Abstract
Modeling complex eye diseases like age-related macular degeneration (AMD) and glaucoma poses significant challenges, since these conditions depend highly on age-related changes that occur over several decades, with many contributing factors remaining unknown. Although both diseases exhibit a relatively high heritability of >50%, a large proportion of individuals carrying AMD- or glaucoma-associated genetic risk variants will never develop these diseases. Furthermore, several environmental and lifestyle factors contribute to and modulate the pathogenesis and progression of AMD and glaucoma.
Several strategies replicate the impact of genetic risk variants, pathobiological pathways and environmental and lifestyle factors in AMD and glaucoma in mice and other species. In this review we will primarily discuss the most commonly available mouse models, which have and will likely continue to improve our understanding of the pathobiology of age-related eye diseases. Uncertainties persist whether small animal models can truly recapitulate disease progression and vision loss in patients, raising doubts regarding their usefulness when testing novel gene or drug therapies. We will elaborate on concerns that relate to shorter lifespan, body size and allometries, lack of macula and a true lamina cribrosa, as well as absence and sequence disparities of certain genes and differences in their chromosomal location in mice.
Since biological, rather than chronological, age likely predisposes an organism for both glaucoma and AMD, more rapidly aging organisms like small rodents may open up possibilities that will make research of these diseases more timely and financially feasible. On the other hand, due to the above-mentioned anatomical and physiological features, as well as pharmacokinetic and -dynamic differences small animal models are not ideal to study the natural progression of vision loss or the efficacy and safety of novel therapies. In this context, we will also discuss the advantages and pitfalls of alternative models that include larger species, such as non-human primates and rabbits, patient-derived retinal organoids, and human organ donor eyes.
Keywords: Age-related macular degeneration, Glaucoma, Mouse model, Non-human primate model, Rabbit model, Age-related disease, Eye disease, Retina, Drusen, Geographic atrophy, Optic nerve degeneration
1. Introduction
Vision loss negatively impacts the quality of life of affected patients and their caregivers: Physically, through falls and injuries (Crews et al., 2016), economically since they may suffer the loss of employment, education and income and bear the burden of higher healthcare costs (Rein et al., 2022) and mentally, through increased psychological distress (Lundeen et al., 2022). Age-related macular degeneration (AMD) and glaucoma represent the leading causes of irreversible blindness worldwide. Flaxman et al. estimated that globally in 2020, 8.8 million patients with AMD and 4.5 million individuals with glaucoma experienced moderate or severe vision impairment (Flaxman et al., 2017). The National Eye Institute projected in 2014 that the number of patients with advanced AMD in the United States will increase from 2.1 million to 3.7 million and those with glaucoma from 2.7 to 4.3 million by 2030 (Eye Disease Statistics, 2014). Since the prevalence of AMD and glaucoma increases with age, the resulting burden on our aging societies makes it ever more urgent to investigate the underlying causes and to find and improve treatments.
At first glance, the natural histories of AMD and glaucoma appear to differ greatly. AMD initiates and progresses at the outer blood-retina barrier, which mainly consists of the retinal pigment epithelium (RPE), Bruch’s membrane (BrM) and choriocapillaris. Dry AMD represents the more common manifestation of the disease, with mechanisms of onset that remain elusive. It commonly includes accumulation of deposits of extracellular material underneath the RPE that can aggregate into drusen (Fig. 1), thinning of the macula, atrophy of the RPE and fragility of the photoreceptor pigment interface with pigment epithelial detachments. Patients with pigmentary abnormalities, drusen and/or RPE detachment more likely advance to geographic atrophy (GA), which involves the formation and development of atrophic regions in the retina characterized by photoreceptor loss and/or to neovascular AMD with abnormal growth of structurally impaired choroidal and/or retinal blood vessels that can become leaky, leading to subretinal hemorrhages ((Fleckenstein et al., 2021), Fig. 2). Functionally, early or intermediate AMD affects the speed by which patients can adapt to darkness after bright light exposure, a phenomenon believed to be mainly due to compromised visual pigment chromophore delivery from the RPE ((Lee et al., 2020a; Owsley et al., 2016), Fig. 3A) specifically in the macula. In addition, a slower activation phase of light flash responses has been observed in both patients with dry and wet AMD (Fig. 3B). Although retinal remodeling, histological and metabolomic pathologies are also observed in regions adjacent to drusen outside of the central macula (Jones et al., 2016), the late forms of AMD substantially diminish patients’ quality of life due to loss of central vision, since AMD specifically affects the macula.
Fig. 1. Fundus and OCT images from early/intermediate AMD patient (A, B) and control subject (C, D).

Drusen can be seen as yellow spots in the fundus image (A) and as protrusions, as indicated by arrow heads, in the OCT image (B).
Fig. 2. Natural History of age-related macular degeneration (AMD).
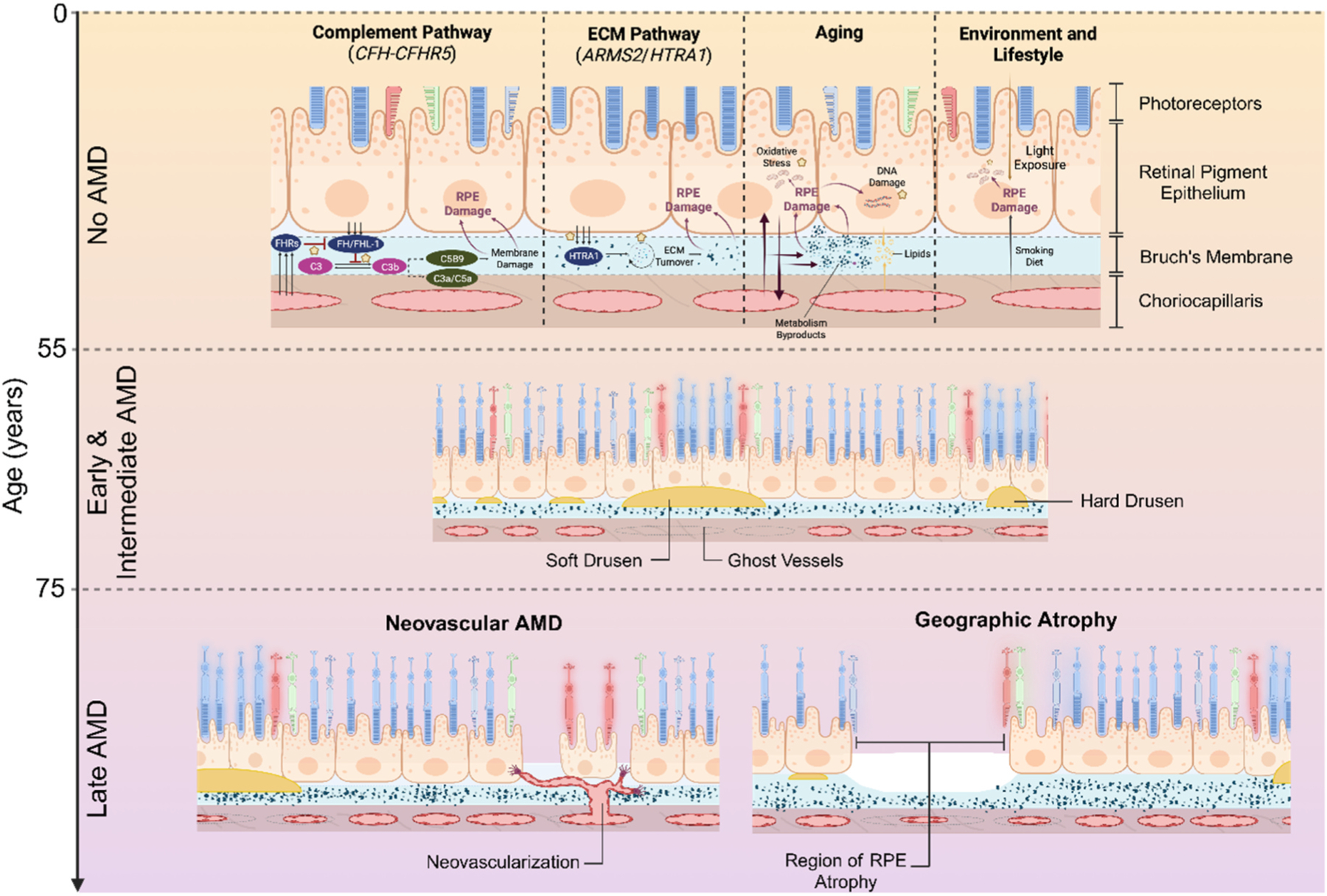
Schematic showing pathways driving AMD-associated pathology, which develops primarily at the interface between retinal pigment epithelium (RPE), Bruch’s membrane (BrM) and choriocapillaris. These pathways are at play prior to the clinical onset of early and intermediate AMD and subsequent progression to late AMD.
Fig. 3. Altered dark adaptation (A) and dark-adapted ERG light responses in human AMD patients (B) and a mouse model of CFH variant (C).

(A) Recovery of visual sensitivity after bleach from control subjects (black) and from AMD patients (grey). (B) Leading edge of ERG light flash response from control (black) and dry (blue) and wet (red) AMD patients. (C) ERG light flash response amplitudes as a function of flash intensity in control (no high-fat diet, black; high-fat cholesterol-enriched diet, green) and in human CFH risk variant expressing mice (no high-fat diet, blue; high-fat cholesterol-enriched diet, pink). Adapted from (Murray et al., 2022), (A) (Dimopoulos et al., 2013), (B) (Landowski et al., 2019), (C).
Glaucoma refers to a group of diseases characterized by retinal ganglion cell (RGC) death and damage to the optic nerve (Fig. 4). In this review, we will focus on primary open-angle glaucoma (POAG) as the most common type of the disease that affects the aging population and refer to it as glaucoma. In contrast to AMD that deprives patients of central vision, patients with glaucoma initially lose peripheral vision, which often goes unnoticed in the early disease stages and only later extends to the central retina. Moreover, unlike AMD in which the pathological progression involves the RPE or vascular components, the death of retinal ganglion cells (RGCs) that form the optic nerve primarily characterizes glaucoma and often correlates with increased intraocular pressure (IOP) (Guo et al., 2005). However, despite their different pathophysiological presentation, both AMD and glaucoma mainly affect patients over 65 years and present as progressive conditions, in which advanced age, genetic risk variants, environmental factors and lifestyle combine to increase the odds of developing these debilitating diseases.
Fig. 4. Optic nerve head and retinal nerve fiber changes in glaucoma patients diagnosed using fundus photography.

Glaucomatous eye (A) has larger cup (bright center) to rim ratio compared to control eye (B). Images in C–F are from the same subject who developed glaucoma within 4-year period. In C the nerve fibers look normal but start to disappear from regions indicated in arrows in D-F. At the time when image F was photographed, patient had developed visual field deficits. (A, B) from (Abramoff et al., 2007), and (C–F) from (Quigley et al., 1992).
For many decades, vascular defects secondary to neovascular AMD were managed with pan-retinal laser photocoagulation, which by its very nature destroys retinal tissue and thus makes loss of vision unavoidable (Macular Photocoagulation Study Group, 1982; The Moorfields Macular Study Group, 1982), although new approaches limit the degree of vision impairment linked to photocoagulation (Sher et al., 2013). Drugs that target vascular endothelial growth factor (VEGF) first became available in the early 2000s and have massively improved the prognosis and treatment of patients with neovascular AMD (Meyer and Holz, 2011). Nevertheless, a significant proportion of patients fail to respond to anti-VEGF drugs and many do not maintain initial vision gains (Comparison of Age-related Macular Degeneration Treatments Trials Research, 2016). Treatment for GA is currently limited to pegcetacoplan and avacincaptad pegol, the first available treatments for GA, which were only recently approved by The Food and Drug Administration (FDA). These drugs only modestly reduce lesion growth, but unlike anti-VEGF therapies neither drug meaningfully improves vision within the first 18 months to two years (Patel et al., 2023; Apellis, 2022).
Glaucoma treatment centers on pharmacologically and/or surgically lowering IOP to slow the disease, although many patients continue to lose vision post-intervention (Weinreb et al., 2014). Disease progression despite treatment may result from IOP remaining higher than expected even with successful surgeries or 100% patient compliance with pharmacological intervention. Neuroprotective treatments to increase RGC survival in glaucoma remain unavailable despite many previous and ongoing studies (Hernandez et al., 2008; Johnson et al., 2010; Khatib and Martin, 2020; Osborne et al., 2018; WoldeMussie et al., 2001). Clearly, significant challenges to developing effective sight-saving therapies for AMD and glaucoma patients remain and many small molecule and gene therapies for AMD and glaucoma are currently under investigation, which require testing in animal models with human disease relevance. This review will complement others on AMD (e.g., (de Jong et al., 2021; Fleckenstein et al., 2021; Merle et al., 2022; Pan et al., 2022; Rozing et al., 2020)) and glaucoma (e.g., (Ekici and Moghimi, 2023; Krizaj, 1995; Shalaby et al., 2022; Stein et al., 2021; Tezel, 2022; Wang et al., 2022)) by discussing the strengths and weaknesses of currently available animal models to elucidate the roles of genetics, aging and environment in disease pathogenesis and progression of AMD and glaucoma.
2. Modeling multifactorial age-related eye disease in animals
Suitable animal models help us understand retinal diseases and allow the development of effective treatments. Mouse and other animal models of inherited retinal degenerative diseases (IRDs, recently reviewed in (Moshiri, 2021)), have given us profound mechanistic insight and have recently resulted in a successful, FDA-approved gene therapy for retinal dystrophies due to two mutations in the RPE65 gene (Russell et al., 2017), with many other potential treatments in late-stage clinical trials (NCT00999609, NCT03913143, NCT04855045, NCT05158296, NCT04671433, NCT04794101, NCT03153293, NCT04912843). In stark contrast, AMD and glaucoma lack animal models that faithfully recapitulate the multifactorial mechanisms and full patient phenotypes, thus hindering our ability to develop effective treatments. Cultured human cells, cell lines, organoids and tissues from human donor eyes, which will be discussed in Chapter 7, have been intensely investigated in the past two decades for their potential to replicate retinal disease, since they maintain genetic and many epigenetic traits that contribute to donors’ diseases, avoid inter-species differences in animal experiments, minimize costs and can be relatively easy to use. However, in vitro and ex vivo studies do not account for complex systemic influences that impact disease development and progression, nor do they allow us to study age- and lifestyle-dependent disease processes or help us understand how treatments affect the whole organism.
Many animal models of AMD or glaucoma reproduce symptoms and disease traits without mimicking their root causes in a process known as phenocopying. To gain mechanistic understanding in a simplified system, researchers have introduced genetic mutations and developed acute injury models, which we will discuss below (Cameron et al., 2020; Frank et al., 1989; Kim et al., 2016; Li et al., 1999; Marc et al., 2008; Miller et al., 1986; Noell et al., 1966; Organisciak and Vaughan, 2010; Urcola et al., 2006; Woodell et al., 2013). These simpler models will necessarily fall short, however, when we try to understand the age-dependent progression of a complex disease with overlapping contributions from multiple risk factors (Figs. 2 and 5) and predict the success of new therapies in patients.
Fig. 5.

The major modeling strategies of AMD (A) and glaucoma (B) based on genetic variants (purple), environmental/lifestyle stressors (green) and pathological pathways (yellow).
Small animal models, such as mice or zebrafish, have been widely employed in in vivo studies, particularly when investigating monogenetic IRDs, since they can be genetically modified comparatively easily, reproduce with a short gestation time and are cheap to maintain. On the other hand, these animals have a short life span of only up to two years and they may not faithfully model cellular insults and disease progression due to lifestyle and environmental factors that accumulate in human patients with AMD and glaucoma for 65 years or more. Rabbits have a long history in ophthalmological research from IRD (Jones et al., 2011b) to glaucoma (Gherezghiher et al., 1986; Johnson et al., 1999; Seidehamel and Dungan, 1974) with strong potential for researchers to leverage them more heavily. Non-human primate (NHP) models can often fill the gap between small mammals and humans, although this research is expensive, fraught with ethical concerns and currently lacks the versatile genetic tools and disease models available in mice and zebrafish. Research into AMD and glaucoma, therefore, mainly relies on small mammals, on which we will focus in this review. However, it is important to understand that as one of their main disadvantages small mammals such as mice, rats or rabbits lack a key anatomical feature, namely the macula, which is primarily affected in AMD. Therefore, we will also briefly discuss prospects for large animal models, retina organoids and donor human eye tissues in Chapter 7.
3. Mouse models based on major genetic risk factors in AMD and glaucoma
Early reports concluded that genetics play an overwhelming role in AMD and glaucoma based on their high concordance in monozygotic twins (Gottfredsdottir et al., 1999; Grizzard et al., 2003; Meyers et al., 1995; Meyers and Zachary, 1988; Teikari, 1990). Since then, genome-wide association studies (GWAS) have linked 52 variants across 34 loci with AMD (Fritsche et al., 2013, 2016) (Fig. 6). The broad range of genes associated with AMD indicates that multiple pathways may be implicated in disease initiation and progression. These include the complement pathway (CFH-CFHR1–5, CFB, CFI, C2, C3, and C9), lipid metabolism and transport pathways (APOE, LIPC, CETP, and BAIAP2L2), angiogenesis (VEGFA, TGFBR1, ADAMTS9, MMP9), the vitamin A cycle, extracellular matrix (ECM) remodeling and degradation (COL8A1, COL10A1, TIMP3, ADAMTS9, TGFBR1, HTRA1, and B3GALTL) and cell survival, oxidative stress response, apoptosis and DNA repair (ARMS2, RAD51B, and TNFRSF10A) (Fritsche et al., 2016; Pool et al., 2020). Variants and haplotypes associated with the chromosome 1q32 and 10q26 regions account for approximately 70% of disease variability explained by additive genetic effects (see Fig. 7), while the remaining 32 associated loci contribute comparatively little to disease course and outcome (Fritsche et al., 2014; Fritsche et al., 2016), making it, therefore, difficult to determine their respective differential effect on disease. Because of their prominence in AMD etiology, this review focuses on mechanisms associated with the 1q32 and 10q26 loci.
Fig. 6. Manhattan plot taken with permission from Fritsche et al., 2016 showing the fifty-two independent variants across 34 loci independently associated with AMD through genome-wide association studies.

The two most common contributors to disease are the CFH-CFHR5 region on chromosome 1q32 and the ARMS2/HTRA1 locus on chromosome 1q26. Other variants have a comparatively smaller effect on disease outcome.
Fig. 7.

A large part of the population-attributable risk for AMD can be traced to risk at the CFH-CFHR5 or ARMS2/HTRA1 loci, smoking and BMI. Additional factors including minor AMD-associated variants, hypertension, diet and light exposure have a comparatively smaller contribution to disease.
Conversely, when considered individually, each of the more than 120 single nucleotide polymorphisms (SNPs) that have been associated with glaucoma (Gharahkhani et al., 2021), increased IOP, vertical cup-to-disc ratio, central corneal thickness, cup area and disc area only marginally increase disease risk and the mechanisms by which these genetic variants cause glaucoma onset and progression remain poorly understood (Liu and Allingham, 2017). Polygenic risk scores (PRS), which combine the effect of variants associated with an increased risk for developing glaucoma into a single score, have a limited ability to predict the onset of the disease. Although individuals at highest risk with a PRS in the top decile have a 15-fold increased risk of developing advanced glaucoma as compared to the bottom decile, the PRS decile does not strongly correlate with mean age at diagnosis and surviving proportion of baseline retinal nerve fibre layer (Craig et al., 2020). To avoid exhaustive lists, we will review only a selection of genetically engineered mouse models for the major known genetic risk variants for AMD and glaucoma and instead focus on strategies to model these complex diseases.
3.1. Complement factor H (CFH) in AMD
Complement factor H (CFH), a key regulator of the alternative pathway of the complement system, has been heavily researched since it was identified as the first gene associated with AMD in 2005. By secreting proteins encoded by the CFH gene, factor H (FH) and its splice variant factor h-like protein 1 (FHL-1), the RPE prevents intraocular complement activation from the systemic circulation and, therefore, maintains the eye’s immune-privileged status. Polymorphisms of CFH and the highly homologous complement factor H-related (CFHR) gene family on chromosome 1q32 can confer either risk for or protection from AMD (Pappas et al., 2021). All common CFH-CFHR5 risk haplotypes contain a polymorphism encoding a tyrosine to histidine substitution at position 402 (Y402H) of FH and FHL-1 (Hageman et al., 2005), which affects approximately 55% of AMD patients in the United States. Refined genetic association analyses have shown that the spectrum of AMD susceptibility associated with the 1q32 locus ranges from protection to neutrality and risk, with odds ratios for developing AMD among individuals carrying combinations of CFH-CFHR5 risk and neutral haplotypes from 1.7 to 2.9. Haplotypes associated with protection against the development of AMD mitigate risk haplotypes in a 1-to-1 manner (Pappas et al., 2021; Zouache et al., 2024). These relatively small odds ratios indicate that other genetic and/or environmental risk factors likely contribute to the onset and progression of the disease.
Many of the mechanisms by which FH/FHL-1 Y402H substitution increases AMD risk are well described: Reduced binding of mutant CFH to C-reactive protein (CRP) upregulates the cytokines interleukin-8 and CCL2 and thus potentially increases inflammatory damage (Giannakis et al., 2003), while reduced binding to oxidized phospholipids and malondialdehyde, a common lipid peroxidation product, may exacerbate oxidative stress (Shaw et al., 2012; Weismann et al., 2011). In addition, FH/FHL-1 Y402H substitution overactivates the complement pathway, explaining the deposition of complement proteins in the region of Bruch’s membrane and increased drusen formation (Giannakis et al., 2003; Magnusson et al., 2006; Ufret-Vincenty et al., 2010). Aged CFH-deficient (Cfh−/−) mice partly recapitulate these phenotypes, with only autofluorescent subretinal deposits that resemble lipofuscin (Fig. 8) and complement C3 accumulation (Anderson et al., 2002a) in the neural retina generally observed. While deformed and degenerated photoreceptor outer segments in Cfh−/− mice likely explain their decreased light response amplitudes and visual acuity, Coffey et al. did not observe appreciable photoreceptor loss, indicating that lack of CFH alone does not cause late-stage AMD (Coffey et al., 2007). Similarly to patients with AMD, environmental stressors, such as high-fat cholesterol-enriched diet, smoking or hydroquinone, a major component of cigarette smoke, markedly accelerate and exacerbate AMD-like pathology in Cfh+/− mice or Cfh−/− mice with mutated human CFH Y402H (Aredo et al., 2015; Feng et al., 2022; Landowski et al., 2019), while a high-fat cholesterol-enriched diet reduces light response amplitudes in mice expressing human CFH Y402H (Fig. 3C, from (Landowski et al., 2019)).
Fig. 8. Autofluorescence deposits in Cfh−/− mouse retinas.

From (Coffey et al., 2007).
3.2. High-temperature requirement factor A1 (HTRA1) and age-related maculopathy susceptibility 2 (ARMS2) in AMD
Although AMD was found to be associated with the age-related maculopathy susceptibility 2 (ARMS2) and high-temperature requirement factor A1 (HTRA1) genes at locus 10q26 only after CFH-CFHR5 had been identified, their higher penetrance impacts individuals substantially more. Sixty-four percent of patients with late AMD carry an ARMS2/HTRA1 risk allele and homozygous individuals have odds ratios of 8.6 for developing GA and 11.2 for neovascular AMD (Thee et al., 2022). Due to strong linkage disequilibrium, i.e., the non-random association of closely positioned genes on the same chromosome during meiosis, at the 10q26 locus (Jakobsdottir et al., 2005; Rivera et al., 2005), the disease-causing gene(s) or variant(s) of ARMS2 and HTRA1 cannot be resolved through GWAS studies, making the differential contribution of these two genes and associated encoded proteins in the pathophysiology of AMD controversial. NHPs, such as rhesus monkeys, possess ARMS2 and HTRA1, variants of which are associated with soft drusen as an indicator of AMD pathology (Francis et al., 2008). However, since the linkage disequilibrium within these genes in monkeys is reduced compared to humans, the different gene structure of this region in NHPs indicates that they may not represent a good model organism to determine AMD-causal variants and elucidate underlying mechanisms.
Variant rs1049024 in exon 1 and an insertion-deletion (del443ins54) in the 3’ untranslated region of ARMS2 demonstrate that SNPs in both coding and non-coding regions contribute to AMD risk (Pan et al., 2022). Only recently have analyses carried out among individuals with rare recombinant haplotypes helped narrow the region associated with AMD risk to a block of SNPs that overlap ARMS2 exon 1 and intron 1 (Grassmann et al., 2017). Recent work performed using a large number of human donor eyes showed that the level of the HtrA1 protein at the primary location of AMD pathology increases with age among individuals with no risk at the 10q26 locus and that this increase is significantly lower among individuals with ARMS2/HTRA1 risk alleles, resulting in significantly lower HTRA1 mRNA levels. Reduced HTRA1 expression is RPE-specific and mediated by a non-coding, cis-regulatory element located in a region almost identical to that identified by Grassman et al. and Williams et al. (Grassmann et al., 2017; Williams et al., 2021). While several investigations have reported that HTRA1 expression and protein levels were in fact higher in patients with AMD, most of these studies, however, did not consider genetic risk status at the 10q26 locus (Merle et al., 2022; Pan et al., 2022). Based on these recent findings, we expect HTRA1 levels to be higher among AMD patients with no risk at the chromosome 10q26 locus as compared to controls.
Reliable detection and localization of the ARMS2 protein in human tissue and cells remain elusive, resulting in many conflicting reports to date (Fritsche et al., 2008; Kanda et al., 2007; Kortvely et al., 2010; Wang et al., 2009). Since mice do not carry the ARMS2 gene and ubiquitous postnatal expression of ARMS2 in aged mice does not confer abnormal retinal phenotypes (Liu and Hoh, 2015) (Table 1), our understanding largely relies on in vitro studies, in which ARMS2 protein localizes to mitochondria (Kanda et al., 2007) and extracellular matrix (ECM) (Kortvely et al., 2010). In a complex with the complement activator properdin, ARMS2 increases C3b surface opsonization, which tags apoptotic and necrotic cells for phagocytosis (Micklisch et al., 2017). Likely due to increased turnover of ARMS2 mRNA (Fritsche et al., 2008), monocytes from patients with two high-risk alleles of the ARMS2 variants rs10490924 and del443ins54 do not express ARMS2 protein (Micklisch et al., 2017), which reduces phagocytosis and cellular debris removal and may increase the risk for drusen deposits over time (Micklisch et al., 2017). Of note, the high-risk del443ins54 and rs10490924 ARMS2 mutations decrease the expression of mitochondrial superoxide dismutase (SOD) 2 and SOD activity and thus increase oxidative stress in patient-induced pluripotent stem cell (iPSC)-derived RPE cells (Chang et al., 2023; Yang et al., 2014), potentially linking these mutations to environmental factors and aging that contribute to AMD pathology (see Chapter 4.1).
Table 1.
Common AMD animal models. Pathologies indicate phenotypes present in each model that are common in human AMD patients.
| Genetic risk models of AMD | Gene of Interest | Model | Pathway | Pathology Autofluorescence | Deposits/plaque | PRC atrophy/changes | RPE atrophy/changes | Reduced ERG | CNV |
|---|---|---|---|---|---|---|---|---|---|
| Chimeric CFH tg + hydroquinone/light | Mouse | Complement/oxidative stress | ✓ | ✓ | N/A | N/A | N/A | N/A | |
| Human CFH−/− Y402H variant + high fat diet | Mouse | Complement/lipid metabolism | N/A | ✓ | N/A | ✓ | ✓ | N/A | |
| CFH+/− + cigarette smoke | Mouse | Complement/oxidative stress | ✓ | ✓ | ✓ | ✓ | ✓ | X | |
| CFH−/− | Mouse | Complement | ✓ | X | ✓ | ✓ | ✓ | X | |
| ARMS2 risk variants | Non-human primates | Transcription factor and oxidative stress | N/A | ✓ | N/A | ✓ | N/A | N/A | |
| HTRA1−/− | Mouse | Serine protease and extracellular chaperone | ✓ | N/A | ✓ | N/A | ✓ | N/A | |
| HTRA1 Ubiquitous Overexpression | Mouse | X | ✓ | ✓ | ✓ | N/A | ✓ | ||
| hHTRA1 expression in RPE | Mouse | ✓ | ✓ | N/A | ✓ | N/A | ✓ | ||
| hHTRA1 expression in PRC | Zebrafish | ✓ | X | ✓ | X | N/A | X | ||
| Human apoE4, high fat-cholesterol diet, aged | Mouse | Lipid metabolism | N/A | ✓ | N/A | ✓ | N/A | ✓ |
Legend.
X Phenotype not present.
✓ Phenotype present.
N/A Unknown phenotype.
ARMS2 not only resides closely to HTRA1, but the del443ins54 ARMS2 mutation also increases HTRA1 transcription by binding Gtf2i-β/δ transcription factors in AMD patient-derived cell lines (Pan et al., 2021). HTRA1 binds various proteins, including transforming growth factor (TGF-β) 2, which regulates angiogenesis (Pan et al., 2021), however, it mainly functions as a secreted serine protease by cleaving several proteins associated with AMD, like retinol binding protein (RBP) 3 which prevents toxic lipofuscin formation around photoreceptors (Tom et al., 2020). HTRA1 also cleaves clusterin, present in soft Drusen from AMD patients (Sakaguchi et al., 2002), vitronectin and fibromodulin that all modulate the complement cascade, as well as other RPE-secreted proteins involved in amyloid deposition (An et al., 2010; Choi et al., 1989). As perhaps one of its most important roles in AMD, HTRA1 cleaves ECM components, such as fibronectin and nidogen 1 and 2, but not laminin, type IV collagen and elastin, resulting in a discontinuous elastic layer of BrM in mice that overexpress murine Htra1 in RPE cells (Vierkotten et al., 2011).
Multiple existing animal models may elucidate some of the mechanisms by which HTRA1 contributes to AMD (Table 1): In line with its role as a serine protease that cleaves ECM proteins, overexpression of mouse Htra1 in the entire body (Nakayama et al., 2014) and human HTRA1 in RPE cells (Nakayama et al., 2014) caused choroidal neovascularization, likely by degrading the elastic lamina of BrM and compromising underlying choroidal blood vessels (Jones et al., 2011a; Kumar et al., 2014). Considering that genetic risk variants that increase HTRA1 expression in patients are associated with neovascular AMD, particularly the transgenic mice that selectively overexpress HTRA1 in the RPE described by Jones et al. and Kumar et al. represent promising models to investigate polypoidal choroid vasculopathy (Jones et al., 2011a; Kumar et al., 2014), an anti-VEGF resistant form of neovascular AMD (Gomi et al., 2008; Kokame et al., 2010). While overexpression of human HTRA1 in zebrafish photoreceptors accumulates lipofuscin and induces photoreceptor cell death (Oura et al., 2018), RPE cell loss may be abrogated since this species not only repairs full thickness retinal injury, but also regenerates RPE, likely from remaining RPE cells (Hanovice et al., 2019). Htra1 knockout mice develop strong retinal phenotypes with loss of photoreceptors and RPE dysfunction according to a recent conference abstract, but no peer-reviewed paper has been published yet that describes this model (Biswas et al., 2022).
3.3. Myocilin (MYOC) in glaucoma
Of the currently four genes specifically associated with Mendelian inheritance of glaucoma, myocilin, WDR36, optineurin, and TBK1, we have chosen to discuss the former in detail for this review. Myocilin (MYOC) mutations as one of the major genetic risk factors for glaucoma were first identified as a causative element in patients with juvenile open-angle glaucoma (JOAG) and subsequently associated with POAG in at least two families (Alward et al., 1998; Stone et al., 1997). Approximately 70 MYOC mutations link to various forms of glaucoma (http://myocilin.com/) and with a prevalence of 3–5 % among adult-onset patients they form the most common form of inherited glaucoma (Alward et al., 1998; Stone et al., 1997; Suzuki et al., 1997), some of which associate with familial forms of glaucoma, including TYR437HIS and ILE477ASN (Alward et al., 1998). While the function of MYOC currently remains unknown, most tissues of the body, including ocular tissues, trabecular meshwork (TM), ciliary body, retina, sclera, cornea, lens and vitreous, express this protein (Adam et al., 1997; Karali et al., 2000; Kubota et al., 1997; Ortego et al., 1997; Swiderski et al., 2000; Wang and Johnson, 2000). As a component of aqueous humor (AH), secreted MYOC circulates throughout the anterior segment and thus reaches all tissues in the conventional outflow pathway (Russell et al., 2001). Although glucocorticoids increase MYOC expression, no “classic” glucocorticoid response element (GRE) sequences were found in the MYOC promoter (Fingert et al., 1998; Nguyen et al., 1998), but they contain multiple GRE “half-sites” with similar sequences that bind glucocorticoid receptors and mediate delayed responses to steroids (Chan et al., 1991), which may induce MYOC protein expression in cultured human TM cells (Shepard et al., 2001) and thus provide a mechanism by which glucocorticoids can induce glaucoma.
Two main hypotheses have been put forward to explain the role for MYOC in glaucoma pathogenesis: 1) overproduced and secreted MYOC may accumulate within the TM and increase outflow resistance (Polansky et al., 1997) or 2) mutations may decrease MYOC secretion from TM cells, which accumulates in cellular secretory compartments (Caballero et al., 2000). While MYOC perfused into human anterior segments increases the outflow resistance and, therefore, IOP (Fautsch et al., 2000) and spontaneously elevated IOP and glaucoma are associated with increased MYOC in rats and canines (Hart et al., 2007; MacKay et al., 2008a; Mackay et al., 2008b; Naskar and Thanos, 2006), reverse findings in human cells and tissues complicate the issue. Primary human TM cells release less mutant compared to wildtype MYOC (Caballero et al., 2000; Jacobson et al., 2001) and the AH in glaucoma patients contains no detectable mutant MYOC Q368X (Jacobson et al., 2001). Conversely, increased MYOC in the TM of glaucoma patients (Lutjen--Drecoll et al., 1998) suggests that the protein, especially when misfolded, overexpressed and aggregated in the endoplasmic reticulum, initiates a response to remove the misfolded protein from the cells’ secretory machinery (Liu and Vollrath, 2004; Wang and Kaufman, 2016). While in non-diseased cells autophagy can clear wildtype MYOC buildup (Aroca-Aguilar et al., 2005; Qiu et al., 2014), dysregulated glaucomatous TM cells likely accumulate mutated MYOC in the secretory pathways (Aroca-Aguilar et al., 2005; Jacobson et al., 2001; Porter et al., 2015), causing ER stress and ultimately resulting in cell death and tissue degradation (Liu and Vollrath, 2004; Wang and Kaufman, 2016). In addition, mutant MYOC also causes intracellular accumulation of ECM proteins such as fibronectin, elastin and type IV collagen due to ER stress caused by misfolded MYOC (Kasetti et al., 2016). Due to this multitude of experimental evidence the second hypothesis that mutant MYOC variants aggregate in the endoplasmic reticulum (ER), creating ER stress and causing cell death, has become widely accepted.
The importance of MYOC in glaucoma led Senatorov et al. to develop a mutant mouse with the same Tyr423His point mutation seen in humans (Senatorov et al., 2006) (Table 2), which associates with the most severe forms of glaucoma and a younger age of onset. While the TM and sclera in both wildtype and transgenic mice expressed MYOC, it was consistently found at elevated levels in the TM of transgenic mice, which also displayed increased IOP compared to wildtype at 12–18 months of age. By 12–20 months the optic nerve of transgenic mice was damaged, with increased GFAP expression, patches of “empty” space and dark axonal profiles and ~20 % loss of RGCs in the peripheral retina. While we do not necessarily understand how mutated MYOC increases IOP, degenerates the optic nerve and causes RGC death, these changes in transgenic mice are consistent with POAG progression in humans, making Tyr423His Myoc mice and effective animal model to study glaucoma.
Table 2. Common genetic animal models in glaucoma.
Pathology indicates phenotypes present in animal models characteristic of glaucoma. IOP, intraocular pressure, RGCs, retinal ganglion cells.
| Genetic models of glaucoma | Gene of Interest | Model | Pathway | Pathology Elevated IOP | Degenerating RGCs | Decreased retinal function | Reduced RGC number | Reduced retinal thickness | Optic nerve changes |
|---|---|---|---|---|---|---|---|---|---|
| Tyr423 His Myoc | Mouse | Myocilin mutation | ✓ | ✓ | ✓ | ✓ | N/A | ✓ | |
| LOXL1−/− | Mouse | Elastosis | ✓ | N/A | N/A | N/A | N/A | ✓ | |
| 9p21 locus−/− | Mouse | Cell cycle progression | X | X | ✓ | X | N/A | ✓ |
Legend.
X Phenotype not present.
✓ Phenotype present.
N/A Unknown phenotype.
3.4. Lysyl oxidase-like 1 (LOXL1) in glaucoma
In addition to the above mentioned MYOC, WDR36, optineurin and TBK1, which cause monogenic glaucoma, a number of genes have been associated with the disease through genome-wide association studies. Although an exhaustive list would exceed the scope of this review, we have included several of these genes in Table 2 and will discuss lysyl oxidase-like 1 (LOXL1) and the 9p21 locus (below) as important examples in detail. First discovered in a GWAS by Thorleifsson et al. (2007), mutations in LOXL1 are associated with pseudoexfoliation glaucoma (XFG), a subtype of open-angle glaucoma. While the specific responsible genetic variants remain under investigation, numerous identified SNPs are associated with decreased or increased risk depending on the variant (Eliseeva et al., 2021) and can even differ among ethnic populations (Chen et al., 2009, 2010; Fuse et al., 2008; Lee et al., 2009; Ramprasad et al., 2008; Rautenbach et al., 2011; Williams et al., 2010). Thus far, the causative element of the LOXL1 gene in glaucoma remains unknown and studies to examine whether non-coding regions, such as those encoding long non-coding RNAs (lncRNAs), regulate LOXL1 expression are underway (Hauser et al., 2015; Schmitt et al., 2023).
The lysyl oxidase family comprises enzymes that regulate, synthesize and maintain elastic fibers (Csiszar, 2001; Kagan and Li, 2003), as demonstrated by the strong colocalization of LOXL1 with elastin (Liu et al., 2004). Differing reports of LOXL1 expression in patients with XFG, ranging from increased protein in the AH (Greene et al., 2020) to reduced mRNA in ocular tissues (Pasutto et al., 2017), may be reconciled by the observation that LOXL1 expression depends on the disease stage, with early increased LOXL1 in ocular tissues which declines in advanced disease (Schlotzer-Schrehardt et al., 2008). LOXL1 likely contributes to IOP control, since knockout mice with severe elastic tissue abnormalities (Liu et al., 2004) exhibit a strong ocular phenotype with decreased ocular compliance, increased AH outflow capacity and possibly increased episcleral venous pressure that prevents collapse of Schlemm’s Canal (SC) during acute IOP elevation (Li et al., 2020). The phenotype of LOXL1−/− mice depends on their genetic background (Schmitt et al., 2021; Suarez et al., 2022), which will surely lend fundamental insight into the human genes that confer susceptibility to XFG. Future studies that develop mouse lines with specific risk haplotypes rather than complete knockouts would allow researchers to specifically determine the pathophysiological pathways affected within the eye.
3.5. 9p21 locus in glaucoma
In addition, GWAS in glaucoma patients have identified multiple SNPs at locus 9p21.3 (Ng et al., 2014), initially in a European and an American Caucasian cohort and subsequently across different continents. On chromosome 9p21 the cyclin-dependent kinase inhibitor (CDKN) 2A and CDKN2B genes lie adjacent in a region known as the Ink4 locus and encode tumor suppression proteins that inhibit cell cycle progression (Hannon and Beach, 1994; Ng et al., 2014; Seoane et al., 2001), including TGFβ, an important cytokine in glaucoma pathogenesis (reviewed e.g. by (Prendes et al., 2013)) that upregulates CDKN2B. A lncRNA, known as CDKN2B-AS, overlaps CDKN2B and is transcribed in the opposite direction, but thus far its function remains elusive. Interestingly, the glaucoma-associated SNPs reside in the lncRNA CDKN2B-AS or its introns, indicating that this lncRNA plays a role in glaucoma pathogenesis, likely by influencing RNA expression levels and splicing patterns (Burd et al., 2010; Wiggs et al., 2012).
Variants in the Ink4 locus could conceivably contribute to glaucoma pathogenesis through several mechanisms: Since TGFβ upregulates CDKN2B, while CDKN2B-AS silences transcription within the Ink4 locus, rendering both molecules antagonists, dysregulated CDKN2B-AS could increase expression of CDKN2B and thus increase susceptibility of RGCs to apoptosis (Ng et al., 2014). In addition, CDKN2B-AS may regulate genes outside of the Ink4 locus that influence RGC function and fate. SNPs in this locus may also affect transcription factor binding independently of CDKN2B and/or CDKN2B-AS (Hannou et al., 2015; Harismendy et al., 2011) or affect post-transcriptional regulation of RNA (Zhang et al., 2014). Visel et al. created a transgenic mouse model to investigate a 70 kb deletion in the murine equivalent of the 9p21 locus, which lacks three exons in the murine counterpart of CDKN2B-AS (Zheng et al., 2013). Although the retina functions as normal with typical histological morphology in heterozygous and homozygous CDKN2B-AS knockout mice, glial cells in the retina and optic nerve head were activated and IOP elevation resulted in greater loss of RGCs in homozygotes (Gao and Jakobs, 2016).
4. Modeling pathological pathways in AMD and glaucoma
While GWAS have identified numerous gene variants associated with AMD and glaucoma, the mechanism(s) by which high or low-risk alleles modify the probability of these diseases developing and progressing remain unclear. AMD and glaucoma likely represent multifaceted diseases in which multiple unknown molecular changes converge on a common, clinically observable pathophysiology. In other words, individual patients are likely to experience AMD and glaucoma due to different underlying causes, making it impractical to study one model system that recapitulates all the known hallmarks of both eye diseases. On the other hand, changes to the same downstream processes in the eye contribute to AMD and glaucoma pathology, even if their link to human genetic risk factors remains unknown or can only be speculated. This has resulted in the development and use of animal models that phenocopy pathways implicated in AMD and glaucoma to study specific aspects of the disease (Tables 3, 4 and 5), sometimes in transgenic animals with mutations that have never been found in patients, but which, nevertheless, offer valuable information about pathobiological mechanisms. In this chapter, we will review the main pathological pathways and animal models used to study their role in AMD and glaucoma.
Table 3.
Genetic models of AMD that phenocopy pathological pathways in human patients.
| Oxidative stress models of AMD | Gene of Interest | Pathway | Pathology Autofluorescence | Deposits/plaque | PRC atrophy/changes | RPE atrophy/changes | Reduced ERG | CNV |
|---|---|---|---|---|---|---|---|---|
| Sod1−/− mice | Superoxide breakdown | N/A | ✓ | ✓ | ✓ | N/A | ✓ | |
| Sod1−/− Park7−/− Prkn−/− mice | Superoxide breakdown | N/A | ✓ | N/A | N/A | ✓ | N/A | |
| Sod2 RPE knockdown mice | Superoxide breakdown | ✓ | ✓ | ✓ | ✓ | ✓ | N/A | |
| MEFD+/− mice + light | Antioxidant gene expression | N/A | N/A | ✓ | N/A | ✓ | N/A | |
| Nrf2−/− mice + light | Antioxidant gene expression | ✓ | ✓ | ✓ | ✓ | ✓ | X | |
| Abca4−/− Rdh8−/− | Compromised all-trans-retinal clearance | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
Legend.
X Phenotype not present.
✓ Phenotype present.
N/A Unknown phenotype.
Table 4.
Genetic models that link oxidative stress with retinal ganglion cell loss due to excitotoxicity.
| Oxidative stress models of glaucoma | Gene of Interest | Species | Pathway | Pathology IOP elevation | RGC loss | Glial cell activation | Altered mitochondrial fission/fusion | Optic nerve degeneration |
|---|---|---|---|---|---|---|---|---|
| OPAenu/+ | Mouse | Mitochondrial inner membrane structure | N/A | ✓ | ✓ | ✓ | N/A | |
| GLAST−/− | Mouse | Glutamate transporter | X | ✓ | ✓ | N/A | ✓ | |
| EAAC1−/− | Mouse | Glutamate transporter | X | ✓ | ✓ | N/A | ✓ |
Legend.
X Phenotype not present.
✓ Phenotype present.
N/A Unknown phenotype.
Table 5.
Genetic models that connect ECM changes with glaucoma.
| ECM models of glaucoma | Gene of Interest | Species | Pathway | Pathology IOP elevation | Decreased outflow capacity | RGC loss | Optic nerve degeneration | Increased ECM protein expression |
|---|---|---|---|---|---|---|---|---|
| Col1a1r/r | Mouse | Collagen hydrolysis inhibition | ✓ | ✓ | ✓ | N/A | N/A | |
| SPARC−/− | Mouse | ECM synthesis, collagen regulation | X | X | N/A | N/A | N/A | |
| Col4a1 C57/BL6 | Mouse | Basement membrane components | X | N/A | X | X | N/A | |
| Col4a1 129B6 | Mouse | Basement membrane components | ✓ | N/A | ✓ | ✓ | N/A | |
| PTP-Meg2 (−/+) | Mouse | Tyrosine-phosphatase | ✓ | N/A | ✓ | ✓ | ✓ |
Legend.
X Phenotype not present.
✓ Phenotype present.
N/A Unknown phenotype.
4.1. Modeling oxidative stress in AMD
The retina provides a unique environment that favors reactive oxygen species (ROS) (Beatty et al., 2000) due to multiple factors: the retina consumes vast quantities of oxygen, even compared to the brain (Carlisle et al., 1964), contains polyunsaturated fatty acids that readily oxidize and photosensitive molecules such as rhodopsin and directly experiences light, including short wavelengths which inflict tissue damage (Ham et al., 1976). Multiple observations have long linked AMD with oxidative stress, including higher AMD risk in current and former smokers (Clemons et al., 2005; Klein et al., 1998; Mitchell et al., 2002), mildly protective effects of antioxidant vitamins and minerals in the AREDS and AREDS2 supplements which slow the advance of AMD (AREDS2 Research Group, 2012; Chew et al., 2022; Chew et al., 2013), and higher levels of the macromolecular oxidative damage markers malondialdehyde, protein carbonyl and 8-OHdG in the serum from patients with neovascular AMD (Totan et al., 2009). In fact, aging of the body increases oxidative stress and reduces antioxidant defense mechanisms (Zhang et al., 2015), which may at least in part explain the age dependency of AMD.
Many genetic mouse models that increase oxidative stress target antioxidant proteins (Table 3), such as SOD, although some studies also affect the nuclear factor erythroid 2-related factor (NRF2) transcription factor that regulates the expression of various antioxidant proteins, including SOD and its upstream activator myocyte enhancer factor 2D (MEF2D). Photoreceptors in homozygous Mef2d knockout mice fail to develop outer segments and eventually degenerate (Andzelm et al., 2015; Omori et al., 2015), but retinas from haploinsufficient Mef2d+/− mice with otherwise normal retinal morphology express approximately 50 % less MEF2D protein, rendering their retinas more susceptible to oxidative stress. A single dose of low light exposure to induce oxidative stress no longer increases Nrf2 transcription and protein translation in Mef2d+/− mice, reducing the expression of multiple antioxidant genes, including all forms of SOD, and resulting in photoreceptor apoptosis (Nagar et al., 2017). Similarly, Nrf2−/− mice lose photoreceptors following light exposure (Wang et al., 2019), but since Nagar et al. mainly evaluated transcription factors and expression of their downstream targets, whereas Wang et al. focused on retinal structure and both studies overlap only when showing reduced photoreceptor light responses, it remains unclear which phenotype is more severe and whether other transcription factors besides MEF2D contribute to light-induced NRF2 activation. Although it takes over a year for symptoms to develop naturally in these mice, it is noteworthy that, like patients who require multiple insults to develop AMD, a diminished antioxidant response combined with light as an environmental insult considerably accelerates AMD-like morphology with subretinal deposits and lesions due to RPE and photoreceptor loss (Wang et al., 2019). In addition, deletion of PGC-1α, a transcription factor that controls mitochondrial biogenesis and respiration, in Nrf2−/− mice also leads to dry AMD-like phenotype including RPE degeneration and loss of photoreceptor function (Felszeghy et al., 2019).
SOD1 or Cu–Zn SOD-deficient (Sod1−/−) mice are more susceptible to oxidative damage, as SOD1 is a major antioxidant enzyme that eliminates superoxide and accounts for most of the SOD activity in the retina (Behndig et al., 1998; Valentine et al., 2005). Aged Sod1−/− mice, one of few available AMD models with both drusen (Fig. 9) and CNV, exhibit, thinning of the RPE, compromised integrity of the RPE barrier, thickening of Bruch’s membrane and CNV at approximately 10 months of age, with light exposure in at least five-month-old mice inducing drusen earlier (Imamura et al., 2006). The Sod1−/− DJ-1(Park7)−/− Parkin (Prkn)−/− triple knockout mouse, which also targets the antioxidant, transcriptional regulator and molecular chaperone PARK7 as well as Parkin, a ubiquitin ligase that marks proteins for degradation, significantly shortens the time until it develops drusen by three to six months, accumulation of microglia and thinning of the RPE at nine months and compromised retinal light responses at one year of age (Zhu et al., 2019). Injection of adeno-associated virus targeting SOD2 or manganese-dependent SOD in RPE cells accelerates the appearance of symptoms to approximately 4.5 months and may represent a more convenient model to study signs of AMD, such as drusen-like deposits, RPE atrophy and increased BrM thickness. These mice also develop photoreceptor disorganization and attenuated photoreceptor responses on electroretinogram, which allow researchers to study disrupted photoreceptor function and structure that normally occur late in AMD (Justilien et al., 2007). An important AMD mouse model combines deletion of two enzymes, ATP-binding cassette, sub-family A, member 4 (ABCA4) and retinol dehydrogenase 8 (RDH8), that rapidly clear all--trans-retinal from the retina. Abca4−/− Rdh8−/− mice accumulate all--trans-retinal in the retina, leading to accumulation of toxic conjugate products (Maeda et al., 2009). This model phenocopies most of the AMD pathology, including lipofuscin, drusen, RPE and photoreceptor atrophy, as well as complement activation and CNV (Table 3) and has been used to test efficacy of antioxidative and anti-VEGF drugs for treatment of AMD (Maeda et al., 2009).
Fig. 9. Drusen formation in old Sod1−/− mouse eyes.

Yellowish deposits in fundus photograph (A) and dome-shaped deposit between RPE (*) and Bruch’s membrane (**) (B). (Imamura et al., 2006).
4.2. Modeling oxidative stress in glaucoma
Increased mitochondrial DNA (mtDNA) damage and lipid peroxidation byproducts indicate oxidative stress in patients with glaucoma (Izzotti et al., 2003; Zanon-Moreno et al., 2008). A combination of endogenous and exogenous factors contributes to oxidative stress-induced damage in the anterior segment of the eye: In addition to ROS generated as part of normal metabolism and cell functions in mitochondria, peroxisomes, endoplasmic reticulum and plasma membrane (Bae et al., 2011; Block et al., 2009; Fransen et al., 2012; Krause, 2004; Nauseef, 2008; Rinnerthaler et al., 2015; Turrens, 2003), light with wavelengths longer than 295 nm passes through the cornea into the anterior chamber (Norren and Vos, 1974), where it can cause oxidative damage. In addition, a higher number of mtDNA mutations in glaucoma patients results in mitochondrial dysfunction with decreased ATP synthesis and increased production of reactive oxygen species (ROS) (Abu-Amero et al., 2006; Lee et al., 2012). Thus, many in vitro and rodent glaucoma models connect elevated pressure or IOP with mitochondrial damage and oxidative stress through mitochondrial pathways (Ferreira et al., 2010; Ju et al., 2007; Ko et al., 2005; Kong et al., 2009, 2012; Moreno et al., 2004; Tezel et al., 2005).
Oxidative stress can likely alter the morphology and function of the TM outflow pathway and thus increase IOP by affecting ECM structure and accumulation (Knepper et al., 1996a, 1996b), cell-matrix adhesion through the cytoskeleton (Zhou et al., 1999), mitochondrial activity (Clopton and Saltman, 1995), cellular DNA (Izzotti et al., 2011), cell cycle progression (Giancotti, 1997) and apoptosis (Droge, 2002; Frisch and Ruoslahti, 1997), as well as other forms of cell death (Martindale and Holbrook, 2002). Interestingly, increased IOP and visual field defects caused by RGC degeneration in patients with glaucoma correlate with oxidative damage in the TM (Izzotti et al., 2003; Sacca et al., 2005). Taken together, multiple studies indicate that ROS alter the morphology and function of the TM outflow pathway leading to an increase in IOP (Alvarado et al., 1981; De La Paz and Epstein, 1996; Freedman et al., 1985; Gabelt and Kaufman, 2005; Izzotti et al., 2003; Kahn et al., 1983; Kumar and Agarwal, 2007; Sacca et al., 2005; Tan et al., 2006; Wielgus and Sarna, 2008; Zhou et al., 1999).
Several mouse models have also provided a possible mechanism by which oxidative stress may link to RGCs loss (Table 4). Although previous studies questioned whether glutamate excitotoxicity contributes to RGC death in glaucoma (Lotery, 2005), more recent work suggested that it likely plays a role at least in glaucomatous mice (Atorf et al., 2013; Ju et al., 2009). Retinas from OPA1-deficient mice with increased mitochondrial fission experience elevated oxidative stress and upregulate expression of N-methyl-D-aspartate glutamate receptors in the RGC layer (Nguyen et al., 2011). Together with the observation that mice lacking the glutamate/aspartate transporter (GLAST) primarily found in Müller glia or the excitatory amino acid carrier 1 (EAAC1) expressed in retinal neurons lose RGCs (Harada et al., 2007), possibly due to decreased glutathione production putting RGCs at risk (Maher and Hanneken, 2005), this suggests that a vicious cycle of mitochondrial dysfunction, oxidative stress and excitotoxicity via upregulated glutamate receptors may cause RGC death in glaucoma. Intriguingly, a recent clinical study demonstrated that eight rare variants in EAAT1, the human GLAST homologue, are overrepresented in patients with glaucoma, with two loss-of-function variants being more than five-fold more common compared to the control population (Yanagisawa et al., 2020). This observation indicates that, although the glutamate receptor antagonist memantine failed to prevent glaucoma progression in a large-scale clinical trial (Weinreb et al., 2018), a follow up study of the drug specifically in patients with EAAT1 mutations may be worthwhile and that glutamate excitotoxicity in rodents likely represents a relevant model to develop new therapies at least for this small patient population.
4.3. Modeling extracellular matrix (ECM) alterations in AMD
Multiple studies have associated the risks of glaucoma and AMD with mutations in genes that encode extracellular matrix (ECM) proteins, matrix metalloproteinases (MMPs) that degrade ECM or tissue inhibitors of MMPs (TIMPs) (Corominas et al., 2018; Desronvil et al., 2010; Fu et al., 2007; Ji and Jia, 2019; Mackay et al., 2015; Vilkeviciute et al., 2019). Highly specialized functions within the eye require different ocular tissues to tightly control the composition and structure of their ECM: As BrM the ECM forms the scaffold for the RPE, through which nutrients, waste products and oxygen diffuse to and from the choroid and which restricts the migration of choroidal and retinal cells. The ECM in the TM regulates the outflow of AH and thus intraocular pressure, while connective tissue in the optic nerve head provides support against mechanical strain from intraocular pressure at the point where RGC axons exit the eye. Instead of coexisting as inert structural support that persists constant throughout life and remains independent from nearby tissues, the ECM closely interacts with its surrounding cells, carries out important signaling functions and changes extensively throughout life and in disease.
BrM lies between the RPE and the choriocapillaris, a fenestrated capillary bed that allows circulating molecules to easily enter the surrounding extracellular space (Fields et al., 2020). Diffusion through BrM depends on many factors, such as hydrostatic pressure, the concentration of diffusing molecules on both sides and the composition and structure of BrM, which affect the availability of e.g., nutrients, lipids, oxygen, vitamins and antioxidant components to the RPE and photoreceptors and the disposal of CO2, water, ions, oxidized lipids and cholesterol and other waste products (Booij et al., 2010). Proteins secreted from RPE and choriocapillaris endothelial cells continuously turn over BrM to ensure its structural integrity and function in the healthy eye (Zouache, 2022).
BrM thickens with both increasing age and in patients with AMD (Ramrattan et al., 1994), while age also changes its composition, e.g., protein (Beattie et al., 2010) and proteoglycan content (Hewitt et al., 1989), deposition of lipids (Beattie et al., 2010), advanced glycation end products (Glenn et al., 2009; Ishibashi et al., 1998) and mineralization (Spraul et al., 1999). As a result, BrM loses much of its permeability to macromolecules throughout life (Moore and Clover, 2001), particularly in the macula (Hussain et al., 2010). Patients with AMD have sustained macular damage that prevents measurements, but permeability of BrM in the periphery is consistently lower than that of donors without AMD (Hussain et al., 2010). These changes may trigger and drive AMD pathology by depriving RPE and photoreceptors of nutrients and oxygen while accumulating waste products may contribute to drusen formation.
Several transgenic mouse models recapitulate genetic variations in the ECM-related proteins TIMP3 and epidermal growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1) that GWAS studies have associated with risk in both glaucoma and AMD (Fu et al., 2007; Ji and Jia, 2019; Mackay et al., 2015). The Timp3S179C6/S179C mutation, which is found in several patients with Sorsby Fundus Dystrophy, an inherited macular degenerative disease that encompasses deposits below the RPE and within BrM, GA and neovascularization, decreases inhibition of MMPs, resulting in MMP2 activation and increased neovascularization in laser-induced CNV (Qi et al., 2019), which supports a role of TIMP3, especially in neovascular AMD. Variants in the EFEMP1 gene can cause familial juvenile-onset open-angle glaucoma (Collantes et al., 2022) and the EFEMP1 gene increasingly expresses in retinal-choroidal tissue from patients with AMD, primarily those affected by the neovascular form (Cheng et al., 2020). Although EFEMP1 contributes relatively little risk to AMD, mice with the R345W EFEMP1 mutation develop sub-RPE deposits and buildup of proteoglycans, which diminish diffusion across BrM (Zayas-Santiago et al., 2017). While Timp3S179C6/S179C and R345W EFEMP1 mice may not effectively replicate the complexities of AMD and do not develop many characteristics of the late disease stages, they, nevertheless, represent valuable models of chronic damage to BrM that results in neovascular AMD.
4.4. Modeling extracellular matrix (ECM) alterations in glaucoma
Dysregulated ECM affects two major tissues in glaucoma: the TM in the conventional AH outflow pathway and the lamina cribrosa (LC) at the optic nerve head, which deposits ECM materials in glaucoma. In the TM, the buildup of ECM materials, primarily in the juxtacanalicular region adjacent to SC, increases outflow resistance which elevates IOP (McMenamin et al., 1986; Miyazaki et al., 1987). Glaucoma alters the ECM of the LC in two stages, with initial thickening and posterior migration in early disease (Yang et al., 2011a, 2011b) followed by collapse of the cribriform plates along with increased collagen and elastin deposition (Burgoyne et al., 2005; Hernandez and Pena, 1997; Jonas et al., 2003). A recent study by Reinhard et al. set out to determine how increased IOP changes ECM proteins at the optic nerve head: Mice heterozygous for megakaryocyte protein tyrosine phosphatase 2 (PTP-Meg2) exhibit not only chronically and progressively increased IOP through an unknown mechanism, RGC loss and optic nerve damage, but also increased expression of the ECM components fibronectin, laminin and tenascin-C in the optic nerve head (Reinhard et al., 2021).
Multiple transgenic and knockout mouse models (Table 5) illustrate that changes in ECM composition likely contribute to different aspects of glaucoma, with those in the TM elevating IOP by affecting outflow mechanics, although those at the optic nerve head are known, but less well understood. The Col1a1r/r mutation, which confers collagenase-resistance to type I collagen, reduces the outflow capacity for AH, increases IOP and significantly decreases the number of RGC axons in the optic nerves of mice (Aihara et al., 2003; Dai et al., 2009; Mabuchi et al., 2004). Human optic nerve heads from patients with glaucoma increase collagen type IV mRNA and presumably protein biosynthesis (Hernandez et al., 1994), while mice with a heterozygous Col4a1 mutation develop highly variable IOP, including lower IOP in some animals, but, nevertheless, exhibit optic nerve hypoplasia, possibly due to altered composition of the inner limiting membrane (ILM) that stressed the immediately adjacent RGCs (Gould et al., 2007). Interestingly, further studies examining Col4a1 mutations demonstrated a strain-dependent effect, with elevated IOP, RGC loss and optic nerve head degeneration in the 129S6/SvEvTac strain, while CAST/EiJ mice did not develop glaucomatous pathologies (Mao et al., 2015). Conversely, knockout of a matricellular protein called secreted protein acidic and rich in cysteine (SPARC), which is highly expressed in the TM and regulates ECM composition, decreases IOP by reducing collagen fibril diameter in the juxtacanalicular region of the TM (Swaminathan et al., 2013).
Constitutively active TGFβ1, which mediates fibrosis, or knockout of MMP9, which degrades ECM proteins to maintain homeostasis, elevate IOP either individually or synergistically in double-transgenic animals (Robertson et al., 2013). The TGFβ signaling pathway may also link these ECM-related changes with cellular senescence: Cultured human TM cells treated with dexamethasone enrich cellular senescence pathways (Li et al., 2023), while TGFβ2 upregulates markers of senescence, possibly through mechanisms involving reactive oxygen species production (Yu et al., 2010). Integrin β3 serves as a cellular ECM receptor (Tietze et al., 1999) with a key role in ECM homeostasis and remodeling in glaucoma in the TM and at the optic nerve head (Filla et al., 2017). Stiffening of the TM due to cellular senescence and/or ECM dysregulation plays a key role in outflow pathology in glaucoma and TM stiffness increases with senescence (Morgan et al., 2015).
4.5. Modeling complement pathway and immune reaction in AMD
The blood-retina barrier grants immune-privilege to the healthy eye (Taylor et al., 2021), with RPE cells secreting immunosuppressants, including CFH that regulates complement activation (Taylor et al., 2021). The identification of CFH mutations as prominent genetic risk factors has highlighted the role of the complement system in disease etiology (Kuchroo et al., 2023), with complement over-activation increasing AMD risk, while complement under-activation protects from AMD (Armento et al., 2021; Heurich et al., 2011; Pappas et al., 2021). The choriocapillaris, which represents the main blood supply for the outer retina, relies mostly on CFH for complement protection (Mullins et al., 2011). Increased accumulation of complement membrane attack complex (MAC) in the choriocapillaris of patients with CFH risk genotypes (Mullins et al., 2011) likely contributes to cytolysis of the choriocapillaris endothelium in early AMD (Mullins et al., 2014), resulting in increased choriocapillaris dropout. Although accumulation of MAC (Mullins et al., 2011) and thinning of the choriocapillaris, especially in the subfovea (Wakatsuki et al., 2015), form a normal part of aging, increased choriocapillaris dropout in patients with CFH risk variants may raise cellular stress from accumulating visual cycle waste products and thus exacerbate immune activation. In addition, the complement system may react to inflammatory components of drusen, such as APOE, C1q, C3, C5 and C5b-9 (Ambati et al., 2013). While the RPE relies on CD46 secretion to protect itself from complement damage, its release diminishes in end-stage AMD, potentially putting RPE cells at risk (Ebrahimi et al., 2013). For a more in-depth understanding of complement system mechanisms and AMD, please refer to other excellent reviews (Armento et al., 2021).
While Ambati et al. suggested that the complement component C5 accumulates in senescent RPE and choroids from mice deficient in the chemokine ligand CCL2 or its receptor CCR2, resulting in a phenotype with many features of AMD, such as lipofuscin and drusen-like deposits, Bruch’s membrane thickening, photoreceptor and RPE degeneration and CNV (Ambati et al., 2003), these findings were not replicated by Luhmann et al. (2009). The observation that CCL2 and CX3CR1 double knockout mice develop drusen-like deposits around 6–9 weeks of age, Bruch’s membrane thickening, photoreceptor and RPE atrophy and CNV (Tuo et al., 2007) was later questioned when this mouse line was found to be contaminated with an Rd8 mutation, in the absence of which mice only showed mild inner retinal changes dissimilar to AMD (Vessey et al., 2012). Lacking accurate small animal models based on aberrant complement activation in AMD, we think that future research with complement models should instead focus on careful description of complement pathway activation in relation to multiple other risk factors to more closely approximate the pathology of AMD. In view of AMD as a chronic disease, in which prolonged immune activation may differ from short-term immune responses to injury, such as laser burns used to induce neovascularization in animal models (Lin et al., 2019), it is, therefore, imperative to closely consider which animal models best represent long-term activation of the complement system in response to environmental stressors, age and genotype risk.
4.6. Modeling ocular hypertension
Common strategies to model glaucoma elevate IOP in animals by artificially decreasing AH outflow and/or intracamerally introducing fluids. Successful early animal models used medium-sized or large species and e.g., injected kaolin into the anterior chamber of rabbits (Voronina, 1954) or α-chymotrypsin into owl monkeys (Kalvin et al., 1966). Technical advancements in small rodent glaucoma models have allowed researchers to increasingly opt for mice and rats as their favored model organisms due to low acquisition and maintenance costs and the additional advantage that mice allow for genetic manipulation. Furthermore, the conventional outflow pathways in mice and rats resemble those in humans, with a wedge-shaped trabecular meshwork consisting of cell-lined trabecular beams, a true, continuous SC and intra- and episcleral vessels (Johnson and Tomarev, 2010; McKinnon et al., 2009; Pang and Clark, 2007). Although the structure of the optic nerve head differs between small rodents and humans, with e.g., mice and rats replacing laminar sheets of connective tissue that support the optic nerve head with a glial lamina (May and Lutjen-Drecoll, 2002; Morrison et al., 1995), they, nevertheless, share many features of glaucoma and the successful development of many IOP-lowering drugs has relied on these animal models.
IOP relies on the equilibrium between the production of AH, which nourishes the cornea and lens and maintains the shape of the eye, and its drainage through the outflow pathways. Thus, IOP can be experimentally elevated by 1) occluding the TM or 2) reducing venous outflow or increasing episcleral venous pressure. The latex microbead model initially developed in the NHP (Weber and Zelenak, 2001) has been adapted to small rodents, in which intracameral injections of polystyrene microbeads physically block outflow pathways in the TM and SC (Chen et al., 2011). Restricting the injected volume to 2 μl in the mouse and 10 μl in the rat minimizes IOP spikes and thus protects the eye from reduced retinal blood flow and ocular injuries during the procedure (Pang and Clark, 2020). Other methods that occlude the TM include physical block of the TM with cross-linked hydrogel (Lin et al., 2022) or destroying the TM tissue by laser photocoagulation (Gaasterland and Kupfer, 1974; Pang and Clark, 2020).
Multiple laboratories have attempted to increase and prolong IOP elevation by varying the bead size, repeating injections or combining them with viscoelastic substances, such as sodium hyaluronate and hydroxypropylmethylcellulose, which reduce reflux when the injection needle is withdrawn and further block the outflow pathways (Chen et al., 2011; Cone et al., 2012; Sappington et al., 2010; Urcola et al., 2006). One method uses magnetic microbeads and magnets placed around the limbus of the eye which allows experimentalists to draw the microbeads from the injection site to the target tissues, thus minimizing loss of the microbeads when the needle is withdrawn and distributing them throughout the TM (Bahrani Fard et al., 2023; Bunker et al., 2015; Ito et al., 2016; Samsel et al., 2011). In these models elevated IOP is sustained for up to 4 weeks, along with RGC loss of ~20–50 % within the same time frame, although both depend on the size and number of introduced microbeads, which allows researchers to tailor IOP increases and expected RGC damage to their needs.
Investigators who wish to study changes in the conventional outflow tissues of the TM and SC in addition to observing glaucomatous changes at the optic nerve head can cauterize episcleral veins or inject hypertonic saline to sclerose them in order to increase AH outflow resistance. Cauterizing episcleral veins elevates IOP for approximately 4 weeks in 87% of the treated mice with 20% RGC loss two weeks after the procedure (Ruiz-Ederra and Verkman, 2006). Sclerosing the veins with saline results in a lesser IOP increase, but similar ~20–50 % loss of RGCs (McKinnon et al., 2003). Since these methods achieve different levels of elevated IOP, it is likely that they rely on different anterior segment responses and should not be used interchangeably (Nissirios et al., 2008). Due to the technical difficulty of sclerosing episcleral veins, this method is used more rarely than cauterization.
Ex vivo cannulation of mouse eyes represents a commonly used method to assess outflow facility and outflow tissue function, which decreases the photopic negative ERG response (Fig. 10C) similar to patients with glaucoma (Fig. 10A and B), although it lacks AH production and episcleral venous pressure (Madekurozwa et al., 2017, 2022; Sherwood et al., 2016). In order to address these limitations, the pressure in mouse eyes can be elevated in vivo, but long-term assessment and precise control of IOP in this procedure to prevent obstruction of blood flow and retinal ischemia remains challenging. Only one device, the iPump by Bello and co-workers (Bello et al., 2017), can monitor IOP over extended periods of time, since it was developed for freely moving rats that undergo cannulation for several months with no signs of significant anatomical or physiological damage while the sensor accurately measures intracameral IOP.
Fig. 10. Photopic negative ERG response (PhNR) in control (A) and glaucoma (B) patient, and in control (black, C) and OHT mouse model (grey, C).

A and B from (Preiser et al., 2013) and C from (Chrysostomou and Crowston, 2013).
In addition to these invasive models, inbred DBA/2J mice spontaneously develop pigmentary glaucoma with elevated IOP caused by compromised ocular drainage (Anderson et al., 2002b, 2008; John et al., 1998). These mice experience RGC death and optic nerve atrophy similar to that observed in human glaucoma patients, although they also develop other pathological symptoms, including corneal calcification and a small iris that complicate in vivo assessment of the ocular phenotypes (Turner et al., 2017), and frequently succumb to early death due to systemic diseases that impedes studying the age-dependent progression of glaucoma.
4.7. Modeling optic neuropathy in glaucoma
At least in animal models of glaucoma, early functional changes and RGC dendrite remodeling likely precede RGC death (Della Santina et al., 2013; El-Danaf and Huberman, 2015; Williams et al., 2016), indicating that these pathological alterations may, therefore, also occur before patients receive glaucoma treatments. Nevertheless, even when patients receive IOP-lowering therapies, they frequently continue to lose vision due to RGC axon degeneration and death, rendering relevant models to study neuroprotection of RGCs critically important (Chang and Goldberg, 2012; Doozandeh and Yazdani, 2016; Gauthier and Liu, 2016; Sena and Lindsley, 2017; Xuejiao and Junwei, 2022). In addition to effects of increased IOP on RGCs, glaucoma as a neurodegenerative disease requires us to also consider the role of the immune system and glial cell activation, similar to other neurodegenerative diseases like AMD and Alzheimer’s disease: Ocular hypertension models (discussed in Chapter 4.6) not only lead to RGC degeneration, but also activate Müller glia, microglia and astrocytes in animal models (de Hoz et al., 2018; Gallego et al., 2012; Guttenplan et al., 2020; Krizaj et al., 2014; Naskar et al., 2002; Schuettauf et al., 2004; Tezel, 2022), while ONHs from patients with glaucoma contained activated microglia (Yuan and Neufeld, 2001).
In order to investigate the pathobiology of optic neuropathy in glaucoma and to test the efficacy and safety of neuroprotective and -regenerative therapies that prevent vision loss independently of IOP elevation, scientists have developed several models that directly injure RGCs in in vivo animal models and compared these to ocular hypertension models described above. The optic nerve can be either crushed or transected without seriously compromising inner retinal circulation (Frank and Wolburg, 1996; Guttenplan et al., 2020; Levkovitch-Verbin et al., 2013a; Tang et al., 2011). Within several days this injury will result in RGC death, astrocyte and microglia activation and changes in RGC gene expression, including upregulation of pro-survival or anti-apoptotic factors (Levkovitch-Verbin et al., 2013a). Alternatively, intravitreal injection of N-methyl-D-aspartate will activate glutamate receptors and cause acute excitotoxicity due to excessive influx of Ca2+ and overactivity of RGCs (Levkovitch-Verbin et al., 2013a).
Both optic nerve crush and intravitreal NMDA injection differ significantly from ocular hypertension models or glaucoma in human patients, since they rapidly cause acute damage compared to long-term low intensity stress in glaucoma or chronically elevated IOP. In addition, the primary injury site of NMDA injection differs from axonal damage, since NMDA receptors mainly localize to RGC dendrites. Side-by-side comparison of these models has shown anti-apoptotic factors to be upregulated only in the retina in the ocular hypertension model, whereas intravitreal NMDA injection and optic nerve crush-induced injury increased pro-survival genes in both the retina and the optic nerve (Levkovitch-Verbin et al., 2013a). Thus, chronic ocular hypertension models might be better suited to understanding the pathobiology of and pre-clinical evaluation of neuroprotective or –regenerative treatments for POAG, while acute RGC injury may serve as a model of normal tension glaucoma.
5. Modeling the effect of environmental and lifestyle factors in AMD and glaucoma
Although genetics play an important role in AMD and glaucoma, explaining >50% of disease variability (Gottfredsdottir et al., 1999; Grizzard et al., 2003; Meyers et al., 1995; Meyers and Zachary, 1988; Teikari, 1990; Zukerman et al., 2021), many people carrying genetic risk factors never suffer from vision disorders despite reaching old age. For example, close to 50% of the population in certain ethnicities carry the CFH Y402H risk variant (Chowers et al., 2008; Edwards et al., 2005; Haines et al., 2005; Klein et al., 2005; Nazari Khanamiri et al., 2014; Seitsonen et al., 2008; Soysal et al., 2012), but this number vastly exceeds patients with AMD, suggesting that environment and lifestyle play an important role in its pathogenesis (Fig. 7). In comparison, the contribution of environmental factors to glaucoma are far less well understood, but IOP as the only clear modifiable factor in glaucoma can be impacted by external stressors, as discussed below. In this chapter, we will review the current knowledge regarding environmental and lifestyle factors that contribute to AMD and glaucoma and discuss animal models based on exposure to external stressors such as smoking, obesity and poor diet, as well as light exposure.
The Age-Related Eye Disease Study (AREDS) recognized the overwhelming contribution of smoking to the progression of neovascular AMD or geographic atrophy (Clemons et al., 2005), with smokers at greater risk of developing large drusen (Klein et al., 1998) and late AMD at a younger age (Mitchell et al., 2002). Light exposure, high metabolism and the abundance of unsaturated fatty acids naturally predispose the retina to oxidative stress, i.e., an imbalance between reactive oxygen and nitrogen species and protective antioxidants in the body. Harmful chemicals and toxins in cigarette smoke promote AMD at least in part by increasing oxidative stress, whereas the lower abundance of antioxidant proteins in the central compared to the peripheral retina (Velez et al., 2018) likely puts the macula at greater risk of cumulative damage. The observation that the antioxidant AREDS and AREDS2 supplements provide AMD patients with limited protection from progressing to late disease stages (AREDS2 Research Group, 2012; Chew et al., 2022; Chew et al., 2013) strengthens the notion that oxidative stress contributes to AMD.
The known link between cigarette smoke and vascular dysfunction supports the theory that smoking reduces choroidal thickness in patients with early AMD (Sigler et al., 2014; Ting et al., 2016) and decreases choroidal vascularity (Wei et al., 2019). Even exposure to secondhand smoke in 6- to 8-year-old children thins the choroid (Yuan et al., 2019), although it remains unclear whether this damage persists and raises AMD risk later in life. In addition, nicotine in cigarette smoke constricts blood vessels by stimulating α-adrenergic receptors, while carbon monoxide binds to hemoglobin in erythrocytes and diminishes their capacity to transport oxygen. While the relative contribution of these mechanisms remains unclear, smoking likely reduces oxygen and nutrient delivery to the RPE and retina and hampers the removal of waste products. In addition, chronic smoking activates long-term inflammation with the production of inflammatory mediators and cytokines that have been shown to contribute to the development and progression of AMD.
Most animal studies on cigarette smoke employ smoke machines or vacuum exposure systems that subject small rodents to smoke (Miliano et al., 2020; Morales-Mantilla et al., 2020), while hydroquinone, one of cigarette smoke’s toxic components, can be given in drinking water (Pons and Marin-Castano, 2011). Cigarette smoke or hydroquinone, administered to animals, can replicate some features of AMD, particularly drusen and changes to Bruch’s membrane. Although mice do not possess a macula, they can accumulate proteins on Bruch’s membrane that resemble drusen in patients (Wang et al., 2009). Complement factor H-deficient (Cfh+/−) mice exposed to smoke develop drusen-like deposits, hyperreflective spots, microglia activation, waste accumulation and impaired RPE tight junctions faster than non-smoking mice (Feng et al., 2022). Even electronic cigarette smoke creates an inflammatory and angiogenic environment in the retina, possibly promoting neovascular AMD in patients (Wang et al., 2021).
The contribution of light to AMD remains contested: Sunlight increased AMD risk in several studies (e.g. (Hirakawa et al., 2008; Schick et al., 2016),), but had no effect in others (e.g., (Khan et al., 2006; Park et al., 2014). While one meta-analysis concluded that sunlight (or its avoidance) does not significantly alter the risk of AMD (Zhou et al., 2018), another reported an odds ratio of 1.38 (Sui et al., 2013). Interestingly, however, several studies with a positive correlation between sunlight and AMD used e.g., skin wrinkling as a surrogate for UV exposure (Hirakawa et al., 2008) or investigated subjects that lived in an environment with plenty of blue light (Plestina-Borjan and Klinger-Lasic, 2007; Vojnikovic et al., 2007). Individuals with the lowest antioxidant levels were also most prone to AMD due to sunlight (Fletcher et al., 2008), supporting in vitro studies that blue-violet light causes maximum oxidative stress in RPE cells loaded with A2E, a component of lipofuscin in macular deposits (Marie et al., 2018).
Light-induced retinal damage has been widely applied to mimic late-stage photoreceptor death in small rodents (Marc et al., 2008) and Nrf2−/− mice, which lack basal and induced expression of a large number of antioxidant response genes, lose photoreceptors and RPE cells, form basal linear deposits that resemble drusen, thicken Bruch’s membrane and accumulate autophagosomes and lipofuscin in the RPE following white light exposure (Wang et al., 2019). These models rapidly replicate dry AMD-like symptoms when exposed to additional stressors such as (blue) light or a high fat diet (Fig. 5) that have been proposed to contribute to AMD pathology (Chapman et al., 2019; Di Carlo and Augustin, 2021; Dighe et al., 2020; Schick et al., 2016; Taylor et al., 1990; van Leeuwen et al., 2018). Chronic blue light exposure and high fat diet have been used in isolation or combined with, e.g. APOE, Cfh+/−, Nrf2−/− or cigarette smoke models to generate AMD-like symptoms in mice or rats (Cousins et al., 2002; Ding et al., 2011; Dithmar et al., 2001; Espinosa-Heidmann et al., 2006; Keeling et al., 2022; Kim et al., 2016; Roddy et al., 2020; Stanton et al., 2017; Toomey et al., 2018; Wielgus et al., 2010; Zhang et al., 2018; Zhao et al., 2014). Animal models that combine even normally low penetrant mutations in APOE, a high-fat diet and advanced age can replicate human-like AMD symptoms more effectively, as described in (Malek et al., 2005), a finding of particular interest since none of these factors individually lead to AMD, but together form an effective mouse model.
Conversely, environmental factors remain less well understood in glaucoma: Consuming 5 or more cups of coffee per day increases the risk of developing glaucoma 1.6-fold (Kang et al., 2008), likely by modestly raising IOP (Avisar et al., 2002; Chandrasekaran et al., 2005; Higginbotham et al., 1989). Although exercise acutely elevates IOP, regular exercise and/or physical fitness lower IOP in both young, healthy individuals and suspected glaucoma patients (Buckingham and Young, 1986; Martin et al., 1999; Passo et al., 1991; Qureshi et al., 1996, 1997), making regular exercise neither a risk factor nor protective. Smoking significantly elevates IOP in current and previous smokers, particularly in patients with glaucoma (Lee et al., 2020b) and increases the risk of progressing visual field defects in heavy smokers more than twofold (Mahmoudinezhad et al., 2022). Instead of replicating environmental factors, animal models generally increase IOP to mimic glaucoma, as discussed above in Chapter 4.6.
6. Modeling aging in AMD and glaucoma
Even patients with multiple risk factors seldomly contract AMD or glaucoma before age 55, and both diseases become vastly more prevalent with advancing age. Although the natural aging process appears necessary, many older patients never progress to advanced AMD or glaucoma and the age of disease onset varies widely. We will explore below how aging may contribute to the pathogenesis and progression of these two blinding diseases and how it could be modeled in animals.
In general, aging represents a complex phenomenon that remains under intensive research and debate and several models of aging at the molecular, cellular and systems level have been proposed. These include nuclear DNA damage, telomere shortening, epigenetic changes (e.g., through accumulation of DNA methylation), cellular senescence and exhaustion of stem cells, compromised proteostasis and autophagy, loss of intercellular communication, dysregulated nutrient sensing (e.g., through growth hormone, insulin and insulin-like growth factor and mTOR pathways), mitochondrial damage and chronic inflammation (recently reviewed in (Lopez-Otin et al., 2023)). Many of these processes also contribute to the pathologies of AMD and glaucoma and overlap with those that we described above in chapters 4 on pathological pathways and 5 on environmental and lifestyle factors.
In principle, age could contribute to AMD and glaucoma in three ways: 1) Chronological age represents a major risk factor in addition to, but independently of environment, lifestyle and genetic variants, 2) genetic risk variants make an individual more susceptible to biological aging in the eye and/or at the systemic level wherein the biological age is influenced by environment and lifestyle and 3) biological age is the main driver of disease, which genetic variants, lifestyle and environment can accelerate. The latter two hypotheses propose that environmental and lifestyle-induced stress accelerates aging of the eye/organism and genetic risk factors would either render cells more vulnerable to these stresses (2) or accelerate the biological age of the cells and tissues in the eye and/or at system level (3). For the development of models that can account for age dependence of AMD or glaucoma, it will be crucial to understand whether the main risk factor is chronological age or biological age of the eye system versus the whole organism.
Several studies provide evidence to suggest that the biological age of the organism, specifically of the eye, plays a critical role in AMD and glaucoma. As an example, some AMD risk variants are more prevalent in patients with advanced compared to early AMD (Magnusson et al., 2006), indicating that the disease mechanism of the risk variant itself is linked to aging. Moreover, scientists have already modified specific hallmarks of aging by removing senescent cells (Rocha et al., 2020; Xu et al., 2022) or reversing age-associated epigenetic changes in mice to treat models of glaucoma (Lu et al., 2020b). Modifying the biological age or removing senescent cells systemically to cure disease as in these two studies does not necessarily entail that the systemic, rather than the local age, drives the pathology, since systemic aging will also lead to local aging. Nevertheless, multiple lines of evidence suggest that the ocular environment drives AMD: Khandhadia et al. discovered that hepatic production of a high-risk allele of complement factor H does not alter the prevalence of AMD in patients with liver transplants (Khandhadia et al., 2013). In addition, plasma levels of CFH risk variants do not appear to play a significant role in AMD pathology whereas the CFH variant produced locally in the RPE correlates strongly with AMD (Feng et al., 2022). Biological age also increases susceptibility to IOP-induced retinal damage including RGC morphology (Di Pierdomenico et al., 2022), RGC number (Levkovitch-Verbin et al., 2013b), retinal nerve fiber layer loss (Jammal et al., 2020), as well as functional and molecular changes in the retina (Xu et al., 2022). In fact, Xu et al. effectively demonstrated that elevated IOP causes changes similar to natural aging and that repetitive stress to a young retina results in accelerated aging (Xu et al., 2022). These data suggest that modifying the biological age of the eye to treat AMD and glaucoma may be feasible and hint at new ways to generate animal models of age-related eye disease, as discussed below.
Its central role in AMD and glaucoma makes aging an imperative factor to consider when developing good animal models. Since small animals like mice or zebrafish live for approximately 2 years, limited lifespan is often cited as a potential reason why these models do not fully recapitulate AMD or glaucoma pathologies. As an alternative perspective, however, mice undergo a full life cycle, including senescence, within a briefer period of time compared to humans, although damage due to environmental stressors throughout their shorter lifespan may not accumulate to the same extent. Mice that carry the P23H rhodopsin mutation serve as an excellent illustration of this argument since they recapitulate photoreceptor degeneration within a few months compared to several decades in human patients with the same mutation (Sakami et al., 2011, 2014). In addition, rhesus monkeys with their significantly shorter lifespan than humans show drusen already at 19 years of age (Yiu et al., 2017). Thus, compared to studying aging in NHPs or humans with longer lifespans, faster disease onset due to accelerated aging in small rodent models will benefit patients by speeding up research at a reduced cost.
Many genetic mouse models that accelerate aging replicate genomic instability and telomer shortening associated with progeroid syndromes, rare genetic disorders that cause premature aging, including typical age-dependent ocular diseases like cataracts early in life (e.g., reviewed in (Folgueras et al., 2018). Htra2mnd2/mnd2/Tg-Htra2 mice express functional HTRA2, a mitochondrial serine protease, specifically in neural cells, but lack HTRA2 protease activity in non-neural tissues and have previously been used as a model of accelerated aging due to mitochondrial dysfunction (Kang et al., 2013). However, HtrA2/Omi also mediates oxidative stress-induced apoptosis of ARPE19 cells, a human RPE cell line (Ding et al., 2009) and a HTRA2-targeting peptide protected axotomized RGCs in retinal explants (Schmelter et al., 2019), indicating that further studies are warranted into whether Htra2mnd2/mnd2/Tg-Htra2 mice represent a useful aging model for AMD and glaucoma studies. Other genetic modifications that result in loss of proteostasis, such as in Stub1−/−, Tg-β5t, Psme3−/− and Gsk3a−/− mice or mitochondrial dysfunction, such as in Sqstm1−/− and Cisd2−/−mice, may, however, hold promise given that AMD and glaucoma are associated with acceleration of these age-associated pathways (Folgueras et al., 2018). Notably, SAMP8 mice, a common Alzheimer’s disease model vulnerable to accelerated aging, show susceptibility to photoreceptor damage following acute bright light exposure (Liu et al., 2017), although a comparison to SAMP8 mice without light damage remains outstanding. In our opinion, researchers should systematically investigate the potentially beneficial strategy of combining mouse models of accelerated aging with the genetic and environmental risk factor models that we reviewed above.
As a possible caveat of animal models with shorter lifespans, aging processes in the eye may not have enough time to manifest in glaucoma or AMD. To overcome this concern, conditional eye-specific mutations or knockout models could be generated in which accelerated aging would only happen in the eyes of a mouse with a normal lifespan. This, combined with mice expressing the human genetic risk variants and exposure to environmental risk factors, could revolutionize our ability to not only understand the disease pathways in AMD and glaucoma, but also test effectiveness of novel therapies.
7. Conclusions and future directions
Animal models have been critical to understanding the mechanisms of eye diseases and developing therapies to treat them, but, unlike in IRDs, complex age-related blinding diseases like AMD and glaucoma have proven challenging. We have reviewed mouse models of AMD and glaucoma based on 1) gene variants from GWAS studies (Chapter 3), 2) genetic or induced phenocopies of known pathological pathways (Chapter 4), as well as combinations of these models with known environmental and lifestyle risk factors (Chapter 5). Moreover, we have discussed the role of chronological and biological age of the organism versus the eye and the challenges and advantages of modeling aging in AMD and glaucoma using small animal models, particularly mice (Chapter 6). Overall, despite significant gaps in our understanding of complex age-related eye diseases, our current, suboptimal mouse models have provided insight and newly emerging models will critically advance our knowledge of specific pathobiological pathways in AMD and glaucoma.
Although we cannot deny the importance of small animal models in investigating eye diseases, studying disease progression from early to late stages and testing the effectiveness of therapies remains not without problems. Firstly, the size and structure of the mouse and primate eye differ greatly, with an 8-fold difference between mouse and human eye diameters of ~3 and ~24 mm, respectively (Bekerman et al., 2014; Park et al., 2012). In addition, the ILM allows for example intravitreally injected adeno-associated viruses to permeate and transduce RGCs in mice, but is impervious in primates except where the ILM thins around the fovea (Yin et al., 2011). Although pores in the ILM, especially along retinal blood vessels (Wolter, 1964), are commonly found in patients with vitreomaculopathies and their reported diameter should allow permeation of AAVs (Schumann et al., 2023), the ILM nevertheless represents a major obstacle for successful AAV delivery and has been suggested to require surgical removal, especially if repeat applications are necessary (Teo et al., 2018). Moreover, most mammalian species, including mice, lack two key structures for AMD and glaucoma, namely the macula and true lamina cribrosa. To overcome limitations imposed by these differences when testing pharmacokinetic and pharmacodynamic properties of gene and drug therapies, pre-clinical testing often relies on larger animals like rabbits and dogs. Interestingly, glaucoma naturally occurs in dogs, cats and rabbits (Gelatt et al., 1977; Kolker et al., 1963; McLellan and Miller, 2011).
AMD primarily affects the central retina with the fovea and surrounding para- and perifovea that together constitute the macula. Although many non-mammalian species possess a fovea, together with the macula it remains a primate-specific feature among mammals. As a sieve-like ECM structure the lamina cribrosa resides in the optic nerve head where it supports RGC axons. Unlike many mammalian species such as mice and rats, macaques and humans have a clearly defined lamina cribrosa (Chen et al., 2016). Since the macula and lamina cribrosa play central roles in AMD and glaucoma, respectively, mouse models of these diseases necessarily cannot fully recapitulate the progression from macular drusen formation to RPE and photoreceptor atrophy in GA or RGC axonal damage and optic neuropathy in glaucoma.
Non-human primates (NHPs) represent an expensive and rarely used, but valuable additional tool to investigate AMD and glaucoma. A significant fraction of aged (>19 year-old) rhesus monkeys surveyed at the California National Primate Research Center had drusen (Yiu et al., 2017), while some colonies contain animals that naturally develop optic neuropathy or AMD and sometimes share human genetic risk variants (Chen et al., 2014; Dawson et al., 1993; Francis et al., 2008; Singh et al., 2009). In addition, experimental disease models in NHPs include laser-induced CNV (Ryan, 1979) or light-induced stress to replicate AMD (Aredo et al., 2015; Kumar et al., 2022; Weber and Zelenak, 2001) and acute or chronic elevation of IOP with manometric or pharmacological methods, microbead injections or photocoagulation of the limbal region to mimic glaucoma (Aredo et al., 2015; Gaasterland and Kupfer, 1974; Kumar et al., 2022; Weber and Zelenak, 2001). Several recent reviews describe non-human primate models for AMD and glaucoma (Burgoyne, 2015; Fletcher et al., 2014; Pennesi et al., 2012). Although NHPs are useful for pre-clinical testing and to better understand AMD and glaucoma in human patients, we cannot, however, expect them to replace small animal models, since (1) most models use acute insults to mimic slowly progressing age-related diseases like AMD and glaucoma and (2) time, money and ethical considerations limit the progress that NHP models can achieve. While new technologies allow us to generate germline gene-edited NHP models of AMD and glaucoma, this approach may not be practical due to long gestation times, but ocular gene manipulation with viral vectors or other delivery methods, such as lipid nanoparticles or engineered virus-like particles, could conceivably locally deliver transgenes or gene editors (Choi et al., 2022; Gautam et al., 2023; Mancuso et al., 2009; Suh et al., 2022; Li et al., 2021).
Biological differences between primates and other species as well as the challenges of NHP research described above have caused scientists to also rely on research in human subjects. Clinical trials with patients importantly ensure safety and efficacy of treatments before they can be approved for standard care. However, conducting basic research with human subjects often remains not ethically justifiable and expensive and cannot take advantage of invasive experimental techniques that require in vivo anesthesia and surgery or euthanasia for ex vivo and in vitro experiments. Alternatively, immortalized or primary RPE cells, BrM and TM cells from human donor eyes or fetal tissues or cells derived from iPSCs have facilitated studying the biology of human ocular cells, as well as AMD and glaucoma for decades (Bharti et al., 2022; Flood et al., 1980; Hu and Bok, 2001; Mulfaul et al., 2020; Pfeffer, 1991; Polansky et al., 1979; Sharma et al., 2020). While these cells thrive in culture for several months, thus enabling gene editing and relatively long-term testing of therapies, especially immortalized cell lines have generally been cryopreserved and passaged repeatedly so that their identities may no longer correspond to primary human cells. In addition, cultured cells remain disconnected from their normal environment and lack cell-cell interactions and systemic inputs that determine their normal biology as well as AMD and glaucoma pathobiology. Further discussion of this topic exceeds the purview of this review, but excellent recent discussion of this topic can be found (e.g. (Bharti et al., 2022; Lu et al., 2020a)).
The use of fresh human tissue circumvents at least some of these drawbacks, but access remains limited and most human donor eyes for research are collected several hours postmortem, which affects their gene expression, structure and function (Abbas et al., 2022; Cowan et al., 2020). While the retina, which forms part of the central nervous system, no longer responds to light within minutes after cardiac death (Abbas et al., 2022), recent work from our laboratory has established the necessary infrastructure and protocols to obtain human eyes with functionally viable retinas from organ donors on a large scale (Fig. 11). This approach holds promise to study the basic physiology and pathophysiology of the retina and could allow testing of the efficiency of gene therapies on human genes in their correct chromosomal location and structural and cellular environment (Muller et al., 2023). Although researchers could perceivably source eyes from donors who were previously diagnosed with AMD or glaucoma, it will not be feasible to obtain a significant number of eyes from donors with a specific mutation except perhaps for the most common AMD risk variants, CFH-Y402H and ARMS2-A69S. Challenging work is currently underway to extend the viability of human donor retinas for longer periods of time.
Fig. 11. Retinal electrophysiology from human donor eyes.
Eyes are collected from research donors or from organ donation between 0 and 4 h postmortem (A) and transported to laboratory in oxygenated Ames’ media (B). Ex vivo ERG responses (D) recorded using custom-build specimen holder (C) from macular punches obtained from macaque, research or organ donors as indicated in D. A, B and C adapted from (Abbas et al., 2022). Data in top and bottom left panel in D is from (Abbas et al., 2022).
As an alternative approach, which has significantly advanced during the past decade, iPSCs derived from e.g., skin fibroblasts can be grown into retinal organoids (Kruczek and Swaroop, 2020; Nakano et al., 2012). With current protocols these can generate light responses and form the different retinal layers (Cowan et al., 2020; Onyak et al., 2022; Saha et al., 2022; Zhong et al., 2014), but culture conditions that facilitate formation of neural retina do not favor maturation of RPE cells or deposition of BrM. These tissues typically originate in separate cultures, although efforts have been made to combine organoid retinas and RPE cells (Achberger et al., 2019). Importantly, the usefulness of retinal organoids in AMD and glaucoma research may be limited, however, since they recapitulate the development of the retina over a similar time frame as during gestation and, therefore, may not represent good models of the aging retina, but also do not contain vascular and microglial cells (Vielle et al., 2021). However, it is possible to recapitulate some aspects of retinal degenerative disease in organoids by acute application of proinflammatory factors or activators of mechanosensitive channels (Volkner et al., 2022). Overall, eye tissues from human organ donors and human iPSC-derived organoids represent promising approaches that will advance our understanding of human eye biology, AMD and glaucoma, as well as allow testing of therapies against these blinding diseases, but they will not likely replace animal experiments in the foreseeable future.
Acknowledgements
This work was supported in part by the John A. Moran Eye Center (JMEC CORE) under Grant P30 EY014800; The work of Frans Vinberg is supported by R01s EY031706 and EY034986 from the National Eye Institute/NIH as well as by a Career Development Award from Research to Prevent Blindness; The work of Silke Becker is supported by the Diabetes Research Connection and a seed grant from the University of Utah School of Medicine; The work of Fiona McDonnell is supported by R00 EY031737 from the National Eye Institute/NIH; The work of Bryan W. Jones was supported in part by the National Institute of Health R01 EY028927, in part by the National Science Foundation under Grant 2014862 and in part by an Unrestricted Research Grant from Gabe Newell. This work was also made possible by an Unrestricted Research Grant from Research to Prevent Blindness, New York, NY, to the Department of Ophthalmology and Visual Sciences, University of Utah.
Abbreviations
- ABCA4
ATP binding cassette, subfamily A, member 4
- AH
Aqueous humor
- AMD
Age-related macular degeneration
- APOE2
Apolipoprotein E2
- ARMS2
Age-related maculopathy susceptibility 2
- BrM
Bruch’s membrane
- CCL2
C–C motif chemokine ligand 2
- CFH
Complement factor H
- CFHR
Complement factor H-related
- CRP
C-reactive protein
- EAAC1
Excitatory amino acid carrier 1
- ECM
Extracellular matrix
- EFEMP1
Epidermal growth factor-containing fibrillin-like extracellular matrix protein 1
- ER
Endoplasmic reticulum
- ERG
Electroretinogram
- FDA
Food and Drug Administration
- FH
Factor H
- FHL-1
Factor H-like protein 1
- GA
Geographic atrophy
- GLAST
Glutamate-aspartate transporter
- GFAP
Glial fibrillary acidic protein
- GWAS
Genome-wide association study
- HTRA1
High Temperature Requirement A Serine Peptidase 1
- ILM
Inner limiting membrane
- IOP
Intraocular pressure
- iPSC
Induced pluripotent stem cell
- IRD
Inherited retinal disease
- lncRNA
Long non-coding RNA
- LC
Lamina cribrosa
- LOXL1
Lysyl oxidase like-1
- MEF2D
Myocyte-specific enhancer factor 2D
- MMP
Matrix metalloproteinase
- mtDNA
Mitochondrial DNA
- MYOC
Myocilin
- NHP
Non-human primate
- NRF2
Nuclear factor erythroid 2-related factor 2
- POAG
Primary open-angle glaucoma
- PhNR
Photopic negative response
- PRS
Polygenic risk score
- PTP-Meg2
Megakaryocyte protein tyrosine phosphatase 2
- RBP3
Retinol-binding protein 3
- RDH8
Retinol Dehydrogenase 8
- RGC
Retinal ganglion cell
- ROS
Reactive oxygen species
- RPE
Retinal pigment epithelium
- SC
Schlemm’s canal
- SNP
Single nucleotide polymorphism
- SPARC
Secreted protein acidic and rich in cysteine
- SOD
Superoxide dismutase
- Tg
Transgenic
- TIMP
Tissue inhibitor of matrix metalloproteinases
- TGF-β
Transforming growth factor β
- TM
Trabecular meshwork
- VEGF
Vascular endothelial growth factor
- WT
Wildtype
- XFG
Pseudoexfoliation glaucoma
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator-1 alpha
Footnotes
CRediT authorship contribution statement
Silke Becker: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Zia L’Ecuyer: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Bryan W. Jones: Funding acquisition, Investigation, Methodology, Resources, Validation, Writing – review & editing. Moussa A. Zouache: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. Fiona S. McDonnell: Conceptualization, Funding acquisition, Investigation, Resources, Validation, Writing – original draft, Writing – review & editing. Frans Vinberg: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Data availability
Data will be made available on request.
References
- Abbas F, Becker S, Jones BW, Mure LS, Panda S, Hanneken A, Vinberg F, 2022. Revival of light signalling in the postmortem mouse and human retina. Nature 606, 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff MD, Alward WL, Greenlee EC, Shuba L, Kim CY, Fingert JH, Kwon YH, 2007. Automated segmentation of the optic disc from stereo color photographs using physiologically plausible features. Invest. Ophthalmol. Vis. Sci 48, 1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Amero KK, Morales J, Bosley TM, 2006. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci 47, 2533–2541. [DOI] [PubMed] [Google Scholar]
- Achberger K, Probst C, Haderspeck J, Bolz S, Rogal J, Chuchuy J, Nikolova M, Cora V, Antkowiak L, Haq W, Shen N, Schenke-Layland K, Ueffing M, Liebau S, Loskill P, 2019. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam MF, Belmouden A, Binisti P, Brezin AP, Valtot F, Bechetoille A, Dascotte JC, Copin B, Gomez L, Chaventre A, Bach JF, Garchon HJ, 1997. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum. Mol. Genet 6, 2091–2097. [DOI] [PubMed] [Google Scholar]
- Aihara M, Lindsey JD, Weinreb RN, 2003. Ocular hypertension in mice with a targeted type I collagen mutation. Invest. Ophthalmol. Vis. Sci 44, 1581–1585. [DOI] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Polansky J, Juster R, 1981. Age-related changes in trabecular meshwork cellularity. Invest. Ophthalmol. Vis. Sci 21, 714–727. [PubMed] [Google Scholar]
- Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM, 1998. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A). N. Engl. J. Med 338, 1022–1027. [DOI] [PubMed] [Google Scholar]
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK, 2003. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med 9, 1390–1397. [DOI] [PubMed] [Google Scholar]
- Ambati J, Atkinson JP, Gelfand BD, 2013. Immunology of age-related macular degeneration. Nat. Rev. Immunol 13, 438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An E, Sen S, Park SK, Gordish-Dressman H, Hathout Y, 2010. Identification of novel substrates for the serine protease HTRA1 in the human RPE secretome. Invest. Ophthalmol. Vis. Sci 51, 3379–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Mullins RF, Hageman GS, Johnson LV, 2002a. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol 134, 411–431. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW, 2002b. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat. Genet 30, 81–85. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Nair KS, Amonoo LA, Mehalow A, Trantow CM, Masli S, John SW, 2008. GpnmbR150X allele must be present in bone marrow derived cells to mediate DBA/2J glaucoma. BMC Genet 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andzelm MM, Cherry TJ, Harmin DA, Boeke AC, Lee C, Hemberg M, Pawlyk B, Malik AN, Flavell SW, Sandberg MA, Raviola E, Greenberg ME, 2015. MEF2D drives photoreceptor development through a genome-wide competition for tissue-specific enhancers. Neuron 86, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apellis Pharmaceutical, Inc., August 24, 2022. 24-Month Results Showing Increased Effects Over Time with Pegcetacoplan in Phase 3 DERBY and OAKS Studies in Geographic Atrophy (GA). https://investors.apellis.com/news-releases/news-release-details/apellis-announces-24-month-results-showing-increased-effects. [Google Scholar]
- Aredo B, Li T, Chen X, Zhang K, Wang CX, Gou D, Zhao B, He Y, Ufret-Vincenty RL, 2015. A chimeric Cfh transgene leads to increased retinal oxidative stress, inflammation, and accumulation of activated subretinal microglia in mice. Invest. Ophthalmol. Vis. Sci 56, 3427–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AREDS2_Research_Group, Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, Sperduto R, Ferris FL, 2012. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 119, 2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armento A, Ueffing M, Clark SJ, 2021. The complement system in age-related macular degeneration. Cell. Mol. Life Sci 78, 4487–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca-Aguilar JD, Sanchez-Sanchez F, Ghosh S, Coca-Prados M, Escribano J, 2005. Myocilin mutations causing glaucoma inhibit the intracellular endoproteolytic cleavage of myocilin between amino acids Arg226 and Ile227. J. Biol. Chem 280, 21043–21051. [DOI] [PubMed] [Google Scholar]
- Atorf J, Scholz M, Garreis F, Lehmann J, Brauer L, Kremers J, 2013. Functional protective effects of long-term memantine treatment in the DBA/2J mouse. Doc. Ophthalmol 126, 221–232. [DOI] [PubMed] [Google Scholar]
- Avisar R, Avisar E, Weinberger D, 2002. Effect of coffee consumption on intraocular pressure. Ann. Pharmacother 36, 992–995. [DOI] [PubMed] [Google Scholar]
- Bae YS, Oh H, Rhee SG, Yoo YD, 2011. Regulation of reactive oxygen species generation in cell signaling. Mol. Cell 32, 491–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani Fard MR, Chan J, Sanchez Rodriguez G, Yonk M, Kuturu SR, Read AT, Emelianov SY, Kuehn MH, Ethier CR, 2023. Improved magnetic delivery of cells to the trabecular meshwork in mice. Exp. Eye Res 234, 109602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie JR, Pawlak AM, Boulton ME, Zhang J, Monnier VM, McGarvey JJ, Stitt AW, 2010. Multiplex analysis of age-related protein and lipid modifications in human Bruch’s membrane. Faseb. J 24, 4816–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty S, Koh H, Phil M, Henson D, Boulton M, 2000. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol 45, 115–134. [DOI] [PubMed] [Google Scholar]
- Behndig A, Svensson B, Marklund SL, Karlsson K, 1998. Superoxide dismutase isoenzymes in the human eye. Invest. Ophthalmol. Vis. Sci 39, 471–475. [PubMed] [Google Scholar]
- Bekerman I, Gottlieb P, Vaiman M, 2014. Variations in eyeball diameters of the healthy adults. J Ophthalmol, 503645, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello SA, Malavade S, Passaglia CL, 2017. Development of a smart pump for monitoring and controlling intraocular pressure. Ann. Biomed. Eng 45, 990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, den Hollander AI, Lakkaraju A, Sinha D, Williams DS, Finnemann SC, Bowes-Rickman C, Malek G, D’Amore PA, 2022. Cell culture models to study retinal pigment epithelium-related pathogenesis in age-related macular degeneration. Exp. Eye Res 222, 109170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P, Berry AM, Pachauri S, Dagar M, Khan NW, Shaw P, Garland D, Jablonski MM, Ayyagari R, 2022. Htra1 KO mice develop severe photoreceptor loss and RPE abnormalities. Invest. Ophthalmol. Vis. Sci 63, 1592. [Google Scholar]
- Block K, Gorin Y, Abboud HE, 2009. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. U. S. A 106, 14385–14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA, 2010. The dynamic nature of Bruch’s membrane. Prog. Retin. Eye Res 29, 1–18. [DOI] [PubMed] [Google Scholar]
- Buckingham T, Young R, 1986. The rise and fall of intra-ocular pressure: the influence of physiological factors. Ophthalmic Physiol. Opt 6, 95–99. [PubMed] [Google Scholar]
- Bunker S, Holeniewska J, Vijay S, Dahlmann-Noor A, Khaw P, Ng YS, Shima D, Foxton R, 2015. Experimental glaucoma induced by ocular injection of magnetic microspheres. J. Vis. Exp. 96 10.3791/52400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE, 2010. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet 6, e1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, 2015. The non-human primate experimental glaucoma model. Exp. Eye Res 141, 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT, 2005. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res 24, 39–73. [DOI] [PubMed] [Google Scholar]
- Caballero M, Rowlette LL, Borras T, 2000. Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim. Biophys. Acta 1502, 447–460. [DOI] [PubMed] [Google Scholar]
- Cameron EG, Xia X, Galvao J, Ashouri M, Kapiloff MS, Goldberg JL, 2020. Optic nerve crush in mice to study retinal ganglion cell survival and regeneration. Bio Protoc 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle R, Lanphier EH, Rahn H, 1964. Hyperbaric oxygen and persistence of vision in retinal ischemia. J. Appl. Physiol 19, 914–918. [DOI] [PubMed] [Google Scholar]
- Chan GC, Hess P, Meenakshi T, Carlstedt-Duke J, Gustafsson JA, Payvar F, 1991. Delayed secondary glucocorticoid response elements. Unusual nucleotide motifs specify glucocorticoid receptor binding to transcribed regions of alpha 2u-globulin DNA. J. Biol. Chem 266, 22634–22644. [PubMed] [Google Scholar]
- Chandrasekaran S, Rochtchina E, Mitchell P, 2005. Effects of caffeine on intraocular pressure: the blue mountains eye study. J. Glaucoma 14, 504–507. [DOI] [PubMed] [Google Scholar]
- Chang EE, Goldberg JL, 2012. Glaucoma 2.0: neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 119, 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Jenny LA, Li YS, Cui X, Kong Y, Li Y, Sparrow JR, Tsang SH, 2023. CRISPR editing demonstrates rs10490924 raised oxidative stress in iPSC-derived retinal cells from patients with ARMS2/HTRA1-related AMD. Proc. Natl. Acad. Sci. U. S. A 120, e2215005120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman NA, Jacobs RJ, Braakhuis AJ, 2019. Role of diet and food intake in age-related macular degeneration: a systematic review. Clin. Exp. Ophthalmol 47, 106–127. [DOI] [PubMed] [Google Scholar]
- Chen L, Jia L, Wang N, Tang G, Zhang C, Fan S, Liu W, Meng H, Zeng W, Liu N, Wang H, Jia H, 2009. Evaluation of LOXL1 polymorphisms in exfoliation syndrome in a Chinese population. Mol. Vis 15, 2349–2357. [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chen LJ, Zhang M, Gong W, Tam PO, Lam DS, Pang CP, 2010. Ethnicity-based subgroup meta-analysis of the association of LOXL1 polymorphisms with glaucoma. Mol. Vis 16, 167–177. [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wei X, Cho KS, Chen G, Sappington R, Calkins DJ, Chen DF, 2011. Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Invest. Ophthalmol. Vis. Sci 52, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Popp NA, Chan CC, 2014. Animal models of age-related macular degeneration and their translatability into the clinic. Expet Rev. Ophthalmol 9, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhao Y, Zhang H, 2016. Comparative anatomy of the trabecular meshwork, the optic nerve head and the inner retina in rodent and primate models used for glaucoma research. Vision 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Chen C, Guo W, Liu K, Zhao Q, Lu P, Yu F, Xu X, 2020. EFEMP1 overexpression contributes to neovascularization in age-related macular degeneration. Front. Pharmacol 11, 547436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Agron E, Sperduto RD, Sangiovanni JP, Kurinij N, Davis MD, Group A-REDSR, 2013. Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 120, 1604–1611 e1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Agron E, Domalpally A, Keenan TDL, Vitale S, Weber C, Smith DC, Christen W, Group AR, 2022. Long-term outcomes of adding lutein/zeaxanthin and omega-3 fatty acids to the AREDS supplements on age-related macular degeneration progression: AREDS2 report 28. JAMA Ophthalmol 140, 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Mazda T, Tomita M, 1989. A serum protein SP40,40 modulates the formation of membrane attack complex of complement on erythrocytes. Mol. Immunol 26, 835–840. [DOI] [PubMed] [Google Scholar]
- Choi EH, Suh S, Foik AT, Leinonen H, Newby GA, Gao XD, Banskota S, Hoang T, Du SW, Dong Z, Raguram A, Kohli S, Blackshaw S, Lyon DC, Liu DR, Palczewski K, 2022. In vivo base editing rescues cone photoreceptors in a mouse model of early-onset inherited retinal degeneration. Nat. Commun 13, 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowers I, Cohen Y, Goldenberg-Cohen N, Vicuna-Kojchen J, Lichtinger A, Weinstein O, Pollack A, Axer-Siegel R, Hemo I, Averbukh E, Banin E, Meir T, Lederman M, 2008. Association of complement factor H Y402H polymorphism with phenotype of neovascular age related macular degeneration in Israel. Mol. Vis 14, 1829–1834. [PMC free article] [PubMed] [Google Scholar]
- Chrysostomou V, Crowston JG, 2013. The photopic negative response of the mouse electroretinogram: reduction by acute elevation of intraocular pressure. Invest. Ophthalmol. Vis. Sci 54, 4691–4697. [DOI] [PubMed] [Google Scholar]
- Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL 3rd, Age-Related Eye Disease Study Research, G, 2005. Risk factors for the incidence of advanced age-related macular degeneration in the age-related eye disease study (AREDS) AREDS report no. 19. Ophthalmology 112, 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopton DA, Saltman P, 1995. Low-level oxidative stress causes cell-cycle specific arrest in cultured cells. Biochem. Biophys. Res. Commun 210, 189–196. [DOI] [PubMed] [Google Scholar]
- Coffey PJ, Gias C, McDermott CJ, Lundh P, Pickering MC, Sethi C, Bird A, Fitzke FW, Maass A, Chen LL, Holder GE, Luthert PJ, Salt TE, Moss SE, Greenwood J, 2007. Complement factor H deficiency in aged mice causes retinal abnormalities and visual dysfunction. Proc. Natl. Acad. Sci. U. S. A 104, 16651–16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collantes ERA, Delfin MS, Fan B, Torregosa JMR, Siguan-Bell C, Florcruz NVG, Martinez JMD, Masna-Hidalgo BJ, Guzman VPT, Anotado-Flores JF, Levina FD, Hernandez SRC, Collantes AA, Sibulo MC, Rong S, Wiggs JL, 2022. EFEMP1 rare variants cause familial juvenile-onset open-angle glaucoma. Hum. Mutat 43, 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comparison of Age-related Macular Degeneration Treatments Trials Research, G., Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE, Toth CA, Ferris FL 3rd, Fine SL, 2016. Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology 123, 1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone FE, Steinhart MR, Oglesby EN, Kalesnykas G, Pease ME, Quigley HA, 2012. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp. Eye Res 99, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas J, Colijn JM, Geerlings MJ, Pauper M, Bakker B, Amin N, Lores Motta L, Kersten E, Garanto A, Verlouw JAM, van Rooij JGJ, Kraaij R, de Jong P, Hofman A, Vingerling JR, Schick T, Fauser S, de Jong EK, van Duijn CM, Hoyng CB, Klaver CCW, den Hollander AI, 2018. Whole-exome sequencing in age-related macular degeneration identifies rare variants in COL8A1, a component of Bruch’s membrane. Ophthalmology 125, 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins SW, Espinosa-Heidmann DG, Alexandridou A, Sall J, Dubovy S, Csaky K, 2002. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp. Eye Res 75, 543–553. [DOI] [PubMed] [Google Scholar]
- Cowan CS, Renner M, De Gennaro M, Gross-Scherf B, Goldblum D, Hou Y, Munz M, Rodrigues TM, Krol J, Szikra T, Cuttat R, Waldt A, Papasaikas P, Diggelmann R, Patino-Alvarez CP, Galliker P, Spirig SE, Pavlinic D, Gerber-Hollbach N, Schuierer S, Srdanovic A, Balogh M, Panero R, Kusnyerik A, Szabo A, Stadler MB, Orgul S, Picelli S, Hasler PW, Hierlemann A, Scholl HPN, Roma G, Nigsch F, Roska B, 2020. Cell types of the human retina and its organoids at single-cell resolution. Cell 182, 1623–1640 e1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JE, Han X, Qassim A, Hassall M, Cooke Bailey JN, Kinzy TG, Khawaja AP, An J, Marshall H, Gharahkhani P, Igo RP Jr., Graham SL, Healey PR, Ong JS, Zhou T, Siggs O, Law MH, Souzeau E, Ridge B, Hysi PG, Burdon KP, Mills RA, Landers J, Ruddle JB, Agar A, Galanopoulos A, White AJR, Willoughby CE, Andrew NH, Best S, Vincent AL, Goldberg I, Radford-Smith G, Martin NG, Montgomery GW, Vitart V, Hoehn R, Wojciechowski R, Jonas JB, Aung T, Pasquale LR, Cree AJ, Sivaprasad S, Vallabh NA, consortium N, Eye UKB, Vision C, Viswanathan AC, Pasutto F, Haines JL, Klaver CCW, van Duijn CM, Casson RJ, Foster PJ, Khaw PT, Hammond CJ, Mackey DA, Mitchell P, Lotery AJ, Wiggs JL, Hewitt AW, MacGregor S, 2020. Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat. Genet 52, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews JE, Dpa, Chou CF, Stevens JA, Saaddine JB, 2016. Falls among persons aged >/65 Years with and without severe vision impairment - United States, 2014. MMWR Morb. Mortal. Wkly. Rep 65, 433–437. [DOI] [PubMed] [Google Scholar]
- Csiszar K, 2001. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol 70, 1–32. [DOI] [PubMed] [Google Scholar]
- Dai Y, Lindsey JD, Duong-Polk X, Nguyen D, Hofer A, Weinreb RN, 2009. Outflow facility in mice with a targeted type I collagen mutation. Invest. Ophthalmol. Vis. Sci 50, 5749–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson WW, Brooks DE, Hope GM, Samuelson DA, Sherwood MB, Engel HM, Kessler MJ, 1993. Primary open angle glaucomas in the rhesus monkey. Br. J. Ophthalmol 77, 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoz R, Ramirez AI, Gonzalez-Martin R, Ajoy D, Rojas B, Salobrar-Garcia E, Valiente-Soriano FJ, Aviles-Trigueros M, Villegas-Perez MP, Vidal-Sanz M, Trivino A, Ramirez JM, Salazar JJ, 2018. Bilateral early activation of retinal microglial cells in a mouse model of unilateral laser-induced experimental ocular hypertension. Exp. Eye Res 171, 12–29. [DOI] [PubMed] [Google Scholar]
- de Jong S, Gagliardi G, Garanto A, de Breuk A, Lechanteur YTE, Katti S, van den Heuvel LP, Volokhina EB, den Hollander AI, 2021. Implications of genetic variation in the complement system in age-related macular degeneration. Prog. Retin. Eye Res 84, 100952. [DOI] [PubMed] [Google Scholar]
- De La Paz MA, Epstein DL, 1996. Effect of age on superoxide dismutase activity of human trabecular meshwork. Invest. Ophthalmol. Vis. Sci 37, 1849–1853. [PubMed] [Google Scholar]
- Della Santina L, Inman DM, Lupien CB, Horner PJ, Wong RO, 2013. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J. Neurosci 33, 17444–17457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desronvil T, Logan-Wyatt D, Abdrabou W, Triana M, Jones R, Taheri S, Del Bono E, Pasquale LR, Olivier M, Haines JL, Fan BJ, Wiggs JL, 2010. Distribution of COL8A2 and COL8A1 gene variants in Caucasian primary open angle glaucoma patients with thin central corneal thickness. Mol. Vis 16, 2185–2191. [PMC free article] [PubMed] [Google Scholar]
- Di Carlo E, Augustin AJ, 2021. Prevention of the onset of age-related macular degeneration. J. Clin. Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierdomenico J, Henderson DCM, Giammaria S, Smith VL, Jamet AJ, Smith CA, Hooper ML, Chauhan BC, 2022. Age and intraocular pressure in murine experimental glaucoma. Prog. Retin. Eye Res 88, 101021. [DOI] [PubMed] [Google Scholar]
- Dighe S, Zhao J, Steffen L, Mares JA, Meuer SM, Klein BEK, Klein R, Millen AE, 2020. Diet patterns and the incidence of age-related macular degeneration in the Atherosclerosis Risk in Communities (ARIC) study. Br. J. Ophthalmol 104, 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos IS, Tennant M, Johnson A, Fisher S, Freund PR, Sauve Y, 2013. Subjects with unilateral neovascular AMD have bilateral delays in rod-mediated phototransduction activation kinetics and in dark adaptation recovery. Invest. Ophthalmol. Vis. Sci 54, 5186–5195. [DOI] [PubMed] [Google Scholar]
- Ding X, Patel M, Shen D, Herzlich AA, Cao X, Villasmil R, Klupsch K, Tuo J, Downward J, Chan CC, 2009. Enhanced HtrA2/Omi expression in oxidative injury to retinal pigment epithelial cells and murine models of neurodegeneration. Invest. Ophthalmol. Vis. Sci 50, 4957–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding JD, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, Mace BE, Sullivan P, Jamison JA, Kelly U, Harrabi O, Bollini SS, Dilley J, Kobayashi D, Kuang B, Li W, Pons J, Lin JC, Bowes Rickman C, 2011. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A 108, E279–E287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dithmar S, Sharara NA, Curcio CA, Le NA, Zhang Y, Brown S, Grossniklaus HE, 2001. Murine high-fat diet and laser photochemical model of basal deposits in Bruch membrane. Arch. Ophthalmol 119, 1643–1649. [DOI] [PubMed] [Google Scholar]
- Doozandeh A, Yazdani S, 2016. Neuroprotection in glaucoma. J. Ophthalmic Vis. Res 11, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W, 2002. Free radicals in the physiological control of cell function. Physiol. Rev 82, 47–95. [DOI] [PubMed] [Google Scholar]
- Ebrahimi KB, Fijalkowski N, Cano M, Handa JT, 2013. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J. Pathol 229, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA, 2005. Complement factor H polymorphism and age-related macular degeneration. Science 308, 421–424. [DOI] [PubMed] [Google Scholar]
- Ekici E, Moghimi S, 2023. Advances in understanding glaucoma pathogenesis: a multifaceted molecular approach for clinician scientists. Mol. Aspect. Med 94, 101223. [Google Scholar]
- El-Danaf RN, Huberman AD, 2015. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J. Neurosci 35, 2329–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliseeva N, Ponomarenko I, Reshetnikov E, Dvornyk V, Churnosov M, 2021. LOXL1 gene polymorphism candidates for exfoliation glaucoma are also associated with a risk for primary open-angle glaucoma in a Caucasian population from central Russia. Mol. Vis 27, 262–269. [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Catanuto P, Hernandez EP, Marin-Castano ME, Cousins SW, 2006. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Invest. Ophthalmol. Vis. Sci 47, 729–737. [DOI] [PubMed] [Google Scholar]
- Fautsch MP, Bahler CK, Jewison DJ, Johnson DH, 2000. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest. Ophthalmol. Vis. Sci 41, 4163–4168. [PubMed] [Google Scholar]
- Felszeghy S, Viiri J, Paterno JJ, Hyttinen JMT, Koskela A, Chen M, Leinonen H, Tanila H, Kivinen N, Koistinen A, Toropainen E, Amadio M, Smedowski A, Reinisalo M, Winiarczyk M, Mackiewicz J, Mutikainen M, Ruotsalainen AK, Kettunen M, Jokivarsi K, Sinha D, Kinnunen K, Petrovski G, Blasiak J, Bjorkoy G, Koskelainen A, Skottman H, Urtti A, Salminen A, Kannan R, Ferrington DA, Xu H, Levonen AL, Tavi P, Kauppinen A, Kaarniranta K, 2019. Loss of NRF-2 and PGC-1alpha genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol 20, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Nie K, Huang Q, Fan W, 2022. Complement factor H deficiency combined with smoking promotes retinal degeneration in a novel mouse model. Exp. Biol. Med 247, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SM, Lerner SF, Brunzini R, Reides CG, Evelson PA, Llesuy SF, 2010. Time course changes of oxidative stress markers in a rat experimental glaucoma model. Invest. Ophthalmol. Vis. Sci 51, 4635–4640. [DOI] [PubMed] [Google Scholar]
- Fields MA, Del Priore LV, Adelman RA, Rizzolo LJ, 2020. Interactions of the choroid, Bruch’s membrane, retinal pigment epithelium, and neurosensory retina collaborate to form the outer blood-retinal-barrier. Prog. Retin. Eye Res 76, 100803. [DOI] [PubMed] [Google Scholar]
- Filla MS, Faralli JA, Peotter JL, Peters DM, 2017. The role of integrins in glaucoma. Exp. Eye Res 158, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingert JH, Ying L, Swiderski RE, Nystuen AM, Arbour NC, Alward WL, Sheffield VC, Stone EM, 1998. Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Res 8, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease, S., 2017. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Global Health 5, e1221–e1234. [DOI] [PubMed] [Google Scholar]
- Fleckenstein M, Keenan TDL, Guymer RH, Chakravarthy U, Schmitz-Valckenberg S, Klaver CC, Wong WT, Chew EY, 2021. Age-related macular degeneration. Nat. Rev. Dis. Prim 7, 31. [DOI] [PubMed] [Google Scholar]
- Fletcher AE, Bentham GC, Agnew M, Young IS, Augood C, Chakravarthy U, de Jong PT, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, Vioque J, 2008. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch. Ophthalmol 126, 1396–1403. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Jobling AI, Greferath U, Mills SA, Waugh M, Ho T, de Iongh RU, Phipps JA, Vessey KA, 2014. Studying age-related macular degeneration using animal models. Optom. Vis. Sci 91, 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood MT, Gouras P, Kjeldbye H, 1980. Growth characteristics and ultrastructure of human retinal pigment epithelium in vitro. Invest. Ophthalmol. Vis. Sci 19, 1309–1320. [PubMed] [Google Scholar]
- Folgueras AR, Freitas-Rodriguez S, Velasco G, Lopez-Otin C, 2018. Mouse models to disentangle the hallmarks of human aging. Circ. Res 123, 905–924. [DOI] [PubMed] [Google Scholar]
- Francis PJ, Appukuttan B, Simmons E, Landauer N, Stoddard J, Hamon S, Ott J, Ferguson B, Klein M, Stout JT, Neuringer M, 2008. Rhesus monkeys and humans share common susceptibility genes for age-related macular disease. Hum. Mol. Genet 17, 2673–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Wolburg H, 1996. Cellular reactions at the lesion site after crushing of the rat optic nerve. Glia 16, 227–240. [DOI] [PubMed] [Google Scholar]
- Frank RN, Das A, Weber ML, 1989. A model of subretinal neovascularization in the pigmented rat. Curr. Eye Res 8, 239–247. [DOI] [PubMed] [Google Scholar]
- Fransen M, Nordgren M, Wang B, Apanasets O, 2012. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim. Biophys. Acta 1822, 1363–1373. [DOI] [PubMed] [Google Scholar]
- Freedman SF, Anderson PJ, Epstein DL, 1985. Superoxide dismutase and catalase of calf trabecular meshwork. Invest. Ophthalmol. Vis. Sci 26, 1330–1335. [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E, 1997. Integrins and anoikis. Curr. Opin. Cell Biol 9, 701–706. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Loenhardt T, Janssen A, Fisher SA, Rivera A, Keilhauer CN, Weber BH, 2008. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat. Genet 40, 892–896. [DOI] [PubMed] [Google Scholar]
- Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S, Igo RP Jr., Buitendijk GH, Sim X, Weeks DE, Guymer RH, Merriam JE, Francis PJ, Hannum G, Agarwal A, Armbrecht AM, Audo I, Aung T, Barile GR, Benchaboune M, Bird AC, Bishop PN, Branham KE, Brooks M, Brucker AJ, Cade WH, Cain MS, Campochiaro PA, Chan CC, Cheng CY, Chew EY, Chin KA, Chowers I, Clayton DG, Cojocaru R, Conley YP, Cornes BK, Daly MJ, Dhillon B, Edwards AO, Evangelou E, Fagerness J, Ferreyra HA, Friedman JS, Geirsdottir A, George RJ, Gieger C, Gupta N, Hagstrom SA, Harding SP, Haritoglou C, Heckenlively JR, Holz FG, Hughes G, Ioannidis JP, Ishibashi T, Joseph P, Jun G, Kamatani Y, Katsanis N, C NK, Khan JC, Kim IK, Kiyohara Y, Klein BE, Klein R, Kovach JL, Kozak I, Lee CJ, Lee KE, Lichtner P, Lotery AJ, Meitinger T, Mitchell P, Mohand-Said S, Moore AT, Morgan DJ, Morrison MA, Myers CE, Naj AC, Nakamura Y, Okada Y, Orlin A, Ortube MC, Othman MI, Pappas C, Park KH, Pauer GJ, Peachey NS, Poch O, Priya RR, Reynolds R, Richardson AJ, Ripp R, Rudolph G, Ryu E, Sahel JA, Schaumberg DA, Scholl HP, Schwartz SG, Scott WK, Shahid H, Sigurdsson H, Silvestri G, Sivakumaran TA, Smith RT, Sobrin L, Souied EH, Stambolian DE, Stefansson H, Sturgill-Short GM, Takahashi A, Tosakulwong N, Truitt BJ, Tsironi EE, Uitterlinden AG, van Duijn CM, Vijaya L, Vingerling JR, Vithana EN, Webster AR, Wichmann HE, Winkler TW, Wong TY, Wright AF, Zelenika D, Zhang M, Zhao L, Zhang K, Klein ML, Hageman GS, Lathrop GM, Stefansson K, Allikmets R, Baird PN, Gorin MB, Wang JJ, Klaver CC, Seddon JM, Pericak-Vance MA, Iyengar SK, Yates JR, Swaroop A, Weber BH, Kubo M, Deangelis MM, Leveillard T, Thorsteinsdottir U, Haines JL, Farrer LA, Heid IM, Abecasis GR, Consortium AMDG, 2013. Seven new loci associated with age-related macular degeneration. Nat. Genet 45, 433–439, 439e431–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, Burdon KP, Hebbring SJ, Wen C, Gorski M, Kim IK, Cho D, Zack D, Souied E, Scholl HP, Bala E, Lee KE, Hunter DJ, Sardell RJ, Mitchell P, Merriam JE, Cipriani V, Hoffman JD, Schick T, Lechanteur YT, Guymer RH, Johnson MP, Jiang Y, Stanton CM, Buitendijk GH, Zhan X, Kwong AM, Boleda A, Brooks M, Gieser L, Ratnapriya R, Branham KE, Foerster JR, Heckenlively JR, Othman MI, Vote BJ, Liang HH, Souzeau E, McAllister IL, Isaacs T, Hall J, Lake S, Mackey DA, Constable IJ, Craig JE, Kitchner TE, Yang Z, Su Z, Luo H, Chen D, Ouyang H, Flagg K, Lin D, Mao G, Ferreyra H, Stark K, von Strachwitz CN, Wolf A, Brandl C, Rudolph G, Olden M, Morrison MA, Morgan DJ, Schu M, Ahn J, Silvestri G, Tsironi EE, Park KH, Farrer LA, Orlin A, Brucker A, Li M, Curcio CA, Mohand-Said S, Sahel JA, Audo I, Benchaboune M, Cree AJ, Rennie CA, Goverdhan SV, Grunin M, Hagbi-Levi S, Campochiaro P, Katsanis N, Holz FG, Blond F, Blanche H, Deleuze JF, Igo RP Jr., Truitt B, Peachey NS, Meuer SM, Myers CE, Moore EL, Klein R, Hauser MA, Postel EA, Courtenay MD, Schwartz SG, Kovach JL, Scott WK, Liew G, Tan AG, Gopinath B, Merriam JC, Smith RT, Khan JC, Shahid H, Moore AT, McGrath JA, Laux R, Brantley MA Jr., Agarwal A, Ersoy L, Caramoy A, Langmann T, Saksens NT, de Jong EK, Hoyng CB, Cain MS, Richardson AJ, Martin TM, Blangero J, Weeks DE, Dhillon B, van Duijn CM, Doheny KF, Romm J, Klaver CC, Hayward C, Gorin MB, Klein ML, Baird PN, den Hollander AI, Fauser S, Yates JR, Allikmets R, Wang JJ, Schaumberg DA, Klein BE, Hagstrom SA, Chowers I, Lotery AJ, Leveillard T, Zhang K, Brilliant MH, Hewitt AW, Swaroop A, Chew EY, Pericak-Vance MA, DeAngelis M, Stambolian D, Haines JL, Iyengar SK, Weber BH, Abecasis GR, Heid IM, 2016. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet 48, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Garland D, Yang Z, Shukla D, Rajendran A, Pearson E, Stone EM, Zhang K, Pierce EA, 2007. The R345W mutation in EFEMP1 is pathogenic and causes AMD-like deposits in mice. Hum. Mol. Genet 16, 2411–2422. [DOI] [PubMed] [Google Scholar]
- Fuse N, Miyazawa A, Nakazawa T, Mengkegale M, Otomo T, Nishida K, 2008. Evaluation of LOXL1 polymorphisms in eyes with exfoliation glaucoma in Japanese. Mol. Vis 14, 1338–1343. [PMC free article] [PubMed] [Google Scholar]
- Gaasterland D, Kupfer C, 1974. Experimental glaucoma in the rhesus monkey. Invest. Ophthalmol 13, 455–457. [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL, 2005. Changes in aqueous humor dynamics with age and glaucoma. Prog. Retin. Eye Res 24, 612–637. [DOI] [PubMed] [Google Scholar]
- Gallego BI, Salazar JJ, de Hoz R, Rojas B, Ramirez AI, Salinas-Navarro M, Ortin-Martinez A, Valiente-Soriano FJ, Aviles-Trigueros M, Villegas-Perez MP, Vidal-Sanz M, Trivino A, Ramirez JM, 2012. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J. Neuroinflammation 9, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Jakobs TC, 2016. Mice homozygous for a deletion in the glaucoma susceptibility locus INK4 show increased vulnerability of retinal ganglion cells to elevated intraocular pressure. Am. J. Pathol 186, 985–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Jozic A, Li-Na G, Herrera-Barrera M, Curtis A, Arrizabalaga S, Tschetter W, Ryals RC, Sahay G, 2023. Lipid nanoparticles with PEG-variant surface modifications mediate genome editing in the mouse retina. Nat. Commun 14, 6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier AC, Liu J, 2016. Neurodegeneration and neuroprotection in glaucoma. Yale J. Biol. Med 89, 73–79. [PMC free article] [PubMed] [Google Scholar]
- Gelatt KN, Peiffer RL Jr., Gwin RM, Gum GG, Williams LW, 1977. Clinical manifestations of inherited glaucoma in the beagle. Invest. Ophthalmol. Vis. Sci 16, 1135–1142. [PubMed] [Google Scholar]
- Gharahkhani P, Jorgenson E, Hysi P, Khawaja AP, Pendergrass S, Han X, Ong JS, Hewitt AW, Segre AV, Rouhana JM, Hamel AR, Igo RP Jr., Choquet H, Qassim A, Josyula NS, Cooke Bailey JN, Bonnemaijer PWM, Iglesias A, Siggs OM, Young TL, Vitart V, Thiadens A, Karjalainen J, Uebe S, Melles RB, Nair KS, Luben R, Simcoe M, Amersinghe N, Cree AJ, Hohn R, Poplawski A, Chen LJ, Rong SS, Aung T, Vithana EN, consortium N, consortium A, Biobank Japan, p., FinnGen, s., Eye, U.K.B., Vision, C., group, G.s., Me Research, T., Tamiya G, Shiga Y, Yamamoto M, Nakazawa T, Currant H, Birney E, Wang X, Auton A, Lupton MK, Martin NG, Ashaye A, Olawoye O, Williams SE, Akafo S, Ramsay M, Hashimoto K, Kamatani Y, Akiyama M, Momozawa Y, Foster PJ, Khaw PT, Morgan JE, Strouthidis NG, Kraft P, Kang JH, Pang CP, Pasutto F, Mitchell P, Lotery AJ, Palotie A, van Duijn C, Haines JL, Hammond C, Pasquale LR, Klaver CCW, Hauser M, Khor CC, Mackey DA, Kubo M, Cheng CY, Craig JE, MacGregor S, Wiggs JL, 2021. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun 12, 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherezghiher T, March WF, Nordquist RE, Koss MC, 1986. Laser-induced glaucoma in rabbits. Exp. Eye Res 43, 885–894. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, 1997. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr. Opin. Cell Biol 9, 691–700. [DOI] [PubMed] [Google Scholar]
- Giannakis E, Jokiranta TS, Male DA, Ranganathan S, Ormsby RJ, Fischetti VA, Mold C, Gordon DL, 2003. A common site within factor H SCR 7 responsible for binding heparin, C-reactive protein and streptococcal M protein. Eur. J. Immunol 33, 962–969. [DOI] [PubMed] [Google Scholar]
- Glenn JV, Mahaffy H, Wu K, Smith G, Nagai R, Simpson DA, Boulton ME, Stitt AW, 2009. Advanced glycation end product (AGE) accumulation on Bruch’s membrane: links to age-related RPE dysfunction. Invest. Ophthalmol. Vis. Sci 50, 441–451. [DOI] [PubMed] [Google Scholar]
- Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M, Tano Y, 2008. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br. J. Ophthalmol 92, 70–73. [DOI] [PubMed] [Google Scholar]
- Gottfredsdottir MS, Sverrisson T, Musch DC, Stefansson E, 1999. Chronic open-angle glaucoma and associated ophthalmic findings in monozygotic twins and their spouses in Iceland. J. Glaucoma 8, 134–139. [PubMed] [Google Scholar]
- Gould DB, Marchant JK, Savinova OV, Smith RS, John SW, 2007. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum. Mol. Genet 16, 798–807. [DOI] [PubMed] [Google Scholar]
- Grassmann F, Heid IM, Weber BH, International AMDGC, 2017. Recombinant haplotypes narrow the ARMS2/HTRA1 association signal for age-related macular degeneration. Genetics 205, 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AG, Eivers SB, McDonnell F, Dervan EWJ, O’Brien CJ, Wallace DM, 2020. Differential Lysyl oxidase like 1 expression in pseudoexfoliation glaucoma is orchestrated via DNA methylation. Exp. Eye Res 201, 108349. [DOI] [PubMed] [Google Scholar]
- Grizzard SW, Arnett D, Haag SL, 2003. Twin study of age-related macular degeneration. Ophthalmic Epidemiol 10, 315–322. [DOI] [PubMed] [Google Scholar]
- Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF, 2005. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest. Ophthalmol. Vis. Sci 46, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenplan KA, Stafford BK, El-Danaf RN, Adler DI, Munch AE, Weigel MK, Huberman AD, Liddelow SA, 2020. Neurotoxic reactive astrocytes drive neuronal death after retinal injury. Cell Rep 31, 107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R, 2005. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A 102, 7227–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA, 2005. Complement factor H variant increases the risk of age-related macular degeneration. Science 308, 419–421. [DOI] [PubMed] [Google Scholar]
- Ham WT Jr., Mueller HA, Sliney DH, 1976. Retinal sensitivity to damage from short wavelength light. Nature 260, 153–155. [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Beach D, 1994. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371, 257–261. [DOI] [PubMed] [Google Scholar]
- Hannou SA, Wouters K, Paumelle R, Staels B, 2015. Functional genomics of the CDKN2A/B locus in cardiovascular and metabolic disease: what have we learned from GWASs? Trends Endocrinol. Metabol 26, 176–184. [DOI] [PubMed] [Google Scholar]
- Hanovice NJ, Leach LL, Slater K, Gabriel AE, Romanovicz D, Shao E, Collery R, Burton EA, Lathrop KL, Link BA, Gross JM, 2019. Regeneration of the zebrafish retinal pigment epithelium after widespread genetic ablation. PLoS Genet 15, e1007939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Nakamura K, Quah HM, Okumura A, Namekata K, Saeki T, Aihara M, Yoshida H, Mitani A, Tanaka K, 2007. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J. Clin. Invest 117, 1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harismendy O, Notani D, Song X, Rahim NG, Tanasa B, Heintzman N, Ren B, Fu XD, Topol EJ, Rosenfeld MG, Frazer KA, 2011. 9p21 DNA variants associated with coronary artery disease impair interferon-gamma signaling response. Nature 470, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Samuelson DA, Tajwar H, MacKay EO, Lewis PA, Kallberg M, Gelatt KN, 2007. Immunolocalization of myocilin protein in the anterior eye of normal and primary open-angle glaucomatous dogs. Vet. Ophthalmol 10 (Suppl. 1), 28–37. [DOI] [PubMed] [Google Scholar]
- Hauser MA, Aboobakar IF, Liu Y, Miura S, Whigham BT, Challa P, Wheeler J, Williams A, Santiago-Turla C, Qin X, Rautenbach RM, Ziskind A, Ramsay M, Uebe S, Song L, Safi A, Vithana EN, Mizoguchi T, Nakano S, Kubota T, Hayashi K, Manabe S, Kazama S, Mori Y, Miyata K, Yoshimura N, Reis A, Crawford GE, Pasutto F, Carmichael TR, Williams SE, Ozaki M, Aung T, Khor CC, Stamer WD, Ashley-Koch AE, Allingham RR, 2015. Genetic variants and cellular stressors associated with exfoliation syndrome modulate promoter activity of a lncRNA within the LOXL1 locus. Hum. Mol. Genet 24, 6552–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez MR, Pena JD, 1997. The optic nerve head in glaucomatous optic neuropathy. Arch. Ophthalmol 115, 389–395. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Ye H, Roy S, 1994. Collagen type IV gene expression in human optic nerve heads with primary open angle glaucoma. Exp. Eye Res 59, 41–51. [DOI] [PubMed] [Google Scholar]
- Hernandez M, Urcola JH, Vecino E, 2008. Retinal ganglion cell neuroprotection in a rat model of glaucoma following brimonidine, latanoprost or combined treatments. Exp. Eye Res 86, 798–806. [DOI] [PubMed] [Google Scholar]
- Heurich M, Martinez-Barricarte R, Francis NJ, Roberts DL, Rodriguez de Cordoba S, Morgan BP, Harris CL, 2011. Common polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease risk. Proc. Natl. Acad. Sci. U. S. A 108, 8761–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt AT, Nakazawa K, Newsome DA, 1989. Analysis of newly synthesized Bruch’s membrane proteoglycans. Invest. Ophthalmol. Vis. Sci 30, 478–486. [PubMed] [Google Scholar]
- Higginbotham EJ, Kilimanjaro HA, Wilensky JT, Batenhorst RL, Hermann D, 1989. The effect of caffeine on intraocular pressure in glaucoma patients. Ophthalmology 96, 624–626. [DOI] [PubMed] [Google Scholar]
- Hirakawa M, Tanaka M, Tanaka Y, Okubo A, Koriyama C, Tsuji M, Akiba S, Miyamoto K, Hillebrand G, Yamashita T, Sakamoto T, 2008. Age-related maculopathy and sunlight exposure evaluated by objective measurement. Br. J. Ophthalmol 92, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Bok D, 2001. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol. Vis 7, 14–19. [PubMed] [Google Scholar]
- Hussain AA, Starita C, Hodgetts A, Marshall J, 2010. Macromolecular diffusion characteristics of ageing human Bruch’s membrane: implications for age-related macular degeneration (AMD). Exp. Eye Res 90, 703–710. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, Shimizu T, Mizushima Y, Shirasawa T, Tsubota K, 2006. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A 103, 11282–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Murata T, Hangai M, Nagai R, Horiuchi S, Lopez PF, Hinton DR, Ryan SJ, 1998. Advanced glycation end products in age-related macular degeneration. Arch. Ophthalmol 116, 1629–1632. [DOI] [PubMed] [Google Scholar]
- Ito YA, Belforte N, Cueva Vargas JL, Di Polo A, 2016. A magnetic microbead occlusion model to induce ocular hypertension-dependent glaucoma in mice. J. Vis. Exp, e53731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A, Sacca SC, Cartiglia C, De Flora S, 2003. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am. J. Med 114, 638–646. [DOI] [PubMed] [Google Scholar]
- Izzotti A, Longobardi M, Cartiglia C, Sacca SC, 2011. Mitochondrial damage in the trabecular meshwork occurs only in primary open-angle glaucoma and in pseudoexfoliative glaucoma. PLoS One 6, e14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC, 2001. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum. Mol. Genet 10, 117–125. [DOI] [PubMed] [Google Scholar]
- Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB, 2005. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am. J. Hum. Genet 77, 389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammal AA, Berchuck SI, Thompson AC, Costa VP, Medeiros FA, 2020. The effect of age on increasing susceptibility to retinal nerve fiber layer loss in glaucoma. Invest. Ophthalmol. Vis. Sci 61, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji ML, Jia J, 2019. Correlations of TIMP2 and TIMP3 gene polymorphisms with primary open-angle glaucoma. Eur. Rev. Med. Pharmacol. Sci 23, 5542–5547. [DOI] [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR, 1998. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest. Ophthalmol. Vis. Sci 39, 951–962. [PubMed] [Google Scholar]
- Johnson TV, Tomarev SI, 2010. Rodent models of glaucoma. Brain Res. Bull 81, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B, House P, Morgan W, Sun X, Yu DY, 1999. Developing laser-induced glaucoma in rabbits. Aust. N. Z. J. Ophthalmol 27, 180–183. [DOI] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR, 2010. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest. Ophthalmol. Vis. Sci 51, 2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JB, Berenshtein E, Holbach L, 2003. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest. Ophthalmol. Vis. Sci 44, 5189–5195. [DOI] [PubMed] [Google Scholar]
- Jones A, Kumar S, Zhang N, Tong Z, Yang JH, Watt C, Anderson J, Amrita, Fillerup H, McCloskey M, Luo L, Yang Z, Ambati B, Marc R, Oka C, Zhang K, Fu Y, 2011a. Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Proc. Natl. Acad. Sci. U. S. A 108, 14578–14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Kondo M, Terasaki H, Watt CB, Rapp K, Anderson J, Lin Y, Shaw MV, Yang JH, Marc RE, 2011b. Retinal remodeling in the Tg P347L rabbit, a large-eye model of retinal degeneration. J. Comp. Neurol 519, 2713–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Tucker J, Marc RE, 2016. Retinal remodeling and metabolic alterations in human AMD. Front. Cell. Neurosci 10, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju WK, Liu Q, Kim KY, Crowston JG, Lindsey JD, Agarwal N, Ellisman MH, Perkins GA, Weinreb RN, 2007. Elevated hydrostatic pressure triggers mitochondrial fission and decreases cellular ATP in differentiated RGC-5 cells. Invest. Ophthalmol. Vis. Sci 48, 2145–2151. [DOI] [PubMed] [Google Scholar]
- Ju WK, Kim KY, Angert M, Duong-Polk KX, Lindsey JD, Ellisman MH, Weinreb RN, 2009. Memantine blocks mitochondrial OPA1 and cytochrome c release and subsequent apoptotic cell death in glaucomatous retina. Invest. Ophthalmol. Vis. Sci 50, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justilien V, Pang JJ, Renganathan K, Zhan X, Crabb JW, Kim SR, Sparrow JR, Hauswirth WW, Lewin AS, 2007. SOD2 knockdown mouse model of early AMD. Invest. Ophthalmol. Vis. Sci 48, 4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan HM, Li W, 2003. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem 88, 660–672. [DOI] [PubMed] [Google Scholar]
- Kahn MG, Giblin FJ, Epstein DL, 1983. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Invest. Ophthalmol. Vis. Sci 24, 1283–1287. [PubMed] [Google Scholar]
- Kalvin NH, Hamasaki DI, Gass JD, 1966. Experimental glaucoma in monkeys. I. Relationship between intraocular pressure and cupping of the optic disc and cavernous atrophy of the optic nerve. Arch. Ophthalmol 76, 82–93. [DOI] [PubMed] [Google Scholar]
- Kanda A, Chen W, Othman M, Branham KE, Brooks M, Khanna R, He S, Lyons R, Abecasis GR, Swaroop A, 2007. A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A 104, 16227–16232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Willett WC, Rosner BA, Hankinson SE, Pasquale LR, 2008. Caffeine consumption and the risk of primary open-angle glaucoma: a prospective cohort study. Invest. Ophthalmol. Vis. Sci 49, 1924–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Louboutin JP, Datta P, Landel CP, Martinez D, Zervos AS, Strayer DS, Fernandes-Alnemri T, Alnemri ES, 2013. Loss of HtrA2/Omi activity in non-neuronal tissues of adult mice causes premature aging. Cell Death Differ 20, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karali A, Russell P, Stefani FH, Tamm ER, 2000. Localization of myocilin/trabecular meshwork–inducible glucocorticoid response protein in the human eye. Invest. Ophthalmol. Vis. Sci 41, 729–740. [PubMed] [Google Scholar]
- Kasetti RB, Phan TN, Millar JC, Zode GS, 2016. Expression of mutant myocilin induces abnormal intracellular accumulation of selected extracellular matrix proteins in the trabecular meshwork. Invest. Ophthalmol. Vis. Sci 57, 6058–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling E, Lynn SA, Koh YM, Scott JA, Kendall A, Gatherer M, Page A, Cagampang FR, Lotery AJ, Ratnayaka JA, 2022. A high fat "Western-style" diet induces AMD-like features in wildtype mice. Mol. Nutr. Food Res 66, e2100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JC, Shahid H, Thurlby DA, Bradley M, Clayton DG, Moore AT, Bird AC, Yates JR, Genetic Factors in AMDS, 2006. Age related macular degeneration and sun exposure, iris colour, and skin sensitivity to sunlight. Br. J. Ophthalmol 90, 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandhadia S, Hakobyan S, Heng LZ, Gibson J, Adams DH, Alexander GJ, Gibson JM, Martin KR, Menon G, Nash K, Sivaprasad S, Ennis S, Cree AJ, Morgan BP, Lotery AJ, 2013. Age-related macular degeneration and modification of systemic complement factor H production through liver transplantation. Ophthalmology 120, 1612–1618. [DOI] [PubMed] [Google Scholar]
- Khatib TZ, Martin KR, 2020. Neuroprotection in glaucoma: towards clinical trials and precision medicine. Curr. Eye Res 45, 327–338. [DOI] [PubMed] [Google Scholar]
- Kim GH, Kim HI, Paik SS, Jung SW, Kang S, Kim IB, 2016. Functional and morphological evaluation of blue light-emitting diode-induced retinal degeneration in mice. Graefes Arch. Clin. Exp. Ophthalmol 254, 705–716. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, 1998. Relation of smoking to the incidence of age-related maculopathy. The beaver dam eye study. Am. J. Epidemiol 147, 103–110. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J, 2005. Complement factor H polymorphism in age-related macular degeneration. Science 308, 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper PA, Goossens W, Hvizd M, Palmberg PF, 1996a. Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci 37, 1360–1367. [PubMed] [Google Scholar]
- Knepper PA, Goossens W, Palmberg PF, 1996b. Glycosaminoglycan stratification of the juxtacanalicular tissue in normal and primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci 37, 2414–2425. [PubMed] [Google Scholar]
- Ko ML, Peng PH, Ma MC, Ritch R, Chen CF, 2005. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic. Biol. Med 39, 365–373. [DOI] [PubMed] [Google Scholar]
- Kokame GT, Yeung L, Lai JC, 2010. Continuous anti-VEGF treatment with ranibizumab for polypoidal choroidal vasculopathy: 6-month results. Br. J. Ophthalmol 94, 297–301. [DOI] [PubMed] [Google Scholar]
- Kolker AE, Moses RA, Constant MA, Becker B, 1963. The development of glaucoma in rabbits. Invest. Ophthalmol 2, 316–321. [PubMed] [Google Scholar]
- Kong YX, Crowston JG, Vingrys AJ, Trounce IA, Bui VB, 2009. Functional changes in the retina during and after acute intraocular pressure elevation in mice. Invest. Ophthalmol. Vis. Sci 50, 5732–5740. [DOI] [PubMed] [Google Scholar]
- Kong YX, van Bergen N, Bui BV, Chrysostomou V, Vingrys AJ, Trounce IA, Crowston JG, 2012. Impact of aging and diet restriction on retinal function during and after acute intraocular pressure injury. Neurobiol. Aging 33, 1126 e1115–1125. [DOI] [PubMed] [Google Scholar]
- Kortvely E, Hauck SM, Duetsch G, Gloeckner CJ, Kremmer E, Alge-Priglinger CS, Deeg CA, Ueffing M, 2010. ARMS2 is a constituent of the extracellular matrix providing a link between familial and sporadic age-related macular degenerations. Invest. Ophthalmol. Vis. Sci 51, 79–88. [DOI] [PubMed] [Google Scholar]
- Krause KH, 2004. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn. J. Infect. Dis 57, S28–S29. [PubMed] [Google Scholar]
- Krizaj D, 1995. What is glaucoma? In: Kolb H, Fernandez E, Nelson R (Eds.), Webvision: the Organization of the Retina and Visual System. Salt Lake City (UT). [PubMed] [Google Scholar]
- Krizaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, Shestopalov VI, 2014. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr. Eye Res 39, 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruczek K, Swaroop A, 2020. Pluripotent stem cell-derived retinal organoids for disease modeling and development of therapies. Stem Cell 38, 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota R, Noda S, Wang Y, Minoshima S, Asakawa S, Kudoh J, Mashima Y, Oguchi Y, Shimizu N, 1997. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: molecular cloning, tissue expression, and chromosomal mapping. Genomics 41, 360–369. [DOI] [PubMed] [Google Scholar]
- Kuchroo M, DiStasio M, Song E, Calapkulu E, Zhang L, Ige M, Sheth AH, Majdoubi A, Menon M, Tong A, Godavarthi A, Xing Y, Gigante S, Steach H, Huang J, Huguet G, Narain J, You K, Mourgkos G, Dhodapkar RM, Hirn MJ, Rieck B, Wolf G, Krishnaswamy S, Hafler BP, 2023. Single-cell analysis reveals inflammatory interactions driving macular degeneration. Nat. Commun 14, 2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar DM, Agarwal N, 2007. Oxidative stress in glaucoma: a burden of evidence. J. Glaucoma 16, 334–343. [DOI] [PubMed] [Google Scholar]
- Kumar S, Berriochoa Z, Ambati BK, Fu Y, 2014. Angiographic features of transgenic mice with increased expression of human serine protease HTRA1 in retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci 55, 3842–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Benavente-Perez A, Ablordeppey R, Lin C, Viswanathan S, Akopian A, Bloomfield SA, 2022. A robust microbead occlusion model of glaucoma for the common marmoset. Transl Vis Sci Technol 11, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowski M, Kelly U, Klingeborn M, Groelle M, Ding JD, Grigsby D, Bowes Rickman C, 2019. Human complement factor H Y402H polymorphism causes an age-related macular degeneration phenotype and lipoprotein dysregulation in mice. Proc. Natl. Acad. Sci. U. S. A 116, 3703–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Ho SL, Thalamuthu A, Venkatraman A, Venkataraman D, Pek DC, Aung T, Vithana EN, 2009. Association of LOXL1 polymorphisms with pseudoexfoliation in the Chinese. Mol. Vis 15, 1120–1126. [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sheck L, Crowston JG, Van Bergen NJ, O’Neill EC, O’Hare F, Kong YX, Chrysostomou V, Vincent AL, Trounce IA, 2012. Impaired complex-I-linked respiration and ATP synthesis in primary open-angle glaucoma patient lymphoblasts. Invest. Ophthalmol. Vis. Sci 53, 2431–2437. [DOI] [PubMed] [Google Scholar]
- Lee AY, Lee CS, Blazes MS, Owen JP, Bagdasarova Y, Wu Y, Spaide T, Yanagihara RT, Kihara Y, Clark ME, Kwon M, Owsley C, Curcio CA, 2020a. Exploring a structural basis for delayed rod-mediated dark adaptation in age-related macular degeneration via deep learning. Transl Vis Sci Technol 9, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Owen JP, Yanagihara RT, Lorch A, Pershing S, Hyman L, Miller JW, Haller JA, Chiang MF, Lum F, Lee AY, 2020b. Smoking is associated with higher intraocular pressure regardless of glaucoma: a retrospective study of 12.5 million patients using the intelligent research in sight (IRIS(R)) registry. Ophthalmol Glaucoma 3, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Makarovsky D, Vander S, 2013a. Comparison between axonal and retinal ganglion cell gene expression in various optic nerve injuries including glaucoma. Mol. Vis 19, 2526–2541. [PMC free article] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Vander S, Makarovsky D, Lavinsky F, 2013b. Increase in retinal ganglion cells’ susceptibility to elevated intraocular pressure and impairment of their endogenous neuroprotective mechanism by age. Mol. Vis 19, 2011–2022. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Schlamp CL, Nickells RW, 1999. Experimental induction of retinal ganglion cell death in adult mice. Invest. Ophthalmol. Vis. Sci 40, 1004–1008. [PubMed] [Google Scholar]
- Li G, Schmitt H, Johnson WM, Lee C, Navarro I, Cui J, Fleming T, Gomez-Caraballo M, Elliott MH, Sherwood JM, Hauser MA, Farsiu S, Ethier CR, Stamer WD, 2020. Integral role for lysyl oxidase-like-1 in conventional outflow tissue function and behavior. Faseb. J 34, 10762–10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Hu Y, Li Y, Hu M, Wang W, Ma Y, Cai Y, Wei M, Yao Y, Wang Y, Dong K, Gu Y, Zhao H, Bao J, Qiu Z, Zhang M, Hu X, Xue T, 2021. Generation of nonhuman primate retinitis pigmentosa model by in situ knockout of RHO in rhesus macaque retina. Sci. Bull 66 (4), 374–385. [DOI] [PubMed] [Google Scholar]
- Li H, Ren J, Cui H, Wang D, Zhao R, Liu X, Tian S, Wang J, Zhang J, Li P, Thorne RF, Duan S, 2023. Dexamethasone induces senescence-associated changes in trabecular meshwork cells by increasing ROS levels via the TGFbeta/smad3-NOX4 Axis. Cell Transplant 32, 9636897231177356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Wang Q, He M, 2019. Repeated retinal photocoagulation in monkeys for the optimization of a laser-induced choroidal neovascularization model. Exp. Eye Res 184, 1–7. [DOI] [PubMed] [Google Scholar]
- Lin J, Xue J, Xu Q, Liu Z, Zhao C, Tang J, Han J, A S, Wang W, Zhuo Y, Li Y, 2022. In situ-crosslinked hydrogel-induced experimental glaucoma model with persistent ocular hypertension and neurodegeneration. Biomater. Sci 10, 5006–5017. [DOI] [PubMed] [Google Scholar]
- Liu Y, Allingham RR, 2017. Major review: molecular genetics of primary open-angle glaucoma. Exp. Eye Res 160, 62–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Hoh J, 2015. Postnatal overexpression of the human ARMS2 gene does not induce abnormalities in retina and choroid in transgenic mouse models. Invest. Ophthalmol. Vis. Sci 56, 1387–1388. [DOI] [PubMed] [Google Scholar]
- Liu Y, Vollrath D, 2004. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum. Mol. Genet 13, 1193–1204. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T, 2004. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet 36, 178–182. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wu Z, Li J, Marmalidou A, Zhang R, Yu M, 2017. Protective effect of resveratrol against light-induced retinal degeneration in aged SAMP8 mice. Oncotarget 8, 65778–65788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2023. Hallmarks of aging: an expanding universe. Cell 186, 243–278. [DOI] [PubMed] [Google Scholar]
- Lotery AJ, 2005. Glutamate excitotoxicity in glaucoma: truth or fiction? Eye 19, 369–370. [DOI] [PubMed] [Google Scholar]
- Lu R, Soden PA, Lee E, 2020a. Tissue-engineered models for glaucoma research. Micromachines 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Brommer B, Tian X, Krishnan A, Meer M, Wang C, Vera DL, Zeng Q, Yu D, Bonkowski MS, Yang JH, Zhou S, Hoffmann EM, Karg MM, Schultz MB, Kane AE, Davidsohn N, Korobkina E, Chwalek K, Rajman LA, Church GM, Hochedlinger K, Gladyshev VN, Horvath S, Levine ME, Gregory-Ksander MS, Ksander BR, He Z, Sinclair DA, 2020b. Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann UF, Robbie S, Munro PM, Barker SE, Duran Y, Luong V, Fitzke FW, Bainbridge JW, Ali RR, MacLaren RE, 2009. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest. Ophthalmol. Vis. Sci 50, 5934–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeen EA, Saydah S, Ehrlich JR, Saaddine J, 2022. Self-reported vision impairment and psychological distress in U.S. Adults. Ophthalmic Epidemiol 29, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjen-Drecoll E, May CA, Polansky JR, Johnson DH, Bloemendal H, Nguyen TD, 1998. Localization of the stress proteins alpha B-crystallin and trabecular meshwork inducible glucocorticoid response protein in normal and glaucomatous trabecular meshwork. Invest. Ophthalmol. Vis. Sci 39, 517–525. [PubMed] [Google Scholar]
- Mabuchi F, Lindsey JD, Aihara M, Mackey MR, Weinreb RN, 2004. Optic nerve damage in mice with a targeted type I collagen mutation. Invest. Ophthalmol. Vis. Sci 45, 1841–1845. [DOI] [PubMed] [Google Scholar]
- MacKay EO, Kallberg ME, Barrie KP, Miller W, Sapienza JS, Denis H, Ollivier FJ, Plummer C, Rinkoski T, Scotty N, Gelatt KN, 2008a. Myocilin protein levels in the aqueous humor of the glaucomas in selected canine breeds. Vet. Ophthalmol 11, 234–241. [DOI] [PubMed] [Google Scholar]
- Mackay EO, Kallberg ME, Gelatt KN, 2008b. Aqueous humor myocilin protein levels in normal, genetic carriers, and glaucoma Beagles. Vet. Ophthalmol 11, 177–185. [DOI] [PubMed] [Google Scholar]
- Mackay DS, Bennett TM, Shiels A, 2015. Exome sequencing identifies a missense variant in EFEMP1 Co-segregating in a family with autosomal dominant primary open-angle glaucoma. PLoS One 10, e0132529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macular Photocoagulation Study Group, 1982. Argon laser photocoagulation for senile macular degeneration. Results of a randomized clinical trial. Arch. Ophthalmol 100, 912–918. [DOI] [PubMed] [Google Scholar]
- Madekurozwa M, Reina-Torres E, Overby DR, Sherwood JM, 2017. Direct measurement of pressure-independent aqueous humour flow using iPerfusion. Exp. Eye Res 162, 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madekurozwa M, Reina-Torres E, Overby DR, van Batenburg-Sherwood J, 2022. Measurement of postmortem outflow facility using iPerfusion. Exp. Eye Res 220, 109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Maeda A, Matosky M, Okano K, Roos S, Tang J, Palczewski K, 2009. Evaluation of Potential Therapies for a Mouse Model of Human Age-Related Macular Degeneration Caused by Delayed all-trans-Retinal Clearance. Invest. Ophthalmol. Vis. Sci 50, 4917–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KP, Duan S, Sigurdsson H, Petursson H, Yang Z, Zhao Y, Bernstein PS, Ge J, Jonasson F, Stefansson E, Helgadottir G, Zabriskie NA, Jonsson T, Bjornsson A, Thorlacius T, Jonsson PV, Thorleifsson G, Kong A, Stefansson H, Zhang K, Stefansson K, Gulcher JR, 2006. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med 3, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Hanneken A, 2005. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Invest. Ophthalmol. Vis. Sci 46, 4796–4803. [DOI] [PubMed] [Google Scholar]
- Mahmoudinezhad G, Nishida T, Weinreb RN, Baxter SL, Eslani M, Micheletti E, Liebmann JM, Fazio MA, Girkin CA, Zangwill LM, Moghimi S, 2022. Impact of smoking on visual field progression in a long-term clinical follow-up. Ophthalmology 129, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek G, Johnson LV, Mace BE, Saloupis P, Schmechel DE, Rickman DW, Toth CA, Sullivan PM, Bowes Rickman C, 2005. Apolipoprotein E allele-dependent pathogenesis: a model for age-related retinal degeneration. Proc. Natl. Acad. Sci. U. S. A 102, 11900–11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso K, Hauswirth WW, Li Q, Connor TB, Kuchenbecker JA, Mauck MC, Neitz J, Neitz M, 2009. Gene therapy for red-green colour blindness in adult primates. Nature 461, 784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao M, Smith RS, Alavi MV, Marchant JK, Cosma M, Libby RT, John SW, Gould DB, 2015. Strain-dependent anterior segment dysgenesis and progression to glaucoma in Col4a1 mutant mice. Invest. Ophthalmol. Vis. Sci 56, 6823–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT, 2008. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol. Vis 14, 782–806. [PMC free article] [PubMed] [Google Scholar]
- Marie M, Bigot K, Angebault C, Barrau C, Gondouin P, Pagan D, Fouquet S, Villette T, Sahel JA, Lenaers G, Picaud S, 2018. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis 9, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Harris A, Hammel T, Malinovsky V, 1999. Mechanism of exercise-induced ocular hypotension. Invest. Ophthalmol. Vis. Sci 40, 1011–1015. [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ, 2002. Cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol 192, 1–15. [DOI] [PubMed] [Google Scholar]
- May CA, Lutjen-Drecoll E, 2002. Morphology of the murine optic nerve. Invest. Ophthalmol. Vis. Sci 43, 2206–2212. [PubMed] [Google Scholar]
- McKinnon SJ, Reitsamer HA, Ransom NL, Caldwell M, Harrison JM, Kiel JW, 2003. Induction and Tonopen Measurement of Ocular Hypertension in C57BL/6 Mice. ARVO Annual Meeting. [Google Scholar]
- McKinnon SJ, Schlamp CL, Nickells RW, 2009. Mouse models of retinal ganglion cell death and glaucoma. Exp. Eye Res 88, 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan GJ, Miller PE, 2011. Feline glaucoma–a comprehensive review. Vet. Ophthalmol 14 (Suppl. 1), 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin PG, Lee WR, Aitken DA, 1986. Age-related changes in the human outflow apparatus. Ophthalmology 93, 194–209. [DOI] [PubMed] [Google Scholar]
- Merle DA, Sen M, Armento A, Stanton CM, Thee EF, Meester-Smoor MA, Kaiser M, Clark SJ, Klaver CCW, Keane PA, Wright AF, Ehrmann M, Ueffing M, 2022. 10q26 - the enigma in age-related macular degeneration. Prog. Retin. Eye Res 101154. [DOI] [PubMed] [Google Scholar]
- Meyer CH, Holz FG, 2011. Preclinical aspects of anti-VEGF agents for the treatment of wet AMD: ranibizumab and bevacizumab. Eye 25, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers SM, Zachary AA, 1988. Monozygotic twins with age-related macular degeneration. Arch. Ophthalmol 106, 651–653. [DOI] [PubMed] [Google Scholar]
- Meyers SM, Greene T, Gutman FA, 1995. A twin study of age-related macular degeneration. Am. J. Ophthalmol 120, 757–766. [DOI] [PubMed] [Google Scholar]
- Micklisch S, Lin Y, Jacob S, Karlstetter M, Dannhausen K, Dasari P, von der Heide M, Dahse HM, Schmolz L, Grassmann F, Alene M, Fauser S, Neumann H, Lorkowski S, Pauly D, Weber BH, Joussen AM, Langmann T, Zipfel PF, Skerka C, 2017. Age-related macular degeneration associated polymorphism rs10490924 in ARMS2 results in deficiency of a complement activator. J. Neuroinflammation 14, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliano C, Scott ER, Murdaugh LB, Gnatowski ER, Faunce CL, Anderson MS, Reyes MM, Gregus AM, Buczynski MW, 2020. Modeling drug exposure in rodents using e-cigarettes and other electronic nicotine delivery systems. J. Neurosci. Methods 330, 108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H, Miller B, Ryan SJ, 1986. The role of retinal pigment epithelium in the involution of subretinal neovascularization. Invest. Ophthalmol. Vis. Sci 27, 1644–1652. [PubMed] [Google Scholar]
- Mitchell P, Wang JJ, Smith W, Leeder SR, 2002. Smoking and the 5-year incidence of age-related maculopathy: the blue mountains eye study. Arch. Ophthalmol 120, 1357–1363. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Segawa K, Urakawa Y, 1987. Age-related changes in the trabecular meshwork of the normal human eye. Jpn. J. Ophthalmol 31, 558–569. [PubMed] [Google Scholar]
- Moore DJ, Clover GM, 2001. The effect of age on the macromolecular permeability of human Bruch’s membrane. Invest. Ophthalmol. Vis. Sci 42, 2970–2975. [PubMed] [Google Scholar]
- Morales-Mantilla DE, Huang X, Erice P, Porter P, Zhang Y, Figueroa M, Chandra J, King KY, Kheradmand F, Rodriguez A, 2020. Cigarette smoke exposure in mice using a whole-body inhalation system. J. Vis. Exp 164 10.3791/61793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MC, Campanelli J, Sande P, Sanez DA, Keller Sarmiento MI, Rosenstein RE, 2004. Retinal oxidative stress induced by high intraocular pressure. Free Radic. Biol. Med 37, 803–812. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Raghunathan VK, Chang YR, Murphy CJ, Russell P, 2015. The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget 6, 15362–15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J, Farrell S, Johnson E, Deppmeier L, Moore CG, Grossmann E, 1995. Structure and composition of the rodent lamina cribrosa. Exp. Eye Res 60, 127–135. [DOI] [PubMed] [Google Scholar]
- Moshiri A, 2021. Animals models of inherited retinal disease. Int. Ophthalmol. Clin 61, 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulfaul K, Giacalone JC, Voigt AP, Riker MJ, Ochoa D, Han IC, Stone EM, Mullins RF, Tucker BA, 2020. Stepwise differentiation and functional characterization of human induced pluripotent stem cell-derived choroidal endothelial cells. Stem Cell Res. Ther 11, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Sullivan J, Schwazer W, Wang M, Park-Windhol C, Klinger B, Matsell J, Hostettler S, Galliker P, Duman M, Hou Y, Balmer P, Virag T, Barrera LA, Xu Q, Magda DP, Kilin F, Khadka A, Quinodoz M, Hasler PW, Moreau P, Fellman L, Azolay T, Cattaneo M, Picelli S, Grison A, Cowan CS, Janeschitz-Kriegl L, Kusnyerik A, Renner M, Nagy ZZ, Szabo A, Rivolta C, Scholl HPN, Bryson D, Ciaramella G, Roska B, Gyorgy B, 2023. High-efficiency base editing for Stargardt disease in mice, non-human primates, and human retina tissue. bioRxiv. 10.1101/2023.04.17.535579. [DOI] [Google Scholar]
- Mullins RF, Dewald AD, Streb LM, Wang K, Kuehn MH, Stone EM, 2011. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Exp. Eye Res 93, 565–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Schoo DP, Sohn EH, Flamme-Wiese MJ, Workamelahu G, Johnston RM, Wang K, Tucker BA, Stone EM, 2014. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am. J. Pathol 184, 3142–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IJ, Rodrigo-Diaz E, Kelly JMF, Aslam TM, Tahir HJ, Carden D, Patryas L, Parry NRA, 2022. The role of dark adaptation in understanding early AMD. Prog. Retin. Eye Res 88, 101015. [DOI] [PubMed] [Google Scholar]
- Nagar S, Noveral SM, Trudler D, Lopez KM, McKercher SR, Han X, Yates JR 3rd, Pina-Crespo JC, Nakanishi N, Satoh T, Okamoto SI, Lipton SA, 2017. MEF2D haploinsufficiency downregulates the NRF2 pathway and renders photoreceptors susceptible to light-induced oxidative stress. Proc. Natl. Acad. Sci. U. S. A 114, E4048–E4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y, 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Iejima D, Akahori M, Kamei J, Goto A, Iwata T, 2014. Overexpression of HtrA1 and exposure to mainstream cigarette smoke leads to choroidal neovascularization and subretinal deposits in aged mice. Invest. Ophthalmol. Vis. Sci 55, 6514–6523. [DOI] [PubMed] [Google Scholar]
- Naskar R, Thanos S, 2006. Retinal gene profiling in a hereditary rodent model of elevated intraocular pressure. Mol. Vis 12, 1199–1210. [PubMed] [Google Scholar]
- Naskar R, Wissing M, Thanos S, 2002. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest. Ophthalmol. Vis. Sci 43, 2962–2968. [PubMed] [Google Scholar]
- National Eye Institute, Eye Disease Statistics, March 2014. https://www.nei.nih.gov/sites/default/files/2019-04/NEI_Eye_Disease_Statistics_Factsheet_2014_V10.pdf.
- Nauseef WM, 2008. Biological roles for the NOX family NADPH oxidases. J. Biol. Chem 283, 16961–16965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari Khanamiri H, Ghasemi Falavarjani K, Sanati MH, Aryan H, Irani A, Hashemi M, Modarres M, Parvaresh MM, Nikeghbali A, 2014. Complement factor H Y402H and LOC387715 A69S polymorphisms in association with age-related macular degeneration in Iran. J. Ophthalmic Vis. Res 9, 181–187. [PMC free article] [PubMed] [Google Scholar]
- Ng SK, Casson RJ, Burdon KP, Craig JE, 2014. Chromosome 9p21 primary open-angle glaucoma susceptibility locus: a review. Clin. Exp. Ophthalmol 42, 25–32. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR, 1998. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J. Biol. Chem 273, 6341–6350. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Alavi MV, Kim KY, Kang T, Scott RT, Noh YH, Lindsey JD, Wissinger B, Ellisman MH, Weinreb RN, Perkins GA, Ju WK, 2011. A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis 2, e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissirios N, Chanis R, Johnson E, Morrison J, Cepurna WO, Jia L, Mittag T, Danias J, 2008. Comparison of anterior segment structures in two rat glaucoma models: an ultrasound biomicroscopic study. Invest. Ophthalmol. Vis. Sci 49, 2478–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell WK, Walker VS, Kang BS, Berman S, 1966. Retinal damage by light in rats. Invest. Ophthalmol 5, 450–473. [PubMed] [Google Scholar]
- Norren DV, Vos JJ, 1974. Spectral transmission of the human ocular media. Vis. Res 14, 1237–1244. [DOI] [PubMed] [Google Scholar]
- Omori Y, Kitamura T, Yoshida S, Kuwahara R, Chaya T, Irie S, Furukawa T, 2015. Mef2d is essential for the maturation and integrity of retinal photoreceptor and bipolar cells. Gene Cell 20, 408–426. [DOI] [PubMed] [Google Scholar]
- Onyak JR, Vergara MN, Renna JM, 2022. Retinal organoid light responsivity: current status and future opportunities. Transl. Res 250, 98–111. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Vaughan DK, 2010. Retinal light damage: mechanisms and protection. Prog. Retin. Eye Res 29, 113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortego J, Escribano J, Coca-Prados M, 1997. Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Lett 413, 349–353. [DOI] [PubMed] [Google Scholar]
- Osborne A, Sanderson J, Martin KR, 2018. Neuroprotective effects of human mesenchymal stem cells and platelet-derived growth factor on human retinal ganglion cells. Stem Cell 36, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oura Y, Nakamura M, Takigawa T, Fukushima Y, Wakabayashi T, Tsujikawa M, Nishida K, 2018. High-temperature requirement A 1 causes photoreceptor cell death in zebrafish disease models. Am. J. Pathol 188, 2729–2744. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G Jr., Clark ME, Jackson GR, Callahan MA, Kline LB, Witherspoon CD, Curcio CA, 2016. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology 123, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Iejima D, Nakayama M, Suga A, Noda T, Kaur I, Das T, Chakrabarti S, Guymer RH, DeAngelis MM, Yamamoto M, Baird PN, Iwata T, 2021. Binding of Gtf2i-beta/delta transcription factors to the ARMS2 gene leads to increased circulating HTRA1 in AMD patients and in vitro. J. Biol. Chem 296, 100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Fu Y, Baird PN, Guymer RH, Das T, Iwata T, 2022. Exploring the contribution of ARMS2 and HTRA1 genetic risk factors in age-related macular degeneration. Prog. Retin. Eye Res 101159. [DOI] [PubMed] [Google Scholar]
- Pang IH, Clark AF, 2007. Rodent models for glaucoma retinopathy and optic neuropathy. J. Glaucoma 16, 483–505. [DOI] [PubMed] [Google Scholar]
- Pang IH, Clark AF, 2020. Inducible rodent models of glaucoma. Prog. Retin. Eye Res 75, 100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas CM, Zouache MA, Matthews S, Faust CD, Hageman JL, Williams BL, Richards BT, Hageman GS, 2021. Protective chromosome 1q32 haplotypes mitigate risk for age-related macular degeneration associated with the CFH-CFHR5 and ARMS2/HTRA1 loci. Hum. Genom 15, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Qazi Y, Tan C, Jabbar SB, Cao Y, Schmid G, Pardue MT, 2012. Assessment of axial length measurements in mouse eyes. Optom. Vis. Sci 89, 296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Lee JH, Woo SJ, Ahn J, Shin JP, Song SJ, Kang SW, Park KH, Epidemiologic Survey Committee of the Korean Ophthalmologic, S., 2014. Age-related macular degeneration: prevalence and risk factors from Korean national Health and nutrition examination survey, 2008 through 2011. Ophthalmology 121, 1756–1765. [DOI] [PubMed] [Google Scholar]
- Passo MS, Goldberg L, Elliot DL, Van Buskirk EM, 1991. Exercise training reduces intraocular pressure among subjects suspected of having glaucoma. Arch. Ophthalmol 109, 1096–1098. [DOI] [PubMed] [Google Scholar]
- Pasutto F, Zenkel M, Hoja U, Berner D, Uebe S, Ferrazzi F, Schodel J, Liravi P, Ozaki M, Paoli D, Frezzotti P, Mizoguchi T, Nakano S, Kubota T, Manabe S, Salvi E, Manunta P, Cusi D, Gieger C, Wichmann HE, Aung T, Khor CC, Kruse FE, Reis A, Schlotzer-Schrehardt U, 2017. Pseudoexfoliation syndrome-associated genetic variants affect transcription factor binding and alternative splicing of LOXL1. Nat. Commun 8, 15466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SS, Lally DR, Hsu J, Wykoff CC, Eichenbaum D, Heier JS, Jaffe GJ, Westby K, Desai D, Zhu L, Khanani AM, 2023. Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye (Lond). 37 (17), 3551–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi ME, Neuringer M, Courtney RJ, 2012. Animal models of age related macular degeneration. Mol. Aspect. Med 33, 487–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer BA, 1991. Chapter 10 Improved methodology for cell culture of human and monkey retinal pigment epithelium. Prog. Retin. Res 10, 251–291. [Google Scholar]
- Plestina-Borjan I, Klinger-Lasic M, 2007. Long-term exposure to solar ultraviolet radiation as a risk factor for age-related macular degeneration. Coll. Antropol 31 (Suppl. 1), 33–38. [PubMed] [Google Scholar]
- Polansky JR, Weinreb RN, Baxter JD, Alvarado J, 1979. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest. Ophthalmol. Vis. Sci 18, 1043–1049. [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, Lutjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD, 1997. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 211, 126–139. [DOI] [PubMed] [Google Scholar]
- Pons M, Marin-Castano ME, 2011. Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PLoS One 6, e16722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool FM, Kiel C, Serrano L, Luthert PJ, 2020. Repository of proposed pathways and protein-protein interaction networks in age-related macular degeneration. NPJ Aging Mech Dis 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Hirt J, Stamer WD, Liton PB, 2015. Autophagic dysregulation in glaucomatous trabecular meshwork cells. Biochim. Biophys. Acta 1852, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiser D, Lagreze WA, Bach M, Poloschek CM, 2013. Photopic negative response versus pattern electroretinogram in early glaucoma. Invest. Ophthalmol. Vis. Sci 54, 1182–1191. [DOI] [PubMed] [Google Scholar]
- Prendes MA, Harris A, Wirostko BM, Gerber AL, Siesky B, 2013. The role of transforming growth factor beta in glaucoma and the therapeutic implications. Br. J. Ophthalmol 97, 680–686. [DOI] [PubMed] [Google Scholar]
- Qi JH, Bell B, Singh R, Batoki J, Wolk A, Cutler A, Prayson N, Ali M, Stoehr H, Anand-Apte B, 2019. Sorsby fundus dystrophy mutation in tissue inhibitor of metalloproteinase 3 (TIMP3) promotes choroidal neovascularization via a fibroblast growth factor-dependent mechanism. Sci. Rep 9, 17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Shen X, Shyam R, Yue BY, Ying H, 2014. Cellular processing of myocilin. PLoS One 9, e92845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA, Katz J, Derick RJ, Gilbert D, Sommer A, 1992. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology 99, 19–28. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Xi XR, Huang YB, Wu XD, 1996. Magnitude of decrease in intraocular pressure depends upon intensity of exercise. Kor. J. Ophthalmol 10, 109–115. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Wu XD, Xi XR, Yang J, Huang YB, 1997. Resting intraocular pressure of steel factory workers is related to their physical fitness. Ind. Health 35, 259–263. [DOI] [PubMed] [Google Scholar]
- Ramprasad VL, George R, Soumittra N, Sharmila F, Vijaya L, Kumaramanickavel G, 2008. Association of non-synonymous single nucleotide polymorphisms in the LOXL1 gene with pseudoexfoliation syndrome in India. Mol. Vis 14, 318–322. [PMC free article] [PubMed] [Google Scholar]
- Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT, 1994. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest. Ophthalmol. Vis. Sci 35, 2857–2864. [PubMed] [Google Scholar]
- Rautenbach RM, Bardien S, Harvey J, Ziskind A, 2011. An investigation into LOXL1 variants in black South African individuals with exfoliation syndrome. Arch. Ophthalmol 129, 206–210. [DOI] [PubMed] [Google Scholar]
- Rein DB, Wittenborn JS, Zhang P, Sublett F, Lamuda PA, Lundeen EA, Saaddine J, 2022. The economic burden of vision loss and blindness in the United States. Ophthalmology 129, 369–378. [DOI] [PubMed] [Google Scholar]
- Reinhard J, Wiemann S, Hildebrandt S, Faissner A, 2021. Extracellular matrix remodeling in the retina and optic nerve of a novel glaucoma mouse model. Biology 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K, 2015. Oxidative stress in aging human skin. Biomolecules 5, 545–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T, Weber BH, 2005. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum. Mol. Genet 14, 3227–3236. [DOI] [PubMed] [Google Scholar]
- Robertson JV, Siwakoti A, West-Mays JA, 2013. Altered expression of transforming growth factor beta 1 and matrix metalloproteinase-9 results in elevated intraocular pressure in mice. Mol. Vis 19, 684–695. [PMC free article] [PubMed] [Google Scholar]
- Rocha LR, Nguyen Huu VA, Palomino La Torre C, Xu Q, Jabari M, Krawczyk M, Weinreb RN, Skowronska-Krawczyk D, 2020. Early removal of senescent cells protects retinal ganglion cells loss in experimental ocular hypertension. Aging Cell 19, e13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy GW, Rosa RH, Viker KB, Holman BH, Hann CR, Krishnan A, Gores GJ, Bakri SJ, Fautsch MP, 2020. Diet mimicking "fast food" causes structural changes to the retina relevant to age-related macular degeneration. Curr. Eye Res 45, 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozing MP, Durhuus JA, Krogh Nielsen M, Subhi Y, Kirkwood TB, Westendorp RG, Sorensen TL, 2020. Age-related macular degeneration: a two-level model hypothesis. Prog. Retin. Eye Res 76, 100825. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ederra J, Verkman AS, 2006. Mouse model of sustained elevation in intraocular pressure produced by episcleral vein occlusion. Exp. Eye Res 82, 879–884. [DOI] [PubMed] [Google Scholar]
- Russell P, Tamm ER, Grehn FJ, Picht G, Johnson M, 2001. The presence and properties of myocilin in the aqueous humor. Invest. Ophthalmol. Vis. Sci 42, 983–986. [PubMed] [Google Scholar]
- Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, Wittes J, Pappas J, Elci O, McCague S, Cross D, Marshall KA, Walshire J, Kehoe TL, Reichert H, Davis M, Raffini L, George LA, Hudson FP, Dingfield L, Zhu X, Haller JA, Sohn EH, Mahajan VB, Pfeifer W, Weckmann M, Johnson C, Gewaily D, Drack A, Stone E, Wachtel K, Simonelli F, Leroy BP, Wright JF, High KA, Maguire AM, 2017. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, 1979. The development of an experimental model of subretinal neovascularization in disciform macular degeneration. Trans. Am. Ophthalmol. Soc 77, 707–745. [PMC free article] [PubMed] [Google Scholar]
- Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A, 2005. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch. Ophthalmol 123, 458–463. [DOI] [PubMed] [Google Scholar]
- Saha A, Capowski E, Fernandez Zepeda MA, Nelson EC, Gamm DM, Sinha R, 2022. Cone photoreceptors in human stem cell-derived retinal organoids demonstrate intrinsic light responses that mimic those of primate fovea. Cell Stem Cell 29, 460–471 e463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H, Miyagi M, Shadrach KG, Rayborn ME, Crabb JW, Hollyfield JG, 2002. Clusterin is present in drusen in age-related macular degeneration. Exp. Eye Res 74, 547–549. [DOI] [PubMed] [Google Scholar]
- Sakami S, Maeda T, Bereta G, Okano K, Golczak M, Sumaroka A, Roman AJ, Cideciyan AV, Jacobson SG, Palczewski K, 2011. Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J. Biol. Chem 286, 10551–10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakami S, Kolesnikov AV, Kefalov VJ, Palczewski K, 2014. P23H opsin knock-in mice reveal a novel step in retinal rod disc morphogenesis. Hum. Mol. Genet 23, 1723–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsel PA, Kisiswa L, Erichsen JT, Cross SD, Morgan JE, 2011. A novel method for the induction of experimental glaucoma using magnetic microspheres. Invest. Ophthalmol. Vis. Sci 52, 1671–1675. [DOI] [PubMed] [Google Scholar]
- Sappington RM, Carlson BJ, Crish SD, Calkins DJ, 2010. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest. Ophthalmol. Vis. Sci 51, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick T, Ersoy L, Lechanteur YT, Saksens NT, Hoyng CB, den Hollander AI, Kirchhof B, Fauser S, 2016. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina 36, 787–790. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Pasutto F, Sommer P, Hornstra I, Kruse FE, Naumann GO, Reis A, Zenkel M, 2008. Genotype-correlated expression of lysyl oxidase-like 1 in ocular tissues of patients with pseudoexfoliation syndrome/glaucoma and normal patients. Am. J. Pathol 173, 1724–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelter C, Fomo KN, Perumal N, Manicam C, Bell K, Pfeiffer N, Grus FH, 2019. Synthetic polyclonal-derived CDR peptides as an innovative strategy in glaucoma therapy. J. Clin. Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H, Watkins T, Hake K, Kuhn M, Li G, Stamer DW, Hauser MA, 2021. Genetic susceptibility to Loxl1 knockout-induced elastosis in a mouse model. ARVO Annual Meeting, Invest. Ophthalmol. Vis. Sci 62, 468. [Google Scholar]
- Schmitt HM, Hake KM, Perkumas KM, Le BM, Suarez MF, De Ieso ML, Rahman RS, Johnson WM, Gomez-Caraballo M, Ashley-Koch AE, Hauser MA, Stamer WD, 2023. LOXL1-AS1 lncRNA differentially regulates gene and protein expression, signaling, and morphology of human ocular cells. Hum. Mol. Genet 32 (21), 3053–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettauf F, Rejdak R, Walski M, Frontczak-Baniewicz M, Voelker M, Blatsios G, Shinoda K, Zagorski Z, Zrenner E, Grieb P, 2004. Retinal neurodegeneration in the DBA/2J mouse-a model for ocular hypertension. Acta Neuropathol 107, 352–358. [DOI] [PubMed] [Google Scholar]
- Schumann RG, Banyai D, Hagenau F, Mautone L, Hammer T, Wolf A, Priglinger SG, Vogt D, 2023. Pores of the internal limiting membrane: a common finding in vitreomaculopathies. Retina 43, 1773–1779. [DOI] [PubMed] [Google Scholar]
- Seidehamel RJ, Dungan KW, 1974. Characteristics and pharmacologic utility of an intraocular pressure (IOP) model in unanesthetized rabbits. Invest. Ophthalmol 13, 319–322. [PubMed] [Google Scholar]
- Seitsonen SP, Onkamo P, Peng G, Xiong M, Tommila PV, Ranta PH, Holopainen JM, Moilanen JA, Palosaari T, Kaarniranta K, Meri S, Immonen IR, Jarvela IE, 2008. Multifactor effects and evidence of potential interaction between complement factor H Y402H and LOC387715 A69S in age-related macular degeneration. PLoS One 3, e3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena DF, Lindsley K, 2017. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. Rev 1, CD006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov V, Malyukova I, Fariss R, Wawrousek EF, Swaminathan S, Sharan SK, Tomarev S, 2006. Expression of mutated mouse myocilin induces open-angle glaucoma in transgenic mice. J. Neurosci 26, 11903–11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, Massague J, 2001. TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat. Cell Biol 3, 400–408. [DOI] [PubMed] [Google Scholar]
- Shalaby WS, Ahmed OM, Waisbourd M, Katz LJ, 2022. A review of potential novel glaucoma therapeutic options independent of intraocular pressure. Surv. Ophthalmol 67, 1062–1080. [DOI] [PubMed] [Google Scholar]
- Sharma R, Bose D, Maminishkis A, Bharti K, 2020. Retinal pigment epithelium replacement therapy for age-related macular degeneration: are we there yet? Annu. Rev. Pharmacol. Toxicol 60, 553–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PX, Zhang L, Zhang M, Du H, Zhao L, Lee C, Grob S, Lim SL, Hughes G, Lee J, Bedell M, Nelson MH, Lu F, Krupa M, Luo J, Ouyang H, Tu Z, Su Z, Zhu J, Wei X, Feng Z, Duan Y, Yang Z, Ferreyra H, Bartsch DU, Kozak I, Zhang L, Lin F, Sun H, Feng H, Zhang K, 2012. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc. Natl. Acad. Sci. U. S. A 109, 13757–13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Fingert JH, Stone EM, Sheffield VC, Clark AF, 2001. Delayed secondary glucocorticoid responsiveness of MYOC in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci 42, 3173–3181. [PubMed] [Google Scholar]
- Sher A, Jones BW, Huie P, Paulus YM, Lavinsky D, Leung LS, Nomoto H, Beier C, Marc RE, Palanker D, 2013. Restoration of retinal structure and function after selective photocoagulation. J. Neurosci 33, 6800–6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR, 2016. Measurement of outflow facility using iPerfusion. PLoS One 11, e0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler EJ, Randolph JC, Calzada JI, Charles S, 2014. Smoking and choroidal thickness in patients over 65 with early-atrophic age-related macular degeneration and normals. Eye 28, 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Krawczak M, Dawson WW, Schmidtke J, 2009. Association of HTRA1 and ARMS2 gene variation with drusen formation in rhesus macaques. Exp. Eye Res 88, 479–482. [DOI] [PubMed] [Google Scholar]
- Soysal Y, Inan UU, Kusbeci T, Imirzalioglu N, 2012. Age-related macular degeneration and association of CFH Y402H and LOC387715 A69S polymorphisms in a Turkish population. DNA Cell Biol 31, 323–330. [DOI] [PubMed] [Google Scholar]
- Spraul CW, Lang GE, Grossniklaus HE, Lang GK, 1999. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv. Ophthalmol 44 (Suppl. 1), S10–S32. [DOI] [PubMed] [Google Scholar]
- Stanton JB, Marmorstein AD, Zhang Y, Marmorstein LY, 2017. Deletion of Efemp1 is protective against the development of sub-RPE deposits in mouse eyes. Invest. Ophthalmol. Vis. Sci 58, 1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JD, Khawaja AP, Weizer JS, 2021. Glaucoma in adults-screening, diagnosis, and management: a review. JAMA 325, 164–174. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC, 1997. Identification of a gene that causes primary open angle glaucoma. Science 275, 668–670. [DOI] [PubMed] [Google Scholar]
- Suarez MF, Schmitt H, Hake K, Kuhn M, Watkins T, Elliott MH, Hauser MA, Stamer DW, 2022. Characterization of Loxl1-knockout-induced elastosis in mice with different genetic backgrounds. ARVO Annual Meeting, Invest. Ophthalmol. Vis. Sci 63, 446. [Google Scholar]
- Suh S, Choi EH, Raguram A, Liu DR, Palczewski K, 2022. Precision genome editing in the eye. Proc. Natl. Acad. Sci 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui GY, Liu GC, Liu GY, Gao YY, Deng Y, Wang WY, Tong SH, Wang L, 2013. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br. J. Ophthalmol 97, 389–394. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Shirato S, Taniguchi F, Ohara K, Nishimaki K, Ohta S, 1997. Mutations in the TIGR gene in familial primary open-angle glaucoma in Japan. Am. J. Hum. Genet 61, 1202–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan SS, Oh DJ, Kang MH, Ren R, Jin R, Gong H, Rhee DJ, 2013. Secreted protein acidic and rich in cysteine (SPARC)-null mice exhibit more uniform outflow. Invest. Ophthalmol. Vis. Sci 54, 2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiderski RE, Ross JL, Fingert JH, Clark AF, Alward WL, Stone EM, Sheffield VC, 2000. Localization of MYOC transcripts in human eye and optic nerve by in situ hybridization. Invest. Ophthalmol. Vis. Sci 41, 3420–3428. [PubMed] [Google Scholar]
- Tan JC, Peters DM, Kaufman PL, 2006. Recent developments in understanding the pathophysiology of elevated intraocular pressure. Curr. Opin. Ophthalmol 17, 168–174. [DOI] [PubMed] [Google Scholar]
- Tang Z, Zhang S, Lee C, Kumar A, Arjunan P, Li Y, Zhang F, Li X, 2011. An optic nerve crush injury murine model to study retinal ganglion cell survival. J. Vis. Exp 50, 2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HR, Munoz B, West S, Bressler NM, Bressler SB, Rosenthal FS, 1990. Visible light and risk of age-related macular degeneration. Trans. Am. Ophthalmol. Soc 88, 163–173.; discussion 173–168. [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, Hsu S, Ng TF, 2021. The role of retinal pigment epithelial cells in regulation of macrophages/microglial cells in retinal immunobiology. Front. Immunol 12, 724601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teikari JM, 1990. Genetic influences in open-angle glaucoma. Int. Ophthalmol. Clin 30, 161–168. [DOI] [PubMed] [Google Scholar]
- Teo KYC, Lee SY, Barathi AV, Tun SBB, Tan L, Constable IJ, 2018. Surgical removal of internal limiting membrane and layering of AAV vector on the retina under air enhances gene transfection in a nonhuman primate. Invest. Ophthalmol. Vis. Sci 59, 3574–3583. [DOI] [PubMed] [Google Scholar]
- Tezel G, 2022. Molecular regulation of neuroinflammation in glaucoma: current knowledge and the ongoing search for new treatment targets. Prog. Retin. Eye Res 87, 100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Cai J, 2005. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest. Ophthalmol. Vis. Sci 46, 3177–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Moorfields Macular Study Group, 1982. Treatment of senile disciform macular degeneration: a single-blind randomised trial by argon laser photocoagulation. The Moorfields Macular Study Group. Br. J. Ophthalmol 66, 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thee EF, Colijn JM, Cougnard-Gregoire A, Meester-Smoor MA, Verzijden T, Hoyng CB, Fauser S, Hense HW, Silva R, Creuzot-Garcher C, Ueffing M, Delcourt C, den Hollander AI, Klaver CCW, European Eye Epidemiology C, Project E-R, 2022. The phenotypic course of age-related macular degeneration for ARMS2/HTRA1: the EYE-RISK consortium. Ophthalmology 129, 752–764. [DOI] [PubMed] [Google Scholar]
- Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K, 2007. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 317, 1397–1400. [DOI] [PubMed] [Google Scholar]
- Tietze L, Borntraeger J, Klosterhalfen B, Amo-Takyi B, Handt S, Gunther K, Merkelbach-Bruse S, 1999. Expression and function of beta(1) and beta(3) integrins of human mesothelial cells in vitro. Exp. Mol. Pathol 66, 131–139. [DOI] [PubMed] [Google Scholar]
- Ting DS, Ng WY, Ng SR, Tan SP, Yeo IY, Mathur R, Chan CM, Tan AC, Tan GS, Wong TY, Cheung CM, 2016. Choroidal thickness changes in age-related macular degeneration and polypoidal choroidal vasculopathy: a 12-month prospective study. Am. J. Ophthalmol 164, 128–136 e121. [DOI] [PubMed] [Google Scholar]
- Tom I, Pham VC, Katschke KJ Jr., Li W, Liang WC, Gutierrez J, Ah Young A, Figueroa I, Eshghi ST, Lee CV, Kanodia J, Snipas SJ, Salvesen GS, Lai P, Honigberg L, van Lookeren Campagne M, Kirchhofer D, Baruch A, Lill JR, 2020. Development of a therapeutic anti-HtrA1 antibody and the identification of DKK3 as a pharmacodynamic biomarker in geographic atrophy. Proc. Natl. Acad. Sci. U. S. A 117, 9952–9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey CB, Landowski M, Klingeborn M, Kelly U, Deans J, Dong H, Harrabi O, Van Blarcom T, Yeung YA, Grishanin R, Lin JC, Saban DR, Bowes Rickman C, 2018. Effect of anti-C5a therapy in a murine model of early/intermediate dry age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 59, 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totan Y, Yagci R, Bardak Y, Ozyurt H, Kendir F, Yilmaz G, Sahin S, Sahin Tig U, 2009. Oxidative macromolecular damage in age-related macular degeneration. Curr. Eye Res 34, 1089–1093. [DOI] [PubMed] [Google Scholar]
- Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, Zhang K, Chan CC, 2007. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 48, 3827–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Vander Wall R, Gupta V, Klistorner A, Graham SL, 2017. DBA/2J mouse model for experimental glaucoma: pitfalls and problems. Clin. Exp. Ophthalmol 45, 911–922. [DOI] [PubMed] [Google Scholar]
- Turrens JF, 2003. Mitochondrial formation of reactive oxygen species. J. Physiol 552, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufret-Vincenty RL, Aredo B, Liu X, McMahon A, Chen PW, Sun H, Niederkorn JY, Kedzierski W, 2010. Transgenic mice expressing variants of complement factor H develop AMD-like retinal findings. Invest. Ophthalmol. Vis. Sci 51, 5878–5887. [DOI] [PubMed] [Google Scholar]
- Urcola JH, Hernandez M, Vecino E, 2006. Three experimental glaucoma models in rats: comparison of the effects of intraocular pressure elevation on retinal ganglion cell size and death. Exp. Eye Res 83, 429–437. [DOI] [PubMed] [Google Scholar]
- Valentine JS, Doucette PA, Zittin Potter S, 2005. Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis. Annu. Rev. Biochem 74, 563–593. [DOI] [PubMed] [Google Scholar]
- van Leeuwen EM, Emri E, Merle BMJ, Colijn JM, Kersten E, Cougnard-Gregoire A, Dammeier S, Meester-Smoor M, Pool FM, de Jong EK, Delcourt C, Rodrigez-Bocanegra E, Biarnes M, Luthert PJ, Ueffing M, Klaver CCW, Nogoceke E, den Hollander AI, Lengyel I, 2018. A new perspective on lipid research in age-related macular degeneration. Prog. Retin. Eye Res 67, 56–86. [DOI] [PubMed] [Google Scholar]
- Velez G, Machlab DA, Tang PH, Sun Y, Tsang SH, Bassuk AG, Mahajan VB, 2018. Proteomic analysis of the human retina reveals region-specific susceptibilities to metabolic- and oxidative stress-related diseases. PLoS One 13, e0193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey KA, Greferath U, Jobling AI, Phipps JA, Ho T, Waugh M, Fletcher EL, 2012. Ccl2/Cx3cr1 knockout mice have inner retinal dysfunction but are not an accelerated model of AMD. Invest. Ophthalmol. Vis. Sci 53, 7833–7846. [DOI] [PubMed] [Google Scholar]
- Vielle A, Park YK, Secora C, Vergara MN, 2021. Organoids for the study of retinal development and developmental abnormalities. Front. Cell. Neurosci 15, 667880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierkotten S, Muether PS, Fauser S, 2011. Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch’s membrane via cleavage of extracellular matrix components. PLoS One 6, e22959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilkeviciute A, Kriauciuniene L, Chaleckis R, Deltuva VP, Liutkeviciene R, 2019. RAD51B (rs8017304 and rs2588809), TRIB1 (rs6987702, rs4351379, and rs4351376), COL8A1 (rs13095226), and COL10A1 (rs1064583) Gene Variants with Predisposition to Age-Related Macular Degeneration. Dis. Markers, 5631083, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojnikovic B, Njiric S, Coklo M, Spanjol J, 2007. Ultraviolet sun radiation and incidence of age-related macular degeneration on Croatian Island Rab. Coll. Antropol 31 (Suppl. 1), 43–44. [PubMed] [Google Scholar]
- Volkner M, Wagner F, Steinheuer LM, Carido M, Kurth T, Yazbeck A, Schor J, Wieneke S, Ebner LJA, Del Toro Runzer C, Taborsky D, Zoschke K, Vogt M, Canzler S, Hermann A, Khattak S, Hackermuller J, Karl MO, 2022. HBEGF-TNF induce a complex outer retinal pathology with photoreceptor cell extrusion in human organoids, 13, 6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina EG, 1954. [Production of experimental glaucoma and its clinical aspects]. Biull Eksp Biol Med 37, 30–32. [PubMed] [Google Scholar]
- Wakatsuki Y, Shinojima A, Kawamura A, Yuzawa M, 2015. Correlation of aging and segmental choroidal thickness measurement using swept source optical coherence tomography in healthy eyes. PLoS One 10, e0144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Johnson DH, 2000. mRNA in situ hybridization of TIGR/MYOC in human trabecular meshwork. Invest. Ophthalmol. Vis. Sci 41, 1724–1729. [PubMed] [Google Scholar]
- Wang M, Kaufman RJ, 2016. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529, 326–335. [DOI] [PubMed] [Google Scholar]
- Wang G, Spencer KL, Court BL, Olson LM, Scott WK, Haines JL, Pericak-Vance MA, 2009. Localization of age-related macular degeneration-associated ARMS2 in cytosol, not mitochondria. Invest. Ophthalmol. Vis. Sci 50, 3084–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zheng M, Lester KL, Han Z, 2019. Light-induced Nrf2(-/-) mice as atrophic age-related macular degeneration model and treatment with nanoceria laden injectable hydrogel. Sci. Rep 9, 14573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Hadzic S, Roxlau ET, Fuehler B, Janise-Libawski A, Wimmer T, Lei B, Li SW, Weissmann N, Stieger K, 2021. Retinal tissue develops an inflammatory reaction to tobacco smoke and electronic cigarette vapor in mice. J. Mol. Med. (Berl.) 99, 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wiggs JL, Aung T, Khawaja AP, Khor CC, 2022. The genetic basis for adult onset glaucoma: recent advances and future directions. Prog. Retin. Eye Res 90, 101066. [DOI] [PubMed] [Google Scholar]
- Weber AJ, Zelenak D, 2001. Experimental glaucoma in the primate induced by latex microspheres. J. Neurosci. Methods 111, 39–48. [DOI] [PubMed] [Google Scholar]
- Wei X, Kumar S, Ding J, Khandelwal N, Agarwal M, Agrawal R, 2019. Choroidal structural changes in smokers measured using choroidal vascularity index. Invest. Ophthalmol. Vis. Sci 60, 1316–1320. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA, 2014. The pathophysiology and treatment of glaucoma: a review. JAMA 311, 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Liebmann JM, Cioffi GA, Goldberg I, Brandt JD, Johnson CA, Zangwill LM, Schneider S, Badger H, Bejanian M, 2018. Oral memantine for the treatment of glaucoma: design and results of 2 randomized, placebo-controlled, phase 3 studies. Ophthalmology 125, 1874–1885. [DOI] [PubMed] [Google Scholar]
- Weismann D, Hartvigsen K, Lauer N, Bennett KL, Scholl HP, Charbel Issa P, Cano M, Brandstatter H, Tsimikas S, Skerka C, Superti-Furga G, Handa JT, Zipfel PF, Witztum JL, Binder CJ, 2011. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 478, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielgus AR, Sarna T, 2008. Ascorbate enhances photogeneration of hydrogen peroxide mediated by the iris melanin. Photochem. Photobiol 84, 683–691. [DOI] [PubMed] [Google Scholar]
- Wielgus AR, Collier RJ, Martin E, Lih FB, Tomer KB, Chignell CF, Roberts JE, 2010. Blue light induced A2E oxidation in rat eyes–experimental animal model of dry AMD. Photochem. Photobiol. Sci 9, 1505–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Yaspan BL, Hauser MA, Kang JH, Allingham RR, Olson LM, Abdrabou W, Fan BJ, Wang DY, Brodeur W, Budenz DL, Caprioli J, Crenshaw A, Crooks K, Delbono E, Doheny KF, Friedman DS, Gaasterland D, Gaasterland T, Laurie C, Lee RK, Lichter PR, Loomis S, Liu Y, Medeiros FA, McCarty C, Mirel D, Moroi SE, Musch DC, Realini A, Rozsa FW, Schuman JS, Scott K, Singh K, Stein JD, Trager EH, Vanveldhuisen P, Vollrath D, Wollstein G, Yoneyama S, Zhang K, Weinreb RN, Ernst J, Kellis M, Masuda T, Zack D, Richards JE, Pericak-Vance M, Pasquale LR, Haines JL, 2012. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet 8, e1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Whigham BT, Liu Y, Carmichael TR, Qin X, Schmidt S, Ramsay M, Hauser MA, Allingham RR, 2010. Major LOXL1 risk allele is reversed in exfoliation glaucoma in a black South African population. Mol. Vis 16, 705–712. [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Tribble JR, Pepper KW, Cross SD, Morgan BP, Morgan JE, John SW, Howell GR, 2016. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol. Neurodegener 11, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Seager NA, Gardiner JD, Pappas CM, Cronin MC, Amat di San Filippo C, Anstadt RA, Liu J, Toso MA, Nichols L, Parnell TJ, Eve JR, Heinz S, Hayes MGB, Bartel PL, Zouache MA, Richards BT, Hageman GS, 2021. Chromosome 10q26-driven age-related macular degeneration is associated with reduced levels of HTRA1 in human retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WoldeMussie E, Ruiz G, Wijono M, Wheeler LA, 2001. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest. Ophthalmol. Vis. Sci 42, 2849–2855. [PubMed] [Google Scholar]
- Wolter JR, 1964. Pores in the internal limiting membrane of the human retina. Acta Ophthalmol 42, 971–974. [DOI] [PubMed] [Google Scholar]
- Woodell A, Coughlin B, Kunchithapautham K, Casey S, Williamson T, Ferrell WD, Atkinson C, Jones BW, Rohrer B, 2013. Alternative complement pathway deficiency ameliorates chronic smoke-induced functional and morphological ocular injury. PLoS One 8, e67894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Rydz C, Nguyen Huu VA, Rocha L, Palomino La Torre C, Lee I, Cho W, Jabari M, Donello J, Lyon DC, Brooke RT, Horvath S, Weinreb RN, Ju WK, Foik A, Skowronska-Krawczyk D, 2022. Stress induced aging in mouse eye. Aging Cell 21, e13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuejiao Y, Junwei Y, 2022. New strategies for neuro protection in glaucoma. Front. Cell Dev. Biol 10, 983195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Namekata K, Aida T, Katou S, Takeda T, Harada T, Fuse N, The Glaucoma Gene Research, G., Tanaka K, 2020. EAAT1 variants associated with glaucoma. Biochem. Biophys. Res. Commun 529, 943–949. [DOI] [PubMed] [Google Scholar]
- Yang H, Thompson H, Roberts MD, Sigal IA, Downs JC, Burgoyne CF, 2011a. Deformation of the early glaucomatous monkey optic nerve head connective tissue after acute IOP elevation in 3-D histomorphometric reconstructions. Invest. Ophthalmol. Vis. Sci 52, 345–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Williams G, Downs JC, Sigal IA, Roberts MD, Thompson H, Burgoyne CF, 2011b. Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma. Invest. Ophthalmol. Vis. Sci 52, 7109–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li Y, Chan L, Tsai YT, Wu WH, Nguyen HV, Hsu CW, Li X, Brown LM, Egli D, Sparrow JR, Tsang SH, 2014. Validation of genome-wide association study (GWAS)-identified disease risk alleles with patient-specific stem cell lines. Hum. Mol. Genet 23, 3445–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Greenberg K, Hunter JJ, Dalkara D, Kolstad KD, Masella BD, Wolfe R, Visel M, Stone D, Libby RT, Diloreto D Jr., Schaffer D, Flannery J, Williams DR, Merigan WH, 2011. Intravitreal injection of AAV2 transduces macaque inner retina. Invest. Ophthalmol. Vis. Sci 52, 2775–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, Tieu E, Munevar C, Wong B, Cunefare D, Farsiu S, Garzel L, Roberts J, Thomasy SM, 2017. In vivo multimodal imaging of drusenoid lesions in rhesus macaques. Sci. Rep 7, 15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AL, Birke K, Moriniere J, Welge-Lussen U, 2010. TGF-beta2 induces senescence-associated changes in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci 51, 5718–5723. [DOI] [PubMed] [Google Scholar]
- Yuan L, Neufeld AH, 2001. Activated microglia in the human glaucomatous optic nerve head. J. Neurosci. Res 64, 523–532. [DOI] [PubMed] [Google Scholar]
- Yuan N, Li J, Tang S, Li FF, Lee CO, Ng MPH, Cheung CY, Tham CC, Pang CP, Chen LJ, Yam JC, 2019. Association of secondhand smoking exposure with choroidal thinning in children aged 6 to 8 Years: the Hong Kong children eye study. JAMA Ophthalmol 137, 1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanon-Moreno V, Marco-Ventura P, Lleo-Perez A, Pons-Vazquez S, Garcia-Medina JJ, Vinuesa-Silva I, Moreno-Nadal MA, Pinazo-Duran MD, 2008. Oxidative stress in primary open-angle glaucoma. J. Glaucoma 17, 263–268. [DOI] [PubMed] [Google Scholar]
- Zayas-Santiago A, Cross SD, Stanton JB, Marmorstein AD, Marmorstein LY, 2017. Mutant fibulin-3 causes proteoglycan accumulation and impaired diffusion across Bruch’s membrane. Invest. Ophthalmol. Vis. Sci 58, 3046–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EB, Kong R, Yin DD, You LH, Sun M, Han L, Xu TP, Xia R, Yang JS, De W, Chen J, 2014. Long noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449a. Oncotarget 5, 2276–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Davies KJA, Forman HJ, 2015. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med 88, 314–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chu Y, Mowery J, Konkel B, Galli S, Theos AC, Golestaneh N, 2018. Pgc-1alpha repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice. Dis Model Mech 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Xu P, Jie Z, Zuo Y, Yu B, Soong L, Sun J, Chen Y, Cai J, 2014. Gammadelta T cells as a major source of IL-17 production during age-dependent RPE degeneration. Invest. Ophthalmol. Vis. Sci 55, 6580–6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Devitt C, Liu J, Mei J, Skapek SX, 2013. A distant, cis-acting enhancer drives induction of Arf by Tgfbeta in the developing eye. Dev. Biol 380, 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV, 2014. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun 5, 4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li Y, Yue BY, 1999. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J. Cell. Physiol 180, 182–189. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang H, Yu A, Xie J, 2018. Association between sunlight exposure and risk of age-related macular degeneration: a meta-analysis. BMC Ophthalmol 18, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Aredo B, Chen B, Zhao CX, He YG, Ufret-Vincenty RL, 2019. Mice with a combined deficiency of superoxide dismutase 1 (Sod1), DJ-1 (Park7), and Parkin (prkn) develop spontaneous retinal degeneration with aging. Invest. Ophthalmol. Vis. Sci 60, 3740–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouache MA, 2022. Variability in retinal neuron populations and associated variations in mass transport systems of the retina in Health and aging. Front. Aging Neurosci 14, 778404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouache MA, Richards BT, Pappas CM, Anstadt RA, Liu J, Corsetti T, Matthews S, Seager NA, Schmitz-Valckenberg S, Fleckenstein M, Hubbard WC, Thomas J, Hageman JL, Williams BL, Hageman GS, 2024. Levels of complement factor H-related 4 protein do not influence susceptibility to age-related macular degeneration or its course of progression. Nat. Commun 15, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman R, Harris A, Oddone F, Siesky B, Verticchio Vercellin A, Ciulla TA, 2021. Glaucoma heritability: molecular mechanisms of disease. Genes 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


