Abstract
Structural variants are responsible for a large part of genomic variation between individuals and play a role in both common and rare diseases. Databases cataloguing structural variants notably do not represent the full spectrum of global diversity, particularly missing information from most African populations. To address this representation gap, we analysed 1,091 high-coverage African genomes, 545 of which are public data sets, and 546 which have been analysed for structural variants for the first time. Variants were called using five different tools and datasets merged and jointly called using SURVIVOR. We identified 67,795 structural variants throughout the genome, with 10,421 genes having at least one variant. Using a conservative overlap in merged data, 6,414 of the structural variants (9.5%) are novel compared to the Database of Genomic Variants. This study contributes to knowledge of the landscape of structural variant diversity in Africa and presents a reliable dataset for potential applications in population genetics and health-related research.
Keywords: Structural variants, African diversity, copy number variants, genomic variation
1. Introduction
Structural variants (SVs) are rearrangements of genomic content that are typically greater than 50bp in size, including deletions, duplications, insertions, inversions, translocations, and more complex rearrangements. Copy number variants (CNVs) are a subset of SVs that result in a change in the number of copies of a genomic region. SVs are responsible for a large part of genomic variation between individuals [1, 2, 3] but also play a role in disease, having been implicated in both common diseases such as cancer [4], and rare diseases, including many neurodevelopmental disorders (NDDs) [5, 6]. Unfortunately, the full characterisation of SVs has been slowed by the complexity and technical challenges of SV discovery. These challenges have resulted in a delay in publicly available high-quality SV databases from large population studies, which in turn has limited our understanding of the precise role of SV in human variation and disease [1].
Understanding the variation of the population informs research on genetic disorders. Early studies to map human SVs and understand their role in human diseases were performed using microarray genotyping technology [7, 8, 9]. These studies began to uncover the role of SVs in disease [10], but did not have the break-point accuracy and high resolution required for the discovery of small SVs [11]. With the increasing number of human genomes sequenced using next-generation sequencing (NGS) approaches, methods to detect SVs from these data were developed. Studies such as the 1000 Genomes Project[11] and the Genome Aggregation Database (gnomAD) [2] have produced landmark SV datasets that greatly contributed to understanding the global distribution of SVs.
Current SV reference databases are biased towards European populations and contain few African genomes, especially from continental Africans, thus representing only a subset of global genetic diversity [12, 13]. The most comprehensive African study to date is the study of 232 individuals using medium-coverage (10x) data [14]. The lack of knowledge of African genomes hampers the goal of access to genomic medicine initiatives for all global populations. As such, high-quality data sets of baseline variation in African populations will enrich our knowledge of the human genome [13] and help to interpret potentially pathogenic variants in the study of genetic diseases [15, 16]. Through our comprehensive dataset, we build on prior work to explore the breadth of SV diversity in Africa and establish a reliable reference of African SVs for potential applications in population genetics and health-related research.
2. Results
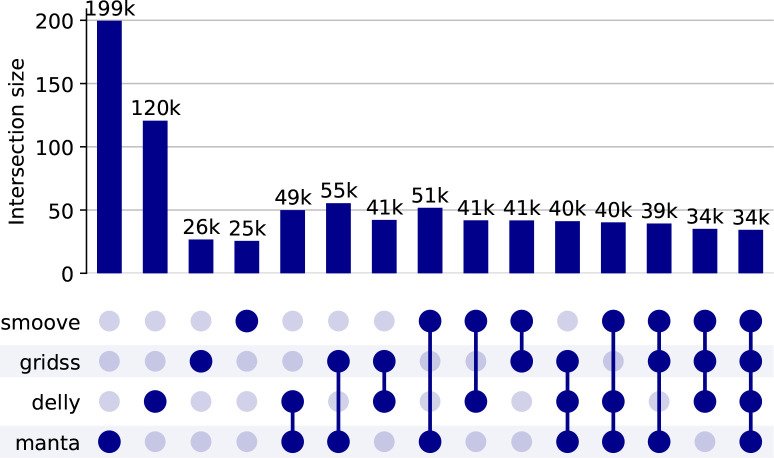
We ran five different SV tools – Manta (1.6.0–3) [17], Delly (1.1.6) [18], GRIDSS2 (v2.13,2)[19], smoove (v0.2.8) [20], and CNVPytor (v1.2.1) [21]) on the 1,091 high coverage WGS. Figure 1 shows an overview of the calls found by each tool and combinations of the tools. As discussed in the Methods, we use as our consensus set any SVs found by at least three tools, and this consensus set is discussed in the rest of the section.
Fig. 1:
Overview of the calls found by each SV caller, and combining them using SURVIVOR. See the methods section for details of choices. Note that in this graph we abuse the standard UpsetPlot representation – for example, 49,000 variants are detected by both Delly and Manta which may or may not be detected by other algorithms (in the standard UpsetPlot the implication would be that they are not detected by other approaches). Note also the anomalous results for gridss and smoove is caused by the fact that joint calling is not done by these tools and so estimating the actual number of SVs is very difficult.
2.1. Overview of calls of length ≤10,000bp
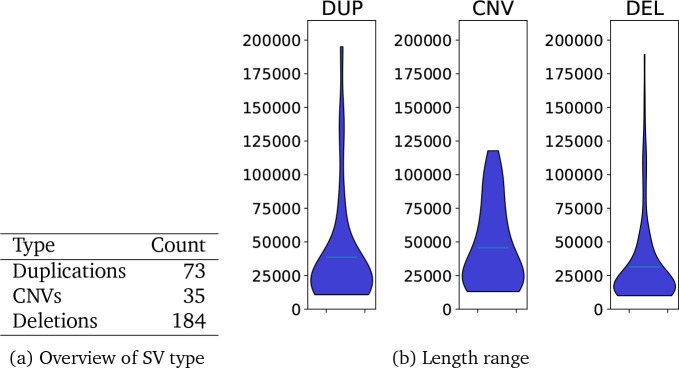
We first reviewed the calls that are ≤10,000bp in length and are so validated by three of the callers. There are 67,795 SVs ≤10,000bp in length, 36,981 in intergenic regions, and 30,814 in gene regions (including the untranslated regions). A total of 10,421 genes contained SVs. Categorised by type of structural variant, the number of variants are shown below in Table 1. However, note that due to the differences in the way that different callers and SURVIVOR call and handle variants that this is only a rough guide, determined as much by the power of the tools as the actual range of variants.
Table 1:
Number of variants by SV type
| Deletions | 50740 |
| Duplications | 13000 |
| Insertions | 2684 |
| Inversions | 1359 |
| Translocations | 12 |
As is expected most SVs occur at low frequency (22,551 singletons), 38% have a frequency less than 1%, however, 17% occur at greater than 10% frequency. More variants occured at higher frequencies compared to the gnomAD SV study where only 10% of variants had frequencies >1%. Table 2 below gives an overview of frequency.
Table 2:
Overview of frequency
| Range | Count |
|---|---|
|
| |
| (0–1%] | 41863 |
| (1–2%] | 5114 |
| (2–5%] | 5770 |
| (5–10%] | 3812 |
| (10–20%] | 3507 |
| (20–30%] | 1857 |
| (30–40%] | 1294 |
| (40–50%] | 987 |
| (50–60%] | 820 |
| (60–70%] | 666 |
| (70–80%] | 619 |
| (80–90%] | 545 |
| (90–100%] | 941 |
Novel SVs discovered
Comparison with the Database of Genomic Variants (DGV)[22] was performed using a cut-off of a reciprocal overlap of 70/90/95% and showed 6,414/9,483/11,673 new SVs respectively. Using a conservative 90% reciprocal overlap, 14% (9,483) of the dataset were novel African SVs, not previously described in DGV. This large number of unique variants highlights that African individuals harbour a substantial amount of previously undescribed structural variation. The population structure analysis described below further shows that not only do African individuals have unique variation overall, but many of these variants differ between African regions in sub-Saharan Africa.
Length spectrum
Showing the spectrum of lengths is complex. Multiple samples may have SVs that are slightly different or that the SV caller reports as slightly different. Furthermore, different tools estimate SVs of different lengths. Figure 2 and Table 3 show the distribution of lengths of SV calls and as expected we see the majority of SVs are small with 70% being less than 1,000bp.
Fig. 2:

Violin plot of distribution of lengths
Table 3:
Count by range
| Range | Count |
|---|---|
|
| |
| (0–250] | 30366 |
| (250–500] | 9012 |
| (500–1000] | 7966 |
| (1000–2000] | 6811 |
| (2000–5000] | 8761 |
| (5000–10000] | 4879 |
Per-individual variants.
On average there were 3073 variants per individual (range [2429–3572], standard deviation = 123). Figure 3 shows an overview.
Fig. 3:
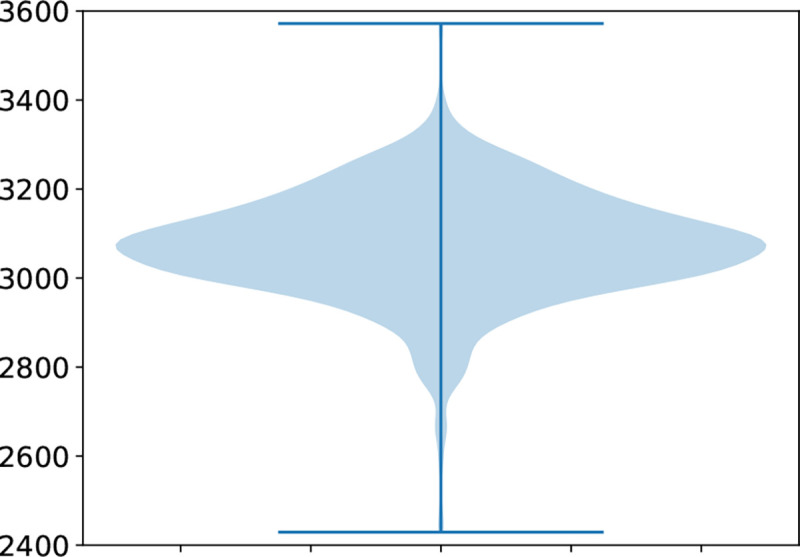
Overview of number of variants per individual.
2.2. Annotation and impact of the calls ≤ 10,000bp in length
Annotation by AnnotSV[23] shows that the majority of the SVs are likely benign, as they do not include genes previously implicated in disease, but that there are a number that may be deleterious. Table 4 summarises predictions made regarding American College of Medical Genetics (ACMG) classification. Table 5 shows the 15 variants predicted to be pathogenic. Of the 15 variants, nine of them are present in population SV databases and almost all these were found uniquely in African individuals in previous studies. With our large African cohort we can add additional frequency data to these variants. The deletion involving PTCH2 present in one individual in this cohort, is an interesting finding. It is accepted that PTCH2 a homolog of PTCH1 may also cause nevoid basal cell carcinoma syndrome, and one such variant is reported in ClinVar as a pathogenic, one-star classified variant (VCV000059967.1). However this association is under dispute as reports of healthy indiviudals with these variants have been published[24, 25]. This deletion involving PTCH2 may be another such case. The remaining five variants have not been previously reported in population databases and are all singletons except the deletion involving most of GNPTG and the beginning of UNKL. This variant was found in three individuals in the cohort and partially overlaps a variant reported in ClinVar as pathogenic, but is not present in any population databases. Most SNVs in GNPTG are associated with mucolipidosis III an autosomal recessive condition. With a deletion of most of the GNPTG gene these individuals are likely heterozygous carriers for a novel pathogenic African variant.
Table 4:
Number of SVs by ACMG predicted impact
| Count | |
|---|---|
| ACMG | |
|
| |
| Benign | 17078 |
| Uncertain | 46509 |
| Likely pathogenic | 138 |
| Pathogenic | 15 |
Table 5:
Genes with predicted pathogenic variants. In some cases there are two variants in a gene, which are listed in separate rows. In some cases, a variants overlaps two genes, listed in the same row.
| Chrom | Location | Length | SV Type | Genes | Disease | Significance |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 44837779 | 4532 | DEL | PTCH2 | ?Nevoid Basal cell carcinoma syndrome | Disputed[24, 25] |
| 5 | 78813976 | 2071 | DEL | ARSB | Mucopolysaccharidosis type VI | Known population variant |
| 6 | 64386321 | 5678 | DUP | EYS | Retinitis Pigmentosa | Novel |
| 6 | 109687391 | 6364 | DEL | AK9;FIG4 | Spermatogenic failure | Novel |
| 10 | 26171085 | 5164 | DEL | MYO3A | Deafness | Known African population variant |
| 11 | 5226163 | 1392 | DEL | HBB | B-Thalassemia | Known African population variant |
| 11 | 5226626 | 7464 | DEL | HBB;HBD | B-Thalassemia;Delta-Thalassemia | Known population variant |
| 12 | 102865716 | 5349 | DEL | PAH | Phenylketonuria | Known African population variant |
| 14 | 74298906 | 4595 | DEL | ABCD4 | Methylmalonic aciduria and homocystinuria | Known African population variant |
| 15 | 27975441 | 8930 | DUP | OCA2 | Albinism | Novel |
| 15 | 28017719 | 2958 | DEL | OCA2 | Albinism | Known African population variant |
| 16 | 173579 | 3773 | DEL | HBA1;HBA2 | Alpha-Thalassemia | Known African population variant |
| 16 | 1356771 | 8145 | DEL | GNPTG;UNKL | Mucolipidosis III gamma; N/A | Novel |
| 18 | 62152637 | 5065 | DEL | PIGN | Multiple congenital anomalies-hypotonia- seizures syndrome 1 | Known African population variant |
| 19 | 41417846 | 5857 | DEL | BCKDHA | Maple Syrup urine disease | Novel |
Table 6 summarises how structural variants overlap with genomic regions. Compared to population SNVs an unexpectedly high proportion of variants falls within the range of a gene. Almost all of these are however intronic variants. In their paper Collins et al. [2] showed that selection against variants is highly correlated to size of the SV and the functional impact. They calculated an adjusted proportion of singleton score to compare selection against different SV types, taking into account genic location. Intronic variants like other non-coding variants score close to null, second to intergenic variants, indicating little selection against them.
Table 6:
Genomic location of variants
| Count | |
|---|---|
| SV Category | |
|
| |
| coding | 3085 |
| full gene | 60 |
| intergenic | 36981 |
| intronic | 29105 |
2.3. Population Structure
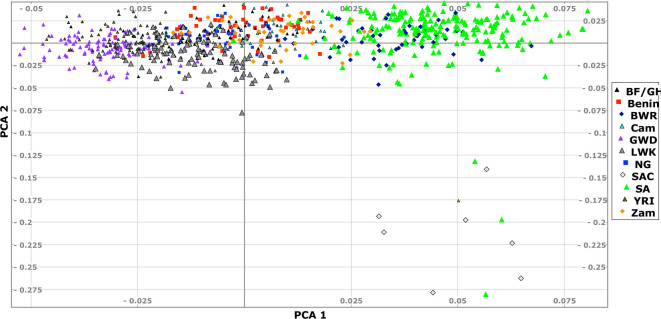
As expected, SVs differ across the continent. A principal component analysis (PCA) using PLINK2 [26] shows that populations can be differentiated with significant variation from south to centre and east to west – Figure 4 shows the PCA. Although the structure is not as defined (first two eigenvalues are 4.3 and 3.7) as for the SNVs shown in Figure A1 (first two eigenvalues 57 and 13), the structure is clearly visible.
Fig. 4:
Principal component analysis of the SVs less than 10,000 in length. Some populations are omitted for clarity. Key: ACB – 1000G African Caribbean in Barbados; Benin – H3Africa; BF/GH – H3Africa Burkina Faso and Ghana; BWR – H3Africa Botswana; CAM – H3Afica Cameroon; GWD – 1000G Gambian Western Districts; LWK – 1000H Luhya from Kenya; MSL – 1000G Mandinka Sierra Leone; NG – H3Africa Berom from Nigeria; SA – CBRL, H3Africa, Bantu-speakers from SAHGP South Africa; SAC – SA Coloureds in SAHGP; YRI — 1000G Yoruba; Zam – H3Africa Zambians.
2.4. Calls that are >10,000bp in length
This class of calls is reported separately because a different method was used for combining results, and the consequences of false positives with very long SV regions in annotation are more serious. Preliminary investigation showed that there were many SV regions much greater than 10,000bp, some with very strong evidence. While some of these calls are indicative of some structural rearrangement it is highly unlikely that they are as long as the callers indicate. Therefore, a different approach was taken.
First, we required that one of the depth-based approaches and another method validate the call. Second, we only analysed calls that are less than 200kbp long, since incorrect longer calls are likely to overlap many genes and pollute downstream analysis. We have however, made BED files available for all calls.
There were 291 SV regions in total. More detail can be found in Figure 5. A disproportionate number of these longer SV regions occur on chromosome X (158). Although with a few exceptions, the SV regions do not occur in the pseudoautosomal regions, this may be due to failure of the methods to properly account for chromosome X. Of the SV regions that do not appear on chromosome X, 41 completely covered a gene. The bed file of all the longer SV regions is available as supplementary data.
Fig. 5:
Overview of SV regions between 10,000bp and 200,000bp in length
3. Discussion
Structural variation research worldwide has been slowed by the fact that SVs are highly varied in nature and technically challenging to identify with high confidence. Given the universal under-representation of African genomic data in the public domain, baseline population SVs have been understudied in African populations. We analysed 1,091 high-coverage African genomes to address this representation gap.
Applying an ensemble call approach, we identified 67,795 SVs throughout the genome, with 10,421 genes having at least one SV. Categorising SVs by subtype is complex and biased by the capabilities of SV-detection algorithms, but with that caveat, 75% of the variants were deletions, 19% duplications, 4% insertions, 2% inversions, and 12 translocations.
There was considerable variation in SV size, with an expected majority of variants being small. About 41% are between 50–200bp, 25% between 200–800bp, 20% between 800–3,200bp, and 13% between 3,200–12,800bp. No SVs longer than that met our criteria for SV-detection although individual methods were able to detect longer SVs. These proportions support the premise that size is one of the greatest factors affecting selection against SVs [2]. Again, the numbers we report are biased by the ability of different algorithms to detect different length SVs, but they do align with expected proportions.
When looking at the frequency of variants detected we see a higher number of common variants than expected. 17% occur at greater than 10% frequency. More variants occured at higher frequencies compared to the gnomAD SV study where only 10% of variants had frequencies >1%. Given that African ancestry individuals have been known to have a greater number of SVs, this greater number of common variants may be a feature of the African SV landscape.
On average, the individuals had ≈ 3000 SVs; very few individuals varied more than ≈10% from this number. This number is lower than that reported in the gnomAD-SV study for African ancestry genomes. This could be due to the smaller size range included in the analysis. In this study, no variants larger than 12,800bp were included, where in the gnomAD-SV study they resolved variants from 50bp–10Mb. They also utilised different tools which will affect variants detected.
At first glance a surprising proportion of SVs were near genes – 42% occurred in introns, 4% affected coding regions with 53% being intergenic. As mentioned earlier, when considering the location of SVs their functional impact and size have to be taken into account. If SVs are small enough to only involve non-coding introns they are unlikely to be highly selected against and therefoore will be found at relatively high proportions. Collins et al. [2] utilised normalised singleton proportions to estimate these selection pressures and indicate that intronic variants are almost as unlikely to be selected against as intergenic regions.
With respect to predicted functionality, 68% of SVs have uncertain impact according to ACMG classification, 25% are predicted as benign, 0.2% likely pathogenic and only 15 are predicted as pathogenic. Given that the participants were all adults recruited as part of population cross-sectional or infectious disease studies, the relatively small number of pathogenic predictions is expected. Some of the pathogenic and likely pathogenic variants, identified here occur in a heterozygous form, and are therefore not disease causing. However, recording the frequency of these variants more accuratley remain important, as it gives a clearer indication of the mutation spectrum for the respective diseases that these variants in homozygous form contribute towards.
Using a conservative overlap, 6,414 of the 67,795 variants (9.5%) are new SVs compared to the DGV. One of the aims of our study was to add to the catalogue of known SVs in current references databases, as such databases have an underrepresentation of African genomic data. This reduces their usefulness to researchers studying individuals of African ancestry. High quality datasets of baseline population African variation, and analysis thereof, are of critical importance as they aid in the interpretation of potentially pathogenic variants in the study of genetic diseases in Africa.
In this study we encountered the multiple reasons why SV research has lagged behind SNV research. Technical and analysis challenges complicate accurate calling of SVs using short read sequencing technologies. Short read data (e.g., 150bp even if paired end) is not ideal for studying SVs. There are several signals that can be used – e.g., read depth, different parts of reads aligning to different places on a genome, unexpected difference where two paired-end reads align to the reference – but variants of increased length are more difficult to detect accurately. While moves to pan-genomes and reference graph representations may partially assist, there is no doubt that high quality long-read sequencing data is much better suited for SV detection, especially in understudied populations [27].n
Different SV-detection algorithms have different advantages as different approaches are employed. Given what is known of the relatively high error-rate (false positives and –negatives), ensemble calling has become common. How to combine callers is not straight-forward as different tools work better for different SV lengths. For example, algorithms that use read-depth as a criterion for CNVs are suitable for detecting variants several thousand bp in length, while other tools that use read content work well for SVs that are 50–70bp in length. Thus simply combining two such tools by requiring that both tools must detect a variant is bound to miss the variant while allowing either tool to detect the variant improves sensitivity but does not impact on false positive rate. We took a conservative strategy – as discussed in Section 5.3.3. Even SV calls with strong evidence using two different tools may lead to incorrect conclusions. For variants less than 1,000bp we requiried that three of four tools must detect an SV. This strict approach meant that in some cases variants called by fewer than three tools in an individual did not proceed to the final merging step, resulting in lower variant frequencies for known common variants.
Each SV-caller is very computationally intensive. Some tools only accept BAM files with individual samples in the range 70–90GB in size. Tools often produce intermediate data files that require significant extra disk space. All algorithms require processing the BAM files once and some twice, and the detection algorithms are complex, so this project has used several hundred thousand CPU hours and hundreds of TB of data. Exploring different approaches and parameters with a significant sample size is therefore a computational challenge.These challenges have significantly hindered the discovery of the African SV landscape and therefore we are making this SV dataset available to the global research community as an important resource to enhance the discovery of functional impact of SVs in the genome.
4. Conclusion
This paper introducing a database of SVs in African populations, contributes to knowledge of the landscape of human diversity with a focus on Africa. We have identified a large number of SVs previously undiscovered in other populations that, together with existing data sets, will assist future work into understanding the functional impact of genomic variation and will become a valuable resource for researchers and clinicians studying individuals with African ancestry.
5. Methods
5.1. Data sets
We used high coverage (> 30×), short read, WGS data from 1,091 individuals obtained from various sources, including the Human Heredity and Health in Africa (H3Africa) consortium [28], the 1000 Genomes Project [11], Simons Genome Diversity Project [29], the South African Human Genome Programme [30] and other unpublished African resources. Table 7 gives an overview of the populations represented in this study, and is further described in Appendix A. Figure A1 shows the regional distribution of the included datasets.
Table 7:
Sources of high-coverage WGS data for this study on 1,091 participants from multiple African populations. Depth=median depth of mapped sections of chromosomes 1–22. Data access details are given in the Data availability section.
| Countries | Data Source | n | Depth |
|---|---|---|---|
|
| |||
| H3Africa — 446 samples | |||
| Benin | University of Montréal | 50 | 37 |
| Burkina Faso, Ghana | AWI-Gen | 60 | 37 |
| Botswana | CAfGEN | 47 | 37 |
| Cameroon | University of Dschang | 26 | 35 |
| Cameroon | IFGeneRA | 24 | 36 |
| Mali | Clinical and Genetic Studies of Hereditary Neurological Disorders in Mali | 50 | 31 |
| Nigeria | Institute of Human Virology | 49 | 36 |
| Zambia | University of Zambia | 41 | 36 |
| South Africa | AWI-Gen | 100 | 37 |
|
| |||
| Other African collaborators: High coverage – 100 samples | |||
|
| |||
| South Africa | SA Human Genome Programme | 15 | |
| South Africa | Cell Biology Research Lab, NICD/Wits | 85 | 40 |
|
| |||
| Public data sets – 545 samples | |||
|
| |||
| Various | Simons Foundation | 31 | 37 |
|
|
|||
| Gambia | 1000 Genomes | 113 |
 35 35 |
| Kenya | 99 | ||
| Nigeria | 217 | ||
| Sierra Leone | 85 | ||
|
| |||
| Total | 1091 | ||
5.2. Data alignment to reference
The 1000 Genome Project VCFs were created using the protocol described in [31]. We applied the same protocol to all other samples. Briefly, reads were aligned with build GRCh38 using BWA (v.0.17- r1188) [32]. Finally, we performed alignment duplicate marking with GATK MarkDuplicates (v4.1.3) and quality score recalibration with GATK Quality Score Recalibrator (v4.1.3) to improve the quality of the alignment BAM file.
5.3. Variant calling
We used several SV and CNV calling tools and produced a consensus dataset, with the Nextflow scripts used accessible here: (https://github.com/shaze/h3acnvalls). We first describe the methodological approach for consensus calling and then detail the calling and merging steps.
5.3.1. Methodological approach
Producing a dataset from many samples using multiple tools requires merging both across samples and tools. Ideally, calling across samples would be done jointly to improve quality of calls and produce cleaner datasets. However, not all calling tools are capable of joint calling, especially on larger datasets. There are three complications in producing a merged dataset. First, SVs and CNVs vary more than single nucleotide variants (SNVs) – both in the type of variant and in the amount of the genome affected by the variant. For example, two samples may have insertions in overlapping regions of the genome that may not be identical. Whether these should be recorded as one or as two SV regions depends in principle on how similar the insertions are (do they differ in content very slightly and so likely are products of the same evolutionary event, or very differently and to what degree do the called regions overlap). Second, the difficulties in calling and limitations of calling tools and random variation in the sequencing of different samples (especially with short reads) means that even if two samples have exactly the same variant, a caller may report them slightly differently. Third, SVs and CNVs are complex, and the various tools used for merging have limits and are not always regularly updated. A simple case is that one caller may call a duplication (DUP) and another an insertion (INS), CNV, or breakpoint (BND) with minor differences in the actual sequencing. More complicated variant annotations (BND, translocation [TRA], inter-chromosomal translocation [CTX], intra-chromosomal translocation [ITX]) are challenging because a caller may produce an annotation that the other does not recognise, and the rules for how combinations are performed may be hidden in code. These considerations impact the decisions made in the protocols described below.
Furthermore, even tools capable of “joint calling” produce outputs that require postprocessing. Joint calling is generally done by first running the tool independently on each sample, merging all the sites where SVs were detected across all samples, and then regenotyping the individual BAMs on the merged site list. For example, a tool detects a duplication in sample A on chromosome 1 at location 10,000 to 10,300, and also detects a duplication in sample B on chromosome 1 at location 10,010 to 10,298. In reality, these two SVs are likely the same SV, but the tools treat them as independent SVs. In the re-genotyping step both sites are checked for all samples – there is a high-probability that both SVs will be called on both samples. On sample A, the first deletion may have a better quality score than the second deletion, but the second deletion will also have a very good score. To remedy this, we use ‘stitching’ as described below.
5.3.2. Calling
Many tools have been developed to call SVs from short-read WGS data. As each tool has its own limitations and advantages, the use of a variety of SV/CNV-calling algorithms can generate a more comprehensive dataset. As such, SV/CNV calling was performed using several different tools. We then combined the results from the different tools and performed joint calling using SURVIVOR (Figure 6).
Fig. 6:
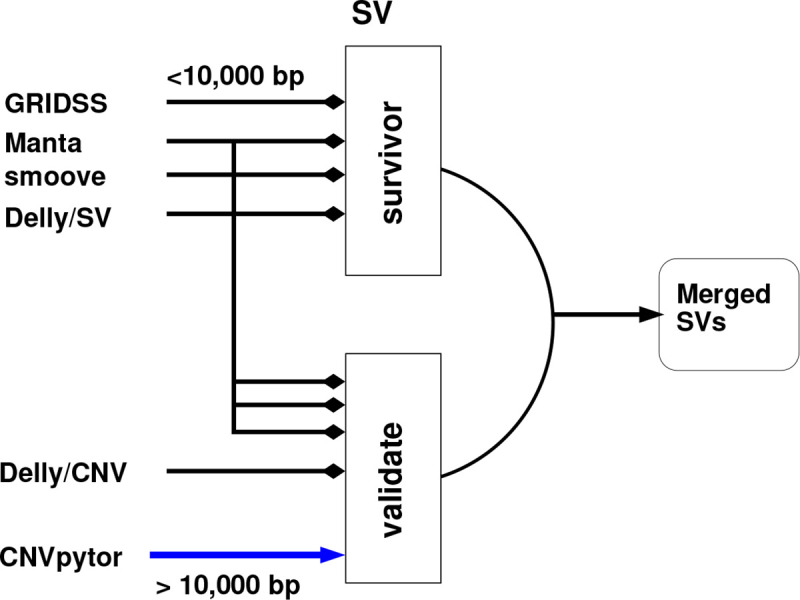
Overview of merging process: Variants that are less than 10,000bp in length must be supported by at least 3 tools. Variants greater than 10,000 bp must be supported by CNVPytor, a depth-based method, and one other tool.
Five tools were used because they make use of different combinations of approaches for calling SVs and CNVs – Manta (1.6.0–3) [17], Delly (1.1.6) [18], GRIDSS2 (v2.13,2)[19], smoove (v0.2.8) [20], and CNVPytor (v1.2.1) [21]). Delly has two modes of operation: SV detection and CNV detection (which will be referred to as Delly/SV and Delly/CNV, respectively). CNVPytor detects CNVs while the other tools detect SVs.
GRIDSS2 extracts reads that are potentially indicative of SVs from BAM files (split reads, discordantly aligned reads, soft-clipped reads and reads anchored only on side of the read). A positional de Bruijn graph is constructed and analysed to identify the SVs. We followed the recommended approach for calling germline SVs except that (a) we did not do joint calling (since this is done with SURVIVOR and the GRIDSS developers suggest that joint calling of hundreds of samples is computationally infeasible) or exclude any regions; and (b) we did not exclude the ENCODE Data Analysis Center blacklist of problematic regions in the genome [33] since we did this jointly across all data calls. We used the GRIDSS2-recommended post-analysis of break-end (BND) variants to determine simple variant types like INS, DEL and DUP and used Truvari [34] to merge variants that were close to each other.
Delly performs SV calling using a combination of split-read and read-pair information. Briefly, for both Delly algorithms individually, the process is: (a) each sample is separately called; (b) all sites found across all individuals are merged; (b) each sample is called again using the common sites to improve accuracy; (c) the resultant calls are merged and (d) filtered using Delly’s SV filter, and then (e) split into individual files for later processing. Delly has a separate read-depth CNV calling method –– the results of the SV caller are used to refine the start and end points of the variant. We used the recommended protocol for detecting germline SVs with Delly: SVs are called using the BAM files of individual samples, and the call sites between samples are merged. The individual BAM files are regenotyped using the combined merged list. For CNV calling, each BAM is called independently using the SV calls from that sample as auxiliary information, the sites are merged and then each BAM is re-genotyped on the combined site list.
Manta [17] uses break-end information as its primary SV detection mechanism. SVs are detected per sample; there is no type of joint calling. The first phase of the algorithm builds a break-end graph using the aligned reads. Once the break-end graph is created, reads aligning to each break are analysed and assembled, and evidence for different types of SVs is evaluated.
Smoove [20] is a wrapper and workflow for LUMPY [35]. LUMPY uses read-pair, split-read, and read-depth information to make probabilistic calls of SVs. Joint calling is supported. We followed the recommended workflow of (a) calling SVs on each BAM separately; (b) merging all called sites; (c) re-genotyping each BAM using the merged site list; and (d) combining (“smoove paste”). We then annotated and filtered out poor quality calls.
Counts of variants for individual tools
For tools that support joint calling, this can be computed directly from the joint output. For GRIDSS and Manta, for which joint calling is not supported, after removing poor quality calls the method proposed by Truvari [34] was followed: the VCF files were merged using bcftools 1.15 [36]. The resulting file was split into deletions and non-deletions and then Truvari was used on each file to merge SVs from different samples that overlap. The resulting files were then merged and sorted. The parameters can be found on our Nextflow scripts.
CNVPytor is primarily a depth-based CNV caller. We computed read-depth and called CNVs independently on each BAM and using a window size of 1,000 bp. Although, CNVPytor’s merge in principle supports some sort of joint calling we were unable to produce useful output. The results of CNVPytor’s calling is in windows of size 1,000 bp resulting in imprecise variant boundaries, which impacted how we combined results.
5.3.3. Merging results
When combining the subsequent variant lists from each tool, we only used variants that passed quality control according to each tool specification. Although there is an argument for including variants with weaker evidence when doing ensemble calling, we chose to be strict. After preliminary experimentation, we used the hybrid approach for ensemble calling, depending on variant length, shown in Figure 6. After preliminary analysis, we filtered out insertions greater than 1,000bp, deletions greater than 10,000bp, inversions greater than 20,000bp and duplications greater than 20,000bp.
For variants that are ≤ 10,000bp, we use SURVIVOR [37] to select variants that were supported by any three of the following tools: Manta, Delly/SV, GRIDSS and smoove. There are relatively few variants larger than this cutoff point. However, we excluded longer variants: manual inspection of examples showed very high-quality evidence for some sort of SV, but the lengths were implausible and in some cases caused thousands of genes to be implicated in an SV. The risk of false positives or misinterpretation outweighs the value of such calls.
Different support values s (2, 3, 4) were tested, which means how many tools called the same variant. For each sample, SURVIVOR was used to combine the results of the four different tools so that at least s of the tools supported the call for a variant. SURVIVOR then combines the per-sample results to produce an overall jointly called file. Finally, the ENCODE DAC regions are excluded. For the main analyses, a variant must be called by at least three tool (s = 3) was used. Preliminary analysis showed that different tools annotate variants differently (e.g., INS, DUP, BND). As such, we used SURVIVOR’s mode of ignoring the variant type to decide to call a consensus.
For longer variants (>10,000bp), we required that they be called by CNVPytor and at least one of the other callers. This means that such calls are supported by both read-depth and sequence content. As CNVPytor has very imprecise calls, SURVIVOR was inappropriate, and we used our own script validate call. Therefore, we took all variants longer than 10,000 bp found by Manta, Delly/SV, Delly/CNV, and smoove and selected those that had at least a 90% overlap with a call from CNVPytor. GRIDSS calls were all shorter than 10,000bp.
5.4. Variant annotation
Annotation of the SVs ≤ 10,000 bp was performed using AnnotSV [23]. AnnotSV reports whether SV regionss are intergenic, coding, or in another genic or non-genic region. It also predicts variant classification according to the ACMG guidelines [38]. We merged SV regions that overlapped by at least 70%, covered the same genes, and had the predicted SV type (either identical or both [DUP, CNV] or [DEL, CNV]).For larger SV regions, AnnotSV was unable to successfully complete the annotation.
5.5. Comparison to Established Databases
To determine which variants in our data set are novel, we compared them with the DGV [22], version (GRCh38 hg38 variants 2020-02-25.txt) as it is the most comprehensive publicly available source of SV/CNV, and all variants in the database align with GRCh38. We used a reciprocal overlap of 70/90/95%.
Supplementary Material
Funding
Whole genome sequencing of H3Africa participants was supported by a grant from the National Human Genome Research Institute, National Institutes of Health (NIH/NHGRI, Grant U54HG003273). The AWI-Gen Collaborative Center is funded by the NIH/NHGRI (Grant U54HG006938) as part of the H3Africa Consortium. MR is a South African Research Chair in Genomics and Bioinformatics of African Populations hosted by the University of the Witwatersrand, funded by the Department of Science and Innovation (South Africa), and administered by National Research Foundation of South Africa (NRF). The TrypanoGEN project was funded by the Wellcome Trust (study number 099310/Z/12/Z). The Collaborative African Genetics Network (CAfGEN) is funded by the NIH/NHGRI (Grant 1U54AI110398). The African Collaborative Center for Microbiome and Genomics Research is funded by the NIH/NHGRI (Grant U54HG006947). The primary work relating to DNA sample processing and WGS at the Cell Biology Research Lab is based on research supported by grant awards from the Strategic Health Innovation Partnerships (SHIP) Unit of the South African Medical Research Council, a grantee of the Bill and Melinda Gates Foundation, and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (84177). LC was supported by a grant from GlaxoSmithKline R&D Ltd to Wits Health Consortium. GR, SH and compute costs were partially supported by grants by the NIH/NHGRI to the the Pan-African Bioinformatics Network for H3Africa (U24HG006941). EKW and ZL were supported by the National Institute Of Mental Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Numbers U01MH115483 and U01HD114537. The funders had no role in design, collection, analysis, interpretation or writing of this manuscript, and the opinions, findings, and conclusions or recommendations expressed in this manuscript are solely the responsibility of the authors and not necessarily to be attributed to GSK, NRF, Wellcome Trust, or the NIH.
Footnotes
Additional Declarations: There is NO Competing Interest.
Ethics Statement
No new data was generated specifically for this project — this is secondary analysis of data that had been generated for other purposes. The H3A AWI-Gen Study (H3A data from Ghana, Burkina Faso and South Africa) was approved by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (Wits) (protocol numbers M121029 and M170880), and each contributing Centre obtained additional local ethics approval, as required. The H3A Benin study was approved by the Comité d’éthique de la recherche, Université de Montréal. The H3A CAfGEN study (Botswana) was approved by the IRB of the Ministry of Health of the Republic of Botswana (PPPME-13/18/1). The H3A TrypanoGEN Study (Cameroon component) was approved by the Comité National D’Ethique de la Recherche pour la Santé Humaine of the Republic of Cameroon (No 2013/11/364/L/CNERSH/SP). The H3A ACCME Study (Nigeria) was approved by the National Health Research Ethics Committee of Nigeria (NHREC/01/01/2007–29/11/2016). The H3A TrypanoGEN Study (Zambian component) was approved by the Biomedical Research Ethics Committee of the University of Zambia (FWA00000338). The data from the Cell Biology Research Lab, NICD/Wits was generated by a study approved by the Wits Human Research Ethics (Medical) Committee (protocol number M140926).
Data availability
The results of our analysis are available at https://github.com/shaze/h3acnvcalls: BED files are available identifying all SV regions from all the analyses shown in Figure 1.
All data are described in more detail in the Supplementary Material. The 1000 Genomes data and the SGDP data are publicly available. The other datasets used in this study are available from the European Genome-Phenome Archive (https://ega-archive.org/) on application to the relevant Data Access Committees: H3Africa project — EGAD00001006418 (South Africa), EGAD00001004220 (ACCME/Berom), EGAD00001004316 & EGAD00001004393 (Cameroon), EGAD00001004334 (Mali), EGAD00001004448 (AWI-Gen West Africa), EGAD00001004505 (Botswana), EGAD00001004557 (Benin); EGAD00001004220 (Zambia); Wits — EGAD00001007589 (South Africa); SAHGP — EGAD00001003791 (South Africa).
References
- [1].Abel H, DE L, Regier Aea. Mapping and characterization of structural variation in 17,795 human genomes. Nature. 2020;583:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Collins R, Brand H, Karczewski K, Zhao X, Alföldi J, Francioli L, et al. A structural variation reference for medical and population genetics. Nature. 2020. 5;581:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sudmant P, Mallick S, Nelson BJ, Hormozdiari F, Krumm N, Huddleston J, et al. Global diversity, population stratification, and selection of human copy-number variation. Science. 2015. 9;349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Y, Glessner J, Coe B, Li J, Mohebnasab M, Chang X, et al. Rare copy number variants in over 100,000 European ancestry subjects reveal multiple disease associations. Nature Communications. 2020. 12;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Coe B, Witherspoon K, Rosenfeld J, van Bon B, Vulto-van Silfhout A, Bosco P, et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nature Genetics. 2014;46(10):1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Collins R, Glessner J, Porcu E, Lepamets M, Brandon R, Lauricella C, et al. A cross-disorder dosage sensitivity map of the human genome. Cell. 2022;185(16):3041–55.e25. Available from: https://www.sciencedirect.com/science/article/pii/S0092867422007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Conrad D, Pinto D, Rea Redon. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McCarroll S, Altshuler D. Copy-number variation and association studies of human disease. Nature Genetics. 2007;39(S7):S37–S42. [DOI] [PubMed] [Google Scholar]
- [9].Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews T, et al. Global variation in copy number in the human genome. Nature. 2006. 11;444:444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Prasad A, Merico D, Thiruvahindrapuram B, Wei J, Lionel AC, Sato D, et al. A Discovery resource of rare copy number variations in individuals with autism spectrum disorder. G3: Genes, Genomes, Genetics. 2012. 12;2:1665–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sudmant PH, Rausch T, Gardner EJ, Handsaker RE, Abyzov A, Huddleston J, et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. Available from: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tishkoff S. The Genetic Structure and History of Africans and African Americans. Science. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Choudhury A, Aron S, Botigué LR, Sengupta D, Botha G, Bensellak T, et al. High-depth African genomes inform human migration and health. Nature. 2020;586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nyangiri O, Noyes H, Mulindwa J, Ilboudo H, Kabore JW, Ahouty B, et al. Copy number variation in human genomes from three major ethno-linguistic groups in Africa. BMC Genomics. 2020. 4;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bope CD, Chimusa ER, V N, K MG, de Vries J, Wonkam A. Dissecting in silico Mutation Prediction of Variants in African Genomes: Challenges and Perspectives. Frontiers in Genetics. 2019;10. Available from: 10.3389/fgene.2019.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lumaka A, Carstens N, Devriendt K, Krause A, Kulohoma B, Kumuthini J, et al. Increasing African genomic data generation and sharing to resolve rare and undiagnosed diseases in Africa: a call-to-action by the H3Africa rare diseases working group. Orphanet Journal of Rare Diseases. 2022;17(230). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen X, Shulz-Trieglaff O, Shaw R, Barnes B, Schlesinger F, Kalberg M, et al. Manta: rapid¨ detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32(8):1220–2. [DOI] [PubMed] [Google Scholar]
- [18].Rausch T, Zichner T, Schlattl A, Stuetz AM, Benes V, Korbel JO. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics. 2012;28(18):i333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cameron D, Baber J, Shale C, Valle-Inclan J, Besselink N, van Hoeck A, et al. GRIDSS2: comprehensive characterisation of somatic structural variation using single breakend variants and structural variant phasing. Genome Biology. 2021. 12;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pedersen BS, Layer R, Quinlan AR. smoove: structural-variant calling and genotyping with existing tools; 2020. https://github.com/brentp/smoove.
- [21].Suvakov M, Panda A, Diesh C, Holmes I, Abyzov A. CNVpytor: a tool for copy number variation detection and analysis from read depth and allele imbalance in whole-genome sequencing. GigaScience. 2021;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].MacDonald J, Ziman R, Yuen R, Feuk L, Scherer S. The database of genomic variants: a curated collection of structural variation in the human genome. Nucleic Acids Research. 2013. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Geoffroy V, Guignard T, Kress A, Gaillard J, Solli-Nowlan T, Schalk A, et al. AnnotSV and knotAnnotSV: a web server for human structural variations annotations, ranking and analysis. Nucleic Acids Research. 2021. May;48:W21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Altaraihi M, Wadt K, Ek J, Gerdes A, Ostergaard E. A healthy individual with a homozygous PTCH2 frameshift variant: Are variants of PTCH2 associated with nevoid basal cell carcinoma syndrome? Human Genome Variation. 2019. 02;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smith M, Evans D. PTCH2 is not a strong candidate gene for gorlin syndrome predisposition. Familial Cancer. 2022;21(3):343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Purcell S, Chang C. PLINK 2; 2023. www.cog-genomics.org/plink/2.0/, accessed July 2023.
- [27].Reis A. The landscape of genomic structural variation in Indigenous Australians. Nature. 2023;624(7992):602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Consortium” TH. Enabling the genomic revolution in Africa. Science. 2014;344(6190):1346–8. Available from: 10.1126/science.1251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mallick S, Li H, Lipson M, Mathieson I, Gymrek M, Racimo F, et al. The Simons Genome Diversity Project: 300 genomes from 142 diverse populations. Nature. 2016;538(7624):201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Choudhury A, Ramsay M, Hazelhurst S, Aron S, Bardien S, Botha G, et al. Whole-genome sequencing for an enhanced understanding of genetic variation among South Africans. Nature Communications. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Regier A, Farjoun Y, Larson D, Krasheninina O, Kang H, Howrigan D, et al. Functional equivalence of genome sequencing analysis pipelines enables harmonized variant calling across human genetics projects. Nature Communications. 2018;9(4038). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arχiv; 2013. arXiv:1303.3997v2. [Google Scholar]
- [33].Amemiya HM, Kundaje A, Boyle AP. The ENCODE Blacklist: Identification of Problematic Regions of the Genome. Scientific Reports. 2019;9(9354). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].English A, Menon V, Gibbs R, Metcalf G, Sedlazeck F. Truvari: refined structural variant comparison preserves allelic diversity. Genome Biology. 2022;23(271). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Layer RM, Chiang C, Quinlan AR, Hall IM. LUMPY: a probabilistic framework for structural variant discovery. Genome Biology. 2014;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Danecek P, Bonfield J, Liddle J, Marshall J, Ohan V, Pollard MO, et al. Twelve years of SAMtools and BCFtools. GigaScience. 2021. 02;10(2). Giab008. Available from: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jeffares D. Transient structural variations have strong effects on quantitative traits and reproductive isolation in fission yeast. Nature Communications. 2017. 1;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Riggs E, Andersen E, Cherry A, Kantarci S, Kearney H, Patel A, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genetics in Medicine. Available from: 10.1038/s41436-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].M R, N C, E T, G A, V B, S D, et al. H3Africa AWI-Gen Collaborative Centre: a resource to study the interplay between genomic and environmental risk factors for cardiometabolic diseases in four sub-Saharan African countries. Global Health Epidemiology and Genomics. 2016;1(e20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Byrska-Bishop M, Evani U, Zhao X, Basile A, Abel H, Regier A, et al. High-coverage whole-genome sequencing of the expanded 1000 Genomes Project cohort including 602 trios. Cell. 2022;185(18):3426–40.e19. Available from: https://www.sciencedirect.com/science/article/pii/S0092867422009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yun T, Li H, Chang PC, Lin M, Carroll A, McLean C. Accurate, scalable cohort variant calls using DeepVariant and GLnexus. Bioinformatics. 2021. 01;36(24):5582–9. Available from: 10.1093/bioinformatics/btaa1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chang C, Chow C, Tellier L, Vattikuti S, Purcell S, JJ L. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The results of our analysis are available at https://github.com/shaze/h3acnvcalls: BED files are available identifying all SV regions from all the analyses shown in Figure 1.
All data are described in more detail in the Supplementary Material. The 1000 Genomes data and the SGDP data are publicly available. The other datasets used in this study are available from the European Genome-Phenome Archive (https://ega-archive.org/) on application to the relevant Data Access Committees: H3Africa project — EGAD00001006418 (South Africa), EGAD00001004220 (ACCME/Berom), EGAD00001004316 & EGAD00001004393 (Cameroon), EGAD00001004334 (Mali), EGAD00001004448 (AWI-Gen West Africa), EGAD00001004505 (Botswana), EGAD00001004557 (Benin); EGAD00001004220 (Zambia); Wits — EGAD00001007589 (South Africa); SAHGP — EGAD00001003791 (South Africa).