Abstract
Background
The mineralocorticoid receptor plays a significant role in the development of chronic kidney disease (CKD) and associated cardiovascular complications. Classic steroidal mineralocorticoid receptor antagonists are a therapeutic option, but their use in the clinic is limited due to the associated risk of hyperkalemia in patients with CKD. Finerenone is a nonsteroidal mineralocorticoid receptor antagonist that has been recently investigated in 2 large phase III clinical trials (FIDELIO‐DKD [Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease] and FIGARO‐DKD [Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease]), showing reductions in kidney and cardiovascular outcomes.
Methods and Results
We tested whether finerenone improves renal and cardiac function in a preclinical nondiabetic CKD model. Twelve weeks after 5/6 nephrectomy, the rats showed classic signs of CKD characterized by a reduced glomerular filtration rate and increased kidney weight, associated with left ventricular (LV) diastolic dysfunction and decreased LV perfusion. These changes were associated with increased cardiac fibrosis and reduced endothelial nitric oxide synthase activating phosphorylation (ser 1177). Treatment with finerenone prevented LV diastolic dysfunction and increased LV tissue perfusion associated with a reduction in cardiac fibrosis and increased endothelial nitric oxide synthase phosphorylation. Curative treatment with finerenone improves nondiabetic CKD‐related LV diastolic function associated with a reduction in cardiac fibrosis and increased cardiac phosphorylated endothelial nitric oxide synthase independently from changes in kidney function. Short‐term finerenone treatment decreased LV end‐diastolic pressure volume relationship and increased phosphorylated endothelial nitric oxide synthase and nitric oxide synthase activity.
Conclusions
We showed that the nonsteroidal mineralocorticoid receptor antagonist finerenone reduces renal hypertrophy and albuminuria, attenuates cardiac diastolic dysfunction and cardiac fibrosis, and improves cardiac perfusion in a preclinical nondiabetic CKD model.
Keywords: 5/6 nephrectomy, cardiorenal syndrome, chronic kidney disease, diastolic dysfunction, mineralocorticoid antagonist
Subject Categories: ACE/Angiotension Receptors/Renin Angiotensin System, Animal Models of Human Disease, Cardiorenal Syndrome, Endothelium/Vascular Type/Nitric Oxide
Nonstandard Abbreviations and Acronyms
- eNOS
endothelial nitric oxide synthase
- FINEARTS‐HF
Finerenone Trial to Investigate Efficacy and Safety Superior to Placebo in Patients With Heart Failure
- HFpEF
heart failure with preserved ejection fraction
- LVEDP
LV end‐diastolic pressure
- LVEDPVR
LV end‐diastolic pressure–volume relationship
- LVESP
LV end‐systolic pressure
- MR
mineralocorticoid receptor
- MRA
mineralocorticoid receptor antagonist
- NOS
nitric oxide synthases
- peNOS ser 1177
endothelial nitric oxide synthase and its activating phosphorylation
Clinical Perspective.
What Is New?
The nonsteroidal mineralocorticoid receptor antagonist finerenone reduces renal hypertrophy and albuminuria, attenuates cardiac diastolic dysfunction and cardiac fibrosis, and improves cardiac perfusion in a preclinical nondiabetic chronic kidney disease model.
What Are the Clinical Implications?
Our data suggest that finerenone may be effective in patients with and without diabetes with chronic kidney disease in reducing cardiovascular outcomes.
Chronic kidney disease (CKD) affects 15% to 25% of the adult global population. 1 , 2 CKD is characterized by the progressive loss of nephrons and thus a decline in the glomerular filtration rate (GFR). 3 Among the diverse adverse outcomes during the evolution of the disease, cardiovascular disease is particularly important and relevant because it is the leading cause of death in the general population. 4 , 5 , 6 Most patients with CKD and end‐stage renal disease develop uremic cardiomyopathy, characterized by left ventricular (LV) hypertrophy and diastolic dysfunction, together with severe fibrosis. 4 The pathogenesis of these complications is multifactorial and includes alterations in ion homeostasis, hemodynamic overload, insulin resistance, inflammation, oxidative stress, and the accumulation of uremic toxins. 4 The severity of CKD positively correlates with the presence of heart failure and the progression of heart failure with preserved ejection fraction (HFpEF). 7 Conversely, among patients with HFpEF, ≈50% have CKD. 8
The mineralocorticoid receptor (MR) is expressed not only by epithelial renal cells but also by other cell types, including cardiomyocytes, endothelial cells, and cardiac fibroblasts. 9 MR overactivation in the heart is proposed to play an important role in the progression of cardiovascular disease in the context of CKD by promoting inflammation and fibrosis. 10 Several preclinical studies have shown that the use of MR antagonists (MRAs) has a beneficial effect by delaying the progression of the disease in both kidney and cardiovascular disease models. 11 , 12 , 13 , 14 However, the use of steroidal MRAs is restricted in certain patient populations due to the risk of hyperkalemia. 15
Finerenone is a new selective nonsteroidal MRA associated with a minimal risk of hyperkalemia that has been recently studied in 2 large clinical outcome trials in patients with CKD and type 2 diabetes: FIDELIO‐DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) and FIGARO‐DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease). 16 , 17 In the FIDELIO‐DKD trial, which included 5734 patients with advanced CKD and type 2 diabetes, finerenone significantly reduced CKD progression by 18% and death from cardiovascular causes by 14% relative to placebo after a mean follow‐up of 2.6 years. 16 FIGARO‐DKD included 7437 patients with type 2 diabetes and less advanced CKD. Finerenone significantly decreased the primary outcome, which was a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure by 13% and showed a trend toward less CKD progression relative to placebo after a mean follow‐up of 3.4 years. 17 We recently reported the beneficial effect of MR blockade by finerenone in the Zucker fatty/spontaneously hypertensive rat, a model associating hypertension and features of HFpEF such as diastolic dysfunction, cardiac fibrosis, and reduced exercise capacity in a context of obesity and type 2 diabetes. Finerenone improved diastolic function and cardiac perfusion with reduced cardiac fibrosis in the Zucker fatty/spontaneously hypertensive rats. 18 The aim of this study was to test whether finerenone improves cardiac and renal function in a preclinical model of CKD induced by 5/6 nephrectomy in the absence of metabolic disease.
Methods
The data generated or analyzed during the current study are available from the corresponding author upon reasonable request. All animal studies were conducted in accordance with the National Institutes of Health Guide and European Community directives for the Care and Use of Laboratory Animals (European Directive 2010/63/UE), approved by the local animal ethics committee (APAFIS No. 18317–20 190 104 115 86 046 v5), and conducted according to the French National Institute of Health and Medical Research animal care and use committee guidelines.
Experimental Design
Experiments were approved by the Darwin Ethics Committee of Sorbonne University (APAFIS No. 18317‐20 190 104 115 86 046 v5) and conducted according to French National Institute of Health and Medical Research animal care and use committee guidelines. Fifty‐three 6‐week‐old male Sprague Dawley rats were purchased from Charles River, and 3 separate protocols were performed (Figure 1). In protocol 1, sham (n=8) and CKD (n=7) animals were included to characterize the cardiac complications 1 month after surgery (Figure 1). In protocol 2, the impact of curative finerenone treatment in CKD rats was evaluated using sham animals (n=8), CKD animals without treatment (n=9), and CKD animals treated (n=7) with finerenone in the food (10 mg/kg per day). Finerenone treatment started 1 month after 5/6 nephrectomy and lasted for 2 months before analysis (Figure 1). In protocol 3, the early impact of curative finerenone treatment in CKD rats was evaluated using CKD animals without treatment (n=6) and CKD animals treated (n=8) with finerenone in the food (10 mg/kg per day). Finerenone treatment started 83 days after 5/6 nephrectomy for 7 days at the end of the experimental period (Figure 1). The dose of finerenone we used (10 mg/kg per day) was in accordance with our previous study in Zucker fatty/spontaneously hypertensive model 18 and Lattenist et al. 19 The dose of 10 mg/kg per day was previously shown to be a maximum efficacious dose in cardiorenal rat models. 20
Figure 1. Schematic presentation of experimental protocols.
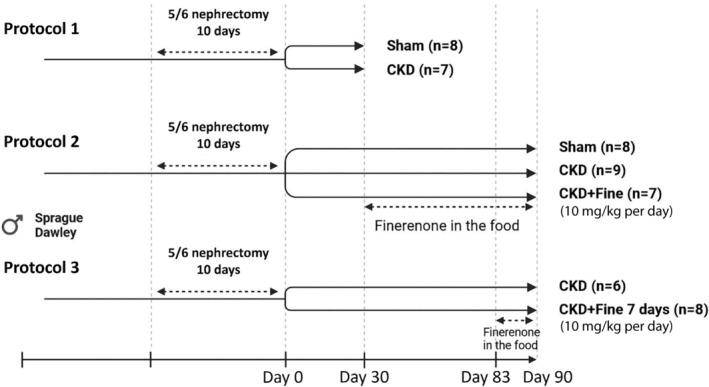
Protocol 1: Early CKD, Fine: finerenone effect, Protocol 2: finerenone treatment long‐term effect, Protocol 3: finerenone treatment short‐term effect. CKD indicates chronic kidney disease.
CKD was induced by 5/6 nephrectomy in 6‐week‐old male rats. Surgeries were performed under ketamine/xylazine anesthesia (100 mg/kg) with the animals placed on a heating pad to maintain a body temperature at 37 °C. The left kidney was exposed and clamped, and two‐thirds (upper and lower poles) tied and cut with a polyglycolic acid suture line, and absorbable hemostat (Surgicel, Johnson and Johnson Medical, Saint Priest, France) was applied before clamp removal. After the muscle and skin were sutured, the animals were returned to their cages and observed until they recovered. Sham surgery was carried out on anesthetized rats in which the left kidney was exposed for the same amount of time as that of the nephrectomized rats without tying the poles. The muscle and skin were then sutured and the animals returned to their cages and observed until they recovered. Seven days after recovery from the first surgery, the right kidney was removed. Time 0 was defined as the day of the second surgery.
The animals were followed for 12 weeks and housed in a climate‐controlled facility with a 12‐hour light/12‐hour dark cycle and provided free access to water and food. The welfare of the rats was monitored throughout the study. Physiological analyses were performed between 11 and 12 weeks of follow‐up. Animals were euthanized at the end of the study. Tissues were collected, weighed, and rinsed in ice‐cold Dulbecco's phosphate buffered saline (Thermo Fisher, Waltham, MA). The kidney and heart were cut into 2 parts, one for histology and the other for molecular analysis, for which the tissue was frozen in liquid nitrogen and stored at −80 °C.
Biochemical Studies
Twenty‐four‐hour urine collection was performed using metabolic cages for all studied groups at the end of each study. We determined the plasma urea and creatinine and urinary albumin levels using an automatic analyzer (Catalyst One; IDEXX, Westbrook, ME).
Glomerular Filtration Rate
GFR was measured by transdermal analysis using the fluorescein isothiocyanate–sinistrin method. 21 Briefly, animals under anesthesia were shaved on the dorsal side and the optical device (Transdermal Mini GFR Monitor, MediBeacon) attached to measure fluorescein isothiocyanate–sinistrin clearance as an estimate of GFR, as described below. Background readings were recorded for 3 minutes before administration of the sinistrin (7 mg/100 g bodyweight bolus). The fluorescence emitted by the fluorescein isothiocyanate–sinistrin was measured through the skin by the optical device for 1.5 hours and the measurements recorded. The data were analyzed using MB_Studio2 software (MediBeacon, St. Louis, MO).
Echocardiography
Transthoracic Doppler echocardiographic studies were performed on anesthetized rats at the end of the study using isoflurane (3% for induction, 2% for maintenance; ISO‐VET, Loughrea, Ireland) and an echocardiographic system (VIVID 7, GE Healthcare, Chicago, IL) equipped with an 8 to 5 MHz transducer. Briefly, a 2‐dimensional short‐axis view of the left ventricle was obtained at the level of the papillary muscle to record M‐mode tracings. Left ventricular (LV) diameters were measured following the American Society of Echocardiology's leading‐edge method from at least 3 consecutive cardiac cycles. The LV outflow velocity was measured by pulsed‐wave Doppler and cardiac output was calculated as CO=aortic VTI · [π · (left ventricular outflow diameter/2)2] · heart rate, where VTI is the velocity–time integral. 22 Echocardiography was done in blinded manner and the same day for all animals.
Magnetic Resonance Imaging
LV tissue perfusion was measured in anesthetized animals (Brietal, 50 mg/kg IP) at the end of the study by magnetic resonance imaging (Biospec 4.7 Tesla; Bruker Corp., Billerica, MA) using the arterial spin labeling technique. Perfusion images were analyzed using ParaVision 5.0 software (Bruker AG Germany). 23
Hemodynamic Studies
Hemodynamic studies were performed in the left ventricle. LV hemodynamics were determined by measuring the LV pressure–volume curves at the end of the study. The right carotid artery of anesthetized rats (Brietal; 50 mg·kg−1 IP) was cannulated with a micromanometer‐tipped catheter (SPR 838, Millar Instruments, Houston, TX). The heart rate and arterial blood pressure were recorded and the LV pressure by introduction of the catheter into the LV. We gently occluded the abdominal aorta with a cotton swab to obtain LV pressure–volume loops at baseline and during loading. Data were stored and analyzed using Millar conductance data acquisition and analysis software (IOX; EMKA, Velbert, Germany). Finally, we measured or calculated the LV end‐systolic pressure (LVESP) and LV end‐diastolic pressure (LVEDP), dP/dtmax per minute, LV relaxation constant τ, and the slopes of the LV end‐systolic and end‐diastolic pressure–volume relationships.
Histology
The LV tissue and kidney sections were collected and immersed in paraformaldehyde fixative solution (Sigma‐Aldrich, St. Louis, MO). After fixation, the sections were dehydrated and embedded in paraffin. From these sections, 5‐μm thick histological slices were obtained and stained with Sirius red for collagen determination in the kidney and heart and with Masson Trichrome for kidney structure. For the measurement of kidney fibrosis and structure, slides were examined and 10 microphotographs per sample were obtained under a microscope (Zeiss, Oberkochen, Germany) at 20× magnification. Injured tubules and glomeruli were counted using ZEN 3.1 software (Zeiss). A semiquantitative score of 0 to 4 was given. Renal and LV fibrosis was calculated as the percentage of the area containing collagen to the total area of the image of the staining. All analyses were blinded.
Western Blot Analysis and Antibodies
Heart samples were homogenized in lysis buffer with complete proteases and phosphatase inhibitor cocktail (Roche, Basel, Switzerland). Extracts were centrifuged at 13 000 rpm for 10 minutes at 4 °C, and the protein concentration in the supernatant was determined via the Lowry method. Total protein (40 μg) was loaded into 8% to 15% SDS‐PAGE gels and transferred to nitrocellulose membranes. Blocking was performed with 5% albumin. Primary antibodies were incubated at 4 °C overnight, and secondary antibodies were incubated for 90 minutes at room temperature. Membranes were stripped after the detection of the phosphorylated protein to detect total proteins. Antibodies and dilutions were used as follows: phospho‐Ser1177 endothelial nitric oxide synthase (eNOS; Cell Signaling, 1:1000), eNOS (1:3000; BD Transduction Laboratories; BD Biosciences, San Jose, CA). Total protein loading controls were done using strain‐free straining, and the amount of protein was detected using a chemiluminescence kit (Millipore, St. Louis, MO) with a ChemiDoc image analyzer (Bio‐Rad Laboratories, Hercules, CA). Relative densitometry was performed using Image Lab (Bio‐Rad Laboratories) software. Protein normalization was performed using stain‐free imaging technology (Bio‐Rad Laboratories).
Analysis of NOS Activity
We estimated global Nitric Oxide Synthase Activity Assay Kit (ab211083; Abcam, Cambridge, UK) with or without inducible nitric oxide synthase (NOS) inhibition (1400W 10 μmol/L; Sigma‐Aldrich).
Statistical Analysis
The results are presented as the mean±SEM. Differences in the means between 2 groups for nonrepeated variables were compared by Student's t test (protocols 1 and 3) and 1‐way repeated‐measures ANOVA (protocol 2) to compare 3 groups. All comparisons passed the normality test (Shapiro–Wilk normality test, in Prism 7.04 [GraphPad Software, La Jolla, CA]). The analysis was performed using GraphPad Prism 7.4. Results were considered significant at a P value <0.05.
Results
CKD by 5/6 Nephrectomy Induces Cardiovascular Alterations After 1 Month
CKD was induced by 5/6 nephrectomy in male Sprague Dawley rats. CKD induced renal alterations from the first month after induction of the disease, as determined by an increase in blood urea nitrogen, without a significant change in serum creatinine levels (Table 1). We observed no changes in systolic blood pressure 1 month after the induction of CKD (Table 1). There were also no differences in the LVESP, dP/dtmax, or LV end‐systolic pressure–volume relationship between the sham and CKD groups (Table 1). There was a trend toward an increase in LVEDP in the CKD group relative to the sham group (P=0.08 versus sham), and the dP/dtmin and LV end‐diastolic pressure–volume relationship (LVEDPVR) were significantly higher in CKD than sham rats, but not the relaxation time constant τ (Table 1). There were no differences in heart rate between the 2 groups (Table 1). These results suggest impaired LV contractility and diastolic dysfunction in CKD relative to sham animals as early as 1 month after CKD induction.
Table 1.
CKD by 5/6 Nephrectomy Induces Cardiovascular Alterations After 1 Month (Protocol 1)
| Sham (n=8) | CKD (n=7) | |
|---|---|---|
| BUN, mg/dL | 17±0.9 | 36±1.8* |
| Creatinine, μmol/L | 0.55±0.03 | 0.61±0.03 |
| SBP, mm Hg | 139±3 | 156±9 |
| LVESP, mm Hg | 128±5 | 154±12 |
| dP/dtmax, mm Hg/s | 9653±456 | 10 819±716 |
| LVESPVR, RVU/mm Hg | 24±1.0 | 25±2.0 |
| LVEDP, mm Hg | 5.3±0.4 | 7.4±0.9 |
| dP/dtmin, mm Hg/s | 10 545±437 | 13735±730* |
| τ, ms | 9.2±0.1 | 9±0.3 |
| LVEDPVR, RVU/mm Hg | 1.5±0.2 | 2.6±0.2* |
| HR, bpm | 359±9 | 364±12 |
Data are presented as the mean±SEM; n=7–8. Student's t test was used for statistical analysis. BUN indicates blood urea nitrogen; CKD, chronic kidney disease; HR, heart rate; LVEDP, left ventricular end‐diastolic pressure; LVEDPVR, left ventricular end‐diastolic pressure–volume relationship; LVESP, left ventricular end‐systolic pressure; LVESPVR, left ventricular end‐systolic pressure volume relationship; SBP, systolic blood pressure; and τ, time constant of relaxation.
P<0.05 vs sham.
Impact of Finerenone on Renal Parameters in Nondiabetic CKD Rats
We evaluated the impact of 2 months of finerenone treatment in CKD rats starting 1 month after CKD induction. CKD rats showed renal hypertrophy of the remanent kidney relative to the left kidney of sham rats (Figure 2A), decreased measured GFR (−57%) (Figure 2B), and increased 24‐hour albuminuria (+46%) (Figure 2C). Finerenone treatment prevented renal hypertrophy and showed a trend toward decreased albuminuria (−22%) (P=0.145 versus CKD) but did not change GFR (Figure 2A through 2C). Interstitial kidney fibrosis (Figure 2D and 2E) was significantly higher in CKD than sham rats (+206%), and finerenone treatment reduced renal fibrosis to sham levels (−57%), although the reduction was not statistically significant (P=0.095 versus CKD) (Figure 2D and 2E). Tubular (Figure 2F and 2G) and glomerular damage (Figure 2F and 2H) was higher in CKD than sham rats and finerenone did not reduce such damage (Figure 2F and 2H).
Figure 2. Renal injury that develops in CKD rats after 12 weeks is partially improved by finerenone.
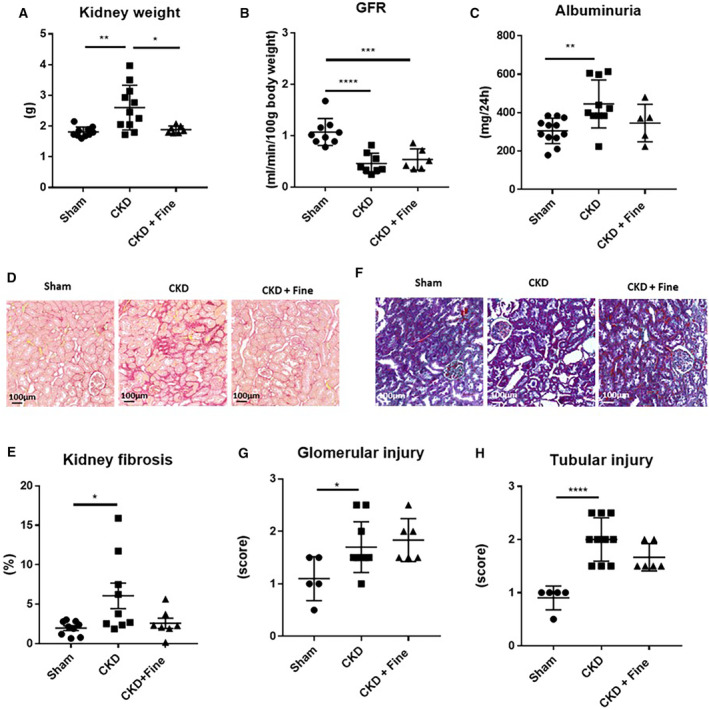
Kidney weight (A); GFR (B); and albuminuria (C). Representative light microphotographs (20× magnification) of rat kidney sections stained with Sirius red (D) and quantification of kidney fibrosis (E). Representative light microphotographs (20× magnification) of rat kidney sections stained with Masson Trichrome (F); quantification of tubular (G) and glomerular injury (H). ANOVA was used for statistical analysis, n=7–9. *P<0.05, **P<0.001, ***P<0.0001. CKD indicates chronic kidney disease; Fine, finerenone; and GFR, glomerular filtration rate.
Overall, these results show that finerenone reduced renal hypertrophy, albuminuria, and renal fibrosis, without altering the GFR or renal damage after 2 months of treatment.
Impact of Finerenone on Cardiac Parameters in Nondiabetic CKD Rats
We performed echocardiography 12 weeks after the beginning of the experimental period (including 2 months of finerenone treatment). CKD rats had similar LV end‐diastolic diameters (Figure 3A) and LV end‐systolic diameters as sham rats (Figure 3B) but showed reduced fractional shortening (−11%; P=0.05 versus sham) (Figure 3C), a reduced stroke volume (−12%) (Figure 3D), and reduced cardiac output (−15%) (Figure 3E). Finerenone showed a trend toward reducing LV end‐diastolic diameter (Figure 3A) and normalized the LV end‐systolic diameter (Figure 3B). It increased fractional shortening (+16%) (Figure 3C) relative to that in CKD rats, without modifying the stroke volume (Figure 3D) or cardiac output (Figure 3E). The cardiac/bodyweight ratio is not altered by nephrectomy or by finerenone treatment (Figure S1).
Figure 3. Echocardiographic parameters in CKD rats are improved by finerenone.
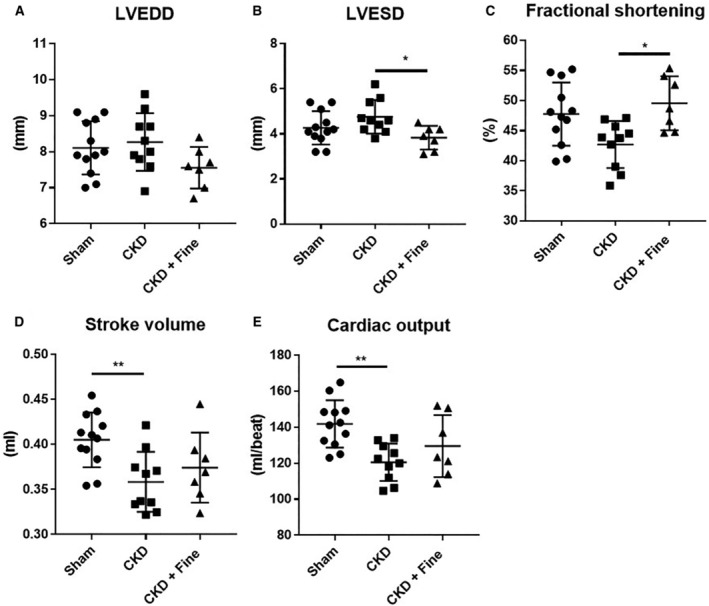
LVEDD, (A); LVESD (B); fractional shortening (C); stroke volume (D); and cardiac output (E) (Protocol 2). ANOVA was used for statistical analysis, n=7–9. *P<0.05, **P<0.001, ***P<0.0001. CKD indicates chronic kidney disease; Fine, finerenone; LVEDD, left ventricular end‐diastolic diameter; and LVESD, left ventricular end‐systolic diameter.
Hemodynamic studies were performed at the end of the 12‐week experimental period after 2 months of finerenone treatment. There was no statistical difference in the systolic blood pressure between CKD, Sham, and finerenone‐treated rats. However, there is a trend for CKD to increase blood pressure while finerenone normalized it close to sham levels (Figure 4A). LV pressure volume curves showed no differences in the LVESP (Figure 4B) or LVESP volume relationship between groups (Figure 4C). The LV end‐diastolic pressure (LVEDP) was higher in the CKD than sham rats (+77%) but no longer different from that of the sham rats for the rats treated with finerenone (Not Significant versus sham) (Figure 4D). The LVEDPVR was higher in the CKD than sham rats (+300%) but was normalized by finerenone treatment (−55% versus CKD) (Figure 4E). The relaxation time constant τ was higher in the CKD than sham rats (+16%), but the effect was blunted by finerenone (−18% versus CKD) (Figure 4F). There was no difference in the heart rate, dP/dtmax, or dP/dtmin between the groups (Figure S2). These results indicate impaired LV contractility and compliance in nondiabetic CKD rats relative to sham animals that was improved by finerenone treatment.
Figure 4. Hemodynamic parameters, fibrosis, and cardiac tissue perfusion in CKD rats are improved by finerenone after 12 weeks.
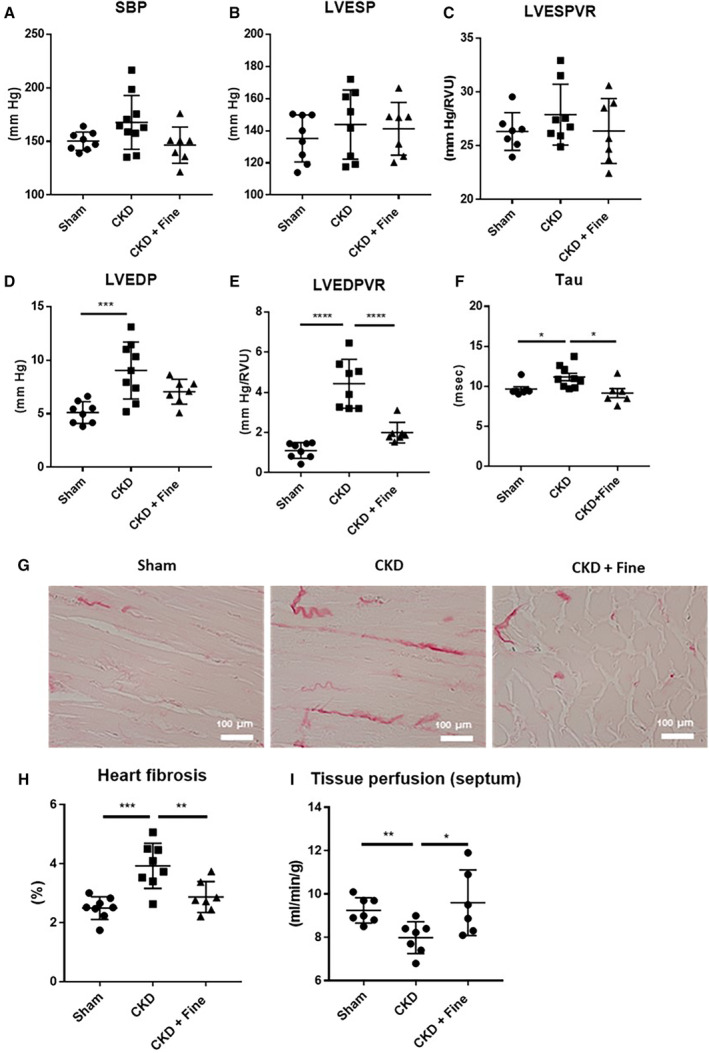
SBP (A); LVESP (B); LVESPVR (C); LVEDP (D); LVEDPVR (E); and tau (F). Representative light microphotographs (20× magnification) of rat kidney sections stained with Sirius red (G) and kidney fibrosis (H). Septum tissue perfusion (I) (Protocol 2). ANOVA was used for statistical analysis, n=7–9. *P<0.05, **P<0.001, ***P<0.0001. CKD indicates chronic kidney disease; Fine, finerenone; LVEDP, left ventricular end‐diastolic pressure; LVEDPVR, left ventricular end‐diastolic pressure–volume relationship; LVESP, left ventricular end‐systolic pressure; LVESPVR, left ventricular end‐systolic pressure–volume relationship; and SBP, systolic blood pressure.
Worsening of LV diastolic function was associated with myocardial interstitial fibrosis in the CKD rats relative to the sham animals (+57%) (Figure 4G and 4H). Myocardial perfusion was assessed by magnetic resonance imaging, showing lower perfusion of the left ventricle in the CKD than sham rats (+14%) (Figure 4I). Two months of finerenone treatment normalized cardiac fibrosis (−27%) (Figure 4H) and perfusion (−20% versus CKD) (Figure 4I) to sham levels.
Overall, these results show that the LV diastolic dysfunction (ie, increase in LVEDP, LVEDPVR, and τ parameters) associated with CKD was improved by 2 moths of finerenone treatment. CKD is associated with a reduction in cardiac perfusion that could contribute to the development of fibrosis and diastolic dysfunction seen in nondiabetic CKD rats. Finerenone improved cardiac perfusion and reduced interstitial fibrosis. Protocol 2 results for GFR, albuminuria, and cardiac function are also summarized in Table 2.
Table 2.
CKD by 5/6 Nephrectomy Induces Cardiovascular Alterations After 3 Months (Protocol 2)
| Sham (n=8) | CKD (n=9) | CKD+finerenone (n=7) | |
|---|---|---|---|
| GFR, mL/min per 100 g of body weight | 1.07±0.08 | 0.46±0.07* | 0.54±0.08 |
| Albuminuria, mg/24 h | 305±19 | 446±39* | 346±44 |
| SBP, mm Hg | 150±3 | 168±8 | 146±6 |
| LVESP, mm Hg | 135±5 | 144±8 | 141±6 |
| dP/dtmax, mm Hg/s | 10 535±658 | 10 501±421 | 10.106±781 |
| LVESPVR, RVU/mm Hg | 26.3±0.6 | 27.9±1.0* | 26.4±1.1† |
| LVEDP, mm Hg | 5.1±0.4 | 9.0±0.9* | 7.1±0.4 |
| dP/dtmin, mm Hg/s | 11 148±559 | 12178±1410* | 13 358±1236 |
| τ, ms | 9.6±0.3 | 11.1±0.5 | 9.2±0.6† |
| LVEDPVR, RVU/mm Hg | 1.1±0.1 | 4.4±0.4* | 1.9±0.2† |
| HR, bpm | 368±6 | 364±10 | 371±7 |
ANOVA was used for statical analysis, Data are presented as the mean±SEM; n=7–9. CKD indicates chronic kidney disease; GFR, glomerular filtration rate; HR, heart rate; LVEDP, left ventricular end‐diastolic pressure; LVEDPVR, left ventricular end‐diastolic pressure volume relationship; LVESP, left ventricular end‐systolic pressure; LVESPVR, left ventricle end‐systolic pressure volume relationship; SBP, systolic blood pressure; and τ, time constant of relaxation.
P<0.05 vs sham.
P<0.05 vs CKD.
Impact of Short‐Term Finerenone Treatment
We next assessed the early regulation by short‐term finerenone treatment administered for 7 days in untreated CKD rats. We observed no changes in systolic blood pressure after 7 days of finerenone treatment (Table 3). There was no difference in LVESP or dP/dtmax between the CKD and CKD+finerenone 7 days groups, but, interestingly, the LV end‐systolic pressure–volume relationship was increased by finerenone treatment (Table 3). There was no difference in LVEDP or dP/dtmin between groups, whereas the τ and LVEDPVR were significantly higher in CKD than CKD+finerenone 7‐day rats (Table 3). There were no differences in heart rate between groups (Table 3). There was a trend toward higher perfusion in the LV tissue in CKD rats treated with finerenone than in untreated CKD rats (P=0.08 versus CKD). These results suggest an early effect of finerenone treatment on the LV contractility and diastolic dysfunction observed in CKD. Cardiac fibrosis was estimated by Sirius red staining. No difference was observed between CKD and CKD rats treated with finerenone for 7 days (mean±SEM, %; CKD: 4.1±0.1; CKD+finerenone 7 days: 4.2±0.2; n=6–8; CKD versus CKD+finerenone 7 days; P=0.9).
Table 3.
Finerenone Induces Early Amelioration of Cardiac Function in CKD (Protocol 3)
| CKD (n=6) | CKD+finerenone 7 days (n=8) | |
|---|---|---|
| SBP, mm Hg | 145±12.5 | 131±9 |
| LVESP, mm Hg | 142±12 | 126±9 |
| dP/dtmax, mm Hg/s | 9133±814 | 9051±1042 |
| LVESPVR, RVU/mm Hg | 23.2±1.6 | 26.9±0.8* |
| LVEDP, mm Hg | 7.5±0.6 | 6.4±0.4 |
| dP/dtmin, mm Hg/s | 12 364±1360 | 11 848±1440 |
| τ, ms | 11±0.5 | 8.5±0.3* |
| LVEDPVR, RVU/mm Hg | 3.9±0.2 | 2.9±0.3* |
| HR, bpm | 346±15 | 374±5 |
| Septum tissue perfusion, (mL/min) | 5.3±0.7 | 7.2±0.7 |
Data are presented as the mean±SEM, n=6–8. Student's t test was used for statistical analysis. CKD indicates chronic kidney disease; HR, heart rate; LVEDP, left ventricular end‐diastolic pressure; LVEDPVR, left ventricular end‐diastolic pressure volume relationship; LVESP, left ventricular end‐systolic pressure; LVESPVR, left ventricular end‐systolic pressure volume relationship; SBP, systolic blood pressure; and τ, time constant of relaxation.
P<0.05 vs CKD.
Impact of Finerenone on Cardiac eNOS Activation
We evaluated the cardiac levels of eNOS and its activating phosphorylation (peNOS ser 1177) to identify the mechanism involved in the cardioprotection conferred by finerenone in nondiabetic CKD rats. The peNOS ser 1177 protein levels were not modified by CKD but were markedly increased by 2 months of finerenone (+360% versus CKD) (Figure 5A and 5B). Total eNOS protein levels were lower in CKD than sham rats but were restored by finerenone treatment (Figure 5A and 5C). The peNOS ser 1177/total eNOS ratio was also higher in the finerenone group than in the CKD and sham groups (+175% versus CKD) (Figure 5D).
Figure 5. Amelioration of diastolic dysfunction, myocardial perfusion, and fibrosis are associated with increased levels of eNOS and its activating phosphorylation.
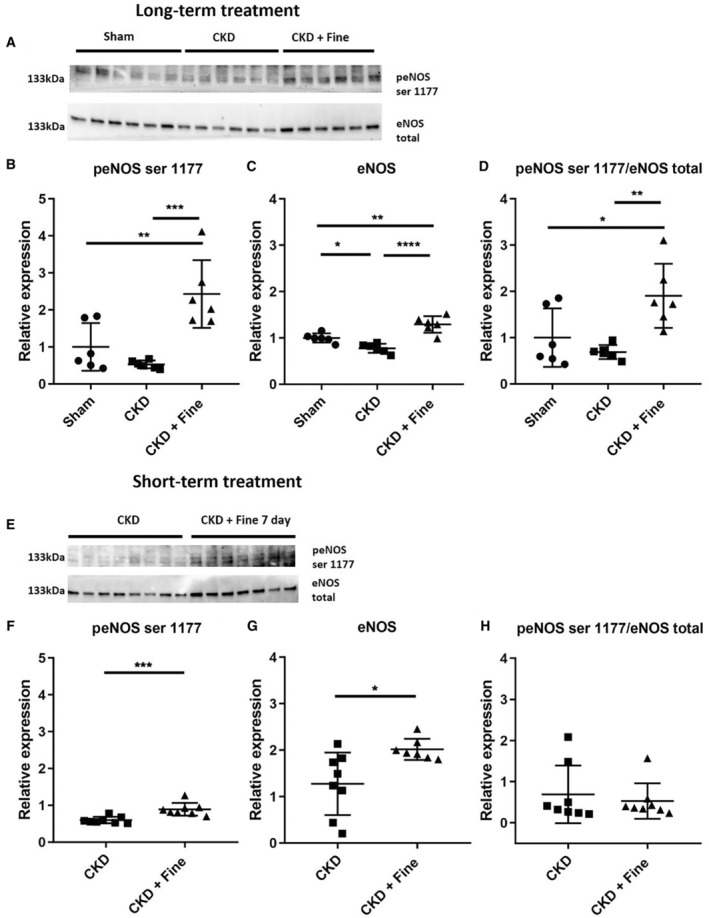
LV tissue levels of peNOS ser 1177 by Western blot analysis (A, upper part), densitometric analysis of peNOS ser 1177 levels (B), LV tissue levels of total eNOS by Western blot analysis (A lower part), densitometric analysis of eNOS levels (C), and the peNOS ser 1177/eNOS total ratio (D) from long‐term treatment (Protocol 2). LV tissue levels of peNOS ser 1177 by Western blot analysis (E, upper part), densitometric analysis of peNOS ser 1177 levels (F), LV tissue levels of total eNOS by Western blot analysis (E, lower part), densitometric analysis of eNOS levels (G), and the peNOS ser 1177/eNOS total ratio (H) from short‐term treatment (Protocol 3). ANOVA was used for statistical analysis of long‐term treatment and Student's t test for short‐term treatment, n=7–9. *P<0.05, **P<0.001, ***P<0.0001. CKD indicates chronic kidney disease; eNOS, endothelial nitric oxide synthase; Fine, finerenone; LV, left ventricular; and peNOS ser 1177, endothelial nitric oxide synthase and its activating phosphorylation.
The activating eNOS phosphorylation at residue ser 1177 was estimated in CKD rats treated with finerenone for 7 days and compared with nontreated CKD rats. We observed an increase of both peNOS ser 1177 and total eNOS levels (+50% and +58%, respectively, versus CKD) but the ratio was unchanged (Figure 5E through 5H). To analyze whether the increase in peNOS ser 1177 resulted in increased activity, we estimated global NOS activity with or without inducible NOS inhibition. We showed that 7 days of finerenone treatment increased total NOS activity. (mean±SEM, pmol/min per μg; CKD: 0.34±0.03; CKD+finerenone 7 days: 0.45±0.09; n=6–8; t test CKD versus CKD+ 7 days; P=0.018) with no effect of inducible NOS inhibition (1400 W), suggesting an increase of eNOS activity (mean±SEM, pmol/min per μg; CKD+finerenone 7 days: 0.79±0.03; CKD+finerenone 7 days +1400 W: 0.92±0.06; n=6–8; t test CKD+ 7 days versus CKD+ 7 days +1400 W; P=0.07).
Discussion
Our results show that finerenone treatment improves cardiac diastolic function and perfusion, together with reducing cardiac fibrosis, in nondiabetic CKD rats.
Cardiovascular complications are common in the context of CKD. Approximately 40% of patients with CKD show LV hypertrophy, increasing to 75% for patients with kidney failure. 5 , 24 The severity of CKD is associated with the presence of heart failure and the progression of HFpEF, 7 which is the most prevalent marker of cardiovascular risk of patients with CKD. LV hypertrophy develops as an adaptive process to regulate the vascular stress associated with CKD in response to hypertension or hypervolemia. However, it results in a pathological process that leads to cardiomyocyte hypertrophy and eventually cardiomyocyte loss by apoptosis and cardiac fibrosis. 4 Cardiomyocyte loss in CKD contributes to heart failure that, together with cardiac fibrosis, leads to the development of diastolic dysfunction. 4 , 25 , 26 The rodent 5/6 nephrectomy model presents diastolic dysfunction with a decreased early/late (atrial) diastolic transmitral flow velocity ratio, altered endothelial function, and increased cardiac fibrosis, 27 , 28 , 29 , 30 , 31 indicating that this CKD model is appropriate for studying nondiabetic CKD‐associated cardiovascular disease and testing our hypothesis. A number of preclinical studies have shown MR overactivation in cardiac dysfunction. 32 , 33 , 34 , 35
The mechanisms involved in the therapeutic effect of MR blockade are mainly related to a reduction in inflammation, oxidative stress, and fibrosis, which have a beneficial impact on cardiorenal disease. 36 MR antagonism by the steroidal MRAs spironolactone and eplerenone has been assessed in HFpEF in several clinical studies. 37 , 38 , 39 , 40 , 41 The cardiac benefits observed following finerenone treatment in Zucker fatty/spontaneously hypertensive rats 18 are consistent with the results documented in 2 recent large clinical outcome studies: FIDELIO‐DKD and FIGARO‐DKD. Both studies showed that finerenone improves cardiovascular outcomes relative to placebo in patients with diabetes with CKD. 16 , 17 However, studies in patients with CKD without diabetes are needed, as nondiabetic CKD represents ≈50% of patients with CKD. Preclinical studies in nondiabetic CKD models have been performed using steroidal MRAs. In a 5/6 nephrectomized model, spironolactone has been shown to reduce endothelial dysfunction through inhibition of the advanced glycation end‐products (AGE)/AGE receptor axis and the reduction of intracellular oxidative stress 42 and to attenuate cardiovascular hypertrophy and fibrosis. 43 In rats with unilateral ureteral obstruction–induced CKD, eplerenone significantly attenuates MR activation and cardiac fibrosis. 44 The nonsteroidal MRA finerenone was shown to reduce endothelial dysfunction in the Munich Wistar Frömter rat, a CKD model, through enhancement of nitric oxide bioavailability. 45 However, these studies did not assess the impact on diastolic dysfunction. As reported in the present study, 5/6 nephrectomy CKD rats develop characteristics shared with HFpEF, such as decreased stroke volume and cardiac output and increased LVEDP, LVEDPVR, and τ, features that have been previously reported in various preclinical rodent HFpEF models. 27 , 28 , 29 , 30 , 31 , 46 Here, we show that MR blockade with finerenone in 5/6 nephrectomy CKD rats improves LVEDP, LVEDPVR, and τ parameters.
Adequate heart tissue perfusion is crucial for maintaining the physiological state in the cardiovascular system. Several studies have shown that CKD induces microvascular rarefaction 47 and endothelial dysfunction, 48 leading to dysfunctional microcirculation, with reduced tissue perfusion and hypoxia. Several studies of animal CKD models have reported that the loss of microvasculature precedes fibrosis and that fibrosis is closely related to vascular rarefaction. 47 , 49 , 50 , 51 A systemic proinflammatory state causes coronary microvascular endothelial inflammation, which reduces nitric oxide bioavailability to adjacent cardiomyocytes, favoring the development of hypertrophy and increasing resting blood pressure. Both stiff cardiomyocytes and interstitial fibrosis contribute to high diastolic LV stiffness and the development of heart failure. 52 MR overactivation promotes cardiac fibrosis. 53 The strong reduction in cardiac fibrosis by finerenone treatment described in our study is consistent with the results obtained in studies using several other preclinical models, which reported a beneficial effect of finerenone due to a reduction in the development of fibrotic tissue in the heart. 54 , 55 , 56 , 57
Evidence of low nitric oxide bioavailability and decreased cyclic cGMP is present in patients with HFrEF, resulting in impaired vasodilation and aerobic exercise capacity, as well as decreased muscle power. 58 Nitric oxide is a paracrine signaling molecule that can diffuse from endothelial cells to vascular smooth muscle cells, vessel lumen, or cardiac myocytes; but it can also act as an autocrine signal, especially in cardiac myocytes. 59 Nitric oxide/cGMP signaling therefore has a crucial role in relaxation of vascular smooth muscle cells and in cardiac contraction and relaxation as well as coronary perfusion. 60 The beneficial effect of finerenone treatment on myocardial perfusion reported here could be related to the increase in peNOS ser1177 that was observed at the end of finerenone long‐term treatment. The relatively rapid impact of short‐term (7 days) finerenone treatment on the activating eNOS ser1177 phosphorylation and NOS activity suggests a direct role of the MR blockade by finerenone rather than a long‐term adaptation. Therefore, one of the possible beneficial mechanisms modulated by finerenone that contributes to the decrease in fibrosis and improvement of diastolic function could be improved myocardial perfusion and function by enhancing the NOS‐dependent cardioprotective and vasodilatory effects.
The clinical relevance of our findings (which will need to be confirmed in a prospective clinical trial) is as follows: (1) Our data suggest that finerenone will be effective in patients without diabetes as well as in patients with diabetes in reducing cardiovascular outcomes; (2) the reduction in cardiac fibrosis and reduction in LV hypertrophy suggests an important effect on the prevention of heart failure in patients with CKD, who have been shown to have an increase in serum aldosterone levels that predict progression to end‐stage renal disease 61 ; and (3) the reduction in myocardial fibrosis would also predict a reduction in ventricular arrythmias and sudden cardiac death, as well as improved exercise performance and quality of life.
A major, yet unsolved, question is whether a combination therapy that associates an MRA and sodium‐glucose cotransporter 2 inhibitor would be beneficial over monotherapy treatment for patients with CKD. A meta‐analysis of clinical trials reported a decrease in the incidence of MRA‐induced hyperkalemia for patients with diabetes treated with both an MRA and sodium‐glucose cotransporter 2 inhibitor. 62 In a nondiabetic rodent model, a recent study showed an improved survival rate and decreased creatinine levels, proteinuria, and blood pressure in hypertensive mice treated with the combination, whereas a low dose of empagliflozin or finerenone alone had an intermediate or no impact. 55 The triple renin–angiotensin system/sodium‐glucose cotransporter 2/MR blockade (using ramipril, empagliflozin, and finerenone, respectively) also improved survival and renal outcomes in a mouse model of Alport syndrome with an additive effect of the MRA finerenone on interstitial fibrosis over other treatment. 63 Taken together, these reports suggest a benefit of combining an MRA and a sodium‐glucose cotransporter 2 inhibitor.
Conclusions
In conclusion, we show that the nonsteroidal MR antagonist finerenone reduces renal hypertrophy and albuminuria, without changing the GFR, attenuates cardiac diastolic dysfunction and cardiac fibrosis, and improves cardiac perfusion associated with an increase in activating phosphorylation of eNOS in a preclinical nondiabetic CKD model. The ongoing FINEARTS‐HF (Finerenone Trial to Investigate Efficacy and Safety Superior to Placebo in Patients With Heart Failure) study is currently evaluating the efficacy and safety of finerenone in patients with HF and an LV ejection fraction ≥40%. 64
Sources of Funding
This research was funded by grants from the Institut National de la Santé et de la Recherche Médicale, the ANR NGAL‐HT (ANR‐19‐CE14‐0013), and a research grant from Bayer‐AG.
Disclosures
P. Kolkhof is a full‐time employee of Bayer‐AG. The remaining authors have no disclosures to report.
Supporting information
Figures S1–S2
Acknowledgments
The authors thank the team of the Functional Exploration Center of the Cordeliers Research Center for their support with animal care. Conceptualization: Drs Jaisser and Mulder; methodology: Drs Posada, Stephan, Soulié, Ramirez, and Bonnard; software: Drs Stephan, Soulié, and Nicol; formal analysis: Drs Posada, Stephan, and Soulié; investigation resources: Drs Jaisser and Mulder; data curation: Drs Posada, Stephan, and Soulié; writing—original draft preparation: Drs Posada, Kolkhof, Jaisser, and Mulder; writing review and editing: Drs Posada, Stephan, Soulié, Jaisser, and Mulder; supervision: Drs Jaisser and Mulder; project administration: Drs Jaisser and Mulder; funding acquisition: Drs Jaisser and Mulder. All authors have read and agreed to the published version of the manuscript.
This manuscript was sent to Julie K. Freed, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032971
For Sources of Funding and Disclosures, see page 13.
References
- 1. Huang R, Fu P, Ma L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct Target Ther. 2023;8:129. doi: 10.1038/s41392-023-01379-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bikbov B, Purcell AC, Levey SA, Smith M, Abdoli A, Abebe M, Adebayo MO, Afarideh M, Agarwal SK, Agudelo‐Botero M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet (London, England). 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaidya SR, Aeddula NR, Doerr C. Chronic renal failure (nursing). StatPearls. 2021. [Google Scholar]
- 4. Wang X, Shapiro JI. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol. 2019;15:159–175. doi: 10.1038/s41581-018-0101-8 [DOI] [PubMed] [Google Scholar]
- 5. Patel N, Yaqoob MM, Aksentijevic D. Cardiac metabolic remodelling in chronic kidney disease. Nat Rev Nephrol. 2022;18:524–537. doi: 10.1038/s41581-022-00576-x [DOI] [PubMed] [Google Scholar]
- 6. Law JP, Pickup L, Pavlovic D, Townend JN, Ferro CJ. Hypertension and cardiomyopathy associated with chronic kidney disease: epidemiology, pathogenesis and treatment considerations. J Hum Hypertens. 2023;37:1–19. doi: 10.1038/s41371-022-00751-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van de Wouw J, Broekhuizen M, Sorop O, Joles JA, Verhaar MC, Duncker DJ, Danser AHJ, Merkus D. Chronic kidney disease as a risk factor for heart failure with preserved ejection fraction: a focus on microcirculatory factors and therapeutic targets. Front Physiol. 2019;10:1108. doi: 10.3389/fphys.2019.01108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin. 2008;4:387–399. doi: 10.1016/j.hfc.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rahman A, Jahan N, Rahman MT, Nishiyama A. Potential impact of non‐steroidal mineralocorticoid receptor antagonists in cardiovascular disease. Int J Mol Sci. 2023;24:1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. González‐Juanatey JR, Górriz JL, Ortiz A, Valle A, Soler MJ, Facila L. Cardiorenal benefits of finerenone: protecting kidney and heart. Ann Med. 2023;55:502–513. doi: 10.1080/07853890.2023.2171110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsai CH, Pan CT, Chang YY, Peng SY, Lee PC, Liao CW, Shun CT, Li PT, Wu VC, Chou CH, et al. Aldosterone excess induced mitochondria decrease and dysfunction via mineralocorticoid receptor and oxidative stress in vitro and in vivo. Biomedicine. 2021;9:946. doi: 10.3390/biomedicines9080946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gutiérrez‐Tenorio J, Marín‐Royo G, Martínez‐Martínez E, Martín R, Miana M, López‐Andrés N, Jurado‐López R, Gallardo I, Luaces M, San Román JA, et al. The role of oxidative stress in the crosstalk between leptin and mineralocorticoid receptor in the cardiac fibrosis associated with obesity. Sci Rep. 2017;7:16802. doi: 10.1038/s41598-017-17103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palacios‐Ramirez R, Lima‐Posada I, Bonnard B, Genty M, Fernandez‐Celis A, Hartleib‐Geschwindner J, Foufelle F, Lopez‐Andres N, Bamberg K, Jaisser F. Mineralocorticoid receptor antagonism prevents the synergistic effect of metabolic challenge and chronic kidney disease on renal fibrosis and inflammation in mice. Front Physiol. 2022;13:852812. doi: 10.3389/fphys.2022.859812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barrera‐Chimal J, André‐Grégoire G, Nguyen Dinh Cat A, Lechner SM, Cau J, Prince S, Kolkhof P, Loirand G, Sauzeau V, Hauet T, et al. Benefit of mineralocorticoid receptor antagonism in AKI: role of vascular smooth muscle Rac1. J Am Soc Nephrol. 2017;28:1216–1226. doi: 10.1681/ASN.2016040477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Georgianos PI, Agarwal R. The nonsteroidal mineralocorticoid‐receptor‐antagonist finerenone in cardiorenal medicine: a state‐of‐the‐art review of the literature. Am J Hypertens. 2023;36:135–143. doi: 10.1093/ajh/hpac124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956 [DOI] [PubMed] [Google Scholar]
- 18. Lima‐Posada I, Stephan Y, Soulié M, Palacios‐Ramirez R, Bonnard B, Nicol L, Kolkhof P, Jaisser F, Mulder P. Benefits of the non‐steroidal mineralocorticoid receptor antagonist finerenone in metabolic syndrome‐related heart failure with preserved ejection fraction. Int J Mol Sci. 2023;24:2536. doi: 10.3390/ijms24032536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lattenist L, Lechner SM, Messaoudi S, Le Mercier A, El Moghrabi S, Prince S, Bobadilla NA, Kolkhof P, Jaisser F, Barrera‐Chimal J. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury‐mediated chronic kidney disease: role of oxidative stress. Hypertens (Dallas, Tex 1979). 2017;69:870–878. doi: 10.1161/HYPERTENSIONAHA.116.08526 [DOI] [PubMed] [Google Scholar]
- 20. Kolkhof P, Delbeck M, Kretschmer A, Steinke W, Hartmann E, Bärfacker L, Eitner F, Albrecht‐Küpper B, Schäfer S. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64:69–78. doi: 10.1097/FJC.0000000000000091 [DOI] [PubMed] [Google Scholar]
- 21. Herrera Pérez Z, Weinfurter S, Gretz N. Transcutaneous assessment of renal function in conscious rodents. J Vis Exp. 2016;109:e53767. doi: 10.3791/53767-v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fang Y, Nicol L, Harouki N, Monteil C, Wecker D, Debunne M, Bauer F, Lallemand F, Richard V, Thuillez C, et al. Improvement of left ventricular diastolic function induced by β‐blockade: a comparison between nebivolol and metoprolol. J Mol Cell Cardiol. 2011;51:168–176. doi: 10.1016/j.yjmcc.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 23. Peschanski N, Harouki N, Soulie M, Lachaux M, Nicol L, Remy‐Jouet I, Henry JP, Dumesnil A, Renet S, Fougerousse F, et al. Transient heart rate reduction improves acute decompensated heart failure‐induced left ventricular and coronary dysfunction. ESC Hear Fail. 2021;8:1085–1095. doi: 10.1002/ehf2.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001;12:1079–1084. doi: 10.1681/ASN.V1251079 [DOI] [PubMed] [Google Scholar]
- 25. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gross ML, Ritz E. Hypertrophy and fibrosis in the cardiomyopathy of uremia—beyond coronary heart disease. Semin Dial. 2008;21:308–318. doi: 10.1111/j.1525-139X.2008.00454.x [DOI] [PubMed] [Google Scholar]
- 27. Hamzaoui M, Djerada Z, Brunel V, Mulder P, Richard V, Bellien J, Guerrot D. 5/6 nephrectomy induces different renal, cardiac and vascular consequences in 129/Sv and C57BL/6JRj mice. Sci Rep. 2020;10:1524. doi: 10.1038/s41598-020-58393-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adams V, Schauer A, Augstein A, Kirchhoff V, Draskowski R, Jannasch A, Goto K, Lyall G, Männel A, Barthel P, et al. Targeting MuRF1 by small molecules in a HFpEF rat model improves myocardial diastolic function and skeletal muscle contractility. J Cachexia Sarcopenia Muscle. 2022;13:1565–1581. doi: 10.1002/jcsm.12968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Winzer EB, Schauer A, Langner E, Augstein A, Goto K, Männel A, Barthel P, Jannasch A, Labeit S, Mangner N, et al. Empagliflozin preserves skeletal muscle function in a HFpEF rat model. Int J Mol Sci. 2022;23:10989. doi: 10.3390/ijms231910989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang D, Xu TT, Zhang SJ, Cai Y, Min SD, Zhao Z, Lu CQ, Wang YC, Ju S. Telmisartan ameliorates cardiac fibrosis and diastolic function in cardiorenal heart failure with preserved ejection fraction. Exp Biol Med (Maywood). 2021;246:2511–2521. doi: 10.1177/15353702211035058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sárközy M, Gáspár R, Zvara Á, Siska A, Kővári B, Szűcs G, Márványkövi F, Kovács MG, Diószegi P, Bodai L, et al. Chronic kidney disease induces left ventricular overexpression of the pro‐hypertrophic microRNA‐212. Sci Rep. 2019;9:1302. doi: 10.3389/fonc.2019.00598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jia G, Jia Y, Sowers JR. Role of mineralocorticoid receptor activation in cardiac diastolic dysfunction. Biochim Biophys Acta Mol basis Dis. 2017;1863:2012–2018. doi: 10.1016/j.bbadis.2016.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jia G, Habibi J, DeMarco VG, Martinez‐Lemus LA, Ma L, Whaley‐Connell AT, Aroor AR, Domeier TL, Zhu Y, Meininger GA, et al. Endothelial mineralocorticoid receptor deletion prevents diet‐induced cardiac diastolic dysfunction in females. Hypertens (Dallas, Tex 1979). 2015;66:1159–1167. doi: 10.1161/HYPERTENSIONAHA.115.06015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang WT, Lin YW, Chen CY, Chen ZC, Shih JY, Wu CC, Luo CY, Liu PY. Mineralocorticoid receptor antagonists mitigate mitral regurgitation‐induced myocardial dysfunction. Cells. 2022;11:2750. doi: 10.3390/cells11172750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bender SB, Demarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, Pulakat L, Aroor AR, Jaffe IZ, Sowers JR. Mineralocorticoid receptor antagonism treats obesity‐associated cardiac diastolic dysfunction. Hypertens (Dallas, Tex 1979). 2015;65:1082–1088. doi: 10.1161/HYPERTENSIONAHA.114.04912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138:929–944. doi: 10.1161/CIRCULATIONAHA.117.028814 [DOI] [PubMed] [Google Scholar]
- 37. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:10–1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 38. Imamura T, Oshima A, Narang N, Kinugawa K. Implication of mineralocorticoid receptor antagonist Esaxerenone in patients with heart failure with preserved ejection fraction. Circ Rep. 2021;3:660–665. doi: 10.1253/circrep.CR-21-0115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suzuki S, Motoki H, Kanzaki Y, Maruyama T, Hashizume N, Kozuka A, Yahikozawa K, Kuwahara K. Prognostic impact of mineralocorticoid receptor antagonist in patients with heart failure with preserved ejection fraction. ESC Hear Fail. 2020;7:2752–2761. doi: 10.1002/ehf2.12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kampourides N, Tziakas D, Chalikias G, Papazoglou D, Maltezos E, Symeonides D, Konstantinides S. Usefulness of matrix metalloproteinase‐9 plasma levels to identify patients with preserved left ventricular systolic function after acute myocardial infarction who could benefit from eplerenone. Am J Cardiol. 2012;110:1085–1091. doi: 10.1016/j.amjcard.2012.05.049 [DOI] [PubMed] [Google Scholar]
- 41. Mares A, Rodriguez T, Deoker A, Lehker A, Mukherjee D. Effect of mineralocorticoid receptor antagonists in heart failure with preserved ejection fraction and with reduced ejection fraction—a narrative review. Curr Vasc Pharmacol. 2022;20:46–51. doi: 10.2174/1570161119666210720120439 [DOI] [PubMed] [Google Scholar]
- 42. Wang CC, Lee AS, Liu SH, Chang KC, Shen MY, Chang CT. Spironolactone ameliorates endothelial dysfunction through inhibition of the AGE/RAGE axis in a chronic renal failure rat model. BMC Nephrol. 2019;20:351. doi: 10.1186/S12882-019-1534-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Böckmann I, Lischka J, Richter B, Deppe J, Rahn A, Fischer DC, Heineke J, Haffner D, Leifheit‐Nestler M. FGF23‐mediated activation of local RAAS promotes cardiac hypertrophy and fibrosis. Int J Mol Sci. 2019;20:4634. doi: 10.3390/ijms20184634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang Y, Ben Y, Li H, Xiong Y, Chen G, Hao J, Ma X, Gao X, Qiang P, Shimosawa T, et al. Eplerenone prevents cardiac fibrosis by inhibiting angiogenesis in unilateral urinary obstruction rats. J Renin‐Angiotensin‐Aldosterone Syst. 2022;1;1283729. doi: 10.1155/2022/1283729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. González‐Blázquez R, Somoza B, Gil‐Ortega M, Ramos MM, Ramiro‐Cortijo D, Vega‐Martín E, Schulz A, Ruilope LM, Kolkhof P, Kreutz R, et al. Finerenone attenuates endothelial dysfunction and albuminuria in a chronic kidney disease model by a reduction in oxidative stress. Front Pharmacol. 2018;9:1131. doi: 10.3389/fphar.2018.01131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Y, Wu J, He Q, Shou Z, Zhang P, Pen W, Zhu Y, Chen J. Angiotensin (1‐7) prevent heart dysfunction and left ventricular remodeling caused by renal dysfunction in 5/6 nephrectomy mice. Hypertens Res. 2009;32:369–374. doi: 10.1038/hr.2009.25 [DOI] [PubMed] [Google Scholar]
- 47. Prommer HU, Maurer J, Von Websky K, Freise C, Sommer K, Nasser H, Samapati R, Reglin B, Guimarães P, Pries AR, et al. Chronic kidney disease induces a systemic microangiopathy, tissue hypoxia and dysfunctional angiogenesis. Sci Rep. 2018;8:5317. doi: 10.1038/s41598-018-23663-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lagrange J, Li Z, Fassot C, Bourhim M, Louis H, Nguyen Dinh Cat A, Parlakian A, Wahl D, Lacolley P, Jaisser F, et al. Endothelial mineralocorticoid receptor activation enhances endothelial protein C receptor and decreases vascular thrombosis in mice. FASEB J. 2014;28:2062–2072. doi: 10.1096/fj.13-238188 [DOI] [PubMed] [Google Scholar]
- 49. Querfeld U, Mak RH, Pries AR. Microvascular disease in chronic kidney disease: the base of the iceberg in cardiovascular comorbidity. Clin Sci (Lond). 2020;134:1333–1356. doi: 10.1042/CS20200279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi‐Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol. 2011;300:F721–F733. doi: 10.1152/ajprenal.00546.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ehling J, Bábícková J, Gremse F, Klinkhammer BM, Baetke S, Knuechel R, Kiessling F, Floege J, Lammers T, Boor P. Quantitative micro‐computed tomography imaging of vascular dysfunction in progressive kidney diseases. J Am Soc Nephrol. 2016;27:520–532. doi: 10.1681/ASN.2015020204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 53. Kolkhof P, Lawatscheck R, Filippatos G, Bakris GL. Nonsteroidal mineralocorticoid receptor antagonism by finerenone‐translational aspects and clinical perspectives across multiple organ systems. Int J Mol Sci. 2022;23:9243. doi: 10.3390/ijms23169243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Droebner K, Pavkovic M, Grundmann M, Hartmann E, Goea L, Nordlohne J, Klar J, Eitner F, Kolkhof P. Direct blood pressure‐independent anti‐fibrotic effects by the selective nonsteroidal mineralocorticoid receptor antagonist finerenone in progressive models of kidney fibrosis. Am J Nephrol. 2021;52:588–601. doi: 10.1159/000518254 [DOI] [PubMed] [Google Scholar]
- 55. Kolkhof P, Hartmann E, Freyberger A, Pavkovic M, Mathar I, Sandner P, Droebner K, Joseph A, Hüser J, Eitner F. Effects of finerenone combined with empagliflozin in a model of hypertension‐induced end‐organ damage. Am J Nephrol. 2021;52:642–652. doi: 10.1159/000516213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grune J, Beyhoff N, Smeir E, Chudek R, Blumrich A, Ban Z, Brix S, Betz IR, Schupp M, Foryst‐Ludwig A, et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone's antifibrotic activity. Hypertens (Dallas, Tex 1979). 2018;71:599–608. doi: 10.1161/HYPERTENSIONAHA.117.10360 [DOI] [PubMed] [Google Scholar]
- 57. Lavall D, Jacobs N, Mahfoud F, Kolkhof P, Böhm M, Laufs U. The non‐steroidal mineralocorticoid receptor antagonist finerenone prevents cardiac fibrotic remodeling. Biochem Pharmacol. 2019;168:173–183. doi: 10.1016/j.bcp.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 58. Mulkareddy V, Racette SB, Coggan AR, Peterson LR. Dietary nitrate's effects on exercise performance in heart failure with reduced ejection fraction (HFrEF). Biochim Biophys Acta Mol basis Dis. 2019;1865:735–740. doi: 10.1016/j.bbadis.2018.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 2000;86:49–86. doi: 10.1016/S0163-7258(99)00072-8 [DOI] [PubMed] [Google Scholar]
- 60. Blanton RM. cGMP signaling and modulation in heart failure. J Cardiovasc Pharmacol. 2020;75:385–398. doi: 10.1097/FJC.0000000000000749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Verma A, Vaidya A, Subudhi S, Waikar SS. Aldosterone in chronic kidney disease and renal outcomes. Eur Heart J. 2022;43:3781–3791. doi: 10.1093/eurheartj/ehac352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsukamoto S, Morita R, Yamada T, Urate S, Azushima K, Uneda K, Kobayashi R, Kanaoka T, Wakui H, Tamura K. Cardiovascular and kidney outcomes of combination therapy with sodium‐glucose cotransporter‐2 inhibitors and mineralocorticoid receptor antagonists in patients with type 2 diabetes and chronic kidney disease: a systematic review and network meta‐analysis. Diabetes Res Clin Pract. 2022;194:110161. doi: 10.1016/j.diabres.2022.110161 [DOI] [PubMed] [Google Scholar]
- 63. Zhu Z, Rosenkranz KAT, Kusunoki Y, Li C, Klaus M, Gross O, Angelotti ML, Antonelli G, Cirillo L, Romagnani P, et al. Finerenone added to RAS/SGLT2 blockade for CKD in Alport syndrome. Results of a randomized controlled trial with Col4a3−/− mice. J Am Soc Nephrol. 2023;34:1513–1520. doi: 10.1681/ASN.0000000000000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bayer . A multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled study to evaluate the efficacy and safety of finerenone on morbidity and mortality in participants with heart failure (NYHA II‐IV) and left ventricular ejection fraction ≥40% (LVEF ≥40%). clinicaltrials.gov 2023. Report No: NCT04435626.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2


