Abstract
Background
Previous studies have shown that serotonin and its receptors are widely distributed in mammalian reproductive tisssues and play an important role in embryonic development. However, the specific effects of the serotonergic system on embryonic arrest (EA) and the underlying mechanism require further investigation.
Methods
Chorionic villi were collected from patients with EA and healthy pregnant women. Western blotting (WB) and immunohistochemistry (IHC) were used to detect serotonin receptor 1B (HTR1B) levels and evaluate mitochondrial function. Additionally, HTR-8/SVneo cells were transfected with an HTR1B overexpression plasmid. Quantitative real-time polymerase chain reaction(qRT-PCR), Cell Counting Kit-8 (CCK-8), and wound healing assays were utilized to evaluate mitophagy level, cell proliferation and cell migration, respectively.
Results
We discovered elevated HTR1B levels in the chorionic villi of the patients with EA compared to controls. Concurrently, we observed enhanced levels of nucleus-encoded proteins including mitofilin, succinate dehydrogenase complex subunit A (SDHA), and cytochrome c oxidase subunit 4 (COXIV), along with the mitochondrial fusion protein optic atrophy 1(OPA1), fission proteins mitochondrial fission protein 1(FIS1) and mitochondrial fission factor (MFF) in the EA group. Additionally, there was an excessive mitophagy levels in EA group. Furthermore, a notable activation of mitogen-activated protein kinase (MAPK) signaling pathway proteins including extracellular regulating kinase (ERK), c-Jun N-terminal kinase (JNK), and P38 was observed in the EA group. By overexpressing HTR1B in HTR-8/SVneo cells, we observed a significant reduction in cell proliferation and migration. HTR1B overexpression also caused an increase in levels of SDHA and FIS1, as well as an upregulation of mitophagy. Notably, the ERK inhibitor U0126 effectively mitigated these effects.
Conclusion
These findings show that HTR1B influences mitochondrial homeostasis, promoting excessive mitophagy and impairing cell proliferation and migration by activating the MAPK signalling pathway during post-implantation EA. Therefore, HTR1B may serve as a potential therapeutic target for patients with EA.
Keywords: HTR1B, Embryonic arrest, MAPK, Excessive mitophagy, Mitochondrial fusion protein, Mitochondrial fission protein
Highlights
-
•
An association between serotonin receptor 1B and post-implantation embryonic development was discovered in our study.
-
•
Serotonin receptor 1B regulates mitochondrial homeostasis and mitophagy through the MAPK signalling pathway.
-
•
Serotonin receptor 1B could be a potential therapeutic target for patients experiencing embryonic arrest.
1. Introduction
Embryonic arrest (EA) is a pathological process that occurs during pregnancy in which various unfavourable factors after embryo implantation lead to the termination of embryo development and subsequent embryo death [1]. It's a specific form of spontaneous abortion, accounting for 15 % of all clinical pregnancies and showing an increasing trend over time [2]. Some recognized causes of EA include chromosomal abnormalities, immune factors, endocrine factors, infectious factors, reproductive organ malformations, environmental factors, and maternal behavior and lifestyle. EA can occur at any stage of pregnancy, with 80 % of cases occurring in the early stages [3,4]. There are no effective diagnostic methods for pregnant women at risk of EA or treatments for those with EA. Currently, there is a lack of comprehensive studies and reports that provide a precise understanding of the causes and mechanisms underlying EA.
Placental trophoblast cells play a crucial role in embryo health and implantation. During blastocyst formation, trophoblast cells differentiate into three major cell populations: cytotrophoblast (CTs), syncytiotrophoblast (STs), and extravillous trophoblast (EVTs) [5]. Maintaining homeostasis within the basal chorionic trophoblast cell layer is essential for fetal health and successful pregnancy. The migration and invasion of trophoblast cells at the maternal-foetal interface are critical processes for successful embryo implantation. Therefore, insufficient invasion of trophoblasts or failure of uterine and placental vascular remodelling can lead to common obstetric complications such as miscarriage, preterm labour, foetal growth restriction, foetal death, and pre-eclampsia [6,7]. Moreover, dysfunctional trophoblast cells are unable to provide sufficient nutrients to meet the needs of the embryo, leading to the cessation of embryonic development [8].
Mitochondria are the most abundant organelles in mammalian oocytes and early embryos, and play a crucial role in the process of embryonic development. Under certain circumstances, such as exposue to external stimuli and environmental changes, the structure of mitochondria can be altered, leading to functional impairments. Mitochondrial abnormalities can disrupt various pathways, and mitochondrial fission and fusion directly impact mitochondrial metabolism, apoptosis, and autophagy [9]. Autophagy is a self-degrading process that maintains cellular and organismal homeostasis by eliminating excess or damaged subcellular and/or cytoplasmic proteins [10]. Autophagy can regulate both cell survival and death in various cell types. Additionally, previous studies have shown abnormal expression of proteins associated with apoptosis and autophagy in the chorionic tissues of patients who experience recurrent spontaneous abortion (RSA) [11]. In particular, autophagy-related factors such as Beclin-1 and LC3 II/I are upregulated in the chorionic cells of RSA patients [12]. It has been previously demonstrated that autophagy is essential for embryo implantation during early pregnancy [13], and that foetal growth restriction induced by environmental cadmium exposure is associated with mitophagy [14]. These results suggest that maintaining normal levels of autophagy is essential for embryonic development.
Recently, it has been found that 5-HT is widely distributed in the reproductive tissues of mammals and plays an important role in cell proliferation, differentiation, follicular development, and embryonic development. Additionally, early human placental chorionic trophoblast cells have the ability to produce 5-HT. In recent years, several studies have explored the role of 5-HT in embryonic development. For example, a 2021 study revealed that parthenogenetically activated porcine embryos exhibited a reduced blastocyst rate when treated with exogenous 5-HT. Furthermore, researchers have observed that 5-HT is present at relatively high levels within mitochondria and colocalizes with them. The addition of exogenous 5-HT also impacts the mitochondrial potential of cells. 5-HT primarily exerts its functions by binding to its receptors, which can be categorized into seven subfamilies: HTR1, HTR2, HTR3, HTR4, HTR5, HTR6, and HTR7. Among these receptors, only HTR3 functions as a ligand-gated ion channels, while the others are G-protein coupled receptors. Notably, the human placenta expresses multiple serotonin receptor subtypes including HTR1,HTR2,HTR3, HTR4, and HTR7 [15]. In both healthy ovaries and ovarian malignancies, the expression of HTR1A, HTR1B, HTR2B, and HTR4 has been detected [16]. In pregnant rats, intraamniotic and subcutaneous injections of 5-HT resulted in developmental abnormalities and in certain instances, even embryonic death [17]. Additionally, activation of the HTR2A has been found to promote cell viability, influence cell cycle progression, and activate the MEK-ERK1/2 and JAK2-STAT3 signalling pathways in human choriocarcinoma cell lines [18]. The MAPK signalling pathway plays a critical role in regulating cell proliferation and differentiation. Moreover, the MAPK/ERK signalling pathway is closely linked to mitochondrial calcium imbalance and its impact on the potential of embryonic development [19]. In this study, we examined the expression of HTR1B in the chorionic tissue of patients with EA for the first time, and conducted a comprehensive analysis to elucidate the role of HTR1B in post-implantation embryonic development. We posited that HTR1B may exert a significant influence on mitochondrial homeostasis and the process of mitophagy, potentially through the mediation of the MAPK signalling pathway. The current study provides insights into the mechanisms underlying EA and suggests that HTR1B may be a viable therapeutic target for the treatment of patients experiencing EA.
2. Materials and methods
2.1. Patient enrolment and sample collection
EA is diagnosed using transvaginal ultrasound, which relies on three criteria: an embryo length ≤5 mm with no cardiac tube pulsation, with the cessation of pulsation persisting upon reevaluation after 7–10 days; an embryo length >5 mm and no cardiac tube pulsation, or a mean internal diameter of the gestational sac >20 mm and no yolk sac or embryo; and a mean internal diameter of the gestational sac ≤20 mm and no yolk sac or embryo, with absence of the yolk sac or embryo persisting upon reevaluation after 1–2 weeks. Patients with any other diseases affecting the hypothalamus, the pituitary gland, or ovaries, such as hypothyroidism, were excluded from this study. Additionally, those who had received insulin or hormone therapy within the previous 3 months were excluded. Gestational age-matched healthy pregnant women who voluntarily opted for abortion were selected as a control group. The study protocol was approved by the Human Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No:2022309). Chorionic villus samples were collected from all subjects following the guidelines of the Declaration of Helsinki, and written informed consent was obtained from all subjects prior to enrolment in this study. Individuals who electively terminated their pregnancies and patients with EA included in this study were 20–38 years old with a gestational age of 7–9 weeks. A total of 30 women with healthy pregnancies and 30 patients with EA were included in this study. The women with normal pregnancies had no previous adverse pregnancy history, such as spontaneous abortion or stillbirth. Their blood routine and leucorrhoea examination results were within normal ranges. All participants reported no vaginal bleeding, abdominal pain, fever, pathogen infection, or signs of obvious local inflammation.
2.2. Immunohistochemistry
The collected samples were fixed in 4 % buffered formalin overnight, followed by paraffin-embedding. The paraffin-embedded tissue blocks were deparaffinized, dehydrated, and subjected to high-pressure antigen repair with 0.01 % sodium citrate buffer (pH = 6.0) for 20 min. Immunohistochemical staining was performed using Histostain™-SP Kits (ZSGB-BIO, Cat#PV-9000) and DAB peroxidase substrate kits (ZSGB-BIO, Cat#ZLI-9018) according to the manufacturer's protocols. Nuclei were stained with haematoxylin (Solarbio, Cat#G4070).Primary antibodies against HTR1B (Proteintech, 22189-1-AP), MFN1 (Proteintech, 66776-1-IG), DRP1 (Proteintech, 12957-1-AP), OPA1 (Proteintech, 27733-1-AP), Fis1 (Proteintech, 10956-1-AP) and MFF (Proteintech, 17090-1-AP) were used. Tissue sections were examined and photographed using an OLYMPUS VS200 microscope. For each sample, 3–5 random 20 × fields of view were scored. Digital images were inputted into Image J software for quantitative image analysis [20].
2.3. Cell culture and transfection
The human extravillous trophoblast HTR-8/SVneo cell line were obtained from the Chinese Academy of Sciences Cell Bank (SCSP-5203, CSTR:19375.09.3101HUMSCSP5203) and maintained in DMEM + GlutaMAXTM-1 medium (Gibco, 10569-010) supplemented with 10 % foetal bovine serum (Sigma, F7524) and 100 U/mL penicillin/streptomycin (Beyotime, C0222). The cells were cultured at 37 °C with 5 % CO2 in a humidified incubator. Cells were passaged when the cell density reached 85 %. After digestion with 0.25 % Trypsin-EDTA solution (Beyotime, C0201) for 1 min, the digestion was terminated by the addition of serum-containing medium, and the cell suspension was centrifuged for 3 min. The centrifuged cells were resuspended and inoculated into culture dishes or 6-well plates.
Cells were transfected with jetPRIME transfection reagent (Polyplus, 0000000806) when they reached a density of 60–80 % in 6-well plates. mRNA expression analysis was performed 24 h after transfection, while protein expression analysis was conducted 48 h post-transfection. The overexpression plasmids utilized in this study were obtained from Miaoling Biology. Before transfection, thecells were treated with the ERK inhibitor U0126 (Selleck, S1102) (10 μM) for 2 h [21].
2.4. Cell Counting Kit-8 (CCK-8) cell viability assay
A CCK-8 assay was used to evaluate the effect of HTR1B overexpression on the relative viability of cells. HTR-8/SVneo cells were incubated for 24 h after transfection. Then the control and HTR1B-overexpressing cells were digested, centrifuged, and resuspended. The cells were seeded at a density of 5000 per well in 96-well plates, with 3 replicates per treatment group. Incubation was carried out at 37 °C with 5 % CO2 for 0, 24, 48, or 72 h. At each time point, 10 μl CCK-8 solution was added per well following the manufacturer's instructions of the CCK-8 Cell Viability Assay Kit (Beyotime, C0039). The absorbance was measured at 450 nm after 2 h of incubation, and relative cell viability was calculated [22].
2.5. Wound healing experiment
Approximately 1-2 × 106 transfected HTR-8/SVneo cells were seeded into 6-well plates. When the transfected HTR-8/SVneo cells reached 90%–100 % confluence, wounds were made in each well using a sterilized 200 μl pipette tip, and the detached cells were washed away with PBS (Biosharp, BL550A). Subsequently, the cells were incubated with medium containing 1 % serum at 37 °C with 5 % CO2. The medium was changed at 0, 24, 48 and 72 h, and photographs were taken with a microscope (NIKON) to observe the progress of wound healing [23].
2.6. Western blotting
Cells and tissues were washed thrice with PBS and lysed with RIPA lysis buffer (Beyotime, P0013B) containing Protease Inhibitor Cocktail (MCE, HY-K0010) and Phosphatase Inhibitor Cocktail I (MCE, HY-K0021). The mixture was placed on ice for 30 min, shaken every few minutes, and centrifuged at 12,000 rpm at 4 °C for 10 min. The supernatant was collected, and SDS-PAGE Sample Loading Buffer (5 × ) (Beyotime, P0015L) at a ratio of 1 μl of Protein Sampling Buffer (5 × ) for every 4 μl of protein samples was added. The samples were boiled in a 100 °C water bath for 10 min to adequately denature the proteins. The appropriate gels were prepared, and the protein samples were separated on a 12 % SDS-PAGE gel and transferred to polyvinylidene fluoride (PVDF) blot membranes (Immobilon-P, Millipore, IPVH00010) in an ice bath. The membrane was blocked with 5 % skim milk in TBST(100 mM Tris-HCl[pH 7.5], 137 mM NaCl, and 0.1 % Tween 20) for 2 h and incubated overnight at 4 °C with the appropriate diluted primary antibody. The primary antibodies used in this experiment are detailed in Table 1. On the next day, the membrane was washed thrice with TBST. Then, the membrane was incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (1:10000, Proteintech) for 2 h at room temperature and washed again with TBST. Finally, the membrane was incubated with the AB mixture provided in the chemiluminescence substrate kit (Biosharp, BL520A), and the protein bands were detected with a Tanon 1600 Series Multifunctional Gel Image Analysis System. Each experiment was repeated three times, and ACTIN or GAPDH was used as an internal reference protein to determine the expression of each protein. ImageJ was used to calculate grayscale values of the bands [24]. Detailed information on the antibodies used is given in Table 1.
Table 1.
Detailed information on antibodies.
| Antibody | Host spices | Vendor | Catalog No. | Working Dilution |
|---|---|---|---|---|
| Actin | Mouse | ZSGB-BIO | TA-09 | 1:5000 |
| GAPDH | Mouse | Proteintech | 60004-1-Ig | 1:10,000 |
| HTR1B | Rabbit | Proteintech | 22189-1-AP | 1:1000 |
| mitofilin | Rabbit | Proteintech | 10179-1-AP | 1:1000 |
| SDHA | Rabbit | CST | 11998S | 1:1000 |
| COX IV | Rabbit | CST | 4850S | 1:1000 |
| MFN1 | Rabbit | Proteintech | 66776-1-IG | 1:1000 |
| DRP1 | Rabbit | Proteintech | 12957-1-AP | 1:1000 |
| OPA1 | Rabbit | Proteintech | 27733-1-AP | 1:1000 |
| Fis1 | Rabbit | Proteintech | 10956-1-AP | 1:1000 |
| MFF | Rabbit | Proteintech | 17090-1-AP | 1:1000 |
| Bax | Rabbit | Proteintech | 50599-2-Ig | 1:1000 |
| Bcl2 | Rabbit | Proteintech | 26593-1-AP | 1:1000 |
| LC3B | Rabbit | NOVUS | NB100-2220 | 1:1000 |
| Beclin1 | Rabbit | Proteintech | 11306-1-AP | 1:1000 |
| Parkin | Rabbit | Proteintech | 66674-1-Ig | 1:1000 |
| PINK1 | Rabbit | Proteintech | 23274-1-AP | 1:1000 |
| T-ERK | Rabbit | Proteintech | 11257-1-AP | 1:1000 |
| P-ERK | Rabbit | Proteintech | 28733-1-AP | 1:1000 |
| T-JNK | Rabbit | Proteintech | 24164-1-AP | 1:1000 |
| P-JNK | Rabbit | CST | 9255S | 1:1000 |
| T-P38 | Rabbit | Proteintech | 66234-1-Ig | 1:1000 |
| P–P38 | Rabbit | Proteintech | 287996-1-AP | 1:1000 |
2.7. RNA extraction, cDNA synthesis and qRT–PCR amplification
Total RNA was extracted from the control and treated cells using TRIzol reagent (Invitrogen,15596018CN) and the concentrations of the obtained RNA samples were determined with a spectrophotometer (Nanodrop 2000, Thermo Fisher Scientific). The reverse transcription reaction was carried out using HyperScript III RT SuperMix for qPCR with gDNA Remover (EnzyArtisan, R202). One microgram of RNA in a reaction mixture with a total volume of 20 μl was reverse transcirbed into cDNA according to the manufacture's instructions. RT- PCR reactions were carried out using 10 μl of SYBR Green qPCR Master Mix (2 × ) (Roche,04887352001), 2 μl of the cDNA product, 0.5 μl of each antisense and sense primer (10 pmol) as shown in Table 2, and 7 μl nuclease-free water on a Roche Diagnostic instrument. The regimen for qRT-PCR consisted of an initial activation step at 95 °C for 10 min, followed by 40 cycles at 94 °C for 10 s, 1 min for each primer pair at the proper annealing temperature, 60 s at 72 °C, and finally 10 min for the extension at 72 °C. The ΔΔCT method was applied to quantify differences in the expression of the target genes between the experimental and control groups [24].
Table 2.
Related primer sequences.
| Genes | Primer sequences (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| actin | CCTGGCACCCAGCACAAT | GGGCCGGACTCGTCATAC |
| htr1b | GCCAATAGCATAACCAGCAGT | GGGTTCCTCAAGCCAACTTATC |
| drp1 | CTGCCTCAAATCGTCGTAGTG | GAGGTCTCCGGGTGACAATTC |
| opa1 | TGTGAGGTCTGCCAGTCTTTA | TGTCCTTAATTGGGGTCGTTG |
| sdha | CAGCTTGGTAACACATGCTGTAT | CAAACAGGAACCCGAGGTTTT |
| cox iv | CAGGGTATTTAGCCTAGTTGGC | GCCGATCCATATAAGCTGGGA |
| fis1 | GTCCAAGAGCACGCAGTTTG | ATGCCTTTACGGATGTCATCATT |
2.8. Statistical analysis
SPSS 25.0 software was used for statistical analyses. Data are expressed as the mean ± standard error of the mean (SEM) from at least three independent experiments. Where the data met the criteria for normality distribution, we proceeded with a homogeneity of variance test followed by an independent samples t-test. In cases where the data did not conform to normality distribution, a non-parametric test (Mann-Whitney U) was employed. Significance was defined as p < 0.05.
3. Results
3.1. The expression of 5-HT receptors is upregulated in the chorionic villi of patients with EA
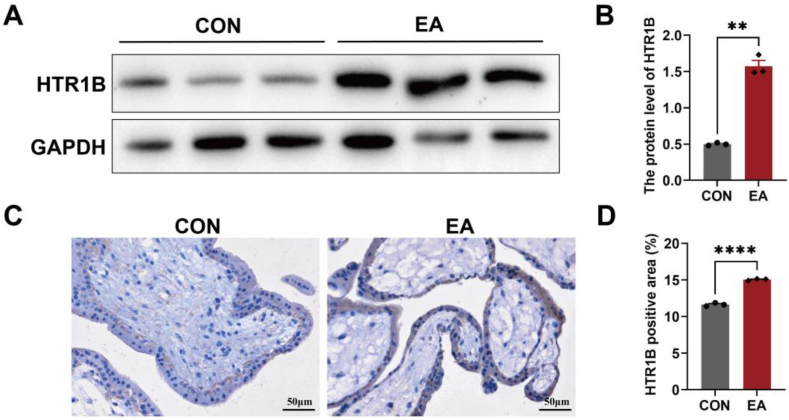
In order to investigate the expression of 5-HT1B in thechorionic villi of patients with EA, we employed Western blotting (WB) and immunohistochemistry. Specifically, we examined the expression of HTR1B in both the control group and EA group. As shown in Fig. 1A and B, HTR1B expression was significantly elevated in the chorionic villi of patients with EA compared to those with healthy pregnancies. Additionally, HTR1B was localized in trophoblast cells within chorionic villi, specifically in syncytiotrophoblast and cytotrophoblast cells. Notably, the morphology of chorionic villi tissue exhibited significant alterations in the EA group compared to the control group. Specifically, some villi transitioned from a two-layer structure to a single layer (Fig. 1C and D). Therefore, these findings indicate that HTR1B plays a significant role in embryonic development.
Fig. 1.
The expression of 5-HT receptors is upregulated in the chorionic villi of patients with EA. (A–B) The expression level of HTR1B was investigated in the chorion villi of patients with EA and control individuals by WB. GAPDH served as a loading control. (C–D) IHC staining of HTR1B in human chorionic villi. Scale bar: 50 μm. Data are shown as the mean ± SEM. **p < 0.01, ****p < 0.0001.
3.2. Mitochondrial homeostasis was out of balance in the chorionic villi of patients with EA
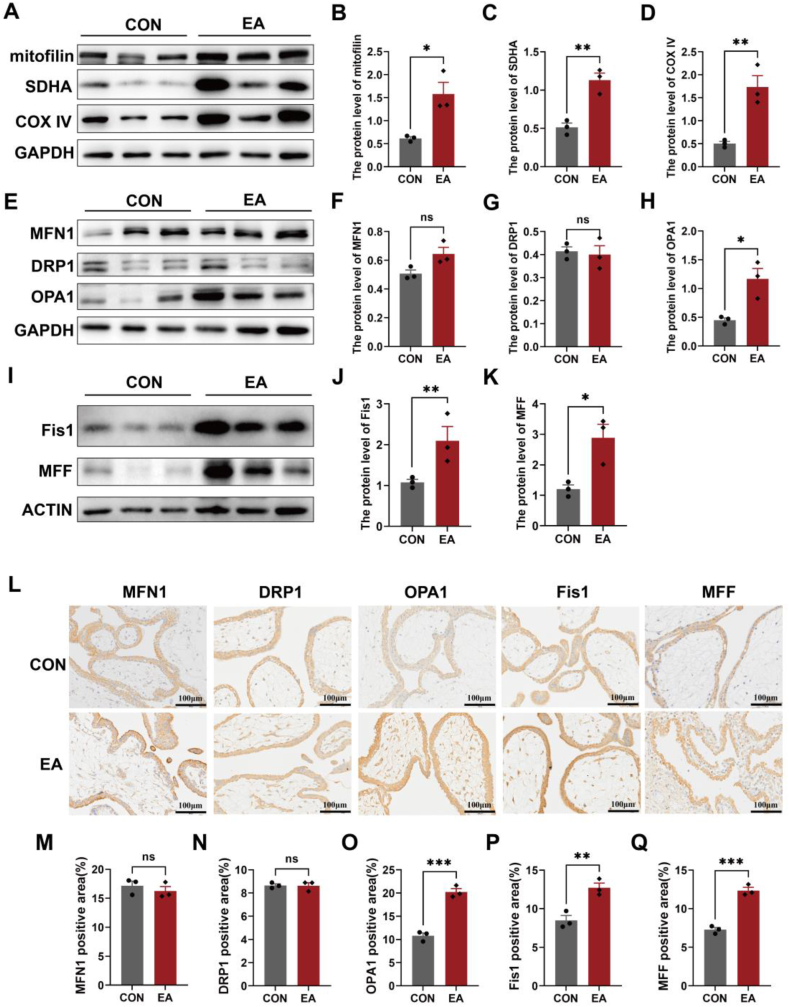
The proliferative form of trophoblast cells shares similarities with tumour cells, as both rely on mitochondria to generate adequate energy for normal cellular activity. Western blotting and immunohistochemistry were used to investigate the expression of mitochondria-related proteins in the thechorionic villi of patients with EA to determine whether there was any change in mitochondrial function. The WB findings showed varying degrees of alteration in nucleus-encoded proteins (mitofilin, SDHA, COX IV), as well as mitochondrial fusion (OPA1) and fission (DRP1, FIS1, MFF) proteins (Fig. 2A–K). Additionally, the IHC staining results showed increased levels of OPA1, Fis1 and MFF (Fig. 2, L-Q). These findings provide strong evidence of mitochondrial dyshomeostasis in the chorionic villi of patients with EA.
Fig. 2.
Mitochondrial homeostasis was out of balance in the chorionic villi of patients with EA. (A–K) The expression levels of nucleus-encoded proteins (mitofilin, SDHA, COXIV), as well as mitochondrial fusion (MFN1, and OPA1) and fission (DRP1, Fis1, MFF) proteins in the chorionic villi were assessed using WB analysis in both the EA group and the control group. GAPDH and ACTIN were used as a loading control. (L–Q) IHC staining was performed to detect the expression of MFN1, DRP1, OPA1, Fis1, and MFF in human chorionic villi from both the EA group and the control group. Scale bar: 100 μm. Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
4. Mitophagy was overactivated in the chorionic villi of patients with EA
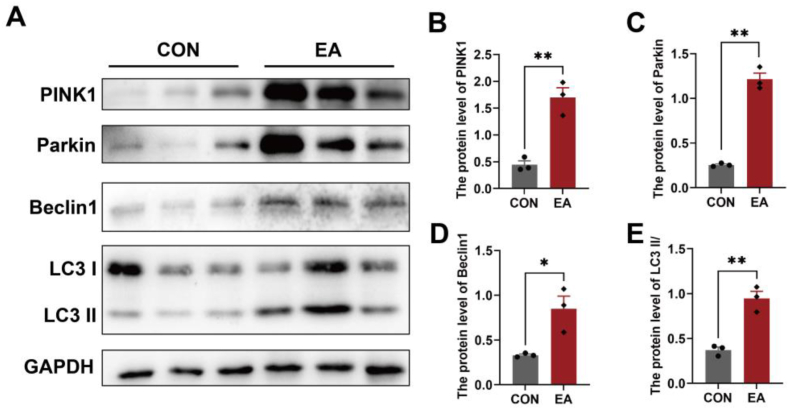
While autophagy is generally considered an adaptive process that regulates both cell survival and cell death [25], our group previously found that excessive autophagy induced mitochondrial dysfunction and impaired ovarian reserve function in Chinese hamster ovary (CHO) cells. In this study, our results indicated that the expression level of Beclin1 and the ratio of LC3 II/I were significantly increased in the chorionic villi of patients with EA (Fig. 3A–E). Notably, we found significant activation of the mitophagy-related proteins PINK1 and Parkin, suggesting mitophagy was overactivated in the chorionic villi of patients with EA (Fig. 3, A-C).
Fig. 3.
Mitophagy was overactivated in the chorionic villi of patients with EA. (A) The characteristic bands of mitophagy-related proteins, including PINK1, Parkin, Beclin1, and LC3. (B–E) The protein expression levels of PINK1(B), Parkin(C), Beclin1(D), and the ratio of LC3 II/I(E) in the chorion villi of the two groups. GAPDH served as a loading control. Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01.
4.1. Activation of the MAPK signalling pathway in the chorionic villi of patients with EA
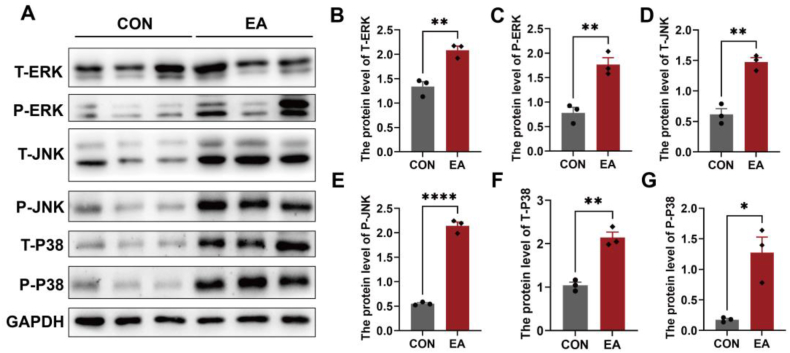
The MAPK signalling pathway has been shown to play an important role in cell growth, embryonic development, and various pathological processes. Its three key components are P38, ERK, and JNK. We observed a significant upregulation in both the total and phosphorylated levels of ERK, JNK, and P38 in the chorionic villi of patients with EA(Fig. 4, A-G). This finding indicates that the MAPK signalling pathway is significantly activated in the chorionic villi of patients with EA.
Fig. 4.
Activation of the MAPK signalling pathway in the chorionic villi from patients with EA. (A) The characteristic bands of MAPK signalling pathway-related proteins, including T-ERK, P-ERK, T-JNK, P-JNK,T-P38, and P–P38. (B-G)The expression levels of total ERK (B), P-ERK (C), total JNK (D), P-JNK (E), total P38 (F), and P–P38 (G) in the chorionic villi of two groups. GAPDH served as a loading control. Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01, ****p < 0.0001.
4.2. Overexpression of HTR1B inhibited cell proliferation, and migration, disrupted mitochondrial homeostasis, and overactivated mitophagy in HTR-8/SVneo cells
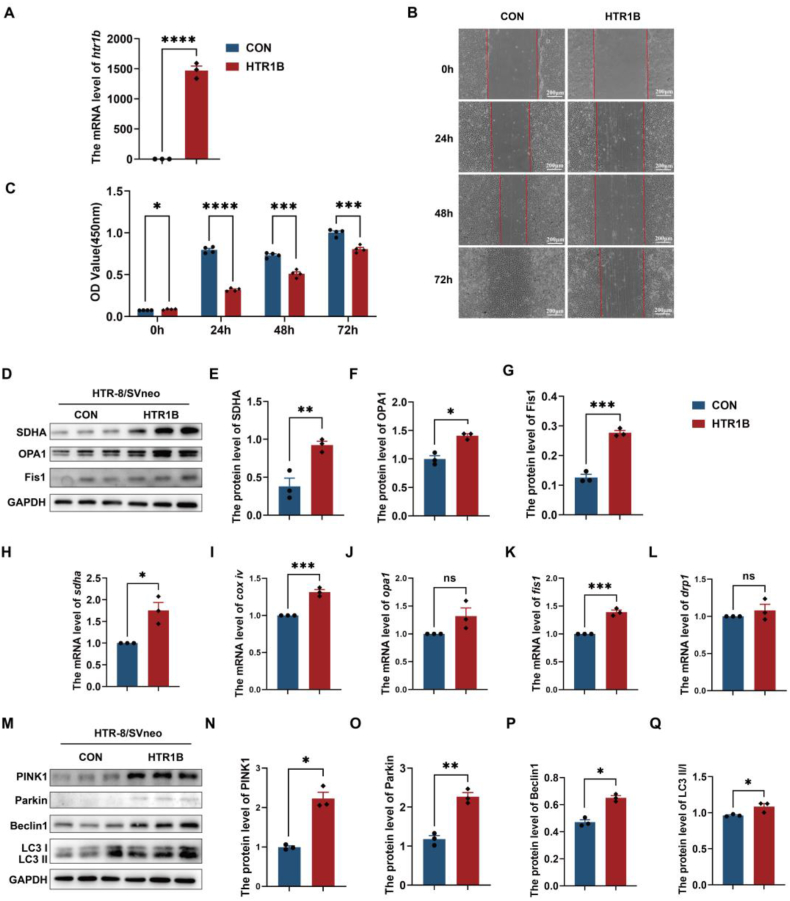
The proliferation and differentiation of placental trophoblast cells are critical during embryonic development. In order to investigate the underlying mechanisms of HTR1B in embryonic development, we generated HTR1B-overexpressing HTR-8/SVneo cells via transfection (Fig. 5A). Our in vitro study results demonstrated that overexpression of HTR1B significantly suppressed both the cell proliferation and migration ability of HTR-8/SVneo cells (Fig. 5B and C).
Fig. 5.
Overexpression of HTR1B inhibited cell proliferation and migration, disrupted mitochondrial homeostasis, and overactivated mitophagy in HTR-8/SVneo cells. (A) q-PCR assays for mRNA levels of htr1b in HTR-8/SVneo cells. GAPDH served as a loading control. (B) Wound healing experiments were conducted in both groups of HTR-8/SVneo cells. Scale bar: 200 μm. (C) Cell proliferation was assessed at various time points following HTR1B transfection. (D–G) Cellular mitochondrial function was assessed following HTR1B overexpression. The protein levels of nucleus-encoded proteins (SDHA) and mitochondrial dynamic-related proteins (OPA1and Fis1). (H–L) The mRNA levels of sdha, cox iv, opa1, fis1, and drp1. (M–Q) The protein levels of mitophagy-associated proteins, such as PINK1, Parkin, Beclin1, and the ratio of LC3 II/I. The Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
To investigate the potential impact of HTR1B on mitochondrial function and mitophagy, we performed qRT-PCR and WB analyses to assess the expression levels of mitochondrial dynamic-related proteins, as well as markers of cell autophagy and apoptosis, in HTR1B-overexpressing HTR-8/SVneo cells. The results indicated that following the overexpression of HTR1B, there was an increase in the levels of SDHA, OPA1 and Fis1, along with alterations in other mitochondrial markers (Fig. 5D-G). Additionally, qRT-PCR analysis revealed an increase in the mRNA levels of sdha, cox iv, and fis1, while no changes were observed in other mitochondrial markers such as opa1, and drp1 (Fig. 5H–L). Furthermore, our observations revealed an elevation in the expression of the autophagy-related markers PINK1, Parkin, Beclin1 and LC3II/I (Fig. 5M–Q). These findings demonstrated that HTR1B overexpression adversely affected mitochondrial function, triggered excessive autophagy, and induced cell apoptosis.
4.3. The ERK inhibitor U0126 abrogated the changes in mitochondrial homeostasis and cell autophagy in HTR-8/SVneo cells induced by HTR1B overexpression
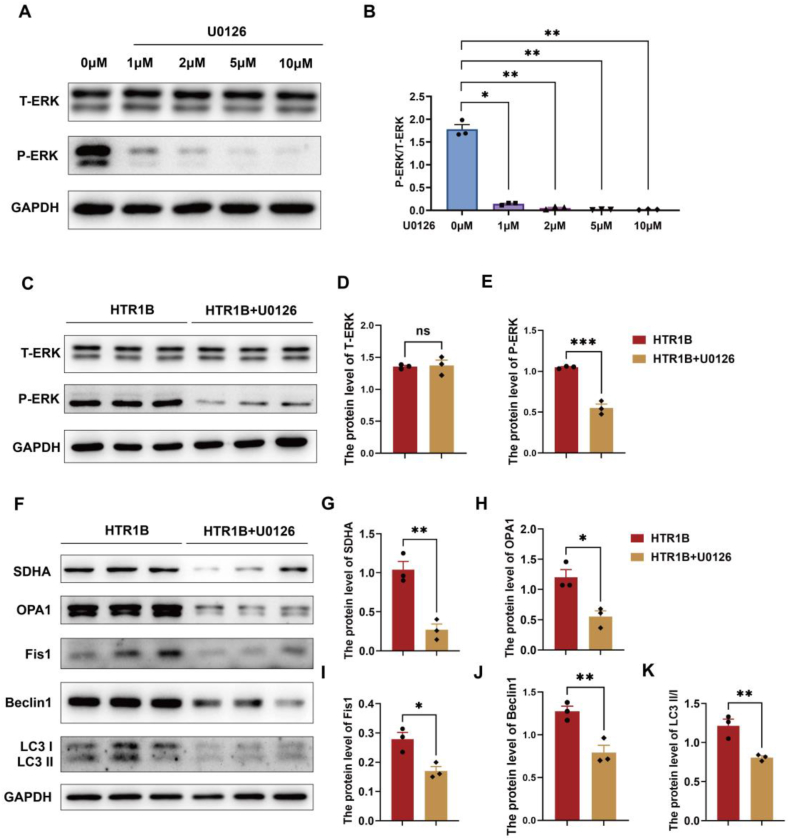
We found that HTR1B expression was elevated and the MAPK signalling pathway was significantly activated in the chorionic villi of patients with EA. Therefore, we speculated that HTR1B activates the MAPK signalling pathway, and we used a cell model to test this hypothesis. We also examined the activation of the MAPK signalling pathway after HTR1B overexpression in HTR-8/SVneo cells. The expression of MAPK signalling pathway-related proteins (P-ERK, T-P38 and P–P38) was elevated in cells transfected with HTR1B compared to control cells, while the expression of other proteins was unchanged (Fig. S1, A-G).
To verify the potential impact of the MAPK signalling pathway on embryonic development, the ERK inhibitor U0126 was used to assess the expression levels of mitochondrial dynamic-related proteins, as well as markers of cell mitophagy, following the overexpression of HTR1B in HTR-8/SVneo cells. To investigate the effect of the MAPK signalling pathway on post-implantation embryonic development, we exposed HTR-8/SVneo cells to different concentrations of U0126 (1 μM, 2 μM, 5 μM, and 10 μM). Our results revealed that treatment with all concentrations of U0126 (1 μM, 2 μM, 5 μM, and 10 μM) resulted in the inhibition of P-ERK expression (Fig. 6A and B). Subsequently, a concentration of 10 μM for U0126 was chosen for further experiments. We pretreated the cells with U0126 for 2 h and found that P-ERK was significantly inhibited (Fig. 6C–E). The results indicated that 10 μM U0126 reversed the levels of nucleus-encoded proteins SDHA, and mitochondrial dynamic-related proteins including OPA1 and Fis1(Fig. 6F–I). In addition, the level of Beclin1 and the ratio of LC3 II/I were reduced by U0126 (Fig. 6F,J,6K). Our in vitro experimental results further verified that the role of HTR1B in EA is regulated through the MAPK signalling pathway.
Fig. 6.
The ERK inhibitor U0126 abrogated the changes in mitochondrial homeostasis and cell autophagy in HTR-8/SVneo cells induced by HTR1B overexpression. (A–B) WB were performed to examine the protein levels of P-ERK/ERK in HTR-8/SVneo cells exposed to different concentrations of U0126 (1 μM, 2 μM, 5 μM, and 10 μM). GAPDH served as a loading control. (C–E) The protein levels of T-ERK and P-ERK in HTR-8/SVneo cells exposed to 10 μM U0126. (F–K) The protein levels of SDHA, OPA1, Fis1, Beclin1, and the ratio of LC3II/I in HTR-8/SVneo cells exposed to 10 μM U0126. Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001.
5. Discussion
The incidence of EA is escalating annually, posing a pressing clinical challenge. Consequently, the etiology and pathogenesis of EA have become a prominent research area in reproductive science [26]. Our research endeavours have provided valuable insights into the prevention and treatment of this condition. In this study, we have, for the first time, confirmed a significant upregulation of HTR1B in the chorionic villi of patients with EA. This novel finding suggests a potential involvement of HTR1B in the pathogenesis of EA.
The placenta serves as the pivotal interface between the maternal and foetal systems, with placental mitochondria playing an indispensable role in sustaining the viability of the pregnancy [27]. In MFN2-deficient embryos, lethality is attributed to trophectoderm deficiency. MFN1 and MFN2 deletions are lethal at different developmental stages, and death occurs earlier in MFN1 and MFN2 double-knockout mice than in single-knockout mice, supporting the alternative function of these proteins in early embryogenesis. However, approximately 33 % of mice with embryo-specific MFN2 deficiency die within the first day of life, and the surviving pups exhibit significant motor impairments [28]. Additionally, deletion of DRP1 disrupts trophoblast giant cell development and leads to embryonic mortality [29,30]. These results indicate that the deletion of proteins associated with mitochondrial dynamics including DRP1, MFN1, and MFN2 exert a significant impact on embryonic development. Conversely, in our study, we observed an elevation in the levels of mitochondrial fission- and fusion-related proteins, including SDHA, OPA1, and Fis1, within the EA group. It is important to note that mitochondrial homeostasis is a multifaceted process, intricately regulated by a myriad of proteins. Perturbations in the expression levels of these proteins can lead to the disruption of mitochondrial homeostasis. Collectively, these observations underscore the indispensable role of mitochondrial homeostasis in the critical stages of early embryonic development.
Recent studies have demonstrated a correlation between autophagy in trophoblast cells and RSA [31]. While an appropriate level of autophagy is advantageous for embryonic development, deviations from this optimal state, whether through insufficient or excessive autophagy, can have adverse effects on the developmental process. Although the relationship between autophagy and preimplantation embryonic development has been extensively investigated [32], there is a lack of research on the involvement of autophagy, particularly mitophagy, in post-implantation embryo development. Autophagy inhibition results in abnormal decidualization, and the administration of rapamycin, an autophagy inducer, prevents spontaneous abortion by triggering decidual stromal cell autophagy-mediated NK cell residence [33]. Previous research has shown that diminished expression of MFN2 is associated with mitochondrial dysfunction, elevated autophagy levels, and impaired trophoblast cell function, which may contribute to early unexplained miscarriage [34]. Additionally, another research has indicated that exposure to zearalenone during the critical period of embryo implantation triggers autophagy, disrupting the uterine environment and ultimately resulting in failed embryo implantation and dysontogenesis in gilts [35]. These findings are congruent with our observations that mitochondrial dysfunction, induced by HTR1B, subsequently leads to excessive autophagy and is implicated in the pathogenesis of EA. Notably, our findings revealed a significant increase in the expression levels of Beclin1 and LC3II/I, particularly the mitophagy-associated proteins PINK1 and Parkin, in the chorionic villi of patients with EA. This result suggests that mitophagy is indeed involved in embryo development after implantation.
A recent study has demonstrated an association between the activation of the MAPK signalling pathway and impaired trophoblast function, indicating a potential role in embryonic development [36]. In this study, we observed the activation of the MAPK signalling pathway in human chorionic villi for the first time, highlighting its crucial role during human embryonic development.
Subsequently, we investigated the underlying mechanism by utilizing the human choriotrophoblast cell line HTR-8/SVneo. Among the various 5-HT receptors, the HTR1 family is coupled to the intracellular G-protein. These receptors inhibit adenylate cyclase, resulting in elevated ERK phosphorylation in the PI3K/AKT-dependent pathway of the medial prefrontal cortex [37]. This suggests that the HTR1 family may modulate the MAPK signalling pathway. In the current study, we discovered that the overexpression of HTR1B notably activated the MAPK signalling pathway and suppressed the proliferation and invasion of trophoblast cells, suggesting that HTR1B may influence human embryonic development by regulating the MAPK/ERK pathway. Furthermore, prior investigations have shown that reducing the phosphorylation of ERK, JNK, and P38 markedly improved mitochondrial function and increased the blastocyst formation rate in porcine models, which could positively impact early embryonic development [38]. Significantly, our group identified that the overexpression of HTR1B led to elevated levels of SDHA and FIS1, along with an upregulation in mitophagy, evidenced by the increased levels of PINK1, Parkin, Beclin1, and the elevated ratio of LC3II/I. These findings suggest that HTR1B may induce mitochondrial dyshomeostasis and excessive mitophagy, potentially affecting human embryonic development.
Subsequently, to confirm the influence of HTR1B on embryonic development through the MAPK signalling pathway, the ERK inhibitor U0126 was introduced into the HTR1B-overexpressing HTR-8/SVneo cells. Our results indicated that the inhibition of the ERK signalling pathway substantially restored mitochondrial dyshomeostasis and excessive autophagy in the human trophoblast cell line HTR-8/SVneo, corroborating our speculations and highlighting the pivotal role of the MAPK/ERK pathway in human embryonic development. The current study has shed light on the underlying pathogenic mechanisms that may lead to EA. Our findings indicate that the overexpression of HTR1B triggers excessive mitophagy and disrupts mitochondrial homeostasis, consequently diminishing cell proliferation and impeding cell invasion. These insights are instrumental for comprehending the complex molecular processes underlying embryonic development and highlight the essential function of the MAPK signalling pathway in this process.
This study has several limitations. Firstly, the scope of our cell models was limited to the human choriotrophoblast cell line HTR-8/SVneo. Future research will include a broader range of cell models, such as the human choriocarcinoma cell line JEG-3, particularly in the context of HTR1B overexpression, which is of considerable interest to our group. Additionally, our investigation on the influence of serotonin receptors on embryonic development and the potential molecular mechanisms was confined to the study of HTR1B alone. The potential contributions of other serotonin receptors to these processes remain to be elucidated. Furthermore, the role of the MAPK signalling pathway in embryonic development was explored using only an ERK inhibitor. The impact of P38 and JNK inhibitors on embryonic development was not assessed and will be a focus of our future research. Lastly, while it is recognized that cell cycle regulation, oxidative stress, and apoptosis are critical for embryonic development, we did not explore how HTR1B influences these processes during postimplantation embryonic development. This represents a significant area for further investigation.
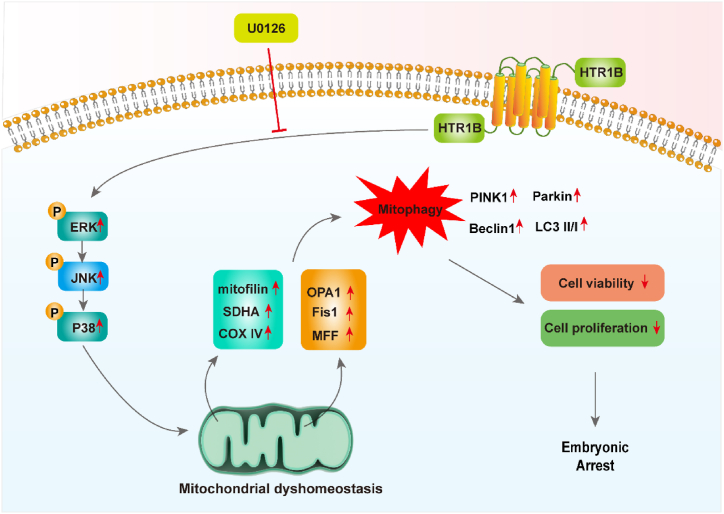
In summary, the activation of the MAPK signalling pathway by HTR1B disrupts mitophagy and mitochondrial homeostasis, resulting in impaired cell proliferation and migration (Fig. 7). This ultimately affects post-implantation embryonic development. Based on our findings, HTR1B could be a potential therapeutic target for patients experiencing EA.
Fig. 7.
This schematic representation illustrates the impact of HTR1B on mitochondrial homeostasis and mitophagy through the activation of the ERK/MAPK signalling pathway during post-implantation embryonic development. HTR1B upregulates the MAPK pathway, leading to mitochondrial dyshomeostasis, excessive mitophagy, and subsequent effects on cell viability and proliferation. Treatment with the ERK inhibitor U0126 rescues mitochondrial dyshomeostasis and excessive mitophagy.
Additional information
No additional information is available for this paper.
Ethics approval statement and consent statement
All patients in this study were anonymous, following the principles of the Declaration of Helsinki and approved by the Human Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No:2022309). All patients signed an informed consent form.
Data availability statement
The data associated with this study have not been deposited into a publicly accessible database, but is available on request.
CRediT authorship contribution statement
Si-min Ding: Writing – original draft, Software, Formal analysis, Data curation. Ling-ge Shi: Writing – original draft, Software, Formal analysis, Data curation. Zhen-ping Cao: Writing – review & editing, Resources. Na-na Zhu: Resources. Yun-yun Liu: Resources. Meng-yao Wang: Resources, Data curation. Shuang-shuang Cui: Resources. Hui-ru Cheng: Data curation. Dan Liang: Writing – review & editing, Project administration, Investigation, Data curation. Yun-xia Cao: Writing – review & editing, Supervision, Project administration, Investigation. Ya-jing Liu: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The present work was supported by the National Natural Science Foundation of China (82271675), and Research Fund of Anhui Institute of Translational Medicine (2022zhyx-B05, ZHYX2020A001).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e33132.
Contributor Information
Dan Liang, Email: ahmuxl@sina.com.
Yun-xia Cao, Email: caoyunxia6@126.com.
Ya-jing Liu, Email: yjl@ustc.edu.cn.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Hussein A.M., Balachandar N., Mathieu J., Ruohola-Baker H. Molecular regulators of embryonic diapause and cancer diapause-like state. Cells. 2022;11(19):2929. doi: 10.3390/cells11192929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh J., Papadopoulou A., Devall A.J., Jeffery H.C., Beeson L.E., Do V., Price M.J., Tobias A., Tuncalp O., Lavelanet A., Gulmezoglu A.M., Coomarasamy A., Gallos I.D. Methods for managing miscarriage: a network meta-analysis. Cochrane Database Syst. Rev. 2021;6(6):CD012602. doi: 10.1002/14651858.CD012602.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong G., Yin C., Huang Y., Yang Y., Hu T., Zhu Z., Shi X., Lin Y. A survey of influencing factors of missed abortion during the two-child peak period. J Obstet Gynaecol. 2021;41(6):977–980. doi: 10.1080/01443615.2020.1821616. [DOI] [PubMed] [Google Scholar]
- 4.Jiang W.Z., Yang X.L., Luo J.R. Risk factors for missed abortion: retrospective analysis of a single institution's experience. Reprod. Biol. Endocrinol. 2022;20(1):115. doi: 10.1186/s12958-022-00987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Z., Yan L., Liang X., Wang H. Progress in deciphering trophoblast cell differentiation during human placentation. Curr. Opin. Cell Biol. 2020;67:86–91. doi: 10.1016/j.ceb.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Liu J., Zhang Z., Zhu W., Shen Y., Gu Y., Zhang X., He L., DU J. CircFBXW4 regulates human trophoblast cell proliferation and invasion via targeting miR-324-3p/TJP1 axis in recurrent spontaneous abortion. Placenta. 2022;126:1–11. doi: 10.1016/j.placenta.2022.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Jeyarajah M.J., Jaju B.H.A.T.T.A.D.G., Kelly R.D., Baines K.J., Jaremek A., Yang F.P., Okae H., Arima T., Dumeaux V., Renaud S.J. The multifaceted role of GCM1 during trophoblast differentiation in the human placenta. Proc Natl Acad Sci U S A. 2022;119(49) doi: 10.1073/pnas.2203071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawamura E., Hamilton G.B., Miskiewicz E.I., Macphee D.J. Examination of FERMT1 expression in placental chorionic villi and its role in HTR8-SVneo cell invasion. Histochem. Cell Biol. 2021;155(6):669–681. doi: 10.1007/s00418-021-01977-y. [DOI] [PubMed] [Google Scholar]
- 9.Faas M.M., DE Vos P. Mitochondrial function in immune cells in health and disease. Biochim. Biophys. Acta, Mol. Basis Dis. 2020;1866(10) doi: 10.1016/j.bbadis.2020.165845. [DOI] [PubMed] [Google Scholar]
- 10.Klionsky D.J., Petroni G., Amaravadi R.K., Baehrecke E.H., Ballabio A., Boya P., Bravo-San Pedro J.M., Cadwell K., Cecconi F., Choi A.M.K., Choi M.E., Chu C.T., Codogno P., Colombo M.I., Cuervo A.M., Deretic V., Dikic I., Elazar Z., Eskelinen E.L., Fimia G.M., Gewirtz D.A., Green D.R., Hansen M., Jaattela M., Johansen T., Juhasz G., Karantza V., Kraft C., Kroemer G., Ktistakis N.T., Kumar S., Lopez-Otin C., Macleod K.F., Madeo F., Martinez J., Melendez A., Mizushima N., Munz C., Penninger J.M., Perera R.M., Piacentini M., Reggiori F., Rubinsztein D.C., Ryan K.M., Sadoshima J., Santambrogio L., Scorrano L., Simon H.U., Simon A.K., Simonsen A., Stolz A., Tavernarakis N., Tooze S.A., Yoshimori T., Yuan J., Yue Z., Zhong Q., Galluzzi L., Pietrocola F. Autophagy in major human diseases. EMBO J. 2021;40(19) doi: 10.15252/embj.2021108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin X.Y., Shen H.H., Zhou W.J., Mei J., Lu H., Tan X.F., Zhu R., Zhou W.H., Li D.J., Zhang T., Ye J.F., Li M.Q. Insight of autophagy in spontaneous miscarriage. Int. J. Biol. Sci. 2022;18(3):1150–1170. doi: 10.7150/ijbs.68335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H.L., Lai Z.Z., Shi J.W., Zhou W.J., Mei J., Ye J.F., Zhang T., Wang J., Zhao J.Y., Li D.J., Li M.Q. A defective lysophosphatidic acid-autophagy axis increases miscarriage risk by restricting decidual macrophage residence. Autophagy. 2022;18(10):2459–2480. doi: 10.1080/15548627.2022.2039000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su Y., Zhang J.J., He J.L., Liu X.Q., Chen X.M., Ding Y.B., Tong C., Peng C., Geng Y.Q., Wang Y.X., Gao R.F. Endometrial autophagy is essential for embryo implantation during early pregnancy. J. Mol. Med. (Berl.) 2020;98(4):555–567. doi: 10.1007/s00109-019-01849-y. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H.L., Shi X.T., Xu X.F., Xiong Y.W., Yi S.J., Zhou G.X., Liu W.B., Huang M.M., Gao L., Zhang C., Zhao L.L., Xu D.X., Wang H. Environmental cadmium exposure induces fetal growth restriction via triggering PERK-regulated mitophagy in placental trophoblasts. Environ. Int. 2021;147 doi: 10.1016/j.envint.2020.106319. [DOI] [PubMed] [Google Scholar]
- 15.Peric M., Beceheli I., Cicin-Sain L., Desoye G., Stefulj J. Serotonin system in the human placenta - the knowns and unknowns. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.1061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karmakar S., Lal G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics. 2021;11(11):5296–5312. doi: 10.7150/thno.55986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenfeld C.S. Placental serotonin signaling, pregnancy outcomes, and regulation of fetal brain developmentdagger. Biol. Reprod. 2020;102(3):532–538. doi: 10.1093/biolre/ioz204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oufkir T., Arseneault M., Sanderson J.T., Vaillancourt C. The 5-HT 2A serotonin receptor enhances cell viability, affects cell cycle progression and activates MEK-ERK1/2 and JAK2-STAT3 signalling pathways in human choriocarcinoma cell lines. Placenta. 2010;31(5):439–447. doi: 10.1016/j.placenta.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Liu K., Zhuan Q., Liu Z., Meng L., Fu X., Jia G., Hou Y. Mitochondrial calcium disorder affects early embryonic development in mice through regulating the ERK/MAPK pathway. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/8221361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao M., Zhang T., Chen T., Chen Z., Zhu Z., Wen Y., Qin S., Bao Y., Zhao T., Li H., Liu L., Hao M., Wang J., Wang F., Wang H., Zhou B., Zhang H., Xia G., Wang C. Polycomb repressive complex 1 modulates granulosa cell proliferation in early folliculogenesis to support female reproduction. Theranostics. 2024;14(4):1371–1389. doi: 10.7150/thno.89878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M., Wang R.B., Xing J.H., Tang Y.X. Atractylenolide inhibits apoptosis and oxidative stress of HTR-8/SVneo cells by activating MAPK/ERK signalling in preeclampsia. Phytomedicine. 2021;93 doi: 10.1016/j.phymed.2021.153773. [DOI] [PubMed] [Google Scholar]
- 22.Fan Z., Wang Q., Deng H. Circ_0011460 upregulates HTRA1 expression by sponging miR-762 to suppress HTR8/SVneo cell growth, migration, and invasion. Am. J. Reprod. Immunol. 2021;86(5) doi: 10.1111/aji.13485. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y., Ma X., Liu Y. Hsa_circ_0001326 regulates proliferation, migration, invasion, and EMT of HTR-8/SVneo cells via increasing IL16 expression. Am. J. Reprod. Immunol. 2021;86(5) doi: 10.1111/aji.13484. [DOI] [PubMed] [Google Scholar]
- 24.Rafat A., Dizaji A.S.L.K., Mazloumi Z., Movassaghpour A.A., Talebi M., Shanehbandi D., Farahzadi R., Nejati B., Nozad Charoudeh H. Telomerase inhibition on acute myeloid leukemia stem cell induced apoptosis with both intrinsic and extrinsic pathways. Life Sci. 2022;295 doi: 10.1016/j.lfs.2022.120402. [DOI] [PubMed] [Google Scholar]
- 25.Liu S., Yao S., Yang H., Liu S., Wang Y. Autophagy: regulator of cell death. Cell Death Dis. 2023;14(10):648. doi: 10.1038/s41419-023-06154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu T., Yan M., Liu F., Ma Y., Fang Y. The role of p53-MDM2 signaling in missed abortion and possible pathogenesis. J. Obstet. Gynaecol. Res. 2022;48(11):2686–2696. doi: 10.1111/jog.15385. [DOI] [PubMed] [Google Scholar]
- 27.Fisher J.J., Bartho L.A., Perkins A.V., Holland O.J. Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin. Exp. Pharmacol. Physiol. 2020;47(1):176–184. doi: 10.1111/1440-1681.13172. [DOI] [PubMed] [Google Scholar]
- 28.Yildirim R.M., Seli E. The role of mitochondrial dynamics in oocyte and early embryo development. Semin. Cell Dev. Biol. 2024;159–160:52–61. doi: 10.1016/j.semcdb.2024.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Guo S.M., Zhang Y.R., Ma B.X., Zhou L.Q., Yin Y. Regulation of cleavage embryo genes upon DRP1 inhibition in mouse embryonic stem cells. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1191797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y., Mei N.H., Cheng G.P., Yang J., Zhou L.Q. Inhibition of DRP1 impedes zygotic genome activation and preimplantation development in mice. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.788512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M., Shi J.-L., Zheng Z.-M., Lin Z., Li M.-Q., Shao J. An abnormal LPA/LPAR1–NHE1 axis leads to the autophagy deficiency of trophoblast cells in recurrent spontaneous abortion. Reproduction. 2023;166(5):12. doi: 10.1530/rep. [DOI] [PubMed] [Google Scholar]
- 32.Moura M.T., Latorraca L.B., Paula-Lopes F.F. Contextualizing autophagy during gametogenesis and preimplantation embryonic development. Int. J. Mol. Sci. 2021;22(12):6313. doi: 10.3390/ijms22126313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H., Yang H.L., Zhou W.J., Lai Z.Z., Qiu X.M., Fu Q., Zhao J.Y., Wang J., Li D.J., Li M.Q. Rapamycin prevents spontaneous abortion by triggering decidual stromal cell autophagy-mediated NK cell residence. Autophagy. 2021;17(9):2511–2527. doi: 10.1080/15548627.2020.1833515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai H., Chen L., Zhang M., Xiang W., Su P. Low expression of MFN2 is associated with early unexplained miscarriage by regulating autophagy of trophoblast cells. Placenta. 2018;70:34–40. doi: 10.1016/j.placenta.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Wu L., Duan Q., Gao D., Wang Y., Xue S., Li W., Lei M. Zearalenone blocks autophagy flow and induces cell apoptosis during embryo implantation in gilts. Toxicol. Sci. 2020;175(1):126–139. doi: 10.1093/toxsci/kfaa018. [DOI] [PubMed] [Google Scholar]
- 36.Li Y., Zhao H., Li N., Yuan C., Dong N., Wen J., Li Z., Wang Q., Wang L., Mao H. BBOX1-AS1 mediates trophoblast cells dysfunction via regulating hnRNPK/GADD45A axisdagger. Biol. Reprod. 2023;108(3):408–422. doi: 10.1093/biolre/ioad002. [DOI] [PubMed] [Google Scholar]
- 37.Zhou S., Shen Y., Zang S., Yin X., Li P. The epigenetic role of HTR1A antagonist in facilitating GnRH expression for pubertal initiation control. Mol. Ther. Nucleic Acids. 2021;25:198–206. doi: 10.1016/j.omtn.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu W.J., Chen C.Z., Peng Y.X., Li Z., Gao Y., Liang S., Yuan B., Kim N.H., Jiang H., Zhang J.B. Schisanhenol improves early porcine embryo development by regulating the phosphorylation level of MAPK. Theriogenology. 2021;175:34–43. doi: 10.1016/j.theriogenology.2021.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with this study have not been deposited into a publicly accessible database, but is available on request.