Abstract
In the delta region of Bangladesh, Sonneratia apetala, also known as Keora and mangrove apple, is widely recognized for its dual role as a source of both food and medicine. Seasonal S. apetala fruits were gathered from Hatiya, Noakhali, in October 2021. The samples were segregated into pericarps and seeds, then fractionated into methanol segments. The anti-proliferative activities of these samples against lung A549 cells were evaluated using the Trypan blue exclusion method. Additionally, High-Performance Liquid Chromatography (HPLC) was employed to quantify phenolic compounds, while standard protocols facilitated the identification of specific phytochemical constituents. Chemical profiling via Gas Chromatography-Mass Spectrometry (GC-MS) and the isolation and detection of bioactive compounds through column chromatography and Nuclear Magnetic Resonance (NMR) analysis were undertaken. The methanol fractions of the seeds and pericarp were found to contain carbohydrates, tannins, flavonoids, steroids, alkaloids, glycosides, and terpenoids, with the absence of saponins and anthraquinones. Notably, the anti-proliferative effect demonstrated statistical significance at a concentration of 300 μg/mL for both extracts. Furthermore, HPLC analysis identified and quantified six polyphenols: catechin hydrate, (−)-epicatechin, rutin hydrate, trans-ferulic acid, trans-cinnamic acid, myricetin, and kaempferol, with the following concentrations: 46.65 and 12.72; 349.29 and 140.39; 5.26 and 33.06; 10.35 and 29.28; ND and 11.93; and 10.03 and 7.90 mg/100 g in the methanol fraction of the seed and pericarp, respectively. GC-MS analysis of S. apetala fruit revealed five notable compounds with significant peak areas (%): 2-methyltetracosane, tetratetracontane, heptacosane, 1-chloro-2-hexyl-1-octanol, and phenol, 3,5-bis(1,1-dimethylethyl), exhibiting peak areas of 43.96, 35.8, and 15.95, respectively. Meanwhile, the notable peak in S. apetala seeds was 1,3-benzenedicarboxylic acid, bis(2-ethylhexyl) ester, with a peak area (%) of 100. These compounds are known for their anticancer and antioxidant properties. Therefore, S. apetala, particularly its seeds and fruits, shows promising potential for development into dietary supplements and functional foods.
Keywords: Sonneratiaapetala, Bioactive compounds, Cytotoxicity, Antioxidant, Isolation
1. Introduction
Sonneratia apetala (Buch.-Ham.) belongs to the Lythraceae family [1]. S. apetala is a rapidly expanding and pioneering tree species in ecological succession in the Sundarbans, along the coast of Bangladesh, India, Australia, Malaysia, and other countries [2,3]. The fruits of S. apetala are widely consumed by local coastal communities [2,4]. The ripened fruit of S. apetala tastes sour and contains a greenish-yellow pulp [5]. It produces berry-like fruits that are 1.5–2 cm in diameter, globose, and have a long, leathery calyx. Each fruit contains many seeds, sometimes up to 100, which are yellowish, compactly arranged into 6 to 8 locules, and are primarily U- or V-shaped [6]. The fruit of S. apetala has cytotoxic, analgesic, antidiarrheal, and antihelmintic properties, in addition to antioxidant, antibacterial, and antifungal characteristics [2,7]. Leaf and bark extracts of S. apetala have been found to possess antioxidant, antibacterial, antidiabetic, and anticancer properties [8]. It can be used to treat asthma, ulcers, fever, sprains, swellings, hemorrhages, bleeding, and piles.
The absence of phytonutrients in the human diet contributes to various disorders. Polyphenols are among the most consumed phytochemicals in the diet, with more than 5000 polyphenols identified to date [9]. The seed extract of Sonneratia apetala demonstrates strong antioxidant properties, attributed to its high reducing power, DPPH scavenging ability, NO free radicals scavenging ability, metal chelating ability, and overall antioxidant capacity [2,9]. Among the different extracts, the methanolic extract exhibits the strongest antioxidant capacity [9]. The bark and leaf extract also possesses significant antioxidant properties [[10], [11], [12]]. The ethanol extract of the bark is particularly rich in phenols and ascorbic acid [8]. The pericarp extract displays a noticeable antioxidant capacity, though not as potent as that of the seeds [2]. The methanolic extract of the seeds shows inhibitory effects on both gram-positive and gram-negative bacteria [2]. This supports the traditional use of S. apetala fruit juice by coastal communities as a tonic to treat ailments, such as diarrhea [2]. Moreover, the acetone extract of the bark and leaves also exhibits significant antibacterial properties [8], whereas the pericarp extract does not show significant antibacterial effects [2]. The JAK/STAT signaling pathway is suppressed, and renal uric acid transporters are regulated in mice with hyperuricemia treated with an extract of S. apetala leaves and branches [13,14]. Despite the fact that over 7000 plant species are grown or harvested for food production globally, apart from the wild (IPGRI, 2001–05) [15], the fruit has not received substantial scientific attention.
The purpose of the study on Sonneratia apetala fruit extracts is to investigate and understand the phytochemical composition, antioxidant, and antiproliferative activities of the fruit extracts. By examining the methanol extracts of the pericarp and seeds, the research aims to identify bioactive compounds such as polyphenols, flavonoids, and other phytochemicals that contribute to these activities. Additionally, it aims to contribute to the development of functional foods, dietary supplements, and pharmaceuticals that harness the therapeutic properties of these natural extracts. Through detailed chemical profiling and bioactivity assays, the research endeavors to add valuable knowledge to the field of natural products and their applications in enhancing human health and well-being.
2. Materials and methods
2.1. Chemical reagents for phytochemical constituent screening and polyphenol analysis
To analyze the phytochemicals in the methanolic extracts of S. apetala seed and pericarp, various chemicals and reagents were employed, including ethyl acetate, sulfuric acid, olive oil, phenol, dimethyl sulfoxide (DMSO), acetic acid, perchloric acid, nitric acid, hydrochloric acid, Hager's reagent, Mayer's reagent, Wagner's reagent, sodium chloride, acetic anhydride, alcoholic solution of α-naphthol, lead acetate, and ammonia solution. All solvents were of analytical grade and procured from commercial suppliers, such as Sigma Aldrich, E. Merck, and BDH in England (Germany). Additionally, the following chemicals were sourced from Sigma-Aldrich (St. Louis, MO, USA): Gallic Acid (GA), 3,4-Dihydroxybenzoic Acid (DHBA), Caffeic Acid (CA), Vanillic Acid (VA), Syringic Acid (SA), p-Coumaric Acid (PCA), Rosmarinic Acid (RA), Quercetin (QU), Catechol (CT), Catechin Hydrate (CH), (−)-Epicatechin (ET), and Rutin Hydrate (RH).
2.2. Collection, identification, and authentication of samples
In October 2021, 1.0 kg of S. apetala was collected from Hatiya, Noakhali, Bangladesh. Following collection, the pericarps and seeds were dried. Once dried, the samples were ground into powder and stored in airtight conditions. A taxonomist at the Bangladesh National Herbarium in Dhaka identified the plant, and a voucher specimen was preserved (DACB No. 66955).
2.3. Extraction and processing
Utilizing advanced methods and protocols [9], the air-dried and powdered pericarp and seed parts were macerated for 72 h at room temperature in 2.5 L of methanol, with intermittent shaking and stirring. The mixture was then filtered through a clean cotton plug and Whatman No. 1 filter paper (125 mm), before being concentrated under reduced pressure and temperature (below 40 °C) using a rotary evaporator to yield the crude extracts. This same process was applied to macerate the air-dried and powdered seed material (250 g) in 2.5 L of methanol for 72 h at room temperature. Following this, the methanol extract was filtered and the solvent was removed under low pressure using a rotary evaporator. Subsequently, the extracts were accurately weighed and stored in a refrigerator.
2.4. Phytochemical screening
To identify the constituents of phytochemicals, specific chemical tests were conducted using standard techniques as reported in the literature [[16], [17], [18]] to confirm their exclusive presence in the methanol extracts (refer to Table 1).
Table 1.
Several standard phytochemical tests were conducted to identify the plant metabolites in the pericarp and seed extracts of S. apetala.
| P.chemical group | Tests | Observations |
|---|---|---|
| Saponins |
|
|
| Flavonoids |
|
|
| Alkaloids |
|
|
| Carbohydrates |
|
|
| Anthraquinones |
|
|
| Steroids | Liebermann-Burchard's test: 1 mL of acetic acid and 1 mL of chloroform mixed with 0.0 °C. Then, the combined solution was mixed with each crude extract and conc. H2SO4 was added. |
|
| Terpenoids |
|
|
| Tannins |
|
|
| Glycosides |
|
|
| Coumarins |
|
|
2.5. GC‒MS analysis
GC-MS analysis was conducted using a GC-MS-QP 2010 Ultra instrument equipped with an AB Innowax column (30 m × 0.25 mm i.d., 0.25 μm film thickness). The decision to inject a 0.5 μL sample for GC-MS analysis likely considered factors such as sensitivity, concentration, analyte stability, column capacity, and instrument specifications. Initially, the oven temperature was set to 120 °C for 1 min, followed by an increase to 270 °C over 25 min. Helium was used as the carrier gas at a flow rate of 1.15 mL/min. The split ratio was set at 200, while the sample injector and mass transfer line temperatures were maintained at 200 °C and 250 °C, respectively, throughout the experiment. The ionization energy for the mass spectroscopy study was set at 70 eV. Mass spectra were recorded for 40.75 min, spanning a range of 50–650 m/z. The retention indices of the components were compared with those in the Wiley and NIST Libraries, as well as literature values [19].
2.6. Analysis of antiproliferative activity using ATCC cell lines
The antiproliferative activity of the samples was evaluated in lung A549 cells using the Trypan blue exclusion method. Lung A549 cells (passage 92) were obtained from ATCC. The cells were cultured in high-glucose Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10 % (v/v) fetal bovine serum and 1 % (v/v) antibiotic solution (10,000 U/mL penicillin and 10,000 μg/mL streptomycin, HyClone Laboratories, USA). Cultures were maintained in a CO2 incubator at 37 °C with 5 % CO2. Cell passaging involved aspirating the old media, washing off the media residue with phosphate-buffered saline (PBS, pH 7.4), and trypsinizing with 0.25 % trypsin-0.5 mM EDTA solution (HyClone Laboratories, USA). Prior to treatment, cells were seeded at approximately 1 × 104 cells/mL in T25 flasks using trypsin-EDTA solution in the aforementioned growth media. After 24 h, achieving 60–80 % confluency, the medium was aspirated and replaced with 3 mL of fresh medium. Approximately 15 mg of each sample was dissolved in DMSO to achieve a concentration of 15,000 μg/mL and was sterilized through a 0.2 μm filter. The samples were then added to each flask at final concentrations of 50, 100, 200, and 300 μg/mL in triplicate, using a maximum of 0.5 % DMSO as the solvent. DMSO also served as a negative control in the experiment. The flasks were incubated at 37 °C with 5 % CO2 for 24 h. Following incubation, cells were harvested using 1 mL of 0.2 g/l EDTA solution. Cell morphology was observed under an inverted microscope and compared with the control. Viable cells were detached and counted using Trypan Blue in an automated cell counter (Luna II, UK). The antiproliferative activity was calculated using the equation no. 1 [20].
| (1) |
The antiproliferative activity of the test samples on the cells was expressed in IC100 values determined by a best-fit curve between % of viable cells to sample concentration.
2.7. Identification and quantification of polyphenols using HPLC
HPLC-DAD was utilized to identify and quantify phenolic compounds in the fraction [21]. Experiments were carried out using a Dionex UltiMate 3000 system equipped with a quaternary rapid separation pump (LPG-3400RS) and a photodiode array detector (DAD-3000RS). Compounds were separated on a 4.6 × 250 mm Acclaim® C18 (5 μm) Dionex column at 30 °C, employing a flow rate of 1 mL/min and an injection volume of 0.5 μL [9]. The mobile phase comprised acetic acid, acetonitrile, and methanol. Post-run, the system was reequilibrated with 5 % A/95 % B for 5 min. The DAD was set to scan from 200 to 700 nm, while the UV detector was programmed at 280 nm for 18 min, 320 nm for 6 min, and 380 nm for the remainder of the analysis. To establish the calibration curve and ensure accurate quantitative analysis of target compounds, a standard stock solution was prepared in methanol with concentrations of 20 μg/mL for GA, VA, CH, EC, PCA, RH, and EA, 8 μg/mL for CA, and 6 μg/mL for QU. The S. apetala methanol seed (MS) solution concentration was 5 mg/mL. Prior to HPLC analysis, all solutions were filtered through a 0.20 μm nylon syringe filter (Sartorius, Germany) and degassed in an ultrasonic bath (Hwashin, Korea) for 15 min. Data collection, peak integration, and calibration were conducted using Dionex Chromeleon Software (Version 6.80 RS 10) [22].
2.8. Isolation of compound ASD-1 from methanol extract of S. apetala seeds using column chromatography
For the initial fractionation, a 5.0 g methanolic extract from the seeds and pericarp of S. apetala was processed using column chromatography. The extract was subjected to TLC analysis, which displayed multiple spots across various solvent systems under UV light and after being sprayed with a detecting reagent on a TLC plate. Notably, the resolution was enhanced when using an n-hexane-dichloromethane mixture as the mobile phase. To prepare the methanolic extract for chromatography, it was first diluted in a minimal volume of ethanol and then adsorbed onto silica gel (Kieselgel 60, mesh 70–230). This mixture was then introduced into a column packed with analytical-grade silica gel. The column, measuring 35 cm in length and 10 cm in diameter, was filled with a slurry of silica gel in n-hexane. After ensuring the adsorbent bed was at the desired height, several hundred milliliters of n-hexane were passed through to achieve proper packing. The adsorbed extract was carefully applied at the top of the column. Elution was performed using solvents of gradually increasing polarity: n-hexane, dichloromethane, a mixture of dichloromethane and ethyl acetate, ethyl acetate, a mixture of ethyl acetate and methanol, and ultimately 100 % methanol. This process yielded eight fractions, ranging from 60 to 100 mL each, which were further purified based on their TLC profiles. From fraction 5 of the VLC methanol extract (collection numbers 1–3), white crystals were isolated by decantation after three days at room temperature. These crystals were then rinsed with a few drops of methanol to yield the compound ASD-1 in its pure form (5.0 mg).
2.9. NMR spectra analysis
At the Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, 1D (1H) NMR spectra were recorded using CDCl₃ solvents on a BRUKER WH 400 MHz NMR spectrometer. All spectra were obtained at room temperature (RT). Chemical shifts are expressed in parts per million (ppm). Prior to use, common laboratory solvents were distilled in glass.
2.10. Statistical analysis
All experiments were conducted in triplicate, with results presented as the mean ± standard error of the mean (SEM). The antiproliferative assay assessed the percentage of inhibition at each concentration relative to the negative control. Statistical significance was determined using Dunnett's multiple comparison test following a one-way analysis of variance (ANOVA) conducted in GraphPad Prism version 9.00. Results were considered statistically significant at P values less than 0.01. Data are reported as mean (average) ± standard deviation (SD) for the specified number of observations. A P-value threshold of 0.05 was set as the criterion for statistical significance.
3. Results and discussion
Plants have medicinal properties owing to their distinctive chemical compounds, which have specific physiological effects on the human body. Principal bioactive components available in the investigated plant include alkaloids, anthraquinones, carbohydrates, coumarins, flavonoids, glycosides, steroids, saponins, and tannins. These secondary metabolites also referred to as phytochemicals, are produced during the plant's normal metabolic processes and serve as a means of defense. Consequently, the medicinal efficacy of a plant is not due solely to a single active ingredient but rather to the synergistic effects of multiple compounds contained within the plant. The phytochemical screening results for the qualitative analysis of methanol extracts of S. apetala pericarp and seed, presented in Table 2, revealed the presence of carbohydrates, tannins, flavonoids, steroids, alkaloids, glycosides, and terpenoids. Conversely, the extracts from both the pericarp and seed were found to lack saponins and anthraquinones.
Table 2.
Screening results of extracts from the pericarp and seed of S. apetala.
| Tests for | Test Name | Pericap extracts | Seed extracts |
|---|---|---|---|
| Saponins |
|
b | b |
| b | b | ||
| Flavonoids |
|
a | a |
| a | a | ||
| Alkaloids |
|
a | a |
| a | a | ||
| a | a | ||
| Carbohydrates | Molisch's test | a | a |
| Anthraquinones | Borntrager'stest | b | b |
| Steroids |
|
a | a |
| a | a | ||
| Terpenoids |
|
a | a |
| a | a | ||
| Tannins |
|
a | a |
| a | a | ||
| Glycosides |
|
a | a |
| a | a | ||
| Coumarins | MeOH test | a | a |
= present.
= absent.
The biological activities of carbohydrates encompass antitumor, antioxidant, antidiabetic, antiviral, hypolipidemic, and immunomodulatory effects [23]. Furthermore, plant tannins are recognized for their antioxidant, antibacterial, antiviral, antiparasitic, anti-inflammatory, anti-HIV, and antidiarrheal properties [24]. Saponins are noted for their pharmacological, antibacterial, fungicidal, and insecticidal impacts [25]. Glycosides exhibit a range of activities including laxative, diuretic, antiseptic effects, and actions against microorganisms, insects, and herbivores [26]. Flavonoids are significant for their antimicrobial, hypoglycemic, antidiabetic, anti-inflammatory, antioxidant, antitumor, anticarcinogenic, antimutagenic properties, and free radical-scavenging activities, besides aiding in the reduction of high blood pressure [[27], [28], [29]]. Alkaloids, naturally occurring nitrogen-containing compounds, have antimicrobial capabilities due to their ability to intercalate with microbial DNA [30]. The hypoglycemic effects are also observed with glycosides, flavonoids, and alkaloids. Terpenoids indicate potential cytotoxic properties in the methanol extract [31]. Pharmaceuticals utilize steroids such as corticosteroids, sex hormones, neurosteroids, vitamin D, bile acids, and cardiotonic steroids for therapy [32]. The pharmacological actions of coumarin, including antitumor, anticoagulant, anti-inflammatory, anticancer, antioxidant, anti-HIV, and antibacterial activities, have garnered significant interest [[33], [34], [35], [36]].
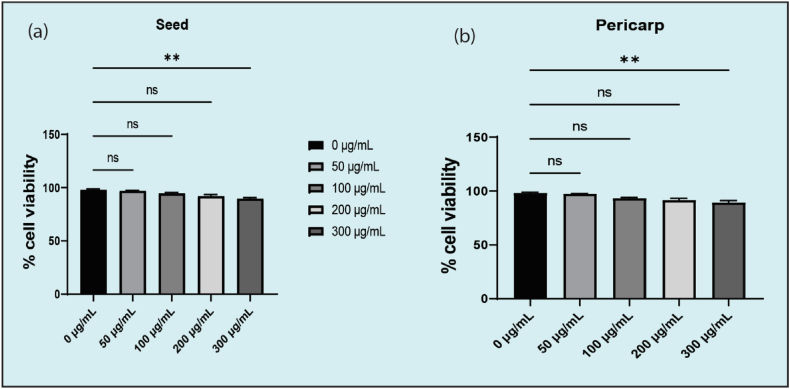
The study on Sonneratia apetala fruit extracts revealed promising cytotoxic potential against cancer cell lines, notably the lung A549 cells. Utilizing the trypan blue exclusion method, the research demonstrated that the extracts, particularly at a concentration of 300 μg/mL (refer to Fig. 1, Fig. 2 (a,b)), exhibited significant antiproliferative effects, underscoring the potential of S. apetala as a source of anticancer compounds. These findings align with the growing body of evidence supporting the use of natural plant extracts in cancer treatment and prevention strategies, suggesting that the bioactive compounds within S. apetala, such as polyphenols, may play a critical role in its cytotoxic activity.
Fig. 1.
Inhibition percent of lung A549 cells by pericarp and seed extracts.
Fig. 2.
Inhibition percent of lung A549 cells by seed extract (a) P < 0.001 at 300 μg/mL; (b) P < 0.001 at 300 μg/mL.
3.1. Measurement of polyphenols in S. apetala's seed and pericarp methanol fractions
The chromatographic separation of polyphenols present in MS and MP extracts, as performed by High-Performance Liquid Chromatography (HPLC), is depicted in Fig. 3 (A, B).
Fig. 3.
(A): HPLC chromatogram of MS Peaks: 1.Catechin hydrate (CH); 2. (−) Epicatechin (ET); 3. Rutin hydrate (RH); 4. Trans-Ferulic acid (TFA); 5. Myricetin (MT) and 6. Kaempferol (KF).
(B): HPLC chromatogram of MP Peaks: 1. Catechin hydrate (CH); 2. (−) Epicatechin (ET); 3. Rutin hydrate (RH); 4. Trans-Ferulic acid (TFA); 5. Trans-Cinnamic acid (TCA); 6. Myricetin (MT) and 7. Kaempferol (KF).
The average of five measurements was utilized to calculate the level of each phenolic compound, which is presented in Table 3 along with the respective calibration curves. High concentrations of (−)-epicatechin (ET) were detected in MS and MP (349.29 and 140.39 mg/100 g of fraction, respectively). Catechin hydrate (CH), rutin hydrate (RH), trans-ferulic acid (TFA), trans-cinnamic acid (TCA), myricetin (MT), and kaempferol (KF) were also identified in lesser amounts (46.65 and 12.72; 5.26 and 33.06; 10.35 and 29.28; ND and 11.93; 10.03 and 7.90; 55.02 and 1.20 mg/100 g of fraction, respectively) for both fractions. Furthermore, the HPLC chromatogram shown in Fig. 3A, B revealed peaks corresponding to simple flavonoid aglycones, anthocyanins, polyphenols, and flavonoid glycosides.
Table 3.
The quantity of polyphenolic compounds in the methanol fraction of seeds and pericarp.
| Name of Standard | Pericarp (mg/100 g dry extract) | Seed (mg/100 g dry extract) |
|---|---|---|
| Caffeic acid (CA), Gallic acid (GA), Vanillic acid (VA), 3,4 dihydroxy benzoic acid (DHBA), Syringic acid (SA), Rosmarinic acid (RA), p-Coumaric acid (PCA), Catechol (CT) and Quercetin (QU) | ND | ND |
| Catechin hydrate (CH) | 46.65 ± 0.26 | 12.72 ± 0.52 |
| (−) Epicatechin (ET) | 349.29 ± 0.98 | 140.39 ± 0.55 |
| Rutin hydrate (RH) | 5.26 ± 0.43 | 33.06 ± 0.02 |
| Trans-Ferulic acid (TFA) | 10.35 ± 0.37 | 29.28 ± 0.06 |
| Trans-Cinnamic acid (TCA) | ND | 11.93 ± 0.10 |
| Myricetin (MT) | 10.03 ± 0.24 | 7.90 ± 0.20 |
| Kaempferol (KF) | 55.02 ± 0.30 | 1.20 ± 0.02 |
Catechins, phytochemicals found in tea leaves, beans, black grapes, cherries, and cacao, exhibit diverse physiological effects. Research indicates that catechins contribute to health promotion and disease prevention. Antioxidant activity, liver damage prevention, cholesterol reduction, and anti-obesity effects have been established through animal and clinical studies [37]. Foods rich in (−)-epicatechin may help to lower blood pressure, addressing a risk factor for heart and brain complications [38]. Rutin (rutoside) hydrate, a flavonoid present in many plants, possesses anti-inflammatory, antidiabetic, antioxidant, neuroprotective, nephroprotective, hepatoprotective, and amyloid oligomer-reducing properties, and is capable of crossing the blood-brain barrier [39]. Trans-ferulic acid (TFA) showcases anti-inflammatory, antibacterial, and antifungal activities through its phenoxy radical scavenging [40]. Cinnamic acid derivatives are employed in the treatment of cancer, bacterial infections, diabetes, and neurological disorders [41]. Myricetin is known for its hepatoprotective, anti-inflammatory, anti-obesity, anti-diabetes, anticancer, and antidiabetic properties [42]. Kaempferol, a flavonol, supports the structural and functional integrity of organs and cells by reducing lipid oxidation. The antioxidant, antibacterial, anticancer, neuroprotective, and hepatoprotective actions of kaempferol have been extensively highlighted in this study [43].
Polyphenols, saponins, flavonoids, tannins, steroids, alkaloids, and other compounds found in mangroves hold significant value for industry and medicine [44]. Polyphenolic compounds contribute to the flavor, color, and taste of foods, vegetables, fruits, and beverages. The methanol seed (MS) and methanol pericarp (MP) extracts displayed high levels of (−)-epicatechin (ET) and lower concentrations of catechin hydrate (CH), rutin hydrate (RH), trans-ferulic acid (TFA), trans-cinnamic acid (TCA), myricetin (MT), and kaempferol (KF). These compounds are beneficial polyphenols known for their rich antioxidant properties. Most exhibited synergistic effects, with their antioxidant efficacy varying according to structure [45]. It was found that pineapple peels did not exhibit synergistic effects among polyphenols [46]. Fig. 3B displays peaks that remained unidentified due to the use of only nine phenolic standards for quantification in the Sonneratia apetala fruit (MS) fraction. These unidentified peaks were observed in MS regions typically associated with simple polyphenols, catechins, anthocyanins, and flavonoid glycosides [47].
The methanolic pericarp extract of Sonneratia apetala contained five distinct chemicals, as identified by GC-MS analysis (refer to Fig. 4 and Table 4). The structure of the identified compounds (refer to Table 4) was illustrated using ChemDraw software and is shown in Fig. 5. The chromatogram for the methanolic pericarp extract (Fig. 4A) showed five peaks. In contrast, the chromatogram for the seed extract (Fig. 4B) displayed only one peak. Table 4 details the structures of the most prevalent compounds found in the methanolic extract of S. apetala fruit. Specifically, the analysis of the methanolic pericarp extract (Fig. 4A) identified five peaks: 2-Methyltetracosane with a retention time of 26.830 min and a peak area of 43.96 %, Tetratetracontane with a retention time of 30.306 min and a peak area of 35.81 %, Heptacosane, 1-chloro, with a retention time of 35.194 min and a peak area of 15.95 %, 2-Hexyl-1-octanol with a retention time of 23.871 min and a peak area of 3.44 %, and Phenol, 3,5-bis(1,1-dimethylethyl), with a retention time of 21.184 min. Conversely, the analysis of the methanolic seed extract (Fig. 4B) revealed a single peak corresponding to 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester, which accounted for 100 % of the peak area.
Fig. 4.
Chromatogram (GC/MS) of the methanol extract (A) Pericarp and (B) seed of S. apetala.
Table 4.
Tentative compounds identified from the methanol extract of S. apetala by GC-MS analysis.
| Sl No | Name of compound | Nature | M. weight | M. formula | P. Area | P. area (%) | m/z | RT |
|---|---|---|---|---|---|---|---|---|
| 01 | 1,3 Benzendicarboxylic acid, bis (2-ethylhexyl) ester | Ester | 390.5561 | C24H38O4 | 85911742 | 100 | 57 | 35.468 |
| 02 | 2methyltetracosane | Fatty acid | 352.680 | C25H52 | 813691133 | 43.96 | 57 | 26.830 |
| 03 | Tetratetracontane | Chain alkane | 619.2 | C44H90 | 66274922 | 35.81 | 57 | 30.306 |
| 04 | Heptacosane, 1-chloro | Chain alkane | 415.2 | C27H55Cl | 29515430 | 15.95 | 57 | 35.194 |
| 05 | 2-Hexyl-1-octanol | aliphatic alcohol | 214.3874 | C14H30O | 6361140 | 3.44 | 57 | 23.871 |
| 06 | Phenol,3,5-bis(1,1-dimethylethyl) | Phenol | 206.3239 | C14H22O | 1560711 | 0.84 | 57 | 21.184 |
Fig. 5.
Chemical structures of the compounds in the methanol extract of the seed and pericarp.
Bioactivity tests have demonstrated that the pure compound 1,2-benzene dicarboxylic acid ester exhibits notable anticancer activity [48] and antibacterial properties [[48], [49], [50]]. Additionally, 2-methyltetracosane is known for its free radical scavenging properties [48]. Tetratetracontane has been found to possess antioxidant, hypoglycemic, hypolipidemic [[51], [52], [53]], and antibacterial properties [50]. Phenol, 3,5-bis(1,1-dimethylethyl), is recognized for its antibacterial and anti-inflammatory activities [53,54]. Heptacosane, 1-chloro, exhibits antibacterial and antifungal activities [55]. Hexyl-1-octanol (2-OH) demonstrates antimicrobial properties [56].
3.2. Spectral data of isolated compound (ASD-1)
The isolated compound, designated ASD-1, was obtained from the seed extract. The 1H NMR spectrum of ASD-1, displayed in Fig. 6, offers insightful information about the molecule's hydrogen atoms. Chemical shifts (δ), expressed in parts per million (ppm), reflect the electronic environment surrounding the hydrogen atoms, aiding in the identification of proton types present (e.g., aliphatic, aromatic, hydroxyl, etc.). According to Fig. 5, the observed chemical shifts (δ) values are as follows: δ 8.51 (1H, s, H-5), 4.87 (1H, s, H-4), 4.86 (1H, s, H-2), 4.59 (1H, s, H-8), 4.21 (3H, s, -OCH3 at C-1), 3.49 (3H, s, -OCH3 at C-3), 3.32 (3H, s, -CH3 at C-7), with additional shifts observed at 3.31, 2.70, 2.16, 1.98, 1.29, and 0.90 ppm, respectively.
Fig. 6.
1H NMR of isolated compound (ASD-1).
4. Conclusions
S. apetala is an evergreen pioneer tree species that grows rapidly and is widely distributed along the coast of South Asia. Extracts from the plant components of S. apetala have been found to exhibit significant antioxidant and cytotoxic effects. The edible mangrove fruit of S. apetala is a natural bounty, rich in bioactive properties and functional phytochemicals. Both the methanol seed (MS) and methanol pericarp (MP) extracts of S. apetala demonstrated remarkable antioxidant activity, attributed to the exceptionally high levels of polyphenolic compounds they contain. The various biological properties of the fruit may be partially due to the synergistic effects of identified polyphenols such as (−)-epicatechin (ET), catechin hydrate (CH), rutin hydrate (RH), trans-ferulic acid (TFA), trans-cinnamic acid (TCA), myricetin (MT), and kaempferol (KF). Furthermore, the seeds of the S. apetala plant have the potential to serve as an enhanced source of both hydrophilic and lipophilic functional food components, aiming to improve human physiological health. Additionally, the current HPLC method offers an efficient approach for identifying and quantifying polyphenols in dietary products.
The fruit could be cultivated in tidal seawater across large tropical coastal areas worldwide, contributing to food security, primary health care, environmental protection, revenue generation, and the development of functional foods or dietary supplements. However, inadequate access to food supplies remains a primary cause of hunger and malnutrition among populations in tropical coastal regions, suggesting the fruit could serve as alternative medicine for deltaic communities. The wood of S. apetala has potential applications in various manufacturing processes, including pulping, veneering, hardboard, strand board, and plywood production. Additionally, the fruits could be utilized in fish and animal feed formulations.
Data availability
All data are available in the manuscript.
CRediT authorship contribution statement
Md Ripaj Uddin: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Fatema Akhter: Writing – original draft, Methodology. Md Jainal Abedin: Writing – review & editing, Supervision. Md Aftab Ali Shaikh: Methodology. Muhammad Abdullah Al Mansur: Methodology, Formal analysis. Mohammad Saydur Rahman: Methodology. AHM Shofiul Islam Molla Jamal: Writing – review & editing, Methodology. Md Ahedul Akbor: Methodology, Formal analysis. Md Hemayet Hossain: Methodology, Formal analysis. Suriya Sharmin: Methodology, Formal analysis. Abubakr M. Idris: Methodology, Formal analysis. Mayeen Uddin Khandaker: Writing – review & editing, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was carried out under the Research and Development (R & D) project entitled “Isolation of Bioactive Compounds and Biological Studies on Mangrove Species, Sonneratiaapetala Fruit and Nipafruticans Fruit and Sap.” approved in the financial year of 2022–2023 by the authority of BCSIR, Ministry of Science and Technology, The people's Republic of Bangladesh (Reference no.39.02.0000.011.14.134.2021/900, Date: 12.07.2022, p-9, Serial No. 254). The authors are grateful to the authority of the Institute of National Analytical Research and Service (INARS), BCSIR, Dhaka, 470 Bangladesh for providing analytical, technical, and other logistic support for conducting this research work. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group research project under grant number (R.G.P.2/430/44).
Contributor Information
Md Ripaj Uddin, Email: md.ripajuddin@gmail.com.
Fatema Akhter, Email: fatemaaktherjenny@gmail.com.
Md Jainal Abedin, Email: abedinj88@yahoo.com.
Md Aftab Ali Shaikh, Email: chairman@bcsir.gov.bd.
Muhammad Abdullah Al Mansur, Email: nayeembcsir@gmail.com.
Mohammad Saydur Rahman, Email: saydur_pr@yahoo.com.
AHM Shofiul Islam Molla Jamal, Email: shofiuljamal@yahoo.com.
Md Ahedul Akbor, Email: akborbcsir@gmail.com.
Md Hemayet Hossain, Email: hemayet.hossain02@gmail.com.
Suriya Sharmin, Email: suriya_sharmin@bcsir.gov.bd.
Abubakr M. Idris, Email: dramidris@gmail.com.
Mayeen Uddin Khandaker, Email: mayeenk@sunway.edu.my.
References
- 1.Hossain S.J., Islam M.R., Pervin T., Iftekharuzzaman M., Hamdi O.A., Mubassara S., Shilpi J.A. Antibacterial, anti-diarrheal, analgesic, cytotoxic activities, and GC‒MS profiling of Sonneratiaapetala (Buch.-Ham.) Seed. Preventive nutrition and food science. 2017;22(3):157. doi: 10.3746/pnf.2017.22.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain S.J., Basar M.H., Rokeya B., Arif K.M.T., Sultana M.S., Rahman M.H. Evaluation of antioxidant, antidiabetic and antibacterial activities of the fruit of Sonneratiaapetala (Buch.-Ham.) Orient Pharm Exp Med. 2013;13:95–102. [Google Scholar]
- 3.Uddin M.R., Khandaker M.U., Akter N., Ahmed M.F., Hossain S.M.M., Gafur A., Idris A.M. Identification and economic potentiality of mineral sands resources of Hatiya island, Bangladesh. Minerals. 2022;12(11):1436. [Google Scholar]
- 4.Pramanick P., Zaman S., Bera D., Raha A.K., Mitra A. Mangrove fruit Products: a search for alternative livelihood for Island dwellers of Gangetic delta. Int. J. Pharm. Res. Sch. 2014;3(1):131–137. [Google Scholar]
- 5.Pramanick P., Zaman S., Mitra A. Mangrove fruit-based jelly as roadmap to alternative livelihood for island dwellers of Indian Sundarbans. Natural Resources And Their Ecosystem Services. 2021;2:121–126. [Google Scholar]
- 6.Das S., Ghose M. Seed structure and germination pattern of some Indian mangroves with taxonomic relevance. Taiwania. 2003;48:287–298. [Google Scholar]
- 7.Sunita A., Ganesh K., Sonam M. Screening and evaluation of bioactive components of Cenchrusciliaris L. by GC‒MS analysis. Int. Res. J. Pharm. 2017;8(6) [Google Scholar]
- 8.Patra J.K., Das S.K., Thatoi H. Phytochemical profiling and bioactivity of a mangrove plant, SonneratiaApetala,from odisha coast of India. Chinese Journal of Integrated Medicine. 2015;21:274–285. doi: 10.1007/s11655-014-1854-y. [DOI] [PubMed] [Google Scholar]
- 9.Hossain S.J., Pervin T., Suma S.A. Effects of cooking methods at different time durations on total phenolics and antioxidant activities of fresh and dried-stored fruits of Sonneratiaapetala(Buch.-Ham.) Int. Food Res. J. 2016;23:556–563. [Google Scholar]
- 10.Islam T., Islam M.N., Zzaman W., Billah M.M. StudyofAntimicrobial,Antioxidantand cytotoxicity properties of selected plant extracts for food preservative applications. International Journal of Food Studies. 2021;10(February):SI95–SI111. doi: 10.7455/ijfs/10.SI.2021.a8. [DOI] [Google Scholar]
- 11.Sarkar Kumar B., Sarkar Kumar B., Das J., Modak P., Das A., Halder S., Islam F., Chowdhury Rani A., Kundu Kumar S. Phytochemical screening and antioxidant profiling of two mangrove species of sundarbans: heritierafomes and sonneratiaapetala. Pharmacologyonline. 2020;2(September):163–174. [Google Scholar]
- 12.Mukul M.E.H., Hossain M.S., Ahamed S.K., Debnath P., Akter M. Antioxidant and membrane stabilizing activities of bark of sonneratiaapetala. Bangladesh Pharmaceutical Journal. 2016;19(2):147–151. doi: 10.3329/bpj.v19i2.29272. [DOI] [Google Scholar]
- 13.Wu Y.L., Chen J.F., Jiang L.Y., Wu X.L., Liu Y.H., Gao C.J., Chen J.N. The extract of Sonneratiaapetala leaves and branches ameliorates hyperuricemia in mice by regulating renal uric acid transporters and suppressing the activation of the JAK/STAT signaling pathway. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.698219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang L., Wu Y., Qu C., Lin Y., Yi X., Gao C., Zeng H. Hypouricemic effect of gallic acid, a bioactive compound from Sonneratiaapetala leaves and branches, on hyperuricemic mice. Food Funct. 2022;13(19):10275–10290. doi: 10.1039/d2fo02068h. [DOI] [PubMed] [Google Scholar]
- 15.International Plant Genetic Reesources Institute (IPGRI) Conserving and increasing the use of neglected and underutilized crop species. 2001–2005. http://www.ipgri.cgiar.org/institute/siteinfo.html
- 16.Harborne J.B. London chapman and hall, Ltd; 1973. Phytochemical Methods; pp. 49–188. [Google Scholar]
- 17.Trease G.E., Evans W.C. Macmillan publishers; 1989. Pharmacognosy. 11thEdn. Brailliartiridel Can; p. 530. [Google Scholar]
- 18.Software A. Spectrum books ltd., Ibadan; Nigeria: 1993. Medicinal Plants and Traditional Medicines in Africa; p. 289. [Google Scholar]
- 19.Adams R. Allured publishing Co., Carol Stream, II; 1995. Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry. [Google Scholar]
- 20.Hoque N., Afroz F., Khatun F., Rony S.R., Hasan C.M., Rana M.S., Sohrab M.H. Physicochemical, pharmacokinetic and cytotoxicity of the compounds isolated from an endophyte fusariumoxysporum: in vitro and in silico approaches. Toxins. 2022;14(3):159. doi: 10.3390/toxins14030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuanphongpanich S., Phanichphant S. Method development and determination of phenolic compounds in broccoli seeds samples. Chiang Mai J. Sci. 2006;33:103–107. [Google Scholar]
- 22.Hossain S.J., Iftekharuzzaman M., Haque M.A., Saha B., Moniruzzaman M., Rahman M.M., Hossain H. Nutrient compositions, antioxidant activity, and common phenolics of Sonneratiaapetala (Buch.-Ham.) fruit. Int. J. Food Prop. 2015;19:1080–1092. [Google Scholar]
- 23.Oyedepo T.A., Kayode A.A. Functional Foods and Nutraceuticals. Springer; Cham: 2020. Bioactive carbohydrates, biological activities, and sources; pp. 39–74. [Google Scholar]
- 24.Tong Z., He W., Fan X., Guo A. Biological function of plant tannin and its application in animal health. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.803657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sparg S., Light M.E., Van Staden J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004;94(2–3):219–243. doi: 10.1016/j.jep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Ghislain O., Sandra L., Nele B. Vol. 38. RSC Publishing; London, UK: 2012. (Applications of Glycobiology: Biological and Immunological Effects of a Chemically Modified Amylose-Derivative). [Google Scholar]
- 27.Narayana K.R., Reddy M.R., Chaluvadi-Krishna D.R. Bioflavonoids classification, pharmacology, biochemical effects and therapeutic potential. Ind. J.Pharmacol. 2001;33:2–16. [Google Scholar]
- 28.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;2013 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad M., Aktar M.S., Malik T. Hypoglycemic action of the flavonoids fraction of cuminumseeds. Phytother Res. 2000;14:103–106. doi: 10.1002/(sici)1099-1573(200003)14:2<103::aid-ptr578>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.Garba S., Okeniyi S.O. Antimicrobial activities of total alkaloids extracted from some Nigerian medicinal plants. J. Microbiol. Antimicrob. 2012;4(3):60–63. [Google Scholar]
- 31.Ozçelik B., Kartal M., Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2001;49:396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- 32.Salvador J.A.R., Carvalho J.F.S., Neves M.A.C., Silvestre S.M., Leitão A.J., Silva M.M.C., et al. Anticancer steroids: linking natural and semisynthetic compounds. Nat. Prod. Rep. 2013;30(2):324–374. doi: 10.1039/c2np20082a. [DOI] [PubMed] [Google Scholar]
- 33.Nasr T., Bondock S., Youns M. Anticancer activity of new coumarin substituted hydrazide–hydrazone derivatives. Eur. J. Med. Chem. 2014;76:539–548. doi: 10.1016/j.ejmech.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Bronikowska J., Szliszka E., Jaworska D., Czuba Z.P., Krol W. The coumarinpsoralidin enhances anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Molecules. 2012;17(6):6449–6464. doi: 10.3390/molecules17066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L., Zhao X.Y., Wu Y.L., Zhang W. 2015 Asia-Pacific Energy Equipment Engineering Research Conference. Atlantis Press; 2015, June. The study on biological and pharmacological activity of coumarins; pp. 135–138. [Google Scholar]
- 36.Canning C., Sun S., Ji X., Gupta S., Zhou K. Antibacterial and cytotoxic activity of isoprenylatedcoumarinmammea A/AA isolated from Mammeaafricana. J. Ethnopharmacol. 2013;147(1):259–262. doi: 10.1016/j.jep.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Kim J.M., Heo H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022:1–14. doi: 10.1007/s10068-022-01069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernatova I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018;36(3):666–681. doi: 10.1016/j.biotechadv.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Ghorbani A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017;96:305–312. doi: 10.1016/j.biopha.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Rezaeiroshan A., Saeedi M., Morteza-Semnani K., Akbari J., Hedayatizadeh-Omran A., Goli H., Nokhodchi A. Vesicular formation of trans-ferulic acid: an efficient approach to improve the radical scavenging and antimicrobial properties. Journal of Pharmaceutical Innovation. 2021:1–10. [Google Scholar]
- 41.Ruwizhi N., Aderibigbe B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020;21(16):5712. doi: 10.3390/ijms21165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imran M., Saeed F., Hussain G., Imran A., Mehmood Z., Gondal T.A., Islam S. Myricetin: a comprehensive review on its biological potentials. Food Sci. Nutr. 2021;9(10):5854–5868. doi: 10.1002/fsn3.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bangar S.P., Chaudhary V., Sharma N., Bansal V., Ozogul F., Lorenzo J.M. Kaempferol: a flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022:1–25. doi: 10.1080/10408398.2022.2067121. [DOI] [PubMed] [Google Scholar]
- 44.Bandaranayake W.M. Bioactivities. Bioactive compounds, and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002;10:421–452. [Google Scholar]
- 45.Meyer A.S., Heinonen M., Frankel E.N. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and EllagicAcidon human LDL oxidation. Food Chem. 1998;61:71–75. [Google Scholar]
- 46.Li T., Shen P., Liu W., Liu C., Liang R., Yan N., Chen J. Major polyphenolics in pineapple peels and their antioxidant interactions. Int. J. Food Prop. 2014;17:1805–1817. [Google Scholar]
- 47.Sakakibara H., Honda Y., Nakagawa S., Ashida H., Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003;51:571–581. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- 48.Sunita S., Joshi M. Pharmacognostic evaluation of medicinally important mangroves Avicennia marina and Sonneratiaapetala. World Journal of Pharmaceutical Science. 2015;3(7):1403–1412. [Google Scholar]
- 49.Ibrahim N.B.B.R., Puchooa D., Govinden-Soulange J., Facknath S. Chemical profiling and biological activity of Cassia abbreviate Oliv. South Afr. J. Bot. 2022;146:325–339. [Google Scholar]
- 50.Albratty M., Alhazmi H.A., Meraya A.M., Najmi A., Alam M.S., Rehman Z., Moni S.S. Spectral analysis and antibacterial activity of the bioactive principles of sargassumtenerrimum J. Agardh collected from the red sea, jazan, kingdom of Saudi arabia. Braz. J. Biol. 2021;83 doi: 10.1590/1519-6984.249536. [DOI] [PubMed] [Google Scholar]
- 51.Kalsum N., Sulaeman A., Setiawan B., Wibawan I.W.T. Phytochemical profiles of propolisTrigona spp. from three regions in Indonesia using GC‒MS. Journal of Biology, Agriculture and Healthcare. 2016;6(14):31–37. [Google Scholar]
- 52.Mallick S.S., Dighe V.V. Detection and estimation of alpha-amyrin, beta-sitosterol, lupeol and n-tricontane in two medicinal plants by high performance thin layer chromatography. Adv. Chem. 2014 doi: 10.1155/2014/143948. [DOI] [Google Scholar]
- 53.Amudha P., Jayalakshmi M., Pushpabharathi N., Vanitha V. Identification of bioactive components in Enhalusacoroidesseagrass extract by gas chromatography‒mass spectrometry. Asian J. Pharmaceut. Clin. Res. 2018;11(10):313–315. [Google Scholar]
- 54.Amaral A.C., Gomes L.A., Sila J.R., Ferreira J.L., Ade S.R., Mdo S.R., et al. Liposomal formulation of turmerone-rich hexane fractions from Curcuma longa enhances their antileishmanial activity. BioMed Res. Int. 2014 doi: 10.1155/2014/694934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isaiah S., Arun Kumar C., SenthamizhSelvan N. Phytochemical screening, antimicrobial activity and GC‒MS analysis of Corchorustridens L. L. International Journal of Pharmacological Research. 2016;6(12):353–357. [Google Scholar]
- 56.Sarmad M., Mahalakshmipriya A., Senthil K. Chemical composition and in vitro antimicrobial activity of Barlerialupulina essential oil. J. Herbs, Spices, Med. Plants. 2012;18(1):101–109. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the manuscript.