Abstract
Background
Although cavitating ultrasonic aspirators are commonly used in neurosurgical procedures, the suitability of ultrasonic aspirator-derived tumor material for diagnostic procedures is still controversial. Here, we explore the feasibility of using ultrasonic aspirator-resected tumor tissue to classify otherwise discarded sample material by fast DNA methylation-based analysis using low pass nanopore whole genome sequencing.
Methods
Ultrasonic aspirator-derived specimens from pediatric patients undergoing brain tumor resection were subjected to low-pass nanopore whole genome sequencing. DNA methylation-based classification using a neural network classifier and copy number variation analysis were performed. Tumor purity was estimated from copy number profiles. Results were compared to microarray (EPIC)-based routine neuropathological histomorphological and molecular evaluation.
Results
19 samples with confirmed neuropathological diagnosis were evaluated. All samples were successfully sequenced and passed quality control for further analysis. DNA and sequencing characteristics from ultrasonic aspirator-derived specimens were comparable to routinely processed tumor tissue. Classification of both methods was concordant regarding methylation class in 17/19 (89%) cases. Application of a platform-specific threshold for nanopore-based classification ensured a specificity of 100%, whereas sensitivity was 79%. Copy number variation profiles were generated for all cases and matched EPIC results in 18/19 (95%) samples, even allowing the identification of diagnostically or therapeutically relevant genomic alterations.
Conclusion
Methylation-based classification of pediatric CNS tumors based on ultrasonic aspirator-reduced and otherwise discarded tissue is feasible using time- and cost-efficient nanopore sequencing.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11060-024-04702-6.
Keywords: Nanopore sequencing, Pediatric brain cancer, Ultrasonic aspirator
Introduction
Ultrasonic aspirator devices are frequently used in pediatric neurosurgery for efficient microsurgical resection of brain tumors while minimizing tissue damage to surrounding healthy brain [1]. With ultrasonic aspirator, tumor tissue is fragmented in situ by ultrasound-induced vibration and tissue debris is aspirated using suction. To date, ultrasonic aspirator tissue specimens have not been used for routine neuropathological examinations. At the same time, molecular profiling is increasingly used and required in addition to histomorphology for diagnostic workup and comprehensive characterization of pediatric brain tumors [2]. In particular, molecular classification based on DNA methylation signatures has proven to be a powerful and elegant unbiased approach to identifying tumor type [3] and has been adopted in the current World Health Organization (WHO) classification of central nervous system (CNS) tumors [4]. For DNA extraction, however, additional tissue is needed which may be scarce in pediatric neurosurgery. While for histological examination it appears necessary to preserve the integrity of the tissue, DNA methylation profiling (as any nucleic acid-based method) only relies on the integrity of tumor DNA. Repurposing ultrasonic aspirator tissue specimens as a source of tumor DNA for molecular diagnostics would maximize use of tumor tissue. To date, only detection of focal amplifications [5] and gene expression profiling by RNA sequencing [6] in ultrasonic aspirator tissue samples have been explored.
The growth patterns of pediatric brain tumors differ from those of adult tumors in that they are more likely to spread in the neuraxis [7]. Furthermore, highly aggressive rare embryonal and sarcomatous pediatric CNS tumors for which there are limited therapeutic recommendations and for which immediate initiation of therapy is essential have only recently been molecularly redefined [8]. The overall time to integrated diagnosis in pediatric oncology is therefore of considerable importance, and any delay in initiating first-line therapy may be critical. Indeed, the presence of molecular markers defining risk groups in therapy trials also leads to different therapeutic approaches.
Recently, we have demonstrated, that low-pass nanopore whole genome sequencing (WGS) is non-inferior to microarray-based DNA methylation profiling of CNS tumors [9]. In addition, real-time analysis is feasible, enabling a reliable intraoperative diagnosis within a surgically relevant time frame at low cost [10]. In addition, adaptive sequencing allows to enrich genomic regions of interest in the same WGS run to detect clinically relevant SNV and SV [11].
In the present study, we studied whether DNA methylation-based classification can be reliably performed using DNA from tumor tissue fragments obtained by ultrasonic aspirator devices using low-pass nanopore whole genome sequencing in order to overcome time-consuming tissue processing and maximize use of limited material in pediatric neuro-oncology.
Methods
Study design
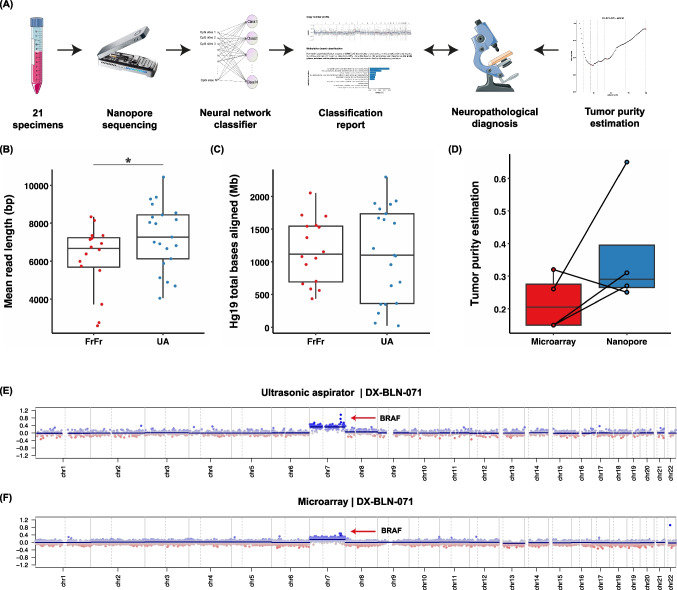
We conducted a prospective, proof-of-concept single-center study using ultrasonic aspirator tissue specimens for molecular characterization of pediatric CNS tumors using nanopore WGS (Fig. 1 A). All patients < 18 years who underwent surgery for tumor resection using an ultrasonic aspiration device at the Department of Pediatric Neurosurgery, Charité-Universitätsmedizin Berlin, Germany, between February 6th, 2020, and October 5th, 2020, were screened. Informed written consent was obtained from patients and/or guardians. The study was approved by the local ethics committee (Charité –Universitätsmedizin Berlin, Berlin, Germany; EA2/041/18) and performed according to the guidelines for Good Scientific Practice. Ultrasonic aspirator tissue samples taken with the LEVICS device (Söring, Quickborn, Germany), which are normally discarded after surgery, are collected using a bronchoalveolar lavage trap, which is connected to the suction tubing coming from the sonotrode instruments and connected to the suction reservoir. Thereby about 5 ml of fluid including fragmented tumor tissue could be collected. In parallel, regularly resected tumor tissue was processed for routine neuropathological procedures including phenotypic-genotypic diagnostics. Pseudonymized study data were recorded using REDCap [12], which was provided by the Berlin Institute of Health’s Clinical Research Unit in a certified computing environment.
Fig. 1.
(A) Overview of the study design including workflow using ultrasonic aspirator tissue specimens for nanopore sequencing for DNA methylation-based classification using a neural network classifier and copy number variation analysis, comparison to microarray-based routine neuropathological profiling and assessment of tumor purity by absolute copy number estimation using ACE. Suitability of ultrasonic aspirator-derived tumor tissue (UA) for nanopore sequencing: (B,C) Comparison of (B) mean read length and (C) read yield obtained from standard nanopore protocol using fresh-frozen (FrFr) tumor tissue vs. ultrasonic aspirator-derived sample material indicates similar sequencing performance. Asterisk indicates p-value < 0.05 for group comparison by Student’s t-test. (D) In silico tumor purity estimation between nanopore ultrasonic aspirator tissue samples and microarray FFPE tissue. (E,F) Representative illustration of matched copy number variation profiles obtained from (E) ultrasonic aspirator tissue samples and nanopore sequencing and (F) FFPE tumor tissue subjected to EPIC microarray (850 K). Red marker indicates a low-level gain of the BRAF locus suggestive of a BRAF gene fusion
Ultrasonic aspirator tissue sample processing
Fresh ultrasonic aspiratior fluid aliquots were centrifuged at 1.000 rpm for 5 min, supernatant was discarded and pellets stored at -40 °C. DNA was extracted from ~ 25 mg thawed aspirate and purified using the DNeasy Blood & Tissue Kit (Qiagen, NL). Based on the 260/280 ratio (NanoDrop, Thermo Fisher, USA), DNA quality was determined, followed by DNA quantification with a Qubit 4.0 fluorometer using a dsDNA BR Assay (Thermo Fisher, USA).
Nanopore low-pass whole genome sequencing
All samples were subjected to low-pass whole genome sequencing as described previously [9]. Preprocessing of raw data for sequencing was performed using the publicly available nanoDx pipeline (https://gitlab.com/pesk/nanoDx, v0.5.1). Briefly, basecalling of nanopore FAST5 raw data was performed using guppy v5.0.16 (Oxford Nanopore Technologies, UK) and aligned to the hg19 human reference genome using minimap2 v2.15 [13]. In order to assess the feasibility of ultrasonic aspirator-derived nanopore sequencing, the aligned sequencing data was normalized to a six hour sequencing window and were compared to a previously published dataset of 16 brain tumor samples obtained during routine nanopore sequencing from fresh-frozen tumor tissue [9]. DNA methylation was assessed using nanopolish v0.11.1 [14]. After binarization of beta values with threshold of ≥ 0.6 [9], features with zero variance were filtered out, leading to 366,263 CpG sites retained. These were used to train the neural network model with randomly masked features [15]. PyTorch, an open source deep-learning framework, was used to develop the model [16]. To obtain class probability estimates that can be used to guide diagnostic decision-making, a normalization function and a Softmax layer was used to convert the raw values into a probability that measures the confidence in the brain tumor class assignment (the classification score). For reporting on methylation class family (MCF) level, scores of individual methylation classes to the same family were added [9]. Complementarily, data was visualized using t-distributed Stochastic Neighborhood Embedding (t-SNE) [17].
CNS tumor classification
All cases were classified according to the 2016 WHO CNS classification during routine neuropathological examination at the Department of Neuropathology, Charité Universitätsmedizin Berlin. The recent 2021 edition [4] was not yet available during the study period of this patient cohort. Nanopore classification results were compared to the reference diagnosis as well as microarray-based classification results of the same tumor considering the established cut-off values for the probability score.
Copy number analysis
Copy number variation (CNV) analysis from nanopore WGS data was performed using the QDNAseq package v1.8.0 and R/Bioconductor v3.3 as described before [18, 19]. To account for region- and technology-specific artifacts, public nanopore WGS data for the NA12878 human reference genome were processed and subtracted from the normalized bin counts of the tumor samples for case reports. To estimate tumor purity in aneuploid tumors, absolute copy number estimation of nanopore- and microarray-based data was performed using the ACE software package (v1.6.0) [20]. All estimates were verified manually.
Methylation array processing
Infinium MethylationEPIC BeadChip microarrays (Illumina) were used to obtain genome-wide DNA methylation profiles for tumor samples during routine neuropathological diagnostic examination. Data were generated following the manufacturer’s protocol at the Department of Neuropathology, Charité—Universitätsmedizin Berlin, using > 250 ng of DNA from FFPE tissues as input material. For classification, IDAT files were uploaded to the public Heidelberg brain tumor classifier available at https://www.molecularneuropathology.org (v.11b4).
Statistical analysis
Reproducible and easy-to-deploy pipelines were implemented using snakemake (v.7.15.2) [21]. Data analysis was mainly performed using R (v.4.0.2). Figures were designed using ggplot2 (v.3.3.2). Statistical analyses were performed using IBM SPSS® 29 (Armonk, N.Y., USA).
Data and code availability
The nanoDx analysis pipeline for end-to-end analysis of nanopore WGS data is available at https://gitlab.com/pesk/nanoDx. Source code to reproduce all analyses and sequencing data based figures in this work is provided at https://gitlab.com/pesk/nanoCUSA. Raw sequencing data have been deposited at the European Genome-phenome archive (accession no. EGA50000000180), while methylation microarray data and nanopore methylation calls are available from Gene Omnibus Express (accession no. GSE255044).
Results
Patient characteristics
A total of 21 children undergoing surgery participated in the study. 2/21 of patients were excluded from analysis due to a final diagnosis of non-neoplastic disease (n = 1) or both histologically and molecularly non-classifiable tumor (n = 1) (Suppl. Table 1). Eventually, our cohort comprised 19 tumor aspirates from 19 pediatric patients (Table 1). 37% of patients (n = 7) were male. Median age at surgery was 8 years (range 1 to 17 years). Twelve patients suffered from a newly diagnosed cerebral lesion, whereas seven samples were obtained from a second or further intervention. Five patients had received previous treatment with vincristine/carboplatin according to the European guidelines for low-grade glioma (LGG) (n = 4) or cyclophosphamide/vincristine/methotrexate/carboplatin/etoposide according to the current treatment recommendation of the German Society of Pediatric Oncology and Hematology (GPOH) for newly diagnosed medulloblastoma, ependymoma, and pineoblastoma (n = 1). One patient with LGG was previously treated with vinblastine monotherapy and targeted therapy using a MEK inhibitor. The most frequent diagnosis was pilocytic astrocytoma (47%, n = 9).
Table 1.
Overview of the patient cohort, including epidemiology (age, sex, tumor location, type of lesion, type of non-surgical pre-treatment, routine neuropathological results (microarray methylation class) and nanopore sequencing results (nanopore methylation class and methylation class family with scores, tSNE concordance)
| Patient ID | Age | Sex | Tumor location | Reference diagnosis | Type of lesion | Type of non-surgical pre-treatment | Microarray methylation class (MC) | Nanopore methylation class (MC) | Score MC | Nanopore methylation class family (MCF) | Score MCF | tSNE concordance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DX-BLN-025 | 8 | F | Parieto-occipital right | CNS neuroblastoma | Primary | None | CNS neuroblastoma with FOXR2 activation | CNS neuroblastoma with FOXR2 activation | 0.68 | CNS neuroblastoma with FOXR2 activation | 0.68 | + |
| DX-BLN-027 | 17 | F | Right trigeminal nerve | Schwannoma | Primary | None | schwannoma | schwannoma | 0.85 | schwannoma | 0.85 | + |
| DX-BLN-028 | 9 | F | Right cerebellar hemisphere | Pilocytic astrocytoma | Primary | None | low grade glioma, subclass posterior fossa pilocytic astrocytoma | low grade glioma, subclass posterior fossa pilocytic astrocytoma | 0.44 | pilocytic astrocytoma | 0.58 | + |
| DX-BLN-029 | 1 | F | Fronto-temporo-parietal right | Ependymoma | Secondary | Cyclophosphamide/vincristine/methotrexate/carboplatin/etoposide | ependymoma, YAP fusion | ependymoma, YAP fusion | 0.07 | glioblastoma, IDH wildtype | 0.10 | + |
| DX-BLN-037 | 5 | F | Right cerebellar hemisphere | Pilocytic astrocytoma | Secondary | Vincristine/carboplatin | low grade glioma, subclass posterior fossa pilocytic astrocytoma | low grade glioma, subclass posterior fossa pilocytic astrocytoma | 0.33 | pilocytic astrocytoma | 0.54 | + |
| DX-BLN-042 | 9 | F | Left cerebellar hemisphere | Pilocytic astrocytoma | Primary | None | low grade glioma, subclass posterior fossa pilocytic astrocytoma | low grade glioma, subclass posterior fossa pilocytic astrocytoma | 0.46 | pilocytic astrocytoma | 0.56 | + |
| DX-BLN-043 | 10 | M | Left frontal | Ependymoma, RELA fusion-positive | Primary | None | ependymoma, RELA fusion | ependymoma, RELA fusion | 0.43 | ependymoma, RELA fusion | 0.43 | + |
| DX-BLN-044 | 9 | F | Left temporo-parieto-thalamic | High grade glioma, H3 K27M mutated | Secondary | None | Diffuse midline glioma, K27-mutant | Diffuse midline glioma, K27-mutant | 0.66 | Diffuse midline glioma, K27-mutant | 0.66 | + |
| DX-BLN-047 | 8 | F | Left mesencephalic | Pilocytic astrocytoma | Secondary | Vincristine/carboplatin | low grade glioma, subclass posterior fossa pilocytic astrocytoma | low grade glioma, subclass posterior fossa pilocytic astrocytoma | 0.34 | pilocytic astrocytoma | 0.50 | + |
| DX-BLN-048 | 3 | M | IV. Ventricle | Anaplastic Ependymoma | Primary | None | ependymoma, posterior fossa group A | ependymoma, posterior fossa group A | 0.75 | ependymoma, posterior fossa group A | 0.75 | + |
| DX-BLN-049 | 3 | F | Thoracic spine | Pilocytic astrocytoma | Primary | None | low grade glioma, subclass posterior fossa pilocytic astrocytoma | low grade glioma, subclass midline pilocytic astrocytoma | 0.07 | pilocytic astrocytoma | 0.12 | - |
| DX-BLN-050 | 15 | F | Right lateral ventricle | Atypical central neurocytoma | Primary | None | central neurocytoma | central neurocytoma | 0.72 | central neurocytoma | 0.72 | + |
| DX-BLN-054 | 16 | F | Left frontal | Diffuse astrocytoma, IDH-mutant | Primary | None | IDH glioma, subclass astrocytoma | control tissue, hemispheric cortex | 0.08 | pilocytic astrocytoma | 0.17 | - |
| DX-BLN-064 | 6 | F | Foramen Monroi right | Subependymal giant cell astrocytoma | Primary | None | N/A | low grade glioma, subependymal giant cell astrocytoma | 0.15 | low grade glioma, subependymal giant cell astrocytoma | 0.15 | - |
| DX-BLN-065 | 6 | M | Right mesencephalic | Pilocytic astrocytoma | Secondary | Vincristine/carboplatin | low grade glioma, subclass posterior fossa pilocytic astrocytoma | low grade glioma, subclass posterior fossa pilocytic astrocytoma | 0.24 | pilocytic astrocytoma | 0.47 | + |
| DX-BLN-071 | 7 | F | Optic pathway | Pilocytic astrocytoma | Secondary | Vincristine/carboplatin, vinblastine, MEK inhibitor | N/A | low grade glioma, subclass midline pilocytic astrocytoma | 0.37 | pilocytic astrocytoma | 0.48 | + |
| DX-BLN-073 | 7 | M | Sella turcica | Craniopharyngioma | Primary | None | craniopharyngioma, adamantinomatous | craniopharyngioma, adamantinomatous | 0.56 | craniopharyngioma, adamantinomatous | 0.56 | + |
| DX-BLN-074 | 6 | M | Right cerebellar hemisphere | Pilocytic astrocytoma | Primary | None | low grade glioma, subclass posterior fossa pilocytic astrocytoma | low grade glioma, subclass posterior fossa pilocytic astrocytoma | 0.58 | pilocytic astrocytoma | 0.69 | + |
| DX-BLN-075 | 9 | M | Right cerebellar hemisphere | Pilocytic astrocytoma | Secondary | None | low grade glioma, subclass posterior fossa pilocytic astrocytoma | low grade glioma, subclass posterior fossa pilocytic astrocytoma | 0.32 | pilocytic astrocytoma | 0.40 | + |
Sequencing characteristics of tumor DNA from ultrasonic aspirator tissue samples
Low-pass whole genome sequencing performed for at least 6 h resulted in a mean genome coverage of 0.47X (range 0.01X to 1.5X, Suppl. Table 1). The mean read length ranged between 4,048 and 10,441 base pairs with an overall mean of 7,439 base pairs and was significantly higher in UA compared to reads obtained in an external cohort of sequencing runs of tumor DNA extracted from fresh-frozen tissue (Fig. 1B). The mean number of CpG sites overlapping the reference atlas was 107,280 CpGs (range: 2,275 to 295,872 CpG sites), exceeding the minimum number of 1,000 overlapping CpG sites for meaningful analysis in 19/19 (100%) samples. In two cases, the minimum number of CpG sites was not achieved initially and required an additional sequencing run. On average, after six hours of sequencing, 1,087 Mb of aligned base pairs were obtained (range: 23.76 Mb—2299.12 Mb), which again was comparable to sequencing runs from fresh-frozen tissue (Fig. 1C). Tumor cell content was reliably estimated from copy number alterations in 4/20 (20%) tumors with a mean tumor purity of 0.37 (range 0.25 – 0.65) (Fig. 1D). Tumor purity was higher in ultrasonic aspirator tissue samples compared to FFPE tissue for routine workup in 3 out of 4 (75%) cases.
DNA methylation-based classification
Tumors were classified based on DNA methylation profiles using a neural network model which has been trained using the Heidelberg brain tumor reference cohort with CNS tumor methylation datasets of 2,801 samples and predictions were made with respect to the 91 methylation classes (MC) or methylation class family (MCF), respectively, as defined in the 11b4 version [3]. A single nanopore-specific cut-off value was determined to identify valid predictions. Receiver operating characteristic curve analysis of classification scores was used to determine an optimal cut-off value > 0.2.
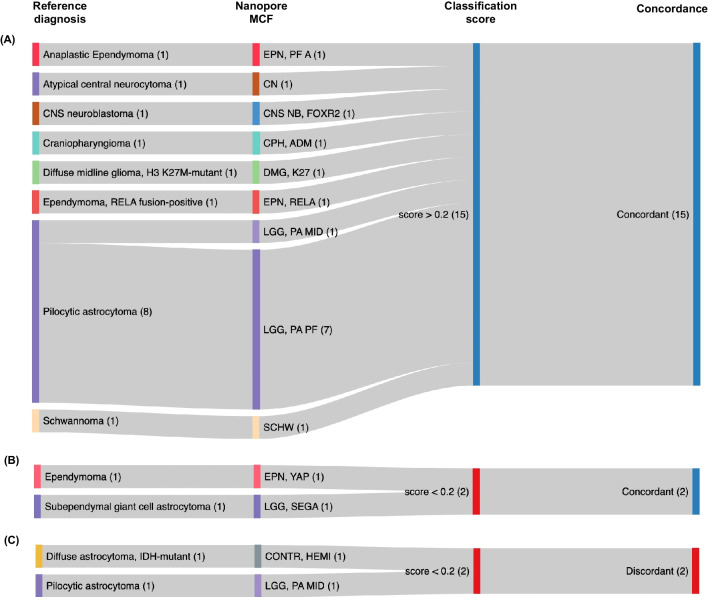
Classification results were identical to microarray in 17/19 (89%) of cases and compatible with the integrative histopathological reference diagnosis in 17/19 (89%) cases (Fig. 2). Application of the optimal calibrated score threshold of > 0.2 resulted in 15/19 cases passing the cut-off all of which were correctly classified, corresponding to a specificity of 100% and a sensitivity of 79% on both MC and MCF level (Fig. 2, Suppl. Table 1).
Fig. 2.
Concordance of nanopore sequencing-based classification of ultrasonic aspirator-derived tumor material with the corresponding 2016 WHO CNS reference diagnosis. (A) All samples with classification scores meeting the platform-specific cut-off of 0.2 (15/19, 79%) were correctly classified (true positives). (B) In 2/19 (11%) cases, the score was below the recommended cut-off but classification was still correct (false negatives). (C) 2/19 (11%) cases had discordant results with scores that were below the nanopore-specific threshold (true negatives), resulting in 100% specificity and 79% sensitivity for a classification score above the cut-off to predict correct classification
At the MC level, in 2/19 (11%) cases, the score was below the threshold but classification was still correct (one YAP-fusion positive ependymoma, one subependymal giant-cell astrocytoma (SEGA)). 2/19 (11%) cases had discordant results with scores that were below the nanopore-specific threshold: In the first case, pilocytic astrocytoma (PA) subtype was incorrect (classifying the case as midline PA instead of posterior fossa PA) while correctly classifying the sample as PA on MCF level. The other sample was an IDH-mutant astrocytoma classified as control tissue. Here, additional Sanger sequencing revealed no IDH mutation in the ultrasonic aspirator tissue sample, potentially indicating a sampling issue.
For comparison, the microarray-based analysis from matched FFPE tissue yielded a diagnostic score in 17/19 (89%) cases. Of note, the two cases with nondiagnostic score included one case of SEGA that also received a low score in nanopore/ultrasonic aspirator tissue profiling.
Copy number profiling
Copy number profiles obtained from WGS closely resembled matched array-based profiles in 18/19 (95%) cases and enabled the detection of large chromosomal alterations. One nanopore-based CNV profile from a case with very low sequencing yield (DX-BLN-049, pilocytic astrocytomas) was of insufficient quality for interpretation (Suppl. Figure 1). Low-level focal gains such as tandem duplications resulting in BRAF fusion genes in the PA samples were visually identified in 1/9 (11%) cases from nanopore CNV profiles while detectable in 8/9 (89%) in matched CNV profiles from methylation microarrays (Suppl. Figure 1).
Discussion
Molecular testing is an essential component of state-of-the-art integrated neuropathological diagnostics for most pediatric brain tumor types. Due to the increasing number of pathological examinations required (such as DNA and RNA gene panel sequencing or methylation microarray), tissue is valuable. This proof-of-principle study reports, to our knowledge, the first application of ultrasonic aspirator-derived tumor tissue for molecular classification of pediatric CNS tumors using low-pass nanopore whole genome sequencing. We show that ultrasonic aspirator-derived tumor fragments are a representative source of tumor DNA with tumor cell content sufficient to DNA methylation-based classification and yielding identical classification results.
Tissue characteristics
Although the use of ultrasonically minced tumor tissue for histopathological analysis of brain tumor tissue has been demonstrated in some studies, its suitability is still matter of debate [22–25]. In particular, the grading of glial tumors has been reported difficult as histomorphology was only partly recapitulated.
Using read length distribution of mapped nanopore reads as a proxy of DNA fragment length, we find significantly longer reads in DNA extracted from fresh ultrasonic aspirator tissue aspirates compared to routinely prepared fresh-frozen tissue. Shearing of DNA fragments during freeze–thaw cycles for fresh-frozen tissue might explain this finding. Importantly, the results show that high molecular weight genomic DNA suitable for nanopore sequencing can be extracted when ultrasonic aspirator-derived tissue is used. Additionally, similar aligned base yields indicate comparable sequencing performance. Tumor purity estimations for microarray/nanopore pairs indicate a tendency towards higher tumor purity in ultrasonic aspirator tissue samples. However, the estimation depends on the existence of numerical chromosomal alterations. As expected, the majority of cases within a pediatric cohort are pilocytic astrocytomas which lack relevant aneuploidy. Therefore, tumor purity could only be determined in 4 cases. Nevertheless, successful methylation-based classification in the majority of cases indicates that tumor purity is sufficient for the analysis despite the lack of control over tissue.
Indeed, one of the major advantages of using ultrasonic aspirator tissue samples is that multiple areas of the excised tumor are sampled [22]. This is particularly important because analyses based on single biopsies may have potential consequences for treatment decisions in spatially and temporally heterogeneous pediatric tumors [26, 27]. In contrast, DNA extraction for methylome profiling is usually done after microdissection of a representative area of the tumor sample with an anticipated tumor cell content of ≥ 70% and therefore reflects only a subset of the entire tumor [3].
Comparison to microarray-based classification
It was recently demonstrated, that the application of nanopore technology can be used with comparable reliability for processing of fresh frozen tissue compared to microarray-based analysis of FFPE material [9]. Our analysis confirms its suitability when using ultrasonic aspirator-derived tissue. Similar to the observed sensitivity of 88% in a well-defined validation cohort for microarray-based classification [3], our approach reaches a sensitity of 79% for the > 0.2 cut-off while retaining 100% specificity. In contrast, in a real-world cohort enriched for challenging cases a sensitivity as low as 56% was reported for EPIC-based microarray analysis [28].
Especially low-tumor cell content, like in the infiltration zone of diffuse glioma, can be challenging for the performance of DNA methylation-based classification [29] and was likely the cause for the discordantly classified cases (before application of diagnostic cut-offs) in this cohort. Therefore, low tumor cell content poses a challenge to methylation-based classification in general, independent of the processed tissue type or technology platform used for methylome profiling.
Copy number profiles can be derived both from nanopore low-pass WGS and methylation microarray datasets. While the resolution of microarray-based CNV plots is fixed due to the probe set of the chip, resolution of low-pass WGS-based CNV plots correlates with read yield. In our cohort, the quality of nanopore CNV plots was frequently inferior to the matched microarray-based ones. However, next generation flow cells and chemistries for nanopore sequencing devices offer better yields and are likely to resolve these issues.
The initial hardware investment for nanopore sequencing amounts to €1000 for the sequencing device and €2000 for a dedicated workstation or laptop (with a graphics processing unit [GPU] for hardware acceleration, if a high-performance computing environment is not available). An end-to-end analysis software, available at https://gitlab.com/pesk/nanoDx, which encapsulates bioinformatic analysis from the end user, can directly generate a PDF-format diagnostic report from the raw sequencing data. Together with a per assay price of 250€ or less, the method is accessible, scalable and cost-effective even in low-throughput settings.
Conclusion
DNA methylation-based classification of pediatric CNS tumors from ultrasonic aspirator-fragmented tissue is feasible using nanopore sequencing. A neural network classifier with nanopore-specific diagnostic score thresholds assures high specificity while achieving acceptable sensitivity. Generation of CNVprofiles is possible and allows for detection of chromosomal changes but was currently inferior in detection of focal changes (e.g. BRAF tandem duplication) compared to microarray approaches. This approach allows maximum exploitation of available tissue for diagnostics. Since advanced molecular techniques have limited benefit for patients in ressource-challenged centers, our time- and cost-efficient approach may be of particular interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Comparison of copy number variation profiles obtained from ultrasonic aspirator tissue samples and nanopore sequencing versus FFPE tumor tissue subjected to EPIC microarray (850K). (PDF 4486 KB)
Supplementary file2 Summary of all patient information, including clinical data (age, sex, tumor location, reference diagnosis, preoperative treatment, previous surgeries, compatible methylation class), microarray-based classification data (methylation class and class family with scores), nanopore-based prediction (majority votes for methylation class and class family with scores and concordance to microarray-based data, tSNE clustering results), sequencing statistics and tumor purity estimation. (XLSX 23 KB)
Acknowledgements
We thank Aydah Sabah for expert technical assistance. Computation has been performed on the HPC for Research cluster of the Berlin Institute of Health. Figures were created in part using Servier Medical Art provided by Servier and licensed under a Creative Commons Attribution 3.0 unported license. In addition, images illustrating nanopore sequencing were reproduced with permission from Oxford Nanopore Technologies Plc, United Kingdom.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Luis Kuschel, Michèle Simon, Matthias Schulz, Ulrich-Wilhelm Thomale and Philipp Euskirchen. The first draft of the manuscript was written by Michèle Simon and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by The Brain Tumour Charity, UK (Grant number GN-000694). Author P.E. has been a participant in the BIH-Charité Clinical Scientist Program funded by the Charité – Universitätsmedizin Berlin and BIH.
Data Availability
A Data and code availability statement has been made in the Methods section.
Declarations
Competing interests
PHD is ICI of the Sprinkle study and advisory board member for Alexion. DC declares a patent for a method to classify tumors according to DNA methylation signature. All other authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Michèle Simon and Luis P. Kuschel contributed equally to this work. Ulrich-Wilhelm Thomale and Philipp Euskirchen jointly supervised this work.
References
- 1.Zebian B, Vergani F, Lavrador JP, Mukherjee S, Kitchen WJ, Stagno V et al (2017) Recent technological advances in pediatric brain tumor surgery. CNS Oncol 6(1):71–82 10.2217/cns-2016-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sturm D, Capper D, Andreiuolo F, Gessi M, Kolsche C, Reinhardt A et al (2023) Multiomic neuropathology improves diagnostic accuracy in pediatric neuro-oncology. Nat Med 29(4):917–926 10.1038/s41591-023-02255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D et al (2018) DNA methylation-based classification of central nervous system tumours. Nature 555(7697):469–474 10.1038/nature26000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23(8):1231–1251 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truong LN, Patil S, Martin SS, LeBlanc JF, Nanda A, Nordberg ML, Beckner ME (2012) Rapid detection of high-level oncogene amplifications in ultrasonic surgical aspirations of brain tumors. Diagn Pathol 7:66 10.1186/1746-1596-7-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alenda C, Rojas E, Valor LM (2021) FFPE samples from cavitational ultrasonic surgical aspirates are suitable for RNA profiling of gliomas. PLoS ONE 16(7):e0255168 10.1371/journal.pone.0255168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merchant TE, Pollack IF, Loeffler JS (2010) Brain tumors across the age spectrum: biology, therapy, and late effects. Semin Radiat Oncol 20(1):58–66 10.1016/j.semradonc.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gojo J, Kjaersgaard M, Zezschwitz BV, Capper D, Tietze A, Kool M et al (2023) Rare embryonal and sarcomatous central nervous system tumours: State-of-the art and future directions. Eur J Med Genet 66(1):104660 10.1016/j.ejmg.2022.104660 [DOI] [PubMed] [Google Scholar]
- 9.Kuschel LP, Hench J, Frank S, Hench IB, Girard E, Blanluet M, et al. Robust methylation-based classification of brain tumours using nanopore sequencing. Neuropathol Appl Neurobiol. 2022:e12856 [DOI] [PubMed]
- 10.Djirackor L, Halldorsson S, Niehusmann P, Leske H, Capper D, Kuschel LP, et al. Intraoperative DNA methylation classification of brain tumors impacts neurosurgical strategy. Neurooncol Adv. 2021;3(1):vdab149 [DOI] [PMC free article] [PubMed]
- 11.Patel A, Dogan H, Payne A, Krause E, Sievers P, Schoebe N et al (2022) Rapid-CNS(2): rapid comprehensive adaptive nanopore-sequencing of CNS tumors, a proof-of-concept study. Acta Neuropathol 143(5):609–612 10.1007/s00401-022-02415-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H (2018) Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34(18):3094–3100 10.1093/bioinformatics/bty191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson JT, Workman RE, Zuzarte PC, David M, Dursi LJ, Timp W (2017) Detecting DNA cytosine methylation using nanopore sequencing. Nat Methods 14(4):407–410 10.1038/nmeth.4184 [DOI] [PubMed] [Google Scholar]
- 15.Yuan D, Jugas, R, Pokorna P, et al. crossNN: an explainable framework for cross-platform DNA methylation-based classification of cancer. medRxiv. medRxiv 2024.01.22.24301523; doi: 10.1101/2024.01.22.24301523, preprint: not peer reviewed
- 16.Paszke A, Gross S, Massa F, Lerer A, Bradbury J, Chanan G, et al. Pytorch: An imperative style, high-performance deep learning library. Advances in neural information processing systems. 2019;32
- 17.van der Maaten L; Hinton G. Visualizing data using t-sne. Journal of Machine Learning Research, 9(Nov):2579–2605, 2008
- 18.Euskirchen P, Bielle F, Labreche K, Kloosterman WP, Rosenberg S, Daniau M et al (2017) Same-day genomic and epigenomic diagnosis of brain tumors using real-time nanopore sequencing. Acta Neuropathol 134(5):691–703 10.1007/s00401-017-1743-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheinin I, Sie D, Bengtsson H, van de Wiel MA, Olshen AB, van Thuijl HF et al (2014) DNA copy number analysis of fresh and formalin-fixed specimens by shallow whole-genome sequencing with identification and exclusion of problematic regions in the genome assembly. Genome Res 24(12):2022–2032 10.1101/gr.175141.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poell JB, Mendeville M, Sie D, Brink A, Brakenhoff RH, Ylstra B (2019) ACE: absolute copy number estimation from low-coverage whole-genome sequencing data. Bioinformatics 35(16):2847–2849 10.1093/bioinformatics/bty1055 [DOI] [PubMed] [Google Scholar]
- 21.Koster J, Rahmann S (2012) Snakemake–a scalable bioinformatics workflow engine. Bioinformatics 28(19):2520–2522 10.1093/bioinformatics/bts480 [DOI] [PubMed] [Google Scholar]
- 22.Rao S, Vazhayil V, Nandeesh BN, Beniwal M, Rao K, Yasha TC et al (2020) Diagnostic Utility of CUSA Specimen in Histopathological Evaluation of Tumors of Central Nervous System. Neurol India 68(6):1385–1388 10.4103/0028-3886.304072 [DOI] [PubMed] [Google Scholar]
- 23.Finley JL, Silverman JF, Dickens MA (1990) Immunocytochemical evaluation of central nervous system tumors obtained by the Cavitron ultrasonic surgical aspirator. Diagn Cytopathol 6(5):308–312 10.1002/dc.2840060504 [DOI] [PubMed] [Google Scholar]
- 24.Silverman JF, Jones FD, Unverferth M, Berns L (1989) Cytopathology of neoplasms of the central nervous system in specimens obtained by the Cavitron Ultrasonic Surgical Aspirator. Acta Cytol 33(5):576–582 [PubMed] [Google Scholar]
- 25.Richmond IL, Hawksley CA (1983) Evaluation of the histopathology of brain tumor tissue obtained by ultrasonic aspiration. Neurosurgery 13(4):415–419 10.1227/00006123-198310000-00012 [DOI] [PubMed] [Google Scholar]
- 26.Schmelz K, Toedling J, Huska M, Cwikla MC, Kruetzfeldt LM, Proba J et al (2021) Spatial and temporal intratumour heterogeneity has potential consequences for single biopsy-based neuroblastoma treatment decisions. Nat Commun 12(1):6804 10.1038/s41467-021-26870-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazow MA, Hoffman L, Schafer A, Osorio DS, Boue DR, Rush S et al (2020) Characterizing temporal genomic heterogeneity in pediatric low-grade gliomas. Acta Neuropathol Commun 8(1):182 10.1186/s40478-020-01054-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaunmuktane Z, Capper D, Jones DTW, Schrimpf D, Sill M, Dutt M et al (2019) Methylation array profiling of adult brain tumours: diagnostic outcomes in a large, single centre. Acta Neuropathol Commun 7(1):24 10.1186/s40478-019-0668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capper D, Stichel D, Sahm F, Jones DTW, Schrimpf D, Sill M et al (2018) Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol 136(2):181–210 10.1007/s00401-018-1879-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Comparison of copy number variation profiles obtained from ultrasonic aspirator tissue samples and nanopore sequencing versus FFPE tumor tissue subjected to EPIC microarray (850K). (PDF 4486 KB)
Supplementary file2 Summary of all patient information, including clinical data (age, sex, tumor location, reference diagnosis, preoperative treatment, previous surgeries, compatible methylation class), microarray-based classification data (methylation class and class family with scores), nanopore-based prediction (majority votes for methylation class and class family with scores and concordance to microarray-based data, tSNE clustering results), sequencing statistics and tumor purity estimation. (XLSX 23 KB)
Data Availability Statement
The nanoDx analysis pipeline for end-to-end analysis of nanopore WGS data is available at https://gitlab.com/pesk/nanoDx. Source code to reproduce all analyses and sequencing data based figures in this work is provided at https://gitlab.com/pesk/nanoCUSA. Raw sequencing data have been deposited at the European Genome-phenome archive (accession no. EGA50000000180), while methylation microarray data and nanopore methylation calls are available from Gene Omnibus Express (accession no. GSE255044).
A Data and code availability statement has been made in the Methods section.