Abstract
Sepsis, a condition characterized by life-threatening organ dysfunction due to a dysregulated host response to infection, significantly impacts global health, with mortality rates varying widely across regions. Traditional therapeutic strategies that target hyperinflammation and immunosuppression have largely failed to improve outcomes, underscoring the need for innovative approaches. This review examines the development of therapeutic agents for sepsis, with a focus on clinical trials addressing hyperinflammation and immunosuppression. It highlights the frequent failures of these trials, explores the underlying reasons, and outlines current research efforts aimed at bridging the gap between theoretical advancements and clinical applications. Although personalized medicine and phenotypic categorization present promising directions, this review emphasizes the importance of understanding the complex pathogenesis of sepsis and developing targeted, effective therapies to enhance patient outcomes. By addressing the multifaceted nature of sepsis, future research can pave the way for more precise and individualized treatment strategies, ultimately improving the management and prognosis of sepsis patients.
Keywords: sepsis, hyperinflammation, immune paralysis
1. Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. Alarmingly, it accounts for nearly 19.7% of all global deaths and thus severely impacts health outcomes [2,3]. Mortality rates for sepsis vary widely, from 15% to higher than 50%, depending on the region and healthcare system. For example, the MOSAICS study reported a hospital mortality rate of 44.5% for severe sepsis in Asian countries, compared with 28.6% in the U.S. and 18.4% in Australia and New Zealand [4]. These statistics underscore the urgent need for innovative treatment approaches, especially given the lack of any FDA-approved treatments for sepsis [5].
Historically, the development of treatments for sepsis was based on the classical view of its pathogenesis, focusing on the systemic inflammatory response to infection causing widespread tissue damage and organ failure. Despite great research efforts aimed at mitigating this exaggerated immune response, the outcomes have been largely unsatisfactory [6,7]. However, recent shifts in understanding sepsis have led to new research avenues, challenging the classical view by suggesting that alternative mechanisms and pathways participate in its development. Studies now focus on modulating the immune system rather than suppressing it, and recognize sepsis as a heterogeneous syndrome, which has led to more personalized treatment approaches [5,8].
This review delineates the development of therapeutic agents targeting hyperinflammation and immunosuppression in sepsis, highlighting the range of clinical trials conducted thus far. Additionally, it discusses the predominance of failures in these trials, investigates the reasons behind these failures, and outlines the current research focus aiming to bridge the gaps between theoretical advancements and clinical applications.
2. Results
2.1. Hyperinflammation Matters
2.1.1. Pathogenesis of Sepsis According to the Classical View
When bacteria or viruses invade the human body, Toll-like receptors (TLRs) on sentinel cells act as an alarm system, swiftly recognizing pathogen-associated molecular patterns (PAMPs) and triggering the body’s initial line of defense [9,10]. The immune system comprises key components known as pathogen recognition receptors (PRRs), which include TLRs, RIG-I-like receptors (RLRs), nucleotide-binding oligomerization domain-like receptors (NLRs), absent in melanoma 2-like receptors (ALRs), C-type lectin receptors, and sensors for internal DNA and RNA [11]. The recognition of bacteria and viruses by PRRs is significantly influenced by the localization and structure of these receptors [11]. For instance, TLRs are categorized based on their cellular localization, which determines the types of ligands they recognize and their recognition mechanisms [12]. Bacteria are typically recognized by surface-expressed TLRs such as TLR2, TLR4, and TLR5 on innate immune cells, while viruses, due to their nucleic acid-based structures, are often identified by endosome-located TLRs, including TLR3, TLR7, and TLR9 [13,14,15,16,17]. The binding of ligands to these TLRs triggers the recruitment of adaptor molecules such as MyD88 and TRIF, initiating signaling pathways that result in the transcription and secretion of key pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), which are essential for coordinating the immune response and enhancing the body’s defense against pathogens [18].
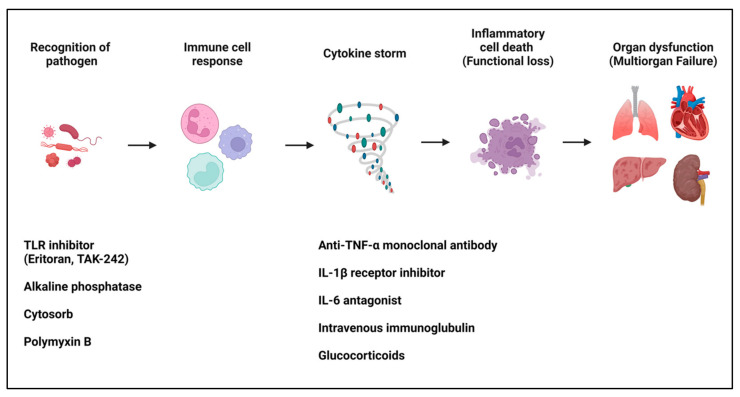
While the response of the innate immune system to PAMPs is crucial for combating infections, an excessive response can lead to a cytokine storm [19,20,21]. The pathogenesis of a cytokine storm involves excessive cytokine production, leading to systemic inflammation and extensive tissue damage, which undermines cellular and tissue integrity, impairs the function of vital organs, and ultimately results in multiorgan failure, a hallmark of sepsis [21,22]. The pathogenesis of sepsis also involves hypoperfusion or hypotension, conditions characterized by significantly reduced blood flow to tissues and markedly decreased blood pressure, respectively [22]. These conditions compound the effects of a cytokine storm by further decreasing the delivery of oxygen and nutrients to tissues, exacerbating tissue damage, and contributing to the failure of multiple organs [20]. This classical view of the pathogenesis of sepsis highlights the critical impact of an overactive immune response in severe infections (Figure 1).
Figure 1.
Simplified diagram explaining the pathogenesis of sepsis from a classical perspective and treatment strategies. This diagram depicts the classical pathogenesis of sepsis, underscoring the activation of the innate immune system by PAMPs via TLRs. It delineates how this activation can escalate into cytokine storms, causing systemic inflammation and multiorgan failure. Various therapeutic strategies based on this classical understanding are illustrated below, including TLR inhibitors, alkaline phosphatase, CytoSorb, polymyxin B, anti-TNF-α monoclonal antibodies, IL-1β receptor inhibitors, IL-6 antagonists, and glucocorticoids. Each treatment is positioned underneath the process it targets.
2.1.2. Strategies for Inhibiting Pathogen Recognition and Targeting Hyperinflammation in Sepsis
Addressing the root causes of sepsis by removing initiating factors and controlling hyperinflammation is a compelling treatment strategy. This approach, focusing on clearing pathogens, inhibiting pathogen recognition, and directly targeting pro-inflammatory cytokines or their receptors, aims to fundamentally alter the course of sepsis. By preventing the overactivation of the immune system, this strategy seeks to avert a cytokine storm and subsequent multiorgan failure, and has attracted significant interest. This logic has garnered significant interest among researchers and pharmaceutical companies, leading to the initiation of various clinical trials [23].
Inhibiting Pathogen Recognition in Sepsis Management
In the context of targeting hyperinflammation in sepsis, the strategy of inhibiting the recognition of PAMPs using TLR inhibitors is a notable approach [24,25,26,27]. TLR4, a receptor that mediates endotoxic shock and cytokine storms associated with sepsis, has been a particular focus of interest. Eritoran, a synthetic lipid A analog, binds to the TLR4-MD-2 complex, effectively blocking the interaction between lipopolysaccharide (LPS) and TLR4 and thus inhibiting the pro-inflammatory signaling pathway [28]. This mechanism demonstrated the efficient blockade of LPS-induced cytokine production both in vitro and in animal models [28]. However, despite promising results in phase I/II clinical trials in terms of safety and tolerability, Eritoran failed to demonstrate efficacy in the ACCESS phase III randomized trial, which involved 1961 patients with severe sepsis. This led to the discontinuation of its clinical development in 2011 [29].
TAK-242 is a molecular inhibitor of TLR4 that was developed to treat sepsis by inhibiting TLR4 signaling, thereby suppressing the excessive immune responses [30]. Despite showing efficacy in in vitro and animal models by reducing fibrosis and inflammatory responses, TAK-242 failed to meet its primary endpoint of reducing mortality in patients with severe sepsis during phase III clinical trials conducted in 2013 [30,31].
Pathogen Clearance in Sepsis Management
In addition to inhibiting the recognition of PAMPs using TLR inhibitors, another pivotal approach to mitigate hyperinflammation in sepsis involves directly neutralizing pathogen-derived components. This strategy targets the removal of elements that drive the inflammatory response, thereby reducing sepsis-induced hyperinflammation. Devices such as CytoSorb and therapeutic strategies like alkaline phosphatase (AP) administration and polymyxin B hemoperfusion exemplify this approach and aim to lessen the burden of systemic inflammation by directly eliminating cytokines, toxins, and endotoxins from the bloodstream. While the inhibition of TLR-mediated recognition targets the upstream initiation of the inflammatory cascade, pathogen clearance addresses the downstream effects, providing a broad spectrum of interventions in the complex pathophysiology of sepsis.
AP is an enzyme recognized for its role in detoxifying LPS and endotoxins produced by Gram-negative bacteria, especially in the context of sepsis [32]. Its capability to dephosphorylate and neutralize LPS can significantly mitigate inflammation and the systemic response to infection [32]. Phase II clinical trials yielded promising results, with AP improving kidney function in sepsis patients, suggesting its potential as a therapeutic agent for sepsis-associated acute kidney injury (AKI) [33]. However, despite the initial promise, the subsequent REVIVAL phase III trial did not demonstrate a significant reduction in mortality rates among patients with sepsis-associated AKI using AP [34].
Polymyxin B hemoperfusion aims to treat sepsis by focusing on the clearance of circulating endotoxins, primarily those released by Gram-negative bacteria [35]. The EUPHRATES trial, a pivotal study in this area, evaluated the efficacy of polymyxin B hemoperfusion in reducing mortality among septic shock patients with high endotoxin levels. Conducted across 55 tertiary hospitals in North America from September 2010 to June 2016, this multicenter, randomized, blinded, sham-controlled study enrolled 450 critically ill adult patients [36]. Despite the theoretical benefit of lowering endotoxin levels to improve outcomes in sepsis patients, the EUPHRATES trial concluded that polymyxin B hemoperfusion, when added to conventional therapy, did not significantly reduce 28-day mortality rates compared with the sham treatment plus conventional therapy [36].
Strategies for Inhibiting Pro-inflammatory Cytokines or Receptor Activation
Until now, strategies have primarily focused on preventing pathogen recognition and blocking pathogen invasion or TLR signaling; however, the discussion is now shifting toward treating sepsis through immunosuppression by inhibiting the cytokines produced host–pathogen recognition. In this section, we will briefly explain the significance of each cytokine in the pathogenesis of sepsis and describe how strategies to inhibit these cytokines have been investigated, using representative clinical trials as examples.
TNF-α is one of the earliest and most extensively studied cytokines in the context of sepsis [37,38]. In murine models of sepsis, TNF-α levels sharply rise and peak remarkably early, within just 1–3 h, after LPS injection [39]. Elevated TNF-α serum levels have received significant attention in clinical settings due to their correlation with the severity of sepsis and increased mortality rates [40,41]. Preclinical studies utilizing anti-TNF-α antibodies demonstrated promising results in terms of reducing the mortality and ameliorating the symptoms of sepsis [42,43,44]. Encouraged by these findings, clinical trials targeting TNF-α have been conducted. However, despite initial optimism, these trials failed to conclusively demonstrate the efficacy of TNF-α inhibition in sepsis treatment [45,46,47]. The NORASEPT II study, as reported in The Lancet in 1998, and a 1995 JAMA study explored the potential of an anti-TNF-α monoclonal antibody (TNF-α MAb) for treating sepsis and septic shock, yielding critical insights into the challenges of targeting TNF-α in sepsis therapy [46,47]. Despite encompassing a large cohort across numerous hospitals in the USA and Canada, the NORASEPT II trial found no significant improvement in 28-day mortality rates for septic shock patients treated with TNF-α MAb compared with placebo [46]. Similarly, the JAMA study also failed to demonstrate a long-term mortality benefit at 28 days, despite showing a temporary reduction in mortality among septic shock patients shortly after treatment [47]. Both studies highlighted a crucial detail: while initial responses to TNF-α MAb suggested potential benefits, these did not translate into sustained survival improvements. Alongside these findings, the trial of the TNF-α mAb afelimomab, known as the RAMESES study, provides a nuanced perspective by demonstrating a modest but significant reduction in 28-day mortality in sepsis patients with IL-6 serum levels higher than 1000 pg/mL [45].
In the context of sepsis, IL-1β plays a crucial role beyond the initial cytokine response and significantly influences the complex cytokine network [37,48]. IL-1β is not only upregulated early in sepsis but also acts as a key player in triggering the release of other cytokines [37]. According to research conducted by Matsumoto et al., IL-1β is a key instigator within the cytokine network, initiating a sequence of events involving other critical cytokines such as IL-6 and IL-8 [37]. IL-1β is an independent prognostic factor in sepsis, with evidence that it is significantly upregulated in sepsis patients compared with patients with systemic inflammatory response syndrome, and its levels are even higher in septic shock cases, emphasizing its critical role in enhancing the inflammatory response and marking it as a key indicator of sepsis severity and prognosis [49,50]. Evidence supporting the efficacy of IL-1β inhibition in sepsis has predominantly been derived from NLRP3-deficient models. Lee et al. and Jin et al. demonstrated that NLRP3 inflammasome deficiency protects against microbial sepsis in mouse models by increasing lipoxin B4 synthesis and by enhancing survival, decreasing autophagy, and augmenting phagocytosis, respectively [51,52]. Despite the promising theoretical framework suggesting the potential of targeting IL-1β for sepsis treatment, clinical trials involving anakinra, an IL-1β receptor antagonist, have not demonstrated a significant improvement in patient outcomes. Randomized, double-blind, placebo-controlled trials that aimed to assess the efficacy of anakinra in sepsis syndrome and severe sepsis unfortunately failed to show a definitive improvement in survival rates for sepsis patients treated with anakinra [53,54].
While TNF-α and IL-1 drive the initial responses during the early stages of sepsis, the blood levels of IL-6 can be maintained over a longer period [37]. The persistent presence of IL-6 is inversely correlated with survival rates in patients with bacterial sepsis and septic shock, indicating that IL-6 plays a significant role in the progression and poor outcome of sepsis [55,56,57]. Evidence from preclinical studies supports the therapeutic potential of IL-6 blockade, showing that antibodies targeting IL-6 can improve survival and physiological responses in sepsis models [58,59]. However, despite these promising findings, the results are not uniformly positive. Contradictory results have emerged, such as the finding that high doses of recombinant IL-6 did not elicit adverse effects in healthy dogs, indicating that IL-6 plays a complex role in sepsis [60]. Furthermore, IL-6-knockout mice do not demonstrate enhanced survival upon the induction of sepsis by cecal ligation and puncture, adding to the debate about the dual role of IL-6 in sepsis dynamics [61]. The role of IL-6 in immunity has dual significance, not solely due to the mixed outcomes described above but because of its inherent pro-inflammatory and anti-inflammatory functions, marking it as a double-edged sword in the immune response [62,63]. This characterization stems from the unique capacity of IL-6 to both initiate pro-inflammatory reactions, potentially exacerbating conditions like sepsis, and concurrently trigger anti-inflammatory pathways, which are essential for the body’s healing and recovery processes [62]. Due to inconsistent preclinical results, few clinical studies initially targeted IL-6 in classical sepsis. However, recent findings of elevated IL-6 levels in COVID-19 patients have spurred numerous clinical trials exploring IL-6-targeted strategies [64,65,66,67]. These findings suggest that, while IL-6 antagonists do not benefit all hospitalized patients with mild-to-moderate COVID-19 symptoms, they may offer advantages in severe COVID-19 cases [67].
Intravenous immunoglobulins (IVIg), particularly those enriched with IgM (IgGAM), are being explored to manage sepsis-induced immune dysregulation [68]. IVIg aim to mitigate inflammation and tissue damage by interacting with excess cytokines and complement factors involved in cytokine storms. The clinical rationale for IVIg therapy encompasses the recognition and clearance of pathogens, the inhibition of mediator gene transcription, and the exertion of anti-apoptotic effects in immune cells [69]. Despite its theoretical benefits, clinical evidence, including guidelines of the Surviving Sepsis Campaign, points to inconsistent results with IVIg, leading to recommendations against their routine use [69]. Research studies such as SBITS and ESSICS have further demonstrated the challenges, showing no significant benefits in mortality rates among IVIg-treated patients, which underscores the need for a cautious approach to IVIg application in sepsis therapy [70,71].
Glucocorticoids have been considered as potential treatment options for sepsis due to their broad anti-inflammatory effects [72]. As pan-inflammation inhibitors, glucocorticoids exert their effects by interacting with glucocorticoid receptors, leading to the modulation of various inflammatory pathways. This interaction primarily results in the inhibition of transcription factors such as NF-κB and AP-1, which are crucial for the synthesis of pro-inflammatory cytokines like TNF-α, IL-1, and IL-6 [73]. The suppression of these cytokines is critical to manage the systemic inflammation observed in sepsis. However, the efficacy of glucocorticoid treatment in sepsis remains highly controversial, as evidenced by the divergent outcomes in major clinical trials [74,75,76,77,78,79]. A 2002 French trial and the 2018 APROCCHHS trial both reported reduced mortality in septic shock patients treated with a combination of hydrocortisone and fludrocortisone [74,76]. In contrast, the CORTICUS trial showed that, while hydrocortisone sped up septic shock reversal, it did not improve the mortality rates, and the HYPRESS trial found no significant prevention of septic shock or improvement in survival outcomes [74,78]. Further complicating the picture, the VANISH trial found that adding hydrocortisone to vasopressor treatments did not improve the mortality or affect kidney failure rates [79]. Similarly, the ADRENAL trial observed that, despite hastening septic shock resolution and reducing the need for blood transfusions, hydrocortisone did not significantly improve the 28- or 90-day mortality rates [75]. Overall, the role of glucocorticoids in sepsis remains a complex and debated topic in critical care, and further research is needed to clarify their place in sepsis management protocols.
Strategies for Inhibiting Damage-Associated Molecular Patterns (DAMPs)
As sepsis treatment strategies focusing on cytokine inhibition encountered setbacks, researchers discovered that cytokines do not merely surge momentarily; rather, they are part of a sustained immune response in sepsis. It has now been recognized that not only is there an initial response to infections, but multiple secondary cytokine releases also occur [80,81,82]. These subsequent releases can be triggered by the destruction of cell membranes during the initial cytokine storm, which releases materials that serve as further immune triggers [82]. In this sequence of septic responses, the complement system, particularly through the activation of components such as C3a and C5a, plays a crucial role in destructive processes during sepsis [83]. Key substances include high mobility group box 1 (HMGB1) and heat shock proteins (HSPs), which are DAMPs released from cells during sepsis. Following the release of such DAMPs during sepsis, neutrophil extracellular traps (NETs), which are webs of DNA, histones, and antimicrobial proteins formed by neutrophils, also contribute to the inflammatory cascade, exacerbating the response and potentially leading to further tissue damage in the septic environment [84]. While inhibitors of HMGB1 and HSPs have demonstrated efficacy in murine experimental models, these findings have not been translated into clinical studies [39,85,86,87,88].
2.2. Immunosuppression Matters
2.2.1. Mechanisms and Consequences of Immunosuppression in Sepsis
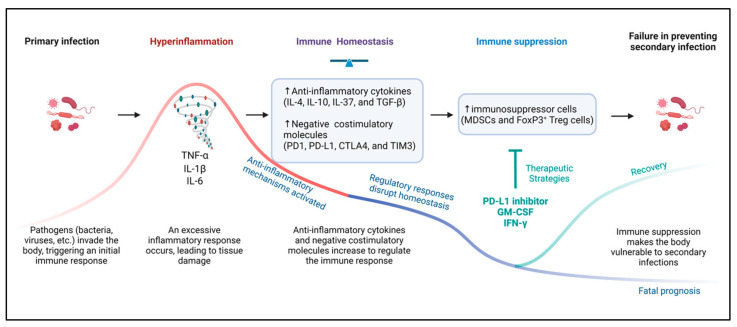
The classical view of sepsis pathogenesis emphasizes the need to inhibit hyperinflammation, which is thought to drive organ damage during the early stages of infection. However, an alternative concept has emerged, notably represented by the Hotchkiss group in the early 2000s, which focuses on the role of immunosuppression in the later stages of sepsis [89]. This view posits that, while hyperinflammation causes initial organ damage, the subsequent immunosuppression prevents the body from effectively combating secondary infections, leading to further deterioration in the patient’s condition. This perspective highlights the importance of understanding the dual phases of sepsis—initial hyperinflammation followed by immunosuppression—to develop more effective management strategies (Figure 2).
Figure 2.
Mechanisms and consequences of immunosuppression in sepsis and potential therapeutic strategies. During hyperinflammatory states, several anti-inflammatory mechanisms are activated to mitigate inflammation and promote tissue recovery, aiming to restore immune homeostasis. These include the upregulation of negative costimulatory molecules such as PD-L1 and PD-1 on immune cells, the increased production of anti-inflammatory cytokines like IL-4, IL-10, IL-37, and TGF-β, and the expansion of the myeloid-derived suppressor cell (MDSC) population together with an increase in FoxP3+ T cells. These regulatory responses can disrupt immune homeostasis, leading to immunosuppression characterized by suppressed T cell responses and increased apoptosis. This immunosuppression can result in failure in preventing secondary infection. Therapeutic strategies targeting these pathways include GM-CSF to enhance neutrophil activity, anti-PD-L1 antibodies to alleviate T cell exhaustion, and IFN-γ to boost macrophage capabilities.
In hyperinflammatory states, several anti-inflammatory mechanisms are activated to mitigate inflammation and promote tissue recovery [90,91]. These include the upregulation of negative costimulatory molecules such as PD-L1 and PD-1 on immune cells, the increased production of anti-inflammatory cytokines like IL-4, IL-10, IL-37, and TGF-β, and the expansion of the myeloid-derived suppressor cell (MDSC) population together with an increase in FoxP3+ T cells [91,92,93,94,95,96,97]. The expansion of MDSCs and regulatory T cells plays a critical role in the suppression of T cell responses. MDSCs exert their suppressive effects by releasing arginase, nitric oxide, and reactive oxygen species (ROS), which inhibit T cell receptor signaling and induce metabolic changes that impair T cell function and promote T cell apoptosis [98]. Similarly, expanded regulatory T cells contribute to immune paralysis by secreting immunosuppressive cytokines such as IL-10 and TGF-β, which further inhibit the activation and function of effector T cells [99]. Although TGF-β is an anti-inflammatory cytokine that suppresses T-cell responses and inhibits the effector T cell function, which has detrimental effects on disease outcomes by promoting fibrosis [97].
In sepsis, similarly to its role in cancer, the interaction between PD-1 and PD-L1 impairs T cell function by inhibiting cytokine secretion, reducing proliferation, and inducing apoptosis [100]. This interaction leads to the recruitment of the phosphatase SHP-2, which dephosphorylates components of the TCR signaling pathway, leading to the reduced activation and increased apoptosis of T cells [100]. These changes contribute to the disrupted immune homeostasis and prolonged immunosuppression observed in sepsis patients, ultimately exacerbating disease progression [91].
While these immune responses initially serve a protective function, their prolonged activation can disrupt immune homeostasis, leading to sepsis-induced immunosuppression. This sequence of regulatory responses and their consequences are effectively described by the “two-hit model” of sepsis, which illustrates how strong initial immune responses can lead to subsequent immunological vulnerabilities [101]. This model highlights the delicate balance required to manage the immune response during sepsis in order to prevent the transitioning from a protective to a harmful state.
The perspective on immunosuppression in sepsis shifted when it was revealed that many sepsis patients exhibit reduced immune activity, correlating with decreased survival rates. Specifically, a reduction in both naïve and memory CD8+ T cells, together with an increased expression of exhaustion markers like PD-1, compromises immune defenses and is associated with higher mortality rates [93,102,103]. The complexity of immunosuppression in sepsis is underscored by outcomes in clinical trials such as the CORTICUS trial [74]. In that trial, the use of glucocorticoids for the pan-inhibition of the immune response did not improve 28-day survival rates and actually increased the incidence of superinfections, including new cases of sepsis and septic shock [74]. These results highlight the risks associated with the over-suppression of the immune system and underscore the necessity of finely tuned immunomodulation strategies for sepsis management.
2.2.2. Inhibition of Immune Paralysis as a Therapeutic Strategy for Sepsis
Strategies targeting immunosuppression were implemented after those targeting inflammation, meaning that fewer clinical trials have been conducted. Among the promising treatments, granulocyte–macrophage colony-stimulating factor (GM-CSF) and anti-PD-L1 antibodies are noteworthy (Table 1).
Table 1.
Characteristics of immune dysregulation in sepsis.
| Hyperinflammation | Immunosuppression | |
|---|---|---|
| Factors | Pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) DAMPs (HMGB1, HSPs, DNA, and RNA) NETosis Complement activation |
Anti-inflammatory cytokines (IL-4, IL-10, IL-37, and TGF-β) Upregulation of negative costimulatory molecules (PD1, PD-L1, CTLA4, and TIM3) Proliferation of immunosuppressor cells (MDSCs and FoxP3+ T regulatory cells) |
| Main cause of death 1 | Cytokine storm-induced organ dysfunction | Weakened clearance of infection Activation of secondary infection |
| Clinical trial | TLR inhibitors (Eritoran and TAK-242) AP CytoSorb Polymyxin B Anti-TNF-α monoclonal antibody IL-1β receptor inhibitor IL-6 antagonist IVIg Glucocorticoids |
PD-L1 inhibitor GM-CSF |
1 Disease-aggravating factors.
The interactions between PD-1 and PD-L1 have attracted considerable interest due to their critical role in promoting T-cell death and exhaustion, thereby exacerbating immune paralysis [92]. Extensive preclinical research has underscored the therapeutic potential of anti-PD-L1 antibodies in alleviating immunosuppression and bolstering immune function [93]. These studies have consistently shown positive results in murine models, suggesting that manipulating this pathway could improve clinical outcomes [104,105]. Additionally, this approach has been expanded to target other inhibitory molecules such as CTLA-4, which achieved similarly encouraging outcomes in diminishing the immunosuppressive environment [106].
However, not all strategies targeting inhibitory molecules have proven effective for treating sepsis. For example, in sepsis patients, elevated levels of T cell immunoglobulin and mucin domain protein 3 (Tim-3) are associated with increased mortality [92,107]. The use of soluble Tim-3 immunoglobulin (sTim-3-IgG) in murine models showed that blocking Tim-3 exacerbates early hyperinflammatory responses and lymphocyte apoptosis, and subsequently promotes a shift toward anti-inflammatory responses [108]. Furthermore, transitioning from the promising preclinical findings of PD-L1 inhibitors as a treatment for sepsis to definitive clinical outcomes remains a complex challenge. Recent initiatives such as the phase Ib trial by the Hotchkiss group focused on PD-L1 inhibition have not conclusively established the effectiveness of such interventions in human subjects [109,110,111].
GM-CSF has demonstrated promise in enhancing neutrophil phagocytic activities in critically ill patients with compromised immune responses, potentially reducing their risk of nosocomial infections [91]. A multicenter phase IIa study indicated that, while GM-CSF did not significantly increase the mean phagocytosis rates, it increased the proportion of patients achieving adequate phagocytosis levels [112]. In a phase II study, GM-CSF therapy significantly improved the Pa(O2)/FI(O2) ratio over 5 days (p = 0.02), indicative of enhanced gas exchange and pulmonary function, but did not increase the 30-day survival rates [113]. Additionally, a randomized, double-blind, placebo-controlled clinical trial found that GM-CSF reduced the duration of antibiotic treatment in patients with nontraumatic abdominal sepsis, but did not significantly affect the in-hospital mortality rates of these patients [114].
Treatment with IFN-γ has also been proposed as a strategy to correct immunosuppression in sepsis. IFN-γ, which enhances the macrophage phagocytic and bactericidal capabilities, significantly boosts monocyte HLA-DR expression and TNF-α secretion, aiding the eradication of pathogens [115]. However, no clinical trials have validated the efficacy of IFN-γ in the treatment of sepsis.
While the inhibition of immune paralysis using agents like GM-CSF and anti-PD-L1 antibodies shows substantial promise, the pathway from promising preclinical results to effective clinical applications remains fraught with challenges. Ongoing research and clinical trials are crucial for establishing reliable therapies to effectively combat sepsis-induced immunosuppression. To aid in understanding, we summarized the pro-inflammatory and anti-inflammatory cytokines discussed in this paper in Table 2.
Table 2.
Summary of the role of pro- and anti-inflammatory cytokines in sepsis.
| Cytokine | Role in Sepsis | References |
|---|---|---|
| TNF-α | Pro-inflammatory cytokine, produced shortly after infection onset | [37,38,39] |
| IL-1β | Pro-inflammatory cytokine, triggers release of other cytokines | [37,48] |
| IL-6 | Plays a dual role by initiating both pro- and anti-inflammatory reactions, presents in prolonged period | [37,62,63] |
| IL-4 | Anti-inflammatory cytokine, upregulated to mitigate inflammation and promote tissue recovery | [94] |
| IL-10 | Anti-inflammatory cytokine, suppresses T cell responses, inhibits effector T cell function | [95] |
| IL-37 | Anti-inflammatory cytokine, upregulated to mitigate inflammation and promote tissue recovery | [96] |
| TGF-β | Anti-inflammatory cytokine, suppresses effector T cell function, but has detrimental effects on disease outcomes by promoting fibrosis | [97] |
2.3. Strategies for Mitigating Organ Damage in Sepsis
Targeting immune dysregulation addresses the causes of sepsis, while there is also research focused on strategies to manage the resulting organ dysfunction. After experiencing a cytokine storm, the target organs of sepsis patients suffer from internal endothelial disruption, metabolic derangement, and mitochondrial damage, leading to a loss of function [116,117]. This section will cover strategies that target specific organ cells to mitigate organ damage in sepsis.
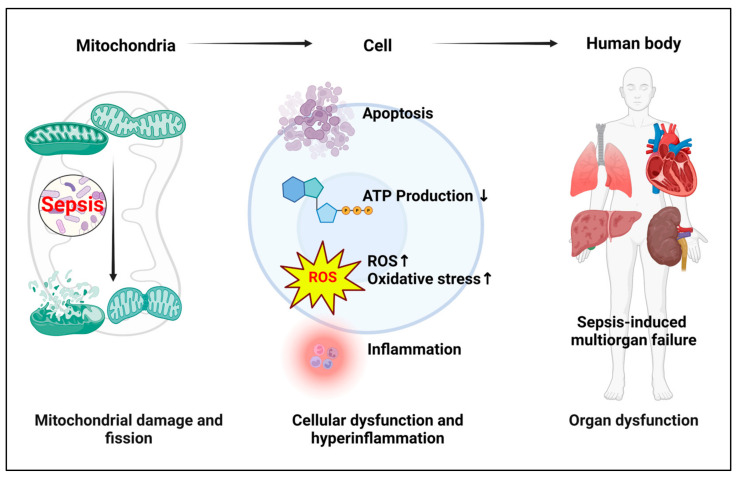
Recent studies have increasingly focused on mitochondrial damage as a key mechanism in sepsis, particularly evident in various organs such as the kidneys, liver, and heart, where specific cell types—renal tubular epithelial cells, hepatocytes, and cardiomyocytes—are targeted [118,119,120,121,122,123]. The promotion of cell death signaling, increased oxidative stress, and disruptions in mitochondrial dynamics are some of the key factors contributing to mitochondrial dysfunction, which has been identified as a major cause of organ damage in sepsis [118]. One primary factor is that of oxidative stress, which leads to the overproduction of reactive oxygen species (ROS) that damage mitochondrial components, including lipids, proteins, and DNA, thereby impairing mitochondrial function and biogenesis [124]. This oxidative stress is exacerbated by the dysregulation of enzymes such as NADPH/NADH oxidase, cyclooxygenase, and xanthine oxidase, which further inhibit critical mitochondrial functions like the sarco/endoplasmic reticulum Ca2+ ATP-ase (SERCA) [125,126]. Additionally, mitochondrial Ca2+ overload disrupts the balance of mitochondrial dynamics, including fusion and fission processes, leading to further mitochondrial fragmentation and energy deficiency [125,126]. Thus, for example, Mdivi-1, a mitochondrial fission inhibitor, preserves mitochondrial integrity and attenuates cell death pathways during sepsis [127,128]. This approach offers a viable strategy for mitigating sepsis-induced organ damage by stabilizing mitochondrial dynamics, reducing ROS production, and lessening hyperinflammation (Figure 3).
Figure 3.
Impact of mitochondrial damage on sepsis-related organ failure. In sepsis, mitochondrial damage leads to increased fragmentation, which boosts apoptotic signaling and impairs the primary function of mitochondria, namely energy production. This failure results in heightened ROS production, exacerbating hyperinflammation. Ultimately, these disruptions contribute to cellular dysfunction and death, culminating in organ dysfunction.
Another example is in the context of sepsis-related acute kidney injury (AKI), where receptor-interacting protein kinase 3 (RIPK3) is significantly elevated and contributes to oxidative stress and mitochondrial dysfunction by upregulating NADPH oxidase-4 (NOX4) and inhibiting mitochondrial complexes I and III [122]. Additionally, RIPK3 exacerbates the kidney tubular injury by facilitating the mitochondrial translocation of NOX4 in response to proinflammatory stimuli, highlighting its role in the necroptotic pathway and inflammation in AKI [122]. Therapeutic strategies that aim to recover mitochondrial function have shown promising results in several preclinical studies for sepsis treatment. Although clinical trials are yet to commence, this remains a highly anticipated area of research.
3. Discussion
In this review, we explored the development of therapeutic agents aimed at addressing the pathogenesis of hyperinflammation and immune paralysis in sepsis, focusing on the progress of these treatments in clinical trials. Despite extensive efforts by pharmaceutical companies to modulate immune responses through methods such as blocking immune cell sensitization and employing monoclonal antibodies to reduce cytokine production, clinical trials have largely failed to improve the survival rates of sepsis patients. This disappointing outcome underscores the complexities involved in effectively treating sepsis and raises critical questions regarding the efficacy of current therapeutic strategies.
A primary reason why clinical trials in sepsis have failed stems from the inherent heterogeneity of the immune response among patients [129]. A clinical trial might focus on neutralizing a specific cytokine, such as TNF, based on the assumption that its upregulation contributes uniformly to disease progression in all patients [130]. However, this approach overlooks the broad range of cytokine responses, as illustrated by the finding that IL-6 concentrations vary from 8 to higher than 1.5 million pg/mL among sepsis patients [130,131]. Such variability indicates that targeting a single cytokine or pathway is unlikely to address the multifaceted and robust immune response, which is necessary to effectively tackle sepsis.
The variability in immune responses among sepsis patients also extends to the type of infecting microorganism, which adds complexity to the development of effective treatments. The type and nature of the pathogen can significantly influence the immune response, which current sepsis therapies should account for but often do not adequately address. For instance, while anti-TNF therapies might improve outcomes in cases involving endotoxins or specific bacteria like Staphylococcus aureus, they show limitations in more complex scenarios involving polymicrobial infections, as seen in models of sepsis induced by cecal ligation and puncture [132]. Additionally, the contrasting performance of IL-6 antagonists, which have shown limited effectiveness in bacterial infections but more success in COVID-19 clinical trials, further illustrates how the efficacy of treatments can vary according to the type of infection [59,133,134].
In addition to the heterogeneity in immune responses related to the type of infecting microorganism, there is also significant variability across different sites of infection and the related host responses. The immune response can vary greatly depending on whether the infection is localized to organs such as the lungs, urinary tract, or abdominal cavity [135]. This site-specific immune response adds another layer of complexity to developing effective sepsis treatments, as different sites of infection can trigger distinct immune pathways and outcomes.
Another reason why clinical trials in sepsis have failed is due to the complexities of immune dysregulation in septic patients with other preexisting immune-related diseases. Recent studies have highlighted the potential for better mortality outcomes in septic patients with certain autoimmune diseases compared to the general septic population. For example, patients with autoimmune conditions like systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis tend to exhibit lower short-term mortality rates during sepsis hospitalizations [136,137,138]. It is hypothesized that pre-existing immune dysfunction in these patients alters their immune response to infection, leading to better outcomes. Analyzing the molecular differences in infection responses between these patients and the general population could enhance our understanding of the complexities of immune dysregulation in sepsis and its implications for future therapies.
In addressing the challenges of sepsis research, the diagnosis of sepsis itself poses a significant hurdle. Misclassification, where patients diagnosed with sepsis may in fact have different, noninfectious conditions, is a substantial issue. This includes scenarios where clinical diagnoses are confused with other serious conditions that mimic sepsis symptoms but are not caused by an infection, or where severe organ dysfunction occurs in the presence of an infection but is not directly attributable to a dysregulated host response. For instance, a case of acute hypoxic respiratory failure might be mistakenly attributed to sepsis when it is in fact caused by cardiogenic pulmonary edema from congestive heart failure exacerbated by a urinary tract infection. Such misdiagnoses can lead to the inclusion of inappropriate subjects in clinical trials, potentially obscuring the beneficial effects of treatments under study and contributing to the trials’ failures.
Moreover, the concept of a dysregulated host response to infection, a cornerstone of the Sepsis-3 framework, remains poorly defined. Despite the extensive descriptions of host response variations in sepsis, there is still no clear pathobiological explanation for the transition to a dysregulated response. Understanding these dynamics more clearly is crucial for advancing treatment strategies and improving patient outcomes in sepsis.
Another significant barrier to translating the findings of preclinical experiments into clinical success is the timing of intervention. Preclinical studies often benefit from the controlled and preemptive administration of immunosuppressive therapies. However, this level of control does not translate to clinical trials, where treatments typically begin only after sepsis is formally diagnosed. The diagnosis is based on the “Sepsis-3” consensus definitions and the sepsis-related organ failure assessment (SOFA) score, which evaluates criteria including the PaO2/FiO2 ratio, Glasgow Coma Scale score, mean arterial pressure, administration of vasopressors, serum creatinine level, urine output, and bilirubin level [1]. The manifestation of these criteria signifies that significant organ dysfunction has already occurred. Despite ongoing research efforts, the precise mechanisms of organ dysfunction in sepsis remain incompletely understood, and the concept of a cytokine storm is not uniformly applicable to all cases of sepsis. For example, in COVID-19-related sepsis, the role of cytokines is still being studied and understood. This gap in our understanding of sepsis pathobiology is partly responsible for the failure of many clinical studies.
In addition to the challenges of timing interventions in clinical trials, the diversity of patient characteristics presents another significant hurdle. This diversity is not just confined to the reaction of the immune system but extends to the genetic makeup and comorbid conditions of patients [131,139]. The transition from the controlled environment of preclinical studies to the unpredictable and varied conditions of clinical settings introduces numerous variables that can impact the efficacy of a therapy. Patients in clinical trials have a wide range of genetic backgrounds, underlying conditions, and stages of disease progression, all of which can influence the outcome of the treatment.
Additionally, the immune response in humans, particularly in patients with sepsis, is more complex and can significantly differ from that in animal models. There are substantial cross-species differences in response to infection or lipopolysaccharide (LPS) in sepsis models. For instance, the timing and dosage of LPS administration, the type of insult, and specific interventions can vary significantly between animal models and human sepsis, as sepsis in animal models is often induced rapidly with high doses of LPS or by the direct introduction of bacteria, which may not accurately replicate the progression of sepsis in humans [140]. Moreover, the progression and timeline of sepsis can be markedly different, with human sepsis often developing over days to weeks, whereas animal models typically show a more acute course. Furthermore, factors such as glucose levels and temperature, which are managed in preclinical sepsis models, can vary widely in human patients and affect the course of the disease and its response to treatment [141]. These complexities underscore the difficulties of applying findings from preclinical studies directly to clinical practice and highlight the need for innovative approaches to bridge this gap. Both the design and implementation of clinical trials must be reevaluated to ensure that they more accurately reflect the heterogenous nature of human sepsis.
As the limitations of traditional, uniform approaches in sepsis therapy become apparent, there is a growing emphasis on personalized therapy [142,143]. This approach underscores the potential for adapting treatments to meet individual patient needs, demonstrating the critical importance of personalized interventions in sepsis management. The efficacy of glucocorticoids, as noted in the results section, varies across clinical trials. However, glucocorticoids consistently yield positive outcomes in patients with heightened immune responses, especially when cytokine levels are elevated. Additionally, corticosteroids effectively reduce the duration of vasopressor infusion in patients requiring vasopressor support, showcasing their capability to manage septic shock under specific inflammatory conditions [144]. A meta-analysis further confirmed that corticosteroids enhance outcomes in sepsis, especially when cytokine levels are elevated [145]. The effectiveness of TNF-α inhibitors for improving treatment outcomes in patients with high IL-6 levels further underscores the value of personalized medicine [45]. These findings emphasize the importance of personalized therapy, suggesting that categorizing patients based on their immune status can lead to more predictable therapeutic responses.
Recent advancements in sepsis management have highlighted the importance of profiling and phenotyping approaches, which enable the development of personalized therapies specifically designed for individual patient characteristics. Considering the heterogeneity of the disease, these methods have become increasingly vital, as they allow for the more precise targeting of treatments that can significantly enhance both the precision of care and patient outcomes.
Cytokine profiling is a fundamental tool in the personalized management of sepsis, enabling the precise customization of therapies based on distinct cytokine profiles to effectively meet individual patient needs [146]. The study by Matsumoto et al. demonstrates the strong association of cytokines like IL-6, IL-8, MCP-1, and IL-10 with disease severity and prognosis, evidenced by their significant correlations with SOFA scores and markers of disseminated intravascular coagulation [37]. Furthermore, multiplex cytokine profiling has been employed to link specific cytokine levels with varying degrees of sepsis severity, where cytokines serve not only as prognosis markers but also as targets for therapeutic intervention [147,148]. For example, IL-8 and MCP-1 are strongly correlated with organ dysfunction and mortality, proving useful as early markers for risk stratification [147]. Additionally, cytokine levels such as IL-6, IL-8, and G-CSF within the first 24 h can predict worsening organ dysfunction, aiding clinicians in monitoring disease progression and adjusting treatments accordingly [148].
Research on sepsis phenotyping extends to areas such as transcriptomics and broader immunophenotyping, which encompass not only cytokines but also various protein biomarkers and cell surface markers. Techniques such as high-dimensional flow cytometry and transcriptomic analyses delineate specific immune phenotypes, such as hypo-responsiveness and hyper-responsiveness. These phenotypes correlate strongly with clinical outcomes and can guide the application of specialized treatments, reducing the one-size-fits-all approach, and increasing the likelihood of positive outcomes [149]. This approach was illustrated in the PROVIDE randomized clinical trial, which highlighted the importance of personalized therapy in managing immune paralysis, identified through notably low HLA-DR expression on monocytes (less than 5000 receptors per monocyte). Patients with this phenotype showed a distinct response profile to treatment interventions, which underscores the value of specifically customizing therapies to such profound immunosuppression [150]. However, it is important to acknowledge the limitations of these phenotypes, especially those generated by post hoc analyses. Such analyses often suffer from imbalances in patient characteristics, unlike the controlled settings of whole-cohort randomizations in randomized clinical trials. This imbalance can skew the accuracy and applicability of the phenotypes derived, potentially limiting their utility in clinical practice.
The integration of artificial intelligence (AI) and machine learning into sepsis management has significantly advanced personalized medicine, enhancing the precision of treatments. For example, the application of the deep deterministic policy gradient (DDPG) algorithm in AI-based medical decision-making systems has been shown to reduce patient mortality rates by providing optimal dosing combinations that closely align with those recommended by professional clinicians [151]. Additionally, the development of real-time, personalized sepsis prediction frameworks combining electrocardiogram data with electronic medical records enables accurate prediction of sepsis onset up to four hours before clinical symptoms appear, making them suitable for at-home monitoring and reducing the need for invasive laboratory tests [152]. Despite these advancements, challenges remain in the implementation and generalization of these models, including the need for data enrichment from sources beyond the electronic health record and the necessity for rigorous prospective studies to validate these algorithms in clinical settings [151].
These findings underscore the necessity of phenotypic categorization and personalized therapeutic approaches, enabling clinicians to better predict patient responses to treatment and ultimately improve outcomes.
4. Conclusions
The frequent failures of sepsis therapies point to the need for a paradigm shift toward more personalized and timely interventions. As we continue to dissect the complex cytokine network and varied immune responses in sepsis patients, future research should prioritize phenotype-driven, personalized approaches that consider the unique profiles of individual patients to enhance treatment outcomes in sepsis management.
Acknowledgments
Figures were created using BioRender.com. Language editing and sentence corrections were performed with the help of OpenAI’s ChatGPT.
Author Contributions
Conceptualization, E.-J.C. (Eun-Jung Choi) and M.-J.K.; methodology, E.-J.C. (Eun-Joo Choi); software, M.-J.K.; validation, E.-J.C. (Eun-Joo Choi) and E.-J.C. (Eun-Jung Choi); formal analysis, E.-J.C. (Eun-Jung Choi); investigation, E.-J.C. (Eun-Jung Choi); resources, E.-J.C. (Eun-Jung Choi); data curation, E.-J.C. (Eun-Jung Choi); writing—original draft preparation, E.-J.C. (Eun-Jung Choi) and M.-J.K.; writing—review and editing, E.-J.C. (Eun-Jung Choi); visualization, E.-J.C. (Eun-Joo Choi) and M.-J.K.; supervision, E.-J.C. (Eun-Jung Choi) and M.-J.K.; project administration, E.-J.C. (Eun-Jung Choi); funding acquisition, E.-J.C. (Eun-Jung Choi). All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI23C1563). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1I1A1A01059721).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleischmann C., Scherag A., Adhikari N.K., Hartog C.S., Tsaganos T., Schlattmann P., Angus D.C., Reinhart K., International Forum of Acute Care Trialists Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 4.Phua J., Koh Y., Du B., Tang Y.Q., Divatia J.V., Tan C.C., Gomersall C.D., Faruq M.O., Shrestha B.R., Gia Binh N., et al. Management of severe sepsis in patients admitted to Asian intensive care units: Prospective cohort study. BMJ. 2011;342:d3245. doi: 10.1136/bmj.d3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bode C., Weis S., Sauer A., Wendel-Garcia P., David S. Targeting the host response in sepsis: Current approaches and future evidence. Crit. Care. 2023;27:478. doi: 10.1186/s13054-023-04762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarczak D., Kluge S., Nierhaus A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021;8:628302. doi: 10.3389/fmed.2021.628302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stasi A., Honore P.M. Editorial: New insights in sepsis pathogenesis and renal dysfunction: Immune mechanisms and novel management strategies. Front. Immunol. 2023;14:1176620. doi: 10.3389/fimmu.2023.1176620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H., Jie Z., Gao P., Zhou Y., Zhang D. Editorial: Immune regulation in sepsis. Front. Immunol. 2023;14:1298777. doi: 10.3389/fimmu.2023.1298777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 10.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Li D., Wu M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021;6:291. doi: 10.1038/s41392-021-00687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai T., Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J., et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 14.Duan T., Du Y., Xing C., Wang H.Y., Wang R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022;13:812774. doi: 10.3389/fimmu.2022.812774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akira S., Takeda K., Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 16.Nemati M., Larussa T., Khorramdelazad H., Mahmoodi M., Jafarzadeh A. Toll-like receptor 2: An important immunomodulatory molecule during Helicobacter pylori infection. Life Sci. 2017;178:17–29. doi: 10.1016/j.lfs.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Yoon S.I., Kurnasov O., Natarajan V., Hong M., Gudkov A.V., Osterman A.L., Wilson I.A. Structural basis of TLR5-flagellin recognition and signaling. Science. 2012;335:859–864. doi: 10.1126/science.1215584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kay E., Scotland R.S., Whiteford J.R. Toll-like receptors: Role in inflammation and therapeutic potential. Biofactors. 2014;40:284–294. doi: 10.1002/biof.1156. [DOI] [PubMed] [Google Scholar]
- 19.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fajgenbaum D.C., June C.H. Cytokine Storm. N. Engl. J. Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deutschman C.S., Tracey K.J. Sepsis: Current dogma and new perspectives. Immunity. 2014;40:463–475. doi: 10.1016/j.immuni.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss R.S., Moldawer L.L., Opal S.M., Reinhart K., Turnbull I.R., Vincent J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinhagen F., Schmidt S.V., Schewe J.C., Peukert K., Klinman D.M., Bode C. Immunotherapy in sepsis-brake or accelerate? Pharmacol. Ther. 2020;208:107476. doi: 10.1016/j.pharmthera.2020.107476. [DOI] [PubMed] [Google Scholar]
- 24.D’Arpa P., Leung K.P. Toll-Like Receptor Signaling in Burn Wound Healing and Scarring. Adv. Wound Care. 2017;6:330–343. doi: 10.1089/wound.2017.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai S.Y., Segovia J.A., Chang T.H., Morris I.R., Berton M.T., Tessier P.A., Tardif M.R., Cesaro A., Bose S. DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: Role of DDX21-TRIF-TLR4-MyD88 pathway. PLoS Pathog. 2014;10:e1003848. doi: 10.1371/journal.ppat.1003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice T.W., Wheeler A.P., Bernard G.R., Vincent J.L., Angus D.C., Aikawa N., Demeyer I., Sainati S., Amlot N., Cao C., et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 2010;38:1685–1694. doi: 10.1097/CCM.0b013e3181e7c5c9. [DOI] [PubMed] [Google Scholar]
- 28.Barochia A., Solomon S., Cui X., Natanson C., Eichacker P.Q. Eritoran tetrasodium (E5564) treatment for sepsis: Review of preclinical and clinical studies. Expert Opin. Drug Metab. Toxicol. 2011;7:479–494. doi: 10.1517/17425255.2011.558190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opal S.M., Laterre P.F., Francois B., LaRosa S.P., Angus D.C., Mira J.P., Wittebole X., Dugernier T., Perrotin D., Tidswell M., et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA. 2013;309:1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 30.Ono Y., Maejima Y., Saito M., Sakamoto K., Horita S., Shimomura K., Inoue S., Kotani J. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci. Rep. 2020;10:694. doi: 10.1038/s41598-020-57714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Zhang D., Li C., Wu X., He C., Zhu X., Zhao H., Mu L. Toll-like receptor 4-mediated endoplasmic reticulum stress induces intestinal paneth cell damage in mice following CLP-induced sepsis. Sci. Rep. 2022;12:15256. doi: 10.1038/s41598-022-19614-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hummeke-Oppers F., Hemelaar P., Pickkers P. Innovative Drugs to Target Renal Inflammation in Sepsis: Alkaline Phosphatase. Front. Pharmacol. 2019;10:919. doi: 10.3389/fphar.2019.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickkers P., Mehta R.L., Murray P.T., Joannidis M., Molitoris B.A., Kellum J.A., Bachler M., Hoste E.A.J., Hoiting O., Krell K., et al. Effect of Human Recombinant Alkaline Phosphatase on 7-Day Creatinine Clearance in Patients with Sepsis-Associated Acute Kidney Injury: A Randomized Clinical Trial. JAMA. 2018;320:1998–2009. doi: 10.1001/jama.2018.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickkers P., Angus D.C., Bass K., Bellomo R., van den Berg E., Bernholz J., Bestle M.H., Doi K., Doig C.J., Ferrer R., et al. Phase-3 trial of recombinant human alkaline phosphatase for patients with sepsis-associated acute kidney injury (REVIVAL) Intensiv. Care Med. 2024;50:68–78. doi: 10.1007/s00134-023-07271-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronco C., Klein D.J. Polymyxin B hemoperfusion: A mechanistic perspective. Crit. Care. 2014;18:309. doi: 10.1186/cc13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dellinger R.P., Bagshaw S.M., Antonelli M., Foster D.M., Klein D.J., Marshall J.C., Palevsky P.M., Weisberg L.S., Schorr C.A., Trzeciak S., et al. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients with Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA. 2018;320:1455–1463. doi: 10.1001/jama.2018.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto H., Ogura H., Shimizu K., Ikeda M., Hirose T., Matsuura H., Kang S., Takahashi K., Tanaka T., Shimazu T. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 2018;8:13995. doi: 10.1038/s41598-018-32275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steeland S., Libert C., Vandenbroucke R.E. A New Venue of TNF Targeting. Int. J. Mol. Sci. 2018;19:1442. doi: 10.3390/ijms19051442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J.H., Jang J.H., Choi E.J., Kim Y.S., Lee E.J., Jung I.D., Han H.D., Wu T.C., Hung C.F., Kang T.H., et al. Annexin A5 increases survival in murine sepsis model by inhibiting HMGB1-mediated pro-inflammation and coagulation. Mol. Med. 2016;22:424–436. doi: 10.2119/molmed.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;1:355–357. doi: 10.1016/S0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 41.Debets J.M., Kampmeijer R., van der Linden M.P., Buurman W.A., van der Linden C.J. Plasma tumor necrosis factor and mortality in critically ill septic patients. Crit. Care Med. 1989;17:489–494. doi: 10.1097/00003246-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Moller A., Emling F., Blohm D., Schlick E., Schollmeier K. Monoclonal antibodies to human tumor necrosis factor alpha: In vitro and in vivo application. Cytokine. 1990;2:162–169. doi: 10.1016/1043-4666(90)90011-H. [DOI] [PubMed] [Google Scholar]
- 43.Pennington J.E. Therapy with antibody to tumor necrosis factor in sepsis. Clin. Infect. Dis. 1993;17((Suppl. 2)):S515–S519. doi: 10.1093/clinids/17.Supplement_2.S515. [DOI] [PubMed] [Google Scholar]
- 44.Tracey K.J., Fong Y., Hesse D.G., Manogue K.R., Lee A.T., Kuo G.C., Lowry S.F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 45.Reinhart K., Menges T., Gardlund B., Harm Zwaveling J., Smithes M., Vincent J.L., Tellado J.M., Salgado-Remigio A., Zimlichman R., Withington S., et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit. Care Med. 2001;29:765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 46.Abraham E., Wunderink R., Silverman H., Perl T.M., Nasraway S., Levy H., Bone R., Wenzel R.P., Balk R., Allred R., et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–941. doi: 10.1001/jama.1995.03520360048038. [DOI] [PubMed] [Google Scholar]
- 47.Abraham E., Anzueto A., Gutierrez G., Tessler S., San Pedro G., Wunderink R., Dal Nogare A., Nasraway S., Berman S., Cooney R., et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998;351:929–933. doi: 10.1016/S0140-6736(05)60602-2. [DOI] [PubMed] [Google Scholar]
- 48.Dinarello C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watany M.M., Elmazny M.I., Nasif E.M. Interleukin-31 interaction with inflammasome: A promising diagnostic and prognostic panel for early sepsis identification in critically ill patients. Cytokine. 2020;131:155102. doi: 10.1016/j.cyto.2020.155102. [DOI] [PubMed] [Google Scholar]
- 50.Pruitt J.H., Copeland E.M., 3rd, Moldawer L.L. Interleukin-1 and interleukin-1 antagonism in sepsis, systemic inflammatory response syndrome, and septic shock. Shock. 1995;3:235–251. doi: 10.1097/00024382-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Jin L., Batra S., Jeyaseelan S. Deletion of Nlrp3 Augments Survival during Polymicrobial Sepsis by Decreasing Autophagy and Enhancing Phagocytosis. J. Immunol. 2017;198:1253–1262. doi: 10.4049/jimmunol.1601745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S., Nakahira K., Dalli J., Siempos I.I., Norris P.C., Colas R.A., Moon J.S., Shinohara M., Hisata S., Howrylak J.A., et al. NLRP3 Inflammasome Deficiency Protects against Microbial Sepsis via Increased Lipoxin B4 Synthesis. Am. J. Respir. Crit. Care Med. 2017;196:713–726. doi: 10.1164/rccm.201604-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher C.J., Jr., Dhainaut J.F., Opal S.M., Pribble J.P., Balk R.A., Slotman G.J., Iberti T.J., Rackow E.C., Shapiro M.J., Greenman R.L., et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271:1836–1843. doi: 10.1001/jama.1994.03510470040032. [DOI] [PubMed] [Google Scholar]
- 54.Opal S.M., Fisher C.J., Dhainaut J.-F.A., Vincent J.-L., Brase R., Lowry S.F., Sadoff J.C., Slotman G.J., Levy H., Balk R.A., et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: A phase III, randomized, doubleblind, placebo-controlled, multicenter trial. Crit. Care Med. 1997;25:1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Vivas M.C., Villamarin Guerrero H.F., Tascon A.J., Valderrama-Aguirre A. Plasma interleukin-6 levels correlate with survival in patients with bacterial sepsis and septic shock. Interv. Med. Appl. Sci. 2021;11:224–230. doi: 10.1556/1646.2020.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardlund B., Sjolin J., Nilsson A., Roll M., Wickerts C.J., Wretlind B. Plasma levels of cytokines in primary septic shock in humans: Correlation with disease severity. J. Infect. Dis. 1995;172:296–301. doi: 10.1093/infdis/172.1.296. [DOI] [PubMed] [Google Scholar]
- 57.Pettila V., Hynninen M., Takkunen O., Kuusela P., Valtonen M. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensiv. Care Med. 2002;28:1220–1225. doi: 10.1007/s00134-002-1416-1. [DOI] [PubMed] [Google Scholar]
- 58.Riedemann N.C., Neff T.A., Guo R.F., Bernacki K.D., Laudes I.J., Sarma J.V., Lambris J.D., Ward P.A. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J. Immunol. 2003;170:503–507. doi: 10.4049/jimmunol.170.1.503. [DOI] [PubMed] [Google Scholar]
- 59.Hamilton F.W., Thomas M., Arnold D., Palmer T., Moran E., Mentzer A.J., Maskell N., Baillie K., Summers C., Hingorani A., et al. Therapeutic potential of IL6R blockade for the treatment of sepsis and sepsis-related death: A Mendelian randomisation study. PLoS Med. 2023;20:e1004174. doi: 10.1371/journal.pmed.1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preiser J.C., Schmartz D., Van der Linden P., Content J., Vanden Bussche P., Buurman W., Sebald W., Dupont E., Pinsky M.R., Vincent J.L. Interleukin-6 administration has no acute hemodynamic or hematologic effect in the dog. Cytokine. 1991;3:1–4. doi: 10.1016/1043-4666(91)90002-U. [DOI] [PubMed] [Google Scholar]
- 61.Leon L.R., White A.A., Kluger M.J. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am. J. Physiol. 1998;275:R269–S277. doi: 10.1152/ajpregu.1998.275.1.R269. [DOI] [PubMed] [Google Scholar]
- 62.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 63.Monsour M., Croci D.M., Agazzi S., Borlongan C.V. Contemplating IL-6, a double-edged sword cytokine: Which side to use for stroke pathology? CNS Neurosci. Ther. 2023;29:493–497. doi: 10.1111/cns.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Investigators R.-C., Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M., Annane D., Beane A., van Bentum-Puijk W., et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Group R.C. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., et al. Efficacy of Tocilizumab in Patients Hospitalized with COVID-19. N. Engl. J. Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones S.A., Hunter C.A. Is IL-6 a key cytokine target for therapy in COVID-19? Nat. Rev. Immunol. 2021;21:337–339. doi: 10.1038/s41577-021-00553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui J., Wei X., Lv H., Li Y., Li P., Chen Z., Liu G. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: A meta-analysis with trial sequential analysis. Ann. Intensiv. Care. 2019;9:27. doi: 10.1186/s13613-019-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jarczak D., Kluge S., Nierhaus A. Use of Intravenous Immunoglobulins in Sepsis Therapy-A Clinical View. Int. J. Mol. Sci. 2020;21:5543. doi: 10.3390/ijms21155543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Werdan K., Pilz G., Bujdoso O., Fraunberger P., Neeser G., Schmieder R.E., Viell B., Marget W., Seewald M., Walger P., et al. Score-based immunoglobulin G therapy of patients with sepsis: The SBITS study. Crit. Care Med. 2007;35:2693–2701. [PubMed] [Google Scholar]
- 71.Werdan K., Pilz G., Muller-Werdan U., Maas Enriquez M., Schmitt D.V., Mohr F.W., Neeser G., Schondube F., Schafers H.J., Haverich A., et al. Immunoglobulin G treatment of postcardiac surgery patients with score-identified severe systemic inflammatory response syndrome--the ESSICS study. Crit. Care Med. 2008;36:716–723. doi: 10.1097/01.CCM.0B013E3181611F62F. [DOI] [PubMed] [Google Scholar]
- 72.Annane D. Corticosteroids for severe sepsis: An evidence-based guide for physicians. Ann. Intensiv. Care. 2011;1:7. doi: 10.1186/2110-5820-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheschowitsch K., Leite J.A., Assreuy J. New Insights in Glucocorticoid Receptor Signaling-More Than Just a Ligand-Binding Receptor. Front. Endocrinol. 2017;8:16. doi: 10.3389/fendo.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sprung C.L., Annane D., Keh D., Moreno R., Singer M., Freivogel K., Weiss Y.G., Benbenishty J., Kalenka A., Forst H., et al. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 75.Venkatesh B., Finfer S., Cohen J., Rajbhandari D., Arabi Y., Bellomo R., Billot L., Correa M., Glass P., Harward M., et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 2018;378:797–808. doi: 10.1056/NEJMoa1705835. [DOI] [PubMed] [Google Scholar]
- 76.Annane D., Renault A., Brun-Buisson C., Megarbane B., Quenot J.P., Siami S., Cariou A., Forceville X., Schwebel C., Martin C., et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N. Engl. J. Med. 2018;378:809–818. doi: 10.1056/NEJMoa1705716. [DOI] [PubMed] [Google Scholar]
- 77.Annane D., Sebille V., Charpentier C., Bollaert P.E., Francois B., Korach J.M., Capellier G., Cohen Y., Azoulay E., Troche G., et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 78.Keh D., Trips E., Marx G., Wirtz S.P., Abduljawwad E., Bercker S., Bogatsch H., Briegel J., Engel C., Gerlach H., et al. Effect of Hydrocortisone on Development of Shock Among Patients with Severe Sepsis: The HYPRESS Randomized Clinical Trial. JAMA. 2016;316:1775–1785. doi: 10.1001/jama.2016.14799. [DOI] [PubMed] [Google Scholar]
- 79.Gordon A.C., Mason A.J., Thirunavukkarasu N., Perkins G.D., Cecconi M., Cepkova M., Pogson D.G., Aya H.D., Anjum A., Frazier G.J., et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients with Septic Shock: The VANISH Randomized Clinical Trial. JAMA. 2016;316:509–518. doi: 10.1001/jama.2016.10485. [DOI] [PubMed] [Google Scholar]
- 80.Mira J.C., Gentile L.F., Mathias B.J., Efron P.A., Brakenridge S.C., Mohr A.M., Moore F.A., Moldawer L.L. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit. Care Med. 2017;45:253–262. doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cicchinelli S., Pignataro G., Gemma S., Piccioni A., Picozzi D., Ojetti V., Franceschi F., Candelli M. PAMPs and DAMPs in Sepsis: A Review of Their Molecular Features and Potential Clinical Implications. Int. J. Mol. Sci. 2024;25:962. doi: 10.3390/ijms25020962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang X.D., Ji T.T., Dong J.R., Feng H., Chen F.Q., Chen X., Zhao H.Y., Chen D.K., Ma W.T. Pathogenesis and Treatment of Cytokine Storm Induced by Infectious Diseases. Int. J. Mol. Sci. 2021;22:13009. doi: 10.3390/ijms222313009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang R., Chen R., Zhang Q., Hou W., Wu S., Cao L., Huang J., Yu Y., Fan X.G., Yan Z., et al. HMGB1 in health and disease. Mol. Asp. Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Denning N.L., Aziz M., Gurien S.D., Wang P. DAMPs and NETs in Sepsis. Front. Immunol. 2019;10:2536. doi: 10.3389/fimmu.2019.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gentile L.F., Moldawer L.L. HMGB1 as a therapeutic target for sepsis: It’s all in the timing! Expert. Opin. Ther. Targets. 2014;18:243–245. doi: 10.1517/14728222.2014.883380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DeWulf B., Minsart L., Verdonk F., Kruys V., Piagnerelli M., Maze M., Saxena S. High Mobility Group Box 1 (HMGB1): Potential Target in Sepsis-Associated Encephalopathy. Cells. 2023;12:1088. doi: 10.3390/cells12071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stevens N.E., Chapman M.J., Fraser C.K., Kuchel T.R., Hayball J.D., Diener K.R. Therapeutic targeting of HMGB1 during experimental sepsis modulates the inflammatory cytokine profile to one associated with improved clinical outcomes. Sci. Rep. 2017;7:5850. doi: 10.1038/s41598-017-06205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vulczak A., Catalao C.H.R., Freitas L.A.P., Rocha M.J.A. HSP-Target of Therapeutic Agents in Sepsis Treatment. Int. J. Mol. Sci. 2019;20:4255. doi: 10.3390/ijms20174255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y., Guo D.Z., Zhu C.L., Ren S.C., Sun C.Y., Wang Y., Wang J.F. The implication of targeting PD-1:PD-L1 pathway in treating sepsis through immunostimulatory and anti-inflammatory pathways. Front. Immunol. 2023;14:1323797. doi: 10.3389/fimmu.2023.1323797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu D., Huang S.Y., Sun J.H., Zhang H.C., Cai Q.L., Gao C., Li L., Cao J., Xu F., Zhou Y., et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil. Med. Res. 2022;9:56. doi: 10.1186/s40779-022-00422-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McBride M.A., Patil T.K., Bohannon J.K., Hernandez A., Sherwood E.R., Patil N.K. Immune Checkpoints: Novel Therapeutic Targets to Attenuate Sepsis-Induced Immunosuppression. Front. Immunol. 2020;11:624272. doi: 10.3389/fimmu.2020.624272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y., Li J., Lou J., Zhou Y., Bo L., Zhu J., Zhu K., Wan X., Cai Z., Deng X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit. Care. 2011;15:R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schrijver D.P., Roring R.J., Deckers J., de Dreu A., Toner Y.C., Prevot G., Priem B., Munitz J., Nugraha E.G., van Elsas Y., et al. Resolving sepsis-induced immunoparalysis via trained immunity by targeting interleukin-4 to myeloid cells. Nat. Biomed. Eng. 2023;7:1097–1112. doi: 10.1038/s41551-023-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bazzoni F., Tamassia N., Rossato M., Cassatella M.A. Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: Lessons from neutrophils. Eur. J. Immunol. 2010;40:2360–2368. doi: 10.1002/eji.200940294. [DOI] [PubMed] [Google Scholar]
- 96.Nold-Petry C.A., Nold M.F. Rationale for IL-37 as a novel therapeutic agent in inflammation. Expert Rev. Clin. Immunol. 2022;18:1203–1206. doi: 10.1080/1744666X.2022.2108792. [DOI] [PubMed] [Google Scholar]
- 97.Thomas B.J., Kan O.K., Loveland K.L., Elias J.A., Bardin P.G. In the Shadow of Fibrosis: Innate Immune Suppression Mediated by Transforming Growth Factor-beta. Am. J. Respir. Cell Mol. Biol. 2016;55:759–766. doi: 10.1165/rcmb.2016-0248PS. [DOI] [PubMed] [Google Scholar]
- 98.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao Y.L., Liu Y.C., Zhang X., Shou S.T., Chai Y.F. Insight Into Regulatory T Cells in Sepsis-Associated Encephalopathy. Front. Neurol. 2022;13:830784. doi: 10.3389/fneur.2022.830784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muenzer J.T., Davis C.G., Dunne B.S., Unsinger J., Dunne W.M., Hotchkiss R.S. Pneumonia after cecal ligation and puncture: A clinically relevant “two-hit” model of sepsis. Shock. 2006;26:565–570. doi: 10.1097/01.shk.0000235130.82363.ed. [DOI] [PubMed] [Google Scholar]
- 102.Luperto M., Zafrani L. T cell dysregulation in inflammatory diseases in ICU. Intensiv. Care Med. Exp. 2022;10:43. doi: 10.1186/s40635-022-00471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boomer J.S., To K., Chang K.C., Takasu O., Osborne D.F., Walton A.H., Bricker T.L., Jarman S.D., 2nd, Kreisel D., Krupnick A.S., et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y., Zhou Y., Lou J., Li J., Bo L., Zhu K., Wan X., Deng X., Cai Z. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit. Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brahmamdam P., Inoue S., Unsinger J., Chang K.C., McDunn J.E., Hotchkiss R.S. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J. Leukoc. Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang K.C., Burnham C.A., Compton S.M., Rasche D.P., Mazuski R.J., McDonough J.S., Unsinger J., Korman A.J., Green J.M., Hotchkiss R.S. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit. Care. 2013;17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang C., Liu J., Wu Q., Wang Z., Hu B., Bo L. The role of TIM-3 in sepsis: A promising target for immunotherapy? Front. Immunol. 2024;15:1328667. doi: 10.3389/fimmu.2024.1328667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Z., Jiang X., Kang C., Xiao Y., Hou C., Yu J., Wang R., Xiao H., Zhou T., Wen Z., et al. Blockade of the T cell immunoglobulin and mucin domain protein 3 pathway exacerbates sepsis-induced immune deviation and immunosuppression. Clin. Exp. Immunol. 2014;178:279–291. doi: 10.1111/cei.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hotchkiss R.S., Colston E., Yende S., Crouser E.D., Martin G.S., Albertson T., Bartz R.R., Brakenridge S.C., Delano M.J., Park P.K., et al. Immune checkpoint inhibition in sepsis: A Phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensiv. Care Med. 2019;45:1360–1371. doi: 10.1007/s00134-019-05704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hotchkiss R.S., Colston E., Yende S., Angus D.C., Moldawer L.L., Crouser E.D., Martin G.S., Coopersmith C.M., Brakenridge S., Mayr F.B., et al. Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Antiprogrammed Cell Death-Ligand 1 Antibody (BMS-936559) Crit. Care Med. 2019;47:632–642. doi: 10.1097/CCM.0000000000003685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang T., Yu-Jing L., Ma T. Role of regulation of PD-1 and PD-L1 expression in sepsis. Front. Immunol. 2023;14:1029438. doi: 10.3389/fimmu.2023.1029438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pinder E.M., Rostron A.J., Hellyer T.P., Ruchaud-Sparagano M.H., Scott J., Macfarlane J.G., Wiscombe S., Widdrington J.D., Roy A.I., Linnett V.C., et al. Randomised controlled trial of GM-CSF in critically ill patients with impaired neutrophil phagocytosis. Thorax. 2018;73:918–925. doi: 10.1136/thoraxjnl-2017-211323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Presneill J.J., Harris T., Stewart A.G., Cade J.F., Wilson J.W. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am. J. Respir. Crit. Care Med. 2002;166:138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 114.Orozco H., Arch J., Medina-Franco H., Pantoja J.P., Gonzalez Q.H., Vilatoba M., Hinojosa C., Vargas-Vorackova F., Sifuentes-Osornio J. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: A randomized, double-blind, placebo-controlled clinical trial. Arch. Surg. 2006;141:150–153; discussion 154. doi: 10.1001/archsurg.141.2.150. [DOI] [PubMed] [Google Scholar]
- 115.Docke W.D., Randow F., Syrbe U., Krausch D., Asadullah K., Reinke P., Volk H.D., Kox W. Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nat. Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 116.Rossi M.T., Langston J.C., Singh N., Merali C., Yang Q., Merali S., Prabhakarpandian B., Kilpatrick L.E., Kiani M.F. Molecular Framework of Mouse Endothelial Cell Dysfunction during Inflammation: A Proteomics Approach. Int. J. Mol. Sci. 2022;23:8399. doi: 10.3390/ijms23158399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Preau S., Vodovar D., Jung B., Lancel S., Zafrani L., Flatres A., Oualha M., Voiriot G., Jouan Y., Joffre J., et al. Energetic dysfunction in sepsis: A narrative review. Ann. Intensiv. Care. 2021;11:104. doi: 10.1186/s13613-021-00893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lira Chavez F.M., Gartzke L.P., van Beuningen F.E., Wink S.E., Henning R.H., Krenning G., Bouma H.R. Restoring the infected powerhouse: Mitochondrial quality control in sepsis. Redox. Biol. 2023;68:102968. doi: 10.1016/j.redox.2023.102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van der Slikke E.C., Star B.S., van Meurs M., Henning R.H., Moser J., Bouma H.R. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI. Crit. Care. 2021;25:36. doi: 10.1186/s13054-020-03424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huo L., Liu C., Yuan Y., Liu X., Cao Q. Pharmacological inhibition of ferroptosis as a therapeutic target for sepsis-associated organ damage. Eur. J. Med. Chem. 2023;257:115438. doi: 10.1016/j.ejmech.2023.115438. [DOI] [PubMed] [Google Scholar]
- 121.Wang P.F., Xie K., Cao Y.X., Zhang A. Hepatoprotective Effect of Mitochondria-Targeted Antioxidant Mito-TEMPO against Lipopolysaccharide-Induced Liver Injury in Mouse. Mediat. Inflamm. 2022;2022:6394199. doi: 10.1155/2022/6394199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sureshbabu A., Patino E., Ma K.C., Laursen K., Finkelsztein E.J., Akchurin O., Muthukumar T., Ryter S.W., Gudas L., Choi A.M.K., et al. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight. 2018;3:e98411. doi: 10.1172/jci.insight.98411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shang X., Li J., Yu R., Zhu P., Zhang Y., Xu J., Chen K., Li M. Sepsis-related myocardial injury is associated with Mst1 upregulation, mitochondrial dysfunction and the Drp1/F-actin signaling pathway. J. Mol. Histol. 2019;50:91–103. doi: 10.1007/s10735-018-09809-5. [DOI] [PubMed] [Google Scholar]
- 124.Nedel W., Deutschendorf C., Portela L.V.C. Sepsis-induced mitochondrial dysfunction: A narrative review. World J. Crit. Care Med. 2023;12:139–152. doi: 10.5492/wjccm.v12.i3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hobai I.A. Mechanisms of Cardiac Dysfunction in Sepsis. Shock. 2023;59:515–539. doi: 10.1097/SHK.0000000000001997. [DOI] [PubMed] [Google Scholar]
- 126.Bar-Or D., Carrick M.M., Mains C.W., Rael L.T., Slone D., Brody E.N. Sepsis, oxidative stress, and hypoxia: Are there clues to better treatment? Redox. Rep. 2015;20:193–197. doi: 10.1179/1351000215Y.0000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhu Y., Kuang L., Wu Y., Deng H., She H., Zhou Y., Zhang J., Liu L., Li T. Protective Effects of Inhibition of Mitochondrial Fission on Organ Function after Sepsis. Front. Pharmacol. 2021;12:712489. doi: 10.3389/fphar.2021.712489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu R., Wang S.C., Li M., Ma X.H., Jia X.N., Bu Y., Sun L., Yu K.J. An Inhibitor of DRP1 (Mdivi-1) Alleviates LPS-Induced Septic AKI by Inhibiting NLRP3 Inflammasome Activation. Biomed. Res. Int. 2020;2020:2398420. doi: 10.1155/2020/2398420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rizvi M.S., Gallo De Moraes A. New Decade, Old Debate: Blocking the Cytokine Pathways in Infection-Induced Cytokine Cascade. Crit. Care Explor. 2021;3:e0364. doi: 10.1097/CCE.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Panacek E.A., Marshall J.C., Albertson T.E., Johnson D.H., Johnson S., MacArthur R.D., Miller M., Barchuk W.T., Fischkoff S., Kaul M., et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit. Care Med. 2004;32:2173–2182. doi: 10.1097/01.CCM.0000145229.59014.6C. [DOI] [PubMed] [Google Scholar]
- 131.Marshall J.C. Why have clinical trials in sepsis failed? Trends Mol. Med. 2014;20:195–203. doi: 10.1016/j.molmed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 132.Lorente J.A., Marshall J.C. Neutralization of tumor necrosis factor in preclinical models of sepsis. Shock. 2005;24((Suppl. 1)):107–119. doi: 10.1097/01.shk.0000191343.21228.78. [DOI] [PubMed] [Google Scholar]
- 133.Yu S.Y., Koh D.H., Choi M., Ryoo S., Huh K., Yeom J.S., Yoon Y.K. Clinical efficacy and safety of interleukin-6 receptor antagonists (tocilizumab and sarilumab) in patients with COVID-19: A systematic review and meta-analysis. Emerg. Microbes Infect. 2022;11:1154–1165. doi: 10.1080/22221751.2022.2059405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shapiro L., Scherger S., Franco-Paredes C., Gharamti A., Henao-Martinez A.F. Anakinra authorized to treat severe coronavirus disease 2019; Sepsis breakthrough or time to reflect? Front. Microbiol. 2023;14:1250483. doi: 10.3389/fmicb.2023.1250483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brunham R.C., Plummer F.A., Stephens R.S. Bacterial antigenic variation, host immune response, and pathogen-host coevolution. Infect. Immun. 1993;61:2273–2276. doi: 10.1128/iai.61.6.2273-2276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oud L., Garza J. The association of systemic lupus erythematosus with short-term mortality in sepsis: A population-level analysis. J. Investig. Med. 2023;71:419–428. doi: 10.1177/10815589221150641. [DOI] [PubMed] [Google Scholar]
- 137.Oud L., Garza J. Association of multiple sclerosis with mortality in sepsis: A population-level analysis. J. Intensiv. Care. 2022;10:36. doi: 10.1186/s40560-022-00628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oud L., Garza J. The prognostic impact of rheumatoid arthritis in sepsis: A population-based analysis. Acute Crit. Care. 2022;37:533–542. doi: 10.4266/acc.2022.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sorensen T.I., Nielsen G.G., Andersen P.K., Teasdale T.W. Genetic and environmental influences on premature death in adult adoptees. N. Engl. J. Med. 1988;318:727–732. doi: 10.1056/NEJM198803243181202. [DOI] [PubMed] [Google Scholar]
- 140.Cai L., Rodgers E., Schoenmann N., Raju R.P. Advances in Rodent Experimental Models of Sepsis. Int. J. Mol. Sci. 2023;24:9578. doi: 10.3390/ijms24119578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cavaillon J.M., Singer M., Skirecki T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020;12:e10128. doi: 10.15252/emmm.201810128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cao M., Wang G., Xie J. Immune dysregulation in sepsis: Experiences, lessons and perspectives. Cell Death Discov. 2023;9:465. doi: 10.1038/s41420-023-01766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Santacroce E., D’Angerio M., Ciobanu A.L., Masini L., Lo Tartaro D., Coloretti I., Busani S., Rubio I., Meschiari M., Franceschini E., et al. Advances and Challenges in Sepsis Management: Modern Tools and Future Directions. Cells. 2024;13:439. doi: 10.3390/cells13050439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu X.J., Tang Y.M., Song H., Yang S.L., Xu W.Q., Shi S.W. Corticosteroid administration is associated with improved outcome of patients presenting high inflammatory cytokine levels during septic shock. Pediatr. Blood Cancer. 2014;61:2243–2248. doi: 10.1002/pbc.25132. [DOI] [PubMed] [Google Scholar]
- 145.Liang H., Song H., Zhai R., Song G., Li H., Ding X., Kan Q., Sun T. Corticosteroids for Treating Sepsis in Adult Patients: A Systematic Review and Meta-Analysis. Front. Immunol. 2021;12:709155. doi: 10.3389/fimmu.2021.709155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gomez H., Anderko R.R., Carcillo J.A. Identifying inflammatory phenotypes to target mechanism-specific treatments in sepsis. Cell Rep. Med. 2022;3:100823. doi: 10.1016/j.xcrm.2022.100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bozza F.A., Salluh J.I., Japiassu A.M., Soares M., Assis E.F., Gomes R.N., Bozza M.T., Castro-Faria-Neto H.C., Bozza P.T. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit. Care. 2007;11:R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mera S., Tatulescu D., Cismaru C., Bondor C., Slavcovici A., Zanc V., Carstina D., Oltean M. Multiplex cytokine profiling in patients with sepsis. APMIS. 2011;119:155–163. doi: 10.1111/j.1600-0463.2010.02705.x. [DOI] [PubMed] [Google Scholar]
- 149.Seymour C.W., Kennedy J.N., Wang S., Chang C.H., Elliott C.F., Xu Z., Berry S., Clermont G., Cooper G., Gomez H., et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019;321:2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leventogiannis K., Kyriazopoulou E., Antonakos N., Kotsaki A., Tsangaris I., Markopoulou D., Grondman I., Rovina N., Theodorou V., Antoniadou E., et al. Toward personalized immunotherapy in sepsis: The PROVIDE randomized clinical trial. Cell Rep. Med. 2022;3:100817. doi: 10.1016/j.xcrm.2022.100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wardi G., Owens R., Josef C., Malhotra A., Longhurst C., Nemati S. Bringing the Promise of Artificial Intelligence to Critical Care: What the Experience with Sepsis Analytics Can Teach Us. Crit. Care Med. 2023;51:985–991. doi: 10.1097/CCM.0000000000005894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sadasivuni S., Saha M., Bhanushali S.P., Banerjee I., Sanyal A. In-Sensor Artificial Intelligence and Fusion with Electronic Medical Records for At-Home Monitoring. IEEE Trans. Biomed. Circuits Syst. 2023;17:312–322. doi: 10.1109/TBCAS.2023.3251310. [DOI] [PubMed] [Google Scholar]