Abstract
Dittrichia viscosa is a perennial herb that has been used for generations in traditional medicine to address a variety of diseases, including diabetes, hypertension, cancer, microbial disorders, inflammatory conditions, and wound healing. The objective of this review is to provide an overview of existing knowledge on D. viscosa with regards to its botanical description, ethnomedicinal uses, and pharmacological properties. Databases such as Scopus, Wiley-Online, PubMed, Springer, Google Scholar, and ScienceDirect were used to select relevant articles based on their title and abstract.
The reviewed studies found a strong correlation between D. viscosa's traditional uses and its observed biological effects. Pharmacological research has shown that the essential oils and extracts from D. viscosa possess a variety of biological activities, such as anti-inflammatory, anticancer, antibacterial, antifungal, analgesic, and antioxidant properties. The chemical compounds found in D. viscosa include sesquiterpenes, monoterpenes, flavonoids, and phenolic acids; some of these compounds, such as tometosin and inuviscolide, have been isolated and displayed promising cytotoxic and anti-inflammatory activity.
The present review suggests that the pharmacological properties of D. viscosa align well with its ethnomedicinal uses. These findings support the traditional use of D. viscosa in treating various illnesses. Additionally, toxicological examinations of D. viscosa extracts and essential oil have demonstrated the plant's safety, which supports the need for comprehensive pharmacological studies, in vivo studies, and clinical trials to evaluate the best doses for optimal medicinal effects. This work underscores the medicinal value of D. viscosa and its potential in developing new pharmacological agents to address major health challenges like antibiotic resistance and cancers.
Keywords: Dittrichia viscosa, Ethnomedicine, Pharmacology, Phytochemistry, Toxicology
Graphical abstract
Abbreviations
- AAE
Ascorbic Acid Equivalent
- AST
Aspartate aminotransferase
- ATCC
American Type Culture Collection
- BHT
Butylated Hydroxy-Toluene
- COX1
Cyclooxygenase 1
- DMBA
7,12-Dimethylbenz(a)anthracene
- DPPH
2,2-Diphenyl-1-picrylhydrazyl radical
- EC50
Half maximal effective concentration
- ED50
Median effective Dose
- EO
Essential oil
- ESBL/BLSE
Extended spectrum beta-lactamases
- FRAP
Ferric Reducing Antioxidant Power
- GC-FID
Gas Chromatography with flame-ionization detection
- GC-MS
Gas Chromatography Mass Spectrometry
- HeLa Cell
Henrietta Lacks cervical cancer cell
- HPLC
High Performance Liquid Chromatography
- IC50
Half Maximal Inhibitory Concentration
- IL‐1β
Interleukin 1β
- IL‐6
Interleukin 6
- iNOS
Inducible nitric oxide synthase
- LD50
Median lethal dose
- LPS
Lipopolysaccharide
- MBC
Minimum Bactericidal Concentration
- MFC
Minimum Fongicide Concentration
- MIC
Minimum Inhibitory Concentration
- MMP
Mitochondrial membrane potential
- MRSA
Methicillin-Resistant Staphylococcus Aureus
- mTOR
Mammalian target of rapamycin
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5 diphenyltetrazolium
- MyD88
Myeloid differentiation primary response gene 88
- NF‐κB
Nuclear factor κB
- NO
Nitric oxide
- ROS
Reactive oxygen species
- sPLA2
Secretory phospholipase A2
- STAT1
Signal transducer and activator of transcription 1
- STZ
Streptozotocin
- TAC
Total antioxidant capacity
- TCP
Tail cuff plethysmography
- TE
Trolox Equivalent
- TLR-4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor alpha
- TPA
12-O-tetradecanoylphorbol-13-acetate
- WHO
World Health Organization
1. Introduction
Traditional medicine has played a pivotal role in the management and treatment of diseases and illnesses throughout human history. The utilization of medicinal plants, a practice widespread among indigenous tribes globally, has been integral in promoting health and in the prevention, amelioration, or treatment of both physical and mental disorders.1 According to reports from the World Health Organization (WHO), approximately 80% of the global population continues to rely on plant-based medicines for their healthcare needs. Furthermore, numerous herbal remedies have successfully transitioned to clinical applications within contemporary medicine, underscoring their enduring relevance and potential in therapeutic interventions.2 (see Fig. 5)
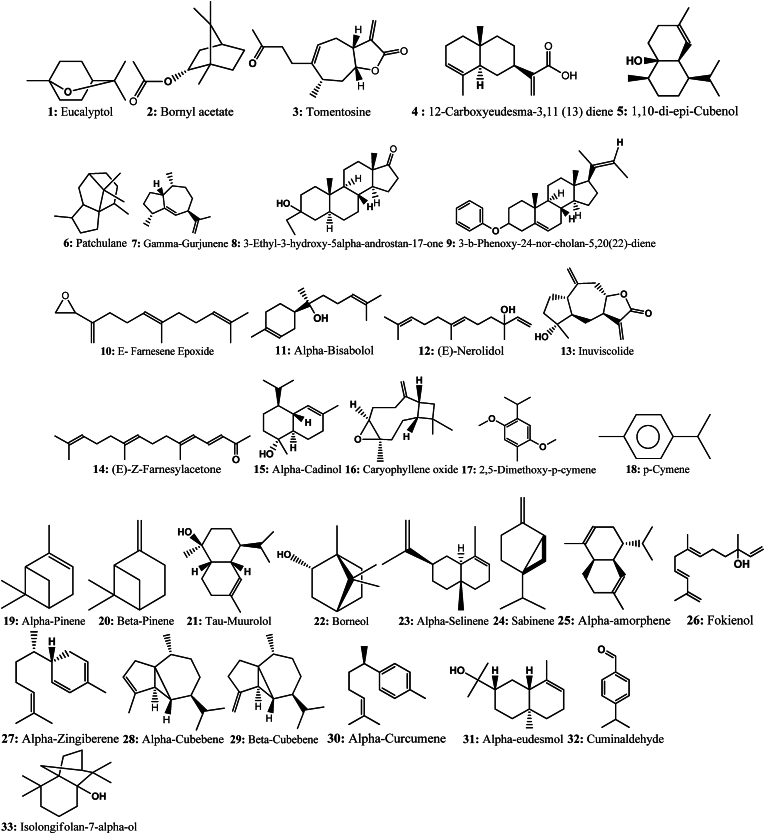
Fig. 5.
Structure of terpenoids compounds identified in D. viscosa.
Plants represent a crucial natural reservoir of diverse underexplored bioactive compounds. Therefore, the investigation of plant metabolites and their biological effects remains a focal point of scientific interest. The ultimate goal is to discover bioactive natural compounds and to advance the development of alternative, green, and sustainable technologies that can reduce or eliminate the reliance on hazardous substances in everyday life.3
A significant number of plant species have garnered attention in recent phytochemical and pharmacological research. Among these, the Asteraceae family, comprising approximately 33,000 species, is renowned for its medicinal properties, which have been recognized for centuries. Despite their diversity, species within this family exhibit similar chemical compositions.4 The genus Dittrichia, belonging to this family, includes five species: D. viscosa, D. graveolens, D. maritima, D. revoluta, and D. orientalis.5 This genus, which was formerly classified under the genus Inula, has been revised and is known for its beneficial medicinal uses. The former genus is abundant in bioactive phytochemicals and more than 300 new compounds have been isolated from the Inula genus in the last decade, most of which have demonstrated pharmacological properties and shown promising results in managing various illnesses.6 Among plants in Dittrichia (Inula) genus, D. viscosa, formerly known as Inula viscosa (Fig. 1), is one of the most commonly used plants in the mediterranean region.5 This perennial shrub, native to the mediterranean basin, is characterized by its sticky leaves with a distinctive smell and its bright yellow flowers that blossom from late summer through early autumn (August to October).7,8 In folk medicine, D. viscosa is used for its biological and medicinal effects like antimicrobial, antipyretic and anti-inflammatory activities.9, 10, 11, 12, 13, 14, 15 The chemical composition of D. viscosa includes a variety of compounds such as terpenoids, sesquiterpene lactones and flavonoids. These phytochemicals are known to have various medicinal properties, such as anti-inflammatory, antioxidant, antimicrobial and antiproliferative effects.16, 17, 18, 19, 20
Fig. 1.
D. viscosa leaves and flowers.
Despite the growing number of studies on D. viscosa aimed at elucidating its phytochemical composition, biological activities, and ethnopharmacological uses, to the best of our knowledge, no comprehensive review has yet been conducted that consolidates all contributions regarding the botany, ethnopharmacology, toxicology, chemical composition, and pharmacological activities of this species. Therefore, this literature review aims to examine the current state of research on D. viscosa. It will cover studies on the botanical description, toxicological investigations, phytochemical analyses of the plant, as well as its traditional and contemporary uses in medicine. The findings of this review are expected to provide insights into the potential therapeutic benefits of D. viscosa and its active compounds, potentially leading to the identification and development of novel medications for a variety of illnesses.
2. Research methodology
In this paper, a review of literature was conducted to gather all the papers published in the last two decades (between 2000 and 2022) on Dittrichia viscosa. These studies encompassed botanical description, ethnomedicinal uses, phytochemical compositions, secondary metabolites, pharmacological activities, and toxicological evaluations. To collect these papers, we utilized several scientific databases and search engines, including Scopus, Wiley Online, PubMed, SpringerLink, Science Direct, and Google Scholar. The authors searched for the information using several keywords and their combinations, such as Dittrichia viscosa, Inula viscosa, D. viscosa essential oils, antioxidant effects of D. viscosa, anticancer activity of D. viscosa, cytotoxicity of D. viscosa, antifungal and antibacterial activity of D. viscosa, antidiabetic activity of D. viscosa, analgesic and anti-inflammatory effects of D. viscosa, anti-hypertensive activity of D. viscosa, dermatological effects of D. viscosa, chemical composition of D. viscosa essential oils, traditional and ethnomedicinal uses of D. viscosa, and toxicity of D. viscosa.
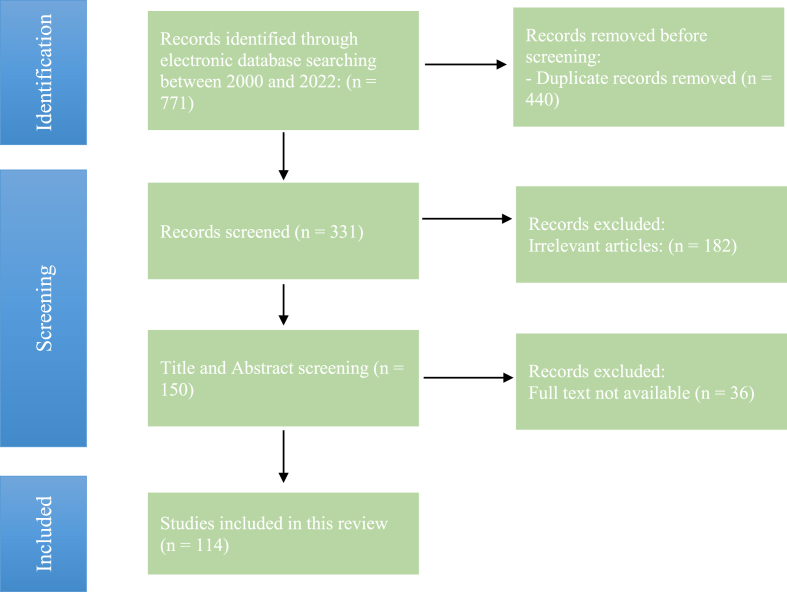
The search results yielded numerous articles that were then assessed for relevance based on their title and abstract. We also scrutinized the reference lists of these papers to identify any other pertinent articles for this literature review. The inclusion criteria for papers were: discussions on Dittrichia viscosa or Inula viscosa; and relevance to the review scope, including botanical description and taxonomy, ethnomedicinal uses, toxicology, chemical composition, and pharmacological activities of D. viscosa targeting human health. The exclusion criteria were articles without full texts, articles not published between 2000 and 2022, and irrelevant articles that fell outside the scope of this review. The search methodology is summarized in Fig. 2. Chemical structures and IUPAC names were sourced from the PubChem database, and ChemDraw Pro 12.0 software was utilized to draw the chemical structures.
Fig. 2.
PRISMA diagram for the research methodology used in the review.
3. Results and discussion
3.1. Botanical description
D. viscosa (L.) Greuter known as false yellowhead, sticky fleabane and Elecampane,21,22 has many vernacular names based on the region where is used. In north Africa, the plant is known as “Tarehla”, “Safsag”, “Magramane” and “Amagramane”.23 In Spain, it is reffered to as “olivarda”,24 in Italy as “brucara”, “purcara”, “vrucara”, erva santa, and pulicara,25,26 and in Turkey as “Yapışkan Andız Out” or Sarı ot.22,27 It is a perennial shrub that belongs to the Asteraceae family (Compositae). This glandular, slimy shrub emits a distinctive, strong odor. Its height varies between 50 cm and 1.50 m, and it has several yellow flowers at the upper part of the stem.5
The stems are frutescent at the base, erect in a fan shape, quite branched and provided with dense foliage. Overtime, the stems undergo lignification and exhibit a pronounced darkening at their basal regions. The foliage demonstrates a tacky consistency, justifying the appellation “viscosa”. The leaves are configured in an alternate sequence, showcasing an elongated to lanceolate morphology, and are directly affixed to the stem, a characteristic indicative of cauline leaf arrangement. (without a petiole or clasping leaves). They are glandular on both sides. The margin is smooth or toothed and the apex acute.28
The plant is sticky and very fragrant, with a camphor smell, considered by some as unpleasant. The whole plant is enveloped in glandular trichomes, which release a viscous, aromatic resin. (Araniti et al., 2017). The roots are solid lignified tap-roots that can measure up to 30 cm in length.29
Typically, the herbaceous plant blossoms from August to October.7,8 D. viscosa has several yellow flowers (capitula) grouped as a composite cluster. Its inflorescences are long and pyramidal, and the corolla of its flowers is about 2 cm.8
The plant exhibits a dichotomy in floral morphology: one variant possesses petals that are fused into yellow bands adorning the periphery of the capitulum (ligulate flowers), while the other variant encompasses tubular blossoms (tubulated flowers), which display a yellow-orange hue at the center of the capitulum. The fruits, which are hairy achenes (dry fruits), are surmounted by a small grayish pappus.29
3.2. Taxonomy and geographic distribution
D. viscosa (L.) Greuter, also known by its homotypic synonym Inula viscosa (L.) Aiton, is the accepted name of a species in the genus Dittrichia (family Compositae). The plant names were checked with The Plant List database: link http://www.theplantlist.org/tpl1.1/record/gcc-16305, World of Flora database https://wfoplantlist.org (WFO ID: wfo-0000059,214) and the National Center of Biotechnology Information (NCBI taxonomy ID: 56525; Link: https://www.ncbi.nlm.nih.gov/data-hub/taxonomy/56525). The plant was first described by Greuter, Werner Rodolfo in 1973. Dittrichia belongs to the Asteraceae family. It is a significant group of dicotyledonous plants that has roughly 20,000 species. These plants are herbaceous perennials with alternate leaves, yellow blooming heads that have both tubular and ligulate flowers, bracts in many series of flowers with pistillate peripherals, with tridentate ligules, sagitted anthers at the base, and chains with coastlines and simple egrets.5
D. viscosa's predominantly occupies mountain slopes, gravel riverbeds, and volcanic scoria, occasionally on sandy soils and along rocky shores.5,30 However, walls, deserted fields, and roadsides serve as examples of its secondary habitats.5,30 Overall, D. viscosa exhibits a pronounced pioneering characteristic; as a result, it colonizes areas where there is little to no plant competition.5,30
Regarding its distribution, D. viscosa is primarily present in the Western Mediterranean regions (Morocco, Tunisia, Algeria, Albania, Yugoslavia, Italy, France, Spain, Portugal Sicily, Corse, Sardinia and Baleares). It is also sporadically located in Greece and Bulgaria.5
3.3. Traditional uses
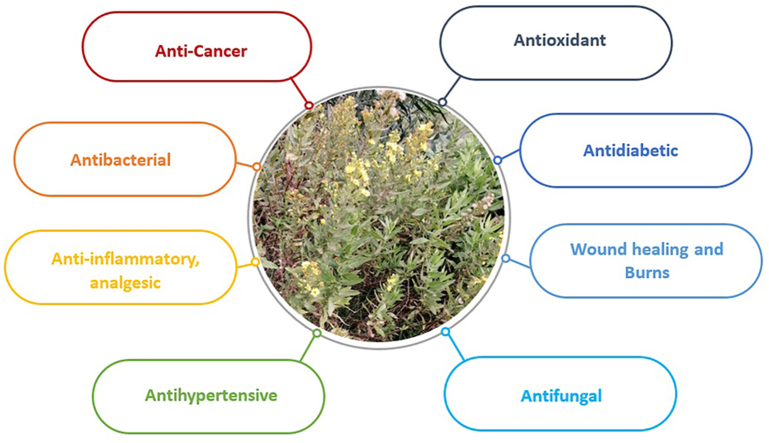
D. viscosa is renowned for its various applications in traditional medicine throughout the world particularly the mediterranean region and north Africa, which have been the focus of most studies on the ethnomedicinal uses of the species. Table 1 and Fig. 3summarize the findings of the most common uses in folk medicine as reported in the literature (see Fig. 4).
Table 1.
Ethno-medicinal uses of D. viscosa.
| Used part | Mode of preparation | Traditional use | Location | References |
|---|---|---|---|---|
| Leaves | Cataplasm Powdered leaves |
Rheumatic pains and headaches Wounds healing and burns |
Algeria Morocco |
9,11 |
| Compresses Powder |
Rheumatic pains and headache Wounds and burns. |
Algeria | 10 | |
| Decoction | Diabetes | Morocco | 14,15 | |
| Decoction | Diabetes, heart disease and antihypertension | Morocco | 13 | |
| Fumigation | Cardiac disease | Algeria | 31 | |
| Infusion Decoction |
Wounds, antidiarrheic and vermifuge | Algeria | 33 | |
| Not reported | Gastrointestinal disorders and hypertension | Algeria | 32 | |
| Not reported | Dermal Wound | Morocco | 37 | |
| Not reported | Skin diseases, wounds, hypertension, diabetes, Cancer, infertility, rheumatic pains, bronchitis, tuberculosis, lung and gastro-duodenal disorders | Turkey | 22 | |
| Not reported | Skin diseases, wounds, cutaneous abscesses, bronchial infections and tuberculosis | Morocco | 35 | |
| Warm leaves: External uses | Injury, Edema, Ulcers. | Algeria | 38 | |
| Direct application/Cataplasm | Swelling, wound healing, hematoma | Italy | 26,40 | |
| Direct application | Hemostasis, wound healing and bruises | Italy | 25 39 | |
| Not reported | Rheumatisms, colds | Israel | 21 | |
| Not reported | Muscle relaxant, infertility, skin diseases | Palestine | 36 34 | |
| Leaves and stems | Infusion | Diabetes | Algeria Morocco |
41,42 |
| Leaves and seeds | Not reported | Hypertension and cardiovascular disorders. | Morocco | 43 |
| Leaves and roots | Decoction Cataplasm Powdered leaves |
Antitussive, diuretic, vermifuge, plant insecticide. Soothing for rheumatic pains, hemostatic, healing skin wounds, purulent dermatoses, and to ripen the abscess, weight gain. |
Morocco | 46 |
| Not reported | Antipyretic, antiseptic, diabetes Anti-diuretic, against bronchitis, gastro-intestinal conditions, anthelmintic, Insecticide | Morocco | 44 | |
| Decoction | Diabetes, digestive system and cancer | Morocco | 45 | |
| Decoction Powdered leaves |
For external use, relieves rheumatic pains. Healing effect for sores |
Morocco | 48 | |
| Decoction | Allergic skin irritations, diabetes mellitus and hypertension | Algeria | 47 | |
| Decoction cataplasm | Diarrhea, Rheumatism |
Algeria | 12 | |
| Leaves and Flowers | Infusion Decoction |
Headache Gastrointestinal diseases: Diarrhea |
Algeria | 57 |
| Roots | Decoction | Allergy, Asthma, Inflammation | Morocco | 50 |
| Decoction Fumigation |
Allergic skin irritations Nasal decongestant |
Italy | 51 | |
| Flowers | Decoction | Anthelminthic, for lung cancer, Muscle relaxant | Jordan | 53 |
| Decoction, essential oil | Respiratory diseases, Injuries, Calluses, Fractures and contusions | Spain | 54 | |
| Aerial part | Decoction | Diabetes, hypertension and renal diseases | Morocco | 52 |
| Direct application | Hemostasis | Italy | 51 | |
| Whole plant | Infusion | Anthelmintic, for lung disorders | Jordan | 56 |
| Not reported | Cataplasm and other | Anti-inflammatory, antipyretic, antiseptic, antiphlogistic, diabetes, treating gastroduodenal disorders, anthelmintic, treatment of tuberculosis, bronchitis, expectorant, anemia, rheumatic pain and diuretic | Jordan | 49 |
| Not reported | Topical application | Wound healing, anti-inflammatory and anti-scabies | Spain | 55 |
Fig. 3.
Ethno-pharmacological activities of D. viscosa.
Fig. 4.
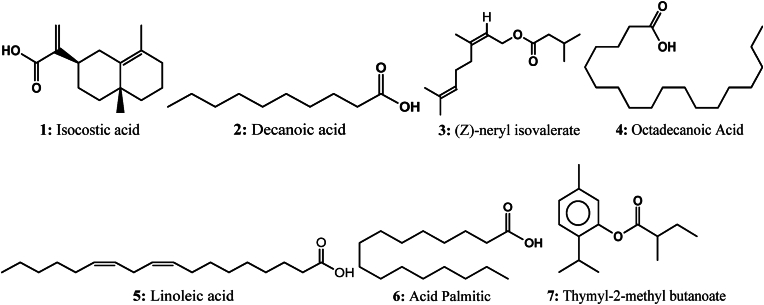
Chemical structures of fatty acids identified in D. viscosa.
Different parts of D. viscosa are prepared in different ways to treat several illnesses. The leaves, used either as a powder or cataplasm, are noted for their efficacy in healing wounds and burns and in treating rheumatic pain and headaches.9, 10, 11, 12 As a decoction, the foliage is utilized therapeutically for diabetes, heart diseases, and hypertension.13, 14, 15 Boughrara and Belgacem (2016) found that in Algeria, fumigation of the leaves is a preparation method for treating heart diseases.31 In several studies where the preparation method was unspecified, leaves were reported to treat respiratory infections such as bronchitis and tuberculosis, gastrointestinal disorders including diarrhea and antiparasitic infections, infertility, cancer, and as a muscle relaxant.21,22,32, 33, 34, 35, 36 The literature most commonly reports the use of D. viscosa leaves for treating skin conditions, such as wounds, injuries, ulcers, and cutaneous abscesses.22,25,26,34,35,37, 38, 39, 40
Furthermore, the leaves are combined with other plant parts, such as roots, seeds, stems, or flowers, to treat a variety of diseases. In Morocco and Algeria, leaves mixed with roots or stems are used against diabetes,41,42 and in combination with seeds, they manage hypertension and cardiovascular diseases.43 Other preparations involving leaves and roots of D. viscosa serve as anthelmintic44 and antidiarrheal remedies,12 or for treating other gastrointestinal conditions,44,45 skin conditions including purulent dermatoses, wounds, skin irritations of allergic origin, and to ripen abscesses.46,47 Additionally, mixtures of D. viscosa roots and leaves are reported to aid in weight gain, act as diuretics, or treat respiratory conditions,44,46 and are used as an antipyretics,44 analgesics for rheumatic pain,12,44,46,48 antiseptic,44 or even as insecticides.46 Preparations from leaves and roots are also noted for their use against diabetes,44,45,47 hypertension47 and cancer.45
Another study, conducted in Jordan by Al-Dissi et al. (2001), did not specify which part of the plant was used. This study revealed that D. viscosa is conventionally used as an antipyretic; anti-inflammatory; antiseptic; antidiabetic; antiphlogistic; and anthelmintic. In addition, it is sometimes employed to treat respiratory pathologies, such as bronchitis and tuberculosis, or used as an expectorant. Furthermore, the study reported that the plant is utilized to manage anemia and rheumatic pain, and as a diuretic.49
Other studies focused on investigating the use of different parts of D. viscosa when used alone. Youbi et al. (2016) reported that a decoction of the roots alone is utilized in Morocco for managing allergies, asthma, and inflammation.50 The same preparation is employed in Italy to treat allergic skin irritations and as a nasal decongestant through fumigation.51 A decoction prepared from the aerial components of D. viscosa has been documented as a traditional remedy for renal disorders, hypertension, and diabetes.52 These aerial parts are also applied directly on the skin for their hemostatic effect to stop bleeding.51 In Jordan, flowers prepared in a decoction are used as an anthelminthic, a muscle relaxant, and against lung cancer.53 Additionally, in Spain, Flowers are used for treating respiratory diseases, injuries, calluses, fractures and contusions.54 Another study in Spain reported the topical application of the plant for wound healing, inflammation and for its anti-scabies properties.55 Al-Qura'n (2009) found that the whole plant is used in Jordanian folk medicine as an anthelmintic and for the treatment of lung disorders.56
These results indicate that D. viscosa is traditionally employed in ethnomedicine to address wide range of health conditions, particularly diabetes, cancer, gastrointestinal, cardiovascular and dermatologic conditions. However, its application varies by geographic location, local practices, and the specific plant part utilized. Despite this variability, D. viscosa is thought to possess some pharmacological actions that can be investigated to determine the active substances responsible for these activities. This is supported by the plant's use in traditional medicine. Moreover, the widespread use of this plant in traditional medicine underscores the importance of conducting toxicological studies to assess its safety profile.
3.4. Toxicological investigation
D. viscosa has many uses in Moroccan traditional medicine. Therefore, conducting research on its toxicity is mandatory to determine the potential toxicologic properties that the plant might exhibit. Table 2 summarizes the findings of the toxicological investigation on D. viscosa reported in the literature.
Table 2.
Toxicological properties of D. viscosa.
| Use parts | Extracts | Dose administration | Route of administration | Model | Effects | References |
|---|---|---|---|---|---|---|
| Aerial parts | Acetone extract | 200 mg/kg to 1000 mg/kg for 60 days | Intraperitoneally | Toxicity | The doses investigated had no death and no toxic effect | 60 |
| Leaves and flowers | Methanolic extracts | 400 and 800 mg/kg for over 14 days. 400 and 800 mg/kg for over 28 days |
Orally | Acute toxicity Sub-chronic toxicity |
None of the doses caused death. No signs of both acute and sub-chronic toxicity. |
59 |
| Leaves and flowers | - Methanolic extracts - Essential oils |
−300, 600, 1000, 1500, 2000, 2500 mg/kg of methanolic extract. −0.3, 0.5, 1, 1.5, 2, 3 mL/kg of essential oils. For 14 days |
Orogastric route | Acute toxicity | No death during the observation period and no change the behavior of animals. | 58 |
| Leaves | Petroleum ether Dichloromethane Methanol extract Aqueous extract |
DL50: 626 mg/kg DL50: 1288 mg/kg DL50: 2958 mg/kg DL50: >8000 mg/kg |
Intraperitoneally | Acute toxicity | No maternal mortality and all the dosages administered in this study were sublethal, | 49 |
Al-Dissi et al. (2001) conducted one of the first studies on the toxicological effects of D. viscosa in rats. The primary objective of the study is to scrutinize the abortifacient and anti-implantation impacts of D. viscosa leaf extracts on rats. In the DL50 experiments, the subjects were divided into six groups, each consisting of six rats per dosage category. Various types of leaf extracts were administered intraperitoneally, followed by meticulous monitoring of the subjects. The DL50 values were determined after a 24-h period. According to the data presented in Table 2, the petroleum ether extract exhibited the highest toxicity with a DL50 of 626 mg/kg, whereas the aqueous extract was deemed the safest, showing no toxic effects up to a dosage of 8000 mg/kg of body weight. The acute toxicity study was conducted to establish safe dosage levels for subsequent investigations into the abortifacient and anti-implantation effects of D. viscosa in rats. Consequently, the dosages applied in this study were sublethal, ensuring no maternal mortality occurred throughout the duration of the trial.49
In 2016, another investigation was conducted to evaluate the acute toxicity of the methanolic extract and essential oil derived from D. viscosa. In this study, the researchers divided the animals into sets of ten rats (five females and five males) that received both methanolic extract and essential oil by orogastric route. The administered doses of the methanolic extract are 300, 600, 1000, 1500, 2000, and 2500 mg/kg given consecutively, whereas the administered doses of the essential oil are 0.3, 0.5, 1, 1.5, 2, and 3 mL/kg. A dose of 10 mL/kg of saline was given to the control group. The groups of rats were monitored from the third hour following the administration of the substances until the 14th day that followed. The results found that the extracts did not cause any deaths or produce any physical or behavioral changes. Therefore, the study determined that the median lethal dose (DL50) was 3 mg/kg for the essential oil, and 2500 mg/kg for the methanolic extract.58 This last finding on the methanolic extract's DL50 is in agreement with the results of Al-Dissi et al. (2001) in which they also found that the DL50 for the methanolic extract is estimated at 2958 mg/kg of body weight.49
Ouahchia et al. (2017) conducted further research on the methanolic extract derived from the leaves and flowers of D. viscosa, centering their investigation on assessing both the acute and chronic toxicity of these extracts in rat subjects. To evaluate the acute toxicity, two groups of rats received a dose of 400 mg/kg of either flower or leaf extract, and an extra two groups received a dose of 800 mg/kg of the methanolic extract of leaves and flowers for a period of 14 days. For the evaluation of the chronic toxicity, four other groups of rats were given the same doses of 400 and 800 mg/kg of either flower or leaf extract, but over an extended period of time of 28 days. Similar to the previous works, the study of acute toxicity concluded that the methanolic extracts did not result in any deaths in the animals. Identical results on the mortality rate were also obtained by the study of sub-chronic toxicity. However, some slight biochemical changes were observed. The liver AST decreased significantly in groups that received a dose of 800 mg/kg for 28 days, 116,17 mg/kg ± 1,27 for the group who received leaves extract. And urea levels increased significantly 0,50 mg/kg ± 0,05 in flower extract the group. The study concluded that methanolic extracts of leaves and flowers of D. viscosa displayed no acute or sub-chronic toxicity at the studied doses..59
The previous data is backed up by one more study that proved the absence of signs of toxicity in the acetonic extracts of D. viscosa. Abbas et al. (2017) delved into the toxicological attributes of the acetone extract from the aerial components of D. viscosa when tested on rats. In this experiment, animals received a dose ranging from 200 mg/kg to 1000 mg/kg of the acetonic extract derived from the aerial parts over a period of 60 days. The doses were administered intraperitoneally, and the animals were observed for physical and behavioral changes. The total of dead rats was counted after 24 h to determine the LD50. Data from this study suggests that the LD50 for the acetonic extracts of D. viscosa is 829.5 mg/kg. Moreover, rats did not display any behavioral or skin alterations such as hair loss, bowel movement, or other abnormal physiological indicators.60
3.5. Chemical composition
Many studies have explored the chemical composition of various extracts derived from different components of D. viscosa. The essential oil was the most commonly used extract for studying chemical composition. However, some researchers also analyzed the extracts obtained using multiple solvents such as ethanol, methanol, and n-hexane. The table below (Table 3) summarizes the most relevant findings that were reported in these papers.
Table 3.
Chemical composition of D. viscosa.
| Part Used | Extracts/Essential oil | Compounds groups | Compounds | References |
|---|---|---|---|---|
| Leaves (Morocco) | Essential Oil | Monoterpenes, Sesquiterpenes. | Bornyl acetate (41.0%), Borneol (9.33%), α-amorphene (6.60%), Caryophyllene oxide (5.73%), Naphthalene (3.25%), Camphene (2.78%), Caryophyllenol (2.49%), Thujopsene (2.25%), Isodrimenin (1.79%), Farnesyl bromide (1.28%), Andrographolide (1.20%), Spathulenol (1.19%), α-Cadinol (1.12%), Ledol (1.09%), τ-Muurolol (1.09%), Isoborneol (1.05%), a-Bulnesene (1.0%), 9-cis-Retinal (0.92%), Epizonarene (0.90%), Bicyclosesquiphellandrene (0.89%), Limonene (0.85%), Fenchyl acetate (0.74%), 3-Carene (0.79%), α-Pinene (0.76%), Pentacosane (0.73%), 11-Hexadecynal (0.57%), τ-Cadinol (0.56%), Naphthalen-2-ol (0.56%), Caryophyllene (0.68%), Verbenol (0.49%), γ-Himachalene (0.45%), Aristolene epoxide (0.44%), Isoaromadendrene epoxide (0.44%), Lupan-3-ol, acetate (0.43%).Humulen-(v1) (0.42%), Longifolenaldehyde (0.38%), Santolina triene (0.28%), Isoledene (0.27%), γ-Elemene (0.26%), Aromadendrene oxide-(2) (0.22%), Isoaromadendrene epoxide (0.19%). | 16 |
| Leaves (Morocco) | EtOH extract | Phenol, Flavonoid, Tannins. |
Trimethylsilyl-mesoinositol (20.54%), 5(4H)-Thebenidinone (16.80%),bis (methylthio)-4-(2-phenylethenyl) (9.76%), Proline 2TMS (8.03 %), Acrylic acid,2,3-bis [(trimethylsilyl) oxy]-,trimethylsilyl ester (6.26%), bis-DMTBS (6.42%), Cyanuric acid, 3TMS derivative (5.50%), d-Ribofuranose,1,2,3,5-tetrakis-O- (trimethylsilyl)- (CAS) (4.46%),L (+)-Bornesitol, TMS (3.14%), Dimethylmercury (2.84%).Octamethylcyclotetrasiloxane (2.79%),α-Galactopyranose, (5TMS) (2.78%), [(1,1-dimethyl-2-propenyl) oxy]trimethyl- (2.62%), p-Methylcinnamic acid (2.28%), Propanephosphonic acid, bis(trimethylsilyl) ester (1.41%), 6-deoxy 1,2,3,5-tetrakis-O-(trimethylsilyl)- (1.56%), Crinan-11-ol (0.99%), d-gluconic acid 6TMS (1.73%), Silane, Succinic acid, β-l-Mannofuranose. |
17 |
| Leaves (Palestine) | Essential oil | Monoterpenoids, Sesquiterpenoides, Steroids. |
γ-Gurjunene (22.82%), Patchulane (22.82%),3-Ethyl-3-hydroxy-5alpha-androstan-17-one (22.82%), 3-b-Phenoxy-24-nor-cholan-5,20 (22)-diene (22.82%), (28Z)−28-Heptatriaconten-2-one (1.71%), 4-methoxy-6-methyl-6,7-dihydro-4H-furo (3,2-c) pyran (1.71%), 1-Methylene-2b-hydroxymethyl-3,3-dimethyl-4b-(3-methylbut-2-enyl)-cyclohexane (0.86%), Tricyclo [4.4.1.0 (1,6)]undecane (0.85%), Spiro [4.5]decane-6-One (0.85%),Naphthalene, 1,2,3,4,4a,5,6,7-octahydro-4a-methyl-2,2-diphenyl-(0.85%), 2-methyl-2-hydroxy-decalin-4A-carboxylic acid,2,4A-lactone (0.85%),Tritetracontane (0.20%), butyl heptadecyl ester (0.20%), Hentriacontane (0.20%),β-Damascenone (0.01%), Pent-1-yn-1-ylcyclohexane (0.03%), L-camphor (0.03%), Chloroacetic acid, dodec-9-ynyl ester (0.03%), 1,3,3-Trimethyl-2-hydroxymethyl-3,3-dimethyl-4-(3-methylbut-2-enyl)-cyclohexene (0.12%), 1-Formyl-2,2-dimethyl-3-trans-(3-methyl-but-2-enyl)−6-methylidene-cyclohexane (0.12%), 2-Methyl-3-(3-methyl-2-butenyl)−2-(4-methyl-3-pentenyl)oxetane (0.12%), Sulfurous acid. |
66 |
| Leaves (Morocco) | n-hexane fraction | Monoterpenes Sesquiterpenes Polyphenols |
Cuminaldehyde, Phenylacetic acid, β-Selinene, α-Terpinen-7-al, α-Cubebene, Eugenol, Germacrene D, β-Cubebene, (E)-Caryophyllene, Caryophyllene oxide, α-Copaene,α-Curcumene, α-Zingiberene, α-Muurolene, (E,E)-, α-Farnesene, epi-Cubebol, β-Bisabolene, β-Sesquiphellandrene, α-Cadinene, δ-Cadinene, γ-Cadinene, Fokienol, β-Oplopenone, δ-Cadinol, α-, epi-Muurolol, Cadin-4-en-10-ol, Oplopanone, Neophytadiene, Phytone, n-Hexadecanoic acid, (Z,Z,Z)-9,12,15-Octadecatrienoic acid, (Z,Z)-9,12-Octadecadienoic acid, n-Tricosane, n-Tetracosane, n-Pentacosane, n-Hexacosane, n-Heptacosane, methyl-Tetracosanoate, n-Octacosane, 2-methyl-Octacosane, n-Nonacosane, Methyl hexacosanoate, Methyl hexacosanoate, n-Dotriacontane, n-Pentatriacontane, n-Tetratriacontane, n-Hentriacontane, n-Tritriacontane. | 61 |
| Aerial parts (Algeria) | Essential oil | Sesquiterpene hydrocarbons, Oxygenated sesquiterpenes. |
α-Bisabolol (16.0%),(E)-Nerolidol (15.5%), (E)-Z-Farnesylacetone (13.2%), α-Cadinol (11.6%), Caryophyllene oxide (10.6%), τ-Muurolol (9.8%), Ledol (4.5%), Zingiberenol (3.2%), Globulol (2.9%), (E)-β-Farnesene (2.6%), allo-Aromadendrene (1.8%), δ-Cadinene (1.5%), γ-Cadinene (0.9%), cis-α-Bergamotene (0.9%), β-Copaene (0.8%), Germacrene-D (0.5%), Bicyclogermacrene (0.5%), (E)-β-Caryophyllene (0.3%), Zingibrene (0.1%). | 68 |
| Roots parts (Tunisia) | Essential oil and its fractions | Sesquiterpene hydrocarbons, Oxygenated sesquiterpenes, Oxygenated monoterpenes, Non-terpene derivatives |
Germacrene D-4-ol, Neryl isovalerate (26.2%), Allocedrol, Humulene epoxide II, 1,10-di-epi-Cubenol (24.7%), 2,5-Dimethoxy-p-cymene (8.9%),Ledol, Thymyl-2-methyl butanoate (5.2%),Copaen-15-ol (3.8%),7-epi-α-Eudesmol, 14-Hydroxy-9-epi-(E)-caryophyllene, Aromadendrene epoxide II, 6-Methoxythymyl isobutyrate (3.7%), epi-α-Cadinol (syn.τ-Cadinol) (3.5%), Humulane-1,6-dien-3-ol, epi-α-Cadinol (syn.τ-Cadinol) (3.2%), γ-Muurolene, Thymyl isobutyrate (3.0%), Alloaromadendrene (2.4%), Neryl isobutyrate (1.9%),α-Cadinol (1.7%), 9-epi-(E)-Caryophyllene, trans-Cadina-1 (6),4-diene, γ-Gurjunene, γ-Himachalene (1.4%), Silphiperfol-6-ene, β-Caryophyllene (1.3%),Viridiflorol (0.8%),Longiborneol (0.8%), (Z)-Nerolidol acetate, (Z)-α-Santalol, epi-α-Bisabolol, Eudesma-4 (15),7-dien-1-β-ol, Guaiol acetate (0.7%).Thymol methylether (0.7%), δ-Cadinene (0.7%), β-Selinene, α-Bulnesene (syn. δ-Guaiene), trans-γ-Cadinene (0.6%), Globulol (0.6%),Himachala-2,4-diene (0.4%),trans-Cubebol (0.4%), 8,14-Cedrane oxide (0.5%), Geranyl-2-methyl butyrate (0.5%), Linalool, (Z)-Tagetone (0.3%), Thymol, Cyclosativene (0.3%), epi-β-Santalene (0.3%), α-Calacorene, (E)-Nerolidol (0.3%), α-Himachalene (0.2%), Thymol methylether (0.2%), | 63 |
| Leaves, flowers and aerial parts (Tunisia) | Essential oils | oxygenated sesquiterpenes, Oxygenated monoterpenes, Sesquiterpenes hydrocarbons |
(E)-caryophyllene, (E)-nerolidol, (E)-β-damascenone,(Z)-β-damascenone, 1,8-dehydro-cineole, 10,11-epoxy-calamenene, 13-hydroxy-valencene, 14-hydroxy-(Z)-, 14-hydroxy-9-epi-(E)-, ishwarone, 1-epi-cubenol, 1-hexadecene, 1-octadecene.1-tetradecene, 8-α-11-elemodiol, allo-aromadendrene, aromadendrene, caryophyllene oxide, cedr-8 (15)-en-9-α-ol, cis-thujopsadiene, cis-β-guaiene, epi-nootkatol, epizonarene, epi-α-cadinol, fokienol, geranyl acetone, guaiol, gymnomitrone, isolongifolan-7-α-ol, muurola-4,10 (14)- dien-1-β-, n-nonanal, para-mentha −1,5-dien-8-ol, α-terpineol, α-cadinene, α-cadinol, α-calacorene, α-cedrene, α-copaen-11-ol, α-copaene, α-cuprenene, α-eudesmol, α-muurolene, β-chamigrene, β-costol, β-selienene, γ-muurolene, δ-cadinene, δ-selienene, | 65 |
| Leaves parts (Tunisia) | Essential oil | Phenol Flavonoids |
3-Hexen-1-ol, 2-Hexenal, Hexanal, 1.8-Cineole, Linalool, p-Cymen-7-ol, Eugenol, trans-Carveol, Nerol, Methyl-eugenol, trans-Caryophyllene, α-Ionone, β-Humulene, α-Humulene, γ-Selinene, Germacrene D, β-Selinene, β-Ionone, α-Farnesene, α-selinene, Delta-Selinene, delta-Cadinene, Nerolidol, Caryophyllene oxide, Cadinene, Dodecane, Tridecane, α-Costol. | 64 |
| Aerial parts (Italy) | Volatile organic compounds (VOCs) | Terpenoids Monoterpenes Sesquiterpenes |
Eucalyptol (43.24%), α-Pinene (9.88%), Sabinene (6.83%), β-Pinene (3.37%), α-Thujene (3.26), α-Cadinene (2.99%), Guaia-6,9-diene (2.63%), Ylangene (1.84%),(E)-Caryophyllene (1.72%), γ-Terpinene (1.47%), o-Cymene (1.31%), α-Copaene (1.36%), α-Muurolene (1.01%), cis-Sabinene hydrate (0.93%), 1,8-Cineole (0.89%), Myrcene (0.81%), α-Methylbutanal (0.56%), 3-Hexen-1-ol (0.52%), (E)-Nerolidol (0.51%), α-Panasinsen (0.44%), Leaf aldehyde (0.39%), α-Humulene (0.34%),Isovaleraldehyde (0.33%), n-Hexanal (0.18%), 3-Methylpentanol (0.17%), Camphene (0.3%), Methyl benzoate (0.08%), 4-Terpinenyl acetate (0.47%), (3E)-4,8-Dimethyl-1,3,7-nonatriene (0.18%), p-Menth-2-en-1-ol (0.06%), Camphor (0.18%), L-terpinen-4-ol (0.3%), α-Terpineol (0.11%), Sativen (0.12%), Isolongifolene (0.14%), α-Gurjunene (0.22%), Alloaromadendrene (0.22%), α-Selinene (0.2%), Δ-Cadinene (0.1%). |
67. |
| Leaves (Tunisia) | Essential oil | Oxygenated sesquiterpenes, Oxygenated monoterpenes, Sesquiterpenes hydrocarbons, Monoterpene hydrocarbons. | Decanoic acid (26.39%),α-Gurjunene (11.12%), α-Selinene (7.46%),Caryophyllene oxide (6.67%), p-Cymene (6.11%), Pentacosane (4.04%),Bicyclogermacrene (3.24%), Aromadendrene (3.09%), Eicosane (2.97%), Valencene (2.37%), Phenylacetaldehyde (2.92%), Tricosane (2.87%), Hexacosane (2.73%), β-Caryophyllene (1.95%), Butylated hydroxytoluene (ional) (1.81%), β-Guaiene (1.52%), β-Cubebene (1.47%), Alloaromadendrene (1.33%), Nonacosane (1.33%), Hotrienol (1.21%), α-Terpineol (1.11%), Docosane (0.93%), Octacosane (0.83%), α-Cedrene (0.68%), Tetracosane (0.52%), Heneicosane (0.42%). | 62 |
| Leaves (Algeria) | Essential oil | Alcohols, Alkanes, Fatty acids, Oxygenated sesquiterpenes, Sesquiterpenes hydrocarbons. |
12-Carboxyeudesma-3,11 (13) diene (28.88%), Linolenic acid (7.80%), Pentacosane (5.43%), 2,3-Didehydrocostic acid, n-Hexadecanoic acid (5.38%), Heptacosane (4.82%), C15H22O2 (4.65%), Butyl hydroxy toluene (4.11%), Fokienol (3.37%), Phytol (2.96%), Eicosanol (2.46%), 9,12-Octadecadienoic acid (2.03%), Pentadecanoic acid (1.85%), C15H22O (1.79%), C15H22O (1.14%), Tricosane (1.50%), Hexacosane (0.89%), C15H22O (0.89%), C15H22O (0.85%), 3,7,11-Trimethyl dodeca-1,6,10 trie'ne,3,9-diol (0.85%), Tetracosane (0.80%), C15H24 (0.77%), Isobutyrate de 3-methoxycuminyl (0.71%), 1,6,10-Dodecatrien-3-ol,3,7,11-trimethyl (0.63%), C15H24O (0.52%), C15H22 (0.32%), C15H24O (0.33%), Phytone (0.31%), Menthol (0.22%), Caryophyllene oxide (0.17%),C15H24 (0.14%), Octadecanoic acid, Cubenol. |
20 |
| Leaves (Algeria) | Essential oil | oxygenated sesquiterpenes Oxygenated monoterpenes Hydroxy-acids |
Isocostic acid (56.83%), Fokienol (14.60%), Hydroxy-acids, p-Mentha-1.5-dien-8-ol, Eugenol (0.14%), β-Damascenone, α-Copaene, β-Patchoulene, Butyl Hydroxy-Toluene (2.26%), Nerolidol (0.56%), trans-Nerolidyl acetate (0.43%), Palmitic acid (1.90%), C23H48 ramified (0.17%), C24H50 ramified (0.10%), Diisooctyl phthalate, C25H52ramified (1.17%), Hexacosane, C26H54 ramified (0.16%), 3-Ethyl tetracosane, Heptacosane (0.17%), C27H56ramified (1.70%), C29H60 ramified (0.26%). | 19 |
| Leaves (Morocco) | n-hexane extract and Methanol extract | Sesquiterpene acid Flavonoids |
Isocostic acid (46.05%), Tomentosin (33.27%), Inuviscolide (13.04%), Iso-velleral (1.87%), 3-(4′-Methoxyphenyl)-1-acetyl-2-phenylindolizine (1.68%),Isoaromadendrene epoxide (1.44%), Tetracosane (0.77%), Phenanthrene, 7-ethenyl-1,2,3,4,4α,4 β,5,6,7,8,10,10α - dodecahydro-4α,7-dimethyl-1-methylene-, [4αS- (4αα′,4 β α′,7α′,10αα′)]- (0.69%), 6-Imino-8-(3′,5′-dichlorolphenyl)-3,4-dihydro-2H, 6H-pyrimido[2,1- β][1,3]thiazine-7-carbonitrile (0.39%), 6,9,12,15, Docosatetraenoic acid, methyl ester (0.37%), Quercetin 7,3′,4′-trimethoxy (0.22%),1-Amino-1-ortho-chlorophenyl-2-(2-quinoxalinyl)ethene (0.21%), | 18 |
The GC–MS study of the essential oil extracted through hydro-distillation of D. viscosa leaves confirmed the existence of 41 chemicals, accounting for 97% of the total mass. The E. O was predominantly made of monoterpenes and sesquiterpenes, with the most predominant compounds being Bornyl acetate (41%), borneol (9.3%), α-amorphene (6.6 %), and caryophyllene oxide (5.7%).16 The same authors conducted a study on the ethanolic extracts of D. viscosa leaves. The GC-MS study of the extract identified 18 phytochemicals, accounting for a total of 99.1%. Trimethylsilyl-mesoinositol was the most prevalent compound, which accounted for 20.54% of the extract, followed by 5(4H)-Thebenidinone at 16.80% and bis(methylthio)-4-(2-phenylethenyl) at 9.76%. In addition to these three compounds, the ethanolic extract contained 2-Chloroquinone (8.03%), Succinic acid, bis-DMTBS (6.42%), and Acrylic acid, 2,3-bis [(trimethylsilyl) oxy]-,trimethylsilyl ester (6.26%).17 Another Moroccan study analyzed the composition of the Hexanic fraction derived from leaves of D. viscosa demonstrated that sesquiterpenes are the main components of this fraction. These sesquiterpene derivates are mainly Isocostic acid, representing 46.05%, besides two other sesquiterpene lactones, Tomentosin (33.27%) and Inuviscolide (13.04%).18 Asraoui et al. (2021) did the same study on the hexanic fraction of Moroccan D. viscosa leaves. The study confirmed that the fraction is composed of forty-eight compounds, which are mostly monoterpenes like Cuminaldehyde and sesquiterpenes such as α-Zingiberene, α-Cubebene, β-Cubebene and α-Curcumene.61
In Algeria, Nadia et al. (2020) researched the phytochemical composition of the essential oil extracted from the aerial parts of D. viscosa collected in ten regions of Algeria using GC-MS. The study revealed the presence of nineteen components, representing 90.1%–98.8% of oils. The most abundant compounds are ten oxygenated sesquiterpenes, accounting for 87.3% and nine sesquiterpenes hydrocarbons. The oxygenated sesquiterpenes are represented by α-bisabolol (16.0%), (E)-Z-Farnesylacetone (13.2%), (E)-nerolidol (15.5%), α-Cadinol (11.6%), Caryophyllene oxide (10.6%) and τ-Muurolol (9.8%), whereas the group of sesquiterpenes hydrocarbons present in small percentages comprised (E)- β-farnesene (2.6%), Allo-aromadendrene (1.8%) and γ-cadinene (1.5%). The findings of this study were also confirmed by the outcomes of Madani et al. (2014) who investigated the chemical composition of Algerian D. viscosa leaves. The research found that the essential oil contains 23 phytochemicals with a percentage of 80%. The main compounds are oxygenated sesquiterpenes, accounting for 75%, and are mainly represented by isocostic acid (56.83%) and Fokienol (14.60%). The oil also contained hydroxy acids representing 1.90% and very small amounts of oxygenated monoterpenes (0.14%) composed essentially of eugenol, cineole and p-mentha-1,5-dien-8-ol.19
Another study used hydro-distillation and steam distillation as essential oil extraction methods for D. viscosa leaves from Algeria. The analysis identified thirty-three compounds, accounting for 83.66% and 86.57% for steam distillation and hydro distillation, respectively. The major compounds of the two oils are oxygenated sesquiterpenes (46.7–72.98%), and this in harmony with the results of the previous two Algerian studies. The most important phytochemicals found in the steam distillation oil are 12-carbo-xyeudesma-3,11 (13)diene (56.81%), 2,3-didehydrocostic acid (3.25%), butyl hydroxy toluene (2.63%), pentacosane (2.31%), heptacosane (2.09%), n-hexadecanoic acid (1.91%) and fokienol (1.89%). On the other hand, the major compounds found in the hydro distillation oil are 12-carboxyeudesma-3,11 (13)diene (28.88%), linolenic acid (7.80%), pentacosane (5.43%), n-hexadecanoic acid (5.38%), heptacosane (4.82%), butyl hydroxy toluene (4.11%) and fokienol (3.37%).20
Additional research has been conducted in Tunisia on the chemical composition of the essential oil of D. viscosa. Mahmoudi et al. (2016) identified 27 phytochemicals in D. viscosa leaves’ essential oil, using HPLC-PDA-ESI-MS/MS. The most predominant groups are nonterpenic components (45.74%) and sesquiterpene hydrocarbons (34.23%), whereas the groups that were present in small percentages are oxygenated sesquiterpenes (6.67%), monoterpene hydrocarbons (6.11%) and oxygenated monoterpenes (5.24%). The major group was composed mainly of decanoic acid (26,39%), pentacosane (4.04%) and hexacosane (2.73%), while the second group was dominated by α-gurjunene (11.12%) and α-selinene (7.46%).62 The essential oil and its fractions that were extracted from D. viscosa roots were also analyzed by I Aissa et al. (2019). The results of the GC-FID and GC-MS of the oil and its fractions revealed the presence of fifty-three compounds. The principal phytochemicals were oxygenated monoterpenes (50.5%), oxygenated sesquiterpenes (37.5%), and sesquiterpene hydrocarbons (7.6%). The oxygenated monoterpenes were dominated by (Z)-neryl isovalerate (17.5–29.8%) and 2,5-dimethoxy-p-cymene (5.9–17.7%), while the oxygenated sesquiterpenes were dominated by 1,10-di-epi-Cubenol (19.1–27.2%).63
To gain insight into how the extraction process affects the composition of the essential oil, Sriti Eljazi et al. (2018) compared three methods: hydro-distillation, solvent extraction and ultrasonic extraction followed by hydro distillation. The major constituents of the essential oil obtained by hydro-distillation were discovered to be aryophyllene oxide (3.11%), -selinene (3.09%), 2-hexenal (3.70%), 3-hexen-1-ol (2.00%), and eugenol (1.70%). On the other hand, the EO isolated using hexane extraction contained tridecane (3.89%), dodecane (3.08%), trans-caryophyllene (2.94%), caryophyllene oxide (2.56%) and nerolidol (2.53%). The constituents of the latter essential oil (obtained through ultrasonic extraction followed by hydro-distillation) were dominated by -selinene (5.68%), caryophyllene oxide (4.87%), trans-caryophyllene (1.9%), and nerolidol (1.74%).64
Gharred et al. (2019) successfully detected forty-seven different phytochemical components through GC/MS and GC/FID examinations of the essential oil derived from various parts of D. viscosa, which was harvested in Tunisia. The most predominant compounds were oxygenated sesquiterpenes, which accounted for 45.8%–64.7% in flowers and leaves, respectively. The major compounds in each oil were (E)-nerolidol (40.7%) for flowers and caryophyllene oxide (9.9%), isolongifo-lan-7-α-ol (10.3%) and α-eudesmol (9.1%) for the essential oil obtained from leaves.65
Other studies were conducted in other countries, such as Palestine and Italy. The study conducted in Palestine revealed that Sesquiterpenoides were the predominant compounds in the essential oil extracted from the leaves of D. viscosa, representing 46.75% of the oil components, followed by steroids accounting for 45.64%. The study identified Twenty-one compounds where Patchulane, 3-b-Phenoxy- 24-nor-cholan-5,20 (22)-diene, 3-Ethyl-3‐hydroxy-5alpha-androstan-17-one and γ-Gurjunene were the most abundant phytochemicals representing 22.82% each respectively.66 Conversely, the GC-MS analysis conducted in Italy on the volatile organic compounds of D. viscosa identified a total of thirty-nine different compounds. Most of these compounds are terpenoids, and in contrast to the findings in the previous studies, Monoterpenes were the most predominant class found in this research (71.29%). Sesquiterpenes that were the major phytochemicals in other studies, represented only 13.84% of the phytochemical ingredients identified.67
The chemical composition of D. viscosa extracts has been extensively studied, revealing a complex array of phytochemicals influenced by extraction methods and geographical variations. Essential oils from Moroccan D. viscosa leaves, primarily consisting of monoterpenes and sesquiterpenes, were found to contain significant amounts of bornyl acetate, borneol, α-amorphene, and caryophyllene oxide.17 In contrast, the hexanic fractions were rich in sesquiterpene derivatives such as isocostic acid, tomentosin, and inuviscolide.18 Algerian studies highlighted the predominance of oxygenated sesquiterpenes, including α-bisabolol, (E)-Z-Farnesylacetone, and (E)-nerolidol, with the extraction method impacting the concentration of specific compounds like 12-carboxyeudesma-3,11 (13)diene and linolenic acid.20,68 Tunisian research identified decanoic acid as a major nonterpenic component, alongside sesquiterpene hydrocarbons such as α-gurjunene and α-selinene.62 Variations in essential oil composition were also noted when comparing different extraction processes, such as solvent extraction and ultrasonic extraction, indicating methodological influences on the resulting phytochemical profiles.64 Studies in Palestine and Italy further underscored the diversity, with sesquiterpenoids and monoterpenes being the most predominant classes, respectively, showcasing the complex and variable phytochemical composition of D. viscosa.66,67
Many compounds of D. viscosa have been studied for their pharmacological effects. Bornyl acetate, one of the major compounds in leaf oils, displayed significant in vitro effects on the inhibition of the proliferation of various cancer cells, including cervical, colon, lung, and breast cancer.69 This compound is also reported to possess antibacterial, antioxidant, and anti-inflammatory properties.16 Other compounds found in the essential oil of D. viscosa, like Borneol, are known for their antimicrobial, antioxidant, insecticidal properties,16 and efficiency in the treatment and prevention of ischemic strokes.70 Tomentosin, isolated from D. viscosa, has shown important antiproliferative activities against leukemia,71 cervical cancer72 and significant antidiabetic properties.73 Other compounds in D. viscosa such as neptin, hispidulin, caryophyllene oxide, 1,8-Cineole, p-menth-1-en-9-ol, 3,4-dihydroxybenzoic acid and methylated quercetins (3,3′-di-O-Methylquercetin and 3-O-methylquercetin) have shown anticancer and antimicrobial potentials.3,27,74 Overall, the investigation of the chemical composition of D. viscosa has unveiled its wealth of biologically active compounds, which can be useful in treating various illnesses.
3.6. Pharmacological properties of D. viscosa
3.6.1. Antioxidant activity
Numerous researchers have undertaken the task of assessing the antioxidant properties of extracts and essential oil obtained from D. viscosa, with a significant number of these investigations affirming substantial antioxidant activity. Various screening techniques, including 2,2-diphenyl-1-picrylhydrazyl (DPPH), Ferric reducing antioxidant power (FRAP), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), and Total antioxidant capacity (TAC), were employed across different parts of the D. viscosa plant to conduct these evaluations. The table below (Table 4) summarizes the findings of the antioxidant activity investigation reported in the literature.
Table 4.
Antioxidant activity of D. viscosa extracts and essential oils.
| Used parts | Extracts | Used Method | Key results | References | |
|---|---|---|---|---|---|
| Leaves | Essential oil | DPPH | IC50 = 1.290 ± 0.055 mg mL−1 (EOD) | 16 | |
| IC50 = 0.007 ± 0.001 mg mL−1(BHT) | |||||
| IC50 = 0.001 ± 0.001 mg/mL (Ascorbic acid) | |||||
| FRAP | EC50 = 35.585 ± 2.520 mg mL−1 (EOD) | ||||
| EC50 = 1.256 ± 0.164 mg mL−1(BHT) | |||||
| EC50 = 0.764 ± 0.125 mg mL−1 (Ascorbic acid) | |||||
| TAC | IC50 = 192.1 ± 0.8 mg AAE/g E (1/10) | ||||
| IC50 = 190.1 ± 0.1 mg AAE/g E (1/20) | |||||
| IC50 = 166.4 ± 0.6 mg AAE/g E (1/40) | |||||
| IC50 = 152.8 ± 0.1 mg AAE/g E (1/80) | |||||
| IC50 = 108.4 ± 0.4 mg AAE/g E (1/160) | |||||
| IC50 = 77.2 ± 1.0 mg AAE/g E (1/320) | |||||
| IC50 = 39.8 ± 0.7 mg AAE/g E (1/640) | |||||
| Leaves | Essential oil | DPPH | IC50 = 13.5 ± 0.44 μg mL−1 (EO) | 66 | |
| IC50 = 3.23 ± 0.92 μg mL−1 (Trolox) | |||||
| FRAP | IC50 = 139.8 ± 2.3 mg Fe/g dry extract | ||||
| Flowers, leaves and aerial parts | Essential oil | DPPH | IC50 = 9.25 mg mL−1 (Aerial parts) | 65 | |
| IC50 = 9.50 mg mL−1(Flowers) | |||||
| IC50 = 9.75 mg mL−1 (Leaves) | |||||
| IC50 = 0.50 mg mL−1 (Quercetine) | |||||
| Leaves | EtOH extract | DPPH | IC50 = 768.06 ± 0.50 μg mL−1 | 75 | |
| IC50 = 58.43 ± 1.74 μg mL−1 (Ascrobic Acid) | |||||
| ABTS | IC50 = 452.08 ± 0.50 μg mL−1 μg/mL | ||||
| IC50 = 65.36 ± 2.34 μg mL−1 (Ascrobic Acid) | |||||
| Leaves and flower buds Parts | EtOH extracts MeOH extracts H2O extracts | DPPH | Leaves | IC50 = 148.79 ± 0.11 μg mL−1 (Mac-MeOH) | 77 |
| IC50 = 77.48 ± 0.16 μg mL−1 (Mac-H2O) | |||||
| IC50 = 75.17 ± 0.60 μg mL−1 (hot-MeOH) | |||||
| IC50 = 59.65 ± 0.68 μg mL−1 (hot-H2O) | |||||
| IC50 = 54.24 ± 0.21 μg mL−1 (Soxhlet-EtOH) | |||||
| IC50 = 48.47 ± 0.44 μg mL−1 (BHT) | |||||
| Flower buds | IC50 = 86.06 ± 0.25 μg mL−1 (Mac-MeOH) | ||||
| IC50 = 54.63 ± 0.85 μg mL−1 (Mac-H2O) | |||||
| IC50 = 74.44 ± 0.32 μg mL−1 (hot-MeOH) | |||||
| IC50 = 47.45 ± 0.62 μg mL−1 (hot-H2O) | |||||
| IC50 = 39.77 ± 0.23 μg mL−1 (Soxhlet-EtOH) | |||||
| IC50 = 48.47 ± 0.44 μg mL−1 (BHT) | |||||
| Reducing power | Leaves | IC50 = 7.21 ± 0.19ASE.mL−1 (Mac-MeOH) | |||
| IC50 = 8.20 ± 0.63 ASE.mL−1 (Mac-H2O) | |||||
| IC50 = 5.05 ± 0.17 ASE.mL−1 (hot-MeOH) | |||||
| IC50 = 5.20 ± 1.27 ASE.mL−1 (hot-H2O) | |||||
| IC50 = 7.56 ± 0.72 ASE.mL−1 (Soxhlet-EtOH) | |||||
| IC50 = 1.97 ± 0.08 ASE.mL−1 (BHT) | |||||
| Flower buds | IC50 = 9.03 ± 0.64ASE.mL−1 (Mac-MeOH) | ||||
| IC50 = 5.51 ± 0.17 ASE.mL−1 (Mac-H2O) | |||||
| IC50 = 5.02 ± 0.12ASE.mL−1 (hot-MeOH) | |||||
| IC50 = 4.65 ± 0.45 ASE.mL−1 (hot-H2O) | |||||
| IC50 = 5.45 ± 0.12 ASE.mL−1 (Soxhlet-EtOH) | |||||
| IC50 = 1.97 ± 0.08 ASE.mL−1 (BHT) | |||||
| Ferrous ions chelating | Leaves | IC50 = 450.85 ± 5.23 μg mL−1 (Mac-H2O) | |||
| IC50 = 6.68 ± 0.04 μg mL−1 (EDTA) | |||||
| Flower buds | IC50 = 199.08 ± 2.14 μg mL−1 (Mac-H2O) | ||||
| IC50 = 549.57 ± 0.31 μg mL−1 (hot-H2O) | |||||
| IC50 = 6.68 ± 0.04 μg mL−1 (EDTA) | |||||
| Leaves | EtOH Extracts | TAC | IC50 = 133.02 ± 3.1 mg AAE/g of sample (Ethanolic extract) | 76 | |
| DPPH | EC50 = 56.25 ± 1.2 μg/mL (Ethanolic extract) | ||||
| EC50 = 26.92 ± 1.22 μg/mL (BHT) | |||||
| ABTS | EC50 = 147.26 ± 1.5 μg/mL (Ethanolic extract) | ||||
| EC50 = 42.64 ± 0.12 μg/mL (BHT) | |||||
| FRAP | IC50 = 296.425 ± 3.3 mg TE/g of sample (Ethanolic extract) | ||||
| Aerial parts | Methanolic extract | DPPH | IC50 = 1.36 mg/L | 78 | |
| IC50 = 0.04 mg/L (Ascorbic Acid) | |||||
| Aerial parts | EtOAc extract Methanol extract Chloroform extract | DPPH | EtOAC | IC50 = 0.6 ± 0.03 μg mL−1 | 61 |
| Methanol | IC50 = 8.2 ± 1.16 μg mL−1 | ||||
| Chloroform | IC50 = 40.8 ± 0.88 μg mL−1 | ||||
| BHT | IC50 = 0.3 ± 0.11 μg mL−1 | ||||
| ABTS | EtOAC | IC50 = 8.6 ± 0.08 μg mL−1 | |||
| Methanol | IC50 = 25.5 ± 0.45 μg mL−1 | ||||
| Chloroform | IC50 = 81.6 ± 0.05 μg mL−1 | ||||
| Ascorbic acid | IC50 = 16.9 ± 4.77 μg mL−1 | ||||
| FRAP | EtOAC | IC50 = 634.8 ± 1.45 mg EAA/g DW | |||
| Methanol | IC50 = 552.1 ± 0.88 mg EAA/g DW | ||||
| Chloroform | IC50 = 90.1 ± 0.66 mg EAA/g DW | ||||
| Whole plant, leaves, steams, flower, and roots | Ethanolic extract Methanolic extract | DPPH | Stem | EC50 = 38.22 μg/mL | 79 |
| Leaves | EC50 = 20.42 μg/mL | ||||
| Flower | EC50 = 23.62 μg/mL | ||||
| Whole plant | EC50 = 17.12 μg/mL | ||||
| Stem | EC50 = 17.42 μg/mL | ||||
| Leaves | EC50 = 18.52 μg/mL | ||||
| Flower | EC50 = 12.52 μg/mL | ||||
| Whole plant | EC50 = 14.62 μg/mL | ||||
| Leaves | Methanolic extract | DPPH | EC50 = 23.33 ± 1.56 μg/mL | 62 | |
| ABTS | EC50 = 16.75 ± 0.26 μg/mL | ||||
| Aerial parts | Ethanol and ethyl acetate extract | DPPH | EtOAc | IC50 = 1.86 g L−1 (Immouzer) | 81 |
| EtOH | IC50 = 0.27 g L−1 (Immouzer) | ||||
| EtOAc | IC50 = 0.28 g L−1 (Sefrou) | ||||
| EtOH | IC50 = 0.20 g L−1 (Sefrou) | ||||
| EtOAc | IC50 = 0.63 g L−1 (Taounate) | ||||
| EtOH | IC50 = 0.18 g L−1 (Taounate) | ||||
| IC50 = 0.15 g L−1 (BHT) | |||||
| IC50 = 0.12 g L−1 (Ascorbic acid) | |||||
| TAC | EtOAc | 91.84 ± 1.52 mg AAE/g dry extract (Immouzer) | |||
| EtOH | 13.61 ± 0.09 mg AAE/g dry extract (Immouzer) | ||||
| EtOAc | 139.31 ± 3.47 mg AAE/g dry extract (Sefrou) | ||||
| EtOH | 103.33 ± 3.17 mg AAE/g dry extract (Sefrou) | ||||
| EtOAc | 103.71 ± 2.78 mg AAE/g dry extract (Taounate) | ||||
| EtOH | 84.85 ± 1.38 mg AAE/g dry extract (Taounate) | ||||
| TAC | EtOAc | 99.79 ± 1.49 mg BHTE/g dry extract (Immouzer) | |||
| EtOH | 8.16 ± 0.02 mg BHTE/g dry extract (Immouzer) | ||||
| EtOAc | 155.42 ± 3.54 mg BHTE/g dry extract (Sefrou) | ||||
| EtOH | 113.25 ± 3.22 mg BHTE/g dry extract (Sefrou) | ||||
| EtOAc | 113.70 ± 3.31 mg BHTE/g dry extract (Taounate) | ||||
| EtOH | 91.61 ± 1.36 mg BHTE/g dry extract (Taounate) | ||||
| Aerial parts | Ethyl acetate extract Diethyl ether extract | DPPH | IC50 = 0.5 μg mL−1 (ethyl acetate) | 82 | |
| IC50 = 0.85 μg mL−1 (diethyl ether) | |||||
| IC50 = 0.97 μg mL−1 (ascorbic acid) | |||||
| Flower, leaves, root parts | Water extract Methanol extract Ethyl acetate extract | DPPH | Water Methanol Ethyl Acetate |
IC50 = 0.28 ± 0.03 mg mL−1 (Flower) | 80 |
| IC50 = 0.47 ± 0.03 mg mL−1 (Leaf) | |||||
| IC50 = 1.07 ± 0.09 mg mL−1 (Root) | |||||
| IC50 = 0.36 ± 0.04 mg mL−1(Flower) | |||||
| IC50 = 0.42 ± 0.02 mg mL−1 (Leaf) | |||||
| IC50 = 0.40 ± 0.08 mg mL−1 (Root) | |||||
| IC50 = 0.99 ± 0.09 mg mL−1(Flower) | |||||
| IC50 = 1.05 ± 0.11 mg mL−1 (Leaf) | |||||
| IC50 = 2.90 ± 0.13 mg mL−1 (Root) | |||||
| ABTS | Water Methanol Ethyl Acetate |
IC50 = 0.17 ± 0.03 mg mL−1(Flower) | |||
| IC50 = 0.21 ± 0.07 mg mL−1 (Leaf) | |||||
| IC50 = 0.23 ± 0.03 mg mL−1 (Root) | |||||
| IC50 = 0.47 ± 0.07 mg mL−1(Flower) | |||||
| IC50 = 0.50 ± 0.09 mg mL−1 (Leaf) | |||||
| IC50 = 0.50 ± 0.01 mg mL−1 (Root) | |||||
| IC50 = 0.55 ± 0.02 mg mL−1(Flower) | |||||
| IC50 = 0.65 ± 0.09 mg mL−1 (Leaf) | |||||
| IC50 = 1.17 ± 0.09 mg mL−1 (Root) | |||||
In a research endeavor carried out in Fez, Morocco, Mssilou, Agoour, Allali et al. (2022) discovered that the essential oil (EO) derived from the leaves of D. viscosa demonstrated a decent ability to reduce DPPH radicals, achieving a Half Maximal Inhibitory Concentration (IC50) of 1.290 ± 0.055 mg/mL. However, when juxtaposed with the control substances BHT (Butylated Hydroxytoluene) and Ascorbic acid, which exhibited IC50 values of 0.007 ± 0.001 mg/mL and 0.001 ± 0.001 mg/mL respectively, the essential oil's antioxidant potency was found to be comparatively lower. The same results were confirmed using the FRAP assay: the essential oil showed less antioxidant activity (35.585 ± 2.52 mg/mL) in comparison to ascorbic acid (Half maximal effective Concentration EC50 = 0.764 ± 0.125 mg/mL) and Butylated Hydroxy-Toluene (BHT) (EC50 = 1.256 ± 0.164 mg/mL). The researchers also assessed the total antioxidant capacity (TAC) of the essential oil at varying concentrations. They discovered that the essential oil exhibited significant antioxidant potential. Specifically, at the lowest dilutions (1/10), the antioxidant capacity was equivalent to 192.1 ± 0.8 mg AAE/g E (Ascorbic Acid Equivalent), and even at the highest dilution tested (1/640), the essential oil maintained a noteworthy antioxidant activity, equivalent to 39.8 ± 0.7 mg AAE/g E..16
In Tunisia, a separate study was conducted to evaluate the antioxidant activity of the essential oil (EO) extracted from various parts of D. viscosa. The findings indicated that the EO from the aerial parts, flowers, and leaves all demonstrated a comparable potential to neutralize DPPH radicals, with an IC50 value of 9.50 mg/mL ± 0.25 mg. However, this antioxidant activity was found to be less pronounced when compared to Quercetin, a known antioxidant, which had an IC50 value of 0.50 mg/mL and was used as a positive control in the assay.65
Similarly, Qneibi et al. (2021) conducted a study in Palestine to investigate the antioxidant properties of the EO from D. viscosa leaves. The results showed that the EO possessed significant DPPH radical scavenging activity, with an IC50 value of 13.5 ± 0.44 μg/mL, although it was less potent than Trolox (IC50 = 3.23 ± 0.92 μg/mL), which served as a positive control. The antioxidant capacity of the EO was further validated using the Ferric Reducing Antioxidant Power (FRAP) assay, yielding a substantial result of 139.8 ± 2.3 mg Fe/g dry extract, confirming the EO's antioxidant potential.66
Beyond the previously mentioned results concerning the essential oil of D. viscosa, additional studies have delved into the antioxidant activity of various parts of the plant using a variety of solvents for extraction. Zeouk et al. (2022) conducted research on the ethanolic extract of D. viscosa leaves collected in Morocco. The findings indicated that the ethanol extract had a potential to reduce the DPPH radical with an IC50 of 768.06 ± 0.5 μg/mL compared to an IC50 of 58.43 ± 1.74 μg/mL for Ascrobic acid. Identical results were confirmed using the ABTS method, in which the ethanolic extract exhibited less antioxidant ability IC50 = 452.08 ± 0.5 μg/mL μg/mL, compared to ascorbic acid that was used as a reference, where IC50 = 65.36 ± 2.34 μg/mL.75 Rhimi el al. (2019) found that the EtOH extracts of D. viscosa leaves in Tunisia had better antioxidant activity compared to Zeouk et al. (2022) findings. The ethanolic extract in this case demonstrated a radical scavenging activity with an EC50 value of 56.25 ± 1.2 μg/mL for DPPH and 147.26 ± 1.5 μg/mL for the ABTS radical. The results for TAC and FRAP were IC50 = 133.02 ± 3.1 mg AAE/g and IC50 = 296.425 ± 3.3 mg TE/g of sample, consecutively.76
Additionally, a study conducted in Morocco used two solvents and three distinct extraction methods: the researchers used water (H2O) and methanol as solvents for hot extraction and maceration, while ethanol was used for Soxhlet extractions. The DPPH assay results revealed that the extracts demonstrated substantial free radical scavenging activity, in comparison to BHT utilized as a reference standard (IC50 = 48.47 ± 0.44 μg/mL). The free radical neutralizing capacity of the leaf extracts ranged from 54.24 ± 0.21 μg/mL in the Soxhlet method to 148.79 ± 0.11 μg/mL in the methanolic maceration method. In contrast, the IC50 values for the flower bud extracts varied between 39.77 ± 0.23 μg/mL and 86.06 ± 0.25 μg/mL when extracted using the Soxhlet method. The extracts exhibited reducing power, with Ascorbic acid equivalents per ml (ASE/ml) values fluctuating from 5.05 ± 0.17 (hot-MeOH) to 8.20 ± 0.63 (Mac-H2O) for the leaves, and from 4.65 ± 0.45 (hot-H2O) to 9.03 ± 0.64 (Mac-H2O) for the flower buds. Regarding the iron chelating activity experiment, only the aqueous maceration extracts of flower buds and leaves and the hot extraction of flower buds with water (hot-H2O) had mild activity.77
To assess the antioxidant potential of the aerial parts of D. viscosa collected in Morocco, Asraoui et al. (2021) used three distinct assays: DPPH, ABTS, and FRAP. The extraction of the samples was performed using methanol, ethyl acetate (EtOAc), and chloroform. All the various extracts showed a significant antioxidant activity. However, the EtOAc extract displayed the highest antioxidant capacity, with Methanol and Chloroform extracts following in effectiveness. However, the EtOAc extract demonstrated superior antioxidant capabilities compared to the Methanol and Chloroform extracts. Specifically, the EtOAc extract achieved an IC50 value of 0.6 μg/mL in the DPPH assay, 8.6 μg/mL in the ABTS assay, and showed a FRAP assay result of 634.8 mg EAA/g DW. On the other hand, the Methanol extract presented IC50 values of 8.2 ± 1.16 μg/mL for DPPH and 25.5 ± 0.45 μg/mL for ABTS. In addition to a reducing power of 552.1 ± 0.88 mg EAA/g DW in the FRAP test. Finally, the Chloroform extract presented values of 40.8 ± 0.88 μg/mL for the DPPH radical, 81.6 ± 0.05 μg/mL for ABTS and 90.1 ± 0.66 mg EAA/g DW for the FRAP test.61 The Methanol extract derived from the aerial parts of D. viscosa showed a better antiradical potential in a study done in Algeria by Ounaissia (2021); in which the IC50 for DPPH was 1.36 mg/L compared to 8.2 ± 1.16 mg/L found by Asraoui et al. (2021).61,78
Salim et al. (2017) conducted a study to assess the antioxidant capacities of different parts of D. viscosa collected in Palestine, utilizing ethanol and methanol for the extraction process. The DPPH free radical scavenging method was employed to evaluate the findings, with results expressed in terms of the minimum extract concentration needed to achieve 50% inhibition of DPPH radicals. The results indicated that the plant parts extracted using methanol as a solvent exhibited higher antioxidant potential compared to the ethanolic extracts. The flowers’ methanolic extract displayed the best antiradical activity (EC50 = 12.52 μg/mL), followed by the whole plant, stems, and leaves (EC50 = 18.52 μg/mL). Regarding the results for the ethanolic extracts, the whole plant displayed the best antioxidant activity (EC50 = 17.12 μg/mL) followed by leaves, flowers, and stems (EC50 = 38.22 μg/mL).79 Mahmoudi et al. (2016) also studied the antioxidant activity of the leaf methanolic extract and found the EC50 to be 23.33 and 16.75 μg/mL for the DPPH and ABTS assays, respectively.62
In another study carried out in Turkey. Different parts of D. viscosa were analyzed using water, methanol and ethyl acetate as extraction solvents. The study revealed that the water extracts displayed the highest antioxidant activity, followed by the methanolic and EtOAc extracts. For all the solvents’ extracts, flowers exhibited the best antiradical activity followed by leaves and roots. The DPPH free radical scavenging activity for water extracts ranged from IC50 = 0.28 ± 0.03 mg/mL for flowers to IC50 = 1.07 ± 0.09 mg/mL for roots. Whereas the IC50 for methanol extracts ranged from 0.36 ± 0.04 mg/mL to 0.42 ± 0.02 mg/mL for flower and root extracts, respectively. The ethyl acetate extracts that showed the lowest antioxidant potential had an IC50 of 0.99 ± 0.09 mg/mL for flower and 2.90 ± 0.13 mg/mL for root extracts. The same findings were confirmed using the ABTS free radical scavenging activity. The results of the ABTS ranged from IC50 = 0.17 ± 0.03 mg/mL being the best for flower water extract to IC50 = 1.17 ± 0.09 mg/mL being the lowest for ethyl Acetate root extracts.80
Chahmi et al. (2015) conducted a study in Morocco to compare the antioxidant activity of D. viscosa aerial parts from three different regions, utilizing ethanol and ethyl acetate for extraction. The ethanol extract from Taounate exhibited superior DPPH free radical scavenging activity, with an IC50 value of 0.18 g/L, closely matching the inhibition potential of positive controls BHT (IC50 = 0.15 g/L) and ascorbic acid (IC50 = 0.12 g/L). This was followed by ethanol samples from Sefrou and Imouzzer. In terms of ethyl acetate extracts, Sefrou's extract demonstrated the highest antiradical capacity with an IC50 value of 0.28 g/L, followed by Taounate (IC50 = 0.63 g/L) and Imouzzer (1.86 g/L). The total antioxidant capacity assay revealed that samples extracted with ethyl acetate had a superior capacity compared to those extracted with ethanol, and antioxidant capacities varied across the three regions. Sefrou exhibited the highest value with (139.31 ± 3.47) mg/g equivalent to ascorbic acid and (155.42 ± 3.54) mg/g equivalent to BHT per gram of dry extract, followed by Taounate and Imouzzer.81 Additionally, a study focusing on the antioxidant activity of D. viscosa from Al Houceima (North Morocco) revealed that the ethyl acetate extract of the aerial parts had better DPPH free radical scavenging activity than the diethyl ether extract, with IC50 values of 0.5 μg/mL and 0.85 μg/mL, respectively. However, all extracts showcased superior antiradical activity compared to ascorbic acid, used as a positive control, which had an IC50 of 0.97 μg/mL.82
3.6.2. Antidiabetic activity
Among other pharmacological activities possessed by D. viscosa, some studies focused on studying its antidiabetic properties. Indeed, D. viscosa extracts were found to have a promising antidiabetic properies through their ability to inhibit α-amylase and α-glucosidase enzymes. These enzymes play a crucial role in elevating postprandial blood sugar levels, subsequently increasing the risk of diabetes onset. Table 5 below summarizes the most relevant literature found regarding the antidiabetic activity of D. viscosa.
Table 5.
In vitro and In vivo Antidiabetic effects of D. viscosa.
| Part used | Extracts |
In vitro/In vivo Assay method |
Keys results | References | |
|---|---|---|---|---|---|
| Leaves | Methanolic extract | In vitro anti-hyperglycemic potential | α-amylase α-glucosidase |
IC50 = 1.381 ± 0.085 (mg mL−1) IC50 = 0.046 ± 0.001 (mg mL−1) Acarbose IC50 = 0.118 ± 0.02 (mg mL−1) IC50 = 0.329 ± 0.041 (mg mL−1) Acarbose |
83 |
| Aerial parts | Dichloromethane extract Ethanol extract |
In vitro anti-hyperglycemic potential | α-amylase α-glucosidase |
IC50 = 26.89 ± 1.54 μM (Tomentosin) IC50 = 0.01 ± 0.00 μM (Acarbose) IC50 = 26.61 ± 0.236 μM (Tomentosin) IC50 = 22.80 ± 0.00 μM (Acarbose) |
73 |
| Leaves | EtOAc extract Methanol extract Chloroform extract |
In vitro anti-hyperglycemic potential | α-glucosidase α-amylase |
IC50 = 29.9 ± 1.04 μg mL−1 (EtOAc extract) IC50 = 22.3 ± 2.82 μg mL−1 (Methanol extract) IC50 = 39.8 ± 0.76 μg mL−1 (CHCl3 extract) IC50 = 33.0 ± 0.00 μg mL−1 (acarbose) I = 22 % 1 g/mL (EtOAc extract) I = 27 % 1 g/mL (MeOH extract) I = 17 % 1 g/mL (CHCl3 extract) |
61 |
| Leaves | EtOH extract | In vivo: measurement of blood glucose of diabetic rats (Alloxan induced diabetes) | Neutral anti-hyperglycemic effect on blood sugar levels | 85 | |
| Flowers, leaves and roots | Aqueous extract MeOH extract EtOAc extract |
In vitro anti-hyperglycemic potential |
Root extract: α-glucosidase α-amylase |
I = 5.16 % (3000 μg mL−1 - Water extract) I = 50.65 % (3000 μg mL−1 -MeOH extract) I = 16.56 % (3000 μg/mL-MeOH extract) |
84 |
|
Flower extract: α-glucosidase α-amylase |
I = 54.33 % (3000 μg mL−1 - Water extract) I = 15.47 % (1000 μg mL−1 - Water extract) I = 6.27 % (570 μg mL−1 - Water extract) I = 90.90 % (3000 μg mL−1 - MeOH extract) I = 36.43 % (1000 μg.mL−1-MeOH extract) I = 20.52 % (570 μg.mL−1-MeOH extract) I = 1.22 % (300 μg.mL−1-MeOH extract) I = 4.62 % (3000 μg/mL-MeOH extract) I = 4.23 % (3000 μg/mL-EtOAc extract) |
||||
|
leave parts: α-glucosidase α-amylase |
I = 63.37 % (3000 μg mL−1 - Water extract) I = 31.47 % (1000 μg mL−1 - Water extract) I = 15.58 % (570 μg mL−1 - Water extract) I = 6.54 % (300 μg mL−1 - Water extract) I = 92.87 % (3000 μg mL−1 - MeOH extract) I = 51.70 % (1000 μg mL−1 - MeOH extract) I = 20.51 % (570 μg mL−1 - MeOH extract) I = 8.84 % (300 μg mL−1 - MeOH extract) I = 2.30 % (100 μg mL−1 - MeOH extract) I = 15.77 % (3000 μg.mL−1-MeOH extract) I = 4.35 % (3000 μg.mL−1-EtOAc extract) |
||||
| Aerial parts | Ethanolic extracts dichloromethane extract Ethyl acetate extract |
In vivo: Measurement of blood glucose after administration of 12.5, 25 and 50 mg/kg of D. viscosa extracts to Alloxan induced diabetic rats | - The administration of high doses of D. viscosa extracts is associated with significant hypoglycemic effect. 50 mg/kg of the extract decreased the blood sugar from 1.8000 to 1.2033 g/L - Significant and exaggerated hypoglycemic action after the eighth day from 1.7150 to 0.9750 g/L. |
86 | |
| Aerial parts | Aqueous extract | In vivo: Measurement of blood glucose after administration of 20 mg/kg of D. viscosa extracts to Streptozotocin (STZ) induced diabetic rats | Remarkable hypoglycemic activity without effect on plasma insulin in diabetic and normal rats. The exhibited hypoglycemic effect seems to be independent of insulin secretion. | 52 | |
Mrid et al. (2022) studied the α-amylase and α-glucosidase inhibitory action of D. viscosa leaf methanolic extract. The study confirmed that the extracts exhibit a high inhibitory effect on both enzymes. The half maximal inhibitory concentration (IC50) of α-amylase was 1.381 mg/mL compared to 0.046 mg/mL for acarbose that is used as a positive control, whereas the IC50 of α-glucosidase was 0.118 mg/mL against 0.329 mg/mL for acarbose.83 In line with these findings, Asraoui et al. (2021) discovered that the methanolic extract possessed a better anti α-glucosidase activity than the ethyl acetate and chloroform extracts, with an IC50 of 0.030 mg/mL and 0.040 mg/mL, respectively, compared to an IC50 of 0.033 μg/mL for the control drug (acarbose). The anti α-amylase activity follows the same pattern. At 1 mg/mL, Methanol, Ethyl Acetate, and Chloroform inhibited α-amylase by 27%, 22%, and 17%, respectively.61 Other research has found that methanolic extracts from different parts of D. viscosa have higher anti-diabetic activity than other solvent extracts. At a concentration of 3000 g/mL, the inhibition percentage for α-glucosidase in methanolic extracts of different parts of the plant ranges from 50.65% for roots to 90.90% and 92.87% for flowers and leaves, respectively. On the other hand, the inhibition for water extracts fluctuates between 5% and 65% depending of the parts used.84
Knowing that the D. viscosa extract are rich sesquiterpenes, Aydin et al. (2022) attempted to isolate Tomentosin; a sesquiterpene lactone found in the ethanolic and dichloromethanic extract of D. viscosa. The analysis of the antidiabetic activity of this compound showed that its IC50 for α-amylase is IC50 = 26.89 ± 1.54 μM compared to IC50 = 0.01 μM for Acarbose, whereas its IC50 for α-glucosidase is IC50 = 26.61 ± 0.236 μM compared to IC50 = 22.80 μM for Acarbose. These results confirm that the isolated phytochemical ingredient has an antidiabetic activity.73
Indeed, there have been varying results from both in vitro and in vivo studies regarding the antidiabetic effects of D. viscosa. Alkofahi et al. (2017) administered a 1 mg/kg dose of the ethanolic extract of D. viscosa to both normal and alloxan-induced diabetic rats, but found no significant impact on blood sugar levels in either group.85 On the other hand, Assi et al. (2015) observed a substantial decrease in blood glucose levels in diabetic rats given a higher dose of 50 mg/kg D. viscosa, with levels dropping from 180.00 to 120.33 mg/dl. This suggests that the antidiabetic effects of D. viscosa may be dose-dependent.86 Similarly, Zeggwagh et al. (2006) reported a notable hypoglycemic effect at a dose of 20 mg/kg, yet interestingly, this effect was not accompanied by changes in plasma insulin levels in either diabetic or normal rats. This indicates that the hypoglycemic activity of D. viscosa may operate independently of insulin secretion.73
3.6.3. Antimicrobial and antifungal activity
The antibacterial properties of D. viscosa extracts and essential oil have been extensively studied, with a variety of results. A summary of the most pertinent findings is presented in Table 6.
Table 6.
Antibacterial, and antifungal effects of D. viscosa.
| Use Parts | Extracts | Used Method | Tested strains | Key results | References |
|---|---|---|---|---|---|
| Aerial Parts |
Methanolic extract | Disc diffusion method (2 mg/disc) Broth dilution method |
Staphylococcus aureus ATCC 43300 Escherichia coli ATCC 25922 Bacillus subtilis ATCC 6633 Micrococcus luteus ATCC 10240 Klebsiella pneumoniae ATCC 43816 |
Ф = 25 ± 1 mm MIC = 0.25 mg mL−1 Ф = 11.33 ± 0.58 mm MIC = 1 mg/mL−1 Ф = 22 ± 1 mm MIC = 0.25 mg/mL−1 Ф = 18.5 ± 0.5 mm MIC = 0.5 mg mL−1 Ф = 10.67 ± 0.58 mm MIC = 2 mg mL−1 |
87 |
| Essential oil | Microdilution assay |
Staphylococcus aureus ATCC 29213 Staphylococcus aureus Clinical/MRSA Staphylococcus epidermidis Human Streptococcus pyogenes ATCC 19615 Streptococcus agalactiae Clinical Enterococcus faecalis ATCC 29212 Listeria monocytogenes ATCC 19111 (1/2a) Bacillus cereus Food Clostridium perfringens Food Escherichia coli ATCC 25922 Acinetobacter baumannii ATCC 19606 Candida albicans ATCC 90029 Aspergillus niger Food |
MIC = 2.8 mg mL−1 MBC = 2.8 mg mL−1 MIC = 5.6 mg mL−1 MBC = 5.6 mg mL−1 MIC = 1.4 mg mL−1 MBC = 1.4 mg mL−1 MIC = 0.09 mg mL−1 MBC = 0.09 mg mL−1 MIC = 0.09 mg mL−1 MBC = 0.09 mg mL−1 MIC = 1.4 mg mL−1 MBC = 2.8 mg mL−1 MIC = 2.8 mg mL−1 MBC = 2.8 mg mL−1 MIC = 0.7 mg mL−1 MBC = 0.7 mg mL−1 MIC = 0.09 mg mL−1 MBC = 0.09 mg mL−1 MIC = 2.8 mg mL−1 MBC = 2.8 mg mL−1 MIC = 5.6 mg mL−1 MBC = 5.6 mg mL−1 MIC50 = 2.8 mg mL−1 MBC50 = 5.6 mg mL−1 MIC50 = 0.09 mg mL−1 MBC50 = 5.6 mg mL−1 |
3 | |
| Aerial parts | Essential oil | Disk diffusion method |
Staphylococcus aureus E. enterica Klebsiella pneumoniae Staphylococcus aureus (SARM) ESBL/BLSE Escherichia coli Pseudomonas aeruginosa Listeria monocytogenes Proteus mirabilis Enterobacter cloacae Acetobacter sp Serratia marcescens Bacillus cereus Enterococcus faecalis Serratia liquefaciens |
Ф = 26.84 (mm) Ф = 24.94 (mm) Ф = 21.43 (mm) Ф = 21.04 (mm) Ф = 19.54 (mm) Ф = 19.04 (mm) Ф = 17.47 (mm) Ф = 17.31 (mm) Ф = 16.85 (mm) Ф = 14.67 (mm) Ф = 14.51 (mm) Ф = 14.32 (mm) Ф = 14.11 (mm) Ф = 13.30 (mm) Ф = 13.18 (mm) |
88 |
| Aqueous extract Methanol extract |
Disc diffusion assay |
Streptococcus pyogenes ATCC19615 Staphylococcus aureus ATCC25923 Staphylococcus epidermidis ATCC12228 Escherichia coli ATCC 25922 Pseudomonas aeruginosa ATCC27853 Salmonella typhimurium ATCC14028 Serratia marcescens ATCC 8100 Proteus vulgaris ATCC 13315 Enterobacter cloacae ATCC23355 Klebsiella pneumoniae ATCC 13883 |
Ф = 16.8 mm (Water) Ф = 16.8 mm (MeOH) Ф = 14.0 mm (Water) Ф = 14.0 mm (MeOH) Ф = 10.4 mm (Water) Ф = 16.4 mm (MeOH) No inhibition No inhibition No inhibition No inhibition No inhibition No inhibition No inhibition |
22 | |
| Leaves | Essential oil | Disk Diffusion Method Microdilution method |
Escherichia coli Pseudomonas aeruginosa Klebsiella pneumonia Staphylococcus aureus Candida albicans Saccharomyces cerevisiae |
Ф = 9.5 ± 0.5 (mm) MIC = 0.406 (mg/mL) Resistant MIC = 1.625 (mg/mL) Resistant MIC = 0.406 (mg/mL) Ф = 31.0 ± 1.5 (mm) MIC = 0.101 (mg/mL) Ф = 20.4 ± 0.5 (mm) MIC = 0.203 (mg/mL) Ф = 28.0 ± 1.0 (mm) MIC = 3.250 (mg/mL) |
16 |
| polyphenolic compounds were extracted from the leaves by maceration in methanol and hexane fractionation | Disk diffusion method Microdilution method |
Morganella morganii Staphylococcus aureus ATCC 29213 Staphylococcus aureus Escherichia coli ATCC 25922 Klebsiella pneumonia Pseudomonas aeruginosa Escherichia coli |
Ф = 21.0 (mm) MIC = 25 mg/mL Ф = 21.0 (mm) MIC = 50 mg/mL Ф = 20.5 (mm) MIC = 0.39 mg/mL Ф = 17.0 (mm) MIC = 100 mg/mL Ф = 19.0 (mm) MIC = 50 mg/mL Ф = 15.0 (mm) MIC = 0.39 mg/mL Ф = 10.8 (mm) MIC = 100 mg/mL |
89 | |
| Lipid extract of D. viscosa leaves | Disk diffusion method (50 mg/mL LLE) Broth Microdilution method |
Candida prapsilosis ATCC 22019 Candida krusei ATCC 6258 Candida albicans CD1378 Candida albicans CD1407 Candida albicans CD1408 Malassezia pachydermatis CD 112 Malassezia pachydermatis CD 90 Malassezia furfur CBS1978 Malassezia furfur CD 1006 Malassezia furfur CD1029 |
Ф = 8.0 mm MIC = 5.0 μg/mL MFC = 10.0 μg/mL Ф = 10.5 mm MIC = 5.0 μg/mL MFC = 5.0 μg/mL Ф = 9.5 mm MIC = 5.0 μg/mL MFC = 10.0 μg/mL Ф = 10.2 mm MIC = 5.0 μg/mL MFC = 10.0 μg/mL Ф = 9.25 mm MIC = 5.0 μg/mL MFC = 10.0 μg/mL Ф = 16.5 mm MIC = 5.0 μg/mL MFC = 5.0 μg/mL Ф = 17.5 mm MIC = 5.0 μg/mL MFC = 5.0 μg/mL Ф = 12.0 mm MIC = 5.0 μg/mL MFC = 5.0 μg/mL Ф = 10.3 mm MIC = 5.0 μg/mL MFC = 5.0 μg/mL Ф = 8.5 mm MIC = 5.0 μg/mL MFC = 5.0 μg/mL |
90 | |
| Leaves Flower buds' parts |
Ethanol extract Methanol extract Aqueous extract |
– |
Candida albicans ATCC 10231 Staphylococcus aureus ATCC 6538 Escherichia coli ATCC 25922 Klebsiella pneumoniae S20/16 food |
MICs = 125 mg/mL (leaves) MICs = 250 mg/mL (flowers) MIC = 250 mg/mL (leaves) No inhibition (flowers) MIC = 250 mg/mL (leaves) No inhibition (flowers) MIC = 250 mg/mL (leaves) No inhibition (flowers) |
77 |
Alfarrayeh et al. (2022) discovered that the methanolic extract of D. viscosa was more potent against gram-positive bacteria such as Staphylococcus aureus, Bacillus subtilis, and Micrococcus luteus than against gram-negative bacteria like Escherichia coli and Klebsiella pneumoniae. The methanolic extract demonstrated the highest inhibition against Staphylococcus aureus and Bacillus Subtilis, with inhibition diameters of 25 ± 1 mm and 22 ± 1 mm, respectively. The minimum inhibitory concentration (MIC) values ranged from 0.25 to 2 mg/mL.87 In terms of the essential oil's antibacterial activity, Ounoughi et al. (2020) reported a significant antibacterial effect against Staphylococcus aureus, with an average inhibition diameter of 26.84 mm. However, Enterococcus faecalis and Serratia liquefaciens were less susceptible, showing average inhibition diameters of 13.30 mm and 13.18 mm, respectively.88 On the other hand, Vuko et al. (2021) found that the essential oil of D. viscosa exhibited a strong concentration-dependent bactericidal effect against both Gram-positive and Gram-negative bacteria. The most pronounced effects were observed against Streptococcus pyogenes ATCC 19615, Streptococcus agalactiae, and Clostridium perfringens, all of which had a MIC of 0.09 mg/mL. These bacteria are known to play a significant role in skin and soft tissue infections in adults. Furthermore, the essential oil was found to be effective against Staphylococcus aureus ATCC 29213 and a clinical strain of methicillin-resistant Staphylococcus aureus (MRSA), eliminating them at dilutions of 2.81 and 5.62 mg/mL, respectively.3
Ozkan et al. (2019) conducted a study revealing that the ethanolic and aqueous extracts of D. viscosa demonstrated effectiveness selectively against specific strains of gram-positive bacteria. The bacteria types that were affected include Staphylococcus epidermidis, Staphylococcus aureus, and Streptococcus pyogenes. The most effective antibacterial activity was recorded against Streptococcus pyogenes showcasing an inhibition diameter measuring 16.8 mm. The methanol extract also had a strong effect on Staphylococcus epidermidis (16.4 mm). However, the three types of extracts did not have any antibacterial effects on other types of gram-positive bacteria.22 Conversely, Mssillou et al. (2022) found that the essential oil from D. viscosa displayed some activity on Escherichia Coli with an inhibition diameter of 9.5 ± 0.5 mm. The oil was also more effective against Staphylococcus aureus with an inhibition zone of 31.0 ± 1.5 mm. Nevertheless, Pseudomonas aeruginosa, and Klebsiella pneumoniae showed resistance to the oil.16 In addition, Mohti et al. (2020) observed that the ethanol extract of D. viscosa was effective against Escherichia Coli and Klebsiella pneumoniae. The extract demonstrated considerable effectiveness against Staphylococcus aureus ATCC 6538, as well as various other bacteria strains originating from both ATCC and food-related sources.77
In a study investigating the antibacterial properties of polyphenolic compounds from D. viscosa leaves, the compounds were extracted through maceration in methanol and subsequent hexane fractionation. The bacterial strains tested showed sensitivity to the polyphenolic extract, with inhibition diameters ranging between 10.8 and 21 mm. The extract was particularly effective against Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus, surpassing the efficacy of other tested antibiotics. The most favorable minimal inhibitory concentration (MIC) values were recorded against Staphylococcus aureus, Morganella morganii, and Pseudomonas aeruginosa, each at 0.39 mg/mL. On the other hand, both strains of Escherichia coli exhibited the highest MIC values. The polyphenolic extract demonstrated a bacteriostatic effect on Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Staphylococcus aureus ATCC29213. Conversely, it showed a bactericidal effect against Staphylococcus aureus, Morganella morganii, and Escherichia coli ATCC25922.89
Regarding the anti-fungal properties of D. viscosa, Rhimi et al. (2018) found that the lipid extracts are effective against various strains of Candida and Malassezia. The size of the inhibition zone varied between 8 and 14 mm for Candida strains and 8.5–20 mm for Malassezia strains. The lipid extract at concentrations of 50 mg/mL and 100 mg/mL was found to be more effective than the drug Fluconazole against certain strains of Candida krusei and Malassezia, with inhibition diameters ranging from 0 to 16.5 mm. The lipid extract of D. viscosa showed a consistent minimum inhibitory concentration (MIC) of 5 mg/mL for all examined yeast species. However, the minimum fungicidal concentration (MFC) values ranged from 5 to 10 mg/mL. Some Candida strains exhibited the highest MFC values and also displayed reduced susceptibility to Fluconazole, with MIC and MFC values exceeding 64 μg/mL and 128 μg/mL, respectively.90 Same results for Candida albicans were confirmed by Mohti el al. (2020) where the extract from leaves had a significant effectiveness against the species with a minimum inhibitory concentration of 125 mg/mL.77 The latter results were also backed by the findings of Mssilou et al. (2022), where the essential oil derived from the leaves demonstrated antifungal activity against Candida albicans and Saccharomyces cerevisiae. The inhibition diameters were measured at 20.4 ± 0.5 mm for Candida albicans and 28.0 ± 1.0 mm for Saccharomyces cerevisiae.16
3.6.4. Analgesic effect
Many studies have been done on the pharmacological activities of D. viscosa extracts and its bioactive compounds, showing a wide range of potential therapeutic effects. Table 7 reports a series of experimental investigations into the analgesic properties of D. viscosa. Mssillou et al. (2022) conducted an experiment where rats were subjected to intraperitoneal injections of acetic acid to induce a writhing response. Following this induction, the rats were treated with an oral administration of 500 mg/kg of D. viscosa's hydroethanolic extract. The treatment resulted in a significant analgesic effect, evidenced by a decrease in abdominal contractions to 52.6 ± 7.68, compared to 97.8 ± 6.24 for the negative control group, which did not receive any treatment.91 Identical results were also found by Ouahchia et al. (2020) where the administration of D. viscosa extracts at different doses was associated with a remarkable analgesic effect. The maximum inhibition for writhing was observed with a dose of 800 mg/kg of the ethanolic extract obtained from leaves (93.39%).92 The conclusions of the last studies are backed by the findings of Side Larbi et al. (2016) who investigated the effect of both the methanol extract and essential oil on central analgesia in rats. The study revealed that both samples had a significant central analgesia by increasing the latency time in the tail immersion test, with a clear dose-dependent relationship in the induced analgesic effect.58
Table 7.
Analgesic activity of D. viscosa.
| Part Used | Extracts | Experimental approach | Key results | References |
|---|---|---|---|---|
| Leaves | Hydroethanolic extract | Acetic acid method. Intraperitoneally (10 mg/mL) | Important analgesic effect of D. viscosa (52.6 ± 7.68) against Acetic acid induced pain. | 91 |
| Leaves and flowers | Methanolic extracts and decoctions of D. viscosa | Acetic acid-induced writhes test. (Intraperitoneally) | Significant pain relief was observed at the tested doses, with the highest degree of inhibition of writhing occurring at a dose of 800 mg/kg (93.39%) using the methanolic extract of the leaves. | 92 |
| Leaves and flower | Methanolic extracts (300 and 500 mg/kg), Essential oils (0.06 and 0.1 mL/kg) | Tail immersion test. Oral administration (0.06 mL/kg) 0.1 mL/kg) |
The methanolic extracts had a significant and dose dependent central analgesic effect which was evident by an increase in the reaction time of rats when subjected to a thermal stimulus. Important analgesic effect of D. viscosa methanolic extract, the best activity was observed at a dosage of 500 mg/kg (83%) with a latency time of 15.76 ± 0.03 Seconds. |
58 |
3.6.5. Wound healing activity
In addition to its analgesic effect, D. viscosa is also thought to possess wound healing potential, as shown in Table 8. Burn wounds were induced in a group of rats, which were then treated with ointments based on a 10% hydroethanolic extract of D. viscosa, a 10% extract of Marrubium vulgare, a mixture of both, and Madecasol®. The wound size was observed for 21 days, and the diameter of the wounds was compared among the different groups and with the negative control group, which received only Vaseline®. The results demonstrated that the group treated with the D. viscosa ointment exhibited better wound closure at day 21 (99.28 ± 0.44%) compared to all other groups.91 In agreement with these findings, Rhimi et al. (2019) observed that the group treated with an ointment based on 5% D. viscosa experienced a significant decrease in wound diameter compared with the negative control group.76 Further supporting these outcomes, another study involved the induction of full-thickness wounds in the dorsal area of mice, followed by treatment for 16 days with extracts from five different medicinal plants, namely Parieteria diffusa, Laurus nobilis, Ajuga chia, and Rubia taenifolia, in addition to D. viscosa. The aqueous extract of Inula viscosa exhibited the most significant wound healing activity, evidenced by a wound area of 0.54 ± 0.12 at day 16, compared to 1.00 ± 0.43 for the control group. This same extract also showed superior histological results, evidenced by full-thickness coverage of the wound area with an organized epidermis and the presence of mature scar tissue in the dermis.93
Table 8.
Wound healing activity of D. viscosa.
| Part Used | Extracts | Experimental approach | Key results | References |
|---|---|---|---|---|
| Leaves | Hydroethanolic extract | Wound Healing Test, Burn Wound Induction (on dorsal part) | Wound closure D. viscosa(10%) = 99.28 ± 0.44% (Day 21) Wound closure M. vulgare(10%) = 97.78 ± 4.95% (Day 21) Wound closure the mixture = 97.96 ± 2.91% (Day 21) Wound closure Madecassol® = 86.74 ± 9.9% (Day 21) The groups treated with D. viscosa and M. vulgare and their mixture had significant wound decrease after 21st day at a dosage of 500 mg/kg in comparison to the negative control, group and Madecassol® at (1%) as a positive control group. |
91 |
| Ethanolic Extract | Wound Healing test: - Ointments Preparation |
The wound in groups treated with the ointment containing D. viscosa 5% had significantly decreased the wound area on the 3, 9, and 12 days, in comparison to the negative control group, the ointment containing D. viscosa 2.5% and ointment containing (vehiculum) group. | 76 | |
| Aerial parts | Aqueous extract | Wound induction in the dorsal area of the mice Observation of the wound diameter for 16 days |
Wound area: 0.54 ± 0.12 at day 16 compared to 1.11 ± 0.18 at day 5 Control group 1.00 ± 0.15 at day 5 and 1.00 ± 0.43 at day 16 Histological analysis showed complete epithelialization and mature scar formation in the dermis. D. viscosa extract significantly outperformed Parietaria diffusa, Laurus nobilis, and Ajuga chia in wound healing efficacy, with Rubia tinctorum extract being the least effective. |
93 |
3.6.6. Anti-inflammatory activity
The results of studies conducted on the anti-inflammatory activity of D. viscosa are reported in Table 9. The anti-inflammatory properties of D. viscosa's methanolic extract were evaluated by measuring protein denaturation inhibition, revealing an inhibition range of 18.16%–44.44% at concentrations of 1.2 mg/mL to 2 mg/mL, respectively. This contrasts with Acetylsalicylic acid, which exhibited 30.18%–66.51% inhibition at the same concentrations.78 Another study of the anti-inflammatory effect of D. viscosa found that the rate of Elastase enzyme inhibition was clearly concentration-dependent, achieving up to 72% inhibition at a concentration of 10 mg/mL. This level of inhibition is comparable to that of Epigallocatechin Gallate, known as a very potent inhibitor of both collagenase and elastase.90 Furthermore, Lounis et al. (2018) investigated the in vivo and in vitro anti-inflammatory potential of the aqueous extract derived from D. viscosa leaves. Their study used carrageenan to induce inflammation in mice paws and measured the levels of inducible nitric oxide synthase (INOS), MyD88, TLR-4, TNF-α and nitric oxide (NO) release from LPS-stimulated J774A.1 macrophages. Both in vivo and in vitro studies demonstrated that the extract decreased Nitric Oxide production and inhibited iNOS expression in a dose-dependent manner, and moderately reduced paw edema 6 h post-carrageenan stimulation. The observed in vitro effect is attributed to the extract's ability to inhibit LPS-induced NO production by suppressing iNOS expression.94 Additionally, Two further studies confirmed that the ethanolic extract and decoction of D. viscosa leaves and flowers decreased the paw edema in rats.91,92
Table 9.
Anti-inflammatory activity of D. viscosa.
| Part Used | Extracts | Experimental approach | Key results | References |
|---|---|---|---|---|
| Leaves | Hydro-ethanolic extract | Carrageenan-induced paw edema (500 mg/kg) | The D. viscosa extract had significantly inhibited the paw edema mice after 6 h at a dose of 500 mg/kg body weight in comparison to the other groups. | 91 |
| Aqueous extract | - Carrageenan-induced paw inflammation - measured the expression of inducible iNOS, MyD88, TLR-4, TNF-α and nitric oxide (NO) release from LPS-stimulated J774A.1 macrophages |
- Decreased the production of NO - Dose-dependent in vivo and in vitro inhibition of the expression of iNOS - Moderate reduction in paw edema after 6 h of carrageenan |
94 | |
| Lipid extract (LLE) | Elastase enzyme Inhibition Activity | I = 25% (1 mg/mL) I = 45% (5 mg/mL) I = 72% (10 mg/mL) I = 82% (0.2 mg/mL) Epigallocatechin Gallate |
90 | |
| Aerial parts | Methanolic extracts at different concentrations | In-vitro anti-inflammatory activity inhibition of albumin denaturation | I = 18.16 % (1.2 mg/mL) I = 35.43 % (1.6 mg/mL) I = 44.44 % (2 mg/mL) |
78 |
| Leaves and Flowers | Methanolic extracts. Decoctions of leaves and flowers. | Carrageenan-induced paw edema | Major reduction of the paw edema after 4 h. | 92 |
3.6.7. Antipyretic, antihypertensive and vasodilator effects
It was also found that D. viscosa exhibits antipyretic activity (Table 10). The Brewer's yeast induced fever was used on rats, who were then given methanolic extracts of D. viscosa at different concentrations (400,600 and 800 mg/kg). The treatment group showed a decrease in rectal temperature compared to the control group. This confirms that the extract had a good antipyretic effect.92 Furthermore, the antihypertensive potential of D. viscosa was evaluated in 2009 by Kattouf et al. The findings indicate that the aqueous extract of D. viscosa leaves effectively countered the increase of blood pressure induced by l-NAME. Additionally, the examination of the extract's impact on the hearts of isolated and perfused rats, according to Langendorff method, revealed a dose-dependent negative inotropic action, suggesting a role in its hypertensive reduction capabilities.95 Similarly, Hakkou el al. (2017) discovered that the methanol extract of D. viscosa has a blood pressure-lowering effect, primarily through an endothelium-dependent vasodilatory effect.96
Table 10.
Antipyretic, antihypertensive and vasodilator effects of D. viscosa.
| Activities | Part Used | Extracts | Experimental approach | Key results | References |
|---|---|---|---|---|---|
| Antipyretic effect | Leaves and flowers | Methanolic extracts and decoctions of D. viscosa | Brewer's yeast-induced pyrexia method | Important reduction of the rectal temperature of the rats after 4 h compared to control groups treated with leaves and flowers extracts at the doses of 600 and 800 mg/kg. | 92 |
| Antihypertensive and vasodilator effects | Leaves | Methanolic extract (40 mg/kg/day) | Non-invasive indirect tail-cuff plethysmographic method. In vitro vasorelaxant effect. |
- The MeOH extract has been found to possess antihypertensive properties. When administered together with l-NAME, it prevented an increase in SBP, which remained steady at 115 ± 1 mmHg after a treatment period of four weeks. - In ex-vivo experiments, the MeOH extract induced relaxation in pre-contracted ring aortas (resulting in 54 ± 2% relaxation at a concentration of 3 g/L). However, when the rings were denuded, the MeOH extract was unable to relax the pre-contracted aortic rings. |
96 |
| Aqueous extract (250 mg/kg/day) | Indirect tail-cuff plethysmographic method (TCP) | It demonstrated a substantial capacity to inhibit the progression of l-NAME-induced hypertension and exhibited a negative dose dependent inotropic effect in cardiac muscle. | 95 |
3.6.8. Anticancer activity
Cancer is one of the leading causes of mortality and morbidity worldwide. In 2020, 19.3 million new cases and 10 million deaths due to cancer were declared.97 The current cancer treatments have many side effects and tumors started developing resistance to them.98 Therefore, finding new innovative treatment that are less aggressive and more efficient becomes a necessity. Many researchers focused on investigating plants as a natural source of various bioactive compounds that showed promising results in combating different cancers while reducing the side effect.98 D. viscosa has been widely investigated for its antineoplastic effects on various cancers. Table 11 summarizes the key findings reported in the literature on the anticancer activity of D. viscosa.
Table 11.
Antineoplastic activity of D. viscosa.
| Part used | Extracts/essential oil | In vivo/In vitro | Cell lines | Key results | Geographic area | References |
|---|---|---|---|---|---|---|
| Leaves | - Aqueous extract - Methanolic extract |
In vitro | - Malignant melanoma cell lines (A2058 and MeWo) - Normal fibroblasts |
The methanolic extract of D. viscosa inhibited growth in A2058 and MeWo melanoma cells, preferentially triggered apoptosis in cancerous cells over fibroblasts, and modulated miRNA expression within melanoma cells | Turkey | 99 |
| Aqueous extract | In vitro/In vivo | - colorectal cancer: HCT116 and Colo320 - Mouse murine adenocarcinoma cell line (MC38) |
In vitro: - The extract decreased cell viability and induced apoptosis at 30 μg/mL in HCT116 and colo320 cells. - Cell death is due to the activation of caspases by the extract In vivo: - The extract suppressed tumor growth in mice at 150–300 mg/kg, significantly reducing tumor weight and volume without side effects on liver and kidney function or causing weight, hair loss, or behavioral changes. |
Israel | 103 | |
| - Hexanic extract - Dichloromethane fractions |
In vitro | Cervical cancer: SiHa and HeLa cell lines | The extracts of D. viscosa demonstrated cytotoxicity against cervical cancer cell lines SiHa and HeLa, with IC50 values of 6.54 ± 1.46 μg/mL (Dichloromethane extract) and 13.17 ± 0.79 μg/mL (Hexane extract), respectively. This effect is attributed to the inhibition of cell proliferation and the induction of caspase-dependent apoptosis via a mitochondria-mediated pathway. | Morocco | 18 | |
| Methanolic extract | In vitro | - Breast cancer: MCF-7 MDA-MB-46 - PBMCs |
- Significant cytotoxic activity against MCF-7 (IC50 = 2.75 ± 1.2 μg/mL) and MDA-MB-468 (IC50 = 20.43 ± 2.99 μg/mL) - No cytotoxic effect on normal cells PBMCs (IC50 > 50 μg/mL) |
Morocco | 83 | |
| ethanolic extract | In vivo/in vitro | - Colon carcinoma: HT29 - Nondifferentiated colorectal adenocarcinoma cells: Caco-2 |
- Inhibition of the proliferation of HT29 cancer cells (EC50 = 62.39 ± 0.34 μg/mL) - No toxicity on Caco-2 cells - Protective effect against ulcerative colitis. |
Algeria | 104 | |
| Aerial parts | Methanol extract Aqueous extract |
In vitro | Breast adenocarcinoma: MCF-7 Brain cancer: T98-G |
MCF-7: - Aqueous extract: IC50 > 200 μg/mL - Methanol extract: IC50 = 179.5 ± 2.0 μg/mL T98-G: - Aqueous extract: IC50 > 200 μg/mL - Methanol extract: IC50 = 121.1 ± 3.0 μg/mL |
Turkey | 22 |
| Ethanol extract | In vivo | Skin carcinoma induced in mice using DMBA and croton oil | D. viscosa extracts exhibited antitumor effects on skin carcinoma, suppressed papilloma development in mice, and delayed skin papilloma formation, reducing their size and count, with changes observable in treated mice skin histology | Morocco | 100 | |
| Ethanolic extract | In vitro | Burkitt lymphoma Raji cell line | The extract displayed strong antiproliferative and cytotoxic effects on the Raji cell line, reducing cell viability in a dose- and time-dependent manner through G2/M phase arrest and increased apoptosis. The extract downregulated genes linked to cell cycle and proliferation while inhibiting apoptosis. The antineoplastic action rooted in the targeted downregulation of genes governing cell cycle and apoptosis. |
Italy | 105 | |
| - Ethanol extract - Ethyl Acetate extract |
In vitro | Breast cancer MCF-7 and MDA-MB231 cell lines | - Both extracts showed a moderate, dose-dependent cytotoxic effect on breast cancer cell lines. The highest growth inhibition was observed for Ethyl acetate extract: MCF-7 (IC50 = 186.20 ± 2.57 μg/mL) and MDA-MB231 (IC50 = 112.20 ± 1.28 μg/mL). - The toxicity is proportionate to the presence of tomentosin, inuviscolide, and isocostic acid in the extracts. |
Morocco | 102 | |
| - Aqueous extract - Ethanolic extract |
In vitro | Breast cancer cells MDA-MB-231 Prostate cancer cell PC3 |
- Dose and time dependent effect, - Significant reduction in cell viability was particularly observed in MDA-MB-231 cells, which were sensitive to ethanolic extracts (12–22%), while PC3 cell lines were more sensitive to aqueous extracts (12–16%) after 72 h. - Ethanol extraction of aerial parts had a higher cytotoxic effect against PC3 cell lines with an IC50 of 2.32–5.34 μg/mL |
Turkey | 27 | |
| Essential oil | In vitro | - Cervical cancer: HeLa - Colon Cancer: HCT116 - Osteosarcoma: U2OS |
- The essential oil of Croatian D. viscosa exhibits potent antiproliferative activity on HeLa, HCT116, and U2OS cancer cell lines, with IC50 values of 0.66, 0.12, and 0.7 mg/mL, respectively. - The hydrosol fraction significantly inhibits cell division with IC50 values indicating 21.70% for HeLa, 37.73% for HCT116, and 54.51% for U2OS. A decrease in GSH levels in hydrosol-treated HeLa cells suggests oxidative stress as a mechanism for tumor cell growth inhibition. - Key compounds identified include 1,8-Cineole, caryophyllene oxide, p-menth-1-en-9-ol, and 3,4-dihydroxybenzoic acid. |
Croatia | 3 | |
| Flowers | Ethanolic extract | In vitro | Vero cell line | IC50 = 202.43 ± 3.70 μg/mL | Jordan | 106 |
| ND | Aqueous Extract | In vitro | - Breast carcinoma: MCF-7 - Glioblastoma cancer: C6 - Bone osteosarcoma: MG63 |
The extract showed significant cytotoxicity against MCF-7 (IC50 = 18.76 ± 1.64 μg/mL), compared to MG63 (IC50 = 20.67 ± 1.11 μg/mL), and C6 (IC50 = 25.47 ± 0.69 μg/mL) cell lines, with tomentosin largely contributing to this effect. | Turkey | 101 |
| 80% methanol and ethanol | In vitro | Breast cancer: MDA-MB-231 | The extract exhibited peak cytotoxic activity at 1 mg/mL concentration, as determined by MTT analysis, with reduced efficacy at concentrations below or above this level. This pattern, commonly reported for flavonoid extracts, suggests they act as antioxidants or pro-oxidants based on concentration and physiological context. | Turkey | 98 |
ND: Not determined.
Extracts from D. viscosa were tested against various skin cancer cell lines, showing promising results against skin carcinoma and malignant melanoma. Unlike the aqueous extract, which had no effect on cell proliferation, the methanolic extract of D. viscosa inhibited the growth of both A2058 and MeWo melanoma cells in a time- and dose-dependent manner. The highest rates of cell death at 24 h were observed to be between 53% and 56% for A2058 cells and 55%–59% for MeWo cells. These cell viability rates were achieved with doses ranging from 80 to 120 μg/mL. The methanolic extract preferentially induced apoptosis in cancer cells compared to fibroblasts and modulated miRNA expression within melanoma cells.99 In a separate study, skin carcinoma was induced in mice using DMBA and croton oil, and the animals were treated with 100 μL of D. viscosa ethanol extracts. The treatment exhibited antitumor effects, evidenced by the inhibited development of papillomas, delayed formation of skin papillomas, and reductions in their size and number, alongside improved skin histology.100
With an estimated number of new cases at 2.3 million (11.7%) in 2020, breast cancer is reported to be the most commonly diagnosed cancer worldwide.97 The extracts derived from D. viscosa showed antiproliferative activity against various breast cancer cells including MCF-7, MDA-MB-231 and MDA-MB-468. The methanolic extract from the leaves of D. viscosa in Morocco exhibited highly significant and selective cytotoxic activity against both MCF-7 and MDA-MB-468 cancer cells with IC50 = 2.75 ± 1.2 μg/mL and IC50 = 20.43 ± 2.99 μg/mL, respectively, without inducing an effect on normal PBMCs cells.83 Similarly, the aqueous extract from Turkey showed significant cytotoxicity against MCF-7 with an IC50 = 18.76 ± 1.64 μg/mL.101 The ethanolic extract exhibited a time and dose dependent cytotoxic effect and significant reduction in MDA-MB-231 cell viability (12–22%).27 Conversely, Messaoudi et al. (2016) reported a moderate anticancer activity for the ethanolic and ethyl acetate aerial parts extract, with the highest growth inhibition observed for ethyl acetate extract at IC50 = 186.20 ± 2.57 μg/mL and IC50 = 112.20 ± 1.28 μg/mL) for MCF-7 and MDA-MB231, respectively.102 Similar results were reported for the water and methanol extract of the aerial parts in Turkey. Ozkan et al. (2019) recorded the inhibition of MCF-7 cell growth at IC50 = 179.5 ± 2.0 μg/mL for the methanol extract, and IC50 > 200 μg/mL for the aqueous extract.22 The extract obtained by 80% ethanol and methanol exhibited peak cytotoxic activity at 1 mg/mL concentration, as determined by MTT analysis, with reduced efficacy at concentrations below or above this level. This pattern, commonly reported for flavonoid extracts, suggests they act as antioxidants or pro-oxidants based on concentration and physiological context.98
Many studies have been conducted to investigate the cytotoxic effect of D. viscosa on colorectal cancer, considering that this cancer is the second lethal cancer worldwide after lung cancer.97 The aqueous extract of D. viscosa leaves from Israel showed promising results against colon cancer HCT116, colo320 and MC38 cells. The in vitro investigation revealed that the extract decreased cell viability and induced apoptosis at 30 μg/mL in both HCT116 and colo320 cell lines in a dose and time dependent manner. The mechanism of cell death is due to the activation of caspases by the extract. The in vivo study found that the extract suppressed tumor growth in mice transplanted with MC38 cells at 150–300 mg/kg and significantly reduced tumor weight and volume without inducing side effects on liver and kidney function or causing weight, hair loss, or behavioral changes.103 Similarly, the ethanolic extract of leaves from Algeria inhibited the proliferation of HT29 colon cancer cells (EC50 = 62.39 ± 0.34 μg/mL), without producing cytotoxicity on nondifferentiated Caco-2 cells. The extract is also reported to possess a protective effect against ulcerative colitis.104 The essential oil and hydrosol extract are also reported to exert a potent anticancer effect on HCT116 cancer cells, evidenced by an antiproliferation effect at IC50 = 0.12 mg/mL and inhibition of cell division at IC50 = 37.37%.3
Regarding cervical cancer, D. viscosa extracts were tested on SiHa and HeLa cell lines. The dichloromethane and hexane extract of leaves in Morocco demonstrated cytotoxicity against both cell lines, with IC50 values of 6.54 ± 1.46 μg/mL and 13.17 ± 0.79 μg/mL respectively. This effect is attributed to the inhibition of cell proliferation and the induction of caspase-dependent apoptosis via a mitochondria-mediated pathway.18 Vuko et al. (2021) found that the essential oil derived from the aerial parts of D. viscosa in Croatia is also active on HeLa cancer cells with an inhibition of proliferation recorded at 0.66 mg/mL. The same study found that the hydrosol extracts also inhibited cell division at IC50 value of 27%. The study concluded that a decrease in GSH levels in hydrosol-treated HeLa cells suggests oxidative stress as a mechanism for tumor cell growth inhibition.3
D. viscosa extract showed promising results against various types of other cancers. The ethanolic extract of the aerial parts from Italy displayed strong antiproliferative and cytotoxic effects against Burkitt lymphoma (Raji cell line), reducing cell viability in a dose- and time-dependent manner through G2/M phase arrest and increased apoptosis. The extract downregulated genes linked to cell cycle and proliferation while inhibiting apoptosis.105 D. viscosa extracts were also tested against bone osteosarcoma, and showed higher cytotoxic activity against MC63 at IC50 = 20.67 ± 1.11 μg/mL,101 compared to IC50 = 0.7 mg/mL for U2OS cell line.3 The results were obtained using the essential oil and the aqueous extract from Croatia and Turkey, respectively.3,101 The ethanol extract from the aerial parts in Turkey displayed higher cytotoxic activity on prostate cancer PC3 cell lines compared to the aqueous extract, with an IC50 of 2.32–5.34 μg/mL.27 The same extract of D. viscosa flowers showed moderate growth inhibition on Vero cell line with IC50 = 202.43 ± 3.70 μg/mL.106 For brain cancer, the aqueous extract showed high antiproliferative activity against glioblastoma C6 cell line with an IC50 = 25.47 ± 0.69 μg/mL,101 while exerting moderate effect on T98-G glioblastoma cell line (IC50 > 200 μg/mL) along with methanol extract IC50 = 121.1 ± 3.0 μg/mL22
The anticancer activity in different extracts of D. viscosa against various cancer types can be attributed to the differences in chemical composition of the plant in different geographical locations and the extraction methods used and the solvent used. The key compounds found in extracts that can be responsible for the antiproliferative activity against cervical cancer, colon and osteosarcoma are 1,8-Cineole, caryophyllene oxide, p-menth-1-en-9-ol, and 3,4-dihydroxybenzoic acid..3 Messaoudi et al. (2016) found that the cytotoxic activity of the plant against Breast cancer cells MCF-7 and MDA-MB231 is proportionate to the presence of isocostic acid, inuviscolide and tomentosin in the extracts..102 The later compound is reported to be a large contributor to the cytotoxic effect of the aqueous extract of D. viscosa against breast carcinoma (MCF-7), bone osteosarcoma (MG63), glioblostoma cancer (C6).101
3.6.9. Pharmacological activities of compounds isolated from D. viscosa
The chemical profiles of D. viscosa extracts, analyzed via various extraction methods and analytical studies from different regions, reveal a diverse array of phytochemicals. As illustrated in Table 12, numerous compounds from D. viscosa have been studied for their pharmacological effects. Tomentosin, a sesquiterpene lactone isolated from different parts of D. viscosa using various solvents, has shown multiple pharmacological activities. This compound has displayed antidiabetic activity by inhibiting α-amylase and α-glucosidase, the key enzymes involved in diabetes.73 Additionally, tomentosin has exhibited a cytotoxic effect on HeLa and SiHa cervical cancer cells by disrupting telomeres, arresting the cell cycle at the G2/M phase, and inducing apoptosis through a decrease in mitochondrial membrane potential and the activation of caspase enzymes.72 Tomentosin also exhibited antifungal activity against Microsporum canis, Microsporum gypseum, and Trichophyton mentagrophytes at a concentration of 1 mg/mL.107 Furthermore, tomentosin has demonstrated an anti-inflammatory effect by inhibiting phospholipase A2 (sPLA2), cyclooxygenase 1 (COX1), and leukocyte elastase,108 as well as suppressing the production of inflammatory mediators such as IL-6, iNOS, NO, COX2, and TNF-α.109 The anti-inflammatory activity of other phytochemicals from D. viscosa, including dehydrocostic acid, ilicic acid, and inuviscolide, was also evaluated. These compounds showed promising in vivo results by reducing edema in rat paws and ears, inhibiting proinflammatory enzymes, and downregulating leukotriene B4 release.55,110 Hernández et al. (2007) investigated the anti-inflammatory effects of three compounds from a dichloromethane extract of the flowering aerial parts of D. viscosa in Spain. The study identified 7-O-methylaromadendrin and sakuranetin as effective against PLA2-induced edema with ED50 values of 8 mg/kg and 18 mg/kg, respectively. For TPA-induced ear edema, 3-acetyl-7-O-methylaromadendrin (ED50 = 185 μg/ear) and sakuranetin (ED50 = 205 μg/ear) were potent, with sakuranetin notably inhibiting leukotriene B4 production (IC50 = 9 μM) and being the only compound to directly suppress 5-lipoxygenase activity. Conversely, 3-acetyl-7-O-methylaromadendrin also inhibited LTB4 (IC50 = 15 μM) but did not affect 5-LOX. Notably, 7-O-methylaromadendrin uniquely inhibited secretory PLA2 in vitro, and sakuranetin at 100 μM reduced elastase release, suggesting its selectivity for 5-LOX inhibition.111 A mixture of tomentosin and inuviscolide also exhibited anti-inflammatory effects by inhibiting the secretion of inflammatory cytokines and downregulating NFκB and STAT1.112 This mixture further showed antiproliferative and cytotoxic activities on skin melanoma cell lines (1363 mel, 624 mel, and SK-28) by inhibiting cell proliferation and inducing cell cycle arrest and apoptosis.113 Another compound with anticancer activity, bornyl acetate, has shown promise against various cancers, including cervical (HeLa), colon (HT29), lung (A549), and breast (MCF7).69
Table 12.
Pharmacological activities of phytochemicals isolated from D. viscosa.
| Phytochemicals | Part used | Extracts/essential oil | Analytical method | In vivo/In vitro | Pharmacological activity | Geographic area | References |
|---|---|---|---|---|---|---|---|
| Tomentosin | Aerial parts | -Dichloromethane extract - Ethanol extract |
LC-MS/MS, TLC | In vitro | - Antidiabetic effect by inhibition of α-amylase and α-glucosidase | Turkey | 73 |
| Aerial parts | - Hexane extract - Methanolic extract |
GC/MS, 1H and 13C NMR | In vitro | - Cytotoxic effects on HeLa and SiHa cell lines in cervical cancer by induction of apoptosis through mitochondria-mediated signaling pathway | Morocco | 72 | |
| Inulae flos | Ethanolic extract | HPLC, 1H and 13C NMR | In vitro | - Anti-inflammatory effects by suppressing the production of inflammatory mediators in RAW264.7 cells. | Korea | 109 | |
| Flowered twigs | ND | ND | In vitro | Anti-inflammatory activity by inhibition of secretory phospholipase A2 (sPLA2) from bee venom, cyclooxygenase 1 (COX1) and leukocyte elastase. | Spain | 108 | |
| Flowers | Petroleum ether | 1H 13C NMR, TLC, GC-MS | In vitro | Antifungal activity against Microsporum canis, Microsporum gypseum and Trichophyton mentagrophytes at a dose of 1 mg/mL | Italy | 107 | |
| Inuviscolide | Aerial parts | Acetonic extract | TLC, 1H and 13C NMR, UV, LC | In vivo/in vivo | Anti-inflammatory effect by reduction of PLA2 induced edema in rat paws, and a reduction of Leukotriene B4 production in rat neutrophils. | Spain | 55 |
| Inuviscolide + Tomentosin | Leaves | Aqueous extract | TLC, 1H and 13C NMR | In vitro | - Antitumoral effect against human Melanoma SK-28, 624 mel, and 1363 mel cell lines by inhibited the proliferation and inducing cell cycle arrest at G2/M and apoptosis | Israel | 113 |
| Inuviscolide + Tomentosin | Leaves | Aqueous extract | TLC, 1H and 13C NMR | In vitro | Anti-inflammatory effect by the inhibition of the secretion of inflammatory cytokines and downregulation of NFκB and STAT1. | Israel | 112 |
| Dehydrocostic acid | Flowered aerial parts | Actonic extract | ND | In vivo/in vitro | Anti-inflammatory activity evidenced by inhibition of pro-inflammatory enzymes (elastase and sPLA2) and control of leukotriene B4 release | Spain | 110 |
| Ilicic Acid | Aerial parts | Acetonic extract | TLC, 1H and 13C NMR, UV, LC | In vivo/in vitro | Anti-inflammatory effect by inhibiting ear edema induced by TPA in rats | Spain | 55 |
| Bornyl Acetate | Aerial parts | Essential oil | ND | In vitro | Antineoplastic activity against various cancers: Cervix (HeLa), Colon (HT29), Lung (A549), Breast (MCF7). | Turkey | 69 |
| Isocostic acid | Leaves | Essential oil | LC, 1H and 13C NMR, GC-FID and GC/MS | In vitro | - Antityrosinase activity, antibacterial activity and anti-inflammatory proprieties by inhibition of 5-lipoxygenase. | Tunisia | 63 |
| Nepetin, Hispidulin 3-O-methylquercetin 3,3′-di-O-methylquercetin |
Aerial parts | - Ethanol extract | Column Chromatography, TLC, 1H NMR, 13C NMR | In vitro | - Antiproliferative activity against MCF-7 cells and antimicrobial effects | Jordan | 74 |
| 1,3-dicaffeoylquinic acid | Leaves | Methanolic extract | HPLC, 1Hand 13C NMR | In vitro | Antioxidant activity by direct scavenging multiple types of free radicals (FeSO4, AAPH, ROS, hydroxyl and superoxide radicals) | Israel | 114 |
| sakuranetin, 7-O-methylaromadendrin, and 3-acetyl-7-O- methylaromadendrin |
Flowering aerial parts | Dichloromethane extract | TLC, 1H and 13c NMR | In vivo/in vitro | - Inflammatory activity: 7-O-methylaromadendrin: Potent against PLA2-edema (ED50 = 8 mg/kg). Sakuranetin: Effective on PLA2-edema (ED50 = 18 mg/kg); TPA-edema (ED50 = 205 μg/ear); inhibits LTB4 (IC50 = 9 μM) and 5-LOX. 3-Acetyl-7-O-methylaromadendrin: Leads TPA-edema reduction (ED50 = 185 μg/ear); inhibits LTB4 (IC50 = 15 μM) without affecting 5-LOX. Elastase Release: Sakuranetin at 100 μM inhibits release, indicating 5-LOX selectivity. |
Spain | 111 |
| Taxifolin, Quercetin |
Leaves | Ethanolic extract | Column chromatography, TLC, 1D & 2D NMR | In vitro | Antioxidant activity: - ABTS: * Quercetin. IC50 = 41.27 μg/mL * Taxifolin IC50 = 142.58 μg/mL - DPPH: * Quercetin. IC50 = 62.53 μg/mL * Taxifolin IC50 = 103.46 μg/mL |
Morocco | 75 |
ND: Not determined.
Two compounds isolated from D. viscosa have been assessed for their antioxidant activity against various free radicals. Significant activities were reported for taxifolin and quercetin,75 as well as for 1,3-dicaffeoylquinic acid.114 Additionally, other molecules isolated from D. viscosa have exhibited substantial antiproliferative effects against multiple cell lines. Four flavonoids, namely, neptine, hispidulin, 3,3′-di-O-methylquercetin, and 3-O-methylquercetin, inhibited cell growth. Specifically, 3,3′-di-O-methylquercetin and 3-O-methylquercetin displayed selective inhibitory activity against MCF-7 cells, with IC50 values of 11.23 μg/mL and 10.11 μg/mL, respectively. The antiproliferative effect was attributed to the induction of apoptosis, as indicated by nuclear condensation, DNA fragmentation, and the formation of apoptotic bodies in the treated cancer cells (Talib et al., 2012). Isocostic acid, another compound from D. viscosa, demonstrated a range of pharmacological activities, including antityrosinase, antibacterial, and anti-inflammatory properties, the latter through the inhibition of 5-lipoxygenase.63
Overall, the investigation of the chemical composition of D. viscosa has unveiled its wealth of biologically active compounds, which can be useful in treating various illnesses and in finding new drug candidates to help overcome some of today's most significant pharmacological challenges, such as the resistance to cancer treatments and their side effects.
4. Conclusion
This review assessed the ethnomedicinal uses, toxicological investigation, botanical description, taxonomy, chemical composition, and pharmacological activities of the medicinal plant D. viscosa. Ethnobotanical studies revealed a broad variety of traditional uses for D. viscosa against various illnesses, depending upon the geographical location, the local population, and the parts used. Most of the ethnobotanical studies were conducted in the mediterranean region, especially north Africa, which signals the need for additional surveys in other regions to fully investigate the unreported medical uses of the plant worldwide.
The chemical analyses of D. viscosa extracts across various regions have identified a diverse range of phytochemicals, predominantly monoterpenes and sesquiterpenes. The concentration in phytochemical components varied significantly with the extraction method, the plant's origin and the solvent used. Differences in extraction techniques, including hydrodistillation and solvent extraction, notably influenced the essential oil's composition. These variations underscore the complexity of D. viscosa's phytochemical profile, as demonstrated by studies from different regions.
The extracts and the essential oil of D. viscosa exhibited promising pharmacological activities, possessing antioxidant, antibacterial, anticancer, antidiabetic, antihypertensive, anti-inflammatory, wound healing activities and analgesic effects.
The extensive phytochemical analysis of D. viscosa has revealed a plethora of compounds with significant pharmacological potential. Sesquiterpene lactones like tomentosin have demonstrated remarkable antidiabetic, antifungal, and anticancer activities. Additionally, anti-inflammatory properties have been attributed to various extracts, showing efficacy in inhibiting key enzymes and cytokines involved in inflammatory responses. Notably, the anti-cancer properties of compounds such as inuviscolide and bornyl acetate offer promising avenues for developing new treatments against a range of cancers. Antioxidant compounds like taxifolin and quercetin contribute to the plant's potential therapeutic profile. This wealth of bioactive substances positions D. viscosa as a valuable source for novel drug discovery and therapeutics, addressing current global challenges like antibiotic resistance and the search for more effective cancer treatments. However, the transition from traditional medicine to clinical practice will require a concerted effort to bridge the gap through comprehensive pharmacological, toxicological, and clinical studies. Embracing the challenges and opportunities presented by D. viscosa could pave the way for new, sustainable, and effective therapeutic options, aligning with the global shift towards natural and green medicine.
Funding
This study was not financially supported by any public, commercial, or non-profit funding organizations.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Informed consent statement
None.
Ethical committee approval
Not Applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Not Applicable.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Rania Jerada, Email: rania.jerada@um5r.ac.ma.
Abdeljalil Er-Rakibi, Email: abdeljalil.errakibi-etu@etu.univh2c.ma.
Abha Cherkani Hassani, Email: abha.cher@gmail.com.
Hanane Benzeid, Email: benzeid_hanane@yahoo.fr.
Abdelmoula El Ouardi, Email: abdoelouardi@yahoo.fr.
Hicham Harhar, Email: h.harhar@um5r.ac.ma.
Bey Hing Goh, Email: beyhingg@sunway.edu.my, goh.bey.hing@monash.edu.
Yoon-Yen Yow, Email: yoonyeny@sunway.edu.my.
Hooi-Leng Ser, Email: hooilengs@sunway.edu.my.
Abdelhakim Bouyahya, Email: a.bouyahya@um5r.ac.ma.
Brahim Mojemmi, Email: brahimpharma@gmail.com.
Anass Doukkali, Email: doukkali73@gmail.com.
References
- 1.WHO . World Health Organization; 2007. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues. [Google Scholar]
- 2.Seca A.M., Grigore A., Pinto D.C., Silva A.M. The genus Inula and their metabolites: from ethnopharmacological to medicinal uses. J Ethnopharmacol. 2014;154(2):286–310. doi: 10.1016/j.jep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Vuko E., Dunkić V., Maravić A., et al. Not only a weed plant—biological activities of essential oil and hydrosol of Dittrichia viscosa (L.) greuter. Plants. 2021;10(9):1837. doi: 10.3390/plants10091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma M., Sharma M., Bithel N., Sharma M. Ethnobotany, phytochemistry, pharmacology and nutritional potential of medicinal plants from asteraceae family. J Mt Res. 2022;17(2):67–83. doi: 10.51220/jmr.v17i2.7. [DOI] [Google Scholar]
- 5.Brullo S., de Marco G. Taxonomical revision of the genus Dittrichia (asteraceae) Port Acta Biol. 2000;19(1):341–354. [Google Scholar]
- 6.Sun C.-P., Jia Z.-L., Huo X.-K., et al. Medicinal inula species: phytochemistry, biosynthesis, and bioactivities. Am J Chin Med. 2021;49(2):315–358. doi: 10.1142/S0192415X21500166. [DOI] [PubMed] [Google Scholar]
- 7.Alcalá Herrera R., Castro-Rodríguez J., Fernández-Sierra M.L., Campos M. Dittrichia viscosa (asterales: asteraceae) as an arthropod reservoir in olive groves. Front Sustain Food Syst. 2019;3:64. doi: 10.3389/fsufs.2019.00064. [DOI] [Google Scholar]
- 8.Parolin P., Ion Scotta M., Bresch C. Biology of Dittrichia viscosa, a Mediterranean ruderal plant: a review. Phyton. 2014;83(2):251–262. doi: 10.32604/phyton.2014.83.251. [DOI] [Google Scholar]
- 9.Rebbas K., Bounar R., Gharzouli R., Ramdani M., Djellouli Y., Alatou D. Plantes d’intérêt médicinale et écologique dans la région d'Ouanougha (M’sila, Algérie) Phytothérapie. 2012;10(2):131–142. [Google Scholar]
- 10.Benderradji L., Rebbas K., Ghadbane M., Bounar R., Brini F., Bouzerzour H. Ethnobotanical study of medicinal plants in Djebel messaad region (M'sila, Algeria) Glob J Res Med Plants Indig Med. 2014;3(12):445–459. [Google Scholar]
- 11.Aziz Z., Lotfi A. Ethnobotanical survey of medicinal and aromatic plants used by the people of Targuist in the North of Morocco. Der Pharma Chem. 2018;10(5) [Google Scholar]
- 12.Sarri M., Boudjelal A., Hendel N., Sarri D., Benkhaled A. Flora and ethnobotany of medicinal plants in the southeast of the capital of Hodna (Algeria) Arabian J Med Aromatic Plants. 2015;1(1):24–30. [Google Scholar]
- 13.Orch H., Douira A., Zidane L. Étude ethnobotanique des plantes médicinales utilisées dans le traitement du diabète, et des maladies cardiaques dans la région d'Izarène (Nord du Maroc) J Appl Biosci. 2015;86:7940–7956. 7940–7956. [Google Scholar]
- 14.Benkhnigue O., Ben Akka F., Salhi S., Fadli M., Douira A., Zidane L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d’Al Haouz-Rhamna (Maroc) J Anim Plant Sci. 2014;23(1):3539–3568. [Google Scholar]
- 15.Katiri A., Barkaoui M., Msanda F., Boubaker H. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in the Tizi n'Test region (Taroudant Province, Morocco) J pharmacogn nat prod. 2017;3(1) doi: 10.1016/j.jep.2017.01.023. 2472-0992. [DOI] [PubMed] [Google Scholar]
- 16.Mssillou I., Agour A., Allali A., et al. Antioxidant, antimicrobial, and insecticidal properties of a chemically characterized essential oil from the leaves of Dittrichia viscosa L. Molecules. 2022;27(7):2282. doi: 10.3390/molecules27072282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mssillou I., Agour A., Slighoua M., et al. Phytochemical characterization, antioxidant activity, and in vitro investigation of antimicrobial potential of Dittrichia viscosa L. leaf extracts against nosocomial infections. Acta Ecol Sin. 2021;42:661–669. [Google Scholar]
- 18.Benbacer L., Merghoub N., El Btaouri H., et al. 2012. Antiproliferative effect and induction of apoptosis by Inula viscosa L. and Retama monosperma L. extracts in human cervical cancer cells. (Topics on Cervical Cancer with an Advocacy for Prevention). InTech. InTech ed. 267-284. [DOI] [PubMed] [Google Scholar]
- 19.Madani L., Derriche R., Haoui I.E. Essential oil of Algerian Inula viscosa leaves. J Essential Oil Bearing Plants. 2014;17(1):164–168. [Google Scholar]
- 20.Haoui I.E., Derriche R., Madani L., Oukali Z. Analysis of the chemical composition of essential oil from Algerian Inula viscosa (L.) Aiton. Arab J Chem. 2015;8(4):587–590. [Google Scholar]
- 21.Lev E., Amar Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J Ethnopharmacol. 2000;72(1-2):191–205. doi: 10.1016/s0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 22.Ozkan E., Karakas F.P., Yildirim A., et al. Promising medicinal plant Inula viscosa L.: antiproliferative, antioxidant, antibacterial and phenolic profiles. Prog Nutr. 2019;21(3):652–661. [Google Scholar]
- 23.Boulos L. Medicinal Plants of North Africa. 1983. Medicinal plants of north Africa. [Google Scholar]
- 24.Barrero A.F., Herrador M.M., Arteaga P., Catalán J.V., Dittrichia viscosa L. Greuter: phytochemistry and biological activity. Nat Prod Commun. 2008;3(11) 1934578X0800301110. [Google Scholar]
- 25.Leto C., Tuttolomondo T., La Bella S., Licata M. Ethnobotanical study in the Madonie Regional Park (Central Sicily, Italy)—medicinal use of wild shrub and herbaceous plant species. J Ethnopharmacol. 2013;146(1):90–112. doi: 10.1016/j.jep.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 26.Tuttolomondo T., Licata M., Leto C., et al. Ethnobotanical investigation on wild medicinal plants in the monti sicani regional park (sicily, Italy) J Ethnopharmacol. 2014;153(3):568–586. doi: 10.1016/j.jep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 27.Sevgi E., Dag A., Kızılarslan-Hançer Ç., Atasoy S., Kurt B.Z., Aksakal Ö. Evaluation of cytotoxic and antioxidant potential of Dittrichia viscosa (L.) Greuter used in traditional medicine. J Ethnopharmacol. 2021;276 doi: 10.1016/j.jep.2021.114211. [DOI] [PubMed] [Google Scholar]
- 28.Sell P., Murrell G. Campanulaceae-Asteraceae. vol. 4. Cambridge University Press; 2006. (Flora of Great Britain and Ireland). [Google Scholar]
- 29.Quezel P., Santa S. Tome II; 1963. Nouvelle flore de l'Algérie et des régions désertiques méridionales. [Google Scholar]
- 30.Topakci N. Sticky elecampane, Inula viscosa (L.) aiton (Asteraceae) Int J Agric Innov Res. 2016;5:413–416. [Google Scholar]
- 31.Boughrara B., Belgacem L. Ethnobotanical study close to the population of the extreme north east of Algeria: the municipalities of El Kala National Park (EKNP) Ind Crop Prod. 2016;88:2–7. doi: 10.1016/j.indcrop.2016.03.009. [DOI] [Google Scholar]
- 32.Djidel S, Khennouf S, Baghiani A, Harzallah D, Arrar L. Medicinal plants used traditionally in the Algerian folk medicine for gastrointestinal disorders and hypertension: total polyphenols, flavonoids and antioxidant activity. Paper Presented at: XIII International Conference on Medicinal and Aromatic Plants 8542009..
- 33.Miara M.D., Bendif H., Rebbas K., Rabah B., Hammou M.A., Maggi F. Medicinal plants and their traditional uses in the highland region of Bordj Bou Arreridj (Northeast Algeria) J Herb Med. 2019;16 [Google Scholar]
- 34.Ali-Shtayeh M.S., Yaniv Z., Mahajna J. Ethnobotanical survey in the Palestinian area: a classification of the healing potential of medicinal plants. J Ethnopharmacol. 2000;73(1-2):221–232. doi: 10.1016/s0378-8741(00)00316-0. [DOI] [PubMed] [Google Scholar]
- 35.Belayachi L., Aceves-Luquero C., Merghoub N., et al. Screening of North African medicinal plant extracts for cytotoxic activity against tumor cell lines. Eur J Med Plants. 2013;3:310–332. [Google Scholar]
- 36.Kaileh M., Berghe W.V., Boone E., Essawi T., Haegeman G. Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity. J Ethnopharmacol. 2007;113(3):510–516. doi: 10.1016/j.jep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Chaachouay N., Benkhnigue O., Zidane L. 2022. Ethnobotanical and Ethnomedicinal Study of Medicinal and Aromatic Plants Used against Dermatological Diseases by the People of Rif, Morocco. [Google Scholar]
- 38.Bouasla A., Bouasla I. Ethnobotanical survey of medicinal plants in northeastern of Algeria. Phytomedicine. 2017;36:68–81. doi: 10.1016/j.phymed.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Tavilla G., Crisafulli A., Ranno V., Picone R.M., Redouan F.Z., del Galdo G.G. First contribution to the ethnobotanical knowledge in the peloritani mounts (NE sicily) Res J Ecol Environ Sci. 2022:1–34. doi: 10.31586/rjees.2022.201. [DOI] [Google Scholar]
- 40.Cornara L., La Rocca A., Marsili S., Mariotti M. Traditional uses of plants in the eastern riviera (liguria, Italy) J Ethnopharmacol. 2009;125(1):16–30. doi: 10.1016/j.jep.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Allali H., Benmehdi H., Dib M., Tabti B., Ghalem S., Benabadji N. Phytotherapy of diabetes in west Algeria. Asian J Chem. 2008;20(4):2701. [Google Scholar]
- 42.Belhaj S., Chaachouay N., Zidane L. Ethnobotanical and toxicology study of medicinal plants used for the treatment of diabetes in the High Atlas Central of Morocco. J Pharm & Pharmacognosy Res. 2021;9(5):619–662. [Google Scholar]
- 43.Eddouks M., Maghrani M., Lemhadri A., Ouahidi M.-L., Jouad H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet) J Ethnopharmacol. 2002;82(2-3):97–103. doi: 10.1016/s0378-8741(02)00164-2. [DOI] [PubMed] [Google Scholar]
- 44.Midaoui M.E., Maataoui A., Benbella M., Houssa A.A., Labazi N. Ethnobotanical study of some aromatic and medicinal plants in the Middle Atlas mountains of Morocco. Nat Prod Commun. 2011;6(10) 1934578X1100601011. [PubMed] [Google Scholar]
- 45.El-Hilaly J., Hmammouchi M., Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco) J Ethnopharmacol. 2003;86(2-3):149–158. doi: 10.1016/s0378-8741(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 46.Tahri N., El Basti A., Zidane L., Rochdi A., Douira A. Etude ethnobotanique des plantes medicinales dans la province de Settat (Maroc) Kastamonu Univ J Forestry Faculty. 2012;12(2):192–208. [Google Scholar]
- 47.Brahmi-Chendouh N., Piccolella S., Crescente G., et al. A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. J Food Drug Anal. 2019;27(3):692–702. doi: 10.1016/j.jfda.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salhi S., Fadli M., Zidane L., Douira A. Etudes floristique et ethnobotanique des plantes médicinales de la ville de Kénitra (Maroc) Mediterranean Botany. 2010;31:133. [Google Scholar]
- 49.Al-Dissi N.M., Salhab A.S., Al-Hajj H.A. Effects of Inula viscosa leaf extracts on abortion and implantation in rats. J Ethnopharmacol. 2001;77(1):117–121. doi: 10.1016/s0378-8741(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 50.Youbi A.E.H.E., Ouahidi I., Mansouri L.E., Daoudi A., Bousta D. Ethnopharmacological survey of plants used for immunological diseases in four regions of Morocco. Eur J Med Plants. 2016;13(1):1. [Google Scholar]
- 51.Passalacqua N.G., Guarrera P.M., De Fine G. Contribution to the knowledge of the folk plant medicine in Calabria region (Southern Italy) Fitoterapia. 2007;78(1):52–68. doi: 10.1016/j.fitote.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Zeggwagh N.-A., Ouahidi M.-L., Lemhadri A., Eddouks M. Study of hypoglycaemic and hypolipidemic effects of Inula viscosa L. aqueous extract in normal and diabetic rats. J Ethnopharmacol. 2006;108(2):223–227. doi: 10.1016/j.jep.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Talib W.H., Mahasneh A.M. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci Pharm. 2010;78(1):33–46. doi: 10.3797/scipharm.0912-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benítez G., González-Tejero M., Molero-Mesa J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): ethnopharmacological synthesis. J Ethnopharmacol. 2010;129(1):87–105. doi: 10.1016/j.jep.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Hernández V., del Carmen Recio M., Máñez S., Prieto J.M., Giner R.M., Ríos J.L. A mechanistic approach to the in vivo anti-inflammatory activity of sesquiterpenoid compounds isolated from Inula viscosa. Planta Med. 2001;67(8):726–731. doi: 10.1055/s-2001-18342. [DOI] [PubMed] [Google Scholar]
- 56.Al-Qura’n S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J Ethnopharmacol. 2009;123(1):45–50. doi: 10.1016/j.jep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 57.Boubekeur A.C., Biche M. First ethnobotanical study relating to usage of medicinal plants in province of ain defla region, south-west of Algeria. Indian J Ecol. 2022;49(3):655–664. [Google Scholar]
- 58.Larbi K.S., Meddah B., Meddah A.T.T., Sonnet P. Central analgesic property of extracts and essential oils from inula viscosa and anacyclus valentinus (asteraceae) in wistar rats. J Appl Environ Biol Sci. 2016;6(9):72–77. [Google Scholar]
- 59.Ouahchia C., Cherif H., Hamaidi-Chergui F., et al. Acute and subacute toxicity of Inula viscosa L.(Dittrichia viscosa L.) methanolic extracts. AgroBiologia. 2017;7(2):562–573. [Google Scholar]
- 60.Abbas M.A., Hameed N.A., Al-Khateeb E.H., Al Haj R. Evaluation of toxicity and fertility effects of Inula viscosa aerial parts extract in male rats. Jordan J Biol Sci. 2017;11(2):147–154. [Google Scholar]
- 61.Asraoui F., Kounnoun A., Cacciola F., et al. Phytochemical profile, antioxidant capacity, α-amylase and α-glucosidase inhibitory potential of wild Moroccan inula viscosa (L.) aiton leaves. Molecules. 2021;26(11):3134. doi: 10.3390/molecules26113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahmoudi H., Hosni K., Zaouali W., et al. Comprehensive phytochemical analysis, antioxidant and antifungal activities of Inula viscosa aiton leaves. J Food Saf. 2016;36(1):77–88. [Google Scholar]
- 63.Aissa I., Znati M., Zardi-Bergaoui A., Flamini G., Ascrizzi R., Jannet H.B. GC and GC-MS integrated analyses and in vitro antibacterial, anticholinesterase, anti-tyrosinase, and anti-5-lipoxygenase potential of Inula viscosa root fractionated essential oil. South Afr J Bot. 2019;125:386–392. [Google Scholar]
- 64.Sriti Eljazi J., Selmi S., Zarroug Y., et al. Essential oil composition, phenolic compound, and antioxidant potential of Inulaviscosa as affected by extraction process. Int J Food Prop. 2018;21(1):2309–2319. [Google Scholar]
- 65.Gharred N., Dbeibia A., Falconieri D., Hammami S., Piras A., Dridi-Dhaouadi S. Chemical composition, antibacterial and antioxidant activities of essential oils from flowers, leaves and aerial parts of Tunisian Dittrichia Viscosa. J Essent Oil Res. 2019;31(6):582–589. [Google Scholar]
- 66.Qneibi M., Hanania M., Jaradat N., Emwas N., Radwan S. Inula viscosa (L.) Greuter, phytochemical composition, antioxidant, total phenolic content, total flavonoids content and neuroprotective effects. Eur J Integr Med. 2021;42 [Google Scholar]
- 67.Araniti F., Lupini A., Sunseri F., Abenavoli M.R. Allelopatic potential of Dittrichia viscosa (L.) W. Greuter mediated by VOCs: a physiological and metabolomic approach. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nadia B., Mesli F., Zahra B.F., et al. Chemical composition variability and vascular endothelial growth factor receptors inhibitory activity of Inulaviscosa essential oils from Algeria. J Biomol Struct Dyn. 2020:1–19. doi: 10.1080/07391102.2020.1847686. Taylor & Francis. [DOI] [PubMed] [Google Scholar]
- 69.Karan T., Yildiz I., Aydin A., Erenler R. Inhibition of various cancer cells proliferation of bornyl acetate and essential oil from Inula graveolens (Linnaeus) Desf. Record Nat Prod. 2018;12(3) doi: 10.25135/rnp.30.17.09.057. [DOI] [Google Scholar]
- 70.Li Y., Ren M., Wang J., et al. Progress in borneol intervention for ischemic stroke: a systematic review. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.606682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang L., Xie J., Almoallim H.S., Alharbi S.A., Chen Y. Tomentosin inhibits cell proliferation and induces apoptosis in MOLT‐4 leukemia cancer cells through the inhibition of mTOR/PI3K/Akt signaling pathway. J Biochem Mol Toxicol. 2021;35(4) doi: 10.1002/jbt.22719. [DOI] [PubMed] [Google Scholar]
- 72.Merghoub N., El Btaouri H., Benbacer L., et al. Tomentosin induces telomere shortening and caspase‐dependant apoptosis in cervical cancer cells. J Cell Biochem. 2017;118(7):1689–1698. doi: 10.1002/jcb.25826. [DOI] [PubMed] [Google Scholar]
- 73.Aydin T., Saglamtas R., Dogan B., Kostekci E., Durmus R., Cakir A. Phytochemical Analysis; 2022. A New Specific Method for Isolation of Tomentosin with a High Yield from Inula Viscosa (L.) and Determination of its Bioactivities. [DOI] [PubMed] [Google Scholar]
- 74.Talib W.H., Zarga M.H.A., Mahasneh A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules. 2012;17(3):3291–3303. doi: 10.3390/molecules17033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeouk I., Sifaoui I., Jalloul A.B., et al. Isolation, identification, and activity evaluation of antioxidant components from Inula viscosa: a bioguided approach. Bioorg Chem. 2022;119 doi: 10.1016/j.bioorg.2021.105551. [DOI] [PubMed] [Google Scholar]
- 76.Rhimi W., Hlel R., Ben Salem I., et al. Ethanolic extract based ointment with antiradical, antioxidant, and healing wound activities. BioMed Res Int. 2019;2019 doi: 10.1155/2019/4081253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohti H., Taviano M., Cacciola F., et al. Inula viscosa (L.) Aiton leaves and flower buds: effect of extraction solvent/technique on their antioxidant ability, antimicrobial properties and phenolic profile. Nat Prod Res. 2020;34(1):46–52. doi: 10.1080/14786419.2019.1569659. [DOI] [PubMed] [Google Scholar]
- 78.Ounaissia K. Total phenol content, antioxidant and anti-inflammatory activities of inula viscosa from guelma-Algeria. Int J Agric Nat Sci. 2021;14(3):186–193. [Google Scholar]
- 79.Salim H., Rimawi W.H., Mjahed A. 2017. Analysis of Extracts from Palestinian Inula Viscosa for Their Phenolic, Flavonoid and Lipid Contents, Antioxidant and Antibacterial Activity. [Google Scholar]
- 80.Gökbulut A., Özhana O., Satılmiş B., Batçioğlu K., Günal S., Şarer E. Antioxidant and antimicrobial activities, and phenolic compounds of selected Inula species from Turkey. Nat Prod Commun. 2013;8(4) [PubMed] [Google Scholar]
- 81.Chahmi N., Anissi J., Jennan S., Farah A., Sendide K., El Hassouni M. Antioxidant activities and total phenol content of Inula viscosa extracts selected from three regions of Morocco. Asian Pac J Trop Biomed. 2015;5(3):228–233. [Google Scholar]
- 82.El Ouariachi E., Bouyanzer A. Antioxidant activity of solvent extracts of Inula viscosa from Morocco. Arabian J Chem Environ Res. 2014;1(1):33–40. [Google Scholar]
- 83.Mrid R.B., Bouchmaa N., Kabach I., et al. Dittrichia viscosa L. Leaves: a valuable source of bioactive compounds with multiple pharmacological effects. Molecules. 2022;27(7):2108. doi: 10.3390/molecules27072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orhan N., Gökbulut A., Deliorman Orhan D. Antioxidant potential and carbohydrate digestive enzyme inhibitory effects of five Inula species and their major compounds. South Afr J Bot. 2017;111:86–92. doi: 10.1016/j.sajb.2017.03.040. [DOI] [Google Scholar]
- 85.Alkofahi A.S., Abdul-Razzak K.K., Alzoubi K.H., Khabour O.F. Screening of the Anti-hyperglycemic activity of some medicinal plants of Jordan. Pak J Pharm Sci. 2017;30(3):907–913. [PubMed] [Google Scholar]
- 86.Assi M., Ela M., Raafat K., El-Lakany A. Role of antioxidants in the antidiabetic potential of two indigenous Lebanese Inula species. Med Aromatic Plants S. 2015;2 2167-0412. [Google Scholar]
- 87.Alfarrayeh I., Tarawneh K., Almajali D., Al-Awaida W. Evaluation of the antibacterial and antioxidant properties of the methanolic extracts of four medicinal plants selected from Wadi Al-Karak/Jordan related to their phenolic contents. Res J Pharm Technol. 2022;15(5):2110–2116. [Google Scholar]
- 88.Ounoughi A., Ramdani M., Lograda T., Chalard P. Chemotypes and antibacterial activities of Inula viscosa essential oils from Algeria. Biodiversitas J Biol Diversity. 2020;21(4) [Google Scholar]
- 89.Chekroud Z., Kheffif A., Bassout R. A Future Source of Antibacterial Drugs; 2020. Research Article Valorisation of Algerian Medicinal Plants: Inula Viscosa L. [Google Scholar]
- 90.Rhimi W., Salem I.B., Iatta R., et al. Dittrichia viscosa L. leaves lipid extract: an unexploited source of essential fatty acids and tocopherols with antifungal and anti-inflammatory properties. Ind Crop Prod. 2018;113:196–201. [Google Scholar]
- 91.Mssillou I., Agour A., Slighoua M., et al. Ointment-based combination of Dittrichia viscosa L. And Marrubium vulgare L. Accelerate burn wound healing. Pharmaceuticals. 2022;15(3):289. doi: 10.3390/ph15030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ouahchia C., Hamaidi-Chergui F., Cherif H.-S., et al. Total phenolic content, anti-inflammatory, analgesic, and antipyretic activities of some extracts of inula viscosa (L.) from Algeria. Phytothérapie. 2020;18(2):81–91. [Google Scholar]
- 93.Khalil E.A., Afifi F.U., Al-Hussaini M. Evaluation of the wound healing effect of some Jordanian traditional medicinal plants formulated in Pluronic F127 using mice (Mus musculus) J Ethnopharmacol. 2007;109(1):104–112. doi: 10.1016/j.jep.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Lounis H., Bergheim I., Bouhaimi A., Guigonis J.-M., Belhamel K. Anti-inflammatory and antioxidant activities of Inula viscosa and Senecio anteuphorbium. Oriental Pharm Exper Med. 2018;18(3):225–236. [Google Scholar]
- 95.Kattouf J., Belmoukhtar M., Harnafi H., et al. Effet antihypertenseur des feuilles d'Inula viscosa. Phytothérapie. 2009;7(6):309–312. [Google Scholar]
- 96.Hakkou Z., Maciuk A., Leblais V., et al. Antihypertensive and vasodilator effects of methanolic extract of Inula viscosa: biological evaluation and POM analysis of cynarin, chlorogenic acid as potential hypertensive. Biomed Pharmacother. 2017;93:62–69. doi: 10.1016/j.biopha.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 97.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 98.Tepe H.D., Uğurlu A., Yazgan İ. Determination of phenolic compounds, organic volatile molecules and anti-cancer properties in inula viscosa L., viscum album L. and raphanus sativus L. Sakarya Univ J Sci. 2021;25(3):647–662. [Google Scholar]
- 99.Colak D.K., Egeli U., Eryilmaz I.E., et al. The anticancer effect of inula viscosa methanol extract by miRNAs' Re-regulation: an in vitro study on human malignant melanoma cells. Nutr Cancer. 2022;74(1):211–224. doi: 10.1080/01635581.2020.1869791. [DOI] [PubMed] [Google Scholar]
- 100.El Yaagoubi O.M., Lahmadi A., Bouyahya A., et al. Antitumor effect of Inula viscosa extracts on DMBA-induced skin carcinoma are mediated by proteasome inhibition. BioMed Res Int. 2021:2021. doi: 10.1155/2021/6687589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hepokur C., Budak Y., Karayel H.B., Selvi B., Yaylim İ. Investigation of cytotoxic effects of Inula viscosa extract. Cumhuriyet Sci J. 2019;40(3):578–582. [Google Scholar]
- 102.Messaoudi M., Chahmi N., El Mzibri M., et al. Cytotoxic effect and chemical composition of Inula viscosa from three different regions of Morocco. Eur J Med Plants. 2016;16(4):1–9. [Google Scholar]
- 103.Bar-Shalom R., Bergman M., Grossman S., Azzam N., Sharvit L., Fares F. Inula viscosa extract inhibits growth of colorectal cancer cells in vitro and in vivo through induction of apoptosis. Front Oncol. 2019;9:227. doi: 10.3389/fonc.2019.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kheyar N., Bellik Y., Serra A.T., Kheyar F., Bedjou F. Inula viscosa phenolic extract suppresses colon cancer cell proliferation and ulcerative colitis by modulating oxidative stress biomarkers. Biotechnologia. 2022;103(3):269–281. doi: 10.5114/bta.2022.118670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Virdis P., Migheli R., Galleri G., et al. Antiproliferative and proapoptotic effects of Inula viscosa extract on Burkitt lymphoma cell line. Tumor Biol. 2020;42(2) doi: 10.1177/1010428319901061. [DOI] [PubMed] [Google Scholar]
- 106.Talib W.H., Mahasneh A.M. Antimicrobial, cytotoxicity and phytochemical screening of Jordanian plants used in traditional medicine. Molecules. 2010;15(3):1811–1824. doi: 10.3390/molecules15031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cafarchia C., De Laurentis N., Milillo M.A., Losacco V., Puccini V. [Fungistatic activity of a sesquiterpene lactone (tomentosin) isolated from fresh Inula viscosa (Asteraceae) flowers from the Puglia region] Parassitologia. 2001;43(3):117–121. [PubMed] [Google Scholar]
- 108.Máñez S., Hernández V., Giner R.-M., Ríos J.-L., Recio MdC. Inhibition of pro-inflammatory enzymes by inuviscolide, a sesquiterpene lactone from Inula viscosa. Fitoterapia. 2007;78(4):329–331. doi: 10.1016/j.fitote.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 109.Park H.-H., Kim S.-G., Kim M.J., et al. Suppressive effect of tomentosin on the production of inflammatory mediators in RAW264. 7 cells. Biol Pharm Bull. 2014;37(7):1177–1183. doi: 10.1248/bpb.b14-00050. [DOI] [PubMed] [Google Scholar]
- 110.Hernández V., Máñez S., Recio M.C., Giner R.M., Ríos J.L. Anti-inflammatory profile of dehydrocostic acid, a novel sesquiterpene acid with a pharmacophoric conjugated diene. Eur J Pharmaceut Sci. 2005;26(2):162–169. doi: 10.1016/j.ejps.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 111.Hernández V., Recio M.C., Máñez S., Giner R.M., Ríos J.L. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007;81(6):480–488. doi: 10.1016/j.lfs.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 112.Abrham G., Dovrat S., Bessler H., Grossman S., Uri N., Bergman M. Inhibition of inflammatory cytokine secretion by plant-derived compounds inuviscolide and tomentosin: the role of NFκB and STAT1. Open Pharmacol J. 2010;4(1) [Google Scholar]
- 113.Rozenblat S., Grossman S., Bergman M., Gottlieb H., Cohen Y., Dovrat S. Induction of G2/M arrest and apoptosis by sesquiterpene lactones in human melanoma cell lines. Biochem Pharmacol. 2008;75(2):369–382. doi: 10.1016/j.bcp.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 114.Danino O., Gottlieb H.E., Grossman S., Bergman M. Antioxidant activity of 1, 3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res Int. 2009;42(9):1273–1280. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.