Abstract
Background:
Larotrectinib, a first-in-class, highly selective tropomyosin receptor kinase (TRK) inhibitor, has demonstrated efficacy in adult and pediatric patients with various solid tumors harboring NTRK gene fusions. This subset analysis focuses on the efficacy and safety of larotrectinib in an expanded cohort of adult patients with TRK fusion sarcomas.
Methods:
Patients (aged ≥18 years) with sarcomas harboring NTRK gene fusions were identified from 3 clinical trials. Patients received larotrectinib 100 mg orally twice daily. Response was investigator-assessed per RECIST v1.1. Data cut-off was July 20, 2021.
Results:
At the data cut-off, 36 adult patients with TRK fusion sarcomas had initiated larotrectinib therapy: 2 (6%) patients had bone sarcomas, 4 (11%) had gastrointestinal stromal tumors, and 30 (83%) had soft tissue sarcomas. All patients were evaluable for response and demonstrated an objective response rate of 58% (95% confidence interval, 41-74). Patients responded well to larotrectinib regardless of number of prior lines of therapy. Adverse events (AEs) were mostly grade 1/2. Grade 3 treatment-emergent AEs (TEAEs) occurred in 15 (42%) patients. There were no grade 4 TEAEs. Two grade 5 TEAEs were reported, neither of which were considered related to larotrectinib. Four (11%) patients permanently discontinued treatment due to TEAEs.
Conclusions:
Larotrectinib demonstrated robust and durable responses, extended survival benefit, and a favorable safety profile in adult patients with TRK fusion sarcomas with longer follow-up. These results continue to demonstrate that testing for NTRK gene fusions should be incorporated into the clinical management of adult patients with various types of sarcomas.
Keywords: larotrectinib, sarcoma, TRK, TRK inhibitors, NTRK gene fusion
PLAIN LANGUAGE SUMMARY
Tropomyosin receptor kinase (TRK) fusion proteins result from translocations involving the NTRK gene, and cause cancer in a range of tumor types.
Larotrectinib is an agent that specifically targets TRK fusion proteins and is approved for the treatment of patients with TRK fusion cancer.
This study looked at how well larotrectinib worked in adult patients with sarcomas caused by TRK fusion proteins.
Over half of patients had a rapid, durable response to larotrectinib, with no unexpected side effects.
These results show that larotrectinib is safe and effective in adult patients with TRK fusion sarcomas.
PRECIS
Larotrectinib, a first-in-class TRK inhibitor, demonstrated robust and durable responses, extended survival benefit, and a favorable safety profile in adult patients with TRK fusion sarcomas. Testing for NTRK gene fusions should be incorporated into the clinical management of adult patients with various types of sarcomas to identify those suitable for targeted therapy.
INTRODUCTION
Neurotrophic tyrosine receptor kinase (NTRK) gene fusions arise from inter- and intrachromosomal rearrangements involving the 3′ region of the NTRK gene and the 5′ end of a partner gene. This results in the expression of a constitutively active tropomyosin receptor kinase (TRK) fusion protein which is the oncogenic driver in a diverse range of adult and pediatric tumor types.1,2 NTRK gene fusions have been reported in ≤5% of adult patients with sarcomas.2-5
Adult sarcomas are a heterogenous group of cancers that include bone sarcomas and gastrointestinal stromal tumors (GIST), as well as soft tissue sarcomas (STS) of various histologies.1,6,7 This heterogeneity makes management difficult, with limited treatment options for advanced sarcomas following failure of first-line cytotoxic chemotherapy.6 The prognosis of adult patients with locally advanced or metastatic sarcoma is poor, with median overall survival (OS) ranging from 12 to 18 months,7-9 demonstrating the unmet need for effective treatment options. As NTRK gene fusions can arise in a variety of sarcoma subtypes, TRK fusion proteins are a potential therapeutic target in sarcomas.6
Larotrectinib is a first-in-class, highly selective, and central nervous system (CNS)-active TRK inhibitor.10-13 Larotrectinib has received tumor-agnostic approval for the treatment of adult and pediatric patients with TRK fusion solid tumors based on the robust and durable antitumor efficacy, regardless of patient age or tumor type, observed in a pooled analysis of 3 phase 1/2 trials.10,14,15 In an expanded dataset of 153 evaluable adult and pediatric patients with non-CNS primary TRK solid tumors, including sarcomas of various subtypes and histologies (data cut-off February 19, 2019), the objective response rate (ORR) was 79% (95% confidence interval [CI], 72-85), the median progression-free survival (PFS) was 28.3 months (95% CI, 22.1-not estimable [NE]), and the OS was 44.4 months (95% CI, 36.5-NE).11 In the expanded safety population of 260 patients, treatment-related adverse events (AEs) were predominantly grade 1 or 2. Two percent of patients discontinued treatment due to AEs.11 These results show that larotrectinib demonstrated significant activity in patients with TRK fusion cancer, regardless of tumor type, and was well-tolerated with minimal safety concerns. The aim of this subset analysis was to evaluate the efficacy and safety of larotrectinib in an expanded dataset of adult patients with TRK fusion sarcomas with longer follow-up from the dataset of the 3 clinical trials: NCT02122913, NCT02637687 (SCOUT), and NCT02576431 (NAVIGATE).
MATERIALS AND METHODS
Study Design
Adult patients aged ≥18 years were eligible for inclusion in this analysis if they had a sarcoma harboring an NTRK gene fusion and had participated in 1 of 3 trials of larotrectinib: NCT02122913, a phase 1 trial in adults, SCOUT (NCT02637687), a phase 1/2 trial involving individuals ≤21 years, and NAVIGATE (NCT02576431), a phase 2 “basket” trial in individuals ≥12 years. Details of these trials have been published previously.10,11 Protocols were approved by the institutional review board or independent ethics committee at each site, and all the protocols complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the 1964 Declaration of Helsinki and amendments, and local laws. All patients provided written informed consent. The data cut-off for the current analysis was July 20, 2021, providing an additional 2 years of follow-up compared to the previous report.11
In brief, patients in the NCT02122913 phase 1 trial and NAVIGATE were eligible if they had a locally advanced or metastatic solid tumor, had received standard therapy previously (if available), had an Eastern Cooperative Oncology Group performance status of 0-3, and had adequate major organ function. Patients were eligible for inclusion in the SCOUT trial if they had a locally advanced or metastatic solid tumor, a Karnofsky or Lansky performance score of at least 50, adequate major organ function, and had relapsed, progressed, or were nonresponsive to available therapies. Adult patients received 100 mg larotrectinib orally twice daily (BID); 1 patient received 150 mg BID in the dose escalation phase. Treatment beyond progression was permitted if the patient continued to derive clinical benefit in the opinion of the investigator.
Study Endpoints
The primary endpoint in this analysis was ORR, measured using Response Evaluation Criteria in Solid Tumours version 1.1 based on investigator assessment; secondary endpoints included duration of response (DoR), PFS (defined as the time from the date of the first dose to the earliest date of documented disease progression or death, based on investigator assessment), and OS.10,11
The occurrence of AEs, including treatment discontinuation and dose modifications, was also assessed.10
Study Assessments
Tumor assessments were performed using computed tomography (CT), magnetic resonance imaging (MRI), and clinical measurement with electronic calipers (where appropriate) in the case of cutaneous lesions, at baseline, and every 8 weeks for 1 year, then every 12 weeks thereafter until disease progression. All tumor responses were confirmed at least 4 weeks after the initial response.10
AEs were assessed per National Cancer Institute Common Terminology Criteria for AEs, version 4.03, from the date that informed consent was obtained until at least 28 days after the last dose of larotrectinib was administered. AEs were deemed to be treatment-related based on the judgement of the investigator.10
Statistical Analysis
ORR was calculated based on patients who achieved best overall responses of confirmed complete response (CR) or partial response (PR). Best overall response for each patient was derived from the time the response was first determined by the investigators, with 95% CIs calculated using the Clopper-Pearson method. DoR, PFS, and OS were summarized descriptively using the Kaplan-Meier method, with 95% CIs calculated using Clopper-Pearson method. Median follow-up for the time-to-event endpoints was estimated based on the Kaplan-Meier estimate of potential follow-up. For DoR and PFS, patients were right-censored if they met at least 1 of the following conditions: no baseline or post-baseline disease assessments unless death occurred prior to the first planned assessment (in which case the death will be considered a PFS event); initiation of subsequent anticancer therapy in the absence of documented progression; death or disease progression after missing 2 or more consecutively scheduled disease assessment visits; and last known to be alive and progression-free on or before the data cut-off date (July 20, 2021). For these patients, the progression or censoring date was determined based on conventions described by the U.S. Food and Drug Administration.
RESULTS
Patients
As of July 20, 2021, an expanded cohort of 36 adult patients with locally advanced or metastatic TRK fusion sarcomas were enrolled in the study and had initiated larotrectinib treatment, including 23 with 2 additional years of follow-up from the previous analysis. Patients were identified from NAVIGATE (n = 29; 81%), the adult phase 1 trial (n = 4; 11%), and SCOUT (n = 3; 8%). Patient characteristics are shown in Table 1. Two (6%) patients had bone sarcomas (1 chondrosarcoma and 1 not otherwise specified), 4 (11%) had GIST, and 30 (83%) had STS. At enrolment, 29 (81%) patients had metastatic disease and 7 (19%) had locally advanced disease.
Table 1.
Demographic and clinical characteristics
| Characteristic | N = 36 |
|---|---|
| Age, median (range), years | 41 (19-70) |
| Sex, n (%) | |
| Male | 19 (53) |
| Female | 17 (47) |
| Race, n (%) | |
| White | 20 (56) |
| Asian | 12 (33) |
| Other | 4 (11) |
| ECOG performance status, n (%) | |
| 0 | 14 (39) |
| 1 | 17 (47) |
| 2 | 5 (14) |
| Disease status at enrollment, n (%) | |
| Locally advanced | 7 (19) |
| Metastatic | 29 (81) |
| Years since initial diagnosis, median (range) | 1.1 (0.0-13.0) |
| NTRK gene fusion, n (%) | |
| NTRK1 | 19 (53) |
| NTRK2 | 1 (3) |
| NTRK3 | 16 (44) |
| Subtype, n (%) | |
| Bone sarcoma | |
| Chondrosarcoma | 1 (3) |
| NOS | 1 (3) |
| Gastrointestinal stromal tumors | 4 (11) |
| Soft tissue sarcoma | |
| NOS | 8 (22) |
| Malignant peripheral nerve sheath tumor | 6 (17) |
| Spindle cell sarcoma | 4 (11) |
| Epithelioid spindle sarcoma | 3 (8) |
| Inflammatory myofibroblastic tumor | 2 (6) |
| Myopericytoma | 2 (6) |
| Stromal tumor | 2 (6) |
| Dedifferentiated liposarcoma | 1 (3) |
| Fibrosarcoma | 1 (3) |
| Synovial sarcoma | 1 (3) |
| Prior therapies, n (%)a | |
| Surgery | 31 (86) |
| Radiotherapy | 21 (58) |
| Systemic therapy | 25 (69) |
| Number of prior systemic therapies, n (%) | |
| 0 | 10 (28) |
| 1 | 12 (33)b |
| 2 | 7 (19) |
| ≥3 | 7 (19) |
| Best response to prior therapy, n (%) | |
| Complete response | 1 (4) |
| Stable disease | 5 (20) |
| Progressive disease | 7 (28) |
| Otherc | 11 (44) |
ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified; NTRK, neurotrophic tyrosine receptor kinase.
Patients may have received more than 1 type of prior therapy.
One patient who reported “No” to prior systemic therapies had the number of prior systemic therapies actually reported as “1”.
Other includes unknown and not evaluable.
NTRK gene fusions were identified locally by a variety of testing methods: DNA and RNA next-generation sequencing (NGS; n = 18), DNA NGS (n = 8), RNA NGS (n = 8), fluorescence in situ hybridization (n = 1), and unknown NGS (n = 1). NTRK1, NTRK2, and NTRK3 gene fusions were identified in 19 (53%), 1 (3%), and 16 (44%) patient(s), respectively. A total of 13 fusion partners were identified, with the most common being LMNA::NTRK1 and ETV6::NTRK3 detected in 9 (25%) and 8 (22%) patients each, respectively.
All patients were previously treated. Thirty-one (86%) had prior surgery, 21 (58%) had prior radiotherapy, and 25 (69%) had prior systemic therapy (Table 1). Duration on most recent prior systemic therapy was available for 13 of the 25 patients; median time on most recent prior therapy was 0.49 months (range 0.0-24.8) for these patients. Best response to prior therapy was CR in 1 patient, stable disease in 5, and progressive disease in 7 (Table 1).
Efficacy Outcomes
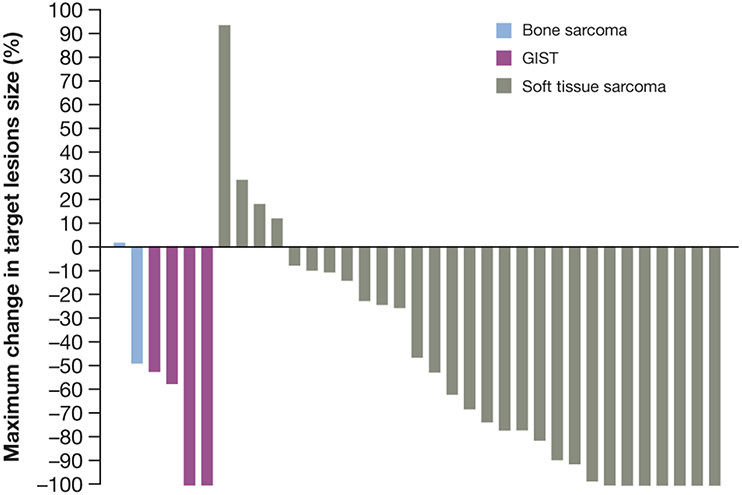
The ORR for all patients was 58% (95% CI, 41-74). Seven (19%) patients had CR, 14 (39%) had PR, 12 (33%) had stable disease (of which 6 had stable disease for ≥24 weeks), 2 (6%) had progressive disease, and 1 (3%) was not determined as they discontinued treatment without evaluable post-baseline assessments (Table 2, Fig. 1). Treatment response by histology is shown in Table 2. Median time to response was 1.8 months (range 0.9-3.5) for all patients. Of the 21 patients with a confirmed best response, 20 (95%) responded to treatment within 2 months. ORR for patients with 0, 1, 2, and ≥3 prior lines of therapy was 80% (95% CI, 44-97), 33% (95% CI, 10-65), 71% (95% CI, 29-96), and 57% (95% CI, 18-90), respectively.
Table 2.
Best response to larotrectinib
| Response | Bone sarcoma | GIST | STS | All sarcomas |
|---|---|---|---|---|
| Evaluable patients | 2 | 4 | 30 | 36 |
| Objective response rate, % (95% CI) | 50 (1-99) | 100 (40-100) | 53 (34-72) | 58 (41-74) |
| Best response, n (%) | ||||
| Complete response | 0 (0) | 1 (25) | 6 (20) | 7 (19) |
| Partial response | 1 (50) | 3 (75) | 10 (33) | 14 (39) |
| Stable disease | 1 (50) | 0 (0) | 11 (37) | 12 (33) |
| Progressive disease | 0 (0) | 0 (0) | 2 (7) | 2 (6) |
| Not determineda | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
CI, confidence interval; GIST, gastrointestinal stromal tumors; STS, soft tissue sarcoma.
Patients that discontinued treatment without evaluable post-baseline assessments.
Figure 1.
Maximum change in target lesions.
GIST, gastrointestinal stromal tumors.
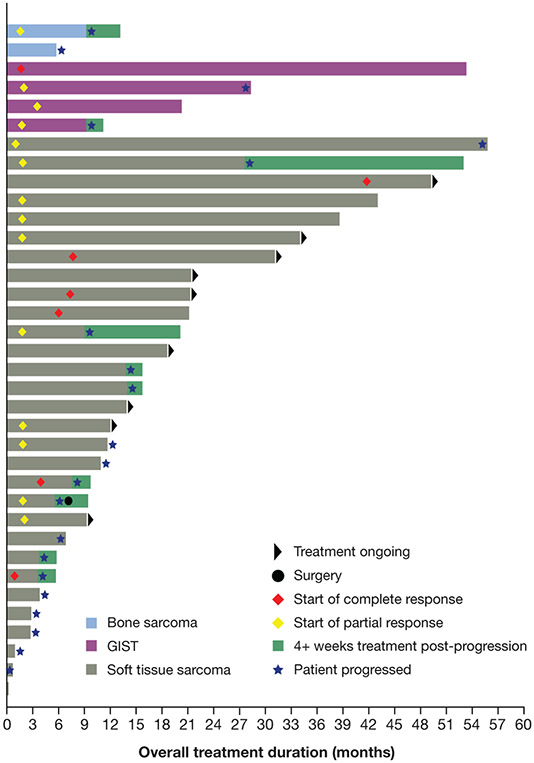
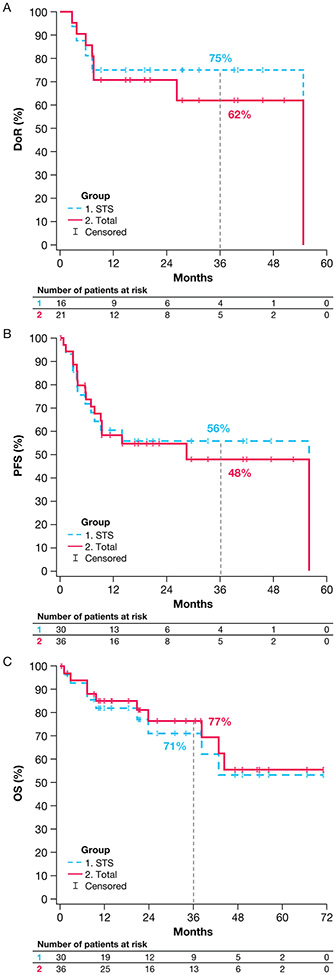
Overall, treatment duration ranged from 0.10 to 55.7 months (Fig. 2). Twenty-one (58%) patients experienced disease progression, with 10 (28%) continuing treatment post-progression. At data cut-off, 9 (25%) patients had treatment ongoing. Median DoRs for the full sarcoma cohort and patients with STS were not reliably estimable at median follow-up periods of 27.5 and 23.9 months. The 36-month DoR rates for all patients and patients with STS were 62% (95% CI, 38-86; Fig. 3A) and 75% (95% CI, 54-96; Fig. 3A), respectively. For the 4 patients with GIST, the median DoR was 26.3 months (95% CI, 7.6-NE) at a median follow-up of 50.4 months, with a 36-month DoR rate of 38% (95% CI, 0-94). The median DoR for the 2 patients with bone sarcoma with a response was 7.7 months (95% CI, NE-NE).
Figure 2.
Treatment duration and time to response.
GIST, gastrointestinal stromal tumors.
Figure 3.
Kaplan-Meier plots showing (A) DoR for the full sarcoma cohort and the STS subgroup, (B) PFS for the full sarcoma cohort and the STS subgroup, and (C) OS for the full sarcoma cohort and the STS subgroup.
DoR, duration of response; OS, overall survival; PFS, progression-free survival; STS, soft tissue sarcoma.
Median PFS was 28.3 months (95% CI, 7.6-55.7) at a median follow-up of 20.7 months for the full sarcoma cohort. The 36-month PFS rate was 48% (95% CI, 28-67; Fig. 3B). For patients with STS, the 36-month PFS rate was 56% (95% CI, 37-75; Fig. 3B). Median OS was not reached for the full sarcoma cohort (median follow-up: 34.0 months) or any of the subtype cohorts. The 36-month OS rate was 77% (95% CI, 61-92; Fig. 3C) for all patients and 71% (95% CI, 53-90; Fig. 3C) for patients with STS.
Safety and Tolerability
Treatment-emergent AEs (TEAEs) that occurred in ≥10% of patients are shown in Table 3. All patients experienced a TEAE; 30 (83%) experienced AEs that were related to larotrectinib. The majority of AEs were grade 1 or 2. Grade 3 TEAEs occurred in 15 (42%) patients, with 1 (3%) considered by the investigator to be related to larotrectinib (increased weight). There were 2 grade 5 TEAEs (neurofibrosarcoma and malignant neoplasm progression), neither of which were considered related to larotrectinib. Four (11%) patients permanently discontinued treatment due to TEAEs not deemed to be treatment-related by the investigators: 1 patient had gait disturbance (grade 3), 1 patient had spinal cord compression (grade 3), 1 patient had pain in extremity (grade 2), and 1 patient had viral infection (grade 3) and malignant neoplasm progression (grade 5). No patients discontinued treatment due to a larotrectinib-related AE. A total of 3 patients reported fractures, 2 grade 1 and 1 grade 2, none were related to larotrectinib.
Table 3.
Adverse events occurring in ≥10% of patients (N = 36)
| MedDRA preferred term | Treatment-emergent adverse events, n (%) |
Treatment-related adverse events, n (%)a | ||||
|---|---|---|---|---|---|---|
| Grade 1 or 2 | Grade 3 | Any gradeb | Grade 1 or 2 | Grade 3 | Any gradec | |
| Any event | 19 (53) | 15 (42) | 36 (100) | 29 (81) | 1 (3) | 30 (83) |
| Constipation | 15 (42) | 0 (0) | 15 (42) | 7 (19) | 0 (0) | 7 (19) |
| Anemia | 9 (25) | 2 (6) | 11 (31) | 2 (6) | 0 (0) | 2 (6) |
| Nausea | 11 (31) | 0 (0) | 11 (31) | 6 (17) | 0 (0) | 6 (17) |
| ALT increased | 9 (25) | 1 (3) | 10 (28) | 5 (14) | 0 (0) | 5 (14) |
| Dizziness | 10 (28) | 0 (0) | 10 (28) | 6 (17) | 0 (0) | 6 17) |
| Fatigue | 9 (25) | 1 (3) | 10 (28) | 4 (11) | 0 (0) | 4 (11) |
| Abdominal pain | 8 (22) | 1 (3) | 9 (25) | 1 (3) | 0 (0) | 1 (3) |
| Oedema peripheral | 9 (25) | 0 (0) | 9 (25) | 3 (8) | 0 (0) | 3 (8) |
| Weight increased | 7 (19) | 2 (6) | 9 (25) | 6 (17) | 1 (3) | 7 (19) |
| Diarrhea | 7 (19) | 1 (3) | 8 (22) | 1 (3) | 0 (0) | 1 (3) |
| Anxiety | 6 (17) | 1 (3) | 7 (19) | 1 (3) | 0 (0) | 1 (3) |
| Arthralgia | 7 (19) | 0 (0) | 7 (19) | 1 (3) | 0 (0) | 1 (3) |
| Back pain | 7 (19) | 0 (0) | 7 (19) | – | – | – |
| Headache | 7 (19) | 0 (0) | 7 (19) | 4 (11) | 0 (0) | 4 (11) |
| Myalgia | 7 (19) | 0 (0) | 7 (19) | 6 (17) | 0 (0) | 6 (17) |
| Pain in extremity | 7 (19) | 0 (0) | 7 (19) | 1 (3) | 0 (0) | 1 (3) |
| AST increased | 5 (14) | 1 (3) | 6 (17) | 5 (14) | 0 (0) | 5 (14) |
| Cough | 6 (17) | 0 (0) | 6 (17) | – | – | – |
| Abdominal distension | 5 (14) | 0 (0) | 5 (14) | 1 (3) | 0 (0) | 1 (3) |
| Dyspnea | 4 (11) | 1 (3) | 5 (14) | 2 (6) | 0 (0) | 2 (6) |
| Pain | 5 (14) | 0 (0) | 5 (14) | 2 (6) | 0 (0) | 2 (6) |
| Upper respiratory tract infection | 5 (14) | 0 (0) | 5 (14) | – | – | – |
| Vomiting | 5 (14) | 0 (0) | 5 (14) | 2 (6) | 0 (0) | 2 (6) |
| Abdominal pain upper | 4 (11) | 0 (0) | 4 (11) | 2 (6) | 0 (0) | 2 (6) |
| Insomnia | 4 (11) | 0 (0) | 4 (11) | – | – | – |
| Muscular weakness | 4 (11) | 0 (0) | 4 (11) | 1 (3) | 0 (0) | 1 (3) |
| Musculoskeletal chest pain | 4 (11) | 0 (0) | 4 (11) | – | – | – |
| Nasopharyngitis | 4 (11) | 0 (0) | 4 (11) | – | – | – |
ALT, alanine aminotransferase; AST, aspartate transaminase; MedDRA; medical dictionary for regulatory activities.
Dashes indicate AEs that were not reported to be treatment-related in any patients.
There were no grade 4 and 2 grade 5 treatment-emergent adverse events (malignant neoplasm progression and neurofibrosarcoma).
There were no grade 4 or 5 treatment-related adverse events.
DISCUSSION
This expanded subset analysis of adult patients with TRK fusion sarcoma with longer follow-up demonstrates the survival benefit and favorable safety profile of larotrectinib. Larotrectinib demonstrated a 58% ORR in adult patients with TRK fusion sarcomas of various histologies. Although 21 (58%) patients experienced disease progression while on treatment, 10 (28%) remained on treatment post-progression because they continued to derive clinical benefit in the opinion of the investigator. Safety findings were consistent with previous reports.10,11 Although larotrectinib was generally well-tolerated, there were a number of AEs reported that are potentially on-target effects of TRK inhibition. Wild-type TRKA/B/C receptors are predominantly expressed in neuronal tissue and are essential for the functioning of the nervous system.1 The 1 grade 3 larotrectinib-related AE reported in this study was increased weight, which can be anticipated due to the role of TRKB in appetite regulation.16,17 Loss-of-function mutations in NTRK1 and NTRK3 may result in dizziness, paresthesia, headaches, and gait disturbances due to their normal functioning in sensory neurons.1,2,12 The AEs seen in this analysis have also been reported in previous studies of larotrectinib and were mostly grade 1 and 2.13,18,19 Of note, no patients discontinued treatment due to an AE related to larotrectinib in this study.
In adult patients with sarcomas for which surgical resection would result in unreasonable morbidity or those with metastatic disease, effective and tolerable therapeutic options are limited and include radiotherapy, isolated limb perfusion, surgery, and systemic therapy. Systemic therapy options vary according to histologic subtype and include cytotoxic chemotherapies (doxorubicin and/or ifosfamide-based regimens), anti-angiogenic multikinase inhibitors (such as pazopanib, regorafenib, sunitinib, and cabozantinib), and multikinase inhibitors that target NTRK, ROS1, and ALK (entrectinib).3,8,20-22 Despite the availability of multiple treatment options, there remains an unmet need for effective targeted treatments. Response rates for chemotherapy in STS vary from 10% to 50% depending on the drugs used, patient selection, and histological subtype.7 Conventional chemotherapy with doxorubicin and/or ifosfamide represents the backbone of systemic treatment. However, no statistically significant OS benefit could be demonstrated by the addition of other chemotherapy to doxorubicin.8 Moreover, a recent partitioned survival modeling study that estimated the long-term comparative effectiveness of larotrectinib and standard of care in adult patients with STS showed that patients receiving larotrectinib gained 5.56 additional life years and 1.99 quality-adjusted life years compared with doxorubicin/ifosfamide.23 Pazopanib, indicated for the treatment of adults with advanced STS,24 demonstrated an ORR of 9%, median PFS of 4.6 months, and OS of 12.5 months in a phase 3 trial.25 Regorafenib is indicated for locally advanced, unresectable, or metastatic GIST that has failed prior treatment with the tyrosine kinase inhibitors imatinib mesylate and sunitinib malate.26 In a phase 3 trial, ORR was 4.5% and median PFS was 7.4 months.27 Entrectinib is a multikinase inhibitor approved for patients aged ≥12 years with TRK fusion solid tumors or adult patients with ROS1-positive metastatic non-small cell lung cancer.28 In 3 phase 1/2 trials in patients with sarcomas treated with entrectinib, median DoR, PFS, and OS were 10.3, 11.0, and 16.8 months, respectively.29 In our current study, larotrectinib demonstrated an ORR of 58%, median PFS of 28.3 months (95% CI, 7.6-55.7), and 36-month OS rate of 77% for all patients and 71% for patients with STS.
The prognosis of adults with advanced sarcomas has improved in the last few years but is still relatively poor, with a median OS of 15-18 months.7,8 There is also the possibility that resistance to first-generation TRK inhibitors might develop in patients.10,30-32 On-target resistance can result from amino acid substitutions involving the solvent front, activation loop xDFG motif, or so-called gatekeeper residue of TRKA or TRKC.2 In anticipation of this, next-generation TRK inhibitors are being developed to overcome this resistance. Repotrectinib and taletrectinib were designed to overcome NTRK resistance mutations; however, data on these agents in patients with sarcoma are limited.22,32,33 Off-target resistance can be mediated by genomic alterations that converge to activate the mitogen-activated protein kinase pathway. Data suggest that off-target resistance will not be adequately addressed by next-generation TRK inhibitors alone and combination therapy may be required.34
Single-arm studies such as this current analysis do not provide comparative data. However, in rare diseases such as TRK fusion cancer, randomized control trials are not feasible due to the low number of patients available for recruitment. The growth module index (GMI) is an intra-patient comparison that uses patients as their own control by comparing PFS on their current therapy against time to progression or treatment failure on their most recent prior therapy.35 A GMI of ≥1.33 indicates a ≥33% improvement in PFS over the previous line of therapy and has been proposed as a threshold of meaningful clinical activity, with the European Medicines Agency endorsing the use of GMI as an endpoint for truly rare tumors or very narrow indications.36,37 In a cohort of 72 patients treated with larotrectinib, 65% of patients met this threshold, indicating they had prolonged PFS compared with the most recent prior therapy, irrespective of the number of prior lines of therapy received.35
The findings reported here support the guideline recommendations from the World Sarcoma Network that consider disease stage and histologic and molecular subtypes to facilitate routine testing for TRK expression and subsequent testing for NTRK gene fusions.3 This subset analysis demonstrates that larotrectinib achieved robust and durable responses, extended survival benefit consistent with prior reports of larotrectinib, and had a favorable safety profile in adult patients with TRK fusion sarcomas. The optimal duration of treatment with larotrectinib is unknown and long-term safety data remain limited. While further studies are needed to assess continued response and to determine the long-term safety profile of larotrectinib, these data continue to highlight the importance of testing for NTRK gene fusions in patients with sarcomas, especially in those with no canonical genomic drivers.
ACKNOWLEDGMENTS
The authors would like to thank the patients, their families, and all investigators involved in these studies. Medical writing support was provided by Cindy Cheung, MBBS (MD) and Luke Springall, PhD, editorial support was provided by George Chappell, MSc, and Melissa Ward, BA, all of Scion, London UK, supported by Bayer according to Good Publication Practice guidelines (Link). The sponsor was involved in the study design and collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
FUNDING SUPPORT
These studies were funded by Bayer Healthcare and Loxo Oncology, Inc., a wholly owned subsidiary of Eli Lilly and Company. Dr. Alexander Drilon was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
CLINICAL TRIAL REGISTRATION
CONFLICT OF INTEREST DISCLOSURES
Shivaani Kummar has served as as a consultant/advisor for Boehringer Ingelheim, Springworks Therapeutics, Gilead, EcoR1, Mirati, Oxford Biotherapeutics, Mundibiopharma Ltd, Bayer, Seagen, Genome & Company, Genome Insight, and HarbourBiomed; as co-founder and equity holder of PathomlQ; and declares a spouse who has served as a scientific advisor for Cadila Pharmaceuticals Ltd., and is a founder of Arxeon, Inc.
Lin Shen has received funding grants from Beijing Xiantong Biomedical Technology Co., Ltd., Qilu Pharmaceutical Co., Ltd., Zaiding Pharmaceutical (Shanghai) Co., Ltd., Jacobio Pharmaceuticals Co., Ltd., and Beihai Kangcheng (Beijing) Medical Technology Co., Ltd.; and consulting fees from MSD, Merck, Boehringer Ingelheim, and Harbour; and has served as a speaker for Hutchison Whampoa, Hengrui Therapeutics, ZaiLab, and CStone.
David S. Hong has received research/grant funding from AbbVie, Adaptimmune, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Eisai, Fate Therapeutics, Genentech, Genmab, Ignyta, Infinity, Kite, Kyowa, Lilly, Loxo Oncology, Merck, MedImmune, Mirati, MiRNA, Molecular Templates, Mologen, NCI-CTEP, Novartis, Pfizer, Seagen, and Takeda; travel/accommodation/expense fees from Loxo Oncology, MiRNA, ASCO, AACR, SITC, and Genmab; served as a consultant/advisor for Alpha Insights, Axiom, Adaptimmune, Baxter, Bayer, Genentech, GLG, Group H, Guidepoint Global, Infinity, Janssen, Merrimack, Medscape, Numab, Pfizer, Seagen, Takeda, and Trieza Therapeutics; and has ownership interests in Molecular Match (advisor), OncoResponse (founder), and Presagia, Inc (advisor).
Ray McDermott has served on advisory boards for Amgen, Bayer, Bristol-Myers Squibb, Clovis, Janssen, and Pfizer; been an invited speaker for Astellas, Ipsen, and MSD; and received research funding (to their institution) from Astellas, Bayer, Bristol-Myers Squibb, Clovis, MSD, and Regeneron.
Vicki L. Keedy has served as a consultant for Karyopharm and Daiichi-Sankyo; and received research funding (to their institution) from Medpacto, Inc., Plexxikon, Daiichi-Sankyo, Roche, AstraZeneca, Immune Design, Adaptimmune, Lilly, Biomed Valley, Tracon, Advenchen, and GSK.
George D. Demetri has served as a scientific consultant (with sponsored research paid to their institution) for Bayer, Pfizer, Novartis, Roche/Genentech, Epizyme, Loxo Oncology, AbbVie, GSK, Janssen, PharmaMar, ZioPharm, Daiichi-Sankyo, Adaptimmune, and Mirati; been a scientific consultant for GSK, EMD Serono, Sanofi, ICON plc, WCG/Arsenal Capital, Polaris Pharmaceuticals, MJ Hennessey/OncLive, C4 Therapeutics, Synlogic, and MEDSCAPE; been a consultant/advisory board member with minor equity holding for G1 Therapeutics, Caris Life Sciences, Champions Biotechnology, Bessor Pharmaceuticals, Erasca Pharmaceuticals, RELAY Therapeutics, and Caprion/HistoGeneX; been on the board of directors and a scientific advisory board consultant with minor equity for Blueprint Medicines, Merrimack Pharmaceuticals (ended Oct 2019), and Translate BIO; and received royalties (to their institution) from Novartis; and declares non-financial interests in McCann Health, Alexandria Real Estate Equities, the AACR Science Policy, and Government Affairs Committee (chair).
Afshin Dowlati has received consulting and advisory fees from Takeda, AbbVie, Seagen, AstraZeneca, and Bristol Myers Squibb; and research funding from Loxo Oncology, Bayer, Incuron, Takeda, Regeneron, Tesaro, Amgen, Seagen, Symphogen, AbbVie, and Ipsen.
Soledad Gallego Melcón has received honoraria from and served on advisory boards for Loxo Oncology, Bayer, and EusaPharma.
Ulrik N. Lassen has served on advisory boards for Bayer, Pfizer, and Novartis; and received research funding from Bristol-Myers Squibb, GSK, Pfizer, and Roche.
Serge Leyvraz has served as an advisor for and received travel grant support from Bayer.
Victor Moreno has received consulting fees from Bayer, Pieris, Bristol-Myers Squibb, and Janssen; travel support from Regeneron/Sanofi, Bristol-Myers Squibb, and Bayer; served on a speaker bureau for Nanobiotix, Bristol-Myers Squibb, and Bayer; and received an educational grant from Medscape/Bayer.
Jyoti Patel has served as a consultant for ARIAD, AbbVie, AstraZeneca, and Takeda Science Foundation.
Tejas Patil has received honoraria from Roche/Genentech, AstraZeneca, PRIME Oncology, and Aptitude Health, LLC; and consulting fees from Guidepoint Global and Axiom.
Atrayee Basu Mallick has served as a consultant for Daiichi-Sankyo.
Makoto Tahara has received consulting/advisory fees from Bayer, Merck Serono, MSD, Ono Pharmaceutical, Rakuten Medical, Pfizer, Lilly, AstraZeneca, and Loxo Oncology; honoraria from Eisai, MSD, Ono Pharmaceutical, and Bristol-Myers Squibb; and research funding from Bayer and Ono Pharmaceutical.
David S. Ziegler has received consulting/advisory board fees from Bayer, Astra Zeneca, Accendatech, Novartis, Day One, FivePhusion, Amgen, Alexion, and Norgine; and research funding from Accendatech.
Ricarda Norenberg is an employee of Bayer.
Pierre Arvis is an employee of Bayer.
Nicoletta Brega is an employee of Bayer.
Alexander Drilon has received honoraria/served on advisory boards for Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, AbbVie, 14ner/Elevation Oncology, ArcherDX, Monopteros, Novartis, EMD Serono, Medendi, Repare RX, Nuvalent, Merus, Chugai Pharmaceutical, Remedica Ltd., mBrace, AXIS, EPG Health, Harborside Nexus, Liberum, RV More, Ology, Amgen, TouchIME, Janssen, Entos, Treeline Bio, Prelude, Applied Pharmaceutical Science, Inc., AiCME, i3 Health, and MonteRosa; and associated research funds (to their institution) from Pfizer, Exelixis, GSK, Teva, Taiho, and PharmaMar; royalties from Wolters Kluwer; CME honoraria from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences, Med Learning, Imedex, Answers in CME, Clinical Care Options, EPG Health, JNCC/Harborside, Liberum, and Remedica Ltd.; and declares equity in Treeline Bio; and a copyright for Selpercatinib-Osimertinib (filed/pending).
Daniel S. W. Tan has received travel fees from Pfizer, Boehringer Ingelheim, and Roche; honoraria from Bristol-Myers Squibb, Takeda, Novartis, Roche, and Pfizer; research funding from Novartis, GSK, and AstraZeneca; and served as a consultant for Novartis, Merck, Loxo, AstraZeneca, Roche, and Pfizer.
All other authors have no conflicts to disclose.
DATA AVAILABILITY
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, timepoint and process of data access.
As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014.
Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal.
Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
REFERENCES
- 1.Amatu A, Sartore-Bianchi A, Bencardino K, Pizzutilo EG, Tosi F, Siena S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol. 2019;30(Suppl_8):viii5–viii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demetri GD, Antonescu CR, Bjerkehagen B, Bovee J, Boye K, Chacon M, et al. Diagnosis and management of tropomyosin receptor kinase (TRK) fusion sarcomas: expert recommendations from the World Sarcoma Network. Ann Oncol. 2020;31(11):1506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsythe A, Zhang W, Phillip Strauss U, Fellous M, Korei M, Keating K. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther Adv Med Oncol. 2020;12:1758835920975613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westphalen CB, Krebs MG, Le Tourneau C, Sokol ES, Maund SL, Wilson TR, et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis Oncol. 2021;5(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilding CP, Loong HH, Huang PH, Jones RL. Tropomyosin receptor kinase inhibitors in the management of sarcomas. Curr Opin Oncol. 2020;32(4):307–13. [DOI] [PubMed] [Google Scholar]
- 7.Dangoor A, Seddon B, Gerrand C, Grimer R, Whelan J, Judson I. UK guidelines for the management of soft tissue sarcomas. Clin Sarcoma Res. 2016;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasper B. The challenge of finding new therapeutic avenues in soft tissue sarcomas. Clin Sarcoma Res. 2019;9(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucchesi C, Khalifa E, Laizet Y, Soubeyran I, Mathoulin-Pelissier S, Chomienne C, et al. Targetable alterations in adult patients with soft-tissue sarcomas: Insights for personalized therapy. JAMA Oncol. 2018;4(10):1398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019;30:viii23–viii30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doz F, van Tilburg CM, Geoerger B, Hojgaard M, Ora I, Boni V, et al. Efficacy and safety of larotrectinib in TRK fusion-positive primary central nervous system tumors. Neuro Oncol. 2022;24:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayer HealthCare Pharmaceuticals Inc. VITRAKVI prescribing information. Updated 2021. Accessed 13 December. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210861s006lbl.pdf.
- 15.Bayer AG. VITRAKVI summary of product characteristics. Accessed 13 December. https://www.ema.europa.eu/en/documents/product-information/vitrakvi-epar-product-information_en.pdf. [Google Scholar]
- 16.Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7(11):1187–9. [DOI] [PubMed] [Google Scholar]
- 17.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong DS, Kummar S, Farago AF, Lassen UN, Berlin J, Schilder RJ, et al. Larotrectinib efficacy and safety in adult TRK fusion cancer patients. J Clin Oncol. 2019;37 (15_suppl):3122. [Google Scholar]
- 19.Drilon A, Tan DSW, Lassen UN, Leyvraz S, Liu Y, Patel JD, et al. Efficacy and safety of larotrectinib in patients with tropomyosin receptor kinase fusion-positive lung cancers. JCO Precis Oncol. 2022;6:e2100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv51–iv67. [DOI] [PubMed] [Google Scholar]
- 21.Cren PY, Lebellec L, Ryckewaert T, Penel N. Anti-angiogenic agents in management of sarcoma patients: Overview of published trials. Front Oncol. 2020;10:594445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laetsch TW, Hong DS. Tropomyosin receptor kinase inhibitors for the treatment of TRK fusion cancer. Clin Cancer Res. 2021;27:4974–82. [DOI] [PubMed] [Google Scholar]
- 23.Suh K, Carlson JJ, Xia F, Williamson T, Sullivan SD. The potential long-term comparative effectiveness of larotrectinib vs standard of care for treatment of metastatic TRK fusion thyroid cancer, colorectal cancer, and soft tissue sarcoma. J Manag Care Spec Pharm. 2022;28(6):622–30. [DOI] [PubMed] [Google Scholar]
- 24.Novartis. VOTRIENT® (pazopanib) prescribing information. Accessed 13 December 2022. https://www.novartis.us/sites/www.novartis.us/files/votrient.pdf.
- 25.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–86. [DOI] [PubMed] [Google Scholar]
- 26.Bayer HealthCare Pharmaceuticals Inc. STIVARGA® (regorafenib) prescribing information. Accessed 13 December 2022. https://labeling.bayerhealthcare.com/html/products/pi/Stivarga_PI.pdf.
- 27.Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genentech I. ROZLYTREK (entrectinib) prescribing information. Accessed 13 December. https://www.gene.com/download/pdf/rozlytrek_prescribing.pdf.
- 29.Liu SV, Paz-Ares L, Hu J, Wolf J, Cho BC, Krzakowski M, et al. Paper #11. Entrectinib in NTRK fusion-positive sarcoma: integrated analysis of patients enrolled in STARTRK-2, STARTRK-1 AND ALKA-372-001. 2019: [Google Scholar]
- 30.Drilon A, Nagasubramanian R, Blake JF, Ku N, Tuch BB, Ebata K, et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 2017;7(9):963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol. 2016;27(5):920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drilon A, Ou SHI, Cho BC, Kim DW, Lee J, Lin JJ, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent- front mutations. Cancer Discov. 2018;8(10):1227–36. [DOI] [PubMed] [Google Scholar]
- 33.Ou SI, Fujiwara Y, Shaw AT, Yamamoto N, Nakagawa K, Fan F, et al. Efficacy of taletrectinib (AB-106/DS-6051b) in ROS1+ NSCLC: An updated pooled analysis of U.S. and Japan phase 1 studies. JTO Clin Res Rep. 2021;2(1):100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cocco E, Schram AM, Kulick A, Misale S, Won HH, Yaeger R, et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med. 2019;25(9):1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Italiano A, Nanda S, Briggs A, Garcia-Foncillas J, Lassen U, Vassal G, et al. Larotrectinib versus Prior Therapies in Tropomyosin Receptor Kinase Fusion Cancer: An Intra-Patient Comparative Analysis. Cancers (Basel). 2020;12(11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Von Hoff DD. There are no bad anticancer agents, only bad clinical trial designs--twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin Cancer Res. 1998;4(5):1079–86. [PubMed] [Google Scholar]
- 37.European Medicines Agency. Guideline on the evaluation of anticancer medicinal products in man. Accessed September 9, 2019. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-anticancer-medicinal-products-man-revision-5_en.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, timepoint and process of data access.
As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014.
Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal.
Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.