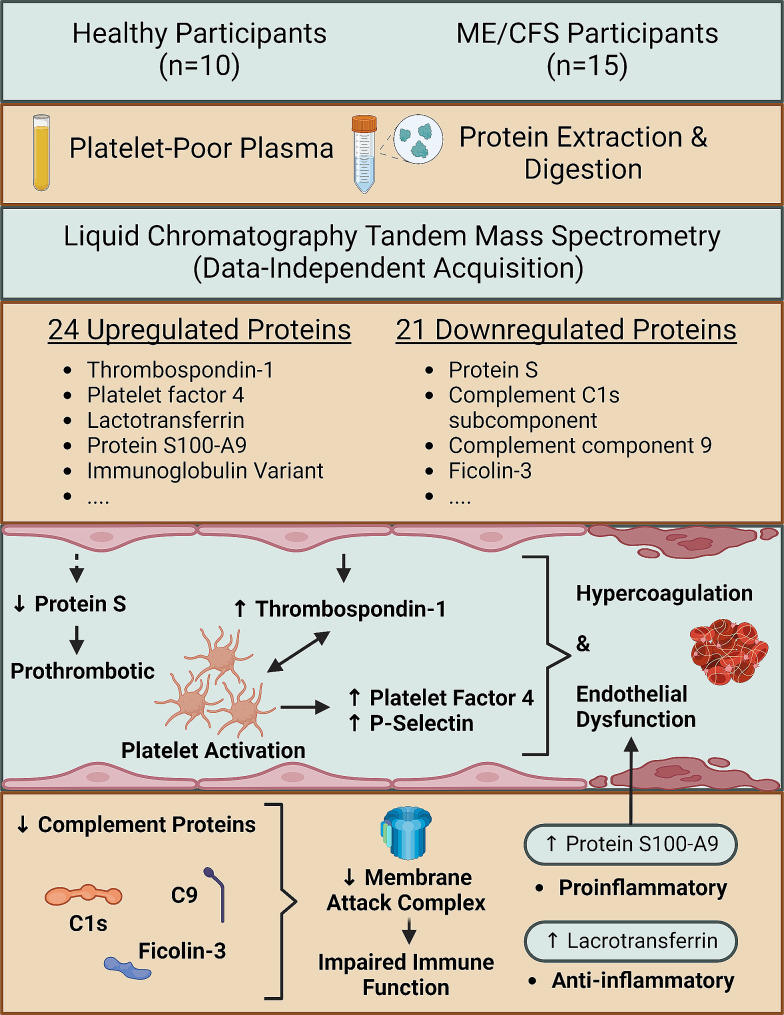
Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating chronic condition that is characterized by unresolved fatigue, post-exertion symptom exacerbation (PESE), cognitive dysfunction, orthostatic intolerance, and other symptoms. ME/CFS lacks established clinical biomarkers and requires further elucidation of disease mechanisms. A growing number of studies demonstrate signs of hematological and cardiovascular pathology in ME/CFS cohorts, including hyperactivated platelets, endothelial dysfunction, vascular dysregulation, and anomalous clotting processes. To build on these findings, and to identify potential biomarkers that can be related to pathophysiology, we measured differences in protein expression in platelet-poor plasma (PPP) samples from 15 ME/CFS study participants and 10 controls not previously infected with SARS-CoV-2, using DIA LC-MS/MS. We identified 24 proteins that are significantly increased in the ME/CFS group compared to the controls, and 21 proteins that are significantly downregulated. Proteins related to clotting processes – thrombospondin-1 (important in platelet activation), platelet factor 4, and protein S – were differentially expressed in the ME/CFS group, suggestive of a dysregulated coagulation system and abnormal endothelial function. Complement machinery was also significantly downregulated, including C9 which forms part of the membrane attack complex. Additionally, we identified a significant upregulation of lactotransferrin, protein S100-A9, and an immunoglobulin variant. The findings from this experiment further implicate the coagulation and immune system in ME/CFS, and bring to attention the pathology of or imposed on the endothelium. This study highlights potential systems and proteins that require further research with regards to their contribution to the pathogenesis of ME/CFS, symptom manifestation, and biomarker potential, and also gives insight into the hematological and cardiovascular risk for ME/CFS individuals affected by diabetes mellitus.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02315-x.
Keywords: Myalgic encephalomyelitis/Chronic fatigue syndrome (ME/CFS), Proteomics, Thrombotic pathology, Endothelial pathology
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating chronic condition that manifests in various physiological systems and is characterized by unresolved fatigue and post-exertional symptom exacerbation (PESE) [1, 2]. The onset of ME/CFS is often connected to viral/bacterial infection [3, 4], with the herpesviruses being most implicated [5–7]. However, there are no widely-established clinical biomarkers for the condition and much of the disease pathogenesis remains unknown.
We have previously shown that whole blood and platelet-poor plasma (PPP) from ME/CFS study participants showed hypercoagulability as measured via thromboelastography and platelet visualization [8]. PPP from the ME/CFS group also contained significant levels of amyloid fibrin(ogen) (some 10× in area of that of the controls), albeit less and of smaller size than observed in Long COVID PPP samples [9, 10].
Along with our study, a growing body of research suggests that cardiovascular and hematological abnormalities, such as endothelial dysfunction [11–18], abnormal blood flow and hence vascular dysregulation [19–21], and hyperactivated platelets [8, 22, 23] may contribute to the ME/CFS disease process [24]. Furthermore, it has been demonstrated that plasma from ME/CFS individuals cause dysfunction of healthy endothelial cells [12], and prompts the assessment of the blood for potential diagnostic and mechanism-related biomarkers.
To expand on these findings, we randomly selected a subset of ME/CFS and healthy control PPP samples from our previous study cohort and used data-independent acquisition (DIA) mass spectrometry (MS) (DIA LC-MS/MS) to identify differentially expressed proteins. DIA LC-MS/MS possesses the capabilities to capture nearly the entire proteome of a sample, enabling global and nominally unbiased detection and quantification of peptides [25]. Using this untargeted approach, we identified statistically significant differences in the levels of 45 proteins between ME/CFS and healthy controls. A select few came with both strong statistical significance and exhibit compelling fold changes, and, consistent with other data [8, 16, 18, 22, 23, 26], these pertain to the endothelium and coagulation system, and immune function.
Methods
Ethical statement
Ethical clearance was issued by the Health Research Ethics Committee (HREC) of Stellenbosch University (South Africa) (N19/03/043, project ID #9521). Strict compliance to ethical guidelines were carried out, as guided by the Declaration of Helsinki, South African Guidelines for Good Clinical Practice, and Medical Research Council Ethical Guidelines for Research.
Blood collection and demographics
Stored PPP samples from 10 healthy individuals were used as controls for this study. The exclusion principles applied to the controls include smoking, pregnancy, contraceptives, cardiovascular disease, coagulopathy, and a previous SARS-CoV-2 infection. 15 stored ME/CFS PPP samples, which were a part of a larger sample group that was collected for a previous study [8], were included in this experiment. ME/CFS blood samples were recruited via the ME/CFS Foundation of South Africa. All ME/CFS participants in this study had not experienced a SARS-CoV-2 infection prior to the date of sample collection. Sodium citrate tubes were used for blood collection, and centrifuged at 3000×g for 15 min at room temperature. The PPP was collected, and stored at –80 °C. ME/CFS individuals were asked to complete the ICC Symptom Questionnaire [27] to further describe the types and severity of symptoms experienced by this cohort.
Sample preparation for proteomics
10 control and 15 ME/CFS stored platelet-poor plasma (PPP) samples were used for the proteomics analysis. Protein determination was performed using a Jenway 7415 Nano Micro-Volume Spectrophotometer. Samples were diluted 20× with ammonium bicarbonate. 50 µg of protein was obtained from each sample and the volume was readjusted to 50 µL with Tris-Buffer (0.1 M; 1% DS). Disulphide bridges were reduced using 5 mM TCEP (tris(2-carboxyethyl)phosphine) at room temperature for 1 h. Cysteine residues were then blocked with 10mM MMTS (Methyl methanethiosulfonate) for 30 min at room temperature. Interfering substances were removed prior to digestion by washing on bead using MagResyn HILIC (https://resynbio.com/wp-content/uploads/2019/12/IFU_HILIC.pdf). Samples were digested on MagResyn HILIC particles with 1 µg trypsin (Pierce) at an enzyme: substrate of 1:50. Samples were left to incubate for 18 h at 37 °C. The supernatant was then collected, and the MagResyn HILIC particles were washed with 50 µL 1% TFA (trifluoroacetic acid) to remove any peptides bound to the particles. This supernatant was then also collected. Supernatants were combined, dried, and the peptides were resuspended in 50µL of 50% acetonitrile and then centrifuged at 10,000g for 5 min to remove any particulate. 20 µL of supernatant was then removed and dried. The samples were resuspended in loading solvent (See Liquid Chromatography section below) containing Biognosys 11 iRTs (indexed retention time standards) at 0.05/µL in preparation for mass spectrometry.
Liquid chromatography (Dionex nano-RSLC)
Liquid chromatography experiments were conducted using the Thermo Scientific Ultimate 3000 RSLC equipped with a 20 mm × 100 μm C18 trap column (Thermo Scientific) and a CSH 25 cm × 75 μm, 1.7 μm particle size C18 column (Waters) analytical column. The loading solvent was constituted by 2% acetonitrile: water and 0.1% formic acid; Solvent A: water and 0.1% formic acid; and Solvent B: 100% acetonitrile containing 0.1% formic acid. Samples were transferred onto the trap column from an autosampler (set to 7 °C) at a flow rate of 2µL/min, for 5 min prior to the samples being eluted onto the analytical column. 300nL/min defined the flow rate, and the gradient occurred as follows: 5–30% B over 60 min and 30–50% B from 60 to 80 min. The experiment was performed at 45 °C.
Library building: data-dependent acquisition (DDA)
Samples were pooled for the construction of the library, to obtain a complete (as possible) protein database from our samples. Samples were reduced and cysteine residues blocked as described above, and the MagResyn HILIC protocol (https://resynbio.com/wp-content/uploads/2019/12/IFU_HILIC.pdf) was again used for protein clean-up. Protein digest was performed on MagResyn HILIC particles with 1 µg trypsin as previously described. A peptide clean-up was performed using HILIC Clean-Up of Peptides Post Protein Digestion (https://resynbio.com/wp-content/uploads/2021/12/HILIC_PEPCLU.pdf). Samples were then cleaned and further fractionated using Pierce™ C18 Spin Tips & Columns (catalogue number: 89,870) with acetonitrile (50%, 20%, 17.5%, 15%, 12.5%, 10%, 7.5%, and 5%). LC experiments were carried out as described previously. The mass spectrometry analysis was performed using a Thermo Scientifc Fusion mass spectrometer equipped with a Nanospray Flex ionization source. Positive mode was chosen with spray voltage set to 2 kV and the ion transfer capillary set to 290 °C. Internal calibration of spectra was conducted using polysiloxane ions at m/z = 445.12003. MS1 scans were performed using the Orbitrap detector set at 60,000 resolution over the scan range 375–1500 with AGC target at 4E5, and maximum injection time of 50 ms. Data was acquired in profile mode. MS2 acquisitions were carried out using monoisotopic precursor selection for ion with charges + 2 to +7 with error tolerance set to ± 10 ppm. Precursor ions were excluded from fragmentation once for a period of 60 s. Precursor ions were selected for fragmentation in HCD mode using the quadrupole mass analyser with HCD energy set to 30%. Fragment ions were detected in the Orbitrap mass analyzer set to 30,000 resolution. The AGC target was set to 5E4 and the maximum injection time to 60 ms. The data was acquired in centroid mode.
Mass spectrometry: data independent acquisition (DIA)/SWATH
A Thermo Scientifc Fusion mass spectrometer equipped with a Nanospray Flex ionization source was used in this study, as previously mentioned. Samples entered via a stainless-steel nano-bore emitter. Positive mode was used for data collection with the spray voltage set to 2 kV and ion transfer capillary set at 290 °C. Alignment of chromatograms were done with the aid of the iRTs kit (Biognosys). MS1 scans were performed using the Orbitrap detector set at 60,000 resolution over a m/z range of 375–1500. The automatic gain control (AGC) target was set to standard and maximum injection time at 100 ms. Data were acquired in profile mode. Precursor ions were selected for fragmentation in higher-energy C-trap dissociation (HCD) mode using the quadrupole mass analyzer with HCD energy set to 30%. Precursor ions were scanned in three windows, 355–555, 555–755, and 755–955 m/z (which were saved as three separate raw files), with an isolation window of 10 m/z and an overlap of 1 m/z. Ions were detected in the Orbitrap mass analyzer set to 30,000 resolution. The AGC target was set to custom and the maximum injection time mode set to custom. The data were acquired in centroid mode.
Data analysis
The raw files generated by the mass spectrometer were imported into Skyline (version 22.2.0.312) using the DIA wizard. Precursor and ion charges were set to 2, 3 4 and 1, 2, 3, respectively, and shuffle sequence chosen as the decoy generation method. Semi-tryptic cleavage with 1 missed cleavage was allowed for. Precursor mass tolerance was set to 10 ppm and fragment mass tolerance set to 0.02 Da. Deamidation (NQ), oxidation (M), and methylthio (C) were allowed as dynamic modifications. Equalize medians was chosen as the normalization method with a 95% confidence interval. mProphet, which automatically adapts the error model for each data set and assigns a confidence measure to each peak group for quality control, was included in the analysis to score peptide identifications using its linear model [28]. A Q value of 0.05 was chosen. A database was constructed from UniProt using the keywords ‘plasma’, ‘immune system’, and ‘herpesviruses’. We also ran the analysis against the spectral library created from patient samples.
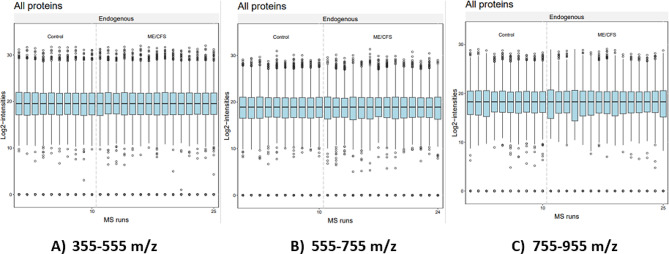
Quality control plots for all proteins (to assess system performance) were obtained using MSstatsShiny, whereby the Skyline output and annotation files, after rearranging the layout, were used as MSstatsShiny input files. Proteins with only 1 feature were not removed from the analysis, and a Q value of 0.05 was selected. For normalization, equalize medians were chosen. Missing values were censored and model-based imputation utilized. Runs with over 50% missing values were also removed.
Results
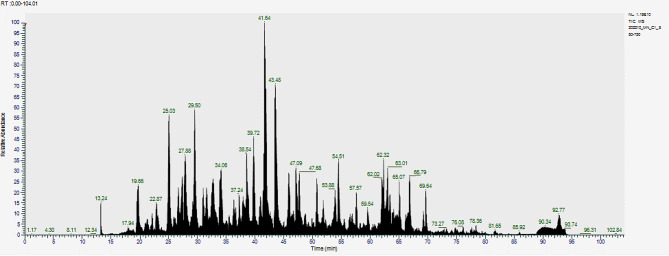
The demographics of the participant groups are contained within Table 1, along with the symptom severity score averages [27] of the ME/CFS population. A portion of the ME/CFS cohort presents with symptoms and comorbidities that are intimately associated with ME/CFS, specifically gastrointestinal issues (6/15 participants) [29], orthostatic symptoms (5/15 participants) [30–32], and fibromyalgia (3/15 participants) [33]. Proteomics data from the analysis of control (n = 10) and ME/CFS (n = 15) PPP using Skyline is represented in Table 2 and the total ion chromatogram (TIC) is depicted in Fig. 1. Figure 2 is a representation of the quality plots for all proteins/peptides across the three m/z ranges, which was obtained from MSstatsShiny. Our experiment indicates that 24 proteins are significantly increased in the ME/CFS group compared to the controls, and that 21 proteins are significantly downregulated. However, only a select few hold strong statistical significance. These will be presented and discussed, while the other identified protein data can be perused in Supplementary Material 1. In some cases, only a limited number of peptides from a protein are significant, which may reflect regulated peptides or be related to the number of ions entering the mass spectrometer at a given time point. The transitions for proteins detected by a single peptide are contained within Supplementary Material 2.
Table 1.
Demographics of the ME/CFS cohort and symptom score averages for the ICC questionnaire
| Age | |
| Age of control population (n=10; 7 females) | 59.3 ± 7.5 |
| Age of ME/CFS population (n=15; 11 females) | 48.9 ± 14.9 |
| P Value (parametric) | 0.054 |
| Comorbidities of ME/CFS Population (%) | |
| Gastrointestinal Symptoms | 40 |
| POTS | 33 |
| Gingivitis/Periodontitis | 20 |
| Hypercholesterolemia | 20 |
| Fibromyalgia | 20 |
| Psoriasis | 13 |
| Rheumatoid Arthritis | 13 |
| Hypertension | 13 |
| Mast Cell Activation Syndrome | 7 |
| Rosacea | 7 |
| ICC Questionnaire Results of ME/CFS Population (mean SD) | |
| Post-Exertional Neuroimmune exhaustion | 7.7 ± 1.9 |
| Neurological Impairments | 6.5 ± 2.8 |
| Immuno, Gastrointestinal, and Genitourinary impairments | 5.9 ± 2.8 |
| Energy Production/Transportation Impairments | 6.5 ± 2.8 |
The ICC questionnaire and the comorbidities are both self-reported by the patients
Statistical significance was determined at p < 0.05
Data are represented as mean ± SD
Table 2.
Selected significant protein and peptide data from the Skyline analysis (all data can be found in Supplementary Material 1)
| Protein name | Peptide sequence | Fold Change (ME/CFS raised) | p value | UniProt accession number | No. of peptides | m/z range |
|---|---|---|---|---|---|---|
| Thrombospondin-1 | GPDPSSPAFR | 3.55 | 0.00009 | P07996 | 2 | 355–555 m/z |
| TIVTTLQDSIR | 3.48 | 0.0002 | P07996 | 2 | 555–755 m/z | |
| IEDANLIPPVPDDKFQDLVDAVR | 3.75 | 0.00009 | P07996 | 3 | 755–955 m/z | |
| Platelet factor 4 | HITSLEVIK | 3.11 | 0.00009 | P02776 | 1 | 355–555 m/z |
| Vitamin K-dependent protein S | QSTNAYPDLR | 0.48 | 0.0006 | P07225 | 2 | 555–755 m/z |
| Complement C1s subcomponent | MLTPEHVFIHPGWK | 0.7 | 0.0069 | P09871 | 7 | 555–755 m/z |
| MLTPEHVFIHPGWK | 0.53 | 0.0013 | P09871 | 5 | 755–955 m/z | |
| Complement component C9 | ISEGLPALEFPNEK | 0.17 | 0.0001 | P02748 | 6 | 755–955 m/z |
| Ficolin-3 | YGIDWASGR | 0.65 | 0.0006 | O75636 | 5 | 355–555 m/z |
| QDGSVDFFR | 0.45 | 0.0086 | O75636 | 5 | 355–555 m/z | |
| LLGEVDHYQLALGK | 0.49 | 0.0348 | O75636 | 5 | 355–555 m/z | |
| LLGEVDHYQLALGK | 0.53 | 0.0084 | O75636 | 1 | 755–955 m/z | |
| Lactotransferrin | LRPVAAEVYGTER | 7.05 | 0.00009 | P02788 | 5 | 355–555 m/z |
| FQLFGSPSGQK | 8.38 | 0.00009 | P02788 | 3 | 555–755 m/z | |
| IDSGLYLGSGYFTAIQNLR | 4.52 | 0.0118 | P02788 | 3 | 555–755 m/z | |
| Protein S100-A9 | NIETIINTFHQYSVK | 2.08 | 0.0159 | P06702 | 1 | 555–755 m/z |
| NIETIINTFHQYSVK | 2.89 | 0.0046 | P06702 | 1 | 755–955 m/z | |
| Immunoglobulin heavy constant gamma 1 | DTLMISR | 1.51 | 0.0094 | P01857 | 6 | 355–555 m/z |
| GFYPSDIAVEWESNGQPENNYK | 5.64 | 0.0031 | P01857 | 20 | 755–955 m/z |
Fold change is expressed with reference to the ME/CFS group
Significance was determined at p < 0.05
Fig. 1.
Total Ion Chromatogram (TIC)
Fig. 2.
Quality control plots of protein levels of all samples across the three m/z ranges. Data were processed on MSstatsShiny. Normalization was performed using equalize medians
Discussion
In the present study, we employed DIA LC-MS/MS to search for any significant differences in protein levels between control and ME/CFS blood (PPP) samples. Albeit a small sample size, we offer insight into the differential protein levels between ME/CFS and control cohorts, and provide direction for future studies with larger cohorts. We identified 45 proteins whose differences in expression level are statistically significant (p < 0.05), but only a select few – the ones with strong statistical significance and a notable fold change – are discussed here.
Proteins related to the coagulation system
We identified three significant proteins related to the coagulation system that deserves discussion: thrombospondin-1; platelet factor 4; and vitamin K-dependent protein S. When interpreting these findings, there are a number of recently published articles implicating platelets and other components of and processes related to the coagulation system in ME/CFS cohorts [8, 22–24, 34, 35], as well as endothelial dysfunction [11–14, 16–18, 36]. The links between platelets, coagulation, and endothelial cells, and dysfunction thereof are discussed elsewhere [37–39]. Further work is now required to elucidate the impact of clotting and endothelial dysfunction on ME/CFS pathology and symptom presentation, and determine if any treatment can result from these findings.
Thrombospondin-1 (TSP-1) is a glycoprotein, part of a family of 5 thrombospondins [40], that is involved in platelet activation [41, 42], clot formation [43–45], haemostasis [46], vascular control [47, 48], inflammation [49, 50], and tissue repair [51]. TSP-1 is found within the extracellular matrix [52], α-granules of platelets [53], endothelial cells and macrophages [51, 54], and plasma [55]. Elevations in TSP-1 induce endothelial dysfunction and interfere with vascular control via a number of mechanisms, including modulation of nitric oxide [47, 48, 56–58]. The potential of TSP-1 to influence vascular control might have relevance to impaired blood flow observed in ME/CFS [19, 59]. In the context of platelets, TSP-1 leads to platelet activation via signalling of inhibitory cyclic adenosine monophosphate (cAMP) [46]. Furthermore, TSP-1-deficient mice exhibit signs of excessive bleeding and impaired coagulation [46]. The researchers also discovered that transfusion of wild-type platelets into TSP-1−/− mice improved clot formation and stability. Hence, TSP-1 plays an important role in platelet function and clot formation.
The exact cellular source of TSP-1 in our ME/CFS population is uncertain, as immune cells [60] and endothelial cells [54] are capable of increasing plasma levels of this protein. The increase in TSP-1 in our ME/CFS cohort might be related to the active states of platelets observed in a subset of these individuals [8, 22, 23], and might help explain endothelial dysregulation and impaired vascular control in this disease population. Interestingly, TSP-1 levels increase and originate from activated platelets during SARS-CoV-2 infection [61].
Platelet factor 4 (PF4) is a CXC chemokine that, much like TSP-1 (and P-selectin), is released from α-granules of activated platelets [62, 63]. Although we only detected a single peptide for PF4 (transition depicted in Supplementary Material 2), its presence in excess is expected when one reviews the evidence of platelet hyperactivity in ME/CFS [8, 22, 23, 35] and related chronic, inflammatory diseases [64]. Its primary function is to facilitate coagulation by neutralizing glycosaminoglycans on endothelial and platelet membranes, prompting platelet aggregation and monocyte recruitment [65–67]. In addition to clotting-specific functions, PF4 also seems to be involved in immune functioning, with its secretion and serum levels increasing during infection – which is expected as platelet activation increases in response to infection [68–70]. PF4 is protective against numerous microorganisms and exerts notable antiviral effects [70–73].
PF4 has been implicated in cardiovascular disease [74–76] and gastrointestinal conditions [77, 78], and has the potential to promote oxidative and nitrosative stress, and subsequent inflammatory sequelae [79–81], although some studies argue otherwise [82]. PF4 modulates endothelial and vascular smooth muscle cells in a proinflammatory manner [66, 83] and is likely an ongoing consequence of platelet hyperactivity in ME/CFS (along with elevations in TSP-1), potentially accounting for signs of endothelial damage observed in patients [12, 16, 18]. In Long COVID, plasma PF4 levels are significantly increased compared to controls [84].
Vitamin K-dependent protein S (PROS) is an endogenous anticoagulant which exists both free in plasma and as a complex where it is non-covalently bound to complement C4-B [85, 86], which, interestingly, is significantly downregulated in the ME/CFS group. PROS functions as a cofactor to another endogenous anticoagulant, protein C, and is required for the inactivation of activated clotting factor VIII [87]. Deficiencies of PROS leads to overzealous clot formation which confers an increased risk for venous thrombosis and, in severe cases, death [86]. Apart from its role in coagulation, PROS is also involved in bone metabolism [88, 89]. Within the hematological system, endothelial cells – a cell-type intimately involved in the regulation of hemostasis – are known to secrete PROS [90]. The downregulation of PROS in the ME/CFS group, along with increases in TSP-1 and PF4, further emphasize a procoagulant phenotype in this ME/CFS population, as well as dysregulated endothelial function.
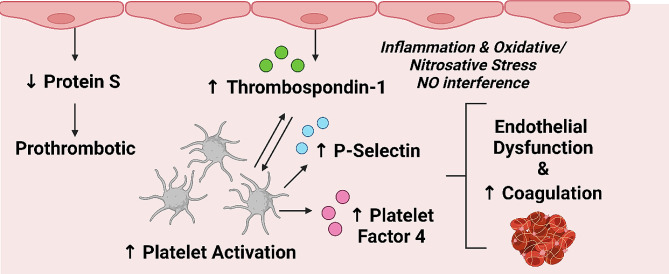
Besides the decreased anticoagulant capacity conferred by decreased levels of PROS, fibrinolysis may also be hindered as PROS facilitates clot degradation via a protein C-dependent mechanism [91]. Relevant to this finding of decreased PROS in the ME/CFS group, individuals suffering from acute COVID-19 and presenting with respiratory distress exhibit decreased serum levels of PROS [92], which likely contribute to the procoagulant pathology associated with SARS-CoV-2 infection [93, 94]. Furthermore, a proteomics analysis of extracellular vesicles obtained from ME/CFS plasma samples identified high levels of SERPINA5, which is involved in hemostasis, particularly by inhibiting protein C [95]. This finding is also indicative of a prothrombotic tendency that occurs by regulating the activity of protein C. A summary of these coagulation-related findings is given within Fig. 3.
Fig. 3.
Representation of the dysregulated coagulation system in the ME/CFS group as inferred from the results of this study (Table 2). The ME/CFS group exhibits an increased propensity for clotting due to decreased levels of vitamin K-dependent protein S (an endogenous anticoagulant). Increased levels of thrombospondin-1 (originating from endothelial cells and platelets) activate platelets, as well as contribute to endothelial dysfunction via proinflammatory and oxidative/nitrosative mechanisms – as does increased levels of platelet factor 4 and P-selectin [8]. The end result is a prothrombotic state, potentially resulting from and contributing to endothelial dysfunction
Proteins related to the Immune System and inflammation
Leukocyte dysfunction [96–99] and dysregulated inflammatory processes [100–106] are documented characteristics of ME/CFS. While we did not identify any significant differences between the two groups with regards to notorious proinflammatory cytokines, such as TNF-α, NF-κB, and IL-1β, we did identify a dysregulation of complement factors and other inflammation-related proteins, including lactotransferrin, protein S100-A9, and an immunoglobulin variant.
The complement system is a well-established element of the innate immune system, with more recent studies revealing its participation in adaptive immunity [107]. There are three different pathways, namely the classical, lecithin, and alternative pathway, which are discussed elsewhere [108]. Dysregulation of the complement system has been documented in both COVID-19 [109] and ME/CFS [110–113].
A previous study showed that a subgroup of ME/CFS individuals (107/250) expressed significantly higher levels of complement factor C1q [110], which, in our study, is not significantly different between groups. Rather, a subunit of the C1 complex, C1s, is downregulated in the ME/CFS group. C9 forms part of the membrane attack complex (or C5b-9) that is used to lyse targeted cells [114, 115], and C9 also contributes to inflammasome activation during infection [116]. In the ME/CFS group this complement protein is significantly downregulated, exhibiting a fold change of 0.17. Impaired complement function characterized by a reduced capacity to form membrane attack complexes and aid in inflammasome activation will certainly result in shortcomings in immune defence.
Ficolin-3 is a pattern recognition receptor which functions within the lectin complement pathway and exerts antibacterial and antiviral effects [117]. Importantly, deficiency of ficolin-3 results in immunodeficiency [118] and is associated with an higher risk (8-fold) of developing a disease and autoimmunity [119]. What may be of relevance is that ficolin dysfunction or under-expression is associated with viral infection and disease [117], and hence may have a major role to play in the ME/CFS disease process [3, 5], related to herpesviruses and other microorganisms. In contrast to our finding of decreased ficolin-3 levels, a previous study found an increase in ficolin-3 expression in leukocytes from ME/CFS patients [120].
The downregulation of these complement proteins corroborates the notion of immune dysfunction in ME/CFS and may confer a susceptibility to infections, and perhaps contribute to the symptoms of malaise and fatigue [1]. Furthermore, viruses are known to have evolved strategies to bypass host defences, and the complement system is a target of such evasive processes [108]. It is speculative, but plausible to propose that these results are a consequence of such viral infection and subsequent maladaptation of the immune system. Further investigation of complement function in ME/CFS is required.
Lactotransferrin (LF) is a non-hematic iron-binding, pleiotropic glycoprotein that is found in mammalian milk, and is produced by a variety of cells, including immune cells [121, 122]. LF, apart from its ability to bind Fe3+ ions and prevent Fe3+-induced oxidative stress and inflammation [123–125], is well known for its antimicrobial, antioxidant, anti-inflammatory, prebiotic, and probiotic effects, and, hence, therapeutic potential [122, 126–129].
The physiological protection offered by LF extends into multiple organ systems, especially the immune system. It acts as a mediator of immune function, whereby, apart from enhancing certain aspects inflammation, serves to prevent an exaggerated inflammatory response and subsequent tissue damage [130–132]. It exerts chemotactic effects on leukocytes [133] and prevents the release of proinflammatory cytokines [134], likely by inhibiting TLR4 activity [135]. Due to its antimicrobial and immunomodulatory effects, LF forms part of the innate defence, and even bridges components of the innate branch to the acquired branch of the immune system [130, 133, 136].
As a biomarker, LF is useful at monitoring inflammation [137–139]. In our ME/CFS population, the increase in serum LF might be an indicator of ongoing inflammation, and even an attempt to counter proinflammatory processes associated with pathology. With speculation aside, the large fold change in this protein might be suggestive of reason for further investigation.
Protein S100-A9 – not to be confused with the endogenous anticoagulant, protein S – is a member of the S100 protein family and is known for its role in inflammation [140]. S100-A9 is predominantly expressed by immune cells, such as monocytes, macrophages, and neutrophils [141, 142], and is recognised as a damage-associated molecular pattern molecule and antimicrobial involved in the innate response [143–145]. Additionally, it acts as a chemotactic agent for phagocytes [146, 147] and is also essential for the translocation of leukocytes across the endothelium, due to its role in microtubule reorganisation [148].
S100-A9 is upregulated during infection, inflammation, and disease, and is highly expressed at sites of inflammation and injury [149–155]. Studies have shown that S100-A9 is proinflammatory, disrupts endocrine signalling, activates NF-κB, and interacts with TLR4 and the receptor for advanced glycation end products [146, 156–160]. Overexpression of S100-A9 can be more damaging than beneficial, as it can result in overzealous immune activity and subsequent inflammatory and oxidative damage, and even toxic shock [157].
S100-A9 promotes a proinflammatory and prothrombotic phenotype in endothelial cells, impairs cell-adhesion processes of the endothelium (thereby increasing vascular permeability, which coincides with the function of leukocyte recruitment and migration), and upregulates endothelial TSP-1 expression [143, 161] – a phenomenon which perhaps underlies the increase in TSP-1 exhibited by the ME/CFS group in this study. The potential of S100-A9 to cause endothelial dysfunction and damage, as well as its overexpression during inflammatory states and its proinflammatory nature, may be of relevance to ME/CFS, a disease which is characterized by endothelial dysfunction [11, 12, 14, 16, 17, 162] and (dysregulated) inflammation [26, 101, 104, 106]. Even more so, S100-A9 can directly activate platelets and promote procoagulant functions [163]. Data regarding S100-A9 in ME/CFS cohorts is scarce, and hence requires investigation, especially since it plays important roles in immune and vascular function. With regards to SARS-CoV-2, S100-A9 is increased during infection [164, 165].
A comment on viral proteins
With regards to viral involvement, a study published in late 2022 discovered signs of active human herpesvirus 6 (HHV6) and Epstein-Barr Virus (EBV) in neurological tissue from deceased ME/CFS patients [6], thereby supporting previous hypotheses implicating herpesviruses in this condition [3, 5]. A more recent study also highlighted the presence and role of active herpes infection in a much larger cohort [7], as have other studies in the past [166–168]. There are also indications that viral reactivation of these herpesviruses is central to ME/CFS and Long COVID pathology [6, 169]. However, because much of the human population harbors latent herpesviruses (and indeed Mycobacterium tuberculosis and Helicobacter pylori and other dormant bacteria) without overt disease, the specific mechanisms by which their activity may contribute to ME/CFS pathology requires further study.
Our only significant DIA LC-MS/MS findings related to herpesviruses is the downregulation of protein UL29 from HHV6-H in the ME/CFS group. This is not conclusive, and most likely reflects the difficulty of detecting low-abundance proteins in plasma samples with a high dynamic range, especially when using global, untargeted proteomics approaches. Future studies aiming to confidently detect viral proteins should aim to decrease the dynamic range within plasma samples if doing DIA analyses, or perform targeted proteomics experiments. We also noticed that several viral proteins were phosphorylated; planning an experiment with this is in mind might offer information about viral activity.
Conclusion
Identification of potential ME/CFS biomarkers is imperative for improved diagnosis, mechanism elucidation, and clinical care. Using DIA LC-MS/MS we show a significant, differential expression of proteins in PPP samples from ME/CFS and healthy individuals, involving the coagulation system, endothelium, and immune system.
Significant increases in TSP-1 and PF4, and a significant decrease in PROS suggest that the coagulation system is dysregulated in the present ME/CFS cohort. Our present and previous data [8] point to a procoagulant profile in ME/CFS, revolving around platelet hyperactivity and potentially a dysregulated endothelium. Related and recent studies are in accord with these inferences [11–14, 16, 17, 22, 23]. Incidentally, Long COVID suffers benefit from anticoagulant and antiplatelet therapy [170, 171]; further research is required to determine if this form of therapy will benefit ME/CFS patients exhibiting clotting pathology and thrombotic endothelialitis.
Beyond the coagulation system, our data further support immunological dysfunction in ME/CFS, including alterations in the complement system, as well as increases in LF and S100-A9. Deficits in the complement system, including a downregulation of the complement proteins that constitute the membrane-attack complex, could be related to viral infection and immune dysregulation in ME/CFS [3, 26], and deserves further study.
Our results highlight physiological systems – namely the cardiovascular, coagulation, and immune system – and proteins that require further research with regards to their contribution to the pathogenesis of ME/CFS, symptom manifestation, and biomarker potential. Furthermore, individuals from the ME/CFS study population were not diagnosed with diabetes mellitus, and hence this study gives insight into the thrombotic and cardiovascular risk associated with ME/CFS individuals also affected by diabetes mellitus.
Study limitations
A major limitation of using DIA LC-MS/MS on samples that contain a large dynamic range is the detection and quantitation of low abundance proteins, such as herpesvirus proteins (not to mention the computational power and efficiency needed to identify and annotate each peptide detected). Future studies can aim to decrease the dynamic range when assessing viral proteins via this method, or employ targeted proteomics techniques (for the sake of identifying low-abundance proteins, as well as corroborating what was identified in the present, untargeted experiment).
Furthermore, a similar approach will benefit from a larger sample size and a further subdivision of the study groups by gender. The inclusion of a study population that experiences a similar lifestyle to that of ME/CFS individuals, i.e. a bed-bound population without ME/CFS, such as post-bone fracture patients, will also be beneficial. Another important point to raise is the influence of pre- and post-menopausal physiology in the female population [172, 173], which was not accounted for in our recruitment process.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We wish to thank the patients and their families who participated in this study. We specifically like to thank Retha Viviers, founder of the South African ME/CFS foundation. Without her perseverance and support, this study would not have been possible. Sadly, Retha passed away recently.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Stellenbosch University (HREC) (N19/03/043, project ID #9521; yearly reapproval). Written informed consent has been obtained from all the participants. Participants or the public were not involved in the design, conduct, reporting, or dissemination plans of our research.
Author contributions
JMN: Proteomics analysis, Investigation, writing review and editing; MV: Proteomics analysis support and analysis; AP: Editing of paper; DBK: editing and co-corresponding author. All authors have read and agreed to the published version of the manuscript. EP: Conceptualization, editing, data curation funding, study leader and co-corresponding author.
Funding
AP: Funding was provided by PolyBio Research Foundation. EP: Laboratory research supported by NRF of South Africa (grant number 142142) and SA MRC (self-initiated research (SIR) grant). DBK: thanks the Novo Nordisk Foundation for funding (grant NNF10CC1016517). The content and findings reported and illustrated are the sole deduction, view and responsibility of the researchers and do not reflect the official position and sentiments of the funders.
Data availability
Supporting proteomics data is available on request.
Declarations
Consent for publication
Written informed consent for the study has been obtained from all participants.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Douglas B. Kell, Email: dbk@liv.ac.uk
Etheresia Pretorius, Email: resiap@sun.ac.za.
References
- 1.Lim E-J, Son C-G. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) J Transl Med. 2020;18(1):289. doi: 10.1186/s12967-020-02455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komaroff AL, Lipkin WI. ME/CFS and Long COVID share similar symptoms and biological abnormalities: road map to the literature. Front Med. 2023;10:1187163. doi: 10.3389/fmed.2023.1187163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasa S, Nora-Krukle Z, Henning N, Eliassen E, Shikova E, Harrer T, Scheibenbogen C, Murovska M, Prusty BK. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) J Transl Med. 2018;16(1):268. doi: 10.1186/s12967-018-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stallmach A, Quickert S, Puta C, Reuken PA. The gastrointestinal microbiota in the development of ME/CFS: a critical view and potential perspectives. Front Immunol. 2024;15:1352744. doi: 10.3389/fimmu.2024.1352744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariza ME. Myalgic encephalomyelitis/chronic fatigue syndrome: the human herpesviruses are back! Biomolecules. 2021;11(2):185. doi: 10.3390/biom11020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasimir F, Toomey D, Liu Z, Kaiping AC, Ariza ME, Prusty BK. Tissue specific signature of HHV-6 infection in ME/CFS. Front Mol Biosci. 2022;9:1044964. doi: 10.3389/fmolb.2022.1044964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasa-Dzelzkaleja S, Krumina A, Capenko S, Nora-Krukle Z, Gravelsina S, Vilmane A, Ievina L, Shoenfeld Y, Murovska M. The Vir ap: the persistent viral infections in the development and severity of myalgic encephalomyelitis/chronic fatigue syndrome. J Transl Med. 2023;21(1):33. doi: 10.1186/s12967-023-03887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunes JM, Kruger A, Proal A, Kell DB, Pretorius E. The occurrence of Hyperactivated platelets and fibrinaloid microclots in myalgic Encephalomyelitis/Chronic fatigue syndrome (ME/CFS) Pharmaceuticals. 2022;15(8):931. doi: 10.3390/ph15080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, Kell DB. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20(1):172. doi: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo SO, Watson LR, Rajaratnam K, Watson BW, Kell DB. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with long COVID/Post-Acute sequelae of COVID-19 (PASC) Cardiovasc Diabetol. 2022;21(1):148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blauensteiner J, Bertinat R, León LE, Riederer M, Sepúlveda N, Westermeier F. Altered endothelial dysfunction-related miRs in plasma from ME/CFS patients. Sci Rep. 2021;11(1):10604. doi: 10.1038/s41598-021-89834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertinat R, Villalobos-Labra R, Hofmann L, Blauensteiner J, Sepúlveda N, Westermeier F. Decreased NO production in endothelial cells exposed to plasma from ME/CFS patients. Vascul Pharmacol. 2022;143:106953. doi: 10.1016/j.vph.2022.106953. [DOI] [PubMed] [Google Scholar]
- 13.Haffke M, Freitag H, Rudolf G, Seifert M, Doehner W, Scherbakov N, Hanitsch L, Wittke K, Bauer S, Konietschke F, et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) J Transl Med. 2022;20(1):138. doi: 10.1186/s12967-022-03346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scherbakov N, Szklarski M, Hartwig J, Sotzny F, Lorenz S, Meyer A, Grabowski P, Doehner W, Scheibenbogen C. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020;7(3):1064–71. doi: 10.1002/ehf2.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanculescu D, Bergquist J. Perspective: drawing on findings from critical illness to explain myalgic encephalomyelitis/chronic fatigue syndrome. Front Med. 2022;9:818728. doi: 10.3389/fmed.2022.818728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sørland K, Sandvik MK, Rekeland IG, Ribu L, Småstuen MC, Mella O, Fluge Ø. Reduced endothelial function in myalgic Encephalomyelitis/Chronic fatigue syndrome-results from open-label cyclophosphamide intervention study. Front Med. 2021;8:642710. doi: 10.3389/fmed.2021.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaskamp L, Roubal C, Uddin S, Sotzny F, Kedor C, Bauer S, Scheibenbogen C, Seifert M. Serum of Post-COVID-19 syndrome patients with or without ME/CFS differentially affects endothelial cell function in Vitro. Cells. 2022;11(15):2376. doi: 10.3390/cells11152376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandvik MK, Sørland K, Leirgul E, Rekeland IG, Stavland CS, Mella O, Fluge Ø. Endothelial dysfunction in ME/CFS patients. PLoS ONE. 2023;18(2):e0280942. doi: 10.1371/journal.pone.0280942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Campen C, Verheugt FWA, Rowe PC, Visser FC. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: a quantitative, controlled study using Doppler Echography. Clin Neurophysiol Pract. 2020;5:50–8. doi: 10.1016/j.cnp.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph P, Arevalo C, Oliveira RKF, Faria-Urbina M, Felsenstein D, Oaklander AL, Systrom DM. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest. 2021;160(2):642–51. doi: 10.1016/j.chest.2021.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wirth K, Scheibenbogen C. A unifying hypothesis of the pathophysiology of myalgic Encephalomyelitis/Chronic fatigue syndrome (ME/CFS): recognitions from the finding of autoantibodies against ß2-adrenergic receptors. Autoimmun rev. 2020;19(6):102527. doi: 10.1016/j.autrev.2020.102527. [DOI] [PubMed] [Google Scholar]
- 22.Bonilla H, Hampton D, Marques de Menezes EG, Deng X, Montoya JG, Anderson J, Norris PJ. Comparative analysis of extracellular vesicles in patients with severe and mild myalgic encephalomyelitis/chronic fatigue syndrome. Front Immunol. 2022;13:841910. doi: 10.3389/fimmu.2022.841910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahanbani F, Maynard RD, Sing JC, Jahanbani S, Perrino JJ, Spacek DV, Davis RW, Snyder MP. Phenotypic characteristics of peripheral immune cells of myalgic encephalomyelitis/chronic fatigue syndrome via transmission electron microscopy: a pilot study. PLoS ONE. 2022;17(8):e0272703. doi: 10.1371/journal.pone.0272703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunes JM, Kell DB, Pretorius E. Cardiovascular and haematological pathology in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a role for viruses. Blood Rev. 2023;60:101075. doi: 10.1016/j.blre.2023.101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krasny L, Bland P, Kogata N, Wai P, Howard BA, Natrajan RC, Huang PH. SWATH mass spectrometry as a tool for quantitative profiling of the matrisome. J Proteom. 2018;189:11–22. doi: 10.1016/j.jprot.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bansal A. What Causes ME/CFS: the role of the dysfunctional immune system and viral infections. J Immunol Allergy. 2022;3:1–4. [Google Scholar]
- 27.Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles ACP, Speight N, Vallings R, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270(4):327–38. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiter L, Rinner O, Picotti P, Hüttenhain R, Beck M, Brusniak M-Y, Hengartner MO, Aebersold R. mProphet: automated data processing and statistical validation for large-scale SRM experiments. Nat Methods. 2011;8(5):430–5. doi: 10.1038/nmeth.1584. [DOI] [PubMed] [Google Scholar]
- 29.Steinsvik EK, Hausken T, Fluge Ø, Mella O, Gilja OH. Gastric dysmotility and gastrointestinal symptoms in myalgic encephalomyelitis/chronic fatigue syndrome. Scand J Gastroenterol. 2023;58(7):718–25. doi: 10.1080/00365521.2023.2173533. [DOI] [PubMed] [Google Scholar]
- 30.van Campen CMC, Rowe PC, Visser FC. Blood volume status in ME/CFS correlates with the presence or absence of orthostatic symptoms: preliminary results. Front Pead. 2018;6:352. doi: 10.3389/fped.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Campen CMC, Rowe PC, Visser FC. Deconditioning does not explain orthostatic intolerance in ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome) J Transl Med. 2021;19(1):193. doi: 10.1186/s12967-021-02819-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miwa K. Orthostatic intolerance and chronotropic incompetence in patients with myalgic encephalomyelitis or chronic fatigue syndrome. Circulation Rep. 2023;5(2):55–61. doi: 10.1253/circrep.CR-22-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faro M, Sáez-Francàs N, Castro-Marrero J, Aliste L, Collado A, Alegre J. [Impact of the fibromyalgia in the chronic fatigue syndrome] Med Clin. 2014;142(12):519–25. doi: 10.1016/j.medcli.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 34.Nacul L, de Barros B, Kingdon CC, Cliff JM, Clark TG, Mudie K, Dockrell HM, Lacerda EM. Evidence of Clinical Pathology abnormalities in people with myalgic Encephalomyelitis/Chronic fatigue syndrome (ME/CFS) from an Analytic Cross-sectional Study. Diagnostics. 2019;9(2):41. doi: 10.3390/diagnostics9020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmed F, Vu LT, Zhu H, Iu DSH, Fogarty EA, Kwak Y, Chen W, Franconi CJ, Munn PR, Levine SM et al. Single-cell transcriptomics of the immune system in ME/CFS at baseline and following symptom provocation. bioRxiv 2022:2022.2010.2013.512091. [DOI] [PMC free article] [PubMed]
- 36.Renz-Polster H, Tremblay M-E, Bienzle D, Fischer JE. The pathobiology of myalgic encephalomyelitis/chronic fatigue syndrome: the case for neuroglial failure. Front Cell Neurosci. 2022;16:888232. doi: 10.3389/fncel.2022.888232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231–41. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Tan M, Xiang Q, Zhou Z, Yan H. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb Res. 2017;154:96–105. doi: 10.1016/j.thromres.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 39.Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15(1):130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kale A, Rogers NM, Ghimire K. Thrombospondin-1 CD47 signalling: from mechanisms to Medicine. Int J Mol Sci. 2021;22(8):4062. doi: 10.3390/ijms22084062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111(2):613–23. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts W, Magwenzi S, Aburima A, Naseem KM. Thrombospondin-1 induces platelet activation through CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade. Blood. 2010;116(20):4297–306. doi: 10.1182/blood-2010-01-265561. [DOI] [PubMed] [Google Scholar]
- 43.Kuijpers MJE, Witt Sd, Nergiz-Unal R, Kruchten Rv, Korporaal SJA, Verhamme P, Febbraio M, Tjwa M, Voshol PJ, Hoylaerts MF, et al. Supporting roles of platelet Thrombospondin-1 and CD36 in Thrombus formation on collagen. Arterioscler Thromb Vasc Biol. 2014;34(6):1187–92. doi: 10.1161/ATVBAHA.113.302917. [DOI] [PubMed] [Google Scholar]
- 44.Prakash P, Kulkarni PP, Chauhan AK. Thrombospondin 1 requires Von Willebrand factor to modulate arterial thrombosis in mice. Blood. 2015;125(2):399–406. doi: 10.1182/blood-2014-06-581942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnefoy A, Daenens K, Feys HB, De Vos R, Vandervoort P, Vermylen J, Lawler J, Hoylaerts MF. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107(3):955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aburima A, Berger M, Spurgeon BEJ, Webb BA, Wraith KS, Febbraio M, Poole AW, Naseem KM. Thrombospondin-1 promotes hemostasis through modulation of cAMP signaling in blood platelets. Blood. 2021;137(5):678–89. doi: 10.1182/blood.2020005382. [DOI] [PubMed] [Google Scholar]
- 47.Rogers NM, Sharifi-Sanjani M, Yao M, Ghimire K, Bienes-Martinez R, Mutchler SM, Knupp HE, Baust J, Novelli EM, Ross M, et al. TSP1-CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc Res. 2017;113(1):15–29. doi: 10.1093/cvr/cvw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res. 2010;88(3):471–81. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z, Morgan S, Ren J, Wang Q, Annis DS, Mosher DF, Zhang J, Sorenson CM, Sheibani N, Liu B. Thrombospondin-1 (TSP1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circul Res. 2015;117(2):129–41. doi: 10.1161/CIRCRESAHA.117.305262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediat Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148(6):1851–60. [PMC free article] [PubMed] [Google Scholar]
- 52.Lin MK, Yang J, Hsu CW, Gore A, Bassuk AG, Brown LM, Colligan R, Sengillo JD, Mahajan VB, Tsang SH. HTRA1, an age-related macular degeneration protease, processes extracellular matrix proteins EFEMP1 and TSP1. Aging Cell. 2018;17(4):e12710. doi: 10.1111/acel.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawler JW, Slayter HS, Coligan JE. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978;253(23):8609–16. doi: 10.1016/S0021-9258(17)34336-3. [DOI] [PubMed] [Google Scholar]
- 54.Reed MJ, Iruela-Arispe L, O’Brien ER, Truong T, LaBell T, Bornstein P, Sage EH. Expression of thrombospondins by endothelial cells. Injury is correlated with TSP-1. Am J Pathol. 1995;147(4):1068–80. [PMC free article] [PubMed] [Google Scholar]
- 55.Bergseth G, Lappegård KT, Videm V, Mollnes TE. A novel enzyme immunoassay for plasma thrombospondin. Comparison with beta-thromboglobulin as platelet activation marker in vitro and in vivo. Thromb Res. 2000;99(1):41–50. doi: 10.1016/S0049-3848(00)00226-7. [DOI] [PubMed] [Google Scholar]
- 56.Do HS, Park SW, Im I, Seo D, Yoo HW, Go H, Kim YH, Koh GY, Lee BH, Han YM. Enhanced thrombospondin-1 causes dysfunction of vascular endothelial cells derived from fabry disease-induced pluripotent stem cells. EBioMedicine. 2020;52:102633. doi: 10.1016/j.ebiom.2020.102633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nör JE, Mitra RS, Sutorik MM, Mooney DJ, Castle VP, Polverini PJ. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res. 2000;37(3):209–18. doi: 10.1159/000025733. [DOI] [PubMed] [Google Scholar]
- 58.Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65(5):728–42. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campen CMCv, Rowe PC, Visser FC. Orthostatic symptoms and reductions in cerebral blood Flow in Long-Haul COVID-19 patients: similarities with myalgic Encephalomyelitis/Chronic fatigue syndrome. Medicina. 2022;58(1):28. doi: 10.3390/medicina58010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaffe EA, Ruggiero JT, Falcone DJ. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985;65(1):79–84. doi: 10.1182/blood.V65.1.79.79. [DOI] [PubMed] [Google Scholar]
- 61.Kim IS, Lee S-G, Shin SG, Jeong H, Sohn KM, Park K-S, Silwal P, Cheon S, Kim J, Kym S, et al. Dysregulated thrombospondin 1 and miRNA-29a-3p in severe COVID-19. Sci Rep. 2022;12(1):21227. doi: 10.1038/s41598-022-23533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rucinski B, Niewiarowski S, Strzyzewski M, Holt JC, Mayo KH. Human platelet factor 4 and its C-terminal peptides: heparin binding and clearance from the circulation. Thromb Haemost. 1990;63(3):493–8. doi: 10.1055/s-0038-1645072. [DOI] [PubMed] [Google Scholar]
- 63.Capitanio AM, Niewiarowski S, Rucinski B, Tuszynski GP, Cierniewski CS, Hershock D, Kornecki E. Interaction of platelet factor 4 with human platelets. Biochim Biophys Acta. 1985;839(2):161–73. doi: 10.1016/0304-4165(85)90033-9. [DOI] [PubMed] [Google Scholar]
- 64.Scherlinger M, Richez C, Tsokos GC, Boilard E, Blanco P. The role of platelets in immune-mediated inflammatory diseases. Nat Rev Immunol. 2023;23(8):495–510. doi: 10.1038/s41577-023-00834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cai Z, Greene MI, Zhu Z, Zhang H. Structural features and PF4 functions that occur in Heparin-Induced Thrombocytopenia (HIT) complicated by COVID-19. Antibodies. 2020;9(4):52. doi: 10.3390/antib9040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu G, Rux AH, Ma P, Bdeir K, Sachais BS. Endothelial expression of E-selectin is induced by the platelet-specific chemokine platelet factor 4 through LRP in an NF-kappaB-dependent manner. Blood. 2005;105(9):3545–51. doi: 10.1182/blood-2004-07-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aidoudi S, Bikfalvi A. Interaction of PF4 (CXCL4) with the vasculature: a role in atherosclerosis and angiogenesis. Thromb Haemost. 2010;104(11):941–8. doi: 10.1160/TH10-03-0193. [DOI] [PubMed] [Google Scholar]
- 68.Assinger A. Platelets and infection? An emerging role of platelets in viral infection. Front Immunol. 2014;5:124104. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elizalde JI, Gómez J, Panés J, Lozano M, Casadevall M, Ramírez J, Pizcueta P, Marco F, Rojas FD, Granger DN, et al. Platelet activation in mice and human Helicobacter pylori infection. J Clin Investig. 1997;100(5):996–1005. doi: 10.1172/JCI119650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solomon Tsegaye T, Gnirß K, Rahe-Meyer N, Kiene M, Krämer-Kühl A, Behrens G, Münch J, Pöhlmann S. Platelet activation suppresses HIV-1 infection of T cells. Retrovirology. 2013;10(1):48. doi: 10.1186/1742-4690-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Auerbach DJ, Lin Y, Miao H, Cimbro R, Difiore MJ, Gianolini ME, Furci L, Biswas P, Fauci AS, Lusso P. Identification of the platelet-derived chemokine CXCL4/PF-4 as a broad-spectrum HIV-1 inhibitor. Proc Natl Acad Sci U S A. 2012;109(24):9569–74. doi: 10.1073/pnas.1207314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McMorran BJ, Wieczorski L, Drysdale KE, Chan JA, Huang HM, Smith C, Mitiku C, Beeson JG, Burgio G, Foote SJ. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science. 2012;338(6112):1348–51. doi: 10.1126/science.1228892. [DOI] [PubMed] [Google Scholar]
- 73.Love MS, Millholland MG, Mishra S, Kulkarni S, Freeman KB, Pan W, Kavash RW, Costanzo MJ, Jo H, Daly TM, et al. Platelet factor 4 activity against P. Falciparum and its translation to nonpeptidic mimics as antimalarials. Cell Host Microbe. 2012;12(6):815–23. doi: 10.1016/j.chom.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu C-C, Chou L-P, Chen T-S, Chen C-A, Tsai Y-F. Significant association of anti-platelet factor 4/heparin antibody with cardiovascular disease in hemodialysis patients: a longitudinal 7-year study. Int Urol Nephrol. 2018;50(12):2289–97. doi: 10.1007/s11255-018-2002-y. [DOI] [PubMed] [Google Scholar]
- 75.Blanchet X, Cesarek K, Brandt J, Herwald H, Teupser D, Küchenhoff H, Karshovska E, Mause SF, Siess W, Wasmuth H, et al. Inflammatory role and prognostic value of platelet chemokines in acute coronary syndrome. Thromb Haemost. 2014;112(6):1277–87. doi: 10.1160/TH14-02-0139. [DOI] [PubMed] [Google Scholar]
- 76.Pitsilos S, Hunt J, Mohler ER, Prabhakar AM, Poncz M, Dawicki J, Khalapyan TZ, Wolfe ML, Fairman R, Mitchell M, et al. Platelet factor 4 localization in carotid atherosclerotic plaques: correlation with clinical parameters. Thromb Haemost. 2003;90(6):1112–20. doi: 10.1160/TH03-02-0069. [DOI] [PubMed] [Google Scholar]
- 77.Vrij R, Wersch V, Stockbrügger Platelet factor 4 and β-thromboglobulin in inflammatory bowel disease and giant cell arteritis. Eur J Clin Invest. 2000;30(3):188–94. doi: 10.1046/j.1365-2362.2000.00616.x. [DOI] [PubMed] [Google Scholar]
- 78.Ye L, Zhang YP, Yu N, Jia YX, Wan SJ, Wang FY. Serum platelet factor 4 is a reliable activity parameter in adult patients with inflammatory bowel disease: a pilot study. Med. 2017;96(11):e6323. doi: 10.1097/MD.0000000000006323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martí F, Bertran E, Llucià M, Villén E, Peiró M, Garcia J, Rueda F. Platelet factor 4 induces human natural killer cells to synthesize and release interleukin-8. J Leukoc Biol. 2002;72(3):590–7. doi: 10.1189/jlb.72.3.590. [DOI] [PubMed] [Google Scholar]
- 80.Pervushina O, Scheuerer B, Reiling N, Behnke L, Schröder JM, Kasper B, Brandt E, Bulfone-Paus S, Petersen F. Platelet factor 4/CXCL4 induces phagocytosis and the generation of reactive oxygen metabolites in mononuclear phagocytes independently of Gi protein activation or intracellular calcium transients. J Immunol. 2004;173(3):2060–7. doi: 10.4049/jimmunol.173.3.2060. [DOI] [PubMed] [Google Scholar]
- 81.Scheuerer B, Ernst M, Dürrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, Flad HD, Petersen F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood. 2000;95(4):1158–66. doi: 10.1182/blood.V95.4.1158.004k31_1158_1166. [DOI] [PubMed] [Google Scholar]
- 82.Kaczor DM, Kramann R, Hackeng TM, Schurgers LJ, Koenen RR. Differential effects of platelet factor 4 (CXCL4) and its non-allelic variant (CXCL4L1) on cultured human vascular smooth muscle cells. Int J Mol Sci. 2022;23(2):580. doi: 10.3390/ijms23020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi G, Field DJ, Long X, Mickelsen D, Ko KA, Ture S, Korshunov VA, Miano JM, Morrell CN. Platelet factor 4 mediates vascular smooth muscle cell injury responses. Blood. 2013;121(21):4417–27. doi: 10.1182/blood-2012-09-454710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turner S, Naidoo CA, Usher TJ, Kruger A, Venter C, Laubscher GJ, Khan MA, Kell DB, Pretorius E. Increased levels of inflammatory molecules in blood of long COVID patients point to thrombotic endotheliitis. medRxiv 2022:2022.2010.2013.22281055. [DOI] [PubMed]
- 85.Dahlbäck B, Stenflo J. High molecular weight complex in human plasma between vitamin K-dependent protein S and complement component C4b-binding protein. Proc Natl Acad Sci U S A. 1981;78(4):2512–6. doi: 10.1073/pnas.78.4.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dahlbäck B. Vitamin K-Dependent protein S: beyond the protein C pathway. Semin Thromb Hemost. 2018;44(2):176–84. doi: 10.1055/s-0037-1604092. [DOI] [PubMed] [Google Scholar]
- 87.Esmon CT. Protein S and protein C: Biochemistry, physiology, and clinical manifestation of deficiencies. Trends Cardiovasc Med. 1992;2(6):214–9. doi: 10.1016/1050-1738(92)90027-P. [DOI] [PubMed] [Google Scholar]
- 88.Maillard C, Berruyer M, Serre CM, Dechavanne M, Delmas PD. Protein-S, a vitamin K-dependent protein, is a bone matrix component synthesized and secreted by osteoblasts. Endocrinology. 1992;130(3):1599–604. doi: 10.1210/endo.130.3.1531628. [DOI] [PubMed] [Google Scholar]
- 89.Fusaro M, Cianciolo G, Brandi ML, Ferrari S, Nickolas TL, Tripepi G, Plebani M, Zaninotto M, Iervasi G, La Manna G, et al. Vitamin K and osteoporosis. Nutrients. 2020;12(12):3625. doi: 10.3390/nu12123625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fair DS, Marlar RA, Levin EG. Human endothelial cells synthesize protein S. Blood. 1986;67(4):1168–71. doi: 10.1182/blood.V67.4.1168.1168. [DOI] [PubMed] [Google Scholar]
- 91.de Fouw NJ, Haverkate F, Bertina RM, Koopman J, van Wijngaarden A, van Hinsbergh VW. The cofactor role of protein S in the acceleration of whole blood clot lysis by activated protein C in vitro. Blood. 1986;67(4):1189–92. doi: 10.1182/blood.V67.4.1189.1189. [DOI] [PubMed] [Google Scholar]
- 92.Şik N, Duman M, Küme T, Gürsoy Doruk Ö, Yilmaz D, Ören H. Roles of vitamin-K-dependent factors protein S and GAS6 with TAM receptors and HMGB1 in Pediatric COVID-19 Disease. J Pediatr Hematol Oncol. 2023;45(3):e298–e303. doi: 10.1097/MPH.0000000000002528. [DOI] [PubMed] [Google Scholar]
- 93.Teimury A, Khameneh MT, Khaledi EM. Major coagulation disorders and parameters in COVID-19 patients. Eur J Med Res. 2022;27(1):25. doi: 10.1186/s40001-022-00655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bunch CM, Moore EE, Moore HB, Neal MD, Thomas AV, Zackariya N, Zhao J, Zackariya S, Brenner TJ, Berquist M, et al. Immuno-Thrombotic complications of COVID-19: implications for timing of surgery and anticoagulation. Front Surg. 2022;9:889999. doi: 10.3389/fsurg.2022.889999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giloteaux L, Li J, Hornig M, Lipkin WI, Ruppert D, Hanson MR. Proteomics and cytokine analyses distinguish myalgic encephalomyelitis/chronic fatigue syndrome cases from controls. J Transl Med. 2023;21(1):322. doi: 10.1186/s12967-023-04179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hardcastle SL, Brenu EW, Johnston S, Nguyen T, Huth T, Wong N, Ramos S, Staines D, Marshall-Gradisnik S. Characterisation of cell functions and receptors in chronic fatigue Syndrome/Myalgic encephalomyelitis (CFS/ME) BMC Immunol. 2015;16(1):35. doi: 10.1186/s12865-015-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brenu EW, Staines DR, Baskurt OK, Ashton KJ, Ramos SB, Christy RM, Marshall-Gradisnik SM. Immune and hemorheological changes in chronic fatigue syndrome. J Transl Med. 2010;8(1):1. doi: 10.1186/1479-5876-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen T, Staines D, Johnston S, Marshall-Gradisnik S. Reduced glycolytic reserve in isolated natural killer cells from myalgic encephalomyelitis/ chronic fatigue syndrome patients: a preliminary investigation. Asian Pac J Allergy Immunol. 2019;37(2):102–8. doi: 10.12932/AP-011117-0188. [DOI] [PubMed] [Google Scholar]
- 99.Eaton-Fitch N, du Preez S, Cabanas H, Staines D, Marshall-Gradisnik S. A systematic review of natural killer cells profile and cytotoxic function in myalgic encephalomyelitis/chronic fatigue syndrome. Syst Rev. 2019;8(1):279. doi: 10.1186/s13643-019-1202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sotzny F, Blanco J, Capelli E, Castro-Marrero J, Steiner S, Murovska M, Scheibenbogen C. Myalgic Encephalomyelitis/Chronic fatigue syndrome– evidence for an autoimmune disease. Autoimmun rev. 2018;17(6):601–9. doi: 10.1016/j.autrev.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 101.Maes M, Twisk FNM, Kubera M, Ringel K. Evidence for inflammation and activation of cell-mediated immunity in myalgic Encephalomyelitis/Chronic fatigue syndrome (ME/CFS): increased interleukin-1, tumor necrosis factor-α, PMN-elastase, lysozyme and neopterin. J Affect Disord. 2012;136(3):933–9. doi: 10.1016/j.jad.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Morris G, Maes M. Increased nuclear factor-κB and loss of p53 are key mechanisms in myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS) Med Hypotheses. 2012;79(5):607–13. doi: 10.1016/j.mehy.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 103.Morris G, Maes M. Mitochondrial dysfunctions in myalgic encephalomyelitis / chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis. 2014;29(1):19–36. doi: 10.1007/s11011-013-9435-x. [DOI] [PubMed] [Google Scholar]
- 104.Tate W, Walker M, Sweetman E, Helliwell A, Peppercorn K, Edgar C, Blair A, Chatterjee A. Molecular mechanisms of Neuroinflammation in ME/CFS and Long COVID to Sustain Disease and promote relapses. Front Neurol 2022, 13. [DOI] [PMC free article] [PubMed]
- 105.Jonsjö MA, Olsson GL, Wicksell RK, Alving K, Holmström L, Andreasson A. The role of low-grade inflammation in ME/CFS (myalgic Encephalomyelitis/Chronic fatigue Syndrome) - associations with symptoms. Psychoneuroendocrinology. 2020;113:104578. doi: 10.1016/j.psyneuen.2019.104578. [DOI] [PubMed] [Google Scholar]
- 106.Maes M, Twisk FN. Why myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) may kill you: disorders in the inflammatory and oxidative and nitrosative stress (IO&NS) pathways may explain cardiovascular disorders in ME/CFS. Neuro Endocrinol Lett. 2009;30(6):677–93. [PubMed] [Google Scholar]
- 107.Killick J, Morisse G, Sieger D, Astier AL. Complement as a regulator of adaptive immunity. Semin Immunopathol. 2018;40(1):37–48. doi: 10.1007/s00281-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, Roumenina LT. Complement system part II: role in immunity. Front Immunol 2015, 6. [DOI] [PMC free article] [PubMed]
- 109.Java A, Apicelli AJ, Liszewski MK, Coler-Reilly A, Atkinson JP, Kim AH, Kulkarni HS. The complement system in COVID-19: friend and foe? JCI Insight 2020, 5(15). [DOI] [PMC free article] [PubMed]
- 110.Castro-Marrero J, Zacares M, Almenar-Pérez E, Alegre-Martín J, Oltra E. Complement component C1q as a potential Diagnostic Tool for myalgic Encephalomyelitis/Chronic fatigue syndrome subtyping. J Clin Med. 2021;10(18):4171. doi: 10.3390/jcm10184171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guenther S, Loebel M, Mooslechner AA, Knops M, Hanitsch LG, Grabowski P, Wittke K, Meisel C, Unterwalder N, Volk HD, et al. Frequent IgG subclass and mannose binding lectin deficiency in patients with chronic fatigue syndrome. Hum Immunol. 2015;76(10):729–35. doi: 10.1016/j.humimm.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 112.Lutz L, Rohrhofer J, Zehetmayer S, Stingl M, Untersmayr E. Evaluation of Immune Dysregulation in an Austrian patient cohort suffering from myalgic Encephalomyelitis/Chronic fatigue syndrome. Biomolecules. 2021;11(9):1359. doi: 10.3390/biom11091359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rajeevan MS, Dimulescu I, Murray J, Falkenberg VR, Unger ER. Pathway-focused genetic evaluation of immune and inflammation related genes with chronic fatigue syndrome. Hum Immunol. 2015;76(8):553–60. doi: 10.1016/j.humimm.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 114.Dudkina NV, Spicer BA, Reboul CF, Conroy PJ, Lukoyanova N, Elmlund H, Law RH, Ekkel SM, Kondos SC, Goode RJ, et al. Structure of the poly-C9 component of the complement membrane attack complex. Nat Commun. 2016;7:10588. doi: 10.1038/ncomms10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sodetz JM. Cytotoxic effector mechanisms: 1989// 1989; Berlin. Heidelberg: Springer Berlin Heidelberg; 1989. Structure and function of C8 in the membrane attack sequence of complement; pp. 19–31. [DOI] [PubMed] [Google Scholar]
- 116.Fu X, Ju J, Lin Z, Xiao W, Li X, Zhuang B, Zhang T, Ma X, Li X, Ma C, et al. Target deletion of complement component 9 attenuates antibody-mediated hemolysis and lipopolysaccharide (LPS)-induced acute shock in mice. Sci Rep. 2016;6:30239. doi: 10.1038/srep30239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ren Y, Ding Q, Zhang X. Ficolins and infectious diseases. Virol Sin. 2014;29(1):25–32. doi: 10.1007/s12250-014-3421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Babaha F, Abolhassani H, Hamidi Esfahani Z, Yazdani R, Aghamohammadi A. A new case of congenital ficolin-3 deficiency with primary immunodeficiency. Expert Rev Clin Immunol. 2020;16(7):733–8. doi: 10.1080/1744666X.2020.1792779. [DOI] [PubMed] [Google Scholar]
- 119.Troldborg A, Steffensen R, Trendelenburg M, Hauser T, Winther KG, Hansen AG, Stengaard-Pedersen K, Voss A, Thiel S. Ficolin-3 Deficiency is Associated with Disease and an increased risk of systemic Lupus Erythematosus. J Clin Immunol. 2019;39(4):421–9. doi: 10.1007/s10875-019-00627-2. [DOI] [PubMed] [Google Scholar]
- 120.Keech A, Vollmer-Conna U, Barry BK, Lloyd AR. Gene expression in response to exercise in patients with chronic fatigue syndrome: a pilot study. Front Physiol. 2016;7:218390. doi: 10.3389/fphys.2016.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kowalczyk P, Kaczyńska K, Kleczkowska P, Bukowska-Ośko I, Kramkowski K, Sulejczak D. The lactoferrin phenomenon: a miracle molecule. Molecules. 2022;27(9):2941. doi: 10.3390/molecules27092941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rascón-Cruz Q, Espinoza-Sánchez EA, Siqueiros-Cendón TS, Nakamura-Bencomo SI, Arévalo-Gallegos S, Iglesias-Figueroa BF. Lactoferrin: a glycoprotein involved in Immunomodulation, Anticancer, and antimicrobial processes. Molecules. 2021;26(1):205. doi: 10.3390/molecules26010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosa L, Cutone A, Lepanto MS, Paesano R, Valenti P. Lactoferrin: a natural glycoprotein involved in iron and inflammatory homeostasis. Int J Mol Sci. 2017;18(9):1985. doi: 10.3390/ijms18091985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Safaeian L, Javanmard SH, Mollanoori Y, Dana N. Cytoprotective and antioxidant effects of human lactoferrin against H2O2-induced oxidative stress in human umbilical vein endothelial cells. Adv Biomed Res. 2015;4:188. doi: 10.4103/2277-9175.164010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shoji H, Oguchi S, Shinohara K, Shimizu T, Yamashiro Y. Effects of iron-unsaturated human lactoferrin on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells. Pediatr Res. 2007;61(1):89–92. doi: 10.1203/01.pdr.0000250198.22735.20. [DOI] [PubMed] [Google Scholar]
- 126.Embleton ND, Berrington JE, McGuire W, Stewart CJ, Cummings SP. Lactoferrin: antimicrobial activity and therapeutic potential. Semin Fetal Neonatal Med. 2013;18(3):143–9. doi: 10.1016/j.siny.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 127.Artym J, Zimecki M. Antimicrobial and prebiotic activity of lactoferrin in the female reproductive tract: a comprehensive review. Biomedicines. 2021;9(12):1940. doi: 10.3390/biomedicines9121940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen PW, Ku YW, Chu FY. Influence of bovine lactoferrin on the growth of selected probiotic bacteria under aerobic conditions. Biometals. 2014;27(5):905–14. doi: 10.1007/s10534-014-9758-z. [DOI] [PubMed] [Google Scholar]
- 129.Kell DB, Heyden EL, Pretorius E. The Biology of Lactoferrin, an Iron-binding protein that can help defend against viruses and Bacteria. Front Immunol. 2020;11:1221. doi: 10.3389/fimmu.2020.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des. 2009;15(17):1956–73. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Siqueiros-Cendón T, Arévalo-Gallegos S, Iglesias-Figueroa BF, García-Montoya IA, Salazar-Martínez J, Rascón-Cruz Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin. 2014;35(5):557–66. doi: 10.1038/aps.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lu J, Francis J, Doster RS, Haley KP, Craft KM, Moore RE, Chambers SA, Aronoff DM, Osteen K, Damo SM, et al. Lactoferrin: a critical mediator of both Host Immune Response and Antimicrobial Activity in response to streptococcal infections. ACS Infect Dis. 2020;6(7):1615–23. doi: 10.1021/acsinfecdis.0c00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de la Rosa G, Yang D, Tewary P, Varadhachary A, Oppenheim JJ. Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. J Immunol. 2008;180(10):6868–76. doi: 10.4049/jimmunol.180.10.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conneely OM. Antiinflammatory activities of lactoferrin. J Am Coll Nutr. 2001;20(5 Suppl):389S–395S. doi: 10.1080/07315724.2001.10719173. [DOI] [PubMed] [Google Scholar]
- 135.He Y, Lawlor NT, Newburg DS. Human milk components modulate toll-like receptor-mediated inflammation. Adv Nutr. 2016;7(1):102–11. doi: 10.3945/an.115.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int Immunopharmacol. 2005;5(3):591–9. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 137.Kane SV, Sandborn WJ, Rufo PA, Zholudev A, Boone J, Lyerly D, Camilleri M, Hanauer SB. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am J Gastroenterol. 2003;98(6):1309–14. doi: 10.1111/j.1572-0241.2003.07458.x. [DOI] [PubMed] [Google Scholar]
- 138.González-Sánchez M, Bartolome F, Antequera D, Puertas-Martín V, González P, Gómez-Grande A, Llamas-Velasco S, Herrero-San Martín A, Pérez-Martínez D, Villarejo-Galende A, et al. Decreased salivary lactoferrin levels are specific to Alzheimer’s disease. EBioMedicine. 2020;57:102834. doi: 10.1016/j.ebiom.2020.102834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bermejo-Pareja F, del Ser T, Valentí M, de la Fuente M, Bartolome F, Carro E. Salivary lactoferrin as biomarker for Alzheimer’s disease: brain-immunity interactions. Alzheimer’s Dement. 2020;16(8):1196–204. doi: 10.1002/alz.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in Inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zwadlo G, Brüggen J, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin Exp Immunol. 1988;72(3):510–5. [PMC free article] [PubMed] [Google Scholar]
- 142.Lagasse E, Clerc RG. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol Cell Biol. 1988;8(6):2402–10. doi: 10.1128/mcb.8.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81(1):28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 144.Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86(3):557–66. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 145.Zaia AA, Sappington KJ, Nisapakultorn K, Chazin WJ, Dietrich EA, Ross KF, Herzberg MC. Subversion of antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm of TR146 epithelial cells after invasion by Listeria monocytogenes. Mucosal Immunol. 2009;2(1):43–53. doi: 10.1038/mi.2008.63. [DOI] [PubMed] [Google Scholar]
- 146.Wang J, Vodovotz Y, Fan L, Li Y, Liu Z, Namas R, Barclay D, Zamora R, Billiar TR, Wilson MA, et al. Injury-induced MRP8/MRP14 stimulates IP-10/CXCL10 in monocytes/macrophages. Faseb J. 2015;29(1):250–62. doi: 10.1096/fj.14-255992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Filippo K, Neill DR, Mathies M, Bangert M, McNeill E, Kadioglu A, Hogg N. A new protective role for S100A9 in regulation of neutrophil recruitment during invasive pneumococcal pneumonia. Faseb j. 2014;28(8):3600–8. doi: 10.1096/fj.13-247460. [DOI] [PubMed] [Google Scholar]
- 148.Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, Gerke V, Manitz MP, Nacken W, et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104(13):4260–8. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- 149.Mizobuchi H, Yamakoshi S, Omachi S, Osada Y, Sanjoba C, Goto Y, Matsumoto Y. The accumulation of macrophages expressing myeloid-related protein 8 (MRP8) and MRP14 in the spleen of BALB/cA mice during infection with Plasmodium berghei. Exp Parasitol. 2014;138:1–8. doi: 10.1016/j.exppara.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 150.Tsai SY, Segovia JA, Chang TH, Morris IR, Berton MT, Tessier PA, Tardif MR, Cesaro A, Bose S. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza a virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 2014;10(1):e1003848. doi: 10.1371/journal.ppat.1003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.De Jong HK, Achouiti A, Koh GCKW, Parry CM, Baker S, Faiz MA, van Dissel JT, Vollaard AM, van Leeuwen EMM, Roelofs JJTH, et al. Expression and function of S100A8/A9 (calprotectin) in human typhoid fever and the Murine Salmonella Model. PLoS Negl Trop Dis. 2015;9(4):e0003663. doi: 10.1371/journal.pntd.0003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baillet A, Trocmé C, Berthier S, Arlotto M, Grange L, Chenau J, Q uétant S, Sève M, Berger F, Juvin R, et al. Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatology. 2010;49(4):671–82. doi: 10.1093/rheumatology/kep452. [DOI] [PubMed] [Google Scholar]
- 153.Pouwels SD, Nawijn MC, Bathoorn E, Riezebos-Brilman A, van Oosterhout AJ, Kerstjens HA, Heijink IH. Increased serum levels of LL37, HMGB1 and S100A9 during exacerbation in COPD patients. Eur Respir J. 2015;45(5):1482–5. doi: 10.1183/09031936.00158414. [DOI] [PubMed] [Google Scholar]
- 154.Holzinger D, Nippe N, Vogl T, Marketon K, Mysore V, Weinhage T, Dalbeth N, Pool B, Merriman T, Baeten D, et al. Myeloid-related proteins 8 and 14 contribute to monosodium urate monohydrate crystal-induced inflammation in gout. Arthritis Rheumatol. 2014;66(5):1327–39. doi: 10.1002/art.38369. [DOI] [PubMed] [Google Scholar]
- 155.Foell D, Hernández-Rodríguez J, Sánchez M, Vogl T, Cid MC, Roth J. Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. J Pathol. 2004;204(3):311–6. doi: 10.1002/path.1660. [DOI] [PubMed] [Google Scholar]
- 156.Li H, Huang X, Chang X, Yao J, He Q, Shen Z, Ji Y, Wang K. S100-A9 protein in exosomes derived from follicular fluid promotes inflammation via activation of NF-κB pathway in polycystic ovary syndrome. J Cell Mol Med. 2020;24(1):114–25. doi: 10.1111/jcmm.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MAD, Nacken W, Foell D, van der Poll T, Sorg C, et al. Mrp8 and Mrp14 are endogenous activators of toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13(9):1042–9. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 158.Yonekawa K, Neidhart M, Altwegg LA, Wyss CA, Corti R, Vogl T, Grigorian M, Gay S, Lüscher TF, Maier W. Myeloid related proteins activate toll-like receptor 4 in human acute coronary syndromes. Atherosclerosis. 2011;218(2):486–92. doi: 10.1016/j.atherosclerosis.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 159.Roth J, Vogl T, Sorg C, Sunderkötter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24(4):155–8. doi: 10.1016/S1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 160.Chen B, Miller AL, Rebelatto M, Brewah Y, Rowe DC, Clarke L, Czapiga M, Rosenthal K, Imamichi T, Chen Y, et al. S100A9 induced inflammatory responses are mediated by distinct damage associated molecular patterns (DAMP) receptors in vitro and in vivo. PLoS ONE. 2015;10(2):e0115828. doi: 10.1371/journal.pone.0115828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B, et al. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood. 2005;105(7):2955–62. doi: 10.1182/blood-2004-07-2520. [DOI] [PubMed] [Google Scholar]
- 162.Sfera A, Osorio C, Zapata Martin del Campo CM, Pereida S, Maurer S, Maldonado JC, Kozlakidis Z. Endothelial senescence and chronic fatigue syndrome, a COVID-19 based hypothesis. Front Cell Neurosci. 2021;15:673217. doi: 10.3389/fncel.2021.673217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Colicchia M, Schrottmaier WC, Perrella G, Reyat JS, Begum J, Slater A, Price J, Clark JC, Zhi Z, Simpson MJ, et al. S100A8/A9 drives the formation of procoagulant platelets through GPIbα. Blood. 2022;140(24):2626–43. doi: 10.1182/blood.2021014966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mellett L, Khader SA. S100A8/A9 in COVID-19 pathogenesis: impact on clinical outcomes. Cytokine Growth Factor Rev. 2022;63:90–7. doi: 10.1016/j.cytogfr.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen L, Long X, Xu Q, Tan J, Wang G, Cao Y, Wei J, Luo H, Zhu H, Huang L, et al. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell Mol Immunol. 2020;17(9):992–4. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chapenko S, Krumina A, Kozireva S, Nora Z, Sultanova A, Viksna L, Murovska M. Activation of human herpesviruses 6 and 7 in patients with chronic fatigue syndrome. J Clin Virol. 2006;37:S47–51. doi: 10.1016/S1386-6532(06)70011-7. [DOI] [PubMed] [Google Scholar]
- 167.Maltsev D. A comparative study of valaciclovir, valganciclovir, and artesunate efficacy in reactivated HHV-6 and HHV-7 infections associated with chronic fatigue syndrome/myalgic encephalomyelitis. Microbiol Immunol. 2022;66(4):193–9. doi: 10.1111/1348-0421.12966. [DOI] [PubMed] [Google Scholar]
- 168.Shikova E, Reshkova V, Kumanova А, Raleva S, Alexandrova D, Capo N, Murovska M, obotENo MECFS. Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic еncephalomyelitis/chronic fatigue syndrome. J Med Virol. 2020;92(12):3682–8. doi: 10.1002/jmv.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schreiner P, Harrer T, Scheibenbogen C, Lamer S, Schlosser A, Naviaux RK, Prusty BK. Human Herpesvirus-6 reactivation, mitochondrial fragmentation, and the coordination of antiviral and metabolic phenotypes in myalgic encephalomyelitis/chronic fatigue syndrome. ImmunoHorizons. 2020;4(4):201–215. doi: 10.4049/immunohorizons.2000006. [DOI] [PubMed] [Google Scholar]
- 170.Pretorius E, Venter C, Laubscher GJ, Kotze M, Moremi K, Oladejo S, Watson L, Rajaratnam K, Watson B, Kell D. Combined triple treatment of fibrin amyloid microclots and platelet pathology in individuals with long COVID/ Post-Acute Sequelae of COVID-19 (PASC) can resolve their persistent symptoms. Research Square; 2021. [Google Scholar]
- 171.Laubscher GJ. Treatment of long COVID symptoms with triple anticoagulant therapy. Research Square; 2023.
- 172.Hart DA. Sex differences in Biological Systems and the Conundrum of Menopause: potential commonalities in Post-menopausal Disease mechanisms. Int J Mol Sci. 2022;23(8):4119. doi: 10.3390/ijms23084119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang Y, Mishra A, Brinton RD. Transitions in metabolic and immune systems from pre-menopause to post-menopause: implications for age-associated neurodegenerative diseases. F1000Res. 2020 doi: 10.12688/f1000research.21599.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting proteomics data is available on request.