Abstract
Accurately and promptly assessing pain in experimental animals is extremely important to avoid unnecessary suffering of the animals and to enhance the reproducibility of experiments. This is a key concern for veterinarians, animal caretakers, and researchers from the perspectives of veterinary care and animal welfare. Various methods including ethology, immunohistochemistry, electrophysiology, and molecular biology are used for pain assessment. However, the grimace scale, which was developed by taking cues from interpreting pain through facial expressions of non-verbal infants, has become recognized as a very simple and practical method for objectively evaluating pain levels by scoring changes in an animal’s expressions. This method, which was first implemented with mice approximately 10 years ago, is now being applied to various experimental animals and is widely used in research settings. This review focuses on the usability of the grimace scale from the “cage-side” perspective, aiming to make it a more user-friendly tool for those involved in animal experiments. Differences in facial expressions in response to pain in various animals, examples of applying the grimace scale, current automated analytical methods, and future prospects are discussed.
Keywords: animal welfare, facial expressions, grimace scale, laboratory animal, pain assessment
Introduction
Accurately and promptly assessing the pain of laboratory animals is crucial to avoid unnecessary animal suffering and to enhance the reproducibility of experiments. This is of utmost importance from the perspectives of veterinary care and animal welfare, which are daily priorities for veterinarians, caretakers, and researchers [1,2,3]. Laboratory animal professionals (LAPs) involved in animal experimentation are often perceived to possess extensive knowledge about animal behavior, emotions, and cognition. However, such perceptions can be misleading. In reality, LAPs do not have the ability to converse directly with animals like Dr. Dolittle in Hugh Lofting’s creation or exist in a scenario in which animals communicate directly, as seen in Disney’s movie Zootopia. As a result, accurately understanding the thoughts and feelings of animals can be challenging. Nevertheless, through meticulous daily observations, LAPs can develop the ability to discern meaning in animal behavior and speculate about the underlying emotions and thoughts. By paying attention to subtle changes in animal behavior, LAPs can quickly detect abnormalities in their health. This ability plays a crucial role in managing the wellbeing of laboratory animals, forming the foundation for the reliability and ethical posture of animal experiments. In this way, LAPs can contribute to scientific research by providing deep insights into animal behavior and psychology.
On the other hand, experiments involving laboratory animals to study conditions such as infections and cancer require the establishment of endpoints for scientific purposes. There is a simultaneous general demand to set humane endpoints based on animal welfare. Humane endpoints refer to the decision point where the suffering of animals is deemed to outweigh the benefits of the experiment, and, as such, the experiment is terminated [4, 5]. This concept is based on harm-benefit analysis and emphasizes the ethical aspects of animal experiments [5]. However, accurately quantifying animal pain can be challenging. Researchers cannot directly assess the degree of pain in animals, and pain assessment relies on their behavior and physiological indicators. This situation poses risks of either subjecting laboratory animals to excessive pain or prematurely terminating experiments without sufficient confirmation of the efficacy of the drug being evaluated.
Currently, the principles of the 3Rs (replacement, reduction, and refinement) are widely recognized and practiced in animal experimentation. These principles aim to minimize the use of animals while maximizing animal welfare. Recently, the concept of “responsibility” has also been considered as the fourth R [6]. The concept of “responsibility” not only enhances the direct welfare of animals but also affects the health and mental wellbeing of veterinarians and researchers involved in experiments. While animal experiments may potentially cause pain to animals, particularly meaningless experiments can result in unforeseen excessive animal pain and inadequate results and induce stress and compassion fatigue in practitioners [7,8,9].
Accurately and promptly assessing the degree and duration of pain felt by experimental animals after procedures is of paramount importance in veterinary care and animal welfare practices. Pain in animals has traditionally been an area of ambiguous recognition because animals lack language, making pain assessment particularly challenging. To address this issue, methods for evaluating facial expressions in infant communication have been referenced [10]. In 2010, Langford et al. developed a new method called the “Mouse grimace scale (MGS)” for assessing pain in mice. This scale quantitatively evaluates the degree of pain based on the facial expressions of mice. The development of the MGS has deepened our understanding of the pain experienced by laboratory animals [11]. The MGS scores elements such as eye narrowing (orbital tightening), nose position, cheek bulge, ear position, and whisker changes as action units (AUs) to objectively assess pain. Individuals trained to use this scale can accurately judge the degree of pain with 80% accuracy in photographs and 97% accuracy in high-quality videos. Of the five elements of the MGS, eye narrowing, nose position, and cheek bulging are common to humans, supporting the notion that animal expressions are rooted in evolutionary processes, consistent with Charles Darwin’s predictions. Initially developed for mice, this pain scale assessment method has now been applied to a variety of animal species including rats, rabbits, horses, sheep, pigs, ferrets, cats, and others [12,13,14,15,16,17,18]. On the other hand, in the case of dogs, pain has traditionally been assessed based on behavior and overall physical condition, so the grimace scale is not used. The scale uses a three-level scoring system for AUs, with 0 representing no signs of pain, 1 indicating the presence of some degree of pain, and 2 denoting clear evidence of pain.
In 2020, the International Association for the Study of Pain redefined pain as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage, or described in terms of such damage” [19]. According to this definition, pain is closely related to the presence of physical damage as well as to an individual’s sensory and emotional experience. Pain is classified into acute pain and chronic pain. Acute pain has a clear cause that has occurred within three months and typically improves during the wound healing process but can potentially recur. In contrast, chronic pain may not have identifiable causes based on imaging findings and can persist for more than three months or recur, resulting in an unpleasant sensory experience [20]. There are various methods for evaluating pain including behavioral studies as well as immunohistochemistry, electrophysiology, and molecular biology assessments. Among these, pain assessment using the grimace scale serves as a supplementary means but holds significant importance in complementing other evaluation methods. In this paper, we focus on the utility of the MGS at the “cage-side” from the perspective of animal experimenters and examine its usefulness.
Species Differences in Animals
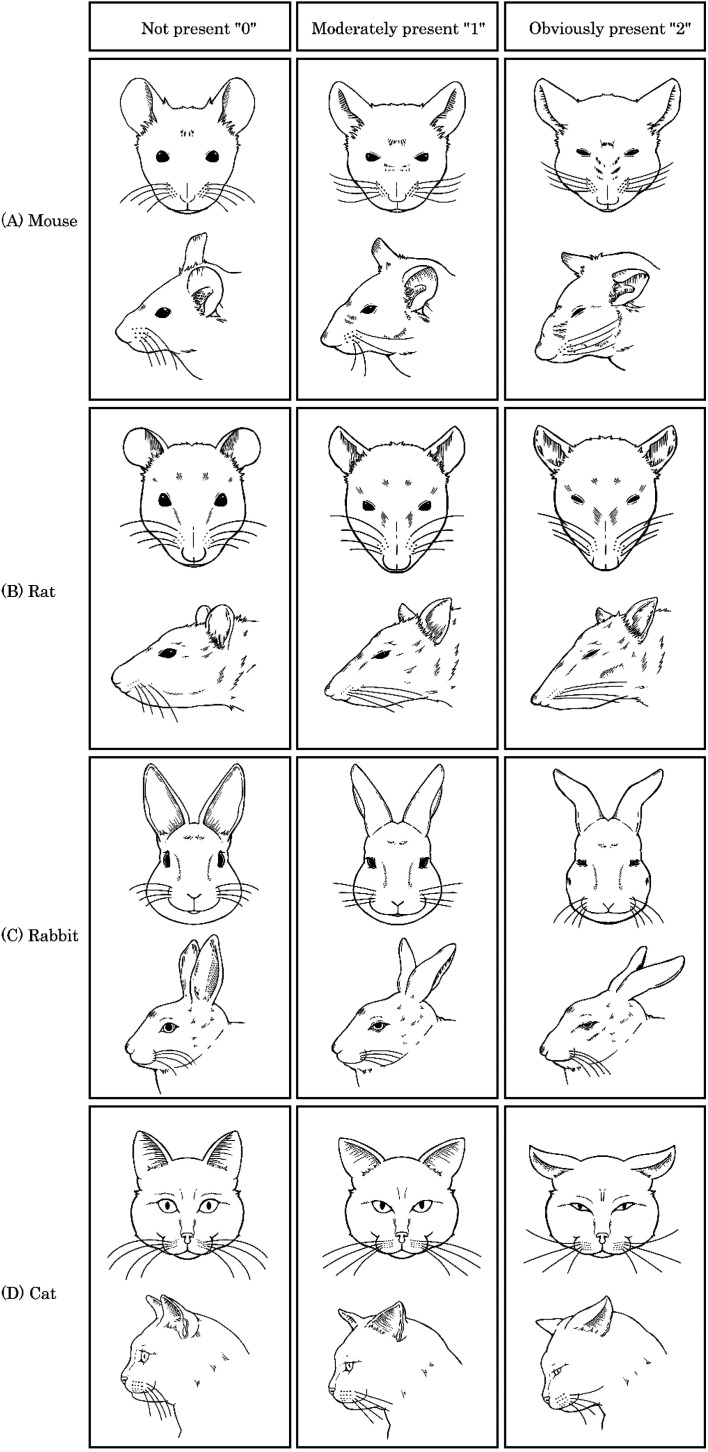
While it may simply be that humans have not yet fully recognized the nuances of animal expressions, the assessment of pain using the grimace scale cannot be universally applied across species; instead, different approaches are proposed for each species. In this paper, we will focus on mice, rats, rabbits, pigs, dogs, and cats, which are commonly used as experimental animals, and introduce their differences. The original studies for each animal are summarized in Table 1, and the differences in facial expressions due to pain in mice, rats, rabbits, and cats are illustrated in Fig. 1. The differences in pain-induced facial expressions in various animals are categorized into three levels based on the intensity of pain: none (0), moderate (1), and obvious (2). A common behavior among animals in response to pain is squinting of the eyes. In mice, the nose and cheeks bulge, the ears do not face forward and spread apart, and the whiskers either point backward along the cheeks or stand up and forward. In rats, the nose and cheeks flatten, the ears become pointed and spread apart, and the whiskers stiffen at an angle along the face. In rabbits, the cheeks flatten, and the nostrils change from a “U” to a “V” shape. The ears fold inward into a cylindrical shape, and the whiskers stand up and away from the cheeks, pointing downwards. In cats, the muzzle area becomes taut and oval-shaped laterally, the ears become pointed, and the whiskers straighten and face forward. The position of the head tilts below or in front of the shoulder line. It should be noted that the facial muscles of mammals are evolutionarily conserved, and there are many common facial movements among various animal species, including humans [21]. Therefore, it is conceivable that some AUs can be universally applied. However, it is also important to consider that facial expressions serve species-specific functions in social communication and intention transmission, which may differ across species [21, 22] Bearing this in mind, it is necessary to understand the species-specific characteristics in pain assessment.
Table 1. Original research on the grimace scale in various species.
| Species | Verification method | Action unit | Decision | Reference No. |
|---|---|---|---|---|
| (Type of pain elicited) | ||||
| Mouse | Intraperitoneal administration of 0.9 % acetic acid | Orbital tightning, Nose bulge, Cheek bulge, Ear position and Whiker changes | Video image, Real-time | 11 |
| Rat | Intraplantar administration of Complete Freund’s adjuvant, caolin and carrageenin, Laparotomy (abdominal surgery) | Orbital tightning, Nose/Cheek flattening, Ear position and Whiker changes | Video image, Real-time | 12 |
| Rabbit | Ear tatooing | Orbital tightning, Nose bulge, Cheek bulge, Ear position and Whiker changes | Video image, Real-time | 13 |
| Piglet | Castration | Orbital tightning, Nose/Cheek bulge and Ear position | Video image | 14 |
| Cat | Spontaneous acute pain, Post-operative pain | Ear position, Orbital tightning, Muzzle tension, Whisker change and Head position | Video image, Real-time | 15 |
| Horse | Castration | Ear position, Orbital tightning, Tension above the eye area, Prominent strained chewing muscles, Mouth strained and pronounced chin and Strained nostrils and flattening of the profile | Real-time | 16 |
| Sheep | Footrot and mastitis | Orbital tightning, Cheek (masseter muscle) tightening, Ear position, Lip and Jaw profile and Nostril and Philtrum shape | Video image | 17 |
| Ferret | Telemetry device implantation | Orbital tightning, Nose bulge, Cheek bulge, Ear position and Whiker changes | Video image | 18 |
Fig. 1.
Differences in facial expressions due to pain in various animals. Differences in facial expressions due to pain in various animals were categorized into three levels of pain intensity: none (0), moderate (1), and clearly present (2). Commonly observed behavior in animals when experiencing pain includes squinting of the eyes. (A) In mice, the nose and cheeks bulge, the ears do not face forward, and there is an increased gap between them. The whiskers either curve backward along the cheeks or stand up forward. (B) In rats, the nose and cheeks become flatter, the ears take on a pointed shape, and the gap widens. The whiskers become stiff and angle along the face. (C) In rabbits, the cheeks become flatter, the nostrils change from a “U” to a “V” shape, the ears fold inwards to a cylindrical shape, and the whiskers stand away from the cheeks and point downwards. (D) In cats, the snout widens into a horizontal elliptical shape, the ears become pointed, the whiskers remain straight and forward, and the head tilts below or in front of the shoulder line.
Mice
In addition to the fact that the development of the animal grimace scale started with mice, research on the MGS is more advanced than that involving other animals, as mice are extensively used in animal experiments. The methods used to induce pain include vasectomy, ear clipping, myocardial infarction and thoracotomy, laparotomy, colitis, dental pulp injury, nerve damage, and tendon injury [23,24,25,26,27,28,29]. Analgesic effects have been tested with opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), and local anesthetics. The final score, calculated as the average of five AUs, ranges from 0–2, with the maximum being 2. Few studies have recommended analgesic intervention levels, but a score of approximately 0.5 has been reported [26]. In this case, animals with grimace scale scores of 0.5 or higher are experiencing pain, and within the limits that do not interfere with the experiment, it implies the necessity of implementing analgesic measures from an animal welfare perspective. Different strains of mice, such as CD-1, BALB/c, and C57BL/6 mice, are used in experiments, and they have been reported to show varying sensitivities to pain in behavioral assays due to differences in nociceptor expression [30,31,32]. Therefore, it might be necessary to consider that the baseline MGS scores can differ among strains. Supporting this, male C57BL/6 mice have been found to have significantly lower MGS scores baseline than C3H or CD-1 mice [33]. In addition, from a different perspective, a systematic review has investigated the relationship between the MGS scores related to pain and nest-building behaviors [34]. These indicators may complement each other and provide a more comprehensive assessment of animal welfare, but their correlation and interaction in specific situations remain unclear. Moreover, comparisons with existing pain scales are not straightforward because the measurements were not obtained simultaneously or over a short period [35]; thus, further research is needed to determine their superiority and accuracy. There are also efforts to further simplify the MGS in mice. Ernst et al. developed a chronic liver fibrosis model by abdominal injection of tetrachloromethane (CCl4) into mice over four weeks and evaluated the relative importance of the AUs of the MGS [36]. This study revealed that the tightening of the eyes has the most significant impact on the MGS score. Based on these results, they suggest using eye tension as the primary evaluation parameter to simplify the MGS. This proposal could be an effective method when the implementation of MGS is challenging or when a rapid assessment is necessary.
Rats
In addition to their frequent use in animal experiments, rats are often preferred over mice due to their larger size and more easily interpretable facial expressions. Consequently, there are numerous reports on the Rat grimace scale (RGS). The methods for inducing pain include acute and chronic colitis models, intraplantar carrageenan injection, fibromyalgia models, neuropathic pain models, plantar incision, spinal cord injury models, laparotomy, and telemetry implantation surgery [37,38,39,40,41,42,43,44]. Analgesic efficacy in these models is tested using opioids, NSAIDs, local anesthetics, anticonvulsants, and antidepressants. In mice, the nose and cheeks normally appear smooth, but distinct bulging is observed in both when in pain. In contrast, rats typically exhibit noticeable bulging of the nose and cheeks under normal conditions, which flattens and elongates along the bridge of the nose and the vibrissal pads during pain [12]. No strain differences have been confirmed with the RGS [43]. Many papers calculate the final score as the average of four AUs in a three-tier system (0-1-2), with the maximum score being 2. The analgesic intervention score is considered to be 0.67 out of 2 [44]. In contrast to rats, the analgesic intervention score for mice is set at 0.5; this discrepancy is attributed to variations in facial expressions based on animal species and developmental stages. Facial expressions are developmentally plastic features, and their neural basis originates from neural circuits in the brainstem and midbrain. Therefore, the function of facial expressions not only conveys the intensity and nature of pain but also affects social interactions and the efficacy of analgesics, leading to different intervention scores across species [45]. Moreover, rats are commonly used in safety testing [46], and incorporating the RGS score as an evaluation criterion can facilitate a more accurate assessment of animal discomfort, potentially accelerating the development of pharmaceuticals.
Rabbits
In rabbits, the evaluation of ear tattoos for individual identification and spontaneous pain following craniotomy surgery has been assessed [13, 47]. The analgesics used include local anesthetics (EMLA cream; a eutectic mixture of lidocaine and propitocaine), meloxicam, and buprenorphine. The AUs employed are similar to those used in mice, comprising five items. The final score is either the average or the sum of these values, and the maximum is 2 (average) or 10 (sum). The intervention score for pain relief is reported to be 4/10 [47].
Pigs
Although reports regarding pigs are scarce, pain assessment during piglet castration is conducted based on facial expressions. The analgesics used include local anesthetics, opioids, and NSAIDs. The AUs consist of orbital tightening, nose and cheek bulge, and ear position. Orbital tightening is scored on a two-point scale (0-1), while the others are on a three-point scale (0-1-2). The highest score is 5, based on the sum of these stages [14, 48].
Dogs
To assess pain in dogs, the Glasgow Composite Measure Pain Scale (CMPS) was developed [49], which evaluates pain through a comprehensive observation of behavior and overall physical condition rather than merely using facial expressions, as in the grimace scale. A modified version, the Short-Form CMPS (CMPS-SF), is currently widely used [50]. The CMPS-SF consists of six behavioral categories, each with multiple options: vocalization, attention to the wound, mobility, response to palpation, demeanor, and posture/activity. The options within each category are numerically ranked according to the severity of the associated pain. The most appropriate option matching the dog’s behavior and condition is selected and scored on a five-point scale, ranging from 0 to 4. The total score is calculated by summing the individual rank scores. The maximum score is 24 points (or 20 points if mobility cannot be assessed). The total score serves as a useful indicator for the need for analgesics, with a recommended analgesic intervention level of 6/24 (or 5/20). There have been no reports of pain assessment in dogs using the grimace scale, and evaluating the correlation between the grimace scale and CMPS-SF remains a task for the future.
Cats
For cats, which are also companion animals, the CMPS-feline (CMPS-F) was developed in 2007 by Reid et al.[51], and its modified version, the rCMPS-F, was reported by Calvo et al. in 2014 [52]. In addition, the Feline grimace scale (FGS), which focuses on cat facial expressions, has been developed [15]. Although changes in behavior due to pain in cats are subtle and unique, the FGS and rCMPS-F have a very strong correlation. Moreover, the FGS has demonstrated high interobserver reliability, temporal consistency, and internal consistency, making grimace scale-based pain assessment particularly useful in cats [15]. Its clinical application has been demonstrated in a study of 65 cats undergoing ovariohysterectomy; responsiveness to analgesics was observed both in a real-time assessment and a retrospective evaluation using images [53]. The final score is based on a three-point scale (0-1-2) across five AUs, with the highest possible score being 10. The recommended level for analgesic intervention is 4/10.
Other animals
The grimace scale has been used for various animals that are not commonly employed as experimental subjects, including horses, sheep, donkeys, and ferrets. Among these, horses, which are known for their expressive faces, have been included in numerous reports [17, 18, 54,55,56]. Significant differences in grimace scale scores have been observed between painful and non-painful states in horses, and a significant reduction in pain scores following analgesic intervention has been noted. However, for animals commonly used in experiments, such as hamsters, guinea pigs, and non-human primates (NHPs), pain assessment using the grimace scale has not yet been conducted; thus, further research is needed. Given that some animals, such as guinea pigs, show only subtle behavioral changes in response to pain [57] and that NHPs tend to conceal their pain in the presence of humans or due to ethical considerations, it is difficult to establish a negative control [58, 59]; therefore, universally applying the grimace scale to all animals presents a significant challenge.
Applications
The grimace scale, which began as an assessment of distress levels in mice in 2010, has now been increasingly applied to various animal species. Many animal researchers have recognized its utility and have started incorporating it into their work. The overwhelming simplicity of the grimace scale is one of its key strengths. Much of the research on the grimace scale involves scoring the distress of animals based on images or videos taken. With trained staff using these scores as a basis, it might be possible to quickly assess animal distress and make real-time judgments. This would enable interventions in veterinary care, such as assessing and alleviating animal distress from the cage side, even in simplified experiments lacking vital monitors. In particular, actions such as administering pain relief medication to animals exceeding a pain intervention score, recognizing pain (i.e., applying humane endpoints to animals with sustained pain beyond a certain threshold), and providing euthanasia when necessary, can be considered. By obtaining prior approval from animal experimentation committees and researchers, it is possible to minimize the time and extent of animal distress and potentially reduce stress for animal researchers. However, it is important to note that real-time assessment is not feasible for everyone and is not always straightforward. Miller et al. reported that real-time AU scores are significantly lower than those determined from captured images [33]. This discrepancy could be due to the timing of observations; for example, a photograph taken when a mouse is facing forward might coincidentally capture it blinking, potentially leading to a higher score for orbital tightening. In contrast, live scoring does not increase the score for orbital tightening if the mouse blinks during a 5-second observation period. This highlights how scoring methods can influence the grimace scale scores. In contrast, some studies have reported that real-time evaluations yield scores comparable to video image analysis. Leung et al. compared real-time RGS score evaluations using a point method (brief checks at certain intervals and scores based on those moments) and an interval method (adding a 15-second observation period to these checks) with traditional video image analysis. The real-time RGS scores showed high concordance with standard scores and could distinguish between analgesic and saline treatments. The interval method was more sensitive than the point method and remained effective even when the observation times were reduced to less than five min [38]. Beyond sensitivity issues in real-time evaluations, the gender of the assessor entering the laboratory may need to be considered. It has been reported that mice and rats exposed to male odors exhibit a significant reduction in facial expressions and behaviors indicative of pain due to stress-induced analgesia [60], leading to lower grimace scale scores. This effect was not observed with female assessors. In addition, women might demonstrate more empathy toward pain, potentially leading to higher FGS scores than male assessors [61].
Traditionally, the grimace scale was considered applicable only for assessing acute pain, such as post-surgical pain, and not suited for evaluating chronic pain [34]. However, significant reductions in the grimace scale scores of rats and mice with neuropathic pain models following fentanyl administration suggest that the grimace scale might also be meaningful for assessing chronic pain [62]. While the advantages and disadvantages of the grimace scale itself have been discussed, its utility as a tool for assessing animal welfare has also been recognized. Miller et al. investigated whether there were differences in MGS scores when CBA and DBA/2 mice were transferred from cage to cage either by tail handling or using a tube [63]. However, this study did not find differences in the MGS scores due to the handling method. Swan et al. demonstrated that training CD-1 mice to be transported using cups instead of traditional handling for 3–5 weeks resulted in lower MGS scores during subcutaneous injections and tail vein blood collection [64]. Notably, the ear score within the MGS was particularly sensitive, suggesting the potential of the scale to assess stress as well as pain.
The grimace scale has also been used to evaluate euthanasia methods in rats. Domínguez-Oliva et al. employed the RGS to assess pain associated with six euthanasia methods (injection, inhalation, and physical methods). They found that decapitation and intraperitoneal injection of ketamine/xylazine scored the highest during euthanasia, while inhalation of CO2 and isoflurane scored high post-euthanasia [65]. While the RGS is based on specific facial muscle units and should be used in combination with other pain indicators for a complete picture, it might provide objective criteria for choosing humane euthanasia methods in other animal species. Thus, the grimace scale can potentially be used to assess animal pain and as a tool for real-time, cage-side determination of animal welfare. However, various conditions, such as the combination of the evaluator and animal, need to be considered. In large-scale experiments requiring multiple scorers, refined scoring sheets are necessary. Bugnon et al. discussed scoring sheet designs [66] and suggested that appropriately designed sheets could help avoid unnecessary pain in animals.
Furthermore, in addition to the specific applications of the grimace scale in mice and rats mentioned earlier (strain, gender, age, method of pain induction, and effectiveness of the grimace scale), we have also included other examples in Tables 2and 3 [67,68,69,70,71,72,73,74,75,76,77,78,79,80].
Table 2. Experimental conditions on mouse grimace scale.
| Species | Strain | Sex | Age (weeks) | Verification method | Results | Reference No. |
|---|---|---|---|---|---|---|
| (Type of pain elicited) | (Positive or Negative) | |||||
| Mouse | CD-1 (ICR) | Both | 6–18 | Intraperitoneal administration of 0.9 % acetic acid | P | 11 |
| Mouse | CD-1 (ICR) | Male | - (B.W. 30–40 g) | Vasectomy | P | 23 |
| Mouse | C57BL/6 | Male | 8 | Ear notching | P | 24 |
| Mouse | C57BL/6J | Female | 12–16 | Myocardial infarction and thoracotomy | P | 25 |
| Mouse | CD-1 (ICR) | Both | 6–8 | Sham ventral ovariectomy (laparotomy) | P | 26 |
| Mouse | C57BL/6J | Female | 8 | Colitis | N | 27 |
| Mouse | CD-1 and C57BL/6J | Both | 17–21 | Injury of the tooth pulp | P | 28 |
| Mouse | C57BL/6 | Male | - | Tendon injury | P | 29 |
| Mouse | C57BL/6N | Male | 8 | CCl4 administration | P | 36 |
| Mouse | CD-1 and C57BL/6J | Both and Female | 6–12 and 12–14 | Olfactory exposure to males, including men | P | 60 |
| Mouse | C57BL/6 | Male | 10–12 | Trigeminal neuropathic pain | P | 62 |
| Mouse | CBA and DBA/2 | Male | - (B.W. 25.6–28.7 g and 23.3–26.3 g) | Handling method (tail versus tube) | P | 63 |
| Mouse | CD-1 and C57BL/6N | Both | 6–8 | Craniotomy | P | 67 |
| Mouse | CD-1 (ICR) | Male | 8–9 | Carotid artery catheterization | P | 68 |
| Mouse | C57BL/6N | Both | 7–9 | Roux-en-Y gastric bypass (Bariatric surgery) | N | 69 |
| Mouse | C57BL/6J | Male | 8–12 | Cecal ligation and Puncture sepsis | N | 70 |
| Mouse | C57BL/6N | Male | 8–9 | Spinal cord injury | N | 71 |
Table 3. Experimental conditions on rat grimace scale.
| Species | Strain | Sex | Age (weeks) | Verification method | Results | Reference No. |
|---|---|---|---|---|---|---|
| (Type of pain elicited) | (Positive or Negative) | |||||
| Rat | Wistar | Both | 6–8 | Intraplantar complete Freund’s adjuvant, intraarticular kaolin-carrageenan, and laparotomy | P | 12 |
| Rat | SD | Both | 6 | Dextran sulfate sodium colitis | P | 37 |
| Rat | SD | Both | - (B.W. 224–435 g) | Intraplantar carrageenan | P | 38 |
| Rat | SD | Male | - (B.W. 200–250 g) | Fibromyalgia | P | 39 |
| Rat | SD | Male | - (B.W. 275–349 g) | Neuropathic pain | P | 40 |
| Rat | Wistar | Male | 8 | Intraplantar carrageenan, intraplantar complete Freund’s adjuvant, plantar incision | P | 41 |
| Rat | SD | Male | - (B.W. 275–349 g) | Spinal cord injury | P | 42 |
| Rat | Wistar and SD | Female | 6 | Laparotomy | P | 43 |
| Rat | SD | Female | - (B.W. 284–420 g) | Implantation of a telemetric radio-transmitter device | P | 44 |
| Rat | SD | Female | - (B.W. 225–250 g) | Olfactory exposure to males, including men | P | 60 |
| Rat | SD | Male | 10–13 | Trigeminal neuropathic pain | P | 62 |
| Rat | Wistar | Male | 96–100 | Laparotomy | P | 72 |
| Rat | SD | Male | 8 | Hind-paw surgery | P | 73 |
| Rat | Wistar | Female | 9–12 | Laminectomy on spinal cord contusion | P | 74 |
| Rat | Wistar | Male | - (B.W. 340–492 g) | Sepsis | P | 75 |
| Rat | Wistar | Male | - (B.W. 275–325 g) | Liver biopsy by laparotomy and laparoscopy | P | 76 |
| Rat | SD | Male | - (B.W. 300–380 g) | Intracerebral hemorrhage | P | 77 |
| Rat | Wistar | Both | - (B.W. 250–300 g) | Hot-plate test and laparotomy | N | 78 |
| Rat | Wistar | Male | 5–6 | Endotoxin-induced exacerbated post-incisional pain | P | 79 |
| Rat | SD | - | - (B.W. 225–250 g) | Sciatic nerve resection | P | 80 |
Automated Analytical Methods
While the scoring of the grimace scale by trained staff is straightforward and quick, it is not easily performed by an inexperienced observer, as it requires a deep understanding of animal expressions and experienced personnel. To address this issue, recent rapid developments have been made in the automated analysis of the grimace scale. From its inception, the reliance of the scale on the observer’s judgement was a known limitation, leading to the swift initiation of automated analytical methods following the public release of the MGS. Sotocinal et al. developed the RGS, a method for quantifying laboratory pain using rat facial expressions, and partially automated it to verify its reliability, accuracy, analgesic sensitivity, and utility. The automation technique used at the time was basic; it involved the detection and extraction of images from video footage where the rat’s eyes and ears are visible. However, it was a significant step forward from the labor-intensive evaluations of the past [12].
Tuttle et al. retrained Google’s InceptionV3, a deep convolutional neural network (CNN) primarily used for image recognition, to build a binary model that classifies mouse facial images as ‘pain’ or ‘no pain’. This model achieved a high accuracy of 94% in determining ‘pain’ or ‘no pain’ for a new set of images that were not included in the training set [81]. Andresen et al. used two types of CNNs, ResNet50 and InceptionV3, to construct a binary model that classifies mouse images as either ‘affected by anesthesia or surgery’ or ‘not affected’. They successfully classified images with over 80% accuracy. To confirm that the model was predicting based on mouse facial expressions, they used a method called Deep Taylor Decomposition to display the contribution of each pixel in a heatmap and found that the facial areas used in the MGS such as the ears, eyes, and nose as well as body parts such as the back and neck were important for classification [82].
Automated analysis is not limited to binary pain detection. In 2022, to avoid labor and time-intensive nature of scoring the grimace scale, Chiang et al. developed a model (DeepMGS) to automatically assess mouse migraine using deep learning [83]. They created a mouse migraine model through repetitive nitroglycerin injections, recorded facial expressions via video, and scored the five action units of the MGS (orbital tightening, nose bulge, cheek bulge, ear position, whisker change) with both a human and deep learning model (DeepMGS) from 0 to 2. DeepMGS scored each action unit with 70–90% accuracy. It also showed scores comparable to those of experienced staff and superior to those of inexperienced staff. Heatmaps generated by gradient-weighted class activation mapping demonstrated that DeepMGS accurately focused on MGS-related areas of the mouse facial images.
On the other hand, there are limitations to automated analytical methods. The facial expressions of animals captured in images or videos can vary significantly depending on the direction of capture, even when taken at the same time, which could potentially influence the scoring. Furthermore, even for a single species such as mice, there can be significant differences in appearance and fur color due to different genetic strains, and the expression of pain may also vary with age and gender. In fact, when measuring facial pain induced by the neuropeptides calcitonin gene-related peptide (CGRP) and amylin, which are associated with migraines, squinting of the eyes more strongly elicited this response in female mice, while in male mice, it had no effect [84]. In addition, attempting to extrapolate certain types of automated analytical models under different conditions, such as variations in administration methods or different drugs, has resulted in low accuracy when tested with actual test data [82]. Other CNNs in use include You Only Look Once (YOLOv3, YOLOv5), Contrastive Language-Image Pretraining (CLIP), Matlab 2016b, ShuffleNetV2, EfficentNetB0, and MobileNetV3, among others [85,86,87,88,89]. With these limitations in mind, it is currently challenging to achieve a perfect assessment of animal pain using automated technology. However, considering the current advancements in image recognition technology and artificial intelligence, these issues related to automation may be resolved in the near future. Moreover, looking ahead, factors such as the recruitment of researchers who have never worked with animals and the retirement of experienced staff capable of distinguishing differences in animal expressions will coincide, making human resources even more valuable. Therefore, the need for automation technology is expected to continue to increase. In this regard, there is no doubt that automated analytical methods will become a powerful tool supporting animal welfare. Furthermore, among the software applications discussed in this section, free software options are summarized in Table 4.
Table 4. Software used for automated analysis methods.
| Software Name | Species | Criteria / Evaluation items | URL | Reference No. |
|---|---|---|---|---|
| Inception V3 | Mouse | Presence or absence of pain | https://cloud.google.com/tpu/docs/inception-v3-advanced | 81 |
| ResNet50 | Mouse | Whether anaesthesia or surgery is affected | https://keras.io/api/applications/resnet/ | 82 |
| Deep Taylor Decomposition | Mouse | Heatmap display of facial expressions | https://github.com/myc159/Deep-Taylor-Decomposition | 82 |
| YOLOv3 | Mouse | Presence or absence of pain | https://github.com/ultralytics/yolov3 | 85 |
| YOLOv5 | Rat | Presence or absence of pain | https://github.com/ultralytics/yolov5 | 86 |
| CLIP | Rabbit | Presence or absence of pain | https://github.com/openai/CLIP | 87 |
| Matlab 2016b | Horse | Presence or absence of pain | https://www.mathworks.com/company/newsroom/mathworks-announces-release-2016b-of-the-matlab-and-simulink-pro.html | 88 |
| ShuffleNetV2 | Cat | Presence or absence of pain | https://pytorch.org/hub/pytorch_vision_shufflenet_v2/ | 89 |
| EfficientNetB0 | Cat | Presence or absence of pain | https://www.tensorflow.org/api_docs/python/tf/keras/applications/efficientnet/EfficientNetB0 | 89 |
| MobileNetV3 | Cat | Presence or absence of pain | https://arxiv.org/abs/1905.02244 | 89 |
Future Perspectives
In animal experimentation, it is generally agreed that except in specific cases such as creating disease models or verifying the effects of analgesics, animals’ pain should be minimized as much as possible. While more accurate pain assessment can be achieved through various monitoring tools or blood tests, these methods are not easily implementable in a noninvasive manner. It is not the intention of animal experimenters to inflict additional suffering on animals through surgical procedures or restraint for the purpose of pain alleviation. In this context, cage-side pain assessment using the grimace scale, which involves no invasiveness, is highly desirable for animal welfare. Animal experimenters have long been vaguely aware of this, but the quantification and visualization of pain levels inferred from animal expressions through the grimace scale are highly significant. The grimace scale has become synonymous with assessing animals’ pain levels. However, it can be challenging for those who do not regularly interact with animals, especially beginners, to interpret and apply these assessments based on textual information from papers. In response, the UK’s National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs) has created posters of the grimace scale for mice, rats, and rabbits to raise awareness and help experimenters familiarize themselves with the specific expressions of pain in different animal species (https://www.nc3rs.org.uk/3rs-resources/grimace-scales). These posters, which capture a snapshot of animals in pain, are extremely useful for beginners who may find it difficult to visualize animal suffering. These grimace scale-related posters have been translated into various languages including Japanese. In Japan, the Laboratory Animal Welfare and Communication (LAWCom) is involved in producing and distributing these posters and supporting universities and companies engaged in research, education, and testing for the realization of animal welfare (https://www.lab-awcom.org). In this regard, before implementing grimace scale assessments, experimenters may benefit from utilizing such materials and aligning perspectives among themselves using online learning tools.
A quick and cage-side pain assessment using the grimace scale is likely to advance significantly towards automation with the improvement of image recognition technology. Due to current processing capacity issues, automated technology is employed primarily retrospectively using recorded videos or images. However, future systems might assess the total behavior and expressions of animals, alerting responsible persons in real-time if an animal’s condition deteriorates. It is premature to assume that such systems will replace the jobs of animal caretakers. When alerts indicate deteriorating conditions, it is still the cage-side humans who will rush to the scene, re-evaluate the animal’s condition, and make decisions on analgesic administration or humane endpoints. By adeptly utilizing these systems, more sophisticated and reproducible animal experiments can be realized.
Conclusion
The grimace scale, which is based on animal expressions for pain assessment, is characterized by its rapidity and is now applicable to various animal species. Although there are differences in assessment items across species and some variations due to breed or sex, the grimace scale has achieved a certain level of validation for pain assessment. Future developments, supported by improved image recognition technologies, will likely facilitate real-time, cage-side pain assessment, making the grimace scale an increasingly valuable tool in refined animal experimentation.
Conflict of Interest
The authors declare no conflict interest.
References
- 1.Cheleuitte-Nieves C, Lipman NS. Improving replicability, reproducibility, and reliability In preclinical research: A shared responsibility. ILAR J. 2019; 60: 113–119. doi: 10.1093/ilar/ilaa009 [DOI] [PubMed] [Google Scholar]
- 2.Loss CM, Melleu FF, Domingues K, Lino-de-Oliveira C, Viola GG. Combining animal welfare with experimental rigor to improve reproducibility in behavioral neuroscience. Front Behav Neurosci. 2021; 15: 763428. doi: 10.3389/fnbeh.2021.763428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pritt SL, Hammer RE. The interplay of ethics, animal welfare, and IACUC oversight on the reproducibility of animal studies. Comp Med. 2017; 67: 101–105. [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace J. Humane endpoints and cancer research. ILAR J. 2000; 41: 87–93. doi: 10.1093/ilar.41.2.87 [DOI] [PubMed] [Google Scholar]
- 5.Olfert ED, Godson DL. Humane endpoints for infectious disease animal models. ILAR J. 2000; 41: 99–104. doi: 10.1093/ilar.41.2.99 [DOI] [PubMed] [Google Scholar]
- 6.Lee KH, Lee DW, Kang BC. The ‘R’ principles in laboratory animal experiments. Lab Anim Res. 2020; 36: 45. doi: 10.1186/s42826-020-00078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randall MS, Moody CM, Turner PV. Mental wellbeing in laboratory animal professionals: A cross-sectional study of compassion fatigue, contributing factors, and coping mechanisms. J Am Assoc Lab Anim Sci. 2021; 60: 54–63. doi: 10.30802/AALAS-JAALAS-20-000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaFollette MR, Riley MC, Cloutier S, Brady CM, O’Haire ME, Gaskill BN. Laboratory animal welfare meets human welfare: A cross-sectional study of professional quality of life, including compassion fatigue in laboratory animal personnel. Front Vet Sci. 2020; 7: 114. doi: 10.3389/fvets.2020.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Hooser JP, Pekow C, Nguyen HM, D’Urso DM, Kerner SE, Thompson-Iritani S. Caring for the animal caregiver—Ocupational health, human-animal bond and compassion fatigue. Front Vet Sci. 2021; 8: 731003. doi: 10.3389/fvets.2021.731003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams AC. Facial expression of pain: an evolutionary account. Behav Brain Sci. 2002; 25: 439–455, discussion 455–488. [DOI] [PubMed] [Google Scholar]
- 11.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010; 7: 447–449. doi: 10.1038/nmeth.1455 [DOI] [PubMed] [Google Scholar]
- 12.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011; 7: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating SCJ, Thomas AA, Flecknell PA, Leach MC. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PLoS One. 2012; 7: e44437. doi: 10.1371/journal.pone.0044437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Giminiani P, Brierley VLMH, Scollo A, Gottardo F, Malcolm EM, Edwards SA, et al. The assessment of facial expressions in piglets undergoing tail docking and castration: toward the development of the piglet grimace scale. Front Vet Sci. 2016; 3: 100. doi: 10.3389/fvets.2016.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evangelista MC, Watanabe R, Leung VSY, Monteiro BP, O’Toole E, Pang DSJ, et al. Facial expressions of pain in cats: the development and validation of a Feline Grimace Scale. Sci Rep. 2019; 9: 19128. doi: 10.1038/s41598-019-55693-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalla Costa E, Minero M, Lebelt D, Stucke D, Canali E, Leach MC. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS One. 2014; 9: e92281. doi: 10.1371/journal.pone.0092281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLennan K, Mahmoud M. Development of an automated pain facial expression detection system for sheep (Ovis Aries). Animals (Basel). 2019; 9: 196. doi: 10.3390/ani9040196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reijgwart ML, Schoemaker NJ, Pascuzzo R, Leach MC, Stodel M, de Nies L, et al. The composition and initial evaluation of a grimace scale in ferrets after surgical implantation of a telemetry probe. PLoS One. 2017; 12: e0187986. doi: 10.1371/journal.pone.0187986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020; 161: 1976–1982. doi: 10.1097/j.pain.0000000000001939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diogo R, Wood BA, Aziz MA, Burrows A. On the origin, homologies and evolution of primate facial muscles, with a particular focus on hominoids and a suggested unifying nomenclature for the facial muscles of the Mammalia. J Anat. 2009; 215: 300–319. doi: 10.1111/j.1469-7580.2009.01111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Descovich KA, Wathan J, Leach MC, Buchanan-Smith HM, Flecknell P, Farningham D, et al. Facial expression: An under-utilised tool for the assessment of welfare in mammals. Altern Anim Exp. 2017; 34: 409–429. [DOI] [PubMed] [Google Scholar]
- 22.Parr LA, Heintz M. Facial expression recognition in rhesus monkeys, Macaca mulatta. Anim Behav. 2009; 77: 1507–1513. doi: 10.1016/j.anbehav.2009.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS One. 2012; 7: e35656. doi: 10.1371/journal.pone.0035656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller AL, Leach MC. Using the mouse grimace scale to assess pain associated with routine ear notching and the effect of analgesia in laboratory mice. Lab Anim. 2015; 49: 117–120. doi: 10.1177/0023677214559084 [DOI] [PubMed] [Google Scholar]
- 25.Faller KME, McAndrew DJ, Schneider JE, Lygate CA. Refinement of analgesia following thoracotomy and experimental myocardial infarction using the Mouse Grimace Scale. Exp Physiol. 2015; 100: 164–172. doi: 10.1113/expphysiol.2014.083139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, et al. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci. 2012; 51: 42–49. [PMC free article] [PubMed] [Google Scholar]
- 27.Chartier LC, Hebart ML, Howarth GS, Whittaker AL, Mashtoub S. Affective state determination in a mouse model of colitis-associated colorectal cancer. PLoS One. 2020; 15: e0228413. doi: 10.1371/journal.pone.0228413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi HL, See LP, Foster W, Pitake S, Gibbs J, Schmidt B, et al. Evoked and spontaneous pain assessment during tooth pulp injury. Sci Rep. 2020; 10: 2759–2765. doi: 10.1038/s41598-020-59742-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moser HL, Abraham AC, Howell K, Laudier D, Zumstein MA, Galatz LM, et al. Cell lineage tracing and functional assessment of supraspinatus tendon healing in an acute repair murine model. J Orthop Res. 2021; 39: 1789–1799. doi: 10.1002/jor.24769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999; 80: 67–82. doi: 10.1016/S0304-3959(98)00197-3 [DOI] [PubMed] [Google Scholar]
- 31.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, et al. Heritability of nociception II. ‘Types’ of nociception revealed by genetic correlation analysis. Pain. 1999; 80: 83–93. doi: 10.1016/S0304-3959(98)00196-1 [DOI] [PubMed] [Google Scholar]
- 32.Minett MS, Eijkelkamp N, Wood JN. Significant determinants of mouse pain behaviour. PLoS One. 2014; 9: e104458. doi: 10.1371/journal.pone.0104458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller AL, Leach MC. The mouse grimace scale: A clinically useful tool? PLoS One. 2015; 10: e0136000. doi: 10.1371/journal.pone.0136000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aulehner K, Leenaars C, Buchecker V, Stirling H, Schönhoff K, King H, et al. Grimace scale, burrowing, and nest building for the assessment of post-surgical pain in mice and rats-A systematic review. Front Vet Sci. 2022; 9: 930005. doi: 10.3389/fvets.2022.930005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evangelista MC, Monteiro BP, Steagall PV. Measurement properties of grimace scales for pain assessment in nonhuman mammals: a systematic review. Pain. 2022; 163: e697–e714. doi: 10.1097/j.pain.0000000000002474 [DOI] [PubMed] [Google Scholar]
- 36.Ernst L, Bruch S, Kopaczka M, Merhof D, Bleich A, Tolba RH, et al. A model-specific simplification of the Mouse Grimace Scale based on the pain response of intraperitoneal CCl4 injections. Sci Rep. 2022; 12: 10910. doi: 10.1038/s41598-022-14852-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung VSY, Benoit-Biancamano MO, Pang DSJ. Performance of behavioral assays: the Rat Grimace Scale, burrowing activity and a composite behavior score to identify visceral pain in an acute and chronic colitis model. Pain Rep. 2019; 4: e718. doi: 10.1097/PR9.0000000000000712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung V, Zhang E, Pang DSJ. Real-time application of the Rat Grimace Scale as a welfare refinement in laboratory rats. Sci Rep. 2016; 6: 31667. doi: 10.1038/srep31667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagakura Y, Miwa M, Yoshida M, Miura R, Tanei S, Tsuji M, et al. Spontaneous pain-associated facial expression and efficacy of clinically used drugs in the reserpine-induced rat model of fibromyalgia. Eur J Pharmacol. 2019; 864: 172716. doi: 10.1016/j.ejphar.2019.172716 [DOI] [PubMed] [Google Scholar]
- 40.Philips BH, Weisshaar CL, Winkelstein BA. Use of the rat grimace scale to evaluate neuropathic pain in a model of cervical radiculopathy. Comp Med. 2017; 67: 34–42. [PMC free article] [PubMed] [Google Scholar]
- 41.De Rantere D, Schuster CJ, Reimer JN, Pang DSJ. The relationship between the Rat Grimace Scale and mechanical hypersensitivity testing in three experimental pain models. Eur J Pain. 2016; 20: 417–426. doi: 10.1002/ejp.742 [DOI] [PubMed] [Google Scholar]
- 42.Schneider LE, Henley KY, Turner OA, Pat B, Niedzielko TL, Floyd CL. Application of the rat grimace scale as a marker of supraspinal pain sensation after cervical spinal cord injury. J Neurotrauma. 2017; 34: 2982–2993. doi: 10.1089/neu.2016.4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klune CB, Larkin AE, Leung VSY, Pang D. Comparing the Rat Grimace Scale and a composite behaviour score in rats. PLoS One. 2019; 14: e0209467. doi: 10.1371/journal.pone.0209467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliver V, De Rantere D, Ritchie R, Chisholm J, Hecker KG, Pang DSJ. Psychometric assessment of the Rat Grimace Scale and development of an analgesic intervention score. PLoS One. 2014; 9: e97882. doi: 10.1371/journal.pone.0097882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chambers CT, Mogil JS. Ontogeny and phylogeny of facial expression of pain. Pain. 2015; 156: 798–799. doi: 10.1097/j.pain.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 46.Prior H, Haworth R, Labram B, Roberts R, Wolfreys A, Sewell F. Justification for species selection for pharmaceutical toxicity studies. Toxicol Res (Camb). 2020; 9: 758–770. doi: 10.1093/toxres/tfaa081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raillard M, Detotto C, Grepper S, Beslac O, Fujioka-Kobayashi M, Schaller B, et al. Anaesthetic and perioperative management of 14 male New Zealand white rabbits for calvarial bone surgery. Animals (Basel). 2019; 9: 896. doi: 10.3390/ani9110896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viscardi AV, Turner PV. Use of meloxicam or ketoprofen for piglet pain control following surgical castration. Front Vet Sci. 2018; 5: 299. doi: 10.3389/fvets.2018.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melzack R, Casey K. Sensory, motivational, and central control determinants of pain;In: Dan R. Kenshalo, editor. The Skin Senses. St, Springfield; Charles C; 1968. 140–154. [Google Scholar]
- 50.Reid J, Nolan A, Hughes L, Lascelles BD, Pawson P, Scott EM. Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Anim Welf. 2007; 16: 97–104. doi: 10.1017/S096272860003178X [DOI] [Google Scholar]
- 51.Reid J, Scott EM, Calvo G, Nolan AM. Definitive Glasgow acute pain scale for cats: validation and intervention level. Vet Rec. 2017; 180: 449. doi: 10.1136/vr.104208 [DOI] [PubMed] [Google Scholar]
- 52.Calvo G, Holden E, Reid J, Scott EM, Firth A, Bell A, et al. Development of a behaviour-based measurement tool with defined intervention level for assessing acute pain in cats. J Small Anim Pract. 2014; 55: 622–629. doi: 10.1111/jsap.12280 [DOI] [PubMed] [Google Scholar]
- 53.Evangelista MC, Benito J, Monteiro BP, Watanabe R, Doodnaught GM, Pang DSJ, et al. Clinical applicability of the Feline Grimace Scale: real-time versus image scoring and the influence of sedation and surgery. PeerJ. 2020; 8: e8967. doi: 10.7717/peerj.8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dai F, Leach M, MacRae AM, Minero M, Costa ED. Does thirty-minute standardised training improve the inter-observer reliability of the horse grimace scale (HGS)? A case study. Animals (Basel). 2020; 10: 781. doi: 10.3390/ani10050781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalla Costa E, Pascuzzo R, Leach MC, Dai F, Lebelt D, Vantini S, et al. Can grimace scales estimate the pain status in horses and mice? A statistical approach to identify a classifier. PLoS One. 2018; 13: e0200339. doi: 10.1371/journal.pone.0200339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalla Costa E, Stucke D, Dai F, Minero M, Leach MC, Lebelt D. Using the Horse grimace scale (HGS) to assess pain associated with acute laminitis in horses (Equus caballus). Animals (Basel). 2016; 6: 47. doi: 10.3390/ani6080047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellen Y, Flecknell P, Leach M. Evaluation of using behavioural changes to assess post-operative pain in the Guinea Pig (Cavia porcellus). PLoS One. 2016; 11: e0161941. doi: 10.1371/journal.pone.0161941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Descovich KA, Richmond SE, Leach MC, Buchanan-Smith HM, Flecknell P, Farningham DAH, et al. Opportunities for refinement in neuroscience: Indicators of wellness and post-operative pain in laboratory macaques. Altern Anim Exp. 2019; 36: 535–554. [DOI] [PubMed] [Google Scholar]
- 59.Miyabe-Nishiwaki T, Gris VN, Muta K, Nishimura R, Mills DS. Primate veterinarians’ knowledge and attitudes regarding pain in macaques. J Med Primatol. 2021; 50: 259–269. doi: 10.1111/jmp.12537 [DOI] [PubMed] [Google Scholar]
- 60.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014; 11: 629–632. doi: 10.1038/nmeth.2935 [DOI] [PubMed] [Google Scholar]
- 61.Evangelista MC, Steagall PV. Agreement and reliability of the Feline Grimace Scale among cat owners, veterinarians, veterinary students and nurses. Sci Rep. 2021; 11: 5262. doi: 10.1038/s41598-021-84696-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akintola T, Raver C, Studlack P, Uddin O, Masri R, Keller A. The grimace scale reliably assesses chronic pain in a rodent model of trigeminal neuropathic pain. Neurobiol Pain. 2017; 2: 13–17. doi: 10.1016/j.ynpai.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller AL, Leach MC. The effect of handling method on the mouse grimace scale in two strains of laboratory mice. Lab Anim. 2016; 50: 305–307. doi: 10.1177/0023677215622144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swan J, Boyer S, Westlund K, Bengtsson C, Nordahl G, Törnqvist E. Decreased levels of discomfort in repeatedly handled mice during experimental procedures, assessed by facial expressions. Front Behav Neurosci. 2023; 17: 1109886. doi: 10.3389/fnbeh.2023.1109886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Domínguez-Oliva A, Olmos-Hernández A, Hernández-Ávalos I, Lecona-Butrón H, Mora-Medina P, Mota-Rojas D. Rat grimace scale as a method to evaluate animal welfare, nociception, and quality of the euthanasia method of Wistar Rats. Animals (Basel). 2023; 13: 3161. doi: 10.3390/ani13203161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bugnon P, Heimann M, Thallmair M. What the literature tells us about score sheet design. Lab Anim. 2016; 50: 414–417. doi: 10.1177/0023677216671552 [DOI] [PubMed] [Google Scholar]
- 67.Cho C, Michailidis V, Lecker I, Collymore C, Hanwell D, Loka M, et al. Evaluating analgesic efficacy and administration route following craniotomy in mice using the grimace scale. Sci Rep. 2019; 9: 359. doi: 10.1038/s41598-018-36897-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallo MS, Karas AZ, Pritchett-Corning K, Garner Guy Mulder JP, Gaskill BN. Tell-tale TINT: Does the time to incorporate into nest test evaluate postsurgical pain or welfare in mice? J Am Assoc Lab Anim Sci. 2020; 59: 37–45. doi: 10.30802/AALAS-JAALAS-19-000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsi ZY, Stewart LA, Lloyd KCK, Grimsrud KN, Hypoglycemia after bariatric surgery in mice and optimal dosage and efficacy of glucose supplementation. Comp Med. 2020; 70: 111–118. doi: 10.30802/AALAS-CM-19-000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mai SHC, Sharma N, Kwong AC, Dwivedi DJ, Khan M, Grin PM, et al. Body temperature and mouse scoring systems as surrogate markers of death in cecal ligation and puncture sepsis. Intensive Care Med Exp. 2018; 6: 20–23. doi: 10.1186/s40635-018-0184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Redaelli V, Papa S, Marsella G, Grignaschi G, Bosi A, Ludwig N, et al. A refinement approach in a mouse model of rehabilitation research. Analgesia strategy, reduction approach and infrared thermography in spinal cord injury. PLoS One. 2019; 14: e0224337. doi: 10.1371/journal.pone.0224337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chi H, Kawano T, Tamura T, Iwata H, Takahashi Y, Eguchi S, et al. Postoperative pain impairs subsequent performance on a spatial memory task via effects on N-methyl-D-aspartate receptor in aged rats. Life Sci. 2013; 93: 986–993. doi: 10.1016/j.lfs.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 73.Clemensen J, Rasmussen LV, Abelson KSP. Transdermal fentanyl solution provides long-term analgesia in the hind-paw incisional model of postoperative pain in male rats. In Vivo. 2018; 32: 713–719. doi: 10.21873/invivo.11299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harikrishnan VS, Krishnan LK, Abelson KSP. A novel technique to develop thoracic spinal laminectomy and a methodology to assess the functionality and welfare of the contusion spinal cord injury (SCI) rat model. PLoS One. 2019; 14: e0219001. doi: 10.1371/journal.pone.0219001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jeger V, Arrigo M, Hildenbrand FF, Müller D, Jirkof P, Hauffe T, et al. Improving animal welfare using continuous nalbuphine infusion in a long-term rat model of sepsis. Intensive Care Med Exp. 2017; 5: 23. doi: 10.1186/s40635-017-0137-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Préfontaine L, Hélie P, Vachon P. Postoperative pain in Sprague Dawley rats after liver biopsy by laparotomy versus laparoscopy. Lab Anim (NY). 2015; 44: 174–178. doi: 10.1038/laban.731 [DOI] [PubMed] [Google Scholar]
- 77.Saine L, Hélie P, Vachon P. Effects of fentanyl on pain and motor behaviors following a collagenase-induced intracerebral hemorrhage in rats. J Pain Res. 2016; 9: 1039–1048. doi: 10.2147/JPR.S121415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waite ME, Tomkovich A, Quinn TL, Schumann AP, Dewberry LS, Totsch SK, et al. Efficacy of common analgesics for postsurgical pain in rats. J Am Assoc Lab Anim Sci. 2015; 54: 420–425. [PMC free article] [PubMed] [Google Scholar]
- 79.Yamanaka D, Kawano T, Nishigaki A, Aoyama B, Tateiwa H, Shigematsu-Locatelli M, et al. The preventive effects of dexmedetomidine on endotoxin-induced exacerbated post-incisional pain in rats. J Anesth. 2017; 31: 664–671. doi: 10.1007/s00540-017-2374-7 [DOI] [PubMed] [Google Scholar]
- 80.Yousef MAA, Dionigi P, Marconi S, Calligaro A, Cornaglia AI, Alfonsi E, et al. Successful reconstruction of nerve defects using distraction neurogenesis with a new experimental device. Basic Clin Neurosci. 2015; 6: 253–264. [PMC free article] [PubMed] [Google Scholar]
- 81.Tuttle AH, Molinaro MJ, Jethwa JF, Sotocinal SG, Prieto JC, Styner MA, et al. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol Pain. 2018; 14: 1744806918763658. doi: 10.1177/1744806918763658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andresen N, Wöllhaf M, Hohlbaum K, Lewejohann L, Hellwich O, Thöne-Reineke C, et al. Towards a fully automated surveillance of well-being status in laboratory mice using deep learning: Starting with facial expression analysis. PLoS One. 2020; 15: e0228059. doi: 10.1371/journal.pone.0228059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiang CY, Chen YP, Tzeng HR, Chang MH, Chiou LC, Pei YC. Deep learning-based grimace scoring is comparable to human scoring in a mouse migraine model. J Pers Med. 2022; 12: 851. doi: 10.3390/jpm12060851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rea BJ, Davison A, Ketcha MJ, Smith KJ, Fairbanks AM, Wattiez AS, et al. Automated detection of squint as a sensitive assay of sex-dependent calcitonin gene-related peptide and amylin-induced pain in mice. Pain. 2022; 163: 1511–1519. doi: 10.1097/j.pain.0000000000002537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vidal A, Jha S, Hassler S, Price T, Busso C. Face detection and grimace scale prediction of white furred mice. Mach Learn Appl. 2022; 8: 100312. [Google Scholar]
- 86.Arnold B, Ramakrishnan R, Wright A, Wilson K, VandeVord PJ. An automated rat grimace scale for the assessment of pain. Sci Rep. 2023; 13: 18859. doi: 10.1038/s41598-023-46123-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Feighelstein M, Ehrlich Y, Naftaly L, Alpin M, Nadir S, Shimshoni I, et al. Deep learning for video-based automated pain recognition in rabbits. Sci Rep. 2023; 13: 14679. doi: 10.1038/s41598-023-41774-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lencioni GC, de Sousa RV, de Souza Sardinha EJ, Corrêa RR, Zanella AJ. Pain assessment in horses using automatic facial expression recognition through deep learning-based modeling. PLoS One. 2021; 16: e0258672. doi: 10.1371/journal.pone.0258672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steagall PV, Monteiro BP, Marangoni S, Moussa M, Sautié M. Fully automated deep learning models with smartphone applicability for prediction of pain using the Feline Grimace Scale. Sci Rep. 2023; 13: 21584. doi: 10.1038/s41598-023-49031-2 [DOI] [PMC free article] [PubMed] [Google Scholar]