Abstract
Gallbladder polypoid lesions (GPLs) refer to any elevated lesion of the mucosal surface of the gallbladder wall, and the prevalence is estimated to be between 0.9% and 12.1%. GPLs include benign polyps and malignant polyps. Benign polyps are further classified as non-neoplastic polyps and neoplastic polyps. Cholesterol polyps are the most common benign polyps and adenocarcinoma is the main type of malignant polyp. Hepatitis B virus infection, liver function abnormalities, dyslipidemia, and obesity are the main risk factors for GPLs. Studies of biological mechanisms have focused on malignant gallbladder polyps, the development of which is regulated by hormone levels in vivo, gut microbiota, inflammation, oxidative stress, Salmonella typhimurium, and related molecules. Diagnostic modalities include chemical examination and imaging examination, with imaging examination currently being the mainstay. Treatment of patients with GPLs is based on the presence or absence of symptoms, age, size of the polyps, tendency of the polyp to increase, and risk factors for symptomatic malignancy to determine whether surgery should be performed.
Keywords: Gallbladder neoplasms, Benign polyps, Malignant polyps, Risk factors, Biological mechanism, Diagnosis, Treatment
Introduction
Gallbladder polypoid lesions (GPLs) refer to any elevated lesion of the mucosal surface of the gallbladder wall. The disease was first pathologically defined in 1970, and was eventually classified as both benign and malignant.[1,2] Benign lesions are subdivided into non-neoplastic polyps and neoplastic polyps.[1,3,4] Non-neoplastic polyps include cholesterol polyps, inflammatory polyps, hyperplastic polyps, and adenomyomatosis, while neoplastic polyps mainly refer to adenomas.[1,3,4,5] Gallbladder adenocarcinoma is the main type of malignant lesion.[1,3,4]
With the development of imaging technology in recent years, the detection rate of GPLs is also improving.[6,7,8] Although polypoid lesions of the gallbladder are becoming more common, the current literature has not reached a consensus on many aspects of this disease. For example, the risk factors for GPLs are not fully understood, and there are differences in clinical management in particular. Therefore, this article is intended to review the current status of the diagnosis and treatment of GPLs with the aim of playing a positive role in clinical management.
Epidemiological Features
Studies have shown that the prevalence of GPLs varies between regions and populations. A Netherlands study showed a prevalence of GPLs of 0.9% in the Netherlands population.[5] Lee et al[8] found a 5.4% prevalence of GPLs in a study of 48,591 Korean individuals, with a higher prevalence in men (6.3%) than in women (4.5%). Another study by Choi et al[6] of 23,827 healthy Korean individuals reported a GPL prevalence of 9.96%. Kratzer et al[9] showed a prevalence of 6.1% for GPLs in Germany and Heitz et al[7] showed that the prevalence of GPLs in German individuals increased from 6.1% in 2002 to 12.1% in 2013. A study by Ali et al[10] in Qatar that included 7156 individuals showed an overall prevalence of 7.4% for GPLs, with a prevalence of 4.2% for monogenic GPLs and 3.2% for multiple GPLs. There have been many related studies in China as well. In a study of 34,669 Taiwanese (China) people, Lin et al[11] showed that the overall prevalence of GPLs was 9.5%, with the highest prevalence in middle-aged men. Xu et al[12] examined 60,064 healthy individuals, and the prevalence of GPLs was 6.9%. Mao et al[13] found a 7.4% prevalence of GPLs in a study of 48,591 Chinese petrochemical employees, and it was more common in the middle-aged population, peaking in the 40–59 years age group. Yamin et al[14] recruited 97,117 Chinese individuals. They showed that the prevalence of GPLs was 7.3% and that male sex was a risk factor for the formation of GPLs. A study by Yang et al[15] that included 11,816 healthy subjects yielded an overall prevalence of 4.2% for GPLs, with a prevalence of 4.6% in men and 3.5% in women. In conclusion, the incidence of GPLs varies around the world and is not precise, with estimates ranging from 0.9% to 12.1% according to the available literature. However, there is much research evidence to support that men have a higher prevalence than women.[5,6,7,8,9,10,11,12,13,14]
Histopathologic Classification
GPLs are pathologically classified into benign and malignant types. Benign polyps include cholesterol polyps, inflammatory polyps, hyperplastic polyps, adenomyomatosis, and adenomas, while malignant polyps mainly refer to adenocarcinoma of the gallbladder.
Benign polyps
Non-neoplastic polyps
Cholesterol polyps: Cholesterol polyps are the most common type of GPLs, with an incidence of 50–70% and have no malignant potential.[6] Cholesterol polyp diameters are usually less than 10 mm (2–10 mm) and they may be pedicled.[16] They are yellow in appearance, friable, and attached to the mucosal surface of the gallbladder cavity by a pedicle.[3] Some researchers believe that the formation of cholesterol polyps may be caused by cholesterol deposition within macrophages in the lamina propria of the gallbladder wall and is not related to cellular proliferation.[3,17]
Adenomyomatosis: Adenomyoma accounts for approximately 25% of GPLs, second only to cholesterol polyps.[18,19] Usually, it localizes to the gallbladder fundus, appearing as a solitary polyp ranging in size from 10 mm to 20 mm.[18] Adenomyoma includes three morphologic types: focal, segmental, and diffuse.[17] The formation of adenomyoma is thought to be the result of the thickening of the mucosa and muscularis propria of the gallbladder wall and is associated with cholesterol crystals or stone deposits without an inflammatory response.[17,19]
Inflammatory polyps: Inflammatory polyps account for approximately 10% of GPLs. They are usually less than 10 mm in diameter and have no malignant potential.[4,16,18,19] They are usually associated with chronic inflammation and are caused by granulation formation and fibrous tissue secondary to chronic inflammation.[4,16,19]
Hyperplastic polyps: Hyperplastic polyps are lesions characterized by papillary hyperplasia.[19] The manifestation is single or multiple, usually in the body and base of the gallbladder, without a malignant tendency. Hyperplastic polyps are histologically characterized by epithelial cell hyperplasia with mucinous glandular metaplasia, smooth muscle fibers connected to the muscular layer in the interstitial fibers, and cupped cells.
Neoplastic polyps
Adenomas: Gallbladder adenomas are rare, accounting for only 4–7% of all gallbladder polyps. They are usually 0.1–2.5 cm in size, nonpedicled solitary polyps with a well-established potential for progression to carcinoma.[16,17] Histologically, gallbladder adenomas can be tubular (most prevalent), papillary, or tubulopapillary.[16,17] Cytologically, gallbladder adenomas may be classified as having a pyloric (most common), intestinal, foveolar, or biliary subtype.[20] It has been suggested that it originates from flattened and dysplastic epithelial cells in any part of the gallbladder wall.[19]
Malignant polyps
Adenocarcinoma: The most common type of malignant gallbladder polyp is adenocarcinoma of the gallbladder.[4,17] There are various subtypes of gallbladder adenocarcinoma, the most common of which is papillary, but there are also signet-ring and mucinous adenocarcinomas.[4] Papillary adenocarcinoma presents pathologically as a dense papillary lobe of cells protruding into the gallbladder lumen, with dysplasia and increased mitoses.[4]
Risk Factors
Sex and age
Previous studies have shown that sex is an independent risk factor for GPLs, with the prevalence being higher in men than in women.[8,11,12,13,14,21,22] Yamin et al[21] reported that age was not associated with the occurrence of GPLs. However, more available results suggest that age is a risk factor for GPLs, although there is no consensus at the age group level.[10,11,12,13,23] Ali et al[10] concluded that the risk of developing GPLs increased significantly with age, with the risk for patients aged greater than or equal to 65 years being 4.5 times higher than that for patients aged 18 years. Other findings tend to support the prevalence of GPLs commonly in the middle-aged population aged 40–59 years.[11,12,13,23]
Hepatitis B virus (HBV) infection
There is a large number of HBV carriers worldwide. The hepatitis B surface antigen (HbsAg) positivity rate in China already accounts for 7.2% of its total population, although some studies put the rate at 10.0%.[24] There is some controversy in the published literature regarding the association between HBV and GPLs. Lim et al[22] reported that HBsAg positivity was not associated with the incidence of GPLs. Evidence from most scholars, however, suggested not only that HbsAg positivity was an independent risk factor for GPLs but also that the risk of GPLs in HBsAg-positive subjects was approximately 2.5 times higher than that in HBsAg-negative subjects.[6,8,10,11,13,21] Some authors have attempted to elucidate the mechanism between the two, suggesting that it may be related to HBV causing changes in the gallbladder mucosa, cholesterol deposition, and increased bile viscosity.[6] However, the overall correlation between HBV and GPLs is still unclear, so further studies are needed to clarify the pathophysiological mechanisms of the association between HBV and GPLs.
Liver function abnormalities and fatty liver
Several findings reported that high levels of aspartate aminotransferase (AST) were a risk factor for inducing the development of GPLs.[8,23] Ahn et al[25] showed that both fatty liver and low levels of alanine aminotransferase (ALT) were independent risk factors for GPLs and noted a correlation between fatty liver and large GPLs (≥5 mm). This is consistent with the previous results published by Lim et al.[26] In addition, some studies have shown that ALT, AST, and fatty liver are not associated with GPLs.[21,22] To resolve this controversy, additional studies are needed to determine whether liver function abnormalities and fatty liver are risk factors for GPLs, as there is currently a limited number of studies on this topic.
Dyslipidemia
Blood lipids are a general term for neutral fats (triglycerides) and lipids (phospholipids, glycolipids, sterols, and steroids) in blood plasma that bind to proteins to form lipoproteins and are widely present in the body. Cholesterol polyps are the most common type of GPLs.[6] Studies on the correlation between dyslipidemia and GPLs are also currently common. There is much evidence in the literature supporting that high triglyceride (TG) levels, high total cholesterol (TC) levels, low high-density lipoprotein (HDL) levels, and high low-density lipoprotein (LDL) levels are risk factors for the development of GPLs.[6,8,14,21,23] Additionally, low HDL levels and high LDL levels are key factors in dyslipidemia and significantly increase the prevalence of GPLs in the population.[14] This may be caused by HDL and LDL, which are the main carriers of cholesterol, by affecting the concentration of bile and the metabolism of cholesterol in bile. In addition, Deng et al[27] noted that dyslipidemia was an independent risk factor for malignancy, as it increased the risk of malignancy 2.674-fold in patients with gallbladder lesions.
Overweight and obesity
Overweight and obesity have become problems that cannot be ignored in today’s society, and some scholars have performed research on whether they increase the risk of developing GPLs. It was found that the formation of GPLs was closely related to the overweight or obese status of the patients, and the prevalence rose with an increase in body mass index (BMI).[8,21,22,23] Lee et al[28] further suggested that visceral adipose tissue might be more important in the pathogenesis of GPLs than being overweight and obesity itself simply expressed by BMI and pointed out that obesity was an important risk factor for gallbladder cancer. The main drivers of being overweight and obesity include the excessive intake of high-calorie foods as a result of increasing wealth and the emergence of a large number of sedentary jobs as a result of urbanization.[29] Therefore, weight control by reducing high-calorie dietary intake, avoiding a sedentary lifestyle, and increasing exercise may help reduce the risk of GPL formation and malignant transformation.
Number, shape, and size of GPLs
GPLs have been increasingly recognized as a predisposing factor for gallbladder cancer. In terms of number and shape, benign polyps of the gallbladder are predominantly multiple-pedicle polyps, while malignant polyps are predominantly solitary non-pedicle polyps.[30] In terms of size, polyp size ≥10 mm is a significant predictor of polypoid lesions with a high risk of neoplastic polyps.[5] For small GPLs less than 10 mm, studies have shown that most of these lesions are benign.[31] In conclusion, solitary non-pedicular polyps larger than 10 mm in size are an important risk factor for malignant polyps of the gallbladder.
Metabolic syndrome (MS) and ethnicity
In addition, MS and ethnicity have also been mentioned as risk factors for GPLs in some of the relevant literature studies. Lim et al[22] suggested that MS is a risk factor for GPLs. Choi et al[6] also reported that MS was a significant independent risk factor for multiple GB polypoid lesions. Another study similarly noted that the presence of MS also appears to be associated with the risk of GPLs.[32] Regarding ethnicity, studies have shown that the prevalence of GPLs is 5.8 times higher in Southeast Asian populations than in sub-Saharan African populations and that the probability of gallbladder malignancy is significantly higher in Indian populations than in Caucasian populations with GPLs.[10]
Biological Mechanism
Current studies on the mechanisms of GPLs are dominated by malignant polyps, with little involvement of benign polyps. It has been suggested that obese individuals may be at increased risk for gallbladder cancer through a variety of mechanisms, including increased sex and metabolic hormones, altered gut microbiota, inflammation, and oxidative stress.[33,34,35] In terms of microorganisms, in recent years, bacterial infections have been increasingly recognized as risk factors for the development of adenocarcinoma, and Salmonella enterica serovars Typhi and Paratyphi A are clinically strongly associated with gallbladder cancer.[36,37] In addition to these studies, there have been many investigations on gallbladder cancer mechanisms at the molecular level. Tian et al[38] showed that apoptosis-stimulating of p53 protein 2 (ASPP2) affected the expression of atypical protein kinase C (aPKC)-ι and glioma-associated oncogene homolog 1 (GLI1) and that downregulation of ASPP2 promoted gallbladder cancer invasion and metastasis through the aPKC-ι/GLI1 pathway. Liu et al[39] showed that long-chain noncoding RNA (lncRNA) maternally expressed gene 3 (MEG3) inhibited adenocarcinoma cell proliferation and promoted apoptosis. However, antisense noncoding RNA at the INK4 locus (ANRIL) promoted gallbladder cell proliferation and inhibited apoptosis. Therefore, the therapeutic strategies of upregulating MEG3 and downregulating ANRIL might be clinically relevant for inhibiting gallbladder cancer progression. Wen et al[40] showed that NLR family pyrin domain containing 3 (NLRP3) inflammatory vesicles could promote adenocarcinoma proliferation through the activation of caspase-1, the production of mature interleukin (IL)-1b and IL-18, and thus the enhancement of phosphorylation of Akt, extracellular regulated protein kinase (ERK), and cAMP responsive element-binding protein (CREB), suggesting that cellular pyroptosis induced by NLRP3 inflammatory vesicles might play a role in the promotion of adenocarcinoma growth. Wang et al[41] indicated that metastasis-associated gene 1 (MTA1) and tumor metastasis-suppression genes (KAI-1 and KiSS-1) might be important biomarkers involved in carcinogenesis, metastasis, and invasion of gallbladder cancer, with MTA1 being an independent prognostic factor. Lin et al[42] proposed that silencing of long noncoding RNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) or overexpression of ABI family member 3 binding protein (ABI3BP) inhibited adenocarcinoma cell growth in vitro and in vivo. MALAT1 promoted ABI3BP expression by binding enhancer of zeste homolog 2 (EZH2) to the ABI3BP promoter region, thereby silencing MALAT1 or inhibiting H3K27 methylation. Wu et al[43] also demonstrated that MALAT1 might act as an oncogenic lncRNA that promoted the proliferation and metastasis of adenocarcinoma cells by activating the ERK/MAPK pathway. The above molecular studies suggest that ASPP2, MEG3, ANRIL, NLRP3, MTA1, and MALAT1 have the potential to be therapeutic targets for gallbladder cancer in the future.
Natural History and Coexisting Diseases
Natural history
The current studies on GPLs are mainly retrospective and lack prospective. Thus, little is known about their natural history and pathophysiological mechanisms. The results of a prospective study indicated that the natural history of small GPLs was benign and that the number and size of polyps did not change over a 5-year period.[44] Research by Beck et al[45] on the natural history of childhood illness also supported this view. However, the results of the study by Colecchia et al[44] showed that in 111 patients with small GPLs, 50% of the GPLs remained similar in size in the late follow-up period, 26.5% increased in number and size, and 23.5% shrank or disappeared.[46] These results may be explained by differences in study design or in the characteristics of the studied populations.
Coexisting diseases
Gallstones
The most important risk factor for the development of gallbladder cancer is cholelithiasis. A cohort study of Taiwan (China) patients hospitalized for gallstones reported that participants with gallstones had a 59 times higher risk of gallbladder cancer than those without gallstones.[47] Another cohort study reported a positive association between gallstones and an increased risk of gallbladder cancer mortality.[48] The increased risk is most likely attributable to greater local epithelial irritation and chronic inflammation leading to dysplasia caused by gallstones.[49]
Primary sclerosing cholangitis (PSC)
PSC is an idiopathic, heterogeneous, cholestatic liver disease that is characterized by persistent, progressive, biliary inflammation and fibrosis.[50] Most gallbladder polyps in patients with PSC are benign. For patients with PSC without high-risk imaging features, monitoring and observation prior to cholecystectomy is recommended.[51] It is reasonable to perform cholecystectomy in patients with small polyps when the underlying liver disease permits.
Diagnosis
GPLs are asymptomatic in most cases and are usually discovered incidentally by imaging.[3,19,52] Some patients with advanced malignant polyps may present with abdominal pain, dyspepsia, and jaundice, but polyps are usually not the direct cause of the complaints.[19] The diagnosis of GPLs is divided into chemical examination and imaging examination. Due to the poor specificity and low accuracy of chemical examination for the diagnosis of GPLs, imaging examination is mainly used at present, including ultrasound (US), endoscopic ultrasound (EUS), computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), elastography, microflow imaging (MFI), and other examination methods.
Imaging examination
Transabdominal ultrasonography
US is currently the imaging method of choice for the diagnosis of GPLs.[53] Transabdominal ultrasound (TAUS) includes conventional ultrasound (CUS), high-resolution ultrasound (HRUS), contrast-enhanced ultrasound (CEUS), and three-dimensional ultrasound (3DUS).[54] Although CUS is helpful in differentiating cholesterol polyps from adenomas, the diagnostic accuracy is poor in GPLs with polyps <1 cm.[52,55] The sensitivity and specificity of CUS for polyps <1 cm were reported to be 20% and 95.1%, respectively, while the sensitivity and specificity for polyps >1 cm were 80% and 99.3%, respectively.[52] In contrast, the magnified images obtained using HRUS are very helpful in identifying small lesions, and therefore, HRUS is considered to have the potential to be an important diagnostic modality for the differential diagnosis of benign polypoid lesions and early-stage gallbladder cancer, as well as for cancer staging.[56] CEUS improves the diagnosis of GPLs compared to CUS.[57] CEUS can detect dynamic microvascularization of PLG >1.0 cm in diameter and facilitate the differentiation between benign and malignant gallbladder tumors and non-neoplastic GPLs.[58] Disruption of gallbladder wall integrity in CEUS is a major sign of gallbladder cancer.[59] In addition to the two-dimensional ultrasound (2DUS) described above, 3DUS plays a role in the clinical diagnosis of GPLs. It has been suggested that 3DUS diagnosis correlates well with 2DUS and could be used as a stand-alone technique for the diagnosis of GPLs.[60] TAUS can also provide a basis for decisions related to surgery for GPLs, where polyp size ≥15 mm is the strongest predictor of tumor polyps.[61] It has also been reported in the literature that despite improvements in TAUS technology, it is still important to take into account that its accuracy is still poor when dealing with patients with GPLs detected by TAUS.[62]
EUS
EUS is routinely used in the differential diagnosis of GPLs because of its high resolution and specificity.[63] The most notable feature of EUS is that it combines ultrasonography with endoscopy, enabling a close examination of the lesion and surrounding tissues and improving the resolution of the images. Compared with US, EUS is more suitable for lesions <1 cm and has a higher diagnostic rate and safety.[64] Some literature suggests that EUS is helpful to distinguish neoplastic from non-neoplastic polyps.[65] Non-neoplastic polyps on EUS appear as hyperechoic spots and aggregates of multiple microcysts.[66] For example, cholesterol polyps and adenomyomatosis are characterized by a tiny or aggregated echogenic spot and multiple microcysts or comet tail artifacts, whereas polypoid lesions without these findings suggest a possible adenoma or adenocarcinoma.[67] In addition, EUS can help determine the treatment strategy for gallbladder polyps and avoid unnecessary surgery.[68] EUS combined with fine needle aspiration is also playing an increasingly important role in the histological diagnosis of gallbladder tumors.[63] However, some investigators have indicated that the accuracy of EUS in identifying neoplastic and non-neoplastic GPLs smaller than 1.0 cm is still low and insufficient to determine their treatment strategy.[69] There are limitations to any invasive test, and EUS is no exception. The most important limitations of EUS are the high requirements for operators, the long learning time, and the invasiveness of the test, which may lead to a series of complications, such as postoperative bleeding, infection, and biliary pancreatitis.[64] Second, calcification and bile duct pneumoperitoneum will affect the image quality. Furthermore, EUS is expensive, which will increase the financial burden of patients.[70]
CT
CT scans are more often used as a first-line imaging method for patients with abdominal symptoms or those undergoing regular monitoring.[3] It is possible to distinguish between neoplastic and non-neoplastic GPLs.[71] Some literature states that in three-phase dynamic enhanced CT scans, plain CT values, delayed phase CT values, and ∆CT (∆CT = portal venous phase CT value minus delayed phase CT value) values can detect malignant lesions of gallbladder polyps, with ∆CT having the highest sensitivity and specificity.[72] Other investigators have questioned the use of CT imaging for the diagnosis and follow-up of GPLs, as CUS has greater advantages.[54]
MRI
MRI has not been widely used to evaluate gallbladder disease or to identify benign and malignant GPLs due to poor spatial resolution and contrast.[19,49,54] One study reported that MRI could be used to differentiate gallbladder cancer from adenomyomatosis by depicting the Rockitansky–Aschoff sinuses.[19] It has also been suggested that adenomas, although more homogeneous in texture, are not easily distinguishable from gallbladder carcinoma because of the enhancing polypoid lesions that appear on MRI.[17] Additional research evidence supports the potential of high b-value diffusion-weighted magnetic resonance imaging (DWI) as a new method for identifying benign and malignant gallbladder disease.[73]
PET
The study and application of PET to distinguish benign and malignant GPLs has become more common in recent years.[19,49] The uptake of 18F-fluorodeoxyglucose (18F-FDG) in GPLs has been found to be a useful predictor of risk stratification for surgical intervention, but mainly applies primarily to polyps 1-2 cm in diameter.[74] This provides a theoretical rationale for fluorodeoxyglucose positron emission tomography (FDG-PET) as a possible method for accurately diagnosing malignant gallbladder polyps preoperatively.
Elastography
Real-time elastography is a dynamic technique that objectively assesses the elastic properties of tissues by measuring the degree of deformation under the action of external forces. Teber et al[75] initially assessed that real-time elastography is feasible in the diagnosis of GPLs and benign lesions with high-strain elastography patterns. However, there are few studies and clinical applications of elastography, and more clinical studies are needed to understand the effectiveness of this technology in diagnosing GPLs.
Photoacoustic (PA) imaging
PA imaging is an emerging biomedical imaging modality that images optical absorbers in tissues through acoustic detectors.[76] Chae et al[77] noted that benign and malignant GPLs showed distinct PA spectral patterns and that multispectral PA imaging could be used to identify them. Further studies are needed to explore this due to the lack of additional evidence on PA imaging in the diagnosis of PLA.
MFI
MFI is a new high-resolution flow US imaging technique that discriminates Doppler signals from blood flow and tissue sources, thus enabling accurate evaluation of microvascular and low-velocity blood flow without the use of US contrast agents.[78,79] MFI can more accurately reflect the morphology of the blood vessels within the polyp and improve the ability to identify the nature of the polypoid lesion of the gallbladder, thus providing a US diagnostic basis for choosing the appropriate treatment for the patient.[80]
Chemical examination
Liver function abnormalities and dyslipidemia are risk factors for GPLs, but blood biochemical tests have no significant diagnostic value for GPLs. Tumor markers include carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and carbohydrate antigen 125 (CA125). Mondal et al[81] reported that CEA expression was negative in benign GPLs, whereas in most primary gallbladder cancers, CEA expression was focal or diffuse. Bind et al[82] stated that CA125 could be a good adjunct to the diagnosis of gallbladder cancer. Some studies have also suggested CA19-9 as a diagnostic and prognostic marker for gallbladder cancer.[83] When comparing the three, CA19-9 was superior to CEA and CA125.[82,83] The poor specificity of tumor markers in the differential diagnosis may be because most malignant GPLs are early-stage gallbladder cancers.[49]
Treatment
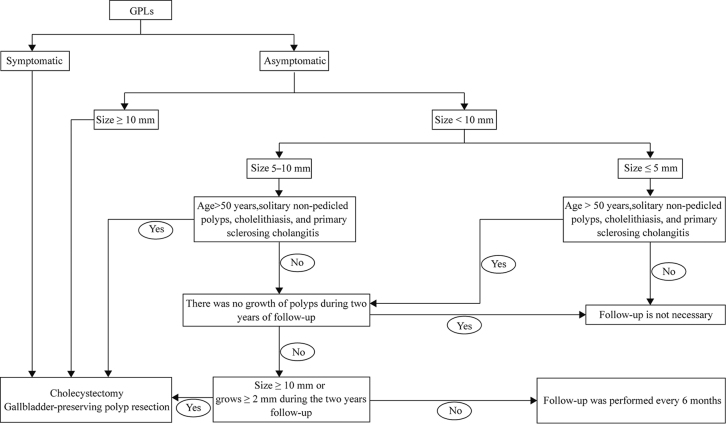
The current treatment of GPLs is mainly based on the patient’s choice of whether to have surgery based on the presence or absence of symptoms. Surgical treatment is required for all symptomatic patients.[84] Asymptomatic patients are treated according to the size of the polyps, the tendency of the polyps to increase rapidly, and the risk factors for malignancy. It has been suggested that age >50 years, solitary non-pedicled polyps, cholelithiasis, and PSC are risk factors for polyp malignancy.[30,85,86] In patients with asymptomatic polyps ≤5 mm in diameter without risk factors for malignancy, follow-up is not considered necessary.[85,86] Patients with polyps ≤5 mm in diameter with risk factors for malignancy and those with polyps of 5–10 mm in diameter without risk factors for malignancy can be followed up first.[85,86] Surgery is recommended if the GPLs grow to ≥10 mm during follow-up or increase 2 mm or more during the 2-year follow-up period.[85,86] Patients with polyps of 5–10 mm in diameter with risk factors for malignancy and those ≥1 cm in diameter (with or without risk factors for malignancy) should undergo surgery promptly [Figure 1].[1,84,85,86]
Figure 1.
Recommended treatment strategies for GPLs. GPLs: Gallbladder polypoid lesions.
Surgery is the main treatment option for GPLs.[87] The surgical treatment of GPLs is mainly divided into cholecystectomy and gallbladder-preserving polyp resection. Cholecystectomy includes open cholecystectomy, laparoscopic cholecystectomy, and transvaginal laparoscopically assisted endoscopic cholecystectomy. Gallbladder-preserving polyp resection mainly refers to natural orifice transluminal endoscopic surgery (NOTES). NOTES is an emerging endoscopic surgery designed to reduce or eliminate abdominal incisions and incision-related complications.
In previous studies, GPLs were mainly performed by cholecystectomy, of which laparoscopic cholecystectomy was the major surgical method.[84,85,86] Compared with open surgery, laparoscopic cholecystectomy has the advantages of less trauma, less intraoperative blood loss, and shorter postoperative hospitalization.[88] However, studies have shown that adhesive disease occurs most often in abdominal wall incisions.[89] This prompted investigators to choose a better surgical approach to avoid postoperative complications. Some studies have explored transvaginal laparoscopically assisted endoscopic cholecystectomy and have shown that this surgery is feasible for being more effective in reducing or eliminating postoperative complications such as wound infections, incisional hernias, esthetic disdain, and adhesions.[90,91] However, transvaginal laparoscopically assisted endoscopic cholecystectomy has not become widespread in clinical practice due to sex restrictions and insufficient evidence to support safety.
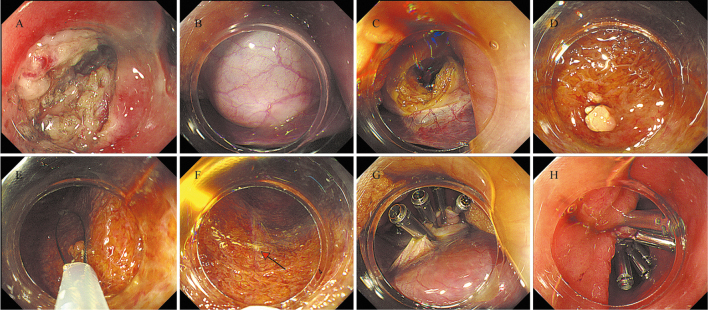
Several studies have reported the feasibility of transgastric NOTES in experimental pig models.[92,93] Bai et al[94] indicated that transgastric NOTES can be used to diagnose ascites of unknown origin. These studies provide an important exploration of NOTES as a new technology for the minimally invasive treatment of clinical abdominal diseases. With the rapid development of endoscopic technology, NOTES is becoming possible as a new approach to treating gallbladder polyps.[95] Gallbladder-preserving polyp resection is performed for patients with benign gallbladder polyps with good gallbladder function and involves transgastric or transduodenal endoscopic access to the gallbladder for resection of the polyps.[96,97,98] Tian et al[96] reported the first case of resection of gallbladder polyps following EUS-guided cholecystoduodenostomy using a lumen-apposing metal stent. The first case of endoscopic polypectomy through a cholecystogastrostomy was reported by Chen et al.[97] Li and Han[95] reported for the first time the successful use of gallbladder-preserving polyp resection and concluded that this procedure was a promising new surgical option for patients who wished to preserve the gallbladder and its function without scarring of the abdominal wall. Zhang et al[98] performed a follow-up of 12 patients treated with transgastric gallbladder-preserving polyp resection for a period of 4 months to determine recurrence or death in any of the patients, and they concluded that transgastric gallbladder-preserving polyp resection was a viable treatment for patients with benign gallbladder disease. The transgastric gallbladder-preserving polyp resection procedure was as follows: gastric sinusotomy, finding the gallbladder, incision of the gallbladder, finding the polyp, looper removal, clamping of the gallbladder incision, and clamping of the gastric incision [Figure 2]. The advantage of gallbladder-preserving polyp resection is that it preserves a functioning gallbladder and can prevent the development of postcholecystectomy syndrome. However, because this is a new technique, the inherent limitations and possible risk factors are not known. Further studies are needed to demonstrate its safety and long-term effectiveness and to determine which patients will benefit the most from the surgery.
Figure 2.
Transgastric gallbladder-preserving polyp resection. (A) A hybrid knife or an insulated tip knife was used to make a full-thickness incision on the anterior wall and greater curvature of the gastric antrum. (B) An endoscope was inserted through the incision and the gallbladder was found. (C) A small incision was made at the bottom of the gallbladder, and the endoscope was used to probe the gallbladder lumen after the bile was completely suctioned. (D) Endoscopy access to the gallbladder reveals polyp. (E) The gallbladder polyp was resected using a looper. (F) Image of location where a polyp was removed. (G) Several clips were used to close the gallbladder incision. (H) Closure of the gastric incision site was achieved with clips.
Conclusion
GPLs are common in populations. GPLs are classified as benign and malignant polyps, with the most common type being cholesterol polyps, and as such, most GPLs have a low malignant potential. The important features of GPLs are shown in [Table 1]. HBV infection, liver function abnormalities, dyslipidemia, and obesity are the main risk factors for GPLs. Studies of biological mechanisms have focused on malignant gallbladder polyps, the development of which is regulated by hormone levels in vivo, gut microbiota, inflammation, oxidative stress, Salmonella typhimurium, and related molecules. The diagnosis of GPLs includes imaging and chemical examination, and imaging is currently the main examination due to the poor specificity and sensitivity of chemical examination. US is currently the most commonly used imaging examination, and the application of other imaging techniques deserves further exploration especially US endoscopy in GPLs. Treatment of patients with GPLs is based on the presence or absence of symptoms, age, size of the polyps, tendency of the polyp to increase rapidly, and risk factors for symptomatic malignancy to determine whether surgery should be performed. Surgical options include laparoscopic-based cholecystectomy and endoscopic-based gallbladder-preserving polyp resection. Further studies are needed to demonstrate the safety and long-term effectiveness of the new technique of endoscopic gallbladder-preserving polyp resection.
Table 1.
Important features of GPLs.
| Features | Description |
|---|---|
| Prevalence | 0.9%–12.1% |
| Histopathology | Most common type (about two-thirds) is cholesterol polyps. Other lesions include inflammatory polyps, hyperplastic polyps, adenomyomatosis, adenomas, adenocarcinoma. |
| Risk factors | Gender, age, hepatitis B virus infection, liver function abnormalities, fatty liver, dyslipidemia, overweight and obesity, number, shape and size of GPLs, metabolic syndrome, and ethnicity. |
| Biological mechanism | Hormone levels in vivo, gut microbiota, inflammation, oxidative stress, Salmonella typhimurium, and related molecules. |
| Natural history | Most of the small polyps were benign, with no change in number and size. |
| Coexisting diseases | GallstonesPrimary sclerosing cholangitis |
| Diagnosis | Including chemical examination and imaging examination. Imaging examination is mainly used at present, ultrasound is the common imaging method. |
| Treatment | Cholecystectomy or gallbladder-preserving polyp resection was performed in patients with symptoms, large lesions (≥10 mm in diameter), or features associated with malignancy. |
GPLs: Gallbladder polypoid lesions.
Acknowledgments
Thanks to Ms. Chen Li for revising and touching up this article.
Funding
This review was funded by Jiangsu Province-Establishment of an endoscopic diagnosis and treatment system for submucosal tumors of the digestive tract and optimization of key technologies (No. BE2019667).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang K, Xu QP, Xia L, Sun JN, Shen KE, Liu HR, Xu LN, Li R. Gallbladder polypoid lesions: Current practices and future prospects. Chin Med J 2024;137:1674–1683. doi: 10.1097/CM9.0000000000003019
References
- 1.Chou SC, Chen SC, Shyr YM, Wang SE. Polypoid lesions of the gallbladder: Analysis of 1204 patients with long-term follow-up. Surg Endosc 2017;31:2776–2782. doi: 10.1007/s00464-016-5286-y. [DOI] [PubMed] [Google Scholar]
- 2.Christensen AH, Ishak KG. Benign tumors and pseudotumors of the gallbladder. Report of 180 cases. Arch Pathol. 1970. Nov;90:423–32. [PubMed] [Google Scholar]
- 3.Zemour J, Marty M, Lapuyade B, Collet D, Chiche L. Gallbladder tumor and pseudotumor: Diagnosis and management. J Visc Surg 2014;151:289–300. doi: 10.1016/j.jviscsurg.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Mellnick VM Menias CO Sandrasegaran K Hara AK Kielar AZ Brunt EM, et al. Polypoid lesions of the gallbladder: Disease spectrum with pathologic correlation. Radiographics 2015;35:387–399. doi: 10.1148/rg.352140095. [DOI] [PubMed] [Google Scholar]
- 5.Wennmacker SZ van Dijk AH Raessens JHJ van Laarhoven CJHM Drenth JPH de Reuver PR, et al. Polyp size of 1 cm is insufficient to discriminate neoplastic and non-neoplastic gallbladder polyps. Surg Endosc 2019;33:1564–1571. doi: 10.1007/s00464-018-6444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YS Do JH Seo SW Lee SE Oh HC Min YJ, et al. Prevalence and risk factors of gallbladder polypoid lesions in a healthy population. Yonsei Med J 2016;57:1370–1375. doi: 10.3349/ymj.2016.57.6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heitz L Kratzer W Gräter T Schmidberger J; EMIL study group . Gallbladder polyps – A follow-up study after 11 years. BMC Gastroenterol 2019;19:42. doi: 10.1186/s12876-019-0959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YJ Park KS Cho KB Kim ES Jang BK Chung WJ, et al. Shifting prevalence of gallbladder polyps in Korea. J Korean Med Sci 2014;29:1247–1252. doi: 10.3346/jkms.2014.29.9.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kratzer W Schmid A Akinli AS Thiel R Mason RA Schuler A, et al. Gallbladder polyps: Prevalence and risk factors. Ultraschall Med 2011;32(Suppl 1):S68–S73. doi: 10.1055/s-0029-1245265. [DOI] [PubMed] [Google Scholar]
- 10.Ali TA, Abougazia AS, Alnuaimi AS, Mohammed MAM. Prevalence and risk factors of gallbladder polyps in primary health care centers among patients examined by abdominal ultrasonography in Qatar: A case-control study. Qatar Med J 2021;2021:48. doi: 10.5339/qmj.2021.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin WR Lin DY Tai DI Hsieh SY Lin CY Sheen IS, et al. Prevalence of and risk factors for gallbladder polyps detected by ultrasonography among healthy Chinese: Analysis of 34 669 cases. J Gastroenterol Hepatol 2008;23:965–969. doi: 10.1111/j.1440-1746.2007.05071.x. [DOI] [PubMed] [Google Scholar]
- 12.Xu Q Tao LY Wu Q Gao F Zhang FL Yuan L, et al. Prevalences of and risk factors for biliary stones and gallbladder polyps in a large Chinese population. HPB (Oxford) 2012;14:373–381. doi: 10.1111/j.1477-2574.2012.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao YS Mai YF Li FJ Zhang YM Hu KM Hong ZL, et al. Prevalence and risk factors of gallbladder polypoid lesions in Chinese petrochemical employees. World J Gastroenterol 2013;19:4393–4399. doi: 10.3748/wjg.v19.i27.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamin Z, Xuesong B, Zhen Z, Yue H, Liwei L, Fei L. Correlation of dyslipidemias and gallbladder polyps-A large retrospective study among Chinese population. Asian J Surg 2020;43:181–185. doi: 10.1016/j.asjsur.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Yang HL Kong L Hou LL Shen HF Wang Y Gu XG, et al. Analysis of risk factors for polypoid lesions of gallbladder among health examinees. World J Gastroenterol 2012;18:3015–3019. doi: 10.3748/wjg.v18.i23.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrén-Sandberg A. Diagnosis and management of gallbladder polyps. N Am J Med Sci 2012;4:203–211. doi: 10.4103/1947-2714.95897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee A Lopes Vendrami C Nikolaidis P Mittal PK Bandy AJ Menias CO, et al. Uncommon intraluminal tumors of the gallbladder and biliary tract: Spectrum of imaging appearances. Radiographics 2019;39:388–412. doi: 10.1148/rg.2019180164. [DOI] [PubMed] [Google Scholar]
- 18.Gallahan WC, Conway JD. Diagnosis and management of gallbladder polyps. Gastroenterol Clin North Am 2010;39:359–367. doi: 10.1016/j.gtc.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Dilek ON, Karasu S, Dilek FH. Diagnosis and treatment of gallbladder polyps: Current perspectives. Euroasian J Hepatogastroenterol 2019;9:40–48. doi: 10.5005/jp-journals-10018-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albores-Saavedra J, Chablé-Montero F, González-Romo MA, Ramírez Jaramillo M, Henson DE. Adenomas of the gallbladder. Morphologic features, expression of gastric and intestinal mucins, and incidence of high-grade dysplasia/carcinoma in situ and invasive carcinoma. Hum Pathol 2012;43:1506–1513. doi: 10.1016/j.humpath.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Yamin Z, Xuesong B, Guibin Y, Liwei L, Fei L. Risk factors of gallbladder polyps formation in East Asian population: A meta-analysis and systematic review. Asian J Surg 2020;43:52–59. doi: 10.1016/j.asjsur.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Lim SH Kim DH Park MJ Kim YS Kim CH Yim JY, et al. Is metabolic syndrome one of the risk factors for gallbladder polyps found by ultrasonography during health screening? Gut Liver 2007;1:138–144. doi: 10.5009/gnl.2007.1.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Z Yang C Bai X Yao G Qian X Gao W, et al. Risk factors for cholesterol polyp formation in the gallbladder are closely related to lipid metabolism. Lipids Health Dis 2021;20:26. doi: 10.1186/s12944-021-01452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Zhang H, Elizabeth A, Liu XQ. Epidemiology of hepatitis B and associated liver diseases in China. Chin Med Sci J 2013;27:243–248. doi: 10.1016/s1001-9294(13)60009-7. [DOI] [PubMed] [Google Scholar]
- 25.Ahn DW Jeong JB Kang J Kim SH Kim JW Kim BG, et al. Fatty liver is an independent risk factor for gallbladder polyps. World J Gastroenterol 2020;26:6979–6992. doi: 10.3748/wjg.v26.i44.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim SH Kim D Kang JH Song JH Yang SY Yim JY, et al. Hepatic fat, not visceral fat, is associated with gallbladder polyps: A study of 2643 healthy subjects. J Gastroenterol Hepatol 2015;30:767–774. doi: 10.1111/jgh.12841. [DOI] [PubMed] [Google Scholar]
- 27.Deng Z Xuan Y Li X Crawford WJ Yuan Z Chen Z, et al. Effect of metabolic syndrome components on the risk of malignancy in patients with gallbladder lesions. J Cancer 2021;12:1531–1537. doi: 10.7150/jca.54617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JK Hahn SJ Kang HW Jung JG Choi HS Lee JH, et al. Visceral obesity is associated with gallbladder polyps. Gut Liver 2016;10:133–139. doi: 10.5009/gnl14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GBD 2015 Obesity Collaborators; Afshin A Forouzanfar MH Reitsma MB Sur P Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon W, Jang JY, Lee SE, Hwang DW, Kim SW. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci 2009;24:481–487. doi: 10.3346/jkms.2009.24.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito H Hann LE D’Angelica M Allen P Fong Y Dematteo RP, et al. Polypoid lesions of the gallbladder: Diagnosis and followup. J Am Coll Surg 2009;208:570–575. doi: 10.1016/j.jamcollsurg.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Park EJ Lee HS Lee SH Chun HJ Kim SY Choi YK, et al. Association between metabolic syndrome and gallbladder polyps in healthy Korean adults. J Korean Med Sci 2013;28:876–880. doi: 10.3346/jkms.2013.28.6.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osório-Costa F, Rocha GZ, Dias MM, Carvalheira JB. Epidemiological and molecular mechanisms aspects linking obesity and cancer. Arq Bras Endocrinol Metabol 2009;53:213–226. doi: 10.1590/s0004-27302009000200013. [DOI] [PubMed] [Google Scholar]
- 34.Lathigara D, Kaushal D, Wilson RB. Molecular mechanisms of western diet-induced obesity and obesity-related carcinogenesis-a narrative review. Metabolites 2023;13:675. doi: 10.3390/metabo13050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Sepe LP Hartl K Iftekhar A Berger H Kumar N Goosmann C, et al. Genotoxic effect of salmonella paratyphi a infection on human primary gallbladder cells. mBio 2020;11:e1911–e1920. doi: 10.1128/mBio.01911-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheweita SA, Alsamghan AS. Molecular mechanisms contributing bacterial infections to the incidence of various types of cancer. Mediators Inflamm 2020;2020:4070419. doi: 10.1155/2020/4070419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian L Deng Z Xu L Yang T Yao W Ji L, et al. Downregulation of ASPP2 promotes gallbladder cancer metastasis and macrophage recruitment via aPKC-iota/GLI1 pathway. Cell Death Dis 2018;9:1115. doi: 10.1038/s41419-018-1145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu B Shen ED Liao MM Hu YB Wu K Yang P, et al. Expression and mechanisms of long non-coding RNA genes MEG3 and ANRIL in gallbladder cancer. Tumour Biol 2016;37:9875–9886. doi: 10.1007/s13277-016-4863-y. [DOI] [PubMed] [Google Scholar]
- 40.Wen J Xuan B Liu Y Wang L He L Meng X, et al. NLRP3 inflammasome-induced pyroptosis in digestive system tumors. Front Immunol 2023;14:1074606. doi: 10.3389/fimmu.2023.1074606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Yang ZL, Liu JQ, Yang LP, Yang XJ, Fu X. Overexpression of MTA1 and loss of KAI-1 and KiSS-1 expressions are associated with invasion, metastasis, and poor-prognosis of gallbladder adenocarcinoma. Tumori 2014;100:667–674. doi: 10.1700/1778.19276. [DOI] [PubMed] [Google Scholar]
- 42.Lin N Yao Z Xu M Chen J Lu Y Yuan L, et al. Long noncoding RNA MALAT1 potentiates growth and inhibits senescence by antagonizing ABI3BP in gallbladder cancer cells. J Exp Clin Cancer Res 2019;38:244. doi: 10.1186/s13046-019-1237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu XS Wang XA Wu WG Hu YP Li ML Ding Q, et al. MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway. Cancer Biol Ther 2014;15:806–814. doi: 10.4161/cbt.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colecchia A Larocca A Scaioli E Bacchi-Reggiani ML Di Biase AR Azzaroli F, et al. Natural history of small gallbladder polyps is benign: Evidence from a clinical and pathogenetic study. Am J Gastroenterol 2009;104:624–629. doi: 10.1038/ajg.2008.99. [DOI] [PubMed] [Google Scholar]
- 45.Beck PL, Shaffer EA, Gall DG, Sherman PM. The natural history and significance of ultrasonographically defined polypoid lesions of the gallbladder in children. J Pediatr Surg 2007;42:1907–1912. doi: 10.1016/j.jpedsurg.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Csendes A, Burgos AM, Csendes P, Smok G, Rojas J. Late follow-up of polypoid lesions of the gallbladder smaller than 10 mm. Ann Surg 2001;234:657–660. doi: 10.1097/00000658-200111000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YK Yeh JH Lin CL Peng CL Sung FC Hwang IM, et al. Cancer risk in patients with cholelithiasis and after cholecystectomy: A nationwide cohort study. J Gastroenterol 2014;49:923–931. doi: 10.1007/s00535-013-0846-6. [DOI] [PubMed] [Google Scholar]
- 48.Ryu S, Chang Y, Yun KE, Jung HS, Shin JH, Shin H. Gallstones and the risk of gallbladder cancer mortality: A cohort study. Am J Gastroenterol 2016;111:1476–1487. doi: 10.1038/ajg.2016.345. [DOI] [PubMed] [Google Scholar]
- 49.Xu A, Hu H. The gallbladder polypoid-lesions conundrum: Moving forward with controversy by looking back. Expert Rev Gastroenterol Hepatol 2017;11:1071–1080. doi: 10.1080/17474124.2017.1372188. [DOI] [PubMed] [Google Scholar]
- 50.Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med 2016;375:1161–1170. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Erp LW Cunningham M Narasimman M Ale Ali H Jhaveri K Drenth JPH, et al. Risk of gallbladder cancer in patients with primary sclerosing cholangitis and radiographically detected gallbladder polyps. Liver Int 2020;40:382–392. doi: 10.1111/liv.14326. [DOI] [PubMed] [Google Scholar]
- 52.Akyürek N, Salman B, Irkörücü O, Sare M, Tatlicioglu E. Ultrasonography in the diagnosis of true gallbladder polyps: The contradiction in the literature. HPB (Oxford) 2005;7:155–158. doi: 10.1080/13651820510003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cocco G Basilico R Delli Pizzi A Cocco N Boccatonda A D’Ardes D, et al. Gallbladder polyps ultrasound: What the sonographer needs to know. J Ultrasound 2021;24:131–142. doi: 10.1007/s40477-021-00563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCain RS, Diamond A, Jones C, Coleman HG. Current practices and future prospects for the management of gallbladder polyps: A topical review. World J Gastroenterol 2018;24:2844–2852. doi: 10.3748/wjg.v24.i26.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu L Han P Jiang B Li N Jiao Z Zhu Y, et al. Value of conventional ultrasound-based scoring system in distinguishing adenomatous polyps from cholesterol polyps. J Clin Gastroenterol 2022;56:895–901. doi: 10.1097/MCG.0000000000001639. [DOI] [PubMed] [Google Scholar]
- 56.Jang JY Kim SW Lee SE Hwang DW Kim EJ Lee JY, et al. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg 2009;250:943–949. doi: 10.1097/SLA.0b013e3181b5d5fc. [DOI] [PubMed] [Google Scholar]
- 57.Tsuji S Sofuni A Moriyasu F Itokawa F Ishii K Kurihara T, et al. Contrast-enhanced ultrasonography in the diagnosis of gallbladder disease. Hepatogastroenterology 2012;59:336–340. doi: 10.5754/hge11447. [DOI] [PubMed] [Google Scholar]
- 58.Miwa H Numata K Sugimori K Sanga K Hirotani A Tezuka S, et al. Differential diagnosis of gallbladder polypoid lesions using contrast-enhanced ultrasound. Abdom Radiol (NY) 2019;44:1367–1378. doi: 10.1007/s00261-018-1833-4. [DOI] [PubMed] [Google Scholar]
- 59.Xie XH Xu HX Xie XY Lu MD Kuang M Xu ZF, et al. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol 2010;20:239–248. doi: 10.1007/s00330-009-1538-8. [DOI] [PubMed] [Google Scholar]
- 60.Stenberg B, Elliott S. Diagnosis of gallbladder problems using three-dimensional ultrasound. Eur Radiol 2010;20:908–914. doi: 10.1007/s00330-009-1614-0. [DOI] [PubMed] [Google Scholar]
- 61.Kim JS, Lee JK, Kim Y, Lee SM. US characteristics for the prediction of neoplasm in gallbladder polyps 10 mm or larger. Eur Radiol 2016;26:1134–1140. doi: 10.1007/s00330-015-3910-1. [DOI] [PubMed] [Google Scholar]
- 62.French DG, Allen PD, Ellsmere JC. The diagnostic accuracy of transabdominal ultrasonography needs to be considered when managing gallbladder polyps. Surg Endosc 2013;27:4021–4025. doi: 10.1007/s00464-013-3033-1. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto S Nakaoka K Kawabe N Kuzuya T Funasaka K Nagasaka M, et al. The role of endoscopic ultrasound in the diagnosis of gallbladder lesions. Diagnostics (Basel) 2021;11:1789. doi: 10.3390/diagnostics11101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ichim VA, Chira RI, Mircea PA, Nagy GA, Crisan D, Socaciu MA. Accuracy of endoscopic ultrasound-guided biopsy of focal liver lesions. Med Ultrason 2020;22:20–25. doi: 10.11152/mu-2078. [DOI] [PubMed] [Google Scholar]
- 65.Sadamoto Y Oda S Tanaka M Harada N Kubo H Eguchi T, et al. A useful approach to the differential diagnosis of small polypoid lesions of the gallbladder, utilizing an endoscopic ultrasound scoring system. Endoscopy 2002;34:959–965. doi: 10.1055/s-2002-35859. [DOI] [PubMed] [Google Scholar]
- 66.Akatsu T Aiura K Shimazu M Ueda M Wakabayashi G Tanabe M, et al. Can endoscopic ultrasonography differentiate nonneoplastic from neoplastic gallbladder polyps? Dig Dis Sci 2006;51:416–421. doi: 10.1007/s10620-006-3146-7. [DOI] [PubMed] [Google Scholar]
- 67.Sugiyama M, Xie XY, Atomi Y, Saito M. Differential diagnosis of small polypoid lesions of the gallbladder: The value of endoscopic ultrasonography. Ann Surg 1999;229:498–504. doi: 10.1097/00000658-199904000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azuma T, Yoshikawa T, Araida T, Takasaki K. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am J Surg 2001;181:65–70. doi: 10.1016/s0002-9610(00)00526-2. [DOI] [PubMed] [Google Scholar]
- 69.Cheon YK Cho WY Lee TH Cho YD Moon JH Lee JS, et al. Endoscopic ultrasonography does not differentiate neoplastic from non-neoplastic small gallbladder polyps. World J Gastroenterol 2009;15:2361–2366. doi: 10.3748/wjg.15.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campos S, Poley JW, van Driel L, Bruno MJ. The role of EUS in diagnosis and treatment of liver disorders. Endosc Int Open 2019;7:E1262–E1275. doi: 10.1055/a-0958-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang X, Liu Y, Guo Y, Chai R, Niu M, Xu K. Utility of radiomics based on contrast-enhanced CT and clinical data in the differentiation of benign and malignant gallbladder polypoid lesions. Abdom Radiol (NY) 2020;45:2449–2458. doi: 10.1007/s00261-020-02461-2. [DOI] [PubMed] [Google Scholar]
- 72.Zhou W, Li G, Ren L. Triphasic dynamic contrast-enhanced computed tomography in the differentiation of benign and malignant gallbladder polypoid lesions. J Am Coll Surg 2017;225:243–248. doi: 10.1016/j.jamcollsurg.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Irie H, Kamochi N, Nojiri J, Egashira Y, Sasaguri K, Kudo S. High b-value diffusion-weighted MRI in differentiation between benign and malignant polypoid gallbladder lesions. Acta Radiol 2011;52:236–240. doi: 10.1258/ar.2010.100234. [DOI] [PubMed] [Google Scholar]
- 74.Lee J, Yun M, Kim KS, Lee JD, Kim CK. Risk stratification of gallbladder polyps (1-2 cm) for surgical intervention with 18F-FDG PET/CT. J Nucl Med 2012;53:353–358. doi: 10.2967/jnumed.111.093948. [DOI] [PubMed] [Google Scholar]
- 75.Teber MA Tan S Dönmez U İpek A Uçar AE Yıldırım H, et al. The use of real-time elastography in the assessment of gallbladder polyps: Preliminary observations. Med Ultrason 2014;16:304–308. doi: 10.11152/mu.201.3.2066.164.1mat. [DOI] [PubMed] [Google Scholar]
- 76.Steinberg I, Huland DM, Vermesh O, Frostig HE, Tummers WS, Gambhir SS. Photoacoustic clinical imaging. Photoacoustics 2019;14:77–98. doi: 10.1016/j.pacs.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chae HD Lee JY Jang JY Chang JH Kang J Kang MJ, et al. Photoacoustic imaging for differential diagnosis of benign polyps versus malignant polyps of the gallbladder: A preliminary study. Korean J Radiol 2017;18:821–827. doi: 10.3348/kjr.2017.18.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Koekkoek-Doll PK Roberti S van den Brekel MW Maas M Smit L Beets-Tan R, et al. Value of assessing peripheral vascularization with micro-flow imaging, resistive index and absent hilum sign as predictor for malignancy in lymph nodes in head and neck squamous cell carcinoma. Cancers (Basel) 2021;13:5071. doi: 10.3390/cancers13205071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin J, Lin W, Xu L, Lin T. Diagnostic value of various vascular features of breast cancer by age. Clin Hemorheol Microcirc 2022;80:317–325. doi: 10.3233/ch-211258. [DOI] [PubMed] [Google Scholar]
- 80.Zhu L, Han P, Jiang B, Li N, Fei X. [Differential diagnosis of gallbladder polypoid lesions by micro-flow imaging]. Nan Fang Yi Ke Da Xue Xue Bao 2022;42:922–928. doi: 10.12122/j.issn.1673-4254.2022.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mondal SK, Bhattacharjee D, Mandal PK, Biswas S. Histopathological study of gallbladder carcinoma and its mimics with role of carcinoembryonic antigen immunomarker in resolving diagnostic difficulties. Indian J Med Paediatr Oncol 2017;38:411–415. doi: 10.4103/ijmpo.ijmpo_230_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bind MK, Mishra RR, Kumar V, Misra V, Singh PA. Serum CA 19-9 and CA 125 as a diagnostic marker in carcinoma of gallbladder. Indian J Pathol Microbiol 2021;64:65–68. doi: 10.4103/IJPM.IJPM_494_19. [DOI] [PubMed] [Google Scholar]
- 83.Sachan A Saluja SS Nekarakanti PK Nimisha Mahajan B Nag HH, et al. Raised CA19-9 and CEA have prognostic relevance in gallbladder carcinoma. BMC Cancer 2020;20:826. doi: 10.1186/s12885-020-07334-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valibouze C El Amrani M Truant S Leroy C Millet G Pruvot FR, et al. The management of gallbladder polyps. J Visc Surg 2020;157:410–417. doi: 10.1016/j.jviscsurg.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 85.Wiles R Thoeni RF Barbu ST Vashist YK Rafaelsen SR Dewhurst C, et al. Management and follow-up of gallbladder polyps: Joint guidelines between the European Society of Gastrointestinal and Abdominal Radiology (ESGAR), European Association for Endoscopic Surgery and other Interventional Techniques (EAES), International Society of Digestive Surgery – European Federation (EFISDS) and European Society of Gastrointestinal Endoscopy (ESGE). Eur Radiol 2017;27:3856–3866. doi: 10.1007/s00330-017-4742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foley KG Lahaye MJ Thoeni RF Soltes M Dewhurst C Barbu ST, et al. Management and follow-up of gallbladder polyps: Updated joint guidelines between the ESGAR, EAES, EFISDS and ESGE. Eur Radiol 2022;32:3358–3368. doi: 10.1007/s00330-021-08384-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, Wang Y, Chi M. The diagnostic value of high-frequency ultrasound combined with color Doppler ultrasound versus surgical pathology in gallbladder polyps. Am J Transl Res 2021;13:7990–7996. [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao X, Li XY, Ji W. Laparoscopic versus open treatment of gallbladder cancer: A systematic review and meta-analysis. J Minim Access Surg 2018;14:185–191. doi: 10.4103/jmas.JMAS_223_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Menzies D, Ellis H. Intestinal obstruction from adhesions – How big is the problem? Ann R Coll Surg Engl 1990;72:60–63. [PMC free article] [PubMed] [Google Scholar]
- 90.Zorrón R, Filgueiras M, Maggioni LC, Pombo L, Lopes Carvalho G, Lacerda Oliveira A. NOTES. Transvaginal cholecystectomy: Report of the first case. Surg Innov 2007;14:279–283. doi: 10.1177/1553350607311090. [DOI] [PubMed] [Google Scholar]
- 91.de Sousa LH de Sousa JA de Sousa Filho LH de Sousa MM de Sousa VM de Sousa AP, et al. Totally NOTES (T-NOTES) transvaginal cholecystectomy using two endoscopes: Preliminary report. Surg Endosc 2009;23:2550–2555. doi: 10.1007/s00464-009-0453-z. [DOI] [PubMed] [Google Scholar]
- 92.Simopoulos C Kouklakis G Zezos P Ypsilantis P Botaitis S Tsalikidis C, et al. Peroral transgastric endoscopic procedures in pigs: Feasibility, survival, questionings, and pitfalls. Surg Endosc 2009;23:394–402. doi: 10.1007/s00464-008-9930-z. [DOI] [PubMed] [Google Scholar]
- 93.Guo XW, Liang YX, Huang PY, Liang LX, Zeng YQ, Ding Z. Snare-assisted flexible endoscope in trans-gastric endoscopic gallbladder-preserving surgery: A pilot animal study. World J Gastroenterol 2022;28:2112–2122. doi: 10.3748/wjg.v28.i19.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bai Y Qiao WG Zhu HM He Q Wang N Cai JQ, et al. Role of transgastric natural orifice transluminal endoscopic surgery in the diagnosis of ascites of unknown origin (with videos). Gastrointest Endosc 2014;80:807–816. doi: 10.1016/j.gie.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Han S. Transgastric endoscopic gallbladder polypectomy and cholecystolithiasis: A case report. Exp Ther Med 2020;19:95–98. doi: 10.3892/etm.2019.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian L Yang Y Xiao D Wang R Li X Shen S, et al. Resection of gallbladder polyps following endoscopic ultrasound-guided cholecystoduodenostomy using a lumen-apposing metal stent. Endoscopy 2018;50:E307–E308. doi: 10.1055/a-0631-7970. [DOI] [PubMed] [Google Scholar]
- 97.Chen YY, Su PY, Shen WC. Gallbladder polyp treated with endoscopic polypectomy through a cholecystogastrostomy. Endoscopy 2011;43 (Suppl 2 UCTN):E88–E89. doi: 10.1055/s-0030-1255983. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y Mao XL Zhou XB You NN Xu SW Zhu LH, et al. Feasibility of transgastric endoscopic gallbladder-preserving surgery for benign gallbladder diseases (with video). Surg Endosc 2022;36:2705–2711. doi: 10.1007/s00464-021-08890-4. [DOI] [PubMed] [Google Scholar]