Abstract
The food safety of livestock is a critical issue between animals and humans due to their complex interactions. Pathogens have the potential to spread at every stage of the animal food handling process, including breeding, processing, packaging, storage, transportation, marketing and consumption. In addition, application of the antibiotic usage in domestic animals is a controversial issue because, while they can combat food-borne zoonotic pathogens and promote animal growth and productivity, they can also lead to the transmission of antibiotic-resistant microorganisms and antibiotic-resistant genes across species and habitats. Coevolution of microbiomes may occur in humans and animals as well which may alter the structure of the human microbiome through animal food consumption. One Health is a holistic approach to systematically understand the complex relationships among humans, animals and environments which may provide effective countermeasures to solve food safety problems aforementioned. This paper depicts the main pathogen spectrum of livestock and animal products, summarizes the flow of antibiotic-resistant bacteria and genes between humans and livestock along the food-chain production, and the correlation of their microbiome is reviewed as well to advocate for deeper interdisciplinary communication and collaboration among researchers in medicine, epidemiology, veterinary medicine and ecology to promote One Health approaches to address the global food safety challenges.
Keywords: Food Safety, Livestock, Microorganism, Antibiotic-resistant bacteria and genes, One Health
1. Introduction
In the entire ecosystem, rich diversities of microorganisms exist at the human-animal interface, some of which cause human and/or animal diseases. There are 58% of human pathogens that are zoonotic and cause diseases in both humans and animals [1]. A huge number of microorganisms and their products are frequently exchanged in the interface of humans and animals, which may raise the possibility of cross-species transmission of pathogens, antibiotic-resistant genes, virulence genes, etc. The worldwide increase in the population of poultry and livestock since the industrial revolution has been closely associated with an increase in the prevalence of zoonotic infections in humans [2]. The close relationship between humans and animals have also led to the similarity in the structure and function of microorganisms between the two. Through direct or indirect contact, humans and animals can be exposed to pathogens and veterinary antibiotic residues. In addition to that, humans can be exposed to pathogens through the consumption of various animal products, such as meat, eggs, milk, and their by-products, as well as animal hides and skin products. A closer scrutiny of such relationships reveals that food safety, the complex system from farming and slaughter to retail and cooking, is caused by the human-animal-environment interface and where it intersects with each other.
Antibiotics have undoubtedly played an indelible role in helping humans to combat pathogens, but their overuse has inadvertently contributed to microorganisms becoming resistant to antibiotics. The spillover and evolution of these antibiotic-resistant microorganisms is an excellent example of the interface of co-evolution for human and animal microbiomes.
The concept of One Health is increasingly recognized as a critical approach to addressing complex issues related to the interdependence of human, animal and environmental health. In order to protect human health, animal health and welfare, and ecological health, it is important to understand how pathogens interact with antibiotic-resistant genes and microorganisms at the human-animal interface [3]. The One Health approach emphasizes the harmony between humans, animals and the environment, and encourages the interdisciplinary communication and multi-sectoral collaboration that plays an important role in promoting the health of humans, animals and the ecosystems within the animal food-supply chain. On December 1st 2021, the One Health High-Level Expert Panel (OHHLEP) further refined the definition of One Health, which pointed out that One Health is an integrated, coalition-building approach that seeks to sustainably balance and optimize the health of people, animals and ecosystems, recognizing that the three are inextricably linked and interdependent, and it takes a multidisciplinary, multisectoral approach to address global challenges, such as food security issues and antibiotic resistance prevention [4]. Moreover, in the context of coevolution, One Health is particularly important because it highlights the role of genetic variation in driving the evolutionary trajectories of both hosts and pathogens [5]. Mutations, recombination and gene flow are key drivers of genetic diversity that facilitate coevolutionary dynamics. However, human activities such as habitat destruction, environmental changes, and the expansion of livestock farming can indirectly affect gene flow by altering the behavior of vectors and host populations. Therefore, regulating gene flow is crucial for preventing the emergence and spread of infectious diseases that can affect human and animal health. However, this requires careful consideration of the ecological, socioeconomic and ethical implications of different policy options, as they affect multiple stakeholders and values. The previous reviews on food safety have limitations in that they have either focused on the review of pathogens at the human-animal interface [6], summarized the flow of antibiotic resistance between humans and livestock [7] or highlighted the importance of One Health for zoonotic diseases [8]. To overcome these limitations, this review summarizes the main pathogen spectrums of livestock and animal products; the coevolution processes that occurs between the human-animal-environment interface; pathways of antibiotic-resistant bacteria and genes with food, animals and humans from a One Health perspective—considering that these pathogens may cause a high burden of food-borne diseases in the population, inducing higher mortality, or being transmitters of antibiotic-resistant genes.
2. The main pathogen spectrum in livestock and animal products
2.1. Bacteria
According to the World Health Organization (WHO), food contamination caused around 600 million outbreaks of disease, with 350 million caused by pathogenic bacteria, including Campylobacter spp., Salmonella spp., and others in livestock, fish, pets and wildlife [9]. These bacteria pose a significant health and economic burden to both developed and developing countries [10]. This review will focus on nine species of bacteria associated with domestic animals, specifically those documented by the WHO or the World Organization for Animal Health (WOAH). Although some bacteria may infect food animals through contact with wildlife, they are not the main focus of this article.
2.1.1. Campylobacter spp.
Campylobacter spp. is a common bacterial food-borne diarrheal disease worldwide [11]. Humans are at risk of contracting Campylobacteriosis from almost all livestock products, including unpasteurized milk and milk products, raw eggs, mayonnaise, undercooked meat and raw seafood [12]. The bacterium can persist throughout the entire poultry food chain, from growing to consumption, and is commonly found in broilers, broiler carcasses and retail chickens [13]. Most strains are multidrug-resistant, with prevalence of resistance to ciprofloxacin, enrofloxacin, nalidixic acid and/or tetracycline [14]. Campylobacter is also commonly found in poultry, cattle, swine and on the surfaces of hoof and mane in ruminants such as cattle and sheep [15]. Animals are primarily infected with Campylobacter through contact with other animals, farm workers, as well as environmental media such as water, soil and air [16]. This requires collaboration between physicians, veterinarians, public health professionals, scientists and government officials to develop a holistic system, including strict biosecurity measures, to prevent the introduction of Campylobacter to concentrated livestock through other animals, farm staff or visitors [17]. Enhancing information sharing between countries and authorities, such as genome sequences and epidemiological information, would reduce the impact on human and animal health following the One Health perspective.
2.1.2. Salmonella spp.
Non-typhoidal Salmonella spp. is a common cause of food poisoning in humans, with eggs, egg products and poultry meat being the primary sources of infection, followed by pork, beef and dairy products. Salmonella can spread both vertically and horizontally in animals. For example, eggs can become contaminated through contact with feces and the environment or by the production process of diseased hens [18]. People can become infected with Salmonella directly or indirectly through contact with animals in various settings, but most human cases result from food-borne transmission or human fecal contamination [19,20]. The emergence of antibiotic-resistant strains of Salmonella is also a major public health concern, with the food chain being responsible for the primary transmission of these antibiotic-resistant genes at the human-animal-environment interface [[21], [22], [23], [24]]. Consequently, given the epidemiological complexity of salmonellosis, in parallel to the implementation of strict health policies throughout the food safety process, an integrated surveillance system with a multisectoral approach, like the One Health actions in Canada [25], could help to control this enteric disease and antimicrobial resistance in the long-term.
2.1.3. Bacillus anthracis
Anthrax is a fatal zoonotic infection caused by Bacillus anthracis, which is endemic in Western Africa [26] and threatens humans, domestic animals and wildlife. Humans can contract the disease through contact with infected animals or their remains, or by consuming contaminated meat [27]. Domestic animals, including cattle, sheep, horses, pigs and dogs, have varying degrees of susceptibility [28]. The global anthrax epidemic is a complex one with a lot of impact factors, such as agriculture, veterinary medicine and health education. Limited surveillance in resource-poor countries further complicates epidemiological data collection. Additionally, antibiotic resistance in B. anthracis is concerning [29], with 10 antimicrobial-resistant genes identified in a global genome-wide dataset [30]. The One Health concept emphasizes collaboration between experts in clinical and veterinary medicine, epidemiology, microbiology, genetics, immunology and ecology to develop interdisciplinary plans to combat this challenging, multi-host and deadly zoonotic pathogen [31].
2.1.4. Listeria monocytogenes
Listeria monocytogenes causes aggressive and uncommon listeriosis, with a mortality rate of 20–30% [32]. Animal products, including meat, milk, dairy and fish are the main sources of infection [33]. Pregnant women, children, elderly individuals and immunocompromised populations are more susceptible [34,35]. The development of antibiotic-resistant L. monocytogenes has made treatment more challenging [36,37]. However, hygienic food processing can reduce contamination and ensure food safety [38]. One Health initiatives have shown promise in reducing the potential threat of L. monocytogenes by fostering collaboration between medicine, public health, veterinary medicine, food safety and policy-making sectors and integrating regional data [39].
2.1.5. Methicillin-resistantStaphylococcus aureus (MRSA)
MRSA is a zoonotic pathogen that spreads from animals to humans through contact with infected animal food. Livestock-associated MRSA is a widespread strain that is derived from a human MRSA strain and can infect a variety of animals [[40], [41], [42], [43]]. Raw meat handling without gloves can allow MRSA to colonize the skin and soft tissues, which can then be transmitted through direct contact with others. It can also spread through contaminated animal products [44,45]. Good Manufacturing Practices (GMP), Good Hygiene Practices (GHP), Hazard Analysis and Critical Control Points (HACCP) systems, as well as increased monitoring of veterinary antibiotic use, can limit S. aureus contamination in food [46]. Despite the existing surveillance systems around the world [47], including collaborative surveillance systems between countries [48,49], the fragmentation of data sets, particularly heterogeneous data collections, has a negative impact on MRSA research [47]. A well-coordinated MRSA surveillance system based on the One Health concept, which integrates microbiological and epidemiological data, would be of great benefit to reducing the risks of food safety [50].
2.1.6. Brucellaspp.
Brucellosis, also known as “Undulant fever”, is one of the major zoonotic diseases [51], and its prevalence is also associated with the prevalence in livestock and wildlife [52]. It can be transmitted to humans through the consumption of contaminated raw milk and dairy products or contact with infected animals [53]. The WOAH, WHO and Food and Agriculture Organization of the United Nations (FAO) rank brucellosis as one of the most important neglected occupational hazards [54]. The severity of brucellosis in human populations depends on factors such as dietary habits, processing methods of milk and dairy products, feeding practices and environmental hygiene [55]. Non-standard treatment can lead to antibiotic resistance, with isolates from livestock and their products showing resistance to commonly used antibiotics in human treatment [56,57]. The complexity of solving the brucellosis epidemic in these countries necessitates the application of the One Health approach, to achieve a strategic layout of multisectoral involvement and multidisciplinary action, all stakeholders including legislators, national and local authorities, veterinary practitioners and workers, doctors and health care providers, livestock producers, dairy processors, suppliers, remote nomadic and rural smallholder farmers and customers should all be involved in policy planning and implementation together [58].
2.2. Viruses, fungi and parasites
Pathogens of viruses, fungi and parasites can also pose a threat to human and animal health at the human-animal interface during feed preparation, mass production, slaughter and food processing. Some examples of these pathogens include avian influenza viruses, Nipah viruses and viral hand-foot-and-mouth disease. Mycotoxin residues can cause chronic health problems in humans and animals. Parasites such as Cryptosporidium spp. and Toxoplasma gondii can cause significant morbidity and mortality in both animals and humans. Although it is not possible to provide a comprehensive list of all potential viral, fungal and parasitic threats, understanding the dynamic nature of the human-animal interface is crucial in preventing and controlling their spread.
2.2.1. Viruses
Food-borne viruses, including norovirus and hepatitis A virus, have become a growing concern at the human-animal interface due to contamination during food production and processing. While viruses cannot grow in animal products, they can survive during processing and storage [59], leading to illness in humans. Swine Influenza (SI) is a respiratory disease caused by the influenza A virus and has led to global infection rates of 10–20% [[60], [61], [62]]. Environmental factors, such as livestock and population density, play a crucial role in the emergence or pandemics of human influenza [63]. The One Health approach can play an important role in raising awareness and preventing zoonotic disease outbreaks. Apart from food-borne viruses, other viruses like the Ebola virus, coronaviruses, monkeypox virus and Nipah virus can also infect humans through direct or indirect contact with contaminated animal products [64]. The risk of virus spillovers and transmission changes with human activities and virus evolution [65]. To mitigate such complex issues, broader surveillance and the One Health approach are required to develop holistic frameworks [66].
2.2.2. Fungi
In animal products, mycotoxin residues result primarily from contaminated feed or pasture, with aflatoxin being the most concerned [67]. Because aflatoxins are highly toxic hepatocarcinogens, mainly residing in animal liver, kidney, blood, milk, eggs and meat, the consumption of these animal products becomes one of the most important sources of mycotoxin intake in humans. In addition, the approach of controlling feed safety by avoiding toxin contamination through blockchain technology and food sensor technologies is in line with One Health's approach of encouraging interdisciplinary collaboration [65].
2.2.3. Parasites
Parasites in animal products can cause public health issues. In China, Clonorchis sinensis and Gnathostoma spp. in raw or cured fish and crustacean products, as well as Trichinella spp. and Taenia spp. in pigs and beef, pose a significant threat to human health. Cestode-caused echinococcosis and cysticercosis are the most severe food-borne parasitic diseases in China, resulting in severe damage to the human liver, lungs, brain and bones [68]. Worldwide, animal products contaminated with T. gondii, Cryptosporidium spp. and Giardia duodenalis are also a major concern [69,70]. Toxocara spp. eggs excreted in feces can persist in soil, contaminate vegetables, or be consumed by farm animals, affecting food safety and endangering public health [71]. A comprehensive and integrated control strategy, adopting the One Health approach, should be developed by professionals, scientists, practitioners in the field of veterinary medicine, human medicine, public health, agriculture and husbandry [72].
3. Transmission of antibiotic resistance genes between livestock and human
The reasons for antibiotic use in livestock farming include increased productivity, treatment of animal diseases, prevention of disease outbreaks, and treatment of diseases for the entire herd in large intensive farms. They are more commonly used in intensively farmed species such as pigs and poultry in Europe than in extensively farmed species such as cattle and sheep, which leads to an increase in microorganism selection pressure [73,74]. The overuse of antibiotics in livestock, particularly the last-line defense against antibiotic resistance, mucin, accelerates the emergence of antibiotic resistance in zoonotic pathogens, a significant challenge to public health [75]. It is encouraging to note that this issue has received much attention and support, and Table 1 demonstrates the discoveries of antibiotic-resistant studies conducted in various countries concerning farmed animals. Microorganisms can acquire antibiotic-resistant genes through horizontal gene transfer of mobile genetic elements and further cause their mobile transmission in the human-animal-environment interface [[76], [77], [78], [79], [80]]. Since antibiotic-resistant bacteria and genes develop trans-habitat and cross-species transmission, an integrated understanding of the linkages between human, animal and environmental microbiota with the One Health approach [81]. The One Health approach is essential in addressing this global health challenge, exploring the complex relationships between humans, animals and the environment at the local, regional and global levels through interdisciplinary and cross-sectoral collaboration [82].
Table 1.
Detected antibiotic resistance (associated with farm animals).
| Region/Country | Animals involved in the study | Strains involved in the study | Antibiotics for resistance | Ref |
|---|---|---|---|---|
| United States | Swine | Salmonella | tetracyclines, sulfonamides, penicillins, fosfomycin, and quinolones | [122] |
| Sheep | the extended-spectrum beta-lactamases- (ESBL) producing Escherichia coli and Salmonella | ampicillin, chloramphenicol, sulfisoxazole, streptomycin, and tetracycline | [123] | |
| Cattle | Salmonella Dublin | amoxicillin/clavulanic acid, ampicillin, ceftriaxone, chloramphenicol, sulfisoxazole, cefoxitin, gentamicin, nalidixic acid, streptomycin, tetracycline, and ceftiofur | [124] | |
| Dairy products | Staphylococcus aureus | aminoglycoside, beta-lactam, fluoroquinolone, lincosamide, macrolide, streptogramin B, phenicol, and tetracycline | [125] | |
| Poultry and its products | Campylobacter | ciprofloxacin, clindamycin, erythromycin, florfenicol, gentamicin, nalidixic acid, telithromycin, and tetracycline | [126] | |
| Canada | Swine | Salmonella | streptomycin, sulfisoxazole, tetracycline, and gentamicin | [127] |
| Sheep | Campylobacter | tetracycline, ciprofloxacin, nalidixic acid, telithromycin, azithromycin, clindamycin, erythromycin | [128] | |
| Dairy products | Escherichia coli | streptomycin, tetracycline, ampicillin, cefotaxime, and cefazolin | [129] | |
| Poultry and its products | Salmonella | ampicillin, amoxicillin-clavulanic acid, ceftiofur, cefoxitin, and ceftriaxone | [130] | |
| Mexico | Swine | Escherichia coli | cefalotin, ampicillin, cefotaxime, nitrofurantoin, and tetracycline | [131] |
| Sheep | Shiga toxin-producing E. coli (STEC), enteropathogenic E. coli (EPEC), and enterotoxigenic E. coli (ETEC) | amoxicillin-clavulanic acid, amikacin, erythromycin, gentamicin, colistin, kanamycin, neomycin, streptomycin, trimethoprim-sulfamethoxazole, ampicillin, tetracycline, ciprofloxacin, sulfisoxazole, and chloramphenicol | [132] | |
| Cattle | non-typhoidal Salmonella | tetracycline, carbenicillin, amoxicillin-clavulanic acid, chloramphenicol, and trimethoprim-sulfamethoxazole | [133] | |
| Brazil | Swine | Escherichia coli | ampicillin, amoxicillin/clavulanic acid, cefoxitin, and ceftiofur | [134] |
| Dairy products | Staphylococcus spp, Salmonella sp., Escherichia coli, and Listeria monocytogenes | beta-lactams, macrolides, tetracycline, quinolones, and sulfonamides | [134] | |
| Poultry and its products | non-typhoidal Salmonella | sulfonamide, trimethoprim-sulfamethoxazole, nalidixic acid, streptomycin, gentamicin, and tetracycline | [134] | |
| Argentina | Swine | Salmonella | ampicillin, cefalotin, tilmicosin, gentamicin, chloramphenicol, tetracycline, nalidixic acid, ciprofloxacin, enrofloxacin | [135] |
| Cattle | Escherichia coli | Escherichia coli: authors reported high resistance to polymyxin B52, very high resistance to gentamicin52, amoxicillin/clavulanic acid79 and extremely high antimicrobial resistance to neomycin52, 79, enrofloxacin79 and tetracycline52 | [136] | |
| Poultry and its products | Campylobacter | tetracyclines, nalidixic acid, ciprofloxacin, doxycycline, and ampicillin | [137] | |
| Peru | Poultry and its products | Escherichia coli | trimethoprim/sulfamethoxazole, amoxicillin, nalidixic acid, tetracycline, and cefalotin | [138] |
| Chile | Aquatic animals | / | oxytetracycline and florfenicol | [139] |
| China | Swine | Escherichia coli | ciprofloxacin, gentamicin, tetracycline, ampicillin, and florfenicol | [140] |
| Sheep | / | Antibiotic residues: sulfadiazine, sulfamethoxazole, sulfamerazine, sulfamethazine, sulfamonomethoxine, trimethoprim, norfloxacin, enoxacin, ciprofloxacin, danofloxacin, enrofloxacin, fleroxacin, difloxacin, tetracycline, oxytetracycline, chlortetracycline | [141] | |
| Cattle | / | tetracyclines, quinolones, β-lactam, and aminoglycosides | [142] | |
| Dairy products | Staphylococcus aureus and MRSA | penicillin G, ampicillin, erythromycin, and oxacillin | [143] | |
| Poultry and its products | Salmonella | streptomycin, phenicol, β-lactams, tetracycline, and sulfonamides | [144] | |
| Aquatic animals | / | tetracyclines, aminoglycosides, chloramphenicol, and sulfonamides | [145] | |
| Japan | Aquatic animals | Listeria monocytogenes | penicillins (ampicillin and penicillin), rifampicin, lincomycin, and tetracycline | [146] |
| India | Cattle | Campylobacter fetus | nalidixic acid, fluoroquinolones, and tetracyclines | [147] |
| Dairy products | / | Antibiotic residues: enrofloxacin, oxytetracycline, and sulphadiazine | [148] | |
| Poultry and its products | Escherichia coli | ampicillin, amoxicillin, amikacin, and ofloxacin | [149] | |
| Aquatic animals | Vibrio parahaemolyticus and Vibrio vulnificus | ampicillin, colistin and cephalothin, amoxycilin, carbenicillin, and ceftazidime | [150] | |
| Thailand | Swine | Salmonella | streptomycin, tetracycline, and sulfisoxazole | [151] |
| Pakistan | Sheep | Clostridium perfringens Type A and D | rifampin, ceftiofur, teicoplanin, chloramphenicol, amoxicillin, linezolid, enrofloxacin, and ciprofloxacin | [152] |
| Iran | Sheep | Acinetobacter baumannii | streptomycin, gentamycin, co-trimoxazole, tetracycline, and trimethoprim | [153] |
| Cattle | Salmonella enteritidis | streptomycin, gentamicin, sulfamethoxazole, amikacin, chloramphenicol, and imipenem | [154] | |
| Dairy products | Arcobacter butzleri and Arcobacter cryaerophilus | amoxicillin-clavulanic acid, tetracycline, gentamycin, streptomycin, erythromycin, and ciprofloxacin | [155] | |
| Turkey | Sheep | Staphylococcus aureus | kanamycin, telithromycin, penicillin G, streptomycin, erythromycin, cloxacillin, ampicillin, pristinamycin, nalidixic acid, azithromycin, and ciprofloxacin | [156] |
| Cattle | Salmonella enteritidis | streptomycin, tetracycline, trimethoprim/sulfamethoxazole, and chloramphenicol | [157] | |
| Poultry and its products | Salmonella | ampicillin, tetracycline, trimethoprim-sulfamethoxazole, and chloramphenicol | [158] | |
| European Union | Cattle, swine, and poultry | / | ampicillin, trimethoprim/sulfamethoxazole, nalidixic acid, ciprofloxacin, cefotaxime, chloramphenicol, colistin gentamicin, and tetracycline | [159] |
| Spain | Cattle | Escherichia coli | amoxicillin-clavulanic acid, chloramphenicol, ciprofloxacin, gentamicin, cefotaxime, cefepime, cefoxitin, cefalotin, cefazolin, nalidixic acid, penicillin G, ampicillin-sulbactam, tetracycline | [160] |
| Italy | Aquatic animals | Vibrio parahaemolyticus | ampicillin, amoxicillin, tetracycline, oxytetracycline, and trimethoprim/sulfamethoxazole | [161] |
| South Africa | Sheep | Salmonella | ampicillin, tetracycline, amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and ceftriaxone | [162] |
| Cattle | Campylobacter | clindamycin, nalidixic acid, tetracycline, and erythromycin. | [163] | |
| Dairy products | Staphylococcus aureus | ampicillin, cloxacillin, penicillin G, clindamycin, oxy-tetracycline, cephalexin, cefuroxime, and tylosin | [164] | |
| Poultry and its products | Salmonella | aminoglycosides, β-lactam, fluoroquinolones, phenicols, sulphonamides, tetracyclines, and trimethoprim | [165] |
3.1. Transmission of antibiotic resistance genes between livestock and humans through direct contact and food products
In small-scale poultry and livestock farm or animal products processing plants that lack biosecurity measures, workers’ involvement can also facilitate the transmission of antibiotic resistance from animals to humans through direct contact. In Ethiopia, disease-prevented materials, such as slaughterhouses, soap, tap water and disinfectant, are not used during the slaughter process, which may be exposing workers to antibiotic-resistant bacteria directly [83]. Moreover, in less developed or rural areas, family members often share living and sleeping quarters with livestock [84], which provides a possibility for the spread of antibiotic resistance [85]. The appearance of quinolone-resistant strains of Campylobacter spp. and Salmonella spp. suggests that antibiotic resistance may also be present in food-borne pathogens ingested through animal products [[86], [87], [88]].
3.2. Transmission of antibiotic resistance genes through the soil
Soil is a potential pathway for the spread of antibiotic-resistant bacteria from livestock to crops, animals and humans, and plays an important role in human, animal, plant and environmental health [89,90]. Aside from the direct application of antibiotics to prevent or treat crop diseases, animal manure can also introduce antibiotics to agricultural soils. In large numbers, animals exposed to antibiotics excrete them in urine and feces, and 58% are released into the environment through the soil, surface runoff and groundwater [91]. As a result, antibiotic-resistant microorganisms are selected in the environment [92], which in turn leads to the spread of antibiotic-resistant bacteria and genes [93]. In this mode, manure, especially composted manure, plays a greater role than direct contact [94,95].
3.3. Airborne transmission of antibiotic resistant genes in livestock
Besides physical and chemical components, airborne particulate matter may also contain microorganisms or fractions of them, known as biological particulate matter (BioPM), which may also lead to adverse health effects [96]. According to several studies, PM2.5 and PM10 containing antibiotic-resistant microorganisms and genes were detected in both indoor and outdoor environments, especially in hospital air particles containing carbapenemase genes such as blaNDM, blaKPC, blaIMP, blaVIM and blaOXA-48 [97]. Furthermore, airborne transmission of antibiotic-resistant bacteria can be influenced by weather conditions such as ultraviolet radiation intensity, temperature, and humidity [98]. Researchers found that farms also experience this, with 100% of airborne bacteria isolated from swine farms being resistant to erythromycin (100%), penicillin (100%), spectinomycin (100%), clindamycin (67%) and tetracycline (21%) [99]. Farms such as these pose a threat to human health, exposing residential areas up to 3000 m away to antibiotic-resistant genes like mecA and tetW [100].
4. Understanding the correlation of livestock and human microbiome with One Health
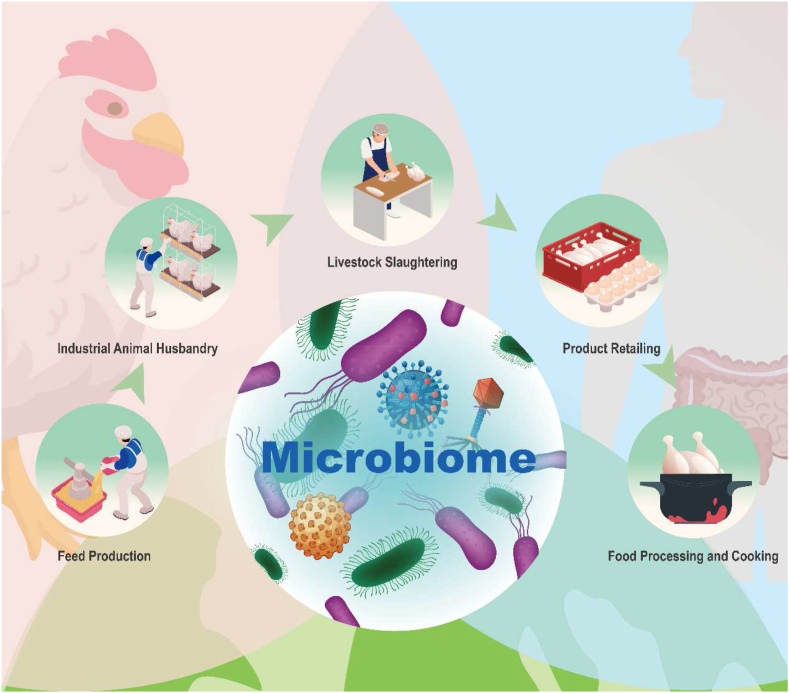
Livestock has an inextricable connection to the human microbiome in the food safety chain. Fig. 1 shows an example of the feeding, breeding, slaughtering, handling, retailing and cooking of poultry. At each stage of this chain, there must be contact between humans and poultry, and the microbiome is dynamically changing through the human-avian-environment interface, where it may spillover and evolve, such as the cross-species transmission of antibiotic-resistant microorganisms. Breaking a difficult topic into multiple smaller issues is undoubtedly a good option when faced with such a complex and dynamic interface, but inevitably a global perspective will be lost in the process. Medicine, veterinary medicine and modern ecology all play a part in the relationship between human health and livestock health. However, researchers in these disciplines have tended to isolate themselves into discipline-specific “silos”, in which the internal research communities are robust, but interaction with each other is limited [101]. As the microbiome, specifically the gut microbiome has grown in prominence, researchers with diverse disciplines have joined forces, the human gut microbiome were considered to contribute to human health and maintains homeostasis by regulating nutrient and pharmaceutical metabolism, synthesizing essential vitamins, defending against pathogens, regulating immunity and altering susceptibility [102].
Fig. 1.
Correlation of livestock and the human microbiome from the “One Health” perspective. Taking poultry feeding, rearing, slaughtering, retailing, and cooking as examples, this illustration demonstrates the interaction of humans with livestock and their products, and the environment in the food safety chain from the One Health perspective, suggesting the interaction, sharing, and evolution of pathogens, antibiotic resistance bacteria carrying antibiotic resistance genes, and the microbiome of all three.
In terms of diet, Animal-Source Foods (ASF) greatly affect the human intestinal microbiome. For example, it was found that the levels of Firmicutes bacteria which metabolize plant polysaccharides, were lower in people who consumed animal protein and fat [103]. Besides, short-term high intake of animal foods rapidly alters the intestinal microbial community structure, increasing deoxycholic acid concentration levels in the intestinal tract and making inflammatory bowel disease more likely to occur [103]. The effects of animal foods on human health vary widely, depending on the type and amount of food. For instance, saturated fats (from pigs, cows, sheep, etc.) and unsaturated fats (from fish) influence the human intestinal microbiome in different ways, which may affect metabolism, the development of obesity, cancer and other aspects of health. Tryptophan is an essential amino acid that can only be obtained from red meat, fish and eggs. When commensal bacteria express tryptophanase, tryptophan is broken down into indole, which activates aromatic hydrocarbon receptors and regulates the production of cytokine IL-22, both of which are essential in mucosal immunity response against pathogens, an important conversion in which Lactobacillus spp. and Clostridium spp. are involved [104]. In addition, dairy products such as yogurt and cheese contain prebiotics, such as lactic acid bacteria and galacto-oligosaccharides, which support the growth of beneficial gastrointestinal bacteria (e.g. Bifidobacterium) [105]. Therefore, the relationship between animal-food microbiota and health is complex, and it is difficult to judge whether they are beneficial or not to human health based on the regulation of intestinal microbiota by food.
Furthermore, from a phylogenetic perspective, the close linkage between the microbiomes of humans and livestock has been noticed [106]. It has been found that people occupationally exposed to antibiotic-resistant genes due to livestock farming (swine and poultry farmers, staff, family members, and slaughterhouse workers) have a higher abundance of antibiotic-resistant genes in their fecal microbiome [107]. The human gut microbiome has also been associated with zoonotic diseases. Infection of Plasmodium spp., the causative agent of malaria, alters the composition of the gut microbiota of the host, subsequently affecting the clinical outcome [108].
There is also a link between the microbiome of humans and livestock based on their environments. Researchers have found antibiotic-resistant genes in airborne particulate matter collected from swine and poultry farms [109]. Additionally, land-use change by humans affects not only the diversity of animal species but also their gut microbes [110]. Moreover, as aquaculture develops, humans and animals are also influencing the microbiome of the environment they live, such as the microbiome of well water, fish ponds, vegetable farming and field soils [111].
The microbiome can be thought of as shaping host characteristics and influencing the evolutionary potential of the host, whether in humans, animals or the environment, by changing the mean host phenotype and altering the variance in host phenotype in the population [112]. This is consistent with the concept of coevolution, which emphasizes how the interaction between the host and its beneficial symbionts can lead to the reciprocal adaptation of one lineage to another [113,114]. Although hosts can sometimes evolve mechanisms to resist such evolution through interactions with microbes, it has been found that even when such resistance exists, microbes can continue to co-evolve through “rock-paper-scissors” dynamics [115]. Research in microbiomes similar to that aforementioned will have the opportunity to be at the intersection of human, animal and environmental microbiology research. However, by considering the following three factors: (i) the complex process of coevolution of microbiome at the human-animal-environmental interface; (ii) mutualistic interactions and syntropy between microorganisms also have an impact on their evolution [116], microbial communities change even without external perturbations [117]; and (iii) more factors such as the impact of climate change on zoonotic diseases [118], facing such a complex situation, relying only on each field alone cannot well solve real-world topics, it is important for us to consider the needs of both developed and developing countries and taking actions on One Health approach in sustainable ways. In order to do this, the One Health connotation which treats humans, animals and the environment as an organic whole, is an important tool for interdisciplinary dialogue to address how pathogens, resistant genes and microbiomes evolve synergistically. And the plan of action for One Health needs to be designed from an interdisciplinary perspective, taking into account environmental, sociocultural, and sociopolitical investigations and implementation strategies. Applying One Health principles to the creation and implementation of evidence-based policy is not a novel idea, but over the past decade, awareness of One Health has increased of the importance of involving interdisciplinary teams, including veterinarians, physicians, ecologists and agricultural economists, among others, to address complex problems. By creating strong connections between scientists, industry stakeholders, and policymakers, communication, which is crucial to successful policy acceptance, can also be strengthened.
5. Conclusion and prospects
Livestock is the animal group most closely related to humans in terms of infections with pathogens and microbiomes. In the coevolution of the human and livestock microbiomes, direct contact, animal products, and the environment, influence and shape the composition and diversity of the microbiome. The “shaping” of livestock's microbiome by humans ultimately affects the composition of the human microbiome through direct contact with food or food-safety chains, which is essentially the “self-shaping” of the human microbiome [119]. Every step of the food safety chain, whether feeding, slaughtering or processing, has the potential to affect human and animal health [120]. One striking challenge of food safety is antibiotic resistance. To ensure food safety and promote human and animal health, in addition to the improvement of hygiene measures throughout the chain, it would be helpful to establish more comprehensive monitoring strategies and systems based on the One Health concept, promote the use of biosafety methods and promote vaccine research and development, among other approaches [121].
This review reveals the necessity to bridge the multidisciplinary research silos through the One Health approach. We are progressively studying the relationship between pathogens, microbiota and antibiotic-resistant genes and relevant policies are gradually being implemented. One Health is a promising avenue to create a breakthrough for future research on the relationship between livestock and their products and human on the microorganism level.
Author contributions
J.Q. and Z.Y.W. wrote the first draft of the manuscript under the supervision of C.L. and Y.Z.Z. J.Q. contributed substantially to revisions and performed the figures and visualization. C.L. acquired funding for this project (The National Natural Science Foundation of China: 32170141).
Disclosure statement
The authors state no conflict of interest.
Acknowledgment
The authors extend their thanks to Professor Xiao-Nong Zhou of the National Institute of Parasitic Diseases at the Chinese Center for Disease Control and Prevention & Chinese Center for Tropical Diseases Research for helpful support and to the anonymous reviewers for their insightful comments that helped improve the quality of this manuscript. This study was supported by a grant from the National Natural Science Foundation of China (32170141). The funding body had no role in the design of the study and the writing of the manuscript.
References
- 1.Woolhouse M.E., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11(12):1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reperant L.A., Cornaglia G., Osterhaus A.D. The importance of understanding the human-animal interface : from early hominins to global citizens. Curr. Top. Microbiol. Immunol. 2013;365:49–81. doi: 10.1007/82_2012_269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zinsstag J., Schelling E., Wyss K., Mahamat M.B. Potential of cooperation between human and animal health to strengthen health systems. Lancet. 2005;366(9503):2142–2145. doi: 10.1016/S0140-6736(05)67731-8. [DOI] [PubMed] [Google Scholar]
- 4.WHO Tripartite and UNEP support OHHLEP's definition of “one health”. 2021. https://www.who.int/news/item/01-12-2021-tripartite-and-unep-support-ohhlep-s-definition-of-one-health [Available from:
- 5.Van Oosterhout C. Mitigating the threat of emerging infectious diseases; a coevolutionary perspective. Virulence. 2021;12(1):1288–1295. doi: 10.1080/21505594.2021.1920741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klous G., Huss A., Heederik D.J.J., Coutinho R.A. Human-livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Health. 2016;2:65–76. doi: 10.1016/j.onehlt.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Rodríguez F., Mercanoglu Taban B. A state-of-art review on multi-drug resistant pathogens in foods of animal origin: risk factors and mitigation strategies. Front. Microbiol. 2019;10:2091. doi: 10.3389/fmicb.2019.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslam B., Khurshid M., Arshad M.I., Muzammil S., Rasool M., Yasmeen N., et al. Antibiotic resistance: one health one world outlook. Front. Cell. Infect. Microbiol. 2021;11:771510. doi: 10.3389/fcimb.2021.771510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health O . Vol. 9. World Health Organization; Geneva: 2015. (WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007-2015). [Google Scholar]
- 10.Chlebicz A., Śliżewska K. Campylobacteriosis, salmonellosis, yersiniosis, and listeriosis as zoonotic foodborne diseases: a review. Int. J. Environ. Res. Publ. Health. 2018;15(5):863. doi: 10.3390/ijerph15050863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havelaar A.H., Haagsma J.A., Mangen M.J., Kemmeren J.M., Verhoef L.P., Vijgen S.M., et al. Disease burden of foodborne pathogens in The Netherlands, 2009. Int. J. Food Microbiol. 2012;156(3):231–238. doi: 10.1016/j.ijfoodmicro.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Fravalo P., Kooh P., Mughini-Gras L., David J., Thébault A., Cadavez V., et al. Risk factors for sporadic campylobacteriosis: a systematic review and meta-analysis. Microb. Risk. Anal. 2021;17:100118. [Google Scholar]
- 13.EPoB Hazards. Scientific opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 2010;8(1):1437. [Google Scholar]
- 14.Abay S., Kayman T., Otlu B., Hizlisoy H., Aydin F., Ertas N. Genetic diversity and antibiotic resistance profiles of Campylobacter jejuni isolates from poultry and humans in Turkey. Int. J. Food Microbiol. 2014;178:29–38. doi: 10.1016/j.ijfoodmicro.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Hald B., Skov M.N., Nielsen E.M., Rahbek C., Madsen J.J., Wainø M., et al. Campylobacter jejuni and Campylobacter coli in wild birds on Danish livestock farms. Acta Vet. Scand. 2016;58(1):11. doi: 10.1186/s13028-016-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin O., Kassem, Shen Z., Lin J., Rajashekara G., Zhang Q. Campylobacter in poultry: ecology and potential interventions. Avian Dis. 2015;59(2):185–200. doi: 10.1637/11072-032315-Review. [DOI] [PubMed] [Google Scholar]
- 17.Fraser R.W., Williams N.T., Powell L.F., Cook A.J.C. Reducing Campylobacter and Salmonella infection: two studies of the economic cost and attitude to adoption of on-farm biosecurity measures. Zoonoses Public Health. 2010;57(7–8):e109–e115. doi: 10.1111/j.1863-2378.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- 18.Whiley H., Ross K. Salmonella and eggs: from production to plate. Int. J. Environ. Res. Publ. Health. 2015;12(3):2543–2556. doi: 10.3390/ijerph120302543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oueslati W., Rjeibi M.R., Mhadhbi M., Jbeli M., Zrelli S., Ettriqui A. Prevalence, virulence and antibiotic susceptibility of Salmonella spp. strains, isolated from beef in Greater Tunis (Tunisia) Meat Sci. 2016;119:154–159. doi: 10.1016/j.meatsci.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 20.Parry C.M., Threlfall E.J. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr. Opin. Infect. Dis. 2008;21(5):531–538. doi: 10.1097/QCO.0b013e32830f453a. [DOI] [PubMed] [Google Scholar]
- 21.Elbediwi M., Li Y., Paudyal N., Pan H., Li X., Xie S., et al. Global burden of colistin-resistant bacteria: mobilized colistin resistance genes study (1980–2018) Microorganisms. 2019;7(10):461. doi: 10.3390/microorganisms7100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Exner M., Bhattacharya S., Christiansen B., Gebel J., Goroncy-Bermes P., Hartemann P., et al. Antibiotic resistance: what is so special about multidrug-resistant Gram-negative bacteria? GMS Hygiene and Infection Control. 2017;12:Doc05. doi: 10.3205/dgkh000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elbediwi M., Beibei W., Pan H., Jiang Z., Biswas S., Li Y., et al. Genomic characterization of mcr-1-carrying Salmonella enterica serovar 4,[5],12:i:- ST 34 clone isolated from pigs in China. Front. Bioeng. Biotechnol. 2020;8:663. doi: 10.3389/fbioe.2020.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwu C.J., Iweriebor B.C., Obi L.C., Basson A.K., Okoh A.I. Multidrug-resistant Salmonella isolates from swine in the eastern cape province, South Africa. J. Food Protect. 2016;79(7):1234–1239. doi: 10.4315/0362-028X.JFP-15-224. [DOI] [PubMed] [Google Scholar]
- 25.Parmley E.J., Pintar K., Majowicz S., Avery B., Cook A., Jokinen C., et al. A Canadian application of one health: integration of Salmonella data from various Canadian surveillance programs (2005-2010) Foodb. Pathog. Dis. 2013;10(9):747–756. doi: 10.1089/fpd.2012.1438. [DOI] [PubMed] [Google Scholar]
- 26.Pittiglio C., Shadomy S., El Idrissi A., Soumare B., Lubroth J., Makonnen Y. Seasonality and ecological suitability modelling for anthrax (Bacillus anthracis) in western Africa. Animals. 2022;12(9):1146. doi: 10.3390/ani12091146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehman M.W., Craig A.S., Malama C., Kapina-Kany'anga M., Malenga P., Munsaka F., et al. Role of food insecurity in outbreak of anthrax infections among humans and hippopotamuses living in a game reserve area, rural Zambia. Emerg. Infect. Dis. 2017;23(9):1471–1477. doi: 10.3201/eid2309.161597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gachohi J.M., Gakuya F., Lekolool I., Osoro E., Nderitu L., Munyua P., et al. Temporal and spatial distribution of anthrax outbreaks among Kenyan wildlife, 1999–2017. Epidemiol. Infect. 2019;147:e249. [Google Scholar]
- 29.Maxson T., Kongphet-Tran T., Mongkolrattanothai T., Travis T., Hendricks K., Parker C., et al. Systematic review of in vitro antimicrobial susceptibility testing for Bacillus anthracis, 1947-2019. Clin. Infect. Dis. 2022;75(Suppl 3):S373–S378. doi: 10.1093/cid/ciac520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruce S.A., Huang Y.H., Kamath P.L., van Heerden H., Turner W.C. The roles of antimicrobial resistance, phage diversity, isolation source and selection in shaping the genomic architecture of Bacillus anthracis. Microb. Genom. 2021;7(8):616. doi: 10.1099/mgen.0.000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson C.J., Getz W.M., Kausrud K.L., Cizauskas C.A., Blackburn J.K., Bustos Carrillo F.A., et al. Spores and soil from six sides: interdisciplinarity and the environmental biology of anthrax (Bacillus anthracis) Biol. Rev. Camb. Phil. Soc. 2018;93(4):1813–1831. doi: 10.1111/brv.12420. [DOI] [PubMed] [Google Scholar]
- 32.Ramaswamy V., Cresence V.M., Rejitha J.S., Lekshmi M.U., Dharsana K.S., Prasad S.P., et al. Listeria--review of epidemiology and pathogenesis. J. Microbiol. Immunol. Infect. 2007;40(1):4–13. [PubMed] [Google Scholar]
- 33.Dos Santos J.S., Biduski B., Dos Santos L.R. Listeria monocytogenes: health risk and a challenge for food processing establishments. Arch. Microbiol. 2021;203(10):5907–5919. doi: 10.1007/s00203-021-02590-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Q., Gooneratne R., Hussain M.A. Listeria monocytogenes in fresh produce: outbreaks, prevalence and contamination levels. Foods. 2017;6(3):21. doi: 10.3390/foods6030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pizarro-Cerdá J., Cossart P. Microbe Profile: Listeria monocytogenes: a paradigm among intracellular bacterial pathogens. Microbiology (Read.) 2019;165(7):719–721. doi: 10.1099/mic.0.000800. [DOI] [PubMed] [Google Scholar]
- 36.Keet R., Rip D. Listeria monocytogenes isolates from Western Cape, South Africa exhibit resistance to multiple antibiotics and contradicts certain global resistance patterns. AIMS Microbiol. 2021;7(1):40–58. doi: 10.3934/microbiol.2021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kayode A.J., Okoh A.I. Assessment of multidrug-resistant Listeria monocytogenes in milk and milk product and One Health perspective. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0270993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Que F., Xu B., Sun L., Zhu Y., Chen W., et al. Identification of Listeria monocytogenes contamination in a ready-to-eat meat processing plant in China. Front. Microbiol. 2021;12:628204. doi: 10.3389/fmicb.2021.628204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jibo G.G., Raji Y.E., Salawudeen A., Amin-Nordin S., Mansor R., Jamaluddin T.Z.M.T. A systematic review and meta-analysis of the prevalence of Listeria monocytogenes in South-East Asia; a one-health approach of human-animal-food-environment. One Health. 2022;15:100417. doi: 10.1016/j.onehlt.2022.100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graveland H., Wagenaar J.A., Bergs K., Heesterbeek H., Heederik D. Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuny C., Friedrich A., Kozytska S., Layer F., Nübel U., Ohlsen K., et al. Emergence of methicillin-resistant Staphylococcus aureus (MRSA) in different animal species. Int. J. Med. Microbiol. 2010;300(2–3):109–117. doi: 10.1016/j.ijmm.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Devriese L.A., Van Damme L.R., Fameree L. Methicillin (cloxacillin)-resistant Staphylococcus aureus strains isolated from bovine mastitis cases. Zentralblatt für Veterinarmed. B. 1972;19(7):598–605. doi: 10.1111/j.1439-0450.1972.tb00439.x. [DOI] [PubMed] [Google Scholar]
- 43.Price L.B., Stegger M., Hasman H., Aziz M., Larsen J., Andersen P.S., et al. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio. 2012;3(1) doi: 10.1128/mBio.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rijen M.M., Kluytmans-van den Bergh M.F., Verkade E.J., Ten Ham P.B., Feingold B.J., Kluytmans J.A. Lifestyle-associated risk factors for community-acquired methicillin-resistant Staphylococcus aureus carriage in The Netherlands: an exploratory hospital-based case-control study. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Boer E., Zwartkruis-Nahuis J., Wit B., Huijsdens X., De Neeling A., Bosch T., et al. Prevalence of methicillin-resistant Staphylococcus aureus in meat. Int. J. Food Microbiol. 2009;134(1–2):52–56. doi: 10.1016/j.ijfoodmicro.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Esiovwa R., Connolly J., Hursthouse A., Mukherji S., Mukherji S., Parasnis A., et al. Bridging the gaps in the global governance of antimicrobial resistance: the UN sustainable development goals and global health security agenda. Routledge Open Research. 2022;1(8):8. [Google Scholar]
- 47.Diallo O.O., Baron S.A., Abat C., Colson P., Chaudet H., Rolain J.-M. Antibiotic resistance surveillance systems: a review. J. Glob. Antimicrob. Resist. 2020;23:430–438. doi: 10.1016/j.jgar.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Mader R., Eu J., Bourély C., Amat J.-P., Broens E.M., Busani L., et al. Defining the scope of the European antimicrobial resistance surveillance network in veterinary medicine (EARS-Vet): a bottom-up and one health approach. J. Antimicrob. Chemother. 2022;77(3):816–826. doi: 10.1093/jac/dkab462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song J.-H., Hsueh P.-R., Chung D.R., Ko K.S., Kang C.-I., Peck K.R., et al. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J. Antimicrob. Chemother. 2011;66(5):1061–1069. doi: 10.1093/jac/dkr024. [DOI] [PubMed] [Google Scholar]
- 50.Baede V.O., David M.Z., Andrasevic A.T., Blanc D.S., Borg M., Brennan G., et al. MRSA surveillance programmes worldwide: moving towards a harmonised international approach. Int. J. Antimicrob. Agents. 2022;59(3):106538. doi: 10.1016/j.ijantimicag.2022.106538. [DOI] [PubMed] [Google Scholar]
- 51.Negrón M.E., Kharod G.A., Bower W.A., Walke H. Notes from the field: human Brucella abortus RB51 infections caused by consumption of unpasteurized domestic dairy products - United States, 2017-2019. MMWR Morb. Mortal. Wkly. Rep. 2019;68(7):185. doi: 10.15585/mmwr.mm6807a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dadar M., Alamian S., Behrozikhah A.M., Yazdani F., Kalantari A., Etemadi A., et al. Molecular identification of Brucella species and biovars associated with animal and human infection in Iran. Vet. Res. Forum. 2019;10(4):315–321. doi: 10.30466/vrf.2018.89680.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dadar M., Shahali Y., Whatmore A.M. Human brucellosis caused by raw dairy products: a review on the occurrence, major risk factors and prevention. Int. J. Food Microbiol. 2019;292:39–47. doi: 10.1016/j.ijfoodmicro.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Franc K.A., Krecek R.C., Häsler B.N., Arenas-Gamboa A.M. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Publ. Health. 2018;18(1):125. doi: 10.1186/s12889-017-5016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gwida M., Al Dahouk S., Melzer F., Rösler U., Neubauer H., Tomaso H. Brucellosis - regionally emerging zoonotic disease? Croat. Med. J. 2010;51(4):289–295. doi: 10.3325/cmj.2010.51.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wareth G., El-Diasty M., Abdel-Hamid N.H., Holzer K., Hamdy M.E.R., Moustafa S., et al. Molecular characterization and antimicrobial susceptibility testing of clinical and non-clinical Brucella melitensis and Brucella abortus isolates from Egypt. One Health. 2021;13:100255. doi: 10.1016/j.onehlt.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wareth G., Dadar M., Ali H., Hamdy M.E.R., Al-Talhy A.M., Elkharsawi A.R., et al. The perspective of antibiotic therapeutic challenges of brucellosis in the Middle East and North African countries: current situation and therapeutic management. Transbound. Emerg. Dis. 2022;69(5):e1253–e1268. doi: 10.1111/tbed.14502. [DOI] [PubMed] [Google Scholar]
- 58.Hermesh B., Rosenthal A., Davidovitch N. Rethinking "one health" through brucellosis: ethics, boundaries and politics. Monash Bioeth. Rev. 2019;37(1–2):22–37. doi: 10.1007/s40592-018-0079-9. [DOI] [PubMed] [Google Scholar]
- 59.Hemmink J.D., Morgan S.B., Aramouni M., Everett H., Salguero F.J., Canini L., et al. Distinct immune responses and virus shedding in pigs following aerosol, intra-nasal and contact infection with pandemic swine influenza A virus, A(H1N1)09. Vet. Res. 2016;47(1):103. doi: 10.1186/s13567-016-0390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abe H., Mine J., Parchariyanon S., Takemae N., Boonpornprasert P., Ubonyaem N., et al. Co-infection of influenza A viruses of swine contributes to effective shuffling of gene segments in a naturally reared pig. Virology. 2015;484:203–212. doi: 10.1016/j.virol.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Kong W., Wang F., Dong B., Ou C., Meng D., Liu J., et al. Novel reassortant influenza viruses between pandemic (H1N1) 2009 and other influenza viruses pose a risk to public health. Microb. Pathog. 2015;89:62–72. doi: 10.1016/j.micpath.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Kothalawala H., Toussaint M.J., Gruys E. An overview of swine influenza. Vet. Q. 2006;28(2):46–53. [PubMed] [Google Scholar]
- 63.Ding F., Li Y., Huang B., Edwards J., Cai C., Zhang G., et al. Infection and risk factors of human and avian influenza in pigs in south China. Prev. Vet. Med. 2021;190:105317. doi: 10.1016/j.prevetmed.2021.105317. [DOI] [PubMed] [Google Scholar]
- 64.Naguib M.M., Li R., Ling J., Grace D., Nguyen-Viet H., Lindahl J.F. Live and wet markets: food access versus the risk of disease emergence. Trends Microbiol. 2021;29(7):573–581. doi: 10.1016/j.tim.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harvey E., Holmes E.C. Diversity and evolution of the animal virome. Nat. Rev. Microbiol. 2022;20(6):321–334. doi: 10.1038/s41579-021-00665-x. [DOI] [PubMed] [Google Scholar]
- 66.Short K.R., Richard M., Verhagen J.H., van Riel D., Schrauwen E.J.A., van den Brand J.M.A., et al. One health, multiple challenges: the inter-species transmission of influenza A virus. One Health. 2015;1:1–13. doi: 10.1016/j.onehlt.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adegbeye M.J., Reddy P.R.K., Chilaka C.A., Balogun O.B., Elghandour M.M.M.Y., Rivas-Caceres R.R., et al. Mycotoxin toxicity and residue in animal products: prevalence, consumer exposure and reduction strategies – a review. Toxicon. 2020;177:96–108. doi: 10.1016/j.toxicon.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Song L., Xie Q., Lv Z. Foodborne parasitic diseases in China: a scoping review on current situation, epidemiological trends, prevention and control. Asian Pac. J. Tropical Med. 2021;14(9):385. [Google Scholar]
- 69.Stelzer S., Basso W., Benavides Silván J., Ortega-Mora L.M., Maksimov P., Gethmann J., et al. Toxoplasma gondii infection and toxoplasmosis in farm animals: risk factors and economic impact. Food Waterborne Parasitol. 2019;15 doi: 10.1016/j.fawpar.2019.e00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Aquino M.C.C., Inácio S.V., Rodrigues F.S., de Barros L.D., Garcia J.L., Headley S.A., et al. Cryptosporidiosis and giardiasis in buffaloes (Bubalus bubalis) Front. Vet. Sci. 2020;7:557967. doi: 10.3389/fvets.2020.557967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Healy S.R., Morgan E.R., Prada J.M., Betson M. Brain food: rethinking food-borne toxocariasis. Parasitology. 2022;149(1):1–9. doi: 10.1017/S0031182021001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Picot S., Beugnet F., Leboucher G., Bienvenu A.-L. Drug resistant parasites and fungi from a one-health perspective: a global concern that needs transdisciplinary stewardship programs. One Health. 2022;14:100368. doi: 10.1016/j.onehlt.2021.100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Esvac . 2011. Sales of Veterinary Antimicrobial Agents in 25 EU/EEA Countries in 2011 Third ESVAC Report.https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-25-european-union/european-economic-area-countries-2011-third-european-surveillance-veterinary-antimicrobial_en.pdf [Available from: [Google Scholar]
- 74.Laxminarayan R., Duse A., Wattal C., Zaidi A.K.M., Wertheim H.F.L., Sumpradit N., et al. Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 2013;13(12):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y.Y., Wang Y., Walsh T.R., Yi L.X., Zhang R., Spencer J., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 76.Spratt B.G. Resistance to antibiotics mediated by target alterations. Science. 1994;264(5157):388–393. doi: 10.1126/science.8153626. [DOI] [PubMed] [Google Scholar]
- 77.Peterson E., Kaur P. Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018;9:2928. doi: 10.3389/fmicb.2018.02928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brañas P., Villa J., Viedma E., Mingorance J., Orellana M.A., Chaves F. Molecular epidemiology of carbapenemase-producing Klebsiella pneumoniae in a hospital in Madrid: successful establishment of an OXA-48 ST11 clone. Int. J. Antimicrob. Agents. 2015;46(1):111–116. doi: 10.1016/j.ijantimicag.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 79.Forsberg K.J., Reyes A., Wang B., Selleck E.M., Sommer M.O.A., Dantas G. The shared antibiotic resistome of soil bacteria and human pathogens. Science. 2012;337(6098):1107–1111. doi: 10.1126/science.1220761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stoesser N., Sheppard A.E., Pankhurst L., De Maio N., Moore C.E., Sebra R., et al. Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131. mBio. 2016;7(2) doi: 10.1128/mBio.02162-15. 021622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackenzie J.S., Jeggo M. The one health approach-why is it so important? Trav. Med. Infect. Dis. 2019;4(2):88. doi: 10.3390/tropicalmed4020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Z., Zhang Q., Wang T., Xu N., Lu T., Hong W., et al. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022;13(1):1533. doi: 10.1038/s41467-022-29283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shanta I.S., Hasnat M.A., Zeidner N., Gurley E.S., Azziz-Baumgartner E., Sharker M.A.Y., et al. Raising backyard poultry in rural Bangladesh: financial and nutritional benefits, but persistent risky practices. Transbound. Emerg. Dis. 2017;64(5):1454–1464. doi: 10.1111/tbed.12536. [DOI] [PubMed] [Google Scholar]
- 84.Stålsby Lundborg C., Diwan V., Pathak A., Purohit M.R., Shah H., Sharma M., et al. Protocol: a 'One health' two year follow-up, mixed methods study on antibiotic resistance, focusing children under 5 and their environment in rural India. BMC Publ. Health. 2015;15:1321. doi: 10.1186/s12889-015-2632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roess A.A., Winch P.J., Akhter A., Afroz D., Ali N.A., Shah R., et al. Household animal and human medicine use and animal husbandry practices in rural Bangladesh: risk factors for emerging zoonotic disease and antibiotic resistance. Zoonoses Public Health. 2015;62(7):569–578. doi: 10.1111/zph.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Engberg J. Quinolone and macrolide resistance in Campylobacter jejuni and C. Coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 2001;7(1):24–34. doi: 10.3201/eid0701.010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.White D.G., Zhao S., Sudler R., Ayers S., Friedman S., Chen S., et al. The isolation of antibiotic-resistant Salmonella from retail ground meats. N. Engl. J. Med. 2001;345(16):1147–1154. doi: 10.1056/NEJMoa010315. [DOI] [PubMed] [Google Scholar]
- 88.Doster E., Thomas K.M., Weinroth M.D., Parker J.K., Crone K.K., Arthur T.M., et al. Metagenomic characterization of the microbiome and resistome of retail ground beef products. Front. Microbiol. 2020;11:541972. doi: 10.3389/fmicb.2020.541972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tyrrell C., Burgess C.M., Brennan F.P., Walsh F. Antibiotic resistance in grass and soil. Biochem. Soc. Trans. 2019;47(1):477–486. doi: 10.1042/BST20180552. [DOI] [PubMed] [Google Scholar]
- 90.Wu N., Qiao M., Zhang B., Cheng W.D., Zhu Y.G. Abundance and diversity of tetracycline resistance genes in soils adjacent to representative swine feedlots in China. Environ. Sci. Technol. 2010;44(18):6933–6939. doi: 10.1021/es1007802. [DOI] [PubMed] [Google Scholar]
- 91.Xie W.Y., Shen Q., Zhao F.J. Antibiotics and antibiotic resistance from animal manures to soil: a review. Eur. J. Soil Sci. 2018;69(1):181–195. [Google Scholar]
- 92.Bartlett J.G., Gilbert D.N., Spellberg B. Seven ways to preserve the miracle of antibiotics. Clin. Infect. Dis. 2013;56(10):1445–1450. doi: 10.1093/cid/cit070. [DOI] [PubMed] [Google Scholar]
- 93.Van Boeckel T.P., Glennon E.E., Chen D., Gilbert M., Robinson T.P., Grenfell B.T., et al. Reducing antimicrobial use in food animals. Science. 2017;357(6358):1350–1352. doi: 10.1126/science.aao1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muloi D., Kiiru J., Ward M.J., Hassell J.M., Bettridge J.M., Robinson T.P., et al. Epidemiology of antimicrobial-resistant Escherichia coli carriage in sympatric humans and livestock in a rapidly urbanizing city. Int. J. Antimicrob. Agents. 2019;54(5):531–537. doi: 10.1016/j.ijantimicag.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X., Ma C., Zhang W., Li W., Yu J., Xue D., et al. Shifts in microbial community, pathogenicity-related genes and antibiotic resistance genes during dairy manure piled up. Microb. Biotechnol. 2020;13(4):1039–1053. doi: 10.1111/1751-7915.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu D., Mariman R., Gerlofs-Nijland M.E., Boere J.F., Folkerts G., Cassee F.R., et al. Microbiome composition of airborne particulate matter from livestock farms and their effect on innate immune receptors and cells. Sci. Total Environ. 2019;688:1298–1307. doi: 10.1016/j.scitotenv.2019.06.217. [DOI] [PubMed] [Google Scholar]
- 97.He P., Wu Y., Huang W., Wu X., Lv J., Liu P., et al. Characteristics of and variation in airborne ARGs among urban hospitals and adjacent urban and suburban communities: a metagenomic approach. Environ. Int. 2020;139:105625. doi: 10.1016/j.envint.2020.105625. [DOI] [PubMed] [Google Scholar]
- 98.Karimi H., Nikaeen M., Gholipour S., Hatamzadeh M., Hassanzadeh A., Hajizadeh Y. PM(2.5)-associated bacteria in ambient air: is PM(2.5) exposure associated with the acquisition of community-acquired staphylococcal infections? J Environ Health Sci Eng. 2020;18(2):1007–1013. doi: 10.1007/s40201-020-00522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davis M.F., Pisanic N., Rhodes S.M., Brown A., Keller H., Nadimpalli M., et al. Occurrence of Staphylococcus aureus in swine and swine workplace environments on industrial and antibiotic-free hog operations in North Carolina, USA: a One Health pilot study. Environ. Res. 2018;163:88–96. doi: 10.1016/j.envres.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Rooij M.M.T., Hoek G., Schmitt H., Janse I., Swart A., Maassen C.B.M., et al. Insights into livestock-related microbial concentrations in air at residential level in a livestock dense area. Environ. Sci. Technol. 2019;53(13):7746–7758. doi: 10.1021/acs.est.8b07029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Manlove K.R., Walker J.G., Craft M.E., Huyvaert K.P., Joseph M.B., Miller R.S., et al. "One health" or three? Publication silos among the one health disciplines. PLoS Biol. 2016;14(4) doi: 10.1371/journal.pbio.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trinh P., Zaneveld J.R., Safranek S., Rabinowitz P.M. One health relationships between human, animal, and environmental microbiomes: a mini-review. Front. Public Health. 2018;6:235. doi: 10.3389/fpubh.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu Y., Wan J., Choe U., Pham Q., Schoene N.W., He Q., et al. Interactions between food and gut microbiota: impact on human health. Annu. Rev. Food Sci. Technol. 2019;10:389–408. doi: 10.1146/annurev-food-032818-121303. [DOI] [PubMed] [Google Scholar]
- 105.Petrova P., Ivanov I., Tsigoriyna L., Valcheva N., Vasileva E., Parvanova-Mancheva T., et al. Traditional Bulgarian dairy products: ethnic foods with health benefits. Microorganisms. 2021;9(3):480. doi: 10.3390/microorganisms9030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berry D., Loy A. Stable-isotope probing of human and animal microbiome function. Trends Microbiol. 2018;26(12):999–1007. doi: 10.1016/j.tim.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Gompel L., Luiken R.E.C., Hansen R.B., Munk P., Bouwknegt M., Heres L., et al. Description and determinants of the faecal resistome and microbiome of farmers and slaughterhouse workers: a metagenome-wide cross-sectional study. Environ. Int. 2020;143:105939. doi: 10.1016/j.envint.2020.105939. [DOI] [PubMed] [Google Scholar]
- 108.Wilkinson J.E., Franzosa E.A., Everett C., Li C., Bae S., Berzansky I., et al. A framework for microbiome science in public health. Nat. Med. 2021;27(5):766–774. doi: 10.1038/s41591-021-01258-0. [DOI] [PubMed] [Google Scholar]
- 109.Luiken R.E.C., Van Gompel L., Bossers A., Munk P., Joosten P., Hansen R.B., et al. Farm dust resistomes and bacterial microbiomes in European poultry and pig farms. Environ. Int. 2020;143:105971. doi: 10.1016/j.envint.2020.105971. [DOI] [PubMed] [Google Scholar]
- 110.San Juan P.A., Hendershot J.N., Daily G.C., Fukami T. Land-use change has host-specific influences on avian gut microbiomes. ISME J. 2020;14(1):318–321. doi: 10.1038/s41396-019-0535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.He L.Y., He L.K., Liu Y.S., Zhang M., Zhao J.L., Zhang Q.Q., et al. Microbial diversity and antibiotic resistome in swine farm environments. Sci. Total Environ. 2019;685:197–207. doi: 10.1016/j.scitotenv.2019.05.369. [DOI] [PubMed] [Google Scholar]
- 112.Henry L.P., Bruijning M., Forsberg S.K.G., Ayroles J.F. The microbiome extends host evolutionary potential. Nat. Commun. 2021;12(1):5141. doi: 10.1038/s41467-021-25315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O'Brien P.A., Webster N.S., Miller D.J., Bourne D.G. Host-microbe coevolution: applying evidence from model systems to complex marine invertebrate holobionts. mBio. 2019;10(1) doi: 10.1128/mBio.02241-18. 022411-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koskella B., Bergelson J. The study of host–microbiome (co)evolution across levels of selection. Phil. Trans. Biol. Sci. 2020;375(1808):20190604. doi: 10.1098/rstb.2019.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lewin-Epstein O., Hadany L. Host–microbiome coevolution can promote cooperation in a rock–paper–scissors dynamics. Proc. Royal Soc. B. 2020;287(1920):20192754. doi: 10.1098/rspb.2019.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hillesland K.L. Evolution on the bright side of life: microorganisms and the evolution of mutualism. Ann. N. Y. Acad. Sci. 2018;1422(1):88–103. doi: 10.1111/nyas.13515. [DOI] [PubMed] [Google Scholar]
- 117.Meroz N., Tovi N., Sorokin Y., Friedman J. Community composition of microbial microcosms follows simple assembly rules at evolutionary timescales. Nat. Commun. 2021;12(1):2891. doi: 10.1038/s41467-021-23247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mills J.N., Gage K.L., Khan A.S. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environ. Health Perspect. 2010;118(11):1507–1514. doi: 10.1289/ehp.0901389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith H.J. An ethical investigation into the microbiome: the intersection of agriculture, genetics, and the obesity epidemic. Gut Microb. 2020;12(1):1760712. doi: 10.1080/19490976.2020.1760712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sapkota A.R., Lefferts L.Y., McKenzie S., Walker P. What do we feed to food-production animals? A review of animal feed ingredients and their potential impacts on human health. Environ. Health Perspect. 2007;115(5):663–670. doi: 10.1289/ehp.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Capita R., Alonso-Calleja C. Antibiotic-resistant bacteria: a challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013;53(1):11–48. doi: 10.1080/10408398.2010.519837. [DOI] [PubMed] [Google Scholar]
- 122.Pires J., Huisman J.S., Bonhoeffer S., Van Boeckel T.P. Multidrug resistance dynamics in Salmonella in food animals in the United States: an analysis of genomes from public databases. Microbiol. Spectr. 2021;9(2) doi: 10.1128/Spectrum.00495-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Atlaw N.A., Keelara S., Correa M., Foster D., Gebreyes W., Aidara-Kane A., et al. Evidence of sheep and abattoir environment as important reservoirs of multidrug resistant Salmonella and extended-spectrum beta-lactamase Escherichia coli. Int. J. Food Microbiol. 2022;363:109516. doi: 10.1016/j.ijfoodmicro.2021.109516. [DOI] [PubMed] [Google Scholar]
- 124.Hsu C.H., Li C., Hoffmann M., McDermott P., Abbott J., Ayers S., et al. Comparative genomic analysis of virulence, antimicrobial resistance, and plasmid profiles of Salmonella dublin isolated from sick cattle, retail beef, and humans in the United States. Microb. Drug Resist. 2019;25(8):1238–1249. doi: 10.1089/mdr.2019.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patel K., Godden S.M., Royster E.E., Crooker B.A., Johnson T.J., Smith E.A., et al. Prevalence, antibiotic resistance, virulence and genetic diversity of Staphylococcus aureus isolated from bulk tank milk samples of U.S. dairy herds. BMC Genom. 2021;22(1):367. doi: 10.1186/s12864-021-07603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bailey M.A., Taylor R.M., Brar J.S., Corkran S.C., Velásquez C., Novoa Rama E., et al. Prevalence and antimicrobial resistance of Campylobacterfrom antibiotic-free broilers during organic and conventional processing. Poultry Sci. 2019;98(3):1447–1454. doi: 10.3382/ps/pey486. [DOI] [PubMed] [Google Scholar]
- 127.Sanchez-Maldonado A.F., Aslam M., Service C., Narváez-Bravo C., Avery B.P., Johnson R., et al. Prevalence and antimicrobial resistance of Salmonella isolated from two pork processing plants in Alberta, Canada. Int. J. Food Microbiol. 2017;241:49–59. doi: 10.1016/j.ijfoodmicro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 128.Scott L., Menzies P., Reid-Smith R.J., Avery B.P., McEwen S.A., Moon C.S., et al. Antimicrobial resistance in Campylobacter spp. isolated from Ontario sheep flocks and associations between antimicrobial use and antimicrobial resistance. Zoonoses Public Health. 2012;59(4):294–301. doi: 10.1111/j.1863-2378.2011.01450.x. [DOI] [PubMed] [Google Scholar]
- 129.Majumder S., Jung D., Ronholm J., George S. Prevalence and mechanisms of antibiotic resistance in Escherichia coli isolated from mastitic dairy cattle in Canada. BMC Microbiol. 2021;21(1):222. doi: 10.1186/s12866-021-02280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Diarra M.S., Delaquis P., Rempel H., Bach S., Harlton C., Aslam M., et al. Antibiotic resistance and diversity of Salmonella enterica serovars associated with broiler chickens. J. Food Protect. 2014;77(1):40–49. doi: 10.4315/0362-028.JFP-13-251. [DOI] [PubMed] [Google Scholar]
- 131.Martínez-Vázquez A.V., Rivera-Sánchez G., Lira-Méndez K., Reyes-López M.Á., Bocanegra-García V. Prevalence, antimicrobial resistance and virulence genes of Escherichia coli isolated from retail meat in Tamaulipas, Mexico. J. Glob. Antimicrob. Resist. 2018;14:266–272. doi: 10.1016/j.jgar.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 132.de la Rosa-HernÁNdez M.C., Cadena-RamÍRez A., TÉLlez-Jurado A., GÓMez-Aldapa C.A., Rangel-Vargas E., ChÁVez-Urbiola E.A., et al. Presence of multidrug-resistant shiga toxin–producing Escherichia coli, enteropathogenic Escherichia coli, and enterotoxigenic Escherichia coli on fresh cheeses from local retail markets in Mexico. J. Food Protect. 2018;81(11):1748–1754. doi: 10.4315/0362-028X.JFP-18-166. [DOI] [PubMed] [Google Scholar]
- 133.Delgado-Suárez E.J., Palós-Guitérrez T., Ruíz-López F.A., Hernández Pérez C.F., Ballesteros-Nova N.E., Soberanis-Ramos O., et al. Genomic surveillance of antimicrobial resistance shows cattle and poultry are a moderate source of multi-drug resistant non-typhoidal Salmonella in Mexico. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0243681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rabello R.F., Bonelli R.R., Penna B.A., Albuquerque J.P., Souza R.M., Cerqueira A.M.F. Antimicrobial resistance in farm animals in Brazil: an update overview. Animals. 2020;10(4):552. doi: 10.3390/ani10040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joaquim P., Herrera M., Dupuis A., Chacana P. Virulence genes and antimicrobial susceptibility in Salmonella enterica serotypes isolated from swine production in Argentina. Rev. Argent. Microbiol. 2021;53(3):233–239. doi: 10.1016/j.ram.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 136.Prack McCormick B., Quiroga M.P., Álvarez V.E., Centrón D., Tittonell P. Antimicrobial resistance dissemination associated with intensive animal production practices in Argentina: a systematic review and meta-analysis. Rev. Argent. Microbiol. 2022;55(1):25–42. doi: 10.1016/j.ram.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 137.Zbrun M.V., Olivero C., Romero-Scharpen A., Rossler E., Soto L.P., Astesana D.M., et al. Antimicrobial resistance in thermotolerant Campylobacter isolated from different stages of the poultry meat supply chain in Argentina. Food Control. 2015;57:136–141. [Google Scholar]
- 138.Dávalos-Almeyda M., Guerrero A., Medina G., Dávila-Barclay A., Salvatierra G., Calderón M., et al. Antibiotic use and resistance knowledge assessment of personnel on chicken farms with high levels of antimicrobial resistance: a cross-sectional survey in ica, Peru. Antibiotics. 2022;11(2):190. doi: 10.3390/antibiotics11020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bueno I., Travis D., Gonzalez-Rocha G., Alvarez J., Lima C., Benitez C.G., et al. Antibiotic resistance genes in freshwater trout farms in a watershed in Chile. J. Environ. Qual. 2019;48(5):1462–1471. doi: 10.2134/jeq2018.12.0431. [DOI] [PubMed] [Google Scholar]
- 140.Li M., Li Z., Zhong Q., Liu J., Han G., Li Y., et al. Antibiotic resistance of fecal carriage of Escherichia coli from pig farms in China: a meta-analysis. Environ. Sci. Pollut. Control Ser. 2022;29(16):22989–23000. doi: 10.1007/s11356-021-17339-z. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Y., Lu J., Yan Y., Liu J., Wang M. Antibiotic residues in cattle and sheep meat and human exposure assessment in southern Xinjiang, China. Food Sci. Nutr. 2021;9(11):6152–6161. doi: 10.1002/fsn3.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang W., Wei X., Wu L., Shang X., Cheng F., Li B., et al. The occurrence of antibiotic resistance genes in the microbiota of yak, beef and dairy cattle characterized by a metagenomic approach. J. Antibiot. (Tokyo) 2021;74(8):508–518. doi: 10.1038/s41429-021-00425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jia K., Fang T., Wang X., Liu Y., Sun W., Wang Y., et al. Antibiotic resistance patterns of Staphylococcus aureus isolates from retail foods in mainland China: a meta-analysis. Foodb. Pathog. Dis. 2020;17(5):296–307. doi: 10.1089/fpd.2019.2686. [DOI] [PubMed] [Google Scholar]
- 144.Li C., Wang Y., Gao Y., Li C., Ma B., Wang H. Antimicrobial resistance and CRISPR typing among Salmonella isolates from poultry farms in China. Front. Microbiol. 2021;12:730046. doi: 10.3389/fmicb.2021.730046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hong B., Ba Y., Niu L., Lou F., Zhang Z., Liu H., et al. A comprehensive research on antibiotic resistance genes in microbiota of aquatic animals. Front. Microbiol. 2018;9:1617. doi: 10.3389/fmicb.2018.01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Beleneva I.A. Incidence and characteristics of Staphylococcus aureus and Listeria monocytogenes from the Japan and South China seas. Mar. Pollut. Bull. 2011;62(2):382–387. doi: 10.1016/j.marpolbul.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 147.Ishtifaq A., Qureshi S., Farooq S., Kashoo Z.A., Malik M.Z., Alam M.R., et al. Genotyping and antibiotic resistance patterns of Campylobacter fetus subsp.venerealis from cattle farms in India. Lett. Appl. Microbiol. 2020;71(6):627–636. doi: 10.1111/lam.13378. [DOI] [PubMed] [Google Scholar]
- 148.Jindal P., Bedi J., Singh R., Aulakh R., Gill J.P. Epidemiological assessment of antibiotic residues in dairy farm milk and farm waste and water in northern India. Environ. Sci. Pollut. Control Ser. 2021;28(23):29455–29466. doi: 10.1007/s11356-020-12057-4. [DOI] [PubMed] [Google Scholar]
- 149.Sebastian S., Tom A.A., Babu J.A., Joshy M. Antibiotic resistance in Escherichia coli isolates from poultry environment and UTI patients in Kerala, India: a comparison study. Comp. Immunol. Microbiol. Infect. Dis. 2021;75:101614. doi: 10.1016/j.cimid.2021.101614. [DOI] [PubMed] [Google Scholar]
- 150.Elmahdi S., DaSilva L.V., Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 2016;57:128–134. doi: 10.1016/j.fm.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 151.Pornsukarom S., van Vliet A.H.M., Thakur S. Whole genome sequencing analysis of multiple Salmonella serovars provides insights into phylogenetic relatedness, antimicrobial resistance, and virulence markers across humans, food animals and agriculture environmental sources. BMC Genom. 2018;19(1):801. doi: 10.1186/s12864-018-5137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mohiuddin M., Iqbal Z., Siddique A., Liao S., Salamat M.K.F., Qi N., et al. Prevalence, genotypic and phenotypic characterization and antibiotic resistance profile of Clostridium perfringens type A and D isolated from feces of sheep (Ovis aries) and goats (Capra hircus) in Punjab, Pakistan. Toxins. 2020;12(10):657. doi: 10.3390/toxins12100657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Askari N., Momtaz H., Tajbakhsh E. Acinetobacter baumannii in sheep, goat, and camel raw meat: virulence and antibiotic resistance pattern. AIMS Microbiol. 2019;5(3):272–284. doi: 10.3934/microbiol.2019.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ranjbar R., Safarpoor Dehkordi F., Heiat M. The frequency of resistance genes in Salmonella enteritidis strains isolated from cattle. Iran. J. Public Health. 2020;49(5):968–974. [PMC free article] [PubMed] [Google Scholar]
- 155.Lameei A., Rahimi E., Shakerian A., Momtaz H. Genotyping, antibiotic resistance and prevalence of Arcobacter species in milk and dairy products. Veterinary Medicine and Science. 2022;8(4):1841–1849. doi: 10.1002/vms3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Şanlıbaba P. Prevalence, antibiotic resistance, and enterotoxin production of Staphylococcus aureus isolated from retail raw beef, sheep, and lamb meat in Turkey. Int. J. Food Microbiol. 2022;361:109461. doi: 10.1016/j.ijfoodmicro.2021.109461. [DOI] [PubMed] [Google Scholar]
- 157.Siriken B., Al G., Erol I. Prevalence and antibiotic resistance of Salmonella enteritidis and Salmonella typhimurium in ground beef and meatball samples in samsun, Turkey. Microb. Drug Res. 2020;26(2):136–144. doi: 10.1089/mdr.2018.0481. [DOI] [PubMed] [Google Scholar]
- 158.Arkali A., Çetinkaya B. Molecular identification and antibiotic resistance profiling of Salmonella species isolated from chickens in eastern Turkey. BMC Vet. Res. 2020;16(1):205. doi: 10.1186/s12917-020-02425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.De Jong A., El Garch F., Hocquet D., Prenger-Berninghoff E., Dewulf J., Migura-Garcia L., et al. European-wide antimicrobial resistance monitoring in commensal Escherichia coli isolated from healthy food animals between 2004 and 2018. J. Antimicrob. Chemother. 2022;77(12):3301–3311. doi: 10.1093/jac/dkac318. [DOI] [PubMed] [Google Scholar]
- 160.González-Gutiérrez M., García-Fernández C., Alonso-Calleja C., Capita R. Microbial load and antibiotic resistance in raw beef preparations from northwest Spain. Food Sci. Nutr. 2020;8(2):777–785. doi: 10.1002/fsn3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ottaviani D., Leoni F., Talevi G., Masini L., Santarelli S., Rocchegiani E., et al. Extensive investigation of antimicrobial resistance in Vibrio parahaemolyticus from shellfish and clinical sources. Italy. Int. J. Antimicrob. Agents. 2013;42(2):191–193. doi: 10.1016/j.ijantimicag.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 162.Mthembu T.P., Zishiri O.T., El Zowalaty M.E. Molecular detection of multidrug-resistant Salmonella isolated from livestock production systems in South Africa. Infect. Drug Resist. 2019;12:3537–3548. doi: 10.2147/IDR.S211618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Karama M., Kambuyi K., Cenci-Goga B.T., Malahlela M., Jonker A., He C., et al. Occurrence and antimicrobial resistance profiles of Campylobacter jejuni, Campylobacter coli, and Campylobacter upsaliensis in beef cattle on cow-calf operations in South Africa. Foodb. Pathog. Dis. 2020;17(7):440–446. doi: 10.1089/fpd.2019.2703. [DOI] [PubMed] [Google Scholar]
- 164.Karzis J., Petzer I.M., Donkin E.F., Naidoo V., Etter E.M.C. Climatic and regional antibiotic resistance patterns of Staphylococcus aureus in South African dairy herds. Onderstepoort J. Vet. Res. 2019;86(1):e1–e9. doi: 10.4102/ojvr.v86i1.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Siddique A., Ullah N., Ali A., Patel A., Moore T., Kenney S.M., et al. Draft genome sequences of 25 Salmonella enterica serovar Agona strains isolated from poultry and associated food products harbouring multiple antibiotic resistance genes. J. Glob. Antimicrob. Resist. 2022;29:131–135. doi: 10.1016/j.jgar.2022.02.013. [DOI] [PubMed] [Google Scholar]