Abstract
The development of gene delivery vectors based on feline immunodeficiency virus (FIV) is an attractive alternative to vectors based on primate sources for the delivery of genes into humans. To investigate the requirements for efficient transduction of dividing and nondividing cells by vector particles based on FIV, a series of packaging and vector constructs was generated for which viral gene expression was minimized and from which unnecessary cis-acting sequences were deleted. Pseudotyped vector particles produced in 293T cells were used to transduce various target cells, including contact-inhibited human skin fibroblasts and growth-arrested HT1080 cells. FIV vectors in which the U3 promoter was replaced with the cytomegalovirus promoter gave rise to over 50-fold-higher titers than FIV vectors containing the complete FIV 5′ long terminal repeat (LTR). Comparison of the transduction efficiencies of vectors containing different portions of the FIV Gag coding region indicates that at least a functional part of the FIV packaging signal (Ψ) is located within an area which includes the 5′ LTR and the first 350 bp of gag. Transduction efficiencies of vectors prepared without FIV vif and orf2 accessory gene expression did not differ substantially from those of vectors prepared with accessory gene expression in either dividing or nondividing cells. The requirement for FIV rev-RRE was, however, demonstrated by the inefficient production of vector particles in the absence of rev expression. Together, these results demonstrate the efficient transduction of nondividing cells in vitro by a multiply attenuated FIV vector and contribute to an understanding of the minimum requirements for efficient vector production and infectivity. In addition, we describe the ability of an FIV vector to deliver genes in vivo into hamster muscle tissue.
Retroviral vectors, such as those based on murine leukemia virus (MLV), are attractive vehicles for delivering genes due to their relatively large coding capacity, efficient gene transfer, long-term expression of foreign genes, and lack of virus-encoded proteins which could elicit undesirable immune responses. The major drawback of MLV vectors is their inability to transduce nondividing cells (28, 33, 52), many of which are clinically relevant targets. To overcome this considerable limitation, vectors have recently been developed from primate lentiviruses, most notably, human immunodeficiency virus type 1 (HIV-1), which have the capacity to infect cells which are not actively dividing (37, 49). The transduction of nonproliferating cells by HIV vectors stems from the ability of the lentiviral preintegration complex to interact with the cellular nuclear import machinery and become actively transported to the nucleus in the absence of mitosis (5, 17–21, 67). HIV vectors have been demonstrated to transduce growth-arrested cells and terminally differentiated primary macrophages in vitro, as well as to deliver genes to postmitotic neurons, terminally differentiated retinal cells, and adult liver and muscle cells in vivo (3, 23, 36, 37, 49, 72).
Although primate lentiviruses such as HIV have been extensively characterized, vectors based on nonprimate lentiviruses may be equally potent but more readily accepted by researchers, clinicians, and patients. It is important to emphasize, however, that no data presently exist regarding the relative safety of primate versus nonprimate lentivirus vectors. Feline immunodeficiency virus (FIV), a model for lentiviral vaccine development and antiviral therapy, is one particularly appealing candidate for vector development. Phylogenetic analysis suggests FIV is only distantly related to the primate lentiviruses (2, 13, 40, 41, 43, 60), and epidemiologic evidence indicates that there has been no occurrence of seroconversion in human populations, despite exposure via the same route of transmission that occurs in natural infections (biting and scratching) (10, 29, 38, 43, 70). However, many aspects of the lentiviral life cycle, including entry, nuclear import, integration, promoter recognition, and RNA export, are mediated, in part, by cellular factors which may not interact with primate elements. Indeed, the inability of FIV to productively infect human cells is attributed not only to the cell tropism governed by the FIV envelope but also to the low transcriptional activity of the FIV long terminal repeat (LTR) (22, 34, 35, 58, 61, 64) and the diminished function of FIV Rev in human cells (63). Recently, Poeschla et al. (46) developed FIV vectors containing the human cytomegalovirus (CMV) immediate early gene promoter in place of the entire FIV LTR U3 region in order to overcome the low transcriptional activity of the FIV LTR in human cells. This single modification allowed the efficient production of FIV vector particles in human cells, a likely necessity for producing vectors which are resistant to inactivation by human complement (59).
In the present study, we describe a second generation of FIV vectors in which the components necessary for efficient vector production as well as efficient transduction of dividing and nondividing target cells have been analyzed. Current retroviral vector systems are designed such that viral cis-acting sequences and viral coding regions are located on separate plasmids to avoid generation and packaging of replication-competent retroviruses. An ideal vector system would also contain minimal cis-acting sequences to discourage homologous recombination and would eliminate production of nonessential viral proteins which could contaminate vector particle preparations and elicit immune responses. The FIV genome, intermediate in complexity between the simple MLV genome and the complex genome of HIV, appears to encode only three accessory and regulatory genes, vif, orf2, and rev (12, 62). FIV Vif appears to be functionally equivalent to that of HIV and necessary for productive infection in certain feline cells (54, 65). FIV Rev is also analogous to HIV Rev in enabling expression of late genes encoded by unspliced or singly spliced mRNAs containing the cis-acting Rev-responsive element (RRE [45, 66]). FIV orf2 (open reading frame 2) encodes a transactivator of the FIV LTR, albeit a weaker transactivator than its HIV Tat counterpart and one which does not appear to interact with a Tar-like element (9, 58, 64, 69). To determine the minimum components necessary for the production of high-titer pseudotyped FIV vector particles capable of infecting both dividing and nondividing cells, the requirements for certain cis-acting sequences in FIV packaging and vector constructs, as well as expression of FIV accessory and regulatory proteins, was investigated. Our results indicate that the FIV accessory genes, vif and orf2, are dispensable for efficient transduction of both dividing and nondividing cells and that rev-RRE is required for high-titer vector particle production, presumably to allow efficient nuclear export of viral RNA.
MATERIALS AND METHODS
Plasmid construction.
FIV vector constructs were generated in a series of steps from FIV-34TF10 (60). The pTFIV vector backbone was constructed by PCR amplification of regions corresponding to the FIV 5′ and 3′ LTRs. DNA corresponding to the 5′ LTR plus 0.3 kb of gag was amplified with primer pair FIV 13 and FIV 14 (primer sequences provided upon request). Likewise, DNA corresponding to the 5′ LTR plus 0.5 kb of gag was amplified with primer pair FIV 13 and FIV 15. DNA corresponding to the 3′ LTR plus the FIV RRE was amplified with primer set FIV 16 and FIV 18. The resulting PCR products were digested with appropriate enzymes included in the primer sequences and separately ligated into similarly digested pBlueScript KSII(+) (Stratagene, San Diego, Calif.) to yield constructs containing both a 5′ and a 3′ LTR. Ligation of FIV 13-14 and FIV 16-18 PCR products into the appropriately digested pBlueScript vector generated an FIV backbone construct containing a short (0.3-kb) region of gag, designated pTFIVS. Ligation of FIV 13-15 and FIV 16-18 PCR products into the appropriately digested pBlueScript vector generated an FIV backbone construct containing a long (0.55-kb) region of gag, designated pTFIVL.
The pTC/FL vector backbone, in which the FIV U3 region is replaced by the CMV promoter-enhancer, was generated by the PCR method described previously (8). For the first-round PCR, primers FIV 19 and FIV 20 were used to amplify the region corresponding to the CMV promoter. In a separate reaction, primers FIV 21 and FIV 15 were used to generate the FIV U3 and R regions from FIV-34TF10. For the second-round PCR, the FIV 19-20 and FIV 21-15 PCR products served as template DNA for the amplification of a CMV-FIV hybrid LTR with FIV 19 and FIV 15 as primers. The FIV 19-15 PCR product was then digested with SacII and NotI (sites included in the primer sequences) and substituted for the similar fragment from the pTFIVL backbone described above. The pTC/FS vector backbone was generated in a similar manner, with FIV 14 substituting for FIV 15.
FIV vectors expressing β-galactosidase (β-Gal) or the enhanced green fluorescent protein (EGFP) gene under the control of a heterologous promoter were generated by insertion of a reporter gene cassette into the appropriate vector backbone. A CMV promoter-β-Gal expression plasmid, pCMVβgal, was generated by combining an XbaI/SalI fragment corresponding to the CMV promoter from pCMV-G (71) and a SalI/SmaI fragment corresponding to the β-Gal gene from pSP6-β-GAL (48) into pBlueScript SK(−). pTFIVLCβ, pTC/FLCβ, and pTC/FSCβ were then generated by insertion of the NotI/SmaI CMV-β-Gal expression cassette from pCMVβgal into similarly digested pTFIVL, pTC/FL, and pTC/FS vector backbones, respectively. These constructs were renamed pTFIVLCβ, pVETLCβ, and pVETSCβ, respectively. A pCMVβgalCTE expression plasmid (kindly provided by Andrew T. Watt) was used to generate an FIV expression vector containing the constitutive RNA transport element (CTE) from Mason-Pfizer monkey virus (MPMV) (57). pCMVβgalCTE was constructed in part from pSK-CTE (kindly provided by Shin-Tai Chen). pSK-CTE was generated by PCR amplification of the CTE with the primers CTEH5 and CTEH3, which harbor HindIII sites near their 5′ ends. The resulting PCR product was digested with HindIII and inserted into similarly digested pBlueScript SK(−) to generate pSK-CTE. pSK-CTE was then digested with SmaI and XhoI, and the insert was ligated into similarly digested pCMVβgal to generate pCMVβgalCTE. A NotI/XhoI fragment containing the CMVβgalCTE expression cassette from pCMVβgalCTE was then ligated into NotI/SalI-digested pTC/FL to create pTC/FLCβCTE (now referred to as pVETLCβCTE). A Moloney murine leukemia virus (MoMLV) promoter-EGFP expression cassette was created by combining an EcoRI/SmaI fragment containing the MoMLV promoter and an Eco47III/XhoI fragment corresponding to the EGFP coding region from pEGFP-C (Clontech Laboratories, Inc., Palo Alto, Calif.) into pBlueScript KSII(+). The MoMLV LTR-egfp expression cassette was then liberated by NheI/XhoI digestion and inserted into an XbaI/SalI-digested pTC/FL vector backbone to create pTC/FLMegfp (now referred to as pVETLMEGFP).
FIV vector constructs in which the FIV RRE was repositioned and, in some cases, replaced with that of HIV-1 were generated in a sequence from pTC/FSCβ. The FIV 3′ LTR was amplified by PCR from pTC/FSCβ or pTC/FSCβCTE with primers FIV LTR and FIV 18, which include ApaI and KpnI sites, respectively. pTC/FSCβ or pTC/FSCβCTE was then digested with ApaI and KpnI, and the original 3′ LTR fragments were replaced with the similarly digested PCR product to create pTC/FSCβΔRRE and pTC/FSCβCTEΔRRE (now referred to as pVETSCβΔRRE and pVETSCβΔRRE+C). To insert RREs upstream of the internal CMV promoter, RREs from either FIV-34TF10 or pNL4-3 (1) were first amplified by PCR with the FIV primers FRRE(+) and FRRE(−) or HIV primers HRRE(+) and HRRE(−). The resulting PCR products were digested with Tth111I and NotI and inserted into similarly digested pTC/FSCβΔRRE and pTC/FSCβCTEΔRRE to create pTC/FSCβFR, pTC/FSCβHR, pTC/FSCβFRC, and pTC/FSCβHRC. Each of these constructs contains either the FIV or HIV-1 RRE upstream of the internal CMV promoter, and the last two constructs contain an additional CTE downstream of the internal cassette. These constructs were renamed pVETSCβFRRE, pVETSCβHRRE, pVETSCβFRRE+C, and pVETSCβHRRE+C, respectively.
FIV packaging constructs, designated pCFIV, were generated in a series of steps beginning with the deletion of a 1.6-kb region corresponding to the FIV env gene in FIV-34TF10. A 1.9-kb KpnI/SpeI fragment from FIV-34TF10 was inserted into similarly digested pBlueScript IIKS(+), which was then digested with AvrII and SpeI and religated to generate a Δenv intermediate plasmid. This intermediate plasmid was digested with KpnI and XbaI, and the resulting fragment was ligated into KpnI/SpeI-digested FIV-34TF10 to create pF34Δenv. pF34Δenv was then used as the source of FIV sequences for constructing the following pCFIV packaging cassettes. pCFIVX was created by first introducing a unique NotI site into pF34Δenv at nucleotide (nt) 9168 by oligonucleotide-directed in vitro mutagenesis in two rounds of PCR. The first-round PCR contained primers FIV 5 and FIV 6 or, in a separate reaction, primers FIV 7 and FIV 8. Second-round PCRs contained the above-mentioned PCR products serving as the template DNA and oligonucleotides FIV 5 and FIV 8 serving as primers. The second-round PCR product was digested with NdeI and SalI, and the resulting product was ligated into similarly digested pF34Δenv to generate pF34NΔenv. pF34NΔenv was then separately digested with either Tth111I and NotI or XhoI and Tth111I, and the resulting products were combined in a three-way ligation together with a NotI/XhoI fragment from pCMVβ to create pCFIVX. pCFIVΔorf2 was created by first introducing a SalI site into pF34NΔenv by in vitro mutagenesis as described above. The first-round PCR contained either oligonucleotides FIV 1 and FIV 9 or oligonucleotides FIV 3 and FIV 4. The second-round PCR contained the purified first-round products serving as the template DNA and oligonucleotides FIV 1 and FIV 4 serving as primers. The second-round product was digested with Tth111I and SacI, and the resulting product was ligated into similarly digested pF34NΔenv to create pF34NSΔenv. pF34NSΔenv was then cleaved with SalI and NotI and ligated into XhoI/NotI-digested pCMVβ to create pCFIVΔorf2. pCFIV was created to replace the orf2 of pCFIVΔorf2 (which contains the premature stop codon found in FIV-34TF10) with the complete orf2 from FIV14 (National Institutes of Health AIDS Research and Reference Reagent Program). FIV14 was used as the template in a PCR similar to that described above. The first-round PCR contained primers F14-1 and F14-2, and the second-round PCR contained primers F34-4 and F34-5. The second-round PCR product was digested with NgoMI and KpnI, and the resulting fragment was ligated into similarly digested pCFIVΔorf2 to generate pCFIV. pCFIVΔvif was generated by digesting pCFIV with Eco47III and dephosphorylating the blunt ends by calf intestinal phosphatase (CIP) treatment. The phosphorylated complementary oligonucleotides AGE(+) and AGE(−) were then inserted into the Eco47III site of pCFIV, creating pCFIVΔvif. pCFIVΔorf2Δvif was generated by digesting pCFIVΔorf2 with Eco47III and dephosphorylating the blunt ends by calf intestinal phosphatase (CIP) treatment. The phosphorylated complementary oligonucleotides AGE(+) and AGE(−) were then inserted into the Eco47III site of pCFIVΔorf2, as described above, to create pCFIVΔorf2Δvif.
FIV packaging constructs which either do not express rev or lack the Rev coding regions were generated from pCFIVΔorf2Δvif. To generate pCFIVΔrev, the splice acceptor site and basic amino acid domain of the second exon of rev were deleted in a manner similar to that described previously (45). A region corresponding to nt 9022 to 9168 of FIV 34TF10 was PCR amplified with primers ΔSA and FIV 6. The ΔSA-FIV6 PCR fragment was digested with BstBI and NotI and inserted into similarly digested pCFIVΔvifΔorf2, resulting in a 100-bp deletion and creating pCFIVΔrev. pCFIVΔrevFRRE was created by first generating a fragment corresponding to the FIV RRE by PCR amplification. Primers FRRE+ and FRRE−, which contain AgeI and NotI sites, respectively, were used to amplify the FIV RRE from nt 8701 to 8952 from FIV 34TF10. The resulting PCR fragment was digested with AgeI and NotI and inserted into similarly digested pCFIVΔorf2Δvif, resulting in the simultaneous insertion of the FIV RRE and the deletion of all FIV sequences extending past the 5′ end of vif. pCFIVΔrevHRRE was created in a similar manner with primers HRRE+ and HRRE− (which contain AgeI and NotI sites, respectively) to generate a region corresponding to the HIV RRE from nt 7744 to 8004 of NL4-3. As described above, the resulting PCR fragment was digested with AgeI and NotI and inserted into similarly digested pCFIVΔorf2Δvif to create pCFIVΔrevHRRE. All constructs were screened by restriction enzyme digestion, and the sequence of regions was amplified by PCR confirmed by sequence analysis. Oligonucleotides were synthesized by Operon Technologies, Inc. (Alameda, Calif.), and the sequences are available upon request.
Construction of the FIV rev expression plasmid, pCFIVrev, has been described elsewhere (9). The HIV rev expression plasmid, pCMV-rev (27) (referred to here as pCHIVrev to distinguish between HIV and FIV rev) was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. Construction of the vesicular stomatitis virus G (VSV-G) envelope expression plasmid, pCMV-G, has also been described previously (71).
Cells.
Human kidney 293T cells (11), HT1080 cells (ATCC CCL 121), and primary human skin fibroblast (HSF) CCD 1059sk cells (ATCC CRL 2070) were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Quiescent HSF cells were obtained by growing passage 5, 6, or 9 cells to confluency and maintaining the cells for 21 days in DMEM containing 10% FBS (49). The arrested state of the cells at the G0/G1 phase of the cell cycle was verified by propidium iodide staining of the DNA and flow cytometry prior to transduction. Dividing HSF cells were obtained by maintaining subconfluent cultures and plating 5 × 104 cells in each well of a 12-well plate 1 day before transduction. Quiescent HT1080 cells were obtained by plating 5 × 105 cells in each well of a six-well plate prior to γ-irradiation (24) at a dose of 6,000 rads. HT1080 cells were analyzed 3 days after irradiation by flow cytometry to confirm growth arrest at the G2 phase of the cell cycle. Dividing HT1080 cells were plated at 5 × 105 per well in a six-well plate 1 day before transduction.
Vector production.
Pseudotyped FIV vector particles were generated by transient transfection of plasmid DNA into 293T cells plated 1 days prior to transfection at a density of 2.8 × 106 per 10-cm-diameter culture dish. Three plasmid cotransfections were performed at a 1:2:1 molar ratio of FIV packaging construct, FIV vector construct, and VSV-G envelope-expressing plasmid. Four plasmid cotransfections were carried out at a 1:2:1:1 molar ratio of FIV packaging construct, FIV vector construct, env plasmid, and rev expression plasmid. DNA complexes were prepared with calcium phosphate (Profectin kit; Promega Corp., Madison, Wis.) and transfected into cells according to the manufacturer’s instructions. The medium was replaced 8 to 16 h after transfection, and the supernatant was harvested 42 to 48 h after the start of transfection, filtered through a 0.45 μm Nalgene filter, and stored at −70°C or concentrated prior to storage. The supernatants were concentrated by precipitation in a 10% solution of polyethylene glycol in phosphate-buffered saline (PBS) and pelleted by centrifugation for 15 min at 3,000 rpm in a Sorvall H-1000B rotor. The pellet was resuspended in DMEM with 10% FBS and stored at −70°C.
In vitro transduction and determination of titer.
To determine the viral titer, HT1080 cells were seeded at a density of 4 × 104 per well in a 24-well plate 1 day prior to transduction. Serial dilutions of FIV vector preparations were added to the cells in the presence of 8 μg of Polybrene/ml, and the cultures were incubated for 48 h after transduction. Growth-arrested HSF and HT1080 cells (as well as the dividing control cells) were transduced in 12- and 6-well plates, respectively. Growth-arrested cells were assayed for reporter gene expression 3 days after transduction. β-Gal expression was assayed after the cells were fixed in a solution of 3% formaldehyde and 1.25% glutaraldehyde in PBS and stained for 4 h at 37°C in a solution containing 400 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; Sigma, St. Louis, Mo.)/ml (53). The titer was determined by counting the number of blue foci per well and was reported as LacZ-forming units (LFU) per milliliter of vector stock. EGFP gene expression was determined by fluorescence-activated cell sorter (FACS) analysis following fixing of the cells in 4% formaldehyde in PBS and was reported as the mean percentage of expressing cells.
In vivo gene delivery and detection of transgene expression.
All animal procedures were performed in accordance with protocols approved by the University of Iowa Animal Care and Use Committee. Five-week-old F1B hamsters were anesthetized by intraperitoneal injection of sodium pentobarbital (Nembutal; Abbott Laboratories) at a dose of 75 mg/kg of body weight. A 1-cm incision was made over the quadriceps femoris muscle, 25 μl of vector (2 × 106 IU) was injected into the muscle, and the incision was closed. Two weeks after injection, the hamsters were euthanized by CO2 asphyxiation. The injected muscle was removed by dissection, embedded in Tissue-Tek compound, and frozen in liquid-N2-cooled isopentane. To assay for β-Gal, 10-μm-thick cross sections were fixed in 0.5% glutaraldehyde, washed with PBS, and stained for 2 h at 37°C in a solution of 1 mg of X-Gal/ml–20 mM K3Fe(CN)6–20 mM K4Fe(CN)6 · 3H2O–2 mM MgCl2 in PBS. The sections were counterstained for 2 min in eosin, mounted with Permount, and examined by light microscopy.
RESULTS
Production of FIV vector.
A three-plasmid expression system similar to that developed for HIV-1 vectors (28, 42, 47) was designed for the production of pseudotyped FIV particles by transient transfection. The system consists of an FIV packaging construct, an FIV vector construct, and a plasmid encoding the surface glycoprotein of VSV-G. The envelope plasmid encoding VSV-G confers a broad tropism on the viral particles as well as greater stability than would be offered by the amphotropic envelope of MLV, enabling particle concentration by ultracentrifugation (6, 71). FIV particles were produced by cotransfection of the three construct components into 293T cells, and the titer of vector particles contained in the supernatant was determined. Vector titers as high as 3 × 106 infectious particles/ml were obtained in HT1080 cells transduced with 293T cell supernatants (Table 1).
TABLE 1.
Effect of vector and packaging construct on vector titer
| Packaging construct | Vector construct | Mean titera (LFU/ml of virus stock) ± SD |
|---|---|---|
| pCFIVX | pVETLCβ | (3.2 ± 0.40) × 106 (100%) |
| pCFIVX | pVETLCβCTE | (3.1 ± 0.35) × 106 (99%) |
| pCFIVX | pVETSCβ | (3.1 ± 0.43) × 106 (97%) |
| pCFIVX | pTFIVLCβ | (5.3 ± 0.65) × 104 (2%) |
| pCFIV | pVETLCβ | (3.1 ± 0.26) × 106 (97%) |
| pCFIVΔorf2 | pVETLCβ | (2.9 ± 0.27) × 106 (93%) |
| pCFIVΔvif | pVETLCβ | (3.3 ± 0.43) × 106 (102%) |
| pCFIVΔorf2Δvif | pVETLCβ | (3.0 ± 0.43) × 106 (96%) |
Results are from a representative experiment, with each value representing the average titer of three replicate vector preparations. At least three experiments done in triplicate were performed. Vector titer is expressed as LacZ-forming units per milliliter of virus stock. Pseudotyped FIV vector stocks were generated by transient transfection in 293T cells, and the resulting supernatant was used to transduce HT1080 cells. The titer of vector stocks was measured by counting the number of blue foci following X-Gal staining. The titer of FIV vector stocks prepared with the packaging construct pCFIVX and the vector construct pVETLCβ was arbitrarily given a value of 100%, to which the titers of vector stocks prepared with the remaining constructs were compared.
Requirements for FIV vector constructs.
The poor transcriptional activity of the FIV LTR in human cells may be overcome by use of a hybrid LTR strategy in which the FIV U3 promoter region is replaced with the CMV promoter-enhancer (46). In addition to containing cis-acting signals necessary for transcription, however, FIV vector constructs must also contain signals to direct reverse transcription and integration as well as incorporation of vector genomic RNA into viral particles. The location of the FIV packaging signal (Ψ) is not known; however, if it is analogous to HIV, some portion of the packaging signal may be situated in the noncoding region adjacent to the 5′ LTR and may extend into the Gag coding region. To delineate the minimum cis-acting vector construct requirements for efficient transduction of dividing cells, four FIV vector constructs expressing β-Gal from an internal CMV promoter were tested in conjunction with an FIV packaging construct (pCFIVX [Fig. 1B]) and the VSV-G env plasmid, pCMV-G. The FIV vector, pTFIVLCβ, contains the authentic FIV 5′ LTR, the adjacent noncoding region, and approximately 550 bp corresponding to the Gag coding region (Fig. 1C). pVETLCβ is similar to pTFIVLCβ except that the entire U3 region of the FIV 5′ LTR is replaced with the CMV promoter. pVETSCβ differs from pVETLCβ only in containing a shortened region of approximately 350 bp corresponding to gag. pVETLCβCTE is identical to pVETLCβ with the addition of a CTE from MPMV (4).
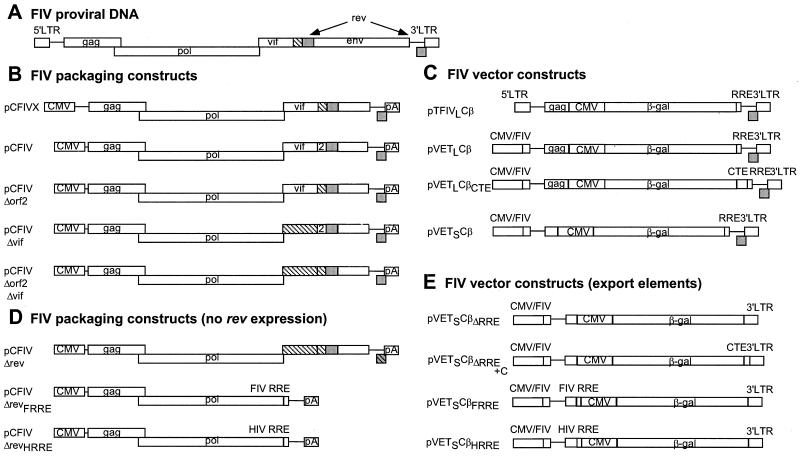
FIG. 1.
Schematic representation of the FIV provirus and FIV-based packaging and vector constructs used to generate pseudotyped FIV-based vector particles by transient transfection. (A) FIV provirus based on FIV-34TF10 molecular clone. (B) FIV-based packaging constructs derived from FIV-34TF10. FIV coding regions are flanked by the CMV promoter (CMV) and the simian virus 40 polyadenylation signal (pA). pCFIVX contains approximately 100 additional base pairs of the FIV 5′ noncoding region compared to pCFIV. pCFIV contains the complete orf2 corresponding to FIV14. Δorf2 and Δvif denote mutations which disrupt the orf2 and vif reading frames (hatched regions). FIV rev is shaded. (C) FIV-based vector constructs expressing the β-Gal gene from an internal CMV promoter. The FIV RRE in these constructs is located downstream of the β-Gal gene. pTFIVLCβ contains the complete FIV 5′ LTR, whereas pVET constructs carry a CMV promoter in place of the FIV U3 region. (D) FIV packaging constructs with a mutated or deleted rev (as well as mutated or deleted vif and orf2 [hatched boxes]). pCFIVΔrev lacks the splice acceptor and basic binding domain of the second exon of rev (hatched and shaded box). pCFIVΔrevFRRE and pCFIVΔrevHRRE lack vif, orf2, and rev but contain the FIV RRE or HIV RRE, respectively. (E) FIV vector constructs lacking the FIV RRE or containing the FIV RRE upstream of the CMV promoter driving expression of the β-Gal gene. The FIV vector construct denoted by +C also contains the MPMV CTE downstream of the β-Gal gene.
Supernatants from 293T cells transfected with the FIV packaging construct pCMV-G and one of the four FIV vector constructs were assayed for vector particle titer in HT1080 cells (Table 1). Particles produced from the vector containing the authentic FIV 5′ LTR (pTFIVLCβ) yielded titers of 5 × 104 LFU/ml, while the CMV promoter-FIV hybrid LTR vectors (pVETLCβ, pVETLCβCTE, and pVETSCβ) all yielded titers of approximately 3 × 106 LFU/ml of vector stock. Analysis of FIV p24 capsid levels with the IDEXX (Portland, Maine) FIV antigen test kit indicated no significant differences between p24 levels in the supernatants of cells transfected with the hybrid vectors and those of cells transfected with nonhybrid LTR vectors (data not shown). However, since FIV p24 is produced from the packaging construct, any differences would likely be minimal with the same packaging construct being used for all of the transfections. The greater-than-50-fold drop in titer repeatedly observed in experiments with vectors containing the complete FIV 5′ LTR suggests a requirement in human cells for additional cis-acting signals not normally present in the FIV LTR for enhanced transcriptional activity.
To verify that the β-Gal activity observed in the transduced cells was due to expression following reverse transcription and not the result of pseudotransduction of β-Gal activity present in the vector preparations, HT1080 cells were transduced in the presence or absence of 3′-azido-3′-deoxythymidine (AZT) (zidovudine; GlaxoWellcome). The cells were incubated with 50 μM AZT for 24 h prior to transduction, and fresh AZT was added at the time of transduction. The titer resulting from transduction of cells in the presence of AZT was 0.5% or less of that observed from cells transduced in the absence of AZT, in the case of both vectors containing hybrid promoters and those containing nonhybrid promoters (data not shown). These data suggest that nearly all of the β-Gal activity is the result of true transduction by the FIV vectors.
Requirements for FIV packaging constructs.
FIV packaging constructs were designed to minimize the amount of sequence homology between packaging and vector constructs as well as to eliminate the production of proteins dispensable for efficient vector production and infectivity (Fig. 1B). Since it is desirable that the packaging construct not be copackaged with the vector transcript, packaging constructs containing minimal 5′ noncoding regions were generated. In addition, packaging constructs lacking one or both of the FIV accessory genes, vif and orf2, were generated. Five packaging constructs (Fig. 1B) differing either in 5′ noncoding sequence or ability to express accessory genes were analyzed in conjunction with an FIV vector construct expressing β-Gal (pVETLCβ) and the env plasmid, pCMV-G. The FIV packaging construct pCFIVX contains approximately 100 bp of noncoding sequence upstream of the major splice donor site, while the packaging construct pCFIV contains only 6 bp of noncoding sequence upstream of the splice donor site and lacks 17 bp normally located between the splice donor site and the start codon for gag (Fig. 1B). In addition, the pCFIV packaging construct contains an intact orf2 corresponding to that of FIV14 (40). In contrast, the pCFIVΔorf2 packaging construct, derived from FIV-34TF10, contains a premature stop codon within orf2 and thus does not give rise to a functional Orf2 product (44, 60). pCFIVΔvif contains a premature stop codon in the Vif coding region followed by a frameshift mutation corresponding to amino acid 22. pCFIVΔorf2Δvif contains the same mutations in both the Orf2 and Vif coding regions (Fig. 1B).
Each of the packaging plasmids was separately transfected into 293T cells together with FIV vector (pVETLCβ) and env plasmid pCMV-G, and the supernatants were assayed by transduction of HT1080 cells (Table 1). All five packaging constructs yielded similar titers, ranging between 2.9 × 106 and 3.2 × 106 LFU/ml. Analysis of p24 levels in the supernatants also indicated similar levels of p24 production (data not shown). Comparison of transduction efficiencies of FIV vector particles prepared from the packaging constructs containing differing lengths of FIV 5′ noncoding sequence (pCFIVX and pCFIV) indicated that the deleted sequences are not required for the efficient translation of FIV proteins required in trans for particle production. In addition, the introduction of mutations in the coding regions for the FIV accessory genes, orf2 and vif, either alone or in combination, did not have a substantial effect on transduction efficiency in HT1080 cells (Table 1).
Requirement for FIV Rev-RRE.
To ascertain whether the FIV rev regulatory gene and the RRE to which it binds are necessary for efficient particle production and subsequent transduction, FIV packaging constructs which do not express rev were generated (Fig. 1D). In the FIV packaging construct pCFIVΔrev, the splice acceptor site and the basic amino acid domain of the second exon of rev were deleted (Fig. 1D). A similar deletion of the splice acceptor site and binding domain to create an FIVΔrev construct was previously demonstrated to yield insignificant levels of infectious virus (45). In the FIV packaging constructs pCFIVΔrevFRRE and pCFIVΔrevHRRE, the coding regions for Rev (as well as Vif and Orf2) were deleted and the RRE from FIV or HIV, respectively, was introduced (Fig. 1D). FIV packaging constructs containing an additional MPMV CTE export element were also generated (not shown). The Δrev FIV packaging constructs were tested together with various FIV vector constructs designed to investigate whether the type, location, and inclusion of an additional export element could influence the relative FIV vector particle production. To complement the FIV packaging constructs with rev deleted, a construct expressing FIV rev from a CMV promoter (pCFIVrev) was included in the cotransfections. A construct expressing the HIV rev from a CMV promoter (pCHIVrev) was cotransfected into producer cells to complement the HIV packaging construct with rev deleted.
To determine the requirement for an export element in the FIV vector constructs, an FIV vector lacking the FIV RRE (pVETSCβΔRRE) was generated. In addition, FIV vector constructs were generated that contain the FIV or HIV RRE upstream of the internal CMV promoter driving expression of the β-Gal gene (pVETSCβFRRE and pVETSCβHRRE, respectively [Fig. 1E]). FIV vectors that contain the MPMV CTE alone (e.g., pVETSCβΔRRE+C [Fig. 1E]) or together with other export elements (not shown) were also generated. The location of the FIV and HIV RREs in these constructs differs from that in the previous constructs, in which the FIV RRE is located downstream of the β-Gal gene (e.g., pVETSCβ [Fig. 1C]).
The requirement for rev for FIV vector and packaging constructs as well as the effect of the type and location of the export element were analyzed by transduction of HT1080 cells with FIV particles produced from various combinations of FIV constructs (Table 2). The titer of vector particles generated in the absence of FIV rev dropped dramatically compared to that of particles prepared in the presence of FIV rev (Table 2, compare the first and fourth lines). Titers of vector particles generated in the presence of FIV rev with a vector construct lacking the FIV RRE were reduced to less than 40% of those generated with an FIV vector containing the FIV RRE (Table 2, compare the first two lines). Titers of FIV vector constructs containing the FIV RRE upstream of the internal CMV promoter were slightly higher (30 to 50% higher in repeated experiments) than those generated with vector constructs containing the FIV RRE downstream of the β-Gal gene (Table 2, compare the first and third lines). The last result indicates that the location of the export element in the vector construct can have a moderate impact on transduction efficiency.
TABLE 2.
Requirement for rev-RRE for efficient transduction of HT1080 cells
| Packaging construct | Vector construct | Rev construct | Mean titera (LFU/ml of virus stock) ± SD |
|---|---|---|---|
| pCFIV | pVETSCβ | None | (2.6 ± 0.38) × 106 (100%) |
| pCFIV | pVETSCβΔRRE | None | (9.3 ± 0.22) × 105 (36%) |
| pCFIV | pVETSCβFRRE | None | (3.4 ± 0.50) × 106 (132%) |
| pCFIVΔrev | pVETSCβ | None | (2.2 ± 0.94) × 101 (<0.01%) |
| pCFIVΔrev | pVETSCβ | pCFIVrev | (1.1 ± 0.34) × 106 (42%) |
| pCFIVΔrevFRRE | pVETSCβ | pCFIVrev | (1.0 ± 0.43) × 105 (4%) |
| pCFIVΔrevFRRE | pVETSCβFRRE | pCFIVrev | (1.6 ± 0.32) × 105 (6%) |
| pCFIVΔrevHRRE | pVETSCβHRRE | pCFIVrev | (1.0 ± 0.20) × 106 (39%) |
Results are from a representative experiment, with each value representing the average titer of three replicate vector preparations. At least three experiments done in triplicate were performed. Titer is expressed as LacZ-forming units per milliliter of virus stock. Pseudotyped FIV vector stocks were generated by transient transfection in 293T cells, and the resulting supernatant was used to transduce HT1080 cells. The titer of vector stocks was measured by counting the number of blue foci following X-Gal staining. The titer of vector stocks prepared with pCFIV and pVETLCβ was arbitrarily given a value of 100%, to which the titers of vector stocks prepared with the remaining constructs were compared.
To further analyze the production of FIV vector particles by FIV packaging constructs lacking rev, a separate rev expression plasmid (pCFIVrev) was utilized (Table 2). Titers resulting from cotransfection of the rev expression plasmid together with an FIV packaging construct which does not express rev were approximately 40% of titers resulting from cotransfection with the pCFIV rev-expressing packaging construct (Table 2, compare the first and fifth lines). Titers resulting from a similar cotransfection with an FIV packaging construct lacking both exons of rev (pCFIVΔrevFRRE), however, were reduced to approximately 4% of those resulting from cotransfection with the pCFIV rev-expressing packaging construct (Table 2, compare the first and sixth lines). This considerable reduction in titer was not significantly compensated for by the use of an FIV vector construct containing the FIV RRE upstream of the internal cassette (pVETSCβFRRE) (Table 2, compare the sixth and seventh lines). Replacement of the FIV RRE with that of HIV in the FIV packaging construct with rev deleted restored titers to the level observed with an FIV packaging construct lacking only the rev splice acceptor and basic binding domain (pCFIVΔrev) (Table 2, compare the fifth and eighth lines). These data indicate that the HIV rev/RRE combination might give rise to higher titers than the FIV rev/RRE combination in human cells. The addition of a CTE in either FIV vector or packaging constructs already containing an RRE had no measurable effect on titer (not shown), although the addition of a CTE in an FIV vector construct lacking an RRE resulted in a slight increase in titer compared to one which contained no export element at all (Fig. 1E, compare pVETSCβΔRRE to pVETSCβΔRRE +C, and data not shown). The observation that the addition of a CTE had little or no effect on titer may be due to the greater-than-200-bp optimal distance between the CTE and the polyadenylation signal (50).
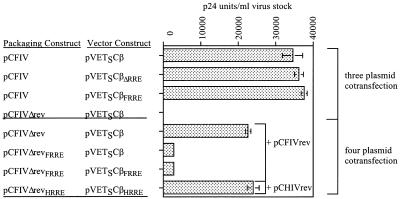
Analysis of FIV p24 levels of supernatants used in the above-mentioned study, in which rev was expressed from the FIV packaging construct (three-plasmid cotransfection) or from a separate expression plasmid (four-plasmid cotransfection), indicated similar trends in vector particle production and in transduction efficiency (Fig. 2). Supernatants generated with the rev-expressing FIV packaging construct, pCFIV, all contained comparable levels of FIV p24 antigen (Fig. 2, lines 1 through 3). Supernatants generated in the absence of rev with an FIV packaging construct lacking the splice acceptor site and basic binding domain (pCFIVΔrev) contained undetectable levels of FIV p24 antigen (Fig. 2, line 4). Where rev was supplied by a separate expression plasmid (four-plasmid cotransfection), FIV p24 levels were reduced compared to those resulting from cotransfections with an FIV packaging construct expressing rev (three-plasmid cotransfection) (Fig. 2, lines 1 and 5). Supernatants prepared with an FIV packaging construct lacking the splice acceptor site and basic binding domain of Rev (pCFIVΔrev) contained significantly higher p24 levels than those prepared with an FIV packaging construct lacking both exons of rev (pCFIVΔrevFRRE) (Fig. 2, lines 5 and 6). Supernatants prepared with an FIV packaging construct lacking both exons of rev but containing the HIV RRE (pCFIVΔrevHRRE) contained higher levels of FIV p24 antigen than supernatants generated with a similar FIV packaging construct containing the FIV RRE (pCFIVΔrevFRRE) (Fig. 2, lines 7 and 8). Taken together, these observations are consistent with the reduction in titer of supernatants generated in the absence of rev being due to the inefficient export of unspliced viral RNA and subsequent poor production of vector particles.
FIG. 2.
Analysis of relative FIV p24 antigen levels in pseudotyped FIV-based vector stocks prepared by transient transfection in 293T cells. Relative FIV p24 levels are expressed as p24 units/ml of virus stock as determined by comparison with a dilution series of the positive control included in the antigen capture enzyme-linked immunosorbent assay (IDEXX). The results shown are from a representative experiment performed in triplicate. Similar relative values were observed in repeated experiments.
Requirement for accessory gene expression in transduction of nondividing cells.
To determine whether expression of the FIV accessory genes, vif and orf2, is required for the transduction of nondividing cells, primary HSF cells were arrested at the G0/G1 phase of the cell cycle by density-dependent inhibition of growth. The arrested cells were then transduced with FIV vectors prepared with packaging constructs expressing FIV orf2 and/or vif or neither (pCFIV, pCFIVΔorf2, pCFIVΔvif, and pCFIVΔorf2Δvif, respectively [Table 1]) together with an FIV β-Gal-expressing vector construct (pVETLCβ) and pCMV-G envelope plasmid. Transduction efficiencies of HSF cells overall were lower than those for HT1080 cells, and transduction efficiencies of growth-arrested HSF cells were approximately twofold lower than those for proliferating HSF cells (e.g., 3.2 × 104 for dividing cells compared to 1.6 × 104 for nondividing cells [Table 3]). However, comparison of transduction efficiencies of vectors which did or did not express FIV vif and/or orf2 accessory genes revealed similar results in dividing as well as nondividing HSF cells (2.9 × 106 to 3.3 × 106 in dividing cells and 1.6 × 106 to 1.7 × 106 in nondividing cells [Table 3]). An MLV vector, also containing an internal CMV promoter driving expression of a β-Gal gene, efficiently transduced dividing HSF cells. In contrast to transduction of nondividing HSF cells by FIV vectors, however, the transduction efficiency of the MLV vector in nondividing HSF cells was dramatically reduced (from 3.8 × 104 in dividing cells to 7.3 × 101 in nondividing cells [Table 3]). These data indicate that, unlike MLV vectors, FIV vectors are capable of efficiently transducing nonproliferating HSF cells and, in addition, that FIV vif and orf2 accessory gene expression is not required for efficient transduction.
TABLE 3.
Effect of accessory gene expression on transduction of human primary skin fibroblasts
| Packaging construct | Vector construct | Mean transduction efficiencya (LFU/ml of virus stock) ± SD
|
|
|---|---|---|---|
| Dividing | Nondividing | ||
| pCFIV | pVETLCβ | (3.2 ± 0.36) × 104 (100%) | (1.6 ± 0.27) × 104 (100%) |
| pCFIVΔorf2 | pVETLCβ | (3.3 ± 0.47) × 104 (104%) | (1.7 ± 0.36) × 104 (106%) |
| pCFIVΔvif | pVETLCβ | (3.3 ± 0.11) × 104 (104%) | (1.7 ± 0.37) × 104 (106%) |
| pCFIVΔorf2Δvif | pVETLCβ | (2.9 ± 0.30) × 104 (93%) | (1.7 ± 0.16) × 104 (108%) |
| pMLVgagpol | pMLVCMVβ | (3.8 ± 0.26) × 104 (121%) | (7.3 ± 0.90) × 101 (0.5%) |
Results are from a representative experiment, with each value representing the average transduction efficiency of three replicate vector preparations. At least three experiments done in triplicate were performed. Transduction efficiency is expressed as LacZ-forming units per milliliter of virus stock. Pseudotyped FIV vector stocks prepared in 293T cells were standardized after their titers in HT1080 cells were determined. The standardized vector stocks were then used to transduce dividing and nondividing HSF cells at an MOI of 2.0. The transduction efficiency in HSF cells was measured by counting the number of blue foci following X-Gal staining. The transduction efficiency of vector stocks prepared from pCFIV and pVETLCβ was arbitrarily given a value of 100%, to which the transduction efficiencies of vector stocks prepared with the remaining constructs were compared in HSF cells.
Effect of MOI and accessory gene expression in transduction of nondividing cells.
To study the effect of vif and orf2 accessory gene expression on transduction of another nondividing cell type at various multiplicities of infection (MOIs), HT1080 cells were arrested at the G2 phase of the cell cycle by exposure to γ-irradiation. Proliferating or growth-arrested HT1080 cells were transduced with vector prepared from packaging constructs expressing vif and/or orf2 or neither accessory gene (pCFIV, pCFIVΔorf2, pCFIVΔvif, or pCFIVΔorf2Δvif, respectively) together with pCMV-G and a vector construct expressing the EGFP gene under the control of the MLV promoter (pVETLMEGFP). The cells were infected at an MOI of 2.0, 0.2, or 0.02, and the percentage of cells expressing the EGFP gene was determined by flow cytometry. At all MOIs, little difference in transduction efficiency was observed in dividing or nondividing cells infected with FIV vector prepared with or without accessory gene expression (Table 4). An MLV vector expressing the EGFP gene also efficiently transduced dividing HT1080 cells at all MOIs tested. Even at a very high MOI (i.e., MOI = 10), however, the MLV vector failed to efficiently transduce growth-arrested HT1080 cells. These data again demonstrate that FIV vectors can efficiently transduce quiescent cells and that FIV vif and orf2 accessory gene expression is not required to transduce these cells even at a low MOI (i.e., MOI = 0.02). In addition, because the transduction efficiency of the VSV-G-pseudotyped MLV vector is comparable to background levels in nondividing cells (i.e., the level observed in the absence of vector), these data indicate that the observed transduction is not due to pseudotransduction of EGFP activity which could be present in the vector preparations.
TABLE 4.
Effect of accessory gene expression on transduction of γ-irradiated HT1080 cells
| Packaging construct | Vector construct | MOIc | Mean % transduced cellsa
|
FACS analysis for % expressing cellsb
|
||
|---|---|---|---|---|---|---|
| Dividing | Irradiated | Dividing | Irradiated | |||
| pCFIV | pVETLMEGFP | 2.0 | 81.1 | 79.1 | 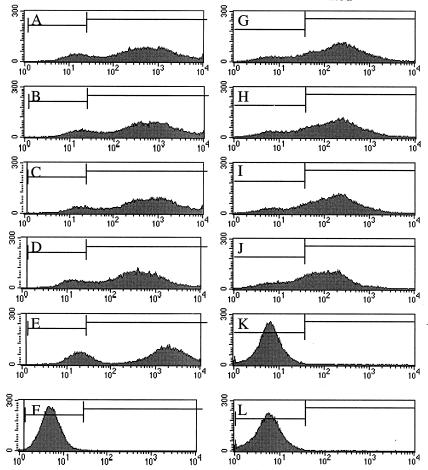 |
|
| 0.2 | 38.5 | 45.5 | ||||
| 0.02 | 4.8 | 6.0 | ||||
| pCFIVΔorf2 | pVETLMEGFP | 2.0 | 74.8 | 78.9 | ||
| 0.2 | 24.0 | 40.2 | ||||
| 0.02 | 3.3 | 8.3 | ||||
| pCFIVΔvif | pVETLMEGFP | 2.0 | 76.2 | 76.3 | ||
| 0.2 | 22.2 | 39.0 | ||||
| 0.02 | 3.8 | 7.6 | ||||
| pCFIVΔorf2Δvif | pVETLMEGFP | 2.0 | 74.5 | 72.5 | ||
| 0.2 | 23.2 | 41.4 | ||||
| 0.02 | 3.5 | 6.3 | ||||
| pMLVgagpol | pMLVEGFP | 10 | 97.9 | 0.5 | ||
| 2.0 | 79.4 | 0.5 | ||||
| 0.2 | 27.3 | 0.4 | ||||
| 0.02 | 4.0 | 0.4 | ||||
| None | None | NA | 0.2 | 0.2 | ||
Mean percentage of transduced cells as determined by FACS analysis for detection of EGFP. The results are from a representative experiment, with each value representing the average transduction efficiency of three replicate vector preparations. The standard deviation for each value did not exceed 10%. At least three experiments were performed, using at least one of the three MOIs indicated.
FACS analysis of a separate experiment with an MOI of 2.0 resulted in the following percentages of transduced cells from each panel: A, 87.4; B, 89.4; C, 91.7; D, 87.2; E, 85.0; F, 0.56; G, 81.6; H, 76.9; I, 79.7; J, 70.0; K, 0.55; L, 0.21.
Vector stocks were standardized after the titer of each on dividing HT1080 cells was determined by FACS analysis, and they were used to transduce growth-arrested HT1080 cells at the MOIs indicated. NA, not available.
In vivo transduction of hamster muscle tissue.
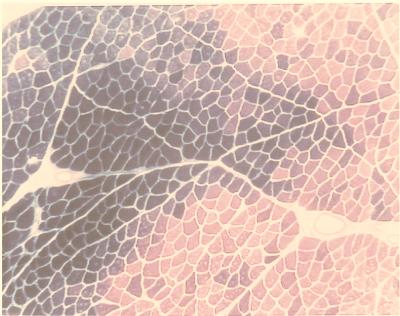
To test the ability of FIV vectors to deliver genes in vivo in muscle, FIV vectors encoding the β-Gal gene were directly injected into the hind-leg muscles of hamsters. FIV vector was generated with a packaging construct, pCFIV, expressing both FIV accessory genes, and with a vector construct, pVETLCβ, expressing the β-Gal gene. F1B hamster hind-leg muscle was injected with 2 × 106 IU of vector, and transgene expression was detected near the injection site after 2 weeks (Fig. 3). Studies to determine the requirement for FIV accessory proteins, Vif and Orf2, for efficient gene delivery into muscle are under way.
FIG. 3.
In vivo transduction of hamster muscle by a VSV-G-pseudotyped FIV vector. F1B hamster hind-leg muscle was injected with 2 × 106 IU of FIV vector generated with a packaging construct expressing FIV accessory genes (pCFIV) and a vector construct expressing the β-Gal gene (pTVETLCβ). Two weeks after injection, the transduced muscle was sectioned and assayed for transgene expression (β-Gal staining is evident as blue color). The figure is representative of samples from three independent experiments performed in duplicate.
DISCUSSION
The advantages of an FIV-based gene delivery system include those inherent in lentivirus vectors, such as a large coding capacity, stable gene transfer, and ability to infect nondividing targets. In addition, FIV vectors may offer a more readily acceptable alternative to vectors derived from human lentiviruses. The feasibility of developing FIV as a vehicle for gene delivery in primates was recently demonstrated by Poeschla et al. (46). Our results expanded on the previous work by describing the development of a minimal FIV vector system that retains its ability to efficiently transduce a variety of dividing and nondividing cell types. This system excludes unnecessary cis-acting sequences, which might increase the likelihood of generating a replication-competent retrovirus, and eliminates nonessential viral gene expression, which could lead to protein contamination and undesired immune responses. Minimal vector systems have been developed for HIV-1-based vectors which lack tat, vif, vpr, vpu, and nef, although the requirement for rev, the remaining auxiliary gene necessary for efficient RNA export, has not yet been demonstrated to be effectively overcome through the use of heterologous export elements (25, 49, 72). In our investigation of the minimum requirements for the efficient production and infectivity of FIV vectors, we have examined requirements for cis-acting sequences, accessory and regulatory genes, and the use of heterologous export elements for the transduction of both dividing and nondividing cells.
The strategy of replacing the U3 regions of retroviral LTRs with the CMV promoter has been utilized to increase MLV vector titers (14, 56), to overcome the requirement for Tat in HIV-1 vectors (7, 25, 51), and, more recently, to enhance the titer of FIV vectors in human cells (46). In the present study, comparison of titers for vectors containing the authentic FIV LTR with those for vectors containing the hybrid FIV LTR indicates that those containing the CMV promoter were over 50 times more efficient in transducing human cells than those without a hybrid LTR. The increase in titer of vectors containing the hybrid promoter is presumably due to more efficient transcription in the producer cell, since the CMV promoter replacing the U3 region is no longer present in the target cell.
Additional cis-acting sequences examined in the FIV vector construct include those within the 5′ LTR and Gag coding region, which likely contain some part of the FIV packaging signal required for efficient encapsidation of genomic RNA. The packaging signal for HIV-1 appears to be a multipartite element containing several subdomains located upstream of the splice donor site and extending to gag (30). Packaging signals within the FIV genome have not yet been characterized; however, the present study reveals that FIV vector constructs containing the FIV R and U5 regions followed by the authentic 5′ noncoding region and as little as 350 bp corresponding to the Gag coding region were able to transduce cells efficiently. These results indicate that at least a functional portion of the FIV packaging signal is located within this region.
In examining the requirement for FIV accessory and regulatory gene expression, we find that expression of FIV vif and orf2 is dispensable for transduction of various dividing and nondividing cell types. Although FIV vif is required for productive infection of certain feline cells, most notably, lymphoid cell lines and peripheral blood lymphocytes, it is not required in others (53, 65). Studies of HIV-1 vif have revealed that the cell type used to produce vif-defective virus determines viral infectivity (15, 16, 68). Vif mutant virus produced in permissive cells can infect nonpermissive cells; however, the resulting virions are only weakly infectious. Recent evidence indicates that nonpermissive cells contain an endogenous inhibitor of HIV replication that is overcome by Vif (30, 55). It is not known whether FIV Vif has a similar function; however, as in the case of HIV vectors, where vif is dispensable for infectivity when produced in 293T cells (25, 72), these cells also appear to compensate for any requirement for vif in the FIV vector system. The requirement for orf2 is likewise observed only in certain cell types (64, 69) and may also be overcome with the use of established producer cell lines. The need for orf2 in certain cells is consistent with at least one role, as a transactivator of the FIV LTR (58, 69). The use of a hybrid LTR in which the entire FIV U3 promoter is replaced with the strong CMV promoter, then, would also negate any requirement for orf2 transactivator function in the producer cell.
The requirement for rev-RRE for both productive FIV infection and transient reporter gene expression has been demonstrated (31, 45, 63). Our study establishes the requirement for rev-RRE in the FIV-based vector system as well, presumably for efficient RNA export and subsequent FIV particle production. The results indicate that FIV rev is required in trans; however, it is unclear why transduction efficiencies are lower when FIV Rev is translated from a separate construct rather than the FIV packaging construct. There are at least four differences between the three-plasmid transfection system and the four-plasmid transfection system, where FIV Rev is supplied from a separate rev expression construct. First, the FIV RRE present in the FIV packaging constructs corresponds to that of the FIV-34TF10 molecular clone whereas the rev gene of pCFIVrev was cloned from the FIV PPR strain (9). The secondary structures of the 34TF10 and PPR strains, however, are highly conserved, with nucleotide differences occurring only in predicted loops or otherwise maintaining the base pairing of the calculated minimal energy structure (45). Second, the FIV packaging construct with rev deleted (pCFIVΔrevFRRE) lacks small open reading frames to which no functions have been attributed but which are present, nonetheless, in the packaging constructs which also encode Rev (e.g., pCFIV). However, a packaging construct which does not express rev but which maintains most of the Rev coding regions (pCFIVΔrev) was also associated with a moderate decrease in titer when complemented in trans by FIV Rev. This observation indicates that the absence of the small open reading frames in the packaging construct with rev deleted might be partially, but not fully, responsible for the drop in titer associated with the four-plasmid transfection. Third, the context of the FIV RRE differs between the rev-expressing packaging construct (pCFIV) and the minimal packaging construct with rev deleted, with the context of the former being closer to that observed in wild-type FIV. The substitution of HIV Rev-RRE for FIV Rev-RRE, where HIV Rev is also translated from a separate construct, does appear to overcome some deficiency in the minimal FIV packaging construct with rev deleted. FIV Rev has been reported to contain sequences that can complement the loss of HIV-1 Rev effector function (30), and it is conceivable that an interaction between Rev and a cellular export factor might be more effective with HIV Rev than with FIV Rev in human cells. Fourth, a four-plasmid transfection might be inherently less efficient than a three-plasmid transfection, since the likelihood of delivering four plasmids to a given cell is decreased.
FIV vectors not only provide a nonprimate alternative to current lentivirus-based gene delivery systems but also represent a convenient system for gaining valuable insight into the molecular biology and pathogenicity of FIV. The present work demonstrates the feasibility of producing high-titer FIV vector particles by transient transfection of human cells and, in addition, demonstrates the dispensable nature of the FIV accessory genes, vif and orf2, for efficient transduction of both dividing and nondividing cells in vitro. It appears that FIV vif is, indeed, functionally analogous to HIV vif, not just in terms of the requirement for it in productive viral infection only in certain cell types but also in terms of its dispensability for vector production and transduction, at least in the cells investigated. FIV orf2 appears to have no additional function (other than its reported transactivating function) necessary for efficient particle production and subsequent transduction in the context of an FIV-based gene delivery system with a hybrid CMV/FIV LTR vector. The FIV regulatory gene rev together with the FIV RRE were found to be essential for efficient FIV-based particle production, although additional heterologous export elements may be found to be capable of overcoming the FIV rev deletion phenotype.
Preliminary results indicate that FIV vectors are capable of delivering genes in vivo into hamster muscle cells. Sustained expression of genes delivered directly into liver and muscle tissue by HIV vectors has been demonstrated, with maximum transduction into liver tissue dependent upon the HIV accessory proteins Vpr and/or Vif (23). It will, therefore, be important to determine whether the FIV accessory proteins, Orf2 and Vif, are also required for maximum transduction of specific tissues by FIV vectors. Additional studies to establish the duration of transgene expression in transduced cells as well as to determine the requirement for FIV accessory proteins for efficient transduction are under way.
ACKNOWLEDGMENTS
A portion of this research was supported by grant NS34568 (B.L.D.) from the National Institutes of Health as well as by grant R01AI25825 (J.H.E.) from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health. Kevin P. Campbell is an investigator of the Howard Hughes Medical Institute.
We thank Moti Bodner for pMLVEGFP, Andrea Lynn for technical assistance, and Cynthia Leveille for animal-handling assistance, as well as Tom Dubensky and Aymeric deParseval for critical reading of the manuscript.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann M H, Mathiason-Dubard C, Learn G H, Rodrigo A G, Sodora D L, Mazzetti P, Hoover E A, Mullins J I. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol. 1997;71:4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blomer U, Naldini L, Kafri T, Trono D, Verma I, Gage F H. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray M, Prasad S, Dubay J W, Hunter E, Jeang K T, Rekosh D, Hammarskjold M L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci USA. 1994;91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J-K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to a very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang L-J, McNulty E, Martin M. Human immunodeficiency viruses containing heterologous enhancer/promoters are replication competent and exhibit different lymphocyte tropisms. J Virol. 1993;67:743–752. doi: 10.1128/jvi.67.2.743-752.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deminie C A, Emerman M. Incorporation of human immunodeficiency virus type 1 Gag proteins into murine leukemia virions. J Virol. 1993;67:6499–6506. doi: 10.1128/jvi.67.11.6499-6506.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Parseval A, Elder J H. Demonstration that orf2 encodes the feline immunodeficiency virus transactivating (Tat) protein and characterization of a unique gene product with partial Rev activity. J Virol. 1999;73:608–617. doi: 10.1128/jvi.73.1.608-617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donaldson L J, Rankin J, Proctor S. Is it possible to catch leukemia from a cat? Lancet. 1994;344:971–972. doi: 10.1016/s0140-6736(94)91636-5. [DOI] [PubMed] [Google Scholar]
- 11.DuBridge R B, Tang P, Hsia H C, Leong P-M, Miller J H, Calos M P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elder J H, Phillips T R. Feline immunodeficiency virus as a model for development of molecular approaches to intervention strategies against lentivirus infections. Adv Virus Res. 1995;45:225–247. doi: 10.1016/s0065-3527(08)60062-7. [DOI] [PubMed] [Google Scholar]
- 13.Elder J H, Phillips T R. Molecular properties of feline immunodeficiency virus (FIV) Infect Agents Dis. 1993;2:361–374. [PubMed] [Google Scholar]
- 14.Finer M H, Dull T J, Qin L, Farson K, Roberts M R. kat: a high-efficiency retroviral transduction system for primary human T lymphocytes. Blood. 1994;83:43–50. [PubMed] [Google Scholar]
- 15.Fisher A G, Ensoli B, Ivanoff I, Chamberlain M, Petteway S, Ratner L, Gallo R C, Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987;237:888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- 16.Gabuzda D H, Lawrence K, Langhoff E, Terwilliger E, Dorfman T, Haseltine W A, Sodroski J. Role of vif in replication of human immunodeficiency virus type 1 in CD4+ T lymphocytes. J Virol. 1992;66:6489–6495. doi: 10.1128/jvi.66.11.6489-6495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 20.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 21.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeda Y, Tomonaga K, Kawaguchi Y, Kohmoto M, Inoshima Y, Tohya Y, Miyazawa T, Kai C, Mikami T. Feline immunodeficiency virus can infect a human cell line (MOLT-4) but establishes a state of latency in the cells. J Gen Virol. 1996;77:1623–1630. doi: 10.1099/0022-1317-77-8-1623. [DOI] [PubMed] [Google Scholar]
- 23.Kafri T, Blomer U, Peterson D A, Gage F H, Verma I M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 24.Kastan M B, Zhan Q, el-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 25.Kim V N, Mitrophanous K, Kingsman S, Kingsman A J. Minimal requirement for a lentivirus vector based on human immunodeficiency virus. J Virol. 1998;72:811–816. doi: 10.1128/jvi.72.1.811-816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landau N R, Page K A, Littman D R. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis N, Williams J, Rekosh D, Hammarskjöld M-L. Identification of a cis-acting element in human immunodeficiency virus type 2 (HIV-2) that is responsive to the HIV-1 rev and human T-cell leukemia virus types I and II rex proteins. J Virol. 1990;64:1690–1697. doi: 10.1128/jvi.64.4.1690-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis P F, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz H. Feline retroviruses: a brief review. Vet Microbiol. 1990;23:131–146. doi: 10.1016/0378-1135(90)90143-j. [DOI] [PubMed] [Google Scholar]
- 30.Madani N, Kabat D. An endogenous inhibitor of human immunodeficiency virus in human lymphocytes is overcome by the viral Vif protein. J Virol. 1998;72:10251–10255. doi: 10.1128/jvi.72.12.10251-10255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancuso V A, Hope T J, Zhu I, Derse D, Phillips T, Parslow T B. Posttranscriptional effector domains in the Rev proteins of feline immunodeficiency virus and equine infectious anemia virus. J Virol. 1994;68:1998–2001. doi: 10.1128/jvi.68.3.1998-2001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride M S, Panganiban A T. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J Virol. 1996;70:2963–2973. doi: 10.1128/jvi.70.5.2963-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller D G, Adam M A, Miller A D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazawa T, Kawaguchi Y, Kohmoto M, Tomonaga K, Mikami T. Comparative functional analysis of the various lentivirus long terminal repeats in human colon carcinoma cell line (SW480 cells) and feline renal cell line (CRFK cells) J Vet Med Sci. 1994;56:895–899. doi: 10.1292/jvms.56.895. [DOI] [PubMed] [Google Scholar]
- 35.Miyazawa T, Kawaguchi Y, Kohmoto M, Sakuragi J, Adachi A, Fukasawa M, Mikami T. Production of feline immunodeficiency virus in feline and non-feline non-lymphoid cell lines by transfection of an infectious molecular clone. J Gen Virol. 1992;73:1543–1546. doi: 10.1099/0022-1317-73-6-1543. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi H, Takahashi M, Gage F H, Verma I M. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 38.Nowotny N, Uthman A, Haas O A, Borkhardt A, Lechner K, Egberink H F, Mostl K, Horzinek M C. Is it possible to catch leukemia from a cat? Lancet. 1995;346:252–253. doi: 10.1016/s0140-6736(95)91300-9. [DOI] [PubMed] [Google Scholar]
- 39.Olmsted R A, Barnes A K, Yamamoto J K, Hirsch V M, Purcell R H, Johnston P R. Molecular cloning of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:2448–2452. doi: 10.1073/pnas.86.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olmsted R A, Hirsch V M, Purcell R H, Johnson P R. Nucleotide sequence analysis of feline immunodeficiency virus: genome organization and relationship to other lentiviruses. Proc Natl Acad Sci USA. 1989;86:8088–8092. doi: 10.1073/pnas.86.20.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olmsted R A, Langley R, Roelke M E, Goeken R M, Adger-Johnson D, Goff J P, Albert J P, Packer C, Laurenson M K, Caro T M, et al. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J Virol. 1992;66:6008–6018. doi: 10.1128/jvi.66.10.6008-6018.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Page K A, Landau N R, Littman D R. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990;64:5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen N C. The feline immunodeficiency virus. In: Levy J A, editor. The Retroviridae. New York, N.Y: Plenum Press; 1993. pp. 181–228. [Google Scholar]
- 44.Phillips T R, Talbott R L, Lamont C, Muir S, Lovelace K, Elder J H. Comparison of two host cell range variants of feline immunodeficiency virus. J Virol. 1990;64:4605–4613. doi: 10.1128/jvi.64.10.4605-4613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips T R, Lamont C, Konings A M, Shacklett B L, Hamson C A, Luciw P A, Elder J H. Identification of the Rev transactivation and Rev-responsive elements of feline immunodeficiency virus. J Virol. 1992;66:5464–5471. doi: 10.1128/jvi.66.9.5464-5471.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poeschla E M, Wong-Staal F, Looney D J. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–356. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 47.Poznansky M, Lever A, Bergeron L, Haseltine W, Sodroski J. Gene transfer into human lymphocytes by a defective human immunodeficiency virus type 1 vector. J Virol. 1991;65:532–536. doi: 10.1128/jvi.65.1.532-536.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price J, Turner D, Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci USA. 1987;84:156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiser J, Harmison G, Kluepfel-Stahl S, Brady R O, Karlsson S, Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizwi T A, Schmidt R D, Lew K A. Mason-Pfizer monkey virus (MPMV) constitutive transport element (CTE) functions in a position-dependent manner. Virology. 1997;236:118–129. doi: 10.1006/viro.1997.8728. [DOI] [PubMed] [Google Scholar]
- 51.Robinson D, Elliot J F, Chang L-J. Retroviral vector with a CMV-IE/HIV-TAR hybrid LTR gives high basal expression levels and is up-regulated by HIV-1 Tat. Gene Ther. 1995;2:269–278. [PubMed] [Google Scholar]
- 52.Roe T, Reynolds T C, Yu G, Brown P O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanes J R, Rubenstein J L, Nicolas J F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shacklett B L, Luciw P A. Analysis of the VIF gene of feline immunodeficiency virus. Virology. 1994;204:860–867. doi: 10.1006/viro.1994.1609. [DOI] [PubMed] [Google Scholar]
- 55.Simon J H, Gaddis N C, Fouchier R A, Malim M H. Evidence for a newly discovered cellular anti-HIV-1 phenotype. Nat Med. 1998;4:1397–1400. doi: 10.1038/3987. [DOI] [PubMed] [Google Scholar]
- 56.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonigo P, Barker C, Hunter E, Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986;45:375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- 58.Sparger E E, Shacklett B L, Renshaw-Gegg L, Barry P A, Pedersen N C, Elder J H, Luciw P A. Regulation of gene expression directed by the long terminal repeat of the feline immunodeficiency virus. Virology. 1992;187:165–177. doi: 10.1016/0042-6822(92)90305-9. [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi T, Cosset F L, Lachmann P J, Okada H, Weiss R A, Collins M K. Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. J Virol. 1994;68:8001–8007. doi: 10.1128/jvi.68.12.8001-8007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talbott R L, Sparger E E, Lovelace K M, Fitch W M, Pedersen N C, Luciw P A, Elder J H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tochikura T S, Tanabe-Tochikura A, Hayes K A, Lazo A, Bailer R T, Blakeslee J R, Lafrado L J, Roy-Burman P, Pandey R, Olsen R G, Mathes L E. Fusion activity dissociated from replication ability in feline immunodeficiency virus (FIV) in human cells. J Acquired Immune Defic Syndr. 1993;6:1301–1310. [PubMed] [Google Scholar]
- 62.Tomonaga K, Takeshi M. Molecular biology of the feline immunodeficiency virus auxiliary genes. J Gen Virol. 1996;77:1611–1621. doi: 10.1099/0022-1317-77-8-1611. [DOI] [PubMed] [Google Scholar]
- 63.Tomonaga K, Miyazawa T, Kawaguchi Y, Kohmoto M, Inoshima Y, Mikami T. Comparison of the Rev transactivation of feline immunodeficiency virus in feline and non-feline cell lines. J Vet Med Sci. 1994;56:199–201. doi: 10.1292/jvms.56.199. [DOI] [PubMed] [Google Scholar]
- 64.Tomonaga K, Miyazawa T, Sakuragi J-I, Mori T, Adachi A, Mikami T. The feline immunodeficiency virus ORF-A gene facilitates efficient viral replication in established T-cell lines and peripheral blood lymphocytes. J Virol. 1993;67:5889–5895. doi: 10.1128/jvi.67.10.5889-5895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomonaga K, Norimine J, Shin Y S, Fukasawa M, Miyazawa T, Adachi A, Toyosaki T, Kawaguchi Y, Kai C, Mikami T. Identification of a feline immunodeficiency virus gene which is essential for cell-free virus infectivity. J Virol. 1992;66:6181–6185. doi: 10.1128/jvi.66.10.6181-6185.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomonaga K, Shin Y-S, Fukasawa M, Miyazawa T, Adachi A, Mikami T. Feline immunodeficiency virus gene expression: analysis of the RNA splicing pattern and the monocistronic rev mRNA. J Gen Virol. 1993;74:2409–2417. doi: 10.1099/0022-1317-74-11-2409. [DOI] [PubMed] [Google Scholar]
- 67.von Schwedler U, Kornbluth R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.von Schwedler U, Song J, Aiken C, Trono D. vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waters A K, De Parseval A P, Lerner D L, Neil N C, Thompson F J, Elder J H. Influence of ORF2 on host cell tropism of feline immunodeficiency virus. Virology. 1996;215:10–16. doi: 10.1006/viro.1996.0002. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto J K, Hansen H, Ho E W, Morishita T Y, Okuda T, Sawa T R, Nakamura R M, Pedersen N C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989;194:213–220. [PubMed] [Google Scholar]
- 71.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]