Abstract
Parkinson’s disease (PD) is one of the most common human neurodegenerative diseases. Belated diagnoses of PD and late treatment are caused by its elongated prodromal phase. Thus, searching for new candidate genes participating in the development of the pathological process in the early stages of the disease in patients who have not yet received therapy is relevant. Changes in mRNA and protein levels have been described both in the peripheral blood and in the brain of patients with PD. Thus, analysis of changes in the mRNA expression in peripheral blood is of great importance in studying the early stages of PD. This work aimed to analyze the changes in MEF2C, SLC22A4, P2RY12, and LRRN3 gene expression in the peripheral blood of patients in the early stages of PD. We found a statistically relevant and PD-specific change in the expression of the LRRN3 gene, indicating a disruption in the processes of neuronal regeneration and the functioning of synapses. The data obtained during the study indicate that this gene can be considered a potential biomarker of the early stages of PD.
Keywords: Parkinson’s disease, gene expression, peripheral blood
1. Introduction
Parkinson’s disease (PD) is one of the most common human neurodegenerative diseases, belonging to the class of multifactorial pathologies. In most cases, this disease is sporadic and is associated with a complex interaction of genetic and environmental factors [1,2]. According to forecasts, the expected number of patients with PD will reach 12 million people worldwide by 2030 [3,4].
The development of PD is primarily associated with the slow and steadily progressive death of dopaminergic neurons in the substantia nigra pars compacta (SN), gradually leading to the appearance of such classical motor symptoms as tremor, rigidity, bradykinesia, and postural instability [5]. Moreover, the manifestation of the first motor symptoms can occur several decades after the onset of the neurodegenerative process [6,7]. A belated diagnosis of PD and late treatment are caused by its elongated prodromal phase. Thus, searching for new candidate genes and pathways participating in the onset and progression of PD in patients who have not yet received therapy continues.
It is known that the CNS is the predominant place where the main pathological processes in PD occur. However, peripheral blood cells are also suitable for the investigation of the processes occurring in the brain in PD. It has been shown that both dopaminergic neurons and blood cells express genes associated with dopaminergic signal transmission [8,9,10,11,12]. Proteins expressed in the peripheral blood may also reflect molecular processes associated with the pathogenesis of PD. Changes in mRNA and protein levels associated with protein degradation [13,14,15,16], mitochondrial dysfunction [14,15,16,17], oxidative stress [16,18], apoptosis [14,15,19,20], and autophagy [21] have been found both in the peripheral blood and in the brains of patients with PD. Thus, analysis of changes in individual mRNA expression in the peripheral blood is potentially relevant for studying the early stages of PD.
This work aimed to analyze changes in MEF2C, SLC22A4, P2RY12, and LRRN3 gene expression in the peripheral blood of patients in the early stages of PD who received or did not receive therapy. The P2RY12 gene was selected as a result of a whole-transcriptome study of neuronal progenitor cells and iPSCs from monozygotic twins discordant for PD [22]. The MEF2C and SLC22A4 genes were selected based on the results of analysis of whole-transcriptomic data obtained from mice in a chronic MPTP-induced PD model. The LRRN3 gene was selected based on the published data [23,24].
2. Materials and Methods
2.1. Patients
The study was conducted on two cohorts of patients newly diagnosed with PD: those who received and those who did not receive therapy. The diagnoses were made at the Scientific Center of Neurology (Moscow) using the international unified PD rating scale (Unified Parkinson’s Disease Rating Scale, UPDRS) [25] and the Hoehn–Yahr scale [26]. Cohorts of 35 patients with various neurological diseases and 40 healthy volunteers without a history of neurological diseases selected at the Scientific Center of Neurology (Moscow) were used for comparison. The most complete description of the healthy control cohort was given by us earlier in [27]. Both groups of patients with PD are described in detail in Table S1.
The study was approved by the ethical committee of the Scientific Center of Neurology (Moscow, Russia), protocol 22/5, dated 16 January 2022 [28]. Patients and neurologically healthy volunteers (Russians living in the European part of Russia) were recruited from the Scientific Center of Neurology (Moscow, Russia). All the participants were screened for common PD mutations and had no familial PD. None of the participants had serious comorbidities.
2.2. Isolation of Total RNA from Peripheral Blood
Peripheral blood samples were collected at 8 a.m. in the fasting state for subsequent total RNA extraction. Total RNA was isolated from 200 μL of whole blood using the Whole-Blood Total RNA Kit (Zymo Research Corp., Irvine, CA, USA) and the Quick RNA Whole Blood kit (Zymo Research Corp., Irvine, CA, USA) according to the manufacturer’s recommendations. The concentration of isolated total RNA was measured using the Quant-iT RNA BR Assay Kit on a Qubit 3.0 fluorometer (Invitrogen, Carlsbad, CA, USA). Then, yeast tRNA was added [29].
2.3. Expression Analysis of Selected Candidate Genes
Analysis of changes in the relative levels of mRNA of the genes was carried out using reverse transcription and real-time PCR with TaqMan probes. The reverse transcription reaction was performed using the RevertAid™ H Minus Reverse Transcriptase kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s recommendations in a T3 Thermocycler amplifier (T3 Thermoblock, Biometra, Göttingen, Germany). cDNA obtained through a reverse transcription reaction and diluted in a solution of yeast tRNA was used as a template for performing the real-time PCR. Real-time PCR was carried out on a QuantStudio 3 amplifier (Applied Biosystems, Foster City, CA, USA) using PCR reagents (Synthol, Moscow, Russia). To carry out amplification, the following temperature regime was used: 50 °C–60 s, then 40 cycles at 95 °C–15 s and 61 °C–30 s, then 25 °C–30 s. Each sample was analyzed in triplicate.
2.4. Data Processing
The sequences of the gene-specific primers and probes for the studied genes are given in Table S2. The specificity of the primers and probes was checked using BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/, accessed on 22 September 2023) [30,31].
The ΔΔCt amplification threshold comparison method was used for calculation [32]. Statistical processing of the obtained data was carried out using the software package Statistica v. 8.0 (StatSoft Inc., Tulsa, OK, USA) and MS Excel 2019 (Microsoft, Redmond, WA, USA). The data were analyzed using the nonparametric Mann–Whitney U-test.
3. Results
The results of the expression analysis in the peripheral blood of patients in the early stages of PD as well as in the neurological control group are presented in Table 1.
Table 1.
Results of analysis of changes in the relative levels of mRNA of LRRN3, MEF2C, SLC22A4, and P2RY12 genes in the peripheral blood of patients in early stages of PD (fold change relative to healthy control).
| Gene | Patients with PD Who Did Not Receive Therapy | Patients with PD Who Received Therapy | Neurological Control |
|---|---|---|---|
| LRRN3 | 0.32 1 (0.18; 0.51) 2 | 0.50 (0.39; 0.56) | 0.91 (0.62; 1.31) |
| MEF2C | 2.56 (2.09; 2.74) | 2.36 (1.84; 2.93) | 3.14 (2.48; 3.75) |
| SLC22A4 | 3.26 (2.45; 4.14) | 2.99 (1.93; 3.33) | 2.34 (1.50; 3.04) |
| P2RY12 | 1.06 (0.85-1.33) | 0.59 (0.54; 1.33) | 0.91 (0.71; 1.08) |
1 Median, 2 25–75 percentiles. Values with p < 0.05 are shown in bold. The expression level in the control was set to 1.
As can be seen from Table 1, significant changes in expression in the peripheral blood of patients in the early stages of PD were obtained for three of the four genes studied. However, changes specific to PD were observed only for the LRRN3 gene since only this gene did not show changes in the neurological control group. A significant decrease in the expression level of the LRRN3 gene by more than two and three times was shown for both groups of patients with PD (both those who received and those who did not receive therapy), respectively. A considerable and statistically significant increase in expression by more than two times was detected for the MEF2C and SLC22A4 genes in both groups of patients with PD. However, the identified changes are not specific to PD since a similar increase in expression was shown for these genes in the neurological control group.
4. Discussion
PD is characterized by a prodromal period of development, and the treatment of patients usually begins immediately upon diagnosis of PD. In this regard, analysis of the gene expression in patients who are in the early stages of PD but have not yet received therapy is a complex and urgent task. Studying changes in gene expression in the peripheral blood allows us not only to search for biomarkers of the early stages of PD but also to study the effect of therapy on gene expression. As can be seen from Table 1, significant changes in expression were obtained in the peripheral blood of patients in the early stages of PD for three of the four studied genes.
The most significant data were obtained for the LRRN3 gene. This gene encodes leucine repeat-rich neuronal protein 3, which is involved in the development and regeneration of neurons, as well as in the regulation of synaptic connections [33,34]. This gene was isolated as a potential diagnostic biomarker based on the results of whole-transcriptome metadata analysis in two independent studies [23,24]. Our work is the first to show a significant decrease in LRRN3 expression. It should be noted that a statistically significant decrease in the expression of this gene in patients with PD does not depend on the therapy since similar changes were detected in patients with PD both who received and who did not receive therapy. Moreover, the decrease in the expression of this gene is specific to PD since no statistically significant changes were detected in the neurological control group. Thus, the LRRN3 gene may be involved in the development of pathological processes in the earliest clinical stages of PD. Apparently, a decrease in the expression of this gene can lead to disruption of the processes of neuronal regeneration and the functioning of synapses [33,34]. In addition, LRRN3 can be considered a potential biomarker for the earliest stages of PD.
We also detected a significant increase in the expression of the MEF2C gene, which encodes a transcription factor involved in the regulation of the functioning of immune cells of myeloid origin, including the microglia, in the formation and differentiation of neurons, and in the growth of axons and dendrites [35,36]. Disruption of the MEF2C-PGC1α neuroprotective pathway has been shown to promote mitochondrial dysfunction and lead to apoptotic cell death [35,37,38]. At the same time, an increased level of MEF2C expression in humans is positively correlated with improved cognitive function, and in rats, it led to increased differentiation of neuronal progenitor cells into dopaminergic neurons and had a positive effect on motor function [39,40]. The elevated level of MEF2C, identified both in the PD group and in the neurological control group, may be a consequence of the development of general compensatory mechanisms aimed at reducing microglial activation, as well as having a positive effect on cognitive and motor functions.
We also found a significant, by more than two times, increase in the expression of the SLC22A4 gene in all the groups of patients studied. SLC22A4 is expressed in neurons and neuronal stem cells and encodes the OCTN1 protein [41]. This protein is involved in the transport of various neuroprotective compounds, in particular the antioxidant ergothioneine, thereby helping to reduce oxidative stress [42]. Increased SLC22A4 expression in both PD patients and neurological controls may be a protective mechanism that alleviates the symptoms of neurological disorders.
P2RY12 encodes the purinergic receptor P2Y12, which is expressed predominantly by platelets and microglia. Its function has been widely studied in relation to platelet activation and blood coagulation [43], but the role of P2Y12 in neuroinflammation requires further study [44]. Activation of microglial P2RY12 by ADP/ATP promotes microglial chemotaxis, and increased amounts of these molecules are released by necrotic and apoptotic cells [45]. No significant changes in the expression of P2RY12 were obtained in our study, which may indicate that it does not participate in the onset and early development of PD and cannot be considered a potential biomarker of this disease.
5. Conclusions
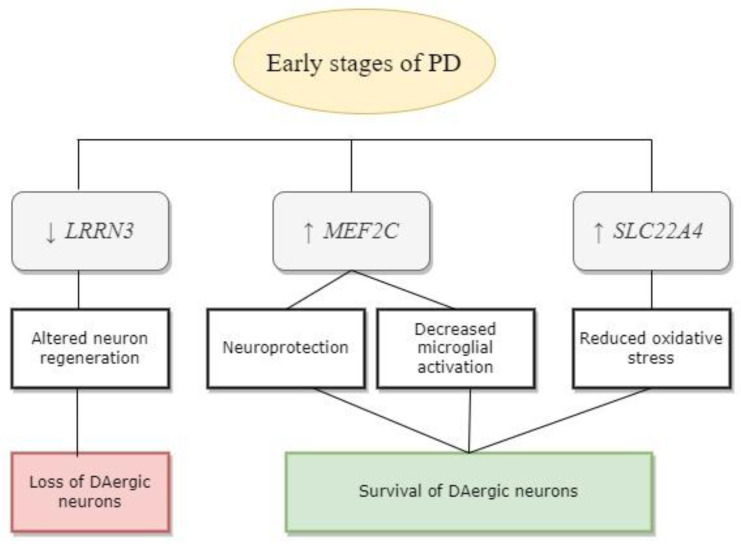
Changes in gene expression in the peripheral blood of patients in the early stages of PD, both those who received and those who did not receive therapy, were analyzed in our study. As a result, a statistically significant and PD-specific decrease in the expression of the LRRN3 gene, which may indicate a disruption in the processes of neuronal regeneration and the functioning of synapses, was shown. The data obtained during the study indicate that this gene can be considered a potential biomarker of the early stages of PD. Apparently, LRRN3 may be involved in the development of neurodegenerative processes in PD, while the MEF2C and SLC22A4 genes might be involved in the development of general compensatory processes typical for various neurodegenerative diseases (Figure 1).
Figure 1.
Possible role of the studied genes in PD pathogenesis. The arrows show up- or downregulated gene expression.
The limitations of the study include the relatively small size of the analyzed samples, but this limitation is explained by the extremely labor-intensive and time-consuming process of collecting peripheral blood samples from patients in stages 1–2 of PD who have not received therapy.
The findings suggest that LRRN3 can be considered a potential biomarker only in the early stages of Parkinson’s disease. For other stages of PD pathogenesis, including the preclinical stage and Hoehn–Yahr stages 3–4, additional research is necessary. In addition, the data from this study suggest that LRRN3 can only be considered a potential biomarker based on its relative mRNA levels using RT-qPCR. Additional studies are also needed to evaluate the corresponding protein as a biomarker for PD.
Acknowledgments
The study was partially performed in the Center “Cellular and Genetic Technologies” of the NRC “Kurchatov Institute”.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines12071391/s1, Figure S1: Results of analysis of changes in the relative levels of mRNA of LRRN3, MEF2C, SLC22A4, and P2RY12 genes in the peripheral blood of patients with early stages of PD; Table S1: Characteristics of cohorts of patients with PD and neurological control used in the study; Table S2: Sequences of gene-specific primers and probes.
Author Contributions
Conceptualization, M.I.S., P.A.S., and A.K.A.; methodology, A.K.A. and E.I.S.; validation, M.V.S., M.V.L., and S.A.P.; investigation, M.V.S., E.I.S., A.K.A., and M.I.S.; resources, A.V.K., E.Y.F., and S.N.I.; data curation, E.I.S. and M.M.R.; writing—original draft preparation, M.V.S. and E.I.S.; writing—review and editing, A.K.A., M.I.S., and P.A.S.; visualization, M.V.S.; supervision, A.K.A., M.I.S., and P.A.S.; project administration, A.K.A., M.I.S., and P.A.S.; funding acquisition, M.I.S. and P.A.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the World Medical Assembly Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. All the blood samples were collected with the informed consent of the subjects. The study was approved by the ethical committee of the Scientific Center of Neurology (Moscow, Russia), protocol 22/5 dated 16 January 2022.
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author. The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
The work was funded by the state task of the National Research Center “Kurchatov Institute” (GZ-5F-IMG.9) with partial financial support from the Russian Science Foundation (RSF) [agreement No. 20-15-00262-P].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Balestrino R., Schapira A.H.V. Parkinson disease. Eur. J. Neurol. 2020;27:27–42. doi: 10.1111/ene.14108. [DOI] [PubMed] [Google Scholar]
- 2.Lee A., Gilbert R.M. Epidemiology of Parkinson Disease. Neurol. Clin. 2016;34:955–965. doi: 10.1016/j.ncl.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2015 Neurological Disorders Collaborator Group Neurological Disorders Collaborator GrouGlobal, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877–897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorsey E.R., Constantinescu R., Thompson J.P., Biglan K.M., Holloway R.G., Kieburtz K., Marshall F.J., Ravina B.M., Schifitto G., Siderowf A., et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 5.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson disease. Nat. Rev. Dis. Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 6.Zeng X.S., Geng W.S., Jia J.J., Chen L., Zhang P. Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front. Aging Neurosci. 2018;10:109. doi: 10.3389/fnagi.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 8.Amenta F., Bronzetti E., Cantalamessa F., El-Assouad D., Felici L., Ricci A., Tayebati S.K. Identification of dopamine plasma membrane and vesicular transporters in human peripheral blood lymphocytes. J. Neuroimmunol. 2001;117:133–142. doi: 10.1016/S0165-5728(01)00317-4. [DOI] [PubMed] [Google Scholar]
- 9.Barbanti P., Fabbrini G., Ricci A., Cerbo R., Bronzetti E., Caronti B., Calderaro C., Felici L., Stocchi F., Meco G., et al. Increased expression of dopamine receptors on lymphocytes in Parkinson’s disease. Mov. Disord. 1999;14:764–771. doi: 10.1002/1531-8257(199909)14:5<764::AID-MDS1008>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Caronti B., Tanda G., Colosimo C., Ruggieri S., Calderaro C., Palladini G., Pontieri F.E., Di Chiara G. Reduced dopamine in peripheral blood lymphocytes in Parkinson’s disease. Neuroreport. 1999;10:2907–2910. doi: 10.1097/00001756-199909290-00006. [DOI] [PubMed] [Google Scholar]
- 11.Pellicano C., Buttarelli F.R., Circella A., Tiple D., Giovannelli M., Benincasa D., Colosimo C., Pontieri F.E. Dopamine transporter immunoreactivity in peripheral blood lymphocytes discriminates Parkinson’s disease from essential tremor. J. Neural Transm. 2007;114:935–938. doi: 10.1007/s00702-006-0623-2. [DOI] [PubMed] [Google Scholar]
- 12.Buttarelli F.R., Fanciulli A., Pellicano C., Pontieri F.E. The dopaminergic system in peripheral blood lymphocytes: From physiology to pharmacology and potential applications to neuropsychiatric disorders. Curr. Neuropharmacol. 2011;9:278–288. doi: 10.2174/157015911795596612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blandini F., Sinforiani E., Pacchetti C., Samuele A., Bazzini E., Zangaglia R., Nappi G., Martignoni E. Peripheral proteasome and caspase activity in Parkinson disease and Alzheimer disease. Neurology. 2006;66:529–534. doi: 10.1212/01.wnl.0000198511.09968.b3. [DOI] [PubMed] [Google Scholar]
- 14.Mutez E., Larvor L., Leprêtre F., Mouroux V., Hamalek D., Kerckaert J.P., Pérez-Tur J., Waucquier N., Vanbesien-Mailliot C., Duflot A., et al. Transcriptional profile of Parkinson blood mononuclear cells with LRRK2 mutation. Neurobiol. Aging. 2011;32:1839–1848. doi: 10.1016/j.neurobiolaging.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson M.K., Sharma P., Aasly J., Toft M., Skogar O., Saebo S., Lonneborg A. Found in transcription: Accurate Parkinson’s disease classification in peripheral blood. J. Parkinsons Dis. 2013;3:19–29. doi: 10.3233/JPD-120159. [DOI] [PubMed] [Google Scholar]
- 16.Shamir R., Klein C., Amar D., Vollstedt E.J., Bonin M., Usenovic M., Wong Y.C., Maver A., Poths S., Safer H., et al. Analysis of blood-based gene expression in idiopathic Parkinson disease. Neurology. 2017;89:1676–1683. doi: 10.1212/WNL.0000000000004516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinde S., Pasupathy K. Respiratory-chain enzyme activities in isolated mitochondria of lymphocytes from patients with Parkinson’s disease: Preliminary study. Neurol. India. 2006;54:390–393. doi: 10.4103/0028-3886.28112. [DOI] [PubMed] [Google Scholar]
- 18.Migliore L., Petrozzi L., Lucetti C., Gambaccini G., Bernardini S., Scarpato R., Trippi F., Barale R., Frenzilli G., Rodilla V., et al. Oxidative damage and cytogenetic analysis in leukocytes of Parkinson’s disease patients. Neurology. 2002;58:1809–1815. doi: 10.1212/WNL.58.12.1809. [DOI] [PubMed] [Google Scholar]
- 19.Blandini F., Cosentino M., Mangiagalli A., Marino F., Samuele A., Rasini E., Fancellu R., Tassorelli C., Pacchetti C., Martignoni E., et al. Modifications of apoptosis-related protein levels in lymphocytes of patients with Parkinson’s disease. The effect of dopaminergic treatment. J. Neural Transm. 2004;111:1017–1030. doi: 10.1007/s00702-004-0123-1. [DOI] [PubMed] [Google Scholar]
- 20.Calligaris R., Banica M., Roncaglia P., Robotti E., Finaurini S., Vlachouli C., Antonutti L., Iorio F., Carissimo A., Cattaruzza T., et al. Blood transcriptomics of drug-naive sporadic Parkinson’s disease patients. BMC Genom. 2015;16:876. doi: 10.1186/s12864-015-2058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Haddad S., Serrano A., Moal F., Normand T., Robin C., Charpentier S., Valery A., Brule-Morabito F., Auzou P., Mollet L., et al. Disturbed expression of autophagy genes in blood of Parkinson’s disease patients. Gene. 2020;738:144454. doi: 10.1016/j.gene.2020.144454. [DOI] [PubMed] [Google Scholar]
- 22.Vlasov I.N., Alieva A.K., Novosadova E.V., Arsenyeva E.L., Rosinskaya A.V., Partevian S.A., Grivennikov I.A., Shadrina M.I. Transcriptome Analysis of Induced Pluripotent Stem Cells and Neuronal Progenitor Cells, Derived from Discordant Monozygotic Twins with Parkinson’s Disease. Cells. 2021;10:3478. doi: 10.3390/cells10123478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo X., Hu W., Gao Z., Fan Y., Wu Q., Li W. Identification of PLOD3 and LRRN3 as potential biomarkers for Parkinson’s disease based on integrative analysis. npj Park. Dis. 2023;9:82. doi: 10.1038/s41531-023-00527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang F., Wu Q., Sun S., Bi G., Guo L. Identification of potential diagnostic biomarkers for Parkinson’s disease. FEBS Open Bio. 2019;9:1460–1468. doi: 10.1002/2211-5463.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov. Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 26.Goetz C.G., Poewe W., Rascol O., Sampaio C., Stebbins G.T., Counsell C., Giladi N., Holloway R.G., Moore C.G., Wenning G.K. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 27.Alieva A., Rudenok M., Filatova E., Karabanov A., Doronina O., Doronina K., Kolacheva A., Ugrumov M., Illarioshkin S., Slominsky P., et al. VCP expression decrease as a biomarker of preclinical and early clinical stages of Parkinson’s disease. Sci. Rep. 2020;10:827. doi: 10.1038/s41598-020-57938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 29.Suslov O., Steindler D.A. PCR inhibition by reverse transcriptase leads to an overestimation of amplification efficiency. Nucleic Acids Res. 2005;33:e181. doi: 10.1093/nar/gni176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alieva A.K., Filatova E.V., Rudenok M.M., Slominsky P.A., Shadrina M.I. Housekeeping Genes for Parkinson’s Disease in Humans and Mice. Cells. 2021;10:2252. doi: 10.3390/cells10092252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler D.L., Church D.M., Federhen S., Lash A.E., Madden T.L., Pontius J.U., Schuler G.D., Schriml L.M., Sequeira E., Tatusova T.A., et al. Database resources of the National Center for Biotechnology. Nucleic Acids Res. 2003;31:28–33. doi: 10.1093/nar/gkg033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Haines B.P., Gupta R., Jones C.M., Summerbell D., Rigby P.W. The NLRR gene family and mouse development: Modified differential display PCR identifies NLRR-1 as a gene expressed in early somitic myoblasts. Dev. Biol. 2005;281:145–159. doi: 10.1016/j.ydbio.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Hong M.G., Myers A.J., Magnusson P.K., Prince J.A. Transcriptome-wide assessment of human brain and lymphocyte senescence. PLoS ONE. 2008;3:e3024. doi: 10.1371/journal.pone.0003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deczkowska A., Matcovitch-Natan O., Tsitsou-Kampeli A., Ben-Hamo S., Dvir-Szternfeld R., Spinrad A., Singer O., David E., Winter D.R., Smith L.K. Mef2C restrains microglial inflammatory response and is lost in brain ageing in an IFN-I-dependent manner. Nat. Commun. 2017;8:717. doi: 10.1038/s41467-017-00769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu X., Fu C., Lin L., Liu S., Su X., Li A., Wu Q., Jia C., Zhang P., Chen L. miR-124 and miR-9 mediated downregulation of HDAC5 promotes neurite development through activating MEF2C-GPM6A pathway. J. Cell Physiol. 2018;233:673–687. doi: 10.1002/jcp.25927. [DOI] [PubMed] [Google Scholar]
- 37.Ryan S.D., Dolatabadi N., Chan S.F., Zhang X., Akhtar M.W., Parker J., Soldner F., Sunico C.R., Nagar S., Talantova M. Isogenic human iPSC Parkinson’s model shows nitrosative stress-induced dysfunction in MEF2-PGC1alpha transcription. Cell. 2013;155:1351–1364. doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue F., Tian J., Yu C., Du H., Guo L. Type I interferon response-related microglial Mef2c deregulation at the onset of Alzheimer’s pathology in 5xFAD mice. Neurobiol. Dis. 2021;152:105272. doi: 10.1016/j.nbd.2021.105272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho E.G., Zaremba J.D., McKercher S.R., Talantova M., Tu S., Masliah E., Chan S.F., Nakanishi N., Terskikh A., Lipton S.A. MEF2C enhances dopaminergic neuron differentiation of human embryonic stem cells in a parkinsonian rat model. PLoS ONE. 2011;6:e24027. doi: 10.1371/journal.pone.0024027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barker S.J., Raju R.M., Milman N.E.P., Wang J., Davila-Velderrain J., Gunter-Rahman F., Parro C.C., Bozzelli P.L., Abdurrob F., Abdelaal K. MEF2 is a key regulator of cognitive potential and confers resilience to neurodegeneration. Sci. Transl. Med. 2021;13:eabd7695. doi: 10.1126/scitranslmed.abd7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamichi N., Kato Y. Physiological Roles of Carnitine/Organic Cation Transporter OCTN1/SLC22A4 in Neural Cells. Biol. Pharm. Bull. 2017;40:1146–1152. doi: 10.1248/bpb.b17-00099. [DOI] [PubMed] [Google Scholar]
- 42.Nakamichi N., Tsuzuku S., Shibagaki F. Ergothioneine and central nervous system diseases. Neurochem. Res. 2022;47:2513–2521. doi: 10.1007/s11064-022-03665-2. [DOI] [PubMed] [Google Scholar]
- 43.Gachet C. P2Y(12) receptors in platelets and other hematopoietic and non-hematopoietic cells. Purinergic Signal. 2012;8:609–619. doi: 10.1007/s11302-012-9303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cattaneo M. P2Y12 receptors: Structure and function. J. Thromb. Haemost. 2015;13:S10–S16. doi: 10.1111/jth.12952. [DOI] [PubMed] [Google Scholar]
- 45.Franco-Bocanegra D.K., McAuley C., Nicoll J.A.R., Boche D. Molecular Mechanisms of Microglial Motility: Changes in Ageing and Alzheimer’s Disease. Cells. 2019;8:639. doi: 10.3390/cells8060639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author. The raw data supporting the conclusions of this article will be made available by the authors on request.