Abstract
In recent decades, immunoglobulin E (IgE) mediated food allergy has become a growing public health concern. Converging evidence from cross-sectional prevalence studies, health care utilization records, and cohort studies indicate that food allergies are increasingly prevalent and often severe. Although IgE-mediated food allergy has long been considered a predominantly pediatric concern, analysis of recent self-reported data suggests that food allergies may be more prevalent among adult populations than previously acknowledged, with many reported cases of adult-onset allergies as well as persistent childhood-onset allergies. Results of studies also suggest that food allergy–related health care utilization is increasing as more individuals seek emergency treatment for food-induced anaphylaxis. Analysis of epidemiologic data also indicates that the burden of food allergies is unequally distributed. Published prevalence rates are highest in Western countries, e.g., the United States, United Kingdom, and Australia. Within these countries, there also is heterogeneity across racial and/or ethnic groups, with non-White and second-generation immigrant populations disproportionately affected. Importantly, such observations can shed light on the etiology of food allergy and inform improved clinical management, treatment, and prevention efforts. For example, there is a growing consensus that earlier introduction of allergenic foods, e.g., peanut, promotes oral tolerance and can dramatically reduce food allergy risk. In addition, much attention has been paid to the potentially deleterious effects of cutaneous allergen exposure, e.g., through eczematous skin, which can skew the immune response away from tolerance and toward allergic sensitization, thereby increasing food allergy risk. Furthermore, there is a growing appreciation for the potential protective effects of diverse microbial exposures, given mounting evidence for the immunomodulatory effects of the human microbiome. Also, when considering the geographic variability in the prevalence of certain food and environmental allergies as well as their structural similarities at the molecular level, it is believed that co-sensitization between food and environmental allergens may be a key driver of rising food allergy prevalence.
Immunoglobulin E (IgE) mediated food allergy is a growing global public health concern1 that can dramatically impact quality of life2 and impose considerable economic burden on patients and their families. Analysis of data also indicates that food allergic reactions result in rising numbers of emergency department (ED) visits and hospitalizations. There is converging evidence from cross-sectional prevalence studies, health care utilization data, and cohort studies that food allergies are increasingly prevalent and often severe. However, analysis of the current epidemiologic data suggests that the population-level burden of food allergies varies substantially around the world, not only between countries but within them as well. This review aimed to summarize the growing epidemiologic literature that characterizes the distribution of IgE-mediated food allergy, both in terms of disease prevalence and other key food allergy outcomes, including how these outcomes vary among patients of different racial and/or ethnic groups. Current hypotheses on mechanisms of food allergy development believed to underlie the observed patterns of global food allergy prevalence are also reviewed.
FOOD ALLERGY PREVALENCE
Worldwide Food Allergy Prevalence
In 2012, the World Allergy Organization systematically reviewed global studies on the prevalence, patterns, and burden of food allergy.3 However, the studies from which these data were gleaned were highly heterogeneous, given that they were conducted over a wide range of time periods, used a variety of study designs, observed varying pediatric age ranges, and applied different criteria to define and/or measure food allergy (oral food challenge [OFC], symptoms and sensitization, or parental reporting). Among the 89 countries that provided prevalence data, only 9 countries had any estimates in which food allergy cases were confirmed in patients via OFC, the most conclusive diagnostic approach.3 Among these nine countries, the highest rates of food allergy were reported among Australian infants, 10% of whom were food allergic (mostly to egg) at 12 months. In contrast, an OFC-confirmed food allergy prevalence rate of 1% (mostly to shrimp) was observed among Thai pre-school children, which indicated a remarkable degree of geographic variability in food allergy prevalence.3
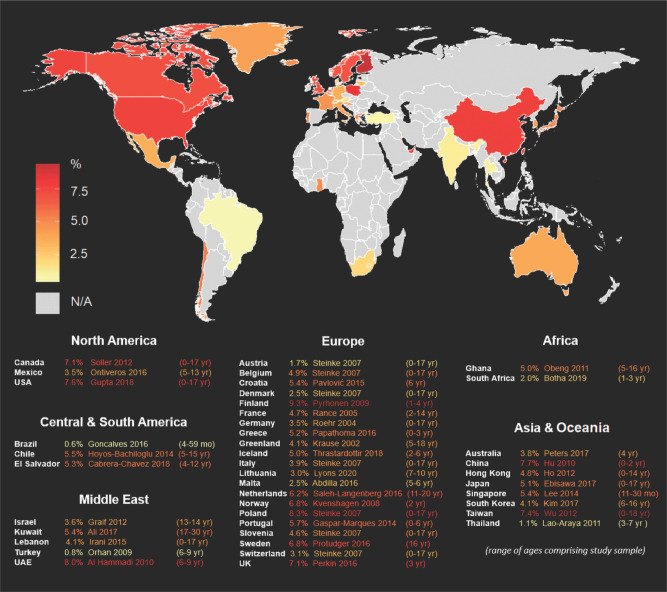
In recent years our understanding of the global distribution of food allergies has grown substantially. The most up-to-date country-specific pediatric food allergy prevalence estimates from published population-based epidemiologic studies are summarized in Fig. 1.4 However, these studies are also heterogeneous in study design. In addition, much remains unknown about the current prevalence of food allergy, particularly in non-Western countries. It is important to note that this map only shows pediatric estimates because there is a paucity of available data on the current food allergy burden among adults, particularly outside of Europe and North America.4
Figure 1.
Available food allergy prevalence estimates around the world, varying in study design. From Ref. 4. Permission obtained from Springer Nature to reuse the figure.
Food Allergy in the United States
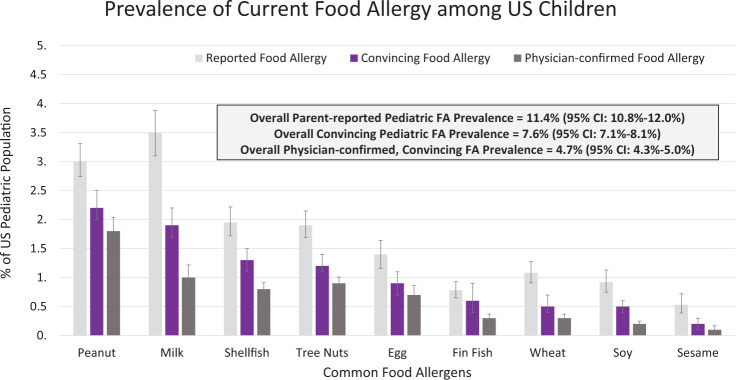
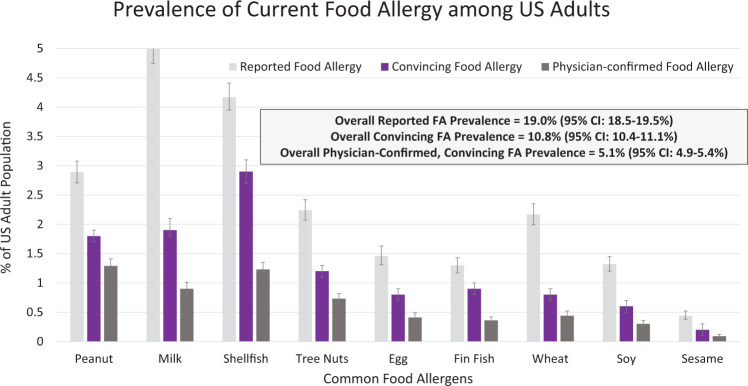
In 2018, a population-based cross-sectional prevalence survey was administered to >50,000 U.S. households.5,6 Findings demonstrate that food allergy affects a much larger number of Americans than previously acknowledged. Specifically, this survey estimated that 10.8% of U.S. adults5 and 7.6% of children6 have at least one current food allergy. In this study, food allergy was defined as having a self- or parent-reported food allergy, along with a self- or parent-reported history of at least one convincingly IgE-mediated reaction symptom.5,6 These symptoms were identified by a panel of food allergy experts and include the following: hives, swelling, difficulty swallowing, throat tightening, chest tightening, trouble breathing, wheezing, vomiting, chest pain, rapid heart rate, fainting and/or dizziness and/or feeling light-headed, and low blood pressure. Because food allergies are often underdiagnosed and overreported by individuals,5,6 application of the strict symptom parameters to estimate food allergy better reflects the current prevalence of “true IgE-mediated food allergy” in the United States. Allergen-specific estimates of current pediatric and adult food allergy prevalence are provided in Figs. 2 and 3, respectively. Both Figs. 2 and 3 indicate that many Americans, including nearly one in five adults, believe themselves to be food allergic, even if they do not report reaction symptoms consistent with an IgE-mediated reaction on ingestion of a suspected allergen.
Figure 2.
The prevalence of current food allergy among U.S. children from a survey of 38,408 children. From Ref. 6. FA = Food allergy.
Figure 3.
Prevalence of current food allergy among U.S. adults from a survey of 40,443 adults. From Ref. 5. FA = Food allergy.
The aforementioned prevalence data build on previous national studies, which describe a growing burden of food allergy over recent decades. Results of a series of cross-sectional, random digit dial telephone survey that assessed self-reported peanut, tree nut, and sesame allergy, suggested that peanut allergy prevalence among U.S. children was 1.4% in 2008, a marked increase from 1997 (0.4%) and 2002 (0.8%).7 The tree nut allergy prevalence was estimated to be 1.1% in 2008, compared with 0.5% in 2002 and 0.2% in 1997, also indicative of a substantial increase in food allergy prevalence.7 In general, common food allergens among children and adults are similar (peanut, tree nut, milk, shellfish, fin fish, egg, wheat, soy, and sesame) but the prevalence of each allergen varies. As seen in Fig. 2, peanut is the most common allergen among children, whereas shellfish is the most common allergen among adults (Fig. 3).5,6,8 Notably, 40% of children with a food allergy and 45% of adults with a food allergy report convincing allergies to multiple foods.
In 2010, food allergy was estimated to be more prevalent in urban areas as opposed to rural areas of the United States, whereas the odds of having a food allergy were higher in southern states compared with northern states.9 In addition, two in five children and half of adults with a food allergy report experiencing a severe food-triggered reaction, with nearly one in five children and one in ten adults with food allergy reporting an ED visit for an allergic reaction within the past year.5,6 In addition, 42.0% of children and 38.3% of adults with a food allergy report at least one food allergy–related ED visit within their lifetime.5,6 These increasing levels of health care utilization are consistent with other U.S. data, which indicated increasing food allergy–related health care utilization.4 For instance a recent analysis of national health care claims data found that, from 2007–2016, the percentage of all claim lines with a diagnosis related to food-induced anaphylaxis increased by 377%.10
RACIAL AND/OR ETHNIC DIFFERENCES IN FOOD ALLERGY
Differences in food allergy prevalence have been observed by race and/or ethnicity; however, there remains a paucity of data from around the world. Data from the Australian HealthNuts study demonstrate that rates of peanut allergy and peanut sensitization are threefold more prevalent among infants with East Asian–born parents compared with their peers with Australian-born parents.11 In a study of South Asian Indian immigrants in the United States, tree nuts were the most commonly self-reported food allergen, whereas legumes (excluding peanut and soy) were the sixth most common allergen.12 However, legumes (excluding peanut and soy) are not among the nine most common food allergens reported by the general U.S. population.5,6 Differences in diet could explain this variation in prevalence. In addition, in the United States, food allergies seem to be particularly more prevalent among Black children compared with White children, similar to other atopic conditions, e.g., asthma and eczema.
Remarkably, analysis of epidemiologic data indicates that, in the United States, there are more non-White children with food allergy than there are White children with food allergy (2.9 versus 2.7 million),6 despite common misconceptions that food allergies disproportionately impact White youth. Analysis of recent survey data also show that White adults have lower rates of food allergy compared with their Black, Hispanic, Asian, and multiracial peers, and that rates are similar among adults in the highest and lowest income strata.5 Sicherer et al.13 also obtained peanut specific IgE levels on children across multiple allergy clinics (N = 503). In observing differences in race, Black children were more likely to have a peanut specific IgE level of >5 kUa/L compared with their White counterparts.13 When studying other allergens (milk, egg, peanut, and shrimp) through the U.S. Centers for Disease Control and Prevention 2005–2006 National Health and Nutrition Examination Survey, Black children were more likely to be sensitized over the diagnostic cutoff values (0.35 kUa/L).14 Reported food sensitization prevalence among Black individuals (regardless of age) was 27% compared with 13.8% for White and 21.2% for Hispanic individuals. The estimated clinical food allergy rates were 5.9% among Black, 1.9% among White, and 2.7% among Hispanic individuals.14
Interestingly, previous epidemiologic studies reported that Hispanic children may be less likely to have a food allergy relative to White children.15,16 However, more recent data have not identified significant differences in disease prevalence among White and Hispanic respondents. In general, the distribution of food allergy among U.S. Hispanic populations has been undercharacterized, and further work is needed to better understand the burden of food allergy among this diverse and growing demographic group.
A multi-site retrospective cohort study conducted in Chicago and Cincinnati concluded that African-American and Hispanic children have higher rates of food-induced anaphylaxis and ED visits than their White counterparts.17 Another population-based study, conducted in Florida, examined ED visits (N = 2751) and used the International Classification of Diseases, Ninth Revision codes to identify food-induced anaphylaxis. The investigators concluded that Black children had higher odds of food-induced anaphylaxis than White children.18 The aforementioned findings are consistent with more recent national survey data, which estimate that Black pediatric patients with food allergy report higher rates of severe reactions as well as higher rates of food allergy–related ED visits than White children, even after adjusting for comorbid atopic conditions and many other relevant sociodemographic factors.6 Adult data from the same nationally-representative survey also identified elevated rates of food allergy-related ED visits among Black and Hispanic adults after adjusting for a similar comprehensive set of covariates.5
Although analysis of the above data suggests that the physical health burden of food allergy may fall disproportionately on communities of color, considerably less is known about how the psychosocial impact of living with a food allergy varies across racial and/or ethnic strata.19 One recent study, of 103 families, found that Asian American caregivers of children with food-allergy reported a higher perceived risk of allergen exposure compared with their Black, White, and Hispanic counterparts.20 In this study, African American caregivers reported the lowest perceived risk of allergen exposure of all racial and/or ethnic groups but the highest worry. At the same time, Hispanic caregivers reported greater perceived food allergy severity yet the lowest perceived parental burden with regard to their child's food allergy management.20
However, little remains known about the (likely) complex dynamics that underlie these observed differences, and further work is needed, not just to replicate these findings in larger, more nationally representative samples but to better understand whether racial differences exist with respect to the psychosocial burden experienced by patients with food allergy themselves, not just their caregivers. When considered together, these data underline the importance of systematic efforts to better understand and address the root causes of these apparent racial differences in food allergy prevalence, health care utilization, and other key patient-reported outcomes.
POTENTIAL DRIVERS OF INCREASING FOOD ALLERGY PREVALENCE
Although the reason for observed increases in food allergy prevalence remains unclear, a growing number of plausible causal factors have been identified. As with other chronic, noninfectious, inflammatory diseases, the fast rise in the prevalence and severity of food allergy cannot be attributed to genetics alone. Although a genetic predisposition to food allergy is apparent, one's environment and lifestyle may cause the epigenetic phenomena that allow for the expression of this allergic phenotype. In exploring food allergy development and the increase in food allergy prevalence, racial differences have yet to be extensively studied.
The presence of atopic dermatitis and the timing of introducing common food allergens to infants are two of the more widely accepted hypotheses for the development of food allergy, and, subsequently, the increase in food allergy prevalence overall. These two hypotheses are based on the observation that, when food is introduced orally, the food proteins are processed by the immune system in a deliberate and specific way to induce tolerance. Briefly, food antigens are actively transported across the gut endothelium, ingested by CD103+ dendritic cells, which induce the production of Forkhead box P3 and transforming growth factor beta (Foxp3+) T-regulatory cells via a TGFβ and retinoic acid dependent mechanism in local lymph nodes.21 When this mechanism of oral tolerance is interrupted and food is first introduced to the immune system by an alternate mechanism, then food allergic sensitization may occur.
Atopic dermatitis is frequently found to be comorbid to food allergy and has increased in prevalence at a rate similar to food allergy.22 In atopic dermatitis, the skin barrier function is disrupted, which accelerates water loss from the skin and leads to excoriation-induced lesions.23 These lesions may provide a pathway of introduction for food more similar to that of bacterial pathogens that, therefore, results in allergic sensitization rather than oral tolerance. Food antigens, such as peanut protein, have been shown to be present in house dust.24 Infants who crawl on the ground through peanut-containing dust, therefore, may be “introduced” peanut for the first time through lesional skin rather than orally where deliberate immune mechanisms can induce tolerance.25 The method of allergen exposure may also explain why the early oral introduction of peanut has been shown to decrease the incidence of food allergy. Early introduction of peanut orally and application of barrier cream to atopic lesional skin may be key elements in decreasing the development of food allergy.26
Another complementary hypothesis for the increase of food allergy prevalence is the microbiome hypothesis. This hypothesis states that the intestinal microbes that have co-evolved with humans over the past several millennia influence the development of the intestinal immune system and, therefore, the development of oral tolerance. Modern practices in Western cultures, such as diet, antibiotic use, and sanitization, may have skewed the types and diversity of microbes in the gut. Loss of certain key bacteria have been shown to allow the disruption of the endothelium and leakage of food antigens from the gut lumen into the blood stream where, as in the atopic dermatitis hypothesis, it can be first presented to the immune system outside the normal pathways of tolerance.27 This hypothesis may explain why the presence of dogs in the home may decrease the risk of allergic sensitization because dogs may help colonize the home environment with microbes conducive to the development of a healthy intestinal immune system.28 Also, co-sensitization with aeroallergens that are structurally similar to certain foods (homologues) may be a key trigger for food allergic sensitization. Examples of this include tropomyosin found in shellfish, mites, and other insects as well as lipid transfer proteins and profilins found in tree and weed pollen as well as vegetables, fruits, and seeds.29 This hypothesis is supported by key observations, particularly in adults who developed shellfish allergy later in life and in close temporal proximity to the development of insect allergy.
CLINICAL PEARLS
Rates of IgE-mediated food allergy vary worldwide, with Western countries, such as the United States, United Kingdom, and Australia, exhibiting some of the highest observed rates.
IgE-mediated food allergy impacts nearly 8% of children and 11% of adults in the United States, with ~40% reporting receiving food allergy treatment in the ED.
Racial differences exist in IgE-mediated food allergy prevalence and health care utilization but further work is needed to elucidate racial differences, especially in the adult population.
In addition to genetics, the Western lifestyle may be causing epigenetic phenomena that contribute to IgE-mediated food allergy development.
Timing of food allergen introduction, eczema, the microbiome, and co-sensitization with aeroallergens may contribute to the increasing prevalence of IgE-mediated food allergy.
Footnotes
R.S. Gupta reports receiving grants from Rho Inc, Stanford Sean N. Parker Center for Allergy Research, UnitedHealth Group, Thermo Fisher Scientific, Genentech, and the National Confectioners Association; and serves as a medical consultant/advisor for Before Brands, Kaléo Inc, Genentech, Institute for Clinical and Economic Review, Food Allergy Research and Education, Aimmune Therapeutics, and DBV Technologies. C.E. Ciaccio serves as a medical consultant/advisor for Aimmune Therapeutics and DBV Technologies; and holds stock from Clostrabio. The remaining authors have no conflicts of interest to declare pertaining to this article
Co-senior authors: C.E. Ciaccio and R.S. Gupta
Funded by Food Allergy Research & Education (FARE)
REFERENCES
- 1.Sicherer SH, Sampson HA.. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018; 141:41–58. [DOI] [PubMed] [Google Scholar]
- 2.Kachru R. Psychosocial issues and quality of life associated with food allergy. J Food Allergy. 2020; 2:95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott SL, Pawankar R, Allen KJet al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013; 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren CM, Jiang J, Gupta RS.. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep. 2020; 20:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta RS, Warren CM, Smith BMet al. Prevalence and severity of food allergies among US adults. JAMA Network Open. 2019; 2:e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta RS, Warren CM, Smith BMet al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018; 142:e20181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sicherer SH, Muñoz-Furlong A, Godbold JHet al. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010; 125:1322–1326. [DOI] [PubMed] [Google Scholar]
- 8.Francis OL, Wang F, Kim EHet al. Common food allergens and cross-reactivity. J Food Allergy. 2020; 2:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta RS, Springston EE, Smith Bet al. Geographic variability of childhood food allergy in the United States. Clin Pediatr. 2012; 51:856–861. [DOI] [PubMed] [Google Scholar]
- 10.FAIR Health, Inc. Food allergy in the United States: recent trends and costs an analysis of private claims data. A FAIR Health White Paper. FAIR Health Inc. 2017. [Google Scholar]
- 11.Koplin JJ, Peters RL, Ponsonby A-Let al. Increased risk of peanut allergy in infants of Asian-born parents compared to those of Australian-born parents. Allergy. 2014; 69:1639–1647. [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, Dinakar C, Fierstein JLet al. Food allergy among Asian Indian immigrants in the United States. J Allergy Clin Immunol Pract. 2020; 8:1740–1742. [DOI] [PubMed] [Google Scholar]
- 13.Sicherer SH, Wood RA, Stablein Det al. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J Allergy Clin Immunol. 2010; 126:1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu AH, Jaramillo R, Sicherer SHet al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010; 126:798–806.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta RS, Springston EE, Warrier MRet al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011; 128:e9–e17. [DOI] [PubMed] [Google Scholar]
- 16.McGowan EC, Keet CA.. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. J Allergy Clin Immunol. 2013; 132:1216–1219.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahdavinia M, Fox SR, Smith BMet al. Racial differences in food allergy phenotype and health care utilization among US children. J Allergy Clin Immunol Pract. 2017; 5:352–357.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harduar-Morano L, Simon MR, Watkins Set al. A population-based epidemiologic study of emergency department visits for anaphylaxis in Florida. J Allergy Clin Immunol. 2011; 128:594–600.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McQuaid EL, Farrow ML, Esteban CAet al. Topical review: pediatric food allergies among diverse children. J Pediatr Psychol. 2016; 41:391–396. [DOI] [PubMed] [Google Scholar]
- 20.Widge AT, Flory E, Sharma H.. Food allergy perceptions and health-related quality of life in a racially diverse sample. Children (Basel). 2018; 5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tordesillas L, Berin MC.. Mechanisms of oral tolerance. Clin Rev Allergy Immunol. 2018; 55:107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017; 35:283–289. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Kim BE, Leung DYM.. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019; 40:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Toit G, Roberts G, Sayre PHet al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015; 372:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banzon T, Leung DYM, Schneider LC.. Food allergy and atopic dermatitis. J Food Allergy. 2020; 2:35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard S. Food allergy prevention including early food introduction. J Food Allergy. 2020; 2:39–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefka AT, Feehley T, Tripathi Pet al. Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci USA. 2014; 111:13145–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Havstad S, Wegienka G, Zoratti EMet al. Effect of prenatal indoor pet exposure on the trajectory of total IgE levels in early childhood. J Allergy Clin Immunol. 2011; 128:880–885.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popescu F-D. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015; 5:31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]