Abstract
This study was designed to test the effect of antioxidant supplementation on feline immunodeficiency virus (FIV)-infected felines. Six acutely FIV-infected cats (≥16 weeks post-inoculation) were given a propriety oral superoxide dismutase (SOD) supplement (Oxstrin; Nutramax Laboratories) for 30 days. Following supplementation, the erythrocyte SOD enzyme concentration was significantly greater in the supplemented FIV-infected group than the uninfected control group or the unsupplemented FIV-infected group. The CD4+ to CD8+ ratio increased significantly (0.66–0.88) in the SOD supplemented FIV-infected cats but not in the unsupplemented FIV-infected cats. Proviral load and reduced glutathione (GSH) levels in leukocyte cell types did not change significantly following supplementation. Antioxidant supplementation resulted in an increase in SOD levels, confirming the oral bioavailability of the compound in FIV-infected cats. This result warrants further investigation with trials of antioxidant therapy in FIV-infected cats that are showing clinical manifestations of their disease, as well as in other feline patients where oxidative stress likely contributes to disease pathogenesis, such as diabetes mellitus and chronic renal failure.
Despite the significance of oxidative stress in felines, there are very few reports documenting the effect of antioxidant supplementation in cats with naturally occurring disease. Recently it has been shown that supplementation of vitamins E and C and β-carotene decreased serum levels of 8-OHdG in cats with chronic renal failure, consistent with a reduction in free radical-induced damage to DNA (Yu and Paetau-Robinson 2006).
Oxidative stress plays a significant role in the pathogenesis and progression of human immunodeficiency virus (HIV) infection. Reduced glutathione (GSH), the predominant endogenous antioxidant in mammalian cells, is significantly reduced in lymphocyte subsets of patients with HIV. In vitro studies show that decreasing intracellular GSH concentration decreases murine hepatocyte survival, effects T-cell signal transduction, and increases HIV replication (Staal et al 1990, 1994, Adamson and Billings 1992). Clinical studies show that a GSH deficiency is correlated with a poor survival in AIDS patients (Herzenberg et al 1997). Glutathione homeostasis relies on the activity of a number of antioxidant enzymes including superoxide dismutase (SOD). SOD enzyme catalyzes the metabolism of the superoxide anion to hydrogen peroxide, and eventually, through a reaction catalyzed by the catalase enzyme, water. Oral supplementation of SOD would be susceptible to gastric degradation. A proprietary plant-derived SOD, complexed to the protein gliadin, has been developed. The gliadin serves to protect the SOD from degradation, allows for delayed release under simulated digestive conditions and adherence to the enterocytes. When given orally, this complex increased SOD, glutathione peroxidase (GPx) and catalase levels in erythrocytes and hepatocytes in mice (Vouldoukis et al 2004a,b).
Because of the importance of oxidative stress in HIV-infected humans and the inherent susceptibility of felines to oxidative stress, the antioxidant effects of short-term supplementation of a propriety SOD complex given to feline immunodeficiency virus (FIV)-infected cats were investigated.
Materials and methods
Cats
All study cats were born within 1 month of each other at the Andrea D. Lauerman specific pathogen-free (SPF) Cat Colony, Department of Microbiology, Immunology, and Pathology Building, Colorado State University. There were 10 males and eight females used in the study. All of the FIV-infected study cats were inoculated within 40 days of each other with a molecular clone of FIV clade C, FIV-PG (clade C). Six 14-month-old SPF cats (three males and three females) were each inoculated intraperitoneally while under sedation with 500 μl pooled plasma from FIV clade C infected cats (Dow et al 1999). Infection was confirmed by both serologic and polymerase chain reaction (PCR) detection within 1 month of inoculation. Six additional 15-month-old FIV-infected cats (same protocol; three males and three females) and six 15-month-old uninfected cats (four males and two females) served as untreated control groups. All study cats were housed under identical conditions in a pathogen-free facility under controlled temperature, humidity, and a 12-h light:dark cycle with dry kibble (Iams Adult Maintenance Dry) and water available ad libitum, in accordance with Colorado State University Animal Care and Use approved protocols. Four months following inoculation, with all cats 18–19 months old, the mean body weight for the cats in the uninfected control group (4.2±1.5 kg) was significantly less than the mean weight of both the supplemented FIV-infected group (5.4±1.5 kg) and the unsupplemented FIV-infected group (5.8±1.0 kg) (P<0.05). All cats received approximately 25 g of the same canned commercial cat food (9 Lives, Del Monte Foods) daily during the study. The SOD supplement was mixed into the canned food for the supplemented FIV-infected group and these cats were monitored to ensure that they finished their entire portion. An identical volume of the same vehicle (corn starch) used in the SOD supplement was mixed into a similar amount of canned food for each cat in the uninfected control group. Nothing was added to the canned food for the cats in the unsupplemented FIV-infected group. None of the cats displayed any clinical signs of illness during the study. Samples were drawn from all cats at day 0 (pre-supplementation) and day 30 (post-supplementation).
Study design
For the SOD supplemented FIV-infected group, day 0 and day 30 measures included proviral load, lymphocyte CD4:CD8 ratio, erythrocyte SOD, whole blood GPx, and malondialdehyde (MDA) concentrations, and intracellular GSH in neutrophils, monocytes, CD4+ and CD8+ T-cells. For the uninfected control group, the same parameters were measured at the same time points. For the unsupplemented FIV-infected control group the SOD enzyme concentration was also determined on day 0 and day 30. All samples from the supplemented FIV-infected and uninfected control group were run together in two batches (day 0 batch and day 30 batch) by the same laboratory technician, who was blinded as to group identity. All the unsupplemented FIV-infected control samples were run 2–3 months later in two batches (day 0 batch and day 30 batch) by the same laboratory technician.
Treatment
The FIV-infected cats were treated with 100 mg of a proprietary oral SOD complex once daily for 30 days (Oxstrin, Nutramax Laboratories, Edgewood, MD). The supplement was supplied as a powder and mixed completely into 25 g of canned cat food. Cats were fed individually and observed until they had consumed the entire dose. The uninfected control group received a placebo identical to the supplementation but without the actual SOD complex. The unsupplemented FIV-infected cats were also given 25 g of canned cat food daily, but with nothing added.
Complete blood counts
Complete blood counts (CBCs) were run on a Siemens Advia 120. The plasma protein is performed using a refractometer and the 100 cell differentials are performed manually along with a morphology review.
Viral load
Blood was collected from each cat in accordance with Colorado State University Animal Care and Use Committee-approved procedures for viral quantification. Proviral load was determined with real-time DNA PCR. Methods including primers and probes were adapted from those developed by Leutenegger et al (1999). Cellular DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA). PCR reactions were performed in a 25 μl volume containing 12.5 μl TaqMan Universal PCR Mastermix (Applied Biosystems, Forest City, CA), 400 nM of each primer (MWG Biotech, High Point, NC), 80 nM of probe (MWG Biotech, High Point, NC), and 5 μl of sample DNA or plasmid FIV DNA standard. Real-time PCR was performed on an iCycler iQ Real-time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Starting quantities of viral DNA were extrapolated from the plasmid DNA standard curve.
SOD and GPx
The SOD enzyme catalyzes the reaction that takes the superoxide free radical to hydrogen peroxide. The GPx enzyme catalyzes the reaction that takes hydrogen peroxide to water (Fig 1). Commercially available colorimetric kits for the measurement of erythrocyte SOD and whole blood GPx were previously validated for use in the domestic cat (Ransod & Ransel, Randox Laboratories, Oceanside, CA) (Marshall et al 2002). In that study, intra- and interassay coefficients of variation were determined (∼10%), and it was shown that freezing samples at −80°C did not affect results measured at day 28. The kit used to determine the pre-supplementation SOD levels for the FIV-infected and uninfected control cats was used in its entirety following completion of the pre-supplementation assays, and a new kit was employed for all subsequent measurements. This appears to have significantly affected the absolute values for the remaining SOD level measurements. Although this inter-kit variation precludes the meaningful comparison of changes in SOD levels within individual groups over time (before and after supplementation), it does not affect the comparison of SOD levels between groups at each time point. The insignificant difference between day 0 and day 30 SOD levels in the unsupplemented FIV-infected group (both samples run with the same newly acquired kit) confirms that results from each individual kit are reliable, although the inter-kit variation was large (Table 1).
Fig 1.
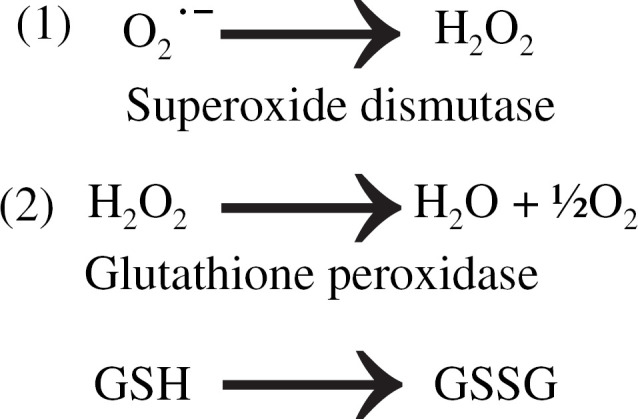
The reaction taking the superoxide free radical (O2−) to hydrogen peroxide (H2O2) (1) is catalyzed by the SOD enzyme. The reaction taking hydrogen peroxide to water (2) is catalyzed by the GPx enzyme using glutathione as a cofactor. During the GPx catalyzed reaction, GSH undergoes oxidation to glutathione disulphide (GSSG).
Table 1.
SOD levels in the three groups (supplemented FIV-infected, uninfected control, unsupplemented FIV-infected); significant differences between groups and significant variation between time points as assayed using two kits
| Group | Day 0 U/ml (old kit) | Day 30 (new kit) | Day 0 (new kit) | Day 30 (new kit) |
|---|---|---|---|---|
| Suppl FIV-inf | 220 ** | 123 * | N/A | N/A |
| Control | 184 ** | 68 | N/A | N/A |
| FIV-inf no. suppl | N/A | N/A | 64.5 | 58 |
P<0.05 suppl (supplemented) FIV-inf (infected) day 30 compared to control day 30;
P<0.05 day 0 (old kit) compared to day 30 suppl (new kit). The kit used to determine day 0 SOD levels was used in its entirety following completion of those assays, and a new kit was employed for all subsequent measurements. N/A=not applicable; assay was not run at that time point for that group.
MDA
MDA is an end-product of free radical-induced cell membrane lipid peroxidation. Heparinized whole blood (250 μl) is transferred into an ethylenediaminetetraacetic acid (EDTA) tube, which is maintained at −70°C until analysis. The whole blood sample is thawed and pipetted into a solution of 0.9 mM butylated hydroxytoluene in absolute alcohol. A 0.03 mM solution of thiobarbituric acid in 50% glacial acetic acid is added, and the resulting solution is heated for approximately 1 h. The reaction mixture is cooled and acidified prior to adding n-butanol. The MDA is extracted into the n-butanol and the fluorescent emission at 550 nm is compared to that of standardizing solutions to determine the micromoles MDA per liter of whole blood.
Determination of leukocyte GSH concentrations using flow cytometry
GSH is a ubiquitous intracellular thiol-containing antioxidant. The intracellular conjugation of monochlorobimane and GSH produces a fluorescent product that can be detected using flow cytometry and allows quantification of GSH levels in specific lymphocyte subsets. Erythrocytes were lysed (NH4Cl) from 500 μl of heparinized whole blood and the remaining leukocyte suspension was washed twice in Hanks Balanced Salt Solution (HBSS) solution and resuspended in FACS buffer (Phosphate Buffered Saline (PBS) with 2% Fetal Bovine Serum (FBS) and 0.1% sodium azide). Monochlorobimane (Molecular Probes Division of Invitrogen Corporation, Carlsbad, CA) was added to a leukocyte suspension (1×107 cells/μl) to a final concentration of 20 μM and the cells were maintained at room temperature in the dark for 20 min before analysis. Monochlorobimane fluorescence was assessed by flow cytometry (Cyan MLE flow cytometer, Dako, Fort Collins, CO) using an ultraviolet laser with excitation wavelength at 350 nm. Neutrophils and monocytes were gated based on their specific forward and side-scatter characteristics (Byrne et al 2000). Antibodies specific for feline leukocyte cell surface determinants were used to confirm the identity of CD4+ and CD8+ lymphocytes as described previously (Webb et al 2006). Immediately after cell surface staining, samples were treated with mBCl as described above and analyzed by flow cytometry. Data were analyzed using commercially available software (Summit Software, Dako, Fort Collins, CO).
Statistical analyses
Following confirmation of a Gaussian distribution using the Kolmogorov–Smirnov test, a comparison of the means for determination of statistically significant differences between groups was performed using either a paired or an unpaired, two-tailed Student's t-test where appropriate, assuming unequal variance with a Welch correction. For non-parametric data the Kruskal–Wallis test was applied, followed by Dunn's multiple comparison test where appropriate. Statistical analysis was done using commercially available computer software (Summit software, Dako, Fort Collins, CO). A P value<0.05 was considered significant for all statistical analyses performed in this study. Results are presented as means±standard deviation (SD) where appropriate.
Results
None of the cats showed any clinical signs of illness or changes in appetite, activity or behavior during the course of the study.
CBC
Prior to SOD supplementation the absolute number of lymphocytes was 4.78×103 cells/μl and 3.78×103 cells/μl in the FIV-infected and control groups, respectively, and none of the cats had any remarkable hematologic abnormalities. The total lymphocyte count did not change significantly in either group after 30 days of supplementation; 5.33×103 cells/μl and 3.54×103 cells/μl for the supplemented FIV-infected and control groups, respectively. The total lymphocyte count for the unsupplemented FIV-infected group was 4.1×103 cells/μl and did not change significantly during the course of the study.
Measures of oxidative stress
There was no significant difference in the SOD enzyme concentration between the supplemented FIV-infected group and the uninfected control group prior to supplementation (Table 1). Following supplementation of the supplemented FIV-infected cats with 30 days of SOD complex, the mean±SD SOD enzyme concentration was significantly greater in supplemented FIV-infected cats than the uninfected control group or the unsupplemented FIV-infected cats; 123.4±15, 67.6±34, and 58.0±11.5 U/ml, respectively (P<0.05) (Fig 2). There was a significant decrease in the SOD enzyme concentration in both the supplemented FIV-infected group and the uninfected control group from day 0 to day 30 time points (Table 1); this difference is attributed to inter-kit variation (see Materials and methods). There was no significant change in the SOD concentration in the unsupplemented FIV-infected group from day 0 to day 30 (64.5±7.5 U/ml; 58.0±11.5 U/ml; P=0.4; Table 1). There was no statistically significant difference in the means between the supplemented FIV-infected and uninfected control groups for GPx or MDA levels (Table 2). These assays were not performed in the unsupplemented FIV-infected group.
Fig 2.
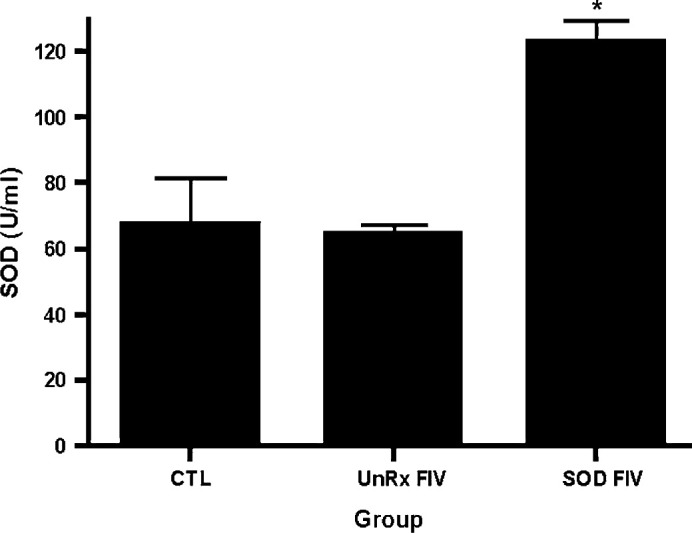
SOD activity in uninfected control and FIV-infected cats. The whole blood SOD enzyme concentration in six FIV-infected cats given oral SOD complex for 4 weeks was compared to six uninfected control cats and six untreated FIV-infected cats. The SOD supplemented FIV-infected cats had significantly greater SOD levels following supplementation. Kruskal–Wallis non-parametric test demonstrated a significant difference (P=0.0062) and Dunn's multiple comparison test confirmed that the SOD supplemented FIV-infected group was significantly different than both the uninfected control and UnRx FIV-infected groups (*P<0.05).
Table 2.
MDA and GPx concentrations in the supplemented FIV-infected and uninfected control groups pre- and post-supplementation
| Group | MDA (μmol/l) | GPx (U/l) | ||
| Day 0 | Day 30 | Day 0 | Day 30 | |
| Suppl FIV-inf | 36.8 | 33.5 | 96,217 | 119,800 |
| Uninfected control | 30.3 | 32.5 | 109,500 | 92,167 |
No significant differences found between suppl (supplemented). FIV-inf (infected) and uninfected control groups.
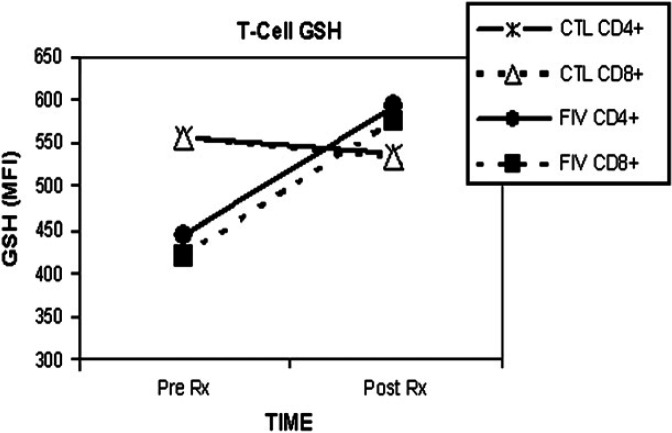
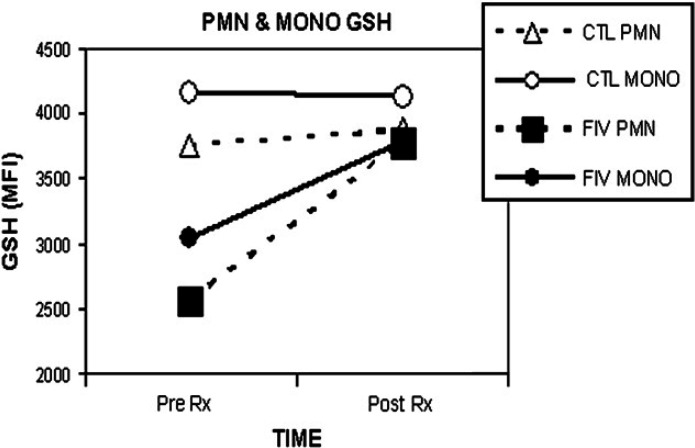
There was no significant change in specific leukocyte GSH levels, as assessed with mBCl using flow cytometry following SOD enzyme supplementation (Table 3; Figs 3 and 4).
Table 3.
Intracellular GSH levels in different leukocytes pre- and post-supplementation with SOD
| Group | CD4+ GSH (MFI) | CD8+ GSH (MFI) | PMN GSH (MFI) | MONO GSH (MFI) | ||||
| Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | |
| Suppl FIV-inf | 445 | 593 | 420 | 570 | 2571 | 3776 | 3050 | 3792 |
| Uninfected control | 559 | 539 | 556 | 534 | 3764 | 3871 | 4169 | 4124 |
No significant differences found between groups for GSH levels measures as MFI using monochlorobimane and flow cytometry. MONO=monocytes, PMN=neutrophils.
Fig 3.
GSH for specific lymphocytes following SOD supplementation. The mean GSH level in CD4+ and CD8+ T-cells is shown for the FIV-infected and the uninfected control group (n=6 per group). Pre Rx is prior to SOD enzyme supplementation; Post Rx is following 30 days of SOD enzyme supplementation to the FIV-infected (closed symbols) cats, compared to uninfected control cats (CTL, open symbols). GSH; MFI=mean fluorescence intensity as measured with flow cytometry. The CD4+ to CD8+ ratio increased significantly in the supplemented FIV-infected group (*P=0.015).
Fig 4.
GSH for specific leukocytes following SOD supplementation. The mean GSH level in neutrophils (PMN) and monocytes (MONO) is shown for the FIV-infected and the uninfected control group (n=6 per group). Pre Rx is prior to SOD enzyme supplementation; Post Rx is following 30 days of SOD enzyme supplementation to the FIV-infected (closed symbols) cats, compared to uninfected control cats (CTL, open symbols). GSH; MFI=mean fluorescence intensity as measured with flow cytometry.
Proviral load
Proviral load was defined as the number of FIV C gag copies per 106 Peripheral Blood Mononuclear Cells (PBMCs) as determined by real-time PCR. Proviral load within the SOD supplemented FIV-infected cat group did not change significantly (mean number of copies prior to and following supplementation was 12,543±7976 and 9759±5202, respectively).
CD4:CD8 ratio
Before supplementation the CD4+ to CD8+ lymphocyte ratio for both the supplemented FIV-infected cats and the unsupplemented FIV-infected cats was significantly less than the CD4+ to CD8+ lymphocyte ratio in the uninfected control cats (0.66±0.17, 0.77±0.25, and 1.79±0.39, respectively; P<0.0005). Following 30 days of supplementation the CD4+ to CD8+ ratio in the supplemented FIV-infected cats had increased significantly (0.66–0.88) (P=0.015). The CD4+ to CD8+ ratio for the unsupplemented FIV-infected control group and the uninfected control cats did not change significantly over the 30-day period (day 0, 0.77±0.25 and 1.79±0.39, respectively; day 30, 0.67±0.28 and 1.62±0.32, respectively).
Discussion
This study was designed to test the hypothesis that oral supplementation with a specific SOD complex would affect parameters of oxidative stress in FIV-infected cats. Oxidative stress is an important component of the pathogenesis of HIV infection in humans, and cats appear to be particularly susceptible to the effects of oxidative stress, making antioxidant supplementation in FIV-infected felines of important potential clinical relevance. The cats in this study never appeared ill and were never challenged by concurrent disease, and so their actual level of oxidative stress over the course of this study may have been minimal.
The erythrocyte SOD concentration in six unsupplemented FIV-infected cats did not change significantly over a 30-day period. Although the unsupplemented FIV-infected group was not a placebo control group this result suggests that the significantly greater concentration of SOD in the supplemented FIV-infected cats was due to the supplementation and not some change in the progression of the disease (unsupplemented FIV-infected cats) or some random environmental or management condition over 30 days. The normal physiologic response to chronic oxidative stress likely involves changes in either the translation of antioxidant genes or the transcription of those gene products. Although gene therapy for conditions of chronic oxidative stress is under investigation, supplementation of the particular antioxidant enzyme complex studied may afford a more rapid and reversible form of therapy. None of the supplemented cats showed any adverse clinical signs while receiving the supplement, and administration of the supplement in the cat's food was very well tolerated, fulfilling several important requirements if long-term antioxidant supplementation is going to be considered for the therapy of feline diseases.
The CD4+ to CD8+ lymphocyte ratio is considered an indicator of prognosis in chronically FIV-infected cats, and as the disease progresses CD4+ cells are lost (Holznagel et al 1998, Kohmoto et al 1998, Paillot et al 2005). The CD4+ to CD8+ ratio was, as expected, low in the supplemented FIV-infected group of cats before supplementation. With SOD enzyme supplementation this ratio increased significantly, possibly representing a decrease in the FIV effect on T-cell survival.
GSH is a ubiquitous non-enzymatic antioxidant that plays a central role in maintaining intracellular oxidative balance (Valko et al 2007). No significant change was seen in intracellular GSH levels following SOD supplementation in the supplemented FIV-infected group. However, prior to supplementation, all of the cats in the supplemented FIV-infected group had GSH levels in all leukocyte subtypes that were lower than in their corresponding uninfected control group. Following supplementation, the GSH level in the CD4+ and CD8+ cells of the supplemented FIV-infected group was higher than their corresponding uninfected control cell types. Determination of intracellular GSH levels following a longer course of supplementation appears warranted as this tripeptide is an important part of cellular defense against oxidative stress. In an earlier study on the use of mBCl to detect GSH in feline leukocytes it was reported that chronically FIV-infected cats had significantly lower levels of GSH than control cats (Webb et al 2006). That was not found to be the case in this study. Potential explanations for differences in the two studies include a difference in the numbers of cats, a difference in the acute versus chronic nature of the infection, or a difference in the infecting virus (clade B instead of clade C).
Although there was a significantly greater concentration of SOD enzyme in the supplemented FIV-infected cats compared to the uninfected control group following SOD enzyme supplementation, the level of GPx enzyme was not different between groups either before or after treatment. Humans with HIV/AIDS have decreased levels of activity of this selenoenzyme, but the role of the GPx enzyme in HIV infection is complex (Baum and Shor-Posner 1998). It appears to be active in stimulating the replication associated with an acutely spreading HIV infection (Diamond and Hu 2001). It is possible that long-term SOD supplementation may increase GPx activity, which would stimulate viral replication, although that did not appear to be the case in the time frame reported in this study. The lack of a difference in GPx between groups in this study may be a reflection of the number of cats per group, the lack of severity or chronicity of disease, or other species differences. It is not surprising that the activities of these two antioxidant enzymes are not bound together in a linear fashion, although the potential for interaction warrants further study.
MDA is a metabolic end-product of free radical-induced lipid peroxidation, a deleterious consequence of oxidative stress on cell membranes. The lack of a change in MDA levels may be a reflection of the fact that MDA is only one of many lipid peroxidation end-products, or it may reflect disease severity. None of the FIV-infected cats was ever anemic.
Although the treatment group and the unsupplemented group consisted of FIV-infected cats, none of the animals showed any signs of illness during the study. Although FIV is a naturally occurring feline disease, these cats were housed in a pathogen-free environment and did not face any of the infectious disease challenges to which client-owned cats are exposed. It is possible that had the cats been challenged by concurrent diseases their antioxidant defenses would have been called upon to a much greater degree and larger differences between supplemented and unsupplemented control groups would have emerged.
In addition to the potential benefits of antioxidant supplementation in cats with viral infections, there are a number of other feline diseases where effective antioxidant therapy would likely be helpful and warrant further investigation.
Acknowledgements
The authors wish to acknowledge Nutramax Laboratories for their generous support of research into oxidative stress and veterinary diseases. Nutramax sponsored a portion of the current study and provided the supplementation and placebo used in this work.
References
- Adamson G.M., Billings R.E. Tumor necrosis factor induced oxidative stress in isolated mouse hepatocytes, Archives of Biochemistry and Biophysics 294, 1992, 223–229. [DOI] [PubMed] [Google Scholar]
- Baum M.K., Shor-Posner G. Micronutrient status in relationship to mortality in HIV-1 disease, Nutrition Reviews 56, 1998, S135–S139. [DOI] [PubMed] [Google Scholar]
- Byrne I.M., Kim H.W., Chew B.P., Reinhart G.A., Hayek M.G. A standardized gating technique for the generation of flow cytometry data for normal canine and normal feline blood lymphocytes, Veterinary Immunology and Immunopathology 73, 2000, 167–182. [DOI] [PubMed] [Google Scholar]
- Diamond A.M., Hu Y.J. Glutathione peroxidase and viral replication: implications for viral evolution and chemoprevention, Biofactors 14, 2001, 205–210. [DOI] [PubMed] [Google Scholar]
- Dow S.W., Mathiason C.K., Hoover E.A. In vivo monocyte tropism of pathogenic feline immunodeficiency viruses, Journal of Virology 73, 1999, 6852–6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L.A., De Rosa S.C., Dubs J.G., Roederer M., Anderson M.T., Ela S.W., Deresinski S.C., Herzenberg L.A. Glutathione deficiency is associated with impaired survival in HIV disease, Proceedings of the National Academy of Sciences of the United States of America 94, 1997, 1967–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holznagel E., Hofmann-Lehmann R., Leutenegger C.M., Allenspach K., Huettner S., Forster U., Niederer E., Joller H., Willett B.J., Hummel U., Rossi G.L., Schupbach J., Lutz H. The role of in vitro-induced lymphocyte apoptosis in feline immunodeficiency virus infection: correlation with different markers of disease progression, Journal of Virology 72, 1998, 9025–9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmoto M., Uetsuka K., Ikeda Y., Inoshima Y., Shimojima M., Sato E., Inada G., Toyosaki T., Miyazawa T., Doi K., Mikami T. Eight-year observation and comparative study of specific pathogen-free cats experimentally infected with feline immunodeficiency virus (FIV) subtypes A and B: terminal acquired immunodeficiency syndrome in a cat infected with FIV petaluma strain, Journal of Veterinary Medical Science 60, 1998, 315–321. [DOI] [PubMed] [Google Scholar]
- Leutenegger C.M., Klein D., Hofmann-Lehmann R., Mislin C., Hummel U., Boni J., Boretti F., Guenzburg W.H., Lutz H. Rapid feline immunodeficiency virus provirus quantitation by polymerase chain reaction using the TaqMan fluorogenic real-time detection system, Journal of Virological Methods 78, 1999, 105–116. [DOI] [PubMed] [Google Scholar]
- Marshall M.D., Samantha L.W., Skinner N.D., Charlton C.J., Harper E.J. Feline antioxidant enzyme activity: the effect of sample storage on stability, Journal of Nutrition 132, 2002, 1733S–1734S. [DOI] [PubMed] [Google Scholar]
- Paillot R., Richard S., Bloas F., Piras F., Poulet H., Brunet S., Andreoni C., Juillard V. Toward a detailed characterization of feline immunodeficiency virus-specific T cell immune responses and mediated immune disorders, Veterinary Immunology and Immunopathology 106, 2005, 1–14. [DOI] [PubMed] [Google Scholar]
- Staal F.J.T., Roederer M., Herzenberg L.A., Herzenberg L.A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus, Proceedings of the National Academy of Sciences of the United States of America 87, 1990, 9943–9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal F.J.T., Anderson M.T., Staal G.E.J., Hersenberg L.A., Gitler C., Herzenberg L.A. Redox regulation of signal transduction: tyrosine phosphorylation and calcium influx, Proceedings of the National Academy of Sciences of the United States of America 91, 1994, 3619–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronan M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease, International Journal of Biochemistry and Cell Biology 39, 2007, 44–84. [DOI] [PubMed] [Google Scholar]
- Vouldoukis I., Lacan D., Kamate C., Coste P., Calenda A., Mazier D., Conti M., Dugas B. Antioxidant and anti-inflammatory properties of a Cucumis melo LC. extract rich in superoxide dismutase activity, Journal of Ethnopharmacology 94, 2004a, 67–75. [DOI] [PubMed] [Google Scholar]
- Vouldoukis I., Conti M., Krauss P., Kamate C., Blazquez S., Tefit M., Mazier D., Calenda A., Dugas B. Supplementation with gliadin-combined plant superoxide dismutase extract promotes antioxidant defences and protects against oxidative stress, Phytotherapy Research 18, 2004b, 957–962. [DOI] [PubMed] [Google Scholar]
- Webb C.B., Bedwell C., Guth A., Avery P., Dow S. Use of flow cytometry and monochlorobimane to quantitate intracellular glutathione concentrations in feline leukocytes, Veterinary Immunology and Immunopathology 112, 2006, 129–140. [DOI] [PubMed] [Google Scholar]
- Yu S., Paetau-Robinson I. Dietary supplements of vitamins E and C and beta-carotene reduced oxidative stress in cats with renal insufficiency, Veterinary Resident Communication 30, 2006, 403–413. [DOI] [PubMed] [Google Scholar]