Abstract
At-risk alcohol use is a major contributor to the global health care burden and leads to preventable deaths and diseases including alcohol addiction, alcoholic liver disease, cardiovascular disease, diabetes, traumatic injuries, gastrointestinal diseases, cancers, and fetal alcohol syndrome. Excessive and frequent alcohol consumption has increasingly been linked to alcohol-associated tissue injury and pathophysiology, which have significant adverse effects on multiple organ systems. Extensive research in animal and in vitro models has elucidated the salient mechanisms involved in alcohol-induced tissue and organ injury. In some cases, these pathophysiological mechanisms are shared across organ systems. The major alcohol- and alcohol metabolite–mediated mechanisms include oxidative stress, inflammation and immunometabolic dysregulation, gut leak and dysbiosis, cell death, extracellular matrix remodeling, endoplasmic reticulum stress, mitochondrial dysfunction, and epigenomic modifications. These mechanisms are complex and interrelated, and determining the interplay among them will make it possible to identify how they synergistically or additively interact to cause alcohol-mediated multiorgan injury. In this article, we review the current understanding of pathophysiological mechanisms involved in alcohol-induced tissue injury.
Keywords: alcohol, metabolites, tissue injury, pathophysiological mechanisms, oxidative stress, epigenomics
INTRODUCTION
Worldwide, 5.3% of all deaths are attributable to alcohol use, and in the 20–39-year age group, 13.5% of total deaths are attributable to alcohol (1). In 2016, alcohol-associated deaths in the United States represented 9.8% of all deaths and the estimated alcohol-related costs were $249 billion, of which 77% is attributable to binge drinking. The major causes of alcohol-associated deaths are cardiovascular disease and diabetes (33.4%), traumatic injuries (17.1%), gastrointestinal diseases (16.2%), and cancers (12.5%) (2, 3) (Figure 1). Alcohol use disorder (AUD), defined as an impaired ability to stop or control alcohol use (6), leads to or exacerbates psychiatric diseases (e.g., drug use, depression, personality disorders, bipolar disorder), chronic somatic diseases [e.g., alcoholic liver disease (ALD), pancreatitis, cancers], and psychosocial problems (e.g., unintentional injuries, aggression, violence, suicide) and shortens life expectancy by more than a decade (2, 7). Compounding this list of morbidities are low treatment prevalence due to stigma and inadequate training of health care providers in identification and treatment of AUD (8, 9).
Figure 1.
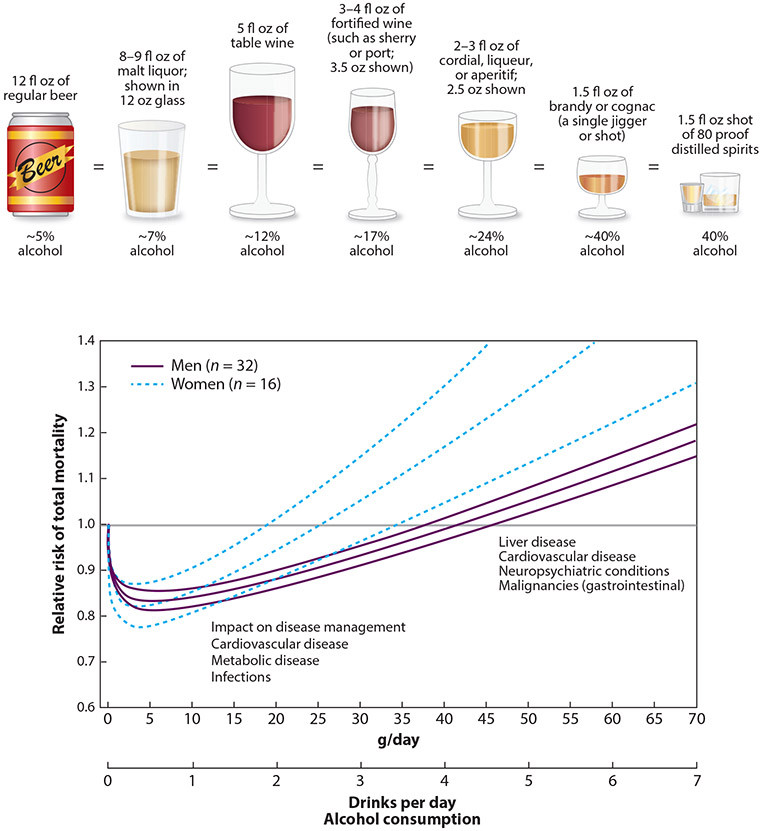
Number of alcoholic drinks consumed per day and alcohol content (grams per day) according to beverage type and relative risk of total mortality (99% confidence interval) in men and women in the United States, countries in Europe, and other countries (Australia, Japan, and/or China), extracted from adjusted curves. Lower alcohol consumption rates may not increase mortality risk but do affect management of cardiovascular, metabolic, and infectious disease (4). Standard alcohol drink contents and comparisons from the US National Institute on Alcohol Abuse and Alcoholism (4). Figure adapted with permission from Reference 5; copyright 2006, American Medical Association.
Historical Perspective
Evidence of consumption of alcoholic beverages dates to 7000–6600 bce, when humans settled in communities and organized agriculture. Alcohol production from plants is believed to have originated in the Fertile Crescent, an area between the Mediterranean Sea and the Persian Gulf. Migration, including to the Americas, dispersed the practice of alcohol consumption to various sections of society (10). Today, alcohol is widely used worldwide for ceremonial and recreational purposes because of its anxiolytic, mood-enhancing, and rewarding effects on the brain (11). A better understanding of the consequences of excess alcohol use has informed policy and public service messaging to decrease overall alcohol use. Cultural and religious beliefs have also influenced rates and patterns of consumption in different populations, as illustrated by Iain Gately (12) in Drink: A Cultural History of Alcohol, discussed by Saira Khaderi (10) in a historical account of alcohol use.
Drinking Patterns
Data from the World Health Organization (WHO) reveal a variety of patterns and types of alcoholic beverages consumed across populations and geographical regions. Individuals are categorized on the basis of drinking patterns into lifetime abstainers, former drinkers, abstainers in the past 12 months, consumers in the past 12 months, and consumers with heavy episodic drinking (60 g or more of alcohol on at least one occasion) in the past month (3). The Middle East and North Africa have the lowest rates of per capita alcohol consumption, and the highest percentages of heavy episodic drinking are reported from the Russian Federation and some European and sub-Saharan African countries (7). Luxembourg has the highest worldwide average per capita consumption (13 L/year; 1 L ≈ 998 g of alcohol)—approximately 40% higher than that reported for North America (8.5 L/year). Globally, spirits are the most common form of alcoholic drink consumed (44.8%), followed by beer (34.3%) and wine (11.7%) (3).
Efforts to guide consumers in gauging their alcohol consumption have led to the definition of drinking levels that are considered “at risk” or “unhealthy.” The National Institute on Alcohol Abuse and Alcoholism (NIAAA) defines low-risk drinking for women as no more than 3 drinks (Figure 1) on any single day and no more than 7 drinks per week (13). For men, low-risk drinking is defined as no more than 4 drinks on any single day and no more than 14 drinks per week. Binge drinking, defined as drinking that elevates blood alcohol concentration (BAC) to 0.08% (80 mg/dL) or higher, generally occurs following consumption of 4 or 5 drinks over a 2-h time frame. This level of alcohol intoxication is considered the legal limit for driving in the United States. Binge drinking as a pattern of alcohol consumption is considered unhealthy and is categorized as extreme when consumption is twofold or greater than the gender-specific binge drinking thresholds (i.e., 8 or more standard drinks for women, 10 or more standard drinks for men). The Substance Abuse and Mental Health Services Administration defines heavy drinking as binge drinking on 5 or more days in the past 30 days (13). Approximately 18% of the adult population worldwide report heavy episodic drinking in the past month; 6.6% of the US adult population report heavy alcohol use, and 26.2% report at least one episode of binge drinking (3).
Alcohol Use Across the Life Span
The pattern and pathophysiological consequences of alcohol use vary across the life span. Prenatal alcohol exposure can result in fetal alcohol syndrome (FAS), a cluster of birth defects including craniofacial abnormalities, growth restriction, and intellectual disabilities (14). Alcohol use begins at a young age, with 50–70% of both boys and girls having used alcohol before 15 years of age (3). Among adolescents and young adults in the United States, binge drinking is the most common form of excessive alcohol drinking (15, 16). Drinking at an earlier age and escalation from first drink to first intoxication are risk factors that predict a transition to alcohol dependence (15). Moreover, binge drinking during adolescence harms the developing brain; impairs cognitive function, motivational responsiveness, and reward processing of social and emotional stimuli; and increases the risk of affective disorders (17). Peak alcohol consumption is reported around 25 years of age, with most individuals decreasing alcohol intake thereafter. However, alcohol consumption prevails throughout adulthood, and more than 50% of adults older than 65 years report drinking in the past year (18).
Multimorbidity, polypharmacy, decreased alcohol metabolic rate, and age-associated physical and mental decline are among the factors that contribute to enhanced susceptibility of elderly adults to alcohol-related pathologies (19-21). Thus, alcohol use among healthy adults older than 65 is recommended to not exceed 3 drinks on a given day and 7 drinks in a week (22). Social interactions and environment strongly influence the amount and patterns of alcohol consumption (22, 23). Social isolation increases at-risk alcohol use in adolescents (24) and the elderly (25), and expectancies for social facilitation and social enhancement predict hazardous drinking (26, 27).
Excessive and frequent alcohol consumption have increasingly been linked to alcohol-associated tissue injury and pathophysiology, leading to significant adverse effects on multiple organ systems. The onset, severity, and prognosis of AUD-related problems vary among individuals and depend on several factors, including genetic makeup, metabolism, age, gender, ethnicity, environment, and lifestyle. At-risk alcohol use is linked to more than 60 acute and chronic diseases, with men having a higher incidence of alcohol-related health problems than women. Several factors can contribute to alcohol-associated diseases. For example, chronic and cumulative consumption can cause organ and tissue injury, acute intoxication can lead to injuries and poisoning, and dependent drinking can cause mental health problems (28, 29). Alcohol metabolism and generation of metabolites play a significant role in alcohol-associated pathophysiology, as discussed in the next section.
ALCOHOL METABOLISM AND METABOLITE GENERATION
BAC is determined by the amount of alcohol consumed, rate of alcohol oxidation, gastric food content, and rate of gastric emptying. The caloric content of alcohol is 7.2 kcal/g, but unlike calories from fat, proteins, and carbohydrates, alcohol calories are considered empty calories (i.e., calories lacking nutritional value) and contribute to long-term nutritional deficits in subjects with excessive alcohol consumption. Several factors contribute to alcohol-associated nutritional deficits, including maldigestion, malabsorption, early satiety, nausea, anorexia, and dysgeusia. Zinc is one critical micronutrient that is decreased due to alcohol use and associated with alcohol-induced organ injury. In addition, levels of other micronutrients (calcium, magnesium, and iron) and vitamins (A, C, D, E, K, and B) are compromised by excessive alcohol use (30).
Alcohol metabolism and elimination through various pathways generate toxic by-products that, in turn, can promote tissue and cell injury. Virtually all tissues can metabolize alcohol, but the liver is the main organ responsible for alcohol metabolism. A small fraction of ingested alcohol is metabolized in the stomach via first-pass metabolism (FPM), which determines the bioavailability of alcohol in the blood. In the fasted state, alcohol rapidly leaves the stomach and enters the duodenum, minimizing FPM and thereby resulting in higher BAC (21, 31). The main oxidative metabolic pathway of alcohol (92–95%) involves the enzymes alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), and the by-products thus formed are acetaldehyde and acetate. Alcohol conversion into acetaldehyde by ADH involves nicotinamide adenine dinucleotide (NAD+), an intermediate carrier of electrons, which is reduced to NADH. The decreased NAD+:NADH redox ratio and acetaldehyde formation reduce levels of mitochondrial glutathione (mGSH), diminishing the antioxidant reserve of the cell. The alcohol-induced decreased mGSH levels are due in part to impaired transport of glutathione (GSH) through the inner mitochondrial membrane and are associated with increased microviscosity and endoplasmic reticulum (ER) stress (32). Acetaldehyde, formed in this first oxidative step of alcohol metabolism, can react with various proteins [albumin, hemoglobin, red blood cell membrane proteins, cytochrome P450 2E1 (CYP2E1), and other serum proteins], resulting in acetaldehyde–protein adduct formation. These acetaldehyde products can contribute to tissue injury. Acetaldehyde is metabolized to acetate by ALDH2 in the mitochondria, further contributing to the decreased cellular NAD+:NADH ratio. Acetate is oxidized into carbon dioxide in tissues such as the brain, heart, and skeletal muscle and can be metabolized in the mitochondria to acetyl-CoA, a substrate for cholesterol and lipid biosynthesis (33).
In the liver and in extrahepatic tissues, alcohol can also be metabolized by the cytochrome P450–dependent ethanol-oxidizing system and by catalase. Alcohol metabolism by cytochrome P450s, including CYP2E1, produces reactive oxygen species (ROS) such as hydroxyl radicals, superoxide anions, and hydroxyethyl, increasing the risk of tissue damage. Alcohol metabolism to acetaldehyde by catalase, located in peroxisomes, occurs in the presence of hydrogen peroxide. Many of the adverse and toxic consequences of alcohol consumption are attributable to acetaldehyde metabolism, the generation of ROS (discussed in the section titled Major Alcohol-Mediated Pathophysiological Mechanisms Contributing to Organ Injury, below), and antioxidant depletion.
Alcohol metabolism varies considerably in terms of absorption, distribution, and elimination according to amount of ethanol ingested, fast/fed state, sex, drug interactions, and genetic variants. Independent of the amount a person drinks, the body can only metabolize the equivalent of 1 drink per hour. Therefore, people who drink large amounts of alcohol in a short period of time can have high BAC (300–450 mg/dL) and consequent central nervous system depression manifesting as lethargy, respiratory depression, coma, and even death (34). Women have lower gastric ADH activity, decreasing FPM of alcohol and leading to higher BAC (35). Moreover, the distribution of alcohol is directly proportional to body water content. Females have a greater percentage of body fat and less body water per kilogram of body weight compared with males. Because alcohol is water soluble, when administered the same amount per kilogram, females have a higher BAC because the volume of distribution is smaller than for males (36). In addition, some common drugs decrease gastric ADH activity, leading to higher BAC (37). Most importantly, multiple enzyme classes encoded by different genes of ADH and ALDH and their genetic variants are associated with drinking patterns, AUD risk, and alcohol dependence (38). ALDH and ADH isoforms are expressed differently among races, influencing alcohol elimination rates. The inactive ALDH2*2 allele is commonly found in people of Asian heritage, in whom even small amounts of alcohol lead to alcohol flush reactions due to a rapid increase in blood acetaldehyde levels (39). The presence of the ADH1B*2 allele, found in East Asians, is known to be protective against development of AUD (40, 41). Another ADH allele, ADH1B*3, found primarily among some people of African descent and in low frequency among Native Americans, is associated with reduced drinking behavior and decreased likelihood of alcohol dependence. Although in vitro studies show that alcohol oxidation increases in the presence of ADH1B*3, research on alcohol metabolism in African Americans is limited, and no studies have investigated the protective mechanism of this allele in this population (42).
Alcohol can also be metabolized, albeit minimally, via nonoxidative pathways, resulting in enzymatic conjugation of ethanol to endogenous metabolites (fatty acids, phospholipids, sulfate, and glucuronic acid). This process leads to the generation of other metabolites [fatty acid ethyl esters (FAEEs), phosphatidylethanol (PEth), ethyl sulfate, and ethyl glucuronide]. The generation of nonoxidative ethanol metabolites can interfere with cell signaling pathways, impacting organs with restricted oxidative capacity. FAEEs are generated by a reaction catalyzed by the enzyme FAEE synthase, present especially in the brain, heart, liver, and pancreas (43). Formation of fatty acid–activating enzymes leads to lysosome destabilization, cell proliferation inhibition, mitochondrial depolarization, and apoptosis. Infusion of FAEE elicits pancreatic damage such as alcoholic pancreatitis, and inhibition of FAEE ameliorates ethanol-induced pancreatitis. Additionally, nonoxidative fatty acid metabolites increase Ca2+, mediating ethanol-induced toxicity of pancreatic acinar cells (44). PEth is produced by breakdown of phospholipids by the action of phospholipase D. PEth formed upon alcohol exposure in red blood cell membranes can be detected for an extended period of time (half-life, 1–13 days) because red blood cells lack the enzyme that degrades PEth (45). Thus, PEth is used as a biomarker of recent alcohol use.
Compelling evidence identifies alcohol metabolism and metabolite generation as a critical cause of alcohol-associated tissue and organ damage, predominantly via ROS generation and oxidative stress. The contribution of ROS generation and oxidative stress to tissue injury in various target organs is discussed in the following subsections. The liver is significantly affected by alcohol metabolism and its toxic effects. Investigation of mechanisms involved in ALD has provided a strong foundation for the understanding of the pathophysiological mechanisms involved in extrahepatic organ injury. Next, we provide a brief overview of the salient pathophysiology underlying ALD and subsequently expand on specific mechanisms identified to contribute to alcohol-induced tissue injury.
Alcoholic Liver Disease
ALD is a spectrum of diseases including fatty liver; alcoholic steatohepatitis; alcoholic hepatitis; and cirrhosis and its complications, including hepatocellular carcinoma (HCC). The global burden due to ALD, in terms of both deaths and years of life lost, is among the top 30 causes of mortality. Worldwide, the rate of alcohol-related cirrhosis deaths is 7.2 per 100,000 people, and 45% of all ALD-related deaths occur in five countries (India, United States, Mexico, China, and Russia). It is estimated that approximately one-third of subjects with at-risk alcohol use progress to alcoholic steatohepatitis, up to 20% develop cirrhosis, and ~2% of people with cirrhosis develop HCC (46, 47). Several factors contribute to the high disease burden of ALD, including sex (women are more sensitive); drinking patterns; and existing comorbidities, including smoking, obesity, and underlying liver diseases (e.g., hepatitis B and C, nonalcoholic steatohepatitis) (46-49).
One of the earliest pathologies of ALD is fatty liver disease (steatosis), which is the accumulation of lipids, mainly triglycerides, phospholipids, and cholesterol esters. Lipid droplets, appearing initially in perivenular hepatocytes, progressively appear in midlobular and periportal hepatocytes. Some of the mechanisms that lead to fatty liver are a reduced hepatocyte NAD+:NADH ratio and an associated decrease in mitochondrial β-oxidation, stimulation of lipogenic genes, increased acetyl-CoA carboxylase, and increased fat mobilization from adipose tissue and intestines. Steatosis can progress to hepatic inflammation, often referred to as alcoholic steatohepatitis. Alcoholic steatohepatitis is triggered by gut-derived pathogen-associated molecular patterns (PAMPs) that stimulate release of inflammatory cytokines from resident macrophages or Kupffer cells and infiltrating macrophages, release of damage-associated molecular patterns (DAMPs) from dying hepatocytes, and increased adaptive immune responses (48). Severe alcoholic steatohepatitis may develop into what is clinically defined as alcoholic hepatitis, characterized by rapid onset of jaundice (bilirubin >3 mg/dL) with high levels of aspartate aminotransferase (>50 IU/mL) and a ratio of aspartate aminotransferase to alanine aminotransferase of >1.5. Continuing inflammation and hepatic injury can eventually cause fibrosis, which is a wound-healing response resulting from the accumulation of extracellular matrix (ECM) proteins. Quiescent hepatic stellate cells (HSCs) are activated and are the principal source for increased and irregular ECM deposition. HSCs also contribute to inflammatory responses by recruiting leukocytes that not only attack hepatocytes but also activate adjacent quiescent HSCs. Portal fibroblasts and bone marrow–derived myofibroblasts also contribute to the fibrogenic response. Fibrosis is thought to initiate in the perivenular area, and continued fibrosis leads to scar tissue formation that replaces liver parenchyma. This process ultimately leads to cirrhosis, which pathologically is constituted by micro- and macronodules surrounded by fibrous septa. In some cases, individuals may present with acute alcoholic hepatitis in the presence of cirrhosis, which is often described as acute-on-chronic disease (48-51).
MAJOR ALCOHOL-MEDIATED PATHOPHYSIOLOGICAL MECHANISMS CONTRIBUTING TO ORGAN INJURY
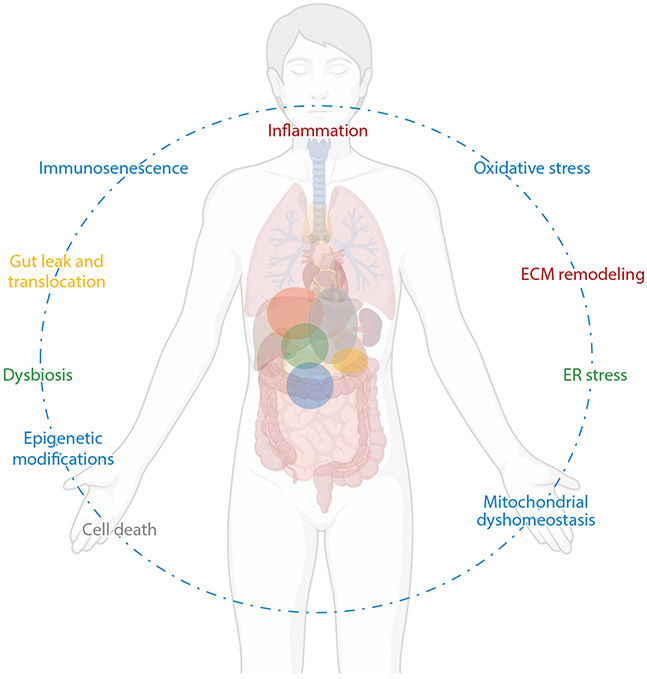
At-risk alcohol use has pathophysiological consequences for most organ systems, including the liver (46, 49), heart (52), muscle (53), gastrointestinal tract (54, 55), pancreas (44, 56), endocrine system (57), and immune system (58, 59). Several mechanisms are implicated in alcohol-induced multiorgan injury (Figure 2). In this section, we focus on some of the major alcohol-induced mechanisms and recent advances that will help inform how these mechanisms interact and lead to tissue dysfunction.
Figure 2.
Principal pathophysiological mechanisms underlying alcohol-induced tissue injury. The dotted line reflects the interconnectedness of the pathophysiological mechanisms of alcohol-induced tissue injury. The colored spheres within the body reflect the overlapping aspects of the pathophysiological mechanisms identified in alcohol-induced tissue injury. Abbreviations: ECM; extracellular matrix, ER; endoplasmic reticulum. Figure adapted from images created with BioRender.com.
Oxidative Stress
As discussed above, alcohol metabolism increases ROS generation, especially in the liver, and decreases antioxidant capacity, leading to oxidative stress (21). ROS molecules can react with most cellular macromolecules (DNA, protein, lipids), interfering with their physiological functions (60). The pathways that increase ROS are alcohol metabolism caused by ADH and ALDH changing the cells’ redox state, hypoxia associated with alcohol metabolism, activation of CYP2E1 metabolism at higher alcohol concentrations, acetaldehyde formation, depletion of antioxidants (cytosolic GSH and mGSH), mitochondrial damage, and activation of Kupffer cells (31). Oxidation of alcohol into acetaldehyde in the cytosol and acetaldehyde into acetate in the mitochondria results in a decreased NAD+:NADH redox ratio and increased NADH availability to the electron transport chain. The electrons are transferred to oxygen in the mitochondrial electron transport chain, oxygen is reduced to water, and a small fraction of the reduced oxygen is released as superoxide anions. Moreover, an imbalance in mitochondrial electron transport activity due to decreased oxygen supply or ATP availability affects alcohol and acetaldehyde metabolism, generating harmful ROS. The decreased alcohol-induced NAD+:NADH redox ratio has profound effects on liver metabolic pathways, affecting glycolysis, fatty acid oxidation, gluconeogenesis, pyruvate dehydrogenase, and the citric acid cycle as well as promoting a highly reduced cytosolic environment that, in turn, increases susceptibility to damage by alcohol metabolites (21).
ROS promote cell toxicity directly, via lipid peroxidation and adduct protein generation, and serve as a redox signal for proinflammatory cytokines (61). Alcohol-induced hepatic ROS play an important role in activation of transcription factors including heat shock factor, a molecular chaperone controlling activity of various kinases, and SREBP-1c/2, which together increase lipid accumulation, upregulate inflammatory promoters [nuclear factor κB, signal transducer and activator of transcription 3, peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α), and hypoxia-inducible factor 1α], activate intracellular kinases, and upregulate receptors on innate immune cells, all of which can lead to inflammation and pathogenesis of ALD (61).
The CYP2E1 microsome-mediated alcohol oxidative pathway generates ROS and highly reactive free radicals, such as hydroxyl radicals and superoxide anions, which promote oxidative stress and cell death (62). CYP2E1 is found in extrahepatic tissues, including the heart, lungs, and brain, as well as in some immune cells (macrophages and neutrophils). Alcohol metabolism through this pathway contributes to tissue injury. CYP2E1 inhibition improves alcohol-mediated cardiac dysfunction, reflected by improved cardiac output, contractility, and intracellular Ca2+ handling (63). In the brain, CYP2E1 is the major alcohol-metabolizing enzyme, with a critical role in lipid peroxidation, blood–brain barrier disruption, and mitochondrial dysfunction. In human astrocytes and monocyte cell lines, CYP2E1 is responsible for alcohol-mediated oxidative stress and apoptosis (64). CYP2E1-derived ROS could be critical in the development of FAS, as CYP2E1 is expressed in the placenta, fetal liver, and fetal brain (65). Lastly, the main oxidative stress protective system in the mitochondria utilizes GSH, a critical cellular antioxidant, and scavenges reactive nitrogen species (RNS) such as peroxynitrite. Alcohol depletes GSH levels and inhibits the GSH mitochondrial transporter, increasing the vulnerability of the cell to ROS (62).
Oxidative stress is considered one of the major pathophysiological mechanisms contributing to pathogenesis of diseases including ALD. Oxidative stress triggers cell damage by altering proteins, lipids, and DNA content, thereby affecting protein expression, gene transcription, cell apoptosis, and immune cell activation. Additionally, ALD-induced systemic oxidative stress can be detrimental to extrahepatic organs such as the kidneys and brain. Prospective studies using antioxidant therapies or drugs that increase intrinsic antioxidant production may further demonstrate the importance of ameliorating oxidative injury in alcohol-associated comorbidities (66).
Alcohol metabolism is associated with alterations in cell redox homeostasis, leading to several metabolic disturbances. Most biological structures can be modified by ROS, and increased ROS can affect cells by damaging biomolecules and organelle structure and promoting a sustained inflammatory response, which is known to be the mechanism underlying the pathogenesis of numerous alcohol-related diseases.
Alterations in the Gut Microbiome (Dysbiosis)
The gut microbiome plays a critical role in endocrine and neurological functions. It preserves barrier integrity, limiting pathogen dissemination; promotes maturation; maintains and stimulates host defense; contributes to amino acid and vitamin synthesis; and is involved in toxin and drug metabolism and macromolecule catabolism. Moreover, through generation of metabolites, it contributes to immunological changes in the gut mucosa and beyond (67, 68). Alcohol consumption can directly change the gut microbiome composition qualitatively, producing dysbiosis, mainly in the upper small intestine. In addition, quantitatively, alcohol promotes gut bacteria overgrowth, decreases α- and β-diversity and abundance of lactobacilli, and alters levels of Bacteroidetes in humans and rodents (69-71). Among the most-studied consequences of alcohol-induced gut dysbiosis is the disruption of gut barrier integrity, which causes increased permeability to bacteria and toxin translocation into the systemic circulation that, in turn, disrupt organ and tissue physiology. Additionally, alcohol-mediated bacterial overgrowth may contribute to increased alcohol metabolism and generation of high concentrations of acetaldehyde in the intestinal lumen, which in turn can impair intestinal intercellular junctions by decreasing tight and adherens junction protein expression and promoting gut permeability (72). Studies have shown that alcohol-mediated changes in gut microbiota function and composition affect other organ systems. For example, alcohol affects bacterial biotransformation, hepatic bile production, and bile acid physiology, contributing to liver injury. Alcohol-fed, germ-free (no gut microbiota) mice receiving gut microbiota from alcoholic hepatitis subjects develop liver inflammation and necrosis (73). In addition, germ-free mice are protected from liver injury compared with conventional mice receiving equal amounts of alcohol (74). Recent studies using different approaches, including germ-free animal models, antibiotics, and fecal microbiota transplantation (FMT), have provided evidence for central nervous system alterations resulting from alcohol-induced dysbiosis. Alcohol-induced gut dysbiosis is associated with depression, emotional behavior, altered memory, and sleep changes arising from changes in neurotransmitters produced by gut microbes (75). Behavior changes have been linked to specific microbial species. For example, Bifidobacterium and Lactobacillus species can alleviate depressive-like and anxiety symptoms, and Lactobacillus species improve social interactions in stressed mice (76). Advances in behavior and gut microbiome research have shown that the effects of the microbiota on the brain are the result of multiple mechanisms, including the production of neuroactive chemicals in the gut that can trigger the vagus nerve and travel directly to the brain via blood and the lymphatic system or by modulation of the immune system. The relative relevance of these routes of gut–brain interaction and how they communicate remain unclear. A study using FMT from alcohol in animals showed increased susceptibility to Klebsiella pneumonia infection in naïve animals, suggesting that alcohol-induced dysbiosis is an underlying mechanism in impaired host defense against pulmonary bacterial infections (77).
The composition of intestinal bacteria influences the type and number of bacterial metabolites produced. Subjects with a history of alcohol use have lower levels of fecal short-chain fatty acids. Short-chain fatty acids promote barrier integrity, suggesting that this may be an additional mechanism by which alcohol consumption impairs intestinal permeability (70). Manipulation of gut microbiota to restore eubiosis through diet; probiotics, prebiotics, and symbiotics; antibiotics; and FMT (70) improve liver metabolism and decrease gut dysbiosis, intestinal permeability, and endotoxemia in alcohol-treated animals (78). With the potential to modulate the gut microbiota and improve homeostasis, FMT has been investigated in clinical trials for several stages of liver disease. These interventions utilize FMT from a healthy donor to modify the gut microbiome of subjects in various stages of liver disease and alleviate gut dysbiosis and immune dysfunction (see https://clinicaltrials.gov/ct2/show/NCT02862249, https://clinicaltrials.gov/ct2/show/NCT02400216, and https://clinicaltrials.gov/ct2/show/NCT02496390). In fact, a pilot study has shown promising results of FMT in subjects with hepatic encephalopathy (79).
While we have gained some insight into alcohol’s effects on gut microbial communities and their interaction with the host, we need a better understanding of alcohol-induced changes in the intestinal microbiome, metabolome, and host response and their interaction with other organs in the spectrum of AUD. Furthermore, microbiome and metabolome screening in subjects with alcohol-related diseases, such as ALD, could be a useful diagnostic approach and a potential way for microbiome manipulation to rebuild microbial balance. Comprehending how alcohol affects the routes of bacterial translocation/activation (blood or lymphatic system, vagal stimulation, endocrine and immune system activation) and how they interact with one another will provide insights into alcohol-induced gut microbiome dyshomeostasis.
Inflammation and Immunometabolism
Among the oldest recognized pathophysiological consequences of at-risk alcohol use are impaired host immune defense mechanisms. Alcohol decreases host immune response to infections and increases morbidity and mortality from viral and bacterial infections, including pneumonia (80). Alcohol-induced immunomodulation is dose and time dependent, and the contrasting effects of chronic, moderate, and acute alcohol consumption can be attributed to transcriptional and post-transcriptional changes in circulating immune cells, which, in turn, affect inflammatory responses to microbial products. Moderate, low-dose alcohol consumption promotes an anti-inflammatory milieu and improves cardiovascular health. In contrast, chronic heavy alcohol consumption is associated with a proinflammatory milieu, innate immune cell activation, decreased T cell numbers, and increased CD8+ T cell activation and proliferation (80). Chronic alcohol exposure shifts the immune cell secretome to a proinflammatory phenotype, resulting in inflammation associated with high proinflammatory and low anti-inflammatory cytokine levels in several organs and tissues, including plasma, liver, adipose tissue, muscle, lungs, and brain (81). Indeed, inflammation is a major contributor to several alcohol-related comorbidities, including ALD and pancreatitis (58). An additional mechanism of alcohol-induced modulation of immune function is through alterations in the gut microbiome, disruption of gut barrier integrity, and translocation of bacterial products and metabolites into the systemic circulation. Gut luminal products, including lipopolysaccharide (LPS), can contribute to inflammation and organ damage, particularly in the liver (80). Alcohol metabolism and ROS generation sensitize hepatocytes and Kupffer cells to proinflammatory cytokines. Moreover, ROS sensitize macrophages to LPS, leading to increased tumor necrosis factor α (TNF-α), and interacts with Toll-like receptor 4 (TLR4) to activate downstream kinases and transcription factors, thereby contributing to inflammatory responses in the liver (61). Altogether, alcohol-induced immune dyshomeostasis and inflammation impair the body’s defense against a range of pathogens, enhancing susceptibility to viral and bacterial infections. Alterations in immune function have recently begun to be studied in the context of alterations in cellular energetics resulting from alcohol exposure.
In 1958, Otto Warburg and colleagues (82) reported that increased cellular glycolysis is a hallmark of leukocyte activation, and many decades later immunologists recognized metabolic adaptations as crucial steps for immune responses, establishing the emerging field of immunometabolism (83). The interrelationship between cellular metabolism and immune responses has begun to unravel the metabolic needs of immune cells and the possible impact of immune cell activation on whole-body metabolism (83). Alcohol affects whole-body energy homeostasis by impairing glucose metabolism in liver, muscle, and adipose tissue; dysregulating lipid metabolism and adipokine secretion; and promoting insulin resistance (53, 84, 85). The effect of alcohol on the immune system and energy metabolism is dependent on dose and drinking pattern. Moderate drinking is associated with enhanced insulin sensitivity and decreased inflammation, and binge and heavy drinking are associated with metabolic impairments, including inflammation and insulin resistance, and increased risk for microbial infections (85, 86). Several reports link alcohol-induced immune cell dysfunction with metabolic dysregulation in adipose tissue, liver, pancreas, brain, and muscle. Alcohol dysregulates adipose tissue metabolism and the adipose secretome and can induce adipocyte death. Studies have demonstrated that alcohol decreases circulating adiponectin levels and increases immune cell recruitment and inflammation in mesenteric adipose tissue, impairing insulin signaling and decreasing adipose tissue glucose uptake; these findings support the concept of alcohol-induced immunometabolic dysregulation (85, 87). Moreover, other studies have shown that alcohol increases lipolysis, adipocyte hypotrophy, and fatty acid efflux, leading to lipid deposition in the liver that contributes to hepatic steatosis (88). Although some advances in understanding alcohol interaction with immunometabolism have been made, much remains to be discovered. Because immune cell responses are highly energy dependent, understanding how alcohol affects energy utilization by immune cells, their increased metabolic demands when activated, and the consequences of impaired energy metabolism of immune cells will provide insight into alcohol-induced host defense disturbances, including excessive inflammation and impaired response to infections.
Mitochondrial Homeostasis
Mitochondrial dysfunction contributes to alcohol-mediated pathophysiology in mitochondriarich tissues (52, 89-92). Mitochondria not only are critical for meeting energy requirements but also are involved in the regulation of redox homeostasis and integration of cell death signaling, and mitochondrial remodeling and biogenesis are adaptive mechanisms of cells to stress and metabolic changes. Whether alcohol-mediated mitochondrial changes are reflective of mitochondrial damage or an adaptive response to alcohol-induced stress is still unclear. Chronic alcohol use results in enlarged morphology and degeneration of inner mitochondrial membrane folds; increases mitochondrial fragmentation and mitochondrial DNA damage; and decreases mitochondrial numbers, ATP levels, mitochondrial protein synthesis, and mitochondrial membrane potential. Alcohol-induced increased ROS/RNS production leads to modifications of the mitochondrial genome and proteome, significantly contributing to mitochondrial structural and functional changes (93). Chronic alcohol use increases cell death in the liver and heart (52, 94). In skeletal muscle, alcohol dysregulates expression of genes involved in bioenergetics and mitochondrial function (92, 95) and reduces maximal oxygen consumption rate in myoblasts and succinate dehydrogenase activity in muscle (96). Mitochondrial dysfunction has also been reported in the brain following adolescent and adult alcohol exposure, with alcohol decreasing expression of the mitochondrial respiration complexes, increasing mitochondrial calcium levels, and decreasing ATP production (90). Alcohol decreases hepatocyte and alveolar mitochondrial maximal oxygen consumption (97, 98) but increases PGC-1-mediated mitochondrial biogenesis (89). In contrast, alcohol decreases PGC-1, uncoupling protein 2, and estrogen-related receptor α in the heart and skeletal muscle (52, 92). These adaptive mechanisms and mitochondrial plasticity suggest that there is a balance between alcohol-induced mitochondrial damage and repair/biogenesis that depends on genetics and pattern of alcohol use. In the short term, mitochondrial remodeling by alcohol is an adaptive response, but when prolonged, it may lead to mitochondrial damage and tissue injury.
Cell Death Pathways
A major consequence of alcohol-induced oxidative stress and impaired immune responses is the dysregulation of cell death/survival pathways, including apoptosis, necrosis, necroptosis, pyroptosis, ferroptosis, and autophagy, which leads to the observed pathophysiology and multiorgan injury (99). Here, we briefly discuss advances in our understanding of alcohol’s effects on programmed cell death pathways.
Apoptosis is triggered by intrinsic mitochondrial and extrinsic death receptor pathways and is characterized by nuclear fragmentation, chromatin condensation, and cellular shrinkage. Alcohol triggers activation of apoptotic pathways in multiple cell types, contributing to pancreatic β cell dysfunction (100) and cardiotoxicity (101); disrupting placentation; and contributing to intrauterine growth restriction in FAS (102), brain pathology (103), and—most significantly—ALD (99, 104). The primary alcohol-mediated mechanism that activates the intrinsic mitochondrial pathway results from alcohol metabolism and generation of ROS. Other causes are mitochondrial DNA and ribosomal damage, reduced mitochondrial protein and ATP synthesis, increased sensitivity to Ca2+-mediated mitochondrial permeability transition, and release of apoptotic factors including cytochrome c and Smac/DIABLO (second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low isoelectric point) (104). Conversely, alcohol activates the extrinsic apoptotic pathway by increasing PAMPs and endogenous DAMPs and by activating the TNF-α and Fas ligand pathways (99).
Necrosis is unregulated cell death characterized by cell swelling, membrane rupture, and release of cell contents, leading to an inflammatory response. Necroptosis is programmed necrosis involving the receptor-interacting protein (RIP)1–RIP3–MLKL (mixed-lineage kinase domain–like protein) cascade (99, 104). Activated RIP3 recruits and phosphorylates MLKL to promote its oligomerization and translocation to the plasma membrane, leading to membrane rupture and necrosis (104). In contrast, RIP1, RIP3, FADD (Fas-associated protein with death domain), caspase-8, and c-FLIP [cellular FLICE (FADD-like interleukin-1β-converting enzyme)-inhibitory protein] form a large complex known as the ripoptosome (also called complex IIb), which activates caspase-8 to trigger apoptosis. Thus, RIP1 and RIP3 are involved in regulating both apoptosis and necroptosis. Studies have demonstrated that RIP3-mediated necroptosis contributes to alcohol-induced liver injury in preclinical models and in ALD subjects (105). Furthermore, alcohol-induced necroptosis is implicated as a cell death pathway in pancreatic cells and in the brain (106, 107). It is possible that different cell death pathways occur depending on the tissue and stage of disease. For example, programmed cell death may occur in early stages of ALD, while necrosis may occur during the later stages.
Additional cell death pathways activated by alcohol include pyroptosis and ferroptosis. Pyroptosis is programmed cell death through activation of caspase-1 by the inflammasome complex; it is associated with cell swelling and rapid plasma membrane lysis (108, 109). Ferroptosis is regulated necrosis that is dependent on intracellular iron levels resulting from loss of activity of the lipid repair enzyme, glutathione peroxidase 4, iron overload, and increased oxidative stress (104).
Autophagy is a cell survival mechanism regulated by the serine/threonine protein kinase PINK1/Parkin (PTEN-induced kinase 1/Parkinson protein 2, E3 ubiquitin protein ligase) pathway. It is characterized by the formation of double-membrane autophagosomes that fuse with lysosomes to form autolysosomes, where the autophagic cargo is degraded (104). Alcohol-mediated activation of autophagy removes damaged mitochondria (mitophagy) and lipid droplets (lipophagy) and acts as a protective mechanism in ALD (110). However, when autophagic mechanisms are dysregulated, injury can result. Dysregulation of autophagy in cortical microvessels is implicated in defective angiogenesis in FAS (111). It increases apoptosis; decreases mitochondrial respiration in the brain, liver, and skeletal muscle (112); and contributes to alcoholic pancreatitis. Preclinical models of alcoholic pancreatitis demonstrate accumulation of autophagy vacuoles and positive LC3 puncta staining in pancreatic acinar cells and transcription factor EB–mediated lysosomal biogenesis, resulting in insufficient autophagy. Alcohol also impairs autophagic flux mediated through the AMP-activated protein kinase signaling pathway, suggesting that lysosomal biogenesis and autophagy play critical roles in promoting the pathogenesis of alcoholic pancreatitis (56).
Thus, the fate of a cell following alcohol exposure is dependent on a complex regulatory network and the balance of autophagy, apoptosis, necroptosis, and necrosis. The literature indicates that alcohol activates cell death pathways, and understanding the cellular and molecular underpinnings provides critical knowledge of how these specific mechanisms synergistically or additively interact to cause tissue injury.
Endoplasmic Reticulum Stress
Being highly reactive, alcohol and its metabolites allow for increased formation of protein adducts, misfolded protein accumulation, and induction of ER stress. Alcohol-induced increased ER stress is observed in major organs including the liver (113), pancreas (114), lungs (115), and heart (113). The ER regulates processing and trafficking of secretory and membrane proteins and degrades misfolded proteins by autophagy and the ubiquitin–proteasome system. This process leads to activation of the three ER stress/unfolded protein response (UPR) transducers, namely inositol-requiring enzyme 1α (IRE-1α), activating transcription factor 6, and PERK [protein kinase R–like ER-localized eukaryotic initiation factor 2α (eIF2α) kinase] (115). With alcohol use, the ER is in an oxidative environment, increasing expression of IRE-1α and X-box binding protein 1, which leads to dysregulated UPR. The increased ER stress triggers cell death by activating caspases, inducing Janus kinase activation, or through C/EBP homologous protein–mediated transcription of proapoptotic cell death genes (99). ER stress is a major alcohol-mediated mechanism of injury in the liver and pancreas, as liver and pancreatic cells have high secretory and membrane protein synthesis demands (56, 114).
As discussed above, alcohol adversely affects multiple cell organelles, such as mitochondria, ER, lysosomes, and nucleus. Efforts to understand the interplay among all alcohol-induced organelle dyshomeostases will make it possible to identify the subcellular mechanisms leading to tissue injury.
Extracellular Matrix Remodeling
Alcohol dysregulates matrisome proteins in multiple organs (52, 116-118), favoring a profibrotic phenotype predisposing to fibrosis. Several studies demonstrate that ECM remodeling is one of the major mechanisms underlying the development of ALD (48, 116, 119). The ECM provides structure and support, acts as a reservoir for growth factors and cytokines, and helps cells communicate with their environment. Alcohol-induced changes in integrins mediate inflammation by interaction of neutrophils and the ECM protein osteopontin, activating HSCs (120, 121). Alcohol increases plasminogen activator 1 (PAI-1) expression and thus impairs fibrinolysis (116). It also increases cellular fibronectin by both increasing its production and decreasing clearance (120). Together, these mechanisms contribute to increased liver fibrosis. There is also compelling evidence that these alcohol-induced mechanisms occur in other organs, like the lung. For example, alcohol-mediated oxidative stress induces transforming growth factor β1 (TGF-β1) and produces significant fibrosis in bleomycin-treated mice (118). Moreover, TGF-β can induce apoptosis via a TNF-α-related pathway by activating the transcription factor activator protein 1 and Smad signaling. Among other proteins, Smads target genes that include ECM-related molecules such as PAI-1, urokinase plasminogen activator, and collagen (122). Studies have shown that alcohol induces ECM remodeling and increased collagen expression in skeletal muscle (95, 123) and adipose tissue (84). The increased profibrotic milieu is associated with decreased differentiation of myoblasts (124) and adipose-derived stem cells (84) that potentially affects the metabolic and regenerative capacity of these tissues. In the brain, interstitial ECM and perineuronal nets are required for stabilization of synapses, regulation of extracellular ion concentrations, and control of neurotransmitter receptor diffusion in the plasma membrane (125). Studies using both in vivo and in vitro preclinical models as well as postmortem samples of people with AUD have shown that alcohol dysregulates ECM proteins including laminins, collagen, PAI-1, and tissue plasminogen activator, contributing to neurodegeneration (125-128). Moreover, alcohol increases Wisteria floribunda agglutinin reactivity, a marker for the presence of perineuronal nets that is associated with increased synaptic plasticity and alcohol consumption (129, 130).
Thus, the dysregulation of ECM plays a significant role in alcohol-induced multiorgan injury, affects alcohol-induced behaviors, and—as discussed in the next section—influences stem cell function and tissue regeneration. Most ECM-related studies of alcohol-related organ injury have focused on collagen expression and fibrosis; however, there is emerging evidence of alterations in nonfibrillar matrisome proteins that influence ECM remodeling and cell adhesion and signaling, and allow the ECM to act as a reservoir for inflammatory mediators. Omics investigations, studies of the interactions among intracellular transduction signaling pathways, and functional approaches of the dynamic matrisome will allow identification of the target proteins, and their functional interactions, that lead to alcohol-associated tissue injury (125, 131).
Tissue Regeneration and Proliferation
Tissue regeneration and repopulation by stem cells are essential for the maintenance of normal structure and physiology of organs, and alcohol perturbs these complex regenerative mechanisms (132-134). In adult tissues, stem cells are in a quiescent state, reside in specialized niches, are activated, self-renew, and differentiate into specific lineages in response to stimulus or injury. The ECM niche provides physical, structural, and biochemical support, and it is the dynamic cross talk between stem cells and the ECM that decides the fate of stem cells. Investigators have proposed that alcohol, its metabolites, and alcohol-induced ROS dysregulate signal transduction, induce transcriptional and epigenomic changes, and cause DNA damage that adversely affects stem cell self-renewal, differentiation, and regeneration (133). In partial hepatectomy models, the best experimental system for the study of liver regeneration, transcriptomic profiling indicates that chronic alcohol shifts HSCs from a proregenerative to an antiregenerative phenotype (135) and delays the peak of liver regenerative activity (136). Additionally, alcohol decreases the proliferation and differentiation of hepatic stem cells and changes the lineage to induce a mesenchymal transition (133). Chronic alcohol–mediated gut-derived proinflammatory signals and immune cell infiltration activate HSCs and portal fibroblasts, leading to progressive fibrosis that contributes significantly to decreased liver regenerative capacity (136, 137). Alcohol treatment both in vivo and in vitro decreases the differentiation potential of myoblasts into myotubes, which is required for the maintenance and repair of skeletal muscle. In a rodent model of hind-limb immobilization, chronic alcohol decreased skeletal muscle expression of myogenic genes and impaired regeneration (138). Alcohol-mediated epigenomic mechanisms and impaired metabolic function could mechanistically contribute to this observed decrease in regenerative capacity (53, 124, 138-141). Alcohol also decreases differentiation of primary adipose and hematopoietic stem cells (84, 142) and decreases neural stem cell proliferation, which is mechanistically mediated by impaired mammalian target of rapamycin and brain-derived neurotrophic factor signaling (133). In contrast, alcohol stimulates proliferation in the highly proliferative gastrointestinal tract. Chronic alcohol use increases proliferation of esophageal, small intestinal (143), colonic (144), and rectal (145) crypt cells, and these hyperproliferative effects can contribute to alcohol-mediated carcinogenicity (145, 146). Thus, compelling evidence demonstrates that alcohol consumption can decrease stem cell renewal, differentiation, and regenerative capacity. These effects add to the complex mechanisms underlying the ability of alcohol not only to affect adult tissue regeneration but also to play a critical role in reprogramming fetal tissue development due to prenatal alcohol exposure. Alcohol-mediated effects that contribute to impaired stem cell function are complex and directly or indirectly involve most of the mechanisms discussed in this review.
Epigenomic Mechanisms
Alcohol-mediated oxidative stress is a major regulator of epigenetic processes. Alcohol exposure both in vivo and in vitro modifies the epigenome in several tissues, including the liver, brain, skeletal muscle, lung, and immune system (147-150). Emerging evidence also indicates that fetal alcohol exposure significantly alters the epigenome and contributes to the observed pathophysiology of FAS (151, 152). Epigenetics, or the modification of gene expression without altering the DNA sequence, includes three major modifications: DNA methylation, posttranslational histone modification, and noncoding RNA interference of transcription and translation. DNA methylation and histone modification alter the chromatin, and the disrupted open nucleosome structure allows for increased gene transcription and expression, while condensation decreases gene expression (150).
DNA methylation, the most common epigenetic modification, is investigated through the use of epigenome-wide association studies (EWASs) and candidate gene methylation and global methylation studies. In an EWAS in Finnish twin cohorts, alcohol consumption was significantly associated with DNA methylation at 24 CpG sites (153). In a combination of candidate gene studies and EWASs, the most-replicated finding was alcohol-mediated differential regulation of heterogeneous nuclear ribonucleoprotein A1, leucine-rich repeat–containing 20, and lipase maturation factor 1, all of which have a role in immune regulation (154). Additionally, hypermethylation of ALDH2 and opioid receptor μ1 (154) and H3K27 trimethylation of specific genes have been reported in people with AUD (155, 156). Moreover, the genomic region that includes KDM4C, a histone demethylase gene, is a hot spot associated with alcohol withdrawal symptoms (157). Chronic alcohol use also decreases S-adenosyl methionine levels and global DNA differential methylation in peripheral blood and liver (149). Thus, alcohol use renders DNA methylation a significant epigenetic modifier, and DNA methyltransferases, DNA methylation readers, or DNA demethylation erasers can serve as potential epigenetic targets (158).
Histone modifications by histone deacetylases (HDACs) and histone acetyl transferases result in inactivation and activation, respectively, of gene transcription. The first study that demonstrated alcohol-induced epigenetic modification showed increased histone acetylation of genes that activated apoptosis (159). Since then, numerous studies have shown histone modifications as major mediators of alcohol pathologies (159-161). Increased expression and activity of class II HDACs are two of the mechanisms by which alcohol decreases skeletal muscle stem cell differentiation, by decreasing expression of myocyte enhancer factor 2 (139, 140). Furthermore, the anxiolytic properties of alcohol are partly due to increased H3K9 and H4K8 acetylation of specific genes in adult and adolescent rats (161, 162), and these epigenetic changes are observed in different brain regions that are critical for affective behaviors. Sirtuin 1 (a NAD+-dependent class III HDAC) converts the alcohol metabolite acetic acid to acetyl-CoA, which, in turn, acetylates histones of genes implicated in fibrogenesis and alcohol metabolism (149). Sirtuin 1 also regulates SREBP-1c and PGC-1α, thereby increasing fatty acid synthesis and decreasing fatty acid β-oxidation. These effects contribute to alcohol-induced fatty liver (93).
Noncoding RNAs affect transcription or prevent translation by binding to mRNAs. microRNAs (miRNAs) are the most-studied noncoding RNAs in alcohol-related pathologies (163-166). A single miRNA can target multiple genes, and one gene can be targeted by multiple miRNAs, making miRNA-mediated fine-tuning of gene regulation complex and significant. Cellular miRNAs are released into circulation in response to physiological or pathological conditions, are stable in body fluids, and modulate cell–cell signal transduction, making circulating miRNAs reliable biomarkers for alcohol-related pathologies (167, 168). miRNAs that are dysregulated due to alcohol have been extensively reviewed by Natarajan et al. (164); some of the main miRNAs that regulate genes implicated in ALD are miR-34a, miR-155, and miR-122 (166), and the miRNAs that regulate genes implicated in regulating intestinal permeability are miR-212 and miR-122 (166, 169). Moreover, miR-132 and miR-155 are increased in the liver, brain, and intestine, promoting a proinflammatory milieu (164). Evidence of miRNA-mediated activation of apoptosis (165, 170) suggests that alcohol-mediated epigenomic mechanisms contribute to activation of cell death pathways as well. Chronic alcohol–mediated miR-206 alterations have also been shown to mechanistically contribute to impaired skeletal muscle stem cell differentiation in simian immunodeficiency virus–infected rhesus macaques (95).
Several published reports indicate that alcohol use across the life span leads to epigenomic changes. Although clinical studies are needed to validate the tremendous progress made in preclinical studies to understand epigenetic modulation by alcohol, there is convincing evidence that these epigenetic marks are at least partly responsible for the pathophysiology of alcohol injury (150). Available state-of-the-art technologies including sequencing, single-cell technology, genome editing, imaging, and transgenic models should facilitate improved identification of the epigenetic mechanisms that mediate AUD-associated pathologies.
Extracellular Vesicles as Endocrine Mediators
Extracellular vesicles (EVs) have emerged as promising biomarkers and therapeutic targets. EVs mediate cellular changes in numerous pathologic and physiologic states and have been proposed as an endocrine mechanism of alcohol-induced pathologies, especially in ALD. EVs are nanosized vesicles ranging in size from 30 to 5,000 nm, bound by a lipid bilayer. EVs with a size range of 30–150 nm are termed exosomes and carry bioactive cargo including miRNAs, proteins, and mRNAs. All cells can release EVs constitutively or in response to an insult. EVs can travel through biological fluids and communicate with recipient cells in distant sites while maintaining high specificity to their destination (171). Alcohol increases both EV release from hepatocytes in vitro (172) and total circulating EV levels in alcohol-administered mice and in people with alcoholic hepatitis (173). Alcohol alters the miRNA cargo in EVs released by hepatocytes (173, 174), monocytes (175), neural stem cells (176), and intestinal epithelial cells (177) and by amniotic cells following fetal alcohol exposure (178). Studies have demonstrated that in ALD, miRNAs are associated with the EV-rich fraction. Conversely, in drug-induced liver injury, miRNAs are associated with the protein-rich fraction, suggesting that the fraction specificity of circulating miRNAs may provide insight into the pathophysiological mechanism of liver injury or may be used as a sensitive diagnostic biomarker (168). The fact that circulating EV–associated miRNAs are more stable and resistant to ribonuclease enzymatic activity compared with non-EV-associated miRNAs, coupled with the ability of surface receptor proteins to allow capture of EVs and incorporation of their bioactive cargo into specific target recipient cells, makes EVs attractive as vehicles for intercellular communication. Few studies have examined how alcohol-mediated EV cargo alterations influence recipient cell function. However, recent findings suggest that alcohol alters tissue-specific and circulating miRNA expression and dysregulates tissue specific phenotype and function, supporting the role of EVs as endocrine mediators of interorgan communication and alcohol-induced pathology.
CONCLUSION AND PERSPECTIVES
The pathophysiological mechanisms of at-risk alcohol use are complex and depend on several factors, including genetics, sex, tissue and cell type, and patterns of alcohol use (179). The health care burden due to at-risk alcohol use is influenced not only by the well-recognized risk for the development of alcohol addiction and ALD but also by its effects on the development of organ injuries and diseases, including cardiovascular disease and diabetes, FAS, pancreatic disease, respiratory disease, and cancer.
The most critical pathophysiological mechanisms of alcohol-induced organ injury are oxidative stress and inflammation, and therapeutic strategies targeting these mechanisms are in use. Nevertheless, incredible progress has been made in identifying other alcohol-induced mechanisms that either are direct alcohol effects or are indirectly activated by oxidative stress and inflammation. Research over the past 20 years has provided evidence for gut microbiome dysbiosis and increased intestinal permeability as an alcohol-induced mechanism for organ injury. Similarly, research over the past 30 years has demonstrated how alcohol modulates the epigenome, leading to multiorgan injury, addiction, and FAS. Alcohol-induced cellular pathogenesis is complex and involves multiple organ systems. Abstinence and moderation in alcohol use are the best interventions to reduce the disease burden associated with alcohol use. However, several emerging mechanisms warrant attention, as they may provide therapeutic targets to ameliorate disease burden. These include personalized gut microbiota interventions, modulation of immunometabolism, druggable epigenetic modulators, and the use of EVs as therapeutic vehicles.
SUMMARY POINTS.
The mechanisms implicated in alcohol-induced multiorgan injury are multifactorial and are frequently shared across organ systems.
Alcohol metabolism and metabolite generation lead to reactive oxygen species (ROS) generation and oxidative stress, which in turn cause biomolecule and organelle structural alterations that span the mitochondria, endoplasmic reticulum (ER), lysosomes, and nucleus. Together, these effects contribute to alcohol-associated tissue injury.
Alcohol-mediated modification of the epigenome in the liver, brain, skeletal muscle, lungs, and immune system contributes to the pathophysiology of alcohol injury.
Alcohol impairs host immune defense mechanisms, increasing morbidity and mortality from infectious diseases.
Alcohol dysregulates multiple mechanisms involved in energy metabolism homeostasis at the cellular and whole-body levels.
Cell death and survival pathways are significantly altered by at-risk alcohol use and contribute to impaired immune responses and multiorgan injury.
Alcohol-mediated dysbiosis contributes to altered behavioral and physiological function in organs including brain and liver.
FUTURE ISSUES.
Further research is needed to identify the immune consequences of bioenergetic dysregulation by excess alcohol use in order to determine how alcohol dysregulates immunometabolism.
Omics and computational modeling may be used to identify alcohol-associated dysbiosis and its modulation through the use of personalized pre- and probiotic manipulations to ameliorate alcohol-induced tissue injury.
Studies to identify dynamic interactions between extracellular matrisome proteins and cellular signal transduction pathways are warranted to understand the alcohol-mediated tissue profibrotic phenotype and its contribution to organ injury.
The translational relevance of preclinical evidence of alcohol-induced epigenomic modulation in mediating pathophysiology of alcohol use disorder (AUD) and multiorgan injury should be established.
The contribution of extracellular vesicle (EV) cargo to interorgan communication and tissue injury should be validated, and the utility of EVs as biomarkers and/or therapeutic vehicles in the treatment of alcohol-induced organ injury should be established.
ACKNOWLEDGMENTS
The authors acknowledge editorial support from Rebecca Gonzales and research support provided by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award numbers P60 AA009803, K01 AA026640, and K01 AA024494.
Glossary
- AUD
alcohol use disorder
- ALD
alcoholic liver disease
- FAS
fetal alcohol syndrome
- FPM
first-pass metabolism
- ADH
alcohol dehydrogenase
- ALDH
aldehyde dehydrogenase
- ER
endoplasmic reticulum
- ROS
reactive oxygen species
- ECM
extracellular matrix
- FMT
fecal microbiota transplantation
- EV
extracellular vesicle
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.World Health Organ. 2018. Alcohol. Fact Sheet, World Health Organ., Geneva. https://www.who.int/news-room/fact-sheets/detail/alcohol [Google Scholar]
- 2.Kranzler HR, Soyka M. 2018. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 320:815–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poznyak P, Rekve D. 2018. Global Status Report on Alcohol and Health 2018. Geneva: World Health Organ. https://www.who.int/publications/i/item/9789241565639 [Google Scholar]
- 4.NIH (Natl. Inst. Health). 2021. What is a standard drink? Fact Sheet, NIH, Bethesda, MD. http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink [Google Scholar]
- 5.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. 2006. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. JAMA Int. Med 166:2437–45 [DOI] [PubMed] [Google Scholar]
- 6.Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. 2019. Alcohol use disorders. Lancet 394:781–92 [DOI] [PubMed] [Google Scholar]
- 7.Peacock A, Leung J, Larney S, Colledge S, Hickman M, et al. 2018. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 113:1905–26 [DOI] [PubMed] [Google Scholar]
- 8.van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. 2013. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 131:23–35 [DOI] [PubMed] [Google Scholar]
- 9.Schomerus G, Matschinger H, Angermeyer MC. 2014. Attitudes towards alcohol dependence and affected individuals: persistence of negative stereotypes and illness beliefs between 1990 and 2011. Eur. Addict. Res 20:293–99 [DOI] [PubMed] [Google Scholar]
- 10.Khaderi SA. 2019. Introduction: alcohol and alcoholism. Clin. Liver Dis 23:1–10 [DOI] [PubMed] [Google Scholar]
- 11.Wackernah RC, Minnick MJ, Clapp P. 2014. Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst. Abuse Rehabil 5:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gately I. 2009. Drink: A Cultural History of Alcohol. New York: Gotham [Google Scholar]
- 13.Alcohol Res. Ed. Staff. 2018. Drinking patterns and their definitions. Alcohol Res. 39:17–18 [PMC free article] [PubMed] [Google Scholar]
- 14.Cook JL, Green CR, Lilley CM, Anderson SM, Baldwin ME, et al. 2016. Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. CMAJ 188:191–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. 2008. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol. Clin. Exp. Res 32:2149–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrick ME, Evans-Polce R, Kloska DD, Maggs JL, Lanza ST. 2017. Age-related changes in associations between reasons for alcohol use and high-intensity drinking across young adulthood. J. Stud. Alcohol Drugs 78:558–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt NB, Buckner JD, Keough ME. 2007. Anxiety sensitivity as a prospective predictor of alcohol use disorders. Behav. Modif 31:202–19 [DOI] [PubMed] [Google Scholar]
- 18.Kuerbis A, Sacco P, Blazer DG, Moore AA. 2014. Substance abuse among older adults. Clin. Geriatr. Med 30:629–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawabe M, Saito M, Naka M, Kasahara I, Saito Y, et al. 2006. Standard organ weights among elderly Japanese who died in hospital, including 50 centenarians. Pathol. Int 56:315–23 [DOI] [PubMed] [Google Scholar]
- 20.Meier P, Seitz HK. 2008. Age, alcohol metabolism and liver disease. Curr. Opin. Clin. Nutr. Metab. Care 11:21–26 [DOI] [PubMed] [Google Scholar]
- 21.Cederbaum AI. 2012. Alcohol metabolism. Clin. Liver Dis 16:667–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NIAAA (Natl. Inst. Alcohol Abuse Alcohol.). 2021. Alcohol’s effects on health. Fact Sheet, NIAAA, Rockville, MD. https://www.niaaa.nih.gov/alcohols-effects-health/special-populations-co-occurring-disorders/older-adults [Google Scholar]
- 23.Perkins AE, Varlinskaya EI, Deak T. 2019. From adolescence to late aging: a comprehensive review of social behavior, alcohol, and neuroinflammation across the lifespan. Int. Rev. Neurobiol 148:231–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meisel SN, Colder CR, Bowker JC, Hussong AM. 2018. A longitudinal examination of mediational pathways linking chronic victimization and exclusion to adolescent alcohol use. Dev. Psychol 54:1795–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacco P, Burruss K, Smith CA, Kuerbis A, Harrington D, et al. 2015. Drinking behavior among older adults at a continuing care retirement community: affective and motivational influences. Aging Ment. Health 19:279–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackintosh MA, Earleywine M, Dunn ME. 2006. Alcohol expectancies for social facilitation: a short form with decreased bias. Addict. Behav 31:1536–46 [DOI] [PubMed] [Google Scholar]
- 27.Kelly S, Olanrewaju O, Cowan A, Brayne C, Lafortune L. 2018. Alcohol and older people: a systematic review of barriers, facilitators and context of drinking in older people and implications for intervention design. PLOS ONE 13:e0191189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehm J, Room R, Monteiro M, Gmel G, Graham K, et al. 2003. Alcohol as a risk factor for global burden of disease. Eur. Addict. Res 9:157–64 [DOI] [PubMed] [Google Scholar]
- 29.GBD 2016 Alcohol Collab. 2018. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392:1015–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClain C, Vatsalya V, Cave M. 2017. Role of zinc in the development/progression of alcoholic liver disease. Curr. Treat. Options Gastroenterol 15:285–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakhari S. 2006. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 29:245–54 [PMC free article] [PubMed] [Google Scholar]
- 32.Lluis JM, Colell A, García-Ruiz C, Kaplowitz N, Fernández-Checa JC. 2003. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology 124:708–24 [DOI] [PubMed] [Google Scholar]
- 33.Molina PE, Gardner JD, Souza-Smith FM, Whitaker AM. 2014. Alcohol abuse: critical pathophysiological processes and contribution to disease burden. Physiology 29:203–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NIAAA (Natl. Inst. Alcohol Abuse Alcohol.). 2007. Alcohol metabolism: an update. Rep. 72, NIAAA, Rockville, MD. https://pubs.niaaa.nih.gov/publications/aa72/aa72.htm [Google Scholar]
- 35.Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, et al. 2001. Gender differences in pharmacokinetics of alcohol. Alcohol. Clin. Exp. Res 25:502–7 [PubMed] [Google Scholar]
- 36.Cowan JM Jr., Weathermon A, McCutcheon JR, Oliver RD. 1996. Determination of volume of distribution for ethanol in male and female subjects. J. Anal. Toxicol 20:287–90 [DOI] [PubMed] [Google Scholar]
- 37.Hernández-Muñoz R, Caballeria J, Baraona E, Uppal R, Greenstein R, Lieber CS. 1990. Human gastric alcohol dehydrogenase: its inhibition by H2-receptor antagonists, and its effect on the bioavailability of ethanol. Alcohol. Clin. Exp. Res 14:946–50 [DOI] [PubMed] [Google Scholar]
- 38.Jornvall H, Hoog JO. 1995. Nomenclature of alcohol dehydrogenases. Alcohol Alcohol. 30:153–61 [PubMed] [Google Scholar]
- 39.Peng GS, Yin JH, Wang MF, Lee JT, Hsu YD, Yin SJ. 2002. Alcohol sensitivity in Taiwanese men with different alcohol and aldehyde dehydrogenase genotypes. J. Formos. Med. Assoc 101:769–74 [PubMed] [Google Scholar]
- 40.Luczak SE, Glatt SJ, Wall TL. 2006. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol. Bull 132:607–21 [DOI] [PubMed] [Google Scholar]
- 41.Whitfield JB. 2002. Alcohol dehydrogenase and alcohol dependence: variation in genotype-associated risk between populations. Am. J. Hum. Genet 71:1247–50; author reply 1250–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wall TL, Carr LG, Ehlers CL. 2003. Protective association of genetic variation in alcohol dehydrogenase with alcohol dependence in Native American Mission Indians. Am. J. Psychiatry 160:41–46 [DOI] [PubMed] [Google Scholar]
- 43.Heier C, Xie H, Zimmermann R. 2016. Nonoxidative ethanol metabolism in humans—from biomarkers to bioactive lipids. IUBMB Life 68:916–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Criddle DN, Raraty MG, Neoptolemos JP, Tepikin AV, Petersen OH, Sutton R. 2004. Ethanol toxicity in pancreatic acinar cells: mediation by nonoxidative fatty acid metabolites. PNAS 101:10738–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon TW. 2018. Providing context for phosphatidylethanol as a biomarker of alcohol consumption with a pharmacokinetic model. Regul. Toxicol. Pharmacol 94:163–71 [DOI] [PubMed] [Google Scholar]
- 46.Avila MA, Dufour JF, Gerbes AL, Zoulim F, Bataller R, et al. 2020. Recent advances in alcohol-related liver disease (ALD): summary of a gut round table meeting. Gut 69:764–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thursz M, Kamath PS, Mathurin P, Szabo G, Shah VH. 2019. Alcohol-related liver disease: areas of consensus, unmet needs and opportunities for further study. J. Hepatol 70:521–30 [DOI] [PubMed] [Google Scholar]
- 48.Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, et al. 2018. Alcoholic liver disease. Nat. Rev. Dis. Primers 4:16. [DOI] [PubMed] [Google Scholar]
- 49.Asrani SK, Mellinger J, Arab JP, Shah VH. 2021. Reducing the global burden of alcohol-associated liver disease: a blueprint for action. Hepatology 73:2039–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osna NA, Donohue TM Jr., Kharbanda KK. 2017. Alcoholic liver disease: pathogenesis and current management. Alcohol. Res 38:147–61 [PMC free article] [PubMed] [Google Scholar]
- 51.O’Shea RS, Dasarathy S, McCullough AJ, Pract. Guid. Comm. Am. Assoc. Study Liver Dis., Pract. Parameters Comm. Am. Coll. Gastroenterol. 2010. Alcoholic liver disease. Hepatology 51:307–28 [DOI] [PubMed] [Google Scholar]
- 52.Steiner JL, Lang CH. 2017. Etiology of alcoholic cardiomyopathy: mitochondria, oxidative stress and apoptosis. Int. J. Biochem. Cell Biol 89:125–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon L, Jolley SE, Molina PE. 2017. Alcoholic myopathy: pathophysiologic mechanisms and clinical implications. Alcohol. Res 38:207–17 [PMC free article] [PubMed] [Google Scholar]
- 54.Fairfield B, Schnabl B. 2021. Gut dysbiosis as a driver in alcohol-induced liver injury. JHEP Rep. 3:100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bishehsari F, Magno E, Swanson G, Desai V, Voigt RM, et al. 2017. Alcohol and gut-derived inflammation. Alcohol. Res 38:163–71 [PMC free article] [PubMed] [Google Scholar]
- 56.Rasineni K, Srinivasan MP, Balamurugan AN, Kaphalia BS, Wang S, et al. 2020. Recent advances in understanding the complexity of alcohol-induced pancreatic dysfunction and pancreatitis development. Biomolecules 10:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rachdaoui N, Sarkar DK. 2017. Pathophysiology of the effects of alcohol abuse on the endocrine system. Alcohol. Res 38:255–76 [PMC free article] [PubMed] [Google Scholar]
- 58.Sureshchandra S, Raus A, Jankeel A, Ligh BJK, Walter NAR, et al. 2019. Dose-dependent effects of chronic alcohol drinking on peripheral immune responses. Sci. Rep 9:7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boule LA, Kovacs EJ. 2017. Alcohol, aging, and innate immunity. J. Leukoc. Biol 102:41–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halliwell B. 1999. Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic. Res 31:261–72 [DOI] [PubMed] [Google Scholar]
- 61.Ambade A, Mandrekar P. 2012. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int. J. Hepatol 2012:853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y, Cederbaum AI. 2008. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol. Med 44:723–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang RH, Gao JY, Guo HT, Scott GI, Eason AR, et al. 2013. Inhibition of CYP2E1 attenuates chronic alcohol intake–induced myocardial contractile dysfunction and apoptosis. Biochim. Biophys. Acta Mol. Basis Dis 1832:128–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin M, Ande A, Kumar A, Kumar S. 2013. Regulation of cytochrome P450 2E1 expression by ethanol: role of oxidative stress–mediated PKC/JNK/SP1 pathway. Cell Death Dis. 4:e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zakhari S. 2013. Alcohol metabolism and epigenetics changes. Alcohol. Res 35:6–16 [PMC free article] [PubMed] [Google Scholar]
- 66.Li S, Tan HY, Wang N, Zhang ZJ, Lao L, et al. 2015. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci 16:26087–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meroni M, Longo M, Dongiovanni P. 2019. Alcohol or gut microbiota: Who is the guilty? Int. J. Mol. Sci 20:4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamada N, Seo SU, Chen GY, Núñez G. 2013. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol 13:321–35 [DOI] [PubMed] [Google Scholar]
- 69.Lee E, Lee J-E. 2021. Impact of drinking alcohol on gut microbiota: recent perspectives on ethanol and alcoholic beverage. Curr. Opin. Food Sci 37:91–97 [Google Scholar]
- 70.Sarin SK, Pande A, Schnabl B. 2019. Microbiome as a therapeutic target in alcohol-related liver disease. J. Hepatol 70:260–72 [DOI] [PubMed] [Google Scholar]
- 71.Starkel P, Leclercq S, de Timary P, Schnabl B. 2018. Intestinal dysbiosis and permeability: the yin and yang in alcohol dependence and alcoholic liver disease. Clin. Sci 132:199–212 [DOI] [PubMed] [Google Scholar]
- 72.Kirpich IA, Petrosino J, Ajami N, Feng W, Wang Y, et al. 2016. Saturated and unsaturated dietary fats differentially modulate ethanol-induced changes in gut microbiome and metabolome in a mouse model of alcoholic liver disease. Am. J. Pathol 186:765–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, et al. 2016. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 65:830–39 [DOI] [PubMed] [Google Scholar]
- 74.Canesso MCC, Lacerda NL, Ferreira CM, Gonçalves JL, Almeida D, et al. 2014. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC Microbiol. 14:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leclercq S, de Timary P, Delzenne NM, Starkel P. 2017. The link between inflammation, bugs, the intestine and the brain in alcohol dependence. Transl. Psychiatry 7:e1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson KV, Foster KR. 2018. Why does the microbiome affect behaviour? Nat. Rev. Microbiol 16:647–55 [DOI] [PubMed] [Google Scholar]
- 77.Samuelson DR, Shellito JE, Maffei VJ, Tague ED, Campagna SR, et al. 2017. Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae. PLOS Pathog. 13:e1006426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. 2015. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol. Res 37:223–36 [PMC free article] [PubMed] [Google Scholar]
- 79.Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, et al. 2017. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 66:1727–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Molina PE, Happel KI, Zhang P, Kolls JK, Nelson S. 2010. Focus on: alcohol and the immune system. Alcohol Res. Health 33:97–108 [PMC free article] [PubMed] [Google Scholar]
- 81.Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, et al. 2006. Cytokines and alcohol. Alcohol. Clin. Exp. Res 30:720–30 [DOI] [PubMed] [Google Scholar]
- 82.Warburg O, Gawehn K, Geissler AW. 1958. Stoffwechsel der weißen Blutzellen [Metabolism of leukocytes]. Z. Naturforsch. B 13(8):515–16 [PubMed] [Google Scholar]
- 83.Lercher A, Baazim H, Bergthaler A. 2020. Systemic immunometabolism: challenges and opportunities. Immunity 53:496–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ford SM Jr., Simon Peter L, Berner P, Cook G, Vande Stouwe C, et al. 2018. Differential contribution of chronic binge alcohol and antiretroviral therapy to metabolic dysregulation in SIV-infected male macaques. Am. J. Physiol. Endocrinol. Metab 315:E892–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Souza-Smith FM, Ford SM Jr., Simon L, Molina PE. 2017. Repeated binge-like alcohol intoxication: depot-specific adipose tissue immuno-metabolic dysregulation. Shock 48:243–50 [DOI] [PubMed] [Google Scholar]
- 86.Barr T, Helms C, Grant K, Messaoudi I. 2016. Opposing effects of alcohol on the immune system. Prog. Neuropsychopharmacol. Biol. Psychiatry 65:242–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Souza-Smith FM, Simon L, Siggins R, Molina PE. 2019. Alcohol-induced mesenteric lymphatic permeability: link to immunometabolic modulation of perilymphatic adipose tissue. Int. J. Mol. Sci 20:4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steiner JL, Lang CH. 2017. Alcohol, adipose tissue and lipid dysregulation. Biomolecules 7:1628212318 [Google Scholar]
- 89.Prasun P, Ginevic I, Oishi K. 2021. Mitochondrial dysfunction in nonalcoholic fatty liver disease and alcohol related liver disease. Transl. Gastroenterol. Hepatol 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tapia-Rojas C, Torres AK, Quintanilla RA. 2019. Adolescence binge alcohol consumption induces hippocampal mitochondrial impairment that persists during the adulthood. Neuroscience 406:356–68 [DOI] [PubMed] [Google Scholar]
- 91.Sadikot RT, Bedi B, Li J, Yeligar SM. 2019. Alcohol-induced mitochondrial DNA damage promotes injurious crosstalk between alveolar epithelial cells and alveolar macrophages. Alcohol 80:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duplanty AA, Simon L, Molina PE. 2017. Chronic binge alcohol–induced dysregulation of mitochondrial-related genes in skeletal muscle of simian immunodeficiency virus–infected rhesus macaques at end-stage disease. Alcohol Alcohol. 52:298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nassir F, Ibdah JA. 2014. Role of mitochondria in alcoholic liver disease. World J. Gastroenterol 20:2136–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tan HK, Yates E, Lilly K, Dhanda AD. 2020. Oxidative stress in alcohol-related liver disease. World J. Hepatol 12:332–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simon L, Hollenbach AD, Zabaleta J, Molina PE. 2015. Chronic binge alcohol administration dysregulates global regulatory gene networks associated with skeletal muscle wasting in simian immunodeficiency virus–infected macaques. BMC Genom. 16:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duplanty AA, Siggins RW, Allerton T, Simon L, Molina PE. 2018. Myoblast mitochondrial respiration is decreased in chronic binge alcohol administered simian immunodeficiency virus–infected antiretroviraltreated rhesus macaques. Physiol. Rep 6:e13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han D, Ybanez MD, Johnson HS, McDonald JN, Mesropyan L, et al. 2012. Dynamic adaptation of liver mitochondria to chronic alcohol feeding in mice: biogenesis, remodeling, and functional alterations. J. Biol. Chem 287:42165–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morris NL, Harris FL, Brown LAS, Yeligar SM. 2021. Alcohol induces mitochondrial derangements in alveolar macrophages by upregulating NADPH oxidase 4. Alcohol 90:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miyata T, Nagy LE. 2020. Programmed cell death in alcohol-associated liver disease. Clin. Mol. Hepatol 26:618–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang BC, Wu SY, Leung PS. 2020. Alcohol ingestion induces pancreatic islet dysfunction and apoptosis via mediation of FGF21 resistance. Ann. Transl. Med 8:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hajnoczky G, Buzas CJ, Pacher P, Hoek JB, Rubin E. 2005. Alcohol and mitochondria in cardiac apoptosis: mechanisms and visualization. Alcohol. Clin. Exp. Res 29:693–701 [DOI] [PubMed] [Google Scholar]
- 102.Bolnick JM, Karana R, Chiang PJ, Kilburn BA, Romero R, et al. 2014. Apoptosis of alcohol-exposed human placental cytotrophoblast cells is downstream of intracellular calcium signaling. Alcohol. Clin. Exp. Res 38:1646–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Freund G. 1994. Apoptosis and gene expression: perspectives on alcohol-induced brain damage. Alcohol 11:385–87 [DOI] [PubMed] [Google Scholar]
- 104.Wang S, Pacher P, De Lisle RC, Huang H, Ding WX. 2016. A mechanistic review of cell death in alcohol-induced liver injury. Alcohol. Clin. Exp. Res 40:1215–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. 2013. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 57:1773–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ye L, Li S, Liu X, Zhang D, Li L, Jiang Y. 2021. CB1R promotes chronic alcohol–induced neuronal necroptosis in mice prefrontal cortex. Alcohol Alcohol. 56:230–39 [DOI] [PubMed] [Google Scholar]
- 107.Gukovskaya AS, Mareninova OA, Odinokova IV, Sung KF, Lugea A, et al. 2006. Cell death in pancreatitis: effects of alcohol. J. Gastroenterol. Hepatol 21(Suppl. 3):S10–13 [DOI] [PubMed] [Google Scholar]
- 108.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, et al. 2012. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Investig 122:3476–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang F, Li G, Ning J, Chen L, Xu H, et al. 2018. Alcohol accumulation promotes esophagitis via pyroptosis activation. Int. J. Biol. Sci 14:1245–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Williams JA, Ding WX. 2020. Role of autophagy in alcohol and drug-induced liver injury. Food Chem. Toxicol 136:111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Girault V, Gilard V, Marguet F, Lesueur C, Hauchecorne M, et al. 2017. Prenatal alcohol exposure impairs autophagy in neonatal brain cortical microvessels. Cell Death Dis. 8:e2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng H, Qin X, Chen S, Ceylan AF, Dong M, et al. 2020. Parkin deficiency accentuates chronic alcohol intake–induced tissue injury and autophagy defects in brain, liver and skeletal muscle. Acta Biochim. Biophys. Sin 52:665–74 [DOI] [PubMed] [Google Scholar]
- 113.Ji C. 2012. Mechanisms of alcohol-induced endoplasmic reticulum stress and organ injuries. Biochem. Res. Int 2012:216450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pandol SJ, Gorelick FS, Gerloff A, Lugea A. 2010. Alcohol abuse, endoplasmic reticulum stress and pancreatitis. Dig. Dis 28:776–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaphalia L, Boroumand N, Hyunsu J, Kaphalia BS, Calhoun WJ. 2014. Ethanol metabolism, oxidative stress, and endoplasmic reticulum stress responses in the lungs of hepatic alcohol dehydrogenase deficient deer mice after chronic ethanol feeding. Toxicol. Appl. Pharmacol 277:109–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dolin CE, Arteel GE. 2020. The matrisome, inflammation, and liver disease. Semin. Liver Dis 40:180–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ninh VK, El Hajj EC, Mouton AJ, Gardner JD. 2019. Prenatal alcohol exposure causes adverse cardiac extracellular matrix changes and dysfunction in neonatal mice. Cardiovasc. Toxicol 19:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sueblinvong V, Kerchberger VE, Saghafi R, Mills ST, Fan X, Guidot DM. 2014. Chronic alcohol ingestion primes the lung for bleomycin-induced fibrosis in mice. Alcohol. Clin. Exp. Res 38:336–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arteel GE, Naba A. 2020. The liver matrisome—looking beyond collagens. JHEP Rep. 2:100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aziz-Seible RS, Casey CA. 2011. Fibronectin: functional character and role in alcoholic liver disease. World J. Gastroenterol 17:2482–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seth D, Duly A, Kuo PC, McCaughan GW, Haber PS. 2014. Osteopontin is an important mediator of alcoholic liver disease via hepatic stellate cell activation. World J. Gastroenterol 20:13088–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seth D, D’Souza El-Guindy NB, Apte M, Mari M, Dooley S, et al. 2010. Alcohol, signaling, and ECM turnover. Alcohol. Clin. Exp. Res 34:4–18 [DOI] [PubMed] [Google Scholar]
- 123.Dodd T, Simon L, LeCapitaine NJ, Zabaleta J, Mussell J, et al. 2014. Chronic binge alcohol administration accentuates expression of pro-fibrotic and inflammatory genes in the skeletal muscle of simian immunodeficiency virus–infected macaques. Alcohol. Clin. Exp. Res 38:2697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simon L, LeCapitaine N, Berner P, Vande Stouwe C, Mussell JC, et al. 2014. Chronic binge alcohol consumption alters myogenic gene expression and reduces in vitro myogenic differentiation potential of myoblasts from rhesus macaques. Am. J. Physiol. Regul. Integr. Comp. Physiol 306:R837–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lasek AW. 2016. Effects of ethanol on brain extracellular matrix: implications for alcohol use disorder. Alcohol. Clin. Exp. Res 40:2030–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trindade P, Hampton B, Manhães AC, Medina AE. 2016. Developmental alcohol exposure leads to a persistent change on astrocyte secretome. J. Neurochem 137:730–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rubio-Araiz A, Porcu F, Pérez-Hernández M, García-Gutiérrez MS, Aracil-Fernández MA, et al. 2017. Disruption of blood–brain barrier integrity in postmortem alcoholic brain: preclinical evidence of TLR4 involvement from a binge-like drinking model. Addict. Biol 22:1103–16 [DOI] [PubMed] [Google Scholar]
- 128.Go BS, Sirohi S, Walker BM. 2020. The role of matrix metalloproteinase-9 in negative reinforcement learning and plasticity in alcohol dependence. Addict. Biol 25:e12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen H, Lasek AW. 2020. Perineuronal nets in the insula regulate aversion-resistant alcohol drinking. Addict. Biol 25:e12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coleman LG Jr., Liu W, Oguz I, Styner M, Crews FT. 2014. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol. Biochem. Behav 116:142–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Poole LG, Arteel GE. 2016. Transitional remodeling of the hepatic extracellular matrix in alcohol-induced liver injury. Biomed. Res. Int 2016:3162670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.About I. 2018. “The stem cell fashion”: Do we need only stem cells for tissue regeneration? Clin. Oral Investig 22:553–54 [DOI] [PubMed] [Google Scholar]
- 133.Di Rocco G, Baldari S, Pani G, Toietta G. 2019. Stem cells under the influence of alcohol: effects of ethanol consumption on stem/progenitor cells. Cell Mol. Life Sci 76:231–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Michalopoulos GK, Bhushan B. 2021. Liver regeneration: biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol 18:40–55 [DOI] [PubMed] [Google Scholar]
- 135.Kuttippurathu L, Juskeviciute E, Dippold RP, Hoek JB, Vadigepalli R. 2016. A novel comparative pattern analysis approach identifies chronic alcohol mediated dysregulation of transcriptomic dynamics during liver regeneration. BMC Genom. 17:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duguay L, Coutu D, Hetu C, Joly JG. 1982. Inhibition of liver regeneration by chronic alcohol administration. Gut 23:8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hosseini N, Shor J, Szabo G. 2019. Alcoholic hepatitis: a review. Alcohol Alcohol. 54:408–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Levitt DE, Yeh AY, Prendergast MJ, Budnar RG Jr., Adler KA, et al. 2020. Chronic alcohol dysregulates skeletal muscle myogenic gene expression after hind limb immobilization in female rats. Biomolecules 10:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adler K, Molina PE, Simon L. 2019. Epigenomic mechanisms of alcohol-induced impaired differentiation of skeletal muscle stem cells: role of class IIA histone deacetylases. Physiol. Genom 51:471–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Simon L, Ford SM Jr., Song K, Berner P, Vande Stouwe C, et al. 2017. Decreased myoblast differentiation in chronic binge alcohol–administered simian immunodeficiency virus–infected male macaques: role of decreased miR-206. Am. J. Physiol. Regul. Integr. Comp. Physiol 313:R240–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levitt DE, Chalapati N, Prendergast MJ, Simon L, Molina PE. 2020. Ethanol-impaired myogenic differentiation is associated with decreased myoblast glycolytic function. Alcohol. Clin. Exp. Res 44:2166–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Siggins RW, Molina P, Zhang P, Bagby GJ, Nelson S, et al. 2014. Dysregulation of myelopoiesis by chronic alcohol administration during early SIV infection of rhesus macaques. Alcohol. Clin. Exp. Res 38:1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Seitz HK, Poschl G. 1997. The role of gastrointestinal factors in alcohol metabolism. Alcohol Alcohol. 32:543–49 [DOI] [PubMed] [Google Scholar]
- 144.Vincon P, Wunderer J, Simanowski UA, Koll M, Preedy VR, et al. 2003. Inhibition of alcohol-associated colonic hyperregeneration by α-tocopherol in the rat. Alcohol. Clin. Exp. Res 27:100–6 [DOI] [PubMed] [Google Scholar]
- 145.Simanowski UA, Homann N, Knuhl M, Arce L, Waldherr R, et al. 2001. Increased rectal cell proliferation following alcohol abuse. Gut 49:418–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Simanowski UA, Stickel F, Maier H, Gartner U, Seitz HK. 1995. Effect of alcohol on gastrointestinal cell regeneration as a possible mechanism in alcohol-associated carcinogenesis. Alcohol 12:111–15 [DOI] [PubMed] [Google Scholar]
- 147.Rodriguez FD. 2021. Targeting epigenetic mechanisms to treat alcohol use disorders (AUD). Curr. Pharm. Des 27:3252–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pal-Bhadra M, Bhadra U, Jackson DE, Mamatha L, Park PH, Shukla SD. 2007. Distinct methylation patterns in histone H3 at Lys-4 and Lys-9 correlate with up- and down-regulation of genes by ethanol in hepatocytes. Life Sci. 81:979–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin XX, Lian GH, Peng SF, Zhao Q, Xu Y, et al. 2018. Reversing epigenetic alterations caused by alcohol: a promising therapeutic direction for alcoholic liver disease. Alcohol. Clin. Exp. Res 42:1863–73 [DOI] [PubMed] [Google Scholar]
- 150.Bohnsack JP, Pandey SC. 2021. Histone modifications, DNA methylation, and the epigenetic code of alcohol use disorder. Int. Rev. Neurobiol 156:1–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sarkar DK. 2016. Male germline transmits fetal alcohol epigenetic marks for multiple generations: a review. Addict. Biol 21:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaminen-Ahola N. 2020. Fetal alcohol spectrum disorders: genetic and epigenetic mechanisms. Prenat. Diagn 40:1185–92 [DOI] [PubMed] [Google Scholar]
- 153.Stephenson M, Bollepalli S, Cazaly E, Salvatore JE, Barr P, et al. 2021. Associations of alcohol consumption with epigenome-wide DNA methylation and epigenetic age acceleration: individual-level and co-twin comparison analyses. Alcohol. Clin. Exp. Res 45:318–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Longley MJ, Lee J, Jung J, Lohoff FW. 2021. Epigenetics of alcohol use disorder—a review of recent advances in DNA methylation profiling. Addict. Biol 26:e13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bohnsack JP, Teppen T, Kyzar EJ, Dzitoyeva S, Pandey SC. 2019. The lncRNA BDNF-AS is an epigenetic regulator in the human amygdala in early onset alcohol use disorders. Transl. Psychiatry 9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Johnstone AL, Andrade NS, Barbier E, Khomtchouk BB, Rienas CA, et al. 2021. Dysregulation of the histone demethylase KDM6B in alcohol dependence is associated with epigenetic regulation of inflammatory signaling pathways. Addict. Biol 26:e12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang KS, Liu X, Zhang Q, Wu LY, Zeng M. 2012. Genome-wide association study identifies 5q21 and 9p24.1 (KDM4C) loci associated with alcohol withdrawal symptoms. J. Neural. Transm 119:425–33 [DOI] [PubMed] [Google Scholar]
- 158.Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. 2012. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J. Neurosci 32:1884–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Parira T, Laverde A, Agudelo M. 2017. Epigenetic interactions between alcohol and cannabinergic effects: focus on histone modification and DNA methylation. J. Alcohol. Drug Depend 5:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Agudelo M, Figueroa G, Parira T, Yndart A, Muñoz K, et al. 2016. Profile of class I histone deacetylases (HDAC) by human dendritic cells after alcohol consumption and in vitro alcohol treatment and their implication in oxidative stress: role of HDAC inhibitors trichostatin A and mocetinostat. PLOS ONE 11:e0156421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. 2013. Aberrant histone deacetylase 2–mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol. Psychiatry 73:763–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. 2012. Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol. Clin. Exp. Res 36:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Torres JL, Novo-Veleiro I, Manzanedo L, Alvela-Suárez L, Macías R, et al. 2018. Role of microRNAs in alcohol-induced liver disorders and non-alcoholic fatty liver disease. World J. Gastroenterol 24:4104–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Natarajan SK, Pachunka JM, Mott JL. 2015. Role of microRNAs in alcohol-induced multi-organ injury. Biomolecules 5:3309–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Iwagami Y, Zou J, Zhang H, Cao K, Ji C, et al. 2018. Alcohol-mediated miR-34a modulates hepatocyte growth and apoptosis. J. Cell Mol. Med 22:3987–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bala S, Szabo G. 2012. MicroRNA signature in alcoholic liver disease. Int. J. Hepatol 2012:498232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ibáñez F, Ureña-Peralta JR, Costa-Alba P, Torres JL, Laso FJ, et al. 2020. Circulating microRNAs in extracellular vesicles as potential biomarkers of alcohol-induced neuroinflammation in adolescence: gender differences. Int. J. Mol. Sci 21:6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, et al. 2012. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 56:1946–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, et al. 2008. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol. Clin. Exp. Res 32:355–64 [DOI] [PubMed] [Google Scholar]
- 170.Pan JH, Kim H, Tang J, Beane KE, Park JW, et al. 2020. Acute alcohol consumption–induced let-7a inhibition exacerbates hepatic apoptosis by regulating Rb1 in mice. Alcohol 85:13–20 [DOI] [PubMed] [Google Scholar]
- 171.Banales JM, Feldstein AE, Sanger H, Lukacs-Kornek V, Szabo G, Kornek M. 2019. Extracellular vesicles in liver diseases:meeting report from the International Liver Congress 2018. Hepatol. Commun 3:305–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Verma VK, Li H, Wang R, Hirsova P, Mushref M, et al. 2016. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J. Hepatol 64:651–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Momen-Heravi F, Bala S, Kodys K, Szabo G. 2015. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep 5:9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eguchi A, Lazaro RG, Wang J, Kim J, Povero D, et al. 2017. Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a microRNA barcode that can be detected in blood. Hepatology 65:475–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Saha B, Momen-Heravi F, Kodys K, Szabo G. 2016. MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J. Biol. Chem 291:149–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tseng AM, Chung DD, Pinson MR, Salem NA, Eaves SE, Miranda RC. 2019. Ethanol exposure increases miR-140 in extracellular vesicles: implications for fetal neural stem cell proliferation and maturation. Alcohol. Clin. Exp. Res 43:1414–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lamas-Paz A, Morán L, Peng J, Salinas B, López-Alcantara N, et al. 2020. Intestinal epithelial cell-derived extracellular vesicles modulate hepatic injury via the gut–liver axis during acute alcohol injury. Front. Pharmacol 11:603771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tavanasefat H, Li F, Koyano K, Gourtani BK, Marty V, et al. 2020. Molecular consequences of fetal alcohol exposure on amniotic exosomal miRNAs with functional implications for stem cell potency and differentiation. PLOS ONE 15:e0242276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tulisiak CT, Harris RA, Ponomarev I. 2017. DNA modifications in models of alcohol use disorders. Alcohol 60:19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]