Important concepts in obstetrics and their clinical implications are increasingly guided by evidence and in many cases by the gold standard—randomised clinical trials. This article focuses on key areas of changing practice chosen because they represent important changes in approach and demonstrate the increasing use of evidence in obstetrics.
Methods
The information I selected for this article was chosen on the basis of a poll I made of the 12 practitioners in maternal fetal medicine at the University of Pittsburgh and from conversations with colleagues from around the United States.
Infections in obstetrics
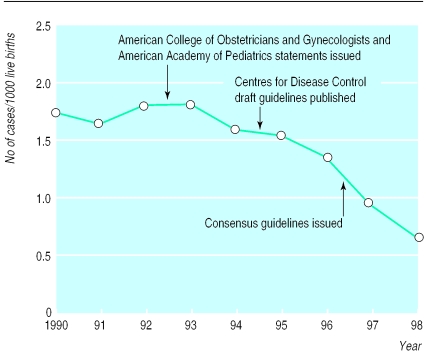
Group B streptococcal infections are a major source of morbidity for full term and preterm infants.1 Neonatal infection is caused by pathogens passed from the mother by vertical transmission. These organisms are exquisitely sensitive to penicillin. These observations have prompted several strategies to treat mothers during childbirth. The approach currently recommended by the American College of Obstetrics and Gynecology is intrapartum treatment with intravenous benzylpenicillin (5 million units, then 2.5 million units every 4 hours) or ampicillin (2 g, then 1 g every 4 hours) until delivery, based on either intrapartum risk (fever, prolonged rupture of membranes, or imminent preterm delivery) or screening for carriage of streptococci at 35-37 weeks' gestation. A recent survey indicates that intrapartum antibiotic treatment guided by this identification of risk has reduced the incidence of early onset neonatal infection with group B streptococci by 65% (figure).1 This is projected to prevent 200 neonatal deaths a year in the United States.1 Thus, a major educational effort can modify obstetric practice and save lives. The implications of the potential modification of maternal, neonatal, and hospital flora await longer term evaluation.
Recent advances
Intrapartum prophylaxis for group B streptococcus prevents neonatal deaths
Subclinical intrauterine infection is associated with premature birth, especially births before 28 weeks' gestation, and with cerebral palsy in children born full term or preterm
Intrauterine growth restriction is associated with increased risks of insulin resistance, obesity, and cardiovascular morbidity in later life
Pre-eclampsia requires maternal-fetal interactions that may converge at the level of oxidative stress
Infection has long been suspected to be important in the pathogenesis of preterm birth. Several landmark studies indicate that preterm birth, especially in early gestation (less than 28 weeks), is associated with pathological signs of infection not accounted for by infection acquired intrapartum.2 These pathological findings often occur in the absence of clinical evidence of infection. A large cohort study recently showed that markers of infection (cytokines and basement membrane components) obtained at 24 and 26 weeks' gestation in asymptomatic women predicted early preterm delivery and pathological evidence of infection at the time of birth (often weeks to months later).3 These findings are consistent with the recognised association of vaginal infections (trichomoniasis and bacterial vaginosis) with preterm birth.4 Unfortunately, treating these vaginal infections in low risk women did not reduce the risk of preterm birth.5 In fact, metronidazole treatment of asymptomatic women infected with Trichomonas vaginalis was associated with increased risk of preterm birth.6 Whether more predictive markers will allow development of antibiotic treatment to prevent preterm birth is currently being tested, but the results of the trichomoniasis trial clearly indicate that association does not prove causality.
There is also evidence emerging that the association of cerebral palsy with premature birth may be heavily influenced by intrauterine infection. Histological evidence of chorioamnionitis is correlated with later cerebral palsy.7 Cerebral injury is felt to be secondary to a fetal inflammatory response. Raised concentrations of cytokine were present in the blood of neonates who subsequently developed cerebral palsy, often without evident maternal infection.8 This is unlikely to be confounded by other components of infant immaturity, as many of these cases were in full term infants. The relation of subclinical infection, preterm birth, and cerebral palsy mandates careful follow up studies to assess the impact of prolonging pregnancies complicated by preterm labour.
Fetal origins of adult diseases
Almost a decade ago, seminal studies by Barker and associates suggested that a fetus that did not achieve its growth potential in utero was at increased risk of cardiovascular disease in later life.9 These studies initially met with scepticism because of numerous obvious confounders.10 However, the same observations have now been made with several datasets, allowing many confounders to be controlled.11,12 Perhaps more convincing are the accumulating experimental findings indicating that nutritional restriction of pregnant animals results in hypertension and insulin resistance in offspring in later life.13,14 The implications for human disease are being investigated. Is it possible that modification of the intrauterine environment can reduce cardiovascular disease in later life? Special attention must be given to mechanisms of human fetal growth restriction that are only rarely nutritional.
New insights in pre-eclampsia
Our understanding of pre-eclampsia progresses rapidly. Attention to the panoply of pathophysiological changes in the disorder (rather than just hypertension) has led to recent insights.15 Although it is evident that reduced perfusion of the placenta leads to pre-eclampsia, it is clear that this is not sufficient to explain the disease. Reduced placental perfusion is a feature of intrauterine growth restriction16 and preterm birth,17 yet these do not give rise to a maternal syndrome. Thus, it seems that the development of pre-eclampsia requires interaction of reduced placental perfusion with maternal factors.
Several maternal factors are recognised, including hyperhomocysteinaemia, obesity, and insulin resistance.15 All of these conditions also predispose to atherosclerosis, and it is increasingly evident that endothelial cells are targets in both pre-eclampsia and atherosclerosis. These similarities raise the possibility that oxidative stress, known to be important in the pathogenesis of atherosclerosis, may also play a role in pre-eclampsia. Oxidative stress may provide the linkage between the placenta and the maternal syndrome, with reduced placental perfusion generating excess reactive oxygen species which interact with maternal factors to alter endothelial function.18
Several lines of evidence support this hypothesis, including increased levels of markers of oxidative stress in blood and tissues of pre-eclamptic women. In a small randomised controlled trial (fewer than 80 women took the drugs throughout pregnancy) administration of pharmacological doses of vitamins C and E from early pregnancy to women at moderate risk significantly reduced the incidence of pre-eclampsia19 and reduced evidence of endothelial dysfunction.15 The results are exciting as this management may be an effective prevention, but past experience with aspirin and calcium indicates that effects evident in small trials may not survive the test of larger trials. More importantly, there is no guarantee of safety for the infant with this small number of patients. Larger trials of prophylaxis with vitamins C and E are about to begin in the United States and Britain.
Changing attitudes toward caesarean delivery
For the past decade efforts to reduce caesarean section rates have intimated that this form of management was a failure of the obstetric team. However, in the past few years several findings may lead to increased rates of caesarean section.
In addition to the question of the safety of vaginal birth after caesarean section,20 recent studies question the safety of vaginal delivery for any woman or infant. A major problem of the ageing female population is urinary and faecal incontinence because of loss of pelvic support and injury to pelvic structures. Increased attention to the consequences of obstetric trauma indicates a previously unsuspected high rate of anal sphincter injury after vaginal births. Anal endosonography indicated that anal sphincter defects appeared in 35% of 150 women after their first delivery.21 Forceps deliveries and episiotomies seem to be the major culprits, but even spontaneous delivery is associated with increased risk. Furthermore, general pelvic support may be compromised by the stretching and neural injury associated with labour and vaginal delivery.22 Thus, the decreased use of routine episiotomy and forceps delivery will reduce perineal trauma, but only the elimination of vaginal birth would prevent this completely.
A study of neonatal outcome in more than 500 000 deliveries indicated that abnormal labour, regardless of management (forceps, vacuum, or caesarean delivery), was associated with a twofold to threefold increased risk of subdural or cerebral haemorrhage compared with spontaneous vaginal delivery or caesarean section without labour.23 Less severe problems, depression of the central nervous system, feeding difficulties, and mechanical ventilation were more common in neonates delivered by caesarean section without labour than in those delivered spontaneously. However, one certain way of preventing abnormal labour is by elective caesarean section before the onset of labour.
Ongoing research with potential impact on clinical care
Antibiotic trials to assess whether markers of intrauterine infection more definitive than vaginal infections will identify women for whom treatment will reduce the incidence of preterm birth
Trials to determine if multiple doses of corticosteroids given to mothers at risk of preterm delivery have beneficial or adverse effects on neonates compared with administration of single dose
Trials of prophylactic antioxidant treatment to prevent morbidity from pre-eclampsia will test efficacy and safety
Registry of all births by caesarean section in 14 US medical centres to assess risks and benefits of caesarean section, including vaginal birth after caesarean section
Should women be made aware of these facts to allow them to choose caesarean section and vaginal birth, and should they have the right to elect caesarean section? It has been argued that this is the right of an informed woman.24 However, women must also be informed that, in addition to the above risks, the best available data indicate that elective caesarean section has a threefold increased death rate compared with spontaneous vaginal birth.25
Elective caesarean delivery for women infected with HIV
Caesarean delivery for women infected with HIV has profound implications for maternal safety, especially in developing countries. A randomised trial26 and a meta-analysis27 suggested that the rate of transmission of HIV from mother to infant was reduced by elective caesarean section. On the basis of these studies, it was recommended that infected women be informed of the greater safety of caesarean section for infants during counselling about delivery. The implications of this are profound since these immunocompromised women are at increased risk of perioperative morbidity.
Others emphasised that this approach must be part of a package: elective caesarean section was unlikely to substantially reduce HIV transmission if infants were subsequently exposed to HIV by breast feeding.28 This point is especially pertinent to developing countries where nursing is mandatory for neonatal survival. The relevance of these recommendations to developed countries has also been questioned. The use of combination therapy for HIV infection has strikingly reduced transmission rates. Letters written in response to the meta-analysis stated that transmission rates were likely to be less than 1% and that, extrapolating from the results of the meta-analysis, at least 100 caesarean sections (and perhaps many more) would be necessary to prevent one transmission.29
Figure 1.
Incidence of early onset neonatal infection with group B streptococci. This decreased 60% between 1993, when preventive antibiotic treatment was advocated, and 1998 in surveillance areas of more than 12 million births. (Adapted from Schrag et al1)
Figure.
JAMES STEVENSON/SPL
Recent findings about the risks of vaginal delivery may lead to increased rates of caesarean section
Footnotes
Competing interests: None declared.
References
- 1.Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med. 1998;339:313–320. doi: 10.1056/NEJM199807303390506. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Mercer BM, Meis PJ, Copper RL, Das A, McNellis D.for the NICHD Maternal Fetal Medicine Units Network. The preterm prediction study: fetal fibronectin testing and spontaneous preterm birth Obstet Gynecol 199687643–648. [DOI] [PubMed] [Google Scholar]
- 4.Cotch MF, Pastorek JG, 2nd, Nugent RP, Hillier SL, Gibbs RS, Martin DH, et al. for the Vaginal Infections and Prematurity Study Group. Trichomonas vaginalis associated with low birth weight and preterm delivery Sex Transm Dis 199724353–360. [DOI] [PubMed] [Google Scholar]
- 5.Carey J, Klebanoff M, Hauth J, Hillier SL, Thorn EA, Ernest JM, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med (in press). [DOI] [PubMed]
- 6.Carey J, Klebanoff M Network ftNM. Metronidazole treatment increased the risk of preterm birth in asymptomatic women with trichomonas. Am J Obstet Gynecol. 2000;182(1):Ss13. [Google Scholar]
- 7.Nelson KB, Ellenberg JH. Predictors of low and very low birth weight and the relation of these to cerebral palsy. JAMA. 1985;254:1473–1479. [PubMed] [Google Scholar]
- 8.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;ii:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 10.Paneth N, Ahmed F, Stein AD. Early nutritional origins of hypertension: a hypothesis still lacking support. J Hypertens. 1996;14(suppl):S121–S129. [PubMed] [Google Scholar]
- 11.Barker DJ. Fetal origins of cardiovascular disease. Ann Med. 1999;31(suppl1):3–6. [PubMed] [Google Scholar]
- 12.Klebanoff MA, Secher NJ, Mednick BR, Schulsinger C. Maternal size at birth and the development of hypertension during pregnancy: a test of the Barker hypothesis. Arch Intern Med. 1999;159:1607–1612. doi: 10.1001/archinte.159.14.1607. [DOI] [PubMed] [Google Scholar]
- 13.Langley-Evans SC, Sherman RC, Welham SJ, Nwagwu MO, Gardner DS, Jackson AA. Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem Soc Trans. 1999;27:88–93. doi: 10.1042/bst0270088. [DOI] [PubMed] [Google Scholar]
- 14.Gluckman PD, Harding JE. Fetal growth retardation: underlying endocrine mechanisms and postnatal consequences. Acta Paediatr. 1997;422(suppl):69–72. doi: 10.1111/j.1651-2227.1997.tb18349.x. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JM. Endothelial dysfunction in preeclampsia [review] Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 16.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 17.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 18.Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet. 1999;354:788–789. doi: 10.1016/S0140-6736(99)80002-6. [DOI] [PubMed] [Google Scholar]
- 19.Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354:810–816. doi: 10.1016/S0140-6736(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 20.Phelan JP. Point/counterpoint: II. The VBAC “con” game. Obstet Gynecol Surv. 1998;53:662–663. doi: 10.1097/00006254-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Sultan AH. Anal incontinence after childbirth. Curr Opin Obstet Gynecol. 1997;9:320–324. [PubMed] [Google Scholar]
- 22.Sultan AH, Monga AK, Stanton SL. The pelvic floor sequelae of childbirth. Br J Hosp Med. 1996;55:575–579. [PubMed] [Google Scholar]
- 23.Towner D, Castro MA, Eby-Wilkens E, Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709–1714. doi: 10.1056/NEJM199912023412301. [DOI] [PubMed] [Google Scholar]
- 24.Paterson-Brown S. Should doctors perform an elective caesarean section on request? Yes, as long as the woman is fully informed. BMJ. 1998;317:462–463. doi: 10.1136/bmj.317.7156.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall MH, Bewley S. Maternal mortality and mode of delivery [letter] Lancet. 1999;354:776. doi: 10.1016/S0140-6736(05)76016-5. [DOI] [PubMed] [Google Scholar]
- 26.European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial [published erratum appears in Lancet 1999;353:1714] Lancet. 1999;353:1035–1039. doi: 10.1016/s0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 27.International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1—a meta-analysis of 15 prospective cohort studies. N Engl J Med. 1999;340:977–987. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 28.Hudson CN. Elective caesarean section for prevention of vertical transmission of HIV-1 infection [commentary] Lancet. 1999;353:1030–1031. doi: 10.1016/s0140-6736(99)00066-5. [DOI] [PubMed] [Google Scholar]
- 29.Stringer JS, Rouse DJ, Goldenberg RL. Prophylactic cesarean delivery for the prevention of perinatal human immunodeficiency virus transmission: the case for restraint. JAMA. 1999;281:1946–1949. doi: 10.1001/jama.281.20.1946. [DOI] [PubMed] [Google Scholar]