Abstract
In this study, cottontail rabbit papillomavirus infection of domestic rabbits was used as an animal model to develop papillomavirus early gene-based vaccines. Groups of rabbits were intracutaneously vaccinated with single papillomavirus early genes E1, E2, E6, and E7 or with a combination of these four genes. Only a fraction of rabbits were protected from subsequent viral challenge when vaccinated with the E1 or E6 gene. Viral tumor growth in those rabbits vaccinated with the E1 or E2 gene was suppressed compared to that in controls. In contrast, seven of nine rabbits vaccinated with the combination of the E1, E2, E6, and E7 genes were completely protected against viral challenge. These data indicated that intracutaneous genetic vaccination with the combination of the E1, E2, E6, and E7 genes can be an effective strategy for immunoprophylaxis of papillomavirus infection.
Human papillomavirus (HPV) infection induces mucosal and/or cutaneous hyperproliferative lesions which persist for months or years. Certain HPV types are linked to the development of skin cancer (21), tumors of the head and neck (8), and anogenital carcinomas (30). Development and testing of papillomavirus vaccines have been conducted extensively in animal models, such as bovine papillomavirus (BPV) infection of cattle (3) and cottontail rabbit papillomavirus (CRPV) infection of domestic rabbits (16). Currently, several strategies have been utilized to develop papillomavirus vaccines. One strategy involves the induction of neutralizing antibodies by immunization with the viral structural protein L1 or L2, particularly, virus-like particles (VLPs) assembled from L1 or L1/L2. Another strategy involves the induction of cell-mediated immunity by papillomavirus early gene/protein-based vaccination. Recently, VLPs containing L1 and chimeric molecules of L1 and early proteins were used as immunogens. This strategy has been applied to elicit concurrent viral neutralizing antibodies and cell-mediated immunity specific for viral early proteins (11).
A number of studies have demonstrated that VLP immunization protected animals against experimental virus challenge in the CRPV-infected rabbit model (2, 6, 13), in the BPV-infected cow model (15), and in the canine oral papillomavirus (COPV)-infected beagle dog model (28). Furthermore, genetic vaccination with CRPV L1 (9, 26) or immunization with CRPV L1 proteins expressed as bacterial fusion proteins (18) also protected rabbits from viral challenge. Protection has also been achieved by immunization with L2 proteins (5, 10, 19). One caveat of these animal model systems is that protection from natural papillomavirus infection has not been determined. In experimental infection models, sites to be infected are vigorously scarified or wounded, resulting in damage to local blood vessels and the release of circulating neutralizing antibodies at the sites of infection. In contrast, natural infection may occur following microtrauma to the epithelium without significant damage to blood vessels and subsequent direct exposure of virus to circulating neutralizing antibodies. Thus, circulating neutralizing antibodies may be unable to protect against natural papillomavirus infection. Protective vaccines targeting virus-infected epithelium via cell-mediated immunity would overcome these potential limitations.
Induction of protective cell-mediated immunity by immunization with papillomavirus early gene/proteins is expected to prevent the establishment of new lesions (immunoprophylaxis) as well as to eliminate existing lesions (immunotherapy). However, early studies disclosed variable results. In the CRPV-infected rabbit model, different papillomavirus early antigens and several methods of antigen delivery have been applied in an attempt to elicit protective antipapillomavirus immunity: (i) immunization with bacterial fusion proteins of CRPV E1 and/or E2 (24); (ii) immunization with recombinant Listeria monocytogenes expressing CRPV E1 (14); (iii) intracutaneous genetic vaccination of rabbits with CRPV E6 (27); and (iv) intramuscular injection of plasmid DNA encoding CRPV E1, E2, E6, or E7 (12). The immunity so induced stimulated papilloma regression in a fraction of vaccinated rabbits (14, 24) and partially protected rabbits from subsequent virus challenge (27). However, none of these studies revealed complete protection. In the BPV-infected cow model, immunization with BPV E6 and E7 proteins delayed papilloma formation, reduced papilloma size, and promoted papilloma regression but also did not lead to complete protection (3, 4). BPV E2 immunizations were ineffective (4). In the present study, we immunized rabbits by gene gun-mediated intracutaneous vaccination with individual CRPV E1, E2, E6, and E7 genes or with a combination of all four genes. We report that vaccination with the combination of CRPV E1, E2, E6, and E7 genes provided strong and complete protection of outbred rabbits against CRPV challenge.
Preparation of DNA vectors and gene gun-mediated immunization.
CRPV E1, E2, E6, and E7 DNA genes were amplified by PCR and cloned into V1Jns expression vector (generous gift of M. A. Liu, Merck & Co, Westpoint, Pa.) at the BglII site individually (20). The resultant recombinant plasmids were referred to as V1JnsE1, V1JnsE2, V1JnsE6, and V1JnsE7, respectively. Plasmid DNA was precipitated onto 1.6-μm-diameter gold microparticles at a ratio of 1 μg of DNA/0.5 mg of gold particles as described by the manufacturer (Bio-Rad, Hercules, Calif.). Outbred New Zealand White rabbits (Covance Research Products Inc., Denver, Pa.) were immunized by gene gun-mediated intracutaneous delivery of DNA/gold particles onto dorsal skin sites at 400 lb/in2.
Protection of rabbits against challenge with cloned CRPV viral DNA.
In our laboratory, we currently use a CRPV stock which was obtained originally from cottontail rabbit viral papillomas and then expanded by using the nude mouse xenograft system. It is possible that several CRPV variants exist in this virus stock (23). In this study, the E1, E2, E6, and E7 genes were cloned from a single clone or a variant. Theoretically, immunity to these early proteins will generate protection against the matching CRPV variant but not to other variants if critical epitopes are antigenically unrelated. Rabbits were thus challenged with the same cloned viral DNA (17) from which the E1, E2, E6, and E7 genes were amplified. In the first experiment, 24 rabbits were divided into six groups (Table 1). For the groups vaccinated with a single gene or vector, rabbits received three immunizations at 3-week intervals: 20 μg of DNA for each of the first and the second immunizations, and 10 μg of DNA for the final booster immunization. For the group vaccinated with the combination of the four genes, rabbits received only two immunizations at 3-week intervals, 10 μg of each E1-, E2-, E6-, and E7-encoding plasmid DNA. Numbers of challenged sites in each rabbit that produced papillomas are shown in Table 1. Papillomas grew at all challenged sites in control rabbits that were vaccinated with V1Jns vector, indicating a high efficiency of papilloma induction by viral DNA (100%) in this experiment. Among the groups vaccinated with single genes, 63% and 44% of challenged sites had no papilloma growth for E1-immunized rabbits and for E6-immunized rabbits, respectively. In contrast, only 13% of challenged sites for E2-vaccinated rabbits and 7% of sites for E7-vaccinated rabbits had no papilloma growth (Table 1). Two of four rabbits vaccinated with the E1 gene were completely protected, and one of four E6-vaccinated rabbits was also completely protected. These results demonstrated that individual E1 or E6 vaccination provided protection in a subset of rabbits following infection with cloned viral DNA. In contrast, E2 and E7 vaccinations did not induce protective immunity. Among four rabbits vaccinated with the combination of E1, E2, E6, and E7 genes, two rabbits had no papillomas, and on the remaining two rabbits, there were only small papillomas which completely regressed within three weeks. Systemic regression of papillomas was not observed on rabbits in the other groups, indicating that vaccination with all four genes induced the strongest protective antiviral immunity in this experiment. It is important to note that the rabbits in the combination vaccine group received two immunizations with a total of 20 μg of DNA of each gene in contrast to the rabbits in the other groups, which received three immunizations with a total of 50 μg of DNA for individual gene vaccination.
TABLE 1.
Papilloma growth in vaccinated and control rabbits
| Groupa | Vaccination dose (μg)
|
No. of sites with papillomasb
|
% Sites with papillomas | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | R1 | R2 | R3 | R4 | R5 | ||
| First expt | |||||||||
| V1JnsE1 | 20 | 20 | 10 | 4 | 0 | 2 | 0 | ND | 37.5 |
| V1JnsE2 | 20 | 20 | 10 | 2 | 4 | 4 | 4 | ND | 87.5 |
| V1JnsE6 | 20 | 20 | 10 | 0 | 4 | 2 | 3 | ND | 56.3 |
| V1JnsE7 | 20 | 20 | 10 | 3 | 4 | 4 | 4 | ND | 93.8 |
| Combined | 10 | 10 | NDd | 2c | 4c | 0 | 0 | ND | 37.5 |
| Vector | 20 | 20 | 10 | 4 | 4 | 4 | 4 | ND | 100 |
| Second expt | |||||||||
| Combined | 10 | 10 | 6 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| Vector | 10 | 10 | 6 | 4 | 4 | 4 | 4 | 4 | 100 |
Rabbits in each group were vaccinated with one type of plasmid DNA, as indicated, except that rabbits in the combined group were vaccinated with four genes, V1JnsE1, V1JnsE2, V1JnsE6, and V1JnsE7.
R1 through R5 denote rabbit numbers in each group. R5 was not used in the first experiment.
Papillomas regressed in 3 weeks.
ND, not done.
In the second experiment (Table 1), 10 rabbits were divided into two groups. One group was vaccinated with the combination of the E1, E2, E6, and E7 genes. The second group received vector DNA only as a control. In addition to the first and second immunizations, rabbits also received a third, booster immunization containing 6 μg of DNA of each gene (Table 1). Papillomas grew at all challenged sites in all five control rabbits. In contrast, all five rabbits vaccinated with the combination of E1, E2, E6, and E7 genes were completely protected (Table 1). These results demonstrated that intracutaneous genetic vaccination with the combination of E1, E2, E6, and E7 protected all rabbits from challenge with cloned viral DNA.
Protection of rabbits against challenge with infectious virions.
To investigate whether these protected rabbits were also resistant to challenge with virus particles, we infected all protected rabbits with infectious virions (two E1-vaccinated, one E6-vaccinated, and nine combination gene-vaccinated rabbits; Table 1). Five rabbits that were previously vaccinated with V1Jns vector were also challenged with virus particles as a control. All control rabbits grew papillomas at virion-challenged sites, and all protected rabbits were resistant to the viral challenge except one rabbit which was vaccinated with the E1 gene alone. Papillomas on the latter rabbit appeared at 4 weeks after virus challenge and persisted without regression until the termination of this experiment (4 months). Vaccination with the combination of E1, E2, E6, and E7 genes therefore completely protected rabbits from virus particle infection.
Papilloma growth in nonprotected and partially protected rabbits.
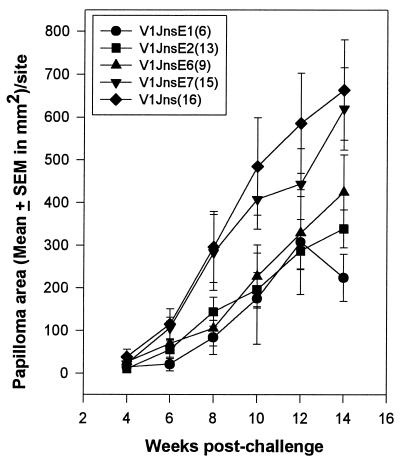
Papilloma size was measured for a total of 14 weeks following viral DNA challenge in those nonprotected and partially protected rabbits. Mean papilloma sizes per site for rabbits vaccinated with the E1 or E2 genes were significantly smaller than those in the vector-injected rabbits (vector group versus E1 group, t test, P < 0.05; vector group versus E2 group, t test, P < 0.05). Papilloma size per site for E6-vaccinated rabbits was somewhat smaller than that for vector-vaccinated control rabbits, but size differences were not statistically significant at 14 weeks postchallenge with virus (Fig. 1). E7 vaccination has little effect on papilloma growth rates (Fig. 1).
FIG. 1.
Papilloma growth for nonprotected and control rabbits in the first experiment. Data represent the means ± standard errors of the means of papilloma areas that were calculated from the total numbers of papillomas, which are indicated in parentheses, for each group. Individual papilloma size was calculated by multiplying length (millimeters) and width (millimeters). Mean papilloma areas were significantly different in the V1Jns group and V1JnsE1 group (t test, P < 0.05) and in the V1Jns group and V1Jns group and V1JnsE2 group (t test, P < 0.05) but not in the V1Jns group and V1JnsE6 group and in the V1Jns group and V1JnsE7 group at 14 weeks postchallenge.
Systemic regression of papillomas was observed only for two rabbits that were vaccinated with the combination of the E1, E2, E6, and E7 genes in the first experiment (rabbits 1 and 2). For one partially protected rabbit in the E2 group (rabbit 1; Table 1), papillomas at one challenged site rapidly regressed and papilloma growth at another site was significantly suppressed (9 mm2 at 14 weeks) but did not regress. One papilloma on one E6-vaccinated rabbit (rabbit 3; Table 1) appeared at week 4, then regressed, and reappeared at week 8. However, this papilloma regressed again, regrew at 14 weeks, and then became persistent. These observations indicated that immunity induced by single gene vaccinations by using the protocol described for the first experiment had an impact on papilloma growth but was insufficient to completely eliminate all viral papillomas. It is possible that additional booster vaccinations with single genes prior to viral DNA challenge may have led to an increased level of protection.
T-cell-mediated immune responses.
In vitro peripheral blood mononuclear cell (PBMC) proliferation assays were conducted to evaluate T-cell-mediated immune responses (12). PBMCs were isolated from ear arterial blood one week after the final booster immunization and stimulated with the specific antigens, CRPV E1, E2, E6 and E7, as histidine-tagged fusion proteins. Control antigens for all rabbits were CRPV L2 histidine-tagged fusion proteins. All fusion proteins were prepared by using recombinant baculoviruses and purified by using nickel columns under denaturing conditions (Invitrogen, San Diego, Calif.) as previously described (12). All four E2-vaccinated rabbits showed strong antigen-specific proliferative responses (Table 2). Three of four rabbits in each of the E1, E6, and E7 groups also showed antigen-specific proliferative responses (Table 2). These data indicated that genetic vaccination with CRPV E1, E2, E6, and E7 genes induced T-cell-mediated immunity. Interestingly, in vitro antigen-specific proliferative responses were strongest in the E2- and E7-vaccinated rabbits despite a lack of protection against viral DNA challenge in these two groups (Table 2). Furthermore, papilloma sizes in rabbits vaccinated with E7 were substantially larger than the papillomas of the E6-vaccinated group (Fig. 1). These data suggest that in vitro antigen-specific proliferative responses do not correlate with in vivo protective immunity.
TABLE 2.
PBMC proliferative responses to in vitro stimulation with antigens
| Rabbit | Vaccine | Timea | PBMC proliferation (cpm × 10−3 ± SEM)b in response to:
|
Stimulation index
|
|||
|---|---|---|---|---|---|---|---|
| No antigenc | Nonspecific antigend | Specific antigene | Nonspecificf | Specificg | |||
| 1 | V1JnsE1 | Day 5 | 0.366 ± 0.027 | 0.441 ± 0.021 | 14.237 ± 1.566 | 1.2 | 38.9 |
| 2 | V1JnsE1 | Day 5 | 0.437 ± 0.012 | 0.640 ± 0.055 | 3.598 ± 0.293 | 1.4 | 8.2 |
| 3 | V1JnsE1 | Day 4 | 0.181 ± 0.040 | 0.234 ± 0.067 | 0.820 ± 0.125 | 1.3 | 4.5 |
| 4 | V1JnsE1 | Day 4 | 2.201 ± 0.312 | 2.145 ± 0.235 | 9.683 ± 0.702 | 0.9 | 4.3 |
| 5 | V1JnsE2 | Day 6 | 0.163 ± 0.014 | 0.355 ± 0.045 | 18.639 ± 2.104 | 2.1 | 114 |
| 6 | V1JnsE2 | Day 6 | 0.270 ± 0.040 | 0.585 ± 0.015 | 5.319 ± 0.404 | 2.1 | 19.7 |
| 7 | V1JnsE2 | Day 5 | 0.239 ± 0.022 | 0.324 ± 0.031 | 32.785 ± 2.639 | 1.3 | 137.1 |
| 8 | V1JnsE2 | Day 6 | 0.155 ± 0.013 | 0.402 ± 0.059 | 1.040 ± 2.500 | 2.5 | 6.7 |
| 9 | V1JnsE6 | Day 6 | 0.179 ± 0.019 | 0.567 ± 0.040 | 1.360 ± 0.078 | 3.1 | 7.5 |
| 10 | V1JnsE6 | Day 5 | 0.245 ± 0.047 | 0.448 ± 0.059 | 1.841 ± 0.000 | 1.8 | 7.5 |
| 11 | V1JnsE6 | Day 5 | 0.160 ± 0.017 | 0.239 ± 0.010 | 0.390 ± 0.050 | 1.4 | 2.4 |
| 12 | V1JnsE6 | Day 4 | 0.775 ± 0.069 | 1.092 ± 0.176 | 6.861 ± 0.218 | 1.4 | 8.8 |
| 13 | V1JnsE7 | Day 5 | 0.336 ± 0.037 | 0.600 ± 0.091 | 5.600 ± 0.000 | 1.7 | 16.6 |
| 14 | V1JnsE7 | Day 4 | 0.482 ± 0.057 | 1.959 ± 0.399 | 6.318 ± 1.380 | 4.0 | 13.1 |
| 15 | V1JnsE7 | Day 5 | 0.307 ± 0.013 | 0.352 ± 0.022 | 0.465 ± 0.135 | 1.1 | 1.5 |
| 16 | V1JnsE7 | Day 5 | 2.450 ± 0.310 | 5.136 ± 0.313 | 17.029 ± 3.159 | 2.0 | 6.9 |
Day indicated is day with highest value of response among day 4 to day 6.
Of triplicate wells.
Without addition of any antigen.
Addition of CRPV L2 protein.
Antigen corresponded to vaccinated antigen.
Ratio of counts per minute in response to nonspecific antigen to counts per minute in response to no antigen.
Ratio of counts per minute in response to specific antigen to counts per minute in response to no antigen.
Humoral immune responses.
Rabbit serum was collected before vaccination and after the first, second, and third vaccinations (Fig. 1). Western blot analysis did not detect specific anti-E1, -E2, -E6, or -E7 antibodies in those vaccinated rabbits (data not shown).
Papillomavirus-induced lesions are maintained by hyperproliferation of virus-infected, undifferentiated cells in the basal and suprabasal layers of the epithelium. Immune-mediated cure of papillomavirus-induced lesions can be successful only when the latter cells are eliminated by effector cells, such as cytotoxic T lymphocytes. A potential vaccine candidate gene/protein(s) therefore must be expressed in the infected, undifferentiated cells and, in addition, must be immunogenic. Experimental evidence for the viral proteins E1, E2, E6, and E7 in basal and suprabasal layers of infected epithelium has been indirectly established by RNA-RNA in situ hybridization studies (1, 25, 29). These four genes therefore are potential candidate vaccines. In the present study, E1 was the most effective of the four early genes for protection by vaccination of animals against viral challenge. These results are consistent with the findings of Jensen and coworkers (14). Immunization with the E6 gene only provided partial protection. A similar result was obtained by other investigators (27). Unexpectedly, T-cell-mediated immune responses for the E2 immunization group were the strongest of any of the vaccinated groups as determined by in vitro PBMC proliferation assays. However, none of the E2-vaccinated rabbits were completely protected from viral challenge, although viral tumor growth was significantly suppressed. E7 vaccination also induced cell-mediated immunity but neither induced protection of rabbits from viral challenge nor significantly suppressed viral tumor growth. In contrast, vaccination with the combination of the E1, E2, E6, and E7 genes provided complete protection of rabbits against subsequent viral challenge. It is possible that the expression levels of the E1, E2, E6, and E7 proteins are low in the basal and suprabasal layers of the infected epithelium. Low expression would result in a low density of virus-derived peptide/major histocompatibility complex (MHC) class I complexes on the cell surface. Efficient elimination of these virus-infected cells may be possible only if there is a pool of CD8+ T lymphocytes specific for E1, E2, E6, and E7 in certain MHC genetic backgrounds. In this study, although the immunity induced by E7 vaccination alone did not affect viral infection, E7 vaccination may still play a role in protection and/or suppression of viral tumor growth in some rabbits with certain MHC genotypes which can efficiently process and present E7 antigen to T cells. E7 vaccination may also play a role in the prevention of cervical cancer.
We have also immunized rabbits by (i) subcutaneous injection of recombinant baculovirus-expressed E1 and E2 proteins (unpublished observations), (ii) subcutaneous injection of recombinant retroviruses expressing E1 or E2 proteins (unpublished observations), and (iii) intramuscular injection of plasmid DNA encoding E1, E2, E6, or E7, respectively (12). Cell-mediated immunity was detectable in these vaccinated rabbits, but none of the above-listed vaccination strategies provided protection of rabbits against subsequent viral challenge. Based on our observations, gene gun-mediated intracutaneous genetic immunization is the most effective strategy for a papillomavirus early-gene-based vaccine. It has been demonstrated that gene gun-mediated intracutaneous inoculation of naked plasmid DNA directly delivered the DNA into skin-derived dendritic cells in vivo (7, 22). The proteins encoded by the plasmid DNA were expressed in the cytoplasm of dendritic cells in the draining lymph nodes. These procedures produced potent, antigen-specific cytotoxic T-lymphocyte-mediated protective tumor immunity (7).
In vivo delivery of an oncogene-expressing plasmid DNA always raises safety concerns. Papillomavirus E6 and E7 are multifunctional oncogenes based on in vitro assays. Gene gun-mediated delivery can directly introduce E6- and E7-expressing plasmid into cells. Whether they can transform the in vivo transfected cells, such as epithelial cells and Langerhans cells, is still an important issue to be studied. Genetic engineering of E6 and E7 genes to inactivate their transforming functions may provide a solution to this potential problem.
Our results provide two important considerations for HPV vaccine development. First, vaccination with a combination of several early genes was an effective strategy for achieving complete protection in outbred populations. Second, gene gun-mediated intracutaneous genetic vaccination induced protective antipapillomavirus immunity. This strategy has great potential for therapeutic approaches to the treatment of HPV infections.
Acknowledgments
This study was supported by grant RO1 CA47622 and the Jake Gittlen Memorial Golf Tournament. R. Han is the recipient of the 1996–1998 American Social Health Association/Merck Foundation Research Fellowship in sexually transmitted diseases.
REFERENCES
- 1.Beyer-Finkler E, Stoler M H, Girardi F, Pfister H. Cell differentiation-related gene expression of human papillomavirus 33. Med Microbiol Immunol. 1990;179:185–192. doi: 10.1007/BF00195249. [DOI] [PubMed] [Google Scholar]
- 2.Breitburd F, Kirnbauer R, Hubbert N L, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller J T, Lowy D R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campo M S. Vaccination against papillomavirus in cattle. Clin Dermatol. 1997;15:275–283. doi: 10.1016/s0738-081x(96)00165-4. [DOI] [PubMed] [Google Scholar]
- 4.Campo M S, Grindlay G J, O’Neil B W, Chandrachud L M, McGarvie G M, Jarrett W F H. Prophylactic and therapeutic vaccination against a mucosal papillomavirus. J Gen Virol. 1993;74:945–953. doi: 10.1099/0022-1317-74-6-945. [DOI] [PubMed] [Google Scholar]
- 5.Christensen N D, Kreider J W, Kan N C, DiAngelo S L. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181:572–579. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- 6.Christensen N D, Reed C A, Cladel N M, Han R, Kreider J W. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol. 1996;70:960–964. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 8.de Villiers M-E. Papillomavirus and HPV typing. Clin Dermatol. 1997;15:199–206. doi: 10.1016/s0738-081x(96)00164-2. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly J J, Martinez D, Jansen K U, Ellis R W, Montgomery D L, Liu M A. Protection against papillomavirus with a polynucleotide vaccine. J Infect Dis. 1996;173:314–320. doi: 10.1093/infdis/173.2.314. [DOI] [PubMed] [Google Scholar]
- 10.Gaukroger J, Chandrachud L M, O’Neil B W, Grindlay G J, Knowles G, Campo M S. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the protection of virus-neutralizing antibodies. J Gen Virol. 1996;77:1577–1583. doi: 10.1099/0022-1317-77-7-1577. [DOI] [PubMed] [Google Scholar]
- 11.Greenstone H L, Nieland J D, de Visser K E, De Bruijn M L H, Kirnbauer R, Roden R B S, Lowy D R, Kast W M, Schiller J T. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han R, Cladel N M, Reed C, Christensen N D. Intramuscular injection of plasmid DNA encoding cottontail rabbit papillomavirus E1, E2, E6 and E7 induces cell-mediated but not humoral immune responses in rabbits. Vaccine. 1999;17:1558–1566. doi: 10.1016/s0264-410x(98)00356-9. [DOI] [PubMed] [Google Scholar]
- 13.Jansen K U, Rosolowsky M, Schultz L D, Markus H Z, Cook J C, Donnelly J J, Martinez D, Ellis R W, Shaw A R. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine. 1995;13:1509–1514. doi: 10.1016/0264-410x(95)00103-8. [DOI] [PubMed] [Google Scholar]
- 14.Jensen E R, Selvakumar R, Shen H, Ahmed R, Wettstein F O, Miller J F. Recombinant Listeria monocytogenes vaccination eliminates papillomavirus-induced tumors and prevents papilloma formation from viral DNA. J Virol. 1997;71:8467–8474. doi: 10.1128/jvi.71.11.8467-8474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirnbauer R, Chandrachud L M, O’Neil B W, Wagner E R, Grindlay G J, Armstrong A, McGarvie G M, Schiller J T, Lowy D R, Campo M S. Virus-like particles of bovine papillomavirus type-4 in prophylactic and therapeutic immunization. Virology. 1996;219:37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- 16.Kreider J W, Bartlett G L. Shope rabbit papilloma-carcinoma complex—a model system of HPV infections. Clin Dermatol. 1985;3:20–26. doi: 10.1016/0738-081x(85)90046-x. [DOI] [PubMed] [Google Scholar]
- 17.Kreider J W, Cladel N M, Patrick S D, Welsh P A, DiAngelo S L, Bower J M, Christensen N D. High efficiency induction of papillomas in vivo using recombinant cottontail rabbit papillomavirus DNA. J Virol Methods. 1995;55:233–244. doi: 10.1016/0166-0934(95)00062-y. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y-L, Borenstein L A, Ahmed R, Wettstein F O. Cottontail rabbit papillomavirus L1 protein-based vaccines: protection is achieved only with a full-length, nondenatured product. J Virol. 1993;67:4154–4162. doi: 10.1128/jvi.67.7.4154-4162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Y L, Borenstein L A, Selvakumar R, Ahmed R, Wettstein F O. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992;187:612–619. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- 20.Montgomery D L, Shiver J W, Leander K R, Perry H C, Friedman A, Martinez D, Ulmer J B, Donnelly J J, Liu M A. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993;12:777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 21.Orth G. Epidermodysplasia verruciformis. In: Salzman N P, Howley P M, editors. The Papovaviridae. 2. The papillomaviruses. New York, N.Y: Plenum Press; 1987. pp. 199–243. [Google Scholar]
- 22.Porgador A, Irvine K R, Iwasaki A, Barber B H, Restifo N P, Germain R N. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salmon J, Ramoz N, Cassonnet P, Orth G, Breitburd F. A cottontail rabbit papillomavirus strain (CRPVb) with strikingly divergent E6 and E7 oncoproteins: an insight in the evolution of papillomaviruses. Virology. 1997;235:228–234. doi: 10.1006/viro.1997.8680. [DOI] [PubMed] [Google Scholar]
- 24.Selvakumar R, Borenstein L A, Lin Y-L, Ahmed R, Wettstein F O. Immunization with nonstructural proteins E1 and E2 of cottontail rabbit papillomavirus stimulates regression of virus-induced papillomas. J Virol. 1995;69:602–605. doi: 10.1128/jvi.69.1.602-605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoler M H, Rhodes C R, Whitbeck A, Wolinsky S M, Chow L T, Broker T R. Human papillomavirus 16 and 18 gene expression in cervical neoplasia. Hum Pathol. 1992;23:117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- 26.Sundaram P, Tigelaar R E, Brandsma J L. Intracutaneous vaccination of rabbits with the cottontail rabbit papillomavirus (CRPV) L1 gene products against virus challenge. Vaccine. 1997;15:664–671. doi: 10.1016/s0264-410x(96)00237-x. [DOI] [PubMed] [Google Scholar]
- 27.Sundaram P, Tigelaar R E, Xiao W, Brandsma J L. Intracutaneous vaccination of rabbits with the E6 gene of cottontail rabbit papillomavirus provides partial protection against virus challenge. Vaccine. 1998;16:613–623. doi: 10.1016/s0264-410x(97)84510-0. [DOI] [PubMed] [Google Scholar]
- 28.Suzich J A, Ghim S-J, Palmer-Hill F J, White W I, Tamura J K, Bell J A, Newsome J A, Jenson A B, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeltner R, Borenstein L A, Wettstein F O, Iftner T. Changes in RNA expression pattern during the malignant progression of cottontail rabbit papillomavirus-induced tumors in rabbits. J Virol. 1994;68:3620–3630. doi: 10.1128/jvi.68.6.3620-3630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.zur Hausen H, de Villiers E-M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]