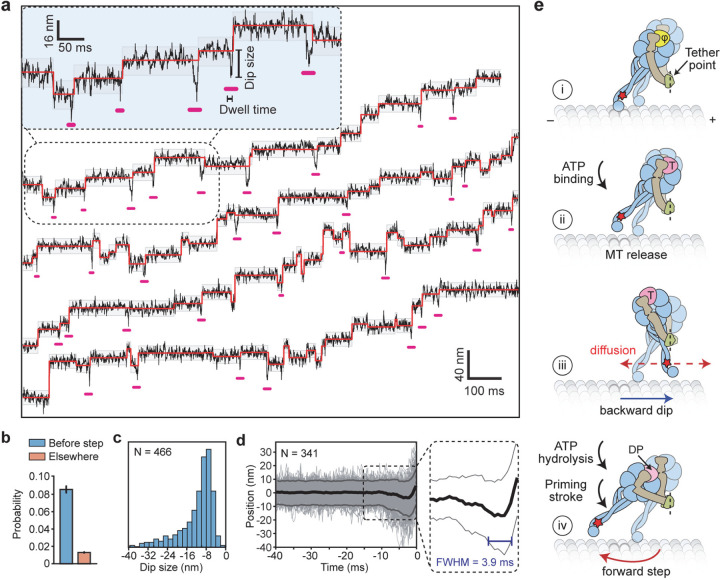
Figure 4 |. MINFLUX observes the dynamics of dynein between successive steps.
a, Example trajectories of dynein in 1 mM ATP at 0.28 ms temporal resolution show brief dips (underlined in pink) before a step. Shaded regions represent ±2σ of the dwelling position between successive steps. b, The probability of −2σ deviation from the dwelling position within 7 ms before a step or elsewhere in trajectories. c, Dips occur in the backward direction and range from 8–40 nm in size. d, The cumulative overlay of dynein before a step (t = 0 s) demonstrates the presence of a backward dip. Black and grey curves represent mean ± 2σ, respectively. The average duration of the dips was measured from the full-width half minimum (FWHM) of the −2σ deviation before steps (blue line in the zoomed region). e, Interpretation of the dips observed in MINFLUX trajectories. (i) Dynein is strongly bound to the MT in the nucleotide-free state (φ). (ii) Upon ATP binding (T), a dynein monomer releases from the MT and (iii) moves backward toward the tethering point at the dimerization domain before ATP is hydrolyzed (dashed red line). (iv) The linker undergoes the priming stroke after ATP hydrolysis (DP) and moves the MTBD towards the minus end before dynein rebinds the MT.