Abstract
Context.
Inequities and gaps in palliative care access are a serious impediment to health systems especially in low- and middle-income countries and the accurate measurement of need across health conditions is a critical step to understanding and addressing the issue. Serious Health-related Suffering (SHS) is a novel methodology to measure the palliative care need and was originally developed by The Lancet Commission on Global Access to Palliative Care and Pain Relief. In 2015, the first iteration – SHS 1.0 – was estimated at over 61 million people worldwide experiencing at least 6 billion days of SHS annually as a result of life-limiting and life-threatening conditions.
Objectives.
In this paper, an updated methodology - SHS 2.0 - is presented building on the work of the Lancet Commission and detailing calculations, data requirements, limitations, and assumptions.
Methods and Results.
The updates to the original methodology focus on measuring the number of people who die with (decedents) or live with (non-decedents) SHS in a given year to assess the number of people in need of palliative care across health conditions and populations. Detail on the methodology for measuring the number of days of SHS that was pioneered by the Lancet Commission, is also shared, as this second measure is essential for determining the health system responses that are necessary to address palliative care need and must be a priority for future methodological work on SHS.
Conclusions.
The methodology encompasses opportunities for applying SHS to future policy making assessment of future research priorities particularly in light of the dearth of data from low- and middle-income countries, and sharing of directions for future work to develop SHS 3.0.
Keywords: Serious health-related suffering, palliative care, suffering measurement, palliative care need
Background
Over 60 million people annually experience serious health-related suffering (SHS) that is amenable to palliative care. However, most reside in low-resource and rural areas with nonexistent or inadequate palliative care services and limited access to medicines and technologies that can reduce SHS,1 emblematic of the tragedy and injustice of overall disparities in healthcare. Palliative care is a core component of universal health coverage (UHC), making the lack of access to palliative care a serious impediment to Sustainable Development Goal (SDG) 3, namely, to “ensure healthy lives and promote well-being for all at all ages”2,3 and to achieving SDG 10 focused on reducing inequality within and among all countries.1,2
Efforts to address this global health failing and to close the divide in access to palliative care have been thwarted by various factors.1,4 One is the dearth of methods and data to quantify global palliative care need and this was a major area of work of The Lancet Commission on Global Access to Palliative Care and Pain Relief (hereafter referred to as Lancet Commission or the Commission) in developing SHS. Although evidence is required to develop appropriate and targeted recommendations for closing gaps in access to palliative care, measurement of the burden of SHS has not kept pace with progress in measuring the burden of disease.1,5 A scientific focus on measurement of SHS6,7 is a necessary complement to existing measures of the burden of disease such as quality-adjusted life years (QALYs) and disability adjusted life years (DALYs). Further, measurement of SHS has value and purpose in its own right as a global health issue and as part of efforts to achieve the SDGs.
The Lancet Commission report presented a breakthrough by introducing the concept of serious health-related suffering (SHS) to quantify the global and country-specific need for palliative care and pain relief in terms of both the number of individuals who experience SHS (population in need of palliative care services), and the number of days of each type of SHS (as an input to develop more effective health system responses to address palliative care need) in a given year. Building on more limited efforts to measure population-based need for palliative care in previous publications,4 the Commission estimated the 2015 global burden of SHS at 61 million: 25.5 million people who died—45% of the 56.2 million deaths worldwide and an additional 35.5 million people who experienced an SHS-associated condition and did not die in that year, with at least 6 billion symptom days experienced by those people. The estimates were calculated by a systematic process documented briefly in the Lancet Commission report and in its entirety in a white paper.1,8
The Lancet Commission Report has been cited by over 1000 research article publications as of this writing, and the data has been used by various international organizations and initiatives including the International Narcotic Control Board (INCB), the Worldwide Hospice Palliative Care Alliance (WHPCA), and the Disease Control Priorities (3rd edition), as well as various country champions of palliative care in their evidence generation, policy making and advocacy endeavors.9–11
The Lancet Commission Secretariat was transformed into an interdisciplinary Research Hub on Global Access to Palliative Care and Pain Relief—jointly led by the University of Miami Institute for Advanced Study of the Americas and the International Association for Hospice and Palliative Care to promote evidence generation, dissemination, and translation to policy and practice to achieve universal access to palliative care. The research hub built on the original Commission methodology—SHS 1.0 to generate the next iteration—SHS 2.0.
In this paper, the SHS 2.0 methodology is summarized, exclusively dealing with measuring the number of people who die with (decedents) or live with (non-decedents) SHS. The assumptions, strengths, and weaknesses of both the original and the 2.0 iteration for measuring people with SHS are discussed. The methodology for measuring the number of days of SHS is also detailed. Pioneered by the Lancet Commission, measuring days with SHS is essential for determining the health system responses to palliative care need and although not undertaken as part of SHS 2.0, must be a priority for future methodological work on SHS. A guide to calculating the burden of SHS is provided, including specific instructions on measuring the number of people who die with (decedents) or live with (non-decedents) SHS and the number of symptom days they experience annually, as well as secondary indicators that may be constructed with the SHS database. The paper concludes with a discussion on the potential applications of SHS data for researchers, policymakers, and practitioners as well as directions for future work and priorities for developing SHS 3.0. It is linked to another methods paper on measuring distributed opioid morphine equivalent (DOME) and comparing DOME against the need for palliative care (SHS).
Defining and Measuring SHS
Serious health-related suffering, as defined by the Lancet Commission, is the “pain, suffering, and severe distress associated with life-threatening or life-limiting health conditions and with end of life”1 that cannot be relieved without medical intervention and that is potentially amenable to relief through palliative care. SHS is not bound by time or prognosis and includes complex, chronic or acute, life threatening, or life-limiting health conditions.12
The definition of palliative care adopted by the Lancet Commission is the one used by the World Health Organization (WHO) at the time: “an approach that improves the quality of life of patients and their families facing the problems associated with life-threatening illness through the prevention and relief of suffering by means of early identification and assessment and treatment of pain and other problems, physical, psychosocial, and spiritual.”1,13 SHS 2.0 adopts the consensus-based definition spearheaded by the IAHPC that was initiated as one of the recommendations of the Commission report and engaged a group of global stakeholders from low, middle, and high-income countries. Specifically, “palliative care is the active holistic care of individuals across all ages with serious health-related suffering due to severe illness and especially of those near the end of life. It aims to improve the quality of life of patients, their families, and their caregivers.”12
The SHS burden is presented both as the number of people experiencing SHS due to life-limiting or life-threatening conditions and as the number of symptomdays of SHS experienced. Individuals experiencing SHS are distinguished as either decedents or non-decedents and the conditions, multipliers, and estimates in each differ. Decedents are defined as individuals who died within the year of calculation and are thus captured in the mortality database. Non-decedents are individuals who did not die within the year of calculation and are thus captured in the prevalence database. Non-decedent categories of SHS include conditions (1) that may have been cured but from which SHS persists; (2) from which patients recover but that nonetheless caused SHS; (3) with survival with chronic severe disability and with SHS symptoms; and (4) have a slowly progressive course. Symptom-days are defined as the number of days decedents and non-decedents lived with any symptoms and are calculated for each symptom and aggregated to measure total palliative care need. The latter is key to analyzing the response to SHS, for example in DOME for specific symptoms such as pain or dyspnea.
General Considerations in the Selection Processes
The selection of conditions, development of multipliers, and calculation of the number of people and days of SHS was informed by a literature search, individual and group expert discussions, and Delphi processes with online surveys for SHS 1.0 as described in the Appendix to the Commission report. Expert panel (s) of palliative care clinicians with experience providing clinical care in different parts of the world, especially in LMICs were engaged in the process.
To estimate symptoms and symptom duration (days of SHS), as part of the work of the Commission and SHS1.0, experts were asked to consider a typical patient with each of the conditions and based on their daily experience, to generate an estimate of the prevalence and duration of each symptom. During the expert consultation stage, including focus group discussions and semi-structured interviews, results from the literature review were presented. Experts were asked to provide responses and feedback based on their work experiences even when those experiences were contrary to the evidence presented to them. Either individually or in groups, all data and estimates were vetted, considering assumptions and limitations or gaps to ensure that all relevant aspects or scenarios are reasonably accounted for when possible. It is expected that these data will serve to provide content validity for estimation of the global burden of remediable suffering.14 See Appendix Table 1 for a full list of the experts’ consensus building practices undertaken by the Lancet Commission.
Finally, the Delphi method for consensus-building also was used to determine the duration (average number of days requiring palliative care) for which palliative care was needed for each of the conditions included in the database.15 Experts were purposively sampled and were considered to be “informed individuals”16 and “specialists”17 within the field of palliative care, in this case palliative care.18 Both rounds of the Delphi requested 18 palliative care experts living in LMICs to estimate the number of days of palliative care that would be required for a patient with each of the given conditions. The responses from the first round were pooled to identify a group average range and standard deviation for each condition. The second round of the Delphi presented respondents with the average range of days of palliative care with confidence intervals for each parameter. Experts were asked to respond again to the same questions based on knowledge of the group’s prior responses. The response rate for round one was 83% and for round two was 27%. Results from each round are presented in Table 2. See Appendix Table 2 for the results from rounds 1 and 2 of the Delphi study. Due to limited resources, estimation for symptom-days is only available from the Lancet Commission (SHS 1.0) and was not updated for SHS 2.0.
Table 2.
Core Assumptions for Estimating Decedents and Non-Decedents in Need of Palliative Care
| Hemorrhagic Fever |
|---|
|
|
| Decedents |
| • 100% of deaths from hemorrhagic fever, which is about 5% of other infectious diseases.) |
| Non-decedents |
| • Approximately the same number of patients who recover from the disease as those who die from it.22–26) |
| Tuberculosis (TB) |
| Decedents |
| • 100% of patients who die from MDR-TB.27–29 MDR-TB deaths estimates were provided by the GBD database separately from the drug susceptible TB deaths, so we no longer need to estimate the deaths from MDR-TB using the proportion calculated from global reports as we did for SHS 1.0. |
| • 90% of drug-susceptible TB deaths. Regular TB deaths were calculated using total TB deaths minus MDR-TB deaths as described above.) |
| Non-decedents |
| • Given the natural history of TB as a consition of relatively short duration as compared to other SHS conditions, especially in the case of MDR- TB and XDR-TB, we estimated the number of MDR and XDR TB patients living with SHS in any given year to be the incidence number minus the deaths number. Subsequently, the non-decedents in need of palliative care for tuberculosis was estimated to be 100% of XDR-TB patients plus 50% ofMDR-TB patients living with SHS: Total TB-nondec = 100% * XDR-TB (incidence-deaths) + 50% * MDR-TB (incidence- deaths)) |
| HIV/AIDS |
| Decedents |
| • 100% of people who die from HIV/AIDs.30–34) |
| Non-decedents not on treatment |
| • 50% of people living with HIV (PLHIV) (non-decedents in 2015) required some type of palliative care. 35–38) |
| Non-decedents on treatment |
| • For PLHIV who are on ART, the percentage with SHS was estimated at approximately 15%, much lower than those without ART. GBD country-specific prevalence of HIV/AIDs was used to estimate PLHIV. Data on percentage of HIV patients on anti-retroviral therapy (ART) (ART coverage) was obtained from the World Bank Group based on UNAIDS estimates, and average levels of ART coverage for each income group were calculated using country-specific data available for each time point. Actual country income group classifications for each respective year were utilized to generate ART coverage averages by income group. We used the below assumption: Number of HIV patients living with SHS = (HIVprevalence*proportionofHIVpatientsonART* 15%) + (HIVprevalence* [1-proportionof HIVpatientsonART] *50%) |
| • Due to the timeline of the advent of antiretroviral (ARV) drugs and combination therapy, widespread introduction of ARVs after the surge of HIV prevalence, time delay in rollout of ARVs in low-income countries as compared to lower-middle, upper-middle, and high-income countries and data availability on ART coverage, ARV adjustment was only made for the years of 2000 (all income groups except low-income countries), 2010, and 2019. For 1990, based on unavailability of ART or ART coverage at 0%, the number of HIV patients with SHS equals total prevalence multiplied by 50%.) |
| Malignant neoplasms (except leukemia) |
| Decedents |
| • 90% of patients who die from malignant neoplasms (except leukemia).30,39–41) |
| Non-decedents |
| • According to International Agency for Research on Cancer (IARC), there were 32.6 million people older than 15 who were alive with a cancer diagnosis within the previous 5 years in 2012.42 Shi et al.,43 report that 28% of people who survive one year with cancer have a “high-symptom burden.” We assumed that people with a high-symptom burden need palliative care. Zucca et al.,44 report that few people who survive cancer for more than five years have symptoms that require palliative care unless they have a recurrence or another disease. Data on the percentage of the 32.6 million non-decedents who survive 1, 2, 3, 4, and 5 years, and on the need for palliative care at years 2, 3, 4, or 5 was unavailable. The International Agency for Research on Cancer (IARC) has data on survivorship from selected cancers in selected countries,42 but in the absence of global data, we estimated the number of non-decedents by year since their cancer diagnosis and the percentage of these non-decedents who need palliative by year since cancer diagnosis (Table 4). IHME prevalence data on malignant neoplasms includes all persons with a cancer diagnosis, regardless of their years, so we decided not to use their cancer prevalence data. The IARC has data on survivorship from selected cancers in selected countries within 5 years of diagnosis,42 but only for 1, 3, and 5 years of diagnosis. Thus, IARC data was used, and a linear distribution was assumed to impute for patients with 2 and 4 years of diagnosis, respectively. (Table 4. Multipliers for cancer survivors at 1, 2, 3, 4, and 5 years of diagnosis) |
| • Since cancer mortality data in GLOBOCAN is not available for the previous four years for each country, the following was assumed: |
| Mort5years = Mort2018 * 5 |
| • Mort2018 corresponds to cancer mortality in 2018 (GLOBOCAN database). Countries were grouped by income level, based on World Bank 2017 classifications. Five-year survival by income group was estimated, generating the following: |
| • Low income = 0.30 |
| • Lower-middle income = 0.361 |
| • Upper-middle income = 0.459 |
| • High-income = 0.574 |
| • Similarly, each income group was divided into quintiles according to its 5-year survival rate. Table 5 shows the assumptions for calculating the survival rate for each year that is not available from the GLOBOCAN database. |
| • Based on Table 5 below, it is assumed that survival in the low-income region in 1990 is similar to what survival in the first quintile of that region is today. In 2000, the same group of low-income countries had what the first two survival quintiles for low-income countries have today; that is, 0.24. Meanwhile, in 2010 the first three quintiles correspond to 0.26. For 2017, the current distribution reported by GLOBOCAN was used. (Table 5. Percentiles used to impute number cancer survivors at 1, 2, 3, 4, and 5 years of diagnosis in historical years)) |
| Leukemia |
| Decedents |
| • 90% of patients who die from leukemia; needs of people with leukemia are of shorter duration or lower intensity than those of people with solid tumors. An exception is some patients in HICs with chronic, difficult-to-control graft-versus-host disease. This globally unusual need was taken into consideration when estimating the duration of need among leukemia patients.) |
| Non-decedents |
| • Non-decedent category for leukemia was added for SHS 2.0 and was calculated separately for children and adults. For children, we estimate that 85% of total survivors living in low-income and lower-middle income countries, 60% of total survivors living in upper-middle income countries, and 25% of total survivors living in high-income countries are living with SHS. Overall, that constitutes 65% of the global total survivors. The childrens expert group placed particular emphasis on the burden of leukemia in low-income countries and the differentials across countries and this is reflected in the multipliers. This approach innovates on previous estimates and is an ongoing area of discussion for alignment with measuring SHS for other conditions. For adults, the calculation is the same as for other malignant neoplasms.) |
| Dementia |
| Decedents |
| • Approximately 80% of people who die from Alzheimer’s disease or other dementias in the year they die.30,45–47) |
| Non-decedents |
| • Approximately 25% of these people had advanced or late dementia. Moens et al. 30 found that 40% of persons with advanced or late dementia had symptoms requiring palliative care (the need for psychological and social support for caregivers likely would yield a higher percentage of need for palliative care, but data on this need are lacking). We thus estimated that 10% (25% * 40%) of people living with dementia are experiencing SHS. The number of people living with dementia came from GBD’s prevalence database.) |
| Inflammatory disease of central neural system |
| Decedents |
| • (70% of patients who die from syphilis) + (50% of patients who die from measles) + (100% of patients who die from tetanus) + (30% of patients who die from meningitis) + (30% of patients who die from encephalitis) + (100% of patients who die of trypanosomiasis) + (90% of patients who die from rabies).) |
| Non-decedents |
| • For every two patients who die from tetanus and require palliative care, there will be one patient who survives tetanus that requires palliative care.) |
| Extrapyramidal & movement disorders; other degenerative disease of CNS; demyelinating disease of CNS; Epilepsy; Cerebral palsy & other paralytic syndromes Decedents |
| • (65% of patients who die from Parkinson’s disease) + (50% of patients who die from epilepsy) + (100% of patients who die from multiple sclerosis) + (65% of patients who die from other neurological conditions).48–60) |
| Non-decedents |
| • Parkinson’s disease: Advanced disease and the attendant distressing symptoms occur approximately nine years after symptoms first appear,61 and we estimate conservatively that 25% of patients survive long enough to have advanced disease and do not die in a given year. Based on the work of Moens et al.,30 we estimate that 40% of these patients require palliative care. We thus estimated that 10% (25% * 40%) of people living with Parkinson’s disease are experiencing SHS and thus need palliative care. |
| • Multiple sclerosis (MS): MS has a long prognosis and shortens life by only 0–6 years. Thus, we estimated that 5% of people with MS who do not die in a given year have end stage disease. Based on the work of Moens et al.,30 we estimated that 34% of these patients—about 2% of total survivors require palliative care. The number of people living with multiple sclerosis was calculated by applying the ratio of global survivors: deaths.) |
| Cerebrovascular diseases |
| Decedents |
| • 65% of people die from stroke.62–70) |
| Non-decedents |
| • The mortality number for the next year was subtracted from the proportion of deaths expected to be within 1 year of diagnosis to approximate the number of cerebrovascular patients living with SHS. Mortality from three sub-categories of stroke, i.e., ischemic stroke, hemorrhagic stroke, and subarachnoid hemorrhage were summed, each subtracted from the proportion of deaths expected to be within the first year of diagnosis. Since actual data for the number of deaths for each year of patients diagnosed within the last year was not available, cohort survival data from literature review were used. In other words, we used the possibility that newly diagnosed patients would die within a year as the percentage among all deaths that would be from the newly diagnosed. |
| • As literature that covered all income groups across all historical years of interest was not available, missing years and income groups were imputed with the closest income group and/all year. The new method limited the estimation of SHS to only patients within the last 1–2 years of their life, since the majority of patients living with cerebrovascular disease can spend years living without SHS. While this method gives us a more realistic estimation of the suffering endured by cerebrovascular disease patients, there is scarce literature to inform an estimate of the percentage of total cerebrovascular disease patients who are within the last 1–2 years of their lives. Thus, a series of assumptions plus a limited compilation of data from our literature review were applied to construct the matrix of percentages of cerebrovascular disease patients living within the last 1–2 years of their lives by income group, for 1990, 2000, 2010 and 2017. These assumptions are limitations of this study given the varying strength of the underlying data. See Tables 4–6 for details. (Table 6: Estimation model used in calculation of cerebrovascular disease patients living with SHS—part 1); (Table 7: Estimation model used in calculation of cerebrovascular disease patients living with SHS—part 2); (Table 8. List of literature review used in calculating the 5-year survival by income group and by year).) |
| Chronic rheumatic heart disease; cardiomyopathy & heart failure |
| Decedents |
| • (65% of patients who die from rheumatic heart disease) + (70% of patients who die from hypertensive heart disease) + (40% of patients who die from cardiomyopathy, myocarditis and endocarditis) + (30% of patients who die from Chagas disease).30,71–76) |
| Chronic ischemic heart disease |
| Decedents |
| • 5% of patients who die from ischemic heart disease.77) |
| Chronic lower respiratory disease; lung disease due to external agents; interstitial lung disease; other disease of respiratory system |
| Decedents |
| • (80% of patients who die from COPD) + (50% of patients who die from other respiratory diseases except asthma).30,78–80) |
| Diseases of liver |
| Decedents |
| • (95% of patients who die from cirrhosis of liver) + (28% of patients who die from other digestive diseases).81–85) |
| Non-decedents |
| • There is little literature describing the suffering of the general liver patients population. We found a recent publication of patients with endstage liver disease but the inclusion criteria included decompensated liver diseases,86 while the vast majority of patients living with liver disease are mild or well compensated. In another study, D’Amico et al observed that patients with decompensated cirrhosis (or end stage liver disease ESLD), this is those who have complications and who cannot access to a liver transplant, have a median survival of 2 years.87 We thus estimated that for adults, if patients with end-stage disease may have SHS for two years before deaths, so the non-decedents number equal that of the decedents. For children, the early onset of liver diseases can cause more damage to the growing organ and thus generate more suffering. So we estimated that the number of pediatric patients living with liver diseases that cause serious health-related suffering is about 3 time that of the deaths every year.) |
| Renal failure |
| Decedents |
| • 45% of patients who die from kidney disease.30,88–90) |
| Non-decedents |
| • We couldn’t find any literature on the suffering of a “typical” or “average” patients living with chronic kidney diseases. We thus took a similar approach as other conditions: we assumed an average of 3 years from onset of SHS to deaths for a “typical” or “average” patient. Thus, the number of non-decedents were calculated as twice the number of decedents with SHS. For children, the early onset of kidney diseases can cause more damage to the growing organ and thus generate more suffering. So we estimated that the number of pediatric patients living with chronic kidney diseases that cause serious health-related suffering is about 3 time that of the deaths every year.) |
| Low birth weight & prematurity; birth trauma |
| Decedents |
| • (75% of patients who die from preterm birth complications) + (40% of patients who die from birth asphyxia and birth trauma).91–95) |
| Non-decedents |
| • The non-decedent category for low birth weight and birth trauma was only added for children. We estimate that about 1% of children under 5 low birth weight survivors, 20% of children under 5 birth trauma survivors, and 10% of 5–19-year-old birth trauma survivors experience SHS.) |
| Congenital malformations/anomalies |
| Decedents |
| • 60% of patients die from congenital anomalies.91,95–97) |
| Non-decedents |
| • As data was not found on the prevalence or longevity of patients with severe congenital malformations, an annual estimate of at least the same number of patients who die of congenital malformations was used for those who do not die, which equals 60% of total deaths.) |
| Injury, poisoning, external causes |
| Decedents |
| • 30% of patients die from injuries (intentional and unintentional).98,99 Many patients die so quickly that there is no time to institute palliative care or pain control.) |
| Non-decedents |
| • Each year, at least twice the number of patients who die of injuries do not die yet need palliative care or pain control.) |
| Atherosclerosis |
| Decedents |
| • 35% of patients who die from other circulatory diseases require palliative care.100,101) |
| Musculoskeletal disorders |
| Decedents |
| • 70% of patients who die from musculoskeletal diseases require palliative care.102) |
| Non-decedents |
| • Each year, at least twice the number of patients who die of musculoskeletal disorders do not die yet need palliative care. This category did not include patients with mild pain or with symptoms that do not significantly disrupt social or occupational functioning.) |
| Malnutrition |
| Decedents |
| • 100% of deaths from protein-energy malnutrition.103,104) |
| Endocrine, metabolic, blood, and immune disorders |
| • Diabetes mellitus: Although diabetes mellitus in adults is not included due to the high overlap with conditions of other key organs that were already included in the estimate, the expert panel on children’s palliative care needs decided to include this condition due to the fact that most of the deaths from diabetes in children are from type-1 diabetes, a congenital condition that can cause SHS without any complication of other key organs. 67% of deaths from diabetes and 10% of survivors with diabetes in children require palliative care. Diabetes mellitus in adults was not included. |
| • Thalassemia: 100% of deaths from thalassemia in children require palliative care. For non-decedents, the expert panel acknowledged that the proportion of patients in need is highly related to the access to treatment, quality of treatment, ability to do transplant and/or regular transfusion. Also, major thalassemia presents different suffering patterns from minor thalassemia. Finally, the panel decided to differentiate the suffering pattern by age groups: for children under 5, 70% and for children 5–19,10%. |
| • Sickle cell disorders: For children, 100% of deaths and 70% of survivors experience SHS. For adults, previous scholars have found that between 30% and 50% patients living with sickle cell disorders experience pain in most of the days surveyed.105 Considering other physical and psychological sufferings, 50% of all adult patients living with sickle cell disorders were estimated to experience SHS.) |
Taking Children in Account in SHS 2.0
The initial SHS database from the Commission work did not differentiate the SHS burden experienced by adults and children. Hence for SHS 2.0 and in collaboration with and under the leadership of the International Childrens Palliative Care Network (ICPCN) with the engagement of IAHPC and WHPCA, an additional expert panel was convened for SHS 2.0 comprised of 8 pediatric palliative care specialists from both high-income and low- and middle-income settings around the world. Literature review and analysis,19 an online survey, two virtual meetings each lasting at least 90 minutes, and various internal discussions were conducted to differentiate the calculation of palliative care needs for children and adults in select conditions.
Time-Series Analysis
A major improvement for SHS 2.0 is the time-series analysis to incorporate the sensitivity of SHS to changes in disease trajectories, changes in pathogens, emergence of new diseases, and with the evolution of and advancements in medical technologies to address the burden of disease, each of which impacts the SHS burden. This gap was identified through the incorporation of time series mortality and prevalence data to analyze historical trends in the SHS burden. Data for 1990, 2000, 2010, and 2019 are presented in the updated calculations. Those years were selected to represent the earliest obtainable evidence, and data points every 10 years, and 2019 was selected as the most recent year since it was the most updated year of data at the time of the commencement of this analysis. The need to account for endogenous variables was particularly evident for people living with human immunodeficiency viruses (PLHIV), as well as patients living with tuberculosis, cancer, or cerebrovascular disease, and for children.
Switching From WHO’s Global Health Estimates (GHE) to IHME’s Global Burden of Diseases (GBD) Database
The Lancet Commission estimated the SHS burden in the most recent year of available data at the time (2015) and using WHO’s global mortality database, Global Health Estimates (GHE). However, due to the lack of prevalence data in GHE, non-decedents were computed using fixed survivor-to-deaths ratios generated from global disease-specific reports. This assumed that all countries experience the global average survivor-to-deaths ratio for all conditions with non-decedents categories, not accounting for country-level variation in the epidemiological profile of survivors and limiting the applicability of country-specific analyses.
SHS 2.0 was improved in several dimensions by using the GBD database released by the Institute for Health Metrics and Evaluation (IHME). Firstly, the GBD includes country-specific data on mortality and prevalence. The prevalence of data strengthens the calculation of non-decedents with SHS. In addition, GBD data dates back to 1990, permitting the calculation of the burden of SHS over three decades. Further, the Lancet Commission report defined children as being 0–15 years of age as more disaggregated data was not available. For SHS 2.0, children are defined as 0–19-year-old to be consistent with other key publications on children’s palliative care need around the world using the GBD data break-down of age groups.19
Several other global databases have also been used for SHS 2.0 in order to compile better, country- and disease-specific mortality or prevalence data. Specifically, the UNAIDS database for ART coverage20 and the International Agency for Research on Cancer (IARC)21 for data on cancer patients by years of diagnosis.
Selection of SHS-Associated Conditions
The first step in estimating the SHS burden was to identify the health conditions that most commonly cause SHS from the ICD-10 classification list that require palliative care at the end-of-life due to life-threatening conditions or living with a life-limiting condition (SHS 1.0). The global SHS 2.0 database includes 21 conditions, and these are presented in Table 1 with their corresponding ICD-10 codes and GBD codes. All 21 groups of conditions include decedent categories, considering that at least a proportion of people dying from those conditions suffer from serious health-related suffering. In addition, non-decedent categories of SHS are included for some of the 21 conditions that: may have been cured but from which SHS persists (drug-resistant tuberculosis, some hemorrhagic fevers such as Ebola, some malignancies, some inflammatory diseases of the central nervous system); from which patients recover but that caused SHS (serious injuries, renal failures, preterm birth complications, and birth trauma); with survival with chronic severe disability and with SHS symptoms (cerebrovascular disease, leukemia, congenital malformations, injury, birth trauma, human immunodeficiency viruses/acquired immunodeficiency syndrome (HIV/AIDS), some musculoskeletal disorders, liver diseases); and, have a slowly progressive course (malignancies, dementia, Parkinson’s disease, multiple sclerosis, type-1 diabetes, thalassemia, and sickle cell disorders).
Table 1.
Twenty-One Conditions That Most Often Generate a Need for Palliative Care (Ranked by ICD-10 Codes) and Their Corresponding GBD Codes
| ICD 10 Conditions That Most Often Generate a Need for PC | Short Names | GBD Sub-Conditions Used | GBD Codes |
|---|---|---|---|
|
| |||
| A96,98,99: Hemorrhagic fevers | HF | Other infectious disease | 408 |
| A15–19: TB | Tuberculosis | TB-MDR/XDR | 946, 947 |
| TB (non-MDR) | 934 | ||
| B20–24: HIV disease | HIV | HIV/AIDs | 298 |
| C00–97: Malignant neoplasms (except C91–95) | Cancer | Malignant neoplasms (- Leukemia) | 410 (−487) |
| C91–95: Leukemia | Leukemia | Leukemia | 487 |
| D50–89, E00–89: Endocrine, metabolic, blood and immune disorders | EMBID | Diabetes mellitus | 587 |
| Thalassaemias | 614 | ||
| Sickle cell disorders | 615 | ||
| E40–46: Protein-energy malnutrition | Malnutrition | Protein-energy malnutrition | 387 |
| F00–04: Dementia | Dementia | Alzheimer’s disease and other dementias | 543 |
| G00–09: Inflammatory disease of the central neural system | Inflammatory disease of CNS | Syphilis | 394 |
| Measles | 341 | ||
| Tetanus | 340 | ||
| Meningitis | 332 | ||
| Encephalitis | 337 | ||
| Trypanosomiasis | 350 | ||
| Rabies | 359 | ||
| G20–26; G30–32; G35–37; G40–41; G80 –83 Extrapyramidal & mvt disorders; other degen dz of CNS; Demyelinating dz of CNS; Epilepsy; Cerebral palsy & other paralytic syndromes | Degen disease of CNS | Parkinson’s disease | 544 |
| Epilepsy | 545 | ||
| Multiple sclerosis | 546 | ||
| Other neurological conditions | 557 | ||
| I60–69: Cerebrovascular diseases | Cerebrovascular diseases | Stroke | 494 |
| I05–09; I25; I42 & I50: Chronic rheumatic heart diseases; cardiomyopathy & heart failure | NIHD (Non-ischemic heart diseases) | Rheumatic heart disease | 492 |
| Hypertensive heart disease | 498 | ||
| Cardiomyopathy, myocarditis and endocarditis | 499, 503 | ||
| Chagas disease | 346 | ||
| I25: Chronic ischemic heart disease | IHD | Ischemic heart disease | 493 |
| I70: Athrosclerosis | Athrosclerosis | Other circulatory disease | 507 |
| J40–47; J60–70; J80–84; J95–99: Chronic lower respiratory dz; lung dz due to external agents; interstitial lung dz; other dz of resp system | Lung diseases | COPD | 509 |
| Other respiratory dz except asthma | 520 | ||
| K70–77: Diseases of liver | Diseases of liver | Cirrhosis of liver | 521 |
| Other digestive disease | 541 | ||
| Schistosomiasis | 351 | ||
| M00–97: Musculoskeletal disorders | MSD | Musculoskeletal diseases | 626 |
| N17–19: Renal failure | Renal failure | Kidney diseases | 589 |
| P07; P10–15: Low birthweight & prematurity; Birth trauma | Low birth weight | Preterm birth complications | 381 |
| Birth asphyxia and birth trauma | 382 | ||
| Q00–99: Congenital malformations | CM | Congenital anomalies | 641 |
| S00–99; T00–98; V01-Y98: Injury, poisoning, external causes | Injury | Injuries | 687 |
In the original Lancet Commission report, the non-decedents category for 11 conditions were considered. In SHS 2.0, non-decedents categories for four more conditions were added and differentiating factors were used that are important to estimating suffering patterns. Table 2 provides a detailed description of how decedents and non-decedents in need of palliative care are estimated for each condition as well as key literature and extra databases used to calculate the decedents and non-decedents with SHS. Conditions are ranked using the alphabetical order of their ICD-10 codes.
As the result of the exercise to estimate palliative care needs for children, there was consensus that the following conditions be added due to their substantive contribution to SHS among children for both decedents and non-decedents: (1) diabetes mellitus, (2) sickle cell disorders, (3) thalassemia, and the following conditions for non-decedents categories of: (1) leukemia, (2) liver diseases, (3) chronic kidney diseases, (4) neonatal preterm birth and birth trauma. Hence, while SHS 1.0 included 20 conditions, SHS 2.0 includes 21 groups of conditions with the addition of endocrine, metabolic, blood, and immune disorders which include diabetes mellitus, sickle cell disorders, and thalassemia for decedents and non-decedents.
The review of the case of diabetes in children prompted an overall review of the included conditions. For diabetes mellitus in adults, deaths from sequelae are attributed to the proximal cause and hence considered captured in other conditions included in the SHS database and specifically, cerebrovascular disease, cardiomyopathy and/or heart failure, chronic ischemic heart disease, renal failure, and atherosclerosis. Because deaths from diabetic ketoacidosis or hyperglycemic hyperosmotic non-ketotic syndrome typically result in death so rapidly that there is no time to institute quality palliative care services, these conditions are not included. In the pediatric population, diabetes mellitus is added due to the concerns over pain and suffering caused by type-1 diabetes even in the absence of organ complications.
Efforts to alleviate SHS experienced by a newborn, the assurance of the newborn’s comfort and that of distraught parents should accompany aggressive life-sustaining treatments if they are to reasonably provide more benefit than burden. Palliative care must also be available as an alternative to potentially harmful life-sustaining interventions when a newborn is moribund. Hence, in both SHS 1.0 and SHS 2.0, extremely premature and very low birth weight newborns whose survival is unlikely, and babies born with severe hypoxic ischemic encephalopathy or congenital anomalies not compatible with life are included in the list of SHS conditions.
In both SHS 1.0 and SHS 2.0, leukemia is considered a separate condition than the rest of the malignancies due to its distinctive patients’ demographics and suffering patterns.
Selection of Types or Symptoms of Suffering
Patients’ suffering varies by type, severity, and duration and a clinically, economically, and strategically useful measure of SHS requires estimation of not only the number of patients who suffer, but also the type of suffering and duration of suffering. Therefore, overarching categories of suffering were identified in SHS 1.0 and then within those categories, the types or symptoms were associated with each condition.
Palliative care literature typically divides suffering into four categories—physical, psychological, social, and spiritual to encompass the full spectrum of human suffering. While the Lancet Commission accepted and adopted all four categories as SHS, the focus was on estimating the prevalence and duration of only physical and psychological categories of suffering and corresponding symptoms. The empirical evidence from published literature or expertise to produce reasonable estimates of the prevalence and duration of each type of social and spiritual suffering were not sufficient.
To estimate SHS as precisely as possible, the Commissions expert group identified the most common symptoms of physical and psychological suffering, and then estimated the prevalence and duration of each type of suffering associated with each condition or its treatment. Through literature review and evidence-informed expert consensus building exercises, physical and psychological types of suffering (symptoms), their frequencies and durations for each condition were identified as part of Commission work. See Fig. 1 for details. Specifically, the types of physical suffering include moderate or severe pain, mild pain, weakness, fatigue, shortness of breath, nausea and vomiting, constipation, diarrhea, dry mouth, itching, wounds, and bleeding. The types of psychological suffering identified include anxiety and worry, depressed mood, delirium or confusion, and dementia with disorientation, agitation, or memory loss. Table 3 summarizes the duration of each type of physical and psychological suffering and Appendix Table 3 lists the results from the literature review on prevalence of the most commonly reported type of physical suffering among patients with serious, complex, or life-limiting health problem.
Fig. 1.
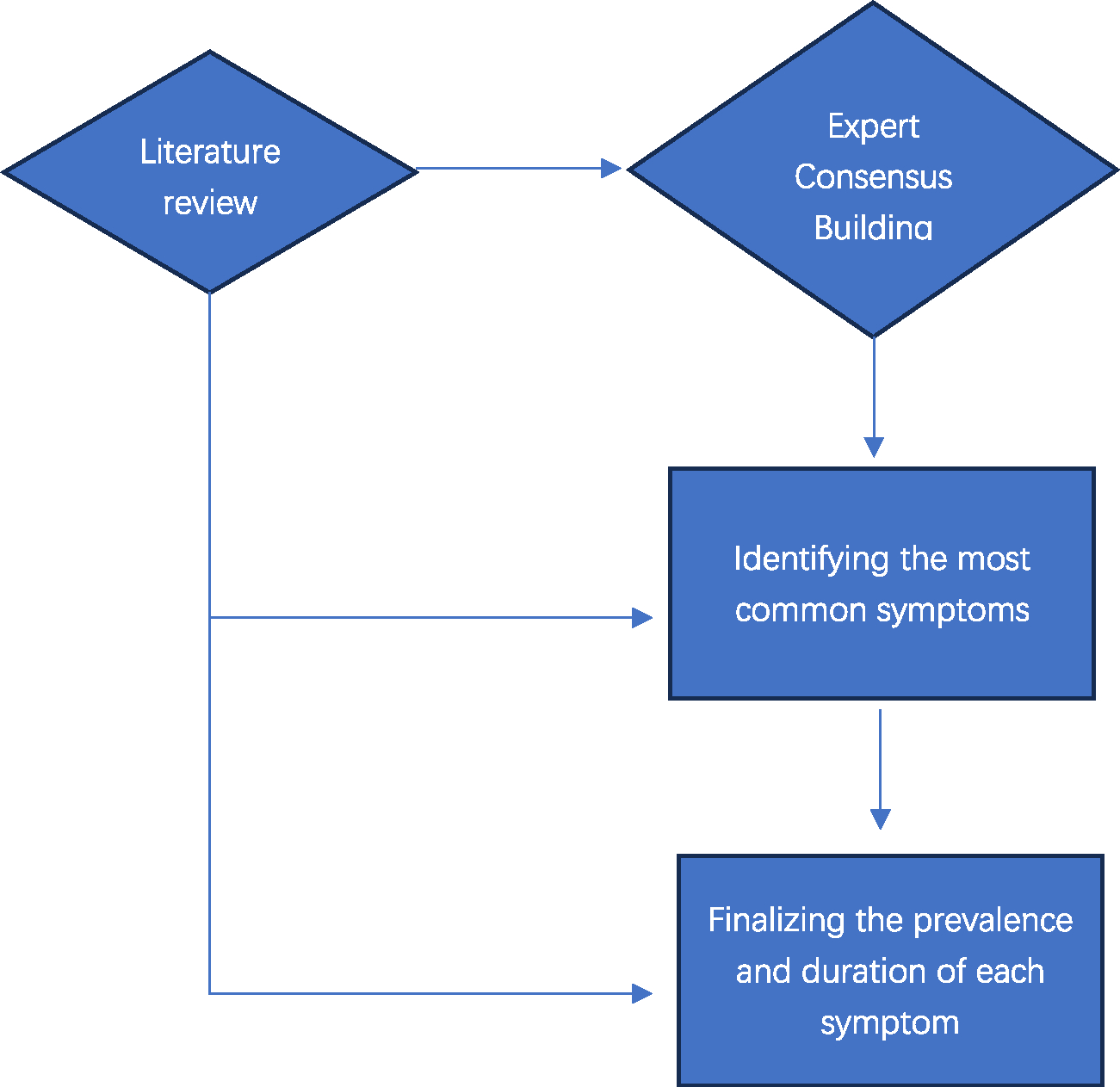
Flowchart of the process to finalize the symptom burden in patients with SHS.
Table 3.
The Final Estimates of Prevalence and Duration of Each Type of Physical and Psychological Suffering by Condition
| Pain Chronic Mild |
Pain Chronic Moderate Severe |
Dyspnea |
Fatigue |
Weakness |
Nausea and/or vomiting |
Diarrhea |
Constipation |
Dry Mouth |
Pruritus |
Bleeding |
Wounds |
Anxiety / worry |
Depressed mood |
Confusion / delirium |
Dementia |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Conditions | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | % | Days | |
|
| |||||||||||||||||||||||||||||||||
| 1 | Hemorrhagic fevers | 60% | 7 | 30% | 5 | 46% | 4 | 84% | 7 | 84% | 7 | 79% | 7 | 77% | 7 | 0% | 0 | 20% | 7 | 0% | 0 | 35% | 5 | 0% | 0 | 80% | 9 | 0% | 0 | 9% | 4 | 0% | 0 |
| 2 | M/XDR TB - decedents | 75% | 270 | 40% | 270 | 70% | 180 | 100% | 360 | 100% | 360 | 50% | 270 | 20% | 270 | 0% | 0 | 0% | 0 | 10% | 180 | 15% | 90 | 10% | 180 | 42% | 180 | 52% | 180 | 12% | 180 | 0% | 0 |
| 2b | M/XDR TB - nondecedents | 60% | 45 | 25% | 30 | 40% | 45 | 80% | 90 | 80% | 90 | 50% | 270 | 20% | 270 | 0% | 0 | 0% | 0 | 10% | 180 | 5% | 30 | 5% | 45 | 42% | 180 | 52% | 180 | 12% | 180 | 0% | 0 |
| 2c | Non-M/XDR TB - decedents | 20% | 14 | 10% | 14 | 85% | 21 | 100% | 21 | 100% | 21 | 10% | 14 | 10% | 14 | 0% | 0 | 0% | 0 | 5% | 14 | 15% | 21 | 10% | 21 | 42% | 180 | 43% | 180 | 0% | 0 | 0% | 0 |
| 3 | HIV | 90% | 160 | 45% | 90 | 70% | 30 | 100% | 180 | 100% | 180 | 30% | 150 | 60% | 180 | 0% | 0 | 50% | 30 | 30% | 90 | 0% | 0 | 35% | 60 | 68% | 180 | 49% | 150 | 47% | 14 | 25% | 120 |
| 3b | HIV - nondecedents | 50% | 160 | 15% | 90 | 10% | 30 | 25% | 90 | 25% | 90 | 10% | 30 | 15% | 45 | 0% | 0 | 10% | 30 | 20% | 60 | 0% | 0 | 5% | 30 | 50% | 150 | 30% | 150 | 2% | 7 | 2% | 30 |
| 4 | Malignant neoplasms (except Leukimia) | 90% | 150 | 80% | 90 | 35% | 90 | 90% | 180 | 90% | 180 | 20% | 120 | 5% | 90 | 35% | 90 | 50% | 30 | 5% | 90 | 10% | 90 | 20% | 90 | 38% | 150 | 47% | 150 | 35% | 14 | 0% | 0 |
| 4b | Malignant neoplasms (except Leukimia) - nondecedents | 35% | 150 | 20% | 90 | 15% | 90 | 50% | 120 | 50% | 120 | 15% | 21 | 5% | 21 | 20% | 90 | 5% | 60 | 2% | 30 | 2% | 30 | 5% | 90 | 25% | 150 | 18% | 150 | 1% | 5 | 0% | 0 |
| 5 | Leukemia | 90% | 90 | 35% | 60 | 50% | 60 | 100% | 120 | 100% | 120 | 20% | 60 | 5% | 60 | 25% | 60 | 50% | 30 | 15% | 60 | 25% | 60 | 5% | 60 | 38% | 90 | 47% | 90 | 35% | 14 | 0% | 0 |
| 6 | Dementia | 30% | 120 | 15% | 60 | 40% | 30 | 65% | 150 | 90% | 90 | 0% | 0 | 0% | 0 | 15% | 60 | 30% | 60 | 0% | 0 | 0% | 0 | 15% | 60 | 40% | 150 | 46% | 150 | 100% | 150 | 0% | 0 |
| 6b | Dementia - nondecedents | 15% | 60 | 5% | 30 | 10% | 20 | 35% | 90 | 45% | 45 | 0% | 0 | 0% | 0 | 10% | 60 | 5% | 30 | 0% | 0 | 0% | 0 | 2% | 30 | 30% | 120 | 25% | 90 | 100% | 120 | 0% | 0 |
| 7 | Inflammatory dz of CNS | 35% | 15 | 10% | 15 | 15% | 15 | 20% | 30 | 40% | 120 | 20% | 15 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 10% | 60 | 0% | 0 | 0% | 0 | 33% | 14 | 0% | 0 |
| 8 | Degen dz ofCNS; | 50% | 120 | 25% | 120 | 50% | 30 | 80% | 120 | 100% | 150 | 5% | 30 | 0% | 0 | 5% | 90 | 0% | 0 | 0% | 0 | 0% | 0 | 25% | 120 | 38% | 150 | 34% | 150 | 10% | 7 | 19% | 150 |
| 8b | Parkinsons – non decedents | 33% | 90 | 10% | 60 | 10% | 30 | 50% | 90 | 75% | 120 | 0% | 0 | 0% | 0 | 15% | 90 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 25% | 120 | 20% | 120 | 24% | 7 | 10% | 90 |
| 8c | Multiple sclerosis - nondecedents | 50% | 120 | 20% | 90 | 10% | 30 | 40% | 90 | 65% | 120 | 5% | 20 | 0% | 0 | 15% | 60 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 20% | 120 | 7% | 60 | 5% | 7 | 0% | 0 |
| 9 | Cerebrovascular diseases | 50% | 60 | 20% | 30 | 35% | 15 | 80% | 90 | 90% | 90 | 0% | 0 | 0% | 0 | 20% | 90 | 50% | 30 | 0% | 0 | 0% | 0 | 35% | 60 | 15% | 21 | 18% | 21 | 29% | 21 | 18% | 150 |
| 9b | Cerebrovascular diseases - nondecedents | 33% | 90 | 5% | 90 | 5% | 30 | 50% | 120 | 80% | 120 | 0% | 0 | 0% | 0 | 15% | 120 | 2% | 60 | 0% | 0 | 0% | 0 | 20% | 120 | 5% | 60 | 10% | 60 | 15% | 21 | 10% | 120 |
| 10 | Non-Ischemic Heart Diseases | 65% | 60 | 20% | 30 | 80% | 90 | 100% | 90 | 90% | 60 | 10% | 60 | 0% | 0 | 15% | 60 | 20% | 15 | 0% | 0 | 0% | 0 | 0% | 0 | 25% | 120 | 32% | 150 | 31% | 14 | 0% | 0 |
| 11 | Chronic ischemic heart disease | 90% | 120 | 75% | 30 | 75% | 30 | 80% | 90 | 50% | 30 | 20% | 30 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 53% | 120 | 60% | 150 | 0% | 0 | 0% | 0 |
| 12 | Lung Diseases | 25% | 120 | 10% | 30 | 100% | 120 | 95% | 90 | 50% | 30 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 38% | 120 | 47% | 120 | 23% | 14 | 0% | 0 |
| 13 | Diseases of liver | 65% | 90 | 30% | 30 | 50% | 60 | 80% | 60 | 70% | 30 | 35% | 30 | 0% | 0 | 0% | 0 | 50% | 15 | 20% | 30 | 15% | 90 | 0% | 0 | 26% | 30 | 25% | 60 | 70% | 21 | 0% | 0 |
| 14 | Renal failure | 40% | 30 | 5% | 15 | 25% | 15 | 90% | 90 | 50% | 30 | 20% | 30 | 0% | 0 | 15% | 30 | 30% | 15 | 10% | 90 | 5% | 30 | 0% | 0 | 29% | 90 | 31% | 90 | 52% | 14 | 0% | 0 |
| 15 | Low birth weight & prematurity; Birth trauma | 50% | 15 | 25% | 15 | 50% | 15 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 |
| 16 | Congenital malformations | 50% | 30 | 25% | 30 | 20% | 30 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 |
| 16b | Congenital malformations - nondecedents | 25% | 60 | 5% | 60 | 5% | 60 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 |
| 17 | Injury | 80% | 15 | 65% | 15 | 10% | 15 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 39% | 14 | 27% | 14 | 0% | 0 | 0% | 0 |
| 18 | Athrosclerosis | 67% | 120 | 50% | 60 | 10% | 30 | 20% | 30 | 20% | 30 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 70% | 2 | 30% | 60 | 35% | 60 | 0% | 0 | 0% | 0 |
| 19 | Musculoskeletal disorders | 70% | 360 | 30% | 360 | 5% | 90 | 30% | 30 | 30% | 60 | 0% | 0 | 0% | 0 | 20% | 210 | 20% | 10 | 0% | 0 | 0% | 0 | 15% | 60 | 13% | 180 | 24% | 180 | 0% | 0 | 0% | 0 |
| 20 | Malnutrition | 20% | 5 | 0% | 0 | 50% | 3 | 0% | 0 | 0% | 0 | 0% | 0 | 33% | 5 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 20% | 10 | 0% | 0 | 0% | 0 | 0% | 0 | 0% | 0 |
Most published data on symptom prevalence comes from high or upper-middle income countries where both disease-modifying and palliative treatments are most accessible. Furthermore, most of the literature either focused on physical and psychological symptoms among a single group of patients (such as cancer), or a single symptom (such as pain) in patients with various conditions. Data, mostly from high income countries, indicates that well over 50% of people who die of or live with malignant neoplasms and AIDS experience pain, and that pain is also common among those who live with heart disease, chronic obstructive pulmonary disease (COPD), renal failure, neurologic disease and dementia.106,107 Dyspnea (shortness of breath) is especially common among people who live with COPD and heart failure and only slightly less common among those who live with malignant neoplasms and AIDS.30 Depressed mood and anxiety are widespread among patients with a variety of advanced life-threatening illnesses including metastatic cancer and trauma.108,109 There are fewer studies among patients with most other serious, complex, or life-limiting health problems.
Of note, dementia appears both in the list of conditions (Alzheimer’s disease and other primary dementias) and as a symptom of other conditions (HIV/AIDS, cerebrovascular disease, and other neurologic conditions). The term dementia is therefore used in two ways, and the distinction in use of each instance is required.
Identifying Multipliers for Each Condition
The next step in measuring SHS was to determine the proportion of people with each condition who experience SHS. These are called multipliers. Multipliers are mathematical factors that estimate number of people dying or living with SHS based on different data sources. They reflect different strategies applied in the estimation and are provided separately for decedents and non-decedents. For decedents, the multipliers are always a percentage between 0 and 100%, to be applied to total deaths. For non-decedents, the multipliers take one of the three different forms: (1) a percentage between 0 and 100% to be applied to total number of patients living with the disease; (2) a ratio that can go over 100% to be applied to total deaths; or (3) a ratio that can go over 100% to be applied to total decedents in need of palliative care. See Table 4 with more details.
Table 4.
Multipliers of Cancer Survivors at 1, 2, 3, 4, and 5 Years of Diagnosis
| Years of Diagnosis | Estimated Percentage of Non-Decedents in Need of Palliative Care |
|---|---|
|
| |
| 1 | 28% |
| 2 | 20% |
| 3 | 15% |
| 4 | 10% |
| 5 | 5% |
To identify the proportion of people with each condition who experience SHS for the different conditions and sub-conditions and therefore identify appropriate multipliers to use for each, an extensive literature review was conducted for both decedents and non-decedents. Empirical evidence of symptom burden for some conditions was identified, but most studies were conducted in high-income settings. Evidence identified from the literature could not directly be used as multipliers since much of it was focused on patients in a certain stage of care whilst the SHS calculation requires multipliers for both people who die within that year—decedents and another for people who live with a condition—non-decedents. As a result, empirical evidence on percentage of patients with each condition experiencing SHS from the literature review were summarized and presented as the basis of discussion in various expert consensus building exercises. When estimating the SHS burden of non-decedents, experts were asked to consider the SHS burden of an “average” patient for each condition among all patients living with that condition who are not in their last year of life.
Because SHS 2.0 incorporates analysis across a number of years, it was possible to implement improvements to the multipliers for HIV and tuberculosis (TB). SHS stemming from HIV among non-decedents was differentiated between individuals undergoing anti-retroviral treatment (ART) from those who are not, reflecting how the advent of ART and increased access to such treatment revolutionized care for PLWHIV and in turn, SHS associated with HIV. Furthermore, extensively drug-resistant TB (XDR-TB) is differentiated from multidrug-resistant TB (MDR-TB), because antimicrobial resistance and the rise of XDR TB pose major challenges to treatment of tuberculosis which is different from MDR-TB.
For cancer, SHS 2.0 also incorporates data across additional years for the estimation of multipliers. In SHS 2.0, unlike for the Commission report, five-year survival data were used to estimate non-decedent SHS for malignant neoplasms and leukemia. The GBD data reports only overall survival and does not further disaggregate by years since diagnosis. Hence, the GBD data were adjusted based on the prevalence and mortality data extracted from the Global Cancer Observatory (GLOBOCAN) 2018 (see Panel 1 and Table 4) that report cancer survivorship for 1, 3, and 5 years from diagnosis.
A country-specific linearly interpolated trend was applied to estimate prevalence for year 2 and 4 post diagnosis. The approximation of survival was estimated as the ratio between the total deaths and the prevalence in the same period. Last, to estimate non-decedent burden for 1990, 2000, and 2010 given that information on 5-year prevalence and survival is not available by year since diagnosis, the GLOBOCAN 2018 data are adjusted using country-income specific quintile distribution data on percentages of all cancer survivors being with each year of diagnosis (see Table 5 for detail).
Table 5.
Percentiles Used to Impute Number Cancer Survivors at 1, 2, 3, 4, and 5 Years of Diagnosis in Historical Years
| Years |
|||||
|---|---|---|---|---|---|
| 1990 | 2000 | 2010 | 2017 | Note | |
|
| |||||
| Low income | 0.21 | Quintile 1 | |||
| 0.24 | Quintile 1–2 | ||||
| 0.26 | Quintile 1–3 | ||||
| 0.3 | Actual | ||||
| Lower-middle income | 0.25 | Quintile 1 | |||
| 0.28 | Quintile 1–2 | ||||
| 0.3 | Quintile 1 – 3 | ||||
| 0.361 | Actual | ||||
| Upper-middle income | 0.37 | Quintile 1 | |||
| 0.4 | Quintile 1–2 | ||||
| 0.42 | Quintile 1 – 3 | ||||
| 0.459 | Actual | ||||
| High income | 0.47 | Quintile 1 | |||
| 0.501 | Quintile 1–2 | ||||
| 0.521 | Quintile 1 – 3 | ||||
| 0.574 | Actual | ||||
Cerebrovascular diseases constitute a major component of overall SHS, yet its non-decedent category was a limitation in SHS 1.0. For SHS 2.0, non-decedent SHS was calculated for patients living in the year prior to their last year of life, assuming that most patients who live for extended periods with this condition do not experience SHS (as the condition is largely asymptomatic until it becomes serious enough to result in death). Still, data are scarce on the proportion of cerebrovascular disease patients in the final years of life and hence with SHS. An estimate of the proportion of patients who are diagnosed and die in the same year was developed based on a literature search focusing on differences by country income level and this was applied to two years of cerebrovascular disease mortality (see Tables 6, 7, and Appendix Table 4). Because data were not available on the number of deaths per year of patients diagnosed in the last year, a literature search was carried out on the survival of these patients in countries by income level. In other words, the calculation factored in the percentage of newly diagnosed patients that would die within one year as the percentage among all deaths that would occur due to newly diagnosed patients. As literature covering all income groups was not available in all years of interest, i.e., 1990, 2000, 2010 and 2019, missing years and income groups were imputed to the nearest income group and/or to all the year (Tables 8 and 9). The new method limited the estimation of SHS only to patients within the last 1–2 years of their life, since most patients living with cerebrovascular disease can spend years living without SHS. While this method gives us a more realistic estimate of the suffering endured by cerebrovascular disease patients, there is little literature to report an estimate of the percentage of total cerebrovascular disease patients who are in the last 1–2 years of their life (Appendix Table 4). Therefore, we applied a series of assumptions plus a limited compilation of data from our literature review to construct the matrix of percentages of cerebrovascular disease patients living within the last 1–2 years of their life by income group, to 1990, 2000, 2010, and 2019. These assumptions are limitations of this study, given the varying strength of the underlying data.
Table 6.
Estimation Model Used in Calculation of Cerebrovascular Disease Patients Living With SHS—Part 1
| 1990 | 2000 | 2010 | 2017 | |
|---|---|---|---|---|
|
| ||||
| Low income | Lower-middle 1991–2000 | Lower-middle 1991–2000 | Lower-middle 1991–2000 | Lower-middle 1991–2000 |
| Lower-middle | Lower-middle 1991–2000 | Lower-middle 1991–2000 | Lower-middle 1991–2000 | Lower-middle 1991–2000 |
| Upper-middle | Upper-middle 2001–2010 | Upper-middle 2001–2010 | Upper-middle 2001–2010 | Upper-middle 2001–2010 |
| High income | High income <1990 (worst-case mortality scenario in high-income countries from the literature) | High income 1991– 2000 | High income 2001– 2010 | High income 2001–2010 |
Table 7.
Estimation Model Used in Calculation of Cerebrovascular Disease Patients Living With SHS—Part 2
| 1990 | 2000 | 2010 | 2017 | ||
|---|---|---|---|---|---|
|
| |||||
| Low income | Ischemic | 41% | 41% | 41% | 41% |
| Hemorrhagic | 62% | 62% | 62% | 62% | |
| Subcranial | 62% | 62% | 62% | 62% | |
| Lower-middle | Ischemic | 41% | 41% | 41% | 41% |
| Hemorrhagic | 62% | 62% | 62% | 62% | |
| Subcranial | 62% | 62% | 62% | 62% | |
| Upper-middle | Ischemic | 41% | 41% | 28% | 28% |
| Hemorrhagic | 62% | 62% | 49% | 49% | |
| Subcranial | 62% | 62% | 48% | 48% | |
| High income | Ischemic | 31% | 31% | 11% | 11% |
| Hemorrhagic | 62% | 59% | 49% | 49% | |
| Subcranial | 58% | 50% | 48% | 48% | |
Table 8.
Multipliers Used to Calculate SHS Burden for 21 Conditions
| Decedents | Non-Decedents |
|||||
|---|---|---|---|---|---|---|
| Conditions That Most Often Generate a Need for PC | GBD Sub-Conditions Used | Multiplier | Non-Decedents Needing PC Relative to Decedents Needing PC_Updated | Non-Decedents Needing PC Relative to Total Non-Decedents | Non-Decedents Needing PC Relative to Total Mortality | |
|
| ||||||
| 1 | Hemorrhagic fevers | Other infectious disease | 5% | 100% | n.a | n.a |
| 2 | TB/deaths from M/ XDR TB | TB-MDR | 100% | n.a | 50%–100% | n.a |
| 2b | TB/deaths from TB that was NOT MDR | TB (non-MDR) | 90% | n.a | n.a | n.a |
| 3 | HIV | HIV/AIDs | 100% | n.a | 15%–50%a | n.a |
| 4 | Malignant neoplasms (except leukemia) | Malignant neoplasms (-Leukemia) | 90% | n.a | 5%–28%b | n.a |
| 5 | Leukemia | Leukemia | 90% | n.a | 65% for 0–19, and 5%-28% for 20+ | n.a |
| 6 | Dementia | Alzheimer’s disease and other dementias | 80% | n.a | 10% | n.a |
| 7 | Inflammatory dz of CNS | Syphilis | 70% | n.a | n.a | n.a |
| Measles | 50% | n.a | n.a | n.a | ||
| Tetanus | 100% | 50% | n.a | n.a | ||
| Meningitis | 30% | n.a | n.a | n.a | ||
| Encephalitis | 30% | n.a | n.a | n.a | ||
| Trypanosomiasis | 100% | n.a | n.a | n.a | ||
| Rabies | 90% | n.a | n.a | n.a | ||
| 8 | Degen dz of CNS | Parkinson’s disease | 65% | n.a | 10% | n.a |
| Epilepsy | 50% | n.a | n.a | n.a | ||
| Multiple sclerosis | 100% | n.a | 2% | n.a | ||
| Other neurological conditions | 65% | n.a | n.a | n.a | ||
| 9 | CVD | Stroke | 65% | n.a | lit reviewc | n.a |
| 10 | NIHD | Rheumatic heart disease | 65% | n.a | n.a | n.a |
| Hypertensive heart disease | 70% | n.a | n.a | n.a | ||
| Cardiomyopathy, myocarditis, and endocarditis | 40% | n.a | n.a | n.a | ||
| Chagas disease | 30% | n.a | n.a | n.a | ||
| 11 | IHD | Ischemic heart disease | 5% | n.a | n.a | n.a |
| 12 | Lung dz | COPD | 80% | n.a | n.a | n.a |
| Other respiratory dz except asthma | 50% | n.a | n.a | n.a | ||
| 13 | Diseases of liver | Cirrhosis of liver | 95% | 100% for 20+ | n.a | 300% for 0–19 |
| Other digestive disease | 30% | 100% for 20+ | n.a | n.a | ||
| Schistosomiasis | 70% | 100% for 20+ | n.a | n.a | ||
| 14 | Renal failure | Kidney diseases | 45% | 200% for 20+ | n.a | 300% for 0–19 |
| 15 | Low birth weight | Preterm birth complications | 75% | n.a | 1% for under 5 | n.a |
| Birth asphyxia and birth trauma | 40% | n.a | 20% for under 5 and 10% for 5–19 | n.a | ||
| 16 | Congenital malformations | Congenital anomalies | 60% | 100% | n.a | n.a |
| 17 | Injury | Injuries | 30% | 200% | n.a | n.a |
| 18 | Athrosclerosis | Other circulatory disease | 35% | n.a | n.a | n.a |
| 19 | MSD | Musculoskeletal diseases | 70% | 200% | n.a | n.a |
| 20 | Malnutrition | Protein-energy malnutrition | 100% | n.a | n.a | n.a |
| 21 | EMBID | Diabetes mellitus | 67% for 0–19 | n.a | 10% for 0–19 | n.a |
| Thalassaemias | 100% for 0–19 | n.a | n.a | |||
| Sickle cell disorders | 100% | n.a | 70% for under 5 and 10% for 5–19 70% for 0–19 and 50% for 20+ |
n.a | ||
n.a: not all condition groups have non-decedent categories, and for those who do, only one of the three approaches was taken to calculate the non-decedents. We have noted “n.a” for “not applicable” in places where multipliers are not applicable.
HIV non-decedents = HIV prevalence on ART * 15% + HIV prevalence not on ART * 50%.
based on the year ofcancer diagnosis.
Table 9.
Indicator-Specific Descriptions, Assumptions, and Limitations
| Indicator 1: Total symptom-days by condition |
| • Description: The sum of the symptom-days from each symptom by condition. |
| • Assumptions and limitations: No weighting of tolerability of symptoms. Assumption that coinciding symptoms make the suffering worse and thus that the symptom-days from each coinciding symptom should be added together. This assumption generates an overestimation of the total number of days of a patient’s suffering. |
| Indicator 2: AT LEAST symptom-days by condition |
| • Description: The symptom-days of the one symptom of longest duration. This would be the LEAST or minimal number of symptom-days experienced by the patient. |
| • Assumption and limitation: Assumes that any other symptoms began and ended during period of the symptom of longest duration. In most cases, this will be an underestimate of the total number of days of a patient’s suffering. |
| Indicator 3: AT LEAST non-pain symptom-days by condition |
| • Description: The symptom-days of the one non-pain symptom of longest duration. This would be the LEAST or minimal number of non-pain symptom-days experienced by the patient. |
| • Assumption and limitation: Assumes that any other non-pain symptoms began and ended during period of the non-pain symptom of longest duration. In many cases, this will be an underestimate of the total number of days of a patient’s suffering from non-pain symptoms. |
| Indicator 4: Total pain-days by condition |
| • Description: The sum of mild pain-days and moderate to severe pain-days. |
| • Assumption and limitation: The mild pain days do not overlap the moderate to severe pain-days. Thus, this indicator shows total days in pain. |
| However, it does not include any other symptoms. |
| Indicator 5: Pain plus At LEAST non-pain symptom-days by condition |
| • Description: This indicator was generated by adding the total pain-days and the AT LEAST non-pain symptom-days (indicator 3). |
| • Assumption and limitation: This is one possible indicator of the burden of suffering for a patient. |
| Indicator 6: Total days in need of palliative care by condition |
| • Description: An estimation of days requiring palliative care by condition by palliative care experts with experience treating patients in LMICs using a Delphi process. |
| • Assumption and limitation: Based only on the opinion of clinical palliative care experts from LMICs in each region. |
Table 7 presents the multipliers used to calculate SHS for all 21 conditions, separating decedents and non-decedents.
Data Limitations and Future Iterations
The measurement of the global burden of SHS presented in the Lancet Commission report set a precedent and the update to SHS 2.0 is an important move forward in measuring the number of people in need of palliative care. However, there are important limitations and there remains work to refine the estimation strategy and hence the estimates.
Data Limitations
First, although a literature review was conducted by condition and symptoms, due to a dearth of reliable empirical data on the types, prevalence, and duration of suffering caused by each SHS associated health condition, both SHS 1.0 and 2.0 rely heavily on expert opinion. Moreover, research on palliative care has so far concentrated on Europe and the United States accounting for over 90% of all publications on palliative care but only 15% of the global population. The fact that 85% of the global population produced only 6.5% research publications points to the glaring lack of information on the elements of suffering for the majority of people in the world.110
Further, the expert groups are relatively small, reflecting limitations in available funding to develop the field of SHS. This makes it especially difficult to develop either disease, region, or country income-specific estimates. The reliance on identifying an “average” patient limits the possibility of exploring regional, cultural or other differences, as well as the effect of providing differential levels of palliative care. The next step in the SHS work is to undertake disease-specific expert panels to refine estimates of people with SHS and especially symptoms and symptom days. This is the focus of research planned for SHS 3.0 and has been piloted for breast cancer and will soon commence on HIV.111
Second, there are conditions which generate SHS but are not included in the analysis to-date due to limited scope. For example, chronic paranoid schizophrenia and other severe chronic psychiatric disorders generate severe suffering but are not included in the methods presented here. Another important example is people living in the context of humanitarian crisis,112 including armed confiict113 but also climate emergencies, communicable disease outbreaks or those under threat of political, sexual, or ethnic violence who suffer from various types of physical and psychological suffering.
Similarly, our work to date extends to 2019. Estimating the shorter-term SHS that was associated with the coronavirus disease 2019 (COVID-19) pandemic, and the longer-term sequelae for those who suffered the disease should be a key next step in the analysis. This should include the suffering associated with bereavement and the lack of access to palliative care support for caregivers, family members and the community during COVID-19 lockdowns. The wealth of data and publications on the pandemic will make this analysis more feasible.
Family caregivers who experience various kinds of physical, psychological, social, and spiritual suffering as a result of their care work are not included in the estimates. While methods to estimate the types, prevalence, or duration of physical, psychological, social, or spiritual suffering of the main family or informal caregiver have not been within the scope of SHS calculations to-date, this is an important area of future SHS methodological development. Family caregivers typically provide many hours of daily care to patients with serious, chronic, complex, or life-limiting health problems and in many health care settings, especially in LMICs, where they must remain with the patient when admitted to the hospital. Across the world, caregiving work at home and in the communities is predominantly provided by women, and often uncompensated or undercompensated.114 It has been shown that caregiving can itself represent a source of suffering.115,116 Family caregivers may have their own need for palliative care and support in managing bereavement.
Expert opinion provides important information, but a patient-centered approach needs to be included in future work on SHS. Confirmatory research on symptom prevalence and severity with patient and caregiver reported real-life data must complement future work. This limitation applies to the symptoms as well as many dimensions of suffering that are important for patients, caregivers, and practitioners. The expert panel identified 11 physical and 4 psychological symptoms, but this is far from an exhaustive list of all possible physical and psychological symptoms patients can experience. Social or spiritual suffering is also not estimated despite being a source of grave concern due to the impact on overall quality of life.117,118 In the context of paucity of resources, of poorly organized healthcare systems and of marginalization of large chunks of the population, the impact on the burden of suffering is likely to be considerable.
Further, the quantity of suffering is estimated only in terms of number of people who died from or lived with SHS (SHS 1.0 and SHS 2.0), or the number of symptom days they each experience (SHS 1.0). This approach neglects the intensity or tolerability of suffering experienced. In SHS 3.0, opportunities for understanding the scope and intensity of social and spiritual suffering for patients in need of palliative care will be explored. Gathering patient and caregiver reported data is the optimal solution to fill in these gaps and should be a priority for donors and foundations interested in improving access to palliative care and achieving the SDGs. To date, only a few pilot and exploratory surveys have been undertaken.119,120
Another important area for future work is to determine to what extent suffering can be alleviated with existing practices and techniques at various resource levels. This also means that the multipliers percentage of deaths or survivors in need of palliative care by condition are time-period specific and should change over time based on previously noted endogenous variables, including the change in disease trajectories and their suffering patterns, as health care technologies and systems evolve.
Last but not least, our work looks at one side of the issue: the demand side. It is equally important, if not more, to measure how much of the need for palliative care is fulfilled, by whom, in what quality, and where. Combined with analysis of the actual provision of palliative care, we will be able to identify gaps and provide more tailored policy recommendations.
Future Iterations
The methods described in this paper are pioneering in the field. However, our exploration has only expanded our vision of the bigger, unknown world, leaving more gaps to be filled with future research. Even the more detailed estimate of “symptom-days” as opposed to number of people has limitations as a measure of the burden of SHS experienced by patients in the absence of a method to weigh the tolerability or intensity of each symptom. Specifically, the number of days is calculated for each symptom using the available information on symptom prevalences and duration for each condition. Simple aggregation of days with each symptom may lead to overestimation from double counting, as many patients with advanced disease will suffer from more than one symptom at the same time. As such, the Commission report presented two aggregate indicators to evaluate the total symptom burden: (1) the “at least” SHS-days, which equals the symptom-days from the single most prevalent symptom, in most case, pain, of each condition, and (2) the total symptom days should is the sum total of all symptoms. The actual days of suffering experienced by people with SHS should be a number between these two bounds. Ongoing refinement of the calculation of the number of days of SHS experienced by the population in a given year is a core area for SHS 3.0. Moreover, and as described, it is important to note that the calculation of the number of days of SHS is derived from the calculation of the number of people with SHS, not the other way around. As a contribution to measurement of burden, several “summary indicators” or ways to characterize the suffering experienced by patients were developed. Table 9 presents these secondary indicators that were constructed for the Lancet Commission report. Another dimension that has not been measured to date is to match SHS to an estimate of palliative care need assessment such as the estimated number of required “palliative care visit-days”—the number of days in which a palliative care provider should see the patient, family or caregiver. Symptom days measures only the days during which the symptom(s) persist(s), regardless of whether a visit by or with a palliative care provider is needed. Severe, refractory, or poorly tolerated symptoms may require daily visits while well-controlled symptoms may require a visit only every 2 to 4 weeks. Indeed, provision of effective palliative care can, and should, reduce the number of symptom days as well as the severity of the symptoms. In doing this, palliative care reduces the SHS burden. This remains an area for future discussion and analysis.
Discussion
This paper is designed to serve as a reference document for calculating SHS. Detailing the methodology is also intended to promote transparency in ongoing efforts to measure the burden of SHS and to promote wider discourse on the assessment of SHS burden that will inform future iterations of SHS measurement and data strengthening. Improving the science of the measurement of SHS will support policies that increase palliative care access and infrastructure as a component of UHC and improve population health.
The estimates generated from this methodology can be used independently or can serve as an input to the development of composite metrics that compare interventions in terms of suffering averted. Researchers can apply the methods presented using country-specific data (i.e., not GBD estimates, which are used here) to generate national and sub-national calculations of SHS.121,122 Researchers can also use our methods to project trends and examine the future scale of the burden of SHS overall or by condition.123 The SHS burden data is also a necessary input to calculating the cost of an essential package of palliative care services, as introduced by the Lancet Commission.121
Data on SHS burden is critical to evaluating health status and as such, for the monitoring and evaluation of health systems performance to achieving universal access to palliative care.124 The number of people with SHS (calculated without a threshold or cutoff in terms of days of SHS experienced) provides a specific insight on palliative care need—an estimated number of patients that need access to palliative care services. Policymakers and practitioners can be guided by the magnitude of SHS within their countries, the distribution of SHS across conditions, age ranges, and geographical locations, and the corresponding need for palliative care, so that they may examine it against the availability of palliative care service. SHS data are hence useful in assessing the need and efficacy of approaches to health system strengthening and UHC, health reforms or across health insurance schemes. Further, the evidence on need can further the argument for adoption of the packages of palliative care services, as was begun with work on the essential package by the Lancet Commission with the Disease Control Priorities (DCP)-3.11 The number of days of SHS is therefore also essential and particularly to measure how need must translate into a health system response such as through an essential package of palliative care services.
Acknowledging this and the previously presented limitations, this paper provides a starting point for further scientific inquiry and consensus-building. The methods described in this paper pave the way forward for future research that examines both the demand side—suffering patterns—and the supply side—ways to address them—for people worldwide. With the methodology to measure SHS, as established by this paper, what’s needed next are better tools to measure the responses to relief, building on existing efforts such as DOME. The next step and complement to this paper is another on DOME that begins to identify access to one fact of palliative care, pain relief medicine, plus a paper looking specifically at SHS in children. Matching DOME and SHS provides an indicator of health system performance and progress over time in delivering palliative care and reducing the unmet burden of SHS.
Estimating the burden of SHS should be a continual endeavor to incorporate scientific, societal, economic, and health care system change into the quest to reduce suffering and improve population health. This must include monitoring advances, but also the challenges that pose a risk to human health and quality of life, including climate change, war, and humanitarian crises. The measurement of serious health-related suffering can serve as a basis for promoting people-centered health systems and analyzing progress toward SDG3 and for future iterations of global health goals and the quest for UHC. It also has the potential to change the focus of today’s healthcare system from diseases alone to suffering. The tools shared in this paper and its contributions toward better conceptualization and measurement of the burden and alleviation of SHS should catalyze this work.
Disclosures and Acknowledgments
We acknowledge support from the University of Miami and U.S. Cancer Pain Relief for this work. The authors are grateful to the Lancet Commission on Palliative Care and Pain Relief Study Group and acknowledged contributors in the Lancet Commission report for their inputs to an earlier iteration of this work. We would also like to thank all palliative care specialists who contributed to expert panels and related Delphi processes for the Lancet Commission and subsequent pediatric specific expert reviews for SHS 2.0. We thank Kathy Foley for her various inputs to the Lancet Commission and beyond to help make this work a reality. We thank Stéphane Verguet for his engagement on related topics as co-chair of the Lancet Commission Working Group on Economic Evaluation. Finally, we thank all individuals who have supported this work in different ways and at varying points. XK and AB report consulting fees from the University of Miami Institute for the Advanced Study of the Americas for part of the submitted work and consulting fees through a research grant from the Medical Research Council to the University of Edinburgh for work related to palliative care outside the submitted work. FMK reports research grant funding to the University of Miami from the U.S. Cancer Pain Relief for part of the submitted work and from ABC Global Alliance outside the submitted work; research grant funding from the Medical Research Council to the University of Miami and FUNSALUD (Mexican Health Foundation) for work related to palliative care outside the submitted work; research grant funding to Tómatelo a Pecho, A.C. from Breast Cancer Now related to palliative care outside the submitted work; research grant funding to Tómatelo a Pecho, A.C. outside submitted work from Merck Sharp & Dohme, Avon Cosmetics; research grant funding to the University of Miami outside submitted work from Merck KGaA/EMD Serono; and personal fees from Merck KGaA/EMD Serono and Tecnológico de Monterrey. FMK is on the board of the IAHPC, Founding President of Tómatelo a Pecho, A.C, and Senior Economist for FUNSALUD. All other authors declare no competing interests.
Acronyms
- AIDS
Acquired immunodeficiency syndrome
- ART
Anti-retroviral treatment
- COPD
Chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease 2019
- DALYs
Disability adjusted life years
- DCP
Disease control priorities
- DOME
Distributed opioid morphine equivalent
- GBD
Global Burden of Disease
- GHE
Global Health Estimates
- GLOBOCAN
Global Cancer Observatory
- HIV
Human immunodeficiency viruses
- IAHPC
International Association for Hospice and Palliative Care
- ICD
International Classification of Diseases
- ICPCN
International Children’s Palliative Care Network
- IHME
Institute for Health Metrics and Evaluation
- LMICs
Low- and middle-income countries
- MDR-TB
Multidrug-resistant tuberculosis
- PLHIV
People Living with HIV
- QALYs
Quality-adjusted life years
- SDG
Sustainable Development Goal
- SHS
Serious health-related suffering
- TB
Tuberculosis
- UHC
Universal health coverage
- UMIA
University of Miami Institute for Advanced Study of the Americas
- WHO
World Health Organization
- WHPCA
Worldwide Hospice Palliative Care Alliance
- XDR-TB
Extensively drug-resistant tuberculosis
Data Appendix
Section 1. Additional materials supporting the construction of the Global SHS database:
Appendix Table 1.
Experts’ consensus building practices undertaken by the LC
| Dates | Activity | Participants | Location | Content Consulted |
|---|---|---|---|---|
|
| ||||
| Between and 2016 | Experts consultation using in-person one-on- one in-depth interviews | 10 palliative care practitioners from 7 LMICs | Rwanda, Vietnam, Mexico, India, Malaysia, Jamaica, Brazil | Multipliers of SHS, types of symptoms, design of the essential package |
| Aug-16 | Group discussion at a Lancet GAPCPR commission meeting | Commissioners, Scientific Advisory Committee Members, Collaborators | Cuernavaca, Mexico | Multipliers of SHS, frequency and duration of symptoms, design of the essential and augmented package, costing framework, etc. |
| ? | A two-stage Delphi | 18 palliative care practitioners from LMICs | virtually | Design of the essential and the augmented package and days in need of palliative care |
| Aug-16 | Expert panel discussions in a virtual meeting and following up in emails | 8 pediatric palliative care specialists from both HICs and LMICs | virtually | Multipliers of SHS for children, additional conditions for children |
Appendix Table2–1.
Delphi Process Results from Round1
| Participant Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1. Should any medicines, equipment, or social supports be added to, or removed from, the essential/highest priority package? If yes, please provide details below. | ||||||||
|
| ||||||||
| Please provide information on what medicines should be added to or rammed from the essential/highest priority package: | should add codein, tramadol, carbamazepine | added: Diclofenac tabs,Tramadol tabs | It would be important to add Acid Tranexamic in my opinion, I do not see so important Fluoxetin or other SSRI if we have Amitriptillin in the list. | added: Midazolan I.V. and Lorazepam oral, Ketorolaco I.V., Metamizol I.V. Olanzapina IV and oral. Paracetmol parenteral. Removed: Diazepam | Nebulization medicines for breathlessness | Should be added: Fentanyl patch and Octeotride parenteral / Should be removed: Loperamide | Entacyd (with Simethicone) oral Body Lotion 3. Antifungal oral 4. Anti fungal ointment | |
| Please provide information on what equipment should be added to or removed from the essential/highest priority package: | convert a suspention cot | NonSterile Gloves, thermometer, BP machine, stethoscope | Antidecubitus materials or medications like Duoderma and Colostoma I do see as high priority to be added | equipment of paracentesis,equipment healing of ulcers and wounds butterfly needles for subcutaneously | nebulizer | Should be added: wheelchairs and 3 positions beds. Bathroom and bath chairs. / Should be removed: Nasogastric drainage or feedin tube | Small kit containing few gauze pads, 1 nail cutter, 1 scissor, 3 syringes - 3 cc, 5 cc, 10 cc, one adhesive micropore | |
| Please provide information on what social support should be added to or removed from the essential/highest priority package: | enough | None | I don’t have any suggestion in this respect | chapel, hostel for families, relaxation room for families, | Ok | Costa Rica provides a special license (Law 7756) for the patient relative who takes care of the terminal patient, during the time he stays alive at home / Should be removed: Cash payment and housing | Education support for 1/2 dependants of the patient | |
|
| ||||||||
| 2. Should any medicines, equipment, or social supports be added to, or removed from, the augmented (second tier) package? If yes, please provide details below. | ||||||||
|
| ||||||||
| Please provide information on what medicines should be added to or remwed from the augmented package: | should add endocrine therapy | added: fentanyl patches, Imodium tabs, Multivitamin, Ion tabs | Not anything to say | added: Midazolan I.V. and Lorazepam oral, Paracetmol parenteral. Removed: Diazepam | Some alternative medicines like prednisolone,pheniramine,spironolactone, | Should be added: Fentanyl patch and Octeotride parenteral / Should be removed: Loperamide | Same as 1 | |
| Please provide information on what equipment should be added to or removed from the augmented package: | convert a suspention cot | added: Wounds dressing packages, NonSterile Gloves | Not anything to say | equipment of paracentesis,equipment healing of ulcers and wounds butterfly needles for subcutaneously | Pulse oximetry | Should be added: wheelchairs and 3 positions beds. Bathroom and bath chairs. / Should be removed: Nasogastric drainage or feedin tube | Same as 1 | |
| Please provide information on what social support should be added to or removed from the augmented packaqe: | should add psychologic support | Not anything to say | chapel, hostel for families, relaxation room for families | Ok | Costa Rica provides a special license (Law 7756) for the patient relative who takes care of the terminal patient, during the time he stays alive at home / Should be removed: Cash payment and housing | Same as 1 | ||
| 3. Please state any suggestions, comments, or concerns you have about the essential/highest priority package. | delivering and using morphine at home | Very good list especially for LMICs needs in PC | ondansetron oral, scopolamine parenteral | Really it would be great if our governments will adopt this package, it would be great for patients and families also for our teams and for the health system | in my country is very important human resource, often exists but there is no way to pay it. Institutions could be responsible for paying the salaries of doctor, nurse and psychologist in palliative care. We also have a small space in the units to meet. | This is highly appreciating work. But I am 100% sure that this will not be universally accessible by everyone everywhere by 2020. | Suggestion: The creation of a “goverment opiod law deliver” for the whole population of terminal patients in low/medium income countries. | Essential/highest priority package should be distributed through a committee formatted by government and non-govern ment members |
| 4. Please state any suggestions, comments, or concerns you have about the augmented package. | control breakthrough pain with rapid onset opioids | I would suggest to add package of education program & activities ( counseling, nutrition education, advocacy...) | Radiology tests, CT Scan or MRI would be very important when these are indicated or needed | in my country is very important human resource, often exists but there is no way to pay it. Institutions could be responsible for paying the salaries of doctor, nurse and psychologist in palliative care. We also have a small space in the units to meet. | There should be action oriented plan! | The principal concern is about the use of Nasogastric drenaige (an invasive method in these stage of a patien illnes) as an option for nutrition. | Same as above | |
| 5. For questions 5.15.22, please provide your estimated range of the number of days that patients with each condition noted below would require palliative care for any reason. NO RESEARCH REQUIRED, ONLY YOUR EXPERT OPINION. | OK but the questions need to be discussed in a session to define at which moment "Palliative care" should start. LMICs & HICs have different approaches. | 15 dias | During the whole process of the patients decease | |||||
| 5.1 Hemorrhagic fevers (includes patients who do not die) | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 3 | 7 | 1 | 7 | 2 | 7 | 15 | 10 |
| Upper bound for average number of days requiring palliative care | 7 | 30 | 10 | 60 | 12 | 90 | 60 | 30 |
| 5.2 Tuberculosis (TB) (Death from TB) | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | N/A | 15 | 30 | 60 | 15 | 90 | 60 | 180 |
| Upper bound for average number of days requiring palliative care | N/A | 90 | 120 | 180–270 | 60 | 365 | 180 | 730 |
| 5.3 Tuberculosis (Death from MDR TB / XDR TB) | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | N/A | 10 | 30 | few weeks | 2 | 30 | 240 | 180 |
| Upper bound for average number of days requiring palliative care | N/A | 60 | 90 | few months | 12 | 180 | 1825–3650 | 730 |
| 5.4 Tuberculosis (On-treatment for MDR TB / XDR TB (outcome uncertain, both cure and death possible) | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | N/A | 8 | 30 | 30 | 2 | 180 | 730 | 180 |
| Upper bound for average number of days requiring palliative care | N/A | 90 | 180 | 180 | 12 | 365 | 3650 | 1000 |
| 5.5 HIV/AIDS | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | N/A | 15 | 30 | 180 | 2 | 180 | 730 | 1000 |
| Upper bound for average number of days requiring palliative care | N/A | 90 | 180 | 365 | 10 | 365 | 3650 | 1000 |
| 5.6 Malignant neoplasms (Death from malignant neoplasms) | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 5 | 8 | 30 | 180 | 5 | 30 | 120 | 10 |
| Upper bound for average number of days requiring palliative care | 30 | 30 | 90 | 365 | 20 | 365 | 730 | 100 |
| 5.7 Malignant neoplasms (Survivors of malignant neoplasms) | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 7 | 7 | 30 | 90 | 10 | 365 | 730 | 10 |
| Upper bound for average number of days requiring palliative care | 90 | 60 | 180 | 730 | 60 | 1825 | 3650 | 1500 |
| 5.8 Leukemia | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 5 | 8 | 30 | 180 | 15 | 30 | 365 | 10 |
| Upper bound for average number of days requiring palliative care | 30 | 60 | 180 | 365 | 120 | 90 | 1825 | 1000 |
| 5.9 Dementia | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 30 | 10 | 30 | 180 | 30 | 30 | 1825 | 30 |
| Upper bound for average number of days requiring palliative care | 180 | 60 | 365 | 730–1095 | 90 | 180 | 3650 | 2000 |
| 5.10 Inflammatory disease of CNS | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 14 | 7 | 30 | 7–14 | 90 | 30 | 1825 | 10 |
| Upper bound for average number of days requiring palliative care | 30 | 30 | 365 | 150–180 | 180 | 90 | 3650 | 30 |
| 5.11 Following disease types: (a) Extrapyramidal & movement disorders, (b) Other degenerative diseases of the CNS, (c) Demyelinating disease of the CNS, (d) Epilepsy, (e) Cerebral palsy & other paralytic syndromes | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 20 | 30 | 30 | 30 | 90 | 180 | 1825 | 30 |
| Upper bound for average number of days requiring palliative care | 90 | 120 | 365 | 730–1095 | 180 | 365 | 3650 | 1000 |
| 5.12 Cerebrovascular disease | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 20 | 12 | 30 | 7 | 15 | 90 | 1825 | 30 |
| Upper bound for average number of days requiring palliative care | 90 | 30 | 180 | 120–150 | 30 | 180 | 3650 | 1000 |
| 5.13 Following disease types: (a) Chronic rheumatic heart diseases, (b) Cardiomyopathy & Heart failure | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 15 | 7 | 1 | 14–21 | 30 | 30 | 365 | 30 |
| Upper bound for average number of days requiring palliative care | 60 | 21 | 90 | 365 | 90 | 90 | 3650 | 1000 |
| 5.14 Chronic ischemic heart disease | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 15 | 7 | 30 | 14–21 | 90 | 180 | 365 | 30 |
| Upper bound for average number of days requiring palliative care | 30 | 20 | 180 | 365 | 180 | 365 | 3650 | 3000 |
| 5.15 Following disease types: (a) Chronic lower respiratory disease, (b) Lung disease due to external agents, (c) Interstitial lung disease, (d) Other diseases of the respiratory system | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 7 | 30 | 30 | 30 | 15 | 30 | 1095 | 30 |
| Upper bound for average number of days requiring palliative care | 60 | 120 | 700 | 365 | 90 | 365 | 3650 | 3000 |
| 5.16 Diseases of the liver | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 7 | 30 | 30 | 30 | 15 | 180 | 730 | 30 |
| Upper bound for average number of days requiring palliative care | 60 | 120 | 120 | 270 | 60 | 365 | 3650 | 3000 |
| 5.17 Renal failure | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 7 | 30 | 30 | 30 | 7 | 30 | 730 | 30 |
| Upper bound for average number of days requiring palliative care | 90 | 120 | 90 | 180 | 15 | 180 | 1825 | 3000 |
| 5.18 Following disease types: (a) Low birth weight & prematurity, (b) Birth trauma | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | N/A | 14 | 1 | 30 | no data | 7 | 180 | 30 |
| Upper bound for average number of days requiring palliative care | N/A | 30 | 30 | some months | no data | 90 | 365 | 4000 |
| 5.19 Congenital malformations | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | N/A | 5 | 1 | 30 | no data | 180 | 365 | 30 |
| Upper bound for average number of days requiring palliative care | N/A | 14 | 30 | several years | no data | 365 | 7300 | life long |
| 5.20 Injury, poisoning, external causes | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 7 | 7 | 1 | 7 | 15 | 7 | 365 | 10 |
| Upper bound for average number of days requiring palliative care | 30 | 20 | 30 | 60–90 | 30 | 30 | 1825 | 30 |
| 5.21 Atherosclerosis | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | N/A | 30 | 30 | 30 | 180 | 90 | 365 | 30 |
| Upper bound for average number of days requiring palliative care | N/A | 60 | 700 | 365 | 365 | 180 | 3650 | 3000 |
| 5.22 Musculoskeletal disorders | ||||||||
|
| ||||||||
| Lower bound for average number of days requiring palliative care | 15 | 30 | 30 | 30 | 90 | 90 | 1095 | 30 |
| Upper bound for average number of days requiring palliative care | 30 | 120 | 700 | several years | 180 | 365 | 3650 | 1000 |
Appendix Table2–2.
Delphi Process Results from Round2
| DELPHI ROUND 2 RESULTS: DURATION PALLIATIVE CARE IS REQUIRED CONDITION | Mean lower bound (days) | SD +/− | Mean upper bound(days) | SD +/− |
|---|---|---|---|---|
| Hemorrhagic fevers (includes patients who do not die) | 13 | 12 | 40 | 35 |
| Tuberculosis (TB) (Death from TB) | 80 | 68 | 184 | 133 |
| Tuberculosis (Death from MDR TB / XDR TB) | 75 | 31 | 168 | 97 |
| Tuberculosis (On-treatment for MDR TB / XDR TB (outcome uncertain, both cure and death possible) | 83 | 39 | 288 | 283 |
| HIV/AIDS | 150 | 117 | 316 | 216 |
| Malignant neoplasms (Death from malignant neoplasms) | 44 | 26 | 178 | 76 |
| Malignant neoplasms (Survivors of malignant neoplasms) | 195 | 140 | 768 | 634 |
| Leukemia | 85 | 56 | 249 | 132 |
| Dementia | 148 | 79 | 599 | 328 |
| Inflammatory disease of CNS | 78 | 32 | 241 | 111 |
| Following disease types: (a) Extrapyramidal & movement disorders, (b) Other degenerative diseases of the CNS, (c) Demyelinating disease of the CNS, (d) Epilepsy, (e) Cerebral palsy & other paralytic syndromes |
173 | 111 | 578 | 361 |
| Cerebrovascular disease | 150 | 117 | 419 | 229 |
| Following disease types: (a) Chronic rheumatic heart diseases, (b) Cardiomyopathy & Heart failure | 95 | 62 | 433 | 327 |
| Chronic ischemic heart disease | 83 | 67 | 349 | 275 |
| Following disease types: (a) Chronic lower respiratory disease, (b) Lung disease due to external agents, (c) Interstitial lung disease, (d) Other diseases of the respiratory system | 83 | 67 | 696 | 800 |
| Diseases of the liver | 85 | 66 | 433 | 327 |
| Renal failure | 88 | 65 | 436 | 219 |
| Following disease types: (a) Low birth weight & prematurity, (b) Birth trauma | 32 | 22 | 443 | 705 |
| Congenital malformations | 123 | 111 | 430 | 332 |
| Injury, poisoning, external causes | 27 | 7 | 202 | 98 |
| Atherosclerosis | 88 | 65 | 324 | 83 |
| Musculoskeletal disorders | 83 | 67 | 479 | 291 |
Appendix Table 3.
Literature review on prevalence of the most commonly reported types of physical suffering among patients with serious, complex or life-limiting health problems.
| Pain Mild | Pain Mod/Severe | Dyspnea | Fatigue | Weakness | Nausea and/or vomiting | Diarrhea | Constipation | Dry Mouth | Pruritus | Bleeding | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | ref | p | ref | p | ref | p | ref | p | ref | p | ref | p | ref | p | ref | p | ref | p | ref | p | ref | |
|
| ||||||||||||||||||||||
| Hemorrh agic fevers | 81% | Qin, E, 2015 | 14.30% | Qin, E, 2015 | 71.40% | Qin, E, 2015 | 100% | Qin, E, 2015 | 24.2% – 57% | Qin, E, 2015; Thomas, E, 2007 | 66.70% | Qin, E, 2015 | 27.60 % | Thomas, E, 2007 | 8–57% | Qin, E, 2015; Thomas, E, 2007 | ||||||
| TB/M/XD R TB | 5.6–30% | Marais, B. J, 2005; Bark, C. M. 2011 | 16.70% | Marais, B. J, 2005 | ||||||||||||||||||
| HIV disease | 30–98% | VietNam National Palliative Care Report; Harding, R., 2012; Solano, J. P., 2006; Moens, K., 2014; Vogl, D., 1999 | 64–64.70% | VietNam National Palliative Care Report; Harding, R., 2012 | 11–62% | Harding, R., 2012; Solano, J. P., 2006; Vogl, D., 1999 | 43–95% | Harding, R., 2012; Solano, J. P., 2006; Moens, K., 2014 | 71.9–85.5% | Harding, R., 2012; Vogl, D., 1999 | 21–60.7% | VietNam National Palliative Care Report; Harding, R., 2012; Solano, J. P., 2006; Moens, K., 2014; Vogl, D., 1999 | 24.6–90% | Harding, R., 2012; Solano, J. P., 2006; Moens, K., 2014; Vogl, D., 1999 | 34–38.1% | Harding, R., 2012; Solano, J. P., 2006; Vogl, D., 1999 | 61.6–67.6% | Harding, R., 2012; Vogl, D., 1999 | 49–58.9% | VietNam National Palliative Care Report; Harding, R., 2012; Vogl, D., 1999 | ||
| Malignant neoplasms (except C91–95) | 30–96% | VietNam National Palliative Care Report; Teunissen, S. C., 2007; Solano, J. P., 2006; Moens, K., 2014; Tranmer, J. E., 2003 | 66% | VietNam National Palliative Care Report | 10–77% | Teunissen, S. C., 2007; Dudgeon, D. J., 2001; Solano, J. P., 2006; Moens, K., 2014; Tranmer, J. E., 2003 | 23–100% | Teunissen, S. C., 2007; Solano, J. P., 2006; Moens, K., 2014 | 60% | Teunissen, S. C., 2007 | 2–78% | VietNam National Palliative Care Report; Teunissen, S. C., 2007; Solano, J. P., 2006; Moens, K., 2014; Tranmer, J. E., 2003 | 3–29% | Teunissen, S. C., 2007; Solano, J. P., 2006; Moens, K., 2014; Tranmer, J. E., 2003 | 4–65% | Teunissen, S. C., 2007; Solano, J. P., 2006; Moens, K., 2014; Tranmer, J. E., 2003 | 40–82% | Teunissen, S. C., 2007; Tranmer, J. E., 2003 | 0–24% | VietNam National Palliative Care Report; Teunissen, S. C., 2007; Tranmer, J. E., 2003 | 15% | Teunissen, S. C., 2007 |
| Leukemia | 28.2–54.3% | Collins, J. J., 2000; Huljer, H. A. S., 2013 | 21.90% | Collins, J. J., 2000 | 15.4–63% | Collins, J. J., 2000; Huljer, H. A. S., 2013 | 43.8+12.5% – 39.1+23.9% | Collins, J. J., 2000; Huijer, H. A. S., 2013 | 21.90% | Collins, J. J., 2000 | 6.30% | Collins, J. J., 2000 | 21.9–26.1% | Collins, J. J., 2000; Huijer, H. A. S., 2013 | 15.4–40.6% | Collins, J. J., 2000; Huijer, H. A. S., 2013 | ||||||
| Dementia | 14–63% | Moens, K., 2014 | 12–52% | Moens, K., 2014 | 22% | Moens, K., 2014 | 8% | Moens, K., 2014 | 40% | Moens, K., 2014 | ||||||||||||
| Degenera tion of CNS | 42–85% | Moens, K., 2014 | 26% | Moens, K., 2014 | 42–80% | Moens, K., 2014 | 26% | Moens, K., 2014 | 24–46% | Moens, K., 2014 | ||||||||||||
| Non-ischemic heart disease | 14–78% | Solano, J. P., 2006; Moens, K., 2014 | 18–88% | Ahmed, A., 2006; Solano, J. P., 2006; Moens, K., 2014 | 42–82% | Solano, J. P., 2006; Moens, K., 2014 | 2–48% | Solano, J. P., 2006; Moens, K., 2014 | 12% | Solano, J. P., 2006 | 12–42% | Solano, J. P., 2006; Moens, K., 2014 | ||||||||||
| Chronic ischemic heart disease | 41–77% | Solano, J. P., 2006 | 60–88% | Solano, J. P., 2006 | 69–82% | Solano, J. P., 2006 | 17–48% | Solano, J. P., 2006 | 12% | Solano, J. P., 2006 | 38–42% | Solano, J. P., 2006 | ||||||||||
| Lung Diseases | 21–77% | Solano, J. P., 2006; Moens, K., 2014 | 56–98% | Solano, J. P., 2006; Moens, K., 2014 | 32–90% | Solano, J. P., 2006; Moens, K., 2014 | 4% | Moens, K., 2014 | 12–44% | Solano, J. P., 2006; Moens, K., 2014 | ||||||||||||
| N17–19: Renal failure | 11–83% | Solano, J. P., 2006; Murtagh, F. E., 2010; Moens, K., 2014 | 11–82% | Solano, J. P., 2006; Murtagh, F. E., 2010; Moens, K., 2014 | 13–100% | Solano, J. P., 2006; Murtagh, F. E., 2010; Moens, K., 2014 | 8–59% | Solano, J. P., 2006; Murtagh, F. E., 2010; Moens, K., 2014 | 8–36% | Solano, J. P., 2006; Murtagh, F. E., 2010; Moens, K., 2014 | 8–70% | Solano, J. P., 2006; Murtagh, F. E., 2010; Moens, K., 2014 | 69% | Murtagh, F. E., 2010 | 84% | Murtagh, F. E., 2010 | ||||||
Appendix Table 4.
List of literature review used in calculating the 1-year survival of patients with cerebrovascular disease by income group and by year
| Article | Year | Population | Subtype | N | Probability of death within 28 days | Probability of death at 1 year | ||
|---|---|---|---|---|---|---|---|---|
| Anderson, C. S., Jamrozik, K. D., Broadhurst, R. J., & Stewart-Wynne, E. G. (1994) | Ischemic | 247 | 30 | 12% | 63 | 26% | ||
| Heomrragic | 44 | 13 | 30% | 17 | 39% | |||
| 1994 | Perth, Western Australia | Subcranial | 18 | 6 | 33% | 8 | 44% | |
| Dennis, M. S., Burn, J. P., Sandercock, P. A., Bamford, J. M., Wade, D. T., & Warlow, C. P. (1993) | Ischemic | 545 | 57 | 10% | 125 | 23% | ||
| Heomrragic | 66 | 34 | 52% | 41 | 62% | |||
| 1993 | Comunidad de Oxfordshire, UK | Subcranial | 33 | 15 | 45% | 16 | 48% | |
| Hankey, G. J., Jamrozik, K., Broadhurst, R. J., Forbes, S., Burvill, P. W., Anderson, C. S., & Stewart-Wynne, E. G. (2000) | 12% | |||||||
| 32% | ||||||||
| 2000 | Perth, Western Australia | 38% | ||||||
| Ischemic | 613 | 171 | 28% | 252 | 41% | |||
| Heomrragic | 223 | 130 | 58% | 138 | 62% | |||
| 2006 | Bosnia-Hz | Subcranial | ||||||
| Sacco, R. L., Wolf, P. A., Kannel, W. B., & McNamara, P. M. (1982) | Ischemic | 111 | 17 | 15% | ||||
| Heomrragic | 10 | 8 | 82% | |||||
| 1982 | The Framingham study | Subcranial | 16 | 7 | 46% | |||
| petty, G. W., Brown Jr, R. D., Whisnant, J. P., Sicks, J. D., O’fallon, W. M., & Wiebers, D. O. (2000) | Ischemic | 16% | ||||||
| 1996 | Rochester, Minnesota | Heomrragic | 58% | |||||
| Subcranial | 37% | |||||||
| Brønnum-Hansen, H., Davidsen, M., & Thorvaldsen, P. (2001) | Ischemic | |||||||
| Heomrragic | 4162 | 28% | 41% | |||||
| Subcranial | ||||||||
| Hardie, K., Hankey, G. J., Jamrozik, K., Broadhurst, R. J., & Anderson, C. (2003) | Ischemic | 173 | 16 | 9% | 45 | 26% | ||
| Heomrragic | 32 | 12 | 38% | 14 | 44% | |||
| 2003 | Perth, Western Australia | Subcranial | 10 | 5 | 50% | 5 | 50% | |
| Feng, W., Hendry, R. M., & Adams, R. J. (2010) | 8848 | 1062 | 12% | 1947 | 22% | |||
| 1227 | 380 | 31% | 503 | 41% | ||||
| 2010 | South Carolina | 324 | 75 | 23% | 100 | 31% | ||
Section 2. Additional data processing: Data aggregation, adjustments, and confidence intervals
2.1. Grouping conditions
All 21 groups of conditions associated with SHS are further grouped into communicable diseases, non-communicable diseases, and injuries using the categorization of IHME i.e.: 1) communicable diseases, which includes hemorrhagic fever, tuberculosis, HIV, and infectious diseases of the central neural system, 2) injuries, and 3) non-communicable diseases, which includes malignant neoplasms (except leukemia), leukemia, dementia, degenerative diseases of the central neuron system such as Parkinson’s diseases and multiple sclerosis, cerebrovascular diseases, chronic rheumatic heart diseases, cardiomyopathy and heart failure, chronic ischemic heart diseases, lung diseases, diseases of liver, renal failure, low birth weight and prematurity, birth trauma, congenital malformations, atherosclerosis, musculoskeletal disorders, protein-energy malnutrition, and endocrine, metabolic, blood and immune disorders. Unlike in IHME/GBD data, maternal, child and nutritional causes are grouped together with other non-communicable diseases for the purpose of analyzing SHS2.0.
2.2. Double counting
When calculating the total burden of SHS in non-decedents, double counting could be a major cause of over-estimation since country data aggregate numbers across all conditions. This is addressed in SHS2.0 for co-morbidities in non-decedent categories of four conditions identified as the most likely sources of double counting: Kaposi sarcoma in HIV patients, cancer, cerebrovascular diseases, and dementia. After analyzing the data, the following adjustments were undertaken: 1) Kaposi sarcoma patients are only counted for HIV but not for malignant neoplasms; 2) cancer patients with dementia and/or cerebrovascular disease are counted for cancer but not for the other two conditions; and 3) among non-cancer patients, those with cerebrovascular disease and dementia are counted for cerebrovascular disease but not for dementia. Details of the algorithm for these adjustments are presented in Appendix Panel 1.
2.3. Confidence intervals
Another improvement in SHS 2.0 is the use of relevant data from the IHME/GBD database to generate confidence intervals. Confidence intervals were constructed based on the IHME data for each of the 21 health conditions considered according to mortality and prevalence in the corresponding GBD codes for each year. For this, based on the mean values and the limits of variation reported by the IHME itself, 1,000 iterations of assuming a normal distribution around the mean and with standard deviation equal to where is the standard deviation with respect to the mean of condition , in country , in age group , and sex were carried out. Likewise, corresponds to the estimated mean value of SHS based on the data reported by IHME in the GBD data for condition , in country , in age group , and sex ; while represents the upper limit of SHS; and 1.96 corresponds to the z-score for a 95% confidence interval in a standard normal distribution. This process was stratified by year, health condition, country, age group, and sex. Following this, SHS multipliers were applied to either the prevalence of mortality in each iteration to derive a specific SHS value. The mean of these values across the 1,000 iterations was considered the expected value. The 95% confidence interval was then defined by the range from the 2.5th to the 97.5th percentiles of these values.
Appendix Panel 1:
Details on two adjustments to remove overlap between conditions and related double counting
| Removing Kaposi Sarcoma (KS) from cancer survivors experiencing SHS |
| Almost all KS patients are also PLWHIVs, and they can account for up to 26% of total cancer survivors in some countries, causing a considerable overestimation of the number of patients in need of palliative care. Since KS patients’ experiences of suffering are more closely aligned with typical HIV patients than cancer patients, KS patients are removed from the cancer non-decedents. |
| Country-specific KS prevalence data are available from GLOBOCAN IARC cancer registries (note that GBD does not separately estimate KS). The 2018 data are used since only the most recent GLOBOCAN data include KS prevalence. Country-specific total cancer prevalence can also be downloaded from GLOBOCAN for consistency reasons. A KS % is calculated for each country: KS % = country-specific KS prevalence / country-specific total cancer prevalence. This percentage is then used to adjust the cancer non-decedents in need of PC for each country such that: Cancer non-decedents after adjustment = Cancer non-decedents before adjustment * (1-KS%). No KS adjustment is made to the years 1990, 2000 and 2010 due to data availability limitations. |
| Removing overlap among cancer, dementia and stroke survivors experiencing SHS |
| Cancer, dementia, and stroke are selected among all non-decedent categories to adjust for overlaps because: 1) they share patients with similar age profiles; and 2) there is a large number of total global survivors thus the overestimation caused by double counting could pose as serious issue. The following two assumptions are applied: 1) the prevalence of each disease is independent of each other, meaning that a cancer patient has the same possibility of living with dementia as the general population; 2) the suffering hierarchy is as follows: cancer > cerebrovascular disease > dementia, meaning that people with more than one condition will only be counted under the one condition causing the greatest suffering. Prevalence rate is calculated for each country, which is downloaded from the IHME database. The recommended formula. to remove double counting among cancer, cerebrovascular disease and dementia patients is as follows: |
| Cancer(C) | cerebrovascular disease (V) | Dementia (D) | |
|---|---|---|---|
| Non-decedents category include: | C, C-D, C-V, C-D-V | V, V-D, C-V, C-D-V | D, C-D, V-D, C-D-V |
| Non-decedents category include adjusted: | C, C-D, C-V, C-D-V | V, V-D | D |
| Adjustment | No changes | Pv*(1-Pc) | Pd*(1-Pc)*(1-Pv) |
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jpainsymman.2024.03.027.
References
- 1.Knaul FM, Farmer PE, Krakauer EL, et al. Alleviating the access abyss in palliative care and pain relief—an imperative of universal health coverage: the Lancet Commission report. Lancet 2018;391:1391–1454. [DOI] [PubMed] [Google Scholar]
- 2.United Nations Department of Economic and Social Affairs. Available at: https://sdgs.un.org/goals. Accessed March 5, 2024.
- 3.World Health Organization. Strengthening of palliative care as a component of comprehensive care throughout the life course. 2014. Available at: https://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R19-en.pdf. Accessed March 5, 2024. [DOI] [PubMed] [Google Scholar]
- 4.Connor S, Bermedo M, Baxter S, et al. Global Atlas of Palliative Care at the End of Life. World Health Organization and Worldwide Palliative Care Alliance 2014; 2014. [Google Scholar]
- 5.Global Burden of Disease Study 2019 (GBD 2019) Results. In: Seattle, United States: Institute for Health Metrics and Evaluation (IHME), 2019. Available at: https://www.healthdata.org/data-tools-practices/interactive-visuals/gbd-results. Accessed March 5, 2021. [Google Scholar]
- 6.Krikorian A, Limonero JT. An integrated view of suffering in palliative care. J Palliat Care 2012;28:41–49. [PubMed] [Google Scholar]
- 7.Gutiérrez-Sánchez D, Gómez-García R, Cuesta-Varga s AI, Pérez-Cruzado D. The suffering measurement instruments in palliative care: a systematic review of psychometric properties. Int J Nurs Studies 2020;110:103704. [DOI] [PubMed] [Google Scholar]
- 8.Knaul FM, Farmer PE, Krakauer EL, et al. Technical note and data appendix for “alleviating the access abyss in palliative care and pain relief—an imperative of universal health coverage: the Lancet Commission report”. 2017. Available at: https://www.mia.as.miami.edu/_assets/pdf/data-appendix-lcgapcpc-oct122017_xk-4-22-201.pdf. Accessed March 5, 2024. [DOI] [PubMed] [Google Scholar]
- 9.International Narcotic Control Board. Progress in ensuring adequate access to internationally controlled substances for medical and scientific purposes. In: Vienna: UN, 2019. Available at: https://digitallibrary.un.org/record/3825815?ln=en&v=pdf, Accessed March 5, 2024. [Google Scholar]
- 10.Connor S, Morris C, Jaramillo E, et al. Global atlas of palliative care. 2nd ed. London, UK: Worldwide palliative care alliance; 2020. Available at: https://www.paho.org/en/node/75063 Accessed March 5, 2024. [Google Scholar]
- 11.Krakauer EL, Kwete X, Verguet S, Arreola-Ornelas H, Bhadelia A, Mendez O, Rodriguez NM, Ali Z, Allende S, Cleary JF, Connor S, Danforth K, Lima LD, Gwyther L, Hamzah E, Jamison DT, Khanh QT, Kumar S, Luyirika E, Merriman A, Mpanumusingo E, Nevzorova D, Ntizimira C, Osman H, Perez-Cruz P, Rajagopal MR, Radbruch L, Spence D, Stoltenberg M, Tapela N, Watkins DA, Knaul F. Palliative care and pain control. In: Jamison DT, Gelband H, Horton S, Jha P, Laxminarayan R, Mock CN, Nugent R, eds. Disease Control Priorities: Improving Health and Reducing Poverty, 3rd ed., Washington DC: The International Bank for Reconstruction and Development / The World Bank; 2017. Chapter 12. [Google Scholar]
- 12.Radbruch L, De Lima L, Knaul F, et al. Redefining palliative care—a new consensus-based definition. J Pain Symptom Manag 2020;60:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Palliative care. 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/palliative-care. Accessed January 15, 2024.
- 14.Almanasreh E, Moles R, Chen TF. Evaluation of methods used for estimating content validity. Res Social Administr Pharmacy 2019;15:214–221. [DOI] [PubMed] [Google Scholar]
- 15.Dalkey N, Helmer O. An experimental application of the Delphi method to the use of experts. Manag Sci 1963;9:458–467. [Google Scholar]
- 16.McKenna HP. The Delphi technique: a worthwhile research approach for nursing? J Adv Nurs 1994;19:1221–1225. [DOI] [PubMed] [Google Scholar]
- 17.Goodman CM. The Delphi technique: a critique. J Adv Nurs 1987;12:729–734. [DOI] [PubMed] [Google Scholar]
- 18.Keeney S, Hasson F, McKenna HP. A critical review of the Delphi technique as a research methodology for nursing. Int J Nurs Studies 2001;38:195–200. [DOI] [PubMed] [Google Scholar]
- 19.Connor SR, Downing J, Marston J. Estimating the global need for palliative care for children: a cross-sectional analysis. J Pain Symptom Manag 2017;53:171–177. [DOI] [PubMed] [Google Scholar]
- 20.United Nations. UNAIDS data 2019. 2019. Available at: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf. Accessed January 15, 2024. [Google Scholar]
- 21.International Agency for Research on Cancer. Cancer Today. 2019. Available at: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1. Accessed January 15, 2024. [Google Scholar]
- 22.West TE, von Saint Andre-von Arnim A. Clinical presentation and management of severe Ebola virus disease. Annals Am Thor Soc 2014;11:1341–1350. [DOI] [PubMed] [Google Scholar]
- 23.Schieffelin JS, Shaffer JG, Goba A, et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. New Engl J Med 2014;371:2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dallatomasina S, Crestani R, Sylvester Squire J, et al. Ebola outbreak in rural West Africa: epidemiology, clinical features and outcomes. Trop Med Int Health 2015;20:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacNeil A, Farnon EC, Wamala J, et al. Proportion of deaths and clinical features in Bundibugyo Ebola virus infection, Uganda. Emerging infectious diseases 2010;16:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boozary AS, Farmer PE, Jha AK. The Ebola outbreak, fragile health systems, and quality as a cure. Jama 2014;312:1859–1860. [DOI] [PubMed] [Google Scholar]
- 27.Harding R, Foley KM, Connor SR, Jaramillo E. Palliative and end-of-life care in the global response to multidrug-resistant tuberculosis. Lancet Infect Dis 2012;12:643–646. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. (2014). Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization. Available at: https://www.who.int/publications/i/item/9789241548809. Accessed May 2, 2024. [PubMed] [Google Scholar]
- 29.Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tubercul Lung Dis 2004;8:1382–1384. [PubMed] [Google Scholar]
- 30.Moens K, Higginson IJ, Harding R, et al. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manag 2014;48:660–677. [DOI] [PubMed] [Google Scholar]
- 31.Harding R, Selman L, Agupio G, et al. Prevalence, burden, and correlates of physical and psychological symptoms among HIV palliative care patients in sub-Saharan Africa: an international multicenter study. J Pain Symptom Manag 2012;44:1–9. [DOI] [PubMed] [Google Scholar]
- 32.Vogl D, Rosenfeld B, Breitbart W, et al. Symptom prevalence, characteristics, and distress in AIDS outpatients. J Pain Symptom Manag 1999;18:253–262. [DOI] [PubMed] [Google Scholar]
- 33.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manag 2006;31:58–69. [DOI] [PubMed] [Google Scholar]
- 34.McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? Aids 2010;24:1367–1370. [DOI] [PubMed] [Google Scholar]
- 35.Namisango E, Harding R, Atuhaire L, et al. Pain among ambulatory HIV/AIDS patients: multicenter study of prevalence, intensity, associated factors, and effect. J Pain 2012;13:704–713. [DOI] [PubMed] [Google Scholar]
- 36.Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: a systematic review. J Int AIDS Soc 2014;17:18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sims A, Hadigan C. Cardiovascular complications in children with HIV infection. Current HIV/AIDS Rep 2011;8:209–214. [DOI] [PubMed] [Google Scholar]
- 38.Simms V, Higginson IJ, Harding R. Integration of palliative care throughout HIV disease. Lancet Infect Dis 2012;12:571–575. [DOI] [PubMed] [Google Scholar]
- 39.Teunissen SCCM, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manag 2007;34:94–104. [DOI] [PubMed] [Google Scholar]
- 40.Tranmer JE, Heyland D, Dudgeon D, et al. Measuring the symptom experience of seriously ill cancer and non-cancer hospitalized patients near the end of life with the Memorial Symptom Assessment Scale. J Pain Symptom Manag 2003;25:420–429. [DOI] [PubMed] [Google Scholar]
- 41.van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen, Janssen DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag 2016;51:1070–1090.e9. [DOI] [PubMed] [Google Scholar]
- 42.International Agency for Research on Cancer. Global Cancer Observatory, Cancer Facts Sheet. 2019. Available at: https://gco.iarc.fr/. Accessed January 15, 2024. [Google Scholar]
- 43.Shi Q, Smith TG, Michonski JD, et al. Symptom burden in cancer survivors 1 year after diagnosis. Cancer 2011;117:2779–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zucca AC, Boyes AW, Linden W, Girgis A. All’s well that ends well? Quality of life and physical symptom clusters in long-term cancer survivors across cancer types. J Pain Symptom Manag 2012;43:720–731. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. New Engl J Med 2009;361:1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Geriatrics Society Ethics C, Clinical P, Models of Care C. American Geriatrics Society feeding tubes in advanced dementia position statement. J Am Geriatr Soc 2014;62:1590–1593. [DOI] [PubMed] [Google Scholar]
- 47.Teno JM, Gozalo PL, Lee IC, et al. Does hospice improve quality of care for persons dying from dementia? J Am Geriatr Soc 2011;59:1531–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riedel O, Klotsche J, Spottke A, et al. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson’s disease. J Neurol 2010;257:1073–1082. [DOI] [PubMed] [Google Scholar]
- 49.Dissanayaka NNW, Sellbach A, Matheson S, et al. Anxiety disorders in Parkinson’s disease: prevalence and risk factors. Movement Disord 2010;25:838–845. [DOI] [PubMed] [Google Scholar]
- 50.Rosenbaum RB. Understanding parkinson’s disease: a personal and professional view. USA: Bloomsbury Publishing; 2006. [Google Scholar]
- 51.Téllez-Zenteno JF, Ronquillo LH, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 2010;89:310–318. [DOI] [PubMed] [Google Scholar]
- 52.de Cerqueira AC, Semionato de Andrade P, Godoy Barreiros JM, Teixeira AL, Nardi AE. Psychiatric disorders in patients with multiple sclerosis. Compreh Psychiatry 2015;63:10–14. [DOI] [PubMed] [Google Scholar]
- 53.Patterson K, Marshall JC, Coltheart M. Surface dyslexia. In: Neuropsychological and Cognitive Studies of Phonological Reading, London: Routledge; 2017. [Google Scholar]
- 54.Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 2014;83:1022–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rolak LA. Multiple sclerosis: it’s not the disease you thought it was. Clin Med Res 2003;1:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis. Neurology 2014;83:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brønnum-Hansen H, Koch-Henriksen N, Hyllested K. Survival of patients with multiple sclerosis in Denmark: a nationwide, long–term epidemiologic survey. Neurology 1994;44:1901–1907. [DOI] [PubMed] [Google Scholar]
- 58.Kalia LV, Kalia SK, Lang AE. Disease-modifying strategies for Parkinson’s disease. Movement Disord 2015;30:1442–1450. [DOI] [PubMed] [Google Scholar]
- 59.Shang Q, Ma CY, Lv N, et al. Clinical study of cerebral palsy in 408 children with periventricular leukomalacia. Exp Ther Med 2015;9:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirsh AT, Gallegos JC, Gertz KJ, Engel JM, Jensen MP. Symptom burden in individuals with cerebral palsy. J Rehabil Res Dev 2010;47:863–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.About Parkinson’s. 2024. Available at: http://www.epda.eu.com/about-parkinson-s/. Accessed January 15, 2024.
- 62.Schnitzler A, Woimant F, Nicolau J, Tuppin P, de Peretti C. Effect of rehabilitation setting on dependence following stroke: an analysis of the French Inpatient Database. Neurorehabilit Neural Repair 2013;28:36–44. [DOI] [PubMed] [Google Scholar]
- 63.Krishnamurthi RV, Moran AE, Feigin VL, et al. Stroke prevalence, mortality and disability-adjusted life years in adults aged 20–64 years in 1990–2013: data from the Global Burden of Disease 2013 study. Neuroepidemiology 2015;45:190–202. [DOI] [PubMed] [Google Scholar]
- 64.Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology 2015;45:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boysen G, Marott JL, Grønbæk M, Hassanpour H, Truelsen T. Long-term survival after stroke: 30 years of follow-up in a cohort, the Copenhagen City Heart study. Neuroepidemiology 2009;33:254–260. [DOI] [PubMed] [Google Scholar]
- 66.Brønnum-Hansen H, Davidsen M, Thorvaldsen P. Long-term survival and causes of death after stroke. Stroke 2001;32:2131–2136. [DOI] [PubMed] [Google Scholar]
- 67.De Wit L, Putman K, Baert I, et al. Anxiety and depression in the first six months after stroke. A longitudinal multicentre study. Disab Rehabilit 2008;30:1858–1866. [DOI] [PubMed] [Google Scholar]
- 68.Broomfield NM, Quinn TJ, Abdul-Rahim AH, Walters MR, Evans JJ. Depression and anxiety symptoms post-stroke/TIA: prevalence and associations in cross-sectional data from a regional stroke registry. BMC Neurol 2014;14:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klimiec E, Dziedzic T, Kowalska K, et al. PRospective Observational POLIsh study on post-stroke delirium (PROPOLIS): methodology of hospital-based cohort study on delirium prevalence, predictors and diagnostic tools. BMC Neurol 2015;15:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brainin M, Heiss W-D. Textbook of stroke medicine. Cambridge, England: Cambridge University Press; 2019. [Google Scholar]
- 71.Rustad JK, Stern TA, Hebert KA, Musselman DL. Diagnosis and treatment of depression in patients with congestive heart failure: a review of the literature. Prim Care Compan CNS Disord 2013;15:26254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure. Circulation 2006;114:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cully JA, Johnson M, Moffett ML, Khan M, Deswal A. Depression and anxiety in ambulatory patients with heart failure. Psychosomatics 2009;50:592–598. [DOI] [PubMed] [Google Scholar]
- 74.Jiang W, Kuchibhatla M, Cuffe MS, et al. Prognostic value of anxiety and depression in patients with chronic heart failure. Circulation 2004;110:3452–3456. [DOI] [PubMed] [Google Scholar]
- 75.Havranek EP, Ware MG, Lowes BD. Prevalence of depression in congestive heart failure. Am J Cardiol 1999;84:348–350. [DOI] [PubMed] [Google Scholar]
- 76.Vaccarino V, Kasl Stanislav V, Abramson J, Krumholz Harlan M. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am College Cardiol 2001;38:199–205. [DOI] [PubMed] [Google Scholar]
- 77.Bankier B, Januzzi JL, Littman AB. The high prevalence of multiple psychiatric disorders in stable outpatients with coronary heart disease. Psychosomatic Med 2004;66:645–650. [DOI] [PubMed] [Google Scholar]
- 78.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Resp Crit Care Med 2011;183:431–440. [DOI] [PubMed] [Google Scholar]
- 79.Lanken PN, Terry PB, DeLisser HM, et al. An Official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Resp Crit Care Med 2008;177:912–927. [DOI] [PubMed] [Google Scholar]
- 80.John H, Kevin G-J, June R, et al. The distribution of COPD in UK general practice using the new GOLD classification. Eur Resp J 2014;43:993. [DOI] [PubMed] [Google Scholar]
- 81.Nusrat S, Khan MS, Fazili J, Madhoun MF. Cirrhosis and its complications: evidence based treatment. World J Gastroenterol 2014;20:5442–5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bianchi G, Marchesini G, Nicolino F, et al. Psychological status and depression in patients with liver cirrhosis. Digest Liver Dis 2005;37:593–600. [DOI] [PubMed] [Google Scholar]
- 83.Aghanwa HS, Ndububa D. Specific psychiatric morbidity in liver cirrhosis in a Nigerian general hospital setting. Gen Hosp Psychiatry 2002;24:436–441. [DOI] [PubMed] [Google Scholar]
- 84.Weissenborn K, Bokemeyer M, Krause J, Ennen J, Ahl B. Neurological and neuropsychiatric syndromes associated with liver disease. AIDS 2005;19:S93–S98. [DOI] [PubMed] [Google Scholar]
- 85.Nardelli S, Pentassuglio I, Pasquale C, et al. Depression, anxiety and alexithymia symptoms are major determinants of health related quality of life (HRQoL) in cirrhotic patients. Metabol Brain Dis 2013;28:239–243. [DOI] [PubMed] [Google Scholar]
- 86.Peng J-K, Hepgul N, Higginson IJ, Gao W. Symptom prevalence and quality of life of patients with end-stage liver disease: a systematic review and meta-analysis. Palliat Med 2018;33:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217–231. [DOI] [PubMed] [Google Scholar]
- 88.Murtagh FEM, Addington-Hall JM, Edmonds PM, et al. Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med 2007;10:1266–1276. [DOI] [PubMed] [Google Scholar]
- 89.Weisbord SD, Carmody SS, Bruns FJ, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dialysis Transplant 2003;18:1345–1352. [DOI] [PubMed] [Google Scholar]
- 90.Cohen LM, Moss AH, Weisbord SD, Germain MJ. Renal Palliative Care. J Palliat Med 2006;9:977–992. [DOI] [PubMed] [Google Scholar]
- 91.Himelstein BP, Hilden JM, Boldt AM, Weissman D. Pediatr Palliat Care. New Engl J Med 2004;350:1752–1762. [DOI] [PubMed] [Google Scholar]
- 92.Connor SR, Sisimay C. Assessment of the need for palliative care for children: three country report: South Africa, Kenya and Zimbabwe. .London: United Nations Children’s Fund (UNICEF), International Children’s Palliative Care Network (ICPCN); 2013. Available at: https://www.icpcn.org/wp-content/uploads/2013/11/Assessment-of-the-Need-for-Palliative-Care-for-Children.-Three-Country-Report-South-Africa-Kenya-and-Zimbabawe.pdf Accessed May 2, 2024. [Google Scholar]
- 93.Kenner C, Press J, Ryan D. Recommendations for palliative and bereavement care in the NICU: a family-centered integrative approach. J Perinatol 2015;35:S19–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Madden K, Wolfe J, Collura C. Pediatric palliative care in the intensive care unit. Crit Care Nurs Clin 2015;27:341–354. [DOI] [PubMed] [Google Scholar]
- 95.McCormick MC, Brooks-Gunn J, Buka SL, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the infant health and development program. Pediatrics 2006;117:771–780. [DOI] [PubMed] [Google Scholar]
- 96.Dastgiri S, Gilmour WH, Stone DH. Survival of children born with congenital anomalies. Arch Dis Child 2003;88:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.World Health Organization. Birth defects: report by the Secretariat. .Geneva: WHO; 2010. Available at: https://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_10-en.pdf Accessed May 2, 2024. [Google Scholar]
- 98.Mosenthal AC, Murphy PA. Trauma care and palliative care: time to integrate the two? J Am College Surg 2003;197:509–516. [DOI] [PubMed] [Google Scholar]
- 99.Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine use after combat injury in Iraq and post-traumatic stress disorder. New Engl J Med 2010;362:110–117. [DOI] [PubMed] [Google Scholar]
- 100.Jones WS, Schmit KM, Vemulapalli S, et al. Treatment strategies for patients with peripheral artery disease. Agency for Healthcare Research and Quality (US), Rockville (MD), 2013. [PubMed] [Google Scholar]
- 101.Bendermacher BLW, Willigendael EM, Teijink JAW, Prins MH. Medical management of peripheral arterial disease. J Thromb Haemost 2005;3:1628–1637. [DOI] [PubMed] [Google Scholar]
- 102.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bullet World Health Organization; 2003;2003:646–656. [PMC free article] [PubMed] [Google Scholar]
- 103.Belachew T, Nekatibeb H. Assessment of outpatient therapeutic programme for severe acture malnutrition in three regions of Ethiopia. East African Med J 2007;84:577–588. [DOI] [PubMed] [Google Scholar]
- 104.World health organization. The treatment and management of severe protein-energy malnutrition. .Geneva, Switzerland: WHO; 1981. Available at: https://kohahq.searo.who.int/cgi-bin/koha/opac-detail.pl?biblionumber=11731&shelf-browse_itemnumber=17997 Accessed May 2, 2024. [Google Scholar]
- 105.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Annals Intern Med 2008;148:94–101. [DOI] [PubMed] [Google Scholar]
- 106.Alnajar M, Darawad M, Khater W, et al. Exploring palliative care needs among patients with cancer and non-cancer serious chronic diseases: a comparison study. Am J Hosp Palliat Care 2024:10499091241235920. 10.1177/10499091241235920. [DOI] [PubMed] [Google Scholar]
- 107.Calsina-Berna A, Amblàs Novellas J, González-Barboteo J, et al. Prevalence and clinical characteristics of patients with advanced chronic illness and palliative care needs, identified with the NECPAL CCOMS-ICO© Tool at a Tertiary Care Hospital. BMC Palliat Care 2022;21:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Islam N, Biswas J, Kowshik MM, et al. Depression, anxiety, and performance status among the women with metastatic breast cancer receiving palliative care in Bangladesh: a cross sectional study. Health Sci Rep 2022;5:e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abbas M, Reich AJ, Wang Y, et al. The burden of preadmission pain, depression, and caregiving on palliative care needs for seriously ill trauma patients. J Am Geriatr Soc 2023;71:2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pastrana T, Vallath N, Mastrojohn J, et al. Disparities in the contribution of low-and middle-income countries to palliative care research. J Pain Symptom Manag 2010;39:54–68. [DOI] [PubMed] [Google Scholar]
- 111.Coles CE, Earl H, Anderson BO, Barrios CH, Bienz M, Bliss JM, Cameron DA, Cardoso F, Cui W, Francis PA, Jagsi R, Knaul FM, Mcintosh SA, Phillips K-A, Radbruch L, Thompson MK, Andre F, Abraham JE, Bhattacharya IS, Zikmund-Fisher B. The Lancet Breast Cancer Commission; 2024. https://www.research.ed.ac.uk/en/publications/the-lancet-breastcancer-commission. [DOI] [PubMed]
- 112.Nouvet E, Sivaram M, Bezanson K, et al. Palliative care in humanitarian crises: a review of the literature. J Int Humanit Action 2018;3:1–14. [Google Scholar]
- 113.Rosa WE, Grant L. Focus: climate change and environmental health: Palliative Justice Post-COP27. Yale J Biol Med 2023;96:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.The Unpaid Care Work and the Labour Market. An analysis of time use data based on the latest World Compilation of Time-use Surveys / Jacques Charmes; International Labour Office; – Geneva: ILO, 2019. Available at: https://www.bollettinoadapt.it/wp-content/uploads/2020/01/wcms_732791.pdf. Accessed May 2, 2024. [Google Scholar]
- 115.Oechsle K, Ullrich A, Marx G, et al. Psychological burden in family caregivers of patients with advanced cancer at initiation of specialist inpatient palliative care. BMC Palliat Care 2019;18:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dipio R, Acuda W, Namisango E, Nalubega-Mbowa MG. Prevalence and factors associated with depressive symptoms among family caregivers of palliative care patients at Hospice Africa Uganda. Palliat Support Care 2022;20:375–382. [DOI] [PubMed] [Google Scholar]
- 117.Rattner M Increasing our understanding of nonphysical suffering within palliative care: a scoping review. Palliat Support Care 2022;20:417–432. [DOI] [PubMed] [Google Scholar]
- 118.VanderWeele TJ. Suffering and response: directions in empirical research. Social Sci Med 2019;224:58–66. [DOI] [PubMed] [Google Scholar]
- 119.Bhadelia A, Greaves N, Doubova S, Knaul FM. Understanding the value of alleviating health-related suffering and palliative care centered in lived experience: the SAVE Toolkit. Research Square; 2023; published online Dec 6. Available at: https://www.researchsquare.com/article/rs-3716807 (preprint). Accessed May 2, 2024. [Google Scholar]
- 120.Doubova SV, Bhadelia A, Pérez-Moran D, et al. Dimensions of suffering and the need for palliative care: experiences and expectations of patients living with cancer and diabetes and their caregivers in Mexico–a qualitative study. BMJ open 2023;13:e075691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pérez-Cruz PE, Undurraga E, Arreola-Ornelas H, et al. Bridging gaps to universal palliative care access in Chile: serious health-related suffering and the cost of expanding the package of care services.. Lancet Region Health Am 2023;19. Available at: https://www.thelancet.com/action/showPdf?pii=S2667-193X%2822%2900242-3 Accessed May 2, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krakauer EL, Kwete XJ, Rassouli M, et al. Palliative care need in the Eastern Mediterranean region and human resource requirements for effective response. PLOS Global Public Health 2023;3:e0001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sleeman KE, Gomes B, de Brito M, Shamieh O, Harding R. The burden of serious health-related suffering among cancer decedents: global projections study to 2060. Palliat Med 2021;35:231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.World Health Organization. Assessing the development of palliative care worldwide: a set of actionable indicators. 2021. Available at: https://www.who.int/publications/i/item/9789240033351. Accessed January 15, 2024.


