Abstract
Chimeric antigen receptor-T cell therapy, a groundbreaking cancer treatment, has achieved remarkable success against hematologic malignancies. However, CAR-T monotherapy faces challenges in certain cases, including treatment tolerance and relapse rates. To overcome these challenges, researchers are investigating combining CAR-T cells with other treatments to enhance therapeutic efficacy. Therefore, this review aims to investigate the progress of research in combining CAR-T cells for hematologic malignancies. It covers the basic principles and clinical applications of CAR-T cell therapy, detailing combinations with chemotherapy, immune checkpoint inhibitors, targeted drugs, radiotherapy, hematopoietic stem cell transplantation, and other treatments. These combinations synergistically enhance the antitumor effects of CAR-T cells and comprehensively target tumors through different mechanisms, improving patient response and survival rates.
Keywords: Combination therapy, Chimeric antigen receptor T cell therapy, Hematologic malignancies, Hematopoietic stem cell transplantation, Complete remission rate, Multiple myeloma, Malignant tumor
Introduction
The principle of chimeric antigen receptor (CAR) T-cell therapy is to genetically modify T cells to recognize specific unique targets on tumor surfaces and exert cytotoxic effects [1–3]. CAR-T cell therapy has been highly successful in treating various hematologic malignancies and solid tumors [4–7]. The complete remission rate (CRR) in diffuse large B-cell lymphoma (DLBCL) is 43%, while in follicular lymphoma, it stands at an impressive 71% with sustained remissions [8]. Studies on Idecabtagene vicleucel for multiple myeloma (MM) involving 128 patients showed a 73% objective response rate(ORR), with 33% achieving complete remission (CR) [9]. The CRR for patients with B-cell acute lymphoblastic leukemia (B-ALL) can reach 81% [10]. Manageable adverse effects underscore the remarkable success of CAR-T products in treating hematologic malignancies, offering patients new treatment options and renewed hope. Currently, the FDA has approved six CAR-T cell products for hematologic malignancies [11–14], all with excellent treatment outcomes. CAR-T has become a reliable approach for treating various hematologic malignancies.
However, comprehensive comparative reports and studies evaluating the safety, tolerability, and efficacy of different combination therapies involving CAR-T cells for hematologic malignancies are lacking. Therefore, this review aims to investigate and analyze the progress in research concerning the combination of CAR-T cells with various treatments for hematologic malignancies. This encompasses an exploration of the fundamental principles and clinical applications of CAR-T cell therapy, along with detailed discussions on combinations involving chemotherapy, immune checkpoint inhibitors, targeted drugs, radiotherapy, hematopoietic stem cell transplantation (HSCT), and other therapeutic modalities.
CAR-T resistance and relapse
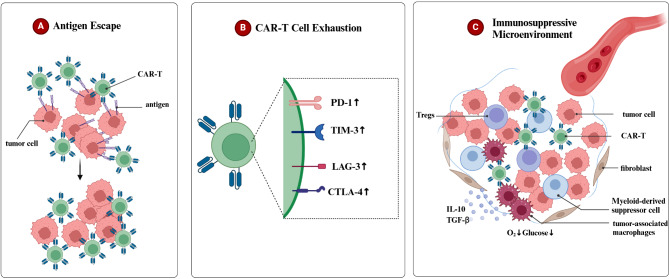
Despite CAR-T therapy demonstrating significant efficacy in some patients, it faces challenges such as tumor relapse and drug resistance in certain individuals [15–17]. Relapse rates range from 10 to 30% in B-ALL [18] and can reach 50% in DLBCL [19]. Factors contributing to this include antigen escape, CAR-T cell exhaustion, and an immune-suppressive microenvironment (Fig. 1).
Fig. 1.
Mechanisms of CAR-T cell resistance and relapse. (A) Antigen Escape: Tumor cell surface antigens escape, preventing effective recognition and destruction. (B) CAR-T Cell Exhaustion: Increased expression of inhibitory receptors (PD-1, TIM-3, LAG-3, CTLA-4) on CAR-T cells leads to exhaustion and reduced anti-tumor function. (C) Immunosuppressive Microenvironment: The tumor microenvironment contains immunosuppressive cells (TAMS, Tregs, myeloid-derived suppressor cells), cytokines (TGF-β, IL-10), and the extracellular matrix. These components inhibit immune cells, weaken CAR-T cell function, and limit infiltration. Tumor cells also consume oxygen and glucose, causing nutrient deprivation and hypoxia, further reducing CAR-T cell activity
Antigen escape
During CAR-T cell therapy, tumor cells evade CAR-T cell attacks through mechanisms such as acquired mutations [20], selective splicing [21, 22], and lineage switching [23–25], resulting in mutated or reduced surface antigen targets. Common surface targets, such as CD19, CD20, CD22, and B-cell maturation antigen (BCMA), among others, are susceptible to evasion [26–28]. Clinical analysis of relapsed samples has revealed genetic mutations in CD19, found in most resistant tumor cells, potentially causing protein truncation and subsequent loss of surface antigen [29]. Several studies indicate that patients with relapsed MM treated with BCMA CAR-T therapy exhibit decreased surface expression of BCMA on tumor cells [30–32]. This antigen escape phenomenon can shorten treatment duration and patient survival while complicating therapy. Consequently, novel treatment strategies are needed to address these challenges effectively.
CAR-T cell exhaustion
CAR-T cell exhaustion results in reduced CAR-T cell cytotoxicity, characterized by increased expression of inhibitory receptors, such as programmed cell death protein-1 (PD-1), lymphocyte activation gene-3 (LAG-3), T cell immunoglobulin and mucin domain-containing protein-3 (TIM-3), and cytotoxic T lymphocyte antigen-4 (CTLA-4), on CAR-T cell surfaces [33–38]. In treating chronic lymphocytic leukemia (CLL) with CD19 CAR-T cells, while some patients exhibit favorable responses, most do not benefit significantly from CAR-T therapy. Transcriptomic sequencing reveals that T cells in non-responsive patients upregulate programs associated with effector differentiation, glycolysis, exhaustion, and apoptosis [39]. This exhaustion significantly affects CAR-T cell functionality, suggesting that reducing exhaustion could enhance therapeutic efficacy. Research should aim to mitigate exhaustion to improve treatment outcomes.
Immunosuppressive microenvironment
The tumor microenvironment is where CAR-T cells exert their effects. It contains numerous immunosuppressive cells (such as tumor-associated macrophages (TAMS), regulatory T cells (Tregs), and myeloid suppressor cells), suppressor cytokines (such as TGF-β and IL-10), and an extracellular matrix [40]. These elements inhibit immune-activated cells through different mechanisms, weakening CAR-T cell function and limiting their infiltration [41, 42]. Studying the influence of the immunosuppressive microenvironment on CAR-T therapy and developing strategies to counter these effects are crucial for enhancing CAR-T treatment efficacy in hematologic malignancies.
Researchers are actively exploring new approaches to address these challenges [43]. One such approach involves combining CAR-T cell therapy with other drugs, leveraging the strengths of various treatment modalities. Through this combined approach, the efficacy of CAR-T cells can be preserved and compensate for the limitations of CAR-T monotherapy, extending the survival of the patients. This comprehensive therapeutic strategy offers more holistic and effective treatment options, enhancing hope and opportunities in cancer treatment. Tables 1 and 2 present details on preclinical research and clinical studies.
Table 1.
Preclinical research on CAR-T combination therapy
| Target | Combination therapy | Type of disease | Time | Mechanism | Refs |
|---|---|---|---|---|---|
| CD19 | PI3K inhibitors | CLL | 2022 |
i. Inhibit PI3K-mediated cell apoptosis by blocking Fas signal transduction. ii. Suppress cellular exhaustion. iii .Increasing the expression of mitochondrial fusion protein MFN2. |
[44] |
| CD19 | venetoclax | R/R NHL | 2022 | Enhance CAR-T cell cytotoxicity. | [45] |
| BCMA | GSI | MM | 2019 | Inhibit BCMA degradation. | [46] |
| CS1 | lenalidomide | MM | 2018 |
i.Increase the proportion of CD8 + CAR-T cells and decrease the proportion of CD4 + CAR-T cells. ii.Promote Th1 cytokine expression in CAR-T cells and inhibit Th2 cytokines. iii.Lenalidomide improves the formation of immune synapses between CAR-T cells and tumor cells, enhancing cytotoxicity against tumor cells. |
[47] |
| CD19 | lenalidomide | DLBCL | 2023 |
i.Polarize CD8 + CAR-T cells into CD8 + central memory cells and Th1 type. ii.Delay CAR-T exhaustion. iii.Promote CAR-T cell expansion. |
[48] |
| BCMA | lenalidomide | MM | 2019 |
i.Alter Th1 cell response, T cell activation, cytokine production, cell cycle control, and cytoskeletal remodeling-associated pathways. ii.In murine models, increased the number of circulating CAR-T cells in the bloodstream. |
[49] |
PI3K: Phosphatidylinositol 3-Kinase; CLL: chronic lymphocytic leukemia; R/R NHL: Relapsed/Refractory non-Hodgkin lymphoma; BCMA: B-cell maturation antigen; GSI: γ-secretase inhibitors; MM: multiple myeloma; DLBCL: diffuse large B-cell lymphoma
Table 2.
Clinical research on CAR-T combination therapy
| Target | Combination therapy | Disea-se | Authors | Time | Outcome | Adverse events | Clinical trials |
|---|---|---|---|---|---|---|---|
| CD19 | ibrutinib | CLL | Saar Gill et al. [50] | 2022 |
3monthsCR:44%; 48monthsOS:84%; PFS:70% |
≥ 3gradeCRS:20%; ICANS:26% |
NCT02640209 |
| CD19 | ibrutinib | CLL | Jordan Gauthier et al. [51] | 2020 | Con-ibr cohort vs. No-ibr cohort:1 year PFS :38% and 50% (P = 0.91) | CRS: Con-ibr cohort < No-ibr cohort | NCT01865617 |
| CD19 | lenalidomide | DLBCL | Nana Ping at al [52] | 2021 | C + Len cohort vs. C + cohort: 1 year OS: 100% vs. 33.3%; ORR 85.7% vs. 77.8% CR 42.9% vs. 33.3% | ≥ 3gradeCRS: 43.8%; | NA |
| CD19 | nivolumab | NHL | Yaqing Cao et al. [53] | 2019 |
ORR:81.81%; CR:45.45%; PFS:6 months |
1grade CRS:25%; 2gradeCRS:50%; ICANS:9% |
NA |
| CD19/CD22 | PD-1 inhibitor | R/R NHL | Xiangke Xin et al. [54] | 2024 |
In CD19/CD22 CAR-Tcohort with PD-1 inhibitors vs. CAR-Talone: ORR :82.9% vs. 60% ; 2 year PFS:59.8% vs. 21.3% |
≥ 3 grade CRS:13.8%, ICANS:6.2% | ChiCTR-OPN − 16,008,526 ChiCTR-OPN-16,009,847 |
| CD19 | Radiation therapy | R/R NHL | TagedPOmran Saifi et al [55] | 2022 | LC: bRT vs. sRT:84%vs.62% | NA | NA |
| CD19 | Radiation therapy | DLBCL |
Austin J. Sim et al. [56] |
2019 |
ORR:81.8%; CR:45% |
≥ 3 grade CRS and ICANS:27% | NA |
| BCMA | Radiation therapy | R/R MM | Shwetha H. Manjunath et al. [57] | 2021 |
Group A(receive no RT < 1 year)vs. Group B(receive RT < 1 year)vs .Group C(receive bridging-RT): PR or better: 54%vs.38%vs.50% |
GroupA vs. Group B vs. GroupC: G4 hematologic toxicities:61.5%vs.62.5%vs.25%; G3–4 neurotoxicity: 7.7%vs.25%vs.25%; G3-4CRS: 38.5%vs.25%vs.25% |
NCT02546167 |
| CD19/CD22 | ASCT | BCL | Yang Cao et al. [58] | 2021 |
ORR:90.5%; 2yearPFS:83.3% |
≥ 3 grade CRS: 3.8%;ICANS:21%; ≥ 3 grade ICANS:5% |
NA |
| CD19/CD22 | ASCT | DHL | Jia Wei et al. [59] | 2020 |
A cohort(CAR-T alone)ORR:75%; B cohort (CAR-T and ASCT)ORR:100% |
NA | ChiCTR-OPN-16,008,526 ChiCTR-OPN- 16,009,847 |
| CD19/CD20/CD22 | ASCT | R/R CNSL | Fei Xue et al. [60] | 2022 | CAR-T alone ORR:44.4%;CAR-T and ASCT ORR:100% |
≥ 3 grade CRS: 41%; ≥ 3 grade ICANS: 29% |
NA |
| CD19/CD20 | ASCT | R/R NHL(TP53 gene alteration) | Jia Wei et al. [61] | 2022 |
CAR-Talone vs. CAR-T and ASCT: ORR:87.1% vs. 92.9% ; CRR: 45.2% vs. 82.1% ; 24-month OS : 56.3% vs. 89.3% |
CRS:90.9%vs.94.7% ICANS:9.1%vs.19.3% |
ChiCTR-OPN-16,008,526 |
CLL: chronic lymphocytic leukemia; CR: complete remission; OS: overall survival; PFS: progression-free survival rate; CRS: cytokine release syndrome; ICANS: immune effector cell-associated neurotoxicity syndrome; DLBCL: diffuse large B-cell lymphoma; ORR: overall response rate; NHL: non-Hodgkin lymphoma; PD-1: programmed cell death protein-1; bRT: bridging radiation therapy; sRT: salvage radiation therapy; R/R MM: Relapsed/Refractory multiple myeloma; RT: radiation therapy; ASCT: Autologous Stem Cell Transplantation; DHL: double-hit lymphoma; R/R CNSL: relapsed/refractory central nervous system lymphoma
Combined therapeutic approaches
Bruton tyrosine kinase inhibitors
Bruton’s tyrosine kinase (BTK) is a pivotal tyrosine kinase in the B-cell signaling pathway, regulating B-cell development, maturation, and function. BTK inhibitors are categorized as covalent [62, 63] and noncovalent. Covalent inhibitors, such as ibrutinib [64], zanubrutinib [65, 66], and acalabrutinib, [67] irreversibly inhibit the BTK enzyme. Conversely, noncovalent inhibitors, such as pirtobrutinib, reversibly inhibit the BTK enzyme [68, 69]. These inhibitors disrupt the B-cell signaling pathway, suppressing B-cell activation, proliferation, and differentiation, thereby affecting disease progression therapeutically. When combined with CAR-T therapy, BTK inhibitors enhance the efficacy of CAR-T therapy against malignant tumors by modulating T cell function and remodeling the tumor immune microenvironment.
Preclinical studies show that BTK inhibitors can enhance CAR-T cell expansion [70], reduce CD19 CAR-T cell depletion, prolong in vivo persistence, increase CD4 + and CD8 + effector memory T cells [71], promote T cell differentiation toward Th1 cells [72], decrease immunosuppressive cell populations in patients [73], and enhance cytotoxicity [74]. This effect is primarily achieved by inhibiting CD3ζ phosphorylation of CAR and downregulating genes related to the T-cell activation pathway [75], demonstrating a synergistic effect when co-administered. BTK inhibitors enhance CAR-T cell function, which is crucial for treating CLL. In in vivo animal models, combining CD19 CAR-T with ibrutinib achieves 80 ~ 100% long-term disease control. Adding ibrutinib to CAR-T cell-target cell co-cultures significantly reduces exhaustion marker expression, such as PD-1, TIM-3, LAG-3, and CTLA-4 [76], indicating CAR-T cell exhaustion reduction is key in enhancing anti-tumor efficacy in combination therapy.
Clinical studies demonstrate that administering BTK inhibitors before or concurrently with CAR-T infusion enhances clinical efficacy. Pre-treatment with BTK inhibitors reduces tumor size, decreases burden, and maintains a healthy immune microenvironment. Using CAR-T and BTK inhibitors concurrently suppresses in vivo CAR-T cell exhaustion, alleviates dysfunction, and improves expansion capacity [77]. Single-cell sequencing of 15 patients with mantle cell lymphoma (MCL) receiving combined BTK inhibitor and CAR-T therapy revealed the importance of the HSP90-MYC-CDK9 axis in this treatment strategy [78]. Further research could investigate the functional characterization of this axis and its effect on treatment responses. In a single-center study, clinical efficacy was evaluated by combining BTK inhibitors with CAR-T cells. Nineteen patients who had received ibrutinib treatment for over 6 months without achieving CR underwent CD19 CAR-T therapy. The 3-month CRR was 44%, with 15 patients showing no detectable minimal residual disease (MRD) at 6 months. The overall survival rate at 48 months reached 84%, and the progression-free survival rate (PFS) was 70%. Thirteen patients maintained CR at the follow-up endpoint. Regarding safety, grade ≥ 3 cytokine release syndrome (CRS) occurred in 20% of cases, while immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 26% [50]. These findings confirm the synergistic effect between CAR-T and BTK inhibitors, offering a novel treatment option with high and sustained remission rates. When CAR-T is administered concurrently with ibrutinib, CRS severity decreases, while ICANS occurrence remains comparable. Data indicate reduced cytokine release, including MCP-1 and IL-2a, among others, with IL-6 levels showing no significant difference from the CAR-T alone group. The 1-year PFS probabilities after CD19 CAR T-cell therapy, with or without concurrent ibrutinib, were 38% and 50%, respectively, but this difference was not statistically significant [51]. This study discovered that combining CD19 CAR T-cell therapy and ibrutinib is well-tolerated and yields lasting therapeutic effects in patients with relapsed/refractory CLL. Patients undergoing concurrent ibrutinib treatment have milder CRS severity and reduced serum cytokine concentrations during treatment. Moreover, no significant difference in PFS is observed compared to those solely on CD19 CAR T-cell therapy.
Phosphatidylinositol 3-kinase inhibitors
The phosphatidylinositol 3-kinase (PI3K)-Akt-mTOR-c-myc signaling pathway is crucial in cancer and metabolic diseases due to its involvement in numerous protein-protein interactions and molecular crosstalk in B and T cells [79]. Studies show that adding PI3K inhibitors (PI3Ki), such as idelalisib (selective for PI3Kδ) [80] or duvelisib (a dual inhibitor of PI3Kδ and PI3Kγ), to in vitro cell experiments increases T cell numbers without hindering proliferation. Speculation suggests that PI3K inhibitors prevent PI3K-mediated cell apoptosis by blocking Fas signaling, thus inhibiting cell exhaustion [44]. During subsequent CAR-T cell manufacturing processes, incorporating duvelisib to create Duv-CART cells (CAR-T cells manufactured with duvelisib) resulted in a significant increase in CD8 + CAR-T cell number, accompanied by enhanced cytotoxicity. Bioinformatics analysis indicates that Duv-CART cells undergo epigenetic reprogramming toward stem cell-like properties. This includes upregulation of the mitochondrial fusion protein MFN2, along with increased expression of SIRT1 and TCF1/7. These changes enhance the efficacy of CAR-T cells against CLL both in vitro and in vivo [44]. Additionally, inhibiting PI3K increases stem cell central memory T cells (Tscm) and central memory T lymphocytes (Tcm) cell numbers, promoting cytotoxicity [44]. These findings underscore the crucial role of the PI3K signaling pathway in immune cell function and tumor immunity, providing valuable insights for new cancer immunotherapy strategies.
B cell lymphoma-2 antagonists
B cell lymphoma-2 (BCL-2), a key regulator of apoptosis, belongs to the BCL-2 protein family [81], comprising two subtypes: BCL-2α and BCL-2β. The difference lies in BCL-2β lacking the transmembrane domain. This protein exerts strong antiapoptotic effects and is often overexpressed in various tumor cells, contributing significantly to cancer resistance and insensitivity [82]. Elevated BCL-2 levels promote cancer cell survival and growth, correlating with disease progression, metastasis, and adverse clinical outcomes across several malignancies [83–86], including acute myeloid leukemia (AML) and B-cell non-Hodgkin lymphoma (B-NHL). Studies indicate that BCL-2 overexpression can induce resistance to tyrosine kinase inhibitors in chronic myeloid leukemia (CML) [84]. Inhibiting BCL-2 activity or reducing BCL-2 protein levels can effectively promote apoptosis in malignant tumor cells and increase sensitivity to radiotherapy and chemotherapy [87]. Venetoclax, a BCL-2 antagonist, induces apoptosis in cancer cells overexpressing BCL-2 and is approved for patients contraindicated for frontline treatment with kinase inhibitors [88, 89]. Combining BCL-2 inhibitors with CAR-T cells is crucial for enhancing therapeutic efficacy against malignant tumors, warranting further exploration of their mechanisms of action.
Researchers investigated the effect of BCL-2 inhibitors on CAR-T cell immunotherapy in leukemia and lymphoma mouse models. They revealed, through targeted proapoptotic small molecule screening, that CAR-T cells alone achieved a killing rate of 47~63%, which increased to 75 ~ 88% when combined with BCL-2 inhibitors. This study showed that BCL-2 inhibitors enhance CAR-T cell cytotoxicity by enhancing Caspase-3/7 cleavage. However, higher doses and longer exposure times of venetoclax showed toxicity to CAR-T cells, reducing CD19 CAR-T cell numbers and inducing CAR-T cell apoptosis in the combination therapy compared to the CAR-T alone group. To address this, a venetoclax-resistant CAR-T cell was designed, targeting a specific point mutation at amino acid residue (Phe104Leu or F104L). In the MINO xenograft model, combining venetoclax with CART19-BCL-2 (F104L) achieved a 100% [45] survival rate with a cut-off of approximately 90 days. These findings underscore the potential of pairing BCL-2 inhibitors with CAR-T cell therapy and suggest strategies to mitigate venetoclax toxicity, offering valuable insights for optimizing CAR-T cell therapy further.
γ-secretase inhibitors
The BCMA on MM cell surfaces is activated by ligands BAFF and APRIL [90–92]. BCMA expression is regulated by γ-secretase (GS), which cleaves BCMA to release soluble BCMA (sBCMA), neutralizes APRIL, and inhibits BCMA-mediated NF-κB pathway activation. This process reduces target antigen density, affecting the targeted killing ability of CAR-T cells [93, 94]. Studies using in vitro cell experiments and MM NOD/SCID mouse models showed that γ-secretase inhibitors (GSI, LY3039478) dose-dependently prevent BCMA cleavage, increasing BCMA surface expression levels on MM cells. This enhancement improves the ability of CAR-T cells to recognize tumors [46].
Based on preclinical data, a clinical study was conducted by combining BCMA CAR-T with the γ-secretase inhibitor crenigacestat (LY3039478) for treating relapsed/refractory MM. The results showed that crenigacestat increased target antigen density and was well-tolerated [95]. γ-Secretase inhibitors enhance anti-BCMA CAR-T efficacy by preventing BCMA shedding from multiple myeloma cells.
Immunomodulatory drugs
The immunomodulator lenalidomide effectively combats hematological malignancies. Studies show that lenalidomide enhances the efficacy of CS1 CAR T cells [47] or BCMA CAR-T cells [49] in killing MM cells in vivo and in vitro when combined with CAR T cells. This effect is specifically illustrated as follows: (i) CD8 + CAR T cell subsets increased dose-dependently, while CD4 + CAR T cell subsets decreased [47]; (ii) After lenalidomide treatment on CAR T cells, autocrine cytokine IL-2 levels, which mainly determines CAR T cell efficacy and persistence, increased, and immunosuppressive Th2 cytokine levels (IL-4, IL-5, and IL-10) decreased [47]; (iii) Increasing CAR-T cell numbers [49]; (iv) Lenalidomide enhances immune synapse formation between CAR-T cells and tumors, increasing CAR-T cell lytic activity against tumors [47]. From these findings, the combination of lenalidomide and CAR-T cell therapy directly inhibits tumor-initiating cells and enhances the anti-tumor activity of CAR-T cells. Another study demonstrated that lenalidomide enhances the anti-tumor capacity of CAR-T cells by promoting CD8 + CAR-T cell differentiation into CD8 + central memory T cells and helper T cells [48], modulating the tumor microenvironment for enhanced CAR-T cell infiltration and delaying CAR-T cell depletion. Preclinical studies demonstrated that lenalidomide enhances the targeted-killing ability of CAR-T cells through various mechanisms, providing a solid foundation for further clinical trials.
A case report using piggyBac-generated CD19 CAR-T cells combined with lenalidomide to treat relapsed/refractory triple-hit DLBCL showed CR just 2 months after CAR-T cell infusion. Subsequently, they received lenalidomide maintenance therapy in the 4th month, maintaining CR for over 2 years [96]. Combining lenalidomide significantly extends the CR time. A study compared CAR-T therapy alone in nine patients with relapsed/refractory DLBCL to seven patients treated with lenalidomide maintenance therapy post-CAR-T therapy. The lenalidomide maintenance therapy group showed significantly higher survival and overall remission rates than the CAR-T therapy alone group (1-year overall survival (OS) rate 100% vs. 33.3%, ORR 85.7% vs. 77.8%, CR 42.9% vs. 33.3%). Additionally, a patient who initially responded briefly to CAR-T treatment but later relapsed achieved CR after lenalidomide therapy. PCR analysis was used to detect an increase in the CAR-T cell count [52], suggesting that lenalidomide can reverse CAR-T decline. Combining lenalidomide with CAR-T in therapy has demonstrated safe and effective clinical effects, benefiting several patients with tumors.
Immune checkpoint inhibitors
T cells primarily harbor immune checkpoint molecules such as PD-1, CTLA-4, LAG-3, and TIM-3. These immune inhibitory molecules are crucial for preventing damage to normal tissues but can lead to T-cell dysfunction, allowing tumor cells to evade immune system attacks, a primary factor in immune tolerance [97, 98]. PD-1, expressed on various immune cells such as activated T cells, NK cells, B lymphocytes, macrophages, dendritic cells, and monocytes, is a widely studied checkpoint molecule [37]. Its ligands, PD-L1/PD-L2, are predominantly overexpressed on tumor cell surfaces. When bound, they inhibit pathways such as PI3K/protein kinase B (Akt) and RAS/MEK/ERK, block protein kinase C-theta) function and glycolysis, and impede ZAP70 phosphatidylinositol signaling [99]. This inhibition compromises T cell function, allowing tumor cells to evade T cell attacks and promoting tumor growth. Therefore, immune checkpoint inhibitors are commonly used in clinical practice to block inhibitory receptor-ligand interactions, effectively treating hematological malignancies. Combining CAR-T cell therapy with checkpoint inhibitors offers a strategy to enhance the survival rate of patients with cancer.
Combining CAR-T with PD-1/PD-L1 inhibitors
Cao et al. conducted a single-center study involving 11 patients with relapsed/refractory B-NHL who underwent CD19 CAR-T therapy combined with the PD-1 antibody nivolumab. The results confirmed the efficacy and safety of this combination, showing an overall remission rate of 81.81%, CRR of 45.45%, median PFS of 6 months, 75% incidence of grade 1–2 CRS, and 9% incidence of ICANS [53]. This combination demonstrates potent antitumor activity. Clinical data demonstrate that PD-1 blockade significantly enhances the anti-tumor effect. Co-expression of PD-1 and Eomes decreases on CAR-T cells, enhancing CAR-T cell expansion. This approach proves effective for patients with malignant tumors with limited CAR-T therapy response [100]. Another study retrospectively analyzed 154 patients who underwent CD19/CD22 CAR-T cell therapy with or without Autologous Stem Cell Transplantation (ASCT). It assessed the PD-1 inhibitor used for subsequent maintenance therapy. The results showed that patients receiving CD19/CD22 CAR-T therapy followed by PD-1 inhibitor maintenance had a higher ORR of 82.9% and a 2-year PFS rate of 59.8% than those not receiving PD-1 inhibitors (with an ORR of 60% and 2-year PFS rate of 21.3%). Among patients who underwent ASCT before CD19/CD22 CAR-T therapy, no significant statistical difference in ORR and 2-year PFS was observed between the two groups [54]. This suggests that PD-1 inhibitors post CD19/CD22 CAR-T therapy may benefit non-ACST patients. However, the efficacy of PD-1 inhibitors in patients with ASCT could be influenced by other factors, such as the ACST efficacy.
The timing of PD-1/PD-L1 inhibitor therapy affects CD19 CAR-T cell immunotherapy efficacy. Patients receiving durvalumab before CAR-T cell infusion show worse outcomes despite delayed and shorter CRS. Conversely, durvalumab maintenance post-CAR-T infusion enhances re-expansion, CAR-T cell numbers in the blood, and tumor cell killing [101]. Studies confirm the reliability of PD-L1 inhibitors combination therapy in terms of safety and efficacy. The combination therapy shows a 67% duration of response (DOR), exceeding the 20% DOR observed in patients receiving CAR-T therapy alone. The reduced efficacy observed with durvalumab before CAR-T therapy may be due to the early increase in soluble PD-L1 (sPD-L1) levels, which dose-dependently inhibit CAR-T cell effector function [102–104]. Clinically, careful timing of PD-1/PD-L1 inhibitor therapy is crucial for effective intratumoral lesion targeting.
Interfering with the PD-1 gene in CAR-T cells
CRISPR/Cas9 gene-editing technology can precisely interfere with the PD-1 gene by designing specific gRNAs to target the Cas9 protein targeted to the PD-1 gene. This induces mutations, insertions, or deletions in the genome, disrupting the function of PD-1 [105–107]. Alternative gene editing methods, such as Transcription Activator-Like Effector Nucleases and Zinc Finger Nucleases [108], can also be used to cleave and edit the PD-1 gene. RNA molecules such as small interfering RNA [109] and short hairpin RNA [110, 111] are used to inhibit gene expression by targeting PD-1 mRNA, reducing PD-1 protein levels, and swiftly inhibiting PD-1 function, although not directly editing genes.
Constructing CAR-T cells secreting PD-1-blocking scFv
CAR-T cells modified with PD-1-blocking scFv were used in xenograft mouse models featuring PD-L1+ expression in hematological malignancies or solid tumors [112]. This modification increases cytotoxicity, promotes cytokine secretion, and enhances in vivo antitumor effects compared to unmodified CAR-expressing [113–115]. However, a significant limitation is that this PD-1 blockade targets only CAR-T cells, leaving PD-1 on T lymphocytes in patients still immunosuppressive.
Radiation therapy
Radiation therapy (RT) is effective for treating localized lesions in the hematologic system, including lymphoma and MM [116]. Combining CAR-T therapy with RT is a complex process but promises a treatment strategy [117, 118]. Radiotherapy induces immunostimulatory genes in lymphoma cells, enhancing signals for CAR-T cell activation and proliferation (CD70, OX40L) [119]. Gupta et al. noted that low-dose radiation (0.5–2.0 Gy) increased CD20 expression on Burkitt’s lymphoma cell lines (Raji and Daudi) with low CD20 expression [120]. This elevated CD20 expression potentially enhances CAR-T treatment efficacy by providing more targets for subsequent CAR-T cells.
Before CD19 CAR-T (Axicabtagene ciloleucel) therapy for DLBCL, using RT as a bridging treatment showed manageable toxicity. 83% of patients had reduced lymphocyte counts, leading to an ORR of 81.8%. The CRR was 45%, indicating the safety and efficacy of using RT as a bridging treatment [56]. In a retrospective analysis of 83 cases involving relapsed/refractory B-NHL patients who received RT and CAR-T therapy, researchers found that 35 patients underwent bridging RT (bRT) before CAR-T infusion, while 48 underwent salvage RT (sRT) post-CAR-T infusion. The bRT group demonstrated an 84% local control rate, significantly higher than the 62% observed in the sRT group during efficacy assessment. Additionally, the bRT group had a significantly higher remission rate than the sRT group [55]. This suggests a relationship between bRT and the local control rate in patients, while sRT offers salvage options for patients who relapse post-CAR-T infusion. DeSelm et al. documented a case involving a relapsed/refractory DLBCL patient with tumor infiltration into the skin of the right lower leg who underwent CD19 CAR-T cell therapy after local radiotherapy as scheduled. The patient had positive PET-CT results 1 month post-treatment, no toxicity at the irradiated site, and grade 1 CRS without neurotoxicity. At the one-year follow-up, the irradiated area showed no disease progression, while other areas exhibited varying degrees of recurrence or progression [121]. In a clinical trial assessing bridging radiotherapy before BCMA CAR-T therapy, radiotherapy was deemed safe and viable as a bridging treatment, demonstrating lower hematological toxicity than the no-radiation group [57]. RT can enhance CAR-T cell recognition by damaging the DNA structure of tumor cells, controlling local tumor growth, and reducing tumor burden. Furthermore, RT may reduce immune escape mechanisms in the tumor microenvironment, such as immune inhibitory factor expression, potentially inducing tumor cell apoptosis and releasing tumor-associated antigens to enhance the immune system response. This strengthens the antitumor efficacy of CAR-T cells. Therefore, selecting the appropriate timing for radiation therapy is crucial in clinical practice.
Hematopoietic stem cell transplantation
Hematologic malignancies can achieve long-term remission or even potential cure through HSCT [122–127]. A recent study shows the efficacy of sequential CAR-T cell therapy post-HSCT. In a study of 42 patients undergoing sequential transplantation, the ORR reached 90.5%, with a 2-year PFS rate of 83.3%. Grade ≤ 3 CRS was observed in 4.8% of cases, while 21% experienced ICANS and 5% experienced severe grade 3 neurotoxicity [58]. Adverse reactions were reversible, indicating a high remission rate with a good safety profile. Additionally, researchers compared CAR-T therapy alone (Group A, eight patients) to sequential CAR-T therapy following ASCT (Group B, six patients, including two patients transitioning from Group A). The overall ORR at 3 months was 83.3%, with Group A having an ORR of 75% and Group B at 100% [59]. This study focused on relapsed/refractory double-hit lymphoma, showing that combining CAR-T with ASCT significantly increased survival, response rates, and duration of CAR-T persistence compared to CAR-T therapy alone while reducing adverse reactions and recurrence rates.
In patients with central nervous system lymphoma, combining ASCT with CAR-T cell therapy can enhance the prognosis of patients with relapsed/refractory central nervous system lymphoma. Researchers analyzed 17 such cases, with 9 receiving only CAR-T cell therapy and 8 receiving ASCT combined with CAR-T cell therapy. The efficacy assessment revealed a CRR of 100% with CAR-T cell therapy combined with ASCT and 44.4% without ASCT. Compared to standalone CAR-T cell therapy, patients undergoing combined ASCT demonstrated significantly extended PFS and OS. The incidence rates of grade ≥ 3 CRS and ICANS were 41% and 29%, respectively, with no treatment-related deaths reported [60], indicating manageable toxicity.
Patients with lymphoma that have TP53 mutations often have a worse prognosis, rendering enhancement of the efficacy in this disease subtype a key focus of clinical research. Studies show that combining CD19/22 CAR-T cell therapy with ASCT is more effective for patients with TP53 gene mutations, enhancing CRR, PFS, and OS. Clinical data from 60 patients with TP53-mutations showed that the objective response rate (ORR) was 87.1% and 92.9%, while CRR was 45.2% and 82.1% in the CAR-T alone and CAR-T combined with ASCT groups, respectively. The 24-month OS rates were 56.3% vs. 89.3% [61], indicating a significant improvement in patient outcomes with this combination therapy.
Combining CAR-T cell therapy with HSCT can be an effective and safe strategy for treating certain hematologic malignancies, leading to enhanced treatment outcomes and prognosis for specific patient subgroups. While some patients may experience adverse reactions, they are typically manageable, indicating the good safety profile of the combination therapy. However, further clinical research is necessary to establish the optimal treatment approach for different types of tumors and individual patients.
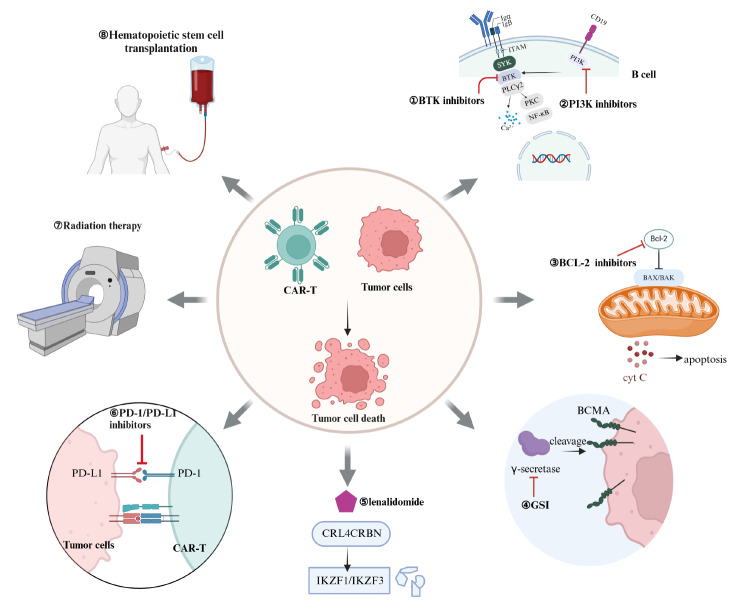
With thorough research and continuous advancements in CAR-T technology, combining CAR-T with other treatments has expanded its applicability and increased treatment options for patients with cancer. Research on various combination therapy strategies in the evolving treatment landscape is bringing new hope for clinical practice and patient well-being (Fig. 2).
Fig. 2.
Combination Therapies with CAR-T Cells. ①Combining CAR-T cells with BTK inhibitors inhibits BTK in the B-cell signaling pathway, reducing malignant B-cell proliferation. ②Combining CAR-T cells with PI3K inhibitors interferes with tumor cell growth and survival by inhibiting the PI3K signaling pathway. ③Combining CAR-T cells with BCL-2 inhibitors promotes tumor cell apoptosis by inhibiting the BCL-2 protein. ④Combining CAR-T cells with GSI prevents the shedding of BCMA from the surface of tumor cells by inhibiting γ-secretase. ⑤Combining CAR-T cells with Lenalidomide recruits the E3 ubiquitin ligase CRL4CRBN, inducing the degradation of IKZF1 and IKZF3. ⑥Combining CAR-T cells with PD-1/PD-L1 inhibitors blocks the PD-1/PD-L1 signaling pathway to counteract the tumor cells’ immune evasion mechanism. ⑦Combining CAR-T cells with Radiotherapy directly kills tumor cells, enhancing the effectiveness of CAR-T therapy. ⑧Combining CAR-T cells with Hematopoietic Stem Cell Transplantation rebuilds the patient’s immune system to support the sustained anti-tumor activity of CAR-T cells.
Conclusion
CAR-T cell therapy, an emerging cancer treatment modality, shows great promise in treating hematologic malignancies. However, some limitations, such as patient tolerance and post-treatment relapse, are associated with this strategy. To address these limitations, researchers are investigating combining CAR-T cell therapy with other treatment modalities to enhance therapeutic efficacy and reduce side effects. Several studies on combination therapies have significantly advanced clinical effectiveness and reduced relapse rates in patients with relapsed/refractory. Detailed insights into combining CAR-T cells with chemotherapy, immune checkpoint inhibitors, targeted drugs, radiotherapy, HSCT, and other treatments are provided in this study. These combination therapies enhance the anti-tumor effects of CAR-T cells and comprehensively target tumors through diverse mechanisms, improving patient response and survival rates. This offers novel insights and strategies for enhancing the clinical application of CAR-T cell therapy. Comparative reports on the safety and tolerability of different combination therapies are lacking, which is a potential starting point for future research to identify the most suitable combination therapy. Furthermore, optimizing protocols for CAR-T combination therapy is necessary to accommodate the diverse needs of patients, including timing, dosages, and post-treatment care. Further clinical studies are needed to validate the safety and efficacy of these combination treatment strategies. With continuous advancements in science, technology, and clinical experience, CAR-T cell combination therapy is expected to play an increasingly vital role in the future, improving treatment outcomes and survival prospects for patients with hematologic malignancies.
Acknowledgements
The figures are created with BioRender.com.
Abbreviations
- CAR
Chimeric antigen receptor
- CRR
Complete remission rate
- DLBCL
Diffuse large B-cell lymphoma
- MM
Multiple myeloma
- ORR
Objective response rate
- CR
Complete remission
- B-ALL
B-cell acute lymphoblastic leukemia
- HSCT
Hematopoietic stem cell transplantation
- BCMA
B-cell maturation antigen
- PD-1
Programmed cell death protein-1
- LAG-3
Lymphocyte activation gene-3
- TIM-3
T cell immunoglobulin and mucin domain-containing protein-3
- CTLA-4
Cytotoxic T lymphocyte antigen-4
- CLL
Chronic lymphocytic leukemia
- TAMS
Tumor-associated macrophages
- Tregs
Regulatory T cells
- BTK
Bruton tyrosine kinase
- MCL
Mantle cell lymphoma
- MRD
Minimal residual disease
- PFS
Progression-free survival rate
- CRS
Cytokine release syndrome
- ICANS
Immune effector cell-associated neurotoxicity syndrome
- PI3K
Phosphatidylinositol 3-kinase
- PI3Ki
PI3K inhibitors
- Tscm
Stem cell central memory T cells
- Tcm
Central memory T lymphocytes
- BCL-2
B cell lymphoma-2
- AML
Acute myeloid leukemia
- B-NHL
B-cell non-Hodgkin lymphoma
- CML
Chronic myeloid leukemia
- GS
γ-secretase
- GSI
γ-secretase inhibitors
- sBCMA
Soluble BCMA
- OS
Overall survival
- ORR
Overall response rate
- ASCT
Autologous Stem Cell Transplantation
- DOR
Duration of response
- sPD-L1
Soluble PD-L1
- scFv
Single-chain variable fragments
- RT
Radiation therapy
- bRT
Bridging radiation therapy
- sRT
Salvage radiation therapy
Author contributions
Conceptualization, YX and XJZ; Writing – Original Draft, DLZ; Writing – Review & Editing, DLZ, YX and XJZ; Funding Acquisition, YX. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82070213, No. 82370196).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaojian Zhu, Email: zhuxiaojian@hust.edu.cn.
Yi Xiao, Email: yixiao@tjh.tjmu.edu.cn.
References
- 1.Levin A, Shah NN. Chimeric antigen receptor modified T cell therapy in B cell non-hodgkin lymphomas. Am J Hematol. 2019; 94(S1). [DOI] [PubMed]
- 2.Song EZ, Milone MC. Pharmacology of chimeric Antigen receptor–modified T cells. Annu Rev Pharmacol Toxicol. 2021;61(1):805–29. 10.1146/annurev-pharmtox-031720-102211 [DOI] [PubMed] [Google Scholar]
- 3.Sadelain M, Brentjens R, Rivière I. The Basic principles of chimeric Antigen receptor design. Cancer Discov. 2013;3(4):388–98. 10.1158/2159-8290.CD-12-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson CA, Hunter BD, Redd R, et al. Axicabtagene Ciloleucel in the Non-trial setting: outcomes and correlates of response, resistance, and toxicity. J Clin Oncol. 2020;38(27):3095–106. 10.1200/JCO.19.02103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419–31. 10.1038/s41591-021-01436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan T, Zhu L, Chen J. Current advances and challenges in CAR T-Cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment. Experimental Hematol Oncol. 2023;12(1):14. 10.1186/s40164-023-00373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403–15. 10.1016/S1470-2045(21)00375-2 [DOI] [PubMed] [Google Scholar]
- 8.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017;377(26):2545–54. 10.1056/NEJMoa1708566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munshi NC, Anderson D, Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory multiple myeloma. N Engl J Med. 2021;384(8):705–16. 10.1056/NEJMoa2024850 [DOI] [PubMed] [Google Scholar]
- 10.Laetsch TW, Maude SL, Rives S, et al. Three-year update of Tisagenlecleucel in Pediatric and Young Adult patients with Relapsed/Refractory Acute Lymphoblastic Leukemia in the ELIANA Trial. J Clin Oncol. 2023;41(9):1664–9. 10.1200/JCO.22.00642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory large B-Cell lymphoma. N Engl J Med. 2017;377(26):2531–44. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med. 2020;382(14):1331–42. 10.1056/NEJMoa1914347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52. 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]
- 14.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young adults with B-Cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson CA, Chavez JC, Sehgal R, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91–103. 10.1016/S1470-2045(21)00591-X [DOI] [PubMed] [Google Scholar]
- 16.Chong EA, Ruella M, Schuster SJ. Five-year outcomes for refractory B-Cell lymphomas with CAR T-Cell therapy. N Engl J Med. 2021;384(7):673–4. 10.1056/NEJMc2030164 [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Munoz J, Goy A, et al. Three-year Follow-Up of KTE-X19 in patients with Relapsed/Refractory Mantle Cell Lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. 2023;41(3):555–67. 10.1200/JCO.21.02370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Wang X, Sauter C, et al. Long-term follow-up of CD19 CAR therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449–59. 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care Axicabtagene Ciloleucel for relapsed or refractory large B-Cell lymphoma: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2020;38(27):3119–28. 10.1200/JCO.19.02104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–95. 10.1158/2159-8290.CD-15-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aparicio-Pérez C, Carmona M, Benabdellah K, et al. Failure of ALL recognition by CAR T cells: a review of CD19-negative relapses after anti-CD19 CAR-T treatment in B-ALL. Front Immunol. 2023;14:1165870. 10.3389/fimmu.2023.1165870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majzner RG, Mackall CL. Tumor Antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8(10):1219–26. 10.1158/2159-8290.CD-18-0442 [DOI] [PubMed] [Google Scholar]
- 23.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406–10. 10.1182/blood-2015-08-665547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans AG, Rothberg PG, Burack WR, et al. Evolution to plasmablastic lymphoma evades CD19-directed chimeric antigen receptor T cells. Br J Haematol. 2015;171(2):205–9. 10.1111/bjh.13562 [DOI] [PubMed] [Google Scholar]
- 25.Majzner RG, Heitzeneder S, Mackall CL. Harnessing the Immunotherapy Revolution for the treatment of Childhood Cancers. Cancer Cell. 2017;31(4):476–85. 10.1016/j.ccell.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 26.O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399):eaaa0984. 10.1126/scitranslmed.aaa0984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grupp SA, Aplenc R, Teachey DT, et al. Chimeric Antigen receptor–modified T cells for Acute Lymphoid Leukemia. N Engl J Med. 2013;368(16):1509–18. 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8. 10.1038/nm.4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24(10):1504–6. 10.1038/s41591-018-0146-z [DOI] [PubMed] [Google Scholar]
- 30.Brudno JN, Maric I, Hartman SD, et al. T cells genetically modified to Express an Anti–B-Cell maturation Antigen chimeric Antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267–80. 10.1200/JCO.2018.77.8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen AD, Garfall AL, Stadtmauer EA, et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210–21. 10.1172/JCI126397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Ahn S, Maity R, et al. Mechanisms of antigen escape from BCMA- or GPRC5D-targeted immunotherapies in multiple myeloma. Nat Med. 2023;29(9):2295–306. 10.1038/s41591-023-02491-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng X, Liu X, Guo X, et al. FBXO38 mediates PD-1 ubiquitination and regulates anti-tumour immunity of T cells. Nature. 2018;564(7734):130–5. 10.1038/s41586-018-0756-0 [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal V, Workman CJ, Vignali DAA. LAG-3 as the third checkpoint inhibitor. Nat Immunol. 2023;24(9):1415–22. 10.1038/s41590-023-01569-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal S, Aznar MA, Rech AJ, et al. Deletion of the inhibitory co-receptor CTLA-4 enhances and invigorates chimeric antigen receptor T cells. Immunity. 2023;56(10):2388–407. 10.1016/j.immuni.2023.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang S, Liu D, Sun J, et al. Tim-3 regulates sepsis-induced immunosuppression by inhibiting the NF-κB signaling pathway in CD4 T cells. Mol Ther. 2022;30(3):1227–38. 10.1016/j.ymthe.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44. 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo SR, Turnis ME, Goldberg MV, et al. Immune Inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote Tumoral Immune escape. Cancer Res. 2012;72(4):917–27. 10.1158/0008-5472.CAN-11-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–71. 10.1038/s41591-018-0010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arner EN, Rathmell JC. Metabolic programming and immune suppression in the tumor microenvironment. Cancer Cell. 2023;41(3):421–33. 10.1016/j.ccell.2023.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riaz N, Havel JJ, Makarov V et al. Tumor and Microenvironment Evolution during Immunotherapy with NivolumabCell. 2017;171(4):934–49. [DOI] [PMC free article] [PubMed]
- 42.Allen GM, Frankel NW, Reddy NR et al. Synthetic cytokine circuits that drive T cells into immune-excluded tumors. Science. 2022; 378(6625). [DOI] [PMC free article] [PubMed]
- 43.Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–67. 10.1038/s41571-019-0297-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funk CR, Wang S, Chen KZ, et al. PI3Kδ/γ inhibition promotes human CART cell epigenetic and metabolic reprogramming to enhance antitumor cytotoxicity. Blood. 2022;139(4):523–37. 10.1182/blood.2021011597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YG, Guruprasad P, Ghilardi G, et al. Modulation of BCL-2 in both T Cells and Tumor Cells to enhance chimeric Antigen receptor T-cell immunotherapy against Cancer. Cancer Discov. 2022;12(10):2372–91. 10.1158/2159-8290.CD-21-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pont MJ, Hill T, Cole GO, et al. γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood. 2019;134(19):1585–97. 10.1182/blood.2019000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Walter M, Urak R, et al. Lenalidomide enhances the function of CS1 chimeric Antigen receptor–redirected T cells against multiple myeloma. Clin Cancer Res. 2018;24(1):106–19. 10.1158/1078-0432.CCR-17-0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin Z, Xiang R, Qing K, et al. Lenalidomide overcomes the resistance to third-generation CD19-CAR-T cell therapy in preclinical models of diffuse large B-cell lymphoma. Cell Oncol. 2023;46(4):1143–57. 10.1007/s13402-023-00833-6 [DOI] [PubMed] [Google Scholar]
- 49.Works M, Soni N, Hauskins C, et al. Anti–B-cell maturation Antigen chimeric Antigen receptor T cell function against multiple myeloma is enhanced in the Presence of Lenalidomide. Mol Cancer Ther. 2019;18(12):2246–57. 10.1158/1535-7163.MCT-18-1146 [DOI] [PubMed] [Google Scholar]
- 50.Gill S, Vides V, Frey NV, et al. Anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia. Blood Adv. 2022;6(21):5774–85. 10.1182/bloodadvances.2022007317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gauthier J, Hirayama AV, Purushe J, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135(19):1650–60. 10.1182/blood.2019002936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ping N, Qu C, Li M, et al. Overall survival benefits provided by lenalidomide maintenance after chimeric antigen receptor T cell therapy in patients with refractory/relapsed diffuse large B-cell lymphoma. Annals Translational Med. 2022;10(6):298. 10.21037/atm-22-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao Y, Lu W, Sun R, et al. Anti-CD19 Chimeric Antigen Receptor T Cells in Combination with Nivolumab are safe and effective against Relapsed/Refractory B-Cell non-hodgkin Lymphoma. Front Oncol. 2019;9:767. 10.3389/fonc.2019.00767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xin X, Zhu X, Yang Y et al. Efficacy of programmed cell death 1 inhibitor maintenance after chimeric antigen receptor T cells in patients with relapsed/refractory B-cell non-hodgkin-lymphoma. Cell Oncol. 2024. [DOI] [PubMed]
- 55.Saifi O, Breen WG, Lester SC, et al. Don’t put the CART before the horse: the role of Radiation Therapy in Peri-CAR T-cell therapy for aggressive B-cell Non-hodgkin Lymphoma. Int J Radiat Oncol Biol Phys. 2023;116(5):999–1007. 10.1016/j.ijrobp.2022.12.017 [DOI] [PubMed] [Google Scholar]
- 56.Sim AJ, Jain MD, Figura NB, et al. Radiation Therapy as a bridging strategy for CAR T cell therapy with axicabtagene ciloleucel in diffuse large B-Cell lymphoma. Int J Radiat Oncol Biol Phys. 2019;105(5):1012–21. 10.1016/j.ijrobp.2019.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manjunath SH, Cohen AD, Lacey SF, et al. The safety of bridging Radiation with Anti-BCMA CAR T-Cell therapy for multiple myeloma. Clin Cancer Res. 2021;27(23):6580–90. 10.1158/1078-0432.CCR-21-0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao Y, Xiao Y, Wang N et al. CD19/CD22 Chimeric Antigen Receptor T Cell Cocktail Therapy following Autologous Transplantation in Patients with Relapsed/Refractory Aggressive B Cell Lymphomas. Transplantation and Cellular Therapy. 2021; 27(11): 910.e1-910.e11. [DOI] [PubMed]
- 59.Wei J, Mao Z, Wang N, et al. Long-term outcomes of relapsed/refractory double-hit lymphoma (r/r DHL) treated with CD19/22 CAR T-cell cocktail therapy. Clin Translational Med. 2020;10(5):e176. 10.1002/ctm2.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xue F, Zheng P, Liu R et al. The Autologous Hematopoietic Stem Cells Transplantation Combination-Based Chimeric Antigen Receptor T-Cell Therapy Improves Outcomes of Relapsed/Refractory Central Nervous System B-Cell Lymphoma. Journal of Oncology. 2022; 2022:2900310. [DOI] [PMC free article] [PubMed]
- 61.Wei J, Xiao M, Mao Z, et al. Outcome of aggressive B-cell lymphoma with TP53 alterations administered with CAR T-cell cocktail alone or in combination with ASCT. Signal Transduct Target Therapy. 2022;7(1):101. 10.1038/s41392-022-00924-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang E, Mi X, Thompson MC, et al. Mechanisms of resistance to Noncovalent Bruton’s tyrosine kinase inhibitors. N Engl J Med. 2022;386(8):735–43. 10.1056/NEJMoa2114110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci. 2010;107(29):13075–80. [DOI] [PMC free article] [PubMed]
- 64.Gomez EB, Ebata K, Randeria HS, et al. Preclinical characterization of pirtobrutinib, a highly selective, noncovalent (reversible) BTK inhibitor. Blood. 2023;142(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or Ibrutinib in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2023;388(4):319–32. 10.1056/NEJMoa2211582 [DOI] [PubMed] [Google Scholar]
- 66.Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 2022;23(8):1031–43. 10.1016/S1470-2045(22)00293-5 [DOI] [PubMed] [Google Scholar]
- 67.Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol. 2016;9(1):21. 10.1186/s13045-016-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mato AR, Woyach JA, Brown R, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33–44. 10.1056/NEJMoa2300696 [DOI] [PubMed] [Google Scholar]
- 69.Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892–901. 10.1016/S0140-6736(21)00224-5 [DOI] [PubMed] [Google Scholar]
- 70.Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9):1117–27. 10.1182/blood-2015-11-679134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052–64. 10.1172/JCI89756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubovsky JA, Beckwith KA, Natarajan G et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. 2013; 122(15):2539–49. [DOI] [PMC free article] [PubMed]
- 73.Stiff A, Trikha P, Wesolowski R, et al. Myeloid-derived suppressor cells Express Bruton’s tyrosine kinase and can be depleted in Tumor-Bearing hosts by Ibrutinib Treatment. Cancer Res. 2016;76(8):2125–36. 10.1158/0008-5472.CAN-15-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fan F, Yoo HJ, Stock S, et al. Ibrutinib for improved chimeric antigen receptor T-cell production for chronic lymphocytic leukemia patients. Int J Cancer. 2021;148(2):419–28. 10.1002/ijc.33212 [DOI] [PubMed] [Google Scholar]
- 75.Luo W, Li C, Wu J, et al. Bruton tyrosine kinase inhibitors preserve anti-CD19 chimeric antigen receptor T-cell functionality and reprogram tumor micro-environment in B-cell lymphoma. Cytotherapy. 2023;25(7):739–49. 10.1016/j.jcyt.2023.03.005 [DOI] [PubMed] [Google Scholar]
- 76.Ruella M, Kenderian SS, Shestova O, et al. The addition of the BTK inhibitor Ibrutinib to Anti-CD19 Chimeric Antigen Receptor T Cells (CART19) improves responses against Mantle Cell Lymphoma. Clin Cancer Res. 2016;22(11):2684–96. 10.1158/1078-0432.CCR-15-1527 [DOI] [PubMed] [Google Scholar]
- 77.Qin JS, Johnstone TG, Baturevych A, et al. Antitumor Potency of an Anti-CD19 chimeric Antigen receptor T-Cell therapy, Lisocabtagene Maraleucel in Combination with Ibrutinib or Acalabrutinib. J Immunother. 2020;43(4):107–20. 10.1097/CJI.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan F, Jiang V, Jordan A, et al. The HSP90-MYC-CDK9 network drives therapeutic resistance in mantle cell lymphoma. Exp Hematol Oncol. 2024;13(1):14. 10.1186/s40164-024-00484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abu Eid R, Ahmad S, Lin Y, et al. Enhanced therapeutic efficacy and memory of Tumor-Specific CD8 T cells by Ex vivo PI3K-δ inhibition. Cancer Res. 2017;77(15):4135–45. 10.1158/0008-5472.CAN-16-1925 [DOI] [PubMed] [Google Scholar]
- 80.Zheng W, O’Hear CE, Alli R, et al. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia. 2018;32(5):1157–67. 10.1038/s41375-017-0008-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Delbridge AR, Strasser A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015;22(7):1071–80. 10.1038/cdd.2015.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fujita N, Tsuruo T. In vivo veritas: Bcl-2 and Bcl-XL mediate tumor cell resistance to chemotherapy. Drug Resist Updat. 2000;3(3):149–54. 10.1054/drup.2000.0142 [DOI] [PubMed] [Google Scholar]
- 83.Goff DJ, Recart C, Sadarangani A, et al. A Pan-BCL2 inhibitor renders bone-marrow-Resident Human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell. 2013;12(3):316–28. 10.1016/j.stem.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Letai A, Sorcinelli MD, Beard C, et al. Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell. 2004;6(3):241–9. 10.1016/j.ccr.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 85.Lee J, Sohn EJ, Yoon SW, et al. Anti-metastatic effect of Dehydrocorydaline on H1299 Non-small Cell Lung Carcinoma cells via inhibition of Matrix metalloproteinases and B Cell Lymphoma 2: Antimetastatic Effect of Dehydrocorydaline on H1299 cells. Phytother Res. 2017;31(3):441–8. 10.1002/ptr.5766 [DOI] [PubMed] [Google Scholar]
- 86.Delbridge AR, Grabow S, Strasser A, et al. Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat Rev Cancer. 2016;16(2):99–109. 10.1038/nrc.2015.17 [DOI] [PubMed] [Google Scholar]
- 87.Davids MS, Letai A. Targeting the B-Cell Lymphoma/Leukemia 2 family in Cancer. J Clin Oncol. 2012;30(25):3127–35. 10.1200/JCO.2011.37.0981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. 10.1182/blood-2018-08-868752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pollyea DA, Stevens BM, Jones CL, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia. Nat Med. 2018;24(12):1859–66. 10.1038/s41591-018-0233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu G, Boone T, Delaney J, et al. APRIL and TALL-1 and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1(3):2526. 10.1038/79802 [DOI] [PubMed] [Google Scholar]
- 91.Meinl E, Thaler FS, Lichtenthaler SF. Shedding of BAFF/APRIL receptors controls B cells. Trends Immunol. 2018;39(9):6736. 10.1016/j.it.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 92.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17(3):282–9. 10.1016/j.coi.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 93.Laurent SA, Hoffmann FS, Kuhn PH, et al. γ-secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun. 2015;6:7333. 10.1038/ncomms8333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan X, Wu H, Xu H, et al. Notch signaling: an emerging therapeutic target for cancer treatment. Cancer Lett. 2015;369(1):20–7. 10.1016/j.canlet.2015.07.048 [DOI] [PubMed] [Google Scholar]
- 95.Mailankody S, Matous JV, Chhabra S, et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat Med. 2023;29(2):422–9. 10.1038/s41591-022-02182-7 [DOI] [PubMed] [Google Scholar]
- 96.Li C, Sun Y, Wang J, et al. PiggyBac-Generated CAR19-T cells plus Lenalidomide cause durable complete remission of triple-hit Refractory/Relapsed DLBCL: a Case Report. Front Immunol. 2021;12:599493. 10.3389/fimmu.2021.599493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and Cancer. Annu Rev Immunol. 2019;37:457–95. 10.1146/annurev-immunol-041015-055318 [DOI] [PubMed] [Google Scholar]
- 98.Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16(10):599–611. 10.1038/nri.2016.80 [DOI] [PubMed] [Google Scholar]
- 99.Ok CY, Young KH. Targeting the programmed death-1 pathway in lymphoid neoplasms. Cancer Treat Rev. 2017;54:99–109. 10.1016/j.ctrv.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)–modified T cells: refueling the CAR. Blood. 2017;129(8):1039–41. 10.1182/blood-2016-09-738245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirayama AV, Kimble EL, Wright JH, et al. Timing of anti-PD-L1 antibody initiation affects efficacy/toxicity of CD19 CAR T-cell therapy for large B-cell lymphoma. Blood Adv. 2024;8(2):453–67. 10.1182/bloodadvances.2023011287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu L, Risch E, Halaban R, et al. Dynamic changes of circulating soluble PD-1/PD-L1 and its association with patient survival in immune checkpoint blockade-treated melanoma. Int Immunopharmacol. 2023;118:110092. 10.1016/j.intimp.2023.110092 [DOI] [PubMed] [Google Scholar]
- 103.Oh SY, Kim S, Keam B, et al. Soluble PD-L1 is a predictive and prognostic biomarker in advanced cancer patients who receive immune checkpoint blockade treatment. Sci Rep. 2021;11(1):19712. 10.1038/s41598-021-99311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen L, Liu S, Adah D, et al. Soluble programmed death ligand-1-induced immunosuppressive effects on chimeric antigen receptor-natural killer cells targeting Glypican-3 in hepatocellular carcinoma. Immunology. 2023;169(2):204–18. 10.1111/imm.13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu X, Zhang Y, Cheng C, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017;27(1):154–7. 10.1038/cr.2016.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rupp LJ, Schumann K, Roybal KT, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. 10.1038/s41598-017-00462-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu W, Zi Z, Jin Y, et al. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunotherapy. 2019;68(3):365–77. 10.1007/s00262-018-2281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang R, Li X, He Y, et al. Recent advances in CAR-T cell engineering. J Hematol Oncol. 2020;13(1):86. 10.1186/s13045-020-00910-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamilton AG, Swingle KL, Joseph RA, et al. Ionizable lipid nanoparticles with Integrated Immune Checkpoint Inhibition for mRNA CAR T cell Engineering. Adv Healthc Mater. 2023;12(30):2301515. 10.1002/adhm.202301515 [DOI] [PubMed] [Google Scholar]
- 110.Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126(8):3130–44. 10.1172/JCI83092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ouyang W, Jin SW, Xu N, et al. PD-1 downregulation enhances CAR-T cell antitumor efficiency by preserving a cell memory phenotype and reducing exhaustion. J Immunother Cancer. 2024;12(4):e008429. 10.1136/jitc-2023-008429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suarez ER, Chang DK, Sun J, et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7(23):34341–55. 10.18632/oncotarget.9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li S, Siriwon N, Zhang X, et al. Enhanced Cancer Immunotherapy by chimeric Antigen receptor–modified T cells Engineered to secrete checkpoint inhibitors. Clin Cancer Res. 2017;23(22):6982–92. 10.1158/1078-0432.CCR-17-0867 [DOI] [PubMed] [Google Scholar]
- 114.Chen X, Yang S, Li S, et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-β trap enhances antitumor efficacy of CAR-T cell therapy. Mol Therapy Oncolytics. 2021;21:144–57. 10.1016/j.omto.2021.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen J, Zhu T, Jiang G, et al. Target delivery of a PD-1-TREM2 scFv by CAR-T cells enhances anti-tumor efficacy in colorectal cancer. Mol Cancer. 2023;22(1):131. 10.1186/s12943-023-01830-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Son SH, Choi BO, Kim GW, et al. Primary Radiation Therapy in patients with localized Orbital marginal zone B-Cell lymphoma of Mucosa-Associated Lymphoid tissue (MALT lymphoma). Int J Radiat Oncol Biol Phys. 2010;77(1):86–91. 10.1016/j.ijrobp.2009.04.018 [DOI] [PubMed] [Google Scholar]
- 117.Zhang Z, Liu X, Chen D, et al. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. 2022;7(1):258. 10.1038/s41392-022-01102-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fang PQ, Gunther JR, Wu SY, et al. Radiation and CAR T-cell therapy in Lymphoma: future frontiers and potential opportunities for Synergy. Front Oncol. 2021;11:648655. 10.3389/fonc.2021.648655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lindgren T, Stigbrand T, Riklund K, et al. Gene expression profiling in MOLT-4 cells during gamma-radiation-induced apoptosis. Tumor Biology. 2012;33(3):689–700. 10.1007/s13277-012-0329-z [DOI] [PubMed] [Google Scholar]
- 120.Gupta D, Crosby ME, Almasan A, et al. Regulation of CD20 expression by radiation-induced changes in intracellular redox status. Free Radic Biol Med. 2008;44(4):614–23. 10.1016/j.freeradbiomed.2007.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.DeSelm C, Palomba ML, Yahalom J, et al. Low-dose Radiation Conditioning enables CAR T cells to mitigate Antigen escape. Mol Ther. 2018;26(11):2542–52. 10.1016/j.ymthe.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang YJ, Wang Y, Liu YR, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol. 2017;10(1):134. 10.1186/s13045-017-0502-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang YJ, Wang Y, Xu P, et al. Haploidentical donor is preferred over matched sibling donor for pre-transplantation MRD positive ALL: a phase 3 genetically randomized study. J Hematol Oncol. 2020;13(1):27. 10.1186/s13045-020-00860-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y, Liu QF, Xu LP, et al. Haploidentical versus matched-sibling transplant in adults with Philadelphia-negative high-risk Acute Lymphoblastic Leukemia: a biologically phase III randomized study. Clin Cancer Res. 2016;22(14):3467–76. 10.1158/1078-0432.CCR-15-2335 [DOI] [PubMed] [Google Scholar]
- 125.Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015;125(25):3956–62. 10.1182/blood-2015-02-627786 [DOI] [PubMed] [Google Scholar]
- 126.Hourigan CS, Dillon LW, Gui G, et al. Impact of conditioning intensity of allogeneic transplantation for Acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. 2020;38(12):1273–83. 10.1200/JCO.19.03011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang X, Chen J, Han MZ, et al. The consensus from the Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. 2021;14(1):145. 10.1186/s13045-021-01159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.