Abstract
Acamprosate is a Food and Drug Administration (FDA) approved medication for the treatment of alcohol use disorder (AUD). However, only a subset of patients achieves optimal treatment outcomes. Currently, no biological measures are utilized to predict response to acamprosate treatment. We applied our established pharmaco-omics informed genomics strategy to identify potential biomarkers associated with acamprosate treatment response. Specifically, our previous open-label acamprosate clinical trial recruited 442 patients with AUD who were treated with acamprosate for three months. We first performed proteomics using baseline plasma samples to identify potential biomarkers associated with acamprosate treatment outcomes. Next, we applied our established “proteomics-informed genome-wide association study (GWAS)” research strategy, and identified 12 proteins, including interleukin-17 receptor B (IL17RB), associated with acamprosate treatment response. A GWAS for IL17RB concentrations identified several genome-wide significant signals. Specifically, the top hit single nucleotide polymorphism (SNP) rs6801605 with a minor allele frequency of 38% in the European American population mapped 4 kilobase (Kb) upstream of IL17RB, and intron 1 of the choline dehydrogenase (CHDH) gene on chromosome 3 (p: 4.8E-20). The variant genotype (AA) for the SNP rs6801605 was associated with lower IL17RB protein expression. In addition, we identified a series of genetic variants in IL17RB that were associated with acamprosate treatment outcomes. Furthermore, the variant genotypes for all of those IL17RB SNPs were protective for alcohol relapse. Finally, we demonstrated that the basal level of mRNA expression of IL17RB was inversely correlated with those of nuclear factor-κB (NF-κB) subunits, and a significantly higher expression of NF-κB subunits was observed in AUD patients who relapsed to alcohol use. In summary, this study illustrates that IL17RB genetic variants might contribute to acamprosate treatment outcomes. This series of studies represents an important step toward generating functional hypotheses that could be tested to gain insight into mechanisms underlying acamprosate treatment response phenotypes.
(The ClinicalTrials.gov Identifier: NCT00662571).
Keywords: Acamprosate treatment response, Alcohol use disorder, IL17RB, Biomarker, Multi-omics
1. Introduction
Alcohol use disorder (AUD) is the most common substance use disorder (SUD) worldwide (Grant et al., 2015). Three medications, including acamprosate, naltrexone, and disulfiram, have been approved by the Food and Drug Administration (FDA) of the United States for the treatment of AUD. However, the treatment response varies among individuals, and approximately half of patients respond to acamprosate treatment (Ho et al., 2022). It is critically important to understand the mechanisms underlying variation in acamprosate efficacy and their relationship with the biological changes associated with alcohol use. That knowledge would facilitate both the development of individualized acamprosate treatment programs as well as contribute to identifying novel and improved medications for the treatment of AUD (Litten et al., 2020). It is noteworthy that present diagnostic instruments for most psychiatric disorders, including SUDs, are based on symptoms included in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) rather than biological measures. Except for AUD, opioid use disorder and nicotine use disorder, most substance use disorders do not have FDA approved medications for pharmacotherapy. In psychiatry, we often lack objective biochemical and molecular indicators to assist with diagnosis or the evaluation of treatment response. No biomarkers for the treatment response to substance use disorders have yet been identified. It would be beneficial if we could effectively address that knowledge gap by utilizing biomarkers to identify individuals who may respond to a medication before prescribing it (Litten et al., 2020; Witkiewitz et al., 2019). Therefore, we designed the present study to pinpoint biomarkers linked to acamprosate treatment response through multi-omics approaches.
Disulfiram, acamprosate, and naltrexone can be used to treat AUD. Disulfiram deters alcohol consumption by inducing severe side effects upon alcohol use, making it suitable for closely monitored AUD patients with a strong motivation to maintain sobriety (Kalra et al., 2014; Skinner et al., 2014). Acamprosate and naltrexone are commonly characterized as anti-craving medications (Hendershot et al., 2017; McHugh et al., 2016). Naltrexone, functioning as a μ opioid receptor antagonist, has FDA approval for both AUD and opioid use disorder pharmacotherapy (Oesterle et al., 2019). Its impacts on neurotransmission, neuroinflammation, and the hypothalamic–pituitary–adrenal axis have been reported (Ray et al., 2010; Hillemacher et al., 2011). Acamprosate, sharing a chemical structure with the neurotransmitter gamma-aminobutyric acid (GABA) and the amino acid taurine, is often discussed in the context of its effects on the GABAergic inhibitory and glutamatergic excitatory balance in the brain (Mason and Heyser, 2010; Shen, 2018). Acamprosate is excreted unchanged in the urine without undergoing metabolism (Más-Serrano et al., 2000; Kalk and Lingford-Hughes, 2014). Acamprosate has effects on restoring disrupted neurotransmission by mitigating overexcitation triggered by alcohol (Witkiewitz et al., 2012). However, similar to naltrexone, specific molecular mechanisms governing acamprosate’s efficacy as an AUD treatment remain unclear. Previously, we conducted an open-label acamprosate clinical trial to identify biomarkers for acamprosate treatment response. That study is one of the largest acamprosate clinical trials ever conducted and the only existing acamprosate study cohort with multi-omics data to date.
We have recently reported that genetic variation may contribute to individual differences in AUD treatment response by performing a genome-wide association study (GWAS) meta-analysis based on data from three of the largest studies of acamprosate and naltrexone completed to date, including COMBINE (Anton et al., 2006), PREDICT (Mann et al., 2009) and the Mayo Clinic Center for Individualized Treatment of Alcoholism study (Ho et al., 2022; Karpyak et al., 2014). This GWAS meta-analysis identified several genome-wide significant signals in the BRE gene that were associated with time until relapse to heavy drinking during the first three months of treatment regardless of AUD pharmacological intervention. In addition, two intergenic genome-wide significant signals were associated with medication-specific outcomes, i.e., rs12749274 (p: 3.9E-08) in the time until heavy drinking analysis of naltrexone-treated patients, and rs77583603 (p: 3.1E-09) in the time until relapse to alcohol use analysis of acamprosate-treated patients. This series of studies has provided further insight into possible genetic contributions to AUD pharmacotherapy (Biernacka et al., 2021). In addition to genomics, we now set out to explore peripheral protein markers which might contribute to acamprosate treatment outcome phenotypes.
Because of the heterogeneity of AUD, the inclusion of other omics data may offer a more comprehensive view of overall molecular variation that contributes to individual differences in response to drug therapy or disease susceptibility than would genomics alone (Ho et al., 2022). For example, we previously reported that genetic variants in the TSPAN5 gene were associated with plasma serotonin concentrations which can contribute to selective serotonin reuptake inhibitor (SSRI) treatment response in patients with major depressive disorder (MDD) (Gupta et al., 2016). That study was a successful example of the application of a metabolomics-informed genomics strategy—i.e., we first associated metabolites with outcomes and then performed a GWAS for the concentration of that metabolite—not for the clinical outcome (Gupta et al., 2016). A GWAS meta-analysis then confirmed that the same TSPAN5 SNPs were associated with AUD (Kranzler et al., 2019). We subsequently extended our original observations with regard to TSPAN5 and serotonin regulation and those results revealed a novel molecular mechanism related to acamprosate treatment response in patients with AUD (Ho et al., 2021). We then applied a metabolomics-informed genomics approach to identify a series of single nucleotide polymorphisms (SNPs) in the PTPRD gene that might be associated with acamprosate treatment response (Ho et al., 2022). Our functional genomic studies demonstrated that PTPRD is ethanol inducible, and that the expression can be reversed by treatment with anti-craving drugs i.e. acamprosate and naltrexone (Ho et al., 2022). The success of this “pharmaco-omics informed genomics” approach led us to study the proteomic signature of exposure and response to acamprosate in patients with AUD in the present study (Ho et al., 2022; Ho et al., 2023; Ho et al., 2022).
A growing body of evidence indicates that persistent and excessive alcohol consumption may trigger immune responses and inflammation (Karoly et al., 2021; Lowe et al., 2020). Both single-cell RNA-seq and bulk RNA-seq suggested that immune signaling pathways were upre-gulated in the brain tissues of patients with AUD (Kapoor et al., 2019; Brenner et al., 2020). Moreover, our recent functional genomic study showed a remarkable gene expression pattern in which a large number of expression signals enriched in immune-related pathways were inversely correlated with response to ethanol and the anti-craving drugs used in AUD pharmacotherapy, i.e., acamprosate and naltrexone (Ho et al., 2024). This series of observations emphasized possible relationships between AUD and inflammation. The present study was designed to identify inflammatory markers associated with response to acamprosate treatment using baseline plasma samples collected during the Mayo Clinic Center for Individualized Treatment of Alcoholism study. We hypothesized that baseline proteomic profiles might differ between patients who relapsed and those who remained sober. The utilization of a proteomics-informed genomics approach helped us to identify genetic variants in IL17RB which may contribute to variations in plasma proteomic profiles associated with acamprosate treatment outcomes. Our results might also provide novel insight into mechanisms involved in individual variation in drug treatment response.
2. Materials and methods
2.1. Study participants and study design
The Mayo Clinic Center for Individualized Treatment of Alcoholism study is an open-label acamprosate clinical trial, which we recruited 442 patients with AUD. All participants underwent a three-month treatment period with acamprosate, an FDA-approved medication for the pharmacotherapy of AUD (Table 1). All participants in the study were initially given acamprosate (one 333 mg tablet three times a day for the first week) to determine their tolerance to the treatment. A standard dose of acamprosate was then prescribed (two 333 mg tablets three times a day). It is essential to highlight that our clinical trial was not specifically structured to assess the effectiveness of acamprosate. Instead, its primary objective was to collect diverse omics data for subsequent analysis and to pinpoint biomarkers linked to the outcomes of acamprosate treatment.
Table 1.
Demographic and clinical characteristics of the subjects with alcohol use disorder in the Mayo Clinic acamprosate trial (n = 442).
| All 442 AUD subjects | Relapse (n = 110) | Non-relapse (n = 157) | |||||
|---|---|---|---|---|---|---|---|
| Mean/n | SD or % | Mean | SD or % | Mean | SD or % | p value# | |
| Men/Women (n) | 286/156 | 65 %/35 % | 67/43 | 60.9 %/39.1 % | 112/45 | 71.3 %/28.7 % | 0.743 |
| European American (n) | 412 | 93.20 % | 100 | 90.90 % | 147 | 93.60 % | 0.400 |
| Age | 42.1 | 11.8 | 41.59 | 12.04 | 42.39 | 11.58 | 0.600 |
| Number of sober days prior to enrollment | 24.8 | 16.1 | 18 | 14.18 | 25.71 | 14.45 | <0.0001 |
| Baseline PHQ-9 score | 9.38 | 6.11 | 10.24 | 6.14 | 8.79 | 6.04 | 0.016 |
| Baseline PACS score | 13.4 | 8.02 | 15.44 | 8.66 | 11.91 | 7.37 | <0.0001 |
| Baseline GADS score | 8.93 | 5.84 | 9.35 | 5.88 | 8.66 | 5.80 | 0.047 |
| Total drinks (90 days prior to enrollment)+ | 562 | 506 | 565.7 | 475.9 | 590.9 | 527.1 | 0.956 |
| Number of drinking days+ | 45.8 | 26.9 | 51.59 | 27.47 | 46.44 | 27.23 | 0.105 |
| Number of heavy drinking days+ | 41.5 | 26.9 | 46.78 | 27.61 | 41.84 | 27.58 | 0.140 |
| Average drinks per day+ | 11.8 | 7.74 | 10.63 | 6.009 | 11.74 | 8.577 | 0.515 |
| Average drinks per week+ | 43.6 | 39.4 | 43.61 | 36.63 | 45.9 | 41.41 | 0.911 |
| Average drinks per month+ | 187 | 169 | 186.9 | 157 | 196.7 | 177.5 | 0.911 |
| Number of days until relapse to alcohol use | 59.6 | 33.2 | 26.56 | 25.33 | 89.82 | 1.03 | <0.0001 |
| Number of days until relapse to heavy drinking | 60.5 | 32.4 | 29.38 | 25.64 | 89.82 | 1.03 | <0.0001 |
Note:
90 days prior to enrollment.
p value: relapse vs non-relapse. Relapse was defined as having a standard drink during three months of acamprosate treatment, whereas non-relapse was defined as the maintenance of abstinence from alcohol during the three months of acamprosate treatment. PHQ-9: patient health questionnaire 9, PACS: Penn alcohol craving scale, GAD-7, general anxiety disorder 7.
The Mayo Clinic Center for the Individualized Treatment of Alcoholism study (ClinicalTrials.gov Identifier: NCT00662571) was conducted under a protocol approved by the Mayo Clinic Institutional Review Board (IRB number: 07–007204) (Ho et al., 2022; Ho et al., 2022). Written informed consent was obtained from all participants. We recruited 442 patients with AUD and all were treated with acamprosate for three months. The eligibility criteria are described elsewhere (Biernacka et al., 2021; Ho et al., 2022). Study subject inclusion criteria included 1) age 18 to 70, 2) DSM-IV diagnosis of AUD as determined by the psychiatric research interview for substance and mental disorders (PRISM), 3) completion of alcohol detoxification as indicated by a clinical institute withdrawal assessment score < 5 and abstinence from alcohol for at least seven days, 4) ability to provide informed consent, and 5) willingness to use the study medications for three months and to attend follow-up visits. Exclusion criteria included 1) hypersensitivity or allergy to acamprosate, 2) renal impairment (creatinine level > 1.5 mg/dL), 3) diagnosis of advanced liver disease indicated in the medical record or by a model for end-stage liver disease (MELD) score of above 10, 4) women who were pregnant, breastfeeding, or planning to become pregnant during the next year, 5) primary diagnosis of substance use disorder other than alcohol as determined by PRISM, 6) current use of benzodiazepines, opioids or any other addictive medications, and 7) active suicidal ideation or any unstable medical or psychiatric condition as determined by responses to PRISM or by the primary care physicians. Specifically, any findings from PRISM assessment raising safety concerns, including positive response to suicide questions would trigger immediate notification to study psychiatrists and the patient’s clinician. In addition, if a participant expressed suicidal thoughts, either as determined by the investigators or by scoring 1 or higher on question 9 of the patient health questionnaire-9 (PHQ-9), or if they acknowledged thoughts of harming themselves or others, the research team would immediately contact the study psychiatrist to refer for appropriate treatment. We collected comprehensive clinical information for this study cohort, including PHQ-9, Penn alcohol craving scale (PACS), general anxiety disorder 7 (GAD-7). Alcohol consumption was measured using the self-report timeline follow-back (TLFB) method 90 days before and after acamprosate treatment. Treatment outcomes were derived from TLFB data collected after treatment initiation. In the present study, primary treatment outcome measures included 1) relapse to alcohol use during the three months of acamprosate treatment (relapse was defined as having one drink during three months of acamprosate treatment, while non-relapse during this time was defined as abstinence), 2) relapse to heavy drinking (≥4 drinks for women, ≥5 drinks for men in a day) during the three months of acamprosate treatment, 3) time until relapse to alcohol use during the first three months of treatment, and 4) time until relapse to heavy drinking during the three months of acamprosate treatment.
2.2. Plasma proteomics
The OLINK explore 384 inflammation I panel was used in this study (Supplementary Table 1). We assayed all plasma samples in a single batch using baseline (pre-acamprosate treatment) plasma samples. Briefly, the assay required 1 μL of plasma sample from each subject to incubate in the presence of proximity antibody pairs linked to DNA reporter molecules. Data pre-processing was performed by OLINK® using NPX Manager Software. Data were normalized to standard EDTA plasma controls to produce relative protein abundance information. Normalized protein expression (NPX) reflects protein concentration on a log2 scale. The average intra-assay and inter-assay coefficients of variation (CV) based on quality control samples were 9 % and 14 % across the five plates, respectively. Protein concentrations were adjusted for age and sex. Results of the differential expression analyses are displayed in Fig. 1B and have not been adjusted for multiple testing.
Fig. 1.
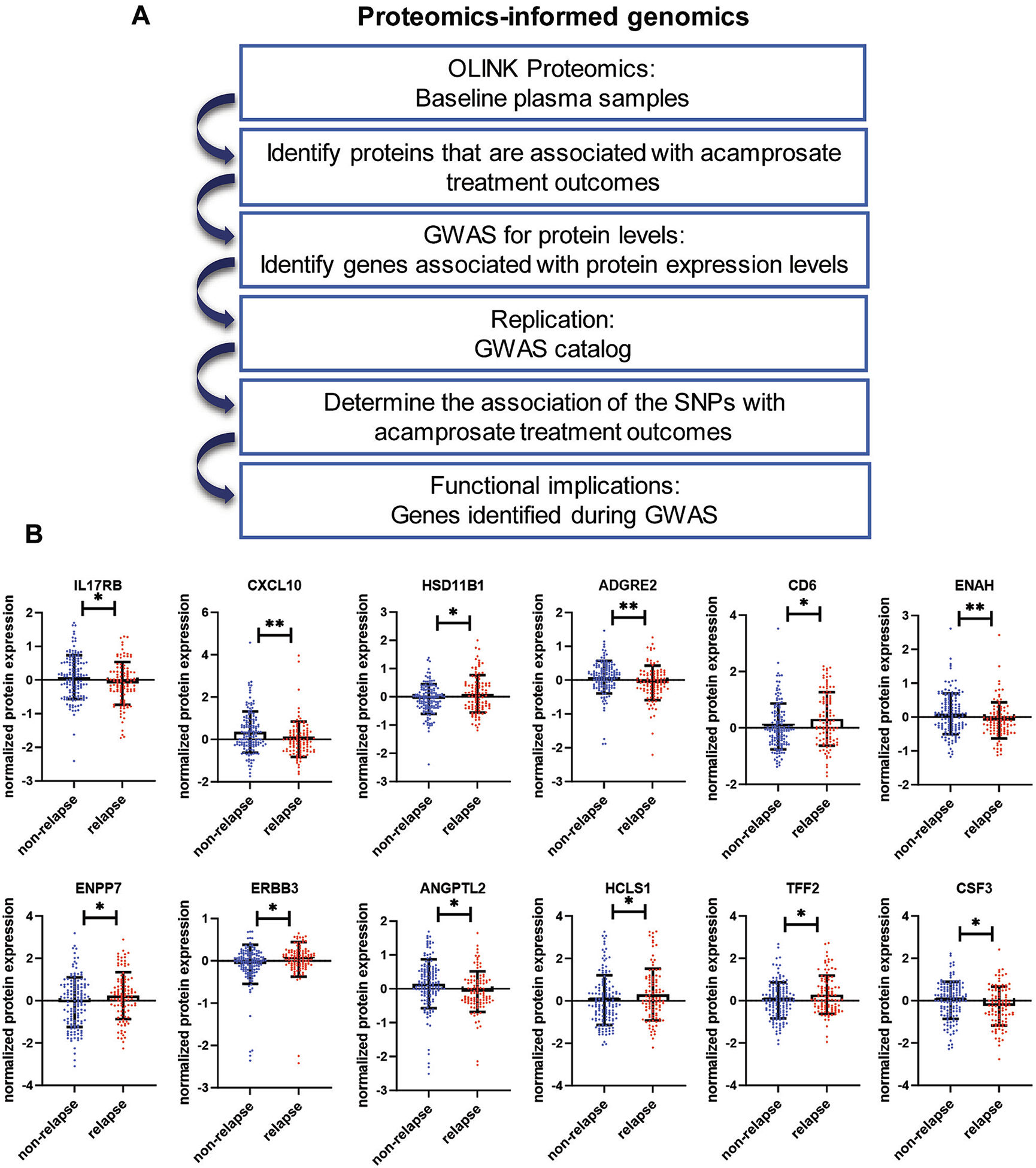
Identification of biomarkers for acamprosate treatment response in patients with alcohol use disorder. (A) Schematic outline of the proteomics-informed genomics research strategy. (B) Plasma proteins associated with acamprosate treatment response. Relapse was defined as having a standard drink during three months of acamprosate treatment, whereas non-relapse was defined as the maintenance of abstinence from alcohol during three months of acamprosate treatment. *p < 0.05. **p < 0.01.
2.3. Genome-wide association study (GWAS)
DNA samples were genotyped using baseline whole blood samples, see supplementary methods (Ho et al., 2022; Ho et al., 2022). Associations between variants and normalized protein expression for each protein marker were tested using linear regression, adjusted for sex, age, program site, and baseline Penn alcohol craving scale (PACS) score. These analyses were done in R version 4.3.1 and PLINK 2.0. We obtained additional data on AUD treatment outcomes from our recent AUD treatment GWAS meta-analysis (Biernacka et al., 2021) to test the association of AUD treatment outcomes with the SNPs identified in the proteomics-informed GWAS. Participants who had at least one week of follow-up were included in the analysis. Outcomes for patients lost to follow-up in the first three months prior to relapse (or heavy relapse) were treated as censored observations in the survival analyses. Single SNPs were tested individually as predictors of time until alcohol use or time until heavy drinking after initiating acamprosate therapy using multivariable Cox proportional hazard models. Models were adjusted for the number of days sober prior to treatment, baseline PACS, and study sites. Results of the Cox proportional hazard analyses are displayed in Fig. 3B using Kaplan-Meier curves and have not been adjusted for multiple testing.
Fig. 3.

IL17RB SNPs were associated with acamprosate treatment outcomes. (A) The locus zoom plot shows the top SNPs of the GWAS for IL17RB on chromosome 3. (B) Kaplan–Meier curves for the time until first alcohol use during three months of acamprosate therapy. IL17RB SNPs were associated with acamprosate treatment response. AUD patients who had at least one week of follow-up were included in the analysis. Variant genotypes were associated with better outcomes i.e., longer abstinence length until the first drink during three months of acamprosate treatment. (C) SNP-dependent plasma IL17RB levels in patients with AUD. MAF: minor allele frequency. SNP: single nucleotide polymorphism.
2.4. RNA sequencing (RNA-seq)
We performed RNA-seq using the PBMC samples from AUD patients (relapse: n = 27, non-relapse: n = 26) as described previously, see supplementary methods (Litten et al., 2020). RNA-seq experiments were conducted by GENEWIZ using an Illumina HiSeq 4000 with eight samples in each lane using 100 bp paired end index reads. Fastq files containing paired RNA-Seq reads were aligned with STAR (Witkiewitz et al., 2019;5(9):eaax4043.) using 2-pass mode (command line parameter–-twopassMode Basic) against the UCSC human reference genome (hg19). Uniquely mapped reads ranged from 62 % to 77 % among all the samples, see Supplementary Table 2. Gene level counts from read pairs were obtained using the subRead featureCounts program (v1.4.6) (Kalra et al., 2014;Vol.) (command line parameter −p) and gene models from the UCSC hg19 Illumina iGenomes annotation package. We filtered out genes with raw counts less than 10 and then performed differential expression analysis using the DESeq2 package (Skinner et al., 2014), with independent filtering turned off. DESeq2 uses linear modeling and variance-reduction techniques for estimated coefficients to test individual null hypotheses of zero log2-fold changes between the two groups (relapse vs non-relapse). To get better estimation of log2 fold change values, DESeq2 function lfcShirnk was used to shrink the log2 fold change values toward zero when the information indicated a gene with low counts or high dispersion. The Wald test was used to compare the beta estimate divided by its estimated standard error to a standard normal distribution to derive p-values. P values were then adjusted for multiple testing using the Benjamini and Hochberg method to produce false discovery rate (FDR) values. A false discovery rate less than 0.05 was considered statistically significant.
2.5. Statistical analysis
R Statistical Software (version 4.3.2; R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis. Data distributions were tested using a Shapiro–Wilk test. Clinical variables as shown in Table 1 were tested using unpaired t-tests or Mann–Whitney U-tests (when the data sets were not normally distributed) between the relapse and non-relapse groups. Data are shown as mean ± S.E.M. P value less than 0.05 was considered statistically significant.
2.6. Data availability
All data supporting our findings can be found in the main paper or in supplementary files. RNA-seq data are available via the GEO accession number: GSE208132 (Ho et al., 2022).
3. Results
3.1. Baseline plasma proteomics markers were associated with acamprosate treatment outcomes
We previously recruited 442 AUD patients to participate in an open-label acamprosate clinical trial. All participants received acamprosate for three months (Table 1). Notably, 267 patients returned for 3-month follow-up, and about 50 % of those patients exhibited a positive response to acamprosate. Among the 175 study participants who did not return for the 3-month follow-up, 70 % of the participants ended the study because they were “lost to follow up”, while a small subset of patients (~5%) ended the study due to medication side effects or travel/work/schedule concerns. The primary objective of our clinical trial was to identify biomarkers associated with the outcomes of acamprosate treatment. We hypothesized that baseline proteomic profiles might be associated with response to acamprosate treatment and that those data might provide valuable insights into mechanisms underlying variation in acamprosate response phenotypes. We assayed baseline plasma samples using the OLINK Explore Inflammation Panel and identified 12 proteins (CXCL10, ENAH, ADGRE2, CSF3, ENPP7, ANGPT2, HSD11B1, CD6, TFF2, ERBB3, IL17RB, and HCLS1) that were associated with relapse to alcohol use during the first three months of acamprosate treatment (Fig. 1B, with complete results in Supplementary Table 1).
3.2. Proteomics-informed GWAS
Next, we applied a proteomics-informed GWAS approach, as outlined schematically in Fig. 1A. Specifically, we performed GWAS using baseline proteomic profiles as quantitative biological traits to identify genetic variants associated with variations in concentrations of proteins that were associated with acamprosate treatment response. The proteomics-informed GWAS identified several genome-wide significant signals (p ≤ 5E-08) in the GWAS for the concentrations of CXCL10, IL17RB, CD6, ENAH, ENPP7, ERBB3, ADGRE2, and ANGPTL2 (Fig. 2). However, no genome-wide significant signals were identified in the GWAS for HSD11B1, HCLS1, TFF2, or CSF3 (Supplementary Fig. 1). We should point out that the levels of these proteins were associated with acamprosate treatment outcomes in patients with AUD. As a result, we hypothesized that the genome-wide significant SNPs identified in the proteomics-informed GWAS might be associated with acamprosate treatment response phenotypes (Supplementary Table 3). Except for IL17RB, there was no association of acamprosate treatment response with the top SNPs in the GWAS for the concentrations of CXCL10, ENAH, ADGRE2, ANGTPL2, ENPP7, CD6, and ERBB3. Specifically, the GWAS for IL17RB concentrations identified several genome-wide significant signals, with the lowest p value of 4.8E-20 for the rs6801605 SNP on chromosome 3 (Fig. 3A). The rs6801605 SNP maps 4 Kb upstream of IL17RB, and intron 1 of the CHDH gene (Fig. 3A). The minor allele frequency (allele A) for the rs6801605 SNP was 38 % in the European American population. In line with our observation, the rs6801605 SNP has been associated with the concentration of plasma IL17RB concentrations with a p value of 1.75E-18 in a Swedish population-based cohort (n = 1005) (Enroth et al., 2014), and in a German population with a p value of 7.48E-19 (n = 997) (Suhre et al., 2017). Our results indicated that the rs6801605 SNP was associated with time until relapse to alcohol use during the three months of acamprosate treatment (p: 0.038) (Fig. 3B). The variant genotype for the rs6801605 SNP was associated with higher IL17RB expression in the baseline plasma samples from our patients with AUD (Fig. 3C). We also identified a series of genome-wide significant SNPs, i.e., rs2289205 in the GWAS for IL17RB concentrations that were associated with the response to acamprosate treatment (Fig. 3B, and Supplementary Fig. 2). Several genome-wide significant SNPs identified in our GWAS for IL17RB concentrations were in linkage disequilibrium (LD) with the rs6801605 SNP (Fig. 3A), which was also associated with the time until relapse to alcohol use during the three-month period of acamprosate treatment (Supplementary Fig. 1). As anticipated, the variant genotype of those IL17RB SNPs was protective against alcohol relapse. The Mayo Clinic Center for the Individualized Treatment of Alcoholism study is one of the largest acamprosate studies ever conducted and is the only existing AUD study cohort with multi-omics data. Therefore, we set out to confirm those findings using our recent GWAS meta-analysis of our AUD treatment response study (Biernacka et al., 2021).
Fig. 2.

Manhattan plots for GWAS of plasma levels of CXCL10, IL17RB, CD6, ENAH, ENPP7, ERBB3, ADGRE2, and ANGPTL2 that were associated with acamprosate treatment outcomes.
3.3. IL17RB genetic variants were associated with acamprosate treatment response
We previously conducted a GWAS meta-analysis to determine genetic contributions to AUD treatment outcomes, using data from 1083 European ancestry AUD patients (Biernacka et al., 2021). We should point out that among the 1083 AUD patients in the meta-analysis of GWAS for AUD pharmacological treatment response, 652 AUD patients received acamprosate treatment (Biernacka et al., 2021). That study set the stage for the present study to confirm the association of acamprosate treatment response with the IL17RB SNP loci identified in our proteomics-informed GWAS. As expected, the genome-wide significant IL17RB SNPs in the GWAS for IL17RB concentrations were associated with time until relapse to alcohol use, and time until relapse to heavy drinking during three months of acamprosate treatment (Table 2). The minor alleles for all 25 genome-wide significant SNPs in the GWAS for plasma IL17RB levels showed a decreased risk of relapse to alcohol use or heavy drinking (Table 2). The rs2289205 SNP was in LD (r = 0.67) with the rs6801605 SNP that was associated with time until relapse to alcohol use (p: 0.0049), and time until relapse to heavy drinking (p: 0.0026) during the first three months of acamprosate treatment (n = 652, see Table 2, and Supplementary Table 3). These observations led us to investigate the possible role of IL17RB in possible molecular mechanisms underlying the acamprosate treatment response.
Table 2.
Genetic variants in IL17RB identified in the GWAS for plasma IL17RB levels and their associations with acamprosate treatment outcomes (n = 652). We studied the association of those genome-wide significant SNPs identified in the GWAS for plasma IL17RB levels with acamprosate treatment outcomes using the data from our recent GWAS meta-analysis of AUD pharmacotherapy (Biernacka, et.al., 2021).
| Time till heavy relapse## | Time till relapse### | |||||||
|---|---|---|---|---|---|---|---|---|
| RS ID | P value IL17RB GWAS# | LD (r2) | Minor allele | Minor allele frequency | Effect | P-value | Effect | P-value |
| rs2289205 | 1.74E-13 | 0.67 | T | 0.333 | −0.291 | 0.0026 | −0.272 | 0.0049 |
| rs3755817 | 1.66E-13 | 0.67 | C | 0.332 | −0.300 | 0.0020 | −0.273 | 0.0050 |
| rs6804251 | 5.75E-11 | 0.41 | G | 0.441 | −0.296 | 0.0014 | −0.254 | 0.0053 |
| rs4687752 | 1.91E-17 | 0.78 | G | 0.388 | −0.272 | 0.0031 | −0.255 | 0.0056 |
| rs1025689 | 2.25E-17 | 0.78 | G | 0.388 | −0.272 | 0.0031 | −0.253 | 0.0057 |
| rs4687751 | 2.40E-17 | 0.77 | C | 0.387 | −0.279 | 0.0024 | −0.253 | 0.0058 |
| rs4546140 | 7.65E-16 | 0.74 | G | 0.327 | −0.297 | 0.0027 | −0.273 | 0.0060 |
| rs7627178 | 2.00E-16 | 0.78 | A | 0.386 | −0.280 | 0.0022 | −0.251 | 0.0061 |
| rs2289206 | 1.93E-16 | 0.78 | T | 0.387 | −0.269 | 0.0032 | 0.245 | 0.0072 |
| rs17641133 | 8.11E-15 | 0.72 | A | 0.317 | −0.301 | 0.0026 | −0.267 | 0.0074 |
| rs2232333 | 8.66E-16 | 0.72 | G | 0.317 | −0.301 | 0.0026 | −0.267 | 0.0075 |
| rs45616236 | 7.92E-15 | 0.72 | G | 0.317 | −0.301 | 0.0026 | −0.265 | 0.0078 |
| rs45560831 | 7.96E-15 | 0.72 | T | 0.317 | −0.301 | 0.0026 | −0.265 | 0.0079 |
| rs2118421 | 7.51E-15 | 0.72 | T | 0.317 | −0.298 | 0.0029 | −0.264 | 0.0082 |
| rs1025690 | 2.32E-15 | 0.76 | G | 0.329 | −0.279 | 0.0045 | −0.257 | 0.0091 |
| rs2276839 | 1.36E-15 | 0.7 | C | 0.319 | −0.282 | 0.0047 | −0.259 | 0.0094 |
| rs6445607 | 4.89E-20 | 1 | G | 0.384 | −0.271 | 0.0036 | −0.231 | 0.0128 |
| rs6801605 | 4.80E-20 | 1 | A | 0.386 | −0.256 | 0.0059 | −0.225 | 0.0150 |
| rs11718497 | 2.25E-17 | 0.84 | G | 0.411 | −0.249 | 0.0066 | −0.220 | 0.0159 |
| rs2196663 | 2.02E-14 | 0.53 | A | 0.335 | −0.267 | 0.0082 | −0.233 | 0.0207 |
| rs35518479 | 3.39E-19 | 0.91 | G | 0.392 | −0.260 | 0.0054 | −0.214 | 0.0213 |
| rs4687750 | 1.19E-18 | 0.78 | G | 0.363 | −0.286 | 0.0031 | 0.213 | 0.0263 |
| rs55974100 | 5.82E-15 | 0.69 | C | 0.323 | −0.256 | 0.0101 | −0.212 | 0.0325 |
| rs56052250 | 1.15E-14 | 0.69 | C | 0.326 | −0.251 | 0.0111 | 0.209 | 0.0341 |
| rs55754695 | 3.16E-13 | 0.61 | C | 0.340 | −0.218 | 0.0288 | −0.210 | 0.0361 |
Note:
Proteomics-informed GWAS was performed using the genomics and proteomics data from the Mayo Clinic Center for Individualized Treatment of Alcoholism study, see Fig. 3A. LD: linkage disequilibrium.
Heavy relapse was defined as having ≥ 4 drinks for women, ≥5 drinks for men in a day during the three months of pharmacotherapy.
Relapse was defined as having a standard drink during three months of pharmacotherapy, whereas heavy relapse was defined as having four drinks a day for a woman, and five drinks a day for a man during three months of pharmacotherapy.
3.4. IL17RB signaling and acamprosate treatment response
IL17RB protein levels were significantly lower in the baseline plasma sample of AUD patients who relapsed to alcohol use during the three months of acamprosate treatment (Fig. 1B), thus raising the question of underlying biology that might drive the differences in IL17RB expression that we observed. The biological function of IL17RB is not well understood. However, IL17RB is known to interact with TRAF6 and ACT1 (also known as TRAF3IP2) through its SEFIR (Similar Expression to Fibroblast growth factor genes and IL17 Receptor) domains, which in turn activate NF-κB and downstream signaling (Fig. 4A) (Wu et al., 2015; Iwakura et al., 2011). We performed RNA-seq on baseline (pre-acamprosate treatment) in peripheral blood mononuclear cells (PBMCs) obtained from a subset of AUD patients who relapsed to alcohol use (n = 27) and a separate group who continued to be abstinent from alcohol during the three-month course of acamprosate treatment (n = 26) (Ho et al., 2022). We then consulted our RNA-seq for baseline PBMC samples from patients with AUD and observed very significant negative correlations between basal expression levels of IL17RB, TRAF6, ACT1 and several NF-κB subunits including RELA, RELB, REL and NF-κB1 (Fig. 4B). Furthermore, IL17RB mRNA expression was significantly lower in AUD patients who relapsed to alcohol use during three months of acamprosate treatment (Fig. 4C, and Supplementary Table 4). In contrast, the mRNA expression of TRAF6, TRAF3IP2, and NF-κB subunits in the relapse group was significantly higher than in the non-relapse group (Fig. 4D). These findings are compatible with the results shown in Fig. 4B. Taken together, this series of studies demonstrates that IL17RB genetic variants may contribute to acamprosate treatment outcomes, and our results suggest that multi-omics approaches may be a feasible strategy for identifying biomarkers that may help predict acamprosate treatment response (Ho et al., 2022; Ho et al., 2022; Ho et al., 2020).
Fig. 4.

IL17RB signaling may contribute to acamprosate treatment response. (A) A schematic outline of the IL17RB signaling pathway. (B) Basal mRNA expression levels of IL17RB were negatively correlated with the expression of TRAF6, TRAF3IP2 (also known as ACT1) and several NF-κB subunits. (C) IL17RB mRNA expression was significantly lower in AUD patients who relapsed to alcohol use during three months of acamprosate treatment. In contrast, the mRNA expression of TRAF6, TRAF3IP2, and NF-κB subunits (NFκB1, RELA, RELB, REL) in the relapse group were significantly higher than those observed in the non-relapse group. *p < 0.05.
4. Discussion
Acamprosate and naltrexone are first-line pharmacotherapies for AUD (McPheeters et al., 2023). Despite studies showing that those medications are effective in treating AUD, some patients are not able to achieve optimal efficacy (Maisel et al., 2013; Jonas et al., 2014; Cheng et al., 2020). Pharmacogenomics has held promise for precision medicine in several aspects of medicine, especially in oncology. However, the field of precision medicine in psychiatry and addiction medicine is still in its infancy (Manchia et al., 2020). One of the reasons is the lack of objective quantitative measures to capture the multifaceted nature of clinical drug treatment responses. AUD is the most common SUD worldwide and there is a pressing need for the development of more individualized treatment programs in the context of the application of precision medicine to SUDs. Our group has attempted to enhance our understanding with regard to acamprosate treatment response and the underlying molecular mechanisms using a series of omics data including genomics (Biernacka et al., 2021), metabolomics (Ho et al., 2022), transcriptomics (Ho et al., 2022), and functional genomics (Ho et al., 2020). We designed the present study to use proteomics data as a quantitative trait as a step toward identifying peripheral biomarkers that might be associated with acamprosate treatment outcomes. We have applied this approach repeatedly and successfully to study response to SSRIs in patients with MDD (Gupta et al., 2016; Liu et al., 2018; Ji et al., 2014), and response to acamprosate in patients with AUD (Ho et al., 2022; Ho et al., 2022).
The role of inflammation in AUD is well-documented (Adams et al., 2020; Moura et al., 1999). 2022). We previously reported that plasma TNFSF10 levels were associated with acamprosate treatment response in patients with AUD. In the present study, we identified 12 plasma protein markers including IL17RB that were associated with acamprosate treatment response (Fig. 1B). It should be noted that acamprosate blood drug levels during the three month follow-up visit were similar between the relapse and non-relapse group, see Supplementary Fig. 4 (Ho et al., 2022). However, 12 plasma protein markers revealed distinct biological function (Supplementary Table 5). We then performed GWAS for those 12 plasma proteins to identify SNPs correlated with their concentrations.
Our results show that IL17RB levels might be associated with acamprosate treatment outcome measures in the Mayo Clinic Center for Individualized Treatment of Alcoholism study, including relapse to alcohol use, relapse to heavy drinking, time until relapse to alcohol use, and time until relapse to heavy drinking during three months of acamprosate treatment (p < 0.05). The IL17 receptor family is composed of five members (IL17RA-IL17RE) (Li et al., 2019). It has been reported that genetic deletion of IL17RA or pharmacological blockade of IL17A signaling could effectively suppress increased voluntary alcohol drinking in alcohol-dependent mice and could block alcohol-induced hepatocellular and neurological damage (Xu et al., 2020). However, to our knowledge, no prior study has suggested that IL17RB plays a role in either the pathophysiology of AUD or the response to acamprosate treatment of patients with AUD. The OLINK Inflammation panel did not cover other IL17 receptors. However, IL17A, IL17C, IL17D, and IL17F were included. Those proinflammatory cytokines were not associated with acamprosate treatment outcomes (Supplementary Fig. 1). Our GWAS for IL17RB concentrations identified several genome-wide significant SNPs that were associated with variation in IL17RB expression, and those IL17RB SNPs were also associated with acamprosate treatment response (Fig. 2A). The rs6801605 SNP was the top hit SNP in the GWAS for IL17RB levels, with a p value of 4.8E-20. We consulted the GWAS Catalog and found that this SNP has been associated with IL17RB concentrations in two independent studies (Enroth et al., 2014; Suhre et al., 2017). Furthermore, the rs6801605 SNP was associated with acamprosate treatment response in AUD patients recruited from the Mayo Clinic Center for Individualized Treatment of Alcoholism study (Fig. 3A). We confirmed that the rs6801605 SNP was associated with time until relapse (p = 0.02), and time until heavy relapse (p = 0.005) during three months of acamprosate therapy using our recent GWAS meta-analysis data (Table 2) (Biernacka et al., 2021); raising the possibility that IL17RB genetic variants might be biomarkers for acamprosate treatment outcomes. Among the 1083 AUD patients in our GWAS meta-analysis of AUD treatment response, some patients were treated with acamprosate, naltrexone or acamprosate and naltrexone in combination. We explored the association of those IL17RB SNPs with treatment outcomes regardless of pharmacological intervention. Interestingly, the p values were more significant than for acamprosate alone, which might be due to the sample size. For example, the rs2289205 SNP was associated with time until relapse to alcohol use (p: 0.0044), and time until relapse to heavy drinking (p: 0.0008) during three months of treatment, regardless of pharmacological intervention (n = 1083, see Supplementary Table 3). We also observed that some of the clinical variables such as baseline PHQ9, PACS, and GAD-7 scores differed significantly between two groups, which could potentially confound the interpretation of genomic observations. However, those clinical variables are difficult to control due to the nature of the study population. Our findings indicate that baseline plasma IL17RB levels showed no significant correlation with initial alcohol consumption or baseline PACS, PHQ-9, and GAD-7 scores. These observations imply that baseline PHQ9, PACS, and GAD-7 scores are linked to the outcome of acamprosate treatment. However, IL17RB concentrations appeared to be independent of these rating scales. The results of this series of studies could represent an important step toward the generation of functional hypotheses that could be tested to gain insight into molecular mechanisms underlying acamprosate treatment response phenotypes. Future studies will be required to replicate our findings in AUD patients and to investigate their significance in other substance use disorders. That would enable us to determine whether the IL17RB genetic variants that we identified might be utilized as biomarkers to monitor patients with diverse substance use disorders.
We observed that the top hit SNP in the GWAS for plasma CXCL10 levels maps to IL17RC and that it is a missense variant with a p value of 1.56E-08 (Fig. 2). In addition, the top hit SNP in the GWAS for CD6 concentrations maps to CD6, and it is also a missense variant with a p value of 2.77E-09 (Fig. 2). The CD6 variant has been associated with a series of autoimmune disorders i.e. Sjögren’s syndrome (Casadó-Llombart et al., 2022), psoriasis (Consuegra-Fernández et al., 2018), multiple sclerosis (Johnson et al., 2010), and inflammatory bowel disease (Di Narzo et al., 2016). Our proteomics findings indicate that these protein markers may be associated with outcomes for acamprosate treatment, despite the fact that missense variants may not play a significant role in the response to acamprosate treatment. We also observed a series of genome-wide significant signals in the GWAS for ENPP7 levels, but none of them were associated with acamprosate treatment outcomes. Therefore, further investigation is needed to explore the possible functional significance of these protein markers in acamprosate treatment response.
Our plasma proteomics data indicated that an increase in the concentration of HSD11B1 (hydroxysteroid 11-beta dehydrogenase 1) was associated with relapse in alcohol use (Fig. 1B). HSD11B1 converts cortisone to cortisol, a major “stress hormone” that is highly expressed in the liver (Dodd et al., 2022; Morgan et al., 2014). This enzyme has been known to play a role in alcohol-related liver disease (ALD) (Ahmed et al., 2008; Stefan et al., 2014). Specifically, mRNA expression of HSD11B1 in the liver is significantly induced in patients with ALD as compared to unaffected subjects (Ahmed et al., 2008). HSD11B1 inhibitors are potential therapeutic agents for AUD, as previous preclinical studies have demonstrated that HSD11B1 inhibitors could significantly reduce excessive alcohol consumption in mice and rats (Sanna et al., 2016). It has been shown that elevation of the mRNA expression of HSD11B1 in the human dorsal prefrontal cortex is associated with MDD and bipolar disorder which are common psychiatric comorbidities in AUD (Qi et al., 2018; Castillo-Carniglia et al., 2019). We found that plasma HSD11B1 levels were significantly higher in AUD patients who relapsed during three months of acamprosate treatment. However, we did not identify any genome-wide significant signals in the GWAS for HSD11B1 concentrations which might be, at least in part, due to a small effect size that could not be detected in our study. Further investigation is warranted to study the effect of HSD11B1 in acamprosate treatment response using different approaches.
Limitations of our study should also be noted. Several factors contributed to the absence of a placebo arm in this open-label acamprosate clinical trial. Firstly, acamprosate has already received FDA approval for treating AUD. Secondly, the primary objective of this trial was not to evaluate the efficacy of acamprosate, but rather to explore biomarkers associated with individual variation in responses to acamprosate treatment. Lastly, it is important to note that the trial was specifically structured to concentrate on acamprosate treatment due to challenges in recruiting and retaining patients. This study focused on one inflammation panel from OLINK which only allowed us to assay a small subset of plasma proteins. However, OLINK proteomics is currently one of the most sensitive high-performance protein biomarker panels available for inflammation studies. Our analyses addressed the association of levels of peripheral protein markers with acamprosate treatment outcomes. However, we were not able to address the causality between peripheral inflammation and alcohol relapse. Furthermore, we do not have plasma samples after acamprosate treatment because the Mayo Clinic Center for Individualized Treatment of Alcoholism study was initially designed to study genomic biomarkers associated with acamprosate treatment response. The clinical trial did not include a placebo arm so, as a result, this study was unable to address placebo effects. We were also unable to account for all the possible confounding variables, such as chronic inflammation conditions, cancer, and current medication use, all of which might influence plasma IL17RB levels. We observed that the quantity of alcohol consumption three months prior to commencing acamprosate treatment did not reveal a correlation with the baseline plasma IL17RB levels. It should also be noted that blood alcohol concentrations at the time of sample collection were not available, even though we collected self-reported alcohol use 90 days before and after enrollment using TLFB to obtain alcohol exposure data. Finally, we should emphasize that our study participants are European Americans. Therefore, further research involving additional racial groups is needed in order to demonstrate the generalizability of our findings. Despite these limitations, our results revealed that the application of multi-omics approaches may be a feasible and helpful strategy for identifying biomarkers that could potentially aid in predicting acamprosate treatment response.
5. Conclusions
Our goal was to identify biomarkers associated with acamprosate treatment outcomes and to improve our understanding of mechanisms responsible for variation in response to acamprosate using a variety of omics datasets. This series of studies demonstrates that genetic variants in IL17RB may potentially contribute to acamprosate outcomes. Our findings also highlight a potentially novel pharmacogenomic mechanism related to response to acamprosate, which could potentially help us move toward the goal of individualized therapy of AUD.
Supplementary Material
Acknowledgements
We extend our sincere gratitude to Rene Kelly-Jambor and Lori Solmonson for providing administrative support for our research.
Funding
This work was supported in part by National Institutes of Health [grant numbers P20 AA17830, R01 AA27486, R01 DA57928 and K01 AA28050]; the Brain & Behavior Research Foundation [grant number: 31329], the Mayo Clinic Research Pipeline K2R Program, James Myron Smith, M.D., Kristine Jensen Smith, the Terrance and Bette Noble Foundation and the Mayo Clinic Center for Individualized Medicine.
Conflict of Interest
Dr. Weinshilboum is a cofounder of and stockholder in OneOme LLC, a pharmacogenomics decision-support company. Dr. Croarkin has received research grant support from Neuronetics, NeoSync and Pfizer, Inc. He has also received grants-in-kind (equipment support for research studies) from Assurex; MagVenture, Inc; and Neuronetics, Inc. Dr. Croarkin has also served on advisory boards for Engrail Therapeutics, Myriad Neuroscience, Procter & Gamble, and Sunovion. All other authors have no conflicts to declare.
Abbreviations:
- AUD
Alcohol use disorder
- CHDH
Choline dehydrogenase
- CV
Coefficient of variation
- FDA
Food and drug administration
- FDR
False discovery rate
- GABA
Gamma-aminobutyric acid
- GAD-7
General anxiety disorder 7
- GWAS
Genome-wide association study
- IL17RB
Interleukin-17 receptor B
- Kb
Kilobase
- LD
Linkage disequilibrium
- MDD
Major depressive disorder
- MELD
Model For End-Stage Liver Disease
- NPX
Normalized protein expression
- NF-κB
Nuclear factor-κB
- PBMCs
Peripheral blood mononuclear cells
- PHQ-9
Patient health questionnaire 9
- PACS
Penn alcohol craving scale
- RNA-seq
RNA sequencing
- SSRI
Selective serotonin reuptake inhibitor
- SEFIR
Similar Expression to Fibroblast growth factor genes and IL17 Receptor
- SUD
Substance use disorder
- SNP
Single nucleotide polymorphism
- DSM
The Diagnostic and Statistical Manual of Mental Disorders
- PRISM
The Psychiatric Research Interview for Substance and Mental Disorders
- TLFB
Timeline follow-back
Footnotes
CRediT authorship contribution statement
Ming-Fen Ho: Writing – original draft, Validation, Supervision, Methodology, Investigation, Funding acquisition, Conceptualization. Cheng Zhang: Formal analysis, Data curation. James S. Cohan: Writing – review & editing. Mustafa Tuncturk: Writing – review & editing. Robin M. Heider: Writing – review & editing. Brandon J. Coombes: Formal analysis, Data curation. Joanna Biernacka: Formal analysis, Data curation. Irene Moon: Project administration, Methodology, Investigation. Michelle Skime: Writing – review & editing, Project administration. Ada M Ho: Project administration. Quyen Ngo: Project administration. Cedric Skillon: Project administration. Paul E. Croarkin: Writing – review & editing. Tyler S. Oesterle: Writing – review & editing. Victor M. Karpyak: Project administration, Investigation. Hu Li: Formal analysis, Data curation. Richard M. Weinshilboum: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2024.06.007.
Data availability
The authors do not have permission to share data.
References
- Adams C, Conigrave JH, Lewohl J, Haber P, Morley KC, 2020. Alcohol use disorder and circulating cytokines: A systematic review and meta-analysis. Brain Behav. Immun 89, 501–512. [DOI] [PubMed] [Google Scholar]
- Ahmed A, Saksena S, Sherlock M, Olliff SP, Elias E, Stewart PM, 2008. Induction of hepatic 11beta-hydroxysteroid dehydrogenase type 1 in patients with alcoholic liver disease. Clin. Endocrinol 68 (6), 898–903. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. , 2006. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The combine study: a randomized controlled trial. JAMA 295 (17), 2003–2017. [DOI] [PubMed] [Google Scholar]
- Biernacka JM, Coombes BJ, Batzler A, Ho A-M-C, Geske JR, Frank J, et al. , 2021. Genetic contributions to alcohol use disorder treatment outcomes: a genome-wide pharmacogenomics study. Neuropsychopharmacology 46 (12), 2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner E, Tiwari GR, Kapoor M, Liu Y, Brock A, Mayfield RD, 2020. Single cell transcriptome profiling of the human alcohol-dependent brain. Hum. Mol. Genet 29 (7), 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadó-Llombart S, Gheitasi H, Ariño S, Consuegra-Fernández M, Armiger-Borrás N, Kostov B, et al. , 2022. Gene Variation at Immunomodulatory and Cell Adhesion Molecules Loci Impacts Primary Sjögren’s Syndrome. Front. Med 9, 822290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Carniglia A, Keyes KM, Hasin DS, Cerdá M, 2019. Psychiatric comorbidities in alcohol use disorder. Lancet Psychiatry 6 (12), 1068–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H-Y, McGuinness LA, Elbers RG, MacArthur GJ, Taylor A, McAleenan A, et al. , 2020. Treatment interventions to maintain abstinence from alcohol in primary care: systematic review and network meta-analysis. BMJ (clinical Research Ed). 371, m3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consuegra-Fernández M, Julìa M, Martínez-Florensa M, Aranda F, Català C, Armiger-Borràs N, et al. , 2018. Genetic and experimental evidence for the involvement of the CD6 lymphocyte receptor in psoriasis. Cell. Mol. Immunol 15 (10), 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Narzo AF, Peters LA, Argmann C, Stojmirovic A, Perrigoue J, Li K, et al. , 2016. Blood and Intestine eQTLs from an Anti-TNF-Resistant Crohn’s Disease Cohort Inform IBD Genetic Association Loci. Clin. Transl. Gastroenterol 7 (6), e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd S, Skvarc DR, Dean OM, Anderson A, Kotowicz M, Berk M, 2022. Effect of Glucocorticoid and 11β-Hydroxysteroid-Dehydrogenase Type 1 (11β-HSD1) in Neurological and Psychiatric Disorders. Int. J. Neuropsychopharmacol 25 (5), 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth S, Johansson Å, Enroth SB, Gyllensten U, 2014. Strong effects of genetic and lifestyle factors on biomarker variation and use of personalized cutoffs. Nat. Commun 5 (1), 4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. , 2015. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiat. 72 (8), 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Neavin D, Liu D, Biernacka J, Hall-Flavin D, Bobo WV, et al. , 2016. TSPAN5, ERICH3 and selective serotonin reuptake inhibitors in major depressive disorder: pharmacometabolomics-informed pharmacogenomics. Mol Psychiatry. 21 (12), 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Samokhvalov AV, Rehm J, 2017. Effects of naltrexone on alcohol self-administration and craving: meta-analysis of human laboratory studies. Addict. Biol 22 (6), 1515–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillemacher T, Heberlein A, Muschler MA, Bleich S, Frieling H, 2011. Opioid modulators for alcohol dependence. Expert Opin. Invest. Drugs 20 (8), 1073–1086. [DOI] [PubMed] [Google Scholar]
- Ho M-F, Zhang C, Zhang L, Wei L, Zhou Y, Moon I, et al. , 2020. TSPAN5 influences serotonin and kynurenine: pharmacogenomic mechanisms related to alcohol use disorder and acamprosate treatment response. Mol. Psychiatry 26, 3122–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MF, Zhang C, Zhang L, Wei L, Zhou Y, Moon I, et al. , 2021. TSPAN5 influences serotonin and kynurenine: pharmacogenomic mechanisms related to alcohol use disorder and acamprosate treatment response. Mol Psychiatry. 26 (7), 3122–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M-F, Zhang C, Moon I, Coombes BJ, Biernacka J, Skime M, et al. , 2022. Plasma TNFSF10 levels associated with acamprosate treatment response in patients with alcohol use disorder. Front. Pharmacol 13 (986238). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MF, Zhang C, Moon I, Wei L, Coombes B, Biernacka J, et al. , 2022. Genome-wide association study for circulating FGF21 in patients with alcohol use disorder: molecular links between the SNHG16 locus and catecholamine metabolism. Molecular Metabolism. 63, 101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M-F, Zhang C, Wei L, Zhang L, Moon I, Geske JR, et al. , 2022. Genetic variants associated with acamprosate treatment response in alcohol use disorder patients: A multiple omics study. Br. J. Pharmacol 173 (13), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M-F, Zhang C, Moon I, Biernacka J, Coombes B, Ngo Q, et al. , 2023. Epigenetic regulation of GABA catabolism in iPSC-derived neurons: the molecular links between FGF21 and histone methylation. Molecular Metabolism. 101798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M-F, Zhang C, Moon I, Tuncturk M, Coombes BJ, Biernacka J, et al. , 2024. Molecular mechanisms involved in alcohol craving, IRF3, and endoplasmic reticulum stress: a multi-omics study. Transl. Psychiatry 14 (1), 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S, Nakae S, 2011. Functional Specialization of Interleukin-17 Family Members. Immunity 34 (2), 149–162. [DOI] [PubMed] [Google Scholar]
- Ji Y, Schaid DJ, Desta Z, Kubo M, Batzler AJ, Snyder K, et al. , 2014. Citalopram and escitalopram plasma drug and metabolite concentrations: genome-wide associations. Br. J. Clin. Pharmacol 78 (2), 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Wang J, Taylor EM, Caillier SJ, Herbert J, Khan OA, et al. , 2010. Multiple sclerosis susceptibility alleles in African Americans. Genes Immun. 11 (4), 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, et al. , 2014. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: A systematic review and meta-analysis. JAMA 311 (18), 1889–1900. [DOI] [PubMed] [Google Scholar]
- Kalk NJ, Lingford-Hughes AR, 2014. The clinical pharmacology of acamprosate. Br. J. Clin. Pharmacol 77 (2), 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra G, Sousa AD, Shrivastava A, 2014;Vol.. Disulfiram in the management of alcohol dependence: A comprehensive clinical review. Open. J. Psychiatry 04 No.01: 10. [Google Scholar]
- Kapoor M, Wang J-C, Farris SP, Liu Y, McClintick J, Gupta I, et al. , 2019. Analysis of whole genome-transcriptomic organization in brain to identify genes associated with alcoholism. Transl. Psychiatry 9 (1), 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly HC, Skrzynski CJ, Moe EN, Bryan AD, Hutchison KE, 2021. Exploring relationships between alcohol consumption, inflammation, and brain structure in a heavy drinking sample. Alcohol. Clin. Exp. Res 45 (11), 2256–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpyak VM, Biernacka JM, Geske JR, Jenkins GD, Cunningham JM, Rüegg J, et al. , 2014. Genetic markers associated with abstinence length in alcohol-dependent subjects treated with acamprosate. Transl. Psychiatry 4, e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Zhou H, Kember RL, Vickers Smith R, Justice AC, Damrauer S, et al. , 2019. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 10 (1), 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL, 2019. IL-17 receptor–based signaling and implications for disease. Nat. Immunol 20 (12), 1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, Fertig J, Leggio L, 2020. Five Priority Areas for Improving Medications Development for Alcohol Use Disorder and Promoting Their Routine Use in Clinical Practice. Alcohol. Clin. Exp. Res 44 (1), 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Ray B, Neavin DR, Zhang J, Athreya AP, Biernacka JM, et al. , 2018. Beta-defensin 1, aryl hydrocarbon receptor and plasma kynurenine in major depressive disorder: metabolomics-informed genomics. Transl. Psychiatry 8 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe PP, Morel C, Ambade A, Iracheta-Vellve A, Kwiatkowski E, Satishchandran A, et al. , 2020. Chronic alcohol-induced neuroinflammation involves CCR2/5-dependent peripheral macrophage infiltration and microglia alterations. J Neuroinflammation. 17 (1), 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW, 2013. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 108 (2), 275–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchia M, Pisanu C, Squassina A, Carpiniello B, 2020. Challenges and Future Prospects of Precision Medicine in Psychiatry. Pharmgenomics Pers Med. 13, 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Smolka M, Gann H, Wellek S, Heinz A, 2009. Searching for responders to acamprosate and naltrexone in alcoholism treatment: rationale and design of the PREDICT study. Alcohol Clin Exp Res. 33 (4), 674–683. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Heyser CJ, 2010. Acamprosate: a prototypic neuromodulator in the treatment of alcohol dependence. CNS Neurol Disord Drug Targets. 9 (1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Más-Serrano P, Granero L, Martín-Algarra RV, Guerri C, Polache A, 2000. KINETIC STUDY OF ACAMPROSATE ABSORPTION IN RAT SMALL INTESTINE. Alcohol Alcohol. 35 (4), 324–330. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Fitzmaurice GM, Griffin ML, Anton RF, Weiss RD, 2016. Association between a brief alcohol craving measure and drinking in the following week. Addiction 111 (6), 1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters M, O’Connor EA, Riley S, Kennedy SM, Voisin C, Kuznacic K, et al. , 2023. Pharmacotherapy for Alcohol Use Disorder: A Systematic Review and Meta-Analysis. JAMA 330 (17), 1653–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SA, McCabe EL, Gathercole LL, Hassan-Smith ZK, Larner DP, Bujalska IJ, et al. 11β-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proceedings of the National Academy of Sciences. 2014;111 (24):E2482–E91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura HF, Hansen F, Galland F, Silvelo D, Rebelatto FP, Ornell F, et al. Inflammatory cytokines and alcohol use disorder: systematic review and meta-analysis. Revista brasileira de psiquiatria (Sao Paulo, Brazil: 1999). 2022;44(5):548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle TS, Thusius NJ, Rummans TA, Gold MS, 2019. Medication-Assisted Treatment for Opioid-Use Disorder. Mayo Clin. Proc 94 (10), 2072–2086. [DOI] [PubMed] [Google Scholar]
- Qi X-R, Luchetti S, Verwer RWH, Sluiter AA, Mason MRJ, Zhou J-N, et al. , 2018. Alterations in the steroid biosynthetic pathways in the human prefrontal cortex in mood disorders: A post-mortem study. Brain Pathol. 28 (4), 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K, 2010. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 9 (1), 13–22. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Kawamura T, Chen J, Koob GF, Roberts AJ, Vendruscolo LF, et al. , 2016. 11β-hydroxysteroid dehydrogenase inhibition as a new potential therapeutic target for alcohol abuse. Transl Psychiatry. 6 (3), e760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WW, 2018. Anticraving therapy for alcohol use disorder: A clinical review. Neuropsychopharmacology Reports. 38 (3), 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MD, Lahmek P, Pham H, Aubin H-J, 2014. Disulfiram Efficacy in the Treatment of Alcohol Dependence: A Meta-Analysis. PLoS One 9 (2), e87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N, Ramsauer M, Jordan P, Nowotny B, Kantartzis K, Machann J, et al. , 2014. Inhibition of 11β-HSD1 with RO5093151 for non-alcoholic fatty liver disease: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2 (5), 406–416. [DOI] [PubMed] [Google Scholar]
- Suhre K, Arnold M, Bhagwat AM, Cotton RJ, Engelke R, Raffler J, et al. , 2017. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun. 8, 14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Saville K, Hamreus K, 2012. Acamprosate for treatment of alcohol dependence: mechanisms, efficacy, and clinical utility. Ther. Clin. Risk Manag 8, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Litten RZ, Leggio L, 2019;5(9):eaax4043.. Advances in the science and treatment of alcohol use disorder. Sci. Adv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H-H, Hwang-Verslues WW, Lee W-H, Huang C-K, Wei P-C, Chen C-L, et al. , 2015. Targeting IL-17B–IL-17RB signaling with an anti–IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J. Exp. Med 212 (3), 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Ma HY, Liu X, Rosenthal S, Baglieri J, McCubbin R, et al. , 2020. Blockade of IL-17 signaling reverses alcohol-induced liver injury and excessive alcohol drinking in mice. JCI Insight. 5 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting our findings can be found in the main paper or in supplementary files. RNA-seq data are available via the GEO accession number: GSE208132 (Ho et al., 2022).
The authors do not have permission to share data.


