Abstract
Introduction
Hypertension, the clinical condition of persistent high blood pressure (BP), is preventable yet remains a significant contributor to poor cardiovascular outcomes. Digital self-management support tools can increase patient self-care behaviours to improve BP. We created a patient-facing and provider-facing clinical decision support (CDS) application, called the Collaboration Oriented Approach to Controlling High BP (COACH), to integrate home BP data, guideline recommendations and patient-centred goals with primary care workflows. We leverage social cognitive theory principles to support enhanced engagement, shared decision-making and self-management support. This study aims to measure the effectiveness of the COACH intervention and evaluate its adoption as part of BP management.
Methods and analysis
The study design is a multisite, two-arm hybrid type III implementation randomised controlled trial set within primary care practices across three health systems. Randomised participants are adults with high BP for whom home BP monitoring is indicated. The intervention arm will receive COACH, a digital web-based intervention with effectively enhanced alerts and displays intended to drive engagement with BP lowering; the control arm will receive COACH without the alerts and a simple display. Outcome measures include BP lowering (primary) and self-efficacy (secondary). Implementation preplanning and postevaluation use the Consolidated Framework for Implementation Research and Reach-Effectiveness-Adoption-Implementation-Maintenance metrics with iterative cycles for qualitative integration into the trial and its quantitative evaluation. The trial analysis includes logistic regression and constrained longitudinal data analysis.
Ethics and dissemination
The trial is approved under a single IRB through the University of Missouri-Columbia, #2091483. Dissemination of the intervention specifications and results will be through open-source mechanisms.
Trial registration number
Keywords: Implementation Science, Hypertension, Primary Care, QUALITATIVE RESEARCH, Clinical Trial, Health informatics
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The study’s utilisation of participants solely from three academic health centres in the USA might restrict the applicability of the results to wider demographics.
The eligibility criteria’s exclusion of non-English speakers and those not enrolled in patient health portals might introduce selection bias, potentially limiting the sample’s representativeness.
The study employs a pragmatic trial design, which allows for the evaluation of interventions in real-world clinical settings, enhancing the generalisability of the findings to routine practice.
Statisticians, investigators and auditors collecting data are blinded to allocation status, reducing the risk of bias in outcome assessment.
The study design, including recruitment strategies, aligns with clinic preferences and involves both routine office visits and population-based identification, providing flexibility and maximising the potential participant pool.
Introduction
High blood pressure (BP) caused by essential hypertension is one of the most common conditions among adults in the USA).1 High BP alone rarely has significant symptoms, but sustained high BP, or hypertension, increases the risk of heart attack, stroke, heart and kidney failure.2 The likelihood of adverse cardiovascular outcomes begins to rise at low BP of 115/75 mm Hg, and for every 12-point increase in average BP, the risk doubles.3 In the USA, rates of uncontrolled BP continue to increase, with 46% of adults having stage 1 (130/80–139/89 mm Hg) or worse hypertension.1 Despite advancements in overall health outcomes, BP control has remained poor, with less than half of adults with hypertension meeting a goal of <140/90 mm Hg.1
Recent studies indicate that effectively managing BP involves navigating a narrow therapeutic window. Overly aggressive treatment increases the risk of significant comorbidities like kidney damage, hypotension and mood disorders.4 Evidence suggests that engaging patients directly in intensive goal setting shared care planning around non-pharmacological and pharmacological treatments, and self-monitoring for effectiveness and adverse events reduces the risk of cardiovascular events.5 Appropriately lowering BP without minimising adverse events is essential for optimising cardiovascular health and improving patient outcomes. Protocols for BP have remained largely driven by manual decision-making and existing clinical workflows, improving processes but not outcomes. Digital interventions involving Clinical Decision Support (CDS) systems can broaden efforts, but the complexity of high BP has led to mixed successes.6–10 Hicks et al 9 showed no significant difference in high BP management using a CDS intervention with providers while several other CDS trials using multidisciplinary, multifaceted interventions, often including patient engagement and support, have shown reductions in BP and improved control.6 7 10 11
An essential component of controlling high BP involves empowering patients to manage their condition themselves by regularly monitoring their BP at home and adjusting their approach based on frequent readings. Review of these home data by the patient’s healthcare team is an evidence-based component of hypertension management but has been historically difficult to integrate into the care team’s workflow.12 13 Personalisation of BP care plans based on patient needs and experience is also required.12 14–17 Patients are encouraged to change health behaviours such as limiting salt intake,18 losing weight,16 stopping smoking,16 adhering to pharmacological treatment plans and simultaneously self-monitoring for adverse effects. Engaging patients in a process to self-monitor and manage conditions has been extensively studied. Team-based interventions with consistent support for motivation, a focus on self-efficacy and consideration of affective, or emotional language, may be the key to enhancing engagement.19 However, uptake is limited, and these approaches are expensive.20 Digital interventions may be able to provide similar effects, but so far have had less success.21 This protocol describes a digital intervention study that combines motivational messages with education, counselling and support to increase patient BP knowledge and self-management capacity.13 22 Given our enhanced capability to provide patient-facing CDS23 and enhanced electronic care planning, integrating CDS thoughtfully into patients’ self-management routines is expected to improve their self-efficacy and improve control of chronic illness. Integration requires addressing traditional barriers to CDS integration addressing five rights—right person, right format, right time, right channel and right information—and avoiding reminder fatigue and enhancing motivation with digital interventions.19
This intervention is a patient-facing high BP CDS web-based digital tool known as the Collaboration Oriented Application for Controlling High BP (COACH). COACH uses the Fast Healthcare Interoperable Resource (FHIR) standard to incorporate eight extant national and international guidelines23 into standardised, interoperable CDS and uses the Agency for Healthcare Research and Quality (AHRQ) Patient-Centred CDS framework24 to engage patients, caregivers and care teams in a collaborative implementation process. The trial will implement COACH across multiple clinic sites spanning three major health systems and in the nation’s two leading electronic health record (EHR) vendor platforms: EPIC and Oracle. Our primary objective is to evaluate the effectiveness of the application at lowering BP via a randomised controlled trial (RCT) comparing two versions of COACH that provide reminders and displays with high affective content (enhanced COACH) versus low affective content (basic COACH) to test improved engagement and results. We will employ a mixed-methods design, with qualitative inquiry nested within the RCT, secondary Reach-Effectiveness-Adoption-Implementation-Maintenance (RE-AIM), and social cognitive theory (SCT) outcomes, and iterative qualitative evaluation of implementations across sites.
COACH was developed with a broad range of patient and clinician viewpoints by (1) incorporating input from patients and providers throughout the entire lifecycle of CDS25 26; (2) customising the COACH CDS to align with patient and care team preferences, values and objectives and (3) disseminating the open source application and underlying logic. The application is intended to be scalable through standard implementation frameworks, CDS artefacts and implementation guides27 that can be adopted beyond this protocol. For interoperability, we use a standard-based, structured process that re-uses concept and value sets from standard terminologies whenever possible while using robust techniques to develop new sets and make them available for future innovators.
Trial design
The COACH study is a patient randomised multisite, single-blind, hybrid type III implementation design28 pragmatic trial leveraging mixed methods29 using implementation science and informed by SCT to test the effectiveness of the enhanced COACH application versus basic COACH at lowering BP. The trial plans to enrol 550 participants who will be randomly assigned in a 1:1 ratio to the intervention or the control arm (275 per arm), stratifying by 3 enrolment sites. Outcomes will be collected from home BP measurements entered manually or via Bluetooth link into the COACH application by study participants and via electronic questionnaires completed by participants at baseline, 8 weeks (2 months) and 24 weeks (6 months).
Methods
Pragmatic design
The pragmatic trial aspects include broad inclusion criteria, no scheduled research visits, tailored workflows within clinic care teams, no clinical staff responsibilities to deliver the intervention and flexibility in delivery within each site. We employed the PRECIS-2 tool (Pragmatic Explanatory Continuum Indicator Summary) to compare the trial to routine care settings.30 Figure 1 highlights scores from 9 PRECIS-2 domains, where 1 is explanatory, idealised clinical trial conditions and 5 is pragmatic, closely matching routine care conditions. Eligibility (5) and recruitment (4): All patients with high BP seen in primary care in the last year will be eligible; these thresholds are standard for determining ongoing home BP monitoring. Setting (4): Primary care practices at the participating sites. Organisational impact (5): The trial will require no additional staff or modifications to usual care. Flexible delivery for the practice (5) and adherence for the patient (5): The delivery of the application aligns with standard practice for home BP monitoring. The intervention offers initial training for providers to orient them to referral and clinical workflows. Follow-up (4): There are no scheduled research visits. Most measurements (clinic encounters, BP data, events and messaging) will be gathered from the EHR. Some additional measures outside usual care (eg, self-efficacy) will be collected on remotely administered surveys. Measurement (4): Measurements will be part of routine care and the COACH application and will not require additional care team time or effort.
Figure 1.
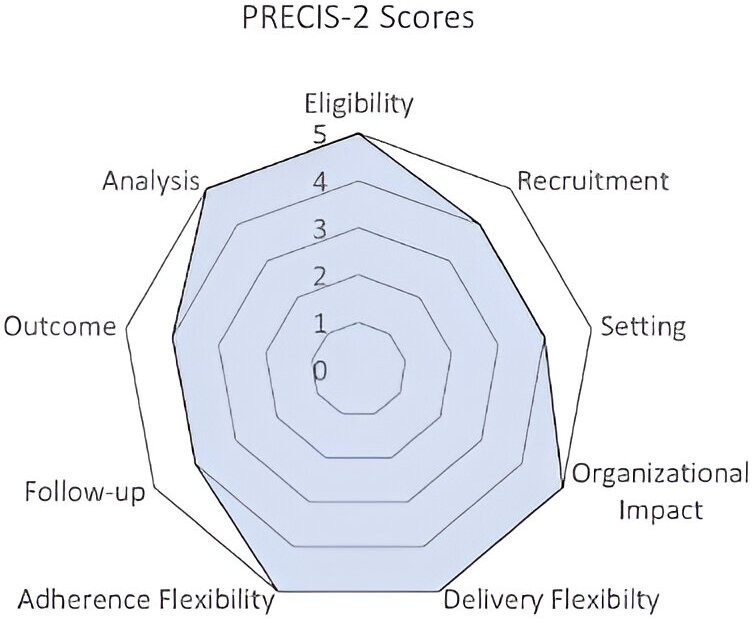
PRagmatic Explanatory Continuum Indicator Summary Tool-2 (PRECIS) diagram.
Preimplementation implementation science evaluation
In preparation for the trial, we conducted a pre-evaluation to explore implementation readiness at each intervention site, including patient perspectives (see online supplemental appendix 1, 2). We used a qualitative design and employed patient coinvestigators, informed by the Consolidation Framework for Implementation Research (CFIR)31 domains: innovation, outer setting, inner setting, individual and implementation process. Results from this evaluation included dozens of programming and implementation recommendations to improve COACH integration. The research and development teams made programming modifications to the COACH application and applied the implementation recommendations, where possible, to ensure the protocol was pragmatic. Implementation recommendations included referral, intervention design, safety monitoring, integration into care and ongoing monitoring. Programming changes included more guidance for patients, simpler text, more streamlined workflow and higher contrast display screens.
bmjopen-2024-085898supp001.pdf (128.6KB, pdf)
bmjopen-2024-085898supp002.pdf (89KB, pdf)
Patient involvement
Patient involvement was integral to the development of the COACH clinical trial, as our funded patient co-investigators (Co-Is) actively contributed to incorporating patient preferences. Through focus groups in the preimplementation phases, patient feedback refined the development process, enhancing the usability of the COACH app and facilitating smoother implementation. Co-Is also played a pivotal role in grant writing, offering essential insights into app usability and priorities. Their involvement extended to building recruitment materials and enhancing the COACH app. Continuously engaged, they shape plans for disseminating study results to linked communities, ensuring a patient-centred and inclusive approach throughout the study process, from planning dissemination to sharing findings.
Study setting
Participants are identified from primary care practices associated with three academic health centres (sites) in the USA. The three sites are Oregon Health & Science University (OHSU), University of Missouri-Columbia (MU) and Vanderbilt University Medical Center (VUMC). Participating primary care practices will include family medicine and internal medicine practices affiliated with each institution. Funding for the study spans from July 2022 to June 2025, with enrolment scheduled to begin in January 2024.
Eligibility criteria
Eligible participants are adults aged 18–100 years who communicate in English, receive care at a participating primary care clinic, enrolled in the site’s patient health portal and with a visit in the last year. The participant must have elevated BP, defined as a single BP of >140 (>135 home) systolic (SBP) or >90 (>85 home) diastolic at the current visit or the average of the last 4 BPs is >140 SBP or >90 diastolic and have a clinician recommendation for a home BP monitoring programme. Participants are excluded if they are pregnant at the time of consent, have severe cognitive impairment in the opinion of the clinician, are on hospice care and/or have a life expectancy of less than 2 years, have end-stage renal disease or for whom tight BP control presents a greater than average risk for falls, dizziness, electrolyte disturbances, hypotension or active heart failure or patient has any other disease or disorder that in the opinion of the investigator or the patient’s primary care clinician, could put participants at risk and affect trial results, or hinder participation will exclude them from participating. COACH includes participants with secondary hypertension as the main objective is to control hypertension, no matter what the cause.
Interventions
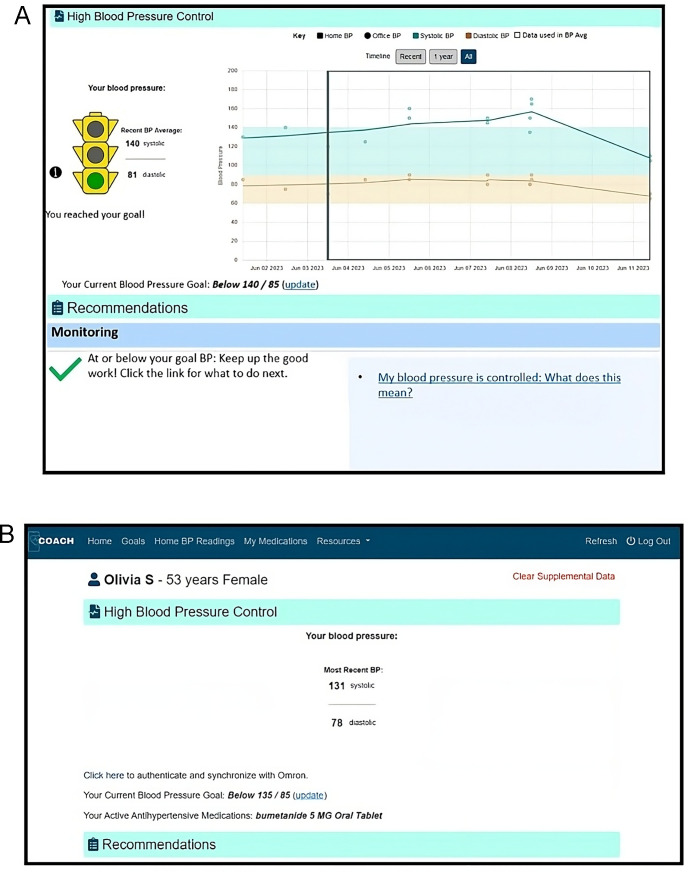
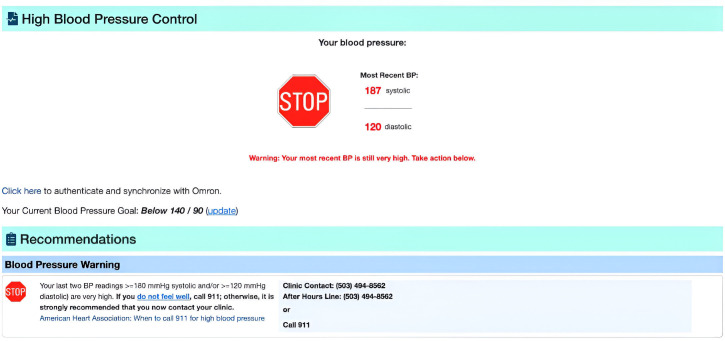
Intervention and control groups will have access to the COACH application: intervention will receive enhanced features (figure 2), including affective reminders and visualisations while the control group will receive simpler displays (figure 2). All groups will receive safety-related reminders. Reminders include screening, monitoring, self-management goal setting and prompts to discuss medications (intervention) and significantly high or low BP alerts and suspected adverse events (both groups; see figure 3). Participants in both groups receive a validated dual-channel, Bluetooth-enabled, home BP monitor (Omron 7 Series Wireless Upper Arm BP Monitor) with instructions for use.
Figure 2.
(A) COACH home page for enhanced arm. (B) COACH home page for control arm. COACH, Collaboration Oriented Approach to Controlling High.
Figure 3.
High BP warning (similar for low BP) for both groups. BP, blood pressure.
Outcomes
The primary outcome measure is BP control, defined as office <140/90 or home <135/85 average of the last set of BPs (defined as 12 home or 4 office, whichever are most recent) at 6 months as recorded by participants via home BP measurement and/or at scheduled clinic encounters. Control levels for home and office come from the American College of Cardiology and the American Heart Association (ACC/AHA) guidelines.5 Secondary outcomes include the average reduction in SBP and diastolic BP after 6 months from the initial BP measures at enrolment, evaluate demographic factors contributing to BP control and changes in key SCT measures using a health beliefs survey from baseline to 6 months.32 33 Technology acceptance and usability will be measured from the Unified Theory of Acceptance and Use of Technology (UTAUT) model.34 The UTAUT model was developed as an extension of the Technology Acceptance Model and is routinely used in health science research to understand factors associated with successful adoption and sustained use of mHealth interventions. UTAUT domains include performance expectancy (ie, the belief that using the system will be useful or create gains), effort expectancy (ie, the perception that the system is easy to use), social influence (ie, belief others think they should use the system) and facilitating conditions (ie, belief there is sufficient organisational and technical support to use the system).
RE-AIM outcomes: As part of the implementation evaluation, we will assess the RE-AIM metrics and concepts shown in table 1. Using the CFIR framework as a guideline, the evaluation will take a mixed-methods approach, with iterative cycles of qualitative and quantitative assessments to understand overall implementation successes and challenges. To measure Reach, the total primary care populations from each clinic will be compared with enrolled participants to understand differences in eligible and enrolled populations. Adoption will be measured via the COACH app for both patients and clinicians: log-ins, consistent BP tracking and interaction with the recommendations. For Implementation, semistructured interviews and a clinician survey will be used to understand the barriers and facilitators of using COACH in practice. Finally, Maintenance will assess the sustainability of the application through semistructured interviews.
Table 1.
RE-AIM measures and outcomes
| RE-AIM evaluation measures | ||
| Component | Outcome measures | Source |
| Reach | ||
| Number of eligible enrolled patients | CONSORT | REDCap—baseline survey |
| Differences from the eligible population | CONSORT | REDCap—baseline survey |
| Effectiveness | ||
| Intervention effects on outcome | Primary | Electronic health record, patient portal |
| Secondary | Electronic health record, patient portal | |
| Increase in patient: | Social cognitive theory | Patient 2/6 months follow-up survey |
| Adoption | ||
| Number of home BP recordings entered | Counts | Electronic health record, patient portal |
| Number of encountered study blocks | App usage | Electronic health record, patient portal |
| Implementation | ||
| Number of portal/phone messages about BP during 6-month intervention period | Electronic health record, patient portal | |
| Barriers to implementation | Interviews | CFIR evaluation |
| Increased/decreased burden of intervention | Interviews | CFIR evaluation |
| Physician/nurse/patient suggestions | Interviews | CFIR evaluation |
| Participant support needs | Email, phone outreach to study staff | REDCap ongoing events |
| Maintenance | ||
| Number of patients who continue to use the app and BP cuff | Study participant tracking | |
| Institutional use of tools beyond trial | CFIR Evaluation |
BP, blood pressure; CFIR, Consolidation Framework for Implementation Research; CONSORT, Consolidated Standards of Reporting Trials; RE-AIM, Reach-Effectiveness-Adoption-Implementation-Maintenance.
Participant timeline
Procedures for the study are in table 2. Consent is obtained through a REDCap e-consent module, and patient training is completed online with support by phone and email. Once enrolled and randomised, patients are placed in the monitoring block for 4 weeks or until they have 12 BPs. Reminders are provided through the monitoring block, with augmented reminders for the intervention arm. Once the monitoring block is over, patients are prompted to reflect on goals, including contacting their care team. This cycle repeats for up to 6 months. Patient surveys occur at baseline, week 8 and week 24; adverse event survey links are constantly available.
Table 2.
COACH study visit schedule
| Procedure | Baseline | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 8 | Week 24 |
| Information sheet, consent, screening, randomisation | X | |||||||
| Receive COACH-related Instructions/materials | X | |||||||
| Monitoring block (2–4 weeks) | If needed post 2 weeks | |||||||
| Goal setting block (1–2 weeks) | If monitoring block ends in 2 weeks | If monitoring block ends in more than 2 weeks | ||||||
| Health Belief Survey | X | |||||||
| Digital and Health Literacy | X | |||||||
| UTAUT | X | X | ||||||
| REDCap Alerts | ||||||||
| REDCap Alerts (Control) | ||||||||
| Adverse Event form* | X | X | ||||||
*AE form continuously available.
COACH, Collaboration Oriented Approach to Controlling High; UTAUT, Unified Theory of Acceptance and Use of Technology.
Sample size and power
The number of participants we plan to enrol and randomise is 550 across all three sites. We anticipate that 40% of participants will come from OHSU (n=225), 40% from MU (n=225) and 20% (n=100) from VUMC. Actual enrolment may differ, and enrolment will continue until 550 participants are randomised.
The total sample size was determined based on a test of two independent proportions (per cent with controlled BP at the end of the trial) assuming a level of significance equal to 0.05 (two sided) and power equal to 90%. We anticipate the intervention arm will increase from 0% controlled at baseline to 40% at 6 months while the control arm will increase from 0% to 25%. Under these assumptions, 406 evaluable participants are required, meaning those with complete data at the 6-month time point. We increased the total enrolment projection to account for attrition and/or uncertainty in projected changes.
Recruitment
Recruitment will take place at primary care practices affiliated with the three sites via two methods: population-based identification using registries and visit-based identification. Consistent with our pragmatic approach, individual sites and practices can prioritise the recruitment method that best suits their environment and resources. The target recruitment period is from January 2024 to June 2025.
Recruitment mechanisms:
Routine office visit (primary): Clinicians will identify a potential participant during a routine office visit where the patient’s BP would prompt pharmacological treatment according to the ACA/AHA guidelines. The clinician can recommend home BP monitoring and initiate a standard patient portal recruitment message with information and links to complete screening, consent and enrolment.
Population-based recruitment: Clinicians or team members identify empaneled, active individuals with high BP through an EHR-based report. The responsible clinician or authorised care team member will send patient portal messages in bulk to a set of selected patients, informing them of the study and providing links to complete screening, consent and enrolment. Population-based chronic condition identification and management are employed to varying degrees at each of our study sites.
Methods: assignment of interventions
Enrolment
After receiving an invitation to participate, patients are directed to a REDCap survey for study information. The patient reviews an information sheet and then begins the eligibility screening process, as defined above. Eligible participants must give informed consent (see online supplemental appendix 3) by signing an electronic form in REDCap, then proceed to the baseline survey.
bmjopen-2024-085898supp003.pdf (131.7KB, pdf)
Randomisation
On enrolment and after completing the baseline survey, participants are randomised with a stratification by site to the COACH enhanced intervention arm or COACH basic control arm by the central coordinating team at OHSU using the randomisation tool in REDCap. The randomisation scheme is stratified by the study site and implemented using a blocking strategy to ensure equal numbers of participants assigned to intervention and control arms within each site.
Allocation: concealment mechanism
The automated randomisation system in REDCap can obfuscate the allocation of all patients, and—other than stratification by site—does not depend on time or previous allocation.
Blinding (masking)
Statisticians, investigators and auditors collecting BP data from the EHR will be blinded to allocation status. Study participants will be told that the study is testing a home BP monitoring programme’s effectiveness, but not that it is comparing two care models. Clinicians and care team members will see the enhanced (or intervention) version of COACH. Patients will not be blinded since the COACH display is different for each arm. Once the trial is over, analysts will be provided data with an obfuscated study arm.
Methods: data collection, management and analysis
Data collection
We will use several techniques to gather data. First, COACH itself will gather data through FHIR connections to the EHR, pulling all relevant clinical information about the patient. COACH will track home BPs received through manual entry and electronic connections to the Omron mobile application. COACH will also store key information about use, including logins and interaction with recommendations. Second, all surveys will be captured through REDCap hosted at OHSU.33 35 REDCap is a secure, broadly used research survey tool that has been integrated with COACH. Standard surveys include the Health Belief Survey; Digital36 and Health Literacy37 and a modified UTAUT survey focused on COACH; the schedule is provided in table 2. Patient demographic collection and adverse events will be collected via REDCap. Finally, as part of implementation evaluation, we will gather qualitative data through clinic site visits, observation, focus groups and interviews; these data will be recorded and transcribed verbatim.
Data management
All tools used in the study have secure access to the underlying data with auditing capabilities for use; in this secure network, we will store all versions of the study data, and manage secure storage. In addition to extracting data directly from the EHR, patients will enter their self-reported data. For qualitative transcripts, the original recordings will be kept until study completion.
Qualitative data collection
During the implementation and trial, we will conduct bimonthly video calls with implementation sites. Implementation site-identified stakeholders and champions will be the participants in the calls. We will use a template approach to guide the call to ensure coverage of relevant topics, altering the template for the stage of implementation and known/evolving context and concerns. We will encourage all participants to voice unique concerns, allowing us to monitor implementation progress, identify barriers, troubleshoot problems and identify any new and unexpected uses of the tool, including both adverse and beneficial outcomes.
Quantitative analysis
The intervention effect on the primary outcome of BP control will be tested using a logistic regression model38 with a binary variable for the intervention arm and health system as a categorical variable,39 adjusting for baseline BP, as defined above.40 Rates of control in each arm and treatment differences will be calculated using mean predicted probabilities from the logistic model.41 Because randomisation is stratified by site, we will include this as a categorical variable for accurate variance estimates and will also adjust for baseline BP. Estimates of the probability of control in each arm and treatment differences will be calculated using mean predicted probabilities from the logistic model.
The secondary outcomes of reduction in SBP and diastolic BP after 6 months will be evaluated with a model sometimes described as constrained longitudinal data analysis, in which the two-time points are treated as panel data with an observation for each. The model will include a term for time (baseline/final) and an interaction term for the study arm, which constrains the arms to the same baseline mean as expected in a randomised trial but estimates different changes over time. A random effect for patients will be included for the correlation between baseline/final measurements. This model is statistically efficient and accommodates missing measurements, so it is a good fit for the intention-to-treat approach.
Continuous secondary outcomes, such as reduction in SBP and diastolic BP after 6 months, will be evaluated with a mixed-effects regression model38 in which the baseline and 6 months time points are treated as panel data with an observation for each. This model includes a term for time (baseline/final) and an interaction term for the study arm. Baseline means are thus constrained to be equal as expected in a randomised trial but changes over time differ. A random effect for patients will model the correlation between baseline/final measurements. This model is statistically efficient and accommodates missing measurements. No adjustments for multiplicity are planned because outcomes are prespecified and correlated.
Adherence
Participants will be included in the analysis once the application and/or home BP monitoring once randomised. Their use of the application will be encouraged by the intervention, but analysis will not depend on their use.
Qualitative analysis
After all site visits are complete, we will arrange the transcription of the audio using Rev.com. All recorded interviews will be transcribed, verbatim and deidentified for qualitative analysis using the web-based analysis software Dedoose V.9.42 Investigators will create case memos with the CFIR construct ratings for each site across all three health systems and identify CFIR constructs most relevant to the planned implementation across all sites. We will use the method described by Damschroder and Lowery43: (1) assign each site transcript to a pair of analysts who will each independently code the transcripts using the CFIR framework as a coding template with a deductive qualitative analytical approach, (2) develop/build-on case memo for each site, (3) large group discussion with investigators, (4) refine case memo, (5) large group assigns construct ratings and (6) case memo with construct ratings. In step 5, the large group will come to a consensus on CFIR construct ratings and score each case/clinic on the identified constructs. It is conventional to rate each CFIR construct from −2 (strong negative influence on implementation) to +2 (strong positive influence implementation), including 0 (neutral influence on implementation). This process will result in a high-level summary matrix of clinic implementation sites rated on multiple CFIR domains representing positive and negative influences of implementation influences across the three health systems.44
Data from bimonthly implementation calls will be analysed in the same manner and will be added to our existing preimplementation CFIR case memos to provide a rich description of the course of the implementation in each context, allowing comparison across sites, giving insights into both common themes across sites and context-specific differences. This robust synthesis and comparison of experiences across sites and EHR platforms will provide valuable system-level information to inform new implementations, emphasising common experiences and highlighting relevant context-specific facilitators and barriers. In addition to the traditional publication of findings, we will leverage the affiliations of our advisory group and the AHRQ CDS Connect Community as outlets for dissemination.
Data monitoring
The principal investigators (PIs) will be responsible for ensuring participants’ safety daily. In addition, the study has an empaneled a 12-member advisory board composed of national experts, including patient experts, to act as a data and safety monitoring board (DSMB) and to evaluate the progress of the study, including periodic assessments of data quality and timeliness, participant recruitment, accrual and retention, participant risk versus benefit, performance of trial sites and other factors that can affect study outcome. The DSMB will make recommendations to the funder and the PIs concerning the continuation, modification or conclusion of the trial. The DSMB will review the informed consent and make recommendations on any changes to the protocol. During the 18-month trial period, the DSMB will meet each quarter to review these data.
Harms
We used the Systolic Blood Pressure Intervention Trial (SPRINT)4 adverse events (hypotension, dizziness, ED visits, acute kidney injury) for our study adverse events and provided an alert for when these are detected in the EHR to the study team, the patient and the care team. Risks to participants include (1) known adverse events from increased treatment and lowered BP, (2) psychological harm and (3) loss of confidentiality. The adverse events are those common with medical and non-medical treatment of high BP and will be monitored by their primary care clinician.
Auditing
This study uses a single IRB through the University of Missouri-Columbia, #2091483.
Discussion
The Collaboration Oriented Approach to Controlling High BP (COACH) trial intends to understand how BP control can be improved by increasing adherence to patient-facing guideline-based recommendations. The focus of the trial is on home BP monitoring, as the starting recommendation underscores the key aspect of home-based efforts to assess and self-manage BP. The intervention also ‘closes the loop’ between patient home monitoring, and getting patient data to the care team in a way that provides actionable information for hypertension management designed to fit the care team preferred workflows and management goals. The intervention, an enhanced version of COACH that includes BP data visualisation, effectively enhanced visual summaries, and reminders about BP management, is based on core principles of SCT, including self-efficacy, social support, outcome expectations and self-regulation.32 45–49 The underlying premise is that higher affective alerting will increase accuracy in judgments about hypertension control and risk perception and motivate patients out of goal range to appropriate action, without creating excess anxiety, which will allow safe, efficient and effective behavioural and medication changes.32 50–52
There have been several other trials that address home BP monitoring. Bosworth et al 53 54 showed that nurse-based telephone support coupled with home BP monitoring was more effective for BP control than usual care or telephone support alone. This effect was strongest for non-white patients. However, individual team-based coaching support is expensive and challenging to scale. Mobile health applications have shown promise in providing this support for key populations, including those with high BP, showing a −4.1 mm Hg drop in SBP in a systematic review.20 These trials generally depend on revised systems of care to enhance patient engagement, with the digital tool a part of the overall intervention COACH is designed to be a scalable electronic patient-facing and care team-facing intervention that does not require additional personnel to implement.
This trial intends to test whether a more advanced, tailored digital intervention can overcome these barriers in a pragmatic way—in essence, with minimal care redesign. To accomplish this goal, we leverage SCT to drive improved patient engagement to measure BPs and accomplish goals for BP lowering. We incorporate these principles along with tailored messaging informed by decision psychology into the intervention to drive engagement and uptake.
We also adapted the trial design based on substantial preimplementation evaluation. To increase the likelihood of generalisable evidence from the trial, we performed a multimodal qualitative study. Results from the analysis were used to (1) offer ad hoc and population-based referral techniques that matched high BP quality improvement initiatives in the practices; (2) change the intervention itself to ensure care teams were getting the information they needed; (3) identify additional personnel that participate in BP management, including pharmacists, panel coordinators and medical assistants and (4) ensure we had good bidirectional communication with the practices. Our implementation blueprint incorporates these elements and will be useful for researchers looking to implement studies that interdigitate with clinic workflow and minimise burden while maximising benefit.
This trial is timely. Information exchange standards and regulations are advancing our ability to create highly functional digital interventions that can integrate directly into care. The 21st Century Cures Act and related regulations require healthcare organisations to release information to patients using relevant standards without extra effort. The COACH tool uses these standards (FHIR) to build a comprehensive and tailored tool that can incorporate specific patient contexts, overcoming previous gaps and enabling easier guidance. Our previous work showed that the available data through the EHR FHIR server was not sufficient to use standard guideline recommendations without adaptations needed to consider missing and inaccurate data24; these adaptations may help expand available data since we are exchanging data back to the EHR.
The results of this trial will help to understand how engagement with digital interventions can be enhanced with affective alerts and other design changes; and whether these changes can help lower BP in a pragmatic way through home monitoring. The flexibility of the tool, its adherence to standards and its incorporation into a carefully designed implementation blueprint to fit into the workflow will be helpful to future researchers and innovators. In addition, the explicit goal of placing minimal burden on care teams and engaging patients in achieving safe and effective BP control is likely to generate knowledge useful about how to optimally redesign primary care processes.
Supplementary Material
Footnotes
Collaborators: Emma Chase (Oregon Health & Science University, Portland, Oregon, USA); Amy Yates (Oregon Health & Science University, Portland, Oregon, USA); Michelle Bobo (Oregon Health & Science University, Portland, Oregon, USA); Donald Casey (Rush Medical College of Rush University, Chicago, Illinois, USA); Jonathan Soffer (Oregon Health & Science University, Portland, Oregon, USA); Guilherme Del Fiol (Department of Biomedical Informatics, University of Utah, Salt Lake City, Utah, USA); Katherine Putnam (Oregon Health & Science University, Portland, Oregon, USA); James Paul (University of Washington School of Medicine, Seattle, Washington, USA); Matthew Storer (Oregon Health & Science University, Portland, Oregon, USA); Sheila Markwardt (Oregon Health & Science University, Portland, Oregon, USA); Richelle Koopman (University of Missouri, Columbia, Missouri, USA).
Contributors: DD, RK, AR, SC, VS and WM collectively planned the study, collaborated on grant writing, and jointly drafted and reviewed this manuscript. VS conducted Qualtrics studies to gather feedback on the study components. EM and PG, acting as study coordinators at the Missouri site, facilitated study submissions, leveraging Missouri’s role as the single IRB. They played integral roles in manuscript drafting and review. AJG, the study coordinator at the Oregon site, contributed to the creation of site-specific documents and educational materials within the COACH application, in addition to drafting and reviewing the manuscript. LM established the REDCap survey database, significantly contributed to manuscript editing and serves as the program manager for the study. BJ and ML, serving as patient coinvestigators, provided valuable feedback on recruitment materials, the COACH interface, and overall study design. Authors affiliated with the COACH Consortium meet the authorship criteria established by the International Committee of Medical Journal Editors (ICMJE). EC provided project administration. AY contributed to software development, validation and visualisation. MB engaged in data curation, software development, supervision, validation and visualisation for the COACH application. DC supported the conceptualisation of the study, contributed to visualisation, writing and manuscript review and served as a member of the COACH advisory board. JS assisted with project administration at one of the clinics and provided resources essential for successful implementation. GDF contributed to COACH methodology, software development, manuscript writing and review and also serves on the COACH Advisory Board. KP aided in project administration at one of the clinics and participated in manuscript review. JP contributed to methodology, manuscript writing and review. MS served as an engineer on the COACH team, assisting with software development. SM served as the statistician, contributing to formal analysis, visualisation, initial manuscript drafting and review.
Funding: This study was funded by The Agency for Healthcare Research and Quality (AHRQ) R18HS028579.
Competing interests: No, there are no competing interests for any author.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: COACH Consortium, Emma Chase, Amy Yates, Michelle Bobo, Donald Casey, Jonathan R Soffer, Guilherme Del Fiol, Katherine Putnam, James Paul, Matthew B Storer, and Sheila Markwardt
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015-2016. NCHS Data Brief 2017;289:1–8. [PubMed] [Google Scholar]
- 2. Zonneveld TP, Richard E, Vergouwen MD, et al. Blood pressure-lowering treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of stroke or transient ischaemic attack. Cochrane Database Syst Rev 2018;7. 10.1002/14651858.CD007858.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13. 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 4. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the systolic blood pressure intervention trial (SPRINT). Clin Trials 2014;11:532–46. 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whelton PK. ACC/AHA/AAPA/ABC/ACPM/AGS/Apha/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart Association task force on clinical practice guidelines. Hypertension 2018;71:e13–115. 10.1161/HYP.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 6. Persson M, Mjörndal T, Carlberg B, et al. Evaluation of a computer-based decision support system for treatment of hypertension with drugs: retrospective, nonintervention testing of cost and guideline adherence. J Intern Med 2000;247:87–93. 10.1046/j.1365-2796.2000.00581.x [DOI] [PubMed] [Google Scholar]
- 7. Roumie CL, Elasy TA, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med 2006;145:165–75. 10.7326/0003-4819-145-3-200608010-00004 [DOI] [PubMed] [Google Scholar]
- 8. Bosworth HB, Olsen MK, McCant F, et al. Hypertension intervention nurse telemedicine study (HINTS): testing a multifactorial tailored behavioral/educational and a medication management intervention for blood pressure control. Am Heart J 2007;153:918–24. 10.1016/j.ahj.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 9. Hicks LS, Sequist TD, Ayanian JZ, et al. Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med 2008;23:429–41. 10.1007/s11606-007-0403-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anchala R, Kaptoge S, Pant H, et al. Evaluation of effectiveness and cost-effectiveness of a clinical decision support system in managing hypertension in resource constrained primary health care settings: results from a cluster randomized trial. J Am Heart Assoc 2015;4:e001213. 10.1161/JAHA.114.001213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rinfret S, Lussier M-T, Peirce A, et al. The impact of a multidisciplinary information technology-supported program on blood pressure control in primary care. Circ Cardiovasc Qual Outcomes 2009;2:170–7. 10.1161/CIRCOUTCOMES.108.823765 [DOI] [PubMed] [Google Scholar]
- 12. Fletcher BR, Hinton L, Hartmann-Boyce J, et al. Self-monitoring blood pressure in hypertension, patient and provider perspectives: a systematic review and thematic synthesis. Patient Educ Couns 2016;99:210–9. 10.1016/j.pec.2015.08.026 [DOI] [PubMed] [Google Scholar]
- 13. Glynn L, Casey M, Walsh J, et al. Patients' views and experiences of technology based self-management tools for the treatment of hypertension in the community: a qualitative study. BMC Fam Pract 2015;16:119. 10.1186/s12875-015-0333-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nair M, Ali MK, Ajay VS, et al. CARRS surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health 2012;12:701. 10.1186/1471-2458-12-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fix GM, Cohn ES, Solomon JL, et al. The role of Comorbidities in patients' hypertension self-management. Chronic Illn 2014;10:81–92. 10.1177/1742395313496591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrera PA, Moncada L, Defey D. Understanding non-adherence from the inside: hypertensive patients' motivations for adhering and not adhering. Qual Health Res 2017;27:1023–34. 10.1177/1049732316652529 [DOI] [PubMed] [Google Scholar]
- 17. Ma C. An investigation of factors influencing self-care behaviors in young and middle-aged adults with hypertension based on a health belief model. Heart Lung 2018;47:136–41. 10.1016/j.hrtlng.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 18. Ghimire S, Shrestha N, Callahan K. Barriers to dietary salt reduction among hypertensive patients. J Nepal Health Res Counc 2018;16:124–30. [PubMed] [Google Scholar]
- 19. Nahum-Shani I, Shaw SD, Carpenter SM, et al. Engagement in digital interventions. Am Psychol 2022;77:836–52. 10.1037/amp0000983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoong EC, Olazo K, Rivadeneira NA, et al. Mobile health strategies for blood pressure self-management in urban populations with digital barriers: systematic review and meta-analyses. NPJ Digit Med 2021;4:114. 10.1038/s41746-021-00486-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Omboni S, Ferrari R. The role of telemedicine in hypertension management: focus on blood pressure telemonitoring. Curr Hypertens Rep 2015;17:535. 10.1007/s11906-015-0535-3 [DOI] [PubMed] [Google Scholar]
- 22. Johnson RA, Huntley A, Hughes RA, et al. Interventions to support shared decision making for hypertension: a systematic review of controlled studies. Health Expect 2018;21:1191–207. 10.1111/hex.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. International HLS . FHIR® –Fast Healthcare Interoperability Resources 2019, Available: http://hl7.org/fhir
- 24. Dorr DA, D’Autremont C, Pizzimenti C, et al. Assessing data adequacy for high blood pressure clinical decision support: a quantitative analysis. Appl Clin Inform 2021;12:710–20. 10.1055/s-0041-1732401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dorr DA, Richardson JE, Bobo M, et al. Provider perspectives on patient- and provider-facing high blood pressure clinical decision support. Appl Clin Inform 2022;13:1131–40. 10.1055/a-1926-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dorr D, D’Autremont C, Richardson JE, et al. Patient-facing clinical decision support for high blood pressure control: patient survey. JMIR Cardio 2023;7:e39490. 10.2196/39490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. OHSU . OHSU hypertension implementation guide, Available: from: http://fhir.org/guides/ohsuhypertensionig/ImplementationGuide/hl7.fhir.us.ohsuhypertensionig [Accessed 5 Nov 2023].
- 28. Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012;50:217–26. 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogink PA, de Jong JM, Koeneman M, et al. Feasibility of a new cuffless device for ambulatory blood pressure measurement in patients with hypertension: mixed methods study. J Med Internet Res 2019;21:e11164. 10.2196/11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147. 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 31. Kirk MA, Kelley C, Yankey N, et al. A systematic review of the use of the consolidated framework for implementation research. Implement Sci 2016;11:72. 10.1186/s13012-016-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson ES, Winett RA, Wojcik JR. Social-cognitive determinants of nutrition behavior among supermarket food shoppers: a structural equation analysis. Health Psychol 2000;19:479–86. [PubMed] [Google Scholar]
- 33. Harris PA, Taylor R, Minor BL, et al. The redcap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rouidi M, Elouadi AE, Hamdoune A, et al. TAM-UTAUT and the acceptance of remote Healthcare Technologies by Healthcare professionals: a systematic review. Inform Med Unlocked 2022;32:101008. 10.1016/j.imu.2022.101008 [DOI] [Google Scholar]
- 35. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a Metadata-driven methodology and Workflow process for providing Translational research Informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nelson LA, Pennings JS, Sommer EC, et al. A 3-item measure of Digital health care literacy: development and validation study. JMIR Form Res 2022;6:e36043. 10.2196/36043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wallston KA, Cawthon C, McNaughton CD, et al. Psychometric properties of the brief health literacy screen in clinical practice. J Gen Intern Med 2014;29:119–26. 10.1007/s11606-013-2568-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu GF, Lu K, Mogg R, et al. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials Stat Med 2009;28:2509–30. 10.1002/sim.3639 [DOI] [PubMed] [Google Scholar]
- 39. Kernan WN, Viscoli CM, Makuch RW, et al. Stratified randomization for clinical trials. J Clin Epidemiol 1999;52:19–26. 10.1016/s0895-4356(98)00138-3 [DOI] [PubMed] [Google Scholar]
- 40. Pocock SJ, Assmann SE, Enos LE, et al. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 2002;21:2917–30. 10.1002/sim.1296 [DOI] [PubMed] [Google Scholar]
- 41. Kleinman LC, Norton EC. What’s the risk? a simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res 2009;44:288–302. 10.1111/j.1475-6773.2008.00900.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dedoose. 2022. Available: https://www.dedoose.com/
- 43. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci 2013;8:51. 10.1186/1748-5908-8-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koopman RJ, Canfield SM, Belden JL, et al. Home blood pressure data visualization for the management of hypertension: designing for patient and physician information needs. BMC Med Inform Decis Mak 2020;20:195. 10.1186/s12911-020-01194-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191–215. 10.1037//0033-295x.84.2.191 [DOI] [PubMed] [Google Scholar]
- 46. Bandura A. Self-efficacy_ the exercise of control. 1997. [Google Scholar]
- 47. Anderson ES, Winett RA, Wojcik JR, et al. Social cognitive mediators of change in a group randomized nutrition and physical activity intervention: social support, self-efficacy, outcome expectations and self-regulation in the guide-to-health trial. J Health Psychol 2010;15:21–32. 10.1177/1359105309342297 [DOI] [PubMed] [Google Scholar]
- 48. Ashford S, Edmunds J, French DP. What is the best way to change self-efficacy to promote lifestyle and recreational physical activity? A systematic review with meta-analysis. Br J Health Psychol 2010;15:265–88. 10.1348/135910709X461752 [DOI] [PubMed] [Google Scholar]
- 49. Tang MY, Smith DM, Mc Sharry J, et al. Behavior change techniques associated with changes in postintervention and maintained changes in self-efficacy for physical activity: a systematic review with meta-analysis. Ann Behav Med 2019;53:801–15. 10.1093/abm/kay090 [DOI] [PubMed] [Google Scholar]
- 50. Anderson ES, Winett RA, Wojcik JR. Self-regulation, self-efficacy, outcome expectations, and social support: social cognitive theory and nutrition behavior. Ann Behav Med 2007;34:304–12. 10.1007/BF02874555 [DOI] [PubMed] [Google Scholar]
- 51. Breaux-Shropshire TL, Brown KC, Pryor ER, et al. Relationship of blood pressure self-monitoring, medication adherence, self-efficacy, stage of change, and blood pressure control among municipal workers with hypertension. Workplace Health Saf 2012;60:303–11. 10.3928/21650799-20120625-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hacihasanoğlu R, Gözüm S. The effect of patient education and home monitoring on medication compliance, hypertension management, healthy lifestyle behaviours and BMI in a primary health care setting. J Clin Nurs 2011;20:692–705. 10.1111/j.1365-2702.2010.03534.x [DOI] [PubMed] [Google Scholar]
- 53. Bosworth HB, Olsen MK, Grubber JM, et al. Racial differences in two self-management hypertension interventions. Am J Med 2011;124:468. 10.1016/j.amjmed.2010.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jackson GL, Oddone EZ, Olsen MK, et al. Racial differences in the effect of a telephone-delivered hypertension disease management program. J Gen Intern Med 2012;27:1682–9. 10.1007/s11606-012-2138-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. CMP O. COACH GitHub Repository. 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2024-085898supp001.pdf (128.6KB, pdf)
bmjopen-2024-085898supp002.pdf (89KB, pdf)
bmjopen-2024-085898supp003.pdf (131.7KB, pdf)