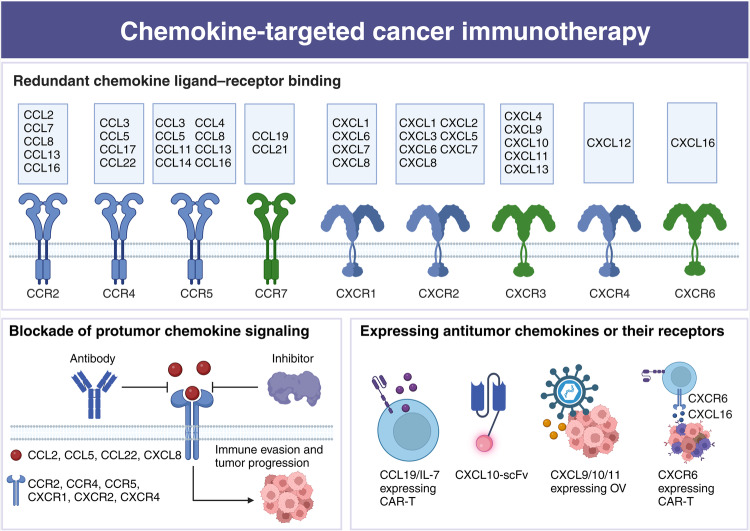
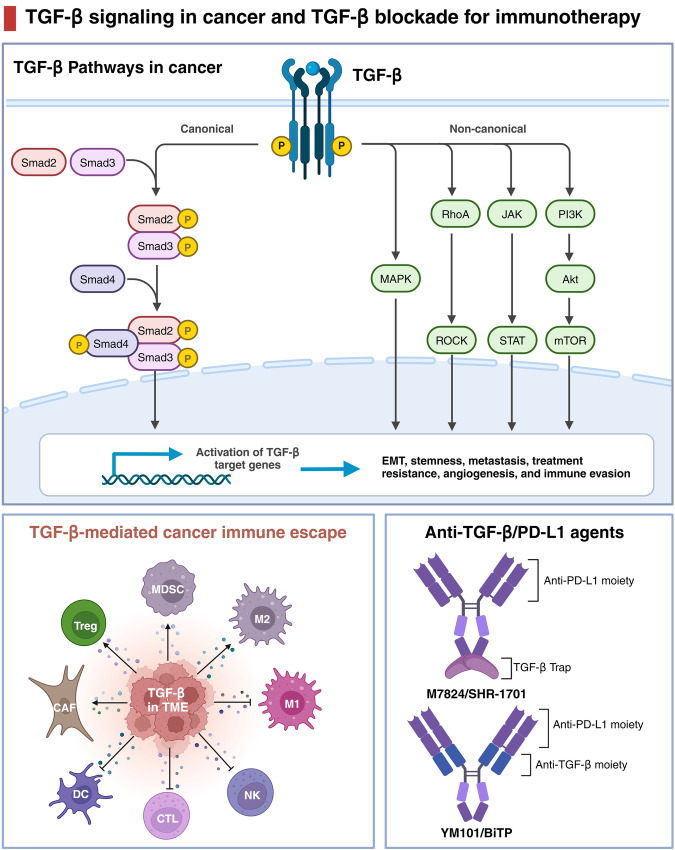
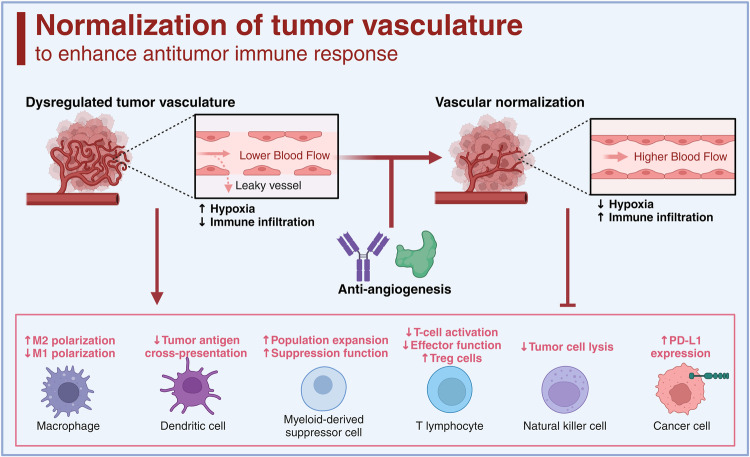
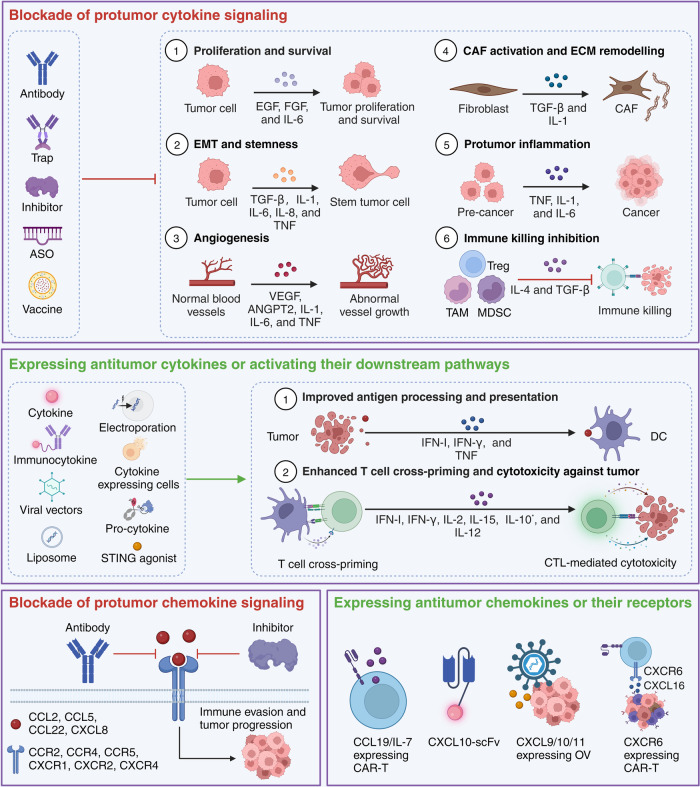
Abstract
Cytokines are critical in regulating immune responses and cellular behavior, playing dual roles in both normal physiology and the pathology of diseases such as cancer. These molecules, including interleukins, interferons, tumor necrosis factors, chemokines, and growth factors like TGF-β, VEGF, and EGF, can promote or inhibit tumor growth, influence the tumor microenvironment, and impact the efficacy of cancer treatments. Recent advances in targeting these pathways have shown promising therapeutic potential, offering new strategies to modulate the immune system, inhibit tumor progression, and overcome resistance to conventional therapies. In this review, we summarized the current understanding and therapeutic implications of targeting cytokine and chemokine signaling pathways in cancer. By exploring the roles of these molecules in tumor biology and the immune response, we highlighted the development of novel therapeutic agents aimed at modulating these pathways to combat cancer. The review elaborated on the dual nature of cytokines as both promoters and suppressors of tumorigenesis, depending on the context, and discussed the challenges and opportunities this presents for therapeutic intervention. We also examined the latest advancements in targeted therapies, including monoclonal antibodies, bispecific antibodies, receptor inhibitors, fusion proteins, engineered cytokine variants, and their impact on tumor growth, metastasis, and the tumor microenvironment. Additionally, we evaluated the potential of combining these targeted therapies with other treatment modalities to overcome resistance and improve patient outcomes. Besides, we also focused on the ongoing research and clinical trials that are pivotal in advancing our understanding and application of cytokine- and chemokine-targeted therapies for cancer patients.
Subject terms: Cancer microenvironment, Cancer therapy
Introduction
Cytokines, which are typically polypeptides or glycoproteins with relatively small molecular weights (usually in the range of 6 to 70 kDa), regulate the functions, differentiation, proliferation, apoptosis, and survival of their target cells.1 When cytokines bind to receptors on target cells, they trigger intracellular signaling pathways to modulate gene transcription, thereby modifying various biological activities. Target cells expressing specific sets of receptors interpret the information from different cytokines based on their concentration and timing of exposure.2 Diverse classes of cytokines, including interferons (IFNs), interleukins (ILs), tumor necrosis factor (TNF) superfamily, chemokines, and growth factors, play pivotal roles in homeostasis and diseases.3 It is well-established that an imbalanced cytokine profile contributes to cancer initiation and progression by inciting chronic inflammation and immune evasion (Fig. 1).4 Consequently, the manipulation or neutralization of abnormal cytokines in the tumor microenvironment (TME) presents a promising approach for the treatment of cancer patients.5,6
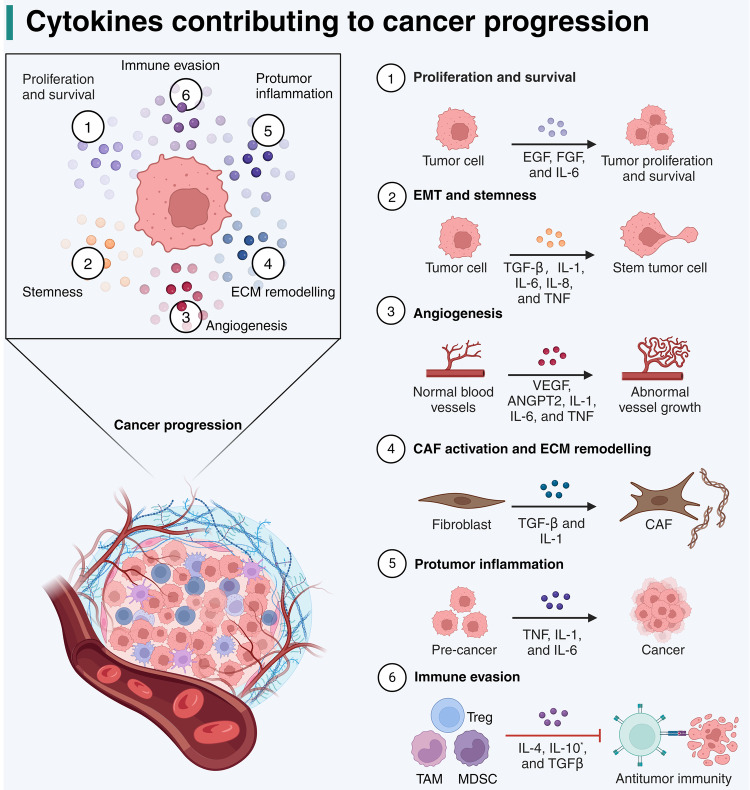
Fig. 1.
Mechanisms of action of cytokines contributing to cancer progression. This figure illustrates the multifaceted roles of cytokines in cancer. The central diagram shows a tumor microenvironment with key processes labeled 1 through 6, indicating different aspects of cancer progression influenced by cytokines. Firstly, cytokines such as EGF, FGF, and IL-6 promote the proliferation and survival of tumor cells. Secondly, TGF-β, IL-1, IL-6, IL-8, and TNF contribute to the epithelial-mesenchymal transition (EMT) and maintenance of stemness in tumor cells, facilitating a more invasive phenotype. Thirdly, VEGF, ANGPT2, IL-1, IL-6, and TNF drive the formation of new blood vessels (angiogenesis), supplying the tumor with nutrients and oxygen. Moreover, TGF-β and IL-1 are involved in activating fibroblasts to cancer-associated fibroblasts (CAFs) and in extracellular matrix (ECM) remodeling, which promotes tumor immune evasion and treatment resistance. Fifthly, proinflammatory cytokines like TNF, IL-1, and IL-6 create the dysregulated inflammation that can support tumor development and progression. Lastly, anti-inflammatory cytokines including IL-4, IL-10, and TGF-β are implicated in the suppression of CD8+ T cell activity and the accumulation of regulatory T cells (Treg), myeloid-derived suppressor cells (MDSC), and tumor-associated macrophages (TAM), which help the tumor evade immune surveillance. Notably, IL-10 generally suppresses immune response, but some studies suggest that it promotes the activation of tumor-resident CD8+ T cells. Adapted from “The Tumor Microenvironment: Overview of Cancer-Associated Changes”, by BioRender.com (2024). Retrieved from https://app.biorender.com/biorender-templates
Several cytokines, including IFN-α, IFN-γ, IL-2, IL-12, IL-15, and granulocyte-macrophage colony-stimulating factor (GM-CSF), exhibit antitumor properties in preclinical models.7 These cytokines slow tumor growth either by directly inhibiting proliferation and promoting apoptosis, or indirectly by mobilizing an antitumor immune response. For example, IFN-α, originally recognized for its capacity to interfere with viral replication, was discovered to possess antitumor potential five decades ago.8 It is now widely accepted that IFN-α not only exerts cytostatic, cytotoxic, and anti-angiogenic effects on tumors but also enhances tumor antigen presentation, primes and activates T cells, boosts the cytotoxic activity of natural killer (NK) cells, improves the maturation and functions of dendritic cells (DCs), and reduces the accumulation of regulatory T cells (Tregs) (Fig. 2).9 The positive outcomes in preclinical studies have fostered exploration into employing these cytokine-based immunotherapies for patients with solid and hematologic malignancies. Currently, the Food and Drug Administration (FDA) has granted approval for IFN-α and IL-2 in the treatment of a wide spectrum of cancers, including melanoma, follicular lymphoma, hairy cell leukemia, acquired immunodeficiency syndrome (AIDS)-associated Kaposi’s sarcoma, and renal cell carcinoma.10–15 Nevertheless, in clinical practice, these cytokines have largely been superseded by alternative immunotherapies, particularly immune checkpoint blockade (ICB), which offers superior efficacy and more favorable safety profiles.16–18 Nonetheless, the potential of combining cytokines with other immunotherapies, along with advances in drug delivery and protein engineering, has reignited interest in cytokines as agents against cancer.19
Fig. 2.
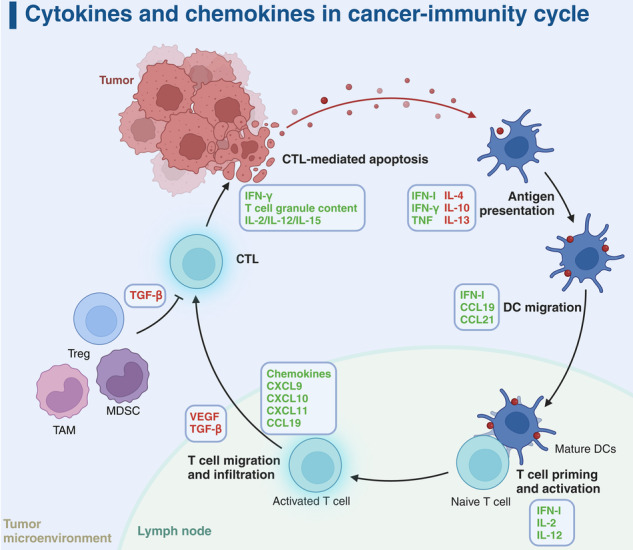
Cytokine dynamics in the cancer-immunity cycle. The figure presents a comprehensive view of cytokine interactions within the cancer-immunity cycle, illustrating the dual role of cytokines in both tumor suppression and promotion. Key features include the promotion of cytotoxic T lymphocyte (CTL)-mediated apoptosis by IFN-γ and various interleukins (IL-2, IL-12, IL-15) within the tumor microenvironment. In contrast, regulatory elements such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) secrete IL-10 and transforming growth factor-beta (TGF-β) to mitigate CTL efficacy and assist in immune evasion. The lymph node emerges as a pivotal site for antigen presentation by dendritic cells (DCs), orchestrated by a suite of cytokines including type I interferon (IFN-I), IFN-γ, tumor necrosis factor (TNF), along with IL-4, IL-10, and IL-13. DC migration to lymph nodes, necessary for T cell priming and activation, is enhanced by IFN-I, chemokine (C-C motif) ligand 19 (CCL19), and CCL21. Subsequently, activated T cells are drawn back to the tumor via a gradient of chemokines, including C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, CXCL11, and CCL19. Nonetheless, the tumor microenvironment, influenced by vascular endothelial growth factor (VEGF) and TGF-β, can counteract T cell infiltration and activation, underscoring the delicate equilibrium between immune defense and tumor immune evasion. Cytokines are distinctly labeled with red and green to denote their immunosuppressive and immunostimulatory functions for antitumor immunity, respectively. Adapted from “Tumor-Specific T Cell Induction and Function”, by BioRender.com (2024). Retrieved from https://app.biorender.com/biorender-templates
On the contrary, certain cytokines could be hijacked to facilitate cancer progression, such as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), TNF-α, IL-1β, IL-6, colony stimulating factor-1 (CSF-1), C-C motif chemokine ligand 2 (CCL2), CCL5, and C-X-C motif chemokine ligand 8 (CXCL8).20 These protumor cytokines actively contribute to various aspects of cancer development, such as growth, metastasis, extracellular matrix remodeling, immune evasion, and resistance to treatment.21 Consequently, the neutralization of these protumor cytokines or the blockade of their receptors could potentially enhance the effectiveness of cancer immunotherapy. Currently, several strategies for blocking these cytokines have been developed, encompassing neutralizing antibodies, bispecific antibodies, small-molecule inhibitors, cytokine traps, small interfering RNA (siRNA), and polypeptides.3 Some cytokine antagonists, like anti-TGF-β and anti-VEGF antibodies, have shown significant promise in augmenting various immunotherapies, particularly ICB, and alleviating treatment resistance.22,23 It is essential to note that most cytokines exhibit versatility, playing diverse roles during different stages of tumor development. As a result, precise patient selection is a crucial prerequisite for optimizing cytokine-targeted therapies. In this comprehensive review, we provide an overview of the role of cytokines in cancer progression, with a particular focus on their involvement in immune evasion. Additionally, we highlight combination strategies involving cytokines or their antagonists, drawing from both preclinical and clinical studies.
Interferons and their agonists
Type 1 IFN (IFN-I)
The biology of IFN-I
IFN-Is stand as a pivotal group of proteins central to the immune response to a wide array of challenges.24 Among these, subtypes like IFN-α and IFN-β interact with a receptor complex, IFNR, composed of IFNαR1 and IFNαR2. This interaction sets off a cascade of signaling events involving Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2), triggering the phosphorylation of signal transducer and activator of transcription (STAT)1 and STAT2. Beyond STAT1 and STAT2, IFN-Is also engage other Stat proteins, mitogen-activated protein kinases (MAPK), and phosphatidylinositide 3-kinase (PI3K), thereby activating various IFN regulatory factors (IRFs) and IFN-stimulated genes (ISGs).25 These processes create an inflammatory environment conducive to immune clearance.
In the context of cancer, IFN-Is have traditionally been viewed as beneficial, as they have shown the capacity to induce senescence, halt the cell cycle, and promote apoptosis in tumor cells, while also enhancing the antitumor T cell response.26 IFN-Is play a pivotal role in supporting cytotoxic T lymphocytes (CTLs) through various mechanisms.27 They enhance DC maturation, facilitate antigen presentation, and promote DC migration to lymph nodes, thereby enhancing cross-priming.28 IFN-Is augment the effector functions of immune cells, increase the expression of cytotoxic molecules, and facilitate the survival of memory CTLs.29–31 Additionally, they prevent the elimination of activated CTLs by NK cells, reduce the ratio of activating versus inhibitory NK cell receptor ligands expressed by CTLs, and stimulate the release of pro-inflammatory cytokines.32 Furthermore, IFN-Is curtail the number and functions of Tregs, partially by disturbing cyclic AMP expression.33
Notably, interferon epsilon (IFN-ε), a recently discovered member of the IFN-I family, has been identified as an intrinsic suppressor of ovarian cancer. Discovered later than other members of the IFN-I family, IFN-ε is uniquely characterized by its constitutive expression in the female reproductive tract, where it plays a crucial role in defending against sexually transmitted infections.34 Notably, IFN-ε expression decreases as ovarian cancer develops, underscoring its potential protective role against tumor progression.35 Detailed investigations into IFN-ε have shed light on its complex antitumor activities, which extend beyond its direct impact on tumor cells, including dose-dependent anti-proliferation and apoptosis induction.35 Critically, IFN-ε enhances antitumor immunity, evidenced by the activation of T cells and NK cells and the suppression of myeloid-derived suppressor cells (MDSCs) and Tregs.35
However, emerging evidence indicates that the impact of IFN-Is on cancer is complex and significantly influenced by the context. While acute and robust IFN-I responses, typically elicited by chemotherapy, radiation therapy, and targeted therapy, have been documented to suppress malignant cell proliferation, playing a crucial role in tumor immunosurveillance, the scenario drastically changes with persistent, weak, and chronic IFN-I signaling. Such prolonged activation paradoxically promotes tumorigenesis and treatment resistance through various cancer cell-intrinsic and immunological mechanisms.36 This dual effect mirrors observations in chronic viral infections where sustained IFN-I signaling not only fails in viral clearance but also shifts from immunostimulation to immunosuppression.
Early and adequate IFN-I production in tumors can stimulate DC activation and T-cell cross-priming within the TME, reinforcing antitumor immune responses. Conversely, suboptimal IFN-Is can inadvertently support cancer progression, notably by upregulating immunosuppressive molecules, including immune checkpoints, thus undermining the effectiveness of antitumor T-cell responses.37 Chronic IFN-I signaling further modifies the TME by inducing nitric oxide synthase 2 (NOS2) expression, which fosters the recruitment of MDSCs and Tregs, thereby amplifying local immunosuppression.38 Additionally, prolonged IFN-I exposure has been linked to increased IL-6 expression by tumor cells, a pro-inflammatory cytokine often associated with mechanisms that facilitate tumor immune evasion.39 Moreover, IFN-Is have been identified as drivers of malignant behaviors, such as epithelial-to-mesenchymal transition (EMT) and stemness in cancer cells, factors known to exacerbate tumor progression and resistance to therapy.40,41 This complex interplay underlines the imperative for precise modulation of IFN-I signaling within therapeutic strategies. By leveraging IFN-I’s immunostimulatory potential while circumventing its protumor consequences, it is feasible to overcome treatment resistance and enhance therapeutic outcomes. Notably, many cancer treatment strategies, such as chemotherapy, radiotherapy, targeted therapy, and immunotherapy, highly rely on the activation of IFN-I signaling pathways, especially the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway.3
IFN-α and engineered IFN-α administration
Given the fundamental importance of IFN-Is in both innate and adaptive immunity, IFN-Is hold remarkable potential in the realm of cancer therapy.42 The late 1970s marked the beginning of an extensive wave of clinical research that ultimately led to the approval of IFN-α2a and IFN-α2b, both in their standard and pegylated forms, for the treatment of various cancers.43 For example, pegylated IFN-α2b has demonstrated efficacy in melanoma by promoting immune infiltration into tumor beds.44,45 Besides, combining pegylated IFN-α with the tyrosine kinase inhibitor imatinib has shown promise in increasing molecular responses among patients with chronic myeloid leukemia (CML).46,47 Also, combination therapy involving the administration of IFN-α and ICB has shown synergistic effects in patients with liver cancer and melanoma. This synergy can be attributed to the inhibition of glycolysis in tumor cells and enhanced T-cell activation.48,49 These encouraging results have led to over 100 ongoing clinical studies worldwide, assessing the safety and efficacy of recombinant IFN-α in a range of hematological and solid tumors.50–52
However, despite the potential of IFN-α, its systemic administration can have paradoxical immunosuppressive effects, accompanied by adverse outcomes such as hepatotoxicity, flu-like symptoms, fatigue, gastrointestinal disorders, and depression.53 To mitigate these side effects, innovative strategies aim to deliver IFN-Is specifically to the TME.54 One such approach is the development of immunocytokine, where IFNs are linked to monoclonal antibodies to target specific cell populations, including malignant cells or leukocyte subsets.55 Also, some novel agents, such as ProIFN, increase the tumor-targeting effect by masking IFN-α with its receptor, linked through a cleavable connector, which can be selectively activated by proteases present in the TME.56 Another promising strategy involves the genetic engineering of various cell types to express IFN-Is, enhancing their antitumor activity or supporting immune effector cells.57,58 For instance, NK cells genetically engineered to express human IFN-α exhibit improved cytotoxicity against hepatocellular carcinoma cells.59 Additionally, direct injection of IFN-α-encoding vectors into tumors has shown promise as well. It has been reported that an adenovirus encoding IFN-α reduces tumor-infiltrating Tregs and promotes the accumulation of Th17 cells in colorectal cancers.60
Increasing IFN-Is by STING agonist and other agents
The development of tumor-specific adaptive immune responses, including the activation of CD8+ T cells with tumor-killing capabilities, relies on IFN-I signaling in antigen-presenting cells (APCs). In the TME, the cGAS-STING signaling pathway represents an evolutionarily conserved innate immune mechanism responsible for regulating the transcription of IFN-I.61,62 STING is a cellular DNA sensor located in the endoplasmic reticulum (ER) and is primarily activated by cyclic dinucleotides (CDNs) generated by cGAS rather than direct activation by double-stranded DNA (dsDNA).63 Cytosolic dsDNA binds to cGAS, leading to the production of cyclic GMP-AMP (cGAMP) and a change in the conformation of STING (Fig. 3).64,65 STING dimers are then translocated from the ER to perinuclear microsomes via the Golgi apparatus. STING recruits and activates TBK1, which phosphorylates IRF3 and upregulates the expression of IFN-I.66 STING can also activate the NF-κB pathway by binding to IKK and NIK, collaborating with the TBK1-IRF3 pathway to induce IFN-I expression, which plays a vital role in immune cell maturation and activation.67 Pharmacological activation of the cGAS/STING pathway has shown promising results in significantly retarding tumor growth and prolonging the survival of tumor-bearing mice.68–71
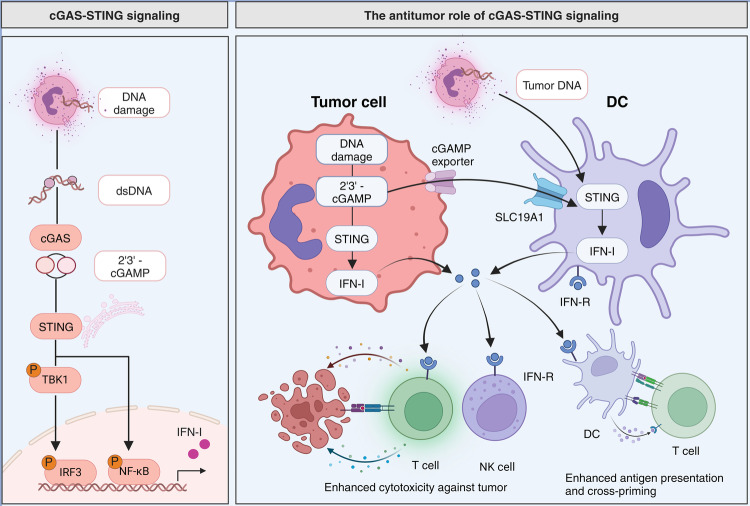
Fig. 3.
The cGAS-STING signaling pathway and its antitumor effects. The left panel delineates the cGAS-STING signaling cascade initiated by DNA damage, resulting in the production of double-stranded DNA (dsDNA). The enzyme cGAS detects dsDNA and synthesizes 2'3’-cGAMP, which in turn activates STING. Subsequent phosphorylation of TBK1 and IRF3, and activation of NF-κB, leads to the expression of type I interferons (IFN-I). The right panel illustrates the antitumor role of cGAS-STING signaling. Tumor cells undergoing DNA damage could produce 2'3’-cGAMP which activates STING and results in IFN-I release. Besides, tumor-derived DNA and cGAMP can be taken up by dendritic cells (DCs) via the SLC19A1 transporter, leading to STING activation and IFN-I production. Increased IFN-I enhances the cytotoxic activity of T cells and natural killer (NK) cells against the tumor and improves antigen presentation and cross-priming, further promoting T cell activation. (Created with BioRender.com)
Besides, accumulated evidence has demonstrated that STING agonists could improve ICB efficacy and overcome immunotherapy resistance.72–77 In a phase I clinical trial (NCT03172936), the combination of intratumoral injection of STING agonist ADU-S100 and anti-PD-1 therapy was well tolerated in patients with advanced tumors, with an overall response rate of 10.4%.78,79 Besides, intratumoral administration of SYNB1891, a probiotic strain of E. coli engineered to activate STING in the TME, combined with anti-PD-L1 antibody atezolizumab also showed local and systemic safety in patients with advanced or metastatic cancers (NCT04167137).80 Moreover, ICB plus intranasal or inhalation administration of natural STING agonist manganese achieved promising efficacy, with the best disease control rate (DCR) of 90.9% and the best objective response rate (ORR) of 45.5%.81 Other IFN-I signaling-associated agents, such as polyinosinic-polycytidylic acid (poly I:C) and CpG oligodeoxynucleotide (ODN) multimers, also exhibited the potential to stimulate innate immunity and improve immunotherapy performance (Table 1).82–86
Table 1.
STING agonists for cancer therapy
| Category | Agents | Combination partners | Clinical trials | Cancer types | Phase | Status |
|---|---|---|---|---|---|---|
| CDN analog | ADU-S100 | Pembrolizumab | NCT03937141 | HNSCC | II | Terminated |
| Ipilimumab | NCT02675439 | Solid tumors or lymphomas | I | Terminated | ||
| PDR001 | NCT03172936 | Solid tumors or lymphomas | I | Terminated | ||
| MK-1454 | Pembrolizumab | NCT04220866 | HNSCC | II | Completed | |
| Pembrolizumab | NCT03010176 | Solid tumors or lymphomas | I | Completed | ||
| SB11285 | Atezolizumab | NCT04096638 | Solid tumors | I | Recruiting | |
| BMS-986301 | Nivolumab or Ipilimumab | NCT03956680 | Solid tumors | I | Active, not recruiting | |
| BI 1387446 | Ezabenlimab | NCT04147234 | Solid tumors | I | Active, not recruiting | |
| TAK-676 | Pembrolizumab | NCT04879849 | Solid tumors | I | Active, not recruiting | |
| Pembrolizumab | NCT04420884 | Solid tumors | I | Recruiting | ||
| Chemotherapy | NCT06062602 | HNSCC | I | Completed | ||
| Non-CDN | MK-2118 | Pembrolizumab | NCT03249792 | Solid tumors or lymphomas | I | Completed |
| GSK3745417 | Monotherapy | NCT05424380 | Myeloid malignancies | I | Active, not recruiting | |
| Dostarlimab | NCT03843359 | Solid tumors | I | Active, not recruiting | ||
| Manganese | Radiotherapy | NCT04873440 | Solid tumors or lymphomas | I/II | Unknown | |
| Anti-PD-1 | NCT03991559 | Solid tumors or lymphomas | I | Unknown | ||
| E7766 | Monotherapy | NCT04144140 | Solid tumors or lymphomas | I | Terminated | |
| Monotherapy | NCT04109092 | Bladder cancer | I | Withdrawn | ||
| SNX281 | Pembrolizumab | NCT04609579 | Solid tumors or lymphomas | I | Terminated | |
| Engineered bacteria | SYNB1891 | Atezolizumab | NCT04167137 | Solid tumors or lymphomas | I | Unknown |
| ADC | TAK-500 | Pembrolizumab | NCT05070247 | Solid tumors | I | Recruiting |
| XMT-2056 | Monotherapy | NCT05514717 | Her-2 positive solid tumors | I | Recruiting |
Note: ADC antibody-drug conjugate, CDN cyclic dinucleotide, HNSCC head and neck squamous cell carcinoma. The specifics of the clinical trials were sourced in January 2024 from the ClinicalTrials.gov website
IFN-γ
IFN-γ signaling and its dual role in cancer
IFN-γ, the exclusive member of the IFN-II family, plays a versatile role encompassing antiviral, antitumor, and immunomodulatory functions. It holds a central position in orchestrating both innate and adaptive immune responses.87 Within an inflammatory milieu, IFN-γ contributes to activating the immune response, aiding pathogen clearance, while also preventing excessive immune activation and tissue damage.88 In the TME, IFN-γ exhibits both protumor and antitumor activities, which are largely dependent on the duration and magnitude of the signaling.89 Initially identified as a cytotoxic cytokine, along with perforin, granzyme, and TNF, IFN-γ is known for inducing apoptosis in tumor cells.90,91 Furthermore, IFN-γ can impede angiogenesis in tumors, induce apoptosis in Tregs, improve the maturation of DCs, and enhance the activity of M1-like macrophages, effectively impeding tumor progression.92 Generally, given its cytostatic, pro-apoptotic, and anti-proliferative properties, IFN-γ emerges as a promising candidate for adjuvant immunotherapy in diverse cancers (Table 2). However, recent studies have revealed the antitumor effect of IFN-γ. Similar to IFN-Is, prolonged IFN-γ exposure facilitates the upregulation of immune inhibitory molecules such as PD-L1, PD-L2, CTLA-4, and indoleamine-2,3-dioxygenase (IDO), thus promoting cancer immune evasion.93 Additionally, some tumor cells evade the antitumor effects of IFN-γ through modifications in the receptor or downstream JAK/STAT signaling pathway, alongside the constitutive activation of JAK inhibitors such as SOCS1 and SOCS3.94
Table 2.
Clinical trials harnessing IFN-γ for cancer therapy
| NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|
| NCT03112590 | HER2-positive Breast Cancer | Paclitaxel, Trastuzumab, and Pertuzumab | I/II | Completed |
| NCT00002637 | Prostate Cancer | Gene-modified tumor cell vaccine therapy | I/II | Completed |
| NCT00786643 | Colorectal Cancer | 5-Fluorouracil, Leucovorin, and Bevacizumab | II | Completed |
| NCT00002796 | Colorectal Cancer | Fluorouracil, Sodium phenylbutyrate, and Indomethacin | I/II | Terminated |
| NCT00047632 | Ovarian/Peritoneal Carcinoma | Monotherapy | III | Terminated |
| NCT00001296 | Melanoma | Melphalan and TNF | III | Completed |
| NCT00501644 | Ovarian/Fallopian Tube/Peritoneal Cancer | Carboplatin and GM-CSF | II | Completed |
| NCT00002505 | Solid Tumors | Tumor cell lysate vaccine | II | Completed |
| NCT00616720 | Multiple Myeloma and Plasma Cell Neoplasm | Autologous dendritic cell vaccine APC8020 | II | Completed |
| NCT01082887 | Melanoma | Adoptive transfer of TIL and IFN-γ-adenovirus | I/II | Terminated |
| NCT00057447 | Non-Hodgkin’s Lymphoma | Rituximab | I/II | Terminated |
| NCT00394693 | B-Cell Lymphoma | IFN-γ-adenovirus | II | Completed |
| NCT00002475 | Solid Tumors | Allogeneic tumor cell vaccine and cyclophosphamide | II | Completed |
| NCT00070187 | Lymphoma | Aldesleukin, Filgrastim, Chemotherapy, and Bone marrow transplantation | II/III | Completed |
| NCT02380443 | Colorectal Cancer | In-Situ Cancer Vaccine, and Cryoablation | II | Completed |
| NCT00006113 | Melanoma | Cancer vaccine therapy, and Aldesleukin | II | Terminated |
| NCT00024271 | Malignant Mesothelioma | Surgery, Chemotherapy, and Radiation therapy | II | Unknown |
| NCT02550678 | Skin Neoplasm | ASN-002 (adenovirus) and 5-FU | I/II | Completed |
| NCT00002761 | Leukemia | Aldesleukin, Filgrastim, Chemotherapy, and Bone marrow transplantation | I/II | Withdrawn |
Note: TIL tumor-infiltrating lymphocyte, GM-CSF granulocyte-macrophage colony-stimulating factor, TNF tumor necrosis factor
IFN-γ therapy
In both basic and clinical investigations, IFN-γ has emerged as a factor contributing to the direct or indirect eradication of tumors through collaboration with other components of the TME. The intraperitoneal administration of recombinant human IFN-γ yielded a 23% complete regression (CR) rate in ovarian cancer patients with residual diseases.95 In the first-line therapy for ovarian cancer, the combination of chemotherapy with subcutaneous IFN-γ treatment demonstrated a superior therapeutic efficacy compared to chemotherapy alone. Key outcomes included a 3-year progression-free survival (PFS) rate of 51% versus 38%, median times to progression of 48 versus 17 months, and a complete clinical response rate of 68% versus 56%.96 However, in expansive phase III clinical trials involving advanced ovarian and primary peritoneal carcinomas, IFN-γ failed to confer additional survival benefits. Instead, interim analysis revealed that patients receiving chemotherapy combined with subcutaneous IFN-γ therapy experienced a shorter overall survival (OS) and an elevated risk of serious hematological toxicities.97 Furthermore, the administration of IFN-γ in various other cancers, including renal-cell carcinoma, melanoma, and colon cancer, did not achieve positive results.98–100 Given its generally modest clinical efficacy, IFN-γ treatment has not gained approval for any solid tumor indication. These findings underscore the nuanced and context-dependent nature of therapeutic effects of IFN-γ, emphasizing the need for a cautious approach in its application for solid tumor indications.
Significantly, IFN-γ is recognized as a pivotal determinant for the success of immunotherapy. Recent advances highlight the critical role of interferon-γ receptor (IFNγR) signaling in modulating the efficacy of chimeric antigen receptor (CAR) T cell therapy, particularly in solid tumors. A pivotal study employing a genome-wide CRISPR knockout screen revealed a marked increase in resistance to CAR-T cell therapy in solid tumors upon disruption of key genes within the IFNγR signaling pathway, such as IFNGR1, JAK1, or JAK2.101 This phenomenon is notably absent in hematologic malignancies like leukemia and lymphoma, underscoring a distinct mechanism of interaction between CAR-T cells and solid tumor cells.101 Specifically, the study illuminated that IFNGR1-deficient glioblastoma cells exhibited significantly reduced adhesion and subsequent cytotoxicity by CAR-T cells.101 This finding stresses the indispensability of IFNγR signaling for the effective targeting of solid tumors by CAR-T therapy. Also, in patients responsive to anti-PD-1 therapy, there was a notable upregulation of the IFN-γ-related gene signature, distinguishing them from non-responders.102–104 Moreover, resistance to anti-CTLA-4 in melanoma patients is often associated with deficiencies in the IFN-γ pathway, including the loss of IFNGR, JAK2, IFIT, MTAP, and IRF1 genes. In murine melanoma models, silencing the IFNGR1 gene nullified the efficacy of anti-CTLA-4.105 IFN-γ has been validated as a promoter of T cell infiltration, upregulating major histocompatibility complex class (MHC) and PD-L1 expression in tumors while limiting the accumulation of immunosuppressive components, such as CXCR2+CD68+ macrophages, in the TME.106,107 Consequently, it is rational to combine IFN-γ with anti-PD-1/PD-L1 for optimal cancer immunotherapy. In a phase I study (NCT02614456), the combination of IFN-γ and nivolumab exhibited modest clinical benefits, with an ORR of 4.3% and a DCR of 26.1% in advanced solid tumors.108 Presently, several ongoing clinical studies are exploring the effects of systemic IFN-γ therapies.109
Interleukins and their agonists or inhibitors
IL-2
IL-2 signaling and its role in cancer immunology
IL-2, initially identified in the supernatants of activated T cells and formerly labeled as T-cell growth factor, plays a pivotal role in immune regulation.110 The IL-2 receptor is a trimeric complex consisting of IL-2Rα (CD25), IL-2Rβ (CD122), and IL-2Rγ (CD132), each exhibiting distinct affinities for IL-2. IL-2 demonstrates low affinity for IL-2Rα, intermediate affinity for IL-2Rβ and IL-2Rγ, and high affinity for heterotrimeric receptors containing all three subunits.111 Generally, Tregs primarily express the high-affinity trimeric IL-2 receptor, whereas CD8+ T cells and NK cells predominantly express the intermediate-affinity dimeric IL-2 receptor (IL-2Rβ/γ complex).112 The interaction between IL-2 and IL-2R triggers downstream JAK-STAT, MAPK, and PI3K signaling pathways by the intracellular domains of IL-2Rβ/γ complex (Fig. 4).113 It has been well established that IL-2 is a core cytokine maintaining adaptive immunity. Primarily, IL-2 promotes the proliferation, differentiation, and cytotoxic activity of T cells.114,115 Also, IL-2 contributes to immune homeostasis by supporting the expansion of Tregs.116 Accumulating evidence underscores the critical role of IL-2 in cancer immunology. Impaired IL-2 signaling is associated with poor outcomes in various cancers, while IL-2-based therapies show promise in stimulating antitumor immune response and improving immunotherapy efficacy in cancer patients.117,118
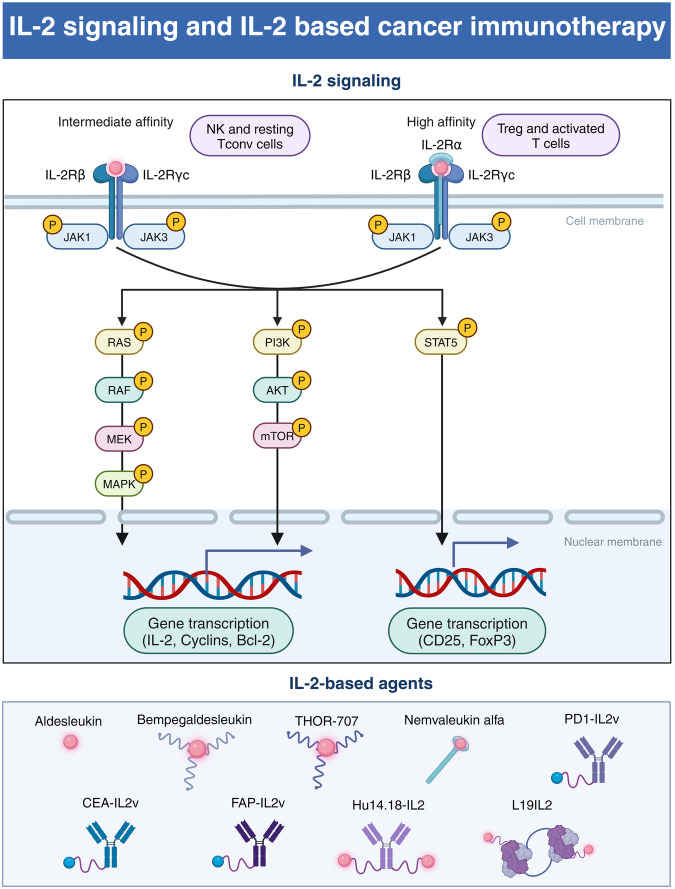
Fig. 4.
IL-2 signaling pathway and IL-2 based cancer immunotherapy. This schematic representation illustrates the differential signaling pathways activated by the binding of IL-2 to its receptor complexes of varying affinities. On the left, the intermediate affinity IL-2 receptor, composed of IL-2Rβ and IL-2Rγc subunits, is primarily found on natural killer (NK) cells and resting conventional T (Tconv) cells. On the right, the high affinity IL-2 receptor, which includes the IL-2Rα subunit in addition to IL-2Rβ and IL-2Rγc, is expressed on regulatory T (Treg) cells and activated T cells. The binding of IL-2 to it receptor activates the RAS/RAF/MEK/MAPK, PI3K/AKT/mTOR and JAK/STAT pathways, which leads to gene transcription of IL-2, Cyclins, and Bcl-2, CD25 and FoxP3. The lower portion of the figure showcases a selection of IL-2-based agents used in cancer immunotherapy, including Aldesleukin, Bempegaldesleukin, THOR-707, Nemvaleukin alfa, PD1-IL2v, CEA-IL2v, FAP-IL2v, Hu14.18-IL2, and L19IL2, each designed to leverage the IL-2 signaling pathways for therapeutic purposes. (Created with BioRender.com)
IL-2 and engineered IL-2 administration
In 1985, Steven Rosenberg first reported a patient with metastatic melanoma experiencing complete regression despite severe toxicities after high-dose intravenous IL-2 treatment.119 Subsequent studies confirmed the antitumor potential of high-dose IL-2 in patients with metastatic melanoma and renal cell cancers.120 These positive data led to FDA approval of high-dose IL-2 therapy for metastatic renal cell carcinoma in 1992 and metastatic melanoma in 1998.121 Despite its efficacy, high-dose IL-2 therapy has limitations, including a short half-life and severe toxicities such as vascular leak syndrome. Besides, patients not responding to high-dose IL-2 exhibited increased Treg cell expansion, which might promote tumor progression in turn.122 To address these limitations, new therapies are being designed to selectively enhance immune activation but avoid Treg accumulation and high IL-2 dosing.
The next generation of IL-2-based antitumor agents has biased affinities to IL-2R subunits (Table 3). For instance, Bempegaldesleukin, a PEGylated IL-2 variant, selectively activates CD8+ T cells and NK cells by preferentially binding to IL-2Rβγ, minimizing impact on Tregs. This PEGylation modification aims to reduce toxicities and extend IL-2 half-life.123 In the phase I study, Bempegaldesleukin induced tumor regression in advanced or metastatic solid tumors as a monotherapy.124 Combined with nivolumab, it achieves an ORR of 59.5% and a complete response rate of 18.9% in immunotherapy-naïve advanced solid tumors, with tolerable adverse events.125 Besides, Nemvaleukin alfa, an engineered fusion protein comprising IL-2 and the extracellular domain of IL-2Rα, is designed to selectively activate effector lymphocytes by binding to intermediate-affinity IL-2 receptors, excluding high-affinity receptors present on Tregs and endothelial cells.126 The protein’s preferential expansion of CD8+ T cells and NK cells, coupled with minimal expansion of immunosuppressive Tregs, underscores its potential to evoke robust systemic antitumor immunity127. Preclinical models demonstrated the outstanding antitumor efficacy of Nemvaleukin alfa, whether administered intravenously or subcutaneously, showcasing superior effectiveness and a notable reduction in distant metastasis.128,129 In a phase I/II clinical trial (NCT02799095), both Nemvaleukin alfa monotherapy and its combination with pembrolizumab exhibited promising antitumor activity in patients with advanced solid tumors.130
Table 3.
Clinical trials harnessing IL-2 and its engineered variants for cancer therapy
| Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|
| Aldesleukin | NCT00018941 | Kidney Cancer | Monotherapy | III | Completed |
| NCT00416871 | Kidney Cancer | IFN-α | III | Completed | |
| NCT00002702 | Head and Neck Cancer | Surgery and Radiation Therapy | III | Unknown | |
| NCT00003126 | Kidney Cancer | Monotherapy | III | Completed | |
| NCT00039234 | Melanoma | Histamine Dihydrochloride | III | Unknown | |
| Nemvaleukin alfa | NCT04592653 | Solid Tumors | Pembrolizumab | I/II | Recruiting |
| NCT05092360 | Ovarian/Fallopian Tube/Peritoneal Cancer | Pembrolizumab | III | Recruiting | |
| NCT03861793 | Solid Tumors | Pembrolizumab | I/II | Completed | |
| NCT02799095 | Solid Tumors | Pembrolizumab | I/II | Completed | |
| NCT04144517 | HNSCC | Pembrolizumab | II | Completed | |
| NCT04830124 | Melanoma | Monotherapy | II | Recruiting | |
| Bempegaldesleukin | NCT03785925 | Bladder Cancer | Nivolumab | II | Completed |
| NCT03548467 | Solid Tumors | VB10.NEO | I/II | Completed | |
| NCT04209114 | Bladder Cancer | Nivolumab | III | Completed | |
| NCT04969861 | HNSCC | Pembrolizumab | II/III | Terminated | |
| NCT04052204 | HNSCC and mCRPC | Avelumab, Talazoparib, and Enzalutamide | I/II | Terminated | |
| NCT03138889 | NSCLC | Pembrolizumab and Chemotherapy | I/II | Terminated | |
| NCT04730349 | Solid tumors | Nivolumab | I/II | Terminated | |
| NCT03435640 | Solid tumors | NKTR-262 and Nivolumab | I/II | Terminated | |
| NCT04936841 | HNSCC | Radiation and Pembrolizumab | II | Terminated | |
| NCT03745807 | Solid tumors | Nivolumab | I | Completed | |
| NCT02983045 | Solid tumors | Nivolumab | I/II | Completed | |
| NCT04540705 | RCC | Nivolumab | I | Active, not recruiting | |
| NCT03729245 | RCC | Nivolumab | III | Terminated | |
| NCT04410445 | Melanoma | Nivolumab | III | Terminated | |
| NCT03635983 | Melanoma | Nivolumab | III | Completed | |
| THOR-707 | NCT04914897 | Pleural Mesothelioma and NSCLC | Pembrolizumab | II | Active, not recruiting |
| NCT04009681 | Solid tumors | ICB and Anti-EGFR antibody | I/II | Recruiting | |
| NCT05104567 | Gastrointestinal Cancers | Pembrolizumab and Cetuximab | II | Active, not recruiting | |
| NCT04913220 | Skin Cancers | Cemiplimab | I/II | Active, not recruiting | |
| NCT05061420 | HNSCC | Pembrolizumab and Cetuximab | II | Active, not recruiting | |
| NCT05179603 | Lymphoma | Pembrolizumab | II | Active, not recruiting | |
| RO6895882 (CEA-IL2v) | NCT02004106 | Solid Tumors | Monotherapy | I | Completed |
| NCT02350673 | Solid Tumors | Atezolizumab | I | Completed | |
| Eciskafusp alfa (PD1-IL2v) | NCT04303858 | Solid Tumors | Atezolizumab | I | Recruiting |
| Simlukafusp alfa (FAP-IL2v) | NCT03386721 | Solid Tumors | Atezolizumab, Gemcitabine, and Vinorelbine | II | Terminated |
| NCT02627274 | Solid Tumors | Trastuzumab and Cetuximab | I | Completed | |
| NCT03875079 | Melanoma | Pembrolizumab | I | Completed | |
| NCT03063762 | RCC | Atezolizumab and Bevacizumab | I | Completed | |
| L19IL2 | NCT01198522 | Pancreatic Cancer | Gemcitabine | I | Terminated |
| NCT01058538 | Solid Tumors | Monotherapy | I/II | Completed | |
| NCT02086721 | Solid Tumors | Monotherapy | I | Completed | |
| NCT05329792 | Skin Cancers | L19TNF | II | Recruiting | |
| NCT02735850 | NSCLC | Radiotherapy | II | Withdrawn | |
| NCT04362722 | Skin Cancers | L19TNF | II | Recruiting | |
| NCT02076646 | Melanoma | Dacarbazine | I/II | Active, not recruiting | |
| NCT02957019 | DLBCL | Rituximab | I/II | Terminated | |
| NCT01055522 | Melanoma | Dacarbazine | II | Terminated | |
| NCT03705403 | NSCLC | Radiation | II | Unknown | |
| NCT01253096 | Melanoma | Monotherapy | II | Completed | |
| NCT02076633 | Melanoma | L19TNF | II | Completed | |
| NCT02938299 | Melanoma | L19TNF | III | Recruiting | |
| NCT03567889 | Melanoma | Monotherapy | III | Recruiting | |
| Hu14.18-IL2 | NCT00003750 | Melanoma | Monotherapy | I | Completed |
| NCT00590824 | Melanoma | Monotherapy | II | Completed | |
| NCT00109863 | Melanoma | Monotherapy | II | Completed | |
| NCT03209869 | Neuroblastoma | Donor NK Cell | I | Withdrawn | |
| NCT00082758 | Neuroblastoma | Monotherapy | II | Completed | |
| NCT01334515 | Neuroblastoma | Sargramostim and Isotretinoin | II | Completed |
Note: HNSCC head and neck squamous cell carcinoma, NSCLC non-small cell lung cancer, RCC renal cell cancer, mCRPC metastatic castration resistant prostate cancer, DLBCL diffuse large B-cell lymphoma
Moreover, some recent studies reported the potent antitumor effects of an engineered variant of IL-2 (IL-2v), specifically PD1-IL2v, in various preclinical tumor models.131–133 PD1-IL2v demonstrates multifaceted molecular mechanisms of action, including targeting IL-2v to PD-1+ tumor-specific T cells, IL-2Rα-independent binding to IL-2R, prolonged interaction with IL-2R through PD-1 anchoring, and partial PD-1 signaling blockade.134 Single-cell RNA-seq data have demonstrated that PD1-IL2v treatment increases the frequency of optimally activated T cells, particularly tumor-infiltrating GZMB+TIM-3−PD-1+TCF7−CD8+ cells.135 Additionally, TransCon IL-2β/γ, a sustained-release drug of IL-2Rβ/γ-selective IL-2v, effectively increased the proliferation and cytotoxicity of primary CD8+ T cells, NK cells, and γδ T cells without severe toxicities, especially vascular leak syndrome and cytokine storm.136 Generally, the selective expansion of CD8+ T cells and NK cells, alongside a manageable safety profile, positions IL-2-based therapy as a compelling therapeutic candidate in the dynamic realm of immunotherapy for advanced solid tumors.
IL-10
The dual role of IL-10: general immunosuppression but tumor-resident CD8+ T cell activation
IL-10, a dimeric protein encoded by the IL10 gene on chromosome 1, is primarily produced by a variety of immune cell types, including T cells, B cells, NK cells, and mast cells.137 Notably, certain tumor cells, such as those associated with human papilloma virus (HPV)-related cervical cancers, can also generate IL-10.138 The IL-10 receptor (IL-10R), expressed on hematopoietic cells, comprises two subunits, IL-10Rα and IL-10Rβ, initiating downstream STAT1 or STAT3 signaling through the phosphorylation of JAK1 and Tyk2.139 Subsequently, STAT3 translocates to the nucleus, prompting the expression of genes responsive to STAT3, including SOCS3 and IL1RN.140 SOCS3 exerts its inhibitory effect on inflammatory gene expression by impeding MAPK and NF-κB pathways, while IL1RN functions as a decoy protein, interfering with IL-1β signaling by binding to its receptor and suppressing inflammatory responses.141
In a broader context, IL-10 assumes a pivotal role in curbing excessive inflammatory responses, contributing to immune tolerance, and mitigating autoimmune diseases.142 By downregulating MHC-II, IL-10 attenuates DC responses to antigen stimulation, leading to the reduction of various immunostimulatory cytokines.143 Furthermore, IL-10 impedes the proliferation and function of CD4+ T cells, thereby contributing to an immunosuppressive TME.144 Conversely, its impact on CD8+ T cells is distinctive,145 as preclinical studies indicate its role in activating tumor-resident CD8+ T cells, retarding tumor growth in murine tumor models.146 IL-10 induces STAT1/3 phosphorylation specifically in tumor-resident CD8+ T cells, enhancing IFN-γ expression and granzyme production, thereby promoting an augmented immune response and facilitating antiproliferative and proapoptotic pathways.146 These findings have stimulated interest in investigating the therapeutic potential of IL-10 in cancer patients, with emerging results demonstrating promising efficacy in specific tumor types, such as renal cell carcinoma, though its activity in other tumors varies.147
Engineered IL-10 treatment
Pegilodecakin, the first pegylated form of IL-10, exhibited promising activity and a reasonable safety profile in the phase I trial NCT02009449 (Table 4).148 The dose-escalation and -expansion cohorts included 51 patients with various solid tumors, and the drug, administered through daily subcutaneous injections, demonstrated good tolerability with no maximum-tolerated dose reached in the dose-escalation cohort.148 Notable adverse events were generally mild, including anemia, fatigue, fever, injection-site reactions, and thrombocytopenia. One patient with uveal melanoma and four out of 15 evaluable patients with RCC exhibited partial responses when treated at a dosage of 20 μg/kg, even in those who had received prior immunotherapy.148 In the other two cohorts of phase I trial NCT02009449, Pegilodecakin was combined with anti-PD-1 antibodies (pembrolizumab or nivolumab).149 Response rates varied by tumor type, with notable responses observed in NSCLC (ORR: 43%), renal cell carcinoma (ORR: 40%), and melanoma (ORR: 10%).149 The combination therapy achieved a favorable response in NSCLC and renal cell carcinoma, but with manageable toxicity of thrombocytopenia and anemia relative to anti-PD-1 monotherapy.149 However, in phase II trials (NCT03382899 and NCT03382912), combining Pegilodecakin with anti-PD-1 therapy in metastatic NSCLC did not improve ORR, PFS, or OS compared to anti-PD-1 therapy alone.150 The combination led to more frequent overall and serious adverse events.150 Similarly, in a phase III trial for pancreatic ductal adenocarcinoma (NCT02923921), the addition of Pegilodecakin to FOLFOX chemotherapy did not improve ORR and survival, while increased adverse events were noted in the combination arm.151
Table 4.
Clinical trials targeting IL-10 for cancer therapy
| Products | NCT number | Cancer types | Combination partners | Phases | Status |
|---|---|---|---|---|---|
| Pegilodecakin (PEGylated IL-10) | NCT02923921 | Pancreatic Cancer | FOLFOX | III | Completed |
| NCT03382912 | NSCLC | Nivolumab | II | Terminated | |
| NCT03382899 | NSCLC | Pembrolizumab | II | Terminated | |
| NCT02009449 | Solid tumors | Chemotherapy | I | Active, not recruiting | |
| IBB0979 (B7H3-IL10 immunocytokine) | NCT05991583 | Solid tumors | Monotherapy | I/II | Recruiting |
| IAE0972 (EGFR/IL10 immunocytokine) | NCT05396339 | Solid tumors | Monotherapy | I/II | Recruiting |
Note: NSCLC non-small cell lung cancer, FOLFOX 5-fluorouracil and oxaliplatin, EGFR epidermal growth factor receptor
Several strategies have been explored to enhance the therapeutic potential of IL-10 beyond PEGylation. One approach involved the development of a bispecific fusion protein by combining cetuximab with the IL-10 dimer to enhance drug delivery to tumors expressing epidermal growth factor receptor (EGFR).152 This fusion protein exhibited an extended half-life without increased toxicity and demonstrated significant antitumor effects in murine tumor models.152 Other IL-10-based strategies, such as engineered IL-10 variants with increased affinity toward IL-10Rβ, incorporating IL-10 into oncolytic viruses, and conjugating IL-10 to nanoparticles, also demonstrated potent antitumor potency.153–155 Generally, although IL-10 monotherapy demonstrated good tolerability, its clinical efficacy in large-scale clinical trials was modest. Nevertheless, the exploration of IL-10 in cancer immunotherapy remains a topic of clinical interest, urging further investigation into potential combination strategies or IL-10 modifications.
IL-12
IL-12 signaling and its role in cancer immunology
IL-12 is the first identified member of the IL-12 family, constituted by two distinctive subunits: the p35 α-chain and the p40 β-chain.156 Correspondingly, its receptor exhibits a dimeric structure, comprising IL-12Rβ1 and IL-12Rβ2 subunits.157 APCs, including DCs, phagocytes, and B cells, primarily produce IL-12. Concurrently, NK and T cells serve as the main targets for IL-12.158 APCs, upon detection of pathogen-associated molecular patterns (PAMPs) through toll-like receptors (TLRs), trigger the transcription of IL-12p35 and IL-12p40.159 The binding of the IL-12 to the IL-12 receptor subunits initiates the JAK-STAT pathway for signal transduction. Tyrosine kinases JAK2 and TYK2 are recruited and undergo phosphorylation, subsequently phosphorylating the IL-12Rβ2 subunit.160 This signaling cascade initiates gene transcription, particularly facilitating STAT4-mediated expression of IFN-γ. It has been substantiated that IL-12 occupies a central role in the differentiation of T helper 1 (Th1) cells and the transcription of IFN-γ in effector cells (Fig. 5).161 Conversely, IL-12 hinders the differentiation of Th2 cells by suppressing the Th2-associated transcription factor GATA3 within T cell populations.162
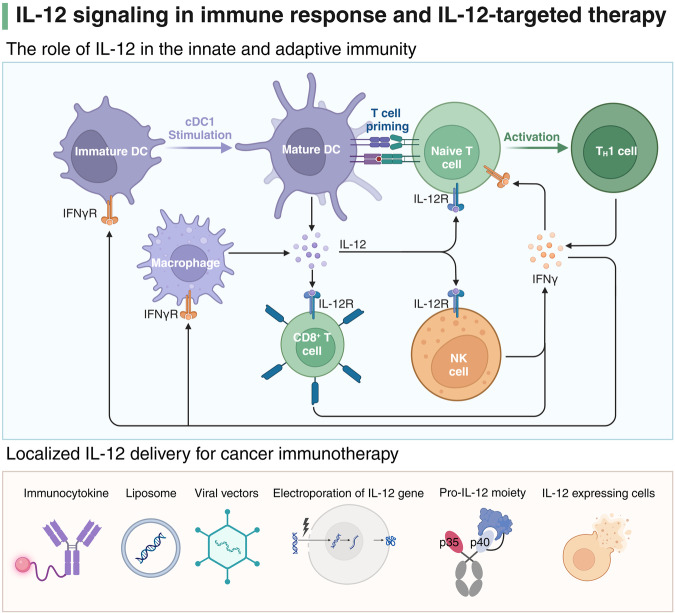
Fig. 5.
IL-12 signaling pathway and therapeutic applications. The upper panel illustrates the role of IL-12 in both innate and adaptive immunity. When exposed to stimuli such as pathogen-associated molecular patterns, immature dendritic cells (DCs) undergo transformation into their mature form, subsequently leading to the production of interleukin-12 (IL-12). This cytokine mainly acts on T cells and natural killer (NK) cells via the IL-12 receptor (IL-12R). IL-12 is pivotal for T cell priming and the subsequent differentiation of naive T cells into Th1 cells, with IFN-γ acting as a critical feedback enhancer of this immune response. The lower panel depicts strategies for localized IL-12 delivery in cancer immunotherapy, including immunocytokines, liposomes, viral vector, electroporation of the IL-12 gene, pro-IL-12 moieties, and cells engineered to express IL-12. Adapted from “Differentiation of TH17 Cells - Indirect and direct activation of T cells by TLR agonists”, by BioRender.com (2024). Retrieved from https://app.biorender.com/biorender-templates
Innovative IL-12-based therapies: localized IL-12 delivery
Although IL-12 has shown promising antitumor effects in preclinical studies, its efficacy at tolerated doses has been limited in clinical trials (ORR: 5%).163 Besides, the later phase II clinical trial of rhIL-12 was halted due to serious safety concerns, with two fatalities reported.164 Despite unsatisfactory initial clinical outcomes, IL-12 remained a compelling target for enhancing anti-cancer immunity. Researchers explored various preclinical strategies to improve IL-12-based therapy efficacy while mitigating its systemic toxicity. Numerous approaches have been assessed to achieve localized delivery of IL-12, aiming to maximize IL-12 abundance in the TME and minimize peripheral leakage and toxicity.165 At present, some of these novel methods are progressing toward clinical applications.
Various viral vectors, such as adenovirus, adeno-associated virus (AAV), Semliki Forest virus (SFV), and herpes simplex virus (HSV), have been employed for localized IL-12 delivery, demonstrating therapeutic efficacy in murine tumor models.166–169 While retroviral vector-based approaches effectively express IL-12 in transfected cells,170 their limited use for in vivo applications arises from safety concerns associated with random genome integration.171 In response to these concerns, non-integrative vectors such as adenovirus and AAV have been developed, which exhibit promise in preclinical models and are undergoing clinical evaluation.172,173 A notable advantage conferred by viral vectors resides in the capacity of oncolytic viruses to kill tumor cells directly. Furthermore, viral infection could activate PAMPs and enhance the functions of APCs, further improving antitumor immune response.165 Moreover, an alternative method for inducing localized expression of IL-12 involves the use of non-viral vectors. This includes the administration of nucleic acids, either in their naked form or intricately combined with polymers or lipid-based delivery systems.174,175 Multiple phase I/II clinical trials, utilizing in-vivo electroporation for IL-12 gene transfer, documented heightened IFN-γ level, increased infiltration of T cells, and effective tumor control in patients with ovarian cancer and melanoma (Table 5).176–178 Furthermore, combination therapy of IL-12 plasmid (Tavo) and pembrolizumab yielded promising outcomes in patients with metastatic melanoma.179 Apart from DNA, mRNA-based IL-12 delivery, particularly using lipid nanoparticles (LPNs), has proven both safe and effective in preclinical models.180 Notably, human IL-12 mRNA LPN products like MEDI1191 have progressed into clinical trials.
Table 5.
Clinical trials involving IL-12 for cancer therapy
| Products | NCT number | Cancer types | Combination partners | Phases | Status |
|---|---|---|---|---|---|
| M032 | NCT02062827 | Glioblastoma | Monotherapy | I | Active, not recruiting |
| NCT05084430 | Glioblastoma | Pembrolizumab | I/II | Recruiting | |
| MEDI1191 | NCT03946800 | Solid Tumors | Durvalumab | I | Completed |
| MEDI9253 | NCT04613492 | Solid Tumors | Durvalumab | I | Active, not recruiting |
| M9241 | NCT05361798 | Prostate Cancer | SBRT | II | Recruiting |
| NCT06096870 | Prostate Cancer | Enzalutamide | II | Not yet recruiting | |
| NCT04633252 | Prostate Cancer | Docetaxel | I/II | Recruiting | |
| NCT04327986 | Pancreatic Cancer | M7824 and SBRT | I/II | Terminated | |
| NCT04235777 | Genitourinary Malignancies | M7824 and SBRT | I | Recruiting | |
| NCT05286814 | Colorectal Cancer or Intrahepatic Cholangiocarcinoma | Chemotherapy | II | Recruiting | |
| NCT02994953 | Solid Tumors | Avelumab | I | Terminated | |
| NCT04756505 | Breast Cancer | M7824 and SBRT | I | Withdrawn | |
| NCT04708470 | HPV-Associated Malignancies, Small Bowel, and Colon Cancers | M7824 and Entinostat | I/II | Recruiting | |
| NCT04491955 | Small Bowel and Colorectal Cancers | CV301, MSB0011359C, and N-803 | II | Active, not recruiting | |
| SAR441000 | NCT03871348 | Solid Tumors | Cemiplimab | I | Active, not recruiting |
Note: SBRT stereotactic body radiation therapy, HPV human papillomavirus
Moreover, immunocytokines represent an innovative strategy for targeted IL-12 delivery to the TME. Most immunocytokine products are chimeric constructs combining an antibody with a cytokine, with the cytokines fused either to the N-term or the C-term of complete IgG antibodies or smaller antibody fragments.181 Several IL-12 immunocytokines, such as BC1-IL12 and NHS-IL12, are now undergoing clinical trials. BC1-IL12 utilizes the single chain fragment variable (scFv) of the L19 antibody (recognizing fibronectin) to target the TME,182 while NHS-IL12, created using the NHS76 antibody (targeting DNA-histone complexes), shows efficacy in inducing tumor regression.183 Additionally, pro-cytokines, where IL-12 is shielded by peptides and unmasked by matrix metalloproteinase 9 (MMP9) in the TME, present another avenue.184 The pro-IL-12 moiety, employing an MMP14 cleavable substrate linker, has shown localized cleavage and accumulation of active IL-12 in the tumor bed, displaying robust efficacy in controlling murine tumor growth.185 Furthermore, engineered mutant forms of IL-12 p40 retain antitumor activity while exhibiting enhanced safety, showcasing diverse and promising strategies in the development of IL-12-based therapies for cancer treatment.186
IL-15
IL-15 vs. IL-2: shared receptors with unique trans-presentation mode
IL-15 is a member of the four-α-helix bundle cytokine family, alongside cytokines such as IL-2, IL-4, and IL-7.187 A distinguishing feature of IL-15 within this family lies in its trans-presentation mode. Commonly, IL-15 exists stably in conjunction with its high-affinity receptor α (IL-15Rα), forming IL-15/IL-15Rα complex on APCs.188 Under this circumstance, IL-15 is trans-presented by IL-15Rα to target cells, including NK, NKT, and memory CD8+ T cells, by binding IL-2Rβ/γc receptor complex.189 While the predominant IL-15 signaling pathway involves the IL-15/IL-15Rα complex, IL-15 could independently bind to the IL-2Rβ/γc complex in the absence of IL-15Rα, with lower binding affinity.190 Upon activation, the β and γc chains initiate intracellular JAK-STAT signaling.191 Despite IL-15 and other four-helix bundle cytokines engaging with common receptor subunits, the unique trans-presentation mode contributes to IL-15’s distinctive functions. For example, both IL-15 and IL-2 bind to and stimulate NK and CD8+ T cells, but Tregs are primarily stimulated by IL-2.192 Furthermore, in contrast to IL-2, IL-15 plays a critical role in inhibiting activation-induced cell death, thereby promoting the survival of memory cells.193 It has been confirmed that IL-15 is indispensable for the proliferation, maintenance, and survival of NK and CD8+ T cells.194
IL-15 and engineered IL-15 treatment
The potential of recombinant human IL-15 (rhIL-15) has been extensively explored as an immunomodulator against cancers. In preclinical studies, rhIL-15 has demonstrated superiority over IL-2 in reducing tumor burden and prolonging survival in tumor-bearing mice.195 In patients with renal cell carcinoma and melanoma, rhIL-15 injection induced a significant increase in circulating NK and CD8+ T cells with moderate toxicity.196 However, challenges persist in achieving sustained IL-15 exposure due to its short serum half-life, which restricts its immunostimulatory potency. The biostability of IL-15 is predominantly restricted by the availability of IL-15Rα. Consequently, various strategies have been employed to surmount these obstacles, involving the development of IL-15/IL-15Rα complexes or IL-15 superagonists.197
Notably, hetIL-15, which is designed based on the natural heterodimeric state of IL-15 and IL-15Rα for higher biostability, exhibits promising outcomes in preclinical models and ongoing clinical trials (Table 6).198 Its sustained plasma IL-15 levels and robust expansion of NK and T cells underscore its potential as a monotherapy for patients with metastatic or unresectable solid tumors.199,200 Likewise, hetIL-15Fc, a glycosylated form covalently linked to the Fc region of human IgG1, demonstrates superior efficacy in murine models.201,202 N-803, an IL-15 superagonist consisting of IL-15 variant fused with an IL-15Rα sushi domain and an Fc fragment, stands out with a remarkable half-life and increased bioactivity, showcasing its potential to eliminate established tumors and enhance NK cell cytotoxicity.203 Clinical trials further support the tolerability and efficacy of N-803, positioning it as a promising candidate for advanced cancer treatment.204–208 The continued exploration of IL-15 variants, including receptor-linker-IL-15 (RLI) and NKTR-255, further diversifies the therapeutic landscape, holding the potential to rescue NK cell activity and exhibit enhanced antitumor activity in various malignancies.209–213
Table 6.
Clinical trials involving IL-15 for cancer therapy
| Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|
| N-803 | NCT03022825 | Bladder Cancer | BCG | II/III | Recruiting |
| NCT04847466 | GEJC and HNSCC | Pembrolizumab and PD-L1 t-haNK | II | Recruiting | |
| NCT05445882 | CRPC | M7824 and BN-Brachyury | II | Not yet recruiting | |
| NCT02138734 | Bladder Cancer | BCG | I/II | Recruiting | |
| NCT06149481 | Colorectal Cancer | SX-682, TriAdeno Vaccine, and Retifanlimab | I/II | Not yet recruiting | |
| NCT06253494 | Endometrial Cancer | Pembrolizumab, Lenvatinib and HER2 Targeting Autologous Dendritic Cell (AdHER2DC) Vaccine | I/II | Not yet recruiting | |
| NCT05642195 | NSCLC | Cancer Lysate Vaccine and Montanide ISA-51 VG | I/II | Recruiting | |
| NCT04491955 | Colorectal Cancer | CV301, MSB0011359C, and NHS-IL12 | II | Active, not recruiting | |
| NCT04247282 | HNSCC | M7824 and TriAd vaccine | I/II | Completed | |
| NCT04927884 | TNBC | PD-L1 t-haNK, Sacituzumab, and Cyclophosphamide | I/II | Terminated | |
| NCT05007769 | NSCLC | Ramucirumab and Atezolizumab | II | Withdrawn | |
| NCT03493945 | Prostate Cancer | BN-Brachyury Vaccine, M7824, and Epacadostat | I/II | Recruiting | |
| NCT03520686 | NSCLC | Pembrolizumab and Chemotherapy | III | Active, not recruiting | |
| NCT06239220 | HNSCC | PD-L1 t-haNK and Cetuximab | II | Not yet recruiting | |
| NCT04290546 | HNSCC | CIML NK cell Infusion, Ipilimumab, and Cetuximab | I | Recruiting | |
| NCT04390399 | Pancreatic Cancer | SBRT, Cyclophosphamide, Gemcitabine, Nab-paclitaxel, Aldoxorubicin, and PD-L1 t-haNK | II | Active, not recruiting | |
| NCT03228667 | NSCLC | Anti-PD-1/PD-L1 + PD-L1 t-haNK | II | Active, not recruiting | |
| NCT02989844 | AML | Monotherapy | II | Completed | |
| NCT06161545 | HNSCC | Pembrolizumab and PD-L1 t-haNK Cells | II | Not yet recruiting | |
| NCT06061809 | Glioblastoma | PD-L1 t-haNK and Bevacizumab | II | Not yet recruiting | |
| NCT05618925 | Non-Hodgkin’s Lymphoma | CD19t-haNK suspension, Cyclophosphamide, Fludarabine, and Rituximab | I | Not yet recruiting | |
| BJ-001 | NCT04294576 | Solid Tumors | Pembrolizumab | I | Active, not recruiting |
| NKTR-255 | NCT05632809 | Lung Cancer | Durvalumab | II | Recruiting |
| NCT05676749 | NSCLC | C-TIL051 and Pembrolizumab | I | Not yet recruiting | |
| NCT04616196 | HNSCC | Cetuximab | I/II | Completed | |
| NCT03233854 | B Acute Lymphoblastic Leukemia | Anti-CD19/CD22 CAR-T therapy | I | Recruiting | |
| NCT04136756 | MM and Non-Hodgkin Lymphoma | Rituximab/Daratumumab | I | Completed | |
| NCT05327530 | Urothelial Carcinoma | Avelumab | II | Recruiting | |
| NCT05664217 | Non-Hodgkin Lymphoma and DLBL | Anti-CD19 CAR-T Therapy | II/III | Recruiting | |
| NCT05359211 | DLBL | Anti-CD19 CAR-T Therapy | I | Recruiting |
Note: GEJC gastroesophageal junction cancer, CRPC castration resistant prostate cancer, TNBC triple negative breast cancer, AML acute myelogenous leukemia, MM multiple myeloma, DLBL diffuse large B-cell lymphoma, HNSCC head and neck squamous cell carcinoma
Moreover, IL-15 is widely used to improve the efficacy of adoptive cell therapies against cancer, especially CAR-T cells.214 This novel approach involves not only ex vivo precultures but also the incorporation of IL-15 and its receptor within CAR engineering.197 IL-15-armored CAR-T cells have shown promising results, with enhanced expansion, prolonged persistence, and reduced cell death, leading to superior antitumor effects.215,216 Membrane-bound IL-15 (mbIL-15) signaling enhanced the persistence of T-memory stem cells and CAR-T cell efficacy.217 Clinical trials involving CAR T cells expressing transgenic mbIL-15 have demonstrated both effectiveness and safety, showcasing potential in treating hematological malignancies.218,219 Additionally, IL-15 or IL-15/IL-15Rα complex has been successfully integrated into NK cells, overcoming their short lifespan and improving NK cell survival.220–223 The application of IL-15 in unconventional T cells, such as invariant natural killer T (iNKT) and gamma delta T (γδT) cells, further extends its application, with IL-15-armed iNKT and γδT cells demonstrating enhanced proliferation ability and antitumor activity.224,225 Despite the encouraging outcomes, safety concerns have been raised, particularly in IL-15-armed NK cell therapy, emphasizing the need for careful evaluation and refinement of these innovative approaches in cancer immunotherapy.220 Moreover, emerging strategies like IL-15-armed oncolytic viruses and tumor-conditional IL-15 pro-cytokines offer the capability to induce localized expansion of NK cells and T cells with minimal systemic toxicity.226–228 These innovative approaches highlight the promising potential of IL-15-based therapies in reshaping the landscape of cancer immunotherapy.
IL-1
IL-1 signaling and its protumor role
IL-1 is a potent DAMP, which was initially identified as a neutrophil-derived endogenous pyrogen.229 Subsequent investigations have elucidated its membership in a superfamily comprising 11 analogous molecules, each contributing to the intricate balance of pro-inflammatory and anti-inflammatory processes, particularly in the regulation of innate immune function.230 This family includes pro-inflammatory cytokines such as IL-1α, IL-1β, IL-18, IL-33, and IL-36α/β/γ, alongside anti-inflammatory counterparts like IL-1Ra, IL-33, IL-36Ra, IL-37, and IL-38.230 Notably, despite their significant homology and shared signaling redundancy, IL-1α and IL-1β exhibit distinct cellular origins, molecular regulations, and physiological roles in promoting inflammation.231 IL-1α serves as a paracrine DAMP, primarily released from cells undergoing severe physiologic stress or death, activating nearby cells to initiate a robust damage response.232 On the contrary, IL-1β functions as a systemic mediator of inflammation, triggered in response to distinct danger signals.233 IL-1α predominantly exerts its biological functions by binding to IL-1R1, a receptor featuring three primary ligands: IL-1α, IL-1β, and IL-1Ra.234 While IL-1α and IL-1β activate downstream signal transduction pathways, IL-1Ra acts as an endogenous inhibitor of IL-1R1 activity. Binding of either IL-1α or IL-1β to IL-1R1 initiates potent inflammation by canonical NF-κB and MAPK signaling pathways.235 This cascade involves the recruitment of IL-1RAcP, followed by the association of MYD88 and IRAK4.236,237 Subsequent autophosphorylation of IRAK4, phosphorylation of IRAK1/2, and the activation of TRAF6 trigger downstream signal transduction.238–241 TRAF6, an E3 ubiquitin ligase, forms K63-linked polyubiquitin chains crucial for activating NF-κB and MAPK pathways.242 As a results, the transcription of multiple IL-1-dependent pro-inflammatory mediators is upregulated, such as CXCL1/2, IL-6, and IL-8.243
IL-1 plays a multifaceted role in cancer, influencing various stages from carcinogenesis to metastasis. Elevated IL-1 levels are associated with poor prognosis in different cancers,244 and its production can be initiated by some oncogenic pathways, such as RAS signaling.245 IL-1 participates in carcinogenesis by promoting chronic inflammation and fostering a protumor cytokine network.245,246 It also mediates tumor angiogenesis by enhancing pro-angiogenic factor expression and endothelial cell activation.247,248 The involvement of IL-1 extends to therapy resistance, where it is linked to poor responses to EGFR tyrosine kinase inhibitor (TKI), radiotherapy, and other targeted therapies.249–252 Notably, the influences of IL-1 on antitumor immunity are paradoxical. While it exhibits antitumor effects by promoting the activation of NK and T cells, IL-1 contributes to cancer immunosuppression by improving the expansion and mobilization of immune cells such as MDSCs.253–255 These contradictory investigations underscore the pleiotropic nature of IL-1 signaling, confirming its dual impact in both promoting and suppressing tumors during cancer initiation and progression.233 Nevertheless, a substantial body of preclinical and clinical data overwhelmingly supports the notion that IL-1 predominantly operates in a protumor manner.235 Consequently, targeting IL-1 emerges as a potential therapeutic strategy, with ongoing clinical trials exploring the efficacy of anti-IL-1 therapies in various cancer types.
Anti-IL-1 therapy
At present, IL-1-based therapy has revealed promising avenues for therapeutic intervention in clinical trials. The strategies employed to target IL-1 signaling include direct inhibition of the IL-1 receptor, selective neutralization of IL-1α or IL-1β ligands with blocking antibodies, and targeted therapies against downstream molecules activated by the IL-1R1/MyD88 complex.235 Anakinra, a recombinant IL-1Ra, has secured FDA approval for rheumatoid arthritis and rare disorders.256 Beyond its established role in inflammatory diseases, anakinra has undergone small-scale clinical trials in solid tumors, exhibiting notable outcomes. Clinical studies using daily subcutaneous anakinra in patients with HER2-negative metastatic breast cancer demonstrated IL-1 receptor blockade-induced downregulation of genes involved in IL-1 and NF-κB signaling among circulating blood leukocytes.257 Additionally, anakinra in combination with standard chemotherapy and bevacizumab in metastatic colorectal cancer patients displayed well-tolerated results, with radiographic responses and stable disease observed.258 Notably, ongoing trials exploring isunakinra (an alternative form of rhIL-1Ra) plus anti-PD-1/L1 antibodies in solid tumors hold promise for further insights into IL-1Ra efficacy.259
Bermekimab/MABp1, an anti-IL-1α monoclonal antibody, has shown encouraging results in advanced colorectal cancer, as demonstrated in multiple clinical trials (Table 7).260 The phase I study exhibited a substantial reduction in serum IL-6 levels and an increase in lean body mass in patients, with notable responses observed, particularly in KRAS-mutant colon adenocarcinoma.261 Despite promising results, a phase III study, focusing on the improvement of quality-of-life metrics and lean body mass rather than traditional tumor-specific endpoints, showed some negative results.262 While patients treated with MABp1 demonstrated a significant improvement in the composite primary endpoint compared to placebo, post-hoc analysis revealed no significant improvements in individual quality-of-life scores with IL-1α neutralization.262 Furthermore, the termination of a subsequent phase III study (NCT01767857) due to treatment futility underscores the challenges of IL-1α inhibitor monotherapy in solid tumors, raising crucial questions about potential combinatorial treatment strategies in different clinical settings.262 Moreover, canakinumab, an anti-IL-1β monoclonal antibody, has emerged as a compelling therapeutic agent.263 The CANTOS trial demonstrated its efficacy in reducing cancer mortality (3.7 years post-treatment, hazard ratio [HR]: 0.49; P = 0.0009), particularly in lung cancer (canakinumab dose: 300 mg; HR: 0.23; P = 0.0002).264 Ongoing trials in advanced NSCLC explore canakinumab in combination with chemotherapy and immunotherapy, presenting a potential breakthrough in cancer treatment.265,266 These studies collectively underscore the intricate role of IL-1β blockade in impeding active disease progression and emphasize the need for further research into canakinumab efficacy as a pivotal element in IL-1-based cancer therapies.
Table 7.
Clinical trials inhibiting IL-1 for cancer therapy
| Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|
| Anakinra (IL-1 receptor antagonist) | NCT01802970 | Breast Cancer | Chemotherapy | I | Completed |
| NCT02090101 | Colorectal Cancer | LV5FU2 and Bevacizumab | II | Completed | |
| NCT04942626 | Rectal Cancer | Capecitabine-based Chemoradiotherapy | I | Active, not recruiting | |
| NCT00072111 | Solid Tumors | Monotherapy | I | Completed | |
| NCT02021422 | Pancreas Cancer | Oxaliplatin, Irinotecan, and Fluorouracil | I | Unknown status | |
| NCT01624766 | Solid Tumors | Everolimus | I | Completed | |
| NCT00635154 | MM | Dexamethasone | II | Completed | |
| NCT04227275 | mCRPC | CART-PSMA-TGFβRDN genetically modified T cells, Cyclophosphamide, and Fludarabine | I | Terminated | |
| NCT02550327 | Pancreatic Adenocarcinoma | Gemcitabine, Nab-Paclitaxel, and Cisplatin | I | Completed | |
| NCT03430011 | MM | JCARH125 | I/II | Completed | |
| NCT02492750 | MM | Lenalidomide and Dexamethasone | I | Completed | |
| NCT04432506 | B-Cell Lymphoma | Axicabtagene Ciloleucel, Cyclophosphamide, and Fludarabine | II | Active, not recruiting | |
| NCT04926467 | Pancreatic Adenocarcinoma | Chemotherapy | II | Not yet recruiting | |
| NCT04150913 | Non Hodgkin’s Lymphoma | Axicabtagene Ciloleucel | II | Active, not recruiting | |
| NCT04691765 | Chronic Lymphocytic Leukemia | Monotherapy | I | Unknown status | |
| NCT04205838 | DLBCL | Axicabtagene Ciloleucel, Cyclophosphamide, and Fludarabine | II | Recruiting | |
| Canakinumab (Anti-IL-1β mAb) | NCT05725343 | Lung Cancer | Monotherapy | III | Terminated |
| NCT05984602 | Pancreatic Cancer | Tislelizumab, Nab-Paclitaxel, and Gemcitabine | I | Recruiting | |
| NCT03447769 | NSCLC | Monotherapy | III | Terminated | |
| NCT04905316 | NSCLC | Chemotherapy, Radiation Therapy, and Durvalumab | II | Active, not recruiting | |
| NCT03968419 | NSCLC | Pembrolizumab | II | Terminated | |
| NCT03631199 | NSCLC | Pembrolizumab Plus Platinum-based Doublet Chemotherapy | III | Active, not recruiting | |
| NCT03626545 | NSCLC | Docetaxel | III | Terminated | |
| NCT03742349 | TNBC | Spartalizumab and LAG525 | I | Terminated | |
| NCT02900664 | Colorectal Cancer, TNBC, and NSCLC | Spartalizumab | I | Completed | |
| NCT04229004 | Pancreatic Adenocarcinoma | Spartalizumab, Nab-paclitaxel, and Gemcitabine | III | Active, not recruiting | |
| NCT04581343 | Pancreatic Ductal Adenocarcinoma | Spartalizumab, Nab-paclitaxel, and Gemcitabine | I | Active, not recruiting | |
| NCT03064854 | NSCLC | Spartalizumab Plus Platinum-doublet Chemotherapy | I | Terminated | |
| NCT04028245 | ccRCC | Spartalizumab | I | Recruiting | |
| NCT03484923 | Melanoma | Spartalizumab | II | Completed | |
| MABp1 (Anti-IL-1α mAb) | NCT01021072 | Solid Tumors | Monotherapy | I | Completed |
| NCT01767857 | Colorectal Cancer | Monotherapy | III | Terminated |
Note: MM multiple myeloma, mCRPC metastatic castration-resistant prostate cancer, DLBCL diffuse large B-cell lymphoma, NSCLC non-small cell lung cancer, TNBC triple negative breast cancer, ccRCC clear cell renal cell carcinoma
IL-6
The role of IL-6 signaling in cancer progression and immune-related adverse events
IL-6 is a multifaceted cytokine playing critical roles in immune responses, inflammation, and a range of physiological processes such as hematopoiesis, bone metabolism, and embryonic development.267 Its significance is particularly noted in the pathophysiology of various diseases, including cancer.268 IL-6 signals through three distinct pathways: classical, trans-signaling, and trans-presentation signaling.269 Classical signaling involves IL-6 binding to its membrane-bound receptor (mIL-6R), leading to gp130 receptor dimerization and signal transduction.270 Trans-signaling allows cells without mIL-6R to respond to IL-6 via the soluble form of IL-6R (sIL-6R).271 Trans-presentation signaling facilitates IL-6 presentation from mIL-6R on one cell to gp130 on another, broadening cellular responses.272 Classical signaling is crucial for acute-phase immune responses, hematopoiesis, and homeostasis.273 Trans-signaling plays a vital role in the TME by modulating immune cell recruitment and stromal cell inflammatory responses.273 Trans-presentation signaling is essential for pathogenic Th17 cell priming.272
The dysregulation of IL-6 signaling, particularly via the JAK-STAT3 pathway, has been identified as a pivotal contributor to tumorigenesis.274 The JAK-STAT3 pathway is initiated by the formation of hexameric IL-6/IL-6Rα/gp130 complex, subsequently ensuing in gp130 phosphorylation and STAT3 activation.275 The activated STAT3 then migrates to the nucleus, where it modulates gene expression related to cell cycle progression, survival, and angiogenesis, including cyclin-D1, Bcl-2, c-Myc, Bcl-xL, survivin, VEGF, MMP-2, and IL-6 itself.276–285 Importantly, this signaling pathway not only directly fosters tumor growth but also significantly contributes to immune evasion by altering the TME.286 IL-6 undermines immune surveillance by regulating the immunosuppressive capacity of MDSCs, inhibiting antigen presentation, and upregulating immune checkpoint molecules.287–290 Consequently, IL-6-mediated immune suppression diminishes the efficacy of ICB therapies, with IL-6 levels serving as predictive markers for ICB response.291,292 Preclinical investigations have shown that IL-6 inhibition, in synergy with ICB, amplifies antitumor immunity and curtails tumor progression across various cancer models.293 Additionally, IL-6 has been implicated in intensifying immune-related adverse events (irAEs) associated with ICB, suggesting its significant impact on patient management beyond mere tumor suppression.294 The strategic combination of IL-6 targeting agents with ICB not only holds promise for augmenting cancer treatment efficacy but also for managing irAEs, as demonstrated by the effective application of the anti-IL-6R antibody tocilizumab in clinical practice.295
IL-6 blockade to improve immunotherapy efficacy and mitigate adverse events
Therapeutic approaches to inhibit IL-6 signaling are principally divided into two main categories: antibodies targeting IL-6 or its receptor, and small-molecule inhibitors of JAK and STAT3. In addition to these conventional strategies, innovative blockade techniques have emerged, including the development of sgp130-Fc fusion proteins, STAT3 antisense oligonucleotides, and cyclic STAT3 decoys.296 These novel approaches offer alternative mechanisms to modulate the IL-6 signaling axis, potentially overcoming the limitations of existing therapies and providing new avenues for the treatment of diseases mediated by aberrant IL-6 signaling.
Anti-IL-6/IL-6R monoclonal antibodies such as tocilizumab, sarilumab, and siltuximab, initially approved for indications like rheumatoid arthritis and Castleman disease, have been repurposed with promising implications for cancer, particularly in managing cytokine release syndrome associated with CAR-T cell therapy (Table 8).297 The development of novel blockade strategies, including sgp130-Fc fusion proteins, has expanded the therapeutic arsenal, aiming to selectively inhibit IL-6 trans-signaling without compromising immune defense mechanisms.298–300 Despite the therapeutic potential, challenges such as increased risk of bacterial infections and limited efficacy in unselected patient populations highlight the complexity of targeting IL-6 in cancer. Clinical trials investigating the antitumor efficacy of siltuximab have shown mixed results, underscoring the necessity for combination therapies and the identification of predictive biomarkers to enhance treatment outcomes.301–303 The exploration of tocilizumab in various cancers through early-phase trials further exemplifies ongoing efforts to harness anti-IL-6 strategies, potentially offering new avenues for cancer therapy by mitigating pro-inflammatory effects while preserving immune surveillance.304,305
Table 8.
Clinical trials of IL-6 blocking antibodies for cancer therapy
| Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|
| Siltuximab | NCT00311545 | Kidney Cancer | Monotherapy | II | Withdrawn |
| NCT00433446 | Prostate Cancer | Monotherapy | II | Completed | |
| NCT00385827 | Prostate Cancer | Mitoxantrone and Prednison | II | Terminated | |
| NCT04191421 | Pancreatic Cancer | Spartalizumab | I/II | Completed | |
| NCT00401765 | Prostate Cancer | Docetaxel | I | Completed | |
| NCT00841191 | Solid Tumors | Monotherapy | I/II | Completed | |
| NCT01309412 | MM | Monotherapy | I | Terminated | |
| NCT00402181 | MM | Dexamethasone | II | Completed | |
| NCT01266811 | MM | Velcade and Dexamethasone | III | Withdrawn | |
| NCT00401843 | MM | Bortezomib and Dexamethasone | II | Completed | |
| NCT01531998 | MM | Lenalidomide, Bortezomib, and Dexamethasone | I/II | Completed | |
| NCT00911859 | MM | Velcade, Melphalan, and Prednisone | II | Completed | |
| NCT01484275 | MM | Monotherapy | II | Completed | |
| NCT05697510 | AML | Monotherapy | I | Recruiting | |
| NCT00265135 | RCC | Monotherapy | I/II | Completed | |
| NCT00412321 | Non-Hodgkin’s Lymphoma and MM | Monotherapy | I | Completed | |
| NCT05316116 | LGLL | Monotherapy | I | Recruiting | |
| NCT05665725 | Non-Hodgkin’s Lymphoma | Monotherapy | I | Recruiting | |
| Tocilizumab | NCT06016179 | Metastatic Cancer | Monotherapy | I | Recruiting |
| NCT05846789 | Breast Cancer | Carboplatin | II | Recruiting | |
| NCT05619744 | SCLC and Neuroendocrine Carcinoma | RO7616789 | I | Recruiting | |
| NCT05129280 | Solid Tumors | RO7444973 | I | Terminated | |
| NCT04940299 | Melanoma, NSCLC, or Urothelial Carcinoma | Ipilimumab and Nivolumab | II | Active, not recruiting | |
| NCT04691817 | NSCLC | Atezolizumab | I/II | Recruiting | |
| NCT04547062 | AML | Monotherapy | I | Completed | |
| NCT04375228 | Solid Tumors | Monotherapy | II | Recruiting | |
| NCT04338685 | Hepatocellular Carcinoma, Biliary Tract Cancer, Or Tumors with Hepatic Metastases | RO7119929 | I | Completed | |
| NCT04258150 | Pancreatic Cancer | Nivolumab, Ipilimumab, and SBRT | II | Terminated | |
| NCT03999749 | Melanoma | Ipilimumab and Nivolumab | II | Active, not recruiting | |
| NCT03866239 | Colorectal Cancer | Obinutuzumab, Atezolizumab, and Cibisatamab | I | Active, not recruiting | |
| NCT03821246 | Prostate Cancer | Atezolizumab and Etrumadenant | II | Recruiting | |
| NCT03708224 | HNSCC | Atezolizumab and Tiragolumab | II | Recruiting | |
| NCT03588936 | Hematological Malignancy | Nivolumab | I | Terminated | |
| NCT03424005 | Breast Cancer | Atezolizumab and Nab-Paclitaxel | I/II | Recruiting | |
| NCT03135171 | Breast Cancer | Trastuzumab and Pertuzumab | I | Completed | |
| NCT02997956 | Hepatocellular Carcinoma | Transcatheter Arterial Chemoembolization | I/II | Withdrawn | |
| NCT02906371 | Lymphoblastic Leukemia | CART19 Therapy | I | Completed | |
| NCT02767557 | Pancreatic Carcinoma | Nab-Paclitaxel and Gemcitabine | II | Completed | |
| NCT01637532 | Ovarian Cancer | Chemotherapy and Peg-Intron | I/II | Completed | |
| Sarilumab | NCT05704634 | NSCLC | Cemiplimab | I | Recruiting |
| NCT04333706 | TNBC | Capecitabine | I/II | Recruiting | |
| NCT03972657 | mCRPC and ccRCC | REGN5678 and Cemiplimab | I/II | Recruiting | |
| NCT03564340 | Ovarian Cancer or Other MUC16+ Cancers | REGN4018 | I/II | Recruiting | |
| NCT05125016 | mCRPC | REGN4336 and Cemiplimab/REGN5678 | I/II | Recruiting | |
| NCT05428007 | Melanoma | Ipilimumab and Nivolumab/Relatlimab | II | Recruiting |
Note: AML acute myeloid leukemia, RCC renal cell carcinoma, LGLL large granular lymphocytic leukemia, mCRPC metastatic castration-resistant prostate cancer, ccRCC clear cell renal cell carcinoma, NSCLC non-small cell lung cancer, SCLC small cell lung cancer, MM multiple myeloma
Besides, small-molecule inhibitors targeting downstream elements of the IL-6 signaling pathway, such as JAK and STAT3, show promise in cancer treatment as well. JAK inhibitors, such as tofacitinib and ruxolitinib, have been approved for various inflammatory diseases and myeloproliferative neoplasms, demonstrating their potential to modulate immune responses.306,307 Despite preclinical data suggesting JAK inhibitors could retard solid tumor growth, clinical evidence supporting their use in solid tumors is limited.308 At present, ongoing early-phase trials continue to evaluate the safety and potential efficacy of JAK inhibitors in various solid cancers, aiming to identify therapeutic windows that balance efficacy with tolerability.309,310 For instance, antisense oligonucleotides like AZD9150 have shown activity against treatment-refractory lymphoma and NSCLC, with a maximum-tolerated dose established at 3 mg/kg, showcasing a favorable safety profile.311 Moreover, early-phase clinical trials for nonpeptide SH2 domain antagonists such as OPB-31121 and OPB-51602 have provided evidence of antitumor activity, particularly in hepatocellular carcinoma and NSCLC, despite facing tolerability challenges like peripheral neuropathy and pneumonitis.312–315
Notably, integrating anti-IL-6 therapies with ICB represents a promising approach to overcoming immunosuppression driven by cancer-promoting inflammation. The complexity of chronic inflammation, regulated by numerous pathways and compensatory mechanisms, has limited the efficacy of cytokine-targeting drugs as monotherapies. However, robust preclinical evidence supports the combination of IL-6 signaling blockade with ICB as an attractive strategy for enhancing treatment efficacy in solid tumors, potentially boosting ICB effectiveness and mitigating irAEs.294,316 The efficacy of tocilizumab in treating ICB-induced colitis and arthritis was evaluated in the COLAR study.317 Nineteen patients received tocilizumab treatment (8 mg/kg) every four weeks until symptoms worsened or unacceptable toxicity, without the use of systemic glucocorticoids or other immunosuppressive drugs within a 14-day follow-up period.317 The primary endpoint, clinical improvement in colitis and arthritis, specifically achieving a reduction of at least one grade in the CTCAE within an 8-week period, was achieved by 79% of the patients, with ongoing improvement or complete remission in 12 patients at week 24, without the need for glucocorticoids. The trial supports the feasibility of randomized trials for tocilizumab as a treatment for ICB-induced colitis and arthritis.317 Additionally, the use of JAK and STAT3 inhibitors combined with ICB in advanced cancers, exemplified by ruxolitinib-alleviated ICB-associated myocarditis, underscores the potential of targeting the IL-6/JAK/STAT3 signaling pathway to augment antitumor immunity and address the adverse inflammatory effects of ICB treatment.318,319 This evolving paradigm suggests a synergistic potential that could redefine treatment strategies for patients with advanced-stage cancers.
TNF signaling and TNF blockade for immunotherapy
TNF signaling: from direct tumoricidal effects to multifaceted protumor activities
TNF was first isolated as a crucial factor responsible for endotoxin-induced hemorrhagic necrosis of tumors.320 The cloning of the TNF gene in the 1980s expanded the understanding of its role, revealing its identity as cachectin, a key player in the physiological responses to infection, including acute shock and chronic cachexia.321 Subsequent research highlighted the complex role of TNF in cancer, initially seen as a promising anti-cancer agent due to its ability to induce tumor necrosis.322 However, its potential as a therapeutic has been limited by a narrow therapeutic window. At physiologically tolerable levels, TNF alone is not directly cytotoxic to cancer cells.323 Currently, our understanding of the biological functions of TNF has undergone significant evolution. Beyond its direct tumoricidal effects under specific conditions, TNF has been implicated in promoting tumor progression. The protumor activities of TNF are multifaceted, involving the modulation of the TME to favor cancer cell proliferation, survival, and metastasis.324 This includes the induction of angiogenesis, a process crucial for tumor growth and metastasis, whereby TNF stimulates the formation of new blood vessels, ensuring a steady supply of nutrients and oxygen to rapidly growing tumors.325
Furthermore, TNF has been shown to contribute to cancer immune evasion. Preclinical studies have revealed that TNF hinders the accumulation of CD8+ T cells in tumor-draining lymph nodes and tumors through TNFR-mediated activation-induced cell death (AICD) in CD8+ T cells.326 Moreover, TNF undermines the antitumor activity of NK cells by upregulating TIM-3 and downregulating NKp46.327,328 Furthermore, TNF promotes Treg proliferation and suppressive functions, which in turn dampens the overall immune response against tumors. This effect is particularly pronounced in Treg cells that express TNFR2, which are found in high densities within the TME and contribute to tumor growth by suppressing non-Treg cell proliferation.329,330 Conversely, TNF enhances Th cell proliferation and pro-inflammatory cytokine production, but this effect is complicated by TNF inhibitors potentially promoting Th1 cell function indirectly by restraining Treg cells.331 TNF also plays a role in the survival and immunosuppressive activity of MDSCs.332,333 Additionally, TNF stimulates mesenchymal stem cells (MSCs) to recruit CCR2-positive tumor-associated macrophages (TAMs) into the TME, further supporting tumor growth.334 Also, TNF increases PD-L1 surface expression on cancer cells by stabilization of PD-L1.335 Therefore, inhibiting TNF presents a promising strategy not only to enhance the antitumor immune response by improving T cell and NK cell function and restraining immunosuppressive Treg, MDSCs, and MSCs but also to directly inhibit cancer cell survival and proliferation, illustrating the multifaceted role of TNF in cancer immunology and the potential benefits of its inhibition. Preclinical studies have demonstrated that TNF blockade enhances the therapeutic effect of anti-PD-1 treatment, elevating tumor rejection rates from 20% with anti-PD-1 alone to 75% when combined with TNF inhibition.336,337
TNF blockade to improve immunotherapy efficacy and alleviate adverse events
In addition to synergistic antitumor effects, of greater interest is the value of TNF blockade in mitigating irAEs, especially IBD-induced colitis. Elevated TNF levels were found in patients with colitis after treatment with ipilimumab and nivolumab. In the xenograft model, preventive TNF blockade not only alleviates colitis and hepatitis in the mice but also maintains the efficacy of immunotherapy.338 Actually, anti-TNF antibodies such as infliximab and adalimumab have been widely used for the treatment of inflammatory bowel disease and some autoimmune diseases such as rheumatoid arthritis.339,340 Badran et al. reported five cancer patients treated with ICB developed immune-related enterocolitis (irEC) within 40 days of treatment onset, confirmed by endoscopy to be acute inflammation. Initial treatment with steroids was supplemented by adding infliximab to avoid long-term steroid use and gastrointestinal symptom recurrence. This combination therapy allowed continued ICB treatment, with follow-up checks showing inflammation resolution and no cancer progression. This suggests that combining anti-TNF-α with ICB is a promising strategy for safely managing irEC.341 Moreover, the TICIMEL phase Ib clinical trial (NTC03293784) evaluated the combination of TNF blockers (infliximab or certolizumab) with ICB in 14 advanced melanoma patients (Table 9).342 This trial aimed to assess the safety and antitumor efficacy of these combinations, with a particular focus on managing gastrointestinal side effects. The trial found both combinations to be safe, with only one dose-limiting toxicity reported in the infliximab group and generally lower treatment-related adverse events for infliximab compared to certolizumab.342 The certolizumab cohort had a notable response rate: 7 of 7 evaluable patients showed an objective response, including four complete responses. In contrast, the infliximab cohort recorded one complete response, two partial responses, and three progressive diseases. The results suggest the safety and potential antitumor benefits of these combinations.342
Table 9.
Clinical trials involving TNF antagonist for cancer therapy
| Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|
| Infliximab | NCT05034536 | Melanoma | Pembrolizumab | II | Recruiting |
| NCT04407247 | Genitourinary Cancer or Melanoma | Monotherapy | I/II | Recruiting | |
| NCT04305145 | Melanoma | Monotherapy | II | Unknown status | |
| NCT04082910 | Solid and Hematological Malignancy | Metoprolol | I/II | Recruiting | |
| NCT03293784 | Melanoma | Nivolumab and Ipilimumab | I | Completed | |
| NCT02763761 | RCC, Melanoma, and Lung Cancer | Monotherapy | II | Withdrawn | |
| NCT00112749 | Breast Cancer | Monotherapy | II | Terminated | |
| NCT00060502 | Pancreatic Neoplasms | Gemcitabine | II | Completed | |
| NCT00040885 | Lung Cancer | Docetaxel | III | Completed | |
| Etanercept | NCT00201812 | Solid Tumors | Docetaxel and Dexamethasone | I | Completed |
| NCT00046904 | Solid Tumors | Monotherapy | III | Completed | |
| NCT00201838 | Pancreatic Neoplasms | Gemcitabine | I/II | Completed | |
| NCT03792841 | Prostate Cancer | Acapatamab | I | Completed | |
| NCT00127387 | Solid Tumors | Monotherapy | II/III | Terminated | |
| NCT04082910 | Solid and Hematological Malignancy | Metoprolol | I/II | Recruiting | |
| Adalimumab | NCT02516774 | Anaplastic Thyroid Cancers | Monotherapy | I | Withdrawn |
| Golimumab | NCT05960578 | Prostate Cancer | Apalutamide | II | Recruiting |
Note: RCC renal cell carcinoma
Utilizing chemokines in cancer therapy
The role of chemokines in cancer involves a complex interplay among cancer cells, tissue-resident cells, and immune cells. These chemokines influence tumor cell behavior by affecting their stemness, proliferation, and invasiveness, as well as impacting stromal cells to modulate processes like angiogenesis and fibrogenesis.343 Importantly, chemokines also shape the phenotype and function of immune cells within both lymphoid tissues and the TME. On the one hand, they orchestrate the recruitment and spatial organization of immune cells, facilitating their interactions within tissues, which is crucial for triggering antitumor immune response. On the other hand, chemokines also contribute to the formation of protumor microenvironment.343 The balance between antitumor and protumor roles of chemokines depends on tumorigenesis stages, immune cell activation states, and the specific chemokine receptors expressed on target cells. Targeting chemokines that facilitate antitumor immune cell recruitment, or inhibiting those that enhance the suppressive immune cell function, presents promising strategies to enhance the efficacy of cancer therapies (Fig. 6).344
Fig. 6.
Chemokine-targeted cancer immunotherapy. The diagram presents the complexity of chemokine ligand-receptor interactions and their implications for cancer immunotherapy. The top section identifies the chemokine ligands (e.g., CCL2, CCL7, CXCL9) and their corresponding receptors, categorized by their role in tumor progression, with antitumor receptors labeled in green (e.g., CXCR3, CXCR6) and protumor receptors in blue (e.g., CCR2, CXCR1, CXCR2). The bottom left panel highlights the blockade of protumor chemokine signaling using antibodies and inhibitors targeting specific CCL and CXCL chemokines and their receptors to prevent immune evasion and tumor progression. The bottom right panel showcases the expression of antitumor chemokines or their receptors, such as CCL19/IL-7 expressing CAR-T cells, CXCL10-scFv, CXCL9/CXCL10/CXCL11 expressing oncolytic viruses (OVs), and CXCR6 expressing CAR-T cells, as innovative strategies to enhance antitumor immunity. This figure encapsulates the dual approach of inhibiting tumor-promoting chemokines and augmenting antitumor chemokines to therapeutically modulate the tumor microenvironment. (Created with BioRender.com)
CCL2-CCR2 axis
The protumor role of CCL2-CCR2 axis
The CCL2-CCR2 signaling axis plays a pivotal role in tumorigenesis, promoting the initiation, progression, and metastasis of various malignancies, including breast, lung, hepatocellular, gastric, esophageal, prostate, ovarian, and bladder cancers.345–352 It supports tumor growth and proliferation at the primary site and facilitates tumor metastasis.353 Moreover, CCL2-CCR2 signaling orchestrates an immunosuppressive TME by recruiting MDSCs, Tregs, TAMs, and other immune cells.354–356 This axis also significantly contributes to tumor angiogenesis by directly stimulating vascular endothelial cells and indirectly through the recruitment of inflammatory cells that express angiogenic factors.357–359 In addition to its role in recruiting immunosuppressive cell types, tumor-derived CCL2 impacts the function of effector T cells.360 Targeting the CCL2-CCR2 axis has emerged as a potential therapeutic strategy, aiming to inhibit the recruitment of protumor immune cells and disrupt the protumor TME, thus opening new avenues for cancer therapy.
CCL2-CCR2 axis blockade for cancer therapy
Agents targeting the CCL2-CCR2 axis have demonstrated promising antitumor activity in preclinical studies. Inhibition of CCL2, using various inhibitors or antibodies like C1142, bindarit, and curcumin, has been shown to suppress tumor growth by blocking CCL2-mediated signaling pathways, reducing immunosuppressive cell recruitment, and increasing effector T cell numbers.361–363 Similarly, targeting CCR2 with antagonists such as RS-504393 and RS-102895 has been effective in delaying tumor progression by inhibiting the infiltration of immunosuppressive cells into tumors.364–366 Moreover, combined therapy approaches, integrating CCL2-CCR2 axis blockade with existing cancer treatments, have been explored to overcome the complexity of cancer pathogenesis and minimize side effects. For instance, dual targeting of CCL2/CCR2 and PD-1 has yielded notable tumor suppression and improved survival of tumor-bearing mice.367–369 These advances underscore the importance of the CCL2-CCR2 axis in cancer immunology and its potential as a therapeutic target.
Encouraged by the positive results of preclinical studies, the antitumor activity and safety profile of CCL2/CCR2 antagonists have been intensively explored in clinical trials, particularly with agents such as Carlumab and PF-04136309 (Table 10). Carlumab, a human anti-CCL2 antibody, was well-tolerated in a phase I study involving patients with advanced solid tumors, showing no dose-limiting toxicity.370 However, its therapeutic impact was modest, with stable disease observed in a minority of patients but without any achieving an objective response.370 In a phase II study for metastatic castration-resistant prostate cancer, Carlumab did not lead to any prostate-specific antigen (PSA) response, and only 34% of patients maintained stable disease beyond three months.371 PF-04136309, a CCR2 inhibitor, exhibited promising antitumor activity in a phase Ib study when combined with FOLFIRINOX chemotherapy for pancreatic cancer, achieving tumor control in 97% of patients and objective tumor response in 49%.372 Moreover, CCX872-B, another CCR2 antagonist, combined with FOLFIRINOX for pancreatic adenocarcinoma, showed an 18-month OS rate of 29%, better than historical data of FOLFIRINOX regimen alone, suggesting a potential survival benefit.373 Notably, the study of PF-04136309 reported treatment-related serious adverse events in 66.7% of patients, especially synergistic pulmonary toxicity when combined with nab-paclitaxel/gemcitabine,374 highlighting the need for careful consideration of safety alongside therapeutic benefits.
Table 10.
Clinical trials involving CCL2/CCR2 inhibitors for cancer therapy
| Classification | Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|---|
| CCR2/5i | BMS-813160 | NCT04123379 | NSCLC and HCC | Nivolumab | II | Active, not recruiting |
| NCT03184870 | Colorectal and Pancreatic Cancer | Chemotherapy or Nivolumab | I/II | Completed | ||
| NCT02996110 | RCC | Nivolumab | II | Completed | ||
| NCT03767582 | Pancreatic Ductal Adenocarcinoma | SBRT, Nivolumab, and GVAX | I/II | Recruiting | ||
| NCT03496662 | Pancreatic Ductal Adenocarcinoma | Nivolumab, Gemcitabine, and Nab-paclitaxel | I/II | Active, not recruiting | ||
| CCR2i | CCX872-B | NCT03778879 | Pancreatic Cancer | SBRT | I/II | Withdrawn |
| NCT02345408 | Pancreatic Cancer | Monotherapy | I | Completed | ||
| PF-04136309 | NCT01413022 | Pancreatic Cancer | Oxaliplatin, Irinotecan, Leucovorin, and Fluorouracil | I | Completed | |
| NCT02732938 | Pancreatic Ductal Adenocarcinoma | Nab-paclitaxel and Gemcitabine | II | Terminated | ||
| Anti-CCR2 mAb | MLN1202 | NCT01015560 | Solid Tumors | Monotherapy | II | Completed |
| NCT02723006 | Melanoma | Nivolumab | I | Terminated | ||
| Anti-CCL2 mAb | Carlumab | NCT00992186 | Prostate Cancer | Monotherapy | II | Completed |
Note: NSCLC non-small cell lung cancer, HCC hepatocellular carcinoma, SBRT stereotactic body radiation therapy, RCC renal cell carcinoma, mAb monoclonal antibody
CCR4 signaling pathway
CCR4 signaling-mediated cancer immune evasion
The CCR4 signaling pathway plays a pivotal role in the TME, primarily through its expression on a majority of human Tregs (>90%).375 In various cancers, tumor cells, TAMs, and DCs secrete high levels of CCR4 ligands, CCL17 and CCL22, which facilitate Treg infiltration into tumor sites.376,377 This infiltration, driven by the interaction between CCR4 on Tregs and ligands produced by the tumor, has been correlated with a poor prognosis.378 The strategic blockade of this pathway, either through targeting CCL22 with monoclonal antibodies to reduce Treg migration into tumors or by directly inhibiting CCR4 to prevent its interaction with multiple chemokines, has shown promise.379 Direct CCR4 blockade, has demonstrated its efficacy by not only reducing Treg infiltration but also inhibiting tumor growth in xenograft mouse models, indicating the potential of CCR4 as a therapeutic target in cancer treatment.380,381 Apart from Tregs, CCR4 blockade regulates the TAM phenotype and decreases the presence of immature myeloid cells in the TME.382,383 Furthermore, CCR4-dependent Treg accumulation is a core factor contributing to ICB resistance. In Pan02 and CT26 mouse tumor models, CCR4 blockade decreases Treg migration, thereby improving ICB performance, particularly in tumors with high baseline CCR4 ligand expression or in those where ICB treatment upregulates CCR4 ligands.384 Consequently, inhibiting CCR4 not only reduces Treg frequency but also amplifies the efficacy of ICB, highlighting the importance of CCR4-dependent Treg recruitment in immunotherapy resistance and supporting the use of CCR4 inhibitors alongside ICB in cancer treatment strategies.384
CCR4 antagonists improving immunotherapy effectiveness especially ICB
At present, several CCR4 antagonists have undergone evaluation in clinical trials, however, mogamulizumab is the sole CCR4 antagonist approved for cancer treatment, specifically for treating T cell lymphomas.385 Besides, mogamulizumab effectively induced depletion of FoxP3+ Tregs in patients with solid tumors.386 In a phase I clinical trial, mogamulizumab was safe and well-tolerated, without any dose-limiting toxicity (Table 11). Notably, four out of ten patients exhibited stable disease and were categorized as long survivors. Treatment resulted in effective Tregs depletion at even the lowest dose, with minimal impact on Th1 T cells but significant reductions in Th2 and Th17 CD4+ T cells.386 Then in the multicenter phase I study (NCT02301130), the safety, antitumor efficacy, and pharmacodynamics of mogamulizumab combined with ICB (durvalumab or tremelimumab) were evaluated in patients with advanced solid tumors. No dose-limiting toxicities were reported across the 64 participants, and the treatment was found to be tolerable.387 However, the ORR stood at a mere 5.3%, indicating limited antitumor efficacy despite the effective depletion of peripheral and intratumoral Tregs by mogamulizumab. There was also no apparent correlation between the clinical response and the reduction in CCR4+ Tregs or baseline CCR4 expression.387 On the contrary, in another phase I clinical study NCT02476123, the combination of mogamulizumab and anti-PD-1 antibody nivolumab exhibited an acceptable safety profile and meaningful antitumor activity in solid tumors. In this trial, no dose-limiting toxicities were observed in the dose-escalation part.388 Grade 3/4 treatment-related adverse events occurred in 29% of patients in the expansion part. Besides, 27% of hepatocellular carcinoma patients (4 out of 15) showed confirmed tumor responses, and in the pancreatic adenocarcinoma cohort, there was one confirmed and two unconfirmed responses among 15 patients.388 This regimen also led to decreased populations of effector Tregs and increased CD8+ T cells within the TME.388 The discrepancy between the two trials underscores the critical role of the tumor microenvironment and the specific mechanisms of action of the therapeutic agents used. It suggests that the success of combining Treg depletion with ICB may be contingent on selecting the right combination of therapeutic agents, the cancer type, and understanding the underlying tumor immunobiology. Furthermore, these trials highlight the need for biomarker-driven patient selection and personalized approaches to immunotherapy. Identifying patients who are more likely to benefit from Treg depletion in combination with checkpoint inhibition could enhance the efficacy of such treatments and provide valuable insights into optimizing cancer immunotherapy strategies.
Table 11.
Clinical trials involving Anti-CCR4 antibody mogamulizumab for cancer therapy
| NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|
| NCT02358473 | NSCLC | Docetaxel | I | Completed |
| NCT02867007 | Solid Tumors | KHK2455 | I | Completed |
| NCT02946671 | Solid Tumors | Nivolumab | I | Completed |
| NCT02281409 | Solid Tumors | Monotherapy | I/II | Completed |
| NCT02301130 | Solid Tumors | Durvalumab/Tremelimumab | I | Completed |
| NCT02476123 | Solid Tumors | Nivolumab | I | Completed |
| NCT02444793 | Solid Tumors | PF-05082566 | I | Terminated |
| NCT01929486 | Solid Tumors | Monotherapy | I | Unknown status |
| NCT02705105 | Solid Tumors | Nivolumab | I/II | Completed |
| NCT01611142 | T-Cell Lymphoma | Monotherapy | II | Completed |
| NCT04745234 | T-Cell Lymphoma | Monotherapy | II | Active, not recruiting |
| NCT04128072 | T-Cell Lymphoma | Total Skin Electron Beam Therapy | II | Recruiting |
| NCT05996185 | T-Cell Lymphoma | DA-EPOCH Chemotherapy | II | Not yet recruiting |
| NCT00920790 | T-cell Leukemia/lymphoma | Monotherapy | II | Completed |
| NCT03309878 | DLBCL | Pembrolizumab | I/II | Completed |
| NCT01728805 | T-Cell Lymphoma | Monotherapy | III | Completed |
| NCT05414500 | T-Cell Lymphoma | Brentuximab vedotin | I | Recruiting |
| NCT05956041 | T-Cell Lymphoma | Pembrolizumab | II | Recruiting |
| NCT04185220 | T-Cell Lymphoma | Recombinant Human IL-15 | I | Completed |
| NCT04930653 | T-Cell Lymphoma | Extracorporeal Photopheresis | II | Recruiting |
| NCT04676087 | Non-Hodgkin’s Lymphoma | Extracorporeal Photopheresis | I/II | Recruiting |
| NCT01226472 | T-Cell Lymphoma | Monotherapy | II | Completed |
| NCT01192984 | T/NK-cell Lymphoma | Monotherapy | II | Completed |
| NCT04848064 | Lymphoma | NK cell infusion and Chemotherapy | I | Recruiting |
| NCT00355472 | T-Cell Lymphoma | Monotherapy | I | Completed |
| NCT01173887 | T-Cell Lymphoma | VCAP/AMP/VECP(mLSG15) Chemotherapy Strategy | II | Completed |
| NCT01626664 | T-Cell Lymphoma | Monotherapy | II | Completed |
| NCT00888927 | T-Cell Lymphoma | Monotherapy | I/II | Completed |
Note: NSCLC non-small cell lung cancer, DLBCL diffuse large B cell lymphoma
CCL5/CCR5 signaling pathway
CCL5/CCR5 signaling supporting tumor development
The CCL5/CCR5 signaling pathway plays a pivotal role in cancer development and progression.389 CCL5, also known as RANTES, is a chemokine primarily expressed by inflammatory cells, notably T cells and monocytes.390 It binds with the highest affinity to CCR5, a G-protein-coupled receptor (GPCR) found in various cell types, including T cells, smooth muscle, epithelial, and endothelial cells.391 The CCL5/CCR5 axis is involved in numerous physiological and pathological processes, such as HIV infection, cell proliferation, migration, angiogenesis, metastasis, and survival, making it a focal point of study in inflammation, cancer, and viral infections.392,393 The signaling pathways activated downstream of CCL5/CCR5 signaling, such as PI3K/AKT, MAPK, JAK-STAT, NF-κB, HIF-1α, and TGF-β-Smad, are implicated in promoting uncontrolled tumor cell proliferation, angiogenesis, apoptosis resistance, invasion, and metastasis.392 Recent research highlights the significant role of CCL5/CCR5 signaling in creating a protumor TME by recruiting Tregs, MDSCs, and TAMs, thereby contributing to tumor immunosuppression.394–396
CCL5/CCR5 blockade: from HIV infection treatment to cancer therapy
The CCL5/CCR5 axis has been identified as a target for therapeutic intervention, especially cancers like breast cancer.397 Current strategies focus on developing small molecule inhibitors like maraviroc, cenicriviroc, anibamine, vicriviroc, and MET-CCL5, which have shown potential in clinical evaluations for their anti-inflammatory and anti-cancer properties (Table 12).398–402 Maraviroc, an FDA-approved drug for HIV infection, repurposed in cancer therapy, competes with CCL5 for CCR5 binding, inhibiting the recruitment of cancer-promoting cells, thus hindering tumor growth and metastasis.403–405 Besides, preclinical results demonstrate that maraviroc could enhance the efficacy of other antitumor agents such as temozolomide and ICB.406 Pericyte-derived CCL5 activates CCR5 in glioblastoma cells, triggering DNA-PKcs-mediated DNA damage repair when exposed to temozolomide. Hereto, blocking this CCL5-CCR5 interaction with maraviroc significantly reduces DDR promoted by pericytes and enhances TMZ efficacy in GBM-2 xenografts.406 In the phase I trial PICCASSO, the safety and potential antitumor effects of the combination of pembrolizumab and maraviroc were evaluated in patients with refractory mismatch repair proficient colorectal cancer.407 Although pembrolizumab combined with maraviroc treatment exhibited a favorable toxicity profile, the ORR was low at 5.3%, and the median PFS was only 2.10 months, with a median OS of 9.83 months.407 This early-phase clinical trial suggests the need for further research to enhance therapeutic strategies for this challenging patient population.
Table 12.
Clinical trials involving CCR5 inhibitors for cancer therapy
| Classification | Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|---|
| CCR5 antagonist | Maraviroc | NCT04721301 | Colorectal and Pancreatic Cancer | Nivolumab and Ipilimumab | I | Completed |
| NCT01736813 | Colorectal Cancer | Monotherapy | I | Completed | ||
| NCT01785810 | Hematologic Malignancy | Monotherapy | II | Completed | ||
| NCT03274804 | Colorectal Cancer | Pembrolizumab | I | Completed | ||
| NCT01276236 | HIV-related Kaposi’s Sarcoma | Monotherapy | II | Completed | ||
| Vicriviroc | NCT03631407 | Colorectal Cancer | Pembrolizumab | II | Completed | |
| Anti-CCR5 mAb | Leronlimab | NCT05730673 | CCR5+ Colorectal Cancer | Regorafenib | II | Withdrawn |
| NCT04504942 | CCR5+ Solid Tumors | Monotherapy | II | Unknown status | ||
| NCT04313075 | TNBC | Monotherapy | CU | No longer available | ||
| NCT03838367 | TNBC | Monotherapy | I/II | Unknown status |
Note: TNBC triple negative breast cancer, CU compassionate use, mAb monoclonal antibody. Clinical trials involving BMS-813160 (CCR2/5 dual antagonist) are present in Table 10
CXCL8-CXCR1/2 axis blockade
CXCL8, known as IL-8, is produced by a variety of cells including macrophages, epithelial cells, and endothelial cells.408 This chemokine, through its cleaved active forms, interacts with its receptors, CXCR1 and CXCR2, to mediate various intracellular signaling pathways such as PI3K-Akt, MAPK, and PLC, influencing cell survival, migration, and angiogenesis.409–411 The CXCL8-CXCR1/2 axis plays a pivotal role in cancer by promoting tumor growth, metastasis, and angiogenesis, largely by affecting the TME.412 This includes recruiting N2 tumor-associated neutrophils (TANs) and TAMs, influencing the infiltration and function of MDSCs, and promoting the recruitment and proliferation of cancer stem cells, contributing to tumor maintenance, metastasis, and resistance to therapies.413 Given its comprehensive role in tumor progression and immune evasion, the CXCL8-CXCR1/2 signaling axis emerges as a promising target for cancer therapy. This is evidenced by the potential benefits of combining anti-CXCL8 antibodies or CXCR1/2 antagonists with conventional anticancer therapies in preclinical models and ongoing clinical trials.414
Given the upregulation of CXCL8 and its receptors in various cancers, targeting this axis represents a promising therapeutic strategy to counteract immune suppression within the TME. Small molecule inhibitors and monoclonal antibodies against CXCL8-CXCR1/2 axis such as SB225002, reparixin, navarixin, AZD5069, SX-682, ABX-IL8, and HuMax-IL8 have shown potential in inhibiting tumor progression and enhancing cancer therapy by impairing the recruitment of immunosuppressive cells and angiogenesis (Table 13).415,416 For instance, reparixin, targeting CXCR1/2, has inhibited polymorphonuclear cell recruitment and demonstrated a 100-fold higher activity on CXCR1 than CXCR2, highlighting its specificity and potential therapeutic benefit.417 In gastric cancer, CXCL8 disrupts CD8+ T cell functions by promoting PD-L1 expression on macrophages, while reparixin reduces PD-L1+ macrophages and boosts antitumor immunity.418 Besides, in the phase I study of anti-CXCL8 antibody HuMax-IL8, while no objective tumor responses were noted, most patients (73%) experienced stable disease, with some maintaining treatment for up to 54 weeks.419 Additionally, treatment with HuMax-IL8 led to a significant reduction in serum CXCL8 levels.419 These findings underscore the potential of CXCL8 blockade as a strategy to enhance outcomes in cancer therapy, particularly in combination with other immunotherapies. Notably, inspired by the synergistic antitumor activity of CXCL8-CXCR1/2 and ICB in murine tumor models, clinical trials exploring combinations of these inhibitors with PD-1/PD-L1 blockade are underway.420 These combination strategies offer new avenues to enhance the efficacy of existing and emerging treatments.
Table 13.
CXCL8-CXCR1/2 axis blockade for cancer therapy
| Classification | Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|---|
| Anti-CXCL8 mAb | HuMax-IL8 | NCT02536469 | Solid Tumor | Monotherapy | I | Completed |
| NCT03689699 | Prostate Cancer | Nivolumab and Degarelix | I/II | Active, not recruiting | ||
| NCT04848116 | HNSCC | Nivolumab | II | Recruiting | ||
| NCT02451982 | Pancreatic Cancer | Nivolumab | II | Recruiting | ||
| CXCR1/2i | SX-682 | NCT05604560 | Pancreatic Cancer | Tislelizumab | II | Recruiting |
| NCT06228053 | mCRPC | Enzalutamide | II | Not yet recruiting | ||
| NCT04574583 | Solid Tumors | M7824, MVA-BN-CV301, and FPV-CV301 | I/II | Active, not recruiting | ||
| NCT06149481 | Colorectal Cancer | Retifanlimab, TriAdeno Vaccine, and N-803 | I/II | Not yet recruiting | ||
| NCT04599140 | Colorectal Cancer | Nivolumab | I/II | Recruiting | ||
| NCT05570825 | NSCLC | Pembrolizumab | II | Recruiting | ||
| NCT04477343 | Pancreatic Cancer | Nivolumab | I | Recruiting | ||
| NCT03161431 | Melanoma | Pembrolizumab | I | Recruiting | ||
| Ladarixin | NCT05815186 | NSCLC With KRAS G12C Mutation | Sotorasib | II | Withdrawn | |
| NCT05815173 | NSCLC With KRAS G12C Mutation | Sotorasib | I | Recruiting | ||
| Selective CXCR1i | Reparixin | NCT01861054 | Breast Cancer | Monotherapy | II | Terminated |
| NCT02001974 | Breast Cancer | Paclitaxel | I | Completed | ||
| NCT02370238 | Breast Cancer | Paclitaxel | II | Completed | ||
| NCT05212701 | Breast Cancer | Monotherapy | II | Withdrawn | ||
| Selective CXCR2i | AZD5069 | NCT03177187 | mCRPC | Enzalutamide | I/II | Terminated |
| NCT02499328 | Solid Tumors | MEDI4736 and Tremelimumab | I/II | Active, not recruiting | ||
| NCT02583477 | Pancreatic Cancer | MEDI4736 | I/II | Completed | ||
| Navarixin | NCT03473925 | Solid Tumors | Pembrolizumab | II | Completed |
Note: HNSCC head and neck squamous cell carcinoma, mCRPC metastatic castration-resistant prostate cancer, NSCLC non-small cell lung cancer
CXCL12-CXCR4 axis
CXCL12-CXCR4 axis promoting tumor growth, metastasis and immune evasion
The CXCL12-CXCR4 axis is pivotal in cancer biology, orchestrating a wide range of processes from tumor growth to metastasis.421 CXCL12, also known as stromal cell-derived factor-1 (SDF-1), is a key chemokine that regulates leukocyte trafficking, stem cell homing, and tissue regeneration.422 Its interaction with CXCR4, a G-protein coupled receptor expressed on various cell types including cancer cells, activates downstream signaling pathways like Ras, PI3K, and PLC, leading to enhanced cell survival, proliferation, and chemotaxis.423 This signaling also involves the activation of JAK-STAT, Wnt-β-catenin, and other pathways, contributing to tumor progression and metastasis.424,425 Notably, the CXCL12-CXCR4 axis is pivotal in the intricate regulation of TME, driving the recruitment and infiltration of immunosuppressive cells such as Treg, TAM, and MDSC. These cells contribute to the creation of an immunosuppressive milieu.426–428 For instance, the CXCL12-CXCR4 mediated recruitment of TAMs has been linked to increased tumor progression and angiogenesis, while the interaction of CXCR4 with CXCL12 attracts Treg cells, further enhancing the immunosuppressive microenvironment.429–431 Targeted inhibition of this signaling pathway, such as the use of the CXCR4 antagonist AMD3100, has shown potential in disrupting these processes, suggesting that modulation of the CXCL12-CXCR4 axis could be a strategic approach to counteract tumor growth, metastasis, and immune evasion mechanisms in cancer therapy.432,433
CXCL12-CXCR4 inhibitors for cancer therapy
CXCR4 antagonists, initially developed for HIV treatment, have shown promise in the treatment of hematological and solid tumors (Table 14). These inhibitors are categorized into non-peptide antagonists like AMD3100 (Plerixafor), peptide antagonists such as LY2510924, and antibodies like ulocuplumab. AMD3100, the first FDA-approved CXCR4 small-molecule inhibitor, is widely used for stem cell mobilization and harvesting, which has evolved from an immunomodulator to a promising anticancer agent.434 Its utility extends beyond monotherapy, showing significant synergies when combined with other anticancer agents, thereby amplifying therapeutic efficacy.435 For example, in pancreatic cancer, Feig et al. identified CXCL12 as a critical factor in immunosuppression, produced mainly by FAP+ CAFs and preventing T-cell infiltration into tumor regions. Treatment with AMD3100 in combination with anti-PD-L1 led to a significant reduction in tumor growth.436 Moreover, in a mouse model of human prostate carcinoma, combining docetaxel with AMD3100 showed a superior antitumor effect compared to docetaxel alone, suggesting that CXCR4 inhibition can effectively chemo-sensitize prostate cancer cells. Further analysis of human prostate cancer samples revealed that cells from bone metastatic lesions exhibited higher levels of CXCR4 than those in primary tumors and lymph node metastases, highlighting the potential of CXCR4 inhibitors as chemo-sensitizing agents.437 Furthermore, in vivo models of human TNBC xenografts, AMD3100 treatment notably increased the radiosensitivity of TNBC cells by upregulating Bax, decreasing Bcl-2 levels, inducing prolonged G2-M phase arrest, and elevating apoptosis.438 In a phase I/II clinical trial aimed at evaluating the safety and effectiveness of Macrophage Exclusion after Radiation Therapy (MERT) through the administration of AMD3100 in newly diagnosed glioblastoma patients, AMD3100 demonstrated a favorable safety profile with no severe toxicities reported. The median OS was 21.3 months, with the PFS of 14.5 months, suggesting that AMD3100 combined with standard chemo-irradiation could potentially enhance local tumor control in glioblastoma patients.439
Table 14.
CXCL12-CXCR4 axis blockade for cancer therapy
| Target | Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|---|
| CXCL12 | Olaptesed | NCT03168139 | Colorectal and Pancreatic Cancer | Pembrolizumab | I/II | Completed |
| NCT04901741 | Pancreatic Cancer | Pembrolizumab and Chemotherapy | II | Not yet recruiting | ||
| NCT04121455 | Glioblastoma | Radiotherapy and Bevacizumab/Pembrolizumab | I/II | Active, not recruiting | ||
| NCT01486797 | Chronic Lymphocytic Leukemia | Bendamustine and Rituximab | II | Completed | ||
| CXCR4 | Plerixafor | NCT04177810 | Pancreatic Cancer | Cemiplimab | II | Completed |
| NCT00914849 | Hematologic Neoplasms | Monotherapy | II | Completed | ||
| NCT04058145 | Head and Neck Cancer | Pembrolizumab | II | Withdrawn | ||
| NCT02179970 | Pancreatic, Ovarian and Colorectal Cancers | Monotherapy | I | Completed | ||
| NCT01753453 | MM | G-CSF | II | Completed | ||
| NCT03277209 | Pancreatic Cancer | Monotherapy | I | Terminated | ||
| NCT01288573 | Pediatric Cancer | Monotherapy | I/II | Completed | ||
| NCT00241358 | Hematological Malignancies | Monotherapy | I/II | Completed | ||
| NCT01225419 | Pediatric Cancer | Monotherapy | II | Completed | ||
| MSX-122 | NCT00591682 | Solid Tumors | Monotherapy | I | Suspended | |
| BL-8040 | NCT02907099 | Pancreatic Cancer | Pembrolizumab | II | Completed | |
| NCT02826486 | Pancreatic Cancer | Pembrolizumab and Onivyde | II | Completed | ||
| NCT02639559 | Hematological Malignancies | Monotherapy | II | Completed | ||
| NCT04543071 | Pancreatic Cancer | Cemiplimab, Gemcitabine, and Nab-Paclitaxel | II | Recruiting | ||
| NCT03246529 | MM | G-CSF | III | Active, not recruiting | ||
| NCT03281369 | Gastric or Gastroesophageal Junction or Esophageal Cancer | Atezolizumab | I/II | Active, not recruiting | ||
| NCT03193190 | Pancreatic Cancer | Atezolizumab | I/II | Active, not recruiting | ||
| NCT01838395 | Acute Myeloid Leukemia | Ara-C | II | Completed | ||
| NCT03154827 | Acute Myeloid Leukemia | Atezolizumab | I/II | Terminated | ||
| NCT02763384 | T-Acute Lymphoblastic Leukemia | Nelarabine | II | Terminated | ||
| NCT02115672 | Chronic Myeloid Leukemia | Imatinib | I/II | Withdrawn | ||
| LY2510924 | NCT02737072 | Solid Tumor | Durvalumab | I | Terminated | |
| NCT01439568 | Extensive Stage Small Cell Lung Carcinoma | Carboplatin and Etoposide | II | Completed | ||
| NCT01391130 | ccRCC | Sunitinib | II | Terminated | ||
| NCT02652871 | Leukemia | Idarubicin and Cytarabine | I | Completed |
Note: ccRCC clear cell renal cell carcinoma, SCLC small cell lung carcinoma, MM multiple myeloma
Additionally, in a phase II trial aimed at enhancing the efficacy of PD-1 inhibitors in pancreatic ductal adenocarcinoma, combining the CXCR4 antagonist BL-8040 (motixafortide) with pembrolizumab and chemotherapy showed promise.440 In the first cohort of 37 chemotherapy-resistant patients treated with BL-8040 and pembrolizumab, the DCR of 34.5% was observed, with one individual showing partial response and several others achieving stable disease, leading to the median OS of 3.3 months that extended to 7.5 months for those treated as a second-line option. This treatment also enhanced CD8+ effector T cell infiltration, reduced MDSCs, and lowered circulating Tregs. The second cohort, involving 22 patients receiving the triple combination, reported an ORR of 32%, a DCR of 77%, and a median response duration of 7.8 months. These findings indicate that the dual blockade of CXCR4 and PD-1, alongside chemotherapy, could significantly improve outcomes for PDAC patients.440 Besides, in a phase IIa clinical trial, the safety and effectiveness of combining BL-8040 with high-dose cytarabine (HiDAC) were assessed in patients with relapsed and refractory acute myelogenous leukemia (AML).441 The study explored six escalating doses of BL-8040, ultimately selecting 1.5 mg/kg for an extended evaluation based on safety and tolerability across all levels. Notably, clinical responses were primarily seen at doses of BL-8040 ≥ 1.0 mg/kg, with the composite response rate of 29% across all participants and 39% in those receiving the 1.5 mg/kg dose. The median OS reached 8.4 months across the cohort, extending to 10.8 months for those in the 1.5 mg/kg group and peaking at 21.8 months among responders at this dose.441 Initial BL-8040 monotherapy notably mobilized leukemia blasts into the bloodstream, especially in responders, and reduced bone marrow blast counts. These findings highlight the potential of CXCR4 inhibition with BL-8040 as a promising approach for AML treatment, warranting further clinical exploration.441
Also, peptide CXCR4 antagonists have similarly blocked CXCR4 in diverse cancer types, showing potential in enhancing immune function and reducing tumor proliferation. In a phase I clinical trial, the safety and efficacy of the peptide antagonist LY2510924 were evaluated in patients with advanced cancers.442 Although the best outcome observed was stable disease in 20% of patients, LY2510924 notably increased CD34+ cell counts in a dose-dependent manner, achieving up to an 18-fold rise at doses as low as 2.5 mg/day. The findings support LY2510924’s potential for stem cell mobilization with a manageable safety profile, justifying further exploration in phase II trials.442 Besides, the combination of peptide CXCR4 antagonist balixafortide and eribulin (chemotherapy agent) demonstrated a safety profile consistent with their monotherapy counterparts and showed promising efficacy in heavily pretreated metastatic breast cancer patients.443 Among the 54 evaluable patients, 16 (30%) showed partial responses to the treatment.443 Moreover, CXCR4 monoclonal antibodies, including ulocuplumab, have been explored primarily in hematological malignancies, showing the ability to potentiate the effects of other treatments.444 Additionally, targeting CXCL12 directly with agents like NOX-A12 impedes the CXCL12-driven movement of CLL cells and renders CLL cells more vulnerable to the chemotherapeutic agents bendamustine and fludarabine in BMSC cocultures.445 Overall, the development of CXCL12-CXCR4 axis inhibitors represents a significant advancement in cancer therapy, with ongoing research required to fully understand their potential and integrate them into clinical practice effectively.
Overexpressing antitumor chemokines or chemokine receptors
Apart from blocking protumor chemokines, overexpressing antitumor chemokines is also a feasible approach to enhancing antitumor immune responses and overcoming the protective mechanisms that tumors use to evade the immune system (Table 15).344 One strategy involves increasing the concentration of antitumorigenic chemokines within the TME, either directly or through combination therapies. For instance, chemokines can be synergistically paired with oncolytic viruses (OVs) to boost the recruitment of endogenous effector cells to the tumor site, thereby amplifying the anticancer effects of concurrent therapies.446 Preclinical studies have demonstrated the effectiveness of OVs engineered to express chemokines such as CXCL9 or CXCL11, leading to increased infiltration of T and NK cells into tumors, reduced tumor growth, and prolonged survival.447,448 Additionally, the development of OVs like NG-641, designed to express a combination of CXCL9, CXCL10, and IFN-α, aims to further enhance the recruitment of immune cells, with clinical trials currently investigating its efficacy in patients with advanced solid tumors.449,450
Table 15.
Overexpressing antitumor chemokines or chemokine receptors for cancer therapy
| Products | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|
| NG-641 (Oncolytic adenoviral producing a FAP-targeting bispecific T cell activator and cytokines CXCL9, CXCL10, and IFN-α2) | NCT05043714 | Epithelial Tumor | Nivolumab | I | Recruiting |
| NCT04053283 | Epithelial Tumor | Monotherapy | I | Recruiting | |
| NCT04830592 | HNSCC | Pembrolizumab | I | Recruiting | |
| CD19-7×19 CAR-T (Anti-CD19 CAR-T Expressing IL-7 and CCL19) | NCT04833504 | B Cell Lymphoma | Monotherapy | I | Completed |
| NCT05659628 | DLBCL | Tislelizumab | I | Recruiting | |
| NCT04381741 | DLBCL | Anti-PD1 mAb | I | Enrolling by invitation | |
| CCL21-Gene Modified Dendritic Cell Vaccine | NCT03546361 | NSCLC | Pembrolizumab | I | Recruiting |
| NCT01574222 | NSCLC | Monotherapy | I | Terminated | |
| NCT00601094 | Lung Cancer | Monotherapy | I | Completed | |
| NCT00798629 | Melanoma | Monotherapy | I | Completed | |
| CCL21 protein | NCT01433172 | Lung Cancer | GM.CD40L Vaccine | I/II | Completed |
| CXCR4 Modified Anti-CD30 CAR-T | NCT03602157 | CD30+ Lymphoma | Monotherapy | I | Recruiting |
| CXCR4 Modified Anti-BCMA CAR-T | NCT04727008 | Multiple Myeloma | Monotherapy | I | Recruiting |
| CXCR4 Modified Anti-CD19 CAR-T | NCT04684472 | CD19+ B-cell Malignancies | Monotherapy | I | Recruiting |
| CXCR5 modified Anti-EGFR CAR-T | NCT04153799 | NSCLC | Monotherapy | I | Unknown status |
| CXCR5 modified Anti-EGFR CAR-T | NCT05060796 | NSCLC | Monotherapy | I | Recruiting |
Note: HNSCC head and neck squamous cell carcinoma, DLBCL diffuse large B-cell lymphoma, NSCLC non-small cell lung cancer, CAR-T chimeric antigen receptor T-cell
Another promising avenue involves the administration of fusion proteins that link chemokines with antibodies or other targeting molecules, directing these immune-modulating agents specifically to tumor cells or the tumor stroma. This approach has led to the development of chemokine-antibody fusion proteins that target specific tumor antigens, such as CXCL10-EGFRvIII for glioma or an anti-human endoglin scFv fused to CXCL10 for hepatocellular carcinoma, showing promising results in enhancing intratumoral effector cell recruitment and improving antitumor activity in preclinical models.451 Similarly, the use of chemokines as adjuvants in cancer vaccines has been explored, with chemokines like CCL21 being employed to boost the recruitment and activation of DCs and T cells, enhancing the efficacy of cancer vaccines in preclinical models, and leading to clinical trials assessing their utility in various cancer types.452–454 Moreover, the direct genetic modification of therapeutic cells to overexpress chemokines or chemokine receptors has emerged as a novel strategy to improve cellular therapies for cancer. By engineering CAR-T cells to co-express chemokines such as CCL19 or chemokine receptors like CXCR6, these modified cells can more effectively home to tumor sites and interact with endogenous immune cells, leading to enhanced antitumor responses.455–457 Notably, synthetic biology provides an innovative approach for the targeted delivery of chemokines directly into the TME. This novel strategy overcomes immune cell exclusion by deploying engineered bacteria that intratumorally release specific chemokines, like an activating mutant of human CXCL16 (hCXCL16K42A), to attract adaptive immune cells to tumors.458 This hCXCL16K42A expressing bacteria (eSLC-hCXCL16K42A) showed significant therapeutic potential in multiple tumor models, primarily by recruiting CD8+ T cells.458 Additionally, the eSLC-hCXCL16K42A strain synergized with CCL20-expressing bacteria (eSLC-CCL20) to boost antitumor immunity, by simultaneously improving the recruitment of cDC1 and CD8+ T cells, eventually overcoming immunotherapy resistance in immune-excluded tumors.458
In a phase I clinical trial of NCT03198546, the safety and efficacy of CAR-T cells secreting IL-7 and CCL19 (7×19) were evaluated in patients with advanced hepatocellular carcinoma, pancreatic carcinoma, and ovarian carcinoma expressing glypican-3 (GPC3) or mesothelin (MSLN).459 Notably, one hepatocellular carcinoma patient treated with anti-GPC3-7×19 CAR-T cells achieved complete tumor remission 30 days after intratumoral injection, and a pancreatic carcinoma patient treated with anti-MSLN-7×19 CAR-T cells experienced almost complete tumor remission 240 days after intravenous infusion.459 These findings suggest that incorporating IL-7 and CCL19 into CAR-T cell therapy significantly boosts its efficacy against solid tumors, marking a significant advancement in the field. Currently, more clinical trials are underway to evaluate the efficacy of these modified CAR-T cells in treating a range of hematological and solid tumors, demonstrating the potential of chemokines to significantly improve the therapeutic landscape of cancer treatment through various innovative approaches.
Growth factor blockade
The growth factor is a type of cytokine that specifically plays a role in the regulation of cell growth, proliferation, and differentiation. Growth factors like TGF-β, VEGF, and EGF play pivotal roles in cancer progression through the promotion of angiogenesis, tumorigenesis, and metastasis.460–463 The investigation into these growth factors has been instrumental in developing targeted therapies, offering a more personalized treatment approach for cancer patients. Inhibitors targeting TGF-β, VEGF, and EGFR have shown significant promise in clinical settings.
TGF-β inhibition
TGF-β signaling and its dual role in cancer
TGF-β is a key cytokine in the TGF-β superfamily, encompassing TGF-βs, Activins, Nodals, BMPs, and GDFs, pivotal in embryogenesis and adult physiological homeostasis.464 It exists as three mammalian isoforms (TGF-βI-III).465 For clarity, discussions around TGF-β typically refer to TGF-βI unless specified otherwise. TGF-β is synthesized and secreted into the extracellular matrix (ECM) predominantly in a latent complex form.466,467 The molecule undergoes a sophisticated activation process, initiated by cleavage via the convertase enzyme furin within the Golgi apparatus, which separates the latency-associated peptide (LAP) from the mature TGF-β cytokine, albeit maintaining a non-covalent association that keeps TGF-β inactive until further activation cues are met.468 Then, with the assistance of mechanical forces and αβ integrins, inactive TGF-β is activated and binds to the receptor complex, initiating the regulation of gene transcription via SMAD and non-SMAD pathways.469,470
Specifically, TGF-β signaling initiates when TGF-β ligands bind to type II receptors (TGFβRII), leading to the activation and phosphorylation of type I receptors (TGFβRI).471 This triggers the phosphorylation of SMAD2 and SMAD3, which then form trimeric complexes with SMAD4.472 These complexes enter the nucleus to regulate genes such as TWIST1, SNAI1, and SNAI2, impacting cellular functions like proliferation and differentiation.473,474 Beyond this canonical pathway, TGF-β also activates non-SMAD pathways, including the PI3K-AKT, MAPK, and RHO signaling (Fig. 7).475,476 The dysregulation of TGF-β signaling is implicated in a myriad of pathological conditions, including metabolic dysfunctions, excessive ECM deposition, immune dysfunction, fibrosis, and various cancers.477 In cancer, TGF-β exhibits dual roles, initially suppressing tumor formation by halting the cell cycle, but in advanced stages, it aids tumor growth by promoting EMT, increasing metastasis, chemoresistance, angiogenesis, and immune evasion.478 This switch from a tumor suppressor to a promoter is a key feature in the progression of advanced cancers, underscoring the complex nature of TGF-β in oncogenesis.479
Fig. 7.
TGF-β signaling in cancer and TGF-β blockade for immunotherapy. The top panel illustrates the TGF-β signaling pathway in cancer cells, including the canonical Smad-dependent pathway and the non-canonical pathways involving various intracellular mediators such as MAPK, PI3K/Akt, and mTOR, leading to cellular processes like EMT, stemness, metastasis, treatment resistance, angiogenesis, and immune evasion. The bottom left panel depicts the role of TGF-β in the tumor microenvironment (TME), highlighting its immunosuppressive effects that facilitate cancer immune escape by interacting with various immune cells such as Treg, MDSC, M1/M2 macrophages, DC, NK, and CTL. The bottom right panel presents a schematic representation of innovative anti-TGF-β/PD-L1 therapeutic agents, demonstrating dual blockade strategies, as exemplified by M7824/SHR-1701, which combines a TGF-β trap with an anti-PD-L1 moiety, and YM101/BITP, which features both anti-TGF-β and anti-PD-L1 moieties for enhanced immunotherapy efficacy. Adapted from “Canonical and Non-canonical TGF-β Pathways in EMT”, by BioRender.com (2024). Retrieved from https://app.biorender.com/biorender-templates
TGF-β inhibition for improved cancer immunotherapy response
The targeting of TGF-β signaling has become a focal point in cancer therapy, given its role in fostering immune evasion and resistance to immunotherapies by altering the TME.480 Multiple TGF-β-targeted therapies, including monoclonal antibodies, ligand traps, receptor kinase inhibitors, antisense oligonucleotides, and vaccines, are currently under clinical investigation (Table 16).5,481,482 Fresolimumab (also known as GC-1008), a monoclonal antibody against TGF-β, demonstrated promising antitumor activities in renal cell carcinoma and melanoma (NCT00356460).483 Besides, the safety, efficacy, and immune responses of fresolimumab combined with radiotherapy were investigated in patients with metastatic breast cancer.484 Participants were assigned to receive either 1 mg/kg or 10 mg/kg fresolimumab every three weeks for five cycles, alongside focal radiotherapy targeting a metastatic site. Patients administered the 10 mg/kg dose of fresolimumab exhibited a significantly longer median OS compared to those on the 1 mg/kg dose, with the HR of 2.73 (95% CI, 1.02–7.30; P = 0.039).484 Additionally, the higher dose was associated with enhanced peripheral blood mononuclear cell counts and a notable increase in the CD8+ central memory T cell pool.484 The results suggest that TGF-β blockade combined with radiotherapy is a viable and safe strategy, with the higher fresolimumab dose prompting a more favorable systemic immune response and improved survival outcomes.484
Table 16.
Agents targeting TGF-β in preclinical or clinical studies
| Target | Molecular type | Agent | Company |
|---|---|---|---|
| TGFβRII | mAb | LY3022859 | Eli Lilly |
| TGFβRI/II | Receptor kinase inhibitor | LY2109761 | Eli Lilly |
| TGFβRI | Receptor kinase inhibitor | Vactosertib | MedPacto |
| Galunisertib | Eli Lilly | ||
| LY3200882 | Eli Lilly | ||
| LY573636 | Eli Lilly | ||
| SB-431542 | GlaxoSmithKline | ||
| SB-505124 | GlaxoSmithKline | ||
| IN-1130 | In2Gen | ||
| TGF-β and PD-L1 | BsAb | BiTP/YM101 | YZY Biopharma |
| TGFβRII and PD-L1 | Bifunctional fusion protein | TQB2858 | Chia-Tai Tianqing |
| M7824 | Merck KGaA | ||
| PM8001 | Pumis Biotechnology | ||
| SHR-1701 | Hengrui | ||
| TGFβRII and PD-1 | Bifunctional fusion protein | JS201 | Junshi |
| TGF-β and VEGF | BsAb | Y332 | YZY Biopharma |
| TGF-β | mAb | Fresolimumab | Genzyme |
| SRK181 | Scholar Rock | ||
| 1D11 | Genzyme | ||
| 2G7 | Genentech | ||
| Trap | AVID200 | Forbius | |
| Luspatercept | Acceleron | ||
| Antisense oligonucleotides | AP 12009 | Antisense Pharma | |
| AP 11014 | Antisense Pharma | ||
| Cancer vaccine | Vigil | Gradalis | |
| Lucanix | NovaRx | ||
| Integrin αvβ6 | mAb | 264RAD | AstraZeneca |
Note: BsAb bispecific antibody, mAb monoclonal antibody
Notably, galunisertib, a TGFβRI inhibitor, when combined with gemcitabine, enhanced OS in pancreatic cancer patients, marking a significant advancement over gemcitabine monotherapy.485 In this clinical trial for patients with unresectable pancreatic cancer, the primary endpoint of OS was achieved, with median OS of 8.9 months for the combination group and 7.1 months for the gemcitabine group (HR = 0.79).485 Moreover, galunisertib combined with neoadjuvant chemoradiotherapy was effective in patients with locally advanced rectal adenocarcinoma.486 In this phase II trial, out of 38 enrolled patients, 25 proceeded to surgery after completing chemoradiotherapy, with 20% achieving pathological complete responses.486 Ten patients opted for non-operative management, with 71% showing clinical complete responses after one year. Overall, 32% of patients achieved a complete response. The treatment was generally well-tolerated, with common grade 3 adverse events being diarrhea and hematological toxicity.486 However, in a study evaluating the combination of galunisertib and lomustine in patients with glioblastoma, no improvement in OS was observed compared to placebo plus lomustine.487 Similarly, the phase Ib study on advanced hepatocellular carcinoma patients combining galunisertib and ramucirumab found the treatment safe but with limited efficacy, leading to the discontinuation of further clinical development of this combination.488
The disparate outcomes of clinical trials exploring galunisertib combinations can be attributed to tumor heterogeneity, variations in patient demographics and disease stages, differences in drug dosing and pharmacokinetics, interactions between TGF-β and other cellular pathways, and study design specifics.489 These factors highlight the complexity of TGF-β targeted therapies and the necessity for tailored treatment strategies and further mechanistic studies. At present, there are more than ten TGFβRI inhibitors are undergoing clinical evaluation. For instance, despite tolerable toxicity in clinical trials, LY573636 by Eli Lilly showed only modest antitumor effects in NSCLC patients, highlighting the challenge of translating TGF-β receptor kinase inhibitors’ preclinical success into clinical efficacy.490
Novel bifunctional antibodies simultaneously targeting PD-L1 and TGF-β
Moreover, M7824, a bifunctional fusion protein targeting both PD-L1 and TGF-β pathways, has shown promising antitumor activity in preclinical and early clinical trials, highlighting its potential in reprogramming the TME and reversing immunotherapy resistance (Table 17).491 In the phase I trial of M7824 (NCT02517398), 19 heavily pretreated patients with advanced solid tumors were treated with doses up to 20 mg/kg every 2 weeks.492 Efficacy signals included one ongoing complete response in cervical cancer, two confirmed partial responses in pancreatic and anal cancers, one near-partial response in cervical cancer, and two instances of prolonged stable disease in pancreatic cancer and carcinoid.492 Besides, in expansion cohort of NCT02517398, 80 patients with advanced NSCLC received either 500 mg or 1200 mg doses, achieving an overall response rate of 21.3%.493 The 1200 mg dose showed a higher response rate, especially in PD-L1-positive patients, with an ORR of 36.0% and 85.7% in those with PD-L1-high expression.493 Treatment-related adverse events were reported in 69% of patients, with 29% experiencing grade 3 or higher events, and 10% discontinued treatment due to adverse events.493 These results highlight M7824’s manageable safety profile and its promising early signs of efficacy in advanced solid tumors. Similarly, SHR-1701, another fusion protein combining anti-PD-L1 antibody with a TGF-β trap, has shown promising antitumor effects in various cancers especially gastric cancer and cervical cancer.494,495
Table 17.
Clinical trials of bifunctional fusion protein M7824 targeting PD-L1 and TGF-β
| NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|
| NCT03833661 | Biliary Tract Cancer | Monotherapy | II | Completed |
| NCT03631706 | NSCLC | Monotherapy | III | Active, not recruiting |
| NCT04835896 | Gastric Cancer | Paclitaxel | I/II | Not yet recruiting |
| NCT03840902 | NSCLC | Concurrent Chemoradiotherapy | II | Terminated |
| NCT03554473 | SCLC | Topotecan or Temozolomide | I/II | Recruiting |
| NCT04574583 | Solid Tumors | SX-682 and CV301 | I/II | Active, not recruiting |
| NCT04296942 | Breast Cancer | BN-Brachyury, Entinostat, and Adotrastuzumab Emtansine | I | Terminated |
| NCT05145569 | Ovarian Cancer | Carboplatin AUC 5 and paclitaxel | I | Not yet recruiting |
| NCT02699515 | Solid Tumors | Monotherapy | I | Completed |
| NCT04327986 | Pancreatic Cancer | M9241 and SBRT | I/II | Terminated |
| NCT02517398 | Solid Tumors | Monotherapy | I | Completed |
| NCT03524170 | Breast Cancer | Radiation Therapy | I | Completed |
| NCT03427411 | HPV Associated Malignancies | Monotherapy | II | Completed |
| NCT03620201 | HER2 Positive Breast Cancer | Monotherapy | I | Active, not recruiting |
| NCT03436563 | Solid Tumors With Microsatellite Instability | Monotherapy | I/II | Active, not recruiting |
| NCT04489940 | TNBC | Monotherapy | II | Terminated |
| NCT04246489 | Cervical Cancer | Monotherapy | II | Completed |
| NCT03579472 | TNBC | Eribulin Mesylate | I | Terminated |
| NCT04432597 | HPV Associated Malignancies | HPV Vaccine PRGN-2009 | I/II | Active, not recruiting |
| NCT04235777 | Non-Prostate Genitourinary Malignancies | M9241 and SBRT | I | Recruiting |
| NCT04066491 | Biliary Tract Cancer | Gemcitabine and Cisplati | II/III | Terminated |
| NCT05445882 | Castration Resistant Prostate Cancer | N-803 and BN-Brachyury | II | Not yet recruiting |
| NCT04417660 | Thymic Cancer | Monotherapy | II | Recruiting |
| NCT04501094 | Urothelial Cancer | Monotherapy | II | Terminated |
| NCT04247282 | HNSCC | TriAd Vaccine and N-803 | I/II | Completed |
| NCT03840915 | NSCLC | Chemotherapy | I/II | Completed |
| NCT04287868 | HPV Associated Malignancies | PDS0101 and NHS-IL12 | I/II | Active, not recruiting |
| NCT04551950 | Cervical Cancer | Cisplatin/Carboplatin, Paclitaxel, and Bevacizumab | I | Completed |
| NCT04727541 | Biliary Tract Cancer | Monotherapy | II | Terminated |
| NCT04560686 | NSCLC | Surgery | II | Terminated |
| NCT05061823 | Lung Cancer | Monotherapy | III | Active, not recruiting |
| NCT03451773 | Pancreatic Cancer | Gemcitabine | I/II | Terminated |
| NCT03493945 | Solid Tumor | BN-Brachyury Vaccine, N-803 and Epacadostat | I/II | Recruiting |
| NCT04349280 | Urothelial Cancer | Monotherapy | I | Active, not recruiting |
| NCT04633252 | Prostate Cancer | ADT, Prednisone, Docetaxel and M9241 | I/II | Recruiting |
| NCT04491955 | Small Bowel and Colorectal Cancers | CEA/MUC1 Vaccines, N-803, and NHSIL12 | II | Active, not recruiting |
| NCT03315871 | Prostate Cancer | PROSTVAC-V and CV301 | II | Active, not recruiting |
| NCT05012098 | Olfactory Neuroblastoma | Monotherapy | II | Active, not recruiting |
| NCT04220775 | HNSCC | SBRT | I/II | Completed |
| NCT04756505 | Breast Cancer | NHS-IL12 and Radiotherapy | I | Withdrawn |
| NCT04396535 | NSCLC | Docetaxel | II | Terminated |
| NCT04789668 | Brain Metastases | Pimasertib | I/II | Completed |
| NCT04708470 | HPV-Associated Malignancies, Small Bowel, and Colon Cancers | NHS-IL12 and Entinostat | I/II | Recruiting |
| NCT04648826 | Pulmonary Metastases | Azacytidine | I/II | Withdrawn |
| NCT04971187 | TKI-Resistant EGFR-Mutant NSCLC | Pemetrexed and Carboplatin/Cisplatin | II | Terminated |
| NCT05005429 | Malignant Pleural Mesothelioma | Monotherapy | II | Recruiting |
| NCT04303117 | Kaposi Sarcoma | NHS-IL12 | I/II | Recruiting |
| NCT04428047 | HNSCC | Monotherapy | II | Terminated |
| NCT04708067 | Intrahepatic Cholangiocarcinoma | Hypofractionated Radiotherapy | I | Active, not recruiting |
Note: SBRT stereotactic body radiation therapy, ADT androgen deprivation therapy, NSCLC non-small cell lung cancer, SCLC small cell lung cancer, TNBC triple negative breast cancer, HNSCC head and neck squamous cell carcinoma, TKI tyrosine kinase inhibitor
In parallel with the fusion protein, YM101, an innovative anti-TGF-β/PD-L1 bispecific antibody developed from the Check-BODY™ technology platform, has demonstrated the capacity to specifically target TGF-β and PD-L1, counteracting their immunosuppressive effects in vitro and showing superior antitumor activity in vivo compared to monotherapies targeting either pathway alone.496,497 By promoting an immune-supportive TME, which was characterized by increased infiltration of lymphocytes and dendritic cells, a higher M1/M2 macrophage ratio, and elevated cytokine production in T cells, YM101 effectively enhances the antitumor immune response, offering a promising strategy to overcome resistance and enhance the efficacy of anti-PD-1/PD-L1 therapies.496 Besides, the combination of bivalent manganese, a natural STING agonist, with YM101 has demonstrated a synergistic antitumor effect in preclinical studies, effectively transforming immune-excluded and immune-desert tumor models into immune-inflamed ones by activating both innate and adaptive immune responses, enhancing DC maturation, T cell activation, and antigen presentation.73 Similarly, MSA-2, another novel STING agonist, when combined with YM101, significantly improved antitumor activity in these resistant tumor models by promoting proinflammatory cytokine and chemokine production, boosting antigen presentation, and increasing tumor-infiltrating lymphocytes, showcasing the potential of these combinations as universal regimens for treating various tumor immune landscapes.72
Lastly, antisense oligonucleotides and cancer vaccines offer innovative strategies targeting TGF-β in cancer therapy. AP 12009, an antisense oligodeoxynucleotide developed by Antisense Pharma, targets TGF-β2 and has shown improved OS for high-grade glioma.498 Notably, in a prespecified subgroup analysis of patients with anaplastic astrocytoma, the 10 µM trabedersen group demonstrated a significant improvement in the 14-month tumor control rate compared to chemotherapy (P = 0.0032).498 Additionally, this group showed a trend towards superior 2-year survival (P = 0.10), with median OS of 39.1 months for 10 µM trabedersen, 35.2 months for 80 µM trabedersen, and 21.7 months for chemotherapy.498 In the realm of cancer vaccines, belagenpumatucel-L, a vaccine by NovaRx composed of NSCLC cells with a TGF-β2 antisense gene, demonstrated improved survival in NSCLC patients in a phase III trial.499 The overall trial did not meet its primary endpoint, with median survival at 20.3 months for belagenpumatucel-L versus 17.8 months for placebo.499 However, prespecified analyses showed patients randomized within 12 weeks after chemotherapy and those who received prior radiation benefited from belagenpumatucel-L, with median survival extending to 28.4 months compared to 16.0 months for placebo recipients in the radiation subgroup.499 In sum, these clinical outcomes underscore the critical role of TGF-β-targeted therapies in the evolving cancer treatment paradigm.
Blocking pro-angiogenic factors
Abnormal angiogenesis in cancer and its role in immune evasion
Angiogenesis, the formation of new blood vessels from pre-existing vasculature, is a crucial process in both physiological conditions, such as wound healing, and pathological conditions, including cancer development and metastasis.500 The rapid proliferation of tumor cells increases the demand for oxygen and nutrients, leading to hypoxia and acidosis within the TME.501 This condition triggers the secretion of various pro-angiogenic factors like VEGF, MMPs, and basic fibroblast growth factor (bFGF), disrupting the balance between pro-angiogenic and anti-angiogenic factors and activating angiogenic pathways.502 However, the continuous overproduction of these factors results in the formation of abnormal, immature blood vessels characterized by a lack of pericyte coverage and increased leakiness, which contributes to elevated vascular permeability and interstitial fluid pressure, further hampering drug delivery and immune cell infiltration into tumors.503–506
While the primary goal of anti-angiogenic therapy was to deprive tumor cells of their blood supply, standalone treatments have not significantly improved patient outcomes, suggesting a need for combined therapeutic strategies.507 The concept of vessel normalization has emerged, proposing a synergistic effect when anti-angiogenic therapies are used in combination with other treatments, such as ICB (Fig. 8).508 This approach aims to modulate the tumor vasculature to improve perfusion and oxygenation, reducing hypoxia-induced immunosuppression and enhancing the efficacy of immunotherapies.509 Abnormal angiogenesis not only supports tumor growth and metastasis but also plays a pivotal role in immune evasion by hindering the infiltration and function of immune cells within the TME.501 The excessive production of angiogenic factors not only promotes the growth of leaky and disorganized blood vessels but also directly contributes to the suppression of antitumor immune responses. VEGF, for instance, can directly inhibit the trafficking, proliferation, and effector functions of CTLs.510 Furthermore, VEGF impedes the maturation and antigen-presenting capability of DCs, crippling the activation of T cells and, consequently, dampening the immune response to tumor antigens.511 Besides, the hypoxia TME promotes the accumulation of immunosuppressive cells like Tregs, MDSCs, and TAMs that exhibit protumor activities.512–514 These immunosuppressive cells further secrete cytokines and growth factors, including more VEGF and TGF-β, reinforcing the cycle of angiogenesis and immunosuppression. Additionally, angiogenic molecules can modulate the expression of immune checkpoint molecules, such as PD-L1 on tumor and immune cells, and adhesion molecules on endothelial cells, which further diminish the effectiveness of CTLs and facilitate tumor immune evasion.515–518 This complex interplay between angiogenesis and immune suppression underscores the challenges in treating cancers solely with anti-angiogenic or immunotherapeutic agents and highlights the potential benefits of combining these therapeutic strategies to normalize tumor vasculature, alleviate immunosuppression, and enhance the efficacy of cancer immunotherapy.519
Fig. 8.
Anti-angiogenesis therapy to improve antitumor immune response. The left side of the figure depicts the consequences of dysregulated tumor vasculature, characterized by lower blood flow, leaky vessels, and resulting hypoxia that subsequently leads to decreased immune infiltration, increased M2 macrophage polarization, reduced M1 polarization, diminished tumor antigen cross-presentation by dendritic cells, expansion and enhanced suppression function of myeloid-derived suppressor cells, along with inhibited T-cell activation, effector function, and increased regulatory T (Treg) cell populations. The introduction of anti-angiogenesis treatment targets these aberrant vessels to shift the balance towards vascular normalization, as shown on the right. This normalization results in improved blood flow, reduced hypoxia, and increased immune infiltration, thereby potentially increasing T-cell activation, enhancing effector function, promoting tumor cell lysis by natural killer cells, and reducing PD-L1 expression on cancer cells, collectively creating an optimized microenvironment for the antitumor immune response. (Created with BioRender.com)
Anti-angiogenesis agents and their applications in cancer immunotherapy
In preclinical studies, the synergistic effect of anti-angiogenesis therapy combined with ICB has been increasingly recognized as a potent strategy against cancer. Anti-angiogenesis therapy, aimed at normalizing tumor vasculature, not only inhibits tumor growth by disrupting blood supply but also enhances the efficacy of ICB.520 This synergy has been observed in various preclinical models, including melanoma, colon, kidney, breast, and lung cancers, where combination therapy significantly prolonged the OS of tumor-bearing mice.521–524 Key mechanisms underlying this synergy include the normalization of tumor vessels, which improves T cell infiltration and reprograms the TME from immunosuppressive to immune-supportive phenotype.525 This effect is achieved by reducing hypoxia and downregulating immune checkpoint expression on T cells and tumor cells.510,522,526 Furthermore, the formation of high endothelial venules (HEV) after combination therapy has been identified as a novel mechanism that promotes lymphocyte homing and infiltration into the tumor.527 These findings underscore the complexity and potential of combining ICB with anti-angiogenesis therapy for cancer treatment, highlighting the need for further exploration to fully understand and optimize this therapeutic strategy.
Encouraged by promising preclinical findings, extensive clinical research has been undertaken to explore the combined efficacy of ICB with anti-angiogenesis treatments (Table 18). Clinical trials, such as the phase I study NCT00790010, have begun to unveil the potential of combining ICB like ipilimumab (anti-CTLA-4) with anti-VEGF agents such as bevacizumab in treating metastatic melanoma,528 demonstrating significant improvements in prognosis and enhanced immune responses. Administering ipilimumab combined with bevacizumab across four dosing cohorts to forty-six patients, the research observed inflammatory responses, enhanced endothelial activation, and significant immune cell infiltration within tumors.528 Additionally, improvements in peripheral blood markers, including increased CCR7+/−CD45RO+ cells and anti-galectin antibodies, were detected.528,529 The combination treatment yielded a DCR of 67.4%, with 8 partial responses and 22 instances of stable disease, culminating in a median survival of 25.1 months. These outcomes underscore the potential of bevacizumab to modify tumor vasculature and immune dynamics in concert with ipilimumab, offering a promising therapeutic strategy that combines angiogenesis inhibition with ICB.528
Table 18.
Representative clinical trials of the combination therapy of anti-angiogenesis and immune checkpoint blockade
| Combination strategy | NCT number | Cancer types | Phases | Status |
|---|---|---|---|---|
| Atezolizumab combined with Bevacizumab | NCT05063552 | HNSCC | II/III | Recruiting |
| NCT02366143 | NSCLC | III | Completed | |
| NCT04732598 | Breast Cancer | III | Active, not recruiting | |
| NCT03038100 | Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | III | Completed | |
| NCT03353831 | Ovarian Cancer | III | Active, not recruiting | |
| NCT02997228 | Colorectal Adenocarcinoma | III | Recruiting | |
| NCT05665348 | HCC | II/III | Not yet recruiting | |
| NCT03991403 | NSCLC | III | Active, not recruiting | |
| NCT04194203 | NSCLC | III | Active, not recruiting | |
| NCT03434379 | HCC | III | Completed | |
| NCT04487067 | HCC | III | Active, not recruiting | |
| NCT03693573 | RCC | III | Withdrawn | |
| NCT05904886 | HCC | III | Recruiting | |
| NCT02420821 | RCC | III | Completed | |
| NCT04803994 | HCC | III | Recruiting | |
| NCT04732286 | HCC | III | Active, not recruiting | |
| NCT04712643 | HCC | III | Active, not recruiting | |
| NCT04102098 | HCC | III | Active, not recruiting | |
| NCT03556839 | Cervical Cancer | III | Active, not recruiting | |
| Axitinib Combined with Avelumab | NCT03013946 | RCC | III | Recruiting |
| NCT03472560 | NSCLC and Urothelial Cancer | II | Terminated | |
| NCT02912572 | Endometrial Cancer | II | Active, not recruiting | |
| NCT04258956 | Gastrointestinal Stromal Tumors | II | Unknown status | |
| NCT02684006 | RCC | III | Active, not recruiting | |
| NCT03386929 | NSCLC | I/II | Terminated | |
| NCT04562441 | NPC | II | Active, not recruiting | |
| NCT04698213 | RCC | II | Recruiting | |
| NCT05327686 | RCC | II | Recruiting | |
| NCT03990571 | Adenoid Cystic Carcinoma | II | Completed | |
| NCT03341845 | RCC | II | Recruiting | |
| NCT03291314 | Recurrent Glioblastoma | II | Completed | |
| NCT05176288 | ccRCC | II | Withdrawn | |
| NCT05249569 | HCC | II | Terminated | |
| Pembrolizumab Combined With Lenvatinib | NCT04676412 | NSCLC | III | Active, not recruiting |
| NCT03829332 | NSCLC | III | Active, not recruiting | |
| NCT04716933 | NSCLC | III | Active, not recruiting | |
| NCT03829319 | NSCLC | III | Active, not recruiting | |
| NCT03976375 | NSCLC | III | Active, not recruiting | |
| NCT03898180 | Urothelial Carcinoma | III | Active, not recruiting | |
| NCT03517449 | Endometrial Neoplasms | III | Active, not recruiting | |
| NCT04776148 | Colorectal Neoplasms | III | Active, not recruiting | |
| NCT04949256 | Esophageal Carcinoma | III | Recruiting | |
| NCT05077215 | Endometrial Cancer | III | Not yet recruiting | |
| NCT04865289 | Endometrial Cancer | III | Active, not recruiting | |
| NCT03884101 | Endometrial Cancer | III | Active, not recruiting | |
| NCT03486873 | Solid and Hematologic Malignancies | III | Recruiting | |
| NCT04889118 | Melanoma | III | Active, not recruiting | |
| NCT03820986 | Melanoma | III | Active, not recruiting | |
| NCT05899049 | RCC | III | Recruiting | |
| NCT04736706 | RCC | III | Recruiting | |
| NCT04662710 | Gastroesophageal Adenocarcinoma | III | Active, not recruiting | |
| NCT05523323 | HNSCC | III | Active, not recruiting | |
| NCT04199104 | HNSCC | III | Active, not recruiting | |
| NCT03713593 | HCC | III | Active, not recruiting | |
| NCT04246177 | HCC | III | Active, not recruiting | |
| NCT02811861 | RCC | III | Active, not recruiting | |
| SHR-1210 Combined with Apatinib | NCT03813784 | Gastric Cancer | III | Unknown status |
| NCT04335006 | TNBC | III | Terminated | |
| NCT04342910 | Gastric Cancer | III | Unknown status | |
| NCT05049681 | Esophageal Cancer | III | Unknown status | |
| Nivolumab Combined with Sitravatinib | NCT03906071 | NSCLC | III | Active, not recruiting |
| NCT03015740 | Kidney Cancer | I/II | Completed | |
| NCT04904302 | ccRCC | II | Active, not recruiting | |
| NCT02954991 | NSCLC | II | Completed | |
| NCT04887870 | Solid Malignancies | II/III | Enrolling by invitation | |
| NCT03606174 | Urothelial Carcinoma | II | Terminated | |
| NCT03680521 | ccRCC | II | Completed |
Note: HNSCC head and neck squamous cell carcinoma, NSCLC non-small-cell lung cancer, HCC hepatocellular carcinoma, RCC renal cell carcinoma, ccRCC clear cell renal cell cancer, NPC nasopharyngeal carcinoma, TNBC triple-negative breast cancer
Besides combination therapies with anti-CTLA-4, several clinical trials have investigated the combination of anti-PD-L1 with anti-VEGF therapies across different cancer types, showing promising results. The phase II/III clinical trial ORIENT-32 aimed to assess the efficacy and safety of combining sintilimab, an anti-PD-L1 antibody, with IBI305, a bevacizumab biosimilar, versus sorafenib for the first-line treatment of unresectable HBV-associated hepatocellular carcinoma.530 In the phase II part, 24 patients received at least one dose of the study drugs, achieving the ORR of 25%, with grade 3 or worse treatment-related adverse events in 29% of patients.530 This led to the commencement of the phase III part, where the sintilimab-bevacizumab biosimilar group showed the median PFS of 4.6 months compared to 2.8 months in the sorafenib group (HR: 0.56, P < 0.0001).530 The first interim analysis for OS indicated a significant advantage for the combination therapy, with a median survival not yet reached, versus 10.4 months for sorafenib (HR: 0.57, P < 0.0001).530 Adverse events were manageable, with hypertension (14% vs 6%) and palmar-plantar erythrodysesthesia syndrome (0% vs 12%) being the most common grade 3–4 events in the sintilimab-bevacizumab biosimilar and sorafenib groups, respectively530. These results suggest that sintilimab plus IBI305 could offer a substantial survival benefit with a tolerable safety profile for patients with HBV-associated hepatocellular carcinoma, presenting a promising first-line treatment alternative.530
Also, in the phase II trial NCT02873962, the efficacy of combining nivolumab and bevacizumab was evaluated in 38 women with relapsed epithelial ovarian cancer.531 The primary endpoint ORR was 28.9% overall, with a higher ORR observed in platinum-sensitive patients (40%) compared to platinum-resistant patients (16.7%).531 The study also reported a median PFS of 8.1 months and noted that 89.5% of participants experienced at least one treatment-related adverse event, with 23.7% experiencing a grade 3 or higher event. Interestingly, responses to the combination therapy occurred across PD-L1 expression levels, suggesting that nivolumab with bevacizumab exhibits activity in relapsed ovarian cancer, particularly in the platinum-sensitive subgroup, and highlighting the need for alternative strategies in platinum-resistant cases.531 Similarly, the phase III IMpower150 study (NCT02366143) assessed atezolizumab plus bevacizumab and chemotherapy in non-squamous NSCLC patients, revealing significantly better response rates and survival outcomes compared to control groups, independent of PD-L1 expression and effector T cell status.532
Apart from anti-VEGF antibody such as bevacizumab, VEGFR tyrosine kinase inhibitors (TKIs) blocking intracellular transduction of VEGF signaling are also widely used in clinical practice, including axitinib, sorafenib, vatalanib, apatinib, and sunitinib.533 In the phase III JAVELIN Renal 101 trial, the combination of avelumab (anti-PD-1 antibody) and axitinib was compared to standard-of-care sunitinib in previously untreated patients with advanced renal-cell carcinoma.534 For PD-L1-positive patients, median PFS was significantly longer for the combination therapy (13.8 months) compared to sunitinib (7.2 months), with an HR for disease progression or death at 0.61.534 In the overall patient population, the median PFS was 13.8 months with the combination therapy versus 8.4 months with sunitinib, indicating a benefit across a broader group. The ORR among PD-L1-positive patients was 55.2% with the combination therapy compared to 25.5% with sunitinib.534 These findings demonstrate that avelumab plus axitinib significantly improves PFS over sunitinib in first-line treatment for advanced renal-cell carcinoma, presenting a potent treatment option for this patient population.534,535 Additionally, Xu et al. reported a phase I study (NCT02942329) combining SHR-1210 (anti-PD-1 antibody) with apatinib in various cancers, noting particularly favorable results in hepatocellular carcinoma patients.536 These studies collectively underscore the potential of combining anti-PD-L1 with anti-angiogenesis agents to enhance therapeutic efficacy across multiple cancer types.
Agents targeting EGF/EGFR and other growth factors
Anti-EGF/EGFR therapy
The discovery and subsequent elucidation of the EGFR signaling pathway represent a cornerstone in our understanding of cellular proliferation and oncogenesis. Initially identified in the 1960s through the pioneering work of Cohen, who discovered the EGF and its role in stimulating epithelial cell proliferation,537 and later Carpenter, who identified the specific receptor for EGF, EGFR has been established as a crucial receptor tyrosine kinase (RTK) in mediating cell growth and survival signals.538 As part of the ErbB family of RTKs, which includes HER2, HER3, and HER4, EGFR plays a pivotal role in organ development and tissue repair under physiological conditions.539 However, its deregulation, through mutations or overexpression, activates a cascade of pro-survival and anti-apoptotic signaling pathways leading to tumorigenesis in various cancers, including NSCLC, colorectal cancer, and glioblastoma.540–544 Hereto, targeting the EGFR pathway has emerged as a cornerstone in the treatment of certain cancers, reflecting a strategic shift towards precision oncology. The therapeutic arsenal against EGFR-driven malignancies includes small molecule TKIs and monoclonal antibodies, which respectively target the receptor intracellular kinase domain to prevent activation and its extracellular domain to block ligand binding and receptor dimerization (Table 19).545–547 Despite the clinical benefits of these agents, the challenge of acquired resistance has spurred ongoing research into novel therapeutic strategies.548–551 These include the development of combination therapies, as well as innovative approaches like peptides, nanobodies, and therapeutic vaccines aimed at directly inhibiting the EGFR or its ligands.552–556 Such advancements hold promise for overcoming resistance mechanisms and enhancing treatment outcomes, underscoring the dynamic evolution of cancer therapy in the era of molecular targeting.
Table 19.
EGFR-TKIs targeting common EGFR mutations
| Generation | Target | Agent | Type of ATP-competitive inhibitor | Status |
|---|---|---|---|---|
| First generation | Del19/L858R | Gefitinib | Reversible | FDA approval |
| Erlotinib | Reversible | FDA approval | ||
| Icotinib | Reversible | NMPA approval | ||
| Second generation | Del19/L858R | Afatinib | Irreversible | FDA approval |
| Dacomitinib | Irreversible | FDA approval | ||
| Third generation | T790M | Osimertinib | Irreversible | FDA approval |
| Almonertinib | Irreversible | NMPA approval | ||
| Lazertinib | Irreversible | Phase III (NCT04248829, NCT04487080, NCT05388669, NCT04988295) | ||
| BPI-7711 | Irreversible | Phase III (NCT03866499) | ||
| SH-1028 | Irreversible | Phase III (NCT06080776, NCT04239833) | ||
| ASK120067 | Irreversible | Phase III (NCT04143607) | ||
| ex20ins | Mobocertinib | Irreversible | FDA approval | |
| Sunvozertinib | Irreversible | NMPA approval | ||
| Furmonertinib | Irreversible | NMPA approval | ||
| Poziotinib | Irreversible | Phase III (NCT05378763) | ||
| CLN-081 | Irreversible | Phase III (NCT05973773) |
Note: FDA Food and Drug Administration, NMPA National Medical Products Administration
The exploration and development of anti-EGFR therapies have significantly evolved, offering new avenues for cancer treatment through a deep understanding of EGFR biochemistry and mechanisms underlying therapeutic resistance.557 Conventional EGFR inhibitors, such as TKIs and monoclonal antibodies, have been foundational in treating EGFR-driven tumors, transforming the management of cancers, especially NSCLC and colorectal cancer.558–560 The first-generation TKIs, exemplified by gefitinib, targeted EGFR with a degree of success limited to patients with specific EGFR mutations.561 This limitation, coupled with the emergence of resistance, led to the development of subsequent generations of TKIs aimed at offering more durable control of cancer progression by targeting additional resistance mechanisms, including the T790M mutation.562 However, despite these advancements, resistance remains a significant challenge, prompting research into fourth-generation TKIs and alternative strategies such as blocking the EGFR ligand, EGF, directly with promising early results from therapeutic vaccines, peptides, and single-domain antibodies.563–565
Besides TKIs, monoclonal antibodies against EGFR, such as cetuximab, panitumumab, amivantamab, and necitumumab, have also been approved for treating various cancers, often in combination with chemotherapy.566–571 Yet, the efficacy of these antibodies is hampered by resistance mechanisms similar to those affecting TKIs, including mutations in the EGFR extracellular domain and alterations in downstream signaling pathways.572,573 Strategies are being explored to address the issue of acquired resistance to existing anti-EGFR monoclonal antibodies, including the combination of antibodies that target distinct, non-overlapping regions of EGFR.574 Furthermore, the combination of anti-EGFR therapies with other treatment strategies, such as ICB and chemotherapy, represents a promising strategy to overcome resistance and improve patient outcomes.575–579 For instance, in a phase II trial involving 33 participants with recurrent or metastatic head and neck squamous cell carcinoma, the combination of pembrolizumab and cetuximab achieved the 45% ORR at 6 months, with the most common serious adverse event being oral mucositis.575 The challenge lies in the intricate nature of cancer cell survival mechanisms, requiring a multifaceted approach that includes the simultaneous targeting of EGFR and other critical pathways involved in tumor growth and progression. As the landscape of anti-EGFR therapy continues to expand, future research will likely focus on optimizing combination treatments, developing novel inhibitors that can bypass or prevent resistance, and refining patient selection criteria to maximize therapeutic efficacy and durability.
Anti-HER2 agents
Apart from EGFR, human epithelial growth factor receptor 2 (HER2) also belongs to the EGF receptor tyrosine kinase family.580 HER2 plays a pivotal role in the development, progression, and prognosis of various cancers due to its gene amplification or receptor overexpression.581 Notably, HER2 positivity is observed in a significant percentage of breast and gastric cancers, making HER2 a key target for diagnosis and treatment.582–584 The absence of a natural ligand for HER2 distinguishes it from other family members, with its activation primarily through dimerization with other receptors.585 HER2-targeted therapies, including trastuzumab and pertuzumab monoclonal antibodies, TKIs like lapatinib and afatinib, and the antibody-drug conjugate including T-DM1 and DS8201, have significantly advanced the treatment of HER2-positive cancers, particularly breast cancer.586–593 These therapies work by inhibiting HER2 dimerization, blocking downstream signaling pathways, inducing antibody-dependent cellular cytotoxicity, and delivering cytotoxic agents directly to cancer cells.594–596 Despite the success, resistance to treatments such as trastuzumab remains a challenge, prompting ongoing research into combination therapies and the development of novel anti-HER2 agents to enhance treatment efficacy and overcome resistance.597,598
Anti-FGFR therapy
Additionally, the fibroblast growth factor receptor (FGFR) pathway plays a significant role in cellular functions such as proliferation, differentiation, and survival, which are critical in both development and cancer progression.599 Aberrations in FGFR signaling, including gene amplifications, mutations, and alterations in ligand specificity through alternative splicing, contribute to oncogenesis and cancer progression across various tumors.600 Anti-FGFR therapies have emerged as promising strategies in cancer treatment. These therapies encompass a range of approaches, including TKIs like pemigatinib, futibatinib, erdafitinib and infigratinib, which have shown efficacy in cancers with FGFR genetic alterations, and monoclonal antibodies targeting FGFRs or their ligands to block aberrant signaling pathways (Table 20).601–605 For example, in a phase III trial, erdafitinib significantly improved OS compared to chemotherapy in patients with FGFR-altered metastatic urothelial carcinoma post-ICB, achieving a median survival of 12.1 vs. 7.8 months (HR: 0.64; P = 0.005).606 Despite the potential, challenges such as drug resistance and the intricate role of FGFR in normal physiology necessitate further research to optimize anti-FGFR therapies in cancer.607
Table 20.
Clinical trials of the combination therapy of FGFR blockade and immune checkpoint inhibitor
| FGFR inhibitor | NCT number | Cancer types | Combination partners | Phase | Status |
|---|---|---|---|---|---|
| Pemigatinib | NCT05913661 | Intrahepatic Cholangiocarcinoma | PD-1 Inhibitors | II | Recruiting |
| NCT05004974 | NSCLC | Sintilimab | II | Recruiting | |
| NCT04949191 | Solid Tumors | Pembrolizumab | II | Active, not recruiting | |
| NCT04003610 | Urothelial Carcinoma | Pembrolizumab | II | Terminated | |
| NCT02393248 | Solid Tumors | Pembrolizumab | I/II | Terminated | |
| Futibatinib | NCT05945823 | Solid Tumors | Pembrolizumab and Chemotherapy | II | Recruiting |
| NCT05036681 | Microsatellite Stable Endometrial Carcinoma | Pembrolizumab | II | Recruiting | |
| NCT04828486 | Hepatocellular Carcinoma | Pembrolizumab | II | Recruiting | |
| NCT04601857 | Urothelial Cancer | Pembrolizumab | II | Recruiting | |
| Erdafitinib | NCT05564416 | Bladder Cancer | Atezolizumab | II | Withdrawn |
| Infigratinib | NCT05510427 | Cholangiocarcinoma | Atezolizumab and Bevacizumab | I | Withdrawn |
| HMPL-453 | NCT05173142 | Solid Tumors | Gemcitabine, Cisplatin, Toripalimab, and Docetaxel | I/II | Recruiting |
| Bemarituzumab | NCT05322577 | Gastric or Gastroesophageal Junction Cancer | CAPOX/SOX and Nivolumab | I | Recruiting |
| NCT05267470 | Squamous-Cell NSCLC | Pembrolizumab, Carboplatin and Paclitaxel/Nab-paclitaxel | I | Active, not recruiting | |
| NCT05111626 | Gastric or Gastroesophageal Junction Cancer | Chemotherapy (mFOLFOX6 or CAPOX) and Nivolumab | III | Recruiting |
Note: NSCLC non-small cell lung cancer
Strategies targeting HGF/c-MET signaling
The HGF/c-MET signaling pathway, fundamental in cell growth and organ regeneration, has been implicated in cancer progression and metastasis due to its role in cellular proliferation, survival, and migration.608 HGF, produced by stromal cells, activates the c-MET receptor tyrosine kinase, leading to various downstream effects including cell scattering, invasion, and angiogenesis.609–611 Alterations in this pathway, such as MET gene amplification, overexpression, or activating mutations, have been identified in several cancers, contributing to tumor growth, angiogenesis, and resistance to therapies.612–615 Consequently, targeting the HGF/c-MET axis has emerged as a therapeutic strategy, with development of inhibitors like crizotinib and cabozantinib showing efficacy in certain cancers by blocking MET kinase activity.616–619 In a phase II trial (NCT01945021), crizotinib (inhibitor of ALK, ROS1, and MET) showed significant efficacy and durable responses in patients with ROS1-positive advanced NSCLC, achieving an ORR of 71.7% and median PFS of 15.9 months, with a safety profile consistent with previous studies.620 Besides, in the phase III trial CELESTIAL, cabozantinib (inhibitor of VEGFR, MET, and AXL) extended median OS to 10.2 months compared to 8.0 months with placebo (HR: 0.76; P = 0.005) and improved median PFS to 5.2 months versus 1.9 months (HR: 0.44; P < 0.001) in previously treated advanced hepatocellular carcinoma patients.621 Also, in the phase III trial CheckMate-9ER, nivolumab plus cabozantinib continued to show superior efficacy over sunitinib in first-line treatment of advanced renal cell carcinoma, with the median OS of 37.7 months compared to 34.3 months (HR: 0.70, P = 0.0043) and the median PFS of 16.6 months versus 8.3 months (HR: 0.56, P < 0.0001).622 Additionally, monoclonal antibodies targeting HGF/c-MET signaling are being investigated to inhibit ligand-receptor interactions, further exploring its potential as a target for cancer therapy.623,624 Notably, the HGF/c-MET signaling pathway is frequently hijacked by cancer cells to develop resistance to chemotherapy, radiotherapy, and targeted therapies, such as gefitinib and sotorasib.625–628 Consequently, targeting the HGF/c-MET signaling axis represents a promising strategy in cancer treatment, particularly when used combined with other therapeutic modalities.
Harnessing other growth factors
Besides, other protumor growth factor pathways such as the PDGF/PDGFR signaling, crucial for tumor cell proliferation, invasion, metastasis, and angiogenesis, present potential anti-cancer targets, with emerging therapies showing effectiveness yet facing challenges with efficacy and toxicity in clinical trials.629,630 Conversely, the hematopoietic growth factor GM-CSF acts as a tumor suppressor in most cases by eliciting immune responses against tumors.631 It enhances antitumor immunity primarily through the activation and maturation of DCs and macrophages, Th9 cell responses, and eventually inducing T cell cytotoxicity against tumors.632–635 The therapeutic applications of GM-CSF extend from counteracting neutropenia in cancer patients, to serving as an adjuvant in cancer vaccines where it boosts antigen presentation and T cell activation, thereby improving vaccine efficacy.636 Furthermore, GM-CSF-expressing oncolytic virus therapies and GM-CSF-secreting tumor cell vaccines have shown promise in inducing potent antitumor immune responses.637–640 However, GM-CSF can also promote tumor progression by enhancing MDSCs and TAMs, indicating the complexity of its effects in the TME.641,642 This dual nature of GM-CSF necessitates a careful balance in its application, which underscores the importance of dosage, administration route, and combination with other therapeutic strategies to maximize its antitumor potential while minimizing protumor effects.643
In a pioneering phase I clinical trial, an innovative autologous GM-CSF-secreting breast cancer vaccine was administered to patients with both metastatic (n = 12) and stage II-III breast cancer (n = 7), showcasing limited toxicity alongside variable efficacy.644 Notably, among those with metastatic disease, eight developed disease progression within two months, whereas one remarkable case exhibited no evidence of disease for an extended period of 13 years; patients with stage II-III breast cancer reported a median survival time of 6.24 years following vaccination.644 Furthermore, the phase II trial (ChiCTR1900026175) assessed the efficacy and safety of the PRaG regimen (a combination of radiotherapy, anti-PD-1, and GM-CSF) in patients facing metastatic cancer resistant to conventional treatments.645 This trial, with a median follow-up period of 16.4 months across 54 participants, revealed an ORR of 16.7%, a DCR of 46.3%, and a median PFS of 4.0 months alongside an OS of 10.5 months645. Lastly, the phase II trial (NCT01767194) explored the I/T/DIN/GM-CSF regimen (irinotecan, temozolomide, dintuximab, and GM-CSF) for patients with relapsed/refractory neuroblastoma, confirming its significant antitumor efficacy.646 Out of 53 patients, 22 (41.5%) achieved objective responses, with another 22 maintaining stable disease.646 This regimen showed a one-year PFS rate of 67.9% and an OS rate of 84.9% with a tolerable safety profile, prompting further research into its application in frontline treatments and the exploration of predictive biomarkers.646
Clinical progress and future direction
The clinical progress of cytokine and chemokine-targeted therapies has been marked by both challenges and significant achievements. The journey from preclinical research to clinical application has illuminated the nuanced role these molecules play in cancer biology, offering novel therapeutic avenues that extend beyond traditional treatment modalities.
The approval of cytokine therapies such as IFN-α and IL-2 for the treatment of certain cancers has been a testament to the clinical potential of targeting cytokine pathways. IFN-α, utilized for its immunomodulatory and anti-proliferative properties, has been approved for melanoma, follicular lymphoma, and other malignancies. IL-2, known for its capacity to boost T-cell responses, has been approved for metastatic renal cell carcinoma and metastatic melanoma, demonstrating the feasibility of enhancing the immune system to fight cancer. These approvals were based on clinical trials that highlighted the efficacy of these cytokines in improving patient outcomes, albeit with the recognition of their limitations, including severe side effects and the need for high-dose administration. Besides, a significant area of clinical research has focused on combining cytokine therapies with ICB to overcome resistance mechanisms that limit the efficacy of ICB alone. Numerous trials have evaluated the combination of ICB with cytokines like IFN-α and IL-12, revealing that such combinations can synergistically enhance antitumor immunity. For example, clinical trials combining IFN-α with pembrolizumab in melanoma patients have demonstrated improved response rates compared to pembrolizumab monotherapy, indicating the potential of IFN-α to augment the immune system to recognize and destroy tumor cells.
Moreover, the clinical development of monoclonal antibodies and receptor inhibitors targeting protumor cytokines such as VEGF, IL-6, and TGF-β represents another milestone in cancer therapy. Bevacizumab, an anti-VEGF antibody, has been widely incorporated into treatment regimens for colorectal cancer, NSCLC, hepatocellular carcinoma, and glioblastoma. Similarly, inhibitors targeting the IL-6 pathway, like tocilizumab, have entered clinical trials to evaluate their potential in mitigating cancer-related inflammation and cachexia, showcasing the therapeutic versatility of targeting cytokine pathways. Also, anti-TGF-β/PD-L1 bifunctional antibodies such as M7824 have demonstrated its potential in treating various advanced solid tumors, including NSCLC and cervical cancer. It has been acclaimed as a “next-generation” anti-PD-L1 agent, exemplified by a phase I clinical trial that reported an outstanding response rate of over 85% in patients with PD-L1high NSCLC.
Despite these advances, the clinical application of cytokine and chemokine-targeted therapies is not without challenges. The adverse effects associated with cytokine therapies, such as the capillary leak syndrome seen with high-dose IL-2 treatment, have necessitated the development of strategies to mitigate toxicity while preserving efficacy. Moreover, the heterogeneity of tumors and the complexity of the TME mean that not all patients respond equally to these treatments, underscoring the need for biomarkers to predict response and guide therapy selection. Take the M7824 as an example, despite the promising early results in phase I clinical trials, subsequent larger-scale clinical trials of anti-TGF-β/PD-L1 therapies encountered unforeseen challenges. M7824 did not meet the primary endpoint event in multiple phase II/III clinical trials including biliary tract cancer and NSCLC. The underlying reasons for these setbacks remain unclear, it is generally believed that optimizing patient selection is crucial for the successful clinical translation.
Looking ahead, the clinical development of cytokine and chemokine-targeted therapies is poised to benefit from advancements in precision medicine, biomarker research, and drug delivery systems. The ongoing integration of these therapies with other treatment modalities, coupled with a deeper understanding of their mechanisms of action, promises to expand their therapeutic potential and refine their clinical application, ultimately improving outcomes for patients with cancer.
Conclusion and perspective
The past few decades have witnessed significant advances in understanding the complex interplay between cytokines, chemokines, and their signaling pathways in the context of cancer biology. These insights have paved the way for innovative therapeutic strategies targeting cytokine and chemokine signaling pathways, offering new hope for patients with cancer. The development of cytokine-based therapies, including both antagonists and agonists, has underscored the dual nature of these molecules in cancer, where they can act as both promoters and suppressors of tumorigenesis depending on the context. This duality presents both challenges and opportunities for therapeutic intervention, necessitating a refined approach to harnessing their potential for cancer therapy. The emergence of targeted therapies against specific cytokines, such as IFN-I, IL-2, and IL-12, has demonstrated the feasibility of modulating the immune system to combat cancer. Similarly, the blockade of protumor cytokines, including TGF-β, VEGF, and IL-6, using antibodies and small-molecule inhibitors, has shown promise in inhibiting tumor growth, metastasis, and angiogenesis (Fig. 9) (Table 21). These therapies not only direct their effects on the tumor cells but also remodel the TME to enhance antitumor immunity. Furthermore, the advent of combination strategies, particularly the synergy between cytokine blockade and ICB, has opened new avenues for overcoming resistance to conventional immunotherapies and improving patient outcomes.
Fig. 9.
Cytokine- and chemokine-targeted strategies in cancer therapy. The top panel illustrates various methods of blocking protumor cytokine signaling pathways, including antibody, trap, inhibitor, antisense oligonucleotide (ASO), and vaccine. The middle panel depicts strategies for expressing antitumor cytokines or activating their downstream pathways, including direct cytokine or modified cytokine administration, electroporation, immunocytokines, cytokine-expressing cells, viral vectors, liposome delivery, and STING agonists. Notably, IL-10 generally suppresses immune response, but some studies suggest that it promotes the activation of tumor-resident CD8+ T cells. Therefore, IL-10 administration is used to improve immunotherapy effectiveness in some clinical trials. The bottom left panel highlights the blockade of protumor chemokine signaling using antibodies and inhibitors to disrupt mechanisms contributing to immune evasion and tumor progression. The bottom right panel presents approaches for expressing antitumor chemokines or their receptors to stimulate an immune response, featuring engineered T cells expressing specific chemokine receptors and oncolytic viruses (OVs) designed to deliver chemokine genes directly into the tumor microenvironment. Adapted from “The Tumor Microenvironment: Overview of Cancer-Associated Changes”, by BioRender.com (2024). Retrieved from https://app.biorender.com/biorender-templates
Table 21.
Cytokines targeted for cancer treatment
| Cytokine | Source | Main generation mechanisms | Main biological activities harnessed by cancer therapy |
|---|---|---|---|
| IFN-Is | Immune cells, tumors cells, endothelial cells, CAFs | TLR4-MyD88 pathway, cGAS/STING pathway, and TLR3-TICAM1 pathway | Inducing tumor cell apoptosis; Promoting the maturation and antigen presentation of DCs; Enhancing NK activation (IFN-I therapy or agonist). |
| IFN-γ | NK cells and T cells | Both receptor- and cytokine-dependent mechanisms | Promoting the antigen presentation of macrophages and DCs; Enhancing the activation of T cells and NK cells; Inhibiting Treg functions; Hampering Th2 and Th17 response (IFN-γ therapy). |
| IL-1 | IL-1α: immune cells and non-immune cells; IL-1β: immune cells | IL-1α: constitutively expressed and upregulated by inflammatory stimuli and oxidative stress; IL-1β: induced by inflammatory stimuli | Increasing the accumulation of TAMs and MDSCs (counteracted by IL-1 inhibitors). |
| IL-2 | T cells | TCR stimulation | Promoting the function and activity of T cells and NK cells; Improving memory T cell development (engineered IL-2 proteins). |
| IL-6 | Immune cells and non-immune cells | Cancer-related inflammation | Promoting cancer cell proliferation, survival, metastasis, angiogenesis, and immune evasion (inhibitors of IL-6 and downstream signaling). |
| IL-10 | Immune cells, tumor cells, epithelial cells | PRR stimulation | Generally, IL-10 suppresses immune response, but some studies suggest that it promotes the activation of tumor-resident CD8+ T cells (IL-10 therapy). |
| IL-12 | DCs, macrophages, and B cells | PRR stimulation, IFN-dependent pathway | Enhancing the function and activity of T cells and NK cells; Promoting Th1 response; Reprograming immunosuppressive cells, such as MDSCs and TAMs (localized IL-12 delivery). |
| IL-15 | Mainly expressed by myeloid cells | Regulated at multiple levels: transcription (IRF-E and NF-κB), post-transcription, and trans-presentation (IL-15Rα). | Promoting the function and activity of T cells and NK cells (engineered IL-15 proteins). |
| TNF | Immune cells and non-immune cells | Cancer-related inflammation | Promoting cancer cell proliferation, survival, metastasis, angiogenesis, and immune evasion (TNF blockade). |
| VEGF | Immune cells and non-immune cells | Hypoxia | Supporting tumor angiogenesis, growth, and metastasis; Undermining the functions of effector cells and DCs; Increasing the accumulation of immunosuppressive cells (Blocked by anti-angiogenesis agents) |
| TGF-β | Tumor cells and stromal cells | Latent TGF-β complex is activated by integrins, acids-bases, ROS, proteases | Promoting tumor epithelial-mesenchymal transition, metastasis, treatment resistance, and matrix remodeling; Inducing the differentiation of Tregs, M2-like macrophages, MDSCs; Hampering the functions of NK cells, T cells, and DCs (TGF-β and PD-L1 dual blockade). |
Note: CAF cancer-associated fibroblast, DC dendritic cell, IRF-E interferon regulatory factor element, MDSC myeloid-derived suppressor cell, NK cell natural killer cell, PRR pattern recognition receptor, ROS reactive oxygen species, TAM tumor-associated macrophage, Treg regulatory T cell
Notably, the pleiotropic nature of cytokines and their context-dependent roles in cancer and immunity necessitate a deeper understanding of the TME and the dynamic interactions between different cell types. This complexity underscores the need for precision medicine approaches that consider individual patient characteristics, including the genetic and molecular profiles of tumors, to tailor therapies for optimal efficacy. Moreover, the development of predictive biomarkers to identify patients who are most likely to benefit from specific cytokine-targeted therapies is crucial for advancing personalized cancer treatment. In the future, continued research into the biology of cytokines, along with technological advancements in drug delivery and molecular engineering, holds the promise of developing more effective and less toxic therapeutic options. The integration of cytokine-based therapies with other treatment modalities, such as targeted therapies, chemotherapy, and radiotherapy, offers a comprehensive approach to cancer management. Furthermore, the exploration of novel targets and mechanisms of action, including the modulation of the immune system and the TME, will likely yield additional therapeutic candidates.
In conclusion, targeting cytokine and chemokine signaling pathways represents a frontier in cancer therapy, offering the potential to significantly improve patient outcomes. The successes achieved so far provide a strong foundation for future research and clinical development. By leveraging our growing understanding of cytokine biology, coupled with advancements in biotechnology and precision medicine, we can look forward to more effective, personalized therapies to fight cancer. The translation from the bench to the bedside is fraught with challenges, but the promise of cytokine- and chemokine-targeted therapies in revolutionizing cancer treatment is undeniably within reach.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 82373281 and 82272794), Natural Science Foundation of Zhejiang Province (Nos. LQ24H160007 and LZ22H160005), and China Postdoctoral Science Foundation (Nos. GZB20230642, 2022M722766, and 2023M743016).
Author contributions
M.Y. and T.L. performed the selection of literature, drafted the paper and prepared the figures. M.N., Y.W., and H.Z. collected the related references and participated in discussion. K.W. and Z.D. designed the work. All authors read and approved the final paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Ming Yi, Tianye Li
Contributor Information
Kongming Wu, Email: kmwu_lab@163.com.
Zhijun Dai, Email: dzj0911@126.com.
References
- 1.Liu, C. et al. Cytokines: from clinical significance to quantification. Adv. Sci.8, e2004433 (2021). 10.1002/advs.202004433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer120, 6–15 (2019). 10.1038/s41416-018-0328-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Propper, D. J. & Balkwill, F. R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol.19, 237–253 (2022). 10.1038/s41571-021-00588-9 [DOI] [PubMed] [Google Scholar]
- 4.Lippitz, B. E. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol.14, e218–e228 (2013). 10.1016/S1470-2045(12)70582-X [DOI] [PubMed] [Google Scholar]
- 5.Yi, M. et al. TGF-β: a novel predictor and target for anti-PD-1/PD-L1 therapy. Front. Immunol.13, 1061394 (2022). 10.3389/fimmu.2022.1061394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin, S. et al. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J. Hematol. Oncol.12, 27 (2019). 10.1186/s13045-019-0718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldmann, T. A. Cytokines in cancer immunotherapy. Cold Spring Harb. Perspect. Biol.10, a028472 (2018). 10.1101/cshperspect.a028472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gresser, I. & Bourali, C. Antitumor effects of interferon preparations in mice. J. Natl Cancer Inst.45, 365–376, (1970). [PubMed] [Google Scholar]
- 9.Yu, R., Zhu, B. & Chen, D. Type I interferon-mediated tumor immunity and its role in immunotherapy. Cell Mol. Life Sci.79, 191 (2022). 10.1007/s00018-022-04219-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkwood, J. M. et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J. Clin. Oncol.14, 7–17 (1996). 10.1200/JCO.1996.14.1.7 [DOI] [PubMed] [Google Scholar]
- 11.Groopman, J. E. et al. Recombinant alpha-2 interferon therapy for Kaposi’s sarcoma associated with the acquired immunodeficiency syndrome. Ann. Intern. Med.100, 671–676, (1984). 10.7326/0003-4819-100-5-671 [DOI] [PubMed] [Google Scholar]
- 12.Golomb, H. M. et al. Alpha-2 interferon therapy of hairy-cell leukemia: a multicenter study of 64 patients. J. Clin. Oncol.4, 900–905 (1986). 10.1200/JCO.1986.4.6.900 [DOI] [PubMed] [Google Scholar]
- 13.Solal-Celigny, P. et al. Recombinant interferon alfa-2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. Groupe d’Etude des Lymphomes de l’Adulte. N. Engl. J. Med.329, 1608–1614 (1993). 10.1056/NEJM199311253292203 [DOI] [PubMed] [Google Scholar]
- 14.Atkins, M. B. et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol.17, 2105–2116 (1999). 10.1200/JCO.1999.17.7.2105 [DOI] [PubMed] [Google Scholar]
- 15.Fyfe, G. et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol.13, 688–696 (1995). 10.1200/JCO.1995.13.3.688 [DOI] [PubMed] [Google Scholar]
- 16.Kennedy, L. B. & Salama, A. K. S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin.70, 86–104 (2020). 10.3322/caac.21596 [DOI] [PubMed] [Google Scholar]
- 17.Weng, J. et al. Exploring immunotherapy in colorectal cancer. J. Hematol. Oncol.15, 95 (2022). 10.1186/s13045-022-01294-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, Y. et al. Immune checkpoint modulators in cancer immunotherapy: recent advances and emerging concepts. J. Hematol. Oncol.15, 111 (2022). 10.1186/s13045-022-01325-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atallah-Yunes, S. A. & Robertson, M. J. Cytokine based immunotherapy for cancer and lymphoma: biology, challenges and future perspectives. Front. Immunol.13, 872010 (2022). 10.3389/fimmu.2022.872010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet357, 539–545, (2001). 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 21.Briukhovetska, D. et al. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer21, 481–499 (2021). 10.1038/s41568-021-00363-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, T. et al. Bispecific antibody targeting TGF-β and PD-L1 for synergistic cancer immunotherapy. Front. Immunol.14, 1196970 (2023). 10.3389/fimmu.2023.1196970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi, M. et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer18, 60 (2019). 10.1186/s12943-019-0974-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazear, H. M., Schoggins, J. W. & Diamond, M. S. Shared and distinct functions of type I and type III interferons. Immunity50, 907–923 (2019). 10.1016/j.immuni.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snell, L. M., McGaha, T. L. & Brooks, D. G. Type I interferon in chronic virus infection and cancer. Trends Immunol.38, 542–557 (2017). 10.1016/j.it.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benci, J. L. et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell167, 1540–1554.e1512 (2016). 10.1016/j.cell.2016.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zitvogel, L. et al. Type I interferons in anticancer immunity. Nat. Rev. Immunol.15, 405–414 (2015). 10.1038/nri3845 [DOI] [PubMed] [Google Scholar]
- 28.Duong, E. et al. Type I interferon activates MHC class I-dressed CD11b(+) conventional dendritic cells to promote protective anti-tumor CD8(+) T cell immunity. Immunity55, 308–323.e309 (2022). 10.1016/j.immuni.2021.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilander, M. et al. Enlarged memory T-cell pool and enhanced Th1-type responses in chronic myeloid leukemia patients who have successfully discontinued IFN-α monotherapy. PLoS ONE9, e87794 (2014). 10.1371/journal.pone.0087794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillot, B. et al. The expression of cytotoxic mediators is altered in mononuclear cells of patients with melanoma and increased by interferon-alpha treatment. Br. J. Dermatol.152, 690–696 (2005). 10.1111/j.1365-2133.2005.06512.x [DOI] [PubMed] [Google Scholar]
- 31.Papewalis, C. et al. IFN-alpha skews monocytes into CD56+-expressing dendritic cells with potent functional activities in vitro and in vivo. J. Immunol.180, 1462–1470 (2008). 10.4049/jimmunol.180.3.1462 [DOI] [PubMed] [Google Scholar]
- 32.Crouse, J. et al. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity40, 961–973 (2014). 10.1016/j.immuni.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 33.Bacher, N. et al. Interferon-α suppresses cAMP to disarm human regulatory T cells. Cancer Res.73, 5647–5656 (2013). 10.1158/0008-5472.CAN-12-3788 [DOI] [PubMed] [Google Scholar]
- 34.Fung, K. Y. et al. Interferon-ε protects the female reproductive tract from viral and bacterial infection. Science339, 1088–1092 (2013). 10.1126/science.1233321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks, Z. R. C. et al. Interferon-ε is a tumour suppressor and restricts ovarian cancer. Nature620, 1063–1070 (2023). 10.1038/s41586-023-06421-w [DOI] [PubMed] [Google Scholar]
- 36.Holicek, P. et al. Type I interferon and cancer. Immunol. Rev.321, 115–127 (2024). 10.1111/imr.13272 [DOI] [PubMed] [Google Scholar]
- 37.Terawaki, S. et al. IFN-α directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J. Immunol.186, 2772–2779 (2011). 10.4049/jimmunol.1003208 [DOI] [PubMed] [Google Scholar]
- 38.Jacquelot, N. et al. Sustained type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res.29, 846–861 (2019). 10.1038/s41422-019-0224-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nan, J., Wang, Y., Yang, J. & Stark, G. R. IRF9 and unphosphorylated STAT2 cooperate with NF-κB to drive IL6 expression. Proc. Natl Acad. Sci. USA115, 3906–3911 (2018). 10.1073/pnas.1714102115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musella, M. et al. Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B. Nat. Immunol.23, 1379–1392 (2022). 10.1038/s41590-022-01290-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tjandra, S. S. et al. IFN-{beta} signaling positively regulates tumorigenesis in aggressive fibromatosis, potentially by modulating mesenchymal progenitors. Cancer Res.67, 7124–7131 (2007). 10.1158/0008-5472.CAN-07-0686 [DOI] [PubMed] [Google Scholar]
- 42.Yi, M. et al. Exploiting innate immunity for cancer immunotherapy. Mol. Cancer22, 187 (2023). 10.1186/s12943-023-01885-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Martínez, E. et al. Trial Watch: Immunostimulation with recombinant cytokines for cancer therapy. Oncoimmunology7, e1433982 (2018). 10.1080/2162402X.2018.1433982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eggermont, A. M. et al. Long-term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J. Clin. Oncol.30, 3810–3818 (2012). 10.1200/JCO.2011.41.3799 [DOI] [PubMed] [Google Scholar]
- 45.Moschos, S. J. et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J. Clin. Oncol.24, 3164–3171 (2006). 10.1200/JCO.2005.05.2498 [DOI] [PubMed] [Google Scholar]
- 46.Simonsson, B. et al. Combination of pegylated IFN-α2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood118, 3228–3235 (2011). 10.1182/blood-2011-02-336685 [DOI] [PubMed] [Google Scholar]
- 47.Burchert, A. et al. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J. Clin. Oncol.28, 1429–1435 (2010). 10.1200/JCO.2009.25.5075 [DOI] [PubMed] [Google Scholar]
- 48.Hu, B. et al. IFNα Potentiates Anti-PD-1 Efficacy by Remodeling Glucose Metabolism in the Hepatocellular Carcinoma Microenvironment. Cancer Discov.12, 1718–1741 (2022). 10.1158/2159-8290.CD-21-1022 [DOI] [PubMed] [Google Scholar]
- 49.Davar, D. et al. Phase Ib/II study of pembrolizumab and pegylated-interferon alfa-2b in advanced melanoma. J. Clin. Oncol.36, Jco1800632 (2018). 10.1200/JCO.18.00632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blaauboer, A., Sideras, K., van Eijck, C. H. J. & Hofland, L. J. Type I interferons in pancreatic cancer and development of new therapeutic approaches. Crit. Rev. Oncol. Hematol.159, 103204 (2021). 10.1016/j.critrevonc.2020.103204 [DOI] [PubMed] [Google Scholar]
- 51.Bialek-Waldmann, J. K., Heuser, M., Ganser, A. & Stripecke, R. Monocytes reprogrammed with lentiviral vectors co-expressing GM-CSF, IFN-α2 and antigens for personalized immune therapy of acute leukemia pre- or post-stem cell transplantation. Cancer Immunol. Immunother.68, 1891–1899 (2019). 10.1007/s00262-019-02406-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bracci, L., Proietti, E. & Belardelli, F. IFN-alpha and novel strategies of combination therapy for cancer. Ann. N. Y Acad. Sci.1112, 256–268, (2007). 10.1196/annals.1415.030 [DOI] [PubMed] [Google Scholar]
- 53.Hauschild, A., Kähler, K. C., Schäfer, M. & Fluck, M. Interdisciplinary management recommendations for toxicity associated with interferon-alfa therapy. J. Dtsch Dermatol. Ges.6, 829–837 (2008). 829-838. 10.1111/j.1610-0387.2008.06651.x [DOI] [PubMed] [Google Scholar]
- 54.Fu, Y., Tang, R. & Zhao, X. Engineering cytokines for cancer immunotherapy: a systematic review. Front. Immunol.14, 1218082 (2023). 10.3389/fimmu.2023.1218082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin, S. et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target Ther.7, 39 (2022). 10.1038/s41392-021-00868-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao, X. et al. Next generation of tumor-activating type I IFN enhances anti-tumor immune responses to overcome therapy resistance. Nat. Commun.12, 5866 (2021). 10.1038/s41467-021-26112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Escobar, G. et al. Genetic engineering of hematopoiesis for targeted IFN-α delivery inhibits breast cancer progression. Sci. Transl. Med.6, 217ra213 (2014). 10.1126/scitranslmed.3006353 [DOI] [PubMed] [Google Scholar]
- 58.Carta, L. et al. Engineering of macrophages to produce IFN-gamma in response to hypoxia. J. Immunol.166, 5374–5380 (2001). 10.4049/jimmunol.166.9.5374 [DOI] [PubMed] [Google Scholar]
- 59.Jiang, W., Zhang, C., Tian, Z. & Zhang, J. hIFN-α gene modification augments human natural killer cell line anti-human hepatocellular carcinoma function. Gene Ther.20, 1062–1069 (2013). 10.1038/gt.2013.31 [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto, H. et al. Type I IFN gene delivery suppresses regulatory T cells within tumors. Cancer Gene Ther.21, 532–541 (2014). 10.1038/cgt.2014.60 [DOI] [PubMed] [Google Scholar]
- 61.Chin, E. N., Sulpizio, A. & Lairson, L. L. Targeting STING to promote antitumor immunity. Trends Cell Biol.33, 189–203 (2023). 10.1016/j.tcb.2022.06.010 [DOI] [PubMed] [Google Scholar]
- 62.Yi, M. et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol. Cancer21, 28 (2022). 10.1186/s12943-021-01489-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burdette, D. L. et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature478, 515–518 (2011). 10.1038/nature10429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao, P. et al. Cyclic [G(2’,5’)pA(3’,5’)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell153, 1094–1107 (2013). 10.1016/j.cell.2013.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ablasser, A. et al. cGAS produces a 2’-5’-linked cyclic dinucleotide second messenger that activates STING. Nature498, 380–384 (2013). 10.1038/nature12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science347, aaa2630 (2015). 10.1126/science.aaa2630 [DOI] [PubMed] [Google Scholar]
- 67.Abe, T. & Barber, G. N. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J. Virol.88, 5328–5341 (2014). 10.1128/JVI.00037-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura, T. et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J. Immunother. Cancer9, e002852 (2021). 10.1136/jitc-2021-002852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding, L. et al. STING agonism overcomes STAT3-mediated immunosuppression and adaptive resistance to PARP inhibition in ovarian cancer. J. Immunother. Cancer11, e005627 (2023). 10.1136/jitc-2022-005627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee, S. J. et al. STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer. J. Immunother. Cancer9, e002195 (2021). 10.1136/jitc-2020-002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramanjulu, J. M. et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature564, 439–443 (2018). 10.1038/s41586-018-0705-y [DOI] [PubMed] [Google Scholar]
- 72.Yi, M. et al. Combination of oral STING agonist MSA-2 and anti-TGF-β/PD-L1 bispecific antibody YM101: a novel immune cocktail therapy for non-inflamed tumors. J. Hematol. Oncol.15, 142 (2022). 10.1186/s13045-022-01363-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yi, M. et al. Combine and conquer: manganese synergizing anti-TGF-β/PD-L1 bispecific antibody YM101 to overcome immunotherapy resistance in non-inflamed cancers. J. Hematol. Oncol.14, 146 (2021). 10.1186/s13045-021-01155-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pan, B. S. et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 369, eaba6098 (2020). [DOI] [PubMed]
- 75.Wu, Y. T. et al. Tumor-targeted delivery of a STING agonist improvescancer immunotherapy. Proc. Natl Acad. Sci. USA119, e2214278119 (2022). 10.1073/pnas.2214278119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu, N. et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J. Exp. Med. 218, e20200844 (2021). [DOI] [PMC free article] [PubMed]
- 77.Lu, Q. et al. Activation of the cGAS-STING pathway combined with CRISPR-Cas9 gene editing triggering long-term immunotherapy. Biomaterials291, 121871 (2022). 10.1016/j.biomaterials.2022.121871 [DOI] [PubMed] [Google Scholar]
- 78.Meric-Bernstam, F. et al. Combination of the STING agonist MIW815 (ADU-S100) and PD-1 inhibitor spartalizumab in advanced/metastatic solid tumors or lymphomas: an open-label, multicenter, phase Ib study. Clin. Cancer Res.29, 110–121 (2023). 10.1158/1078-0432.CCR-22-2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meric-Bernstam, F. et al. Phase I dose-escalation trial of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin. Cancer Res.28, 677–688 (2022). 10.1158/1078-0432.CCR-21-1963 [DOI] [PubMed] [Google Scholar]
- 80.Luke, J. J. et al. Phase I study of SYNB1891, an engineered E. coli nissle strain expressing STING agonist, with and without atezolizumab in advanced malignancies. Clin. Cancer Res.29, 2435–2444 (2023). 10.1158/1078-0432.CCR-23-0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lv, M. et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res.30, 966–979 (2020). 10.1038/s41422-020-00395-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fang, L. et al. Light-controllable charge-reversal nanoparticles with polyinosinic-polycytidylic acid for enhancing immunotherapy of triple negative breast cancer. Acta Pharm. Sin. B12, 353–363 (2022). 10.1016/j.apsb.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagato, T., Lee, Y. R., Harabuchi, Y. & Celis, E. Combinatorial immunotherapy of polyinosinic-polycytidylic acid and blockade of programmed death-ligand 1 induce effective CD8 T-cell responses against established tumors. Clin. Cancer Res.20, 1223–1234, (2014). 10.1158/1078-0432.CCR-13-2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li, T. et al. Discrepant antitumor efficacies of three CpG oligodeoxynucleotide classes in monotherapy and co-therapy with PD-1 blockade. Pharm. Res.161, 105293 (2020). 10.1016/j.phrs.2020.105293 [DOI] [PubMed] [Google Scholar]
- 85.Wang, S. et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc. Natl Acad. Sci. USA113, E7240–E7249 (2016). 10.1073/pnas.1608555113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang, Y. et al. Exploiting RIG-I-like receptor pathway for cancer immunotherapy. J. Hematol. Oncol.16, 8 (2023). 10.1186/s13045-023-01405-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han, J., Wu, M. & Liu, Z. Dysregulation in IFN-γ signaling and response: the barricade to tumor immunotherapy. Front. Immunol.14, 1190333 (2023). 10.3389/fimmu.2023.1190333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ivashkiv, L. B. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol.18, 545–558 (2018). 10.1038/s41577-018-0029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gocher, A. M., Workman, C. J. & Vignali, D. A. A. Interferon-γ: teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol.22, 158–172 (2022). 10.1038/s41577-021-00566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tau, G. Z. et al. Regulation of IFN-gamma signaling is essential for the cytotoxic activity of CD8(+) T cells. J. Immunol.167, 5574–5582 (2001). 10.4049/jimmunol.167.10.5574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song, M. et al. Low-dose IFNγ induces tumor cell stemness in tumor microenvironment of non-small cell lung cancer. Cancer Res.79, 3737–3748 (2019). 10.1158/0008-5472.CAN-19-0596 [DOI] [PubMed] [Google Scholar]
- 92.Jorgovanovic, D., Song, M., Wang, L. & Zhang, Y. Roles of IFN-γ in tumor progression and regression: a review. Biomark. Res.8, 49 (2020). 10.1186/s40364-020-00228-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mojic, M., Takeda, K. & Hayakawa, Y. The dark side of IFN-γ: its role in promoting cancer immunoevasion. Int. J. Mol. Sci.19, 89 (2017). 10.3390/ijms19010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castro, F. et al. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol.9, 847 (2018). 10.3389/fimmu.2018.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pujade-Lauraine, E. et al. Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second-look laparotomy. J. Clin. Oncol.14, 343–350 (1996). 10.1200/JCO.1996.14.2.343 [DOI] [PubMed] [Google Scholar]
- 96.Windbichler, G. H. et al. Interferon-gamma in the first-line therapy of ovarian cancer: a randomized phase III trial. Br. J. Cancer82, 1138–1144 (2000). 10.1054/bjoc.1999.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alberts, D. S. et al. Randomized phase 3 trial of interferon gamma-1b plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel alone for first-line treatment of advanced ovarian and primary peritoneal carcinomas: results from a prospectively designed analysis of progression-free survival. Gynecol. Oncol.109, 174–181 (2008). 10.1016/j.ygyno.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 98.Gleave, M. E. et al. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. Canadian Urologic Oncology Group. N. Engl. J. Med.338, 1265–1271 (1998). 10.1056/NEJM199804303381804 [DOI] [PubMed] [Google Scholar]
- 99.Schiller, J. H. et al. Eastern cooperative group trial of interferon gamma in metastatic melanoma: an innovative study design. Clin. Cancer Res.2, 29–36 (1996). [PubMed] [Google Scholar]
- 100.Wiesenfeld, M. et al. Controlled clinical trial of interferon-gamma as postoperative surgical adjuvant therapy for colon cancer. J. Clin. Oncol.13, 2324–2329 (1995). 10.1200/JCO.1995.13.9.2324 [DOI] [PubMed] [Google Scholar]
- 101.Larson, R. C. et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature604, 563–570 (2022). 10.1038/s41586-022-04585-5 [DOI] [PubMed] [Google Scholar]
- 102.Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest.127, 2930–2940 (2017). 10.1172/JCI91190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Higgs, B. W. et al. Interferon gamma messenger RNA signature in tumor biopsies predicts outcomes in patients with non-small cell lung carcinoma or urothelial cancer treated with durvalumab. Clin. Cancer Res.24, 3857–3866 (2018). 10.1158/1078-0432.CCR-17-3451 [DOI] [PubMed] [Google Scholar]
- 104.Reijers, I. L. M. et al. IFN-γ signature enables selection of neoadjuvant treatment in patients with stage III melanoma. J. Exp. Med.220, e20221952 (2023). 10.1084/jem.20221952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao, J. et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell167, 397–404.e399 (2016). 10.1016/j.cell.2016.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang, S. et al. Systemic interferon-γ increases MHC class I expression and T-cell infiltration in cold tumors: results of a phase 0 clinical trial. Cancer Immunol. Res.7, 1237–1243 (2019). 10.1158/2326-6066.CIR-18-0940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang, M. et al. Interferon gamma inhibits CXCL8-CXCR2 axis mediated tumor-associated macrophages tumor trafficking and enhances anti-PD1 efficacy in pancreatic cancer. J. Immunother. Cancer8, e000308 (2020). 10.1136/jitc-2019-000308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zibelman, M. et al. A phase 1 study of nivolumab in combination with interferon-gamma for patients with advanced solid tumors. Nat. Commun.14, 4513 (2023). 10.1038/s41467-023-40028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schroeder, B. A. et al. Histiocyte predominant myocarditis resulting from the addition of interferon gamma to cyclophosphamide-based lymphodepletion for adoptive cellular therapy. J. Immunother. Cancer8, e000247 (2020). 10.1136/jitc-2019-000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morgan, D. A., Ruscetti, F. W. & Gallo, R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science193, 1007–1008 (1976). 10.1126/science.181845 [DOI] [PubMed] [Google Scholar]
- 111.Liao, W., Lin, J. X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity38, 13–25 (2013). 10.1016/j.immuni.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ko, B. et al. Rethinking oncologic treatment strategies with interleukin-2. Cells12, 1316 (2023). 10.3390/cells12091316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ross, S. H. & Cantrell, D. A. Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol.36, 411–433 (2018). 10.1146/annurev-immunol-042617-053352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lanzavecchia, A. & Sallusto, F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science290, 92–97 (2000). 10.1126/science.290.5489.92 [DOI] [PubMed] [Google Scholar]
- 115.Boyman, O. & Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol.12, 180–190 (2012). 10.1038/nri3156 [DOI] [PubMed] [Google Scholar]
- 116.Sakaguchi, S. et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol.155, 1151–1164 (1995). 10.4049/jimmunol.155.3.1151 [DOI] [PubMed] [Google Scholar]
- 117.Hernandez, R., Põder, J., LaPorte, K. M. & Malek, T. R. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat. Rev. Immunol.22, 614–628 (2022). 10.1038/s41577-022-00680-w [DOI] [PubMed] [Google Scholar]
- 118.Lisiero, D. N., Soto, H., Liau, L. M. & Prins, R. M. Enhanced sensitivity to IL-2 signaling regulates the clinical responsiveness of IL-12-primed CD8(+) T cells in a melanoma model. J. Immunol.186, 5068–5077 (2011). 10.4049/jimmunol.1003317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosenberg, S. A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med.313, 1485–1492 (1985). 10.1056/NEJM198512053132327 [DOI] [PubMed] [Google Scholar]
- 120.Rosenberg, S. A., Yang, J. C., White, D. E. & Steinberg, S. M. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann. Surg.228, 307–319 (1998). 10.1097/00000658-199809000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosenberg, S. A. IL-2: the first effective immunotherapy for human cancer. J. Immunol.192, 5451–5458 (2014). 10.4049/jimmunol.1490019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Skrombolas, D. & Frelinger, J. G. Challenges and developing solutions for increasing the benefits of IL-2 treatment in tumor therapy. Expert Rev. Clin. Immunol.10, 207–217 (2014). 10.1586/1744666X.2014.875856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Doberstein, S. K. Bempegaldesleukin (NKTR-214): a CD-122-biased IL-2 receptor agonist for cancer immunotherapy. Expert Opin. Biol. Ther.19, 1223–1228 (2019). 10.1080/14712598.2019.1685489 [DOI] [PubMed] [Google Scholar]
- 124.Bentebibel, S. E. et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov.9, 711–721 (2019). 10.1158/2159-8290.CD-18-1495 [DOI] [PubMed] [Google Scholar]
- 125.Diab, A. et al. Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: phase I dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov.10, 1158–1173 (2020). 10.1158/2159-8290.CD-19-1510 [DOI] [PubMed] [Google Scholar]
- 126.Lopes, J. E. et al. ALKS 4230: a novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy. J. Immunother. Cancer8, e000673 (2020). 10.1136/jitc-2020-000673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lopes, J. E. et al. Pharmacokinetics and pharmacodynamic effects of nemvaleukin alfa, a selective agonist of the intermediate-affinity IL-2 receptor, in cynomolgus monkeys. J. Pharm. Exp. Ther.379, 203–210 (2021). 10.1124/jpet.121.000612 [DOI] [PubMed] [Google Scholar]
- 128.Pan, Y. et al. Nemvaleukin alfa, a novel engineered IL-2 fusion protein, drives antitumor immunity and inhibits tumor growth in small cell lung cancer. J. Immunother. Cancer10, e004913 (2022). 10.1136/jitc-2022-004913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boyman, O. & Arenas-Ramirez, N. Development of a novel class of interleukin-2 immunotherapies for metastatic cancer. Swiss Med. Wkly149, w14697 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Vaishampayan, U. N. et al. Nemvaleukin alfa monotherapy and in combination with pembrolizumab in patients (pts) with advanced solid tumors: ARTISTRY-1. J. Clin. Oncol.40, 2500–2500 (2022). 10.1200/JCO.2022.40.16_suppl.2500 [DOI] [Google Scholar]
- 131.Tichet, M. et al. Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8(+) T cells and reprogramming macrophages. Immunity56, 162–179.e166 (2023). 10.1016/j.immuni.2022.12.006 [DOI] [PubMed] [Google Scholar]
- 132.Piper, M. et al. Simultaneous targeting of PD-1 and IL-2Rβγ with radiation therapy inhibits pancreatic cancer growth and metastasis. Cancer Cell41, 950–969.e956 (2023). 10.1016/j.ccell.2023.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ren, Z. et al. Selective delivery of low-affinity IL-2 to PD-1+ T cells rejuvenates antitumor immunity with reduced toxicity. J. Clin. Invest. 132, e153604 (2022). [DOI] [PMC free article] [PubMed]
- 134.Niederlova, V., Tsyklauri, O., Kovar, M. & Stepanek, O. IL-2-driven CD8(+) T cell phenotypes: implications for immunotherapy. Trends Immunol.44, 890–901 (2023). 10.1016/j.it.2023.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Codarri Deak, L. et al. PD-1-cis IL-2R agonism yields better effectors from stem-like CD8(+) T cells. Nature610, 161–172 (2022). 10.1038/s41586-022-05192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rosen, D. B. et al. TransCon IL-2 β/γ: a novel long-acting prodrug with sustained release of an IL-2Rβ/γ-selective IL-2 variant with improved pharmacokinetics and potent activation of cytotoxic immune cells for the treatment of cancer. J. Immunother. Cancer10, e004991 (2022). 10.1136/jitc-2022-004991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saraiva, M. & O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol.10, 170–181 (2010). 10.1038/nri2711 [DOI] [PubMed] [Google Scholar]
- 138.Bermúdez-Morales, V. H. et al. IL-10 expression is regulated by HPV E2 protein in cervical cancer cells. Mol. Med. Rep.4, 369–375 (2011). [DOI] [PubMed] [Google Scholar]
- 139.Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol.19, 683–765 (2001). 10.1146/annurev.immunol.19.1.683 [DOI] [PubMed] [Google Scholar]
- 140.Murray, P. J. The JAK-STAT signaling pathway: input and output integration. J. Immunol.178, 2623–2629, (2007). 10.4049/jimmunol.178.5.2623 [DOI] [PubMed] [Google Scholar]
- 141.Murray, P. J. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr. Opin. Pharm.6, 379–386 (2006). 10.1016/j.coph.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 142.Wang, X., Wong, K., Ouyang, W. & Rutz, S. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb. Perspect. Biol.11, a028548 (2019). 10.1101/cshperspect.a028548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.de Waal Malefyt, R. et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med.174, 915–924 (1991). 10.1084/jem.174.4.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taga, K. & Tosato, G. IL-10 inhibits human T cell proliferation and IL-2 production. J. Immunol.148, 1143–1148 (1992). 10.4049/jimmunol.148.4.1143 [DOI] [PubMed] [Google Scholar]
- 145.Brooks, D. G., Walsh, K. B., Elsaesser, H. & Oldstone, M. B. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc. Natl Acad. Sci. USA107, 3018–3023 (2010). 10.1073/pnas.0914500107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Emmerich, J. et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res.72, 3570–3581 (2012). 10.1158/0008-5472.CAN-12-0721 [DOI] [PubMed] [Google Scholar]
- 147.Salkeni, M. A. & Naing, A. Interleukin-10 in cancer immunotherapy: from bench to bedside. Trends Cancer9, 716–725 (2023). 10.1016/j.trecan.2023.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Naing, A. et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J. Clin. Oncol.34, 3562–3569 (2016). 10.1200/JCO.2016.68.1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Naing, A. et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol.20, 1544–1555 (2019). 10.1016/S1470-2045(19)30514-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spigel, D. et al. Randomized phase 2 studies of checkpoint inhibitors alone or in combination with pegilodecakin in patients with metastatic NSCLC (CYPRESS 1 and CYPRESS 2). J. Thorac. Oncol.16, 327–333 (2021). 10.1016/j.jtho.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 151.Hecht, J. R. et al. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J. Clin. Oncol.39, 1108–1118 (2021). 10.1200/JCO.20.02232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qiao, J. et al. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8(+) T cell apoptosis. Cancer Cell35, 901–915.e904 (2019). 10.1016/j.ccell.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 153.Gorby, C. et al. Engineered IL-10 variants elicit potent immunomodulatory effects at low ligand doses. Sci. Signal.13, eabc0653 (2020). 10.1126/scisignal.abc0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chard, L. S. et al. A vaccinia virus armed with interleukin-10 is a promising therapeutic agent for treatment of murine pancreatic cancer. Clin. Cancer Res.21, 405–416 (2015). 10.1158/1078-0432.CCR-14-0464 [DOI] [PubMed] [Google Scholar]
- 155.Baganizi, D. R. et al. Interleukin-10 conjugation to carboxylated PVP-coated silver nanoparticles for improved stability and therapeutic efficacy. Nanomaterials (Basel)7, 165 (2017). 10.3390/nano7070165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kobayashi, M. et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med.170, 827–845 (1989). 10.1084/jem.170.3.827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chua, A. O. et al. Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor superfamily with strong homology to gp130. J. Immunol.153, 128–136 (1994). 10.4049/jimmunol.153.1.128 [DOI] [PubMed] [Google Scholar]
- 158.Desai, B. B. et al. IL-12 receptor. II. Distribution and regulation of receptor expression. J. Immunol.148, 3125–3132 (1992). 10.4049/jimmunol.148.10.3125 [DOI] [PubMed] [Google Scholar]
- 159.Goriely, S., Neurath, M. F. & Goldman, M. How microorganisms tip the balance between interleukin-12 family members. Nat. Rev. Immunol.8, 81–86 (2008). 10.1038/nri2225 [DOI] [PubMed] [Google Scholar]
- 160.Zou, J., Presky, D. H., Wu, C. Y. & Gubler, U. Differential associations between the cytoplasmic regions of the interleukin-12 receptor subunits beta1 and beta2 and JAK kinases. J. Biol. Chem.272, 6073–6077 (1997). 10.1074/jbc.272.9.6073 [DOI] [PubMed] [Google Scholar]
- 161.Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol.3, 133–146 (2003). 10.1038/nri1001 [DOI] [PubMed] [Google Scholar]
- 162.Billerbeck, E. et al. Insufficient interleukin-12 signalling favours differentiation of human CD4(+) and CD8(+) T cells into GATA-3(+) and GATA-3(+) T-bet(+) subsets in humanized mice. Immunology143, 202–218 (2014). 10.1111/imm.12304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Atkins, M. B. et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res.3, 409–417 (1997). [PubMed] [Google Scholar]
- 164.Leonard, J. P. et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood90, 2541–2548 (1997). [PubMed] [Google Scholar]
- 165.Cirella, A. et al. Novel strategies exploiting interleukin-12 in cancer immunotherapy. Pharm. Ther.239, 108189 (2022). 10.1016/j.pharmthera.2022.108189 [DOI] [PubMed] [Google Scholar]
- 166.Caruso, M. et al. Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma. Proc. Natl Acad. Sci. USA93, 11302–11306 (1996). 10.1073/pnas.93.21.11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pañeda, A. et al. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum. Gene Ther.20, 908–917 (2009). 10.1089/hum.2009.031 [DOI] [PubMed] [Google Scholar]
- 168.Zhang, J. et al. Cloning of human IL-12 p40 and p35 DNA into the Semliki Forest virus vector: expression of IL-12 in human tumor cells. Gene Ther.4, 367–374 (1997). 10.1038/sj.gt.3300409 [DOI] [PubMed] [Google Scholar]
- 169.Ghouse, S. M. et al. Oncolytic herpes simplex virus encoding IL12 controls triple-negative breast cancer growth and metastasis. Front. Oncol.10, 384 (2020). 10.3389/fonc.2020.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zitvogel, L. et al. Construction and characterization of retroviral vectors expressing biologically active human interleukin-12. Hum. Gene Ther.5, 1493–1506 (1994). 10.1089/hum.1994.5.12-1493 [DOI] [PubMed] [Google Scholar]
- 171.Li, X. et al. Viral vector-based gene therapy. Int J. Mol. Sci.24, 7736 (2023). 10.3390/ijms24097736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barton, K. N. et al. Phase I trial of oncolytic adenovirus-mediated cytotoxic and interleukin-12 gene therapy for the treatment of metastatic pancreatic cancer. Mol. Ther. Oncolyt.20, 94–104 (2021). 10.1016/j.omto.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sangro, B. et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J. Clin. Oncol.22, 1389–1397 (2004). 10.1200/JCO.2004.04.059 [DOI] [PubMed] [Google Scholar]
- 174.Watanabe, M. et al. Intradermal delivery of IL-12 naked DNA induces systemic NK cell activation and Th1 response in vivo that is independent of endogenous IL-12 production. J. Immunol.163, 1943–1950 (1999). 10.4049/jimmunol.163.4.1943 [DOI] [PubMed] [Google Scholar]
- 175.Salem, M. L. et al. Review: novel nonviral delivery approaches for interleukin-12 protein and gene systems: curbing toxicity and enhancing adjuvant activity. J. Interferon Cytokine Res.26, 593–608 (2006). 10.1089/jir.2006.26.593 [DOI] [PubMed] [Google Scholar]
- 176.Anwer, K. et al. Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther.17, 360–369 (2010). 10.1038/gt.2009.159 [DOI] [PubMed] [Google Scholar]
- 177.Algazi, A. et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann. Oncol.31, 532–540 (2020). 10.1016/j.annonc.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 178.Greaney, S. K. et al. Intratumoral plasmid IL12 electroporation therapy in patients with advanced melanoma induces systemic and intratumoral T-cell responses. Cancer Immunol. Res.8, 246–254 (2020). 10.1158/2326-6066.CIR-19-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Algazi, A. P. et al. Phase II trial of IL-12 plasmid transfection and PD-1 BLockade in Immunologically Quiescent Melanoma. Clin. Cancer Res.26, 2827–2837 (2020). 10.1158/1078-0432.CCR-19-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hewitt, S. L. et al. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin. Cancer Res.26, 6284–6298 (2020). 10.1158/1078-0432.CCR-20-0472 [DOI] [PubMed] [Google Scholar]
- 181.Zheng, X. et al. The use of supercytokines, immunocytokines, engager cytokines, and other synthetic cytokines in immunotherapy. Cell Mol. Immunol.19, 192–209 (2022). 10.1038/s41423-021-00786-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ongaro, T. et al. A novel anti-cancer L19-interleukin-12 fusion protein with an optimized peptide linker efficiently localizes in vivo at the site of tumors. J. Biotechnol.291, 17–25 (2019). 10.1016/j.jbiotec.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 183.Strauss, J. et al. First-in-human phase I trial of a tumor-targeted cytokine (NHS-IL12) in subjects with metastatic solid tumors. Clin. Cancer Res.25, 99–109 (2019). 10.1158/1078-0432.CCR-18-1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Skrombolas, D., Sullivan, M. & Frelinger, J. G. Development of an interleukin-12 fusion protein that is activated by cleavage with matrix metalloproteinase 9. J. Interferon Cytokine Res.39, 233–245 (2019). 10.1089/jir.2018.0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xue, D. et al. A tumor-specific pro-IL-12 activates preexisting cytotoxic T cells to control established tumors. Sci. Immunol.7, eabi6899 (2022). 10.1126/sciimmunol.abi6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Glassman, C. R. et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell184, 983–999.e924 (2021). 10.1016/j.cell.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Perera, L. P., Goldman, C. K. & Waldmann, T. A. Comparative assessment of virulence of recombinant vaccinia viruses expressing IL-2 and IL-15 in immunodeficient mice. Proc. Natl Acad. Sci. USA98, 5146–5151, (2001). 10.1073/pnas.081080298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Giri, J. G. et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J.14, 3654–3663 (1995). 10.1002/j.1460-2075.1995.tb00035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dubois, S., Mariner, J., Waldmann, T. A. & Tagaya, Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity17, 537–547 (2002). 10.1016/S1074-7613(02)00429-6 [DOI] [PubMed] [Google Scholar]
- 190.Giri, J. G. et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. Embo j.13, 2822–2830 (1994). 10.1002/j.1460-2075.1994.tb06576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lodolce, J. P. et al. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev.13, 429–439 (2002). 10.1016/S1359-6101(02)00029-1 [DOI] [PubMed] [Google Scholar]
- 192.Hangasky, J. A. et al. A very long-acting IL-15: implications for the immunotherapy of cancer. J. Immunother. Cancer10, e004104 (2022). 10.1136/jitc-2021-004104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marks-Konczalik, J. et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc. Natl Acad. Sci. USA97, 11445–11450 (2000). 10.1073/pnas.200363097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Waldmann, T. A., Dubois, S., Miljkovic, M. D. & Conlon, K. C. IL-15 in the combination immunotherapy of cancer. Front Immunol.11, 868 (2020). 10.3389/fimmu.2020.00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tang, F. et al. Activity of recombinant human interleukin-15 against tumor recurrence and metastasis in mice. Cell Mol. Immunol.5, 189–196 (2008). 10.1038/cmi.2008.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Miller, J. S. et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin. Cancer Res.24, 1525–1535 (2018). 10.1158/1078-0432.CCR-17-2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou, Y. et al. Interleukin 15 in cell-based cancer immunotherapy. Int. J. Mol. Sci.23, 7311 (2022). 10.3390/ijms23137311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chertova, E. et al. Characterization and favorable in vivo properties of heterodimeric soluble IL-15·IL-15Rα cytokine compared to IL-15 monomer. J. Biol. Chem.288, 18093–18103 (2013). 10.1074/jbc.M113.461756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bergamaschi, C. et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10. J. Immunother. Cancer8, e000599 (2020). 10.1136/jitc-2020-000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Watson, D. C. et al. Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog.14, e1006902 (2018). 10.1371/journal.ppat.1006902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu, Z. & Xu, Y. IL-15R alpha-IgG1-Fc enhances IL-2 and IL-15 anti-tumor action through NK and CD8+ T cells proliferation and activation. J. Mol. Cell Biol.2, 217–222, (2010). 10.1093/jmcb/mjq012 [DOI] [PubMed] [Google Scholar]
- 202.Dubois, S. et al. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol.180, 2099–2106 (2008). 10.4049/jimmunol.180.4.2099 [DOI] [PubMed] [Google Scholar]
- 203.Han, K. P. et al. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine56, 804–810 (2011). 10.1016/j.cyto.2011.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Felices, M. et al. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol. Oncol.145, 453–461 (2017). 10.1016/j.ygyno.2017.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Romee, R. et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood131, 2515–2527 (2018). 10.1182/blood-2017-12-823757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Margolin, K. et al. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin. Cancer Res.24, 5552–5561 (2018). 10.1158/1078-0432.CCR-18-0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim, P. S. et al. IL-15 superagonist/IL-15RαSushi-Fc fusion complex (IL-15SA/IL-15RαSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget7, 16130–16145 (2016). 10.18632/oncotarget.7470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chu, Y. et al. Combinatorial immunotherapy of N-803 (IL-15 superagonist) and dinutuximab with ex vivo expanded natural killer cells significantly enhances in vitro cytotoxicity against GD2(+) pediatric solid tumors and in vivo survival of xenografted immunodeficient NSG mice. J. Immunother. Cancer9, e002267 (2021). 10.1136/jitc-2020-002267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mortier, E. et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J. Biol. Chem.281, 1612–1619 (2006). 10.1074/jbc.M508624200 [DOI] [PubMed] [Google Scholar]
- 210.Bessard, A. et al. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol. Cancer Ther.8, 2736–2745 (2009). 10.1158/1535-7163.MCT-09-0275 [DOI] [PubMed] [Google Scholar]
- 211.Desbois, M. et al. IL-15 superagonist RLI has potent immunostimulatory properties on NK cells: implications for antimetastatic treatment. J. Immunother. Cancer8, e000632 (2020). 10.1136/jitc-2020-000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Miyazaki, T. et al. NKTR-255, a novel polymer-conjugated rhIL-15 with potent antitumor efficacy. J. Immunother. Cancer9, e002024 (2021). 10.1136/jitc-2020-002024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Robinson, T. O. et al. NKTR-255 is a polymer-conjugated IL-15 with unique mechanisms of action on T and natural killer cells. J. Clin. Invest.131, e144365 (2021). 10.1172/JCI144365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Van Acker, H. H. et al. The role of the common gamma-chain family cytokines in γδ T cell-based anti-cancer immunotherapy. Cytokine Growth Factor Rev.41, 54–64 (2018). 10.1016/j.cytogfr.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 215.Hoyos, V. et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia24, 1160–1170 (2010). 10.1038/leu.2010.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang, Y. et al. Co-expression IL-15 receptor alpha with IL-15 reduces toxicity via limiting IL-15 systemic exposure during CAR-T immunotherapy. J. Transl. Med.20, 432 (2022). 10.1186/s12967-022-03626-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hurton, L. V. et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl Acad. Sci. USA113, E7788–E7797 (2016). 10.1073/pnas.1610544113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Feng, J. et al. Treatment of aggressive T cell lymphoblastic lymphoma/leukemia using Anti-CD5 CAR T cells. Stem Cell Rev. Rep.17, 652–661 (2021). 10.1007/s12015-020-10092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sun, Y. et al. CD19 CAR-T cells with membrane-bound IL-15 for B-cell acute lymphoblastic leukemia after failure of CD19 and CD22 CAR-T cells: case report. Front. Immunol.12, 728962 (2021). 10.3389/fimmu.2021.728962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Christodoulou, I. et al. Engineering CAR-NK cells to secrete IL-15 sustains their anti-AML functionality but is associated with systemic toxicities. J. Immunother. Cancer9, e003894 (2021). 10.1136/jitc-2021-003894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Du, Z., Ng, Y. Y., Zha, S. & Wang, S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol. Ther. Methods Clin. Dev.23, 582–596 (2021). 10.1016/j.omtm.2021.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med.382, 545–553 (2020). 10.1056/NEJMoa1910607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu, E. et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia32, 520–531 (2018). 10.1038/leu.2017.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Makkouk, A. et al. Off-the-shelf Vδ1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J. Immunother. Cancer9, e003441 (2021). 10.1136/jitc-2021-003441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Heczey, A. et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat. Med.26, 1686–1690 (2020). 10.1038/s41591-020-1074-2 [DOI] [PubMed] [Google Scholar]
- 226.Deng, X. et al. Combination of novel oncolytic herpesvirus with paclitaxel as an efficient strategy for breast cancer therapy. J. Med. Virol.95, e28768 (2023). 10.1002/jmv.28768 [DOI] [PubMed] [Google Scholar]
- 227.Guo, J. et al. Tumor-conditional IL-15 pro-cytokine reactivates anti-tumor immunity with limited toxicity. Cell Res.31, 1190–1198 (2021). 10.1038/s41422-021-00543-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nelson, A., Gebremeskel, S., Lichty, B. D. & Johnston, B. Natural killer T cell immunotherapy combined with IL-15-expressing oncolytic virotherapy and PD-1 blockade mediates pancreatic tumor regression. J. Immunother. Cancer10, e003923 (2022). 10.1136/jitc-2021-003923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dinarello, C. A., Goldin, N. P. & Wolff, S. M. Demonstration and characterization of two distinct human leukocytic pyrogens. J. Exp. Med.139, 1369–1381 (1974). 10.1084/jem.139.6.1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dinarello, C. A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev.281, 8–27 (2018). 10.1111/imr.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mantovani, A., Dinarello, C. A., Molgora, M. & Garlanda, C. Interleukin-1 and Related Cytokines in the regulation of inflammation and immunity. Immunity50, 778–795 (2019). 10.1016/j.immuni.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Malik, A. & Kanneganti, T. D. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol. Rev.281, 124–137 (2018). 10.1111/imr.12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bent, R., Moll, L., Grabbe, S. & Bros, M. Interleukin-1 beta-A friend or foe in malignancies? Int. J. Mol. Sci.19, 2155 (2018). 10.3390/ijms19082155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mantovani, A., Barajon, I. & Garlanda, C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev.281, 57–61 (2018). 10.1111/imr.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Dosch, A. R. et al. Interleukin-1 signaling in solid organ malignancies. Biochim. Biophys. Acta Rev. Cancer1877, 188670 (2022). 10.1016/j.bbcan.2021.188670 [DOI] [PubMed] [Google Scholar]
- 236.Casadio, R. et al. Model of interaction of the IL-1 receptor accessory protein IL-1RAcP with the IL-1beta/IL-1R(I) complex. FEBS Lett.499, 65–68 (2001). 10.1016/S0014-5793(01)02515-7 [DOI] [PubMed] [Google Scholar]
- 237.Brikos, C. et al. Mass spectrometric analysis of the endogenous type I interleukin-1 (IL-1) receptor signaling complex formed after IL-1 binding identifies IL-1RAcP, MyD88, and IRAK-4 as the stable components. Mol. Cell Proteom.6, 1551–1559 (2007). 10.1074/mcp.M600455-MCP200 [DOI] [PubMed] [Google Scholar]
- 238.Yamazaki, K. et al. Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci. Signal2, ra66 (2009). 10.1126/scisignal.2000387 [DOI] [PubMed] [Google Scholar]
- 239.Huang, Q. et al. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat. Immunol.5, 98–103 (2004). 10.1038/ni1014 [DOI] [PubMed] [Google Scholar]
- 240.Schmidt, C. et al. Mechanisms of proinflammatory cytokine-induced biphasic NF-kappaB activation. Mol. Cell12, 1287–1300 (2003). 10.1016/S1097-2765(03)00390-3 [DOI] [PubMed] [Google Scholar]
- 241.Li, X., Commane, M., Jiang, Z. & Stark, G. R. IL-1-induced NFkappa B and c-Jun N-terminal kinase (JNK) activation diverge at IL-1 receptor-associated kinase (IRAK). Proc. Natl Acad. Sci. USA98, 4461–4465 (2001). 10.1073/pnas.071054198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Walsh, M. C., Lee, J. & Choi, Y. Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev.266, 72–92 (2015). 10.1111/imr.12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Weber, A., Wasiliew, P. & Kracht, M. Interleukin-1 (IL-1) pathway. Sci. Signal.3, cm1 (2010). [DOI] [PubMed] [Google Scholar]
- 244.Elaraj, D. M. et al. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin. Cancer Res.12, 1088–1096 (2006). 10.1158/1078-0432.CCR-05-1603 [DOI] [PubMed] [Google Scholar]
- 245.Ling, J. et al. KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell21, 105–120 (2012). 10.1016/j.ccr.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lau, L. et al. Uncoupling the senescence-associated secretory phenotype from cell cycle exit via interleukin-1 inactivation unveils its protumorigenic role. Mol. Cell Biol. 39, e00586–18 (2019). [DOI] [PMC free article] [PubMed]
- 247.Voronov, E. et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl Acad. Sci. USA100, 2645–2650 (2003). 10.1073/pnas.0437939100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Voronov, E., Carmi, Y. & Apte, R. N. The role IL-1 in tumor-mediated angiogenesis. Front. Physiol.5, 114 (2014). 10.3389/fphys.2014.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jiménez-Garduño, A. M. et al. IL-1β induced methylation of the estrogen receptor ERα gene correlates with EMT and chemoresistance in breast cancer cells. Biochem. Biophys. Res. Commun.490, 780–785 (2017). 10.1016/j.bbrc.2017.06.117 [DOI] [PubMed] [Google Scholar]
- 250.Mendoza-Rodríguez, M. G. et al. IL-1β inflammatory cytokine-induced TP63 isoform ∆NP63α signaling cascade contributes to cisplatin resistance in human breast cancer cells. Int. J. Mol. Sci.20, 270 (2019). 10.3390/ijms20020270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Huang, J. et al. Targeting the IL-1β/EHD1/TUBB3 axis overcomes resistance to EGFR-TKI in NSCLC. Oncogene39, 1739–1755 (2020). 10.1038/s41388-019-1099-5 [DOI] [PubMed] [Google Scholar]
- 252.Gelfo, V. et al. A novel role for the interleukin-1 receptor axis in resistance to anti-EGFR therapy. Cancers (Basel)10, 355 (2018). 10.3390/cancers10100355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lin, D. et al. Membrane IL1α inhibits the development of hepatocellular carcinoma via promoting T- and NK-cell activation. Cancer Res.76, 3179–3188 (2016). 10.1158/0008-5472.CAN-15-2658 [DOI] [PubMed] [Google Scholar]
- 254.Tu, S. et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell14, 408–419 (2008). 10.1016/j.ccr.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jiang, H. et al. Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int. J. Cancer136, 2352–2360 (2015). 10.1002/ijc.29297 [DOI] [PubMed] [Google Scholar]
- 256.Mertens, M. & Singh, J. A. Anakinra for rheumatoid arthritis. Cochrane Database Syst. Rev. Cd005121, (2009). [DOI] [PubMed]
- 257.Wu, T. C. et al. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res.78, 5243–5258 (2018). 10.1158/0008-5472.CAN-18-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Isambert, N. et al. Fluorouracil and bevacizumab plus anakinra for patients with metastatic colorectal cancer refractory to standard therapies (IRAFU): a single-arm phase 2 study. Oncoimmunology7, e1474319 (2018). 10.1080/2162402X.2018.1474319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hou, J. et al. Design of a superior cytokine antagonist for topical ophthalmic use. Proc. Natl Acad. Sci. USA110, 3913–3918 (2013). 10.1073/pnas.1217996110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.O’Sullivan Coyne, G. & Burotto, M. MABp1 for the treatment of colorectal cancer. Expert Opin. Biol. Ther.17, 1155–1161 (2017). 10.1080/14712598.2017.1347631 [DOI] [PubMed] [Google Scholar]
- 261.Hong, D. S. et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol.15, 656–666 (2014). 10.1016/S1470-2045(14)70155-X [DOI] [PubMed] [Google Scholar]
- 262.Hickish, T. et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol.18, 192–201 (2017). 10.1016/S1470-2045(17)30006-2 [DOI] [PubMed] [Google Scholar]
- 263.De Benedetti, F. et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N. Engl. J. Med.378, 1908–1919 (2018). 10.1056/NEJMoa1706314 [DOI] [PubMed] [Google Scholar]
- 264.Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet390, 1833–1842 (2017). 10.1016/S0140-6736(17)32247-X [DOI] [PubMed] [Google Scholar]
- 265.Garrido, P. et al. Canakinumab with and without pembrolizumab in patients with resectable non-small-cell lung cancer: CANOPY-N study design. Future Oncol.17, 1459–1472 (2021). 10.2217/fon-2020-1098 [DOI] [PubMed] [Google Scholar]
- 266.Lythgoe, M. P. & Prasad, V. Repositioning canakinumab for non-small cell lung cancer-important lessons for drug repurposing in oncology. Br. J. Cancer127, 785–787 (2022). 10.1038/s41416-022-01893-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci.8, 1237–1247 (2012). 10.7150/ijbs.4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol.33, 127–148 (2021). 10.1093/intimm/dxaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Neurath, M. F. & Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev.22, 83–89 (2011). 10.1016/j.cytogfr.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 270.Mihara, M. et al. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. (Lond.)122, 143–159 (2012). 10.1042/CS20110340 [DOI] [PubMed] [Google Scholar]
- 271.Rose-John, S. et al. Targeting IL-6 trans-signalling: past, present and future prospects. Nat. Rev. Immunol.23, 666–681 (2023). 10.1038/s41577-023-00856-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Heink, S. et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic T(H)17 cells. Nat. Immunol.18, 74–85 (2017). 10.1038/ni.3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol.16, 448–457 (2015). 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 274.Rašková, M. et al. The role of IL-6 in cancer cell invasiveness and metastasis-overview and therapeutic opportunities. Cells11, 3698 (2022). 10.3390/cells11223698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Schaper, F. & Rose-John, S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor Rev.26, 475–487 (2015). 10.1016/j.cytogfr.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 276.Bromberg, J. F. et al. Stat3 as an oncogene. Cell98, 295–303 (1999). 10.1016/S0092-8674(00)81959-5 [DOI] [PubMed] [Google Scholar]
- 277.Tanaka, H. et al. GATA-1 blocks IL-6-induced macrophage differentiation and apoptosis through the sustained expression of cyclin D1 and bcl-2 in a murine myeloid cell line M1. Blood95, 1264–1273 (2000). 10.1182/blood.V95.4.1264.004k09_1264_1273 [DOI] [PubMed] [Google Scholar]
- 278.Petrenko, O. et al. IL-6 promotes MYC-induced B cell lymphomagenesis independent of STAT3. PLoS ONE16, e0247394 (2021). 10.1371/journal.pone.0247394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Puthier, D. et al. Mcl-1 and Bcl-xL are co-regulated by IL-6 in human myeloma cells. Br. J. Haematol.107, 392–395 (1999). 10.1046/j.1365-2141.1999.01705.x [DOI] [PubMed] [Google Scholar]
- 280.Lepiller, Q. et al. HCMV activates the IL-6-JAK-STAT3 axis in HepG2 cells and primary human hepatocytes. PLoS ONE8, e59591 (2013). 10.1371/journal.pone.0059591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhang, R., Roque, D. M., Reader, J. & Lin, J. Combined inhibition of IL‑6 and IL‑8 pathways suppresses ovarian cancer cell viability and migration and tumor growth. Int. J. Oncol. 60, 50 (2022). [DOI] [PMC free article] [PubMed]
- 282.Zhao, G. et al. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol. Rep.35, 1787–1795 (2016). 10.3892/or.2016.4544 [DOI] [PubMed] [Google Scholar]
- 283.Lin, C. M. et al. Silibinin inhibits the invasion of IL-6-stimulated colon cancer cells via selective JNK/AP-1/MMP-2 modulation in vitro. J. Agric. Food Chem.60, 12451–12457 (2012). 10.1021/jf300964f [DOI] [PubMed] [Google Scholar]
- 284.Li, H. et al. IL-6-induced cGGNBP2 encodes a protein to promote cell growth and metastasis in intrahepatic cholangiocarcinoma. Hepatology75, 1402–1419 (2022). 10.1002/hep.32232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Hong, C. et al. cGAS-STING drives the IL-6-dependent survival of chromosomally instable cancers. Nature607, 366–373 (2022). 10.1038/s41586-022-04847-2 [DOI] [PubMed] [Google Scholar]
- 286.Weng, Y. S. et al. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer18, 42 (2019). 10.1186/s12943-019-0988-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Chan, L. C. et al. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J. Clin. Invest.129, 3324–3338 (2019). 10.1172/JCI126022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Weber, R. et al. IL-6 regulates CCR5 expression and immunosuppressive capacity of MDSC in murine melanoma. J. Immunother. Cancer8, e000949 (2020). 10.1136/jitc-2020-000949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Yang, Q. et al. Obesity promotes tumor immune evasion in ovarian cancer through increased production of myeloid-derived suppressor cells via IL-6. Cancer Manag. Res.13, 7355–7363 (2021). 10.2147/CMAR.S303707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ohno, Y. et al. IL-6 down-regulates HLA class II expression and IL-12 production of human dendritic cells to impair activation of antigen-specific CD4(+) T cells. Cancer Immunol. Immunother.65, 193–204 (2016). 10.1007/s00262-015-1791-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Huseni, M. A. et al. CD8(+) T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep. Med.4, 100878 (2023). 10.1016/j.xcrm.2022.100878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Keegan, A. et al. Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J. Immunother. Cancer8, e000678 (2020). 10.1136/jitc-2020-000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Li, W. et al. Blockade of IL-6 inhibits tumor immune evasion and improves anti-PD-1 immunotherapy. Cytokine158, 155976 (2022). 10.1016/j.cyto.2022.155976 [DOI] [PubMed] [Google Scholar]
- 294.Hailemichael, Y. et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell40, 509–523.e506 (2022). 10.1016/j.ccell.2022.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Soler, M. F. et al. New perspectives in cancer immunotherapy: targeting IL-6 cytokine family. J. Immunother. Cancer11, e007530 (2023). 10.1136/jitc-2023-007530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol.15, 234–248 (2018). 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Kishimoto, T. & Kang, S. IL-6 revisited: from rheumatoid arthritis to CAR T cell therapy and COVID-19. Annu. Rev. Immunol.40, 323–348 (2022). 10.1146/annurev-immunol-101220-023458 [DOI] [PubMed] [Google Scholar]
- 298.Yu, L. et al. Development and validation of a reporter-cell-line-based bioassay for therapeutic soluble gp130-Fc. Molecules24, 3845 (2019). 10.3390/molecules24213845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Brooks, G. D. et al. IL6 trans-signaling promotes KRAS-driven lung carcinogenesis. Cancer Res.76, 866–876 (2016). 10.1158/0008-5472.CAN-15-2388 [DOI] [PubMed] [Google Scholar]
- 300.Goumas, F. A. et al. Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int. J. Cancer137, 1035–1046 (2015). 10.1002/ijc.29445 [DOI] [PubMed] [Google Scholar]
- 301.Angevin, E. et al. A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clin. Cancer Res.20, 2192–2204 (2014). 10.1158/1078-0432.CCR-13-2200 [DOI] [PubMed] [Google Scholar]
- 302.Hudes, G. et al. A phase 1 study of a chimeric monoclonal antibody against interleukin-6, siltuximab, combined with docetaxel in patients with metastatic castration-resistant prostate cancer. Invest. N. Drugs31, 669–676 (2013). 10.1007/s10637-012-9857-z [DOI] [PubMed] [Google Scholar]
- 303.Fizazi, K. et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur. J. Cancer48, 85–93 (2012). 10.1016/j.ejca.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 304.Yanaihara, N. et al. Antitumor effects of interleukin-6 (IL-6)/interleukin-6 receptor (IL-6R) signaling pathway inhibition in clear cell carcinoma of the ovary. Mol. Carcinog.55, 832–841 (2016). 10.1002/mc.22325 [DOI] [PubMed] [Google Scholar]
- 305.Dijkgraaf, E. M. et al. A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-α2b in patients with recurrent epithelial ovarian cancer. Ann. Oncol.26, 2141–2149 (2015). 10.1093/annonc/mdv309 [DOI] [PubMed] [Google Scholar]
- 306.Dhillon, S. Tofacitinib: a review in rheumatoid arthritis. Drugs77, 1987–2001 (2017). 10.1007/s40265-017-0835-9 [DOI] [PubMed] [Google Scholar]
- 307.McLornan, D. P., Pope, J. E., Gotlib, J. & Harrison, C. N. Current and future status of JAK inhibitors. Lancet398, 803–816 (2021). 10.1016/S0140-6736(21)00438-4 [DOI] [PubMed] [Google Scholar]
- 308.Hedvat, M. et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell16, 487–497 (2009). 10.1016/j.ccr.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Moskowitz, A. J. et al. A phase 2 biomarker-driven study of ruxolitinib demonstrates effectiveness of JAK/STAT targeting in T-cell lymphomas. Blood138, 2828–2837 (2021). 10.1182/blood.2021013379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lynce, F. et al. Phase I study of JAK1/2 inhibitor ruxolitinib with weekly paclitaxel for the treatment of HER2-negative metastatic breast cancer. Cancer Chemother. Pharm.87, 673–679 (2021). 10.1007/s00280-021-04245-x [DOI] [PubMed] [Google Scholar]
- 311.Hong, D. et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci. Transl. Med.7, 314ra185 (2015). 10.1126/scitranslmed.aac5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Dong, J., Cheng, X. D., Zhang, W. D. & Qin, J. J. Recent update on development of small-molecule STAT3 inhibitors for cancer therapy: from phosphorylation inhibition to protein degradation. J. Med. Chem.64, 8884–8915 (2021). 10.1021/acs.jmedchem.1c00629 [DOI] [PubMed] [Google Scholar]
- 313.Bendell, J. C. et al. Phase 1, open-label, dose-escalation, and pharmacokinetic study of STAT3 inhibitor OPB-31121 in subjects with advanced solid tumors. Cancer Chemother. Pharm.74, 125–130 (2014). 10.1007/s00280-014-2480-2 [DOI] [PubMed] [Google Scholar]
- 314.Oh, D. Y. et al. Phase I study of OPB-31121, an oral STAT3 inhibitor, in patients with advanced solid tumors. Cancer Res. Treat.47, 607–615 (2015). 10.4143/crt.2014.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wong, A. L. et al. Phase I and biomarker study of OPB-51602, a novel signal transducer and activator of transcription (STAT) 3 inhibitor, in patients with refractory solid malignancies. Ann. Oncol.26, 998–1005 (2015). 10.1093/annonc/mdv026 [DOI] [PubMed] [Google Scholar]
- 316.Mace, T. A. et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut67, 320–332 (2018). 10.1136/gutjnl-2016-311585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Holmstroem, R. B. et al. COLAR: open-label clinical study of IL-6 blockade with tocilizumab for the treatment of immune checkpoint inhibitor-induced colitis and arthritis. J. Immunother. Cancer10, e005111 (2022). 10.1136/jitc-2022-005111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Salem, J. E. et al. Abatacept/ruxolitinib and screening for concomitant respiratory muscle failure to mitigate fatality of immune-checkpoint inhibitor myocarditis. Cancer Discov.13, 1100–1115 (2023). 10.1158/2159-8290.CD-22-1180 [DOI] [PubMed] [Google Scholar]
- 319.Nguyen, L. S. et al. Reversal of immune-checkpoint inhibitor fulminant myocarditis using personalized-dose-adjusted abatacept and ruxolitinib: proof of concept. J. Immunother. Cancer10, e004699 (2022). 10.1136/jitc-2022-004699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Carswell, E. A. et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl Acad. Sci. USA72, 3666–3670 (1975). 10.1073/pnas.72.9.3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Beutler, B. et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature316, 552–554 (1985). 10.1038/316552a0 [DOI] [PubMed] [Google Scholar]
- 322.Waters, J. P., Pober, J. S. & Bradley, J. R. Tumour necrosis factor and cancer. J. Pathol.230, 241–248 (2013). 10.1002/path.4188 [DOI] [PubMed] [Google Scholar]
- 323.Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer9, 361–371 (2009). 10.1038/nrc2628 [DOI] [PubMed] [Google Scholar]
- 324.Chen, A. Y., Wolchok, J. D. & Bass, A. R. TNF in the era of immune checkpoint inhibitors: friend or foe? Nat. Rev. Rheumatol.17, 213–223 (2021). 10.1038/s41584-021-00584-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Fràter-Schröder, M. et al. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc. Natl Acad. Sci. USA84, 5277–5281 (1987). 10.1073/pnas.84.15.5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Bertrand, F. et al. Blocking tumor necrosis factor α enhances CD8 T-cell-dependent immunity in experimental melanoma. Cancer Res.75, 2619–2628 (2015). 10.1158/0008-5472.CAN-14-2524 [DOI] [PubMed] [Google Scholar]
- 327.Zheng, Y. et al. TNF-α-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J. Transl. Med.17, 165 (2019). 10.1186/s12967-019-1917-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ivagnès, A. et al. TNFR2/BIRC3-TRAF1 signaling pathway as a novel NK cell immune checkpoint in cancer. Oncoimmunology7, e1386826 (2018). 10.1080/2162402X.2017.1386826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Grinberg-Bleyer, Y. et al. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J. Clin. Invest.120, 4558–4568 (2010). 10.1172/JCI42945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Chen, X. et al. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J. Immunol.180, 6467–6471 (2008). 10.4049/jimmunol.180.10.6467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Chen, X. et al. TNFR2 expression by CD4 effector T cells is required to induce full-fledged experimental colitis. Sci. Rep.6, 32834 (2016). 10.1038/srep32834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhao, X. et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J. Clin. Invest.122, 4094–4104 (2012). 10.1172/JCI64115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sade-Feldman, M. et al. Tumor necrosis factor-α blocks differentiation and enhances suppressive activity of immature myeloid cells during chronic inflammation. Immunity38, 541–554 (2013). 10.1016/j.immuni.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 334.Ren, G. et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell11, 812–824 (2012). 10.1016/j.stem.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lim, S. O. et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell30, 925–939 (2016). 10.1016/j.ccell.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Bertrand, F. et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat. Commun.8, 2256 (2017). 10.1038/s41467-017-02358-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Liu, L. et al. A bacteria-based system expressing anti-TNF-α nanobody for enhanced cancer immunotherapy. Signal Transduct. Target Ther.8, 134 (2023). 10.1038/s41392-023-01364-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Perez-Ruiz, E. et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature569, 428–432 (2019). 10.1038/s41586-019-1162-y [DOI] [PubMed] [Google Scholar]
- 339.D’Haens, G. R. & van Deventer, S. 25 years of anti-TNF treatment for inflammatory bowel disease: lessons from the past and a look to the future. Gut70, 1396–1405 (2021). 10.1136/gutjnl-2019-320022 [DOI] [PubMed] [Google Scholar]
- 340.Bongartz, T. et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. J. Am. Med. Assoc.295, 2275–2285 (2006). 10.1001/jama.295.19.2275 [DOI] [PubMed] [Google Scholar]
- 341.Badran, Y. R. et al. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J. Immunother. Cancer7, 226 (2019). 10.1186/s40425-019-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Montfort, A. et al. Combining nivolumab and ipilimumab with infliximab or certolizumab in patients with advanced melanoma: first results of a phase Ib clinical trial. Clin. Cancer Res.27, 1037–1047 (2021). 10.1158/1078-0432.CCR-20-3449 [DOI] [PubMed] [Google Scholar]
- 343.Ozga, A. J., Chow, M. T. & Luster, A. D. Chemokines and the immune response to cancer. Immunity54, 859–874 (2021). 10.1016/j.immuni.2021.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Märkl, F., Huynh, D., Endres, S. & Kobold, S. Utilizing chemokines in cancer immunotherapy. Trends Cancer8, 670–682 (2022). 10.1016/j.trecan.2022.04.001 [DOI] [PubMed] [Google Scholar]
- 345.Hao, Q., Vadgama, J. V. & Wang, P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun. Signal.18, 82 (2020). 10.1186/s12964-020-00589-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Shen, H. et al. PLEK2 promotes gallbladder cancer invasion and metastasis through EGFR/CCL2 pathway. J. Exp. Clin. Cancer Res.38, 247 (2019). 10.1186/s13046-019-1250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Yoshimura, T., Li, C., Wang, Y. & Matsukawa, A. The chemokine monocyte chemoattractant protein-1/CCL2 is a promoter of breast cancer metastasis. Cell Mol. Immunol.20, 714–738 (2023). 10.1038/s41423-023-01013-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Li, X. et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut66, 157–167 (2017). 10.1136/gutjnl-2015-310514 [DOI] [PubMed] [Google Scholar]
- 349.Yang, H. et al. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol. Cancer19, 41 (2020). 10.1186/s12943-020-01165-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Yang, Y. I. et al. CCL2 overexpression is associated with paclitaxel resistance in ovarian cancer cells via autocrine signaling and macrophage recruitment. Biomed. Pharmacother.153, 113474 (2022). 10.1016/j.biopha.2022.113474 [DOI] [PubMed] [Google Scholar]
- 351.Ma, L., Jiang, Y. & Wu, N. Long non-coding RNA CCL2 promoted gastric cancer function via miR-128/ PARP2 signal pathway. Bioengineered13, 1602–1611 (2022). 10.1080/21655979.2021.2020548 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 352.Zhang, J., Patel, L. & Pienta, K. J. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev.21, 41–48, (2010). 10.1016/j.cytogfr.2009.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Fei, L., Ren, X., Yu, H. & Zhan, Y. Targeting the CCL2/CCR2 axis in cancer immunotherapy: one stone, three birds? Front. Immunol.12, 771210 (2021). 10.3389/fimmu.2021.771210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yang, X. et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res.76, 4124–4135 (2016). 10.1158/0008-5472.CAN-15-2973 [DOI] [PubMed] [Google Scholar]
- 355.Zhao, S. et al. Surgical trauma-induced CCL2 upregulation mediates lung cancer progression by promoting Treg recruitment in mice and patients. Cancer Invest.40, 91–102 (2022). 10.1080/07357907.2021.1977314 [DOI] [PubMed] [Google Scholar]
- 356.Xie, M. et al. FGF19/FGFR4-mediated elevation of ETV4 facilitates hepatocellular carcinoma metastasis by upregulating PD-L1 and CCL2. J. Hepatol.79, 109–125 (2023). 10.1016/j.jhep.2023.02.036 [DOI] [PubMed] [Google Scholar]
- 357.Feng, H. et al. Targeting tumor cell-derived CCL2 as a strategy to overcome Bevacizumab resistance in ETV5(+) colorectal cancer. Cell Death Dis.11, 916 (2020). 10.1038/s41419-020-03111-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Low-Marchelli, J. M. et al. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res.73, 662–671 (2013). 10.1158/0008-5472.CAN-12-0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Bess, S. N., Greening, G. J., Rajaram, N. & Muldoon, T. J. Macrophage-targeted anti-CCL2 immunotherapy enhances tumor sensitivity to 5-fluorouracil in a Balb/c-CT26 murine colon carcinoma model measured using diffuse reflectance spectroscopy. BMC Immunol.23, 20 (2022). 10.1186/s12865-022-00493-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Vitiello, P. F. et al. Impact of tumor-derived CCL2 on T cell effector function. Immunol. Lett.91, 239–245 (2004). 10.1016/j.imlet.2003.12.009 [DOI] [PubMed] [Google Scholar]
- 361.Zhu, X., Fujita, M., Snyder, L. A. & Okada, H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J. Neurooncol.104, 83–92 (2011). 10.1007/s11060-010-0473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zollo, M. et al. Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin. Exp. Metastasis29, 585–601 (2012). 10.1007/s10585-012-9473-5 [DOI] [PubMed] [Google Scholar]
- 363.Herman, J. G., Stadelman, H. L. & Roselli, C. E. Curcumin blocks CCL2-induced adhesion, motility and invasion, in part, through down-regulation of CCL2 expression and proteolytic activity. Int. J. Oncol.34, 1319–1327, (2009). [PMC free article] [PubMed] [Google Scholar]
- 364.Mu, X. Y. et al. RS 504393 inhibits M-MDSCs recruiting in immune microenvironment of bladder cancer after gemcitabine treatment. Mol. Immunol.109, 140–148 (2019). 10.1016/j.molimm.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 365.Yang, Z. et al. CCL2/CCR2 axis promotes the progression of salivary adenoid cystic carcinoma via recruiting and reprogramming the tumor-associated macrophages. Front. Oncol.9, 231 (2019). 10.3389/fonc.2019.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Han, R. et al. Estrogen promotes progression of hormone-dependent breast cancer through CCL2-CCR2 axis by upregulation of Twist via PI3K/AKT/NF-κB signaling. Sci. Rep.8, 9575 (2018). 10.1038/s41598-018-27810-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Zhou, C. et al. Disruption of SLFN11 deficiency-induced CCL2 signaling and macrophage M2 polarization potentiates anti-PD-1 therapy efficacy in hepatocellular carcinoma. Gastroenterology164, 1261–1278 (2023). 10.1053/j.gastro.2023.02.005 [DOI] [PubMed] [Google Scholar]
- 368.Tu, M. M. et al. Inhibition of the CCL2 receptor, CCR2, enhances tumor response to immune checkpoint therapy. Commun. Biol.3, 720 (2020). 10.1038/s42003-020-01441-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Flores-Toro, J. A. et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc. Natl Acad. Sci. USA117, 1129–1138 (2020). 10.1073/pnas.1910856117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Sandhu, S. K. et al. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother. Pharm.71, 1041–1050 (2013). 10.1007/s00280-013-2099-8 [DOI] [PubMed] [Google Scholar]
- 371.Pienta, K. J. et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest. N. Drugs31, 760–768 (2013). 10.1007/s10637-012-9869-8 [DOI] [PubMed] [Google Scholar]
- 372.Nywening, T. M. et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol.17, 651–662 (2016). 10.1016/S1470-2045(16)00078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Xu, M. et al. Role of the CCL2-CCR2 signalling axis in cancer: mechanisms and therapeutic targeting. Cell Prolif.54, e13115 (2021). 10.1111/cpr.13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Noel, M. et al. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Invest. N. Drugs38, 800–811 (2020). 10.1007/s10637-019-00830-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Gobert, M. et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res.69, 2000–2009 (2009). 10.1158/0008-5472.CAN-08-2360 [DOI] [PubMed] [Google Scholar]
- 376.Wiedemann, G. M. et al. Cancer cell-derived IL-1α induces CCL22 and the recruitment of regulatory T cells. Oncoimmunology5, e1175794 (2016). 10.1080/2162402X.2016.1175794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Shabaneh, T. B. et al. Oncogenic BRAF(V600E) governs regulatory t-cell recruitment during melanoma tumorigenesis. Cancer Res.78, 5038–5049 (2018). 10.1158/0008-5472.CAN-18-0365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Bayry, J., Tartour, E. & Tough, D. F. Targeting CCR4 as an emerging strategy for cancer therapy and vaccines. Trends Pharm. Sci.35, 163–165, (2014). 10.1016/j.tips.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 379.Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med.10, 942–949 (2004). 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]
- 380.Maeda, S. et al. CCR4 blockade depletes regulatory T cells and prolongs survival in a canine model of bladder cancer. Cancer Immunol. Res.7, 1175–1187 (2019). 10.1158/2326-6066.CIR-18-0751 [DOI] [PubMed] [Google Scholar]
- 381.Pere, H. et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood118, 4853–4862 (2011). 10.1182/blood-2011-01-329656 [DOI] [PubMed] [Google Scholar]
- 382.Berlato, C. et al. A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer. J. Clin. Invest.127, 801–813 (2017). 10.1172/JCI82976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Khabipov, A. et al. CCR4 blockade diminishes intratumoral macrophage recruitment and augments survival of syngeneic pancreatic cancer-bearing mice. Biomedicines11, 1517 (2023). 10.3390/biomedicines11061517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Marshall, L. A. et al. Tumors establish resistance to immunotherapy by regulating T(reg) recruitment via CCR4. J. Immunother. Cancer8, e000764 (2020). 10.1136/jitc-2020-000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Ogura, M. et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol.32, 1157–1163 (2014). 10.1200/JCO.2013.52.0924 [DOI] [PubMed] [Google Scholar]
- 386.Kurose, K. et al. Phase Ia study of FoxP3+ CD4 Treg depletion by infusion of a humanized Anti-CCR4 antibody, KW-0761, in cancer patients. Clin. Cancer Res21, 4327–4336 (2015). 10.1158/1078-0432.CCR-15-0357 [DOI] [PubMed] [Google Scholar]
- 387.Zamarin, D. et al. Mogamulizumab in combination with durvalumab or tremelimumab in patients with advanced solid tumors: a phase I study. Clin. Cancer Res.26, 4531–4541 (2020). 10.1158/1078-0432.CCR-20-0328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Doi, T. et al. A phase I study of the anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin. Cancer Res.25, 6614–6622 (2019). 10.1158/1078-0432.CCR-19-1090 [DOI] [PubMed] [Google Scholar]
- 389.Aldinucci, D., Borghese, C. & Casagrande, N. The CCL5/CCR5 axis in cancer progression. Cancers (Basel)12, 1765 (2020). 10.3390/cancers12071765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Appay, V. & Rowland-Jones, S. L. RANTES: a versatile and controversial chemokine. Trends Immunol.22, 83–87, (2001). 10.1016/S1471-4906(00)01812-3 [DOI] [PubMed] [Google Scholar]
- 391.Velasco-Velázquez, M., Xolalpa, W. & Pestell, R. G. The potential to target CCL5/CCR5 in breast cancer. Expert Opin. Ther. Targets18, 1265–1275, (2014). 10.1517/14728222.2014.949238 [DOI] [PubMed] [Google Scholar]
- 392.Zeng, Z., Lan, T., Wei, Y. & Wei, X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis.9, 12–27 (2022). 10.1016/j.gendis.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Velasco-Velázquez, M. et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res.72, 3839–3850 (2012). 10.1158/0008-5472.CAN-11-3917 [DOI] [PubMed] [Google Scholar]
- 394.Schlecker, E. et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J. Immunol.189, 5602–5611 (2012). 10.4049/jimmunol.1201018 [DOI] [PubMed] [Google Scholar]
- 395.Wang, H. C. et al. Tumor-associated macrophages promote epigenetic silencing of gelsolin through DNA methyltransferase 1 in gastric cancer cells. Cancer Immunol. Res.5, 885–897 (2017). 10.1158/2326-6066.CIR-16-0295 [DOI] [PubMed] [Google Scholar]
- 396.Yang, L. et al. Blockade of CCR5-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy in gastric cancer. Immunopharmacol. Immunotoxicol.40, 91–97 (2018). 10.1080/08923973.2017.1417997 [DOI] [PubMed] [Google Scholar]
- 397.Brett, E. et al. Naming the barriers between Anti-CCR5 therapy, breast cancer and its microenvironment. Int. J. Mol. Sci.23, 14159 (2022). 10.3390/ijms232214159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Blanco, J. R. & Ochoa-Callejero, L. Off-label use of maraviroc in clinical practice. Expert Rev. Anti Infect. Ther.14, 5–8 (2016). 10.1586/14787210.2016.1100535 [DOI] [PubMed] [Google Scholar]
- 399.Zeng, H. et al. Cancer-associated fibroblasts facilitate premetastatic niche formation through lncRNA SNHG5-mediated angiogenesis and vascular permeability in breast cancer. Theranostics12, 7351–7370 (2022). 10.7150/thno.74753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Jiao, X. et al. CCR5 governs DNA damage repair and breast cancer stem cell expansion. Cancer Res.78, 1657–1671 (2018). 10.1158/0008-5472.CAN-17-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Zhang, F. et al. Structure activity relationship studies of natural product chemokine receptor CCR5 antagonist anibamine toward the development of novel anti prostate cancer agents. Eur. J. Med. Chem.55, 395–408 (2012). 10.1016/j.ejmech.2012.07.049 [DOI] [PubMed] [Google Scholar]
- 402.Robinson, S. C. et al. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res.63, 8360–8365 (2003). [PubMed] [Google Scholar]
- 403.Woollard, S. M. & Kanmogne, G. D. Maraviroc: a review of its use in HIV infection and beyond. Drug Des. Dev. Ther.9, 5447–5468, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Dunbar, K. J. et al. Tumor-derived CCL5 recruits cancer-associated fibroblasts and promotes tumor cell proliferation in esophageal squamous cell carcinoma. Mol. Cancer Res.21, 741–752 (2023). 10.1158/1541-7786.MCR-22-0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Passman, A. M. et al. Maraviroc prevents HCC development by suppressing macrophages and the liver progenitor cell response in a murine chronic liver disease model. Cancers (Basel)13, 4935 (2021). 10.3390/cancers13194935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Zhang, X. N. et al. Pericytes augment glioblastoma cell resistance to temozolomide through CCL5-CCR5 paracrine signaling. Cell Res.31, 1072–1087 (2021). 10.1038/s41422-021-00528-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Haag, G. M. et al. Pembrolizumab and maraviroc in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer—the PICCASSO phase I trial. Eur. J. Cancer167, 112–122 (2022). 10.1016/j.ejca.2022.03.017 [DOI] [PubMed] [Google Scholar]
- 408.Brat, D. J., Bellail, A. C. & Van Meir, E. G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol.7, 122–133 (2005). 10.1215/S1152851704001061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Knall, C., Worthen, G. S. & Johnson, G. L. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc. Natl Acad. Sci. USA94, 3052–3057, (1997). 10.1073/pnas.94.7.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Knall, C. et al. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J. Biol. Chem.271, 2832–2838 (1996). 10.1074/jbc.271.5.2832 [DOI] [PubMed] [Google Scholar]
- 411.Lang, K., Niggemann, B., Zanker, K. S. & Entschladen, F. Signal processing in migrating T24 human bladder carcinoma cells: role of the autocrine interleukin-8 loop. Int. J. Cancer99, 673–680 (2002). 10.1002/ijc.10424 [DOI] [PubMed] [Google Scholar]
- 412.Ha, H., Debnath, B. & Neamati, N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics7, 1543–1588 (2017). 10.7150/thno.15625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Cambier, S., Gouwy, M. & Proost, P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol. Immunol.20, 217–251 (2023). 10.1038/s41423-023-00974-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Liu, Q. et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev.31, 61–71 (2016). 10.1016/j.cytogfr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Han, Z. J. et al. Roles of the CXCL8-CXCR1/2 axis in the tumor microenvironment and immunotherapy. Molecules. 27, 137 (2021). [DOI] [PMC free article] [PubMed]
- 416.Greene, S. et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models. Clin. Cancer Res.26, 1420–1431 (2020). 10.1158/1078-0432.CCR-19-2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Bertini, R. et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc. Natl Acad. Sci. USA101, 11791–11796 (2004). 10.1073/pnas.0402090101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Lin, C. et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut68, 1764–1773 (2019). 10.1136/gutjnl-2018-316324 [DOI] [PubMed] [Google Scholar]
- 419.Bilusic, M. et al. Phase I trial of HuMax-IL8 (BMS-986253), an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J. Immunother. Cancer7, 240 (2019). 10.1186/s40425-019-0706-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Kargl, J. et al. Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC. JCI Insight4, e130850 (2019). 10.1172/jci.insight.130850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Gonçalves, T. L., de Araújo, L. P. & Pereira Ferrer, V. Tamoxifen as a modulator of CXCL12-CXCR4-CXCR7 chemokine axis: a breast cancer and glioblastoma view. Cytokine170, 156344 (2023). 10.1016/j.cyto.2023.156344 [DOI] [PubMed] [Google Scholar]
- 422.Teicher, B. A. & Fricker, S. P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res.16, 2927–2931 (2010). 10.1158/1078-0432.CCR-09-2329 [DOI] [PubMed] [Google Scholar]
- 423.Zhou, W. et al. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr. Med. Chem.26, 3026–3041 (2019). 10.2174/0929867324666170830111531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Khalighfard, S. et al. Breast tumor metastasis following filgrastim administration due to the SDF-1/CXCR4 pathway. Med. Oncol.40, 74 (2023). 10.1007/s12032-022-01935-1 [DOI] [PubMed] [Google Scholar]
- 425.Song, Z. Y. et al. Downregulation of the CXCR4/CXCL12 axis blocks the activation of the Wnt/β-catenin pathway in human colon cancer cells. Biomed. Pharmacother.71, 46–52 (2015). 10.1016/j.biopha.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 426.Daniel, S. K., Seo, Y. D. & Pillarisetty, V. G. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin. Cancer Biol.65, 176–188 (2020). 10.1016/j.semcancer.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 427.Eckert, F. et al. Potential role of CXCR4 targeting in the context of radiotherapy and immunotherapy of cancer. Front. Immunol.9, 3018 (2018). 10.3389/fimmu.2018.03018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Bao, S. et al. CXC chemokine receptor 4 (CXCR4) blockade in cancer treatment. J. Cancer Res. Clin. Oncol.149, 7945–7968 (2023). 10.1007/s00432-022-04444-w [DOI] [PubMed] [Google Scholar]
- 429.Mota, J. M. et al. Post-sepsis state induces tumor-associated macrophage accumulation through CXCR4/CXCL12 and favors tumor progression in mice. Cancer Immunol. Res.4, 312–322 (2016). 10.1158/2326-6066.CIR-15-0170 [DOI] [PubMed] [Google Scholar]
- 430.Fortunato, O. et al. CXCR4 inhibition counteracts immunosuppressive properties of metastatic NSCLC stem cells. Front. Immunol.11, 02168 (2020). 10.3389/fimmu.2020.02168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Dürr, C. et al. CXCL12 mediates immunosuppression in the lymphoma microenvironment after allogeneic transplantation of hematopoietic cells. Cancer Res.70, 10170–10181 (2010). 10.1158/0008-5472.CAN-10-1943 [DOI] [PubMed] [Google Scholar]
- 432.Righi, E. et al. CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Res.71, 5522–5534 (2011). 10.1158/0008-5472.CAN-10-3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Fearon, D. T. & Janowitz, T. AMD3100/Plerixafor overcomes immune inhibition by the CXCL12-KRT19 coating on pancreatic and colorectal cancer cells. Br. J. Cancer125, 149–151 (2021). 10.1038/s41416-021-01315-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Bila, J. et al. Bone marrow microenvironment interplay and current clinical practice in multiple myeloma: a review of the balkan myeloma study group. J. Clin. Med.10, 3940 (2021). 10.3390/jcm10173940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Wang, J., Tannous, B. A., Poznansky, M. C. & Chen, H. CXCR4 antagonist AMD3100 (plerixafor): From an impurity to a therapeutic agent. Pharm. Res.159, 105010 (2020). 10.1016/j.phrs.2020.105010 [DOI] [PubMed] [Google Scholar]
- 436.Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA110, 20212–20217 (2013). 10.1073/pnas.1320318110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Domanska, U. M. et al. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia14, 709–718 (2012). 10.1593/neo.12324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Zhou, K. X. et al. CXCR4 antagonist AMD3100 enhances the response of MDA-MB-231 triple-negative breast cancer cells to ionizing radiation. Cancer Lett.418, 196–203 (2018). 10.1016/j.canlet.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 439.Thomas, R. P. et al. Macrophage exclusion after radiation therapy (MERT): a first in human phase I/II trial using a CXCR4 inhibitor in glioblastoma. Clin. Cancer Res.25, 6948–6957 (2019). 10.1158/1078-0432.CCR-19-1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Bockorny, B. et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat. Med.26, 878–885 (2020). 10.1038/s41591-020-0880-x [DOI] [PubMed] [Google Scholar]
- 441.Borthakur, G. et al. BL-8040 CXCR4 antagonist is safe and demonstrates antileukemic activity in combination with cytarabine for the treatment of relapsed/refractory acute myelogenous leukemia: an open-label safety and efficacy phase 2a study. Cancer127, 1246–1259 (2021). 10.1002/cncr.33338 [DOI] [PubMed] [Google Scholar]
- 442.Galsky, M. D. et al. A phase I trial of LY2510924, a CXCR4 peptide antagonist, in patients with advanced cancer. Clin. Cancer Res.20, 3581–3588 (2014). 10.1158/1078-0432.CCR-13-2686 [DOI] [PubMed] [Google Scholar]
- 443.Pernas, S. et al. Balixafortide plus eribulin in HER2-negative metastatic breast cancer: a phase 1, single-arm, dose-escalation trial. Lancet Oncol.19, 812–824 (2018). 10.1016/S1470-2045(18)30147-5 [DOI] [PubMed] [Google Scholar]
- 444.Ghobrial, I. M. et al. A phase Ib/II trial of the first-in-class anti-CXCR4 antibody ulocuplumab in combination with lenalidomide or bortezomib plus dexamethasone in relapsed multiple myeloma. Clin. Cancer Res.26, 344–353 (2020). 10.1158/1078-0432.CCR-19-0647 [DOI] [PubMed] [Google Scholar]
- 445.Hoellenriegel, J. et al. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood123, 1032–1039 (2014). 10.1182/blood-2013-03-493924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Pol, J. G. et al. Cytokines in oncolytic virotherapy. Cytokine Growth Factor Rev.56, 4–27 (2020). 10.1016/j.cytogfr.2020.10.007 [DOI] [PubMed] [Google Scholar]
- 447.Liu, Z. et al. CXCL11-Armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. Oncoimmunology5, e1091554 (2016). 10.1080/2162402X.2015.1091554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Eckert, E. C. et al. Generation of a tumor-specific chemokine gradient using oncolytic vesicular stomatitis virus encoding CXCL9. Mol. Ther. Oncolyt.16, 63–74 (2020). 10.1016/j.omto.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Simon, G. et al. 762 First-in-human phase 1a study of NG-641, a tumour-selective vector expressing a FAP-TAc bispecific antibody and immune enhancer module, in patients with metastatic/advanced epithelial tumours (STAR). J. Immunother. Cancer10, A794 (2022). [Google Scholar]
- 450.Lillie, T. et al. Abstract CT214: A multicenter phase 1a/b study of NG-641, a tumor-selective transgene-expressing adenoviral vector, and nivolumab in patients with metastatic or advanced epithelial tumors (NEBULA). Cancer Res.82, CT214 (2022). 10.1158/1538-7445.AM2022-CT214 [DOI] [Google Scholar]
- 451.Wang, X. et al. A novel recombinant protein of IP10-EGFRvIIIscFv and CD8(+) cytotoxic T lymphocytes synergistically inhibits the growth of implanted glioma in mice. Cancer Immunol. Immunother.62, 1261–1272 (2013). 10.1007/s00262-013-1426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Yamano, T. et al. Enhancement of immunity by a DNA melanoma vaccine against TRP2 with CCL21 as an adjuvant. Mol. Ther.13, 194–202 (2006). 10.1016/j.ymthe.2005.05.018 [DOI] [PubMed] [Google Scholar]
- 453.Yamano, T. et al. Immunity against breast cancer by TERT DNA vaccine primed with chemokine CCL21. Cancer Gene Ther.14, 451–459 (2007). 10.1038/sj.cgt.7701035 [DOI] [PubMed] [Google Scholar]
- 454.Gray, J. E. et al. A phase I/randomized phase II study of GM.CD40L vaccine in combination with CCL21 in patients with advanced lung adenocarcinoma. Cancer Immunol. Immunother.67, 1853–1862 (2018). 10.1007/s00262-018-2236-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Goto, S. et al. Enhanced anti-tumor efficacy of IL-7/CCL19-producing human CAR-T cells in orthotopic and patient-derived xenograft tumor models. Cancer Immunol. Immunother.70, 2503–2515 (2021). 10.1007/s00262-021-02853-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol.36, 346–351 (2018). 10.1038/nbt.4086 [DOI] [PubMed] [Google Scholar]
- 457.Lesch, S. et al. T cells armed with C-X-C chemokine receptor type 6 enhance adoptive cell therapy for pancreatic tumours. Nat. Biomed. Eng.5, 1246–1260 (2021). 10.1038/s41551-021-00737-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Savage, T. M. et al. Chemokines expressed by engineered bacteria recruit and orchestrate antitumor immunity. Sci. Adv.9, eadc9436 (2023). 10.1126/sciadv.adc9436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Pang, N. et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J. Hematol. Oncol.14, 118 (2021). 10.1186/s13045-021-01128-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Claesson-Welsh, L. & Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med.273, 114–127, (2013). 10.1111/joim.12019 [DOI] [PubMed] [Google Scholar]
- 461.Sigismund, S., Avanzato, D. & Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol.12, 3–20 (2018). 10.1002/1878-0261.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Bai, X. et al. Blocking TGF-β signaling to enhance the efficacy of immune checkpoint inhibitor. Onco Targets Ther.12, 9527–9538 (2019). 10.2147/OTT.S224013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Wozney, J. L. & Antonarakis, E. S. Growth factor and signaling pathways and their relevance to prostate cancer therapeutics. Cancer Metastasis Rev.33, 581–594, (2014). 10.1007/s10555-013-9475-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Derynck, R. & Budi, E. H. Specificity, versatility, and control of TGF-β family signaling. Sci. Signal.12, eaav5183 (2019). 10.1126/scisignal.aav5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Wilson, S. E. TGF beta -1, -2 and -3 in the modulation of fibrosis in the cornea and other organs. Exp. Eye Res.207, 108594 (2021). 10.1016/j.exer.2021.108594 [DOI] [PubMed] [Google Scholar]
- 466.Robertson, I. B. et al. Latent TGF-β-binding proteins. Matrix Biol.47, 44–53 (2015). 10.1016/j.matbio.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Lawrence, D. A. Latent-TGF-beta: an overview. Mol. Cell Biochem.219, 163–170, (2001). 10.1023/A:1010819716023 [DOI] [PubMed] [Google Scholar]
- 468.Kusakabe, M. et al. The structure of the TGF-beta latency associated peptide region determines the ability of the proprotein convertase furin to cleave TGF-betas. J. Cell Biochem.103, 311–320 (2008). 10.1002/jcb.21407 [DOI] [PubMed] [Google Scholar]
- 469.Shi, M. et al. Latent TGF-β structure and activation. Nature474, 343–349 (2011). 10.1038/nature10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Munger, J. S. et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell96, 319–328 (1999). 10.1016/S0092-8674(00)80545-0 [DOI] [PubMed] [Google Scholar]
- 471.Syed, V. TGF-β signaling in cancer. J. Cell Biochem.117, 1279–1287 (2016). 10.1002/jcb.25496 [DOI] [PubMed] [Google Scholar]
- 472.Derynck, R. & Zhang, Y. E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature425, 577–584, (2003). 10.1038/nature02006 [DOI] [PubMed] [Google Scholar]
- 473.Aomatsu, K. et al. TGF-β induces sustained upregulation of SNAI1 and SNAI2 through Smad and non-Smad pathways in a human corneal epithelial cell line. Invest. Ophthalmol. Vis. Sci.52, 2437–2443, (2011). 10.1167/iovs.10-5635 [DOI] [PubMed] [Google Scholar]
- 474.Cho, K. H. et al. STAT3 mediates TGF-β1-induced TWIST1 expression and prostate cancer invasion. Cancer Lett.336, 167–173 (2013). 10.1016/j.canlet.2013.04.024 [DOI] [PubMed] [Google Scholar]
- 475.Zhang, Y. E. Non-smad signaling pathways of the TGF-β family. Cold Spring Harb. Perspect. Biol. 9, a022129 (2017). [DOI] [PMC free article] [PubMed]
- 476.Zhang, Y. E. Non-Smad pathways in TGF-beta signaling. Cell Res19, 128–139 (2009). 10.1038/cr.2008.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Morikawa, M., Derynck, R. & Miyazono, K. TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harb. Perspect. Biol.8, a021873 (2016). 10.1101/cshperspect.a021873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Peng, D. et al. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer21, 104 (2022). 10.1186/s12943-022-01569-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Gough, N. R., Xiang, X. & Mishra, L. TGF-β signaling in liver, pancreas, and gastrointestinal diseases and cancer. Gastroenterology161, 434–452.e415 (2021). 10.1053/j.gastro.2021.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Chen, J., Gingold, J. A. & Su, X. Immunomodulatory TGF-β signaling in hepatocellular carcinoma. Trends Mol. Med.25, 1010–1023 (2019). 10.1016/j.molmed.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 481.Shi, X. et al. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J. Hematol. Oncol.15, 135 (2022). 10.1186/s13045-022-01349-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Niu, M. et al. Synergistic efficacy of simultaneous anti-TGF-β/VEGF bispecific antibody and PD-1 blockade in cancer therapy. J. Hematol. Oncol.16, 94 (2023). 10.1186/s13045-023-01487-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Morris, J. C. et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE9, e90353 (2014). 10.1371/journal.pone.0090353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Formenti, S. C. et al. Focal irradiation and systemic TGFβ blockade in metastatic breast cancer. Clin. Cancer Res.24, 2493–2504 (2018). 10.1158/1078-0432.CCR-17-3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Melisi, D. et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer119, 1208–1214 (2018). 10.1038/s41416-018-0246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Yamazaki, T. et al. Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a single-arm, phase 2 trial. Lancet Oncol.23, 1189–1200 (2022). 10.1016/S1470-2045(22)00446-6 [DOI] [PubMed] [Google Scholar]
- 487.Brandes, A. A. et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol.18, 1146–1156 (2016). 10.1093/neuonc/now009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Harding, J. J. et al. Phase 1b study of galunisertib and ramucirumab in patients with advanced hepatocellular carcinoma. Cancer Med.10, 3059–3067 (2021). 10.1002/cam4.3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Herbertz, S. et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Dev. Ther.9, 4479–4499, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Scagliotti, G. V. et al. Tasisulam sodium (LY573636 sodium) as third-line treatment in patients with unresectable, metastatic non-small-cell lung cancer: a phase-II study. J. Thorac. Oncol.7, 1053–1057 (2012). 10.1097/JTO.0b013e3182519d79 [DOI] [PubMed] [Google Scholar]
- 491.Lan, Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 10, eaan5488 (2018). [DOI] [PubMed]
- 492.Strauss, J. et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin. Cancer Res.24, 1287–1295 (2018). 10.1158/1078-0432.CCR-17-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Paz-Ares, L. et al. Bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: results from an expansion cohort of a phase 1 trial. J. Thorac. Oncol.15, 1210–1222 (2020). 10.1016/j.jtho.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Liu, D. et al. Bifunctional anti-PD-L1/TGF-βRII agent SHR-1701 in advanced solid tumors: a dose-escalation, dose-expansion, and clinical-expansion phase 1 trial. BMC Med.20, 408 (2022). 10.1186/s12916-022-02605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Feng, J. et al. SHR-1701, a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, for Recurrent or Metastatic Cervical Cancer: A Clinical Expansion Cohort of a Phase I Study. Clin. Cancer Res.28, 5297–5305 (2022). 10.1158/1078-0432.CCR-22-0346 [DOI] [PubMed] [Google Scholar]
- 496.Yi, M. et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1. J. Hematol. Oncol.14, 27 (2021). 10.1186/s13045-021-01045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Yi, M. et al. Anti-TGF-β/PD-L1 bispecific antibody promotes T cell infiltration and exhibits enhanced antitumor activity in triple-negative breast cancer. J. Immunother. Cancer10, e005543 (2022). 10.1136/jitc-2022-005543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Bogdahn, U. et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol.13, 132–142 (2011). 10.1093/neuonc/noq142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Giaccone, G. et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur. J. Cancer51, 2321–2329 (2015). 10.1016/j.ejca.2015.07.035 [DOI] [PubMed] [Google Scholar]
- 500.Lugano, R., Ramachandran, M. & Dimberg, A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol. Life Sci.77, 1745–1770 (2020). 10.1007/s00018-019-03351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Fukumura, D. et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol.15, 325–340 (2018). 10.1038/nrclinonc.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Ronca, R. et al. Tumor angiogenesis revisited: Regulators and clinical implications. Med. Res. Rev.37, 1231–1274 (2017). 10.1002/med.21452 [DOI] [PubMed] [Google Scholar]
- 503.Liu, Z. et al. Vascular normalization in immunotherapy: a promising mechanisms combined with radiotherapy. Biomed. Pharmacother.139, 111607 (2021). 10.1016/j.biopha.2021.111607 [DOI] [PubMed] [Google Scholar]
- 504.Goel, S. et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev.91, 1071–1121 (2011). 10.1152/physrev.00038.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Choi, Y. & Jung, K. Normalization of the tumor microenvironment by harnessing vascular and immune modulation to achieve enhanced cancer therapy. Exp. Mol. Med.55, 2308–2319 (2023). 10.1038/s12276-023-01114-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Yu, P. et al. Vascular normalization: reshaping the tumor microenvironment and augmenting antitumor immunity for ovarian cancer. Front. Immunol.14, 1276694 (2023). 10.3389/fimmu.2023.1276694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Ye, W. The complexity of translating anti-angiogenesis therapy from basic science to the clinic. Dev. Cell37, 114–125 (2016). 10.1016/j.devcel.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 508.Viallard, C. & Larrivée, B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis20, 409–426 (2017). 10.1007/s10456-017-9562-9 [DOI] [PubMed] [Google Scholar]
- 509.Huang, Y. et al. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol.18, 195–203 (2018). 10.1038/nri.2017.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med.212, 139–148 (2015). 10.1084/jem.20140559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med.2, 1096–1103 (1996). 10.1038/nm1096-1096 [DOI] [PubMed] [Google Scholar]
- 512.Facciabene, A. et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature475, 226–230 (2011). 10.1038/nature10169 [DOI] [PubMed] [Google Scholar]
- 513.Movahedi, K. et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res.70, 5728–5739 (2010). 10.1158/0008-5472.CAN-09-4672 [DOI] [PubMed] [Google Scholar]
- 514.Du Four, S. et al. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am. J. Cancer Res.6, 2514–2531 (2016). [PMC free article] [PubMed] [Google Scholar]
- 515.Noman, M. Z. et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med.211, 781–790 (2014). 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Jain, R. K. et al. Leukocyte-endothelial adhesion and angiogenesis in tumors. Cancer Metastasis Rev.15, 195–204 (1996). 10.1007/BF00437472 [DOI] [PubMed] [Google Scholar]
- 517.Melder, R. J. et al. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat. Med.2, 992–997 (1996). 10.1038/nm0996-992 [DOI] [PubMed] [Google Scholar]
- 518.Hendry, S. A. et al. The role of the tumor vasculature in the host immune response: implications for therapeutic strategies targeting the tumor microenvironment. Front. Immunol.7, 621 (2016). 10.3389/fimmu.2016.00621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Ramjiawan, R. R., Griffioen, A. W. & Duda, D. G. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis20, 185–204 (2017). 10.1007/s10456-017-9552-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Tu, J. et al. The application and research progress of anti-angiogenesis therapy in tumor immunotherapy. Front. Immunol.14, 1198972 (2023). 10.3389/fimmu.2023.1198972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Wu, F. T. H. et al. Pre- and post-operative anti-PD-L1 plus anti-angiogenic therapies in mouse breast or renal cancer models of micro- or macro-metastatic disease. Br. J. Cancer120, 196–206 (2019). 10.1038/s41416-018-0297-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Meder, L. et al. Combined VEGF and PD-L1 blockade displays synergistic treatment effects in an autochthonous mouse model of small cell lung cancer. Cancer Res.78, 4270–4281 (2018). 10.1158/0008-5472.CAN-17-2176 [DOI] [PubMed] [Google Scholar]
- 523.Yasuda, S. et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin. Exp. Immunol.172, 500–506 (2013). 10.1111/cei.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Tran, T. T. et al. Lenvatinib or anti-VEGF in combination with anti-PD-1 differentially augments antitumor activity in melanoma. JCI Insight8, e157347 (2023). 10.1172/jci.insight.157347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Song, Y. et al. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front. Immunol.11, 1956 (2020). 10.3389/fimmu.2020.01956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Yi, M. et al. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol.14, 10 (2021). 10.1186/s13045-020-01027-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Allen, E. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9, eaak9679 (2017). [DOI] [PMC free article] [PubMed]
- 528.Hodi, F. S. et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol. Res.2, 632–642 (2014). 10.1158/2326-6066.CIR-14-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Wu, X. et al. Combined anti-VEGF and anti-CTLA-4 therapy elicits humoral immunity to galectin-1 which is associated with favorable clinical outcomes. Cancer Immunol. Res.5, 446–454 (2017). 10.1158/2326-6066.CIR-16-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Ren, Z. et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol.22, 977–990 (2021). 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 531.Liu, J. F. et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol.5, 1731–1738 (2019). 10.1001/jamaoncol.2019.3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Socinski, M. A. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med.378, 2288–2301 (2018). 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 533.Wang, K. et al. Recent advances in, and challenges of, anti-angiogenesis agents for tumor chemotherapy based on vascular normalization. Drug Discov. Today26, 2743–2753 (2021). 10.1016/j.drudis.2021.07.024 [DOI] [PubMed] [Google Scholar]
- 534.Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med.380, 1103–1115 (2019). 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Choueiri, T. K. et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol.19, 451–460 (2018). 10.1016/S1470-2045(18)30107-4 [DOI] [PubMed] [Google Scholar]
- 536.Xu, J. et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin. Cancer Res.25, 515–523 (2019). 10.1158/1078-0432.CCR-18-2484 [DOI] [PubMed] [Google Scholar]
- 537.Cohen, S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev. Biol.12, 394–407 (1965). 10.1016/0012-1606(65)90005-9 [DOI] [PubMed] [Google Scholar]
- 538.Carpenter, G., Lembach, K. J., Morrison, M. M. & Cohen, S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J. Biol. Chem.250, 4297–4304, (1975). 10.1016/S0021-9258(19)41417-8 [DOI] [PubMed] [Google Scholar]
- 539.Yarden, Y. & Shilo, B. Z. SnapShot: EGFR signaling pathway. Cell131, 1018 (2007). 10.1016/j.cell.2007.11.013 [DOI] [PubMed] [Google Scholar]
- 540.da Cunha Santos, G., Shepherd, F. A. & Tsao, M. S. EGFR mutations and lung cancer. Annu Rev. Pathol.6, 49–69 (2011). 10.1146/annurev-pathol-011110-130206 [DOI] [PubMed] [Google Scholar]
- 541.Ray, K., Ujvari, B., Ramana, V. & Donald, J. Cross-talk between EGFR and IL-6 drives oncogenic signaling and offers therapeutic opportunities in cancer. Cytokine Growth Factor Rev.41, 18–27 (2018). 10.1016/j.cytogfr.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 542.Lo, H. W., Hsu, S. C. & Hung, M. C. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res Treat.95, 211–218, (2006). 10.1007/s10549-005-9011-0 [DOI] [PubMed] [Google Scholar]
- 543.Hu, T. & Li, C. Convergence between Wnt-β-catenin and EGFR signaling in cancer. Mol. Cancer9, 236 (2010). 10.1186/1476-4598-9-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Liu, Y. et al. Rolling-translated EGFR variants sustain EGFR signaling and promote glioblastoma tumorigenicity. Neuro Oncol.23, 743–756 (2021). 10.1093/neuonc/noaa279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Li, X. et al. Can EGFR be a therapeutic target in breast cancer? Biochim. Biophys. Acta Rev. Cancer1877, 188789 (2022). 10.1016/j.bbcan.2022.188789 [DOI] [PubMed] [Google Scholar]
- 546.Strickler, J. H. et al. Diagnosis and treatment of ERBB2-positive metastatic colorectal cancer: a review. JAMA Oncol.8, 760–769 (2022). 10.1001/jamaoncol.2021.8196 [DOI] [PubMed] [Google Scholar]
- 547.Remon, J., Steuer, C. E., Ramalingam, S. S. & Felip, E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann. Oncol.29, i20–i27 (2018). 10.1093/annonc/mdx704 [DOI] [PubMed] [Google Scholar]
- 548.Wu, S. G. & Shih, J. Y. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol. Cancer17, 38 (2018). 10.1186/s12943-018-0777-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Passaro, A., Jänne, P. A., Mok, T. & Peters, S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat. Cancer2, 377–391 (2021). 10.1038/s43018-021-00195-8 [DOI] [PubMed] [Google Scholar]
- 550.Liu, Q. et al. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol. Cancer17, 53 (2018). 10.1186/s12943-018-0793-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Chong, C. R. & Jänne, P. A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med.19, 1389–1400 (2013). 10.1038/nm.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Guardiola, S., Varese, M., Sánchez-Navarro, M. & Giralt, E. A third shot at EGFR: new opportunities in cancer therapy. Trends Pharm. Sci.40, 941–955 (2019). 10.1016/j.tips.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 553.Hailing, T., Yonghong, P., Yufeng, Z. & Haitao, T. Challenges for the application of EGFR-targeting peptide GE11 in tumor diagnosis and treatment. J. Control Release349, 592–605 (2022). 10.1016/j.jconrel.2022.07.018 [DOI] [PubMed] [Google Scholar]
- 554.Wang, L. et al. Anti-EGFR binding nanobody delivery system to improve the diagnosis and treatment of solid tumours. Recent Pat. Anticancer Drug Discov.15, 200–211 (2020). 10.2174/1574892815666200904111728 [DOI] [PubMed] [Google Scholar]
- 555.Sharifi, J., Khirehgesh, M. R., Safari, F. & Akbari, B. EGFR and anti-EGFR nanobodies: review and update. J. Drug Target29, 387–402 (2021). 10.1080/1061186X.2020.1853756 [DOI] [PubMed] [Google Scholar]
- 556.Huang, M. et al. Targeting glutamine metabolism to enhance immunoprevention of EGFR-driven lung cancer. Adv. Sci.9, e2105885 (2022). 10.1002/advs.202105885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Sabbah, D. A., Hajjo, R. & Sweidan, K. Review on epidermal growth factor receptor (EGFR) structure, signaling pathways, interactions, and recent updates of EGFR inhibitors. Curr. Top. Med. Chem.20, 815–834 (2020). 10.2174/1568026620666200303123102 [DOI] [PubMed] [Google Scholar]
- 558.Ramalingam, S. S. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med.382, 41–50 (2020). 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 559.Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med.378, 113–125 (2018). 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 560.Fakih, M. G. et al. Sotorasib plus panitumumab in refractory colorectal cancer with mutated KRAS G12C. N. Engl. J. Med.389, 2125–2139 (2023). 10.1056/NEJMoa2308795 [DOI] [PubMed] [Google Scholar]
- 561.Huang, L. & Fu, L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm. Sin. B5, 390–401 (2015). 10.1016/j.apsb.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Wang, S., Cang, S. & Liu, D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J. Hematol. Oncol.9, 34 (2016). 10.1186/s13045-016-0268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Lu, X. et al. Targeting EGFR(L858R/T790M) and EGFR(L858R/T790M/C797S) resistance mutations in NSCLC: current developments in medicinal chemistry. Med. Res. Rev.38, 1550–1581 (2018). 10.1002/med.21488 [DOI] [PubMed] [Google Scholar]
- 564.Wang, S., Song, Y. & Liu, D. EAI045: the fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett.385, 51–54 (2017). 10.1016/j.canlet.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 565.Du, X. et al. Acquired resistance to third-generation EGFR-TKIs and emerging next-generation EGFR inhibitors. Innovation2, 100103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Syed, Y. Y. Amivantamab: first approval. Drugs81, 1349–1353 (2021). 10.1007/s40265-021-01561-7 [DOI] [PubMed] [Google Scholar]
- 567.Mazzarella, L., Guida, A. & Curigliano, G. Cetuximab for treating non-small cell lung cancer. Expert Opin. Biol. Ther.18, 483–493 (2018). 10.1080/14712598.2018.1452906 [DOI] [PubMed] [Google Scholar]
- 568.Baysal, H. et al. The right partner in crime: unlocking the potential of the anti-EGFR antibody cetuximab via combination with natural killer cell chartering immunotherapeutic strategies. Front. Immunol.12, 737311 (2021). 10.3389/fimmu.2021.737311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Battaglin, F. et al. Anti-EGFR monoclonal antibody panitumumab for the treatment of patients with metastatic colorectal cancer: an overview of current practice and future perspectives. Expert Opin. Biol. Ther.17, 1297–1308 (2017). 10.1080/14712598.2017.1356815 [DOI] [PubMed] [Google Scholar]
- 570.Garnock-Jones, K. P. Necitumumab: first global approval. Drugs76, 283–289, (2016). 10.1007/s40265-015-0537-0 [DOI] [PubMed] [Google Scholar]
- 571.di Noia, V. et al. Necitumumab in the treatment of non-small-cell lung cancer: clinical controversies. Expert Opin. Biol. Ther.18, 937–945 (2018). 10.1080/14712598.2018.1508445 [DOI] [PubMed] [Google Scholar]
- 572.Cai, W. Q. et al. The latest battles between EGFR monoclonal antibodies and resistant tumor cells. Front. Oncol.10, 1249 (2020). 10.3389/fonc.2020.01249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Brand, T. M., Iida, M. & Wheeler, D. L. Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol. Ther.11, 777–792 (2011). 10.4161/cbt.11.9.15050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Montagut, C. et al. Efficacy of Sym004 in patients with metastatic colorectal cancer with acquired resistance to anti-EGFR therapy and molecularly selected by circulating tumor DNA analyses: a phase 2 randomized clinical trial. JAMA Oncol.4, e175245 (2018). 10.1001/jamaoncol.2017.5245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Sacco, A. G. et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol.22, 883–892 (2021). 10.1016/S1470-2045(21)00136-4 [DOI] [PubMed] [Google Scholar]
- 576.Lu, S. et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir. Med.11, 624–636 (2023). 10.1016/S2213-2600(23)00135-2 [DOI] [PubMed] [Google Scholar]
- 577.Noronha, V. et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J. Clin. Oncol.38, 124–136 (2020). 10.1200/JCO.19.01154 [DOI] [PubMed] [Google Scholar]
- 578.Hosomi, Y. et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J. Clin. Oncol.38, 115–123 (2020). 10.1200/JCO.19.01488 [DOI] [PubMed] [Google Scholar]
- 579.Saito, H. et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol.20, 625–635 (2019). 10.1016/S1470-2045(19)30035-X [DOI] [PubMed] [Google Scholar]
- 580.Raghav, K. P. S. & Moasser, M. M. Molecular pathways and mechanisms of HER2 in cancer therapy. Clin. Cancer Res.29, 2351–2361 (2023). 10.1158/1078-0432.CCR-22-0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Moasser, M. M. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene26, 6469–6487, (2007). 10.1038/sj.onc.1210477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Zhu, Y. et al. HER2-targeted therapies in gastric cancer. Biochim. Biophys. Acta Rev. Cancer1876, 188549 (2021). 10.1016/j.bbcan.2021.188549 [DOI] [PubMed] [Google Scholar]
- 583.Krishnamurti, U. & Silverman, J. F. HER2 in breast cancer: a review and update. Adv. Anat. Pathol.21, 100–107, (2014). 10.1097/PAP.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 584.Zhou, B. P. & Hung, M. C. Dysregulation of cellular signaling by HER2/neu in breast cancer. Semin. Oncol.30, 38–48 (2003). 10.1053/j.seminoncol.2003.08.006 [DOI] [PubMed] [Google Scholar]
- 585.Ménard, S., Tagliabue, E., Campiglio, M. & Pupa, S. M. Role of HER2 gene overexpression in breast carcinoma. J. Cell Physiol.182, 150–162, (2000). [DOI] [PubMed] [Google Scholar]
- 586.Maximiano, S., Magalhães, P., Guerreiro, M. P. & Morgado, M. Trastuzumab in the treatment of breast cancer. BioDrugs30, 75–86 (2016). 10.1007/s40259-016-0162-9 [DOI] [PubMed] [Google Scholar]
- 587.Gerratana, L. et al. Pertuzumab and breast cancer: another piece in the anti-HER2 puzzle. Expert Opin. Biol. Ther.17, 365–374 (2017). 10.1080/14712598.2017.1282944 [DOI] [PubMed] [Google Scholar]
- 588.Bilancia, D. et al. Lapatinib in breast cancer. Ann. Oncol.18, vi26–30 (2007). 10.1093/annonc/mdm220 [DOI] [PubMed] [Google Scholar]
- 589.Laskin, J. et al. NRG1 fusion-driven tumors: biology, detection, and the therapeutic role of afatinib and other ErbB-targeting agents. Ann. Oncol.31, 1693–1703 (2020). 10.1016/j.annonc.2020.08.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Hunter, F. W. et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer122, 603–612 (2020). 10.1038/s41416-019-0635-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Xu, Z. et al. Novel HER2-targeting antibody-drug conjugates of trastuzumab beyond T-DM1 in breast cancer: trastuzumab deruxtecan(DS-8201a) and (Vic-)trastuzumab duocarmazine (SYD985). Eur. J. Med. Chem.183, 111682 (2019). 10.1016/j.ejmech.2019.111682 [DOI] [PubMed] [Google Scholar]
- 592.Tarantino, P. et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J. Clin.72, 165–182 (2022). 10.3322/caac.21705 [DOI] [PubMed] [Google Scholar]
- 593.Swain, S. M. et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis—focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev.106, 102378 (2022). 10.1016/j.ctrv.2022.102378 [DOI] [PubMed] [Google Scholar]
- 594.Yu, S. et al. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp. Hematol. Oncol.6, 31 (2017). 10.1186/s40164-017-0091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Meric-Bernstam, F. et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin. Cancer Res.25, 2033–2041 (2019). 10.1158/1078-0432.CCR-18-2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Swain, S. M., Shastry, M. & Hamilton, E. Targeting HER2-positive breast cancer: advances and future directions. Nat. Rev. Drug Discov.22, 101–126 (2023). 10.1038/s41573-022-00579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Oh, D. Y. & Bang, Y. J. HER2-targeted therapies—a role beyond breast cancer. Nat. Rev. Clin. Oncol.17, 33–48 (2020). 10.1038/s41571-019-0268-3 [DOI] [PubMed] [Google Scholar]
- 598.Lev, S. Targeted therapy and drug resistance in triple-negative breast cancer: the EGFR axis. Biochem. Soc. Trans.48, 657–665 (2020). 10.1042/BST20191055 [DOI] [PubMed] [Google Scholar]
- 599.Chioni, A. M. & Grose, R. P. Biological significance and targeting of the FGFR axis in cancer. Cancers (Basel)13, 5681 (2021). 10.3390/cancers13225681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Xie, Y. et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target Ther.5, 181 (2020). 10.1038/s41392-020-00222-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.De Luca, A., Frezzetti, D., Gallo, M. & Normanno, N. FGFR-targeted therapeutics for the treatment of breast cancer. Expert Opin. Investig. Drugs26, 303–311 (2017). 10.1080/13543784.2017.1287173 [DOI] [PubMed] [Google Scholar]
- 602.Loriot, Y. et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med.381, 338–348 (2019). 10.1056/NEJMoa1817323 [DOI] [PubMed] [Google Scholar]
- 603.Pant, S. et al. Erdafitinib in patients with advanced solid tumours with FGFR alterations (RAGNAR): an international, single-arm, phase 2 study. Lancet Oncol.24, 925–935 (2023). 10.1016/S1470-2045(23)00275-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Javle, M. et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol.6, 803–815 (2021). 10.1016/S2468-1253(21)00196-5 [DOI] [PubMed] [Google Scholar]
- 605.Lassman, A. B. et al. Infigratinib in patients with recurrent gliomas and FGFR alterations: a multicenter phase II study. Clin. Cancer Res.28, 2270–2277 (2022). 10.1158/1078-0432.CCR-21-2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Loriot, Y. et al. Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. N. Engl. J. Med.389, 1961–1971 (2023). 10.1056/NEJMoa2308849 [DOI] [PubMed] [Google Scholar]
- 607.Yue, S. et al. FGFR-TKI resistance in cancer: current status and perspectives. J. Hematol. Oncol.14, 23 (2021). 10.1186/s13045-021-01040-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Fu, J. et al. HGF/c-MET pathway in cancer: from molecular characterization to clinical evidence. Oncogene40, 4625–4651 (2021). 10.1038/s41388-021-01863-w [DOI] [PubMed] [Google Scholar]
- 609.Stella, M. C. & Comoglio, P. M. HGF: a multifunctional growth factor controlling cell scattering. Int. J. Biochem. Cell Biol.31, 1357–1362, (1999). 10.1016/S1357-2725(99)00089-8 [DOI] [PubMed] [Google Scholar]
- 610.Zhang, Y. et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer17, 45 (2018). 10.1186/s12943-018-0796-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Vimalraj, S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol.221, 1428–1438 (2022). 10.1016/j.ijbiomac.2022.09.129 [DOI] [PubMed] [Google Scholar]
- 612.Raghav, K. P., Gonzalez-Angulo, A. M. & Blumenschein, G. R. Jr. Role of HGF/MET axis in resistance of lung cancer to contemporary management. Transl. Lung Cancer Res.1, 179–193 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613.Pasquini, G. & Giaccone, G. C-MET inhibitors for advanced non-small cell lung cancer. Expert Opin. Investig. Drugs27, 363–375 (2018). 10.1080/13543784.2018.1462336 [DOI] [PubMed] [Google Scholar]
- 614.Recondo, G., Che, J., Jänne, P. A. & Awad, M. M. Targeting MET dysregulation in cancer. Cancer Discov.10, 922–934 (2020). 10.1158/2159-8290.CD-19-1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Drilon, A., Cappuzzo, F., Ou, S. I. & Camidge, D. R. Targeting MET in lung cancer: will expectations finally be MET? J. Thorac. Oncol.12, 15–26 (2017). 10.1016/j.jtho.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Forde, P. M. & Rudin, C. M. Crizotinib in the treatment of non-small-cell lung cancer. Expert Opin. Pharmacother.13, 1195–1201, (2012). 10.1517/14656566.2012.688029 [DOI] [PubMed] [Google Scholar]
- 617.Morris, T. A., Khoo, C. & Solomon, B. J. Targeting ROS1 rearrangements in non-small cell lung cancer: crizotinib and newer generation tyrosine kinase inhibitors. Drugs79, 1277–1286 (2019). 10.1007/s40265-019-01164-3 [DOI] [PubMed] [Google Scholar]
- 618.Abdelaziz, A. & Vaishampayan, U. Cabozantinib for the treatment of kidney cancer. Expert Rev. Anticancer Ther.17, 577–584 (2017). 10.1080/14737140.2017.1344553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Brose, M. S. et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.22, 1126–1138 (2021). 10.1016/S1470-2045(21)00332-6 [DOI] [PubMed] [Google Scholar]
- 620.Wu, Y. L. et al. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J. Clin. Oncol.36, 1405–1411 (2018). 10.1200/JCO.2017.75.5587 [DOI] [PubMed] [Google Scholar]
- 621.Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med.379, 54–63 (2018). 10.1056/NEJMoa1717002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622.Motzer, R. J. et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol.23, 888–898 (2022). 10.1016/S1470-2045(22)00290-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Kim, H. et al. Preclinical development of a humanized neutralizing antibody targeting HGF. Exp. Mol. Med.49, e309 (2017). 10.1038/emm.2017.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Modica, C. et al. A receptor-antibody hybrid hampering MET-driven metastatic spread. J. Exp. Clin. Cancer Res.40, 32 (2021). 10.1186/s13046-020-01822-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Huang, X. et al. The HGF-MET axis coordinates liver cancer metabolism and autophagy for chemotherapeutic resistance. Autophagy15, 1258–1279 (2019). 10.1080/15548627.2019.1580105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Engelman, J. A. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science316, 1039–1043 (2007). 10.1126/science.1141478 [DOI] [PubMed] [Google Scholar]
- 627.Suzuki, S. et al. KRAS inhibitor resistance in MET-amplified KRAS (G12C) non-small cell lung cancer induced by RAS- and non-RAS-mediated cell signaling mechanisms. Clin. Cancer Res.27, 5697–5707 (2021). 10.1158/1078-0432.CCR-21-0856 [DOI] [PubMed] [Google Scholar]
- 628.Aebersold, D. M. et al. Prevalence and clinical impact of Met Y1253D-activating point mutation in radiotherapy-treated squamous cell cancer of the oropharynx. Oncogene22, 8519–8523 (2003). 10.1038/sj.onc.1206968 [DOI] [PubMed] [Google Scholar]
- 629.Zou, X. et al. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: a review. Int. J. Biol. Macromol.202, 539–557 (2022). 10.1016/j.ijbiomac.2022.01.113 [DOI] [PubMed] [Google Scholar]
- 630.Pandey, P. et al. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed. Pharmacother.161, 114491 (2023). 10.1016/j.biopha.2023.114491 [DOI] [PubMed] [Google Scholar]
- 631.Conlon, K. C., Miljkovic, M. D. & Waldmann, T. A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res.39, 6–21 (2019). 10.1089/jir.2018.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Kim, I. K. et al. GM-CSF promotes antitumor immunity by inducing Th9 cell responses. Cancer Immunol. Res.7, 498–509 (2019). 10.1158/2326-6066.CIR-18-0518 [DOI] [PubMed] [Google Scholar]
- 633.Ushach, I. & Zlotnik, A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol.100, 481–489 (2016). 10.1189/jlb.3RU0316-144R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Van Overmeire, E. et al. M-CSF and GM-CSF receptor signaling differentially regulate monocyte maturation and macrophage polarization in the tumor microenvironment. Cancer Res.76, 35–42 (2016). 10.1158/0008-5472.CAN-15-0869 [DOI] [PubMed] [Google Scholar]
- 635.Urdinguio, R. G. et al. Immune-dependent and independent antitumor activity of GM-CSF aberrantly expressed by mouse and human colorectal tumors. Cancer Res.73, 395–405 (2013). 10.1158/0008-5472.CAN-12-0806 [DOI] [PubMed] [Google Scholar]
- 636.Parmiani, G. et al. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann. Oncol.18, 226–232 (2007). 10.1093/annonc/mdl158 [DOI] [PubMed] [Google Scholar]
- 637.Tian, H. et al. A novel cancer vaccine with the ability to simultaneously produce anti-PD-1 antibody and GM-CSF in cancer cells and enhance Th1-biased antitumor immunity. Signal Transduct. Target Ther.1, 16025 (2016). 10.1038/sigtrans.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Rangsitratkul, C. et al. Intravesical immunotherapy with a GM-CSF armed oncolytic vesicular stomatitis virus improves outcome in bladder cancer. Mol. Ther. Oncolyt.24, 507–521 (2022). 10.1016/j.omto.2022.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Thomas, S. et al. Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1. J. Immunother. Cancer7, 214 (2019). 10.1186/s40425-019-0682-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Varghese, B. et al. Invariant NKT cell-augmented GM-CSF-secreting tumor vaccine is effective in advanced prostate cancer model. Cancer Immunol. Immunother.71, 2943–2955 (2022). 10.1007/s00262-022-03210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Thorn, M. et al. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Ther.23, 188–198 (2016). 10.1038/cgt.2016.19 [DOI] [PubMed] [Google Scholar]
- 642.Cho, H. et al. Cancer-stimulated CAFs enhance monocyte differentiation and protumoral TAM activation via IL6 and GM-CSF secretion. Clin. Cancer Res.24, 5407–5421 (2018). 10.1158/1078-0432.CCR-18-0125 [DOI] [PubMed] [Google Scholar]
- 643.Kumar, A., Taghi Khani, A., Sanchez Ortiz, A. & Swaminathan, S. GM-CSF: a double-edged sword in cancer immunotherapy. Front. Immunol.13, 901277 (2022). 10.3389/fimmu.2022.901277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Anderson, K. S. et al. The feasibility of using an autologous GM-CSF-secreting breast cancer vaccine to induce immunity in patients with stage II-III and metastatic breast cancers. Breast Cancer Res. Treat.194, 65–78 (2022). 10.1007/s10549-022-06562-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Kong, Y. et al. PD-1 inhibitor combined with radiotherapy and GM-CSF (PRaG) in patients with metastatic solid tumors: an open-label phase II study. Front. Immunol.13, 952066 (2022). 10.3389/fimmu.2022.952066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Mody, R. et al. Irinotecan, temozolomide, and dinutuximab with GM-CSF in children with refractory or relapsed neuroblastoma: a report from the Children’s Oncology Group. J. Clin. Oncol.38, 2160–2169 (2020). 10.1200/JCO.20.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]