Abstract
Background:
Naloxone distribution is central to ongoing efforts to address the opioid overdose crisis. Some critics contend that naloxone expansion may inadvertently promote high-risk substance use behaviors among adolescents, but this question has not been directly investigated.
Methods:
We examined relationships between naloxone access laws and pharmacy naloxone distribution with lifetime heroin and injection drug use (IDU), 2007–2019. Models generating adjusted odds ratios (aOR) and 95% confidence intervals (CI) included year and state fixed effects, controlled for demographics and sources of variation in opioid environments (e.g., fentanyl penetration), as well as additional policies expected to impact substance use (e.g., prescription drug monitoring). Exploratory and sensitivity analyses further examined naloxone law provisions (e.g., third-party prescribing) and applied e-value testing to assess vulnerability to unmeasured confounding.
Results:
Adoption of any naloxone law was not associated with changes in adolescent lifetime heroin or IDU. For pharmacy dispensing, we observed a small decrease in heroin use (aOR: 0.95 [CI: 0.92, 0.99]) and a small increase in IDU (aOR: 1.07 [CI: 1.02, 1.11]). Exploratory analyses of law provisions suggested that third-party prescribing (aOR: 0.80, [CI: 0.66, 0.96]) and non-patient-specific dispensing models (aOR: 0.78, [CI: 0.61, 0.99]) were associated with decreased heroin use but not decreased IDU. Small e-values associated with the pharmacy dispensing and provision estimates indicate that unmeasured confounding may explain observed findings.
Conclusion:
Naloxone access laws and pharmacy naloxone distribution were more consistently associated with decreases rather than increases in lifetime heroin and IDU among adolescents. Our findings therefore do not support concerns that naloxone access promotes high-risk adolescent substance use behaviors. As of 2019, all US states have adopted legislation to improve naloxone access and facilitate use. However, further removal of adolescent naloxone access barriers is an important priority given that the opioid epidemic continues to affect people of all ages.
Keywords: Naloxone, Adolescent, Harm Reduction, Injection Drug Use, Heroin, Policy analysis
Background
Most opioid overdose deaths are preventable with the timely administration of naloxone, an opioid agonist medication that restores normal breathing and prevents death and disability after an overdose has occurred (Chamberlain & Klein, 1994). Because of this lifesaving potential, broadening access to naloxone is a key component of the US opioid overdose epidemic response, including community-level efforts to expand naloxone distribution (Lambdin et al., 2020; McDonald & Strang, 2016; Wheeler et al., 2012), and state-level efforts to improve access through legal reform (Davis & Carr, 2015; Green et al., 2020). Despite the benefits of naloxone, however, some concerns have been raised regarding potential unintended consequences associated with widespread distribution. Often described as ‘risk compensation’ in the public health literature, or ‘moral hazard’ in the economics literature, the concern is that if naloxone is perceived as preventing or minimizing risk of overdose fatality, some individuals may be more likely to initiate or increase opioid use, or to engage in higher risk behaviors, than they would if naloxone were not widely available (Tas et al., 2019). Adolescents in particular are perceived as being especially susceptible to potential risk compensation harms given their greater propensity for risk-taking behaviors and developmental vulnerability to substance use disorder (Conrod & Nikolaou, 2016; Nawi et al., 2021; Substance Abuse and Mental Health Services Administration, 2022).
To date, little existing research on naloxone compensatory behaviors directly focuses on adolescents. Studies in the general population suggest that naloxone receipt is not typically associated with increases in use among people who already use opioids (Tse et al., 2022). However, most existing studies rely on non-experimental designs with use behaviors assessed via self-report, suggesting potential for bias (Tse et al., 2022). To overcome this limitation, recent work has instead leveraged timing in the adoption of state-level naloxone access laws (NALs) to examine the potential population-level impacts of increasing naloxone access over time. While most studies report stable or decreasing overdose trends associated with NALs (Smart et al., 2021), a small number have identified evidence of overall or region-specific overdose increases in the general population (Doleac & Mukherjee, 2018; Erfanian et al., 2019; Lee et al., 2021), leading to speculation that NALs could inadvertently encourage opioid misuse (Doleac & Mukherjee, 2018; Erfanian et al., 2019; Seelye, 2016). However, because few of these existing studies measure changes in individual-level substance use behaviors (as opposed to changes in overdose rates), it is unclear whether findings truly support the risk compensation hypothesis.
We tested whether state-level naloxone access, operationalized as NAL adoption, and as a measure of pharmacy-based naloxone dispensing, were associated with changes in self-reported lifetime heroin use and lifetime injection drug use (IDU) among adolescents. We focus on these outcomes because risk compensation theory predicts that in the presence of naloxone and the protection it affords, adolescents might be more likely to: (1) initiate opioid misuse (i.e., prescription opioid misuse or heroin use), or (2) engage in riskier forms of drug use (i.e., injection use) than they might otherwise. Potential changes in initiation and risk behaviors are theoretically mediated through reductions in perceived harmfulness, a well-established determinant of adolescent substance use (Arria et al., 2008; Kapadia & Bao, 2019; Lipari, 2013; Nawi et al., 2021). Data limitations precluded us from examining prescription opioid misuse and measures of current use or frequency of use. However, given that heroin and IDU initiation in the US typically occur among individuals in their late teens and early twenties (Broz et al., 2014; Volkow et al., 2021), we expected that lifetime use would be relevant to the adolescent population. Evidence of increases in self-reported heroin use or IDU would be consistent with a risk compensation perspective, whereas no association or evidence of decreases would not align with the theory.
Methods
Sample
Data on adolescent substance use were obtained from the Youth Risk Behavior Surveillance System (YRBSS), a national US survey monitoring health risk behaviors among high school students. A two-stage cluster design is used to produce reliable state-level estimates of youth substance use (Underwood, 2020). After obtaining parental consent (passive or active depending on local requirements), students self-administer the survey anonymously in classrooms. From 2007 to 2019, all but three states (MN, OR, WA) participated in the YRBSS. Research files are available for states with representative data, determined by an overall response rate of ≥60% or nonresponse bias analysis (Underwood, 2020).
We restricted our analyses to students 15–18 years old to focus on the age cohort in which substance use is more prevalent, and among whom overdose deaths are rising (Gaither et al., 2018; SAMSHA, 2012). In the primary analysis, we further excluded observations from participants reporting a pattern of extreme responses to limit bias attributable to potential ‘mischievous responders’ (Cimpian et al., 2018), a documented concern among adolescent substance use surveys including the YRBSS (see Supplement). Respondents with missing data were also excluded (Supplemental Table 2). Two states (WI, RI) did not include IDU information in the study period and were excluded from IDU-specific analyses. As all data were de-identified and publicly available, we did not seek explicit institutional review board approval for these secondary data analyses.
Exposure data
We had two primary exposure variables. Our first was state-level NAL adoption, operationalized as a time-varying binary construct, representing presence or absence of any law intended to facilitate naloxone distribution or use (Table 1). Our second primary exposure was pharmacy-based naloxone dispensing. To measure this, we obtained transaction-level data on retail pharmacy dispensed naloxone prescriptions from the IQVIA longitudinal prescription database ([IQVIA LRx], IQVIA Inc., Durham, North Carolina). These data capture dispensing from approximately 92% of US retail pharmacies, including prescriptions from all payers. Counts excluded prescriptions obtained by mail, dispensed within hospitals, and distributed through overdose education and naloxone distribution programs. We aggregated transactions to the state-year level, then converted these to proportions using American Community Survey 1-year total population estimates as denominators.
Table 1.
Naloxone access measures
| Primary exposures | Definition |
|---|---|
|
| |
| Any naloxone access law (NAL) | Any law intended to facilitate naloxone distribution or use |
| Pharmacy distribution | The number of naloxone prescriptions dispensed at retail pharmacies per 1,000 state population in IQVIA LRx |
|
| |
| Secondary exposures | Definition |
|
| |
| Prescriber or dispenser immunity | Laws that provide prescribers or dispensers with immunity from either criminal prosecution or civil liability for prescribing, dispensing, or distributing naloxone to a layperson |
| Layperson immunity | Laws that provide laypersons with immunity from either criminal prosecution or civil liability when administering naloxone |
| Third party prescribing | Laws that authorize naloxone prescriptions to third parties (i.e., persons in a position to assist individuals atrisk of overdose) |
| Non-patient-specific dispensing model | Laws that enable pharmacists to dispense or distribute naloxone without a patient-specific prescription |
| Decriminalized possession | Laws that remove criminal liability for possession of naloxone without a prescription |
In addition to any NAL, we examined NAL provisions, including prescriber or dispenser immunity, layperson immunity, third-party prescribing, non-patient-specific dispensing, and decriminalized possession, as exploratory analyses (Table 1). Prior literature has shown that the relative effectiveness of NALs may depend on the specific provisions included within laws (Smart et al., 2021). Under a risk compensation perspective, any NAL that facilitates greater naloxone access or changes risk perceptions could potentially impact adolescent opioid misuse. However, certain provisions could be more salient within this population. For example, provisions that directly reduce barriers to obtaining or using naloxone, like the removal of penalties for possessing naloxone without a prescription, could be more relevant to adolescents than those targeting prescribers or dispensers (e.g., prescriber and dispenser immunity protections). For the provision-based models, we coded exposures using 3-level variables comparing respondents in states with an overall NAL but not the provision in question, and those in states with NALs including the provision, to those in states with no NAL.
NAL dates were obtained from the Prescription Drug Abuse Policy System and Network for Public Health Law. Laws were coded as in effect in the year of interest if the law became effective Jan-Jun; laws that became effective Jul-Dec were coded as effective in the following year. For the primary NAL exposure, we expected any potential effects of laws to be relatively immediate, however, to relax this assumption we additionally conducted sensitivity analyses using 1-year and 2-year lags to explore the possibility of more delayed impacts. For exploratory provision analyses, we did not include lags as several provisions were first implemented relatively late in the observation period and including lags would reduce power.
Covariates
Covariates were based on prior literature and included other opioid-related policies, specifically: overdose Good Samaritan laws, prescription drug monitoring program (PDMP) laws, cannabis laws, pain clinic regulations, opioid prescription limits, and Medicaid expansion (Supplemental Table 1) (Smart et al., 2021; Tse et al., 2022). We also controlled for other time-varying state-level factors, including child poverty, unemployment rate, prior year total mortality, fentanyl seizures (capturing the unregulated drug environment), and substance use treatment facilities. Individual-level control variables were age, gender (female, male), and race/ethnicity (Hispanic, non-Hispanic Black, non-Hispanic white, and all other races and ethnicities).
Statistical analyses
We used standard logistic regression with state and year fixed effects to test the associations of: (1) NALs, (2) pharmacy dispensing, and (3) NAL provisions [secondary analyses] with lifetime adolescent heroin use and IDU. In addition to the fixed-effects and the covariates described above, analyses accounted for the YRBSS complex survey design and included Huber-adjusted robust standard errors (Abadie et al., 2017). Because we conceptualized the provision analyses as exploratory a priori, we did not further adjust p-values for multiple comparison testing.
To probe the main underlying assumption of the NAL models, namely that the trends in each outcome were uncorrelated with differences in policy introduction across states, we used two strategies. First, to visually inspect unconditional outcome trends pre-NAL introduction, we plotted the prevalence of heroin use and IDU, grouping states by NAL introduction timing (relative to YRBS survey wave). In addition, given multiple periods of NAL implementation, we also used event study regressions to confirm findings, by incorporating 2-year lags and leads reflecting time to, and following, NAL adoption, and evaluating the pre-policy coefficients (Roth et al., 2023).
One methodological challenge associated with drug law evaluation generally (Martins et al., 2021; Schuler et al., 2020) and NALs specifically (Rudolph et al., 2021; Smart et al., 2021), is accounting for potential collinearity in policy adoption. Prior literature shows that NALs are often passed as a package together with overdose Good Samaritan laws (GSLs)—laws that provide protections from some crimes to individuals who report or experience an overdose (Hamilton et al., 2021; Rudolph et al., 2021). Additionally, in our data, we observed multicollinearity between these measures. Because GSLs have not been theorized to increase risk compensation, we attempted to limit this potential source of misclassification bias by excluding observations from 12 states that concurrently implemented GSL and naloxone laws in the main analysis (AL, AR, GA, HI, MS, NC, ND, NV, PA, UT, WI, WV). This conservative approach helps to ensure that the estimates presented are specific to NAL adoption in isolation, rather than the combined effect of NAL/GSL co-implementation. However, because this additional restriction limits the sample, we additionally present models including all states, and models combining NALs/GSLs, as sensitivity analyses. Pharmacy dispensing models included YRBSS respondents from all states with available data.
For additional sensitivity checks, we re-ran our analyses comparing the primary findings to those from models including potential mischievous responders. We additionally lagged the main NAL models by 1 and 2 years to reflect the fact that there may be delays between the time a law goes into effect, and when its impacts occur. We also refined the state inclusion criteria to exclude states that had already adopted NALs prior to the start of the observation period in 2007 (NM [2001], CT [2004], NY [2006]).
Finally, we calculated e-values and Bayes factors to further contextualize significant and null results (Beard et al., 2016; VanderWeele & Ding, 2017). E-values quantify the minimum strength of association that a potential unmeasured confounder would need to exert to artifactually produce the observed association if the true association were null (VanderWeele & Ding, 2017). In other words, the e-value quantifies the extent to which potential residual confounding could threaten observed conclusions. Larger e-values suggest that results are relatively robust, whereas smaller e-values suggest that a small amount of unmeasured confounding could explain the observed associations. Likewise, we calculated Bayes factors to provide additional evidence for null results. Bayes factors represent the ratio of the average likelihood of two hypotheses being correct (Beard et al., 2016). A Bayes factor of <1/3 generally indicates evidence for the null hypothesis. Analyses were conducted using R version 4.1.2. (R Foundation for Statistical Computing).
Results
From 2007 to 2019, 920,333 students aged 15–18 in participated in the YRBSS (Table 2, Supplemental Table 2). Lifetime heroin use was self-reported by 2.75% (95% Confidence Interval [CI]: 2.64, 2.86) and lifetime IDU by 2.48% (95% CI: 2.30, 2.64). There was a small decrease in heroin use over the period; prevalence was 2.72% (95% CI: 2.50, 2.67) in 2007, and decreased to 2.27% (95% CI: 2.04, 2.49) in 2019. Lifetime IDU remained stable (2007: 2.48% [95% CI: 2.28, 2.67]; 2019: 2.65% [95% CI: 1.75, 3.56]).
Table 2.
Characteristics of YRBSS respondents aged 15–18 across 44 states, 2007–2019
| Weighted % (or mean) | N=920,333 | |
|---|---|---|
|
| ||
| Age | 16.29 | |
| Gender | ||
| Female | 48.81 | 460,418 |
| Male | 51.19 | 455,459 |
| Race/ethnicity | ||
| Black | 17.22 | 120,945 |
| Hispanic | 20.97 | 158,256 |
| Other | 7.86 | 114,790 |
| White | 53.94 | 503,235 |
| Lifetime heroin use | ||
| No | 97.25 | 726,764 |
| Yes | 2.75 | 22,344 |
| Lifetime IDU | ||
| No | 97.52 | 620,487 |
| Yes | 2.48 | 17,781 |
Abbreviations: YRBSS, Youth Risk Behavior Surveillance System; IDU, injection drug use
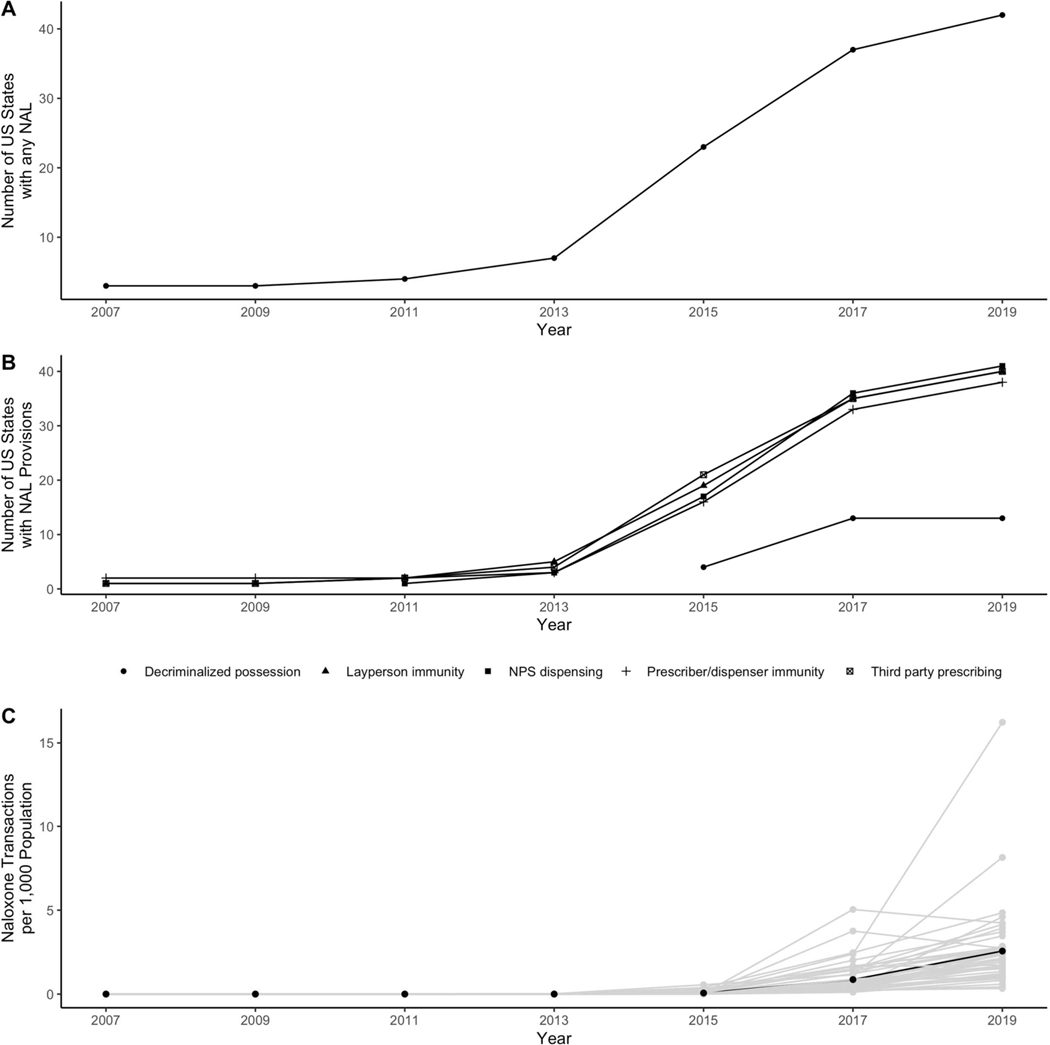
Over the observation period, naloxone access increased substantially (Figure 1). In 2007, only three states had adopted any NAL; however, by 2019, all states had some version of a law in place. Immunity-related provisions tended to be enacted earlier, while naloxone decriminalization tended to be enacted later. Pharmacy-based naloxone dispensing also increased over the period. Approximately 0.002 prescriptions were dispensed nationally per 1,000 population in 2007. By 2019, this number had risen to 2.62 prescriptions per 1,000 population.
Figure 1.
Panels A and B display the number of states with any naloxone access law (NAL) and with specific NAL provisions over the study period (2007–2019). NALs and provisions dates were obtained from the Prescription Drug Abuse Policy System. Panel C displays the number of naloxone prescriptions in IQVIA LRx dispensed at retail pharmacies per 1,000 population over the study period. Grey lines indicate state-specific prescriptions dispensed; the black line indicates the average number of prescriptions dispensed across all states.
Table 3 shows results from our regression models. Regarding the main exposures, adoption of any NAL was not associated with lifetime heroin use (aOR: 0.82 [95% CI: 0.65, 1.04]), or IDU (aOR: 1.10 [95% CI: 0.78, 1.53]). For pharmacy dispensing, a 1-unit increase in dispensing was associated with about a 5% relative decrease in heroin use (aOR: 0.95 [95% CI: 0.92, 0.99]) and about a 7% relative increase in the IDU (aOR: 1.07 [95% CI: 1.02, 1.11]).
Table 3.
Association between naloxone access measures and lifetime heroin and IDU among adolescents aged 15–18 across US states, 2007–2019
| Lifetime heroin use | Lifetime IDU | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||||
|
| ||||||||||||
| Primary exposures | OR | 95% CI | P-value | aOR | 95% CI | P-value | OR | 95% CI | P-value | aOR | 95% CI | P-value |
|
| ||||||||||||
| Any NAL | 0.81 | (0.79, 0.95) | <0.01 | 0.82 | (0.65, 1.04) | 0.10 | 0.89 | (0.75, 1.07) | 0.21 | 1.10 | (0.78, 1.53) | 0.59 |
| Pharmacy dispensingA | 0.94 | (0.90, 0.98) | 0.01 | 0.95 | (0.92, 0.99) | 0.01 | 1.02 | (0.99, 1.04) | 0.23 | 1.07 | (1.02, 1.11) | 0.01 |
|
| ||||||||||||
| Secondary exposures | ||||||||||||
|
| ||||||||||||
| Any NAL | 0.84 | (0.68, 1.05) | 0.12 | 0.75 | (0.70, 1.04) | 0.19 | 0.69 | 0.56, 0.86 | <0.01 | 1.08 | (0.85, 1.38) | 0.51 |
| NAL, prescriber or dispenser immunity | 0.75 | (0.59, 0.96) | 0.02 | 0.96 | (0.77, 1.19) | 0.69 | 0.72 | 0.61, 0.85 | <0.01 | 1.15 | (0.86, 1.55) | 0.35 |
| Any NAL | 0.81 | (0.66, 0.99) | 0.04 | 0.88 | (0.71, 1.09) | 0.24 | 0.67 | 0.57, 0.78 | <0.01 | 1.18 | (0.92, 1.51) | 0.20 |
| NAL, layperson immunity | 0.74 | (0.58, 0.96) | 0.02 | 0.83 | (0.65, 1.05) | 0.11 | 0.79 | 0.64, 0.97 | 0.02 | 1.03 | (0.78, 1.36) | 0.82 |
| Any NAL | 1.00 | (0.81, 1.22) | 0.96 | 1.36 | (0.99, 1.70) | 0.06 | 1.05 | 0.82, 1.35 | 0.69 | 1.13 | (0.84, 1.51) | 0.41 |
| NAL, third party prescribing | 0.76 | (0.60, 0.95) | 0.02 | 0.80 | (0.66, 0.96) | 0.03 | 0.66 | 0.57, 0.77 | <0.01 | 1.11 | (0.87, 1.43) | 0.40 |
| Any NAL | 0.84 | (0.69, 1.03) | 0.09 | 0.89 | (0.73, 1.08) | 0.25 | 0.7 | 0.61, 0.81 | <0.01 | 1.24 | (0.98, 1.56) | 0.07 |
| NAL, Non-patient-specific dispensing | 0.63 | (0.46, 0.86) | <0.01 | 0.78 | (0.61, 0.99) | 0.05 | 0.73 | 0.58, 0.91 | 0.01 | 0.83 | (0.62, 1.13) | 0.24 |
| Any NAL | 0.83 | (0.68, 1.00) | 0.05 | 0.84 | (0.69, 1.03) | 0.09 | 0.72 | 0.61, 0.84 | <0.01 | 1.22 | (0.97, 1.55) | 0.09 |
| NAL, decriminalized possession | 0.72 | (0.54, 0.97) | 0.03 | 0.89 | (0.70, 1.14) | 0.35 | 0.7 | 0.58, 0.83 | <0.01 | 0.91 | (0.63, 1.31) | 0.61 |
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; 95% CI, 95% Confidence Interval; IDU, injection drug use; NAL, Naloxone access law.
Pharmacy dispensing refers to the number of naloxone prescriptions in IQVIA LRx dispensed at retail pharmacies per 1,000 population in a given state-year. Secondary exposure models coded NAL provisions using a 3-level variable comparing a NAL excluding the specific provision, and a NAL including the specific provision to no NAL. NAL primary and secondary exposure models excluded Good Samaritan law co-implementation states (AL, AR, GA, HI, MS, NC, ND, NV, PA, UT, WI, WV), however sensitivity models including all states and states with co-implementation are presented in Supplemental Tables 3 and 4; lifetime IDU models excluded WI and RI which did not assess IDU in the observation period. Unadjusted models excluded potential confounders but included state and year fixed effects. All models include clustered standard errors.
Secondary and sensitivity analyses
For the exploratory analyses of NAL provisions, we observed reductions in heroin use among respondents in NAL states with third-party prescribing (aOR: 0.80 [95% CI: 0.66, 0.96]) and non-patient-specific dispensing models (aOR: 0.78 [95% CI: 0.61, 0.99]) compared to respondents in states without laws. All other associations varied around the null (1.0).
Assumption testing and sensitivity results were as follows. When we examined trends in heroin use and IDU prevalence pre-NAL introduction graphically, we identified reasonably similar patterns across groups though we noted some variability by period (Supplemental Figures 1–2). We therefore formally assessed pre-trends with an event study specification that indicated no statistically significant associations in the pre-NAL period (Supplemental Figures 3–4).
Next, when we included GSL co-implementation states (Supplemental Table 3), estimates and CIs moved farther from the null, such that NALs became significantly associated with decreased odds of heroin use (aOR: 0.71 [95% CI: 0.59, 0.86]) and IDU (aOR: 0.72 [95% CI: 0.57, 0.91]). After including GSL states, 4 of 5 provisions were associated with decreased odds of heroin use in adjusted models (excluding prescriber/dispenser immunity, Supplemental Table 3) and for IDU, 3 of 5 provisions were associated with decreased odds (excluding prescriber/dispenser immunity, third-party prescribing). A broadly similar pattern was also observed when we treated NAL/GSL co-implementation states as an additional law exposure level (Supplemental Table 4). Relative to no law, NAL/GSL co-implementation was associated with decreased IDU (aOR: 0.72 [95% CI: 0.53, 0.97]) but not heroin use (aOR: 0.92 [95% CI: 0.70, 1.20]).
When we included responses from possible mischievous responders, results were highly consistent with the primary analysis (Supplemental Table 5). Third, in lagged models, we observed decreasing odds of heroin use and IDU that was stronger after the first and especially the second year a NAL was in effect, highlighting the potential role of delayed law impacts (2-year lagged heroin aOR: [95% CI: 0.61, 0.88]; IDU aOR: [95% CI: 0.46, 0.89]; Supplemental Tables 6 and 7). For sensitivity analyses excluding the earliest adopting states (NM, CT, NY), results were qualitatively similar to the primary analyses, but with wider confidence intervals including the null (Supplemental Table 8).
Lastly, supplemental Tables 9–12 present e-values and Bayes factors. For pharmacy dispensing, we observed small e-values (heroin point estimate e-value 1.25 [95% CI e-value: 1.11], IDU point estimate e-value 1.34 [95% CI e-value: 1.16]), meaning for example, that an unmeasured confounder associated with pharmacy dispensing and with IDU by ORs of 1.16 each, could push the bounds of the observed CI to include 1 (Table 9–10). Small e-values associated with third party prescribing and non-patient-specific dispensing also suggest that minimal unmeasured confounding could explain significant findings. Calculation of Bayes factors, which ranged from 0.39–0.82, confirmed the frequentist statistical findings. Results indicated anecdotal evidence for the null hypothesis (Supplemental Table 12).
Discussion
We examined whether increasing naloxone access, defined both in terms of state-level NAL adoption, and as a measure of pharmacy naloxone dispensing, was associated with changes in adolescent self-reported lifetime heroin use and IDU. Overall, increasing NAL adoption from 2007 to 2019 was not associated with meaningful changes in either outcome among adolescents at the population-level. Pharmacy naloxone dispensing was associated with decreased odds of heroin use (aOR: 0.95, 95% CI: 0.92, 0.99), and increased odds of IDU (aOR: 1.07, 95% CI: 1.02, 1.11), but at levels indicating minimal to null effects, and associated e-values suggesting strong sensitivity to potential unmeasured confounding. Exploratory and sensitivity analyses further revealed that certain NAL provisions may have been associated with decreases in heroin use, and that 1–2 years post-adoption NALs were associated with decreases in both outcomes. These additional analyses may suggest that our main conclusion—that increasing NAL adoption has not been associated with increases in substance use behaviors—is a conservative interpretation. Overall, we found that naloxone expansion was more consistently associated with decreases rather than increases in adolescent heroin use and IDU.
Even though naloxone is recognized as one of the most valuable tools for reducing opioid overdose deaths, access in many places remains low given the number of people at risk (Guy et al., 2019; Lambdin et al., 2020). NALs are designed to reduce this gap by facilitating access to this lifesaving medication and therefore increasing opportunities to directly intervene in an overdose. However, concerns that naloxone access might inadvertently increase opioid misuse and overdose—the risk compensation hypothesis—appear to be a barrier to distribution efforts, both in the US and internationally (Clauss, 2016; Vergano, 2018; Wong, 2019). The assumption inherent to the risk compensation perspective is that the way in which NALs could influence overdose trends is through changes in behaviors, such as increasing opioid use initiation, or increasing higher-risk behaviors like IDU. However, rising overdose mortality, despite broadening naloxone availability, could also reflect the rapid proliferation of fentanyl and other synthetics into the drug supply post-2014 (Dowell et al., 2017; Smart et al., 2021), rather than behavioral changes. Our findings among adolescents lend greater support to this second explanation.
To the best of our knowledge, only one prior study has examined whether NALs are associated with changes in self-reported substance use behaviors. Using 2002 to 2014 National Survey on Drug Use and Health (NSDUH) data among respondents aged 15 and older, McClellan et al. identified stable prevalence of past month nonmedical opioid use before and after NALs, also in contrast to the risk compensation hypothesis (McClellan et al., 2018). As with McClellan’s findings which capture only past-month use, our results regarding lifetime use do not necessarily rule out the possibility of increasing heroin or IDU frequency among adolescents already engaging in substance use. At present only four states (AK, MI, NM, VA) assess past-month use heroin use, precluding recent use analyses. However, adolescence and early adulthood is typically the period when nonmedical opioid and heroin initiation occur (Lipari et al., 2017; SAMSHA, 2014), so lifetime rather than recency or frequency measures are potentially less problematic in adolescents than older adults, but remain a concern. Although we did not explicitly examine frequency, our findings are consistent with multiple evaluations of naloxone distribution programs that report stable or reduced frequency of opioid use after take-home naloxone receipt among people already using opioids (J. D. Jones et al., 2017; Seal et al., 2005; Wagner et al., 2010).
Our findings regarding pharmacy naloxone dispensing—mainly that increased dispensing was associated with small decreases in heroin use (aOR: 0.95, 95% CI: 0.92, 0.99) and small increases in IDU (aOR: 1.07, 95% CI: 1.02, 1.11)—deserve further investigation. We interpreted these results as failing to provide meaningful support for the risk compensation hypothesis, given the limited magnitude of both associations, and the fact that e-value testing identified strong vulnerability to unmeasured confounding. However, finding that these associations were in opposite directions for heroin use and IDU given the presumed interconnectedness of these measures is somewhat surprising. Moreover, the increase in odds for IDU stands in contrast to the findings of the multiple sensitivity analyses which consistently suggest decreases rather than increases in IDU associated with NAL adoption. Further work is needed to replicate these results in other adolescent and adult data sources and to interrogate potential mechanisms.
In addition, we found that third-party prescribing and non-patient-specific dispensing appear to decrease odds of heroin use. These findings could have several explanations. First, we cannot rule out the possibility that decreases could be related to other changes occurring at the same time as NAL adoption, especially given the small e-values observed. For example, a state’s propensity for adopting more robust naloxone laws could be correlated with other effective state efforts to address the opioid overdose crisis that we could not measure (e.g. law enforcement priorities, ideology, etc.). A second possibility relates to potential synergies between state-level naloxone distribution efforts and more evidence-based substance use prevention priorities. For example, state adoption of NALs has been shown to be associated with higher levels of naloxone distribution program implementation (Lambdin et al., 2018), which often include educational components (Razaghizad et al., 2021). Broader public health messaging associated with naloxone education and distribution efforts may also shift social norms regarding substance use in ways that are beneficial to all adolescents. The potential for changes in knowledge or risk perceptions in response to NALs is supported by evidence that perceived risk of heroin use has increased more among adolescents living in states with NALs than those living in states without NALs (Kelly & Vuolo, 2022). Research examining how packages of opioid-related policies impact adolescents could provide further insights into the most effective ways to reduce opioid-related harms in this population.
Limitations
Limitations are noted. First, due to limited law data, we were unable to examine certain youth-targeted naloxone law provisions that might be relevant. For example, given increasing overdoses among adolescents, states are starting to implement provisions that require schools to stock naloxone, or that explicitly address minimum age requirements for pharmacy naloxone purchases (Jimenez et al., 2019). Second, we did not have available measures of nonmedical opioid use or other prescription drug misuse over the entire analysis period. Although YRBSS now assesses youth prescription pain medicine misuse and has done so since 2017, the prior question on nonmedical prescription drug use included additional drugs (e.g., stimulants) making it less suitable for answering our research question. Further, the public use data do not include responses to this question from 2009 to 2015. Third, as previously noted, we relied on lifetime measures of heroin use and IDU rather than measures capturing current use. Lifetime use overestimates current use, given that adolescents in the sample could have initiated heroin use or IDU prior to being surveyed, therefore contributing to potential outcome misclassification and residual time trend confounding. Additional research should examine naloxone dispensing and NALs associations with current heroin use, IDU and prescription opioid misuse. Fourth, the pharmacy dispensing measure captures only naloxone dispensed in retail pharmacies, so naloxone distributed through community-based harm-reduction programs were not included, and such distribution is a major channel for naloxone distribution (Lambdin et al., 2020; Wheeler & Doe-Simkins, 2020). Lastly, heroin use and IDU are generally rare outcomes that are likely to be underreported given their stigmatized nature (Reuter et al., 2021), especially among adolescents. We identified a relatively high prevalence of both heroin and IDU consistent with other analyses of YRBSS data, and note that non-household surveys of adolescent opioid misuse that are nationally representative are currently unavailable. (C. Jones, 2020; SAMSHA, 2012). Additional research is needed to examine the naloxone risk compensation hypothesis in other adolescent surveys and in other population surveys generally.
Several limitations also relate to the timeframe captured by these analyses. First, in our provision-based exploratory analyses, most of the provisions explored had only been in effect for a few years of the study timeframe. E-value sensitivity testing also suggested that these findings are potentially susceptible to unmeasured confounding, indicating that a longer observation period may be needed to establish more robust trends. Second, our results also span the period where fentanyl and other synthetic opioids were becoming increasingly prevalent in the unregulated drug supply, knowledge of which could have altered adolescents’ substance use behaviors. Lastly, recent methodological literature has highlighted several challenges associated with evaluating policy effects in settings where laws are adopted at multiple timepoints, as with NALs. In this context, bias could have been introduced if the effects of NALs change over time, or differ by policy timing, given that estimated coefficients we present reflect a weighted average over the observation period and across policy groups (Goodman-Bacon, 2018; Roth et al., 2023).
Conclusion
Our findings do not support the hypothesis that broader availability of naloxone between 2007 and 2019 increased heroin use or injection drug use among adolescents and suggest that increased adolescent drug use as an unintended consequences of naloxone availability is an unfounded concern. Efforts to improve naloxone access should continue to be an urgent public health priority, including among adolescents who represent an increasingly vulnerable population at risk for fatal and nonfatal overdose (Chadi & Hadland, 2019).
Supplementary Material
References
- Abadie A, Athey S, Imbens G, & Wooldridge J. (2017). When Should You Adjust Standard Errors for Clustering? (No. w24003; p. w24003). National Bureau of Economic Research. 10.3386/w24003 [DOI] [Google Scholar]
- Arria AM, Caldeira KM, Vincent KB, O’Grady KE, & Wish ED (2008). Perceived harmfulness predicts nonmedical use of prescription drugs among college students: Interactions with sensation-seeking. Prevention Science : The Official Journal of the Society for Prevention Research, 9(3), 191–201. 10.1007/s11121-008-0095-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard E, Dienes Z, Muirhead C, & West R. (2016). Using Bayes factors for testing hypotheses about intervention effectiveness in addictions research. Addiction (Abingdon, England), 111(12), 2230–2247. 10.1111/add.13501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz D, Pham H, Spiller M, Wejnert C, Le B, Neaigus A, & Paz-Bailey G. (2014). Prevalence of HIV Infection and Risk Behaviors Among Younger and Older Injecting Drug Users in the United States, 2009. AIDS and Behavior, 18(3), 284–296. 10.1007/s10461-013-0660-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadi N, & Hadland SE (2019). Youth Access to Naloxone: The Next Frontier? The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine, 65(5), 571–572. 10.1016/j.jadohealth.2019.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain JM, & Klein BL (1994). A comprehensive review of naloxone for the emergency physician. The American Journal of Emergency Medicine, 12(6), 650–660. 10.1016/0735-6757(94)90033-7 [DOI] [PubMed] [Google Scholar]
- Cimpian JR, Timmer JD, Birkett MA, Marro RL, Turner BC, & Phillips GL (2018). Bias From Potentially Mischievous Responders on Large-Scale Estimates of Lesbian, Gay, Bisexual, or Questioning (LGBQ)–Heterosexual Youth Health Disparities. American Journal of Public Health, 108(S4), S258–65. 10.2105/AJPH.2018.304407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss KS (2016, April 21). Maine’s Gov. LePage Vetoes Bill Giving Increased Access to Lifesaving Narcan. Boston Magazine. bostonmagazine.com/news/2016/04/21/maine-lepage-narcan-addicts/ [Google Scholar]
- Conrod PJ, & Nikolaou K. (2016). Annual Research Review: On the developmental neuropsychology of substance use disorders. Journal of Child Psychology and Psychiatry, 57(3), 371–394. 10.1111/jcpp.12516 [DOI] [PubMed] [Google Scholar]
- Davis CS, & Carr D. (2015). Legal changes to increase access to naloxone for opioid overdose reversal in the United States. Drug and Alcohol Dependence, 157, 112–120. 10.1016/j.drugalcdep.2015.10.013 [DOI] [PubMed] [Google Scholar]
- Doleac JL, & Mukherjee A. (2018). The Moral Hazard of Lifesaving Innovations: Naloxone Access, Opioid Abuse, and Crime. SSRN Electronic Journal. 10.2139/ssrn.3135264 [DOI] [Google Scholar]
- Dowell D, Noonan RK, & Houry D. (2017). Underlying Factors in Drug Overdose Deaths. JAMA, 318(23), 2295–2296. 10.1001/jama.2017.15971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfanian E, Grossman D, & Collins AR (2019). The Impact of Naloxone Access Laws on Opioid Overdose Deaths in the US. Review of Regional Studies. 10.52324/001c.7932 [DOI] [Google Scholar]
- Fletcher R, & Ward R. (2019, August 31). Alberta NDP call for firing after miister wonders is naloxone may be an “enabler” of opioid abuse. Canadian Broadcasting Corporation. https://www.cbc.ca/news/canada/calgary/jason-luan-naloxone-enabler-eddy-lang-disagrees-1.5266782#:~:text=Alberta’s%20associate%20minister%20of%20mental,the%20limits%20of%20opioid%20dosing [Google Scholar]
- Gaither JR, Shabanova V, & Leventhal JM (2018). US National Trends in Pediatric Deaths From Prescription and Illicit Opioids, 1999–2016. JAMA Network Open, 1(8), e186558. 10.1001/jamanetworkopen.2018.6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman-Bacon A. (2018). Difference-in-Differences with Variation in Treatment Timing (Working Paper No. 25018). National Bureau of Economic Research. 10.3386/w25018 [DOI] [Google Scholar]
- Green TC, Davis C, Xuan Z, Walley AY, & Bratberg J. (2020). Laws Mandating Coprescription of Naloxone and Their Impact on Naloxone Prescription in Five US States, 2014–2018. American Journal of Public Health, 110(6), 881–887. 10.2105/AJPH.2020.305620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP, Haegerich TM, Evans ME, Losby JL, Young R, & Jones CM (2019). Vital Signs: Pharmacy-Based Naloxone Dispensing — United States, 2012–2018. Morbidity and Mortality Weekly Report, 68(31), 679–686. 10.15585/mmwr.mm6831e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton L, Davis CS, Kravitz-Wirtz N, Ponicki W, & Cerdá M. (2021). Good Samaritan laws and overdose mortality in the United States in the fentanyl era. International Journal of Drug Policy, 97, 103294. 10.1016/j.drugpo.2021.103294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez DE, Singer MR, & Adesman A. (2019). Availability of Naloxone in Pharmacies and Knowledge of Pharmacy Staff Regarding Dispensing Naloxone to Younger Adolescents. Journal of Adolescent Health, 65(5), 698–701. 10.1016/j.jadohealth.2019.07.009 [DOI] [PubMed] [Google Scholar]
- Jones C. (2020). Prescription Opioid Misuse and Use of Alcohol and Other Substances Among High School Students—Youth Risk Behavior Survey, United States, 2019. MMWR Supplements, 69. 10.15585/mmwr.su6901a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Campbell A, Metz VE, & Comer SD (2017). No evidence of compensatory drug use risk behavior among heroin users after receiving take-home naloxone. Addictive Behaviors, 71, 104–106. 10.1016/j.addbeh.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia SN, & Bao Y. (2019). Prescription painkiller misuse and the perceived risk of harm from using heroin. Addictive Behaviors, 93, 141–145. 10.1016/j.addbeh.2019.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BC, & Vuolo M. (2022). Do naloxone access laws affect perceived risk of heroin use? Evidence from national US data. Addiction, 117(3), 666–676. 10.1111/add.15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambdin BH, Bluthenthal RN, Wenger LD, Wheeler E, Garner B, Lakosky P, & Kral AH (2020). Overdose Education and Naloxone Distribution Within Syringe Service Programs—United States, 2019. Morbidity and Mortality Weekly Report, 69(33), 1117–1121. 10.15585/mmwr.mm6933a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambdin BH, Davis CS, Wheeler E, Tueller S, & Kral AH (2018). Naloxone laws facilitate the establishment of overdose education and naloxone distribution programs in the United States. Drug and Alcohol Dependence, 188, 370–376. 10.1016/j.drugalcdep.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Lee B, Zhao W, Yang K-C, Ahn Y-Y, & Perry BL (2021). Systematic Evaluation of State Policy Interventions Targeting the US Opioid Epidemic, 2007–2018. JAMA Network Open, 4(2), e2036687. 10.1001/jamanetworkopen.2020.36687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari RN (2013). Trends in Adolescent Substance Use and Perception of Risk from Substance Use. In The CBHSQ Report. Substance Abuse and Mental Health Services Administration (US). http://www.ncbi.nlm.nih.gov/books/NBK385059/ [PubMed] [Google Scholar]
- Lipari RN, Williams MR, Copello EAP, Pemberton MR, & Porter JD (2017). Risk and Protective Factors and Estimates of Substance Use Initiation: Results from the 2016 National Survey on Drug Use and Health. In CBHSQ Data Review. Substance Abuse and Mental Health Services Administration (US). https://pubmed.ncbi.nlm.nih.gov/29431965/ [PubMed] [Google Scholar]
- Martins SS, Bruzelius E, Stingone JA, Wheeler-Martin K, Akbarnejad H, Mauro CM, Marziali ME, Samples H, Crystal S, Davis CS, Rudolph KE, Keyes KM, Hasin DS, & Cerdá M. (2021). Prescription Opioid Laws and Opioid Dispensing in US Counties: Identifying Salient Law Provisions With Machine Learning. Epidemiology, 32(6), 868–876. 10.1097/EDE.0000000000001404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan C, Lambdin BH, Ali MM, Mutter R, Davis CS, Wheeler E, Pemberton M, & Kral AH (2018). Opioid-overdose laws association with opioid use and overdose mortality. Addictive Behaviors, 86, 90–95. 10.1016/j.addbeh.2018.03.014 [DOI] [PubMed] [Google Scholar]
- McDonald R, & Strang J. (2016). Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction, 111(7), 1177–1187. 10.1111/add.13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawi AM, Ismail R, Ibrahim F, Hassan MR, Manaf MRA, Amit N, Ibrahim N, & Shafurdin NS (2021). Risk and protective factors of drug abuse among adolescents: A systematic review. BMC Public Health, 21, 1–15. 10.1186/s12889-021-11906-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razaghizad A, Windle SB, Filion KB, Gore G, Kudrina I, Paraskevopoulos E, Kimmelman J, Martel MO, & Eisenberg MJ (2021). The Effect of Overdose Education and Naloxone Distribution: An Umbrella Review of Systematic Reviews. American Journal of Public Health, 111(8), e1–e12. 10.2105/AJPH.2021.306306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter P, Caulkins JP, & Midgette G. (2021). Heroin use cannot be measured adequately with a general population survey. Addiction (Abingdon, England), 116(10), 2600–2609. 10.1111/add.15458 [DOI] [PubMed] [Google Scholar]
- Roth J, Sant’Anna PHC, Bilinski A, & Poe J. (2023). What’s Trending in Difference-in-Differences? A Synthesis of the Recent Econometrics Literature (arXiv:2201.01194). arXiv. http://arxiv.org/abs/2201.01194 [Google Scholar]
- Rudolph KE, Gimbrone C, Matthay EC, Diaz I, Davis CS, Pamplin JR, Keyes K, & Cerda M. (2021, May 6). When effects cannot be estimated: Redefining estimands to understand the effects of naloxone access laws. ArXiv.Org http://arxiv.org/abs/2105.02757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMSHA. (2012). Comparing and Evaluating Youth Substance Use Estimates from the National Survey on Drug Use and Health and Other Surveys. Substance Abuse and Mental Health Services Administration, CBHSQ Methodology Report. https://www.ncbi.nlm.nih.gov/books/NBK533887/ [PubMed] [Google Scholar]
- SAMSHA. (2014). Age of Substance Use Initiation Among Treatment Admissions Aged 18 to 30, The TEDS Report. https://www.samhsa.gov/data/sites/default/files/WebFiles_TEDS_SR142_AgeatInit_07-10-14/TEDS-SR142-AgeatInit-2014.htm [PubMed]
- Schuler MS, Heins SE, Smart R, Griffin BA, Powell D, Stuart EA, Pardo B, Smucker S, Patrick SW, Pacula RL, & Stein BD (2020). The state of the science in opioid policy research. Drug and Alcohol Dependence, 214, 108137. 10.1016/j.drugalcdep.2020.108137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Thawley R, Gee L, Bamberger J, Kral AH, Ciccarone D, Downing M, & Edlin BR (2005). Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: A pilot intervention study. Journal of Urban Health, 82(2). 10.1093/jurban/jti053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelye KQ (2016, July 27). Naloxone Saves Lives, but Is No Cure in Heroin Epidemic. The New York Times. https://www.nytimes.com/2016/07/28/us/naloxone-eases-pain-of-heroin-epidemic-but-not-without-consequences.html [Google Scholar]
- Smart R, Pardo B, & Davis CS (2021). Systematic review of the emerging literature on the effectiveness of naloxone access laws in the United States. Addiction, 116(1), 6–17. 10.1111/add.15163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2022). National Survey on Drug Use and Health 2020 NSDUH Detailed Tables. 7/13/2022. https://www.samhsa.gov/data/report/2020-nsduh-detailed-tables
- Tas B, Humphreys K, McDonald R, & Strang J. (2019). Should we worry that take-home naloxone availability may increase opioid use? Addiction, 114(10), 1723–1725. 10.1111/add.14637 [DOI] [PubMed] [Google Scholar]
- Tse WC, Djordjevic F, Borja V, Picco L, Lam T, Olsen A, Larney S, Dietze P, & Nielsen S. (2022). Does naloxone provision lead to increased substance use? A systematic review to assess if there is evidence of a ‘moral hazard’ associated with naloxone supply. International Journal of Drug Policy, 100, 103513. 10.1016/j.drugpo.2021.103513 [DOI] [PubMed] [Google Scholar]
- Underwood JM (2020). Overview and Methods for the Youth Risk Behavior Surveillance System—United States, 2019. MMWR Supplements, 69(1), 1–10. 10.15585/MMWR.SU6901A1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, & Ding P. (2017). Sensitivity Analysis in Observational Research: Introducing the E-Value. Annals of Internal Medicine, 167(4), 268–274. 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- Vergano D. (2018, July 23). A Drug Company Tried To Give Away An Overdose Antidote To Schools. But Most Of Them Declined The Offer. Buzzfeed.News https://www.buzzfeednews.com/article/danvergano/narcan-naloxone-schools [Google Scholar]
- Volkow ND, Han B, Einstein EB, & Compton WM (2021). Prevalence of Substance Use Disorders by Time Since First Substance Use Among Young People in the US. JAMA Pediatrics, 175(6), 640–643. 10.1001/jamapediatrics.2020.6981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KD, Valente TW, Casanova M, Partovi SM, Mendenhall BM, Hundley JH, Gonzalez M, & Unger JB (2010). Evaluation of an overdose prevention and response training programme for injection drug users in the Skid Row area of Los Angeles, CA. International Journal of Drug Policy, 21(3), 186–193. 10.1016/j.drugpo.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler E, Davidson PJ, Jones TS, & Irwin KS (2012). Community-Based Opioid Overdose Prevention Programs Providing Naloxone—United States, 2010. MMWR. Morbidity and Mortality Weekly Report, 61(6), 101–105. [PMC free article] [PubMed] [Google Scholar]
- Wheeler E, & Doe-Simkins M. (2020). Harm Reduction programs distribute one million doses of naloxone in 2019. https://medium.com/@ejwharmreduction/harm-reduction-programs-distribute-one-million-doses-of-naloxone-in-2019-4884d3535256
- Wong J. (2019, August 31). Alberta government reiterates support for naloxone kits after concerns it may enable some drug users. Global News. https://globalnews.ca/news/5840866/alberta-government-reiterates-support-for-naloxone-kits-after-concerns-it-may-enable-some-drug-users/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.