Abstract
Pinostrobin, a key bioactive compound found in the medicinal plant Boesenbergia rotunda (L.), has been noted for its beneficial biological properties including antioxidant, anti-inflammation, anti-cancer and anti-amnesia activities. In view of this, the present study purposed to evaluate the neuroprotective potential of pinostrobin in reversing scopolamine-induced cognitive impairment involving oxidative stress and cholinergic function in rats. A total of 30 male Wistar rats were randomly divided into five groups (n=6): Group 1 received vehicle as a control, group 2 received vehicle + scopolamine (3 mg/kg, i.p.), group 3 received pinostrobin (20 mg/kg, p.o.) + scopolamine, group 4 received pinostrobin (40 mg/kg, p.o.) + scopolamine and group 5 received donepezil (5 mg/kg, p.o.) + scopolamine. Treatments were administered orally to the rats for 14 days. During the final 7 days of treatment, a daily injection of scopolamine was administered. Scopolamine impaired learning and memory performance, as measured by the novel object recognition test and the Y-maze test. Additionally, oxidative stress marker levels, acetylcholinesterase (AChE) activity, choline acetyltransferase (ChAT) and glutamate receptor 1 (GluR1) expression were determined. Consequently, the findings demonstrated that the administration of pinostrobin (20 and 40 mg/kg) markedly improved cognitive function as indicated by an increase in recognition index and by spontaneous alternation behaviour. Pinostrobin also modulated the levels of oxidative stress by causing a decrease in malondialdehyde levels accompanied by increases in superoxide dismutase and glutathione activities. Similarly, pinostrobin markedly enhanced cholinergic function by decreasing AChE activity and promoting ChAT immunoreactivity in the hippocampus. Additionally, the reduction in GluR1 expression due to scopolamine was diminished by treatment with pinostrobin. The findings indicated that pinostrobin exhibited a significant restoration of scopolamine-induced memory impairment by regulating oxidative stress and cholinergic system function. Thus, pinostrobin could serve as a potential therapeutic agent for the management of neurodegenerative diseases such as Alzheimer's disease.
Keywords: pinostrobin, scopolamine, memory deficit, acetylcholinesterase, choline acetyltransferase
Introduction
Due to the progressive increase in the number of patients with Alzheimer's disease (AD), which is currently estimated to be more than 46 million individuals worldwide, AD has become a significant concern for the elderly population (1). AD stands as the primary condition associated with cognitive dysfunction, language difficulties and alterations in personality (2,3). A key neuropathological characteristic linked to AD symptoms is the presence of senile plaques and neurofibrillary tangles, which are significant indicators of neuronal loss. Additionally, various mechanisms have been investigated to further explain the pathogenesis of AD, including cholinergic dysfunction, oxidative damage, deposits of amyloid beta (Aβ), hyperphosphorylation of tau protein and the aggregation of senile plaques (4,5).
The central cholinergic system plays a vital role in memory processing whereby the loss of the cholinergic neurons, neurotransmitters and their receptors results in learning and memory impairment (6,7). Cholinergic dysfunction is caused by an elevation of acetylcholinesterase (AChE) activity and a reduction in acetylcholine (ACh) concentration in the brain, which are the main drivers of cognitive impairment (8). Scopolamine has been widely used in in vivo models to evaluate potential agents for the treatment of dementia (9). Scopolamine, which acts as a blocker of the muscarinic cholinergic receptor, leads to cognitive dysfunction by reducing cholinergic neurotransmission and heightening oxidative stress in the brain (10,11). Furthermore, scopolamine leads to the shrinking of neuronal cells and initiates degenerative alterations within the hippocampus (12).
Glutamate is the major fast-excitatory neurotransmitter in the hippocampal network and is essential for learning and memory. Dysfunctions in this system are considered to be causally linked to neurodegenerative disorders (13). Glutamate receptor subunit 1 (GluR1) is one of the four subunits of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which are critical for synaptic plasticity and learning processes (14). Notably, a reduction in GluR1 is observed in the hippocampal formation of patients with AD, resulting in impaired synaptic transmission and potentially contributing to cognitive dysfunction (15). Additionally, several animal studies have shown that decreased expression of GluR1 in the hippocampus may be associated with learning and memory deficits (16,17). Therefore, acetylcholine and glutamate are now recognised as key contributors to memory processes.
Emerging evidence indicates that the interactions between these neurotransmitters may be crucial for certain memory types, with acetylcholine particularly facilitating glutamate activity by coordinating the acquisition and recall phases in the cortex and hippocampus (18). Glutamatergic neurotransmission, through the AMPA receptor and the N-methyl d-aspartate (NMDA) receptor, induces long-term potentiation (LTP), which strengthens synapses with repeated use and is essential for learning and memory. Besides glutamatergic mechanisms, acetylcholine and its receptors markedly contribute to both the induction and maintenance of LTP (19). Thus, medications capable of enhancing cholinergic transmission while mitigating oxidative stress may potentially reverse memory deficits induced by scopolamine (20). Currently, existing medications can only provide temporary alleviation from AD symptoms, which is accompanied by adverse effects. Hence, there is an imperative need to develop natural compounds for an effective treatment against AD.
Pinostrobin (5-hydroxy-7-methoxy flavanone) is a substituted flavonoid isolated from Boesenbergia rotunda (L.), that has been identified as the main active ingredient responsible for its pharmacological properties, including antioxidant (21,22), anti-inflammatory (23,24) and anti-apoptotic (25) activities. In addition, our previous findings showed that pinostrobin exerts protective effects against chronic restraint stress and nerve crush injury (26,27). Nevertheless, the potential of using pinostrobin to improve scopolamine-induced cognitive impairment has not yet been examined. Thus, the present study was conducted to explore whether pinostrobin could mitigate memory impairment in this model. It also investigated whether pinostrobin could enhance the function of the cholinergic and glutamatergic systems.
Materials and methods
Chemicals
Scopolamine used in the present study was obtained from (cat. no. S1875; MilliporeSigma). Donepezil (Aricept® 10 mg tablet) was purchased from the Eisai Co., Ltd.). Scopolamine was dissolved in 0.9% sodium chloride (NaCl) and administered by i.p. injection to rats (3 mg/kg) as described in a previous study (28). Donepezil was dissolved in water and orally administered at a dose of 5 mg/kg in accordance with an established report (29).
Isolation of pinostrobin
The preparation and structural characterisation of pinostrobin (the purity of which was determined by NMR analysis) was described in our previous study (26). Briefly, fresh rhizomes of B. rotunda were purchased from a market in Phayao, Thailand. A plant specimen was authenticated by the Walai Rukhavej Botanical Research Institute, Mahasarakham University, MahaSarakham, Thailand (No. S. Sedlak 19-1). The ethanolic crude extract (251 g) was dissolved in methanol and then subjected to open column chromatography (Arch. No. 7734, pore size Å, particle size 70-230 mesh, Merck), using silica gel as the absorbent. The column was washed with a gradient of dichloromethane-hexane mixture for separation. Fractions exhibiting similar patterns, as determined by thin-layer chromatography analysis, were combined and recrystallised with methanol to yield pinostrobin. Structural analysis was conducted using 13C- and 1H-NMR spectroscopy (Bruker Avance DRX 500 Spectrometer; Bruker Corporation) and the NMR spectra were compared with the existing literature for identification as follow: 1H NMR (500 MHz, acetone-d6): 2.80 (dd, J=17.2, 3.0 Hz; 1H, H-3a), 3.06 (dd, J=17.2, 13.0 Hz; 1H, H-3b), 3.79 (s, 3H; -OCH3), 5.39 (dd, J=13.0, 3.0 Hz; 1H, H-2), 6.06 (m, 2H, H-6, H-8), 7.41 (m, 5H; H-2', H-3', H-3', H-5', H-6'). 13C NMR (150 MHz, acetone-d6): 43.1 (C-3), 55.7 (C-7-OCH3), 79.2 (C-2), 94.3 (C-8), 95.1 (C-6), 103.1 (C-10), 126.1 (C-2', C-3', C-5', C-6'), 128.9 (C-4'), 138.4 (C-1'), 162.8 (C-5), 164.1 (C-9), 167.9 (C-7), 195.8 (C-4). (Central Science Laboratory, Faculty of Science, Chiang Mai University, Thailand).
Experimental animals and treatments
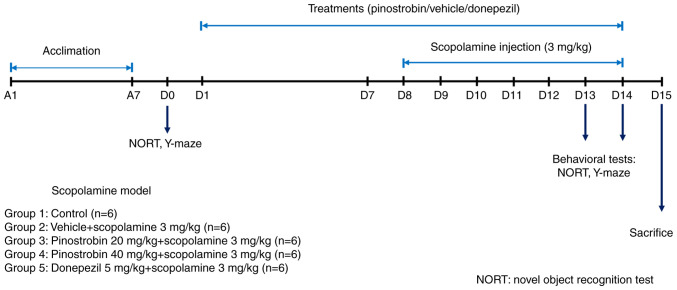
A total of 30 male Wistar rats (aged 8 weeks and weighing 220-250 g) were obtained from Nomura Siam International. The rats were allowed a week for acclimation prior to the commencement of the experiments. All experimental procedures were conducted under a 12-h light/dark cycle with standard chow and water available ad libitum in a room with a constant temperature (21±1˚C) and humidity (35-60%). All experiments were approved by the Ethics Committee of the Laboratory Animal Research Center, University of Phayao, Thailand (approval no. 640104029). All rats were randomly divided into five groups (6 rats per group) as follows: group 1 received vehicle as control, group 2 received vehicle + scopolamine (3 mg/kg, i.p.), group 3 received pinostrobin (20 mg/kg, p.o.) + scopolamine, group 4 received pinostrobin (40 mg/kg, p.o.) + scopolamine and group 5 received donepezil (5 mg/kg, p.o.) + scopolamine. The animals were administered 1% carboxymethylcellulose (cat. no. 21904; MilliporeSigma), which was used as the vehicle or pinostrobin (at doses of 20 or 40 mg/kg) or donepezil (5 mg/kg) via oral gavage daily for 14 days. During the final 7 days of treatment, a daily intraperitoneal injection of scopolamine (3 mg/kg) was administered. The cognitive performance of all animals was assessed by using the novel object recognition test (NORT) and the Y-maze test on day 0 for baseline data and on day 13 and 14 to identify treatment effects (Fig. 1).
Figure 1.
Schematic representation of the experimental design.
Tissue preparation
At the end of the experimental period, all animals were anesthetised using thiopental sodium (70 mg/kg) and transcardially perfused with 0.1 M PBS for the evaluation of biochemical and immunohistochemical parameters. The brains (n=30) were immediately collected and separated into two hemispheres. The left hemisphere of the hippocampus was isolated and homogenised in Tris-buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, protease inhibitor, pH 7.4). After centrifugation at 9,279 x g at 4˚C for 15 min, the supernatant was stored at -80˚C for later biochemical evaluation. The right hemisphere was fixed with ice-cold 4% paraformaldehyde for 24 h and cryopreserved in 30% sucrose for later immunohistochemistry analysis. The brains were cut into 30 µm sections using a Leica CM1950 cryostat (Leica Microsystems GmbH) and stored in an anti-freeze solution (4˚C).
Total protein concentration
The protein concentrations were determined using the method of Lowry (30). Bovine serum albumin (BSA; cat. no. 9048-46-8; MilliporeSigma) was used as a standard and protein concentrations were expressed as mg/ml.
Biochemical analysis. Measurement of the lipid peroxidation product
Estimation of the malondialdehyde (MDA) concentration was performed according to the protocol previously described (31,32) with some modifications. The absorbance was measured at 532 nm using a microplate reader (Synergy H1; BioTek; Agilent Technologies, Inc.). The results were presented as µmol/mg protein.
Measurement of superoxide dismutase (SOD) activity
The activity of SOD was examined by commercial assay kit (cat. no. S19160; MilliporeSigma) according to the manufacturer's instructions. The SOD activity was measured from the curve of the standard solution and expressed as U/mg protein.
Measurement of reduced glutathione (GSH)
A reduced GSH assay was investigated according to the method described by Polycarp et al (33) and Srdjenovic et al (32). The absorbance of each sample mixture was determined at 412 nm. The result was expressed as µmol/mg protein.
Measurement of acetylcholinesterase (AChE) activity
The activity of AChE, which is responsible for the degradation of acetylcholine in tissue samples, was determined using a commercial acetylcholinesterase colorimetric kit (cat. no. ab138871; Abcam). The assay was performed according to the manufacturer's instructions and the activity was measured from the curve of the standard solution. The activity was expressed as U/mg protein.
Immunohistochemical staining
To accomplish immunohistochemistry staining, the free-floating sections were washed and treated in H2O2 followed by blocking solution (5% normal horse serum in 0.1 M PBS) (cat. no. 16050130; Gibco; Thermo Fisher Scientific). Subsequently, sections were incubated with the primary antibody, which was rabbit anti-choline acetyltransferase (ChAT; 1:100; cat. no. AB143; MilliporeSigma) or anti-glutamate receptor 1 (GluR1; 1:200; cat. no. AB1504; MilliporeSigma) at 4˚C overnight. The sections were washed in 0.1 M PBS for 30 min and incubated for 2 h at room temperature with biotinylated donkey anti-rabbit antibody (cat. no. 711-065-152; 1:500; Jackson ImmunoResearch Europe, Ltd.). The sections were rinsed in 0.1 M PBS followed by 1 h of incubation in extravidin peroxidase (1:1,000) and then visualized using the 3,3'-diaminobenzidine (DAB) reaction as previously described (34). For immunohistochemistry evaluation, hippocampal images (six images per group) of subregion cornu ammonis 1 (CA1), cornu ammonis 2 (CA2), cornu ammonis 3 (CA3) and dentate gyrus (DG) were captured at 40x magnification using a Ni-U upright microscope (Nikon Corporation) with NIS Element Imaging Software version 5 (Nikon Corporation). The hippocampus is composed of various subregions: CA1, CA2, CA3 and the DG. Based on the basic anatomical organisation of the hippocampal formation and on the morphological and connectivity differences, ChAT and GluR1 immunostaining was analysed in the pyramidal layer of CA1, CA2 and CA3 subregions and the granular layer of DG.
Evaluation of ChAT and GluR1 immunostaining
The quantitative immunoreactivity of the ChAT and GluR1 proteins within the hippocampus were determined using the thresholding function of Image J software (v1.53; National Institutes of Health). Image thresholding is a commonly utilised method for quantitatively assessing changes in immunolabelled materials, as previously documented (35). The data are presented as the percentage of labelling area (% immunoreactivity).
Behavioural studies. NORT
The experimental process used to assess recognition memory was the same as that described in our previous study (26). One day prior to the experiment, the rats were allowed to explore the empty open field for 5 min. On the experiment day, during the training phase, two identical objects (A1 and A2) were placed in two corners of the open field. Each rat was placed in the middle of the open field and allowed to freely explore these two identical objects for 5 min. After 4 h of post-training phase, one old object was replaced by a new object (B) and the rat was left to inspect the two objects for 5 min. The open field was cleaned with 70% ethanol before the next rat was tested to avoid affecting subsequent test results. The percentage of recognition index (RI) was calculated using a formula as previously described (36).
 |
Y-maze test
The Y-maze test was performed to measure working memory related to the hippocampus in animals according to the instructions in our previous work (26). The maze was Y-shaped with three arms that were designated as regions A, B and C. A single rat was placed at one of three enclosed arms for free exploration for a total of 8 min. A spontaneous alternation occurred when an animal entered all three arms in a sequence of three consecutive entries. The maze was cleaned with 70% ethanol to avoid affecting subsequent sessions. The percentage of spontaneous alternation was considered to indicate working memory and was computed according to the previous study (37) as follows:
 |
Statistical analysis
GraphPad Prism version 9 (Dotmatics) was used to analyse the data. Statistical analysis was performed using the one-way ANOVA test, followed by Tukey's post-hoc test for multiple comparisons. Data are expressed as the mean ± SEM. P<0.05 was considered to indicate a statistically significant difference.
Results
Pinostrobin reverses scopolamine-induced memory impairment in the NORT and Y-maze test
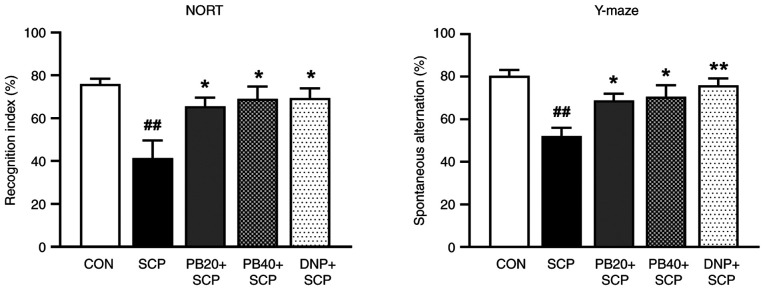
The NORT was used to measure whether pinostrobin could restore scopolamine-induced recognition deficit. The scopolamine-treated group demonstrated a reduction in RI by decreased preference for the novel object compared with the control group (P<0.01). However, the administration of pinostrobin (20 or 40 mg/kg) or donepezil notably enhanced recognition memory compared with the scopolamine-treated group (P<0.05). Moreover, the scopolamine-treated group showed a significant memory deficit by their decreased percentage of spontaneous alternation in the Y-maze test compared with that of the control group (P<0.01). However, oral administration of pinostrobin (20 or 40 mg/kg) markedly improved the cognitive performance as compared with the scopolamine-treated group (P<0.05). Under the same conditions, the standard drug donepezil also showed a reversal of the scopolamine-induced cognitive impairment seen in the scopolamine-treated group (P<0.01) as shown in Fig. 2.
Figure 2.
PB reverses SCP-induced memory impairment in the NORT and Y-maze test. Graphs showed the recognition index assessed by NORT and the percentage of spontaneous alternation using Y-maze test. Data are expressed as the mean ± SEM. ##P<0.01 vs. the control group; *P<0.05, **P<0.01 vs. the SCP-treated group. PB, pinostrobin; SCP, scopolamine; NORT, novel object recognition test; CON, control; DNP, donepezil.
Pinostrobin modulates scopolamine-induced disruption of oxidative stress and antioxidant defence in the hippocampus
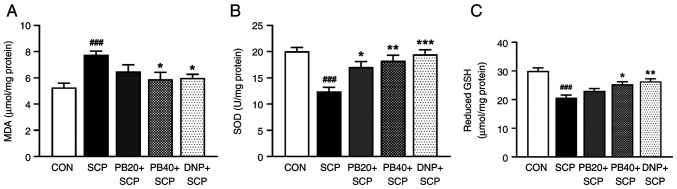
Fig. 3 shows the results for oxidative stress and antioxidant markers in the hippocampus. In the scopolamine-induced group, the level of MDA, which is a key product of lipid peroxidation, was markedly higher than that in the control group (P<0.001). Furthermore, both pinostrobin at a dose of 40 mg/kg and donepezil exhibited significant reductions in the MDA levels when compared with the scopolamine-treated group (P<0.05; Fig. 3A). In addition, the activities of scavenging enzymes, including SOD and GSH, were examined. Notably, there was a significant decrease in the activities of SOD in the scopolamine-treated group compared with the control group (P<0.001), whereas treatment with of pinostrobin or donepezil resulted in a noticeable increase in SOD activity as compared with the scopolamine-treated group (PB 20 mg/kg, P<0.05; PB 40 mg/kg, P<0.01; DNP, P<0.001; Fig. 3B). Moreover, treatment with pinostrobin at a high dose (40 mg/kg) or with donepezil markedly reversed the scopolamine-induced reduction of GSH activity in the hippocampus as compared with the scopolamine-treated group (PB 40 mg/kg, P<0.05; DNP, P<0.01; Fig. 3C).
Figure 3.
PB modulates SCP-induced oxidative stress indicators in the hippocampus. Graphs exhibit the (A) MDA level, (B) SOD activity and (C) reduced GSH activity. Data are expressed as the mean ± SEM. ###P<0.001 vs. the control group; *P<0.05, **P<0.01, ***P<0.001 vs. the SCP-treated group. PB, pinostrobin; SCP, scopolamine; MDA, malondialdehyde; SOD, superoxide dismutase; GSH, reduced glutathione; CON, control; DNP, donepezil.
Pinostrobin attenuates scopolamine-induced AChE hyperactivation in the hippocampus
To evaluate the effect of pinostrobin on cholinergic function, the AChE enzymatic activity in the hippocampus was also determined. As illustrated in Fig. 4, compared with the control group, the levels of AChE in the hippocampus were significantly increased in the scopolamine-induced group (P<0.01). Importantly, the administration of pinostrobin (20 or 40 mg/kg) or donepezil significantly suppressed the scopolamine-induced overactivation of AChE activity in the hippocampus when compared with the scopolamine-treated group (PB 20 mg/kg, P<0.01; PB 40 mg/kg, P<0.001; DNP, P<0.001).
Figure 4.
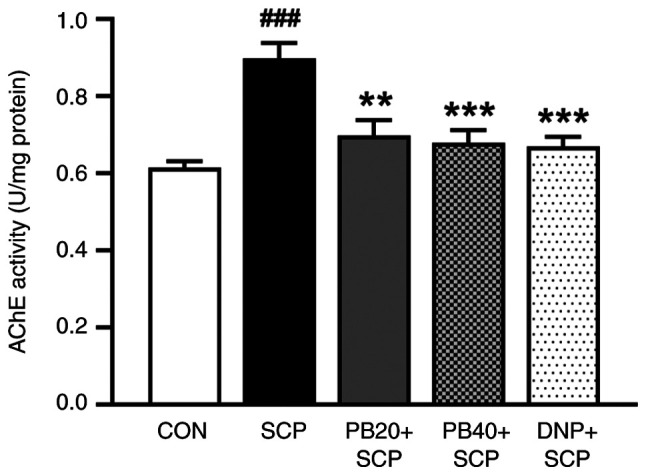
PB attenuates SCP-induced AChE hyperactivation in the hippocampus. Data are expressed as the mean ± SEM. ###P<0.001 vs. the control group; **P<0.01, ***P<0.001 vs. the scopolamine-treated group. PB, pinostrobin; SCP, scopolamine; AChE, acetylcholinesterase; CON, control; DNP, donepezil.
Pinostrobin attenuates scopolamine-induced alteration of ChAT expression in the hippocampus
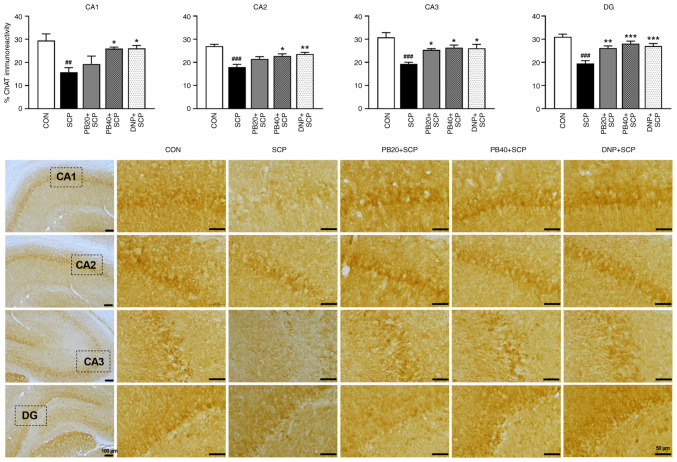
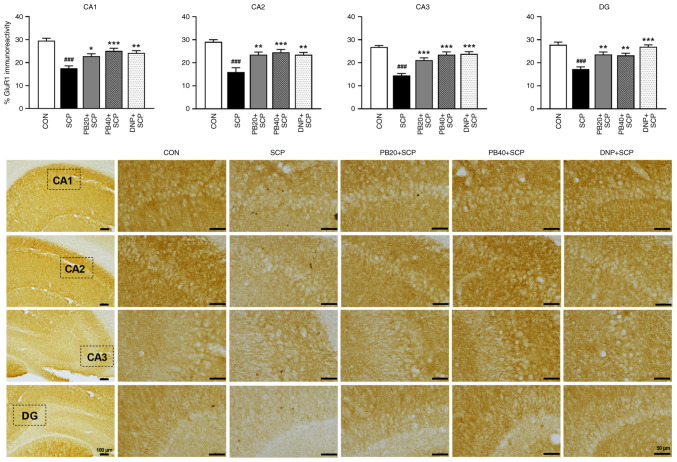
To investigate whether pinostrobin affected the neural response in scopolamine-injected rats, ChAT expression was determined in the hippocampus using immunohistochemistry as shown in Fig. 5. The scopolamine-treated group significantly decreased the expression of ChAT in all subregions of the hippocampus (CA1, P<0.05; CA2, CA3 and DG, P<0.001) compared with the control group. Markedly, treatment with pinostrobin at a dose of 20 mg/kg significantly suppressed the decrease of ChAT immunoreactivity in CA3 and DG of the hippocampus compared with the scopolamine-treated group (CA3; P<0.05, DG; P<0.01). More notably, pinostrobin at a high dose (40 mg/kg) markedly inhibited the reduction of ChAT expression in all subregions of the hippocampus (CA1, CA2 and CA3; P<0.05, DG; P<0.001), which was similar to that observed in the donepezil group (CA1; P<0.05, CA2; P<0.01, CA3; P<0.05, DG; P<0.001) compared with the scopolamine-treated group.
Figure 5.
PB attenuates SCP-induced alteration of ChAT expression in the hippocampus. Representative photomicrographs showed ChAT expression measured by a percentage of immunoreactivity in CA1, CA2, CA3 and DG of the hippocampus. Scale bar, 50 µm; magnification, x40. Data are expressed as the mean ± SEM. ##P<0.01, ###P<0.001 vs. the control group; *P<0.05, **P<0.01, ***P<0.001 vs. the scopolamine-treated group. PB, pinostrobin; SCP, scopolamine; ChAT, choline acetyltransferase; CA1, cornu ammonis 1; CA2, cornu ammonis 2; CA3, cornu ammonis 3; DG, dentate gyrus; CON, control; DNP, donepezil.
Pinostrobin attenuates scopolamine-induced alteration of GluR1 expression in the hippocampus
With regard to the effect of pinostrobin on the activity of glutamate receptors in scopolamine-treated rats, the expression of GluR1 in the hippocampus was further determined using immunohistochemistry analysis as shown in Fig. 6. A reduction in GluR1 expression in the scopolamine-treated group was observed in all subregions of the hippocampus compared with the control group (P<0.001). Markedly, treatments with pinostrobin (20 or 40 mg/kg) significantly enhanced the expression of GluR1 compared with the scopolamine-treated group (PB 20 mg/kg, CA1, P<0.05; CA2, P<0.01; CA3, P<0.001; DG, P<0.01; PB 40 mg/kg, CA1, CA2 and CA3, P<0.001; DG; P<0.01). Moreover, these changes were potentially reversed by treatment with the AChE inhibitor donepezil (CA1 and CA2, P<0.01; CA3 and DG, P<0.001), compared with the scopolamine-treated group.
Figure 6.
PB attenuates SCP-induced alteration of GluR1 expression in the hippocampus. Representative photomicrographs showed GluR1 expression represented by a percentage of immunoreactivity in CA1, CA2, CA3 and DG of the hippocampus. Scale bar, 50 µm; magnification, x40. Data are expressed as the mean ± SEM. ###P<0.001 vs. the control group; *P<0.05, **P<0.01, ***P<0.001 vs. the scopolamine-treated group. PB, pinostrobin; SCP, scopolamine; GluR1, glutamate receptor 1; CA1, cornu ammonis 1; CA2, cornu ammonis 2; CA3, cornu ammonis 3; DG, dentate gyrus; CON, control; DNP, donepezil.
Discussion
The present study demonstrated for the first time, to the best of the authors' knowledge, that pinostrobin, one of the core active compounds of Boesenbergia rotunda, probably attenuates memory impairment by the regulation of the oxidative stress and cholinergic system in an animal model of AD induced by scopolamine. Scopolamine is a muscarinic acetylcholine receptor antagonist, which induces cognitive impairment (38). Scopolamine has been shown to cause surges of oxidative stress including increased reactive oxygen species (ROS) production in the cortex and the hippocampus (39). Several behavioural tasks have been tentatively used to examine learning and memory performance in scopolamine models, including the Morris water maze test, novel objective recognition test, passive avoidance test and Y-maze test (8,10,29). In the current study, the NORT and Y-maze test were selected to probe the effects of pinostrobin on scopolamine-induced memory impairment. From a pragmatic standpoint, both the NORT and Y-maze test proved highly effective, demanding minimal, brief training. The NORT is considered to be a consistent behavioural model based on the time spent exploring new objects that appraises recognition memory (36). Recognition memory is a form of sporadic memory that is regularly observed to be declining during aging in humans and in patients with AD (40). The Y-maze test is a foundation model for evaluating working memory impairment, which is based on the willingness of animals to explore new environments (41). Therefore, controlled animals desire to explore a new arm of the maze rather than the one they have previously visited. The results of the present study demonstrated that scopolamine-treated rats illustrated a significant memory deficit by decreasing the percentages of RI in the NORT and spontaneous alternation in the Y-maze test. These observations align with several previous findings (10,42,43). Unexpectedly, treatment of rats with pinostrobin notably enhanced the cognitive performance that had been impaired by the injection of scopolamine. Additionally, to assess the neuroprotective mechanisms of pinostrobin against scopolamine-induced cognitive impairment, the effects of pinostrobin on the related changes in oxidative stress markers and cholinergic activity were investigated. Oxidative stress can occur when there is excessive production of ROS under various conditions and it contributes to the pathogenesis of several neurodegenerative disorders such as AD (32,44). Studies have demonstrated that scopolamine-induced memory impairment is associated with increased oxidative stress within the brain (45,46). Scopolamine administration decreases the activity of SOD, catalase, glutathione s-transferase and glutathione peroxidase (GPx) (47) and it also increases the level of MDA, a marker of lipid peroxidation (48). Thus, oxidative stress appears to be a pivotal factor in cognitive decline induced by scopolamine. Furthermore, the present study showed a significant increase in the hippocampal MDA concentration that was observed after scopolamine injection. Similarly, it has been reported that the administration of scopolamine for 7 days increased the levels of MDA in the brains of mice (11). Moreover, scopolamine has been associated with elevated Aβ accumulation and heightened oxidative stress within the hippocampus of rats (49). Aβ-mediated oxidative stress can cause metabolic alterations in the brain, such as lipid peroxidation and ROS production (50) leading to synaptic loss and cognitive dysfunction (51). Moreover, previous studies have indicated that injection of scopolamine causes oxidative stress by decreasing the activities of antioxidant enzymes (SOD or GPx) and increasing lipid peroxidation in the brain (52,53). These findings are consistent with the present results; the activities of SOD and GSH were markedly decreased in the scopolamine-treated group. Alternatively, treatment with pinostrobin or donepezil markedly reversed the scopolamine-induced increase in MDA concentration. Furthermore, the reduced activities of SOD and GSH in the hippocampus were markedly increased by pinostrobin. Therefore, the present results indicated that pinostrobin might help to alleviate scopolamine-induced memory deficits by reducing oxidative stress.
Cognitive function is linked to the cholinergic system and acetylcholine, a neurotransmitter that is widely spread across the nervous system and plays a vital role in memory and learning processes (54). Acetylcholine is degraded in the synaptic cleft by AChE, which converts it to acetic acid and choline. Excessive activation of AChE activity can lead to a shortage of ACh and cognitive dysfunction (10). Studies have demonstrated that scopolamine injection enhances the level of AChE (55,56). An elevated level of AChE metabolises more acetylcholine and decreases its level in the synapses and then disturbs cholinergic neurotransmission, which results in memory impairment (8). A previous study suggested that inhibitors of AChE can potentially reverse memory deficit induced by scopolamine (57). However, the precise mechanism of increased AChE activity by scopolamine remains to be elucidated. Some evidence suggests that scopolamine-induced oxidative stress may act as a potential mechanism for increasing AChE activity that results in cognitive impairment (42,58). The present results demonstrated that the significant dysfunction of the cholinergic system was observed in scopolamine-treated rats was accompanied by an increase in AChE activity in the hippocampus along with memory impairment, which is in accordance with previous studies (28,59). However, treatment with pinostrobin and donepezil significantly decreased AChE activity in the hippocampus. Furthermore, ChAT, the enzyme responsible for acetylcholine biosynthesis, is one of the most explicit markers for examining the functional state of cholinergic neurons in the central nervous system (60). Studies have indicated that scopolamine leads to reduced ChAT activity, which is associated with dementia (8,61). Hence, the present study aimed to determine cholinergic function by evaluating the expression of ChAT in the hippocampal CA1, CA2, CA3 and DG regions. The immunohistochemistry results revealed that the scopolamine-treated group showed a significant reduction in ChAT expression in all subregions of the hippocampus. These results are consistent with previous research that demonstrate a reduction in ChAT expression in the CA3 region of the hippocampus following scopolamine administration (62). Similarly, a 7-day regimen of scopolamine injections (3 mg/kg) markedly decreased ChAT expression in the CA1, CA3 and DG regions of the hippocampus (63). However, supplementation with pinostrobin at a low dose (20 mg/kg) markedly enhanced the function of ChAT in the hippocampal CA3 and DG. In addition, a high dose of pinostrobin (40 mg/kg) or of donepezil markedly restored the downregulation of hippocampal ChAT expression in all subregions. Accordingly, the modulation of the cholinergic activity was counteracted by pinostrobin, suggesting that it could play a significant role in restoring cognitive function.
Furthermore, the glutamatergic system also plays a crucial role in cognitive function and working memory in various brain areas such as the prefrontal cortex and the hippocampus (64,65). The Schaffer collateral pathway is a key neural route in the hippocampus, particularly in the CA1 area. It consists of axons from pyramidal neurons in the CA3 region projecting to pyramidal neurons in the CA1 region. Glutamate is essential for the normal functioning of the Schaffer collateral pathway in the hippocampus, mediating fast synaptic transmission through AMPA receptors and synaptic plasticity through NMDA receptors. This relationship is fundamental for processes such as learning and memory (66). The function of AMPA receptors (AMPARs) is important for synaptic plasticity and fundamental circuit of memory and learning (67). The cellular trafficking, synaptic anchoring and overall function of AMPARs are influenced by the specific subunits that make up the core ion channel, which is a tetramer composed of GluA1-GluA4 subunits (also known as GluR1-GluR4). The activity of AMPARs is dependent on neuronal activity. Consequently, a model that specifies subunit-dependent trafficking of AMPARs has been suggested and LTP is considered to require sequences or domains of the GluA1 subunit (68). Alterations in the number and function of AMPARs are crucial for synaptic plasticity and the cognitive decline associated with ageing. AMPARs are highly dynamic proteins, which are precisely regulated through processes of trafficking, recycling, degradation and replacement (69).
Studies have reported the involvement of hippocampal NMDA receptors in the learning and memory process (70,71). However, the critical role of AMPA receptors in cognitive function within the hippocampus, particularly in neurodegenerative animal models, has not been well evaluated. Therefore, the purpose of the present study was to investigate the function of glutamatergic AMPARs following scopolamine administration. The present study revealed that scopolamine injection markedly reduced GluR1 expression within the hippocampus. A previous study indicated that a decrease in GluA1 expression may contribute to cognitive deficits associated with ketamine-induced neurotoxicity (16). However, the present findings demonstrated that pinostrobin and donepezil treatment substantially increased GluR1 expression in the hippocampus of rats treated with scopolamine. Although the alleviating effect of pinostrobin has been determined against oxidative stress, cholinergic and glutamatergic dysfunction related to memory impairment induced by scopolamine in adult male rats, further investigations are recommended to verify the molecular mechanisms of pinostrobin against scopolamine-induced memory deficit involved in cholinergic, glutamatergic and inflammatory signalling pathways.
Pinostrobin demonstrates neuroprotective effects by mitigating cognitive impairment, decreasing oxidative stress and enhancing neuronal density and astrocyte function in the hippocampus (26,72). Although previous studies have not detailed the exact mechanism by which pinostrobin crosses the blood-brain barrier, its pharmacological properties, such as antioxidant and anti-inflammatory effects, suggest that it may interact with transporters or mechanisms that facilitate this process (73). Moreover, molecular dynamics simulations have shown that pinostrobin can form inclusion complexes with beta-cyclodextrin derivatives, potentially improving its stability and solubility for enhanced bioavailability and transport across biological barriers, including the blood-brain barrier (74). Although not explicitly studied for pinostrobin, other flavonoids such as pinocembrin are known to interact with transporters such as P-glycoprotein (P-gp) and multidrug resistance proteins, which could potentially play a role in the transport of pinostrobin (75). However, further research is needed to elucidate the specific transport mechanisms or interactions that enable pinostrobin's penetration of the blood-brain barrier, which would deepen our understanding of its neuroprotective effects.
A limitation of the present study was the lack of hippocampal NMDA receptor measurement, for an improved understanding of the involvement of hippocampal glutamate receptors in cognitive function associated with neurological disorders; defining the underlying molecular mechanisms of NMDA and AMPA glutamate is essential for future research. Furthermore, to provide more robust data on the nootropic effects of pinostrobin or donepezil alone on cognitive function and oxidative stress markers without the interference of scopolamine, future studies should include groups of animals that do not receive scopolamine treatment. The present study also provided recommendations for assessing locomotor activity by using the open field that may influence cognitive function. This is important for distinguishing between cognitive enhancements and changes in general activity levels. Lastly, it is further advised to use scoring methods for histopathological examination that are effective and validate for quantifying histological changes.
The current study revealed that oral administration of pinostrobin could improve the cognitive deficit induced in an AD model by scopolamine. Administration of scopolamine resulted in an increase in oxidative stress and AChE activity was potentially alleviated by the treatment with pinostrobin. Additionally, pinostrobin also enhanced the expression of ChAT and GluR1 in the hippocampus. Based on these results, the present study proposes that pinostrobin may serve as a potential therapeutic agent for restoring memory function by affecting oxidative stress and cholinergic activity in the hippocampus.
Acknowledgements
Not applicable.
Funding Statement
Funding: The current study was financially supported by the Thailand Science Research and Innovation fund and the University of Phayao, Phayao, Thailand (grant no. FF65-RIM121).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
ST was responsible for conceptualization, investigation, methodology, writing, reviewing and editing. TP was responsible for formal analysis, reviewing and editing the manuscript. NS was responsible for methodology, reviewing and editing the manuscript. SS was responsible for methodology and formal analysis. JJ was responsible for formal analysis, reviewing and editing the manuscript. RK was responsible for conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, validation, writing the original draft and writing, reviewing and editing the manuscript. RK and ST confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethical approval and consent to participate
All experiments were approved by the Ethics Committee of the Laboratory Animal Research Center, University of Phayao, Thailand (approval no. 640104029).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mat Nuri TH, Hong YH, Ming LC, Mohd Joffry S, Othman MF, Neoh CF. Knowledge on Alzheimer's disease among public hospitals and health clinics pharmacists in the State of Selangor, Malaysia. Front Pharmacol. 2017;8(739) doi: 10.3389/fphar.2017.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djeuzong E, Kandeda AK, Djiogue S, Stéphanie L, Nguedia D, Ngueguim F, Djientcheu JP, Kouamouo J, Dimo T. Antiamnesic and neuroprotective effects of an aqueous extract of ziziphus jujuba mill. (rhamnaceae) on scopolamine-induced cognitive impairments in rats. Evid Based Complement Alternat Med. 2021;2021(5577163) doi: 10.1155/2021/5577163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shabani S, Mirshekar MA. Diosmin is neuroprotective in a rat model of scopolamine-induced cognitive impairment. Biomed Pharmacother. 2018;108:1376–1383. doi: 10.1016/j.biopha.2018.09.127. [DOI] [PubMed] [Google Scholar]
- 4.Mahdy K, Shaker O, Wafay H, Nassar Y, Hassan H, Hussein A. Effect of some medicinal plant extracts on the oxidative stress status in Alzheimer's disease induced in rats. Eur Rev Med Pharmacol Sci. 2012;16 (Suppl 3):S31–S42. [PubMed] [Google Scholar]
- 5.von Bernhardi R, Eugenin-von Bernhardi L, Eugenin J. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci. 2015;7(124) doi: 10.3389/fnagi.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake MG, Krawczyk MC, Baratti CM, Boccia MM. Neuropharmacology of memory consolidation and reconsolidation: Insights on central cholinergic mechanisms. J Physiol Paris. 2014;108:286–291. doi: 10.1016/j.jphysparis.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, Kim HG, Lee HW, Han JM, Lee SK, Kim DW, Saravanakumar A, Son CG. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci Rep. 2015;5(9651) doi: 10.1038/srep09651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JH, Lee EB, Jang HH, Cha YS, Park YS, Lee SH. Allium hookeri extracts improve scopolamine-induced cognitive impairment via activation of the cholinergic system and anti-neuroinflammation in mice. Nutrients. 2021;13(2890) doi: 10.3390/nu13082890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang KS. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer's biomarkers. Life Sci. 2019;233(116695) doi: 10.1016/j.lfs.2019.116695. [DOI] [PubMed] [Google Scholar]
- 10.Hussain H, Ahmad S, Shah SWA, Ullah A, Ali N, Almehmadi M, Ahmad M, Khalil AAK, Jamal SB, Ahmad H, Halawi M. Attenuation of scopolamine-induced amnesia via cholinergic modulation in mice by synthetic curcumin analogs. Molecules. 2022;27(2468) doi: 10.3390/molecules27082468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, Kim J, He M, Lee A, Cho E. Apigenin ameliorates scopolamine-induced cognitive dysfunction and neuronal damage in mice. Molecules. 2021;26(5192) doi: 10.3390/molecules26175192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safar MM, Arab HH, Rizk SM, El-Maraghy SA. Bone marrow-derived endothelial progenitor cells protect against scopolamine-induced alzheimer-like pathological aberrations. Mol Neurobiol. 2016;53:1403–1418. doi: 10.1007/s12035-014-9051-8. [DOI] [PubMed] [Google Scholar]
- 13.Danysz W, Parsons CG. Alzheimer's disease, β-amyloid, glutamate, NMDA receptors and memantine-searching for the connections. Br J Pharmacol. 2012;167:324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eder P, Reinprecht I, Schreiner E, Skofitsch G, Windisch M. Increased density of glutamate receptor subunit 1 due to Cerebrolysin treatment: An immunohistochemical study on aged rats. Histochem J. 2001;33:605–612. doi: 10.1023/a:1016394031947. [DOI] [PubMed] [Google Scholar]
- 15.Dewar D, Chalmers DT, Graham DI, McCulloch J. Glutamate metabotropic and AMPA binding sites are reduced in Alzheimer's disease: an autoradiographic study of the hippocampus. Brain Res. 1991;553:58–64. doi: 10.1016/0006-8993(91)90230-s. [DOI] [PubMed] [Google Scholar]
- 16.Ding R, Li Y, Du A, Yu H, He B, Shen R, Zhou J, Li L, Cui W, Zhang G, et al. Changes in hippocampal AMPA receptors and cognitive impairments in chronic ketamine addiction models: Another understanding of ketamine CNS toxicity. Sci Rep. 2016;6(38771) doi: 10.1038/srep38771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YJ, Chen HB, Wei B, Wang W, Zhou PL, Zhan JQ, Hu MR, Yan K, Hu B, Yu B. Cognitive decline is associated with reduced surface GluR1 expression in the hippocampus of aged rats. Neurosci Lett. 2015;591:176–181. doi: 10.1016/j.neulet.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 18.Aigner TG. Pharmacology of memory: Cholinergic-glutamatergic interactions. Curr Opin Neurobiol. 1995;5:155–160. doi: 10.1016/0959-4388(95)80021-2. [DOI] [PubMed] [Google Scholar]
- 19.Francis PT, Parsons CG, Jones RW. Rationale for combining glutamatergic and cholinergic approaches in the symptomatic treatment of Alzheimer's disease. Expert Rev Neurother. 2012;12:1351–1365. doi: 10.1586/ern.12.124. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Luo X, Liu X, Liu D, Wang X, Guo Z, Zhu L, Tian Q, Yang X, Wang JZ. Intraperitoneal administration of a novel TAT-BDNF peptide ameliorates cognitive impairments via modulating multiple pathways in two Alzheimer's rodent models. Sci Rep. 2015;5(15032) doi: 10.1038/srep15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahey JW, Stephenson KK. Pinostrobin from honey and Thai ginger (Boesenbergia pandurata): A potent flavonoid inducer of mammalian phase 2 chemoprotective and antioxidant enzymes. J Agric Food Chem. 2002;50:7472–7476. doi: 10.1021/jf025692k. [DOI] [PubMed] [Google Scholar]
- 22.Ijaz MU, Shahzadi S, Hamza A, Azmat R, Anwar H, Afsar T, Shafique H, Bhat MA, Naglah AM, Al-Omar MA, Razak S. Alleviative effects of pinostrobin against cadmium-induced renal toxicity in rats by reducing oxidative stress, apoptosis, inflammation, and mitochondrial dysfunction. Front Nutr. 2023;10(1175008) doi: 10.3389/fnut.2023.1175008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu D, Nair MG, DeWitt DL. Novel compounds from Piper methysticum Forst (Kava Kava) roots and their effect on cyclooxygenase enzyme. J Agric Food Chem. 2002;50:701–705. doi: 10.1021/jf010963x. [DOI] [PubMed] [Google Scholar]
- 24.Patel NK, Bhutani KK. Pinostrobin and Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits TNF-α and IL-1β production: In vitro and in vivo experimentation. Phytomedicine. 2014;21:946–953. doi: 10.1016/j.phymed.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhao LL, Jayeoye TJ, Ashaolu TJ, Olatunji OJ. Pinostrobin, a dietary bioflavonoid exerts antioxidant, anti-inflammatory, and anti-apoptotic protective effects against methotrexate-induced ovarian toxicity in rats. Tissue Cell. 2023;85(102254) doi: 10.1016/j.tice.2023.102254. [DOI] [PubMed] [Google Scholar]
- 26.Thongrong S, Surapinit S, Promsrisuk T, Jittiwat J, Kongsui R. Pinostrobin alleviates chronic restraint stress-induced cognitive impairment by modulating oxidative stress and the function of astrocytes in the hippocampus of rats. Biomed Rep. 2023;18(20) doi: 10.3892/br.2023.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kongsui R, Surapinit S, Promsrisuk T, Thongrong S. Pinostrobin from Boesenbergia rotunda attenuates oxidative stress and promotes functional recovery in rat model of sciatic nerve crush injury. Braz J Med Biol Res. 2023;56(e12578) doi: 10.1590/1414-431X2023e12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J, Huang L, Yu J, Xiang S, Wang J, Zhang J, Yan X, Cui W, He S, Wang Q. Fucoxanthin, a marine carotenoid, reverses scopolamine-induced cognitive impairments in mice and inhibits acetylcholinesterase in vitro. Mar Drugs. 2016;14(67) doi: 10.3390/md14040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo Y, Lim JS, Oh J, Lee JS, Kim JS. Neuroprotective effects of euonymus alatus extract on scopolamine-induced memory deficits in mice. Antioxidants (Basel) 2020;9(449) doi: 10.3390/antiox9050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Nakmareong S, Kukongviriyapan U, Pakdeechote P, Donpunha W, Kukongviriyapan V, Kongyingyoes B, Sompamit K, Phisalaphong C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:519–529. doi: 10.1007/s00210-011-0624-z. [DOI] [PubMed] [Google Scholar]
- 32.Srdjenovic B, Mrdjanovic J, Galovic AJ, Kladar N, Bozin B, Jurisic V, Bogdanovic G. Effect of ELF-EMF on antioxidant status and micronuclei in K562 cells and normal lymphocytes. Open Life Sci. 2014;9:931–940. [Google Scholar]
- 33.Polycarp TN, Obukowho EB, Yusoff SM. Changes in haematological parameters and oxidative stress response of goats subjected to road transport stress in a hot humid tropical environment. Comp Clin Pathol. 2016;25:285–293. [Google Scholar]
- 34.Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic stress induced remodeling of the prefrontal cortex: Structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex. 2013;23:1784–1797. doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- 35.Johnson SJ, Walker FR. Strategies to improve quantitative assessment of immunohistochemical and immunofluorescent labelling. Sci Rep. 2015;5(10607) doi: 10.1038/srep10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batool Z, Sadir S, Liaquat L, Tabassum S, Madiha S, Rafiq S, Tariq S, Batool TS, Saleem S, Naqvi F, et al. Repeated administration of almonds increases brain acetylcholine levels and enhances memory function in healthy rats while attenuates memory deficits in animal model of amnesia. Brain Res Bull. 2016;120:63–74. doi: 10.1016/j.brainresbull.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Mooshekhian A, Sandini T, Wei Z, Van Bruggen R, Li H, Li XM, Zhang Y. Low-field magnetic stimulation improved cuprizone-induced depression-like symptoms and demyelination in female mice. Exp Ther Med. 2022;23(210) doi: 10.3892/etm.2022.11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Rahimzadegan M, Soodi M. Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus. Basic Clin Neurosci. 2018;9:5–14. doi: 10.29252/NIRP.BCN.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terry AV, Callahan PM. Nicotinic acetylcholine receptor ligands, cognitive function, and preclinical approaches to drug discovery. Nicotine Tob Res. 2019;21:383–394. doi: 10.1093/ntr/nty166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraeuter AK, Guest PC, Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol. 2019;1916:105–111. doi: 10.1007/978-1-4939-8994-2_10. [DOI] [PubMed] [Google Scholar]
- 42.Afzal M, Alzarea SI, Alharbi KS, Alzarea AI, Alenezi SK, Alshammari MS, Alquraini AH, Kazmi I. Rosiridin attenuates scopolamine-induced cognitive impairments in rats via inhibition of oxidative and nitrative stress leaded caspase-3/9 and TNF-α signaling pathways. Molecules. 2022;27(5888) doi: 10.3390/molecules27185888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhuvanendran S, Kumari Y, Othman I, Shaikh MF. Amelioration of cognitive deficit by embelin in a scopolamine-induced Alzheimer's disease-like condition in a rat model. Front Pharmacol. 2018;9(665) doi: 10.3389/fphar.2018.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J, O W, Li W, Jiang ZG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013;14:24438–24475. doi: 10.3390/ijms141224438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J, Ding J, Geng M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett. 2005;374:222–226. doi: 10.1016/j.neulet.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 46.Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, Biala G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology (Berl) 2015;232:931–942. doi: 10.1007/s00213-014-3728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uma G, Maheswari SU. Neuroprotective effects of polyherbal formulation (Indian NONI) on scopolamine-induced memory impairment in mice. Int J Pharm Pharm Sci. 2014;6:354–357. [Google Scholar]
- 48.Abd-El-Fattah MA, Abdelakader NF, Zaki HF. Pyrrolidine dithiocarbamate protects against scopolamine-induced cognitive impairment in rats. Eur J Pharmacol. 2014;723:330–338. doi: 10.1016/j.ejphar.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Hernández-Rodríguez M, Arciniega-Martínez IM, García-Marín ID, Correa-Basurto J, Rosales-Hernández MC. Chronic administration of scopolamine increased GSK3βP9, beta secretase, amyloid beta, and oxidative stress in the hippocampus of wistar rats. Mol Neurobiol. 2020;57:3979–3988. doi: 10.1007/s12035-020-02009-x. [DOI] [PubMed] [Google Scholar]
- 50.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 51.Gul S, Attaullah S, Alsugoor MH, Bawazeer S, Shah SA, Khan S, Salahuddin HS, Ullah M. Folicitin abrogates scopolamine induced oxidative stress, hyperlipidemia mediated neuronal synapse and memory dysfunction in mice. Heliyon. 2023;9(e16930) doi: 10.1016/j.heliyon.2023.e16930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo C, Shen J, Meng Z, Yang X, Li F. Neuroprotective effects of polygalacic acid on scopolamine-induced memory deficits in mice. Phytomedicine. 2016;23:149–155. doi: 10.1016/j.phymed.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Liao J, Nai Y, Feng L, Chen Y, Li M, Xu H. Walnut oil prevents scopolamine-induced memory dysfunction in a mouse model. Molecules. 2020;25(1630) doi: 10.3390/molecules25071630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghias M, Shoaib M, Ali Shah SW, Umar MN, Ullah S, Ali N, Shah I, Ullah S. Nootropic effects of synthetic flavonoid derivatives on scopolamine induced memory impairment in mice via cholinesterase inhibition and antioxidant system. Pak J Pharm Sci. 2019;32 (5 Suppl):S2325–S2332. [PubMed] [Google Scholar]
- 55.Soodi M, Naghdi N, Hajimehdipoor H, Choopani S, Sahraei E. Memory-improving activity of Melissa officinalis extract in naive and scopolamine-treated rats. Res Pharm Sci. 2014;9:107–114. [PMC free article] [PubMed] [Google Scholar]
- 56.Yadang FSA, Nguezeye Y, Kom CW, Betote PHD, Mamat A, Tchokouaha LRY, Taiwé GS, Agbor GA, Bum EN. Scopolamine-induced memory impairment in mice: Neuroprotective effects of carissa edulis (forssk.) valh (apocynaceae) aqueous extract. Int J Alzheimers Dis. 2020;2020(6372059) doi: 10.1155/2020/6372059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaudhaery SS, Roy KK, Shakya N, Saxena G, Sammi SR, Nazir A, Nath C, Saxena AK. Novel carbamates as orally active acetylcholinesterase inhibitors found to improve scopolamine-induced cognition impairment: Pharmacophore-based virtual screening, synthesis, and pharmacology. J Med Chem. 2010;53:6490–6505. doi: 10.1021/jm100573q. [DOI] [PubMed] [Google Scholar]
- 58.Härtl R, Gleinich A, Zimmermann M. Dramatic increase in readthrough acetylcholinesterase in a cellular model of oxidative stress. J Neurochem. 2011;116:1088–1096. doi: 10.1111/j.1471-4159.2010.07164.x. [DOI] [PubMed] [Google Scholar]
- 59.Eshaghi Ghalibaf MH, Rajabian A, Parviz M, Akbarian M, Amirahmadi S, Vafaee F, Hosseini M. Minocycline alleviated scopolamine-induced amnesia by regulating antioxidant and cholinergic function. Heliyon. 2023;9(e13452) doi: 10.1016/j.heliyon.2023.e13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oda Y. Choline acetyltransferase: The structure, distribution and pathologic changes in the central nervous system. Pathol Int. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- 61.Sohn E, Lim HS, Kim YJ, Kim BY, Jeong SJ. Annona atemoya leaf extract improves scopolamine-induced memory impairment by preventing hippocampal cholinergic dysfunction and neuronal cell death. Int J Mol Sci. 2019;20(3538) doi: 10.3390/ijms20143538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim DW, Son HJ, Um MY, Kim IH, Han D, Cho S, Lee CH. Enhanced cognitive effects of demethoxycurcumin, a natural derivative of curcumin on scopolamine-induced memory impairment in mice. Molecules. 2016;21(1022) doi: 10.3390/molecules21081022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olayinka JN, Eduviere A, Adeoluwa O, Akinluyi E, Obisesan A, Akawa O, Adebanjo A. Quercetin mitigates scopolamine-induced memory dysfunction: Impact on oxidative stress and cholinergic mechanisms. Metab Brain Dis. 2022;37:265–277. doi: 10.1007/s11011-021-00861-x. [DOI] [PubMed] [Google Scholar]
- 64.Cheng YJ, Lin CH, Lane HY. Involvement of cholinergic, adrenergic, and glutamatergic network modulation with cognitive dysfunction in Alzheimer's disease. Int J Mol Sci. 2021;22(2283) doi: 10.3390/ijms22052283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hascup KN, Findley CA, Sime LN, Hascup ER. Hippocampal alterations in glutamatergic signaling during amyloid progression in AβPP/PS1 mice. Sci Rep. 2020;10(14503) doi: 10.1038/s41598-020-71587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwon O, Feng L, Druckmann S, Kim J. Schaffer collateral inputs to CA1 excitatory and inhibitory neurons follow different connectivity rules. J Neurosci. 2018;38:5140–5152. doi: 10.1523/JNEUROSCI.0155-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Royo M, Escolano BA, Madrigal MP, Jurado S. AMPA receptor function in hypothalamic synapses. Front Synaptic Neurosci. 2022;14(833449) doi: 10.3389/fnsyn.2022.833449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 69.Henley JM, Wilkinson KA. AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin Neurosci. 2013;15:11–27. doi: 10.31887/DCNS.2013.15.1/jhenley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bye CM, McDonald RJ. A specific role of hippocampal NMDA receptors and arc protein in rapid encoding of novel environmental representations and a more general long-term consolidation function. Front Behav Neurosci. 2019;13(8) doi: 10.3389/fnbeh.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada K, Arai M, Suenaga T, Ichitani Y. Involvement of hippocampal NMDA receptors in encoding and consolidation, but not retrieval, processes of spontaneous object location memory in rats. Behav Brain Res. 2017;331:14–19. doi: 10.1016/j.bbr.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 72.Li C, Tang B, Feng Y, Tang F, Pui-Man Hoi M, Su Z, Ming-Yuen Lee S. Pinostrobin exerts neuroprotective actions in neurotoxin-induced Parkinson's disease models through Nrf2 induction. J Agric Food Chem. 2018;66:8307–8318. doi: 10.1021/acs.jafc.8b02607. [DOI] [PubMed] [Google Scholar]
- 73.Gao WY, Chen PY, Chen SF, Wu MJ, Chang HY, Yen JH. Pinostrobin inhibits proprotein convertase subtilisin/kexin-type 9 (PCSK9) gene expression through the modulation of FoxO3a protein in HepG2 cells. J Agric Food Chem. 2018;66:6083–6093. doi: 10.1021/acs.jafc.8b02559. [DOI] [PubMed] [Google Scholar]
- 74.Kicuntod J, Khuntawee W, Wolschann P, Pongsawasdi P, Chavasiri W, Kungwan N, Rungrotmongkol T. Inclusion complexation of pinostrobin with various cyclodextrin derivatives. J Mol Graph Model. 2016;63:91–98. doi: 10.1016/j.jmgm.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Yang ZH, Sun X, Qi Y, Mei C, Sun XB, Du GH. Uptake characteristics of pinocembrin and its effect on p-glycoprotein at the blood-brain barrier in in vitro cell experiments. J Asian Nat Prod Res. 2012;14:14–21. doi: 10.1080/10286020.2011.620393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.