Abstract
OBJECTIVE
To evaluate the efficacy of LX9211 in reducing pain related to diabetic peripheral neuropathy.
RESEARCH DESIGN AND METHODS
In this double-blind, multicenter, proof-of-concept trial, 319 individuals with diabetic peripheral neuropathic pain (DPNP) were randomized (1:1:1) to LX9211 10 mg (n = 106), LX9211 20 mg (n = 106), or matching placebo (n = 107), administered once daily for 6 weeks. DPNP was rated daily with an 11-point numerical rating scale. The primary end point was change from baseline to week 6 in the average daily pain score. The difference between each LX9211 group and placebo was evaluated with mixed-model repeated-measures analysis.
RESULTS
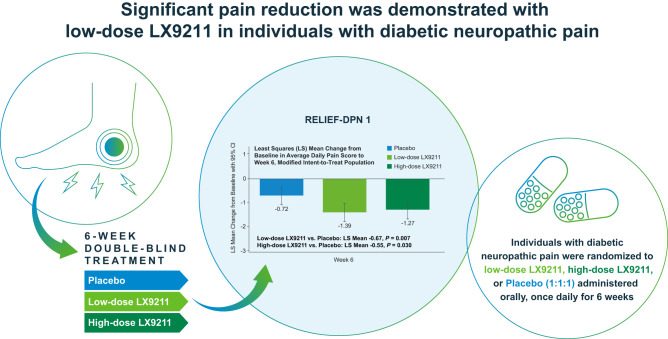
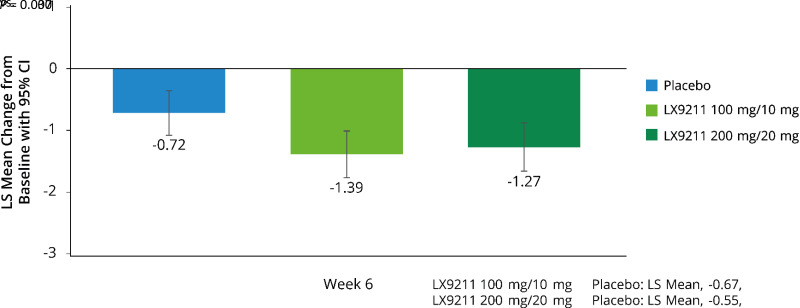
For those on low-dose LX9211 the primary efficacy end point was achieved: −1.39 vs. −0.72 points for placebo, least squares mean (SE) difference −0.67 (0.249), 95% CI −1.16 to −0.18, P = 0.007; results for high-dose LX9211 demonstrated improvement in pain severity versus placebo (−1.27 vs. −0.72 points, respectively), but the between-group LS mean difference did not reach the prespecified statistical significance (−0.55 [0.254], 95% CI −1.06 to −0.05, P = 0.030). Treatment benefit was observed beginning at week 1 and maintained thereafter. Results for LX9211 also demonstrated improvement in several patient-reported secondary outcomes. Most common adverse events (AEs) were dizziness, nausea, and headache. More participants treated with LX9211 (20 mg, n = 28 [26.4%]; 10 mg, 17 [16.0%]) than placebo (3 [2.8%]) discontinued study drug prematurely due to AEs; serious AEs were uncommon (2 [1.9%], 0, and 1 [0.9%], respectively).
CONCLUSIONS
These preliminary findings of improvement in DPNP with LX9211 support further investigation in larger trials.
Graphical Abstract
Introduction
Diabetic peripheral neuropathy (DPN) is a major chronic complication of diabetes (1), affecting approximately 50% of individuals with diabetes (1,2), 30–40% of whom experience neuropathic pain (NP) (3–5). Furthermore, obesity, metabolic syndrome, and prediabetes increase the risk of peripheral neuropathy (6), underscoring the growing numbers of people at risk for peripheral neuropathy with a metabolic etiology. Painful DPN leads to serious comorbidities including anxiety, depression and other mood disorders, insomnia, gait disturbances, and loss of physical function, all adversely affecting patients’ quality of life (1,5,7,8). The polypharmacy often required to treat diabetic peripheral neuropathic pain (DPNP) further exacerbates these problems (1,4,9).
Despite the burden of illness, currently available therapies for DPNP either alone or in combination provide only modest pain relief, with fewer than half of individuals achieving 50% reduction in pain severity (10). Additionally, most people experience undesirable, dose-limiting adverse events (AEs) with existing therapies (e.g., dizziness, lethargy, weight gain) (9,11,12). Taken together, there is a substantial unmet need for effective and well-tolerated treatment options for relief of DPNP.
Adapter protein-2–associated kinase 1 (AAK1) was identified as a novel nonopioid therapeutic target for NP (13). LX9211 is an orally administered, selective, potent inhibitor of AAK1 (14). In phase 1 clinical studies, LX9211 was safe and well tolerated (15). In a single ascending dose study (from 5 to 300 mg), the half-life of LX9211 ranged from 143 to 197 h. In a multiple ascending dose study, in which a loading dose of LX9211 was administered on day 1 followed by a maintenance dose (i.e., 25 and 2.5, 50 and 5.0, 100 and 10.0, 150 and 15.0, or 200 and 20.0 mg) administered once daily in the ensuing 13 days, steady-state plasma concentrations were rapidly attained and maintained, with no accumulation observed. Findings from these studies support the further evaluation of LX9211 for treatment of DPNP.
Herein we report results from a phase 2 proof-of-concept trial, RELIEF-DPN 1, that was conducted to test the hypothesis that LX9211 is more efficacious than placebo for reducing NP among adults with DPNP.
Research Design and Methods
Ethics Practices
Independent review boards approved the study protocol, its amendments, and the patient informed consent form. The study was conducted in accordance with good clinical practice and applicable regulatory requirements. All participants provided written informed consent before participation. The study is registered at ClinicalTrials.gov (clinical trial reg. no. NCT04455633).
Study Population
The trial enrolled adults (aged ≥18 years) with a diagnosis of type 1 or type 2 diabetes (16) (HbA1c ≤11%), a stable antihyperglycemic regimen for at least 1 month prior to screening, BMI between 18 and 40 kg/m2 at screening, confirmed DPN (Michigan Neuropathy Screening Instrument clinical examination score of ≥2.5), and chronic NP (lasting at least 6 months). Additional eligibility criteria required that participants rate their DPNP as moderate to severe (based on average daily pain score [ADPS] ≥5) in the 14 days prior to randomization.
Main exclusions were other painful conditions that could have confounded self-evaluation of DPNP, psychiatric comorbidities that could interfere with pain self-assessments, clinically significant substance or alcohol use disorder, and history of neurolytic or neurosurgical therapy for peripheral neuropathy. Use of opioid medications for managing DPNP within the 2 months prior to screening and/or chronic nonsteroidal anti-inflammatory agents in the 2 weeks prior to screening was also exclusionary. Individuals could continue medications prescribed for DPNP, including pregabalin, gabapentin, and antidepressant medications, that had been taken at stable doses for ≥1 month prior to screening (Supplementary Table 1).
Trial Design, Study Drug, and Randomization
This double-blind, randomized, placebo-controlled, multicenter study was conducted from 3 September 2020 to 28 December 2022 at 40 study sites in the U.S. After a screening period of up to 2 weeks, eligible participants entered a 2-week single-blind placebo run-in period, followed by a 6-week double-blind treatment period and a 5-week single-blind safety follow-up period (Supplementary Fig. 1).
On day 1 of the run-in period, participants were administered the first dose of placebo tablets at a research site. On days 2–14, they were instructed to take their daily dose of study drug before the first meal of the day with water. All eligible participants entered the 6-week double-blind treatment period and were randomized (1:1:1) to placebo or one of two doses of LX9211 (10 or 20 mg, once daily). Balanced randomization was achieved using randomly permuted blocks of six with stratification by pain intensity at baseline. An Interactive Voice/Web Response System was used as a central mechanism to assign patients to study treatment. Investigators, study site personnel, participants, and the sponsor and its designees remained blinded until database lock.
On day 1 of the double-blind period, the first dose of study drug was administered at a research site. Each dose level of LX9211 was initiated with a loading dose (10× the maintenance dose) followed by a daily maintenance dose (Supplementary Fig. 1). Dose adjustments were not permitted. During the safety follow-up period, all participants received a single placebo tablet daily. At the investigator’s discretion, participants were allowed to take up to 3 g acetaminophen/day as rescue medication, if needed.
Assessments and Outcome Measures
Sex, race, other demographics, and medical history at baseline were determined by self-report of participants.
Several DPNP-related patient-reported outcomes were assessed. The Brief Pain Inventory Short Form for Diabetic Peripheral Neuropathy (BPI-DPN) is a nine-item questionnaire for assessment of severity of pain and its impact on functioning in people with DPN. It was administered at baseline and at weeks 2, 4, 6, and 11. The Neuropathic Pain Symptom Inventory (NPSI) instrument (17), which includes 10 items related to different pain descriptors and 2 items on frequency and duration of pain, was also used to assess NP at baseline and weeks 6 and 11. The Patient Global Impression of Change (PGIC) instrument (18), a 7-point numerical rating scale (from 1, very much improved, to 7, very much worse), was used to rate participants’ beliefs about overall improvement at weeks 6 and 11.
Beginning at baseline and through week 11 (or 35 days after end of treatment/premature discontinuation), each evening participants rated the intensity of pain over the previous 24 h. This rating was based on participants responding to question no. 5 of the BPI-DPN (19), “Please rate your pain due to your diabetes by indicating the one number that best describes your pain on the average,” using an 11-point numerical rating scale (from 0, no pain, to 10, pain as bad as you can imagine) and recording the pain severity rating in a daily pain e-diary. Participants also recorded any use of rescue acetaminophen and rated the interference of pain with sleep by responding to question no. 9F of the BPI-DPN (11-point numerical rating scale from 0, does not interfere, to 10, completely interferes).
Efficacy End Points
The primary efficacy end point was the change from baseline to week 6 in ADPS. Secondary efficacy end points included change from baseline to week 6 in BPI-DPN and NPSI scores, and the PGIC at week 6.
Assessments of Safety
Safety was assessed through monitoring of AEs, vital signs, electrocardiogram findings, and laboratory parameters.
An independent data monitoring committee (DMC), whose members did not participate in patient care or efficacy/safety assessments, reviewed unblinded safety data after 25%, 50%, and 75% target enrollment. No safety signal of concern was raised by the DMC.
Statistical Analyses
All randomized participants who received at least one dose of study drug in the double-blind treatment period were included in the safety analysis set. Efficacy data were analyzed in a modified intent-to-treat analysis set, which included all participants in the safety analysis set.
Sample Size Determination
A sample size of 75 patients per treatment group was calculated to yield 80% power to detect a true mean difference of 1 unit in ADPS change from baseline between at least one of the LX9211 treatment groups and placebo, assuming a common SD of 2 and an overall significance level of α = 0.05 (two-sided Dunnett test). With a dropout rate of 20% accounted for, a total of 282 patients (94 patients per treatment group) were enrolled and randomly assigned to treatment in a 1:1:1 ratio. This sample size was adjusted for the multiple comparisons of each LX9211 treatment group with placebo.
Efficacy Analyses
We calculated ADPS using all available daily pain diary data. A minimum of 5 days for baseline based on week 2 of the single-blind run-in period and 4 days from the last week prior to each clinic visit was required for the calculation of weekly averages.
For the primary end point (change from baseline to week 6 in ADPS), a mixed-effects model repeated-measures (MMRM) approach was used to assess the difference between LX9211 and placebo. The MMRM included fixed effects of treatment, week, treatment-by-week interaction, the randomization factor of baseline pain severity, and baseline score as a covariate. An unstructured covariance structure was used to model the within-patient error. If the convergence were not met, other covariance structures could have been explored. A P value of <0.028 was considered statistically significant. This was based on the Dunnett test comparing each of the two treatments versus placebo, with an adjusted significance level to preserve an overall type I error α = 0.05. The primary end point was analyzed for subgroups by sex, by baseline pain severity (moderate [ADPS 5–7] or severe [ADPS 8–9]), and by use of medications for DPNP at baseline.
PGIC response at week 6 was analyzed with an ANOVA model with treatment and randomization stratum of baseline pain severity (moderate, severe) as independent variables. In post hoc analysis, time to initiation of rescue medication was analyzed with a Cox proportional hazards model with stratification by randomized baseline pain severity.
Interim Efficacy Analyses
A prespecified interim efficacy analysis for futility, using a two-sided overall α = 0.05 O’Brien-Fleming group sequential test, was planned after the first 141 participants had been accrued and followed to the end point at week 6 or discontinued the trial—whichever occurred first. The DMC recommended continuing the study without an adjustment of sample size.
Safety Analyses
Safety was assessed through evaluation of all reported AEs, actual and change in clinical laboratory values, vital signs, and electrocardiograms. Baseline for computing the change in safety variables was the observation measure before the first dose of study drug. No formal statistical significance tests were performed for safety data.
In post hoc analysis, time to early termination in the double-blind treatment period was analyzed with a Cox proportional hazards model with stratification by randomized baseline pain severity. Subgroup analysis by baseline use of medications for DPNP was performed with the same model.
Data and Resource Availability
Inquiries regarding specific data and methods can be made to the corresponding author.
Results
Participants and Treatment
A total of 557 participants met the screening criteria for the RELIEF-DPN 1 study and were enrolled into the 2-week placebo run-in period, 319 of whom remained eligible to progress into the double-blind treatment period (106 participants each in the LX9211 groups and 107 participants in the placebo group) (Supplementary Fig. 2). The double-blind treatment period was completed by 80.2% of participants in the low-dose LX9211 group, 66.0% of participants in the high-dose LX9211 group, and 94.4% of participants in the placebo group. All but 1 (in the placebo group) of 295 participants who entered completed the 5-week safety follow-up period.
Overall, the enrolled cohort included more men than women (58.6% vs. 41.4%, respectively), of whom 76.5% were White and 18.5% Black and 16.9% identified as Hispanic. Mean (SD) age was 62 (10) years and BMI 32.1 (4.45) kg/m2. DPN duration was 6.6 (5.43) years. Three-quarters of the participants had moderate pain at baseline, and the others reported severe pain. HbA1c was 7.7% (1.28%). The treatment groups were similar with respect to demographic and clinical characteristics at baseline (Table 1).
Table 1.
Demographic and other characteristics of participants randomized into the double-blind treatment period of the RELIEF-DPN 1 study
| LX9211 10 mg | LX9211 20 mg | Placebo | Total | |
|---|---|---|---|---|
| N | 106 | 106 | 107 | 319 |
| Demographics | ||||
| Age (years), mean (SD) | 63 (9) | 62 (11) | 62 (10) | 62 (10) |
| Sex, n (%) | ||||
| Male | 58 (54.7) | 63 (59.4) | 66 (61.7) | 187 (58.6) |
| Female | 48 (45.3) | 43 (40.6) | 41 (38.3) | 132 (41.4) |
| Race, n (%) | ||||
| White | 79 (74.5) | 83 (78.3) | 82 (76.6) | 244 (76.5) |
| Black | 22 (20.8) | 18 (17.0) | 19 (17.8) | 59 (18.5) |
| Asian | 3 (2.8) | 1 (0.9) | 1 (0.9) | 5 (1.6) |
| Other | 2 (1.9) | 4 (3.8) | 5 (4.7) | 11 (3.4) |
| BMI (kg/m2), mean (SD) | 32.1 (4.27) | 32.1 (4.58) | 32.0 (4.53) | 32.1 (4.45) |
| Diabetes characteristics | ||||
| Type 1, n (%) | 5 (4.7) | 5 (4.7) | 5 (4.7) | 15 (4.7) |
| Type 2, n (%) | 87 (82.1) | 83 (78.3) | 87 (81.3) | 257 (80.6) |
| Type not reported, n (%) | 14 (13.2) | 18 (17.0) | 15 (14.0) | 47 (14.7) |
| HbA1c, % (mmol/mol), mean (SD) | 7.7 (61) | 7.6 (60) | 7.7 (61) | 7.7 (61) |
| Duration of NP (years), mean (SD) | 7.0 (6.15) | 6.5 (5.48) | 6.4 (4.62) | 6.6 (5.43) |
| Medication use for NP at baseline, n (%) | 44 (41.5) | 42 (39.6) | 59 (55.1) | 145 (45.5) |
| Gabapentin | 41 (38.7) | 38 (35.8) | 54 (50.5) | 133 (41.7) |
| Pregabalin | 2 (1.9) | 2 (1.9) | 3 (2.8) | 7 (2.2) |
| Duloxetine | 4 (3.8) | 3 (2.8) | 7 (6.5) | 14 (4.4) |
| Other | 2 (1.9) | 2 (1.9) | 4 (3.7) | 8 (2.5) |
| Pain intensity*† | ||||
| ADPS, mean (SD) | 6.6 (1.09) | 6.5 (1.03) | 6.5 (1.15) | 6.6 (1.09) |
| Moderate pain, n (%)‡ | 82 (77.4) | 82 (77.4) | 82 (76.6) | 246 (77.1) |
| Severe pain, n (%)‡ | 24 (22.6) | 24 (22.6) | 25 (23.4) | 73 (22.9) |
N = 106, N = 104, and N = 106 for the low-dose LX9211, high-dose LX9211, and placebo groups, respectively.
Scale of 0–10 based on patient’s answer to question no. 5 of BPI-DPN; higher scores indicate greater pain intensity.
Moderate, 5–7; severe, 8–9.
Mean (SD) time of exposure to study drug during the double-blind treatment period was 38.1 (12.7), 35.4 (14.3), and 41.7 (6.0) days for the low-dose LX9211, high-dose LX9211, and placebo groups, respectively. Adherence to study drug was 93.7%, 93.0%, and 98.9% for the respective treatment groups. More participants in the placebo group (55.1%) compared with the active treatment groups (41.5% low-dose, 39.6% high-dose LX9211) were on a concomitant drug for relief of DPNP (Table 1).
Efficacy Results
Results for LX9211 showed achievement of the primary efficacy end point of the trial in the low-dose group, demonstrating a statistically significant reduction in pain at week 6 in comparisons with placebo (−1.39 vs. −0.72 for placebo, least squares [LS] mean [SE] difference −0.67 [0.25], 95% CI −1.16 to −0.18, P = 0.007); results in the high-dose LX9211 group demonstrated numerically greater improvement in pain intensity in comparisons with placebo (−1.27 vs. −0.72 points, respectively) but did not reach the prespecified level of statistical significance (LS mean difference −0.55 [0.25], 95% CI −1.06 to −0.05, P = 0.030) (Table 2, Fig. 1). The treatment effect (LX9211 vs. comparator) was consistent in subgroup analyses by sex and by baseline use of medications for DPNP. It was notably larger for those in the low-dose versus high-dose LX9211 group among participants with severe DPNP (Supplementary Fig. 3). Treatment effect was larger in the absence of baseline DPNP medication (Supplementary Fig. 3).
Table 2.
Change in ADPS* from baseline to week 6 of the RELIEF-DPN 1 study
| LX9211 10 mg | LX9211 20 mg | Placebo | |
|---|---|---|---|
| N | 106 | 106 | 107 |
| Baseline† | |||
| N | 106 | 104 | 106 |
| Mean | 6.6 | 6.5 | 6.5 |
| MMRM analysis‡ | |||
| N | 87 | 77 | 99 |
| Change from baseline to week 6 | |||
| LS mean (SE) | −1.39 (0.19) | −1.27 (0.20) | −0.72 (0.18) |
| 95% CI for change from baseline | −1.77 to −1.01 | −1.66 to −0.88 | −1.08 to −0.36 |
| P | <0.001 | <0.001 | <0.001 |
| Between-group comparison | |||
| LS mean (SE) difference vs. placebo | −0.67 (0.25) | −0.55 (0.25) | |
| 95% CI for difference | −1.16 to −0.18 | −1.06 to −0.05 | |
| Two-sided P | 0.007 | 0.030 |
Week 6 ADPS was calculated with use of daily pain diary data between weeks 5 and 6, with a minimum of 4 days (consecutive or nonconsecutive) in the week required for the calculation.
The scale is 0–10 based on patient’s answer to question no. 5 of BPI-DPN; higher scores indicate greater pain intensity. Negative change in score indicates improvement. Negative difference favors LX92111 over placebo.
Baseline value was defined as the average of the week 2 run-in period data collected by patients in the daily pain diary, provided that data for ≥5 days from that period were available for analysis.
MMRM included fixed effects of treatment, week, treatment-by-week interaction, the randomization stratum of baseline pain severity (moderate, severe), and baseline ADPS score as a covariate. Unstructured covariance structure was used to model the within-patient error.
Figure 1.
LS mean difference in change from baseline in ADPS at week 6. ADPS was derived from participants’ answer to question no. 5 of the BPI-DPN (“Please rate your pain due to your diabetes by indicating the one number that best describes your pain on the average,” using an 11-point numerical rating scale [0 = no pain to 10 = pain as bad as you can imagine]). Baseline value was defined as the average of the week 2 run-in period data. A minimum of 4 days (consecutive or nonconsecutive) of daily pain diary data from week 5 to week 6 was required for the calculation of mean weekly ADPS at week 6.
Improvement of pain from baseline ADPS favored both LX9211 groups over the placebo group over the entire 6-week double-blind treatment period, with separation from placebo by week 1 and maintained thereafter (P < 0.05 vs. placebo at weeks 1, 2, 3, and 4 for both dose groups and at week 5 for low-dose group). Rebound pain was not observed during the 5-week single-blind safety follow-up period (Supplementary Fig. 4).
The results for other efficacy end points were generally similar to those observed for the primary end point (Supplementary Table 2). The changes from baseline to week 6 in severity of pain and interference of pain with sleep and other aspects of the patient’s life for the low-dose and high-dose LX9211 groups were nominally significantly different from changes for the placebo group for the categories of worst pain (P = 0.014 and 0.017 vs. placebo, respectively) least pain (P = 0.015 and 0.020 vs. placebo), and interference of pain with sleep (P = 0.005 and 0.002 vs. placebo). The difference was also nominally significantly different from the placebo group for the low-dose LX9211 group for the category of pain right now (P = 0.005 vs. placebo).
Improvement of pain with LX9211 was also demonstrated at week 6 for total NPSI score (significant for the low dose, P = 0.008, and not the high dose, P = 0.064) and burning pain (P < 0.001 and P = 0.017 for low dose and high dose vs. placebo, respectively) (Supplementary Fig. 5).
More than half of participants (low-dose LX9211, 63.2% [67 of 106]; high-dose LX9211, 56.6% [60 of 106]; placebo, 55.1% [59 of 107]) considered overall health status to be improved after 6 weeks of treatment, as assessed with PGIC. Between-group difference versus placebo was nominally significant only for the low-dose group (LS mean [SE] −0.35 [0.16], 95% CI −0.67 to −0.03, P = 0.031; high-dose group −0.15 [0.167], 95% CI −0.48 to 0.17, P = 0.351).
The responder rates for ≥30% and ≥50% reductions in ADPS from baseline to week 6 were numerically greater for the low-dose LX9211 group (27.4% and 15.1%, respectively) than for the placebo group (17.8% and 10.3%) but not for the high-dose LX9211 group (17.0% and 9.4%). No statistically significant differences from placebo were observed.
Analysis of time to initiate rescue acetaminophen showed hazard ratio (HR) <1 for LX9211 versus placebo (low-dose LX9211 HR 0.75, 95% CI 0.45–1.24, and high-dose LX9211 HR 0.81, 95% CI 0.50–1.34), which indicates a lower risk for initiating rescue medication for both LX9211 groups than for the placebo group over the 11-week study period (Supplementary Fig. 6).
Safety Results
Treatment-Emergent AEs: Double-blind Treatment Period
LX9211 was generally well tolerated in the low-dose LX9211 group. The incidence of treatment-emergent adverse events (TEAEs) was higher in the LX9211 groups (low-dose LX9211 53.8% and high-dose LX9211 50.9%) than in the placebo group (29.9%). The most common AEs reported for LX9211-treated participants (incidence >5% for either dose group) were dizziness, nausea, headache, constipation, balance disorder, somnolence, and vomiting, with a dose-dependent trend noted for dizziness, nausea, constipation, and vomiting (Table 3). Falls were reported infrequently: 2 (1.9%) and 1 (0.9%) participants in the low- and high-dose LX9211 groups, respectively. No AEs of changes in blood pressure or AEs suggestive of addictive behavior were reported in the active treatment arms, and treatment with LX9211 did not impact HbA1c or weight. The majority of LX9211-treated participants with AEs had events that were characterized by investigators as mild (51.5% [52 of 101]) or moderate (41.6% [42 of 101]). Onset of most TEAEs was during the first week of treatment, and most resolved during continued treatment with LX9211.
Table 3.
Summary of the most frequently reported* TEAEs
| LX9211 | Placebo | ||
|---|---|---|---|
| 10 mg | 20 mg | ||
| Double-blind treatment period, N | 106 | 106 | 107 |
| Any AE | 57 (53.8) | 54 (50.9) | 32 (29.9) |
| Dizziness | 16 (15.1) | 29 (27.4) | 2 (1.9) |
| Nausea | 9 (8.5) | 12 (11.3) | 3 (2.8) |
| Headache | 9 (8.5) | 10 (9.4) | 4 (3.7) |
| Constipation | 4 (3.8) | 8 (7.5) | 3 (2.8) |
| Balance disorder | 6 (5.7) | 5 (4.7) | 0 |
| Somnolence | 7 (6.6) | 2 (1.9) | 0 |
| Vomiting | 2 (1.9) | 7 (6.6) | 2 (1.9) |
| Placebo safety follow-up period, N† | 99 | 92 | 104 |
| Any AE | 21 (21.2) | 18 (19.6) | 15 (14.4) |
| Dizziness | 1 (1.0) | 1 (1.1) | 2 (1.9) |
| Nausea | 0 | 3 (3.3) | 1 (1.0) |
| Headache | 1 (1.0) | 0 | 2 (1.9) |
| Constipation | 0 | 0 | 0 |
| Somnolence | 0 | 1 (1.1) | 0 |
| Vomiting | 0 | 2 (2.2) | 0 |
Data are n (%) of participants unless otherwise indicated.
Defined as >5% of participants in either LX9211 group during the double-blind treatment period. Data are presented in descending order of incidence for all LX9211-treated participants.
Incidence was <5% for each TEAE reported in the placebo safety follow-up period. Data are presented for the most common TEAEs reported during the double-blind treatment period.
Discontinuations Due to AEs During the Double-blind Treatment Period
The primary reason for early discontinuation was a TEAE (16.0% for low-dose LX9211, 26.4% for high-dose LX9211, and 3.7% for placebo). Events leading to premature discontinuation of more than two participants (≥2.0%) included dizziness, headache, nausea, and fatigue (Supplementary Table 3). Most of the discontinuations in the low-dose LX9211 group occurred early, within the first 2 weeks of the double-blind treatment period. In the high-dose LX9211 group, discontinuations continued over the 6-week treatment period. The likelihood of discontinuation due to an AE during treatment was increased with LX9211 compared with placebo, irrespective of baseline use of DPN medications.
TEAEs: Safety Follow-up Period
The incidence of TEAEs in the 5-week (placebo) follow-up period was similar across the original randomization groups (low-dose LX9211, 21.2%; high-dose LX9211, 19.6%; placebo, 14.4%) (Table 3). The most common events reported during the double-blind treatment period were less common in the follow-up period (all were reported for <5% of participants in each randomization group). None of the events reported for LX9211 were severe. AEs suggestive of withdrawal were not reported.
Serious AEs
There were two deaths during the double-blind treatment period: a participant in the placebo group experienced a TEAE of coronavirus disease 2019 on day 35 that resulted in death on day 42, and a participant in the high-dose LX9211 group experienced a TEAE of complications related to type 1 diabetes on day 41 that resulted in death the same day. Both deaths were considered unrelated to study drug. During the double-blind treatment period, a participant (high-dose LX9211) experienced serious AEs, peripheral edema and orthopnea, that were not considered related to study drug; no action was taken regarding study drug, and the events resolved.
There were no deaths in the 5-week safety follow-up period. Three participants experienced serious AEs: atrial fibrillation and supraventricular tachycardia (one participant in the high-dose LX9211 group), coronavirus disease 2019 (low-dose LX9211 group), and abdominal (lower) pain (placebo group). None of these were considered related to study drug, and all resolved.
Conclusions
Results of this double-blind, multicenter, proof-of-concept trial demonstrated that with low-dose LX9211 the primary efficacy end point was achieved of significant DPNP reduction in comparisons with placebo after 6 weeks of treatment in participants with moderate-to-severe DPNP. While the results of high-dose LX9211 also demonstrated numerical pain reduction, it did not reach the prespecified significance level, which may be due to the higher incidence of TEAEs leading to treatment discontinuation in the high-dose arm. The reduction of ADPS from baseline for both the LX9211 groups separated from the placebo group by week 1 and was maintained through week 6. This effect was consistent irrespective of sex and baseline DPNP drug use. For participants with severe DPNP, a notably larger difference from placebo in change in ADPS from baseline to week 6 was observed for participants who received low-dose LX9211 than for those who received high-dose LX9211. The results for other efficacy end points were generally similar to those observed for the primary end point: for both doses results were often numerically superior to placebo, and low-dose LX9211 was more efficacious. These end points included change from baseline to week 6 in severity of pain and interference of pain with sleep and other aspects of the patient’s life, PGIC at week 6, change from baseline in NPSI total score to week 6, and change from baseline in NPSI burning pain subscore to week 6. Additionally, post hoc analysis showed reduced risk for initiating use of rescue medications for participants in the LX9211 treatment groups.
These findings are clinically meaningful considered in perspective with the challenge of treating painful DPN at the point of care and considered in comparison with findings of other interventional trials. In DPNP clinical trials with pregabalin, for instance, the magnitude of reduction in pain from baseline for the placebo group has been reported between −0.88 and −2.54 (20), while for duloxetine it was −0.90 (21). In a very recent study by Tesfaye et al. (10), monotherapy with amitriptyline, duloxetine, and pregabalin resulted in a change from baseline to week 6 of −2.9, −2.8, and −2.5, respectively, on a daily pain numerical rating scale. In the absence of a placebo group in that study, comparison of the reduction in the magnitude of pain is difficult, as it was previously demonstrated that the placebo response could vary depending on study design. In another key difference, in the study by Tesfaye et al. (10), all participants were washed out of their baseline therapy prior to starting the 6-week long monotherapy arm of the trial.
Although TEAEs were more frequent with both doses of LX9211 than with placebo, the drug was in general well tolerated in the low-dose LX9211 arm, with 79% completing the double-blind treatment period with 94% adherence to study drug. Dizziness was the most reported TEAE (15% in the low-dose arm). In comparison, 18% of the participants in the pregabalin group and 8% in the duloxetine and amitriptyline groups in the study by Tesfaye et al. (10) reported dizziness. It should be noted that in that study, participants were allowed to gradually titrate to a maximum tolerated dose over a 2-week period. In contrast, in the RELIEF-DPN 1 study, participants were administered a high loading dose (10× the maintenance dose) on day 1, which may have contributed to the early incidence of TEAEs in the study.
Currently standard of care for DPNP either as monotherapy or combination therapy provides only modest pain relief for most patients and is often associated with dose-limiting side effects (10). Thus, in this trial, relevant to contemporary patients as seen in clinical care, participants were allowed to continue their baseline medication for DPNP. Approximately 55% of the participants in the placebo group were on baseline DPNP medication (mostly gabapentin) compared with 42% and 40%, respectively, in the low-dose and high-dose LX9211 groups. Treatment with LX9211 resulted in reduction in ADPS both in the presence and absence of baseline DPNP medication use, with the reduction numerically greater in the absence of baseline DPNP medication.
Importantly, DPNP remains very challenging to treat in daily practice, with limited treatment options. Despite a lack of supportive clinical evidence, the use of opioids in the treatment of DPNP remains high, with ∼53% of patients using opioids for pain management and 33% using it as first-line treatment (22). More recent data from large managed care cohorts show that a majority of people with DPNP continue to be treated with opioids as first-line therapy (23) leading to very high associated risks and complications including death (24). This substantiates the need for new therapeutic options in this patient population. It should be noted that the high percentage of participants who entered the trial without baseline DPNP medication is similar to that previously reported (23).
Results of the RELIEF-DPN 1 study support the hypothesis that LX9211 may be an efficacious treatment for moderate-to-severe pain due to DPN. These findings reflect a promising breakthrough in the clinical management of a debilitating condition for which currently used pharmacotherapies provide only modest pain relief for fewer than half of affected patients (10).
These data should be viewed as a phase 2 proof-of-concept study, thus limited by a relatively short treatment duration and enrollment of participants only in the U.S. Notwithstanding these limitations, in this proof-of-concept study the primary efficacy end point was met, supporting the translation of AAK1 as a novel therapeutic target for treating NP, as observed in animal models, to benefits with the AAKI inhibitor LX9211 in painful DPN.
Further investigation is needed to confirm our clinical findings. To this end, a dose-ranging phase 2b clinical study of LX9211 in DPNP is in progress.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25451386.
Article Information
Acknowledgments. The authors acknowledge Sandra Norris of Norris Communications Group, who received compensation from Lexicon Pharmaceuticals, for medical writing assistance. The authors thank Manon Girard for contributions to the statistical analysis. The authors thank the study participants and investigators in the U.S. for participation in this study (states and investigators): AL, Harry Studdard (Alliance for Multispecialty Research, LLC); AZ, Zaffar Iqbal (Revival Research Institute, LLC), Thili Kulatilake (Novak Clinical Research), and Hemant Pandey (MD First Research – Chandler); CA, Linda Gaudiani (Marin Endocrine Care & Research, Inc.), Steve Sitar (Orange County Research Institute), Teresa Sligh (Providence Clinical Research), Joanna Van (University Clinical Investigators, Inc.), and Douglas Young (Northern California Research); FL, Cathy Barnes (Suncoast Clinical Research, Inc.), David Carpenter (Ormond Medical Arts Pharmaceutical Research Center), Dalla Fulop (Neurology Associates of Ormond Beach), Daniel Lorch (Teradan Clinical Trials), James MaGee (East Coast Institute for Research, LLC), Anand Patel (Conquest Research LLC), Guy Strauss (Multi-Specialty Research Associates, Inc), and Pedro Ylisadtigui (Alliance for Multispecialty Research, LLC); GA, Thomas Jones (East Coast Institute for Research, LLC); MA, Mushtaque Chachar (Boston Neuro Research Center) and Christine Sang (Brigham and Women's Hospital); MI, Talal Khader (Pelham Primary Care; Vida Clinical Studies), Rodica Pop-Busui (University of Michigan, Metabolism, Endocrinology and Diabetes Clinic), and Abdulhassan Saad (Revival Research Institute, LLC); MO, Larry Reed (Healthcare Research Network); NE, John Puente (West Omaha Family Physicians/CCT Research); NJ, Michael Hasseman (Hassman Research Institute); NY, Donald Brautigam (Great Lakes Medical Research, LLC) and James Wild (Upstate Clinical Research Associates, LLC), NC, Mrinalini Kodumagulla (Cary Research Group, LLC), Wendy Lane (Mountain Diabetes & Endocrine Center), and Kathryn Lucas (Lucas Research, Inc.); SC, Harlicia Farley (The Research Center of the Upstate); TX, Peter Bressler (North Texas Endocrine Center), Wasim Haque (Prime Revival Research Institute, LLC), John Hudson (FutureSearch Trials of Neurology), Murtaza Mussaji (LinQ Research, LLC), Alan Reichman (Clinical Trial Network), and Aziz Shaibani (Nerve And Muscle Center of Texas); UT, Albert Jarvi (Wasatch Clinical Research, LLC); WA, Leslie Klaff (Rainier Clinical Research Center).
R.P.-B. is an editor of Diabetes Care but was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Funding and Duality of Interest. This study was funded by Lexicon Pharmaceuticals. The study sponsor was involved in the design of the RELIEF-DPN 1 study, collected data from the study investigators, and performed statistical analyses of the data. Authors of this manuscript, including those employed by the sponsor, interpreted the study data, wrote and revised the manuscript, and made the decision to submit the manuscript for publication. R.P.-B. received grant funding to University of Michigan from Lexicon Pharmaceuticals, Novo Nordisk, and Medtronic and consulting fees from Averitas Pharma, Lexicon Pharmaceuticals, Nevro, Novo Nordisk, Roche, and Procter & Gamble. A.P. received consulting fees from Lexicon Pharmaceuticals. C.N.-M.S. received grant funding (to Brigham and Women’s Hospital) from Lexicon Pharmaceuticals, Merck, AbbVie, and Vertex Pharmaceuticals and consulting fees from Ono Pharmaceutical, Creative Bio-Peptides, Pfizer, and Solvd. P.L.B., P.F.P., F.S., C.G., and S.G. are employed by and may have stock or stock options in Lexicon Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.P.-B., A.P., and C.N.-M.S. contributed to the concept or design of the study; acquisition, analysis, or interpretation of data; and the drafting, reviewing, and editing of the manuscript. P.L.B., P.F.P., F.S., C.G., and S.G. contributed to the concept or design of the study; acquisition, analysis, or interpretation of data; the approval of the protocol and amendments; the reviewing of the data quality prior to database lock; the overseeing of the statistical analysis; and the drafting, reviewing, and editing of the manuscript. All authors critically reviewed the manuscript and provided substantive comments. S.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the International Association for the Study of Pain (IASP) 2022 World Congress on Pain, Toronto, Canada, 19–23 September 2022; at a plenary session at the 16th Annual Pain Therapeutics Summit, Washington, DC, 14–15 November 2022; and at the 83rd Scientific Sessions of the American Diabetes Association, San Diego, CA, 23–26 June 2023.
Handling Editors. The journal editor responsible for overseeing the review of the manuscript was Matthew C. Riddle.
Funding Statement
This study was funded by Lexicon Pharmaceuticals. The study sponsor was involved in the design of the RELIEF-DPN 1 study, collected data from the study investigators, and performed statistical analyses of the data. Authors of this manuscript, including those employed by the sponsor, interpreted the study data, wrote and revised the manuscript, and made the decision to submit the manuscript for publication. R.P.-B. received grant funding to University of Michigan from Lexicon Pharmaceuticals, Novo Nordisk, and Medtronic and consulting fees from Averitas Pharma, Lexicon Pharmaceuticals, Nevro, Novo Nordisk, Roche, and Procter & Gamble. A.P. received consulting fees from Lexicon Pharmaceuticals. C.N.-M.S. received grant funding (to Brigham and Women’s Hospital) from Lexicon Pharmaceuticals, Merck, AbbVie, and Vertex Pharmaceuticals and consulting fees from Ono Pharmaceutical, Creative Bio-Peptides, Pfizer, and Solvd. P.L.B., P.F.P., F.S., C.G., and S.G. are employed by and may have stock or stock options in Lexicon Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no. NCT04455633, clinicaltrials.gov
References
- 1. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braffett BH, Gubitosi-Klug RA, Albers JW, et al.; DCCT/EDIC Research Group . Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2020;69:1000–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011;34:2220–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jensen TS, Karlsson P, Gylfadottir SS, et al. Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain 2021;144:1632–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ang L, Mizokami-Stout K, Eid SA, et al. The conundrum of diabetic neuropathies-past, present, and future. J Diabetes Complications 2022;36:108334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig 2017;8:646–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc 2006;81(Suppl.):S3–S11 [DOI] [PubMed] [Google Scholar]
- 8. Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care 2005;28:2378–2383 [DOI] [PubMed] [Google Scholar]
- 9. Pop-Busui R, Ang L, Boulton AJM, et al. Diagnosis and Treatment of Painful Diabetic Peripheral Neuropathy. Arlington, VA, American Diabetes Association, 2022 [PubMed]
- 10. Tesfaye S, Sloan G, Petrie J, et al. Optimal pharmacotherapy pathway in adults with diabetic peripheral neuropathic pain: the OPTION-DM RCT. Health Technol Assess 2022;26:1–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol 2019;33:2058738419838383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kostich W, Hamman BD, Li YW, et al. Inhibition of AAK1 kinase as a novel therapeutic approach to treat neuropathic pain. J Pharmacol Exp Ther 2016;358:371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo G, Chen L, Kostich WA, et al. Discovery of (S)-1-((2′,6-Bis(difluoromethyl)-[2,4′-bipyridin]-5-yl)oxy)-2,4-dimethylpentan-2-amine (BMS-986176/LX-9211): a highly selective, CNS penetrable, and orally active adaptor protein-2 associated kinase 1 inhibitor in clinical trials for the treatment of neuropathic pain. J Med Chem 2022;65:4457–4480 [DOI] [PubMed] [Google Scholar]
- 15. Bundrant L, Hunt TL, Banks P, et al. Results of two phase 1, randomized, double-blind, placebo-controlled, studies (ascending single-dose and multiple-dose studies) to determine the safety, tolerability, and pharmacokinetics of orally administered LX9211 in healthy participants. Clin Ther 2021;43:1029–1050 [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association Professional Practice Committee . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S17–S38 [DOI] [PubMed] [Google Scholar]
- 17. Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004;108:248–257 [DOI] [PubMed] [Google Scholar]
- 18. Perrot S, Lantéri-Minet M. Patients’ global impression of change in the management of peripheral neuropathic pain: clinical relevance and correlations in daily practice. Eur J Pain 2019;23:1117–1128 [DOI] [PubMed] [Google Scholar]
- 19. Zelman DC, Gore M, Dukes E, Tai KS, Brandenburg N. Validation of a modified version of the brief pain inventory for painful diabetic peripheral neuropathy. J Pain Symptom Manage 2005;29:401–410 [DOI] [PubMed] [Google Scholar]
- 20. Freeman R, Emir B, Parsons B. Predictors of placebo response in peripheral neuropathic pain: insights from pregabalin clinical trials. J Pain Res 2015;8:257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005;6:346–356 [DOI] [PubMed] [Google Scholar]
- 22. Patil PR, Wolfe J, Said Q, Thomas J, Martin BC. Opioid use in the management of diabetic peripheral neuropathy (DPN) in a large commercially insured population. Clin J Pain 2015;31:414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Callaghan BC, Reynolds E, Banerjee M, Kerber KA, Skolarus LE, Burke JF. Longitudinal pattern of pain medication utilization in peripheral neuropathy patients. Pain 2019;160:592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nalini M, Khoshnia M, Kamangar F, et al. Joint effect of diabetes and opiate use on all-cause and cause-specific mortality: the Golestan cohort study. Int J Epidemiol 2021;50:314–324 [DOI] [PMC free article] [PubMed] [Google Scholar]