Abstract
Sustaining crop production and food security are threatened by a burgeoning world population and adverse environmental conditions. Traditional breeding methods for vegetable crops are time-consuming, laborious, and untargeted, often taking several years to develop new and improved varieties. The challenges faced by a long breeding cycle need to be overcome. The speed breeding (SB) approach is broadly employed in crop breeding, which greatly shortens breeding cycles and facilities plant growth to obtain new, better-adapted crop varieties as quickly as possible. Potential opportunities are offered by SB in plant factories, where optimal photoperiod, light quality, light intensity, temperature, CO2 concentration, and nutrients are precisely manipulated to enhance the growth of horticultural vegetable crops, holding promise to surmount the long-standing problem of lengthy crop breeding cycles. Additionally, integrated with other breeding technologies, such as genome editing, genomic selection, and high-throughput genotyping, SB in plant factories has emerged as a smart and promising platform to hasten generation turnover and enhance the efficiency of breeding in vegetable crops. This review considers the pivotal opportunities and challenges of SB in plant factories, aiming to accelerate plant generation turnover and improve vegetable crops with precision and efficiency.
Keywords: vegetable, speed breeding, plant factory, smart breeding, breeding cycle
1. Introduction
The current trajectory for crop productivity needs to produce 60% more food to sustainably nourish a burgeoning world population of 10 billion by 2050; therefore, there is an urgent need for higher-yielding varieties and accelerating crop production (Pardey et al., 2014). The ever-increasing human population, urbanization, declining agricultural lands, the rising frequency of destructive weather events, and the high incidence of pests and diseases pose grim threats to global food security (Shrivastava and Kumar, 2015; Alotaibi, 2023). In particular, during the coronavirus disease 2019 (COVID-19) pandemic era, improving crop productivity to alleviate food scarcity problems and ensuring food and nutritional security through modern breeding technologies are crucial for plant breeders and scientists.
2. The demand for faster vegetable crop breeding
The length of the breeding cycle is a major issue for crop genetic improvement (Saxena et al., 2017; Wanga et al., 2021). The development of genetically stable homozygous or novel cultivars with market-preferred traits of crops is time-consuming, which generally takes up to a decade or more via conventional breeding methodologies. Hence, shortening crop breeding cycles through promoting rapid plant growth and early flowering has greatly attracted the attention of plant breeders and researchers worldwide for crop production and productivity improvement (Watson et al., 2018; Bhatta et al., 2021; Guo et al., 2022; Liu et al., 2022). Efficient strategies are being employed to greatly reduce generation time including shuttle breeding, doubled haploid, targeting-induced local lesions in genomes (TILLING), and genome editing technologies ( Table 1 ). However, these molecular and conventional breeding approaches cannot satisfy the ever-increasing demands for agricultural production and more available breeding approaches need to be explored.
Table 1.
Strategies to speed up the breeding process of crops.
| Strategies | Basics of methods | Generation per year | Limitations | Reference |
|---|---|---|---|---|
| Shuttle-breeding | Growing crop material in alternative suitable locations. | 2–3 | ⋄ Not reliable ⋄ High rate of loss of material ⋄ Reducing genetic variance |
Ortiz et al., 2007 |
| Double haploid (DH) | Facilitating the development of entirely homozygous lines in two generations. | 3–4 | ⋄ Genotype dependence ⋄ Requiring special skills and labor ⋄ High cost |
Germanà, 2011 |
| In vitro culture | Using nutritive culture media and controlled aseptic conditions for the growth of plant cells, tissues, organs, or immature embryos. | 12 | ⋄ Specific protocol for each species ⋄ High cost ⋄ Requiring specialized staff ⋄ Labor intensive |
Germanà, 2011 |
| Targeting induced local lesions in genomes | A reverse genetics approach for high-throughput discovery of induced mutations in the desired gene(s) from a mutant population developed through mutagenesis. | Not available | ⋄ High screening costs ⋄ Large insertions or deletions are difficult to detect ⋄ Rely on known genome ⋄ Dependence on random mutagenesis |
Mba, 2013; Chen et al., 2014 |
| Transgenesis or genetic editing | Transgenesis involves the introduction of foreign genes into an organism’s genome, while genetic editing allows for precise modifications to be made to the organism’s existing DNA sequence. | Not available | ⋄ Political and social issues ⋄ The lack of regulatory hurdle ⋄ Specific protocol for each species ⋄ Genotype dependence and requiring specialized staff |
Zhan et al., 2021 |
| Single seed descent (SSD) | Sampling one seed of each plant in a segregating population and continue this process until the desired level of inbreeding has been achieved. | Not available | ⋄ More inferior progenies ⋄ Risks of losing desirable genes ⋄ Requiring appropriate facilities |
Kigoni et al., 2023 |
| Speed breeding (SB) | Using optimal day length, light intensity, light quality, and temperature to stimulate flowering and seed production. | 4–6 | ⋄ Genotype dependence ⋄ High establishment cost ⋄ High energy consumption |
Watson et al., 2018 |
In recent years, a system called speed breeding (SB) has been in the spotlight (Watson et al., 2018). It allows plant breeders to drastically shorten crop life cycles to produce more generations per year by optimized photoperiod, light quality, light intensity, day/night temperature and humidity, and CO2 concentration under controlled environments. This approach allows multiple generations of wheat, barley, rice, and legumes to be produced within a single year, significantly shortening the development cycles (Watson et al., 2018). Vegetable crops are integral to dietary diversity, nutritional sustenance, and economic development. While significant progress has been achieved in vegetable breeding, there remains a pressing need to expedite the development of green, environmentally resilient cultivars characterized by high yield and quality to meet the demands of the global population in a timely manner. Previous extensive reviews have summarized the methods, applications, potential advantages, and key challenges of SB techniques that can enhance crop quality and yield to meet the growing demand for food and ensure global food security. Certain reviews also focused on exploring the integration of SB with new breeding techniques (AI in crop breeding, genomics-assisted breeding, haplotype-based breeding, etc.) to efficiently and rapidly produce stable lines for both basic and applied research purposes. The current review mainly discusses the key technologies used in plant factories for efficient vegetable breeding and highlights the potential advantages of SB in plant factories as a smart and promising platform to hasten generation turnover.
3. Speed breeding technology utilizing plant factories
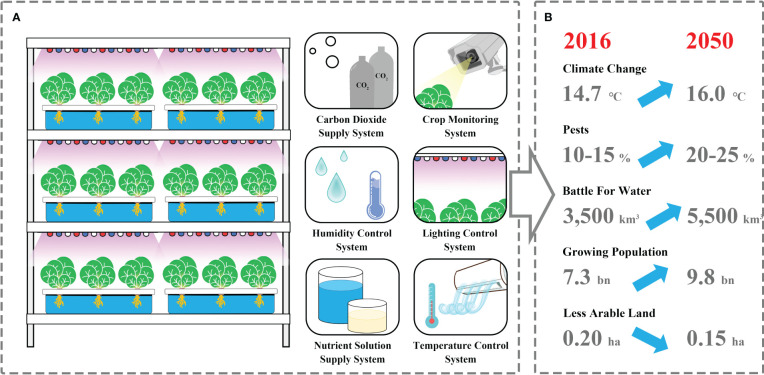
Plant factories are considered as multilayer closed plant production systems in which light, temperature, humidity, CO2, and nutrients are precisely manipulated through various sensors and artificial intelligence systems, allowing the precise control of physiological and developmental processes in plants (Kozai and Niu, 2015a) ( Figure 1A ). Crop production in plant factories can overcome many limitations of pathogens, pests, soil conditions, or climate change, allowing year-round cultivation at any location around the globe ( Figure 1B ). SB in plant factories paves a way to access four or more generations per year by providing optimal light and temperature, accelerating biological processes for rapid generation advancement (Ghosh et al., 2018; Watson et al., 2018). The shift from the vegetative to the flowering stage is one of the most crucial developmental milestones in the plant life cycle. Accelerating plant growth and development and promoting early flowering in plant factories, where various environmental factors, namely, photoperiod, temperature, and light quality and intensity, were regulated precisely and efficiently, are conducive to shortening the breeding cycles of vegetable crops Ghosh et al., 2018; Watson et al., 2018. Vegetables exhibiting short growth cycles and sensitivity to environmental cues are ideal candidates for SB, such as tomato, cucumbers, hot pepper, cabbage, spinach, and mustard greens. Their suitability for this method lies in their swift life cycles, enabling rapid generational turnover and efficient assessment of breeding characteristics. Furthermore, their adaptability to controlled environments permits precise adjustment of factors such as light, temperature, and humidity, facilitating accelerated vegetable growth rates.
Figure 1.
The primary structural components of a plant factory (A) are advantageous for coping with adverse factors (B) while simultaneously fulfilling the requirements for rapid crop breeding.
4. Opportunities for vegetable crops speed breeding in plant factories
4.1. Photoperiod
Photoperiod is a pivotal component of light environmental factors that has a prominent influence on vegetative and phenological development and physiological reactions. The effect of photoperiod on flowering time and seeding of plants varies on crop genotype or cultivar. Plants are classified into long-day, short-day, or day-neutral plants based on their sensitivity response to photoperiod (Jackson, 2009). For long-day plants, the time of flowering was frequently accelerated with a prolonged photoperiod, while in short-day plants, flowering is triggered when photoperiod becomes shorter than their critical photoperiod, or night time becomes longer than the critical night period (Jackson, 2009).
SB is an approach relying on prolonging the photoperiod, that is, providing a longer duration of lighted period to hasten plant growth and accelerate the plant life cycle (Watson et al., 2018). Efforts toward SB of vegetable crops mainly focus on accelerating the transition from vegetative to reproductive development. Optimum photoperiod is critical and responsible for vegetative and reproductive growth. Thus, manipulation of the photoperiod regime has been well applied in many plant species to stimulate early flowering and seeding and shorten breeding cycles, such as in wheat, canola, and hot pepper ( Table 2 ). For long-day plants, although the days of flowering seem to decrease linearly with prolonging the photoperiod, some stress and damage such as foliar chlorosis, leaf burn, flower abortion, wilting, and mortality, caused by an artificially longer photoperiod, should not be neglected (Takahashi and Badger, 2011; Omar, 2020; Mitache et al., 2023). Short-day plants initiate flowering when the photoperiod is shorter than a critical day length, but to date, the sensitive period of short-day crops to flower is less unknown. Therefore, the optimum light/dark ratio that accelerates the plant growth of various stress-free crops still needs more exploration.
Table 2.
Effect of light photoperiod on crop speed breeding.
| Plant species | Light photoperiod | Other experimental details | Influence on speed breeding generations | Reference | |
|---|---|---|---|---|---|
| Effects | Generations/year | ||||
| Spring wheat (Triticum aestivum) |
Natural 12-h control photoperiod as control, extend the photoperiod to 22 h | 22/17°C day/night; immature seed harvest and drying | In a glasshouse with a natural variable photoperiod (10–16 h), only 2–3 generations of wheat, barley, chickpea, and canola per year, speed breeding stimulated early flowering and seed set and shortened breeding cycles, 4–6 generations of these crops per year. | 6 generations per year | Watson et al., 2018 |
| Durum wheat (T. durum) | |||||
| Barley (Hordeum vulgare) | |||||
| Chickpea (Cicer arietinum) | |||||
| Pea (Pisum sativum) | |||||
| Canola (Brassica napus) |
4 generations per year | ||||
| Hot pepper (Capsicum spp. cv. “Xiangyan55”) |
14-h, 16-h, and 18-h photoperiod | 420 µmol·m−2·s−1 | The 20-h photoperiod reduced flowering time, and the shortest day from sowing to flowering was 37 days. The breaker stage was reached at 82 DAS (days after sowing), and the red ripening stage was reached at 86 DAS. | 4 generations per year | Liu et al., 2022 |
| Hot pepper (Capsicum spp. cv. “Xiangla712’) |
Plants under the 20-h photoperiod reached the flowering stage at 43 DAS, the breaker stage at 90 DAS, and the red ripened stage at 95 DAS. | 4 generations per year | |||
| Lentil (Lens culinaris Medik.) cross-derived F2 lines |
A conventional control treatment under a glasshouse of 10-h to 12-h photoperiod as control, extended photoperiod treatment of 22 h in a growth chamber | Using a single seed descent method (SSD) under the extended photoperiod treatment | Days to maturity under extended photoperiod method was 84 days while glasshouse-based conventional method was 172 days. | 3–4 generations per year | Omar, 2020 |
| Wild accessions of L. orientalis |
Days to maturity under an extended photoperiod method was 115 days while the glasshouse-based conventional method was 225 days. | ||||
| Moroccan varieties lentil (Lens culinaris “Bakria” and “L24”) |
22-h, 18-h, and 14-h photoperiod | Temperature of 23–26°C light/12–18°C dark | A photoperiod of 18 h as a balance between 22 h and 14 h was more optimal for speeding up the breeding cycles. | Not available | Mitache et al., 2023 |
| Chickpea (Cicer arietinum “Bouchra” and “Local Chaouia”) | |||||
| Faba bean (Vicia faba L. “Hiba” and “Loubab”) | |||||
| Strawberry (Fragaria × ananassa cv. “Albion”) |
Photoperiodic treatment of 24-h and 18-h continuous light and natural daylight | Using incandescent light as the predominant light source | Flowering response for “Albion” established a similar degree of sensitivity to photoperiod. | Not available | Sidhu et al., 2022 |
| Supplemented for long-day (LD; 24 h) and short-day (SD; 10 h) photoperiods | Using light-emitting diodes (LEDs) as the light source | LD photoperiod exposure of diverse light combinations significantly promoted the inflorescences and flower buds inside the crown. | Not available | ||
4.2. Light quality
Light quality, as another critical signal besides photoperiod, has broad effects on plant growth and developmental processes, including seed germination, photomorphogenesis, and flowering (Cerdán and Chory, 2003; Kami et al., 2010; Jones, 2018; Paradiso and Proietti, 2022). Rapid advances in LEDs make it possible to obtain vegetal material with desired traits through the application of specific light wavelengths and accelerate flowering and seeding to advance to the next breeding generation as quickly as possible (Brazaityte et al., 2016; Al Murad et al., 2021). Using optimal light quality could accelerate photosynthesis and flowering and shorten the length of the breeding cycle ( Table 3 ).
Table 3.
Opportunity of light quality on crop speed breeding in plant factories.
| Plant species | Light quality | Other experimental details | Influence on speed breeding generations | Reference | |
|---|---|---|---|---|---|
| Effects | Generations/year | ||||
| Petunia (Petunia hybrida) |
White light Monochromatic red light Monochromatic blue lights |
70 or 150 µmol·m−2·s−1 | Blue lights hasten plant flowering, whereas red light did not display flowering promotion effect. | Not available | Fukuda et al., 2016 |
| Soybean [Glycine max (L.) Merr] |
1,018 µmol·m−2·s−1 red (80%) and blue (20%) LED 1,190 µmol·m−2·s−1 full-spectrum (FS) white light is considered the control |
25/18°C, day/night, 12-h photoperiod |
RB LED coupled with photothermal conditions can reduce the generation cycle by 56–66 days compared with regular field conditions by 120 days. | 5 generations per year | Harrison et al., 2021 |
|
Hippeastrum hybridum
“Red Lion” |
Red/blue light ratio of 1:9 (R10B90) and 9:1 (R90B10) | 200 µmol·m−2·s−1, 14-h photoperiod | Higher blue light and low (1/10) red light intensity (R10B90) promoted early flowering and shorted flowering period. | Not available | Wang et al., 2022 |
| Wheat (Triticum aestivum L.) |
White light (W), white–green light (W:G = 4:1, W4G1), red–green light (R:G = 4:1, R4G1), red–green–blue light (R:G:B = 4:1:1, R4G1B1), red–blue lights (R:B = 3:1, R3B1; R:B = 2:1, R2B1; R:B = 1:1, R1B1; R:B = 1:6, R1B6) |
360 µmol·m−2·s−1, 22-h photoperiod | Higher blue light ratio, such as R2B1 (38.1%), R1B1 (58.7%), and R1B6 (85.8%) delayed flowering time and produced fewer grains. R4G1 induced early flowering time, high yield, and excellent quality, which could be the recommended light environment for indoor wheat cultivation. | Not available | Guo et al., 2022 |
| Tomato (Solanum lycopersicum L. cv. Micro-Tom) |
Supplementary 100 µmol·m−2·s−1 red light |
Supplementary red light for 12 h per day (6:00−18:00) at the onset of anthesis | Supplementary red light targeted genes that are linked to ripening and promoted the biosynthesis and signaling of ethylene, resulting in the earlier ripening of tomato fruit. | Not available | Zhang et al., 2020 |
| Tomato (Solanum lycopersicum L. cv. Micro-Tom) |
Supplementary 100 µmol·m−2·s−1 blue light |
Supplementary different blue light frequencies 6 h, 8 h, 10 h, and 12 h with the same intensity at the onset of anthesis | Different frequencies of supplemental blue light accelerated flowering and promote fruit ripening approximately 3–4 days early via promoting ethylene evolution of fruits. | Not available | He et al., 2022 |
| Tomato (Solanum lycopersicum L. cv. “Mini Chal”) |
Supplement 0.4, 0.6, 0.8 W·m−2 UV-A | Basal light: R3B7 [red (R):blue (B) = 30:70], 25/18°C, day/night 50 ± 10% RH, 200 µmol·m−2·s−1, 12-h photoperiod |
UV-A (0.4 W·m−2) light intensities promoted faster flowering. | Not available | Kim and Hwang, 2019 |
| Lupin (Lupinus angustifolius L.) |
R:FR ratio of 5.86, 3.42, 2.89, 2.16, and 1.14 | Not available | An R:FR ratio above 3.5 might inhibit flowering while those below 3.5 might induce earlier flowering. | Not available | Croser et al., 2016 |
| Hot pepper (Capsicum spp.) |
Additional FR light intensity was set to 30, 50, 70, and 90 µmol·m−2·s−1 | Basal light: white:red:blue = 3:2:1, 420 µmol·m−2·s−1, 12 h photoperiod |
Supplementation low-intensity far-red light (30 µmol·m−2·s−1, R:FR = 2.1) speed up the flowering and significantly accelerated the red ripening of pepper fruit and improved seed germination rates. | 4 generations per year | Liu et al., 2022 |
| Winter canola (Brassica napus cv. “Darmorbzh”) |
Additional FR 500 µmol·m−2·s−1 | 22-h light period; 22°C, humidity 70% | Generated visible flower buds at 92 DAG and mature seeds at approximately 125 DAG. | 3 generations per year | Song et al., 2022 |
| Spring canola (Brassica napus cv. “Westar”) |
The life cycle accelerated by 12 days and mature seeds at approximately 55 DAG. | 4.5 and 5.5 generations per year | |||
| Semi-winter canola (Brassica napus cv. “ZS11”) |
The life cycle accelerated by 21 days and mature seeds at approximately 66 DAG. | 4.5 and 5.5 generations per year | |||
| Geranium (Pelargonium × hortorum L.H.) |
Supplement 10 µmol·m−2·s−1 green light during night interruption | 20 ± 1°C, 60 ± 10% RH, 140 ± 20 µmol·m−2·s−1, 350 ± 50 µmol·m−2·s−1 |
Hasten flowering. | Not available | Park et al., 2017 |
| Chinese kale (Brassica alboglabra) |
Supplement 3 W·m−2 (FR-3), 6 W·m−2 (FR-6) far-red-light | 21 ± 2°C, 55%–60% RH, CO2 concentration (400–600 µmol·mol−1) Basal light: white LED light (250 µmol·m−2·s−1 PPFD), 10-h photoperiod. |
The budding rate of no-far red light treatment (control group) was only 11.1%, while that of FR-3 and FR-6 reached 30.6% and 45.8%, respectively after 45 days FR supplement. FR accelerated flowering via regulating expression of key genes in the plant circadian rhythm pathway. | Not available | Li et al., 2023 |
| Chinese kale (Brassica alboglabra) |
Supplement 40 µmol·m−2·s−1 UVA | Basal light: white 250 µmol·m−2·s−1, 12-h photoperiod UVA exposure for 6 h/d |
Induced faster flowering of Chinese kale than no-UVA treatment. | Not available | Gao et al., 2022 |
| Petunia (Petunia × hybrida) |
Supplement green light intensity 0, 2, 13, or 25 µmol·m−2·s−1 |
Not available | Accelerated flowering of all long-day plants (Petunia, Ageratum, Snapdragon, and Arabidopsis) and delayed flowering of all short-day plants (Chrysanthemum and Marigold). | Not available | Meng and Runkle, 2019 |
| Ageratum (Ageratum houstonianum) | |||||
| Snapdragon (Antirrhinum majus) | |||||
| Arabidopsis (Arabidopsis thaliana) | |||||
| Chrysanthemum (Chrysanthemum morifolium) | |||||
| Marigold (Tagetes erecta) | |||||
Red and blue LEDs have been widely applied in horticulture, since these two spectral regions are regarded as particularly significant to photosynthetic CO2 assimilation (Chory, 2010). Both blue and red light are involved in the regulation of different processes through the plant life cycle (including seed germination and vegetative and reproductive growth). Blue light is a predominant signal for flower initiation; it can promote floral induction and flowering by regulating the level of hormones and gene expression associated with flowering (Yoshida et al., 2016; He et al., 2022; Wang et al., 2022; Nie et al., 2023). Inversely, red light has been recorded to promote both vegetative and reproductive growth but hinder flowering (Fukuda et al., 2016; Wang et al., 2022). However, it should be noted that flowering responses to blue and red light need to be further explored in various crop species since the research results often contain contradictory information to date (Giliberto et al., 2005; Monostori et al., 2018; Zhang et al., 2020; He et al., 2022).
Acceleration of flowering was well identified in many crops such as Arabidopsis thaliana, Hordeum vulgare, Petunia hybrida, and Zinnia elegans, in response to low R:FR ratios (Demotes-Mainard et al., 2016). In general, an approximate R:FR of 1.2, which occurred under natural sunlight, is suitable for plant growth and flowering (Holmes and Smith, 1975). However, the thresholds of R:FR ratio regarding flowering time vary among plant species and varieties, as well as growing conditions. For example, thresholds of R:FR ratio that promote or delay flowering were 3.5 and 5.3 in Eustoma grandiflora and lupin, respectively (Yamada et al., 2009; Croser et al., 2016). FR was beneficial for plants to facilitate light interception and tremendously accelerate plant growth and development responsible for the wide application in a controlled environment to induce early flowering and shorten breeding cycles (Liu et al., 2022; Song et al., 2022; Li et al., 2023). Besides R and FR light, blue light, in specific conditions, and some other light (i.e., green and ultraviolet-A) are also constructive for regulating the flowering of a few species such as petunia, tomato, and Chinese kale (Smith et al., 2017; Verdaguer et al., 2017; Kim and Hwang, 2019; Gao et al., 2022). To facilitate the optimal utilization of LEDs in the realm of horticultural crop production within plant factories, a comprehensive comprehension of the effects of light wavelengths on plant performance, encompassing the underlying mechanisms and temporal aspects, becomes imperative.
4.3. Light intensity
To date, a considerable number of studies focus on clarifying the impact of light photoperiod and light quality on plants, while those that target plant responses to light intensity are relatively few, even though plants have developed a sophisticated mechanism to alter their morphological features in response to varying intensity. Generally, under the light saturation point, increasing light intensity and the photosynthesis rate, measured as photosynthetic oxygen evolution, increases linearly with the light, because in this phase, light is the limiting factor (Haliapas et al., 2008; Formighieri, 2015; Oh, 2015). Higher light intensity provides more photosynthetic energy required for the synthesis of flowering-promoting substances, which is conducive to the formation and growth of flower primordium (Wimalasekera, 2019). Thus, reasonable light intensity can promote photosynthesis, allowing more light assimilation substances to be distributed to seeds or fruits, leading to rapid growth, higher biomass, and greater seed production ( Table 4 ). Advancements in LED technologies have significantly contributed to achieving optimal light intensity for facilitating plant growth, thereby expediting plant life cycles and enhancing the completion of multiple generations within a shorter time frame (Jähne et al., 2020; Liu et al., 2022). Notably, the augmentation of light intensity, concomitant with escalated energy expenditures, manifests only marginal alterations, necessitating a comprehensive evaluation of the balance between advantages and associated costs.
Table 4.
Effect of light intensity on crop speed breeding.
| Plant species | Light intensity | Other experimental details | Influence on speed breeding generations | Reference | |
|---|---|---|---|---|---|
| Effects | Generations/year | ||||
| Petunias (Petunia × hybrida) |
Low (L) light: 40 µmol·m−2·s−1
Medium (M) light: 120 µmol·m−2·s−1 High (H) light: 360 µmol·m−2·s−1 |
HPS; 16 h·d−1 photoperiod | Flower bud formation was achieved at the end of the 4th, 5th, and 6th week after H, M, and L, respectively. | Not available | Haliapas et al., 2008 |
| Eustoma grandiflorum cv. “Nail Peach Neo” | Light intensity increased from 100 to 400 µmol·m−2·s−1 | 10 h·d−1 photoperiod | Flowering time was shortened by 7 to 10 days. | Not available | Oh, 2015 |
| Soybean (Glycine max) |
480, 720, 960, 1,160, and 1,511 µmol·m−2·s−1 | 10 h·d−1 photoperiod | Above 1,000 µmol·m−2·s−1 flowering at ~21.8 days after planting while 500–900 µmol·m−2·s−1 at 23.9 days. Thus, ~500 µmol·m−2·s−1 should suffice to achieve fast generation times on a moderate budget. |
Generation cycle by 75 days |
Jähne et al., 2020 |
| Hot pepper (Capsicum spp.) cv. “Xiangyan55” |
240, 300, 360, and 420 µmol·m−2·s−1 | 12 h·d−1 photoperiod | 300 to 360 µmol·m−2·s−1 reduced flowering time. Completely red earliest under 360 µmol·m−2·s−1, only 86 days from sowing to full maturity. |
Generation cycle by 86 days 4 generations per year |
Liu et al., 2022 |
| Hot pepper (Capsicum spp.) cv. “Xiangla712” |
The earliest to reach each physiological stage was under 420 µmol·m−2·s−1, which reached the flowering stage at 43 days, the breaker stage at 83 days, and the red ripened stage at 86 days. | ||||
4.4. Temperature
Apart from the light environment, temperature is particularly important to manipulate plant reproductive development and accelerate plant flowering. Each species has a specific temperature regime (maximum and minimum temperatures) for growth and development. The temperature range conducive to the germination of most crops typically falls within 12°C to 30°C, while the optimal temperature range for growth, flowering, and seed setting is generally observed between 25°C and 30°C (Hatfield and Prueger, 2015). Within the temperature range that does not cause heat stress to plants, higher temperature might facilitate vegetative growth while lower temperature is more suitable for grain production in the reproductive stages (Hickey et al., 2019). Optimum temperature is beneficial to accelerate photosynthesis and flowering, coupled with early seed harvest to shorten the breeding cycle, such as the SB approach applied in peanut, chickpea, lupin, lentil, pea, soybean, and faba bean (O’Connor et al., 2013; Croser et al., 2016) ( Table 5 ). Notably, some crops must undergo a period of low temperature to transition from vegetative growth to reproductive growth, that is, vernalization. For instance, to achieve typical flower initiation and formation and accelerate plant growth, winter wheat requires long exposure to low temperatures (Cha et al., 2022). Hence, overcoming the rapid vernalization of winter crops stands as a pivotal technological advancement for expediting breeding processes in plant factories.
Table 5.
Effect of temperature on crop speed breeding.
| Plant species | Temperature | Other experimental details |
Influence on speed breeding generations | Reference | |
|---|---|---|---|---|---|
| Effects | Generations/year | ||||
| Sorghum (Sorghum bicolor L. cv.) “Moench” |
31.9/21.0°C (day/night) 32.8/21.0°C (day/night) 36.1/21.0°C (day/night) 38.0/21.0°C (day/night) |
14 h·d−1 photoperiod, 600 µmol·m−2·s−1 |
Average seed set percentage decreased significantly from 80% and 69% to 59% and 31% as maximum temperature increased across the four temperature regimes. | Not available | Singh et al., 2015 |
| Grain amaranths (Amaranthus spp.) |
35°C/30°C, 16-h day length 30°C/25°C, 8-h day length |
Single-nucleotide polymorphism (SNP) markers |
Maintained at 30°C induced early flowering 4 weeks after planting and allowing amaranth to complete one breeding generation for 2 months, while approximately 6 months are needed to take in the field. | 6 generations per year |
Stetter et al., 2016 |
| Early flowering pea cultivars (Pisum sativum L. cv.) “PBA Twilight” |
20/18°C (day/night) 24/20°C (day/night) |
13–14 h·d−1 photoperiod, 600 µmol·m−2·s−1, in vivo/in vitro SSD | Increasing temperature resulting in early flowering by 6.58–10 days. Growing plants under optimized temperature 24/20°C (day/night) allowed up to 2.5 times faster floral onset compared to field conditions and up to 1.7 times compared to the conventional SSD system across the entire range of pea genotypes tested. | Not available | Ribalta et al., 2017 |
| Mid-flowering pea cultivars (Pisum sativum L. cv.) “PBA Pearl” | |||||
| Late-flowering pea cultivars (Pisum sativum L. cv.) “Kaspa” | |||||
4.5. CO2 concentrations
Carbon dioxide (CO2) serves as the principal substrate for both photosynthesis and photorespiration in higher plants, thereby playing a pivotal role as a predominant greenhouse gas that regulates crop growth and development. Plant growth and flowering exhibit sensitivity to varying CO2 levels, whose response depends on the species and environmental conditions (McGrath and Lobell, 2013). Overall, elevated concentrations of CO2 have generally facilitated expedited plant growth and hastened the transition from vegetative to reproductive stages across most species (Jagadish et al., 2016). This effect is particularly pronounced among C3 plants, which exhibit greater sensitivity to heightened CO2 levels compared to C4 plants. Supplemental CO2 or the remaining higher CO2 concentration in growth chambers contributes to the reduction in the days to flowering of rice and Arabidopsis (Ohnishi et al., 2011; Li et al., 2014; Tanaka et al., 2016) ( Table 6 ). Regulation of CO2 level often combines with other optimal light, temperature, and innovative breeding technologies (i.e., embryo rescue, biotron breeding system, and using immature seeds) to shorten the breeding cycle length in the controlled environment (Tanaka et al., 2016; Nagatoshi and Fujita, 2019).
Table 6.
Effect of CO2 concentrations on crop speed breeding.
| Plant species | CO2 concentrations | Other experimental details | Influence on speed breeding generations | Reference | |
|---|---|---|---|---|---|
| Effects | Generations/year | ||||
| Arabidopsis thaliana | Ambient CO2 (380 ppm), low CO2 (100 ppm) | 8 h·d−1 photoperiod, 150 µmol·m−2·s−1, 70% relative humidity, 21°C | Flowering time was delayed by 7 days under low CO2 (100 ppm) compared to normal CO2 (380 ppm) conditions | Not available | Li et al., 2014 |
| Rice (Oryza sativa cv. Nipponbare) |
Supplemental 475 ppm CO2 | 30°C/25°C, light/dark, 14 h·d−1 photoperiod, regulation of CO2 level, embryo rescue, removal of tillers | Mean flowering time was 6 d earlier under regulated CO2 conditions compared with no CO2 regulation. Shorten the generation time of cv. Nipponbare plants to approximately 2 months. | 6 generations per year | Ohnishi et al., 2011 |
| Rice (Oryza sativa L. cv. “Nipponbare”) |
Supplemental 600 ppm CO2 | 27°C/25°C, light/dark, 14 h·d−1 photoperiod, 230 µmol·m−2·s−1. Biotron breeding system (BBS), without requiring tiller removal |
Higher CO2 treatment shortened the days to heading by 2.7 days and 9.3 days in “Nipponbare” and “Yamadawara”, respectively, and shortened the generation cycle of rice to less than 90 days, enabling 4 generations of rice to be obtained per annum, instead of only 1–2 generations per year in the field and/or greenhouse. | 4 generations per year | Tanaka et al., 2016 |
| Rice (Oryza sativa L. cv. “Yamadawara”) | |||||
| Soybean (Glycine max L. cv. “Merr”) |
Supplemental CO2 >400 ppm | 14 h·d−1 photoperiod, 27°C/25°C, light/dark, use of immature seeds | CO2 supplementation promoted the number of high-quality flowers and crossing efficiency, but did not affect the days to flowering. Utilizes CO2 in combination with optimal light shortened the breeding cycle length from 102–132 d to 70 d, which permits 4–5 generations a year compared to 1–2 generations on the field. | 4–5 generations per year |
Nagatoshi and Fujita, 2019 |
4.6. Density of plant populations
Planting density is an important agronomic management, and optimization of density will determine the productivity and economic returns of plant factory with artificial lighting (PFAL). Higher planting density promotes shoot positioned upward and leads to the elongation of stems and leaves to outcompete its neighbors for enough sunlight, ultimately accelerating the transition from a vegetative stage to a reproductive stage and triggering early flowering and maturation (Warnasooriya and Brutnell, 2014). Additionally, higher cultivation density provides more sufficient leaf area to absorb sunlight efficiently or improve symbiotic nitrogen fixation by root nodules, thus benefiting early flowering or improving grain yield (Abubaker, 2008; Makoi et al., 2009; Biosci et al., 2013; Rahmanet, 2019; Asemanrafat and Honar, 2023) ( Table 7 ). In contrast, the greater availability of fertilizers per plant due to low plant density results in more vigorous vegetative growth and late flowering (Abubaker, 2008). However, biomass increase was not linear with the planting density. Excessive planting density results in an intra-specific competition for space, nutrients, and light, causing the source–sink ratio to be not reasonable and ultimately hinder plant growth and development (Khan et al., 2017).
Table 7.
Effect of plant density on crop speed breeding.
| Plant species | Density of plant populations | Other experimental details | Influence on speed breeding generations | Reference | |
|---|---|---|---|---|---|
| Effects | Generations/year | ||||
| Bean (Phaseolus vulgaris L.) |
Planting densities (10*30 cm, 20*30 cm, 30*30 cm, 40*30 cm, 50*30 cm, 60*30 cm) | 8 h·d−1 photoperiod, 150 µmol·m−2·s−1, 70% relative humidity, 21°C | Planting density of 20*30 cm results in the highest yields compared to other planting densities. Greater availability of fertilizers per plant due to low plant density results in more vigorous vegetative growth and late flowering. | Not available | Abubaker, 2008 |
| Cowpea (Vigna unguiculata L. cv. “Walp”) |
High plant density: row-to-row spacing of 60 cm, and plant-to-plant distance of 20 cm. Low plant density: row-to-row spacing of 90 cm, and plant-to-plant distance of 40 cm |
Not available | The earliest flowering date was observed in high-density cropping that improved symbiotic nitrogen fixation by root nodules in cowpea. | Not available | Makoi et al., 2009 |
| Spotted bean (Phaseolus vulgaris L.) |
15, 25, 35, and 45 plants/m2 | Not available | The maximum grain yield was related to the density of 45 plants/m2 while the minimum yield was in the density of 25 plants/m2; grain yield increased with plant density increasing might be attributed to sub branches, which are placed at the lower part of the plant, exerting obvious influence on biological yield increase. | Not available | Biosci et al., 2013 |
| Rice (Oryza sativa L.) |
5 cm × 5 cm spacing (over 4,000,000 plants/hectare), 20 cm × 15 cm spacing (300,000 plants/hectare) |
Not available | A high density triggered early flowering, and shortened the growth duration of rice plants compared to the traditional plant density. | Not available | Rahmanet, 2019 |
| Red beans (Phaseolus vulgaris L. cv. Akhtar) |
Row spacing of 5 cm, 10 cm, and 15 cm | Not available | Higher density (row spacing of 5 cm, 66 plants/m2) provides more sufficient leaf area to absorb sunlight efficiently and achieved the highest yield when compared to the row spacing of 10 and 15 cm, whose plant density was 33 and 22 plants/m2, respectively. | Not available | Asemanrafat and Honar, 2023 |
4.7. Nutrition and hormones
Flowering is an important phase in the life cycle of plants, which is profoundly impacted by numerous exogenous and endogenous factors. Five different pathways, namely, photoperiod, vernalization, gibberellin acids (GAs), autonomous, and endogenous pathways, have been reported to regulate flowering time (Mouradov et al., 2002; Blümel et al., 2015). Five major types of plant hormones, namely, auxin, gibberellin, cytokinin, ethylene, and abscisic acid, are important to induce flowering and seeding as well as germination of immature seed in vitro working together or independently (Bermejo et al., 2016). Exogenous application of GAs accelerated flowering in wild-type Arabidopsis (Blázquez et al., 2015). Additionally, cytokinins (5.7 μM) in conjunction with auxin (2.3 μM) led to the highest rate of flowering (100%) and seeding (91%) with the shortest flowering time (32 d) in lentil and faba bean; this combination promoted plant flowering and seeding rates by 4.9 d in advance (Mobini et al., 2015). Moreover, applying 10–5 M of 6-benzylaminopurine (BAP) accelerated flowering in faba bean, with the generation time reduced by 20–24 days (Mobini et al., 2020). Despite the widespread demonstration of phytohormone-mediated flowering regulation potential, plant hormone application in breeding is constrained by several limitations. On the one hand, plant hormones exhibit intricate and extensive regulatory effects on growth and development, potentially leading to unanticipated physiological and morphological changes during the regulation of flowering. This complexity poses challenges for the precise control of hormone-mediated regulation in breeding and may result in unforeseen side effects. On the other hand, phytohormone-mediated flowering regulation is heavily influenced by environmental factors such as light, temperature, and water availability, leading to inconsistent effects in varying growth environments and limiting its stability and reliability in breeding practice. Additionally, the impact of hormones on genetic stability and safety further discourages their widespread use in breeding practices.
Among essential nutrients, nitrogen (N), phosphorus (P), and potassium (K) are the most prominent nutrients required in greater amounts for the proper growth and development of crops (Ye et al., 2019). Nitrogen (N) as a most abundant essential nutrient plays critical roles in the process of flowering. The influence of N on flowering time greatly varies with different N forms and concentrations. In general, flowering is often accelerated by low N concentration, because limited N concentration enhances the export rate of assimilates from vegetative tissues to grains during the post-anthesis period. In contrast, excessive ammonium N supply delayed flowering by hindering N transport in the phloem at the flowering stage and decreased the efficiency of N remobilization (Marín et al., 2011; Zhang et al., 2021). A hypothetical U-shaped flowering curve was proposed, in that an appropriate concentration of nitrate promotes flowering, while both limited and excessive nitrate supply result in late flowering (Lin and Tsay, 2017). Phosphorus (P) fertilizer is a crucial nutrient for normal plant growth, development and yield, which is taken up by plant roots as inorganic phosphate (Pi) transporters (Muchhal et al., 1996). The flowering of rice and Arabidopsis grown under P deficiency conditions was prolonged (Kant et al., 2011; Jiaying et al., 2022). A longer time to flower was shown in low P levels, which might have contributed to the low gibberellin levels, where P level was positively correlated with gibberellin levels (Jiang et al., 2007). Notably, an antagonistic crosstalk was observed on the flowering between NO3 − and Pi, in which low NO3 − caused earlier flowering while low Pi postponed flowering time in Arabidopsis (Linkohr et al., 2002; Kant et al., 2011). Otherwise, the application of K fertilization led to flowering 1–3 days earlier in rice, and flowering time was faster as the application rate of K fertilizer increased (Ye et al., 2019). Thus, a robust and intelligent water and fertilizer control system needs to be created based on the demand for water and fertilizer for horticultural crops’ SB in a plant factory.
5. Challenges and perspectives
Remarkable breeding approaches in the last few decades have allowed researchers to shorten the breeding cycles, but they were limited in scale and cost and insufficient to cope with the ever-increasing population and radical changes under adverse environmental conditions. Integrating SB in plant factories with biology, information science, and breeding science to accelerate rapid growth, development, flowering, and seeding and establishing a rapid crop breeding technology system are a promising option to shorten the breeding cycle. However, every coin has two sides; thus, SB in plant factories has its advantages and limitations.
5.1. Challenges of speed breeding in plant factories
Tremendous advancements have been achieved in smart breeding recently, which is driven by big data, artificial intelligence, and integrated genomic–environmental prediction, but considerable challenges remain to be addressed for SB in plant factories. Firstly, the high cost of energy and considerable investment in setup and operation are major drawbacks of an artificially lighted plant factory. The electricity cost typically accounts for 25% of the total production cost (Kozai and Niu, 2015b), with the lighting, air conditioning, and other information control system depending on electricity. Advances in LED technology have provided more efficient power usage and reduced heat than other lighting types. Investing in solar panels is a good strategy to offset the erratic electricity supply in SB in plant factories.
Secondly, there is a lack of rapid vernalization techniques for long-day crops, and determining the short photoperiod for short-day crops remains challenging. Therefore, longer time needs to be taken to obtain the optimal environmental conditions (light intensity, light quality, temperature, etc.) for the rapid generation advancement of target crops.
The expression of the same phenotypes in both controlled environments and field conditions because of genotype × environment interactions is considered a challenge in SB. Thus, smart breeding driven by big data, artificial intelligence, and integrated genomic–enviromic prediction is an area that requires further enhancement in the future. Although phenotyping technology and platform have been widely applied in controlled environments for crop genetic improvement and breeding, compared with genomics, transcriptomics, and proteomics, phenomics is lagging behind. At present, a lack of in vivo dynamic detection technology for crop phenotypes, a poor ability to obtain high-throughput crop phenotypes, and the unclear relationship between genotype, phenotype, and environmental metrics limit the acquisition and analysis of crop phenotypes in plant factories. Therefore, with the targeted goal of horticultural crop breeding, a collaborative breeding framework of various branches of science is paramount to speed up breeding to accelerate crop improvement for a rapidly expanding global population.
5.2. Smart breeding: speed breeding in plant factories integrates with innovative breeding technologies
In the short-term or long-term future, SB in plant factories where the light environment (light quality, light intensity, and photoperiod), temperature, and nutrient are modulated accurately and efficiently holds promise to relieve the long-standing problem of lengthy crop breeding cycles. Notably, processes as diverse as seed germination, flower bud differentiation, flowering and fruiting, and seed development are closely regulated by light, temperature, phytohormone, and nutrition, and accelerating the above process is conducive to shortening the growth cycle of crops using the conditions provided by the smart system in plant factories.
Interdisciplinary strategies consisting of plant breeding, genome sequencing, genomic selection (GS), and CRISPR-based genome editing help plant breeders improve existing crops and develop new crops with good agronomic traits by minimizing time, cost, and space and maximizing resource efficiency. SB in plant factories is a promising approach that allows breeders to rapidly develop lines with the desired trait by advancing generations quickly within a controlled environment. Numerous steps are required for SB to effectively work in plant factories. Optimization of light formula and nutrient solution formula for species specificity and the establishment of rapid generation advancement technology within plant factories were the cornerstone for SB in plant factories. Moreover, overcoming physiological seed dormancy, combined with early seed harvest, is critical to reduce the length of the breeding cycle.
Phenotypic performance or target trait observation was the first step in plant breeding, and identifying desirable traits for selective breeding has become the crucial problem restricting crop breeding owing to the presence of masking. Recent advances in in vivo imaging, hyperspectral imaging, and graphic and spectral analysis technology, which dramatically help to measure crop phenotypic traits rapidly and nondestructively in controlled environments, make high-throughput phenotyping a powerful approach to solving future crop breeding problems.
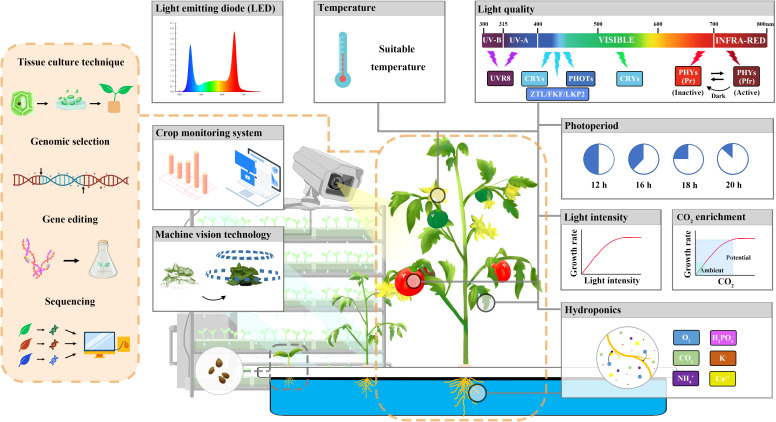
Overall, the integration of innovative breeding technologies such as embryo rescue and immature seed harvesting, TILLING, gene editing, high-throughput phenotyping and genotyping, genomic selection, marker-assisted selection (MAS), machine learning, deep learning, and omics technologies to form a smart breeding platform offers an attractive opportunity to promote the transformation of breeding technology from the traditional “experience breeding” to a directional and more efficient “precision breeding” ( Figure 2 ).
Figure 2.
Vegetable speed breeding in a plant factory.
Author contributions
RH: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. JJ: Conceptualization, Data curation, Validation, Writing – original draft. KL: Conceptualization, Methodology, Writing – original draft. JS: Conceptualization, Supervision, Validation, Writing – review & editing. XL: Conceptualization, Methodology, Writing – original draft. YL: Conceptualization, Methodology, Writing – original draft. SZ: Data curation, Investigation, Methodology, Writing – original draft. MZ: Conceptualization, Methodology, Writing – review & editing. YH: Conceptualization, Methodology, Writing – original draft. HL: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research is supported by a grant from the Key Research and Development Program of Ningxia (2021BBF02024) and the National Key Research and Development Program of China (2017YFE0131000).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abubaker S. (2008). Effect of plant density on flowering date, yield and quality attribute of bush beans (Phaseolus Vulgaris L.) under center pivot iirrigation system. Am. J. Agric. Biol. Sci. 3, 666–668. doi: 10.3844/ajabssp.2008.666.668 [DOI] [Google Scholar]
- Al Murad M., Razi K., Jeong B. R., Samy P. M. A., Muneer S. (2021). Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustain 13, 1–25. doi: 10.3390/su13041985 [DOI] [Google Scholar]
- Alotaibi M. (2023). Climate change, its impact on crop production, challenges, and possible solutions. Not. Bot. Horti Agrobot. Cluj-Napoca. 51, 1–39. doi: 10.15835/nbha51113020 [DOI] [Google Scholar]
- Asemanrafat M., Honar T. (2023). Effect of plant density and different irrigation strategieson crop yield and canopy cover of red beans, Phaseolusvulgaris L. cv. Akhtar. Iran Agric. Res. 36, 13–22. doi: 10.22099/iar.2017.4145 [DOI] [Google Scholar]
- Bermejo C., Gatti I., Cointry E. (2016). In vitro embryo culture to shorten the breeding cycle in lentil (Lens culinaris Medik). Plant Cell. Tissue Organ Cult. 127, 585–590. doi: 10.1007/s11240-016-1065-7 [DOI] [Google Scholar]
- Bhatta M., Sandro P., Smith M. R., Delaney O., Voss-Fels K. P., Gutierrez L., et al. (2021). Need for speed: manipulating plant growth to accelerate breeding cycles. Curr. Opin. Plant Biol. 60, 101986. doi: 10.1016/j.pbi.2020.101986 [DOI] [PubMed] [Google Scholar]
- Biosci I. J., Ardakani L. G., Farajee H. (2013). The effect of water stress and plant density on yield and some physiologic traits of spotted bean (Phaseolus vulgaris L.), cultivar Talash in Yasouj region. Int. J. Biosci. 3, 175–184. doi: 10.12692/ijb/3.9.175-184 [DOI] [Google Scholar]
- Blázquez M. A., Green R., Nilsson O., Sussman M. R., Weigel D., The S., et al. (2015). Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell 10, 791–800. doi: 10.1105/tpc.10.5.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümel M., Dally N., Jung C. (2015). Flowering time regulation in crops-what did we learn from Arabidopsis? Curr. Opin. Biotechnol. 32, 121–129. doi: 10.1016/j.copbio.2014.11.023 [DOI] [PubMed] [Google Scholar]
- Brazaityte A., Viršile A., Samuoliene G., Jankauskiene J., Sakalauskiene S., Sirtautas R., et al. (2016). Light quality: Growth and nutritional value of microgreens under indoor and greenhouse conditions. Acta Hortic. 1134, 277–284. doi: 10.17660/ActaHortic.2016.1134.37 [DOI] [Google Scholar]
- Cerdán P. D., Chory J. (2003). Regulation of flowering time by light quality. Nature 423, 881–885. doi: 10.1038/nature01636 [DOI] [PubMed] [Google Scholar]
- Cha J. K., O’Connor K., Alahmad S., Lee J. H., Dinglasan E., Park H., et al. (2022). Speed vernalization to accelerate generation advance in winter cereal crops. Mol. Plant 15, 1300–1309. doi: 10.1016/j.molp.2022.06.012 [DOI] [PubMed] [Google Scholar]
- Chen L., Hao L., Parry M. A. J., Phillips A. L., Hu Y. G. (2014). Progress in TILLING as a tool for functional genomics and improvement of crops. J. Integr. Plant Biol. 56, 425–443. doi: 10.1111/jipb.12192 [DOI] [PubMed] [Google Scholar]
- Chory J. (2010). Light signal transduction: An infinite spectrum of possibilities. Plant J. 61, 982–991. doi: 10.1111/j.1365-313X.2009.04105.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croser J. S., Pazos-Navarro M., Bennett R. G., Tschirren S., Edwards K., Erskine W., et al. (2016). Time to flowering of temperate pulses in vivo and generation turnover in vivo–in vitro of narrow-leaf lupin accelerated by low red to far-red ratio and high intensity in the far-red region. Plant Cell. Tissue Organ Cult. 127, 591–599. doi: 10.1007/s11240-016-1092-4 [DOI] [Google Scholar]
- Demotes-Mainard S., Péron T., Corot A., Bertheloot J., Le Gourrierec J., Pelleschi-Travier S., et al. (2016). Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 121, 4–21. doi: 10.1016/j.envexpbot.2015.05.010 [DOI] [Google Scholar]
- Formighieri C. (2015). “Light saturation of photosynthesis,” in Solar-To-Fuel Conversion in Algae and Cyanobacteria (Springer, Cham, Switzerland: ), 55–58. doi: 10.1007/978-3-319-16730-5 [DOI] [Google Scholar]
- Fukuda N., Ajima C., Yukawa T., Olsen J. E. (2016). Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ. Exp. Bot. 121, 102–111. doi: 10.1016/j.envexpbot.2015.06.014 [DOI] [Google Scholar]
- Gao M., Li Y., Jiang H., He R., Shi R., Song S., et al. (2022). UVA-radiation exposure of different Durations promoted the growth, phytochemicals and glucosinolate biosynthesis of Chinese Kale. Int. J. Mol. Sci. 23, 7619. doi: 10.3390/ijms23147619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanà M. A. (2011). Anther culture for haploid and doubled haploid production. Plant Cell. Tissue Organ Cult. 104, 283–300. doi: 10.1007/s11240-010-9852-z [DOI] [Google Scholar]
- Ghosh S., Watson A., Gonzalez-Navarro O. E., Ramirez-Gonzalez R. H., Yanes L., Mendoza-Suárez M., et al. (2018). Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 13, 2944–2963. doi: 10.1038/s41596-018-0072-z [DOI] [PubMed] [Google Scholar]
- Giliberto L., Perrotta G., Pallara P., Weller J. L., Fraser P. D., Bramley P. M., et al. (2005). Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 37, 199–208. doi: 10.1104/pp.104.051987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Xue X., Chen L., Li J., Wang Z., Zhang Y. (2022). Effects of LEDs light spectra on the growth, yield, and quality of winter wheat (Triticum aestivum L.) cultured in plant factory. J. Plant Growth Regul. 42, 2530–2544. doi: 10.1007/s00344-022-10724-z [DOI] [Google Scholar]
- Haliapas S., Yupsanis T. A., Syros T. D., Kofidis G., Economou A. S. (2008). Petunia x hybrida during transition to flowering as affected by light intensity and quality treatments. Acta Physiol. Plant 30, 807–815. doi: 10.1007/s11738-008-0185-z [DOI] [Google Scholar]
- Harrison D., Da Silva M., Wu C., De Oliveira M., Ravelombola F., Florez-Palacios L., et al. (2021). Effect of light wavelength on soybean growth and development in a context of speed breeding. Crop Sci. 61, 917–928. doi: 10.1002/csc2.20327 [DOI] [Google Scholar]
- Hatfield J. L., Prueger J. H. (2015). Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 10, 4–10. doi: 10.1016/j.wace.2015.08.001 [DOI] [Google Scholar]
- He R., Wei J., Zhang J., Tan X., Li Y., Gao M., et al. (2022). Supplemental blue light frequencies improve ripening and nutritional qualities of tomato fruits. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.888976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey L. T., Hafeez N., Robinson H., Jackson S. A., Leal-Bertioli S. C. M., Tester M., et al. (2019). Breeding crops to feed 10 billion. Nat. Biotechnol. 37, 744–754. doi: 10.1038/s41587-019-0152-9 [DOI] [PubMed] [Google Scholar]
- Holmes M. G., Smith H. (1975). Erratum: The function of phytochrome in plants growing in the natural environment. Nature 256, 72. doi: 10.1038/256072a0 [DOI] [Google Scholar]
- Jackson S. D. (2009). Plant responses to photoperiod. New Phytol. 181, 517–531. doi: 10.1111/j.1469-8137.2008.02681.x [DOI] [PubMed] [Google Scholar]
- Jagadish S. V. K., Bahuguna R. N., Djanaguiraman M., Gamuyao R., Prasad P. V. V., Craufurd P. Q. (2016). Implications of high temperature and elevated CO2 on flowering time in plants. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähne F., Hahn V., Würschum T., Leiser W. L. (2020). Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 133, 2335–2342. doi: 10.1007/s00122-020-03601-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Gao X., Liao L., Harberd N. P., Fu X. (2007). Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis . Plant Physiol. 145, 1460–1470. doi: 10.1104/pp.107.103788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiaying M., Tingting C., Jie L., Weimeng F., Baohua F., Guangyan L., et al. (2022). Functions of nitrogen, phosphorus and potassium in energy status and their influences on rice growth and development. Rice Sci. 29, 166–178. doi: 10.1016/j.rsci.2022.01.005 [DOI] [Google Scholar]
- Jones M. A. (2018). Using light to improve commercial value. Hortic. Res. 5, 47. doi: 10.1038/s41438-018-0049-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami C., Lorrain S., Hornitschek P., Fankhauser C. (2010). Light-regulated plant growth and development. Curr. Topics Dev. Biol. 91, 29–66. doi: 10.1016/S0070-2153(10)91002-8 [DOI] [PubMed] [Google Scholar]
- Kant S., Peng M., Rothstein S. J. (2011). Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis . PloS Genet. 7, e1002021. doi: 10.1371/journal.pgen.1002021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Najeeb U., Wang L., Tan D. K. Y., Yang G., Munsif F., et al. (2017). Planting density and sowing date strongly influence growth and lint yield of cotton crops. F. Crop Res. 209, 129–135. doi: 10.1016/j.fcr.2017.04.019 [DOI] [Google Scholar]
- Kigoni M., Choi M., Arbelaez J. D. (2023). ‘Single-Seed-SpeedBulks:’ a protocol that combines ‘speed breeding’ with a cost-efficient modified single-seed descent method for rapid-generation-advancement in oat (Avena sativa L.). Plant Methods 19, 1–9. doi: 10.1186/s13007-023-01067-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. M., Hwang S. J. (2019). The growth and development of ‘mini chal’ tomato plug seedlings grown under various wavelengths using light emitting diodes. Agronomy 9, 190–205. doi: 10.3390/agronomy9030157 [DOI] [Google Scholar]
- Kozai T., Niu G. (2015. a). Role of the plant factory with artificial lighting (PFAL) in urban areas. Plant Factory 2016, 7–33. doi: 10.1016/B978-0-12-801775-3.00002-0 [DOI] [Google Scholar]
- Kozai T., Niu G. (2015. b). Plant Factory as a resource-efficient closed pplant pproduction ssystem. Plant factory: an indoor vertical farming system for efficient quality food production. Plant Factory 16, 69–90. doi: 10.1016/B978-0-12-801775-3.00004-4 [DOI] [Google Scholar]
- Li Y., Jiang H., Gao M., He R., Liu X., Su W., et al. (2023). Far-red-light-induced morphology changes, phytohormone, and transcriptome reprogramming of Chinese kale (Brassica alboglabra Bailey). Int. J. Mol. Sci. 24, 5563. doi: 10.3390/ijms24065563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu J., Haq N. U., Zhang H., Zhu X. G. (2014). Was low CO2 a driving force of C4 evolution: Arabidopsis responses to long-term low CO2 stress. J. Exp. Bot. 65, 3657–3667. doi: 10.1093/jxb/eru193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Tsay Y. F. (2017). Influence of differing nitrate and nitrogen availability on flowering control in Arabidopsis . J. Exp. Bot. 68, 2603–2609. doi: 10.1093/jxb/erx053 [DOI] [PubMed] [Google Scholar]
- Linkohr B. I., Williamson L. C., Fitter A. H., Leyser H. M. O. (2002). Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 29, 751–760. doi: 10.1046/j.1365-313X.2002.01251.x [DOI] [PubMed] [Google Scholar]
- Liu K., He R., He X., Tan J., Chen Y., Li Y., et al. (2022). Speed breeding scheme of hot pepper through light environment modification. Sustain 14, 1–9. doi: 10.3390/su141912225 [DOI] [Google Scholar]
- Makoi J. H. J. R., Chimphango S. B. M., Dakora F. D. (2009). Effect of legume plant density and mixed culture on symbiotic N2 fixation in five cowpea (Vigna unguiculata L. Walp.) genotypes in South Africa. Symbiosis 48, 57–67. doi: 10.1007/BF03179985 [DOI] [Google Scholar]
- Marín I. C., Loef I., Bartetzko L., Searle I., Coupland G., Stitt M., et al. (2011). Nitrate regulates floral induction in Arabidopsis, acting independently of light, gibberellin and autonomous pathways. Planta 233, 539–552. doi: 10.1007/s00425-010-1316-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mba C. (2013). Induced mutations unleash the potentials of plant genetic resources for food and agriculture. Agronomy 3, 200–231. doi: 10.3390/agronomy3010200 [DOI] [Google Scholar]
- McGrath J. M., Lobell D. B. (2013). Regional disparities in the CO2 fertilization effect and implications for crop yields. Environ. Res. Lett. 8, 14054. doi: 10.1088/1748-9326/8/1/014054 [DOI] [Google Scholar]
- Meng Q., Runkle E. S. (2019). Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. (Amsterdam) 255, 269–280. doi: 10.1016/j.scienta.2019.05.030 [DOI] [Google Scholar]
- Mitache M., Baidani A., Houasli C., Khouakhi K., Bencharki B., Idrissi O. (2023). Optimization of light/dark cycle in an extended photoperiod-based speed breeding protocol for grain legumes. Plant Breed. 4, 1–14. doi: 10.1111/pbr.13112 [DOI] [Google Scholar]
- Mobini S., Khazaei H., Warkentin T. D., Vandenberg A. (2020). Shortening the generation cycle in faba bean (Vicia faba) by application of cytokinin ands cold stress to assist speed breeding. Plant Breed. 139, 1181–1189. doi: 10.1111/pbr.12868 [DOI] [Google Scholar]
- Mobini S. H., Lulsdorf M., Warkentin T. D., Vandenberg A. (2015). Plant growth regulators improve in vitro flowering and rapid generation advancement in lentil and faba bean. Vitr. Cell. Dev. Biol. - Plant 51, 71–79. doi: 10.1007/s11627-014-9647-8 [DOI] [Google Scholar]
- Monostori I., Heilmann M., Kocsy G., Rakszegi M., Simon-sarkadi L., Harnos N., et al. (2018). LED lighting–modification of growth, metabolism, yield and flour composition in wheat by spectral quality and intensity. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouradov A., Cremer F., Coupland G. (2002). Control of flowering time: Interacting pathways as a basis for diversity. Plant Cell. 14, 111–130. doi: 10.1105/tpc.001362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhal U. S., Pardo J. M., Raghothama K. G. (1996). Phosphate transporters from the higher plant Arabidopsis thaliana . Proc. Natl. Acad. Sci. U. S. A. 93, 10519–10523. doi: 10.1073/pnas.93.19.10519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatoshi Y., Fujita Y. (2019). Accelerating soybean breeding in a CO2-supplemented growth chamber. Plant Cell Physiol. 60, 77–84. doi: 10.1093/pcp/pcy189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie W. F., Li Y., Chen Y., Zhou Y., Yu T., Zhou Y., et al. (2023). Spectral light quality regulates the morphogenesis, architecture, and flowering in pepper (Capsicum annuum L.). J. Photochem. Photobiol. B Biol. 241, 112673. doi: 10.1016/j.jphotobiol.2023.112673 [DOI] [PubMed] [Google Scholar]
- O’Connor D. J., Wright G. C., Dieters M. J., George D. L., Hunter M. N., Tatnell J. R., et al. (2013). Development and application of speed breeding technologies in a commercial peanut breeding program. Peanut Sci. 40, 107–114. doi: 10.3146/PS12-12.1 [DOI] [Google Scholar]
- Oh W. (2015). Effects of temperature, photoperiod and light intensity on growth and flowering in Eustoma grandiflorum. Korean J. Hortic. Sci. Technol. 33, 349–355. doi: 10.7235/hort.2015.15023 [DOI] [Google Scholar]
- Ohnishi T., Yoshino M., Yamakawa H., Kinoshita T. (2011). The biotron breeding system: A rapid and reliable procedure for genetic studies and breeding in rice. Plant Cell Physiol. 52, 1249–1257. doi: 10.1093/pcp/pcr066 [DOI] [PubMed] [Google Scholar]
- Omar I. (2020). Application of extended photoperiod in lentil: Towards acceleratedgenetic gain in breeding for rapid improved variety development. Moroccan J. Agric. Sci. 1, 14–19. [Google Scholar]
- Ortiz R., Trethowan R., Ferrara G. O., Iwanaga M., Dodds J. H., Crouch J. H., et al. (2007). High yield potential, shuttle breeding, genetic diversity, and a new international wheat improvement strategy. Euphytica 157, 365–384. doi: 10.1007/s10681-007-9375-9 [DOI] [Google Scholar]
- Paradiso R., Proietti S. (2022). Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 41, 742–780. doi: 10.1007/s00344-021-10337-y [DOI] [Google Scholar]
- Pardey P. G., Beddow J. M., Hurley T. M., Beatty T. K. M., Eidman V. R. (2014). A bounds analysis of world food futures: Global agriculture through to 2050. Aust. J. Agric. Resour. Econ. 58, 571–589. doi: 10.1111/1467-8489.12072 [DOI] [Google Scholar]
- Park Y. G., Muneer S., Soundararajan P., Manivnnan A., Jeong B. R. (2017). Light quality during night interruption affects morphogenesis and flowering in geranium. Hortic. Environ. Biotechnol. 58, 212–217. doi: 10.1007/s13580-017-0246-6 [DOI] [Google Scholar]
- Rahmanet M. A. (2019). Field rapid generation advance: An effective technique for industrial scale rice breeding program. Exp 47, 2659–2670. [Google Scholar]
- Ribalta F. M., Pazos-Navarro M., Nelson K., Edwards K., Ross J. J., Bennett R. G., et al. (2017). Precocious floral initiation and identification of exact timing of embryo physiological maturity facilitate germination of immature seeds to truncate the lifecycle of pea. Plant Growth Regul. 81, 345–353. doi: 10.1007/s10725-016-0211-x [DOI] [Google Scholar]
- Saxena K., Saxena R. K., Varshney R. K. (2017). Use of immature seed germination and single seed descent for rapid genetic gains in pigeonpea. Plant Breed. 136, 954–957. doi: 10.1111/pbr.12538 [DOI] [Google Scholar]
- Shrivastava P., Kumar R. (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 22, 123–131. doi: 10.1016/j.sjbs.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu V., Bernier-English V., Lamontagne-Drolet M., Gravel V. (2022). Effect of light quality and extended photoperiod on flower bud induction during transplant production of day-neutral strawberry cultivars. Can. J. Plant Sci. 102, 356–367. doi: 10.1139/cjps-2021-0081 [DOI] [Google Scholar]
- Singh V., Nguyen C. T., van Oosterom E. J., Chapman S. C., Jordan D. R., Hammer G. L. (2015). Sorghum genotypes differ in high temperature responses for seed set. F. Crop Res. 171, 32–40. doi: 10.1016/j.fcr.2014.11.003 [DOI] [Google Scholar]
- Smith H. L., Mcausland L., Murchie E. H. (2017). Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 68, 2099–2110. doi: 10.1093/jxb/erx098 [DOI] [PubMed] [Google Scholar]
- Song Y., Duan X., Wang P., Li X., Yuan X., Wang Z., et al. (2022). Comprehensive speed breeding: a high-throughput and rapid generation system for long-day crops. Plant Biotechnol. J. 20, 13–15. doi: 10.1111/pbi.13726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetter M. G., Zeitler L., Steinhaus A., Kroener K., Biljecki M., Schmid K. J. (2016). Crossing methods and cultivation conditions for rapid production of segregating populations in three grain amaranth species. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Badger M. R. (2011). Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 16, 53–60. doi: 10.1016/j.tplants.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Tanaka J., Hayashi T., Iwata H. (2016). A practical, rapid generation-advancement system for rice breeding using simplified biotron breeding system. Breed. Sci. 66, 542–551. doi: 10.1270/jsbbs.15038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdaguer D., Jansen M. A. K., Llorens L., Morales L. O., Neugart S. (2017). UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 255, 72–81. doi: 10.1016/j.plantsci.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Wang S., Liu X., Liu X., Xue J., Ren X., Zhai Y., et al. (2022). The red/blue light ratios from light-emitting diodes affect growth and flower quality of Hippeastrum hybridum ‘Red Lion.’. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1048770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanga M. A., Shimelis H., Mashilo J., Laing M. D. (2021). Opportunities and challenges of speed breeding: A review. Plant Breed. 140, 185–194. doi: 10.1111/pbr.12909 [DOI] [Google Scholar]
- Warnasooriya S. N., Brutnell T. P. (2014). Enhancing the productivity of grasses under high-density planting by engineering light responses: From model systems to feedstocks. J. Exp. Bot. 65, 2825–2834. doi: 10.1093/jxb/eru221 [DOI] [PubMed] [Google Scholar]
- Watson A., Ghosh S., Williams M. J., Cuddy W. S., Simmonds J., Rey M. D., et al. (2018). Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 4, 23–29. doi: 10.1038/s41477-017-0083-8 [DOI] [PubMed] [Google Scholar]
- Wimalasekera R. (2019). Effect of light intensity on photosynthesis. Photosynthesis productivity Environ. stress 5, 65–73. doi: 10.1002/9781119501800 [DOI] [Google Scholar]
- Yamada A., Tanigawa T., Suyama T., Matsuno T., Kunitake T. (2009). Red:far-red light ratio and far-red light integral promote or retard growth and flowering in Eustoma grandiflorum (Raf.) Shinn . Sci. Hortic. (Amsterdam) 120, 101–106. doi: 10.1016/j.scienta.2008.09.009 [DOI] [Google Scholar]
- Ye T., Li Y., Zhang J., Hou W., Zhou W., Lu J., et al. (2019). Nitrogen, phosphorus, and potassium fertilization affects the flowering time of rice (Oryza sativa L.). Glob. Ecol. Conserv. 20, e00753. doi: 10.1016/j.gecco.2019.e00753 [DOI] [Google Scholar]
- Yoshida H., Mizuta D., Fukuda N., Hikosaka S., Goto E., et al. (2016). Effects of varying light quality from single-peak blue and red light-emitting diodes during nursery period on flowering , photosynthesis , growth , and fruit yield of everbearing strawberry. Plant Biol. 276, 267–276. doi: 10.5511/plantbiotechnology.16.0216a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Lu Y., Zhu J. K., Botella J. R. (2021). Genome editing for plant research and crop improvement. J. Integr. Plant Biol. 63, 3–33. doi: 10.1111/jipb.13063 [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang Y., Song S., Su W., Hao Y., Liu H. (2020). Supplementary red light results in the earlier ripening of tomato fruit depending on ethylene production. Environ. Exp. Bot. 175, 104044. doi: 10.1016/j.envexpbot.2020.104044 [DOI] [Google Scholar]
- Zhang S., Zhang Y., Li K., Yan M., Zhang J., Yu M., et al. (2021). Nitrogen mediates flowering time and nitrogen use efficiency via ffloral rregulators in rice. Curr. Biol. 31, 671–683. doi: 10.1016/j.cub.2020.10.095 [DOI] [PubMed] [Google Scholar]