Abstract
In the frontline high-dose phase 3 FIL-MCL0208 trial (NCT02354313), 8% of enrolled mantle cell lymphoma (MCL) patients could not be randomised to receive lenalidomide (LEN) maintenance vs observation after autologous stem cell transplantation (ASCT) due to inadequate hematological recovery and 52% of those who started LEN, needed a dose reduction due to toxicity. We therefore focused on the role played by CD34 + hematopoietic stem cells (PBSC) harvesting and reinfusion on toxicity and outcome. Overall, 90% (n = 245) of enrolled patients who underwent the first leukapheresis collected ≥ 4 × 106 PBSC/kg, 2.6% (n = 7) mobilized < 4 × 106 PBSC/kg and 7.7% (n = 21) failed the collection. Similar results were obtained for the planned second leukapheresis, with only one patient failing both attempts. Median count of reinfused PBSC was 5 × 106/kg and median time to recovery from neutropenia G4 was 10 days from ASCT. No impact of mobilizing subtype or number of reinfused PBSC on hematological recovery and LEN dose reduction was noted. At a median follow-up of 75 months from ASCT, PFS and OS of transplanted patients were 50% and 73%, respectively. A long lasting G4 neutropenia after ASCT (> 10 days) was associated with a worse outcome, both in terms of PFS and OS. In conclusion, although the harvesting procedures proved feasible for younger MCL patients, long-lasting cytopenia following ASCT remains a significant issue: this can hinder the administration of effective maintenance therapies, potentially increasing the relapse rate and negatively affecting survival outcomes.
Keywords: Mantle cell lymphoma (MCL), Autologous stem cell transplantation (ASCT), Peripheral blood stem cells (PBSC), Leukapheresis (LK), Hematological recovery
Subject terms: Non-hodgkin lymphoma, Non-hodgkin lymphoma
Introduction
Mantle cell lymphoma (MCL) is an infrequent, mature B-cell lymphoproliferative disease characterized by heterogeneous outcome1. Although the introduction of high dose cytarabine-containing chemotherapy schedules (HDS) followed by autologous stem cell transplantation (ASCT) as front-line consolidation, as well as effective maintenance strategies2,3, significantly improved the outcome of the disease, MCL is still considered incurable.
The FIL-MCL0208 trial is a phase 3, multicenter, randomized, controlled trial which demonstrated the efficacy in terms of progression-free survival (PFS) of lenalidomide (LEN) maintenance after HDS and ASCT4. However, 8% of enrolled patients could not be randomly assigned to receive LEN due to inadequate hematological recovery after ASCT. In addition, 52% of those who started LEN, needed a dose reduction due to toxicity. For this reason, a substudy was conducted in order to better define the role played by the ASCT procedures, namely the harvest and the reinfusion of CD34-positive hematopoietic stem cells as well as hematological recovery data, on toxicity and outcome. Moreover, even if a comprehensive analysis of minimal residual disease (MRD) of the trial was recently published, no details regarding MRD in leukapheresis products were provided5.
Materials and methods
Patients and protocol
FIL-MCL0208 is a phase 3, multicenter, open-label, randomized, controlled trial, designed to determine the efficacy of lenalidomide as maintenance versus observation in younger (18–65 years old), fit, advanced stage (Ann Arbor III-IV or stage II plus bulky disease [≥ 5 cm] or B symptoms) MCL patients after first line intensified HDS followed by ASCT. This study is registered with EudraCT (2009-012807-25) and ClinicalTrials.gov (NCT02354313, first registration on 03/02/2015). The clinical trial was approved by the Ethical Committees of all the enrolling Centers. All patients provided written informed consent for the use of their biological samples for research purposes, in accordance with Institutional Review Boards requirements and the Helsinki’s declaration. Clinical results have recently been published by Ladetto et al.4
Treatment plan and stem cells collection
The high-dose chemo-immunotherapy schedule (R-HDS) consisted in 3 cycles of R-CHOP, 1 of R-high dose cyclophosphamide [R-CTX], 2 cycles of R-high dose cytarabine [R-HD-ARA-C] before ASCT, as per protocol (Figure S1)4. Granulocyte colony-stimulating factor (G-CSF–5 μg/kg qd subcutaneously) was administered from day 5 after the first R-HD-ARA-C cycle for CD34 + peripheral blood stem cells (PBSC) mobilization and collection. Harvest was considered sufficient if at least 4 × 106 PBSC/Kg were collected by leukapheresis (LK1), according to local modalities. Of note, no data regarding plerixafor use was available in the eCRF. A second collection of PBSC (LK2) was planned after the second R-HD-ARA-C cycle only in patients who did not reach the harvesting target, did not achieve minimal residual disease (MRD) negativity in the first PBSC harvest by allele-specific oligonucleotide (ASO) nested polymerase chain reaction (N-PCR) centralized determination (see below) or lacked a molecular marker for MRD. Of note, the rationale for collecting a second time in patients lacking MRD markers was to obtain a more purified harvest, as MRD monitoring was not possible. In these cases, the second collection was planned for reinfusion. A BEAM conditioning regimen followed by ASCT was then performed. G-CSF was administered from day 2 until absolute neutrophil count (ANC) was above 1.5 × 109/L.
Responding patients (complete remission or partial remission) with hematological recovery (ANC > 1.5 × 109/L and platelets [PLTs] > 60 × 109/L) within 120 days after autologous HSCT were randomly assigned to receive oral lenalidomide (15 mg per day for patients with platelets > 100 × 109/L or 10 mg per day for platelets 60–100 × 109/L, days 1–21 every 28 days) for 24 months, or observation4.
Minimal residual disease analysis
MRD was monitored on leukapheresis products both by ASO N-PCR and quantitative real-time PCR (RQ-PCR) approaches in a EuroMRD certified lab6, targeting either IGH::BCL-1 or IGH rearrangements5.
Toxicity investigations
Data collection included the number of PBSC harvested (eventually in both harvests) and reinfused. In order to evaluate the eventual leukapheresis influence on hematological recovery, dates of PLTs count and ANC superior to 20 and 0.5 × 109/L respectively, as well as superior to 50 and 1.0 × 109/L from ASCT were recorded. Toxicity data included the frequency and severity of adverse events (based on the National Cancer Institute Common Terminology Criteria for Adverse Events—CTCAE version 4.0).
Statistical analysis
The primary endpoint was the progression-free survival (PFS), defined as the time from ASCT to progression/death from any cause, while the secondary one was the overall survival (OS), defined as the time from ASCT to death from any cause. PFS and OS had been investigated either by the Kaplan–Meier method (comparing survival curves across groups by the log-rank test) or by the Cox proportional hazards model (comparing the two arms by the Wald test and calculating 95% confidence intervals).
The optimal cut-point for continuous covariates (namely days of PLTs and ANC recovery, and number of PBSC harvested and reinfused) was estimated by the maximally selected rank statistics, an outcome-oriented method providing a cut-off value that corresponds to the most significant relation with survival7.
The potential impact on PFS and OS was tested for the following risk factors: lenalidomide maintenance (yes vs no), lenalidomide dose reduction (any vs none), hematological recovery (obtained vs not obtained), first harvest (0–3 vs > 3 × 106 PBSC/kg mobilized), second harvest (0–3 vs > 3 × 106 PBSC/kg mobilized), reinfusion (0–3 vs > 3 × 106 PBSC/kg reinfused), MRD by N-PCR (positive vs negative) and RQ-PCR (positive vs negative), neutrophils recovery (0.5 × 109/L in up to 10 vs > 10 days) and platelets count (20 × 109/L in up to 15 vs > 15 days).
Patients’ characteristics were compared using Fisher’s exact test for categorical variables and the Mann–Whitney test for continuous ones, describing them as median (inter quartile range–IQR).
All reported p-values were obtained by the two-sided exact method, at the conventional 5% significance level. Data were analyzed as of June 2023 (data cut-off: 10/05/2022) by R 4.3.0 (R Foundation for Statistical Computing, Vienna-A, http://www.R-project.org). The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Harvesting and reinfusion
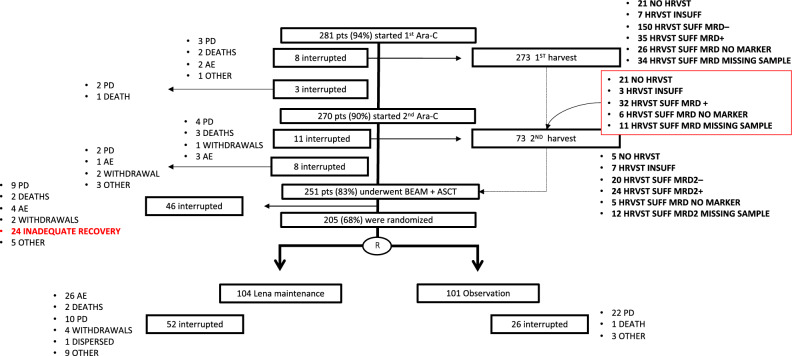
Patients clinical features have been previously reported4. A detailed study flow chart, focused on the leukapheresis procedure, is shown in Fig. 1. Of the 281 enrolled patients who started I R-HD-ARA-C course, 273 (91%) proceeded with the first harvest attempt. Almost 90% (n = 245) of these patients collected ≥ 4 × 106 CD34 + /kg stem cells (median 11, IQR 7–16: “good mobilizers”). Of the remaining 28 patients, 7 (2.6%) mobilized < 4 × 106 CD34 + /kg (median 3, IQR 1.5–3, “poor mobilizers”), while 21 (7.7%) failed the collection (due either to no mobilization [“non mobilizers”; n = 6, 29%], adverse events [n = 4, 9%] or other reasons, including protocol deviations).
Figure 1.
Study flow chart. Consort of the study protocol with focus on harvest results and reasons of interruption. Patients undergoing leukapheresis after the 2 courses of HD-ARA-C and MRD results are depicted on the top-right of the diagram. pts patients; Ara-C cytarabine; HRVST harvest; INSUFF insufficient; SUFF sufficient; MRD minimal residual disease; BEAM carmustine with etoposide, cytarabine and melphalan; ASCT autologous stem cell transplantation; PD progressive disease; AE adverse event; R randomisation; Lena lenalidomide.
Of the 270 patients who started II R-HD-ARA-C course, 73 (27%) proceeded with a second harvest attempt), as per protocol. This group included 24 (33%) patients with no sufficient stem cell collection on first attempt, 32 (46%) MRD-positive patients, 17 patients without MRD data (11 missing sample and 6 without a molecular marker). Among them (n = 73), 61 (83%) patients were good mobilizers (median 6, IQR 4–9), 7 (10%) were poor mobilizers (median 2, IQR 1–2) and 5 (7%) patients failed to harvest (4 non mobilizers and 1 adverse event). Notably, only 1 patient failed to mobilize stem cells on both harvest attempts (Table S1—supplementary material).
Therefore, overall 251 (84%) of the 300 enrolled patients proceeded with ASCT (study interruptions are listed in Ladetto et al. manuscript4 and in Fig. 1). The median count of reinfused CD34 + cells was 5 × 106 PBSC/kg (IQR 4–7), with no significant difference between good and poor mobilizers. Twenty patients out of the 49 who stopped treatment managed to harvest, but interrupted either because of progressive disease (n = 8, 40%), adverse event (n = 3, 15%), death (n = 4, 20%), consent withdrawal (n = 3, 15%) and other reasons (n = 2, 10%).
Hematological recovery after ASCT and survival
Overall, median time to reach an ANC count of 0.5 and 1 × 109/L after PBSC reinfusion was 10 (IQR 10–11) and 11 (IQR 10–13) days, respectively. PLTs count of 20 and 50 × 109/L was reached in 13 (IQR 10–16) and 19 (IQR 15–25) days, respectively. No statistically significant difference in hematological recovery after ASCT was found between poor and good mobilizers; notably, a higher number of reinfused stem cells did not translate into a shorter hematological recovery. Moreover, no impact was seen of the quantity of harvested or reinfused stem cells on dose reduction during subsequent lenalidomide maintenance for patient randomized in the experimental arm.
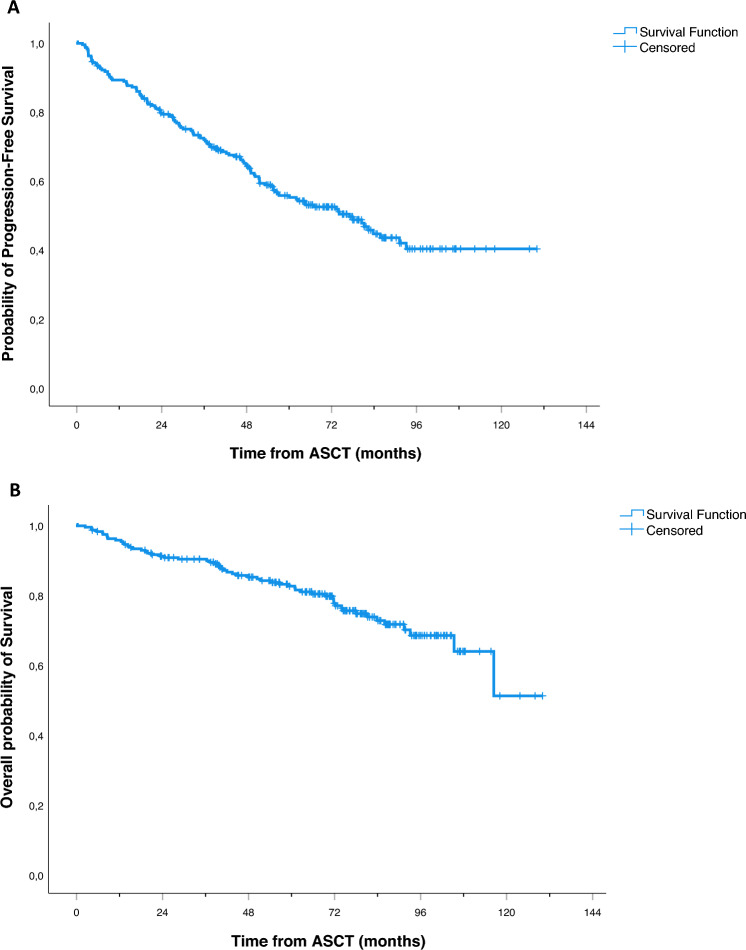
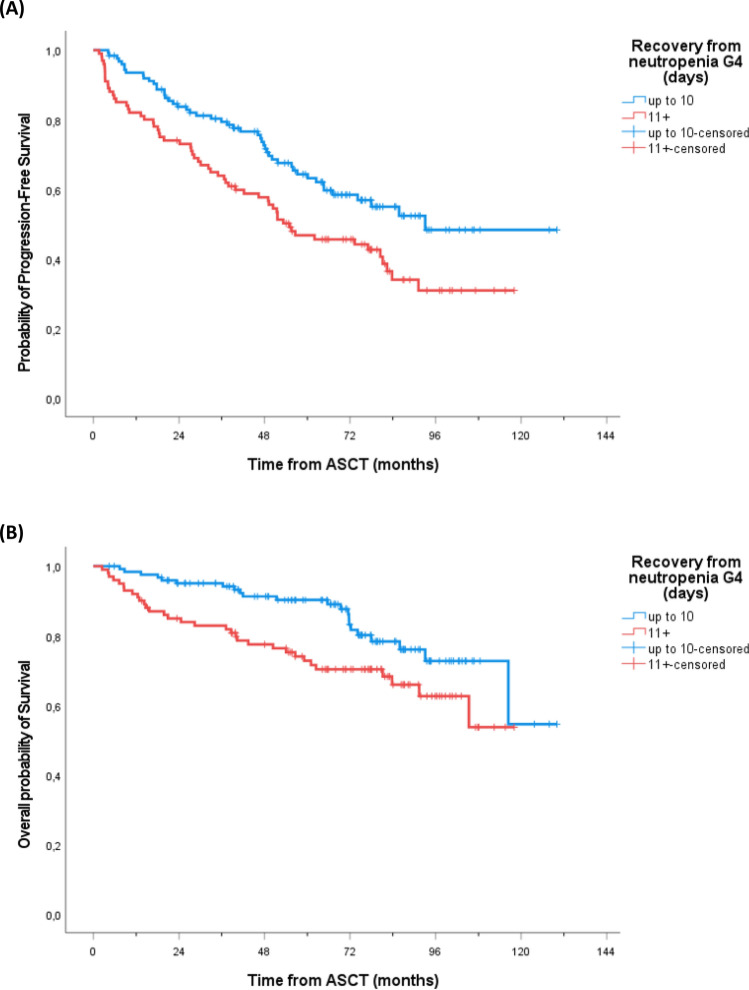
At a median follow-up (FU) of 75 months from ASCT, PFS and OS of transplanted patients were 50% and 73% respectively (Fig. 2A,B). Of note, a long lasting G4 neutropenia after ASCT (> 10 days) was associated with a worse outcome, both in terms of PFS (PFS at median FU 44% vs 57%, p = 0.006—Fig. 3A) and OS (OS at median FU 70% vs 80%, p = 0.018—Fig. 3B). Interestingly, this finding was only observed in patients subsequently randomized to lenalidomide maintenance (p = 0.016), while no outcome difference according to neutropenia recovery was noted in patients in the control arm (p = 0.349; Figure S2A,B). Moreover, a higher incidence of infectious events related to ASCT was reported in the long lasting G4 neutropenia group (45% vs 32% of patients recovering in less than 11 days), including a death related to bacterial infection (G5). A trend towards worse survival outcomes was noted also for PLTs count recovery (> 15 days), although not statistically significant (PFS, p = 0.073 and OS, p = 0.220 respectively; Figure S3A,B). On the other hand, no difference in patients’ clinical outcome was observed between good and poor mobilizers (data not shown).
Figure 2.
Kaplan Meier estimates of PFS (A) and OS (B) of transplanted patients.
Figure 3.
Kaplan–Meier estimates of PFS (A) and OS (B) of all transplanted patients according to days of recovery from neutropenia G4 (up to 10 vs 11 and more) after ASCT. G4 grade 4; ASCT autologous stem cell transplantation.
Minimal residual disease (MRD) analysis
Overall, 81% of patients with an available MRD marker (150/185) reached MRD negativity in nested PCR after the first R-HD-ARAC course, thus allowing 124 patients to spare a second harvesting procedure, as per protocol (the remaining 26 patients proceeded anyways to LK2, according to physician decision). Moreover, of the 32 patients still MRD-positive at the first harvest who faced a second one after the II R-HD-ARAC course, 10 (31%) became MRD negative (the remaining 3 patients MRD + at LK1 interrupted before LK2 due to progressive disease).
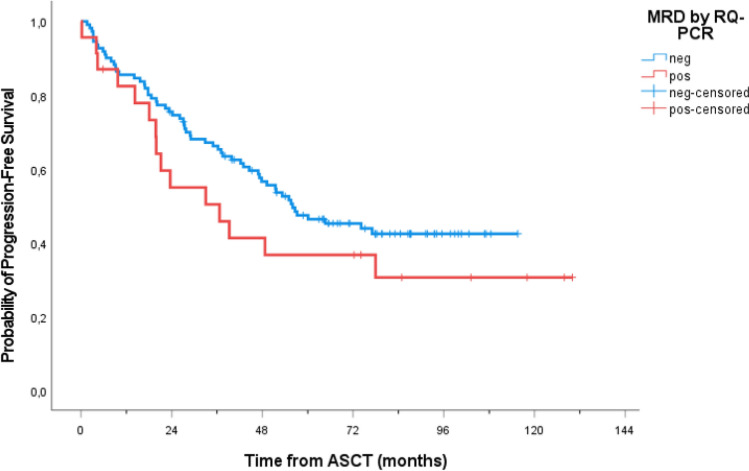
The RQ-PCR analysis, subsequently performed, showed similar results (MRD negativity on 118/148 samples, 79.7%, at first LK and 8/13, 38%, at second LK), with most of the positive samples (73% and 86% of the two leukapheresis, respectively) scoring as “positive not quantifiable” (PNQ)6. Notably, transplanted patients who received RQ-PCR MRD-negative autografts, regardless of whether obtained after the first or the second R-HD-ARA-C course, showed a trend towards PFS advantage, even if not statistically significant (median PFS 56 and 37 months for negative and positive LK MRD samples respectively, p = 0.128) (Fig. 4).
Figure 4.
Kaplan–Meier estimates of PFS of transplanted patients according to MRD by RQ-PCR on leukapheresis samples. MRD minimal residual disease; RQ-PCR quantitative real-time polymerase chain reaction.
Discussion
In this study we describe in detail the harvest and reinfusion data of the FIL MCL0208 trial. The main findings are the following:
even if characterized by non negligible toxicity, the R-HDS was a feasible regimen in younger MCL patients and did not impair an adequate CD34 + harvest;
the R-HDS was highly effective in inducing MRD negativity in LK samples;
LK-related features (namely CD34 + mobilization, harvest and reinfusion) did not correlate with post ASCT hematological recovery times or lenalidomide dose reductions during maintenance;
although no differences in patients’ clinical outcome was observed between good and poor mobilizers, long-lasting G4 neutropenia after ASCT was associated to poorer PFS and OS.
This subanalysis of the FIL MCL0208 trial, focusing on the ASCT procedures, highlights the overall feasibility of an upfront R-HDS regimen8,9 in younger MCL patients, especially showing no impairment on stem cell harvesting. In fact, 5/6 (83%) patients who did not mobilize in first attempt managed to collect in the second one and only one patient failed to mobilize in both leukapheresis attempts (not due to adverse event or other reasons), thus demonstrating an almost complete harvesting success rate (272/273, 99.6%). Actually, less than 10% of the enrolled patients (27/300, 9%) did not start leukapheresis due to other reasons (progressive disease, adverse event, death, consent withdrawal) and, overall, 84% of patients (251/300) safely proceeded to autologous transplantation. Moreover, both harvest procedures showed good mobilization rates, with 83% of patients collecting more than 4 × 106 CD34 + /kg and 90% more than 3 × 106 CD34 + /kg. Interestingly, in the cytarabine arm of the “Younger trial” of the European MCL Network, in which the collection target was ≥ 2 × 106 CD34 + /kg bodyweight in 2 harvests (including a back-up), the harvest success was lower (66%), while it was superimposable in the standard arm (84%)10. The target of ≥ 2 × 106 CD34 + /kg was utilized also in the LyMa trial, with only 2 reported mobilization failures2. Moreover, in the more recent LyMa-101 trial, with a collection target established at ≥ 3 × 106 CD34 + /kg, no stem cell harvest failure was reported11. We can only speculate that the lower harvest rate in the experimental arm of the “Younger trial” was potentially due to plerixafor, which was not yet commonly used at the time of enrollment. Nonetheless, in all these trials, the proportion of patients who received ASCT after the induction was comparable to the results of our trial (between 84 and 92%)4,10,11.
The overall analysis of MRD data in the FIL MCL0208 trial was recently published5, however LK data were not presented in detail. The MRD evaluation of leukapheresis samples confirmed the high efficacy of the R-HDS scheme in inducing a deep MRD negativity already after the first R-HD-ARA-C cycle (81%) and allowing to avoid a second harvesting attempt for most patients. This translated in significant savings, both in terms of pharmacoeconomics and patients compliance. Seminal studies of the pre-rituximab era in follicular lymphoma showed that MRD detection in bone marrow autograft was a significant predictor of 12-year PFS12. In MCL, the efficacy of the R-HDS scheme as a good in vivo purging strategy was demonstrated in the pivotal study published by Magni et al.13 More recently the achievement of MRD negativity in peripheral blood after induction and after ASCT in the EuMCLNet “Younger trial” was an independent PFS predictor10, but no MRD data were available in leukapheresis samples. In our study a trend for PFS advantage was noticed in patient receiving MRD-negative autografts, even though not statistically significant: this is in line with previously published data, suggesting that early MRD evaluation timepoints are less reliable in predicting disease relapse than those after the end of therapy5,14. Therefore, according to the MRD data described in this manuscript, MRD testing in LK products might not be useful for patient clinical management. Notably, the MCL0208 trial was not designed to investigate modifications of MRD-driven therapies (e.g., omitting the second cycle of R-HD-ARA-C in the case of an MRD-negative harvest).
Our study was not able to find any statistically significant association between the number of mobilized, harvested or reinfused stem cells and the lack of hematological recovery after ASCT. Actually, the FIL MCL0208 trial acknowledged a significant hematological toxicity of the R-HDS schedule, with 24 patients (8%) not randomized due to inadequate hematological recovery after ASCT4, irrespective of the high median number of reinfused PBSC. Moreover, 18 patients of the experimental arm (17%) experienced major dose reductions or discontinued lenalidomide during maintenance, but no associations with either low mobilization rates or the quantity of reinjected PBSC was found. Although we recognize that hematological recovery following ASCT might be a more accurate functional marker of PBSC quality than merely the CD34 + cell dose, the putative biological reasons for these major toxicities of the R-HDS regimen still need to be deciphered. Current investigations are targeting both pharmacogenetics15 and clonal hematopoiesis of indeterminate potential (CHIP)16. Interestingly, a prior study showed that the presence of CHIP was associated with a longer time to neutrophil and platelet recovery17. Another study involving a large cohort of lymphoma and multiple myeloma patients found an association between specific CHIP mutations and mobilization failure18. Preliminary data on the role of CHIP in the MCL0208 series have been recently presented19.
Notably, we found an association between long lasting G4 neutropenia after ASCT and worse PFS: we can speculate that this result reflects a slower or inadequate immune reconstitution and, therefore, an augmented susceptibility towards infectious complications and disease relapse. Moreover, this survival trend was evident only in patients randomised to lenalidomide: we might assume that patients with early hematological recovery actually received a more effective maintenance therapy, in terms of less dose reductions or discontinuation. It should be noted that prolonged neutropenia may be influenced by several factors: alteration of the bone marrow microenvironment by chemoimmunotherapy, pre-existing or post-transplant infections delaying recovery, patient-specific factors like age, nutritional status, and genetic variations. To mitigate these risks, strategies should include optimizing conditioning regimens, careful patient selection, early infection management, and the use of G-CSF, despite the lack of consensus on its timing post-ASCT.
We are aware that this study has some limitations. First, it shows only descriptive results of a not pre-planned analysis. A wider, better annotated series focused on immune reconstitution might find other putative reasons of the high incidence of inadequate hematological recovery and toxicity, as well as eventually might confirm the impact of late neutrophils recovery on outcome. Moreover, data on plerixafor use were available only for a minority of cases and were not routinely annotated on the eCRF: this evaluation should be taken into account in further studies for a better analysis of mobilization procedures and impact on patients’ outcomes. Finally, we acknowledge that the role of ASCT in MCL has been recently challenged by the frontline introduction of Bruton’s tyrosine kinase inhibitors3. Our data provide further evidence of the high toxicity and worse outcome of transplanted patient with slower hematological recovery, regardless of a high quantity of reinfused PBSC. Only a longer follow-up will definitely clarify whether ASCT might effectively be skipped or not20; however, our study strengthens the argument that, in presence of alternative, active first-line treatments, the real need of such a consolidation should be reconsidered, as it could potentially hinder the administration of effective therapies in further lines, at least in a considerable subgroup of patients.
In conclusion, despite very high rates of successful PBSC collection, as well as of adequate reinfusions, the present study highlighted that incomplete hematological recovery and long-lasting cytopenias after R-HDS and ASCT are still an issue in younger MCL patients. This risk should be considered when planning an upfront therapy in this setting, as it might hamper the delivery of highly effective maintenance therapies for these patients, eventually impacting on relapse rate and survival.
Supplementary Information
Acknowledgements
The authors would like to thank all the patients who participated in the study. We are grateful to Daniela Drandi, Daniela Barbero, Pier Paola Fenoglio, Barbara Mantoan, Daniele Grimaldi, Mariapia Pironti and Gabriele De Luca for their scientific advice and to Sonia Perticone, Antonella Ferranti, Daniela Gioia, Antonella Fiorillo, and Giulia Bondielli for their assistance.
Author contributions
M.C., S.F., S.C. and M.L. conceived and designed the study; S.F., R.T., F.C., S.H., G.M., A.M.C., C.S., M.T., G.G., J.O., S.V.U., S.G., F.R., M.M., C.C., V.P., B.B., S.C., and M.L. enrolled patients and provided biological samples; B.A. and E.G. performed the experiments; M.C., S.F., G.M.Z., B.A., S.R., A.E. and R.P. collected and analyzed data; RP performed statistical analysis; M.C., S.F., R.P. and M.L. wrote the paper. All the authors approved the final version of the manuscript.
Funding
This work was supported by Progetto di Rilevante Interesse Nazionale (PRIN2009) from the Ministero Italiano dell’Università e della Ricerca (MIUR), Roma, Italy [7.07.02.60 AE01]; Progetto di Ricerca Sanitaria Finalizzata 2009 [RF-2009-1469205] and 2010 [RF-2010-2307262 to S.C.], A.O.S. Maurizio, Bolzano/Bozen, Italy; Fondi di Ricerca Locale, Università degli Studi di Torino, Italy; Fondazione CRT (project codes: 2016.0677 and 2018.1284), Torino, Italy; AL-AIL ODV Sezione Interprovinciale di Alessandria-Asti; Associazione DaRosa, Torino, Italy; the Gilead Fellowship Program 2019, Milano, Italy; Cancer Research UK (C355/A26819) and FC AECC and AIRC under the Accelerator Award Program; Progetto di Ricerca Finalizzata e Giovani Ricercatori 2021 (code: RF-2021-12371972); this paper was funded by the Leukemia and Lymphoma Society Grant No. MCL7005-24.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
SF: Janssen, Recordati, Abbvie, Gilead, Beigene, Morphosys, Incyte, Clinigen, Italfarmaco, Astra Zeneca, Roche, Sandoz, Servier, Gentili; ML: AbbVie, Acerta, Amgen, GSKI, Gentili, Sandoz, Gilead/Kite, Novartis, Roche, Eusapharma, Takeda, Regeneron, Incyte, and Jazz, ADC Therapeutics, BeiGene, Celgene, Janssen; RT: Lilly, Janssen-Cilag; FC: Roche, Astra Zeneca, Servier; GG: Astra Zeneca, BeiGene, Incyte, Janssen, Lilly, Abbvie, Astra-Zeneca, Hikma, Janssen.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-67906-w.
References
- 1.Swerdlow, S.H. World Health Organization, International Agency for Research on Cancer. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Haematopoietic-And-Lymphoid-Tissues-2017. Accessed October 6, 2022.
- 2.Le Gouill, S. et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N. Engl. J. Med.377(13), 1250–1260. 10.1056/nejmoa1701769 (2017). 10.1056/nejmoa1701769 [DOI] [PubMed] [Google Scholar]
- 3.Dreyling, M. et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized triangle trial by the European MCL network. Blood.140(Supplement 1), 1–3. 10.1182/blood-2022-163018 (2022).35797018 10.1182/blood-2022-163018 [DOI] [Google Scholar]
- 4.Ladetto, M. et al. Lenalidomide maintenance after autologous haematopoietic stem-cell transplantation in mantle cell lymphoma: Results of a Fondazione Italiana Linfomi (FIL) multicentre, randomised, phase 3 trial. Lancet Haematol.8(1), e34–e44. 10.1016/S2352-3026(20)30358-6 (2021). 10.1016/S2352-3026(20)30358-6 [DOI] [PubMed] [Google Scholar]
- 5.Ferrero, S. et al. Punctual and kinetic MRD analysis from the Fondazione Italiana Linfomi MCL0208 phase 3 trial in mantle cell lymphoma. Blood.140(12), 1378–1389. 10.1182/blood.2021014270 (2022). 10.1182/blood.2021014270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Velden, V. H. J. et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: Guidelines for interpretation of real-time quantitative PCR data. Leukemia.21(4), 604–611. 10.1038/sj.leu.2404586 (2007). 10.1038/sj.leu.2404586 [DOI] [PubMed] [Google Scholar]
- 7.Lausen, B. & Schumacher, M. Maximally selected rank statistics. Biometrics.48(1), 73. 10.2307/2532740 (1992). 10.2307/2532740 [DOI] [Google Scholar]
- 8.Gianni, A. M. et al. Long-term remission in mantle cell lymphoma following high-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting (R-HDS regimen). Blood.102(2), 749–755. 10.1182/blood-2002-08-2476 (2003). 10.1182/blood-2002-08-2476 [DOI] [PubMed] [Google Scholar]
- 9.Magni, M. et al. High-dose sequential chemotherapy and in vivo rituximab-purged stem cell autografting in mantle cell lymphoma: A 10-year update of the R-HDS regimen. Bone Marrow Transplant.43(6), 509–511. 10.1038/bmt.2008.349 (2009). 10.1038/bmt.2008.349 [DOI] [PubMed] [Google Scholar]
- 10.Hermine, O. et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): A randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma N. Lancet.388(10044), 565–575. 10.1016/S0140-6736(16)00739-X (2016). 10.1016/S0140-6736(16)00739-X [DOI] [PubMed] [Google Scholar]
- 11.Le Gouill, S. et al. Molecular response after obinutuzumab plus high-dose cytarabine induction for transplant-eligible patients with untreated mantle cell lymphoma (LyMa-101): A phase 2 trial of the LYSA group. Lancet Haematol.7(11), e798–e807. 10.1016/S2352-3026(20)30291-X (2020). 10.1016/S2352-3026(20)30291-X [DOI] [PubMed] [Google Scholar]
- 12.Brown, J. R. et al. Long-term survival after autologous bone marrow transplantation for follicular lymphoma in first remission. Biol. Blood Marrow Transplant.13(9), 1057–1065. 10.1016/j.bbmt.2007.05.012 (2007). 10.1016/j.bbmt.2007.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magni, M. et al. Successful in vivo purging of CD34-containing peripheral blood harvests in mantle cell and indolent lymphoma: Evidence for a role of both chemotherapy and rituximab infusion. Blood.96(3), 864–869. 10.1182/blood.v96.3.864 (2000). 10.1182/blood.v96.3.864 [DOI] [PubMed] [Google Scholar]
- 14.Ladetto, M. A comprehensive and systematic analysis of minimal residual disease (MRD) monitoring in follicular lymphoma: Results from the Fondazione Italiana Linfomi (FIL) FOLL12 Trial. December 2021. https://ash.confex.com/ash/2021/webprogram/Paper146773.html. Accessed November 5, 2021.
- 15.Ferrero, S. et al. Candidate germline biomarkers of lenalidomide efficacy in mantle cell lymphoma: The FIL MCL0208 trial. Blood Adv.10.1182/bloodadvances.2022009504 (2023). 10.1182/bloodadvances.2022009504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husby, S. et al. Clinical impact of clonal hematopoiesis in patients with lymphoma undergoing ASCT: A national population-based cohort study. Leukemia.34(12), 3256–3268. 10.1038/s41375-020-0795-z (2020). 10.1038/s41375-020-0795-z [DOI] [PubMed] [Google Scholar]
- 17.Lackraj, T. et al. Clinical significance of clonal hematopoiesis in the setting of autologous stem cell transplantation for lymphoma. Am. J. Hematol.97(12), 1538–1547. 10.1002/ajh.26726 (2022). 10.1002/ajh.26726 [DOI] [PubMed] [Google Scholar]
- 18.Hazenberg, C. L. E. et al. Clonal hematopoiesis in patients with stem cell mobilization failure: A nested case-control study. Blood Adv.7(7), 1269–1278. 10.1182/bloodadvances.2022007497 (2023). 10.1182/bloodadvances.2022007497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ragaini, S., Galli, A., Genuardi, E., Gandossini, M., Alessandria, B. & Maria Civita, A. et al. Myeloid clonal hematopoiesis affects outcome in younger mantle cell lymphoma patients: Updated results from the Fondazione Italiana Linfomi MCL0208 clinical trial. Abstract release date: 05/14/24. EHA Library; 419334; P1247.
- 20.Hermine, O. et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized mantle cell lymphoma younger trial of the European mantle cell lymphoma network. J. Clin. Oncol.41(3), 479–484. 10.1200/JCO.22.01780 (2023). 10.1200/JCO.22.01780 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.