Most neurological problems are dealt with by general practitioners and hospital physicians, not by neurologists.1 Neurological disorders account for 10%-20% of acute hospital admissions. Around 10% of the adult population consult their general practitioner each year with neurological symptoms, but of these less than 10% are referred to hospital clinics. Developments in the management of neurological disorders are therefore relevant to doctors without specialist neurological training.
Methods
We identified references by regular reading of general medical and neurological journals, from searching the electronic literature (Medline, BIDS), and through discussion with general practitioners and neurological colleagues with specialist interests. The final selection of papers was partly subjective.
Cerebrovascular disease
The international stroke trial and the Chinese acute stroke trial, each concerning around 20 000 patients, examined antithrombotic therapy (aspirin, heparin) given within 48 hours of acute ischaemic stroke.2,3 Both found aspirin to be associated with about 10 fewer deaths or recurrent strokes in the first 4 weeks for each 1000 patients treated, but with slightly more haemorrhagic strokes. The international stroke trial reported no benefit from subcutaneous heparin (5000 or 12 500 IU twice daily) given with or without aspirin. Hence it was concluded that aspirin should be started as soon as possible after the onset of an acute ischaemic stroke.2,3 Whether aspirin use is “acute treatment” or simply early secondary prevention remains debatable.
No clinical indicators reliably differentiate ischaemic from haemorrhagic stroke. The recommendation that aspirin be started only after appropriate brain imaging in patients requiring admission to hospital will place a huge burden on acute neurological services (over 100 000 people have a first stroke in England and Wales each year). The issue is still more problematic for thrombolytic therapy (tissue type plasminogen activator, streptokinase, urokinase). An overview of previous trials indicated significant excesses of early and total deaths, and of symptomatic and fatal intracranial haemorrhages, after acute thrombolytic therapy, but a significant reduction in death or dependency in patients randomised to treatment within 3 hours of stroke onset.4 To identify the small number of patients likely to benefit from thrombolysis, early hospital admission and prompt investigation will be necessary. This may be achieved in dedicated stroke units, which have been shown to produce long term reductions in death, dependency, and need for institutional care.5
Carotid artery stenosis is an important predisposing factor for cerebrovascular ischaemic events, the risk increasing with the severity of stenosis and the presence of symptoms. For severe (more than 70% narrowing) symptomatic stenosis, carotid endarterectomy is recommended. For severe symptom free stenosis, optimal management has yet to be defined: a meta-analysis of trials6 showed only a small absolute benefit from surgery in reducing the odds of ipsilateral stroke; hence the procedure cannot be routinely recommended. For mild to moderate symptomatic stenosis (less than 70% narrowing), antiplatelet therapy with aspirin or dipyridamole, or both, is recommended. Persistent symptoms may necessitate use of other treatments such as ticlopidine (named patient basis only); clopidogrel, which reduces the relative risk for further ischaemic events slightly more than aspirin7; or anticoagulation with warfarin.
Cerebrovascular disease
Given within 48 hours of ischaemic stroke, aspirin reduces risk of death and recurrent stroke
Thrombolysis for acute ischaemic stroke is most effective if delivered within 3 hours of stroke onset
Stroke units reduce death, dependency, and need for institutional care after stroke
Carotid endarterectomy is currently recommended only for severe and symptomatic carotid stenoses
Epilepsy
Most individuals with newly diagnosed epilepsy enter prolonged seizure remission and have an excellent prognosis, but seizures remain refractory in 20%-30%.8 Improved evaluation of such patients with magnetic resonance imaging and telemetry may identify the structural and functional abnormalities that give rise to seizures. Up to 75% of patients with refractory partial epilepsy show evidence of abnormalities on magnetic resonance imaging,9 some of which are amenable to surgery.
The first line drugs for epilepsy monotherapy remain carbamazepine and sodium valproate; phenytoin is now less used, and although lamotrigine has a monotherapy licence its place has still to be defined. Several new “add on agents” have been licensed in recent years including vigabatrin, gabapentin, lamotrigine, and topiramate. An overview of trials in patients with refractory partial seizures suggests no major differences between these agents in either efficacy or tolerability.10 Severe visual field defects have recently been reported after prolonged use of vigabatrin, prompting the development of guidelines for monitoring vision.11 Vagal stimulation remains an experimental approach to seizure control.12
Population based studies show that patients with epilepsy have an increased risk of death compared with age and sex matched controls.13 Some of these deaths are related to epilepsy itself, for example, as a consequence of accidents, but others are unexplained. The category of “sudden unexpected death in epilepsy” (SUDEP) has recently been introduced to encompass all such deaths, which are more common in individuals with refractory epilepsy (about 1 per 200 patients per year).13 Many of these deaths may be related to unwitnessed seizures, possibly associated with ventricular fibrillation, asystole, respiratory arrest, or neurologically mediated pulmonary oedema. A proportion of cases of sudden unexpected death in epilespy may therefore be preventable with improved seizure control.
Epilepsy
Evaluation of patients with refractory partial epilepsy with magnetic resonance imaging and telemetry may yield diagnostic information in up to 75%
Second line, “add on,” agents for refractory partial seizures show no significant differences in efficacy or tolerability
Guidelines for monitoring of visual fields in patients receiving long term vigabatrin have been issued
Sudden unexplained death in epilepsy (SUDEP) may be preventable with better seizure control
Multiple sclerosis
Inteferon betas reduce relapse rate in relapsing-remitting multiple sclerosis by about one third in patients experiencing two disabling relapses every 2 years, and may have a small effect in delaying disability progression
Magnetic resonance imaging can help predict which patients with clinically isolated syndromes will progress to multiple sclerosis
Liaison between primary and secondary healthcare services is essential for the appropriate management of established disability in multiple sclerosis
Multiple sclerosis
Interferon betas (interferon beta-1b, Betaferon; interferon beta-1a, Avonex, Rebif) have been shown to reduce relapse rate in relapsing-remitting (non-progressive) multiple sclerosis by about one third.14–16 The Association of British Neurologists recommends (guidelines, June 1999) interferon beta be prescribed for ambulant patients with at least two definite relapses in the previous 2 years followed by recovery (may or may not be complete). Whether reduction in relapse rate reduces or prevents later disability is not known; some evidence has been presented in favour.15,16 The uncertainty reflects the difficulties of treatment trials for multiple sclerosis owing to the variable clinical course of the disease, the validation of disability measurements, and discrepancies between secondary outcome measures such as evidence of changes on magnetic resonance imaging, and clinical behaviour.17 Interferon beta-1b has been reported to delay progression (for 9-12 months in a study period of 2-3 years) in secondarily progressive multiple sclerosis of moderate severity (minimum walking distance of 20 metres with assistance)18 and has been licensed for this indication. The Association of British Neurologists has produced guidelines to aid therapeutic decision making in secondary progressive multiple sclerosis, but these may be altered in the light of a recent large study (SPECTRIMS; to date presented only in abstract), which reported no significant effect of interferon beta-1a in delaying disability in secondary progressive multiple sclerosis. Sorting out this complex area, with profound financial implications, will be a high priority for NICE.
Trials are also in progress to ascertain whether interferon betas are useful in primary progressive multiple sclerosis, or in delaying or preventing the onset of disseminated disease after clinically isolated syndromes such as optic neuritis and transverse myelitis. Defining which of these patients will progress to widespread disease may be facilitated by magnetic resonance imaging; clinically silent lesions are predictive of the long term risk of subsequent development of multiple sclerosis.19
Other possible treatments for multiple sclerosis include copolymer-120 and pulsed intravenous immunoglobulin,21 which, like interferon betas, reduce the relapse rate. However, the place for these immunomodulatory agents remains to be defined. Intravenous methylprednisolone may hasten recovery from acute relapses but has no effect in the long term. A recent trial suggested intravenous methylprednisolone is no better than equivalent oral doses of methylprednisolone for acute relapses.22 If so, then switching from intravenous to oral steroids for acute relapses may make considerable savings.
In advanced multiple sclerosis, management of established disability may be the primary role of the neurologist. Bladder dysfunction usually consists of combined detrusor hyperreflexia and incomplete emptying volumes of less than 100 ml of urine remaining in the bladder after micturition are managed with oxybutinin or detrusitol; volumes greater than 100 ml require clean, intermittent, self catheterisation.23 Sexual dysfunction (erectile failure) may be helped with the phosphodiesterase inhibitor sildenafil citrate (Viagra), or more established agents such as yohimbine or other α adrenoreceptor blockers. Management of limb spasticity requires a multidisciplinary approach ensuring correct posture, prevention of skin ulceration from pressure, management of bladder and bowel dysfunction, as well as pharmacological measures. Tizanidine, an α2 adrenoreceptor agonist, has recently been licensed in the United Kingdom as an antispastic agent.24
Parkinson’s disease
The management of idiopathic Parkinson’s disease is still centred around the use of levodopa preparations since they produce the most effective relief of symptoms in most patients. Distinguishing idiopathic Parkinson’s disease from other parkinsonian syndromes (for example, multiple system atrophy, Steele-Richardson-Olszewski syndrome) is crucial as the latter differ in prognosis and management. With long term treatment with levodopa, response fluctuations develop in around 10% of patients with idiopathic Parkinson’s disease per year. Delaying the use of levodopa in early disease, particularly in young patients (under 50 years), is therefore desirable.
Levodopa sparing agents that may be used as monotherapy in early Parkinson’s disease include amantadine, anticholinergics (particularly if tremor is predominant), selegiline, and dopamine agonists. Bromocriptine alone improves about 50% of patients during the first year of treatment but there is a gradual loss of benefit thereafter, with only 10% responding at 5 years; other dopamine agonists have similar effects. Selegiline delays the need for levodopa by a mean of 8 months. One study found increased mortality in patients taking levodopa in combination with selegilne.25
Parkinson’s disease
Accurate diagnosis of parkinsonian syndromes is important for appropriate management
Use of levodopa sparing agents may be desirable in the treatment of early onset Parkinson’s disease
The late stages of Parkinson’s disease necessitate the use of polypharmacy to manage unpredictable response fluctuations to levodopa, which are an almost invariable feature after several years’ treatment
Functional neurosurgery may improve some of the features of Parkinson’s disease and treatment complications in selected patients
Once levodopa is started it may be several years before response fluctuations develop. These are usually predictable initially, such as end of dose or “wearing off” effects. Strategies to ameliorate these problems include dose fractionation, long acting levodopa preparations (particularly helpful for nocturnal immobility), and addition of dopamine agonists.26 Three new dopamine agonists are now available: ropinirole, cabergoline, and pramipexole.27–29 It is not yet known whether these agents confer significant therapeutic advantages over bromocriptine and pergolide as add on therapy. Add on use of catechol-O-methyltransferase inhibitors (tolcapone, entacapone) may increase total “on” timeby about 20%,30 but tolcapone has been withdrawn in the United Kingdom because of hepatotoxicity.
Sudden unpredictable changes between periods of mobility (“on”) with severe levodopa induced involuntary movements (dyskinesias) and disabling parkinsonism (“off”), the “on-off phenomenon”, present a major management problem. Various strategies may be tried to minimise the severity of dyskinesias31—for example, adjusting the timing of levodopa intake, optimising levodopa absorption, and addition of other antiparkinsonian agents such as dopamine agonists and amantadine thus permitting levodopa dose reduction. Increased use of the dopamine agonist apomorphine, given by subcutaneous injection or infusion, may rescue patients from severe sudden off periods, and improve overall mobility.
Renewed interest has been shown in surgery for the symptoms of Parkinson’s disease, levodopa induced dyskinesias, and disorders of movement such as tremor and dystonia through the use of stereotactically placed lesions or chronic electrical stimulation with indwelling electrodes.32 Thalamotomy or thalamic stimulation helps tremor and often rigidity but not akinesia. Unilateral pallidotomy (figure) significantly improves contralateral dyskinesias; bilateral pallidotomy may, in addition, improve parkinsonian symptoms but carries increased risks.33 Bilateral subthalamic nucleus stimulation34 or subthalamotomy has produced dramatic improvements in parkinsonism, sufficient to allow large reductions in the levodopa dose and thus improvement in levodopa induced dyskinesias. Appropriate patient selection is a key factor in its successful use, and it will be available only in a few specialist centres.
Dementia
The definition of a new variant of Creutzfeldt-Jakob disease35 has attracted huge media attention, because it seems to be caused by the same strain of prion protein that causes bovine spongiform encephalopathy, and hence may have been transmitted to humans through contaminated food. However, this uniformly fatal condition remains extremely rare, with only 41 cases reported to date in the United Kingdom.
For Alzheimer’s disease, the commonest cause of dementia, two cholinesterase inhibitors, donepezil (Aricept)36 and rivastigmine (Exelon),37 are now licensed for the symptomatic treatment of mild to moderate cognitive impairment (minimental state examination score of 10-26). Trials suggest that in some patients these agents improve cognition, global function, and behavioural function, but there are as yet no data as to whether they delay deterioration or improve outcome. Currently it is recommended that these drugs are only commenced on the advice of a specialist. Despite advances in understanding the pathological basis of Alzheimer’s disease, centred around amyloid β peptides, this has not resulted in new treatments although many are in development.38
Migraine
Since the launch of sumatriptan, several triptans have become available for the treatment of acute migraine.39 They differ in pharmacodynamic properties such as time of onset and duration of action. Direct comparisons have not been performed and it is not known whether the differences claimed will have significant impact on clinical practice. Preventive treatments include pizotifen and amitriptyline, but the strongest evidence of efficacy is available for β blockers and sodium valproate.39
Rare disorders
The glutamate antagonist riluzole (Rilutek) remains the only licensed treatment for motor neurone disease. Risk of death or tracheostomy was lower with 100 mg riluzole than placebo in limb or bulbar onset disease,40 but it is debatable whether this translates into an improved quality of remaining life.
Intravenous immunoglobulin has been used in many neurological disorders,21,41 but its place remains to be defined; many of these applications are empirical and the long term effects of intravenous immunoglobulin are unknown. Trials in acute idiopathic neuropathy (Guillain-Barré syndrome) suggest that intravenous immunglobulin is equivalent to plasma exchange in reducing disability at 4 weeks, but that the combination of intravenous immunoglobulin and plasma exchange offers no significant additional advantage.42 Likewise, in myasthenia gravis, intravenous immunglobulin seems as efficacious as plasma exchange,43 and it may also be useful in chronic inflammatory demyelinating polyneuropathy in relapse.44
Dementia, rare disorders
Cholinesterase inhibitors are of symptomatic benefit in about 20% of patients with mild to moderate Alzheimer’s disease
Some peripheral neuropathies are treatable may show profound benefit with intravenous immunoglobulin treatment
Intravenous immunglobulin may be helpful in the acute treatment of Guillain-Barré syndrome and myasthenia gravis
Conclusion
In the past 5 years, new treatments have become available for neurological disorders previously considered untreatable (multiple sclerosis, Alzheimer’s disease, motor neurone disease). Although the high cost of these treatments has sometimes led to antagonism between purchasers and doctors wanting to prescribe, these small, initial, therapeutic inroads have focused on the broader needs of patients with neurological disorders, engendering specialist clinics, specialist nurses, and fostering liaison with community services and patient interest groups.
Figure.
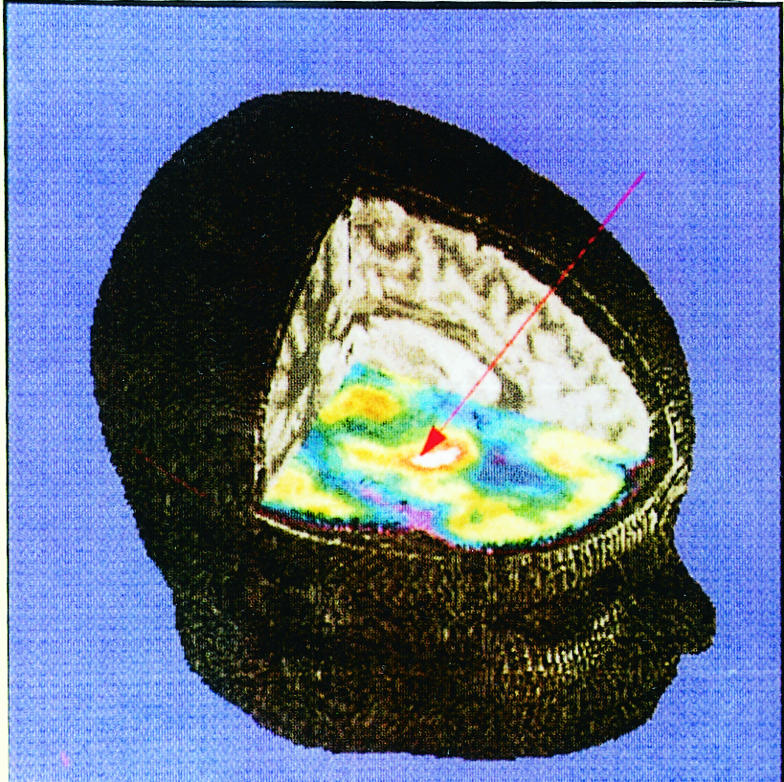
Superimposed 3-dimensional positron emission tomogram and magnetic resonance image of patient with idiopathic Parkinson’s disease showing hypermetabolism of pallidum(white area), target for unilateral stereotaxic pallidotomy
Acknowledgments
We thank Drs DJ Thomas, RJ Butterworth, M Prevett, P Rudge, D Kidd, CAH Fisher, S Fearn, and S Addison, and Professors NP Quinn and MN Rossor for commenting on earlier drafts of the article.
Footnotes
Competing interests: None declared.
References
- 1.Association of British Neurologists. Neurology in the United Kingdom: towards 2000 and beyond. London: ABN; 1997. [Google Scholar]
- 2.International Stroke Trial Collaborative Group. The international stroke trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19 435 patients with acute ischaemic stroke. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 3.Chinese Acute Stroke Trial Collaborative Group. CAST: randomised placebo-controlled trial of early aspirin use in 20 000 patients with acute ischaemic stroke. Lancet. 1997;349:1641–1649. [PubMed] [Google Scholar]
- 4.Wardlaw JM, Warlow CP, Counsell C. Systematic review of evidence on thrombolytic therapy for acute ischaemic stroke. Lancet. 1997;350:607–614. doi: 10.1016/s0140-6736(97)03022-5. [DOI] [PubMed] [Google Scholar]
- 5.Stroke Unit Trialists’ Collaboration. Collaborative systematic review of the randomised trials of organised inpatient (stroke unit) care after stroke. BMJ. 1997;314:1151–1159. [PMC free article] [PubMed] [Google Scholar]
- 6.Benavente O, Moher D, Pham B. Carotid endarterectomy for asymptomatic carotid stenosis: a meta-analysis. BMJ. 1998;317:1477–1480. doi: 10.1136/bmj.317.7171.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CAPRIE steering committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 8.Cockerell OC, Johnson AJ, Goodridge DMG, Sander JWAS, Shorvon SD. The remission of epilepsy: results from the national general practice study of epilepsy. Lancet. 1995;346:140–144. doi: 10.1016/s0140-6736(95)91208-8. [DOI] [PubMed] [Google Scholar]
- 9.Li LM, Fish DR, Sisodiya SM, Shorvon SD, Alsanjari N, Stevens JM. High resolution magnetic resonance imaging in adults with partial or secondary generalised epilepsy attending a tertiary referral unit. J Neurol Neurosurg Psychiatry. 1995;59:384–387. doi: 10.1136/jnnp.59.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marson AG, Kadir ZA, Chadwick DW. New antiepileptic drugs: a systematic review of their efficacy and tolerability. BMJ. 1996;313:1169–1174. doi: 10.1136/bmj.313.7066.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appleton RE. Guideline may help in prescribing vigabatrin. BMJ. 1998;317:1322. doi: 10.1136/bmj.317.7168.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLachlan RS. Vagus nerve stimulation for intractable epilepsy: a review. J Clin Neurophysiol. 1997;14:358–368. doi: 10.1097/00004691-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Nashef L, Sander JWAS, Fish DR, Shorvon SD. Incidence of sudden unexpected death in an adult outpatient cohort with epilepsy at a tertiary referral centre. J Neurol Neurosurg Psychiatry. 1995;58:462–464. doi: 10.1136/jnnp.58.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Interferon Beta Multiple Sclerosis Study Group and the University of British Columbia Multiple Sclerosis/Magnetic Resonance Imaging Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995;45:1277–1285. [PubMed] [Google Scholar]
- 15.Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 16.Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis Study Group (PRISMS) Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. [PubMed] [Google Scholar]
- 17.Noseworthy JH, Miller DH. Measurement of treatment efficacy and new trial results in multiple sclerosis. Curr Opin Neurol. 1997;10:201–210. doi: 10.1097/00019052-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 18.European Study Group on Interferon β-1b in Secondary Progressive Multiple Sclerosis. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352:1491–1497. [PubMed] [Google Scholar]
- 19.O’Riordan JI, Thompson AJ, Kingsley DPE, MacManus DG, Kendall BE, Rudge P, et al. The prognostic value of brain MRI in clinically isolated syndromes of the central nervous system. A 10-year follow-up. Brain. 1998;121:495–503. doi: 10.1093/brain/121.3.495. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 21.Fazekas F, Deisenhammer F, Strasser-Fuchs S, Nahler G, Mamoli B.for the Austrian Immunoglobulin in Multiple Sclerosis Study Group. Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing-remitting multiple sclerosis Lancet 1997349589–593. [DOI] [PubMed] [Google Scholar]
- 22.Barnes D, Hughes RAC, Morris RW, Wade-Jones O, Brown P, Britton T, et al. Randomised trial of oral and intravenous methylprednisolone in acute relapses of multiple sclerosis. Lancet. 1997;349:902–906. doi: 10.1016/s0140-6736(96)06453-7. [DOI] [PubMed] [Google Scholar]
- 23.Fowler CJ. Investigation of the neurogenic bladder. J Neurol Neurosurg Psychiatry. 1996;60:6–13. doi: 10.1136/jnnp.60.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UK Tizanidine Trial Group. A double-blind, placebo-controlled trial of tizanidine in the treatment of spasticity caused by multiple sclerosis. Neurology. 1994;44(suppl 9):70–78S. [PubMed] [Google Scholar]
- 25.Lees AJ.on behalf of the Parkinson’s Disease Research Group of the United Kingdom. Comparison of therapeutic effects and mortality data of levodopa and levodopa combined with selegiline in patients with early, mild Parkinson’s disease BMJ 19953111602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nohria V, Partiot A. A review of the efficacy of the dopamine agonists pergolide and bromocriptine in the treatment of Parkinson’s disease. Eur J Neurol. 1997;4:537–543. [Google Scholar]
- 27.Adler CH, Sethi KD, Hauser RA, Davis TL, Hammerstad JP, Bertoni J, et al. Ropinirole for the treatment of early Parkinson’s disease. Neurology. 1997;49:393–399. doi: 10.1212/wnl.49.2.393. [DOI] [PubMed] [Google Scholar]
- 28.Rinne UK, Bracco F, Chouza C, Dupont E, Gershanik O, Marti Masso JF, et al. Cabergoline in the treatment of early Parkinson’s disease: results of the first year of treatment in a double-blind comparison of cabergoline and levodopa. Neurology. 1997;48:363–368. doi: 10.1212/wnl.48.2.363. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman A, Ranhosky A, Korts D. Clinical evaluation of pramipexole in advanced Parkinson’s disease: results of a double-blind, placebo-controlled, parallel-group study. Neurology. 1997;49:162–168. doi: 10.1212/wnl.49.1.162. [DOI] [PubMed] [Google Scholar]
- 30.Baas H, Beiske AG, Ghika J, Jackson M, Oertel WH, Poewe W, et al. Catechol-O-methyltransferase inhibition with tolcapone reduces the “wearing off” phenomenon and levodopa requirements in fluctuating parkinsonian patients. J Neurol Neurosurg Psychiatry. 1997;63:421–428. doi: 10.1136/jnnp.63.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocchi F, Nordera G, Marsden CD. Strategies for treating patients with advanced Parkinson’s disease with disastrous fluctuations and dyskinesias. Clin Neuropharmacol. 1997;20:95–115. doi: 10.1097/00002826-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Quinn N, Bhatia K. Functional neurosurgery for Parkinson’s disease. BMJ. 1998;316:1259–1260. doi: 10.1136/bmj.316.7140.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel M, Caputo E, Brooks DJ, Schrag A, Scaravilli T, Branston NM, et al. A study of medial pallidotomy for Parkinson’s disease: clinical outcome, MRI location and complications. Brain. 1998;121:59–75. doi: 10.1093/brain/121.1.59. [DOI] [PubMed] [Google Scholar]
- 34.Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas J-F, Broussolle E, et al. Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 35.Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 36.Rogers SL, Farlow MR, Doody RS, Mohs R Friedhoff LT and the Donepezil Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 37.Corey-Bloom J, Anand R, Veach J.for the ENA 713 B352 Study Group. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer’s disease Int J Geriatr Psychopharmacol 1998155–65. [Google Scholar]
- 38.Larner AJ, Rossor MN. Alzheimer’s disease: towards therapeutic manipu-lation of the amyloid precursor protein and amyloid β-peptides. Exp Opin Ther Patents. 1997;7:1115–1127. [Google Scholar]
- 39.Ferrari MD. Migraine. Lancet. 1998;351:1043–1051. doi: 10.1016/S0140-6736(97)11370-8. [DOI] [PubMed] [Google Scholar]
- 40.Lacomblez L, Bensimon G, Leigh PN, Guillet P, meininger V.for the Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Dose-ranging study of riluzole in amyotrophic lateral sclerosis Lancet 19963471425–1431. [DOI] [PubMed] [Google Scholar]
- 41.Wills AJ, Unsworth DJ. A practical approach to the use of intravenous immunoglobulin in neurological disease. Eur Neurol. 1998;39:3–8. doi: 10.1159/000007891. [DOI] [PubMed] [Google Scholar]
- 42.Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet. 1997;349:225–230. [PubMed] [Google Scholar]
- 43.Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C.for the Myasthenia Gravis Clinical Study Group. Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis Ann Neurol 199741789–796. [DOI] [PubMed] [Google Scholar]
- 44.Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double-blind, placebo-controlled, cross-over study. Brain. 1996;119:1067–1077. doi: 10.1093/brain/119.4.1067. [DOI] [PubMed] [Google Scholar]


