Abstract
Diabetes mellitus (DM) is a prevalent metabolic disorder often accompanied by oxidative stress, which contributes to various diabetic complications. Investigating the antioxidant activity of linalool (LIN) is crucial as it may offer a natural therapeutic approach to mitigate oxidative damage in DM. The aim of the present study was to investigate the antioxidant activity of LIN in a DM rat model. A total of 40 male Wistar albino rats (age, 8 weeks; weight, 250-300 g) were used. CONTROL and DM groups were administered physiological saline solution by oral gavage for 21 days. In rats in the DM + LIN and LIN groups, 100 mg/kg LIN was administered intragastrically after streptozotocin injection (n=10 per group). In the first (48 h after STZ injection), second (1 week later), third (2 weeks later), and fourth (3 weeks later) blood glucose measurements, a statistically significant increase was found in the blood glucose values of the DM and DM + LIN groups compared with those of the CONTROL group. During the 21-day experimental period, there was no reduction in blood glucose levels of the DM + LIN group. Consequently, no discernible anti-hyperglycemic effect of LIN was observed. Catalase enzyme activity, superoxide dismutase (SOD) enzyme activity, malondialdehyde (MDA) levels and glutathione (GSH) levels were measured spectrophotometrically. All assays were conducted according to the protocols provided in the respective kits. The results were analyzed to assess the oxidative status and antioxidant capacity in the experimental groups. Catalase (CAT) activity was decreased in the DM group compared with that in the CONTROL group in both the serum and liver. However, LIN administration restored CAT activity in the DM + LIN group to the level of the CONTROL group. In the liver, the DM + LIN-treated group showed a notable reduction in malondialdehyde (MDA) levels compared with those in the DM group. In conclusion, the present results suggest that the antioxidant properties of LIN may have a regulatory effect on the oxidative status in diabetes-affected systems, potentially offering therapeutic benefits in managing oxidative stress associated with diabetes.
Keywords: diabetes mellitus, linalool, catalase, antioxidants
Introduction
Diabetes is a metabolic disease characterized by hyperglycemia due to defects in insulin production/function of insulin. Chronic hyperglycemia can cause damage to the organs, including the kidneys, eyes, nerves and heart. Multiple pathogenic processes contribute to the onset of diabetes, including the autoimmune destruction of pancreatic β-cells leading to insulin deficiency, as well as various abnormalities causing insulin resistance, while deficient insulin function can also lead to abnormalities in metabolism. Insufficient secretion of insulin and tissue responses to insulin often coexist, making the primary cause of hyperglycemia unclear (1).
Reactive oxygen species (ROS) are molecules containing chemically active oxygen that are produced within living systems. They are natural by-products of oxygen metabolism in all aerobic organisms (2,3). Elevated levels of ROS result in oxidative stress, which serves an important role in damaging cellular components, such as lipids, proteins and DNA (4). The antioxidant defense system plays a vital role in protecting biological systems by mitigating the detrimental effects of ROS. Numerous antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase, catalase (CAT) and paraoxonase, actively contribute to this protective mechanism (5). Apart from the enzymatic antioxidants, the non-enzymatic antioxidant defense system [including ascorbate, tocopherols, retinol, carotenoids, reduced glutathione (GSH), melatonin, polyphenols, ceruloplasmin and carnosine, among others] is equally important in the regulation of ROS levels and preservation of normal cellular function (6). Elevated levels of oxygen free radicals are associated with lipid peroxidation, non-enzymatic protein glycation and glucose oxidation, all of which contribute to the development of diabetes mellitus (DM) and its complications (2).
Cellular and animal model studies have revealed that essential oils possess notable anti-inflammatory, antioxidant and anticancer properties (7,8). Researchers are increasingly interested in essential oils due to their natural phenolic content and potential antioxidant and free radical scavenging activities. Essential oils from basil, cinnamon, clove, nutmeg, oregano and thyme have demonstrated significant radical-scavenging and antioxidant effects in the DPPH radical assay at room temperature (9). However, it is important to note that the free radical scavenging ability of these essential oils is not solely attributed to their phenolic components, as monoterpene alcohols, ketones, aldehydes, hydrocarbons and ethers also contribute to their antioxidant activity (10).
Linalool (LIN), an acyclic monoterpene alcohol, is a naturally occurring compound found in aromatic plants and is widely used as a fragrance ingredient in various products. Its versatile application extends from decorative cosmetics, fine fragrances and toiletries to non-cosmetic items, such as household cleaners and detergents (11,12). LIN has been reported to possess pharmacological properties, including sedative, analgesic, anti-inflammatory, antioxidant, antimicrobial and antitumor effects (13,14).
The interest in the antioxidant properties of essential oils has increased recently (15,16). There is a growing trend to substitute synthetic antioxidants with natural compounds, leading to the emergence of their potential use as natural additives (17). Cinnamomum osmophloeum (Lauraceae) oil exhibited 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (IC50, 29.7 µg/ml), which was associated with its major component, LIN (73%) (18). Despite the association between the antioxidant activity of essential oils and the presence of major compounds, such as LIN, in certain plants, van Zyl et al (19) showed that LIN alone has minimal antioxidant activity (IC50 >648 µM) against the DPPH radical.
LIN has been reported to reduce oxidative stress-induced damage by inhibiting superoxide anion and hydroxyl radical formation under in vitro conditions (20). In particular, studies have indicated that its antioxidant effect is mediated by the prevention of H2O2-induced decreases in cell viability, the reduction of LDH release, excessive ROS production, apoptosis and G2/M phase cell cycle arrest (21), as well as the inhibition of NF-κB activation (20).
Streptozotocin (STZ), also known as streptozocin, was identified in 1959 as a natural antibiotic originating from Streptomyces achromogenes (22). The toxic effects of STZ on pancreatic β-cells, known as its diabetogenic action, were first reported in 1963 (23,24). The STZ molecule, with a molecular formula of C8H15N3O7 and a molecular weight of ~265 g/mol, comprises two components: i) A glucopyranosyl group, which aids its absorption by pancreatic β-cells through glucose transporter 2; and ii) a nitrosourea group, which is responsible for the destruction of pancreatic β-cells (25). STZ has been observed to induce diabetic conditions in animal studies due to its selective destruction of insulin-producing β-cells within the pancreatic islets (23). The detailed description of this effect in preclinical rat laboratory models has encouraged the use of STZ to induce diabetes in laboratory animals for research purposes (26).
Numerous studies have reported the diverse biological activities of LIN (27-31). While most of these studies were conducted in vitro or in animal models using different administration methods (i.e. intraperitoneally and inhalation) there is a lack of consistent clinical investigations specifically focused on LIN. Considering that processes such as oxidative stress and inflammation are involved in the Considering that processes such as oxidative stress and inflammation are involved in the pathogenesis of diabetes (32), the antioxidant properties of LIN may support a potential activity of this phytochemical against oxidative stress in diabetes. Therefore, the present study aimed to investigate the antioxidant activity of LIN in an STZ-induced diabetic rat model.
Materials and methods
Chemicals and reagents
LIN and STZ were supplied by Glentham Life Sciences Ltd. The kits for the SOD (cat. no. 706002), CAT (cat. no. 707002), GSH (cat. no. 703002) and malondialdehyde (MDA) (cat. no. 10009055) assays were purchased from Cayman Chemical Company.
Animals
In the present study, 40 male Wistar albino rats (age, 8 weeks; weight, 250-300 g) were used. Care of the rats and experimental procedures were performed at the Experimental Medicine Application and Research Center of Karabük University. Rats were housed in transparent plastic conventional cages (5 rats in each cage) according to the experimental animal care conditions of the research center (21±2˚C, 50±5% humidity), with a 12/12 h light/dark cycle, with standard pellet rat chow and tap water given ad libitum. Ethics approval for the study was obtained from the Karabük University Animal Experiments Local Ethics Committee (approval no. 2022/2/3).
Induction of diabetes
In the present study, rats were intraperitoneally injected with a single dose of STZ (65 mg/kg) to induce experimental diabetes (33). On experimental day 1, all rats were fasted for 6-8 h before STZ administration. Water was given normally. Immediately before injection, STZ was dissolved in 50 mM cold (4˚C) sodium citrate buffer (pH 4.5). Animals in the CONTROL and LIN groups were injected with an equal volume of citrate buffer (pH 4.5). Rats were returned to their cages and were provided with normal food and water containing 5% glucose. On day 2 of the experiment, 5% glucose in the water was replaced with normal water (33,34).
Fasting blood glucose levels were measured with a glucometer device (Accu-Chek Active; Roche Diagnostics) in ~1-2 µl of blood taken from the tail vein by pricking and bleeding the tail 48 h after STZ administration, and rats with glucose levels exceeding 200 mg/dl were considered diabetic (35).
Administration of the experimental drug
Animals in the CONTROL were included in the experiment as healthy controls (n=10). The rats in the DM group (untreated diabetic group) received no drugs (n=10). The CONTROL and DM groups were given physiological saline solution by oral gavage for 21 days. In the DM + LIN group, 48 h after STZ injection, 100 mg/kg LIN (in physiological serum) (36) was administered via oral gavage once a day, every day for 21 days (n=10). In the LIN group, 100 mg/kg LIN was administered via oral gavage. Throughout the 21-day experiment (37), the animals were provided with ad libitum access to food and water. Body weight and fasting blood glucose levels (on the 7th, 14th and 21st days) were measured once a week before and after LIN treatment initiation.
Collection of blood and tissue samples
At the end of the 21-day experimental period, anesthesia was induced in rats using a combination of ketamine (80 mg/kg) and xylazine (10 mg/kg) (38), and euthanasia was performed by cardiac puncture and exsanguination. Blood samples from the heart of the rats were placed in tubes without anticoagulant and were left to coagulate at room temperature for 30 min, followed by centrifugation at 2,000 x g for 15 min at 4˚C.
Following euthanasia, the liver tissues were removed, washed with saline and stored at -80˚C until further analysis.
Analysis of oxidant and antioxidant parameters in blood and tissue
CAT and SOD activities and GSH and MDA levels in serum and liver tissue samples were determined using the aforementioned commercial kits in accordance with the protocols specified by the manufacturer. All measurements were performed using a microplate reader (Multiskan™ GO Microplate Spectrophotometer; Thermo Fisher Scientific Inc.).
Statistical analysis
Data are presented as mean ± SD of multiple animals used in one assay, and were analyzed using SPSS Statistics, version 17.0 (SPSS, Inc.). Normality was checked using the Shapiro-Wilk test. One-way ANOVA followed by Tukey's post hoc test was used for determining significant differences among the studied groups. P<0.05 considered to indicate a statistically significant difference.
Results
Blood glucose measurement
In all groups, blood glucose was measured in the blood collected from the tail veins once a week before and after LIN treatment initiation, four times in total during the experiment. The blood glucose values of the rats in the LIN-treated and untreated groups were assessed (Table I). There was no statistically significant difference in blood glucose levels between the CONTROL and LIN groups and between the DM + LIN and DM groups at all measurement times. This indicated that LIN alone did not have an impact on blood glucose levels in non-diabetic rats, nor did it significantly alter glucose levels in diabetic rats compared with untreated diabetic rats.
Table I.
Blood glucose levels of rats (mg/dl).
| Time point | CONTROL (n=10) | DM (n=10) | DM + LIN (n=10) | LIN (n=10) |
|---|---|---|---|---|
| Before model establishment | 113.40±15.67 | 413.20±46.94a | 465.20±95.20a,b | 98.60±7.71c |
| 1 week after model establishment | 97.56±9.96 | 528.50±81.37a | 547.50±76.77a,b | 102.80±15.75c |
| 2 weeks after model establishment | 104.89±5.88 | 564.70±40.72a | 572.10±56.26a,b | 106.10±11.50c |
| 3 weeks after model establishment | 103.44±6.52 | 566.20±39.47a | 579.90±39.64a,b | 98.70±9.73c |
The data are presented as the mean ± SD.
aP<0.001 vs. CONTROL,
bP<0.001 vs. LIN and
cP<0.001 vs. DM at the same time point. DM, diabetes mellitus; LIN, linalool.
A statistically significant difference was found between the blood glucose values of the CONTROL vs. DM, CONTROL vs. DM + LIN, DM vs. LIN and DM + LIN vs. LIN groups at all time points (P<0.001). This demonstrates that the blood glucose levels of the diabetic groups (DM and DM + LIN) were consistently elevated compared with the non-diabetic groups (CONTROL and LIN), confirming the successful induction of diabetes in these groups.
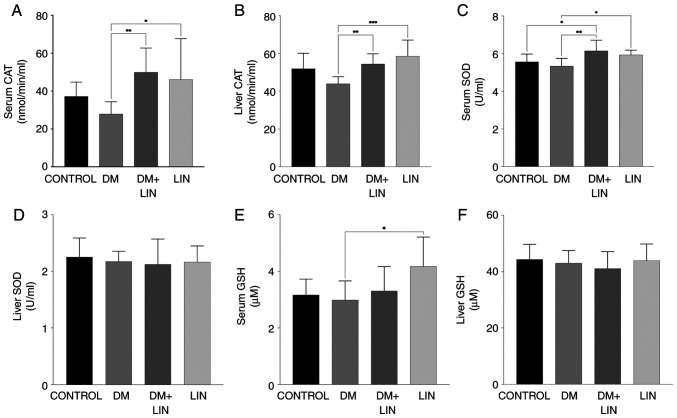
Results of oxidative stress markers. Serum and liver CAT activity
Serum and liver tissue CAT activity levels were evaluated as an indicator of antioxidant status between the groups (Fig. 1A and B).
Figure 1.
Serum and liver antioxidant levels. Levels of catalase enzymatic activity in (A) serum and (B) liver tissue samples. Enzymatic activity of SOD in (C) serum and (D) liver tissue samples. Levels of GSH in (E) serum and (F) liver tissue samples. *P<0.05; **P<0.01 and ***P<0.001. SOD, superoxide dismutase; GSH, reduced glutathione; DM, diabetes mellitus; LIN, linalool.
Serum and tissue CAT activity were significantly higher (P<0.01) in LIN-treated diabetic rats (DM+LIN) compared with untreated diabetic rats (DM). This indicated that LIN treatment enhanced antioxidant enzyme activity in diabetic rats, which suggests a potential protective effect against oxidative stress induced by diabetes. This increase in CAT activity was also observed in the LIN-treated group compared with the DM group, further supporting the antioxidant role of LIN.
However, no significant difference was found in CAT activity levels when DM, DM + LIN and LIN groups were compared with the CONTROL group (P>0.05). This suggests that while LIN treatment significantly improved CAT activity in diabetic rats, it did not lead to a statistically significant increase in non-diabetic rats compared with their respective controls.
Serum and liver tissue CAT activity values indicate the potential of LIN in modulating antioxidant CAT activity in diabetic conditions, although its effects were less pronounced in non-diabetic conditions.
Serum and liver SOD activity. Serum SOD values were assessed at the end of the study (Fig. 1C). There were no statistically significant differences in serum SOD levels between the CONTROL vs. DM, CONTROL vs. LIN or DM + LIN vs. LIN groups (P>0.05). This indicates that LIN treatment alone did not significantly alter serum SOD levels in non-diabetic rats, nor did it result in significant differences when comparing LIN-treated diabetic rats with LIN-treated non-diabetic rats.
However, there were statistically significant differences between the CONTROL vs. DM + LIN (P<0.05), DM vs. LIN (P<0.05) and DM vs. DM + LIN groups (P<0.01). These results suggest that LIN treatment in diabetic rats (DM + LIN) led to significant changes in serum SOD levels compared with untreated diabetic rats (DM), indicating a potential beneficial effect of LIN on antioxidant enzyme activity in diabetic conditions.
No statistically significant difference was found in liver tissue SOD values among the groups (P>0.05; Fig. 1D). This suggests that while LIN may affect serum SOD levels in diabetic rats, its impact on liver tissue SOD activity was not significant under the conditions of the present study. The serum and liver tissue SOD values were presented These data provide a detailed overview of the antioxidant SOD enzyme activity, indicating that LIN specifically enhances serum SOD levels in diabetic conditions while having no significant effect on liver tissue SOD activity.
Serum and liver GSH levels. No statistically significant difference was found in serum GSH levels between the CONTROL vs. DM, CONTROL vs. DM + LIN, CONTROL vs. LIN, DM vs. DM + LIN and DM + LIN vs. LIN groups (P>0.05). A statistically significant difference was found only between the DM and LIN groups (P=0.013; Fig. 1E). This indicates that LIN treatment did not significantly alter serum GSH levels in most group comparisons, with the exception of a notable increase in the LIN group compared with the DM group.
The liver tissue GSH values obtained in the present study are shown in Fig. 1F. No statistically significant difference was found in liver tissue GSH levels between any of the groups (P>0.05). This suggests that LIN treatment did not have a significant impact on liver GSH levels under the conditions of the present study.
Serum and liver tissue GSH values provide a comprehensive overview of GSH levels, highlighting that while LIN increased serum GSH compared with the DM group, its overall impact on liver tissue GSH levels was not significant. These findings contribute to the better understanding of the diabetic conditions under which LIN affects antioxidant defense.
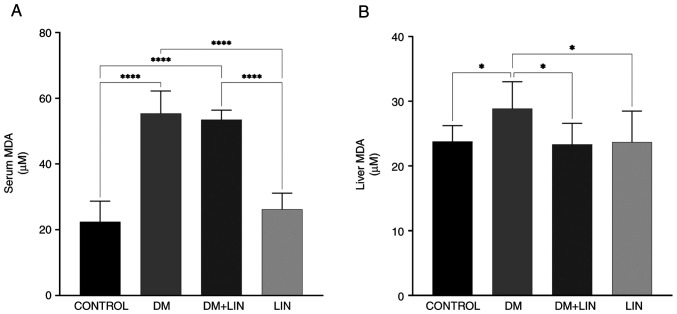
Serum and liver tissue MDA levels. A statistically significant difference in serum MDA levels was found between the CONTROL vs. DM, CONTROL vs. DM + LIN, DM vs. LIN and DM + LIN vs. LIN groups (P<0.0001). However, there was no statistically significant difference between the CONTROL vs. LIN and DM vs. DM + LIN groups (P>0.05) (Fig. 2). This suggests that LIN did not significantly alter serum MDA levels in non-diabetic rats, nor did it result in a significant difference in serum MDA levels between treated and untreated diabetic groups.
Figure 2.
Serum and liver MDA levels. Effects of LIN treatment on MDA levels in (A) serum and (B) liver tissues. *P<0.05 and ****P<0.0001. MDA, malondialdehyde; DM, diabetes mellitus; LIN, linalool.
Liver tissue MDA values were also assessed (Fig. 2). A statistically significant difference was found between the CONTROL vs. DM (P=0.0328), DM vs. DM + LIN (P=0.0174) and DM vs. LIN (P=0.0228) groups. There were no statistically significant differences between the CONTROL vs. DM + LIN, CONTROL vs. LIN and DM + LIN vs. LIN groups (P>0.05). LIN significantly reduced liver MDA levels, specifically in diabetic rats compared with untreated diabetic rats, but this effect was not observed between non-diabetic groups or between certain non-diabetic and treated diabetic groups.
Serum and liver tissue MDA values were assessed and provide a detailed overview of the MDA oxidative stress marker, highlighting the specific conditions under which LIN exerts its antioxidant effects, particularly in reducing MDA levels in diabetic rats.
Discussion
Diabetes is a chronic metabolic disorder characterized by elevated blood sugar levels, resulting from either insufficient insulin production or ineffective insulin utilization by the body. It is a complex disease with various complications (such as retinopathy, nephropathy, hypertension, coronary heart disease and neuropathy) that affect multiple organ systems, in particular the eyes, kidneys, heart and nerves (39). Oxidative stress is a condition that occurs when there is an imbalance between the production of ROS and the body's ability to neutralize them with antioxidants. ROS are highly reactive molecules that contain oxygen and can cause damage to cells and tissues if their levels become too high (40). Persistently high blood glucose levels, known as hyperglycemia, are a hallmark of diabetes. Elevated glucose levels can lead to increased production of ROS through processes such as glucose autoxidation and the formation of advanced glycation end products. These reactions generate ROS as byproducts, which can promote oxidative stress (41,42). To counteract the effects of oxidative stress in diabetes, herbal-based therapeutic strategies to enhance the antioxidant defense and reduce ROS production are being investigated (43). Numerous reports have provided various results on the antidiabetic effects of plant extracts containing LIN (44-47). However, studies on the antioxidant effects of LIN in diabetic rats are scarce.
In the present study, a significant increase in the blood glucose concentration of STZ-induced diabetic rats compared with the CONTROL group was observed. In STZ-treated groups, blood glucose concentrations should typically exceed 200 mg/dl in non-fasting animals, while blood glucose levels should be >150 mg/dl in fasting diabetic animals (33). The statistically significant difference between the STZ-treated and CONTROL groups was one of the most important points in this study, as it confirmed the successful establishment of the diabetic model. Typically, it has been reported that within 3 weeks following STZ injection, >50% of animals develop severe hyperglycemia, with blood glucose concentrations ranging from 300-600 mg/dl. Therefore, a 21-day period was considered for the LIN treatment (48).
However, during the 21-day study period, no significant decrease in the blood glucose levels was observed in the DM + LIN group. Despite LIN administration, the blood glucose concentrations remained elevated and were not statistically different from those in the DM group. This lack of a significant difference suggests that LIN did not exert a notable hypoglycemic effect under the conditions of the present study, indicating the necessity of further investigation into its potential therapeutic benefits for diabetes management. It was concluded that LIN alone, at a dose of 100 mg/kg, did not play an effective role in the control of diabetic hyperglycemia. Lee et al (49) reported that indigenous cinnamon leaf essential oil containing ~40% LIN significantly decreased fasting blood glucose and fructosamine levels at all doses tested (12.5, 25 and 50 mg/kg) and increased plasma and pancreatic insulin levels under fasting conditions in the STZ-induced diabetic rat model. The study attributed the effect of LIN in the leaf essential oil from indigenous cinnamon to its hypoglycemic and pancreas-protective effects. LIN, being the major component of cinnamon leaf essential oil, was suggested to contribute to the hypoglycemic effect observed in diabetic rats. Additionally, LIN was reported to reverse the increase in hepatic pro-inflammatory cytokine levels in diabetic rats (50). These findings suggest that LIN may exert its effects through anti-inflammatory and antioxidant mechanisms, contributing to its potential therapeutic benefits in diabetes management (49). Deepa and Venkatraman (50) reported an improvement in blood glucose levels through inhibition of gluconeogenesis in a study in which 25 mg/kg LIN was administered for 45 days in diabetic rats. Although the results of the aforementioned studies are different from those obtained in the present study, it is hypothesized that these differences may be attributed to the particular diabetes model used, the dose and duration of LIN administration, the enantiomer differences of LIN (51) or the type of experimental animal used.
In the liver tissue of DM group, a decrease in CAT activity was exhibited. Similarly, a notable reduction in serum CAT activity was observed. Both liver and serum CAT activities were significantly increased in DM + LIN and LIN groups compared with those in the DM group Altınok-Yipel et al (36) reported that LIN increased CAT activity in rats with liver damage induced by CCl4. Consistent with this study, the present results demonstrated that LIN can restore impaired CAT activity under stress. The restoration of serum CAT levels suggests that LIN exhibits a systemic effect and can ameliorate oxidative imbalances throughout the body. The results of the present study highlight the efficacy of LIN in combating systemic oxidative stress, as reflected in the serum and its potential regulatory effects on various systems in the organism. This may indicate that the antioxidant capacity of LIN is beneficial for the general oxidative status of the organism beyond the cellular level.
It was found that SOD levels in the liver tissue did not show statistically significant differences among the groups. The level of SOD activity in the LIN group was similar to that in the CONTROL group, suggesting that LIN had no effect on SOD activity under normal physiological conditions. Despite the increase in serum SOD levels in the DM + LIN group compared with those in the DM group, the effects of LIN on SOD activity in the liver were not as pronounced as those in the serum. This suggests that the antioxidant effects of LIN may differ between target tissues. The results of the current study are in accordance with those of previous studies (52-54), which indicated that there were no substantial fluctuations in the tissue activity of SOD in individuals with DM. However, other studies have reported both elevated (55) and decreased (56) levels of SOD in patients with DM.
Antioxidants help protect cells by neutralizing free radicals caused by oxidative stress (57). Reversely, oxidative stress, by increasing the formation of free radicals in cells, depletes antioxidant molecules, such as GSH (58). Both in the serum and liver, no significant effect of LIN treatment on GSH levels was observed in diabetic rats. LIN is a compound with antioxidant properties, and its antioxidant activity may protect GSH in cells from the harmful effects of oxidative stress. However, it has also been indicated that LIN improves GSH levels in brain tissue depending on the time of administration. For example, it was reported that LIN was more effective when it was administered to rats 3 days before or at the same time as the acrylamide-induced neurotoxicity model establishment, while no effect was observed when LIN was administered 3 days after the model was established (59). Even if this model and the diabetes model of the presented study are not the same, LIN was administered 48 h after the onset of diabetes in the present study. Therefore, its efficacy may have varied depending on the time of administration.
MDA is a by-product of lipid peroxidation, indicative of oxidative damage to cell membrane lipids (60). In the present study, a significant increase in serum MDA levels were observed in the diabetic group (DM) compared with the CONTROL group, as well as in the LIN-treated diabetic group (DM + LIN) compared with the CONTROL group. These findings confirm that diabetes induction substantially elevated serum MDA levels, resulting in increased oxidative stress. The results showed that the antioxidant effects of LIN were significantly effective in reducing MDA levels, especially in the liver of diabetic rats. The notable reduction in liver MDA levels in LIN-treated diabetic rats compared with untreated diabetic rats demonstrates the potential therapeutic benefits of LIN in mitigating oxidative stress associated with diabetes. However, the absence of significant differences in serum MDA levels between the treated and untreated diabetic groups, as well as between the non-diabetic groups, indicates that the effects of LIN may be more pronounced in the liver tissue than in the serum. This differential impact suggests that the antioxidant properties of LIN may be tissue specific. Further research is required to elucidate the precise mechanisms through which LIN modulates oxidative stress and to explore its potential applications in other tissues and diseases.
The present study assessed the antioxidant properties of LIN in DM model. In STZ-induced diabetic rats, LIN treatment alone failed to control hyperglycemia. However, a significant restoration of CAT activity was observed in both the liver and serum in diabetic rats treated with LIN. Nonetheless, there was no significant change in liver SOD activity. Additionally, LIN was observed to have a reducing effect on MDA levels, an important indicator of diabetes-related oxidative stress. It was also found that LIN did not affect GSH levels in either the serum or liver in diabetic rats. In summary, the present results suggest that LIN has the potential to reduce diabetes-associated oxidative stress. Overall, the current study may indicate that LIN has a regulatory effect on the overall oxidative status of the organism by reducing liver MDA levels, an important indicator of diabetes-related oxidative stress, while increasing the activity of antioxidant enzymes, such as liver and serum CAT activity, and serum SOD activity in diabetic rats. This suggests that LIN may help in mitigating oxidative damage associated with diabetes.
One of the limitations of the present study is the absence of histopathologic tissue evaluation. Although blood glucose levels and other biochemical parameters were thoroughly assessed, a detailed examination of pancreatic or other relevant tissue samples was not performed. Histopathologic analysis could have provided valuable insights into the cellular and structural changes associated with STZ-induced diabetes and the potential protective effects of LIN. This limitation restricts the understanding of the underlying mechanisms at the tissue level and the broader implications of the present findings. Future studies should incorporate comprehensive histopathologic evaluations to complement biochemical data and offer a more holistic understanding of the therapeutic potential of LIN in diabetic models.
These findings suggest that LIN has potential antioxidant effects in the context of diabetes. Further research is needed to understand the mechanisms underlying these effects and determine the optimal dosage of LIN for diabetes management.
Acknowledgements
Not applicable.
Funding Statement
Funding: This present study was funded by Karabük University Scientific Research Projects Unit (grant no. KBÜBAP-22-YL-076).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
SB and MK conducted the experiments and edited the manuscript. SB designed the study, and collected and processed the data. MK conducted the statistical analysis, and reviewed and revised the article. SB and MK confirm the authenticity of all the raw data. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by Karabük University Animal Experiments Local Ethics Committee (approval no. 2022/2/3).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 (Suppl 1):S81–S90. doi: 10.2337/dc14-S081. American Diabetes Association. [DOI] [PubMed] [Google Scholar]
- 2.Sies H (ed) Oxidative Stress: Eustress and Distress. Academic Press, Cambridge, MA, 2019. [Google Scholar]
- 3.Darenskaya MA, Kolesnikova LI, Kolesnikov SI. Oxidative stress: Pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med. 2021;171:179–189. doi: 10.1007/s10517-021-05191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sifuentes-Franco S, Pacheco-Moisés FP, Rodríguez-Carrizalez AD, Miranda-Díaz AG. The role of oxidative stress, mitochondrial function, and autophagy in diabetic polyneuropathy. J Diabetes Res. 2017;2017(1673081) doi: 10.1155/2017/1673081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozougwu JC. The role of reactive oxygen species and antioxidants in oxidative stress. Int J Res. 2016;3:1–8. [Google Scholar]
- 6.Mirończuk-Chodakowska I, Witkowska AM, Zujko ME. Endogenous non-enzymatic antioxidants in the human body. Adv Med Sci. 2018;63:68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules. 2010;15:9252–9287. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blowman K, Magalhães M, Lemos MFL, Cabral C, Pires IM. Anticancer properties of essential oils and other natural products. Evid Based Complement Alternat Med. 2018;2018(3149362) doi: 10.1155/2018/3149362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomaino A, Cimino F, Zimbalatti V, Venuti V, Sulfaro V, De Pasquale A, Saija A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005;89:549–554. [Google Scholar]
- 10.Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 11.Lapczynski A, Letizia C, Api A. Addendum to fragrance material review on linalool. Food Chem Toxicol. 2008;46 (Suppl 11):S190–S192. doi: 10.1016/j.fct.2008.06.087. [DOI] [PubMed] [Google Scholar]
- 12.Kamatou GPP, Viljoen AM. Linalool-A review of a biologically active compound of commercial importance. Nat Prod Commun. 2008;3(1187) [Google Scholar]
- 13.Aprotosoaie AC, Hăncianu M, Costache II, Miron A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr J. 2014;29:193–219. [Google Scholar]
- 14.de Cássia da Silveira e Sá R, Andrade LN, de Sousa DP. A review on anti-inflammatory activity of monoterpenes. Molecules. 2013;18:1227–1254. doi: 10.3390/molecules18011227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tit DM, Bungau SG. Antioxidant activity of essential oils. Antioxidants (Basel) 2023;12(383) doi: 10.3390/antiox12020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Shang S, Yan F, Jiang H, Zhao G, Tian S, Chen R, Chen D, Dang Y. Antioxidant activities of essential oils and their major components in scavenging free radicals, inhibiting lipid oxidation and reducing cellular oxidative stress. Molecules. 2023;28(4559) doi: 10.3390/molecules28114559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sökmen M, Serkedjieva J, Daferera D, Gulluce M, Polissiou M, Tepe B, Akpulat HA, Sahin F, Sokmen A. In vitro antioxidant, antimicrobial, and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. J Agric Food Chem. 2004;52:3309–3312. doi: 10.1021/jf049859g. [DOI] [PubMed] [Google Scholar]
- 18.Lin KH, Yeh SY, Lin MY, Shih MC, Yang KTU, Hwang SY. Major chemotypes and antioxidative activity of the leaf essential oils of Cinnamomum osmophloeum Kaneh. from a clonal orchard. Food Chem. 2007;105:133–139. [Google Scholar]
- 19.van Zyl RL, Seatlholo ST, van Vuuren SF, Viljoen AM. The Biological activities of 20 nature identical essential oil constituents. Journal of Essential Oil Research. 2006;18 (Suppl 1):S129–S133. [Google Scholar]
- 20.Gunaseelan S, Balupillai A, Govindasamy K, Ramasamy K, Muthusamy G, Shanmugam M, Thangaiyan R, Robert BM, Prasad Nagarajan R, Ponniresan VK, Rathinaraj P. Linalool prevents oxidative stress activated protein kinases in single UVB-exposed human skin cells. PLoS One. 2017;12(e0176699) doi: 10.1371/journal.pone.0176699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migheli R, Lostia G, Galleri G, Rocchitta G, Serra PA, Bassareo V, Acquas E, Peana AT. Neuroprotective effect of (R)-(-)-linalool on oxidative stress in PC12 cells. Phytomed Plus. 2021;1(100073) [Google Scholar]
- 22.Vavra JJ, Deboer C, Dietz A, Hanka LJ, Sokolski WT. Streptozotocin, a new antibacterial antibiotic. Antibiot Annu. 1959;7:230–235. [PubMed] [Google Scholar]
- 23.Rakieten N, Rakieten ML, Nadkarni MV. Studies on the diabetogenic action of streptozotocin (NSC-37917) Cancer Chemother Rep. 1963;29:91–98. [PubMed] [Google Scholar]
- 24.Ghasemi A, Jeddi S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. EXCLI J. 2023;22:274–294. doi: 10.17179/excli2022-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia. 2000;43:1528–1533. doi: 10.1007/s001250051564. [DOI] [PubMed] [Google Scholar]
- 26.Gunnarsson R, Berne C, Hellerström C. Cytotoxic effects of streptozotocin and N-nitrosomethylurea on the pancreatic B cells with special regard to the role of nicotinamide-adenine dinucleotide. Biochem J. 1974;140:487–494. doi: 10.1042/bj1400487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huo M, Cui X, Xue J, Chi G, Gao R, Deng X, Guan S, Wei J, Soromou LW, Feng H, Wang D. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J Surg Res. 2013;180:e47–e54. doi: 10.1016/j.jss.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 28.Sun XB, Wang SM, Li T, Yang Y. Anticancer activity of linalool terpenoid: apoptosis induction and cell cycle arrest in prostate cancer cells. Trop J Pharm Res. 2015;14:619–625. [Google Scholar]
- 29.Cho SY, Jun HJ, Lee JH, Jia Y, Kim KH, Lee SJ. Linalool reduces the expression of 3-hydroxy-3-methylglutaryl CoA reductase via sterol regulatory element binding protein-2- and ubiquitin-dependent mechanisms. FEBS Lett. 2011;585:3289–3296. doi: 10.1016/j.febslet.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Herman A, Tambor K, Herman A. Linalool affects the antimicrobial efficacy of essential oils. Curr Microbiol. 2016;72:165–172. doi: 10.1007/s00284-015-0933-4. [DOI] [PubMed] [Google Scholar]
- 31.Katsuyama S, Kuwahata H, Yagi T, Kishikawa Y, Komatsu T, Sakurada T, Nakamura H. Intraplantar injection of linalool reduces paclitaxel-induced acute pain in mice. Biomed Res. 2012;33:175–181. doi: 10.2220/biomedres.33.175. [DOI] [PubMed] [Google Scholar]
- 32.Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-a concise review. Saudi Pharm J. 2016;24:547–553. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc. 2021;1(e78) doi: 10.1002/cpz1.78. [DOI] [PubMed] [Google Scholar]
- 34.Fararh KM, Shimizu Y, Shiina T, Nikami H, Ghanem MM, Takewaki T. Thymoquinone reduces hepatic glucose production in diabetic hamsters. Res Vet Sci. 2005;79:219–223. doi: 10.1016/j.rvsc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Faisal Lutfi M, Abdel-Moneim AH, Alsharidah AS, Mobark MA, Abdellatif AAH, Saleem IY, Al Rugaie O, Mohany KM, Alsharidah M. Thymoquinone lowers blood glucose and reduces oxidative stress in a rat model of diabetes. Molecules. 2021;26(2348) doi: 10.3390/molecules26082348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altınok-Yipel F, Tekeli İO, Özsoy ŞY, Güvenç M, Kaya A, Yipel M. Hepatoprotective activity of linalool in rats against liver injury induced by carbon tetrachloride. Int J Vitam Nutr Res. 2020;90:302–308. doi: 10.1024/0300-9831/a000581. [DOI] [PubMed] [Google Scholar]
- 37.Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL, Kudva YC. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Lab Anim. 2011;45:131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatia A, Saikia PP, Dkhar B, Pyngrope H. Anesthesia protocol for ear surgery in Wistar rats (animal research) Animal Model Exp Med. 2022;5:183–188. doi: 10.1002/ame2.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banday MZ, Sameer AS, Nissar S. Pathophysiology of diabetes: An overview. Avicenna J Med. 2020;10:174–188. doi: 10.4103/ajm.ajm_53_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev. 2017;2017(8416763) doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caturano A, D'Angelo M, Mormone A, Russo V, Mollica MP, Salvatore T, Galiero R, Rinaldi L, Vetrano E, Marfella R, et al. Oxidative stress in type 2 diabetes: impacts from pathogenesis to lifestyle modifications. Curr Issues Mol Biol. 2023;45:6651–6666. doi: 10.3390/cimb45080420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.González P, Lozano P, Ros G, Solano F. Hyperglycemia and oxidative stress: An integral, updated and critical overview of their metabolic interconnections. Int J Mol Sci. 2023;24(9352) doi: 10.3390/ijms24119352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalid M, Petroianu G, Adem A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules. 2022;12(542) doi: 10.3390/biom12040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vats V, Yadav SP, Grover JK. Ethanolic extract of Ocimum sanctum leaves partially attenuates streptozotocin-induced alterations in glycogen content and carbohydrate metabolism in rats. J Ethnopharmacol. 2004;90:155–160. doi: 10.1016/j.jep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 45.More TA, Kulkarni BR, Nalawade ML, Arvindekar AU. antidiabetic activity of linalool and limonene in streptozotocin-induced diabetic rat: A combinatorial therapy approach. Int J Pharm Pharm Sci. 2014;6:159–163. [Google Scholar]
- 46.Garba HA, Mohammed A, Ibrahim MA, Shuaibu MN. Effect of lemongrass (Cymbopogon citratus Stapf) tea in a type 2 diabetes rat model. Clin Phytosci. 2020;6(19) [Google Scholar]
- 47.Tran N, Pham B, Le L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology (Basel) 2020;9(252) doi: 10.3390/biology9090252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camargo SB, Simões LO, Medeiros CFA, de Melo Jesus A, Fregoneze JB, Evangelista A, Villarreal CF, Araújo AAS, Quintans-Júnior LJ, Silva DF. Antihypertensive potential of linalool and linalool complexed with β-cyclodextrin: Effects of subchronic treatment on blood pressure and vascular reactivity. Biochem Pharmacol. 2018;151:38–46. doi: 10.1016/j.bcp.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Lee SC, Xu WX, Lin LY, Yang JJ, Liu CT. Chemical composition and hypoglycemic and pancreas-protective effect of leaf essential oil from indigenous cinnamon (Cinnamomum osmophloeum Kanehira) J Agric Food Chem. 2013;61:4905–4913. doi: 10.1021/jf401039z. [DOI] [PubMed] [Google Scholar]
- 50.Deepa B, Venkatraman Anuradha C. Effects of linalool on inflammation, matrix accumulation and podocyte loss in kidney of streptozotocin-induced diabetic rats. Toxicol Mech Methods. 2013;23:223–234. doi: 10.3109/15376516.2012.743638. [DOI] [PubMed] [Google Scholar]
- 51.Peana AT, D'Aquila PS, Panin F, Serra G, Pippia P, Moretti MD. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9:721–726. doi: 10.1078/094471102321621322. [DOI] [PubMed] [Google Scholar]
- 52.Peuchant E, Delmas-Beauvieux MC, Couchouron A, Dubourg L, Thomas MJ, Perromat A, Clerc M, Gin H. Short-term insulin therapy and normoglycemia. Effects on erythrocyte lipid peroxidation in NIDDM patients. Diabetes Care. 1997;20:202–207. doi: 10.2337/diacare.20.2.202. [DOI] [PubMed] [Google Scholar]
- 53.Kesavulu M, Rao BK, Giri R, Vijaya J, Subramanyam G, Apparao C. Lipid peroxidation and antioxidant enzyme status in type 2 diabetics with coronary heart disease. Diabetes Res Clin Pract. 2001;53:33–39. doi: 10.1016/s0168-8227(01)00238-8. [DOI] [PubMed] [Google Scholar]
- 54.Sözmen EY, Sözmen B, Delen Y, Onat T. Catalase/superoxide dismutase (SOD) and catalase/paraoxonase (PON) ratios may implicate poor glycemic control. Arch Med Res. 2001;32:283–287. doi: 10.1016/s0188-4409(01)00285-5. [DOI] [PubMed] [Google Scholar]
- 55.Kimura F, Hasegawa G, Obayashi H, Adachi T, Hara H, Ohta M, Fukui M, Kitagawa Y, Park H, Nakamura N, et al. Serum extracellular superoxide dismutase in patients with type 2 diabetes: Relationship to the development of micro- and macrovascular complications. Diabetes Care. 2003;26:1246–1250. doi: 10.2337/diacare.26.4.1246. [DOI] [PubMed] [Google Scholar]
- 56.Ciechanowski K, Kedzierska K, Gołembiewska E, Safranow K, Bober J, Domański L, Rózański J, Myślak M. Impaired synthesis is not the reason for decreased activity of extracellular superoxide dismutase in patients with diabetes. Arch Med Res. 2005;36:148–153. doi: 10.1016/j.arcmed.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehri S, Meshki MA, Hosseinzadeh H. Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wistar rats. Drug Chem Toxicol. 2015;38:162–166. doi: 10.3109/01480545.2014.919585. [DOI] [PubMed] [Google Scholar]
- 60.Gaweł S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004;57:453–455. (In Polish) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.