8.5) |
Precipitation Biosorption Pyromorphite (Pb5(PO4)3Cl) |
Zhu et al., 2019 |
| China: Soybean rhizosphere |
Enterobacter sp. | 4.8 mM Pb [1000mg/L] added to culture after 3 days incubation |
~160 (3 d incubation) | Organic P in growth media and biochar | Production of alkaline matter pH increase (7.2-->8.7) |
Precipitation: Pyromorphite (Pb5(PO4)3Cl), cerussite Biosorption (biochar) |
Chen et al., 2019 |
| China: Heavy metal waste collection & distribution center soil Pb, 137.3 mg/kg (site L1) Pb, 323.7 mg/kg (site F2) |
Leclercia adecarboxylata L1–5 Pseudomonas putida F2–1 |
8 mM Pb(NO3)2 8 mM Pb(NO3)2 Minimum Inhibitory Concentration (MIC) > 1mM |
218 and ~18, respectively (7 d incubation) | Ca3(PO4)2 liquid medium |
Production of organic acid (Acetate, Malonate) and acid phosphatase pH decrease (6.7->2.8) |
Precipitation:HydroxylpyromorphitePb10(PO4)6(OH)2 and Pyromorphite Pb5(PO4)3ClBiosorption (cell wall) • Hydroxyl • Amide • Carboxyl • Phosphate |
Teng et al., 2019 |
| The Netherlands: Canal water* U.S.A.: Human skin‡ |
Citrobacter freundii ATCC 8090† Staphylococcus aureus K4‡ |
1 mM Pb(NO3)2 7 mM Pb(NO3)2 |
ND | ND | ND | ND |
Levinson and Mahler, 1998 Levinson et al., 1996 Werkman and Gillen, 1932 |
| China: Fertilizer plant soil |
Bacillus megaterium | 100–500 mg/L Pb2+ add to log phase cells | ND | Organic P in growth medium | ND | Precipitation/biosorption Pb phosphate Pb3(PO4)2 (Pb5(PO4)3OH intermediate) |
Zhang et al., 2019 |
| India: battery manufacturing industry waste |
Achromobacter xylosoxidans SJ11 | Maximum Tolerance Concentration (MTC): 4 mM Pb(NO3)2 | ND | Organic P in growth medium | 160% increase in phosphatase activity; CheZ & CheA up-regulated |
Precipitation: Pyromorphite Pb5(PO4)3Cl Extracellular accumulation |
Sharma et al., 2018 |
| China: Heavy metal contaminated site (Pb/Zn smelting) Pb, 127 mg/kg Zn, 379 mg/kg Cu, 93.1 mg/kg Ni, 54.6 mg/kg Co, 26.2 mg/kg Mn, 1095 mg/kg Cr, 133 mg/kg V, 206 mg/kg Hg, 0.15 mg/kg As, 16.2 mg/kg Cd, 3.08 mg/kg |
PSB Consortium: Enterobacter spp., 92.65% Bacillus spp., 4.90% Lactococcus spp., 1.65% |
500 mg/kg Pb(NO3)2 400 mg/kg CdCl2 MTC < 500 mg Pb/L |
429.5 | Ca3(PO4)2 in growth medium |
ND | Precipitation Biosorption Intracellular accumulation |
Yuan et al., 2017a; Yuan et al., 2017b |
| Australia: Pb and Zn smelter site soil Pb, 619 mg/kg |
Enterobacter sp. | MIC = 2.23 mM Pb(NO3)2 | 200.3 after 14 d | Rock phosphate added into growth medium | Production of organic acids pH decrease (7->3.8) Increase in acid and alkaline phosphatase activities |
Precipitation Pyromorphite Pb5(PO4)3Cl Biosorption |
Park et al., 2011a; Park et al., 2011b |
| Spain: decayed wood |
Penicillium chrysogenum | MIC = 8 mM Pb(NO3)2 | ND | Organic P in growth medium | ND | Precipitation/bio-sorption Biotic: Pb phosphate Pb3(PO4)2 Abiotic: Pb oxide (Pb3O4, PbO) Hydrocerussite Pb3(CO3)2(OH)2 Cerussite PbCO3 |
Povedano-Priego et al., 2017 |
| China: Root of Zn/Cd hyperaccumulator Sedum alfredii Hance, collected from Pb/Zn mine tailings (Pantoea ananatis) |
Pantoea ananatis HCR2 Bacillus thuringiensis GL-1 |
3–5 mM Pb | 99.6 and 139.1 After 6–8 d |
phosphate rock added into growth medium | Organic acids Strain HCR2: gluconate, citrate, succinate, α-ketoglutarate, pyruvate Strain GL-1: gluconate, citrate, α-ketoglutarate, succinate pH decrease (6.7->2.8) |
Precipitation Strain GL-1: Pb5(PO4)3Cl, Pb3(CO3)2(OH)2, PbAl3PO4SO4(OH)6, Pb3(CO3)2(OH)2 Strain HCR2: Pb5(PO4)3Cl, Pb3(CO3)2(OH)2 |
Xu et al., 2019 |
| China: phosphate mining wasteland. |
Citrobacter farmer CFI-01 in Pikovskaya's liquid medium |
ND, at a minimum 100 mg Pb/L | 351.5 mg/L after 5 d |
Calcium phosphate | pH reduced from 7.0 to 4.9 | Precipitation (Pb5(PO4)3OH) (Pb5(PO4)3Cl) |
Li et al., 2022 |
2.1. Mechanisms of phosphate solubilization
Total phosphorus (P) content in soils is generally less than 0.1% and only 0.1% of total P is reactive or biologically available. Most P in soils is insoluble, bound by Fe and Al in acidic soils and Ca in alkaline soils. Phosphate solubilizing bacteria play a key role in biogeochemical transformations of P in soils. Insoluble P exists in soils in two forms: (i) inorganic P (Pi) as Al-, Fe-, or Ca-phosphates or sorbed by Al, Fe, Ca oxides, and (ii) organically bound P (Po) as soil organic matter and plant/animal residues (Fig. 2). PSB employ different strategies to convert insoluble forms of P into reactive forms (primarily three species of orthophosphate). The literature generally supports two primary mechanisms of microbial mediated P solubilization: (1) Pi -solubilization by secreted organic acids or hydrogen ions that dissolve Pi compounds; and (2) Po-mineralization by enzymatic action such as extracellular phosphatase to mineralize Po compounds (Fig. 2). Most PSB are specific to solubilization of either Pi or Po; yet a PSB isolate from alfalfa rhizosphere soil, Pantoea sp. S32, possessed high P dissolving capacity for both Pi and Po (Chen and Liu, 2019). An Enterobacter cloacae strain also used both mechanisms (organic acid production and phosphatase action) to mobilize P and precipitate Pb to form pyromorphite (Park et al., 2011b).
Fig. 2.
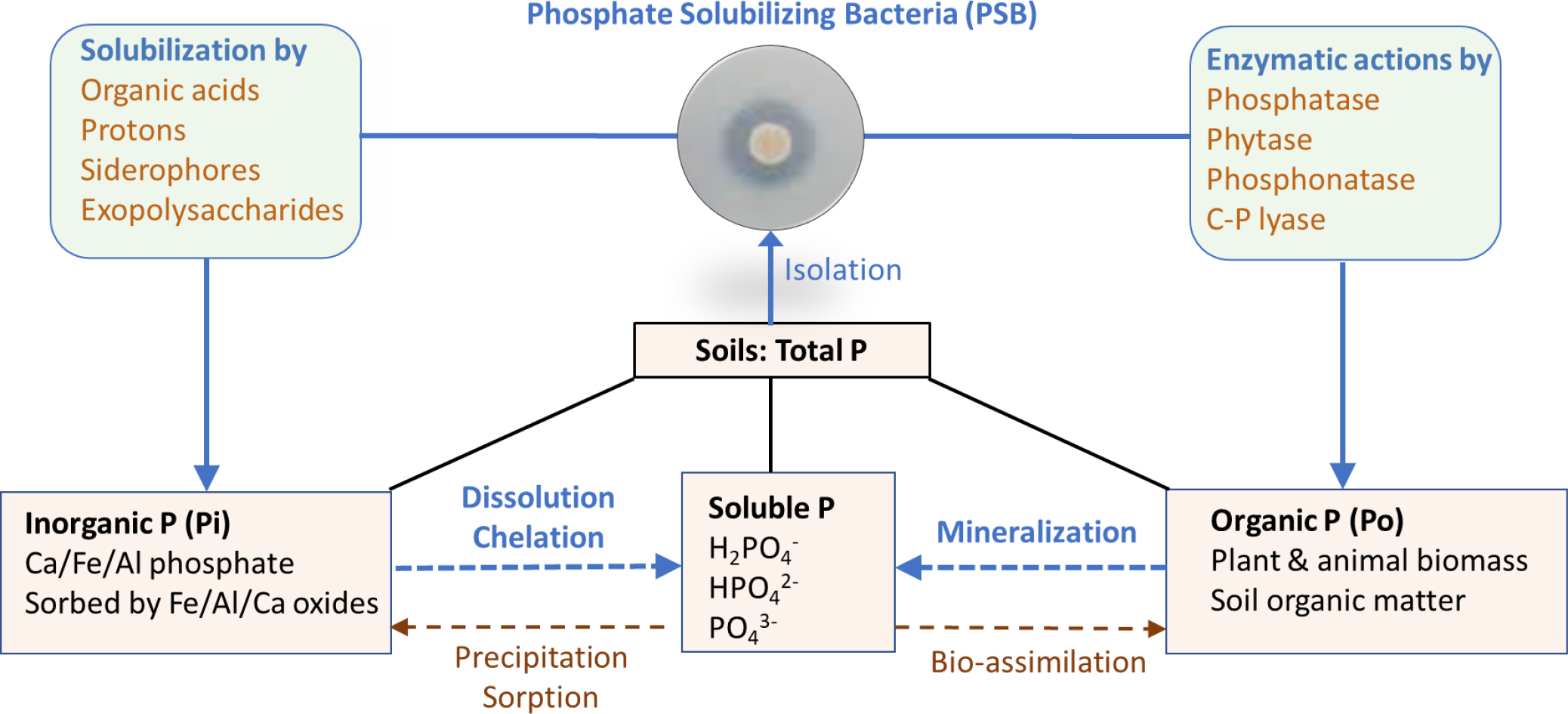
Soil phosphorus transformation and mechanisms of inorganic and organic phosphate solubilization by microorganisms.
2.1.1. Production of organic acids
The formation of low molecular weight organic acids during microbial metabolism of organic carbon is a key process involved in solubilization of inorganic phosphate. Organic acids released by PSB act as good chelators of divalent cations of Ca2+ coupled with the release of phosphate from insoluble complexes (Sashidhar and Podile, 2010). Organic acids also may form soluble complexes with other metal ions that are co-complexed with insoluble P, thereby releasing the P moiety. In addition, inorganic soil phosphates of Ca, Fe, and Al can be solubilized via acidification by organic acids. The foremost mechanism for mineral phosphate solubilization by Gram-negative PSB is through the production of organic acids related to the dissolution of mineral glucose to gluconic acid. These reactions can be represented as follows using gluconic acid as an example:
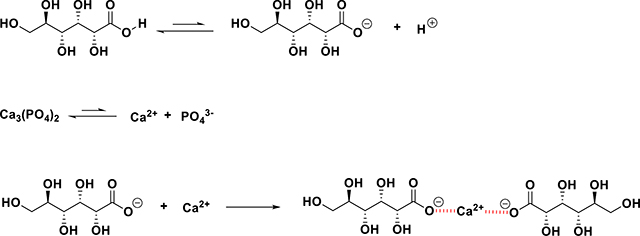
The other commonly produced organic acids by PSB include acetic, pyruvic, fumaric, succinic and citric acids (Bolan et al., 1994). For example, Schneider et al. (2010) suggested that citric acid and oxalic acid produced by Aspergillus niger mobilized P from phosphate rock. Xu et al. (2019) found positive correlations between concentrations of soluble phosphate and citric (r = 0.923), gluconic (r = 0.926), and pyruvic acids secreted by Pantoea ananatis HCR2 and Bacillus thuringiensis GL-1. Pande et al. (2017) noted production of glucuronate, formate, and citrate by Alcaligenes aquatilis and Burkholderia cepacia. Chen et al. (2006) demonstrated the secretion of citric, gluconic, lactic, succinic and propionic acids by several PSB strains (Arthrobacter, Bacillus, Serratia, Chryseobacterium, Pseudomonas, and Delftia), which exhibited the ability to solubilize considerable amounts of tricalcium phosphate.
The low molecular weight organic acids produced by PSB contain functional groups such as hydroxyl and carboxyl that chelate cations bound to phosphate (Fe and Al in acid soils, Ca in alkaline soils) and release soluble phosphate (Sharma et al., 2013). Type and position of the ligand in addition to acid strength determine effectiveness in the solubilization process (Kpomblekou-a and Tabatabai, 1994). Carboxylic anions produced by PSB, have especially high affinity to Ca and solubilize more P than acidification alone (Staunton and Leprince, 1996). Chelation of metal cations is greatly influenced by the molecular structure of organic acids, particularly by the number of carboxyl and hydroxyl groups. Complexation of cations becomes an important mechanism in P solubilization if the organic acid structure favors complexation (Fox et al., 1990). Exopolysaccharides and siderophores secreted by PSB may function similarly to release phosphate. Phosphorus desorption potential decreases with increasing stability of Fe - or Al - organic acid complexes in the order: citrate > oxalate > malonate / malate > tartrate > lactate > gluconate > acetate > formate (Ryan et al., 2001).
2.1.2. Reduction of pH
Many PSB cause reduction in the pH of their surrounding environment either by H+ extrusion or by secretion of organic acids. Thus, phosphate solubilization by lowered pH can be the result of the combined effect of H+ extrusion and organic acids production. However, excretion of H+ may occur in the absence of organic acid production such as in N assimilation and respiratory H2CO3 production (Arvieu et al., 2003). The decrease in pH is an important process regulating the solubilization of Ca3(PO4)2 as well as the enzyme activity involved in biosynthesis and growth. Protonating phosphate is the major pathway enhancing the dissolution of :
Negative correlations between pH and P solubilized have been reported by several researchers (Park et al., 2011b; Teng et al., 2019). Park et al. (2011b) indicated that 88.4% of variability in P solubilization can be explained by pH and organic acid concentration. Kumar et al. (2008) isolated a metal tolerant plant growth promoting bacterium (Enterobacter sp.) which decreased the pH of the growth medium from 7 to 2, thereby achieving the maximum P solubilization of 229 mg/L. Similarly, the effectiveness of phosphate rock as a P source depends mainly on pH in acid soils (pH < 6.5) and as a metal immobilizing agent in acid mine wastes (Harris and Lottermoser, 2006). Thus, effectiveness of rock phosphate amendment for Pb immobilization in soils can be enhanced by adding inorganic or organic acids (Cao et al., 2009; Park et al., 2011b).
It should be noted that pH reduction induced by growth of PSB may inhibit Pb-phosphate formation though the immobilized Pb in solution can increase with reduction in solution pH due to solubilization of phosphate. A near neutral or alkaline pH is most conducive for Pb-phosphate precipitation, which is not favored with decreasing or increasing solution pH (Kopittke et al., 2008). Therefore, it is important to maintain favorable solution pH for both solubilization of P and immobilization of Pb with P compounds. Most soils have a buffering capacity that resists pH change. Thus, the effect of PSB in lowering pH as measured in growth media can be diminished when applying in soils.
2.1.3. Enzymatic actions through phosphatases
PSB can produce or release either acidic or alkaline phosphatases to convert organic into the soluble inorganic form to immobilize Pb and/or augment plant growth (Tarafdar and Claassen, 1988; recently reviewed by Antoun, 2012; Barea and Richardson, 2015; Bi et al., 2018):
These phosphatase enzymes hydrolyze ester phosphate bonds to release phosphate while high-molecular-weight organic compounds are dissociated into low-molecular-weight compounds (Pereira and Castro, 2014). Phytase enzymes, commonly detected and characterized in fungal species, hydrolyze phospho-monoester bonds in phytic acids or phytates (salt form), which are the major pools of phosphorus in plant tissues. Phosphonatase and C-P lyase hydrolyze phosphonates that contain a characteristic carbon–phosphorus (C–P) bond into hydrocarbons and phosphate ions.
Phosphatase enzymes are active over a range of environmental pH. Alkaline materials such as NH3 can be produced when PSB break down proteins and amino acids, a biochemical process called ammonification (Chen et al., 2019). In this case, releases of phosphate from organic material are accompanied by a pH increase. For example, a PSB stain, Serratia marcescens OPDB3–6-1, can produce soluble phosphate and alkaline matter during the degradation of organic phosphorus compounds (Zhu et al., 2019a). Acid phosphatase activity also was found to be positively correlated to solubilized P, serving as an indicator for organic P mineralization (Behera et al., 2014). However, acid phosphatase would not act directly on inorganic P solubilization. Instead, it may participate in lowering the pH by the dephosphorylating action and the production of organic acids (Achal et al., 2007).
2.1.4. PSB induced P cycling gene expression
Phosphate solubilization has been linked with changes in P-cycling gene abundance in soils. Phosphorus mobilizing genes, such as phoX, phoA, and phoD encoding alkaline phosphatase, ptp encoding protein tyrosine phosphatase, hap encoding histidine acid phosphatase, bpp encoding b-propeller phytase, cphy encoding cysteine phytase, gcd encoding glucose dehydrogenase, and pqq encoding pyrroloquinoline-quinone synthase, are involved in varying pathways of P-cycling and production of Pi (Bi et al., 2018; Huang et al., 2009; Neal et al., 2017). Wan et al. (2020) were among the first to relate Pi solubilization and Pb immobilization with changes in P cycling genes. In their experiment, PSB Acinetobacter pittii gp-1 transformed insoluble Ca-bound phosphate into soluble P, and this was correlated to expression of pqq and gcd genes and Pb immobilization. They further noted that Pb immobilization efficiency was positively correlated with gcd- and bpp-harboring bacterial abundance, suggesting that these bacteria might be responsible for Pb immobilization. A slight increase in the abundances of organic P-cycling-related bpp- and phoD-harboring bacterial communities occurred as the indigenous bacterial community changed. Through metagenomic analysis, Liang et al. (2020) also identified a novel PSB gcd gene which had an important role in driving the enhancement of soil P cycling following the restoration of a degraded heavily mined site. The abundance of pqq and gcd genes in highly contaminated environments suggests that bacteria harboring these genes also can develop heavy metal resistance strategies.
2.2. Processes of Pb immobilization mediated by PSB
Immobilization of Pb mediated by PSB is a complex process and can involve both extracellular and intracellular mechanisms. Biomineralization and biosorption are the predominant processes involved in Pb immobilization after soluble P levels are elevated by PSB. Biosorption describes the adherence of Pb to microbial cells and Pb biomineralization is the formation Pb-containing minerals. It should be noted that these two processes often occur concurrently. In biomineralization, bacterial cells may serve as nucleation sites to support the formation of Pb-P crystals, which resembles a self-assembly process involving first dissolution of small particles and then growth of the mineral crystals. From a remediation standpoint, interactions of Pb, P on the microbial cell surface improve the removal rate and makes the mineralized products more stable and compact.
2.2.1. Pb biomineralization
Various Pb-P minerals have been identified as PSB-related through studies with modern analytical techniques such as X-ray diffraction analysis (XRD), scanning electron microscope (SEM), and x-ray absorption near-edge structure (XANES) or X-ray absorption fine structure (XAFS) spectroscopy using synchrotron radiation. Pyromorphite-type Pb-P minerals are perhaps the most commonly observed biomineral precipitate. The mineral phase of pyromorphite [Pb5(PO4)3X, where X = F, Cl, Br, OH] is known as the most stable Pb mineral occurring in the terrestrial environment. Chloropyromorphite has a widely cited solubility product of Ksp = 10−84.4 based on the reaction:
Formation of a specific mineral depends on the soil environment or the experimental conditions, especially ionic composition and soil pH. For example, PSB Pantoea ananatis and Bacillus thuringiensis were assayed in broth medium containing Pb and rock phosphate and produced Pb-P compounds identified as pyromorphite [Pb5(PO4)3Cl] and hindsdalite [PbAl3PO4SO4(OH)6] (Xu et al., 2019). Co-formation of hydrocerussite [Pb3(CO3)2(OH)2] suggested the influence of hydroxides and carbon dioxides. Acetate and malonate, synthesized by Leclercia adecarboxylata and Pseudomonas putida, were associated with Pb precipitation as hydroxylpyromorphite [Pb5(PO4)3OH] and chloropyromorphite (Teng et al., 2019). Bacillus megaterium isolated from soil decomposed phosphate-containing organic compounds in culture medium containing Pb2+ at 100–500 mg/L and formed Pb3(PO4)2 stable minerals such as hydroxylpyromorphite [Pb5(PO4)3OH] (Zhang et al., 2019b). During the 72-h experiment, the rate of crystallization induced by this bacterium and the shapes of the crystals formed varied, suggesting that Pb-P minerals crystalize with time.
Phosphatase activity also may serve as an indicator for Pb biomineralization. In Citrobacter freundii and Staphylococcus aureus, phosphatase activity was detected in both Pb resistant and sensitive strains at comparable levels (Levinson and Mahler, 1998). Lead nitrate [Pb(NO3)2] induced phosphatase activity to increase by 160% in Achromobacter xylosoxidans and resulted in the formation of extracellular pyromorphite (Sharma et al., 2018). Organic phosphate was converted to soluble phosphate by Bacillus megaterium phosphatase, producing a lead phosphate [Pb3(PO4)2] precipitate through the hydroxyl intermediate, hydroxylpyromorphite [Pb5(PO4)3OH] (Zhang et al., 2019b; Zhu et al., 2015). Microbial produced organic acids and phosphatases contribute to the soluble P pool and formation of recalcitrant Pb precipitates (Park et al., 2011a; Teng et al., 2019; Xu et al., 2019). Clearly, multiple sequestration mechanisms follow microbial phosphate generation and Pb immobilization.
2.2.2. Cell Pb biosorption
The Pb biosorption process results from various functional groups presented on the bacterial cell surface or cell wall that serve as sorption sites (Bai et al., 2014; Teng et al., 2019; Yuan et al., 2017). Hydroxyl, amide, carboxyl, and phosphate groups of lipids, polysaccharides, and protein amide, alkyl chain are common functional groups in the surface of cells detected with Fourier transformed infrared spectroscopy (FTIR). Comparative FTIR spectra of bacterial cells before and after Pb adsorption reveal carboxyl, hydroxyl, carbonyl, amido, and phosphate groups as the common functional groups binding Pb2+ on the bacterial surface (Bai et al., 2014; Teng et al., 2019). For example, Yuan et al. (2017) examined a phosphate solubilizing bacterial consortium, comprised of Enterobacter spp. (92.65%), Bacillus spp. (4.90%), and Lactococcus spp. (1.65%), for immobilization of soil Pb and Cd with introduction of calcium phosphate [Ca3(PO4)2] as a source of phosphate and indicated that amide I and amide II bonds and carboxyl are active with sorption of metals.
Biosorption of Pb may involve various mechanisms including physical entrapment, ionic exchange, complexation, and precipitation. The adsorption capacity of intact living cells is generally higher than that of cell debris because intact cells have complete cell surfaces, structural integrity, and functionality, while cell debris has only a simple physical adsorption function. Bai et al. (2014) developed a multi-desorption approach using H2O, 1 M NH4NO3 solution, and 0.1 M EDTA-Na2 solution sequentially, to partition Pb sequestration by cells into (1) physical entrapment by the cell wall mesh structure, (2) ion exchange with cell wall polysaccharides (K2+, Ca2+, Na2+, and Mg2+), and (3) complexation or extracellular binding to cell wall functional groups such as amide, carboxyl and phosphate groups. The remaining fraction that was not desorbed by EDTA was attributed to intracellular accumulation inside the cells. These authors noted that 8.5% was physically entrapped, 43.3% was held by ion-exchange, 9.7% was complexed with functional groups, and 38.5% was accumulated inside cells. Using this approach, Yuan et al. (2017) reported that 8.55% of the adsorbed Pb2+ was by physical entrapment, 35.74% by ion-exchange, 1.78% complexed with functional groups, and 53.93% accumulated inside cells. The large percentage of intracellular accumulation and small percentage of binding by functional groups are problematic. The kinetics of pyromorphite formation process is very rapid once both P and Pb become available (Chrysochoou et al., 2007), and Bai et al. (2014) noted the formation of Pb5(PO4)3OH, Pb5(PO4)3Cl, and Pb10(PO4)6(OH)2 on cell walls. Thus, EDTA is unlikely able to fully account for Pb-P precipitates on the cell surface. Biomass from phosphate accumulating organisms, such as Ochrobactrum cicero, Stenotrophomonas maltophilia, and Pseudomonas putida, has been used as an effective bio-sorbent to immobilize Cd, Cu, Pb, and Zn (Li et al., 2022). Biosorption was both pH and strain dependent with maximum immobilization (averaged 64%, 84%, 45%, and 64% of Cd, Cu, Pb, and Zn, respectively) occuring at pH ~6.
2.3. Practical considerations of PSB for Pb remediation
Concerns of using highly soluble phosphates or phosphoric acids for Pb remediation highlight the importance of using PSB along with soil amendment of poorly soluble P compounds such as rock phosphate. Existing laboratory research generally supports about 10–30% enhanced immobilization of Pb in contaminated soils upon inoculation of PSB (Table 2). For instance, Park et al. (2011b) reported that Pb immobilization in soil was enhanced by 13.7% and 26.4% using PSB strains, Pantoea sp. and Enterobacter sp., respectively, with phosphate rock amendment of 800 mg/kg. Li et al. (2022) reported 7.79% and 22.18% increase in the amount of Pb immobilized in a soil spiked with Pb(NO3)2 at 60 and 100 mg Pb/kg, respectively, due to inoculation with a PSB Citrobacter farmeri CFI-01. In most of the studies referenced in Table 2, Pb mobilization rate was found to be positively correlated with the amount of phosphate solubilized. Apparently, the efficiency of Pb immobilization varied with PSB, soil Pb levels, and the amount of P added into the soil. While these studies showed promising performance for Pb remediation, optimization of PSB is needed for site-specific conditions when applied in the field. The following two criteria are especially germane when selecting PSB for field applications: phosphate solubilization capacity and inhibition of PSB by Pb.
Table 2.
Enhanced lead immobilization by inoculation of phosphate solubilizing bacteria in lead contaminated soils
| Phosphate solubilizing bacteria | Contaminated soil | P amendment rate | Lead immobilization measurement | Enhanced lead immobilization over the control without PSB inoculation | References |
|---|---|---|---|---|---|
| Pantoea sp. | Spiked with Pb(NO3)2 at 2000 mg Pb/kg soil | Rock phosphate amendment at 0, 200, 800 mg/kg soil | NH4NO3 (1M) extractable Pb concentration after 14 day incubation | 8.25–13.7% | Park et al., 2011a |
| Enterobacter sp. | Spiked with Pb(NO3)2 at 2000 mg Pb/kg soil | Rock phosphate amendment at 0, 200, 800 mg/kg soil | NH4NO3 (1M) extractable Pb concentration after 14 day incubation | 4.7–26.4% | Park et al., 2011a |
| PSB Consortium: Enterobacter spp., 92.65% Bacillus spp., 4.90% Lactococcus spp., 1.65% |
435 mg Pb/kg soil taken at a Pb/Zn smelting site | Ca3(PO4)2 amendment at 10.6 mg/g soil | Acetic acid (0.11 M) extractable Pb concentration after 15 day incubation | 10.81% | Yuan et al., 2017 |
| Citrobacter farmeri CFI-01 | Spiked with Pb(NO3)2 at 60 and 100 mg Pb/kg soil | Soil taken from phosphate mining site with total P of 2 mg/kg. No external P was added | NH4NO3 (1M) extractable Pb concentration after 14 day incubation | 7.79% and 22.18%, | Li et al., 2022 |
|
Acinetobacter pittii gp-1 |
Spiked with Pb(NO3)2 at 500 mg Pb/kg soil | 1, 2, 3, 4, 5, and 10% Ca3(PO4)2 | Acetic acid (0.11 M) extractable Pb concentration after 30 day incubation | 8–22% | Wan et al., 2020 |
2.3.1. Phosphate solubilization capacity
Numerous studies screened and isolated highly efficient PSB that can increase phosphate ions for immobilization of Pb (Table 1). Phosphate solubilization capacity is determined by inoculating PSB into a fixed volume of liquid medium (~100 mL) supplemented with an insoluble P source followed by measuring phosphate concentration in the medium during the incubation (typically for 7 d). Agar plates have also been used to screen PSB which produce acids to form a clear zone or halo around the colonies. Screening on agar medium is no longer considered a suitable test, and phosphate solubilization ability of microorganisms must be observed in liquid media. The insoluble P sources include Ca3(PO4)2, powdered phosphate rock, AlPO4, FePO4, and lecithin (Table 1). Use of Ca3(PO4)2 is perhaps the most popular method [e.g., the National Botanical Research Institute’s Phosphate (NBRIP) growth medium] with numerous reports of phosphate solubilization capacity in the range of 100–200 mg/L (Melo et al., 2018; Panhwar et al., 2014). Some PSB possessing extremely high Ca3(PO4)2 measured phosphate solubilization capacity include Pseudomonas trivialis BIHB 745 for 827 mg/L (Vyas and Gulati, 2009), Serratia marcescens RP8 for 974 mg/L (Misra et al., 2012) and Acinetobacter sp. ASL12 for 717 mg/L (Liu et al., 2014). The reported phosphate solubilization capacity measured with FePO4 and AlPO4 were much lower (5–50 mg/L), possibly due to their lower solubility than Ca3(PO4)2 (Chen et al., 2014; Oliveira et al., 2009). In another study, Wan et al. (2020) took a stepwise acclimation approach using Ca3(PO4)2, phytate, FePO4, and AlPO4 and identified a PSB (Acinetobacter pittii gp-1) that can solubilize multiple P sources. Apparently, targeted application of PSB depends on P speciation in the soil. Rock phosphate is an attractive amendment because it may supply phosphate gradually to the soil in aqueous phase for long-term Pb immobilization in the environment (Ma and Rao, 1999; Ma et al., 1995), but few studies examined PSB’s phosphate solubilizing capacity with rock phosphate (Park et al., 2011b).
2.3.2. Minimum inhibitory concentration of Pb
Environmental contamination by heavy metals generally induces morphological and physiological changes within microbial communities. Because Pb is bacteriostatic or bactericidal to many microorganisms, bacteria-induced immobilization of metals is unlikely to occur if heavy metals are present at concentrations that are toxic to the inoculated bacteria. PSB isolated from contaminated soils likely develop resistance mechanisms that enable them to survive and remain active in Pb contaminated environment. Therefore, isolating Pb resistant strains typically involves determination of the minimum inhibitory concentration (MIC), which is defined as the lowest concentration of Pb that inhibits visible growth of the isolate (Muñoz et al., 2012). The MIC value is obtained by incubating the PSB in varying concentrations of soluble Pb in the inoculated growth medium (typically at 28–30± C for 2–7 d) followed by growth measurements of the colony counts or optical density. Strains with high Pb resistance typically have a MIC greater than 1 mM (Teng et al., 2019). For instance, the MIC was found to be 0.6 mM for Bacillus megaterium, 2.5mM for Pseudomonas marginalis (Das et al., 2016; Roane, 1999), 1.21 mM for Agrobacterium tumefaciens, and 3.62 mM for Acinetobacter sp. (Zhang et al., 2011). It should be noted that visual observation of the colony growth can be subjective. Park et al. (2011b) evaluated MIC bacteria growth by measuring optical density at 600 nm wavelength, and they found a linear relationship between Pb concentration and absorbance. In this case, a MIC value cannot be readily obtained for the linearity observed. Several workers used the Maximal Tolerable Concentration (MTC), the highest Pb concentration that allows bacterial growth, as an alternative metric of Pb resistance (Yuan et al., 2017). The sensitivity of PSB to other environmental factors such as pH, temperature, and salinity can be tested in a similar fashion and will be important considerations for field applications, though bacteria have been reported to solubilize phosphate under abiotic stresses such as drought, low or high pH, salinity, and temperature (Chen and Liu, 2019).
3. Immobilization of Lead by Sulfate Reducing Microorganisms
Sulfate reducing microorganisms (SRM) have a major role in the cycling of sulfur in the environment (Leloup et al., 2009; Nakagawa et al., 2012; Vigneron et al., 2021; recently reviewed by Jørgensen et al., 2019). In aquatic systems, anoxic layers accumulate H2S and favor a reducing environment, thus allowing for the production of reduced divalent metals that bond to sulfide to form stable metal sulfides (Leloup et al., 2009; Nakagawa et al., 2012; Niu et al., 2018). Furthermore, the sulfur cycle is inextricably linked to the carbon cycle through remineralization organic matter carbon in sediment as evidenced by the inverse correlation of H2S concentration with organic matter (Holmkvist et al., 2011; Jørgensen, 1977; Westrich and Berner, 1984). SRM thrive in the sulfate reducing zone of sediments and can persist in the methanogenic zone where sulfate concentrations are low; scarce amounts of sulfate are likely produced by the reoxidation of sulfide in the presence of deeply buried oxidized iron in sediments (Holmkvist et al., 2011; Wehrmann et al., 2017). In addition, carbon also can be assimilated by anoxygenic phototroph oxidation of sulfides, thus completing the sulfur cycle (Nakagawa et al., 2012). Many SRM are resistant to heavy metals, can thrive in a harsh acidic or alkaline environment, withstand high salinity, and can use a variety of organic compounds as electron donors to reduce sulfate or sulfite to sulfide and sequester Pb or other metals as metal-sulfides (Cao et al., 2012; Martins et al., 2009; Utgikar et al., 2002; Vavourakis et al., 2019; recently reviewed by Ayangbenro et al., 2018). Thus, engineering applications harness the microbial processes to remediate Pb and other heavy metals from acid mine drainage (Elliott et al., 1998; Liu et al., 2017b; recently reviewed by Munyai et al., 2021); contaminated sediments (Li et al., 2017; Niu et al., 2018); wastewater (Kieu et al., 2015; Zhang et al., 2016a), and other environmental media (Beyenal and Lewandowski, 2004; Lin et al., 2010). This section focuses on Pb and toxic metal sequestration associated with sulfate reduction and molecular insights into these processes.
3.1. Biogeochemical processes of sulfate reduction and Pb immobilization
Sulfate reducing microorganisms (SRM) are ubiquitous in freshwater, brackish and marine environments (Jørgensen, 1977; Leloup et al., 2009; Li et al., 1999; Nakagawa et al., 2012; van Vliet et al., 2021; recently reviewed by Jørgensen et al., 2019; Wasmund et al., 2017) and have been isolated from diverse sources including dairy products, the human gastrointestinal tract, wastewater, sediment (Leloup et al., 2009), and low sulfate ground water (Bell et al., 2020; Feng et al., 2017; Doyle et al., 2018; Martins et al., 2009; Widdel and Pfennig, 1981). SRM live under anoxic conditions and use sulfate as a terminal electron acceptor to produce H2S; the genes associated with these processes are found in anoxic waters. Sulfur cycling relies on both anoxic and oxic conditions where, in addition to sulfate reduction to sulfides by SRMs, chemoautotrophic or photoautotropic sulfur bacteria or abiotic processes oxidize sulfides to sulfates (Fortin et al., 1995).
In aqueous environments, such as lakes and marine systems, pore water and leachates, SRM reduce sulfate in the presence of organic matter, and through a sulfite intermediate, produce hydrogen sulfide (S2−) and bicarbonate, summarized in the following reactions (Neculita et al., 2007):
Sulfide ions can complex with divalent metals, such as Pb2+, to form an insoluble precipitate (Fortin et al., 1995):
In the presence of Pb2+, lead sulfide is formed:
Electron microprobe analysis of marsh sediments revealed that lead sulfide also can aggregate with FeS2 and be sequestered as FePbS2 in an anoxic environment where ferrous iron (Fe2+) is more reactive than Pb2+ and FeS2 is formed preferentially (Moreau et al., 2013). Similarly, As and Cu may be sequestered as FeAsS2 and FeCuS2 whereas the sulfide of Cd may prefer to precipitate with Zn.
The above processes can be limited by the ambient environment, especially carbon sources. In a two-year Danish fjord study, sulfate reduction was coupled with the availability of organic matter and only a fraction (10%) of the produced sulfide formed metal-sulfide precipitates (Jørgensen, 1977). In a monomictic lake, the SRM Algidimarina propionica (Desulfobacteraceae) was correlated with sulfate reduction, a process that was limited by sulfate concentration as well as dissolved oxygen, temperature, pH, and availability of organic matter (Nakagawa et al., 2012; Sánchez-Andrea et al., 2014).
3.2. Functional genes associated with sulfide-mediated heavy metal sequestration
3.2.1. Sulfate and sulfite reduction functional genes and microbes
Gene products for sulfate and sulfite reduction pathways have been well described and genomic analysis provides insights into the reductive metabolism in an anoxic environment (Anantharaman et al., 2018; Vigneron et al., 2021). Of these, dissimilatory sulfate and sulfite reduction are most prevalent in Pb immobilization (Fig. 3). Sulfide is generated by sulfate adenylyltransferase (sat), adenylyl sulfate reductase alpha and beta subunits (aprAB) and which complexes with a quinone-interacting membrane-bound oxidoreductase (qmoABC) complex to transport electrons, and dissimilatory sulfite reductase alpha and beta subunits (dsrAB) (Friedrich, 2002; Fritz et al., 2000; Müller et al., 2015; Ramos et al., 2012; Wagner et al., 1998; Wasmund et al., 2016; Zane Grant et al., 2010). The dsr operon is comprised of dsrAB (catalytic core), dsrC (substrate delivery), dsrT (putative regulation), dsrEFH (sulfur carrier), dsrMKJOP (transmembrane complex involved in electron transport; Anantharaman et al., 2018; Ramos et al., 2012; van Vliet et al., 2021; Wasmund et al., 2016); a subset of these gene products are involved in the reverse reaction, i.e., oxidation of sulfide to sulfite and sulfate. For example, microbial oxidation of sulfide (electron donor) can involve the dsrAB and dsrEFH gene products. While not definitive, presence of dsrD may be indicative of preference for the reduction pathway (Anantharaman et al., 2018; Bell et al., 2020; Umezawa et al., 2020). The dsrD gene product has been shown to be down regulated in a high sulfide (10 mM) environment, possibly as a feedback mechanism to preclude toxic levels of sulfide (Caffrey and Voordouw, 2010). In the presence of Pb, less soluable sulfide precipitates can form in anoxic environments and sequester Pb.
Fig. 3.
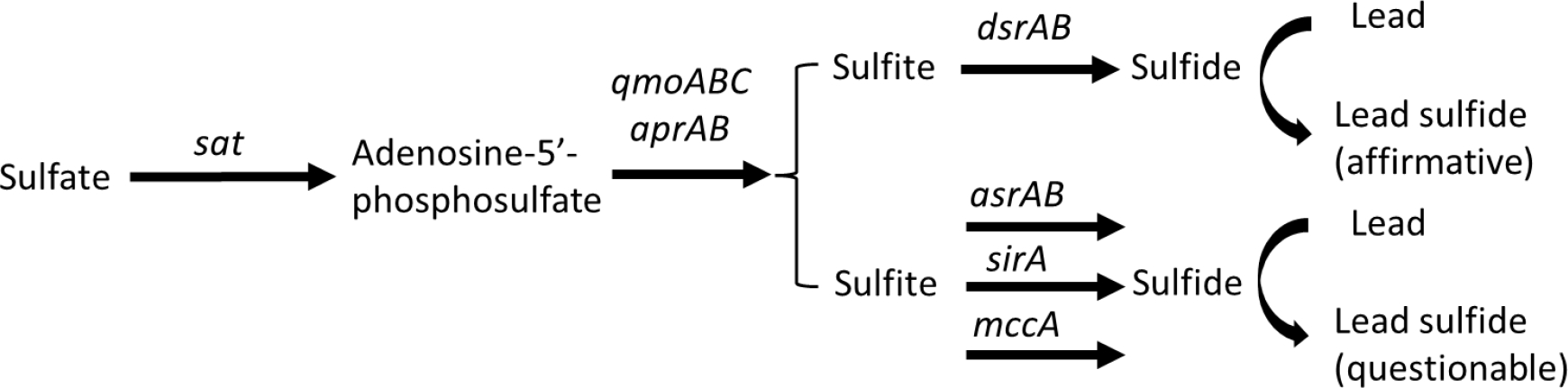
Dissimilatory sulfate and sulfite reduction for lead sulfide production. The functional genes involved include sat, sulfate adenylyl transferase (Fritz et al., 2000); qmoABC, quinone-interacting membrane-bound oxidoreductase (Ramos et al., 2012; Zane et al., 2010); aprAB, adenylyl sulfate reductase (Friedrich et al., 2002); dsrAB, dissimilatory sulfite reductase (sulfate reducing bacteria; Wagner et al., 1998); asrA, anaerobic sulfite reduction (Salmonella typhimurium; Huang and Barrett, 1991); sirA, cytochrome C sulfite reductase (Shewanella oneidensis; Shirodkar et al., 2011); mccA, cytochrome C sulfite reductase (Wolinella succinogenes; Eller et al., 2019; Kern et al., 2010).
The highly described Gram-negative dissimilatory sulfate reducing mesophilic bacteria, which belong to class Deltaproteobacteria, families Desulfovibrionaceae, Desulfobacteraceae, Desulfohalobiaceae, Desulfonatronumaceae, Desulfomicrobiaceae, and Syntrophobacteraceae include genera such as Desulfovibrio, Desulfomicrobiium, Desulfobulbus, and Syntrophobacter (Bao et al., 2021; Leloup et al., 2009; Müller et al., 2015; van Vliet et al., 2021). Other lessor represented sulfate reducers include members from the phyla Caldiserica (Caldiserica sp.), Firmicutes (Thermanaeromonas toyohensis, Desulfotomaculum sp.), Actinobacteria (Gordonibacter pamelaeae and Thermodesulfobium narugense), Thermodesulfobacteria (Thermodesulfobacterium commune), Nitrospirae (Thermodesulfovibrio yellowstonii, and Thermodesulfovibrio islandicus), Thaumarchaeota (Aigarchaeota archaeon), and Euryarchaeota (Archaeoglobus fulgidus, Archaeoglobus profundus) based in part on the presence of dissimilatory sulfate reductase genes dsrAB. Families Desulfobulbaceae and Desulfomicrobiaceae were positively correlated with reduced sulfur species (Bao et al., 2021; Leloup et al., 2009).
Communities in the anoxic zone are not as well described as ones from more oxic zones, however 16S rRNA and sulfate/sulfite reduction related gene sequencing has revealed new SRMs that harbor genes for dissimilatory sulfate (dsrAB) and/or anaerobic sulfite reduction (asrAB), such as members of the Class Dehalococcoidia (Phylum Chloroflexi), Planctomycetes, Candidatus Rokubacteria, Candidatus Woesearchaeota, Candidatus Omnitrophica, and Candidatus Parcubacteria (Candidatus Nealsonbacteria, Ca. Zixibacteria and Ca. Abyssubacteria; Hug et al., 2016; Vavourakis et al., 2019; Vigneron et al., 2020; Wasmund et al., 2016). While the sulfate reducing genes (e.g. sat, apr and dsr) may be detected, their presence does not confirm sulfate reduction activity nor does it rule out the ability to undergo the reverse dissimilatory oxidation of reduced species, such as sulfide (Umezawa et al., 2020).
Several facultative anaerobes harbor alternative dissimilatory sulfite reductase genes (Figure 3). The sirA (Shewanella oneidensis) and mccA (Wolinella succinogenes) gene products, cytochrome c sulfite reductases, also reduce sulfite to sulfide and are generally found in microorganisms that cannot respire sulfate (Hermann et al., 2015; Kern et al., 2011; Sanchez-Andrea et al., 2011; Shirodkar et al., 2011). S. oneidensis was demonstrated to reduce metals using sulfite as an electron acceptor, generating ZnS (Xiao et al., 2015), CuS (Zhou et al., 2016), FeS (Lutterbach et al., 2009), and Ag2S (Voeikova et al., 2016). While the literature is sparse on W. succinogenes metal resistance, the organism tolerated 20 mg L−1 Cd but not Zn or Cu at 30 mg L−1 or 2 mg L−1, respectively (Sinbuathong et al., 2013). Both S. oneidensis and W. succinogenes formed a black precipitate on Pb acetate test strips in vitro which is indicative of H2S production and PbS formation (Tanner et al., 1981; Wu et al., 2015), suggesting the potential for Pb resistant strains to generate PbS.
Sulfite reduction also is catalyzed by the asrAB gene product, siroheme-dependent anaerobic sulfite reductase (Fig. 3), described in Gammaproteobacteria (Salmonella spp. and Edwardsiella spp.), Firmicutes, Spirochaetes, and Fusobacteria, as well as newly identified Candidatus Omnitrophica, Candidatus Planctomycetes, Candidatus Riflebacteria, and Candidatus Parcubacteria (Anantharaman et al., 2018; Huang and Barrett, 1991; Vigneron et al., 2020). Strictly anaerobic sulfite reductase differs from its assimilatory cysteine synthesis analog, as described in studies on S. typhimurium (Haber et al., 1980; Huang and Barrett, 1990; Huang and Barrett, 1991). In assimilatory sulfate reduction, cysteine regulates H2S production with little to no accumulation. However, asrAB sulfite reduction in Salmonella spp. is an anaerobic dissimilatory process and H2S accumulates; H2S is not dependent on cysteine biosynthetic pathway regulation and gene expression (Hallenbeck et al., 1989; Huang and Barrett, 1990). Because genes for sulfate reduction were absent from Candidatus Riflebacteria and Candidatus Omnitrophica (Anantharaman et al., 2018), it is likely that sulfite reduction is a strictly anaerobic process similar to that in S. typhimurium where H2S accumulates and if Pb tolerant, PbS produced.
3.2.2. Use of sulfite reduction genes as environmental genetic markers
The dissimilatory sulfite reductase genes, dsrAB, dsrA, and dsrM, have been used as genetic markers to denote sulfate reduction in environmental systems (Ben-Dov et al., 2007; Müller et al., 2015; Nguyen et al., 2017; Scholten et al., 2005). Quantitative and qualitative enumeration of SRMs in freshwater and marine systems, as well as industrial wastewater, have been done with the adenylyl-sulfate reductase subunit alpha gene (aprA) usually in combination with the dsrA or dsrM genes (Ben-Dov et al., 2007; Keshri et al., 2015; Scholten et al., 2005). Deltaproteobacteria harboring sat, aprAB, dsrAB, and dsrD (dissimilatory sulfite reductase D) genes was unexpectedly found in low sulfate groundwater, which suggests, but not confirms, metabolic activity (Bell et al., 2020).
The dsrA and dsrM genes are present in marine sediments in both the sulfate reducing (20–150 cm) and deeper methanogenic (280–440 cm) zones, with no loss of bacterial community richness throughout the zones, to include the intervening transition zone (Leloup et al., 2009). In the sulfate reducing zone, the dsrA gene was associated with dominant families Desulfobacteraceae and Desulfobulbaceae, Desulfobacterium anilini, which was present in the transition and methane-rich zones, and Firmicutes, in the methane-rich zone. Dissimilatory-type sulfite reductase genes (dsrAB) have been detected in extreme environments including thermal water and sediment from heavy metal mining operations (Bao et al., 2021). Using polymerase chain reaction amplification and 16S rRNA gene analysis, hydrothermal water from a Cu-Pb-Zn mine was shown to contain the dsrAB genes which were attributed to Desulfotomaculum spp., Desulfovibrio spp., Desulfococcus spp., Thermodesulforhabdus norvegica, and Thermodesulfobacterium spp., among others (Nakagawa et al., 2012). The dsrB gene was detected in an acid mine drainage contaminated river (Hengshi River, China); classes Clostridia and Deltaproterobacteria and families Syntrophobacteraceae and Desulfobacteraceae were dominant in sediment (Bao et al., 2021).
Desulfovibrio spp. and Desulfotomaculum spp. have been isolated from Kidd Creek Cu-Zn sulphide mine tailings (Fortin et al., 1995). Tailings from the mine contained pyrite, pyrrhotite, sphalerite, chalcopyrite and galena; metals including Zn, Pb, Cd, and Cu, were released into the associated pore water as part of the refinery process (Al et al., 1994). The SRMs reduced sulfate to hydrogen sulfide which, upon dissolution, caused the formation of metal-sulfide precipitates when present (Al et al., 1994; Fortin et al., 1995). Dissimilatory-type sulfite reductase subunit β (dsrM) gene also was detected in metal contaminated sediments (Fe, Mn, V, Cr, Ni, Cu, Zn, Sr, Cd, Pb) and was more prevalent in the anaerobic rhizosphere of the marsh plant, Scirpus triqueter, than in surrounding sediment, suggesting a presence of SRM (Niu et al., 2018). Even though SRM abundance and SO42− concentration and Pb and sulfide concentration were positively correlated, nanoparticles observed throughout the rhizosphere sediments only contained S and Fe, Ag-Ca, or Pt-Cu.
3.3. Lead and toxic metal remediation by sulfate reduction sequestration
Leveraging the sulfur cycle and adapting bacterial sulfate reduction to alleviate anthropogenic derived metal contamination has been explored by many researchers (Fortin et al., 1995; Foucher et al., 2001; Liu et al., 2017a; Nguyen et al., 2017; Yin et al., 2020a; Yin et al., 2020b; recently reviewed by Ayangbenro et al., 2018). SRM biogenic sulfide production may be a less expensive and more environmentally friendly approach for Pb sequestration as PbS (Tong et al., 2021; Table 3). Generally, through a plethora of engineering approaches designed for specific matrices, the microorganisms and their metabolism are optimized to maximize precipitation of PbS. Formation of these insoluble metal-sulfide precipitates are detected by X-ray diffraction analysis (XRD), scanning electron microscopy (SEM), and various spectroscopic analytical approaches such as energy dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), fourier transform infrared spectroscopy (FTRI), induced coupled plasma optical emission spectroscopy or mass spectroscopy (ICP-OES/MS), atomic absorption spectroscopy (AAS), and synchrotron-based x-ray diffraction spectroscopy (SXRD) or synchrotron-based X-ray absorption fine structure spectroscopy (XAFS; Beyenal and Lewandowski, 2004; Karna et al., 2016; Li et al., 2016b; Nguyen et al., 2017; Pérez-López et al., 2018; Sinharoy and Pakshirajan, 2019).
Table 3.
Summary results of selected studies on removal of Pb and other heavy metals by sulfate reducing bacteria. All identified are from the phylum Proteobacteria.
| Source | Microorganisms | Lead Concentration | Reactor | Removal Efficacy | References |
|---|---|---|---|---|---|
| USA: Culture collection |
Desulfovibrio desulfuricans G20 | 30 mg L−1 | Flat-plate flow reactor with hematite or quartz containing sulfate reducing biofilm, pH 7.2 | 100% Pb + hematite <100% Pb + quartz | (Beyenal and Lewandowski, 2004) |
| Vietnam: Industrial wastewater from battery storage |
Consortium: Desulfovibrio vulgaris Desulfovibrio carbinolicus* Desulfobacterium autotrophicum Desulfomicrobium salsugmis Desulfocmicrobium escambiense* *dominant |
Loading rate 20, 30, 40 mgl−day−1 Pb Stock 0, 100, 150, 200 mg L−1 |
Moving bed biofilm reactor, pH 7.5 optimum (pH 6.5–8.5) | 99–100% Pb | (Kieu et al., 2015) (Nguyen et al., 2017) |
| China: Sewage treatment plant landfill leachate |
Desulfobulbus propionicus
Desulfovibrio vulgaris |
Cu, 17 mg L−1
Cd, 9.4 mg L−1 Zn, 131 mg L−1 Pb, 38 mg L−1 (Sediment) |
Reaction bottle +/- micro zero valent iron (mFe0) pH 6.2–7.5 |
100% Cu 98.5% Cd 90.7% Zn 100% Pb |
(Li et al., 2016b) |
| Thailand: Anaerobic lagoon treating tapioca wastewater |
Unidentified Sulfate Reducing Bacteria & Methane Producing Bacteria | 45–50 mg L−1 Pb | Two-Stage Reactor 1) Sulfide production (Anaerobic sludge blanket reactor, pH 7.5–8.5) 2) Lead precipitation (Chamber containing Pb(NO3)2; no SRB present) |
85–95% Pb | (Hien Hoa et al., 2007) 7.5–8.5 optimum 85–95% Pb removal |
| The Netherlands: Domestic wastewater anaerobic sludge |
Consortium: Not identified |
Cu, 10 mg ml−1 Zn, 10 mg ml−1 Pb, 10 mg ml−1 Cd, 10 mg ml−1 |
Inversed fluidized bed reactor, influent pH 7.0, effluent pH 6.6–7.5 | 98.4% Cu 96.5% Zn 96% Pb 97.9% Cd |
(Villa-Gomez et al., 2011) |
| China: Culture collection |
Not Identified | Cu, 7.1 mg kg−1 Zn, 339.6 mg kg−1 Pb, 12.7 mg kg−1 Cd, 80.2 mgkg−1 (Sediment) |
SRB immobilized on polyvinyl alcohol beads, pH7.2 | 91.2% Cu 95.6% Zn 100% Pb 76.3% Cd |
(Li et al., 2017) |
3.3.1. SRM-assisted biofilm reactor
The use of sulfate reducing biofilms is an effective approach for precipitating Pb. Desulfovibrio desulfuricans biofilms grown anaerobically on both quartz (SiO2) and hematite (Fe2O3) effectively immobilized Pb (Beyenal and Lewandowski, 2004). Less H2S production and Pb immobilization occurred on the hematite-grown biofilm, probably due to elevated concentrations of Fe2+ in the system competing for the scarcer sulfide. In an inversed fluidized bed reactor, Pb precipitation and recovery from synthetic wastewater, which contained 50 mg/L iron sulfate, was influenced by sulfide concentration (Villa-Gomez et al., 2011). Lead sulfide precipitates were suspended in the liquid phase in the higher sulfide concentration (648 mg/L) reactor whereas Pb precipitates were associated with the biomass at lower sulfide (44 mg/L) and thus, harder to recover, even though both concentrations had similar Pb recovery (58% versus 60%). In the presence of micro-sized Fe0 particles designed to scavenge available O2, Desulfobulbus propionicus and Desulfovibrio vulgaris were able to immobilize sediment Pb (380.6 mg kg−1), Cu (174.8 mg/kg), Cd (94.4 mg/kg), and Zn (1311.3 mg/kg) by 90.69% to 100%, depending on the metal (Li et al., 2016b). These authors suggested that the heavy metals combined with reduced sulfate metabolites to form insoluble compounds.
Nguyen et al. (2017) used a continuous moving bed biofilm reactor, populated with Desulfomicrobium escambiense, Desulfovibrio carbinolicusto, Desulfobacterium autotrophicum, Desulfomicrobium salsugmis, and Desulfovibrio vulgaris to determine optimal SRM communities and Pb concentrations to maximize removal rate of Pb from the wastewater system. Following dsrM gene-based denaturing gradient gel electrophoresis, fluorescent in situ hybridization, and chemical analysis, it was determined that Desulfomicrobium escambiense and Desulfovibrio carbinolicusto were instrumental in Pb removal and tolerated up to 40 mg L−1 day−1 for 20 days, however populations decreased by 40 days, contributing to reduced Pb removal compared to 20 mg L−1 day−1 and 30 mg L−1 day−1 removal was observed (91% versus 99%–100%). Desulfobacterium autotrophicum and Desulfomicrobium salsugmis tolerated up to 20 mg L−1day−1 for 40 days however Desulfovibrio vulgaris was inhibited by Pb concentrations used in the study. Lead precipitates attached to the cells were observed by scanning electron microscopy. Hoa et al. (2007) described a two-phase up flow anaerobic sludge blanket reactor system for Pb removal. In the first phase, SRM converted sulfate to sulfide in the absence of Pb, and in the second phase, Pb (Pb[NO3]2; 50 mg/L) was added to the sulfide-rich phase and 85–95% of the Pb was precipitated to lead sulfide and then removed from the system. The stoichiometric concentrations of Pb+2 and SH− were maintained to maximize PbS precipitation. Using highly controlled systems, i.e., optimizing sulfate, sulfide, Pb, and carbon source, as well as pH and temperature, or through less engineered approaches, Pb can effectively be precipitated as a sulfide and removed from the environment.
3.3.2. Applications with acid mine drainage
Both abiotic and biotic processes have been used to remediate acid mine drainage (AMD), which is typically contaminated with a variety of toxic metals (see more in-depth reviews on specific related topics by Papirio et al., 2013; Rambabu et al., 2020; Simate and Ndlovu, 2014; Tong et al., 2021). Although chemical processes are effective, some create a toxic environmental footprint that requires additional remediation (Tong et al., 2021). For the biotic processes, finding SRM that can survive in the extreme pH range (2–4) and are resistant to the toxic metals is challenging. Several configurations that enrich for SRM and the production of sulfide in the presence of toxic metals have been demonstrated (Fig. 4): 1) separate biogenic H2S production and toxic metal precipitation phases (Foucher et al., 2001); 2) inoculate the system with SRM (e.g. activated sludge) and demonstrate sulfate removal or H2S production prior to introducing AMD with toxic metals (Villa-Gomez et al., 2011); 3) increase the pH of the solution with lime or other alkaline material and then inoculate with SRM with or without the toxic metals (Sato et al., 2022; Sato et al., 2019); 4) inoculate the system with SRM and sequentially lower pH and then add AMD (acid/toxic metals; Elliott et al., 1998); 5) select acid and/or toxic metal tolerant SRM (e.g. mining site sediment, battery industry wastewater; enrichment and isolated cultures) and add to the system (Dev et al., 2021; Ňancucheo and Johnson, 2012; Nguyen et al., 2017; Zhang et al., 2016b); or 6) inoculate the system with a SRM source (e.g., mud from under coal gangue pile, pond sediment, activated sludge, isolated cultures) and concurrently introduce aqueous toxic metals (Chen et al., 2021; Liu et al., 2017b; Makhathini et al., 2021; Wang et al., 2021; Zhang et al., 2016a). Optimized systems have been demonstrated to remove greater than 99.5% Pb (loading rate 9.2 g/m3-d Pb(II)) and Hg (loading rate 2.6 g/m3-d Hg(II)) as PbS and HgS (Zhang et al., 2016a).
Fig. 4.
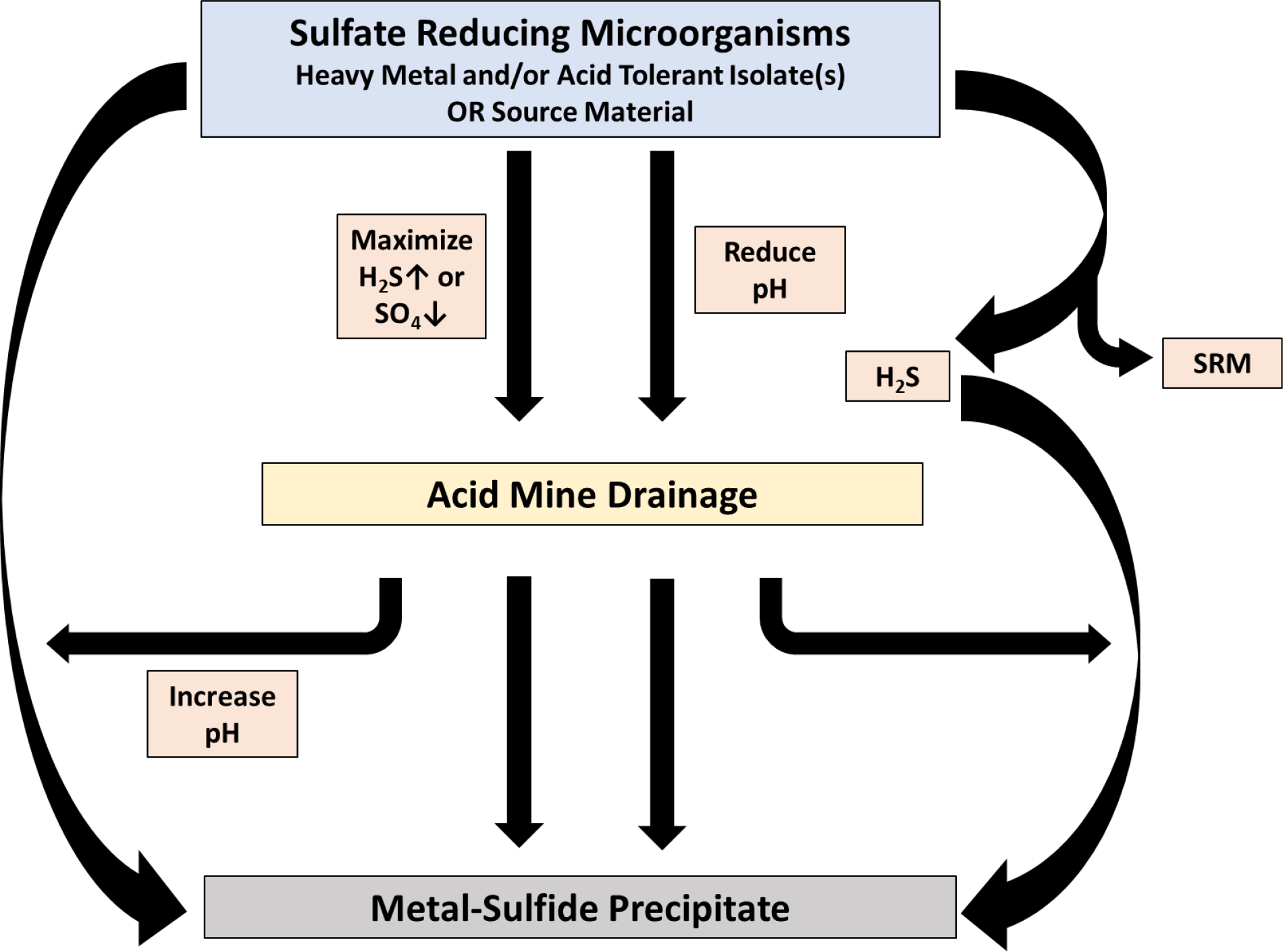
Applications that enrich for sulfate reducing microorganisms (SRM) or sulfide production in acid mine drainage and immobilization through metal sulfide precipitation.
Identifying economical nitrogen and carbon sources also are required to make using SRM advantageous. In one study, a packed bed reactor with introduced ground marine waste (dead crustaceans and mollusks and fish scales) as an organic nitrogen source was used to select for sulfate reducers and remove metals from acid mine waste (Dev et al., 2016). The marine waste performed better than tryptone, yeast extract, corn steep liquor, ammonium bicarbonate (NH4HCO3) and ammonium chloride (NH4Cl) and when introduced into the reactor; 62–66% of the sulfate and 66–75% of the divalent metals (Fe, Cu, Zn, Ni, Mg) were removed. The marine waste selected for sulfate reducers which accounted for 87–89% of the bacterial community and the genera Desulfovibrio (Deltaproteobacteria), Desulfotomaculum (Firmicutes), Desulfobacter (Deltaproteobacteria), classes Clostridia and Synergistia (Synergistetes), and members of the phylum Bacteroidetes dominated (Dev et al., 2016). Other engineered systems designed to remove toxic metals, which may be transferable to Pb remediation, have experimented with less costly carbon sources, such as rice husk (Sato et al., 2022; Sato et al., 2019), rice bran (Sato et al., 2022), sugar cane slag (Wang et al., 2021), corn cob (Wang et al., 2021), sunflower straw (Wang et al., 2021), walnut shells (Chen et al., 2021), and anaerobic sludge (Makhathini et al., 2021). In one study with three different reactor scenarios using vertical flow sulfate reducing bioreactors, AMD was neutralized with limestone, rice bran served as a carbon source, and the system was inoculated with soil as a source for SRM and Fe was removed from the system as applicable (Sato et al., 2019). After approximately four to five months, Desulfosporosinus meridiei dominated and other Desulfosporosinus (D. fructosivorans), SRM-like Clostridium, Ruminococcus, Desulfovibrio and Desulfobulbus were prevalent in each system. In a previous study using a similar system, Zn (83–100%), Cd (93–100%), and Cu (99–100%) were removed and Desulfatirhabdium butyrativorans was dominant (Sato et al., 2018). Sulfate reduction was correlated with metal recovery rate. In another system using dynamic columns inoculated with SRM to evaluate the efficacy of different carbon sources, iron scrap was added to enhance the system performance (Wang et al., 2021). All three carbon sources (sugarcane slag, corn cob, sunflower straw) supported efficient removal of Cr6+ (95–97%); the corn cob carbon source yielded maximum Cr3+ (86%) and SO42− (75%) removal.
Passive systems, which may be more economical and easier to implement at the source, also can be effective at removing Pb from mine waste. In an aqueous laboratory scale open tank system, SRM and iron reducing bacteria were added to sulfidic mine waste (chalcopyrite tailings) and incubated for 60 days, resulting in decreased dissolved metals (Fe, Cu, Zn), including an 85% reduction in dissolved Pb (Liu et al., 2017b). At the end of the incubation period, Desulfosporosinus medirie (Firmicutes) was the dominant sulfate reducer. Seven months after the SRM and iron-reducing bacteria were applied to Pb-Zn sulfide mine tailings in the field, Fe, As, and Sb, as well as S, were reduced however Pb, Zn, and Cd were indistinguishable from initial concentrations. However, toxicity was reduced as evidenced by moss and plant growth over the initially barren area.
In an effort to assess efficiency of constructed wetlands to remove metals, microcosm tanks containing walnut shells as a carbon source were inoculated with pond sediment and activated sludge and seedlings (Iris pseudoacorus L.) were planted in the microcosm (Chen et al., 2021). The synthetic AMD (pH 4), containing FeSO4 (50 mg L−1), and K2Cr2O7, CuCl2, CdCl2, and ZnSO4 (concentration at 5 mg L−1 for each) was introduced into the microcosm which was reinoculated 30 days later. Sequential “fill and drain” batch operations occurred over a period of 200 days with several nutrient and carbon source deviations. Over 50% of Cu was sequestered as copper sulfide; lower concentrations of other metal sulfides were identified, primarily in the walnut shell substrate. Few SRM were detected in Phase I when acidic and oxygenic conditions prevailed; Desulforegula and Desulfovibrio were detectable at low numbers. However, Desulfobulbus, Desulfosporosinus, and Desulfatirhabdium were selected for in the control and treated constructed wetland microcosms following inoculation of both with wastewater. Batch bioreactors supplemented with various carbon sources and artificial AMD, successfully removed up to 100% of Al (61 mg/L), Fe (171 mg/L), and Mn (2 mg/L), presumably as metal sulfides (Neculita et al., 2011). During the 35-day period, elevated SRM counts positively correlated with sulfide production and increase in pH; a 1:1 sawdust and cow manure mixture was most effective at pH 3. While not all studies report results in terms of Pb removal, the processes involved are applicable because formation of metal sulfides from toxic divalent metals is demonstrated as the removal mechanism. Furthermore, metal resistance genes have been shown to be co-located, thus SRM Pb tolerance is plausible (Kang and So, 2016; Leedjarv et al., 2008; Pal et al., 2017).
4. Immobilization of Lead through Microbial Induced Carbonate Precipitation
Microbial induced carbonate precipitation (MICP) is well documented and has been shown to be facilitated by Bacteria, Archaea, and Eukaryotes under favorable conditions, i.e., an alkaline environment, presence of a nucleation site, and adequate concentration of substrates. The process has shown promise for soil and sand stabilization (Graddy et al., 2021; Montoya et al., 2014; Ohan et al., 2020), stone and cement repair (Pei et al., 2013; Su et al., 2021), and toxic metal remediation (Liu et al., 2021; Xue et al., 2022; Zeng et al., 2021). MICP, which has been reported to modulate Ca2+ levels intracellularly and extracellularly to optimize conditions for growth, is connected to carbon, nitrogen, and sulfur metabolism (Hammes and Verstraete, 2002; Schultze-Lam et al., 1996; Wright, 1999; recently reviewed by Castro-Alonso et al., 2019; Görgen et al., 2021). While most inorganic carbon generating mechanisms under alkaline conditions can produce calcium carbonate, potential engineering applications have selected for strains with high urease activity which results in higher yields of calcium carbonate from urea and maximizes performance for the intended purpose such as Pb immobilization (Table 4). Various polymorphisms, i.e., calcite, aragonite, vaterite and dolomite can accumulate, a process governed by species and/or biomineralization mechanism (Ferrer et al., 1988; Roberts et al., 2004; Zhang et al., 2019a). This section focuses on lead and toxic metal sequestration associated with MICP.
Table 4.
Summary results of selected studies on immobilization of lead by microbial induced carbonate precipitation.
| Source | Microorganism (Phylum) | Metal Tolerance | Precipitate | Immobilization Efficacy | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
| United Kingdom: Culture collection |
Sporosarcina pasteurii (Firmicutes) |
PbCl2, 1.0 mM ZnCl2, 0.5 mM CuCl2, 0.5 mM CdSO4, 0.06 mM |
PbCO3 CaCO3 Biotic/Abiotic ~pH9.0 |
(Mugwar and Harbottle, 2016) | |
| USA: Culture collection |
Sporosarcina pasterii (ATCC 6452) | Pb(NO3)2, 10–40 mM | (PbCl)2CO3 PbCl(OH) PbCO3 Pb3(CO3)2(OH)2 CaCO3 PbCl2 (Abiotic) |
(Jiang et al., 2019) | |
| South Korea: Culture collection |
Sporosarcina pasterii (KCTC 3558) | Pb(CH3COO2), 0.01–1 mM CuCl2, 0.01–1 mM ZnCl2, 0.01–1 mM Cd(C2H3O2)2, 0.01–1 mM SrCl2, 1–30 mM |
PbCO3 (Cerussite) CaCO3 (Aragonite, Calcite, Vaterite) pH >9.0 ZnCO3 pH >8.5 CdCO3 pH >9.0 (Sr,Ca)CO3 pH>9.0 |
>99% Pb 30% Zn 60% Cu 43.2–60% Cd >99% Sr |
(Kim et al., 2021) |
| Germany: Culture collection |
Sporosarcina pasteurii (DSM 33) | Pb, 342 mg kg−1 Zn, 235 mg kg−1 Cd, 6.8 mg kg−1 |
PbCO3 CaCO3 ZnCO3 CdCO3 |
(Liu et al., 2021) | |
| USA: Culture collection |
Sporosarcina pasterii (ATCC 11859) | Pb(NO3)2, 25 mg/L CdCl2, 5.6 mg/L |
Pb3(CO3)2(OH)2 (Hydrocerussite) PbCO3 CdCO3 CaCO3 (Calcite) |
(Zeng et al., 2021) | |
| China: Culture collection |
Sporosarcina pasterii | PbCl2, 10–50 mM | ~100% Pb | (Xue et al., 2022) | |
| China & USA: Culture collection & garden soil |
Sporosarcina pasteurii ATCC 11859 Sporosarcina globispora UR53 Sporosarcina Koreensis UR47 Sporosarcina sp. R-31323 (UR31) Terrabacter tumescens AS.1.2690 (Actinobacteria) Bacillus lentus UR41 (Firmicutes) |
PbCl2, 2 mg ml−1 NiCl2, 2 mg ml−1 CuCl2, 2 mg ml−1 CoCl2, 2 mg ml−1 ZnCl2, 2 mg ml−1 CdCl2, 2 mg ml−1 |
PbCO3 NiCO3 CuCO3 CoCO3 ZnCO3 CdCO3 pH 8–9 |
100% Pb 88% Ni 91% Cu 91% Co 95% Zn 97% Cd |
(Li et al., 2013) |
| South Korea: Abandoned mine soil |
(Firmicutes) Sporosarcina soli B-22 Viridibacillus arenosi B-21 Lysinibacillus sphaericus KJ-64 Sporsacina pasteurii WJ-2 (Proteobacteria, Gamma-) Enterobacter cloacae KJ-46 |
PbCl2, 3000 mg L−1 CoCl2, 300–3000 mg L−1 CoSO4, 300–3000 mg L−1 CuSO4, 300–3000 mg L−1 CuCl2, 300–1000 mg L−1 FeCl3, 3000–1000 mg L−1 FeSO4, 300–3000 mg L−1 CdCl2, 300–3000 mg L−1 BaCl2, 2000–3000 mg L−1 SrCl2, 3000 mg L−1 ZnSO4, 300–3000 mg L−1 |
PbCO3 CaCO3 C4CuO4 Zn(OH)2 |
(Kang and So, 2016) | |
| Mexico: Mine tailings (mg/kg) As, 1140–11,800 Pb, 10100–43,700 Zn, 780–10,000 Cd, 8–780 Cu, 72–1320 Fe, 6000–12,300 |
Sporosarcina luteola (UB3 & UB5) | AsV, CrVI, Fe3+, Fe2+, Co2+, Rb+ Sb3+; 25–50 mM Mn2+, AsIII, Cu2+, Ni2+, Ba2+; 5–10mM Pb2+, Cd2+, Zn2+, Hg2+, Te4+, Ag+; <1mM |
PbCO3 (Cerussite) CaCO3 (Calcite, Vaterite) MnCO3 (rhodochrosite) Mg5(CO3)4(OH)2 (hydromagnesite) CdCO3 (otavite) SrCO3 (strontianite) BaCO3 (witherite) Zn5(CO3)2(OH)6 (hydrozincite) pH 8.7–9.0 |
(Cuaxinque-Flores et al., 2020) | |
| USA: Mine discharge sediment ~102 mg/L As ~101 Cd ~103 Cu ~103 Zn |
Consortium Sporosarcina spp., 95% Acidovorax spp., 3% (Proteobacteria, -Beta) |
Pb2+, ~103-104 mg L−1 | Precipitation (not identified) pH>8.0 |
(Proudfoot et al., 2022) | |
| China: Culture collection |
Terrabacter tumescens A12 (Actinobacteria) |
PbCl2, 2 mg ml−1 NiCl2, 2 mg ml−1 CuCl2, 2 mg ml−1 CoCl2, 2 mg ml−1 ZnCl2, 2 mg ml−1 CdCl2, 2 mg ml−1 |
PbCO3 CaCO3 NiCO3 CuCO3 CoCO3 ZnCO3 CdCO3 pH 9.0 |
99% Pb 90% Ni 90% Cu 91% Co 97% Zn 99% Cd 99% Ca |
(Li et al., 2016a) |
| China: Electronic waste |
Lysinibacillus sp. (GY-3) | PbCl2, 20–1000 ppm PbCl2, 20–200 ppm CuCl2, 20–100 |
Precipitation (not identified) | 87.5–100% Pb 80.6–98.7% Cu |
(Li et al., 2021) |
| China: Mining area soil |
Kocuria flava CR1 (Actinobacteria) | Pb(NO3)2, 50 mM Pb, 100 mg kg−1 |
PbCO3 CaCO3 (Calcite, Vatarite) |
83.4% Pb | (Achal et al., 2012) |
| South Korea: Pb contaminated mine tailings soil Pb, 1050 mg/kg Zn, 431 mg/kg Cu, 93 mg/kg As, 65 mg/kg |
Bacillus sp. KK1 | Pb(NO3)2, 300 mg/L ZnCl2, 150 mg/L CuCl2, 650 mg/L As, 150 mg/L (Mine tailing soil) |
PbCO3 PbSiO3 (Alamosite) PbS |
(Govarthanan et al., 2013) | |
| China: Lake water |
Exiguobacterium sp. (JBHLT-3; Firmicutes) | PbCl2, 1mM | PbCO3 CaCO3 (Calcite, Vaterite (saltwater) |
89% Pb | (Bai et al., 2021) |
| South Korea: Abandoned metal mine soil |
Enterobacter cloacae KJ-47 & KJ-46 | 100 Mm PbCl2 (MIC) | PbCO3 | 60% Pb | (Kang et al., 2015) |
| Japan: Soil near beachrock |
Pararhodobacter sp. (Proteobacteria, -Alpha) |
PbCl2, 1036 mg L−1 | PbCO3 CaCO3 (Calcite & Vaterite) Biotic/Abiotic pH 8.8 |
100% PB | (Mwandira et al., 2017) |
| Egypt: Soil |
Micrococcus sp. NCTC-1716 or WD-9 (Actinobacteria) |
Pb(NO3)2, 5 mM CdSO4, 2mM ZnCl2, 3 mM FeSO4, 3 mM |
PbCO3 CaCO3 ZnCO3 CdCO3 FeCO3 pH 9 |
61% Cd 97% Pb 75% Zn 88% Fe |
(Gomaa, 2019) |
| China: Heavy metal contaminated soil from industrial area |
Staphylococcus epidermis HJ2 (Firmicutes) | PbCl2, 50 mg l−1 K2Cr2O7, 50 mg l−1 |
PbCO3 CaCO3 pH >9.0 |
86% Pb 76.8% Cr |
(He et al., 2019) |
| Egypt: Culture collection |
Proteus mirabilis (10B) | Pb, 350 ppm Hg, 350 ppm |
PbCO3 Pb2O CaPbO3 Hg2O (Aerobic/Anaerobic) pH 8.4 |
95.2% Pb 92% Hg 98.4% Ca |
(Eltarahony et al., 2020) |
| Bacteria & Eukaryota | |||||
| Nigeria: Coal mine drainage |
Proteobacteria (51%) Bacteroidetes (19%) Ascomycota (61%) Ciliophora (13%) |
Pb, 326 mg L−1 Cd, 95.0 As, 307.6 Ni, 28.8 Co, 27.3 acid mine drainage |
Heavy metal carbonate precipitates pH >8.2 |
94.8% Pb 96.3% Cd 88.9% As 90.6% Ni 27.3% Co |
(Oyetibo et al., 2021) |
| Egypt: Culture collection |
Metschnikowia pulcherrima (29A) Raoultella planticola (VIP; Proteobacteria, Gamma-) Alcaligenes faecalis (46N) Bacillus aryabhattai (39A) Ochrobactrum sp. (CNE2) Streptomyces cyaneofuscatus (EM3) |
VIP & 29A: Pb(CH3COO)2 & HgCl2, 700 ppm 39A, 46N, EM3, CNE2: inhibited by Pb(CH3COO)2 & HgCl2, 175 ppm |
CaPbO3 PbCO3 (Cerussite) CaHgO2 HgO |
100% Pb 100% Hg >95% Ca |
(Eltarahony et al., 2021) |
| Eukaryota | |||||
| Australia: Karstic cave |
Aspergillus sp. (UF3) Fusarium oxysporum (UF8) |
PbNO3, 10–100 mM SrCl2, 10–100 mM |
PbCO3 Pb2OCO3 CaCO3 (Aragonite, Vaterite, Calcite) SrCO3 |
(Dhami et al., 2017) | |
| China: Contaminated cement sludge Pb, 366 mg/kg Cr, 91.4 mg/kg |
Penicillium chrysogenum (CS1) | Pb(CH3COO)2, 400 mg l−1 K2Cr2O7, 100 mg l−1 |
Pb3(CO3)2(OH)2 (Hydrocerussite) CaCO3 (Calcite & Vaterite) Calcium chromium oxide carbonate pH 9.22 & 8.47 |
98% Pb 39–65% Cr |
(Qian et al., 2017) |
4.1. Biogenesis of carbonate for Pb immobilization
The most prevalent biotic scenarios with MICP involve ureolytic organisms, which have urease enzymatic activity that can convert urea to ammonium (NH4+) and carbonate (CO32−) accompanied with increase in pH (Fig. 5). In turn, divalent cations, such as Pb2+ and Ca2+ can bind to carbonate and form precipitates, lead carbonate (PbCO3) and calcium carbonate (CaCO3). These processes are well tested and verified in the laboratory setting. Both active and passive biogenesis mechanisms have been described (Ferrer et al., 1988; Hammes et al., 2003; Roberts et al., 2004; Zhang et al., 2019a). Highest yields of microbial mediated PbCO3 and CaCO3 precipitates occur when urea is hydrolyzed by urease creating an alkaline environment through the reaction below as described by (Schultze-Lam et al., 1996) and adapted from (Stocks-Fischer et al., 1999).
Fig. 5.
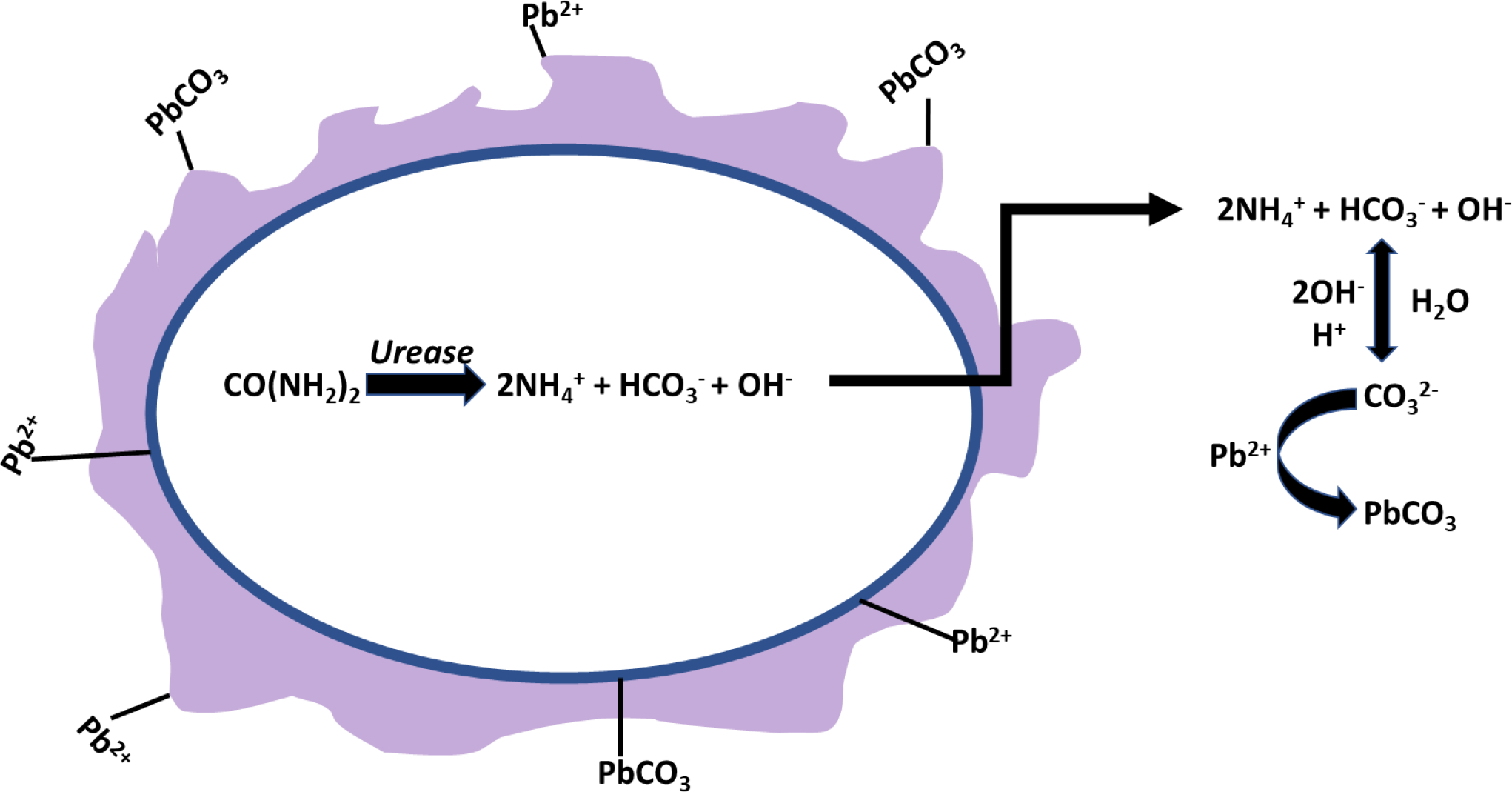
Urease mediated biogenesis of carbonate and for lead immobilization through biomineralization and biosorption with a bacterium cell. Purple shade represents exopolysaccharide produced by the bacterium and blue line is the cell wall.
In the presence of Pb2+ (and other divalent metals):
In addition to urea hydrolysis (Graddy et al., 2021; Zhu et al., 2019b), other mechanisms generating or utilizing inorganic carbon have been described for MICP such as dissimilatory sulfate reduction (Lin et al., 2018; Wright, 1999), nitrification/denitrification (Eltarahony et al., 2020; Erşan et al., 2016; Zhu et al., 2019b), iron reduction (Kenward et al., 2009), methanogenesis (Kenward et al., 2009; Roberts et al., 2004; Su and Yang, 2021), fatty acid metabolism (Barabesi et al., 2007) and photosynthesis (Benzerara et al., 2014). Urease activity has been correlated with the ureC gene (Urease subunit alpha) in mining site isolates Sporosarcina luteola UB-3 and UB-5, Sporosarcina soli B-22, Sporosarcina pastuerii WJ-2, Viridibacillus arenosi B-21, Enterobacter cloacae Kj46 and KJ-47, and Lysinibacillus sphaericus KJ-64 (Cuaxinque-Flores et al., 2020; Kang and So, 2016). Carbonic anhydrase, which catalyzes reversable hydration of CO2 forming HCO3−, also has been implicated in calcite formation in Bacillus sp. AP4, Bacillus megaterium AP6, and Bacillus simplex AP-9 (Achal and Pan, 2011). In Bacillus subtillis PB-19, targeted mutations in the lcfA operon (lcfA, ysiA[fadR], ysiB [fadB], etfB, and etfA), which is linked to fatty acid metabolism impaired CaCO3 formation indirectly suggesting a role of this operon in formation of the Pb+2- Ca2+ carbonate matrix (Barabesi et al., 2007; Frandi et al., 2011; Fujihashi et al., 2014).
Mechanisms linked to carbonate production do not occur in isolation. Isolates from Pb, As, and Zn contaminated mine tailing soil identified as Bacillus sp., linked a reduction in exchangeable Pb and increase in Pb carbonate to urease, dehydrogenase and phosphatase activities, suggesting multiple mechanisms involved in Pb immobilization (Govarthanan et al., 2013). Dehydrogenase activity, which is involved in organic compound oxidation-reduction reactions, provided insight into metabolic status under the PbCO3 synthesis conditions. Kocuria flava, a Pb resistant isolate from a Pb mining area, also formed Pb carbonate, which positively correlated to urease and dehydrogenase activities and negatively correlated to toxicity (Achal et al., 2012).
4.2. Mechanism of Pb immobilization through carbonate biomineralization
Precipitating Pb in its carbonate form has been described extensively as a potential Pb remediation strategy with both biotic and abiotic processes involved in Pb carbonate synthesis. While this section focuses on the biotic process, one cannot ignore the abiotic mechanisms because both can co-occur with ion exchange of Pb2+ for Ca2+. Because heavy metals of similar ionic radius to Ca2+, such as Pb2+, can co-precipitate with and/or substitute for Ca2+, this principal can be applied to Pb remediation. Both natural limestone formations and limestone enriched soil and water field applications can sequester Pb as minerals e.g., PbCO3, Pb3 (CO3)2(OH)2, and PbO (Aziz et al., 2008; Berger et al., 2000; Caraballo et al., 2009; Fuchida et al., 2020). In addition, Pb, Cd, Cu, and Zn can bind to calcite surfaces such as aragonite and calcite mollusk shells (Chada et al., 2005; Du et al., 2011; Elzinga et al., 2006). Calcite crystals have been reported to preferentially incorporate metals (Cu2+, Zn2+, Cr4+) during formation and Pb2+ has a high affinity to calcite, strongly sorbing to the surface (Chada et al., 2005; Elzinga and Reeder, 2002; Elzinga et al., 2006; Mugwar and Harbottle, 2016; Rouff et al., 2006; Tang et al., 2007). Because the bacterial cell wall (Kulczycki et al., 2002; Pei et al., 2013; Perito et al., 2014) and associated exopolysaccharide or capsular polysaccharides (Azulay et al., 2018; De Philippis et al., 2011; Ercole et al., 2007; Su et al., 2021; Yin et al., 2020b) are electronegatively charged, they can serve as nucleation sites for calcium carbonate crystals in the presence of Ca2+, inorganic carbon (CO2, CO32−,HCO3−), and alkaline pH (Hammes and Verstraete, 2002; Schultze-Lam et al., 1996). The same is true for biomineralization of cerussite in the presence of Pb2+ (Fig. 5). Thus, biotic and abiotic precipitation as well as biosorption contribute to the sequestration of Pb. The associated mineral products are characterized by X-ray diffraction (XRD) patterns and scanning electron microscopy (SEM) images or through other modern analytical approaches.
Biomineralization of lead carbonate through MICP has been observed with a variety of species. Sporosarcina globispora, Sporosarcina koreensis, Sporosarcina sp., Sporosarcina pasteurii, Bacillus lentus, and Terrabacter tumescens incubation with PbCl2 resulted in 100% removal of Pb, observed by SEM and XRD as extracellularly-bound Pb crystalline carbonate structures at pH 8–9 (Li et al., 2013; Li et al., 2016a). Greater than 97% Pb removal was observed for Micrococcus sp. and Pararhodobacter sp. resulting from co-precipitation of PbCO3 and CaCO3 (Gomaa, 2019; Mwandira et al., 2017). Both strains formed PbCO3 biotically through the MICP mechanism and abiotically, through Pb2+ substitution for Ca2+ in the calcite crystalline structure. Urease positive Raoultella planticola VIP and the fungus Metschnikowia pulcherrima 29A were resistant to 700 mg/L Pb(CH3COO)2, precipitating it as CaPbO3, lead oxide, and PbCO3 cerussite (Eltarahony et al., 2021). Penicillium chrysogenum, isolated from Cr and Pb contaminated cement sludge, removed 99% of the Pb (Qian et al., 2017). Fungal uptake, through both biosorption and MICP mechanisms, accounted for Pb immobilization as 1–10 μm particles, visualized by SEM. Carbonate products included calcite, vaterite, hydrocerussite [lead hydroxycarbonate; Pb3(CO3)2(OH)2], and lead carbonate (PbCO3); both Ca2+ and Pb2+ containing precipitates occurred in parallel. In another study, a Pb resistant ureolytic Staphylococcus epidermis strain (HJ2), isolated from heavy metal contaminated soil, similarly co-precipitated calcite and Pb carbonate, immobilizing 86% of the Pb present (He et al., 2019). In a yeast extract enriched medium, ureolytic Sporosarcina pasteurii removed almost 100% Pb in solution (Jiang et al., 2019). Geochemical modelling revealed that >85% Pb was removed from the system resulting in co-precipitation of PbCO3 and CaCO3, with Pb carbonate intermediates, (PbCl)2CO3 and Pb3(CO3)2(OH)2.
4.3. Microbial Isolates for removal of Lead and toxic metals as carbonate precipitates
When microbial induced carbonate precipitation (MICP) is the method of choice to explore environmental Pb remediation options, urease producing Pb resistant microbial strains or consortia usually are selected and their efficacy for PbCO3 biogenesis determined experimentally. Microbial strains recovered from toxic metal contaminated sites, e.g., mining soils and leachates, cements, and electronic waste, are good candidates for Pb remediation through the MICP mechanism (Table 4). Represented bacterial phyla include Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria and eukaryotes, Ascomycota and Ciliophora. Clearly, production of PbCO3 has the potential to improve the overall health of the ecosystem by immobilizing Pb and alleviating Pb induced stress. Selecting for strains and consortiums that produce high urease activity and are resistant to toxic metals show much promise for environmental applications.
4.3.1. Single isolates
Ureolytic isolates recovered from environmentally contaminated sites also have been studied for their ability to precipitate metals. In addition to strains mentioned in Section 4.2, Sporosarcina luteola UB3 and UB5 were isolated from mine tailing contaminated with As, Pb, Zn, Cd, Cu, and Fe; the isolates were resistance to 25–50 mM As5+, Cr6+, Fe3+, Fe2+, Co2+, Rb+ Sb3+; 5–10mM: Mn2+, As3+, Cu2+, Ni2+, Ba2+; and <1mM: Pb2+, Cd2+, Zn2+, Hg2+, Te4+ Ag+ (Cuaxinque-Flores et al., 2020). Urease activity was correlated to metal resistance and precipitates included cerussite, calcite, and vaterite. In other studies, Enterobacter cloacae, Viridibacillus arenosi, Sporosarcina soli, Lysinibacillus sphaericus, and Sporosacina pasteurii were isolated from soil recovered from an abandoned mine (Kang et al., 2015; Kang and So, 2016). All strains harbored the ureC gene and had urease activity, were resistant to a variety of heavy metals and antibiotics and produced calcite. Two Enterobacter cloacae isolates produced a PbCO3 precipitate which positively correlated with viability, thus immobilizing 68% of the Pb (Kang et al., 2015).
Bacterial isolates with no apparent previous exposure to heavy metals can mineralize Pb and other metals into carbonate compounds (Bai et al., 2021; Gomaa, 2019; Jiang et al., 2019; Kim et al., 2021; Li et al., 2013; Li et al., 2016a; Liu et al., 2021; Mugwar and Harbottle, 2016) (Xue et al., 2022; Zeng et al., 2021). Soil isolates, Sporosarcina globispora, Sporosarcina koreensis, Sporosarcina pasterii, Sporosarcina sp., Bacillus lentus, and Pararhodobacter sp., and well as culture collection isolates, Terrabacter tumescens, Sporosarcina pasteuria, and Micrococcus sp., effectively biomineralized Pb and/or Fe, Ni, Cu, Co, Zn, Cd, Fe through the MICP mechanism (Jiang et al., 2019; Kim et al., 2021; Li et al., 2013; Li et al., 2016a; Liu et al., 2021; Xue et al., 2022; Zeng et al., 2021).
Sporosarcina pasterii, obtained from various culture collections, is especially efficient precipitating lead and other toxic metals in the laboratory. In cultures containing 10 mM to 50 mM lead nitrate, 95–97% of the Pb was precipitated (Jiang et al., 2019). Similar results were observed when S. pasterii was grown in 0.01–1 mM lead acetate; Cu (60%), Zn (30%), Cd (43%), and Sr (100%) also were removed from solution (Kim et al., 2021). S. pasterii precipitated 100% Pb and Cd from landfill leachate, supplemented with lead nitrate (25 mg/L) and cadmium chloride (5.6 mg/L; Zeng et al., 2021). When a complex gas plant soil, contaminated with Pb (352 mg/kg), Zn (235 mg/kg), and Cd (6.8 mg/kg), was inoculated with S. pasterii in an aqueous medium, 14–68% Pb, 34–79% Zn, and 70–86% Cd were removed, resulting in a decrease in toxicity as evidenced by a bacterial luminescence assay (Liu et al., 2021). Metabolically inactivated S. pasteurii also removed >90% available Pb (0.05 mM to 5 mM PbCl2) by Day 7 of incubation. At concentrations of 0.05 mM to 0.5 mM (PbCl2), this was accompanied by an increase in pH (>8) and reduction of Ca2+ (>95%). However, decrease of Ca2+ lagged at the higher doses (1mM to 5mM PbCl2) and an increase in pH was not observed (Mugwar and Harbottle, 2016). This suggests involvement of an abiotic mechanism and/or spore regeneration.
It should be noted that optimizing urea hydrolysis and nitrate reduction, which generate HCO3−, can help achieve higher levels of carbonate precipitation. Optimized Ralstonia eutropha H16 urease and nitroreduction activities in solution, substantially improved calcium carbonate biomineralization (Zhu et al., 2019b). Optimized activity of periplasmic and membrane-bound nitroreductases in Proteus mirabilis 10B enhanced Pb precipitation resulting in higher removal efficiencies under anoxic (91%) and oxic (95%) conditions following incubation with 350 ppm lead acetate and 350 ppm mercuric chloride (Eltarahony et al., 2020). Lead was precipitated as PbCO3, Pb2O, and CaPbO3. Not only does precipitate formation modulate Ca2+ levels, but it also serves as a resistance mechanism that protects the cell by sequestering Pb2+ and other toxic metals.
4.3.2. Consortia isolates
Consortia isolated from targeted remediation sites may be more adapted to the complex environmental chemical, physical and biological conditions and therefore, more effective in biomineralizing Pb and other toxic metals. A consortium isolated from mine discharge sediment contaminated with Pb, As, Cd, Cu and Zn, comprised of 95% Sporosarcina spp. and 3% Acidovorax spp., precipitated Pb as observed by scanning electron microscopy (Proudfoot et al., 2022). A microbial consortium, isolated from coal mine drainage contaminated with Pb, Cd, Co, Ni, As, Fe, and Cr, was comprised of Proteobacteria and Bacteroidetes, which constituted 51% and 19% of the Bacteria, respectively, and Ascomycota and Cilophora, which accounted for 61% and 13% of the eukaryotes identified (Oyetibo et al., 2021). Seven taxonomic groups, were identified from the coal mine drainage as phylum Proteobacteria (class Gammaproteobacteria) Acinetobacter pittii group (Moraxellaceae), Enterobacteriaceae group (Enterobacteriaceae), unclassified FWNZ species (Enterobacteriaceae), and Pseudomonas citronellolis group (Pseudomonadaceae); and phylum Firmicutes (class Bacilli) Sporosarcina koreensis group (Planococcaceae), Bacillus cereus group (Bacillaceae) and Exiguobacterium aurantiacum group (Exiguobacteriaceae). The consortium was urease positive and formed metal-carbonate precipitates which negatively correlated with metal concentrations.
5. Environmental Factors for Lead Sequestration
Sequestration as phosphate-, sulfide- and carbonate precipitates are viable options for Pb and toxic metal sequestration and have the potential to decrease Pb bioavailability. While PSB has been demonstrated for field applications with contaminated soils (Bradham et al., 2018; Park et al., 2011b), MICP and SRM have primarily been used under carefully controlled conditions (Kim et al., 2021; Sato et al., 2022). However, MICP cementing and soil stabilization properties, have been demonstrated in the field (Gomez et al., 2015; Phillips et al., 2016), suggesting the potential of this microbial process for use as an environmental remediation tool for Pb and other metals. New, more sustainable biomineralization technologies under investigation include incorporating sandstone waste (Sharma et al., 2022), carbide sludge (Yu and Zhang, 2023), or sulfate reducing bacteria containing granules (Chetty et al., 2022). Generating a biological based material that can withstand various matrices, such as freshwater, saltwater, and wastewater results in greater material versatility with a lower carbon footprint that can be employed in more remote locations (Chetty et al., 2022; Yu and Rong, 2022). Field application of these new technologies may inform future Pb sequestration field efforts.
While many microbial strains and consortia isolated for phosphate solubilization, sulfate reduction, or biogenesis of carbonate are Pb tolerant, their metabolic capacity to immobilize Pb is dependent on microbial strains and the environment. The oxygen status and pH are perhaps the most relevant (Fig. 6). For example, strict aerobes cannot survive in anoxic conditions and thus, cannot generate Pb sulfide. While facultative anaerobes have a wider range of oxygen tolerance, their ability to immobilize Pb under anoxic conditions may be limited because low oxygen is not metabolic favorable. The oxidation-reduction potential is dependent on physical, chemical, and biological factors. Because redox impacts compound solubility, it influences and is modulated in part by microbial community cycling of P, S, and C and other elements, all of which are inextricably intertwined (Peralta et al., 2014; Song et al., 2008). By changing the redox potential, Meng et al. (2019) identified a microbial driver of Cd sequestration in the family Anaerolineaceae, that influenced both microbial community composition and Cd solubility, thus having implications on bioaccessibility and a potential candidate to promote Cd sequestration in environmental bioremediation applications. In poorly buffered sediments, increasing oxygen content decreased pH and mobilized Pb and Cu; low pH (3–4.5) resulted in mobilization of more Pb and Cu in oxic compared to anoxic condition (Calmano et al., 1993). Redox cycling, i.e., cycles of anoxic-oxic condition, also influenced Pb, Zn, and Cd sulfide and carbonate formation and dissolution in sediment. In an effort to promote metal sulfide production during mine waste remediation, Karna et al. (2016) explored supplementing with organic carbon to decrease the oxygen concentration through respiration, and thus reduce the redox potential. This provided a more favorable environment for Pb-, Z-, and Cd-sulfide production.
Fig. 6.
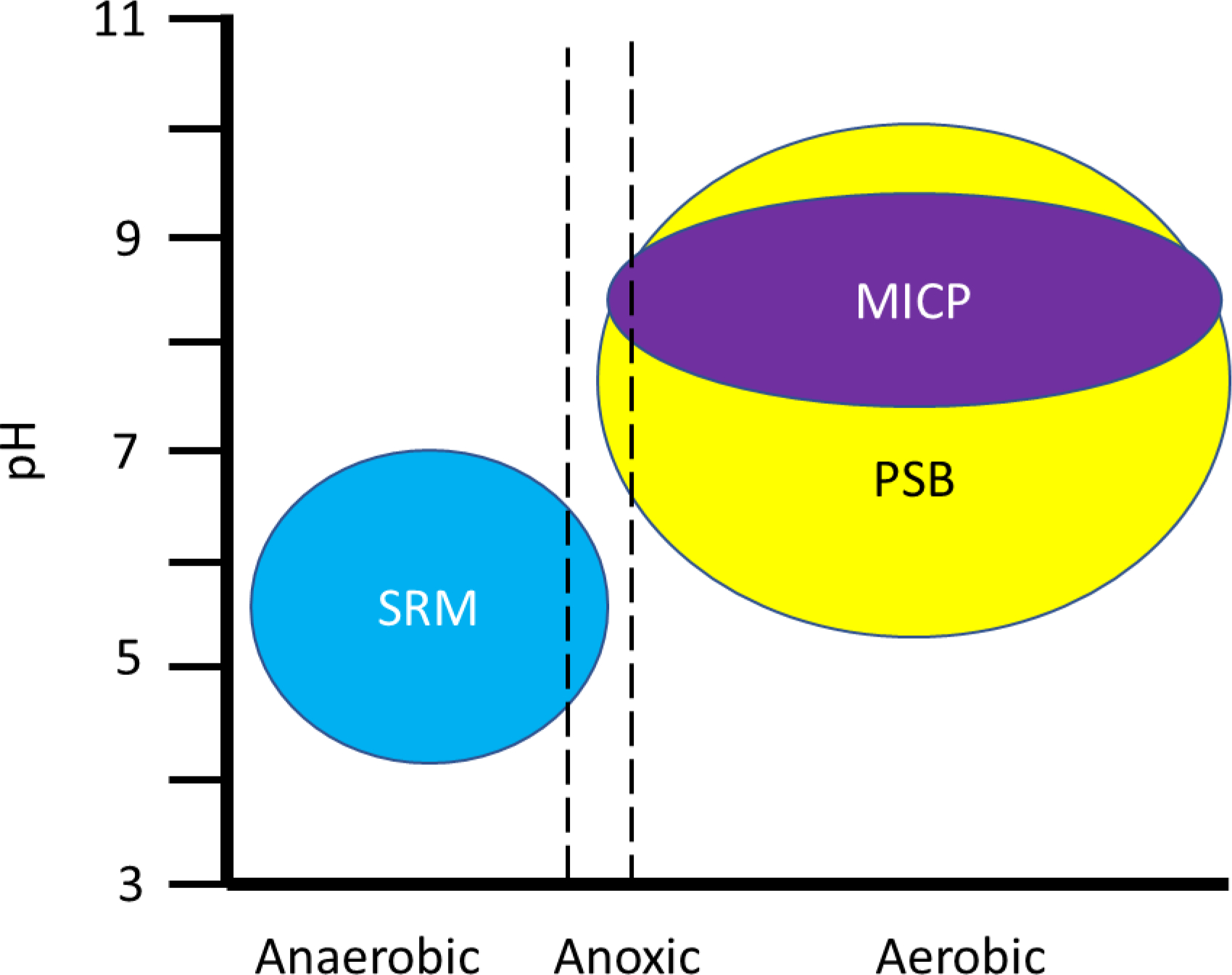
Oxygen and pH range for microbial mediated formation of lead sulfide, phosphate, and carbonate formation. PSB - phosphate solubilization bacteria, SRM -sulfate reducing microorganisms, and MICP - microbially induced calcium carbonate precipitation.
Theoretically, these three precipitation mechanisms might occur simultaneously under anoxic conditions, yet usually one process is optimized for a specific application. Anaerobic conditions (dissolved oxygen <0.1 mg/L; oxygen-reduction potential, −65mV - −198mV) are required for sulfide production from sulfate; phosphatase activity and organic acid production (products dependent on pH) can occur under anoxic conditions however phosphatase activity is lower in the absence of oxygen (Horiuchi et al., 2002; Steenbergh et al., 2011). Bacillus megaterium formed calcium carbonate under anoxic (dissolved oxygen = 0.73–0.93 mg/L) conditions in the absence of Pb, so it is feasible that PbCO3 could be produced by a Pb resistant strain (Jiang et al., 2016). Urease is active under anaerobic conditions, which further supports that PbCO3 formation could occur anaerobically (Wozny et al., 1977). Most applications used to immobilize Pb with PSB and MICP are done aerobically where phosphate or carbonate are more efficiently produced. However, in a CaCO3 rich environment, addition of organic carbon resulted in the formation of both galena (PbS) and cerussite (PbCO3) as observed by synchrotron-based x-ray diffraction spectroscopy; cerussite only was detected in the absence of the organic carbon supplement (Karna et al., 2016).
Immobilization of Pb also is dependent on pH. Pyrophosphate forms at pH 5.3–10.0 (Liang and Lu, 1952), Pb sulfide at pH 7.5–8.5 (Hoa et al., 2007), and Pb carbonate at pH 7.5–9.5 (Xue et al., 2022). In solution with rock phosphate as a phosphate source, Enterobacter sp. produced organic acids that decreased the pH to 3.8, thus solubilizing rock phosphate; acid and alkaline phosphatases also were active (Park et al., 2011a; Park et al., 2011b). At low pH (2.2–3.2), tertiary Pb species were not formed and most remained solubilized because phosphate formation was inefficient. However, due to the buffering effect of soil constituents, the pH decreased to 5.3–5.5 which was conducive for pyrophosphate formation in soil. Conversely, ureolytic bacteria such as Enterobacter cloacae KJ-46 and KJ-47, produced carbonate and ammonia from urea, raising the pH >9.0 and removing 54–68% Pb from solution as PbCO3 (Kang et al., 2015; Kang and So, 2016). Overall, weakly acidic-neutral pH is preferable for Pb phosphate formation whereas Pb carbonate generation occurs in alkaline conditions. When SRM is used to treat acid mine waste, increase the pH with lime or other alkaline material is required prior to inoculation of SRM. Adapting processes that leverage the natural ability of the microbial constituents to maintain pH should reduce the engineering needed to sequester Pb.
6. Future Perspectives
Use of biomineralization and biosorption leverages microbial metabolism and processes to produce recalcitrant transformation products and sequester Pb and other heavy metals. As one looks to the future of microbe-based remediation of environmental Pb, which will likely evolve considerably, several research needs are identified. First, further understanding the growth conditions of microbes and the associated molecular mechanisms responsible for Pb immobilization will inform future engineering applications that maximize Pb and other toxic metal removal from environmental systems. Modern molecular techniques play a key role in such research. DNA microarrays have been used to study the expression of genes under different environmental conditions, such as temperature, salinity and pH, and metabolomics has been used to study Pb induced changes, such as the oxygen stress response (Gao et al., 2017). Metagenomic analysis, or “bioprospecting”, will allow for molecular insight into changes in the microbial community structure, enrichment of related functional abundances e.g., metal metabolism, and activation of functional genes along with Pb immobilization (Li et al., 2022; Wani et al., 2022). A list of such functional genes and enzymes involved in phosphate, sulfide, and carbonate metal precipitation is summarized in Table 5. With these tools in hand, not only can important information on the niche differentiation of microbes be obtained, but they also can be used to predict their behavior in real world applications, thereby allowing for improved performance of Pb immobilization. The novel opportunities that have been created by the genomics revolution will generate enormous opportunities for microbiologists to obtain detailed insights into the ecology and biotechnology of these important microorganisms. Molecular manipulation has potential for desired trait selection which may facilitate a significant process change in Pb and toxic metal remediation.
Table 5.
Representative genes and enzymes involved in phosphate, sulfide, and carbonate metal precipitation
| Gene | Enzyme | Microorganism | Reference |
|---|---|---|---|
|
| |||
| Phosphate | |||
| phoD | alkaline phosphatase | Acinetobacter pittii | Wan et al., 2020 |
| phyK | histidine acid phosphatase | Xanthomonas campestris | Huang et al., 2009 |
| Pseudomonas syringae | Cangussu et al., 2018 | ||
| Pectobacterium carotovorum (formerly Erwinia carotovora) | Cangussu et al., 2018 | ||
| phyA | beta-propeller phytase | Acinetobacter pittii | Wan et al., 2020 |
| Xanthomonas oryzae pv. oryzae | Chatterjee et al., 2007 | ||
| pqq | pyrroloquinoline-quinone synthase | Acinetobacter pittii | Wan et al., 2020 |
| pkk | polyphosphate kinase | Acinetobacter pittii | Wan et al., 2020 |
| Sulfide | |||
| sat | sulfate adenylyltransferase | Thiorhodovibrio | Vavourakis et al., 2019 |
| Thioalkalivibrio sp. | Vavourakis et al., 2019 | ||
| Thohalocapsa sp. | Vavourakis et al., 2019 | ||
| aprAB | adenylyl sulfate reductase alpha & beta subunits | Desulfovibrio spp. | Ramos et al., 2012 |
| qmoABC | quinone-interacting membrane-bound oxidoreductase | Desulfovibrio spp. | Ramos et al., 2012 |
| dsrAB | dissimilatory sulfite reductase alpha & beta subunits | Desulfovibrio vulgaris | Oliveira et al., 2008 |
| Thiorhodovibrio | Vavourakis et al., 2019 | ||
| Thioalkalivibrio sp. | Vavourakis et al., 2019 | ||
| Thohalocapsa sp. | Vavourakis et al., 2019 | ||
| Ca. Rokubacteria | Anantharaman et al., 2018 | ||
| Ca. Hydrothermarchaeota | Anantharaman et al., 2018 | ||
| sirA | dissimilatory sulfite reductase | Shewanella oneidensis | Shirodkar et al., 2011 |
| mccA | dissimilatory sulfite reductase | Wolinella succinogenes | Kern et al., 2011 |
| asrAB | siroheme-dependent anaerobic sulfite reductase | Ca. Woesearchaeota | Vavourakis et al., 2019 |
| Salmonella spp. | Vavourakis et al., 2019 | ||
| Edwardsiella spp. | Vavourakis et al., 2019 | ||
| Carbonate | |||
| ureC | Urease subunit alpha | Sporosarcina luteola | Cuaxinque-Flores et al., 2020 |
| Sporosarcina pastuerii | Kang & So, 2016 | ||
| Viridibacillus arenosi | Kang & So, 2016 | ||
| Enterobacter cloacae | Kang & So, 2016 | ||
| Lysinibacillus sphaericus | Kang & So, 2016 | ||
Second, a multi-disciplinary approach is called for breakthroughs in the science and technology for microbial assisted remediation of Pb and toxic metals in the environment. While a biologist may lead microbial research, it is difficult to break the present obstacles using only microbial approaches. A coordinated effort in integrating biological and molecular theories with advanced analytical techniques and new findings from related disciplines such as material sciences, chemical engineering, and environmental sciences will allow for innovations and breakthroughs to advance the science and technology. One line of research towards this direction exhibits novel use of solid matrices embedded with microorganisms to immobilize metals (Wang et al., 2019a; Yang et al., 2019). For example, the vitality of microorganisms and enzymes can be maintained in alginate or nanomaterials such as hydroxyapatite impregnated polyurethane foam, which effectively bound Pb (Jang et al., 2008; Sone et al., 2009). More recently, metal-organic frameworks have been explored as remediation alternatives. Lead (667–833 mg/g) and Cd (370–714 mg/g) were removed from aqueous solution using two different magnetic metal-organic frameworks, Fe3O4@UiO-66–NH2, and Fe3O4@ZIF-8 (Abdel-Magied et al., 2022); graphene oxide adsorbed 555 mg/g Pb (Bilal et al., 2021; Jun et al., 2019). Nanostructured materials with immobilized enzymes, such as laccases, showed potential for remediation of wastewater and other environmental contaminants.
Third, most studies reviewed in this paper were conducted in laboratory settings. Scaling up for environmental applications requires further investigation because the ambient environment and competition from the indigenous microbial community may have a large role in the functionality of the bacterial consortia or isolates. While carefully controlled pilot runs at the bench level are informative, in situ immobilization will be wrought with uncontrolled variables that need to be managed. Thus, optimization of the microbe functionalities for phosphate solubilization, sulfate reduction, or carbonate synthesis should be evaluated with a wide array of metrics relating to the environmental conditions in the field. Furthermore, biomineralization may form stable Pb precipitates while bio-sorbed Pb can be desorbed depending on the environmental condition. When selecting a process to reduce Pb bioavailability, the physical and chemical properties of the environmental matrix will inform the approach for Pb immobilization and microbial strain or consortium selection. Each aqueous, soil, or sediment matrix is unique, and the process will need to be optimized. Environmental conditions are influenced by physical, chemical, and biological factors which have an effect on the fate and transport of Pb. In a study by Karna et al. (2016), Pb-, Zn-, and Cd-sulfide were the preferred transformation products because they were more stable than their carbonate counterparts which were more likely to form in a CaCO3 rich environment (PbS, 0.01244 g/100 mL vs PbCO3, 0.00011 g/100 mL water at 20 °C). Therefore, the redox potential was manipulated by added organic carbon to drive respiration and remove oxygen from the system to enhance sulfide production. Additionally, because the transformation products are pH sensitive, low pH can lead to solubilization of the phosphate, sulfide, and carbonate compounds as discussed in Section 5, e.g., Pb phosphate, < pH 5.3 (Liang and Lu, 1952), Pb sulfide pH < 7.5 (Hoa et al., 2007), and Pb carbonate at pH < 7.5 (Xue et al., 2022). Biosorption of Pb also is pH dependent (Choińska-Pulit et al., 2018). Clearly, optimizing system biological, physical, and chemical parameters is paramount to a successful outcome, however some of the variables cannot be manipulated.
Furthermore, introduction of a single isolate optimized for a specific function may be met with intense competition from the highly adapted microbial residents, even if it were isolated from a similar contaminated site. For example, urease activity alone for MICP doesn’t necessarily equate with efficacy due to complex environmental variables. This is evidenced by one study where, in the absence of toxic metals, a ureolytic Sporosarcina pasteurii isolate from a culture collection did not persist in soil as long as the community isolated from that soil (Graddy et al., 2021). On many occasions, consortia comprised of several functional species, especially those isolated from contaminated sources, may be a more viable alternative than a single isolate because in theory the consortium has adapted to the harsh environment and successful remediation would not be dependent on one specific isolate’s survival. However, maintaining the percentages of the individual members of the community may be difficult, even though they are fully characterized. Some of the contaminated sites are over 100 years old and the existing microbial community is well adapted to the environment (Beattie et al., 2018). Consideration should be given to enriching for those resident microorganisms that have the functionalities needed to better sequester environmental Pb and other heavy metals.
7. Conclusions
Biological formation of Pb phosphate, sulfide, and carbonate precipitates has been demonstrated to provide effective approaches to immobilize Pb in environmental media such as soil, water and sediment. Each of the approaches addressed in this review has proven useful to immobilize Pb, thus making it less bioavailable, which in turn, reduces exposure to humans, animals, plants and the environment. Because microbial treatment is less harmful to the environment and more cost effective than chemical treatment or physical removal of contaminated soil or water off-site, results on microbial consortia and single isolate applications for phosphate solubilization, sulfate reduction, and MICP exhibit a novel strategy for remediation of Pb and other heavy metal contaminated sites. While many of the approaches are successful under carefully controlled laboratory settings, field application requires optimization for a host of variables to define a wide array of bio-engineering parameters, including microbial competitiveness, soil physical and chemical parameters, concentration, and co-contaminants. This is especially so for environmental factors such as redox potential and pH that have great impacts on microbial induced solubilization or immobilization, microbial activity, product (crystal) stability, and source availability. Large scale applications of microbe-based remediation will benefit from further multidisciplinary research to establish the effectiveness of bioremediation technology, particularly long-term monitoring of microbial survival, establishment, and transport for successful in-situ bioremediation of contaminated soil, water, and sediment.
Acknowledgements
We would like to thank Dr. Richard Devereux for valuable reviews of earlier versions of this manuscript. We also thank Ashley Frith for her assistance in compiling the references. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
CRediT author statement
Yongshan Wan: Conceptualization, Writing - Original Draft, Writing - Review & Editing, Visualization
S. Elizabeth George: Conceptualization, Writing - Original Draft, Writing - Review & Editing, Visualization
References
- Abdel-Magied AF, Abdelhamid HN, Ashour RM, Fu L, Dowaidar M, Xia W, and Forsberg K. 2022. Magnetic metal-organic frameworks for efficient removal of cadmium (II), and lead (II) from aqueous solution. Journal of Environmental Chemical Engineering. 10:107467. [Google Scholar]
- Achal V, and Pan X. 2011. Characterization of urease and carbonic anhydrase producing bacteria and their role in calcite precipitation. Current microbiology. 62:894–902. [DOI] [PubMed] [Google Scholar]
- Achal V, Pan X, Zhang D, and Fu Q. 2012. Bioremediation of Pb-contaminated soil based on microbially induced calcite precipitation. Journal of microbiology and biotechnology. 22:244–247. [DOI] [PubMed] [Google Scholar]
- Achal V, Savant VV, and Reddy MS. 2007. Phosphate solubilization by a wild type strain and UV-induced mutants of Aspergillus tubingensis. Soil Biology and Biochemistry. 39:695–699. [Google Scholar]
- Adey WH, Luckett C, and Smith M. 1996. Purification of industrially contaminated groundwaters using controlled ecosystems. Ecological Engineering. 7:191–212. [Google Scholar]
- Ajayan K, Harilal C, and Selvaraju M. 2018. Phycoremediation resultant lipid production and antioxidant changes in green microalgae Chlorella sp. International journal of phytoremediation. 20:1144–1151. [DOI] [PubMed] [Google Scholar]
- Al T, Blowes D, Jambor J, and Scott J. 1994. The geochemistry of mine-waste pore water affected by the combined disposal of natrojarosite and base-metal sulphide tailings at Kidd Creek, Timmins, Ontario. Canadian geotechnical journal. 31:502–512. [Google Scholar]
- Anantharaman K, Hausmann B, Jungbluth SP, Kantor RS, Lavy A, Warren LA, Rappé MS, Pester M, Loy A, and Thomas BC. 2018. Expanded diversity of microbial groups that shape the dissimilatory sulfur cycle. The ISME journal. 12:1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoun H 2012. Beneficial microorganisms for the sustainable use of phosphates in agriculture. Procedia Engineering. 46:62–67. [Google Scholar]
- Arvieu J-C, Leprince F, and Plassard C. 2003. Release of oxalate and protons by ectomycorrhizal fungi in response to P-deficiency and calcium carbonate in nutrient solution. Annals of Forest Science. 60:815–821. [Google Scholar]
- Ayangbenro AS, Olanrewaju OS, and Babalola OO. 2018. Sulfate-reducing bacteria as an effective tool for sustainable acid mine bioremediation. Frontiers in microbiology:1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz HA, Adlan MN, and Ariffin KS. 2008. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr (III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresource technology. 99:1578–1583. [DOI] [PubMed] [Google Scholar]
- Azulay DN, Abbasi R, Ben Simhon Ktorza I, Remennik S, Reddy M A, and Chai L. 2018. Biopolymers from a bacterial extracellular matrix affect the morphology and structure of calcium carbonate crystals. Crystal Growth & Design. 18:5582–5591. [Google Scholar]
- Bai H, Liu D, Zheng W, Ma L, Yang S, Cao J, Lu X, Wang H, and Mehta N. 2021. Microbially-induced calcium carbonate precipitation by a halophilic ureolytic bacterium and its potential for remediation of heavy metal-contaminated saline environments. International Biodeterioration & Biodegradation. 165:105311. [Google Scholar]
- Bai J, Yang X, Du R, Chen Y, Wang S, and Qiu R. 2014. Biosorption mechanisms involved in immobilization of soil Pb by Bacillus subtilis DBM in a multi-metal-contaminated soil. Journal of Environmental Sciences. 26:2056–2064. [DOI] [PubMed] [Google Scholar]
- Bao Y, Jin X, Guo C, Lu G, and Dang Z. 2021. Sulfate-reducing bacterial community shifts in response to acid mine drainage in the sediment of the Hengshi watershed, South China. Environmental Science and Pollution Research. 28:2822–2834. [DOI] [PubMed] [Google Scholar]
- Barabesi C, Galizzi A, Mastromei G, Rossi M, Tamburini E, and Perito B. 2007. Bacillus subtilis gene cluster involved in calcium carbonate biomineralization. Journal of bacteriology. 189:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea J-M, and Richardson AE. 2015. Phosphate mobilisation by soil microorganisms. In Principles of plant-microbe interactions. Springer. 225–234. [Google Scholar]
- Bell E, Lamminmäki T, Alneberg J, Andersson AF, Qian C, Xiong W, Hettich RL, Frutschi M, and Bernier-Latmani R. 2020. Active sulfur cycling in the terrestrial deep subsurface. The ISME journal. 14:1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dov E, Brenner A, and Kushmaro A. 2007. Quantification of sulfate-reducing bacteria in industrial wastewater, by real-time polymerase chain reaction (PCR) using dsrA and apsA genes. Microbial ecology. 54:439–451. [DOI] [PubMed] [Google Scholar]
- Benzerara K, Skouri-Panet F, Li J, Férard C, Gugger M, Laurent T, Couradeau E, Ragon M, Cosmidis J, and Menguy N. 2014. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proceedings of the National Academy of Sciences. 111:10933–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AC, Bethke CM, and Krumhansl JL. 2000. A process model of natural attenuation in drainage from a historic mining district. Applied geochemistry. 15:655–666. [Google Scholar]
- Beyenal H, and Lewandowski Z. 2004. Dynamics of lead immobilization in sulfate reducing biofilms. Water research. 38:2726–2736. [DOI] [PubMed] [Google Scholar]
- Bi Q-F, Zheng B-X, Lin X-Y, Li K-J, Liu X-P, Hao X-L, Zhang H, Zhang J-B, Jaisi DP, and Zhu Y-G. 2018. The microbial cycling of phosphorus on long-term fertilized soil: Insights from phosphate oxygen isotope ratios. Chemical Geology. 483:56–64. [Google Scholar]
- Bilal M, Ashraf SS, Cui J, Lou W-Y, Franco M, Mulla SI, and Iqbal HM. 2021. Harnessing the biocatalytic attributes and applied perspectives of nanoengineered laccases—A review. International Journal of Biological Macromolecules. 166:352–373. [DOI] [PubMed] [Google Scholar]
- Bolan NS, Naidu R, Mahimairaja S, and Baskaran S. 1994. Influence of low-molecular-weight organic acids on the solubilization of phosphates. Biology and Fertility of Soils. 18:311–319. [Google Scholar]
- Bradham KD, Diamond GL, Nelson CM, Noerpel M, Scheckel KG, Elek B, Chaney RL, Ma Q, and Thomas DJ. 2018. Long-term in situ reduction in soil lead bioavailability measured in a mouse model. Environmental science & technology. 52:13908–13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey SM, and Voordouw G. 2010. Effect of sulfide on growth physiology and gene expression of Desulfovibrio vulgaris Hildenborough. Antonie Van Leeuwenhoek. 97:11–20. [DOI] [PubMed] [Google Scholar]
- Calmano W, Hong J, and Förstner U. 1993. Binding and mobilization of heavy metals in contaminated sediments affected by pH and redox potential. Water science and technology. 28:223–235. [Google Scholar]
- Cao J, Zhang G, Mao Z-S, Li Y, Fang Z, and Yang C. 2012. Influence of electron donors on the growth and activity of sulfate-reducing bacteria. International Journal of Mineral Processing. 106:58–64. [Google Scholar]
- Cao X, Ma L, Liang Y, Gao B, and Harris W. 2011. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environmental science & technology. 45:4884–4889. [DOI] [PubMed] [Google Scholar]
- Cao X, Wahbi A, Ma L, Li B, and Yang Y. 2009. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. Journal of Hazardous Materials. 164:555–564. [DOI] [PubMed] [Google Scholar]
- Caraballo MA, Rötting TS, Macías F, Nieto JM, and Ayora C. 2009. Field multi-step limestone and MgO passive system to treat acid mine drainage with high metal concentrations. Applied Geochemistry. 24:2301–2311. [Google Scholar]
- Castro-Alonso MJ, Montañez-Hernandez LE, Sanchez-Muñoz MA, Macias Franco MR, Narayanasamy R, and Balagurusamy N. 2019. Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Frontiers in Materials. 6:126. [Google Scholar]
- Chada VGR, Hausner DB, Strongin DR, Rouff AA, and Reeder RJ. 2005. Divalent Cd and Pb uptake on calcite {101¯ 4} cleavage faces: An XPS and AFM study. Journal of Colloid and Interface Science. 288:350–360. [DOI] [PubMed] [Google Scholar]
- Chai L, Tang J, Liao Y, Yang Z, Liang L, Li Q, Wang H, and Yang W. 2016. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans and its application in arsenic immobilization in the contaminated soil. Journal of Soils and Sediments. 16:2430–2438. [Google Scholar]
- Chen H, Zhang J, Tang L, Su M, Tian D, Zhang L, Li Z, and Hu S. 2019. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environment International. 127:395–401. [DOI] [PubMed] [Google Scholar]
- Chen J, Li X, Jia W, Shen S, Deng S, Ji B, and Chang J. 2021. Promotion of bioremediation performance in constructed wetland microcosms for acid mine drainage treatment by using organic substrates and supplementing domestic wastewater and plant litter broth. Journal of Hazardous Materials. 404:124125. [DOI] [PubMed] [Google Scholar]
- Chen Q, and Liu S. 2019. Identification and characterization of the phosphate-solubilizing bacterium Pantoea sp. S32 in reclamation soil in Shanxi, China. Frontiers in microbiology. 10:2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fan J-B, Du L, Xu H, Zhang Q-H, and He Y-Q. 2014. The application of phosphate solubilizing endophyte Pantoea dispersa triggers the microbial community in red acidic soil. Applied Soil Ecology. 84:235–244. [Google Scholar]
- Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, and Young CC. 2006. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Applied Soil Ecology. 34:33–41. [Google Scholar]
- Chetty K, Garbe U, Wang Z, Zhang S, McCarthy T, Hai F, and Jiang G. 2022. Bioconcrete based on sulfate-reducing bacteria granules: cultivation, mechanical properties, and self-healing performance. Journal of Sustainable Cement-Based Materials:1–12. [Google Scholar]
- Chrysochoou M, Dermatas D, and Grubb DG. 2007. Phosphate application to firing range soils for Pb immobilization: the unclear role of phosphate. Journal of Hazardous Materials. 144:1–14. [DOI] [PubMed] [Google Scholar]
- Cross G 2012. The World’s worst pollution problems: assessing health risks at hazardous waste sites.
- Cuaxinque-Flores G, Aguirre-Noyola JL, Hernández-Flores G, Martínez-Romero E, Romero-Ramírez Y, and Talavera-Mendoza O. 2020. Bioimmobilization of toxic metals by precipitation of carbonates using Sporosarcina luteola: An in vitro study and application to sulfide-bearing tailings. Science of The Total Environment. 724:138124. [DOI] [PubMed] [Google Scholar]
- Das S, Dash HR, and Chakraborty J. 2016. Genetic basis and importance of metal resistant genes in bacteria for bioremediation of contaminated environments with toxic metal pollutants. Applied microbiology and biotechnology. 100:2967–2984. [DOI] [PubMed] [Google Scholar]
- De Philippis R, Colica G, and Micheletti E. 2011. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: molecular basis and practical applicability of the biosorption process. Applied microbiology and biotechnology. 92:697–708. [DOI] [PubMed] [Google Scholar]
- Dev S, Galey M, Chun CL, Novotny C, Ghosh T, and Aggarwal S. 2021. Enrichment of psychrophilic and acidophilic sulfate-reducing bacterial consortia–a solution toward acid mine drainage treatment in cold regions. Environmental Science: Processes & Impacts. [DOI] [PubMed] [Google Scholar]
- Dev S, Roy S, and Bhattacharya J. 2016. Understanding the performance of sulfate reducing bacteria based packed bed reactor by growth kinetics study and microbial profiling. Journal of Environmental Management. 177:101–110. [DOI] [PubMed] [Google Scholar]
- Ding Z, Hu X, Wan Y, Wang S, and Gao B. 2016. Removal of lead, copper, cadmium, zinc, and nickel from aqueous solutions by alkali-modified biochar: Batch and column tests. Journal of Industrial and Engineering Chemistry. 33:239–245. [Google Scholar]
- Doyle CJ, O’Toole PW, and Cotter PD. 2018. Genomic characterization of sulphite reducing bacteria isolated from the dairy production chain. Frontiers in microbiology. 9:1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Lian F, and Zhu L. 2011. Biosorption of divalent Pb, Cd and Zn on aragonite and calcite mollusk shells. Environmental Pollution. 159:1763–1768. [DOI] [PubMed] [Google Scholar]
- Elliott P, Ragusa S, and Catcheside D. 1998. Growth of sulfate-reducing bacteria under acidic conditions in an upflow anaerobic bioreactor as a treatment system for acid mine drainage. Water Research. 32:3724–3730. [Google Scholar]
- Eltarahony M, Kamal A, Zaki S, and Abd-El-Haleem D. 2021. Heavy metals bioremediation and water softening using ureolytic strains Metschnikowia pulcherrima and Raoultella planticola. Journal of Chemical Technology & Biotechnology. 96:3152–3165. [Google Scholar]
- Eltarahony M, Zaki S, and Abd-El-Haleem D. 2020. Aerobic and anaerobic removal of lead and mercury via calcium carbonate precipitation mediated by statistically optimized nitrate reductases. Scientific reports. 10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga E, and Reeder R. 2002. X-ray absorption spectroscopy study of Cu2+ and Zn2+ adsorption complexes at the calcite surface: Implications for site-specific metal incorporation preferences during calcite crystal growth. Geochimica et Cosmochimica Acta. 66:3943–3954. [Google Scholar]
- Elzinga EJ, Rouff AA, and Reeder RJ. 2006. The long-term fate of Cu2+, Zn2+, and Pb2+ adsorption complexes at the calcite surface: An X-ray absorption spectroscopy study. Geochimica et Cosmochimica Acta. 70:2715–2725. [Google Scholar]
- Ercole C, Cacchio P, Botta AL, Centi V, and Lepidi A. 2007. Bacterially induced mineralization of calcium carbonate: the role of exopolysaccharides and capsular polysaccharides. Microscopy and Microanalysis. 13:42–50. [DOI] [PubMed] [Google Scholar]
- Erşan YÇ, Hernandez-Sanabria E, Boon N, and De Belie N. 2016. Enhanced crack closure performance of microbial mortar through nitrate reduction. Cement and concrete composites. 70:159–170. [Google Scholar]
- Feng Z, Long W, Hao B, Ding D, Ma X, Zhao L, and Pang X. 2017. A human stool-derived Bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut pathogens. 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer MR, Quevedo-Sarmiento J, Rivadeneyra MA, Bejar V, Delgado R, and Ramos-Cormenzana A. 1988. Calcium carbonate precipitation by two groups of moderately halophilic microorganisms at different temperatures and salt concentrations. Current Microbiology. 17:221–227. [Google Scholar]
- Fewtrell LJ, Prüss-Üstün A, Landrigan P, and Ayuso-Mateos JL. 2004. Estimating the global burden of disease of mild mental retardation and cardiovascular diseases from environmental lead exposure. Environmental Research. 94:120–133. [DOI] [PubMed] [Google Scholar]
- Fortin D, Davis B, Southam G, and Beveridge T. 1995. Biogeochemical phenomena induced by bacteria within sulfidic mine tailings. Journal of Industrial Microbiology. 14:178–185. [Google Scholar]
- Foucher S, Battaglia-Brunet F, Ignatiadis I, and Morin D. 2001. Treatment by sulfate-reducing bacteria of Chessy acid-mine drainage and metals recovery. Chemical Engineering Science. 56:1639–1645. [Google Scholar]
- Fox T, Comerford N, and McFee W. 1990. Phosphorus and aluminum release from a spodic horizon mediated by organic acids. Soil Science Society of America Journal. 54:1763–1767. [Google Scholar]
- Frandi A, Zucca P, Marvasi M, Mastromei G, Sanjust E, and Perito B. 2011. Bacillus subtilis fadB (ysiB) gene encodes an enoyl-CoA hydratase. Annals of microbiology. 61:371–374. [Google Scholar]
- Friedrich MW 2002. Phylogenetic analysis reveals multiple lateral transfers of adenosine-5′-phosphosulfate reductase genes among sulfate-reducing microorganisms. Journal of bacteriology. 184:278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz G, Büchert T, Huber H, Stetter KO, and Kroneck PM. 2000. Adenylylsulfate reductases from archaea and bacteria are 1: 1 αβ-heterodimeric iron–sulfur flavoenzymes–high similarity of molecular properties emphasizes their central role in sulfur metabolism. Febs Letters. 473:63–66. [DOI] [PubMed] [Google Scholar]
- Fuchida S, Suzuki K, Kato T, Kadokura M, and Tokoro C. 2020. Understanding the biogeochemical mechanisms of metal removal from acid mine drainage with a subsurface limestone bed at the Motokura Mine, Japan. Scientific reports. 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi M, Nakatani T, Hirooka K, Matsuoka H, Fujita Y, and Miki K. 2014. Structural characterization of a ligand-bound form of Bacillus subtilis FadR involved in the regulation of fatty acid degradation. Proteins: Structure, Function, and Bioinformatics. 82:1301–1310. [DOI] [PubMed] [Google Scholar]
- Gomaa EZ 2019. Biosequestration of heavy metals by microbially induced calcite precipitation of ureolytic bacteria. Rom Biotechnol Lett. 24:147–153. [Google Scholar]
- Gomez MG, Martinez BC, DeJong JT, Hunt CE, deVlaming LA, Major DW, and Dworatzek SM. 2015. Field-scale bio-cementation tests to improve sands. Proceedings of the Institution of Civil Engineers-Ground Improvement. 168:206–216. [Google Scholar]
- Görgen S, Benzerara K, Skouri-Panet F, Gugger M, Chauvat F, and Cassier-Chauvat C. 2021. The diversity of molecular mechanisms of carbonate biomineralization by bacteria. Discover Materials. 1:1–20. [Google Scholar]
- Govarthanan M, Lee K-J, Cho M, Kim JS, Kamala-Kannan S, and Oh B-T. 2013. Significance of autochthonous Bacillus sp. KK1 on biomineralization of lead in mine tailings. Chemosphere. 90:2267–2272. [DOI] [PubMed] [Google Scholar]
- Graddy CM, Gomez MG, DeJong JT, and Nelson DC. 2021. Native bacterial community convergence in augmented and stimulated ureolytic MICP biocementation. Environmental Science & Technology. 55:10784–10793. [DOI] [PubMed] [Google Scholar]
- Haber CL, Tornabene TG, and Skogerboe RK. 1980. Regulation of bacterial abstraction of lead by cell surface charge and chemical equilibria. Chemosphere. 9:21–31. [Google Scholar]
- Hallenbeck PC, Clark MA, and Barrett EL. 1989. Characterization of anaerobic sulfite reduction by Salmonella typhimurium and purification of the anaerobically induced sulfite reductase. Journal of bacteriology. 171:3008–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes F, Boon N, de Villiers J, Verstraete W, and Siciliano SD. 2003. Strain-specific ureolytic microbial calcium carbonate precipitation. Applied and environmental microbiology. 69:4901–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes F, and Verstraete W. 2002. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Reviews in environmental science and biotechnology. 1:3–7. [Google Scholar]
- He J, Chen X, Zhang Q, and Achal V. 2019. More effective immobilization of divalent lead than hexavalent chromium through carbonate mineralization by Staphylococcus epidermidis HJ2. International Biodeterioration & Biodegradation. 140:67–71. [Google Scholar]
- Hermann B, Kern M, La Pietra L, Simon J, and Einsle O. 2015. The octahaem MccA is a haem c–copper sulfite reductase. Nature. 520:706–709. [DOI] [PubMed] [Google Scholar]
- Hoa TTH, Liamleam W, and Annachhatre AP. 2007. Lead removal through biological sulfate reduction process. Bioresource Technology. 98:2538–2548. [DOI] [PubMed] [Google Scholar]
- Holmkvist L, Kamyshny A Jr, Vogt C, Vamvakopoulos K, Ferdelman TG, and Jørgensen BB. 2011. Sulfate reduction below the sulfate–methane transition in Black Sea sediments. Deep Sea Research Part I: Oceanographic Research Papers. 58:493–504. [Google Scholar]
- Horiuchi J-I, Shimizu T, Tada K, Kanno T, and Kobayashi M. 2002. Selective production of organic acids in anaerobic acid reactor by pH control. Bioresource technology. 82:209–213. [DOI] [PubMed] [Google Scholar]
- Huang CJ, and Barrett EL. 1990. Identification and cloning of genes involved in anaerobic sulfite reduction by Salmonella typhimurium. Journal of bacteriology. 172:4100–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CJ, and Barrett EL. 1991. Sequence analysis and expression of the Salmonella typhimurium asr operon encoding production of hydrogen sulfide from sulfite. Journal of bacteriology. 173:1544–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shi P, Wang Y, Luo H, Shao N, Wang G, Yang P, and Yao B. 2009. Diversity of Beta-Propeller Phytase Genes in the Intestinal Contents of Grass Carp Provides Insight into the Release of Major Phosphorus from Phytate in Nature. Applied and Environmental Microbiology. 75:1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug LA, Thomas BC, Sharon I, Brown CT, Sharma R, Hettich RL, Wilkins MJ, Williams KH, Singh A, and Banfield JF. 2016. Critical biogeochemical functions in the subsurface are associated with bacteria from new phyla and little studied lineages. Environmental microbiology. 18:159–173. [DOI] [PubMed] [Google Scholar]
- Jang SH, Min BG, Jeong YG, Lyoo WS, and Lee SC. 2008. Removal of lead ions in aqueous solution by hydroxyapatite/polyurethane composite foams. Journal of hazardous materials. 152:1285–1292. [DOI] [PubMed] [Google Scholar]
- Jaroslawiecka A, and Piotrowska-Seget Z. 2014. Lead resistance in micro-organisms. Microbiology. 160:12–25. [DOI] [PubMed] [Google Scholar]
- Jiang N-J, Liu R, Du Y-J, and Bi Y-Z. 2019. Microbial induced carbonate precipitation for immobilizing Pb contaminants: Toxic effects on bacterial activity and immobilization efficiency. Science of The Total Environment. 672:722–731. [DOI] [PubMed] [Google Scholar]
- Jiang N-J, Yoshioka H, Yamamoto K, and Soga K. 2016. Ureolytic activities of a urease-producing bacterium and purified urease enzyme in the anoxic condition: Implication for subseafloor sand production control by microbially induced carbonate precipitation (MICP). Ecological engineering. 90:96–104. [Google Scholar]
- Jørgensen BB 1977. The sulfur cycle of a coastal marine sediment (Limfjorden, Denmark) 1. Limnology and oceanography. 22:814–832. [Google Scholar]
- Jørgensen BB, Findlay AJ, and Pellerin A. 2019. The biogeochemical sulfur cycle of marine sediments. Frontiers in microbiology. 10:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun B-M, Kim S, Kim Y, Her N, Heo J, Han J, Jang M, Park CM, and Yoon Y. 2019. Comprehensive evaluation on removal of lead by graphene oxide and metal organic framework. Chemosphere. 231:82–92. [DOI] [PubMed] [Google Scholar]
- Kang C-H, Oh SJ, Shin Y, Han S-H, Nam I-H, and So J-S. 2015. Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecological Engineering. 74:402–407. [Google Scholar]
- Kang C-H, and So J-S. 2016. Heavy metal and antibiotic resistance of ureolytic bacteria and their immobilization of heavy metals. Ecological Engineering. 97:304–312. [Google Scholar]
- Karna RR, Hettiarachchi GM, Newville M, Sun C, and Ma Q. 2016. Synchrotron-based X-ray spectroscopy studies for redox-based remediation of lead, zinc, and cadmium in mine waste materials. Journal of environmental quality. 45:1883–1893. [DOI] [PubMed] [Google Scholar]
- Kenward P, Goldstein R, Gonzalez L, and Roberts J. 2009. Precipitation of low-temperature dolomite from an anaerobic microbial consortium: the role of methanogenic Archaea. Geobiology. 7:556–565. [DOI] [PubMed] [Google Scholar]
- Kern M, Klotz MG, and Simon J. 2011. The Wolinella succinogenes mcc gene cluster encodes an unconventional respiratory sulphite reduction system. Molecular microbiology. 82:1515–1530. [DOI] [PubMed] [Google Scholar]
- Keshri J, Yousuf B, Mishra A, and Jha B. 2015. The abundance of functional genes, cbbL, nifH, amoA and apsA, and bacterial community structure of intertidal soil from Arabian Sea. Microbiological Research. 175:57–66. [DOI] [PubMed] [Google Scholar]
- Kieu TQH, Nguyen TY, Dang TY, Nguyen TB, Vuong TN, and Horn H. 2015. Optimization of sulfide production by an indigenous consortium of sulfate-reducing bacteria for the treatment of lead-contaminated wastewater. Bioprocess and biosystems engineering. 38:2003–2011. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kwon S, and Roh Y. 2021. Effect of Divalent Cations (Cu, Zn, Pb, Cd, and Sr) on Microbially Induced Calcium Carbonate Precipitation and Mineralogical Properties. Frontiers in Microbiology. 12:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopittke PM, Asher CJ, and Menzies NW. 2008. Prediction of Pb speciation in concentrated and dilute nutrient solutions. Environmental Pollution. 153:548–554. [DOI] [PubMed] [Google Scholar]
- Kpomblekou-a K, and Tabatabai M. 1994. Effect of organic acids on release of phosphorus from phosphate rocks1. Soil Science. 158:442–453. [Google Scholar]
- Kulczycki E, Ferris F, and Fortin D. 2002. Impact of cell wall structure on the behavior of bacterial cells as sorbents of cadmium and lead. Geomicrobiology Journal. 19:553–565. [Google Scholar]
- Kumar KV, Singh N, Behl HM, and Srivastava S. 2008. Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere. 72:678–683. [DOI] [PubMed] [Google Scholar]
- Leedjarv A, Ivask A, and Virta M. 2008. Interplay of different transporters in the mediation of divalent heavy metal resistance in Pseudomonas putida KT2440. J Bacteriol. 190:2680–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup J, Fossing H, Kohls K, Holmkvist L, Borowski C, and Jørgensen BB. 2009. Sulfate-reducing bacteria in marine sediment (Aarhus Bay, Denmark): abundance and diversity related to geochemical zonation. Environmental Microbiology. 11:1278–1291. [DOI] [PubMed] [Google Scholar]
- Levinson HS, and Mahler I. 1998. Phosphatase activity and lead resistance in Citrobacter freundii and Staphylococcus aureus. FEMS Microbiol Lett. 161:135–138. [DOI] [PubMed] [Google Scholar]
- Li J. h., Purdy KJ, Takii S, and Hayashi H. 1999. Seasonal changes in ribosomal RNA of sulfate-reducing bacteria and sulfate reducing activity in a freshwater lake sediment. FEMS Microbiology Ecology. 28:31–39. [Google Scholar]
- Li M, Cheng X, and Guo H. 2013. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. International Biodeterioration & Biodegradation. 76:81–85. [Google Scholar]
- Li M, Cheng X, Guo H, and Yang Z. 2016a. Biomineralization of carbonate by Terrabacter tumescens for heavy metal removal and biogrouting applications. Journal of Environmental Engineering. 142:C4015005. [Google Scholar]
- Li X, Dai L, Zhang C, Zeng G, Liu Y, Zhou C, Xu W, Wu Y, Tang X, Liu W, and Lan S. 2017. Enhanced biological stabilization of heavy metals in sediment using immobilized sulfate reducing bacteria beads with inner cohesive nutrient. Journal of Hazardous Materials. 324:340–347. [DOI] [PubMed] [Google Scholar]
- Li X, Wu Y, Zhang C, Liu Y, Zeng G, Tang X, Dai L, and Lan S. 2016b. Immobilizing of heavy metals in sediments contaminated by nonferrous metals smelting plant sewage with sulfate reducing bacteria and micro zero valent iron. Chemical Engineering Journal. 306:393–400. [Google Scholar]
- Li Y, Guo S, Zheng Y, Yu J, Chi R, and Xiao C. 2022. Bioimmobilization of lead in phosphate mining wasteland by isolated strain Citrobacter farmeri CFI-01. Environmental Pollution:119485. [DOI] [PubMed] [Google Scholar]
- Li Z, Su M, Duan X, Tian D, Yang M, Guo J, Wang S, and Hu S. 2018. Induced biotransformation of lead (II) by Enterobacter sp. in SO4-PO4-Cl solution. Journal of Hazardous Materials. 357:491–497. [DOI] [PubMed] [Google Scholar]
- Liang J-L, Liu J, Jia P, Yang T.-t., Zeng Q.-w., Zhang S.-c., Liao B, Shu W.-s., and Li J.-t.. 2020. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. The ISME Journal. 14:1600–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S-C, and Lu K-I. 1952. On the determination of lead as normal phosphate. Analytica Chimica Acta. 7:451–457. [Google Scholar]
- Liao Y, Zhou L, Liang J, and Xiong H. 2009. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans cell suspensions under different pH condition. Materials Science and Engineering: C. 29:211–215. [Google Scholar]
- Lin CY, Turchyn AV, Steiner Z, Bots P, Lampronti GI, and Tosca NJ. 2018. The role of microbial sulfate reduction in calcium carbonate polymorph selection. Geochimica et Cosmochimica Acta. 237:184–204. [Google Scholar]
- Lin H, Shi J, Chen X, Yang J, Chen Y, Zhao Y, and Hu T. 2010. Effects of lead upon the actions of sulfate-reducing bacteria in the rice rhizosphere. Soil Biology and Biochemistry. 42:1038–1044. [Google Scholar]
- Liu F-P, Liu H-Q, Zhou H-L, Dong Z-G, Bai X-H, Bai P, and Qiao J-J. 2014. Isolation and characterization of phosphate-solubilizing bacteria from betel nut (Areca catechu) and their effects on plant growth and phosphorus mobilization in tropical soils. Biology and fertility of soils. 50:927–937. [Google Scholar]
- Liu P, Zhang Y, Tang Q, and Shi S. 2021. Bioremediation of metal-contaminated soils by microbially-induced carbonate precipitation and its effects on ecotoxicity and long-term stability. Biochemical Engineering Journal. 166:107856. [Google Scholar]
- Liu X, Zhou Y, Zhang J, Tang L, Luo L, and Zeng G. 2017a. Iron containing metal–organic frameworks: structure, synthesis, and applications in environmental remediation. ACS applied materials & interfaces. 9:20255–20275. [DOI] [PubMed] [Google Scholar]
- Liu XY, Zhang MJ, Li YB, Wang ZN, and Wen JK. 2017b. In situ bioremediation of tailings by sulfate reducing bacteria and iron reducing bacteria: lab-and field-scale remediation of sulfidic mine tailings. In Solid State Phenomena. Vol. 262. Trans Tech Publ. 651–655. [Google Scholar]
- Lutterbach M, Contador LS, Oliveira A, Galvão M, De Franca F, and de Souza Pimenta G. 2009. Iron sulfide production by Shewanella strain isolated from black powder. In CORROSION 2009. OnePetro. [Google Scholar]
- Ma LQ, and Rao GN. 1999. Aqueous Pb Reduction in Pb-Contaminated Soils by Florida Phosphate Rocks. Water, Air, and Soil Pollution. 110:1–16. [Google Scholar]
- Ma QY, Logan TJ, and Traina SJ. 1995. Lead immobilization from aqueous solutions and contaminated soils using phosphate rocks. Environ. Sci. Technol. 29:1118–1126. [DOI] [PubMed] [Google Scholar]
- Makhathini TP, Mulopo J, and Bakare BF. 2021. Sulfidogenic fluidized-bed bioreactor kinetics for co-treatment of hospital wastewater and acid mine drainage. Biotechnology Reports. 32:e00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M, Faleiro ML, Barros RJ, Veríssimo AR, Barreiros MA, and Costa MC. 2009. Characterization and activity studies of highly heavy metal resistant sulphate-reducing bacteria to be used in acid mine drainage decontamination. Journal of Hazardous materials. 166:706–713. [DOI] [PubMed] [Google Scholar]
- Melo J, Carvalho L, Correia P, de Souza SB, Dias T, Santana M, Carolino M, Aguiar NO, Canellas LP, and Cruz C. 2018. Conventional farming disrupts cooperation among phosphate solubilising bacteria isolated from Carica papaya’s rhizosphere. Applied Soil Ecology. 124:284–288. [Google Scholar]
- Meng D, Li J, Liu T, Liu Y, Yan M, Hu J, Li X, Liu X, Liang Y, Liu H, and Yin H. 2019. Effects of redox potential on soil cadmium solubility: Insight into microbial community. Journal of Environmental Sciences. 75:224–232. [DOI] [PubMed] [Google Scholar]
- Min G, Li M.-m., Jian Z, Liu X.-x., Zhu J.-y., Hu Y.-h., and Qiu G.-z.. 2017. Acidithiobacillus ferrooxidans enhanced heavy metals immobilization efficiency in acidic aqueous system through bio-mediated coprecipitation. Transactions of Nonferrous Metals Society of China. 27:1156–1164. [Google Scholar]
- Misra N, Gupta G, and Jha PN. 2012. Assessment of mineral phosphate-solubilizing properties and molecular characterization of zinc-tolerant bacteria. Journal of basic microbiology. 52:549–558. [DOI] [PubMed] [Google Scholar]
- Montoya B, DeJong J, and Boulanger R. 2014. Dynamic response of liquefiable sand improved by microbial-induced calcite precipitation. In Bio-and Chemo-Mechanical Processes in Geotechnical Engineering: Géotechnique Symposium in Print 2013. ICE Publishing. 125–135. [Google Scholar]
- Moreau JW, Fournelle JH, and Banfield JF. 2013. Quantifying heavy metals sequestration by sulfate-reducing bacteria in an acid mine drainage-contaminated natural wetland. Frontiers in microbiology. 4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugwar AJ, and Harbottle MJ. 2016. Toxicity effects on metal sequestration by microbially-induced carbonate precipitation. Journal of hazardous materials. 314:237–248. [DOI] [PubMed] [Google Scholar]
- Müller AL, Kjeldsen KU, Rattei T, Pester M, and Loy A. 2015. Phylogenetic and environmental diversity of DsrAB-type dissimilatory (bi) sulfite reductases. The ISME journal. 9:1152–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz AJ, Ruiz E, Abriouel H, Gálvez A, Ezzouhri L, Lairini K, and Espínola F. 2012. Heavy metal tolerance of microorganisms isolated from wastewaters: Identification and evaluation of its potential for biosorption. Chemical Engineering Journal. 210:325–332. [Google Scholar]
- Munyai R, Ogola HJO, and Modise DM. 2021. Microbial Community Diversity Dynamics in Acid Mine Drainage and Acid Mine Drainage-Polluted Soils: Implication on Mining Water Irrigation Agricultural Sustainability. Frontiers in Sustainable Food Systems. 5:701870. [Google Scholar]
- Muthusaravanan S, Sivarajasekar N, Vivek J, Paramasivan T, Naushad M, Prakashmaran J, Gayathri V, and Al-Duaij OK. 2018. Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environmental chemistry letters. 16:1339–1359. [Google Scholar]
- Mwandira W, Nakashima K, and Kawasaki S. 2017. Bioremediation of lead-contaminated mine waste by Pararhodobacter sp. based on the microbially induced calcium carbonate precipitation technique and its effects on strength of coarse and fine grained sand. Ecological engineering. 109:57–64. [Google Scholar]
- Naik MM, and Dubey SK. 2013. Lead resistant bacteria: lead resistance mechanisms, their applications in lead bioremediation and biomonitoring. Ecotoxicol Environ Saf. 98:1–7. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Ueno Y, Hattori S, Umemura M, Yagi A, Takai K, Koba K, Sasaki Y, Makabe A, and Yoshida N. 2012. Seasonal change in microbial sulfur cycling in monomictic Lake Fukami-ike, Japan. Limnology and oceanography. 57:974–988. [Google Scholar]
- Ňancucheo I, and Johnson DB. 2012. Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microbial biotechnology. 5:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal AL, Rossmann M, Brearley C, Akkari E, Guyomar C, Clark IM, Allen E, and Hirsch PR. 2017. Land-use influences phosphatase gene microdiversity in soils. Environmental Microbiology. 19:2740–2753. [DOI] [PubMed] [Google Scholar]
- Neculita C-M, Zagury GJ, and Bussière B. 2008. Effectiveness of sulfate-reducing passive bioreactors for treating highly contaminated acid mine drainage: II. Metal removal mechanisms and potential mobility. Applied Geochemistry. 23:3545–3560. [Google Scholar]
- Neculita CM, Yim G-J, Lee G, Ji S-W, Jung JW, Park H-S, and Song H. 2011. Comparative effectiveness of mixed organic substrates to mushroom compost for treatment of mine drainage in passive bioreactors. Chemosphere. 83:76–82. [DOI] [PubMed] [Google Scholar]
- Neculita CM, Zagury GJ, and Bussière B. 2007. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: Critical review and research needs. Journal of environmental quality. 36:1–16. [DOI] [PubMed] [Google Scholar]
- Nguyen YT, Kieu HT, West S, Dang YT, and Horn H. 2017. Community structure of a sulfate-reducing consortium in lead-contaminated wastewater treatment process. World Journal of Microbiology and Biotechnology. 33:1–10. [DOI] [PubMed] [Google Scholar]
- Niu Z. s., Pan H, Guo X.-p., Lu D.-p., Feng J.-n., Chen Y.-r., Tou F.-y., Liu M, and Yang Y. 2018. Sulphate-reducing bacteria (SRB) in the Yangtze Estuary sediments: Abundance, distribution and implications for the bioavailibility of metals. Science of The Total Environment. 634:296–304. [DOI] [PubMed] [Google Scholar]
- Ohan J, Saneiyan S, Lee J, Bartlow AW, Ntarlagiannis D, Burns S, and Colwell FS. 2020. Microbial and geochemical dynamics of an aquifer stimulated for microbial induced calcite precipitation (MICP). Frontiers in microbiology. 11:1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C, Alves V, Marriel I, Gomes E, Scotti M, Carneiro N, Guimaraes C, Schaffert R, and Sá N. 2009. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biology and Biochemistry. 41:1782–1787. [Google Scholar]
- Oyetibo GO, Enahoro JA, Ikwubuzo CA, and Ukwuoma CS. 2021. Microbiome of highly polluted coal mine drainage from Onyeama, Nigeria, and its potential for sequestrating toxic heavy metals. Scientific Reports. 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnout C, Présent RM, Billard P, Rotureau E, and Duval JFL. 2018. What do luminescent bacterial metal-sensors probe? Insights from confrontation between experiments and flux-based theory. Sensors and Actuators B: Chemical. 270:482–491. [Google Scholar]
- Pal C, Asiani K, Arya S, Rensing C, Stekel DJ, Larsson DGJ, and Hobman JL. 2017. Chapter Seven - Metal Resistance and Its Association With Antibiotic Resistance. In Advances in Microbial Physiology. Vol. 70. Poole RK, editor. Academic Press. 261–313. [DOI] [PubMed] [Google Scholar]
- Pan X, Chen Z, Li L, Rao W, Xu Z, and Guan X. 2017. Microbial strategy for potential lead remediation: a review study. World J Microbiol Biotechnol. 33:35. [DOI] [PubMed] [Google Scholar]
- Pande A, Pandey P, Mehra S, Singh M, and Kaushik S. 2017. Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. Journal of Genetic Engineering and Biotechnology. 15:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhwar QA, Naher UA, Jusop S, Othman R, Latif MA, and Ismail MR. 2014. Biochemical and molecular characterization of potential phosphate-solubilizing bacteria in acid sulfate soils and their beneficial effects on rice growth. PloS one. 9:e97241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papirio S, Villa-Gomez D, Esposito G, Pirozzi F, and Lens P. 2013. Acid mine drainage treatment in fluidized-bed bioreactors by sulfate-reducing bacteria: a critical review. Critical reviews in environmental science and technology. 43:2545–2580. [Google Scholar]
- Park JH, Bolan N, Megharaj M, and Naidu R. 2011a. Concomitant rock phosphate dissolution and lead immobilization by phosphate solubilizing bacteria (Enterobacter sp.). Journal of environmental management. 92:1115–1120. [DOI] [PubMed] [Google Scholar]
- Park JH, Bolan N, Megharaj M, and Naidu R. 2011b. Isolation of phosphate solubilizing bacteria and their potential for lead immobilization in soil. Journal of hazardous materials. 185:829–836. [DOI] [PubMed] [Google Scholar]
- Pei R, Liu J, Wang S, and Yang M. 2013. Use of bacterial cell walls to improve the mechanical performance of concrete. Cement and Concrete Composites. 39:122–130. [Google Scholar]
- Peralta AL, Ludmer S, Matthews JW, and Kent AD. 2014. Bacterial community response to changes in soil redox potential along a moisture gradient in restored wetlands. Ecological Engineering. 73:246–253. [Google Scholar]
- Pereira SI, and Castro PM. 2014. Phosphate-solubilizing rhizobacteria enhance Zea mays growth in agricultural P-deficient soils. Ecological Engineering. 73:526–535. [Google Scholar]
- Pérez-López R, Carrero S, Cruz-Hernández P, Asta MP, Macías F, Cánovas CR, Guglieri C, and Nieto JM. 2018. Sulfate reduction processes in salt marshes affected by phosphogypsum: Geochemical influences on contaminant mobility. Journal of Hazardous Materials. 350:154–161. [DOI] [PubMed] [Google Scholar]
- Perito B, Marvasi M, Barabesi C, Mastromei G, Bracci S, Vendrell M, and Tiano P. 2014. A Bacillus subtilis cell fraction (BCF) inducing calcium carbonate precipitation: biotechnological perspectives for monumental stone reinforcement. Journal of Cultural Heritage. 15:345–351. [Google Scholar]
- Phillips AJ, Cunningham AB, Gerlach R, Hiebert R, Hwang C, Lomans BP, Westrich J, Mantilla C, Kirksey J, and Esposito R. 2016. Fracture sealing with microbially-induced calcium carbonate precipitation: A field study. Environmental science & technology. 50:4111–4117. [DOI] [PubMed] [Google Scholar]
- Povedano-Priego C, Martín-Sánchez I, Jroundi F, Sánchez-Castro I, and Merroun ML. 2017. Fungal biomineralization of lead phosphates on the surface of lead metal. Minerals engineering. 106:46–54. [Google Scholar]
- Proudfoot D, Brooks L, Gammons CH, Barth E, Bless D, Nagisetty RM, and Lauchnor EG. 2022. Investigating the potential for microbially induced carbonate precipitation to treat mine waste. Journal of hazardous materials. 424:127490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Fang C, Huang M, and Achal V. 2017. Characterization of fungal-mediated carbonate precipitation in the biomineralization of chromate and lead from an aqueous solution and soil. Journal of Cleaner Production. 164:198–208. [Google Scholar]
- Rambabu K, Banat F, Pham QM, Ho S-H, Ren N-Q, and Show PL. 2020. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environmental Science and Ecotechnology. 2:100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos AR, Keller KL, Wall JD, and Pereira IAC. 2012. The membrane QmoABC complex interacts directly with the dissimilatory adenosine 5-phosphosulfate reductase in sulfate reducing bacteria. Frontiers in microbiology. 3:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roane TM 1999. Lead Resistance in Two Bacterial Isolates from Heavy Metal–Contaminated Soils. Microbial Ecology. 37:218–224. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Bennett PC, González LA, Macpherson G, and Milliken KL. 2004. Microbial precipitation of dolomite in methanogenic groundwater. Geology. 32:277–280. [Google Scholar]
- Rodríguez H, and Fraga R. 1999. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances. 17:319–339. [DOI] [PubMed] [Google Scholar]
- Rouff AA, Elzinga EJ, Reeder RJ, and Fisher NS. 2006. The effect of aging and pH on Pb (II) sorption processes at the calcite– water interface. Environmental science & technology. 40:1792–1798. [DOI] [PubMed] [Google Scholar]
- Ryan P, Delhaize E, and Jones D. 2001. Function and mechanism of organic anion exudation from plant roots. Annual review of plant biology. 52:527. [DOI] [PubMed] [Google Scholar]
- Safonova E, Kvitko K, Iankevitch M, Surgko L, Afti I, and Reisser W. 2004. Biotreatment of industrial wastewater by selected algal-bacterial consortia. Engineering in life sciences. 4:347–353. [Google Scholar]
- Sanchez-Andrea I, Rodriguez N, Amils R, and Sanz JL. 2011. Microbial diversity in anaerobic sediments at Rio Tinto, a naturally acidic environment with a high heavy metal content. Appl Environ Microbiol. 77:6085–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Andrea I, Sanz JL, Bijmans MF, and Stams AJ. 2014. Sulfate reduction at low pH to remediate acid mine drainage. Journal of hazardous materials. 269:98–109. [DOI] [PubMed] [Google Scholar]
- Sashidhar B, and Podile AR. 2010. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. Journal of applied microbiology. 109:1–12. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hamai T, Hori T, Aoyagi T, Inaba T, Hayashi K, Kobayashi M, Sakata T, and Habe H. 2022. Optimal start-up conditions for the efficient treatment of acid mine drainage using sulfate-reducing bioreactors based on physicochemical and microbiome analyses. Journal of Hazardous Materials. 423:127089. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hamai T, Hori T, Aoyagi T, Inaba T, Kobayashi M, Habe H, and Sakata T. 2019. Desulfosporosinus spp. were the most predominant sulfate-reducing bacteria in pilot-and laboratory-scale passive bioreactors for acid mine drainage treatment. Applied microbiology and biotechnology. 103:7783–7793. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hamai T, Hori T, Habe H, Kobayashi M, and Sakata T. 2018. Year-round performance of a passive sulfate-reducing bioreactor that uses rice bran as an organic carbon source to treat acid mine drainage. Mine Water and the Environment. 37:586–594. [Google Scholar]
- Scheckel KG, Diamond GL, Burgess MF, Klotzbach JM, Maddaloni M, Miller BW, Partridge CR, and Serda SM. 2013. Amending soils with phosphate as means to mitigate soil lead hazard: a critical review of the state of the science. Journal of Toxicology and Environmental Health, Part B. 16:337–380. [DOI] [PubMed] [Google Scholar]
- Schneider KD, Van Straaten P, De Orduña RM, Glasauer S, Trevors J, Fallow D, and Smith PS. 2010. Comparing phosphorus mobilization strategies using Aspergillus niger for the mineral dissolution of three phosphate rocks. Journal of Applied Microbiology. 108:366–374. [DOI] [PubMed] [Google Scholar]
- Scholten JC, Joye SB, Hollibaugh J, and Murrell J. 2005. Molecular Analysis of the Sulfate Reducing and ArchaealCommunity in a Meromictic Soda Lake (Mono Lake, California) by Targeting 16S rRNA, mcrA, apsA, and dsrAB Genes. Microbial ecology. 50:29–39. [DOI] [PubMed] [Google Scholar]
- Schultze-Lam S, Fortin D, Davis B, and Beveridge T. 1996. Mineralization of bacterial surfaces. Chemical Geology. 132:171–181. [Google Scholar]
- Seo H, and Roh Y. 2015. Biotransformation and its application: biogenic nano-catalyst and metal-reducing-bacteria for remediation of Cr (VI)-contaminated water. Journal of Nanoscience and Nanotechnology. 15:5649–5652. [DOI] [PubMed] [Google Scholar]
- Seo H, Sun E, and Roh Y. 2013. Remediation of chromium-contaminated water using biogenic nano-sized materials and metal-reducing bacteria. Journal of Nanoscience and Nanotechnology. 13:4405–4408. [DOI] [PubMed] [Google Scholar]
- Sevak PI, Pushkar BK, and Kapadne PN. 2021. Lead pollution and bacterial bioremediation: a review. Environmental Chemistry Letters. 19:4463–4488. [Google Scholar]
- Shan B, Hao R, Xu H, Li J, Li Y, Xu X, and Zhang J. 2021. A review on mechanism of biomineralization using microbial-induced precipitation for immobilizing lead ions. Environmental Science and Pollution Research. 28:30486–30498. [DOI] [PubMed] [Google Scholar]
- Sharma B, Singh A, Joshi S, and Reddy MS. 2022. Utilization of sandstone waste in cement mortar for sustainable production of building materials through biomineralization. Journal of Sustainable Cement-Based Materials:1–9. [Google Scholar]
- Sharma J, Shamim K, and Dubey SK. 2018. Phosphatase mediated bioprecipitation of lead as pyromorphite by Achromobacter xylosoxidans. Journal of environmental management. 217:754–761. [DOI] [PubMed] [Google Scholar]
- Sharma SB, Sayyed RZ, Trivedi MH, and Gobi TA. 2013. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodkar S, Reed S, Romine M, and Saffarini D. 2011. The octahaem SirA catalyses dissimilatory sulfite reduction in Shewanella oneidensis MR-1. Environmental microbiology. 13:108–115. [DOI] [PubMed] [Google Scholar]
- Simate GS, and Ndlovu S. 2014. Acid mine drainage: Challenges and opportunities. Journal of Environmental Chemical Engineering. 2:1785–1803. [Google Scholar]
- Sinbuathong N, Sirirote P, Watts D, and Chulalaksananukul S. 2013. Heavy metal resistant anaerobic bacterial strains from brewery digester sludge. International Journal of Global Warming. 5:127–134. [Google Scholar]
- Sinharoy A, and Pakshirajan K. 2019. Heavy metal sequestration by sulfate reduction using carbon monoxide as the sole carbon and energy source. Process Biochemistry. 82:135–143. [Google Scholar]
- Sone H, Fugetsu B, and Tanaka S. 2009. Selective elimination of lead (II) ions by alginate/polyurethane composite foams. Journal of Hazardous Materials. 162:423–429. [DOI] [PubMed] [Google Scholar]
- Song Y, Deng SP, Acosta-Martínez V, and Katsalirou E. 2008. Characterization of redox-related soil microbial communities along a river floodplain continuum by fatty acid methyl ester (FAME) and 16S rRNA genes. Applied Soil Ecology. 40:499–509. [Google Scholar]
- Staunton S, and Leprince F. 1996. Effect of pH and some organic anions on the solubility of soil phosphate: implications for P bioavailability. European Journal of Soil Science. 47:231–239. [Google Scholar]
- Steenbergh AK, Bodelier PL, Hoogveld HL, Slomp CP, and Laanbroek HJ. 2011. Phosphatases relieve carbon limitation of microbial activity in Baltic Sea sediments along a redox-gradient. Limnology and Oceanography. 56:2018–2026. [Google Scholar]
- Stocks-Fischer S, Galinat JK, and Bang SS. 1999. Microbiological precipitation of CaCO3. Soil Biology and Biochemistry. 31:1563–1571. [Google Scholar]
- Su F, and Yang Y. 2021. Microbially induced carbonate precipitation via methanogenesis pathway by a microbial consortium enriched from activated anaerobic sludge. Journal of Applied Microbiology. 131:236–256. [DOI] [PubMed] [Google Scholar]
- Su Y, Qian C, Rui Y, and Feng J. 2021. Exploring the coupled mechanism of fibers and bacteria on self-healing concrete from bacterial extracellular polymeric substances (EPS). Cement and Concrete Composites. 116:103896. [Google Scholar]
- Sukweenadhi J, Kim Y-J, Choi E-S, Koh S-C, Lee S-W, Kim Y-J, and Yang DC. 2015. Paenibacillus yonginensis DCY84T induces changes in Arabidopsis thaliana gene expression against aluminum, drought, and salt stress. Microbiological Research. 172:7–15. [DOI] [PubMed] [Google Scholar]
- Tang Y, Elzinga EJ, Lee YJ, and Reeder RJ. 2007. Coprecipitation of chromate with calcite: batch experiments and X-ray absorption spectroscopy. Geochimica et Cosmochimica Acta. 71:1480–1493. [Google Scholar]
- Tanner AC, Badger S, Lai C-H, Listgarten MA, Visconti RA, and Socransky SS. 1981. Wolinella gen. nov., Wolinella succinogenes (Vibrio succinogenes Wolin et al.) comb. nov., and description of Bacteroides gracilis sp. nov., Wolinella recta sp. nov., Campylobacter concisus sp. nov., and Eikenella corrodens from humans with periodontal disease. International Journal of Systematic and Evolutionary Microbiology. 31:432–445. [Google Scholar]
- Tarafdar J, and Claassen N. 1988. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biology and fertility of soils. 5:308–312. [Google Scholar]
- Teng Z, Shao W, Zhang K, Huo Y, and Li M. 2019. Characterization of phosphate solubilizing bacteria isolated from heavy metal contaminated soils and their potential for lead immobilization. Journal of environmental management. 231:189–197. [DOI] [PubMed] [Google Scholar]
- Tong L, Fan R, Yang S, and Li C. 2021. Development and status of the treatment technology for acid mine drainage. Mining, Metallurgy & Exploration. 38:315–327. [Google Scholar]
- Uchimiya M, Bannon DI, Wartelle LH, Lima IM, and Klasson KT. 2012. Lead retention by broiler litter biochars in small arms range soil: impact of pyrolysis temperature. Journal of agricultural and food chemistry. 60:5035–5044. [DOI] [PubMed] [Google Scholar]
- Umezawa K, Kojima H, Kato Y, and Fukui M. 2020. Disproportionation of inorganic sulfur compounds by a novel autotrophic bacterium belonging to Nitrospirota. Systematic and Applied Microbiology. 43:126110. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency, 2019a. Implementation Status Report for EPA Actions under the December 2018 Federal Action Plan to Reduce Childhood Lead Exposures and Associated Health Impacts. EPA Publication Number: 100-R-19–003. [Google Scholar]
- United States Environmental Protection Agency, 2019b. Lead at Superfund Sites: Risk Assessment. https://www.epa.gov/superfund/lead-superfund-sites-risk-assessment, retrieved 4/14/2023,
- United States Environmental Protection Agency. 2022. EPA Strategy to Reduce Lead Exposures and Disparities in U.S. Communities (Lead Strategy). EPA Publication Number: 540R22006. https://www.epa.gov/lead/final-strategy-reduce-lead-exposures-and-disparities-us-communities, retrieved April 20, 2023. [Google Scholar]
- Utgikar VP, Harmon SM, Chaudhary N, Tabak HH, Govind R, and Haines JR. 2002. Inhibition of sulfate-reducing bacteria by metal sulfide formation in bioremediation of acid mine drainage. Environmental Toxicology: An International Journal. 17:40–48. [DOI] [PubMed] [Google Scholar]
- van Vliet DM, von Meijenfeldt FB, Dutilh BE, Villanueva L, Sinninghe Damsté JS, Stams AJ, and Sánchez-Andrea I. 2021. The bacterial sulfur cycle in expanding dysoxic and euxinic marine waters. Environmental microbiology. 23:2834–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavourakis CD, Mehrshad M, Balkema C, Van Hall R, Andrei A-Ş, Ghai R, Sorokin DY, and Muyzer G. 2019. Metagenomes and metatranscriptomes shed new light on the microbial-mediated sulfur cycle in a Siberian soda lake. BMC biology. 17:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron A, Cruaud P, Culley AI, Couture R-M, Lovejoy C, and Vincent WF. 2020. Sulfur intermediates as new biogeochemical hubs in an aquatic model microbial ecosystem. [DOI] [PMC free article] [PubMed]
- Vigneron A, Cruaud P, Culley AI, Couture R-M, Lovejoy C, and Vincent WF. 2021. Genomic evidence for sulfur intermediates as new biogeochemical hubs in a model aquatic microbial ecosystem. Microbiome. 9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Gomez D, Ababneh H, Papirio S, Rousseau D, and Lens P. 2011. Effect of sulfide concentration on the location of the metal precipitates in inversed fluidized bed reactors. Journal of hazardous materials. 192:200–207. [DOI] [PubMed] [Google Scholar]
- Voeikova T, Shebanova A, Ivanov YD, Kaysheva A, Novikova L, Zhuravliova O, Shumyantseva V, Shaitan K, Kirpichnikov M, and Debabov V. 2016. The role of proteins of the outer membrane of Shewanella oneidensis MR-1 in the formation and stabilization of silver sulfide nanoparticles. Applied Biochemistry and Microbiology. 52:769–775. [Google Scholar]
- Vyas P, and Gulati A. 2009. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC microbiology. 9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Roger AJ, Flax JL, Brusseau GA, and Stahl DA. 1998. Phylogeny of Dissimilatory Sulfite Reductases Supports an Early Origin of Sulfate Respiration. Journal of Bacteriology. 180:2975–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W, Qin Y, Wu H, Zuo W, He H, Tan J, Wang Y, and He D. 2020. Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Frontiers in Microbiology. 11:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wan Y, Zheng Y, Lee X, Liu T, Yu Z, Huang J, Ok YS, Chen J, and Gao B. 2019a. Alginate-based composites for environmental applications: a critical review. Critical reviews in environmental science and technology. 49:318–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Chen S, Han Y, Chen L, and Wang D. 2019b. Responses of soil aggregates and bacterial communities to soil-Pb immobilization induced by biofertilizer. Chemosphere. 220:828–836. [DOI] [PubMed] [Google Scholar]
- Wang X, Di J, Dong Y, Yang Y, Liang B, Meng F, Wang T, An W, Li Z, and Guo J. 2021. The Dynamic Experiment on Treating Acid Mine Drainage with Iron Scrap and Sulfate Reducing Bacteria Using Biomass Materials as Carbon Source. Journal of Renewable Materials. 9:163. [Google Scholar]
- Wani A, Rahayu F, Kadarwati F, Suhara C, Singh R, Dhanjal D, Akhtar N, Mir T, and Chopra C. 2022. Metagenomic screening strategies for bioprospecting enzymes from environmental samples. In IOP Conference Series: Earth and Environmental Science. Vol. 974. IOP Publishing. 012003. [Google Scholar]
- Wasmund K, Cooper M, Schreiber L, Lloyd KG, Baker BJ, Petersen DG, Jørgensen BB, Stepanauskas R, Reinhardt R, and Schramm A. 2016. Single-cell genome and group-specific dsrAB sequencing implicate marine members of the class Dehalococcoidia (phylum Chloroflexi) in sulfur cycling. MBio. 7:e00266–00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmund K, Mußmann M, and Loy A. 2017. The life sulfuric: microbial ecology of sulfur cycling in marine sediments. Environmental microbiology reports. 9:323–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrmann LM, Riedinger N, Brunner B, Kamyshny A Jr, Hubert CR, Herbert LC, Brüchert V, Jørgensen BB, Ferdelman TG, and Formolo MJ. 2017. Iron-controlled oxidative sulfur cycling recorded in the distribution and isotopic composition of sulfur species in glacially influenced fjord sediments of west Svalbard. Chemical Geology. 466:678–695. [Google Scholar]
- Westrich JT, and Berner RA. 1984. The role of sedimentary organic matter in bacterial sulfate reduction: The G model tested 1. Limnology and oceanography. 29:236–249. [Google Scholar]
- Widdel F, and Pfennig N. 1981. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. Archives of microbiology. 129:395–400. [DOI] [PubMed] [Google Scholar]
- Wozny M, Bryant M, Holdeman L.t., and Moore W. 1977. Urease assay and urease-producing species of anaerobes in the bovine rumen and human feces. Applied and Environmental Microbiology. 33:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DT 1999. The role of sulphate-reducing bacteria and cyanobacteria in dolomite formation in distal ephemeral lakes of the Coorong region, South Australia. Sedimentary Geology. 126:147–157. [Google Scholar]
- Wu G, Li N, Mao Y, Zhou G, and Gao H. 2015. Endogenous generation of hydrogen sulfide and its regulation in Shewanella oneidensis. Frontiers in microbiology. 6:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Ma X-B, Yuan H, Liu P-C, Lei Y-B, Xu H, Du D-L, Sun J-F, and Feng Y-J. 2015. Photocatalytic properties of zinc sulfide nanocrystals biofabricated by metal-reducing bacterium Shewanella oneidensis MR-1. Journal of Hazardous Materials. 288:134–139. [DOI] [PubMed] [Google Scholar]
- Xu J. c., Huang L.-m., Chen C, Wang J, and Long X.-x.. 2019. Effective lead immobilization by phosphate rock solubilization mediated by phosphate rock amendment and phosphate solubilizing bacteria. Chemosphere. 237:124540. [DOI] [PubMed] [Google Scholar]
- Xu R, Li Q, Nan X, Yang Y, Xu B, Li K, Wang L, Zhang Y, and Jiang T. 2022a. Synthesis of nano–silica and biogenic iron (oxyhydr) oxides composites mediated by iron oxidizing bacteria to remove antimonite and antimonate from aqueous solution: Performance and mechanisms. Journal of Hazardous Materials. 422:126821. [DOI] [PubMed] [Google Scholar]
- Xu R, Li Q, Yang Y, Jin S, Liao L, Wu Z, Yin Z, Xu B, Nan X, He Y, Zhu B, and Jiang T. 2022b. Removal of heavy metal(loid)s from aqueous solution by biogenic FeS–kaolin composite: Behaviors and mechanisms. Chemosphere. 299:134382. [DOI] [PubMed] [Google Scholar]
- Xue Z-F, Cheng W-C, Wang L, and Hu W. 2022. Effects of bacterial inoculation and calcium source on microbial-induced carbonate precipitation for lead remediation. Journal of Hazardous Materials. 426:128090. [DOI] [PubMed] [Google Scholar]
- Yang X, Wan Y, Zheng Y, He F, Yu Z, Huang J, Wang H, Ok YS, Jiang Y, and Gao B. 2019. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chemical Engineering Journal. 366:608–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Deng C-B, Zhu H, Xiong J, and Sun Z. 2020a. Effective Removal of Lead from Solution by Sulfate Reducing Bacteria Cultured with Sugar Byproducts. Journal of Biobased Materials and Bioenergy. 14:384–395. [Google Scholar]
- Yin X, Weitzel F, Jimenez-Lopez C, Griesshaber E, Fernandez-Diaz L, Rodriguez-Navarro A, Ziegler A, and Schmahl WW. 2020b. Directing effect of bacterial extracellular polymeric substances (EPS) on calcite organization and eps–carbonate composite aggregate formation. Crystal Growth & Design. 20:1467–1484. [Google Scholar]
- Yu X, and Rong H. 2022. Seawater based MICP cements two/one-phase cemented sand blocks. Applied Ocean Research. 118:102972. [Google Scholar]
- Yu X, and Zhang Z. 2023. Calcium carbide sludge activated fly ash mixture for offshore construction and its crack repair using seawater-mixed bioslurry cement. Journal of Cleaner Production. 395:136456. [Google Scholar]
- Yuan Z, Yi H, Wang T, Zhang Y, Zhu X, and Yao J. 2017. Application of phosphate solubilizing bacteria in immobilization of Pb and Cd in soil. Environmental Science and Pollution Research. 24:21877–21884. [DOI] [PubMed] [Google Scholar]
- Zane Grant M, Yen Huei-che B, and Wall Judy D. 2010. Effect of the Deletion of qmoABC and the Promoter-Distal Gene Encoding a Hypothetical Protein on Sulfate Reduction in Desulfovibrio vulgaris Hildenborough. Applied and Environmental Microbiology. 76:5500–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Chen Z, Du Y, Lyu Q, Yang Z, Liu Y, and Yan Z. 2021. Microbiologically induced calcite precipitation technology for mineralizing lead and cadmium in landfill leachate. Journal of Environmental Management. 296:113199. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhao C, Zhou A, Yang C, Zhao L, and Li Z. 2019a. Aragonite formation induced by open cultures of microbial consortia to heal cracks in concrete: Insights into healing mechanisms and crystal polymorphs. Construction and Building Materials. 224:815–822. [Google Scholar]
- Zhang K, Xue Y, Xu H, and Yao Y. 2019b. Lead removal by phosphate solubilizing bacteria isolated from soil through biomineralization. Chemosphere. 224:272–279. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lin X, Wang J, Jiang F, Wei L, Chen G, and Hao X. 2016a. Effects of lead and mercury on sulfate-reducing bacterial activity in a biological process for flue gas desulfurization wastewater treatment. Scientific reports. 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang H, and Han X. 2016b. Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment. Chemosphere. 154:215–223. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liao S.-a., Yu X, Lu H, Xian J.-a., Guo H, Wang A, and Xie J. 2015. Microbial diversity of mangrove sediment in Shenzhen Bay and gene cloning, characterization of an isolated phytase-producing strain of SPC09 B. cereus. Applied microbiology and biotechnology. 99:5339–5350. [DOI] [PubMed] [Google Scholar]
- Zhang Y. f., He L.-y., Chen Z.-j., Zhang W.-h., Wang Q.-y., Qian M, and Sheng X.-f.. 2011. Characterization of lead-resistant and ACC deaminase-producing endophytic bacteria and their potential in promoting lead accumulation of rape. Journal of hazardous materials. 186:1720–1725. [DOI] [PubMed] [Google Scholar]
- Zheng X, Liang C, Miaomiao C, Jinghao C, and Xiaofang L. 2019. Functional metagenomics to mine soil microbiome for novel cadmium resistance genetic determinants. Pedosphere. 29:298–310. [Google Scholar]
- Zhou N-Q, Tian L-J, Wang Y-C, Li D-B, Li P-P, Zhang X, and Yu H-Q. 2016. Extracellular biosynthesis of copper sulfide nanoparticles by Shewanella oneidensis MR-1 as a photothermal agent. Enzyme and Microbial Technology. 95:230–235. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lv B, Shang X, Wang J, Li M, and Yu X. 2019a. The immobilization effects on Pb, Cd and Cu by the inoculation of organic phosphorus-degrading bacteria (OPDB) with rapeseed dregs in acidic soil. Geoderma. 350:1–10. [Google Scholar]
- Zhu X, Wang J, De Belie N, and Boon N. 2019b. Complementing urea hydrolysis and nitrate reduction for improved microbially induced calcium carbonate precipitation. Applied microbiology and biotechnology. 103:8825–8838. [DOI] [PubMed] [Google Scholar]


