Abstract
Background
Current assessments on topical treatment attributes in actinic keratosis (AK) do not evaluate safety, effectiveness, and satisfaction from both clinician and patient perspectives, creating an unmet need for more comprehensive AK-specific measures that fully capture the patient experience.
Objective
To develop an actinic keratosis–specific expert panel questionnaire (AK-EPQ) of patient-reported outcomes and clinician-reported outcomes for use in research studies.
Methods
Using interviews of patients with AK and targeted literature reviews, a 9-person consensus panel of dermatologists with expertise in AK treatment was convened to develop the AK-EPQ to assess AK-specific patient-reported outcomes and clinician-reported outcomes.
Results
Nine expert advisers achieved consensus on 11 AK-EPQ items that encompass patient and clinician perspectives of treatment-related local skin reactions, clinical and cosmetic outcomes associated with AK, and satisfaction with treatment; the AK-EPQ will be first implemented in the Patient-Reported Outcomes for Actinic Keratosis study (NCT05260073).
Limitations
The AK-EPQ does not directly measure quality of life, although it can be used with validated quality of life instruments.
Conclusion
The newly developed AK-EPQ elicits insights into the patient and clinician experience with AK treatments. Comparative probing of these perspectives may help optimize precision medicine in AK treatment.
Key words: adherence, clinical outcomes, clinician-reported outcomes, consensus panel, cosmetic outcomes, health-related quality of life, local skin reactions, patient-reported outcomes, patient satisfaction
Capsule Summary.
-
•
Patient satisfaction, adherence, and local skin reactions are treatment concerns in actinic keratosis; thus, the actinic keratosis expert panel questionnaire was developed to characterize actinic keratosis–specific perspectives on treatment attributes.
-
•
This newly developed questionnaire may be used to supplement validated quality-of-life measures in patient-centered outcomes research in actinic keratosis.
Introduction
Timely, effective treatment of actinic keratoses (AKs) is important because there is no reliable way to predict which of these lesions on chronically sun-exposed skin will become cancerous.1,2 In a study of patients at high risk of skin cancer, 65% of squamous cell carcinomas and 36% of basal cell carcinomas arose from AKs,3 suggesting that many skin cancers may be prevented if AK is recognized and treated early. In addition to the risk of cancer, AKs may impact quality of life (QoL) because they often occur in highly visible areas, primarily sun-exposed areas such as the face, scalp, and arms.1,4,5 Additionally, some topical treatments for AKs such as 5-fluorouracil (5-FU) and imiquimod are associated with uncomfortable and visible local skin reactions (LSRs), which have been linked to poor treatment tolerability and low treatment satisfaction.5, 6, 7 The incidence of severe LSRs, including redness, pain, erosions, and ulcerations, contributes to the poor tolerability of these treatments and may lead to dissatisfaction among patients.7 Patients have reported that the appearance of their AKs affects social interactions.8 This is further evidenced in a recent series of in-depth qualitative interviews with 13 patients (mean age, 58.7 years; 53.8% female and 46.2% male) in the United States who have AK. Nearly all of these patients (92.3%) reported experiencing discomfort and negative effects due to LSRs associated with topical AK treatments (including 5-FU), which were noted to impact both social interactions and work life.4 Patients also reported shyness during work interactions (53.8%), and some effects of AK and AK treatment-related LSRs (46.2%) on work experience.4 Treatment-related adverse effects may persist for weeks depending on the treatment used, leading to lower treatment adherence, lower treatment efficacy, and decreased likelihood of treating future lesions.1,2,9
Because of the chronic nature of AK, long-term achievement of treatment outcomes is important and depends on patient perceptions of the risks and benefits of treatment.10 Most of the patients in the aforementioned series of interviews indicated a desire for future AK treatments that have both less severe LSRs (76.9%) and shorter treatment durations (61.5%).4 Easier and less frequent application (23.1%) were also considered desirable treatment attributes.4 The relatively long duration of treatment (>1 week) and adverse effects of many topical therapies compound the challenges with maximizing patient adherence.1,2,10,11 In a 2021 expert consensus panel, 10 of 10 dermatologists agreed that patients prefer topical treatments for AK that require fewer applications.2
Clinicians agree that concerns regarding patient adherence, satisfaction, and severity of LSRs are important to consider when prescribing topical AK therapy, and the shared perspectives of both clinicians and patients should be taken into account to develop a more personalized treatment strategy.12 Fostering effective clinician-patient communication on perceptions of treatment attributes may reduce patient distress and improve QoL, treatment satisfaction, and adherence13; as seen in other chronic conditions; therefore, assessments that encompass both perspectives and strengthen this communication may provide benefits to patients and clinicians.13
A 2019 review of AK treatment studies found that LSRs were common across topical treatments.1 In 3 studies of 5-FU, the rates of LSRs ranged from 8.8% (in a study of 33 patients using 5-FU and salicylic acid once daily for ≤6 weeks) to 92.0% (in a study of 187 patients using 5-FU and salicylic acid once daily for ≤12 weeks).1 Severe LSRs had a prevalence of up to 27.8%.1 In a study of 185 patients treated with 3% diclofenac twice daily for ≤12 weeks, the overall prevalence of LSRs was 62.7%, and the prevalence of severe LSRs was 11.9%.1 In another study of twice-daily diclofenac treatment for 3 or 6 months (N = 418), 13.6% of patients experienced severe LSRs.1 There were 8 studies of imiquimod included in the review, with rates of all LSRs and severe LSRs ranging from 95.2% to 100% and 8.8% to 58.5%, respectively.1 In clinical studies of 5-FU and diclofenac, discontinuation rates due to LSRs were up to 9.1% and 13.6%, respectively.1 Furthermore, in clinical studies, tirbanibulin 1% ointment demonstrated efficacy, had the shortest treatment course (once daily, 5 consecutive days) compared with AK topical treatments, and was well tolerated with mostly mild-to-moderate LSRs; less than 10% of patients administered tirbanibulin experienced severe LSRs, which all resolved spontaneously.2,14,15 The most common LSRs were mild-to-moderate erythema and flaking/scaling.15 However, these reported rates of adverse outcomes do not fully capture the patient experience and the aspects of treatment that can be targeted for improvement. Table I.
Table I.
Items from the actinic keratosis–specific expert panel questionnaire16
| Cosmetic outcomes |
|
| Effects of LSRs |
|
| Satisfaction and future preferences |
|
| Clinician Reported: effectiveness and photodamage severity |
|
AK, Actinic keratosis; LSR, local skin reaction.
Item asked only among patients who have used another topical treatment, for comparative probing of previous and current treatment satisfaction.
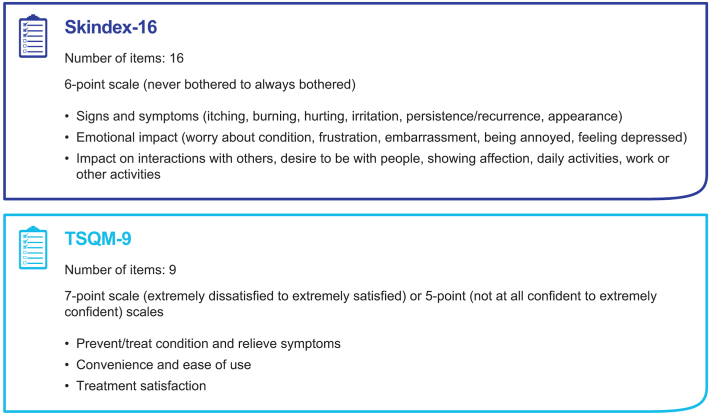
The prevalence of AK is estimated to be ∼14% of all dermatology visits in the United States, and given that AK is the most common visit diagnosis among dermatologists , a specific treatment-related patient-reported outcome (PRO) measure is warranted.2,17,18 Validated PRO measures that may be used to assess AK include the Abbreviated Treatment Satisfaction Questionnaire for Medication and the dermatology-specific Skindex-16 (Fig 1).5
Fig 1.
Comparison of questionnaires used to assess outcomes. TSQM-9, Abbreviated Treatment Satisfaction Questionnaire for Medication.
Skindex-16 is a validated, internally consistent, reliable, and responsive instrument for measuring the impact of dermatologic conditions on patient QoL.19 Similar to the AK QoL questionnaire, Skindex-16 does not differentiate between the burdens of the underlying condition and those of treatment adverse effects. Additionally, it does not capture concerns common with AK, such as skin texture and photodamage and the severity and duration of LSRs. Currently, there are no PRO measures tailored specifically to the adverse effects commonly seen with AK treatment, and therefore the impact of LSRs may not be fully captured. Because AK is a chronic condition, patients often experience recurrence and require repeated treatment, highlighting the need to assess how the treatment itself can influence future willingness to seek repeat treatment.3,5,10 Comparing current and previous AK treatments would be useful in gauging the relative impact of different treatments. An AK-specific PRO instrument may add new insight into future clinical studies of AK treatments. These outcomes could guide clinical recommendations and ensure that patient concerns regarding efficacy, safety, and practicality are all recognized. Moreover, the solicitation of clinician perceptions via the same instrument can provide clinician-reported outcomes (ClinROs). These outcomes could facilitate a comparison of PROs with ClinROs on treatment attributes and may enhance clinician-patient communication, providing great utility among practitioners.13 Clarity of communication between the physician and patient during AK treatment has been shown to contribute to patient satisfaction over the treatment course, and in some treatment scenarios, can improve adherence.13
Thus, an expert panel was convened to develop an AK-specific measure of PROs and select ClinROs for comparative use in future clinical studies of AK treatments; this resulted in a simple and easy-to-use assessment of AK status for clinicians that better characterizes patient burden and can enhance clinician-patient communication. Eleven questions were designed to solicit patient and clinician perspectives of treatment-related LSRs, clinical and cosmetic outcomes associated with AK, and overall satisfaction with treatment. Here, we present the resulting actinic keratosis–specific expert panel questionnaire (AK-EPQ).
Materials and methods
Actinic Keratosis-Expert Panel Questionnaire
Input from targeted literature reviews and interviews of patients with AK4 was used to initially identify 11 AK-EPQ items. In the first round, AK-EPQ items were distributed to the panel for discussion of each item and to solicit comments individually and as a group. A 9-person consensus panel of dermatologists with expertise in the treatment of AK was convened and met via Zoom (Zoom Video Communications) in October 2021 to establish consensus on AK-EPQ items.16,20
The panel discussed 9 PROs regarding cosmetic outcomes, convenience and ease of use of new treatment, overall satisfaction with new treatment, and likelihood of future use (Table I). Refinements were suggested to items pertaining to retreatment to capture relative satisfaction associated use of their current versus previous topical treatment for AK.16 Other changes included the description of skin texture (“how your skin feels in terms of roughness, bumpiness, scaliness”), adding duration of skin reactions, and updating the description of daily activities to “shopping, bathing, social engagements, scheduling vacations, outdoor activities, activities at work, attendance at work, etc.”16 The scales for several of the AK-EPQ items were also refined.16
The panel also discussed 2 ClinROs encompassing Investigator Global Assessment of AK and severity of skin photodamage (Table I). A description of photodamage was also added to item 11 (“alterations in the structure, function, and appearance of the skin as a result of prolonged or repeated exposure to UV radiation from the sun or other UV sources”).16 For the AK Investigator Global Assessment (item 10), the panel suggested defining specific cutoffs for AK clearance based on clinical judgment. It was proposed that the 9 PROs items also be utilized as ClinROs to capture responses on all domains of the AK-EPQ from both the patient and clinician perspective.
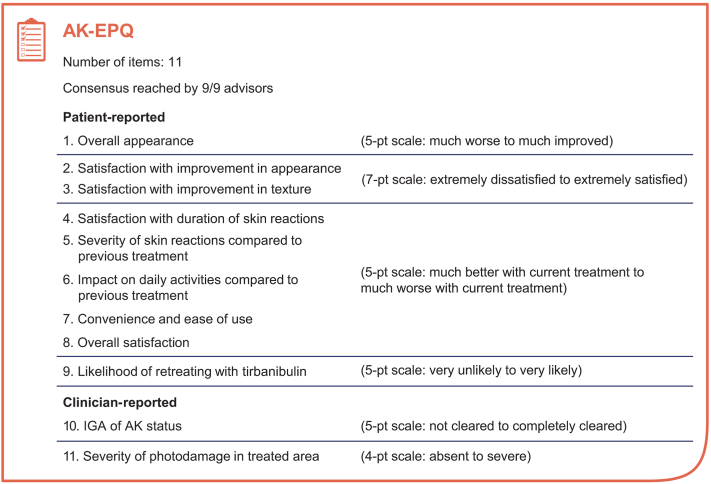
Results
Following feedback and refinement discussions, the panel unanimously achieved consensus and approved each of the 11 items (Table I) on the AK-EPQ (Fig 2), with the intention that the AK-EPQ will be first implemented in the Patient- and Clinician-Reported Outcomes for Tirbanibulin Effectiveness and Safety in Actinic Keratosis study (NCT05260073) and used in future research studies, with the caveat that item 9 would be tailored for the individual study and treatment.21
Fig 2.
Summary of actinic keratosis–specific questionnaire for the Patient- and Clinician-Reported Outcomes for Tirbanibulin Effectiveness and Safety in Actinic Keratosis study. AK, Actinic keratosis; AK-EPQ, actinic keratosis–specific expert panel questionnaire; IGA, Investigator’s Global Assessment.
Discussion
We convened a panel of dermatologists to discuss PROs and ClinROs relevant to AK and the study of AK treatments. The panel reached consensus on the 11-item AK-EPQ that assesses changes in skin appearance and texture, the severity and impact of LSRs, the convenience of the prescribed treatment, and overall treatment satisfaction. Items in the patient and clinician version of the AK-EPQ provide PROs and ClinROs that can be used for comparison. Items 3 through 8 refer to only patients who have previously received another topical treatment and are used to comparatively probe PROs and ClinROs in relation to current treatment and previous treatments. This comparative assessment provides a useful measurement of treatment satisfaction. The clinician version of these questions refers to clinician experience and observations of treatment effects among their patients. Items 10 and 11, which assess skin clearance (Investigator Global Assessment of AK status) and photodamage, respectively, were designed to be answered by only clinicians. The first implementation of the AK-EPQ will be during the Patient- and Clinician-Reported Outcomes for Tirbanibulin Effectiveness and Safety in Actinic Keratosis study of tirbanibulin 1% ointment in patients with AK; specifically, this study will evaluate PROs and ClinROs among patients with AK in the face or scalp who are prescribed tirbanibulin as part of usual care in clinical practice settings in the United States.21
Conclusion
The AK-EPQ does not directly assess QoL but was newly developed to be used alongside general validated QoL instruments (such as Skindex-16 and Abbreviated Treatment Satisfaction Questionnaire for Medication) which will provide the context needed to establish its functionality and reliability as a tool for assessing treatment outcomes in AK. The AK-EPQ elicits insights into the patient and clinician experience with AK treatments to help improve AK management and patient health outcomes.
Conflicts of interest
Dr Berman has served as an investigator, speaker, advisory board member, or consultant for Aclaris, Almirall, AiViva Biopharma, Biofrontera, Evommune, Ferndale Laboratories, Inc, Galderma Laboratories, L.P., GlaxoSmithKline, Lemonex, LEO Pharma, Mediwound, Mino Labs, Miragen, NOVAN, Novartis Pharmaceuticals Corp, Pfizer, Pierre Fabre, PHD Biosciences, Pulse Biosciences, Sensus, and Sirnaomics. Dr Armstrong served as a research investigator and/or scientific adviser to AbbVie, BI, BMS, EPI, Incyte, LEO, UCB, Janssen, Lilly, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, and Pfizer. Dr Lebwohl receives research funding from AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Dermavant Sciences, Eli Lilly, Incyte, Janssen Research & Development, LLC, Ortho Dermatologics, Regeneron, and UCB, Inc, and is a consultant for Aditum Bio, Almirall, AltruBio Inc, AnaptysBio, Arcutis, Inc, Aristea Therapeutics, Arrive Technologies, Avotres Therapeutics, BiomX, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle BioSciences, Corrona, Dermavant Sciences, Dr. Reddy’s Laboratories, Evelo Biosciences, Evommune, Inc, Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Helsinn Therapeutics, Hexima Ltd, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Seanergy, and Verrica. Dr Grada is the former Head of R&D and Medical Affairs at Almirall, US. Dr Bhatia has been an adviser, consultant, and investigator for AbbVie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, J&J, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, P&G, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr Patel has received honoraria from Regeneron and Almirall, has served as a consultant for PhD Biosciences and Jounce Therapeutics, and has served as a speaker for Regeneron and Sanofi. Dr Rigel has served as a consultant for Almirall, Castle BioSciences, DermTech, and SciBase. Dr Del Rosso has served as a research investigator, consultant/adviser, or speaker for AbbVie, Aclaris, Almirall, Amgen (Celgene), AnaptysBio, Arcutis, Athenex, Bausch (Ortho Dermatologics), Biofrontera, BioPharmX, Biorasi, Blue Creek, Botanix, Brickell, Bristol-Myers Squibb, Cara Therapeutics, Cassiopea, Dermata, Dermavant, Encore, EPI Health, Ferndale, Galderma, Genentech, Incyte, Jem Health, LEO Pharma, La Roche-Posay, Lilly (Dermira), MC2, NOVAN, Pfizer, Ralexar, Regeneron, Sanofi-Genzyme, Sente, Solgel, Sonoma (Intraderm), Sun Pharma, UCB, Verrica, and VYNE (Foamix/Menlo). Dr Schlesinger has received grant/research funding from AbbVie, Aclaris, Allergan, Almirall, Anterios, AOBiome, Arcutis Premier Research, Astellas Pharma US, Inc, Athenex, Biofrontera, Biorasi, Boehringer Ingelheim, Brickell Biotech, Bristol-Myers Squibb, Cara Therapeutics, Castle BioScience, Celgene, Centocor Ortho Biotech (now Janssen Biotech), ChemoCentryx, Coherus Biosciences, Concert Pharmaceutical, Corrona, Cutanea Life Sciences, Dermavant, Dermira (now Lilly), DT Pharmacy & DT Collagen, EPI Health, Galderma (Nestle), Janssen Pharmaceuticals, Inc, Kiniksa, LEO, Lilly, Merz, Nimbus, Novartis, Pfizer, Processa, Pulse Biosciences, Regeneron, Sanofi Genzyme, Sisaf, and Trevi; has received honoraria from AbbVie, Allergen, Almirall, Biofrontera AG, Bristol-Myers Squibb, Castle BioScience, EPI Health, Foundation for Research and Education in Dermatology (FRED), Galderma, Merz, Novartis, Regeneron, and Sun Pharma; has served as a speaker, advisory board member, or consultant for AbbVie, Allergan, Almirall, Amgen, Biofrontera AG, Bristol-Meyers Squibb, Castle BioScience, Celgene, CMS Aesthetics DCME, DUSA/Sun Pharma, EPI Health, Foundation for Research and Education in Dermatology (FRED), Genentech, Greenway Therapeutix, Kintor, Merz, Nextphase, Novartis, Pharmatecture, Prolacta Biosciences, Pulse Biosciences, Regeneron, Remedly, Inc, Sanofi Genzyme, Sun Pharma, UCB, and Verrica; has received consulting fees from Lilly, Ortho Dermatologics, Pierre Fabre, Plasmed, Regeneron, and Skinceuticals/L’Oreal; has been involved in the CME Program for MED Learning Group; has been involved with OncLive SSC Insights Filming/Stacy Jaffe for MJH Associates; has served as Co-Chair of AAD AK Guideline Committee and Co-Chair of AAD Performance Measurement Work Group; is a member of AAD Performance Measurement Committee; and owns stock in Remedly, Inc. Dr Kircik has served as an investigator, speaker, advisory board member, or consultant for Abbott Laboratories, Aclaris, Inc, Allergan, Inc, Almirall, Anacor Pharmaceuticals, Inc, Assos Pharma, Astellas Pharma US, Inc, Asubio Pharma Co, Ltd, Berlex Laboratories (Bayer Healthcare Pharmaceuticals), Biogen-Idec, Inc, Biolife, Biopelle, Boehringer Ingelheim, Breckinridge Pharma, Celgene Corporation, Centocor, Inc, Colbar, CollaGenex, Combinatrix, Connetics Corporation, Coria, Dermik Laboratories, Dermira, Inc, Dow Pharmaceutical Sciences, Inc, Dusa Pharmaceuticals, Inc, Eli Lilly & Co, Embil Pharmaceutical Co, Ltd, EOS, Ferndale Laboratories, Inc, Galderma Laboratories, LP, Genentech, Inc, GlaxoSmithKline, PLC, Health Point Ltd, Idera, Inc, Innocutis Medical, LLC, Innovail, Intendis, Inc, Johnson & Johnson, Laboratory Skin Care, Inc, Leo Pharmaceuticals, Inc, L’Oreal SA, 3M, Maruho Co, Ltd, Medical International Technologies, Medicis Pharmaceutical Corp, Merck & Co, Inc, Merz, Nano Bio Corporation, Novartis Pharmaceutical Corporation, Noven Pharmaceuticals, Inc, Nucryst Pharmaceuticals Corporation, Obagi Medical Products, Inc, Onset, Ortho Dermatologics, OrthoNeutrogena, PediaPharma, Inc, Promius Pharma, LLC, PharmaDerm, Pfizer, Inc, PuraCap, QLT, Inc, Quatrix, Quinnova, Serono (Merck-Serono International SA), SkinMedica, Inc, Stiefel Laboratories, Inc, Sun Pharmaceutical Industries, Ltd, Taro, TolerRx, Inc, Triax, UCB, Inc, Valeant Pharmaceuticals North America LLC, Warner-Chilcott, XenoPort, Inc, and ZAGE. Dr Salem is an employee of Almirall, US. Dr Narayanan is an employee of Avant Health LLC, and has served as a consultant for Almirall, Biogen, Johnson & Johnson, Sarepta Therapeutics, SeaGen, and Takeda. Dr Kasujee is an employee of Almirall, Spain.
Acknowledgments
Medical writing and editorial assistance was provided under the direction of the authors by Zade Holloway, PhD, Rebecca E. Slager, PhD, MS, and David Boffa, ELS, of MedThink SciCom.
Footnotes
Funding sources: This study was funded by Almirall SA. Medical writing and editorial assistance was provided under the direction of the authors by Zade Holloway, PhD, Rebecca E. Slager, PhD, MS, and David Boffa, ELS, of MedThink SciCom.
Data reported in this manuscript were presented in part at the Fall Clinical Dermatology Conference; October 19-23, 2022; Las Vegas, NV.
Patient consent: Not applicable.
IRB approval status: Not applicable.
References
- 1.Balcere A., Rone Kupfere M., Čēma I., Krūmiņa A. Prevalence, discontinuation rate, and risk factors for severe local site reactions with topical field treatment options for actinic keratosis of the face and scalp. Medicina (Kaunas) 2019;55(4):92. doi: 10.3390/medicina55040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Rosso J., Armstrong A.W., Berman B., et al. Advances and considerations in the management of actinic keratosis: an expert consensus panel report. J Drugs Dermatol. 2021;20(8):888–893. doi: 10.36849/JDD.6078. [DOI] [PubMed] [Google Scholar]
- 3.Criscione V.D., Weinstock M.A., Naylor M.F., Luque C., Eide M.J., Bingham S.F. Actinic keratoses: natural history and risk of malignant transformation in the veterans affairs topical tretinoin chemoprevention trial. Cancer. 2009;115(11):2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- 4.Kasujee I., Diaz Gallo C., Jose Lambert C., Massana Montejo E., Narayanan S. 2022. Patient Perception of Disease and Treatment Burden and New Treatment Preferences in Actinic Keratosis. Paper presented at: ISPOR; May 15-18, 2022; Washington, DC. [Google Scholar]
- 5.Khanna R., Bakshi A., Amir Y., Goldenberg G. Patient satisfaction and reported outcomes on the management of actinic keratosis. Clin Cosmet Investig Dermatol. 2017;10:179–184. doi: 10.2147/CCID.S121323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emmerich V.K., Cull D., Kelly K.A., Feldman S.R. Patient assessment of 5-fluorouracil and imiquimod for the treatment of actinic keratoses: a retrospective study of real-world effectiveness. J Dermatolog Treat. 2022;33(4):2075–2078. doi: 10.1080/09546634.2021.1917758. [DOI] [PubMed] [Google Scholar]
- 7.Grada A., Feldman S.R., Bragazzi N.L., Damiani G. Patient-reported outcomes of topical therapies in actinic keratosis: a systematic review. Dermatol Ther. 2021;34(2) doi: 10.1111/dth.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmann S., Vinding G.R., Christensen K.B., Jemec G.B. Assessing the influence of actinic keratosis on patients' quality of life: the AKQoL questionnaire. Br J Dermatol. 2013;168(2):277–283. doi: 10.1111/bjd.12036. [DOI] [PubMed] [Google Scholar]
- 9.Del Rosso J.Q., Kircik L., Goldenberg G., Brian B. Comprehensive management of actinic keratoses: practical integration of available therapies with a review of a newer treatment approach. J Clin Aesthet Dermatol. 2014;7(9 Suppl S2-S12):S2–S12. [PMC free article] [PubMed] [Google Scholar]
- 10.Marson J., Rosso J., Bhatia N., Rigel D. Considerations in the management of actinic keratoses: the importance of adherence and persistence to therapy. SKIN: J Cutan Med. 2021;5:83–89. [Google Scholar]
- 11.Mohney L., Singh R., Grada A., Feldman S. Use of topical calcipotriol plus 5-fluorouracil in the treatment of actinic keratosis: a systematic review. J Drugs Dermatol. 2022;21(1):60–65. doi: 10.36849/JDD.2022.6632. [DOI] [PubMed] [Google Scholar]
- 12.Stockfleth E., Peris K., Guillen C., et al. A consensus approach to improving patient adherence and persistence with topical treatment for actinic keratosis. Int J Dermatol. 2015;54(5):509–515. doi: 10.1111/ijd.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neri L., Peris K., Longo C., et al. Physician-patient communication and patient-reported outcomes in the actinic keratosis treatment adherence initiative (AK-TRAIN): a multicenter, prospective, real-life study of treatment satisfaction, quality of life and adherence to topical field-directed therapy for the treatment of actinic keratosis in Italy. J Eur Acad Dermatol Venereol. 2019;33(1):93–107. doi: 10.1111/jdv.15142. [DOI] [PubMed] [Google Scholar]
- 14.Schlesinger T., Stockfleth E., Grada A., Berman B. Tirbanibulin for actinic keratosis: insights into the mechanism of action. Clin Cosmet Investig Dermatol. 2022;15:2495–2506. doi: 10.2147/CCID.S374122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blauvelt A., Kempers S., Lain E., et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384(6):512–520. doi: 10.1056/NEJMoa2024040. [DOI] [PubMed] [Google Scholar]
- 16.Bhatia N, Armstrong A, Schlesinger T, et al. An Expert Panel Questionnaire (EPQ) For Assessing Patient-Reported and Clinician-Reported Outcomes in Actinic Keratosis. Poster presented at the Fall Clinical Dermatology Conference; October 19-23, 2022; Las Vegas, NV.
- 17.Grada A., Muddasani S., Fleischer A.B., Jr., Feldman S.R., Peck G.M. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15(5):E82–E86. [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung H., Baranowski M.L., Swerlick R.A., et al. Use and cost of actinic keratosis destruction in the medicare part B fee-for-service population, 2007 to 2015. JAMA Dermatol. 2018;154(11):1281–1285. doi: 10.1001/jamadermatol.2018.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zug K.A., Aaron D.M., MacKenzie T. Baseline quality of life as measured by skindex-16+5 in patients presenting to a referral center for patch testing. Dermatitis. 2009;20(1):21–28. [PubMed] [Google Scholar]
- 20.Berman B., Armstrong A.W., Lebwohl M., et al. 2022. Patient-Reported Outcomes for Tirbanibulin Effectiveness and Safety in Actinic Keratosis in Real-World Settings: Proak Study Protocol. Paper presented at: Winter Clinical Dermatology Conference; January 14-19, 2022; Kauai, HI. [Google Scholar]
- 21.Schlesinger T., Kircik L., Del Rosso J., et al. Clinician- and patient-reported outcomes with tirbanibulin 1% treatment for actinic keratosis in routine clinical practice across the U.S. (PROAK study) SKIN: J Cutan Med. 2023;7(3):771–787. [Google Scholar]