Abstract
Eating disorders (EDs) commonly co-occur with other psychiatric and neurodevelopmental disorders including attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD); however, the pattern of family history and genetic overlap among them requires clarification. This study investigated the diagnostic, familial, and genetic associations of EDs with ADHD and ASD. The nationwide population-based cohort study included all individuals born in Denmark, 1981–2008, linked to their siblings and cousins. Cox regression was used to estimate associations between EDs and ADHD or ASD, and mediation analysis was used to assess the effects of intermediate mood or anxiety disorders. Polygenic scores (PGSs) were used to investigate the genetic association between anorexia nervosa (AN) and ADHD or ASD. Significantly increased risk for any ED was observed following an ADHD [hazard ratio = 1.97, 95% confidence interval = 1.75–2.22] or ASD diagnosis [2.82, 2.48–3.19]. Mediation analysis suggested that intermediate mood or anxiety disorders could account for 44–100% of the association between ADHD or ASD and ED. Individuals with a full sibling or maternal halfsibling with ASD had increased risk of AN [1.54, 1.33–1.78; 1.45, 1.08–1.94] compared to those with siblings without ASD. A positive association was found between ASD-PGS and AN risk [1.06, 1.02–1.09]. In this study, positive phenotypic associations between EDs and ADHD or ASD, mediation by mood or anxiety disorder, and a genetic association between ASD-PGS and AN were observed. These findings could guide future research in the development of new treatments that can mitigate the development of EDs among individuals with ADHD or ASD.
Introduction
Eating disorders (EDs), including anorexia nervosa (AN), bulimia nervosa (BN), and eating disorder not otherwise specified (EDNOS), are severe psychiatric disorders associated with impaired quality of life and high comorbidity and relative mortality1. EDs co-occur with neurodevelopmental conditions including attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD)2, as well as mood—including major depressive and bipolar disorder—and anxiety disorders3. The lifetime prevalence of ADHD in EDs has been reported to range between 1.6% and 18%, while the lifetime prevalence of ASD in EDs has been reported to be on average 4.7%2. Mood and anxiety disorders have been reported to be the most prevalent psychiatric comorbidities in individuals with EDs3, 4 with comorbidity rates of up to 54% and 62%, respectively4. Individuals with EDs and co-occurring ADHD or ASD often experience more severe ED symptoms, need longer time to recover, and have a longer duration of inpatient treatment than individuals with EDs without co-occurring neurodevelopmental conditions5–7.
The co-occurrence of EDs with ADHD and ASD suggests potential etiologic overlap across these disorders. Familial and genetic studies have investigated the source of the overlap, and results have been mixed. Twin studies have reported low cross-twin, cross-trait correlations between eating problems and ADHD and/or ASD8, and a twin-based genetic correlation of 0.35 between current ADHD symptoms and lifetime binge-eating behaviour was observed9. A Swedish study reported familial co-aggregation and a genetic association between ADHD and EDs, which was weaker for AN than for other EDs10. In recent large genome-wide association studies (GWASs) for ADHD11, 12, ASD13, and AN14, risk loci have been identified for all three disorders. Despite comorbidity between AN and ADHD, a significant negative11 or no12 genetic correlation after Bonferroni correction was reported while no significant genetic correlation after Bonferroni correction was found between AN and ASD13, 14. An ADHD polygenic score (PGS) showed a significant positive association with binge-eating disorder, and an ASD-PGS was negatively associated with AN, although this finding was not significant15. Frequent co-occurrence of mood and anxiety disorders with EDs16, 17, ADHD, and ASD also suggest potential etiologic overlap, but to our knowledge, no studies have examined the role of intermediate mood or anxiety disorders on ED-ADHD-ASD associations.
To address these knowledge gaps, we conducted a Danish register-based cohort study of patterns of diagnostic co-occurrence between EDs and ADHD or ASD, including an exploration of the role of intermediate mood or anxiety disorders on the diagnostic patterns, and shared familial and genetic factors.
Materials and Methods
Study population
The study population comprised all individuals born in Denmark between May 1, 1981, and December 31, 2008, who were alive and residing in Denmark on their sixth birthday (N = 1,690,087). Individuals whose parents could not be identified in the Danish Civil Registration System18 (CRS; N = 11,512) or who were adopted (N = 6,752) were excluded, resulting in a final sample of 1,671,823 individuals. We used data from the CRS to link these individuals to their full siblings, maternal and paternal half siblings, and cousins within the study population. We restricted family linkage to individuals within the study population so that the same diagnostic system (International Classifications of Disease-Tenth Edition, ICD-10) and time frame for diagnosis (1994 and onwards) applied to all family members. Information on diagnoses before 1994 was not included. Consequently, some cases were not captured, and some cases are not incidence. Linkage was achieved via the unique personal identification number assigned to all Danish citizens.
Outcome and covariate data
Each cohort member and relative was followed until December 31, 2016, for a reported ICD-10 diagnosis of AN, BN, EDNOS, any ED, ADHD, ASD, mood disorder, or anxiety disorder as described in Table 1. Individuals were considered being at risk of ADHD and ASD from birth and EDs, mood, and anxiety disorders from age 6. The EDNOS case group includes EDs such as binge-eating disorder, avoidant/restrictive food intake disorder (ARFID), and pica. EDNOS is no longer recognized in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)19, but it is still used in ICD-10. Diagnostic information was obtained from the Danish National Patient Register20, 21 and Danish Psychiatric Central Research Register22. These registers contain diagnoses from all inpatient contacts before 1995 and both in- and outpatient contacts from 1995 onwards. Information on sex, birth year, death, and emigration was obtained from the CRS18.
Genetic data
Genotypes were generated from samples obtained from the Danish Neonatal Screening Biobank, which stores dried blood spots taken after birth from nearly all infants born in Denmark since 1981. The samples used in this study were originally genotyped for The Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH)23, a large-scale genetic study nested within our study population, and Danish cohorts of the Anorexia Nervosa Genetics Initiative (ANGI)24 and the Eating Disorders Genetics Initiative (EDGI)25, which used the same sampling method as iPSYCH. Possible batch effects were captured by a sample cohort variable. For this study, we included individuals with AN, ADHD, and ASD from the iPSYCH2015, ANGI, and EDGI cohorts as described in Table 1. None of these cohorts specifically sampled BN and EDNOS, and genetic analyses therefore focused on associations with AN only. Controls were drawn from a sub-cohort that included a random 2% subsample of the birth population without the disorders of interest. PGSs for AN14, ADHD11, and ASD13 were calculated using LDpred226 and GWAS summary statistics excluding Danish samples. PGSs were standardized by subtracting the mean and dividing by the standard deviation (SD) calculated using controls only.
Statistical analysis
Analyses described in the following paragraphs were conducted using Stata software version 16.
Co-occurrence risks between ADHD or ASD and EDs
We first estimated hazard ratios (HRs) with 95% confidence intervals (CIs) for each ED following ADHD or ASD diagnosis using Cox proportional hazard models, adjusted for age as the underlying time scale, birth year as a categorical covariate, and stratified by the registered sex assigned at birth, except if an individual had their registered sex changed (henceforth referred to as sex) in the underlying hazard. ADHD and ASD were handled as time-varying exposures, meaning individuals were considered unexposed until receiving an ADHD or ASD diagnosis. The rate of an ED diagnosis in the exposed group was compared with a same-age unexposed group. Individuals were followed from their sixth birthday until death, emigration, ED diagnosis, or December 31, 2016, whichever came first. In the registers, ED diagnoses are occasionally registered before ADHD or ASD diagnosis. The analysis was consequently also conducted with EDs as time-varying exposures and ADHD or ASD as outcomes. In all Cox regression models, a cluster-robust sandwich estimator was used for standard errors (SEs) and CIs to account for dependencies between individuals with the same mother.
We performed sensitivity analyses to examine the reliability of our co-occurrence risks results. Analyses with mutually adjusted ADHD and ASD were conducted to assess whether an association between EDs and ADHD was explained by comorbidity with ASD and vice versa. In the iPSYCH2015 cohort, a narrower definition of ADHD (F90.0) was used. Thus, a sensitivity analysis narrowing the definition of ADHD to individuals diagnosed with disturbance of activity and attention was performed.
Influence of mood and anxiety disorders on risk for ED following ADHD or ASD diagnosis
To assess the potential mediating effects of intermediate mood or anxiety disorders on the association between ADHD or ASD as exposures and EDs as outcomes, an analysis using mood or anxiety disorders as an additional time-varying covariate was performed. We performed four-way decomposition mediation analysis27 with mood or anxiety disorders as potential time-dependent mediators between ADHD or ASD (as exposure, assumed to be present from start of follow-up at age 6) and EDs (as outcome) to estimate the direct effect of ADHD or ASD on ED risk, and the indirect effect mediated by intermediate mood or anxiety disorders. Only mood and anxiety disorders diagnosed prior to EDs were included, but they could be present from the start of follow-up.
Familial coaggregation analysis
We identified all possible pairs of full siblings, half siblings, and cousins in the study population. The diagnostic status of each person was evaluated at the end of follow-up. Using logistic regression, we estimated odds ratios (ORs) with 95% CIs of EDs among individuals with each type of relative diagnosed with either ADHD or ASD compared to individuals with the same type of relative not diagnosed with that disorder. All logistic regression models were adjusted for both the index person’s and relative’s sex and birth year.
Cross-disorder polygenic association
We estimated the association between ADHD-PGS or ASD-PGS and the risk of AN diagnosis, and vice versa. ORs per SD increase in PGS and per quintile (with the lowest quintile as reference) were estimated in a weighted logistic regression model using the Kalbfleisch and Lawless estimator28 with robust SEs (where weights are 1 for individuals with AN, ADHD or ASD diagnosis whereas weights for controls from the sub-cohort were calculated as the inverse probability of being selected for the sub-cohort23). All analyses were adjusted for sex, birth year, sample cohort variable, and population stratification using the first five genetic principal components29, 30. We were interested in obtaining estimates of the association between the ADHD-PGS or the ASD-PGS with AN diagnosis accounting for MDD genetics because MDD frequently co-occur with AN, ADHD and ASD. Hence, we carried out sensitivity analysis in which we included MDD-PGS31 as a covariate in the model. AN was split into AN without lifetime BN and AN with lifetime BN as proxy measures of AN subtype to examine whether ADHD or ASD PGS associations varied between these AN diagnostic subtypes (i.e., characterized by the presence/absence of binge eating and purging). The GWASs used to calculate PGSs were based on individuals with European ancestry. Thus, we performed a sensitivity analysis limiting our study population to individuals with European ancestry.
Ethics and data approvals
The iPSYCH201523, ANGI24, and EDGI25 studies were approved by the Danish Scientific Ethics Committee, the Danish Health Data Authority, the Danish Data Protection Agency, and Danish Newborn Screening Biobank Steering Committee. The Danish Scientific Ethics Committee, in accordance with Danish legislation, has waived the need for informed consent in biomedical research based on existing biobanks. The study was approved by the Danish Data Protection Agency, and data access was approved by Statistics Denmark and the Danish Health Data Authority. All data were de-identified and not recognizable at an individual level.
Results
The study population comprised 1,671,823 individuals (814,047 females and 857,776 males). Supplementary Fig. 1 shows the cumulative incidence of EDs, ADHD, and ASD by sex and age. The prevalence of any ED overall was 0.94%; individuals with either ADHD or ASD had a higher prevalence of any ED (2.01% and 2.57%, respectively) compared to individuals without ADHD or ASD (0.91% in both groups). Some case groups are overlapping as individuals could be diagnosed with multiple EDs and both ADHD and ASD. More information on the demographics of the Danish population can be found elsewhere32, 33.
Co-occurrence risk between ADHD or ASD and EDs
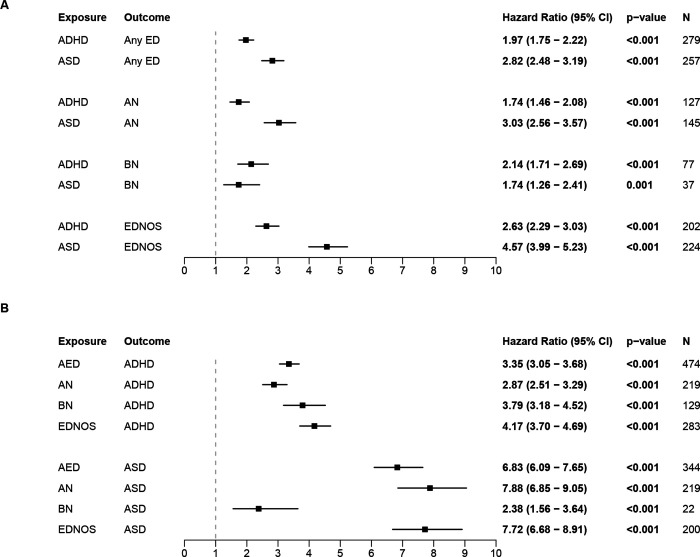
Increased risk for all EDs were observed in individuals first diagnosed with either ADHD or ASD (Fig. 1A). Further, individuals in each ED diagnostic group (i.e., Any ED, AN, BN, EDNOS) had an increased risk of a subsequent ADHD or ASD diagnosis (Fig. 1B). Adjusting for co-occurring ADHD and ASD in individuals only slightly attenuated associations with a later ED diagnosis (Supplementary Table 1). Individuals with co-occurring ADHD and ASD were thus included in both the ADHD and the ASD case groups. The time between first and second diagnoses was shorter among individuals diagnosed with an ED prior to ASD than those diagnosed with ASD first (Supplementary Table 2); this pattern was not observed between ADHD and EDs. Also, narrowing the definition of ADHD to individuals diagnosed with F90.0 did not change the results (Supplementary Table 3).
Figure 1.
Hazard ratios for within-individual associations. A) Association between prior ADHD or ASD and subsequent EDs. B) Association between prior EDs and subsequent ADHD or ASD.
Influence of mood and anxiety disorders on risk for ED following ADHD or ASD diagnosis
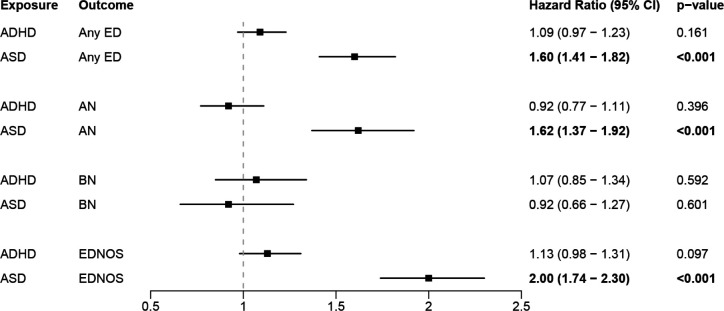
Adding mood or anxiety disorders as a covariate reduced all ED risks following ADHD or ASD diagnosis and led to non-significant associations between all EDs (including the ‘Any ED’ category) following ADHD as time-varying exposure, and between BN following ASD as time-varying exposures (Fig. 2).
Figure 2.
Hazard ratios for within-individual associations between prior ADHD or ASD and subsequent EDs, adjusted for presence of mood disorders and/or anxiety disorders.
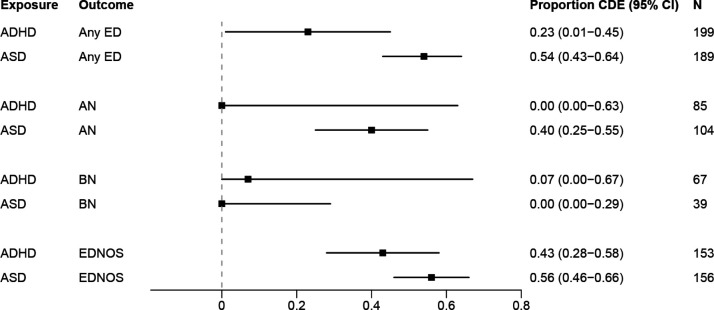
The mediation analysis showed that the estimated direct association between ADHD and AN or BN and between ASD and BN were small when mood or anxiety disorders were included as a mediator (Fig. 3). For associations between all other EDs (including the ‘Any ED’ category) and ADHD or ASD, the estimated direct effects ranged from 23 to 56% when mood or anxiety disorders were included as a mediator between ADHD or ASD diagnosis and later EDs.
Figure 3.
Results from four-way decomposition mediation analysis with mood and/or anxiety disorders as mediator. Proportion of association attributed to the controlled direct effect (CDE).
Familial co-aggregation
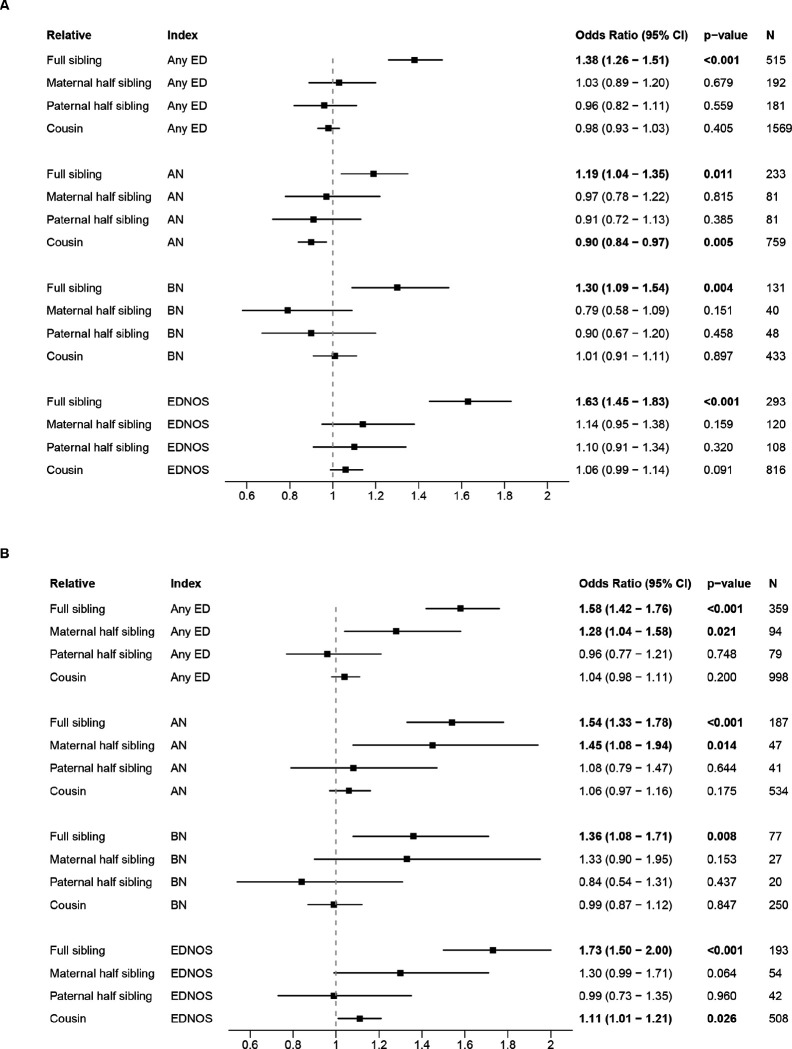
The number of individuals linked to at least one of each relative type was: full sibling, 1,303,017 (77.94%); maternal half sibling, 249,130 (14.90%); paternal half sibling, 270,798 (16.20%); cousin 1,259,351, (75.33%). Individuals from the cohort with a full sibling diagnosed with ADHD or ASD had increased risks of all EDs compared to individuals with a sibling not diagnosed with that disorder (Figs. 4A and 4B, respectively). Also, individuals with a maternal half sibling with ASD had an increased risk of any ED and AN (Fig. 4B). A decreased AN risk for individuals with a cousin diagnosed with ADHD and an increased EDNOS risk for individuals with a cousin diagnosed with ASD was observed.
Figure 4.
Hazard ratios for familial coaggregation associations. A) Association between relative ADHD and index person ED. B) Association between relative ASD and index person ED.
Cross-disorder polygenic association
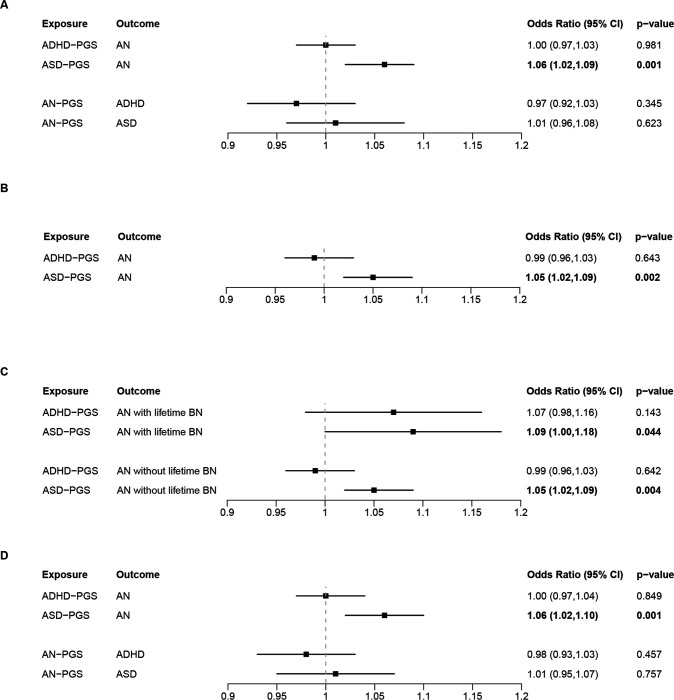
Individuals with AN had a higher ASD-PGS than controls, whereas we found no difference in ADHD-PGS between individuals with AN and controls (Fig. 5A; see Supplementary Figs. 2 for quintile plots). Furthermore, no differences in AN-PGS were observed for ADHD or ASD cases compared with controls. Including MDD-PGS as a covariate did not alter the main results (Fig. 5B). Splitting individuals with AN into those with a lifetime BN diagnosis versus those without did not significantly change the association between ADHD-PGS/ASD-PGS and AN (Fig. 5C). Limiting the cohort to individuals of European ancestry also did not alter the results (Fig. 5D). Of note, the proportion of individuals with European ancestry was 90.09%, 90.47% and 89.74% in the analyses for AN, ADHD or ASD as the outcome, respectively.
Figure 5.
Odds ratios for within-individual associations between PGSs and diagnoses. A) Associations between AN-PGS and diagnosis of ADHD or ASD, and vice versa. B) Associations between ASD-PGS and diagnosis of AN, adjusted for MDD-PGS. C) Associations between ADHD-PGS or ASD-PGS and diagnosis of AN with and without lifetime BN. D) Associations between AN-PGS and diagnosis of ADHD or ASD, and vice versa. Cohort limited to individuals with European ancestry.
Discussion
In this nationwide, population-based study, we found significant co-occurrence risk for EDs with either ADHD or ASD. Further, we report the novel finding that the risk of ADHD or ASD and later EDs is strongly mediated by intermediate mood or anxiety disorders. We also observed consistent cross-disorder risks for all combinations EDs and ADHD or ASD among full siblings, indicating that familial factors may contribute to the co-occurrence risks. Increased risk of AN in individuals with a maternal half sibling with ASD indicates that genetic factors may contribute to the co-occurrence risk. This association between ASD and AN could potentially be explained by current measures of common genetic variation.
We found bidirectional associations between EDs and ADHD or ASD, although the strength of the associations varied by ED diagnosis. Regardless of which disorder was diagnosed first, there was an overall stronger association between ADHD and BN or EDNOS than between ADHD and AN. Similarly, we observed a stronger association between ASD and AN or EDNOS than between ASD and BN. These findings are consistent with a previous study10 that reported a stronger association between ADHD and EDs other than AN, as well as a systematic review demonstrating that ASD is more common in individuals with AN than individuals with other EDs2.
The association between EDNOS and ASD was stronger compared to associations between ASD and AN or BN. This could be explained in part by a lower age at EDNOS diagnosis among individuals with an ASD diagnosis, suggesting that some individuals with ASD likely had symptoms pertaining to feeding from infancy34 that could have been classified as EDNOS. This finding is consistent with clinical studies reporting a high prevalence of ARFID among individuals with ASD35, and higher co-occurrence between ASD and ARFID than between ASD and AN or BN36. This association has important clinical implications, as individuals with ARFID restrict food intake due to sensory aspects of food, a lack of interest in food and/or fear of negative consequences when eating or a combination of these symptoms, but typically restriction is not motivated by a fear of weight gain as seen in AN37. A Swedish twin study estimated the ARFID population prevalence to be 2.0%38, which could be comparable in the Danish population. Although ARFID was formally recognized in DSM-5 in 2013, ARFID has no unique ICD-10 code in the Danish registers and is classified under EDNOS (F50.8-F50.9). It is thus not possible to distinguish ARFID in the Danish registers.
Although we observed strong associations between EDs as exposures and ADHD or ASD as the outcome, we urge caution when interpreting the order of diagnosis. Although ADHD and ASD are shown to be neurodevelopmental in origin and have an early-life onset, there is considerable variation in the age at diagnosis, with additional differences by sex (Supplementary Fig. 1). In females, there is a marked increase in the rate of new diagnoses of ADHD and ASD during early to mid-adolescence, whilst males are more commonly diagnosed during childhood39. As adolescent rates of EDs also increase among females33, the associations between EDs and subsequent ADHD or ASD may reflect these age- and sex-specific diagnostic patterns, influenced by the effects of both social and biological changes in early adolescence. These could also be milder cases of ADHD/ASD that are detected later in life under more careful examination when the patients are evaluated for their ongoing ED. Low counts of individuals in some ED groups prevented sex specific analysis.
A novel finding of this study is that most of the associations observed between ADHD or ASD as exposure and later ED were largely accounted for by the mediating effects of intermediate mood or anxiety disorders. That is, individuals with ADHD or ASD were more likely to develop a mood/anxiety disorder, which in turn increased their risk of developing an ED. Our results suggest that intermediate mood and anxiety disorders could account for 44% or more of subsequent ED diagnoses in people with ADHD or ASD. This is consistent with past research indicating that relationships between EDs and neurodevelopmental disorders can be mediated by mood- and stress-related factors including alexithymia, negative affect, and emotion dysregulation40, 41. Taken together, this association points to a potential intervention opportunity to mitigate ED development in individuals with ADHD or ASD by rapidly detecting and treating co-occurring mood or anxiety disorders.
Our results also showed that individuals who had a full sibling with ADHD or ASD had increased risk for all EDs. Also, individuals with a maternal half sibling with ASD had an increased risk of any ED and AN, which supports the presence of a shared genetic influence between ASD and AN13, 14. Although no statistically significant associations were observed between other relatives diagnosed with ADHD and increased ED risk in the index person, our results do not contradict findings in the Swedish population study showing a positive correlation between individuals who had a maternal half sibling with ADHD and increased risk of EDs other than AN10. The wide confidence intervals should warrant caution when interpreting these results, which could suggest that our analyses of half siblings may be underpowered compared to the Swedish study.
We found no associations between ADHD-PGS and AN diagnosis, or vice versa, indicating that the positive diagnostic correlations do not appear to be due to shared genetic influence, at least as measured by current PGSs. While early studies of genetic correlations between AN and ADHD found a modest inverse correlation11, 14 or a positive correlation15, recent studies with improved statistical power found no such correlation12, 42. The positive association between ASD-PGS and AN diagnosis supports the hypothesis that AN and ASD may have a shared genetic architecture. Our results align with prior studies showing small but positive genetic correlations between AN and ASD13, 14, although a negative correlation has also been reported15. The small genetic correlation between AN and ASD and the lack of a genetic correlation between AN and ADHD could be due to insufficient sample size (both in the discovery GWASs and our study) and PGSs only capturing common genetic variation at single nucleotide polymorphism level. Of note, a recent examination of the genetic architecture of major psychiatric disorders revealed low genetic correlation between AN and ADHD or ASD43, suggesting that AN is not a neurodevelopmental disorder like ADHD and ASD.
We were unable to examine AN restricting versus AN binge-eating/purging subtypes separately since subtype data are not available in Danish registers. Examining individuals with AN with versus without a lifetime BN diagnosis as a proxy measure for AN subtypes yielded slightly higher estimates for associations between AN with lifetime BN and ADHD-PGS, suggesting that ADHD could be more strongly associated with the AN binge-eating/purging subtype than the restricting subtype, as suggested in previous studies7, 44.
Strengths and Limitations
This study used nationwide registers that only capture diagnoses reported by specialists in hospital settings. Therefore, individuals diagnosed in primary care, with subthreshold disorders, or who are not in contact with hospital-based specialists were not captured. The true prevalence of EDs is expected to be higher in the Danish population. Danish registry data on psychiatric diagnoses generally have good validity with high positive predictive values45, 46. ED diagnoses in the Danish registers have not been validated, although EDs from highly similar Swedish register data show good validity47. The registers only provide data on the date of first diagnosis, not the actual time of onset. Some degree of detection bias is probable, and the very high incidence of ASD diagnosis subsequent to ED diagnosis may in part be due to this detection bias or reflect the impact of starvation on cognitive and social functioning in females with undiagnosed ASD.
No large GWASs for EDs other than AN are currently available, therefore not allowing the inclusion of PGSs for BN and EDNOS. Further, current PGSs are underpowered and capture only a fraction of the estimated heritability due to existing GWAS sample sizes. Of note, Danish contributions constitute a large proportion of available GWAS training samples for AN, ADHD, and ASD. Therefore, exclusion of Danish samples from the training samples further limits the power of PGSs based on summary statistics. Replication of the PGS analysis will be necessary as larger GWASs and PGSs with increased power become available.
Conclusion
We observed a strong and consistent diagnostic overlap between EDs and ADHD or ASD, which strongly points to the role of genetics in the association between ASD-PGS and AN. First, in analyses serving as a proxy for genetic liability by PGSs, only the ASD-PGS showed a positive association with AN. Second, we observed significant associations between any ED or AN and ASD for maternal half siblings in family analysis, as well as co-aggregation in full siblings for all EDs and ADHD or ASD. Overall, these results highlight considerable gaps in our knowledge of the neurodevelopmental mechanisms linking EDs to ADHD and ASD. Nevertheless, our mediation analysis results underscore that the associations between EDs and ADHD or ASD appear to be strongly mediated by intermediate mood or anxiety disorders. These findings highlight opportunities to mitigate the development of EDs among individuals with ADHD or ASD by screening and treating emergent mood and anxiety disorders as early as possible.
Acknowledgements
The authors would like to thank the Psychiatric Genomics Consortium and the research participants and employees of 23andMe, Inc. for providing the summary statistics used to generate the polygenic scores.
The study was supported by the Novo Nordisk Foundation (grant no. NNF20OC0064993).
The iPSYCH team was supported by grants from the Lundbeck Foundation (grant no. R102-A9118, R155-2014-1724, and R248-2017-2003), NIH/NIMH (1U01MH109514-01 and 1R01MH124851-01 to ADB) and the Universities and University Hospitals of Aarhus and Copenhagen. High-performance computer capacity for handling and statistical analysis of iPSYCH data on the GenomeDK HPC facility was provided by the Center for Genomics and Personalized Medicine and the Centre for Integrative Sequencing, iSEQ, Aarhus University, Denmark (grant to ADB). The Anorexia Nervosa Genetics Initiative (ANGI) was an initiative of the Klarman Family Foundation, and additional anorexia nervosa genotyping was supported by grant from the Lundbeck Foundation (grant no. R276-2018-4581). ZY acknowledges grant support from the Independent Research Fund Denmark (DFF, Sapere Aude, grant no. 1052-00029B). CMB Is supported by NIMH (R56MH129437; R01MH120170; R01MH124871; R01MH119084; R01MH118278); Swedish Research Council (Vetenskapsrådet, award: 538-2013-8864); Lundbeck Foundation (Grant no. R276-2018-4581). BJV and CA were supported by a Lundbeck Foundation Fellowship (R335-2019-2339) and by the Danish National Research Foundation (Niels Bohr Professorship to Prof. John McGrath). DD is supported by the Novo Nordisk Foundation (NNF20OC0065561 and NNF21SA0072102), the Lundbeck Foundation (R344-2020-1060) the European Union’s Horizon 2020 research and innovation program under grant agreement No. 965381 (TIMESPAN).
Footnotes
Conflict of interest
Dr. Bulik is an author and royalty recipient from Pearson. D.D. had received speaker fee from Medici Nordic. The remaining authors declare no conflict of interest.
Table 1
Table 1 is available in the Supplementary Files section.
Contributor Information
Liselotte Petersen, Aarhus University.
Cynthia Bulik, University of North Carolina at Chapel Hill.
Jakob Grove, Aarhus University.
Isabell Brikell, Karolinska Institutet.
Christopher Hubel, Aahus University, Denmark.
Bjarni Vilhjálmsson, Aarhus University.
Anders Borglum, Aarhus University.
Ditte Demontis, Aarhus University.
Preben Mortensen, Aarhus University.
References
- 1.van Hoeken D, Hoek HW. Review of the burden of eating disorders: mortality, disability, costs, quality of life, and family burden. Curr Opin Psychiatry 2020; 33(6): 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nickel K, Maier S, Endres D, Joos A, Maier V, Tebartz van Elst L et al. Systematic Review: Overlap Between Eating, Autism Spectrum, and Attention-Deficit/Hyperactivity Disorder. Front Psychiatry 2019; 10: 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plana-Ripoll O, Musliner KL, Dalsgaard S, Momen NC, Weye N, Christensen MK et al. Nature and prevalence of combinations of mental disorders and their association with excess mortality in a population-based cohort study. World Psychiatry 2020; 19(3): 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hambleton A, Pepin G, Le A, Maloney D, National Eating Disorder Research C, Touyz S et al. Psychiatric and medical comorbidities of eating disorders: findings from a rapid review of the literature. J Eat Disord 2022; 10(1): 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen S, Anckarsäter H, Gillberg C, Gillberg C, Råstam M, Wentz E. Effects of autism spectrum disorders on outcome in teenage-onset anorexia nervosa evaluated by the Morgan-Russell outcome assessment schedule: a controlled community-based study. Mol Autism 2015; 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchanturia K, Adamson J, Leppanen J, Westwood H. Characteristics of autism spectrum disorder in anorexia nervosa: A naturalistic study in an inpatient treatment programme. Autism 2019; 23(1): 123–130. [DOI] [PubMed] [Google Scholar]
- 7.Svedlund NE, Norring C, Ginsberg Y, von Hausswolff-Juhlin Y. Symptoms of Attention Deficit Hyperactivity Disorder (ADHD) among adult eating disorder patients. BMC Psychiatry 2017; 17(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastam M, Taljemark J, Tajnia A, Lundstrom S, Gustafsson P, Lichtenstein P et al. Eating problems and overlap with ADHD and autism spectrum disorders in a nationwide twin study of 9- and 12-year-old children. TheScientificWorldJournal 2013; 315429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capusan AJ, Yao S, Kuja-Halkola R, Bulik CM, Thornton LM, Bendtsen P et al. Genetic and environmental aspects in the association between attention-deficit hyperactivity disorder symptoms and binge-eating behavior in adults: a twin study. Psychol Med 2017; 47(16): 2866–2878. [DOI] [PubMed] [Google Scholar]
- 10.Yao S, Kuja-Halkola R, Martin J, Lu Y, Lichtenstein P, Norring C et al. Associations Between Attention-Deficit/Hyperactivity Disorder and Various Eating Disorders: A Swedish Nationwide Population Study Using Multiple Genetically Informative Approaches. Biol Psychiatry 2019; 86(8): 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 2019; 51(1): 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demontis D, Walters GB, Athanasiadis G, Walters R, Therrien K, Nielsen TT et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat Genet 2023; 55(2): 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet 2019; 51(3): 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson HJ, Yilmaz Z, Thornton LM, Hubel C, Coleman JRI, Gaspar HA et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet 2019; 51(8): 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubel C, Abdulkadir M, Herle M, Loos RJF, Breen G, Bulik CM et al. One size does not fit all. Genomics differentiates among anorexia nervosa, bulimia nervosa, and binge-eating disorder. Int J Eat Disord 2021; 54(5): 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry 2011; 68(7): 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon-Lipkin E, Marvin AR, Law JK, Lipkin PH. Anxiety and Mood Disorder in Children With Autism Spectrum Disorder and ADHD. Pediatrics 2018; 141(4). [DOI] [PubMed] [Google Scholar]
- 18.Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011; 39(7 Suppl): 22–25. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edn: Washington, DC, 2013.
- 20.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011; 39(7 Suppl): 30–33. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7: 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health 2011; 39(7 Suppl): 54–57. [DOI] [PubMed] [Google Scholar]
- 23.Bybjerg-Grauholm J, Bøcker Pedersen C, Bækvad-Hansen M, Giørtz Pedersen M, Adamsen D, Søholm Hansen C et al. The iPSYCH2015 Case-Cohort sample: updated directions for unravelling genetic and environmental architectures of severe mental disorders. medRxiv 2020: 2020.2011.2030.20237768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton LM, Munn-Chernoff MA, Baker JH, Jureus A, Parker R, Henders AK et al. The Anorexia Nervosa Genetics Initiative (ANGI): Overview and methods. Contemp Clin Trials 2018; 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulik CM, Thornton LM, Parker R, Kennedy H, Baker JH, MacDermod C et al. The Eating Disorders Genetics Initiative (EDGI): study protocol. BMC Psychiatry 2021; 21(1): 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Privé F, Arbel J, Vilhjálmsson BJ. LDpred2: better, faster, stronger. Bioinformatics 2020; 36(22–23): 5424–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 2014; 25(5): 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalbfleisch JD, Lawless JF. Likelihood analysis of multi-state models for disease incidence and mortality. Stat Med 1988; 7(1–2): 149–160. [DOI] [PubMed] [Google Scholar]
- 29.Privé F, Aschard H, Ziyatdinov A, Blum MGB. Efficient analysis of large-scale genome-wide data with two R packages: bigstatsr and bigsnpr. Bioinformatics 2018; 34(16): 2781–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Privé F, Luu K, Blum MGB, McGrath JJ, Vilhjálmsson BJ. Efficient toolkit implementing best practices for principal component analysis of population genetic data. Bioinformatics 2020; 36(16): 4449–4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 2019; 22(3): 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen CB, Mors O, Bertelsen A, Waltoft BL, Agerbo E, McGrath JJ et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry 2014; 71(5): 573–581. [DOI] [PubMed] [Google Scholar]
- 33.Zerwas S, Larsen JT, Petersen L, Thornton LM, Mortensen PB, Bulik CM. The incidence of eating disorders in a Danish register study: Associations with suicide risk and mortality. J Psychiatr Res 2015; 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emond A, Emmett P, Steer C, Golding J. Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics 2010; 126(2): e337–342. [DOI] [PubMed] [Google Scholar]
- 35.Koomar T, Thomas TR, Pottschmidt NR, Lutter M, Michaelson JJ. Estimating the Prevalence and Genetic Risk Mechanisms of ARFID in a Large Autism Cohort. Front Psychiatry 2021; 12: 668297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicely TA, Lane-Loney S, Masciulli E, Hollenbeak CS, Ornstein RM. Prevalence and characteristics of avoidant/restrictive food intake disorder in a cohort of young patients in day treatment for eating disorders. J Eat Disord 2014; 2(1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brede J, Babb C, Jones C, Elliott M, Zanker C, Tchanturia K et al. “For Me, the Anorexia is Just a Symptom, and the Cause is the Autism”: Investigating Restrictive Eating Disorders in Autistic Women. J Autism Dev Disord 2020; 50(12): 4280–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinkler L, Wronski ML, Lichtenstein P, Lundstrom S, Larsson H, Micali N et al. Etiology of the Broad Avoidant Restrictive Food Intake Disorder Phenotype in Swedish Twins Aged 6 to 12 Years. JAMA Psychiatry 2023; 80(3): 260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalsgaard S, Thorsteinsson E, Trabjerg BB, Schullehner J, Plana-Ripoll O, Brikell I et al. Incidence Rates and Cumulative Incidences of the Full Spectrum of Diagnosed Mental Disorders in Childhood and Adolescence. JAMA Psychiatry 2020; 77(2): 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vuillier L, Carter Z, Teixeira AR, Moseley RL. Alexithymia may explain the relationship between autistic traits and eating disorder psychopathology. Mol Autism 2020; 11(1): 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Archi S, Cortese S, Ballon N, Réveillère C, De Luca A, Barrault S et al. Negative Affectivity and Emotion Dysregulation as Mediators between ADHD and Disordered Eating: A Systematic Review. Nutrients 2020; 12(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero C, Werme J, Jansen PR, Gelernter J, Stein MB, Levey D et al. Exploring the genetic overlap between twelve psychiatric disorders. Nat Genet 2022. [DOI] [PubMed] [Google Scholar]
- 43.Grotzinger AD, Mallard TT, Akingbuwa WA, Ip HF, Adams MJ, Lewis CM et al. Genetic architecture of 11 major psychiatric disorders at biobehavioral, functional genomic and molecular genetic levels of analysis. Nat Genet 2022; 54(5): 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sala L, Martinotti G, Carenti ML, Romo L, Oumaya M, Pham-Scottez A et al. Attention-deficit/hyperactivity disorder symptoms and psychological comorbidity in eating disorder patients. Eat Weight Disord 2018; 23(4): 513–519. [DOI] [PubMed] [Google Scholar]
- 45.Lauritsen MB, Jorgensen M, Madsen KM, Lemcke S, Toft S, Grove J et al. Validity of childhood autism in the Danish Psychiatric Central Register: findings from a cohort sample born 1990–1999. J Autism Dev Disord 2010; 40(2): 139–148. [DOI] [PubMed] [Google Scholar]
- 46.Mohr-Jensen C, Vinkel Koch S, Briciet Lauritsen M, Steinhausen HC. The validity and reliability of the diagnosis of hyperkinetic disorders in the Danish Psychiatric Central Research Registry. Eur Psychiatry 2016; 16–24. [DOI] [PubMed] [Google Scholar]
- 47.Birgegård A, Forsén Mantilla E, Dinkler L, Hedlund E, Savva A, Larsson H et al. Validity of eating disorder diagnoses in the Swedish national patient register. J Psychiatr Res 2022; 150: 227–230. [DOI] [PubMed] [Google Scholar]