Abstract
Objective
This umbrella review aimed to summarize (and update) the effectiveness of non-pharmacological and non-surgical interventions for patients with knee osteoarthritis.
Methods
The study followed the PRISMA guidelines. Manual and electronic databases were searched, to identify systematic reviews, following the P (knee osteoarthritis) I (non-pharmacological and non-surgical treatments) C (pharmacological, surgical, placebo, no intervention, or other non-pharmacological/non-surgical conservative treatments) O (pain, function, quality of life, and other knee-specific measures) model. The quality of evidence was assessed using the R-AMSTAR checklist and GRADE principles.
Results
The search yielded 4086 records, of which 61 met the eligibility criteria. After evaluation with R-AMSTAR, four systematic reviews were excluded, resulting in 57 included systematic reviews, with an overall score of 29.6. The systematic reviews were published between 2018 and 2022 (29.8% in 2022), conducted in 19 countries (52.6% in China), and explored 24 distinct interventions. The systematic reviews encompassed 714 trials (mean of 13 ± 7.7 studies per systematic review), and 59,343 participants (mean 1041 ± 1002 per systematic review, and 82 ± 59.2 per study). The majority of participants were older obese women (61.6 ± 4.2 years, 30.2 ± 3.6 kg/m2, 70%, respectively).
Conclusions
Based on the systematic reviews findings, Diet Therapy, Patient Education, and Resistance Training are strongly supported as core interventions for managing patients with knee osteoarthritis. Aquatic Therapy, Balance Training, Balneology, Dietary Supplements, Extracorporeal Shockwave Therapy, and Tai Ji show moderate support. For other interventions, the evidence quality was low, results were mixed or inconclusive, or there was not sufficient efficacy to support their use.
Keywords: Osteoarthritis, Knee, Non-pharmacological, Non-surgical
1. Introduction
Osteoarthritis (OA) is a non-communicable, chronic, and progressive disease characterized by degenerative changes in the joint [1]. OA can impact various joints, with the knee being the most commonly affected location [2]. The development of knee osteoarthritis (KOA) is often related to many factors, including the patient's age, sex, knee joint trauma, obesity, inflammation, muscle mass, menopausal status, occupational labor intensity, exercise intensity, and genetics [[3], [4], [5]]. The incidence of KOA is increasing annually particularly due to the increased aging population and growing rate of obesity [6]. Given that a significant proportion of OA patients have co-existing medical conditions and co-morbidities, they require special attention due to their fragility [[7], [8], [9]].
Managing KOA is challenging and impose billions of dollars per year in costs to healthcare systems (could reach 0.25%–0.50% of a country's Gross Domestic Product) [[10], [11], [12]]. Current strategies to manage KOA patients include conservative (pharmacological and/or non-pharmacological) and surgical interventions [13]. Clinical guidelines recommend conservative non-pharmacological interventions as first line for managing KOA patients [[14], [15], [16], [17], [18], [19]]. Although the paramount importance of conservative non-pharmacological strategies, only 65 to 40% of patients with KOA receive proper treatment approach [20], indicating that the uptake of evidence-based guidelines in clinical practice and rehabilitation is still suboptimal [[21], [22], [23], [24], [25]]. Instead, surgical and pharmacological strategies remain dominant, despite the fact that use of many of these treatments has been associated with adverse side effects or unnecessary procedures and costs [18].
While there are numerous non-pharmacologic and non-surgical interventions for KOA, and integrated models of patient-centered multi-disciplinary care have been shown to improve outcomes, there is no cure or proven strategy for slow, prevent, stop, or reverse the progression from early to end-stage OA [1,23,26,27]. Understanding treatment strategies for KOA is essential for improving rehabilitation outcomes across all stages of management (health promotion; detect and treat early; and reduce the damage) [1,11,23,[28], [29], [30], [31], [32], [33]]. Therefore, continuously updating evidence is essential for optimizing patient care and addressing gaps in knowledge.
There is, to our knowledge, no available update of the last umbrella review [34] on the effectiveness of non-pharmacological and non-surgical interventions for KOA. Therefore, the aim of this study is to summarize and update the available high-quality evidence from systematic reviews on the effectiveness of non-pharmacological and non-surgical interventions for KOA patients.
2. Methods
This review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement [35] (checklist presented in Supplementary Material Table 1). The review protocol was registered prospectively at the PROSPERO (International Prospective Register of Systematic Reviews — www.crd.york.ac.uk/prospero) under identification number CRD42023485026.
2.1. Search strategy
The literature search aimed to identify systematic reviews that evaluated the effect of non-pharmacological and non-surgical interventions for KOA. In January 2024, systematic and comprehensive searches were conducted in electronic databases: PubMed, PEDro, Scopus, EBSCO, The Cochrane Library, Web of Science and Google Scholar. The search strategy was guided using the following patients, intervention, comparison, outcomes, studies (P.I.C.O.S.) model: KOA; non-pharmacological and non-surgical treatments; pharmacological, surgical, placebo, no intervention, or other non-pharmacological/non-surgical conservative treatments; pain, function, quality of life (QOL) and other knee-specific outcomes. For the search strategy, a conjunction of keywords, mesh terms and established search filters were used. The main keywords used to search in the databases were maintained from the previous umbrella review [34], namely: “knee”; “osteoarthritis”; “gonarthosis”; and “systematic review”. The terms (and their associates/derivatives) were then combined with the appropriate truncation and Boolean connectors. There was no language restriction. However, considering the last known umbrella review by Ferreira et al. [34], the search was restricted to systematic reviews of non-pharmacological and non-surgical treatments for KOA published in the electronic databases after January 2018. Additional publications that were not found during the original database search were identified through manual searches of the personal, related studies, website bibliographies and references lists. An online search strategy draft used is presented in Supplementary Material Fig. 1.
2.2. Study selection process
Two independent authors conducted the search in the electronic databases and screened the studies’ titles and abstracts to determine if they met the established eligible criteria. Considering the biomechanical and disease relationship, systematic reviews exploring both hip and KOA could be included, if the results from patients with KOA could be extracted separately. Potential studies were compiled in EndNote, and the duplicates removed using the automated software command “find duplicates”. Beyond this process, all the studies were manually reviewed to ensure that no duplicates remained. The authors then assessed the full-text versions and decided whether they actually met the eligible criteria. In cases where full versions were inaccessible or data were missing, authors of the respective studies were contacted via email. The study selection process was supervised, and the disagreements were solved through verbal discussion or arbitration by a third reviewer. The inclusion and exclusion criteria applied to this review are similar to the previous umbrella review [34] (Table 1).
Table 1.
Inclusion and Exclusion criteria.
| Inclusion | Exclusion |
|---|---|
| at least one of the keywords; | papers with experimental or control group composed by any kind of animal; |
| papers with an intervention group that has primary KOA either clinical or radiological criteria (or a combination); | papers with participants that do not have a KOA (healthy subjects) or who have secondary KOA (traumatic or post-surgical); |
| with or without meta-analysis, exclusively from randomized controlled trials, after January 2018; | with or without meta-analysis of randomized controlled trials prior to January 2018; |
| papers with non-pharmacological and non-surgical interventions; | papers with multi-modal interventions or exclusively surgical, pharmacological (injectable, topical, oral, or inhalation), or herbal interventions; |
| with their full versions, published in peer-reviewed scientific literature journals; | books, controlled trials, case reports, expert opinions, conference papers or academic thesis; |
| papers that evaluate pain, function, overall QOL, or other knee-related symptoms and measures; | papers with subjects with other illnesses namely cancer, heart diseases, kidney diseases, neurological diseases, respiratory diseases, rheumatoid arthritis, gouty arthritis, septic arthritis or Paget's disease; |
| detailed description of the non-pharmacological and non-surgical intervention; | papers with subjects exclusively with osteoarthritis in the hip, foot, shoulder, elbow, wrist and/or fingers. |
| performed under the PRISMA guidelines; | |
| studies that exhibit the highest specificity within each identified intervention MeSH term. |
2.3. Data extraction and syntheses
Data collection and extraction were performed by one author, with another author verifying the process to enhance consistency. The selected study-associated documents (i.e., full document, supplementary material, appendices, and journal publications) were collected for analysis. The extracted data from the selected publications to assess the effectiveness of non-pharmacological and non-surgical interventions included: title, authors' name, year of publication, KOA conditions, participants' sample size and their characteristics, objectives, description of the interventions, description of the control groups, studies' outcomes, assessment times, studies' results and studies’ conclusions. An Excel spreadsheet was created for a proper data analysis.
2.4. Outcomes
Studies were combined using the most adequate qualitative and quantitative evidence synthesis, and maintained most of the previous umbrella terms, such as pain, function, overall QOL, knee-specific outcomes measures (e.g., KOOS [Knee injury and Osteoarthritis Outcome Score], and WOMAC [Western Ontario and McMaster Universities Arthritis Index]), and other knee-related outcomes (e.g., inflammatory markers, and radiological findings).
2.5. Quality assessment
Two authors independently assessed the risk of bias, while a third author arbitrated when needed. The reviews were evaluated using the R-AMSTAR (Revised A MeaSurement Tool to Assess systematic Reviews) 11-item checklist [36]. In R-AMSTAR each domain's score ranges between 1 (minimum) and 4 (maximum), and the total score has a range of 11 (minimum) to 44 (maximum). Based on the overall score, quality grades are assigned as follows: A (high quality: 44-33 score); B (moderate quality: 32-23 score); C (low quality: 22-13 score); and D (very low quality: 12-11 score). Considering the recommendations that only total scores of 23/44 are considered to have at least moderate methodological quality, it was established as the cutting-point for include a systematic review in this study.
Additionally, GRADE (Grading of Recommendations Assessment, Development, and Evaluation) guidelines [[37], [38], [39], [40], [41]] were adapted, following the same principles as Jamtvedt et al. [42], to assess and integrate the strength of evidence for each intervention (Table 2).
Table 2.
Grading quality of evidence.
| Level | Criteria |
|---|---|
| High-quality evidence (A) (Highly recommended) |
One or more high-quality systematic review that are based on at least 2 high-quality primary studies with consistent results |
| Moderate-quality evidence (B) (Moderately recommended) |
One or more systematic reviews of high or moderate quality
|
| Low-quality evidence (C) (Uncertainty) |
One or more systematic reviews of high or moderate quality
|
| Very low-quality evidence (D) (No recommendation) |
No high-quality systematic review identified or supports the intervention |
3. Results
As umbrella reviews are designed to provide an overview of the topic appraised, the results of the search will be presented in the Results section. The conclusions and orientations of the individual papers will be summarized by treatment domain in the Discussion section, with further details provided in a tabular form (Table 4).
Table 4.
Systematic Reviews summaries (n = 57).
| Interventions | Authors (A to Z; year) | No of included RCTs (subjects; grade) | Results/Conclusions |
|---|---|---|---|
| Athletic tape | |||
| Li et al. [68] | 11 (n = 168; B) | Statistical significance was found in self-reported pain during activity (MD = −0.85; 95% CI: −1.55 to −0.14; p = 0.02), knee flexibility (MD = 7.59; 95% CI: 0.61 to 14.57; p = 0.03), knee-related health status (WOMAC scale, MD = −4.10; 95% CI: −7.75 to −0.45; p = 0.03), and proprioceptive sensibility (MD = −4.69; 95% CI: −7.75 to −1.63; p = 0.003). However, no significant enhancement was reported regarding knee muscle strength (MD = 1.25; 95% CI: −0.03 to 2.53; p = 0.06). | |
| Lin et al. [72] | 15 (n = 546; A) | The study suggests that physical therapy combined with kinesio taping is more effective than physical therapy alone, as indicated by a greater reduction in pain scores (MD = −0.70; 95% CI: −1.14 to −0.26; p = 0.002) and functional improvement (MD = −5.45; 95% CI: −10.23 to −0.66; p = 0.03). The results also show significant pain reduction (MD = −0.72; 95% CI: −1.18 to −0.26; p = 0.002) and functional improvement (MD = −6.05; 95% CI: −11.18 to −0.93; p = 0.02) within six weeks after initial treatments. | |
| Lu et al. [75] | 5 (n = 363; B) | Kinesio taping is effective in improving for pain (VAS at rest, WMD = −0.394; 95% CI: −0.759 to −0.029; p = 0.034; VAS during walking, WMD = −0.429; 95% CI: −0.752 to −0.105; p = 0.009), WOMAC index score (WMD = −5.026; 95% CI: −7.649 to −2.403; p < 0.001), and knee flexion ROM (WMD = 6.193; 95% CI: 2.678 to 9.709; p = 0.001). However, it does not improve muscle strenght (WMD = 3.205; 95% CI: −3.141 to 9.550; p = 0.322). | |
| Melese et al. [77] | 18 (n = 876; B) | Differences were found between Kinesio Taping groups and control groups in terms of VAS, WOMAC index scale and flexion ROM. | |
| Wu et al. [96] | 16 (n = 642; A) | There was a significant difference between the Kinesio taping plus exercise group and the exercise-only group in terms of VAS score after the intervention (MD = −0.86; 95% CI: −1.32 to −0.40; p = 0.0003). However, no significant differences were found in terms of VAS at the follow-up period (MD = −0.58; 95% CI: −1.41 to 0.25; p = 0.17), WOMAC score (MD = 0.28; 95% CI: −9.16 to 9.71; p = 0.95), and TUG after the intervention (MD = −0.74; 95% CI: −1.72 to 0.24; p = 0.14). | |
| Ye et al. [101] | 11 (n = 490; B) | The study found statistically significant differences in pain (SMD = −0.78; 95% CI: 1.07 to −0.50; p < 0.00001), physical function (SMD = 0.73; 95% CI: −1.03 to −0.43; p < 0.00001), ROM (MD = 2.04; 95% CI: 0.14 to 3.94; p = 0.04), and quadriceps muscle strength (MD = 2.42; 95% CI: 1.09 to 3.74; p = 0.0004). No significant differences were found for the hamstring muscle strength. | |
| Balneology | |||
| Antonelli et al. [45] | 17 (n = 1599; B) | When comparing balneological interventions with standard treatment, the results showed that the former were more effective in terms of long-term overall QOL (SMD = −1.03; 95% CI: −1.66 to −0.40; p < 0.00001). Additionally, when comparing balneological interventions with sham interventions, the results showed that the former were more effective in terms of long-term pain improvement (SMD = −0.38; 95% CI: −0.74 to −0.02; p = 0.04), while no significant difference was found when considering social function (SMD = −0.16; 95% CI: −0.52 to 0.19; p = 0.36). | |
| Hou et al. [62] | 11 (n = 1106; B) | The study found significant differences in VAS score (SMD = −0.74; 95% CI: −1.08 to −0.41; p < 0.0001) and WOMAC Index (pain, SMD = −0.53; 95% CI: −0.71 to −0.36; p < 0.00001; stiffness, SMD = −0.50; 95% CI: −0.68 to −0.31; p < 0.00001; function, SMD = −0.43; 95% CI: −0.57 to −0.29; p < 0.00001). | |
| Exercise therapies | |||
| Aquatic therapy | Dong et al. [55] | 8 (n = 579; B) | The study found no significant difference in pain relief, physical function, and improvement in QOL between aquatic exercise and land-based exercise for short- and long-term interventions in patients with KOA. However, patients reported higher adherence and satisfaction levels with aquatic exercise compared to land-based exercise. Compared to no intervention, aquatic exercise had a mild effect on elevating activities of daily living (SMD = −0.55; 95% CI: −0.94 to −0.16; p = 0.005) and a high effect on improving sports and recreational activities (SMD = −1.03; 95% CI: −1.82 to −0.25; p = 0.01). |
| Ma et al. [76] | 13 (n = 883; B) | Aquatic physical therapy has been found to significantly reduce pain based on the WOMAC index (SMD = −1.09; 95% CI: −1.97 to −0.21; p = 0.02) and VAS (SMD = −0.55; 95% CI: −0.98 to −0.12; p = 0.01). Additionally, it effectively improved physical function based on the WOMAC physical function score (SMD = −0.57; 95% CI: −1.14 to −0.01; p = 0.05). However, there were no significant improvements in joint symptoms, QOL, flexibility, or body composition for KOA. Aquatic physical therapy has been found to improve knee extension muscle strength (MD = 2.11; 95% CI: 0.02 to 4.20; p = 0.05) and TUG (MD = −0.89; 95% CI: −1.25 to −0.53; p < 0.05), thereby improving walking ability. | |
| Balance training | Pirayeh et al. [80] | 15 (n = 919; B) | The studies revealed that balance training can significantly improve physical function in KOA patients. However, the effect of balance training on muscle strength of the quadriceps and the hamstring remains unclear due to conflicting results. Additionally, the balance training group showed more significant improvement in postural stability and balance compared to the control group. |
| Wang et al. [94] | 24 (n = 1275; A) | In comparison with no intervention, proprioceptive training significantly improved pain, stiffness, physical function, joint position sense, muscle strength, mobility, and knee ROM (P < 0.05) in people with KOA. When compared to other non-proprioceptive training, proprioceptive training yielded superior results in terms of joint position sense (SMD = −1.28; 95% CI: −1.64 to −0.92; p < 0.00001) and mobility (timed walk over spongy surface) (SMD = −0.76; 95% CI: −1.33 to −0.18; p = 0.01), while other outcomes were comparable. When comparing proprioceptive training plus other non-proprioceptive training to other non-proprioceptive training, both groups showed similar outcomes. However, the proprioceptive training group showed greater improvement in joint position sense (SMD −1.54; 95% CI: −2.74 to −0.34; p = 0.01), physical function (SMD -0.34; 95% CI: −0.56 to −0.12; p = 0.003), and knee ROM (p < 0.05). When comparing proprioceptive training plus conventional physiotherapy to conventional physiotherapy alone, both groups demonstrated similar outcomes. However, the proprioceptive training plus conventional physiotherapy group showed a significant improvement in joint position sense (SMD −0.95; 95% CI: −1.73 to −0.18; p = 0.02). | |
| Blood flow restriction therapy | Grantham et al. [58] | 5 (n = 199; A) | There was no statistical difference (p: 0.329–0.880) found between blood flow restriction therapy and traditional resistance training in terms of pain reduction, functional improvement, and TUG improvement. |
| Pitsillides et al. [81] | 3 (n = 117; B) | The blood flow restriction and high intensity training groups demonstrated significant improvements in quadriceps strength in the strength outcome, with an increase from baseline to post-intervention. Additionally, the blood flow restriction and high intensity training groups showed significant strength gains in leg press and leg extension exercises, while the low intensity training group showed minimal improvements. In terms of the pain outcome, all groups experienced a reduction in pain. However, blood flow restriction training was found to be more effective in reducing compared to high-intensity training. While blood flow restriction resulted in decreased scores on physical function scales compared to baseline, there were no significant changes observed in the TUG test among the three groups. Regarding the QOL outcome, there were few studies to draw conclusions from. However, it appears that all three groups can improve WOMAC scores, with no statistically significant differences found in SF-36. | |
| Circuit-based exercise | Al-Mhanna et al. [44] | 7 (n = 346; B) | The intervention group showed a significant improvement in pain level (SMD = −0.96; 95% CI: −1.77 to −0.14; p = 0.02). However, no significant improvement was found in physical function (SMD = 0.03; 95% CI: −0.44 to 0.50; p = 0.89), QOL (SMD = −0.25; 95% CI: −1.18 to 0.68; p = 0.60), activity of daily living (SMD = 0.81; 95% CI: −0.85 to 2.48; p = 0.34), or knee stiffness (SMD = −0.65, 95% CI: −1.96 to 0.66; p = 0.33). |
| Resistance training | Kus and Yeldan [65] | 10 (n = 759; B) | When comparing different exercises to strengthen the quadriceps femoris muscle, no significant difference was found between the training groups. However, exercise training to strengthen the quadriceps femoris muscle was found to be superior to proprioceptive training. Additionally, the use of hot packs along with shortwave diathermy, ultrasound, or transcutaneous electrical nerve stimulation was found to be superior to isokinetic strengthening of the quadriceps femoris muscle alone. Only the additional use of Russian electrical stimulation showed a significant difference compared to the strengthening of the quadriceps femoris muscle exercise. Most of the studies included in this analysis showed that exercises aimed at strengthening the quadriceps femoris muscle have a positive effect on reducing pain and improving function. |
| Liao et al. [69] | 19 (n = 1195; A) | Muscle strength exercise training resulted in a significantly higher gain in lean mass (SMD = 0.49; 95% CI: 0.28 to 0.71; p < 0.00001), muscle thickness (SMD = 0.82; 95% CI: 0.20 to 1.43; p = 0.009), and cross-sectional area (SMD = 0.80; 95% CI: 0.25 to 1.35; p = 0.004) compared to non-exercise controls. No significant effects in favor of muscle strength exercise training were observed for any muscle outcome compared to exercise controls. | |
| Neelapala et al. [78] | 5 (n = 331; B) | Strong, high-quality evidence demonstrated the effectiveness of hip muscle strengthening was assessed in isolation, combination, and comparison with other lower extremity exercise. Overall, the studies reported clear benefits of hip muscle strengthening on knee pain, physical function, and hip muscle strength. However, hip muscle strengthening was ineffective in improving the biomechanical measures such as dynamic alignment and knee adduction moment. | |
| Thomas et al. [87] | 7 (n = 428; B) | Hip abductor strengthening interventions were found to be superior to the control groups. Specifically, hip abductor strengthening significantly reduced the VAS (SMD = −0.60; 95% CI: −0.88 to −0.33; p < 0.0001) and improved the WOMAC scores (SMD = −0.75; 95% CI: −1.05 to −0.45; p < 0.0001). All of the included studies concluded that strengthening the hip abductor muscle had a positive impact on knee pain and functional outcomes. | |
| Thorlund et al. [88] | 13 (n = 1398; A) | The study found that the treatment effect of NSAIDs for KOA pain was comparable to that of opioids (SMD = 0.02; 95% CI: −0.14 to 0.18). Exercise therapy had a larger effect than NSAIDs (SMD = 0.54; 95% CI: 0.19 to 0.89). No estimate could be made for exercise vs opioids due to the lack of studies. | |
| Turner et al. [91] | 12 (n = 1428; B) | Resistance training has been shown to improve pain, QOL, and physical function in individuals with KOA. The study found that 24 total sessions over an 8- to 12-week period had large effect sizes. No optimal number of repetitions, maximum strength, or frequency of sets or repetitions was found. | |
| Whole-body vibration | Qiu et al. [82] | 14 (n = 559; B) | Whole-body vibration combined with strengthening exercises has a significant positive effect on pain score (SMD = 0.46; 95% CI: 0.20 to 0.71; p = 0.0004), WOMAC Index (WOMAC-function, SMD = 0.51; 95% CI: 0.27 to 0.75; p < 0.0001), TUG (SMD = 0.82; 95% CI: 0.46 to 1.18; p < 0.00001), extensor isokinetic peak torque (SMD = 0.65; 95% CI: 0.00 to 1.29; p = 0.05), peak power (SMD = 0.68; 95% CI: 0.26 to 1.10; p = 0.001), and extensor isometric strength (SMD = 0.44; 95% CI: 0.13 to 0.75; p = 0.006). Both low-frequency (10–30 Hz) and high-frequency (30–40 Hz) whole-body vibration resulted in significant changes in pain, physical function, and knee extensor strength (p < 0.05). Whole-body vibration was not associated with significant changes in stiffness, balance, QOL, and knee flexor strength. |
| Mind-body therapies | |||
| Baduanjin | Zeng et al. [103] | 7 (n = 424; A) | Statistically significant differences were found between Baduanjin exercise and waiting list control on WOMAC index scores (pain, MD = −4.40; 95% CI: −7.16 to −1.64; p < 0.01; stiffness, MD = −1.34; 95% CI: −1.64, −1.04; p < 0.01; function, MD = −2.44; 95% CI: −4.33 to −0.55; p < 0.01). Furthermore, when used in isolation, the Baduanjin exercise demonstrated a statistically significant improvement on three domains of WOMAC index scores (pain, MD = −1.69; 95 % CI: −2.03 to −1.35; p < 0.01; stiffness, MD = −0.86; 95 % CI: −1.13 to −0.58; p < 0.01; function, MD = −2.23; 95 % CI: −3.65 to −0.82; p < 0.01) compared to health education. In addition, the combination of Baduanjin exercise and NSAID therapies led to a significant improvement in the total WOMAC score (MD = −10.26; 95% CI: −13.41 to −7.11; p < 0.01) and a reduction in VAS (MD = −1.65; 95% CI: −1.83 to −1.48; p < 0.01) compared to NSAID therapies alone. |
| Tai Ji | Hu et al. [63] | 16 (n = 986; B) | Tai Ji significantly improved patients' outcomes, including pain (SMD = −0.69; 95% CI: −0.95 to −0.44; p < 0.001), stiffness (SMD = −0.59; 95% CI: −0.91 to −0.27, p < 0.001), physical function (SMD = −0.92; 95% CI: −1.16 to −0.69, p < 0.001), dynamic balance (SMD = 0.69; 95% CI: 0.38 to 0.99; P < 0.001), and physiological and psychological health (SF-36 physical, SMD = 0.48; 95% CI: 0.28 to 0.68; p < 0.001; SF-36 mental, SMD = 0.26; 95% CI: 0.06 to 0.45; p = 0.01). |
| You et al. [102] | 11 (n = 603; B) | The results showed that the Tai Ji group was associated with better performance in 6-MWT (MD = 46.67; 95% CI: 36.91 to 56.43; p < 0.001), TUG (MD = −0.89; 95% CI: −1.16 to −0.61; p < 0.001]), and WOMAC Index function score (MD = −11.28; 95% CI: −13.33 to −9.24; p < 0.001) than the control group. | |
| Wu Qin Xi | Guo et al. [59] | 7 (n = 668; B) | Wu Qin Xi exercise showed a significant improvement in WOMAC total score regardless of the intervention of control group (MD = −105.76; 95% CI: −161.38 to −50.14; p < 0.01). Furthermore, Wu Qin Xi exercise significantly improved the pain symptoms (MD = −17.00; 95% CI: −21.41 to −12.58; p < 0.00001), joint stiffness (MD = −3.43; 95% CI: −5.50 to −1.37; p = 0.001), and joint function (MD = −33.45; 95% CI: −48.74 to −18.17; p < 0.0001). Wu Qin Xi can also decrease pain, as VAS scores revealed an improvement (MD = −1.07; 95% CI: −1.97 to −0.17; p = 0.02). |
| Yoga | Lauche et al. [66] | 9 (n = 640; B) | The studies revealed effects of yoga on pain (vs. exercise, SMD = −1.07; 95% CI: −1.92 to −0.21; p = 0.01; vs. non-exercise, SMD = −0.75; 95% CI: −1.18 to −0.31; p < 0.001), physical function (vs. exercise, SMD = 0.80; 95% CI: 0.36 to 1.24; p < 0.001; vs. non-exercise, SMD = 0.60; 95% CI: 0.30 to 0.98; p < 0.001), and stiffness (vs. exercise, SMD = −0.92; 95% CI: −1.69 to −0.14; p = 0.008; vs. non-exercise, SMD = −0.76; 95% CI −1.26 to −0.26; p = 0.003) in KOA individuals. |
| Musculoskeletal manual manipulations | |||
| Anwer et al. [46] | 11 (n = 494; B) | The results indicated a statistically insignificant reduction of VAS score with orthopaedic manual therapy compared with the control group was (SMD = −0.59; 95% CI: −1.54 to −0.36; p = 0.224). However, there was a statistically significant reduction in VAS score with orthopaedic manual therapy compared to exercise therapy (SMD = −0.78; 95% CI: −1.42 to −0.17; p = 0.013). There was a statistically significant reduction in WOMAC pain (SMD = −0.79; 95% CI: −1.14 to −0.43; p = 0.001) and function (SMD = −0.85; 95% CI: −1.20 to −0.50; p = 0.001) with orthopaedic manual therapy compared to the exercise therapy group. However, the reduction in WOMAC global score with orthopaedic manual therapy compared to the exercise therapy group was statistically insignificant (SMD = −0.23; 95% CI: −0.54 to −0.09; p = 0.164). A statistically significant reduction was found in the time taken to ascend and descend stairs in the orthopaedic manual therapy group compared to the exercise therapy group (SMD = −0.88; 95% CI: −1.48 to −0.29; p = 0.004). | |
| Runge et al. [83] | 19 (n = 1394; B) | There was very low- to moderate-certainty evidence that manual therapy when added to exercise, provided benefit in the short-term for pain (SMD = −0.82; 95% CI: −1.22 to −0.43) and WOMAC global score (SMD = −1.05; 95% CI: −1.52 to −0.59), but not for TUG (MD = −0.12; 95% CI: −0.27 to 0.03) and WOMAC function (SMD = −0.27; 95% CI: −0.85 to 0.30). In the medium-term, there was low- to very-low-certainty evidence that MT added benefit for TUG (MD = −2.20; 95% CI: −2.89 to −1.51) and WOMAC global score (MD = −7.40; 95% CI: −10.31 to −4.49), but not for pain (MD = −0.97; 95% CI: −2.02 to 0.09) and WOMAC physical function (MD = 0.23; 95% CI: −6.36 to 6.82). There was high-certainty evidence that manual therapy did not provided any additional benefit in the long-term for pain (MD = −0.14; 95% CI: −0.48 to 0.21), TUG (MD = 0.39; 95% CI: −0.30 to 1.08) and WOMAC global score (MD = 0.56; 95% CI: −8.45 to 9.57; p = 0.90). | |
| Weleslassie et al. [95] | 15 (n = 704; B) | The results suggests that there were significant differences between mobilization with movement groups and control groups in terms of VAS, WOMAC Index scale, and flexion ROM. | |
| Needle-based therapies | |||
| Acupuncture therapy | Gong et al. [57] | 17 (n = 4774; B) | Acutherapy had a significant effect on knee pain (SMD = −0.73; 95% CI: −0.98 to −0.47; p < 0.001), knee stiffness (SMD = −0.66; 95% CI: −0.85 to −0.47; p < 0.001), and physical function (SMD = −1.56; 95% CI: −2.17 to −0.95; p < 0.001) compared to a control condition without any acutherapy intervention. Additionally, acutherapy was found to be more effective than a corresponding sham intervention applied on nonacupoints (SMD = −0.16; 95% CI: −0.32 to −0.01; p = 0.04). However, there were no significant differences found in treatment effects between acutherapy and sham acutherapy at the same acupoints (SMD = −0.09; 95% CI: −0.40 to 0.21; p = 0.55). |
| Sun et al. [86] | 8 (n = 2106; B) | Compared with low- and medium-dosage acupuncture treatments, strong evidence showed that there was a positive correlation between high-dosage acupuncture treatment and positive outcomes. | |
| Dry needling | Jiménez-del-Barrio et al. [64] | 7 (n = 291; A) | In the short-term, dry needling demonstrated significant improvements in pain intensity (SMD = −0.76; 95% CI: −1.24 to −0.29; p = 0.002) and physical function (SMD = −0.98; 95% CI: −1.54 to −0.42; p = 0.0006). However, no significant differences were observed in the medium- or long-term. |
| Ughreja and Prem [92] | 9 (n = 779; A) | Subgroup analysis of moderate-quality evidence shows that periosteal stimulation technique has short-term effects on pain (post-treatment, MD = −1.13; 95% CI: −1.31 to −0.95; p < 0.00001; 3-month follow-up, MD = −1.46; 95% CI: −2.43 to −0.50; p = 0.003) and WOMAC function (post-treatment, MD = −5.47; 95% CI: −7.56 to −3.37; p < 0.00001; 3-month follow-up, MD = −4.95; 95% CI: −9.69 to −0.21; p = 0.04). Intramuscular electrical stimulation has a significant effect on pain (MD = −2.30; 95% CI: −4.4 to 0.20; p = 0.03) in KOA. The myofascial trigger point needling technique showed significant within-group differences in pain and knee function, but no significant differences were found between the dry needling and sham dry needling groups. A meta-analysis was not performed for this technique due to the lack of studies that could be compared. | |
| Nutrition therapies | |||
| Diet therapy | Chu et al. [52] | 7 (n = 1105; B) | The study results indicate that weight loss had a significant positive effect on pain (SMD = 0.33; 95% CI: 0.17 to 0.48; p < 0.0001), self-reported disability (SMD = 0.42; 95% CI: 0.25 to 0.59; p < 0.00001), QOL (physical) (SMD = 0.39; 95% CI: 0.24 to 0.54; p < 0.00001), WOMAC index (SMD = 0.37; 95% CI: 0.11 to 0.62; p = 0.004), and 6-MWT (SMD = 0.23; 95% CI: 0.06 to 0.40; p = 0.009). However, no significant improvements were observed in the timed stair climb test (p = 0.20). |
| Hall et al. [60] | 16 (n = 2142; A) | The study found that diet-only treatments did not result in a reduction of pain (SMD = −0.13; 95% CI: −0.37 to 0.10; p = 0.10). However, a combination of diet and exercise treatments did moderately reduce pain (SMD = −0.37; 95% CI: −0.69 to −0.04; p = 0.112). Physical function showed moderate improvement with both diet treatments (SMD = −0.30; 95% CI: −0.52 to −0.08; p = 0.08) and combined diet and exercise treatments (SMD = −0.32; 95% CI: −0.56 to −0.08; p = 0.265). Of all the inflammatory markers that were assessed, only interleukin-6 showed a reduction with diet-only treatments (SMD = −0.23; 95% CI: −0.45 to −0.02; p = 0.38). | |
| Dietary supplements | Liao et al. [71] | 6 (n = 242; B) | The group that received protein supplementation combined with exercise training showed significant improvements in muscle mass (SMD = 1.13; 95% CI: 0.72 to 1.53; p < 0.00001), pain (SMD = 1.36; 95% CI: 0.68 to 2.03; p < 0.00001), and muscle strength (involved leg, SMD = 0.44; 95% CI: 0.03 to 0.85; p = 0.04; uninvolved leg, SMD = 0.54; 95% CI: 0.13 to 0.95; p = 0.01). |
| Patient education | |||
| Goff et al. [56] | 29 (n = 4107; A) | Patient education was found to be more effective than usual care in improving pain (SMD = 20.35; 95% CI: 20.56 to 20.14) and function in the short-term (SMD = 20.31; 95% CI: 20.62 to 0.00). However, it was less effective than exercise therapy in reducing pain in the short-term (SMD = 0.77; 95% CI: 0.07 to 1.47). Combining patient education with exercise therapy resulted in better outcomes for pain in the short-term (SMD = 0.44; 95% CI: 0.19 to 0.69) and function in the short- (SMD = 0.81; 95% CI: 0.54 to 1.08) and medium-term (SMD = 0.39; 95% CI: 0.15 to 0.62). When comparing results using the WOMAC Index, it was found that exercise therapy was more effective than patient education for short-term pain relief (MD = 1.56; 95% CI: 0.14 to 2.98). Additionally, a combination of patient education and exercise therapy was found to be more effective than patient education alone for short-term improvement in function (MD = 8.94; 95% CI: 6.05 to 11.82). | |
| Safari et al. [84] | 8 (n = 2687; B) | Studies reported that digital-based structured self-management programs compared with the treatment as usual control group resulted in a significant medium reduction in pain (SMD = −0.28; 95% CI: −0.38 to −0.18) and improvement in physical function (SMD = −0.26; 95% CI: −0.35 to −0.16) at post-treatment. Although the effect of digital-based structured self-management programs on pain and function reduced slightly at the 12-month follow-up, it remained medium and significant. The effect of digital-based structured self-management programs after treatment was small and significant for disability (SMD = −0.10; 95% CI: −0.17 to 0.03), but not significant for QOL (SMD = −0.17; 95% CI: −0.47 to 0.14). The intervention's effect at the 12-month follow-up was very small for both disability and QOL. | |
| Uritani et al. [93] | 7 (n = 1123; B) | Group-based and face-to-face self-management education programmes have been found to have beneficial effects on self-efficacy for managing pain and other symptoms, as well as for self-regulating KOA. However, due to the wide range of clinical heterogeneity, most of the information in the systematic review was inconclusive. | |
| Wu et al. [98] | 13 (n = 1610; B) | Meta-analysis revealed significant differences between the self-management and control groups in pain (SMD = −1.51; 95% CI: 2.41 to 0.62; p = 0.001), function (SMD = −0.24; 95% CI: −0.45 to 0.04; p = 0.02), arthritis self-efficacy (pain, MD = 2.82; 95% CI: 0.35 to 5.29; p = 0.03; other symptoms, SMD = 3.99; 95% CI: 1.55 to 6.43; p = 0.001), and mental health (MD = 3.82; 95% CI: 3.31 to 4.32; p < 0.00001). However, no statistically significant differences were found in the WOMAC index. | |
| Xie et al. [99] | 6 (n = 791; B) | The study found that internet-based rehabilitation programs can significantly reduce osteoarthritic pain in patients compared to conventional rehabilitation (SMD = −0.21; 95% CI: −0.4 to −0.01; p = 0.04). However, there was no significant difference in physical function improvement between patients with KOA who underwent internet-based rehabilitation and those who underwent conventional rehabilitation within 2–12 months (SMD = −0.08; 95% CI; −0.27 to 0.12; p = 0.43). | |
| Physical agents | |||
| Cryotherapy | Dantas et al. [53] | 5 (n = 202; B) | Low-quality evidence showed improvements in pain control and functional outcomes. |
| Electric stimulation therapy | Chen et al. [49] | 10 (n = 493; B) | The groups that received interferential current therapy showed significant improvements in short-term pain scores (SMD = −0.64; 95% CI: −1.04 to −0.25; p = 0.001), long-term pain scores (SMD = −0.36; 95% CI: −0.60 to −0.11; p = 0.005), and short-term WOMAC index scores (SMD = −0.39; 95% CI: −0.77 to −0.02; p = 0.04) compared to the control groups. |
| Extracorporeal shockwave therapy | Avendano-Coy et al. [47] | 14 (n = 782; A) | Extracorporeal shockwave therapy caused a decrease on the pain VAS (MD = 1.7; 95% CI: 1.1 to 2.3) and WOMAC (MD = 13.9; 95%CI: 6.9 to 20.8). The effect of extracorporeal shockwave therapy using medium energetic density was greater than with low or high density in the WOMAC (X2 = 9.8; p = 0.002) and bordered statistical significance on the VAS (X2 = 3.8; p = 0.05). Extracorporeal shockwave therapy causes moderate improvement in the knee ROM (MD = 17.5; 95% CI: 9.4 to 25.5) and walking test (SMD = 0.58; 95% CI: 0.35 to 0.81). |
| Chen et al. [51] | 32 (n = 2408; B) | Extracorporeal shockwave therapy demonstrated significant improvement in pain reduction and functional improvement compared to placebo, corticosteroid, hyaluronic acid, medication, and ultrasound (p < 0.05). In terms of functional improvement, shockwave therapy showed statistically significant improvement compared to kinesiotherapy and moxibustion (p < 0.05), but not with acupotomy surgery (p = 0.24). A statistically significant difference was observed in pain reduction (p < 0.05) between shockwave therapy and platelet-rich plasma, but not in functional improvement (p = 0.89). Similarly, a statistical difference was found in functional improvement (p < 0.05) between extracorporeal shockwave therapy and fumigation, but not in pain reduction (p = 0.26). Furthermore, there was no statistically significant difference between extracorporeal shockwave therapy and manipulation in both pain reduction (p = 0.21) and functional improvement (p = 0.45). | |
| Li et al. [67] | 7 (n = 366; B) | The extracorporeal shockwave therapy group exhibited a lower VAS score (MD = −2.35; 95% CI: −2.92 to −1.79; p < 0.00001), larger ROM (MD = 17.58; 95% CI: 12.88 to 22.28; p < 0.00001) and a better Lequesne index (MD = −3.06; 95% CI: −3.90 to −2.21; p < 0.00001) than the placebo group after 1 month of therapy. At 1 month post-therapy, the group that received extracorporeal shockwave therapy had a lower VAS score (MD = −1.98; 95% CI: −2.93 to −1.03; p < 0.00001), a larger ROM (MD = 11.69; 95% CI: 6.40 to 16.98; p < 0.00001), and better WOMAC scores (MD = −15.38; 95% CI: −18.87 to −11.89; p < 0.00001) compared to the group that received physical therapy. | |
| Liao et al. [70] | 50 (n = 4844; B) | Results indicate a significant improvement in the success rate of shockwave therapy (OR = 3.22; 95% CI: 2.21 to 4.69; p < 0.00001), pain reduction (SMD = −2.02; 95% CI: −2.38 to −1.67; p < 0.00001), and WOMAC Index function outcome (SMD = −2.71; 95% CI: −3.50 to −1.92; p < 0.00001). | |
| Laser therapy | Ahmad et al. [43] | 10 (n = 495; B) | Statistically significant improvements were observed in the VAS (SMD = −0.67; 95% CI: −1.05 to −0.29; p < 0.05) and WOMAC function (SMD = −0.70; 95% CI: −1.36 to −0.04; p < 0.05) scores of patients treated with low-level laser therapy plus exercise compared to the control group. However, no significant difference was found between the two groups in the WOMAC pain and stiffness scores. High-level laser therapy was found to be superior to the control group in terms of VAS (SMD = −2.06; 95% CI: −3.14 to −0.98; p < 0.05), WOMAC pain (SMD = −2.03; 95% CI: −3.81 to −0.26; p = 0.02), stiffness (SMD = −0.84; 95% CI: −1.43 to −0.24, p < 0.05), and function (SMD = −3.11; 95% CI: −5.59 to −0.62; p < 0.05) when compared to the control group. |
| Stausholm et al. [85] | 22 (n = 1063; B) | Overall, low-level laser therapy significantly reduced VAS compared to placebo at the end of therapy (MD = 14.23; 95% CI: 7.31 to 21.14; p < 0.0001) and during follow-ups 1–12 weeks later (MD = 15.92; 95% CI: 6.47 to 25.37; p = 0.001). The results of the subgroup analysis indicate that the recommended low-level laser therapy doses significantly reduced pain compared to placebo at the end of therapy (MD = 18.71; 95% CI: 9.42 to 27.99; p < 0.0001) and during follow-ups 1–12 weeks after the end of therapy (MD = 23.23; 95% CI: 10.60 to 35.86; p = 0.0003). The greatest reduction in pain from the recommended low-level laser therapy doses was observed during follow-ups 2–4 weeks after the end of therapy (MD = 31.87; 95% CI: 18.18 to 45.56; p ≤ 0.01). Low-level laser therapy significantly reduced disability compared to placebo at the end of therapy (MD = 0.59; 95% CI: 0.33 to 0.86; p < 0.00001) and during follow-ups 1–12 weeks later (MD = 0.66; 95% CI: 0.23 to 1.09; p = 0.003). The subgroup analysis showed that the recommended low-level laser therapy doses significantly increased disability compared to placebo at the end of therapy (MD = 0.75; 95% CI: 0.46 to 1.03; p < 0.00001) and during follow-ups 1–12 weeks after the end of therapy (MD = 1.31; 95% CI: 0.92 to 1.69; p < 0.00001). | |
| Wu et al. [97] | 10 (n = 580; B) | High-intensity laser demonstrated the highest probability of being among the most effective treatments, compared to a control (placebo laser, exercise, or a combination of both) in the VAS (WMD = 1.66; 95% CI: 1.48 to 1.84; p < 0.00001) and WOMAC (WMD = 10.87; 95% CI: 8.85 to 12.88; p < 0.00001). Comparing low- to high-intensity laser, differences were found in WOMAC (WMD = 6.48; 95% CI: 4.07 to 8.89; p < 0.00001) and pain (WMD = 0.81; 95% CI: 0.44 to 1.18; p < 0.00001), favoring high-intensity laser. | |
| Magnetic field therapy | Chen et al. [50] | 8 (n = 421; B) | Pulsed electromagnetic field therapy improved physical function (WMD = −5.28; 95% CI: −9.45 to −1.11, p = 0.01), but did not show advantage in reducing WOMAC total score (WMD = −7.80; 95% CI: −16.08 to 0.47; p = 0.06), WOMAC pain score (WMD = −1.06; 95% CI −2.30 to 0.17, p = 0.09), VAS pain score (WMD = −0.88; 95% CI: −2.06 to 0.31, p = 0.15), or WOMAC stiffness score (WMD = −0.50; 95% CI: −1.09 to 0.09; p = 0.1). |
| Tong et al. [89] | 11 (n = 614; B) | Compared to the control groups, pulsed electromagnetic field therapy yielded more favorable results. It alleviated pain (SMD = 0.71; 95% CI: 0.08 to 1.34; p = 0.03), improved stiffness (SMD = 1.34; 95% CI: 0.45 to 2.23; p = 0.003), and restored physical function (SMD = 1.52; 95% CI: 0.49 to 2.55; p = 0.004). | |
| Yang et al. [100] | 16 (n = 1078; B) | Compared to the placebo group, electromagnetic field therapy was found to have a beneficial effect on pain (SMD = 1.06; 95% CI = 0.61 to 1.51; p < 0.00001), stiffness (SMD = 0.37; 95% CI: 0.07 to 0.67; p = 0.02), and function (SMD = 0.46; 95% CI: 0.14 to 0.78; p = 0.005). However, no statistically significant differences were found for QOL (SMD = 1.49; 95% CI: −0.06 to 3.04; p = 0.06). Electromagnetic field therapy parameters, such as duration of treatment, may not be a critical factor to influence symptoms. | |
| Ultrasonic therapy | Chen et al. [48] | 13 (n = 807; B) | Low-intensity pulsed ultrasound significantly improved the VAS (MD = −0.95, 95% CI: −1.43 to −0.48; p < 0.001), WOMAC index score (MD = −4.35; 95% CI: −8.30 to −0.40; p = 0.0309), Lysholm score (SMD = 1.59; 95% CI: 1.29 to 1.90; p < 0.001), Lequesne index (MD = −1.33; 95% CI: −1.69 to −0.96; p < 0.001), ROM (MD = 2.43; 95% CI: 0.39 to 4.46; p = 0.0197) and 50-m walking time (SMD = 1.48; 95% CI: 0.46 to 2.49; p = 0.0044). Subgroup analyses revealed that low-intensity pulsed ultrasound monotherapy was more effective in reducing VAS (p = 0.0213), while a shorter therapeutic period (≤4 weeks) was more effective in increasing the WOMAC score (p = 0.0083). |
| Dantas et al. [54] | 5 (n = 234; B) | Therapeutic ultrasonic therapy resulted in statistically significant pain relief (SMD = −0.33; 95% CI: −0.60 to −0.07; p = 0.01) and improved self-reported function (SMD = −0.33; 95% CI: −0.65 to −0.01; p = 0.05) compared to sham treatment. | |
| Liu et al. [73] | 14 (n = 1080; B) | Both pulsed (SMD = 1.11; 95% CI: 0.86 to 1.36; p < 0.00001) and continuous ultrasound (SMD = 1.18; 95% CI: 0.78 to 1.57; p < 0.00001) therapy were found to have significant pain relief effects. High-intensity ultrasound (>1.5 W/cm2) appeared to be more effective than other intensities (SMD = 1.34; 95% CI: 0.94 to 1.73; p < 0.00001). Additionally, therapeutic ultrasound was effective in improving joint function as measured by WOMAC (SMD = 8.18; 95% CI: 5.88 to 10.48; p < 0.00001). |
Abbreviations: 6-MWT, 6-min Walk Test; KOA, knee osteoarthritis; MD, mean difference; NSAID, non-steroidal anti-inflammatory drug; OR, odds ratio; QOL, quality of life; ROM, range of motion; SF-36, Short Form Health Survey 36-item; SMD, standardized mean difference; TUG, Timed Up and Go test; VAS, Visual Analogic Scale; WMD, weighted mean difference; WOMAC, Western Ontario and McMaster Universities Arthritis.
3.1. Selection of the studies
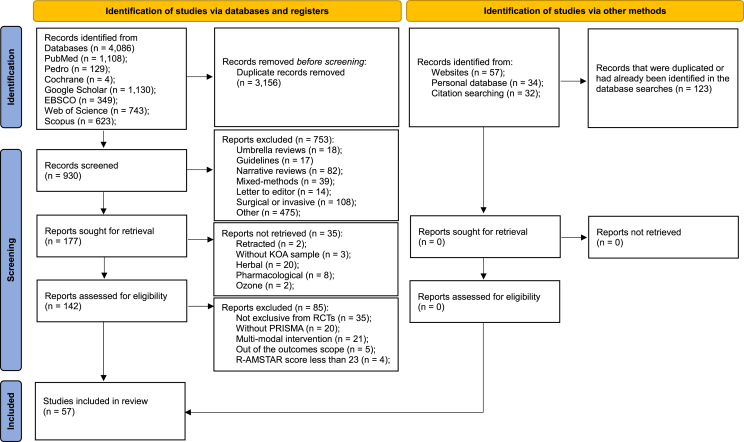
The searches yielded 4086 records, out of which 930 were screened. After the application of the inclusion and exclusion criteria, 57 could be included. The flowdiagram (Fig. 1) and the Supplementary Material Table 2 summarizes the selection process.
Fig. 1.
PRISMA flow diagram highlighting the selection process for the studies included in the systematic review.
3.2. Methodological quality
The methodological quality assessment revealed a mean score of 29.1 (range 21–39) [[43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103]]. Among the assessed studies, the two problematical items were the list of studies, and the of publication bias assessment. The domains characteristics of the included studies, and conflict of interest were less problematic items. Four systematic reviews [61,74,79,90] were excluded because they did not reach 23/44, raising the mean score to 29.6. The classifications obtained are described in Table 3.
Table 3.
Methodological quality of eligible studies (n = 61).
| Study (Author; Year) | R-AMSTAR Items |
R-AMSTAR |
Grade |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score (11–44) | (A–D) | |
| Ahmad et al. [43] | 3 | 4 | 4 | 2 | 4 | 4 | 2 | 3 | 2 | 1 | 3 | 32 | B |
| Al-Mhanna et al. [44] | 3 | 4 | 1 | 2 | 1 | 3 | 4 | 4 | 1 | 1 | 4 | 28 | B |
| Antonelli et al. [45] | 3 | 4 | 4 | 2 | 1 | 3 | 4 | 1 | 2 | 1 | 3 | 28 | B |
| Anwer et al. [46] | 3 | 4 | 4 | 2 | 1 | 4 | 2 | 3 | 2 | 1 | 3 | 29 | B |
| Avendano-Coy et al. [47] | 3 | 4 | 4 | 2 | 2 | 4 | 4 | 3 | 4 | 2 | 4 | 36 | A |
| Chen et al. [48] | 4 | 2 | 4 | 3 | 2 | 4 | 3 | 2 | 4 | 1 | 3 | 32 | B |
| Chen et al. [49] | 4 | 4 | 4 | 3 | 1 | 4 | 3 | 2 | 1 | 1 | 4 | 31 | B |
| Chen et al. [50] | 3 | 4 | 4 | 2 | 1 | 4 | 3 | 2 | 4 | 1 | 4 | 32 | B |
| Chen et al. [51] | 3 | 4 | 3 | 3 | 1 | 3 | 3 | 2 | 3 | 3 | 4 | 32 | B |
| Chu et al. [52] | 3 | 1 | 4 | 2 | 1 | 3 | 3 | 3 | 1 | 1 | 2 | 24 | B |
| Dantas et al. [53] | 3 | 4 | 3 | 3 | 1 | 4 | 4 | 1 | 1 | 1 | 4 | 29 | B |
| Dantas et al. [54] | 3 | 4 | 4 | 2 | 1 | 3 | 4 | 4 | 2 | 1 | 4 | 32 | B |
| Dong et al. [55] | 3 | 4 | 3 | 2 | 1 | 3 | 3 | 1 | 2 | 1 | 4 | 27 | B |
| Goff et al. [56] | 3 | 4 | 4 | 3 | 3 | 4 | 3 | 3 | 4 | 2 | 4 | 37 | A |
| Gong et al. [57] | 3 | 2 | 3 | 2 | 1 | 3 | 3 | 1 | 2 | 2 | 4 | 26 | B |
| Grantham et al. [58] | 4 | 1 | 4 | 2 | 2 | 4 | 4 | 4 | 3 | 1 | 4 | 33 | A |
| Guo et al. [59] | 3 | 2 | 2 | 2 | 1 | 4 | 3 | 2 | 3 | 1 | 4 | 27 | B |
| Hall et al. [60] | 3 | 4 | 4 | 3 | 2 | 4 | 4 | 3 | 3 | 2 | 2 | 34 | A |
| Heddon et al. [61] | 3 | 2 | 3 | 2 | 1 | 1 | 3 | 1 | 1 | 1 | 4 | 22 | C |
| Hou et al. [62] | 2 | 4 | 3 | 2 | 1 | 2 | 3 | 1 | 2 | 2 | 4 | 26 | B |
| Hu et al. [63] | 3 | 4 | 3 | 2 | 1 | 3 | 4 | 2 | 2 | 1 | 4 | 29 | B |
| Jiménez-del-Barrio et al. [64] | 4 | 4 | 4 | 2 | 1 | 4 | 4 | 3 | 1 | 2 | 4 | 33 | A |
| Kus and Yeldan [65] | 3 | 2 | 3 | 2 | 1 | 4 | 3 | 2 | 1 | 1 | 1 | 23 | B |
| Lauche et al. [66] | 3 | 4 | 4 | 3 | 3 | 3 | 4 | 4 | 1 | 1 | 2 | 32 | B |
| Li et al. [67] | 3 | 2 | 3 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 4 | 23 | B |
| Li et al. [68] | 3 | 4 | 4 | 3 | 2 | 4 | 4 | 1 | 1 | 2 | 2 | 30 | B |
| Liao et al. [69] | 3 | 4 | 4 | 3 | 2 | 4 | 3 | 3 | 3 | 3 | 1 | 33 | A |
| Liao et al. [70] | 3 | 4 | 4 | 3 | 1 | 3 | 3 | 1 | 4 | 2 | 4 | 32 | B |
| Liao et al. [71] | 3 | 4 | 2 | 2 | 1 | 4 | 2 | 2 | 2 | 2 | 4 | 28 | B |
| Lin et al. [72] | 3 | 4 | 4 | 3 | 4 | 3 | 3 | 2 | 2 | 2 | 4 | 34 | A |
| Liu et al. [73] | 2 | 4 | 3 | 2 | 1 | 4 | 3 | 1 | 1 | 1 | 2 | 24 | B |
| Long et al. [74] | 3 | 2 | 1 | 3 | 1 | 3 | 3 | 1 | 2 | 2 | 1 | 22 | C |
| Lu et al. [75] | 3 | 4 | 4 | 2 | 1 | 4 | 3 | 2 | 3 | 1 | 4 | 31 | B |
| Ma et al. [76] | 3 | 4 | 4 | 2 | 1 | 3 | 3 | 2 | 2 | 1 | 4 | 29 | B |
| Melese et al. [77] | 3 | 4 | 3 | 2 | 1 | 3 | 4 | 1 | 1 | 1 | 3 | 26 | B |
| Neelapala et al. [78] | 3 | 3 | 4 | 2 | 2 | 4 | 3 | 3 | 1 | 1 | 1 | 27 | B |
| Novak et al. [79] | 3 | 2 | 2 | 2 | 1 | 3 | 3 | 1 | 1 | 1 | 2 | 21 | C |
| Pirayeh et al. [80] | 3 | 4 | 2 | 2 | 1 | 4 | 4 | 1 | 1 | 1 | 2 | 25 | B |
| Pitsillides et al. [81] | 2 | 1 | 4 | 2 | 4 | 4 | 2 | 3 | 1 | 1 | 3 | 27 | B |
| Qiu et al. [82] | 3 | 4 | 2 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 4 | 26 | B |
| Runge et al. [83] | 3 | 2 | 4 | 3 | 1 | 3 | 4 | 4 | 3 | 1 | 2 | 30 | B |
| Safari et al. [84] | 4 | 4 | 4 | 2 | 4 | 2 | 4 | 2 | 1 | 1 | 4 | 32 | B |
| Stausholm et al. [85] | 2 | 4 | 3 | 3 | 3 | 4 | 3 | 2 | 2 | 1 | 2 | 29 | B |
| Sun et al. [86] | 2 | 4 | 2 | 2 | 1 | 3 | 2 | 2 | 1 | 1 | 4 | 24 | B |
| Thomas et al. [87] | 4 | 4 | 3 | 2 | 1 | 4 | 3 | 2 | 3 | 1 | 4 | 31 | B |
| Thorlund et al. [88] | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 2 | 4 | 3 | 4 | 39 | A |
| Tong et al. [89] | 3 | 2 | 3 | 2 | 1 | 1 | 3 | 1 | 2 | 2 | 4 | 24 | B |
| Tsokanos et al. [90] | 2 | 1 | 2 | 3 | 1 | 3 | 3 | 1 | 1 | 1 | 4 | 22 | C |
| Turner et al. [91] | 3 | 4 | 4 | 3 | 1 | 4 | 3 | 2 | 1 | 1 | 3 | 29 | B |
| Ughreja and Prem [92] | 2 | 4 | 4 | 2 | 4 | 4 | 3 | 3 | 2 | 1 | 4 | 33 | A |
| Uritani et al. [93] | 4 | 4 | 4 | 3 | 2 | 4 | 3 | 2 | 1 | 1 | 4 | 32 | B |
| Wang et al. [94] | 3 | 4 | 4 | 2 | 1 | 4 | 4 | 4 | 3 | 3 | 3 | 35 | A |
| Weleslassie et al. [95] | 4 | 2 | 2 | 2 | 1 | 4 | 3 | 2 | 1 | 1 | 2 | 24 | B |
| Wu et al. [96] | 4 | 4 | 4 | 3 | 4 | 3 | 3 | 2 | 3 | 2 | 4 | 36 | A |
| Wu et al. [97] | 3 | 2 | 2 | 3 | 1 | 4 | 3 | 2 | 2 | 1 | 4 | 27 | B |
| Wu et al. [98] | 4 | 3 | 2 | 2 | 1 | 4 | 4 | 3 | 2 | 2 | 4 | 31 | B |
| Xie et al. [99] | 4 | 3 | 4 | 2 | 1 | 3 | 3 | 2 | 2 | 1 | 4 | 29 | B |
| Yang et al. [100] | 4 | 4 | 3 | 2 | 1 | 3 | 4 | 1 | 2 | 1 | 4 | 29 | B |
| Ye et al. [101] | 3 | 2 | 3 | 2 | 1 | 2 | 3 | 1 | 2 | 1 | 4 | 24 | B |
| You et al. [102] | 1 | 4 | 4 | 2 | 1 | 3 | 4 | 2 | 3 | 1 | 4 | 29 | B |
| Zeng et al. [103] | 3 | 4 | 4 | 3 | 2 | 4 | 3 | 4 | 2 | 2 | 4 | 35 | A |
| Average | 3 | 3.3 | 3.3 | 2.3 | 1.6 | 3.4 | 3.2 | 2.1 | 2.0 | 1.4 | 3.3 | 29.1 | B |
R-AMSTAR items: 1 – Was an ‘‘a priori’’ design provided?; 2 – Was there duplicate study selection and data extraction?; 3 – Was a comprehensive literature search performed?; 4 – Was the status of publication used as an inclusion criterion?; 5 – Was a list of studies provided?; 6 – Were the characteristics of the included studies provided?; 7 – Was the scientific quality of the included studies assessed and documented?; 8 – Was the scientific quality of the included studies used appropriately in formulating conclusions?; 9 – Were the methods used to combine the findings of studies appropriate?; 10 – Was the likelihood of publication bias assessed?; 11 – Was the conflict of interest included?.
3.3. Study characteristics
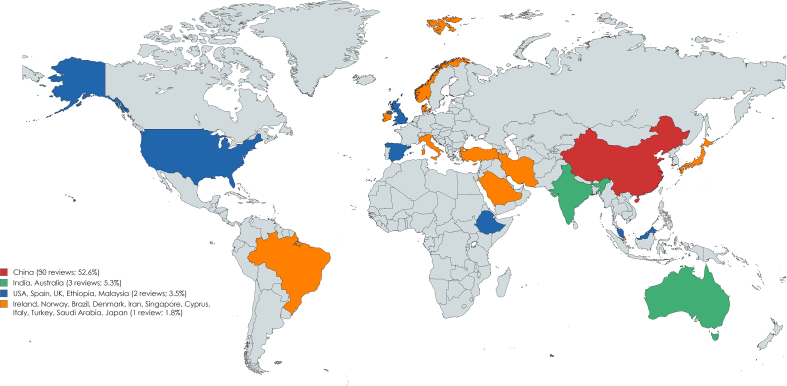
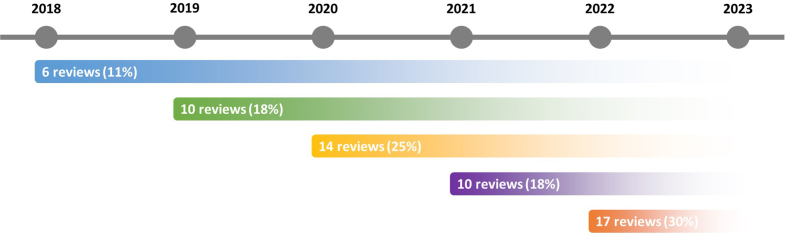
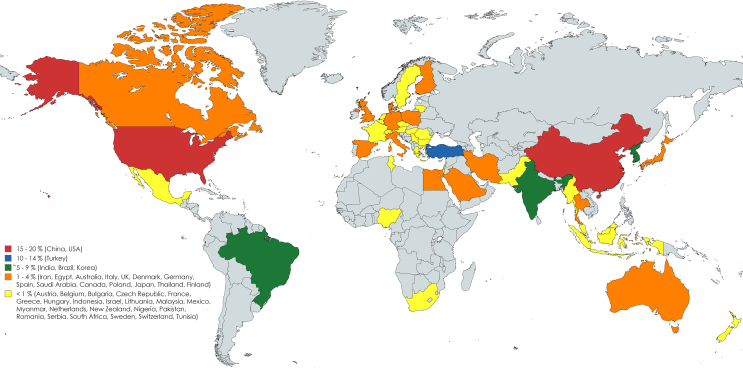
The 57 reviews included [[43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60],[62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73],[75], [76], [77], [78],[80], [81], [82], [83], [84], [85], [86], [87], [88], [89],[91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103]] were published from 2018 [45,46,52,55,68,75] to 2022 [43,44,48,49,59,64,73,76,80,82,83,[87], [88], [89],[96], [97], [98]], being 2022 the most common year (17; 29.8%). The reviews were conducted in 19 different countries, majorly in China [[48], [49], [50], [51],55,57,59,62,63,[68], [69], [70], [71], [72], [73],75,76,82,86,89,94,[96], [97], [98], [99], [100], [101], [102], [103]] (30; 52.6%), followed by India [78,87,92] and Australia [56,60,66] (each with three; 5.3%). Supplemental Figures 2 and 3, show in more detail the years and countries distribution.
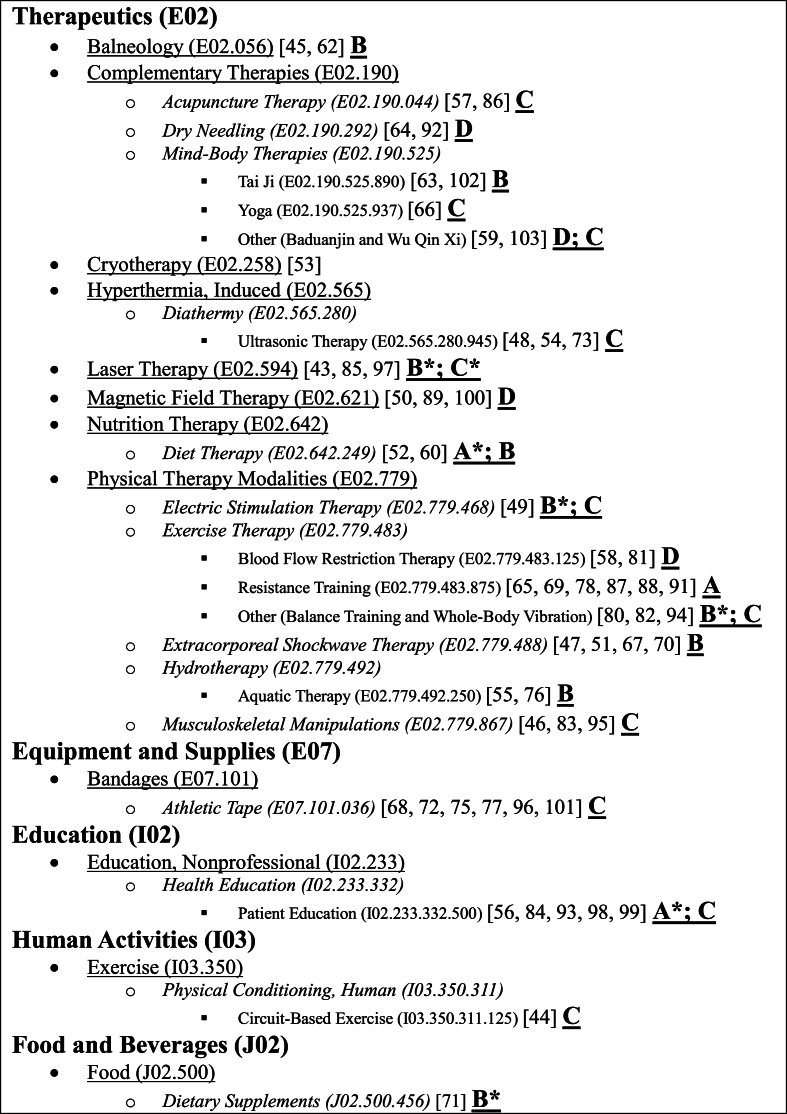
The reviews encompassed a total of 714 clinical trials, yielding a mean of 13 ± 7.7 studies per review (maximum: 50 [70]; minimum: 3 [81]). The number of participants enrolled in the studies was 59,343, averaging 1041 ± 1002 per review (maximum: 4844 [70]; minimum: 117 [81]), and 82 ± 59.2 (maximum: 336 [84]; minimum: 15 [68]) per RCT. Among these participants, approximately 70% were female (male [n,%] – maximum: 1500 [56], 60% [43]; minimum: 17 [81], 3.5% [72]; female [n,%] – maximum: 3787 [70], 96.5% [72]; minimum: 100 [81], 40% [43]), with a mean BMI of 30.2 ± 3.6 (maximum: 40 [60]; minimum: 23 [70]) and 61.6 ± 4.2 years of age (maximum: 74.1 [66]; minimum: 53.4 [95]). The most frequently reported outcomes were physical function (27.1%; e.g., ROM, strength, TUG, and 6MWT), pain (31.6%; e.g., VAS and NPRS), and knee-specific patient-reported (32.9%; e.g., WOMAC and KOOS) related. QOL (e.g., SF-36), radiological (e.g., X-ray), and laboratorial (e.g., erythrocyte sediment rate) outcomes, accounted for only 5.2%, 1.9%, and 1.3%, respectively. The systematic reviews explored 24 distinct non-pharmacological and non-surgical interventions (Fig. 2).
Fig. 2.
The non-pharmacological and non-surgical interventions tree MeSH codes and their hierarchy (n = 57). Note: The letters in bold and underlined are the interventions classification. The “∗” symbolize the classification when the intervention is added to exercise.
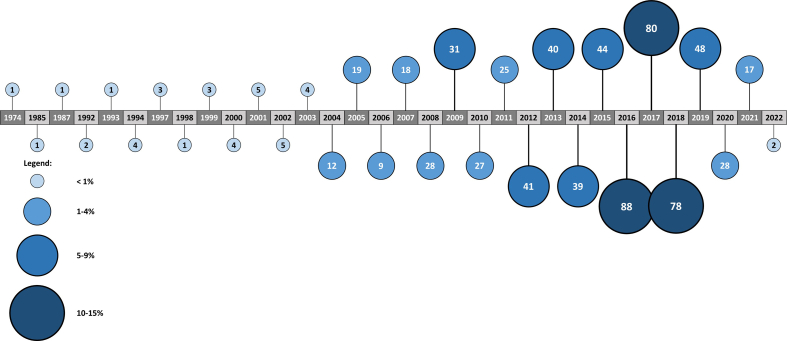
From the individual studies included in the reviews, 43 countries were enlisted, being China (19.4%) and USA (15.4%) the most prominently represented countries. On average, each review covered 6 ± 2.8 countries, with Liao et al. [69] including the most countries (13) and Zeng et al. [103] being the less international (100% China). The years ranged 1974–2022, with the most common years being 2012 (5.8%), 2013 (5.6%), 2014 (5.5%), 2015 (6.2%), 2016 (12.4%), 2017 (11.3%), 2018 (11%), and 2019 (6.8%). On average, each review covered 7.3 ± 2.7 years, with Goff et al. [56], Liao et al. [69], and Gong et al. [57] having the widest distribution (13 different years), and Lu et al. [75] and Pitsillides et al. [81] having the narrowest distribution (3 different years). Supplemental Figures 4 and 5, show in more detail the years and countries distribution. The characteristics of the included reviews are summarized in Table 4.
4. Discussion
The discussion will be presented according to the interventions explored in the selected reviews.
4.1. Athletic tape
Among the different taping techniques, Kinesio Tape (KT) was the only analyzed in the reviews [68,72,75,77,96,101]. Apparently, KT can mitigate pain, enhance function, improve self-reported knee-related health status, and increase knee ROM, in short-term [68,72,75,77,96,101]. Notably, no significant differences were observed in strength-related outcomes [68,75,101]. The evidence suggests a higher degree of certainty regarding the positive impact on pain reduction, particularly when compared with placebo, sham, or no intervention groups [68,75,77,96,101]. Also, combining KT with physical therapy appears to yield superior results compared to physical therapy alone, although additional validation is warranted [72,101]. Despite these findings, a consensus regarding optimal taping methods remains elusive. Notably, employing a Y-shaped configuration with a 120–140% stretching length over a period of 3–4 weeks has been identified as optimal [72,96]. This technique is believed to stimulate the quadriceps femoris and stabilize the patella, by wrapping the tape around it [72]. However, due to the taping methods heterogeneity among the individual studies (such as taping shape, number of tapes, and different intervention durations) [68,72,75,77,96,101], providing definitive clinical guidance proves challenging, and irrefutable conclusions or recommendations are difficult to achieve. By some methodological inconsistencies in the studies and the overall low-level evidence, this intervention is considered as C.
4.2. Balneology
Based on the reviews [45,62], balneology interventions (particularly those involving mud-related therapies) demonstrate significant efficacy in promptly alleviating pain and improving joint function in the short-term. This pain reduction is highlighted by a significant NSAIDs consumption reduction among KOA patients. Moreover, there are indications of long-term enhancements in overall QOL (including social functions), in comparison with sham mud/peloid therapy, or standard treatment. Similarly, it was also found that balneological interventions in adjunct to standard treatment are significantly more effective than standard treatment alone in improving QOL. Interestingly, social function does not seem to be directly influenced by the treatment itself. This could be attributed to the fact that, regardless of the type of intervention (real or sham), patients were asked for a short period to regularly go to a spa center, where they could relax, socialize with other people, and carefully assessed by physicians. Both real and sham balneological interventions may contribute to an improved self-perception of well-being and social function, possibly due to placebo effects (primarily attributed to the ritualistic nature of the intervention, the therapeutic environment, and the patient-clinician relationship dynamics). Due to wide range of interventions included in the umbrella term “balneology”, moderate risk of bias, and publication bias found, these interventions are considered a B.
4.3. Aquatic therapy
From the reviews [55,76], Ma et al. [76] found that compared with no aquatic physical therapy, aquatic physical therapy is associated with a large significant change in pain. Furthermore, for muscle strength, aquatic physical therapies exhibit a small but significant effect on knee extension muscle strength. Mixed results were achieved in physical function and walking ability (although the overall results show a tendency of improvement). Conversely, aquatic physical therapy did not significantly relieve knee stiffness and symptoms, QOL, ROM, and body composition. Dong et al. [55] found that compared to no intervention, the aquatic group had significant enhancements in KOOS activities of daily living and sports & recreational activities, but not for KOOS pain, symptom, or QOL. Despite a higher level of adherence and satisfaction reported in the aquatic therapy group, it did not outperform land-based therapy in all evaluated outcomes.
These mixed results can be attributed to the heterogeneity among the methodologies employed in the individual studies, particularly regarding exercise prescription parameters (such as mode, intensity, duration and frequency, and water characteristics) [55,76]. The studies had sessions typically ranged 40–60 min, conducted 2–3 times per week over a span of 6–18 weeks, with water depths ranging 1.15–1.5 m and temperatures between 30 and 34 °C [55]. These methodological discrepancies likely influenced the obtained results. For instance, due to water resistance and buoyancy, the aquatic exercise program intensity is quite different to land-based [55], therefore these different groups may achieve the same effects under different intensities. Moreover, water properties such as temperature and depth play crucial roles in these interventions. A temperature range 33.5–35.5 °C is best suitable as it allows lengthy immersion and thus enables sufficient exercise to be performed to achieve therapeutic effects without participants becoming cold or over-heating, and may promote muscle relaxation [55,76]. Additionally, varying water depths (xiphoid or cervical) can lead to distinct buoyancy effects, impacting joint load-bearing and influencing outcomes such as pain alleviation, stiffness reduction, strength enhancement, and improvement in physical function [55,76]. Considering the aforementioned factors, these interventions are categorized as B.
4.4. Balance training
The reviews [80,94] suggests that compared to no intervention, balance/proprioceptive training group was superior across all outcome measures. However, when compared to other training methods, the results are less elusive, with differences observed simply in joint position sense, knee ROM, and physical function. Notably, the addition of balance/proprioceptive training to other interventions (such as conventional physiotherapy or other exercise regimens), resulted in sustained superiority solely in joint position sense. Consequently, it is prudent to consider this intervention as a complementary component to standard exercise or physiotherapy protocols, particularly recommended for patients displaying significant clinical impairments in joint position sense. It should not be regarded as an individual intervention. Considering the low publication bias and the very-low to moderate-quality of evidence, these interventions are classified as B (when utilized as a complementary exercise).
4.5. Blood flow restriction therapy (BFR)
Based on the reviews [58,81], it appears that this intervention can reduce pain and improve strength. However, it failed to surpass standard resistance training [58] or high-intensity training [81] regimens. Nonetheless, despite the limited number of studies, a guidance is starting to emerge. Although the precise mechanism of action of this less explored intervention in the KOA context remains unclear, several theories have been proposed. BFR involves performing low-intensity exercises while applying cuffs in the upper third of the thigh, reducing arterial inflow and causing venous occlusion, leading to transient ischemia to the afferent tissues [[104], [105], [106]]. It is hypothesized that this technique will potentially stimulate neovascularization and promote various biochemical and physiological tissue changes such as hypertrophy, increased fast-twitch fiber recruitment, mechanotransduction, muscle damage, systemic and localized hormone production, cell swelling, and the production of reactive oxygen species and its variants (including nitric oxide and heat shock proteins) [107]. Consequently, this intervention appears to have the potential to be applied to KOA patients who require strength improvements and experience pain during exercises, particularly in more intense training regimens [58,81]. In terms of protocol variables, it should be performed at least six weeks, with a session frequency of 2–3 times weekly, with until voluntary failure volume, a rest period of 30–60 s, and a cuff pressure individualized and maintained throughout the session (40–80% of the arterial occlusion pressure more usual) [81]. While this intervention appears relatively safe, awareness among KOA patients with comorbid conditions (specially, cardiovascular [e.g., hypertension or chronic venous insufficiency]) is crucial, due to the potential effect on the exercise pressor reflex (a body's physiologic autonomic sympathetic response to exercise, that increases carbon monoxide, heart rate, contractility, and ultimately mean arterial pressure) [108,109]. Due to pain reporting inconsistencies, disparities in the protocols, inaccuracies in the one-repetition maximum calculation, limited studies, inefficacy compared to more traditional and established training regimens, and overall quality of evidence, this intervention is considered D.
4.6. Circuit-based exercise
Circuit-based training involves repeatedly and sequentially performing sets of several resistance and callisthenic exercises, targeting different body parts, with minimal or no rest intervals [110,111]. By its nature, the heart rate maintains raised throughout the workout and different muscle groups are activated, leading to a high metabolic cost [111]. Thereby, it is expected that it could promote both local (muscle/strength) and systemic (cardiorespiratory/functional capacity/body composition) benefits [110,111]. The brisk transition between exercises, coupled with shorter rest intervals, significantly reduces the session duration, which may encourage participant retention and adherence [110,112]. A recommendation for the elderly population is to engage a minimum of two weekly sessions, lasting 30–50 min (the sets and repetitions per exercise should be scalable by the individual training/fitness level and the clinical status), incorporating different intensity levels (hypertrophy [e.g., 60–85% 1 RM] and high-velocity low-loads [e.g., 40% 1 RM]), with a 1:1 work-to-rest ratio (e.g., 30:30 s) [110]. Despite the anticipated positive effects of this intervention, in the KOA population it fails to demonstrate is effectiveness. In fact, among all outcomes evaluated, circuit-based exercises solely exhibited improvements in the pain, depression, and health-related QOL compared to standard treatment groups [44]. In the other outcomes, no statistically significant differences observed between the groups [44]. Therefore, and due to the limited number of trials, inconsistencies among protocols, heterogeneity found in positive outcomes, and the overall quality of evidence, this intervention is classified as C.
4.7. Resistance training
Similar to findings in other studies [11,113,114], resistance training showed to be a cost-effective intervention for KOA patients. From the reviews [65,69,78,87,88,91], positive results were found in the overall explored outcomes (especially, pain, strength, QOL, function, and knee health-related status). These benefits were particularly evident when compared to non-exercise groups as opposed to other exercise regimens [65]. For example, the exercise group demonstrated superiority over NSAIDs and opioids groups in pain reduction [88]. Additionally, significant increases in lean mass, muscle thickness, and cross-sectional area were observed in the exercise groups compared to non-exercise controls [69]. While most of the studies targeted in strengthening the quadriceps femoris, a holistic approach involving hip-focused exercises (e.g., abductors) is also recommended, as evidence suggests it can improve knee-symptoms [78,87]. Although it was found that 24 total sessions over an 8–12 week period produces large effect sizes, the optimal protocol for resistance training in KOA patients remains undetermined (although, the most common regimens prescribed are 2–3 sets, 8–12 repetitions, starting at a resistance maximum of 50–60% 1 RM, 3 times per week) [91]. Due to the consistency of positive results, and overall low risk of bias, this intervention is A classified.
4.8. Whole-body vibration (WBV)
From the review [82], it was found that this intervention (both low- and high-frequency) when combined with strengthening exercises could improve pain, physical function, and knee extensor strength, compared to a control group performing strengthening exercises alone. However, no significant improvement were found in stiffness, balance, QOL, and knee flexor strength. These results can be explained by the device mechanism of action. WBV involves standing, sitting, or lying on an oscillating platform, that generates vertical or lateral vibrations, at a pre-determined frequency [115]. These vibrations are transferred to the body, in which is thought that can stimulate muscle spindles, influencing the central mechanism and activating the alpha-motor neurons, subsequently triggering the vibration tonic reflex, which may contribute to modulating neuromuscular adaptations [116]. Therefore, is expected that it can improve muscle strength and decrease pain [117]. Nevertheless, current evidence regarding the physiological mechanisms, therapeutic effects, device parameters and usage on KOA remains controversial [82,117]. By these uncertainties, evidence quality, limited studies, and conflicting results, this intervention is classified as C.
4.9. Baduanjin
Baduanjin, a form of Qigong, is an ancient Chinese mind-body therapy that integrates spirit and meditation with slow and gentle postures, musculoskeletal stretching, and deep breathing [118]. Baduanjin exercise involves eight separate postures (support the heaven, draw a bow, hold up the hand, looking back, shake the hand and wag the tail, touch the feet, climbing and relax the back) that may have beneficial body effects, such as muscular strength, weight reduction, and physical, psychosocial, cognitive and spiritual well-being [119]. This intervention has fewer physical and cognitive demands compared to practices (like Tai Ji), making it suitable for the elderly beginner with KOA to practice in a short-time [120]. In fact, findings from the included review [103] suggest that Baduanjin exercise is superior in WOMAC scores when compared with non-exercise groups (such as, waiting list or patient education). When combined with NSAIDS, it not only maintained superiority in WOMAC but also demonstrated a higher pain decrease when compared with NSAIDs alone. However, the therapeutic efficacy of Baduanjin exercise relative to other exercises, interventions, or mind-body therapies remains uncertain. Given these uncertainties, along with the overall weak evidence, limited study availability, and some safety concerns (such as mild muscle pain, falls, and exercise-related injuries during sessions), this intervention is categorized as D.
4.10. Tai Ji
Tai Ji, a traditional Chinese martial art, involves low-intensity exercises characterized by flowing circular, gentle, graceful movements, which requires practitioners to concentrate on exercise and eliminate distractions, while consciously deep breath and relax joints/muscles to the maximum extent possible, attempting to maintain proper posture when weight shifting, thereby emphasizing balance and coordination of the mind and body [121,122]. Presently, there are various training styles, such as Chen, Yang, Wu Hao, Wu, and Sun [121]. Additionally, to meet contemporary needs, adaptations of traditional forms have emerged, such as the 24-form Tai Ji [122]. Tai Ji is considered a suitable exercise for the elderly due to its potential effects for both physical and mental well-being. By practicing Tai Ji exercise, it is expect maintenance or improvement in pain levels, cardiorespiratory capacity, body weight, balance, muscle strength, and ROM, without exacerbating arthritis symptoms [123]. Additionally, by Tai Ji simplicity, safeness, and meditative nature, it is expected that facilitate the reduction of learning failure frustration, fear of falling, depression, and anxiety, among the elderly [124,125]. The findings of the included reviews [63,102] support these statements, as it was found significant improvements in pain, physical function, walking, balance, self-reported knee-related health status, and QOL (physiological and psychological), among Tai Ji groups compared with the control groups (especially no active interventions, such as no-exercise, standard care, waiting list, or education). Although it was not found a specific protocol or style that stood out, the majority of the studies prescribed a regimen consisting of sessions lasting 40–60 min each, conducted 2–3 times per week, over a period of 8–24 weeks. Considering the quality of evidence and the inconsistencies found in the studies, this intervention in considered B.
4.11. Wu Qin Xi (WQX)
WQX, a type of Qigong that mimics animal movements, is an ancient Chinese mind-body therapy [126]. Each routine contains two symmetrically movements and synchronized with controlled breathing, featuring the following movements [126,127]: tiger standing up and lunging forward to eat; deer holding its horns and running; bear shaking its arms and swaying its body; ape lifting and picking things upwards; crane stretching and flying. While WQX exercises are less well-known both internationally and in China compared to other Qigong forms, they offer distinct advantages. From example, compared to the simplified version of Tai Ji, WQX is easier to learn because it only has 10-sets of movements [59]. Furthermore, Tai Ji contains movements with extreme knee flexion, which could be detrimental to the KOA patients where, in contrast, all movements in WQX have knee flexion no greater than 90° [59]. Similar to other Qigong forms, WQX exercises may offer a physiological benefits as, for example, the support and weight shift of the knee joint in a semi-squat position in the tiger and deer movements, and the dynamic flexion and extension of the knee in a single-leg support position in the bird movement, potentially leading to local (strength) and systemic (balance and cardiorespiratory) improvements [59,127]. Beyond physical conditioning, this intervention may also yield psychological benefits by restoring the balance of “Yin” and “Yang” (as known as “Qi”) through specific breathing patterns coupled with the intentional movement, thereby alleviating mental tension, reducing psychological stress, and promoting mental health [127]. From the supposed benefits of these exercises, the review [59] only demonstrated improvements in WOMAC and pain. For the other outcomes, the clinical importance of WQX exercises remains uncertain. Due to the limited evidence, moderate risk of bias, and the low adverse effects associated with this intervention, it is classified as C.
4.12. Yoga
Yoga is a form of mind-body therapy originating in ancient India, and in the Western context constitutes a number of practices, including physical practices (postures, asanas), breath regulation techniques (pranayama), mental practices (meditation, mindfulness), and relaxation [128]. Yoga has become a popular intervention of achieving and maintaining well-being and health [129]. Yoga sequences include a variety of postures (e.g., Mountain, Downward-Facing Dog, Warrior, Tree, Child's, Cobra, Bridge, Seated Forward Bend, Triangle, and Corpse) to improve stiffness, joint function, ROM, and strength [130,131]. Beyond physical activity, yoga also often incorporates breathing/relaxation/meditation exercises, which serve to alleviate stress and pain by releasing muscle tension, countering muscle tightness, and enhancing mental equilibrium [132,133]. The included review [66] found that compared to exercise and non-exercise groups, yoga appears be safe and beneficial in terms of pain intensity, physical function, and stiffness. However, no significant effects were observed concerning QOL or depression. It is important to note that these findings were derived from limited studies, with an overall very-low quality of evidence. Therefore, this intervention is classified as C.
4.13. Musculoskeletal manual manipulations
Musculoskeletal manual manipulations (commonly referred as Manual Therapy (MT)) can be defined as any hands-on therapy that may include soft issue techniques, moving joints in various and specific directions and at various speeds, or having the patient move the body part against the therapist's resistance [134]. Within this broad definition, several techniques are suitable such as massage, manual stretch, myofascial techniques, mobilizations, and manipulations [46,83,95]. They can be utilized either individually or in combination during a session [25]. The choice of technique(s) is influenced by the clinical (e.g., experience), patient (e.g., personal characteristics, clinical status, and preferences) and external factors (e.g., session time) [135]. By applying these techniques, it is expected to Ref. [136]: improve tissues mobility and function; restore movement, stretching, or ROM; improve muscle activation and timing; decrease pain; and improve circulation. However, from the included reviews [46,83,95], some of these benefits were unclear. It was found that MT can be beneficial, when compared with non-active interventions, in the studied outcomes. Similarly, it was also found benefits when combined to an active intervention, however they were less evident. It was found benefits of combined MT and exercise over exercise alone for reducing pain (the larger effect), and improving function and WOMAC in the short- and medium-term. However, these findings were predominantly based on trials with very-low to moderate-level of certainty, thereby should be considered with caution. In long-term, high-certainty evidence showed that combined MT with exercise did not offer additional benefits compared to exercise alone in terms of pain, WOMAC, and function. Due to the polled results, the overall quality of evidence, the lack of protocol standardization (type, dosage, force, amplitude, rate, repetition, and duration), and the inclusion of several MT techniques under the same umbrella term, these interventions are categorized as C.
4.14. Acupuncture therapy
Acutherapy belongs to the Traditional Chinese Medicine, and is based on the principle of acupoint stimulation across meridians through a wide range of modalities, including needle acupuncture, laser acupuncture, acupressure, electroacupuncture, moxibustion, etc [57]. While evidence has been showing potential clinical benefits of acutherapy for KOA, controversy on its role in managing these patients remains [86]. Gong et al. [57] found that acutherapy presented benefits in pain, stiffness and function when compared with the usual care, but the differences were not significant as compared with the sham condition. The authors speculated that since differences existed between sham conditions (sham non-acupoints versus sham true-acupoints) the lack of differences found between sham and acutherapy could be attributed to potential therapeutic effects of sham true-acupoints. However, acutherapy did not demonstrate any apparent therapeutic advantages in pain, stiffness, and function over physiotherapeutic approaches, such as exercise-based interventions (e.g., exercise oriented leg strengthening, stretching, and balance). Consequently, the most plausible explanation for these outcomes is psychological factor dependent, potentially indicating a placebo effect. Another factor to consider is dosage. Sun et al. [86] showed that an adequate acutherapy dosage involved needling of ≥4 points (for each painful knee, for at least 20 min), ≥6 treatment sessions, conducted at least once weekly, with either elicitation of de qi sensation or application of electrical stimulation, and that high-dosages had more benefits compared to low- or medium-dosages. However, the criteria used to define high-dosage were as follows: (1) the number of points needled was ≥9; or (2) there was a de qi response; or (3) frequency of treatment was ≥2 sessions a week; or (4) the total number of treatment sessions was ≥8. These are volatile and unassertive, providing limited assistance in decision-making or clinical guidance. Therefore, and as explored, these interventions are classified as C.
4.15. Dry needling (DN)
DN entails the insertion of fine monofilament needles through the skin to manage various neuromusculoskeletal syndromes [137,138]. These needles can target a variety of tissues, including muscles, subcutaneous fascia, tendons, ligaments, scar tissue, periosteum, teno-osseous junction, peripheral nerves, and even neurovascular bundles [137,138]. There are three primary DN techniques most common used [92]: myofascial trigger point needling, periosteal stimulation, and intramuscular electrical stimulation. Myofascial trigger point needling, is the most common invasive technique, and consists of repeated needle insertion of a single-use acupuncture needle into the trigger point (hyperirritable spot present in taut bands of skeletal muscles or fascia, which produce local and referred pain, stiffness, limited ROM, and muscle spasm, fatigue, and weakness) [137,138]. The periosteal stimulation employs a similar technique but uses needles over the periosteum [92]. The intramuscular electrical stimulation is another needling technique that uses electrical stimulation over motor points, regional, and paravertebral musculature [92]. With these techniques it is expected to reduce pain, relax muscles, and improve ROM, and activate circulation [137]. Nevertheless, the actual mechanisms are still elusive. Ughreja and Prem [92] found that periosteal stimulation could have booster short-term benefits in pain and WOMAC when added to conventional physiotherapy protocols. Intramuscular electrical stimulation, although less explored, demonstrated potential in reducing pain compared to sham intervention. However, caution is warranted in interpreting these findings, as the study yielding these results included an additional intervention in the form of active transcranial direct current stimulation, making it difficult to ascertain the individual effects of each intervention. Finally, the myofascial trigger point needling, was the most explored technique, and reached mixed results. While some studies reported differences in pain and function favoring DN over acupuncture, others found no changes between DN and sham interventions. Jiménez-del-Barrio et al. [64] also reported mixed results concerning this technique. Overall, DN demonstrated efficacy in reducing pain and improving function in the short-term compared to control groups, particularly when contrasted to sham or no intervention. However, when compared to more active interventions (such as, exercise or self-stretching), the results were less obvious. No differences in medium- or long-term were found. Therefore, due to the inconsistency of the results, the very-low to low-quality of evidence, and safety concerns (e.g., post-needling soreness), these interventions are categorized as D.
4.16. Diet therapy
Diet therapy is a form of intervention that adjusts the quantity and quality of food intake to improve health status of an individual. These interventions for KOA patients encompassed various approaches, including: nutritional education and behavioral therapy about reduced-energy diets; partial meal replacements; and nutrition powder to fully replace conventional meals. While some improvements were noted with diet-only interventions (particularly function), more consistent results were observed when diet was combined with exercise (particularly pain). However, it is important not to underestimate the efficacy of these interventions as, for example, significant reductions in total weight and fat mass were observed in the diet groups compared to the control groups (8.5 ± 2.9 kg [7.8 ± 3.1 %] vs 2.7 ± 1.3 kg [2.7 ± 1.2 %]; and 7.6 ± 1.0 kg [3.3 ± 0.4 %] vs 2.1 ± 1.4 kg [0.4 ± 1.3 %], respectively) [52]. Additionally, it appears that these patients have more benefits when these interventions are sustained over longer durations (+12 months), without encountering adverse effects during that time [60]. Consequently, these interventions are dual-classified as both A (as a complement to exercise) and B (as a standalone intervention).
4.17. Dietary supplements
Dietary supplements can be defined as products in capsule, tablet or liquid form that provide dietary ingredients, and that are intended to be taken by mouth to increase the intake of nutrients [139]. Dietary supplements can include macronutrients (such as proteins, carbohydrates, and fats) and/or micronutrients (such as vitamins, minerals, and phytochemicals) [139]. In this subsection, only protein supplements was explored [69]. The protein supplements administered consisted of either whey protein (milk or leucine) or branched-chain amino acids. The essential amino acid doses varied between 3 and 40 g/day, and were administered to a resistance training intervention group. It was found that, compared to groups receiving exercise plus placebo supplementation or exercise alone, the intervention group exhibited statistically significant improvements in pain, muscle mass, strength, and function outcomes. These results are particularly interesting, given that deficits in muscle volume and function are commonly observed in KOA patients, often attributed to the development of sarcopenia (a condition associated with gradual and progressive muscle mass loss in older adults), that frequently result in a poorer health status and QOL [140]. Due to the limited studies, and inconsistencies among protocols and doses, this intervention is classified as B (as a complement to exercise).
4.18. Patient education
Patient education interventions were scrutinized in five reviews [56,84,93,98,99]. This intervention aims to teach or train patients regarding their own health-needs. Therefore, it may be used a range of modalities to achieve its objectives, including exercises guidance, diet or weight management, physical, psychological, and occupational therapies, cognitive or behavioral pain coping skills, as well as encouragement, medication, educational lectures, and medical information. The reviews revealed a short- and medium-term reduction in pain, and improvement in function among patients who received educational interventions, compared to those who received usual care or no intervention. Similarly (but superior), when educational interventions were combined with conventional rehabilitation, outcomes were enhanced compared to conventional rehabilitation alone. However, when compared to more active interventions (such as exercise), the improvements in outcomes were less pronounced. Long-term results were either non-existent or slightly/very small for the majority of the outcomes, regardless of the compared group. Intriguingly, therapist-based educational interventions (whether group or face-to-face) yielded consistently superior positive results compared to internet-based interventions. Therefore, to achieve more consistent results, it is recommended to adopt a more personal approach in these interventions. Consequently, these interventions are dual-classified as both A (as a complement to exercise) and C (as a standalone intervention).
4.19. Cryotherapy
Cryotherapy consists in the local or general use of cold encompassing various modalities, including ice packs, ice cubes, cold compresses, cold sprays, cold tubs, and cold chambers. From low-quality evidence [53], it was found high within-group effects sizes when cryotherapy was combined with other types of therapy (e.g., exercises or analgesics), in pain and function. Moreover, between-group comparisons yielded only small effect sizes. However, when cryotherapy was administered alone, the effect sizes were generally moderate for both within-group and between-group comparisons. Although study protocols varied, the techniques, frequency, and duration of cryotherapy applications did not significantly impact the outcomes. Furthermore, the results should be interpreted with caution, as the small sample sizes may increase the likelihood of encountering a type II error. For the reasons stated before, these interventions are classified as C.
4.20. Electric stimulation therapy
The review [49] that explored electric stimulation therapy focused solely on interferential current therapy (IFC). IFC is a type of electrical stimulation therapy, which involves the use of two or more sinusoidal currents (applied to the body via electrodes), intersecting and “interfering” with each other at the target area to generate a “beat frequency” and induce a therapeutic effect [141]. Findings indicated that this therapy could yield positive outcomes in short-term pain reduction and WOMAC scores compared to control groups. However, in the long-term, only pain reduction remained statistically significant. No statically significant differences were observed for mobility and stiffness. Results were more pronounced when active IFC was compared with sham IFC. Conversely, for other combinations and comparisons (e.g., active IFC plus exercise versus sham IFC plus exercise or exercise alone), outcomes were less evident. Although the studies applied more frequently a carrier frequency of 3850–4000 Hz and an amplitude modulated frequency 80–100 Hz, a consistent treatment protocol for IFC application was not established. Nonetheless, this lack of uniformity may not pose a significant issue, as research suggests that most IFC parameters do not appear to influence its analgesic effects [142]. Although previous studies have reported adverse effects (such as, burns and vasovagal reactions), no adverse effects of similar severity were reported in the selected studies. Therefore, these interventions are double-evaluated as B (as a complement to exercise) and C (as a standalone intervention).
4.21. Extracorporeal shockwave therapy
This therapy operates by generating shockwaves through electromagnetic means, involving the passage of electric current through a coil to produce a strong magnetic field [143]. The waves are then focused using a lens, precisely targeting the therapeutic focal point (determined by the lens length) [143]. As the acoustic wave advances towards the focal point, it initiates mechanotransduction, generating vibrations within tissues (energy that can develop a peak pressure about 1000 times higher than that of ultrasound), achieving its therapeutic effects [144]. Polled findings [47,51,67,70] indicate positive outcomes in pain reduction, ROM improvement, enhanced function, and self-reported knee-health status when compared to either placebo or other interventions. Most of these interventions consisted of 3–5 sessions, with 1000–2500 pulses per session, and a pulse frequency between 4 and 12 Hz. Certain parameters of extracorporeal shockwave therapy interventions merit special consideration, particularly follow-up duration, shockwave energy level, and type [47,67,70]. Results suggest that interventions lasting ≥4 weeks yield superior outcomes compared to <4 weeks [67,70]. Regarding the energetic density dosage, it seems that medium-doses (0.08–0.25 mJ/mm2 or 1.5–2.5 bar) produced a greater effects than low- or high-doses (<0.08 and >0.25 mJ/mm2 or <1.5 and >2.5 bar) [47]. Comparing low-to high-dosages, high-dosages produced better results than low [70]. Concerning the type of shockwaves, both focused and radial shockwaves are beneficial for KOA patients [47,51,67], although the radial shockwave may exert superior effects [70]. Due to overall quality evidence (moderate) and some safety issues found (e.g., pain, minor bruising, soft tissue swelling, redness, burning sensation, or effusion), this intervention is classified as B.
4.22. Laser therapy
Findings form the reviews [43,85,97] indicate that laser therapy is effective in reducing pain and improving WOMAC scores, particularly when compared to placebo or sham interventions. These benefits are further accentuated when laser therapy is combined with exercise [43]. Laser therapy encompasses both low- (LLLT) and high-level laser therapy (HLLT), and works by delivering specific wavelengths of light, that absorbed by the targeted tissues, triggering a cascade of biological responses [145]. LLLT and HLLT have different characteristics, with HLLT appearing to yield superior results for KOA patients compared to low-level [43,97]. Typically, LLLT involves longer continuous concentrated therapy time (e.g., 16 min), with ≤500 mW of energy output, 600–980 nm wavelength, 100 J/cm2 energy density, in 5 cm2 treatment area, having a potential penetration of <2 cm (superficial tissues) [43]. In contrast, HLLT features shorter continuous or pulsed diffuse therapy session (e.g., 2 min), with >500 mW of energy output, 660–1280 nm wavelength, 100 J/cm2 energy density, in 5 cm2 treatment area, having a potential penetration of 5–15 cm (deep tissues) [43]. The higher dosage delivered by HLLT effectively increase local temperature, thereby enhancing tissue metabolism and blood circulation [97]. This results in the rapid removal of inflammatory substances, improved mitochondrial oxidation and adenosine triphosphate production, enhanced absorption of tissue edema, and increased nutrient exchange and tissue regeneration [97]. Conversely, the local temperature increase is less pronounced with LLLT, potentially limiting its efficacy [85]. Despite variations in intervention procedures, studies suggest that the ideal protocol parameters include a wavelength of 1064 nm, an energy density of 15–810 J/cm2, a total dose per session of 1250–3000 J, 10–12 therapy sessions within a 2–6 week intervention period [43]. Consequently, this therapy is double-evaluated as B (HLLT as a complement to exercise) and C (LLLT as a complement to exercise, and laser therapy as a standalone intervention).
4.23. Magnetic field therapy
Pulsed electromagnetic field therapy (PEMF) utilizes a time-varying magnetic field generated by electrical current passing through a conductor, in which provides electrical stimulation piezoelectric scaffolds facilitating the transmission of mechanical impulses, potentially resulting in cellular proliferation, cartilage degeneration prevention, and subchondral trabecular bone microarchitecture stabilization [50,89,100]. This therapy appears to offer potential improvements in pain, stiffness, and function when compared to placebo or sham groups [50,89,100]. However, when contrasted with active controlled groups, these positive effects were less evidenced [89]. The most commonly applied protocol involves sessions lasting between 15 min and 2 h, occurring 3 times per week to daily (or twice daily), over a period of 4–6 weeks [50]. In terms of the therapy's parameters, it appears that low-frequency (i.e., <300 Hz) are more conducive to achieving favorable results when compared to high-frequency [89]. Other factors, such as duration of treatment, may not be critical to influence KOA symptoms [100]. Due to the limited evidence, overall quality, and the modest results obtained, this intervention is classified with D.
4.24. Ultrasonic therapy (US)
US is a therapeutic modality that uses high-frequency sound waves to treat various medical conditions [146]. These sound waves penetrate into the body's tissues, creating thermal and mechanical effects [146]. With these effects is expected to enhance soft tissue healing, decrease the inflammatory response, increase blood flow, increase metabolic activity, decrease pain, and improve cartilage repair [146]. From the reviews [48,54,73], it was found that this therapy could be effective in improving function, alleviating pain, enhancing ROM, and self-reported knee-health status, compared to either placebo or sham interventions. However, the comparison with other interventions yielded limited findings, precluding definitive conclusions. Regarding the US protocol parameters, evidence suggests that longer session durations (i.e., ≥20 min) over shorter periods (i.e., ≤4 weeks) tend to yield more favorable results [48]. Moreover, higher intensities (≥1.5 W/cm2) are associated with superior results compared to low-intensity treatments [73]. In terms of frequency, the US typically range from 0.2 to 3 MHz (1 MHz US is suitable for treating tissues with a 2.3–5 cm depth, and 3 MHz US is suitable for treating tissues with a 0.8–1.6 cm depth) [147]. The choice of frequency will depend on the desired therapeutic effect, though 1 MHz US appears more suitable for pain relief [73]. Unlike previous findings suggesting that pulsed modes are more effective, the included studies indicate no significant difference between pulsed and continuous modes [73]. Consequently, due to the limited outcomes achieved, the low-quality of evidence, and the minimal risk of adverse events associated, this intervention is classified as C.
5. Conclusion
Based in the systematic reviews included, it can be concluded that Diet Therapy, Patient Education, and Resistance Training are strongly supported as core interventions for managing KOA patients. Aquatic Therapy, Balance Training, Balneology, Dietary Supplements, Extracorporeal Shockwave Therapy, and Tai Ji show moderate support for their usage. However, for other interventions, the evidence quality was low, results were mixed or inconclusive, or there was not sufficient efficacy to support their use. Additionally, in comparison to Ferreira et al. [34], eleven new interventions were identified, including Baduanji, Balance Training, BFR, Circuit-based Exercise, Cryotherapy, Diet Therapy, Dietary Supplements, DN, Extracorporeal Shockwave Therapy, Patient Education, and WQX. In the contrary, no systematic reviews were included with the Cupping Therapy, Insoles, Moxibustion, Neuromuscular Electrical Stimulation, and Transcutaneous Electrical Nerve Stimulation interventions. When comparing interventions found in both studies, Aquatic Therapy, KT, Resistance Training, Tai Ji, and WBV, maintained their previous classification. Balneology and Laser Therapy were upgraded (form D to B and C, respectively). On the other hand, Acunpucture, IFC, MT, PEMF, US, and Yoga interventions were downgraded (all from B to C, except PEMF intervention which went from B to D).
6. Limitation
This umbrella review has some limitations that warrant acknowledgment. In an attempt to reach the highest quality evidence, our eligibility criteria were confined to systematic reviews exclusively derived from RCTs. While this approach prioritizes methodological rigor, a broader eligibility criteria (encompassing other review types and primary studies), have the potential to reach additional studies and interventions, consequently altering the results achieved. Furthermore, to attain accuracy and precision, clinical guide and relevance, and efficacy and efficiency of the interventions, it was opted to include only systematic reviews with the highest specificity. Consequently, certain pertinent systematic reviews may have been inadvertently excluded from our analysis, potentially withholding information capable of influencing the overall findings.
Conflict of interest
The authors have no conflicts of interest to declare.
Authors contributions
RMF had the conception and design of the study. RMF and PNM, searched, analyzed and interpreted the data. RSG supervised and arbitrated. RMF wrote the sections of the manuscript. All authors have read, revised, and approved the submitted version.
Acknowledgements
There are no acknowledgments in this study.
Handling Editor: Professor H Madry
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2024.100497.
Contributor Information
Ricardo Maia Ferreira, Email: rferreira@ipmaia.pt.
Pedro Nunes Martins, Email: pmartins@ipmaia.pt.
Rui Soles Gonçalves, Email: ruigoncalves@estesc.ipc.pt.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Fig. S2.
Fig. S3.
Fig. S4.
References
- 1.Kraus V.B., Blanco F.J., Englund M., Karsdal M.A., Lohmander L.S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartil. 2015;23:1233–1241. doi: 10.1016/j.joca.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safiri S., Kolahi A.-A., Smith E., Hill C., Bettampadi D., Mansournia M.A., et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020;79:819–828. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 3.Appleyard T., Thomas M.J., Antcliff D., Peat G. Prediction models to estimate the future risk of osteoarthritis in the general population: a systematic review. Arthritis Care Res. 2023;75:1481–1493. doi: 10.1002/acr.25035. [DOI] [PubMed] [Google Scholar]
- 4.Dong Y., Yan Y., Zhou J., Zhou Q., Wei H. Evidence on risk factors for knee osteoarthritis in middle-older aged: a systematic review and meta analysis. J. Orthop. Surg. Res. 2023;18:634. doi: 10.1186/s13018-023-04089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vina E.R., Kwoh C.K. Epidemiology of osteoarthritis: literature update. Curr. Opin. Rheumatol. 2018;30:160–167. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter D., Bierma-Zeinstra S. Osteoarthritis Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. (CAS) [DOI] [PubMed] [Google Scholar]
- 7.Swain S., Coupland C., Mallen C., Kuo C.F., Sarmanova A., Bierma-Zeinstra S.M., et al. Temporal relationship between osteoarthritis and comorbidities: a combined case control and cohort study in the UK primary care setting. Rheumatology. 2021;60:4327–4339. doi: 10.1093/rheumatology/keab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie Y., Zhou W., Zhong Z., Zhao Z., Yu H., Huang Y., et al. Metabolic syndrome, hypertension, and hyperglycemia were positively associated with knee osteoarthritis, while dyslipidemia showed no association with knee osteoarthritis. Clin. Rheumatol. 2021;40:711–724. doi: 10.1007/s10067-020-05216-y. [DOI] [PubMed] [Google Scholar]
- 9.Calders P., Van Ginckel A. Presence of comorbidities and prognosis of clinical symptoms in knee and/or hip osteoarthritis: a systematic review and meta-analysis. Semin. Arthritis Rheum. 2018;47:805–813. doi: 10.1016/j.semarthrit.2017.10.016. Elsevier. [DOI] [PubMed] [Google Scholar]
- 10.Bowden J.L., Hunter D.J., Deveza L.A., Duong V., Dziedzic K.S., Allen K.D., et al. Core and adjunctive interventions for osteoarthritis: efficacy and models for implementation. Nat. Rev. Rheumatol. 2020;16:434–447. doi: 10.1038/s41584-020-0447-8. [DOI] [PubMed] [Google Scholar]
- 11.Dantas L.O., de Fátima Salvini T., McAlindon T.E. Knee osteoarthritis: key treatments and implications for physical therapy. Braz. J. Phys. Ther. 2021;25:135–146. doi: 10.1016/j.bjpt.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puig-Junoy J., Zamora A.R. Socio-economic costs of osteoarthritis: a systematic review of cost-of-illness studies. Semin. Arthritis Rheum. 2015;44:531–541. doi: 10.1016/j.semarthrit.2014.10.012. Elsevier. [DOI] [PubMed] [Google Scholar]
- 13.Sharma L. Osteoarthritis of the knee. N. Engl. J. Med. 2021;384:51–59. doi: 10.1056/NEJMcp1903768. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L., Wang Y., Ye T., Hu Y., Wang S., Qian T., et al. Quality of clinical practice guidelines relevant to rehabilitation of knee osteoarthritis: a systematic review. Clin. Rehabil. 2023;37:986–1008. doi: 10.1177/02692155221144892. [DOI] [PubMed] [Google Scholar]
- 15.Oral A., Arman S., Tarakci E., Patrini M., Arienti C., Etemadi Y., et al. A systematic review of clinical practice guidelines for persons with osteoarthritis. A “Best Evidence for Rehabilitation”(be4rehab) paper to develop the WHO's Package of Interventions for Rehabilitation. Int. J. Rheumat. Dis. 2022;25:383–393. doi: 10.1111/1756-185X.14292. [DOI] [PubMed] [Google Scholar]
- 16.Gray B., Eyles J.P., Grace S., Hunter D.J., Østerås N., Quicke J., et al. Best evidence Osteoarthritis Care: what are the Recommendations and what is needed to improve practice? Clin. Geriatr. Med. 2022;38:287–302. doi: 10.1016/j.cger.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Tittlemier B.J., Wittmeier K.D., Webber S.C. Quality and content analysis of clinical practice guidelines which include nonpharmacological interventions for knee osteoarthritis. J. Eval. Clin. Pract. 2021;27:93–102. doi: 10.1111/jep.13391. [DOI] [PubMed] [Google Scholar]
- 18.Bichsel D., Liechti F.D., Schlapbach J.M., Wertli M.M. Cross-sectional analysis of recommendations for the treatment of hip and knee osteoarthritis in clinical guidelines. Arch. Phys. Med. Rehabil. 2022;103:559–569. e555. doi: 10.1016/j.apmr.2021.07.801. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs A.J., Gray B., Wallis J.A., Taylor N.F., Kemp J.L., Hunter D.J., et al. Recommendations for the management of hip and knee osteoarthritis: a systematic review of clinical practice guidelines. Osteoarthritis Cartil. 2023;31:1280–1292. doi: 10.1016/j.joca.2023.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Meiyappan K.P., Cote M.P., Bozic K.J., Halawi M.J. Adherence to the American Academy of Orthopaedic Surgeons clinical practice guidelines for nonoperative management of knee osteoarthritis. J. Arthroplasty. 2020;35:347–352. doi: 10.1016/j.arth.2019.08.051. [DOI] [PubMed] [Google Scholar]
- 21.El-Khoury J., Orozco T., Bernatsky S., Desmeules F., Perreault K., Woodhouse L.J., et al. Do Quebec physiotherapists follow evidence-based guidelines for treating knee osteoarthritis? Physiother. Can. 2020;72:374–381. doi: 10.3138/ptc-2019-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackah M., Boakye H., Yeboah C.O., Bello A.I. Physiotherapy practice patterns in the management of patients with knee osteoarthritis: a national survey on the use of clinical practice guidelines. Physiother. Res. Int. 2022;27:e1964. doi: 10.1002/pri.1964. [DOI] [PubMed] [Google Scholar]
- 23.Eyles J.P., Hunter D.J., Bennell K.L., Dziedzic K.S., Hinman R.S., van der Esch M., et al. Priorities for the effective implementation of osteoarthritis management programs: an OARSI international consensus exercise. Osteoarthritis Cartil. 2019;27:1270–1279. doi: 10.1016/j.joca.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Ettlin L., Nast I., Huber E.O., Niedermann K. Does the conservative non-pharmacological management of knee osteoarthritis in Switzerland reflect the clinical guidelines? A survey among general practitioners, rheumatologists, and orthopaedic surgeons. Front. Rehabil. Sci. 2021;2 doi: 10.3389/fresc.2021.658831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira R.M., Martins P.N., Pimenta N., Gonçalves R.S. Physical therapists' choices, views and agreements regarding non-pharmacological and non-surgical interventions for knee osteoarthritis patients: a mixed-methods study. Medit. J. Rheumatol. 2023;34:188. doi: 10.31138/mjr.34.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hummer C.D., Huang Y., Sheehan B. Adherence to the OARSI recommendations for designing, conducting, and reporting of clinical trials in knee osteoarthritis: a targeted literature review. BMC Muscoskel. Disord. 2022;23:171. doi: 10.1186/s12891-022-05116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deveza L.A., Melo L., Yamato T., Mills K., Ravi V., Hunter D. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthritis Cartil. 2017;25:1926–1941. doi: 10.1016/j.joca.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Teo P.L., Hinman R.S., Egerton T., Dziedzic K.S., Bennell K.L. Identifying and prioritizing clinical guideline recommendations most relevant to physical therapy practice for hip and/or knee osteoarthritis. J. Orthop. Sports Phys. Ther. 2019;49:501–512. doi: 10.2519/jospt.2019.8676. [DOI] [PubMed] [Google Scholar]
- 29.Bartholdy C., Nielsen S., Warming S., Hunter D., Christensen R., Henriksen M. Poor replicability of recommended exercise interventions for knee osteoarthritis: a descriptive analysis of evidence informing current guidelines and recommendations. Osteoarthritis Cartilage. 2019;27:3–22. doi: 10.1016/j.joca.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Eyles J.P., Sharma S., Telles R.W., Namane M., Hunter D.J., Bowden J.L. Implementation of best-evidence osteoarthritis care: perspectives on challenges for, and opportunities from, low and middle-income countries. Frontiers in rehabilitation sciences. 2022;2 doi: 10.3389/fresc.2021.826765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michael J.W.-P., Schlüter-Brust K.U., Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Deutsches Arzteblatt International. 2010;107:152. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva M.D.C., Perriman D.M., Fearon A.M., Couldrick J.M., Scarvell J.M. Minimal important change and difference for knee osteoarthritis outcome measurement tools after non-surgical interventions: a systematic review. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-063026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowden J.L., Hunter D.J., Mills K., Allen K., Bennell K., Briggs A.M., et al. The OARSI joint effort initiative: priorities for osteoarthritis management program implementation and research 2024–2028. Osteoarthritis Cartil. Open. 2023;5 doi: 10.1016/j.ocarto.2023.100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira R., Duarte J., Gonçalves R. Non-pharmacological and non-surgical interventions to manage patients with knee osteoarthritis: an umbrella review. Acta Reumatologica Portuguesa. 2018;43:182–200. [PubMed] [Google Scholar]
- 35.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 36.Kung J., Chiappelli F., Cajulis O.O., Avezova R., Kossan G., Chew L., et al. From systematic reviews to clinical recommendations for evidence-based health care: validation of revised assessment of multiple systematic reviews (R-AMSTAR) for grading of clinical relevance. Open Dent. J. 2010;4:84. doi: 10.2174/1874210601004020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Brożek J., Akl E., Compalati E., Kreis J., Terracciano L., Fiocchi A., et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy. 2011;66:588–595. doi: 10.1111/j.1398-9995.2010.02530.x. [DOI] [PubMed] [Google Scholar]
- 39.Brożek J., Akl E.A., Alonso-Coello P., Lang D., Jaeschke R., Williams J.W., et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64:669–677. doi: 10.1111/j.1398-9995.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 40.Group G.W. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollock A., Farmer S.E., Brady M.C., Langhorne P., Mead G.E., Mehrholz J., et al. An algorithm was developed to assign GRADE levels of evidence to comparisons within systematic reviews. J. Clin. Epidemiol. 2016;70:106–110. doi: 10.1016/j.jclinepi.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamtvedt G., Dahm K.T., Christie A., Moe R.H., Haavardsholm E., Holm I., et al. Physical therapy interventions for patients with osteoarthritis of the knee: an overview of systematic reviews. Phys. Ther. 2008;88:123–136. doi: 10.2522/ptj.20070043. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad M.A., Hamid M.S.A., Yusof A. Effects of low-level and high-intensity laser therapy as adjunctive to rehabilitation exercise on pain, stiffness and function in knee osteoarthritis: a systematic review and meta-analysis. Physiotherapy. 2022;114:85–95. doi: 10.1016/j.physio.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Al-Mhanna S.B., Mohamed M., Mohd Noor N., Aldhahi M.I., Afolabi H.A., Mutalub Y.B., et al. Effects of circuit training on patients with knee osteoarthritis: a systematic review and meta-analysis. Healthcare. 2022;10:2041. doi: 10.3390/healthcare10102041. MDPI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonelli M., Donelli D., Fioravanti A. Effects of balneotherapy and spa therapy on quality of life of patients with knee osteoarthritis: a systematic review and meta-analysis. Rheumatol. Int. 2018;38:1807–1824. doi: 10.1007/s00296-018-4081-6. [DOI] [PubMed] [Google Scholar]
- 46.Anwer S., Alghadir A., Zafar H., Brismee J.-M. Effects of orthopaedic manual therapy in knee osteoarthritis: a systematic review and meta-analysis. Physiotherapy. 2018;104:264–276. doi: 10.1016/j.physio.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Avendano-Coy J., Comino-Suárez N., Grande-Munoz J., Avendaño-López C., Gómez-Soriano J. Extracorporeal shockwave therapy improves pain and function in subjects with knee osteoarthritis: a systematic review and meta-analysis of randomized clinical trials. Int. J. Surg. 2020;82:64–75. doi: 10.1016/j.ijsu.2020.07.055. [DOI] [PubMed] [Google Scholar]
- 48.Chen H., Wang Z., Zhang X., Sun M. Effects of low-intensity pulsed ultrasound on knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Clin. Rehabil. 2022;36:1153–1169. doi: 10.1177/02692155221097035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H.-L., Yang F.-A., Lee T.-H., Liou T.-H., Escorpizo R., Chen H.-C. Effectiveness of interferential current therapy in patients with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2022;12:9694. doi: 10.1038/s41598-022-13478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L., Duan X., Xing F., Liu G., Gong M., Li L., et al. Effects of pulsed electromagnetic field therapy on pain, stiffness and physical function in patients with knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2019;51:821–827. doi: 10.2340/16501977-2613. [DOI] [PubMed] [Google Scholar]
- 51.Chen L., Ye L., Liu H., Yang P., Yang B. Extracorporeal shock wave therapy for the treatment of osteoarthritis: a systematic review and meta-analysis. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/1907821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chu I., Lim A., Ng C. Effects of meaningful weight loss beyond symptomatic relief in adults with knee osteoarthritis and obesity: a systematic review and meta-analysis. Obes. Rev. 2018;19:1597–1607. doi: 10.1111/obr.12726. [DOI] [PubMed] [Google Scholar]
- 53.Dantas L.O., Moreira R.d.F.C., Norde F.M., Mendes Silva Serrao P.R., Alburquerque-Sendín F., Salvini T.F. The effects of cryotherapy on pain and function in individuals with knee osteoarthritis: a systematic review of randomized controlled trials. Clin. Rehabil. 2019;33:1310–1319. doi: 10.1177/0269215519840406. [DOI] [PubMed] [Google Scholar]
- 54.Dantas L.O., Osani M.C., Bannuru R.R. Therapeutic ultrasound for knee osteoarthritis: a systematic review and meta-analysis with grade quality assessment. Braz. J. Phys. Ther. 2021;25:688–697. doi: 10.1016/j.bjpt.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong R., Wu Y., Xu S., Zhang L., Ying J., Jin H., et al. Is aquatic exercise more effective than land-based exercise for knee osteoarthritis? Medicine. 2018;97 doi: 10.1097/MD.0000000000013823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goff A.J., Silva D.D.O., Merolli M., Bell E.C., Crossley K.M., Barton C.J. Patient education improves pain and function in people with knee osteoarthritis with better effects when combined with exercise therapy: a systematic review. J. Physiother. 2021;67:177–189. doi: 10.1016/j.jphys.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Gong Z., Liu R., Yu W., Wong T.K.-S., Guo Y., Sun Y. Acutherapy for knee osteoarthritis relief in the elderly: a systematic review and meta-analysis. Evid. base Compl. Alternative Med. 2019;2019 doi: 10.1155/2019/1868107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grantham B., Korakakis V., O'Sullivan K. Does blood flow restriction training enhance clinical outcomes in knee osteoarthritis: a systematic review and meta-analysis. Phys. Ther. Sport. 2021;49:37–49. doi: 10.1016/j.ptsp.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Guo J., Peng C., Hu Z., Guo L., Dai R., Li Y. Effect of Wu Qin Xi exercises on pain and function in people with knee osteoarthritis: a systematic review and meta-analysis. Front. Med. 2022;9 doi: 10.3389/fmed.2022.979207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall M., Castelein B., Wittoek R., Calders P., Van Ginckel A. Diet-induced weight loss alone or combined with exercise in overweight or obese people with knee osteoarthritis: a systematic review and meta-analysis. Semin. Arthritis Rheum. 2019;48:765–777. doi: 10.1016/j.semarthrit.2018.06.005. Elsevier. [DOI] [PubMed] [Google Scholar]
- 61.Heddon S., Saulnier N., Mercado J., Shalmiyev M., Berteau J.-P. Systematic review shows no strong evidence regarding the use of elastic taping for pain improvement in patients with primary knee osteoarthritis. Medicine. 2021;100 doi: 10.1097/MD.0000000000025382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hou C., Liang L., Chu X., Qin W., Li Y., Zhao Y. The short-term efficacy of mud therapy for knee osteoarthritis: a meta-analysis. Medicine. 2020;99 doi: 10.1097/MD.0000000000019761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu L., Wang Y., Liu X., Ji X., Ma Y., Man S., et al. Tai Chi exercise can ameliorate physical and mental health of patients with knee osteoarthritis: systematic review and meta-analysis. Clin. Rehabil. 2020;35:64–79. doi: 10.1177/0269215520954343. [DOI] [PubMed] [Google Scholar]
- 64.Jiménez-del-Barrio S., Medrano-de-la-Fuente R., Hernando-Garijo I., Mingo-Gómez M.T., Estébanez-de-Miguel E., Ceballos-Laita L. The effectiveness of dry needling in patients with hip or knee osteoarthritis: a systematic review and meta-analysis. Life. 2022;12:1575. doi: 10.3390/life12101575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kus G., Yeldan I. Strengthening the quadriceps femoris muscle versus other knee training programs for the treatment of knee osteoarthritis. Rheumatol. Int. 2019;39:203–218. doi: 10.1007/s00296-018-4199-6. [DOI] [PubMed] [Google Scholar]
- 66.Lauche R., Hunter D.J., Adams J., Cramer H. Yoga for osteoarthritis: a systematic review and meta-analysis. Curr. Rheumatol. Rep. 2019;21:1–12. doi: 10.1007/s11926-019-0846-5. [DOI] [PubMed] [Google Scholar]
- 67.Li T., Ma J., Zhao T., Gao F., Sun W. Application and efficacy of extracorporeal shockwave treatment for knee osteoarthritis: a systematic review and meta-analysis. Exp. Ther. Med. 2019;18:2843–2850. doi: 10.3892/etm.2019.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X., Zhou X., Liu H., Chen N., Liang J., Yang X., et al. Effects of elastic therapeutic taping on knee osteoarthritis: a systematic review and meta-analysis. Aging Dis. 2018;9:296. doi: 10.14336/AD.2017.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao C.D., Chen H.C., Kuo Y.C., Tsauo J.Y., Huang S.W., Liou T.H. Effects of muscle strength training on muscle mass gain and hypertrophy in older adults with osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res. 2020;72:1703–1718. doi: 10.1002/acr.24097. [DOI] [PubMed] [Google Scholar]
- 70.Liao C.-D., Tsauo J.-Y., Liou T.-H., Chen H.-C., Huang S.-W. Clinical efficacy of extracorporeal shockwave therapy for knee osteoarthritis: a systematic review and meta-regression of randomized controlled trials. Clin. Rehabil. 2019;33:1419–1430. doi: 10.1177/0269215519846942. [DOI] [PubMed] [Google Scholar]
- 71.Liao C.-D., Wu Y.-T., Tsauo J.-Y., Chen P.-R., Tu Y.-K., Chen H.-C., et al. Effects of protein supplementation combined with exercise training on muscle mass and function in older adults with lower-extremity osteoarthritis: a systematic review and meta-analysis of randomized trials. Nutrients. 2020;12:2422. doi: 10.3390/nu12082422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin C.-H., Lee M., Lu K.-Y., Chang C.-H., Huang S.-S., Chen C.-M. Comparative effects of combined physical therapy with Kinesio taping and physical therapy in patients with knee osteoarthritis: a systematic review and meta-analysis. Clin. Rehabil. 2020;34:1014–1027. doi: 10.1177/0269215520928398. [DOI] [PubMed] [Google Scholar]
- 73.Liu Y., Wang Y., Wang Y., Jia X. A meta-analysis of analgesic effect of ultrasound therapy for patients with knee osteoarthritis. J. Ultrasound Med. 2022;41:1861–1872. doi: 10.1002/jum.15866. [DOI] [PubMed] [Google Scholar]
- 74.Long Y., Yanmin W., Jianzhong Y., Jie W., Zhang Y. Effects of orthopaedic insoles on patients with knee osteoarthritis: a meta-analysis and systematic review. J. Rehabil. Med. 2021;53 doi: 10.2340/16501977-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Z., Li X., Chen R., Guo C. Kinesio taping improves pain and function in patients with knee osteoarthritis: a meta-analysis of randomized controlled trials. Int. J. Surg. 2018;59:27–35. doi: 10.1016/j.ijsu.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 76.Ma J., Chen X., Xin J., Niu X., Liu Z., Zhao Q. Overall treatment effects of aquatic physical therapy in knee osteoarthritis: a systematic review and meta-analysis. J. Orthop. Surg. Res. 2022;17:1–15. doi: 10.1186/s13018-022-03069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melese H., Alamer A., Hailu Temesgen M., Nigussie F. Effectiveness of kinesio taping on the management of knee osteoarthritis: a systematic review of randomized controlled trials. J. Pain Res. 2020:1267–1276. doi: 10.2147/JPR.S249567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neelapala Y.R., Bhagat M., Shah P. Hip muscle strengthening for knee osteoarthritis: a systematic review of literature. J. Geriatr. Phys. Ther. 2020;43:89–98. doi: 10.1519/JPT.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 79.Novak S., Guerron G., Zou Z., Cheung G., Berteau J.-P. New guidelines for electrical stimulation parameters in adult patients with knee osteoarthritis based on a systematic review of the current literature. Am. J. Phys. Med. Rehabil. 2020;99:682–688. doi: 10.1097/PHM.0000000000001409. [DOI] [PubMed] [Google Scholar]
- 80.Pirayeh N., Kazemi K., Rahimi F., Mostafaee N., Shaterzadeh-Yazdi M.-J. The effect of balance training on functional outcomes in patients with knee osteoarthritis: a systematic review. Med. J. Islam. Repub. Iran. 2022;36 doi: 10.47176/mjiri.36.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pitsillides A., Stasinopoulos D., Mamais I. Blood flow restriction training in patients with knee osteoarthritis: systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2021;27:477–486. doi: 10.1016/j.jbmt.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 82.Qiu C.G., Chui C.S., Chow S.K.H., Cheung W.-H., Wong R.M.Y. Effects of whole-body vibration therapy on knee Osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2022;54 doi: 10.2340/jrm.v54.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Runge N., Aina A., May S. The benefits of adding manual therapy to exercise therapy for improving pain and function in patients with knee or hip osteoarthritis: a systematic review with meta-analysis. J. Orthop. Sports Phys. Ther. 2022;52 doi: 10.2519/jospt.2022.11062. 675-A613. [DOI] [PubMed] [Google Scholar]
- 84.Safari R., Jackson J., Sheffield D. Digital self-management interventions for people with osteoarthritis: systematic review with meta-analysis. J. Med. Internet Res. 2020;22 doi: 10.2196/15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stausholm M.B., Joensen J., Lopes-Martins R.Á.B., Lund H., Fersum K.V., Bjordal J.M. Efficacy of low-level laser therapy on pain and disability in knee osteoarthritis: systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-031142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun N., Tu J.F., Lin L.L., Li Y.T., Yang J.W., Shi G.X., et al. Correlation between acupuncture dose and effectiveness in the treatment of knee osteoarthritis: a systematic review. Acupunct. Med. 2019;37:261–267. doi: 10.1136/acupmed-2017-011608. [DOI] [PubMed] [Google Scholar]
- 87.Thomas D.T., Prabhakar A.J., Dineshbhai P.V., Eapen C. Hip abductor strengthening in patients diagnosed with knee osteoarthritis–a systematic review and meta-analysis. BMC Muscoskel. Disord. 2022;23:1–14. doi: 10.1186/s12891-022-05557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thorlund J.B., Simic M., Pihl K., Berthelsen D.B., Day R., Koes B., et al. Similar effects of exercise therapy, nonsteroidal anti-inflammatory drugs, and opioids for knee osteoarthritis pain: a systematic review with network meta-analysis. J. Orthop. Sports Phys. Ther. 2022;52:207–216. doi: 10.2519/jospt.2022.10490. [DOI] [PubMed] [Google Scholar]
- 89.Tong J., Chen Z., Sun G., Zhou J., Zeng Y., Zhong P., et al. The efficacy of pulsed electromagnetic fields on pain, stiffness, and physical function in osteoarthritis: a systematic review and meta-analysis. Pain Res. Manag. 2022;2022 doi: 10.1155/2022/9939891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsokanos A., Livieratou E., Billis E., Tsekoura M., Tatsios P., Tsepis E., et al. The efficacy of manual therapy in patients with knee osteoarthritis: a systematic review. Medicina. 2021;57:696. doi: 10.3390/medicina57070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turner M.N., Hernandez D.O., Cade W., Emerson C.P., Reynolds J.M., Best T.M. The role of resistance training dosing on pain and physical function in individuals with knee osteoarthritis: a systematic review. Sports health. 2020;12:200–206. doi: 10.1177/1941738119887183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ughreja R.A., Prem V. Effectiveness of dry needling techniques in patients with knee osteoarthritis: a systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2021;27:328–338. doi: 10.1016/j.jbmt.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 93.Uritani D., Koda H., Sugita S. Effects of self-management education programmes on self-efficacy for osteoarthritis of the knee: a systematic review of randomised controlled trials. BMC Muscoskel. Disord. 2021;22:515. doi: 10.1186/s12891-021-04399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y., Wu Z., Chen Z., Ye X., Chen G., Yang J., et al. Proprioceptive training for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Front. Med. 2021;8 doi: 10.3389/fmed.2021.699921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weleslassie G.G., Temesgen M.H., Alamer A., Tsegay G.S., Hailemariam T.T., Melese H. Effectiveness of mobilization with movement on the management of knee osteoarthritis: a systematic review of randomized controlled trials. Pain Res. Manag. 2021;2021:1–12. doi: 10.1155/2021/8815682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu H., Yao R., Wu J., Wen G., Wang Y. Does kinesio taping plus exercise improve pain and function in patients with knee osteoarthritis?: a systematic review and meta-analysis of randomized controlled trials. Front. Physiol. 2022:1901. doi: 10.3389/fphys.2022.961264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu M., Luan L., Pranata A., Witchalls J., Adams R., Bousie J., et al. Is high intensity laser therapy more effective than other physical therapy modalities for treating knee osteoarthritis? A systematic review and network meta-analysis. Front. Med. 2022;9 doi: 10.3389/fmed.2022.956188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Z., Zhou R., Zhu Y., Zeng Z., Ye Z., Wang Z., et al. Self-management for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Pain Res. Manag. 2022:2022. doi: 10.1155/2022/2681240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie S.-H., Wang Q., Wang L.-Q., Wang L., Song K.-P., He C.-Q. Effect of internet-based rehabilitation programs on improvement of pain and physical function in patients with knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J. Med. Internet Res. 2021;23 doi: 10.2196/21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang X., He H., Ye W., Perry T.A., He C. Effects of pulsed electromagnetic field therapy on pain, stiffness, physical function, and quality of life in patients with osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Phys. Ther. 2020;100:1118–1131. doi: 10.1093/ptj/pzaa054. [DOI] [PubMed] [Google Scholar]
- 101.Ye W., Jia C., Jiang J., Liang Q., He C. Effectiveness of elastic taping in patients with knee osteoarthritis: a systematic review and meta-analysis. Am. J. Phys. Med. Rehabil. 2020;99:495–503. doi: 10.1097/PHM.0000000000001361. [DOI] [PubMed] [Google Scholar]
- 102.You Y., Liu J., Tang M., Wang D., Ma X. Effects of Tai Chi exercise on improving walking function and posture control in elderly patients with knee osteoarthritis: a systematic review and meta-analysis. Medicine. 2021;100 doi: 10.1097/MD.0000000000025655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeng Z.-p., Liu Y.-b., Fang J., Liu Y., Luo J., Yang M. Effects of Baduanjin exercise for knee osteoarthritis: a systematic review and meta-analysis. Compl. Ther. Med. 2020;48 doi: 10.1016/j.ctim.2019.102279. [DOI] [PubMed] [Google Scholar]
- 104.Bielitzki R., Behrendt T., Behrens M., Schega L. Current techniques used for practical blood flow restriction training: a systematic review. J. Strength Condit Res. 2021;35:2936–2951. doi: 10.1519/JSC.0000000000004104. [DOI] [PubMed] [Google Scholar]
- 105.Wortman R.J., Brown S.M., Savage-Elliott I., Finley Z.J., Mulcahey M.K. Blood flow restriction training for athletes: a systematic review. Am. J. Sports Med. 2021;49:1938–1944. doi: 10.1177/0363546520964454. [DOI] [PubMed] [Google Scholar]
- 106.Scott B.R., Loenneke J.P., Slattery K.M., Dascombe B.J. Exercise with blood flow restriction: an updated evidence-based approach for enhanced muscular development. Sports Med. 2015;45:313–325. doi: 10.1007/s40279-014-0288-1. [DOI] [PubMed] [Google Scholar]
- 107.Pearson S.J., Hussain S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187–200. doi: 10.1007/s40279-014-0264-9. [DOI] [PubMed] [Google Scholar]
- 108.Anderson K.D., Rask D.M., Bates T.J., Nuelle J.A. Overall safety and risks associated with blood flow restriction therapy: a literature review. Mil. Med. 2022;187:1059–1064. doi: 10.1093/milmed/usac055. [DOI] [PubMed] [Google Scholar]
- 109.Vopat B.G., Vopat L.M., Bechtold M.M., Hodge K.A. Blood flow restriction therapy: where we are and where we are going. JAAOS-J. Am. Acad. Orthopaed. Surg. 2020;28:e493–e500. doi: 10.5435/JAAOS-D-19-00347. [DOI] [PubMed] [Google Scholar]
- 110.Romero-Arenas S., Martínez-Pascual M., Alcaraz P.E. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4:256. doi: 10.14336/AD.2013.0400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seo Y.G., Noh H.M., Kim S.Y. Weight loss effects of circuit training interventions: a systematic review and meta-analysis. Obes. Rev. 2019;20:1642–1650. doi: 10.1111/obr.12911. [DOI] [PubMed] [Google Scholar]
- 112.Dobson F., Bennell K.L., French S.D., Nicolson P.J., Klaasman R.N., Holden M.A., et al. Barriers and facilitators to exercise participation in people with hip and/or knee osteoarthritis: synthesis of the literature using behavior change theory. Am. J. Phys. Med. Rehabil. 2016;95:372–389. doi: 10.1097/PHM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 113.Mazzei D., Ademola A., Abbott J., Sajobi T., Hildebrand K., Marshall D. Are education, exercise and diet interventions a cost-effective treatment to manage hip and knee osteoarthritis? A systematic review. Osteoarthritis Cartil. 2021;29:456–470. doi: 10.1016/j.joca.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 114.Verhagen A., Ferreira M., Reijneveld-van de Vendel E., Teirlinck C., Runhaar J., van Middelkoop M., et al. Do we need another trial on exercise in patients with knee osteoarthritis?: No new trials on exercise in knee OA. Osteoarthritis Cartil. 2019;27:1266–1269. doi: 10.1016/j.joca.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 115.Reis-Silva A., Coelho-Oliveira A.C., Moura-Fernandes M.C., Bruno Bessa M.-O., Bernardo-Filho M., de Sá Caputo D.d.C. Evidence of whole-body vibration exercises on body composition changes in older individuals: a systematic review and meta-analysis. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1202613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gonçalves de Oliveira R., Coutinho H.M.E.L., Martins M.N.M., Bernardo-Filho M., de Sá-Caputo D.d.C., Campos de Oliveira L., et al. Impacts of whole-body vibration on muscle strength, power, and endurance in older adults: a systematic review and meta-analysis. J. Clin. Med. 2023;12:4467. doi: 10.3390/jcm12134467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bonanni R., Cariati I., Romagnoli C., D'Arcangelo G., Annino G., Tancredi V. Whole body vibration: a valid alternative strategy to exercise? J. Funct. Morphol. Kinesiol. 2022;7:99. doi: 10.3390/jfmk7040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fang J., Zhang L., Wu F., Ye J., Cai S., Lian X. The safety of Baduanjin exercise: a systematic review. Evid. Base Compl. Alternat. Med. 2021;2021 doi: 10.1155/2021/8867098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zou L., Sasaki J.E., Wang H., Xiao Z., Fang Q., Zhang M. A systematic review and meta-analysis of baduanjin qigong for health benefits: randomized controlled trials. Evid. base Compl. Alternative Med. 2017;2017 doi: 10.1155/2017/4548706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.An B., Dai K., Zhu Z., Wang Y., Hao Y., Tang T., et al. Baduanjin alleviates the symptoms of knee osteoarthritis. J. Alternat. Compl. Med. 2008;14:167–174. doi: 10.1089/acm.2007.0600. [DOI] [PubMed] [Google Scholar]
- 121.Lan C., Lai J.-S., Chen S.-Y. Tai Chi Chuan: an ancient wisdom on exercise and health promotion. Sports Med. 2002;32:217–224. doi: 10.2165/00007256-200232040-00001. [DOI] [PubMed] [Google Scholar]
- 122.Li J., Hong Y., Chan K. Tai chi: physiological characteristics and beneficial effects on health. Br. J. Sports Med. 2001;35:148–156. doi: 10.1136/bjsm.35.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huston P., McFarlane B. Health benefits of tai chi: what is the evidence? Can. Fam. Physic. 2016;62:881–890. [PMC free article] [PubMed] [Google Scholar]
- 124.Webster C.S., Luo A.Y., Krägeloh C., Moir F., Henning M. A systematic review of the health benefits of Tai Chi for students in higher education. Prevent. Med. Rep. 2016;3:103–112. doi: 10.1016/j.pmedr.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chang W.-D., Chen S., Lee C.-L., Lin H.-Y., Lai P.-T. The effects of Tai Chi Chuan on improving mind-body health for knee osteoarthritis patients: a systematic review and meta-analysis. Evid. Base Compl. Alternat. Med. 2016;2016 doi: 10.1155/2016/1813979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wei X., Xu A., Yin Y., Zhang R. The potential effect of Wuqinxi exercise for primary osteoporosis: a systematic review and meta-analysis. Maturitas. 2015;82:346–354. doi: 10.1016/j.maturitas.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 127.Guo Y., Xu M., Wei Z., Hu Q., Chen Y., Yan J., et al. Beneficial effects of qigong wuqinxi in the improvement of health condition, prevention, and treatment of chronic diseases: evidence from a systematic review. Evid. Base Compl. Alternat. Med. 2018;2018 doi: 10.1155/2018/3235950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Feuerstein G. Shambhala Publications; 2014. The Psychology of Yoga: Integrating Eastern and Western Approaches for Understanding the Mind. [Google Scholar]
- 129.Patel N.K., Newstead A.H., Ferrer R.L. The effects of yoga on physical functioning and health related quality of life in older adults: a systematic review and meta-analysis. J. Alternative Compl. Med. 2012;18:902–917. doi: 10.1089/acm.2011.0473. [DOI] [PubMed] [Google Scholar]
- 130.Kan L., Zhang J., Yang Y., Wang P. The effects of yoga on pain, mobility, and quality of life in patients with knee osteoarthritis: a systematic review. Evid. Base Compl. Alternat. Med. 2016;2016 doi: 10.1155/2016/6016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shin S. Meta-analysis of the effect of yoga practice on physical fitness in the elderly. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph182111663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Saeed S.A., Cunningham K., Bloch R.M. Depression and anxiety disorders: benefits of exercise, yoga, and meditation. Am. Fam. Physic. 2019;99:620–627. [PubMed] [Google Scholar]
- 133.D'Alessio L., Korman G.P., Sarudiansky M., Guelman L.R., Scévola L., Pastore A., et al. Reducing allostatic load in depression and anxiety disorders: physical activity and yoga practice as add-on therapies. Front. Psychiatr. 2020;11:501. doi: 10.3389/fpsyt.2020.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mintken P., DeRosa C., Little T., Smith B. American Academy of Orthopaedic Manual Physical T. A model for standardizing manipulation terminology in physical therapy practice. J. Man. Manip. Ther. 2008;16:50–56. doi: 10.1179/106698108790818567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.French H., Brennan A., White B., Cusack T. Manual therapy for osteoarthritis of the hip or knee–a systematic review. Man. Ther. 2011;16:109–117. doi: 10.1016/j.math.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 136.Feng T., Wang X., Jin Z., Qin X., Sun C., Qi B., et al. Effectiveness and safety of manual therapy for knee osteoarthritis: an overview of systematic reviews and meta-analyses. Front. Public Health. 2023;11 doi: 10.3389/fpubh.2023.1081238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sánchez-Infante J., Navarro-Santana M.J., Bravo-Sanchez A., Jimenez-Diaz F., Abian-Vicen J. Is dry needling applied by physical therapists effective for pain in musculoskeletal conditions? A systematic review and meta-analysis. Phys. Ther. 2021;101:pzab070. doi: 10.1093/ptj/pzab070. [DOI] [PubMed] [Google Scholar]
- 138.A.P.T. Association . American Physical Therapy Association; Alexandria, VA: 2013. Description of Dry Needling in Clinical Practice: an Educational Resource Paper. [Google Scholar]
- 139.N.C.f.B.I. (NCBI) National Library of Medicine; 1998. Dietary supplements (MeSH)https://www.ncbi.nlm.nih.gov/mesh/68019587 [Google Scholar]
- 140.Shorter E., Sannicandro A.J., Poulet B., Goljanek-Whysall K. Skeletal muscle wasting and its relationship with osteoarthritis: a mini-review of mechanisms and current interventions. Curr. Rheumatol. Rep. 2019;21:1–8. doi: 10.1007/s11926-019-0839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goats G. Interferential current therapy. Br. J. Sports Med. 1990;24:87. doi: 10.1136/bjsm.24.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rampazo É.P., Liebano R.E. Analgesic effects of interferential current therapy: a narrative review. Medicina. 2022;58:141. doi: 10.3390/medicina58010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang C.-J. Extracorporeal shockwave therapy in musculoskeletal disorders. J. Orthop. Surg. Res. 2012;7:1–8. doi: 10.1186/1749-799X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schroeder A.N., Tenforde A.S., Jelsing E.J. Extracorporeal shockwave therapy in the management of sports medicine injuries. Curr. Sports Med. Rep. 2021;20:298–305. doi: 10.1249/JSR.0000000000000851. [DOI] [PubMed] [Google Scholar]
- 145.Beckerman H., de Bie R.A., Bouter L.M., De Cuyper H.J., Oostendorp R.A. The efficacy of laser therapy for musculoskeletal and skin disorders: a criteria-based meta-analysis of randomized clinical trials. Phys. Ther. 1992;72:483–491. doi: 10.1093/ptj/72.7.483. [DOI] [PubMed] [Google Scholar]
- 146.Loyola-Sánchez A., Richardson J., MacIntyre N. Efficacy of ultrasound therapy for the management of knee osteoarthritis: a systematic review with meta-analysis. Osteoarthritis Cartil. 2010;18:1117–1126. doi: 10.1016/j.joca.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 147.Hayes B.T., Merrick M.A., Sandrey M.A., Cordova M.L. Three-MHz ultrasound heats deeper into the tissues than originally theorized. J. Athl. Train. 2004;39:230. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.