Abstract
Nanoparticle-based systems imbued with both diagnostic and therapeutic functions, known as nanotheranostics, have enabled remarkable progress in guiding focal therapy, inducing active responses to endogenous and exogenous biophysical stimuli, and stratifying patients for optimal treatment. However, although in recent years more nanotechnological platforms and techniques have been implemented in the clinic, several important challenges remain that are specific to nanotheranostics. In this Review, we first discuss some of the many ways of 'constructing’ nanotheranostics, focusing on the different imaging modalities and therapeutic strategies. We then outline nanotheranostics that are currently used in humans at different stages of clinical development, identifying specific advantages and opportunities. Finally, we define critical steps along the winding road of preclinical and clinical development and suggest actions to overcome technical, manufacturing, regulatory and economical challenges for the safe and effective clinical translation of nanotheranostics.
Introduction
The first known intentional use of synthetic nanoparticles is found in the ornamented Roman ‘Lycurgus cup’ from the 4th century AD, which appears green in reflected light but turns ruby red when light is transmitted through it1. This cup is made of dichroic glass in which a colloidal dispersion of gold and silver nanoparticles causes the optical effect, which was ingeniously exploited some 2,000 years before similar nanoparticles were used for optical imaging in biomedical research. Today, nanotechnology is defined foremost by the scale of the involved structures – typically ranging between 1 and 100 nm – and is used in a vast array of applications. Materials at this scale differ vastly in their physical, chemical and biological properties from their bulk counterparts. Innovation in nanotechnology relies on the manipulation of materials and the exploration of their interactions with other systems, including living ones2. The juncture of nanotechnology and medicine gave rise to the field of ‘nanomedicine’ in the archetypal form of a nanoparticle carrying drug molecules, which quickly became one of the most intriguing, but also controversial, branches of a new science3.
A first unique attribute of nanomedicines is the ability to modulate the distribution of a payload, resulting in improved bioavailability with increased deposition at the biological target and diminished systemic toxicity. A nanomedicine can improve the balance between desired efficacy and undesired toxicity (therapeutic index), thereby enabling the delivery of drug amounts that would not be possible if the same drug were administered freely. Another unique attribute of nanomedicines is their ability to create a ‘nanoenvironment’ providing the necessary solubility, stability and protection to the selected payload. This nanoenvironment provides stealthiness to the payload and allows it to reach the target unperturbed avoiding enroute degradation4. Besides their use as drug carriers, nanoparticles themselves interact with the surrounding environment and respond to multiple endogenous and exogenous stimuli5. This aspect motivated the use of nanoparticles for various diagnostic applications and, eventually, for the integration of therapeutic and diagnostic functions and the rise of theranostics6. Notably, the notion of theranostics has its origin in a single atom: iodine. The radioactive isotope of iodine 131I has been used since 1941 for the treatment of thyroid diseases as it emits gamma rays for imaging and highly energetic electrons for killing iodide-accumulating thyroid cancer cells7, therefore allowing both imaging and therapy. More recently, the clinical success and approval by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) of the radiopharmaceuticals 177Lu-DOTATATE (Lutathera®) for the treatment of neuroendocrine tumors, and 68Ga-PSMA-11 (Locametz®) together with 177Lu-PSMA-617 (Pluvicto™) for the positron emission tomography (PET) imaging-guided treatment of prostate cancer are establishing the potential of theranostics in the clinic.8
In the past decade, the concept of theranostics has been extensively applied to nanoparticles leading to nanotheranostics, which in its archetypal form is the combination of imaging agents and therapeutic substances in the same nanostructure (Figure 1). In addition to sharing the attributes listed above, nanotheranostics provide clear advantages over nanomedicines. First, they can be used without drugs to probe the accumulation of the nanoscale carrier at the diseased site and thus stratify patients that would benefit from the nanotherapy. Second, they allow monitoring and predicting outcomes already at the time of administration, or shortly thereafter. Third, nanotheranostics can carry a high-density load of therapeutic and imaging agents to the biological target, thus increasing the signal-to-noise ratio for imaging while improving therapeutic efficacy. Fourth, nanotheranostics can be activated ‘on command’ by endogenous biological stimuli or exogenous energy sources, whereby a signal is produced or drug molecules are released upon interacting with the correct milieu (such as low pH or enzymatic activity). Finally, nanotheranostics can be engineered to report the release of a drug from the system itself9.
Figure 1 |. Theranostic nanoparticles.
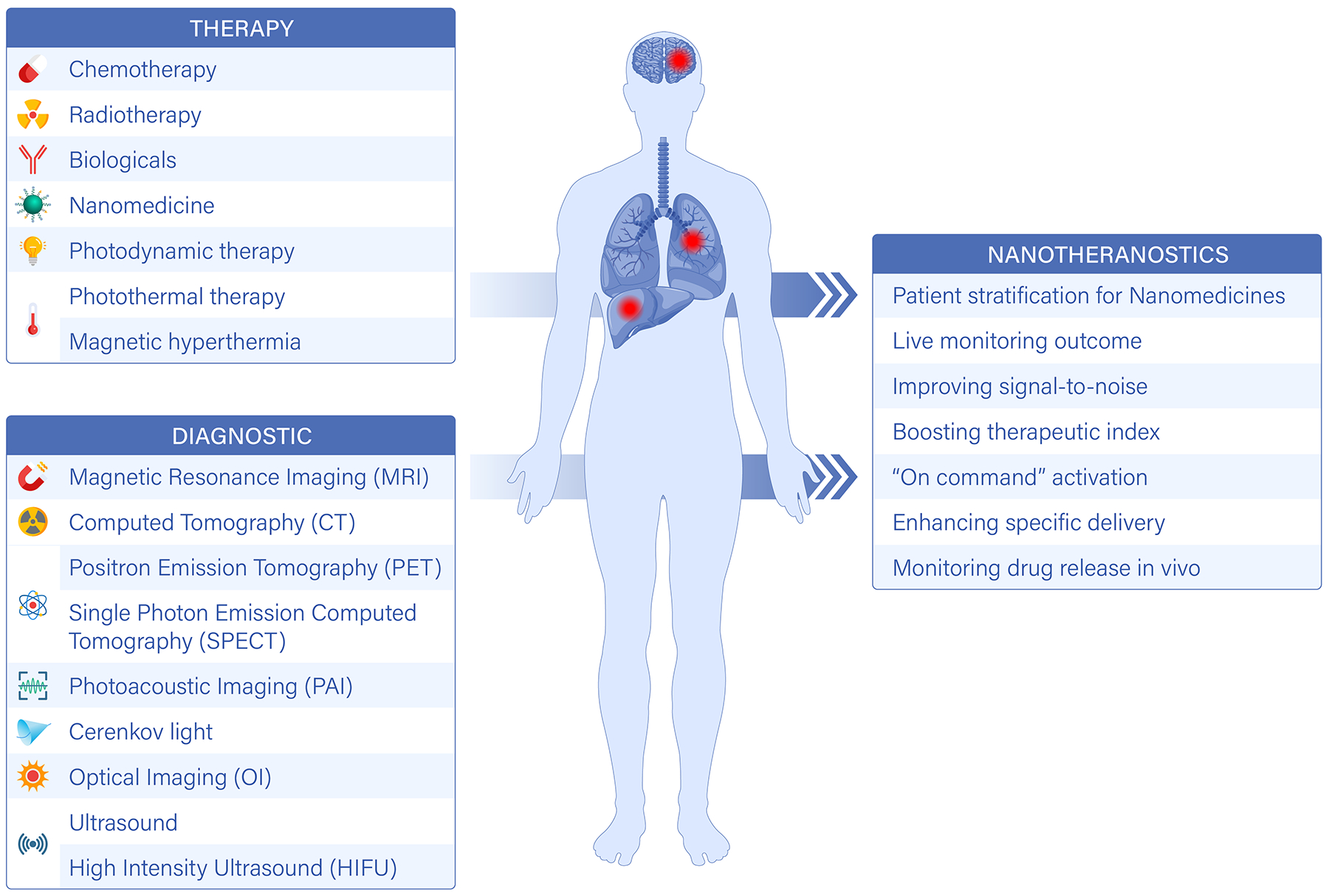
Nanotheranostics combine therapeutic and imaging functions in a nanocarrier. This can be achieved by the incorporation of specific therapeutic and imaging agents into a nanoparticle, or can be a result of the innate properties of the material, or a combination of the two. Targeting molecules (such as small ligands, peptides, aptamers, antibodies and fragments of antibodies) can be incorporated into a nanoparticle to enhance the recognition of specific cellular and subcellular targets. It is also possible to use materials with the ability to specifically recognize the diseased tissue. Moreover, nanotheranostics can be designed to be activated by endogenous and exogenous stimuli. Compared to molecular agents and nanomedicines, nanotheranostics offer several advantages, including patient stratification, ‘on command’ activation and enhanced therapeutic efficacy.
Design and Formulation of Nanotheranostics
An incredible variety of therapeutic and imaging modalities has been integrated into nanoparticles (Table 1) to improve disease detection, boost therapeutic efficacy and monitor the outcome of medical interventions6, 10, 11. Based on the mode of integration, three basic strategies emerge in the design and formulation of nanotheranostics: first, nanocarriers may be formed with materials that themselves have imaging and therapeutic capabilities (innate); second, imaging and therapeutic components may be chemically modified and conjugated to the structure of the nanocarrier (modification); and, finally, imaging and therapeutic components may be dispersed and loaded within the structure of the nanocarrier without any chemical modification (encapsulation). Nanotheranostics can also be targeted to specific cell receptors by encapsulating or modifying the carriers with targeting moieties, or even by using materials with innate affinity for a particular molecule. The literature is filled with examples of innate, encapsulation, modification and targeted nanotheranostics as well as nanotheranostics that combine several of these design strategies, as extensively detailed in the Supplementary Information. A few examples are discussed in this section, highlighting their unique features and mechanisms of action (Figure 2).
Table 1 |.
Most relevant preclinical and clinical nanotheranostics.
| Imaging modality | Therapeutic modality | Imaging agent | Therapeutic agent | Stage | Ref. |
|---|---|---|---|---|---|
| Magnetic Resonance Imaging | nanomedicine | Gd3+, Mn2+, IONP | small molecule | pre-Clinical | 14–16 |
| thermal ablation |
IONP | IONP (magnetic) PTA (photothermal) |
pre-Clinical & Clinical | 12–14, 59, 60, 67 | |
| radiation therapy | Gd3+, Gd2O3, IONP | Au, Bi, Gd-complexes | pre-Clinical &Clinical | 17, 18, 74, 78 | |
| Image-guided cell therapies | IONP (intracellular) |
cellular therapies | Clinical | 69, 70 | |
| Nuclear Imaging | ‘companion’ nanoparticle |
64Cu, 89Zr, … (PET imaging) |
nanomedicine | pre-Clinical &Clinical | 51, 107, 129 |
| radiation therapy |
64Cu, 89Zr, … (PET imaging) |
177Lu, 90Y, 186/188Re | pre-Clinical | 50, 109 | |
| CRIT |
64Cu, 89Zr, … (PET imaging) |
porphyrins, chlorins | pre-Clinical | 21, 23–25 | |
| Optical Imaging | photodynamic therapy | Cy5.5, Cy7; PLNP | porphyrins, chlorins, nanomedicine | pre-Clinical | 30–33 |
| image-guided surgery | Cy5.5, Cy7; PLNP; DCNP | surgery | pre-Clinical &Clinical | 26, 80 | |
| nanomedicine | endogenous, AuNP, CNT | small/macro-molecules | pre-Clinical | 82, 85 | |
| Ultrasound | enhanced delivery | vesicles | small/macro-molecules, nanomedicine | pre-Clinical | 41–44 |
| triggered drug release | vesicles | drug-loaded vesicles | pre-Clinical &Clinical | 37, 38, 40 |
HIFU: high-intensity focused ultrasound; CRIT: Cerenkov radiation-induced therapy; IONP: iron oxide nanoparticle; PET: positron emission tomography; PLNP: persistent luminescence nanoparticle; DCNP: down-conversion nanoparticle; NP: nanoparticle; CNT: carbon nanotube; MRI: magnetic resonance imaging; AuNP: Gold nanoparticle; PTA: photothermal ablation.
Figure 2 |. Examples of nanotheranostics with different imaging, therapeutic and targeting components.
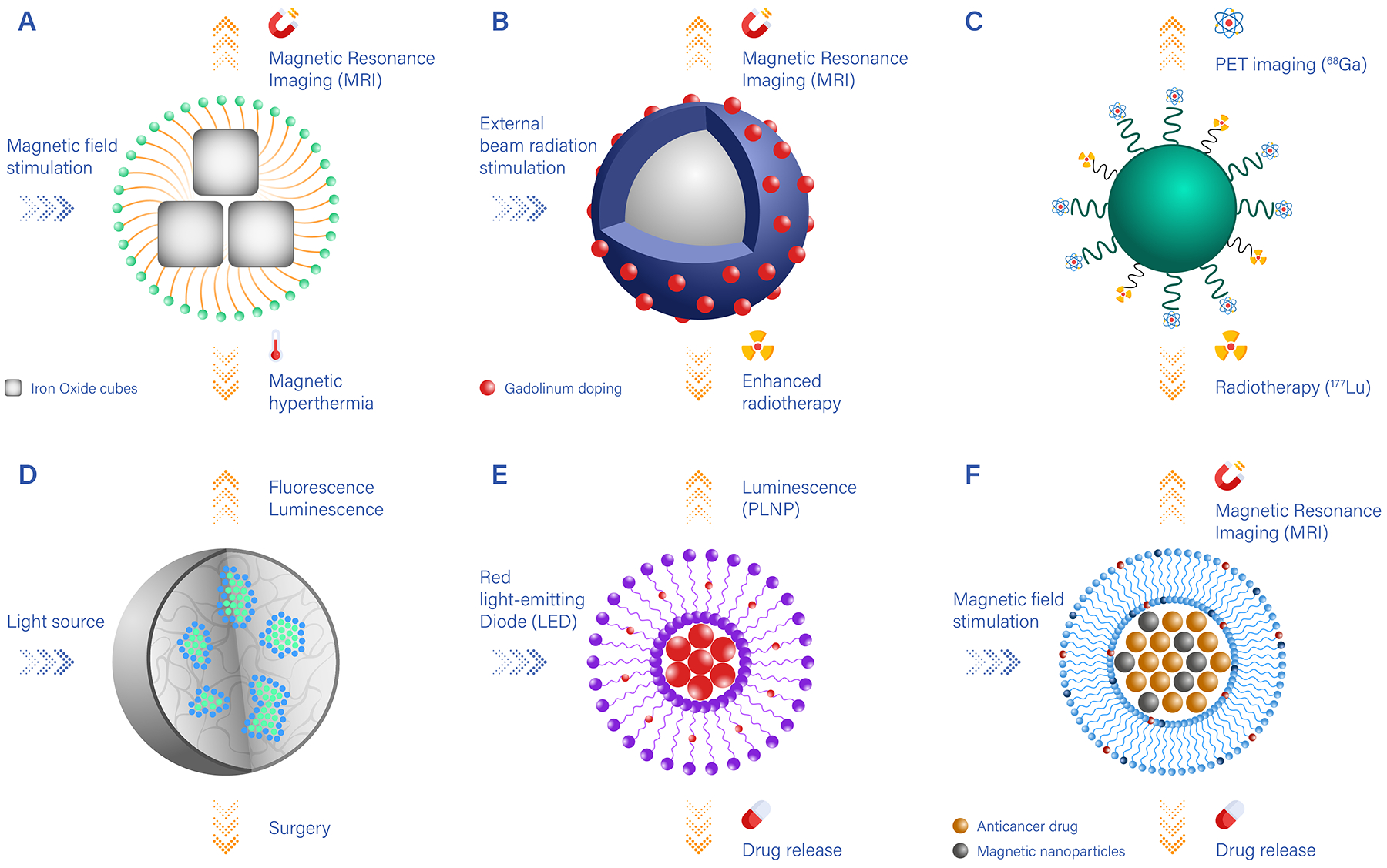
A. Clusters of iron oxide nanocubes coated by polymeric or lipid chains (yellow) and presenting surface-targeting moieties (blue). Upon magnetic stimulation, iron oxide nanocubes generate thermal energy, which can be used for magnetic hyperthermia, and can be visualized via magnetic resonance imaging (MRI); B. Nanocarriers carrying heavy metals (such as Gd) locally enhance the therapeutic efficacy of external beam radiation and can be imaged via MRI; C. Nanocarriers conjugated to two different radionuclides can be used for positron emission tomography (PET) imaging (using 89Zr) and radiotherapy (using 177Lu); D. Nanoparticles encapsulating luminescent/fluorescent molecules, which under external light stimulation can be visualised and guide surgical resection; E. Nanocarriers loaded with persistent luminescence nanoparticles (PLNP) and chemotherapeutic drugs that are released by passive diffusion over time; F. Nanocarriers loaded with iron oxide particles for MR imaging guidance, which under high-intensity focused ultrasound stimulation trigger the release of anti-cancer molecules.
Metallic nanoparticles are arguably the primary source of nanotheranostics with innate properties. Their magnetic, radioactive or plasmonic properties turn them into natural candidates for applications such as diagnostic, imaging and photothermal therapies. Super-paramagnetic iron oxide nanocubes and nanospheres provide a classic example of innate nanotheranostics (Figure 2A)12–14. They can be either used individually or clustered together to boost both the generated signal in magnetic resonance (MR) imaging and the efficacy of magnetic-induced ablation therapy. Combined MR imaging and delivery of chemotherapeutic agents has been also demonstrated by several authors, using gadolinium or manganese as the imaging reporting agent.15, 16 Heavy-metal oxide nanoparticles have also been used as MR imaging agents and enhancers of external beam radiation therapy (Figure 2B)17, 18.
Polymeric nanoparticle and lipid-based nanotheranostics are strongly associated with modification as a method to integrate imaging and therapeutic components. Functional groups present on the surface of the nanocarriers, or introduced during their assembly, can be used to label the surface with different agents. For example, two different radionuclides – one for imaging and one for internal radiotherapy – can be combined within the same nanoparticle (Figure 2C)19, 20. Also, radiolabeled nanoparticles can carry photosensitizers that induce reactive oxygen species via the Cerenkov effect, which enables the nanoparticles to act as an internal light source, locally deploying a potent therapy21–25. A broad variety of nanoparticles has also been used to guide surgeons in the resection of malignant tissues and lymph nodes (Figure 2D)26–29.
Although the surface of polymeric nanoparticles or liposomes can be modified, the encapsulation of the imaging and therapeutic agents has been the method most frequently chosen for the preparation of new theranostic nanoparticles. Typically, these agents are mixed together with the components of the nanocarrier during the assembly process. This preparation method has led to lipid nanocarriers that can be stimulated by external light sources, for instance using persistent luminescence nanoparticles incorporated with chemotherapeutic molecules into liposomes. Upon external irradiation, these systems serve both as anti-cancer and optical imaging agents (Figure 2E)30–36. Also, nanomedicines carrying different drug molecules can be triggered by high-intensity focused ultrasound under MRI guidance (Figure 2F)37, 38. The photoacoustic effect has also been exploited in conjunction with nanomedicines to enhance imaging depth and quantify drug release in vivo39, 40. Finally, focused ultrasound has been used to permeabilize the blood vasculature and facilitate the extravascular deposition of nanomedicines41–44, as well as to burst vesicles loaded with therapeutic and imaging agents45–48.
It is important to highlight that these strategies to incorporate theranostic components are often mixed together to obtain nanocarriers with different physico-chemical properties. For example, the loading of iron oxide nanoparticles for MR imaging into pH-responsive polymeric micelles combines innate nanotheranostic agents and encapsulation nanotheranostic49. Additionally, modifying the surface of nanomedicines with radionuclei for PET and single-photon emission computed tomography (SPECT) enables imaging the biodistribution of the nanomedicines and – in the case of PET – the quantification of the tissue-specific accumulation of nanomedicines in vivo50–54. Besides these examples, an extensive description of preclinical nanotheranostics is provided in the Supplementary Information.
Clinical Nanotheranostics
As scientists, we are not bound to one particle or imaging molecule or drug molecule, and we can devise new sophisticated nanotheranostic agents capable of combining multiple therapeutic and imaging modalities. However, caution must be taken with the huge ‘sandbox’ of imaging and therapeutic nanoparticles. The integration of multiple functions (imaging, therapies, targeting) in a single nanostructure must address a precise unmet clinical need – rather than being inspired by the ‘because we can’ credo – in order to maximize the odds for clinical translation.
Indeed, the variety of configurations shown in Figure 2 clashes against the modest number of theranostics studied in active and completed clinical studies (Table 2, 3). As of today, clinically relevant nanotheranostics are mostly limited to iron oxide nanoparticles, gadolinium-based and hafnium-based nanocomplexes, fluorescent nanoprobes, thermoresponsive liposomes and radiolabeled nanomedicines, which are mostly still undergoing clinical investigation at different levels of development. In this section, these clinically relevant nanotheranostics are described, highlighting their technical features and advantages.
Table 2 |. Active and Recruiting Clinical Trials for Nanotheranostics.
Information from clinicaltrial.gov.
| Imaging modality | Therapy | nano-particle | clinical application | Phase | Start Date |
Ref. |
|---|---|---|---|---|---|---|
| Magnetic Resonance Imaging | surgery | SPIO | sentinel node | NA | 07/2018 05/2020 07/2021 |
NCT05288686
NCT05161507 NCT04803331 |
| Phase1/2 | 11/2021 | NCT05359783 | ||||
| Phase 3 | 03/2020 05/2020 |
NCT04722692
NCT04261777 |
||||
| small molecule | USPIO | neuro-inflammation | Phase 1 | 06/2022 | NCT05357833 | |
| USPIO | vascular inflammation | 09/2021 | NCT03948555 | |||
| nutritional supplement | SPIO | iron deficiency | Phase 3 | 08/2018 02/2020 |
NCT03619850
NCT04268849 |
|
| Phase 4 | 02/2020 01/2020 09/2021 |
NCT04205266
NCT04080908 NCT04278651 |
||||
| radiation therapy | SPIO | hepatocellular carcinomas and liver metastasis | NA | 11/2020 | NCT04682847 | |
| magnetic hyperthermia | NanoTherm | prostate cancer | NA | 11/2021 | NCT05010759 | |
| radiation therapy | AGuIX | brain tumor and metastasis | Phase 1/2 | 03/2022 | NCT04881032 | |
| Phase 2 | 03/2019 09/2021 |
NCT03818386
NCT04899908 |
||||
| lung & pancreatic cancer | Phase 1/2 | 05/2021 | NCT04789486 | |||
| gynecologic cancer | Phase 1 | 05/2018 | NCT03308604 | |||
| photothermal therapy | AuroShell | prostate cancer | NA | 01/2020 | NCT04240639 | |
| Computed Tomography | radiation therapy | NBTXR3 | NSCL | Phase 1 | 02/2021 | NCT04505267 |
| head & neck squamous cell | Phase 2 Phase 3 |
04/2021 01/2022 |
NCT04862455
NCT04892173 |
|||
| pancreatic cancer | Phase 1 | 07/2020 | NCT04484909 | |||
| esophageal cancer | Phase 1 | 11/2020 | NCT04615013 | |||
| Optical Imaging | surgery | Carbon NP | sentinel node | NA | 01/2018 02/2022 |
NCT03550001 NCT05167149 |
| Phase 2 | 01/2022 | NCT05229874 | ||||
| Phase 2/3 | 01/2021 | NCT04759820 | ||||
| surgery | C-dots | prostate cancer | Phase 1 | 02/2021 | NCT04167969 | |
| sentinel node | Phase 1/2 | 04/2014 | NCT02106598 | |||
| surgery | ONM-100 | Peritoneal Carcinomatosis | Phase 2 | 11/2021 | NCT04950166 |
NA means that the trial didn’t have FDA-defined phases. SPIO: super-paramagnetic iron oxide nanoparticles; USPIO: ultra-small SPIO; carbon NP: carbon nanoparticle; C-dots: carbon dots. NanoTherm are iron oxide nanoparticle, AGuIX are polymeric gadolinium complexes, AuroShell are silica gold nanoshells, NBTXR3 are hafnium oxide nanoparticles; ONM-100 are fluorescent imaging nanoparticles.
Table 3 |. Completed Clinical Trials for Nanotheranostics.
Information from clinicaltrial.gov.
| Imaging modality | Therapy | Nano-particle | clinical application | Phase | Last Update | Reference | Results | Comments |
|---|---|---|---|---|---|---|---|---|
| Magnetic Resonance Imaging | surgery | SPIO | sentinel node | NA | 06/2017 06/2019 10/2020 10/2020 09/2022 |
NCT01927887
NCT02739425 NCT01815333 NCT03898687 NCT05054062 |
Y N Y N P |
SPIO for lymph node visualization in melanoma patients |
| small molecule | USPIO | neuro-inflammation | NA | 07/2022 | NCT02549898 | P | no correlation between neuroinflammation and USPIO signal enhancement | |
| Phase 1 | 03/2023 | NCT02511028 | N | - | ||||
| cell therapy | SPIO | cell mobility & retention | NA | 06/2021 | NCT00972946 | P | safety and feasibility of cell labeling with SPIO for MRI tracking | |
| Phase 1 | 08/2018 | NCT03651791 | P | |||||
| Phase 1/2 | 03/2021 | NCT00781872 | N | - | ||||
| Cell tracking | USPIO | inflammation in cardiovascular disorders | NA | 02/2013 12/2014 04/2015 |
NCT01169935
NCT01323296 NCT00368589 |
P P P |
safety and feasibility of cell labeling with SPIO for MRI tracking | |
| Phase 2/3 | 05/2017 | NCT02319278 | P | |||||
| nutritional supplement | SPIO | iron deficiency | NA | 03/2014 | NCT01374919 | P | safety of Ferumoxytol | |
| Phase 2 | 05/2018 | NCT01052779 | P | efficacy of Ferumoxytol vs iron sucrose | ||||
| Phase 3 | 01/2015 01/2015 06/2018 04/2022 04/2022 04/2022 |
NCT00255437
NCT00255424 NCT02694978 NCT01114204 NCT01114139 NCT01114217 |
P P P Y Y Y |
safety of i.v. iron therapy in kidney disease patients | ||||
| Phase 4 | 03/2012 04/2022 |
NCT01148745
NCT01227616 |
Y P |
safety of Ferumoxytol in chronic kidney disease patients | ||||
| magnetic hyperthermia | SPIO | prostate cancer | Phase 0 | 05/2017 | NCT02033447 | N | - | |
| radiation therapy | AGuIX | brain tumor and metastasis | Phase 1 | 06/2019 | NCT02820454 | P77 | AGuIX phase I study protocol in brain metastasis patients | |
| photothermal therapy | AuroShell | prostate cancer head & neck |
NA | 02/2017 03/2021 |
NCT00848042
NCT02680535 |
Y P |
demonstration of a software for mapping tissue ablation and preoperative planning | |
| irinotecan liposomes | SPIO | breast cancer and solid tumors | Phase 1 | 11/2019 | NCT01770353 | P | efficacy of liposomal irinotecan in metastatic breast cancer patients | |
| Nuclear Imaging | Nanomedicine | 89Zr-CPC634 | multiple solid tumors | Phase 1 | 10/2020 | NCT03712423 | P108 | localization of 89Zr-CPC634 in malignant tissue |
| 64Cu-MM-302 | Breast Cancer Brain Tumours |
Phase 1 | 01/2017 n/a |
NCT01304797
NCT02735798 |
P107 N |
localization of 64Cu-MM-302 in malignant tissue product withdrawn (trial never started) |
||
| 188Re-liposome | solid tumors | Phase 1 | 10/2014 | NCT02271516 | N | study terminated for safety concerns | ||
| Computed Tomography | radiation therapy | NBTXR3 | sarcoma | Phase 1 | 10/2020 | NCT01433068 | N | - |
| Phase 2/3 | 04/2021 | NCT02379845 | P | demonstration of NBTXR3 as theramostic nanoparticles | ||||
|
Optical
Imaging |
surgery | carbon NP | sentinel node | NA | 03/2016 | NCT02724176 | P | demonstration of carbon nanoparticles for patient stratification |
| ONM-100 | Cancer (breast, lungs, prostate, ovarian, lungs…) | Phase 2 | 11/2022 | NCT03735680 | N | - | ||
| Lung cancer | Phase 2 | 03/2023 | NCT05048082 | N | - |
NA means that the trial didn’t have FDA-defined phases. Y indicates results posted at clinicaltrials.gov, N results not posted, and P results published in a scientific manuscript. SPIO: super-paramagnetic iron oxide nanoparticles; USPIO: ultra-small SPIO; Carbon NP: carbon nanoparticle; C-dots: carbon dots; NanoTherm: iron oxide nanoparticle, AGuIX: polymeric gadolinium complexes, AuroShell: silica gold nanoshell, NBTXR3: hafnium oxide nanoparticles; ONM-100: fluorescent imaging nanoparticles; 89Zr-CPC634: 89Zr-labeled polymeric nanoparticles carrying docetaxel; 64Cu-MM-302: 64Cu- labeled liposomes carrying doxorubicin; 188Re-liposome: 188Re-labeled liposomes.
Nanoparticles with ‘innate’ imaging capabilities for thermal therapies
As mentioned, several metallic nanoparticles are well suited for theranostic applications as they have the innate ability to serve as imaging contrast enhancers and locally deploy heat upon stimulation by external energy sources. Already in the early 2000s, gold nanoparticles with a core diameter of a few nanometers were proposed as computed tomography (CT) contrast nanoparticles due to their high X-ray attenuation profile55, 56. Gold nanoparticles are also known to generate heat upon stimulation with an external light source via surface plasmon resonance.57 An example is gold-silica nanoshells, which are currently being tested in humans for the photothermal ablation of prostate and head and neck cancer cells (Auroshell – Table 2 and 3)58. The nanoshells have a silica core of ~ 150 nm coated by a ~ 10 nm gold shell designed to optimally resonate with a near-infrared light stimulation (~ 800 nm) and produce sufficient heat already at 10 μg/ml local concentration of gold59, 60. However, nanoparticles for CT imaging typically require concentrations higher than 100 μg/ml to generate sufficient contrast in human applications, and they are ~ 10 nm spheres of solid gold.61 It is then not surprising that, in the clinic, the gold nanoshells are detectable via MR and ultrasound imaging but not CT. The mismatch between the optimal conditions for heat generation and imaging, as well as the cost of the native material, are limiting the clinical development of gold-based true nanotheranostics. However, newer CT scanners able to gather spectral information might be able to change this situation, as the amount of gold required for imaging would be lower and compatible with that of thin nanoshells62, 63.
Iron oxide nanoparticles (IONPs) have been developed preclinically in a myriad of different configurations (Table 1 and Supplementary Information)64, and they have been approved for the treatment of anemia (20 nm ferumoxytol nanoparticles65, 66) and for magnetic hyperthermia of brain tumors (NanoTherm® particles67, Table 2 and 3). In the treatment of high-grade gliomas, NanoTherm® are injected directly in the tumor to overcome the blood-brain barrier and then heated up via external alternating magnetic fields at a frequency of 100 kHz with a field strength of 2.5 – 18 kA/m68. Not only does magnetic hyperthermia with IONPs require dedicated equipment, but also the local high density of iron induces susceptibility artifacts in MR imaging, hence, disease progression is typically monitored using CT scans. As for gold nanoparticles, a truly nanotheranostic use of IONPs is limited by the mismatch between the high specific absorption rate needed for efficient heat generation and the requirements for optimal imaging. Extensive efforts are being dedicated to developing IONPs that overcome this mismatch.13, 14
IONPs have also been tested clinically to target cancer metastases in the lymph nodes and track immune and stem cells in a variety of conditions, including cardiovascular and neurological disorders, in cancer immunotherapy and in regenerative medicine (Table 2 and 3)65, 69–71. However, these studies have mostly focused on developing new methods for stem cell deposition and elucidating the dynamics of immune cells leveraging the good safety profile of IONPs in humans rather than designing novel theranostic approaches.
Nanoparticles with ‘innate’ imaging capabilities for radiosensitization
Gadolinium-based contrast agents are extensively used in MR imaging.72 They are typically preferred to IONPs for their positive contrast, but they are also associated with the risk of transmetallation, which can lead to severe side effects in patients with poor renal clearance73. More recently, complexes of Gd are being actively investigated in the clinic as possible nanotheranostic agents. This is the case of the sub-5 nm AGuIX® particles74–76, comprising a polysiloxane matrix with multiple 1,4,7,10-tetraazacyclododecane,1-glutaric acid-4,7,10-triacetic acid (DOTAGA) ligands carrying gadolinium atoms. The resulting nanocomplexes function both as MR contrast agents (longitudinal relaxivity of ~ 10 mM−1s−1 at 1.5T) and local enhancers for radiation therapy (~ 2-fold increase in DNA damage following irradiation). Notably, in clinical studies, AGuIX nanoparticles have been shown to detect cancer metastasis in the brain under MR imaging and modulate their progression upon whole brain irradiation. In particular, it has been documented that the rate of growth of brain metastasis diminishes with increasing AGuIX concentration within the lesions (Figure 3A)77. Indeed, the larger is the accumulation of gadolinium in the metastasis, the larger is the local enhancement in MRI signal and irradiation-induced damage. This demonstrates the truly theranostic nature of AGuIX, which could not be achieved with molecular-based Gd contrast agents due to insufficient metal concentration and retention at the target.
Figure 3 |. Clinical demonstrations of nanotheranostics.
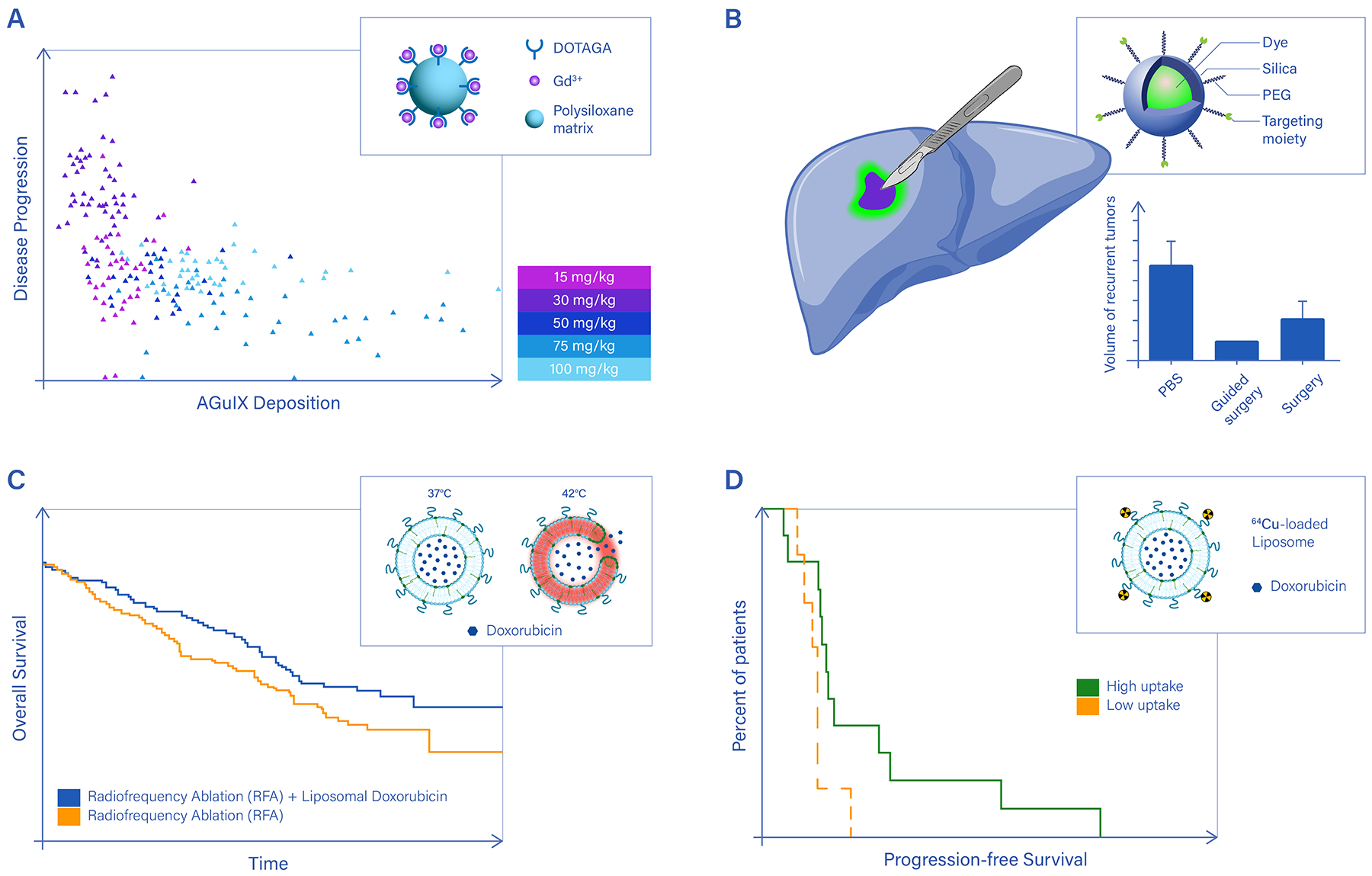
A. Enhancing the efficacy of external beam radiation therapy using Gd-based nanoparticles (AGuIX) under magnetic resonance imaging (MRI). Clinical data on cancer metastases to the brain show a positive correlation between AGuIX deposition within the lesions, as detected via MRI, and disease regression. Upon external beam radiation therapy, brain metastases receiving higher amounts of AGuIX grow less or even reduce in diameter (relative lesion size < 1) over a 28-day observation period. DOTAGA: 1,4,7,10-tetraazacyclododecane,1-glutaric acid-4,7,10-triacetic acid ligands. The legend shows the dose of AGuIX injected in the patients. Each symbol in the plot corresponds to a different injected dose and patient.77 B. Multimodal imaging nanoparticles carrying targeting moieities to specifically recognize cancer cells help visualize tumor margins, inspect lymph nodes, and accurately guide surgical resection of the bulk malignancy. However, to fully unveil the advantage of this approach, future clinical studies will have to address patient survival and disease recurrence, as was done for indocyanine. The Kaplan-Meier plot compares the overall survival for patients with rectal cancer following a laparoscopic lateral pelvic lymph node dissection performed with or without indocyanine green fluorescence imaging89. Image-guided surgery with indocyanine positively correlates with improved survival and reduced risk of local disease relapse. C. Boosting the spatio-temporal specificity of conventional therapies using thermoresponsive nanomedicines. The Kaplan-Meier plot shows a significant improvement in overall survival for hepatocellular carcinoma patients treated with radiofrequency ablation plus thermosensitive doxorubicin liposomes93. The chemotherapeutic agent is released preferentially at the tumor site, following the localized temperature increase and consequent destabilization of the liposomes. Drug release from thermosensitive nanomedicines has been achieved in clinical settings using radiofrequency ablation and high-intensity focused ultrasound; D. 89Zr-labeled, docetaxel loaded polymeric nanoparticles have been used to treat patients with various cancers (primary and metastatic) and image particle deposition in each single lesion for 7 patients. Although at the overall patient level no statistically significant correlation was found between nanoparticle accumulation in the cancer tissue and response to therapy, individual lesions with higher nanoparticle deposition (red lesion in Patient 1 and yellow lesion in Patient 6) underwent a reduction in size of up to 50% within a 96-hour observation period108.
Panel a adapted from Ref.77, panel b from Ref.89, panel c from Ref.93, panel d from Ref.108.
In addition to gadolinium, hafnium is another element with high atomic number and electron density that could be efficiently shaped into nanoparticles and used as a radiation enhancer. Hafnium oxide nanoparticles (NBTXR3) with an average diameter of 50 nm have been shown to locally deposit high energy upon exposure to external ionizing radiation78. In patients affected by advanced sarcoma, an intratumor injection under CT guidance of NBTXR3 before radiotherapy doubled the number of responding patients compared to radiotherapy alone.79 This preliminary success is fostering the launch of multiple clinical studies on other cancers too (Table 2 and 3).
These are two elegant examples of how the innate properties of a chemical element can be harnessed to result in clinically relevant theranostic properties, but only after the element is reshaped into nanoscale objects providing the necessary material density.
Multimodal imaging nanoparticles for guiding surgical therapy
In the operating room, nanoparticles labeling specifically the malignant tissue provide information on its local extension and on the status of the resection margin. Typically, in this application, nanoparticles do not carry therapeutic agents, but rather recognize the diseased cells based on specific receptors and generate a local signal for more accurate detection. Strictly speaking these particles would be considered nanodiagnostics, but the association with surgical intervention (therapy) justifies their listing as nanotheranostics.
Optical imaging is by far the simplest, most cost- and time-effective detection modality in an operating room setting. Nanotheranostics for guiding surgery include silica-based nanoagents (C-Dots, also known as Cornell dots), carbon-based nanoparticles (CNPs, also known as carbon dots) and fluorophore-lipid complexes (Table 2). C-Dots are ~ 10 nm silica spheres containing near-infrared (NIR) fluorophores, typically cyanine 5.5. The surface of C-Dots can be also modified to expose targeting moieties as well as radioisotopes (64Cu, 124I) to enhance specificity and sensitivity with PET imaging. Clinically relevant examples are the cRGDY-PEG-Cy5.5 – C-Dot targeting αvβ3 integrins for the optical detection of lymph nodes80 and the multimodal 64Cu-NOTA-PSMA-PEG-Cy5.5, which are C-Dots targeted against the prostate-specific membrane antigen. C-Dots are systemically injected, reach and accumulate within the diseased tissue, and, upon intraoperative optical or PET imaging, help visualize tumor margins and inspect lymph nodes to assess the stage of the disease and guide surgery (Figure 3B). A similar clinical application is proposed for CNPs, which are ~ 10 nm carbon spheres designed to fluoresce upon UV exposure. As for C-Dots, the CNP surface can be readily derivatized to expose multiple chemical groups and biologically relevant molecules81, 82. CNPs have been tested on a huge variety of tumors, including colon rectal, gastric, rectal, breast, cervical and thyroid cancer (Table 2)83, 84. Another potentially relevant agent for guiding the resection of malignant masses is LipImage 815. This is a ~ 50 nm complex resulting from mixing various lipids with NIR IR780 iodide molecules85. After multiple tests in rodents, LipImage 815 was validated in larger animals to assess toxicity and identify the effective dose86, 87. However, these preliminary feasibility studies have not yet been followed by human trials.
The advantage of nanoparticles over single molecules in guiding surgical intervention mostly resides in the straightforward surface modification that supports the attachment of multiple targeting moieties and different imaging agents to increase specificity and signal-to-noise ratio. However, clinical studies have yet to demonstrate the full advantage of using multimodal, targeted imaging nanoparticles for image-guided surgery in terms of disease-free survival, overall survival, post-operative complications and rate of recurrence, as it has been already shown for indocyanine green and other fluorescent molecules88, 89 (Figure 3B). Furthermore, in the future, newer particles capable to fluoresce in the short-wave infrared region will play an increasingly important role, as tissue absorption, scattering and autofluorescence in this portion of the spectrum (900 – 1700 nm) are negligible compared to visible wavelengths90. Preclinical short-wave infrared imaging with specialized fluorochromes enables high-resolution in-vivo imaging at depths not possible with conventional fluorochromes91.
MRI-guided thermoresponsive liposomes
Because a variety of lipid-based nanoparticles have been already translated into the clinic as nanomedicines, thermosensitive liposomes are promising for nanotheranostics. For example, ThermoDox® is a liposomal formulation of doxorubicin whose lipid bilayer, resulting from mixing in a specific molar ratio three phospholipids, becomes unstable at temperatures higher than ~40°C92. Within the circulatory system, the thermoresponsive liposomes firmly encapsulate doxorubicin, limiting undesired drug leakage and off-site effects but, at the diseased site, the bilayer can be destabilized by localized heating, which can be obtained via a high-intensity focused ultrasound source or via radiofrequency ablation, triggering the release of the therapeutic cargo. Clinical data have shown that the overall survival of patients with hepatocellular carcinomas undergoing conventional radiofrequency ablation can be improved by 40% or even 100% upon exposing thermoresponsive doxorubicin liposomes to 2 or 3 min of ablation per unit tumor volume, respectively93 (Figure 3C). This theranostic approach could only be achieved by integrating a drug into a nanostructure designed to respond to external energy sources.
Companion nanodiagnostics for patient stratification
To lodge within the malignant tissue, most nanomedicines rely on the enhanced permeability and retention effect, a phenomenon that occurs in tumors with leaky vasculature that allows nanoparticles in the bloodstream to selectively accumulate within the malignant tissue. Indeed, due to its rapid and chaotic growth, the tumor vasculature is not continuous but has openings, named fenestrations, with sizes of hundreds of nanometers94. Therefore, most nanomedicines with a diameter smaller than ~ 200 nm could passively accumulate within the malignant tissue by crossing these fenestrations. However, the clinical relevance of the enhanced permeability and retention effect is still debated94. Agents with a high safety profile, like IONPs, could be administered before nanotherapy to probe the vascular permeability of tumors and inform oncologists on the most adequate intervention. This approach has been recently tested in the clinic by characterizing the accumulation of systemically administered ferumoxytol in primary solid tumors95 and metastatic lesions96 via MR imaging. These studies found a positive correlation between the MR contrast enhancement induced by IONPs and the therapeutic response to irinotecan-loaded liposomes (Onivyde®2, Table 3). Despite a major difference in size between the ~20 nm ferumoxytol and the ~100 nm Onyvide®, these studies provide clinical evidence of the usefulness of IONPs as a nanoscale companion diagnostics to select patients who are likely to respond to nanotherapy95,96.
A similar, but potentially more quantitative approach, consists in labeling nanomedicines with a sufficiently long-lived radionuclide. The clinical imaging of radiolabeled nanomedicines was likely first performed in 1976, when Anthony Segal and colleagues labelled liposomes containing bleomycin with 111In and detected them using scintigraphy in patients with liver cancer97. Since then, planar gamma scintigraphy has been used to quantitatively assess in patients the biodistribution and tissue deposition of various liposomal nanomedicines98–103, Ca-Na2EDTA nanoparticles104 and a copolymer-doxorubicin conjugate105. Following this, 3D SPECT imaging was used to detect 99mTc-radiolabeled DOXIL/Caelyx in patients with non–small-cell lung cancer and head and neck cancer to probe the particles’ biodistribution106. More recently, the first clinical PET imaging study of nanomedicines was performed in patients with primary and metastatic breast cancer, who received HER2-targeted liposomal doxorubicin (MM-302) radiolabeled with 64Cu107. PET and CT imaging showed both inter- and intra-patient heterogeneity in uptake of the liposomes in primary tumors and metastases. Despite this, a correlation was observed between the intratumor deposition of 64Cu and doxorubicin, and patients’ disease progression-free survival107. In another important clinical example, a docetaxel-entrapping polymeric nanoparticle (CPC634) was labeled with 89Zr by modifying the carrier’s surface and was imaged in cancer patients108. Again, high inter- and intra-patient heterogeneity in tumor uptake was documented. In the same study, no correlation was found between the tumor deposition of 89Zr-CPC634 nanoparticles and the response to chemotherapy on 7 late-stage, heavily pretreated patients with various forms of cancer. Importantly, however, individual lesions with the highest nanoparticle uptake showed up to 50% reduction in size at 96 hours post treatment (Figure 3D).
The nuclear imaging of nanomedicines is historically the first example of clinical nanotheranostics. All these studies also highlight the advantages of using radiolabeled nanomedicines as a surrogate to test the actual, unlabeled nanomedicine. First, the radiolabeled nanomedicine is comparable in size and surface physico-chemical properties to the native therapeutic nanoparticle, implying that the former should more accurately match the biodistribution and pharmacokinetics of the latter. Second, the tissue-specific deposition of the radiolabeled nanomedicines can be accurately quantified, providing important information on dosing and drug accumulation during disease evolution. Third, accurate quantification enables the objective and precise characterization of inter- and intra-patient heterogeneity in nanomedicine deposition and nanotherapy response, building a database of fundamental information that could help develop more effective nanotheranostics for the future. However, radiolabeling requires extra manufacturing steps and characterization efforts that unavoidably result in higher costs.
Nanoradiotheranostics
Alongside the nuclear imaging of nanomedicines, the use of nanoradiotheranostics in the clinic was also reported. A key exploratory study in patients with metastatic cancer documented the administration of PEGylated liposomes radiolabeled with the β− -emitter 188Re109. A partial therapeutic effect was observed in some metastatic lesions, which also showed uptake of the radiolabeled liposomes by SPECT and CT imaging. However, dosimetry studies showed that the liver and spleen of patients received the highest dose, which caused the termination of the clinical work. Nonetheless, these preliminary studies, together with the fact that regulatory approval for clinical use has been obtained for a number of molecular radiotheranostics110, highlight the potential of nanocarriers in radiotherapy for oncological applications.
Challenges and opportunities in Clinical Translation
Two decades from the launch of the US Cancer Nanotechnology initiative, almost 40 nanoparticle formulations have been clinically approved, and several more are advancing towards clinical translation111–113. Most are used as therapeutic nanomedicines for cancer, iron deficiency and, more recently, rare and infectious diseases. The latter include Onpattro®, the first approved siRNA-encapsulating liposome for the treatment of the rare condition known as hereditary transthyretin-mediated amyloidosis114, and Comirnaty® and Spikevax®, the nanoparticle-based mRNA-vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)115. Whereas some nanoparticle-based medicines have carved a market space worth multiple billions of US$, the same has not yet happened for the many nanotheranostics available on the laboratory benches.
The composition, architecture, imaging modality and therapeutic strategy affect the clinical translatability of nanotheranostics and together contribute to shaping the depth and width of the so called ‘valley of the death’ between laboratory benches and clinical beds (Figure 4). However, it is important to recall that the rate of translation into the clinic continues to be moderate (< 10%) even for more conventional therapeutic agents, such as small molecules, that have been developed by the pharmaceutical industry and reviewed by international regulatory bodies for decades116, 117. The imbalance between the number of preclinical studies and the number of clinical products certainly remains a contentious point, but it should not be taken, just yet, as a measure of the impact of nanomedicines or nanotheranostics. A few companies developing nano-health products have failed to improve outcomes over the current standard treatments, with BIND Therapeutics being the most notable example114, but most nanotheranostics are simply getting lost along the winding road of clinical translation because of the lack of information, technical resources and funding and owing to improper design of clinical trials rather than for their potential or actual performance. In this section, technical, economical and regulatory challenges are critically discussed.
Figure 4. Milestones in the clinical translation of nanotheranostics.
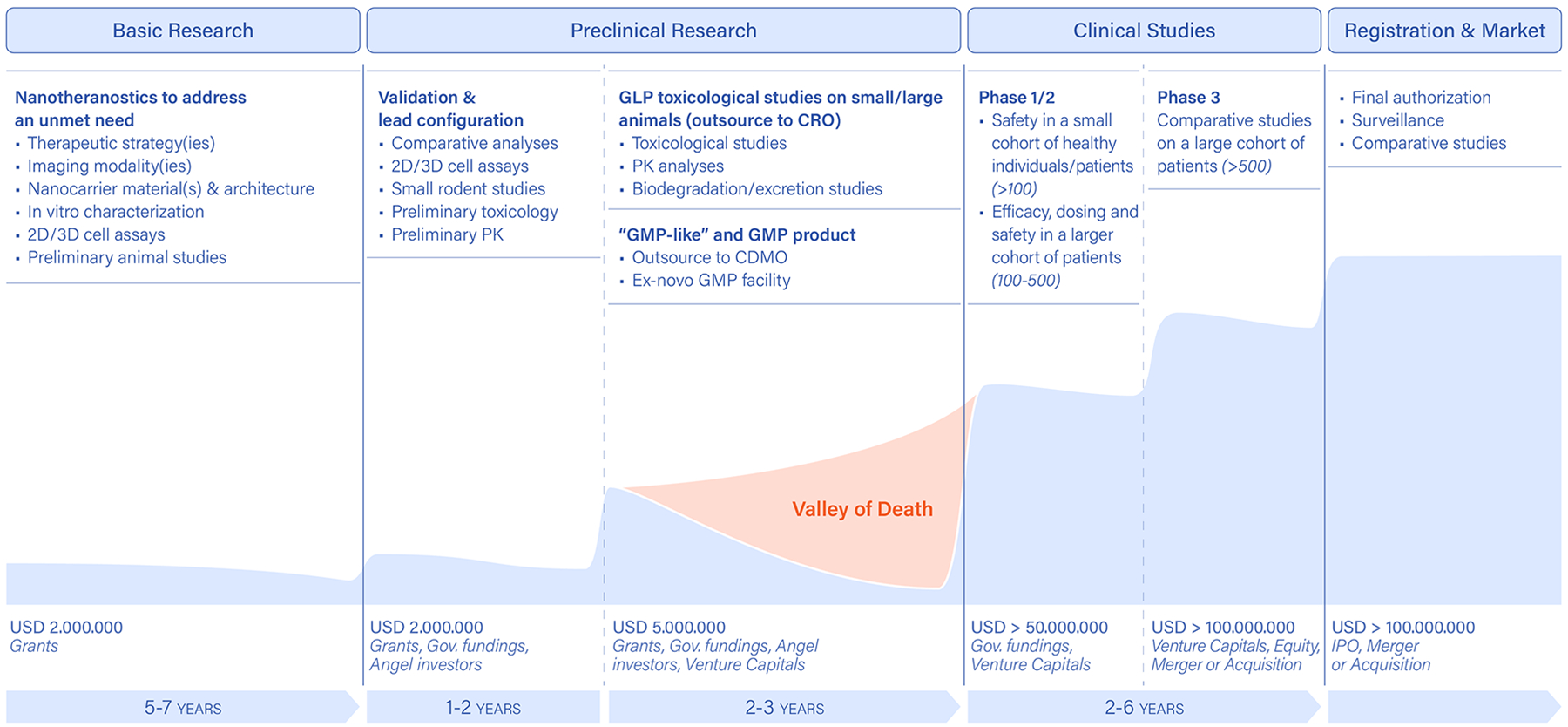
Early design, development and characterization of novel nanotheranostics entirely relies on research grants, which help demonstrating a new idea and technology all the wat up to small-rodent models. This basic research phase is almost exclusively funded by governmental research grants. More systematic analyses and in-vitro/in-vivo characterizations of the new technology are conducted in the preclinical research phase. This aims at identifying a lead product, with a specific imaging/therapeutic agent combination as well as material and architecture configurations. This phase is typically funded by industrial-like governmental grants, such as small business innovation research (SBIR) and small business technology transfer (STTR) programs in the US; European Innovation Council (EIC) Transition and Accelerator programmes in the EU, and angel investors and foundations. A second portion of the preclinical research phase deals with the manufacturing and toxicological testing of the proposed product, following well-coded procedures (good manufacturing practice, GMP, and good laboratory practice, GLP) in agreement with the relevant regulatory bodies (FDA, EMA and others). This phase can be funded by governmental agencies, angel donors and foundations, as well as venture capital firms investing in early-stage products. Finally, the clinical studies aim to assess the safety, efficacy and economic convenience of the proposed product over different phases, depending on the type of product and disease. These activities typically require significant capitals involving multiple venture capital firms as well as pharmaceutical companies. PK: pharmacokinetics; CRO: contract research organization; CDMO: contract development and manufacturing organization).
The fate of systemically administered nanotheranostics
The goal of systemically injected nanoparticles for therapy and imaging is to reach the diseased tissue, deploy the therapeutic cargo, generate a signal for imaging and eventually clear out of the body without inducing any undesired damage. When it comes to nanoparticle accumulation within diseased tissue, nano skeptics often refer to a meta-analysis by Warren Chan and colleagues supporting the notion that tumor uptake of systemically injected nanoparticles is modest and, on average, equal to 0.7% of the injected dose (%ID)118. Although tumor accumulation is certainly important, a proper assessment of nanoparticle performance should also include traditional pharmacokinetic and toxicological parameters119. Indeed, a more recent meta-analysis by William Zamboni and colleagues compared the tumor (AUCtumor) and systemic (AUCblood) exposure (concentration and duration) of nanomedicines using the same dataset of Chan and coworkers. They found that the AUCtumor/AUCblood ratio was ~100 times higher than the tumor ID%120. Incidentally, with the advent of more sophisticated data mining tools and artificial intelligence algorithms, similar meta-analyses could be efficiently conducted on a larger cohort of studies stratifying the results in terms of nanoparticle attributes such as size, shape, surface properties, deformability and composition, as well as disease type and stage, and animal species. This represents an immense opportunity to objectively assess the performance of nanohealth products as well as identify subsets of conditions for which nanomedicines could be more effective than molecular or cellular therapies121–123.
Those nanoparticles that do not accumulate in the diseased tissue can be either directly excreted through the glomerular pores in the kidneys or deposited over time into organs of the reticulo-endothelial system, mainly the liver and spleen. The glomerular pore size in healthy kidneys is ~10 nm, as determined via direct morphometric analysis of electron microscopy images124, which correlates nicely with a cut-off size for kidneys’ filtration of 6 to 8 nanometers, documented for a variety of nanoparticles125, 126. By contrast, in the liver most nanocarriers would degrade, if biodegradable, and be eventually excreted through the hepatobiliary circulation and the gut, or accumulate, if not biodegradable. In this context, it should be highlighted that the mechanisms of excretion and the biodegradation products for nanoparticles are of high concern to regulatory bodies and could impact the preclinical and clinical development of nanotheranostics. Focusing on rapid elimination rather than biodegradation could be a safer and less expensive approach, as the dwelling time of nanocarriers in the body and amount of byproducts would be minimized. This is an important consideration for the 5 nm AGuIX nanocomplexes, for which longer residence times in the body could lead to transmetallation. Different nanoparticles, such as IONPs and liposomes, whose hydrodynamic diameter is significantly larger than 10 nm, are designed to circulate for longer, accumulate within the diseased tissue, and eventually degrade. Interestingly, looking at clinically relevant nanotheranostics, rapid excretion does not seem to be a necessary condition to access and succeed in clinical studies (Table 4). Rather, clinically relevant nanotheranostics are mostly made of well-known and extensively characterized materials (iron oxide, gold, lipids, silica) and are realized following simple manufacturing steps involving mixing, chemical conjugation and filtration. Therefore, investigators should keep in mind that efforts to translate nanotheranostics with augmented complexity and made with ‘exotic’ materials can only be justified by a significant improvement over the current clinical standards.
Table 4 |.
Main attributes of clinically relevant nanotheranostics.
| Nanotheranostic | size (nm) |
composition | blood longeivty | route of Administration | First Trial | Major Application |
|---|---|---|---|---|---|---|
| ferumoxides 64 | 50 – 100 | dextran-coated IONP | < 1 h | iv/local | 09/1999 | liver imaging |
| ferumoxytol 66 | 17 – 31 | polyglucose sorbitol carboxymethylether - coated IONP | ~ 15 h | iv/local | 05/2004 | anemia treatment |
| AuroShell 58 | 150 | Au nanoshell on a silica core | 3 – 6 h | iv | 04/2008 | cancer ablation |
| 64Cu-MM-302 107 | ~ 100 | 64Cu-labeled HER2-targeted PEGylated liposomal doxorubicin | ~ 33 h | iv | 03/2011 | cancer chemotherapy |
| Carbon NP 83 | ~ 10 | carbon pellet (after surface modification ~ 150) | - | local | 01/2012 | lymph node dissection |
| NBTXR3 78 | 50 | functionalized hafnium oxide nanoparticles | - | local | 01/2014 | head & neck cancer |
| Cornell Dots 80 | ~ 10 | radiolabeled molecular targeted PEGylated silica matrix | - | iv/local | 04/2014 | lymph node dissection |
| 188Re-Liposome 109 | ~ 80 | 188Re-labeled PEGylated liposome | ~ 40 h | iv | 10/2014 | terminated |
| AGuIX 77 | ~ 5 | Gd chelated-polysiloxane | > 6h | iv | 03/2016 | cancer radiotherapy |
| 89Zr-CPC634 108 | 65 | radiolabeled docetaxel-loaded nanoparticles | ~ 60 h | iv | 04/2018 | cancer chemotherapy |
IONP: iron oxide nanoparticle; AGuIX: polymeric gadolinium complexes; AuroShell: silica gold nanoshells; NBTXR3: hafnium oxide nanoparticles; Carbon NP: carbon nanoparticle; 89Zr-CPC634: 89Zr-labeled polymeric nanoparticles carrying docetaxel; 64Cu-MM-302: 64Cu-labeled liposomes carrying doxorubicin; 188Re-liposome: 188Re-labeled liposome; iv: intravenous administration.
Certified preclinical development
In the preclinical development of any health product, the definition of good manufacturing practices (GMPs) and the collection of preclinical toxicological data are crucial steps, and represent a large portion of the ‘valley of death’. GMPs are fundamental to comply with the requirements of regulatory bodies and ensure that nanotheranostics are fabricated with the same properties over multiple production batches (reproducibility), while toxicological studies must be conducted under good laboratory practices and be fully certified by regulating bodies. These two steps cannot be undertaken by research laboratories within academic settings, as they require specific expertise, certifications and, most importantly, financial support well beyond the figures commonly granted by governmental agencies for industrial research. Just to put things in perspective, the launch of Onpattro took 15 years of preclinical and clinical development and $2.5B total investments.127, 128 Currently, the medication is sold at ~$400k per patient per year with global net product revenues of $150 millions, as estimated using the Electronic Data Gathering, Analysis, and Retrieval (EDGAR) system.
Defining proper manufacturing protocols for nanotheranostics generally costs hundreds of thousand US$, typically requiring a first phase to realize the so called ‘GMP-like’ product followed by a second phase to obtain the actual GMP product for in-human use. The precise cost and duration of the two phases depend on technical, geographical and economic factors. The complexity of the technology, the number and type of fabrication steps and the too often neglected sterilization process all affect manufacturing costs. For instance, if the resulting product cannot be sterilized following standard procedures because these would impact the material properties and the final performance of the product, then the entire fabrication process should be conducted under sterile conditions, inevitably boosting the cost. Also, there is a lot of variation in costs and expertise among countries as well as within different areas in the same country. Not to mention that there are countries that lack any capacity in GMP manufacturing of nano-products. Finally, the cost of raw materials and the currently ongoing supply chain issues also affect development costs. Whereas small molecules and monoclonal antibodies have reached a level of development that enables most contract research organizations and contract development and manufacturing organizations to deal with GMP manufacturing and toxicological studies, this is not yet the case for nanotheranostics. If a contract development and manufacturing organization with specific expertise does not exist or cannot be involved without compromising the protection of intellectual rights, then a dedicated GMP facility may be needed, resulting in much longer development times, larger investments, much higher risks, and an inevitable widening of the ‘valley of death’. A large portion of nanotheranostics described in the literature falls under this less fortunate scenario. Therefore, in several cases, the lack of specific expertise for manufacturing becomes an insurmountable obstacle that explains the imbalance between the number of nanotheranostic agents described in the scientific literature and the number of clinically approved products. Consequently, it is not surprising that most nanotheranostics currently used in the clinic are based on consolidated technologies – iron oxide and gold nanoparticles, silica nanoparticles and liposomes – or molecular complexes resulting from the mixture of relatively inexpensive and well-characterized materials (Table 4). Fortunately, also thanks to the efforts dedicated to developing the lipid nanoparticles used for the SARS-CoV-2 vaccination campaign, a larger portion of contract development and manufacturing organizations are becoming familiar with a variety of nano- and micro-manufacturing and characterization techniques, which should facilitate the access of nanotheranostics to the clinic.
Once at least a GMP-like product is available, a contract research organization can conduct toxicological analyses, typically on rodent and non-rodent species, taking up to a few million US$. Even in this case, it is difficult to give precise figures, as costs depend on multiple factors. It is however important to remark that the cost of GLP toxicological studies tends to increase quasi-linearly with the number of therapeutic and imaging components associated with the nanotheranostic. For instance, the addition of an imaging component via different appendices (metallic or non-metallic ligands) and in different locations (surface modification or core encapsulation) to a nanomedicine might affect the overall biodistribution of the carrier and therefore the actual imaging accuracy, safety and therapeutic efficacy108, 129, 130. For example, liposomes containing 111In within the lipid bilayer have significantly higher liver trapping than those encapsulating the same molecule in the aqueous core129.
Another important point is that changes often occur in dosage, materials and manufacturing steps during the preclinical development phase. These changes can dramatically impact the performance of the agent and therefore require new toxicological studies, as well as the possible addition of new steps in the GMP workflow. Furthermore, especially in the case of highly innovative products, new ad-hoc analytical techniques may have to be developed, which can be far from current standards and challenging to validate131. Within this context, new opportunities are provided by the convergence of biology with physical sciences, mathematical modelling and organ-on-chip platforms, which could be used to efficiently and accurately fill the gap between clinical validation and animal testing. The development and validation of computational approaches, such as the Quantitative Nanostructure-Toxicity Relationship;132 advances in in-vitro techniques, such as microfluidics and organs-on-chip133; and new approaches to data processing with machine learning and artificial intelligence tools121 134, 135 may facilitate the accurate reproduction of in-vivo conditions.
As costs grow with complexity, nanotheranostics are expected to be more expensive than their therapeutic counterparts136. Selecting innate nanotheranostics could possibly reduce the complexity and contain costs, but it is difficult to generalize. This consideration suggests once again that including multiple imaging modalities, therapeutic molecules and targeting moieties without a clear medical need only widens and deepens the ‘valley of death’. At the same time, it is important to note that adding a nanotheranostic agent upfront could reduce cost further down the road by stratifying patients that will truly benefit from a nanomedicine rather than just applying it and then learning through follow-up imaging studies that the uptake of the agent was too low to achieve an effect. Indeed, this is something that a companion nanotheranostic could have revealed upfront.
Regulatory process for nanotheranostics
It is accepted that the existing criteria based on quality, safety, efficacy, performance and benefit/risk balance, and the two-framework structure of ‘medicinal product’ and ‘medical device’ are adequate for regulating medicines, devices and more complex health products, like nanotheranostics. In the US, the FDA Office of Combination Products selects the most competent experts for the evaluation of nano-based products. In the EU, the centralized procedure of EMA is used for evaluating and supervising nanomedicines, but several nano-based products, especially those intended for the treatment of iron deficiency, are approved at the national level137.
The regulatory classification of all the products intended for use on humans follows the fundamental principle of the ‘main’ mechanism of action (MOA). As nanotheranostics possess both therapeutic and diagnostic capabilities and embody different MOAs, they may fall in different regulatory classifications and, therefore, different nanotheranostics may follow different pathways for regulatory oversight and approval.138 According to current legislation, any product intended for prevention, in-vivo diagnosis or treatment and acting mainly via a metabolic, immunological or pharmacological mode is defined as a medicinal product, and is governed by the pharmaceuticals legislation. In addition, products containing more than one active agent are defined either as ‘fixed combination medicinal products’, if they involve two or more substances acting as medicinal products, or as ‘combined medicinal products’, if one of the components is a medical device integral to the product but the main MOA is exerted by the medicinal substance. By contrast, any product intended for medical purposes and acting mainly via a physical, mechanical or chemical mode is defined as a medical device and is governed by the medical devices regulation. A regulatory classification becomes more complex when the intended action of the product is physical but also pharmacological, which identifies so-called ‘borderline products’, and when new materials, or combinations of materials, are to be introduced into the human body. However, it is important to point out that the legislation foresees well-defined interactions between the medicinal product and medical device frameworks in these borderline cases, so that all the needed expertise is made available.
The decision on the proper classification of a product is crucial, as the regulatory path as well as duration and costs of product development and testing are different. In the US, the decision is taken by the FDA centralized offices, including the Office for Combination Products together with the Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research and Center for Devices and Radiological Health. In the EU, the responsibility resides within the authority of each Member State. However, the new EU regulation on medical devices has reinforced the harmonisation process among the EU Member States, establishing a central body where divergences might be resolved.
The regulations and guidelines cannot address all possible variables that could emerge in the development of innovative and complex health products. When performing studies with nanotechnology-based products, it is recognized that they are closer in complexity to biological structures (such as proteins and viral particles) than to small synthetic molecules, because beyond the identified pharmacological, immunological and metabolic interactions there are multiple additional layers of physical and spatial dynamics between the product and the host. The inherent physico-chemical properties – such as size, surface charge, shape and imaging detectability – and biological activity of nanomaterials, as well as their interaction with plasma or serum proteins (the corona) and blood cells, the stable incorporation of imaging agents and the release profile of the drug molecules should all be ascertained during preclinical studies.
In spite of the regulatory complexity and challenges posed by the convergence of different regulatory frameworks, it is important to recall that investigators have the opportunity to establish an open discussion with regulators early in the product development phase. This interaction can happen with the FDA Office of Combined Products, where the classification of the product takes place and the coordination of the relevant competences for the regulatory review can be identified in a timely manner in the different FDA Centers. In the EU, although the decision on the classification as medicinal product or medical device is officially given at a national level, there is a centralised regulatory framework coordinated by EMA that can provide scientific orientation at a very early stage – the EMA Innovation Task Force. This early interaction with regulatory scientists and officers could mitigate risks and costs and facilitate dialogue during a formal investigational new drug application, reducing the depth and width of the ‘valley of death’.
Harmonisation of expertise and knowledge
Nanotheranostics lie at the intersection of multiple disciplines. Far more than in medicinal chemistry and pharmacology, research laboratories focusing on the design, engineering and validation of nanotheranostics must build and accumulate a critical mass of knowledge and expertise to be effective. Whereas medicinal chemists and pharmaceutical scientists can enjoy spending long hours perfecting the potency and water solubility of their molecules before handling them to a contract research organization for GMP manufacturing and GLP preclinical toxicological analyses and efficacy studies, nanotheranostic laboratories typically have to address internally all the steps in the development process from the synthesis to the in-vitro physico-chemical and biological characterizations and the preclinical validation in small rodents. Therefore, the development of nanotheranostics requires a research team integrating expertise in physics, chemistry, materials science, engineering, biotechnology, biology, pharmacology, medicine and biomedical imaging.
Once the academic laboratory has produced a prototype nanotheranostic, its clinical translation requires a gamut of expertise typically not found within the wet benches of universities and research institutions, revolving around standardized manufacturing practices, accurate analytical and toxicological analyses, regulatory guidelines, clinical trial design, financing and healthcare reimbursement. The number of contract development and manufacturing organizations and contract research organizations with a documented track record in handling the manufacturing and toxicological characterization of nanotheranostics is very limited. The communication among academic laboratories and external contract organizations has to be developed for the specific product at hand. It also commands for reciprocal education and willingness to learn, while protecting intellectual property and know-how.
As mentioned earlier, another crucial connection is that between investigators and regulators. In particular, an early investigator-regulator dialogue helps exchange knowledge on novel developments in nanotechnology, materials science, drug delivery and related fields. This is fundamental to provide all the tools required to critically evaluate a nano-health product and more efficiently advance it towards the clinic. This early dialogue also serves to anticipate and address specific bottlenecks in the preclinical and clinical development of complex products, such as nanotheranostics. This approach offers the opportunity to shift the paradigm in drug and device development, seeing the regulator as an ally rather than an additional hurdle along the ‘valley of death’.
Finally, scientists should learn to resist the temptation to integrate multiple functions – multiple molecules and therapeutic modes, multiple imaging agents for different modalities, multiple targeting moieties – into nanocarriers simply ‘because they can’. This approach can perhaps facilitate the publication of manuscripts, even in high-impact journals. However, its utility to patients and to the scientific community is highly questionable. If the complexity of nanotheranostics is not justified by a clear unmet medical need or a dramatic (meaning orders of magnitude) improvement over the current standards, it is unlikely that the technology would reach the clinic and therefore benefit patients. Also, it is well recognized that true innovation does not derive from adding together known concepts and functions, but rather from encouraging diverse thinking at the interface among multiple disciplines.
Future Perspectives
The abundance of preclinical nanotheranostics demonstrates the tremendous potential of combining imaging, therapy and targeting in one nanostructure. However, strategies are needed to support the clinical integration of these technologies and address the challenges that widen the ‘valley of death’. In terms of technical challenges, more efforts are needed to rationalize and optimize the performance of ‘innate’ theranostic materials, where a significant mismatch still exists between imaging and therapeutic performances, while leveraging lipid and polymeric nanoparticles that are currently being tested, or even already used, in the clinic. More systematic studies on the therapeutic and imaging performances as well as on pharmacokinetics and pharmacodynamics could facilitate the selection of the most appropriate materials and nanostructure configurations. This approach would require a change in research funding schemes, balancing the quest for absolute novelty with that for rational design and optimization, which is often considered as a mere incremental activity. New opportunities are provided by the implementation of funding schemes focusing on different technology readiness levels and following the development of a product from the laboratory bench to clinical testing. Although this is already happening in some countries, more resources should be allocated to unconventional technologies – like nanotheranostics – that are less likely than small molecules to attract the interest of large pharmaceutical companies at an early developmental stage.
Regarding manufacturing, a ‘low hanging’ but promising fruit is the conversion of existing nanomedicines into nanotheranostics. This strategy would not only build on consolidated knowledge and minimize fabrication costs, but it would also help conduct more efficiently, and possibly more successfully, clinical trials by properly stratifying patients. Moreover, the combination of nanotheranostics with artificial intelligence tools would help extract precious information on the disease biology as a function of the specific patient, stage and used technology. Importantly, the impact that the SARS-CoV-2 pandemic has had on the pharmaceutical manufacturing community, favoring the rapid conversion and even the launch of new contract research organizations and contract development and manufacturing organizations for the realization and testing of nanocarriers and unconventional therapeutics, cannot be neglected.
In terms of regulatory approval, most agencies have established protocols to facilitate early engagement, already during the preclinical development phase, with regulatory scientists, although there is no dedicated path for nanotheranostics. This represents a unique opportunity, as early interactions could help set requirements and expectations, instruct the scientists in the regulatory bodies on new materials and technologies, and, more importantly, help shape the proper path (medicinal product vs medical device) for the proposed nanotheranostics. As communication and cross-fertilization between fields are crucial, a new opportunity is provided by the unprecedented proliferation of biotechnological accelerators, launched by academic institutions as well as by private entities. These initiatives have the objective to connect scientists with experts in regulatory sciences, patent attorneys, manufacturers and venture capitalists and help scientists familiarize with communities that are very different from academia. These accelerators serve to build common knowledge and trust around new technologies and should become a new model for funding and overseeing the development of novel medical technologies.
In conclusion, scientific soundness and medical relevance are necessary conditions for the clinical translation of nanotheranostics, but this translation can only occur if academia establishes a symbiotic interaction with other stakeholders involved in the process.
Supplementary Material
Acknowledgements
This work was partially supported by the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 616695, European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement no. 754490 and 872648. PD acknowledges support from the Academic Biotechnology program SPARK Stanford. JG acknowledges support by NIH grants CA218615 and CA215700.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Wagner FE; Haslbeck S; Stievano L; Calogero S; Pankhurst QA; Martinek KP, Before striking gold in gold-ruby glass. Nature 2000, 407 (6805), 691–2. [DOI] [PubMed] [Google Scholar]
- 2.Nano on reflection. Nature Nanotechnology 2016, 11 (10), 828–834. [DOI] [PubMed] [Google Scholar]
- 3.Weber DO, Nanomedicine. Health Forum J 1999, 42 (4), 32, 36–7. [PubMed] [Google Scholar]
- 4.Mitchell MJ; Billingsley MM; Haley RM; Wechsler ME; Peppas NA; Langer R, Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021, 20 (2), 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stater EP; Sonay AY; Hart C; Grimm J, The ancillary effects of nanoparticles and their implications for nanomedicine. Nat Nanotechnol 2021, 16 (11), 1180–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H; Zhang W; Zhu G; Xie J; Chen X, Rethinking cancer nanotheranostics. Nat Rev Mater 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCready VR, Radioiodine - the success story of Nuclear Medicine : 75th Anniversary of the first use of Iodine-131 in humans. Eur J Nucl Med Mol Imaging 2017, 44 (2), 179–182. [DOI] [PubMed] [Google Scholar]
- 8.Bodei L; Herrmann K; Schöder H; Scott AM; Lewis JS, Radiotheranostics in oncology: current challenges and emerging opportunities. Nature Reviews Clinical Oncology 2022, 19 (8), 534–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaittanis C; Shaffer TM; Ogirala A; Santra S; Perez JM; Chiosis G; Li Y; Josephson L; Grimm J, Environment-responsive nanophores for therapy and treatment monitoring via molecular MRI quenching. Nat Commun 2014, 5, 3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Meel R; Sulheim E; Shi Y; Kiessling F; Mulder WJM; Lammers T, Smart cancer nanomedicine. Nat Nanotechnol 2019, 14 (11), 1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasgupta A; Biancacci I; Kiessling F; Lammers T, Imaging-assisted anticancer nanotherapy. Theranostics 2020, 10 (3), 956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho M; Cervadoro A; Ramirez MR; Stigliano C; Brazdeikis A; Colvin VL; Civera P; Key J; Decuzzi P, Assembly of Iron Oxide Nanocubes for Enhanced Cancer Hyperthermia and Magnetic Resonance Imaging. Nanomaterials (Basel) 2017, 7 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Y; Liu X; Liang Q; Liang X-J; Tian J, Optimization and Design of Magnetic Ferrite Nanoparticles with Uniform Tumor Distribution for Highly Sensitive MRI/MPI Performance and Improved Magnetic Hyperthermia Therapy. Nano Letters 2019, 19 (6), 3618–3626. [DOI] [PubMed] [Google Scholar]
- 14.Mai BT; Balakrishnan PB; Barthel MJ; Piccardi F; Niculaes D; Marinaro F; Fernandes S; Curcio A; Kakwere H; Autret G; Cingolani R; Gazeau F; Pellegrino T, Thermoresponsive Iron Oxide Nanocubes for an Effective Clinical Translation of Magnetic Hyperthermia and Heat-Mediated Chemotherapy. ACS Appl Mater Interfaces 2019, 11 (6), 5727–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z; Yang Y; Wei H; Shan X; Wang X; Ou M; Liu Q; Gao N; Chen H; Mei L; Zeng X, Charge-reversal biodegradable MSNs for tumor synergetic chemo/photothermal and visualized therapy. J Control Release 2021, 338, 719–730. [DOI] [PubMed] [Google Scholar]
- 16.Li X; Zhao W; Liu X; Chen K; Zhu S; Shi P; Chen Y; Shi J, Mesoporous manganese silicate coated silica nanoparticles as multi-stimuli-responsive T1-MRI contrast agents and drug delivery carriers. Acta Biomater 2016, 30, 378–387. [DOI] [PubMed] [Google Scholar]
- 17.Wu C; Cai R; Zhao T; Wu L; Zhang L; Jin J; Xu L; Li P; Li T; Zhang M, Hyaluronic acid-functionalized gadolinium oxide nanoparticles for magnetic resonance imaging-guided radiotherapy of tumors. Nanoscale research letters 2020, 15 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma M; Huang Y; Chen H; Jia X; Wang S; Wang Z; Shi J, Bi2S3-embedded mesoporous silica nanoparticles for efficient drug delivery and interstitial radiotherapy sensitization. Biomaterials 2015, 37, 447–455. [DOI] [PubMed] [Google Scholar]
- 19.Herrero Alvarez N; Bauer D; Hernandez-Gil J; Lewis JS, Recent Advances in Radiometals for Combined Imaging and Therapy in Cancer. ChemMedChem 2021, 16 (19), 2909–2941. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira CA; Goel S; Ehlerding EB; Rosenkrans ZT; Jiang D; Sun T; Aluicio-Sarduy E; Engle JW; Ni D; Cai W, Ultrasmall Porous Silica Nanoparticles with Enhanced Pharmacokinetics for Cancer Theranostics. Nano Lett 2021, 21 (11), 4692–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotagiri N; Sudlow GP; Akers WJ; Achilefu S, Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol 2015, 10 (4), 370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang R; Zheleznyak A; Mixdorf M; Ghai A; Prior J; Black KCL; Shokeen M; Reed N; Biswas P; Achilefu S, Osteotropic Radiolabeled Nanophotosensitizer for Imaging and Treating Multiple Myeloma. ACS Nano 2020, 14 (4), 4255–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamkaew A; Cheng L; Goel S; Valdovinos HF; Barnhart TE; Liu Z; Cai W, Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles. ACS Appl Mater Interfaces 2016, 8 (40), 26630–26637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni D; Ferreira CA; Barnhart TE; Quach V; Yu B; Jiang D; Wei W; Liu H; Engle JW; Hu P; Cai W, Magnetic Targeting of Nanotheranostics Enhances Cerenkov Radiation-Induced Photodynamic Therapy. J Am Chem Soc 2018, 140 (44), 14971–14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu B; Ni D; Rosenkrans ZT; Barnhart TE; Wei H; Ferreira CA; Lan X; Engle JW; He Q; Yu F; Cai W, A “Missile-Detonation” Strategy to Precisely Supply and Efficiently Amplify Cerenkov Radiation Energy for Cancer Theranostics. Adv Mater 2019, 31 (52), e1904894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xi L; Jiang H, Image‐guided surgery using multimodality strategy and molecular probes. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2016, 8 (1), 46–60. [DOI] [PubMed] [Google Scholar]
- 27.Shi H; Yan R; Wu L; Sun Y; Liu S; Zhou Z; He J; Ye D, Tumor-targeting CuS nanoparticles for multimodal imaging and guided photothermal therapy of lymph node metastasis. Acta Biomaterialia 2018, 72, 256–265. [DOI] [PubMed] [Google Scholar]
- 28.de Vries M; Jager PL; Suurmeijer AJH; Plukker JTM; van Ginkel RJ; Hoekstra HJ, [Sentinel lymph node biopsy for melanoma: prognostic value and disadvantages in 300 patients]. Ned Tijdschr Geneeskd 2005, 149 (33), 1845–1851. [PubMed] [Google Scholar]
- 29.Rousseau C; Classe JM; Campion L; Curtet C; Dravet F; Pioud R; Sagan C; Bridji B; Resche I, The impact of nonvisualization of sentinel nodes on lymphoscintigraphy in breast cancer. Ann Surg Oncol 2005, 12 (7), 533–8. [DOI] [PubMed] [Google Scholar]
- 30.Chen L-J; Yang C-X; Yan X-P, Liposome-Coated Persistent Luminescence Nanoparticles as Luminescence Trackable Drug Carrier for Chemotherapy. Analytical Chemistry 2017, 89 (13), 6936–6939. [DOI] [PubMed] [Google Scholar]
- 31.Wang J; Ma Q; Hu X-X; Liu H; Zheng W; Chen X; Yuan Q; Tan W, Autofluorescence-free targeted tumor imaging based on luminous nanoparticles with composition-dependent size and persistent luminescence. ACS nano 2017, 11 (8), 8010–8017. [DOI] [PubMed] [Google Scholar]
- 32.Maldiney T; Bessière A; Seguin J; Teston E; Sharma SK; Viana B; Bos AJ; Dorenbos P; Bessodes M; Gourier D, The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nature materials 2014, 13 (4), 418–426. [DOI] [PubMed] [Google Scholar]
- 33.Zheng B; Bai Y; Chen H; Pan H; Ji W; Gong X; Wu X; Wang H; Chang J, Near-infrared light-excited upconverting persistent nanophosphors in vivo for imaging-guided cell therapy. ACS applied materials & interfaces 2018, 10 (23), 19514–19522. [DOI] [PubMed] [Google Scholar]
- 34.Xue Z; Li X; Li Y; Jiang M; Ren G; Liu H; Zeng S; Hao J, A 980 nm laser-activated upconverted persistent probe for NIR-to-NIR rechargeable in vivo bioimaging. Nanoscale 2017, 9 (21), 7276–7283. [DOI] [PubMed] [Google Scholar]
- 35.Zheng S; Shi J; Fu X; Wang C; Sun X; Chen C; Zhuang Y; Zou X; Li Y; Zhang H, X-ray recharged long afterglow luminescent nanoparticles MgGeO 3: Mn 2+, Yb 3+, Li+ in the first and second biological windows for long-term bioimaging. Nanoscale 2020, 12 (26), 14037–14046. [DOI] [PubMed] [Google Scholar]
- 36.Liu N; Shi J; Wang Q; Guo J; Hou Z; Su X; Zhang H; Sun X, In Vivo Repeatedly Activated Persistent Luminescence Nanoparticles by Radiopharmaceuticals for Long‐Lasting Tumor Optical Imaging. Small 2020, 16 (26), 2001494. [DOI] [PubMed] [Google Scholar]
- 37.Thébault CJ; Ramniceanu G; Boumati S; Michel A; Seguin J; Larrat B; Mignet N; Ménager C; Doan B-T, Theranostic MRI liposomes for magnetic targeting and ultrasound triggered release of the antivascular CA4P. Journal of Controlled Release 2020, 322, 137–148. [DOI] [PubMed] [Google Scholar]
- 38.Centelles MN; Wright M; So P-W; Amrahli M; Xu XY; Stebbing J; Miller AD; Gedroyc W; Thanou M, Image-guided thermosensitive liposomes for focused ultrasound drug delivery: Using NIRF-labelled lipids and topotecan to visualise the effects of hyperthermia in tumours. Journal of Controlled Release 2018, 280, 87–98. [DOI] [PubMed] [Google Scholar]
- 39.Han S; Lee D; Kim S; Kim HH; Jeong S; Kim J, Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors (Basel) 2022, 12 (8), 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Z; Wang Y; Li L; Zeng F; Shang Q; Liao Y; Liang C; Nie L, Tumor-Homing and Immune-Reprogramming Cellular Nanovesicles for Photoacoustic Imaging-Guided Phototriggered Precise Chemoimmunotherapy. ACS Nano 2022, 16 (10), 16177–16190. [DOI] [PubMed] [Google Scholar]
- 41.Morse SV; Mishra A; Chan TG; R T. M. d. R.; Choi JJ, Liposome delivery to the brain with rapid short-pulses of focused ultrasound and microbubbles. J Control Release 2022, 341, 605–615. [DOI] [PubMed] [Google Scholar]
- 42.Morse SV; Pouliopoulos AN; Chan TG; Copping MJ; Lin J; Long NJ; Choi JJ, Rapid Short-pulse Ultrasound Delivers Drugs Uniformly across the Murine Blood-Brain Barrier with Negligible Disruption. Radiology 2019, 291 (2), 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan L; Yang L; Jin J; Yang F; Liu D; Hu K; Wang Q; Yue Y; Gu N, Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10 (2), 462–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theek B; Baues M; Ojha T; Mockel D; Veettil SK; Steitz J; van Bloois L; Storm G; Kiessling F; Lammers T, Sonoporation enhances liposome accumulation and penetration in tumors with low EPR. J Control Release 2016, 231, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X; Khorsandi S; Wang Y; Santelli J; Huntoon K; Nguyen N; Yang M; Lee D; Lu Y; Gao R; Kim BYS; de Gracia Lux C; Mattrey RF; Jiang W; Lux J, Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat Nanotechnol 2022, 17 (8), 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandan R; Mehta S; Banerjee R, Ultrasound-Responsive Carriers for Therapeutic Applications. ACS Biomater Sci Eng 2020, 6 (9), 4731–4747. [DOI] [PubMed] [Google Scholar]
- 47.Tayier B; Deng Z; Wang Y; Wang W; Mu Y; Yan F, Biosynthetic nanobubbles for targeted gene delivery by focused ultrasound. Nanoscale 2019, 11 (31), 14757–14768. [DOI] [PubMed] [Google Scholar]
- 48.Rwei AY; Paris JL; Wang B; Wang W; Axon CD; Vallet-Regi M; Langer R; Kohane DS, Ultrasound-triggered local anaesthesia. Nat Biomed Eng 2017, 1, 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen Z; Chen T; Ma X; Ren W; Zhou Z; Zhu G; Zhang A; Liu Y; Song J; Li Z, Multifunctional theranostic nanoparticles based on exceedingly small magnetic iron oxide nanoparticles for T 1-weighted magnetic resonance imaging and chemotherapy. ACS nano 2017, 11 (11), 10992–11004. [DOI] [PubMed] [Google Scholar]
- 50.Pellico J; Gawne PJ; R T. M. d. R., Radiolabelling of nanomaterials for medical imaging and therapy. Chem Soc Rev 2021, 50 (5), 3355–3423. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Medina C; Teunissen AJP; Kluza E; Mulder WJM; van der Meel R, Nuclear imaging approaches facilitating nanomedicine translation. Adv Drug Deliv Rev 2020, 154–155, 123–141. [DOI] [PubMed] [Google Scholar]
- 52.Tang J; Baxter S; Menon A; Alaarg A; Sanchez-Gaytan BL; Fay F; Zhao Y; Ouimet M; Braza MS; Longo VA; Abdel-Atti D; Duivenvoorden R; Calcagno C; Storm G; Tsimikas S; Moore KJ; Swirski FK; Nahrendorf M; Fisher EA; Perez-Medina C; Fayad ZA; Reiner T; Mulder WJ, Immune cell screening of a nanoparticle library improves atherosclerosis therapy. Proc Natl Acad Sci U S A 2016, 113 (44), E6731–E6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X; Cherry SR; Xie Z; Shi H; Badawi RD; Qi J, Subsecond total-body imaging using ultrasensitive positron emission tomography. Proc Natl Acad Sci U S A 2020, 117 (5), 2265–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Binderup T; Duivenvoorden R; Fay F; van Leent MMT; Malkus J; Baxter S; Ishino S; Zhao Y; Sanchez-Gaytan B; Teunissen AJP; Frederico YCA; Tang J; Carlucci G; Lyashchenko S; Calcagno C; Karakatsanis N; Soultanidis G; Senders ML; Robson PM; Mani V; Ramachandran S; Lobatto ME; Hutten BA; Granada JF; Reiner T; Swirski FK; Nahrendorf M; Kjaer A; Fisher EA; Fayad ZA; Perez-Medina C; Mulder WJM, Imaging-assisted nanoimmunotherapy for atherosclerosis in multiple species. Sci Transl Med 2019, 11 (506), eaaw7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hainfeld JF; Slatkin DN; Focella TM; Smilowitz HM, Gold nanoparticles: a new X-ray contrast agent. Br J Radiol 2006, 79 (939), 248–53. [DOI] [PubMed] [Google Scholar]
- 56.Cormode DP; Naha PC; Fayad ZA, Nanoparticle contrast agents for computed tomography: a focus on micelles. Contrast Media Mol Imaging 2014, 9 (1), 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georgy Sergeevich T; Galina NM; Leyla VS; Nikolay Grigorievich K; Boris Nokolaevich K; Garif Gazizovich A; Irina Leonidovna M; Valery VT, Laser-induced tissue hyperthermia mediated by gold nanoparticles: toward cancer phototherapy. Journal of Biomedical Optics 2009, 14 (2), 021016. [DOI] [PubMed] [Google Scholar]
- 58.Stern JM; Kibanov Solomonov VV; Sazykina E; Schwartz JA; Gad SC; Goodrich GP, Initial Evaluation of the Safety of Nanoshell-Directed Photothermal Therapy in the Treatment of Prostate Disease. International Journal of Toxicology 2016, 35 (1), 38–46. [DOI] [PubMed] [Google Scholar]
- 59.Hirsch LR; Stafford RJ; Bankson JA; Sershen SR; Rivera B; Price RE; Hazle JD; Halas NJ; West JL, Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A 2003, 100 (23), 13549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rastinehad AR; Anastos H; Wajswol E; Winoker JS; Sfakianos JP; Doppalapudi SK; Carrick MR; Knauer CJ; Taouli B; Lewis SC; Tewari AK; Schwartz JA; Canfield SE; George AK; West JL; Halas NJ, Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci U S A 2019, 116 (37), 18590–18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oumano M; Russell L; Salehjahromi M; Shanshan L; Sinha N; Ngwa W; Yu H, CT imaging of gold nanoparticles in a human-sized phantom. J Appl Clin Med Phys 2021, 22 (1), 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimm MA; Shevtsov M; Werner C; Sievert W; Zhiyuan W; Schoppe O; Menze BH; Rummeny EJ; Proksa R; Bystrova O; Martynova M; Multhoff G; Stangl S, Gold Nanoparticle Mediated Multi-Modal CT Imaging of Hsp70 Membrane-Positive Tumors. Cancers (Basel) 2020, 12 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cormode DP; Roessl E; Thran A; Skajaa T; Gordon RE; Schlomka JP; Fuster V; Fisher EA; Mulder WJ; Proksa R; Fayad ZA, Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology 2010, 256 (3), 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daldrup-Link HE, Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology 2017, 284 (3), 616–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y; Naha PC; Hwang G; Kim D; Huang Y; Simon-Soro A; Jung HI; Ren Z; Li Y; Gubara S; Alawi F; Zero D; Hara AT; Cormode DP; Koo H, Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat Commun 2018, 9 (1), 2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toth GB; Varallyay CG; Horvath A; Bashir MR; Choyke PL; Daldrup-Link HE; Dosa E; Finn JP; Gahramanov S; Harisinghani M; Macdougall I; Neuwelt A; Vasanawala SS; Ambady P; Barajas R; Cetas JS; Ciporen J; DeLoughery TJ; Doolittle ND; Fu R; Grinstead J; Guimaraes AR; Hamilton BE; Li X; McConnell HL; Muldoon LL; Nesbit G; Netto JP; Petterson D; Rooney WD; Schwartz D; Szidonya L; Neuwelt EA, Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int 2017, 92 (1), 47–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maier-Hauff K; Ulrich F; Nestler D; Niehoff H; Wust P; Thiesen B; Orawa H; Budach V; Jordan A, Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 2011, 103 (2), 317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahmoudi K; Bouras A; Bozec D; Ivkov R; Hadjipanayis C, Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy's history, efficacy and application in humans. Int J Hyperthermia 2018, 34 (8), 1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richards JM; Shaw CA; Lang NN; Williams MC; Semple SI; MacGillivray TJ; Gray C; Crawford JH; Alam SR; Atkinson AP; Forrest EK; Bienek C; Mills NL; Burdess A; Dhaliwal K; Simpson AJ; Wallace WA; Hill AT; Roddie PH; McKillop G; Connolly TA; Feuerstein GZ; Barclay GR; Turner ML; Newby DE, In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: feasibility and safety in humans. Circ Cardiovasc Imaging 2012, 5 (4), 509–17. [DOI] [PubMed] [Google Scholar]
- 70.Karussis D; Karageorgiou C; Vaknin-Dembinsky A; Gowda-Kurkalli B; Gomori JM; Kassis I; Bulte JW; Petrou P; Ben-Hur T; Abramsky O; Slavin S, Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 2010, 67 (10), 1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang TY; Howarth SP; Miller SR; Graves MJ; Patterson AJ; JM UK-I; Li ZY; Walsh SR; Brown AP; Kirkpatrick PJ; Warburton EA; Hayes PD; Varty K; Boyle JR; Gaunt ME; Zalewski A; Gillard JH, The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol 2009, 53 (22), 2039–50. [DOI] [PubMed] [Google Scholar]
- 72.Rogosnitzky M; Branch S, Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 2016, 29 (3), 365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laurent S; Vander Elst L; Henoumont C; Muller RN, How to measure the transmetallation of a gadolinium complex. Contrast Media & Molecular Imaging 2010, 5 (6), 305–308. [DOI] [PubMed] [Google Scholar]
- 74.Bort G; Lux F; Dufort S; Cremillieux Y; Verry C; Tillement O, EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic AGuIX nanoparticles. Theranostics 2020, 10 (3), 1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tran VL; Lux F; Tournier N; Jego B; Maitre X; Anisorac M; Comtat C; Jan S; Selmeczi K; Evans MJ; Tillement O; Kuhnast B; Truillet C, Quantitative Tissue Pharmacokinetics and EPR Effect of AGuIX Nanoparticles: A Multimodal Imaging Study in an Orthotopic Glioblastoma Rat Model and Healthy Macaque. Adv Healthc Mater 2021, 10 (16), e2100656. [DOI] [PubMed] [Google Scholar]
- 76.Miladi I; Aloy M-T; Armandy E; Mowat P; Kryza D; Magné N; Tillement O; Lux F; Billotey C; Janier M; Rodriguez-Lafrasse C, Combining ultrasmall gadolinium-based nanoparticles with photon irradiation overcomes radioresistance of head and neck squamous cell carcinoma. Nanomedicine: Nanotechnology, Biology and Medicine 2015, 11 (1), 247–257. [DOI] [PubMed] [Google Scholar]
- 77.Verry C; Dufort S; Villa J; Gavard M; Iriart C; Grand S; Charles J; Chovelon B; Cracowski J-L; Quesada J-L; Mendoza C; Sancey L; Lehmann A; Jover F; Giraud J-Y; Lux F; Crémillieux Y; McMahon S; Pauwels PJ; Cagney D; Berbeco R; Aizer A; Deutsch E; Loeffler M; Le Duc G; Tillement O; Balosso J, Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiotherapy and Oncology 2021, 160, 159–165. [DOI] [PubMed] [Google Scholar]
- 78.Marill J; Anesary NM; Zhang P; Vivet S; Borghi E; Levy L; Pottier A, Hafnium oxide nanoparticles: toward an in vitro predictive biological effect? Radiat Oncol 2014, 9, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bonvalot S; Rutkowski PL; Thariat J; Carrere S; Ducassou A; Sunyach MP; Agoston P; Hong A; Mervoyer A; Rastrelli M; Moreno V; Li RK; Tiangco B; Herraez AC; Gronchi A; Mangel L; Sy-Ortin T; Hohenberger P; de Baere T; Le Cesne A; Helfre S; Saada-Bouzid E; Borkowska A; Anghel R; Co A; Gebhart M; Kantor G; Montero A; Loong HH; Verges R; Lapeire L; Dema S; Kacso G; Austen L; Moureau-Zabotto L; Servois V; Wardelmann E; Terrier P; Lazar AJ; Bovee J; Le Pechoux C; Papai Z, NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol 2019, 20 (8), 1148–1159. [DOI] [PubMed] [Google Scholar]
- 80.Bradbury MS; Phillips E; Montero PH; Cheal SM; Stambuk H; Durack JC; Sofocleous CT; Meester RJ; Wiesner U; Patel S, Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr Biol (Camb) 2013, 5 (1), 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao L; Yang ST; Wang X; Luo PG; Liu JH; Sahu S; Liu Y; Sun YP, Competitive performance of carbon “quantum” dots in optical bioimaging. Theranostics 2012, 2 (3), 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong G; Diao S; Antaris AL; Dai H, Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem Rev 2015, 115 (19), 10816–906. [DOI] [PubMed] [Google Scholar]
- 83.Wu X; Lin Q; Chen G; Lu J; Zeng Y; Chen X; Yan J, Sentinel Lymph Node Detection Using Carbon Nanoparticles in Patients with Early Breast Cancer. PLoS One 2015, 10 (8), e0135714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Du J; Zhang Y; Ming J; Liu J; Zhong L; Liang Q; Fan L; Jiang J, Evaluation of the tracing effect of carbon nanoparticle and carbon nanoparticle-epirubicin suspension in axillary lymph node dissection for breast cancer treatment. World J Surg Oncol 2016, 14 (1), 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacquart A; Keramidas M; Vollaire J; Boisgard R; Pottier G; Rustique E; Mittler F; Navarro FP; Boutet J; Coll JL; Texier I, LipImage 815: novel dye-loaded lipid nanoparticles for long-term and sensitive in vivo near-infrared fluorescence imaging. J Biomed Opt 2013, 18 (10), 101311. [DOI] [PubMed] [Google Scholar]
- 86.Sayag D; Cabon Q; Texier I; Navarro FP; Boisgard R; Virieux-Watrelot D; Carozzo C; Ponce F, Phase-0/phase-I study of dye-loaded lipid nanoparticles for near-infrared fluorescence imaging in healthy dogs. Eur J Pharm Biopharm 2016, 100, 85–93. [DOI] [PubMed] [Google Scholar]
- 87.Cabon Q; Sayag D; Texier I; Navarro F; Boisgard R; Virieux-Watrelot D; Ponce F; Carozzo C, Evaluation of intraoperative fluorescence imaging-guided surgery in cancer-bearing dogs: a prospective proof-of-concept phase II study in 9 cases. Transl Res 2016, 170, 73–88. [DOI] [PubMed] [Google Scholar]
- 88.Chen QY; Xie JW; Zhong Q; Wang JB; Lin JX; Lu J; Cao LL; Lin M; Tu RH; Huang ZN; Lin JL; Zheng HL; Li P; Zheng CH; Huang CM, Safety and Efficacy of Indocyanine Green Tracer-Guided Lymph Node Dissection During Laparoscopic Radical Gastrectomy in Patients With Gastric Cancer: A Randomized Clinical Trial. JAMA Surg 2020, 155 (4), 300–311. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe J; Ohya H; Sakai J; Suwa Y; Goto K; Nakagawa K; Ozawa M; Ishibe A; Suwa H; Kunisaki C; Endo I, Long-term outcomes of indocyanine green fluorescence imaging-guided laparoscopic lateral pelvic lymph node dissection for clinical stage II/III middle-lower rectal cancer: a propensity score-matched cohort study. Tech Coloproctol 2023. [DOI] [PubMed] [Google Scholar]
- 90.Carr JA; Franke D; Caram JR; Perkinson CF; Saif M; Askoxylakis V; Datta M; Fukumura D; Jain RK; Bawendi MG; Bruns OT, Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc Natl Acad Sci U S A 2018, 115 (17), 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Antaris AL; Chen H; Cheng K; Sun Y; Hong G; Qu C; Diao S; Deng Z; Hu X; Zhang B; Zhang X; Yaghi OK; Alamparambil ZR; Hong X; Cheng Z; Dai H, A small-molecule dye for NIR-II imaging. Nat Mater 2016, 15 (2), 235–42. [DOI] [PubMed] [Google Scholar]
- 92.Borys N; Dewhirst MW, Drug development of lyso-thermosensitive liposomal doxorubicin: Combining hyperthermia and thermosensitive drug delivery. Adv Drug Deliv Rev 2021, 178, 113985. [DOI] [PubMed] [Google Scholar]
- 93.Celik H; Wakim P; Pritchard WF; Castro M; Leonard S; Karanian JW; Dewhirst MW; Lencioni R; Wood BJ, Radiofrequency Ablation Duration per Tumor Volume May Correlate with Overall Survival in Solitary Hepatocellular Carcinoma Patients Treated with Radiofrequency Ablation Plus Lyso-Thermosensitive Liposomal Doxorubicin. J Vasc Interv Radiol 2019, 30 (12), 1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nichols JW; Bae YH, EPR: Evidence and fallacy. J Control Release 2014, 190, 451–64. [DOI] [PubMed] [Google Scholar]
- 95.Ramanathan RK; Korn RL; Raghunand N; Sachdev JC; Newbold RG; Jameson G; Fetterly GJ; Prey J; Klinz SG; Kim J; Cain J; Hendriks BS; Drummond DC; Bayever E; Fitzgerald JB, Correlation between Ferumoxytol Uptake in Tumor Lesions by MRI and Response to Nanoliposomal Irinotecan in Patients with Advanced Solid Tumors: A Pilot Study. Clin Cancer Res 2017, 23 (14), 3638–3648. [DOI] [PubMed] [Google Scholar]
- 96.Ravi H; Arias-Lorza AM; Costello JR; Han HS; Jeong DK; Klinz SG; Sachdev JC; Korn RL; Raghunand N, Pretherapy Ferumoxytol-enhanced MRI to Predict Response to Liposomal Irinotecan in Metastatic Breast Cancer. Radiology: Imaging Cancer 2023, 5 (2), e220022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Segal AW; Gregoriadis G; Lavender JP; Tarin D; Peters TJ, Tissue and hepatic subcellular distribution of liposomes containing bleomycin after intravenous administration to patients with neoplasms. Clin Sci Mol Med 1976, 51 (4), 421–5. [DOI] [PubMed] [Google Scholar]
- 98.Murray JL; Kleinerman ES; Cunningham JE; Tatom JR; Andrejcio K; Lepe-Zuniga J; Lamki LM; Rosenblum MG; Frost H; Gutterman JU, Phase I trial of liposomal muramyl tripeptide phosphatidylethanolamine in cancer patients. Journal of Clinical Oncology 1989, 7 (12), 1915–1925. [DOI] [PubMed] [Google Scholar]
- 99.Weers J; Metzheiser B; Taylor G; Warren S; Meers P; Perkins WR, A gamma scintigraphy study to investigate lung deposition and clearance of inhaled amikacin-loaded liposomes in healthy male volunteers. J Aerosol Med Pulm Drug Deliv 2009, 22 (2), 131–8. [DOI] [PubMed] [Google Scholar]
- 100.Behr J; Zimmermann G; Baumgartner R; Leuchte H; Neurohr C; Brand P; Herpich C; Sommerer K; Seitz J; Menges G; Tillmanns S; Keller M; Munich Lung Transplant G, Lung deposition of a liposomal cyclosporine A inhalation solution in patients after lung transplantation. J Aerosol Med Pulm Drug Deliv 2009, 22 (2), 121–30. [DOI] [PubMed] [Google Scholar]
- 101.Saari M; Vidgren MT; Koskinen MO; Turjanmaa VM; Nieminen MM, Pulmonary distribution and clearance of two beclomethasone liposome formulations in healthy volunteers. Int J Pharm 1999, 181 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- 102.Saari SM; Vidgren MT; Koskinen MO; Turjanmaa VM; Waldrep JC; Nieminen MM, Regional lung deposition and clearance of 99mTc-labeled beclomethasone-DLPC liposomes in mild and severe asthma. Chest 1998, 113 (6), 1573–9. [DOI] [PubMed] [Google Scholar]
- 103.Koukourakis MI; Koukouraki S; Giatromanolaki A; Kakolyris S; Georgoulias V; Velidaki A; Archimandritis S; Karkavitsas NN, High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas--rationale for combination with radiotherapy. Acta Oncol 2000, 39 (2), 207–11. [DOI] [PubMed] [Google Scholar]
- 104.Kumar N; Soni S; Jaimini A; Ahmad FJ; Bhatnagar A; Mittal G, Edetate calcium disodium nanoparticle dry powder inhalation: a novel approach against heavy metal decorporation. Int J Pharm 2011, 416 (1), 376–83. [DOI] [PubMed] [Google Scholar]
- 105.Seymour LW; Ferry DR; Kerr DJ; Rea D; Whitlock M; Poyner R; Boivin C; Hesslewood S; Twelves C; Blackie R; Schatzlein A; Jodrell D; Bissett D; Calvert H; Lind M; Robbins A; Burtles S; Duncan R; Cassidy J, Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int J Oncol 2009, 34 (6), 1629–36. [DOI] [PubMed] [Google Scholar]
- 106.Koukourakis MI; Koukouraki S; Giatromanolaki A; Archimandritis SC; Skarlatos J; Beroukas K; Bizakis JG; Retalis G; Karkavitsas N; Helidonis ES, Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J Clin Oncol 1999, 17 (11), 3512–21. [DOI] [PubMed] [Google Scholar]
- 107.Lee H; Shields AF; Siegel BA; Miller KD; Krop I; Ma CX; LoRusso PM; Munster PN; Campbell K; Gaddy DF; Leonard SC; Geretti E; Blocker SJ; Kirpotin DB; Moyo V; Wickham TJ; Hendriks BS, (64)Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin Cancer Res 2017, 23 (15), 4190–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miedema IHC; Zwezerijnen GJC; Huisman MC; Doeleman E; Mathijssen RHJ; Lammers T; Hu Q; van Dongen G; Rijcken CJF; Vugts DJ; Menke-van der Houven van Oordt, C. W., PET-CT Imaging of Polymeric Nanoparticle Tumor Accumulation in Patients. Adv Mater 2022, 34 (21), e2201043. [DOI] [PubMed] [Google Scholar]
- 109.Wang SJ; Huang WS; Chuang CM; Chang CH; Lee TW; Ting G; Chen MH; Chang PM; Chao TC; Teng HW; Chao Y; Chen YM; Lin TP; Chang YJ; Chen SJ; Huang YR; Lan KL, A phase 0 study of the pharmacokinetics, biodistribution, and dosimetry of (188)Re-liposome in patients with metastatic tumors. EJNMMI Res 2019, 9 (1), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herrmann K; Schwaiger M; Lewis JS; Solomon SB; McNeil BJ; Baumann M; Gambhir SS; Hricak H; Weissleder R, Radiotheranostics: a roadmap for future development. Lancet Oncol 2020, 21 (3), e146–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anselmo AC; Mitragotri S, Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng Transl Med 2021, 6 (3), e10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Namiot ED; Sokolov AV; Chubarev VN; Tarasov VV; Schioth HB, Nanoparticles in Clinical Trials: Analysis of Clinical Trials, FDA Approvals and Use for COVID-19 Vaccines. Int J Mol Sci 2023, 24 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pallares RM; Mottaghy FM; Schulz V; Kiessling F; Lammers T, Nanoparticle Diagnostics and Theranostics in the Clinic. J Nucl Med 2022, 63 (12), 1802–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lammers T; Ferrari M, The success of nanomedicine. Nano Today 2020, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hou X; Zaks T; Langer R; Dong Y, Lipid nanoparticles for mRNA delivery. Nature Reviews Materials 2021, 6 (12), 1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paul SM; Mytelka DS; Dunwiddie CT; Persinger CC; Munos BH; Lindborg SR; Schacht AL, How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov 2010, 9 (3), 203–14. [DOI] [PubMed] [Google Scholar]
- 117.Hay M; Thomas DW; Craighead JL; Economides C; Rosenthal J, Clinical development success rates for investigational drugs. Nat Biotechnol 2014, 32 (1), 40–51. [DOI] [PubMed] [Google Scholar]
- 118.Wilhelm S; Tavares AJ; Dai Q; Ohta S; Audet J; Dvorak HF; Chan WC, Analysis of nanoparticle delivery to tumours. Nature reviews materials 2016, 1 (5), 1–12. [Google Scholar]
- 119.McNeil SE, Evaluation of nanomedicines: stick to the basics. Nature Reviews Materials 2016, 1 (10), 16073. [Google Scholar]
- 120.Price LSL; Stern ST; Deal AM; Kabanov AV; Zamboni WC, A reanalysis of nanoparticle tumor delivery using classical pharmacokinetic metrics. Science Advances 2020, 6 (29), eaay9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin Z; Chou WC; Cheng YH; He C; Monteiro-Riviere NA; Riviere JE, Predicting Nanoparticle Delivery to Tumors Using Machine Learning and Artificial Intelligence Approaches. Int J Nanomedicine 2022, 17, 1365–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamankurt G; Berns EJ; Xue A; Lee A; Bagheri N; Mrksich M; Mirkin CA, Exploration of the nanomedicine-design space with high-throughput screening and machine learning. Nat Biomed Eng 2019, 3 (4), 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Faria M; Bjornmalm M; Thurecht KJ; Kent SJ; Parton RG; Kavallaris M; Johnston APR; Gooding JJ; Corrie SR; Boyd BJ; Thordarson P; Whittaker AK; Stevens MM; Prestidge CA; Porter CJH; Parak WJ; Davis TP; Crampin EJ; Caruso F, Minimum information reporting in bio-nano experimental literature. Nat Nanotechnol 2018, 13 (9), 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gagliardini E; Conti S; Benigni A; Remuzzi G; Remuzzi A, Imaging of the porous ultrastructure of the glomerular epithelial filtration slit. J Am Soc Nephrol 2010, 21 (12), 2081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi HS; Liu W; Misra P; Tanaka E; Zimmer JP; Itty Ipe B; Bawendi MG; Frangioni JV, Renal clearance of quantum dots. Nat Biotechnol 2007, 25 (10), 1165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou C; Hao G; Thomas P; Liu J; Yu M; Sun S; Öz OK; Sun X; Zheng J, Near-Infrared Emitting Radioactive Gold Nanoparticles with Molecular Pharmacokinetics. Angewandte Chemie 2012, 124 (40), 10265–10269. [DOI] [PubMed] [Google Scholar]
- 127.Maraganore J, Reflections on Alnylam. Nature Biotechnology 2022, 40 (5), 641–650. [DOI] [PubMed] [Google Scholar]
- 128.Akinc A; Maier MA; Manoharan M; Fitzgerald K; Jayaraman M; Barros S; Ansell S; Du X; Hope MJ; Madden TD; Mui BL; Semple SC; Tam YK; Ciufolini M; Witzigmann D; Kulkarni JA; van der Meel R; Cullis PR, The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nature Nanotechnology 2019, 14 (12), 1084–1087. [DOI] [PubMed] [Google Scholar]
- 129.Man F; Gawne PJ; R T. M. d. R., Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv Drug Deliv Rev 2019, 143, 134–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van der Geest T; Laverman P; Gerrits D; Franssen GM; Metselaar JM; Storm G; Boerman OC, Comparison of three remote radiolabelling methods for long-circulating liposomes. J Control Release 2015, 220 (Pt A), 239–244. [DOI] [PubMed] [Google Scholar]
- 131.Takechi-Haraya Y; Ohgita T; Demizu Y; Saito H; Izutsu KI; Sakai-Kato K, Current Status and Challenges of Analytical Methods for Evaluation of Size and Surface Modification of Nanoparticle-Based Drug Formulations. AAPS PharmSciTech 2022, 23 (5), 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fourches D; Pu D; Tassa C; Weissleder R; Shaw SY; Mumper RJ; Tropsha A, Quantitative nanostructure-activity relationship modeling. ACS Nano 2010, 4 (10), 5703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu D; Long Q; Xu Y; Xing J, Evaluating Nanoparticles in Preclinical Research Using Microfluidic Systems. Micromachines (Basel) 2019, 10 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang T; Jia Y; Yu Y; Zhang B; Xu F; Guo H, Targeting the tumor biophysical microenvironment to reduce resistance to immunotherapy. Adv Drug Deliv Rev 2022, 186, 114319. [DOI] [PubMed] [Google Scholar]
- 135.Boso DP; Di Mascolo D; Santagiuliana R; Decuzzi P; Schrefler BA, Drug delivery: Experiments, mathematical modelling and machine learning. Comput Biol Med 2020, 123, 103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cheng Z; Al Zaki A; Hui JZ; Muzykantov VR; Tsourkas A, Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338 (6109), 903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Isles MP, Nanomedicines and Nanosimilars-Why a Robust Centralised Regulatory Framework Is Essential to Enhance Patient Safety. Front Pharmacol 2021, 12, 787239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pita R; Ehmann F; Papaluca M, Nanomedicines in the EU-Regulatory Overview. AAPS J 2016, 18 (6), 1576–1582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


