Abstract
Objective:
To determine whether quantitative EEG analysis of burst suppression can predict seizure recurrence in patients with refractory status epilepticus (RSE) being treated with anesthetic doses of continuous IV antiseizure medications (cIVASM).
Methods:
Quantitative assessment of burst suppression (including epileptiform discharges [EDs] and evolution) in 31 occasions (from 27 patients), and correlation with seizure recurrence up to 48 hours post sedative wean.
Results:
Occasions resulting in seizure recurrence (vs. no seizure recurrence) had lower burst (8.4 vs. 10.6 μV) and interburst interval (IBI) (4.2 vs. 4.8 μV) average amplitude, duration (bursts 2.8 vs. 3.6 s: IBIs 3.6 vs. 4.4 s); and burst total power (0.4 vs. 0.7 μV2). Bursts (0.86 vs. 0.60) and IBIs (0.28 vs. 0.07) with EDs, higher number of EDs within bursts (mean 2.1 vs. 1.4) and IBIs (0.6 vs. 0.2), and positive evolution measures all predicted seizure recurrence, although EDs had the greatest adjusted odds ratio on multivariate analysis.
Conclusions:
For patients in burst suppression, successful wean of cIVASM was not determined by classical burst suppression measures, but instead how “epileptiform” bursts and IBIs were, as determined by EDs in both bursts and IBIs and surrogates for evolution within bursts.
Significance:
If confirmed, these objective measures could be used during clinical care to help determine when to wean cIVASM in patients with RSE.
Keywords: burst suppression, status epilepticus, highly-epileptiform bursts (HEBs), quantitative EEG1
Introduction:
Burst suppression on an electroencephalogram (EEG) has historically been a treatment target (a surrogate for seizure control) in the titration of anesthetic doses of continuous IV antiseizure medication (cIVASM) for patients with refractory status epilepticus (RSE).(Rossetti, Logroscino, & Bromfield, 2005; Shorvon, 2001) Despite the practice standing for half a century, there remains little robust evidence to guide clinicians as to the features of the EEG pattern that determines adequate seizure control, and more importantly, the features that would signify that weaning sedative medications would be successful (i.e., RSE would not recur). Historically, arbitrary targets were chosen, such as burst suppression where the interburst intervals (IBIs) were at least 10-30 seconds in duration, or maintaining burst suppression for 12-48 hours.(Shorvon, 2001) The issue with such an approach is that cIVASM, typically involving high-dose IV anesthetics such as pentobarbital, midazolam or propofol, is not benign and several studies have suggested increased harm and mortality with more prolonged therapeutic coma in this context.(Rossetti et al., 2005; Watson et al., 2008) A major problem with the historical approach has been that the amount and duration of sedative administered is often center specific, based on local practices, and not patient specific. This likely exposes many patients to unnecessary total cumulative doses of sedation and in turn the associated harm.(Schmutzhard & Pfausler, 2011)
Recognizing this, there has been a shift towards using the EEG features of burst suppression at a given time to stratify patients into having a high or low risk of seizure recurrence should cIVASM be weaned.(Johnson, Martinez, & Ritzl, 2016; Thompson & Hantus, 2016) By this method, if burst suppression is required in the treatment of RSE, etiology can be corrected and non-sedating anti-seizure medication (ASM) can be optimized, then the EEG can guide the appropriate time for weaning cIVASM for a given individual, thereby minimizing the total sedative exposure and limiting harm. One EEG feature suggestive of seizure recurrence is the presence of highly epileptiform bursts (HEBs).(Thompson & Hantus, 2016) The American Clinical Neurophysiology Society (ACNS) defined the term by consensus in 2011 with a minor revision in 2021. As of 2021 HEBs are defined as: “Present if two or more epileptiform discharges (spikes or sharp waves) are seen within most (>50%) of bursts and occur at an average of 1 Hz or faster within a single burst; Also present if a rhythmic, potentially ictal-appearing pattern occurs within most (>50%) bursts”.(Hirsch et al., 2021; Hirsch et al., 2013; Thompson & Hantus, 2016) This work aims to determine the relative weighting of the two defining criteria for HEBs--epileptiform discharges (EDs) and evolution (defined further in methods)--and how these factor in to all other EEG characteristics of burst suppression (e.g., amplitude, duration, spectral content, etc.) in determining seizure recurrence, using objective quantitative methods.(Johnson et al., 2016; Thompson & Hantus, 2016)
Methods:
Patients
Patients were selected from retrospective review of all cases in a critical care EEG database (Yale New Haven Hospital [YNHH]) between December 2014 and December 2020. During this time, YNHH used the Critical Care EEG Monitoring Research Consortium’s (CCEMRC) publicly available database https://www.acns.org/research/critical-care-eeg-monitoring-research-consortium-ccemrc/ccemrc-public-database). Patients were screened if they were older than 18 years of age, had greater than 6 hours of continuous EEG monitoring (cEEG) and had burst suppression on their EEG present at any time during the admission.
Patients were then excluded if cardiac arrest or hypoxic ischemic encephalopathy were the indications for the EEG, or if the EEG was not burst suppressed at the time of identified wean of cIVASM (e.g., discontinuous, nearly continuous, continuous, or suppressed), using ACNS criteria.(Hirsch et al., 2021) A flowchart with inclusion and exclusion criteria and the steps of the analysis is shown in Figure 1. This analysis was approved under the YNHH Institutional Review Board (IRB) 2011 “Urgent Inpatient EEG and Multimodality Monitoring Databank” (IRB protocol MOD00040247). The requirement for informed consent was waived.
Figure 1:
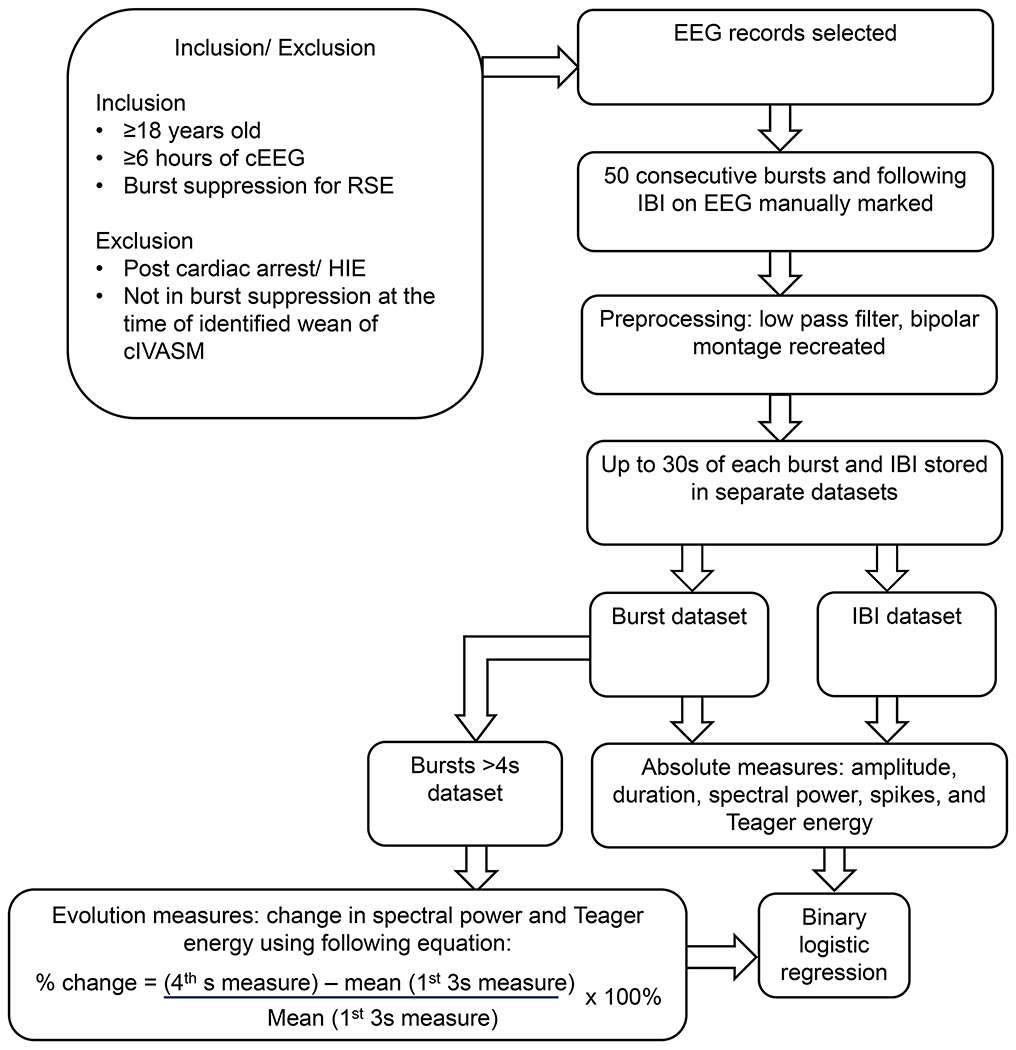
A flowchart illustrating the inclusion and exclusion criteria and the steps of the analysis. Outline of the screening and analysis process including screening, pre-processing, analysis (for absolute measures and those included in evolution measures), leading to binary logistic regression. cEEG: continuous EEG monitoring. RSE: Refractory Status Epilepticus. HIE: hypoxic ischemic encephalopathy. cIVASM: continuous intravenous antiseizure medication. IBI: Interburst interval.
Selection of Bursts/Suppressions for Analysis
Electronic chart review, combined with EEG annotation review, identified occasions where a trial of weaning cIVASM occurred (including infusions of midazolam/lorazepam, propofol, ketamine, and pentobarbital). Over the course of a patient’s admission more than one occasion of weaning cIVASM may have occurred, but for subsequent trials to be included they had to be separated by at least 48 hours from the original trial. As per above, for subsequent trials to be included they also had to qualify as burst suppression at the time of the subsequent wean.
For quantitative analysis, 50 consecutive pairs of bursts and interburst intervals (IBI) were manually marked by author MF who is fellowship trained in critical care EEG, certified in the ACNS terminology, and co-author of the 2021 ACNS terminology.(Hirsch et al., 2021) Interburst intervals consisted of the lower amplitude segment of an alternating burst suppression pattern. By this definition the IBI could have been suppressed or attenuated and could have included sporadic EDs. The 50 pairs of bursts/IBIs were selected from a relatively artifact free epoch of EEG within 1 hour preceding the time of identified cIVASM wean. The onset of 51 bursts and 50 IBIs were marked. Given the alternating pattern, the beginning of the IBIs marked the end of the bursts, and vice versa. The 51st burst needed to be marked to determine the end of the 50th IBI, but the 51st burst itself was not analyzed. Examples of marking of bursts and IBIs has been provided in Supplementary Figure 1.
Primary Outcome
Electrographic seizure recurrence by 48 hours following commencement of weaning cIVASM. Forty-eight hours post wean (vs. 12 or 24 hours) was chosen to provide the most accurate reflection of seizure recurrence. Common clinical practice has been to wean and cease sedation over 24 hours and then discontinue the EEG at that point if seizures have not returned (Note, our center routinely continues cEEG for 24 hours post wean). If taking seizure recurrence at 24 hours as the primary outcome, a proportion of patients would have just ceased cIVASM and most would not have had an adequate duration of cEEG following sedative medication cessation to accurately reflect the true rate of seizure recurrence.
Quantitative Analysis
The quantitative analysis of bursts and IBIs for occasions associated with seizure recurrence vs. occasions that were not (i.e., occasions that were successful) was performed with MATLAB (MathWorks, Natick, MA). Standard measures of EEG were determined including mean amplitude, mean duration, total and band power, and Teager energy of bursts and IBIs. Teager energy is a weighted measure of signal energy such that higher frequency signals have a greater contribution than lower frequency signals. This emphasis on higher frequencies results in a measure which is akin to the line length measure. For consistency, a maximum duration of 30 seconds was set for each EEG segment, either bursts or IBIs. Note: this threshold was set as by definition activity lasting greater than 30 seconds can no longer count as a “burst” and prolonged IBIs (>30 sec) are almost universally suppressed with no background (the first 30 seconds of which were still included in analysis).
In order to include additional measures of how “epileptiform” bursts were, information on EDs (including spikes and sharp-waves) for bursts and IBIs was taken from an established commercial quantitative EEG platform (Persyst™ version 13) with standard/medium sensitivity settings. Quantitative surrogates for “evolution” were used as our measure of “ictal-appearing”. “Change-in” measures were obtained for each power and Teager energy estimate as a positive 50% from baseline cut off. To achieve this, the activity from the first 3 seconds of a burst was averaged and this was used as a baseline to compare to the activity from the 4th second. A 50% increase in the measure in the 4th second compared to baseline qualified as a marker of evolution. By this definition, only bursts lasting at least 4 seconds could be included in this analysis.
Statistical Analysis
Quantitative measures for bursts and IBIs have been shown for occasions that resulted in seizure recurrence vs. those that did not. Measures have been shown with means and 95% confidence intervals (CI). Comparisons between groups (seizure recurrence vs. no seizure recurrence) were achieved with Student’s t-test for parametric data, or Pearson’s Chi-Square test for non-parametric data. Clinically and statistically relevant variables were then added to a binary logistic regression model to determine the relative contribution of each variable to a distinction between seizure recurrence and no seizure recurrence. A result was statistically significant if p values were less than 0.05.
Results:
Inclusion/exclusion
In the study period 4226 patients were entered into the YNHH critical care EEG database. Of these, 312 patients had burst suppression at any time of their admission, and 220 occurred in the post cardiac arrest/hypoxic-ischemic encephalopathy (HIE) setting, leaving 92 that were screened (Figure 1). Out of these 92, 45 had RSE (i.e., the other 47 had burst suppression occurring in another context, e.g., diffuse cerebral dysfunction), and 27 were ultimately included (i.e., they had burst suppression present at the time of identified weaning of cIVASM, compared to 18 that were not in burst suppression at the time of weaning [discontinuous, nearly continuous, etc.]). In these 27 patients that qualified for our inclusion, only 2 met the fairly strict criteria to allow more than 1 occasion per admission (1 patient with 2 occasions and 1 patient with New Onset Refractory Status Epilepticus [NORSE] with 4 occasions). This resulted in 31 unique occasions where a patient was in burst suppression at the time of identified trial of cIVASM wean; 15 occasions resulted in seizure recurrence by 48 hours and 16 did not. Of the 15 occasions with seizure recurrence, the electrographic seizure was generalized in 6 and focal in 9. Two of the 15 occasions (13%) resulted in electroclinical (in addition to electrographic) seizures, with fixed gaze deviation and facial hemiclonus in one patient and the other with rhythmic non-clonic head movement.
Quantitative analysis of bursts/suppressions
The occasions associated with seizure recurrence had lower mean burst amplitude (seizure 8.4 ± 0.2 μV [standard deviation] vs. no seizure 10.6 ± 0.4 μV) and mean IBI amplitude (4.2 vs. 4.8 μV), Table 1 and Figure 2. Occasions resulting in seizures had shorter burst (2.8 vs. 3.6 sec) and IBI (3.6 vs. 4.4 sec) duration; and lower burst total power (0.4 vs. 0.7 μV2), burst delta power (0.2 vs. 0.5 μV2), burst theta power (0.1 vs. 0.2 μV2), and burst alpha power (0.03 vs. 0.04 μV2). The IBI power was low across all spectral power estimates for those with seizure recurrence and those without, but the Teager energy for suppressed components was greater in those with seizure recurrence (0.66 vs 0.39). In occasions resulting in seizure recurrence, bursts were more likely to contain EDs (0.86 vs. 0.60), as were the IBIs (0.28 vs. 0.07), with the average number of EDs per burst greater in those with seizure recurrence (2.1 vs. 1.4), a finding that held true for IBIs as well (0.6 vs. 0.2). Cumulative seizure recurrence probability was plotted against number of EDs in bursts and IBIs, Figure 3. Considering bursts, and to a lesser extent IBIs, as the number of EDs increased so did the probability of seizure recurrence. This effect was most pronounced for a small number of EDs. For bursts, 2 EDs already gave a seizure probability >50% with little additional increase after 5-6 EDs.
Table 1:
Signal characteristics for occasions without seizure recurrence vs. those that resulted in seizure recurrence. Characteristics of bursts and IBIs have been presented as mean ± 95% confidence interval (95%CI) IBI: interburst interval, EDs: epileptiform discharges.
| Segment Characteristic | No seizure recurrence Mean ± 95%CI |
Seizure recurrence Mean ± 95%CI |
p-value |
|---|---|---|---|
| Burst amplitude (μV) | 10.6 ± 0.4 | 8.4 ± 0.2 | < 0.01 |
| IBI amplitude (μV) | 4.8 ± 0.2 | 4.2 ± 0.1 | < 0.01 |
| Burst duration (s) | 3.6 ± 0.1 | 2.8 ± 0.1 | < 0.01 |
| IBI duration (s) | 4.4 ± 0.2 | 3.6 ± 0.2 | < 0.01 |
| Burst total power (μV2) | 0.7 ± 0.1 | 0.4 ± 0.04 | < 0.01 |
| Burst delta power (μV2) | 0.5 ± 0.1 | 0.2 ± 0.02 | < 0.01 |
| Burst theta power (μV2) | 0.2 ±0.02 | 0.1 ± 0.02 | 0.02 |
| Burst alpha power (μV2) | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.02 |
| Burst beta power (μV2) | 0.02 ±0.01 | 0.02 ± 0.01 | 0.97 |
| Burst gamma power (μV2) | 0.004 ± 0.001 | 0.004 ± 0.0004 | 0.67 |
| Burst Teager energy | 2.5 ± 0.20 | 2.3 ± 0.2 | 0.15 |
| IBI total power (μV2) | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.51 |
| IBI delta power (μV2) | 0.03 ±0.01 | 0.02 ± 0.01 | 0.02 |
| IBI theta power (μV2) | 0.004 ± 0.001 | 0.005 ± 0.001 | 0.03 |
| IBI alpha power (μV2) | 0.002 ± 0.001 | 0.003 ± 0.001 | < 0.01 |
| IBI beta power (μV2) | 0.002 ± 0.001 | 0.003 ± 0.001 | < 0.01 |
| IBI gamma power (μV2) | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.01 |
| IBI Teager energy | 0.39 ± 0.03 | 0.66 ± 0.08 | < 0.01 |
| Bursts with EDs | 0.60 ± 0.03 | 0.86 ± 0.03 | < 0.01 |
| IBI with EDs | 0.07 ± 0.02 | 0.28 ± 0.03 | < 0.01 |
| Number of EDs within bursts | 1.4 ± 0.1 | 2.1 ± 0.1 | < 0.01 |
| Number of EDs within IBIs | 0.2 ± 0.1 | 0.6 ± 0.1 | < 0.01 |
Figure 2:
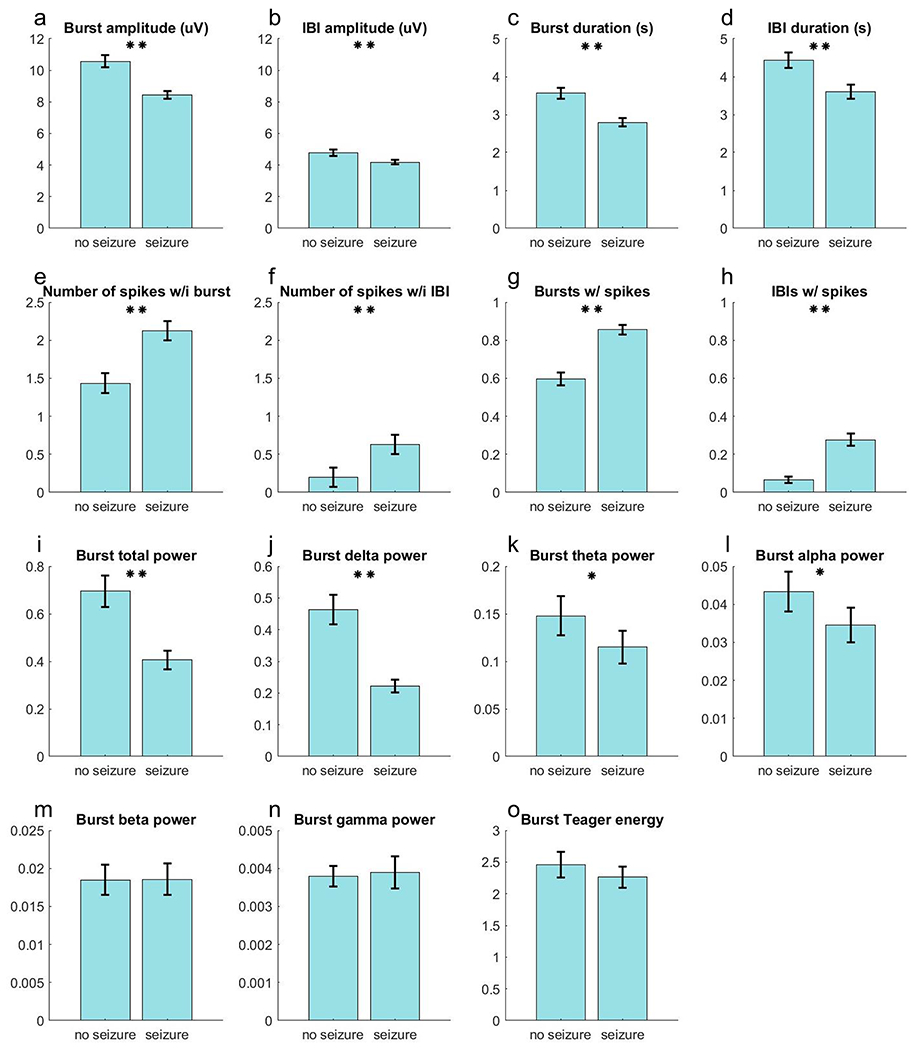
Burst and Interburst Interval (IBI) signal segment characteristics for occasions without seizure recurrence vs. those that resulted in seizure recurrence. The results from Table 1 are plotted as bar graphs to highlight the differences between the two groups. Error margins for each bar represents the 95% confidence interval. One asterisk for p values <0.05, two asterisks for p values <0.01.
Figure 3:
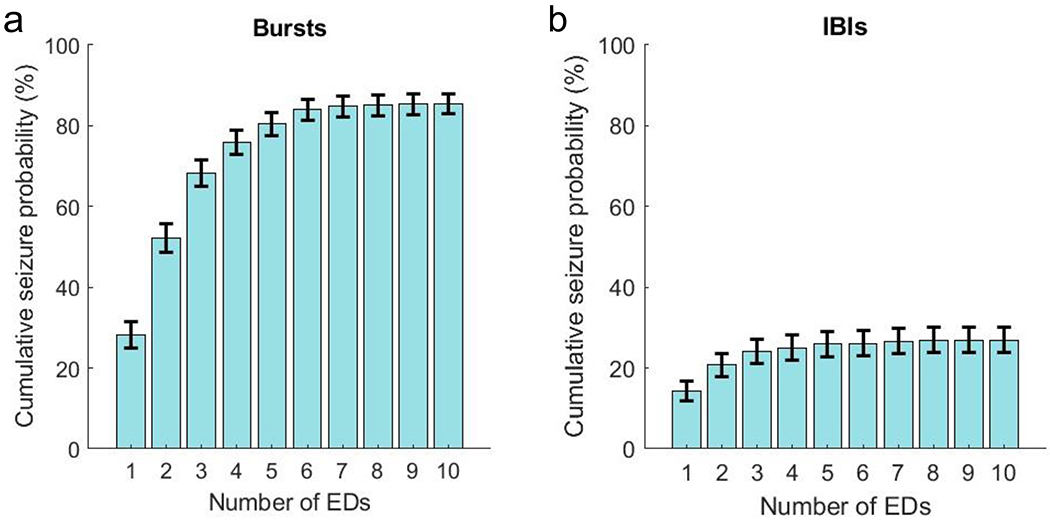
The cumulative probability for seizure recurrence as a function of the number of epileptiform discharges (EDs) in (a) bursts and (b) interburst intervals (IBI). The error margins for each bar represents the 95% confidence interval. A considerable increase in seizure recurrence probability was seen with the first few EDs observed during bursts.
Signal evolution / change-in measures clearly differed between groups by visual inspection of the trends, which was especially true for bursts (Figure 4). Given evolution measures required at least 4 seconds by definition, 274 bursts (out of 1550) were analyzed (106 associated with seizure recurrence and 168 without). For these cases, occasions with seizure recurrence had a greater positive change in total power (seizure 108.4 ± 45.8% vs. no seizure −22.7 ± 5.0%), delta power (129.7 vs. −21.2%); and alpha (81.5 vs. −19.9%), beta (88.4 vs. −16.4%), and gamma (4.6 vs. −17.6%) power (Table 2). Occasions with recurrent seizures were also associated with bursts with greater positive change in Teager energy (27.1 vs. −22.2%, not shown in figure).
Figure 4:
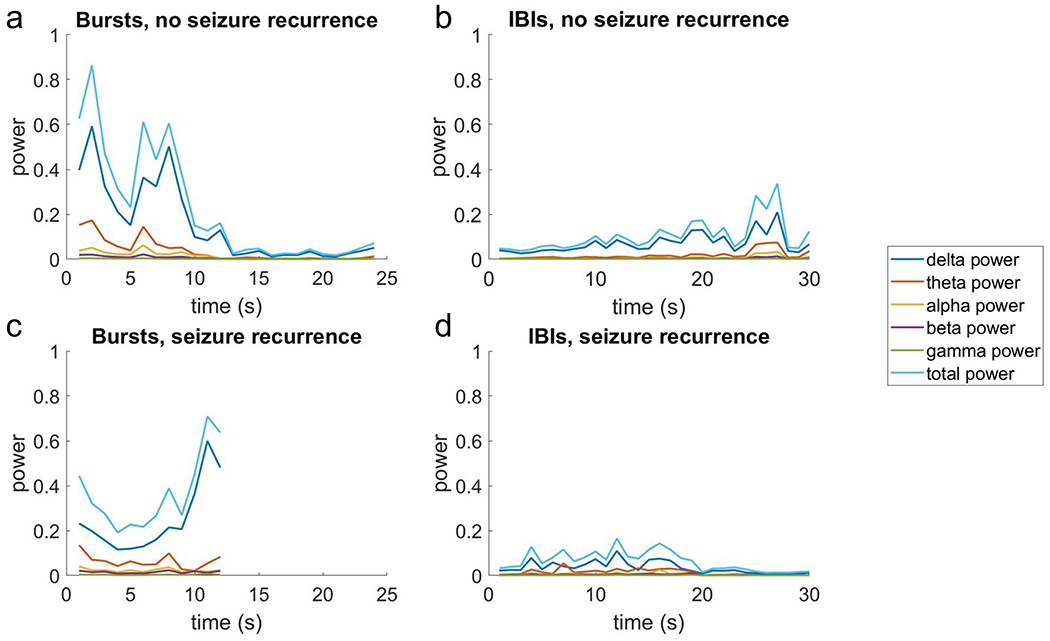
The signal band power measures associated with seizure recurrence or not over the duration of bursts and interburst intervals (IBIs).
Table 2:
Signal measures changing over the bursts, for patients without seizure recurrence vs. those with. Bursts associated with seizure recurrence had significant positive changes (i.e., band power increased by shown percentage over the course of the burst), compared with bursts without seizure recurrence that all reduced over the burst. 95%CI: 95% confidence interval.
| Signal measure | No seizure recurrence Mean ± 95%CI |
Seizure recurrence Mean ± 95%CI |
p-value |
|---|---|---|---|
| Change in total power (%) | −22.7 ± 9.1 | 108.4 ± 83.5 | < 0.01 |
| Change in delta power (%) | −21.2 ± 10.0 | 129.7 ± 94.3 | < 0.01 |
| Change in theta power (%) | −16.8 ± 15.3 | 192.6 ± 193.2 | 0.07 |
| Change in alpha power (%) | −19.9 ± 9.8 | 81.5 ± 67.5 | 0.01 |
| Change in beta power (%) | −16.4 ± 9.3 | 88.4 ± 71.2 | 0.01 |
| Change in gamma power (%) | −17.6 ± 5.9 | 4.6 ± 14.8 | 0.02 |
| Change in Teager energy (%) | −22.2 ± 5.7 | 27.1 ± 25.9 | < 0.01 |
Binary logistic regression
Upon assessment, although many variables were statistically significant, the most clinically significant measures were presence or absence of EDs, number of EDs, and the evolution measures. These were subsequently placed into a binary logistic regression model with the binary outcome being seizure recurrence vs. not (Table 3). This model demonstrated that the presence of EDs within bursts (adjusted odds ratio [aOR] 8.55 [95% CI 7.82 – 9.27]) followed by EDs within IBIs (4.08 [3.59 – 4.58]) were the greatest determinants for seizure recurrence. The number of EDs within bursts or IBIs was not significant in this model. The likelihood of seizure recurrence was greater if there was evolution in theta power (2.72 [2.39 – 3.05]) and beta power (3.04 [2.68 – 3.39]). Evolution in alpha power did reach statistical significance (p=0.02), but with an aOR of 0.29 (−0.19 – 0.78) was of limited clinical significance.
Table 3:
Binary logistic regression model for occasions without seizure recurrence vs. those where seizures recurred. Variables with only 2 outcomes (i.e., dichotomous variables) such as the presence or absence of epileptiform discharges (EDs) or the presence or absence of >50 % evolution have been shown as a proportion of that group. Number of EDs have been presented as the mean. Adjusted odds ratios (95% confidence intervals [95%CI]) and p values for each variable are then shown in the respective columns. IBIs: interburst intervals.
| Signal measure | No seizure recurrence | Seizure recurrence | Adjusted odds ratio (95% CI) | p-value |
|---|---|---|---|---|
| Bursts with EDs | 0.60 | 0.86 | 8.55 (7.82 – 9.27) | <0.01 |
| IBIs with EDs | 0.07 | 0.28 | 4.08 (3.59 – 4.58) | <0.01 |
| Number of EDs within bursts | 1.43 | 2.13 | 0.99 (0.99 – 0.99) | 0.90 |
| Number of EDs within IBIs | 0.20 | 0.63 | 0.94 (0.94 – 0.95) | 0.64 |
| >50% evolution in total power | 0.13 | 0.25 | 2.84 (2.17 – 3.51) | 0.24 |
| >50% evolution in delta power | 0.14 | 0.25 | 0.93 (0.88 – 0.97) | 0.92 |
| >50% evolution in theta power | 0.13 | 0.27 | 2.72 (2.39 – 3.05) | 0.03 |
| >50% evolution in alpha power | 0.15 | 0.20 | 0.29 (−0.19 – 0.78) | 0.02 |
| >50% evolution in beta power | 0.11 | 0.28 | 3.04 (2.68 – 3.39) | 0.01 |
| >50% evolution in gamma power | 0.07 | 0.10 | 0.37 (−0.07 – 0.81) | 0.11 |
| >50% evolution in Teager energy | 0.05 | 0.14 | 1.49 (1.31 – 1.67) | 0.53 |
Discussion:
This study confirmed that for patients in burst suppression for management of RSE, a successful wean of cIVASM was mostly determined by how “epileptiform” bursts and IBIs appear (e.g., complexity of suppressions measured with Teager energy, bursts or IBIs with EDs, and burst evolution measures) as opposed to more classical burst suppression measures (e.g., amplitude, duration, spectral content).
The two most relevant prior works to this study were Johnson et al. and Thompson & Hantus. both in 2016. Johnson et al. analyzed bursts from 19 patients and determined that a successful wean could be predicted if bursts had a maximum amplitude <125 μV (i.e., patients that failed weaning had a higher maximum burst amplitude).(Johnson et al., 2016) Our study did not report on maximum burst amplitude, but occasions resulting in seizure recurrence definitely did not have higher mean burst amplitude, and statistically it was significantly lower in those with seizure recurrence.
Importantly, in the Johnson et al. study, IBI duration (including a dichotomous variable of ≥10 sec vs. <10 sec), burst suppression ratios, length of bursts, relative alpha power, alpha/delta ratio, high component (alpha + beta) power, high component to total power ratio, and measures of spectral edge did not differentiate between the groups.(Johnson et al., 2016) The current study largely supports/reproduces this aspect. It is true that the current study demonstrated many statistically significant differences between the two groups when assessing the classical burst suppression measures, but this is likely due to the vastly increased power generated by the study design performing analysis on a burst level (as opposed to subject level). In our study, burst and IBI amplitude and duration, and burst power (across all spectra) were actually lower in patients with seizure recurrence. One possible explanation for this is that seizure control was more difficult to achieve in these patients and therefore they were administered a greater cumulative dose of sedative medication, resulting in lower EEG amplitude and power. Cumulatively the studies (Johnson et al. and current) make a strong argument that classical burst suppression measures (amplitude, duration, spectral content) are poor differentiators of seizure recurrence.
Instead, it seems that seizure recurrence can be determined by how “epileptiform” bursts are and intuitively how epileptiform IBIs are (although the current work is the first to place an objective measure on the latter). Seizure recurrence was significantly greater if bursts or IBIs had EDs and an increasing cumulative probability with a greater number of EDs. Seizure recurrence was also associated with increases over time within bursts in total, delta, beta, and gamma power as well as Teager energy (surrogates for “evolution” or “ictal appearing”).
Johnson et al. and Thompson & Hantus. both include measures of epileptiform bursts, however both studies used subjective assessment (i.e., consensus between two raters) to determine the bursts that were epileptiform and those that were not.(Johnson et al., 2016; Thompson & Hantus, 2016) The major limitation for both studies is therefore generalizability (i.e., how translatable the findings are to other centers) and subjectivity, which is inherent to all studies using clinical raters. This is highlighted by Johnson et al. where the rating of presence or absence of “epileptiform features” (not further defined in that study) had a kappa value for interrater reliability (IRA) of 0.87 (“almost perfect”), but a determination of the percentage of bursts with epileptiform activity had considerably lower agreement (κ=0.52, “moderate”)(Johnson et al., 2016). Thompson & Hantus used the 2012 ACNS definition of HEBs to report on 24 patients where again the presence of HEBs was associated with a greater rate of seizure recurrence; perhaps more importantly, there was no occasion where HEBs were absent and seizures returned within 24 hours of weaning of anesthesia.(Thompson & Hantus, 2016) The finding remains important, but the limitation was that Thompson & Hantus did not make comment as to the IRA of HEBs between the two raters involved (in fact, to our knowledge no study has determined the IRA for HEBs).(Thompson & Hantus, 2016) The current study overcomes this by using purely objective quantitative measures. The only subjective component was the manual selection of the beginning and end of bursts, which is rarely ambiguous given the majority of the time this is about determining activity vs. no activity. By this method the findings here are universally generalizable.
The current study, based on objective measures, provides clinical validation to the current ACNS definition of HEBs, which until now has been mostly based on expert consensus. The current study confirms that 2 EDs within a burst was sufficient to result in a cumulative seizure recurrence probability >50% and objective surrogates of evolution were similarly associated with seizure recurrence. This is the first time each criterion of the HEBs definition has been independently assessed. Johnson et al. did not provide specific detail as to “epileptiform”, and Thompson et al. utilized HEBs as a whole without specifying if patients qualified as having HEBs because of the presence of EDs or because they were “ictal appearing”.
The multivariate analysis demonstrated that much of the risk of seizure recurrence was determined by the presence of EDs within bursts (aOR 8.55 [95% CI 7.82 – 9.27]) or IBIs (4.08 [3.59 – 4.58]). Although univariate analysis confirmed that the average number of EDs within bursts (2.1 vs. 1.4) and IBIs (0.6 vs. 0.2) was significantly different between groups, and an increasing number of EDs clearly lead to higher cumulative seizure recurrence probability (Figure 3), the significance for number of EDs was lost with multivariate analysis. It is probable that this was lost within the model as the vast majority of this effect was present in the occurrence of only the first 3 EDs and there was no limit on the number of EDs on quantitative assessment. For example, it was true that the seizure probability for a burst with 10 EDs was not that much greater than one with 3 EDs, and this was likely not accommodated for in the model. Evolution in theta (2.72 [2.39 – 3.05]) and beta (3.04 [2.68 – 3.39]) provided significant contributions to greater risk of seizure recurrence, with a non-significant trend for evolution in total power (2.84 [2.17 – 3.51]).
The findings are of considerable clinical significance. It has often been thought that an evolving pattern holds greater clinical significance compared to the presence of EDs (even when occurring in a very brief run). Take for example a pattern similar to HEBs that occur outside of a repeating sequence, such as brief potentially ictal rhythmic discharges (BIRDs). In one study, “evolving BIRDs” universally resulted in seizures occurring in the same EEG session in 30 patients, compared to non-evolving BIRDs where seizures only occurred in 50% of patients (32 out of 64).(Yoo et al., 2021) The current study suggests that this may not be true for bursts within burst suppression. In RSE, the presence of EDs within bursts or IBIs may already indicate sufficient cortical excitability that seizures are likely to recur if sedation is then weaned.
An important limitation with the current study was that the surrogate quantitative markers for evolution were not validated. In clinical practice, a standard but fairly rudimentary measure of seizure onset (for example when programming responsive neurostimulation) has been an increase in the line length by 50%. In our study, we used Teager energy, a measure of signal energy, which is akin to the line length measure. The field of seizure detection is vast in its own right and there is currently no universally agreed upon set of measures for optimal seizure detection (let alone any set of detectors optimized for ictal rhythms in the critical care setting). It was therefore decided to apply the same 50% increase threshold to individual band power to at least determine a change in measures. An argument could have been made for utilizing the commercially available seizure probability calculator and seizure detector to determine if a pattern was “ictal appearing”. The critical flaw with that was of course such algorithms have been optimized for detecting seizures. Although evolution measures are a part of this, electrographic seizures by definition are still required to be at least 10 seconds duration.(Hirsch et al., 2021) In the study design phase, the seizure probability calculator lacked the temporal resolution to be able to output meaningful data on a burst level. Even within our analysis, bursts had to be at least 4 seconds to be included in the assessment for evolution, and our analysis could not differentiate between evolution over 4 seconds vs. evolution over 9 seconds for example. Future work on optimizing detectors for “ictal appearing” rhythms that do not qualify as electrographic seizures, especially in the critical care setting, could lead to further adjustment of the odds ratios determined in our study.
Conclusion:
For patients in RSE where use of cIVASMs has led to burst suppression, successful wean of cIVASM was not determined by classical burst suppression measures (amplitude, duration, spectral content of bursts and IBIs), but instead how “epileptiform” bursts and IBIs were (presence of EDs and positive evolution of bursts).
Supplementary Material
Supplementary Figure 1. Example of marking of highly epileptiform and non-highly epileptiform bursts. Panel A demonstrates the marking of burst suppression with highly epileptiform bursts (HEBs). The automated spike detections can be seen in the bottom comment of the panel (highlighted box). Within each manually marked burst there are consistently 3-4 epileptiform discharges (EDs) occurring at ≥1 Hz. Panel B demonstrates a patient where the bursts were not highly epileptiform. The automated spike detector suggests there is an occasional ED, but these are not consistent across bursts (in this example there are none detected in the first burst shown and 1 detected in the second burst). The occasion in panel A resulted in generalized electrographic status epilepticus following continuous IV antiseizure medication wean, whereas panel B resulted in a successful wean (i.e., did not result in seizure recurrence).
Disclosures:
Dr Fong has received:
• Part of an investigator-initiated grant that led to a co-first author publication unrelated to this work: Upsher-Smith Laboratories, Proximagen, and UCB
• Royalties from Wiley for co-authoring the book “Hirsch and Brenner’s Atlas of EEG in Critical Care”.
• Honoraria for speaking UCB
Kelly Pu has no relevant financial or commercial disclosures
Dr Jadav has no relevant financial or commercial disclosures
Dr Kahn has no relevant financial or commercial disclosures
Dr. Hirsch has received:
• Consultation fees for advising from Accure, Aquestive, Ceribell, Eisai, Marinus, Medtronic, Neurelis, Neuropace, Rafa Laboratories and UCB
• Royalties from Wolters-Kluwer for authoring chapters for UpToDate-Neurology, and from Wiley for co-authoring the book “Hirsch and Brenner’s Atlas of EEG in Critical Care”.
• Honoraria for speaking from Neuropace, Natus, and UCB
Dr Zaveri has no relevant financial or commercial disclosures
Ms Kelly Pu and Dr Hitten P. Zaveri are supported by funding from NIH R01 NS109062-01A1. The content of this manuscript has been presented in abstract form at the American Epilepsy Society Annual Scientific Meeting 2022. It has not been submitted previously to a journal
Footnotes
RSE: refractory status epilepticus, cIVASM: continuous IV antiseizure medications, ASM: antiseizure medications, EDs: epileptiform discharges, IBI: interburst interval, HEBs: highly epileptiform bursts, ACNS: American Clinical Neurophysiology Society, cEEG: continuous EEG monitoring, HIE: hypoxic-ischemic encephalopathy.
References:
- Hirsch LJ, Fong MWK, Leitinger M, LaRoche SM, Beniczky S, Abend NS, … Gaspard N. (2021). American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2021 Version. J Clin Neurophysiol, 38(1), 1–29. doi: 10.1097/WNP.0000000000000806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, … Drislane FW. (2013). American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol, 30(1), 1–27. doi: 10.1097/WNP.0b013e3182784729 [DOI] [PubMed] [Google Scholar]
- Johnson EL, Martinez NC, & Ritzl EK (2016). EEG Characteristics of Successful Burst Suppression for Refractory Status Epilepticus. Neurocrit Care, 25(3), 407–414. doi: 10.1007/s12028-016-0294-2 [DOI] [PubMed] [Google Scholar]
- Rossetti AO, Logroscino G, & Bromfield EB (2005). Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol, 62(11), 1698–1702. doi: 10.1001/archneur.62.11.1698 [DOI] [PubMed] [Google Scholar]
- Schmutzhard E, & Pfausler B (2011). Complications of the management of status epilepticus in the intensive care unit. Epilepsia, 52 Suppl 8(8), 39–41. doi: 10.1111/j.1528-1167.2011.03233.x [DOI] [PubMed] [Google Scholar]
- Shorvon S (2001). The management of status epilepticus. J Neurol Neurosurg Psychiatry, 70 Suppl 2(Suppl 2), II22–27. doi: 10.1136/jnnp.70.suppl_2.ii22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, & Hantus S (2016). Highly Epileptiform Bursts Are Associated With Seizure Recurrence. J Clin Neurophysiol, 33(1), 66–71. doi: 10.1097/WNP.0000000000000232 [DOI] [PubMed] [Google Scholar]
- Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, & Ely EW (2008). Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Critical care medicine, 36(12), 3171–3177. doi: 10.1097/ccm.0b013e318186b9ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JY, Jette N, Kwon CS, Young J, Marcuse LV, Fields MC, … Hirsch LJ. (2021). Brief potentially ictal rhythmic discharges and paroxysmal fast activity as scalp electroencephalographic biomarkers of seizure activity and seizure onset zone. Epilepsia, 62(3), 742–751. doi: 10.1111/epi.16822 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Example of marking of highly epileptiform and non-highly epileptiform bursts. Panel A demonstrates the marking of burst suppression with highly epileptiform bursts (HEBs). The automated spike detections can be seen in the bottom comment of the panel (highlighted box). Within each manually marked burst there are consistently 3-4 epileptiform discharges (EDs) occurring at ≥1 Hz. Panel B demonstrates a patient where the bursts were not highly epileptiform. The automated spike detector suggests there is an occasional ED, but these are not consistent across bursts (in this example there are none detected in the first burst shown and 1 detected in the second burst). The occasion in panel A resulted in generalized electrographic status epilepticus following continuous IV antiseizure medication wean, whereas panel B resulted in a successful wean (i.e., did not result in seizure recurrence).


