Abstract
While studying apoptosis induced by baculovirus transactivator IE1 in SF-21 cells, we found that the levels of IE1-induced apoptosis were increased approximately twofold upon cotransfection with the baculovirus early pe38 gene. However, no apoptotic activity was observed in cells transfected with pe38 alone, even when placed under the control of a constitutive promoter. Thus, pe38 was able to augment IE1-induced apoptosis but was unable to induce apoptosis when expressed in SF-21 cells alone. PE38, the full-length product of pe38, is a nuclear protein with RING finger and leucine zipper motifs. Deletion of the amino-terminal region, which contains a putative nuclear localization motif, resulted in cytoplasmic localization of the PE38 mutants. These N-terminal deletion mutants were unable to enhance IE1-induced apoptosis. Mutation of a single conserved leucine (L242) of the leucine zipper motif also eliminated the ability of PE38 to augment apoptosis induced by IE1. In contrast, PE38 mutants with alanine substitutions for conserved cysteine residues (C109 or C138) of the RING finger motif were able to increase IE1-induced apoptosis to levels equivalent to those of wild-type PE38. We propose that PE38 is one of at least two viral factors which collectively evoke a cellular apoptotic response during baculovirus infection.
During replication in the permissive SF-21 cell line, the baculovirus Autographa californica nuclear polyhedrosis virus (AcMNPV) triggers apoptosis (6, 8) involving the activation of a cellular caspase (cysteine-dependent, aspartate-specific protease), SF-caspase-1 (1, 3, 22, 41). If unchecked, apoptosis results in an abortive infection (6, 7, 17). However, AcMNPV carries the P35 gene (p35), which encodes a general caspase inhibitor (5). P35 can inhibit active SF-caspase-1 and block apoptosis (3, 6, 7, 41).
While p35 is found in AcMNPV and its close relative Bombyx mori NPV (12, 18), at least two other baculoviruses, Orgyia pseudotsugata multicapsid NPV (OpMNPV) and Cydia pomonella granulovirus, appear to lack a p35 homolog but contain a member of the inhibitor of apoptosis (IAP) family of genes capable of functionally replacing p35 during AcMNPV replication (4, 9). In contrast to P35, however, the antiapoptotic Op-IAP and Cp-IAP genes are unable to block active SF-caspase-1 but are able to block the activation of this caspase (27, 41). The mechanism by which this is accomplished is not well understood, but it is known that these IAPs physically interact with and inhibit several known inducers of apoptosis isolated from Drosophila melanogaster: Reaper, Hid, Grim, and Doom (16, 45–47). It is not known whether AcMNPV infection signals apoptosis through inducers of this nature which might directly or indirectly activate SF-caspase-1. Several eukaryotic IAPs both interact with proximal signaling proteins, such as tumor necrosis factor receptor-associated factors and Reaper (29, 40, 48), and associate with and block distal caspases (11, 23, 24).
AcMNPV-induced apoptosis, including the activation of caspases, membrane blebbing, and DNA fragmentation, coincides with the initiation of the late phase of infection (6, 22). Involvement of DNA replication in the signaling of apoptosis during AcMNPV infection is implicated by the following observations: (i) the timing of apoptosis corresponds with the initiation of viral DNA replication (6, 22); (ii) aphidicolin, an inhibitor of both host and viral DNA polymerases (30), inhibits apoptosis following AcMNPV infection (7); and (iii) an AcMNPV mutant defective in viral DNA replication induces only limited apoptosis (22). However, DNA replication may be an indirect trigger since many events during infection depend on viral DNA replication including late viral gene expression and the decline of host RNA levels (26).
Transient overexpression in SF-21 cells of a single AcMNPV gene, the ie-1 gene (IE1), is sufficient to induce apoptosis which can be inhibited by coexpression with either p35 or one of the antiapoptotic baculoviral IAPs (35). IE1 is a potent transactivator of gene expression and may influence viral DNA replication through its interaction with homologous repeat sequences of AcMNPV which appear to serve as origins of DNA replication (13–15, 26). In SF-21 cells, IE1 is expressed immediately following infection, and its level increases throughout infection. Although IE1 is likely to be an important player in the induction of apoptosis during virus infection, it seems unlikely that ie-1 expression is solely responsible, based on the apparent involvement of viral DNA replication in signaling apoptosis (35).
In exploring the possibility that additional factors may be involved in AcMNPV-induced apoptosis, we found and now report that another AcMNPV gene, the PE38 gene (pe38), was able to augment IE1-induced apoptosis in transient assays. The pe38 gene of AcMNPV was first characterized as a gene expressed immediately upon infection (20). PE38 is present during the early phase of infection as a nuclear 38-kDa protein, but during the late phase, it appears to be converted to or expressed as a cytoplasmic 20-kDa protein in a process which is controlled by viral factors (21). Upon transient expression, pe38 generates only a 38-kDa product which localizes to punctate regions of the nucleus. PE38 contains a putative nuclear localization signal near the N terminus, a RING (C3HC4) finger in the central portion of the protein, and a leucine zipper near the C terminus. In this regard, PE38 is very similar in structure to AcMNPV CG30, but in contrast to CG30, PE38 appears to be essential for virus replication (34). PE38 exerts a mild stimulatory effect on transient expression from the promoter of the p143 gene, which encodes a protein with a DNA helicase motif (25). Stimulation of viral origin-dependent plasmid DNA replication and late transient gene expression by pe38 is also observed (19, 33) and may be due to the stimulatory effect of PE38 on p143 expression in these assays. In conjunction with ascribing a new activity of PE38 in augmenting IE1-induced apoptosis, we also describe the effect of mutations in the putative N-terminal nuclear localization signal, the RING finger motif, and the leucine zipper of PE38 on its proapoptotic activity, expression levels, and cellular localization.
MATERIALS AND METHODS
Cells.
Spodoptera frugiperda IPLB-SF-21 (SF-21) (44) cells were cultured at 27°C in TC-100 medium (GIBCO BRL, Gaithersburg, Md.) supplemented with 10% fetal bovine serum and 0.26% tryptose broth, as described previously (32).
Plasmid constructs and site-directed mutagenesis.
Plasmids pBs-H3F, containing the AcMNPV HindIII-F fragment (from 91.0 to 3.4 map units [m.u.]); pBs-IE1/HC; pBs-PE38; and pBE42 were described previously (34). To construct plasmid pBs-PE38hr, pBs-H3F was digested with MscI763 and XhoI restriction enzymes, blunt ended with T4 DNA polymerase, and ligated, followed by digestion with PstI and religation. The resulting plasmid, pBs-PE38hr, contains the PstI-MscI763 fragment of the AcMNPV genome encompassing two complete open reading frames (ORFs), pe38 and orf154. The PstI-MscI763 fragment also contains the hr1 sequence. To construct plasmid pHSPE38, which contains pe38 under the transcriptional control of the D. melanogaster hsp70 promoter, the chloramphenicol acetyltransferase (CAT) gene from the pHSP70CATPLVI+ plasmid (7) was replaced by the PCR-amplified pe38 ORF. Primers used to amplify pe38 were a 5′ primer in the sense orientation (5′-GCCGGATCCAATATGCCAAGGGACACC) and a 3′ primer in the antisense orientation (5′-TCCCCCGGGTTAATTTTCAAACCCAAA). To construct pHSFLAG-PE38, expressing N-terminally FLAG-tagged PE38 under Drosophila hsp70 promoter control, the same PCR product was inserted into the pHSP70FLAGPLVI+ plasmid (38), in frame with and downstream of a sequence encoding a FLAG epitope tag. To construct pHSFLAGPE38D1 and pHSFLAGPE38D2, two truncated forms of FLAG-PE38 lacking the first 38 and 69 amino-terminal amino acids of PE38, respectively, the PCR-amplified pe38D1 and pe38D2 coding sequences were inserted into pHSP70FLAGPLVI+. To PCR amplify pe38D1 and pe38D2, 5′ primers in the sense orientation (5′-GCCGGATCCAATATGCAAGAAGAACAA and 5′-GCCGGATCCAATATGGAACAGCAGCAG, respectively) were used. The 3′ primer in the antisense orientation used in these PCRs was the same as the 3′ primer used to PCR amplify the full-length pe38 gene. Site-specific mutagenesis was performed on pHSFLAGPE38 with a Transformer site-directed mutagenesis kit (Clontech Laboratories, Inc., Palo Alto, Calif.) with the selection primer 5′-CATCAGAGTCGCTAGCGATGTAAACGATGG and the mutagenic primers 5′-GATTCCGACTACGGCCGACCACGGTTTTTG, 5′-GCTGTCCATTGGCCAATACCCCAGGTAAAAATG, and 5′-CAGATTCAAGAGGCGCAGCATCAGGTG to generate the PE38 mutants PE38C109A (pHSFLAG-PE38C109A), PE38C138A (pHSFLAG-PE38C138A), and PE38L242A (pHSFLAG-PE38L242A), containing alanine instead of cysteine at residue 109, cysteine at residue 138, and leucine at residue 242, respectively. To construct pHSFLAG-ORF154, PCR-amplified orf154 coding sequences were inserted into pHSP70FLAGPLVI+ (38). Primers used to PCR amplify orf154 were a 5′ primer in the sense orientation (5′-GCGAGATCTAATATGGATAGTAGTAATTGT) and a 3′ primer in the antisense orientation (5′-TCCCCCGGGTTAAATTTTTATTATGCAAGA).
The pHSEpi-IE1 plasmid, expressing HA.11-tagged IE1 under Drosophila hsp70 promoter control, was described previously (39).
Apoptosis assay and internucleosomal DNA fragmentation.
SF-21 cells (1.0 × 106 per 60-mm-diameter dish) were transfected with 1.0 μg of the indicated plasmid by using Lipofectin reagent (GIBCO BRL). At 18 h posttransfection, medium was removed and the cells were harvested in 1 ml of TC-100 medium (without supplements) containing 0.04% trypan blue. Cell viability was determined as described previously (7). In experiments involving the induction of gene expression by heat shock, cells were transferred at 18 h posttransfection to 42°C for 30 min. Cells were returned to 27°C and analyzed for cell viability 12 h after heat shock. Analysis of cellular DNA degradation was performed as described previously (7).
Immunofluorescence.
SF-21 cells (0.5 × 106) were seeded on glass coverslips placed in 35-mm-diameter dishes and transfected with 1.0 μg of indicated plasmids as described above. Cells were heat shocked and, 3 h after induction, fixed in methanol as described previously (36). Cells were incubated in blocking buffer (5% dry milk, 5% Bacto Peptone [Difco, Detroit, Mich.]) in TTBS (0.4% Tween 20 in Tris-buffered saline, pH 7.6) for 1 h and washed twice with TTBS. To detect FLAG and HA.11 epitope-tagged proteins, mouse M2–anti-FLAG (Eastman Kodak, New Haven, Conn.) and mouse anti-HA.11 (Babco, Richmond, Calif.) monoclonal antibodies were used, respectively, followed by the treatment with lissamine rhodamine-conjugated anti-mouse immunoglobulin G (IgG)-IgM antibodies (Jackson Immunoresearch, West Grove, Pa.). Visualization of the nucleus was accomplished by staining with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, St. Louis, Mo.). Cells were examined and photographed with a confocal microscope.
Immunoblot analysis.
Transfected cells were heat shocked at 18 h posttransfection and harvested 3 h after heat shock. Cells were lysed in sodium dodecyl sulfate buffer as described previously (32). Proteins in lysates were separated on sodium dodecyl sulfate–12% polyacrylamide gels and transferred to Immobilon P membranes (Millipore, Bedford, Mass.). FLAG-tagged proteins were detected with a 1:8,000 dilution of anti-FLAG M2 monoclonal antibodies followed by a 1:10,000 dilution of rabbit anti-mouse Ig-horseradish peroxidase conjugate (Amersham, Chicago, Ill.). Immunoblots were visualized by the chemiluminescence method (Amersham).
RESULTS
The levels of IE1-induced apoptosis are increased by PE38.
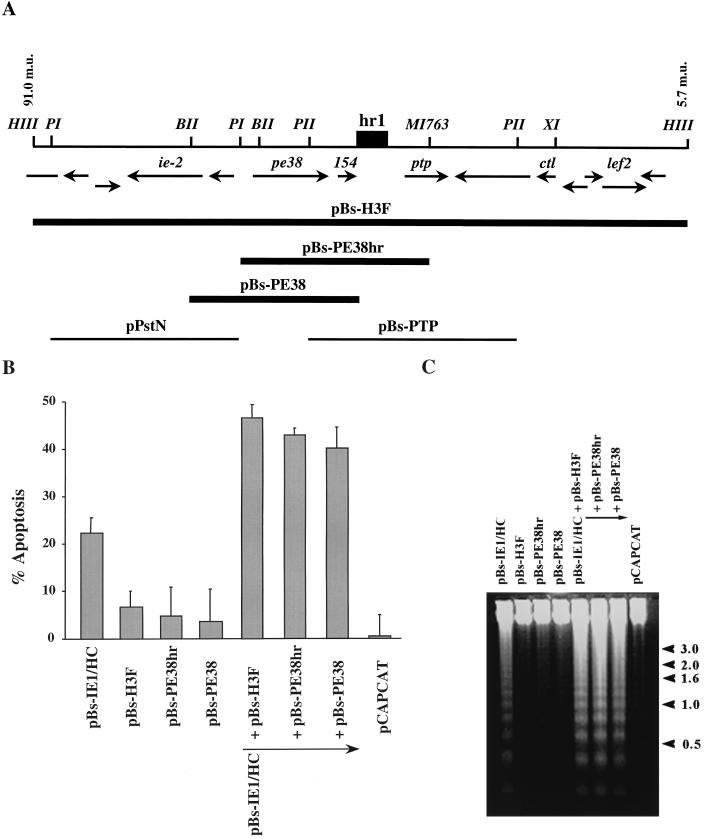
We have been interested in determining how AcMNPV induces apoptosis in SF-21 cells and which genes might be involved in induction. Since the expression of many AcMNPV genes depends on trans activation by IE1, which itself induces apoptosis, we tested the abilities of different regions of the AcMNPV genome to increase the levels of IE1-induced apoptosis by cotransfecting cells with pBs-IE1/HC, expressing IE1, and different clones of the AcMNPV genome. The only genomic clones which showed substantially increased levels of apoptosis, compared to IE1 alone, were clones in the HindIII-F region (97.0 to 5.7 m.u.) of the AcMNPV genome (Fig. 1 and data not shown). When a plasmid containing the HindIII-F fragment of AcMNPV (pBs-H3F) was cotransfected with pBs-IE1/HC, approximately 45% of the cells underwent apoptosis (Fig. 1B). This level was approximately twice as high as the level of apoptosis observed for cells cotransfected with pBs-IE1/HC and pCAPCAT, a plasmid expressing the CAT gene. This latter plasmid was used routinely as a negative control and to balance the plasmid DNA concentration so that the same amount of plasmid DNA was present in each transfection. Plasmids containing viral DNA flanking pe38 (e.g., pPstN and pBs-PTP in Fig. 1A) were unable to enhance IE1-induced apoptosis and exhibited less than a 1.1-fold enhancement of apoptosis (data not shown and Fig. 2 below).
FIG. 1.
Subclones of the AcMNPV HindIII-F fragment including PE38 augment IE1-induced apoptosis. (A) Location and direction of ORFs within the HindIII-F fragment between 91.0 and 3.4 m.u. are those determined by Ayres et al. (2) and are indicated by arrows below a physical map with key restriction enzymes. A dark box indicates the position of the homologous region hr1. Restriction sites are abbreviated as follows: HIII, HindIII; BII, BglII; BHI, BamHI; PI, PstI; XI, XhoI; MI763, MscI763. Solid lines indicate plasmids, which augment IE1-induced apoptosis. Thin lines at the bottom indicate clones of other portions of the region which did not augment IE1-induced apoptosis. (B) Percentage of SF-21 cells undergoing apoptosis by 18 h posttransfection with pBs-IE1/HC and different subclones of the AcMNPV HindIII-F fragment. pCAPCAT was used as a negative control of apoptotic activity (0% of apoptosis) and to balance total plasmid DNA levels to 2.0 μg of plasmid DNA in each transfection. The data represent the averages of two or more experiments, and standard errors are indicated. (C) DNA fragmentation pattern of transfected cells. Total cellular DNA was extracted from SF-21 cells cotransfected with the indicated plasmids and subjected to 1.2% agarose gel electrophoresis. DNA was visualized by ethidium bromide staining. The pCAPCAT-transfected cells served as a negative control for apoptosis. The positions of DNA molecular weight markers (sizes in kilobase pairs) are indicated at the right by arrowheads.
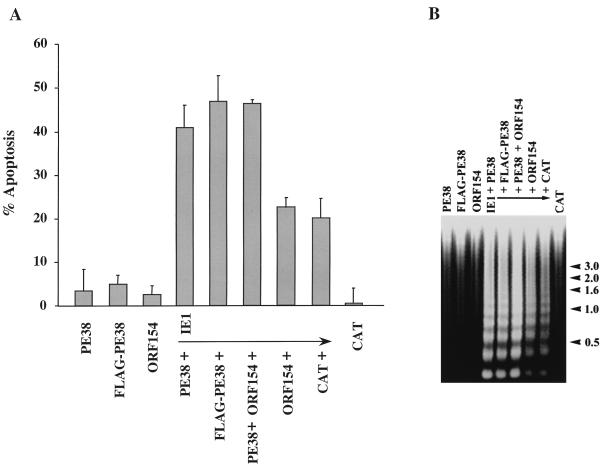
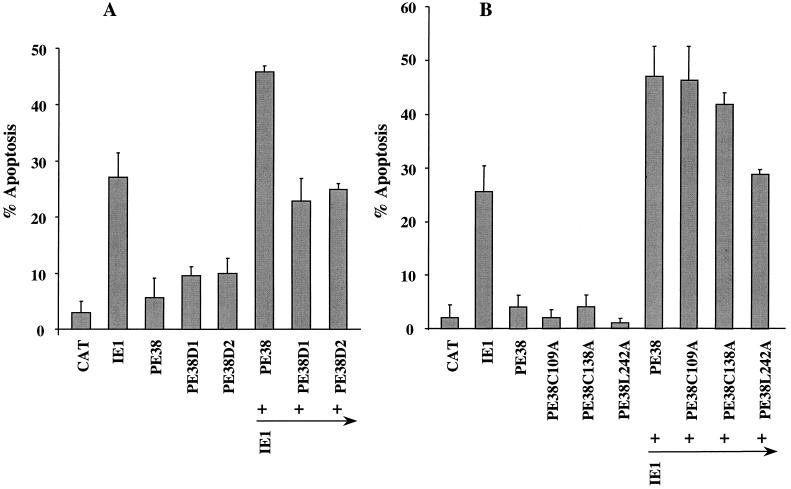
FIG. 2.
Augmentation of IE1-induced apoptosis by PE38. (A) Cells were transfected with plasmids expressing pe38, a FLAG-tagged version of pe38, and orf154, or they were cotransfected with a plasmid expressing an epitope-tagged version of ie-1 and plasmids expressing cat, pe38, FLAG-pe38, orf154, or a combination of pe38 and orf154. All genes were under hsp70 promoter control. At 12 h after induction of gene expression by heat shock, the cells were stained with trypan blue to determine viability. Cells transfected with pHSP70CATPLVI+, a plasmid expressing the cat gene, served as a negative control of apoptotic activity (percentage of apoptosis). The results represent averages of at least three independent experiments, and standard errors are indicated. (B) Cells were transfected with plasmids expressing the hsp70-promoted genes indicated above each lane. At 18 h posttransfection, gene expression was induced by heat shock. Three hours after heat shock, cells were harvested and oligonucleosomal degradation analysis was performed as described previously (7). DNA molecular markers (sizes in kilobase pairs) are indicated at right.
By 24 h posttransfection, SF-21 cells transfected with pBs-IE1/HC and pCAPCAT exhibited characteristic features of apoptosis including membrane blebbing, apoptotic body formation (reference 35 and data not shown), and oligonucleosomal ladder formation (Fig. 1C). No signs of apoptosis were observed in cells transfected with pBs-H3F only, and there was no detectable oligonucleosomal ladder formation, similar to control pCAPCAT-transfected cells. Cells cotransfected with both pBs-IE1/HC and pBs-H3F had an increased level of DNA degradation compared to that in cells cotransfected with pBs-IE1/HC and pCAPCAT (Fig. 1C).
To further define the gene or genes within the HindIII-F fragment that were responsible for the increase in the levels of apoptosis, several subclones of HindIII-F were analyzed. Only those clones spanning PE38 (Fig. 1A) showed increased levels of apoptosis in the presence of IE1: cotransfection of pBs-IE1/HC with pBs-PE38hr or pBs-PE38 resulted in approximately twofold increases in the percentage of cells undergoing apoptosis and the levels of oligonucleosomal DNA compared to those for the cells transfected with pBs-IE1/HC and control pCAPCAT (Fig. 1B and C). No signs of apoptosis were observed when SF-21 cells were transfected with pBs-PE38hr or pBs-PE38 alone (Fig. 1B and C).
The overlapping regions of pBs-PE38hr and pBs-PE38 contain two complete ORFs, pe38 and orf154. To determine which of these genes was responsible for the increase in apoptosis, we constructed plasmids pHSPE38 and pHSORF154 containing the PCR-amplified coding sequences of pe38 and orf154, respectively, under the transcriptional control of the Drosophila hsp70 promoter. This promoter is constitutively active in SF-21 cells and can be further induced by heat shock treatment (31). Little or no apoptotic activity was detected when SF-21 cells were transfected with pHSPE38 or pHSORF154 alone (Fig. 2), whereas 20% of the cells transfected with pHSEpi-IE1, a plasmid expressing ie1 under hsp70 promoter control, underwent apoptosis (Fig. 2A) and oligonucleosomal ladder formation was observed (Fig. 2B). Coexpression of IE1 with PE38 increased the levels of apoptosis twofold (Fig. 2A) and increased the level of oligonucleosomal DNA (Fig. 2B). Similar results were obtained in cells coexpressing IE1 and FLAG-PE38, which contains an amino-terminal FLAG tag. No increase in the number of apoptotic cells was observed when IE1 was coexpressed with ORF154 (Fig. 2A). These results indicated that PE38, not ORF154, increased IE1-induced apoptosis in SF-21 cells.
Mutational analysis of PE38.
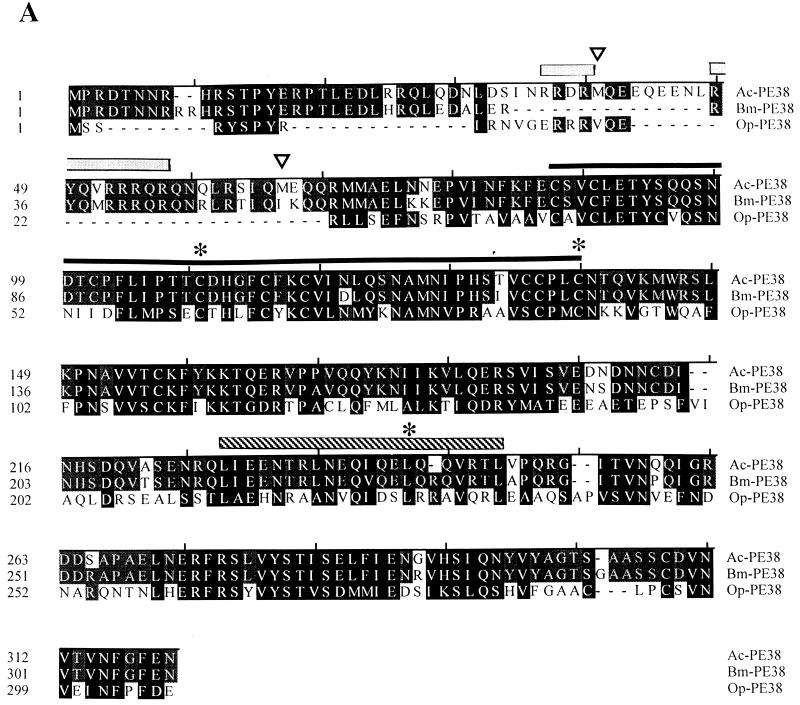
To determine whether specific sequence motifs of PE38 were required for PE38 activity in increasing the levels of IE1-induced apoptosis, we constructed several mutants of pe38 under hsp70 promoter control. Alignment of the sequences of the three known baculovirus PE38 homologs (Fig. 3A) revealed that the arginine-rich N-terminal region of PE38 from AcMNPV and B. mori NPV is largely missing in the PE38 of OpMNPV although two arginine-containing sequences, RYSPYR and RRRVQER, were found. To assess the contribution of the N terminus of PE38 of AcMNPV, two N-terminal deletion mutants, PE38D1 and PE38D2, lacking the first 38 and 64 N-terminal amino acids, respectively, were constructed. The D1 mutant eliminates the sequence RSTPYER while D2 eliminates both RSTPYER and an arginine-rich sequence including RRRQR (Fig. 3A). In addition, mutants PE38C109A and PE38C138A, with alanine substitutions at conserved cysteine residues (Fig. 3A), were constructed to determine the possible involvement of the conserved RING finger motif while mutant PE38L242A tested the contribution of the conserved leucine zipper domain (Fig. 3A) to PE38 proapoptotic activity.
FIG. 3.
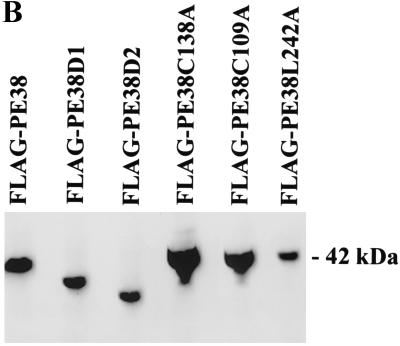
Nature and expression of PE38 mutants. (A) Alignment of the PE38 sequences of AcMNPV, B. mori NPV, and OpMNPV. Solid boxes indicate similar amino acids present in all three proteins, shaded boxes indicate similar amino acids present in two of the three proteins, and dashes indicate gaps introduced for optimal alignment. Two arginine-rich regions are indicated with gray overlining. Black and striped overlinings indicate the RING finger motif and leucine zipper, respectively. Open arrowheads show the positions of the PE38D1 and PE38D2 truncated mutants, each initiating at an existing methionine. The asterisks denote amino acid changes made in the conserved residues within the RING finger or leucine zipper domains in mutational analysis of PE38. (B) Immunoblot analysis of the expression of the PE38 mutants. SF-21 cells transfected for 18 h with plasmids expressing genes indicated above each lane were heat shocked to induce gene expression. Cells were harvested 3 h after heat shock. Equal amounts of cell lysates were analyzed by Western blot analysis with anti-FLAG monoclonal antibody. The position of wild-type FLAG-tagged PE38 (42 kDa) is shown on the right.
Since the presence of an amino-terminal FLAG tag did not significantly affect the ability of PE38 to increase the levels of IE1-induced apoptosis (Fig. 2), all PE38 mutants were N-terminally FLAG tagged and tested for expression in SF-21 cells. Expression of FLAG-tagged PE38 was strong at 3 h after heat shock induction, and comparable or slightly lower levels of expression were observed for the FLAG-PE38D1, FLAG-PE38D2, and FLAG-PE38L242A mutants (Fig. 3B). FLAG-PE38C109A and FLAG-PE38C138A, containing alanine in place of conserved cysteine 109 or 138 of the PE38 RING finger motif, had significantly higher levels of expression than did the wild-type FLAG-PE38, suggesting that mutations in the RING finger increase PE38 expression or stability.
Neither of the N-terminal deletion mutants, FLAG-PE38D1 and FLAG-PE38D2, augmented the levels of IE1-induced apoptosis (Fig. 4A), suggesting that the amino terminus of PE38 is important for its ability to accentuate IE1-induced apoptosis. FLAG-PE38C138A, containing cysteine in place of C138 in the RING finger motif, increased IE1-induced apoptosis to levels comparable to that observed for wild-type FLAG-PE38 (Fig. 4B). Similarly, mutation of C109, another conserved cysteine residue of the RING finger motif, had no observable effect on the ability of PE38 to increase IE1-induced apoptosis. Thus, the RING finger motif does not appear to be essential for this activity of PE38. When L242, a conserved leucine residue of the PE38 leucine zipper domain, was mutated to alanine, the ability of PE38 to increase the levels of IE1-induced apoptosis was severely impaired (Fig. 4B). Thus, the ability of PE38 to increase the levels of IE1-induced apoptosis appears to depend on the integrity of the N-terminal region and leucine zipper but not the integrity of the RING finger.
FIG. 4.
The effect of PE38 mutants on IE1-induced apoptosis. Cells were cotransfected with epitope-tagged version of the genes indicated below each bar. (A) Comparison of wild-type PE38 with truncated forms of PE38 (PE38D1 and PE38D2); (B) comparison of wild-type PE38 with mutants containing point mutations in the RING finger or leucine zipper. Cells were harvested 12 h after heat shock induction, and cell viability was determined by trypan blue staining (7).
Effect of mutations on PE38 subcellular location.
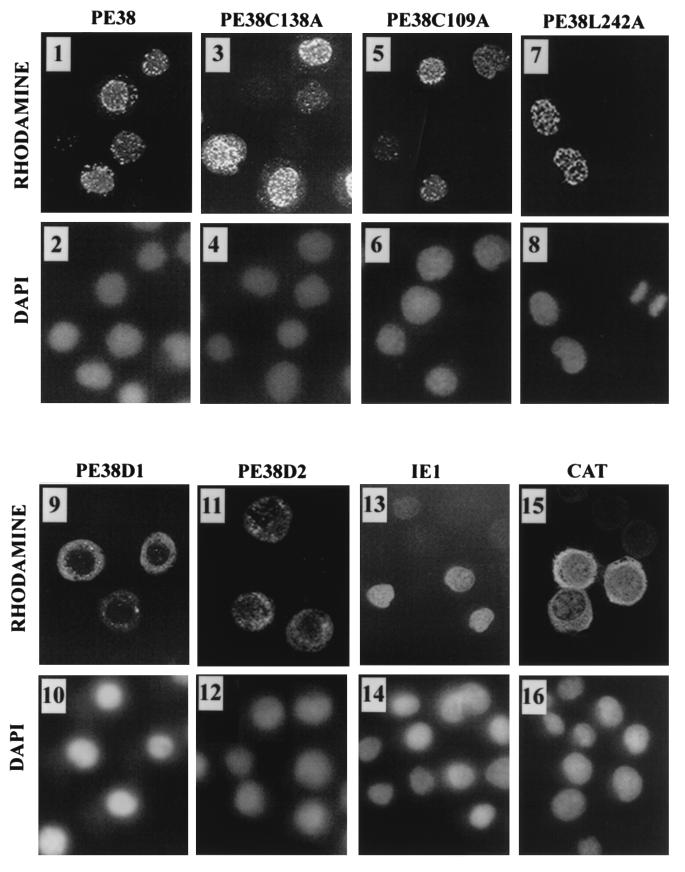
To further define the effect of different PE38 mutations, we compared the subcellular localization of the mutants with wild-type FLAG-tagged PE38. Wild-type PE38 localized predominantly to the nucleus in a punctate pattern (Fig. 5, panels 1 and 2), similar to the pattern previously observed for wild-type PE38 expressed under the transcriptional control of its own promoter (21). The same punctate nuclear localization was observed for FLAG-PE38C109A, FLAG-PE38C138A, and FLAG-PE38L242A (Fig. 5, panels 3 to 8). However, deletions of the N-terminal region altered PE38’s location. FLAG-PE38D1 was localized predominantly to the cytoplasm of the cells (Fig. 5, panels 9 and 10). FLAG-tagged PE38D2 displayed more dispersed localization, although the major portion of the protein was localized to the cytoplasm (Fig. 5, panels 11 and 12). Thus, the N-terminal region of PE38, including the first 38 amino acids, affects the nuclear localization of the protein. Epitope-tagged IE1 and CAT were used as controls for nuclear and cytoplasmic localization, respectively (16, 42). By 3 h after heat shock, Epi-IE1, an HA.11 epitope-tagged version of IE1, was localized to the nucleus of the cells. Coexpression with Epi-IE1 did not affect the subcellular localization of FLAG-tagged wild type or mutants of PE38 (data not shown).
FIG. 5.
Subcellular localization of PE38 mutants. SF-21 cells were transiently transfected with plasmids expressing FLAG-tagged PE38 or FLAG-tagged PE38 mutants. Epitope-tagged versions of IE1 and CAT served as controls for nuclear and cytoplasmic localization, respectively. After 18 h, gene expression was induced by heat shock. At 3 h after heat shock, cells were fixed in methanol and analyzed by indirect immunofluorescence. FLAG-PE38 and FLAG-tagged PE38 mutants were visualized with mouse M2 anti-FLAG monoclonal antibody and lissamine rhodamine-conjugated goat anti-mouse IgG-IgM antibodies (panels 1, 3, 5, 7, 9, and 11). Mouse anti-HA.11 monoclonal antibody was used to localize hemagglutinin epitope-tagged IE1 and CAT followed by incubation with lissamine rhodamine-conjugated anti-mouse IgG-IgM antibody (panels 13 and 15). Nuclei were visualized by staining with DAPI (panels 2, 4, 6, 8, 10, 12, 14, and 16). Micrographs of immunofluorescence and DAPI staining were taken from the same fields of cells.
DISCUSSION
We have examined the AcMNPV genome for genes that can induce apoptosis. In a previous study, we determined whether any clones within an overlapping set of genomic clones of AcMNPV could induce apoptosis and discovered that ie-1 was able to induce apoptosis when transiently expressed individually (35). However, the study was incomplete because many viral genes depend on ie-1 for strong expression in a transient expression assay. Thus, we screened the genomic library for clones that could augment apoptosis in the presence of ie-1. We found only one gene, pe38, which exhibited this type of activity. It is likely that we have surveyed all of the early viral genes in these two studies. Since late viral genes depend on many other viral factors for strong expression in transient expression systems, there may still be additional viral genes which induce apoptosis when individually expressed under a constitutive promoter or when expressed in combination with other genes. However, as with all viral genes found to be proapoptotic when transiently overexpressed, it will be important to establish the relevance of PE38 to the induction of apoptosis during infection.
Augmentation of IE1-induced apoptosis by pe38 does not appear to be due to pe38 regulation of ie-1 expression. Both the ie-1 and pe38 promoters are expressed immediately after infection (15, 20) and are constitutive in the absence of other viral factors (15, 28). When placed under the transcriptional control of the hsp70 promoter, both genes exerted activities similar to those observed under their own promoters. In addition, pe38 did not influence ie-1 expression from the hsp70 promoter nor did ie-1 influence the level of pe38 expression from the hsp70 promoter (reference 37 and data not shown). Although PE38 was expressed at a high level from the hsp70 promoter, it did not induce apoptosis in the absence of IE1. Thus, PE38 is unable to induce apoptosis directly but augments IE1-induced apoptosis.
The mechanism by which PE38 augments IE1-induced apoptosis remains unclear. Full-length PE38 is known to localize in a punctate pattern in the nucleus of transfected or infected cells (21). However, during the late phase of infection, levels of the 38-kDa polypeptide decline and a smaller polypeptide (20 kDa) which cross-reacts with PE38 antiserum is observed only in the cytoplasmic fraction (21). We have observed that two N-terminally truncated mutants which delete a putative nuclear localization signal were cytoplasmically localized and unable to enhance the level of IE1-induced apoptosis, suggesting that nuclear localization of PE38 may be important for this activity. Replacement of the PE38 nuclear localization signal with an alternate nuclear localization signal is necessary to determine whether nuclear localization is the primary property of PE38 which is affected by these mutations. The twofold reduction in the level of these PE38 mutants may also affect their proapoptotic activity but would not be expected to entirely eliminate this activity as observed. The pe38 gene may be multifunctional, and our results indicate that its truncated cytoplasmic product will have different functional properties than those of the full-length nuclear form.
The leucine zipper may also be important for the proapoptotic activity of PE38. Leucine zippers are usually involved in homo- or heterodimerization of proteins, and the observation that this motif of PE38 is required for its activity suggests that the ability of PE38 to interact with itself or another protein is important for this function, although no such interaction has been described yet. Studies looking at PE38 interaction with other proteins have been hampered by its insolubility (38). It seems unlikely that PE38 interacts with IE1, since the nuclear distribution of IE1 is more uniform than that of PE38 and IE1 does not have a leucine zipper, although other interactions may occur.
Mutation of the RING finger motif appeared to have little or no effect on the ability of PE38 to augment IE1-induced apoptosis. The role of RING fingers in protein function remains unclear, although it has been proposed from structural studies that RING fingers may be protein interaction domains. RING mutations increase the level of PE38, substantially suggesting that the RING may decrease PE38 stability. However, increased levels of PE38 do not increase the level of IE1-mediated apoptosis, suggesting that only low levels of PE38 are required for the observed effect or that 50% apoptosis is the maximum effect that can be observed under these conditions.
Although we have found that IE1 induces apoptosis and PE38 augments this effect in transient expression assays, there are still many unanswered questions regarding the mechanism by which baculoviruses induce apoptosis. LaCount and Friesen (22) concluded that early gene expression was sufficient for apoptosis since infection with a temperature-sensitive mutant defective in viral DNA replication results in some apoptosis. Since both PE38 and IE1 are present immediately following infection and in the presence of aphidicolin, these could suffice to induce apoptosis. However, DNA replication is required for a full apoptotic response (6, 22). It is possible that IE1 and PE38, which are known transactivators of gene expression, modify the cellular environment to be conducive for viral replication, but as a result, the cell is precariously poised for apoptosis. Cellular proliferation pathways are intimately entwined with apoptotic pathways sensitive to DNA damage (10), and baculoviruses may need to engage features of proliferative pathways to replicate in a timely and efficient manner. Subsequent DNA replication, viral or host, could be interpreted by the cell under these conditions as DNA damage or unscheduled DNA synthesis, resulting in induction of apoptosis. Alternately, events subsequent to DNA replication such as the shutoff of host RNA synthesis might also trigger apoptosis. Antiapoptotic genes such as p35 or iap genes thus become essential in order to prevent the cell from dying prematurely and thereby aborting infection. The steps connecting IE1 and PE38 expression with the activation of caspases will be important to establish in order to understand the reason for induction of apoptosis by DNA-containing viruses and the role of iap genes in blocking caspase activation.
ACKNOWLEDGMENT
This research was supported in part by Public Health Service grant AI23719 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ahmad M, Srinivasula S M, Wang L, Litwack G, Fernandes-Alnemri T, Alnemri E S. Spodoptera frugiperda caspase-1, a novel insect death protease that cleaves the nuclear immunophilin FKBP48. J Biol Chem. 1997;272:1421–1424. doi: 10.1074/jbc.272.3.1421. [DOI] [PubMed] [Google Scholar]
- 2.Ayres M D, Howard S C, Kuzio J, Lopez-Ferber M, Possee R D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- 3.Bertin J, Mendrysa S M, LaCount D J, Gaur S, Krebs J F, Armstrong R C, Tomaselli K J, Friesen P D. Apoptotic suppression by baculovirus p35 involves cleavage by an inhibition of a virus-induced CED-3/ICE-like protease. J Virol. 1996;70:6251–6259. doi: 10.1128/jvi.70.9.6251-6259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnbaum M J, Clem R J, Miller L K. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Virol. 1994;68:2521–2528. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, Licari P, Mancovich J, Shi L, Greenberg A, Miller L K, Wong W W. Inhibition of ICE family proteases by baculovirus anti-apoptotic protein P35. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 6.Clem R J, Fechheimer M, Miller L K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 7.Clem R J, Miller L K. Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol. 1994;14:5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clem R J. Regulation of programmed cell death by baculoviruses. In: Miller L K, editor. The baculoviruses. New York, N.Y: Plenum Press; 1997. pp. 237–261. [Google Scholar]
- 9.Crook N E, Clem R J, Miller L K. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes-Alnemri T, Armstrong R C, Krebs J, Srinivasula S M, Litwak G, Alnemri E S. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friesen P D, Miller L K. Divergent transcription of early 35- and 94-kilodalton protein genes encoded by the HindIII K genome fragment of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol. 1987;61:2264–2272. doi: 10.1128/jvi.61.7.2264-2272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friesen P D. Regulation of baculovirus early gene expression. In: Miller L K, editor. The baculoviruses. New York, N.Y: Plenum Press; 1997. pp. 141–170. [Google Scholar]
- 14.Guarino L A, Summers M D. Interspersed homologous DNA of Autographa californica nuclear polyhedrosis virus enhances delayed-early gene expression. J Virol. 1986;60:215–223. doi: 10.1128/jvi.60.1.215-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarino L A, Summers M D. Nucleotide sequence and temporal expression of a baculovirus regulatory gene. J Virol. 1987;61:2091–2099. doi: 10.1128/jvi.61.7.2091-2099.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvey A J, Bidwai A P, Miller L K. Doom, a product of the Drosophila mod(mdg4) gene, induces apoptosis and binds to baculovirus inhibitor-of-apoptosis proteins. Mol Cell Biol. 1997;17:2835–2843. doi: 10.1128/mcb.17.5.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershberg P A, Dickson J A, Friesen P D. Site-specific mutagenesis of the 35-kilodalton protein gene encoded by Autographa californica nuclear polyhedrosis virus: cell line-specific effects on virus replication. J Virol. 1992;66:5525–5533. doi: 10.1128/jvi.66.9.5525-5533.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamita S G, Majima K, Maeda S. Identification and characterization of the p35 gene of Bombyx mori nuclear polyhedrosis virus that prevents virus-induced apoptosis. J Virol. 1993;67:455–463. doi: 10.1128/jvi.67.1.455-463.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kool M, Ahrens C, Goldbach R W, Rohrmann G F, Vlak J M. Identification of genes involved in DNA replication of the Autographa californica baculovirus. Proc Natl Acad Sci USA. 1994;91:11212–11216. doi: 10.1073/pnas.91.23.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krappa R, Knebel-Mörsdorf D. Identification of the very early transcribed baculovirus gene PE-38. J Virol. 1991;65:805–812. doi: 10.1128/jvi.65.2.805-812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krappa R, Roncarati R, Knebel-Mörsdorf D. Expression of PE38 and IE2, viral members of the C3HC4 finger family, during baculovirus infection: PE38 and IE2 localize to distinct nuclear regions. J Virol. 1995;69:5287–5293. doi: 10.1128/jvi.69.9.5287-5293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaCount D J, Friesen P D. Role of early and late replication events in induction of apoptosis by baculoviruses. J Virol. 1997;71:1530–1537. doi: 10.1128/jvi.71.2.1530-1537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Bergeron L, Cryns V, Pasternack M S, Zhu H, Shi L, Greenberg A, Yuan J. Activation of caspase-2 in apoptosis. J Biol Chem. 1997;272:21010–21017. doi: 10.1074/jbc.272.34.21010. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Nijhawan D, Budihardjio I, Srinavasula S M, Ahmad M, Alnemri E S, Wang X. Cytochrome c and ATP-dependent formation of Apaf-1/caspase-9 complex initiates in apoptotic cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 25.Lu A, Carsten E B. Immediately-early baculovirus genes transactivate the p143 promoter of Autographa californica nuclear polyhedrosis virus. Virology. 1993;195:710–718. doi: 10.1006/viro.1993.1422. [DOI] [PubMed] [Google Scholar]
- 26.Lu A, Miller L K. Regulation of baculovirus late and very late gene expression. In: Miller L K, editor. The baculoviruses. New York, N.Y: Plenum Press; 1997. pp. 193–216. [Google Scholar]
- 27.Manji G A, Hozak R R, LaCount D J, Friesen P D. Baculovirus inhibitor of apoptosis functions at or upstream of the apoptotic suppressor P35 to prevent programmed cell death. J Virol. 1997;71:4509–4516. doi: 10.1128/jvi.71.6.4509-4516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mans R M, Knebel-Mörsdorf D. In vitro transcription of pe38/polyhedrin hybrid promoters reveals sequences essential for recognition by the baculovirus-induced RNA polymerase and for strength of very late viral promoters. J Virol. 1998;72:2991–2998. doi: 10.1128/jvi.72.4.2991-2998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy J V, Dixit V M. Apoptosis induced by Drosophila reaper and grim in a human system: attenuation by inhibitor of apoptosis proteins. J Biol Chem. 1998;273:24009–24015. doi: 10.1074/jbc.273.37.24009. [DOI] [PubMed] [Google Scholar]
- 30.Miller L K, Jewell J E, Browne D. Baculovirus induction of a DNA polymerase. J Virol. 1981;40:305–308. doi: 10.1128/jvi.40.1.305-308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris T D, Miller L K. Promoter influence of baculovirus-mediated gene expression in permissive and nonpermissive insect cell lines. J Virol. 1992;66:7397–7405. doi: 10.1128/jvi.66.12.7397-7405.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Reilly D A, Miller L K, Luckow V A. Baculovirus expression vectors: a laboratory manual. W. H. New York, N.Y: Freeman and Company; 1992. [Google Scholar]
- 33.Passarelli A L, Miller L K. Three baculovirus genes involved in late and very late gene expression: ie-1, ie-n, and lef-2. J Virol. 1993;67:2149–2158. doi: 10.1128/jvi.67.4.2149-2158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passarelli A L, Miller L K. In vivo and in vitro analysis of recombinant baculoviruses lacking a functional cg30 gene. J Virol. 1994;68:1186–1190. doi: 10.1128/jvi.68.2.1186-1190.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prikhod’ko E A, Miller L K. Induction of apoptosis by baculovirus transactivator IE1. J Virol. 1996;70:7116–7124. doi: 10.1128/jvi.70.10.7116-7124.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prikhod’ko E A, Miller L K. Role of baculovirus IE2 and its RING finger in cell cycle arrest. J Virol. 1998;72:684–692. doi: 10.1128/jvi.72.1.684-692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prikhod’ko, E. A., and L. K. Miller. Unpublished data.
- 38.Prikhod’ko G G, Wang Y, Freulich E, Prives C, Miller L K. Baculovirus p33 binds human p53 and enhances p53-mediated apoptosis. J Virol. 1999;73:1227–1234. doi: 10.1128/jvi.73.2.1227-1234.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rapp J C, Wilson J A, Miller L K. Nineteen baculovirus reading frames, including LEF-12, support late gene expression. J Virol. 1998;72:10197–10206. doi: 10.1128/jvi.72.12.10197-10206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothe M, Pan M G, Henzel W J, Ayres T M, Goeddel D V. The TNFR2-TRAF signaling complex contains 2 novel proteins related to baculoviral-inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 41.Seshagiri S, Miller L K. Baculovirus inhibitors of apoptosis (IAPs) block activation of Sf-caspase-1. Proc Natl Acad Sci USA. 1997;94:13606–13611. doi: 10.1073/pnas.94.25.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slack J M, Blissard G W. Identification of two independent transcriptional activation domains in Autographa californica multicapsid nuclear polyhedrosis virus IE1 protein. J Virol. 1997;71:9579–9587. doi: 10.1128/jvi.71.12.9579-9587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toeroek L, Karch F. Nucleotide sequences of heat shock activated genes in Drosophila melanogaster. I. Sequences in the regions of the 5′ and 3′ ends of the hsp70 gene in the hybrid plasmid 56h8. Nucleic Acids Res. 1980;8:3105–3123. doi: 10.1093/nar/8.14.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaughn J L, Goodwin R H, Tompkins G J, McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera: Noctuidae) In Vitro. 1977;13:213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- 45.Vucic D, Seshagiri S, Miller L K. Characterization of reaper- and FADD-induced apoptosis in a lepidopteran cell line. Mol Cell Biol. 1997;17:2835–2843. doi: 10.1128/mcb.17.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vucic D, Kaiser W J, Harvey A J, Miller L K. Inhibition of reaper-induced apoptosis by interaction with inhibitor of apoptosis proteins (IAPs) Proc Natl Acad Sci USA. 1997;94:10183–10188. doi: 10.1073/pnas.94.19.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vucic D, Kaiser W J, Miller L K. Inhibitor of apoptosis proteins physically interact with and block apoptosis induced by Drosophila proteins HID and GRIM. Mol Cell Biol. 1998;18:3300–3309. doi: 10.1128/mcb.18.6.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C-Y, Mayo M W, Korneluk R G, Goeddel B V, Baldwin A S., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and cIAP1 and cIAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]