Abstract
Background
Cellular adhesion molecules play an important role in the pathogenesis of ulcerative colitis, making selective blockade of these molecules a promising therapeutic strategy. Vedolizumab, a recombinant humanized IgG1 monoclonal antibody, inhibits adhesion and migration of leukocytes into the gastrointestinal tract by binding the alpha4beta7 integrin. Animal studies have suggested that vedolizumab may be a useful therapy for ulcerative colitis. This updated systematic review summarizes the current evidence on the use of vedolizumab for induction and maintenance of remission in ulcerative colitis.
Objectives
The primary objectives were to determine the efficacy and safety of vedolizumab used for induction and maintenance of remission in ulcerative colitis.
Search methods
A computer‐assisted search for relevant studies (inception to 15 June 2014) was performed using PubMed, MEDLINE, EMBASE and CENTRAL. References from published articles and conference proceedings were searched to identify additional citations.
Selection criteria
Randomized controlled trials comparing vedolizumab to placebo or a control therapy for induction or maintenance of remission in ulcerative colitis were included.
Data collection and analysis
Two authors independently extracted data and assessed the risk of bias for each trial. The primary outcomes were failure to induce clinical remission and relapse. Secondary outcomes included failure to induce a clinical response, failure to induce endoscopic remission, failure to induce an endoscopic response, quality of life, adverse events, serious adverse events and withdrawal due to adverse events. We calculated the relative risk (RR) and 95% confidence intervals (CI) for each outcome. Data were analyzed on an intention‐to‐treat basis. The overall quality of the evidence supporting the outcomes was evaluated using the GRADE criteria.
Main results
Four studies (606 patients) were included. All of the studies were rated as having a low risk of bias. Pooled analyses revealed that vedolizumab was significantly superior to placebo for induction of remission, clinical response, and endoscopic remission and prevention of relapse. After 4 to 6 weeks of therapy 77% (293/382) of vedolizumab patients failed to enter clinical remission compared to 92% (205/224) of placebo patients (RR 0.86, 95% CI 0.80 to 0.91; 4 studies 606 patients). After 6 weeks of therapy 48% of vedolizumab patients failed to have a clinical response compared to 72% of placebo patients (RR 0.68, 95% CI 0.59 to 0.78; 3 studies 601 patients). After 4 to 6 weeks of therapy 68% of vedolizumab patients failed to enter endoscopic remission compared to 81% of placebo patients (RR 0.82, 95% CI 0.75 to 0.91; 3 studies, b583 patients). After 52 weeks of therapy, 54% of vedolizumab patients had a clinical relapse compared to 84% of placebo patients (RR 0.67, 95% CI 0.59 to 0.77; 1 study, 373 patients). One small study (28 patients) found no statistically significant difference in endoscopic response (RR 1.00, 95% CI 0.62 to 1.61). GRADE analyses indicated that the overall quality of the evidence for the primary outcomes was high for induction of remission and moderate for relapse (due to sparse data 246 events). There was no statistically significant difference between vedolizumab and placebo in terms of the risk of any adverse event (RR 0.99, 95% CI 0.93 to 1.07), or serious adverse events (RR 1.01, 95% CI 0.72 to 1.42). There was a statistically significant difference in withdrawals due to adverse events. Six per cent of vedolizumab patients withdrew due to an adverse event compared to 11% of placebo patients (RR 0.55, 95% CI 0.35 to 0.87; 2 studies, 941 patients). Adverse events commonly reported across the studies included: worsening ulcerative colitis, headache, nasopharyngitis, upper respiratory tract infection, nausea, and abdominal pain.
Authors' conclusions
Moderate to high quality data from four studies shows that vedolizumab is superior to placebo for induction of clinical remission and response and endoscopic remission in patients with moderate to severely active ulcerative colitis and prevention of relapse in patients with quiescent ulcerative colitis. Moderate quality data from one study suggests that vedolizumab is superior to placebo for prevention of relapse in patients with quiescent ulcerative colitis. Adverse events appear to be similar to placebo. Future trials are needed to define the optimal dose, frequency of administration and long‐term efficacy and safety of vedolizumab used for induction and maintenance therapy of ulcerative colitis. Vedolizumab should be compared to other currently approved therapies for ulcerative colitis in these trials.
Keywords: Humans; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/adverse effects; Antibodies, Monoclonal, Humanized/therapeutic use; Colitis, Ulcerative; Colitis, Ulcerative/prevention & control; Colitis, Ulcerative/therapy; Induction Chemotherapy; Induction Chemotherapy/methods; Maintenance Chemotherapy; Maintenance Chemotherapy/methods; Randomized Controlled Trials as Topic; Secondary Prevention
Plain language summary
Vedolizumab for the treatment of active and inactive ulcerative colitis
Ulcerative colitis is a chronic inflammatory disease of the colon. Vedolizumab (formerly known as MLN‐02) is a synthetic antibody that blocks the adhesion and migration of white blood cells into the gut, reducing intestinal inflammation. This medication is given to patients intravenously. Four studies including 606 patients were included in this review. Pooled analysis of these trials revealed vedolizumab is significantly more effective than placebo (sham infusion) for inducing clinical remission and response (improvement of symptoms), as well as endoscopic remission (healing of inflamed mucosa in the colon) in patients with moderate to severely active ulcerative colitis. Evidence from one study suggests that vedolizumab is superior to placebo for preventing relapse (recurrence of active disease) in patients with ulcerative colitis in remission. Patients receiving vedolizumab were no more likely than placebo patients to experience side effects or serious side effects. Commonly reported side effects included: worsening ulcerative colitis, headache, nasopharyngitis (inflammation of nose and throat), upper respiratory tract infection, nausea, and abdominal pain. Further research is needed in order to define the optimal dose, frequency of drug administration and the long‐term effectiveness and safety of vedolizumab used for induction and maintenance of remission in ulcerative colitis. Vedolizumab should be compared to other currently approved therapies of ulcerative colitis in these studies.
Summary of findings
Summary of findings for the main comparison. Vedolizumab versus placebo for induction of remission in ulcerative colitis.
| Vedolizumab versus placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: Patients with moderately to severely active ulcerative colitis Settings: Outpatients Intervention: Vedolizumab versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vedolizumab versus placebo | |||||
| Failure to induce clinical remission | 915 per 10001 | 787 per 1000 (732 to 833) | RR 0.86 (0.80 to 0.91) | 606 (4 studies) | ⊕⊕⊕⊕ High | |
| Failure to induce clinical response | 720 per 10001 | 490 per 1000 (425 to 562) | RR 0.68 (0.59 to 0.78) | 601 (3 studies) | ⊕⊕⊕⊝ Moderate2 | |
| Failure to induce endoscopic remission | 809 per 10001 | 663 per 1000 (607 to 736) | RR 0.82 (0.75 to 0.91) | 583 (3 studies) | ⊕⊕⊕⊕ High | |
| Clinical relapse | 841 per 10001 | 563 per 1000 (496 to 648) | RR 0.67 (0.59 to 0.77) | 373 (1 study) | ⊕⊕⊕⊝ Moderate3 | |
| Endoscopic relapse | 802 per 10001 | 465 per 1000 (393 to 545) | RR 0.58 (0.49 to 0.68) | 373 (1 study) | ⊕⊕⊕⊝ Moderate4 | |
| Adverse events | 799 per 10001 | 791 per 1000 (743 to 855) | RR 0.99 (0.93 to 1.07) | 941 (2 studies)5 | ⊕⊕⊕⊕ High | |
| Serious adverse events | 121 per 10001 | 122 per 1000 (87 to 172) | RR 1.01 (0.72 to 1.42) | 1122 (3 studies)5 | ⊕⊕⊕⊝ Moderate6 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk estimates come from control arm of meta‐analysis, based on included trials. 2 Sparse data (342 events). 3 Sparse data (246 events). 4 Sparse data (215 events). 5 Includes full safety population from Feagan 2013 study (induction and maintenance phase). 6 Sparse data (136 events).
Background
Ulcerative colitis is a chronic idiopathic inflammatory disease of the colon that is characterized by abdominal pain and bloody diarrhea. Conventional treatments for patients with active disease may include 5‐aminosalicylates, corticosteroids, and azathioprine or 6‐mercaptopurine. Infliximab, a neutralizing mouse or human immunoglobulin (Ig)G1 chimeric anti‐human tumour necrosis factor monoclonal antibody, may be effective for patients with moderate to severely active disease who have an inadequate response to conventional therapy (Akobeng 2006; Kornbluth 2010). The calcineurin inhibitor cyclosporine may be effective for patients with severe steroid‐refractory disease (Shibolet 2005). Despite currently available treatment options, some patients will require colectomy because of intolerance or lack of response to medical therapy. Identification of other treatment strategies for ulcerative colitis is thus an important area of research.
Cellular adhesion molecules play an important role in the pathogenesis of intestinal inflammation, and have been studied as potential therapeutic targets in inflammatory bowel disease. One potential target is the alpha4beta7 integrin, a molecule found on circulating T lymphocytes that is involved in recruitment of leukocytes to the gastrointestinal tract (Erle 1994). The major ligand of alpha4beta7, mucosal adressin‐cell adhesion molecule‐1 (MAdCAM‐1), is selectively expressed on the endothelium of the intestinal vasculature and is present in increased concentrations in areas of intestinal inflammation. Studies evaluating natalizumab, a humanized IgG4 monoclonal antibody that leads to inhibition of the alpha4 integrin, found natalizumab to be effective for the induction and maintenance of remission of Crohn's disease (Sandborn 2005; MacDonald 2007; Targan 2007). However, reports of progressive multifocal leukoencephalopathy (PML) in patients taking natalizumab as part of multi‐drug therapy have raised concerns regarding the safety of broad inhibition of the alpha4 integrin (Yousry 2006; Bloomgren 2012). Therapies directed at alpha4beta7 integrin may be associated with a more selective inhibition of T cell homing, possibly resulting in less impairment of systemic immunity while reducing intestinal inflammation.
Vedolizumab is a humanized monoclonal IgG1 antibody specific for the alpha4beta7 integrin that does not cross‐react with the alpha4 monomer. Treatment with vedolizumab results in reduced intestinal inflammation in a cotton‐top tamarin model of colitis (Hesterberg 1996). Further study in healthy cynomolgus monkeys showed that vedolizumab has selective activity in its effects on gastrointestinal tissue. In the gut it brought changes in leukocytes and β lymphocytes; this was not seen in other organs (Fedyk 2012). Data from earlier dose‐ranging studies in humans suggested vedolizumab is safe and possibly effective in patients with ulcerative colitis (Feagan 2000). This systematic review and meta‐analysis summarizes the current evidence regarding the use of vedolizumab for the induction of remission in ulcerative colitis. This systematic review is an update of a previously published Cochrane review (Behm 2009).
Objectives
The primary objective was to determine the efficacy and safety of vedolizumab used for induction and maintenance of remission in ulcerative colitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials were considered for inclusion.
Types of participants
Adult patients (>18 years of age) with active or quiescent ulcerative colitis as defined by conventional clinical, histological or endoscopic criteria were considered for inclusion.
Types of interventions
Trials where vedolizumab was compared to placebo or a control medication were considered for inclusion.
Types of outcome measures
The primary outcome measures were the proportion of patients who failed to achieve clinical remission and the proportion of patients who relapsed as defined by the included studies. Secondary outcomes included the proportion of patients who failed to have a clinical response, failure to enter endoscopic remission, failure to have an endoscopic response, endoscopic relapse, disease‐specific quality of life, adverse events, withdrawal due to adverse events and serious adverse events.
Search methods for identification of studies
A computer‐assisted search of PubMed (1966 to 15 June 2014), MEDLINE (1966 to 15 June 2014), EMBASE (1980 to 15 June 2014), and CENTRAL (Cochrane Library, Issue 6, 2014) was performed. The search strategies are reported in Appendix 1.
Furthermore, two authors (DJT and JKM) hand‐searched the articles cited in each full text publication obtained. Manual searches to identify key conference abstracts from 1999 onwards were performed [i.e. Gastroenterology (American Gastroenterological Association); Gut (British Society of Gastroenterology); American Journal of Gastroenterology (American College of Gastroenterology)]. References from published articles were searched to identify additional citations. Unpublished data from on‐going trials were identified by correspondence with authors and experts in the field.
Data collection and analysis
Selection criteria
Publications identified by the search strategy were independently assessed by two authors (DJT and JKM). Relevant studies were selected according to the inclusion criteria.
Data collection and analysis
Both authors independently reviewed the full texts independently and assessed the eligibility of the trials based on the inclusion criteria above. The methodological quality of the included studies was assessed using the Cochrane risk of bias tool (Higgins 2011). This tool involves a number of factors:
Sequence generation (i.e. method of randomisation);
Allocation sequence concealment;
Appropriate blinding;
Incomplete outcome reporting (i.e. the investigators had determined methods of dealing with attrition)
Selective outcome reporting (i.e. the investigators reported all a priori outcomes)
Other sources of bias (i.e. anything else in the study that could have increased the risk of bias)
The studies were judged to be of high, low or unclear risk of bias based on these factors.
We used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) criteria to assess the overall quality of evidence used for specific outcomes in this review. Evidence from randomized controlled trials begin as high quality evidence. They can then be downgraded due to: (1) risk of bias from the studies, (2) indirect evidence, (3) inconsistency (unexplained heterogeneity), (4) imprecision in data, and (5) publication bias. The overall quality of evidence for each outcome was determined and classified as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); and very low quality (i.e. we are very uncertain about the estimate) (Guyatt 2008, Schünemann 2011).
Disagreement among authors regarding risk of bias was resolved by consensus. In future updates of this review publication bias will be assessed by examining the authors and institutions involved, journal of publication, funding sources, and the affiliation of authors with manufacturers.
Two authors (DJT and JKM) separately extracted data and results from relevant studies. The extracted data included patient demographics such as age, disease distribution, disease duration, and concomitant medications, the type of disease activity scoring instrument used, treatment and control modalities, the number of subjects randomized into each treatment group, the number of subjects maintaining remission and stopping steroids, the duration of treatment and follow up, and the number of subjects lost to follow up. The corresponding author was contacted in cases where data were not available from the published reports.
Statistical analysis
Data were analyzed using Review Manager (RevMan 5.3). All data were analyzed on an intention‐to‐treat basis. The results were expressed as the risk ratio (RR) and 95% confidence intervals (CI) for dichotomous outcomes. The risk ratio was calculated using a fixed‐effect model. The definitions of clinical remission, clinical response, and endoscopic response were set by the authors of the included studies.
Results
Description of studies
The literature search conducted on 15 June 2014 identified 103 records. Six additional studies were identified through searching of conference abstracts. After duplicates were removed, a total of 82 studies remained for review of titles and abstracts. Two authors (DJT and JKM) independently reviewed the titles and abstracts of these trials and 32 studies were selected for full text review (see Figure 1). Twelve reports of seven studies were excluded (See: Characteristics of excluded studies). Twenty reports of four studies met the pre‐defined inclusion criteria and were included in the review (Feagan 2000; Feagan 2005; Parikh 2012; Feagan 2013).
1.
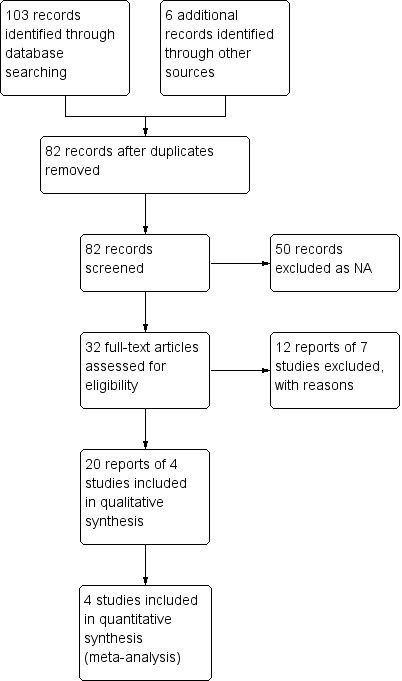
Study flow diagram.
Feagan 2000 was a safety and efficacy evaluation of vedolizumab. It was a randomized, double blind trial where 29 patients with moderately‐severe ulcerative colitis received a single dose of vedolizumab at various doses or a placebo and were followed for 30 days. One patient was excluded before randomization for not meeting inclusion criteria. The inclusion criteria included: a minimum score of five on the Mayo Clinic score, greater than or equal to three bowel movements per day and signs of active disease on endoscopy. Outcomes after 30 days were final Mayo Clinic scores, clinical remission (Mayo Clinic score of zero) and endoscopic response (two grade improvement in modified Baron score) (Feagan 2000).
Feagan 2005, enrolled 181 adult patients with active ulcerative colitis from 20 centers. The study duration was six weeks. Disease activity was quantified using the ulcerative colitis clinical score (Feagan 2000), and the modified Baron score (Baron 1964). Active disease was defined as an ulcerative colitis clinical score of 5 to 9 points, with a score of at least 1 on either stool frequency or rectal bleeding, and a modified Baron score of at least 2 on sigmoidoscopic examination with active disease extending a minimum of 25 cm from the anal verge. Exclusion criteria included patients with current or recent topical therapy, corticosteroids, or immunosuppressives. A total of 181 patients were randomized to vedolizumab at doses of 0.5 mg/kg (n = 58) or 2 mg/kg (n = 60), or identical placebo (n = 63). Patients received study drug or placebo intravenously on day 1 and 29. The primary study outcome was the proportion of patients in clinical remission at week six, defined as an ulcerative colitis clinical score of zero or one and a modified Baron score of zero or one with no evidence of rectal bleeding. Secondary outcomes included the proportion of patients with a clinical response (defined as an improvement of three points or more on the ulcerative colitis clinical score), endoscopic remission and adverse events. Disease‐related quality of life was assessed by the inflammatory bowel disease questionnaire (IBDQ).
Parikh 2012 randomised 47 patients from 11 different centers. The study duration was 253 days. Patients received either vedolizumab at a dose of 2 mg/kg (n = 13), 6 mg/kg (n = 14) or 10 mg/kg (n = 11); or placebo (n = 9). Study medications were given on days 1, 15, 29 and 85. The primary goal of the study was to assess the clinical pharmacology and safety of a new formulation of vedolizumab, with efficacy (i.e. remission and response) being a secondary outcome. This new formulation was made with a substance produced in Chinese hamster ovary. Pharmacological evaluation included measuring serum vedolizumab concentrations and receptor saturation. Immunogenicity was evaluated by measuring the level of human antihuman antibodies. Evaluation of safety involved monitoring for any adverse events, with particular attention to PML. Contrary to Feagan 2005, this study did not involve endoscopic evaluation and used the partial Mayo score (Lewis 2008), as a measure of baseline disease state, remission and response. Remission was defined as a partial Mayo score of two or less with no subscore greater than one. Remission was only reported in a post‐hoc subgroup of patients with active disease (partial Mayo score of four to seven). Response was defined as a decrease of two or more and greater than 25% decrease in the partial Mayo score compared to baseline, along with a decrease in the rectal bleeding subscore of one or greater or an absolute subscore of zero or one for rectal bleeding. Endoscopic remission was not investigated in this study, however fecal calprotectin was used as a surrogate marker of intestinal inflammation (Parikh 2012).
Feagan 2013 randomised 374 patients to receive a 300 mg intravenous dose of vedolizumab (n = 225) or placebo (n = 149). The patients were then followed for 52 weeks. There were two phases to the trial: induction (weeks 1 to 6) and maintenance (weeks 7 to 52). The primary outcome was clinical response defined as a decrease in Mayo score by three or more and a 30% decrease from the baseline score and a decrease in the rectal bleeding subscore of one or greater or an absolute rectal bleeding subscore of one or less. Secondary outcomes included clinical remission (Mayo score of less than two and no subscale scores greater than one) and mucosal healing (Mayo endoscopic subscore of less than or equal to one) (Feagan 2013).
Risk of bias in included studies
The risk of bias assessment is summarized in Figure 2. The studies were of high methodological quality. All four were randomised trials. The sequence generation technique was computer‐generated in all four studies. All four studies utilized a centralized randomization technique and were rated as low risk of bias for allocation concealment. All four studies were double‐blind, and used identical placebo and were rated as low risk of bias for blinding. All four studies used adequate methods to deal with missing data and were rated as low risk for incomplete outcome data. All of the studies reported results for all a priori outcomes and were rated as low risk for selective reporting. All four studies were rated as low risk for other sources of bias.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Effectiveness of vedolizumab
A pooled analysis of four studies (n = 606 patients) showed a statistically significant difference in clinical remission rates favouring vedolizumab over placebo (Feagan 2000; Feagan 2005; Parikh 2012; Feagan 2013). After an induction phase of four to six weeks of therapy 77% (293/382) of vedolizumab patients failed to enter clinical remission compared to 92% (205/224) of placebo patients (RR 0.86, 95% CI 0.80 to 0.91). No significant heterogeneity was detected for this comparison (P = 0.57, I2 = 0%). A GRADE analysis indicated that the overall quality of the evidence for the primary outcome (failure to achieve clinical remission) was high (See Table 1).
A pooled analysis of three studies (n = 601 patients) showed a statistically significant difference in clinical response rates favouring vedolizumab over placebo (Feagan 2005; Parikh 2012; Feagan 2013). After 6 weeks of treatment 48% (183/380) of vedolizumab patients failed to have a clinical response compared to 72% (159/221) of placebo patients (RR 0.68, 95% CI 0.59 to 0.78). No significant heterogeneity was detected for this comparison (P = 0.64, I2 = 0%). A GRADE analysis indicated that the overall quality of the evidence for this outcome was moderate due to sparse data (342 events, See Table 1).
The pooled analysis examining endoscopic remission included three studies and 583 patients. It showed a statistically significant difference in endoscopic remission rates favouring vedolizumab over placebo (Feagan 2000; Feagan 2005; Feagan 2013). Sixty‐eight per cent (246/363) of vedolizumab patients failed to achieve endoscopic remission compared to 81% (178/220) of placebo patients (RR 0.82, 95% CI 0.75 to 0.91). No significant heterogeneity was detected for this comparison (P = 0.27, I2 = 24%). A GRADE analysis indicated that the overall quality of the evidence for this outcome was high (See Table 1). Feagan 2000 (n = 28 patients) reported endoscopic response as an outcome. No statistically significant difference in failure to achieve endoscopic response was found between vedolizumab and placebo patients. In both groups 75% of patients failed to have an endoscopic response (RR 1.00; 95% CI 0.62 to 1.61).
Feagan 2005 reported significantly higher IBDQ scores in patients receiving vedolizumab compared to placebo. The mean IBDQ score was 171.5 in the vedolizumab group compared to 162.5 in placebo patients (P = 0.03).
Feagan 2013 reported on clinical and endoscopic relapse at 52 weeks (n = 373 patients) as outcomes. There was a statistically significant difference in clinical relapse rates favouring vedolizumab over placebo. Fifty‐seven per cent (140/247) of vedolizumab patients relapsed by 52 weeks follow‐up compared to 84% (106/126) of placebo patients (RR 0.67, 95% CI 0.59 to 0.77). A GRADE analysis indicated that the overall quality of the evidence for the primary outcome (clinical relapse) was moderate due to sparse data (246 events, See Table 1). There was a statistically significant difference in endoscopic relapse rates favouring vedolizumab over placebo. Forty‐six per cent (114/247) of vedolizumab patients relapsed endoscopically by 52 weeks follow‐up compared to 80% (101/126) of placebo patients (RR 0.58, 95% CI 0.49 to 0.68). A GRADE analysis indicated that the overall quality of the evidence for this outcome was moderate due to sparse data (215 events, See Table 1).
Two studies evaluated the development of clinically relevant titers of human antihuman antibodies in study patients, which is associated with a decrease in the efficacy of vedolizumab (Feagan 2005; Parikh 2012). Feagan 2005 reported that 44% (52/188) of vedolizumab patients developed detectable human antihuman antibodies at week 8, compared to 11% (4/37) of vedolizumab patients in the Parikh 2012 study. Furthermore, Feagan 2005 reported that 24% (28/118) of patients with high antibody titers (greater than 1:125) had a remission rate similar to placebo patients (12% versus 14% respectively). In these patients the alpha4beta7 binding site was observed to be unsaturated on CD4+CD45RO+ T cells, which could have affected the efficacy of vedolizumab. One of the primary differences between the vedolizumab formulations used in Feagan 2005 and Parikh 2012 was the cell line used to produce the antibody. Feagan 2005 used a NS0 mouse myeloma cell line, whereas Parikh 2012 used a Chinese hamster ovary (CHO) cell‐based system. Parikh 2012 used this CHO cell‐derived vedolizumab in an attempt to reduce the level of human antihuman antibodies. Comparing the two studies, it would appear that patients who received NSO mouse myeloma cell‐derived vedolizumab had a significantly higher chance of developing human antihuman antibodies compared to patients who received CHO‐cell derived vedolizumab (RR 4.08, 95% CI 1.58 to 10.51).
Pooled data from Feagan 2013 and Sandborn 2013 (both ulcerative colitis and Crohn's patients) indicates that concomitant immunosuppressive therapy was associated with decreased immunogenicity in patients who were randomized to placebo for maintenance therapy. Human antihuman antibodies were detected in 3% (1/32) of patients who received concomitant immunosuppressive therapy compared to 18% (44/247) of patients who did not.
Adverse Events
Adverse events occurred in similar numbers of patients treated with vedolizumab and placebo. Two studies reported the proportion of patients who experienced at least one adverse event (Parikh 2012; Feagan 2013). Parikh 2012 collected safety data up to day 253. Safety data from the Feagan 2013 study included the full safety population for both the induction (6 weeks) and maintenance phases (52 weeks, n = 895). A pooled analysis (n = 941 patients) found no statistically significant difference in the incidence of adverse events between vedolizumab and placebo patients. Seventy‐nine per cent (520/657) of vedolizumab patients experienced at least one adverse event compared to 80% (227/284) of placebo patients (RR 0.99, 95% CI 0.93 to 1.07). A GRADE analysis for this outcome showed the overall quality of evidence was high (See Table 1).
Two of the studies reported on withdrawals due to adverse events as an outcome (Feagan 2013; Parikh 2012). Parikh 2012 reported that no patients withdrew due to adverse events. A pooled analysis (2 studies, 941 patients) showed that significantly fewer vedolizumab patients withdrew due to adverse events compared to placebo patients. Six per cent of vedolizumab patients withdrew due to adverse events compared to 11% of placebo patients (RR 0.55, 95% CI 0.35 to 0.87).
A pooled analysis of three studies (Feagan 2005; Parikh 2012; Feagan 2013), including 1122 patients showed that the likelihood of serious adverse events was not significantly increased by the administration of vedolizumab. Twelve per cent (97/775) of vedolizumab patients had a serious adverse event compared to 12% (43/347) of placebo patients (RR 1.02, 95% CI 0.73 to 1.42). A GRADE analysis indicated that the overall quality of the evidence for this outcome was moderate due to sparse data (136 events, See Table 1). Serious adverse events reported in the Feagan 2005 study included exacerbation of colitis, angioedema with an infusion reaction, infection and degenerative disk disease. Parikh 2012 reported two serious adverse events in the vedolizumab group including compression fractures of the thoracic vertebrae in one patient and gastroduodenitis in another patient.
The most commonly reported adverse event in the Feagan 2000 study was headache. Commonly reported adverse events in the Feagan 2005 study included: worsening ulcerative colitis, nausea, headache, frequent bowel movements, fatigue, nasopharyngitis and abdominal pain. In the Feagan 2005 study three notable adverse events occurred in the setting of vedolizumab administration: one patient developed an infusion reaction with hives and angioedema, one patient developed a primary CMV infection, and one patient developed postoperative pneumonia after spine surgery. The patient that developed the infusion reaction had high anti‐vedolizumab antibody titers (1:3125). Commonly reported adverse events in the Parikh 2012 study included: headache, worsening ulcerative colitis, upper respiratory tract infection and nasopharyngitis. Parikh 2012 reported two incidents of pyrexia, but no allergic reactions to the medication. No systemic opportunistic infections or neoplasms were reported (Parikh 2012). Commonly reported adverse events in the Feagan 2013 study included: worsening ulcerative colitis, headache, nasopharyngitis, arthralgia, upper respiratory tract infection, nausea, cough, anemia, abdominal pain, fatigue and influenza. There were no reports of PML in any of the four studies (Feagan 2000; Feagan 2005; Feagan 2013; Parikh 2012). This is substantial finding as this has been observed in other biologic therapies against integrins (e.g. natalizumab) (Parikh 2012).
Discussion
The results of this meta‐analysis show that vedolizumab is significantly superior to placebo for induction of clinical remission, clinical improvement, and endoscopic remission and prevention of clinical relapse and endoscopic relapse in ulcerative colitis. Although vedolizumab did not appear to have any significant effect on endoscopic response (RR 1.00, 95% CI 0.24 to 4.14), only one small inadequately powered study assessed this outcome (Feagan 2000). Furthermore, vedolizumab was not significantly associated with an increased rate of adverse events or serious adverse events relative to placebo. Withdrawal due to adverse events was significantly more likely in placebo patients than vedolizumab patients. This may be due to higher rates of worsening ulcerative colitis in placebo treated patients.
We believe that the methodological basis for these conclusions is sound. The Cochrane risk of bias tool was used to assess the quality of four included trials and the possibility of bias was judged to be low for these studies. Furthermore, the primary outcome failure to induce clinical remission was rated as 'high' using the GRADE criteria indicating that further research is unlikely to change our confidence in the point estimate of effect. The primary outcome relapse was rated as 'moderate' using the GRADE criteria indicating that further research may change the point estimate of effect. The adverse event outcomes data (any adverse event and serious adverse events) were rated as 'moderate'. Further evaluation, particularly long‐term data is necessary to elucidate the risk of adverse events associated with the use of vedolizumab. These findings are summarized in the Table 1.
Future research should consider comparing vedolizumab to other active treatments (e.g. infliximab or natalizumab), as it is difficult to choose between two therapies without a direct comparison of efficacy and adverse events. There have been indirectly observed benefits of vedolizumab over previous less selective alpha4 integrin blockers (e.g. natalizumab). Natalizumab has been associated with the development of PML, a rare infection of the brain caused by reactivated JC virus (Bloomgren 2012). PML is normally seen in immunocompromised patients (Bloomgren 2012). Bloomgren 2012 investigated the risk of PML among patients receiving natalizumab and reported a total of 212 cases of PML among 99,571 patients receiving natalizumab (2.1 cases per 1000 patients). Forty‐six of these patients (22%) died. Bloomgren 2012 identified three risk factors for the development of PML: prior use of immunosuppressants, increased length of therapy (especially beyond two years), and presence of anti‐JC virus antibodies. It is important to note that of the identified cases who had samples available for analysis for these antibodies (n = 54), all of the patients tested positive for anti‐JC virus antibodies. Risk stratification by these factors is applicable for treating patients with natalizumab, as outlined by Bloomgren 2012, and may prove to be helpful in vedolizumab if any cases of PML are identified in vedolizumab treated patients.
Vedolizumab is more selective than previous generations of integrin antagonists, blocking alpha4beta7 integrin specifically. Alpha4beta7 integrin is found on T and B cells and binds to mucosal addressin cell adhesion molecule‐1 (MAd‐CAM‐1) which is expressed primarily on the vascular endothelial cells of the intestines (Parikh 2012). Concerns over the potential for vedolizumab‐associated PML prompted Parikh 2012 to have a special protocol for any patients displaying symptoms of PML. However, there were no reported cases of PML in any of the studies included in this review (Feagan 2000; Feagan 2005; Parikh 2012; Feagan 2013). Vedolizumab likely has little or no risk of PML as a result of its selectivity, as lymphocyte trafficking to the central nervous system is not impaired. At present testing for anti‐JC virus antibodies in patients receiving vedolizumab does not appear to be helpful.
The development of human antihuman antibodies has been reported in patients treated with vedolizumab. Human antihuman antibodies can interfere with drug efficacy. For example, Feagan 2005 reported that 24% of patients had high titers of human antihuman antibodies (>1:125) and these patients had a clinical remission rate similar to the placebo group. Forty‐four per cent of patients who received vedolizumab in this study developed detectable human antihuman antibodies (Feagan 2005). One way of addressing this issue was to change the formulation of vedolizumab from a NS0 mouse myeloma cell line (Feagan 2005), to a Chinese hamster ovary (CHO) cell system (Parikh 2012). Parikh 2012 reported the development of human antihuman antibodies in only 11% of patients who received CHO cell‐derived vedolizumab. We compared the two formulations and it appears that patients who received NSO mouse myeloma cell‐derived vedolizumab had a significantly higher chance of developing human antihuman antibodies compared to patients who received CHO‐cell derived vedolizumab (RR 4.08, 95% CI 1.58 to 10.51). Thus the CHO cell‐derived vedolizumab may be more effective than the NSO formulation. The Feagan 2013 study used a CHO‐cell derived formulation of vedolizumab. Pooled data from the Feagan 2013 and Sandborn 2013 studies (both ulcerative colitis and Crohn's patients) suggests that combination therapy with an immunosuppressive may prevent the formation of human antihuman antibodies. However, this result needs to be confirmed by future research.
In conclusion, vedolizumab has been shown to be significantly more efficacious than placebo. Furthermore, the current CHO cell‐derived version of vedolizumab appears to have a lower incidence of human antihuman antibodies, which should be more efficacious than the previous NS0 cell‐derived version. To date, no cases of PML have been reported in vedolizumab treated patients and the incidence of other adverse events was not statistically different between vedolizumab and placebo. The results of the GRADE analysis indicate that overall quality of the evidence supporting the efficacy and safety of vedolizumab for treatment of active and quiescent ulcerative colitis is moderate to high. Future studies should compare vedolizumab to other active therapies for induction and maintenance of remission in ulcerative colitis.
Authors' conclusions
Implications for practice.
There is now moderate to high quality evidence from four randomized controlled trials that vedolizumab is more effective than placebo for induction of clinical remission and response and endoscopic remission in patients with moderately to severely active ulcerative colitis. Moderate quality data from one study suggests that vedolizumab is superior to placebo for prevention of relapse in patients with quiescent ulcerative colitis. Various doses of vedolizumab were used in these trials. Feagan 2000 observed the greatest rate of endoscopic response in those receiving 0.5 mg/kg. Similarly, Feagan 2005 observed the greatest decrease in ulcerative colitis clinical score using the same dose. Parikh 2012 reported the greatest effect for a 6 mg/kg dose at a similar time point. The Feagan 2013 study used a 300 mg dose.
Implications for research.
Biologic therapies now play a central role in the management of inflammatory bowel diseases. The approval of natalizumab for Crohn's disease can be expected to encourage further development of therapies that target adhesion and integrin mechanisms. The data for vedolizumab are positive and should encourage further investigation. Data from larger trials or databases are needed to confirm the long‐term safety and efficacy of vedolizumab as induction and maintenance therapy for patients with ulcerative colitis. The optimal dose and frequency of administration of vedolizumab needs to be further defined. Ideally, future research should also include active comparators. These trials should include additional patient populations, including patients who are dependent on or refractory to corticosteroids.
In addition, although immunogenicity seems to be decreased with the use of the CHO‐derived vedolizumab, further investigations into whether immunogenicity can be further lowered by dosing strategies or by the use of concomitant immunosuppressives should be explored. Furthermore, the efficacy of combination therapy (i.e. vedolizumab and immunosuppressives) will need to be assessed in future trials. Given that immunosuppressive use has been identified as a risk factor for PML, it seems prudent to use the risk factor stratification outlined by Bloomgren 2012 in the design of such studies.
What's new
| Date | Event | Description |
|---|---|---|
| 16 June 2014 | New search has been performed | New literature searches conducted on June 15, 2014. New studies added. |
| 16 June 2014 | New citation required and conclusions have changed | Substantively updated review with new conclusions and authors. |
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. Search strategies
PubMed (1966 to 15 June 2014)
#1. (singl* OR doubl* OR tripl* OR trebl* OR blind* OR mask* OR placebo* OR single‐blind* OR double‐blind* OR triple‐blind* OR random* OR (controlled clinical)) #2. ((ulcerat* AND colitis) OR proctocolitis OR proctosigmoiditis OR rectocolitis OR rectosigmoiditis OR proctitis) #3. (MLN‐02 OR MLN02 OR (MLN 02) OR LDP‐02 OR LDP02 OR (LDP 02) OR MLN0002 OR MLN‐0002 OR (MLN 0002) OR LDP0002 OR LDP‐0002 OR (LDP 0002) OR vedolizumab) #4. (anti‐alpha4* OR (anti alpha4*) OR antialpha4* OR (alpha4beta7 antibod*)) #5. (#1 AND #2) #6. (#3 OR #4) #7. (#5 AND #6)
EMBASE + EMBASE Classic (1947 – 15 June 2014)
1. random$.tw. 2. factorial$.tw. 3. (crossover$ or cross over$ or cross‐over$).tw. 4. placebo$.tw. 5. single blind.mp. 6. double blind.mp. 7. triple blind.mp. 8. (singl$ adj blind$).tw. 9. (double$ adj blind$).tw. 10. (tripl$ adj blind$).tw. 11 assign$.tw.. 12. allocat$.tw. 13. crossover procedure/ 14. double blind procedure/ 15. single blind procedure/ 16. triple blind procedure/ 17. randomized controlled trial/ 18. or/1‐17 19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 20. 18 not 19 21. ulcerative colitis.mp. or exp ulcerative colitis/ 22. (proctocolitis or proctosigmoiditis or rectocolitis or rectosigmoiditis or proctitis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 23. 21 or 22 24. 20 and 23 25. vedolizumab.mp. or exp vedolizumab/ 26. (MLN‐02 or MLN02 or "MLN 02" or LDP‐02 or LDP02 or "LDP 02" or MLN0002 or MLN‐0002 or "MLN 0002" or LDP0002 or LDP‐0002 or "LDP 0002").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 27. (anti‐alpha4* or "anti alpha4*" or antialpha4* or "alpha4beta7 antibod*").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 28. 25 or 26 or 27 29. 24 and 28
MEDLINE (1946 – 15 June 2014)
1. random$.tw. 2. factorial$.tw. 3. (crossover$ or cross over$ or cross‐over$).tw. 4. placebo$.tw. 5. single blind.mp. 6. double blind.mp. 7. triple blind.mp. 8. (singl$ adj blind$).tw. 9. (double$ adj blind$).tw. 10. (tripl$ adj blind$).tw. 11 assign$.tw.. 12. allocat$.tw. 13. crossover procedure/ 14. double blind procedure/ 15. single blind procedure/ 16. triple blind procedure/ 17. randomized controlled trial/ 18. or/1‐17 19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 20. 18 not 19 21. ulcerative colitis.mp. or exp ulcerative colitis/ 22. (proctocolitis or proctosigmoiditis or rectocolitis or rectosigmoiditis or proctitis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 23. 21 or 22 24. 20 and 23 25. vedolizumab.mp. or exp vedolizumab/ 26. (MLN‐02 or MLN02 or "MLN 02" or LDP‐02 or LDP02 or "LDP 02" or MLN0002 or MLN‐0002 or "MLN 0002" or LDP0002 or LDP‐0002 or "LDP 0002").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 27. (anti‐alpha4* or "anti alpha4*" or antialpha4* or "alpha4beta7 antibod*").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 28. 25 or 26 or 27 29. 24 and 28
CENTRAL (15 June 2014)
#1. (ulcerat* and colitis) or proctocolitis or proctosigmoiditis or rectocolitis or rectosigmoiditis or proctitis #2. MLN‐02 or MLN02 or (MLN 02) or LDP‐02 or LDP02 or (LDP 02) or MLN0002 or MLN‐0002 or (MLN 0002) or LDP0002 or LDP‐0002 or (LDP 0002) or vedolizumab #3. anti‐alpha4* or (anti alpha4*) or antialpha4* or (alpha4beta7 antibod*) #4. #2 or #3 #5. #1 and #4
SR‐IBD (15 June 2014)
“MLN*” “OR LDP*” OR “vedo*” OR “alpha4* OR “anti‐alpha*” OR antialpha*
Data and analyses
Comparison 1. Vedolizumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to induce clinical remission | 4 | 606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.80, 0.91] |
| 2 Failure to induce clinical response | 3 | 601 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.59, 0.78] |
| 3 Failure to induce endoscopic remission | 3 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.75, 0.91] |
| 4 Failure to induce endoscopic response | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.62, 1.61] |
| 5 Clinical relapse at 52 weeks | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.59, 0.77] |
| 6 Endoscopic relapse at 52 weeks | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.49, 0.68] |
| 7 Adverse Events | 2 | 941 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.07] |
| 8 Withdrawal due to adverse events | 2 | 941 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.35, 0.87] |
| 9 Serious Adverse Events | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.73, 1.42] |
1.1. Analysis.
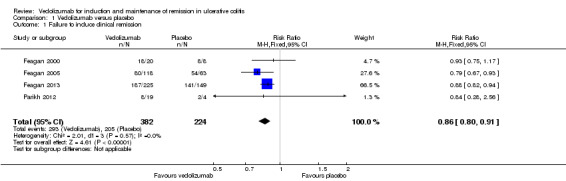
Comparison 1 Vedolizumab versus placebo, Outcome 1 Failure to induce clinical remission.
1.2. Analysis.

Comparison 1 Vedolizumab versus placebo, Outcome 2 Failure to induce clinical response.
1.3. Analysis.
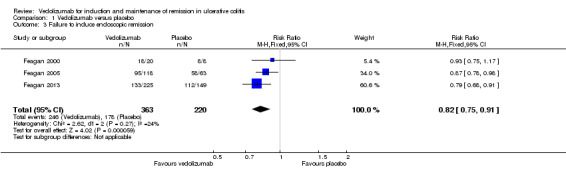
Comparison 1 Vedolizumab versus placebo, Outcome 3 Failure to induce endoscopic remission.
1.4. Analysis.
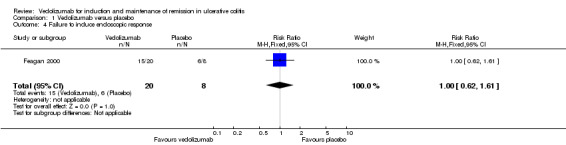
Comparison 1 Vedolizumab versus placebo, Outcome 4 Failure to induce endoscopic response.
1.5. Analysis.
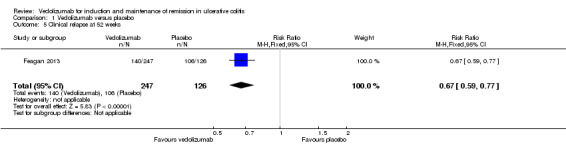
Comparison 1 Vedolizumab versus placebo, Outcome 5 Clinical relapse at 52 weeks.
1.6. Analysis.

Comparison 1 Vedolizumab versus placebo, Outcome 6 Endoscopic relapse at 52 weeks.
1.7. Analysis.
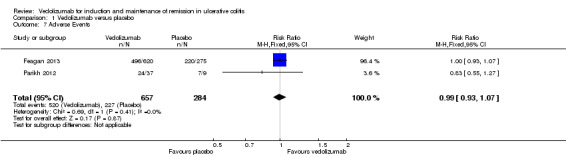
Comparison 1 Vedolizumab versus placebo, Outcome 7 Adverse Events.
1.8. Analysis.
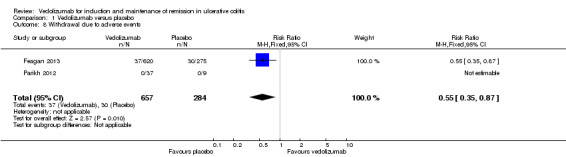
Comparison 1 Vedolizumab versus placebo, Outcome 8 Withdrawal due to adverse events.
1.9. Analysis.
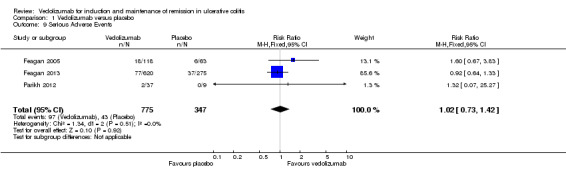
Comparison 1 Vedolizumab versus placebo, Outcome 9 Serious Adverse Events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Feagan 2000.
| Methods | Randomized, double‐blind, placebo controlled trial involving one administration of placebo or vedolizumab at various doses. Evaluated 30 days after administration of study medication. | |
| Participants | 29 patients (17M/12F) with diagnosed ulcerative colitis (moderately severe as defined by a minimum Mayo Clinical score of ≥5, ≥3 bowel movements per day and evidence of active disease on endoscopy). One patient was withdrawn for not meeting inclusion criteria. |
|
| Interventions | Vedolizumab 0.15mg/kg SC (n=5), vedolizumab 0.15mg/kg IV (n=5), vedolizumab 0.5mg/kg IV (n=5), vedolizumab 2.0mg/kg IV (n=5) or placebo (n=8) | |
| Outcomes | Endoscopic response (measured by a modified Baron score), Mayo Clinical score and adverse events | |
| Notes | Available as an abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other issues |
Feagan 2005.
| Methods | Randomised, double‐blind, placebo‐controlled, 8 week induction trial involving 20 centers. Computer‐generated block randomization schedule. | |
| Participants | 181 adult subjects (98M/83F) with moderately active UC (ulcerative colitis clinical score 5 to 9, with either stool frequency or rectal bleeding score at least 1, and modified Baron score of at least 2, with disease minimum 25cm from anal verge). Exclusion criteria: oral corticosteroids within 4 weeks, topical mesalamine or corticosteroids within 1 week, immunosuppressive therapy within 3 months, severe disease, abnormal WBC, platelet, AST, ALT, or creatinine, positive stool test for infectious pathogens, proteinuria. | |
| Interventions | MLN02 0.5mg/kg (n=58), MLN02 2mg/kg (n=60), or placebo (n=63) intravenous infusion on day 1 and day 29. | |
| Outcomes | Primary outcome measure: Clinical remission at week 6, defined as an ulcerative colitis clinical score of 0 or 1 and a modified Baron score of 0 or 1 with no evidence of rectal bleeding. Secondary outcome measures: Changes in ulcerative colitis clinical scores, Riley scores, and IBDQ scores. Proportion of subjects with clinical response (defined as a decrease of 3 or more on the Mayo score) at week 4 and 6, endoscopic remission (defined as a modified Baron score of 0) at week 4 and 6, endoscopic response (defined as a 2 or more grade improvement in the modified Baron score) at week 4 and 6. Patients were evaluated at baseline and one, two, four, and six weeks after randomization. Sigmoidoscopy was performed at weeks 0, 4 and 6. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a computer generated schedule" Comment: The sequence was randomised |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Neither the investigators nor the patients were aware of the treatment assignment. The placebo was identical in appearance to MLN02." Comment: Blinding of assessors and participants was adequate. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates in the groups were 2%, 8% and 5% for the MLN02 0.5mg/kg, MLN02 2.0mg/kg and placebo groups. Quote: "No important differences were observed among the three groups in the reasons for withdrawal." |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported. |
| Other bias | Low risk | No other apparent sources of bias. |
Feagan 2013.
| Methods | Randomised, double‐blind, placebo‐controlled trial, induction (6 weeks) and maintenance (46 weeks) trial Participants were randomised to induction or placebo at week 0, then randomised again at week 6 to placebo or maintenance vedolizumab every four or eight weeks |
|
| Participants | 404 subjects (242M/162F) between the ages of 18 and 80 years with Mayo scores of six or greater and an endoscopic subscore of two or greater despite treatment with one or more of: corticosteroids, purine antimetabolites and TNFα antagonists Exlusion criteria: previous treatment with rituximab, natalizumab, efalizumab, VDZ or precursors; TNFα antagonist use within 60 days; use of cyclosporine, thalidomide and other investigational therapies; patients with toxic megacolon, ostomy, increased risk of infection, abdominal abscess, prior colonic resection, anticipated need for major surgery, colonic stricture, laboratory abnormalities, colonic dysplasia or adenoma, or diagnosed malignant neoplasm, |
|
| Interventions | Induction phase: VDZ 300 mg intravenous (n=225) or placebo (n=149). Maintenance phase: VDZ maintenance dose every 8 weeks (n=122), VDZ maintenance dose every 4 weeks (n=125), or placebo (n=126). |
|
| Outcomes | Primary outcomes: clinical response (defined as a decrease in Mayo score by three or more and a 30% decrease and a decrease in the rectal bleeding subscore of one or greater or an absolute rectal bleeding subscore of one or less) measured at week six and week 52. Secondary outcomes: clinical remission (defined as Mayo score of two or less and no subscores greater than one) and mucosal healing (defined as a Mayo endoscopic subscore of one or less) after week six, durability of the clinical responses and remissions (defined as the endpoint being meet at weeks six and 52), mucosal healing (defined as Mayo endoscopic subscore of one or zero) at week 52, corticosteroid‐free remission at week 52. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was computer‐generated" |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Neither the investigators nor the patients were aware of the treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of subjects who withdrew during the induction phase were 14 and 7 in the placebo and VDZ groups respectively |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
Parikh 2012.
| Methods | Randomised trial comparing different doses of VDZ (2, 6 or 10 mg/kg) to placebo given on days 1, 15, 29 and 85 involving 11 centers. Outcomes were measured at multiple time points between day 1 and 253. | |
| Participants | 47 subjects (20M/27F) between the ages of 18 and 70 with a confirmed diagnosis of UC for at least two years and a partial Mayo score of 1‐7. The subjects were not excluded if the were on stable doses of one or more of: oral 5‐aminosalicyclates, corticosteroids, purine antimetabolites or methotrexate. Exlusion criteria: "anticipation of surgery;hemoglobin <10 g/dL; treatment with cyclosporine, tacrolimus or infliximab within 60 days of enrollment; dysplasia or colorectal cancer on most recent surveillance colonoscopy; colostomy, presence of fistulae or known fixed symptomatic intestinal stenosis; evidence of intestinal infection within four weeks of initial screening; toxic megacolon; history of hepatitis B, hepatitis C, or human immunodeficiency virus (HIV); recurrent or uncontrolled infections; active or latent tuberculosis (TB); or history of major neurological disease or neurological findings upon screening that could potentially confound the safety monitoring." One subject was found to be ineligible after randomisation but before administration of the therapy and removed from analysis. |
|
| Interventions | VDZ 2 mg/kg (n=13), VDZ 6 mg/kg (n=14), VDZ 10 mg/kg (n=11) or placebo (n=9) | |
| Outcomes | Primary outcomes: pharmacokinetics, pharmacodynamics, immunogenicity and safety Secondary outcomes: efficacy measured by partial Mayo score. "Clinical remission defined as partial Mayo score of two or less with no subscore greater than one." "Clinical response was defined as partial Mayo score decreased by two or more points and greater than or equal to 25% with decrease subscore for rectal bleeding of one or more points or absolute subscore for rectal bleeding of 0 or one." Fecal calprotectin levels were measured as an assessment of intestinal inflammation. |
|
| Notes | NCT01177228 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Centralized randomization |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind (protocol described on clinicaltrials.gov) Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition during the study included one subject from the placebo group and the VDZ 2 mg/kg group |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other apparent sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Colombel 2013a | Not a RCT ‐ open label extension study |
| Colombel 2013b | Not a RCT ‐ pooled safety analysis |
| Feagan 2009 | Not a RCT ‐ pooled safety analysis |
| Parikh 2011a | Not a RCT ‐ pooled safety analysis |
| Parikh 2011b | Not a RCT ‐ open label extension study |
| Parikh 2013 | Not a RCT ‐ open label extension study |
| Wyant 2013 | RCT in healthy subjects |
Declarations of interest
Stephen Bickston's institution has received grant funding from Janssen for the PREVENT clinical trial. This activity is outside of the submitted work.
Brian Behm's institution received funding to be a site for the GEMINI clinical trials.
Brian Feagan is an author of three manuscripts that were included in this review. He has received fee(s) from Abbott/AbbVie, Amgen, Astra Zeneca, Avaxia Biologics Inc., Bristol‐Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Ferring, JnJ/Janssen, Merck, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Takeda, Teva, Tillotts Pharma AG, UCB Pharma for Board membership; fee(s) from Abbott/AbbVie, Actogenix, Albireo Pharma, Amgen, Astra Zeneca, Avaxia Biologics Inc., Axcan, Baxter Healthcare Corp., Boehringer‐Ingelheim, Bristol‐Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Ironwood Pharma, Janssen Biotech (Centocor), JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Merck, Millennium, Nektar, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Protagonist, Receptos, Salix Pharma, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Takeda, Teva Pharma, Tillotts, UCB Pharma, Vertex Pharma, Warner‐Chilcott, Wyeth, Zealand, and Zyngenia for consultancy; and lecture fee(s) from: Abbott/AbbVie, JnJ/Janssen, Takeda, Warner‐Chilcott, and UCB Pharma.
Reena Khanna has received fee(s) from AbbVie for Board membership and fee(s) from Takeda for consultancy. All of these activities are outside the submitted work.
The other authors have no known declarations of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Feagan 2000 {published data only}
- Feagan B, McDonald JWD, Greenberg G, Wild G, Pare P, Fedorak RN, et al. An ascending dose trial of a humanised a4b7 antibody in ulcerative colitis. Gastroenterology 2000;118(4 Suppl 2):A874. [Google Scholar]
Feagan 2005 {published data only}
- Feagan BG, Greenberg G, Wild G, McDonald JW, Fedorak R, Pare P, et al. Efficacy and safety of a humanized A4B7 antibody in active Crohn's disease (CD). Gastroenterology 2003;124(4 Suppl 1):A25‐6. [Google Scholar]
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. New England Journal of Medicine 2005;352(24):2499‐507. [DOI] [PubMed] [Google Scholar]
Feagan 2013 {published data only}
- Feagan B, Colombel JF, Rubin D, Mody R, Sankoh S, Lasch K. Improvements in health‐related quality of life in patients with ulcerative colitis treated with vedolizumab. Journal of Crohn's and Colitis 2014;8:S51‐2. [Google Scholar]
- Feagan B, Rutgeerts P, Sands B, Sandborn W, Colombel JF, Hanauer S. Vedolizumab maintenance therapy for ulcerative colitis: Results of gemini I, a randomized, placebo‐controlled, double‐blind, multicenter phase 3 trial. American Journal of Gastroenterology 2012;107:S609‐10. [Google Scholar]
- Feagan B, Sandborn WJ, Smyth M, Sankoh S, Parikh A, Fox I. Effects of continued vedolizumab therapy for ulcerative colitis in week 6 induction therapy nonresponders. Journal of Crohn's and Colitis 2014;8:S276‐7. [Google Scholar]
- Feagan B, Sands B, Sankoh S, Milch C, Fox I. Efficacy of vedolizumab in ulcerative colitis by prior treatment failure in GEMINI I, a randomised, placebo‐controlled, double‐blind, multi‐centre trial. Journal of Crohn's and Colitis 2013;7:S216. [Google Scholar]
- Feagan B, Sands B, Sankoh S, Milch C, Fox I. Efficacy of vedolizumab in ulcerative colitis by prior treatment failure in gemini i, a randomized, placebo‐controlled, double‐blind, multicenter trial. Inflammatory Bowel Diseases 2012;18:S1‐2. [Google Scholar]
- Feagan BG, Colombel JF, Rubin DT, Mody R, Sankoh S, Lasch K. Health‐related quality of life in patients with ulcerative colitis after treatment with vedolizumab: Results from the GEMINI 1 study. Gastroenterology 2014;146(5 Suppl 1):S590. [Google Scholar]
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699‐710. [DOI] [PubMed] [Google Scholar]
- Feagan BG, Rutgeerts PJ, Sands BE, Colombel J, Sandborn WJ, Hanauer SB, et al. Induction therapy for ulcerative colitis: Results of GEMINI I, a randomized, placebo‐controlled, double‐blind, multicenter phase 3 trial. Gastroenterology 2012;142(5 Suppl 1):S160‐1. [Google Scholar]
- Feagan BG, Sandborn W, Smyth MD, Sankoh S, Parikh A, Fox I. Effects of continued vedolizumab therapy for ulcerative colitis in week 6 induction therapy nonresponders. Gastroenterology 2014;146(5 Suppl 1):S590. [Google Scholar]
- Parikh A. Efficacy of vedolizumab in ulcerative colitis by prior treatment failure in gemini i, a randomized, placebo‐controlled, double‐blind, multicenter trial. Inflammatory Bowel Diseases 2012;18:S26. [Google Scholar]
- Rosario M, Fox I, Milch C, Parikh A, Feagan B, Sandborn W, et al. Pharmacokinetic/pharmacodynamic relationship and immunogenicity of vedolizumab in adults with inflammatory bowel disease: Additional results from GEMINI 1 and 2. Inflammatory Bowel Diseases 2013;19:S80. [Google Scholar]
- Rosario M, French J, Dirks N, Milton A, Fox I, Gastonguay M. Exposure response relationship during vedolizumab induction therapy in adults with ulcerative colitis. Journal of Crohn's and Colitis 2014;8:S270‐1. [Google Scholar]
- Rosario M, Wyant T, Milch C, Parikh A, Feagan B, Sandborn WJ, et al. Pharmacokinetic and pharmacodynamic relationship and immunogenicity of vedolizumab in adults with inflammatory bowel disease: Additional results from the GEMINI 1 and 2 studies. Journal of Crohn's and Colitis 2014;8:S42‐3. [Google Scholar]
- Sandborn W, Sands B, Rutgeerts P, Sankoh S, Rosario M, Milch C, et al. Sustained therapeutic benefit of vedolizumab throughout 1 year in ulcerative colitis in GEMINI I, a randomized, placebo‐controlled, double‐blind, multicenter trial. Journal of Crohn's and Colitis 2013;7:S138‐9. [Google Scholar]
- Sandborn W, Sands B, Rutgeerts P, Sankoh S, Rosario M, Milch C, et al. Sustained therapeutic benefit of vedolizumab throughout 1 year in ulcerative colitis in gemini i, a randomized, placebo‐controlled, double‐blind, multicenter trial. Inflammatory Bowel Diseases 2012;18:S1. [Google Scholar]
- Sands B, Hanauer S, Colombel JF, Danese S, Abreu M, Ahuja V, et al. Reductions in corticosteroid use in patients with ulcerative colitis or crohn's disease treated with vedolizumab. American Journal of Gastroenterology 2013;108:S503. [Google Scholar]
Parikh 2012 {published data only}
- Parikh A, Leach T, Wyant T, Scholz C, Sankoh S, Mould DR, et al. Vedolizumab for the treatment of active ulcerative colitis: A randomized controlled phase 2 dose‐ranging study. Inflammatory Bowel Diseases 2012;18(8):1470‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Colombel 2013a {published data only}
- Colombel JF, Sands B, Hanauer S, Rutgeerts P, Sandborn W, Danese S, et al. Long‐term safety of vedolizumab for the treatment of ulcerative colitis or Crohn's disease. American Journal of Gastroenterology 2013;108:S502‐3. [Google Scholar]
Colombel 2013b {published data only}
- Colombel JF, Sands BE, Feagan BG, Loftus EV, Sankoh S, Fox I, et al. Integrated safety analysis of vedolizumab for the treatment of ulcerative colitis or Crohn's disease. Gastroenterology 2013;144(5 Suppl 1):S113. [Google Scholar]
Feagan 2009 {published data only}
- Feagan B, Leach T, Milch C, Parikh A, Fox I. Emerging safety profile of vedolizumab: A novel, selective integrin inhibitor for the treatment of IBD. Inflammatory Bowel Diseases 2009;15:S12. [Google Scholar]
Parikh 2011a {published data only}
- Parikh A, Fedyk E, Clifford D, Berger J, Sankoh S, Paolino J, et al. No association between vedolizumab exposure and serum JC virus levels. Inflammatory Bowel Diseases 2011;17:S56. [Google Scholar]
- Parikh A, Paolino J, Fedy ER, Clifford D, Berger J, Sankoh S, et al. No association between vedolizumab exposure and serum JC virus levels. Journal of Crohn's and Colitis 2011;5:S109‐10. [Google Scholar]
Parikh 2011b {published data only}
- Parikh A, Leach T, Xu J, Feagan B. Long‐Term clinical experience with vedolizumab in patients with mild to moderate ulcerative colitis. American Journal of Gastroenterology 2011;106:S467. [Google Scholar]
- Parikh A, Leach T, Xu J, Feagan B. Long‐term clinical experience with vedolizumab (VDZ) in patients with mild to moderate ulcerative colitis (UC). Journal of Crohn's and Colitis 2012;6:S103. [Google Scholar]
- Parikh A, Leach T, Xu J, Feagan B. Long‐term clinical experience with vedolizumab in patients with mild to moderate ulcerative colitis. Inflammatory Bowel Diseases 2011;17:S23. [DOI] [PubMed] [Google Scholar]
Parikh 2013 {published data only}
- Parikh A, Fox I, Leach T, Xu J, Scholz C, Patella M, et al. Long‐term clinical experience with vedolizumab in patients with inflammatory bowel disease. Inflammatory Bowel Diseases 2013;19(8):1691‐9. [DOI] [PubMed] [Google Scholar]
- Parikh A, Leach T, Fox I, Xu J, Patella M, Feagan B. Long‐term clinical experience with vedolizumab for the treatment of inflammatory bowel disease: A phase 2 open‐label safety extension study. Journal of Crohn's and Colitis 2011;5:S123. [Google Scholar]
- Parikh A, Leach T, Fox I, Xu J, Patella M, Feagan B. Long‐term clinical experience with vedolizumab for the treatment of inflammatory bowel disease: Results of a phase 2 open‐label safety extension study. Inflammatory Bowel Diseases 2011;17:S56. [Google Scholar]
Wyant 2013 {published data only}
- Wyant T, Leach T, Sankoh S, Wang Y, Paolino J, Feagan B, et al. A phase 1 double‐blind placebo‐controlled single‐dose study to determine the immune response to systemic and mucosal antigenic challenge in the presence of vedolizumab. Journal of Crohn's and Colitis 2013;7:S248. [Google Scholar]
Additional references
Akobeng 2006
- Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005112.pub2] [DOI] [PubMed] [Google Scholar]
Baron 1964
- Baron JH, Connell AM, Lennard‐Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. British Medical Journal 1964;1(5375):89‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloomgren 2012
- Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab‐associated progressive multifocal leukoencephalopathy. New England Journal of Medicine 2012;366(20):1870‐80. [DOI] [PubMed] [Google Scholar]
Erle 1994
- Erle DJ, Briskin MJ, Butcher EC, Garcia‐Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM‐1 receptor, integrin alpha 4 beta 7, on human leukocytes. Journal of Immunology 1994;153(2):517‐28. [PubMed] [Google Scholar]
Fedyk 2012
- Fedyk ER, Wyant T, Yang LL, Csizmadia V, Burke K, Yang H, et al. Exclusive antagonism of the α4 β7 integrin by vedolizumab confirms the gut‐selectivity of this pathway in primates. Inflammatory Bowel Diseases 2012;18(11):2107‐19. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesterberg 1996
- Hesterberg PE, Winsor‐Hines D, Briskin MJ, Soler‐Ferran D, Merrill C, Mackay CR, et al. Rapid resolution of chronic colitis in the cotton‐top tamarin with an antibody to a gut‐homing integrin alpha 4 beta 7. Gastroenterology 1996;111(5):1373‐80. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Kornbluth 2010
- Kornbluth A, Sachar D, The Practice Parameters Committee of the American College of Gastroenterology. Ulcerative Colitis Practice Guidelines in Adults: American College of Gastroenterology, Practice Parameters Committee. American Journal of Gastroenterology 2010;105:501–23. [DOI] [PubMed] [Google Scholar]
Lewis 2008
- Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflammatory Bowel Diseases 2008;14(12):1660‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacDonald 2007
- MacDonald JK, McDonald JWD. Natalizumab for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006097.pub2] [DOI] [PubMed] [Google Scholar]
Sandborn 2005
- Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med 2005;353(18):1912‐25. [DOI] [PubMed] [Google Scholar]
Sandborn 2013
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. New England Journal of Medicine 2013;369(8):711‐21. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Shibolet 2005
- Shibolet O, Regushevskaya E, Brezis M, Soares‐Weiser K. Cyclosporine A for induction of remission in severe ulcerative colitis. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004277.pub2] [DOI] [PubMed] [Google Scholar]
Targan 2007
- Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, et al. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology 2007;132(5):1672‐83. [DOI] [PubMed] [Google Scholar]
Yousry 2006
- Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006;354(9):924‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Behm 2009
- Behm BW, Bickston SJ. Humanized antibody to the alpha4beta7 integrin for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007571] [DOI] [PubMed] [Google Scholar]


