Abstract
The presence of perianal fistulae constitutes a more severe phenotype of Crohn’s disease (CD) that often requires intensive medical therapy, wound care, and surgical intervention. Despite therapeutic advances in inflammatory bowel disease, the treatment of perianal fistulae remains challenging. Hyperbaric oxygen therapy (HBOT) has been proposed as an adjunctive treatment modality for induction of fistula healing. We illustrate a case in which HBOT achieved fistula healing in a young patient with severe refractory perianal Crohn’s disease (pCD). We also review the current literature and discuss the role of HBOT in the treatment armamentarium of pCD.
Keywords: hyperbaric medicine, inflammatory bowel disease, perianal fistula, hyperbaric oxygen treatment, crohn’s disease
Introduction
Crohn’s disease (CD) is a chronic disease characterized by recurrent inflammation of the gastrointestinal tract. Perianal fistulae occur in 20-25% of patients with CD and constitute a more severe phenotype that often requires a combination of intensive medical therapy, wound care, and surgical intervention [1,2].
Hyperbaric oxygen therapy (HBOT) is a treatment modality that involves delivering 100% oxygen in a pressurized chamber at two to three times standard atmospheric pressure at sea level, where 1 atmosphere absolute (ATA) is defined as the average atmospheric pressure exerted at sea level. It represents an adjunctive treatment option in perianal Crohn’s disease (pCD) and is postulated to have beneficial effects on wound healing via stem cell mobilization, decrease in inflammatory cytokines, and reduction of wound hypoxia [3,4]. We report a case in which fistula healing was achieved with adjunctive HBOT.
Case presentation
Our patient was a 21-year-old male of South Asian ethnicity with a background of ileocolonic CD with complex perianal fistulae. He was diagnosed with CD at 14 years old and first developed perianal fistulae at the age of 19 years. Since then, he has had multiple hospitalizations for recurrent perianal abscesses requiring both percutaneous and surgical drainage with seton insertion. He underwent two lines of biological treatment, which did not achieve fistula healing. First, he received infliximab but developed antibodies to infliximab after one year, with a secondary loss of response. Next, he received adalimumab but showed no improvement in inflammation and fistula discharge, suggesting primary non-response.
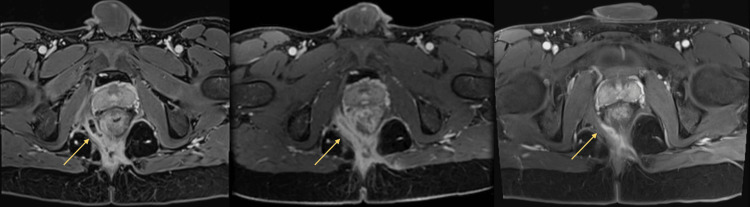
At this time, he was clinically malnourished with a body mass index (BMI) of 16.8. Laboratory results showed a hemoglobin (Hb) level of 9.5 g/dL, serum albumin level of 30 g/L, and C-reactive protein (CRP) level of 113.3 mg/L (normal: <3 mg/L). The perianal disease activity index (PDAI) score was 12. Magnetic resonance imaging of the pelvis showed a complex intersphincteric fistula with multiple internal openings (Figure 1).
Figure 1. Serial axial T1-weighted contrast-enhanced MRI of the perianal region showing interval improvement of a curvilinear fluid-filled track (arrow) in the right ischioanal fossa over time. This track was one component of a complex perianal fistula with multiple openings.
A (left): Appearance at first presentation. B (middle): Two months post treatment. C (right): Five months post treatment.
In view of the refractory and debilitating disease with prior failure of two biologics, various options, including switching biologic therapy to ustekinumab (an interleukin-12/23 inhibitor), surgical treatment such as a diverting colostomy, and adjunctive treatment with HBOT, were considered. After discussion, a shared decision was made for ustekinumab with HBOT. He was started on ustekinumab with adjunctive HBOT concurrently (60 sessions, two-hour duration, 2.0 ATA) over a period of three months. He tolerated the HBOT well with no adverse events.
On review at 16 weeks after HBOT, clinical examination revealed complete fistula healing with the resolution of perineum swelling, closure of the external orifice, the absence of active perianal discharge, and radiological improvement (Figure 1). His PDAI score improved from 12 to 3 and his BMI increased from 16.8 to 19.4, with an improvement in his nutritional status. Biochemical response was demonstrated with CRP decreasing from 113.3 mg/L to 28.9 mg/L. Hb increased from 9.5 g/dL to 12.7 g/dL and albumin increased from 30 g/L to 38 g/L (Table 1). He reported an improvement in his quality of life in terms of his bowel movements, emotional well-being, and social functioning.
Table 1. Indices pre- and post-HBOT.
HBOT: hyperbaric oxygen therapy.
| Prior to HBOT | 16 weeks post HBOT | |
| Hemoglobin (Hb) | 9.5 g/dL | 12.7 g/dL |
| Albumin | 30 g/L | 38 g/L |
| C-reactive protein (CRP) | 113.3 mg/L | 28.9 mg/L |
| Perianal disease activity index (PDAI) | 12 | 3 |
| Body mass index (BMI) | 16.8 | 19.4 |
However, at nine months post-completion of HBOT, he presented with a recurrence of abdominal pain, diarrhea, and active perianal discharge. Repeat imaging showed active intestinal inflammation and recrudescence of a complex perianal fistula with abscess formation. This occurred despite compliance with maintenance therapy with ustekinumab. He was started on antibiotics and subsequently underwent percutaneous drainage of the abscess. While HBOT successfully induced fistula healing initially, the clinical response in this patient was not sustained.
Discussion
Our case highlights a patient with severe refractory fistulizing pCD who achieved clinical remission and radiological improvement of his complex perianal fistulae with combined ustekinumab and adjunctive HBOT. Unfortunately, remission was not sustained after the conclusion of HBOT despite maintenance biologic therapy, suggesting that HBOT, rather than ustekinumab, was the major contributor to his initial therapeutic response.
Despite the multiple advances in the treatment of inflammatory bowel disease over the last decade, the treatment of perianal fistulae remains one of the biggest unmet needs in the treatment of CD. The implications of a diagnosis of pCD are myriad, including increased healthcare utilization, increased need for surgery, greater medication burden, and poorer quality of life [5].
Achieving closure of perianal fistulas is challenging. A recent study showed that this outcome was achieved in only 68% of patients at 18 months who underwent a combination of anti-tumor necrosis factor therapy and surgery [6-9]. HBOT presents the option of a novel adjunctive therapy with minimal adverse effects. Through breathing 100% oxygen under pressure, HBOT increases plasma and tissue oxygen levels, thereby relieving hypoxia and increasing the oxygen content of blood reaching inflamed bowel or chronic nonhealing fistulas and triggering tissue restorative pathways essential for wound healing. Additionally, HBOT has been shown to suppress the production of proinflammatory cytokines (IL-1, IL-6, and tumor necrosis factor-alpha) and modulate inflammatory responses by decreasing the ratio of CD4:CD8 T-cell subsets, reducing neutrophil chemotaxis and enhancing lymphocyte apoptosis. Lastly, relevant to pCD, antimicrobial pathways are also stimulated with the formation of reactive oxygen species. These pathways work in concert and contribute to the healing of the perianal fistula [10-12].
The emerging literature on the efficacy of HBOT in pCD is promising. Lansdorp et al. demonstrated a reduction in median PDAI score from 7.5 to 4 with an overall clinical response of 60% at 16 weeks after 40 daily sessions of HBOT in 20 patients with pCD who had failed conventional treatment [7]. Longer-term evaluation of the same group who received no further HBOT showed an increase in the biochemical indices of CRP and fecal calprotectin from week 16 to week 60, although PDAI scores and clinical closure response at 60 weeks were maintained. Further to this, a subsequent meta-analysis by McCurdy et al. showed that in patients with refractory pCD, HBOT was associated with an overall clinical response rate of 75% and a clinical remission rate of 55% [13].
Efficacy aside, safety remains an important determinant of a patient’s willingness to accept treatment. A recent review of patients undergoing HBOT showed an adverse effect rate of less than 1%. Middle ear or paranasal barotrauma was the most commonly reported adverse effect and was typically mild and self-resolving. Other adverse effects such as hyperbaric myopia, hypoglycemia, and claustrophobia have been reported but are rare. The majority of these events were minor and did not impact the continuation of treatment [14].
Conclusions
The effectiveness of HBOT is heartening, particularly for the induction of healing of fistulizing disease. Achievement of clinical remission remains challenging and HBOT offers an adjunctive treatment option that is safe, well-tolerated, and effective. At present, there remains no consensus on the duration of treatment, and the optimal dose of HBOT remains uncertain. Further research is required to determine the optimal treatment duration, feasibility, cost-effectiveness, and long-term outcomes of HBOT and establish its place in the treatment armamentarium of pCD.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Justin Wen Hao Leong, Tze Tong Tey, Zhi Hao Yan, Fung Joon Foo, Frederick Hong-Xiang Koh
Drafting of the manuscript: Justin Wen Hao Leong, Tze Tong Tey
Acquisition, analysis, or interpretation of data: Tze Tong Tey, Lionel Tim-Ee Cheng, San Choon Kong
Critical review of the manuscript for important intellectual content: Tze Tong Tey, Zhi Hao Yan, Fung Joon Foo, Frederick Hong-Xiang Koh, Lionel Tim-Ee Cheng, San Choon Kong
Supervision: Tze Tong Tey, Zhi Hao Yan
References
- 1.Prevalence of fistulizing Crohn’s disease in the United States: estimate from a systematic literature review attempt and population-based database analysis. Schwartz DA, Tagarro I, Carmen Díez M, Sandborn WJ. Inflamm Bowel Dis. 2019;25:1773–1779. doi: 10.1093/ibd/izz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adjunctive hyperbaric oxygen therapy in refractory Crohn’s disease: an observational study. Feitosa MR, Parra RS, Machado VF, et al. Gastroenterol Res Pract. 2021;2021:6628142. doi: 10.1155/2021/6628142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyperbaric oxygenation in severe perineal Crohn's disease. Colombel JF, Mathieu D, Bouault JM, Lesage X, Zavadil P, Quandalle P, Cortot A. Dis Colon Rectum. 1995;38:609–614. doi: 10.1007/BF02054120. [DOI] [PubMed] [Google Scholar]
- 4.Perianal fistulizing Crohn's disease: pathogenesis, diagnosis and therapy. Panés J, Rimola J. Nat Rev Gastroenterol Hepatol. 2017;14:652–664. doi: 10.1038/nrgastro.2017.104. [DOI] [PubMed] [Google Scholar]
- 5.Characteristics of patients with Crohn’s disease with or without perianal fistulae in the CorEvitas Inflammatory Bowel Disease Registry. Fan Y, Delgado-Aros S, Valdecantos WC, Janak JC, Moore PC, Crabtree MM, Stidham RW. https://doi.org/10.1007/s10620-022-07491-y. Dig Dis Sci. 2023;68:214–222. doi: 10.1007/s10620-022-07491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyperbaric oxygen therapy for the treatment of perianal fistulas in 20 patients with Crohn's disease: results of the HOT-TOPIC trial after 1-year follow-up. Lansdorp CA, Buskens CJ, Gecse KB, et al. United European Gastroenterol J. 2022;10:160–168. doi: 10.1002/ueg2.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyperbaric oxygen therapy for the treatment of perianal fistulas in 20 patients with Crohn's disease. Lansdorp CA, Gecse KB, Buskens CJ, et al. Aliment Pharmacol Ther. 2021;53:587–597. doi: 10.1111/apt.16228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Short-term anti-TNF therapy with surgical closure versus anti-TNF therapy in the treatment of perianal fistulas in Crohn’s disease (PISA-II): a patient preference randomised trial. Meima-van Praag EM, van Rijn KL, Wasmann KATGM, et al. https://pubmed.ncbi.nlm.nih.gov/35427495/ Lancet Gastroenterol Hepatol. 2022;7:617–626. doi: 10.1016/S2468-1253(22)00088-7. [DOI] [PubMed] [Google Scholar]
- 9.Systematic review: the safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Dulai PS, Gleeson MW, Taylor D, Holubar SD, Buckey JC, Siegel CA. Aliment Pharmacol Ther. 2014;39:1266–1275. doi: 10.1111/apt.12753. [DOI] [PubMed] [Google Scholar]
- 10.Hyperbaric oxygen therapy: antimicrobial mechanisms and clinical application for infections. Memar MY, Yekani M, Alizadeh N, Baghi HB. Biomed Pharmacother. 2019;109:440–447. doi: 10.1016/j.biopha.2018.10.142. [DOI] [PubMed] [Google Scholar]
- 11.Effects of hyperbaric oxygen on inflammatory response to wound and trauma: possible mechanism of action. Al-Waili NS, Butler GJ. ScientificWorldJournal. 2006;6:425–441. doi: 10.1100/tsw.2006.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modification of in vivo and in vitro TNF-alpha, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn's disease. Weisz G, Lavy A, Adir Y, Melamed Y, Rubin D, Eidelman S, Pollack S. J Clin Immunol. 1997;17:154–159. doi: 10.1023/a:1027378532003. [DOI] [PubMed] [Google Scholar]
- 13.The effectiveness and safety of hyperbaric oxygen therapy in various phenotypes of inflammatory bowel disease: systematic review with meta-analysis. McCurdy J, Siw KC, Kandel R, Larrigan S, Rosenfeld G, Boet S. Inflamm Bowel Dis. 2022;28:611–621. doi: 10.1093/ibd/izab098. [DOI] [PubMed] [Google Scholar]
- 14.Addition of hyperbaric oxygen therapy versus usual care alone for inflammatory bowel disease: a systematic review and meta-analysis. You JH, Jiang JL, He WB, et al. Heliyon. 2022;8:0. doi: 10.1016/j.heliyon.2022.e11007. [DOI] [PMC free article] [PubMed] [Google Scholar]