Abstract
Trans-resveratrol, a widely used supplement for humans, aims to enhance the body’s antioxidant defense. Studies suggest that it exerts anti-inflammatory and antioxidant effects by activating the nuclear factor erythroid 2-related factor 2 (Nrf2). In order to evaluate this hypothesis, LDLr(−/−) mice were fed a Western diet to induce liver inflammation and oxidative stress. One group was fed a diet containing 0.60 mg/day of trans-resveratrol (RESV), while another group received no dietary supplementation (CONT). Oxidative stress biomarkers and inflammatory cytokines were assessed in liver homogenates. It was observed that trans-resveratrol decreased hepatic oxidative stress by increasing the GSH/GSSG ratio and reducing malondialdehyde (MDA) concentration. However, the RESV group exhibited a reduction in Nrf2 relative expression compared to CONT. Additionally, trans-resveratrol supplementation reduced nuclear factor-κB (NF-κB) expression but led to an increase in IL-6, with no significant changes observed in tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10) concentrations. Overall, these findings indicate that the in vivo antioxidant impact induced by trans-resveratrol supplementation in hepatic tissue did not correlate with increase of inflammatory cytokines and Nrf2 relative expression. Further exploration of alternative mechanisms, such as direct radical scavenger activity, is warranted to elucidate the antioxidant effect.
Keywords: oxidative stress, trans-resveratrol, inflammation, liver, mice
Introduction
The polyphenol trans-resveratrol (3,5,4'-trihydroxy-trans-stilbene) is one of the most studied phytochemicals, due to its multiple molecular mechanisms in immune, redox, and metabolic regulatory pathways.(1,2) Some studies have reported the beneficial effect of trans-resveratrol in order to reduce the atherosclerosis.(3) A classical animal model to induce experimental atherosclerosis is the LDLr(−/−) mice fed a Wester diet.(4) Besides the increase of fatty strakes and plaques,(5) this model also induces inflammation in the liver.(6) It has also been reported that the consumption of a high-fat diet can increase the production of reactive oxygen species (ROS), which in turn, can also activate the systemic inflammation and oxidative stress.(7,8)
A number of studies have shown that trans-resveratrol can stimulate the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and, consequently, decreases oxidative stress and inflammation.(9–11) Additionally, many studies have reported that resveratrol regulates inflammatory response through a variety of signaling pathways, such as arachidonic acid pathway, nuclear factor kappa B (NF-κB), Mitogen-activated protein kinase (MAPK), and activator protein-1 (AP-1).(12)
However, results from clinical trials carried out with trans-resveratrol supplementation vary according to several factors, including the number of participants, health status of the gut microbiota, age, gender, lifestyle, dose, administration medium, and type of administration, becoming difficult to achieve a consensus.(13) In vitro studies performed with different types of cells or others performed in animal models, also bring different results about the antioxidant and anti-inflammatory effects of trans-resveratrol.(12,14) In a recent review, it was highlighted that most of the clinical trials involving trans-resveratrol did not evaluate its antioxidant activity.(15) This fact may be partially justified by the complexity of the methods applied to quantify oxidative markers, the high variability observed among the studies, the lack of reference values, and the weak correlation with clinical endpoints.(16)
Although the evidences are still not enough, trans-resveratrol has been widely commercialized as antioxidant for human supplementation.(17,18) Thus, in view of the elevate consume of trans-resveratrol supplements and the lack of enough information about its physiological effect, it is necessary to better investigate its action on hepatic tissue and potential mechanism involved in this response.
Materials and Methods
Material
Trans-resveratrol was acquired from Eop Eireli Pharmacy (Santo André, Brazil). 1,1,3,3-tetraethoxypropane (TEP), nicotinamide adenine dinucleotide (NADH), superoxide dismutase (SOD), nicotinamide adenine dinucleotide phosphate (NADPH), glutathione peroxidase (GPx) and reduced and oxidized glutathione (GSH and GSSG) were purchased from Sigma-Aldrich (Sigma Chemical Co, St. Louis, MO). Trans-resveratrol standard was also obtained from Sigma-Aldrich (Sigma Chemical Co). All solvents were HPLC grade.
Study design
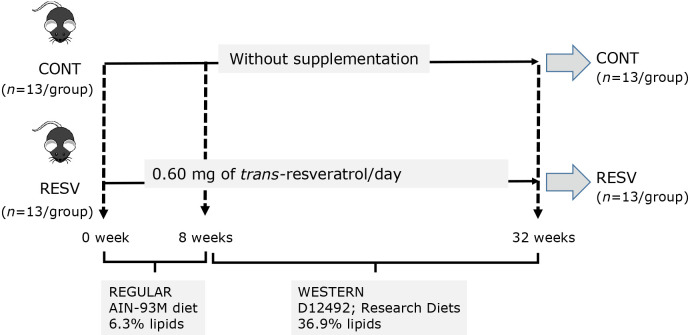
Three-month-old male homozygous LDLr(−/−) mice in the C57BL/6 background were purchased from the Faculty of Pharmaceutical Sciences at the University of São Paulo. The experimental protocol for animal use was approved by the Institutional Animal Care and Use Committee of the Faculty of Pharmaceutical Sciences at the University of São Paulo (CEUA/FCF 595). Mice were housed in plastic cages (4–5 animal cages) at constant room temperature (25 ± 2°C) and relative humidity (55 ± 10%), under a 12 h light–12 h dark cycle. Mice with an initial body weight of 25.49 ± 1.73 g were randomly allocated into two groups (n = 13/group) and fed ad libitum with a standard AIN-93M diet for 8 weeks without supplementation (CONT),(19) or the same diet mixed with 250 mg of trans-resveratrol per kg diet (RESV). After 8 weeks, the standard diet was replaced by a Western diet (D12492; Research Diets, New Brunswink, NJ), keeping the same supplementation. Figure 1 presents the experimental design applied in this study. The trans-resveratrol added to the diets showed a high degree of purity by comparing with the standard (PHL89539; Sigma Chemical Co) (Supplemental Methods, Supplemental Table 1, and Supplemental Fig. 1*). The dose applied in this study (0.60 mg/day) was based on human (70 kg body weight) intake of about 1.0 g/day. Diets formulation and chemical composition is shown in Supplemental Table 2 and Supplemental Table 3*, respectively. Individual body weight and food intake per cage were recorded twice a week. After the experimental period, mice were deprived of food for 8 h, anesthetized with 3% isoflurane and euthanized. Blood was collected for plasma lipid profile analysis. The liver was excised and weighed and small pieces of the larger lobe were stored at −80°C.
Fig. 1.
Experimental design.
Plasma lipids
Plasma lipids [total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL-C)] concentration was quantified using Labtest Diagnóstica SA (Lagoa Santa, Brazil) commercial kits for enzymatic colorimetric tests according to the manufacturer’s instructions.
Antioxidant activity and biomarkers of oxidative stress
Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activity was determined using spectrophotometry method.(20–22) The GSH/GSSG ratio was quantified using a kit for oxidized and reduced glutathione (Sigma Chemical Co). All enzymatic assays were performed in liver homogenate using the Synergy HTX Multi-Detection Microplate Reader (BioTek Instruments Inc., Winooski, VT) and were analyzed using Gen 5 software. Hepatic malondialdehyde (MDA) concentration was determined by High Performance Liquid Chromatography.(23) Protein concentration was determined using the Pierce BCA Protein Assay kit (Thermo Scientific, Waltham, MA), and results were expressed as μM MDA/mg of protein. The analyses were run in triplicate.
Cytokine content
For cytokine analysis, the liver was homogenized in RIPA buffer with a complete protease inhibitor cocktail, and the homogenates were cleared by centrifugation. The protein content was quantified with the Pierce BCA Protein Assay kit. The cytokines IL-6, TNF-α, and IL-10 were determined using the MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel. The values obtained were normalized to the total protein content and expressed as pg/mg protein.
Real-time PCR
Total RNA from liver homogenate samples was extracted with TRIzol (Invitrogen, Carlsbad, CA), followed by incubation in DNase I RNase-free (Roche Applied Science, Indianapolis, IN) and then reverse transcription using 2 μg of total RNA, SuperScript II Reverse Transcriptase (Invitrogen) and random primers p(dN)6 (Roche Applied Science). Real-time PCR was performed using the 7500TM Real-Time PCR System (Applied Biosystems, Warrington, UK), Power SYBR Green Gene Expression PCR Master Mix (Applied Biosystems) and specific primers for target genes: Actb (forward: gctccggcatgtgcaaag; reverse: catcacaccctggtgccta), Gapdh (forward: ctttggcg-gaggtgctagat; reverse: aggactcgtgcagccttacac), Nfe2l2 (forward: tgaccatgagtcgcttgcc; reverse: cctgatgaggggcagtgaag), Nfkb1 (forward: agcaaccaaaacagaggggat; reverse: ctttgcaggccccacatagt), Ppia (forward: ccgttcagctctgggatgac; reverse: ggg-cagcccagaacatcat), Sod1 (forward: ggaaccatccacttcgagca; reverse: cccatgctggccttcag-tta) and Sod2 (forward: gcctgctctaatcaggaccc; reverse: tagtaagcgtgctcccacac). The superoxide dismutase (SOD) family scavenge superoxide radicals (O2•−) by dismutation into hydrogen peroxide (H2O2). SOD1 (Cu, ZnSOD) isoform is a cytosolic enzyme, while SOD2 (MnSOD) is located inside the mitochondrial matrix, being considered the major protective barrier against the superoxide produced during mitochondrial respiration.(24) Data were normalized to the geometric average of Actb, Gapdh and Ppia. Relative quantification of mRNA was calculated by 2−ΔΔCt (n = 8/group).
Statistical analysis
Data were evaluated by t test for independent groups or non-parametric Mann–Whitney test, depending on their distribution and homogeneity of variances evaluated by Shapiro–Wilk and Hartley test, respectively. Values were expressed as mean ± SEM. A p value of 0.05 was adopted to reject the null hypothesis. Calculations were performed using software Statistica ver. 13 (TIBCO Software Inc., Palo Alto, CA) and graphs using GraphPad Prisma ver. 10 (GraphPad Software, San Diego, CA).
Results
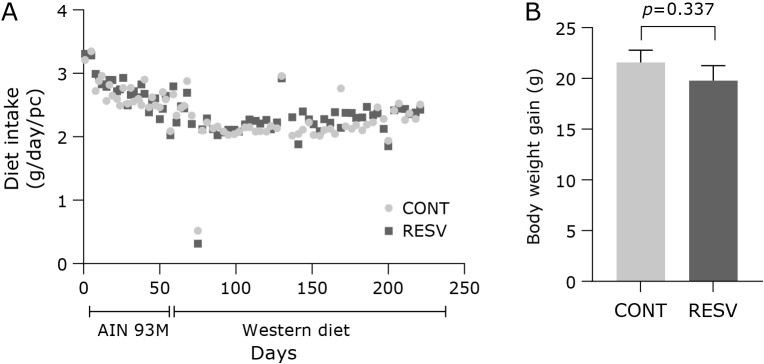
The supplementation with trans-resveratrol (0.60 mg/day) (RESV) during 16 weeks did not change diet intake and body weight gain compared with control group (CONT) (Fig. 2A). In general, the diet intake decreased by 15.7% (p<0.001) after 8 weeks, when the regular diet (AIN-93M) was switched to the Western diet. The body weight of the groups CONT and RESV at the start (25.53 ± 0.34 g; p = 0.877) and at the end (46.20 ± 1.04 g; p = 0.427) of the assay were also similar, as well as the body weight gain (Fig. 2B). The liver weight (1.97 ± 0.09 g; p = 0.230) and relative liver weight (4.21 ± 0.12%; p = 0.170) did not differ between the groups. The lipid profile was not altered by the supplementation of trans-resveratrol, as evidenced in the plasma total cholesterol (954.83 ± 46.94 mg/dl; p = 0.197), LDL-C (690.50 ± 61.04 mg/dl; p = 0.342), HDL-C (52.21 ± 5.09 mg/dl; p = 0.686), and triacylglycerol (213.00 ± 31.41 mg/dl; p = 0.088) concentrations.
Fig. 2.
Effects of trans-resveratrol in diet intake and body weight gain during the assay. (A) diet intake, (B) body weight gain. CONT, control group; RESV, trans-resveratrol group. Values are expressed as mean ± SEM (n = 13/group).
Regarding to the oxidative biomarkers, GSH concentration was not different among the groups (Fig. 3A), while a decrease in GSSG was observed in RESV group (Fig. 3B). As consequence, the GSH/GSSG ratio was higher in RESV group (Fig. 3C). This result was corroborated by malondialdehyde (MDA) concentration evaluated in the liver homogenate. When compared with the control group, RESV group showed 37% less MDA concentration (Fig. 3D).
Fig. 3.
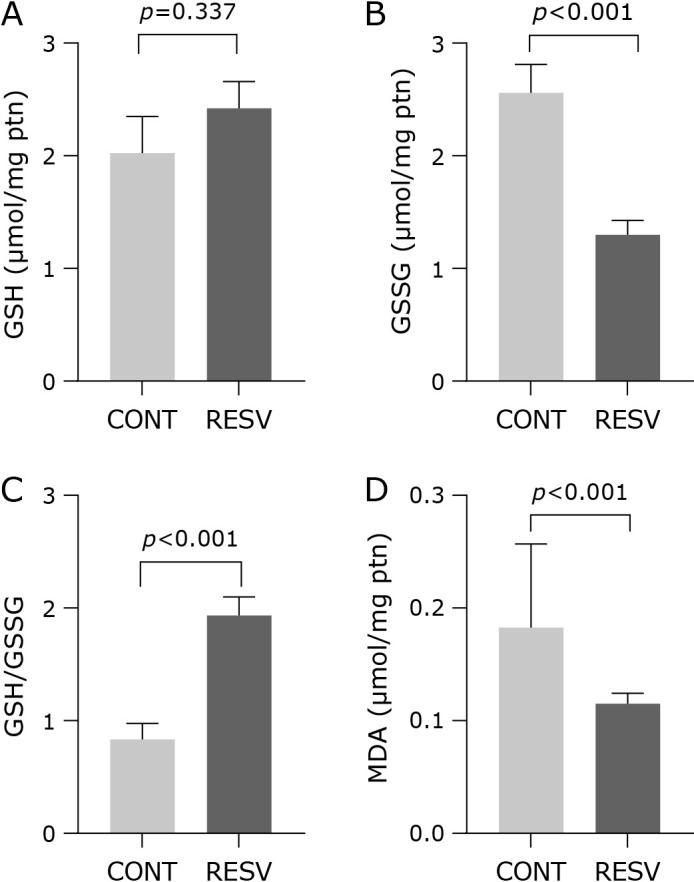
Trans-resveratrol effects in oxidative stress in the liver. (A) GSH, (B) GSSG, (C) GSH/GSSG, and (D) MDA concentration. CONT, control group; RESV, trans-resveratrol group. Values are expressed as mean ± SEM (n = 10/group).
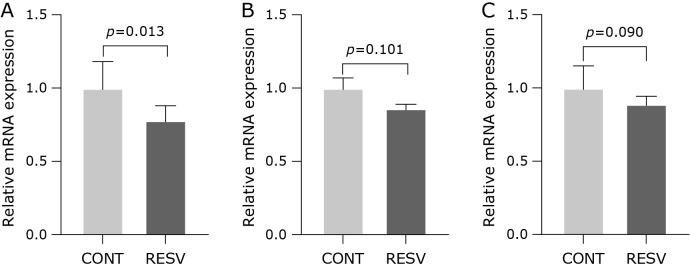
We hypothesized that trans-resveratrol mediates redox protection via antioxidant gene regulation by increasing the Nrf2 expression. However, an opposite result was found in our samples, in which the relative expression of Nrf2 was reduced after trans-resveratrol supplementation (Fig. 4A). Although trans-resveratrol has reduced the expression of Nrf2, no changes were found to the relative expression of SOD1 (Fig. 4B) and SOD2 (Fig. 4C). The activity of SOD (2.91 ± 0.19 U/mg ptn; p = 0.220), GPx (0.90 ± 0.05 U/mg ptn; p = 0.686) and CAT (0.49 ± 0.04 U/mg ptn; p = 0.091) did not change neither. The inflammatory cytokines measured in the hepatic tissue showed that trans-resveratrol supplementation reduced NF-κB (Fig. 5A), increased IL-6 (Fig. 5B) and did not change TNF-α (Fig. 5C) and IL-10 (Fig. 5D).
Fig. 4.
Effect of trans-resveratrol on the mRNA expression levels in the liver homogenate. (A) Nrf2, (B) SOD1, and (C) SOD2. CONT, control group; RESV, trans-resveratrol group. Values are expressed as mean ± SEM (n = 7/group).
Fig. 5.
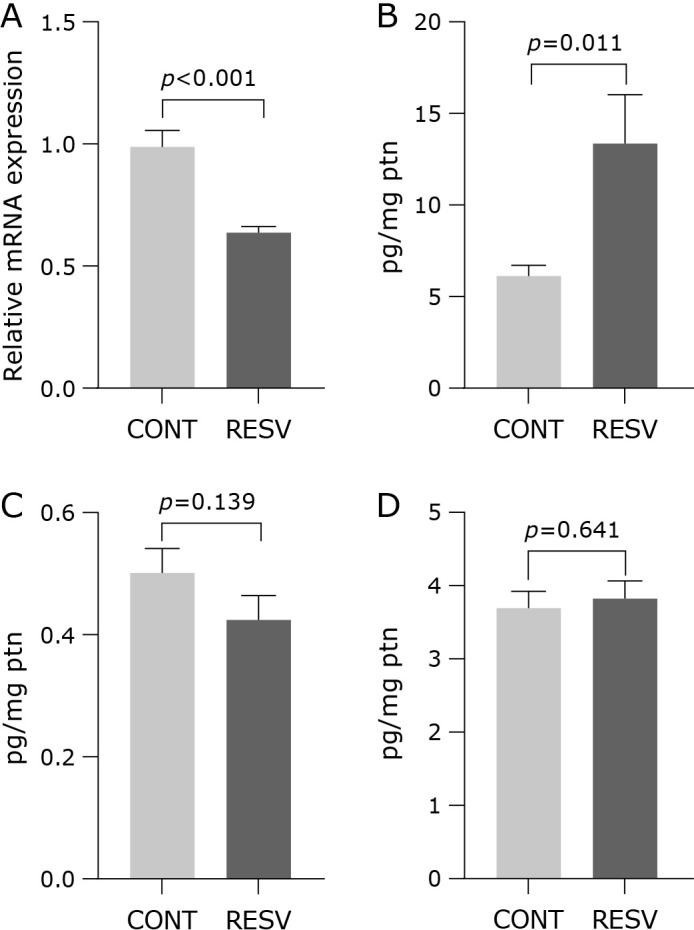
Effects of trans-resveratrol in relative mRNA expression of NF-κB and cytokines concentration measured in the liver homogenate. (A) NF-κB, (B) IL-6, (C) TNF-α, (D) IL-10. CONT, control group; RESV, trans-resveratrol group. Values are expressed as mean ± SEM (n = 7/group).
Discussion
Resveratrol can play a pivotal role in prevention and treatment of liver disorders.(25) Thus, our hypothesis was that trans-resveratrol supplementation could reduce the inflammatory and oxidative damage caused by the Western diet, and that this effect would be promoted by the activation of the transcription factor nuclear factor (erythroid-2)–related factor 2 (Nrf2). Our study showed that a supplementation with trans-resveratrol reduced the oxidative stress in the liver, as measured by GSH/GSSG ratio and MDA concentration, but this effect was not associated to the transcription factors and biomarkers of the inflammatory cycle.
Another mechanism by which trans-resveratrol could reduce oxidative stress could be the decrease of food intake and, consequently, the weight gain. Adiposity leads to oxidative stress via several multiple biochemical processes such as superoxide generation through the action of NADPH oxidase, glyceraldehyde auto-oxidation, oxidative phosphorylation, protein kinase C (PKC) activation, and polyol and hexosamine pathways.(26) A high intake of macronutrients increases oxidative stress through the activation of NADPH oxidase.(27) A meta-analysis, including 36 clinical trials, demonstrated that resveratrol intake significantly reduced weight, body mass index, waist circumference and fat mass, and significantly increased lean mass.(28) However, in our model, trans-resveratrol supplementation did not reduce diet intake and animal weight gain (Fig. 2).
Actually, in our study, instead of increasing Nrf2, RESV group showed a lower expression of this transcription factor. Nfr2 was originally identified as a critical regulator of intracellular anti-oxidants and of phase II detoxification enzymes, through its transcriptional up-regulation of many anti-oxidant response element (ARE)-containing genes.(29) It has been reported that the main mechanism by which trans-resveratrol enhances the Nrf2 expression is the disruption of Nrf2-Keap1 binding and increases the translocation of Nrf2 into the nucleus.(11,30) Therefore, a reduction of Nrf2 expression does not imply a lower Nrf2/ARE activation promoted by trans-resveratrol. This effect could be observed if Nrf2 had been determined in the cytoplasm and nucleus, as shown for example by Kim et al.,(9) that found that male 18-month-old C57BL/6 mice supplemented with trans-resveratrol for 6 months ameliorated oxidative stress and mitochondrial dysfunction due to the activation of the Nrf2 and SIRT1 signaling pathways, measured in the kidney tissues.
On the other side, in our study no difference was observed in the expression or activity of the main antioxidant enzymes, corroborating the absence of trans-resveratrol influence on enzymatic defense. For this reason, it can be supposed that the antioxidant effect found in our model could be promoted by the direct reaction between trans-resveratrol and reactive species. Resveratrol has effective in vitro and in vivo radical scavenging activities, reducing power, and Fe2+ chelating activities.(12,17) In fact, the radical scavenger capacity of the trans-resveratrol is widely known, and has been associated to the antioxidant effect of red wines.(2,31) Rats fed a high-fat diet to induce inflammation and oxidative stress, received supplementation (1.0 ml/day) containing water or three red wine samples characterized by high, medium and low in vitro antioxidant activity. The authors observed that in liver, only the wine containing the highest in vitro antioxidant activity was able to reduce MDA concentration. This wine presented 6.04 mg/L of trans-resveratrol, while the other two wines, that did not change MDA levels, had 1.94 and 1.46 mg/L, respectively.(32)
The supplementation did not contribute to reduce the inflammation promoted by the Western diet, as measured by the concentration of cytokines in the liver. It has been reported that a high fat diet promotes the increase of pro-inflammatory condition in several tissues. In a recent study in which mice were fed a semi-purified high fat diet (HFD) with 39.2 kcal% fat for 24 weeks, the most significant pathways strongly detectable from the start of HFD were TNF-α signaling via NF-κB, interferon-gamma (IFN-γ) and interferon-alpha (IFN-α) responses, IL-6, and Janus kinase/signal transducer and activator of transcription 3 (JAK-STAT3) signaling in liver.(33) In another previous study carried out in our group, mice fed a Western diet for 8 weeks showed an increase of TNF-α in the liver, while the other cytokines did not change.(6) In this study, it was found a reduction of NF-κB (Fig. 5A), but an increase of IL-6 (Fig. 5B), that does not allow to achieve a conclusion about the trans-resveratrol effect in the inflammatory condition, since both NF-κB and IL-6 were expected to conjointly reduce after supplementation. Tian et al.(34) observed that hepatic NF-κB inflammatory pathway was over-induced in high-fat (60% fat) diet mice. Interestingly, resveratrol (30 mg/kg body weight/day) treatment significantly inhibited over activation of NF-κB pathway and improved hepatic steatosis. Moreover, TNFα and IL-6 determined in the primary hepatocytes also were reduced after supplementation. On the other side, rats exposed to 2 Gy dose of gamma radiation and supplemented with resveratrol 100 mg/kg of resveratrol (RSV) intraperitoneally for 30 days showed an upregulation of IL-6 produced mainly by Th2 cells in rat liver.(35) In this study, the authors suggested that this increase in IL-6 may be involved in tissue repair and regeneration post-irradiation. In our study, using a lower dose (0.60 mg/day), it was found a strong enhance of IL-6 concentration: 13.50 ± 2.52 pg/ml in the supplemented group vs 6.28 ± 0.43 pg/ml in the control, that must be further investigated.
No effects of the supplementation were observed in the lipoproteins measured in plasma. This result can be due to the high concentration of LDL-C that characterizes the LDLr(−/−) mice undergoing a Western diet used in our study (690.50 ± 61.04 mg/dl). For example, wild type and LDLr(−/−) C57BL/6J mice receiving a standard cholesterol-free diet for 12 weeks presented a concentration of 74 ± 5 and 251 ± 27 mg/dl, respectively.(36) Thus, the 0.6 mg/day (≈17 mg/kg body weight/day) dose applied in our protocol was not enough to improve the lipoprotein profile, as observed in a previous study using a similar model, but a higher dose (25 mg/kg body weight/day).(37)
Conclusions
Our study showed that the oxidative damage caused by a Western diet was reduced by the supplementation of trans-resveratrol, but this effect was not associated to changes in the inflammatory biomarkers, lipoproteins or Nrf2 expression.
Author Contributions
TMS, SJC, and GCGC carried out the analysis and contributed to discuss the results and edit the manuscript. MMR contributed to discuss the results and edit the manuscript. JDJr and MRT were responsible for the RT-PCR determination. IAC led the funding acquisition, methodology supervision, project administration, and contributed to the conceptualization, statistical analysis and writing of the manuscript.
Acknowledgments
This work was financially supported by the National Council for Scientific and Technological Development (CNPq) (grant 134102/2019-3) and São Paulo Research Foundation – FAPESP (Process: 2013/07914-8, 2019/24023-6, 2021/08196-8).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Pannu N, Bhatnagar A. Resveratrol: from enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed Pharmacother 2019; 109: 2237–2251. [DOI] [PubMed] [Google Scholar]
- 2.Salehi B, Mishra AP, Nigam M, et al. Resveratrol: a double-edged sword in health benefits. Biomedicines 2018; 6: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zordoky BN, Robertson IM, Dyck JR. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta 2015; 1852: 1155–1177. [DOI] [PubMed] [Google Scholar]
- 4.Getz GS, Reardon CA. Diet and murine atherosclerosis. Arterioscler Thromb Vasc Biol 2006; 26: 242–249. [DOI] [PubMed] [Google Scholar]
- 5.Whitman SC. A practical approach to using mice in atherosclerosis research. Clin Biochem Rev 2004; 25: 81–93. [PMC free article] [PubMed] [Google Scholar]
- 6.de Castro Leão M, di Piazza I, Caria SJ, et al. Effect of nanocapsules containing docosahexaenoic acid in mice with chronic inflammation. Biomed Pharmacother 2023; 167: 115474. [DOI] [PubMed] [Google Scholar]
- 7.Aleksandrova K, Koelman L, Rodrigues CE. Dietary patterns and biomarkers of oxidative stress and inflammation: a systematic review of observational and intervention studies. Redox Biol 2021; 42: 101869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oppi S, Lüscher TF, Stein S. Mouse models for atherosclerosis research—Which is my line? Front Cardiovasc Med 2019; 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim EN, Lim JH, Kim MY, et al. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging (Albany NY) 2018; 10: 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izzo C, Annunziata M, Melara G, et al. The role of resveratrol in liver disease: a comprehensive review from in vitro to clinical trials. Nutrients 2021; 13: 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farkhondeh T, Folgado SL, Pourbagher-Shahri AM, Ashrafizadeh M, Samarghandian S. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed Pharmacother 2020; 127: 110234. [DOI] [PubMed] [Google Scholar]
- 12.Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021; 26: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramírez-Garza SL, Laveriano-Santos EP, Marhuenda-Muñoz M, et al. Health effects of resveratrol: results from human intervention trials. Nutrients 2018; 10: 1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu HC, Lei YH, Zhang WH, Luo XQ. Antioxidant and anti-inflammatory properties of resveratrol in diabetic nephropathy: a systematic review and meta-analysis of animal studies. Front Pharmacol 2022; 13: 841818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santana TM, Ogawa LY, Rogero MM, Barroso LP, Alves de Castro I. Effect of resveratrol supplementation on biomarkers associated with atherosclerosis in humans. Complement Ther Clin Pract 2022; 46: 101491. [DOI] [PubMed] [Google Scholar]
- 16.de Mello Barros Pimentel MV, Bertolami A, Fernandes LP, Barroso LP, Castro IA. Could a lipid oxidative biomarker be applied to improve risk stratification in the prevention of cardiovascular disease? Biomed Pharmacother 2023; 160: 114345. [DOI] [PubMed] [Google Scholar]
- 17.Gülçin İ. Antioxidant properties of resveratrol: a structure–activity insight. Innov Food Sci Emerg 2010; 11: 210–218. [Google Scholar]
- 18.Khattar S, Khan SA, Zaidi SAA, et al. Resveratrol from dietary supplement to a drug candidate: an assessment of potential. Pharmaceuticals (Basel) 2022; 15: 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993; 123: 1939–1951. [DOI] [PubMed] [Google Scholar]
- 20.Ewing JF, Janero DR. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem 1995; 232: 243–248. [DOI] [PubMed] [Google Scholar]
- 21.Bonaventura J, Schroeder WA, Fang S. Human erythrocyte catalase: an improved method of isolation and a reevaluation of reported properties. Arch Biochem Biophys 1972; 150: 606–617. [DOI] [PubMed] [Google Scholar]
- 22.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol 1984; 105: 114–120. [DOI] [PubMed] [Google Scholar]
- 23.Hong YL, Yeh SL, Chang CY, Hu ML. Total plasma malondialdehyde levels in 16 Taiwanese college students determined by various thiobarbituric acid tests and an improved high-performance liquid chromatography-based method. Clin Biochem 2000; 33: 619–625. [DOI] [PubMed] [Google Scholar]
- 24.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 2011; 15: 1583–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faghihzadeh F, Hekmatdoost A, Adibi P. Resveratrol and liver: a systematic review. J Res Med Sci 2015; 20: 797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Čolak E, Pap D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J Med Biochem 2021; 40: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ávila-Escalante ML, Coop-Gamas F, Cervantes-Rodríguez M, Méndez-Iturbide D, Aranda-González II. The effect of diet on oxidative stress and metabolic diseases-Clinically controlled trials. J Food Biochem 2020; 44: e13191. [DOI] [PubMed] [Google Scholar]
- 28.Tabrizi R, Tamtaji OR, Lankarani KB, et al. The effects of resveratrol intake on weight loss: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 2020; 60: 375–390. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki Y, Ishigami S, Arigami T, et al. Clinicopathological significance of nuclear factor (erythroid-2)-related factor 2 (Nrf2) expression in gastric cancer. BMC Cancer 2015; 15: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 2009; 284: 13291–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catalgol B, Batirel S, Taga Y, Ozer NK. Resveratrol: French paradox revisited. Front Pharmacol 2012; 3: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macedo LF, Rogero MM, Guimarães JP, Granato D, Lobato LP, Castro IA. Effect of red wines with different in vitro antioxidant activity on oxidative stress of high-fat diet rats. Food Chem 2013; 137: 122–129. [DOI] [PubMed] [Google Scholar]
- 33.Bae HR, Shin SK, Yoo JH, Kim S, Young HA, Kwon EY. Chronic inflammation in high-fat diet-fed mice: unveiling the early pathogenic connection between liver and adipose tissue. J Autoimmun 2023; 139: 103091. [DOI] [PubMed] [Google Scholar]
- 34.Tian Y, Ma J, Wang W, et al. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol Cell Biochem 2016; 422: 75–84. [DOI] [PubMed] [Google Scholar]
- 35.Khalil A, Al-Massarani G, Aljapawe A, Ekhtiar A, Bakir MA. Resveratrol modulates the inflammatory profile of immune responses and circulating endothelial cells’ (CECs’) population during acute whole body gamma irradiation. Front Pharmacol 2020; 11: 528400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunes VS, Cazita PM, Catanozi S, Nakandakare ER, Quintão ECR. Cholesterol metabolism in mice models of genetic hypercholesterolemia. J Physiol Biochem 2020; 76: 437–443. [DOI] [PubMed] [Google Scholar]
- 37.Chassot LN, Scolaro B, Roschel GG, et al. Comparison between red wine and isolated trans-resveratrol on the prevention and regression of atherosclerosis in LDLr(−/−) mice. J Nutr Biochem 2018; 61: 48–55. [DOI] [PubMed] [Google Scholar]
- 38.Piñeiro Z, Palma M, Barroso CG. Determination of trans-resveratrol in grapes by pressurised liquid extraction and fast high-performance liquid chromatography. J Chromatogr A 2006; 1110: 61–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.